User login
Facial Angioedema, Rash, and “Mastitis” in a 31-Year-Old Female
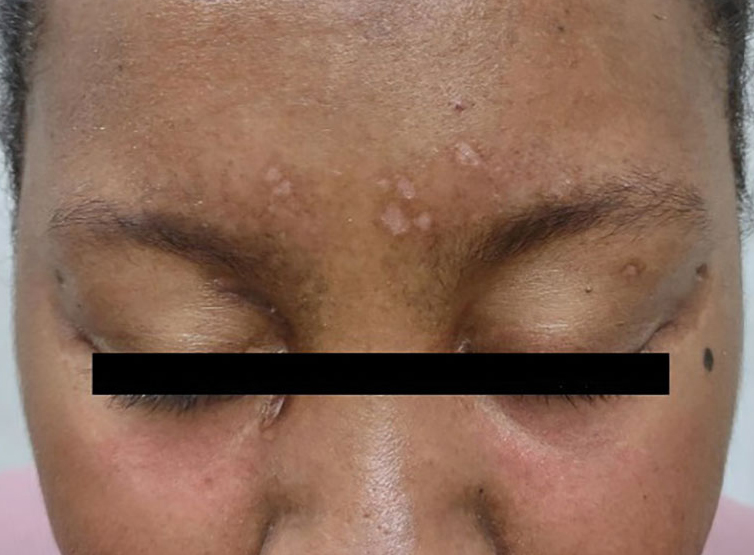
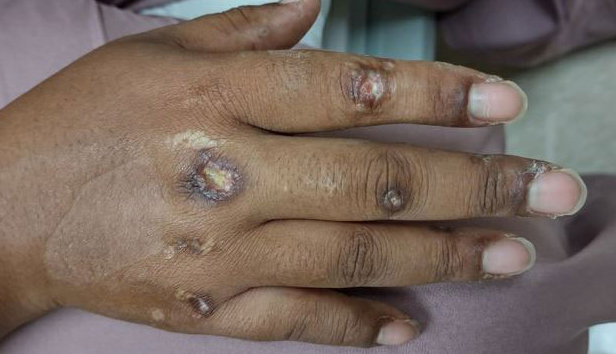
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.

DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.



DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
A previously healthy 31-year-old female active-duty Navy sailor working as a calibration technician developed a painful, erythematous, pruritic, indurated plaque on her left breast. The sailor was not lactating and had no known family history of malignancy. Initially, she was treated by her primary care practitioner for presumed mastitis with oral cephalexin and then with oral clindamycin with no symptom improvement. About 2 weeks after the completion of both antibiotic courses, she developed angioedema and periorbital edema (Figure 1), requiring highdose corticosteroids and antihistamines with a corticosteroid course of prednisone 40 mg daily tapered to 10 mg daily over 12 days and diphenhydramine 25 mg to use up to 4 times daily. Workup for both was acquired and hereditary angioedema was unremarkable. Two months later, the patient developed patches of alopecia, oral ulcerations, and hypopigmented plaques with a peripheral hyperpigmented rim on the central face and bilateral conchal bowls (Figure 2). She also developed hypopigmented papules with peripheral hyperpigmentation on the bilateral dorsal hands overlying the metacarpal and proximal interphalangeal joints, which eventually ulcerated (Figure 3). Laboratory evaluation, including tests for creatine kinase, aldolase, transaminases, lactate dehydrogenase, and autoantibodies (antiJo-1, anti-Mi-2, anti-MDA-5, anti-TIF-1, anti-NXP-2, and anti-SAEP), were unremarkable. A punch biopsy from a papule on the right dorsal hand showed superficial perivascular lymphohistiocytic inflammation with a subtle focal increase in dermal mucin, highlighted by the colloidal iron stain. Further evaluation of the left breast plaque revealed ER/PR+ HER2- stage IIIB inflammatory breast cancer.



DISCUSSION
Based on the clinical presentation and diagnosis of inflammatory breast cancer, the patient was diagnosed with paraneoplastic clinically amyopathic dermatomyositis (CADM). She was treated for her breast cancer with an initial chemotherapy regimen consisting of dose-dense cyclophosphamide and doxorubicin followed by paclitaxel. The patient underwent a mastectomy, axillary lymph node dissection, and 25 sessions of radiation therapy, and is currently continuing therapy with anastrozole 1 mg daily and ovarian suppression with leuprorelin 11.25 mg every 3 months. For the severe angioedema and dermatomyositis-like cutaneous findings, the patient was continued on high-dose corticosteroids at prednisone 60 mg daily with a prolonged taper to prednisone 10 mg daily. After about 10 months, she transitioned from prednisone 10 mg daily to hydrocortisone 30 mg daily and is currently tapering her hydrocortisone dosing. She was additionally started on monthly intravenous immunoglobulin, hydroxychloroquine 300 mg daily, and amlodipine 5 mg daily. The ulcerated papules on her hands were treated with topical clobetasol 0.05% ointment applied daily, topical tacrolimus 0.1% ointment applied daily, and multiple intralesional triamcinolone 5 mg/mL injections. With this regimen, the patient experienced significant improvement in her cutaneous symptoms.
CADM is a rare autoimmune inflammatory disease featuring classic dermatomyositis-like cutaneous findings such as a heliotrope rash and Gottron papules. Ulcerative Gottron papules are less common than the typical erythematous papules and are associated more strongly with amyopathic disease.1 Paraneoplastic myositis poses a diagnostic challenge because it presents like an idiopathic dermatomyositis and often has a heterogeneous clinical presentation with additional manifestations, including periorbital edema, myalgias, dysphagia, and shortness of breath. If clinically suspected, laboratory tests (eg, creatine kinase, aldolase, transaminases, and lactate dehydrogenase) can assist in diagnosing paraneoplastic myositis. Additionally, serologic testing for autoantibodies such as anti-CADM-140, anti-Jo-1, anti-Mi-2, antiMDA-5, anti-TIF-1, anti-NXP-2, and antiSAE can assist the diagnosis and predict disease phenotype.1,2
Malignancy can precede, occur during, or develop after the diagnosis of CADM.3 Malignancies most often associated with CADM include ovarian, breast, and lung cancers.4 Despite the strong correlation with malignancy, there are currently no screening guidelines for malignancy upon inflammatory myositis diagnosis. Therefore, it is important to consider the entirety of a patient’s clinical presentation in establishing further evaluation in the initial diagnostic workup.
There are numerous systemic complications associated with inflammatory myositis and imaging modalities can help to rule out some of these conditions. CADM is strongly associated with the development of interstitial lung disease, so chest radiography and pulmonary function testing are often checked.1 Though cardiac and esophageal involvement are more commonly associated with classic dermatomyositis, it may be useful to obtain an electrocardiogram to rule out conduction abnormalities from myocardial involvement, along with esophageal manometry to evaluate for esophageal dysmotility.1,5
In the management of paraneoplastic CADM, the underlying malignancy should be treated first.6 If symptoms persist after the cancer is in remission, then CADM is treated with immunosuppressive medications such as methotrexate, mycophenolate mofetil, or azathioprine. Physical therapy can also provide further symptom relief for those suffering from proximal weakness.
CONCLUSIONS
Presumed mastitis, angioedema, and eczematous lesions for this patient were dermatologic manifestations of an underlying inflammatory breast cancer. This case highlights the importance of early recognition, the diagnosis of CADM and awareness of its association with underlying malignancy, especially within the primary care setting where most skin concerns are addressed. Early clinical suspicion and a swift diagnostic workup can further optimize multidisciplinary management, which is often required to treat malignancies.
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
- Cao H, Xia Q, Pan M, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. 2016;43(9):1735-1742. doi:10.3899/jrheum.160024
- Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52(1):1-19. doi:10.1007/s12016-015-8510-y
- Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13(3):208-215. doi:10.1007/s11926-011-0169-7
- Udkoff J, Cohen PR. Amyopathic dermatomyositis: a concise review of clinical manifestations and associated malignancies. Am J Clin Dermatol. 2016;17(5): 509-518. doi:10.1007/s40257-016-0199-z
- Fathi M, Lundberg IE, Tornling G. Pulmonary complications of polymyositis and dermatomyositis. Semin Respir Crit Care Med. 2007;28(4):451-458. doi:10.1055/s-2007-985666
- Hendren E, Vinik O, Faragalla H, Haq R. Breast cancer and dermatomyositis: a case study and literature review. Curr Oncol. 2017;24(5):e429-e433. doi:10.3747/co.24.3696
‘Cancer Doesn’t Wait’: How Prior Authorization Harms Care
Fantine Giap, MD, sat across from a 21-year-old with a rare sarcoma at the base of her skull.
Despite the large tumor, nestled in a sensitive area, the Boston-based radiation oncologist could envision a bright future for her patient.
She and the other members of the patient’s care team had an impressive cancer-fighting arsenal at her fingertips. The team had recommended surgery, followed by proton therapy — a sophisticated tool able to deliver concentrated, razor-focused radiation to the once apple-sized growth, while sparing the fragile brain stem, optic nerve, and spinal cord.
Surgery went as planned. But as the days and weeks wore on and insurance prior authorization for the proton therapy never came, the tumor roared back, leading to more surgeries and more complications. Ultimately, the young woman needed a tracheostomy and a feeding tube.
By the time insurance said yes, more than 1 year from her initial visit, the future the team had envisioned seemed out of reach.
“Unfortunately for this patient, it went from a potentially curable situation to a likely not curable situation,” recalled Dr. Giap, a clinician at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. “I wanted to cry every day that she waited.’’
While a stark example, such insurance delays are not uncommon, according to new research published in JAMA Network Open.
Other studies have found that number to be even higher, with more than 86% of prior authorization requests ultimately approved with few changes.
‘’It gives you the idea that this entire process might be a little futile — that it’s just wasting people’s time,’’ said Fumiko Chino, MD, coauthor on the JAMA study and now an assistant professor in radiation oncology at MD Anderson Cancer Center in Houston. ‘’The problem is cancer doesn’t wait for bureaucracy.’’
Barriers at Every Step
As Dr. Chino and her study coauthors explained, advancements like intensity-modulated radiation therapy and stereotactic radiosurgery have allowed a new generation of specialists to treat previously untreatable cancers in ways that maximize tumor-killing power while minimizing collateral damage. But these tools require sophisticated planning, imaging, simulations and execution — all of which are subject to increased insurance scrutiny.
‘’We face barriers pretty much every step of the way for every patient,’’ said Dr. Chino.
To investigate how such barriers impact care, Dr. Chino and colleagues at Memorial Sloan Kettering Cancer Center — where she worked until July — looked at 206 cases in which payers denied prior authorization for radiation therapy from November 1, 2021 to December 8, 2022.
The team found that 62% were ultimately approved without any change to technique or dose, while 28% were authorized, but with lower doses or less sophisticated techniques. Four people, however, never got authorization at all — three abandoned treatment altogether, and one sought treatment at another institution.
Treatment delays ranged from 1 day to 49 days. Eighty-three patients died.
Would some of them have lived if it weren’t for prior authorization?
Dr. Chino cannot say for sure, but did note that certain cancers, like cervical cancer, can grow so quickly that every day of delayed treatment makes them harder to control.
Patients with metastatic or late-stage cancers are often denied more aggressive treatments by insurers who, in essence, “assume that they are going to die from their disease anyway,” Dr. Chino said.
She views this as tragically shortsighted.
‘’There’s actually a strong body of evidence to show that if you treat even metastatic stage IV diseases aggressively, you can prolong not just quality of life but also quantity,’’ she said.
In cases where the cancer is more localized and insurance mandates lower doses or cheaper techniques, the consequences can be equally heartbreaking.
‘’It’s like saying instead of taking an extra-strength Tylenol you can only have a baby aspirin,’’ she said. ‘’Their pain is less likely to be controlled, their disease is less likely to be controlled, and they are more likely to need retreatment.’’
Prior authorization delays can also significantly stress patients at the most vulnerable point of their lives.
In another recent study, Dr. Chino found that 69% of patients with cancer reported prior authorization-related delays in care, with one-third waiting a month or longer. One in five never got the care their doctors recommended, and 20% reported spending more than 11 hours on the phone haggling with their insurance companies.
Most patients rated the process as ‘’bad’’ or ‘’horrible,’’ and said it fueled anxiety.
Such delays can be hard on clinicians and the healthcare system too.
One 2022 study found that a typical academic radiation oncology practice spent about a half-million dollars per year seeking insurance preauthorization. Nationally, that number exceeds $40 million.
Then there is the burnout factor.
Dr. Giap, an early-career physician who specializes in rare, aggressive sarcomas, works at an institution that helped pioneer proton therapy. She says it pains her to tell a desperate patient, like the 21-year-old, who has traveled to her from out of state that they have to wait.
‘’Knowing that the majority of the cases are ultimately approved and that this wait is often unnecessary makes it even tougher,’’ she said.
Dr. Chino, a breast cancer specialist, has taken to warning patients before the alarming insurance letter arrives in the mail that their insurance may delay authorizing their care. But she tells patients that she will do everything she can to fight for them and develops a back-up plan to pivot to quickly, if needed.
‘’No one goes into medicine to spend their time talking to insurance companies,’’ said Dr. Chino.
The national trade group, America’s Health Insurance Plans (AHIP), did not return repeated requests for an interview for this story. But their official position, as stated on their website, is that “prior authorization is one of many tools health insurance providers use to promote safe, timely, evidence-based, affordable, and efficient care.”
Both Dr. Giap and Dr. Chino believe that prior authorization was developed with good intentions: to save healthcare costs and rein in treatments that don’t necessarily benefit patients.
But, in their specialty, the burden has proliferated to a point that Dr. Chino characterizes as ‘’unconscionable.’’
She believes that policy changes like the proposed Improving Seniors’ Timely Access to Care Act — which would require real-time decisions for procedures that are routinely approved — could go a long way in improving patient care.
Meanwhile, Dr. Giap said, more research and professional guidelines are necessary to bolster insurance company confidence in newer technologies, particularly for rare cancers.
Her patient ultimately got her proton therapy and is ‘’doing relatively well, all things considered.’’
But not all the stories end like this.
Dr. Chino will never forget a patient with a cancer growing so rapidly she could see it protruding through her chest wall. She called for an urgent PET scan to see where else in the body the cancer might be brewing and rushed the planning process for radiation therapy, both of which faced prior authorization barriers. That scan — which ultimately showed the cancer had spread — was delayed for months.*
If the team had had those imaging results upfront, she said, they would have recommended a completely different course of treatment.
And her patient might be alive today.
‘’Unfortunately,” Dr. Chino said, “the people with the very worst prior authorization stories aren’t here anymore to tell you about them.”
*Correction, 10/4/24: An earlier version of this article erroneously stated that Dr. Chino called for surgery for her patient. She actually called for a PET scan and an urgent radiation start.
A version of this article first appeared on Medscape.com.
Fantine Giap, MD, sat across from a 21-year-old with a rare sarcoma at the base of her skull.
Despite the large tumor, nestled in a sensitive area, the Boston-based radiation oncologist could envision a bright future for her patient.
She and the other members of the patient’s care team had an impressive cancer-fighting arsenal at her fingertips. The team had recommended surgery, followed by proton therapy — a sophisticated tool able to deliver concentrated, razor-focused radiation to the once apple-sized growth, while sparing the fragile brain stem, optic nerve, and spinal cord.
Surgery went as planned. But as the days and weeks wore on and insurance prior authorization for the proton therapy never came, the tumor roared back, leading to more surgeries and more complications. Ultimately, the young woman needed a tracheostomy and a feeding tube.
By the time insurance said yes, more than 1 year from her initial visit, the future the team had envisioned seemed out of reach.
“Unfortunately for this patient, it went from a potentially curable situation to a likely not curable situation,” recalled Dr. Giap, a clinician at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. “I wanted to cry every day that she waited.’’
While a stark example, such insurance delays are not uncommon, according to new research published in JAMA Network Open.
Other studies have found that number to be even higher, with more than 86% of prior authorization requests ultimately approved with few changes.
‘’It gives you the idea that this entire process might be a little futile — that it’s just wasting people’s time,’’ said Fumiko Chino, MD, coauthor on the JAMA study and now an assistant professor in radiation oncology at MD Anderson Cancer Center in Houston. ‘’The problem is cancer doesn’t wait for bureaucracy.’’
Barriers at Every Step
As Dr. Chino and her study coauthors explained, advancements like intensity-modulated radiation therapy and stereotactic radiosurgery have allowed a new generation of specialists to treat previously untreatable cancers in ways that maximize tumor-killing power while minimizing collateral damage. But these tools require sophisticated planning, imaging, simulations and execution — all of which are subject to increased insurance scrutiny.
‘’We face barriers pretty much every step of the way for every patient,’’ said Dr. Chino.
To investigate how such barriers impact care, Dr. Chino and colleagues at Memorial Sloan Kettering Cancer Center — where she worked until July — looked at 206 cases in which payers denied prior authorization for radiation therapy from November 1, 2021 to December 8, 2022.
The team found that 62% were ultimately approved without any change to technique or dose, while 28% were authorized, but with lower doses or less sophisticated techniques. Four people, however, never got authorization at all — three abandoned treatment altogether, and one sought treatment at another institution.
Treatment delays ranged from 1 day to 49 days. Eighty-three patients died.
Would some of them have lived if it weren’t for prior authorization?
Dr. Chino cannot say for sure, but did note that certain cancers, like cervical cancer, can grow so quickly that every day of delayed treatment makes them harder to control.
Patients with metastatic or late-stage cancers are often denied more aggressive treatments by insurers who, in essence, “assume that they are going to die from their disease anyway,” Dr. Chino said.
She views this as tragically shortsighted.
‘’There’s actually a strong body of evidence to show that if you treat even metastatic stage IV diseases aggressively, you can prolong not just quality of life but also quantity,’’ she said.
In cases where the cancer is more localized and insurance mandates lower doses or cheaper techniques, the consequences can be equally heartbreaking.
‘’It’s like saying instead of taking an extra-strength Tylenol you can only have a baby aspirin,’’ she said. ‘’Their pain is less likely to be controlled, their disease is less likely to be controlled, and they are more likely to need retreatment.’’
Prior authorization delays can also significantly stress patients at the most vulnerable point of their lives.
In another recent study, Dr. Chino found that 69% of patients with cancer reported prior authorization-related delays in care, with one-third waiting a month or longer. One in five never got the care their doctors recommended, and 20% reported spending more than 11 hours on the phone haggling with their insurance companies.
Most patients rated the process as ‘’bad’’ or ‘’horrible,’’ and said it fueled anxiety.
Such delays can be hard on clinicians and the healthcare system too.
One 2022 study found that a typical academic radiation oncology practice spent about a half-million dollars per year seeking insurance preauthorization. Nationally, that number exceeds $40 million.
Then there is the burnout factor.
Dr. Giap, an early-career physician who specializes in rare, aggressive sarcomas, works at an institution that helped pioneer proton therapy. She says it pains her to tell a desperate patient, like the 21-year-old, who has traveled to her from out of state that they have to wait.
‘’Knowing that the majority of the cases are ultimately approved and that this wait is often unnecessary makes it even tougher,’’ she said.
Dr. Chino, a breast cancer specialist, has taken to warning patients before the alarming insurance letter arrives in the mail that their insurance may delay authorizing their care. But she tells patients that she will do everything she can to fight for them and develops a back-up plan to pivot to quickly, if needed.
‘’No one goes into medicine to spend their time talking to insurance companies,’’ said Dr. Chino.
The national trade group, America’s Health Insurance Plans (AHIP), did not return repeated requests for an interview for this story. But their official position, as stated on their website, is that “prior authorization is one of many tools health insurance providers use to promote safe, timely, evidence-based, affordable, and efficient care.”
Both Dr. Giap and Dr. Chino believe that prior authorization was developed with good intentions: to save healthcare costs and rein in treatments that don’t necessarily benefit patients.
But, in their specialty, the burden has proliferated to a point that Dr. Chino characterizes as ‘’unconscionable.’’
She believes that policy changes like the proposed Improving Seniors’ Timely Access to Care Act — which would require real-time decisions for procedures that are routinely approved — could go a long way in improving patient care.
Meanwhile, Dr. Giap said, more research and professional guidelines are necessary to bolster insurance company confidence in newer technologies, particularly for rare cancers.
Her patient ultimately got her proton therapy and is ‘’doing relatively well, all things considered.’’
But not all the stories end like this.
Dr. Chino will never forget a patient with a cancer growing so rapidly she could see it protruding through her chest wall. She called for an urgent PET scan to see where else in the body the cancer might be brewing and rushed the planning process for radiation therapy, both of which faced prior authorization barriers. That scan — which ultimately showed the cancer had spread — was delayed for months.*
If the team had had those imaging results upfront, she said, they would have recommended a completely different course of treatment.
And her patient might be alive today.
‘’Unfortunately,” Dr. Chino said, “the people with the very worst prior authorization stories aren’t here anymore to tell you about them.”
*Correction, 10/4/24: An earlier version of this article erroneously stated that Dr. Chino called for surgery for her patient. She actually called for a PET scan and an urgent radiation start.
A version of this article first appeared on Medscape.com.
Fantine Giap, MD, sat across from a 21-year-old with a rare sarcoma at the base of her skull.
Despite the large tumor, nestled in a sensitive area, the Boston-based radiation oncologist could envision a bright future for her patient.
She and the other members of the patient’s care team had an impressive cancer-fighting arsenal at her fingertips. The team had recommended surgery, followed by proton therapy — a sophisticated tool able to deliver concentrated, razor-focused radiation to the once apple-sized growth, while sparing the fragile brain stem, optic nerve, and spinal cord.
Surgery went as planned. But as the days and weeks wore on and insurance prior authorization for the proton therapy never came, the tumor roared back, leading to more surgeries and more complications. Ultimately, the young woman needed a tracheostomy and a feeding tube.
By the time insurance said yes, more than 1 year from her initial visit, the future the team had envisioned seemed out of reach.
“Unfortunately for this patient, it went from a potentially curable situation to a likely not curable situation,” recalled Dr. Giap, a clinician at Massachusetts General Hospital and instructor at Harvard Medical School, Boston. “I wanted to cry every day that she waited.’’
While a stark example, such insurance delays are not uncommon, according to new research published in JAMA Network Open.
Other studies have found that number to be even higher, with more than 86% of prior authorization requests ultimately approved with few changes.
‘’It gives you the idea that this entire process might be a little futile — that it’s just wasting people’s time,’’ said Fumiko Chino, MD, coauthor on the JAMA study and now an assistant professor in radiation oncology at MD Anderson Cancer Center in Houston. ‘’The problem is cancer doesn’t wait for bureaucracy.’’
Barriers at Every Step
As Dr. Chino and her study coauthors explained, advancements like intensity-modulated radiation therapy and stereotactic radiosurgery have allowed a new generation of specialists to treat previously untreatable cancers in ways that maximize tumor-killing power while minimizing collateral damage. But these tools require sophisticated planning, imaging, simulations and execution — all of which are subject to increased insurance scrutiny.
‘’We face barriers pretty much every step of the way for every patient,’’ said Dr. Chino.
To investigate how such barriers impact care, Dr. Chino and colleagues at Memorial Sloan Kettering Cancer Center — where she worked until July — looked at 206 cases in which payers denied prior authorization for radiation therapy from November 1, 2021 to December 8, 2022.
The team found that 62% were ultimately approved without any change to technique or dose, while 28% were authorized, but with lower doses or less sophisticated techniques. Four people, however, never got authorization at all — three abandoned treatment altogether, and one sought treatment at another institution.
Treatment delays ranged from 1 day to 49 days. Eighty-three patients died.
Would some of them have lived if it weren’t for prior authorization?
Dr. Chino cannot say for sure, but did note that certain cancers, like cervical cancer, can grow so quickly that every day of delayed treatment makes them harder to control.
Patients with metastatic or late-stage cancers are often denied more aggressive treatments by insurers who, in essence, “assume that they are going to die from their disease anyway,” Dr. Chino said.
She views this as tragically shortsighted.
‘’There’s actually a strong body of evidence to show that if you treat even metastatic stage IV diseases aggressively, you can prolong not just quality of life but also quantity,’’ she said.
In cases where the cancer is more localized and insurance mandates lower doses or cheaper techniques, the consequences can be equally heartbreaking.
‘’It’s like saying instead of taking an extra-strength Tylenol you can only have a baby aspirin,’’ she said. ‘’Their pain is less likely to be controlled, their disease is less likely to be controlled, and they are more likely to need retreatment.’’
Prior authorization delays can also significantly stress patients at the most vulnerable point of their lives.
In another recent study, Dr. Chino found that 69% of patients with cancer reported prior authorization-related delays in care, with one-third waiting a month or longer. One in five never got the care their doctors recommended, and 20% reported spending more than 11 hours on the phone haggling with their insurance companies.
Most patients rated the process as ‘’bad’’ or ‘’horrible,’’ and said it fueled anxiety.
Such delays can be hard on clinicians and the healthcare system too.
One 2022 study found that a typical academic radiation oncology practice spent about a half-million dollars per year seeking insurance preauthorization. Nationally, that number exceeds $40 million.
Then there is the burnout factor.
Dr. Giap, an early-career physician who specializes in rare, aggressive sarcomas, works at an institution that helped pioneer proton therapy. She says it pains her to tell a desperate patient, like the 21-year-old, who has traveled to her from out of state that they have to wait.
‘’Knowing that the majority of the cases are ultimately approved and that this wait is often unnecessary makes it even tougher,’’ she said.
Dr. Chino, a breast cancer specialist, has taken to warning patients before the alarming insurance letter arrives in the mail that their insurance may delay authorizing their care. But she tells patients that she will do everything she can to fight for them and develops a back-up plan to pivot to quickly, if needed.
‘’No one goes into medicine to spend their time talking to insurance companies,’’ said Dr. Chino.
The national trade group, America’s Health Insurance Plans (AHIP), did not return repeated requests for an interview for this story. But their official position, as stated on their website, is that “prior authorization is one of many tools health insurance providers use to promote safe, timely, evidence-based, affordable, and efficient care.”
Both Dr. Giap and Dr. Chino believe that prior authorization was developed with good intentions: to save healthcare costs and rein in treatments that don’t necessarily benefit patients.
But, in their specialty, the burden has proliferated to a point that Dr. Chino characterizes as ‘’unconscionable.’’
She believes that policy changes like the proposed Improving Seniors’ Timely Access to Care Act — which would require real-time decisions for procedures that are routinely approved — could go a long way in improving patient care.
Meanwhile, Dr. Giap said, more research and professional guidelines are necessary to bolster insurance company confidence in newer technologies, particularly for rare cancers.
Her patient ultimately got her proton therapy and is ‘’doing relatively well, all things considered.’’
But not all the stories end like this.
Dr. Chino will never forget a patient with a cancer growing so rapidly she could see it protruding through her chest wall. She called for an urgent PET scan to see where else in the body the cancer might be brewing and rushed the planning process for radiation therapy, both of which faced prior authorization barriers. That scan — which ultimately showed the cancer had spread — was delayed for months.*
If the team had had those imaging results upfront, she said, they would have recommended a completely different course of treatment.
And her patient might be alive today.
‘’Unfortunately,” Dr. Chino said, “the people with the very worst prior authorization stories aren’t here anymore to tell you about them.”
*Correction, 10/4/24: An earlier version of this article erroneously stated that Dr. Chino called for surgery for her patient. She actually called for a PET scan and an urgent radiation start.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Popular Weight Loss Drugs Now for Patients With Cancer?
Demand for new weight loss drugs has surged over the past few years.
Led by the antiobesity drugs semaglutide (Wegovy) and tirzepatide (Zepbound), these popular medications — more commonly known as glucagon-like peptide 1 (GLP-1) agonists — have become game changers for shedding excess pounds.
Aside from obesity indications, both drugs have been approved to treat type 2 diabetes under different brand names and have a growing list of other potential benefits, such as reducing inflammation and depression.
While there’s limited data to support the use of GLP-1 agonists for weight loss in cancer, some oncologists have begun carefully integrating the antiobesity agents into care and studying their effects in this patient population.
The reason: Research suggests that obesity can reduce the effectiveness of cancer therapies, especially in patients with breast cancer, and can increase the risk for treatment-related side effects.
The idea is that managing patients’ weight will improve their cancer outcomes, explained Lajos Pusztai, MD, PhD, a breast cancer specialist and professor of medicine at Yale School of Medicine in New Haven, Connecticut.
Although Dr. Pusztai and his oncology peers at Yale don’t yet use GPL-1 agonists, Neil Iyengar, MD, and colleagues have begun doing so to help some patients with breast cancer manage their weight. Dr. Iyengar estimates that a few hundred — almost 40% — of his patients are on the antiobesity drugs.
“For a patient who has really tried to reduce their weight and who is in the obese range, that’s where I think the use of these medications can be considered,” said Dr. Iyengar, a breast cancer oncologist at Memorial Sloan Kettering Cancer Center in New York City.
Why GLP-1s in Cancer?
GLP-1 is a hormone that the small intestine releases after eating. GLP-1 agonists work by mimicking GLP-1 to trigger the release of insulin and reduce the production of glucagon — two processes that help regulate blood sugar.
These agents, such as Wegovy (or Ozempic when prescribed for diabetes), also slow gastric emptying and can make people feel fuller longer.
Zebound (or Mounjaro for type 2 diabetes) is considered a dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, which may enhance its weight loss benefits.
In practice, however, these drugs can increase nausea and vomiting from chemotherapy, so Dr. Iyengar typically has patients use them afterwards, during maintenance treatment.
Oncologists don’t prescribe the drugs themselves but instead refer patients to endocrinologists or weight management centers that then write the prescriptions. Taking these drugs involves weekly subcutaneous injections patients can administer themselves.
Endocrinologist Emily Gallagher, MD, PhD, of Mount Sinai Hospital in New York City, estimates she has prescribed the antiobesity drugs to a few hundred patients with cancer and, like Dr. Iyengar, uses the drugs during maintenance treatment with hormone therapy for breast cancer. She also has used these agents in patients with prostate and endometrial cancers and has found the drugs can help counter steroid weight gain in multiple myeloma.
But, to date, the evidence for using GPL-1 agonists in cancer remains limited and the practice has not yet become widespread.
Research largely comes down to a few small retrospective studies in patients with breast cancer receiving aromatase inhibitors. Although no safety issues have emerged so far, these initial reports suggest that the drugs lead to significantly less weight loss in patients with cancer compared to the general population.
Dr. Iyengar led one recent study, presented at the 2024 annual meeting of the American Society of Clinical Oncology, in which he and his team assessed outcomes in 75 women with breast cancer who received a GLP-1 agonist. Almost 80% of patients had diabetes, and 60% received hormone therapy, most commonly an aromatase inhibitor. Patients’ median body mass index (BMI) at baseline was 34 kg/m2 (range, 23-50 kg/m2).
From baseline, patients lost 6.2 kg, on average, or about 5% of their total body weight, 12 months after initiating GLP-1 therapy.
In contrast, phase 3 trials show much higher mean weight loss — about two times — in patients without cancer.
Another recent study also reported modest weight loss results in patients with breast cancer undergoing endocrine therapy. The researchers reported that, at 12 months, Wegovy led to 4.34% reduction in BMI, compared with a 14% change reported in the general population. Zebound, however, was associated with a 2.31% BMI increase overall — though some patients did experience a decrease — compared with a 15% reduction in the general population.
“These findings indicate a substantially reduced weight loss efficacy in breast cancer patients on endocrine therapy compared to the general population,” the authors concluded.
It’s unclear why the drugs appear to not work as well in patients with cancer. It’s possible that hormone therapy or metabolic changes interfere with their effectiveness, given that some cancer therapies lead to weight gain. Steroids and hormone therapies, for instance, often increase appetite, and some treatments can slow patients’ metabolism or lead to fatigue, which can make it harder to exercise.
Patients with cancer may need a higher dose of GLP-1 agonists to achieve similar weight loss to the general population, Dr. Iyengar noted.
However, Dr. Gallagher said, in her own experience, she hasn’t found the drugs to be less effective in patients with cancer, especially the newer agents, like Wegovy and Zepbound.
As for safety, Wegovy and Zepbound both carry a black box warning for thyroid C-cell tumors, including medullary thyroid carcinoma. (Recent research, however, has found that GLP-1 agonists do not increase thyroid cancer risk).
These antiobesity agents are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients who have multiple endocrine neoplasia syndrome type 2, which is associated with medullary thyroid carcinoma.
Dr. Gallagher hasn’t seen any secondary tumors — thyroid or otherwise — in her patients with cancer, but she follows the labeling contraindications. Dr. Iyengar also noted that more recent and larger data sets have shown no impact on this risk, which may not actually exist, he said
Dr. Gallagher remains cautious about using GPL-1 agonists in patients who have had bariatric surgery because these agents can compound the slower gastric emptying and intestinal transit from surgery, potentially leading to gastrointestinal obstructions.
Looking ahead, GPL-1 manufacturers are interested in adding cancer indications to the drug labeling. Both Dr. Iyengar and Dr. Gallagher said their institutions are in talks with companies to participate in large, multicenter, global phase 3 trials.
Dr. Iyengar welcomes the efforts, not only to test the effectiveness of GPL-1 agonists in oncology but also to “nail down” their safety in cancer.
“I don’t think that there’s mechanistically anything that’s particularly worrisome,” and current observations suggest that these drugs are likely to be safe, Dr. Iyengar said. Even so, “GLP-1 agonists do a lot of things that we don’t fully understand yet.”
The bigger challenge, Dr. Iyengar noted, is that companies will have to show a sizable benefit to using these drugs in patients with cancer to get the Food and Drug Administration’s approval. And to move the needle on cancer-specific outcomes, these antiobesity drugs will need to demonstrate significant, durable weight loss in patients with cancer.
But if these drugs can do that, “I think it’s going to be one of the biggest advances in medicine and oncology given the obesity and cancer epidemic,” Dr. Iyengar said.
Dr. Iyengar has adviser and/or researcher ties with companies that make or are developing GPL-1 agonists, including AstraZeneca, Novartis, Gilead, and Pfizer. Dr. Gallagher is a consultant for Novartis, Flare Therapeutics, Reactive Biosciences, and Seagen.
A version of this article first appeared on Medscape.com.
Demand for new weight loss drugs has surged over the past few years.
Led by the antiobesity drugs semaglutide (Wegovy) and tirzepatide (Zepbound), these popular medications — more commonly known as glucagon-like peptide 1 (GLP-1) agonists — have become game changers for shedding excess pounds.
Aside from obesity indications, both drugs have been approved to treat type 2 diabetes under different brand names and have a growing list of other potential benefits, such as reducing inflammation and depression.
While there’s limited data to support the use of GLP-1 agonists for weight loss in cancer, some oncologists have begun carefully integrating the antiobesity agents into care and studying their effects in this patient population.
The reason: Research suggests that obesity can reduce the effectiveness of cancer therapies, especially in patients with breast cancer, and can increase the risk for treatment-related side effects.
The idea is that managing patients’ weight will improve their cancer outcomes, explained Lajos Pusztai, MD, PhD, a breast cancer specialist and professor of medicine at Yale School of Medicine in New Haven, Connecticut.
Although Dr. Pusztai and his oncology peers at Yale don’t yet use GPL-1 agonists, Neil Iyengar, MD, and colleagues have begun doing so to help some patients with breast cancer manage their weight. Dr. Iyengar estimates that a few hundred — almost 40% — of his patients are on the antiobesity drugs.
“For a patient who has really tried to reduce their weight and who is in the obese range, that’s where I think the use of these medications can be considered,” said Dr. Iyengar, a breast cancer oncologist at Memorial Sloan Kettering Cancer Center in New York City.
Why GLP-1s in Cancer?
GLP-1 is a hormone that the small intestine releases after eating. GLP-1 agonists work by mimicking GLP-1 to trigger the release of insulin and reduce the production of glucagon — two processes that help regulate blood sugar.
These agents, such as Wegovy (or Ozempic when prescribed for diabetes), also slow gastric emptying and can make people feel fuller longer.
Zebound (or Mounjaro for type 2 diabetes) is considered a dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, which may enhance its weight loss benefits.
In practice, however, these drugs can increase nausea and vomiting from chemotherapy, so Dr. Iyengar typically has patients use them afterwards, during maintenance treatment.
Oncologists don’t prescribe the drugs themselves but instead refer patients to endocrinologists or weight management centers that then write the prescriptions. Taking these drugs involves weekly subcutaneous injections patients can administer themselves.
Endocrinologist Emily Gallagher, MD, PhD, of Mount Sinai Hospital in New York City, estimates she has prescribed the antiobesity drugs to a few hundred patients with cancer and, like Dr. Iyengar, uses the drugs during maintenance treatment with hormone therapy for breast cancer. She also has used these agents in patients with prostate and endometrial cancers and has found the drugs can help counter steroid weight gain in multiple myeloma.
But, to date, the evidence for using GPL-1 agonists in cancer remains limited and the practice has not yet become widespread.
Research largely comes down to a few small retrospective studies in patients with breast cancer receiving aromatase inhibitors. Although no safety issues have emerged so far, these initial reports suggest that the drugs lead to significantly less weight loss in patients with cancer compared to the general population.
Dr. Iyengar led one recent study, presented at the 2024 annual meeting of the American Society of Clinical Oncology, in which he and his team assessed outcomes in 75 women with breast cancer who received a GLP-1 agonist. Almost 80% of patients had diabetes, and 60% received hormone therapy, most commonly an aromatase inhibitor. Patients’ median body mass index (BMI) at baseline was 34 kg/m2 (range, 23-50 kg/m2).
From baseline, patients lost 6.2 kg, on average, or about 5% of their total body weight, 12 months after initiating GLP-1 therapy.
In contrast, phase 3 trials show much higher mean weight loss — about two times — in patients without cancer.
Another recent study also reported modest weight loss results in patients with breast cancer undergoing endocrine therapy. The researchers reported that, at 12 months, Wegovy led to 4.34% reduction in BMI, compared with a 14% change reported in the general population. Zebound, however, was associated with a 2.31% BMI increase overall — though some patients did experience a decrease — compared with a 15% reduction in the general population.
“These findings indicate a substantially reduced weight loss efficacy in breast cancer patients on endocrine therapy compared to the general population,” the authors concluded.
It’s unclear why the drugs appear to not work as well in patients with cancer. It’s possible that hormone therapy or metabolic changes interfere with their effectiveness, given that some cancer therapies lead to weight gain. Steroids and hormone therapies, for instance, often increase appetite, and some treatments can slow patients’ metabolism or lead to fatigue, which can make it harder to exercise.
Patients with cancer may need a higher dose of GLP-1 agonists to achieve similar weight loss to the general population, Dr. Iyengar noted.
However, Dr. Gallagher said, in her own experience, she hasn’t found the drugs to be less effective in patients with cancer, especially the newer agents, like Wegovy and Zepbound.
As for safety, Wegovy and Zepbound both carry a black box warning for thyroid C-cell tumors, including medullary thyroid carcinoma. (Recent research, however, has found that GLP-1 agonists do not increase thyroid cancer risk).
These antiobesity agents are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients who have multiple endocrine neoplasia syndrome type 2, which is associated with medullary thyroid carcinoma.
Dr. Gallagher hasn’t seen any secondary tumors — thyroid or otherwise — in her patients with cancer, but she follows the labeling contraindications. Dr. Iyengar also noted that more recent and larger data sets have shown no impact on this risk, which may not actually exist, he said
Dr. Gallagher remains cautious about using GPL-1 agonists in patients who have had bariatric surgery because these agents can compound the slower gastric emptying and intestinal transit from surgery, potentially leading to gastrointestinal obstructions.
Looking ahead, GPL-1 manufacturers are interested in adding cancer indications to the drug labeling. Both Dr. Iyengar and Dr. Gallagher said their institutions are in talks with companies to participate in large, multicenter, global phase 3 trials.
Dr. Iyengar welcomes the efforts, not only to test the effectiveness of GPL-1 agonists in oncology but also to “nail down” their safety in cancer.
“I don’t think that there’s mechanistically anything that’s particularly worrisome,” and current observations suggest that these drugs are likely to be safe, Dr. Iyengar said. Even so, “GLP-1 agonists do a lot of things that we don’t fully understand yet.”
The bigger challenge, Dr. Iyengar noted, is that companies will have to show a sizable benefit to using these drugs in patients with cancer to get the Food and Drug Administration’s approval. And to move the needle on cancer-specific outcomes, these antiobesity drugs will need to demonstrate significant, durable weight loss in patients with cancer.
But if these drugs can do that, “I think it’s going to be one of the biggest advances in medicine and oncology given the obesity and cancer epidemic,” Dr. Iyengar said.
Dr. Iyengar has adviser and/or researcher ties with companies that make or are developing GPL-1 agonists, including AstraZeneca, Novartis, Gilead, and Pfizer. Dr. Gallagher is a consultant for Novartis, Flare Therapeutics, Reactive Biosciences, and Seagen.
A version of this article first appeared on Medscape.com.
Demand for new weight loss drugs has surged over the past few years.
Led by the antiobesity drugs semaglutide (Wegovy) and tirzepatide (Zepbound), these popular medications — more commonly known as glucagon-like peptide 1 (GLP-1) agonists — have become game changers for shedding excess pounds.
Aside from obesity indications, both drugs have been approved to treat type 2 diabetes under different brand names and have a growing list of other potential benefits, such as reducing inflammation and depression.
While there’s limited data to support the use of GLP-1 agonists for weight loss in cancer, some oncologists have begun carefully integrating the antiobesity agents into care and studying their effects in this patient population.
The reason: Research suggests that obesity can reduce the effectiveness of cancer therapies, especially in patients with breast cancer, and can increase the risk for treatment-related side effects.
The idea is that managing patients’ weight will improve their cancer outcomes, explained Lajos Pusztai, MD, PhD, a breast cancer specialist and professor of medicine at Yale School of Medicine in New Haven, Connecticut.
Although Dr. Pusztai and his oncology peers at Yale don’t yet use GPL-1 agonists, Neil Iyengar, MD, and colleagues have begun doing so to help some patients with breast cancer manage their weight. Dr. Iyengar estimates that a few hundred — almost 40% — of his patients are on the antiobesity drugs.
“For a patient who has really tried to reduce their weight and who is in the obese range, that’s where I think the use of these medications can be considered,” said Dr. Iyengar, a breast cancer oncologist at Memorial Sloan Kettering Cancer Center in New York City.
Why GLP-1s in Cancer?
GLP-1 is a hormone that the small intestine releases after eating. GLP-1 agonists work by mimicking GLP-1 to trigger the release of insulin and reduce the production of glucagon — two processes that help regulate blood sugar.
These agents, such as Wegovy (or Ozempic when prescribed for diabetes), also slow gastric emptying and can make people feel fuller longer.
Zebound (or Mounjaro for type 2 diabetes) is considered a dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, which may enhance its weight loss benefits.
In practice, however, these drugs can increase nausea and vomiting from chemotherapy, so Dr. Iyengar typically has patients use them afterwards, during maintenance treatment.
Oncologists don’t prescribe the drugs themselves but instead refer patients to endocrinologists or weight management centers that then write the prescriptions. Taking these drugs involves weekly subcutaneous injections patients can administer themselves.
Endocrinologist Emily Gallagher, MD, PhD, of Mount Sinai Hospital in New York City, estimates she has prescribed the antiobesity drugs to a few hundred patients with cancer and, like Dr. Iyengar, uses the drugs during maintenance treatment with hormone therapy for breast cancer. She also has used these agents in patients with prostate and endometrial cancers and has found the drugs can help counter steroid weight gain in multiple myeloma.
But, to date, the evidence for using GPL-1 agonists in cancer remains limited and the practice has not yet become widespread.
Research largely comes down to a few small retrospective studies in patients with breast cancer receiving aromatase inhibitors. Although no safety issues have emerged so far, these initial reports suggest that the drugs lead to significantly less weight loss in patients with cancer compared to the general population.
Dr. Iyengar led one recent study, presented at the 2024 annual meeting of the American Society of Clinical Oncology, in which he and his team assessed outcomes in 75 women with breast cancer who received a GLP-1 agonist. Almost 80% of patients had diabetes, and 60% received hormone therapy, most commonly an aromatase inhibitor. Patients’ median body mass index (BMI) at baseline was 34 kg/m2 (range, 23-50 kg/m2).
From baseline, patients lost 6.2 kg, on average, or about 5% of their total body weight, 12 months after initiating GLP-1 therapy.
In contrast, phase 3 trials show much higher mean weight loss — about two times — in patients without cancer.
Another recent study also reported modest weight loss results in patients with breast cancer undergoing endocrine therapy. The researchers reported that, at 12 months, Wegovy led to 4.34% reduction in BMI, compared with a 14% change reported in the general population. Zebound, however, was associated with a 2.31% BMI increase overall — though some patients did experience a decrease — compared with a 15% reduction in the general population.
“These findings indicate a substantially reduced weight loss efficacy in breast cancer patients on endocrine therapy compared to the general population,” the authors concluded.
It’s unclear why the drugs appear to not work as well in patients with cancer. It’s possible that hormone therapy or metabolic changes interfere with their effectiveness, given that some cancer therapies lead to weight gain. Steroids and hormone therapies, for instance, often increase appetite, and some treatments can slow patients’ metabolism or lead to fatigue, which can make it harder to exercise.
Patients with cancer may need a higher dose of GLP-1 agonists to achieve similar weight loss to the general population, Dr. Iyengar noted.
However, Dr. Gallagher said, in her own experience, she hasn’t found the drugs to be less effective in patients with cancer, especially the newer agents, like Wegovy and Zepbound.
As for safety, Wegovy and Zepbound both carry a black box warning for thyroid C-cell tumors, including medullary thyroid carcinoma. (Recent research, however, has found that GLP-1 agonists do not increase thyroid cancer risk).
These antiobesity agents are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients who have multiple endocrine neoplasia syndrome type 2, which is associated with medullary thyroid carcinoma.
Dr. Gallagher hasn’t seen any secondary tumors — thyroid or otherwise — in her patients with cancer, but she follows the labeling contraindications. Dr. Iyengar also noted that more recent and larger data sets have shown no impact on this risk, which may not actually exist, he said
Dr. Gallagher remains cautious about using GPL-1 agonists in patients who have had bariatric surgery because these agents can compound the slower gastric emptying and intestinal transit from surgery, potentially leading to gastrointestinal obstructions.
Looking ahead, GPL-1 manufacturers are interested in adding cancer indications to the drug labeling. Both Dr. Iyengar and Dr. Gallagher said their institutions are in talks with companies to participate in large, multicenter, global phase 3 trials.
Dr. Iyengar welcomes the efforts, not only to test the effectiveness of GPL-1 agonists in oncology but also to “nail down” their safety in cancer.
“I don’t think that there’s mechanistically anything that’s particularly worrisome,” and current observations suggest that these drugs are likely to be safe, Dr. Iyengar said. Even so, “GLP-1 agonists do a lot of things that we don’t fully understand yet.”
The bigger challenge, Dr. Iyengar noted, is that companies will have to show a sizable benefit to using these drugs in patients with cancer to get the Food and Drug Administration’s approval. And to move the needle on cancer-specific outcomes, these antiobesity drugs will need to demonstrate significant, durable weight loss in patients with cancer.
But if these drugs can do that, “I think it’s going to be one of the biggest advances in medicine and oncology given the obesity and cancer epidemic,” Dr. Iyengar said.
Dr. Iyengar has adviser and/or researcher ties with companies that make or are developing GPL-1 agonists, including AstraZeneca, Novartis, Gilead, and Pfizer. Dr. Gallagher is a consultant for Novartis, Flare Therapeutics, Reactive Biosciences, and Seagen.
A version of this article first appeared on Medscape.com.
Does Medicare Advantage Offer Higher-Value Chemotherapy?
TOPLINE:
METHODOLOGY:
- Private Medicare Advantage plans enroll more than half of the Medicare population, but it is unknown if or how the cost restrictions they impose affect chemotherapy, which accounts for a large portion of cancer care costs.
- Researchers conducted a cohort study using national Medicare data from January 2015 to December 2019 to look at Medicare Advantage enrollment and treatment patterns for patients with cancer receiving chemotherapy.
- The study included 96,501 Medicare Advantage enrollees and 206,274 traditional Medicare beneficiaries who initiated chemotherapy between January 2016 and December 2019 (mean age, ~73 years; ~56% women; Hispanic individuals, 15% and 8%; Black individuals, 15% and 8%; and White individuals, 75% and 86%, respectively).
- Resource use and care quality were measured during a 6-month period following chemotherapy initiation, and survival days were measured 18 months after beginning chemotherapy.
- Resource use measures included hospital inpatient services, outpatient care, prescription drugs, hospice services, and chemotherapy services. Quality measures included chemotherapy-related emergency visits and hospital admissions, as well as avoidable emergency visits and preventable hospitalizations.
TAKEAWAY:
- Medicare Advantage plans had lower resource use than traditional Medicare per enrollee with cancer undergoing chemotherapy ($8718 lower; 95% CI, $8343-$9094).
- The lower resource use was largely caused by fewer chemotherapy visits and less expensive chemotherapy per visit in Medicare Advantage plans ($5032 lower; 95% CI, $4772-$5293).
- Medicare Advantage enrollees had 2.5 percentage points fewer chemotherapy-related emergency department visits and 0.7 percentage points fewer chemotherapy-related hospitalizations than traditional Medicare beneficiaries.
- There was no clinically meaningful difference in survival between Medicare Advantage and traditional Medicare beneficiaries during the 18 months following chemotherapy initiation.
IN PRACTICE:
“Our new finding is that MA [Medicare Advantage] plans had lower resource use than TM [traditional Medicare] among enrollees with cancer undergoing chemotherapy — a serious condition managed by specialists and requiring expensive treatments. This suggests that MA’s cost advantages over TM are not limited to conditions for which low-cost primary care management can avoid costly services,” the authors wrote.
SOURCE:
The study was led by Yamini Kalidindi, PhD, McDermott+ Consulting, Washington, DC. It was published online on September 20, 2024, in JAMA Network Open (doi: 10.1001/jamanetworkopen.2024.34707), with a commentary.
LIMITATIONS:
The study’s findings may be affected by unobserved patient characteristics despite the use of inverse-probability weighting. The exclusion of Medicare Advantage enrollees in contracts with incomplete encounter data limits the generalizability of the results. The study does not apply to beneficiaries without Part D drug coverage. Quality measures were limited to those available from claims and encounter data, lacking information on patients’ cancer stage. The 18-month measure of survival might not adequately capture survival differences associated with early-stage cancers. The study did not measure whether patient care followed recommended guidelines.
DISCLOSURES:
Various authors reported grants from the National Institute on Aging, the National Institutes of Health, The Commonwealth Fund, Arnold Ventures, the National Cancer Institute, the Department of Defense, and the National Institute of Health Care Management. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Private Medicare Advantage plans enroll more than half of the Medicare population, but it is unknown if or how the cost restrictions they impose affect chemotherapy, which accounts for a large portion of cancer care costs.
- Researchers conducted a cohort study using national Medicare data from January 2015 to December 2019 to look at Medicare Advantage enrollment and treatment patterns for patients with cancer receiving chemotherapy.
- The study included 96,501 Medicare Advantage enrollees and 206,274 traditional Medicare beneficiaries who initiated chemotherapy between January 2016 and December 2019 (mean age, ~73 years; ~56% women; Hispanic individuals, 15% and 8%; Black individuals, 15% and 8%; and White individuals, 75% and 86%, respectively).
- Resource use and care quality were measured during a 6-month period following chemotherapy initiation, and survival days were measured 18 months after beginning chemotherapy.
- Resource use measures included hospital inpatient services, outpatient care, prescription drugs, hospice services, and chemotherapy services. Quality measures included chemotherapy-related emergency visits and hospital admissions, as well as avoidable emergency visits and preventable hospitalizations.
TAKEAWAY:
- Medicare Advantage plans had lower resource use than traditional Medicare per enrollee with cancer undergoing chemotherapy ($8718 lower; 95% CI, $8343-$9094).
- The lower resource use was largely caused by fewer chemotherapy visits and less expensive chemotherapy per visit in Medicare Advantage plans ($5032 lower; 95% CI, $4772-$5293).
- Medicare Advantage enrollees had 2.5 percentage points fewer chemotherapy-related emergency department visits and 0.7 percentage points fewer chemotherapy-related hospitalizations than traditional Medicare beneficiaries.
- There was no clinically meaningful difference in survival between Medicare Advantage and traditional Medicare beneficiaries during the 18 months following chemotherapy initiation.
IN PRACTICE:
“Our new finding is that MA [Medicare Advantage] plans had lower resource use than TM [traditional Medicare] among enrollees with cancer undergoing chemotherapy — a serious condition managed by specialists and requiring expensive treatments. This suggests that MA’s cost advantages over TM are not limited to conditions for which low-cost primary care management can avoid costly services,” the authors wrote.
SOURCE:
The study was led by Yamini Kalidindi, PhD, McDermott+ Consulting, Washington, DC. It was published online on September 20, 2024, in JAMA Network Open (doi: 10.1001/jamanetworkopen.2024.34707), with a commentary.
LIMITATIONS:
The study’s findings may be affected by unobserved patient characteristics despite the use of inverse-probability weighting. The exclusion of Medicare Advantage enrollees in contracts with incomplete encounter data limits the generalizability of the results. The study does not apply to beneficiaries without Part D drug coverage. Quality measures were limited to those available from claims and encounter data, lacking information on patients’ cancer stage. The 18-month measure of survival might not adequately capture survival differences associated with early-stage cancers. The study did not measure whether patient care followed recommended guidelines.
DISCLOSURES:
Various authors reported grants from the National Institute on Aging, the National Institutes of Health, The Commonwealth Fund, Arnold Ventures, the National Cancer Institute, the Department of Defense, and the National Institute of Health Care Management. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Private Medicare Advantage plans enroll more than half of the Medicare population, but it is unknown if or how the cost restrictions they impose affect chemotherapy, which accounts for a large portion of cancer care costs.
- Researchers conducted a cohort study using national Medicare data from January 2015 to December 2019 to look at Medicare Advantage enrollment and treatment patterns for patients with cancer receiving chemotherapy.
- The study included 96,501 Medicare Advantage enrollees and 206,274 traditional Medicare beneficiaries who initiated chemotherapy between January 2016 and December 2019 (mean age, ~73 years; ~56% women; Hispanic individuals, 15% and 8%; Black individuals, 15% and 8%; and White individuals, 75% and 86%, respectively).
- Resource use and care quality were measured during a 6-month period following chemotherapy initiation, and survival days were measured 18 months after beginning chemotherapy.
- Resource use measures included hospital inpatient services, outpatient care, prescription drugs, hospice services, and chemotherapy services. Quality measures included chemotherapy-related emergency visits and hospital admissions, as well as avoidable emergency visits and preventable hospitalizations.
TAKEAWAY:
- Medicare Advantage plans had lower resource use than traditional Medicare per enrollee with cancer undergoing chemotherapy ($8718 lower; 95% CI, $8343-$9094).
- The lower resource use was largely caused by fewer chemotherapy visits and less expensive chemotherapy per visit in Medicare Advantage plans ($5032 lower; 95% CI, $4772-$5293).
- Medicare Advantage enrollees had 2.5 percentage points fewer chemotherapy-related emergency department visits and 0.7 percentage points fewer chemotherapy-related hospitalizations than traditional Medicare beneficiaries.
- There was no clinically meaningful difference in survival between Medicare Advantage and traditional Medicare beneficiaries during the 18 months following chemotherapy initiation.
IN PRACTICE:
“Our new finding is that MA [Medicare Advantage] plans had lower resource use than TM [traditional Medicare] among enrollees with cancer undergoing chemotherapy — a serious condition managed by specialists and requiring expensive treatments. This suggests that MA’s cost advantages over TM are not limited to conditions for which low-cost primary care management can avoid costly services,” the authors wrote.
SOURCE:
The study was led by Yamini Kalidindi, PhD, McDermott+ Consulting, Washington, DC. It was published online on September 20, 2024, in JAMA Network Open (doi: 10.1001/jamanetworkopen.2024.34707), with a commentary.
LIMITATIONS:
The study’s findings may be affected by unobserved patient characteristics despite the use of inverse-probability weighting. The exclusion of Medicare Advantage enrollees in contracts with incomplete encounter data limits the generalizability of the results. The study does not apply to beneficiaries without Part D drug coverage. Quality measures were limited to those available from claims and encounter data, lacking information on patients’ cancer stage. The 18-month measure of survival might not adequately capture survival differences associated with early-stage cancers. The study did not measure whether patient care followed recommended guidelines.
DISCLOSURES:
Various authors reported grants from the National Institute on Aging, the National Institutes of Health, The Commonwealth Fund, Arnold Ventures, the National Cancer Institute, the Department of Defense, and the National Institute of Health Care Management. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
AACR Cancer Progress Report: Big Strides and Big Gaps
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
The AACR’s 216-page report — an annual endeavor now in its 14th year — focused on the “tremendous” strides made in cancer care, prevention, and early detection and highlighted areas where more research and attention are warranted.
One key area is funding. For the first time since 2016, federal funding for the National Institutes of Health (NIH) and National Cancer Institute (NCI) decreased in the past year. The cuts followed nearly a decade of funding increases that saw the NIH budget expand by nearly $15 billion, and that allowed for a “rapid pace and broad scope” of advances in cancer, AACR’s chief executive officer Margaret Foti, MD, PhD, said during a press briefing.
These recent cuts “threaten to curtail the medical progress seen in recent years and stymie future advancements,” said Dr. Foti, who called on Congress to commit to funding cancer research at significant and consistent levels to “maintain the momentum of progress against cancer.”
Inside the Report: Big Progress
Overall, advances in prevention, early detection, and treatment have helped catch more cancers earlier and save lives.
According to the AACR report, the age-adjusted overall cancer death rate in the United States fell by 33% between 1991 and 2021, meaning about 4.1 million cancer deaths were averted. The overall cancer death rate for children and adolescents has declined by 24% in the past 2 decades. The 5-year relative survival rate for children diagnosed with cancer in the US has improved from 58% for those diagnosed in the mid-1970s to 85% for those diagnosed between 2013 and 2019.
The past fiscal year has seen many new approvals for cancer drugs, diagnostics, and screening tests. From July 1, 2023, to June 30, 2024, the Food and Drug Administration (FDA) approved 15 new anticancer therapeutics, as well as 15 new indications for previously approved agents, one new imaging agent, several artificial intelligence (AI) tools to improve early cancer detection and diagnosis, and two minimally invasive tests for assessing inherited cancer risk or early cancer detection, according to the report.
“Cancer diagnostics are becoming more sophisticated,” AACR president Patricia M. LoRusso, DO, PhD, said during the briefing. “New technologies, such as spatial transcriptomics, are helping us study tumors at a cellular level, and helping to unveil things that we did not initially even begin to understand or think of. AI-based approaches are beginning to transform cancer detection, diagnosis, clinical decision-making, and treatment response monitoring.”
The report also highlights the significant progress in many childhood and adolescent/young adult cancers, Dr. LoRusso noted. These include FDA approvals for two new molecularly targeted therapeutics: tovorafenib for children with certain types of brain tumor and repotrectinib for children with a wide array of cancer types that have a specific genetic alteration known as NTRK gene fusion. It also includes an expanded approval for eflornithine to reduce the risk for relapse in children with high-risk neuroblastoma.
“Decades — decades — of basic research discoveries, have led to these clinical breakthroughs,” she stressed. “These gains against cancer are because of the rapid progress in our ability to decode the cancer genome, which has opened new and innovative avenues for drug development.”
The Gaps
Even with progress in cancer prevention, early detection, and treatment, cancer remains a significant issue.
“In 2024, it is estimated that more than 2 million new cases of cancer will be diagnosed in the United States. More than 611,000 people will die from the disease,” according to the report.
The 2024 report shows that incidence rates for some cancers are increasing in the United States, including vaccine-preventable cancers such as human papillomavirus (HPV)–associated oral cancers and, in young adults, cervical cancers. A recent analysis also found that overall cervical cancer incidence among women aged 30-34 years increased by 2.5% a year between 2012 and 2019.
Furthermore, despite clear evidence demonstrating that the HPV vaccine reduces cervical cancer incidence, uptake has remained poor, with only 38.6% of US children and adolescents aged 9-17 years receiving at least one dose of the vaccine in 2022.
Early-onset cancers are also increasing. Rates of breast, colorectal, and other cancers are on the rise in adults younger than 50 years, the report noted.
The report also pointed to data that 40% of all cancer cases in the United States can be attributed to preventable factors, such as smoking, excess body weight, and alcohol. However, our understanding of these risk factors has improved. Excessive levels of alcohol consumption have, for instance, been shown to increase the risk for six different types of cancer: certain types of head and neck cancer, esophageal squamous cell carcinoma, and breast, colorectal, liver, and stomach cancers.
Financial toxicity remains prevalent as well.
The report explains that financial hardship following a cancer diagnosis is widespread, and the effects can last for years. In fact, more than 40% of patients can spend their entire life savings within the first 2 years of cancer treatment. Among adult survivors of childhood cancers, 20.7% had trouble paying their medical bills, 29.9% said they had been sent to debt collection for unpaid bills, 14.1% had forgone medical care, and 26.8% could not afford nutritious meals.
For young cancer survivors, the lifetime costs associated with a diagnosis of cancer are substantial, reaching an average of $259,324 per person.
On a global level, it is estimated that from 2020 to 2050, the cumulative economic burden of cancer will be $25.2 trillion.
The Path Forward
Despite these challenges, Dr. LoRusso said, “it is unquestionable that we are in a time of unparalleled opportunities in cancer research.
“I am excited about what the future holds for cancer research, and especially for patient care,” she said.
However, funding commitments are needed to avoid impeding this momentum and losing a “talented and creative young workforce” that has brought new ideas and new technologies to the table.
Continued robust funding will help “to markedly improve cancer care, increase cancer survivorship, spur economic growth, and maintain the United States’ position as the global leader in science and medical research,” she added.
The AACR report specifically calls on Congress to:
- Appropriate at least $51.3 billion in fiscal year 2025 for the base budget of the NIH and at least $7.934 billion for the NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through fiscal year 2026 in addition to other funding, consistent with the President’s fiscal year 2025 budget.
- Appropriate at least $472.4 million in fiscal year 2025 for the CDC’s Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in fiscal year 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By working together with Congress and other stakeholders, “we will be able to accelerate the pace of progress and make major strides toward the lifesaving goal of preventing and curing all cancers at the earliest possible time,” Dr. Foti said. “I believe if we do that ... one day we will win this war on cancer.”
A version of this article first appeared on Medscape.com.
Screening Identifies Familial Risk for Hereditary Breast and Ovarian Cancer in Large Health System
TOPLINE:
Electronic health record (EHR)–derived family history identified 29,913 patients with familial risk for hereditary breast and ovarian cancer, but 82% had no evidence of genetic testing. Seven-question family history screening (FHS7)–positive status was associated with a threefold increase in BRCA1/2 positivity and a 44% increase in cancer risk among women.
METHODOLOGY:
- A cross-sectional and retrospective cohort analysis used EHR data from Renown Health in northern Nevada. The study period spanned from January 1, 2018, to February 1, 2024, with data on demographic variables, healthcare utilization, and cancer diagnoses.
- The study aimed to use the FHS7 to identify patients meeting family history criteria for genetic testing (familial risk for hereditary breast and ovarian cancer) in their EHRs; patients meeting the FHS7 criteria were deemed to be FHS7-positive.
- A total of 835,727 patients aged 18-79 years were included, with genotype data available for 38,003 participants from the Healthy Nevada Project, which notified 330 individuals with BRCA1/2 variants of their genetic risk.
- The primary outcomes were the presence of pathogenic or likely pathogenic variants in specific genes and the diagnosis of cancer.
TAKEAWAY:
- FHS7-positive status was associated with a 3.34-fold increase in BRCA1/2 positivity among female participants and a 3.35-fold increase among male participants (95% CI, 2.48-4.47 and 1.93-5.56, respectively).
- Female FHS7-positive participants had a 1.62-fold increase in CHEK2 positivity and a 2.84-fold increase in PALB2 positivity (95% CI, 1.05-2.43 and 1.23-6.16, respectively).
- Age-adjusted cancer incidence rates were higher for FHS7-positive patients, with 367.2 cases per 100,000 per year for women and 309.9 cases per 100,000 per year for men.
- The number needed to test to detect one BRCA1/2-positive patient decreased from 128 to 53 for women and from 119 to 42 for men when prescreening with FHS7.
IN PRACTICE:
“EHR-derived FHS7 identified thousands of patients with familial risk for breast cancer, indicating a substantial gap in genetic testing,” the study authors wrote. “Survey results suggest that most patients who are FHS7-positive in their EHR truly meet family history criteria, but that EHR-derived FHS7 may miss many patients who would be FHS7-positive if approached with a direct questionnaire,” the author wrote.
SOURCE:
The study was led by Daniel Kiser, MS, University of Nevada, Reno School of Medicine. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s observational design may introduce self-selection biases, particularly among Healthy Nevada Project participants. The 21.8% response rate to the survey suggests potential self-selection among respondents. The tendency of less healthy patients to have more data available in their EHRs could influence the authors’ analysis of cancer incidence rates, despite adjustments for healthcare utilization levels.
DISCLOSURES:
Daniel Kiser and Joseph J. Grzymski, PhD, reported holding patents outside the submitted work. Dr. Grzymski also disclosed receiving grants from Gilead Sciences. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Electronic health record (EHR)–derived family history identified 29,913 patients with familial risk for hereditary breast and ovarian cancer, but 82% had no evidence of genetic testing. Seven-question family history screening (FHS7)–positive status was associated with a threefold increase in BRCA1/2 positivity and a 44% increase in cancer risk among women.
METHODOLOGY:
- A cross-sectional and retrospective cohort analysis used EHR data from Renown Health in northern Nevada. The study period spanned from January 1, 2018, to February 1, 2024, with data on demographic variables, healthcare utilization, and cancer diagnoses.
- The study aimed to use the FHS7 to identify patients meeting family history criteria for genetic testing (familial risk for hereditary breast and ovarian cancer) in their EHRs; patients meeting the FHS7 criteria were deemed to be FHS7-positive.
- A total of 835,727 patients aged 18-79 years were included, with genotype data available for 38,003 participants from the Healthy Nevada Project, which notified 330 individuals with BRCA1/2 variants of their genetic risk.
- The primary outcomes were the presence of pathogenic or likely pathogenic variants in specific genes and the diagnosis of cancer.
TAKEAWAY:
- FHS7-positive status was associated with a 3.34-fold increase in BRCA1/2 positivity among female participants and a 3.35-fold increase among male participants (95% CI, 2.48-4.47 and 1.93-5.56, respectively).
- Female FHS7-positive participants had a 1.62-fold increase in CHEK2 positivity and a 2.84-fold increase in PALB2 positivity (95% CI, 1.05-2.43 and 1.23-6.16, respectively).
- Age-adjusted cancer incidence rates were higher for FHS7-positive patients, with 367.2 cases per 100,000 per year for women and 309.9 cases per 100,000 per year for men.
- The number needed to test to detect one BRCA1/2-positive patient decreased from 128 to 53 for women and from 119 to 42 for men when prescreening with FHS7.
IN PRACTICE:
“EHR-derived FHS7 identified thousands of patients with familial risk for breast cancer, indicating a substantial gap in genetic testing,” the study authors wrote. “Survey results suggest that most patients who are FHS7-positive in their EHR truly meet family history criteria, but that EHR-derived FHS7 may miss many patients who would be FHS7-positive if approached with a direct questionnaire,” the author wrote.
SOURCE:
The study was led by Daniel Kiser, MS, University of Nevada, Reno School of Medicine. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s observational design may introduce self-selection biases, particularly among Healthy Nevada Project participants. The 21.8% response rate to the survey suggests potential self-selection among respondents. The tendency of less healthy patients to have more data available in their EHRs could influence the authors’ analysis of cancer incidence rates, despite adjustments for healthcare utilization levels.
DISCLOSURES:
Daniel Kiser and Joseph J. Grzymski, PhD, reported holding patents outside the submitted work. Dr. Grzymski also disclosed receiving grants from Gilead Sciences. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Electronic health record (EHR)–derived family history identified 29,913 patients with familial risk for hereditary breast and ovarian cancer, but 82% had no evidence of genetic testing. Seven-question family history screening (FHS7)–positive status was associated with a threefold increase in BRCA1/2 positivity and a 44% increase in cancer risk among women.
METHODOLOGY:
- A cross-sectional and retrospective cohort analysis used EHR data from Renown Health in northern Nevada. The study period spanned from January 1, 2018, to February 1, 2024, with data on demographic variables, healthcare utilization, and cancer diagnoses.
- The study aimed to use the FHS7 to identify patients meeting family history criteria for genetic testing (familial risk for hereditary breast and ovarian cancer) in their EHRs; patients meeting the FHS7 criteria were deemed to be FHS7-positive.
- A total of 835,727 patients aged 18-79 years were included, with genotype data available for 38,003 participants from the Healthy Nevada Project, which notified 330 individuals with BRCA1/2 variants of their genetic risk.
- The primary outcomes were the presence of pathogenic or likely pathogenic variants in specific genes and the diagnosis of cancer.
TAKEAWAY:
- FHS7-positive status was associated with a 3.34-fold increase in BRCA1/2 positivity among female participants and a 3.35-fold increase among male participants (95% CI, 2.48-4.47 and 1.93-5.56, respectively).
- Female FHS7-positive participants had a 1.62-fold increase in CHEK2 positivity and a 2.84-fold increase in PALB2 positivity (95% CI, 1.05-2.43 and 1.23-6.16, respectively).
- Age-adjusted cancer incidence rates were higher for FHS7-positive patients, with 367.2 cases per 100,000 per year for women and 309.9 cases per 100,000 per year for men.
- The number needed to test to detect one BRCA1/2-positive patient decreased from 128 to 53 for women and from 119 to 42 for men when prescreening with FHS7.
IN PRACTICE:
“EHR-derived FHS7 identified thousands of patients with familial risk for breast cancer, indicating a substantial gap in genetic testing,” the study authors wrote. “Survey results suggest that most patients who are FHS7-positive in their EHR truly meet family history criteria, but that EHR-derived FHS7 may miss many patients who would be FHS7-positive if approached with a direct questionnaire,” the author wrote.
SOURCE:
The study was led by Daniel Kiser, MS, University of Nevada, Reno School of Medicine. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s observational design may introduce self-selection biases, particularly among Healthy Nevada Project participants. The 21.8% response rate to the survey suggests potential self-selection among respondents. The tendency of less healthy patients to have more data available in their EHRs could influence the authors’ analysis of cancer incidence rates, despite adjustments for healthcare utilization levels.
DISCLOSURES:
Daniel Kiser and Joseph J. Grzymski, PhD, reported holding patents outside the submitted work. Dr. Grzymski also disclosed receiving grants from Gilead Sciences. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Black Women Have a Higher Risk for Death in BC Subtypes
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The greatest disparity in BC-specific survival was observed in those with hormone receptor-positive (HR+), human epidermal growth factor 2–negative (HER2−) tumors, with Black women having a 50% higher risk for death.
METHODOLOGY:
- US Black women have a 40% higher risk for death from BC than White women, and many cancer specialists believe that disparities are worse among more treatable subtypes, such as HR+ tumors.
- Researchers conducted a systematic review and meta-analysis of 18 US studies published during 2009-2022 that included 228,885 women (34,262 Black women; 182,466 White women) and examined racial differences in BC survival by subtype.
- The analysis included hormone receptor and HER2/neu status to define subtypes: HR+ HER2+, HR+ HER2−, HR− HER2+, and HR− HER2−.
- Random-effects models were used to generate pooled relative risks and 95% CI for BC-specific survival and overall survival.
- The primary outcome was BC-specific survival, with overall survival as a secondary analysis.
TAKEAWAY:
- Black women had a higher risk for BC death across all tumor subtypes than White women, with the greatest disparity observed in HR+ HER2− tumors (hazard ratio [HR], 1.50; 95% CI, 1.30-1.72).
- The risk for BC death was also higher for Black women with HR+ HER2+ tumors (HR, 1.34; 95% CI, 1.10-1.64); HR− HER2+ tumors (HR, 1.20; 95% CI, 1.00-1.43); and HR− HER2− tumors (HR, 1.17; 95% CI, 1.10-1.25).
- Overall survival was poorer for Black women across all subtypes, although estimates for HR− HER2+ tumors did not reach statistical significance.
- In analysis of two subtypes with significant heterogeneity among studies, adjustments for socioeconomic status and number of Black participants explained about half and all the variance for HR+ HER2− and HR− HER2+ tumors, respectively.
IN PRACTICE:
“These results suggest there are both subtype-specific and subtype-independent mechanisms that contribute to disparities in breast cancer survival between Black and White women, which require multilevel interventions to address and achieve health equity,” wrote the authors.
SOURCE:
The study was led by Juliana M. Torres, Dana-Farber/Harvard Cancer Center, CURE Program, Boston. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
The study’s limitations included potential heterogeneity between studies as indicated by significant heterogeneity in some analyses. The use of different subtype definitions and potential overlap in data sets may have also affected the results. Many included studies did not capture the extent to which treatments were completed or detection and treatment of recurrences. Additionally, the study’s findings may not fully capture socioeconomic inequality and other unmeasured factors contributing to disparities. The racial and ethnic disparities analysis focused only on Black and White women.
DISCLOSURES:
Individual authors disclosed financial relationships with Pfizer, Healthix, Merck, AstraZeneca, LabCorp, and Takeda. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
FDA OKs Adjuvant Ribociclib in Earlier Stage Breast Cancer
FDA also approved ribociclib and the aromatase inhibitor letrozole packaged together (Kisqali Femara Co-Pack, Novartis) for the same indication.
A rival cyclin-dependent kinase 4/6 (CDK4/6) inhibitor abemaciclib (Verzenio, Eli Lilly) carries a similar adjuvant indication, but use of this agent requires patients to be lymph node–positive.
There’s no such restriction for the new ribociclib indication, which “allows us to offer treatment with a CDK4/6 inhibitor to a significantly broader group of people,” lead investigator Dennis J. Slamon, MD, breast oncologist at the University of California Los Angeless, said in a Novartis press release.
The new indication joins ribociclib’s previous approval for advanced or metastatic HR-positive, HER2-negative breast cancer in combination with an aromatase inhibitor or fulvestrant.
The current approval was based on data from the NATALEE trial. NATALEE randomized 5101 patients with early-stage HR-positive, HER2-negative disease to either 400 mg ribociclib with an aromatase inhibitor or to an aromatase inhibitor alone following surgery.
Invasive disease-free survival at 36 months was 90.7% in the ribociclib arm vs 87.6% with aromatase inhibitor monotherapy (hazard ratio [HR], 0.749; P = .0006). The trial included patients with and without lymph node involvement.
At 4 years (well beyond NATALEE’s 3-year treatment window), the ribociclib group continued to do better, with an invasive disease-free survival rate of 88.5% vs 83.6% in the control arm.
Overall survival data remain immature but with a trend towards improved survival in the ribociclib arm (HR, 0.715; P < .0001), according to a recent report from the 2024 European Society for Medical Oncology Congress.
There were no new safety signals in the trial. Adverse events in the ribociclib group included neutropenia (62.5% overall; 44.3% grade 3/4), liver-related events (26.4% overall; 8.6% grade 3/4), QT prolongation (5.3% overall; 1.0% grade 3/4), and interstitial lung disease/pneumonitis (1.5% overall; 0.0% grade 3/4), according to Novartis.
Ribociclib dosing for the adjuvant indication is lower than for metastatic disease, but patients are on the same schedule — two 200 mg tablets once daily for 21 days followed by 7 days off in 28-day cycles. Treatment continues for 3 years.
Forty-two 200 mg tablets cost about $15,000, according to drugs.com. A patient assistance program is available through Novartis.
A version of this article appeared on Medscape.com.
FDA also approved ribociclib and the aromatase inhibitor letrozole packaged together (Kisqali Femara Co-Pack, Novartis) for the same indication.
A rival cyclin-dependent kinase 4/6 (CDK4/6) inhibitor abemaciclib (Verzenio, Eli Lilly) carries a similar adjuvant indication, but use of this agent requires patients to be lymph node–positive.
There’s no such restriction for the new ribociclib indication, which “allows us to offer treatment with a CDK4/6 inhibitor to a significantly broader group of people,” lead investigator Dennis J. Slamon, MD, breast oncologist at the University of California Los Angeless, said in a Novartis press release.
The new indication joins ribociclib’s previous approval for advanced or metastatic HR-positive, HER2-negative breast cancer in combination with an aromatase inhibitor or fulvestrant.
The current approval was based on data from the NATALEE trial. NATALEE randomized 5101 patients with early-stage HR-positive, HER2-negative disease to either 400 mg ribociclib with an aromatase inhibitor or to an aromatase inhibitor alone following surgery.
Invasive disease-free survival at 36 months was 90.7% in the ribociclib arm vs 87.6% with aromatase inhibitor monotherapy (hazard ratio [HR], 0.749; P = .0006). The trial included patients with and without lymph node involvement.
At 4 years (well beyond NATALEE’s 3-year treatment window), the ribociclib group continued to do better, with an invasive disease-free survival rate of 88.5% vs 83.6% in the control arm.
Overall survival data remain immature but with a trend towards improved survival in the ribociclib arm (HR, 0.715; P < .0001), according to a recent report from the 2024 European Society for Medical Oncology Congress.
There were no new safety signals in the trial. Adverse events in the ribociclib group included neutropenia (62.5% overall; 44.3% grade 3/4), liver-related events (26.4% overall; 8.6% grade 3/4), QT prolongation (5.3% overall; 1.0% grade 3/4), and interstitial lung disease/pneumonitis (1.5% overall; 0.0% grade 3/4), according to Novartis.
Ribociclib dosing for the adjuvant indication is lower than for metastatic disease, but patients are on the same schedule — two 200 mg tablets once daily for 21 days followed by 7 days off in 28-day cycles. Treatment continues for 3 years.
Forty-two 200 mg tablets cost about $15,000, according to drugs.com. A patient assistance program is available through Novartis.
A version of this article appeared on Medscape.com.
FDA also approved ribociclib and the aromatase inhibitor letrozole packaged together (Kisqali Femara Co-Pack, Novartis) for the same indication.
A rival cyclin-dependent kinase 4/6 (CDK4/6) inhibitor abemaciclib (Verzenio, Eli Lilly) carries a similar adjuvant indication, but use of this agent requires patients to be lymph node–positive.
There’s no such restriction for the new ribociclib indication, which “allows us to offer treatment with a CDK4/6 inhibitor to a significantly broader group of people,” lead investigator Dennis J. Slamon, MD, breast oncologist at the University of California Los Angeless, said in a Novartis press release.
The new indication joins ribociclib’s previous approval for advanced or metastatic HR-positive, HER2-negative breast cancer in combination with an aromatase inhibitor or fulvestrant.
The current approval was based on data from the NATALEE trial. NATALEE randomized 5101 patients with early-stage HR-positive, HER2-negative disease to either 400 mg ribociclib with an aromatase inhibitor or to an aromatase inhibitor alone following surgery.
Invasive disease-free survival at 36 months was 90.7% in the ribociclib arm vs 87.6% with aromatase inhibitor monotherapy (hazard ratio [HR], 0.749; P = .0006). The trial included patients with and without lymph node involvement.
At 4 years (well beyond NATALEE’s 3-year treatment window), the ribociclib group continued to do better, with an invasive disease-free survival rate of 88.5% vs 83.6% in the control arm.
Overall survival data remain immature but with a trend towards improved survival in the ribociclib arm (HR, 0.715; P < .0001), according to a recent report from the 2024 European Society for Medical Oncology Congress.
There were no new safety signals in the trial. Adverse events in the ribociclib group included neutropenia (62.5% overall; 44.3% grade 3/4), liver-related events (26.4% overall; 8.6% grade 3/4), QT prolongation (5.3% overall; 1.0% grade 3/4), and interstitial lung disease/pneumonitis (1.5% overall; 0.0% grade 3/4), according to Novartis.
Ribociclib dosing for the adjuvant indication is lower than for metastatic disease, but patients are on the same schedule — two 200 mg tablets once daily for 21 days followed by 7 days off in 28-day cycles. Treatment continues for 3 years.
Forty-two 200 mg tablets cost about $15,000, according to drugs.com. A patient assistance program is available through Novartis.
A version of this article appeared on Medscape.com.
Cancer Risk: Are Pesticides the New Smoking?
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Starting Mammography at Age 40 May Backfire Due to False Positives
Earlier this year, I wrote a Medscape commentary to explain my disagreement with the US Preventive Services Task Force (USPSTF)’s updated recommendation that all women at average risk for breast cancer start screening mammography at age 40. The bottom line is that when the evidence doesn’t change, the guidelines shouldn’t change. Since then, other screening experts have criticized the USPSTF guideline on similar grounds, and a national survey reported that nearly 4 out of 10 women in their 40s preferred to delay breast cancer screening after viewing a decision aid and a personalized breast cancer risk estimate.
The decision analysis performed for the USPSTF guideline estimated that compared with having mammography beginning at age 50, 1000 women who begin at age 40 experience 519 more false-positive results and 62 more benign breast biopsies. Another study suggested that anxiety and other psychosocial harms resulting from a false-positive test are similar between patients who require a biopsy vs additional imaging only. Of greater concern, women who have false-positive results are less likely to return for their next scheduled screening exam.
A recent analysis of 2005-2017 data from the US Breast Cancer Surveillance Consortium found that about 1 in 10 mammograms had a false-positive result. Sixty percent of these patients underwent immediate additional imaging, 27% were recalled for diagnostic imaging within the next few days to weeks, and 13% were advised to have a biopsy. While patients who had additional imaging at the same visit were only 1.9% less likely to return for screening mammography within 30 months compared with those with normal mammograms, women who were recalled for short-interval follow-up or recommended for biopsy were 15.9% and 10% less likely to return, respectively. For unclear reasons, women who identified as Asian or Hispanic had even lower rates of return screening after false-positive results.
These differences matter because women in their 40s, with the lowest incidence of breast cancer among those undergoing screening, have a lot of false positives. A patient who follows the USPSTF recommendation and starts screening at age 40 has a 42% chance of having at least one false positive with every-other-year screening, or a 61% chance with annual screening, by the time she turns 50. If some of these patients are so turned off by false positives that they don’t return for regular mammography in their 50s and 60s, when screening is the most likely to catch clinically significant cancers at treatable stages, then moving up the starting age may backfire and cause net harm.
The recently implemented FDA rule requiring mammography reports to include breast density could compound this problem. Because younger women are more likely to have dense breasts, more of them will probably decide to have supplemental imaging for cancer. I previously pointed out that we don’t know whether supplemental imaging with breast ultrasonography or MRI reduces cancer deaths, but we do know that it increases false-positive results.
I have personally cared for several patients who abandoned screening mammography for long stretches, or permanently, after having endured one or more benign biopsies prompted by a false-positive result. I vividly recall one woman in her 60s who was very reluctant to have screening tests in general, and mammography in particular, for that reason. After she had been my patient for a few years, I finally persuaded her to resume screening. We were both surprised when her first mammogram in more than a decade revealed an early-stage breast cancer. Fortunately, the tumor was successfully treated, but for her, an earlier false-positive result nearly ended up having critical health consequences.
Dr. Lin is associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has no relevant financial relationships.
A version of this article appeared on Medscape.com.
Earlier this year, I wrote a Medscape commentary to explain my disagreement with the US Preventive Services Task Force (USPSTF)’s updated recommendation that all women at average risk for breast cancer start screening mammography at age 40. The bottom line is that when the evidence doesn’t change, the guidelines shouldn’t change. Since then, other screening experts have criticized the USPSTF guideline on similar grounds, and a national survey reported that nearly 4 out of 10 women in their 40s preferred to delay breast cancer screening after viewing a decision aid and a personalized breast cancer risk estimate.
The decision analysis performed for the USPSTF guideline estimated that compared with having mammography beginning at age 50, 1000 women who begin at age 40 experience 519 more false-positive results and 62 more benign breast biopsies. Another study suggested that anxiety and other psychosocial harms resulting from a false-positive test are similar between patients who require a biopsy vs additional imaging only. Of greater concern, women who have false-positive results are less likely to return for their next scheduled screening exam.
A recent analysis of 2005-2017 data from the US Breast Cancer Surveillance Consortium found that about 1 in 10 mammograms had a false-positive result. Sixty percent of these patients underwent immediate additional imaging, 27% were recalled for diagnostic imaging within the next few days to weeks, and 13% were advised to have a biopsy. While patients who had additional imaging at the same visit were only 1.9% less likely to return for screening mammography within 30 months compared with those with normal mammograms, women who were recalled for short-interval follow-up or recommended for biopsy were 15.9% and 10% less likely to return, respectively. For unclear reasons, women who identified as Asian or Hispanic had even lower rates of return screening after false-positive results.
These differences matter because women in their 40s, with the lowest incidence of breast cancer among those undergoing screening, have a lot of false positives. A patient who follows the USPSTF recommendation and starts screening at age 40 has a 42% chance of having at least one false positive with every-other-year screening, or a 61% chance with annual screening, by the time she turns 50. If some of these patients are so turned off by false positives that they don’t return for regular mammography in their 50s and 60s, when screening is the most likely to catch clinically significant cancers at treatable stages, then moving up the starting age may backfire and cause net harm.
The recently implemented FDA rule requiring mammography reports to include breast density could compound this problem. Because younger women are more likely to have dense breasts, more of them will probably decide to have supplemental imaging for cancer. I previously pointed out that we don’t know whether supplemental imaging with breast ultrasonography or MRI reduces cancer deaths, but we do know that it increases false-positive results.
I have personally cared for several patients who abandoned screening mammography for long stretches, or permanently, after having endured one or more benign biopsies prompted by a false-positive result. I vividly recall one woman in her 60s who was very reluctant to have screening tests in general, and mammography in particular, for that reason. After she had been my patient for a few years, I finally persuaded her to resume screening. We were both surprised when her first mammogram in more than a decade revealed an early-stage breast cancer. Fortunately, the tumor was successfully treated, but for her, an earlier false-positive result nearly ended up having critical health consequences.
Dr. Lin is associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has no relevant financial relationships.
A version of this article appeared on Medscape.com.
Earlier this year, I wrote a Medscape commentary to explain my disagreement with the US Preventive Services Task Force (USPSTF)’s updated recommendation that all women at average risk for breast cancer start screening mammography at age 40. The bottom line is that when the evidence doesn’t change, the guidelines shouldn’t change. Since then, other screening experts have criticized the USPSTF guideline on similar grounds, and a national survey reported that nearly 4 out of 10 women in their 40s preferred to delay breast cancer screening after viewing a decision aid and a personalized breast cancer risk estimate.
The decision analysis performed for the USPSTF guideline estimated that compared with having mammography beginning at age 50, 1000 women who begin at age 40 experience 519 more false-positive results and 62 more benign breast biopsies. Another study suggested that anxiety and other psychosocial harms resulting from a false-positive test are similar between patients who require a biopsy vs additional imaging only. Of greater concern, women who have false-positive results are less likely to return for their next scheduled screening exam.
A recent analysis of 2005-2017 data from the US Breast Cancer Surveillance Consortium found that about 1 in 10 mammograms had a false-positive result. Sixty percent of these patients underwent immediate additional imaging, 27% were recalled for diagnostic imaging within the next few days to weeks, and 13% were advised to have a biopsy. While patients who had additional imaging at the same visit were only 1.9% less likely to return for screening mammography within 30 months compared with those with normal mammograms, women who were recalled for short-interval follow-up or recommended for biopsy were 15.9% and 10% less likely to return, respectively. For unclear reasons, women who identified as Asian or Hispanic had even lower rates of return screening after false-positive results.
These differences matter because women in their 40s, with the lowest incidence of breast cancer among those undergoing screening, have a lot of false positives. A patient who follows the USPSTF recommendation and starts screening at age 40 has a 42% chance of having at least one false positive with every-other-year screening, or a 61% chance with annual screening, by the time she turns 50. If some of these patients are so turned off by false positives that they don’t return for regular mammography in their 50s and 60s, when screening is the most likely to catch clinically significant cancers at treatable stages, then moving up the starting age may backfire and cause net harm.
The recently implemented FDA rule requiring mammography reports to include breast density could compound this problem. Because younger women are more likely to have dense breasts, more of them will probably decide to have supplemental imaging for cancer. I previously pointed out that we don’t know whether supplemental imaging with breast ultrasonography or MRI reduces cancer deaths, but we do know that it increases false-positive results.
I have personally cared for several patients who abandoned screening mammography for long stretches, or permanently, after having endured one or more benign biopsies prompted by a false-positive result. I vividly recall one woman in her 60s who was very reluctant to have screening tests in general, and mammography in particular, for that reason. After she had been my patient for a few years, I finally persuaded her to resume screening. We were both surprised when her first mammogram in more than a decade revealed an early-stage breast cancer. Fortunately, the tumor was successfully treated, but for her, an earlier false-positive result nearly ended up having critical health consequences.
Dr. Lin is associate director, Family Medicine Residency Program, Lancaster General Hospital, Lancaster, Pennsylvania. He blogs at Common Sense Family Doctor. He has no relevant financial relationships.
A version of this article appeared on Medscape.com.