User login
Early-Onset Asthma May Slow Memory Development
Children with asthma scored significantly lower than those without asthma on measures of episodic memory, based on longitudinal data from nearly 500 individuals.
Animal models have shown associations between asthma and memory problems, but data for children are lacking, wrote Nicholas J. Christopher-Hayes, MA, of the University of California, Davis, and colleagues.
“Asthma is very frequent among children, and there is mounting evidence from rodent models that asthma may result in neural injury in the hippocampus, which in turn may cause memory loss,” Christopher-Hayes said in an interview. “Although there is also a good amount of research with older adults, very little research has been done with children, the period that is most frequently linked to asthma onset,” he said. Therefore, the researchers leveraged a large national study on child development to examine development of memory as a function of asthma exposure.
In this study published in JAMA Network Open, the researchers conducted both a longitudinal and cross-sectional analysis of data from the Adolescent Brain Cognitive Development Study, which began in 2015. Children were enrolled at ages 9-10 years with a follow-up assessment 1-2 years later.
The participants were categorized as early childhood-onset asthma (asthma at baseline and follow-up), later childhood-onset asthma (asthma at follow-up only), or no asthma history. The primary outcome of the longitudinal analysis was episodic memory. Approximately half of the participants were boys, and slightly more than half were White.
Overall, those with early-onset asthma showed significantly lower rates of longitudinal memory improvements at follow-up compared with the comparison group (P < .01).
Developmental memory improvement in children with later-onset asthma was not significantly different from the control individuals.
Secondary outcomes included processing speed and inhibition, and attention. In a cross-sectional analysis with a larger sample of 2062 children from the same database (1031 with any asthma), those with asthma scored significantly lower on measures not only of episodic memory but also processing speed and inhibition/attention than children with no asthma, with P values of .04, .01, and .02, respectively.
The results were limited by several factors, including the reliance on parent reports for indicators of asthma and the lack of data on the potential effect of prescription corticosteroid use on neurocognitive development, the researchers noted.
The mechanism behind the association remains unclear; the inflammation associated with asthma may disrupt neural processing and manifest as cognitive dysfunction, as has been seen in rodent models of asthma, the researchers wrote. “It is possible that associations between asthma and developmental trajectories emerge earlier for memory, perhaps due to its sensitivity to subtle hippocampal injury,” they noted.
Longer follow-up studies are needed to fully understand how childhood asthma predicts memory declines or difficulties in childhood and beyond, said Christopher-Hayes. “We also need additional studies to understand why children who were diagnosed earlier and had asthma for longer seem to be particularly affected,” he said.
The results of this study were consistent with previous findings and therefore not surprising, senior author Simona Ghetti, PhD, a professor of psychology at the University of California, Davis, said in an interview. However, the finding that the extent of exposure to asthma was associated with slower memory improvement in childhood was striking, she said. That children with an earlier asthma onset who had disease indicators for a longer period showed a slower development of memory over time, suggests that asthma exposure may affect the developmental trajectory of memory, Ghetti noted.
“Recommendations to clinicians are premature because we need a better understanding of the boundary conditions, such as the minimal level of asthma exposure that might generate memory difficulties,” said Ghetti.
“Nevertheless, our results underscore the importance of looking at asthma as a potential source of cognitive difficulty in children,” she said.
Asthma’s Extensive Effect
Evidence is mounting that a diagnosis of asthma may have implications outside the pulmonary system, Diego J. Maselli, MD, professor and chief of the Division of Pulmonary Diseases & Critical Care at UT Health, San Antonio, said in an interview.
“Asthmatics may be at risk of nasal polyps, allergic rhinitis, and other allergic conditions, but there is emerging of evidence inflammation associated with asthma may affect other organ systems,” said Maselli, who was not involved in the study.
“For example, chronic inflammation in asthmatics may increase the risk of cardiovascular disease,” he said.
Although less is known about the effects of asthma on the nervous system, animal models suggest that inflammation associated with asthma may result in neuronal injury and potential effects on memory, said Maselli.
The findings of this study provide evidence of potential detrimental effects on the memory of children with asthma but should be interpreted with caution, Maselli said. “Children with chronic medical conditions may have an inherent disadvantage compared with their peers due to the burden of their disease, medication utilization and side effects, absenteeism from school, physical limitations, and other disease-specific circumstances,” he noted.
“Uncontrolled asthma, in particular, has strong links to low socioeconomic factors that are closely tied to access to adequate medical care, nutrition, educational institutions, and other relevant contributors to normal cognitive development,” Maselli said. Although the authors account for some of these socioeconomic factors by evaluating income and race, other variables may have influenced the results, he added.
Overall, this study’s findings suggested that the diagnosis of asthma in children may be associated with memory deficits and may influence neurodevelopment; however, more research is needed to determine whether the findings are replicated in other cohorts, said Maselli. “In particular, evaluating the effects of the severity of asthma and different asthma endotypes would be crucial to identify children with a higher risk of memory or cognitive deficits and confirm these associations,” he said.
This study was funded by the Memory and Plasticity Program at the University of California, Davis, and by a Learning, Memory, and Plasticity Training Program Fellowship grant from the National Institutes of Health. The researchers and Maselli had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Children with asthma scored significantly lower than those without asthma on measures of episodic memory, based on longitudinal data from nearly 500 individuals.
Animal models have shown associations between asthma and memory problems, but data for children are lacking, wrote Nicholas J. Christopher-Hayes, MA, of the University of California, Davis, and colleagues.
“Asthma is very frequent among children, and there is mounting evidence from rodent models that asthma may result in neural injury in the hippocampus, which in turn may cause memory loss,” Christopher-Hayes said in an interview. “Although there is also a good amount of research with older adults, very little research has been done with children, the period that is most frequently linked to asthma onset,” he said. Therefore, the researchers leveraged a large national study on child development to examine development of memory as a function of asthma exposure.
In this study published in JAMA Network Open, the researchers conducted both a longitudinal and cross-sectional analysis of data from the Adolescent Brain Cognitive Development Study, which began in 2015. Children were enrolled at ages 9-10 years with a follow-up assessment 1-2 years later.
The participants were categorized as early childhood-onset asthma (asthma at baseline and follow-up), later childhood-onset asthma (asthma at follow-up only), or no asthma history. The primary outcome of the longitudinal analysis was episodic memory. Approximately half of the participants were boys, and slightly more than half were White.
Overall, those with early-onset asthma showed significantly lower rates of longitudinal memory improvements at follow-up compared with the comparison group (P < .01).
Developmental memory improvement in children with later-onset asthma was not significantly different from the control individuals.
Secondary outcomes included processing speed and inhibition, and attention. In a cross-sectional analysis with a larger sample of 2062 children from the same database (1031 with any asthma), those with asthma scored significantly lower on measures not only of episodic memory but also processing speed and inhibition/attention than children with no asthma, with P values of .04, .01, and .02, respectively.
The results were limited by several factors, including the reliance on parent reports for indicators of asthma and the lack of data on the potential effect of prescription corticosteroid use on neurocognitive development, the researchers noted.
The mechanism behind the association remains unclear; the inflammation associated with asthma may disrupt neural processing and manifest as cognitive dysfunction, as has been seen in rodent models of asthma, the researchers wrote. “It is possible that associations between asthma and developmental trajectories emerge earlier for memory, perhaps due to its sensitivity to subtle hippocampal injury,” they noted.
Longer follow-up studies are needed to fully understand how childhood asthma predicts memory declines or difficulties in childhood and beyond, said Christopher-Hayes. “We also need additional studies to understand why children who were diagnosed earlier and had asthma for longer seem to be particularly affected,” he said.
The results of this study were consistent with previous findings and therefore not surprising, senior author Simona Ghetti, PhD, a professor of psychology at the University of California, Davis, said in an interview. However, the finding that the extent of exposure to asthma was associated with slower memory improvement in childhood was striking, she said. That children with an earlier asthma onset who had disease indicators for a longer period showed a slower development of memory over time, suggests that asthma exposure may affect the developmental trajectory of memory, Ghetti noted.
“Recommendations to clinicians are premature because we need a better understanding of the boundary conditions, such as the minimal level of asthma exposure that might generate memory difficulties,” said Ghetti.
“Nevertheless, our results underscore the importance of looking at asthma as a potential source of cognitive difficulty in children,” she said.
Asthma’s Extensive Effect
Evidence is mounting that a diagnosis of asthma may have implications outside the pulmonary system, Diego J. Maselli, MD, professor and chief of the Division of Pulmonary Diseases & Critical Care at UT Health, San Antonio, said in an interview.
“Asthmatics may be at risk of nasal polyps, allergic rhinitis, and other allergic conditions, but there is emerging of evidence inflammation associated with asthma may affect other organ systems,” said Maselli, who was not involved in the study.
“For example, chronic inflammation in asthmatics may increase the risk of cardiovascular disease,” he said.
Although less is known about the effects of asthma on the nervous system, animal models suggest that inflammation associated with asthma may result in neuronal injury and potential effects on memory, said Maselli.
The findings of this study provide evidence of potential detrimental effects on the memory of children with asthma but should be interpreted with caution, Maselli said. “Children with chronic medical conditions may have an inherent disadvantage compared with their peers due to the burden of their disease, medication utilization and side effects, absenteeism from school, physical limitations, and other disease-specific circumstances,” he noted.
“Uncontrolled asthma, in particular, has strong links to low socioeconomic factors that are closely tied to access to adequate medical care, nutrition, educational institutions, and other relevant contributors to normal cognitive development,” Maselli said. Although the authors account for some of these socioeconomic factors by evaluating income and race, other variables may have influenced the results, he added.
Overall, this study’s findings suggested that the diagnosis of asthma in children may be associated with memory deficits and may influence neurodevelopment; however, more research is needed to determine whether the findings are replicated in other cohorts, said Maselli. “In particular, evaluating the effects of the severity of asthma and different asthma endotypes would be crucial to identify children with a higher risk of memory or cognitive deficits and confirm these associations,” he said.
This study was funded by the Memory and Plasticity Program at the University of California, Davis, and by a Learning, Memory, and Plasticity Training Program Fellowship grant from the National Institutes of Health. The researchers and Maselli had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Children with asthma scored significantly lower than those without asthma on measures of episodic memory, based on longitudinal data from nearly 500 individuals.
Animal models have shown associations between asthma and memory problems, but data for children are lacking, wrote Nicholas J. Christopher-Hayes, MA, of the University of California, Davis, and colleagues.
“Asthma is very frequent among children, and there is mounting evidence from rodent models that asthma may result in neural injury in the hippocampus, which in turn may cause memory loss,” Christopher-Hayes said in an interview. “Although there is also a good amount of research with older adults, very little research has been done with children, the period that is most frequently linked to asthma onset,” he said. Therefore, the researchers leveraged a large national study on child development to examine development of memory as a function of asthma exposure.
In this study published in JAMA Network Open, the researchers conducted both a longitudinal and cross-sectional analysis of data from the Adolescent Brain Cognitive Development Study, which began in 2015. Children were enrolled at ages 9-10 years with a follow-up assessment 1-2 years later.
The participants were categorized as early childhood-onset asthma (asthma at baseline and follow-up), later childhood-onset asthma (asthma at follow-up only), or no asthma history. The primary outcome of the longitudinal analysis was episodic memory. Approximately half of the participants were boys, and slightly more than half were White.
Overall, those with early-onset asthma showed significantly lower rates of longitudinal memory improvements at follow-up compared with the comparison group (P < .01).
Developmental memory improvement in children with later-onset asthma was not significantly different from the control individuals.
Secondary outcomes included processing speed and inhibition, and attention. In a cross-sectional analysis with a larger sample of 2062 children from the same database (1031 with any asthma), those with asthma scored significantly lower on measures not only of episodic memory but also processing speed and inhibition/attention than children with no asthma, with P values of .04, .01, and .02, respectively.
The results were limited by several factors, including the reliance on parent reports for indicators of asthma and the lack of data on the potential effect of prescription corticosteroid use on neurocognitive development, the researchers noted.
The mechanism behind the association remains unclear; the inflammation associated with asthma may disrupt neural processing and manifest as cognitive dysfunction, as has been seen in rodent models of asthma, the researchers wrote. “It is possible that associations between asthma and developmental trajectories emerge earlier for memory, perhaps due to its sensitivity to subtle hippocampal injury,” they noted.
Longer follow-up studies are needed to fully understand how childhood asthma predicts memory declines or difficulties in childhood and beyond, said Christopher-Hayes. “We also need additional studies to understand why children who were diagnosed earlier and had asthma for longer seem to be particularly affected,” he said.
The results of this study were consistent with previous findings and therefore not surprising, senior author Simona Ghetti, PhD, a professor of psychology at the University of California, Davis, said in an interview. However, the finding that the extent of exposure to asthma was associated with slower memory improvement in childhood was striking, she said. That children with an earlier asthma onset who had disease indicators for a longer period showed a slower development of memory over time, suggests that asthma exposure may affect the developmental trajectory of memory, Ghetti noted.
“Recommendations to clinicians are premature because we need a better understanding of the boundary conditions, such as the minimal level of asthma exposure that might generate memory difficulties,” said Ghetti.
“Nevertheless, our results underscore the importance of looking at asthma as a potential source of cognitive difficulty in children,” she said.
Asthma’s Extensive Effect
Evidence is mounting that a diagnosis of asthma may have implications outside the pulmonary system, Diego J. Maselli, MD, professor and chief of the Division of Pulmonary Diseases & Critical Care at UT Health, San Antonio, said in an interview.
“Asthmatics may be at risk of nasal polyps, allergic rhinitis, and other allergic conditions, but there is emerging of evidence inflammation associated with asthma may affect other organ systems,” said Maselli, who was not involved in the study.
“For example, chronic inflammation in asthmatics may increase the risk of cardiovascular disease,” he said.
Although less is known about the effects of asthma on the nervous system, animal models suggest that inflammation associated with asthma may result in neuronal injury and potential effects on memory, said Maselli.
The findings of this study provide evidence of potential detrimental effects on the memory of children with asthma but should be interpreted with caution, Maselli said. “Children with chronic medical conditions may have an inherent disadvantage compared with their peers due to the burden of their disease, medication utilization and side effects, absenteeism from school, physical limitations, and other disease-specific circumstances,” he noted.
“Uncontrolled asthma, in particular, has strong links to low socioeconomic factors that are closely tied to access to adequate medical care, nutrition, educational institutions, and other relevant contributors to normal cognitive development,” Maselli said. Although the authors account for some of these socioeconomic factors by evaluating income and race, other variables may have influenced the results, he added.
Overall, this study’s findings suggested that the diagnosis of asthma in children may be associated with memory deficits and may influence neurodevelopment; however, more research is needed to determine whether the findings are replicated in other cohorts, said Maselli. “In particular, evaluating the effects of the severity of asthma and different asthma endotypes would be crucial to identify children with a higher risk of memory or cognitive deficits and confirm these associations,” he said.
This study was funded by the Memory and Plasticity Program at the University of California, Davis, and by a Learning, Memory, and Plasticity Training Program Fellowship grant from the National Institutes of Health. The researchers and Maselli had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Dementia Risk Higher for Stroke Survivors
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Risk for dementia is nearly 80% higher in stroke survivors than in those without stroke, a new study reveals. The data suggest risk declines within 1 year after stroke but remains elevated for up to 20 years.
METHODOLOGY:
- Researchers conducted a population-wide analysis of over 15 million people in Canada between 2002 and 2022. The study focused on adults hospitalized for ischemic stroke, intracerebral hemorrhage, or acute myocardial infarction (AMI).
- Of 175,980 stroke survivors, 99% were matched 1:1 to residents without stroke on the basis of age, sex, rural residence, neighborhood deprivation, and vascular comorbidities. In addition, 90% of patients were matched to those with AMI.
- Incident dementia diagnoses were tracked starting 90 days after stroke until death, emigration, or the end of the study, using a validated algorithm based on hospitalization for dementia, prescriptions for cholinesterase inhibitors, or physician claims within 2 years.
- The mean follow-up duration was 5.6 years.
TAKEAWAY:
- Among stroke survivors, 19% were diagnosed with dementia vs 12.5% in the reference population. The dementia rate per 100 person-years was higher among stroke survivors than in the reference population over the entire follow-up period (3.34 vs 1.89).
- Over the entire study period, dementia was 76% more likely among stroke patients (hazard ratio [HR], 1.76; 95% CI, 1.73-1.79) and 82% more likely in the AMI cohort (HR, 1.82; 95% CI, 1.79-1.85) than in the reference population.
- Time-varying analysis revealed that dementia risk was highest within the first year after stroke, with a > 2.5-fold increase at 6 months (HR, 2.51; 95% CI, 2.42-2.59), which decreased to a 1.5-fold increase at 5 years (HR, 1.51; 95% CI, 1.48-1.56) but remained elevated compared with the reference population even 20 years after the index stroke.
- Recurrent stroke was associated with an approximately threefold increased risk for dementia (single recurrent stroke adjusted HR, 2.64; 95% CI, 2.54-2.74; multiple recurrent strokes adjusted HR, 3.05; 95% CI, 2.81-3.33).
IN PRACTICE:
“While much research has been focused on reducing the risk of a second stroke, our findings make it clear that more research also is needed on developing interventions to help prevent dementia after stroke,” lead author Raed A. Joundi, MD, DPhil, McMaster University, Hamilton, Ontario, Canada, said in a press release.
“There is a need to accelerate the implementation of promising interventions or multipronged approaches into large randomized controlled trials to lower the risk of dementia,” the investigators wrote.
SOURCE:
The study was published online on December 4 in Neurology.
LIMITATIONS:
The study’s limitations included reliance on administrative coding without imaging data, potential underestimation of mild dementia, and lack of granular information on stroke severity, disability, and prestroke cognitive decline. While adjustments were made for healthcare contact and secondary prevention medications, residual biases may have persisted.
DISCLOSURES:
This study received funding from the Canada Brain Research Fund, Heart & Stroke Foundation of Canada, and Canadian Stroke Consortium. Two authors hold awards and positions from national organizations and academic institutions in Canada. Additional details are provided in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Can GLP-1s Reduce Alzheimer’s Disease Risk?
Tina is a lovely 67-year-old woman who was recently found to be an APOE gene carrier (a gene associated with increased risk of developing Alzheimer’s disease as well as an earlier age of disease onset), with diffused amyloid protein deposition her brain.
Her neuropsychiatric testing was consistent with mild cognitive impairment. Although Tina is not a doctor herself, her entire family consists of doctors, and she came to me under their advisement to consider semaglutide (Ozempic) for early Alzheimer’s disease prevention.
This would usually be simple, but in Tina’s case, there was a complicating factor: At 5’ and 90 pounds, she was already considerably underweight and was at risk of becoming severely undernourished.
To understand the potential role for glucagon-like peptide-1 (GLP-1) receptor agonists such as Ozempic in prevention, a quick primer on Alzheimer’s Disease is necessary.
The exact cause of Alzheimer’s disease remains elusive, but it is probably due to a combination of factors, including:
- Buildup of abnormal amyloid and tau proteins around brain cells
- Brain shrinkage, with subsequent damage to blood vessels and mitochondria, and inflammation
- Genetic predisposition
- Lifestyle factors, including obesity, high blood pressure, high cholesterol, and diabetes.
Once in the brain, they can reduce inflammation and improve functioning of the neurons. In early rodent trials, GLP-1 receptor agonists led to reduced amyloid and tau aggregation, downregulation of inflammation, and improved memory.
In 2021, multiple studies showed that liraglutide, an early GLP-1 receptor agonist, improved cognitive function and MRI volume in patients with Alzheimer’s disease.
A study recently published in Alzheimer’s & Dementia analyzed data from 1 million people with type 2 diabetes and no prior Alzheimer’s disease diagnosis. The authors compared Alzheimer’s disease occurrence in patients taking various diabetes medications, including insulin, metformin, and GLP-1 receptor agonists. The study found that participants taking semaglutide had up to a 70% reduction in Alzheimer’s risk. The results were consistent across gender, age, and weight.
Given the reassuring safety profile of GLP-1 receptor agonists and lack of other effective treatment or prophylaxis for Alzheimer’s disease, I agreed to start her on dulaglutide (Trulicity). My rationale was twofold:
1. In studies, dulaglutide has the highest uptake in the brain tissue at 68%. By contrast, there is virtually zero uptake in brain tissue for semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro/Zepbound). Because this class of drugs exert their effects in the brain tissue, I wanted to give her a GLP-1 receptor agonist with a high percent uptake.
2. Trulicity has a minimal effect on weight loss compared with the newer-generation GLP-1 receptor agonists. Even so, I connected Tina to my dietitian to ensure that she would receive a high-protein, high-calorie diet.
Tina has now been taking Trulicity for 6 months. Although it is certainly too early to draw firm conclusions about the efficacy of her treatment, she is not experiencing any weight loss and is cognitively stable, according to her neurologist.
The EVOKE and EVOKE+ phase 3 trials are currently underway to evaluate the efficacy of semaglutide to treat mild cognitive impairment and early Alzheimer’s in amyloid-positive patients. Results are expected in 2025, but in the meantime, I feel comforted knowing that Tina is receiving a potentially beneficial and definitively low-risk treatment.
Dr Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Tina is a lovely 67-year-old woman who was recently found to be an APOE gene carrier (a gene associated with increased risk of developing Alzheimer’s disease as well as an earlier age of disease onset), with diffused amyloid protein deposition her brain.
Her neuropsychiatric testing was consistent with mild cognitive impairment. Although Tina is not a doctor herself, her entire family consists of doctors, and she came to me under their advisement to consider semaglutide (Ozempic) for early Alzheimer’s disease prevention.
This would usually be simple, but in Tina’s case, there was a complicating factor: At 5’ and 90 pounds, she was already considerably underweight and was at risk of becoming severely undernourished.
To understand the potential role for glucagon-like peptide-1 (GLP-1) receptor agonists such as Ozempic in prevention, a quick primer on Alzheimer’s Disease is necessary.
The exact cause of Alzheimer’s disease remains elusive, but it is probably due to a combination of factors, including:
- Buildup of abnormal amyloid and tau proteins around brain cells
- Brain shrinkage, with subsequent damage to blood vessels and mitochondria, and inflammation
- Genetic predisposition
- Lifestyle factors, including obesity, high blood pressure, high cholesterol, and diabetes.
Once in the brain, they can reduce inflammation and improve functioning of the neurons. In early rodent trials, GLP-1 receptor agonists led to reduced amyloid and tau aggregation, downregulation of inflammation, and improved memory.
In 2021, multiple studies showed that liraglutide, an early GLP-1 receptor agonist, improved cognitive function and MRI volume in patients with Alzheimer’s disease.
A study recently published in Alzheimer’s & Dementia analyzed data from 1 million people with type 2 diabetes and no prior Alzheimer’s disease diagnosis. The authors compared Alzheimer’s disease occurrence in patients taking various diabetes medications, including insulin, metformin, and GLP-1 receptor agonists. The study found that participants taking semaglutide had up to a 70% reduction in Alzheimer’s risk. The results were consistent across gender, age, and weight.
Given the reassuring safety profile of GLP-1 receptor agonists and lack of other effective treatment or prophylaxis for Alzheimer’s disease, I agreed to start her on dulaglutide (Trulicity). My rationale was twofold:
1. In studies, dulaglutide has the highest uptake in the brain tissue at 68%. By contrast, there is virtually zero uptake in brain tissue for semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro/Zepbound). Because this class of drugs exert their effects in the brain tissue, I wanted to give her a GLP-1 receptor agonist with a high percent uptake.
2. Trulicity has a minimal effect on weight loss compared with the newer-generation GLP-1 receptor agonists. Even so, I connected Tina to my dietitian to ensure that she would receive a high-protein, high-calorie diet.
Tina has now been taking Trulicity for 6 months. Although it is certainly too early to draw firm conclusions about the efficacy of her treatment, she is not experiencing any weight loss and is cognitively stable, according to her neurologist.
The EVOKE and EVOKE+ phase 3 trials are currently underway to evaluate the efficacy of semaglutide to treat mild cognitive impairment and early Alzheimer’s in amyloid-positive patients. Results are expected in 2025, but in the meantime, I feel comforted knowing that Tina is receiving a potentially beneficial and definitively low-risk treatment.
Dr Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Tina is a lovely 67-year-old woman who was recently found to be an APOE gene carrier (a gene associated with increased risk of developing Alzheimer’s disease as well as an earlier age of disease onset), with diffused amyloid protein deposition her brain.
Her neuropsychiatric testing was consistent with mild cognitive impairment. Although Tina is not a doctor herself, her entire family consists of doctors, and she came to me under their advisement to consider semaglutide (Ozempic) for early Alzheimer’s disease prevention.
This would usually be simple, but in Tina’s case, there was a complicating factor: At 5’ and 90 pounds, she was already considerably underweight and was at risk of becoming severely undernourished.
To understand the potential role for glucagon-like peptide-1 (GLP-1) receptor agonists such as Ozempic in prevention, a quick primer on Alzheimer’s Disease is necessary.
The exact cause of Alzheimer’s disease remains elusive, but it is probably due to a combination of factors, including:
- Buildup of abnormal amyloid and tau proteins around brain cells
- Brain shrinkage, with subsequent damage to blood vessels and mitochondria, and inflammation
- Genetic predisposition
- Lifestyle factors, including obesity, high blood pressure, high cholesterol, and diabetes.
Once in the brain, they can reduce inflammation and improve functioning of the neurons. In early rodent trials, GLP-1 receptor agonists led to reduced amyloid and tau aggregation, downregulation of inflammation, and improved memory.
In 2021, multiple studies showed that liraglutide, an early GLP-1 receptor agonist, improved cognitive function and MRI volume in patients with Alzheimer’s disease.
A study recently published in Alzheimer’s & Dementia analyzed data from 1 million people with type 2 diabetes and no prior Alzheimer’s disease diagnosis. The authors compared Alzheimer’s disease occurrence in patients taking various diabetes medications, including insulin, metformin, and GLP-1 receptor agonists. The study found that participants taking semaglutide had up to a 70% reduction in Alzheimer’s risk. The results were consistent across gender, age, and weight.
Given the reassuring safety profile of GLP-1 receptor agonists and lack of other effective treatment or prophylaxis for Alzheimer’s disease, I agreed to start her on dulaglutide (Trulicity). My rationale was twofold:
1. In studies, dulaglutide has the highest uptake in the brain tissue at 68%. By contrast, there is virtually zero uptake in brain tissue for semaglutide (Ozempic/Wegovy) and tirzepatide (Mounjaro/Zepbound). Because this class of drugs exert their effects in the brain tissue, I wanted to give her a GLP-1 receptor agonist with a high percent uptake.
2. Trulicity has a minimal effect on weight loss compared with the newer-generation GLP-1 receptor agonists. Even so, I connected Tina to my dietitian to ensure that she would receive a high-protein, high-calorie diet.
Tina has now been taking Trulicity for 6 months. Although it is certainly too early to draw firm conclusions about the efficacy of her treatment, she is not experiencing any weight loss and is cognitively stable, according to her neurologist.
The EVOKE and EVOKE+ phase 3 trials are currently underway to evaluate the efficacy of semaglutide to treat mild cognitive impairment and early Alzheimer’s in amyloid-positive patients. Results are expected in 2025, but in the meantime, I feel comforted knowing that Tina is receiving a potentially beneficial and definitively low-risk treatment.
Dr Messer, Clinical Assistant Professor, Mount Sinai School of Medicine; Associate Professor, Hofstra School of Medicine, New York, NY, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Donepezil Shows Promise in TBI Recovery
TOPLINE:
Donepezil was associated with improved verbal memory and enhanced recall and processing speed, compared with placebo, in patients with severe traumatic brain injury (TBI), with a favorable safety profile despite mild to moderate gastrointestinal side effects.
METHODOLOGY:
- A four-site, randomized, parallel-group, double-blind, placebo-controlled, 10-week clinical trial (MEMRI-TBI-D) was conducted between 2013 and 2019 to evaluate the efficacy of donepezil for verbal memory impairments following severe TBI.
- 75 adults (75% men; mean age, 37 years) with complicated mild, moderate, or severe nonpenetrating TBI at least 6 months prior to study participation were included and randomly assigned to receive donepezil (n = 37) or placebo (n = 38).
- Participants received 5 mg donepezil daily or matching placebo for 2 weeks, then donepezil at 10 mg daily or matching placebo for 8 weeks; treatment was discontinued at 10 weeks, with an additional 4-week observation period.
- Verbal memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R). The primary outcome measure was verbal learning, evaluated through the HVLT-R total recall (ie, Total Trials 1-3) score.
TAKEAWAY:
- Compared with placebo, donepezil was associated with significantly greater improvements in verbal learning in both modified intent-to-treat and per-protocol analyses (P = .034 and .036, respectively).
- Treatment-responder rates were significantly higher in the donepezil group than in the placebo group (42 vs 18%; P = .03), with donepezil responders showing significant improvements in delayed recall and processing speed.
- Although there were no serious adverse events in either group, treatment-emergent adverse events were significantly more common in the donepezil group vs placebo (46% vs 8%; P < .001). No serious adverse events occurred in either group.
- Diarrhea and nausea were significantly more common in the donepezil group than in the placebo group (Fisher’s exact test: diarrhea, P = .03; nausea, P = .01).
IN PRACTICE:
“This study demonstrates the efficacy of donepezil on severe, persistent verbal memory impairments after predominantly severe TBI, with significant benefit for a subset of persons with such injuries, as well as a relatively favorable safety and tolerability profile,” the investigators wrote.
SOURCE:
The study was led by David B. Arciniegas, MD, University of Colorado School of Medicine, Aurora. It was published online in The Journal of Neuropsychiatry and Clinical Neurosciences.
LIMITATIONS:
The study included a relatively small sample with predominantly severe TBI requiring hospitalization and inpatient rehabilitation. The sample characteristics limit the generalizability of the findings to persons with other severities of TBI, other types of memory impairments, or more complex neuropsychiatric presentations. The study population had an average of 14 years of education, making generalizability to individuals with lower education levels uncertain. Additionally, while measures of information processing speed and immediate auditory attention were included, specific measures of sustained or selective attention were not, making it difficult to rule out improvements in higher-level attention as potential contributors to the observed verbal memory performance improvements.
DISCLOSURES:
The study was funded by the National Institute on Disability, Independent Living, and Rehabilitation Research, with in-kind support from TIRR Memorial Hermann. Four authors disclosed various financial and professional affiliations, including advisory roles with pharmaceutical and diagnostic companies, support from institutional awards, and involvement in programs funded by external organizations. One author served as the editor of The Journal of Neuropsychiatry and Clinical Neurosciences, with an independent editor overseeing the review and publication process for this article.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Donepezil was associated with improved verbal memory and enhanced recall and processing speed, compared with placebo, in patients with severe traumatic brain injury (TBI), with a favorable safety profile despite mild to moderate gastrointestinal side effects.
METHODOLOGY:
- A four-site, randomized, parallel-group, double-blind, placebo-controlled, 10-week clinical trial (MEMRI-TBI-D) was conducted between 2013 and 2019 to evaluate the efficacy of donepezil for verbal memory impairments following severe TBI.
- 75 adults (75% men; mean age, 37 years) with complicated mild, moderate, or severe nonpenetrating TBI at least 6 months prior to study participation were included and randomly assigned to receive donepezil (n = 37) or placebo (n = 38).
- Participants received 5 mg donepezil daily or matching placebo for 2 weeks, then donepezil at 10 mg daily or matching placebo for 8 weeks; treatment was discontinued at 10 weeks, with an additional 4-week observation period.
- Verbal memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R). The primary outcome measure was verbal learning, evaluated through the HVLT-R total recall (ie, Total Trials 1-3) score.
TAKEAWAY:
- Compared with placebo, donepezil was associated with significantly greater improvements in verbal learning in both modified intent-to-treat and per-protocol analyses (P = .034 and .036, respectively).
- Treatment-responder rates were significantly higher in the donepezil group than in the placebo group (42 vs 18%; P = .03), with donepezil responders showing significant improvements in delayed recall and processing speed.
- Although there were no serious adverse events in either group, treatment-emergent adverse events were significantly more common in the donepezil group vs placebo (46% vs 8%; P < .001). No serious adverse events occurred in either group.
- Diarrhea and nausea were significantly more common in the donepezil group than in the placebo group (Fisher’s exact test: diarrhea, P = .03; nausea, P = .01).
IN PRACTICE:
“This study demonstrates the efficacy of donepezil on severe, persistent verbal memory impairments after predominantly severe TBI, with significant benefit for a subset of persons with such injuries, as well as a relatively favorable safety and tolerability profile,” the investigators wrote.
SOURCE:
The study was led by David B. Arciniegas, MD, University of Colorado School of Medicine, Aurora. It was published online in The Journal of Neuropsychiatry and Clinical Neurosciences.
LIMITATIONS:
The study included a relatively small sample with predominantly severe TBI requiring hospitalization and inpatient rehabilitation. The sample characteristics limit the generalizability of the findings to persons with other severities of TBI, other types of memory impairments, or more complex neuropsychiatric presentations. The study population had an average of 14 years of education, making generalizability to individuals with lower education levels uncertain. Additionally, while measures of information processing speed and immediate auditory attention were included, specific measures of sustained or selective attention were not, making it difficult to rule out improvements in higher-level attention as potential contributors to the observed verbal memory performance improvements.
DISCLOSURES:
The study was funded by the National Institute on Disability, Independent Living, and Rehabilitation Research, with in-kind support from TIRR Memorial Hermann. Four authors disclosed various financial and professional affiliations, including advisory roles with pharmaceutical and diagnostic companies, support from institutional awards, and involvement in programs funded by external organizations. One author served as the editor of The Journal of Neuropsychiatry and Clinical Neurosciences, with an independent editor overseeing the review and publication process for this article.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Donepezil was associated with improved verbal memory and enhanced recall and processing speed, compared with placebo, in patients with severe traumatic brain injury (TBI), with a favorable safety profile despite mild to moderate gastrointestinal side effects.
METHODOLOGY:
- A four-site, randomized, parallel-group, double-blind, placebo-controlled, 10-week clinical trial (MEMRI-TBI-D) was conducted between 2013 and 2019 to evaluate the efficacy of donepezil for verbal memory impairments following severe TBI.
- 75 adults (75% men; mean age, 37 years) with complicated mild, moderate, or severe nonpenetrating TBI at least 6 months prior to study participation were included and randomly assigned to receive donepezil (n = 37) or placebo (n = 38).
- Participants received 5 mg donepezil daily or matching placebo for 2 weeks, then donepezil at 10 mg daily or matching placebo for 8 weeks; treatment was discontinued at 10 weeks, with an additional 4-week observation period.
- Verbal memory was assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R). The primary outcome measure was verbal learning, evaluated through the HVLT-R total recall (ie, Total Trials 1-3) score.
TAKEAWAY:
- Compared with placebo, donepezil was associated with significantly greater improvements in verbal learning in both modified intent-to-treat and per-protocol analyses (P = .034 and .036, respectively).
- Treatment-responder rates were significantly higher in the donepezil group than in the placebo group (42 vs 18%; P = .03), with donepezil responders showing significant improvements in delayed recall and processing speed.
- Although there were no serious adverse events in either group, treatment-emergent adverse events were significantly more common in the donepezil group vs placebo (46% vs 8%; P < .001). No serious adverse events occurred in either group.
- Diarrhea and nausea were significantly more common in the donepezil group than in the placebo group (Fisher’s exact test: diarrhea, P = .03; nausea, P = .01).
IN PRACTICE:
“This study demonstrates the efficacy of donepezil on severe, persistent verbal memory impairments after predominantly severe TBI, with significant benefit for a subset of persons with such injuries, as well as a relatively favorable safety and tolerability profile,” the investigators wrote.
SOURCE:
The study was led by David B. Arciniegas, MD, University of Colorado School of Medicine, Aurora. It was published online in The Journal of Neuropsychiatry and Clinical Neurosciences.
LIMITATIONS:
The study included a relatively small sample with predominantly severe TBI requiring hospitalization and inpatient rehabilitation. The sample characteristics limit the generalizability of the findings to persons with other severities of TBI, other types of memory impairments, or more complex neuropsychiatric presentations. The study population had an average of 14 years of education, making generalizability to individuals with lower education levels uncertain. Additionally, while measures of information processing speed and immediate auditory attention were included, specific measures of sustained or selective attention were not, making it difficult to rule out improvements in higher-level attention as potential contributors to the observed verbal memory performance improvements.
DISCLOSURES:
The study was funded by the National Institute on Disability, Independent Living, and Rehabilitation Research, with in-kind support from TIRR Memorial Hermann. Four authors disclosed various financial and professional affiliations, including advisory roles with pharmaceutical and diagnostic companies, support from institutional awards, and involvement in programs funded by external organizations. One author served as the editor of The Journal of Neuropsychiatry and Clinical Neurosciences, with an independent editor overseeing the review and publication process for this article.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Simufilam: Just Another Placebo
An Alzheimer’s drug trial failing is, unfortunately, nothing new. This one, however, had more baggage behind it than most.
Like all of these things, it was worth a try. It’s an interesting molecule with a reasonable mechanism of action.
But the trials have been raising questions for a few years, with allegations of misconduct against the drug’s co-discoverer Hoau-Yan Wang. He’s been indicted for defrauding the National Institutes of Health of $16 million in grants related to the drug. There have been concerns over doctored images and other not-so-minor issues in trying to move simufilam forward. Cassava itself agreed to pay the Securities and Exchange Commission $40 million in 2024 to settle charges about misleading investors.
Yet, like an innocent child with criminal parents, many of us hoped that the drug would work, regardless of the ethical shenanigans behind it. On the front lines we deal with a tragic disease that robs people of what makes them human and robs the families who have to live with it.
As the wheels started to come off the bus I told a friend, “it would be really sad if this drug is THE ONE and it never gets to finish trials because of everything else.”
Now we know it isn’t. Regardless of the controversy, the final data show that simufilam is just another placebo, joining the ranks of many others in the Alzheimer’s development graveyard.
Yes, there is a vague sense of jubilation behind it. I believe in fair play, and it’s good to know that those who misled investors and falsified data were wrong and will never have their day in the sun.
At the same time, however, I’m disappointed. I’m happy that the drug at least got a chance to prove itself, but when it’s all said and done, it doesn’t do anything.
I feel bad for the innocent people in the company, who had nothing to do with the scheming and were just hoping the drug would go somewhere. The majority, if not all, of them will likely lose their jobs. Like me, they have families, bills, and mortgages.
But I’m even more disappointed for the patients and families who only wanted an effective treatment for Alzheimer’s disease, and were hoping that, regardless of its dirty laundry, simufilam would work.
They’re the ones that I, and many other neurologists, have to face every day when they ask “is there anything new out?” and we sadly shake our heads.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
An Alzheimer’s drug trial failing is, unfortunately, nothing new. This one, however, had more baggage behind it than most.
Like all of these things, it was worth a try. It’s an interesting molecule with a reasonable mechanism of action.
But the trials have been raising questions for a few years, with allegations of misconduct against the drug’s co-discoverer Hoau-Yan Wang. He’s been indicted for defrauding the National Institutes of Health of $16 million in grants related to the drug. There have been concerns over doctored images and other not-so-minor issues in trying to move simufilam forward. Cassava itself agreed to pay the Securities and Exchange Commission $40 million in 2024 to settle charges about misleading investors.
Yet, like an innocent child with criminal parents, many of us hoped that the drug would work, regardless of the ethical shenanigans behind it. On the front lines we deal with a tragic disease that robs people of what makes them human and robs the families who have to live with it.
As the wheels started to come off the bus I told a friend, “it would be really sad if this drug is THE ONE and it never gets to finish trials because of everything else.”
Now we know it isn’t. Regardless of the controversy, the final data show that simufilam is just another placebo, joining the ranks of many others in the Alzheimer’s development graveyard.
Yes, there is a vague sense of jubilation behind it. I believe in fair play, and it’s good to know that those who misled investors and falsified data were wrong and will never have their day in the sun.
At the same time, however, I’m disappointed. I’m happy that the drug at least got a chance to prove itself, but when it’s all said and done, it doesn’t do anything.
I feel bad for the innocent people in the company, who had nothing to do with the scheming and were just hoping the drug would go somewhere. The majority, if not all, of them will likely lose their jobs. Like me, they have families, bills, and mortgages.
But I’m even more disappointed for the patients and families who only wanted an effective treatment for Alzheimer’s disease, and were hoping that, regardless of its dirty laundry, simufilam would work.
They’re the ones that I, and many other neurologists, have to face every day when they ask “is there anything new out?” and we sadly shake our heads.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
An Alzheimer’s drug trial failing is, unfortunately, nothing new. This one, however, had more baggage behind it than most.
Like all of these things, it was worth a try. It’s an interesting molecule with a reasonable mechanism of action.
But the trials have been raising questions for a few years, with allegations of misconduct against the drug’s co-discoverer Hoau-Yan Wang. He’s been indicted for defrauding the National Institutes of Health of $16 million in grants related to the drug. There have been concerns over doctored images and other not-so-minor issues in trying to move simufilam forward. Cassava itself agreed to pay the Securities and Exchange Commission $40 million in 2024 to settle charges about misleading investors.
Yet, like an innocent child with criminal parents, many of us hoped that the drug would work, regardless of the ethical shenanigans behind it. On the front lines we deal with a tragic disease that robs people of what makes them human and robs the families who have to live with it.
As the wheels started to come off the bus I told a friend, “it would be really sad if this drug is THE ONE and it never gets to finish trials because of everything else.”
Now we know it isn’t. Regardless of the controversy, the final data show that simufilam is just another placebo, joining the ranks of many others in the Alzheimer’s development graveyard.
Yes, there is a vague sense of jubilation behind it. I believe in fair play, and it’s good to know that those who misled investors and falsified data were wrong and will never have their day in the sun.
At the same time, however, I’m disappointed. I’m happy that the drug at least got a chance to prove itself, but when it’s all said and done, it doesn’t do anything.
I feel bad for the innocent people in the company, who had nothing to do with the scheming and were just hoping the drug would go somewhere. The majority, if not all, of them will likely lose their jobs. Like me, they have families, bills, and mortgages.
But I’m even more disappointed for the patients and families who only wanted an effective treatment for Alzheimer’s disease, and were hoping that, regardless of its dirty laundry, simufilam would work.
They’re the ones that I, and many other neurologists, have to face every day when they ask “is there anything new out?” and we sadly shake our heads.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Urinary Metals Linked to Increased Dementia Risk
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This multicenter prospective cohort study included 6303 participants from six US study centers from 2000 to 2002, with follow-up through 2018.
- Participants were aged 45-84 years (median age at baseline, 60 years; 52% women) and were free of diagnosed cardiovascular disease.
- Researchers measured urinary levels of arsenic, cadmium, cobalt, copper, lead, manganese, tungsten, uranium, and zinc.
- Neuropsychological assessments included the Digit Symbol Coding, Cognitive Abilities Screening Instrument, and Digit Span tests.
- The median follow-up duration was 11.7 years for participants with dementia and 16.8 years for those without; 559 cases of dementia were identified during the study.
TAKEAWAY:
- Lower Digit Symbol Coding scores were associated with higher urinary concentrations of arsenic (mean difference [MD] in score per interquartile range [IQR] increase, –0.03), cobalt (MD per IQR increase, –0.05), copper (MD per IQR increase, –0.05), uranium (MD per IQR increase, –0.04), and zinc (MD per IQR increase, –0.03).
- Effects for cobalt, uranium, and zinc were stronger in apolipoprotein epsilon 4 allele (APOE4) carriers vs noncarriers.
- Higher urinary levels of copper were associated with lower Digit Span scores (MD, –0.043) and elevated levels of copper (MD, –0.028) and zinc (MD, –0.024) were associated with lower global cognitive scores.
- Individuals with urinary levels of the nine-metal mixture at the 95th percentile had a 71% higher risk for dementia compared to those with levels at the 25th percentile, with the risk more pronounced in APOE4 carriers than in noncarriers (MD, –0.30 vs –0.10, respectively).
IN PRACTICE:
“We found an inverse association of essential and nonessential metals in urine, both individually and as a mixture, with the speed of mental operations, as well as a positive association of urinary metal levels with dementia risk. As metal exposure and levels in the body are modifiable, these findings could inform early screening and precision interventions for dementia prevention based on individuals’ metal exposure and genetic profiles,” the investigators wrote.
SOURCE:
The study was led by Arce Domingo-Relloso, PhD, Columbia University Mailman School of Public Health, New York City. It was published online in JAMA Network Open.
LIMITATIONS:
Data may have been missed for patients with dementia who were never hospitalized, died, or were lost to follow-up. The dementia diagnosis included nonspecific International Classification of Diseases codes, potentially leading to false-positive reports. In addition, the sample size was not sufficient to evaluate the associations between metal exposure and cognitive test scores for carriers of two APOE4 alleles.
DISCLOSURES:
The study was supported by the National Heart, Lung, and Blood Institute. Several authors reported receiving grants from the National Institutes of Health and consulting fees, editorial stipends, teaching fees, or unrelated grant funding from various sources, which are fully listed in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Common Gut Infection Tied to Alzheimer’s Disease
Researchers are gaining new insight into the relationship between the human cytomegalovirus (HCMV), a common herpes virus found in the gut, and the immune response associated with CD83 antibody in some individuals with Alzheimer’s disease (AD).
Using tissue samples from deceased donors with AD, the study showed CD83-positive (CD83+) microglia in the superior frontal gyrus (SFG) are significantly associated with elevated immunoglobulin gamma 4 (IgG4) and HCMV in the transverse colon (TC), increased anti-HCMV IgG4 in the cerebrospinal fluid (CSF), and both HCMV and IgG4 in the SFG and vagus nerve.
“Our results indicate a complex, cross-tissue interaction between HCMV and the host adaptive immune response associated with CD83+ microglia in persons with AD,” noted the investigators, including Benjamin P. Readhead, MBBS, research associate professor, ASU-Banner Neurodegenerative Disease Research Center, Arizona State University, Tempe.
The results suggest antiviral therapy in patients with biomarker evidence of HCMV, IgG4, or CD83+ microglia might ward off dementia.
“We’re preparing to conduct a clinical trial to evaluate whether careful use of existing antivirals might be clinically helpful in preventing or slowing progression of CD83+ associated Alzheimer’s disease,” Readhead said in an interview.
The study was published on December 19, 2024, in Alzheimer’s & Dementia.
Vagus Nerve a Potential Pathway?
CMV is a common virus. In the United States, nearly one in three children are already infected with CMV by age 5 years. Over half the adults have been infected with CMV by age 40 years, the Centers for Disease Control and Prevention reported.
It is typically passed through bodily fluids and spread only when the virus is active. It’s not considered a sexually transmitted disease.
Compared with other IgG subclasses, IgG4 is believed to be a less inflammatory, and therefore less damaging, immune response. But this response may be less effective at clearing infections and allow invasion of HCMV into the brain.
The researchers previously found a CD83+ microglial subtype in the SFG of 47% of brain donors with AD vs 25% of unaffected control individuals. They reported this subtype is associated with increased IgG4 in the TC.
The current analysis extends investigations of the potential etiology and clinicopathologic relevance of CD83+ microglia in the context of AD.
Researchers conducted experiments using donated tissue samples from deceased patients with AD and control individuals. Sources for these samples included the Banner cohort, for whom classifications for the presence of CD83+ microglia were available, as were tissue samples from the SFG, TC, and vagus nerve, and the Religious Orders Study and Rush Memory and Aging Project (ROSMAP), in which participants without known dementia are evaluated annually.
From the Banner cohort, researchers completed immunohistochemistry (IHC) studies on 34 SFG samples (21 AD and 13 control individuals) and included 25 TC samples (13 AD and 12 control individuals) and 8 vagal nerve samples (6 AD and 2 control individuals) in the study. From the ROSMAP cohort, they completed IHC studies on 27 prefrontal cortex samples from individuals with AD.
They carefully selected these samples to ensure matching for critical factors such as postmortem interval, age, and sex, as well as other relevant covariates, said the authors.
The study verified that CD83+ microglia are associated with IgG4 and HCMV in the TC and showed a significant association between CD83+ microglia and IgG4 immunoreactivity in the TC.
Investigators confirmed HCMV positivity in all nine CD83+ TC samples evaluated and in one CD83– TC sample, indicating a strong positive association between HCMV within the TC and CD83+ microglia within the SFG.
HCMV IgG seroprevalence is common, varies by age and comorbidity, and is present in 79% of 85-year-olds, the investigators noted. “Despite this, we note that HCMV presence in the TC was not ubiquitous and was significantly associated with CD83+ microglia and HCMV in the SFG,” they wrote.
This observation, they added, “may help reconcile how a common pathogen might contribute to a disease that most individuals do not develop.”
The experiments also uncovered increased anti-HCMV IgG4 in the CSF and evidence of HCMV and IgG4 in the vagus nerve.
“Overall, the histochemical staining patterns observed in TC, SFG, and vagus nerve of CD83+ subjects are consistent with active HCMV infection,” the investigators wrote. “Taken together, these results indicate a multiorgan presence of IgG4 and HCMV in subjects with CD83+ microglia within the SFG,” they added.
Accelerated AD Pathology
The team showed HCMV infection accelerates production of two pathologic features of AD — amyloid beta (Abeta) and tau — and causes neuronal death. “We observed high, positive correlations between the abundance of HCMV, and both Abeta42 and pTau-212,” they wrote.
As HCMV histochemistry is consistent with an active HCMV infection, the findings “may indicate an opportunity for the administration of antiviral therapy in subjects with AD and biomarker evidence of HCMV, IgG4, or CD83+ microglia,” they added.
In addition to planning a clinical trial of existing antivirals, the research team is developing a blood test that can help identify patients with an active HCMV infection who might benefit from such an intervention, said Readhead.
But he emphasized that the research is still in its infancy. “Our study is best understood as a series of interesting scientific findings that warrant further exploration, replication, and validation in additional study populations.”
Although it’s too early for the study to impact practice, “we’re motivated to understand whether these findings have implications for clinical care,” he added.
Tipping the Balance
A number of experts have weighed in on the research via the Science Media Center, an independent forum featuring the voices and views on science news from experts in the field.
Andrew Doig, PhD, professor, Division of Neuroscience, University of Manchester in England, said the new work supports the hypothesis that HCMV might be a trigger that tips the balance from a healthy brain to one with dementia. “If so, antiviral drugs against HCMV might be beneficial in reducing the risk of AD.”
Doig noted newly approved drugs for AD are expensive, provide only a small benefit, and have significant risks, such as causing brain hemorrhages. “Antiviral drugs are an attractive alternative that are well worth exploring.”
Richard Oakley, PhD, associate director of research and innovation, Alzheimer’s Society, cautioned the study only established a connection and didn’t directly show the virus leads to AD. “Also, the virus is not found in the brain of everyone with Alzheimer’s disease, the most common form of dementia.”
The significance of the new findings is “far from clear,” commented William McEwan, PhD, group leader at the UK Dementia Research Institute at Cambridge, England. “The study does not address how common this infection is in people without Alzheimer’s and therefore cannot by itself suggest that HCMV infection, or the associated immune response, is a driver of disease.”
The experts agreed follow-up research is needed to confirm these new findings and understand what they mean.
The study received support from the National Institute on Aging, National Institutes of Health, Global Lyme Alliance, National Institute of Neurological Disorders and Stroke, Arizona Alzheimer’s Consortium, The Benter Foundation, and NOMIS Stiftung. Readhead is a coinventor on a patent application for an IgG4-based peripheral biomarker for the detection of CD83+ microglia. Doig is a founder, director, and consultant for PharmaKure, which works on AD drugs and diagnostics, although not on viruses. He has cowritten a review on Viral Involvement in Alzheimer’s Disease. McEwan reported receiving research funding from Takeda Pharmaceuticals and is a founder and consultant to Trimtech Therapeutics.
A version of this article first appeared on Medscape.com.
Researchers are gaining new insight into the relationship between the human cytomegalovirus (HCMV), a common herpes virus found in the gut, and the immune response associated with CD83 antibody in some individuals with Alzheimer’s disease (AD).
Using tissue samples from deceased donors with AD, the study showed CD83-positive (CD83+) microglia in the superior frontal gyrus (SFG) are significantly associated with elevated immunoglobulin gamma 4 (IgG4) and HCMV in the transverse colon (TC), increased anti-HCMV IgG4 in the cerebrospinal fluid (CSF), and both HCMV and IgG4 in the SFG and vagus nerve.
“Our results indicate a complex, cross-tissue interaction between HCMV and the host adaptive immune response associated with CD83+ microglia in persons with AD,” noted the investigators, including Benjamin P. Readhead, MBBS, research associate professor, ASU-Banner Neurodegenerative Disease Research Center, Arizona State University, Tempe.
The results suggest antiviral therapy in patients with biomarker evidence of HCMV, IgG4, or CD83+ microglia might ward off dementia.
“We’re preparing to conduct a clinical trial to evaluate whether careful use of existing antivirals might be clinically helpful in preventing or slowing progression of CD83+ associated Alzheimer’s disease,” Readhead said in an interview.
The study was published on December 19, 2024, in Alzheimer’s & Dementia.
Vagus Nerve a Potential Pathway?
CMV is a common virus. In the United States, nearly one in three children are already infected with CMV by age 5 years. Over half the adults have been infected with CMV by age 40 years, the Centers for Disease Control and Prevention reported.
It is typically passed through bodily fluids and spread only when the virus is active. It’s not considered a sexually transmitted disease.
Compared with other IgG subclasses, IgG4 is believed to be a less inflammatory, and therefore less damaging, immune response. But this response may be less effective at clearing infections and allow invasion of HCMV into the brain.
The researchers previously found a CD83+ microglial subtype in the SFG of 47% of brain donors with AD vs 25% of unaffected control individuals. They reported this subtype is associated with increased IgG4 in the TC.
The current analysis extends investigations of the potential etiology and clinicopathologic relevance of CD83+ microglia in the context of AD.
Researchers conducted experiments using donated tissue samples from deceased patients with AD and control individuals. Sources for these samples included the Banner cohort, for whom classifications for the presence of CD83+ microglia were available, as were tissue samples from the SFG, TC, and vagus nerve, and the Religious Orders Study and Rush Memory and Aging Project (ROSMAP), in which participants without known dementia are evaluated annually.
From the Banner cohort, researchers completed immunohistochemistry (IHC) studies on 34 SFG samples (21 AD and 13 control individuals) and included 25 TC samples (13 AD and 12 control individuals) and 8 vagal nerve samples (6 AD and 2 control individuals) in the study. From the ROSMAP cohort, they completed IHC studies on 27 prefrontal cortex samples from individuals with AD.
They carefully selected these samples to ensure matching for critical factors such as postmortem interval, age, and sex, as well as other relevant covariates, said the authors.
The study verified that CD83+ microglia are associated with IgG4 and HCMV in the TC and showed a significant association between CD83+ microglia and IgG4 immunoreactivity in the TC.
Investigators confirmed HCMV positivity in all nine CD83+ TC samples evaluated and in one CD83– TC sample, indicating a strong positive association between HCMV within the TC and CD83+ microglia within the SFG.
HCMV IgG seroprevalence is common, varies by age and comorbidity, and is present in 79% of 85-year-olds, the investigators noted. “Despite this, we note that HCMV presence in the TC was not ubiquitous and was significantly associated with CD83+ microglia and HCMV in the SFG,” they wrote.
This observation, they added, “may help reconcile how a common pathogen might contribute to a disease that most individuals do not develop.”
The experiments also uncovered increased anti-HCMV IgG4 in the CSF and evidence of HCMV and IgG4 in the vagus nerve.
“Overall, the histochemical staining patterns observed in TC, SFG, and vagus nerve of CD83+ subjects are consistent with active HCMV infection,” the investigators wrote. “Taken together, these results indicate a multiorgan presence of IgG4 and HCMV in subjects with CD83+ microglia within the SFG,” they added.
Accelerated AD Pathology
The team showed HCMV infection accelerates production of two pathologic features of AD — amyloid beta (Abeta) and tau — and causes neuronal death. “We observed high, positive correlations between the abundance of HCMV, and both Abeta42 and pTau-212,” they wrote.
As HCMV histochemistry is consistent with an active HCMV infection, the findings “may indicate an opportunity for the administration of antiviral therapy in subjects with AD and biomarker evidence of HCMV, IgG4, or CD83+ microglia,” they added.
In addition to planning a clinical trial of existing antivirals, the research team is developing a blood test that can help identify patients with an active HCMV infection who might benefit from such an intervention, said Readhead.
But he emphasized that the research is still in its infancy. “Our study is best understood as a series of interesting scientific findings that warrant further exploration, replication, and validation in additional study populations.”
Although it’s too early for the study to impact practice, “we’re motivated to understand whether these findings have implications for clinical care,” he added.
Tipping the Balance
A number of experts have weighed in on the research via the Science Media Center, an independent forum featuring the voices and views on science news from experts in the field.
Andrew Doig, PhD, professor, Division of Neuroscience, University of Manchester in England, said the new work supports the hypothesis that HCMV might be a trigger that tips the balance from a healthy brain to one with dementia. “If so, antiviral drugs against HCMV might be beneficial in reducing the risk of AD.”
Doig noted newly approved drugs for AD are expensive, provide only a small benefit, and have significant risks, such as causing brain hemorrhages. “Antiviral drugs are an attractive alternative that are well worth exploring.”
Richard Oakley, PhD, associate director of research and innovation, Alzheimer’s Society, cautioned the study only established a connection and didn’t directly show the virus leads to AD. “Also, the virus is not found in the brain of everyone with Alzheimer’s disease, the most common form of dementia.”
The significance of the new findings is “far from clear,” commented William McEwan, PhD, group leader at the UK Dementia Research Institute at Cambridge, England. “The study does not address how common this infection is in people without Alzheimer’s and therefore cannot by itself suggest that HCMV infection, or the associated immune response, is a driver of disease.”
The experts agreed follow-up research is needed to confirm these new findings and understand what they mean.
The study received support from the National Institute on Aging, National Institutes of Health, Global Lyme Alliance, National Institute of Neurological Disorders and Stroke, Arizona Alzheimer’s Consortium, The Benter Foundation, and NOMIS Stiftung. Readhead is a coinventor on a patent application for an IgG4-based peripheral biomarker for the detection of CD83+ microglia. Doig is a founder, director, and consultant for PharmaKure, which works on AD drugs and diagnostics, although not on viruses. He has cowritten a review on Viral Involvement in Alzheimer’s Disease. McEwan reported receiving research funding from Takeda Pharmaceuticals and is a founder and consultant to Trimtech Therapeutics.
A version of this article first appeared on Medscape.com.
Researchers are gaining new insight into the relationship between the human cytomegalovirus (HCMV), a common herpes virus found in the gut, and the immune response associated with CD83 antibody in some individuals with Alzheimer’s disease (AD).
Using tissue samples from deceased donors with AD, the study showed CD83-positive (CD83+) microglia in the superior frontal gyrus (SFG) are significantly associated with elevated immunoglobulin gamma 4 (IgG4) and HCMV in the transverse colon (TC), increased anti-HCMV IgG4 in the cerebrospinal fluid (CSF), and both HCMV and IgG4 in the SFG and vagus nerve.
“Our results indicate a complex, cross-tissue interaction between HCMV and the host adaptive immune response associated with CD83+ microglia in persons with AD,” noted the investigators, including Benjamin P. Readhead, MBBS, research associate professor, ASU-Banner Neurodegenerative Disease Research Center, Arizona State University, Tempe.
The results suggest antiviral therapy in patients with biomarker evidence of HCMV, IgG4, or CD83+ microglia might ward off dementia.
“We’re preparing to conduct a clinical trial to evaluate whether careful use of existing antivirals might be clinically helpful in preventing or slowing progression of CD83+ associated Alzheimer’s disease,” Readhead said in an interview.
The study was published on December 19, 2024, in Alzheimer’s & Dementia.
Vagus Nerve a Potential Pathway?
CMV is a common virus. In the United States, nearly one in three children are already infected with CMV by age 5 years. Over half the adults have been infected with CMV by age 40 years, the Centers for Disease Control and Prevention reported.
It is typically passed through bodily fluids and spread only when the virus is active. It’s not considered a sexually transmitted disease.
Compared with other IgG subclasses, IgG4 is believed to be a less inflammatory, and therefore less damaging, immune response. But this response may be less effective at clearing infections and allow invasion of HCMV into the brain.
The researchers previously found a CD83+ microglial subtype in the SFG of 47% of brain donors with AD vs 25% of unaffected control individuals. They reported this subtype is associated with increased IgG4 in the TC.
The current analysis extends investigations of the potential etiology and clinicopathologic relevance of CD83+ microglia in the context of AD.
Researchers conducted experiments using donated tissue samples from deceased patients with AD and control individuals. Sources for these samples included the Banner cohort, for whom classifications for the presence of CD83+ microglia were available, as were tissue samples from the SFG, TC, and vagus nerve, and the Religious Orders Study and Rush Memory and Aging Project (ROSMAP), in which participants without known dementia are evaluated annually.
From the Banner cohort, researchers completed immunohistochemistry (IHC) studies on 34 SFG samples (21 AD and 13 control individuals) and included 25 TC samples (13 AD and 12 control individuals) and 8 vagal nerve samples (6 AD and 2 control individuals) in the study. From the ROSMAP cohort, they completed IHC studies on 27 prefrontal cortex samples from individuals with AD.
They carefully selected these samples to ensure matching for critical factors such as postmortem interval, age, and sex, as well as other relevant covariates, said the authors.
The study verified that CD83+ microglia are associated with IgG4 and HCMV in the TC and showed a significant association between CD83+ microglia and IgG4 immunoreactivity in the TC.
Investigators confirmed HCMV positivity in all nine CD83+ TC samples evaluated and in one CD83– TC sample, indicating a strong positive association between HCMV within the TC and CD83+ microglia within the SFG.
HCMV IgG seroprevalence is common, varies by age and comorbidity, and is present in 79% of 85-year-olds, the investigators noted. “Despite this, we note that HCMV presence in the TC was not ubiquitous and was significantly associated with CD83+ microglia and HCMV in the SFG,” they wrote.
This observation, they added, “may help reconcile how a common pathogen might contribute to a disease that most individuals do not develop.”
The experiments also uncovered increased anti-HCMV IgG4 in the CSF and evidence of HCMV and IgG4 in the vagus nerve.
“Overall, the histochemical staining patterns observed in TC, SFG, and vagus nerve of CD83+ subjects are consistent with active HCMV infection,” the investigators wrote. “Taken together, these results indicate a multiorgan presence of IgG4 and HCMV in subjects with CD83+ microglia within the SFG,” they added.
Accelerated AD Pathology
The team showed HCMV infection accelerates production of two pathologic features of AD — amyloid beta (Abeta) and tau — and causes neuronal death. “We observed high, positive correlations between the abundance of HCMV, and both Abeta42 and pTau-212,” they wrote.
As HCMV histochemistry is consistent with an active HCMV infection, the findings “may indicate an opportunity for the administration of antiviral therapy in subjects with AD and biomarker evidence of HCMV, IgG4, or CD83+ microglia,” they added.
In addition to planning a clinical trial of existing antivirals, the research team is developing a blood test that can help identify patients with an active HCMV infection who might benefit from such an intervention, said Readhead.
But he emphasized that the research is still in its infancy. “Our study is best understood as a series of interesting scientific findings that warrant further exploration, replication, and validation in additional study populations.”
Although it’s too early for the study to impact practice, “we’re motivated to understand whether these findings have implications for clinical care,” he added.
Tipping the Balance
A number of experts have weighed in on the research via the Science Media Center, an independent forum featuring the voices and views on science news from experts in the field.
Andrew Doig, PhD, professor, Division of Neuroscience, University of Manchester in England, said the new work supports the hypothesis that HCMV might be a trigger that tips the balance from a healthy brain to one with dementia. “If so, antiviral drugs against HCMV might be beneficial in reducing the risk of AD.”
Doig noted newly approved drugs for AD are expensive, provide only a small benefit, and have significant risks, such as causing brain hemorrhages. “Antiviral drugs are an attractive alternative that are well worth exploring.”
Richard Oakley, PhD, associate director of research and innovation, Alzheimer’s Society, cautioned the study only established a connection and didn’t directly show the virus leads to AD. “Also, the virus is not found in the brain of everyone with Alzheimer’s disease, the most common form of dementia.”
The significance of the new findings is “far from clear,” commented William McEwan, PhD, group leader at the UK Dementia Research Institute at Cambridge, England. “The study does not address how common this infection is in people without Alzheimer’s and therefore cannot by itself suggest that HCMV infection, or the associated immune response, is a driver of disease.”
The experts agreed follow-up research is needed to confirm these new findings and understand what they mean.
The study received support from the National Institute on Aging, National Institutes of Health, Global Lyme Alliance, National Institute of Neurological Disorders and Stroke, Arizona Alzheimer’s Consortium, The Benter Foundation, and NOMIS Stiftung. Readhead is a coinventor on a patent application for an IgG4-based peripheral biomarker for the detection of CD83+ microglia. Doig is a founder, director, and consultant for PharmaKure, which works on AD drugs and diagnostics, although not on viruses. He has cowritten a review on Viral Involvement in Alzheimer’s Disease. McEwan reported receiving research funding from Takeda Pharmaceuticals and is a founder and consultant to Trimtech Therapeutics.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA
Common Herbicide a Player in Neurodegeneration?
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROINFLAMMATION
How Metals Affect the Brain
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.
Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.
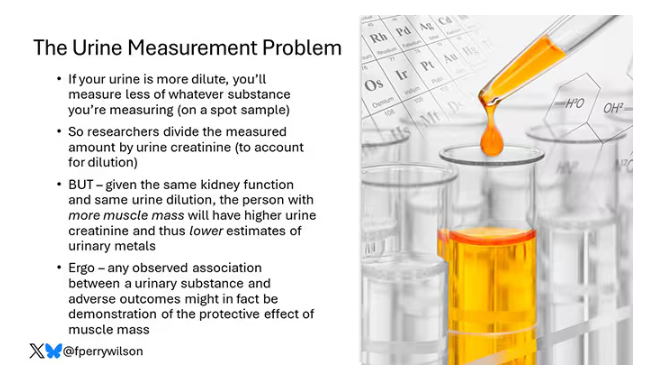
This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.
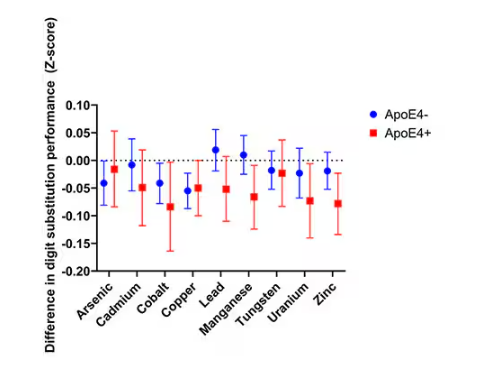
Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.
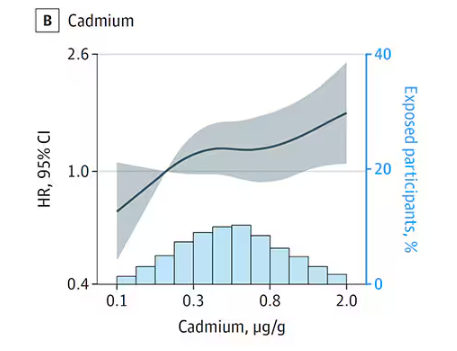
We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).
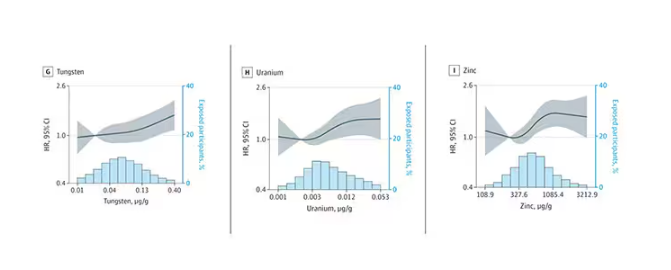
But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.
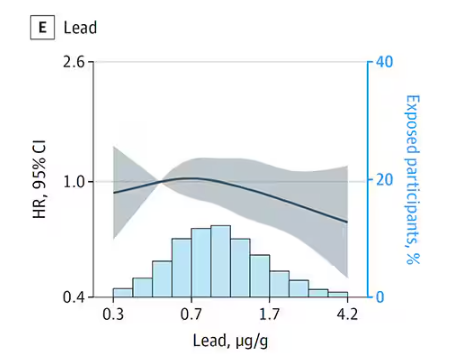
This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.
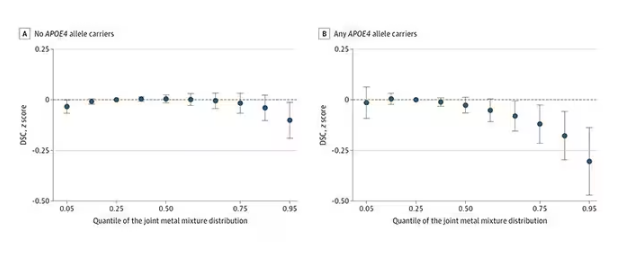
So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Watch That Attitude: Is There Ageism in Healthcare?
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
People are living longer in Europe. Life expectancy increased on the continent by around 12 years between 1960 and 2022. And despite slower progress during the COVID-19 pandemic, the trend appears to be continuing.
Not only are Europeans living longer, their fertility rates are declining. This means that the number of people aged 75-84 years is projected to grow in Europe a full 56.1% by 2050, while the population younger than 55 years is expected to fall by 13.5%.
This means that attitudes toward age need to change, and fast — even among healthcare professionals.
Healthcare Is Not Exempt From Ageist Attitudes
A systematic review published in the journal PLOS ONE in 2020 found that age was a determinant factor in dictating who received certain medical procedures or treatments. For example, a study of 9105 hospitalized patients found that healthcare providers were significantly more likely to withhold life-sustaining treatments from older patients. Another study found evidence that older people are excluded from clinical trials, even when the trials are for diseases that appear later in life, like Parkinson’s.
“In healthcare, there are different levels of ageism,” explained Hannah Swift, PhD, reader in social and organizational psychology at the University of Kent in the United Kingdom.
Ageism is embedded in the laws, rules, and practices of institutions, she explained. This became especially obvious during the pandemic, when health professionals had to decide who to treat, possibly using age as a proxy for making some of these decisions, she said.
“When you categorize people, you might be using stereotypes, assumptions, and expectations about age and that age group to make those decisions, and that’s where errors can occur.”
She added that ageist attitudes also become apparent at the interpersonal level by using patronizing language or offering unnecessary help to older people based on assumptions about their cognitive and physical abilities.
“Older age is often wrongly associated with declining levels of health and activity,” said Ittay Mannheim, PhD, guest postdoctoral researcher on aging and ageism at the Open University of the Netherlands. “However, older adults are a very diverse group, varying widely in many aspects, including health conditions. This stereotype can influence how healthcare professionals interact with them, assuming frailty or memory issues simply based on age. It’s important to recognize that being older doesn’t necessarily mean being ill.”
Mannheim’s research found that healthcare professionals often stand in the way of older people using technology-based treatments due to negative attitudes towards age. “So, actually, a barrier to using these technologies could be that healthcare professionals don’t think that someone can use it or won’t even offer it because someone looks old or is old,” he said.
The Impacts
Discrimination impacts the physical, mental, and social well-being of its victims. This includes attitudes towards age.
The PLOS ONE review of research on the global reach of ageism found that experienced or self-determined ageism was associated with significantly worse health outcomes across all countries examined. The same research team calculated that an estimated 6.3 million cases of depression worldwide are linked to ageism.
Other research has found that exposure to negative age stereotyping impacts willingness to adopt a healthy lifestyle in addition to increasing the risk for cardiovascular events.
What Can Be Done?
“Healthcare professionals frequently interact with older adults at their most vulnerable, which can reinforce negative stereotypes of older people being vulnerable or ill,” said Swift. “However, not all older adults fit these stereotypes. Many can live well and independently. Perhaps healthcare education should include reminders of the diverse experiences of older individuals rather than solely focusing on the moments when they require help.”
Research indicates that although progress has been made in geriatric training and the care of older individuals by healthcare education institutions, improved education and training are still needed at all levels of geriatric healthcare, including hospital administrators, physicians, nurses, personal caregivers, and associated health professions.
“Generally speaking, what healthcare professionals learn about aging tends to focus more on the biological aspects,” said Mannheim. “However, they may not fully understand what it means to be old or how to interact with older individuals, especially regarding technology. It is important to raise awareness about ageism because, in my experience working with healthcare professionals, even a single workshop on ageism can have a profound impact. Participants often respond with surprise, saying something like, ‘Wow, I never thought about this before.’”
Mannheim said that training healthcare providers to understand the aging process better could help to reduce any biases they might have and better prepare them to respond more adequately to the needs of older patients.
“We cannot devalue the lives of older people simply because they are older. It is crucial for all of us, especially governments, to acknowledge our responsibility to protect and promote human rights for individuals of all ages. If we fail to do this, the strategies we’ve witnessed during this pandemic will be repeated in the future,” said Nena Georgantzi, PhD, Barcelona-based human rights manager at AGE Platform Europe, an EU network of organizations of and for older people.
A version of this article appeared on Medscape.com.
