User login
COVID-19 Vaccine Reactions in Dermatology: “Filling” in the Gaps
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
As we marked the 1-year anniversary of the COVID-19 pandemic, nearly 100 million Americans had received their first dose of the COVID-19 vaccine, heralding some sense of relief and enabling us to envision a return to something resembling life before lockdown.1 Amid these breakthroughs and vaccination campaigns forging ahead worldwide, we saw new questions and problems arise. Vaccine hesitancy was already an issue in many segments of society where misinformation and mistrust of the medical establishment have served as barriers to the progress of public health. Once reports of adverse reactions following COVID-19 vaccination—such as those linked to use of facial fillers—made news headlines, many in the dermatology community began facing inquiries from patients questioning if they should wait to receive the vaccine or skip it entirely. As dermatologists, we must be informed and prepared to address these situations, to manage adverse reactions when they arise, and to encourage and promote vaccination during this critical time for public health in our society.
Cutaneous Vaccine Reactions and Facial Fillers
As public COVID-19 vaccinations move forward, dermatologic side effects, which were first noted during clinical trials, have received amplified attention, despite the fact that these cutaneous reactions—including localized injection-site redness and swelling, generalized urticarial and morbilliform eruptions, and even facial filler reactions—have been reported as relatively minor and self-limited.2 The excipient polyethylene glycol has been suspected as a possible etiology of vaccine-related allergic and hypersensitivity reactions, suggesting care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (eg, penicillin, laxatives, makeup, certain dermal fillers).2,3 Although rare, facial and lip swelling reactions in those with a prior history of facial fillers in COVID-19 vaccine trials have drawn particular public concern and potential vaccine hesitancy given that more than 2.7 million Americans seek treatment with dermal fillers annually. There has been continued demand for these treatments during the pandemic, particularly due to aesthetic sensitivity surrounding video conferencing.4
Release of trial data from the Moderna COVID-19 vaccine prompted a discourse around safety and recommended protocols for filler procedures in the community of aesthetic medicine, as 3 participants in the experimental arm—all of whom had a history of treatment with facial filler injections—were reported to have facial or lip swelling shortly following vaccination. Two of these cases were considered to be serious adverse events due to extensive facial swelling, with the participants having received filler injections 6 months and 2 weeks prior to vaccination, respectively.5 A third participant experienced lip swelling only, which according to the US Food and Drug Administration briefing document was considered “medically significant” but not a serious adverse event, with unknown timing of the most recent filler injection. In all cases, symptom onset began 1 or 2 days following vaccination, and all resolved with either no or minimal intervention.6 The US Food and Drug Administration briefing document does not detail which type of fillers each participant had received, but subsequent reports indicated hyaluronic acid (HA) fillers. Of note, one patient in the placebo arm of the trial also developed progressive periorbital and facial edema in the setting of known filler injections performed 5 weeks prior, requiring treatment with corticosteroids and barring her from receiving a second injection in the trial.7
After public vaccination started, additional reports have emerged of facial edema occurring following administration of both the Pfizer and Moderna COVID-19 vaccines.2,8,9 In one series, 4 cases of facial swelling were reported in patients who had HA filler placed more than 1 year prior to vaccination.9 The first patient, who had a history of HA fillers in the temples and cheeks, developed moderate periorbital swelling 2 days following her second dose of the Pfizer vaccine. Another patient who had received a series of filler injections over the last 3 years experienced facial swelling 24 hours after her second dose of the Moderna vaccine and also reported a similar reaction in the past following an upper respiratory tract infection. The third patient developed perioral and infraorbital edema 18 hours after her first dose of the Moderna vaccine. The fourth patient developed inflammation in filler-treated areas 10 days after the first dose of the Pfizer vaccine and notably had a history of filler reaction to an unknown trigger in 2019 that was treated with hyaluronidase, intralesional steroids, and 5-fluorouracil. All cases of facial edema reportedly resolved.9
The observed adverse events have been proposed as delayed-type hypersensitivity reactions (DTRs) to facial fillers and are suspected to be triggered by the COVID-19 spike protein and subsequent immunogenic response. This reaction is not unique to the COVID-19 vaccines; in fact, many inflammatory stimuli such as sinus infections, flulike illnesses, facial injury, dental procedures, and exposure to certain medications and chemotherapeutics have triggered DTRs in filler patients, especially in those with genetic or immunologic risk factors including certain human leukocyte antigen subtypes or autoimmune disorders.3
Counseling Patients and Reducing Risks
As reports of DTRs to facial fillers after COVID-19 vaccination continue to emerge, it is not surprising that patients may become confused by potential side effects and postpone vaccination as a result. This evolving situation has called upon aesthetic physicians to adapt our practice and prepare our patients. Most importantly, we must continue to follow the data and integrate evidence-based COVID-19 vaccine–related counseling into our office visits. It is paramount to encourage vaccination and inform patients that these rare adverse events are both temporary and treatable. Given the currently available data, patients with a history of treatment with dermal fillers should not be discouraged from receiving the vaccine; however, we may provide suggestions to lessen the likelihood of adverse reactions and ease patient concerns. For example, it may be helpful to consider a time frame between vaccination and filler procedures that is longer than 2 weeks, just as would be advised for those having dental procedures or with recent infections, and potentially longer windows for those with risk factors such as prior sensitivity to dermal fillers, autoimmune disorders, or those on immunomodulatory medications. Dilution of fillers with saline or lidocaine or use of non-HA fillers also may be suggested around the time of vaccination to mitigate the risk of DTRs.3
Managing Vaccine Reactions
If facial swelling does occur despite these precautions and lasts longer than 48 hours, treatment with antihistamines, steroids, and/or hyaluronidase has been successful in vaccine trial and posttrial patients, both alone or in combination, and are likely to resolve edema promptly without altering the effectiveness of the vaccine.3,5,9 Angiotensin-converting enzyme inhibitors such as lisinopril more recently have been recommended for treatment of facial edema following COVID-19 vaccination,9 but questions remain regarding the true efficacy in this scenario given that the majority of swelling reactions resolve without this treatment. Additionally, there were no controls to indicate treatment with the angiotensin-converting enzyme inhibitor demonstrated an actual impact. Dermatologists generally are wary of adding medications of questionable utility that are associated with potential side effects and drug reactions, given that we often are tasked with managing the consequences of such mistakes. Thus, to avoid additional harm in the setting of insufficient evidence, as was seen following widespread use of hydroxychloroquine at the outset of the COVID-19 pandemic, well-structured studies are required before such interventions can be recommended.
If symptoms arise following the first vaccine injection, they can be managed if needed while patients are reassured and advised to obtain their second dose, with pretreatment considerations including antihistamines and instruction to present to the emergency department if a more severe reaction is suspected.2 In a larger sense, we also can contribute to the collective knowledge, growth, and preparedness of the medical community by reporting cases of adverse events to vaccine reporting systems and registries, such as the US Department of Health and Human Services’ Vaccine Adverse Event Reporting System, the Centers for Disease Control and Prevention’s V-Safe After Vaccination Health Checker, and the American Academy of Dermatology’s COVID-19 Dermatology Registry.
Final Thoughts
As dermatologists, we now find ourselves in the familiar role of balancing the aesthetic goals of our patients with our primary mission of public health and safety at a time when their health and well-being is particularly vulnerable. Adverse reactions will continue to occur as larger segments of the world’s population become vaccinated. Meanwhile, we must continue to manage symptoms, dispel myths, emphasize that any dermatologic risk posed by the COVID-19 vaccines is far outweighed by the benefits of immunization, and promote health and education, looking ahead to life beyond the pandemic.
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus (COVID-19) vaccinations. Our World in Data website. Accessed May 10, 2021. https://ourworldindata.org/covid-vaccinations
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases [published online April 7, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.092
- Rice SM, Ferree SD, Mesinkovska NA, et al. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021;7:209-212. doi:10.1016/j.ijwd.2021.01.007
- Rice SM, Siegel JA, Libby T, et al. Zooming into cosmetic procedures during the COVID-19 pandemic: the provider’s perspective. Int J Womens Dermatol. 2021;7:213-216.
- FDA Briefing Document: Moderna COVID-19 Vaccine. US Department of Health and Human Services; 2020. Accessed May 11, 2021. https://www.fda.gov/media/144434/download
- Moderna’s COVID-19 vaccine may cause swelling, inflammation in those with facial fillers. American Society of Plastic Surgeons website. Published December 27, 2020. Accessed May 11, 2021. http://www.plasticsurgery.org/for-medical-professionals/publications/psn-extra/news/modernas-covid19-vaccine-may-cause-swelling-inflammation-in-those-with-facial-fillers
- Munavalli GG, Guthridge R, Knutsen-Larson S, et al. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment [published online February 9, 2021]. Arch Dermatol Res. doi:10.1007/s00403-021-02190-6
- Schlessinger J. Update on COVID-19 vaccines and dermal fillers. Practical Dermatol. February 2021:46-47. Accessed May 10, 2021. https://practicaldermatology.com/articles/2021-feb/update-on-covid-19-vaccines-and-dermal-fillers/pdf
- Munavalli GG, Knutsen-Larson S, Lupo MP, et al. Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination—a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep. 2021;10:63-68. doi:10.1016/j.jdcr.2021.02.018
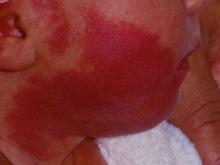
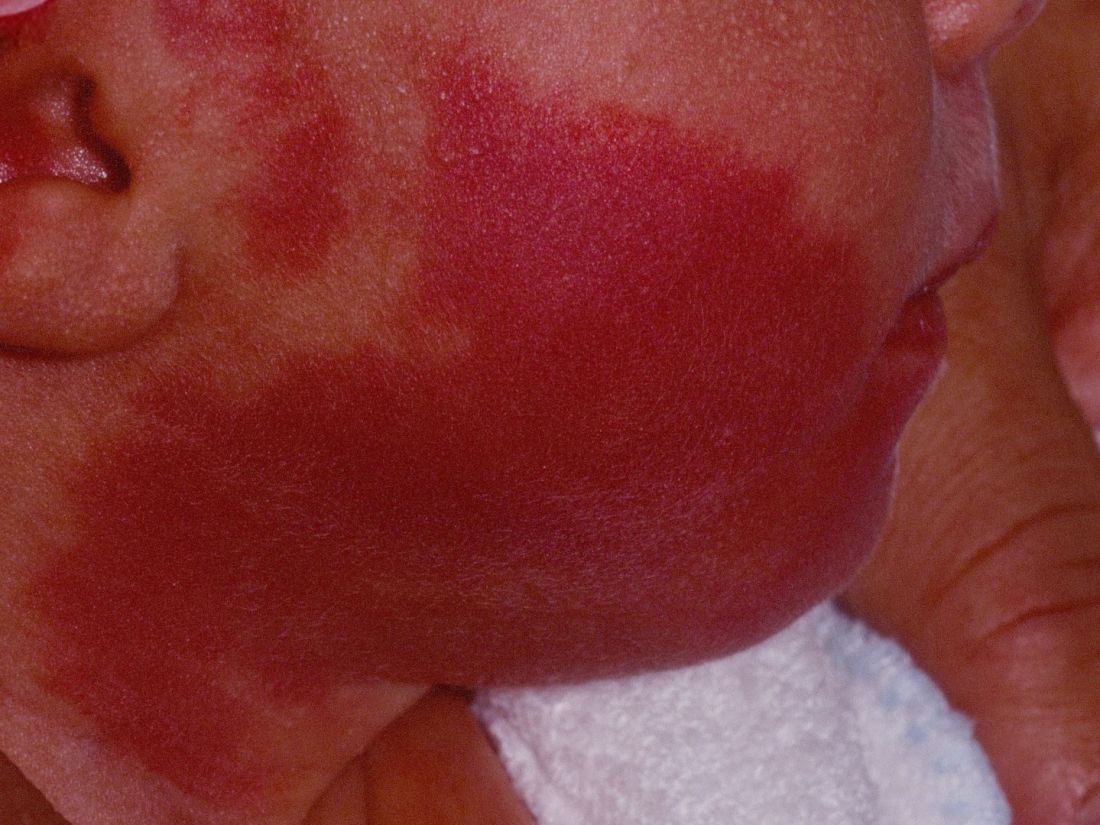
How early can laser treatment for port wine stains in infants be initiated?
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
without any complications, results from a single-center study showed.
“The current modality of choice for the treatment of port wine birthmarks is pulsed dye laser,” Chelsea Grimes Fidai, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. “When performed by a highly trained expert at efficient frequencies, PDL is a safe, effective treatment that is successful in the majority of patients. We know that earlier treatment yields maximal clearance. However, just how early can you initiate treatment?”
To find out, Dr. Fidai, Roy G. Geronemus, MD, and colleagues at the Laser and Skin Surgery Center of New York, conducted a retrospective chart review of 39 infants with port wine birthmarks who were treated with a 595-nm PDL between 2015 and 2020 at the center. Of the 39 infants, the average age at first treatment was 18 days, with a range from 5 to 29 days. The youngest patient was born prematurely at 35 weeks’ gestation and presented for his first treatment even before his expected due date. Most (74%) had facial lesions with the remaining distributed on the trunk or extremities. The average number of treatments was 15 over the course of 15 months.
The initial settings chosen for facial lesions were a 10-mm spot size, a fluence of 8.0 J/cm2, and a 1.5-millisecond pulse duration. For body lesions, the typical initial settings were a 12-mm spot size, a fluence of 6.7 J/cm2, and 1.5-millisecond pulse duration. Corneal eye shields were placed for all cases with port wine birthmarks approaching the eyelid. “We do recommend a treatment interval of every 2-3 weeks, with longer intervals for patients of darker skin type until the child is 2 years old, at which time the interval is increased to every 3-6 months,” said Dr. Fidai.
Patients in the study experienced the expected short-term side effects of erythema, edema, purpura, and mild transient postinflammatory hyperpigmentation, but there were no cases of atrophy, scarring, infection, or permanent pigmentary change.
“Families seeking early treatment of port wine birthmarks can be reassured that it can be safely initiated within the first few days after birth,” Dr. Fidai concluded. “This procedure can be quickly and confidently performed as an in-office procedure without any complications. The early intervention allows for treatment without general anesthesia and it maximizes the chance of significant clearance as early in life as possible.”
During a question-and-answer session, the abstract section chair, Albert Wolkerstorfer, MD, PhD, expressed concern about the effect of PDL on developing infants. “We do repeated treatments at this young age without any type of anesthesia,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam.
“Will that influence the development of the child, especially when I hear there might be 15 or 20 treatments done within the first year of life? I think this is a problem where we need to ask the experts in the field of pain management in children, like pediatric anesthesiologists, to find the right way, because I think that the results that you showed are fantastic. I don’t think we can achieve that at a later age, although there’s no direct comparison at this moment.”
Dr. Fidai said that she understood the concern, but pointed to a 2020 article by Dr. Geronemus and colleagues that assessed treatment tolerance and parental perspective of outpatient PDL treatment for port-wine birthmarks without general anesthesia in infants and toddlers. “The kids recover pretty quickly after the treatment,” she said. “There has never been any longstanding issue from the parents’ perspective.”
Dr. Fidai reported having no financial disclosures. Dr. Geronemus disclosed having financial conflicts with numerous device and pharmaceutical companies. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
One treatment with a 1,060-nm diode laser helped reduce unwanted fat
A a small single-center study showed.
Nonsurgical fat reduction was the third-most common nonsurgical aesthetic procedure in the United States in 2018 and includes lasers, high-intensity focused ultrasound, radiofrequency, photobiomodulation therapy, and cryolipolysis, according to 2018 data from the American Society for Aesthetic Plastic Surgery.
“Our study is unique because we used a 1,060-nm diode laser with integrated skin cooling to evaluate the efficacy and safety of its use for the reduction of unwanted fat of the abdomen and flanks,” lead study author Alison S. Kang, MD, told this news organization following the annual conference of the American Society for Laser Medicine and Surgery, where the data were presented. “A 1,060-nm laser works by delivering controlled thermal energy between 42 °C and 47 °C, temperatures at which adipocytes are permanently destroyed,” she explained.
Dr. Kang and Suzanne Kilmer, MD, both of the Laser & Skin Surgery Center of Northern California, Sacramento, enrolled 28 women and 2 men into the study. Each study participant received a single treatment with Venus Bliss, a 1,060-nm diode laser with four laser applicators and a built-in skin-cooling mechanism. Half received treatment of the flanks delivered at up to 1.4 watts per cm2 on each diode for 25 minutes, while the other 15 received treatment of the abdomen with the same energy settings. Photos and ultrasound images were taken at baseline, 6 weeks, and 12 weeks, and the investigators administered a satisfaction questionnaire upon study exit. The primary endpoint was efficacy, defined as the percentage of correctly identified posttreatment photographs by three blinded reviewers (one plastic surgeon and two dermatologists). Secondary endpoints of interest were change in adipose thickness on ultrasound, subject satisfaction, and adverse events.
After losing 1 patient to follow-up, 29 completed the study. Dr. Kang reported that the blinded evaluators could identify the pretreatment image, compared with the posttreatment image in an average of 67% of patients. Between baseline and 12 weeks, the ultrasound images showed an average reduction in the adipose layer of 9% on the abdomen and 7% on the flank, while the average self-reported pain score based on the Wong-Baker FACES Pain Rating Scale was 2 out of 10 among those in the abdomen treatment group and 2.6 out of 10 among those in the flank treatment group.
In addition, 76% of subjects stated they were “satisfied” to “very satisfied” with the treatment, and 79% stated that they would recommend this treatment to a friend. The most common posttreatment responses in both groups were erythema and trace edema, but no serious or permanent adverse events were observed.
Dr. Kang acknowledged certain limitations of the study, including its small sample size. “Only one treatment was performed in our study, so it is unclear if multiple treatments will improve efficacy or if multiple treatments will have no effect on efficacy,” she said.
The work won a “best of session early career-clinical” abstract award from the ASLMS.
The study was funded by Venus Concept, the manufacturer of the Venus Bliss laser. Dr. Kang reported having no relevant financial disclosures. Dr. Kilmer has received grants and honoraria from Venus Concept.
[email protected]
A a small single-center study showed.
Nonsurgical fat reduction was the third-most common nonsurgical aesthetic procedure in the United States in 2018 and includes lasers, high-intensity focused ultrasound, radiofrequency, photobiomodulation therapy, and cryolipolysis, according to 2018 data from the American Society for Aesthetic Plastic Surgery.
“Our study is unique because we used a 1,060-nm diode laser with integrated skin cooling to evaluate the efficacy and safety of its use for the reduction of unwanted fat of the abdomen and flanks,” lead study author Alison S. Kang, MD, told this news organization following the annual conference of the American Society for Laser Medicine and Surgery, where the data were presented. “A 1,060-nm laser works by delivering controlled thermal energy between 42 °C and 47 °C, temperatures at which adipocytes are permanently destroyed,” she explained.
Dr. Kang and Suzanne Kilmer, MD, both of the Laser & Skin Surgery Center of Northern California, Sacramento, enrolled 28 women and 2 men into the study. Each study participant received a single treatment with Venus Bliss, a 1,060-nm diode laser with four laser applicators and a built-in skin-cooling mechanism. Half received treatment of the flanks delivered at up to 1.4 watts per cm2 on each diode for 25 minutes, while the other 15 received treatment of the abdomen with the same energy settings. Photos and ultrasound images were taken at baseline, 6 weeks, and 12 weeks, and the investigators administered a satisfaction questionnaire upon study exit. The primary endpoint was efficacy, defined as the percentage of correctly identified posttreatment photographs by three blinded reviewers (one plastic surgeon and two dermatologists). Secondary endpoints of interest were change in adipose thickness on ultrasound, subject satisfaction, and adverse events.
After losing 1 patient to follow-up, 29 completed the study. Dr. Kang reported that the blinded evaluators could identify the pretreatment image, compared with the posttreatment image in an average of 67% of patients. Between baseline and 12 weeks, the ultrasound images showed an average reduction in the adipose layer of 9% on the abdomen and 7% on the flank, while the average self-reported pain score based on the Wong-Baker FACES Pain Rating Scale was 2 out of 10 among those in the abdomen treatment group and 2.6 out of 10 among those in the flank treatment group.
In addition, 76% of subjects stated they were “satisfied” to “very satisfied” with the treatment, and 79% stated that they would recommend this treatment to a friend. The most common posttreatment responses in both groups were erythema and trace edema, but no serious or permanent adverse events were observed.
Dr. Kang acknowledged certain limitations of the study, including its small sample size. “Only one treatment was performed in our study, so it is unclear if multiple treatments will improve efficacy or if multiple treatments will have no effect on efficacy,” she said.
The work won a “best of session early career-clinical” abstract award from the ASLMS.
The study was funded by Venus Concept, the manufacturer of the Venus Bliss laser. Dr. Kang reported having no relevant financial disclosures. Dr. Kilmer has received grants and honoraria from Venus Concept.
[email protected]
A a small single-center study showed.
Nonsurgical fat reduction was the third-most common nonsurgical aesthetic procedure in the United States in 2018 and includes lasers, high-intensity focused ultrasound, radiofrequency, photobiomodulation therapy, and cryolipolysis, according to 2018 data from the American Society for Aesthetic Plastic Surgery.
“Our study is unique because we used a 1,060-nm diode laser with integrated skin cooling to evaluate the efficacy and safety of its use for the reduction of unwanted fat of the abdomen and flanks,” lead study author Alison S. Kang, MD, told this news organization following the annual conference of the American Society for Laser Medicine and Surgery, where the data were presented. “A 1,060-nm laser works by delivering controlled thermal energy between 42 °C and 47 °C, temperatures at which adipocytes are permanently destroyed,” she explained.
Dr. Kang and Suzanne Kilmer, MD, both of the Laser & Skin Surgery Center of Northern California, Sacramento, enrolled 28 women and 2 men into the study. Each study participant received a single treatment with Venus Bliss, a 1,060-nm diode laser with four laser applicators and a built-in skin-cooling mechanism. Half received treatment of the flanks delivered at up to 1.4 watts per cm2 on each diode for 25 minutes, while the other 15 received treatment of the abdomen with the same energy settings. Photos and ultrasound images were taken at baseline, 6 weeks, and 12 weeks, and the investigators administered a satisfaction questionnaire upon study exit. The primary endpoint was efficacy, defined as the percentage of correctly identified posttreatment photographs by three blinded reviewers (one plastic surgeon and two dermatologists). Secondary endpoints of interest were change in adipose thickness on ultrasound, subject satisfaction, and adverse events.
After losing 1 patient to follow-up, 29 completed the study. Dr. Kang reported that the blinded evaluators could identify the pretreatment image, compared with the posttreatment image in an average of 67% of patients. Between baseline and 12 weeks, the ultrasound images showed an average reduction in the adipose layer of 9% on the abdomen and 7% on the flank, while the average self-reported pain score based on the Wong-Baker FACES Pain Rating Scale was 2 out of 10 among those in the abdomen treatment group and 2.6 out of 10 among those in the flank treatment group.
In addition, 76% of subjects stated they were “satisfied” to “very satisfied” with the treatment, and 79% stated that they would recommend this treatment to a friend. The most common posttreatment responses in both groups were erythema and trace edema, but no serious or permanent adverse events were observed.
Dr. Kang acknowledged certain limitations of the study, including its small sample size. “Only one treatment was performed in our study, so it is unclear if multiple treatments will improve efficacy or if multiple treatments will have no effect on efficacy,” she said.
The work won a “best of session early career-clinical” abstract award from the ASLMS.
The study was funded by Venus Concept, the manufacturer of the Venus Bliss laser. Dr. Kang reported having no relevant financial disclosures. Dr. Kilmer has received grants and honoraria from Venus Concept.
[email protected]
FROM ASLMS 2021
Systematic review of radiofrequency microneedling studies unveiled
Of the according to results from a new systematic review.
“Most devices for aesthetic purposes induce denaturation and remodeling of collagen, elastin, and other dermal structures through tissue injury and stimulating the body’s wound-healing response,” lead study author Marcus G. Tan, MD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “Radiofrequency microneedling is no exception in this regard. RFMN creates perforations in the skin and delivers radiofrequency-generated thermal energy into the underlying tissue. However, RFMN is unique in that thermal energy is delivered in a fashion that produces a reverse temperature gradient to most ablative lasers.”
When using ablative lasers, which target water as its chromophore through selective photothermolysis, the temperature gradient is highest at the epidermis and papillary dermis, and decreases as it penetrates the deeper structures of the skin. In RFMN, radiofrequency energy is delivered directly to the target depth through the microneedle electrodes, thus creating a temperature gradient that is highest in the deep, target structures and cooler at the superficial structures. “This results in less unwanted epidermal heating and reduces the risk of postinflammatory hyperpigmentation,” explained Dr. Tan, a resident in the division of dermatology at the University of Ottawa.
“Because RFMN is unaffected by skin chromophores, it is essentially a ‘color-blind’ technology and safe for use in patients of all skin phototypes. In comparison to lasers, radiofrequency energy can also be delivered to deeper structures of the skin by increasing the length of microneedle electrodes. Despite these advantages of RFMN, this technology remains utilized less frequently compared to ablative lasers for its skin rejuvenating effects.”
To review high-quality medical literature related to RFMN, Dr. Tan and colleagues searched EMBASE and MEDLINE from inception to May 13, 2020, by using the terms “radiofrequency microneedling,” “fractional radiofrequency,” “radiofrequency needling,” or “radiofrequency percutaneous collagen induction.” They limited the analysis to dermatology-related randomized, split-body, or blinded studies with original data in humans. Of the 42 studies included in the final analysis, there were 14 studies of skin rejuvenation, 7 of acne scars, 6 of acne vulgaris, 5 each of striae and axillary hyperhidrosis, 2 of melasma, and 1 each of rosacea, cellulite, and androgenetic alopecia.
After reviewing the 42 studies, the study authors proposed that a strong recommendation for RFMN be made for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis, and a weak recommendation for the technology to be used for papulopustular rosacea, striae, and male-pattern androgenetic alopecia when used in conjunction with topical 5% minoxidil. There was insufficient evidence to make recommendations for its use in cellulite and melasma.
One finding that Dr. Tan described as “interesting” was the observation that RFMN was superior to Er:YAG fractional ablative lasers for treatment of rhytides on the lower face (i.e., the nasolabial, perioral, jawline and neck regions). “Secondly, we observed that one session of RFMN was able to achieve 37% efficacy of a surgical face-lift, but without any adverse effects,” Dr. Tan said. “Two-thirds of the patients who received surgical face-lift developed hypertrophic scarring requiring further scar management, compared to none of the patients receiving RFMN.”
Based on their review, Dr. Tan and colleagues recommend that RFMN be offered as one of the therapeutic options for patients seeking treatment for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis. “It is usually tolerable with just topical anesthesia applied 30-60 minutes before treatment, and its side effects are transient and usually resolve after 5 days,” he said. “Patients should be counseled that the benefits of RFMN may have a slower onset, compared to other treatments, but it is progressive, durable, and can be used repeatedly and safely in all skin types including darker-skin phenotypes with minimal risk of adverse events.”
One of the abstract section chairs, Fernanda H. Sakamoto, MD, PhD, said that RFMN devices have become increasingly popular in recent years. “The paper presented by Tan et al. is very relevant, as it compares clinical indications, parameters, and results in search for evidence of efficacy and appropriate settings,” said Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, told this news organization. “The paper provides long-needed guidelines to clinicians and helps manage patients’ expectations.”
Dr. Tan acknowledged certain limitations of the study, including the lack of head-to-head studies comparing specific RFMN devices. “There are many RFMN devices available commercially, each with different capabilities and degrees of effectiveness,” he said. “With more research and technological advancements since the first radiofrequency device was approved in 2002, RFMN has made significant improvements. In general, the newer generation devices produce markedly better results.”
Dr. Tan reported having no financial disclosures. Dr. Sakamoto disclosed that she holds intellectual property rights with Accure Acne, Massachusetts General Hospital, and Lightwater Biosciences.
Of the according to results from a new systematic review.
“Most devices for aesthetic purposes induce denaturation and remodeling of collagen, elastin, and other dermal structures through tissue injury and stimulating the body’s wound-healing response,” lead study author Marcus G. Tan, MD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “Radiofrequency microneedling is no exception in this regard. RFMN creates perforations in the skin and delivers radiofrequency-generated thermal energy into the underlying tissue. However, RFMN is unique in that thermal energy is delivered in a fashion that produces a reverse temperature gradient to most ablative lasers.”
When using ablative lasers, which target water as its chromophore through selective photothermolysis, the temperature gradient is highest at the epidermis and papillary dermis, and decreases as it penetrates the deeper structures of the skin. In RFMN, radiofrequency energy is delivered directly to the target depth through the microneedle electrodes, thus creating a temperature gradient that is highest in the deep, target structures and cooler at the superficial structures. “This results in less unwanted epidermal heating and reduces the risk of postinflammatory hyperpigmentation,” explained Dr. Tan, a resident in the division of dermatology at the University of Ottawa.
“Because RFMN is unaffected by skin chromophores, it is essentially a ‘color-blind’ technology and safe for use in patients of all skin phototypes. In comparison to lasers, radiofrequency energy can also be delivered to deeper structures of the skin by increasing the length of microneedle electrodes. Despite these advantages of RFMN, this technology remains utilized less frequently compared to ablative lasers for its skin rejuvenating effects.”
To review high-quality medical literature related to RFMN, Dr. Tan and colleagues searched EMBASE and MEDLINE from inception to May 13, 2020, by using the terms “radiofrequency microneedling,” “fractional radiofrequency,” “radiofrequency needling,” or “radiofrequency percutaneous collagen induction.” They limited the analysis to dermatology-related randomized, split-body, or blinded studies with original data in humans. Of the 42 studies included in the final analysis, there were 14 studies of skin rejuvenation, 7 of acne scars, 6 of acne vulgaris, 5 each of striae and axillary hyperhidrosis, 2 of melasma, and 1 each of rosacea, cellulite, and androgenetic alopecia.
After reviewing the 42 studies, the study authors proposed that a strong recommendation for RFMN be made for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis, and a weak recommendation for the technology to be used for papulopustular rosacea, striae, and male-pattern androgenetic alopecia when used in conjunction with topical 5% minoxidil. There was insufficient evidence to make recommendations for its use in cellulite and melasma.
One finding that Dr. Tan described as “interesting” was the observation that RFMN was superior to Er:YAG fractional ablative lasers for treatment of rhytides on the lower face (i.e., the nasolabial, perioral, jawline and neck regions). “Secondly, we observed that one session of RFMN was able to achieve 37% efficacy of a surgical face-lift, but without any adverse effects,” Dr. Tan said. “Two-thirds of the patients who received surgical face-lift developed hypertrophic scarring requiring further scar management, compared to none of the patients receiving RFMN.”
Based on their review, Dr. Tan and colleagues recommend that RFMN be offered as one of the therapeutic options for patients seeking treatment for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis. “It is usually tolerable with just topical anesthesia applied 30-60 minutes before treatment, and its side effects are transient and usually resolve after 5 days,” he said. “Patients should be counseled that the benefits of RFMN may have a slower onset, compared to other treatments, but it is progressive, durable, and can be used repeatedly and safely in all skin types including darker-skin phenotypes with minimal risk of adverse events.”
One of the abstract section chairs, Fernanda H. Sakamoto, MD, PhD, said that RFMN devices have become increasingly popular in recent years. “The paper presented by Tan et al. is very relevant, as it compares clinical indications, parameters, and results in search for evidence of efficacy and appropriate settings,” said Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, told this news organization. “The paper provides long-needed guidelines to clinicians and helps manage patients’ expectations.”
Dr. Tan acknowledged certain limitations of the study, including the lack of head-to-head studies comparing specific RFMN devices. “There are many RFMN devices available commercially, each with different capabilities and degrees of effectiveness,” he said. “With more research and technological advancements since the first radiofrequency device was approved in 2002, RFMN has made significant improvements. In general, the newer generation devices produce markedly better results.”
Dr. Tan reported having no financial disclosures. Dr. Sakamoto disclosed that she holds intellectual property rights with Accure Acne, Massachusetts General Hospital, and Lightwater Biosciences.
Of the according to results from a new systematic review.
“Most devices for aesthetic purposes induce denaturation and remodeling of collagen, elastin, and other dermal structures through tissue injury and stimulating the body’s wound-healing response,” lead study author Marcus G. Tan, MD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “Radiofrequency microneedling is no exception in this regard. RFMN creates perforations in the skin and delivers radiofrequency-generated thermal energy into the underlying tissue. However, RFMN is unique in that thermal energy is delivered in a fashion that produces a reverse temperature gradient to most ablative lasers.”
When using ablative lasers, which target water as its chromophore through selective photothermolysis, the temperature gradient is highest at the epidermis and papillary dermis, and decreases as it penetrates the deeper structures of the skin. In RFMN, radiofrequency energy is delivered directly to the target depth through the microneedle electrodes, thus creating a temperature gradient that is highest in the deep, target structures and cooler at the superficial structures. “This results in less unwanted epidermal heating and reduces the risk of postinflammatory hyperpigmentation,” explained Dr. Tan, a resident in the division of dermatology at the University of Ottawa.
“Because RFMN is unaffected by skin chromophores, it is essentially a ‘color-blind’ technology and safe for use in patients of all skin phototypes. In comparison to lasers, radiofrequency energy can also be delivered to deeper structures of the skin by increasing the length of microneedle electrodes. Despite these advantages of RFMN, this technology remains utilized less frequently compared to ablative lasers for its skin rejuvenating effects.”
To review high-quality medical literature related to RFMN, Dr. Tan and colleagues searched EMBASE and MEDLINE from inception to May 13, 2020, by using the terms “radiofrequency microneedling,” “fractional radiofrequency,” “radiofrequency needling,” or “radiofrequency percutaneous collagen induction.” They limited the analysis to dermatology-related randomized, split-body, or blinded studies with original data in humans. Of the 42 studies included in the final analysis, there were 14 studies of skin rejuvenation, 7 of acne scars, 6 of acne vulgaris, 5 each of striae and axillary hyperhidrosis, 2 of melasma, and 1 each of rosacea, cellulite, and androgenetic alopecia.
After reviewing the 42 studies, the study authors proposed that a strong recommendation for RFMN be made for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis, and a weak recommendation for the technology to be used for papulopustular rosacea, striae, and male-pattern androgenetic alopecia when used in conjunction with topical 5% minoxidil. There was insufficient evidence to make recommendations for its use in cellulite and melasma.
One finding that Dr. Tan described as “interesting” was the observation that RFMN was superior to Er:YAG fractional ablative lasers for treatment of rhytides on the lower face (i.e., the nasolabial, perioral, jawline and neck regions). “Secondly, we observed that one session of RFMN was able to achieve 37% efficacy of a surgical face-lift, but without any adverse effects,” Dr. Tan said. “Two-thirds of the patients who received surgical face-lift developed hypertrophic scarring requiring further scar management, compared to none of the patients receiving RFMN.”
Based on their review, Dr. Tan and colleagues recommend that RFMN be offered as one of the therapeutic options for patients seeking treatment for skin rejuvenation, acne vulgaris, acne scars, and axillary hyperhidrosis. “It is usually tolerable with just topical anesthesia applied 30-60 minutes before treatment, and its side effects are transient and usually resolve after 5 days,” he said. “Patients should be counseled that the benefits of RFMN may have a slower onset, compared to other treatments, but it is progressive, durable, and can be used repeatedly and safely in all skin types including darker-skin phenotypes with minimal risk of adverse events.”
One of the abstract section chairs, Fernanda H. Sakamoto, MD, PhD, said that RFMN devices have become increasingly popular in recent years. “The paper presented by Tan et al. is very relevant, as it compares clinical indications, parameters, and results in search for evidence of efficacy and appropriate settings,” said Dr. Sakamoto, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, told this news organization. “The paper provides long-needed guidelines to clinicians and helps manage patients’ expectations.”
Dr. Tan acknowledged certain limitations of the study, including the lack of head-to-head studies comparing specific RFMN devices. “There are many RFMN devices available commercially, each with different capabilities and degrees of effectiveness,” he said. “With more research and technological advancements since the first radiofrequency device was approved in 2002, RFMN has made significant improvements. In general, the newer generation devices produce markedly better results.”
Dr. Tan reported having no financial disclosures. Dr. Sakamoto disclosed that she holds intellectual property rights with Accure Acne, Massachusetts General Hospital, and Lightwater Biosciences.
FROM ASLMS 2021
Botulinum toxin and depression
. But confounding factors, such as medications, injection/acupuncture effect, physician interaction or touch, or other life scenarios, have made it difficult to discern botulinum toxin type A’s true effect on mood or psychiatric diagnosis. Now a systematic review and meta-analysis of randomized controlled trials examining botulinum toxin versus placebo provides evidence that botulinum toxin type A (BTX-A) injections are associated with statistically significant improvement in depressive symptoms.
Qian et al. analyzed all randomized controlled trials that investigated the efficacy and safety of facial BTX-A injections on patients with a diagnosis of major depressive disorder in PubMed and Web of Science from inception to June 17, 2020. A meta-analysis of the changes in depressive symptoms 6 weeks after BTX-A injections compared with placebo were the primary outcome of the report, while the safety of injections were also assessed.
A total of 417 patients from five randomized controlled trials (189 patients who received BTX-A injections and 228 in the placebo group) were deemed eligible. There was a statistically significant improvement in depressive symptoms in the BTX-A injections compared with placebo (Hedges’ g, –0.82; 95% confidence interval, –1.38 to 0.27). BTX-A injections were well tolerated with mild and temporary adverse events (headache, eyelid ptosis, and upper respiratory tract infection) reported in three of the five studies.
Limitations to the analysis include publication bias due to the limited number of studies in the analysis, the difficulty of being able to reliably blind participants because of potential noticeable cosmetic effects of BTX-A treatment, and the heterogeneity of symptom severity associated with major depressive disorder.
The authors referred to the Global Burden of Disease Study, which estimated that approximately 216 million people experienced major depressive disorder in 2015, the latest data available. MDD symptoms of sadness, fatigue, and loss of interest or pleasure, “incur a tremendous burden on health and finances,” they wrote. According to the Department of Health and Human Services, it is estimated that about 60% of people who commit suicide have had a mood disorder (major depression, bipolar disorder, dysthymia). The high rate of suicide associated with severe depression is also a serious public health concern. While further analysis is clearly warranted, cosmetic BTX-A injections may provide an alternative option in the treatment of depression.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at [email protected]. They had no relevant disclosures.
. But confounding factors, such as medications, injection/acupuncture effect, physician interaction or touch, or other life scenarios, have made it difficult to discern botulinum toxin type A’s true effect on mood or psychiatric diagnosis. Now a systematic review and meta-analysis of randomized controlled trials examining botulinum toxin versus placebo provides evidence that botulinum toxin type A (BTX-A) injections are associated with statistically significant improvement in depressive symptoms.
Qian et al. analyzed all randomized controlled trials that investigated the efficacy and safety of facial BTX-A injections on patients with a diagnosis of major depressive disorder in PubMed and Web of Science from inception to June 17, 2020. A meta-analysis of the changes in depressive symptoms 6 weeks after BTX-A injections compared with placebo were the primary outcome of the report, while the safety of injections were also assessed.
A total of 417 patients from five randomized controlled trials (189 patients who received BTX-A injections and 228 in the placebo group) were deemed eligible. There was a statistically significant improvement in depressive symptoms in the BTX-A injections compared with placebo (Hedges’ g, –0.82; 95% confidence interval, –1.38 to 0.27). BTX-A injections were well tolerated with mild and temporary adverse events (headache, eyelid ptosis, and upper respiratory tract infection) reported in three of the five studies.
Limitations to the analysis include publication bias due to the limited number of studies in the analysis, the difficulty of being able to reliably blind participants because of potential noticeable cosmetic effects of BTX-A treatment, and the heterogeneity of symptom severity associated with major depressive disorder.
The authors referred to the Global Burden of Disease Study, which estimated that approximately 216 million people experienced major depressive disorder in 2015, the latest data available. MDD symptoms of sadness, fatigue, and loss of interest or pleasure, “incur a tremendous burden on health and finances,” they wrote. According to the Department of Health and Human Services, it is estimated that about 60% of people who commit suicide have had a mood disorder (major depression, bipolar disorder, dysthymia). The high rate of suicide associated with severe depression is also a serious public health concern. While further analysis is clearly warranted, cosmetic BTX-A injections may provide an alternative option in the treatment of depression.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at [email protected]. They had no relevant disclosures.
. But confounding factors, such as medications, injection/acupuncture effect, physician interaction or touch, or other life scenarios, have made it difficult to discern botulinum toxin type A’s true effect on mood or psychiatric diagnosis. Now a systematic review and meta-analysis of randomized controlled trials examining botulinum toxin versus placebo provides evidence that botulinum toxin type A (BTX-A) injections are associated with statistically significant improvement in depressive symptoms.
Qian et al. analyzed all randomized controlled trials that investigated the efficacy and safety of facial BTX-A injections on patients with a diagnosis of major depressive disorder in PubMed and Web of Science from inception to June 17, 2020. A meta-analysis of the changes in depressive symptoms 6 weeks after BTX-A injections compared with placebo were the primary outcome of the report, while the safety of injections were also assessed.
A total of 417 patients from five randomized controlled trials (189 patients who received BTX-A injections and 228 in the placebo group) were deemed eligible. There was a statistically significant improvement in depressive symptoms in the BTX-A injections compared with placebo (Hedges’ g, –0.82; 95% confidence interval, –1.38 to 0.27). BTX-A injections were well tolerated with mild and temporary adverse events (headache, eyelid ptosis, and upper respiratory tract infection) reported in three of the five studies.
Limitations to the analysis include publication bias due to the limited number of studies in the analysis, the difficulty of being able to reliably blind participants because of potential noticeable cosmetic effects of BTX-A treatment, and the heterogeneity of symptom severity associated with major depressive disorder.
The authors referred to the Global Burden of Disease Study, which estimated that approximately 216 million people experienced major depressive disorder in 2015, the latest data available. MDD symptoms of sadness, fatigue, and loss of interest or pleasure, “incur a tremendous burden on health and finances,” they wrote. According to the Department of Health and Human Services, it is estimated that about 60% of people who commit suicide have had a mood disorder (major depression, bipolar disorder, dysthymia). The high rate of suicide associated with severe depression is also a serious public health concern. While further analysis is clearly warranted, cosmetic BTX-A injections may provide an alternative option in the treatment of depression.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at [email protected]. They had no relevant disclosures.
Seaweed and other marine-derived products in skin care, Part II: Cosmetic formulations, fucoidan, and salmon eggs
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
Reconstruction Technique for Defects of the Cutaneous and Mucosal Lip: V-to-flying-Y Closure
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?
Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?

Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?

Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
Pandemic took a cut of cosmetic procedures in 2020
pandemic, according to the American Society of Plastic Surgeons.
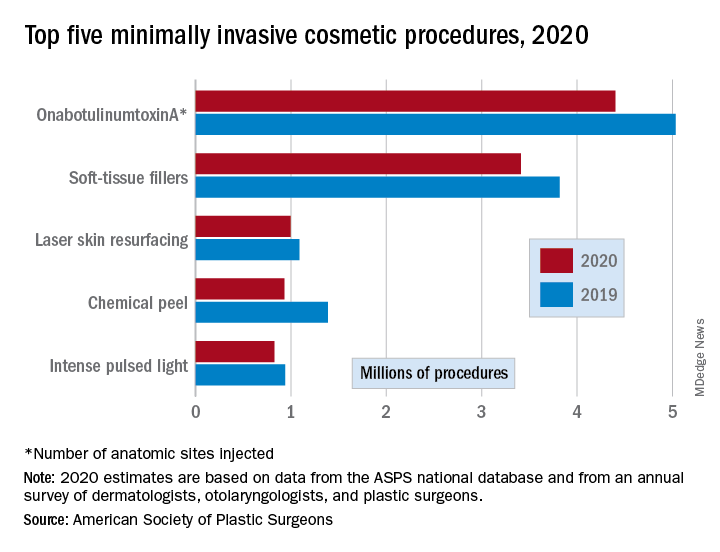
There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
pandemic, according to the American Society of Plastic Surgeons.

There were an estimated 15.6 million cosmetic procedures performed in 2020, compared with 18.4 million in 2019, a drop of 15.2%. Meanwhile, society members reported that they stopped performing elective surgery for an average of 8.1 weeks, which works out to 15.6% of a 52-week year, the ASPS said in its annual statistics report.
“The pandemic isn’t over, but thanks to vaccines, a new normal is starting to define itself – and some surgeons’ offices that were closed or offered only limited services within the last year are seeing higher demand,” Lynn Jeffers, MD, MBA, immediate past president of the ASPS, said in a written statement.
Minimally invasive procedures, which made up the majority of cosmetic procedures in 2020, dropped by a slightly higher 16%, compared with 14% on the surgical side. “Injectables continued to be the most sought-after treatments in 2020,” the ASPS said, with survey respondents citing “a significant uptick in demand during the coronavirus pandemic.”
OnabotuliumtoxinA injection, the most popular form of minimally invasive procedure, was down by 13% from 2019, while use of soft-tissue fillers fell by 11%. Laser skin resurfacing was third in popularity and had the smallest drop, just 8%, among the top five from 2019 to 2020, the ASPS data show.
The drop in volume for chemical peels was large enough (33%), to move it from third place in 2019 to fourth in 2020, and a slightly less than average drop of 12% moved intense pulsed-light treatments from sixth place in 2019 to fifth in 2020, switching places with laser hair removal (down 28%), the ASPS reported.
Among the surgical procedures, rhinoplasty was the most popular in 2020, as it was in 2019, after dropping by just 3%. Blepharoplasty was down by 8% from 2019, but two other common procedures, liposuction and breast augmentation, fell by 20% and 33%, respectively, the ASPS said.
Goodbye, OTC hydroquinone
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
In 1972, an over-the-counter drug review process was established by the Food and Drug Administration to regulate the safety and efficacy of over-the-counter (OTC) drugs. This created a book or “monograph” for each medication category that describes the active ingredients, indications, doses, route of administration, testing, and labeling. If a drug meets the criteria in its therapeutic category, it does not have to undergo an FDA review before being marketed to consumers.
As part of this process, drugs are classified into one of three categories: category I: generally recognized as safe and effective (GRASE) and not misbranded; category II: not GRASE; category III: lacking sufficient data on safety and efficacy to permit classification. This methodology was outdated and made it difficult under the old guidelines to make changes to medications in the evolving world of drug development. Some categories of OTC drugs, including hand sanitizers, hydroquinone, and sunscreens, have been marketed for years without a final monograph.
The signing of the “Coronavirus Aid, Relief, and Economic Security” (CARES) Act in March 2020 included reforms in the FDA monograph process for OTC medications. Under this proceeding, a final monograph determination was made for all OTC categories. While drugs in category I and some in category III may remain on the market, if certain specifications are met, category II drugs had to be removed within 180 days of the enactment of the CARES Act.
Hydroquinone was one of those that fell victim to the ban. This ban is similar to hydroquinone bans in other places, including Europe. However, for manufacturers, this issue was under the radar and packaged in a seemingly irrelevant piece of legislation.
Among dermatologists, there is no consensus as to whether 2% hydroquinone is safe or not. However, the unmonitored use and overuse that is common for this type of medication has led to heightened safety concerns. Common side effects of hydroquinone include irritant and allergic contact dermatitis; the most difficult to treat side effect with long-term use is ochronosis. But there are no reported cancer data in humans with the use of topical hydroquinone as previously thought. Hydroquinone used short term is a very safe and effective treatment for hard to treat hyperpigmentation and is often necessary when other topicals are ineffective, particularly in our patients with skin of color.
The bigger problem however is the legislative process involved, as exemplified by this ban, which only came to light because of the CARES act.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Seaweed and other marine-derived products in skin care, part 1: Current indications
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.