User login
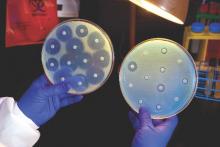
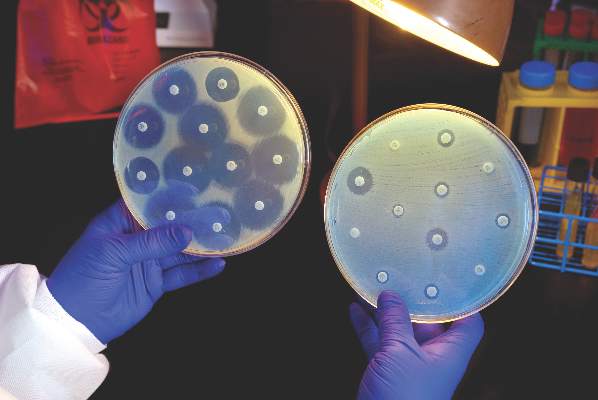
FDA rule will pull many consumer antibacterial soaps from market
Over-the-counter consumer antiseptic wash products with active ingredients such as triclosan and triclocarban will be pulled from the market, following a final rule issued Sept. 2 by the Food and Drug Administration.
Companies will no longer be able to sell antibacterial washes with those ingredients, the FDA said, because manufacturers failed to show the ingredients are safe for long-term daily use and are better than plain soap and water at preventing illness and the spread of infections.
The final rule targets consumer antiseptic wash products containing 1 or more of 19 active ingredients, including the 2 most commonly used ingredients, triclosan and triclocarban. Companies have 1 year to comply with the new rule.
The FDA’s rule does not apply to hand sanitizers, wipes, or antibacterial products used in health care settings.
The agency has deferred for 1 year a decision on the continued use of three other ingredients in consumer wash products: benzalkonium chloride, benzethonium chloride, and chloroxylenol.
The FDA’s decision was driven in part by concerns about the risks posed by long-term exposure to such products, including bacterial resistance or hormonal effects.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, in a statement. “In fact, some data suggest that antibacterial ingredients may do more harm than good over the long term.”
Washing with plain soap and water remains one of the most important steps consumers can take to prevent illness and the spread of infection, the FDA advised. The agency also recommended use of alcohol-based hand sanitizer with at least 60% alcohol.
Read the full press release on the FDA website.
Over-the-counter consumer antiseptic wash products with active ingredients such as triclosan and triclocarban will be pulled from the market, following a final rule issued Sept. 2 by the Food and Drug Administration.
Companies will no longer be able to sell antibacterial washes with those ingredients, the FDA said, because manufacturers failed to show the ingredients are safe for long-term daily use and are better than plain soap and water at preventing illness and the spread of infections.
The final rule targets consumer antiseptic wash products containing 1 or more of 19 active ingredients, including the 2 most commonly used ingredients, triclosan and triclocarban. Companies have 1 year to comply with the new rule.
The FDA’s rule does not apply to hand sanitizers, wipes, or antibacterial products used in health care settings.
The agency has deferred for 1 year a decision on the continued use of three other ingredients in consumer wash products: benzalkonium chloride, benzethonium chloride, and chloroxylenol.
The FDA’s decision was driven in part by concerns about the risks posed by long-term exposure to such products, including bacterial resistance or hormonal effects.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, in a statement. “In fact, some data suggest that antibacterial ingredients may do more harm than good over the long term.”
Washing with plain soap and water remains one of the most important steps consumers can take to prevent illness and the spread of infection, the FDA advised. The agency also recommended use of alcohol-based hand sanitizer with at least 60% alcohol.
Read the full press release on the FDA website.
Over-the-counter consumer antiseptic wash products with active ingredients such as triclosan and triclocarban will be pulled from the market, following a final rule issued Sept. 2 by the Food and Drug Administration.
Companies will no longer be able to sell antibacterial washes with those ingredients, the FDA said, because manufacturers failed to show the ingredients are safe for long-term daily use and are better than plain soap and water at preventing illness and the spread of infections.
The final rule targets consumer antiseptic wash products containing 1 or more of 19 active ingredients, including the 2 most commonly used ingredients, triclosan and triclocarban. Companies have 1 year to comply with the new rule.
The FDA’s rule does not apply to hand sanitizers, wipes, or antibacterial products used in health care settings.
The agency has deferred for 1 year a decision on the continued use of three other ingredients in consumer wash products: benzalkonium chloride, benzethonium chloride, and chloroxylenol.
The FDA’s decision was driven in part by concerns about the risks posed by long-term exposure to such products, including bacterial resistance or hormonal effects.
“Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, in a statement. “In fact, some data suggest that antibacterial ingredients may do more harm than good over the long term.”
Washing with plain soap and water remains one of the most important steps consumers can take to prevent illness and the spread of infection, the FDA advised. The agency also recommended use of alcohol-based hand sanitizer with at least 60% alcohol.
Read the full press release on the FDA website.
When should primary care physicians prescribe antibiotics to children with respiratory infection symptoms?
Duration of illness, age, and the presence of specific symptoms are key predictors of hospitalization risk due to respiratory infection, according to a study published in The Lancet. These demographic and clinical factors should guide a primary care physician’s decision to prescribe antibiotics.
“More than 80% of all health-service antibiotics [are] prescribed by primary care clinicians,” reported Alastair Hay, MD, of the University of Bristol, England, and his associates.
“Antibiotic prescribing in primary care is increasing and directly affects antimicrobial resistance,” the researchers noted, adding that many primary care clinicians prescribe antibiotics to pediatric patients with respiratory tract infections and/or cough to “mitigate perceived risk of future hospital admission and complications.”
A total of 8,394 pediatric patients who presented with acute cough and one or more other symptoms of respiratory tract infection (such as fever and coryza) were enrolled in the study by primary care physicians at 247 clinical sites in England. All eligible patients were between the ages of 3 months and 16 years; children were excluded if they presented with noninfective exacerbation of asthma, were at high risk of serious infection, or required a throat swab. The study’s primary outcome was hospital admission for any respiratory tract infection within 30 days of enrollment; the data were collected from a review of electronic medical records (Lancet. 2016. Sept 1. doi: 10.1016/S2213-2600[16]30223-5).
The median age of the pediatric cohort was 3 years, 52% were male, and 78% were white. A total of 3,121 patients (37%) were prescribed an antibiotic by their primary care physicians, but only 78 patients (0.9%) were admitted to the hospital, and 27% of discharge diagnoses suggested a possible bacterial cause (lower respiratory tract infection, tonsillitis, and pneumonia).
Multivariate modeling with bootstrap validation demonstrated that duration of illness, age, and the presence or absence of specific respiratory symptoms were the key factors that should be used to identify children at low, normal, and high risk for hospitalization due to respiratory infection. Younger patients with shorter illness durations who presented with wheeze, fever, vomiting, intercostal or subcostal recession, and/or asthma were at higher risk for hospitalization.
“Our data show that 1,846 (33%) of the very-low-risk stratum children received antibiotics. Because these children represent the majority (67%) of all the participants, a 10% overall reduction in antibiotic prescription would be achieved if prescription in this group halved, remained static in the normal risk stratum, and increased to 90% in the high risk stratum, resulting in a similar effect size to other contemporary antimicrobial stewardship interventions,” Dr. Hay and his associates concluded.
This study received funding and sponsorship from the National Institute for Health Research and the University of Bristol. Two investigators reported receiving financial compensation or honoraria from multiple companies including companies with an interest in diagnostic microbiology in respiratory tract infections.
On Twitter @jessnicolecraig
Duration of illness, age, and the presence of specific symptoms are key predictors of hospitalization risk due to respiratory infection, according to a study published in The Lancet. These demographic and clinical factors should guide a primary care physician’s decision to prescribe antibiotics.
“More than 80% of all health-service antibiotics [are] prescribed by primary care clinicians,” reported Alastair Hay, MD, of the University of Bristol, England, and his associates.
“Antibiotic prescribing in primary care is increasing and directly affects antimicrobial resistance,” the researchers noted, adding that many primary care clinicians prescribe antibiotics to pediatric patients with respiratory tract infections and/or cough to “mitigate perceived risk of future hospital admission and complications.”
A total of 8,394 pediatric patients who presented with acute cough and one or more other symptoms of respiratory tract infection (such as fever and coryza) were enrolled in the study by primary care physicians at 247 clinical sites in England. All eligible patients were between the ages of 3 months and 16 years; children were excluded if they presented with noninfective exacerbation of asthma, were at high risk of serious infection, or required a throat swab. The study’s primary outcome was hospital admission for any respiratory tract infection within 30 days of enrollment; the data were collected from a review of electronic medical records (Lancet. 2016. Sept 1. doi: 10.1016/S2213-2600[16]30223-5).
The median age of the pediatric cohort was 3 years, 52% were male, and 78% were white. A total of 3,121 patients (37%) were prescribed an antibiotic by their primary care physicians, but only 78 patients (0.9%) were admitted to the hospital, and 27% of discharge diagnoses suggested a possible bacterial cause (lower respiratory tract infection, tonsillitis, and pneumonia).
Multivariate modeling with bootstrap validation demonstrated that duration of illness, age, and the presence or absence of specific respiratory symptoms were the key factors that should be used to identify children at low, normal, and high risk for hospitalization due to respiratory infection. Younger patients with shorter illness durations who presented with wheeze, fever, vomiting, intercostal or subcostal recession, and/or asthma were at higher risk for hospitalization.
“Our data show that 1,846 (33%) of the very-low-risk stratum children received antibiotics. Because these children represent the majority (67%) of all the participants, a 10% overall reduction in antibiotic prescription would be achieved if prescription in this group halved, remained static in the normal risk stratum, and increased to 90% in the high risk stratum, resulting in a similar effect size to other contemporary antimicrobial stewardship interventions,” Dr. Hay and his associates concluded.
This study received funding and sponsorship from the National Institute for Health Research and the University of Bristol. Two investigators reported receiving financial compensation or honoraria from multiple companies including companies with an interest in diagnostic microbiology in respiratory tract infections.
On Twitter @jessnicolecraig
Duration of illness, age, and the presence of specific symptoms are key predictors of hospitalization risk due to respiratory infection, according to a study published in The Lancet. These demographic and clinical factors should guide a primary care physician’s decision to prescribe antibiotics.
“More than 80% of all health-service antibiotics [are] prescribed by primary care clinicians,” reported Alastair Hay, MD, of the University of Bristol, England, and his associates.
“Antibiotic prescribing in primary care is increasing and directly affects antimicrobial resistance,” the researchers noted, adding that many primary care clinicians prescribe antibiotics to pediatric patients with respiratory tract infections and/or cough to “mitigate perceived risk of future hospital admission and complications.”
A total of 8,394 pediatric patients who presented with acute cough and one or more other symptoms of respiratory tract infection (such as fever and coryza) were enrolled in the study by primary care physicians at 247 clinical sites in England. All eligible patients were between the ages of 3 months and 16 years; children were excluded if they presented with noninfective exacerbation of asthma, were at high risk of serious infection, or required a throat swab. The study’s primary outcome was hospital admission for any respiratory tract infection within 30 days of enrollment; the data were collected from a review of electronic medical records (Lancet. 2016. Sept 1. doi: 10.1016/S2213-2600[16]30223-5).
The median age of the pediatric cohort was 3 years, 52% were male, and 78% were white. A total of 3,121 patients (37%) were prescribed an antibiotic by their primary care physicians, but only 78 patients (0.9%) were admitted to the hospital, and 27% of discharge diagnoses suggested a possible bacterial cause (lower respiratory tract infection, tonsillitis, and pneumonia).
Multivariate modeling with bootstrap validation demonstrated that duration of illness, age, and the presence or absence of specific respiratory symptoms were the key factors that should be used to identify children at low, normal, and high risk for hospitalization due to respiratory infection. Younger patients with shorter illness durations who presented with wheeze, fever, vomiting, intercostal or subcostal recession, and/or asthma were at higher risk for hospitalization.
“Our data show that 1,846 (33%) of the very-low-risk stratum children received antibiotics. Because these children represent the majority (67%) of all the participants, a 10% overall reduction in antibiotic prescription would be achieved if prescription in this group halved, remained static in the normal risk stratum, and increased to 90% in the high risk stratum, resulting in a similar effect size to other contemporary antimicrobial stewardship interventions,” Dr. Hay and his associates concluded.
This study received funding and sponsorship from the National Institute for Health Research and the University of Bristol. Two investigators reported receiving financial compensation or honoraria from multiple companies including companies with an interest in diagnostic microbiology in respiratory tract infections.
On Twitter @jessnicolecraig
FROM THE LANCET
Key clinical point: Duration of illness, age, and the presence of specific symptoms are key predictors of hospitalization risk due to respiratory infection. These factors should guide a primary care physician’s decision to prescribe antibiotics.
Major finding: Younger patients with shorter illness durations who presented with wheeze, fever, vomiting, intercostal or subcostal recession, and/or asthma are at higher risk for hospitalization.
Data source: A prospective, prognostic cohort study of 8,394 children.
Disclosures: This study received funding and sponsorship from the National Institute for Health Research and the University of Bristol. Two investigators reported receiving financial compensation or honoraria from multiple companies, including those with an interest in diagnostic microbiology in respiratory tract infections.
Hospitals increase CRE risk when they share patients
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
The more hospitals share patients, the more likely they are to have a problem with carbapenem-resistant Enterobacteriaceae (CRE), especially if long-term acute care hospitals (LTACHs) are in the mix, according to a state-wide investigation from Illinois.
Greater hospital centrality was independently associated with higher rates overall, and sharing four or more patients with a long-term acute care hospital (LTACH) in the 3-month study window doubled the rate of CRE cases.
Although it’s possible that was because of chance (P = 0.11), the link between LTACHs and CRE “is consistent with prior analyses that have shown the central role LTACHs have in” spreading the organism, said the researchers, led by Michael Ray of the Illinois Department of Public Health (Clin Infect Dis. 2016 Aug 2. pii: ciw461).
Patients often spend weeks in LTACH facilities for ongoing, serious health problems. The severity of illness, long stay, and sometimes chronic antibiotic use increase the risk of CRE exposure, and the team found that many LTACH patients are colonized.
“These findings have immediate public health implications. … Early interventions should be focused on the most connected facilities, as well as those with strong connections to LTACHs.” When one hospital has an outbreak, facilities that share its patients need to swing into action screening new admissions and taking other steps to prevent regional spread, the team said.
Meanwhile, “state-wide patient-sharing data, which are now increasingly available through sources like the Healthcare Cost and Utilization Project, provide an important way to assess hospital risk of CRE exposure based on its position in regional patient-sharing networks,” they noted. “Public health can play a critical role in identifying tightly connected hospitals and educating personnel at such facilities about their risk and need for enhanced infection control interventions.”
The team came to their conclusions after linking Illinois’ drug-resistant organisms registry with admissions data for 185 hospitals. About half reported at least one CRE case over 3 months, with a mean of 3.5 cases per hospital.
There was an average of 64 patient-sharing connections per facility, with a minimum of one connection and a maximum of 145 connections. Each additional patient two hospitals shared corresponded to a 3% increase in the CRE rate in urban facilities and a 6% increase in rural ones. The investigators didn’t explain the discrepancy, except to note that rural areas don’t have LTACHs.
Almost two-thirds of hospitals reporting CRE were in Chicago-area counties; almost half had shared at least one patient with an LTACH, and 21% had shared four or more.
CRE cases were an average of 64 years old, and equally distributed between men and women and black and white patients.
The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: The more hospitals share patients, the more likely they are to have a problem with CRE, especially if long-term acute care hospitals are in the mix.
Major finding: Sharing four or more patients with a long-term acute care hospital in the 3-month study window doubled the rate of CRE cases (P = 0.11).
Data source: 185 Illinois hospitals.
Disclosures: The Centers for Disease Control and Prevention funded the work. The authors had no disclosures.
Antibiotics overprescribed during asthma-related hospitalizations
Antibiotics are overprescribed in asthma-related hospitalizations, even though guidelines recommend against prescribing antibiotics during exacerbations of asthma in the absence of concurrent infection, reported Peter K. Lindenauer, MD, MSc, of Baystate Medical Center in Springfield, Mass., and his colleagues.
They examined the hospitalization records of 51,951 individuals admitted to 577 hospitals in the United States between 2013 and 2014 with a principal diagnosis of either asthma or acute respiratory failure combined with asthma as a secondary diagnosis. Each patient type and the timing of antibiotic therapy was noted.
A total of 30,226 of the 51,951 patients (58.2%) were prescribed antibiotics at some point during their hospitalization, while 21,248 (40.9%) were prescribed antibiotics on the first day of hospitalization, without “documentation of an indication for antibiotic therapy.”
Macrolides were most commonly prescribed, given to 9,633 (18.5%) of patients, followed by quinolones (8,632, 16.1%), third-generation cephalosporins (4,420, 8.5%), and tetracyclines (1,858, 3.6%). After adjustment for risk variables, chronic obstructive asthma hospitalizations were found to be those most highly associated with receiving antibiotics (odds ratio 1.6, 95% confidence interval 1.5-1.7).
“Possible explanations for this high rate of potentially inappropriate treatment include the challenge of differentiating bacterial from nonbacterial infections, distinguishing asthma from chronic obstructive pulmonary disease in the acute care setting, and gaps in knowledge about the benefits of antibiotic therapy,” the authors posited, adding that these findings “suggest a significant opportunity to improve patient safety, reduce the spread of resistance, and lower spending through greater adherence to guideline recommendations.”
The National Heart, Lung, and Blood Institute and Veterans Affairs Health Services Research and Development funded the study. Dr. Lindenauer and his coauthors did not report any relevant financial disclosures.
Antibiotics are overprescribed in asthma-related hospitalizations, even though guidelines recommend against prescribing antibiotics during exacerbations of asthma in the absence of concurrent infection, reported Peter K. Lindenauer, MD, MSc, of Baystate Medical Center in Springfield, Mass., and his colleagues.
They examined the hospitalization records of 51,951 individuals admitted to 577 hospitals in the United States between 2013 and 2014 with a principal diagnosis of either asthma or acute respiratory failure combined with asthma as a secondary diagnosis. Each patient type and the timing of antibiotic therapy was noted.
A total of 30,226 of the 51,951 patients (58.2%) were prescribed antibiotics at some point during their hospitalization, while 21,248 (40.9%) were prescribed antibiotics on the first day of hospitalization, without “documentation of an indication for antibiotic therapy.”
Macrolides were most commonly prescribed, given to 9,633 (18.5%) of patients, followed by quinolones (8,632, 16.1%), third-generation cephalosporins (4,420, 8.5%), and tetracyclines (1,858, 3.6%). After adjustment for risk variables, chronic obstructive asthma hospitalizations were found to be those most highly associated with receiving antibiotics (odds ratio 1.6, 95% confidence interval 1.5-1.7).
“Possible explanations for this high rate of potentially inappropriate treatment include the challenge of differentiating bacterial from nonbacterial infections, distinguishing asthma from chronic obstructive pulmonary disease in the acute care setting, and gaps in knowledge about the benefits of antibiotic therapy,” the authors posited, adding that these findings “suggest a significant opportunity to improve patient safety, reduce the spread of resistance, and lower spending through greater adherence to guideline recommendations.”
The National Heart, Lung, and Blood Institute and Veterans Affairs Health Services Research and Development funded the study. Dr. Lindenauer and his coauthors did not report any relevant financial disclosures.
Antibiotics are overprescribed in asthma-related hospitalizations, even though guidelines recommend against prescribing antibiotics during exacerbations of asthma in the absence of concurrent infection, reported Peter K. Lindenauer, MD, MSc, of Baystate Medical Center in Springfield, Mass., and his colleagues.
They examined the hospitalization records of 51,951 individuals admitted to 577 hospitals in the United States between 2013 and 2014 with a principal diagnosis of either asthma or acute respiratory failure combined with asthma as a secondary diagnosis. Each patient type and the timing of antibiotic therapy was noted.
A total of 30,226 of the 51,951 patients (58.2%) were prescribed antibiotics at some point during their hospitalization, while 21,248 (40.9%) were prescribed antibiotics on the first day of hospitalization, without “documentation of an indication for antibiotic therapy.”
Macrolides were most commonly prescribed, given to 9,633 (18.5%) of patients, followed by quinolones (8,632, 16.1%), third-generation cephalosporins (4,420, 8.5%), and tetracyclines (1,858, 3.6%). After adjustment for risk variables, chronic obstructive asthma hospitalizations were found to be those most highly associated with receiving antibiotics (odds ratio 1.6, 95% confidence interval 1.5-1.7).
“Possible explanations for this high rate of potentially inappropriate treatment include the challenge of differentiating bacterial from nonbacterial infections, distinguishing asthma from chronic obstructive pulmonary disease in the acute care setting, and gaps in knowledge about the benefits of antibiotic therapy,” the authors posited, adding that these findings “suggest a significant opportunity to improve patient safety, reduce the spread of resistance, and lower spending through greater adherence to guideline recommendations.”
The National Heart, Lung, and Blood Institute and Veterans Affairs Health Services Research and Development funded the study. Dr. Lindenauer and his coauthors did not report any relevant financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Antibiotics are overprescribed in asthma-related hospitalizations.
Major finding: Among patients hospitalized for asthma, 58.2% had received antibiotics without any documentation or indication for such therapy.
Data source: Retrospective study of 51,951 patients in 577 U.S. hospitals from 2013 to 2014.
Disclosures: The National Heart, Lung, and Blood Institute and Veterans Affairs Health Services Research and Development funded the study. The researchers reported no relevant financial disclosures.
Clinical decision tree pinpointed risk of extended-spectrum beta-lactamase bacteremia
A new classification tool helped guide the treatment of bacteremic patients while clinicians awaited antibiotic resistance results, investigators reported.
The clinical decision tree had a positive predictive value of 91% and a negative predictive value of 92% for determining whether certain gram-negative infections produced extended-spectrum beta-lactamase (ESBL), Catherine Goodman, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and her associates wrote online in Clinical Infectious Diseases. “These predictions may assist empiric treatment decisions in order to optimize clinical outcomes while reducing administration of overly broad antibiotic agents that can select for further resistance emergence,” they added.
Bacteria that produce ESBL can hydrolyze all broad-spectrum beta-lactam antibiotics except carbapenems. Rapid tests for beta-lactamase genes can shorten the lag time between gram-stain identification and antimicrobial resistance results, but are cost prohibitive for most clinical laboratories and often do not assess ESBL gene groups, the researchers said. To find a way to predict which infections are characterized by ESBL production, they studied adults hospitalized at Johns Hopkins from October 2008 to March 2015 with bloodstream isolates of Klebsiella pneumoniae (40% of patients), Klebsiella oxytoca (4% of patients), and Escherichia coli (56% of patients). Most bacteremias began as urinary tract infections (34% of cases), followed by intra-abdominal infections (24%), catheter-related infections (16%), and biliary infections (14%) (Clin Infect Dis. 2016 Jul 26. doi:10.1093/cid/ciw425).
A total of 194 patients (15%) had bacteremias that produced ESBL, according to the investigators. Using a technique called binary recursive partitioning, they compared these patients with ESBL-negative patients to create a clinical decision tree based on five yes-or-no questions. The tree first asked if the patient had been colonized or infected with ESBL-producing bacteria within 6 months, and if so, whether the patient currently had an indwelling catheter. Patients meeting both criteria had a 92% chance of being ESBL positive. Patients with a recent history of ESBL but no catheter had an 81% chance of being ESBL positive if they were at least 43 years old, but a 75% chance of being ESBL negative if they were under age 43 years.
Among patients with no recent history of ESBL, the decision tree asked about hospitalization in a country with a high ESBL burden and antibiotic therapy during the past 6 months. Patients responding “yes” to both questions had a 100% chance of being ESBL positive. Patients with only the geographic risk factor had a 63% chance of being ESBL negative, and patients with neither risk factor had a 93% chance of being ESBL negative.
The decision tree detected only half of ESBL cases because there was a subgroup with no recent ESBL history or geographic exposure, the investigators noted. “The poor predictive nature of health care–associated variables within this patient subset may suggest a high proportion of community-acquired ESBL infections. Indeed, although risk factors for ESBLs have traditionally focused on the health care setting, increasing reports describe the community as an important ESBL reservoir,” they added. Nonetheless, of 194 patients with ESBL bacteremia, 35% received empiric carbapenem treatment within 6 hours after identification of the bacterial genus and species, the investigators emphasized. “Utilization of the decision tree would have increased ESBL case detection during the empiric treatment window by approximately 50%.”
The National Institutes of Health funded the study. The researchers reported having no conflicts of interest.
A new classification tool helped guide the treatment of bacteremic patients while clinicians awaited antibiotic resistance results, investigators reported.
The clinical decision tree had a positive predictive value of 91% and a negative predictive value of 92% for determining whether certain gram-negative infections produced extended-spectrum beta-lactamase (ESBL), Catherine Goodman, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and her associates wrote online in Clinical Infectious Diseases. “These predictions may assist empiric treatment decisions in order to optimize clinical outcomes while reducing administration of overly broad antibiotic agents that can select for further resistance emergence,” they added.
Bacteria that produce ESBL can hydrolyze all broad-spectrum beta-lactam antibiotics except carbapenems. Rapid tests for beta-lactamase genes can shorten the lag time between gram-stain identification and antimicrobial resistance results, but are cost prohibitive for most clinical laboratories and often do not assess ESBL gene groups, the researchers said. To find a way to predict which infections are characterized by ESBL production, they studied adults hospitalized at Johns Hopkins from October 2008 to March 2015 with bloodstream isolates of Klebsiella pneumoniae (40% of patients), Klebsiella oxytoca (4% of patients), and Escherichia coli (56% of patients). Most bacteremias began as urinary tract infections (34% of cases), followed by intra-abdominal infections (24%), catheter-related infections (16%), and biliary infections (14%) (Clin Infect Dis. 2016 Jul 26. doi:10.1093/cid/ciw425).
A total of 194 patients (15%) had bacteremias that produced ESBL, according to the investigators. Using a technique called binary recursive partitioning, they compared these patients with ESBL-negative patients to create a clinical decision tree based on five yes-or-no questions. The tree first asked if the patient had been colonized or infected with ESBL-producing bacteria within 6 months, and if so, whether the patient currently had an indwelling catheter. Patients meeting both criteria had a 92% chance of being ESBL positive. Patients with a recent history of ESBL but no catheter had an 81% chance of being ESBL positive if they were at least 43 years old, but a 75% chance of being ESBL negative if they were under age 43 years.
Among patients with no recent history of ESBL, the decision tree asked about hospitalization in a country with a high ESBL burden and antibiotic therapy during the past 6 months. Patients responding “yes” to both questions had a 100% chance of being ESBL positive. Patients with only the geographic risk factor had a 63% chance of being ESBL negative, and patients with neither risk factor had a 93% chance of being ESBL negative.
The decision tree detected only half of ESBL cases because there was a subgroup with no recent ESBL history or geographic exposure, the investigators noted. “The poor predictive nature of health care–associated variables within this patient subset may suggest a high proportion of community-acquired ESBL infections. Indeed, although risk factors for ESBLs have traditionally focused on the health care setting, increasing reports describe the community as an important ESBL reservoir,” they added. Nonetheless, of 194 patients with ESBL bacteremia, 35% received empiric carbapenem treatment within 6 hours after identification of the bacterial genus and species, the investigators emphasized. “Utilization of the decision tree would have increased ESBL case detection during the empiric treatment window by approximately 50%.”
The National Institutes of Health funded the study. The researchers reported having no conflicts of interest.
A new classification tool helped guide the treatment of bacteremic patients while clinicians awaited antibiotic resistance results, investigators reported.
The clinical decision tree had a positive predictive value of 91% and a negative predictive value of 92% for determining whether certain gram-negative infections produced extended-spectrum beta-lactamase (ESBL), Catherine Goodman, PhD, of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and her associates wrote online in Clinical Infectious Diseases. “These predictions may assist empiric treatment decisions in order to optimize clinical outcomes while reducing administration of overly broad antibiotic agents that can select for further resistance emergence,” they added.
Bacteria that produce ESBL can hydrolyze all broad-spectrum beta-lactam antibiotics except carbapenems. Rapid tests for beta-lactamase genes can shorten the lag time between gram-stain identification and antimicrobial resistance results, but are cost prohibitive for most clinical laboratories and often do not assess ESBL gene groups, the researchers said. To find a way to predict which infections are characterized by ESBL production, they studied adults hospitalized at Johns Hopkins from October 2008 to March 2015 with bloodstream isolates of Klebsiella pneumoniae (40% of patients), Klebsiella oxytoca (4% of patients), and Escherichia coli (56% of patients). Most bacteremias began as urinary tract infections (34% of cases), followed by intra-abdominal infections (24%), catheter-related infections (16%), and biliary infections (14%) (Clin Infect Dis. 2016 Jul 26. doi:10.1093/cid/ciw425).
A total of 194 patients (15%) had bacteremias that produced ESBL, according to the investigators. Using a technique called binary recursive partitioning, they compared these patients with ESBL-negative patients to create a clinical decision tree based on five yes-or-no questions. The tree first asked if the patient had been colonized or infected with ESBL-producing bacteria within 6 months, and if so, whether the patient currently had an indwelling catheter. Patients meeting both criteria had a 92% chance of being ESBL positive. Patients with a recent history of ESBL but no catheter had an 81% chance of being ESBL positive if they were at least 43 years old, but a 75% chance of being ESBL negative if they were under age 43 years.
Among patients with no recent history of ESBL, the decision tree asked about hospitalization in a country with a high ESBL burden and antibiotic therapy during the past 6 months. Patients responding “yes” to both questions had a 100% chance of being ESBL positive. Patients with only the geographic risk factor had a 63% chance of being ESBL negative, and patients with neither risk factor had a 93% chance of being ESBL negative.
The decision tree detected only half of ESBL cases because there was a subgroup with no recent ESBL history or geographic exposure, the investigators noted. “The poor predictive nature of health care–associated variables within this patient subset may suggest a high proportion of community-acquired ESBL infections. Indeed, although risk factors for ESBLs have traditionally focused on the health care setting, increasing reports describe the community as an important ESBL reservoir,” they added. Nonetheless, of 194 patients with ESBL bacteremia, 35% received empiric carbapenem treatment within 6 hours after identification of the bacterial genus and species, the investigators emphasized. “Utilization of the decision tree would have increased ESBL case detection during the empiric treatment window by approximately 50%.”
The National Institutes of Health funded the study. The researchers reported having no conflicts of interest.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: A clinical decision tree helped identify bacteria producing extended-spectrum beta-lactamases.
Major finding: The positive predictive value was 91%, and the negative predictive value was 92%.
Data source: A single-center retrospective study of 1,288 adults with blood isolates of Klebsiella pneumoniae, Klebsiella oxytoca, or Escherichia coli.
Disclosures: The National Institutes of Health funded the study. The researchers reported having no conflicts of interest.
Most sepsis cases begin outside of the hospital
Sepsis is a medical emergency that begins outside of the hospital in 79% of cases. In addition, 72% of patients with sepsis had recently used healthcare services or had chronic diseases that required frequent medical care.
Those are key findings from a special report in Morbidity and Mortality Weekly Report published by the U.S. Centers for Disease Control and Prevention (Morb Mortal Wkly Rep. 2016 Aug 23. doi: 10.15585/mmwr.mm6533e1).
“The treatment of sepsis is a race against time,” CDC Director Tom Frieden, MD, said during a media teleconference about the report. “We can protect more people from sepsis by informing patients and their families, treating infections promptly, and acting fast when sepsis does occur.”
Each year, between 1 and 3 million people in the United States are diagnosed with sepsis, a syndrome marked by the body’s overwhelming and life-threatening response to an infection, Dr. Frieden said. Of these, 15%-30% will die from the condition, for which there is no blood test. “Health care providers are on the front lines of both sepsis prevention and early recognition,” he emphasized. “Prevention really is possible.” For example, he continued, if a patient with diabetes visits their regular doctor and is found to have increased blood sugar and a small wound on their foot, “this is a prime opportunity to think about infection and reduce the risk of sepsis. In addition to treating the infection, the clinician can inform the patient and family members about how to care for the wound, how to recognize the signs that the infection may be getting worse, and when to seek additional medical care. If the infection gets worse the patient could be at risk for sepsis. Taking the opportunity to both treat and inform patients could save their life, and helping patients know to ask, ‘Could this be sepsis?’ empowers patients and families and could save lives.”
In an effort to describe the characteristics of patients with sepsis, researchers from the CDC and from New York State conducted a retrospective review of medical records from 246 adults and 79 children with sepsis who were treated at four New York hospitals. They found that sepsis most often occurs in patients older than age 65 years and in infants younger than 1 year of age, and the median hospital length of stay is 10 days. Six key signs and symptoms of sepsis were shivering or feeling cold; pain or discomfort; clammy or sweaty skin; being confused or disoriented; shortness of breath, and having a rapid heartbeat. “People with chronic diseases such as diabetes or weakened immune systems from things like tobacco use are at higher risk of sepsis,” Dr. Frieden said. “But even healthy people can develop sepsis from an infection, especially if it’s not treated properly and promptly.”
The four types of infections most commonly associated with sepsis include those involving the lungs, urinary tract, skin, and intestines, while the most common germs that can cause sepsis are Staphylococcus aureus, Escherichia coli (E. coli), and some types of Streptococcus. Infection prevention strategies such as increasing vaccination rates for pneumococcal disease and for influenza are likely to reduce the incidence of sepsis, according to the report. “We could also improve infections by improving handwashing at health care facilities as well as in the community,” Dr. Frieden added. “We can [also] improve recognition of sepsis both in the community and in health care facilities and act fast if sepsis is suspected in a patient. We’ve been able to reduce the rates of some infections that cause sepsis in health care facilities by half, but preventing more infections and stopping the spread of antibiotic resistant infections will protect even more patients from sepsis.”
Mitchell Levy, MD, founding member of the Surviving Sepsis Campaign, said during the teleconference that clinicians have made “tremendous progress in sepsis,” despite current challenges. “First, we now understand the importance of early identification and treatment of sepsis,” he said. “Second, we have seen improved survival through routine screening and treatment that is integrated into the work flow of hospitals. And third, frontline health care providers really do make a difference. What’s clear is that we need to expand these successes to other parts of hospitals and to other care locations.”
Forthcoming free CDC webinars related to sepsis for health care providers include one on Sept. 13 at 3 p.m., ET, entitled “Advances in Sepsis: Protecting Patients Throughout the Lifespan.” Another webinar will be offered on Sept. 22 at 2 p.m., ET, entitled “Empowering Nurses for Early Sepsis Recognition.”
Sepsis is a medical emergency that begins outside of the hospital in 79% of cases. In addition, 72% of patients with sepsis had recently used healthcare services or had chronic diseases that required frequent medical care.
Those are key findings from a special report in Morbidity and Mortality Weekly Report published by the U.S. Centers for Disease Control and Prevention (Morb Mortal Wkly Rep. 2016 Aug 23. doi: 10.15585/mmwr.mm6533e1).
“The treatment of sepsis is a race against time,” CDC Director Tom Frieden, MD, said during a media teleconference about the report. “We can protect more people from sepsis by informing patients and their families, treating infections promptly, and acting fast when sepsis does occur.”
Each year, between 1 and 3 million people in the United States are diagnosed with sepsis, a syndrome marked by the body’s overwhelming and life-threatening response to an infection, Dr. Frieden said. Of these, 15%-30% will die from the condition, for which there is no blood test. “Health care providers are on the front lines of both sepsis prevention and early recognition,” he emphasized. “Prevention really is possible.” For example, he continued, if a patient with diabetes visits their regular doctor and is found to have increased blood sugar and a small wound on their foot, “this is a prime opportunity to think about infection and reduce the risk of sepsis. In addition to treating the infection, the clinician can inform the patient and family members about how to care for the wound, how to recognize the signs that the infection may be getting worse, and when to seek additional medical care. If the infection gets worse the patient could be at risk for sepsis. Taking the opportunity to both treat and inform patients could save their life, and helping patients know to ask, ‘Could this be sepsis?’ empowers patients and families and could save lives.”
In an effort to describe the characteristics of patients with sepsis, researchers from the CDC and from New York State conducted a retrospective review of medical records from 246 adults and 79 children with sepsis who were treated at four New York hospitals. They found that sepsis most often occurs in patients older than age 65 years and in infants younger than 1 year of age, and the median hospital length of stay is 10 days. Six key signs and symptoms of sepsis were shivering or feeling cold; pain or discomfort; clammy or sweaty skin; being confused or disoriented; shortness of breath, and having a rapid heartbeat. “People with chronic diseases such as diabetes or weakened immune systems from things like tobacco use are at higher risk of sepsis,” Dr. Frieden said. “But even healthy people can develop sepsis from an infection, especially if it’s not treated properly and promptly.”
The four types of infections most commonly associated with sepsis include those involving the lungs, urinary tract, skin, and intestines, while the most common germs that can cause sepsis are Staphylococcus aureus, Escherichia coli (E. coli), and some types of Streptococcus. Infection prevention strategies such as increasing vaccination rates for pneumococcal disease and for influenza are likely to reduce the incidence of sepsis, according to the report. “We could also improve infections by improving handwashing at health care facilities as well as in the community,” Dr. Frieden added. “We can [also] improve recognition of sepsis both in the community and in health care facilities and act fast if sepsis is suspected in a patient. We’ve been able to reduce the rates of some infections that cause sepsis in health care facilities by half, but preventing more infections and stopping the spread of antibiotic resistant infections will protect even more patients from sepsis.”
Mitchell Levy, MD, founding member of the Surviving Sepsis Campaign, said during the teleconference that clinicians have made “tremendous progress in sepsis,” despite current challenges. “First, we now understand the importance of early identification and treatment of sepsis,” he said. “Second, we have seen improved survival through routine screening and treatment that is integrated into the work flow of hospitals. And third, frontline health care providers really do make a difference. What’s clear is that we need to expand these successes to other parts of hospitals and to other care locations.”
Forthcoming free CDC webinars related to sepsis for health care providers include one on Sept. 13 at 3 p.m., ET, entitled “Advances in Sepsis: Protecting Patients Throughout the Lifespan.” Another webinar will be offered on Sept. 22 at 2 p.m., ET, entitled “Empowering Nurses for Early Sepsis Recognition.”
Sepsis is a medical emergency that begins outside of the hospital in 79% of cases. In addition, 72% of patients with sepsis had recently used healthcare services or had chronic diseases that required frequent medical care.
Those are key findings from a special report in Morbidity and Mortality Weekly Report published by the U.S. Centers for Disease Control and Prevention (Morb Mortal Wkly Rep. 2016 Aug 23. doi: 10.15585/mmwr.mm6533e1).
“The treatment of sepsis is a race against time,” CDC Director Tom Frieden, MD, said during a media teleconference about the report. “We can protect more people from sepsis by informing patients and their families, treating infections promptly, and acting fast when sepsis does occur.”
Each year, between 1 and 3 million people in the United States are diagnosed with sepsis, a syndrome marked by the body’s overwhelming and life-threatening response to an infection, Dr. Frieden said. Of these, 15%-30% will die from the condition, for which there is no blood test. “Health care providers are on the front lines of both sepsis prevention and early recognition,” he emphasized. “Prevention really is possible.” For example, he continued, if a patient with diabetes visits their regular doctor and is found to have increased blood sugar and a small wound on their foot, “this is a prime opportunity to think about infection and reduce the risk of sepsis. In addition to treating the infection, the clinician can inform the patient and family members about how to care for the wound, how to recognize the signs that the infection may be getting worse, and when to seek additional medical care. If the infection gets worse the patient could be at risk for sepsis. Taking the opportunity to both treat and inform patients could save their life, and helping patients know to ask, ‘Could this be sepsis?’ empowers patients and families and could save lives.”
In an effort to describe the characteristics of patients with sepsis, researchers from the CDC and from New York State conducted a retrospective review of medical records from 246 adults and 79 children with sepsis who were treated at four New York hospitals. They found that sepsis most often occurs in patients older than age 65 years and in infants younger than 1 year of age, and the median hospital length of stay is 10 days. Six key signs and symptoms of sepsis were shivering or feeling cold; pain or discomfort; clammy or sweaty skin; being confused or disoriented; shortness of breath, and having a rapid heartbeat. “People with chronic diseases such as diabetes or weakened immune systems from things like tobacco use are at higher risk of sepsis,” Dr. Frieden said. “But even healthy people can develop sepsis from an infection, especially if it’s not treated properly and promptly.”
The four types of infections most commonly associated with sepsis include those involving the lungs, urinary tract, skin, and intestines, while the most common germs that can cause sepsis are Staphylococcus aureus, Escherichia coli (E. coli), and some types of Streptococcus. Infection prevention strategies such as increasing vaccination rates for pneumococcal disease and for influenza are likely to reduce the incidence of sepsis, according to the report. “We could also improve infections by improving handwashing at health care facilities as well as in the community,” Dr. Frieden added. “We can [also] improve recognition of sepsis both in the community and in health care facilities and act fast if sepsis is suspected in a patient. We’ve been able to reduce the rates of some infections that cause sepsis in health care facilities by half, but preventing more infections and stopping the spread of antibiotic resistant infections will protect even more patients from sepsis.”
Mitchell Levy, MD, founding member of the Surviving Sepsis Campaign, said during the teleconference that clinicians have made “tremendous progress in sepsis,” despite current challenges. “First, we now understand the importance of early identification and treatment of sepsis,” he said. “Second, we have seen improved survival through routine screening and treatment that is integrated into the work flow of hospitals. And third, frontline health care providers really do make a difference. What’s clear is that we need to expand these successes to other parts of hospitals and to other care locations.”
Forthcoming free CDC webinars related to sepsis for health care providers include one on Sept. 13 at 3 p.m., ET, entitled “Advances in Sepsis: Protecting Patients Throughout the Lifespan.” Another webinar will be offered on Sept. 22 at 2 p.m., ET, entitled “Empowering Nurses for Early Sepsis Recognition.”
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Key clinical point: Sepsis is a significant public health and clinical management challenge.
Major finding: Sepsis begins outside of the hospital in 79% of cases.
Data source: A retrospective review of medical records from 246 adults and 79 children with sepsis who were treated at four New York hospitals.
Disclosures: The researchers reported having no financial disclosures.
Healthy donor stool safe, effective for recurrent CDI
For patients with recurrent Clostridium difficile infection (CDI), donor stool administered via colonoscopy seemed safe and achieved clinical cure significantly more often than autologous fecal microbiota transplantation (FMT), based on a small trial reported online in Annals of Internal Medicine.
In all, 20 of 22 patients (91%) achieved cure with donor FMT, compared with 63% of patients who received their own markedly dysbiotic stool (P = .04), reported Colleen Kelly, MD, of The Miriam Hospital, Providence, R.I., together with her associates. “Differences in efficacy between sites suggest that some patients with lower risk for CDI recurrence may not benefit from FMT. Further research may help determine the best candidates,” the researchers wrote.
FMT corrects the dysbiosis associated with CDI and is recommended in the event of failed antibiotic therapy leading to a third episode of infection. But this advice is based mainly on case series and open-label trials, the researchers noted. Their dual-center, randomized, controlled, double-blinded study included 46 patients with at least three recurrences of CDI who had completed a course of vancomycin during their most recent episode of infection. Patients older than age 75 years or who were immunocompromised were excluded (Ann Intern Med. 2016 Aug 22. doi: 10.7326/M16-0271).
The overall clinical cure rates reflected the literature, the researchers reported, and all nine patients who developed CDI after autologous FMT were subsequently cured by donor FMT. Indeed, donor FMT “restored normal microbial community structure, with reductions in Proteobacteria and Verrucomicrobia and increases in Bacteroidetes and Firmicutes. In contrast, microbial diversity did not improve after autologous FMT.”
Notably, however, 90% of autologous FMT patients at the center in New York achieved clinical cure, compared with 43% of patients at the center in Rhode Island. Further analyses revealed differences between patients and fecal microbiota at the two sites, the investigators said. Patients in New York typically had CDI for longer, with more recurrences and up to 148 weeks of vancomycin and other antibiotics. Thus, they might have been cured before enrollment. But “autologous FMT patients at the New York site [also] had greater abundances of Clostridia, raising the possibility of emergence of microbial community assemblages inhibitory to C. difficile via competitive niche exclusion, or possibly by emergence of nontoxigenic organisms,” the researchers wrote.
There were no serious adverse effects associated with either type of FMT, they noted.
Dr. Kelly disclosed ties to Seres Health outside the submitted work. Two coauthors had patents or patents pending for “compositions and methods for transplantation of colon microbiota.” A third coauthor disclosed ties to OpenBiome and personal fees from CIPAC/Crestovo outside the submitted work. The remaining coauthors had no conflicts of interest.
Kelly and her colleagues demonstrate that rigorous controlled trials are valuable even when we think we know the answer. Their results prompt us to ask again whether microbial manipulation has any as-yet unappreciated health benefits or risks and whether there are preferred microbiomes for specific human populations or locales.
Careful review of reported adverse events in the current trial is instructive. One participant reported a 9.1-kg weight gain (donor details were not provided), a problem previously described in a separate case report. There is great interest in understanding whether the microbiome can be manipulated to modify weight in humans, as has been clearly shown in mice. In addition, patients receiving donor stool more frequently reported chills. In my own practice, I have rarely observed transient fever after healthy donor FMT delivered orally in encapsulated form, and I hypothesize that this may be due to an immune reaction to a new microbial ecosystem. Patients considering FMT should be informed of both of these possible adverse events.
Elizabeth L. Hohmann, MD, is at Massachusetts General Hospital, Boston. She reported grant support and personal fees from Seres Therapeutics outside the submitted work. These comments are from an editorial accompanying the article (Ann Intern Med. 2016 Aug 22. doi: 10.7326/M16-1784).
AGA Resource
The AGA Center for Gut Microbiome Research and Education was created to serve as a virtual ‘home’ for AGA activities related to the gut microbiome with a mission to advance research and education on the gut microbiome with the goal of improving human health. Learn more at www.gastro.org/microbiome.
Kelly and her colleagues demonstrate that rigorous controlled trials are valuable even when we think we know the answer. Their results prompt us to ask again whether microbial manipulation has any as-yet unappreciated health benefits or risks and whether there are preferred microbiomes for specific human populations or locales.
Careful review of reported adverse events in the current trial is instructive. One participant reported a 9.1-kg weight gain (donor details were not provided), a problem previously described in a separate case report. There is great interest in understanding whether the microbiome can be manipulated to modify weight in humans, as has been clearly shown in mice. In addition, patients receiving donor stool more frequently reported chills. In my own practice, I have rarely observed transient fever after healthy donor FMT delivered orally in encapsulated form, and I hypothesize that this may be due to an immune reaction to a new microbial ecosystem. Patients considering FMT should be informed of both of these possible adverse events.
Elizabeth L. Hohmann, MD, is at Massachusetts General Hospital, Boston. She reported grant support and personal fees from Seres Therapeutics outside the submitted work. These comments are from an editorial accompanying the article (Ann Intern Med. 2016 Aug 22. doi: 10.7326/M16-1784).
AGA Resource
The AGA Center for Gut Microbiome Research and Education was created to serve as a virtual ‘home’ for AGA activities related to the gut microbiome with a mission to advance research and education on the gut microbiome with the goal of improving human health. Learn more at www.gastro.org/microbiome.
Kelly and her colleagues demonstrate that rigorous controlled trials are valuable even when we think we know the answer. Their results prompt us to ask again whether microbial manipulation has any as-yet unappreciated health benefits or risks and whether there are preferred microbiomes for specific human populations or locales.
Careful review of reported adverse events in the current trial is instructive. One participant reported a 9.1-kg weight gain (donor details were not provided), a problem previously described in a separate case report. There is great interest in understanding whether the microbiome can be manipulated to modify weight in humans, as has been clearly shown in mice. In addition, patients receiving donor stool more frequently reported chills. In my own practice, I have rarely observed transient fever after healthy donor FMT delivered orally in encapsulated form, and I hypothesize that this may be due to an immune reaction to a new microbial ecosystem. Patients considering FMT should be informed of both of these possible adverse events.
Elizabeth L. Hohmann, MD, is at Massachusetts General Hospital, Boston. She reported grant support and personal fees from Seres Therapeutics outside the submitted work. These comments are from an editorial accompanying the article (Ann Intern Med. 2016 Aug 22. doi: 10.7326/M16-1784).
AGA Resource
The AGA Center for Gut Microbiome Research and Education was created to serve as a virtual ‘home’ for AGA activities related to the gut microbiome with a mission to advance research and education on the gut microbiome with the goal of improving human health. Learn more at www.gastro.org/microbiome.
For patients with recurrent Clostridium difficile infection (CDI), donor stool administered via colonoscopy seemed safe and achieved clinical cure significantly more often than autologous fecal microbiota transplantation (FMT), based on a small trial reported online in Annals of Internal Medicine.
In all, 20 of 22 patients (91%) achieved cure with donor FMT, compared with 63% of patients who received their own markedly dysbiotic stool (P = .04), reported Colleen Kelly, MD, of The Miriam Hospital, Providence, R.I., together with her associates. “Differences in efficacy between sites suggest that some patients with lower risk for CDI recurrence may not benefit from FMT. Further research may help determine the best candidates,” the researchers wrote.
FMT corrects the dysbiosis associated with CDI and is recommended in the event of failed antibiotic therapy leading to a third episode of infection. But this advice is based mainly on case series and open-label trials, the researchers noted. Their dual-center, randomized, controlled, double-blinded study included 46 patients with at least three recurrences of CDI who had completed a course of vancomycin during their most recent episode of infection. Patients older than age 75 years or who were immunocompromised were excluded (Ann Intern Med. 2016 Aug 22. doi: 10.7326/M16-0271).
The overall clinical cure rates reflected the literature, the researchers reported, and all nine patients who developed CDI after autologous FMT were subsequently cured by donor FMT. Indeed, donor FMT “restored normal microbial community structure, with reductions in Proteobacteria and Verrucomicrobia and increases in Bacteroidetes and Firmicutes. In contrast, microbial diversity did not improve after autologous FMT.”
Notably, however, 90% of autologous FMT patients at the center in New York achieved clinical cure, compared with 43% of patients at the center in Rhode Island. Further analyses revealed differences between patients and fecal microbiota at the two sites, the investigators said. Patients in New York typically had CDI for longer, with more recurrences and up to 148 weeks of vancomycin and other antibiotics. Thus, they might have been cured before enrollment. But “autologous FMT patients at the New York site [also] had greater abundances of Clostridia, raising the possibility of emergence of microbial community assemblages inhibitory to C. difficile via competitive niche exclusion, or possibly by emergence of nontoxigenic organisms,” the researchers wrote.
There were no serious adverse effects associated with either type of FMT, they noted.
Dr. Kelly disclosed ties to Seres Health outside the submitted work. Two coauthors had patents or patents pending for “compositions and methods for transplantation of colon microbiota.” A third coauthor disclosed ties to OpenBiome and personal fees from CIPAC/Crestovo outside the submitted work. The remaining coauthors had no conflicts of interest.
For patients with recurrent Clostridium difficile infection (CDI), donor stool administered via colonoscopy seemed safe and achieved clinical cure significantly more often than autologous fecal microbiota transplantation (FMT), based on a small trial reported online in Annals of Internal Medicine.
In all, 20 of 22 patients (91%) achieved cure with donor FMT, compared with 63% of patients who received their own markedly dysbiotic stool (P = .04), reported Colleen Kelly, MD, of The Miriam Hospital, Providence, R.I., together with her associates. “Differences in efficacy between sites suggest that some patients with lower risk for CDI recurrence may not benefit from FMT. Further research may help determine the best candidates,” the researchers wrote.
FMT corrects the dysbiosis associated with CDI and is recommended in the event of failed antibiotic therapy leading to a third episode of infection. But this advice is based mainly on case series and open-label trials, the researchers noted. Their dual-center, randomized, controlled, double-blinded study included 46 patients with at least three recurrences of CDI who had completed a course of vancomycin during their most recent episode of infection. Patients older than age 75 years or who were immunocompromised were excluded (Ann Intern Med. 2016 Aug 22. doi: 10.7326/M16-0271).
The overall clinical cure rates reflected the literature, the researchers reported, and all nine patients who developed CDI after autologous FMT were subsequently cured by donor FMT. Indeed, donor FMT “restored normal microbial community structure, with reductions in Proteobacteria and Verrucomicrobia and increases in Bacteroidetes and Firmicutes. In contrast, microbial diversity did not improve after autologous FMT.”
Notably, however, 90% of autologous FMT patients at the center in New York achieved clinical cure, compared with 43% of patients at the center in Rhode Island. Further analyses revealed differences between patients and fecal microbiota at the two sites, the investigators said. Patients in New York typically had CDI for longer, with more recurrences and up to 148 weeks of vancomycin and other antibiotics. Thus, they might have been cured before enrollment. But “autologous FMT patients at the New York site [also] had greater abundances of Clostridia, raising the possibility of emergence of microbial community assemblages inhibitory to C. difficile via competitive niche exclusion, or possibly by emergence of nontoxigenic organisms,” the researchers wrote.
There were no serious adverse effects associated with either type of FMT, they noted.
Dr. Kelly disclosed ties to Seres Health outside the submitted work. Two coauthors had patents or patents pending for “compositions and methods for transplantation of colon microbiota.” A third coauthor disclosed ties to OpenBiome and personal fees from CIPAC/Crestovo outside the submitted work. The remaining coauthors had no conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Donor stool administered via colonoscopy seemed safe and achieved clinical cure significantly more often than autologous fecal microbiota transplantation (FMT) in patients with recurrent Clostridium difficile infection (CDI).
Major finding: In all, 91% of donor FMT patients and 63% of autologous FMT patients achieved clinical cure stool (P = .04).
Data source: A prospective, double-blind, randomized trial of 46 patients with at least three episodes of CDI, who had completed a full course of vancomycin during the most recent episode.
Disclosures: Dr. Kelly disclosed ties to Seres Health outside the submitted work. Two coauthors had patents or patents pending for “compositions and methods for transplantation of colon microbiota.” A third coauthor disclosed ties to OpenBiome and personal fees from CIPAC/Crestovo outside the submitted work. The remaining coauthors had no conflicts of interest.
Oral Antibiotics for Infective Endocarditis May Be Safe in Low-Risk Patients
Background: Treating infective endocarditis with four to six weeks of intravenous antibiotics carries a high cost. There are data to support oral antibiotics for right-sided endocarditis due to methicillin-sensitive Staphylococcus aureus (with ciprofloxacin and rifampicin), but experience in using oral antibiotics for infective endocarditis is limited.
Study design: Cohort study.
Setting: Large academic hospital in France.
Synopsis: The researchers included 426 patients with definitive or probable endocarditis by Duke criteria. After an initial period of treatment with intravenous (IV) antibiotics, 50% of the identified group was transitioned to oral antibiotics (amoxicillin alone in 50% and combinations of fluoroquinolones, rifampicin, amoxicillin, and clindamycin in the others).
The risk of death was not increased in the group treated with oral antibiotics when adjusted for the four biggest predictors of death (age >65, type 1 diabetes mellitus, disinsertion of prosthetic valve, and endocarditis due to S. aureus). Nine patients treated with IV antibiotics experienced relapsed endocarditis compared to two patients treated with oral antibiotics.
Patients selected for treatment with oral antibiotics were less likely to have severe disease, significant comorbidities, or infection with S. aureus. The length of treatment with IV antibiotics before switching to oral antibiotics varied widely.
Bottom line: It’s possible low-risk patients with infective endocarditis may be treated with oral antibiotics, but more data are needed.
Citation: Mzabi A, Kernéis S, Richaud C, Podglajen I, Fernandez-Gerlinger MP, Mainardi, JL. Switch to oral antibiotics in the treatment of infective endocarditis is not associated with increased risk of mortality in non-severely ill patients [published online ahead of print April 16, 2016]. Clin Microbiol Infect. doi:10.1016/j.cmi.2016.04.003.
Background: Treating infective endocarditis with four to six weeks of intravenous antibiotics carries a high cost. There are data to support oral antibiotics for right-sided endocarditis due to methicillin-sensitive Staphylococcus aureus (with ciprofloxacin and rifampicin), but experience in using oral antibiotics for infective endocarditis is limited.
Study design: Cohort study.
Setting: Large academic hospital in France.
Synopsis: The researchers included 426 patients with definitive or probable endocarditis by Duke criteria. After an initial period of treatment with intravenous (IV) antibiotics, 50% of the identified group was transitioned to oral antibiotics (amoxicillin alone in 50% and combinations of fluoroquinolones, rifampicin, amoxicillin, and clindamycin in the others).
The risk of death was not increased in the group treated with oral antibiotics when adjusted for the four biggest predictors of death (age >65, type 1 diabetes mellitus, disinsertion of prosthetic valve, and endocarditis due to S. aureus). Nine patients treated with IV antibiotics experienced relapsed endocarditis compared to two patients treated with oral antibiotics.
Patients selected for treatment with oral antibiotics were less likely to have severe disease, significant comorbidities, or infection with S. aureus. The length of treatment with IV antibiotics before switching to oral antibiotics varied widely.
Bottom line: It’s possible low-risk patients with infective endocarditis may be treated with oral antibiotics, but more data are needed.
Citation: Mzabi A, Kernéis S, Richaud C, Podglajen I, Fernandez-Gerlinger MP, Mainardi, JL. Switch to oral antibiotics in the treatment of infective endocarditis is not associated with increased risk of mortality in non-severely ill patients [published online ahead of print April 16, 2016]. Clin Microbiol Infect. doi:10.1016/j.cmi.2016.04.003.
Background: Treating infective endocarditis with four to six weeks of intravenous antibiotics carries a high cost. There are data to support oral antibiotics for right-sided endocarditis due to methicillin-sensitive Staphylococcus aureus (with ciprofloxacin and rifampicin), but experience in using oral antibiotics for infective endocarditis is limited.
Study design: Cohort study.
Setting: Large academic hospital in France.
Synopsis: The researchers included 426 patients with definitive or probable endocarditis by Duke criteria. After an initial period of treatment with intravenous (IV) antibiotics, 50% of the identified group was transitioned to oral antibiotics (amoxicillin alone in 50% and combinations of fluoroquinolones, rifampicin, amoxicillin, and clindamycin in the others).
The risk of death was not increased in the group treated with oral antibiotics when adjusted for the four biggest predictors of death (age >65, type 1 diabetes mellitus, disinsertion of prosthetic valve, and endocarditis due to S. aureus). Nine patients treated with IV antibiotics experienced relapsed endocarditis compared to two patients treated with oral antibiotics.
Patients selected for treatment with oral antibiotics were less likely to have severe disease, significant comorbidities, or infection with S. aureus. The length of treatment with IV antibiotics before switching to oral antibiotics varied widely.
Bottom line: It’s possible low-risk patients with infective endocarditis may be treated with oral antibiotics, but more data are needed.
Citation: Mzabi A, Kernéis S, Richaud C, Podglajen I, Fernandez-Gerlinger MP, Mainardi, JL. Switch to oral antibiotics in the treatment of infective endocarditis is not associated with increased risk of mortality in non-severely ill patients [published online ahead of print April 16, 2016]. Clin Microbiol Infect. doi:10.1016/j.cmi.2016.04.003.
Candida auris in Venezuela outbreak is triazole-resistant, opportunistic
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM 2016
Key clinical point: Isolates in an outbreak of nosocomially acquired Candida auris were fluconazole-resistant.
Major finding: All C. auris isolates were resistant to fluconazole, with geometric mean minimum inhibitory concentrations greater than 64 mcg/mL.
Data source: Retrospective, single-center study of 18 pediatric and adult patients with C. auris infections at a tertiary care center in Venezuela.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
Procalcitonin Guidance Safely Decreases Antibiotic Use in Critically Ill Patients
Clinical question: Can the use of procalcitonin levels to determine when to discontinue antibiotic therapy safely reduce the duration of antibiotic use in critically ill patients?
Bottom line: For patients in the intensive care unit (ICU) who receive antibiotics for presumed or proven bacterial infections, the use of procalcitonin levels to determine when to stop antibiotic therapy results in decreased duration and consumption of antibiotics without increasing mortality.
Reference: De Jong E, Van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016;16(7):819-827.
Design: Randomized controlled trial (nonblinded); LOE: 1b
Setting: Inpatient (ICU only)
Synopsis: To test the efficacy and safety of procalcitonin-guided antibiotic therapy, these investigators recruited patients in the ICU who had received their first doses of antibiotics for a presumed or proven bacterial infection within 24 hours of enrollment. Patients who were severely immunosuppressed and patients requiring prolonged courses of antibiotics (such as those with endocarditis) were excluded.
Using concealed allocation, patients were assigned to procalcitonin-guided treatment (n = 761) or to usual care (n = 785). The usual care group did not have procalcitonin levels drawn. In the procalcitonin group, patients had a procalcitonin level drawn close to the start of antibiotic therapy and daily thereafter until discharge from the ICU or 3 days after stopping antibiotic use. These levels were provided to the attending physician who could then decide whether to stop giving antibiotics.
Although the study protocol recommended that antibiotics be discontinued if the procalcitonin level had decreased by more than 80% of its peak value or reached a level of 0.5 mcg/L, the ultimate decision to do so was at the discretion of the attending physician. Overall, fewer than half the physicians actually discontinued antibiotics within 24 hours of reaching either of these goals. Despite this, the procalcitonin group had decreased number of days of antibiotic treatment (5 days vs 7 days; between group absolute difference = 1.22; 95% CI 0.65-1.78; P < .0001) and decreased consumption of antibiotics (7.5 daily defined doses vs 9.3 daily defined doses; between group absolute difference = 2.69; 1.26-4.12; P < .0001). Additionally, when examining 28-day mortality rates, the procalcitonin group was noninferior to the standard group, and ultimately, had fewer deaths than the standard group (20% vs 25%; between group absolute difference = 5.4%;1.2-9.5; P = .012). This mortality benefit persisted at 1 year.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Can the use of procalcitonin levels to determine when to discontinue antibiotic therapy safely reduce the duration of antibiotic use in critically ill patients?
Bottom line: For patients in the intensive care unit (ICU) who receive antibiotics for presumed or proven bacterial infections, the use of procalcitonin levels to determine when to stop antibiotic therapy results in decreased duration and consumption of antibiotics without increasing mortality.
Reference: De Jong E, Van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016;16(7):819-827.
Design: Randomized controlled trial (nonblinded); LOE: 1b
Setting: Inpatient (ICU only)
Synopsis: To test the efficacy and safety of procalcitonin-guided antibiotic therapy, these investigators recruited patients in the ICU who had received their first doses of antibiotics for a presumed or proven bacterial infection within 24 hours of enrollment. Patients who were severely immunosuppressed and patients requiring prolonged courses of antibiotics (such as those with endocarditis) were excluded.
Using concealed allocation, patients were assigned to procalcitonin-guided treatment (n = 761) or to usual care (n = 785). The usual care group did not have procalcitonin levels drawn. In the procalcitonin group, patients had a procalcitonin level drawn close to the start of antibiotic therapy and daily thereafter until discharge from the ICU or 3 days after stopping antibiotic use. These levels were provided to the attending physician who could then decide whether to stop giving antibiotics.
Although the study protocol recommended that antibiotics be discontinued if the procalcitonin level had decreased by more than 80% of its peak value or reached a level of 0.5 mcg/L, the ultimate decision to do so was at the discretion of the attending physician. Overall, fewer than half the physicians actually discontinued antibiotics within 24 hours of reaching either of these goals. Despite this, the procalcitonin group had decreased number of days of antibiotic treatment (5 days vs 7 days; between group absolute difference = 1.22; 95% CI 0.65-1.78; P < .0001) and decreased consumption of antibiotics (7.5 daily defined doses vs 9.3 daily defined doses; between group absolute difference = 2.69; 1.26-4.12; P < .0001). Additionally, when examining 28-day mortality rates, the procalcitonin group was noninferior to the standard group, and ultimately, had fewer deaths than the standard group (20% vs 25%; between group absolute difference = 5.4%;1.2-9.5; P = .012). This mortality benefit persisted at 1 year.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question: Can the use of procalcitonin levels to determine when to discontinue antibiotic therapy safely reduce the duration of antibiotic use in critically ill patients?
Bottom line: For patients in the intensive care unit (ICU) who receive antibiotics for presumed or proven bacterial infections, the use of procalcitonin levels to determine when to stop antibiotic therapy results in decreased duration and consumption of antibiotics without increasing mortality.
Reference: De Jong E, Van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016;16(7):819-827.
Design: Randomized controlled trial (nonblinded); LOE: 1b
Setting: Inpatient (ICU only)
Synopsis: To test the efficacy and safety of procalcitonin-guided antibiotic therapy, these investigators recruited patients in the ICU who had received their first doses of antibiotics for a presumed or proven bacterial infection within 24 hours of enrollment. Patients who were severely immunosuppressed and patients requiring prolonged courses of antibiotics (such as those with endocarditis) were excluded.
Using concealed allocation, patients were assigned to procalcitonin-guided treatment (n = 761) or to usual care (n = 785). The usual care group did not have procalcitonin levels drawn. In the procalcitonin group, patients had a procalcitonin level drawn close to the start of antibiotic therapy and daily thereafter until discharge from the ICU or 3 days after stopping antibiotic use. These levels were provided to the attending physician who could then decide whether to stop giving antibiotics.
Although the study protocol recommended that antibiotics be discontinued if the procalcitonin level had decreased by more than 80% of its peak value or reached a level of 0.5 mcg/L, the ultimate decision to do so was at the discretion of the attending physician. Overall, fewer than half the physicians actually discontinued antibiotics within 24 hours of reaching either of these goals. Despite this, the procalcitonin group had decreased number of days of antibiotic treatment (5 days vs 7 days; between group absolute difference = 1.22; 95% CI 0.65-1.78; P < .0001) and decreased consumption of antibiotics (7.5 daily defined doses vs 9.3 daily defined doses; between group absolute difference = 2.69; 1.26-4.12; P < .0001). Additionally, when examining 28-day mortality rates, the procalcitonin group was noninferior to the standard group, and ultimately, had fewer deaths than the standard group (20% vs 25%; between group absolute difference = 5.4%;1.2-9.5; P = .012). This mortality benefit persisted at 1 year.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.