User login
Potential dapagliflozin benefit post MI is not a ‘mandate’
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
PHILADELPHIA – Giving the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) to patients with acute myocardial infarction and impaired left ventricular systolic function but no diabetes or chronic heart failure significantly improved a composite of cardiovascular outcomes, a European registry-based randomized trial suggests.
In presenting these results from the DAPA-MI trial, Stefan James, MD, of Uppsala University (Sweden), noted that which the trial described as the hierarchical “win ratio” composite outcomes, compared with patients randomized to placebo plus standard of care.
“The ‘win ratio’ tells us that there’s a 34% higher likelihood of patients having a better cardiometabolic outcome with dapagliflozin vs placebo in terms of the seven components,” James said in an interview. The win ratio was achieved in 32.9% of dapagliflozin patients versus 24.6% of placebo (P < .001).
Dr. James presented the results at the annual scientific sessions of the American Heart Association, and they were published online simultaneously in NEJM Evidence.
Lower-risk patients
DAPA-MI enrolled 4,017 patients from the SWEDEHEART and Myocardial Ischemia National Audit Project registries in Sweden and the United Kingdom, randomly assigning patients to dapagliflozin 10 mg or placebo along with guideline-directed therapy for both groups.
Eligible patients were hemodynamically stable, had an acute MI within 10 days of enrollment, and impaired left ventricular systolic function or a Q-wave MI. Exclusion criteria included history of either type 1 or 2 diabetes, chronic heart failure, poor kidney function, or current treatment with an SGLT2 inhibitor. Baseline demographic characteristics were similar between trial arms.
- The hierarchical seven primary endpoints were:
- Death, with cardiovascular death ranked first followed by noncardiovascular death
- Hospitalization because of heart failure, with adjudicated first followed by investigator-reported HF
- Nonfatal MI
- Atrial fibrillation/flutter event
- New diagnosis of type 2 diabetes
- New York Heart Association functional class at the last visit
- Drop in body weight of at least 5% at the last visit
The key secondary endpoint, Dr. James said, was the primary outcome minus the body weight component, with time to first occurrence of hospitalization for HF or cardiovascular death.
When the seventh factor, body weight decrease, was removed, the differential narrowed: 20.3% versus 16.9% (P = .015). When two or more variables were removed from the composite, the differences were not statistically significant.
For 11 secondary and exploratory outcomes, ranging from CV death or hospitalization for HF to all-cause hospitalization, the outcomes were similar in both the dapagliflozin and placebo groups across the board.
However, the dapagliflozin patients had about half the rate of developing diabetes, compared with the placebo group: 2.1 % versus 3.9%.
The trial initially used the composite of CV death and hospitalization for HF as the primary endpoint, but switched to the seven-item composite endpoint in February because the number of primary composite outcomes was substantially lower than anticipated, Dr. James said.
He acknowledged the study was underpowered for the low-risk population it enrolled. “But if you extended the trial to a larger population and enriched it with a higher-risk population you would probably see an effect,” he said.
“The cardiometabolic benefit was consistent across all prespecified subgroups and there were no new safety concerns,” Dr. James told the attendees. “Clinical event rates were low with no significant difference between randomized groups.”
Not a ringing endorsement
But for invited discussant Stephen D. Wiviott, MD, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, the DAPA-MI trial result isn’t quite a ringing endorsement of SGLT2 inhibition in these patients.
“From my perspective, DAPA-MI does not suggest a new mandate to expand SGLT2 inhibition to an isolated MI population without other SGLT2 inhibitor indications,” Dr. Wiviott told attendees. “But it does support the safety of its use among patients with acute coronary syndromes.”
However, “these results do not indicate a lack of clinical benefit in patients with prior MI and any of those previously identified conditions – a history of diabetes, coronary heart failure or chronic kidney disease – where SGLT2 inhibition remains a pillar of guideline-directed medical therapy,” Dr. Wiviott said.
In an interview, Dr. Wiviott described the trial design as a “hybrid” in that it used a registry but then added, in his words, “some of the bells and whistles that we have with normal cardiovascular clinical trials.” He further explained: “This is a nice combination of those two things, where they use that as part of the endpoint for the trial but they’re able to add in some of the pieces that you would in a regular registration pathway trial.”
The trial design could serve as a model for future pragmatic therapeutic trials in acute MI, he said, but he acknowledged that DAPA-MI was underpowered to discern many key outcomes.
“They anticipated they were going to have a rate of around 11% of events so they needed to enroll about 6,000 people, but somewhere in the middle of the trial they saw the rate was 2.5%, not 11%, so they had to completely change the trial,” he said of the DAPA-MI investigators.
But an appropriately powered study of SGLT2 inhibition in this population would need about 28,000 patients. “This would be an enormous trial to actually clinically power, so in my sense it’s not going to happen,” Dr. Wiviott said.
The DAPA-MI trial was sponsored by AstraZeneca. Dr. James disclosed relationships with AstraZeneca, Janssen, and Amgen. Dr. Wiviott disclosed relationships with Amgen, AstraZeneca, Janssen, Merck, Pfizer, Icon Clinical, Novo Nordisk, and Varian.
AT AHA 2023
Semaglutide ‘a new pathway’ to CVD risk reduction: SELECT
over the approximately 3-year follow-up in patients with overweight or obesity and cardiovascular disease but not diabetes.
“This is a very exciting set of results. I think it is going to have a big impact on a large number of people,” lead investigator A. Michael Lincoff, MD, vice chair for research in the department of cardiovascular medicine at the Cleveland Clinic, said in an interview.
“And from a scientific standpoint, these data show that we now have a new pathway or a new modifiable risk factor for cardiovascular disease that we can use in our patients who have overweight or obesity,” he added.
The trial involved 17,604 patients with a history of cardiovascular disease and a body mass index of 27 kg/m2 or above (mean BMI was 33), who were randomly assigned to the glucagonlike peptide–1 (GLP-1) agonist semaglutide, given by subcutaneous injection once weekly at a gradually escalating dose up to 2.4 mg daily by week 16, or placebo. The mean baseline glycated hemoglobin level was 5.8% and 66.4% of patients met the criteria for prediabetes.
Patients lost a mean of 9.4% of body weight over the first 2 years with semaglutide versus 0.88% with placebo.
The primary cardiovascular endpoint – a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke – was reduced significantly, with a hazard ratio of 0.80 (95% confidence interval, 0.72-0.90; P < .001).
Death from cardiovascular causes, the first confirmatory secondary endpoint, showed a 15% reduction (HR, 0.85; P = .07) but this missed meeting criteria for statistical significance, and because of the hierarchical design of the trial, this meant that superiority testing was not performed for the remaining confirmatory secondary endpoints.
However, results showed reductions of around 20% for the heart failure composite endpoint and for all-cause mortality, with confidence intervals that did not cross 1.0, and directionally consistent effects were observed for all supportive secondary endpoints.
The HR for the heart failure composite endpoint was 0.82 (95% CI, 0.71-0.96), and the HR for death from any cause was 0.81 (95% CI, 0.71-0.93). Nonfatal MI was reduced by 28% (HR 0.72; 95% CI, 0.61-0.85).
The effects of semaglutide on the primary endpoint appeared to be similar across all prespecified subgroups.
Adverse events leading to discontinuation of treatment occurred in 16.6% in the semaglutide group, mostly gastrointestinal effects, and in 8.2% in the placebo group.
The trial results were presented by Dr. Lincoff at the annual scientific sessions of the American Heart Association . They were also simultaneously published online in the New England Journal of Medicine.
Dr. Lincoff explained that there is a growing pandemic of overweight and obesity worldwide with clear evidence for years that these conditions increase the risk of cardiovascular events – and yet there has been no evidence, until now, that any pharmacologic or lifestyle therapy can reduce the increased risk conferred by overweight/obesity.
“Patients in the trial were already taking standard of care therapies for other risk factors, such as hypertension and cholesterol, so this drug is giving additional benefit,” he said.
Dr. Lincoff believes these data will lead to a large increase in use of semaglutide, which is already available for the treatment of obesity and diabetes but can be difficult to get reimbursed.
“There is a lot of difficulty getting payors to pay for this drug for weight management. But with this new data from the SELECT trial there should be more willingness – at least in the population with a history of cardiovascular disease,” he commented. In diabetes, where it is already established that there is a cardiovascular risk reduction, it is easier to get these drugs reimbursed, he noted.
On the outcome data, Dr. Lincoff said he could not explain why cardiovascular death was not significantly reduced while all-cause mortality appeared to be cut more definitively.
“The cardiovascular death curves separated, then merged, then separated again. We don’t really know what is going on there. It may be that some deaths were misclassified. This trial was conducted through the COVID era and there may have been less information available on some patients because of that.”
But he added: “The all-cause mortality is more reassuring, as it doesn’t depend on classifying cause of death. Because of the design of the trial, we can’t formally claim a reduction in all-cause mortality, but the results do suggest there is an effect on this endpoint. And all the different types of cardiovascular events were similarly reduced in a consistent way, with similar effects seen across all subgroups. That is very reassuring.”
‘A new era’ for patients with obesity
Outside experts in the field were also impressed with the data.
Designated discussant of the trial at the AHA meeting, Ania Jastreboff, MD, associate professor medicine (endocrinology) at Yale University, New Haven, Conn., said the SELECT trial was “a turning point in the treatment of obesity and a call to action.
“Now is the time to treat obesity to improve health outcomes in people with cardiovascular disease,” she said.
Dr. Jastreboff noted that high BMI was estimated to have accounted for 4 million deaths worldwide in 2015, two-thirds of which were caused by cardiovascular disease. And she presented data showing that U.S. individuals meeting the SELECT criteria increased from 4.3 million in 2011-12 to 6.6 million in 2017-18.
She highlighted one major limitation of the SELECT trial: it enrolled a low number of women (38%) and ethnic minorities, with only 12% of the trial population being Black.
Deepak L. Bhatt, MD, director of Mount Sinai Fuster Heart Hospital, New York, described the SELECT results as “altogether a compelling package of data.”
“These results are even better than I had expected,” Dr. Bhatt said in an interview. “There is a significant reduction in MI as I had anticipated, but additionally, there is a reduction in all-cause death. One can debate the statistics, though on a common-sense level, I think it is a real finding,” he noted.
“Given that MI, heart failure, nephropathy, and revascularization are all reduced, and even stroke is numerically lower, it makes sense that all-cause mortality would be reduced,” he said. “To me, apart from the GI side effects, this counts as a home run.”
Steve Nissen, MD, chief academic officer at the Cleveland Clinic’s Heart, Vascular and Thoracic Institute, was similarly upbeat.
“These data prove what many of us have long suspected – that losing weight can reduce cardiovascular morbidity and mortality. This is great news for patients living with obesity. The obesity epidemic is out of control,” he added. “We need to have therapies that improve cardiovascular outcomes caused by obesity and this shows that semaglutide can do that. I think this is the beginning of a whole new era for patients with obesity.”
Michelle O’Donoghue, MD, associate professor of medicine at Harvard Medical School, Boston, called the results of SELECT “both intriguing and compelling. Certainly, these findings lend further support to the use of semaglutide in a much broader secondary prevention population of individuals with obesity.”
Christie Ballantyne, MD, director of the center for cardiometabolic disease prevention at Baylor College of Medicine, Houston, described the SELECT study as “a landmark trial which will change the practice of medicine in regard to how we treat obesity.”
He compared it with the landmark 4S trial in 1994, the first study in the area of cholesterol lowering therapy to show a clear benefit in reducing cardiovascular events and total mortality, and “began a drastic change in the way that physicians approached treatment of cholesterol.”
On the more robust reduction in all-cause death, compared with cardiovascular death,
Dr. Ballantyne pointed out: “Adjudication of dead or alive is something that everyone gets right. In contrast, the cause of death is sometime difficult to ascertain. Most importantly, the benefit on total mortality also provides assurance that this therapy does not have some adverse effect on increasing noncardiovascular deaths.”
Gastrointestinal adverse effects
On the side effects seen with semaglutide, Dr. Lincoff reported that 10% of patients in the semaglutide group discontinued treatment because of GI side effects versus 2% in the placebo arm. He said this was “an expected issue.”
“GI effects, such as nausea, vomiting and diarrhea, are known side effects of this whole class of drugs. The dose is slowly escalated to manage these adverse effects but there will be a proportion of patients who can’t tolerate it, although the vast majority are able to continue.”
He noted that, while dose reduction was allowed, of the patients who were still on the drug at 2 years, 77% were on the full dose, and 23% were on a reduced dose.
Dr. Lincoff pointed out that there were no serious adverse events with semaglutide. “This is the largest database by far now on the drug with a long-term follow up and we didn’t see the emergence of any new safety signals, which is very reassuring.”
Dr. Nissen said the 16% rate of patients stopping the drug because of tolerability “is not a trivial number.”
He noted that the semaglutide dose used in this study was larger than that used in diabetes.
“They did this to try to achieve more weight loss but then you get more issues with tolerability. It’s a trade-off. If patients are experiencing adverse effects, the dose can be reduced, but then you will lose some effect. All the GLP-1 agonists have GI side effects – it’s part of the way that they work.”
Just weight loss or other actions too?
Speculating on the mechanism behind the reduction in cardiovascular events with semaglutide, Dr. Lincoff does not think it is just weight reduction.
“The event curves start to diverge very soon after the start of the trial and yet the maximum weight loss doesn’t occur until about 65 weeks. I think something else is going on.”
In the paper, the researchers noted that GLP-1 agonists have been shown in animal studies to reduce inflammation, improve endothelial and left ventricular function, promote plaque stability, and decrease platelet aggregation. In this trial, semaglutide was associated with changes in multiple biomarkers of cardiovascular risk, including blood pressure, waist circumference, glycemic control, nephropathy, and levels of lipids and C-reactive protein.
Dr. Lincoff also pointed out that similar benefits were seen in patients with different levels of overweight, and in those who were prediabetic and those who weren’t, so benefit was not dependent on baseline BMI or glycated hemoglobin levels.
Dr. O’Donoghue agreed that other effects, as well as weight loss, could be involved. “The reduction in events with semaglutide appeared very early after initiation and far preceded the drug’s maximal effects on weight reduction. This might suggest that the drug offers other cardioprotective effects through pathways independent of weight loss. Certainly, semaglutide and the other GLP-1 agonists appear to attenuate inflammation, and the patterns of redistribution of adipose tissue may also be of interest.”
She also pointed out that the reduction in cardiovascular events appeared even earlier in this population of obese nondiabetic patients with cardiovascular disease than in prior studies of patients with diabetes. “It may suggest that there is particular benefit for this type of therapy in patients with an inflammatory milieu. I look forward to seeing further analyses to help tease apart the correlation between changes in inflammation, observed weight loss and cardiovascular benefit.”
Effect on clinical practice
With the majority of patients with cardiovascular disease being overweight, these results are obviously going to increase demand for semaglutide, but cost and availability are going to be an issue.
Dr. Bhatt noted that semaglutide is already very popular. “Weight loss drugs are somewhat different from other medications. I can spend 30 minutes trying to convince a patient to take a statin, but here people realize it’s going to cause weight loss and they come in asking for it even if they don’t strictly need it. I think it’s good to have cardiovascular outcome data because now at least for this population of patients, we have evidence to prescribe it.”
He agreed with Dr. Lincoff that these new data should encourage insurance companies to cover the drug, because in reducing cardiovascular events it should also improve downstream health care costs.
“It is providing clear cardiovascular and kidney benefit, so it is in the best interest to the health care system to fund this drug,” he said. “I hope insurers look at it rationally in this way, but they may also be frightened of the explosion of patients wanting this drug and now doctors wanting to prescribe it and how that would affect their shorter-term costs.”
Dr. Lincoff said it would not be easy to prioritize certain groups. “We couldn’t identify any subgroup who showed particularly more benefit than any others. But in the evolution of any therapy, there is a time period where it is in short supply and prohibitively expensive, then over time when there is some competition and pricing deals occur as more people are advocating for it, they become more available.”
‘A welcome treatment option’
In an editorial accompanying publication of the trial, Amit Khera, MD, University of Texas Southwestern Medical Center, Dallas, and Tiffany Powell-Wiley, MD, MPH, National Institutes of Health, Bethesda, noted that baseline risk factors such as LDL cholesterol (78 mg/dL) and systolic blood pressure (131 mm Hg) were not ideal in the semaglutide group in this trial, and they suggest that the benefits of semaglutide may be attenuated when these measures are better controlled.
But given that more than 20 million people in the United States have coronary artery disease, with the majority having overweight or obesity and only approximately 30% having concomitant diabetes, they said that, even in the context of well-controlled risk factors and very low LDL cholesterol levels, the residual risk of atherosclerotic cardiovascular disease in these persons is unacceptably high. “Thus, the SELECT trial provides a welcome treatment option that can be extended to millions of additional patients.”
However, the editorialists cautioned that semaglutide at current pricing comes with a significant cost to both patients and society, which makes this treatment inaccessible for many.
They added that intensive lifestyle interventions and bariatric surgery remain effective but underutilized options for obesity, and that the prevention of obesity before it develops should be the primary goal.
The SELECT trial was supported by Novo Nordisk, and several coauthors are employees of the company. Dr. Lincoff is a consultant for Novo Nordisk. Dr. Bhatt and Dr. Nissen are involved in a cardiovascular outcomes trial with a new investigational weight loss drug from Lilly. Dr. Bhatt and Dr. Ballantyne are also investigators in a Novo Nordisk trial of a new anti-inflammatory drug.
over the approximately 3-year follow-up in patients with overweight or obesity and cardiovascular disease but not diabetes.
“This is a very exciting set of results. I think it is going to have a big impact on a large number of people,” lead investigator A. Michael Lincoff, MD, vice chair for research in the department of cardiovascular medicine at the Cleveland Clinic, said in an interview.
“And from a scientific standpoint, these data show that we now have a new pathway or a new modifiable risk factor for cardiovascular disease that we can use in our patients who have overweight or obesity,” he added.
The trial involved 17,604 patients with a history of cardiovascular disease and a body mass index of 27 kg/m2 or above (mean BMI was 33), who were randomly assigned to the glucagonlike peptide–1 (GLP-1) agonist semaglutide, given by subcutaneous injection once weekly at a gradually escalating dose up to 2.4 mg daily by week 16, or placebo. The mean baseline glycated hemoglobin level was 5.8% and 66.4% of patients met the criteria for prediabetes.
Patients lost a mean of 9.4% of body weight over the first 2 years with semaglutide versus 0.88% with placebo.
The primary cardiovascular endpoint – a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke – was reduced significantly, with a hazard ratio of 0.80 (95% confidence interval, 0.72-0.90; P < .001).
Death from cardiovascular causes, the first confirmatory secondary endpoint, showed a 15% reduction (HR, 0.85; P = .07) but this missed meeting criteria for statistical significance, and because of the hierarchical design of the trial, this meant that superiority testing was not performed for the remaining confirmatory secondary endpoints.
However, results showed reductions of around 20% for the heart failure composite endpoint and for all-cause mortality, with confidence intervals that did not cross 1.0, and directionally consistent effects were observed for all supportive secondary endpoints.
The HR for the heart failure composite endpoint was 0.82 (95% CI, 0.71-0.96), and the HR for death from any cause was 0.81 (95% CI, 0.71-0.93). Nonfatal MI was reduced by 28% (HR 0.72; 95% CI, 0.61-0.85).
The effects of semaglutide on the primary endpoint appeared to be similar across all prespecified subgroups.
Adverse events leading to discontinuation of treatment occurred in 16.6% in the semaglutide group, mostly gastrointestinal effects, and in 8.2% in the placebo group.
The trial results were presented by Dr. Lincoff at the annual scientific sessions of the American Heart Association . They were also simultaneously published online in the New England Journal of Medicine.
Dr. Lincoff explained that there is a growing pandemic of overweight and obesity worldwide with clear evidence for years that these conditions increase the risk of cardiovascular events – and yet there has been no evidence, until now, that any pharmacologic or lifestyle therapy can reduce the increased risk conferred by overweight/obesity.
“Patients in the trial were already taking standard of care therapies for other risk factors, such as hypertension and cholesterol, so this drug is giving additional benefit,” he said.
Dr. Lincoff believes these data will lead to a large increase in use of semaglutide, which is already available for the treatment of obesity and diabetes but can be difficult to get reimbursed.
“There is a lot of difficulty getting payors to pay for this drug for weight management. But with this new data from the SELECT trial there should be more willingness – at least in the population with a history of cardiovascular disease,” he commented. In diabetes, where it is already established that there is a cardiovascular risk reduction, it is easier to get these drugs reimbursed, he noted.
On the outcome data, Dr. Lincoff said he could not explain why cardiovascular death was not significantly reduced while all-cause mortality appeared to be cut more definitively.
“The cardiovascular death curves separated, then merged, then separated again. We don’t really know what is going on there. It may be that some deaths were misclassified. This trial was conducted through the COVID era and there may have been less information available on some patients because of that.”
But he added: “The all-cause mortality is more reassuring, as it doesn’t depend on classifying cause of death. Because of the design of the trial, we can’t formally claim a reduction in all-cause mortality, but the results do suggest there is an effect on this endpoint. And all the different types of cardiovascular events were similarly reduced in a consistent way, with similar effects seen across all subgroups. That is very reassuring.”
‘A new era’ for patients with obesity
Outside experts in the field were also impressed with the data.
Designated discussant of the trial at the AHA meeting, Ania Jastreboff, MD, associate professor medicine (endocrinology) at Yale University, New Haven, Conn., said the SELECT trial was “a turning point in the treatment of obesity and a call to action.
“Now is the time to treat obesity to improve health outcomes in people with cardiovascular disease,” she said.
Dr. Jastreboff noted that high BMI was estimated to have accounted for 4 million deaths worldwide in 2015, two-thirds of which were caused by cardiovascular disease. And she presented data showing that U.S. individuals meeting the SELECT criteria increased from 4.3 million in 2011-12 to 6.6 million in 2017-18.
She highlighted one major limitation of the SELECT trial: it enrolled a low number of women (38%) and ethnic minorities, with only 12% of the trial population being Black.
Deepak L. Bhatt, MD, director of Mount Sinai Fuster Heart Hospital, New York, described the SELECT results as “altogether a compelling package of data.”
“These results are even better than I had expected,” Dr. Bhatt said in an interview. “There is a significant reduction in MI as I had anticipated, but additionally, there is a reduction in all-cause death. One can debate the statistics, though on a common-sense level, I think it is a real finding,” he noted.
“Given that MI, heart failure, nephropathy, and revascularization are all reduced, and even stroke is numerically lower, it makes sense that all-cause mortality would be reduced,” he said. “To me, apart from the GI side effects, this counts as a home run.”
Steve Nissen, MD, chief academic officer at the Cleveland Clinic’s Heart, Vascular and Thoracic Institute, was similarly upbeat.
“These data prove what many of us have long suspected – that losing weight can reduce cardiovascular morbidity and mortality. This is great news for patients living with obesity. The obesity epidemic is out of control,” he added. “We need to have therapies that improve cardiovascular outcomes caused by obesity and this shows that semaglutide can do that. I think this is the beginning of a whole new era for patients with obesity.”
Michelle O’Donoghue, MD, associate professor of medicine at Harvard Medical School, Boston, called the results of SELECT “both intriguing and compelling. Certainly, these findings lend further support to the use of semaglutide in a much broader secondary prevention population of individuals with obesity.”
Christie Ballantyne, MD, director of the center for cardiometabolic disease prevention at Baylor College of Medicine, Houston, described the SELECT study as “a landmark trial which will change the practice of medicine in regard to how we treat obesity.”
He compared it with the landmark 4S trial in 1994, the first study in the area of cholesterol lowering therapy to show a clear benefit in reducing cardiovascular events and total mortality, and “began a drastic change in the way that physicians approached treatment of cholesterol.”
On the more robust reduction in all-cause death, compared with cardiovascular death,
Dr. Ballantyne pointed out: “Adjudication of dead or alive is something that everyone gets right. In contrast, the cause of death is sometime difficult to ascertain. Most importantly, the benefit on total mortality also provides assurance that this therapy does not have some adverse effect on increasing noncardiovascular deaths.”
Gastrointestinal adverse effects
On the side effects seen with semaglutide, Dr. Lincoff reported that 10% of patients in the semaglutide group discontinued treatment because of GI side effects versus 2% in the placebo arm. He said this was “an expected issue.”
“GI effects, such as nausea, vomiting and diarrhea, are known side effects of this whole class of drugs. The dose is slowly escalated to manage these adverse effects but there will be a proportion of patients who can’t tolerate it, although the vast majority are able to continue.”
He noted that, while dose reduction was allowed, of the patients who were still on the drug at 2 years, 77% were on the full dose, and 23% were on a reduced dose.
Dr. Lincoff pointed out that there were no serious adverse events with semaglutide. “This is the largest database by far now on the drug with a long-term follow up and we didn’t see the emergence of any new safety signals, which is very reassuring.”
Dr. Nissen said the 16% rate of patients stopping the drug because of tolerability “is not a trivial number.”
He noted that the semaglutide dose used in this study was larger than that used in diabetes.
“They did this to try to achieve more weight loss but then you get more issues with tolerability. It’s a trade-off. If patients are experiencing adverse effects, the dose can be reduced, but then you will lose some effect. All the GLP-1 agonists have GI side effects – it’s part of the way that they work.”
Just weight loss or other actions too?
Speculating on the mechanism behind the reduction in cardiovascular events with semaglutide, Dr. Lincoff does not think it is just weight reduction.
“The event curves start to diverge very soon after the start of the trial and yet the maximum weight loss doesn’t occur until about 65 weeks. I think something else is going on.”
In the paper, the researchers noted that GLP-1 agonists have been shown in animal studies to reduce inflammation, improve endothelial and left ventricular function, promote plaque stability, and decrease platelet aggregation. In this trial, semaglutide was associated with changes in multiple biomarkers of cardiovascular risk, including blood pressure, waist circumference, glycemic control, nephropathy, and levels of lipids and C-reactive protein.
Dr. Lincoff also pointed out that similar benefits were seen in patients with different levels of overweight, and in those who were prediabetic and those who weren’t, so benefit was not dependent on baseline BMI or glycated hemoglobin levels.
Dr. O’Donoghue agreed that other effects, as well as weight loss, could be involved. “The reduction in events with semaglutide appeared very early after initiation and far preceded the drug’s maximal effects on weight reduction. This might suggest that the drug offers other cardioprotective effects through pathways independent of weight loss. Certainly, semaglutide and the other GLP-1 agonists appear to attenuate inflammation, and the patterns of redistribution of adipose tissue may also be of interest.”
She also pointed out that the reduction in cardiovascular events appeared even earlier in this population of obese nondiabetic patients with cardiovascular disease than in prior studies of patients with diabetes. “It may suggest that there is particular benefit for this type of therapy in patients with an inflammatory milieu. I look forward to seeing further analyses to help tease apart the correlation between changes in inflammation, observed weight loss and cardiovascular benefit.”
Effect on clinical practice
With the majority of patients with cardiovascular disease being overweight, these results are obviously going to increase demand for semaglutide, but cost and availability are going to be an issue.
Dr. Bhatt noted that semaglutide is already very popular. “Weight loss drugs are somewhat different from other medications. I can spend 30 minutes trying to convince a patient to take a statin, but here people realize it’s going to cause weight loss and they come in asking for it even if they don’t strictly need it. I think it’s good to have cardiovascular outcome data because now at least for this population of patients, we have evidence to prescribe it.”
He agreed with Dr. Lincoff that these new data should encourage insurance companies to cover the drug, because in reducing cardiovascular events it should also improve downstream health care costs.
“It is providing clear cardiovascular and kidney benefit, so it is in the best interest to the health care system to fund this drug,” he said. “I hope insurers look at it rationally in this way, but they may also be frightened of the explosion of patients wanting this drug and now doctors wanting to prescribe it and how that would affect their shorter-term costs.”
Dr. Lincoff said it would not be easy to prioritize certain groups. “We couldn’t identify any subgroup who showed particularly more benefit than any others. But in the evolution of any therapy, there is a time period where it is in short supply and prohibitively expensive, then over time when there is some competition and pricing deals occur as more people are advocating for it, they become more available.”
‘A welcome treatment option’
In an editorial accompanying publication of the trial, Amit Khera, MD, University of Texas Southwestern Medical Center, Dallas, and Tiffany Powell-Wiley, MD, MPH, National Institutes of Health, Bethesda, noted that baseline risk factors such as LDL cholesterol (78 mg/dL) and systolic blood pressure (131 mm Hg) were not ideal in the semaglutide group in this trial, and they suggest that the benefits of semaglutide may be attenuated when these measures are better controlled.
But given that more than 20 million people in the United States have coronary artery disease, with the majority having overweight or obesity and only approximately 30% having concomitant diabetes, they said that, even in the context of well-controlled risk factors and very low LDL cholesterol levels, the residual risk of atherosclerotic cardiovascular disease in these persons is unacceptably high. “Thus, the SELECT trial provides a welcome treatment option that can be extended to millions of additional patients.”
However, the editorialists cautioned that semaglutide at current pricing comes with a significant cost to both patients and society, which makes this treatment inaccessible for many.
They added that intensive lifestyle interventions and bariatric surgery remain effective but underutilized options for obesity, and that the prevention of obesity before it develops should be the primary goal.
The SELECT trial was supported by Novo Nordisk, and several coauthors are employees of the company. Dr. Lincoff is a consultant for Novo Nordisk. Dr. Bhatt and Dr. Nissen are involved in a cardiovascular outcomes trial with a new investigational weight loss drug from Lilly. Dr. Bhatt and Dr. Ballantyne are also investigators in a Novo Nordisk trial of a new anti-inflammatory drug.
over the approximately 3-year follow-up in patients with overweight or obesity and cardiovascular disease but not diabetes.
“This is a very exciting set of results. I think it is going to have a big impact on a large number of people,” lead investigator A. Michael Lincoff, MD, vice chair for research in the department of cardiovascular medicine at the Cleveland Clinic, said in an interview.
“And from a scientific standpoint, these data show that we now have a new pathway or a new modifiable risk factor for cardiovascular disease that we can use in our patients who have overweight or obesity,” he added.
The trial involved 17,604 patients with a history of cardiovascular disease and a body mass index of 27 kg/m2 or above (mean BMI was 33), who were randomly assigned to the glucagonlike peptide–1 (GLP-1) agonist semaglutide, given by subcutaneous injection once weekly at a gradually escalating dose up to 2.4 mg daily by week 16, or placebo. The mean baseline glycated hemoglobin level was 5.8% and 66.4% of patients met the criteria for prediabetes.
Patients lost a mean of 9.4% of body weight over the first 2 years with semaglutide versus 0.88% with placebo.
The primary cardiovascular endpoint – a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke – was reduced significantly, with a hazard ratio of 0.80 (95% confidence interval, 0.72-0.90; P < .001).
Death from cardiovascular causes, the first confirmatory secondary endpoint, showed a 15% reduction (HR, 0.85; P = .07) but this missed meeting criteria for statistical significance, and because of the hierarchical design of the trial, this meant that superiority testing was not performed for the remaining confirmatory secondary endpoints.
However, results showed reductions of around 20% for the heart failure composite endpoint and for all-cause mortality, with confidence intervals that did not cross 1.0, and directionally consistent effects were observed for all supportive secondary endpoints.
The HR for the heart failure composite endpoint was 0.82 (95% CI, 0.71-0.96), and the HR for death from any cause was 0.81 (95% CI, 0.71-0.93). Nonfatal MI was reduced by 28% (HR 0.72; 95% CI, 0.61-0.85).
The effects of semaglutide on the primary endpoint appeared to be similar across all prespecified subgroups.
Adverse events leading to discontinuation of treatment occurred in 16.6% in the semaglutide group, mostly gastrointestinal effects, and in 8.2% in the placebo group.
The trial results were presented by Dr. Lincoff at the annual scientific sessions of the American Heart Association . They were also simultaneously published online in the New England Journal of Medicine.
Dr. Lincoff explained that there is a growing pandemic of overweight and obesity worldwide with clear evidence for years that these conditions increase the risk of cardiovascular events – and yet there has been no evidence, until now, that any pharmacologic or lifestyle therapy can reduce the increased risk conferred by overweight/obesity.
“Patients in the trial were already taking standard of care therapies for other risk factors, such as hypertension and cholesterol, so this drug is giving additional benefit,” he said.
Dr. Lincoff believes these data will lead to a large increase in use of semaglutide, which is already available for the treatment of obesity and diabetes but can be difficult to get reimbursed.
“There is a lot of difficulty getting payors to pay for this drug for weight management. But with this new data from the SELECT trial there should be more willingness – at least in the population with a history of cardiovascular disease,” he commented. In diabetes, where it is already established that there is a cardiovascular risk reduction, it is easier to get these drugs reimbursed, he noted.
On the outcome data, Dr. Lincoff said he could not explain why cardiovascular death was not significantly reduced while all-cause mortality appeared to be cut more definitively.
“The cardiovascular death curves separated, then merged, then separated again. We don’t really know what is going on there. It may be that some deaths were misclassified. This trial was conducted through the COVID era and there may have been less information available on some patients because of that.”
But he added: “The all-cause mortality is more reassuring, as it doesn’t depend on classifying cause of death. Because of the design of the trial, we can’t formally claim a reduction in all-cause mortality, but the results do suggest there is an effect on this endpoint. And all the different types of cardiovascular events were similarly reduced in a consistent way, with similar effects seen across all subgroups. That is very reassuring.”
‘A new era’ for patients with obesity
Outside experts in the field were also impressed with the data.
Designated discussant of the trial at the AHA meeting, Ania Jastreboff, MD, associate professor medicine (endocrinology) at Yale University, New Haven, Conn., said the SELECT trial was “a turning point in the treatment of obesity and a call to action.
“Now is the time to treat obesity to improve health outcomes in people with cardiovascular disease,” she said.
Dr. Jastreboff noted that high BMI was estimated to have accounted for 4 million deaths worldwide in 2015, two-thirds of which were caused by cardiovascular disease. And she presented data showing that U.S. individuals meeting the SELECT criteria increased from 4.3 million in 2011-12 to 6.6 million in 2017-18.
She highlighted one major limitation of the SELECT trial: it enrolled a low number of women (38%) and ethnic minorities, with only 12% of the trial population being Black.
Deepak L. Bhatt, MD, director of Mount Sinai Fuster Heart Hospital, New York, described the SELECT results as “altogether a compelling package of data.”
“These results are even better than I had expected,” Dr. Bhatt said in an interview. “There is a significant reduction in MI as I had anticipated, but additionally, there is a reduction in all-cause death. One can debate the statistics, though on a common-sense level, I think it is a real finding,” he noted.
“Given that MI, heart failure, nephropathy, and revascularization are all reduced, and even stroke is numerically lower, it makes sense that all-cause mortality would be reduced,” he said. “To me, apart from the GI side effects, this counts as a home run.”
Steve Nissen, MD, chief academic officer at the Cleveland Clinic’s Heart, Vascular and Thoracic Institute, was similarly upbeat.
“These data prove what many of us have long suspected – that losing weight can reduce cardiovascular morbidity and mortality. This is great news for patients living with obesity. The obesity epidemic is out of control,” he added. “We need to have therapies that improve cardiovascular outcomes caused by obesity and this shows that semaglutide can do that. I think this is the beginning of a whole new era for patients with obesity.”
Michelle O’Donoghue, MD, associate professor of medicine at Harvard Medical School, Boston, called the results of SELECT “both intriguing and compelling. Certainly, these findings lend further support to the use of semaglutide in a much broader secondary prevention population of individuals with obesity.”
Christie Ballantyne, MD, director of the center for cardiometabolic disease prevention at Baylor College of Medicine, Houston, described the SELECT study as “a landmark trial which will change the practice of medicine in regard to how we treat obesity.”
He compared it with the landmark 4S trial in 1994, the first study in the area of cholesterol lowering therapy to show a clear benefit in reducing cardiovascular events and total mortality, and “began a drastic change in the way that physicians approached treatment of cholesterol.”
On the more robust reduction in all-cause death, compared with cardiovascular death,
Dr. Ballantyne pointed out: “Adjudication of dead or alive is something that everyone gets right. In contrast, the cause of death is sometime difficult to ascertain. Most importantly, the benefit on total mortality also provides assurance that this therapy does not have some adverse effect on increasing noncardiovascular deaths.”
Gastrointestinal adverse effects
On the side effects seen with semaglutide, Dr. Lincoff reported that 10% of patients in the semaglutide group discontinued treatment because of GI side effects versus 2% in the placebo arm. He said this was “an expected issue.”
“GI effects, such as nausea, vomiting and diarrhea, are known side effects of this whole class of drugs. The dose is slowly escalated to manage these adverse effects but there will be a proportion of patients who can’t tolerate it, although the vast majority are able to continue.”
He noted that, while dose reduction was allowed, of the patients who were still on the drug at 2 years, 77% were on the full dose, and 23% were on a reduced dose.
Dr. Lincoff pointed out that there were no serious adverse events with semaglutide. “This is the largest database by far now on the drug with a long-term follow up and we didn’t see the emergence of any new safety signals, which is very reassuring.”
Dr. Nissen said the 16% rate of patients stopping the drug because of tolerability “is not a trivial number.”
He noted that the semaglutide dose used in this study was larger than that used in diabetes.
“They did this to try to achieve more weight loss but then you get more issues with tolerability. It’s a trade-off. If patients are experiencing adverse effects, the dose can be reduced, but then you will lose some effect. All the GLP-1 agonists have GI side effects – it’s part of the way that they work.”
Just weight loss or other actions too?
Speculating on the mechanism behind the reduction in cardiovascular events with semaglutide, Dr. Lincoff does not think it is just weight reduction.
“The event curves start to diverge very soon after the start of the trial and yet the maximum weight loss doesn’t occur until about 65 weeks. I think something else is going on.”
In the paper, the researchers noted that GLP-1 agonists have been shown in animal studies to reduce inflammation, improve endothelial and left ventricular function, promote plaque stability, and decrease platelet aggregation. In this trial, semaglutide was associated with changes in multiple biomarkers of cardiovascular risk, including blood pressure, waist circumference, glycemic control, nephropathy, and levels of lipids and C-reactive protein.
Dr. Lincoff also pointed out that similar benefits were seen in patients with different levels of overweight, and in those who were prediabetic and those who weren’t, so benefit was not dependent on baseline BMI or glycated hemoglobin levels.
Dr. O’Donoghue agreed that other effects, as well as weight loss, could be involved. “The reduction in events with semaglutide appeared very early after initiation and far preceded the drug’s maximal effects on weight reduction. This might suggest that the drug offers other cardioprotective effects through pathways independent of weight loss. Certainly, semaglutide and the other GLP-1 agonists appear to attenuate inflammation, and the patterns of redistribution of adipose tissue may also be of interest.”
She also pointed out that the reduction in cardiovascular events appeared even earlier in this population of obese nondiabetic patients with cardiovascular disease than in prior studies of patients with diabetes. “It may suggest that there is particular benefit for this type of therapy in patients with an inflammatory milieu. I look forward to seeing further analyses to help tease apart the correlation between changes in inflammation, observed weight loss and cardiovascular benefit.”
Effect on clinical practice
With the majority of patients with cardiovascular disease being overweight, these results are obviously going to increase demand for semaglutide, but cost and availability are going to be an issue.
Dr. Bhatt noted that semaglutide is already very popular. “Weight loss drugs are somewhat different from other medications. I can spend 30 minutes trying to convince a patient to take a statin, but here people realize it’s going to cause weight loss and they come in asking for it even if they don’t strictly need it. I think it’s good to have cardiovascular outcome data because now at least for this population of patients, we have evidence to prescribe it.”
He agreed with Dr. Lincoff that these new data should encourage insurance companies to cover the drug, because in reducing cardiovascular events it should also improve downstream health care costs.
“It is providing clear cardiovascular and kidney benefit, so it is in the best interest to the health care system to fund this drug,” he said. “I hope insurers look at it rationally in this way, but they may also be frightened of the explosion of patients wanting this drug and now doctors wanting to prescribe it and how that would affect their shorter-term costs.”
Dr. Lincoff said it would not be easy to prioritize certain groups. “We couldn’t identify any subgroup who showed particularly more benefit than any others. But in the evolution of any therapy, there is a time period where it is in short supply and prohibitively expensive, then over time when there is some competition and pricing deals occur as more people are advocating for it, they become more available.”
‘A welcome treatment option’
In an editorial accompanying publication of the trial, Amit Khera, MD, University of Texas Southwestern Medical Center, Dallas, and Tiffany Powell-Wiley, MD, MPH, National Institutes of Health, Bethesda, noted that baseline risk factors such as LDL cholesterol (78 mg/dL) and systolic blood pressure (131 mm Hg) were not ideal in the semaglutide group in this trial, and they suggest that the benefits of semaglutide may be attenuated when these measures are better controlled.
But given that more than 20 million people in the United States have coronary artery disease, with the majority having overweight or obesity and only approximately 30% having concomitant diabetes, they said that, even in the context of well-controlled risk factors and very low LDL cholesterol levels, the residual risk of atherosclerotic cardiovascular disease in these persons is unacceptably high. “Thus, the SELECT trial provides a welcome treatment option that can be extended to millions of additional patients.”
However, the editorialists cautioned that semaglutide at current pricing comes with a significant cost to both patients and society, which makes this treatment inaccessible for many.
They added that intensive lifestyle interventions and bariatric surgery remain effective but underutilized options for obesity, and that the prevention of obesity before it develops should be the primary goal.
The SELECT trial was supported by Novo Nordisk, and several coauthors are employees of the company. Dr. Lincoff is a consultant for Novo Nordisk. Dr. Bhatt and Dr. Nissen are involved in a cardiovascular outcomes trial with a new investigational weight loss drug from Lilly. Dr. Bhatt and Dr. Ballantyne are also investigators in a Novo Nordisk trial of a new anti-inflammatory drug.
FROM AHA 2023
Does vaginal estrogen use increase the risk for adverse cardiovascular outcomes?
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.
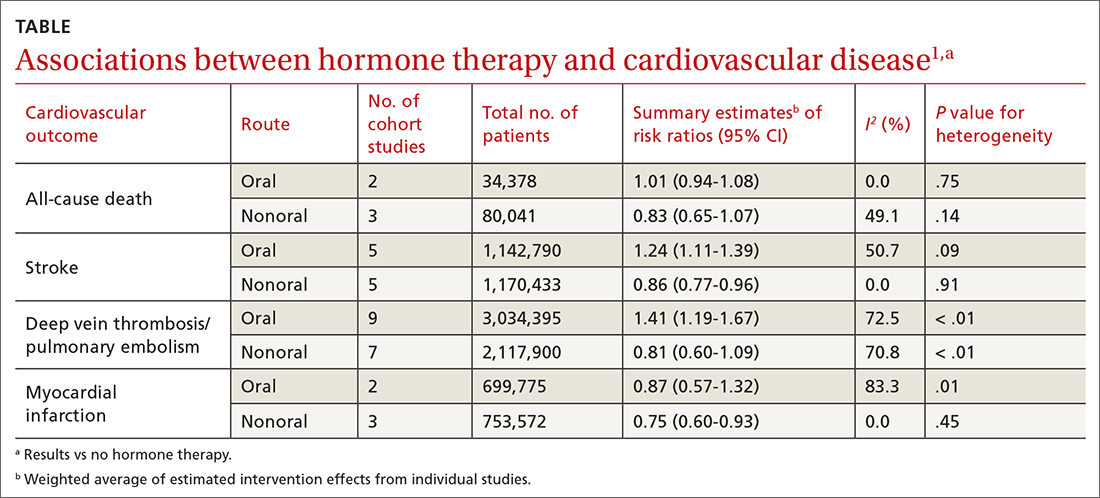
Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
Evidence summary
Cohort studies demonstrate no adverse CV outcomes
A 2020 systematic review and meta-analysis evaluated randomized controlled trials (RCTs) and observational studies to examine the association between menopausal hormone therapy and CV disease.1 The 26 RCTs primarily evaluated oral hormone administration. The observational studies comprised 30 cohort studies, 13 case-control studies, and 5 nested case-control studies, primarily in Europe and North America; 21 reported the route of administration. The trials evaluated women ages 49 to 77 years (mean, 61 years), and follow-up ranged from 1 to 21.5 years (mean, 7 years). In subgroup analyses of the observational studies, nonoral hormone therapy was associated with a lower risk for stroke and MI compared to oral administration (see TABLE1). Study limitations included enrollment of patients with few comorbidities, from limited geographic regions. Results in the meta-analysis were not stratified by the type of nonoral hormone therapy; only 4 studies evaluated vaginal estrogen use.

Two large cohort studies included in the systematic review provided more specific data on vaginal estrogens. The first used data from the Women’s Health Initiative in a subset of women ages 50 to 79 years (n = 46,566) who were not already on systemic hormone therapy and who did not have prior history of breast, endometrial, or ovarian cancer.2 Data were collected from self-assessment questionnaires and medical record reviews. The median duration of vaginal estrogen use was 2 years, and median follow-up duration was 7.2 years. Vaginal estrogen users had a 48% lower risk for CHD (adjusted hazard ratio [aHR] = 0.52; 95% CI, 0.31-0.85) than nonusers. Rates for all-cause mortality (aHR = 0.78; 95% CI, 0.58-1.04), stroke (aHR = 0.78; 95% CI, 0.49-1.24), and DVT/PE (aHR = 0.68; 95% CI, 0.36-1.28) were similar. In this and the other cohort studies to be discussed, outcome data for all vaginal estrogen preparations (eg, cream, ring, tablet) were combined.
The other large cohort study in the systematic review evaluated data on postmenopausal women from the Nurses’ Health Study.3 The authors evaluated health reports on 53,797 women as they transitioned through menopause. Patients with systemic hormone therapy use, history of cancer, and self-reported CV disease were excluded. After adjusting for covariates, the authors found no statistically significant difference between users and nonusers of vaginal estrogen and risk for total MI (aHR = 0.73; 95% CI, 0.47-1.13), stroke (aHR = 0.85; 95% CI, 0.56-1.29), or DVT/PE (aHR = 1.06; 95% CI, 0.58-1.93). Study limitations included low prevalence of vaginal estrogen use (< 3%), short duration of use (mean, 37.5 months), and lack of data on the type or dose of vaginal estrogen used. The study only included health professionals, which limits generalizability.
A Finnish cohort study (excluded from the systematic review because it used historical controls) compared rates of CHD and stroke in postmenopausal women who used vaginal estrogen against an age-matched background population. Researchers collected data from a nationwide prescription registry for women at least 50 years old who had purchased vaginal estrogens between 1994 and 2009 (n = 195,756).4 Women who purchased systemic hormone therapy at any point were excluded. After 3 to 5 years of exposure, use of vaginal estrogen was associated with a decreased risk for mortality from CHD (relative risk [RR] = 0.64; 95% CI, 0.57-0.70) and stroke (RR = 0.79; 95% CI, 0.69-0.91). However, after 10 years, these benefits were not seen (CHD: RR = 0.95; 95% CI, 0.90-1.00; stroke: RR = 0.93; 95% CI, 0.85-1.01). All confidence interval data were presented graphically. Key weaknesses of this study included use of both vaginal and systemic estrogen in the comparator background population, and the failure to collect data for other CV risk variables such as weight, tobacco exposure, and blood pressure.
Recommendations from others
In 2022, the North American Menopause Society issued a Hormone Therapy Position Statement that acknowledged the lack of clinical trials directly comparing risk for adverse CV endpoints with different estrogen administration routes.5 They stated nonoral routes of administration might offer advantages by bypassing first-pass hepatic metabolism.
Similarly, the 2015 Endocrine Society Clinical Practice Guideline on the Treatment of Symptoms of the Menopause also stated that the effects of low-dose vaginal estrogen therapy on CV disease or DVT/PE risk had not been adequately studied.6
A 2013 opinion by the American College of Obstetricians and Gynecologists stated that topical estrogen vaginal creams, tablets, and rings had low levels of systemic absorption and were not associated with an increased risk for DVT/PE.7
Editor’s takeaway
The available evidence on vaginal estrogen replacement reassures us of its safety. After decades spent studying hormone replacement therapy with vacillating conclusions and opinions, these cohorts—the best evidence we may ever get—along with a consensus of expert opinions, consistently demonstrate no adverse CV outcomes.
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
1. Kim JE, Chang JH, Jeong MJ, et al. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci Rep. 2020;10:20631. doi: 10.1038/s41598-020-77534-9
2. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the WHI Observational Study. Menopause. 2018;25:11-20. doi: 10.1097/GME.0000000000000956
3. Bhupathiraju SN, Grodstein F, Stampfer MJ, et al. Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause. 2018;26:603-610. doi: 10.1097/GME.0000000000001284
4. Mikkola TS, Tuomikoski P, Lyytinen H, et al. Vaginal estrogen use and the risk for cardiovascular mortality. Human Reproduction. 2016;31:804-809. doi: 10.1093/humrep/dew014
5. North American Menopause Society. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi: 10.1097/GME.0000000000002028
6. Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236
7. American College of Obstetricians and Gynecologists. Committee Opinion No 565: hormone therapy and heart disease. Obstet Gynecol. 2013;121:1407-1410. doi: 10.1097/01.AOG.0000431053.33593.2d
EVIDENCE-BASED ANSWER:
NO. In general, nonoral estrogen use for menopausal symptoms is associated with a lower cardiovascular (CV) risk profile than oral estrogen use (strength of recommendation [SOR], B; meta-analysis of cohort studies). Vaginal estrogen use is associated with lower risk for coronary heart disease (CHD) and similar risk for myocardial infarction (MI), stroke, and deep vein thrombosis/pulmonary embolism (DVT/PE) compared with nonuse (SOR, B; cohort studies). Vaginal estrogen therapy also is associated with lower CV-related mortality for 3 to 5 years compared with nonuse (SOR, B; cohort study). No high-quality randomized trials address this topic.
In MI with anemia, results may favor liberal transfusion: MINT
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
Two biomarkers promising for preeclampsia prediction
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Two biomarkers – pregnancy-associated plasma protein A2 (PAPP-A2) and activin A – when added to relevant clinical information have a better positive predictive value than and a comparable negative predictive value to the currently used ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF), new research suggests.
The third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia. By contrast, , according to the authors.
Preeclampsia has “potentially devastating maternal and fetal complications, [including] significantly increased cardiovascular risk for affected women later in life,” study author Stella S. Daskalopoulou, MD, PhD, associate professor of medicine at McGill University Health Centre in Montreal, said in an interview.
“A more accurate prediction of preeclampsia is expected to improve risk stratification and clinical care and shape clinical practice guidelines,” she said.
The study was published online in the Canadian Journal of Cardiology.
Better predictive value
For a prospective cohort study, the investigators recruited 192 women with first-trimester high-risk singleton pregnancies from tertiary obstetric clinics in Montreal.
At baseline, they collected clinical information, including height, prepregnancy weight, personal and family medical history, and medication use.
At each trimester, blood pressure was measured, and blood samples were collected to quantify sFlt-1, PlGF, PAPP-A2, PAPP-A, activin A, inhibin A, follistatin, and glycosylated fibronectin. For the sFlt-1:PlGF ratio, the researchers used a cutoff point of 38, based on prior evidence. Because there are no agreed-upon cutoff points for the other biomarkers, they chose cutoff points that maximized sensitivity and specificity.
Pregnancies were considered high risk if the mother had any of the following conditions: prepregnancy BMI ≥ 25, maternal age ≥ 35 years, chronic hypertension, diabetes, renal disease, conception via in vitro fertilization, or maternal or first-degree family history of preeclampsia.
The primary outcome was preeclampsia, which was defined according to the Society of Obstetrics and Gynecology guidelines as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ mm Hg together with either proteinuria or maternal end-organ dysfunction.
A total of 18 women (9.38%) developed preeclampsia. Those women had higher blood pressure at baseline (although it was within normal limits) and were more likely to have preexisting diabetes or a previous pregnancy with preeclampsia. They were also more likely to report Black race. Serum levels of PAPP-A, PAPP-A2, activin A, and inhibin A were significantly different between patients who developed preeclampsia and those who did not. These levels were increased throughout pregnancy.
Alongside the sFlt-1:PlGF ratio, two biomarkers, PAPP-A2 (odds ratio, 1.78) and activin A (OR, 1.84), were significantly associated with the primary outcome after adjustment for age, prepregnancy BMI, race, and mean arterial pressure.
When added to a model that included those clinical factors, a positive third-trimester result for both PAPP-A2 and activin A had a better positive predictive value than the sFlt-1:PlGF ratio added to the clinical model (91.67% vs. 66.67%). The two biomarkers also had a negative predictive value that was comparable to that of the sFlt-1:PlGF ratio (97.69% vs. 96%).
Study limitations include the small sample size and missing covariates for some participants. Furthermore, the findings cannot be generalized to low-risk populations.
“Whereas the third-trimester sFlt-1:PlGF ratio can predict short-term absence of preeclampsia, PAPP-A2 and activin A had both high positive and negative predictive values and thus could serve as biomarkers to predict the occurrence (and absence) of preeclampsia; these findings will be validated in future studies,” the authors concluded.
Dr. Daskalopoulou said that her group is currently performing a large multinational study, PULSE, “which will be the ideal platform to validate and extend our findings. The aim of the study is to predict preeclampsia using a multimodal approach that includes arterial stiffness measurements and blood biomarkers.”
She expanded on the potential benefits of this research. “Finding an accurate predictive tool would not only help design appropriate early care plans for truly high-risk pregnant women, including monitoring and delivery planning, but also facilitate the development of novel strategies for the prevention and treatment of preeclampsia, improving the life of millions of young mothers and their offspring around the world.”
Promising biomarkers
Commenting on the study, Nieca Goldberg, MD, clinical associate professor of medicine at NYU Langone Health and medical director of Atria, both in New York, said, “These biomarkers are promising, as the current biomarker, sFlt-1:PlGF, is good at ruling out preeclampsia in the short term, while the new biomarkers show that they are better at ruling in preeclampsia” as well as ruling it out. Dr. Goldberg was not involved in the research.
“The current study is small, some participant data points are missing, and the researchers only studied high-risk pregnancies,” she added. “We need larger studies of all the risk markers, in both high- and low-risk pregnancies that are followed throughout pregnancy.”
This work was supported by the Fonds de recherche du Québec Santé (FRQS), Heart and Stroke Foundation of Canada, McGill University Department of Obstetrics and Gynecology Academic Enrichment Fund, and Canadian Foundation for Women›s Health. Dr. Daskalopoulou is a senior clinician-scientist supported by a FRQS Clinician Scientist-Senior salary award. Dr. Daskalopoulou and Dr. Goldberg disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
Women have worse outcomes in cardiogenic shock
“These data identify the need for us to continue working to identify barriers in terms of diagnosis, management, and technological innovations for women in cardiogenic shock to resolve these issues and improve outcomes,” the senior author of the study, Navin Kapur, MD, Tufts Medical Center, Boston, said in an interview.
The study is said to be the one of the largest contemporary analyses of real-world registry data on the characteristics and outcomes of women in comparison with men with cardiogenic shock.
It showed sex-specific differences in outcomes that were primarily driven by differences in heart failure–related cardiogenic shock. Women with heart failure–related cardiogenic shock had more severe cardiogenic shock, worse survival at discharge, and more vascular complications than men. Outcomes in cardiogenic shock related to MI were similar for men and women.
The study, which will be presented at the upcoming annual meeting of the American Heart Association, was published online in JACC: Heart Failure.
Dr. Kapur founded the Cardiogenic Shock Working Group in 2017 to collect quality data on the condition.
“We realized our patients were dying, and we didn’t have enough data on how best to manage them. So, we started this registry, and now have detailed data on close to 9,000 patients with cardiogenic shock from 45 hospitals in the U.S., Mexico, Australia, and Japan,” he explained.
“The primary goal is to try to investigate the questions related to cardiogenic shock that can inform management, and one of the key questions that came up was differences in how men and women present with cardiogenic shock and what their outcomes may be. This is what we are reporting in this paper,” he added.
Cardiogenic shock is defined as having a low cardiac output most commonly because of MI or an episode of acute heart failure, Dr. Kapur said. Patients with cardiogenic shock are identified by their low blood pressure or hypoperfusion evidenced by clinical exam or biomarkers, such as elevated lactate levels.
“In this analysis, we’re looking at patients presenting with cardiogenic shock, so were not looking at the incidence of the condition in men versus women,” Dr. Kapur noted. “However, we believe that cardiogenic shock is probably more underrepresented in women, who may present with an MI or acute heart failure and may or may not be identified as having low cardiac output states until quite late. The likelihood is that the incidence is similar in men and women, but women are more often undiagnosed.”
For the current study, the authors analyzed data on 5,083 patients with cardiogenic shock in the registry, of whom 1,522 (30%) were women. Compared with men, women had slightly higher body mass index (BMI) and smaller body surface area.
Results showed that women with heart failure–related cardiogenic shock had worse survival at discharge than men (69.9% vs. 74.4%) and a higher rate of refractory shock (SCAI stage E; 26% vs. 21%). Women were also less likely to undergo pulmonary artery catheterization (52.9% vs. 54.6%), heart transplantation (6.5% vs. 10.3%), or left ventricular assist device implantation (7.8% vs. 10%).
Regardless of cardiogenic shock etiology, women had more vascular complications (8.8% vs. 5.7%), bleeding (7.1% vs. 5.2%), and limb ischemia (6.8% vs. 4.5%).
“This analysis is quite revealing. We identified some important distinctions between men and women,” Dr. Kapur commented.
For many patients who present with MI-related cardiogenic shock, many of the baseline characteristics in men and women were quite similar, he said. “But in heart failure–related cardiogenic shock, we saw more differences, with typical comorbidities associated with cardiogenic shock [e.g., diabetes, chronic kidney disease, hypertension] being less common in women than in men. This suggests there may be phenotypic differences as to why women present with heart failure shock versus men.”
Dr. Kapur pointed out that differences in BMI or body surface area between men and women may play into some of the management decision-making.
“Women having a smaller stature may lead to a selection bias where we don’t want to use large-bore pumps or devices because we’re worried about causing complications. We found in the analysis that vascular complications such as bleeding or ischemia of the lower extremity where these devices typically go were more frequent in women,” he noted.
“We also found that women were less likely to receive invasive therapies in general, including pulmonary artery catheters, temporary mechanical support, and heart replacements, such as LVAD or transplants,” he added.
Further results showed that, after propensity score matching, some of the gender differences disappeared, but women continued to have a higher rate of vascular complications (10.4% women vs. 7.4% men).
But Dr. Kapur warned that the propensity-matched analysis had some caveats.
“Essentially what we are doing with propensity matching is creating two populations that are as similar as possible, and this reduced the number of patients in the analysis down to 25% of the original population,” he said. “One of the things we had to match was body surface area, and in doing this, we are taking out one of the most important differences between men and women, and as a result, a lot of the differences in outcomes go away.
“In this respect, propensity matching can be a bit of a double-edge sword,” he added. “I think the non–propensity-matched results are more interesting, as they are more of a reflection of the real world.”
Dr. Kapur concluded that these findings are compelling enough to suggest that there are important differences between women and men with cardiogenic shock in terms of outcomes as well as complication rates.
“Our decision-making around women seems to be different to that around men. I think this paper should start to trigger more awareness of that.”
Dr. Kapur also emphasized the importance of paying attention to vascular complications in women.
“The higher rates of bleeding and limb ischemia issues in women may explain the rationale for being less aggressive with invasive therapies in women,” he said. “But we need to come up with better solutions or technologies so they can be used more effectively in women. This could include adapting technology for smaller vascular sizes, which should lead to better outcome and fewer complications in women.”
He added that further granular data on this issue are needed. “We have very limited datasets in cardiogenic shock. There are few randomized controlled trials, and women are not well represented in such trials. We need to make sure we enroll women in randomized trials.”
Dr. Kapur said more women physicians who treat cardiogenic shock are also required, which would include cardiologists, critical care specialists, cardiac surgeons, and anesthesia personnel.
He pointed out that the two first authors of the current study are women – Van-Khue Ton, MD, Massachusetts General Hospital, Boston, and Manreet Kanwar, MD, Allegheny Health Network, Pittsburgh.
“We worked hard to involve women as principal investigators. They led the effort. These are investigations led by women, on women, to advance the care of women,” he commented.
Gender-related inequality
In an editorial accompanying publication of the study, Sara Kalantari, MD, and Jonathan Grinstein, MD, University of Chicago, and Robert O. Roswell, MD, Hofstra University, Hempstead, N.Y., said these results “provide valuable information about gender-related inequality in care and outcomes in the management of cardiogenic shock, although the exact mechanisms driving these observed differences still need to be elucidated.
“Broadly speaking, barriers in the care of women with heart failure and cardiogenic shock include a reduced awareness among both patients and providers, a deficiency of sex-specific objective criteria for guiding therapy, and unfavorable temporary mechanical circulatory support devices with higher rates of hemocompatibility-related complications in women,” they added.
“In the era of the multidisciplinary shock team and shock pathways with protocolized management algorithms, it is imperative that we still allow for personalization of care to match the physiologic needs of the patient in order for us to continue to close the gender gap in the care of patients presenting with cardiogenic shock,” the editorialists concluded.
A version of this article appeared on Medscape.com.
“These data identify the need for us to continue working to identify barriers in terms of diagnosis, management, and technological innovations for women in cardiogenic shock to resolve these issues and improve outcomes,” the senior author of the study, Navin Kapur, MD, Tufts Medical Center, Boston, said in an interview.
The study is said to be the one of the largest contemporary analyses of real-world registry data on the characteristics and outcomes of women in comparison with men with cardiogenic shock.
It showed sex-specific differences in outcomes that were primarily driven by differences in heart failure–related cardiogenic shock. Women with heart failure–related cardiogenic shock had more severe cardiogenic shock, worse survival at discharge, and more vascular complications than men. Outcomes in cardiogenic shock related to MI were similar for men and women.
The study, which will be presented at the upcoming annual meeting of the American Heart Association, was published online in JACC: Heart Failure.
Dr. Kapur founded the Cardiogenic Shock Working Group in 2017 to collect quality data on the condition.
“We realized our patients were dying, and we didn’t have enough data on how best to manage them. So, we started this registry, and now have detailed data on close to 9,000 patients with cardiogenic shock from 45 hospitals in the U.S., Mexico, Australia, and Japan,” he explained.
“The primary goal is to try to investigate the questions related to cardiogenic shock that can inform management, and one of the key questions that came up was differences in how men and women present with cardiogenic shock and what their outcomes may be. This is what we are reporting in this paper,” he added.
Cardiogenic shock is defined as having a low cardiac output most commonly because of MI or an episode of acute heart failure, Dr. Kapur said. Patients with cardiogenic shock are identified by their low blood pressure or hypoperfusion evidenced by clinical exam or biomarkers, such as elevated lactate levels.
“In this analysis, we’re looking at patients presenting with cardiogenic shock, so were not looking at the incidence of the condition in men versus women,” Dr. Kapur noted. “However, we believe that cardiogenic shock is probably more underrepresented in women, who may present with an MI or acute heart failure and may or may not be identified as having low cardiac output states until quite late. The likelihood is that the incidence is similar in men and women, but women are more often undiagnosed.”
For the current study, the authors analyzed data on 5,083 patients with cardiogenic shock in the registry, of whom 1,522 (30%) were women. Compared with men, women had slightly higher body mass index (BMI) and smaller body surface area.
Results showed that women with heart failure–related cardiogenic shock had worse survival at discharge than men (69.9% vs. 74.4%) and a higher rate of refractory shock (SCAI stage E; 26% vs. 21%). Women were also less likely to undergo pulmonary artery catheterization (52.9% vs. 54.6%), heart transplantation (6.5% vs. 10.3%), or left ventricular assist device implantation (7.8% vs. 10%).
Regardless of cardiogenic shock etiology, women had more vascular complications (8.8% vs. 5.7%), bleeding (7.1% vs. 5.2%), and limb ischemia (6.8% vs. 4.5%).
“This analysis is quite revealing. We identified some important distinctions between men and women,” Dr. Kapur commented.
For many patients who present with MI-related cardiogenic shock, many of the baseline characteristics in men and women were quite similar, he said. “But in heart failure–related cardiogenic shock, we saw more differences, with typical comorbidities associated with cardiogenic shock [e.g., diabetes, chronic kidney disease, hypertension] being less common in women than in men. This suggests there may be phenotypic differences as to why women present with heart failure shock versus men.”
Dr. Kapur pointed out that differences in BMI or body surface area between men and women may play into some of the management decision-making.
“Women having a smaller stature may lead to a selection bias where we don’t want to use large-bore pumps or devices because we’re worried about causing complications. We found in the analysis that vascular complications such as bleeding or ischemia of the lower extremity where these devices typically go were more frequent in women,” he noted.
“We also found that women were less likely to receive invasive therapies in general, including pulmonary artery catheters, temporary mechanical support, and heart replacements, such as LVAD or transplants,” he added.
Further results showed that, after propensity score matching, some of the gender differences disappeared, but women continued to have a higher rate of vascular complications (10.4% women vs. 7.4% men).
But Dr. Kapur warned that the propensity-matched analysis had some caveats.
“Essentially what we are doing with propensity matching is creating two populations that are as similar as possible, and this reduced the number of patients in the analysis down to 25% of the original population,” he said. “One of the things we had to match was body surface area, and in doing this, we are taking out one of the most important differences between men and women, and as a result, a lot of the differences in outcomes go away.
“In this respect, propensity matching can be a bit of a double-edge sword,” he added. “I think the non–propensity-matched results are more interesting, as they are more of a reflection of the real world.”
Dr. Kapur concluded that these findings are compelling enough to suggest that there are important differences between women and men with cardiogenic shock in terms of outcomes as well as complication rates.
“Our decision-making around women seems to be different to that around men. I think this paper should start to trigger more awareness of that.”
Dr. Kapur also emphasized the importance of paying attention to vascular complications in women.
“The higher rates of bleeding and limb ischemia issues in women may explain the rationale for being less aggressive with invasive therapies in women,” he said. “But we need to come up with better solutions or technologies so they can be used more effectively in women. This could include adapting technology for smaller vascular sizes, which should lead to better outcome and fewer complications in women.”
He added that further granular data on this issue are needed. “We have very limited datasets in cardiogenic shock. There are few randomized controlled trials, and women are not well represented in such trials. We need to make sure we enroll women in randomized trials.”
Dr. Kapur said more women physicians who treat cardiogenic shock are also required, which would include cardiologists, critical care specialists, cardiac surgeons, and anesthesia personnel.
He pointed out that the two first authors of the current study are women – Van-Khue Ton, MD, Massachusetts General Hospital, Boston, and Manreet Kanwar, MD, Allegheny Health Network, Pittsburgh.
“We worked hard to involve women as principal investigators. They led the effort. These are investigations led by women, on women, to advance the care of women,” he commented.
Gender-related inequality
In an editorial accompanying publication of the study, Sara Kalantari, MD, and Jonathan Grinstein, MD, University of Chicago, and Robert O. Roswell, MD, Hofstra University, Hempstead, N.Y., said these results “provide valuable information about gender-related inequality in care and outcomes in the management of cardiogenic shock, although the exact mechanisms driving these observed differences still need to be elucidated.
“Broadly speaking, barriers in the care of women with heart failure and cardiogenic shock include a reduced awareness among both patients and providers, a deficiency of sex-specific objective criteria for guiding therapy, and unfavorable temporary mechanical circulatory support devices with higher rates of hemocompatibility-related complications in women,” they added.
“In the era of the multidisciplinary shock team and shock pathways with protocolized management algorithms, it is imperative that we still allow for personalization of care to match the physiologic needs of the patient in order for us to continue to close the gender gap in the care of patients presenting with cardiogenic shock,” the editorialists concluded.
A version of this article appeared on Medscape.com.
“These data identify the need for us to continue working to identify barriers in terms of diagnosis, management, and technological innovations for women in cardiogenic shock to resolve these issues and improve outcomes,” the senior author of the study, Navin Kapur, MD, Tufts Medical Center, Boston, said in an interview.
The study is said to be the one of the largest contemporary analyses of real-world registry data on the characteristics and outcomes of women in comparison with men with cardiogenic shock.
It showed sex-specific differences in outcomes that were primarily driven by differences in heart failure–related cardiogenic shock. Women with heart failure–related cardiogenic shock had more severe cardiogenic shock, worse survival at discharge, and more vascular complications than men. Outcomes in cardiogenic shock related to MI were similar for men and women.
The study, which will be presented at the upcoming annual meeting of the American Heart Association, was published online in JACC: Heart Failure.
Dr. Kapur founded the Cardiogenic Shock Working Group in 2017 to collect quality data on the condition.
“We realized our patients were dying, and we didn’t have enough data on how best to manage them. So, we started this registry, and now have detailed data on close to 9,000 patients with cardiogenic shock from 45 hospitals in the U.S., Mexico, Australia, and Japan,” he explained.
“The primary goal is to try to investigate the questions related to cardiogenic shock that can inform management, and one of the key questions that came up was differences in how men and women present with cardiogenic shock and what their outcomes may be. This is what we are reporting in this paper,” he added.
Cardiogenic shock is defined as having a low cardiac output most commonly because of MI or an episode of acute heart failure, Dr. Kapur said. Patients with cardiogenic shock are identified by their low blood pressure or hypoperfusion evidenced by clinical exam or biomarkers, such as elevated lactate levels.
“In this analysis, we’re looking at patients presenting with cardiogenic shock, so were not looking at the incidence of the condition in men versus women,” Dr. Kapur noted. “However, we believe that cardiogenic shock is probably more underrepresented in women, who may present with an MI or acute heart failure and may or may not be identified as having low cardiac output states until quite late. The likelihood is that the incidence is similar in men and women, but women are more often undiagnosed.”
For the current study, the authors analyzed data on 5,083 patients with cardiogenic shock in the registry, of whom 1,522 (30%) were women. Compared with men, women had slightly higher body mass index (BMI) and smaller body surface area.
Results showed that women with heart failure–related cardiogenic shock had worse survival at discharge than men (69.9% vs. 74.4%) and a higher rate of refractory shock (SCAI stage E; 26% vs. 21%). Women were also less likely to undergo pulmonary artery catheterization (52.9% vs. 54.6%), heart transplantation (6.5% vs. 10.3%), or left ventricular assist device implantation (7.8% vs. 10%).
Regardless of cardiogenic shock etiology, women had more vascular complications (8.8% vs. 5.7%), bleeding (7.1% vs. 5.2%), and limb ischemia (6.8% vs. 4.5%).
“This analysis is quite revealing. We identified some important distinctions between men and women,” Dr. Kapur commented.
For many patients who present with MI-related cardiogenic shock, many of the baseline characteristics in men and women were quite similar, he said. “But in heart failure–related cardiogenic shock, we saw more differences, with typical comorbidities associated with cardiogenic shock [e.g., diabetes, chronic kidney disease, hypertension] being less common in women than in men. This suggests there may be phenotypic differences as to why women present with heart failure shock versus men.”
Dr. Kapur pointed out that differences in BMI or body surface area between men and women may play into some of the management decision-making.
“Women having a smaller stature may lead to a selection bias where we don’t want to use large-bore pumps or devices because we’re worried about causing complications. We found in the analysis that vascular complications such as bleeding or ischemia of the lower extremity where these devices typically go were more frequent in women,” he noted.
“We also found that women were less likely to receive invasive therapies in general, including pulmonary artery catheters, temporary mechanical support, and heart replacements, such as LVAD or transplants,” he added.
Further results showed that, after propensity score matching, some of the gender differences disappeared, but women continued to have a higher rate of vascular complications (10.4% women vs. 7.4% men).
But Dr. Kapur warned that the propensity-matched analysis had some caveats.
“Essentially what we are doing with propensity matching is creating two populations that are as similar as possible, and this reduced the number of patients in the analysis down to 25% of the original population,” he said. “One of the things we had to match was body surface area, and in doing this, we are taking out one of the most important differences between men and women, and as a result, a lot of the differences in outcomes go away.
“In this respect, propensity matching can be a bit of a double-edge sword,” he added. “I think the non–propensity-matched results are more interesting, as they are more of a reflection of the real world.”
Dr. Kapur concluded that these findings are compelling enough to suggest that there are important differences between women and men with cardiogenic shock in terms of outcomes as well as complication rates.
“Our decision-making around women seems to be different to that around men. I think this paper should start to trigger more awareness of that.”
Dr. Kapur also emphasized the importance of paying attention to vascular complications in women.
“The higher rates of bleeding and limb ischemia issues in women may explain the rationale for being less aggressive with invasive therapies in women,” he said. “But we need to come up with better solutions or technologies so they can be used more effectively in women. This could include adapting technology for smaller vascular sizes, which should lead to better outcome and fewer complications in women.”
He added that further granular data on this issue are needed. “We have very limited datasets in cardiogenic shock. There are few randomized controlled trials, and women are not well represented in such trials. We need to make sure we enroll women in randomized trials.”
Dr. Kapur said more women physicians who treat cardiogenic shock are also required, which would include cardiologists, critical care specialists, cardiac surgeons, and anesthesia personnel.
He pointed out that the two first authors of the current study are women – Van-Khue Ton, MD, Massachusetts General Hospital, Boston, and Manreet Kanwar, MD, Allegheny Health Network, Pittsburgh.
“We worked hard to involve women as principal investigators. They led the effort. These are investigations led by women, on women, to advance the care of women,” he commented.
Gender-related inequality
In an editorial accompanying publication of the study, Sara Kalantari, MD, and Jonathan Grinstein, MD, University of Chicago, and Robert O. Roswell, MD, Hofstra University, Hempstead, N.Y., said these results “provide valuable information about gender-related inequality in care and outcomes in the management of cardiogenic shock, although the exact mechanisms driving these observed differences still need to be elucidated.
“Broadly speaking, barriers in the care of women with heart failure and cardiogenic shock include a reduced awareness among both patients and providers, a deficiency of sex-specific objective criteria for guiding therapy, and unfavorable temporary mechanical circulatory support devices with higher rates of hemocompatibility-related complications in women,” they added.
“In the era of the multidisciplinary shock team and shock pathways with protocolized management algorithms, it is imperative that we still allow for personalization of care to match the physiologic needs of the patient in order for us to continue to close the gender gap in the care of patients presenting with cardiogenic shock,” the editorialists concluded.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Standing BP measures improve hypertension diagnosis
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
results of a new study suggest.
METHODOLOGY:
- The study included 125 adults, mean age 49 years and 62% female, who were free of cardiovascular disease and had no previous history of hypertension.
- Researchers collected data on 24-hour ambulatory blood pressure monitoring (ABPM), and three BP measurements in the seated position, then three in the standing position.
- They assessed overall diagnostic accuracy of seated and standing BP using the area under the receiver operating characteristic (AUROC) curve and considered a Bayes factor (BF) of 3 or greater as significant.
- They defined the presence of hypertension (HTN) by the 2017 American College of Cardiology/American Heart Association and 2023 European Society of Hypertension HTN guidelines based on ABPM.
- Sensitivity and specificity of standing BP was determined using cutoffs derived from Youden index, while sensitivity and specificity of seated BP was determined using the cutoff of 130/80 mm Hg and by 140/90 mm Hg.
TAKEAWAY:
- The AUROC for standing office systolic blood pressure (SBP; 0.81; 0.71-0.92) was significantly higher than for seated office SBP (0.70; 0.49-0.91) in diagnosing HTN when defined as an average 24-hour SBP ≥ 125 mm Hg (BF = 11.8), and significantly higher for seated versus standing office diastolic blood pressure (DBP; 0.65; 0.49-0.82) in diagnosing HTN when defined as an average 24-hour DBP ≥ 75 mm Hg (BF = 4.9).
- The AUROCs for adding standing office BP to seated office BP improved the accuracy of detecting HTN, compared with seated office BP alone when HTN was defined as an average 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg, or when defined as an average 24-hour SBP/DBP ≥ 130/80 mm Hg or daytime SBP/DBP ≥ 135/85 mm Hg (all BFs > 3).
- Sensitivity of standing SBP was 71%, compared with 43% for seated SBP.
IN PRACTICE:
The “excellent diagnostic performance” for standing BP measures revealed by the study “highlights that standing office BP has acceptable discriminative capabilities in identifying the presence of hypertension in adults,” the authors write.
SOURCE:
The study was conducted by John M. Giacona, Hypertension Section, department of internal medicine, University of Texas Southwestern Medical Center, Dallas, and colleagues. It was published online in Scientific Reports.
LIMITATIONS:
As the study enrolled only adults free of comorbidities who were not taking antihypertensive medications, the results may not be applicable to other patients. The study design was retrospective, and the order of BP measurements was not randomized (standing BP measurements were obtained only after seated BP).
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Hypertensive disorders of pregnancy and high stroke risk in Black women
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
I’d like to talk with you about a recent report from the large-scale Black Women’s Health Study, published in the new journal NEJM Evidence.
This study looked at the association between hypertensive disorders of pregnancy, including preeclampsia and gestational hypertension, and the risk for stroke over the next 20 (median, 22) years. Previous studies have linked hypertensive disorders of pregnancy with an increased risk for stroke. However, most of these studies have been done in White women of European ancestry, and evidence in Black women has been very limited, despite a disproportionately high risk of having a hypertensive disorder of pregnancy and also of stroke.
– overall, a 66% increased risk, an 80% increased risk with gestational hypertension, and about a 50% increased risk with preeclampsia.
We know that pregnancy itself can lead to some remodeling of the vascular system, but we don’t know whether a direct causal relationship exists between preeclampsia or gestational hypertension and subsequent stroke. Another potential explanation is that these complications of pregnancy serve as a window into a woman’s future cardiometabolic health and a marker of her cardiovascular risk.
Regardless, the clinical implications are the same. First, we would want to prevent these complications of pregnancy whenever possible. Some women will be candidates for the use of aspirin if they are at high risk for preeclampsia, and certainly for monitoring blood pressure very closely during pregnancy. It will also be important to maintain blood pressure control in the postpartum period and during the subsequent years of adulthood to minimize risk for stroke, because hypertension is such a powerful risk factor for stroke.
It will also be tremendously important to intensify lifestyle modifications such as increasing physical activity and having a heart-healthy diet. These complications of pregnancy have also been linked in other studies to an increased risk for subsequent coronary heart disease events and heart failure.
This transcript has been edited for clarity.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, and chief of the division of preventive medicine, Brigham and Women’s Hospital, both in Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
Aprocitentan reduces resistant hypertension in CKD
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
AT KIDNEY WEEK 2023
Marijuana use dramatically increases risk of heart problems, stroke
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
Regularly using marijuana can significantly increase a person’s risk of heart attack, heart failure, and stroke, according to a pair of new studies that will be presented at a major upcoming medical conference.
People who use marijuana daily have a 34% increased risk of heart failure, compared with people who don’t use the drug, according to one of the new studies.
The new findings leverage health data from 157,000 people in the National Institutes of Health “All of Us” research program. Researchers analyzed whether marijuana users were more likely to experience heart failure than nonusers over the course of nearly 4 years. The results indicated that coronary artery disease was behind marijuana users’ increased risk. (Coronary artery disease is the buildup of plaque on the walls of the arteries that supply blood to the heart.)
The research was conducted by a team at Medstar Health, a large Maryland health care system that operates 10 hospitals plus hundreds of clinics. The findings will be presented at the American Heart Association’s Scientific Sessions 2023 in Philadelphia.
“Our results should encourage more researchers to study the use of marijuana to better understand its health implications, especially on cardiovascular risk,” said researcher Yakubu Bene-Alhasan, MD, MPH, a doctor at Medstar Health in Baltimore. “We want to provide the population with high-quality information on marijuana use and to help inform policy decisions at the state level, to educate patients, and to guide health care professionals.”
About one in five people in the United States use marijuana, according to the Centers for Disease Control and Prevention. The majority of U.S. states allow marijuana to be used legally for medical purposes, and more than 20 states have legalized recreational marijuana, a tracker from the National Conference of State Legislatures shows.
A second study that will be presented at the conference shows that older people with any combination of type 2 diabetes, high blood pressure, and high cholesterol who use marijuana have an increased risk for a major heart or brain event, compared with people who never used the drug.
The researchers analyzed data for more than 28,000 people age 65 and older who had health conditions that put them at risk for heart problems and whose medical records showed they were marijuana users but not tobacco users. The results showed at least a 20% increased risk of heart attack, stroke, cardiac arrest, or arrhythmia (irregular heartbeat).
The findings are significant because medical professionals have long said that research on the long-term health effects of using marijuana are limited.
“The latest research about cannabis use indicates that smoking and inhaling cannabis increases concentrations of blood carboxyhemoglobin (carbon monoxide, a poisonous gas), tar (partly burned combustible matter) similar to the effects of inhaling a tobacco cigarette, both of which have been linked to heart muscle disease, chest pain, heart rhythm disturbances, heart attacks and other serious conditions,” said Robert L. Page II, PharmD, MSPH, chair of the volunteer writing group for the 2020 American Heart Association Scientific Statement: Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, in a statement. “Together with the results of these two research studies, the cardiovascular risks of cannabis use are becoming clearer and should be carefully considered and monitored by health care professionals and the public.”
A version of this article first appeared on WebMD.com.
FROM AHA 2023