User login
Graying of hair: Could it be reversed?
as hair pigment goes through its natural progression of senescence.
However, the recent publication that is a collaboration between the department of psychiatry at Columbia University, New York; and the departments of dermatology at the University College Dublin, University of Miami, and the University of Manchester (England); and the Monasterium Laboratory in Münster, Germany, demonstrates a quantitative mapping of human hair graying – and its reversal – in relation to stress.
In the study, hair color of single strands of hair from seven healthy females and seven healthy males, whose mean age was 35 years (range, 9-65 years), were analyzed. In addition to hair pigment analysis, study subjects documented the stress they were experiencing each week in diaries. Using either high resolution image scanners, electron microscopy, and/or hair shaft proteomics, the investigators were able to evaluate loss of pigment within fragments small enough to have grown over one hour.
When changes in hair color were noted, variations in up to 300 proteins were documented, including an up-regulation of the fatty acid synthesis and metabolism machinery in graying. Recent studies also corroborate that fatty acid synthesis by fatty acid synthase and “transport by CPT1A ... are sufficient drivers of cell senescence, and that fatty acid metabolism regulates melanocyte aging biology” the authors wrote.
Molecularly, the investigators found that gray hairs up-regulate proteins associated with energy metabolism, mitochondria, and antioxidant defenses. The graying correlated with stress was also reversible, “at least temporarily,” based on their retrospective analysis and analysis over the 2.5-year recruitment period, the investigators wrote. Specifically, they found that graying hair “may be acutely triggered by stressful life experiences, the removal of which can trigger reversal.” From the data, they also developed a mathematical model to predict what might happen to human hair over time.
Through this study, proof-of-concept evidence is provided indicating that biobehavioral factors are linked to human hair graying dynamics. Future analysis with larger sample sizes and incorporating neuroendocrine markers may further support these correlations. This is an interesting study that elucidates the mechanisms responsible for how stress and other life exposures manifest in human biology, and, if we as human beings effectively manage that stress, how it may both reverse the negative impact and outcomes affecting our body and health.
The study was supported by the Wharton Fund and grants from the National Institutes of Health.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
as hair pigment goes through its natural progression of senescence.
However, the recent publication that is a collaboration between the department of psychiatry at Columbia University, New York; and the departments of dermatology at the University College Dublin, University of Miami, and the University of Manchester (England); and the Monasterium Laboratory in Münster, Germany, demonstrates a quantitative mapping of human hair graying – and its reversal – in relation to stress.
In the study, hair color of single strands of hair from seven healthy females and seven healthy males, whose mean age was 35 years (range, 9-65 years), were analyzed. In addition to hair pigment analysis, study subjects documented the stress they were experiencing each week in diaries. Using either high resolution image scanners, electron microscopy, and/or hair shaft proteomics, the investigators were able to evaluate loss of pigment within fragments small enough to have grown over one hour.
When changes in hair color were noted, variations in up to 300 proteins were documented, including an up-regulation of the fatty acid synthesis and metabolism machinery in graying. Recent studies also corroborate that fatty acid synthesis by fatty acid synthase and “transport by CPT1A ... are sufficient drivers of cell senescence, and that fatty acid metabolism regulates melanocyte aging biology” the authors wrote.
Molecularly, the investigators found that gray hairs up-regulate proteins associated with energy metabolism, mitochondria, and antioxidant defenses. The graying correlated with stress was also reversible, “at least temporarily,” based on their retrospective analysis and analysis over the 2.5-year recruitment period, the investigators wrote. Specifically, they found that graying hair “may be acutely triggered by stressful life experiences, the removal of which can trigger reversal.” From the data, they also developed a mathematical model to predict what might happen to human hair over time.
Through this study, proof-of-concept evidence is provided indicating that biobehavioral factors are linked to human hair graying dynamics. Future analysis with larger sample sizes and incorporating neuroendocrine markers may further support these correlations. This is an interesting study that elucidates the mechanisms responsible for how stress and other life exposures manifest in human biology, and, if we as human beings effectively manage that stress, how it may both reverse the negative impact and outcomes affecting our body and health.
The study was supported by the Wharton Fund and grants from the National Institutes of Health.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
as hair pigment goes through its natural progression of senescence.
However, the recent publication that is a collaboration between the department of psychiatry at Columbia University, New York; and the departments of dermatology at the University College Dublin, University of Miami, and the University of Manchester (England); and the Monasterium Laboratory in Münster, Germany, demonstrates a quantitative mapping of human hair graying – and its reversal – in relation to stress.
In the study, hair color of single strands of hair from seven healthy females and seven healthy males, whose mean age was 35 years (range, 9-65 years), were analyzed. In addition to hair pigment analysis, study subjects documented the stress they were experiencing each week in diaries. Using either high resolution image scanners, electron microscopy, and/or hair shaft proteomics, the investigators were able to evaluate loss of pigment within fragments small enough to have grown over one hour.
When changes in hair color were noted, variations in up to 300 proteins were documented, including an up-regulation of the fatty acid synthesis and metabolism machinery in graying. Recent studies also corroborate that fatty acid synthesis by fatty acid synthase and “transport by CPT1A ... are sufficient drivers of cell senescence, and that fatty acid metabolism regulates melanocyte aging biology” the authors wrote.
Molecularly, the investigators found that gray hairs up-regulate proteins associated with energy metabolism, mitochondria, and antioxidant defenses. The graying correlated with stress was also reversible, “at least temporarily,” based on their retrospective analysis and analysis over the 2.5-year recruitment period, the investigators wrote. Specifically, they found that graying hair “may be acutely triggered by stressful life experiences, the removal of which can trigger reversal.” From the data, they also developed a mathematical model to predict what might happen to human hair over time.
Through this study, proof-of-concept evidence is provided indicating that biobehavioral factors are linked to human hair graying dynamics. Future analysis with larger sample sizes and incorporating neuroendocrine markers may further support these correlations. This is an interesting study that elucidates the mechanisms responsible for how stress and other life exposures manifest in human biology, and, if we as human beings effectively manage that stress, how it may both reverse the negative impact and outcomes affecting our body and health.
The study was supported by the Wharton Fund and grants from the National Institutes of Health.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
Synthetic snake venom to the rescue? Potential uses in skin health and rejuvenation
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras, four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras, four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras, four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
Understanding the alpha hydroxy acids: Glycolic acid
. The extent of exfoliation with any of the alpha hydroxy acids depends on the type of acid, its concentration, and the pH of the preparations. Glycolic acid inhibits tyrosinase and chelates calcium ion concentration between the cells in the epidermis, which results in exfoliation of the skin.
Over-the-counter glycolic acid is available in concentrations up to 30%, and in professional products up to 70%. Clinically, glycolic acid above a concentration of 30% causes local burning, erythema, and dryness.
However, overuse of glycolic acid among consumers has increased the incidence of skin reactions and hyperpigmentation. Professional-grade products containing up to 70% glycolic acid are widely available on the Internet and without proper guidelines on use and sun avoidance, adverse events and long term scarring are becoming prevalent.
The overuse of acids and overexfoliation of the skin in patients with skin types I-IV is a growing problem as consumers are purchasing more “at-home peels,” peel pads, glow pads, and at-home exfoliation regimens. This overexfoliation of the skin and the resulting erythema induces rapid postinflammatory hyperpigmentation. Consumers then often mistakenly try to self-treat the hyperpigmentation with increasing concentrations of acids, retinols, and/or hydroquinone on top of an already compromised skin barrier, further worsening the problem. In addition, these acids increase sensitivity to UV light and increase the risk of sunburns in all skin types.
Although glycolic acids are generally safe, standardized recommendations for their use in the skin care market are necessary. More is not always better. In our clinic, we do not treat patients with postinflammatory hyperpigmentation from acids or peels with more exfoliation. We focus on repairing the barrier for 1-3 months, which includes use of gentle cleansers and occlusive moisturizers, and avoidance of acids, retinols, or scrubs, and aggressive sun protection, and then using gentle fade ingredients – such as kojic acid, licorice root extract, and vitamin C – at low concentrations to slowly decrease melanin production. Barrier repair is the first and most important step and it is often overlooked when clinicians try to lighten the skin in haste.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
. The extent of exfoliation with any of the alpha hydroxy acids depends on the type of acid, its concentration, and the pH of the preparations. Glycolic acid inhibits tyrosinase and chelates calcium ion concentration between the cells in the epidermis, which results in exfoliation of the skin.
Over-the-counter glycolic acid is available in concentrations up to 30%, and in professional products up to 70%. Clinically, glycolic acid above a concentration of 30% causes local burning, erythema, and dryness.
However, overuse of glycolic acid among consumers has increased the incidence of skin reactions and hyperpigmentation. Professional-grade products containing up to 70% glycolic acid are widely available on the Internet and without proper guidelines on use and sun avoidance, adverse events and long term scarring are becoming prevalent.
The overuse of acids and overexfoliation of the skin in patients with skin types I-IV is a growing problem as consumers are purchasing more “at-home peels,” peel pads, glow pads, and at-home exfoliation regimens. This overexfoliation of the skin and the resulting erythema induces rapid postinflammatory hyperpigmentation. Consumers then often mistakenly try to self-treat the hyperpigmentation with increasing concentrations of acids, retinols, and/or hydroquinone on top of an already compromised skin barrier, further worsening the problem. In addition, these acids increase sensitivity to UV light and increase the risk of sunburns in all skin types.
Although glycolic acids are generally safe, standardized recommendations for their use in the skin care market are necessary. More is not always better. In our clinic, we do not treat patients with postinflammatory hyperpigmentation from acids or peels with more exfoliation. We focus on repairing the barrier for 1-3 months, which includes use of gentle cleansers and occlusive moisturizers, and avoidance of acids, retinols, or scrubs, and aggressive sun protection, and then using gentle fade ingredients – such as kojic acid, licorice root extract, and vitamin C – at low concentrations to slowly decrease melanin production. Barrier repair is the first and most important step and it is often overlooked when clinicians try to lighten the skin in haste.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
. The extent of exfoliation with any of the alpha hydroxy acids depends on the type of acid, its concentration, and the pH of the preparations. Glycolic acid inhibits tyrosinase and chelates calcium ion concentration between the cells in the epidermis, which results in exfoliation of the skin.
Over-the-counter glycolic acid is available in concentrations up to 30%, and in professional products up to 70%. Clinically, glycolic acid above a concentration of 30% causes local burning, erythema, and dryness.
However, overuse of glycolic acid among consumers has increased the incidence of skin reactions and hyperpigmentation. Professional-grade products containing up to 70% glycolic acid are widely available on the Internet and without proper guidelines on use and sun avoidance, adverse events and long term scarring are becoming prevalent.
The overuse of acids and overexfoliation of the skin in patients with skin types I-IV is a growing problem as consumers are purchasing more “at-home peels,” peel pads, glow pads, and at-home exfoliation regimens. This overexfoliation of the skin and the resulting erythema induces rapid postinflammatory hyperpigmentation. Consumers then often mistakenly try to self-treat the hyperpigmentation with increasing concentrations of acids, retinols, and/or hydroquinone on top of an already compromised skin barrier, further worsening the problem. In addition, these acids increase sensitivity to UV light and increase the risk of sunburns in all skin types.
Although glycolic acids are generally safe, standardized recommendations for their use in the skin care market are necessary. More is not always better. In our clinic, we do not treat patients with postinflammatory hyperpigmentation from acids or peels with more exfoliation. We focus on repairing the barrier for 1-3 months, which includes use of gentle cleansers and occlusive moisturizers, and avoidance of acids, retinols, or scrubs, and aggressive sun protection, and then using gentle fade ingredients – such as kojic acid, licorice root extract, and vitamin C – at low concentrations to slowly decrease melanin production. Barrier repair is the first and most important step and it is often overlooked when clinicians try to lighten the skin in haste.
Dr. Lily Talakoub and Dr. Naissan O. Wesley and are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected].
Toxic chemicals found in many cosmetics
People may be absorbing and ingesting potentially toxic chemicals from their cosmetic products, a new study suggests.
– per- and polyfluoroalkyl substances. Many of these chemicals were not included on the product labels, making it difficult for consumers to consciously avoid them.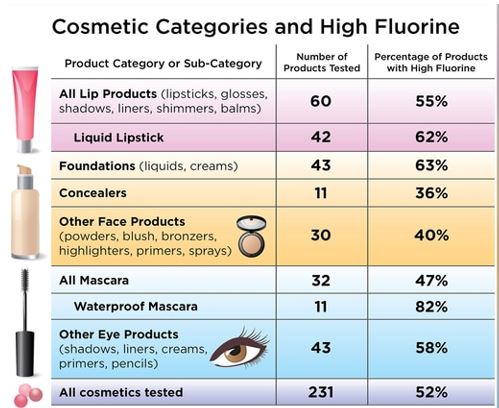
“This study is very helpful for elucidating the PFAS content of different types of cosmetics in the U.S. and Canadian markets,” said Elsie Sunderland, PhD, an environmental scientist who was not involved with the study.
“Previously, all the data had been collected in Europe, and this study shows we are dealing with similar problems in the North American marketplace,” said Dr. Sunderland, a professor of environmental chemistry at the Harvard School of Public Health, Boston.
PFAS are a class of chemicals used in a variety of consumer products, such as nonstick cookware, stain-resistant carpeting, and water-repellent clothing, according to the Centers for Disease Control and Prevention. They are added to cosmetics to make the products more durable and spreadable, researchers said in the study.
“[PFAS] are added to change the properties of surfaces, to make them nonstick or resistant to stay in water or oils,” said study coauthor Tom Bruton, PhD, senior scientist at the Green Science Policy Institute in Berkeley, Calif. “The concerning thing about cosmetics is that these are products that you’re applying to your skin and face every day, so there’s the skin absorption route that’s of concern, but also incidental ingestion of cosmetics is also a concern as well.”
The CDC says some of the potential health effects of PFAS exposure includes increased cholesterol levels, increased risk of kidney and testicular cancer, changes in liver enzymes, decreased vaccine response in children, and a higher risk of high blood pressure or preeclampsia in pregnant women.
“PFAS are a large class of chemicals. In humans, exposure to some of these chemicals has been associated with impaired immune function, certain cancers, increased risks of diabetes, obesity and endocrine disruption,” Dr. Sunderland said. “They appear to be harmful to every major organ system in the human body.”
For the current study, published online in Environmental Science & Technology Letters, Dr. Bruton and colleagues purchased 231 cosmetic products in the United States and Canada from retailers such as Ulta Beauty, Sephora, Target, and Bed Bath & Beyond. They then screened them for fluorine.Three-quarters of waterproof mascara samples contained high fluorine concentrations, as did nearly two-thirds of foundations and liquid lipsticks, and more than half of the eye and lip products tested.
The authors found that different categories of makeup tended to have higher or lower fluorine concentrations. “High fluorine levels were found in products commonly advertised as ‘wear-resistant’ to water and oils or ‘long-lasting,’ including foundations, liquid lipsticks, and waterproof mascaras,” Dr. Bruton and colleagues wrote.
When they further analyzed a subset of 29 products to determine what types of chemicals were present, they found that each cosmetic product contained at least 4 PFAS, with one product containing 13.The PFAS substances found included some that break down into other chemicals that are known to be highly toxic and environmentally harmful.
“It’s concerning that some of the products we tested appear to be intentionally using PFAS, but not listing those ingredients on the label,” Dr. Bruton said. “I do think that it is helpful for consumers to read labels, but beyond that, there’s not a lot of ways that consumers themselves can solve this problem. ... We think that the industry needs to be more proactive about moving away from this group of chemicals.”
Dr. Sunderland said a resource people can use when trying to avoid PFAS is the Environmental Working Group, a nonprofit organization that maintains an extensive database of cosmetics and personal care products.
“At this point, there is very little regulatory activity related to PFAS in cosmetics,” Dr. Sunderland said. “The best thing to happen now would be for consumers to indicate that they prefer products without PFAS and to demand better transparency in product ingredient lists.”
A similar study done in 2018 by the Danish Environmental Protection Agency found high levels of PFAS in nearly one-third of the cosmetics products it tested.
People can also be exposed to PFAS by eating or drinking contaminated food or water and through food packaging. Dr. Sunderland said some wild foods like seafood are known to accumulate these compounds in the environment.
“There are examples of contaminated biosolids leading to accumulation of PFAS in vegetables and milk,” Dr. Sunderland explained. “Food packaging is another concern because it can also result in PFAS accumulation in the foods we eat.”
Although it’s difficult to avoid PFAS altogether, the CDC suggests lowering exposure rates by avoiding contaminated water and food. If you’re not sure if your water is contaminated, you should ask your local or state health and environmental quality departments for fish or water advisories in your area.
A version of this article first appeared on WebMD.com.
People may be absorbing and ingesting potentially toxic chemicals from their cosmetic products, a new study suggests.
– per- and polyfluoroalkyl substances. Many of these chemicals were not included on the product labels, making it difficult for consumers to consciously avoid them.
“This study is very helpful for elucidating the PFAS content of different types of cosmetics in the U.S. and Canadian markets,” said Elsie Sunderland, PhD, an environmental scientist who was not involved with the study.
“Previously, all the data had been collected in Europe, and this study shows we are dealing with similar problems in the North American marketplace,” said Dr. Sunderland, a professor of environmental chemistry at the Harvard School of Public Health, Boston.
PFAS are a class of chemicals used in a variety of consumer products, such as nonstick cookware, stain-resistant carpeting, and water-repellent clothing, according to the Centers for Disease Control and Prevention. They are added to cosmetics to make the products more durable and spreadable, researchers said in the study.
“[PFAS] are added to change the properties of surfaces, to make them nonstick or resistant to stay in water or oils,” said study coauthor Tom Bruton, PhD, senior scientist at the Green Science Policy Institute in Berkeley, Calif. “The concerning thing about cosmetics is that these are products that you’re applying to your skin and face every day, so there’s the skin absorption route that’s of concern, but also incidental ingestion of cosmetics is also a concern as well.”
The CDC says some of the potential health effects of PFAS exposure includes increased cholesterol levels, increased risk of kidney and testicular cancer, changes in liver enzymes, decreased vaccine response in children, and a higher risk of high blood pressure or preeclampsia in pregnant women.
“PFAS are a large class of chemicals. In humans, exposure to some of these chemicals has been associated with impaired immune function, certain cancers, increased risks of diabetes, obesity and endocrine disruption,” Dr. Sunderland said. “They appear to be harmful to every major organ system in the human body.”
For the current study, published online in Environmental Science & Technology Letters, Dr. Bruton and colleagues purchased 231 cosmetic products in the United States and Canada from retailers such as Ulta Beauty, Sephora, Target, and Bed Bath & Beyond. They then screened them for fluorine.Three-quarters of waterproof mascara samples contained high fluorine concentrations, as did nearly two-thirds of foundations and liquid lipsticks, and more than half of the eye and lip products tested.
The authors found that different categories of makeup tended to have higher or lower fluorine concentrations. “High fluorine levels were found in products commonly advertised as ‘wear-resistant’ to water and oils or ‘long-lasting,’ including foundations, liquid lipsticks, and waterproof mascaras,” Dr. Bruton and colleagues wrote.
When they further analyzed a subset of 29 products to determine what types of chemicals were present, they found that each cosmetic product contained at least 4 PFAS, with one product containing 13.The PFAS substances found included some that break down into other chemicals that are known to be highly toxic and environmentally harmful.
“It’s concerning that some of the products we tested appear to be intentionally using PFAS, but not listing those ingredients on the label,” Dr. Bruton said. “I do think that it is helpful for consumers to read labels, but beyond that, there’s not a lot of ways that consumers themselves can solve this problem. ... We think that the industry needs to be more proactive about moving away from this group of chemicals.”
Dr. Sunderland said a resource people can use when trying to avoid PFAS is the Environmental Working Group, a nonprofit organization that maintains an extensive database of cosmetics and personal care products.
“At this point, there is very little regulatory activity related to PFAS in cosmetics,” Dr. Sunderland said. “The best thing to happen now would be for consumers to indicate that they prefer products without PFAS and to demand better transparency in product ingredient lists.”
A similar study done in 2018 by the Danish Environmental Protection Agency found high levels of PFAS in nearly one-third of the cosmetics products it tested.
People can also be exposed to PFAS by eating or drinking contaminated food or water and through food packaging. Dr. Sunderland said some wild foods like seafood are known to accumulate these compounds in the environment.
“There are examples of contaminated biosolids leading to accumulation of PFAS in vegetables and milk,” Dr. Sunderland explained. “Food packaging is another concern because it can also result in PFAS accumulation in the foods we eat.”
Although it’s difficult to avoid PFAS altogether, the CDC suggests lowering exposure rates by avoiding contaminated water and food. If you’re not sure if your water is contaminated, you should ask your local or state health and environmental quality departments for fish or water advisories in your area.
A version of this article first appeared on WebMD.com.
People may be absorbing and ingesting potentially toxic chemicals from their cosmetic products, a new study suggests.
– per- and polyfluoroalkyl substances. Many of these chemicals were not included on the product labels, making it difficult for consumers to consciously avoid them.
“This study is very helpful for elucidating the PFAS content of different types of cosmetics in the U.S. and Canadian markets,” said Elsie Sunderland, PhD, an environmental scientist who was not involved with the study.
“Previously, all the data had been collected in Europe, and this study shows we are dealing with similar problems in the North American marketplace,” said Dr. Sunderland, a professor of environmental chemistry at the Harvard School of Public Health, Boston.
PFAS are a class of chemicals used in a variety of consumer products, such as nonstick cookware, stain-resistant carpeting, and water-repellent clothing, according to the Centers for Disease Control and Prevention. They are added to cosmetics to make the products more durable and spreadable, researchers said in the study.
“[PFAS] are added to change the properties of surfaces, to make them nonstick or resistant to stay in water or oils,” said study coauthor Tom Bruton, PhD, senior scientist at the Green Science Policy Institute in Berkeley, Calif. “The concerning thing about cosmetics is that these are products that you’re applying to your skin and face every day, so there’s the skin absorption route that’s of concern, but also incidental ingestion of cosmetics is also a concern as well.”
The CDC says some of the potential health effects of PFAS exposure includes increased cholesterol levels, increased risk of kidney and testicular cancer, changes in liver enzymes, decreased vaccine response in children, and a higher risk of high blood pressure or preeclampsia in pregnant women.
“PFAS are a large class of chemicals. In humans, exposure to some of these chemicals has been associated with impaired immune function, certain cancers, increased risks of diabetes, obesity and endocrine disruption,” Dr. Sunderland said. “They appear to be harmful to every major organ system in the human body.”
For the current study, published online in Environmental Science & Technology Letters, Dr. Bruton and colleagues purchased 231 cosmetic products in the United States and Canada from retailers such as Ulta Beauty, Sephora, Target, and Bed Bath & Beyond. They then screened them for fluorine.Three-quarters of waterproof mascara samples contained high fluorine concentrations, as did nearly two-thirds of foundations and liquid lipsticks, and more than half of the eye and lip products tested.
The authors found that different categories of makeup tended to have higher or lower fluorine concentrations. “High fluorine levels were found in products commonly advertised as ‘wear-resistant’ to water and oils or ‘long-lasting,’ including foundations, liquid lipsticks, and waterproof mascaras,” Dr. Bruton and colleagues wrote.
When they further analyzed a subset of 29 products to determine what types of chemicals were present, they found that each cosmetic product contained at least 4 PFAS, with one product containing 13.The PFAS substances found included some that break down into other chemicals that are known to be highly toxic and environmentally harmful.
“It’s concerning that some of the products we tested appear to be intentionally using PFAS, but not listing those ingredients on the label,” Dr. Bruton said. “I do think that it is helpful for consumers to read labels, but beyond that, there’s not a lot of ways that consumers themselves can solve this problem. ... We think that the industry needs to be more proactive about moving away from this group of chemicals.”
Dr. Sunderland said a resource people can use when trying to avoid PFAS is the Environmental Working Group, a nonprofit organization that maintains an extensive database of cosmetics and personal care products.
“At this point, there is very little regulatory activity related to PFAS in cosmetics,” Dr. Sunderland said. “The best thing to happen now would be for consumers to indicate that they prefer products without PFAS and to demand better transparency in product ingredient lists.”
A similar study done in 2018 by the Danish Environmental Protection Agency found high levels of PFAS in nearly one-third of the cosmetics products it tested.
People can also be exposed to PFAS by eating or drinking contaminated food or water and through food packaging. Dr. Sunderland said some wild foods like seafood are known to accumulate these compounds in the environment.
“There are examples of contaminated biosolids leading to accumulation of PFAS in vegetables and milk,” Dr. Sunderland explained. “Food packaging is another concern because it can also result in PFAS accumulation in the foods we eat.”
Although it’s difficult to avoid PFAS altogether, the CDC suggests lowering exposure rates by avoiding contaminated water and food. If you’re not sure if your water is contaminated, you should ask your local or state health and environmental quality departments for fish or water advisories in your area.
A version of this article first appeared on WebMD.com.
Photobiomodulation: Evaluation in a wide range of medical specialties underway
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
FROM ASLMS 2021
Expert offers 10 ‘tips and tricks’ for everyday cosmetic practice
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
, based on nearly 10 years of experience treating patients on both coasts of the United States.
They are as follows:
1. Know your clinical endpoints. “One of the things that was drilled into me during my fellowship in lasers and cosmetics at Mass General was to know your clinical endpoints and to avoid a cookbook approach,” said Dr. Jalian, who practices dermatology in Los Angeles. “You should treat based on the pathology that you’re seeing on the skin and let the endpoints be your guide. The skin will tell you what you’re doing right, and the skin will tell you what you’re doing wrong. Picking up on these cues will allow you to deliver a safe and effective treatment to your patients.”
The selection of proper treatment parameters is driven by selective photothermolysis, a microsurgery technique that uses customized wavelengths, pulse durations, and fluences to target a chromophore. “Knowing the size and shape of your target allows you to pick the right pulse duration,” he said. “This is dictated by thermal relaxation time, which is proportional to the size and shape of the target. Smaller targets require a shorter pulse width, while larger targets require a longer pulse width.”
2. Do not perform a procedure for which you cannot recognize and treat the side effects. “I can’t tell you how many times I’ve gotten referrals from outside providers with a side effect that, if it had been recognized and treated, would have been inconsequential long-term for the patient,” Dr. Jalian said. “You can only avoid complications by not firing the laser. The more you practice, the more complications you’re going to have. This is inevitable, but good practice and common sense can reduce complications significantly.”
Even the most skilled clinicians encounter side effects from time to time. “The most important thing is to form a network of physicians you trust that you can call or text when you need help in managing a particular complication,” he continued. “This happens to all of us,” he said. Don’t be afraid to phone a colleague, he advised, “and get a fresh set of eyes because oftentimes they can provide insights, especially when you’re having tunnel vision during a complication, that can ultimately result in better patient care.”
3. Don’t forget your clinical training. “Trust your clinical judgment,” Dr. Jalian said. “If something doesn’t seem right, even if it was a case referred to you by experienced practitioners, you are a clinician first and foremost, and you are allowed to make a clinical judgment on lesions.” He referred to a 2011 report in which the authors described a series of four cases where patients presented for cosmetic evaluation of vascular lesions that turned out to be more significant pathologic disease. “Trust your clinical insight because this will serve you in the long term,” he said.
4. Set realistic expectations. Patients with unrealistically high expectations are likely to express dissatisfaction with their treatment results, “no matter how good of a job you do,” Dr. Jalian said. “In addition to safely treating the patient, we strive for patient satisfaction, because with these elective procedures we’re trying to give a patient a result they’re looking for. But our No. 1 job is also to be realistic about the results we can obtain. If someone comes in wanting treatment with a skin-tightening device but clearly needs a face-lift because they have too much laxity, your job is to tell them that this is not the appropriate device for them. Learn the art of saying no. If handled correctly, the patient will often thank you before she heads out the door. Ultimately, honesty is the best policy. I may say something like, ‘I’m not telling you the answer you want to hear, I’m telling you the truth.’ That often goes over well.”
5. Use proper anesthesia. Patients come to you for results, but they’re also likely to remember how well you controlled their pain during procedures. Strategies favored by Dr. Jalian include applying extra topical anesthetic to “hot spots” and splitting up treatment sessions when tackling a large area. “Consider using adjunctive analgesics such as oral medications and nitrous oxide,” he added. Other options, he said, are cooling techniques and distraction techniques, such as the use of a stress ball, consideration of the gate control theory of pain, and “talkesthesia”(using conversation to distract the patient).
6. Obtain proper informed consent. A lack of informed consent ranks as a common reason why doctors get sued. “This happens when a physician fails to inform the patient of all medically reasonable alternatives and their risks, even for noninvasive procedures prior to administering treatment,” Dr. Jalian said. “All patients have the right to an informed consent prior to any treatment. It doesn’t necessarily have to be in a written form, but it’s important to at least have a discussion and document it for all procedures, including medical and cosmetic procedures and oral and topical treatments. Keep it simple. A written consent is ineffective if the patient does not understand material about the procedure.” Avoid the use of excessive medical terms. For example, use bruising instead of purpura, redness instead of erythema, and drooping instead of ptosis.
7. Lower the laser treatment density for darker skin types. According to Dr. Jalian, several clinical studies have demonstrated that lower densities are associated with less postinflammatory hyperpigmentation in Asian and Black patients, without sacrificing clinical outcomes. “Density determines how ‘aggressive’ a treatment is,” he said. “The greater the density, the more downtime is required.”
8. Have a vascular occlusion emergency kit on hand. At a minimum, the kit should contain at least 1,500 units of hyaluronidase, aspirin, timolol/acetazolamide, a Snellen chart, steroids, and an EpiPen.
9. Use standardized photography. Even the slightest change in lighting can manipulate your results. According to Dr. Jalian, standardized photos enable you to monitor patient progress, minimize liability, and can serve as a marketing tool “so that you can capitalize on your talent.”
10. Consider combination treatments. He combines lasers based on target and depth. For example, prior to resurfacing he often performs a pass or two with a color laser such as intense pulsed light. “Depending on what’s being done, we’ll do soft-tissue augmentation before or after treatment with certain lasers,” Dr. Jalian added. “If you’re performing a toxin treatment on the same day as a laser procedure, do not treat the lower face or neck. Do the laser procedure first and limit that to the upper third of the face.”
He reported having no relevant financial disclosures.
FROM ASLMS 2021
Expert shares her tips for an effective cosmetic consultation
The way Kelly Stankiewicz, MD, sees it,
“I can’t tell you how many times I’ve walked into a room and thought the patient would say they’re concerned about one thing, but they’re concerned about something totally different,” Dr. Stankiewicz, a dermatologist in private practice in Park City, Utah, said during the annual conference of the American Society for Laser Medicine and Surgery. “The first question I ask is, ‘What would you like to improve?’ ‘What’s bothering you?’ ‘What would you like to make look better?’ Frequently, it’s not what you think.”
Next, she tries to get a sense of their lifestyle by asking patients about their occupation, hobbies, and outdoor activities they may engage in. “Here in Park City, it’s very sunny most all the time, so treatments need to be tailored to when those outdoor activities are being done, or perhaps they can be avoided for a period of time,” she said. “This gives you an idea of what kind of downtime people will tolerate. I also like to hear about their history of cosmetic procedures. If someone has had a lot of cosmetic procedures done, you can talk with them on a more detailed level. If someone is completely unaware of treatment options, you have to keep it simple.”
Dr. Stankiewicz also reviews their personal history of cosmetic procedures when considering safety of treatment. “For instance, if somebody has had a neck lift, you want to be very cautious doing any ablative procedures along the jawline,” she said. “I also like to know if anyone has had any reactions to dermal fillers or neuromodulators that they did not like. It’s very helpful to hear from patients what’s worked for them and what hasn’t. I also like to keep my ear open for pricing concerns. Not everyone will bring up the pricing issues, but sometimes they will, and it’s an important piece of information. Lastly, it’s important to look for any warning signs like irrational behavior or unrealistic expectations. These are patients you want to try to avoid treating.”
She shared four other key components to an effective cosmetic consultation, including the examination itself, which she prefers to separate from the discussion portion of the visit. “I lean the patient back in the exam chair and shine the light on their skin, which is important for evaluating for conditions you may not have discussed that could be easily improved,” Dr. Stankiewicz said.
“If the patient is concerned about pigmented lesions, I’ll pull out my dermatoscope to make sure there isn’t any concern for skin cancer. After the examination, I’ll sit the patient up again so that there is a very distinct start and finish to the examination portion of my cosmetic consultation.”
Surgery vs. noninvasive treatments
Step three in her consultative process is to review treatment options with patients. “I never hold back if surgery is their best treatment option,” she said. “I don’t perform surgery, but I have a list of people I can refer them to.”
Once she addresses the potential for surgery, she reviews noninvasive treatment options, including topical products, injectables, lasers, and chemical peels. “Everyone who comes in for a cosmetic consultation leaves with some sort of topical recommendation, even if it’s as simple as a sunscreen I think they would like or a prescription for generic tretinoin,” she said. “I always present options in a framework starting with those that require lower downtime, higher number of treatment options, and lower cost. Then I move up the scale to tell them more about treatments that require higher downtime, a lower number of treatments, but have a higher cost.”
Step four in her consultative process involves discussing her final treatment recommendations. She’ll say something like, “I’ve been through all these options with you and my final recommendation is X,” and the patient walks away with a clear understanding of the recommendations, she said. “When I leave the room after giving my final recommendation, I’ll write everything down outside of the room, or I’ll have a member of my staff write down everything I’ve said outside the room.”
Finally, she and her staff record all the relevant information for the patient as a customized handout, including the treatment options discussed, how many will be required, whether they have to come in early for numbing cream or not, and the per treatment price tag. “Once we’ve written down everything we’ve discussed, I’ll circle or I’ll star my recommended treatment,” Dr. Stankiewicz said. They also have a handout for topical products, and she checks off the topical products that she discussed with the patient. The third handout she provides to patients is a recommended skin care regimen.
Dr. Stankiewicz reported having no relevant financial disclosures.
The way Kelly Stankiewicz, MD, sees it,
“I can’t tell you how many times I’ve walked into a room and thought the patient would say they’re concerned about one thing, but they’re concerned about something totally different,” Dr. Stankiewicz, a dermatologist in private practice in Park City, Utah, said during the annual conference of the American Society for Laser Medicine and Surgery. “The first question I ask is, ‘What would you like to improve?’ ‘What’s bothering you?’ ‘What would you like to make look better?’ Frequently, it’s not what you think.”
Next, she tries to get a sense of their lifestyle by asking patients about their occupation, hobbies, and outdoor activities they may engage in. “Here in Park City, it’s very sunny most all the time, so treatments need to be tailored to when those outdoor activities are being done, or perhaps they can be avoided for a period of time,” she said. “This gives you an idea of what kind of downtime people will tolerate. I also like to hear about their history of cosmetic procedures. If someone has had a lot of cosmetic procedures done, you can talk with them on a more detailed level. If someone is completely unaware of treatment options, you have to keep it simple.”
Dr. Stankiewicz also reviews their personal history of cosmetic procedures when considering safety of treatment. “For instance, if somebody has had a neck lift, you want to be very cautious doing any ablative procedures along the jawline,” she said. “I also like to know if anyone has had any reactions to dermal fillers or neuromodulators that they did not like. It’s very helpful to hear from patients what’s worked for them and what hasn’t. I also like to keep my ear open for pricing concerns. Not everyone will bring up the pricing issues, but sometimes they will, and it’s an important piece of information. Lastly, it’s important to look for any warning signs like irrational behavior or unrealistic expectations. These are patients you want to try to avoid treating.”
She shared four other key components to an effective cosmetic consultation, including the examination itself, which she prefers to separate from the discussion portion of the visit. “I lean the patient back in the exam chair and shine the light on their skin, which is important for evaluating for conditions you may not have discussed that could be easily improved,” Dr. Stankiewicz said.
“If the patient is concerned about pigmented lesions, I’ll pull out my dermatoscope to make sure there isn’t any concern for skin cancer. After the examination, I’ll sit the patient up again so that there is a very distinct start and finish to the examination portion of my cosmetic consultation.”
Surgery vs. noninvasive treatments
Step three in her consultative process is to review treatment options with patients. “I never hold back if surgery is their best treatment option,” she said. “I don’t perform surgery, but I have a list of people I can refer them to.”
Once she addresses the potential for surgery, she reviews noninvasive treatment options, including topical products, injectables, lasers, and chemical peels. “Everyone who comes in for a cosmetic consultation leaves with some sort of topical recommendation, even if it’s as simple as a sunscreen I think they would like or a prescription for generic tretinoin,” she said. “I always present options in a framework starting with those that require lower downtime, higher number of treatment options, and lower cost. Then I move up the scale to tell them more about treatments that require higher downtime, a lower number of treatments, but have a higher cost.”
Step four in her consultative process involves discussing her final treatment recommendations. She’ll say something like, “I’ve been through all these options with you and my final recommendation is X,” and the patient walks away with a clear understanding of the recommendations, she said. “When I leave the room after giving my final recommendation, I’ll write everything down outside of the room, or I’ll have a member of my staff write down everything I’ve said outside the room.”
Finally, she and her staff record all the relevant information for the patient as a customized handout, including the treatment options discussed, how many will be required, whether they have to come in early for numbing cream or not, and the per treatment price tag. “Once we’ve written down everything we’ve discussed, I’ll circle or I’ll star my recommended treatment,” Dr. Stankiewicz said. They also have a handout for topical products, and she checks off the topical products that she discussed with the patient. The third handout she provides to patients is a recommended skin care regimen.
Dr. Stankiewicz reported having no relevant financial disclosures.
The way Kelly Stankiewicz, MD, sees it,
“I can’t tell you how many times I’ve walked into a room and thought the patient would say they’re concerned about one thing, but they’re concerned about something totally different,” Dr. Stankiewicz, a dermatologist in private practice in Park City, Utah, said during the annual conference of the American Society for Laser Medicine and Surgery. “The first question I ask is, ‘What would you like to improve?’ ‘What’s bothering you?’ ‘What would you like to make look better?’ Frequently, it’s not what you think.”
Next, she tries to get a sense of their lifestyle by asking patients about their occupation, hobbies, and outdoor activities they may engage in. “Here in Park City, it’s very sunny most all the time, so treatments need to be tailored to when those outdoor activities are being done, or perhaps they can be avoided for a period of time,” she said. “This gives you an idea of what kind of downtime people will tolerate. I also like to hear about their history of cosmetic procedures. If someone has had a lot of cosmetic procedures done, you can talk with them on a more detailed level. If someone is completely unaware of treatment options, you have to keep it simple.”
Dr. Stankiewicz also reviews their personal history of cosmetic procedures when considering safety of treatment. “For instance, if somebody has had a neck lift, you want to be very cautious doing any ablative procedures along the jawline,” she said. “I also like to know if anyone has had any reactions to dermal fillers or neuromodulators that they did not like. It’s very helpful to hear from patients what’s worked for them and what hasn’t. I also like to keep my ear open for pricing concerns. Not everyone will bring up the pricing issues, but sometimes they will, and it’s an important piece of information. Lastly, it’s important to look for any warning signs like irrational behavior or unrealistic expectations. These are patients you want to try to avoid treating.”
She shared four other key components to an effective cosmetic consultation, including the examination itself, which she prefers to separate from the discussion portion of the visit. “I lean the patient back in the exam chair and shine the light on their skin, which is important for evaluating for conditions you may not have discussed that could be easily improved,” Dr. Stankiewicz said.
“If the patient is concerned about pigmented lesions, I’ll pull out my dermatoscope to make sure there isn’t any concern for skin cancer. After the examination, I’ll sit the patient up again so that there is a very distinct start and finish to the examination portion of my cosmetic consultation.”
Surgery vs. noninvasive treatments
Step three in her consultative process is to review treatment options with patients. “I never hold back if surgery is their best treatment option,” she said. “I don’t perform surgery, but I have a list of people I can refer them to.”
Once she addresses the potential for surgery, she reviews noninvasive treatment options, including topical products, injectables, lasers, and chemical peels. “Everyone who comes in for a cosmetic consultation leaves with some sort of topical recommendation, even if it’s as simple as a sunscreen I think they would like or a prescription for generic tretinoin,” she said. “I always present options in a framework starting with those that require lower downtime, higher number of treatment options, and lower cost. Then I move up the scale to tell them more about treatments that require higher downtime, a lower number of treatments, but have a higher cost.”
Step four in her consultative process involves discussing her final treatment recommendations. She’ll say something like, “I’ve been through all these options with you and my final recommendation is X,” and the patient walks away with a clear understanding of the recommendations, she said. “When I leave the room after giving my final recommendation, I’ll write everything down outside of the room, or I’ll have a member of my staff write down everything I’ve said outside the room.”
Finally, she and her staff record all the relevant information for the patient as a customized handout, including the treatment options discussed, how many will be required, whether they have to come in early for numbing cream or not, and the per treatment price tag. “Once we’ve written down everything we’ve discussed, I’ll circle or I’ll star my recommended treatment,” Dr. Stankiewicz said. They also have a handout for topical products, and she checks off the topical products that she discussed with the patient. The third handout she provides to patients is a recommended skin care regimen.
Dr. Stankiewicz reported having no relevant financial disclosures.
FROM ASLMS 2021
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
Cellular senescence, skin aging, and cosmeceuticals
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.
Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
One year after a single RAP treatment, patients give the results high marks
One year after a single treatment of cellulite with the Rapid Acoustic Pulse (RAP) device, 42 out of 43 patients said that they felt good about the results, an interim analysis from a multicenter study showed.
“I think this speaks to the duration of improvement” associated with this treatment, lead study author Elizabeth L. Tanzi, MD, said during the annual conference of the American Society for Laser Medicine and Surgery.
The study is an extension of a prospective pivotal clinical trial that Dr. Tanzi first presented during late-breaking abstract session at the 2020 virtual annual meeting of the American Academy of Dermatology. For the trial, she and her colleagues at four sites evaluated the safety and effectiveness of the RAP device in 62 women who were treated with a single, rapid acoustic pulse treatment comprised of 1-2 minutes on each identified dimple or large ridge of cellulite. In February 2021, the Food and Drug Administration cleared the device for the short-term improvement in the appearance of cellulite.
“The high peak pressure and fast repetition rate of this device will exploit the viscoelastic tissue compared to other acoustic wave devices that are on the market,” said Dr. Tanzi, associate clinical professor of dermatology at George Washington University, Washington. “Those compressed pulses from electronic filtering and reflector shape eliminate cavitation, heat, and pain. So, what we see physically is fiber septa disruption, as well as other nonthermal physical effects.”
She and her colleagues used a specific photography and fixed lighting setup to record the treated areas at baseline, 12 weeks, and 52 weeks, and administered a patient satisfaction questionnaire at 12 and 52 weeks. Prior to treatment, the investigators marked the dimples and ridges intended for treatment. After placing a hydrogel dressing, it took 45-60 minutes to treat both buttocks and thighs with the RAP device. “It was completely noninvasive,” said Dr. Tanzi, who practices in Chevy Chase, Md. “There was no anesthesia used: no incisions and no needles.”
Among 57 patients who were evaluated at 12 weeks, the pain score on a scale of 0-10 was 2.4, while 61.5% strongly agreed and 35.9% agreed that their cellulite appeared improved. In addition, three blinded assessors were able to correctly identify the treated thigh 96% of the time and physician-graded Global Aesthetic Improvement Scale assessments showed 90% improvement of cellulite.
Follow-up analysis
For the current subject satisfaction analysis, the investigators evaluated results from 43 patients in the trial who were followed for at least 52 weeks (a mean of 60 weeks). Of the 43 patients, 30 (69.8%) strongly agreed and 13 (30.2%) agreed that their cellulite “appears improved.” In addition, 29 (67.4%) strongly agreed, 13 (30.2%) agreed, and 1 (2.3%) disagreed with the statement “I feel there is good improvement” of their cellulite.
“Currently, we are evaluating the blinded assessors’ view of these patients and not just going with [findings from] the patient satisfaction survey, but I think these are encouraging results,” Dr. Tanzi said. “We found that 42 out of 43 patients said, ‘yes; I feel that there was good improvement of the area at 52 weeks.’ ”
Dr. Tanzi disclosed that she is a member of the speakers bureau for Eucerin. She is a member of the advisory board for Allergan, Endo Pharmaceuticals, Pulse Biosciences, Sciton, and Soliton. Soliton markets the RAP device.
One year after a single treatment of cellulite with the Rapid Acoustic Pulse (RAP) device, 42 out of 43 patients said that they felt good about the results, an interim analysis from a multicenter study showed.
“I think this speaks to the duration of improvement” associated with this treatment, lead study author Elizabeth L. Tanzi, MD, said during the annual conference of the American Society for Laser Medicine and Surgery.
The study is an extension of a prospective pivotal clinical trial that Dr. Tanzi first presented during late-breaking abstract session at the 2020 virtual annual meeting of the American Academy of Dermatology. For the trial, she and her colleagues at four sites evaluated the safety and effectiveness of the RAP device in 62 women who were treated with a single, rapid acoustic pulse treatment comprised of 1-2 minutes on each identified dimple or large ridge of cellulite. In February 2021, the Food and Drug Administration cleared the device for the short-term improvement in the appearance of cellulite.
“The high peak pressure and fast repetition rate of this device will exploit the viscoelastic tissue compared to other acoustic wave devices that are on the market,” said Dr. Tanzi, associate clinical professor of dermatology at George Washington University, Washington. “Those compressed pulses from electronic filtering and reflector shape eliminate cavitation, heat, and pain. So, what we see physically is fiber septa disruption, as well as other nonthermal physical effects.”
She and her colleagues used a specific photography and fixed lighting setup to record the treated areas at baseline, 12 weeks, and 52 weeks, and administered a patient satisfaction questionnaire at 12 and 52 weeks. Prior to treatment, the investigators marked the dimples and ridges intended for treatment. After placing a hydrogel dressing, it took 45-60 minutes to treat both buttocks and thighs with the RAP device. “It was completely noninvasive,” said Dr. Tanzi, who practices in Chevy Chase, Md. “There was no anesthesia used: no incisions and no needles.”
Among 57 patients who were evaluated at 12 weeks, the pain score on a scale of 0-10 was 2.4, while 61.5% strongly agreed and 35.9% agreed that their cellulite appeared improved. In addition, three blinded assessors were able to correctly identify the treated thigh 96% of the time and physician-graded Global Aesthetic Improvement Scale assessments showed 90% improvement of cellulite.
Follow-up analysis
For the current subject satisfaction analysis, the investigators evaluated results from 43 patients in the trial who were followed for at least 52 weeks (a mean of 60 weeks). Of the 43 patients, 30 (69.8%) strongly agreed and 13 (30.2%) agreed that their cellulite “appears improved.” In addition, 29 (67.4%) strongly agreed, 13 (30.2%) agreed, and 1 (2.3%) disagreed with the statement “I feel there is good improvement” of their cellulite.
“Currently, we are evaluating the blinded assessors’ view of these patients and not just going with [findings from] the patient satisfaction survey, but I think these are encouraging results,” Dr. Tanzi said. “We found that 42 out of 43 patients said, ‘yes; I feel that there was good improvement of the area at 52 weeks.’ ”
Dr. Tanzi disclosed that she is a member of the speakers bureau for Eucerin. She is a member of the advisory board for Allergan, Endo Pharmaceuticals, Pulse Biosciences, Sciton, and Soliton. Soliton markets the RAP device.
One year after a single treatment of cellulite with the Rapid Acoustic Pulse (RAP) device, 42 out of 43 patients said that they felt good about the results, an interim analysis from a multicenter study showed.
“I think this speaks to the duration of improvement” associated with this treatment, lead study author Elizabeth L. Tanzi, MD, said during the annual conference of the American Society for Laser Medicine and Surgery.
The study is an extension of a prospective pivotal clinical trial that Dr. Tanzi first presented during late-breaking abstract session at the 2020 virtual annual meeting of the American Academy of Dermatology. For the trial, she and her colleagues at four sites evaluated the safety and effectiveness of the RAP device in 62 women who were treated with a single, rapid acoustic pulse treatment comprised of 1-2 minutes on each identified dimple or large ridge of cellulite. In February 2021, the Food and Drug Administration cleared the device for the short-term improvement in the appearance of cellulite.
“The high peak pressure and fast repetition rate of this device will exploit the viscoelastic tissue compared to other acoustic wave devices that are on the market,” said Dr. Tanzi, associate clinical professor of dermatology at George Washington University, Washington. “Those compressed pulses from electronic filtering and reflector shape eliminate cavitation, heat, and pain. So, what we see physically is fiber septa disruption, as well as other nonthermal physical effects.”
She and her colleagues used a specific photography and fixed lighting setup to record the treated areas at baseline, 12 weeks, and 52 weeks, and administered a patient satisfaction questionnaire at 12 and 52 weeks. Prior to treatment, the investigators marked the dimples and ridges intended for treatment. After placing a hydrogel dressing, it took 45-60 minutes to treat both buttocks and thighs with the RAP device. “It was completely noninvasive,” said Dr. Tanzi, who practices in Chevy Chase, Md. “There was no anesthesia used: no incisions and no needles.”
Among 57 patients who were evaluated at 12 weeks, the pain score on a scale of 0-10 was 2.4, while 61.5% strongly agreed and 35.9% agreed that their cellulite appeared improved. In addition, three blinded assessors were able to correctly identify the treated thigh 96% of the time and physician-graded Global Aesthetic Improvement Scale assessments showed 90% improvement of cellulite.
Follow-up analysis
For the current subject satisfaction analysis, the investigators evaluated results from 43 patients in the trial who were followed for at least 52 weeks (a mean of 60 weeks). Of the 43 patients, 30 (69.8%) strongly agreed and 13 (30.2%) agreed that their cellulite “appears improved.” In addition, 29 (67.4%) strongly agreed, 13 (30.2%) agreed, and 1 (2.3%) disagreed with the statement “I feel there is good improvement” of their cellulite.
“Currently, we are evaluating the blinded assessors’ view of these patients and not just going with [findings from] the patient satisfaction survey, but I think these are encouraging results,” Dr. Tanzi said. “We found that 42 out of 43 patients said, ‘yes; I feel that there was good improvement of the area at 52 weeks.’ ”
Dr. Tanzi disclosed that she is a member of the speakers bureau for Eucerin. She is a member of the advisory board for Allergan, Endo Pharmaceuticals, Pulse Biosciences, Sciton, and Soliton. Soliton markets the RAP device.
FROM ASLMS 2021