User login
Gaps persist in awareness, treatment of high LDL cholesterol
TOPLINE:
The prevalence of elevated LDL cholesterol (LDL-C) has declined over the past 2 decades, but 1 in 17 Americans still have a level of 160-189 mg/dL, and 1 in 48 have a level of at least 190 mg/dL, new research shows. Among people with the higher LDL-C level, one in four are both unaware and untreated, the authors report.
METHODOLOGY:
- Using data on 23,667 adult participants in the National Health and Nutrition Examination Survey conducted from 1999 to 2020, researchers identified 1,851 (7.8%) with an LDL-C level of 160-189 mg/dL and 669 (2.8%) with an LDL-C level of at least 190 mg/dL.
- Individuals were classified as “unaware” if they had never had their LDL-C measured or had never been informed of having elevated LDL-C and as “untreated” if their medications didn’t include a statin, ezetimibe, a bile acid sequestrant, or a proprotein convertase subtilisin/kexin type 9 inhibitor.
- The authors compared the prevalence of “unaware” and “untreated” by age, sex, race and ethnicity, educational attainment, poverty index, and insurance status.
TAKEAWAY:
- During the study period, the age-adjusted prevalence of an LDL-C level of 160-189 mg/dL declined from 12.4% (95% confidence interval, 10.0%-15.3%), representing 21.5 million U.S. adults, to 6.1% (95% CI, 4.8%-7.6%), representing 14.0 million adults (P < .001).
- The age-adjusted prevalence of an LDL-C level of at least 190 mg/dL declined from 3.8% (95% CI, 2.8%-5.2%), representing 6.6 million adults, to 2.1% (95% CI, 1.4%-3.0%), representing 4.8 million adults (P = .001).
- Among those with an LDL-C level of 160-189 mg/dL, the proportion of who were unaware and untreated declined from 52.1% to 42.7%, and among those with an LDL-C level of at least 190 mg/dL, it declined from 40.8% to 26.8%.
- Being unaware and untreated was more common in younger adults, men, racial and ethnic minority groups, those with lower educational attainment, those with lower income, and those without health insurance.
IN PRACTICE:
The lack of awareness and treatment of high LDL-C uncovered by the study “may be due to difficulties accessing primary care, low rates of screening in primary care, lack of consensus on screening recommendations, insufficient emphasis on LDL-C as a quality measure, and hesitance to treat asymptomatic individuals,” the authors concluded.
SOURCE:
The research was led by Ahmed Sayed, MBBS, faculty of medicine, Ain Shams University, Cairo, Egypt. It was published online in JAMA Cardiology.
LIMITATIONS:
The analysis was limited by a small number of participants with LDL-C levels of at least 190 mg/dL, possible nonresponse bias, and dependency on participant recall of whether LDL-C was previously measured. The inclusion of pregnant women may have influenced LDL-C levels.
DISCLOSURES:
Dr. Sayed has no relevant conflict of interest. The disclosures of the other authors are listed in the original publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of elevated LDL cholesterol (LDL-C) has declined over the past 2 decades, but 1 in 17 Americans still have a level of 160-189 mg/dL, and 1 in 48 have a level of at least 190 mg/dL, new research shows. Among people with the higher LDL-C level, one in four are both unaware and untreated, the authors report.
METHODOLOGY:
- Using data on 23,667 adult participants in the National Health and Nutrition Examination Survey conducted from 1999 to 2020, researchers identified 1,851 (7.8%) with an LDL-C level of 160-189 mg/dL and 669 (2.8%) with an LDL-C level of at least 190 mg/dL.
- Individuals were classified as “unaware” if they had never had their LDL-C measured or had never been informed of having elevated LDL-C and as “untreated” if their medications didn’t include a statin, ezetimibe, a bile acid sequestrant, or a proprotein convertase subtilisin/kexin type 9 inhibitor.
- The authors compared the prevalence of “unaware” and “untreated” by age, sex, race and ethnicity, educational attainment, poverty index, and insurance status.
TAKEAWAY:
- During the study period, the age-adjusted prevalence of an LDL-C level of 160-189 mg/dL declined from 12.4% (95% confidence interval, 10.0%-15.3%), representing 21.5 million U.S. adults, to 6.1% (95% CI, 4.8%-7.6%), representing 14.0 million adults (P < .001).
- The age-adjusted prevalence of an LDL-C level of at least 190 mg/dL declined from 3.8% (95% CI, 2.8%-5.2%), representing 6.6 million adults, to 2.1% (95% CI, 1.4%-3.0%), representing 4.8 million adults (P = .001).
- Among those with an LDL-C level of 160-189 mg/dL, the proportion of who were unaware and untreated declined from 52.1% to 42.7%, and among those with an LDL-C level of at least 190 mg/dL, it declined from 40.8% to 26.8%.
- Being unaware and untreated was more common in younger adults, men, racial and ethnic minority groups, those with lower educational attainment, those with lower income, and those without health insurance.
IN PRACTICE:
The lack of awareness and treatment of high LDL-C uncovered by the study “may be due to difficulties accessing primary care, low rates of screening in primary care, lack of consensus on screening recommendations, insufficient emphasis on LDL-C as a quality measure, and hesitance to treat asymptomatic individuals,” the authors concluded.
SOURCE:
The research was led by Ahmed Sayed, MBBS, faculty of medicine, Ain Shams University, Cairo, Egypt. It was published online in JAMA Cardiology.
LIMITATIONS:
The analysis was limited by a small number of participants with LDL-C levels of at least 190 mg/dL, possible nonresponse bias, and dependency on participant recall of whether LDL-C was previously measured. The inclusion of pregnant women may have influenced LDL-C levels.
DISCLOSURES:
Dr. Sayed has no relevant conflict of interest. The disclosures of the other authors are listed in the original publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The prevalence of elevated LDL cholesterol (LDL-C) has declined over the past 2 decades, but 1 in 17 Americans still have a level of 160-189 mg/dL, and 1 in 48 have a level of at least 190 mg/dL, new research shows. Among people with the higher LDL-C level, one in four are both unaware and untreated, the authors report.
METHODOLOGY:
- Using data on 23,667 adult participants in the National Health and Nutrition Examination Survey conducted from 1999 to 2020, researchers identified 1,851 (7.8%) with an LDL-C level of 160-189 mg/dL and 669 (2.8%) with an LDL-C level of at least 190 mg/dL.
- Individuals were classified as “unaware” if they had never had their LDL-C measured or had never been informed of having elevated LDL-C and as “untreated” if their medications didn’t include a statin, ezetimibe, a bile acid sequestrant, or a proprotein convertase subtilisin/kexin type 9 inhibitor.
- The authors compared the prevalence of “unaware” and “untreated” by age, sex, race and ethnicity, educational attainment, poverty index, and insurance status.
TAKEAWAY:
- During the study period, the age-adjusted prevalence of an LDL-C level of 160-189 mg/dL declined from 12.4% (95% confidence interval, 10.0%-15.3%), representing 21.5 million U.S. adults, to 6.1% (95% CI, 4.8%-7.6%), representing 14.0 million adults (P < .001).
- The age-adjusted prevalence of an LDL-C level of at least 190 mg/dL declined from 3.8% (95% CI, 2.8%-5.2%), representing 6.6 million adults, to 2.1% (95% CI, 1.4%-3.0%), representing 4.8 million adults (P = .001).
- Among those with an LDL-C level of 160-189 mg/dL, the proportion of who were unaware and untreated declined from 52.1% to 42.7%, and among those with an LDL-C level of at least 190 mg/dL, it declined from 40.8% to 26.8%.
- Being unaware and untreated was more common in younger adults, men, racial and ethnic minority groups, those with lower educational attainment, those with lower income, and those without health insurance.
IN PRACTICE:
The lack of awareness and treatment of high LDL-C uncovered by the study “may be due to difficulties accessing primary care, low rates of screening in primary care, lack of consensus on screening recommendations, insufficient emphasis on LDL-C as a quality measure, and hesitance to treat asymptomatic individuals,” the authors concluded.
SOURCE:
The research was led by Ahmed Sayed, MBBS, faculty of medicine, Ain Shams University, Cairo, Egypt. It was published online in JAMA Cardiology.
LIMITATIONS:
The analysis was limited by a small number of participants with LDL-C levels of at least 190 mg/dL, possible nonresponse bias, and dependency on participant recall of whether LDL-C was previously measured. The inclusion of pregnant women may have influenced LDL-C levels.
DISCLOSURES:
Dr. Sayed has no relevant conflict of interest. The disclosures of the other authors are listed in the original publication.
A version of this article first appeared on Medscape.com.
AF tied to 45% increase in mild cognitive impairment
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- From over 4.3 million people in the UK primary electronic health record (EHR) database, researchers identified 233,833 (5.4%) with AF (mean age, 74.2 years) and randomly selected one age- and sex-matched control person without AF for each AF case patient.
- The primary outcome was incidence of mild cognitive impairment (MCI).
- The authors adjusted for age, sex, year at study entry, socioeconomic status, smoking, and a number of comorbid conditions.
- During a median of 5.3 years of follow-up, there were 4,269 incident MCI cases among both AF and non-AF patients.
TAKEAWAY:
- Individuals with AF had a higher risk of MCI than that of those without AF (adjusted hazard ratio [aHR], 1.45; 95% confidence interval [CI], 1.35-1.56).
- Besides AF, older age (risk ratio [RR], 1.08) and history of depression (RR, 1.44) were associated with greater risk of MCI, as were female sex, greater socioeconomic deprivation, stroke, and multimorbidity, including, for example, diabetes, hypercholesterolemia, and peripheral artery disease (all P < .001).
- Individuals with AF who received oral anticoagulants or amiodarone were not at increased risk of MCI, as was the case for those treated with digoxin.
- Individuals with AF and MCI were at greater risk of dementia (aHR, 1.25; 95% CI, 1.09-1.42). Sex, smoking, chronic kidney disease, and multi-comorbidity were among factors linked to elevated dementia risk.
IN PRACTICE:
The findings emphasize the association of multi-comorbidity and cardiovascular risk factors with development of MCI and progression to dementia in AF patients, the authors wrote. They noted that the data suggest combining anticoagulation and symptom and comorbidity management may prevent cognitive deterioration.
SOURCE:
The study was conducted by Sheng-Chia Chung, PhD, Institute of Health informatics Research, University College London, and colleagues. It was published online Oct. 25, 2023, as a research letter in the Journal of the American College of Cardiology (JACC): Advances.
LIMITATIONS:
The EHR dataset may have lacked granularity and detail, and some risk factors or comorbidities may not have been measured. While those with AF receiving digoxin or amiodarone treatment had no higher risk of MCI than their non-AF peers, the study’s observational design and very wide confidence intervals for these subgroups prevent making solid inferences about causality or a potential protective role of these drugs.
DISCLOSURES:
Dr. Chung is supported by the National Institute of Health and Care Research (NIHR) Author Rui Providencia, MD, PhD, of the Institute of Health informatics Research, University College London, is supported by the University College London British Heart Foundation and NIHR. All other authors report no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- From over 4.3 million people in the UK primary electronic health record (EHR) database, researchers identified 233,833 (5.4%) with AF (mean age, 74.2 years) and randomly selected one age- and sex-matched control person without AF for each AF case patient.
- The primary outcome was incidence of mild cognitive impairment (MCI).
- The authors adjusted for age, sex, year at study entry, socioeconomic status, smoking, and a number of comorbid conditions.
- During a median of 5.3 years of follow-up, there were 4,269 incident MCI cases among both AF and non-AF patients.
TAKEAWAY:
- Individuals with AF had a higher risk of MCI than that of those without AF (adjusted hazard ratio [aHR], 1.45; 95% confidence interval [CI], 1.35-1.56).
- Besides AF, older age (risk ratio [RR], 1.08) and history of depression (RR, 1.44) were associated with greater risk of MCI, as were female sex, greater socioeconomic deprivation, stroke, and multimorbidity, including, for example, diabetes, hypercholesterolemia, and peripheral artery disease (all P < .001).
- Individuals with AF who received oral anticoagulants or amiodarone were not at increased risk of MCI, as was the case for those treated with digoxin.
- Individuals with AF and MCI were at greater risk of dementia (aHR, 1.25; 95% CI, 1.09-1.42). Sex, smoking, chronic kidney disease, and multi-comorbidity were among factors linked to elevated dementia risk.
IN PRACTICE:
The findings emphasize the association of multi-comorbidity and cardiovascular risk factors with development of MCI and progression to dementia in AF patients, the authors wrote. They noted that the data suggest combining anticoagulation and symptom and comorbidity management may prevent cognitive deterioration.
SOURCE:
The study was conducted by Sheng-Chia Chung, PhD, Institute of Health informatics Research, University College London, and colleagues. It was published online Oct. 25, 2023, as a research letter in the Journal of the American College of Cardiology (JACC): Advances.
LIMITATIONS:
The EHR dataset may have lacked granularity and detail, and some risk factors or comorbidities may not have been measured. While those with AF receiving digoxin or amiodarone treatment had no higher risk of MCI than their non-AF peers, the study’s observational design and very wide confidence intervals for these subgroups prevent making solid inferences about causality or a potential protective role of these drugs.
DISCLOSURES:
Dr. Chung is supported by the National Institute of Health and Care Research (NIHR) Author Rui Providencia, MD, PhD, of the Institute of Health informatics Research, University College London, is supported by the University College London British Heart Foundation and NIHR. All other authors report no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- From over 4.3 million people in the UK primary electronic health record (EHR) database, researchers identified 233,833 (5.4%) with AF (mean age, 74.2 years) and randomly selected one age- and sex-matched control person without AF for each AF case patient.
- The primary outcome was incidence of mild cognitive impairment (MCI).
- The authors adjusted for age, sex, year at study entry, socioeconomic status, smoking, and a number of comorbid conditions.
- During a median of 5.3 years of follow-up, there were 4,269 incident MCI cases among both AF and non-AF patients.
TAKEAWAY:
- Individuals with AF had a higher risk of MCI than that of those without AF (adjusted hazard ratio [aHR], 1.45; 95% confidence interval [CI], 1.35-1.56).
- Besides AF, older age (risk ratio [RR], 1.08) and history of depression (RR, 1.44) were associated with greater risk of MCI, as were female sex, greater socioeconomic deprivation, stroke, and multimorbidity, including, for example, diabetes, hypercholesterolemia, and peripheral artery disease (all P < .001).
- Individuals with AF who received oral anticoagulants or amiodarone were not at increased risk of MCI, as was the case for those treated with digoxin.
- Individuals with AF and MCI were at greater risk of dementia (aHR, 1.25; 95% CI, 1.09-1.42). Sex, smoking, chronic kidney disease, and multi-comorbidity were among factors linked to elevated dementia risk.
IN PRACTICE:
The findings emphasize the association of multi-comorbidity and cardiovascular risk factors with development of MCI and progression to dementia in AF patients, the authors wrote. They noted that the data suggest combining anticoagulation and symptom and comorbidity management may prevent cognitive deterioration.
SOURCE:
The study was conducted by Sheng-Chia Chung, PhD, Institute of Health informatics Research, University College London, and colleagues. It was published online Oct. 25, 2023, as a research letter in the Journal of the American College of Cardiology (JACC): Advances.
LIMITATIONS:
The EHR dataset may have lacked granularity and detail, and some risk factors or comorbidities may not have been measured. While those with AF receiving digoxin or amiodarone treatment had no higher risk of MCI than their non-AF peers, the study’s observational design and very wide confidence intervals for these subgroups prevent making solid inferences about causality or a potential protective role of these drugs.
DISCLOSURES:
Dr. Chung is supported by the National Institute of Health and Care Research (NIHR) Author Rui Providencia, MD, PhD, of the Institute of Health informatics Research, University College London, is supported by the University College London British Heart Foundation and NIHR. All other authors report no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Nightmare on CIL Street: A Simulation Series to Increase Confidence and Skill in Responding to Clinical Emergencies
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
The Central Texas Veteran’s Health Care System (CTVHCS) in Temple, Texas, is a 189-bed teaching hospital. CTVHCS opened the Center for Innovation and Learning (CIL) in 2022. The CIL has about 279 m2 of simulation space that includes high- and low-fidelity simulation equipment and multiple laboratories, which can be used to simulate inpatient and outpatient settings. The CIL high-fidelity manikins and environment allow learners to be immersed in the simulation for maximum realism. Computer and video systems provide clear viewing of training, which allows for more in-depth debriefing and learning. CIL simulation training is used by CTVHCS staff, medical residents, and medical and physician assistant students.
The utility of technology in medical education is rapidly evolving. As noted in many studies, simulation creates an environment that can imitate real patients in the format of a lifelike manikin, anatomic regions stations, clinical tasks, and many real-life circumstances.1 Task trainers for procedure simulation have been widely used and studied. A 2020 study noted that simulation training is effective for developing procedural skills in surgery and prevents the decay of surgical skills.2
In reviewing health care education curriculums, we noted that most of the rapid response situations are learned through active patient experiences. Rapid responses are managed by the intensive care unit and primary care teams during the day but at night are run primarily by the postgraduate year 2 (PGY2) night resident and intern. Knowing these logistics and current studies, we decided to build a rapid response simulation curriculum to improve preparedness for PGY1 residents, medical students, and physician assistant students.
Curriculum Planning
Planning the simulation curriculum began with the CTVHCS internal medicine chief resident and registered nurse (RN) educator. CTVHCS data were reviewed to identify the 3 most common rapid response calls from the past 3 years; research on the most common systems affected by rapid responses also was evaluated.
A 2019 study by Lyons and colleagues evaluated 402,023 rapid response activations across 360 hospitals and found that respiratory scenarios made up 38% and cardiac scenarios made up 37%.3 In addition, the CTVHCS has limited support in stroke neurology. Therefore, the internal medicine chief resident and RN educator decided to run 3 evolving rapid response scenarios per session that included cardiac, respiratory, and neurological scenarios. Capabilities and limitations of different high-fidelity manikins were discussed to identify and use the most appropriate simulator for each situation. Objectives that met both general medicine and site-specific education were discussed, and the program was formulated.
Program Description
Nightmare on CIL Street is a simulation-based program designed for new internal medicine residents and students to encounter difficult situations (late at night, on call, or when resources are limited; ie, weekends/holidays) in a controlled simulation environment. During the simulation, learners will be unable to transfer the patient and no additional help is available. Each learner must determine a differential diagnosis and make appropriate medical interventions with only the assistance of a nurse. Scenarios are derived from common rapid response team calls and low-volume/high-impact situations where clinical decisions must be made quickly to ensure the best patient outcomes. High-fidelity manikins that have abilities to respond to questions, simulate breathing, reproduce pathological heart and breath sounds and more are used to create a realistic patient environment.
This program aligns with 2 national Veterans Health Administration priorities: (1) connect veterans to the soonest and best care; and (2) accelerate the Veterans Health Administration journey to be a high-reliability organization (sensitivity to operations, preoccupation with failure, commitment to resilience, and deference to expertise). Nightmare on CIL Street has 3 clinical episodes: 2 cardiac (A Tell-Tale Heart), respiratory (Don’t Breathe), and neurologic (Brain Scan). Additional clinical episodes will be added based on learner feedback and assessed need.
Each simulation event encompassed all 3 episodes that an individual or a team of 2 learners rotate through in a round-robin fashion. The overarching theme for each episode was a rapid response team call with minimal resources that the learner would have to provide care and stabilization. A literature search for rapid response team training programs found few results, but the literature assisted with providing a foundation for Nightmare on CIL Street.4,5 The goal was to completely envelop the learners in a nightmare scenario that required a solution.
After the safety brief and predata collection, learners received a phone call with minimal information about a patient in need of care. The learners responded to the requested area and provided treatment to the emergency over 25 minutes with the bedside nurse (who is an embedded participant). At the conclusion of the scenario, a physician subject matter expert who has been observing, provided a personalized 10-minute debriefing to the learner, which presented specific learning points and opportunities for the learner’s educational development. After the debriefing, learners returned to a conference room and awaited the next call. After all learners completed the 3 episodes, a group debriefing was conducted using the gather, analyze, summarize debriefing framework. The debriefing begins with an open-ended forum for learners to express their thoughts. Then, each scenario is discussed and broken down by key learning objectives. Starting with cardiac and ending with neurology, the logistics of the cases are discussed based on the trajectory of the learners during the scenarios. Each objective is discussed, and learners are allowed to ask questions before moving to the next scenario. After the debriefing, postevent data were gathered.
Objectives
The program objective was to educate residents and students on common rapid response scenarios. We devised each scenario as an evolving simulation where various interventions would improve or worsen vital signs and symptoms. Each scenario had an end goal: cardioversion (cardiac), intubation (respiratory), and transfer (neurologic). Objectives were tailored to the trainees present during the specific simulation (Table).
IMPLEMENTATION
The initial run of the simulation curriculum was implemented on February 22, 2023, and ended on May 17, 2023, with 5 events. Participants included internal medicine PGY1 residents, third-year medical students, and fourth-year physician assistant students. Internal medicine residents ran each scenario with a subject matter expert monitoring; the undergraduate medical trainees partnered with another student. Students were pulled from their ward rotations to attend the simulation, and residents were pulled from electives and wards. Each trainee was able to experience each planned scenario. They were then briefed, participated in each scenario, and ended with a debriefing, discussing each case in detail. Two subject matter experts were always available, and occasionally 4 were present to provide additional knowledge transfer to learners. These included board-certified physicians in internal medicine and pulmonary critical care. Most scenarios were conducted on Wednesday afternoon or Thursday.
The CIL provided 6 staff minimum for every event. The staff controlled the manikins and acted as embedded players for the learners to interact and work with at the bedside. Every embedded RN was provided the same script: They were a new nurse just off orientation and did not know what to do. In addition, they were instructed that no matter who the learner wanted to call/page, that person or service was not answering or unavailable. This forced learners to respond and treat the simulated patient on their own.
Survey Responses
To evaluate the effect of this program on medical education, we administered surveys to the trainees before and after the simulation (Appendix). All questions were evaluated on a 10-point Likert scale (1, minimal comfort; 10, maximum comfort). The postsurvey added an additional Likert scale question and an open-ended question.
Sixteen trainees underwent the simulation curriculum during the 2022 to 2023 academic year, 9 internal medicine PGY1 residents, 4 medical students, and 3 physician assistant students. Postsimulation surveys indicated a mean 2.2 point increase in comfort compared with the presimulation surveys across all questions and participants.
DISCUSSION
The simulation curriculum proved to be successful for all parties, including trainees, medical educators, and simulation staff. Trainees expressed gratitude for the teaching ability of the simulation and the challenge of confronting an evolving scenario. Students also stated that the simulation allowed them to identify knowledge weaknesses.
Medical technology is rapidly advancing. A study evaluating high-fidelity medical simulations between 1969 and 2003 found that they are educationally effective and complement other medical education modalities.6 It is also noted that care provided by junior physicians with a lack of prior exposure to emergencies and unusual clinical syndromes can lead to more adverse effects.7 Simulation curriculums can be used to educate junior physicians as well as trainees on a multitude of medical emergencies, teach systematic approaches to medical scenarios, and increase exposure to unfamiliar experiences.
The goals of this article are to share program details and encourage other training programs with similar capabilities to incorporate simulation into medical education. Using pre- and postsimulation surveys, there was a concrete improvement in the value obtained by participating in this simulation. The Nightmare on CIL Street learners experienced a mean 2.2 point improvement from presimulation survey to postsimulation survey. Some notable improvements were the feelings of preparedness for rapid response situations and developing a systematic approach. As the students who participated in our Nightmare on CIL Street simulation were early in training, we believe the improvement in preparation and developing a systematic approach can be key to their success in their practical environments.
From a site-specific standpoint, improvement in confidence working through cardiac, respiratory, and neurological emergencies will be very useful. The anesthesiology service intubates during respiratory failures and there is no stroke neurologist available at the CTVHCS hospital. Giving trainees experience in these conditions may allow them to better understand their role in coordination during these times and potentially improve patient outcomes. A follow-up questionnaire administered a year after this simulation may be useful in ascertaining the usefulness of the simulation and what items may have been approached differently. We encourage other institutions to build in aspects of their site-specific challenges to improve trainee awareness in approaches to critical scenarios.
Challenges
The greatest challenge for Nightmare on CIL Street was the ability to pull internal medicine residents from their clinical duties to participate in the simulation. As there are many moving parts to their clinical scheduling, residents do not always have sufficient coverage to participate in training. There were also instances where residents needed to cover for another resident preventing them from attending the simulation. In the future, this program will schedule residents months in advance and will have the simulation training built into their rotations.
Medical and physician assistant students were pulled from their ward rotations as well. They rotate on a 2-to-4-week basis and often had already experienced the simulation the week prior, leaving out students for the following week. With more longitudinal planning, students can be pulled on a rotating monthly basis to maximize their participation. Another challenge was deciding whether residents should partner or experience the simulation on their own. After some feedback, it was noted that residents preferred to experience the simulation on their own as this improves their learning value. With the limited resources available, only rotating 3 residents on a scenario limits the number of trainees who can be reached with the program. Running this program throughout an academic year can help to reach more trainees.
CONCLUSIONS
Educating trainees on rapid response scenarios by using a simulation curriculum provides many benefits. Our trainees reported improvement in addressing cardiac, respiratory, and neurological rapid response scenarios after experiencing the simulation. They felt better prepared and had developed a better systematic approach for the future.
Acknowledgments
The authors thank Pawan Sikka, MD, George Martinez, MD and Braden Anderson, MD for participating as physician experts and educating our students. We thank Naomi Devers; Dinetra Jones; Stephanie Garrett; Sara Holton; Evelina Bartnick; Tanelle Smith; Michael Lomax; Shaun Kelemen for their participation as nurses, assistants, and simulation technology experts.
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
1. Guze PA. Using technology to meet the challenges of medical education. Trans Am Clin Climatol Assoc. 2015;126:260-270.
2. Higgins M, Madan C, Patel R. Development and decay of procedural skills in surgery: a systematic review of the effectiveness of simulation-based medical education interventions. Surgeon. 2021;19(4):e67-e77. doi:10.1016/j.surge.2020.07.013
3. Lyons PG, Edelson DP, Carey KA, et al. Characteristics of rapid response calls in the United States: an analysis of the first 402,023 adult cases from the Get With the Guidelines Resuscitation-Medical Emergency Team registry. Crit Care Med. 2019;47(10):1283-1289. doi:10.1097/CCM.0000000000003912
4. McMurray L, Hall AK, Rich J, Merchant S, Chaplin T. The nightmares course: a longitudinal, multidisciplinary, simulation-based curriculum to train and assess resident competence in resuscitation. J Grad Med Educ. 2017;9(4):503-508. doi:10.4300/JGME-D-16-00462.1
5. Gilic F, Schultz K, Sempowski I, Blagojevic A. “Nightmares-Family Medicine” course is an effective acute care teaching tool for family medicine residents. Simul Healthc. 2019;14(3):157-162. doi:10.1097/SIH.0000000000000355
6. Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28. doi:10.1080/01421590500046924
7. Datta R, Upadhyay K, Jaideep C. Simulation and its role in medical education. Med J Armed Forces India. 2012;68(2):167-172. doi:10.1016/S0377-1237(12)60040-9
Second pig heart recipient dies
the University of Maryland Medical Center (UMMC), Baltimore, reported in a statement.
Mr. Faucette, a former lab tech who was turned down repeatedly for a standard allograft transplantation because of his various medical conditions, received the pig heart transplant on Sept. 20, 2023.
He first came to UMMC as a patient on Sept. 14. When he was admitted, he was in end-stage heart failure. Shortly before the surgery, his heart stopped, and he required resuscitation.
On Sept. 15, the Food and Drug Administration granted an emergency authorization for the surgery through its single-patient investigational new drug compassionate use pathway.
“My only real hope left is to go with the pig heart, the xenotransplant,” Mr. Faucette said in an interview from his hospital room a few days before his surgery. “At least now I have hope, and I have a chance.” He made “significant progress” in the month after the surgery, participating in physical therapy and spending time with family, according to the university. But in the days before his death, the heart showed signs of rejection.
“Mr. Faucette’s last wish was for us to make the most of what we have learned from our experience, so others may be guaranteed a chance for a new heart when a human organ is unavailable,” said Bartley P. Griffith, MD, who transplanted the pig heart into Mr. Faucette at UMMC. “He then told the team of doctors and nurses who gathered around him that he loved us. We will miss him tremendously.”
Muhammad M. Mohiuddin, MD, professor of surgery and scientific/program director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine, said that “Mr. Faucette was a scientist who not only read and interpreted his own biopsies, but who understood the important contribution he was making in advancing the field.
“As with the first patient, David Bennett Sr., we intend to conduct an extensive analysis to identify factors that can be prevented in future transplants; this will allow us to continue to move forward and educate our colleagues in the field on our experience,” Dr. Mohiuddin added.
The researchers don’t plan to make further comments until their investigation is complete, a university spokesperson said in an interview.
UMMC performed the first transplant of a genetically modified pig heart in January 2022. Mr. Bennett, the recipient of that heart, survived for 60 days. The researchers published their initial findings in The New England Journal of Medicine, and then the results of their follow-up investigation in The Lancet.
A version of this article first appeared on Medscape.com.
the University of Maryland Medical Center (UMMC), Baltimore, reported in a statement.
Mr. Faucette, a former lab tech who was turned down repeatedly for a standard allograft transplantation because of his various medical conditions, received the pig heart transplant on Sept. 20, 2023.
He first came to UMMC as a patient on Sept. 14. When he was admitted, he was in end-stage heart failure. Shortly before the surgery, his heart stopped, and he required resuscitation.
On Sept. 15, the Food and Drug Administration granted an emergency authorization for the surgery through its single-patient investigational new drug compassionate use pathway.
“My only real hope left is to go with the pig heart, the xenotransplant,” Mr. Faucette said in an interview from his hospital room a few days before his surgery. “At least now I have hope, and I have a chance.” He made “significant progress” in the month after the surgery, participating in physical therapy and spending time with family, according to the university. But in the days before his death, the heart showed signs of rejection.
“Mr. Faucette’s last wish was for us to make the most of what we have learned from our experience, so others may be guaranteed a chance for a new heart when a human organ is unavailable,” said Bartley P. Griffith, MD, who transplanted the pig heart into Mr. Faucette at UMMC. “He then told the team of doctors and nurses who gathered around him that he loved us. We will miss him tremendously.”
Muhammad M. Mohiuddin, MD, professor of surgery and scientific/program director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine, said that “Mr. Faucette was a scientist who not only read and interpreted his own biopsies, but who understood the important contribution he was making in advancing the field.
“As with the first patient, David Bennett Sr., we intend to conduct an extensive analysis to identify factors that can be prevented in future transplants; this will allow us to continue to move forward and educate our colleagues in the field on our experience,” Dr. Mohiuddin added.
The researchers don’t plan to make further comments until their investigation is complete, a university spokesperson said in an interview.
UMMC performed the first transplant of a genetically modified pig heart in January 2022. Mr. Bennett, the recipient of that heart, survived for 60 days. The researchers published their initial findings in The New England Journal of Medicine, and then the results of their follow-up investigation in The Lancet.
A version of this article first appeared on Medscape.com.
the University of Maryland Medical Center (UMMC), Baltimore, reported in a statement.
Mr. Faucette, a former lab tech who was turned down repeatedly for a standard allograft transplantation because of his various medical conditions, received the pig heart transplant on Sept. 20, 2023.
He first came to UMMC as a patient on Sept. 14. When he was admitted, he was in end-stage heart failure. Shortly before the surgery, his heart stopped, and he required resuscitation.
On Sept. 15, the Food and Drug Administration granted an emergency authorization for the surgery through its single-patient investigational new drug compassionate use pathway.
“My only real hope left is to go with the pig heart, the xenotransplant,” Mr. Faucette said in an interview from his hospital room a few days before his surgery. “At least now I have hope, and I have a chance.” He made “significant progress” in the month after the surgery, participating in physical therapy and spending time with family, according to the university. But in the days before his death, the heart showed signs of rejection.
“Mr. Faucette’s last wish was for us to make the most of what we have learned from our experience, so others may be guaranteed a chance for a new heart when a human organ is unavailable,” said Bartley P. Griffith, MD, who transplanted the pig heart into Mr. Faucette at UMMC. “He then told the team of doctors and nurses who gathered around him that he loved us. We will miss him tremendously.”
Muhammad M. Mohiuddin, MD, professor of surgery and scientific/program director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine, said that “Mr. Faucette was a scientist who not only read and interpreted his own biopsies, but who understood the important contribution he was making in advancing the field.
“As with the first patient, David Bennett Sr., we intend to conduct an extensive analysis to identify factors that can be prevented in future transplants; this will allow us to continue to move forward and educate our colleagues in the field on our experience,” Dr. Mohiuddin added.
The researchers don’t plan to make further comments until their investigation is complete, a university spokesperson said in an interview.
UMMC performed the first transplant of a genetically modified pig heart in January 2022. Mr. Bennett, the recipient of that heart, survived for 60 days. The researchers published their initial findings in The New England Journal of Medicine, and then the results of their follow-up investigation in The Lancet.
A version of this article first appeared on Medscape.com.
Another study ties statins to T2D: Should practice change?
Studies have shown links between statin use and type 2 diabetes (T2D) for more than a decade. A U.S. Food and Drug Administration label change for the drugs warned in 2012 about reports of increased risks of high blood glucose and glycosylated hemoglobin (A1c) levels. However, in the same warning, the FDA said it “continues to believe that the cardiovascular benefits of statins outweigh these small increased risks.”
Indeed, although the warning triggered much discussion at the time and a number of meta-analyses and other observational studies in more recent years, that conclusion seems to hold among clinicians and society guidelines.
For example, in a recent practice pointer on the risk of diabetes with statins published in the BMJ, Ishak Mansi, MD, of the Orlando VA Health Care System, and colleagues write, “This potential adverse effect of diabetes with statin use should not be a barrier to starting statin treatment when indicated.”
They also called for further research to answer such questions as, “Is statin-associated diabetes reversible upon statin discontinuation? Would intermittent use minimize this risk while maintaining cardiovascular benefits?”
An earlier study among individuals at high risk for diabetes found significantly higher rates of incident diabetes at 10 years among patients on placebo, metformin, or lifestyle intervention who also initiated statin therapy. Jill Crandall, MD, Albert Einstein College of Medicine, New York, and colleagues conclude, “For individual patients, a potential modest increase in diabetes risk clearly needs to be balanced against the consistent and highly significant reductions in myocardial infarction, stroke, and cardiovascular death associated with statin treatment.”
In the same vein, a recent review by Byron Hoogwerf, MD, Emeritus, department of endocrinology, diabetes, and metabolism, Cleveland Clinic, is titled, “Statins may increase diabetes, but benefit still outweighs risk.”
Rosuvastatin versus Atorvastatin
The latest study in this arena is an analysis of the LODESTAR randomized controlled trial of 4,400 patients with coronary artery disease in 12 hospitals in Korea which compares the risks associated with individual statins.
Senior author Myeong-Ki Hong, MD, PhD, Yonsei University College of Medicine, Severance Cardiovascular Hospital, Seoul, South Korea, said in an interview that the study was prompted by the “limited” studies evaluating clinical outcomes, including diabetes risk, according to statin type.
Dr. Hong and colleagues compared the risk of developing diabetes among those taking rosuvastatin (mean daily dose, 17.1 mg) or atorvastatin (mean daily dose 36 mg) for 3 years. While both statins effectively prevented myocardial infarction, stroke, and death, (2.5% vs. 1.5%; HR, 1.66).
Overall, the HR of new-onset T2D was 1.29 (95% confidence interval, 1.01-1.63; P = .04).
“The percentages of new-onset diabetes and cataract are in line with previous studies regarding statin therapy in patients with atherosclerotic cardiovascular disease,” Dr. Hong said. “Additional research specifically focusing on these outcomes is required, with more frequent measurement of glucose and A1c levels to detect new-onset diabetes and regular ophthalmologic examinations to detect cataracts.”
“However,” he added, “when using rosuvastatin over atorvastatin, we ... emphasize the importance of meticulous monitoring and appropriate lifestyle interventions to mitigate the risk of new-onset diabetes or cataracts.”
Steven Nissen, MD, chief academic officer of Cleveland Clinic’s Heart and Vascular Institute, was not convinced, and said the study “does not provide useful insights into the use of these drugs.”
The investigators used whatever dose they wanted, “and the authors report only the median dose after 3 years,” he said in an interview. “Because there was a slightly greater reduction in low-density lipoprotein (LDL) cholesterol with rosuvastatin, the relative dose was actually higher.”
“We know that new-onset diabetes with statins is dose-dependent,” he said. “The P-values for diabetes incidence were marginal (very close to P = .05). Accordingly, the diabetes data are unconvincing. ... The similar efficacy is not surprising given the open-label dosing with relatively similar effects on lipids.”
Seth Shay Martin, MD, MHS, director of the Advanced Lipid Disorders Program and Digital Health Lab, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Medicine, Baltimore, also commented on the results. The findings are “in line with existing knowledge and current guidelines,” he said. “Therefore, the study should not influence prescribing.”
“Although the study suggests that rosuvastatin was associated with a higher risk of new-onset diabetes mellitus requiring antidiabetics and cataract surgery, compared with atorvastatin, these findings should be interpreted with caution given the open-label nature of the study and require further investigation,” he said.
“The mean daily doses of statins were somewhat below target for secondary prevention,” he noted. “Ideally, patients with coronary artery disease (CAD) take 20-40 mg daily of rosuvastatin or 40-80 mg daily of atorvastatin.”
“Furthermore, the LDL cholesterol levels were not optimized in the patients,” he said. “The mean LDL-C was 1.8-1.9 mmol/L, which is equivalent to 70-73 mg/dL. In the current treatment era, we generally treat to LDL-C levels less than 70 mg/dL and often less than 55 mg/dL in CAD patients.”
“The cataracts finding is particularly odd,” he added. “There was historic concern for cataracts with statin therapy, initially because of studies in beagle dogs. However, high-quality evidence from statin trials has not shown a risk for cataracts.”
So which statin has the lowest risk of triggering new-onset diabetes? As Dr. Hong noted, the literature is sparse when it comes to comparing the risk among specific statins. Some studies suggest that the risk may depend on the individual and their specific risk factors, as well as the dose and intensity of the prescribed statin.
One recent study suggests that while the overall chance of developing diabetes is small, when looking at risk by years of exposure, atorvastatin, rosuvastatin, and lovastatin carried the largest risk, whereas the risk was lower with pravastatin and simvastatin.
Risks also seemed lower with fluvastatin and pitavastatin, but there were too few study patients taking those drugs long-term to include in the subanalysis.
With input from the latest guidelines from the American Heart Association and the American Diabetes Association, as well as findings from a clinical guide on statin-associated diabetes, Dr. Hoogwerf suggests in his review that shared decision-making before starting statin therapy of any type include the following considerations/discussion points:
- For all patients: Screening to determine baseline glycemic status; nonstatin therapies to lower cholesterol; and variables associated with an increased risk of diabetes, including antihypertensive drugs.
- For patients without T2D: The possibility of developing T2D, types and doses of statins, and the fact that statin benefits “generally far outweigh” risks of developing diabetes.
- For patients with T2D: Possible small adverse effects on glycemic control; statin benefits in reducing risk for atherosclerotic cardiovascular disease, which “significantly outweigh” the small increase in A1c; and mitigation of adverse glycemic effects of statins with glucose-lowering therapies.
It’s worth noting that the AHA and ADA guidelines, among others, also emphasize that such discussions should include the importance of weight loss, regular exercise, and adhering to a healthy lifestyle to mitigate risks of both diabetes and heart disease, with or without statins.
Dr. Hong, Dr. Nissen, and Dr. Martin report no relevant financial relationships. Dr. Hoogwerf has disclosed ownership interest in Eli Lilly and consulting for MannKind and Zealand Pharmaceuticals.
A version of this article appeared on Medscape.com.
Studies have shown links between statin use and type 2 diabetes (T2D) for more than a decade. A U.S. Food and Drug Administration label change for the drugs warned in 2012 about reports of increased risks of high blood glucose and glycosylated hemoglobin (A1c) levels. However, in the same warning, the FDA said it “continues to believe that the cardiovascular benefits of statins outweigh these small increased risks.”
Indeed, although the warning triggered much discussion at the time and a number of meta-analyses and other observational studies in more recent years, that conclusion seems to hold among clinicians and society guidelines.
For example, in a recent practice pointer on the risk of diabetes with statins published in the BMJ, Ishak Mansi, MD, of the Orlando VA Health Care System, and colleagues write, “This potential adverse effect of diabetes with statin use should not be a barrier to starting statin treatment when indicated.”
They also called for further research to answer such questions as, “Is statin-associated diabetes reversible upon statin discontinuation? Would intermittent use minimize this risk while maintaining cardiovascular benefits?”
An earlier study among individuals at high risk for diabetes found significantly higher rates of incident diabetes at 10 years among patients on placebo, metformin, or lifestyle intervention who also initiated statin therapy. Jill Crandall, MD, Albert Einstein College of Medicine, New York, and colleagues conclude, “For individual patients, a potential modest increase in diabetes risk clearly needs to be balanced against the consistent and highly significant reductions in myocardial infarction, stroke, and cardiovascular death associated with statin treatment.”
In the same vein, a recent review by Byron Hoogwerf, MD, Emeritus, department of endocrinology, diabetes, and metabolism, Cleveland Clinic, is titled, “Statins may increase diabetes, but benefit still outweighs risk.”
Rosuvastatin versus Atorvastatin
The latest study in this arena is an analysis of the LODESTAR randomized controlled trial of 4,400 patients with coronary artery disease in 12 hospitals in Korea which compares the risks associated with individual statins.
Senior author Myeong-Ki Hong, MD, PhD, Yonsei University College of Medicine, Severance Cardiovascular Hospital, Seoul, South Korea, said in an interview that the study was prompted by the “limited” studies evaluating clinical outcomes, including diabetes risk, according to statin type.
Dr. Hong and colleagues compared the risk of developing diabetes among those taking rosuvastatin (mean daily dose, 17.1 mg) or atorvastatin (mean daily dose 36 mg) for 3 years. While both statins effectively prevented myocardial infarction, stroke, and death, (2.5% vs. 1.5%; HR, 1.66).
Overall, the HR of new-onset T2D was 1.29 (95% confidence interval, 1.01-1.63; P = .04).
“The percentages of new-onset diabetes and cataract are in line with previous studies regarding statin therapy in patients with atherosclerotic cardiovascular disease,” Dr. Hong said. “Additional research specifically focusing on these outcomes is required, with more frequent measurement of glucose and A1c levels to detect new-onset diabetes and regular ophthalmologic examinations to detect cataracts.”
“However,” he added, “when using rosuvastatin over atorvastatin, we ... emphasize the importance of meticulous monitoring and appropriate lifestyle interventions to mitigate the risk of new-onset diabetes or cataracts.”
Steven Nissen, MD, chief academic officer of Cleveland Clinic’s Heart and Vascular Institute, was not convinced, and said the study “does not provide useful insights into the use of these drugs.”
The investigators used whatever dose they wanted, “and the authors report only the median dose after 3 years,” he said in an interview. “Because there was a slightly greater reduction in low-density lipoprotein (LDL) cholesterol with rosuvastatin, the relative dose was actually higher.”
“We know that new-onset diabetes with statins is dose-dependent,” he said. “The P-values for diabetes incidence were marginal (very close to P = .05). Accordingly, the diabetes data are unconvincing. ... The similar efficacy is not surprising given the open-label dosing with relatively similar effects on lipids.”
Seth Shay Martin, MD, MHS, director of the Advanced Lipid Disorders Program and Digital Health Lab, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Medicine, Baltimore, also commented on the results. The findings are “in line with existing knowledge and current guidelines,” he said. “Therefore, the study should not influence prescribing.”
“Although the study suggests that rosuvastatin was associated with a higher risk of new-onset diabetes mellitus requiring antidiabetics and cataract surgery, compared with atorvastatin, these findings should be interpreted with caution given the open-label nature of the study and require further investigation,” he said.
“The mean daily doses of statins were somewhat below target for secondary prevention,” he noted. “Ideally, patients with coronary artery disease (CAD) take 20-40 mg daily of rosuvastatin or 40-80 mg daily of atorvastatin.”
“Furthermore, the LDL cholesterol levels were not optimized in the patients,” he said. “The mean LDL-C was 1.8-1.9 mmol/L, which is equivalent to 70-73 mg/dL. In the current treatment era, we generally treat to LDL-C levels less than 70 mg/dL and often less than 55 mg/dL in CAD patients.”
“The cataracts finding is particularly odd,” he added. “There was historic concern for cataracts with statin therapy, initially because of studies in beagle dogs. However, high-quality evidence from statin trials has not shown a risk for cataracts.”
So which statin has the lowest risk of triggering new-onset diabetes? As Dr. Hong noted, the literature is sparse when it comes to comparing the risk among specific statins. Some studies suggest that the risk may depend on the individual and their specific risk factors, as well as the dose and intensity of the prescribed statin.
One recent study suggests that while the overall chance of developing diabetes is small, when looking at risk by years of exposure, atorvastatin, rosuvastatin, and lovastatin carried the largest risk, whereas the risk was lower with pravastatin and simvastatin.
Risks also seemed lower with fluvastatin and pitavastatin, but there were too few study patients taking those drugs long-term to include in the subanalysis.
With input from the latest guidelines from the American Heart Association and the American Diabetes Association, as well as findings from a clinical guide on statin-associated diabetes, Dr. Hoogwerf suggests in his review that shared decision-making before starting statin therapy of any type include the following considerations/discussion points:
- For all patients: Screening to determine baseline glycemic status; nonstatin therapies to lower cholesterol; and variables associated with an increased risk of diabetes, including antihypertensive drugs.
- For patients without T2D: The possibility of developing T2D, types and doses of statins, and the fact that statin benefits “generally far outweigh” risks of developing diabetes.
- For patients with T2D: Possible small adverse effects on glycemic control; statin benefits in reducing risk for atherosclerotic cardiovascular disease, which “significantly outweigh” the small increase in A1c; and mitigation of adverse glycemic effects of statins with glucose-lowering therapies.
It’s worth noting that the AHA and ADA guidelines, among others, also emphasize that such discussions should include the importance of weight loss, regular exercise, and adhering to a healthy lifestyle to mitigate risks of both diabetes and heart disease, with or without statins.
Dr. Hong, Dr. Nissen, and Dr. Martin report no relevant financial relationships. Dr. Hoogwerf has disclosed ownership interest in Eli Lilly and consulting for MannKind and Zealand Pharmaceuticals.
A version of this article appeared on Medscape.com.
Studies have shown links between statin use and type 2 diabetes (T2D) for more than a decade. A U.S. Food and Drug Administration label change for the drugs warned in 2012 about reports of increased risks of high blood glucose and glycosylated hemoglobin (A1c) levels. However, in the same warning, the FDA said it “continues to believe that the cardiovascular benefits of statins outweigh these small increased risks.”
Indeed, although the warning triggered much discussion at the time and a number of meta-analyses and other observational studies in more recent years, that conclusion seems to hold among clinicians and society guidelines.
For example, in a recent practice pointer on the risk of diabetes with statins published in the BMJ, Ishak Mansi, MD, of the Orlando VA Health Care System, and colleagues write, “This potential adverse effect of diabetes with statin use should not be a barrier to starting statin treatment when indicated.”
They also called for further research to answer such questions as, “Is statin-associated diabetes reversible upon statin discontinuation? Would intermittent use minimize this risk while maintaining cardiovascular benefits?”
An earlier study among individuals at high risk for diabetes found significantly higher rates of incident diabetes at 10 years among patients on placebo, metformin, or lifestyle intervention who also initiated statin therapy. Jill Crandall, MD, Albert Einstein College of Medicine, New York, and colleagues conclude, “For individual patients, a potential modest increase in diabetes risk clearly needs to be balanced against the consistent and highly significant reductions in myocardial infarction, stroke, and cardiovascular death associated with statin treatment.”
In the same vein, a recent review by Byron Hoogwerf, MD, Emeritus, department of endocrinology, diabetes, and metabolism, Cleveland Clinic, is titled, “Statins may increase diabetes, but benefit still outweighs risk.”
Rosuvastatin versus Atorvastatin
The latest study in this arena is an analysis of the LODESTAR randomized controlled trial of 4,400 patients with coronary artery disease in 12 hospitals in Korea which compares the risks associated with individual statins.
Senior author Myeong-Ki Hong, MD, PhD, Yonsei University College of Medicine, Severance Cardiovascular Hospital, Seoul, South Korea, said in an interview that the study was prompted by the “limited” studies evaluating clinical outcomes, including diabetes risk, according to statin type.
Dr. Hong and colleagues compared the risk of developing diabetes among those taking rosuvastatin (mean daily dose, 17.1 mg) or atorvastatin (mean daily dose 36 mg) for 3 years. While both statins effectively prevented myocardial infarction, stroke, and death, (2.5% vs. 1.5%; HR, 1.66).
Overall, the HR of new-onset T2D was 1.29 (95% confidence interval, 1.01-1.63; P = .04).
“The percentages of new-onset diabetes and cataract are in line with previous studies regarding statin therapy in patients with atherosclerotic cardiovascular disease,” Dr. Hong said. “Additional research specifically focusing on these outcomes is required, with more frequent measurement of glucose and A1c levels to detect new-onset diabetes and regular ophthalmologic examinations to detect cataracts.”
“However,” he added, “when using rosuvastatin over atorvastatin, we ... emphasize the importance of meticulous monitoring and appropriate lifestyle interventions to mitigate the risk of new-onset diabetes or cataracts.”
Steven Nissen, MD, chief academic officer of Cleveland Clinic’s Heart and Vascular Institute, was not convinced, and said the study “does not provide useful insights into the use of these drugs.”
The investigators used whatever dose they wanted, “and the authors report only the median dose after 3 years,” he said in an interview. “Because there was a slightly greater reduction in low-density lipoprotein (LDL) cholesterol with rosuvastatin, the relative dose was actually higher.”
“We know that new-onset diabetes with statins is dose-dependent,” he said. “The P-values for diabetes incidence were marginal (very close to P = .05). Accordingly, the diabetes data are unconvincing. ... The similar efficacy is not surprising given the open-label dosing with relatively similar effects on lipids.”
Seth Shay Martin, MD, MHS, director of the Advanced Lipid Disorders Program and Digital Health Lab, Ciccarone Center for the Prevention of Cardiovascular Disease, Johns Hopkins Medicine, Baltimore, also commented on the results. The findings are “in line with existing knowledge and current guidelines,” he said. “Therefore, the study should not influence prescribing.”
“Although the study suggests that rosuvastatin was associated with a higher risk of new-onset diabetes mellitus requiring antidiabetics and cataract surgery, compared with atorvastatin, these findings should be interpreted with caution given the open-label nature of the study and require further investigation,” he said.
“The mean daily doses of statins were somewhat below target for secondary prevention,” he noted. “Ideally, patients with coronary artery disease (CAD) take 20-40 mg daily of rosuvastatin or 40-80 mg daily of atorvastatin.”
“Furthermore, the LDL cholesterol levels were not optimized in the patients,” he said. “The mean LDL-C was 1.8-1.9 mmol/L, which is equivalent to 70-73 mg/dL. In the current treatment era, we generally treat to LDL-C levels less than 70 mg/dL and often less than 55 mg/dL in CAD patients.”
“The cataracts finding is particularly odd,” he added. “There was historic concern for cataracts with statin therapy, initially because of studies in beagle dogs. However, high-quality evidence from statin trials has not shown a risk for cataracts.”
So which statin has the lowest risk of triggering new-onset diabetes? As Dr. Hong noted, the literature is sparse when it comes to comparing the risk among specific statins. Some studies suggest that the risk may depend on the individual and their specific risk factors, as well as the dose and intensity of the prescribed statin.
One recent study suggests that while the overall chance of developing diabetes is small, when looking at risk by years of exposure, atorvastatin, rosuvastatin, and lovastatin carried the largest risk, whereas the risk was lower with pravastatin and simvastatin.
Risks also seemed lower with fluvastatin and pitavastatin, but there were too few study patients taking those drugs long-term to include in the subanalysis.
With input from the latest guidelines from the American Heart Association and the American Diabetes Association, as well as findings from a clinical guide on statin-associated diabetes, Dr. Hoogwerf suggests in his review that shared decision-making before starting statin therapy of any type include the following considerations/discussion points:
- For all patients: Screening to determine baseline glycemic status; nonstatin therapies to lower cholesterol; and variables associated with an increased risk of diabetes, including antihypertensive drugs.
- For patients without T2D: The possibility of developing T2D, types and doses of statins, and the fact that statin benefits “generally far outweigh” risks of developing diabetes.
- For patients with T2D: Possible small adverse effects on glycemic control; statin benefits in reducing risk for atherosclerotic cardiovascular disease, which “significantly outweigh” the small increase in A1c; and mitigation of adverse glycemic effects of statins with glucose-lowering therapies.
It’s worth noting that the AHA and ADA guidelines, among others, also emphasize that such discussions should include the importance of weight loss, regular exercise, and adhering to a healthy lifestyle to mitigate risks of both diabetes and heart disease, with or without statins.
Dr. Hong, Dr. Nissen, and Dr. Martin report no relevant financial relationships. Dr. Hoogwerf has disclosed ownership interest in Eli Lilly and consulting for MannKind and Zealand Pharmaceuticals.
A version of this article appeared on Medscape.com.
Portfolio diet tied to lower risk for CVD, stroke
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
Close adherence to the Portfolio dietary pattern, including foods that have been shown to actively lower cholesterol (for example, plant proteins, nuts, viscous fiber, phytosterols, and plant monounsaturated fats) is associated with a 14% lower risk for total cardiovascular disease (CVD), coronary heart disease (CHD), and stroke, pooled results from three large observational studies suggest.
METHODOLOGY:
- The study included 73,924 women from the Nurses’ Health Study (NHS), 92,346 women from the Nurses’ Health Study II (NHSII), and 43,970 men from the Health Professionals Follow-Up Study (HPFS) without CVD at baseline who were followed biennially on lifestyle, medical history, and other health-related factors.
- From food-frequency questionnaires (FFQs) completed every 4 years, researchers categorized foods into the six components of the Portfolio diet:
- Plant protein such as legumes, beans, tofu, peas, and soymilk
- Nuts and seeds
- Fiber sources such as bran, oats, berries, and eggplant
- Phytosterols
- Monounsaturated fat (MUFA) sources such as olive oil and avocado
- High saturated fat and cholesterol sources such as whole-fat dairy and red and processed meats
- They scored each from 1 (least adherent) to 5 (most adherent), with a higher score indicating higher consumption.
- Researchers examined the association of this Portfolio Diet Score (PDS) with total CVD, CHD, and stroke, in the three cohorts, and associations with plasma levels of lipid and inflammatory biomarkers in a subpopulation of the cohorts.
TAKEAWAY:
- During up to 30 years of follow-up, there were 16,917 incident CVD cases, including 10,666 CHD cases and 6,473 strokes.
- In a pooled analysis of the three cohorts, the fully adjusted hazard ratio for total CVD comparing the highest with the lowest quintile of the PDS was 0.86 (95% confidence interval, 0.81-0.92; P for trend < .001).
- Also comparing extreme quintiles, the pooled HR for CHD was 0.86 (95% CI, 0.80-0.93; P for trend = .0001) and for stroke, it was 0.86 (95% CI, 0.78-0.95; P for trend = .0003).
- A higher PDS was also associated with a more favorable lipid profile and lower levels of inflammation.
IN PRACTICE:
which aligns with American Heart Association guidelines promoting consumption of whole grains, fruits and vegetables, plant-based proteins, minimally processed foods, and healthy unsaturated plant oils, the authors conclude.
SOURCE:
The study was conducted by Andrea J. Glenn, PhD, RD, department of nutrition, Harvard T.H. Chan School of Public Health, Boston, and colleagues. It was published online in the journal Circulation.
LIMITATIONS:
As the study was observational, residual confounding can’t be ruled out. Diet was self-reported, which may have resulted in measurement errors. Consumption of some recommended foods was low, even in the top quintiles, so the association with CVD risk may be underestimated. Information on a few key Portfolio diet foods, including barley and okra, was unavailable, potentially leading to underestimation of intake, which may also attenuate the findings.
DISCLOSURES:
The study was supported by the Diabetes Canada End Diabetes 100 Award. The NH and HPFS studies are supported by the National Institutes of Health. Dr. Glenn is supported by a Canadian Institutes of Health Research fellowship; she has received honoraria or travel support from the Soy Nutrition Institute Global, Vinasoy, and the Academy of Nutrition and Dietetics. See original article for disclosures of other authors.
A version of this article first appeared on Medscape.com.
Heart rate variability: Are we ignoring a harbinger of health?
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
Orthostatic hypotension no deterrent to hypertension treatment
TOPLINE:
Intensive antihypertensive treatment provides the same benefit with regard to cardiovascular disease (CVD) and all-cause mortality regardless of the presence or absence of orthostatic or standing hypotension, new research shows.
METHODOLOGY:
- In response to ongoing concern about the benefits of intensive versus standard blood pressure treatment for adults with orthostatic hypotension (OH), researchers conducted a meta-analysis of individual patient data from nine randomized clinical trials to see whether the benefit of antihypertensive treatment was diminished for patients who had OH at baseline. Benefit was defined as a reduction in nonfatal CVD events and all-cause mortality.
- The included trials assessed BP pharmacologic treatment (more intensive BP goal or active agent) and had data on OH.
TAKEAWAY:
- The nine trials included 29,235 participants (mean age, 69 years; 48% women) who were followed for a median of 4 years; 9% had OH and 5% had standing hypotension at baseline.
- Having OH at baseline was significantly associated with the composite of CVD or all-cause mortality (hazard ratio, 1.14; 95% confidence interval, 1.04-1.26) and with all-cause mortality (HR, 1.24; 95% CI, 1.09-1.41). The same was true for baseline standing hypotension (composite outcome: HR, 1.39; 95% CI, 1.24-1.57; all-cause mortality: HR, 1.38; 95% CI, 1.14-1.66).
- More intensive BP treatment or active therapy significantly and similarly lowered risk of CVD or all-cause mortality among adults who did not have OH at baseline (HR, 0.81; 95% CI, 0.76-0.86) as well as those with OH at baseline (HR, 0.83; 95% CI, 0.70-1.00).
- More intensive BP treatment or active therapy also significantly lowered risk of CVD or all-cause mortality among those without baseline standing hypotension (HR, 0.80; 95% CI, 0.75-0.85) and nonsignificantly lowered the risk among those with baseline standing hypotension (HR, 0.94; 95% CI, 0.75-1.18).
IN PRACTICE:
“These findings suggest that orthostatic hypotension alone (that is, without symptoms) and standing hypotension measured prior to intensification of BP treatment should not deter adoption of more intensive BP treatment in adults with hypertension,” the authors conclude.
The findings should “reassure clinicians that patients with OH (and perhaps standing hypotension) will derive the full expected benefits from antihypertensive therapy,” add the authors of an accompanying editorial. “This also applies to patients treated to lower BP goals, albeit with less certainty.”
SOURCE:
The study, with first author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, and the accompanying editorial were published online in JAMA.
LIMITATIONS:
In the hypertension trials that were included in the analysis, the study populations differed, as did BP measurement procedures, interventions, duration, and CVD outcome ascertainment processes and definitions. Some trials excluded adults with low standing systolic BP, limiting the number of participants with standing hypotension. OH was determined on the basis of a seated-to-standing protocol; supine-to-standing protocols are more sensitive and may not be interchangeable. Medications used in the trials may not reflect current medicine practice, or the trials may not have included agents thought to be more likely to affect OH and falls.
DISCLOSURES:
The study had no specific funding. Dr. Juraschek has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intensive antihypertensive treatment provides the same benefit with regard to cardiovascular disease (CVD) and all-cause mortality regardless of the presence or absence of orthostatic or standing hypotension, new research shows.
METHODOLOGY:
- In response to ongoing concern about the benefits of intensive versus standard blood pressure treatment for adults with orthostatic hypotension (OH), researchers conducted a meta-analysis of individual patient data from nine randomized clinical trials to see whether the benefit of antihypertensive treatment was diminished for patients who had OH at baseline. Benefit was defined as a reduction in nonfatal CVD events and all-cause mortality.
- The included trials assessed BP pharmacologic treatment (more intensive BP goal or active agent) and had data on OH.
TAKEAWAY:
- The nine trials included 29,235 participants (mean age, 69 years; 48% women) who were followed for a median of 4 years; 9% had OH and 5% had standing hypotension at baseline.
- Having OH at baseline was significantly associated with the composite of CVD or all-cause mortality (hazard ratio, 1.14; 95% confidence interval, 1.04-1.26) and with all-cause mortality (HR, 1.24; 95% CI, 1.09-1.41). The same was true for baseline standing hypotension (composite outcome: HR, 1.39; 95% CI, 1.24-1.57; all-cause mortality: HR, 1.38; 95% CI, 1.14-1.66).
- More intensive BP treatment or active therapy significantly and similarly lowered risk of CVD or all-cause mortality among adults who did not have OH at baseline (HR, 0.81; 95% CI, 0.76-0.86) as well as those with OH at baseline (HR, 0.83; 95% CI, 0.70-1.00).
- More intensive BP treatment or active therapy also significantly lowered risk of CVD or all-cause mortality among those without baseline standing hypotension (HR, 0.80; 95% CI, 0.75-0.85) and nonsignificantly lowered the risk among those with baseline standing hypotension (HR, 0.94; 95% CI, 0.75-1.18).
IN PRACTICE:
“These findings suggest that orthostatic hypotension alone (that is, without symptoms) and standing hypotension measured prior to intensification of BP treatment should not deter adoption of more intensive BP treatment in adults with hypertension,” the authors conclude.
The findings should “reassure clinicians that patients with OH (and perhaps standing hypotension) will derive the full expected benefits from antihypertensive therapy,” add the authors of an accompanying editorial. “This also applies to patients treated to lower BP goals, albeit with less certainty.”
SOURCE:
The study, with first author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, and the accompanying editorial were published online in JAMA.
LIMITATIONS:
In the hypertension trials that were included in the analysis, the study populations differed, as did BP measurement procedures, interventions, duration, and CVD outcome ascertainment processes and definitions. Some trials excluded adults with low standing systolic BP, limiting the number of participants with standing hypotension. OH was determined on the basis of a seated-to-standing protocol; supine-to-standing protocols are more sensitive and may not be interchangeable. Medications used in the trials may not reflect current medicine practice, or the trials may not have included agents thought to be more likely to affect OH and falls.
DISCLOSURES:
The study had no specific funding. Dr. Juraschek has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Intensive antihypertensive treatment provides the same benefit with regard to cardiovascular disease (CVD) and all-cause mortality regardless of the presence or absence of orthostatic or standing hypotension, new research shows.
METHODOLOGY:
- In response to ongoing concern about the benefits of intensive versus standard blood pressure treatment for adults with orthostatic hypotension (OH), researchers conducted a meta-analysis of individual patient data from nine randomized clinical trials to see whether the benefit of antihypertensive treatment was diminished for patients who had OH at baseline. Benefit was defined as a reduction in nonfatal CVD events and all-cause mortality.
- The included trials assessed BP pharmacologic treatment (more intensive BP goal or active agent) and had data on OH.
TAKEAWAY:
- The nine trials included 29,235 participants (mean age, 69 years; 48% women) who were followed for a median of 4 years; 9% had OH and 5% had standing hypotension at baseline.
- Having OH at baseline was significantly associated with the composite of CVD or all-cause mortality (hazard ratio, 1.14; 95% confidence interval, 1.04-1.26) and with all-cause mortality (HR, 1.24; 95% CI, 1.09-1.41). The same was true for baseline standing hypotension (composite outcome: HR, 1.39; 95% CI, 1.24-1.57; all-cause mortality: HR, 1.38; 95% CI, 1.14-1.66).
- More intensive BP treatment or active therapy significantly and similarly lowered risk of CVD or all-cause mortality among adults who did not have OH at baseline (HR, 0.81; 95% CI, 0.76-0.86) as well as those with OH at baseline (HR, 0.83; 95% CI, 0.70-1.00).
- More intensive BP treatment or active therapy also significantly lowered risk of CVD or all-cause mortality among those without baseline standing hypotension (HR, 0.80; 95% CI, 0.75-0.85) and nonsignificantly lowered the risk among those with baseline standing hypotension (HR, 0.94; 95% CI, 0.75-1.18).
IN PRACTICE:
“These findings suggest that orthostatic hypotension alone (that is, without symptoms) and standing hypotension measured prior to intensification of BP treatment should not deter adoption of more intensive BP treatment in adults with hypertension,” the authors conclude.
The findings should “reassure clinicians that patients with OH (and perhaps standing hypotension) will derive the full expected benefits from antihypertensive therapy,” add the authors of an accompanying editorial. “This also applies to patients treated to lower BP goals, albeit with less certainty.”
SOURCE:
The study, with first author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, and the accompanying editorial were published online in JAMA.
LIMITATIONS:
In the hypertension trials that were included in the analysis, the study populations differed, as did BP measurement procedures, interventions, duration, and CVD outcome ascertainment processes and definitions. Some trials excluded adults with low standing systolic BP, limiting the number of participants with standing hypotension. OH was determined on the basis of a seated-to-standing protocol; supine-to-standing protocols are more sensitive and may not be interchangeable. Medications used in the trials may not reflect current medicine practice, or the trials may not have included agents thought to be more likely to affect OH and falls.
DISCLOSURES:
The study had no specific funding. Dr. Juraschek has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This drug works, but wait till you hear what’s in it
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.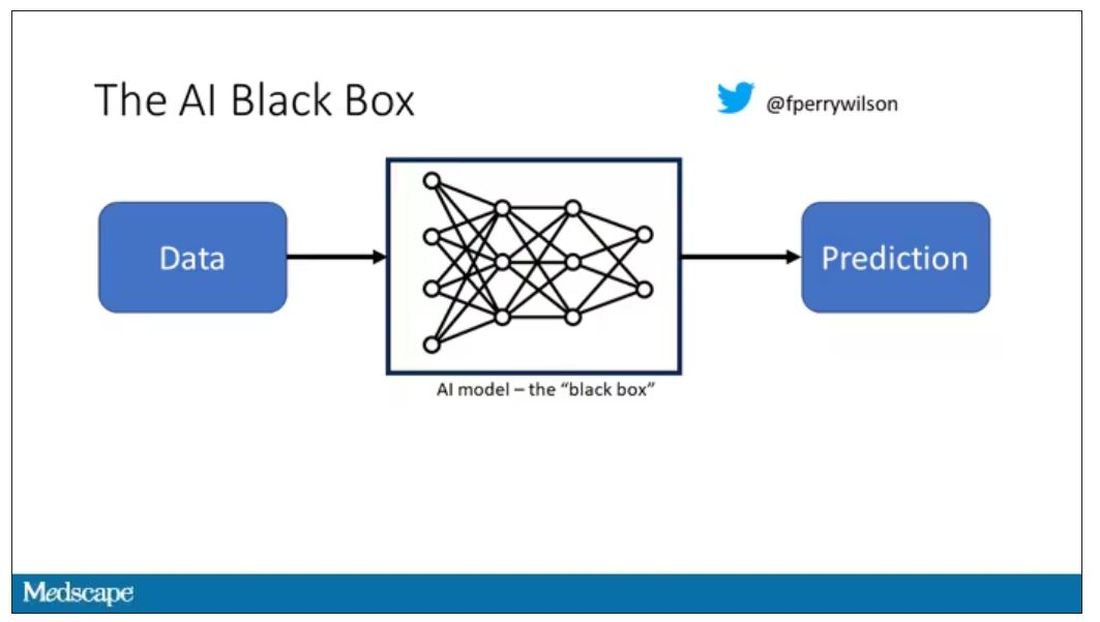
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.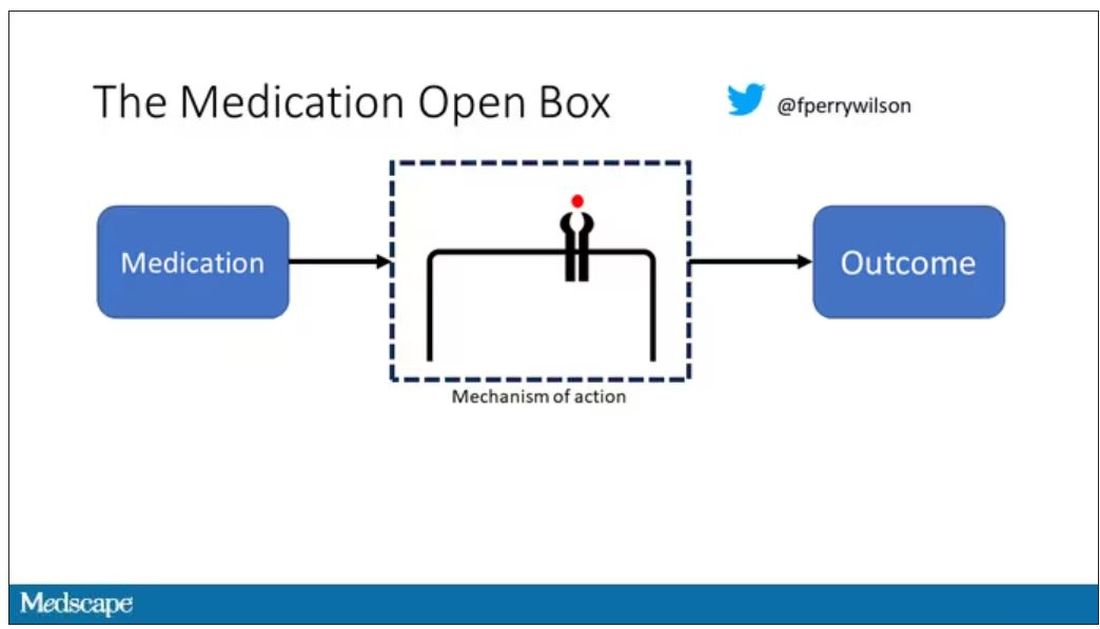
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.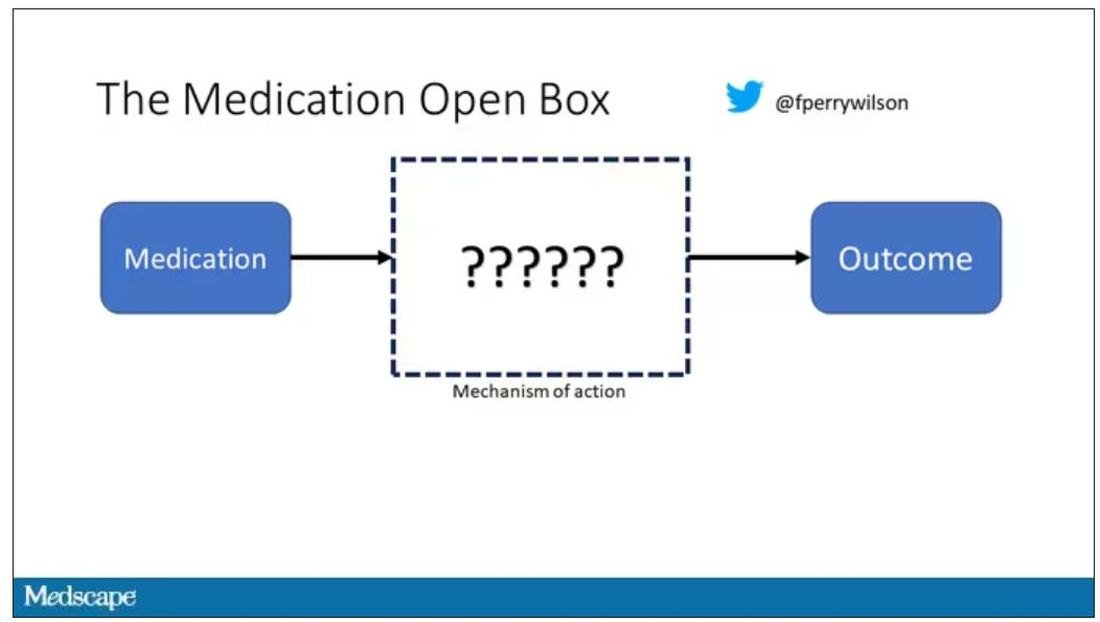
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.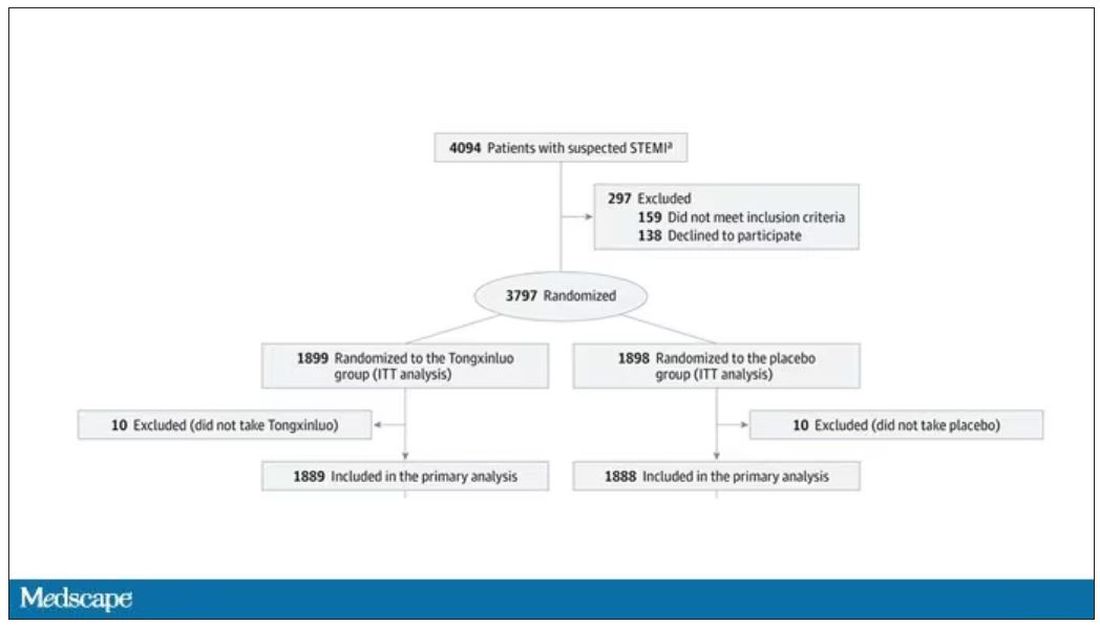
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.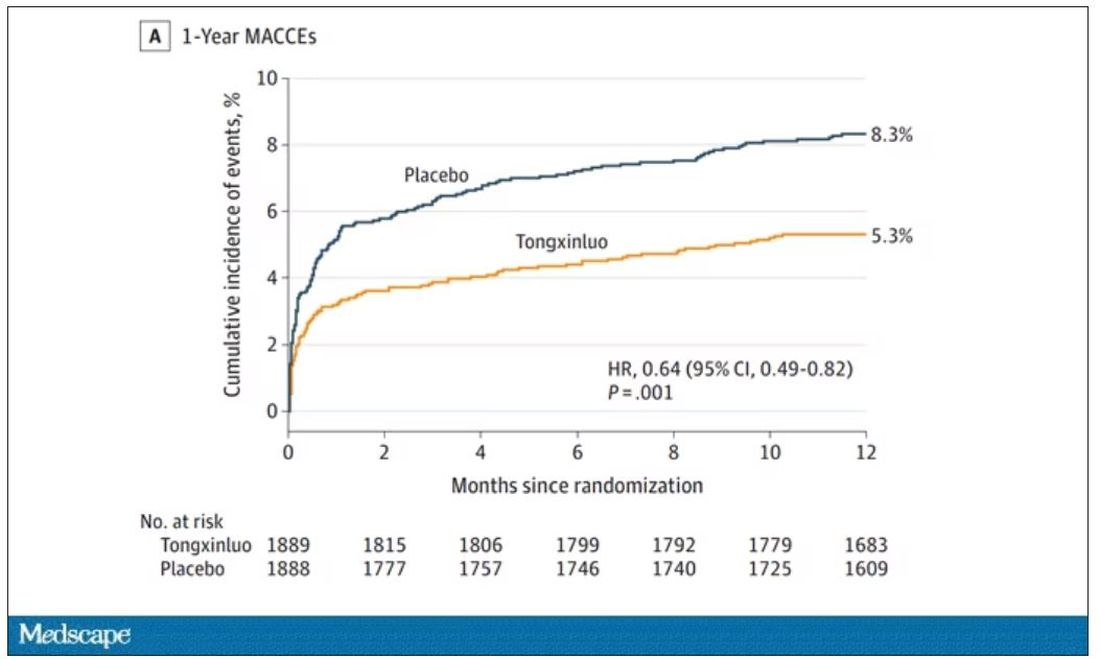
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.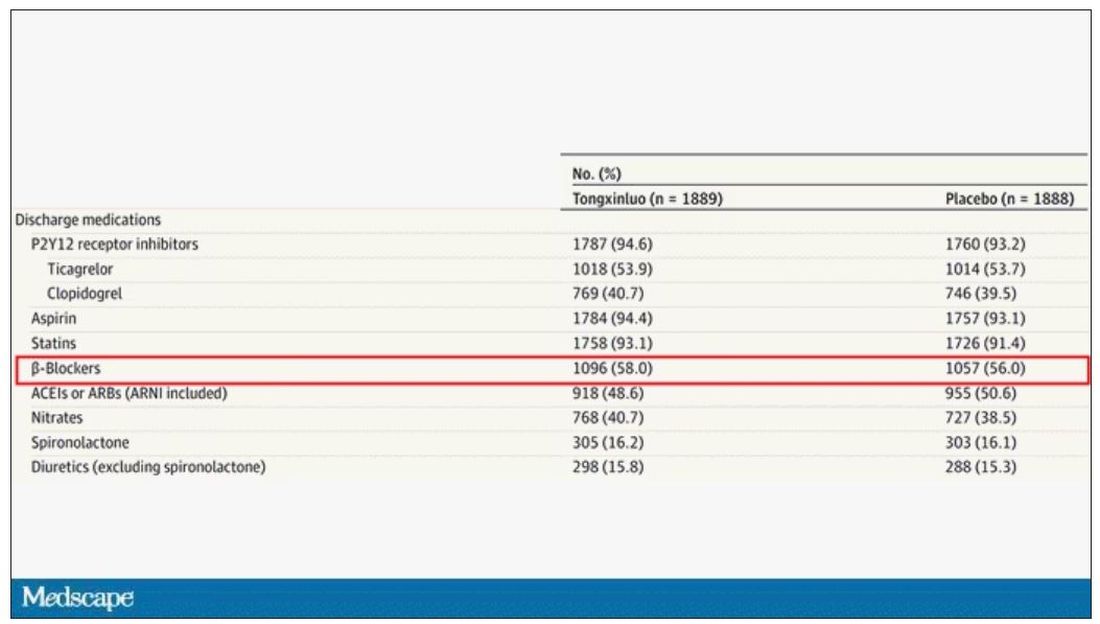
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
COVID coronary plaque infection confirms CV risk
The findings may not only explain the link between COVID and the increased risk of cardiovascular events but mark a starting point for new therapeutic approaches.
“Our study shows there is persistence of viral debris in the artery,” senior investigator Chiara Giannarelli, MD, associate professor of medicine and pathology at NYU Langone Health, New York, said in an interview. “There is an important inflammatory response. We can now look at ways to control this inflammation,” she said.
Dr. Giannarelli says COVID is more than a respiratory virus and that it can affect the whole body. “Our study shows a remarkable ability of the virus to hijack the immune system,” she points out. “Our findings may explain how that happens.”
Dr. Giannarelli says it’s important for doctors and patients to be aware of an increased cardiovascular risk after a SARS-CoV-2 infection and to pay extra attention to traditional risk factors, such as blood pressure and cholesterol.
“This study showing that severe acute respiratory syndrome coronavirus directly infects coronary artery plaques, producing inflammatory substances, really joins the dots and helps our understanding on why we’re seeing so much heart disease in COVID patients,” Peter Hotez, MD, professor of molecular virology and microbiology at Baylor College of Medicine, Houston, said in an interview.
Asked whether this direct infection of vascular plaques was unique to SARS-CoV-2 or whether this may also occur with other viruses, both Dr. Giannarelli and Dr. Hotez said they believe this may be a specific COVID effect.
“I wouldn’t say it is likely that other viruses infect coronary arteries in this way, but I suppose it is possible,” Dr. Giannarelli said.
Dr. Hotez pointed out that other viruses can cause inflammation in the heart, such as myocarditis. “But I can’t think of another virus that stimulates the sequence of events in coronary artery inflammation like we’re seeing here.”
Dr. Giannarelli noted that influenza is also associated with an increased risk of cardiovascular events, but there has been no evidence to date that it directly affects coronary arteries.
Dr. Hotez added that the increased risk of cardiovascular events with influenza has also been reported to be prolonged after the acute infection. “These new findings with SARS-CoV-2 could stimulate a redoubling of efforts to look at this possibility with influenza,” he suggested.
Heart disease after COVID
In a recent article published online in Nature Cardiovascular Research, Dr. Giannarelli and colleagues analyzed human autopsy tissue samples from coronary arterial walls of patients who had died from COVID in the early stages of the pandemic in New York.
They found an accumulation of viral RNA in atherosclerotic plaques in the coronary arteries, which was particularly concentrated in lipid-rich macrophage foam cells present within the plaques.
“Our data conclusively demonstrate that severe acute respiratory syndrome coronavirus is capable of infecting and replicating in macrophages within the coronary vasculature,” the researchers report.
The virus preferentially replicates in foam cells, in comparison with other macrophages, they add, suggesting that these cells might act as a reservoir of viral debris in atherosclerotic plaque.
“We have shown that the virus is targeting lipid-rich macrophages in atherosclerotic lesions. This is the first time this has been shown, and we think this is a very important finding,” Dr. Giannarelli said in an interview.
“We also found that the virus persists in these foam cells that could be responsible for long-term, low-grade inflammation in the vasculature that could contribute to the long-term cardiovascular manifestations in patients who have recovered from COVID,” she said.
Viral reservoirs
Macrophages residing in vascular tissue can undergo self-renewal and can remain in the tissue for many years, the investigators point out. They suggest that these macrophages may act as viral reservoirs of SARS-CoV-2 RNA in atherosclerotic plaques.
Using an ex vivo model, the researchers also found that atherosclerotic tissue could be directly infected by the virus. And just as was seen in cultured macrophages and foam cells, infection of vascular tissue triggered an inflammatory response. That response induced the secretion of key proatherogenic cytokines, such as interleukin-6 and interleukin-1 beta, which have been implicated in the pathogenesis of atherosclerosis and in an increased risk of cardiovascular events.
“Considering that plaque inflammation promotes disease progression and contributes to plaque rupture, our results provide a molecular basis for how infection of coronary lesions can contribute to the acute cardiovascular manifestations of COVID-19, such as myocardial infarction,” the researchers report.
Another interesting finding was a higher accumulation of viral RNA in the coronary vasculature of the three patients with acute ischemic cardiovascular manifestations, which they say adds to evidence that infection may increase cardiovascular risk.
Dr. Giannarelli points out that the patients in their study died in New York early in the pandemic, before vaccines were available. “They were unvaccinated and likely had little immunity against initial viral strains.”
Dr. Hotez says that when COVID-19 first emerged, many in the medical and scientific communities thought it would closely resemble the original SARS viral infection, which was primarily a respiratory pathogen.
“But it became pretty clear early on this virus was causing a lot of cardiovascular and thromboembolic disease,” he says. “This study provides an insight into the mechanisms involved here.”
Affecting more than lungs
Dr. Hotez pointed out that a recent study reported a 5% increase in cardiovascular deaths during the years 2020-2022, compared with before the pandemic.
“Those peaks of cardiovascular deaths corresponded with specific waves of COVID – the first happening at the time of the initial wave with the original virus and second during the Delta wave. So, there’s no question that this virus is contributing to excess cardiovascular mortality, and this paper appears to explain the mechanism.”
Dr. Hotez pointed out that the new findings suggest the cardiovascular risk may be prolonged well after the acute infection resolves.
“In long COVID, a lot of people focus on the neurological effects – brain fog and depression. But cardiac insufficiency and other cardiovascular events can also be considered another element of long COVID,” he said.
Dr. Giannarelli says her group is now studying whether patients with long COVID have virus in their coronary arteries. She points out that the current studies were a result of a team effort between experts in cardiovascular disease and virology and infectious disease. “We need to collaborate more like this to understand better the impact of viral infection in patients and the clinical manifestations,” she said.
Dr. Hotez says he believes these new findings will have implications for the future.
“COVID hasn’t gone away. The numbers have been going up again steadily in the U.S. in the last few months. There are still a significant number of hospitalizations,” he said.
While it would be unwieldy to ask for a cardiology consult for every COVID patient, he acknowledged, “there is probably a subset of people – possibly those of older age and who have had a severe case of COVID – who we suspect are now going to be more prone to cardiovascular disease because of having COVID.
“We should be vigilant in looking for cardiovascular disease in these patients,” Dr. Hotez said, “and perhaps be a bit more aggressive about controlling their cardiovascular risk factors.”
The study was funded by the U.S. National Institutes of Health, the American Heart Association, and the Chan Zuckerberg Initiative.
A version of this article first appeared on Medscape.com .
The findings may not only explain the link between COVID and the increased risk of cardiovascular events but mark a starting point for new therapeutic approaches.
“Our study shows there is persistence of viral debris in the artery,” senior investigator Chiara Giannarelli, MD, associate professor of medicine and pathology at NYU Langone Health, New York, said in an interview. “There is an important inflammatory response. We can now look at ways to control this inflammation,” she said.
Dr. Giannarelli says COVID is more than a respiratory virus and that it can affect the whole body. “Our study shows a remarkable ability of the virus to hijack the immune system,” she points out. “Our findings may explain how that happens.”
Dr. Giannarelli says it’s important for doctors and patients to be aware of an increased cardiovascular risk after a SARS-CoV-2 infection and to pay extra attention to traditional risk factors, such as blood pressure and cholesterol.
“This study showing that severe acute respiratory syndrome coronavirus directly infects coronary artery plaques, producing inflammatory substances, really joins the dots and helps our understanding on why we’re seeing so much heart disease in COVID patients,” Peter Hotez, MD, professor of molecular virology and microbiology at Baylor College of Medicine, Houston, said in an interview.
Asked whether this direct infection of vascular plaques was unique to SARS-CoV-2 or whether this may also occur with other viruses, both Dr. Giannarelli and Dr. Hotez said they believe this may be a specific COVID effect.
“I wouldn’t say it is likely that other viruses infect coronary arteries in this way, but I suppose it is possible,” Dr. Giannarelli said.
Dr. Hotez pointed out that other viruses can cause inflammation in the heart, such as myocarditis. “But I can’t think of another virus that stimulates the sequence of events in coronary artery inflammation like we’re seeing here.”
Dr. Giannarelli noted that influenza is also associated with an increased risk of cardiovascular events, but there has been no evidence to date that it directly affects coronary arteries.
Dr. Hotez added that the increased risk of cardiovascular events with influenza has also been reported to be prolonged after the acute infection. “These new findings with SARS-CoV-2 could stimulate a redoubling of efforts to look at this possibility with influenza,” he suggested.
Heart disease after COVID
In a recent article published online in Nature Cardiovascular Research, Dr. Giannarelli and colleagues analyzed human autopsy tissue samples from coronary arterial walls of patients who had died from COVID in the early stages of the pandemic in New York.
They found an accumulation of viral RNA in atherosclerotic plaques in the coronary arteries, which was particularly concentrated in lipid-rich macrophage foam cells present within the plaques.
“Our data conclusively demonstrate that severe acute respiratory syndrome coronavirus is capable of infecting and replicating in macrophages within the coronary vasculature,” the researchers report.
The virus preferentially replicates in foam cells, in comparison with other macrophages, they add, suggesting that these cells might act as a reservoir of viral debris in atherosclerotic plaque.
“We have shown that the virus is targeting lipid-rich macrophages in atherosclerotic lesions. This is the first time this has been shown, and we think this is a very important finding,” Dr. Giannarelli said in an interview.
“We also found that the virus persists in these foam cells that could be responsible for long-term, low-grade inflammation in the vasculature that could contribute to the long-term cardiovascular manifestations in patients who have recovered from COVID,” she said.
Viral reservoirs
Macrophages residing in vascular tissue can undergo self-renewal and can remain in the tissue for many years, the investigators point out. They suggest that these macrophages may act as viral reservoirs of SARS-CoV-2 RNA in atherosclerotic plaques.
Using an ex vivo model, the researchers also found that atherosclerotic tissue could be directly infected by the virus. And just as was seen in cultured macrophages and foam cells, infection of vascular tissue triggered an inflammatory response. That response induced the secretion of key proatherogenic cytokines, such as interleukin-6 and interleukin-1 beta, which have been implicated in the pathogenesis of atherosclerosis and in an increased risk of cardiovascular events.
“Considering that plaque inflammation promotes disease progression and contributes to plaque rupture, our results provide a molecular basis for how infection of coronary lesions can contribute to the acute cardiovascular manifestations of COVID-19, such as myocardial infarction,” the researchers report.
Another interesting finding was a higher accumulation of viral RNA in the coronary vasculature of the three patients with acute ischemic cardiovascular manifestations, which they say adds to evidence that infection may increase cardiovascular risk.
Dr. Giannarelli points out that the patients in their study died in New York early in the pandemic, before vaccines were available. “They were unvaccinated and likely had little immunity against initial viral strains.”
Dr. Hotez says that when COVID-19 first emerged, many in the medical and scientific communities thought it would closely resemble the original SARS viral infection, which was primarily a respiratory pathogen.
“But it became pretty clear early on this virus was causing a lot of cardiovascular and thromboembolic disease,” he says. “This study provides an insight into the mechanisms involved here.”
Affecting more than lungs
Dr. Hotez pointed out that a recent study reported a 5% increase in cardiovascular deaths during the years 2020-2022, compared with before the pandemic.
“Those peaks of cardiovascular deaths corresponded with specific waves of COVID – the first happening at the time of the initial wave with the original virus and second during the Delta wave. So, there’s no question that this virus is contributing to excess cardiovascular mortality, and this paper appears to explain the mechanism.”
Dr. Hotez pointed out that the new findings suggest the cardiovascular risk may be prolonged well after the acute infection resolves.
“In long COVID, a lot of people focus on the neurological effects – brain fog and depression. But cardiac insufficiency and other cardiovascular events can also be considered another element of long COVID,” he said.
Dr. Giannarelli says her group is now studying whether patients with long COVID have virus in their coronary arteries. She points out that the current studies were a result of a team effort between experts in cardiovascular disease and virology and infectious disease. “We need to collaborate more like this to understand better the impact of viral infection in patients and the clinical manifestations,” she said.
Dr. Hotez says he believes these new findings will have implications for the future.
“COVID hasn’t gone away. The numbers have been going up again steadily in the U.S. in the last few months. There are still a significant number of hospitalizations,” he said.
While it would be unwieldy to ask for a cardiology consult for every COVID patient, he acknowledged, “there is probably a subset of people – possibly those of older age and who have had a severe case of COVID – who we suspect are now going to be more prone to cardiovascular disease because of having COVID.
“We should be vigilant in looking for cardiovascular disease in these patients,” Dr. Hotez said, “and perhaps be a bit more aggressive about controlling their cardiovascular risk factors.”
The study was funded by the U.S. National Institutes of Health, the American Heart Association, and the Chan Zuckerberg Initiative.
A version of this article first appeared on Medscape.com .
The findings may not only explain the link between COVID and the increased risk of cardiovascular events but mark a starting point for new therapeutic approaches.
“Our study shows there is persistence of viral debris in the artery,” senior investigator Chiara Giannarelli, MD, associate professor of medicine and pathology at NYU Langone Health, New York, said in an interview. “There is an important inflammatory response. We can now look at ways to control this inflammation,” she said.
Dr. Giannarelli says COVID is more than a respiratory virus and that it can affect the whole body. “Our study shows a remarkable ability of the virus to hijack the immune system,” she points out. “Our findings may explain how that happens.”
Dr. Giannarelli says it’s important for doctors and patients to be aware of an increased cardiovascular risk after a SARS-CoV-2 infection and to pay extra attention to traditional risk factors, such as blood pressure and cholesterol.
“This study showing that severe acute respiratory syndrome coronavirus directly infects coronary artery plaques, producing inflammatory substances, really joins the dots and helps our understanding on why we’re seeing so much heart disease in COVID patients,” Peter Hotez, MD, professor of molecular virology and microbiology at Baylor College of Medicine, Houston, said in an interview.
Asked whether this direct infection of vascular plaques was unique to SARS-CoV-2 or whether this may also occur with other viruses, both Dr. Giannarelli and Dr. Hotez said they believe this may be a specific COVID effect.
“I wouldn’t say it is likely that other viruses infect coronary arteries in this way, but I suppose it is possible,” Dr. Giannarelli said.
Dr. Hotez pointed out that other viruses can cause inflammation in the heart, such as myocarditis. “But I can’t think of another virus that stimulates the sequence of events in coronary artery inflammation like we’re seeing here.”
Dr. Giannarelli noted that influenza is also associated with an increased risk of cardiovascular events, but there has been no evidence to date that it directly affects coronary arteries.
Dr. Hotez added that the increased risk of cardiovascular events with influenza has also been reported to be prolonged after the acute infection. “These new findings with SARS-CoV-2 could stimulate a redoubling of efforts to look at this possibility with influenza,” he suggested.
Heart disease after COVID
In a recent article published online in Nature Cardiovascular Research, Dr. Giannarelli and colleagues analyzed human autopsy tissue samples from coronary arterial walls of patients who had died from COVID in the early stages of the pandemic in New York.
They found an accumulation of viral RNA in atherosclerotic plaques in the coronary arteries, which was particularly concentrated in lipid-rich macrophage foam cells present within the plaques.
“Our data conclusively demonstrate that severe acute respiratory syndrome coronavirus is capable of infecting and replicating in macrophages within the coronary vasculature,” the researchers report.
The virus preferentially replicates in foam cells, in comparison with other macrophages, they add, suggesting that these cells might act as a reservoir of viral debris in atherosclerotic plaque.
“We have shown that the virus is targeting lipid-rich macrophages in atherosclerotic lesions. This is the first time this has been shown, and we think this is a very important finding,” Dr. Giannarelli said in an interview.
“We also found that the virus persists in these foam cells that could be responsible for long-term, low-grade inflammation in the vasculature that could contribute to the long-term cardiovascular manifestations in patients who have recovered from COVID,” she said.
Viral reservoirs
Macrophages residing in vascular tissue can undergo self-renewal and can remain in the tissue for many years, the investigators point out. They suggest that these macrophages may act as viral reservoirs of SARS-CoV-2 RNA in atherosclerotic plaques.
Using an ex vivo model, the researchers also found that atherosclerotic tissue could be directly infected by the virus. And just as was seen in cultured macrophages and foam cells, infection of vascular tissue triggered an inflammatory response. That response induced the secretion of key proatherogenic cytokines, such as interleukin-6 and interleukin-1 beta, which have been implicated in the pathogenesis of atherosclerosis and in an increased risk of cardiovascular events.
“Considering that plaque inflammation promotes disease progression and contributes to plaque rupture, our results provide a molecular basis for how infection of coronary lesions can contribute to the acute cardiovascular manifestations of COVID-19, such as myocardial infarction,” the researchers report.
Another interesting finding was a higher accumulation of viral RNA in the coronary vasculature of the three patients with acute ischemic cardiovascular manifestations, which they say adds to evidence that infection may increase cardiovascular risk.
Dr. Giannarelli points out that the patients in their study died in New York early in the pandemic, before vaccines were available. “They were unvaccinated and likely had little immunity against initial viral strains.”
Dr. Hotez says that when COVID-19 first emerged, many in the medical and scientific communities thought it would closely resemble the original SARS viral infection, which was primarily a respiratory pathogen.
“But it became pretty clear early on this virus was causing a lot of cardiovascular and thromboembolic disease,” he says. “This study provides an insight into the mechanisms involved here.”
Affecting more than lungs
Dr. Hotez pointed out that a recent study reported a 5% increase in cardiovascular deaths during the years 2020-2022, compared with before the pandemic.
“Those peaks of cardiovascular deaths corresponded with specific waves of COVID – the first happening at the time of the initial wave with the original virus and second during the Delta wave. So, there’s no question that this virus is contributing to excess cardiovascular mortality, and this paper appears to explain the mechanism.”
Dr. Hotez pointed out that the new findings suggest the cardiovascular risk may be prolonged well after the acute infection resolves.
“In long COVID, a lot of people focus on the neurological effects – brain fog and depression. But cardiac insufficiency and other cardiovascular events can also be considered another element of long COVID,” he said.
Dr. Giannarelli says her group is now studying whether patients with long COVID have virus in their coronary arteries. She points out that the current studies were a result of a team effort between experts in cardiovascular disease and virology and infectious disease. “We need to collaborate more like this to understand better the impact of viral infection in patients and the clinical manifestations,” she said.
Dr. Hotez says he believes these new findings will have implications for the future.
“COVID hasn’t gone away. The numbers have been going up again steadily in the U.S. in the last few months. There are still a significant number of hospitalizations,” he said.
While it would be unwieldy to ask for a cardiology consult for every COVID patient, he acknowledged, “there is probably a subset of people – possibly those of older age and who have had a severe case of COVID – who we suspect are now going to be more prone to cardiovascular disease because of having COVID.
“We should be vigilant in looking for cardiovascular disease in these patients,” Dr. Hotez said, “and perhaps be a bit more aggressive about controlling their cardiovascular risk factors.”
The study was funded by the U.S. National Institutes of Health, the American Heart Association, and the Chan Zuckerberg Initiative.
A version of this article first appeared on Medscape.com .
FROM NATURE CARDIOVASCULAR RESEARCH