User login
Circadian rhythms: Does the time of day you use a skin care product matter?
The majority of human cells, including skin and hair cells, keep their own time; that is, they manifest autonomous clocks and the genes that regulate their functioning.1 During the day, one primary function of the skin is protection; at night, repairing any damage (particularly DNA impairment) incurred during the day prevails.2-4 These activities are driven through circadian rhythms using clock genes that exist in all cutaneous cells.2 Important cutaneous functions such as blood flow, transepidermal water loss, and capacitance are affected by circadian rhythms.5 Hydration and inflammation are also among the several functions pertaining to epidermal homeostasis affected by circadian rhythms.6 In addition, some collagens and extracellular matrix proteases are diurnally regulated, and approximately 10% of the transcriptome, including the extracellular matrix, is thought to be controlled by circadian rhythms.7
Cutaneous cell migration and proliferation, wound healing, and tissue vulnerability to harm from UV exposure, oxidative stress, and protease activity, for example, are affected by circadian rhythms, Sherratt et al. noted in suggesting that chronotherapy presents promise for enhancing skin therapy.7 Indeed, recent research has led to the understanding that cutaneous aging, cellular repair, optimal timing for drug delivery to the skin, and skin cancer development are all affected by the chronobiological functioning of the skin.8
We have known for several years that certain types of products should be used at different times of the day. For instance, antioxidants should be used in the morning to protect skin from sun exposure and retinols should be used in the evening because of its induction of light sensitivity. The remainder of this column focuses on research in the last 2 decades that reinforces the notion of circadian rhythms working in the skin, and may alter how we view the timing of skin care. Next month’s column, part two on the circadian rhythms of the skin, will address recent clinical trials and the implications for timing treatments for certain cutaneous conditions.
Emerging data on the circadian rhythms of the skin
In 2001, Le Fur et al. studied the cutaneous circadian rhythms in the facial and forearm skin of eight healthy White women during a 48-hour period. They were able to detect such rhythms in facial sebum excretion, transepidermal water loss (TEWL) in the face and forearm, pH in the face, forearm skin temperature, and forearm capacitance using cosinor or analysis of variance methods. The investigators also observed 8- and 12-hour rhythms in TEWL in both areas, and 12 hours for forearm skin temperature. They verified that such rhythms could be measured and that they vary between skin sites. In addition, they were the first to show that ultradian and/or component rhythms can also be found in TEWL, sebum excretion, and skin temperature.9
A year later, Kawara et al. showed that mRNA of the circadian clock genes Per1, Clock, and bmal1/mop3 are expressed in normal human-cultured keratinocytes and that low-dose UVB down-regulates these genes and changes their express in keratinocyte cell cultures. They concluded that UV targeting of keratinocytes could alter circadian rhythms.10
In 2011, Spörl and colleagues characterized an in vitro functional cell autonomous circadian clock in adult human low calcium temperature keratinocytes, demonstrating that the molecular composition of the keratinocyte clock was comparable with peripheral tissue clocks. Notably, they observed that temperature acts as a robust time cue for epidermal traits, such as cholesterol homeostasis and differentiation.11
The next year, Sandu et al. investigated the kinetics of clock gene expression in epidermal and dermal cells collected from the same donor and compared their characteristics. They were able to reveal the presence of functional circadian machinery in primary cultures of fibroblasts, keratinocytes, and melanocytes, with oscillators identified in all skin cell types and thought to be involved in spurring cutaneous rhythmic functions as they exhibited discrete periods and phase relationships between clock genes.12
Three years later, Sandu et al. characterized the circadian clocks in rat skin and dermal fibroblasts. They found that skin has a self-sustaining circadian clock that experiences age-dependent alterations, and that dermal fibroblasts manifest circadian rhythms that can be modulated by endogenous (e.g., melatonin) and exogenous (e.g., temperature) influences.13
In 2019, Park et al. demonstrated that the diurnal expression of the gene TIMP3, which is thought to evince a circadian rhythm in synchronized human keratinocytes, experiences disruptions in such rhythms by UVB exposure. The inflammation that results can be blocked, they argued, by recovering the circadian expression of TIMP3 using synthetic TIMP3 peptides or bioactive natural ingredients, such as green tea extracts.6
Conclusion
Circadian rhythms and the biological clocks by which most cells, including skin and hair cells, regulate themselves represent a ripe and fascinating area of research. Applying evidence in this realm to skin care has been occurring over time and is likely to enhance our practice even more as we continue to elucidate the behavior of cutaneous cells based on the solar day. Based on this information, my recommendations are to use antioxidants and protective products in the morning, and use DNA repair enzymes, retinoids, and other repair products at night.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Dong K et al. Int J Mol Sci. 2020 Jan 3. doi: 10.3390/ijms21010326.
2. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
3. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
4. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
5. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
6. Park S et al. Int J Mol Sci. 2019 Feb 16. doi: 10.3390/ijms20040862.
7. Sherratt MJ et al. Matrix Biol. 2019 Nov;84:97-110.
8. Luber AJ et al. J Drugs Dermatol. 2014 Feb;13(2):130-4.
9. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
10. Kawara S et al. J Invest Dermatol. 2002 Dec;119(6):1220-3.
11. Spörl F et al. J Invest Dermatol. 2011 Feb;131(2):338-48.
12. Sandu C et al. Cell Mol Life Sci. 2012 Oct;69(19):3329-39.
13. Sandu C et al. Cell Mol Life Sci. 2015 Jun;72(11):2237-48.
The majority of human cells, including skin and hair cells, keep their own time; that is, they manifest autonomous clocks and the genes that regulate their functioning.1 During the day, one primary function of the skin is protection; at night, repairing any damage (particularly DNA impairment) incurred during the day prevails.2-4 These activities are driven through circadian rhythms using clock genes that exist in all cutaneous cells.2 Important cutaneous functions such as blood flow, transepidermal water loss, and capacitance are affected by circadian rhythms.5 Hydration and inflammation are also among the several functions pertaining to epidermal homeostasis affected by circadian rhythms.6 In addition, some collagens and extracellular matrix proteases are diurnally regulated, and approximately 10% of the transcriptome, including the extracellular matrix, is thought to be controlled by circadian rhythms.7
Cutaneous cell migration and proliferation, wound healing, and tissue vulnerability to harm from UV exposure, oxidative stress, and protease activity, for example, are affected by circadian rhythms, Sherratt et al. noted in suggesting that chronotherapy presents promise for enhancing skin therapy.7 Indeed, recent research has led to the understanding that cutaneous aging, cellular repair, optimal timing for drug delivery to the skin, and skin cancer development are all affected by the chronobiological functioning of the skin.8
We have known for several years that certain types of products should be used at different times of the day. For instance, antioxidants should be used in the morning to protect skin from sun exposure and retinols should be used in the evening because of its induction of light sensitivity. The remainder of this column focuses on research in the last 2 decades that reinforces the notion of circadian rhythms working in the skin, and may alter how we view the timing of skin care. Next month’s column, part two on the circadian rhythms of the skin, will address recent clinical trials and the implications for timing treatments for certain cutaneous conditions.
Emerging data on the circadian rhythms of the skin
In 2001, Le Fur et al. studied the cutaneous circadian rhythms in the facial and forearm skin of eight healthy White women during a 48-hour period. They were able to detect such rhythms in facial sebum excretion, transepidermal water loss (TEWL) in the face and forearm, pH in the face, forearm skin temperature, and forearm capacitance using cosinor or analysis of variance methods. The investigators also observed 8- and 12-hour rhythms in TEWL in both areas, and 12 hours for forearm skin temperature. They verified that such rhythms could be measured and that they vary between skin sites. In addition, they were the first to show that ultradian and/or component rhythms can also be found in TEWL, sebum excretion, and skin temperature.9
A year later, Kawara et al. showed that mRNA of the circadian clock genes Per1, Clock, and bmal1/mop3 are expressed in normal human-cultured keratinocytes and that low-dose UVB down-regulates these genes and changes their express in keratinocyte cell cultures. They concluded that UV targeting of keratinocytes could alter circadian rhythms.10
In 2011, Spörl and colleagues characterized an in vitro functional cell autonomous circadian clock in adult human low calcium temperature keratinocytes, demonstrating that the molecular composition of the keratinocyte clock was comparable with peripheral tissue clocks. Notably, they observed that temperature acts as a robust time cue for epidermal traits, such as cholesterol homeostasis and differentiation.11
The next year, Sandu et al. investigated the kinetics of clock gene expression in epidermal and dermal cells collected from the same donor and compared their characteristics. They were able to reveal the presence of functional circadian machinery in primary cultures of fibroblasts, keratinocytes, and melanocytes, with oscillators identified in all skin cell types and thought to be involved in spurring cutaneous rhythmic functions as they exhibited discrete periods and phase relationships between clock genes.12
Three years later, Sandu et al. characterized the circadian clocks in rat skin and dermal fibroblasts. They found that skin has a self-sustaining circadian clock that experiences age-dependent alterations, and that dermal fibroblasts manifest circadian rhythms that can be modulated by endogenous (e.g., melatonin) and exogenous (e.g., temperature) influences.13
In 2019, Park et al. demonstrated that the diurnal expression of the gene TIMP3, which is thought to evince a circadian rhythm in synchronized human keratinocytes, experiences disruptions in such rhythms by UVB exposure. The inflammation that results can be blocked, they argued, by recovering the circadian expression of TIMP3 using synthetic TIMP3 peptides or bioactive natural ingredients, such as green tea extracts.6
Conclusion
Circadian rhythms and the biological clocks by which most cells, including skin and hair cells, regulate themselves represent a ripe and fascinating area of research. Applying evidence in this realm to skin care has been occurring over time and is likely to enhance our practice even more as we continue to elucidate the behavior of cutaneous cells based on the solar day. Based on this information, my recommendations are to use antioxidants and protective products in the morning, and use DNA repair enzymes, retinoids, and other repair products at night.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Dong K et al. Int J Mol Sci. 2020 Jan 3. doi: 10.3390/ijms21010326.
2. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
3. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
4. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
5. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
6. Park S et al. Int J Mol Sci. 2019 Feb 16. doi: 10.3390/ijms20040862.
7. Sherratt MJ et al. Matrix Biol. 2019 Nov;84:97-110.
8. Luber AJ et al. J Drugs Dermatol. 2014 Feb;13(2):130-4.
9. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
10. Kawara S et al. J Invest Dermatol. 2002 Dec;119(6):1220-3.
11. Spörl F et al. J Invest Dermatol. 2011 Feb;131(2):338-48.
12. Sandu C et al. Cell Mol Life Sci. 2012 Oct;69(19):3329-39.
13. Sandu C et al. Cell Mol Life Sci. 2015 Jun;72(11):2237-48.
The majority of human cells, including skin and hair cells, keep their own time; that is, they manifest autonomous clocks and the genes that regulate their functioning.1 During the day, one primary function of the skin is protection; at night, repairing any damage (particularly DNA impairment) incurred during the day prevails.2-4 These activities are driven through circadian rhythms using clock genes that exist in all cutaneous cells.2 Important cutaneous functions such as blood flow, transepidermal water loss, and capacitance are affected by circadian rhythms.5 Hydration and inflammation are also among the several functions pertaining to epidermal homeostasis affected by circadian rhythms.6 In addition, some collagens and extracellular matrix proteases are diurnally regulated, and approximately 10% of the transcriptome, including the extracellular matrix, is thought to be controlled by circadian rhythms.7
Cutaneous cell migration and proliferation, wound healing, and tissue vulnerability to harm from UV exposure, oxidative stress, and protease activity, for example, are affected by circadian rhythms, Sherratt et al. noted in suggesting that chronotherapy presents promise for enhancing skin therapy.7 Indeed, recent research has led to the understanding that cutaneous aging, cellular repair, optimal timing for drug delivery to the skin, and skin cancer development are all affected by the chronobiological functioning of the skin.8
We have known for several years that certain types of products should be used at different times of the day. For instance, antioxidants should be used in the morning to protect skin from sun exposure and retinols should be used in the evening because of its induction of light sensitivity. The remainder of this column focuses on research in the last 2 decades that reinforces the notion of circadian rhythms working in the skin, and may alter how we view the timing of skin care. Next month’s column, part two on the circadian rhythms of the skin, will address recent clinical trials and the implications for timing treatments for certain cutaneous conditions.
Emerging data on the circadian rhythms of the skin
In 2001, Le Fur et al. studied the cutaneous circadian rhythms in the facial and forearm skin of eight healthy White women during a 48-hour period. They were able to detect such rhythms in facial sebum excretion, transepidermal water loss (TEWL) in the face and forearm, pH in the face, forearm skin temperature, and forearm capacitance using cosinor or analysis of variance methods. The investigators also observed 8- and 12-hour rhythms in TEWL in both areas, and 12 hours for forearm skin temperature. They verified that such rhythms could be measured and that they vary between skin sites. In addition, they were the first to show that ultradian and/or component rhythms can also be found in TEWL, sebum excretion, and skin temperature.9
A year later, Kawara et al. showed that mRNA of the circadian clock genes Per1, Clock, and bmal1/mop3 are expressed in normal human-cultured keratinocytes and that low-dose UVB down-regulates these genes and changes their express in keratinocyte cell cultures. They concluded that UV targeting of keratinocytes could alter circadian rhythms.10
In 2011, Spörl and colleagues characterized an in vitro functional cell autonomous circadian clock in adult human low calcium temperature keratinocytes, demonstrating that the molecular composition of the keratinocyte clock was comparable with peripheral tissue clocks. Notably, they observed that temperature acts as a robust time cue for epidermal traits, such as cholesterol homeostasis and differentiation.11
The next year, Sandu et al. investigated the kinetics of clock gene expression in epidermal and dermal cells collected from the same donor and compared their characteristics. They were able to reveal the presence of functional circadian machinery in primary cultures of fibroblasts, keratinocytes, and melanocytes, with oscillators identified in all skin cell types and thought to be involved in spurring cutaneous rhythmic functions as they exhibited discrete periods and phase relationships between clock genes.12
Three years later, Sandu et al. characterized the circadian clocks in rat skin and dermal fibroblasts. They found that skin has a self-sustaining circadian clock that experiences age-dependent alterations, and that dermal fibroblasts manifest circadian rhythms that can be modulated by endogenous (e.g., melatonin) and exogenous (e.g., temperature) influences.13
In 2019, Park et al. demonstrated that the diurnal expression of the gene TIMP3, which is thought to evince a circadian rhythm in synchronized human keratinocytes, experiences disruptions in such rhythms by UVB exposure. The inflammation that results can be blocked, they argued, by recovering the circadian expression of TIMP3 using synthetic TIMP3 peptides or bioactive natural ingredients, such as green tea extracts.6
Conclusion
Circadian rhythms and the biological clocks by which most cells, including skin and hair cells, regulate themselves represent a ripe and fascinating area of research. Applying evidence in this realm to skin care has been occurring over time and is likely to enhance our practice even more as we continue to elucidate the behavior of cutaneous cells based on the solar day. Based on this information, my recommendations are to use antioxidants and protective products in the morning, and use DNA repair enzymes, retinoids, and other repair products at night.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Dong K et al. Int J Mol Sci. 2020 Jan 3. doi: 10.3390/ijms21010326.
2. Dong K et al. Int J Cosmet Sci. 2019 Dec;41(6):558-62.
3. Lyons AB et al. J Clin Aesthet Dermatol. 2019 Sep;12(9):42-5.
4. Wu G et al. Proc Natl Acad Sci U S A. 2018 Nov 27;115(48):12313-8.
5. Vaughn AR et al. Pediatr Dermatol. 2018 Jan;35(1):152-7.
6. Park S et al. Int J Mol Sci. 2019 Feb 16. doi: 10.3390/ijms20040862.
7. Sherratt MJ et al. Matrix Biol. 2019 Nov;84:97-110.
8. Luber AJ et al. J Drugs Dermatol. 2014 Feb;13(2):130-4.
9. Le Fur I et al. J Invest Dermatol. 2001 Sep;117(3):718-24.
10. Kawara S et al. J Invest Dermatol. 2002 Dec;119(6):1220-3.
11. Spörl F et al. J Invest Dermatol. 2011 Feb;131(2):338-48.
12. Sandu C et al. Cell Mol Life Sci. 2012 Oct;69(19):3329-39.
13. Sandu C et al. Cell Mol Life Sci. 2015 Jun;72(11):2237-48.
Tattoo removal techniques continue to be refined
According to a 2016 Harris Poll, 29% of Americans have at least one tattoo, up from 21% in 2012. At the same time, 23% of Americans polled in 2016 regret having their tattoo, which means big business for dermatologists who practice laser tattoo removal.
Prior to the theory of selective photothermolysis, tattoo removal mostly consisted of chemical or mechanical abrasion, surgical removal, or using some sort of caustic chemical or thermal destruction of the tattoo, Omar A. Ibrahimi, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. “The earliest lasers prior to refinement by the theory of selective photothermolysis also fell into these categories: just basically crudely removing the skin and trying to get under to where the tattoo is,” said Dr. Ibrahimi, a dermatologist with the Connecticut Skin Institute in Stamford. “These would often heal with horrible scarring.”
Today, clinicians use Q-switched nanosecond and picosecond lasers for tattoo removal, though appropriate wavelengths need to be selected based on the tattoo ink color. Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be about 10 nanoseconds. Black is the most common color dermatologists will treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “The other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
The clinical endpoint to strive for during tattoo removal is a whitening of the ink. That typically fades after about 20 minutes. “This whitening corresponds to cavitation [the production of gas vacuoles in the cells that were holding the ink],” Dr. Ibrahimi explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “These vacuoles are what lead to the whitening when using a high-gigawatt laser in a very short pulse. This causes highly localized heating, cavitation, and cell rupture. We don’t fully understand how tattoos are removed today, but the working models include some of the residual ink coming out through transepidermal elimination, some of it being removed via lymphatics, and some of it being removed by rephagocytosis.”
For optimal results, determine if the tattoo is professional, amateur, traumatic, or cosmetic. “That’s going to give you some insight as to what kind of expectations to set for the patient,” he said. “Black ink is often the easiest to remove, while certain colors like white are more challenging. Certain colors are more prone to paradoxical ink darkening, like red or orange, or pink. These can undergo a chemical reaction where they darken. This is something important to discuss with patients in advance.”
Older tattoos “tend to be less hearty” and usually respond better to laser, he continued. Location of the tattoo also plays a role. “I find that tattoos below the knee are very slow to respond. Smaller tattoos will respond faster.”
During the focused medical exam, ask patients about any history of keloid scarring, vitiligo or any dermatologic conditions with a Koebner phenomenon, and rule out a history of parental gold salt administration for arthritis. “During your informed consent you want to make sure you address the expected healing time and the risks such as hyper- and hypopigmentation, blistering, and scarring,” Dr. Ibrahimi said. “You also want to set the expectation that this is not going to be a one and done procedure. Laser tattoo removal takes a series of treatments, often more than what we think – sometimes in the range of 15-20. And you may not get complete clearance. I liken it to breaking it up enough so that if somebody sees it, they won’t be able to recognize what the tattoo is. But you won’t be able to erase it 100%.”
Black, dark blue, and red tattoo colors respond best to laser light. Light blue, green, and purple colors are slower to respond, while yellow and orange colors respond poorly. “Now that we have picosecond lasers, we’re a little better at treating these tougher colors, but I think we still have a lot of room for improvement,” Dr. Ibrahimi said.
Melanin is a competing chromophore, which complicates treatment of tanned individuals and those with darker skin types. “The Q-switched 1064-nm laser is the safest device to use for these patients but it’s not effective for many ink colors,” he said.
Options to keep patients comfortable during the procedure include application of ice or forced chilled air. “You can also use topical anesthetics such as EMLA or liposomal lidocaine cream under occlusion,” he said. “You can also use injectable lidocaine. If you go that route, I recommend a ring block. If you inject right into the tattoo sometimes the ink can get leeched out after treatment. As for spot size, a larger spot size will penetrate deeper, so I try to treat tattoos with the biggest spot size. It also results in less bleeding, less splatter, less side effects, and you get better results.”
Common adverse events from tattoo removal include prolonged erythema, blistering, hyperpigmentation, hypopigmentation, and scarring. Less frequent complications include ink darkening, chrysiasis, and transient immunoreactivity. “We don’t really know what’s in a lot of these ink residues,” Dr. Ibrahimi said. “We know they’re getting mobilized and some of it’s going into the lymphatics. What’s happening with these ink particles? We don’t fully know.”
He also warned against using hair-removal devices to treat a tattoo. “It is the wrong pulse duration,” he said. “You need a picosecond or nanosecond device. You cannot use any other pulse durations, or you will horribly scar your patient.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the R20 method, was more effective than a single-pass treatment (P < .01). “Subsequent papers have shown that this result isn’t as impressive as initially reported, but I think it’s a method that persists,” Dr. Ibrahimi said.
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin patch during tattoo removal, which has been shown to reduce epidermal whitening. “So, instead of waiting 20 minutes you wait 0 minutes,” he said. “This is called the R0 method,” he added, noting that there are also some secondary benefits to using this patch, including possibly helping as an optical clearing agent for deeper penetration of the laser. “Often after treatment you can see ink on the underside of the patch, which speaks to the transdermal elimination mechanism of action for removal of tattoos.”
As for future directions, Dr. Ibrahimi predicted that there will be better picosecond lasers coming down the pike. He also anticipates that Soliton’s Rapid Acoustic Pulse (RAP) device will make a significant impact in the field. The device was cleared for tattoo removal in 2019 and is being investigated as an option to improve the appearance of cellulite. The manufacturer anticipates that an upgraded RAP device will be cleared for use by the end of the first quarter of 2021.
Dr. Ibrahimi disclosed that he has received research funding and speaker honorarium from Cutera, Lumenis, Lutronic, and Syneron-Candela. He also holds stock in Soliton.
According to a 2016 Harris Poll, 29% of Americans have at least one tattoo, up from 21% in 2012. At the same time, 23% of Americans polled in 2016 regret having their tattoo, which means big business for dermatologists who practice laser tattoo removal.
Prior to the theory of selective photothermolysis, tattoo removal mostly consisted of chemical or mechanical abrasion, surgical removal, or using some sort of caustic chemical or thermal destruction of the tattoo, Omar A. Ibrahimi, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. “The earliest lasers prior to refinement by the theory of selective photothermolysis also fell into these categories: just basically crudely removing the skin and trying to get under to where the tattoo is,” said Dr. Ibrahimi, a dermatologist with the Connecticut Skin Institute in Stamford. “These would often heal with horrible scarring.”
Today, clinicians use Q-switched nanosecond and picosecond lasers for tattoo removal, though appropriate wavelengths need to be selected based on the tattoo ink color. Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be about 10 nanoseconds. Black is the most common color dermatologists will treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “The other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
The clinical endpoint to strive for during tattoo removal is a whitening of the ink. That typically fades after about 20 minutes. “This whitening corresponds to cavitation [the production of gas vacuoles in the cells that were holding the ink],” Dr. Ibrahimi explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “These vacuoles are what lead to the whitening when using a high-gigawatt laser in a very short pulse. This causes highly localized heating, cavitation, and cell rupture. We don’t fully understand how tattoos are removed today, but the working models include some of the residual ink coming out through transepidermal elimination, some of it being removed via lymphatics, and some of it being removed by rephagocytosis.”
For optimal results, determine if the tattoo is professional, amateur, traumatic, or cosmetic. “That’s going to give you some insight as to what kind of expectations to set for the patient,” he said. “Black ink is often the easiest to remove, while certain colors like white are more challenging. Certain colors are more prone to paradoxical ink darkening, like red or orange, or pink. These can undergo a chemical reaction where they darken. This is something important to discuss with patients in advance.”
Older tattoos “tend to be less hearty” and usually respond better to laser, he continued. Location of the tattoo also plays a role. “I find that tattoos below the knee are very slow to respond. Smaller tattoos will respond faster.”
During the focused medical exam, ask patients about any history of keloid scarring, vitiligo or any dermatologic conditions with a Koebner phenomenon, and rule out a history of parental gold salt administration for arthritis. “During your informed consent you want to make sure you address the expected healing time and the risks such as hyper- and hypopigmentation, blistering, and scarring,” Dr. Ibrahimi said. “You also want to set the expectation that this is not going to be a one and done procedure. Laser tattoo removal takes a series of treatments, often more than what we think – sometimes in the range of 15-20. And you may not get complete clearance. I liken it to breaking it up enough so that if somebody sees it, they won’t be able to recognize what the tattoo is. But you won’t be able to erase it 100%.”
Black, dark blue, and red tattoo colors respond best to laser light. Light blue, green, and purple colors are slower to respond, while yellow and orange colors respond poorly. “Now that we have picosecond lasers, we’re a little better at treating these tougher colors, but I think we still have a lot of room for improvement,” Dr. Ibrahimi said.
Melanin is a competing chromophore, which complicates treatment of tanned individuals and those with darker skin types. “The Q-switched 1064-nm laser is the safest device to use for these patients but it’s not effective for many ink colors,” he said.
Options to keep patients comfortable during the procedure include application of ice or forced chilled air. “You can also use topical anesthetics such as EMLA or liposomal lidocaine cream under occlusion,” he said. “You can also use injectable lidocaine. If you go that route, I recommend a ring block. If you inject right into the tattoo sometimes the ink can get leeched out after treatment. As for spot size, a larger spot size will penetrate deeper, so I try to treat tattoos with the biggest spot size. It also results in less bleeding, less splatter, less side effects, and you get better results.”
Common adverse events from tattoo removal include prolonged erythema, blistering, hyperpigmentation, hypopigmentation, and scarring. Less frequent complications include ink darkening, chrysiasis, and transient immunoreactivity. “We don’t really know what’s in a lot of these ink residues,” Dr. Ibrahimi said. “We know they’re getting mobilized and some of it’s going into the lymphatics. What’s happening with these ink particles? We don’t fully know.”
He also warned against using hair-removal devices to treat a tattoo. “It is the wrong pulse duration,” he said. “You need a picosecond or nanosecond device. You cannot use any other pulse durations, or you will horribly scar your patient.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the R20 method, was more effective than a single-pass treatment (P < .01). “Subsequent papers have shown that this result isn’t as impressive as initially reported, but I think it’s a method that persists,” Dr. Ibrahimi said.
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin patch during tattoo removal, which has been shown to reduce epidermal whitening. “So, instead of waiting 20 minutes you wait 0 minutes,” he said. “This is called the R0 method,” he added, noting that there are also some secondary benefits to using this patch, including possibly helping as an optical clearing agent for deeper penetration of the laser. “Often after treatment you can see ink on the underside of the patch, which speaks to the transdermal elimination mechanism of action for removal of tattoos.”
As for future directions, Dr. Ibrahimi predicted that there will be better picosecond lasers coming down the pike. He also anticipates that Soliton’s Rapid Acoustic Pulse (RAP) device will make a significant impact in the field. The device was cleared for tattoo removal in 2019 and is being investigated as an option to improve the appearance of cellulite. The manufacturer anticipates that an upgraded RAP device will be cleared for use by the end of the first quarter of 2021.
Dr. Ibrahimi disclosed that he has received research funding and speaker honorarium from Cutera, Lumenis, Lutronic, and Syneron-Candela. He also holds stock in Soliton.
According to a 2016 Harris Poll, 29% of Americans have at least one tattoo, up from 21% in 2012. At the same time, 23% of Americans polled in 2016 regret having their tattoo, which means big business for dermatologists who practice laser tattoo removal.
Prior to the theory of selective photothermolysis, tattoo removal mostly consisted of chemical or mechanical abrasion, surgical removal, or using some sort of caustic chemical or thermal destruction of the tattoo, Omar A. Ibrahimi, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. “The earliest lasers prior to refinement by the theory of selective photothermolysis also fell into these categories: just basically crudely removing the skin and trying to get under to where the tattoo is,” said Dr. Ibrahimi, a dermatologist with the Connecticut Skin Institute in Stamford. “These would often heal with horrible scarring.”
Today, clinicians use Q-switched nanosecond and picosecond lasers for tattoo removal, though appropriate wavelengths need to be selected based on the tattoo ink color. Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be about 10 nanoseconds. Black is the most common color dermatologists will treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “The other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
The clinical endpoint to strive for during tattoo removal is a whitening of the ink. That typically fades after about 20 minutes. “This whitening corresponds to cavitation [the production of gas vacuoles in the cells that were holding the ink],” Dr. Ibrahimi explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “These vacuoles are what lead to the whitening when using a high-gigawatt laser in a very short pulse. This causes highly localized heating, cavitation, and cell rupture. We don’t fully understand how tattoos are removed today, but the working models include some of the residual ink coming out through transepidermal elimination, some of it being removed via lymphatics, and some of it being removed by rephagocytosis.”
For optimal results, determine if the tattoo is professional, amateur, traumatic, or cosmetic. “That’s going to give you some insight as to what kind of expectations to set for the patient,” he said. “Black ink is often the easiest to remove, while certain colors like white are more challenging. Certain colors are more prone to paradoxical ink darkening, like red or orange, or pink. These can undergo a chemical reaction where they darken. This is something important to discuss with patients in advance.”
Older tattoos “tend to be less hearty” and usually respond better to laser, he continued. Location of the tattoo also plays a role. “I find that tattoos below the knee are very slow to respond. Smaller tattoos will respond faster.”
During the focused medical exam, ask patients about any history of keloid scarring, vitiligo or any dermatologic conditions with a Koebner phenomenon, and rule out a history of parental gold salt administration for arthritis. “During your informed consent you want to make sure you address the expected healing time and the risks such as hyper- and hypopigmentation, blistering, and scarring,” Dr. Ibrahimi said. “You also want to set the expectation that this is not going to be a one and done procedure. Laser tattoo removal takes a series of treatments, often more than what we think – sometimes in the range of 15-20. And you may not get complete clearance. I liken it to breaking it up enough so that if somebody sees it, they won’t be able to recognize what the tattoo is. But you won’t be able to erase it 100%.”
Black, dark blue, and red tattoo colors respond best to laser light. Light blue, green, and purple colors are slower to respond, while yellow and orange colors respond poorly. “Now that we have picosecond lasers, we’re a little better at treating these tougher colors, but I think we still have a lot of room for improvement,” Dr. Ibrahimi said.
Melanin is a competing chromophore, which complicates treatment of tanned individuals and those with darker skin types. “The Q-switched 1064-nm laser is the safest device to use for these patients but it’s not effective for many ink colors,” he said.
Options to keep patients comfortable during the procedure include application of ice or forced chilled air. “You can also use topical anesthetics such as EMLA or liposomal lidocaine cream under occlusion,” he said. “You can also use injectable lidocaine. If you go that route, I recommend a ring block. If you inject right into the tattoo sometimes the ink can get leeched out after treatment. As for spot size, a larger spot size will penetrate deeper, so I try to treat tattoos with the biggest spot size. It also results in less bleeding, less splatter, less side effects, and you get better results.”
Common adverse events from tattoo removal include prolonged erythema, blistering, hyperpigmentation, hypopigmentation, and scarring. Less frequent complications include ink darkening, chrysiasis, and transient immunoreactivity. “We don’t really know what’s in a lot of these ink residues,” Dr. Ibrahimi said. “We know they’re getting mobilized and some of it’s going into the lymphatics. What’s happening with these ink particles? We don’t fully know.”
He also warned against using hair-removal devices to treat a tattoo. “It is the wrong pulse duration,” he said. “You need a picosecond or nanosecond device. You cannot use any other pulse durations, or you will horribly scar your patient.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the R20 method, was more effective than a single-pass treatment (P < .01). “Subsequent papers have shown that this result isn’t as impressive as initially reported, but I think it’s a method that persists,” Dr. Ibrahimi said.
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin patch during tattoo removal, which has been shown to reduce epidermal whitening. “So, instead of waiting 20 minutes you wait 0 minutes,” he said. “This is called the R0 method,” he added, noting that there are also some secondary benefits to using this patch, including possibly helping as an optical clearing agent for deeper penetration of the laser. “Often after treatment you can see ink on the underside of the patch, which speaks to the transdermal elimination mechanism of action for removal of tattoos.”
As for future directions, Dr. Ibrahimi predicted that there will be better picosecond lasers coming down the pike. He also anticipates that Soliton’s Rapid Acoustic Pulse (RAP) device will make a significant impact in the field. The device was cleared for tattoo removal in 2019 and is being investigated as an option to improve the appearance of cellulite. The manufacturer anticipates that an upgraded RAP device will be cleared for use by the end of the first quarter of 2021.
Dr. Ibrahimi disclosed that he has received research funding and speaker honorarium from Cutera, Lumenis, Lutronic, and Syneron-Candela. He also holds stock in Soliton.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Expert spotlights three emerging technologies for dermatology practice
New technologies being developed at the Wellman Center for Photomedicine, Boston, that .
During a virtual course on laser and aesthetic skin therapy, Lilit Garibyan, MD, PhD, discussed findings from a swine study published online in January 2020 that used an injectable physiologic ice slurry for the nonsurgical removal of fat, a technology that could give CoolSculpting a run for its money. “It does lead to more efficient and effective cryolipolysis,” said Dr. Garibyan, the lead study author who is an assistant professor of dermatology at Harvard University, and director of The Magic Wand Initiative at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The treatment of fat tissue with ice slurry injection can be done in less than 1 minute, as opposed to an hour of cooling with CoolSculpting. In addition, because cooling is delivered directly into target tissue, it is more effective.”
For the study, she and her colleagues at the Wellman Center injected the slurry – a mix of ice, saline, and glycol – into the flanks of swine and followed them for up to 8 weeks. They used ultrasound imaging to show the location of the fat loss and to quantify it. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% fat gain in swine who served as controls. “This is because the pig is growing and gaining weight, so the fat is increasing,” she explained.
Gross histologic images also showed fat loss in the subcutaneous fat tissue of treated swine, but not in controls. “When we quantified this loss, there was about a 60% loss of fat after a single injection of ice slurry in the subcutaneous fat,” Dr. Garibyan said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “On histology there was loss of fat in the subcutaneous area and it was replaced by new collagen. No damage to surrounding skin or muscle tissue was seen.”
She characterized the approach as “a minimally invasive and novel method of adipose tissue removal. It’s very simple, because it’s just a simple injection, and it’s very efficient and effective in fat removal. Most importantly, it can target any anatomic site accessible with a needle.”
Human studies are currently underway.
Another emerging technology Dr. Garibyan discussed is a novel controlled skin cooling device for the treatment of benign pigmented lesions. The approach, known as Cryomodulation, was invented by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry HL Chan, MD, at Massachusetts General Hospital, Boston, and is being commercialized by R2 Technologies. It delivers precise controlled and titratable freezing of benign pigmented lesions without damage to the epidermal barrier. It has been cleared by the Food and Drug Administration, and R2 Technologies plans to launch its first commercial product in the United States in December 2020.
The handpiece of the device, which is placed on top of the skin, provides localized and controlled freezing to targeted benign pigmented lesions. “The cold, or the freeze, is delivered to where the melanocytes reside,” Dr. Garibyan said. “The ice nucleation essentially pauses melanin production. As cell turnover occurs, cells that are melanin-free migrate upward and renew freshly healthy skin. So, melanocyte function is still preserved but there is no destruction to the epidermal barrier. This technology is totally color blind, and there is no persistent inflammatory response.”
After this treatment, histology reveals a reduction of epidermal melanin without destruction of melanocytes. The treatment impairs melanocyte transfer, but not the melanocytes. “Clinically, that is seen as lightening of the skin,” she said. More than 550 patients have been treated with Cryomodulation to demonstrate its safety and effectiveness, described in a study published in 2019, and an ASLMS e-poster.
The final technology Dr. Garibyan discussed is a novel device for removing dermal pigment with a highly focused laser beam. “The problem with current lasers is that the maximum absorption of energy happens at the dermal/epidermal junction,” she said. “This not only increases the risk of epidermal injury, especially in skin of color, but it also leaves very little energy to reach the pigmented target tissue or cells. In addition, there is scattering in the skin, which also reduces the amount of fluence or energy that can reach the target depth, therefore reducing the efficacy of treatment with currently available laser.”
The investigative focused laser beam with high-speed scanning creates a large differential between the fluence at the surface and the fluence at the target, which improves safety. “It’s able to deliver enhanced energy to the target,” she said. “Therefore it’s more effective than destroying the target pigmented cells. There is no injury outside of the focal point, so it offers improved safety, efficacy, and spatial selectivity. The end result on histology is a selective destruction of the pigmented cells, which are typically melanophages.”
Dr. Garibyan predicted that this device will be an ideal therapy for postinflammatory hyperpigmentation and for melasma, “as no effective therapies are available for those conditions.”
She disclosed that she has received royalties/inventorship assigned to MGH. She holds equity in, is a consultant to, and is a member of the scientific advisory board of Brixton Biosciences. She is a consultant to Vyome Therapeutics, Blossom Innovations, Aegle Therapeutics, and ClearifiRx.
New technologies being developed at the Wellman Center for Photomedicine, Boston, that .
During a virtual course on laser and aesthetic skin therapy, Lilit Garibyan, MD, PhD, discussed findings from a swine study published online in January 2020 that used an injectable physiologic ice slurry for the nonsurgical removal of fat, a technology that could give CoolSculpting a run for its money. “It does lead to more efficient and effective cryolipolysis,” said Dr. Garibyan, the lead study author who is an assistant professor of dermatology at Harvard University, and director of The Magic Wand Initiative at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The treatment of fat tissue with ice slurry injection can be done in less than 1 minute, as opposed to an hour of cooling with CoolSculpting. In addition, because cooling is delivered directly into target tissue, it is more effective.”
For the study, she and her colleagues at the Wellman Center injected the slurry – a mix of ice, saline, and glycol – into the flanks of swine and followed them for up to 8 weeks. They used ultrasound imaging to show the location of the fat loss and to quantify it. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% fat gain in swine who served as controls. “This is because the pig is growing and gaining weight, so the fat is increasing,” she explained.
Gross histologic images also showed fat loss in the subcutaneous fat tissue of treated swine, but not in controls. “When we quantified this loss, there was about a 60% loss of fat after a single injection of ice slurry in the subcutaneous fat,” Dr. Garibyan said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “On histology there was loss of fat in the subcutaneous area and it was replaced by new collagen. No damage to surrounding skin or muscle tissue was seen.”
She characterized the approach as “a minimally invasive and novel method of adipose tissue removal. It’s very simple, because it’s just a simple injection, and it’s very efficient and effective in fat removal. Most importantly, it can target any anatomic site accessible with a needle.”
Human studies are currently underway.
Another emerging technology Dr. Garibyan discussed is a novel controlled skin cooling device for the treatment of benign pigmented lesions. The approach, known as Cryomodulation, was invented by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry HL Chan, MD, at Massachusetts General Hospital, Boston, and is being commercialized by R2 Technologies. It delivers precise controlled and titratable freezing of benign pigmented lesions without damage to the epidermal barrier. It has been cleared by the Food and Drug Administration, and R2 Technologies plans to launch its first commercial product in the United States in December 2020.
The handpiece of the device, which is placed on top of the skin, provides localized and controlled freezing to targeted benign pigmented lesions. “The cold, or the freeze, is delivered to where the melanocytes reside,” Dr. Garibyan said. “The ice nucleation essentially pauses melanin production. As cell turnover occurs, cells that are melanin-free migrate upward and renew freshly healthy skin. So, melanocyte function is still preserved but there is no destruction to the epidermal barrier. This technology is totally color blind, and there is no persistent inflammatory response.”
After this treatment, histology reveals a reduction of epidermal melanin without destruction of melanocytes. The treatment impairs melanocyte transfer, but not the melanocytes. “Clinically, that is seen as lightening of the skin,” she said. More than 550 patients have been treated with Cryomodulation to demonstrate its safety and effectiveness, described in a study published in 2019, and an ASLMS e-poster.
The final technology Dr. Garibyan discussed is a novel device for removing dermal pigment with a highly focused laser beam. “The problem with current lasers is that the maximum absorption of energy happens at the dermal/epidermal junction,” she said. “This not only increases the risk of epidermal injury, especially in skin of color, but it also leaves very little energy to reach the pigmented target tissue or cells. In addition, there is scattering in the skin, which also reduces the amount of fluence or energy that can reach the target depth, therefore reducing the efficacy of treatment with currently available laser.”
The investigative focused laser beam with high-speed scanning creates a large differential between the fluence at the surface and the fluence at the target, which improves safety. “It’s able to deliver enhanced energy to the target,” she said. “Therefore it’s more effective than destroying the target pigmented cells. There is no injury outside of the focal point, so it offers improved safety, efficacy, and spatial selectivity. The end result on histology is a selective destruction of the pigmented cells, which are typically melanophages.”
Dr. Garibyan predicted that this device will be an ideal therapy for postinflammatory hyperpigmentation and for melasma, “as no effective therapies are available for those conditions.”
She disclosed that she has received royalties/inventorship assigned to MGH. She holds equity in, is a consultant to, and is a member of the scientific advisory board of Brixton Biosciences. She is a consultant to Vyome Therapeutics, Blossom Innovations, Aegle Therapeutics, and ClearifiRx.
New technologies being developed at the Wellman Center for Photomedicine, Boston, that .
During a virtual course on laser and aesthetic skin therapy, Lilit Garibyan, MD, PhD, discussed findings from a swine study published online in January 2020 that used an injectable physiologic ice slurry for the nonsurgical removal of fat, a technology that could give CoolSculpting a run for its money. “It does lead to more efficient and effective cryolipolysis,” said Dr. Garibyan, the lead study author who is an assistant professor of dermatology at Harvard University, and director of The Magic Wand Initiative at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The treatment of fat tissue with ice slurry injection can be done in less than 1 minute, as opposed to an hour of cooling with CoolSculpting. In addition, because cooling is delivered directly into target tissue, it is more effective.”
For the study, she and her colleagues at the Wellman Center injected the slurry – a mix of ice, saline, and glycol – into the flanks of swine and followed them for up to 8 weeks. They used ultrasound imaging to show the location of the fat loss and to quantify it. The researchers observed about 40%-50% loss of fat in the treated area, compared with a 60% fat gain in swine who served as controls. “This is because the pig is growing and gaining weight, so the fat is increasing,” she explained.
Gross histologic images also showed fat loss in the subcutaneous fat tissue of treated swine, but not in controls. “When we quantified this loss, there was about a 60% loss of fat after a single injection of ice slurry in the subcutaneous fat,” Dr. Garibyan said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “On histology there was loss of fat in the subcutaneous area and it was replaced by new collagen. No damage to surrounding skin or muscle tissue was seen.”
She characterized the approach as “a minimally invasive and novel method of adipose tissue removal. It’s very simple, because it’s just a simple injection, and it’s very efficient and effective in fat removal. Most importantly, it can target any anatomic site accessible with a needle.”
Human studies are currently underway.
Another emerging technology Dr. Garibyan discussed is a novel controlled skin cooling device for the treatment of benign pigmented lesions. The approach, known as Cryomodulation, was invented by R. Rox Anderson, MD, Dieter Manstein, MD, PhD, and Henry HL Chan, MD, at Massachusetts General Hospital, Boston, and is being commercialized by R2 Technologies. It delivers precise controlled and titratable freezing of benign pigmented lesions without damage to the epidermal barrier. It has been cleared by the Food and Drug Administration, and R2 Technologies plans to launch its first commercial product in the United States in December 2020.
The handpiece of the device, which is placed on top of the skin, provides localized and controlled freezing to targeted benign pigmented lesions. “The cold, or the freeze, is delivered to where the melanocytes reside,” Dr. Garibyan said. “The ice nucleation essentially pauses melanin production. As cell turnover occurs, cells that are melanin-free migrate upward and renew freshly healthy skin. So, melanocyte function is still preserved but there is no destruction to the epidermal barrier. This technology is totally color blind, and there is no persistent inflammatory response.”
After this treatment, histology reveals a reduction of epidermal melanin without destruction of melanocytes. The treatment impairs melanocyte transfer, but not the melanocytes. “Clinically, that is seen as lightening of the skin,” she said. More than 550 patients have been treated with Cryomodulation to demonstrate its safety and effectiveness, described in a study published in 2019, and an ASLMS e-poster.
The final technology Dr. Garibyan discussed is a novel device for removing dermal pigment with a highly focused laser beam. “The problem with current lasers is that the maximum absorption of energy happens at the dermal/epidermal junction,” she said. “This not only increases the risk of epidermal injury, especially in skin of color, but it also leaves very little energy to reach the pigmented target tissue or cells. In addition, there is scattering in the skin, which also reduces the amount of fluence or energy that can reach the target depth, therefore reducing the efficacy of treatment with currently available laser.”
The investigative focused laser beam with high-speed scanning creates a large differential between the fluence at the surface and the fluence at the target, which improves safety. “It’s able to deliver enhanced energy to the target,” she said. “Therefore it’s more effective than destroying the target pigmented cells. There is no injury outside of the focal point, so it offers improved safety, efficacy, and spatial selectivity. The end result on histology is a selective destruction of the pigmented cells, which are typically melanophages.”
Dr. Garibyan predicted that this device will be an ideal therapy for postinflammatory hyperpigmentation and for melasma, “as no effective therapies are available for those conditions.”
She disclosed that she has received royalties/inventorship assigned to MGH. She holds equity in, is a consultant to, and is a member of the scientific advisory board of Brixton Biosciences. She is a consultant to Vyome Therapeutics, Blossom Innovations, Aegle Therapeutics, and ClearifiRx.
EXPERT ANALYSIS FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Mobile Apps for Professional Dermatology Education: An Objective Review
With today’s technology, it is easier than ever to access web-based tools that enrich traditional dermatology education. The literature supports the use of these innovative platforms to enhance learning at the student and trainee levels. A controlled study of pediatric residents showed that online modules effectively supplemented clinical experience with atopic dermatitis.1 In a randomized diagnostic study of medical students, practice with an image-based web application (app) that teaches rapid recognition of melanoma proved more effective than learning a rule-based algorithm.2 Given the visual nature of dermatology, pattern recognition is an essential skill that is fostered through experience and is only made more accessible with technology.
With the added benefit of convenience and accessibility, mobile apps can supplement experiential learning. Mirroring the overall growth of mobile apps, the number of available dermatology apps has increased.3 Dermatology mobile apps serve purposes ranging from quick reference tools to comprehensive modules, journals, and question banks. At an academic hospital in Taiwan, both nondermatology and dermatology trainees’ examination performance improved after 3 weeks of using a smartphone-based wallpaper learning module displaying morphologic characteristics of fungi.4 With the expansion of virtual microscopy, mobile apps also have been created as a learning tool for dermatopathology, giving trainees the flexibility and autonomy to view slides on their own time.5 Nevertheless, the literature on dermatology mobile apps designed for the education of medical students and trainees is limited, demonstrating a need for further investigation.
Prior studies have reviewed dermatology apps for patients and practicing dermatologists.6-8 Herein, we focus on mobile apps targeting students and residents learning dermatology. General dermatology reference apps and educational aid apps have grown by 33% and 32%, respectively, from 2014 to 2017.3 As with any resource meant to educate future and current medical providers, there must be an objective review process in place to ensure accurate, unbiased, evidence-based teaching.
Well-organized, comprehensive information and a user-friendly interface are additional factors of importance when selecting an educational mobile app. When discussing supplemental resources, accessibility and affordability also are priorities given the high cost of a medical education at baseline. Overall, there is a need for a standardized method to evaluate the key factors of an educational mobile app that make it appropriate for this demographic. We conducted a search of mobile apps relating to dermatology education for students and residents.
Methods
We searched for publicly available mobile apps relating to dermatology education in the App Store (Apple Inc) from September to November 2019 using the search terms dermatology education, dermoscopy education, melanoma education, skin cancer education, psoriasis education, rosacea education, acne education, eczema education, dermal fillers education, and Mohs surgery education. We excluded apps that were not in English, were created for a conference, cost more than $5 to download, or did not include a specific dermatology education section. In this way, we hoped to evaluate apps that were relevant, accessible, and affordable.
We modeled our study after a review of patient education apps performed by Masud et al6 and utilized their quantified grading rubric (scale of 1 to 4). We found their established criteria—educational objectives, content, accuracy, design, and conflict of interest—to be equally applicable for evaluating apps designed for professional education.6 Each app earned a minimum of 1 point and a maximum of 4 points per criterion. One point was given if the app did not fulfill the criterion, 2 points for minimally fulfilling the criterion, 3 points for mostly fulfilling the criterion, and 4 points if the criterion was completely fulfilled. Two medical students (E.H. and N.C.)—one at the preclinical stage and the other at the clinical stage of medical education—reviewed the apps using the given rubric, then discussed and resolved any discrepancies in points assigned. A dermatology resident (M.A.) independently reviewed the apps using the given rubric.
The mean of the student score and the resident score was calculated for each category. The sum of the averages for each category was considered the final score for an app, determining its overall quality. Apps with a total score of 5 to 10 were considered poor and inadequate for education. A total score of 10.5 to 15 indicated that an app was somewhat adequate (ie, useful for education in some aspects but falling short in others). Apps that were considered adequate for education, across all or most criteria, received a total score ranging from 15.5 to 20.
Results
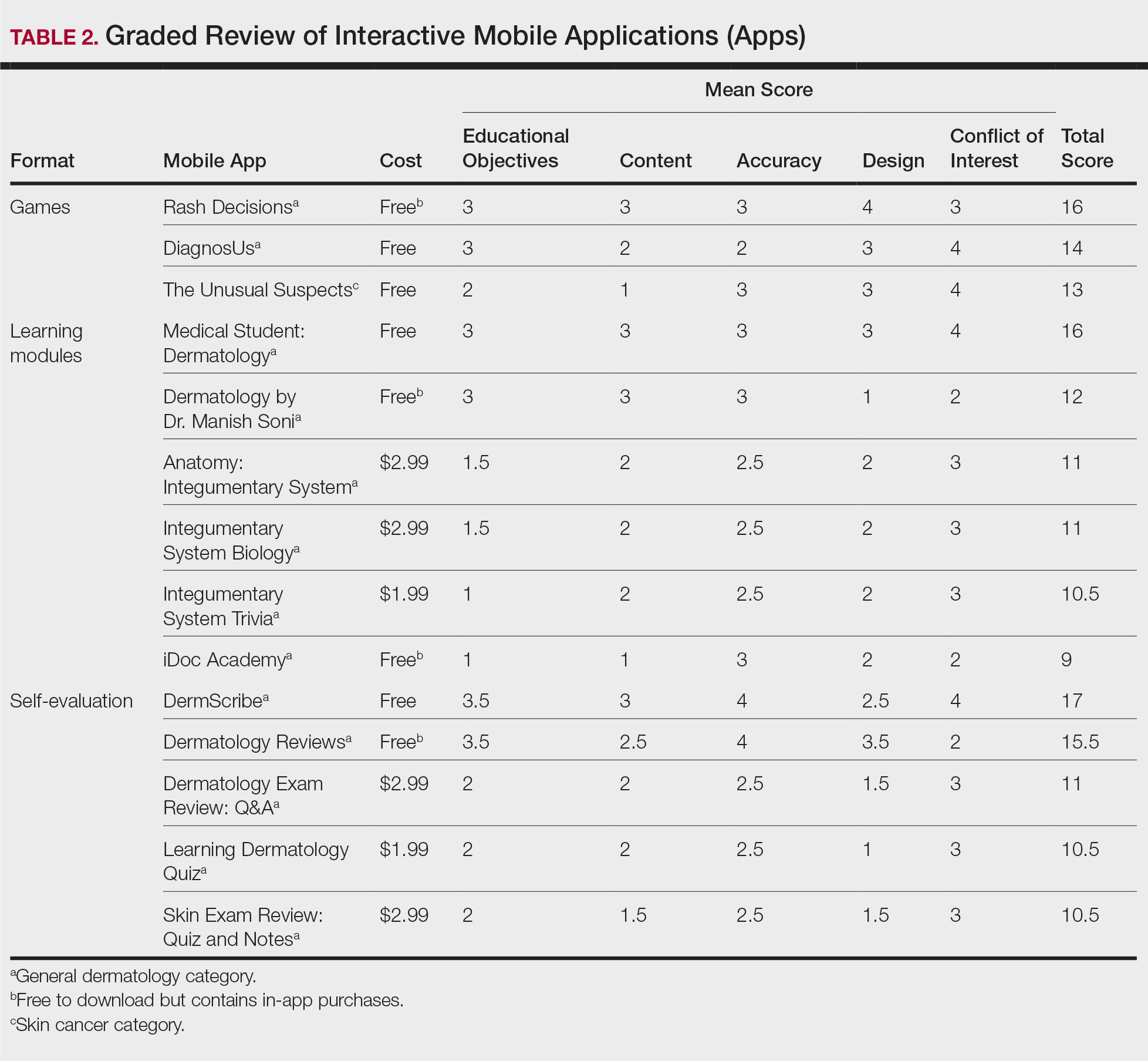
Our search generated 130 apps. After applying exclusion criteria, 42 apps were eligible for review. At the time of publication, 36 of these apps were still available. The possible range of scores based on the rubric was 5 to 20. The actual range of scores was 7 to 20. Of the 36 apps, 2 (5.6%) were poor, 16 (44.4%) were somewhat adequate, and 18 (50%) were adequate. Formats included primary resources, such as clinical decision support tools, journals, references, and a podcast (Table 1). Additionally, interactive learning tools included games, learning modules, and apps for self-evaluation (Table 2). Thirty apps covered general dermatology; others focused on skin cancer (n=5) and cosmetic dermatology (n=1). Regarding cost, 29 apps were free to download, whereas 7 charged a fee (mean price, $2.56).

Comment
In addition to the convenience of having an educational tool in their white-coat pocket, learners of dermatology have been shown to benefit from supplementing their curriculum with mobile apps, which sets the stage for formal integration of mobile apps into dermatology teaching in the future.8 Prior to widespread adoption, mobile apps must be evaluated for content and utility, starting with an objective rubric.
Without official scientific standards in place, it was unsurprising that only half of the dermatology education applications were classified as adequate in this study. Among the types of apps offered—clinical decision support tools, journals, references, podcast, games, learning modules, and self-evaluation—certain categories scored higher than others. App formats with the highest average score (16.5 out of 20) were journals and podcast.
One barrier to utilization of these apps was that a subscription to the journals and podcast was required to obtain access to all available content. Students and trainees can seek out library resources at their academic institutions to take advantage of journal subscriptions available to them at no additional cost. Dermatology residents can take advantage of their complimentary membership in the American Academy of Dermatology for a free subscription to AAD Dialogues in Dermatology (otherwise $179 annually for nonresident members and $320 annually for nonmembers).
On the other hand, learning module was the lowest-rated format (average score, 11.3 out of 20), with only Medical Student: Dermatology qualifying as adequate (total score, 16). This finding is worrisome given that students and residents might look to learning modules for quick targeted lessons on specific topics.
The lowest-scoring app, a clinical decision support tool called Naturelize, received a total score of 7. Although it listed the indications and contraindications for dermal filler types to be used in different locations on the face, there was a clear conflict of interest, oversimplified design, and little evidence-based education, mirroring the current state of cosmetic dermatology training in residency, in which trainees think they are inadequately prepared for aesthetic procedures and comparative effectiveness research is lacking.9-11
At the opposite end of the spectrum, MyDermPath+ was a reference app with a total score of 20. The app cited credible authors with a medical degree (MD) and had an easy-to-use, well-designed interface, including a reference guide, differential builder, and quiz for a range of topics within dermatology. As a free download without in-app purchases or advertisements, there was no evidence of conflict of interest. The position of a dermatopathology app as the top dermatology education mobile app might reflect an increased emphasis on dermatopathology education in residency as well as a transition to digitization of slides.5
The second-highest scoring apps (total score of 19 points) were Dermatology Database and VisualDx. Both were references covering a wide range of dermatology topics. Dermatology Database was a comprehensive search tool for diseases, drugs, procedures, and terms that was simple and entirely free to use but did not cite references. VisualDx, as its name suggests, offered quality clinical images, complete guides with references, and a unique differential builder. An annual subscription is $399.99, but the process to gain free access through a participating academic institution was simple.
Games were a unique mobile app format; however, 2 of 3 games scored in the somewhat adequate range. The game DiagnosUs, which tested users’ ability to differentiate skin cancer and psoriasis from dermatitis on clinical images, would benefit from more comprehensive content as well as professional verification of true diagnoses, which earned the app 2 points in both the content and accuracy categories. The Unusual Suspects tested the ABCDE algorithm in a short learning module, followed by a simple game that involved identification of melanoma in a timed setting. Although the design was novel and interactive, the game was limited to the same 5 melanoma tumors overlaid on pictures of normal skin. The narrow scope earned 1 point for content, the redundancy in the game earned 3 points for design, and the lack of real clinical images earned 2 points for educational objectives. Although game-format mobile apps have the capability to challenge the user’s knowledge with a built-in feedback or reward system, improvements should be made to ensure that apps are equally educational as they are engaging.
AAD Dialogues in Dermatology was the only app in the form of a podcast and provided expert interviews along with disclosures, transcripts, commentary, and references. More than half the content in the app could not be accessed without a subscription, earning 2.5 points in the conflict of interest category. Additionally, several flaws resulted in a design score of 2.5, including inconsistent availability of transcripts, poor quality of sound on some episodes, difficulty distinguishing new episodes from those already played, and a glitch that removed the episode duration. Still, the app was a valuable and comprehensive resource, with clear objectives and cited references. With improvements in content, affordability, and user experience, apps in unique formats such as games and podcasts might appeal to kinesthetic and auditory learners.
An important factor to consider when discussing mobile apps for students and residents is cost. With rising prices of board examinations and preparation materials, supplementary study tools should not come with an exorbitant price tag. Therefore, we limited our evaluation to apps that were free or cost less than $5 to download. Even so, subscriptions and other in-app purchases were an obstacle in one-third of apps, ranging from $4.99 to unlock additional content in Rash Decisions to $69.99 to access most topics in Fitzpatrick’s Color Atlas. The highest-rated app in our study, MyDermPath+, historically cost $19.99 to download but became free with a grant from the Sulzberger Foundation.12 An initial investment to develop quality apps for the purpose of dermatology education might pay off in the end.
To evaluate the apps from the perspective of the target demographic of this study, 2 medical students—one in the preclinical stage and the other in the clinical stage of medical education—and a dermatology resident graded the apps. Certain limitations exist in this type of study, including differing learning styles, which might influence the types of apps that evaluators found most impactful to their education. Interestingly, some apps earned a higher resident score than student score. In particular, RightSite (a reference that helps with anatomically correct labeling) and Mohs Surgery Appropriate Use Criteria (a clinical decision support tool to determine whether to perform Mohs surgery) each had a 3-point discrepancy (data not shown). A resident might benefit from these practical apps in day-to-day practice, but a student would be less likely to find them useful as a learning tool.
Still, by defining adequate teaching value using specific categories of educational objectives, content, accuracy, design, and conflict of interest, we attempted to minimize the effect of personal preference on the grading process. Although we acknowledge a degree of subjectivity, we found that utilizing a previously published rubric with defined criteria was crucial in remaining unbiased.
Conclusion
Further studies should evaluate additional apps available on Apple’s iPad (tablet), as well as those on other operating systems, including Google’s Android. To ensure the existence of mobile apps as adequate education tools, they should be peer reviewed prior to publication or before widespread use by future and current providers at the minimum. To maximize free access to highly valuable resources available in the palm of their hand, students and trainees should contact the library at their academic institution.
- Craddock MF, Blondin HM, Youssef MJ, et al. Online education improves pediatric residents' understanding of atopic dermatitis. Pediatr Dermatol. 2018;35:64-69.
- Lacy FA, Coman GC, Holliday AC, et al. Assessment of smartphone application for teaching intuitive visual diagnosis of melanoma. JAMA Dermatol. 2018;154:730-731.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology--2017 update. Dermatol Online J. 2018;24:13.
- Liu R-F, Wang F-Y, Yen H, et al. A new mobile learning module using smartphone wallpapers in identification of medical fungi for medical students and residents. Int J Dermatol. 2018;57:458-462.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Mercer JM. An array of mobile apps for dermatologists. J Cutan Med Surg. 2014;18:295-297.
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol. 2013;68:e23-e28.
- Waldman A, Sobanko JF, Alam M. Practice and educational gaps in cosmetic dermatologic surgery. Dermatol Clin. 2016;34:341-346.
- Nielson CB, Harb JN, Motaparthi K. Education in cosmetic procedural dermatology: resident experiences and perceptions. J Clin Aesthet Dermatol. 2019;12:E70-E72.
- Hanna MG, Parwani AV, Pantanowitz L, et al. Smartphone applications: a contemporary resource for dermatopathology. J Pathol Inform. 2015;6:44.
With today’s technology, it is easier than ever to access web-based tools that enrich traditional dermatology education. The literature supports the use of these innovative platforms to enhance learning at the student and trainee levels. A controlled study of pediatric residents showed that online modules effectively supplemented clinical experience with atopic dermatitis.1 In a randomized diagnostic study of medical students, practice with an image-based web application (app) that teaches rapid recognition of melanoma proved more effective than learning a rule-based algorithm.2 Given the visual nature of dermatology, pattern recognition is an essential skill that is fostered through experience and is only made more accessible with technology.
With the added benefit of convenience and accessibility, mobile apps can supplement experiential learning. Mirroring the overall growth of mobile apps, the number of available dermatology apps has increased.3 Dermatology mobile apps serve purposes ranging from quick reference tools to comprehensive modules, journals, and question banks. At an academic hospital in Taiwan, both nondermatology and dermatology trainees’ examination performance improved after 3 weeks of using a smartphone-based wallpaper learning module displaying morphologic characteristics of fungi.4 With the expansion of virtual microscopy, mobile apps also have been created as a learning tool for dermatopathology, giving trainees the flexibility and autonomy to view slides on their own time.5 Nevertheless, the literature on dermatology mobile apps designed for the education of medical students and trainees is limited, demonstrating a need for further investigation.
Prior studies have reviewed dermatology apps for patients and practicing dermatologists.6-8 Herein, we focus on mobile apps targeting students and residents learning dermatology. General dermatology reference apps and educational aid apps have grown by 33% and 32%, respectively, from 2014 to 2017.3 As with any resource meant to educate future and current medical providers, there must be an objective review process in place to ensure accurate, unbiased, evidence-based teaching.
Well-organized, comprehensive information and a user-friendly interface are additional factors of importance when selecting an educational mobile app. When discussing supplemental resources, accessibility and affordability also are priorities given the high cost of a medical education at baseline. Overall, there is a need for a standardized method to evaluate the key factors of an educational mobile app that make it appropriate for this demographic. We conducted a search of mobile apps relating to dermatology education for students and residents.
Methods
We searched for publicly available mobile apps relating to dermatology education in the App Store (Apple Inc) from September to November 2019 using the search terms dermatology education, dermoscopy education, melanoma education, skin cancer education, psoriasis education, rosacea education, acne education, eczema education, dermal fillers education, and Mohs surgery education. We excluded apps that were not in English, were created for a conference, cost more than $5 to download, or did not include a specific dermatology education section. In this way, we hoped to evaluate apps that were relevant, accessible, and affordable.
We modeled our study after a review of patient education apps performed by Masud et al6 and utilized their quantified grading rubric (scale of 1 to 4). We found their established criteria—educational objectives, content, accuracy, design, and conflict of interest—to be equally applicable for evaluating apps designed for professional education.6 Each app earned a minimum of 1 point and a maximum of 4 points per criterion. One point was given if the app did not fulfill the criterion, 2 points for minimally fulfilling the criterion, 3 points for mostly fulfilling the criterion, and 4 points if the criterion was completely fulfilled. Two medical students (E.H. and N.C.)—one at the preclinical stage and the other at the clinical stage of medical education—reviewed the apps using the given rubric, then discussed and resolved any discrepancies in points assigned. A dermatology resident (M.A.) independently reviewed the apps using the given rubric.
The mean of the student score and the resident score was calculated for each category. The sum of the averages for each category was considered the final score for an app, determining its overall quality. Apps with a total score of 5 to 10 were considered poor and inadequate for education. A total score of 10.5 to 15 indicated that an app was somewhat adequate (ie, useful for education in some aspects but falling short in others). Apps that were considered adequate for education, across all or most criteria, received a total score ranging from 15.5 to 20.
Results
Our search generated 130 apps. After applying exclusion criteria, 42 apps were eligible for review. At the time of publication, 36 of these apps were still available. The possible range of scores based on the rubric was 5 to 20. The actual range of scores was 7 to 20. Of the 36 apps, 2 (5.6%) were poor, 16 (44.4%) were somewhat adequate, and 18 (50%) were adequate. Formats included primary resources, such as clinical decision support tools, journals, references, and a podcast (Table 1). Additionally, interactive learning tools included games, learning modules, and apps for self-evaluation (Table 2). Thirty apps covered general dermatology; others focused on skin cancer (n=5) and cosmetic dermatology (n=1). Regarding cost, 29 apps were free to download, whereas 7 charged a fee (mean price, $2.56).


Comment
In addition to the convenience of having an educational tool in their white-coat pocket, learners of dermatology have been shown to benefit from supplementing their curriculum with mobile apps, which sets the stage for formal integration of mobile apps into dermatology teaching in the future.8 Prior to widespread adoption, mobile apps must be evaluated for content and utility, starting with an objective rubric.
Without official scientific standards in place, it was unsurprising that only half of the dermatology education applications were classified as adequate in this study. Among the types of apps offered—clinical decision support tools, journals, references, podcast, games, learning modules, and self-evaluation—certain categories scored higher than others. App formats with the highest average score (16.5 out of 20) were journals and podcast.
One barrier to utilization of these apps was that a subscription to the journals and podcast was required to obtain access to all available content. Students and trainees can seek out library resources at their academic institutions to take advantage of journal subscriptions available to them at no additional cost. Dermatology residents can take advantage of their complimentary membership in the American Academy of Dermatology for a free subscription to AAD Dialogues in Dermatology (otherwise $179 annually for nonresident members and $320 annually for nonmembers).
On the other hand, learning module was the lowest-rated format (average score, 11.3 out of 20), with only Medical Student: Dermatology qualifying as adequate (total score, 16). This finding is worrisome given that students and residents might look to learning modules for quick targeted lessons on specific topics.
The lowest-scoring app, a clinical decision support tool called Naturelize, received a total score of 7. Although it listed the indications and contraindications for dermal filler types to be used in different locations on the face, there was a clear conflict of interest, oversimplified design, and little evidence-based education, mirroring the current state of cosmetic dermatology training in residency, in which trainees think they are inadequately prepared for aesthetic procedures and comparative effectiveness research is lacking.9-11
At the opposite end of the spectrum, MyDermPath+ was a reference app with a total score of 20. The app cited credible authors with a medical degree (MD) and had an easy-to-use, well-designed interface, including a reference guide, differential builder, and quiz for a range of topics within dermatology. As a free download without in-app purchases or advertisements, there was no evidence of conflict of interest. The position of a dermatopathology app as the top dermatology education mobile app might reflect an increased emphasis on dermatopathology education in residency as well as a transition to digitization of slides.5
The second-highest scoring apps (total score of 19 points) were Dermatology Database and VisualDx. Both were references covering a wide range of dermatology topics. Dermatology Database was a comprehensive search tool for diseases, drugs, procedures, and terms that was simple and entirely free to use but did not cite references. VisualDx, as its name suggests, offered quality clinical images, complete guides with references, and a unique differential builder. An annual subscription is $399.99, but the process to gain free access through a participating academic institution was simple.
Games were a unique mobile app format; however, 2 of 3 games scored in the somewhat adequate range. The game DiagnosUs, which tested users’ ability to differentiate skin cancer and psoriasis from dermatitis on clinical images, would benefit from more comprehensive content as well as professional verification of true diagnoses, which earned the app 2 points in both the content and accuracy categories. The Unusual Suspects tested the ABCDE algorithm in a short learning module, followed by a simple game that involved identification of melanoma in a timed setting. Although the design was novel and interactive, the game was limited to the same 5 melanoma tumors overlaid on pictures of normal skin. The narrow scope earned 1 point for content, the redundancy in the game earned 3 points for design, and the lack of real clinical images earned 2 points for educational objectives. Although game-format mobile apps have the capability to challenge the user’s knowledge with a built-in feedback or reward system, improvements should be made to ensure that apps are equally educational as they are engaging.
AAD Dialogues in Dermatology was the only app in the form of a podcast and provided expert interviews along with disclosures, transcripts, commentary, and references. More than half the content in the app could not be accessed without a subscription, earning 2.5 points in the conflict of interest category. Additionally, several flaws resulted in a design score of 2.5, including inconsistent availability of transcripts, poor quality of sound on some episodes, difficulty distinguishing new episodes from those already played, and a glitch that removed the episode duration. Still, the app was a valuable and comprehensive resource, with clear objectives and cited references. With improvements in content, affordability, and user experience, apps in unique formats such as games and podcasts might appeal to kinesthetic and auditory learners.
An important factor to consider when discussing mobile apps for students and residents is cost. With rising prices of board examinations and preparation materials, supplementary study tools should not come with an exorbitant price tag. Therefore, we limited our evaluation to apps that were free or cost less than $5 to download. Even so, subscriptions and other in-app purchases were an obstacle in one-third of apps, ranging from $4.99 to unlock additional content in Rash Decisions to $69.99 to access most topics in Fitzpatrick’s Color Atlas. The highest-rated app in our study, MyDermPath+, historically cost $19.99 to download but became free with a grant from the Sulzberger Foundation.12 An initial investment to develop quality apps for the purpose of dermatology education might pay off in the end.
To evaluate the apps from the perspective of the target demographic of this study, 2 medical students—one in the preclinical stage and the other in the clinical stage of medical education—and a dermatology resident graded the apps. Certain limitations exist in this type of study, including differing learning styles, which might influence the types of apps that evaluators found most impactful to their education. Interestingly, some apps earned a higher resident score than student score. In particular, RightSite (a reference that helps with anatomically correct labeling) and Mohs Surgery Appropriate Use Criteria (a clinical decision support tool to determine whether to perform Mohs surgery) each had a 3-point discrepancy (data not shown). A resident might benefit from these practical apps in day-to-day practice, but a student would be less likely to find them useful as a learning tool.
Still, by defining adequate teaching value using specific categories of educational objectives, content, accuracy, design, and conflict of interest, we attempted to minimize the effect of personal preference on the grading process. Although we acknowledge a degree of subjectivity, we found that utilizing a previously published rubric with defined criteria was crucial in remaining unbiased.
Conclusion
Further studies should evaluate additional apps available on Apple’s iPad (tablet), as well as those on other operating systems, including Google’s Android. To ensure the existence of mobile apps as adequate education tools, they should be peer reviewed prior to publication or before widespread use by future and current providers at the minimum. To maximize free access to highly valuable resources available in the palm of their hand, students and trainees should contact the library at their academic institution.
With today’s technology, it is easier than ever to access web-based tools that enrich traditional dermatology education. The literature supports the use of these innovative platforms to enhance learning at the student and trainee levels. A controlled study of pediatric residents showed that online modules effectively supplemented clinical experience with atopic dermatitis.1 In a randomized diagnostic study of medical students, practice with an image-based web application (app) that teaches rapid recognition of melanoma proved more effective than learning a rule-based algorithm.2 Given the visual nature of dermatology, pattern recognition is an essential skill that is fostered through experience and is only made more accessible with technology.
With the added benefit of convenience and accessibility, mobile apps can supplement experiential learning. Mirroring the overall growth of mobile apps, the number of available dermatology apps has increased.3 Dermatology mobile apps serve purposes ranging from quick reference tools to comprehensive modules, journals, and question banks. At an academic hospital in Taiwan, both nondermatology and dermatology trainees’ examination performance improved after 3 weeks of using a smartphone-based wallpaper learning module displaying morphologic characteristics of fungi.4 With the expansion of virtual microscopy, mobile apps also have been created as a learning tool for dermatopathology, giving trainees the flexibility and autonomy to view slides on their own time.5 Nevertheless, the literature on dermatology mobile apps designed for the education of medical students and trainees is limited, demonstrating a need for further investigation.
Prior studies have reviewed dermatology apps for patients and practicing dermatologists.6-8 Herein, we focus on mobile apps targeting students and residents learning dermatology. General dermatology reference apps and educational aid apps have grown by 33% and 32%, respectively, from 2014 to 2017.3 As with any resource meant to educate future and current medical providers, there must be an objective review process in place to ensure accurate, unbiased, evidence-based teaching.
Well-organized, comprehensive information and a user-friendly interface are additional factors of importance when selecting an educational mobile app. When discussing supplemental resources, accessibility and affordability also are priorities given the high cost of a medical education at baseline. Overall, there is a need for a standardized method to evaluate the key factors of an educational mobile app that make it appropriate for this demographic. We conducted a search of mobile apps relating to dermatology education for students and residents.
Methods
We searched for publicly available mobile apps relating to dermatology education in the App Store (Apple Inc) from September to November 2019 using the search terms dermatology education, dermoscopy education, melanoma education, skin cancer education, psoriasis education, rosacea education, acne education, eczema education, dermal fillers education, and Mohs surgery education. We excluded apps that were not in English, were created for a conference, cost more than $5 to download, or did not include a specific dermatology education section. In this way, we hoped to evaluate apps that were relevant, accessible, and affordable.
We modeled our study after a review of patient education apps performed by Masud et al6 and utilized their quantified grading rubric (scale of 1 to 4). We found their established criteria—educational objectives, content, accuracy, design, and conflict of interest—to be equally applicable for evaluating apps designed for professional education.6 Each app earned a minimum of 1 point and a maximum of 4 points per criterion. One point was given if the app did not fulfill the criterion, 2 points for minimally fulfilling the criterion, 3 points for mostly fulfilling the criterion, and 4 points if the criterion was completely fulfilled. Two medical students (E.H. and N.C.)—one at the preclinical stage and the other at the clinical stage of medical education—reviewed the apps using the given rubric, then discussed and resolved any discrepancies in points assigned. A dermatology resident (M.A.) independently reviewed the apps using the given rubric.
The mean of the student score and the resident score was calculated for each category. The sum of the averages for each category was considered the final score for an app, determining its overall quality. Apps with a total score of 5 to 10 were considered poor and inadequate for education. A total score of 10.5 to 15 indicated that an app was somewhat adequate (ie, useful for education in some aspects but falling short in others). Apps that were considered adequate for education, across all or most criteria, received a total score ranging from 15.5 to 20.
Results
Our search generated 130 apps. After applying exclusion criteria, 42 apps were eligible for review. At the time of publication, 36 of these apps were still available. The possible range of scores based on the rubric was 5 to 20. The actual range of scores was 7 to 20. Of the 36 apps, 2 (5.6%) were poor, 16 (44.4%) were somewhat adequate, and 18 (50%) were adequate. Formats included primary resources, such as clinical decision support tools, journals, references, and a podcast (Table 1). Additionally, interactive learning tools included games, learning modules, and apps for self-evaluation (Table 2). Thirty apps covered general dermatology; others focused on skin cancer (n=5) and cosmetic dermatology (n=1). Regarding cost, 29 apps were free to download, whereas 7 charged a fee (mean price, $2.56).


Comment
In addition to the convenience of having an educational tool in their white-coat pocket, learners of dermatology have been shown to benefit from supplementing their curriculum with mobile apps, which sets the stage for formal integration of mobile apps into dermatology teaching in the future.8 Prior to widespread adoption, mobile apps must be evaluated for content and utility, starting with an objective rubric.
Without official scientific standards in place, it was unsurprising that only half of the dermatology education applications were classified as adequate in this study. Among the types of apps offered—clinical decision support tools, journals, references, podcast, games, learning modules, and self-evaluation—certain categories scored higher than others. App formats with the highest average score (16.5 out of 20) were journals and podcast.
One barrier to utilization of these apps was that a subscription to the journals and podcast was required to obtain access to all available content. Students and trainees can seek out library resources at their academic institutions to take advantage of journal subscriptions available to them at no additional cost. Dermatology residents can take advantage of their complimentary membership in the American Academy of Dermatology for a free subscription to AAD Dialogues in Dermatology (otherwise $179 annually for nonresident members and $320 annually for nonmembers).
On the other hand, learning module was the lowest-rated format (average score, 11.3 out of 20), with only Medical Student: Dermatology qualifying as adequate (total score, 16). This finding is worrisome given that students and residents might look to learning modules for quick targeted lessons on specific topics.
The lowest-scoring app, a clinical decision support tool called Naturelize, received a total score of 7. Although it listed the indications and contraindications for dermal filler types to be used in different locations on the face, there was a clear conflict of interest, oversimplified design, and little evidence-based education, mirroring the current state of cosmetic dermatology training in residency, in which trainees think they are inadequately prepared for aesthetic procedures and comparative effectiveness research is lacking.9-11
At the opposite end of the spectrum, MyDermPath+ was a reference app with a total score of 20. The app cited credible authors with a medical degree (MD) and had an easy-to-use, well-designed interface, including a reference guide, differential builder, and quiz for a range of topics within dermatology. As a free download without in-app purchases or advertisements, there was no evidence of conflict of interest. The position of a dermatopathology app as the top dermatology education mobile app might reflect an increased emphasis on dermatopathology education in residency as well as a transition to digitization of slides.5
The second-highest scoring apps (total score of 19 points) were Dermatology Database and VisualDx. Both were references covering a wide range of dermatology topics. Dermatology Database was a comprehensive search tool for diseases, drugs, procedures, and terms that was simple and entirely free to use but did not cite references. VisualDx, as its name suggests, offered quality clinical images, complete guides with references, and a unique differential builder. An annual subscription is $399.99, but the process to gain free access through a participating academic institution was simple.
Games were a unique mobile app format; however, 2 of 3 games scored in the somewhat adequate range. The game DiagnosUs, which tested users’ ability to differentiate skin cancer and psoriasis from dermatitis on clinical images, would benefit from more comprehensive content as well as professional verification of true diagnoses, which earned the app 2 points in both the content and accuracy categories. The Unusual Suspects tested the ABCDE algorithm in a short learning module, followed by a simple game that involved identification of melanoma in a timed setting. Although the design was novel and interactive, the game was limited to the same 5 melanoma tumors overlaid on pictures of normal skin. The narrow scope earned 1 point for content, the redundancy in the game earned 3 points for design, and the lack of real clinical images earned 2 points for educational objectives. Although game-format mobile apps have the capability to challenge the user’s knowledge with a built-in feedback or reward system, improvements should be made to ensure that apps are equally educational as they are engaging.
AAD Dialogues in Dermatology was the only app in the form of a podcast and provided expert interviews along with disclosures, transcripts, commentary, and references. More than half the content in the app could not be accessed without a subscription, earning 2.5 points in the conflict of interest category. Additionally, several flaws resulted in a design score of 2.5, including inconsistent availability of transcripts, poor quality of sound on some episodes, difficulty distinguishing new episodes from those already played, and a glitch that removed the episode duration. Still, the app was a valuable and comprehensive resource, with clear objectives and cited references. With improvements in content, affordability, and user experience, apps in unique formats such as games and podcasts might appeal to kinesthetic and auditory learners.
An important factor to consider when discussing mobile apps for students and residents is cost. With rising prices of board examinations and preparation materials, supplementary study tools should not come with an exorbitant price tag. Therefore, we limited our evaluation to apps that were free or cost less than $5 to download. Even so, subscriptions and other in-app purchases were an obstacle in one-third of apps, ranging from $4.99 to unlock additional content in Rash Decisions to $69.99 to access most topics in Fitzpatrick’s Color Atlas. The highest-rated app in our study, MyDermPath+, historically cost $19.99 to download but became free with a grant from the Sulzberger Foundation.12 An initial investment to develop quality apps for the purpose of dermatology education might pay off in the end.
To evaluate the apps from the perspective of the target demographic of this study, 2 medical students—one in the preclinical stage and the other in the clinical stage of medical education—and a dermatology resident graded the apps. Certain limitations exist in this type of study, including differing learning styles, which might influence the types of apps that evaluators found most impactful to their education. Interestingly, some apps earned a higher resident score than student score. In particular, RightSite (a reference that helps with anatomically correct labeling) and Mohs Surgery Appropriate Use Criteria (a clinical decision support tool to determine whether to perform Mohs surgery) each had a 3-point discrepancy (data not shown). A resident might benefit from these practical apps in day-to-day practice, but a student would be less likely to find them useful as a learning tool.
Still, by defining adequate teaching value using specific categories of educational objectives, content, accuracy, design, and conflict of interest, we attempted to minimize the effect of personal preference on the grading process. Although we acknowledge a degree of subjectivity, we found that utilizing a previously published rubric with defined criteria was crucial in remaining unbiased.
Conclusion
Further studies should evaluate additional apps available on Apple’s iPad (tablet), as well as those on other operating systems, including Google’s Android. To ensure the existence of mobile apps as adequate education tools, they should be peer reviewed prior to publication or before widespread use by future and current providers at the minimum. To maximize free access to highly valuable resources available in the palm of their hand, students and trainees should contact the library at their academic institution.
- Craddock MF, Blondin HM, Youssef MJ, et al. Online education improves pediatric residents' understanding of atopic dermatitis. Pediatr Dermatol. 2018;35:64-69.
- Lacy FA, Coman GC, Holliday AC, et al. Assessment of smartphone application for teaching intuitive visual diagnosis of melanoma. JAMA Dermatol. 2018;154:730-731.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology--2017 update. Dermatol Online J. 2018;24:13.
- Liu R-F, Wang F-Y, Yen H, et al. A new mobile learning module using smartphone wallpapers in identification of medical fungi for medical students and residents. Int J Dermatol. 2018;57:458-462.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Mercer JM. An array of mobile apps for dermatologists. J Cutan Med Surg. 2014;18:295-297.
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol. 2013;68:e23-e28.
- Waldman A, Sobanko JF, Alam M. Practice and educational gaps in cosmetic dermatologic surgery. Dermatol Clin. 2016;34:341-346.
- Nielson CB, Harb JN, Motaparthi K. Education in cosmetic procedural dermatology: resident experiences and perceptions. J Clin Aesthet Dermatol. 2019;12:E70-E72.
- Hanna MG, Parwani AV, Pantanowitz L, et al. Smartphone applications: a contemporary resource for dermatopathology. J Pathol Inform. 2015;6:44.
- Craddock MF, Blondin HM, Youssef MJ, et al. Online education improves pediatric residents' understanding of atopic dermatitis. Pediatr Dermatol. 2018;35:64-69.
- Lacy FA, Coman GC, Holliday AC, et al. Assessment of smartphone application for teaching intuitive visual diagnosis of melanoma. JAMA Dermatol. 2018;154:730-731.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology--2017 update. Dermatol Online J. 2018;24:13.
- Liu R-F, Wang F-Y, Yen H, et al. A new mobile learning module using smartphone wallpapers in identification of medical fungi for medical students and residents. Int J Dermatol. 2018;57:458-462.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Mercer JM. An array of mobile apps for dermatologists. J Cutan Med Surg. 2014;18:295-297.
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol. 2013;68:e23-e28.
- Waldman A, Sobanko JF, Alam M. Practice and educational gaps in cosmetic dermatologic surgery. Dermatol Clin. 2016;34:341-346.
- Nielson CB, Harb JN, Motaparthi K. Education in cosmetic procedural dermatology: resident experiences and perceptions. J Clin Aesthet Dermatol. 2019;12:E70-E72.
- Hanna MG, Parwani AV, Pantanowitz L, et al. Smartphone applications: a contemporary resource for dermatopathology. J Pathol Inform. 2015;6:44.
Practice Points
- Mobile applications (apps) are a convenient way to learn dermatology, but there is no objective method to assess their quality.
- To determine which apps are most useful for education, we performed a graded review of dermatology apps targeted to students and residents.
- By applying a rubric to 36 affordable apps, we identified 18 (50%) with adequate teaching value.
Maintaining credibility when evaluating new dermatologic technologies
A healthy dose of skepticism is reasonable when evaluating new technologies to potentially use in your practice, but being overly critical can backfire, according to E. Victor Ross, MD.
During a virtual course on laser and aesthetic skin therapy, he noted that dermatologists may be evaluating new technologies in roles as an investigator or provider, or as a provider who buys the equipment outright, without any direct compensation from industry. “If you’re doing investigative work, you’re already in a conflicted situation because you’re trying to serve two masters,” said Dr. Ross, who directs the Scripps Clinic Laser and Cosmetic Dermatology Center in San Diego. “You’re trying to advance science and to make sure your reputation is intact and maybe even enhanced, but you’re also kind of at the whim of industry, because they have a goal. Sometimes the goals are similar. Your goal is to advance the technology and advance patient care, but they have a goal of selling equipment. Those goals should be compatible, and they should be in the same pathway; they should be parallel.”
Being too critical as an investigator/researcher of new technologies can hinder further interactions with industry. “Sometimes your criticism can be premature, and small changes in the technology and/or waiting for results can validate the technology,” he said. “Maybe it’s a skin-tightening technology, or maybe there’s something you don’t like about it; you have a prototype and you say: ‘This is not so great,’ but these are studies that take a long time to evaluate, like hair removal. Generally, if you’re a big critic, after a while, nobody wants to hear it. So, you can’t be overly critical. I think you can be skeptical, but not overly critical.”
On the other hand, Dr. Ross continued, if you cheerlead for the device industry, your reputation may be sold to the highest bidder. “You may compromise your ability to be trusted in future work or presentations. I’ve regretted some things I said many years ago, not because I was being dishonest but because I really wasn’t as skeptical as I should have been about the types of results I was getting. You do tend to get on a bandwagon; you want everything to be positive,” he said, adding: “The other thing that can happen is, if things don’t work out with other buyers, they’re going to say, ‘I can’t get the same results.’ If somebody can’t replicate what you’re doing, it’s going to put your reputation on the line to some degree. So, you have to be very careful.”
Before agreeing to evaluate a new technology as an investigator or in your own practice, Dr. Ross recommended asking yourself if the intervention makes sense on a gross or microanatomic level. “There should be a physical basis for how it works, and ideally there should be some histology that backs it up,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Where is the data to support the outcomes? If it is your own data, how skeptical have you been in its acquisition and assessment?”
When evaluating a new technology, Dr. Ross recommended starting with test spots and low settings. “It behooves you not to be too aggressive because there are some untoward things you may not see right away.” He advised evaluating short- and long-term outcomes and being wary of devices that heat very deeply and rely on long-term outcomes, such as hair removal, acne improvement, scar improvement, and skin tightening – all of which require long intervals for assessment. As one of his “Ross Rules” states: “The deeper the heating and the less focused the heating, the less we can expect results.” Another Ross Rule calls for being skeptical about technologies without an immediate finding that can be “seen” on routine histology. “To me, the deeper the procedure, particularly if it’s not fractional, the confidence of my outcome is diminished,” he said. “The exception is any intervention that relies on selective photothermolysis.”
Using new technologies in practice
Dr. Ross also offered tips on how to properly incorporate newly approved technologies into your practice safely, including the use of visual endpoints. “That’s tough for technologies like laser liposuction or fractional technologies, where we rely more on ‘guidelines’ than endpoints,” he said. In addition, he recommended gradually increasing settings and using test areas to stall and/or hone techniques. “It’s exciting, but you’re like a test pilot. You want to be careful that you are doing things that are not likely to risk the patient. Try some off-the-face applications first. When you’re using a new technology, push a little harder with each patient so you can find a safe zone for that technology. You don’t have to get it all in one treatment session. Be conservative. Anticipate that you may be underassessing the immediate response.”
Above all, be careful. “Use your judgment more than laser company-prescribed settings,” he said. “Most companies have the go-by settings on the low side for patient protection, but sometimes efficacy suffers. Use cautiously on friends and family. If you treat a spouse or a friend and things don’t go perfectly, that’s always a recipe for a problem.”
Dr. Ross reported having received financial grants and research grants from Candela, Cutera, Lumenis, and Lutronic; consulting fees from Palomar; and honoraria from Cynosure, Cutera, and Lumenis. He has also received research funding from Venous Concepts, Pulsed Biosciences, and Cynosure.
A healthy dose of skepticism is reasonable when evaluating new technologies to potentially use in your practice, but being overly critical can backfire, according to E. Victor Ross, MD.
During a virtual course on laser and aesthetic skin therapy, he noted that dermatologists may be evaluating new technologies in roles as an investigator or provider, or as a provider who buys the equipment outright, without any direct compensation from industry. “If you’re doing investigative work, you’re already in a conflicted situation because you’re trying to serve two masters,” said Dr. Ross, who directs the Scripps Clinic Laser and Cosmetic Dermatology Center in San Diego. “You’re trying to advance science and to make sure your reputation is intact and maybe even enhanced, but you’re also kind of at the whim of industry, because they have a goal. Sometimes the goals are similar. Your goal is to advance the technology and advance patient care, but they have a goal of selling equipment. Those goals should be compatible, and they should be in the same pathway; they should be parallel.”
Being too critical as an investigator/researcher of new technologies can hinder further interactions with industry. “Sometimes your criticism can be premature, and small changes in the technology and/or waiting for results can validate the technology,” he said. “Maybe it’s a skin-tightening technology, or maybe there’s something you don’t like about it; you have a prototype and you say: ‘This is not so great,’ but these are studies that take a long time to evaluate, like hair removal. Generally, if you’re a big critic, after a while, nobody wants to hear it. So, you can’t be overly critical. I think you can be skeptical, but not overly critical.”
On the other hand, Dr. Ross continued, if you cheerlead for the device industry, your reputation may be sold to the highest bidder. “You may compromise your ability to be trusted in future work or presentations. I’ve regretted some things I said many years ago, not because I was being dishonest but because I really wasn’t as skeptical as I should have been about the types of results I was getting. You do tend to get on a bandwagon; you want everything to be positive,” he said, adding: “The other thing that can happen is, if things don’t work out with other buyers, they’re going to say, ‘I can’t get the same results.’ If somebody can’t replicate what you’re doing, it’s going to put your reputation on the line to some degree. So, you have to be very careful.”
Before agreeing to evaluate a new technology as an investigator or in your own practice, Dr. Ross recommended asking yourself if the intervention makes sense on a gross or microanatomic level. “There should be a physical basis for how it works, and ideally there should be some histology that backs it up,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Where is the data to support the outcomes? If it is your own data, how skeptical have you been in its acquisition and assessment?”
When evaluating a new technology, Dr. Ross recommended starting with test spots and low settings. “It behooves you not to be too aggressive because there are some untoward things you may not see right away.” He advised evaluating short- and long-term outcomes and being wary of devices that heat very deeply and rely on long-term outcomes, such as hair removal, acne improvement, scar improvement, and skin tightening – all of which require long intervals for assessment. As one of his “Ross Rules” states: “The deeper the heating and the less focused the heating, the less we can expect results.” Another Ross Rule calls for being skeptical about technologies without an immediate finding that can be “seen” on routine histology. “To me, the deeper the procedure, particularly if it’s not fractional, the confidence of my outcome is diminished,” he said. “The exception is any intervention that relies on selective photothermolysis.”
Using new technologies in practice
Dr. Ross also offered tips on how to properly incorporate newly approved technologies into your practice safely, including the use of visual endpoints. “That’s tough for technologies like laser liposuction or fractional technologies, where we rely more on ‘guidelines’ than endpoints,” he said. In addition, he recommended gradually increasing settings and using test areas to stall and/or hone techniques. “It’s exciting, but you’re like a test pilot. You want to be careful that you are doing things that are not likely to risk the patient. Try some off-the-face applications first. When you’re using a new technology, push a little harder with each patient so you can find a safe zone for that technology. You don’t have to get it all in one treatment session. Be conservative. Anticipate that you may be underassessing the immediate response.”
Above all, be careful. “Use your judgment more than laser company-prescribed settings,” he said. “Most companies have the go-by settings on the low side for patient protection, but sometimes efficacy suffers. Use cautiously on friends and family. If you treat a spouse or a friend and things don’t go perfectly, that’s always a recipe for a problem.”
Dr. Ross reported having received financial grants and research grants from Candela, Cutera, Lumenis, and Lutronic; consulting fees from Palomar; and honoraria from Cynosure, Cutera, and Lumenis. He has also received research funding from Venous Concepts, Pulsed Biosciences, and Cynosure.
A healthy dose of skepticism is reasonable when evaluating new technologies to potentially use in your practice, but being overly critical can backfire, according to E. Victor Ross, MD.
During a virtual course on laser and aesthetic skin therapy, he noted that dermatologists may be evaluating new technologies in roles as an investigator or provider, or as a provider who buys the equipment outright, without any direct compensation from industry. “If you’re doing investigative work, you’re already in a conflicted situation because you’re trying to serve two masters,” said Dr. Ross, who directs the Scripps Clinic Laser and Cosmetic Dermatology Center in San Diego. “You’re trying to advance science and to make sure your reputation is intact and maybe even enhanced, but you’re also kind of at the whim of industry, because they have a goal. Sometimes the goals are similar. Your goal is to advance the technology and advance patient care, but they have a goal of selling equipment. Those goals should be compatible, and they should be in the same pathway; they should be parallel.”
Being too critical as an investigator/researcher of new technologies can hinder further interactions with industry. “Sometimes your criticism can be premature, and small changes in the technology and/or waiting for results can validate the technology,” he said. “Maybe it’s a skin-tightening technology, or maybe there’s something you don’t like about it; you have a prototype and you say: ‘This is not so great,’ but these are studies that take a long time to evaluate, like hair removal. Generally, if you’re a big critic, after a while, nobody wants to hear it. So, you can’t be overly critical. I think you can be skeptical, but not overly critical.”
On the other hand, Dr. Ross continued, if you cheerlead for the device industry, your reputation may be sold to the highest bidder. “You may compromise your ability to be trusted in future work or presentations. I’ve regretted some things I said many years ago, not because I was being dishonest but because I really wasn’t as skeptical as I should have been about the types of results I was getting. You do tend to get on a bandwagon; you want everything to be positive,” he said, adding: “The other thing that can happen is, if things don’t work out with other buyers, they’re going to say, ‘I can’t get the same results.’ If somebody can’t replicate what you’re doing, it’s going to put your reputation on the line to some degree. So, you have to be very careful.”
Before agreeing to evaluate a new technology as an investigator or in your own practice, Dr. Ross recommended asking yourself if the intervention makes sense on a gross or microanatomic level. “There should be a physical basis for how it works, and ideally there should be some histology that backs it up,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Where is the data to support the outcomes? If it is your own data, how skeptical have you been in its acquisition and assessment?”
When evaluating a new technology, Dr. Ross recommended starting with test spots and low settings. “It behooves you not to be too aggressive because there are some untoward things you may not see right away.” He advised evaluating short- and long-term outcomes and being wary of devices that heat very deeply and rely on long-term outcomes, such as hair removal, acne improvement, scar improvement, and skin tightening – all of which require long intervals for assessment. As one of his “Ross Rules” states: “The deeper the heating and the less focused the heating, the less we can expect results.” Another Ross Rule calls for being skeptical about technologies without an immediate finding that can be “seen” on routine histology. “To me, the deeper the procedure, particularly if it’s not fractional, the confidence of my outcome is diminished,” he said. “The exception is any intervention that relies on selective photothermolysis.”
Using new technologies in practice
Dr. Ross also offered tips on how to properly incorporate newly approved technologies into your practice safely, including the use of visual endpoints. “That’s tough for technologies like laser liposuction or fractional technologies, where we rely more on ‘guidelines’ than endpoints,” he said. In addition, he recommended gradually increasing settings and using test areas to stall and/or hone techniques. “It’s exciting, but you’re like a test pilot. You want to be careful that you are doing things that are not likely to risk the patient. Try some off-the-face applications first. When you’re using a new technology, push a little harder with each patient so you can find a safe zone for that technology. You don’t have to get it all in one treatment session. Be conservative. Anticipate that you may be underassessing the immediate response.”
Above all, be careful. “Use your judgment more than laser company-prescribed settings,” he said. “Most companies have the go-by settings on the low side for patient protection, but sometimes efficacy suffers. Use cautiously on friends and family. If you treat a spouse or a friend and things don’t go perfectly, that’s always a recipe for a problem.”
Dr. Ross reported having received financial grants and research grants from Candela, Cutera, Lumenis, and Lutronic; consulting fees from Palomar; and honoraria from Cynosure, Cutera, and Lumenis. He has also received research funding from Venous Concepts, Pulsed Biosciences, and Cynosure.
REPORTING FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Efforts underway to eradicate racism in photomedicine
For one thing, melanin’s extinction overlaps with common laser lines, which affects the safety and efficacy of laser treatments in dermatology, but also in imaging and wearable devices that use LEDs in the visible range. “Pheomelanin and eumelanin are chemically very similar and both have this property of having very high extinction coefficients in the visible range, meaning that melanins both absorb and scatter light which we commonly use for laser treatments and for wearable medical devices,” Dr. Marks, a research scientist in dermatology at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Melanins also shadow a number of other biological signals that we look for in the skin, such as those from hemoglobin.”
A number of different scales can be used to estimate the amount of eumelanin, or darkly pigmented melanin, in the skin, but the most famous is Fitzpatrick skin typing, the classification system that ranges from I to VI originally intended to quantify the skin’s response to UV light. “It’s so famous that it’s used in the emoji modifier of the Unicode Consortium lookup table,” said Dr. Marks, who spoke on behalf of the Wellman Anti-Racism Effort (WARE), a grassroots working group within the Wellman Center for Photomedicine at Massachusetts General Hospital. (The mission of the group is to eradicate racism in STEM, medicine, and academia starting with its own research and Center.)
Dr. Marks referred to a Northwestern University study published in 2013, which found that both patients and dermatologists failed to accurately determine Fitzpatrick skin type (FST) when compared with reflectance spectrophotometry used to measure melanin index objectively. “There is a need to classify skin type with reliable questions with responses suitable for all skin types,” the authors concluded.
Plenty more can go wrong when clinicians ignore or misunderstand the role of melanin as a background contrast agent, Dr. Marks continued. She cited the common misconception that melanomas do not occur in darker pigmented skin, a topic discussed in an article published online in January 2020 in Cancer Cytopathology.
“While they do occur at a lower rate, this misconception leads to an alarmingly low survival rate for black melanoma patients,” Dr. Marks said. “Acral lentiginous melanoma is one example of this. It is not related to sun exposure, yet it occurs in 30% to 70% of melanomas in black patients. This also exposes a mortality rate of 1 in 3 for Black melanoma patients, compared with 1 in 11 for White patients. In fact, Black patients face a lower survival for most cancers, often attributed to social and economic disparities rather than biological differences.”
Another significant contributing factor may be the lack of data and awareness of clinical research related to patients with skin of color. The Skin of Color Society’s “Find a Doctor” database is attempting to address this by improving patients’ access to board-certified dermatologists who specialize in skin of color. “Some of the discrepancies in dermatology education, screening, and treatment for Black, indigenous, and people of color is likely attributed to the fact that only 4.5% of images in general medical textbooks show darker skin, as they are only 5% of clinical trial participants despite making up 17% of the U.S. population,” Dr. Marks said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Mind The Gap, a handbook of clinical signs and symptoms in black and brown skin, was published in 2020 by students and staff at St. George’s University of London. It can be downloaded for free.
Some 40 years after Kodak was criticized for not acknowledging inherent biases in their film stocks based on its “Kodak Shirley” color correction card, Dr. Marks said that camera makers are still ignoring racial bias in their technologies. “This is likely a ‘garbage in, garbage out’ phenomenon,” she said. “Due to the lack of diverse images, these biases get ingrained into machine learning models themselves, either because patients were not served in the first place, resulting in missing data, or because of mislabeling due to a lack of knowledge in properly classifying these images. So, while machine learning has the potential to step in where dermatologists fall short, we must be very diligent about recognizing any bias we are ingraining into these algorithms,” she said.
“That said, no technology is ‘born racist,’ of course; it is up to us to prevent history from repeating itself and prevent these biases from being ingrained in our work,” she added. “We can start by holding ourselves and others accountable when designing studies that have exclusion criteria, by challenging our sponsors on the exclusion of Fitzpatrick V and VI if you feel it is not scientifically sound, and by ensuring inclusive algorithm development. If these things are not possible, please use a disclaimer to make these limitations clear.”
According to Dr. Marks and WARE, clinicians can increase diversity in clinical trials by widening eligibility criteria, tapping into community-based medical centers, connecting with patient advocacy groups, using point-of-care and telemedicine technologies, supporting diversity-focused public policy on a larger scale, and making diversity an internal mandate, “within your institution, and within yourselves.”
Some community efforts stemming from Wellman inventions so far include the Texas-based Removery INK-nitiative program, which removes racist and hateful tattoos for free via laser tattoo removal technology that was invented at Wellman. Dr. Marks and her WARE colleagues also work with the Dream Beam Foundation, which is a global initiative bringing laser-based technologies to children in Vietnam, Armenia, Israel, Brazil, and Lebanon.
Dr. Marks reported having no financial disclosures.
For one thing, melanin’s extinction overlaps with common laser lines, which affects the safety and efficacy of laser treatments in dermatology, but also in imaging and wearable devices that use LEDs in the visible range. “Pheomelanin and eumelanin are chemically very similar and both have this property of having very high extinction coefficients in the visible range, meaning that melanins both absorb and scatter light which we commonly use for laser treatments and for wearable medical devices,” Dr. Marks, a research scientist in dermatology at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Melanins also shadow a number of other biological signals that we look for in the skin, such as those from hemoglobin.”
A number of different scales can be used to estimate the amount of eumelanin, or darkly pigmented melanin, in the skin, but the most famous is Fitzpatrick skin typing, the classification system that ranges from I to VI originally intended to quantify the skin’s response to UV light. “It’s so famous that it’s used in the emoji modifier of the Unicode Consortium lookup table,” said Dr. Marks, who spoke on behalf of the Wellman Anti-Racism Effort (WARE), a grassroots working group within the Wellman Center for Photomedicine at Massachusetts General Hospital. (The mission of the group is to eradicate racism in STEM, medicine, and academia starting with its own research and Center.)
Dr. Marks referred to a Northwestern University study published in 2013, which found that both patients and dermatologists failed to accurately determine Fitzpatrick skin type (FST) when compared with reflectance spectrophotometry used to measure melanin index objectively. “There is a need to classify skin type with reliable questions with responses suitable for all skin types,” the authors concluded.
Plenty more can go wrong when clinicians ignore or misunderstand the role of melanin as a background contrast agent, Dr. Marks continued. She cited the common misconception that melanomas do not occur in darker pigmented skin, a topic discussed in an article published online in January 2020 in Cancer Cytopathology.
“While they do occur at a lower rate, this misconception leads to an alarmingly low survival rate for black melanoma patients,” Dr. Marks said. “Acral lentiginous melanoma is one example of this. It is not related to sun exposure, yet it occurs in 30% to 70% of melanomas in black patients. This also exposes a mortality rate of 1 in 3 for Black melanoma patients, compared with 1 in 11 for White patients. In fact, Black patients face a lower survival for most cancers, often attributed to social and economic disparities rather than biological differences.”
Another significant contributing factor may be the lack of data and awareness of clinical research related to patients with skin of color. The Skin of Color Society’s “Find a Doctor” database is attempting to address this by improving patients’ access to board-certified dermatologists who specialize in skin of color. “Some of the discrepancies in dermatology education, screening, and treatment for Black, indigenous, and people of color is likely attributed to the fact that only 4.5% of images in general medical textbooks show darker skin, as they are only 5% of clinical trial participants despite making up 17% of the U.S. population,” Dr. Marks said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Mind The Gap, a handbook of clinical signs and symptoms in black and brown skin, was published in 2020 by students and staff at St. George’s University of London. It can be downloaded for free.
Some 40 years after Kodak was criticized for not acknowledging inherent biases in their film stocks based on its “Kodak Shirley” color correction card, Dr. Marks said that camera makers are still ignoring racial bias in their technologies. “This is likely a ‘garbage in, garbage out’ phenomenon,” she said. “Due to the lack of diverse images, these biases get ingrained into machine learning models themselves, either because patients were not served in the first place, resulting in missing data, or because of mislabeling due to a lack of knowledge in properly classifying these images. So, while machine learning has the potential to step in where dermatologists fall short, we must be very diligent about recognizing any bias we are ingraining into these algorithms,” she said.
“That said, no technology is ‘born racist,’ of course; it is up to us to prevent history from repeating itself and prevent these biases from being ingrained in our work,” she added. “We can start by holding ourselves and others accountable when designing studies that have exclusion criteria, by challenging our sponsors on the exclusion of Fitzpatrick V and VI if you feel it is not scientifically sound, and by ensuring inclusive algorithm development. If these things are not possible, please use a disclaimer to make these limitations clear.”
According to Dr. Marks and WARE, clinicians can increase diversity in clinical trials by widening eligibility criteria, tapping into community-based medical centers, connecting with patient advocacy groups, using point-of-care and telemedicine technologies, supporting diversity-focused public policy on a larger scale, and making diversity an internal mandate, “within your institution, and within yourselves.”
Some community efforts stemming from Wellman inventions so far include the Texas-based Removery INK-nitiative program, which removes racist and hateful tattoos for free via laser tattoo removal technology that was invented at Wellman. Dr. Marks and her WARE colleagues also work with the Dream Beam Foundation, which is a global initiative bringing laser-based technologies to children in Vietnam, Armenia, Israel, Brazil, and Lebanon.
Dr. Marks reported having no financial disclosures.
For one thing, melanin’s extinction overlaps with common laser lines, which affects the safety and efficacy of laser treatments in dermatology, but also in imaging and wearable devices that use LEDs in the visible range. “Pheomelanin and eumelanin are chemically very similar and both have this property of having very high extinction coefficients in the visible range, meaning that melanins both absorb and scatter light which we commonly use for laser treatments and for wearable medical devices,” Dr. Marks, a research scientist in dermatology at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “Melanins also shadow a number of other biological signals that we look for in the skin, such as those from hemoglobin.”
A number of different scales can be used to estimate the amount of eumelanin, or darkly pigmented melanin, in the skin, but the most famous is Fitzpatrick skin typing, the classification system that ranges from I to VI originally intended to quantify the skin’s response to UV light. “It’s so famous that it’s used in the emoji modifier of the Unicode Consortium lookup table,” said Dr. Marks, who spoke on behalf of the Wellman Anti-Racism Effort (WARE), a grassroots working group within the Wellman Center for Photomedicine at Massachusetts General Hospital. (The mission of the group is to eradicate racism in STEM, medicine, and academia starting with its own research and Center.)
Dr. Marks referred to a Northwestern University study published in 2013, which found that both patients and dermatologists failed to accurately determine Fitzpatrick skin type (FST) when compared with reflectance spectrophotometry used to measure melanin index objectively. “There is a need to classify skin type with reliable questions with responses suitable for all skin types,” the authors concluded.
Plenty more can go wrong when clinicians ignore or misunderstand the role of melanin as a background contrast agent, Dr. Marks continued. She cited the common misconception that melanomas do not occur in darker pigmented skin, a topic discussed in an article published online in January 2020 in Cancer Cytopathology.
“While they do occur at a lower rate, this misconception leads to an alarmingly low survival rate for black melanoma patients,” Dr. Marks said. “Acral lentiginous melanoma is one example of this. It is not related to sun exposure, yet it occurs in 30% to 70% of melanomas in black patients. This also exposes a mortality rate of 1 in 3 for Black melanoma patients, compared with 1 in 11 for White patients. In fact, Black patients face a lower survival for most cancers, often attributed to social and economic disparities rather than biological differences.”
Another significant contributing factor may be the lack of data and awareness of clinical research related to patients with skin of color. The Skin of Color Society’s “Find a Doctor” database is attempting to address this by improving patients’ access to board-certified dermatologists who specialize in skin of color. “Some of the discrepancies in dermatology education, screening, and treatment for Black, indigenous, and people of color is likely attributed to the fact that only 4.5% of images in general medical textbooks show darker skin, as they are only 5% of clinical trial participants despite making up 17% of the U.S. population,” Dr. Marks said at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Mind The Gap, a handbook of clinical signs and symptoms in black and brown skin, was published in 2020 by students and staff at St. George’s University of London. It can be downloaded for free.
Some 40 years after Kodak was criticized for not acknowledging inherent biases in their film stocks based on its “Kodak Shirley” color correction card, Dr. Marks said that camera makers are still ignoring racial bias in their technologies. “This is likely a ‘garbage in, garbage out’ phenomenon,” she said. “Due to the lack of diverse images, these biases get ingrained into machine learning models themselves, either because patients were not served in the first place, resulting in missing data, or because of mislabeling due to a lack of knowledge in properly classifying these images. So, while machine learning has the potential to step in where dermatologists fall short, we must be very diligent about recognizing any bias we are ingraining into these algorithms,” she said.
“That said, no technology is ‘born racist,’ of course; it is up to us to prevent history from repeating itself and prevent these biases from being ingrained in our work,” she added. “We can start by holding ourselves and others accountable when designing studies that have exclusion criteria, by challenging our sponsors on the exclusion of Fitzpatrick V and VI if you feel it is not scientifically sound, and by ensuring inclusive algorithm development. If these things are not possible, please use a disclaimer to make these limitations clear.”
According to Dr. Marks and WARE, clinicians can increase diversity in clinical trials by widening eligibility criteria, tapping into community-based medical centers, connecting with patient advocacy groups, using point-of-care and telemedicine technologies, supporting diversity-focused public policy on a larger scale, and making diversity an internal mandate, “within your institution, and within yourselves.”
Some community efforts stemming from Wellman inventions so far include the Texas-based Removery INK-nitiative program, which removes racist and hateful tattoos for free via laser tattoo removal technology that was invented at Wellman. Dr. Marks and her WARE colleagues also work with the Dream Beam Foundation, which is a global initiative bringing laser-based technologies to children in Vietnam, Armenia, Israel, Brazil, and Lebanon.
Dr. Marks reported having no financial disclosures.
EXPERT ANALYSIS FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Several approaches recommended to reduce filler, neuromodulator complications
Katie Beleznay, MD, of the University of British Columbia, Vancouver, said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
The number of reported cases of vascular complications in patients receiving fillers has increased in recent years, said Dr. Beleznay, who also treats patients in private practice in Vancouver. However, knowing the facial anatomy and recognizing that there is no “one-size-fits-all” approach goes a long way in preventing and managing complications.
In terms of neuromodulators such as Botox, the upper face is the most common area for treatment, and potential complications include eyelid ptosis, brow ptosis, and the “Spock brow,” Dr. Beleznay noted. For example, patients won’t be able to engage elevator muscles, such as the frontalis, if too much neuromodulator is injected. But, a couple of units in the upper forehead can help make the effect look natural, soften the lines, without being too frozen.
To help avoid eyelid ptosis with neuromodulators, inject at least one centimeter above the supraorbital rim at the midpupillary line, Dr. Beleznay advised. “I will feel the muscle,” because some brows are drawn or microbladed on, she noted. Patients who develop eyelid ptosis can be treated with apraclonidine drops.
To avoid brow ptosis with neuromodulators, it is important to assess the anatomy at baseline, Dr. Beleznay said. Some patients like to be able to lift their brows, and too much Botox will prevent their doing so. In order to mitigate this, it is important to treat brow depressors to balance and provide lift, and staying above the first horizontal forehead rhytid when injecting can help reduce brow ptosis risk.
Remember when injecting the upper face there are several glabellar contraction patterns, so “be sure you are targeting the treatment for the muscle pulling pattern that you see,” she said.
Complications associated with fillers
When injecting fillers, there are rare complications, including blindness, that are worth acknowledging, said Dr. Beleznay, lead author of a study on global cases of blindness caused by fillers published in 2015, including 98 cases up to 2015, and another 48 cases in a study published in 2019.
The highest-risk areas for causing blindness with fillers are the glabella and the nose, but “anywhere you are injecting is at risk for this complication,” she commented.
Explaining the mechanism of action for blindness resulting from filler injections, she said: “When the tip of the needle gets into the vessel, if you put enough pressure on the plunger, the filler can travel retrograde in the vessel back into the ophthalmic artery system, and then travels distally and blocks blood supply to the retina,” causing vision complications.
Understanding the potential mechanism for these complications informs preventive strategies, Dr. Beleznay emphasized.
If vision complications from fillers occur, they are likely to happen immediately, she said. There could be skin involvement or stroke-like features in addition to vision complications, so it is important to screen for these conditions as well if patients complain of vision loss.
Tips for prevention
Knowing the anatomy is the first step to maximize safe placement of fillers, Dr. Beleznay said. For example, the glabella is a high-risk location and includes the supraorbital and supratrochlear arteries, which start deep and become more superficial as they travel up the forehead.
When Dr. Beleznay injects in the glabella area, “I will do a true intradermal injection using tiny microdroplets, because that feels safest to me.” A video with additional details on surface anatomy and safer planes for injecting is available online to members of the American Society of Dermatologic Surgery.
Other tips to reduce the risk of vascular complications include injecting slowly and with a minimal amount of pressure, Dr. Beleznay emphasized. Injecting in small increments, moving the needle tip between injections, and using a cannula also may help reduce risk.
Always ask and use caution if patients have had other recent surgical procedures, she added.
Vascular complications such as blindness can be devastating, but the overall risks remain low. It’s important that clinicians know their anatomy, educate patients, and have prepared treatment protocols in place in the event of serious complications, Dr. Beleznay noted.
Dr. Beleznay disclosed relationships as an investigator, speaker, and/or consultant with AbbVie, Actelion, Allergan, Almirall, Amgen, Bausch Health, Celgene, Cipher, Evolus, Galderma, Johnson & Johnson, L’Oreal, Leo, Merz, Novartis, Procter & Gamble, Prollenium, Revance, Sandoz, Sanofi, Valeant, Vichy, and Zeltiq.
MedscapeLive and this news organization are owned by the same parent company.
Katie Beleznay, MD, of the University of British Columbia, Vancouver, said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
The number of reported cases of vascular complications in patients receiving fillers has increased in recent years, said Dr. Beleznay, who also treats patients in private practice in Vancouver. However, knowing the facial anatomy and recognizing that there is no “one-size-fits-all” approach goes a long way in preventing and managing complications.
In terms of neuromodulators such as Botox, the upper face is the most common area for treatment, and potential complications include eyelid ptosis, brow ptosis, and the “Spock brow,” Dr. Beleznay noted. For example, patients won’t be able to engage elevator muscles, such as the frontalis, if too much neuromodulator is injected. But, a couple of units in the upper forehead can help make the effect look natural, soften the lines, without being too frozen.
To help avoid eyelid ptosis with neuromodulators, inject at least one centimeter above the supraorbital rim at the midpupillary line, Dr. Beleznay advised. “I will feel the muscle,” because some brows are drawn or microbladed on, she noted. Patients who develop eyelid ptosis can be treated with apraclonidine drops.
To avoid brow ptosis with neuromodulators, it is important to assess the anatomy at baseline, Dr. Beleznay said. Some patients like to be able to lift their brows, and too much Botox will prevent their doing so. In order to mitigate this, it is important to treat brow depressors to balance and provide lift, and staying above the first horizontal forehead rhytid when injecting can help reduce brow ptosis risk.
Remember when injecting the upper face there are several glabellar contraction patterns, so “be sure you are targeting the treatment for the muscle pulling pattern that you see,” she said.
Complications associated with fillers
When injecting fillers, there are rare complications, including blindness, that are worth acknowledging, said Dr. Beleznay, lead author of a study on global cases of blindness caused by fillers published in 2015, including 98 cases up to 2015, and another 48 cases in a study published in 2019.
The highest-risk areas for causing blindness with fillers are the glabella and the nose, but “anywhere you are injecting is at risk for this complication,” she commented.
Explaining the mechanism of action for blindness resulting from filler injections, she said: “When the tip of the needle gets into the vessel, if you put enough pressure on the plunger, the filler can travel retrograde in the vessel back into the ophthalmic artery system, and then travels distally and blocks blood supply to the retina,” causing vision complications.
Understanding the potential mechanism for these complications informs preventive strategies, Dr. Beleznay emphasized.
If vision complications from fillers occur, they are likely to happen immediately, she said. There could be skin involvement or stroke-like features in addition to vision complications, so it is important to screen for these conditions as well if patients complain of vision loss.
Tips for prevention
Knowing the anatomy is the first step to maximize safe placement of fillers, Dr. Beleznay said. For example, the glabella is a high-risk location and includes the supraorbital and supratrochlear arteries, which start deep and become more superficial as they travel up the forehead.
When Dr. Beleznay injects in the glabella area, “I will do a true intradermal injection using tiny microdroplets, because that feels safest to me.” A video with additional details on surface anatomy and safer planes for injecting is available online to members of the American Society of Dermatologic Surgery.
Other tips to reduce the risk of vascular complications include injecting slowly and with a minimal amount of pressure, Dr. Beleznay emphasized. Injecting in small increments, moving the needle tip between injections, and using a cannula also may help reduce risk.
Always ask and use caution if patients have had other recent surgical procedures, she added.
Vascular complications such as blindness can be devastating, but the overall risks remain low. It’s important that clinicians know their anatomy, educate patients, and have prepared treatment protocols in place in the event of serious complications, Dr. Beleznay noted.
Dr. Beleznay disclosed relationships as an investigator, speaker, and/or consultant with AbbVie, Actelion, Allergan, Almirall, Amgen, Bausch Health, Celgene, Cipher, Evolus, Galderma, Johnson & Johnson, L’Oreal, Leo, Merz, Novartis, Procter & Gamble, Prollenium, Revance, Sandoz, Sanofi, Valeant, Vichy, and Zeltiq.
MedscapeLive and this news organization are owned by the same parent company.
Katie Beleznay, MD, of the University of British Columbia, Vancouver, said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
The number of reported cases of vascular complications in patients receiving fillers has increased in recent years, said Dr. Beleznay, who also treats patients in private practice in Vancouver. However, knowing the facial anatomy and recognizing that there is no “one-size-fits-all” approach goes a long way in preventing and managing complications.
In terms of neuromodulators such as Botox, the upper face is the most common area for treatment, and potential complications include eyelid ptosis, brow ptosis, and the “Spock brow,” Dr. Beleznay noted. For example, patients won’t be able to engage elevator muscles, such as the frontalis, if too much neuromodulator is injected. But, a couple of units in the upper forehead can help make the effect look natural, soften the lines, without being too frozen.
To help avoid eyelid ptosis with neuromodulators, inject at least one centimeter above the supraorbital rim at the midpupillary line, Dr. Beleznay advised. “I will feel the muscle,” because some brows are drawn or microbladed on, she noted. Patients who develop eyelid ptosis can be treated with apraclonidine drops.
To avoid brow ptosis with neuromodulators, it is important to assess the anatomy at baseline, Dr. Beleznay said. Some patients like to be able to lift their brows, and too much Botox will prevent their doing so. In order to mitigate this, it is important to treat brow depressors to balance and provide lift, and staying above the first horizontal forehead rhytid when injecting can help reduce brow ptosis risk.
Remember when injecting the upper face there are several glabellar contraction patterns, so “be sure you are targeting the treatment for the muscle pulling pattern that you see,” she said.
Complications associated with fillers
When injecting fillers, there are rare complications, including blindness, that are worth acknowledging, said Dr. Beleznay, lead author of a study on global cases of blindness caused by fillers published in 2015, including 98 cases up to 2015, and another 48 cases in a study published in 2019.
The highest-risk areas for causing blindness with fillers are the glabella and the nose, but “anywhere you are injecting is at risk for this complication,” she commented.
Explaining the mechanism of action for blindness resulting from filler injections, she said: “When the tip of the needle gets into the vessel, if you put enough pressure on the plunger, the filler can travel retrograde in the vessel back into the ophthalmic artery system, and then travels distally and blocks blood supply to the retina,” causing vision complications.
Understanding the potential mechanism for these complications informs preventive strategies, Dr. Beleznay emphasized.
If vision complications from fillers occur, they are likely to happen immediately, she said. There could be skin involvement or stroke-like features in addition to vision complications, so it is important to screen for these conditions as well if patients complain of vision loss.
Tips for prevention
Knowing the anatomy is the first step to maximize safe placement of fillers, Dr. Beleznay said. For example, the glabella is a high-risk location and includes the supraorbital and supratrochlear arteries, which start deep and become more superficial as they travel up the forehead.
When Dr. Beleznay injects in the glabella area, “I will do a true intradermal injection using tiny microdroplets, because that feels safest to me.” A video with additional details on surface anatomy and safer planes for injecting is available online to members of the American Society of Dermatologic Surgery.
Other tips to reduce the risk of vascular complications include injecting slowly and with a minimal amount of pressure, Dr. Beleznay emphasized. Injecting in small increments, moving the needle tip between injections, and using a cannula also may help reduce risk.
Always ask and use caution if patients have had other recent surgical procedures, she added.
Vascular complications such as blindness can be devastating, but the overall risks remain low. It’s important that clinicians know their anatomy, educate patients, and have prepared treatment protocols in place in the event of serious complications, Dr. Beleznay noted.
Dr. Beleznay disclosed relationships as an investigator, speaker, and/or consultant with AbbVie, Actelion, Allergan, Almirall, Amgen, Bausch Health, Celgene, Cipher, Evolus, Galderma, Johnson & Johnson, L’Oreal, Leo, Merz, Novartis, Procter & Gamble, Prollenium, Revance, Sandoz, Sanofi, Valeant, Vichy, and Zeltiq.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Role of lasers and light sources in medicine continue to expand
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Microneedling: What’s the truth?
according to Catherine M. DiGiorgio, MD, MS.
During a virtual course on laser and aesthetic skin therapy, Dr. DiGiorgio, a laser and cosmetic dermatologist at the Boston Center for Facial Rejuvenation, provided a state-of-the-art update on microneedling, a procedure in which microneedles are rolled over the skin to create epidermal and dermal microwounds.
“The depths are adjustable and it’s purely mechanical: no energy is being delivered with these treatments,” she said. “The hypothesized mechanism of action is that microneedling creates microwounds which initiate wound healing to stimulate new collagen production. This breaks apart compact collagen in the superficial dermis while stimulating new collagen and elastin,” she explained, adding that it is also hypothesized that this “stimulates growth factors that directly impact collagen and elastin synthesis.”
Conditions that have been reported to be treatable with microneedling in the medical literature include scars – especially acne scars – as well as rhytides, skin laxity, striae, melasma, and enlarged pores. Microneedling can also be used for transdermal drug delivery, although it’s far inferior to microinjection of medications. Contraindications are similar to those with laser surgery, including active infection of the area, history of keloids, inflammatory acne, and immunosuppression; and it should not be performed on the same day as neuromodulator treatment, to avoid diffusion of the neuromodulator. Herpes simplex virus prophylaxis is also indicated prior to microneedling treatment.
Many devices are available for use, including fixed, manual needle rollers and electric-powered pens with single-use sterile cartridges. The devices vary by needle length, quantity, diameter, configuration, and material of which the microneedles are made of. The needle length is not reliable for penetration depth, especially when greater than 1 mm. Treatment guidelines vary based on the area being treated.
“You put tension on the skin and apply the device perpendicularly,” Dr. DiGiorgio said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It should be performed in quadrants, and I prefer to treat in cosmetic units. The endpoint is pinpoint bleeding versus deep purpura. Ice water–soaked sterile gauze can be applied after treatment and skin care can be resumed in 5-7 days.”
In an effort to compare the efficacy and safety of the 2940-nm Er:YAG laser and microneedling for the treatment of atrophic acne scars, researchers in Egypt performed a randomized, split-face study in 30 patients. Study participants were evaluated by two blinded physicians at baseline and at 3 months follow-up. Both modalities showed a significant improvement in acne scars, but those treated with the Er:YAG laser showed a statistically significant greater improvement (70% vs. 30%, respectively; P < .001). Histology revealed a significantly higher increase in the mean quantity of collagen fibers in the Er:YAG-treated patients, compared with those who underwent microneedling, but patients in the microneedling group experienced less erythema and edema. Pain scores were significantly higher in the microneedling group compared with the Er:YAG group.
In a more recent study, researchers performed a systematic review of 37 articles in the medical literature related to microneedling. They found that the procedure provides good results when used on its own, and is preferred by patients because of its minimal downtime and side effects. However, they concluded that, while microneedling is a safe and effective option, methodological shortcomings and further research is required to establish it as an evidence-based therapeutic option.
“There are a limited number of high-quality studies demonstrating the efficacy of microneedling,” Dr. DiGiorgio said. “It is a safe procedure, which could complement laser treatments, so you could perform it between expensive and high-downtime lasers. It is an option for patients who seek measurable results with little to no downtime, and it’s also an option for clinicians who do not use laser-resurfacing devices. Basically, further research is needed to establish microneedling as an evidence-based therapeutic option. Laser continues to remain the gold standard for treatment.”
Another treatment option is fractional microneedling with radiofrequency (RF). These are microneedles which deliver energy in the form of RF at the tip of the needle, which denatures collagen and creates thermal coagulative injury zones at temperatures greater than 65° C. The microneedles can be insulated or noninsulated. “Insulated tips are safer for darker skin types because the epidermis is protected from the heat damage,” Dr. DiGiorgio said.
These treatments are used for the improvement of rhytides and scars and for skin tightening. “The treatments are painful and require topical anesthesia,” she said. “Erythema can range from about 24 hours to 4 days depending on the device being used. Usually monthly treatments are recommended.”
A study by investigators in South Korea and China set out to analyze histometric changes of this approach in pigs. They treated the pigs with a fractional microneedle delivery system at various depths, conduction times, and energies, and performed punch biopsies immediately after treatment, 4 days post treatment, and at 2 weeks post treatment. They noted that depth and conduction time affected the height, width, and volume of the columns of coagulation, but that the energy only affected the level of tissue destruction. “They also noted that RF-induced coagulated columns had a mixed cellular infiltrate, neovascularization, granular tissue formation with fibroblasts, and neocollagenesis and elastogenesis in the dermis,” Dr. DiGiorgio said.
In another study, researchers in Thailand performed a study in two women who were going to undergo abdominoplasty. Participants received six treatments prior to abdominoplasty with biopsies at different time intervals following microneedling with radiofrequency. The researchers tested five energy levels and five test areas; no collagen denaturization was observed with microneedling alone.
“This supports the idea that heat is required to stimulate neocollagenesis, and needles alone do not denature collagen,” Dr. DiGiorgio said. “They also found that neocollagenesis and neoelastogenesis occurred at optimal heating levels.”
In a separate study, researchers from Denmark used a number of different imaging modalities to evaluate the impact of microneedle fractional RF-induced micropores. When they used reflectance confocal microscopy, they observed that the micropores showed a concentric shape. “They contained hyper-reflective granules, and the coagulated tissue was seen from the epidermis to the dermal-epidermal junction,” Dr. DiGiorgio said. “This was not seen in the low energy microneedle RF. On optical coherence tomography, they noted that high-energy needle RF showed deeper, more easily identifiable micropores versus low-energy microneedle RF.” On histology the researchers noted that tissue coagulation reached a depth of 1,500 mcm with high-energy microneedle RF, but low-energy microneedle RF only showed visible damage to the epidermis. “This also supports the idea that microneedles alone without energy do not reach the deeper layers of the dermis,” she said.
Dr. DiGiorgio concluded her presentation by discussing promising results from a split-face study of fractional microneedling RF for the treatment of rosacea. For the 12-week randomized study, researchers from South Korea performed two sessions 4 weeks apart, with no treatment to the control side. Erythema decreased 13.6% and results were maintained for about 2 months after treatment. The researchers also measured inflammatory markers and noticed decreased dermal inflammation and mast cell counts and decreased markers related to angiogenesis, inflammation, innate immunity, and neuronal cation channels. “This could be a promising treatment for inflammatory rosacea in the future,” Dr. DiGiorgio said.
She disclosed that she is a consultant for Allergan Aesthetics.
according to Catherine M. DiGiorgio, MD, MS.
During a virtual course on laser and aesthetic skin therapy, Dr. DiGiorgio, a laser and cosmetic dermatologist at the Boston Center for Facial Rejuvenation, provided a state-of-the-art update on microneedling, a procedure in which microneedles are rolled over the skin to create epidermal and dermal microwounds.
“The depths are adjustable and it’s purely mechanical: no energy is being delivered with these treatments,” she said. “The hypothesized mechanism of action is that microneedling creates microwounds which initiate wound healing to stimulate new collagen production. This breaks apart compact collagen in the superficial dermis while stimulating new collagen and elastin,” she explained, adding that it is also hypothesized that this “stimulates growth factors that directly impact collagen and elastin synthesis.”
Conditions that have been reported to be treatable with microneedling in the medical literature include scars – especially acne scars – as well as rhytides, skin laxity, striae, melasma, and enlarged pores. Microneedling can also be used for transdermal drug delivery, although it’s far inferior to microinjection of medications. Contraindications are similar to those with laser surgery, including active infection of the area, history of keloids, inflammatory acne, and immunosuppression; and it should not be performed on the same day as neuromodulator treatment, to avoid diffusion of the neuromodulator. Herpes simplex virus prophylaxis is also indicated prior to microneedling treatment.
Many devices are available for use, including fixed, manual needle rollers and electric-powered pens with single-use sterile cartridges. The devices vary by needle length, quantity, diameter, configuration, and material of which the microneedles are made of. The needle length is not reliable for penetration depth, especially when greater than 1 mm. Treatment guidelines vary based on the area being treated.
“You put tension on the skin and apply the device perpendicularly,” Dr. DiGiorgio said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It should be performed in quadrants, and I prefer to treat in cosmetic units. The endpoint is pinpoint bleeding versus deep purpura. Ice water–soaked sterile gauze can be applied after treatment and skin care can be resumed in 5-7 days.”
In an effort to compare the efficacy and safety of the 2940-nm Er:YAG laser and microneedling for the treatment of atrophic acne scars, researchers in Egypt performed a randomized, split-face study in 30 patients. Study participants were evaluated by two blinded physicians at baseline and at 3 months follow-up. Both modalities showed a significant improvement in acne scars, but those treated with the Er:YAG laser showed a statistically significant greater improvement (70% vs. 30%, respectively; P < .001). Histology revealed a significantly higher increase in the mean quantity of collagen fibers in the Er:YAG-treated patients, compared with those who underwent microneedling, but patients in the microneedling group experienced less erythema and edema. Pain scores were significantly higher in the microneedling group compared with the Er:YAG group.
In a more recent study, researchers performed a systematic review of 37 articles in the medical literature related to microneedling. They found that the procedure provides good results when used on its own, and is preferred by patients because of its minimal downtime and side effects. However, they concluded that, while microneedling is a safe and effective option, methodological shortcomings and further research is required to establish it as an evidence-based therapeutic option.
“There are a limited number of high-quality studies demonstrating the efficacy of microneedling,” Dr. DiGiorgio said. “It is a safe procedure, which could complement laser treatments, so you could perform it between expensive and high-downtime lasers. It is an option for patients who seek measurable results with little to no downtime, and it’s also an option for clinicians who do not use laser-resurfacing devices. Basically, further research is needed to establish microneedling as an evidence-based therapeutic option. Laser continues to remain the gold standard for treatment.”
Another treatment option is fractional microneedling with radiofrequency (RF). These are microneedles which deliver energy in the form of RF at the tip of the needle, which denatures collagen and creates thermal coagulative injury zones at temperatures greater than 65° C. The microneedles can be insulated or noninsulated. “Insulated tips are safer for darker skin types because the epidermis is protected from the heat damage,” Dr. DiGiorgio said.
These treatments are used for the improvement of rhytides and scars and for skin tightening. “The treatments are painful and require topical anesthesia,” she said. “Erythema can range from about 24 hours to 4 days depending on the device being used. Usually monthly treatments are recommended.”
A study by investigators in South Korea and China set out to analyze histometric changes of this approach in pigs. They treated the pigs with a fractional microneedle delivery system at various depths, conduction times, and energies, and performed punch biopsies immediately after treatment, 4 days post treatment, and at 2 weeks post treatment. They noted that depth and conduction time affected the height, width, and volume of the columns of coagulation, but that the energy only affected the level of tissue destruction. “They also noted that RF-induced coagulated columns had a mixed cellular infiltrate, neovascularization, granular tissue formation with fibroblasts, and neocollagenesis and elastogenesis in the dermis,” Dr. DiGiorgio said.
In another study, researchers in Thailand performed a study in two women who were going to undergo abdominoplasty. Participants received six treatments prior to abdominoplasty with biopsies at different time intervals following microneedling with radiofrequency. The researchers tested five energy levels and five test areas; no collagen denaturization was observed with microneedling alone.
“This supports the idea that heat is required to stimulate neocollagenesis, and needles alone do not denature collagen,” Dr. DiGiorgio said. “They also found that neocollagenesis and neoelastogenesis occurred at optimal heating levels.”
In a separate study, researchers from Denmark used a number of different imaging modalities to evaluate the impact of microneedle fractional RF-induced micropores. When they used reflectance confocal microscopy, they observed that the micropores showed a concentric shape. “They contained hyper-reflective granules, and the coagulated tissue was seen from the epidermis to the dermal-epidermal junction,” Dr. DiGiorgio said. “This was not seen in the low energy microneedle RF. On optical coherence tomography, they noted that high-energy needle RF showed deeper, more easily identifiable micropores versus low-energy microneedle RF.” On histology the researchers noted that tissue coagulation reached a depth of 1,500 mcm with high-energy microneedle RF, but low-energy microneedle RF only showed visible damage to the epidermis. “This also supports the idea that microneedles alone without energy do not reach the deeper layers of the dermis,” she said.
Dr. DiGiorgio concluded her presentation by discussing promising results from a split-face study of fractional microneedling RF for the treatment of rosacea. For the 12-week randomized study, researchers from South Korea performed two sessions 4 weeks apart, with no treatment to the control side. Erythema decreased 13.6% and results were maintained for about 2 months after treatment. The researchers also measured inflammatory markers and noticed decreased dermal inflammation and mast cell counts and decreased markers related to angiogenesis, inflammation, innate immunity, and neuronal cation channels. “This could be a promising treatment for inflammatory rosacea in the future,” Dr. DiGiorgio said.
She disclosed that she is a consultant for Allergan Aesthetics.
according to Catherine M. DiGiorgio, MD, MS.
During a virtual course on laser and aesthetic skin therapy, Dr. DiGiorgio, a laser and cosmetic dermatologist at the Boston Center for Facial Rejuvenation, provided a state-of-the-art update on microneedling, a procedure in which microneedles are rolled over the skin to create epidermal and dermal microwounds.
“The depths are adjustable and it’s purely mechanical: no energy is being delivered with these treatments,” she said. “The hypothesized mechanism of action is that microneedling creates microwounds which initiate wound healing to stimulate new collagen production. This breaks apart compact collagen in the superficial dermis while stimulating new collagen and elastin,” she explained, adding that it is also hypothesized that this “stimulates growth factors that directly impact collagen and elastin synthesis.”
Conditions that have been reported to be treatable with microneedling in the medical literature include scars – especially acne scars – as well as rhytides, skin laxity, striae, melasma, and enlarged pores. Microneedling can also be used for transdermal drug delivery, although it’s far inferior to microinjection of medications. Contraindications are similar to those with laser surgery, including active infection of the area, history of keloids, inflammatory acne, and immunosuppression; and it should not be performed on the same day as neuromodulator treatment, to avoid diffusion of the neuromodulator. Herpes simplex virus prophylaxis is also indicated prior to microneedling treatment.
Many devices are available for use, including fixed, manual needle rollers and electric-powered pens with single-use sterile cartridges. The devices vary by needle length, quantity, diameter, configuration, and material of which the microneedles are made of. The needle length is not reliable for penetration depth, especially when greater than 1 mm. Treatment guidelines vary based on the area being treated.
“You put tension on the skin and apply the device perpendicularly,” Dr. DiGiorgio said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It should be performed in quadrants, and I prefer to treat in cosmetic units. The endpoint is pinpoint bleeding versus deep purpura. Ice water–soaked sterile gauze can be applied after treatment and skin care can be resumed in 5-7 days.”
In an effort to compare the efficacy and safety of the 2940-nm Er:YAG laser and microneedling for the treatment of atrophic acne scars, researchers in Egypt performed a randomized, split-face study in 30 patients. Study participants were evaluated by two blinded physicians at baseline and at 3 months follow-up. Both modalities showed a significant improvement in acne scars, but those treated with the Er:YAG laser showed a statistically significant greater improvement (70% vs. 30%, respectively; P < .001). Histology revealed a significantly higher increase in the mean quantity of collagen fibers in the Er:YAG-treated patients, compared with those who underwent microneedling, but patients in the microneedling group experienced less erythema and edema. Pain scores were significantly higher in the microneedling group compared with the Er:YAG group.
In a more recent study, researchers performed a systematic review of 37 articles in the medical literature related to microneedling. They found that the procedure provides good results when used on its own, and is preferred by patients because of its minimal downtime and side effects. However, they concluded that, while microneedling is a safe and effective option, methodological shortcomings and further research is required to establish it as an evidence-based therapeutic option.
“There are a limited number of high-quality studies demonstrating the efficacy of microneedling,” Dr. DiGiorgio said. “It is a safe procedure, which could complement laser treatments, so you could perform it between expensive and high-downtime lasers. It is an option for patients who seek measurable results with little to no downtime, and it’s also an option for clinicians who do not use laser-resurfacing devices. Basically, further research is needed to establish microneedling as an evidence-based therapeutic option. Laser continues to remain the gold standard for treatment.”
Another treatment option is fractional microneedling with radiofrequency (RF). These are microneedles which deliver energy in the form of RF at the tip of the needle, which denatures collagen and creates thermal coagulative injury zones at temperatures greater than 65° C. The microneedles can be insulated or noninsulated. “Insulated tips are safer for darker skin types because the epidermis is protected from the heat damage,” Dr. DiGiorgio said.
These treatments are used for the improvement of rhytides and scars and for skin tightening. “The treatments are painful and require topical anesthesia,” she said. “Erythema can range from about 24 hours to 4 days depending on the device being used. Usually monthly treatments are recommended.”
A study by investigators in South Korea and China set out to analyze histometric changes of this approach in pigs. They treated the pigs with a fractional microneedle delivery system at various depths, conduction times, and energies, and performed punch biopsies immediately after treatment, 4 days post treatment, and at 2 weeks post treatment. They noted that depth and conduction time affected the height, width, and volume of the columns of coagulation, but that the energy only affected the level of tissue destruction. “They also noted that RF-induced coagulated columns had a mixed cellular infiltrate, neovascularization, granular tissue formation with fibroblasts, and neocollagenesis and elastogenesis in the dermis,” Dr. DiGiorgio said.
In another study, researchers in Thailand performed a study in two women who were going to undergo abdominoplasty. Participants received six treatments prior to abdominoplasty with biopsies at different time intervals following microneedling with radiofrequency. The researchers tested five energy levels and five test areas; no collagen denaturization was observed with microneedling alone.
“This supports the idea that heat is required to stimulate neocollagenesis, and needles alone do not denature collagen,” Dr. DiGiorgio said. “They also found that neocollagenesis and neoelastogenesis occurred at optimal heating levels.”
In a separate study, researchers from Denmark used a number of different imaging modalities to evaluate the impact of microneedle fractional RF-induced micropores. When they used reflectance confocal microscopy, they observed that the micropores showed a concentric shape. “They contained hyper-reflective granules, and the coagulated tissue was seen from the epidermis to the dermal-epidermal junction,” Dr. DiGiorgio said. “This was not seen in the low energy microneedle RF. On optical coherence tomography, they noted that high-energy needle RF showed deeper, more easily identifiable micropores versus low-energy microneedle RF.” On histology the researchers noted that tissue coagulation reached a depth of 1,500 mcm with high-energy microneedle RF, but low-energy microneedle RF only showed visible damage to the epidermis. “This also supports the idea that microneedles alone without energy do not reach the deeper layers of the dermis,” she said.
Dr. DiGiorgio concluded her presentation by discussing promising results from a split-face study of fractional microneedling RF for the treatment of rosacea. For the 12-week randomized study, researchers from South Korea performed two sessions 4 weeks apart, with no treatment to the control side. Erythema decreased 13.6% and results were maintained for about 2 months after treatment. The researchers also measured inflammatory markers and noticed decreased dermal inflammation and mast cell counts and decreased markers related to angiogenesis, inflammation, innate immunity, and neuronal cation channels. “This could be a promising treatment for inflammatory rosacea in the future,” Dr. DiGiorgio said.
She disclosed that she is a consultant for Allergan Aesthetics.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Dermatologists and the history of skin care and beauty devices: Part 4
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.













