User login
Antiamyloids linked to accelerated brain atrophy
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
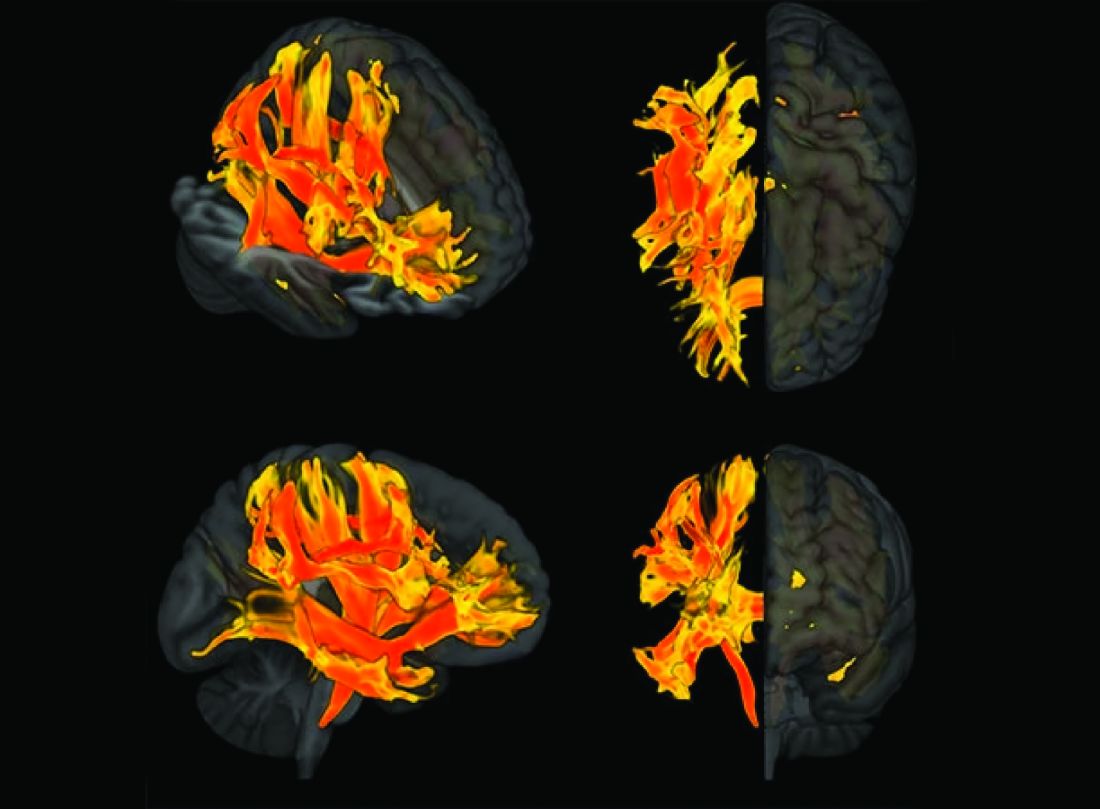
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
a comprehensive meta-analysis of MRI data from clinical trials suggests.
Depending on the anti–amyloid-beta drug class, these agents can accelerate loss of whole brain and hippocampal volume and increase ventricular volume. This has been shown for some of the beta-secretase inhibitors and with several of the antiamyloid monoclonal antibodies, researchers noted.
“These data warrant concern, but we can’t make any firm conclusions yet. It is possible that the finding is not detrimental, but the usual interpretation of this finding is that volume changes are a surrogate for disease progression,” study investigator Scott Ayton, PhD, of the Florey Institute of Neuroscience and Mental Health, University of Melbourne, said in an interview.
“These data should be factored into the decisions by clinicians when they consider prescribing antiamyloid therapies. Like any side effect, clinicians should inform patients regarding the risk of brain atrophy. Patients should be actively monitored for this side effect,” Dr. Ayton said.
The study was published online in Neurology.
Earlier progression from MCI to AD?
Dr. Ayton and colleagues evaluated brain volume changes in 31 clinical trials of anti–amyloid-beta drugs that demonstrated a favorable change in at least one biomarker of pathological amyloid-beta and included detailed MRI data sufficient to assess the volumetric changes in at least one brain region.
A meta-analysis on the highest dose in each trial on the hippocampus, ventricles, and whole brain showed drug-induced acceleration of volume changes that varied by anti–amyloid-beta drug class.
Secretase inhibitors accelerated atrophy in the hippocampus (mean difference –37.1 mcL; –19.6% relative to change in placebo) and whole brain (mean difference –3.3 mL; –21.8% relative to change in placebo), but not ventricles.
Conversely, monoclonal antibodies caused accelerated ventricular enlargement (mean difference +1.3 mL; +23.8% relative to change in placebo), which was driven by the subset of monoclonal antibodies that induce amyloid-related imaging abnormalities (ARIA) (+2.1 mL; +38.7% relative to change in placebo). There was a “striking correlation between ventricular volume and ARIA frequency,” the investigators reported.
The effect of ARIA-inducing monoclonal antibodies on whole brain volume varied, with accelerated whole brain volume loss caused by donanemab (mean difference –4.6 mL; +23% relative to change in placebo) and lecanemab (–5.2 mL; +36.4% relative to change in placebo). This was not observed with aducanumab and bapineuzumab.
Monoclonal antibodies did not cause accelerated volume loss to the hippocampus regardless of whether they caused ARIA.
The researchers also modeled the effect of anti–amyloid-beta drugs on brain volume changes. In this analysis, participants with mild cognitive impairment (MCI) treated with anti–amyloid-beta drugs were projected to have a “material regression” toward brain volumes typical of AD roughly 8 months earlier than untreated peers.
The data, they note, “permit robust conclusions regarding the effect of [anti–amyloid-beta] drug classes on different brain structures, but the lack of individual patient data (which has yet to be released) limits the interpretations of our findings.”
“Questions like which brain regions are impacted by [anti–amyloid-beta] drugs and whether the volume changes are related to ARIA, plaque loss, cognitive/noncognitive outcomes, or clinical factors such as age, sex, and apoE4 genotype can and should be addressed with available data,” said Dr. Ayton.
Dr. Ayton and colleagues called on data safety monitoring boards (DSMBs) for current clinical trials of anti–amyloid-beta drugs to review volumetric data to determine if patient safety is at risk, particularly in patients who develop ARIA.
In addition, they noted ethics boards that approve trials for anti–amyloid-beta drugs “should request that volume changes be actively monitored. Long-term follow-up of brain volumes should be factored into the trial designs to determine if brain atrophy is progressive, particularly in patients who develop ARIA.”
Finally, they added that drug companies that have conducted trials of anti–amyloid-beta drugs should interrogate prior data on brain volume, report the findings, and release the data for researchers to investigate.
“I have been banging on about this for years,” said Dr. Ayton. “Unfortunately, my raising of this issue has not led to any response. The data are not available, and the basic questions haven’t been asked (publicly).”
Commendable research
In an accompanying editorial, Frederik Barkhof, MD, PhD, with Amsterdam University Medical Centers, and David Knopman, MD, with Mayo Clinic Alzheimer’s Disease Research Center, Rochester, Minn., wrote that the investigators should be “commended” for their analysis.
“The reality in 2023 is that the relevance of brain volume reductions in this therapeutic context remains uncertain,” they wrote.
“Longer periods of observation will be needed to know whether the brain volume losses continue at an accelerated rate or if they attenuate or disappear. Ultimately, it’s the clinical outcomes that matter, regardless of the MRI changes,” Barkhof and Knopman concluded.
The research was supported by funds from the Australian National Health & Medical Research Council. Dr. Ayton reported being a consultant for Eisai in the past 3 years. Dr. Barkhof reported serving on the data and safety monitoring board for Prothena and the A45-AHEAD studies; being a steering committee member for Merck, Bayer, and Biogen; and being a consultant for IXICO, Roche, Celltrion, Rewind Therapeutics, and Combinostics. Dr. Knopman reported serving on the DSMB for the Dominantly Inherited Alzheimer Network Treatment Unit study; serving on a DSMB for a tau therapeutic for Biogen; being an investigator for clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He reported consulting with Roche, Samus Therapeutics, Magellan Health, BioVie, and Alzeca Biosciences.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Kidney disease skews Alzheimer’s biomarker testing
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research provides more evidence that
In a cross-sectional study of adults with and those without cognitive impairment, chronic kidney disease was associated with increased plasma concentrations of phosphorylated tau (p-tau) 217 and 181.
However, there were no associations between chronic kidney disease and the ratio of p-tau217 to unphosphorylated tau 217 (pT217/T217), and the associations with p-tau181 to unphosphorylated tau 181 (pT181/T181) were attenuated in patients with cognitive impairment.
“These novel findings suggest that plasma measures of the phosphorylated to unphosphorylated tau ratios are more accurate than p-tau forms alone as they correlate less with individual difference in glomerular filtration rate or impaired kidney function,” reported an investigative team led by Oskar Hansson, MD, PhD, with Lund University in Sweden.
“Thus, to mitigate the effects of non-Alzheimer’s–related comorbidities like chronic kidney disease on the performance of plasma Alzheimer’s disease biomarkers, certain tau ratios, and specifically pT217/T217, should be considered for implementation in clinical practice and drug trials,” they added.
The study was published online in JAMA Neurology to coincide with a presentation at the International Conference on Alzheimer’s and Parkinson’s Diseases in Gothenburg, Sweden.
Skewed tau levels
Plasma biomarkers of amyloid-beta (Abeta) and tau pathologies, and in particular variants in p-tau217 and p-tau181, have shown promise for use in Alzheimer’s disease diagnosis and prognosis. However, previous reports have suggested that chronic kidney disease might influence plasma p-tau217 and p-tau181 concentrations, potentially decreasing their usefulness in the diagnostic workup of dementia.
Researchers investigated associations of chronic kidney disease with plasma ratios of p-tau217 and p-tau181 to the corresponding unphosphorylated peptides in Alzheimer’s disease.
The data came from 473 older adults, with and without cognitive impairment, from the Swedish BioFinder-2 study who had plasma tau assessments and chronic kidney disease status established within 6 months of plasma collection.
The researchers found that lower estimated glomerular filtration rate levels (indicative of kidney dysfunction) were associated with higher plasma concentrations of phosphorylated and unphosphorylated tau peptides measured simultaneously using the tau immunoprecipitation mass spectrometry assay.
However, the correlations with estimated glomerular filtration rate were nonsignificant for the pT217/T217 ratio in individuals with cognitive impairment and were significantly attenuated for pT217/T217 in cognitively unimpaired individuals and for pT181/T181 in both cognitively unimpaired and impaired individuals.
“Importantly, we demonstrate that there were no significant associations between chronic kidney disease and the pT217/T217 ratio and changes in plasma pT181/T181 associated with chronic kidney disease were small or nonsignificant,” the researchers noted.
“Our results indicate that by using p-tau/tau ratios we may be able to reduce the variability in plasma p-tau levels driven by impaired kidney function and consequently such ratios are more robust measures of brain p-tau pathology in individuals with both early- and later-stage Alzheimer’s disease,” they added.
The researchers believe this is likely true for the ratios of other related proteins – a view that is supported by findings of attenuated associations of chronic kidney disease with Abeta42/40, compared with Abeta42 and Abeta40 in the current study and in previous studies.
Important clinical implications
Reached for comment, Rebecca Edelmayer, PhD, Alzheimer’s Association senior director of scientific engagement, noted that research continues to indicate that blood biomarkers have “promise for improving, and possibly even redefining, the clinical workup for Alzheimer’s disease.
“Many of the Alzheimer’s blood tests in development today have focused on core blood biomarkers associated with amyloid accumulation and tau tangle formation in the brain,” Dr. Edelmayer said.
“Before these tests can be used more broadly in the clinic, we need to understand all of the variables that may impact the results of various blood biomarkers, including differences that may be driven by race, ethnicity, sex, and underlying health conditions, such as chronic kidney disease.
“This study corroborates other research suggesting that some Alzheimer’s-associated markers can be affected by chronic kidney disease, but by using ratios of amyloid or tau markers, we may be able to minimize these differences in results caused by underlying disease,” Dr. Edelmayer said.
Howard Fillit, MD, cofounder and chief science officer at the Alzheimer’s Drug Discovery Foundation, said, “Using these ratios makes a lot of sense because the ratios wouldn’t change with kidney function. I think this is an important advance in clinical utilization of these tests.”
Dr. Fillit noted that older people often have declining kidney function, which can be easily measured by glomerular filtration rate. Changes in blood levels of these markers with declining kidney function are “not unexpected, but [it’s] important that it’s been demonstrated.
“As we go forward, maybe the future utilization of these tests will be not only recording the ratios but also reporting the ratios in the context of somebody’s glomerular filtration rate. You could imagine a scenario where when the test is done, it’s automatically done alongside of glomerular filtration rate,” Dr. Fillit said in an interview.
The study was supported by Coins for Alzheimer’s Research Trust, the Tracy Family Stable Isotope Labeling Quantitation Center, and the National Institute of Neurological Disorders and Stroke. Dr. Hansson has received support to his institution from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche; consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens; and personal fees from Eli Lilly, Eisai, Bioarctic, and Biogen outside the submitted work. Dr. Edelmayer and Dr. Fillit have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AD/PD 2023
Magnesium-rich diet linked to lower dementia risk
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
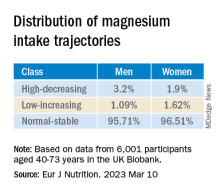
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.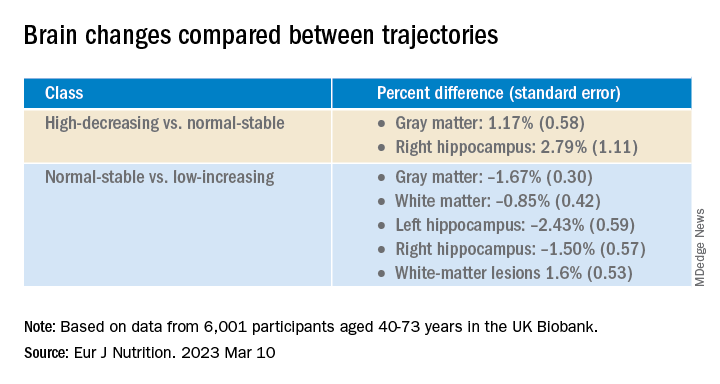
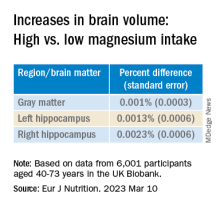
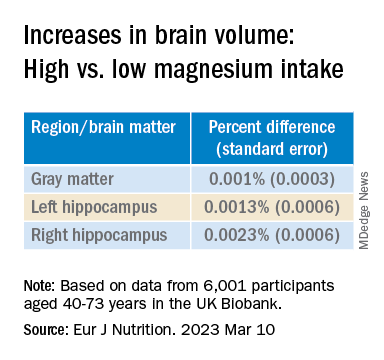
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below: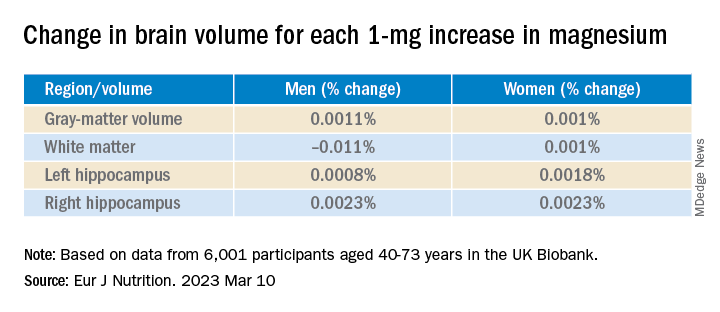
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators studied more than 6,000 cognitively healthy individuals, aged 40-73, and found that those who consumed more than 550 mg of magnesium daily had a brain age approximately 1 year younger by age 55 years, compared with a person who consumed a normal magnesium intake (~360 mg per day).
“This research highlights the potential benefits of a diet high in magnesium and the role it plays in promoting good brain health,” lead author Khawlah Alateeq, a PhD candidate in neuroscience at Australian National University’s National Centre for Epidemiology and Population Health, said in an interview.
Clinicians “can use [the findings] to counsel patients on the benefits of increasing magnesium intake through a healthy diet and monitoring magnesium levels to prevent deficiencies,” she stated.
The study was published online in the European Journal of Nutrition.
Promising target
The researchers were motivated to conduct the study because of “the growing concern over the increasing prevalence of dementia,” Ms. Alateeq said.
“Since there is no cure for dementia, and the development of pharmacological treatment for dementia has been unsuccessful over the last 30 years, prevention has been suggested as an effective approach to address the issue,” she added.
Nutrition, Ms. Alateeq said, is a “modifiable risk factor that can influence brain health and is highly amenable to scalable and cost-effective interventions.” It represents “a promising target” for risk reduction at a population level.
Previous research shows individuals with lower magnesium levels are at higher risk for AD, while those with higher dietary magnesium intake may be at lower risk of progressing from normal aging to cognitive impairment.
Most previous studies, however, included participants older than age 60 years, and it’s “unclear when the neuroprotective effects of dietary magnesium become detectable,” the researchers note.
Moreover, dietary patterns change and fluctuate, potentially leading to changes in magnesium intake over time. These changes may have as much impact as absolute magnesium at any point in time.
In light of the “current lack of understanding of when and to what extent dietary magnesium exerts its protective effects on the brain,” the researchers examined the association between magnesium trajectories over time, brain matter, and white matter lesions.
They also examined the association between magnesium and several different blood pressure measures (mean arterial pressure, systolic blood pressure, diastolic blood pressure, and pulse pressure).
Since cardiovascular health, neurodegeneration, and brain shrinkage patterns differ between men and women, the researchers stratified their analyses by sex.
Brain volume differences
The researchers analyzed the dietary magnesium intake of 6,001 individuals (mean age, 55.3 years) selected from the UK Biobank – a prospective cohort study of participants aged 37-73 at baseline, who were assessed between 2005 and 2023.
For the current study, only participants with baseline DBP and SBP measurements and structural MRI scans were included. Participants were also required to be free of neurologic disorders and to have an available record of dietary magnesium intake.
Covariates included age, sex, education, health conditions, smoking status, body mass index, amount of physical activity, smoking status, and alcohol intake.
Over a 16-month period, participants completed an online questionnaire five times. Their responses were used to calculate daily magnesium intake. Foods of particular interest included leafy green vegetables, legumes, nuts, seeds, and whole grains, all of which are magnesium rich.
They used latent class analysis (LCA) to “identify mutually exclusive subgroup (classes) of magnesium intake trajectory separately for men and women.”
Men had a slightly higher prevalence of BP medication and diabetes, compared with women, and postmenopausal women had a higher prevalence of BP medication and diabetes, compared with premenopausal women.
Compared with lower baseline magnesium intake, higher baseline dietary intake of magnesium was associated with larger brain volumes in several regions in both men and women.
The latent class analysis identified three classes of magnesium intake:
In women in particular, the “high-decreasing” trajectory was significantly associated with larger brain volumes, compared with the “normal-stable” trajectory, while the “low-increasing” trajectory was associated with smaller brain volumes.
Even an increase of 1 mg of magnesium per day (above 350 mg/day) made a difference in brain volume, especially in women. The changes associated with every 1-mg increase are found in the table below:
Associations between magnesium and BP measures were “mostly nonsignificant,” the researchers say, and the neuroprotective effect of higher magnesium intake in the high-decreasing trajectory was greater in postmenopausal versus premenopausal women.
“Our models indicate that compared to somebody with a normal magnesium intake (~350 mg per day), somebody in the top quartile of magnesium intake (≥ 550 mg per day) would be predicted to have a ~0.20% larger GM and ~0.46% larger RHC,” the authors summarize.
“In a population with an average age of 55 years, this effect corresponds to ~1 year of typical aging,” they note. “In other words, if this effect is generalizable to other populations, a 41% increase in magnesium intake may lead to significantly better brain health.”
Although the exact mechanisms underlying magnesium’s protective effects are “not yet clearly understood, there’s considerable evidence that magnesium levels are related to better cardiovascular health. Magnesium supplementation has been found to decrease blood pressure – and high blood pressure is a well-established risk factor for dementia,” said Ms. Alateeq.
Association, not causation
Yuko Hara, PhD, director of Aging and Prevention, Alzheimer’s Drug Discovery Foundation, noted that the study is observational and therefore shows an association, not causation.
“People eating a high-magnesium diet may also be eating a brain-healthy diet and getting high levels of nutrients/minerals other than magnesium alone,” suggested Dr. Hara, who was not involved with the study.
She noted that many foods are good sources of magnesium, including spinach, almonds, cashews, legumes, yogurt, brown rice, and avocados.
“Eating a brain-healthy diet (for example, the Mediterranean diet) is one of the Seven Steps to Protect Your Cognitive Vitality that ADDF’s Cognitive Vitality promotes,” she said.
Open Access funding was enabled and organized by the Council of Australian University Librarians and its Member Institutions. Ms. Alateeq, her co-authors, and Dr. Hara declare no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM EUROPEAN JOURNAL OF NUTRITION
Specific brain damage links hypertension to cognitive impairment
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Poor bone health is a ‘robust’ dementia risk factor
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
After adjusting for relevant factors, adults with the lowest versus highest BMD at the femoral neck were 42% more likely to develop dementia over roughly 10 years.
“Our research has found a link between bone loss and dementia, but further studies are needed to better understand this connection between bone density and memory loss,” study investigator Mohammad Arfan Ikram, MD, PhD, with Erasmus University Medical Center in Rotterdam, the Netherlands, said in a statement.
“It’s possible that bone loss may occur already in the earliest phases of dementia, years before any clinical symptoms manifest themselves. If that were the case, bone loss could be an indicator of risk for dementia and people with bone loss could be targeted for screening and improved care,” Dr. Ikram added.
The study was published online in Neurology.
Common bedfellows
Low BMD and dementia commonly co-occur in the older population, with bone loss accelerating in dementia patients because of physical inactivity and poor nutrition. However, the extent to which bone loss already exists prior to the onset of dementia remains unclear.
The new findings are based on 3,651 adults (mean age 72 years, 58% women) in the Rotterdam Study who were free of dementia between 2002 and 2005. At that time, BMD at the femoral neck, lumbar spine, and total body were obtained using dual-energy radiography absorptiometry (DXA) and the trabecular bone score, which offers further details such as bone microarchitecture, was calculated. Participants were followed up until Jan. 1, 2020.
Analyses were adjusted for age, sex, education, physical activity, smoking status, body mass index, blood pressure, cholesterol, history of comorbidities (stroke and diabetes), and apolipoprotein E genotype.
During follow-up, 688 (19%) participants developed dementia, mostly Alzheimer’s disease (77%).
Throughout the entire follow-up period, lower BMD at the femoral neck (per standard deviation), but not at other bone sites, correlated with a higher risk for all-cause dementia (hazard ratio, 1.12; 95% confidence interval, 1.02-1.23) and Alzheimer’s disease (HR, 1.14; 95% CI, 1.02-1.28).
Within the first 10 years after baseline, the risk for dementia was greatest in individuals with the lowest BMD at the femoral neck (HR, 2.03; 95% CI, 1.39-2.96) and total body (HR, 1.42; 95% CI, 1.01-2.02) and lowest trabecular bone score (HR, 1.59; 95% CI, 1.11-2.28).
Only BMD at the femoral neck was related to incident all-cause dementia in the first 5 years of follow-up (HR, 2.13; 95% CI, 1.28-3.57).
These findings add “extra knowledge to previous findings that associations change with time, with the strength of the effect decreasing with increasing follow-up time,” the investigators noted.
They suggest that total BMD and trabecular bone score might occur as “prodromal features instead of causes of dementia and related toxic protein accumulation in the brain. In other words, persons with subclinical, incipient dementia may have poor bone health due to the dementia process instead of vice versa.”
The investigators noted that further research focusing on the predictive ability of BMD for dementia is necessary. “As an indicator of dementia risk, intervening in BMD may improve clinical care of these persons, especially considering the multicomorbidities and polypharmacy that are highly preventive in this group,” they concluded.
Little known bone-brain axis to blame?
In a comment, Shaheen Lakhan, MD, a neurologist and researcher in Boston, noted that “bone health is increasingly becoming front of mind in older adults. This study confirms an association between poor bone health – low bone mineral density and bone scores – and poor brain health.”
However, it’s unclear whether the link is causal – that is, whether poor bone health actually leads to poor brain health, and whether that can be staved off by directly supporting bone density,” Dr. Lakhan said.
“The link may very well be the little known ‘brain-bone axis’ – where our bones actually regulate our brain,” he added.
“Take for example the bone-generated hormone osteocalcin that crosses the blood-brain barrier and regulates brain functions like memory and cognition. Mice who don’t express the osteocalcin gene or are injected with antibodies that block osteocalcin actually have poor memory and worse anxiety,” Dr. Lakhan said.
“In any event, good bone health begins with healthy habits: a diet with plenty of calcium, vitamin D, and protein; a regimen of not just cardio, but also weight-bearing exercises; and staying clear of smoking and heavy alcohol intake,” he concluded.
The study was funded by Erasmus Medical Center and Erasmus University Rotterdam, the Netherlands Organization for Scientific Research, the Netherlands Organization for Health Research and Development, the Research Institute for Diseases in the Elderly, the Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission, and the Municipality of Rotterdam. Dr. Ikram and Dr. Lakhan report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Longer telomeres tied to better brain health
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
, new research suggests.
“This is the largest and most systematic investigation of telomere length and brain structure and function,” said Anya Topiwala, of the University of Oxford (England). “We found that longer telomeres associated with protection against dementia. The links with brain structure, we think, offer a possible mechanism for this protection. The hope is, by understanding the mechanism, new treatment targets could be uncovered,” Dr. Topiwala said.
The study was published online in PLOS ONE.
UK Biobank cohort
Telomeres form protective caps at the ends of chromosomes, and they progressively shorten with age, which may increase susceptibility to age-related diseases including Alzheimer’s disease. The mechanism underlying this risk is unclear and may involve changes in brain structure and function. However, the relationship between telomere length and neuroimaging markers is poorly characterized.
Dr. Topiwala and colleagues compared telomere length in white blood cells to brain MRI and health record data in 31,661 middle-aged and older adults in UK Biobank. They found that longer leucocyte telomere length (LTL) was associated with a larger volume of global and subcortical grey matter and a larger hippocampus – both of which shrink in patients with Alzheimer’s disease. Longer telomeres were also associated with a thicker cerebral cortex, which thins as Alzheimer’s disease progresses.
Longer LTL was also associated with reduced incidence of dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.91-0.96).
Dr. Topiwala noted that many of the factors related to telomere shortening, such as age, genetics, and sex, can’t be changed. However, in a previous study, her team found that drinking alcohol may shorten telomere length. “So by this logic, reducing your alcohol intake could curb the shortening,” Dr. Topiwala said.
She said that a limitation of the study is that telomere length was measured in blood rather than brain and that it’s not clear at present how closely the two relate. Also, UK Biobank participants are generally more healthy than is the general population. Also, though telomere length and brain measures were associated, “we cannot from this study prove one is causing the other,” she added.
Need for more research
Commenting on the research, Percy Griffin, PhD, Alzheimer’s Association director of scientific engagement, said that it’s been “known for some time that shortened telomeres – the caps at the end of DNA – are associated with increased aging.”
This new study is “interesting,” said Dr. Percy, in that it shows an association between longer telomere length in white blood cells and healthier brain structures in the areas associated with Alzheimer’s disease. The longer telomeres were also associated with lower incidence of all-cause dementia.
But echoing Dr. Topiwala, “association does not mean causation,” Dr. Griffin said. “More research is needed to understand how diverse mechanisms contributing to Alzheimer’s and other dementia can be targeted.”
“The Alzheimer’s Association is accelerating the discovery of novel therapies through its Part the Cloud funding program, which has invested more than $65 million to accelerate the development of 65 drug development programs,” Dr. Griffin said.
The study had no specific funding. Dr. Topiwala and Dr. Griffin report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Tooth loss and diabetes together hasten mental decline
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF DENTAL RESEARCH
Restless legs a new modifiable risk factor for dementia?
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
suggesting the disorder may be a risk factor for dementia or a very early noncognitive sign of dementia, researchers say.
In a large population-based cohort study, adults with RLS were significantly more likely to develop dementia over more than a decade than were their peers without RLS.
If confirmed in future studies, “regular check-ups for cognitive decline in older patients with RLS may facilitate earlier detection and intervention for those with dementia risk,” wrote investigators led by Eosu Kim, MD, PhD, with Yonsei University, Seoul, Republic of Korea.
The study was published online in Alzheimer’s Research and Therapy.
Sleep disorders and dementia
RLS is associated with poor sleep, depression/anxiety, poor diet, microvasculopathy, and hypoxia – all of which are known risk factors for dementia. However, the relationship between RLS and incident dementia has been unclear.
The researchers compared risk for all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) among 2,501 adults with newly diagnosed RLS and 9,977 matched control persons participating in the Korean National Health Insurance Service–Elderly Cohort, a nationwide population-based cohort of adults aged 60 and older.
The mean age of the cohort was 73 years; most of the participants were women (65%). Among all 12,478 participants, 874 (7%) developed all-cause dementia during follow-up – 475 (54%) developed AD, and 194 (22%) developed VaD.
The incidence of all-cause dementia was significantly higher among the RLS group than among the control group (10.4% vs. 6.2%). Incidence rates of AD and VaD (5.6% and 2.6%, respectively) were also higher in the RLS group than in the control group (3.4% and 1.3%, respectively).
In Cox regression analysis, RLS was significantly associated with an increased risk of all-cause dementia (adjusted hazard ratio [aHR], 1.46; 95% confidence interval [CI], 1.24-1.72), AD (aHR 1.38; 95% CI, 1.11-1.72) and VaD (aHR, 1.81; 95% CI, 1.30-2.53).
The researchers noted that RLS may precede deterioration of cognitive function, leading to dementia, and they suggest that RLS could be regarded as a “newly identified” risk factor or prodromal sign of dementia.
Modifiable risk factor
Reached for comment, Thanh Dang-Vu, MD, PhD, professor and research chair in sleep, neuroimaging, and cognitive health at Concordia University in Montreal, said there is now “increasing literature that shows sleep as a modifiable risk factor for cognitive decline.
“Previous evidence indicates that both sleep apnea and insomnia disorder increase the risk for cognitive decline and possibly dementia. Here the study adds to this body of evidence linking sleep disorders to dementia, suggesting that RLS should also be considered as a sleep-related risk factor,” Dr. Dang-Vu told this news organization.
“More evidence is needed, though, as here, all diagnoses were based on national health insurance diagnostic codes, and it is likely there were missed diagnoses for RLS but also for other sleep disorders, as there was no systematic screening for them,” Dr. Dang-Vu cautioned.
Support for the study was provided by the Ministry of Health and Welfare, the Korean government, and Yonsei University. Dr. Kim and Dr. Dang-Vu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S RESEARCH AND THERAPY
Bruce Willis’ frontotemporal dementia is not your grandpa’s dementia
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
What is remarkable about the swamp that we call FTD is that it’s a somewhat rare and unusual type of dementia. We tend to characterize dementia as the erosion of memory, but FTD is more characterized by the loss of control over emotions and other cognitive functions. What›s especially tragic for performers like Mr. Willis is the loss of the verbal fluency required for delivering one’s lines.
Frontotemporal dementia
To this casual observer, Bruce Willis was an almost invincible force, vigorous, vital, one of the “immortals.” Alas, with his FTD diagnosis, we know that even a die-hard like Mr. Willis, now only 67 years of age, may have to endure years of progressive decline. If the disease follows its typical path, that will probably include slowly disconnecting and progressively losing emotional judgment and control as well as losing a reasonable understanding of what or why any of it is happening. He may also experience a progressive deterioration of the control of bodily functions and general health.
Most people with dementia lose their neurocognitive abilities through a number of different pathways, all of which result in brain shrinkage, disconnection, evident neuropathology, neurobehavioral expressions of loss, and forms of befuddlement. Alzheimer’s disease leads the list as the most common form of dementia, but vascular dementias; dementia with Lewy bodies; “mixed” dementias; dementias associated with Parkinson’s, Huntington’s, or other diseases; dementia rising from alcoholic or other brain poisoning, HIV, Lyme disease, or a host of other brain infections; or from traumatic encephalopathy (chronic or more current) may present at any active neurology clinic. These are what you might think of as your “grandpa’s dementia” – the common types often associated with old age.
FTD is a particularly interesting variant for several reasons. First, it usually arises in relatively young individuals, with initial symptoms emerging in one’s 50s or 60s. In most cases, there is no genetic and, with rare exception, any other explanation of origin – except that old medical standby, bad luck.
Second, FTD has little initial impact on a patient’s broader memory and associated cognitive abilities. The patient will stumble to come up with that next word and ultimately slow down their speech as their brain struggles with verbal fluency; they will struggle with translating their feelings and emotions into fast and appropriate actions expressed in their mind and their physical body while their memory will appear intact.
In all other dementias, cognitive losses can be profound, whereas social and emotional control and voluble speech production are generally better sustained. Imagine the impact that these struggles in verbal fluency and in emotional calibration and response must have for an established actor. By all reports, Mr. Willis vigorously pursued the work that he loved right up until the time of his dementia diagnosis, even as his colleagues would almost certainly have seen that he was struggling. Sadly, a lack of that type of self-awareness is an expected consequence of FTD.
The salience network and von Economo neurons
Third and most intriguing to a neuroscientific nerd like me is that patients with FTD experience an initial loss of a special population of cortical neurons located within the salience network in our brains, called the von Economo neurons. That salience network is designed to quickly read and evaluate our complex thoughts and emotions and via those Economo neurons, initiate appropriate neurologic and physical responses.
We share this special von Economo machinery with great apes, whales, elephants, and a handful of other especially social mammalian species.
When we see or hear or otherwise sense something that induces fear, alarm, or a potential reward, the salience network in our brain acts as a kind of gatekeeper. First, it assesses the emergent or changing situation, then it rapidly initiates an emotional and physical response. As I sit with a patient in obvious distress in my office, my salience network turns on an empathetic alarm. My brain and body immediately adjust to initiate appropriately sympathetic reactions. The von Economo neurons – those very neurons that have substantially died off in a brain with FTD – are the linchpins in this fast-response emotion and complex body signal-informed system.
Controlled emotional response is at the heart of our humanity. It’s a sad day when we lose it.
In other neurologic clinical conditions marked by the loss of specific brain cells, different forms of “disuse atrophy” are partly the cause. We don’t know whether that’s the case for FTD. Scientists have shown that specific forms of computerized brain exercises can sharply increase activity levels in the salience network which is linked to improvements in the regulatory control of the autonomic nervous system – one of the key response-mediating targets of the network’s von Economo neurons.
Interestingly, superagers who sustain body and brain health into their 90s (and beyond) die with a full complement of von Economo neurons operating happily in a still-vigorous salience network.
This neuroscientist can foresee a day when we routinely assess the integrity of this important brain system and more reliably maintain its good health. Keeping those very special neurons alive would have probably allowed Mr. Willis to sustain himself on the soundstage and on the grander stage of life for a long time to come. Alas, like so many things in medicine, there is promise. But at this moment for this famous patient, our current medical science appears to be a day late, and a dollar short.
Dr. Merzenichis is professor emeritus at the University of California, San Francisco, and a Kavli Laureate in Neuroscience. He reported conflicts of interest with the National Institutes of Health, Stronger Brains, and Posit Science.
A version of this article first appeared on Medscape.com.
Older men more at risk as dangerous falls rise for all seniors
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.
When Senate Minority Leader Mitch McConnell (R-Ky.) fell recently at a dinner event in Washington, he unfortunately joined a large group of his senior citizen peers.
This wasn’t the first tumble the 81-year-old has taken. In 2019, he fell in his home, fracturing his shoulder. This time, he got a concussion and was recently released to an in-patient rehabilitation facility. While Sen. McConnell didn’t fracture his skull, in falling and hitting his head, he became part of an emerging statistic: One that reveals falls are more dangerous for senior men than senior women.
This new research, which appeared in the American Journal of Emergency Medicine, came as a surprise to lead researcher Scott Alter, MD, associate professor of emergency medicine at the Florida Atlantic University, Boca Raton.
“We always hear about lower bone density rates among females, so we didn’t expect to see males with more skull fractures,” he said.
Dr. Alter said that as a clinician in a southern Florida facility, his emergency department was the perfect study grounds to evaluate incoming geriatric patients due to falls. Older “patients are at higher risk of skull fractures and intercranial bleeding, and we wanted to look at any patient presenting with a head injury. Some 80% were fall related, however.”
The statistics bear out the fact that falls of all types are common among the elderly: Some 800,000 seniors wind up in the hospital each year because of falls.
The numbers show death rates from falls are on the rise in the senior citizen age group, too, up 30% from 2007 to 2016. Falls account for 70% of accidental deaths in people 75 and older. They are the leading cause of injury-related visits to emergency departments in the country, too.
Jennifer Stevens, MD, a gerontologist and executive director at Florida-based Abbey Delray South, is aware of the dire numbers and sees their consequences regularly. “The reasons seniors are at a high fall risk are many,” she said. “They include balance issues, declining strength, diseases like Parkinson’s and Alzheimer’s, side effects of their medications, and more.”
In addition, many seniors live in spaces that are not necessarily equipped for their limitations, and hazards exist all over their homes. Put together, and the risks for falls are everywhere. But there are steps seniors, their families, and even middle-aged people can take to mitigate and hopefully prevent dangerous falls.
Starting early
While in many cases the journey to lessen fall risks begins after a fall, the time to begin addressing the issue is long before you hit your senior years. Mary Therese Cole, a physical therapist and certified dementia practitioner at Manual Edge Physical Therapy in Colorado Springs, Colo., says that age 50 is a good time to start paying attention and addressing physical declines.
“This is an age where your vision might begin deteriorating,” she said. “It’s a big reason why elderly people trip and fall.”
As our brains begin to age in our middle years, the neural pathways from brain to extremities start to decline, too. The result is that many people stop picking up their feet as well as they used to do, making them more likely to trip.
“You’re not elderly yet, but you’re not a spring chicken, either,” Ms. Cole said. “Any issues you have now will only get worse if you’re not working on them.”
A good starting point in middle age, then, is to work on both strength training and balance exercises. A certified personal trainer or physical therapist can help get you on a program to ward off many of these declines.
If you’ve reached your later years, however, and are experiencing physical declines, it’s smart to check in with your primary care doctor for an assessment. “He or she can get your started on regular PT to evaluate any shortcomings and then address them,” Ms. Cole said.
She noted that when she’s working with senior patients, she’ll test their strength getting into and out of a chair, do a manual strength test to check on lower extremities, check their walking stride, and ask about conditions such as diabetes, former surgeries, and other conditions.
From there, Ms. Cole said she can write up a plan for the patient. Likewise, Dr. Stevens uses a program called Be Active that allows her to test seniors on a variety of measurements, including flexibility, balance, hand strength, and more.
“Then we match them with classes to address their shortcomings,” she said. “It’s critical that seniors have the ability to recover and not fall if they get knocked off balance.”
Beyond working on your physical limitations, taking a good look at your home is essential, too. “You can have an occupational therapist come to your home and do an evaluation,” Dr. Stevens said. “They can help you rearrange and reorganize for a safer environment.”
Big, common household fall hazards include throw rugs, lack of nightlights for middle-of-the-night visits to the bathroom, a lack of grab bars in the shower/bathtub, and furniture that blocks pathways.
For his part, Dr. Alter likes to point seniors and their doctors to the CDC’s STEADI program, which is aimed at stopping elderly accidents, deaths, and injuries.
“It includes screening for fall risk, assessing factors you can modify or improve, and more tools,” he said.
Dr. Alter also recommended seniors talk to their doctors about medications, particularly blood thinners.
“At a certain point, you need to weigh the benefits of disease prevention with the risk of injury if you fall,” he said. “The bleeding risk might be too high if the patient is at a high risk of falls.”
A version of this article originally appeared on WebMD.com.