User login
QI initiative reduces antibiotic use in chorioamnionitis-exposed newborns
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
FROM PEDIATRICS
Key clinical point:
Major finding: After a quality improvement initiative was implemented, 55% fewer late-preterm and term, chorioamnionitis-exposed infants received antibiotics without an increase in negative outcomes.
Data source: A study of 310 chorioamnionitis-exposed newborns who were late preterm or term at a California hospital.
Disclosures: The study did not use external funding. The authors had no relevant financial disclosures.
Source: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
Making structural improvements in health care
Every day, hospitalists devote time and energy to the best practices that can limit the spread of infection and the development of antibiotic resistance. Infection Prevention (IP) and Antimicrobial Stewardship (ASP) are two hospital programs that address that same goal.
But there may be a more effective approach possible, according to Jerome A. Leis, MD, MSc, FRCPC, of the Centre for Quality Improvement and Patient Safety at the University of Toronto.
“Despite the high-quality evidence supporting these IP/ASP interventions, our approach to adding these to our current practice sometimes feels like adding scaffolding to a rickety building,” he said. “It supports the underlying structure, but remove the scaffolding without fixing the building, and it may just come tumbling down.” Sometimes the work seems like an uphill battle, he added, as the same problems continue to recur.
That’s because there’s a systemic element to the problems. “Hospitalists know first hand about how the system that we work in makes it difficult to ensure that all the best IP/ASP practices are adhered to all the time,” Dr. Leis said. “Simply reminding staff to remove a urinary catheter in a timely fashion or clean their hands every single time they touch a patient or the environment can only get us so far.” That’s where improvement science comes in.
The relatively new field of improvement science provides a framework for research focused on health care improvement; its goal is to determine which improvement strategies are most effective. Dr. Leis argued that, “when our approach to IP and ASP incorporate principles of improvement science, we are more likely to be successful in achieving sustainable changes in practice.”
Rather than constantly adding extra steps and reminders for hospitalists about patient safety, he said, we need to recognize that there are systemic factors that lead to specific practices. “Our focus should be to use improvement-science methodology to understand these barriers and redesign the processes of care in a way that makes it easier for hospitalists to adhere to the best IP/ASP practices for our patients.”
These structural changes should come from collaboration among content experts in IP/ASP and those with training in improvement science, he said – many IP and ASP programs are already putting this in practice, using improvement science to create safer systems of care.
Reference
Leis J. Advancing infection prevention and antimicrobial stewardship through improvement science. BMJ Qual Saf. 2017 Jun 14. doi: 10.1136/bmjqs-2017-006793.
Every day, hospitalists devote time and energy to the best practices that can limit the spread of infection and the development of antibiotic resistance. Infection Prevention (IP) and Antimicrobial Stewardship (ASP) are two hospital programs that address that same goal.
But there may be a more effective approach possible, according to Jerome A. Leis, MD, MSc, FRCPC, of the Centre for Quality Improvement and Patient Safety at the University of Toronto.
“Despite the high-quality evidence supporting these IP/ASP interventions, our approach to adding these to our current practice sometimes feels like adding scaffolding to a rickety building,” he said. “It supports the underlying structure, but remove the scaffolding without fixing the building, and it may just come tumbling down.” Sometimes the work seems like an uphill battle, he added, as the same problems continue to recur.
That’s because there’s a systemic element to the problems. “Hospitalists know first hand about how the system that we work in makes it difficult to ensure that all the best IP/ASP practices are adhered to all the time,” Dr. Leis said. “Simply reminding staff to remove a urinary catheter in a timely fashion or clean their hands every single time they touch a patient or the environment can only get us so far.” That’s where improvement science comes in.
The relatively new field of improvement science provides a framework for research focused on health care improvement; its goal is to determine which improvement strategies are most effective. Dr. Leis argued that, “when our approach to IP and ASP incorporate principles of improvement science, we are more likely to be successful in achieving sustainable changes in practice.”
Rather than constantly adding extra steps and reminders for hospitalists about patient safety, he said, we need to recognize that there are systemic factors that lead to specific practices. “Our focus should be to use improvement-science methodology to understand these barriers and redesign the processes of care in a way that makes it easier for hospitalists to adhere to the best IP/ASP practices for our patients.”
These structural changes should come from collaboration among content experts in IP/ASP and those with training in improvement science, he said – many IP and ASP programs are already putting this in practice, using improvement science to create safer systems of care.
Reference
Leis J. Advancing infection prevention and antimicrobial stewardship through improvement science. BMJ Qual Saf. 2017 Jun 14. doi: 10.1136/bmjqs-2017-006793.
Every day, hospitalists devote time and energy to the best practices that can limit the spread of infection and the development of antibiotic resistance. Infection Prevention (IP) and Antimicrobial Stewardship (ASP) are two hospital programs that address that same goal.
But there may be a more effective approach possible, according to Jerome A. Leis, MD, MSc, FRCPC, of the Centre for Quality Improvement and Patient Safety at the University of Toronto.
“Despite the high-quality evidence supporting these IP/ASP interventions, our approach to adding these to our current practice sometimes feels like adding scaffolding to a rickety building,” he said. “It supports the underlying structure, but remove the scaffolding without fixing the building, and it may just come tumbling down.” Sometimes the work seems like an uphill battle, he added, as the same problems continue to recur.
That’s because there’s a systemic element to the problems. “Hospitalists know first hand about how the system that we work in makes it difficult to ensure that all the best IP/ASP practices are adhered to all the time,” Dr. Leis said. “Simply reminding staff to remove a urinary catheter in a timely fashion or clean their hands every single time they touch a patient or the environment can only get us so far.” That’s where improvement science comes in.
The relatively new field of improvement science provides a framework for research focused on health care improvement; its goal is to determine which improvement strategies are most effective. Dr. Leis argued that, “when our approach to IP and ASP incorporate principles of improvement science, we are more likely to be successful in achieving sustainable changes in practice.”
Rather than constantly adding extra steps and reminders for hospitalists about patient safety, he said, we need to recognize that there are systemic factors that lead to specific practices. “Our focus should be to use improvement-science methodology to understand these barriers and redesign the processes of care in a way that makes it easier for hospitalists to adhere to the best IP/ASP practices for our patients.”
These structural changes should come from collaboration among content experts in IP/ASP and those with training in improvement science, he said – many IP and ASP programs are already putting this in practice, using improvement science to create safer systems of care.
Reference
Leis J. Advancing infection prevention and antimicrobial stewardship through improvement science. BMJ Qual Saf. 2017 Jun 14. doi: 10.1136/bmjqs-2017-006793.
FDA warns against clarithromycin use in patients with heart disease
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
The Food and Drug Administration has added a new warning for an increased risk of death in patients with heart disease who have used clarithromycin (Biaxin), on the basis of results of a 10-year follow-up from the CLARICOR trial.
The CLARICOR trial followed 4,372 randomized patients for at least 2 years after undergoing 14 days of treatment with daily doses of 500 mg clarithromycin. Among these patients, researchers observed an unexpected increase in deaths in patients with coronary heart disease. (The Feb. 22 FDA statement announcing the alert did not provide data from CLARICOR.) As of yet, there is no clear explanation of how clarithromycin would lead to more deaths, compared with a placebo, the agency said.
Regardless, two of the six observational studies published found a link between clarithromycin use and long-term risks; four did not. The CLARICOR trial provides the strongest evidence of increased health risks, the statement said.
The FDA is recommending that health care professionals be aware of the risks associated with clarithromycin use and consider the benefits and risks of use in patients with heart disease. If at all possible, the use of other antibiotics may be a better option. Doctors should advise patients to be aware of signs and symptoms associated with cardiovascular issues.
Patients are also an important piece of the puzzle and should communicate with their health care providers about heart disease, particularly when taking antibiotics to treat for an infection.
The FDA has added the results of the CLARICOR trial to the clarithromycin drug labels. The agency will continue to monitor the safety reports in patients using clarithromycin.
Serious adverse events associated with clarithromycin should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch/.
Reported penicillin allergies hike inpatient costs
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Total inpatient costs for patients who report being allergic to penicillin are much higher than for those who don’t report an allergy, according to a recent systematic review and meta-analysis.
The review, which eventually included 30 articles, found that total inpatient costs ranged from an average $1,145-$4,254 higher per patient with a reported penicillin allergy compared to nonallergic patients, said T. Joseph Mattingly, PharmD, and his associates. Outpatient prescription costs were also estimated to be steeper, running $14-$93 higher per patient who reported a penicillin allergy.
Although 10%-20% of patients report a penicillin allergy, “[a] majority of patients who report PCN [penicillin] allergy are not truly allergic upon confirmatory testing,” Dr. Mattingly and his colleagues wrote.
This overreporting of penicillin allergies is a problem for the patient and the health care system because “reported antibiotic allergies have been associated with suboptimal antibiotic therapy, increased antimicrobial resistance, increased length of stay, increased antibiotic-related adverse events, increased rates of C. difficile infection, intensive care unit (ICU) admission, death, as well as increased treatment cost,” said Dr. Mattingly and his coauthors.
Health care providers often “tend to take reported allergies at face value,” said coauthor Anne Fulton, suggesting that primary care practices can help by considering skin testing for those patients who carry a label of penicillin allergy, but don’t have a documented confirmatory test. The cost for a commonly used skin test for penicillin allergy runs about $200, said Ms. Fulton, a doctoral candidate at the University of Maryland, Baltimore, in an interview.
When conducting the meta-analysis, Dr. Mattingly and his coauthors converted all figures to 2017 U.S. dollars, using Consumer Price Index figures to adjust for inflation. This yields conservative estimates for cost, as drug and health care prices have far outstripped the general rate of inflation during the period in which the studies occurred, Ms. Fulton acknowledged.
The investigators highlighted the need for ongoing study in this area. “To our knowledge, there are no evaluations of long-term outpatient outcomes related to the effects of PCN allergy and the potential impact of delabeling patients who do not have a true allergy,” they wrote.
Ms. Fulton agreed, noting that the studies covered in the meta-analysis were primarily focused on short-term outcomes, though there are many potential long-term benefits to delabeling patients who are not truly penicillin allergic.
For the patient, this includes the opportunity to receive optimal antimicrobial therapy, as well as potential savings in copays and other out-of-pocket expenses for outpatient medications, she said.
As antimicrobial resistance becomes an ever more pressing problem, there are more opportunities for targeted therapy if inappropriate allergy labeling is addressed, Ms. Fulton added.
Further study should use “cost-effectiveness analysis methods that include societal and health sector perspectives capturing immediate and future outcomes and costs to evaluate the use of skin-testing procedures in either inpatient or outpatient settings,” the investigators wrote.
The study was supported by ALK, the manufacturer of Pre-Pen, a commercially available penicillin allergy skin test.
SOURCE: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Key clinical point: Inpatient costs were $1,145 – $4,254 higher for those reporting penicillin allergy.
Major finding: Though most studies addressed inpatient admissions, outpatient costs were also significantly higher.
Study details: Systematic review and meta-analysis of 30 articles addressing reported penicillin allergy.
Disclosures: The study was sponsored by ALK.
Source: Mattingly TJ et al. J Allergy Clin Immunol Pract. 2018 Jan 31. doi: 10.1016/j.jaip.2017.12.033.
Paring the risk of antibiotic resistance
One unintended consequence of the increased attention to early sepsis identification and intervention can be unnecessary or excessive antibiotic use. Overuse of broad-spectrum antibiotics, in turn, can fuel the emergence of life-threatening infections such as antibiotic-resistant Clostridium difficile, a scourge in many hospitals.
For a sepsis quality improvement (QI) initiative at the University of Utah, Salt Lake City, the hospitalist coleaders took several precautions to lessen the risk of antibiotic overuse. Kencee K. Graves, MD, said she and her colleague Devin J. Horton, MD, designed the hospital’s order sets in collaboration with an infectious disease specialist and pharmacist so they could avoid overly broad antibiotics whenever possible. The project also included an educational effort to get pharmacists in the habit of prompting medical providers to initiate antibiotic de-escalation at 48 hours. The hospital had an antibiotic stewardship program that likely helped as well, she said. As a result of their precautions, the team found no significant difference in the amount of broad-spectrum antibiotics doled out before and after their QI pilot project.
Infection control and antimicrobial specialists also can help; they can monitor an area’s resistance profile, create a antibiogram and reevaluate sepsis pathways and order sets to adjust the recommended antibiotics as the resistance profile changes. “I think we still have a long ways to go,” said Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis. “The initial risk of mortality is so much more dramatic than the long-term risks of developing antimicrobial resistors that unless you have the antimicrobial stewardship people with a seat at the table, that voice can get drowned out very easily.”
The antimicrobial stewardship program at University of Pennsylvania, Philadelphia, has received a boost from technology. The program offers initial guidance on which broad-spectrum antibiotics to consider depending on the suspected source of the sepsis-linked infection. Software by Jackson, Wyo.–based biotech company Teqqa also synthesizes the university hospital’s resistance data based on blood, urine, and sputum cultures. “It can predict the antibiotic sensitivity of a given bug growing out of a given culture on a given unit,” said Craig A. Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine at the university.
The bigger issue, Dr. Umscheid said, is when and how to de-escalate antibiotic treatment. “If somebody is feeling better in 48 hours or 72 hours and no cultures have grown back, they have no more fever, and their white counts have normalized, do you start pulling off the antibiotics slowly and, if so, how do you do that?” Several trials are examining such questions, including a multicenter collaboration called DETOURS (De-Escalating Empiric Treatment: Opting-Out of Rx for Selected Patients With Suspected Sepsis). One of the trial’s chief aims is to set up a new opt-out protocol for acute care patients in the wards.
One unintended consequence of the increased attention to early sepsis identification and intervention can be unnecessary or excessive antibiotic use. Overuse of broad-spectrum antibiotics, in turn, can fuel the emergence of life-threatening infections such as antibiotic-resistant Clostridium difficile, a scourge in many hospitals.
For a sepsis quality improvement (QI) initiative at the University of Utah, Salt Lake City, the hospitalist coleaders took several precautions to lessen the risk of antibiotic overuse. Kencee K. Graves, MD, said she and her colleague Devin J. Horton, MD, designed the hospital’s order sets in collaboration with an infectious disease specialist and pharmacist so they could avoid overly broad antibiotics whenever possible. The project also included an educational effort to get pharmacists in the habit of prompting medical providers to initiate antibiotic de-escalation at 48 hours. The hospital had an antibiotic stewardship program that likely helped as well, she said. As a result of their precautions, the team found no significant difference in the amount of broad-spectrum antibiotics doled out before and after their QI pilot project.
Infection control and antimicrobial specialists also can help; they can monitor an area’s resistance profile, create a antibiogram and reevaluate sepsis pathways and order sets to adjust the recommended antibiotics as the resistance profile changes. “I think we still have a long ways to go,” said Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis. “The initial risk of mortality is so much more dramatic than the long-term risks of developing antimicrobial resistors that unless you have the antimicrobial stewardship people with a seat at the table, that voice can get drowned out very easily.”
The antimicrobial stewardship program at University of Pennsylvania, Philadelphia, has received a boost from technology. The program offers initial guidance on which broad-spectrum antibiotics to consider depending on the suspected source of the sepsis-linked infection. Software by Jackson, Wyo.–based biotech company Teqqa also synthesizes the university hospital’s resistance data based on blood, urine, and sputum cultures. “It can predict the antibiotic sensitivity of a given bug growing out of a given culture on a given unit,” said Craig A. Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine at the university.
The bigger issue, Dr. Umscheid said, is when and how to de-escalate antibiotic treatment. “If somebody is feeling better in 48 hours or 72 hours and no cultures have grown back, they have no more fever, and their white counts have normalized, do you start pulling off the antibiotics slowly and, if so, how do you do that?” Several trials are examining such questions, including a multicenter collaboration called DETOURS (De-Escalating Empiric Treatment: Opting-Out of Rx for Selected Patients With Suspected Sepsis). One of the trial’s chief aims is to set up a new opt-out protocol for acute care patients in the wards.
One unintended consequence of the increased attention to early sepsis identification and intervention can be unnecessary or excessive antibiotic use. Overuse of broad-spectrum antibiotics, in turn, can fuel the emergence of life-threatening infections such as antibiotic-resistant Clostridium difficile, a scourge in many hospitals.
For a sepsis quality improvement (QI) initiative at the University of Utah, Salt Lake City, the hospitalist coleaders took several precautions to lessen the risk of antibiotic overuse. Kencee K. Graves, MD, said she and her colleague Devin J. Horton, MD, designed the hospital’s order sets in collaboration with an infectious disease specialist and pharmacist so they could avoid overly broad antibiotics whenever possible. The project also included an educational effort to get pharmacists in the habit of prompting medical providers to initiate antibiotic de-escalation at 48 hours. The hospital had an antibiotic stewardship program that likely helped as well, she said. As a result of their precautions, the team found no significant difference in the amount of broad-spectrum antibiotics doled out before and after their QI pilot project.
Infection control and antimicrobial specialists also can help; they can monitor an area’s resistance profile, create a antibiogram and reevaluate sepsis pathways and order sets to adjust the recommended antibiotics as the resistance profile changes. “I think we still have a long ways to go,” said Andy Odden, MD, SFHM, patient safety officer in the department of medicine at Washington University in St. Louis. “The initial risk of mortality is so much more dramatic than the long-term risks of developing antimicrobial resistors that unless you have the antimicrobial stewardship people with a seat at the table, that voice can get drowned out very easily.”
The antimicrobial stewardship program at University of Pennsylvania, Philadelphia, has received a boost from technology. The program offers initial guidance on which broad-spectrum antibiotics to consider depending on the suspected source of the sepsis-linked infection. Software by Jackson, Wyo.–based biotech company Teqqa also synthesizes the university hospital’s resistance data based on blood, urine, and sputum cultures. “It can predict the antibiotic sensitivity of a given bug growing out of a given culture on a given unit,” said Craig A. Umscheid, MD, MSCE, of the department of epidemiology and vice chair for quality and safety in the department of medicine at the university.
The bigger issue, Dr. Umscheid said, is when and how to de-escalate antibiotic treatment. “If somebody is feeling better in 48 hours or 72 hours and no cultures have grown back, they have no more fever, and their white counts have normalized, do you start pulling off the antibiotics slowly and, if so, how do you do that?” Several trials are examining such questions, including a multicenter collaboration called DETOURS (De-Escalating Empiric Treatment: Opting-Out of Rx for Selected Patients With Suspected Sepsis). One of the trial’s chief aims is to set up a new opt-out protocol for acute care patients in the wards.
Drug combo indicated for bacterial pneumonia
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
Is it time for health policy M&Ms?
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
What would happen if hospitalists began to incorporate health policy into morbidity and mortality (M&M) conferences? That was a question Chris Moriates, MD, explored in an entry for SHM’s The Hospital Leader blog1 and an idea that caused a minor stir on Twitter when he proposed it last summer.
In late July 2017, the U.S. Senate was debating a bill to repeal the Affordable Care Act, without a clear vision for replacing it. In response, physicians around the country took to Twitter to share their sentiments about repeal under the hashtag #DoctorsSpeakOut. In one such tweet, Dr. Moriates, assistant dean for health care value and an associate professor of internal medicine at Dell Medical School at the University of Texas, Austin, said this, in 140 characters: “We recently had idea: health policy M&M for residents to discuss adverse outcomes we see as result of lack of access.”
The idea began with a conversation Dr. Moriates had with Beth Miller, MD, program director for the Dell Medical School Internal Medicine Residency Program. “We were meeting and talking about revamping the [resident] M&M conference to have more learning objectives and put in place best practices,” Dr. Moriates said. “Dr. Miller suggested it could be a good forum [for health policy] because it’s an area where we all come together and there’s a natural hook to it, since it is case-based, thus we can use it to recognize the drivers within the system that lead to bad outcomes.”
In his SHM blog post, Dr. Moriates said he has increasingly observed adverse events that result from issues related to health policy. He provided an example: “A patient I admitted for ‘expedited work-up’ for rectal bleeding after he told me he had been trying to get a recommended colonoscopy for many months but could not get it scheduled due to his lack of insurance. He had colon cancer that had spread.”
In another example, he conjured a hypothetical (though not impractical) case where a patient prescribed blood thinners upon hospital discharge returns to the hospital soon after with a blood clot. Unable to afford the medication, or seek primary care follow-up, this kind of patient is readmitted through no direct fault of his physicians. Yet, the patient is worse off and the hospital takes the hit on readmissions penalties.
Dr. Moriates believes that viewing a case like this through a health policy lens is not only moving, but critical to better understanding health care delivery, particularly in an environment where physician performance is measured, in part, by outcomes. He now believes health policy M&Ms would be valuable to all hospital-based physicians, not just residents.
“Hospitalists are being asked to hit these value-based performance metrics, like readmissions and length of stay, and while we deal with the consequences, we are not always the best informed” with respect to policy, he said. “We could use this forum to teach health policy topics and continually update people and contribute, in real time, to all these different discussions and understand how things are changing or could change and impact our patients.”
“It’s important for physicians to know the policies that are aligned with, and the policies that may undermine, what they’re doing in their practice to improve their patients’ health,” Pourat said.
This knowledge can benefit physicians, too, Pourat added, because health policy M&Ms could help providers understand the goals of particular policies and in turn adjust their own behaviors and expectations.
“Physicians could discuss, what are the underlying issues or root causes, like the decision not to expand Medicaid here in Texas,” Dr. Moriates said. “Not all of these things you can fix, but you’re exposing those stories and perhaps we can come up with some actionable steps. How do we ensure in the future that our patients are able to fulfill their prescription so we’re not just sending someone out assuming they will but not knowing they’re unable to afford it?”
Similar to other domains in which physician leaders become champions, such as antibiotic stewardship, Dr. Pourat suggested that hospitalists could champion policy awareness through the kind of M&Ms Dr. Moriates proposed.
While journal clubs and lectures are great ways for hospitalists to learn more about health policy, the emotionally gripping nature of M&Ms could inspire more physicians to act in favor of policies that benefit their patients and themselves, Dr. Moriates said.
For example, physicians may write to or visit legislative offices, or author op-eds in their local newspapers. This collective action carries the potential to effect change. And it need not be partisan.
“I believe that if health policy issues were more explicitly integrated into M&Ms then clinicians would be more inclined and prepared to effectively advocate for specific policy changes,” he wrote in his blog post. “Perhaps entire groups would be moved to engage in the political process.”
On Twitter, even before Dr. Moriates’ first tweet about health policy M&Ms, New Jersey–based Jennifer Chuang, MD, an adolescent medicine physician, wrote: “M&M is heart-wrenching in academic hospitals. I dare @SenateGOP to present their role in M&M’s to come if ACA is repealed.”
While Dr. Moriates believes the chances are quite small that legislators and policymakers would attend health policy M&Ms, he called the notion “provocative and intriguing.”
In his blog post, Dr. Moriates invites state legislators and local members of Congress to join him in reviewing M&M cases where patients have been negatively affected by policy. He also emphasized that, like most modern M&Ms, the point should not be derision or finger-pointing, but an opportunity to learn how policy translates into practice.
Physicians may learn from legislators, too, he said in his blog post. “Just as policymakers could see legislation through the eyes of practitioners and their patients, this is where we as physicians could possibly learn from our legislators,” he wrote. “We may recognize the potential trade-offs, downsides, and barriers to proposals that to us may have seemed like no-brainers.”
What’s clear, said Dr. Pourat, who is also a professor in the UCLA Fielding School of Public Health and the School of Dentistry, is that Dr. Moriates’ blog post and tweet are “touching an important point for a lot of physicians during this whole debate over health reform.”
President Donald Trump campaigned on a promise to fully repeal and replace the Affordable Care Act but Republican efforts have thus far been stymied. In the meantime, some physicians are watching closely, knowing that whatever comes next will continue to affect them and their patients.
Source
1. Moriates C. Is it time for health policy M&Ms? The Hospital Leader. Aug 16, 2017. http://thehospitalleader.org/is-it-time-for-health-policy-mms/. Accessed 2017 Sep 14.
Antibiotic choice for acute otitis media 2018
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 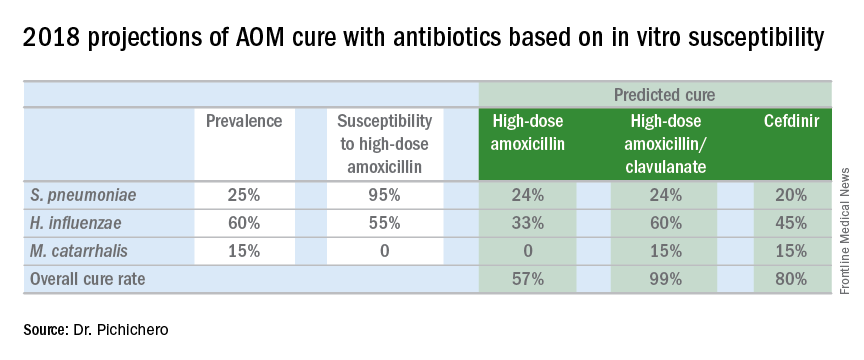
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
Predicting MDR Gram-negative infection mortality risk
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Source control, defined as location and elimination of the source of the infection, was critical for patient survival in the case of multidrug resistant bacterial infection, according to the results of a case-control study of 62 critically ill surgical patients who were assessed between 2011 and 2014.
Researchers examined the characteristics of infected patients surviving to hospital discharge compared with those of nonsurvivors to look for predictive factors. Demographically, patients had an overall mean age of 62 years; 30.6% were women; 69.4% were white. The first culture obtained during a surgical ICU admission that grew a carbapenem-resistant Enterobacteriaceae (CRE), MDR Pseudomonas aeruginosa, or MDR Acinetobacter spp. was defined as the index culture.
“In this study, 33.9% [21/62] of critically ill surgical patients with a culture positive for MDR Gram-negative bacteria died prior to hospital discharge,” according to Andrew S. Jarrell, PharmD, of the Johns Hopkins Hospital, Baltimore, and his colleagues.
With multivariate logistic regression, achievement of source control was the only variable associated with decreased in-hospital mortality (odds ratio 0.04, 95% confidence interval, 0.003-0.52); P = .01).
“Source control status was predictive of in-hospital mortality after controlling for other factors. Specifically, the odds of in-hospital mortality were 97% lower when source control was achieved as compared to when source control was not achieved,” the authors stated (J Crit Care. 2018;43:321-6).
Scenarios in which source control was not applicable (pneumonia and urinary tract infection) were also similarly distributed between survivors and nonsurvivors, they reported.
Other than source control, the only significant risk factors for mortality, as seen in univariate analysis, all occurred prior to index culture. They were: vasopressor use (46.3% of survivors, vs. 76.2% of nonsurvivors, P = .03); mechanical ventilation (63.4% vs. 100%, P = .001); and median ICU length of stay (10 days vs. 18 days, P = .001).
“Achievement of source control stands out as a critical factor for patient survival. Clinicians should take this, along with prior ICU LOS, vasopressor use, and mechanical ventilation status, into consideration when evaluating patient prognosis,” Dr. Jarrell and his colleagues concluded.
The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
FROM THE JOURNAL OF CRITICAL CARE
Key clinical point: Source control was the most important predictor of MDR Gram-negative infection mortality in hospitalized patients.
Major finding: The odds of in-hospital mortality were 97% lower when source control was achieved.
Study details: Case-control study of 62 critically ill surgical patients from 2011 to 2014 who had an MDR infection.
Disclosures: The authors reported that they had no conflicts or source of funding.
Source: Jarrell, A.S., et al. J Crit Care. 2018;43:321-6.
Common food additive makes C. difficile more virulent
, a study showed.
“Out of several carbon sources identified that supported CD2015 growth [epidemic RT027 isolate], we found the disaccharide trehalose increased the growth yield of CD2015 by approximately fivefold, compared with a non-RT027 strain,” according to James Collins, PhD, of Baylor University, Houston, and his colleagues. The increased growth of the epidemic strain of C. difficile observed by Dr. Collins and his team demonstrates that trehalose is a robust carbon source for C. difficile bacterium.
In one experiment, mice with humanized microbiota were infected with two strains of RT027, either R20291 (n = 27) or R20291-delta treA (n = 28), a phosphotrehalase enzyme (TreA) deletion mutant that cannot metabolize trehalose. Mice were then given 5 mM of trehalose ad libitum in their drinking water. Researchers observed that the mice infected with R20291-delta treA had much lower mortality rates than the R20291 group (33.3% vs.78.6%). These findings were then reinforced with a second experiment using mice with humanized microbiota, in which trehalose addition increased mortality in RT027 mice, compared with RT027-infected mice that were not given dietary trehalose.
While Dr. Collins and his team demonstrated the effect of trehalose on C. difficile in mice, they also conducted a limited analysis of ileostomy effluent from three human donors. The researchers found that in two of three samples, treA was strongly induced in CD2015, but not in another ribotype, CD2048. This demonstrates that amounts of trehalose found in food are high enough to be metabolized by certain epidemic strains of C. difficile in humans.
Prior to 2000, trehalose use was limited by a relatively high cost of production, approximately $700 per kilogram. A production innovation that utilized a novel enzymatic method that yielded trehalose from starch brought the price of trehalose to approximately $3 per kilogram, making it a commercially viable food supplement. After being considered “generally recognized as safe” by the U.S. Food and Drug Administration in 2000 and approved for use in Europe, the trehalose concentrations in food skyrocketed from around 2% to 11.25%, and trehalose became widely used in several foods, including ice cream, pasta, and ground beef.
Dr. Collins and his associates said that there is considerable evidence that the widespread use of dietary trehalose has contributed to the spread of epidemic C. difficile ribotypes. First, strains RT027 and RT078 have always had the ability to metabolize trehalose, as evidenced by outbreaks of nonepidemic C. difficile in the 1980s. But no epidemic outbreaks were reported until after 2003, several years after trehalose was approved by the FDA. Second, RT027 and RT078 are phylogenetically distant, but independently evolved the ability to metabolize low levels of trehalose. Third, increased severity of the RT027 strain, which metabolizes trehalose in mice, is consistent with increased virulence of RT078 and RT027 in human patients. Fourth, a competitive advantage is conferred to C. difficile being able to metabolize trehalose in low concentrations in a diverse intestinal setting. Finally, the levels of trehalose in ileostomy fluid from patients who eat a normal diet are high enough to be utilized by RT027 strains.
“On the basis of these observations, we propose that the widespread adoption and use of the disaccharide trehalose in the human diet has played a significant role in the emergence of these epidemic and hypervirulent strains,” Dr. Collins and his colleagues wrote in their article in Nature.
The authors of the study had no relevant financial disclosures to report.
SOURCE: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.
, a study showed.
“Out of several carbon sources identified that supported CD2015 growth [epidemic RT027 isolate], we found the disaccharide trehalose increased the growth yield of CD2015 by approximately fivefold, compared with a non-RT027 strain,” according to James Collins, PhD, of Baylor University, Houston, and his colleagues. The increased growth of the epidemic strain of C. difficile observed by Dr. Collins and his team demonstrates that trehalose is a robust carbon source for C. difficile bacterium.
In one experiment, mice with humanized microbiota were infected with two strains of RT027, either R20291 (n = 27) or R20291-delta treA (n = 28), a phosphotrehalase enzyme (TreA) deletion mutant that cannot metabolize trehalose. Mice were then given 5 mM of trehalose ad libitum in their drinking water. Researchers observed that the mice infected with R20291-delta treA had much lower mortality rates than the R20291 group (33.3% vs.78.6%). These findings were then reinforced with a second experiment using mice with humanized microbiota, in which trehalose addition increased mortality in RT027 mice, compared with RT027-infected mice that were not given dietary trehalose.
While Dr. Collins and his team demonstrated the effect of trehalose on C. difficile in mice, they also conducted a limited analysis of ileostomy effluent from three human donors. The researchers found that in two of three samples, treA was strongly induced in CD2015, but not in another ribotype, CD2048. This demonstrates that amounts of trehalose found in food are high enough to be metabolized by certain epidemic strains of C. difficile in humans.
Prior to 2000, trehalose use was limited by a relatively high cost of production, approximately $700 per kilogram. A production innovation that utilized a novel enzymatic method that yielded trehalose from starch brought the price of trehalose to approximately $3 per kilogram, making it a commercially viable food supplement. After being considered “generally recognized as safe” by the U.S. Food and Drug Administration in 2000 and approved for use in Europe, the trehalose concentrations in food skyrocketed from around 2% to 11.25%, and trehalose became widely used in several foods, including ice cream, pasta, and ground beef.
Dr. Collins and his associates said that there is considerable evidence that the widespread use of dietary trehalose has contributed to the spread of epidemic C. difficile ribotypes. First, strains RT027 and RT078 have always had the ability to metabolize trehalose, as evidenced by outbreaks of nonepidemic C. difficile in the 1980s. But no epidemic outbreaks were reported until after 2003, several years after trehalose was approved by the FDA. Second, RT027 and RT078 are phylogenetically distant, but independently evolved the ability to metabolize low levels of trehalose. Third, increased severity of the RT027 strain, which metabolizes trehalose in mice, is consistent with increased virulence of RT078 and RT027 in human patients. Fourth, a competitive advantage is conferred to C. difficile being able to metabolize trehalose in low concentrations in a diverse intestinal setting. Finally, the levels of trehalose in ileostomy fluid from patients who eat a normal diet are high enough to be utilized by RT027 strains.
“On the basis of these observations, we propose that the widespread adoption and use of the disaccharide trehalose in the human diet has played a significant role in the emergence of these epidemic and hypervirulent strains,” Dr. Collins and his colleagues wrote in their article in Nature.
The authors of the study had no relevant financial disclosures to report.
SOURCE: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.
, a study showed.
“Out of several carbon sources identified that supported CD2015 growth [epidemic RT027 isolate], we found the disaccharide trehalose increased the growth yield of CD2015 by approximately fivefold, compared with a non-RT027 strain,” according to James Collins, PhD, of Baylor University, Houston, and his colleagues. The increased growth of the epidemic strain of C. difficile observed by Dr. Collins and his team demonstrates that trehalose is a robust carbon source for C. difficile bacterium.
In one experiment, mice with humanized microbiota were infected with two strains of RT027, either R20291 (n = 27) or R20291-delta treA (n = 28), a phosphotrehalase enzyme (TreA) deletion mutant that cannot metabolize trehalose. Mice were then given 5 mM of trehalose ad libitum in their drinking water. Researchers observed that the mice infected with R20291-delta treA had much lower mortality rates than the R20291 group (33.3% vs.78.6%). These findings were then reinforced with a second experiment using mice with humanized microbiota, in which trehalose addition increased mortality in RT027 mice, compared with RT027-infected mice that were not given dietary trehalose.
While Dr. Collins and his team demonstrated the effect of trehalose on C. difficile in mice, they also conducted a limited analysis of ileostomy effluent from three human donors. The researchers found that in two of three samples, treA was strongly induced in CD2015, but not in another ribotype, CD2048. This demonstrates that amounts of trehalose found in food are high enough to be metabolized by certain epidemic strains of C. difficile in humans.
Prior to 2000, trehalose use was limited by a relatively high cost of production, approximately $700 per kilogram. A production innovation that utilized a novel enzymatic method that yielded trehalose from starch brought the price of trehalose to approximately $3 per kilogram, making it a commercially viable food supplement. After being considered “generally recognized as safe” by the U.S. Food and Drug Administration in 2000 and approved for use in Europe, the trehalose concentrations in food skyrocketed from around 2% to 11.25%, and trehalose became widely used in several foods, including ice cream, pasta, and ground beef.
Dr. Collins and his associates said that there is considerable evidence that the widespread use of dietary trehalose has contributed to the spread of epidemic C. difficile ribotypes. First, strains RT027 and RT078 have always had the ability to metabolize trehalose, as evidenced by outbreaks of nonepidemic C. difficile in the 1980s. But no epidemic outbreaks were reported until after 2003, several years after trehalose was approved by the FDA. Second, RT027 and RT078 are phylogenetically distant, but independently evolved the ability to metabolize low levels of trehalose. Third, increased severity of the RT027 strain, which metabolizes trehalose in mice, is consistent with increased virulence of RT078 and RT027 in human patients. Fourth, a competitive advantage is conferred to C. difficile being able to metabolize trehalose in low concentrations in a diverse intestinal setting. Finally, the levels of trehalose in ileostomy fluid from patients who eat a normal diet are high enough to be utilized by RT027 strains.
“On the basis of these observations, we propose that the widespread adoption and use of the disaccharide trehalose in the human diet has played a significant role in the emergence of these epidemic and hypervirulent strains,” Dr. Collins and his colleagues wrote in their article in Nature.
The authors of the study had no relevant financial disclosures to report.
SOURCE: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.
FROM NATURE
Key clinical point: Metabolizing trehalose increases the virulence and mortality of C. difficile ribotype 027 (RT027).
Major finding: The ability to metabolize trehalose with the phosphotrehalase enzyme (TreA) increases mortality in RT027.
Study details: Experimental mouse models and an analysis of ileostomy effluent from three anonymous donors.
Disclosures: All authors had no financial disclosures to report.
Source: Collins J et al. Nature. 2018 Jan 3. doi: 10.1038/nature25178.