User login
Dermatologists skeptical of calamine lotion TikTok trend
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Though this may seem to work as a base layer for some people, dermatologists have concerns about this trend, particularly the risk of dryness.
As of Aug. 15, the #calaminelotion tag had more than 20.9 million views on TikTok, with hundreds of videos hailing the cream for its opaque pink tint and matte effect when used under foundation.
Calamine lotion has been used to treat itchy rashes, insect bites, and pain from chickenpox and poison ivy for years. It’s sold over the counter and is a common first-line treatment for skin discomfort that has been used for hundreds of years, says Doris Day, MD, a dermatologist who practices in New York City. It is also on the World Health Organization’s list of essential drugs, she points out in an interview.
“This is something that has been around for a long time. It’s recognized as a drug that has importance. So every now and then, I guess somebody comes across it” and says it’s a “new panacea” for something, “but it’s really not. It’s just an old-time simple product.”
Calamine lotion is made of ferric oxide and zinc oxide, which gives it its antiseptic and anti-itch properties, in addition to its characteristic pink color. Zinc oxide is also commonly used in mineral sunscreens, Dr. Day points out.
Although these ingredients are exceedingly safe with temporary, localized use, high concentrations and chronic use of calamine lotion can be irritating to the skin, says Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington.
At these high concentrations, calamine lotion can be drying, which may cause skin clumping and can be abrasive, says Dr. Sodha. She also cautions that the astringent properties of the zinc and the high pH may disrupt proteins on the skin, which breaks down the skin’s natural defenses. Using calamine lotion all over the face daily can “potentially damage your skin barrier to a point where you’re going to have to do a lot of extra work ... to bring it back,” says Dr. Sodha.
Dr. Day also worries about this trend resulting in dry skin among followers. Even in situations where using calamine lotion is appropriate, like treating poison ivy, its drying effects can sometimes irritate the skin.
And dry skin can be more than an aesthetic issue: It can lead to breaks in the skin, which can result in infections and scarring, she points out. Although this may not occur in someone with extremely oily skin, most people don’t have extremely oily skin, says Dr. Day, so this will be ineffective at best, and at worst, damaging.
If someone is looking for a good makeup base layer, Dr. Sodha recommends something that’s noncomedogenic and nonsensitizing, like silicon-based primers. “The great thing about these products is that they are noncomedogenic, so they won’t clog your pores. They’re synthetic, so they’re not going to cause some sort of allergy,” she says.
In general, both dermatologists warn their patients to be wary of the TikTok trends they see online, and they cautioned about possible effects with long term use of calamine lotion on the face, even if it appears to work with one-time use. “Consumers have to think about this like they do with any sort of product that they come across, just thinking about the long-term effects of something like this and how it works for their own skin,” says Dr. Sodha.
A version of this article first appeared on Medscape.com.
Funding of cosmetic clinical trials linked to racial/ethnic disparity
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.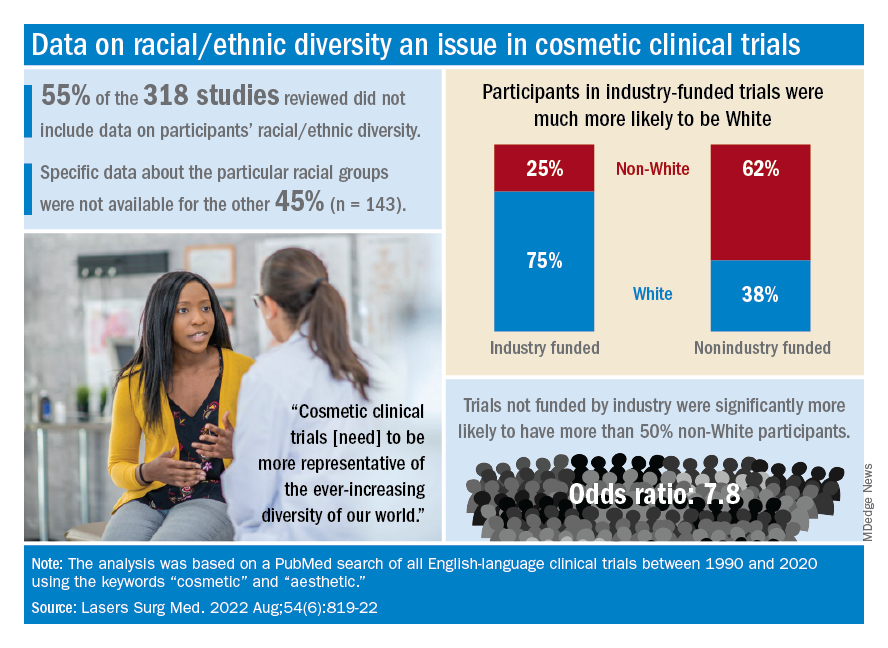
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
FROM LASERS IN SURGERY AND MEDICINE
Aesthetics abound for the aging face
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
How does radiofrequency microneedling work?
Technology in the field of aesthetic dermatology continues to advance over time. Microneedling, largely used to improve textural changes of the skin associated with photoaging and acne scarring, has evolved over time from the use of dermarollers and microneedling skin pens to energy-based devices that deliver radiofrequency (RF) energy though microneedles that are used today.
.
Unlike prior radiofrequency energy-based devices that deliver radiofrequency energy on the skin surface to allow bulk thermal energy (or heat) to stimulate collagen remodeling and tissue tightening, RF microneedling devices deliver the same RF or thermal energy via needles. RF, measured in Hertz (Hz) is part of the electromagnetic spectrum, with most devices delivering thermal energy at around 1-2 MHz, which is less than most typical RF only devices (at around 4-6 MHz), but with potentially more precise depth and delivery. For comparison, the RF of household electrical currents are around 60 Hz; traditional electrosurgical units, 50Hz -300 kHz; AM radio, 500 KHz; and microwaves, 2500 MHz.
When delivered to the skin, RF energy produces a change in the electrical charge of the skin, resulting in movement of electrons. The impedance (or resistance) of the tissue to the electron movement is what generates heat. Different factors, including tissue thickness, pressure applied to the tissue, hydration, bipolar versus monopolar delivery, and the number of needles are several factors than can affect the impedance.
Bipolar RF means that the current passes between two electrodes, whereas monopolar RF means that the electrical current is between an active treatment electrode and a passive grounding electrode (or grounding pad typically placed on the patient’s back). With bipolar RF, the current is limited to the area between the two electrodes. The depth of penetration is half of the distance between the electrodes, thus resulting in shallow (but potentially more aggressive) tissue heating. With monopolar RF, deeper tissue penetration occurs that is also often less uncomfortable to the patient.
The desired result of the energy delivery is collagen remodeling and strengthening of elastin. RF microneedling and microneedling in general may also have potential for use in enhancing topical product delivery.
Depending on the device, settings can be tailored to affect the energy delivery, including the type of needle (insulated vs. uninsulated vs. semi-insulated), Hz, number of needles, depth of needles, and time of exposure. In general, insulated needle tips provide less heat accumulation and potential injury to the skin surface, whereas uninsulated needles allow for more heat accumulation. Insulated needles, longer time of exposure, and lower energies (Hz) are safer options for darker skin types and those who hyperpigment easily.
Immediately after treatment, expected clinical endpoints can include erythema, edema, and possibly pinpoint bleeding that may last approximately several days to 2 weeks depending on the intensity of treatment. Potential side effects include infection, pigmentary alteration, folliculitis, prolonged grid marks, and scarring. Contraindications to treatment include having a pacemaker, history of keloid formation, active skin infections, prior gold threads in the treatment area, pregnancy and breastfeeding, metal implants in the treatment area, embedded electronic devices that cannot be turned off, isotretinoin use in the past 6 months, and allergy to any of the components of treatment.
Caution should be taken with tattoos in the treatment area or grounding pad (including cosmetic tattoos as tattoo ink may often contain metals that may absorb some of the heat, increasing the risk for injury or extrusion of the ink), a history of cold sores or herpes simplex virus in the treatment area (if so, a prophylactic antiviral would be indicated prior to treatment), use of topical retinoids in the past 7 days, having received neurotoxin or fillers in the prior 2 weeks, autoimmune disease, bleeding disorders, neuropathy, and history of poor healing.
Depending on the device and area being treated, most RF microneedling treatments require two to five treatments, typically 4-6 weeks apart. If improvement is seen, it may be noticeable after one to two treatments, and as with laser resurfacing, continued improvement may be noticeable over the following 6-12 months post treatment.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. Dr. Wesley has no relevant disclosures.
Technology in the field of aesthetic dermatology continues to advance over time. Microneedling, largely used to improve textural changes of the skin associated with photoaging and acne scarring, has evolved over time from the use of dermarollers and microneedling skin pens to energy-based devices that deliver radiofrequency (RF) energy though microneedles that are used today.
.
Unlike prior radiofrequency energy-based devices that deliver radiofrequency energy on the skin surface to allow bulk thermal energy (or heat) to stimulate collagen remodeling and tissue tightening, RF microneedling devices deliver the same RF or thermal energy via needles. RF, measured in Hertz (Hz) is part of the electromagnetic spectrum, with most devices delivering thermal energy at around 1-2 MHz, which is less than most typical RF only devices (at around 4-6 MHz), but with potentially more precise depth and delivery. For comparison, the RF of household electrical currents are around 60 Hz; traditional electrosurgical units, 50Hz -300 kHz; AM radio, 500 KHz; and microwaves, 2500 MHz.
When delivered to the skin, RF energy produces a change in the electrical charge of the skin, resulting in movement of electrons. The impedance (or resistance) of the tissue to the electron movement is what generates heat. Different factors, including tissue thickness, pressure applied to the tissue, hydration, bipolar versus monopolar delivery, and the number of needles are several factors than can affect the impedance.
Bipolar RF means that the current passes between two electrodes, whereas monopolar RF means that the electrical current is between an active treatment electrode and a passive grounding electrode (or grounding pad typically placed on the patient’s back). With bipolar RF, the current is limited to the area between the two electrodes. The depth of penetration is half of the distance between the electrodes, thus resulting in shallow (but potentially more aggressive) tissue heating. With monopolar RF, deeper tissue penetration occurs that is also often less uncomfortable to the patient.
The desired result of the energy delivery is collagen remodeling and strengthening of elastin. RF microneedling and microneedling in general may also have potential for use in enhancing topical product delivery.
Depending on the device, settings can be tailored to affect the energy delivery, including the type of needle (insulated vs. uninsulated vs. semi-insulated), Hz, number of needles, depth of needles, and time of exposure. In general, insulated needle tips provide less heat accumulation and potential injury to the skin surface, whereas uninsulated needles allow for more heat accumulation. Insulated needles, longer time of exposure, and lower energies (Hz) are safer options for darker skin types and those who hyperpigment easily.
Immediately after treatment, expected clinical endpoints can include erythema, edema, and possibly pinpoint bleeding that may last approximately several days to 2 weeks depending on the intensity of treatment. Potential side effects include infection, pigmentary alteration, folliculitis, prolonged grid marks, and scarring. Contraindications to treatment include having a pacemaker, history of keloid formation, active skin infections, prior gold threads in the treatment area, pregnancy and breastfeeding, metal implants in the treatment area, embedded electronic devices that cannot be turned off, isotretinoin use in the past 6 months, and allergy to any of the components of treatment.
Caution should be taken with tattoos in the treatment area or grounding pad (including cosmetic tattoos as tattoo ink may often contain metals that may absorb some of the heat, increasing the risk for injury or extrusion of the ink), a history of cold sores or herpes simplex virus in the treatment area (if so, a prophylactic antiviral would be indicated prior to treatment), use of topical retinoids in the past 7 days, having received neurotoxin or fillers in the prior 2 weeks, autoimmune disease, bleeding disorders, neuropathy, and history of poor healing.
Depending on the device and area being treated, most RF microneedling treatments require two to five treatments, typically 4-6 weeks apart. If improvement is seen, it may be noticeable after one to two treatments, and as with laser resurfacing, continued improvement may be noticeable over the following 6-12 months post treatment.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. Dr. Wesley has no relevant disclosures.
Technology in the field of aesthetic dermatology continues to advance over time. Microneedling, largely used to improve textural changes of the skin associated with photoaging and acne scarring, has evolved over time from the use of dermarollers and microneedling skin pens to energy-based devices that deliver radiofrequency (RF) energy though microneedles that are used today.
.
Unlike prior radiofrequency energy-based devices that deliver radiofrequency energy on the skin surface to allow bulk thermal energy (or heat) to stimulate collagen remodeling and tissue tightening, RF microneedling devices deliver the same RF or thermal energy via needles. RF, measured in Hertz (Hz) is part of the electromagnetic spectrum, with most devices delivering thermal energy at around 1-2 MHz, which is less than most typical RF only devices (at around 4-6 MHz), but with potentially more precise depth and delivery. For comparison, the RF of household electrical currents are around 60 Hz; traditional electrosurgical units, 50Hz -300 kHz; AM radio, 500 KHz; and microwaves, 2500 MHz.
When delivered to the skin, RF energy produces a change in the electrical charge of the skin, resulting in movement of electrons. The impedance (or resistance) of the tissue to the electron movement is what generates heat. Different factors, including tissue thickness, pressure applied to the tissue, hydration, bipolar versus monopolar delivery, and the number of needles are several factors than can affect the impedance.
Bipolar RF means that the current passes between two electrodes, whereas monopolar RF means that the electrical current is between an active treatment electrode and a passive grounding electrode (or grounding pad typically placed on the patient’s back). With bipolar RF, the current is limited to the area between the two electrodes. The depth of penetration is half of the distance between the electrodes, thus resulting in shallow (but potentially more aggressive) tissue heating. With monopolar RF, deeper tissue penetration occurs that is also often less uncomfortable to the patient.
The desired result of the energy delivery is collagen remodeling and strengthening of elastin. RF microneedling and microneedling in general may also have potential for use in enhancing topical product delivery.
Depending on the device, settings can be tailored to affect the energy delivery, including the type of needle (insulated vs. uninsulated vs. semi-insulated), Hz, number of needles, depth of needles, and time of exposure. In general, insulated needle tips provide less heat accumulation and potential injury to the skin surface, whereas uninsulated needles allow for more heat accumulation. Insulated needles, longer time of exposure, and lower energies (Hz) are safer options for darker skin types and those who hyperpigment easily.
Immediately after treatment, expected clinical endpoints can include erythema, edema, and possibly pinpoint bleeding that may last approximately several days to 2 weeks depending on the intensity of treatment. Potential side effects include infection, pigmentary alteration, folliculitis, prolonged grid marks, and scarring. Contraindications to treatment include having a pacemaker, history of keloid formation, active skin infections, prior gold threads in the treatment area, pregnancy and breastfeeding, metal implants in the treatment area, embedded electronic devices that cannot be turned off, isotretinoin use in the past 6 months, and allergy to any of the components of treatment.
Caution should be taken with tattoos in the treatment area or grounding pad (including cosmetic tattoos as tattoo ink may often contain metals that may absorb some of the heat, increasing the risk for injury or extrusion of the ink), a history of cold sores or herpes simplex virus in the treatment area (if so, a prophylactic antiviral would be indicated prior to treatment), use of topical retinoids in the past 7 days, having received neurotoxin or fillers in the prior 2 weeks, autoimmune disease, bleeding disorders, neuropathy, and history of poor healing.
Depending on the device and area being treated, most RF microneedling treatments require two to five treatments, typically 4-6 weeks apart. If improvement is seen, it may be noticeable after one to two treatments, and as with laser resurfacing, continued improvement may be noticeable over the following 6-12 months post treatment.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. Dr. Wesley has no relevant disclosures.
Meet Argireline, the neurotoxinlike cosmeceutical
Acetyl hexapeptide-8 (or -3), better known by its brand name, Argireline (Lubrizol; Wickliffe, Ohio), is a synthetic peptide gaining popularity in cosmeceutical products for its antiaging benefits. Argireline was developed by the company Lipotec in 2001. Media, beauty bloggers, and product claims have likened this product to a “Botox [or other neurotoxin] alternative,” or “Botox mimicker.”
Mechanism of action
Understanding how Argireline works requires a brief refresher on the mechanism of action of botulinum neurotoxin (BoNT). BoNT relaxes facial muscles and smooths expression lines by inhibiting acetylcholine release at the neuromuscular junction.1 More specifically, the various serotypes of BoNT are single-chain polypeptides that target members of the SNARE complex: SNAP-25, syntaxin, and Vamp. The proteins within the SNARE complex are involved in the docking and fusion of presynaptic vesicles to the presynaptic membrane, necessary steps for acetylcholine release into the neuromuscular junction and muscle contraction. By blocking the action of the SNARE complex proteins, BoNT inhibits release of acetylcholine in the neuromuscular junction and prevents muscle contraction.
Argireline is a synthetic peptide with the sequence Ac-EEMQRR-NH2.2 It is patterned after the N-terminal domain of SNAP-25, one of the members of the SNARE complex targeted by BoNT, and functions to interfere with the assembly of the SNARE complex. In this manner, Argireline would theoretically inhibit fusion of presynaptic vesicles and release of acetylcholine into the neuromuscular junction, thus impeding muscle movement. For this reason, it has been likened to topical Botox. Unlike Botox and other neurotoxins, Argireline was developed for topical application rather than injection.
Preclinical studies
In vitro work done 20 years ago demonstrated that Argireline can prevent assembly of the SNARE complex and inhibit neurotransmitter release with a potency similar to that of BoNT A (Botox).2
In 2013, Wang et al. evaluated the histologic effects of Argireline in aged mouse skin induced by D-galactose. For 6 weeks, Argireline was applied twice daily, and histological changes were assessed using hematoxylin and eosin (H&E) and picrosirius–polarization (PSP) stains. The researchers found elevated levels of type I collagen (P < .01) and reduced type III collagen (P < .05) with the Argireline treatment. These results demonstrated that Argireline could histologically enhance collagen in a manner consistent with skin rejuvenation.3
Clinical studies
In 2002, Blanes et al. assessed the antiwrinkle activity of Argireline by measuring skin topography from silicone implants in the lateral periorbital region of an oil/water (O/W) emulsion containing 10% of the acetyl-hexapeptide in 10 healthy women volunteers. The hexapeptide emulsion was applied twice daily in one lateral periorbital area, and the emulsion vehicle alone was applied twice daily on the contralateral side. Over 30 days of treatment, wrinkle depth was found to have decreased by 30%. The investigators also found that Argireline significantly hindered neurotransmitter release in vitro as robustly as BoNT A, though with notably lower efficacy. No toxicity or irritation was associated with this treatment.2 However, it should be noted that this small study conducted 2 decades ago evaluated only silicone implants with confocal microscopy to evaluate wrinkle depth. There was no subjective clinical assessment of dynamic facial wrinkles. As such, their study is an insufficient basis for drawing conclusions that Argireline is a BoNT mimic. Botox and other types of BoNT affect dynamic facial wrinkles mostly (i.e., wrinkles created by moving muscles of facial expression). This study primarily considers static wrinkles on periorbital skin. While static wrinkles may result from longstanding dynamic wrinkles, BoNT mainly targets dynamic wrinkles, again not comparing apples to apples.
At the same time that Wang et al. conducted their experiment on the skin of aged mice as noted above, they performed a multicenter clinical trial in 60 human subjects who received a randomized treatment of Argireline or placebo in a ratio of 3:1 to assess its safety and efficacy. For 4 weeks, the test product or placebo was applied to periorbital wrinkles twice daily. The researchers found the total antiwrinkle efficacy in the Argireline group to be 48.9% based on the subjective evaluation, compared with 0% in the placebo group. The objective evaluation indicated that all parameters of roughness were diminished in the Argireline group (P < .01), with no reduction observed in the placebo group (P < .05).4 There was a little more to appreciate from this study compared with the one reported by Blanes et al., insofar as subjective evaluations and objective evaluations with silica replicas were done. However, this study was not blinded, so the 48.9% wrinkle reduction in the Argireline group vs. 0% in the control group seems suspicious. Additionally, there was a greater focus on static rather than dynamic wrinkles.
In 2017, Raikou et al. conducted a prospective, randomized controlled study to assess the effects of acetyl hexapeptide-3 (Argireline) and tripeptide-10 citrulline in 24 healthy female volunteers (aged 30-60 years) and determine if there was any synergistic action between the peptides. Subjects were randomized to receive a combination of the peptides, tripeptide-10 citrulline only, acetyl hexapeptide-3 only, or neither peptide for 60 days. The researchers found a significant reduction in transepidermal water loss (TEWL) in the Argireline group, compared with the placebo group.5 The result of this study makes me question if the decrease in depth of the wrinkles measured in the former studies is really just a measure of increased skin hydration from the Argireline, rather than a neurotoxic effect of Argireline.
Formulation and penetration: Can Argireline get through your skin?
One of the fundamental questions regarding Argireline is whether it can penetrate through the stratum corneum and find its target – the facial muscles – where it is intended to function. Argireline is a charged, hydrophilic, and large–molecular weight peptide, and each of these factors impairs penetration through the stratum corneum. Therefore, studies assessing penetration are particularly important.
In 2015, Kraeling et al. conducted an in vitro evaluation of the skin penetration of acetyl hexapeptide-8 in hairless guinea pig and human cadaver skin. An oil-in-water (O/W) emulsion containing 10% acetyl hexapeptide-8 was applied (2 mg/cm2) and penetration was quantified in skin layers via hydrophilic interaction liquid chromatography with tandem mass spectrometry. Most of the acetyl hexapeptide-8 was found to have been washed from human cadaver, as well as guinea pig, skin. Less than 1% of the peptide penetrated the guinea pig or human skin. Of this small amount that penetrated the skin, most stayed in the stratum corneum of guinea pigs (0.54%) and human cadavers (0.22%). The levels of acetyl hexapeptide-8 declined further with each layer of tape stripping removal. Epidermal levels of the peptide in tested skin were similar at 0.01%, and none of the peptide was found in the dermis.6 These results indicate negligible penetration by this highly touted peptide ingredient.
Some studies have shown that altering the formulation of acetyl hexapeptide-8 can enhance penetration. Hoppel et al. demonstrated that formulations of the peptide, especially in a water-oil-water (W/O/W emulsion [as compared with O/W and W/O emulsions] can increase penetration into the stratum corneum in porcine skin.7 Notably, this is still very superficial relative to the dermis and muscles. Irrespective of formulation, studies have shown that Argireline barely penetrates the stratum corneum, let alone the dermis. Therefore, I would give pause to attributing any clinical impact or benefit of Argireline to its neurotoxinlike effects measured in vitro.
Conclusion
Despite the growing popularity of this ingredient in cosmeceuticals and the praise it gets in media for acting as a topical neurotoxin, there are no rigorous clinical trials or data demonstrating its efficacy in suppressing dynamic facial wrinkles like BoNT does. Most importantly, without penetration into the stratum corneum and deeper layers of the skin, it seems unlikely that Argireline’s clinical benefit derives from a neurotoxiclike mechanism of action. It seems more likely that the Argireline-containing product enhances hydration or imparts some other quality to the skin surface. While there is certainly great appeal for a neurotoxinlike product without injections, I do not believe this ingredient will replace injections of BoNT in the foreseeable future, or at least until scientists can figure out how to enable these products to penetrate into the deeper layers of the skin.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Dr. Goldman has no relevant disclosures. Write to her at [email protected] or message her on Instagram @DrChloeGoldman.
References
1. Reddy BY et al. Exp Dermatol. 2012 Aug;21(8):569-75.
2. Blanes-Mira C et al. Int J Cosmet Sci. 2002 Oct;24(5):303-10.
3. Wang Y et al. J Cosmet Laser Ther. 2013 Aug;15(4):237-41.
4. Wang Y et al. J Cosmet Laser Ther. 2013;14(2):147-53.
5. Raikou V et al. J Cosmet Dermatol. 2017 Jun;16(2):271-8.
6. Kraeling ME et al. Cutan Ocul Toxicol. 2015 Mar;34(1):46-52.
7. Hoppel M et al. Eur J Pharm Sci. 2015 Feb 20;68:27-35.
Acetyl hexapeptide-8 (or -3), better known by its brand name, Argireline (Lubrizol; Wickliffe, Ohio), is a synthetic peptide gaining popularity in cosmeceutical products for its antiaging benefits. Argireline was developed by the company Lipotec in 2001. Media, beauty bloggers, and product claims have likened this product to a “Botox [or other neurotoxin] alternative,” or “Botox mimicker.”
Mechanism of action
Understanding how Argireline works requires a brief refresher on the mechanism of action of botulinum neurotoxin (BoNT). BoNT relaxes facial muscles and smooths expression lines by inhibiting acetylcholine release at the neuromuscular junction.1 More specifically, the various serotypes of BoNT are single-chain polypeptides that target members of the SNARE complex: SNAP-25, syntaxin, and Vamp. The proteins within the SNARE complex are involved in the docking and fusion of presynaptic vesicles to the presynaptic membrane, necessary steps for acetylcholine release into the neuromuscular junction and muscle contraction. By blocking the action of the SNARE complex proteins, BoNT inhibits release of acetylcholine in the neuromuscular junction and prevents muscle contraction.
Argireline is a synthetic peptide with the sequence Ac-EEMQRR-NH2.2 It is patterned after the N-terminal domain of SNAP-25, one of the members of the SNARE complex targeted by BoNT, and functions to interfere with the assembly of the SNARE complex. In this manner, Argireline would theoretically inhibit fusion of presynaptic vesicles and release of acetylcholine into the neuromuscular junction, thus impeding muscle movement. For this reason, it has been likened to topical Botox. Unlike Botox and other neurotoxins, Argireline was developed for topical application rather than injection.
Preclinical studies
In vitro work done 20 years ago demonstrated that Argireline can prevent assembly of the SNARE complex and inhibit neurotransmitter release with a potency similar to that of BoNT A (Botox).2
In 2013, Wang et al. evaluated the histologic effects of Argireline in aged mouse skin induced by D-galactose. For 6 weeks, Argireline was applied twice daily, and histological changes were assessed using hematoxylin and eosin (H&E) and picrosirius–polarization (PSP) stains. The researchers found elevated levels of type I collagen (P < .01) and reduced type III collagen (P < .05) with the Argireline treatment. These results demonstrated that Argireline could histologically enhance collagen in a manner consistent with skin rejuvenation.3
Clinical studies
In 2002, Blanes et al. assessed the antiwrinkle activity of Argireline by measuring skin topography from silicone implants in the lateral periorbital region of an oil/water (O/W) emulsion containing 10% of the acetyl-hexapeptide in 10 healthy women volunteers. The hexapeptide emulsion was applied twice daily in one lateral periorbital area, and the emulsion vehicle alone was applied twice daily on the contralateral side. Over 30 days of treatment, wrinkle depth was found to have decreased by 30%. The investigators also found that Argireline significantly hindered neurotransmitter release in vitro as robustly as BoNT A, though with notably lower efficacy. No toxicity or irritation was associated with this treatment.2 However, it should be noted that this small study conducted 2 decades ago evaluated only silicone implants with confocal microscopy to evaluate wrinkle depth. There was no subjective clinical assessment of dynamic facial wrinkles. As such, their study is an insufficient basis for drawing conclusions that Argireline is a BoNT mimic. Botox and other types of BoNT affect dynamic facial wrinkles mostly (i.e., wrinkles created by moving muscles of facial expression). This study primarily considers static wrinkles on periorbital skin. While static wrinkles may result from longstanding dynamic wrinkles, BoNT mainly targets dynamic wrinkles, again not comparing apples to apples.
At the same time that Wang et al. conducted their experiment on the skin of aged mice as noted above, they performed a multicenter clinical trial in 60 human subjects who received a randomized treatment of Argireline or placebo in a ratio of 3:1 to assess its safety and efficacy. For 4 weeks, the test product or placebo was applied to periorbital wrinkles twice daily. The researchers found the total antiwrinkle efficacy in the Argireline group to be 48.9% based on the subjective evaluation, compared with 0% in the placebo group. The objective evaluation indicated that all parameters of roughness were diminished in the Argireline group (P < .01), with no reduction observed in the placebo group (P < .05).4 There was a little more to appreciate from this study compared with the one reported by Blanes et al., insofar as subjective evaluations and objective evaluations with silica replicas were done. However, this study was not blinded, so the 48.9% wrinkle reduction in the Argireline group vs. 0% in the control group seems suspicious. Additionally, there was a greater focus on static rather than dynamic wrinkles.
In 2017, Raikou et al. conducted a prospective, randomized controlled study to assess the effects of acetyl hexapeptide-3 (Argireline) and tripeptide-10 citrulline in 24 healthy female volunteers (aged 30-60 years) and determine if there was any synergistic action between the peptides. Subjects were randomized to receive a combination of the peptides, tripeptide-10 citrulline only, acetyl hexapeptide-3 only, or neither peptide for 60 days. The researchers found a significant reduction in transepidermal water loss (TEWL) in the Argireline group, compared with the placebo group.5 The result of this study makes me question if the decrease in depth of the wrinkles measured in the former studies is really just a measure of increased skin hydration from the Argireline, rather than a neurotoxic effect of Argireline.
Formulation and penetration: Can Argireline get through your skin?
One of the fundamental questions regarding Argireline is whether it can penetrate through the stratum corneum and find its target – the facial muscles – where it is intended to function. Argireline is a charged, hydrophilic, and large–molecular weight peptide, and each of these factors impairs penetration through the stratum corneum. Therefore, studies assessing penetration are particularly important.
In 2015, Kraeling et al. conducted an in vitro evaluation of the skin penetration of acetyl hexapeptide-8 in hairless guinea pig and human cadaver skin. An oil-in-water (O/W) emulsion containing 10% acetyl hexapeptide-8 was applied (2 mg/cm2) and penetration was quantified in skin layers via hydrophilic interaction liquid chromatography with tandem mass spectrometry. Most of the acetyl hexapeptide-8 was found to have been washed from human cadaver, as well as guinea pig, skin. Less than 1% of the peptide penetrated the guinea pig or human skin. Of this small amount that penetrated the skin, most stayed in the stratum corneum of guinea pigs (0.54%) and human cadavers (0.22%). The levels of acetyl hexapeptide-8 declined further with each layer of tape stripping removal. Epidermal levels of the peptide in tested skin were similar at 0.01%, and none of the peptide was found in the dermis.6 These results indicate negligible penetration by this highly touted peptide ingredient.
Some studies have shown that altering the formulation of acetyl hexapeptide-8 can enhance penetration. Hoppel et al. demonstrated that formulations of the peptide, especially in a water-oil-water (W/O/W emulsion [as compared with O/W and W/O emulsions] can increase penetration into the stratum corneum in porcine skin.7 Notably, this is still very superficial relative to the dermis and muscles. Irrespective of formulation, studies have shown that Argireline barely penetrates the stratum corneum, let alone the dermis. Therefore, I would give pause to attributing any clinical impact or benefit of Argireline to its neurotoxinlike effects measured in vitro.
Conclusion
Despite the growing popularity of this ingredient in cosmeceuticals and the praise it gets in media for acting as a topical neurotoxin, there are no rigorous clinical trials or data demonstrating its efficacy in suppressing dynamic facial wrinkles like BoNT does. Most importantly, without penetration into the stratum corneum and deeper layers of the skin, it seems unlikely that Argireline’s clinical benefit derives from a neurotoxiclike mechanism of action. It seems more likely that the Argireline-containing product enhances hydration or imparts some other quality to the skin surface. While there is certainly great appeal for a neurotoxinlike product without injections, I do not believe this ingredient will replace injections of BoNT in the foreseeable future, or at least until scientists can figure out how to enable these products to penetrate into the deeper layers of the skin.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Dr. Goldman has no relevant disclosures. Write to her at [email protected] or message her on Instagram @DrChloeGoldman.
References
1. Reddy BY et al. Exp Dermatol. 2012 Aug;21(8):569-75.
2. Blanes-Mira C et al. Int J Cosmet Sci. 2002 Oct;24(5):303-10.
3. Wang Y et al. J Cosmet Laser Ther. 2013 Aug;15(4):237-41.
4. Wang Y et al. J Cosmet Laser Ther. 2013;14(2):147-53.
5. Raikou V et al. J Cosmet Dermatol. 2017 Jun;16(2):271-8.
6. Kraeling ME et al. Cutan Ocul Toxicol. 2015 Mar;34(1):46-52.
7. Hoppel M et al. Eur J Pharm Sci. 2015 Feb 20;68:27-35.
Acetyl hexapeptide-8 (or -3), better known by its brand name, Argireline (Lubrizol; Wickliffe, Ohio), is a synthetic peptide gaining popularity in cosmeceutical products for its antiaging benefits. Argireline was developed by the company Lipotec in 2001. Media, beauty bloggers, and product claims have likened this product to a “Botox [or other neurotoxin] alternative,” or “Botox mimicker.”
Mechanism of action
Understanding how Argireline works requires a brief refresher on the mechanism of action of botulinum neurotoxin (BoNT). BoNT relaxes facial muscles and smooths expression lines by inhibiting acetylcholine release at the neuromuscular junction.1 More specifically, the various serotypes of BoNT are single-chain polypeptides that target members of the SNARE complex: SNAP-25, syntaxin, and Vamp. The proteins within the SNARE complex are involved in the docking and fusion of presynaptic vesicles to the presynaptic membrane, necessary steps for acetylcholine release into the neuromuscular junction and muscle contraction. By blocking the action of the SNARE complex proteins, BoNT inhibits release of acetylcholine in the neuromuscular junction and prevents muscle contraction.
Argireline is a synthetic peptide with the sequence Ac-EEMQRR-NH2.2 It is patterned after the N-terminal domain of SNAP-25, one of the members of the SNARE complex targeted by BoNT, and functions to interfere with the assembly of the SNARE complex. In this manner, Argireline would theoretically inhibit fusion of presynaptic vesicles and release of acetylcholine into the neuromuscular junction, thus impeding muscle movement. For this reason, it has been likened to topical Botox. Unlike Botox and other neurotoxins, Argireline was developed for topical application rather than injection.
Preclinical studies
In vitro work done 20 years ago demonstrated that Argireline can prevent assembly of the SNARE complex and inhibit neurotransmitter release with a potency similar to that of BoNT A (Botox).2
In 2013, Wang et al. evaluated the histologic effects of Argireline in aged mouse skin induced by D-galactose. For 6 weeks, Argireline was applied twice daily, and histological changes were assessed using hematoxylin and eosin (H&E) and picrosirius–polarization (PSP) stains. The researchers found elevated levels of type I collagen (P < .01) and reduced type III collagen (P < .05) with the Argireline treatment. These results demonstrated that Argireline could histologically enhance collagen in a manner consistent with skin rejuvenation.3
Clinical studies
In 2002, Blanes et al. assessed the antiwrinkle activity of Argireline by measuring skin topography from silicone implants in the lateral periorbital region of an oil/water (O/W) emulsion containing 10% of the acetyl-hexapeptide in 10 healthy women volunteers. The hexapeptide emulsion was applied twice daily in one lateral periorbital area, and the emulsion vehicle alone was applied twice daily on the contralateral side. Over 30 days of treatment, wrinkle depth was found to have decreased by 30%. The investigators also found that Argireline significantly hindered neurotransmitter release in vitro as robustly as BoNT A, though with notably lower efficacy. No toxicity or irritation was associated with this treatment.2 However, it should be noted that this small study conducted 2 decades ago evaluated only silicone implants with confocal microscopy to evaluate wrinkle depth. There was no subjective clinical assessment of dynamic facial wrinkles. As such, their study is an insufficient basis for drawing conclusions that Argireline is a BoNT mimic. Botox and other types of BoNT affect dynamic facial wrinkles mostly (i.e., wrinkles created by moving muscles of facial expression). This study primarily considers static wrinkles on periorbital skin. While static wrinkles may result from longstanding dynamic wrinkles, BoNT mainly targets dynamic wrinkles, again not comparing apples to apples.
At the same time that Wang et al. conducted their experiment on the skin of aged mice as noted above, they performed a multicenter clinical trial in 60 human subjects who received a randomized treatment of Argireline or placebo in a ratio of 3:1 to assess its safety and efficacy. For 4 weeks, the test product or placebo was applied to periorbital wrinkles twice daily. The researchers found the total antiwrinkle efficacy in the Argireline group to be 48.9% based on the subjective evaluation, compared with 0% in the placebo group. The objective evaluation indicated that all parameters of roughness were diminished in the Argireline group (P < .01), with no reduction observed in the placebo group (P < .05).4 There was a little more to appreciate from this study compared with the one reported by Blanes et al., insofar as subjective evaluations and objective evaluations with silica replicas were done. However, this study was not blinded, so the 48.9% wrinkle reduction in the Argireline group vs. 0% in the control group seems suspicious. Additionally, there was a greater focus on static rather than dynamic wrinkles.
In 2017, Raikou et al. conducted a prospective, randomized controlled study to assess the effects of acetyl hexapeptide-3 (Argireline) and tripeptide-10 citrulline in 24 healthy female volunteers (aged 30-60 years) and determine if there was any synergistic action between the peptides. Subjects were randomized to receive a combination of the peptides, tripeptide-10 citrulline only, acetyl hexapeptide-3 only, or neither peptide for 60 days. The researchers found a significant reduction in transepidermal water loss (TEWL) in the Argireline group, compared with the placebo group.5 The result of this study makes me question if the decrease in depth of the wrinkles measured in the former studies is really just a measure of increased skin hydration from the Argireline, rather than a neurotoxic effect of Argireline.
Formulation and penetration: Can Argireline get through your skin?
One of the fundamental questions regarding Argireline is whether it can penetrate through the stratum corneum and find its target – the facial muscles – where it is intended to function. Argireline is a charged, hydrophilic, and large–molecular weight peptide, and each of these factors impairs penetration through the stratum corneum. Therefore, studies assessing penetration are particularly important.
In 2015, Kraeling et al. conducted an in vitro evaluation of the skin penetration of acetyl hexapeptide-8 in hairless guinea pig and human cadaver skin. An oil-in-water (O/W) emulsion containing 10% acetyl hexapeptide-8 was applied (2 mg/cm2) and penetration was quantified in skin layers via hydrophilic interaction liquid chromatography with tandem mass spectrometry. Most of the acetyl hexapeptide-8 was found to have been washed from human cadaver, as well as guinea pig, skin. Less than 1% of the peptide penetrated the guinea pig or human skin. Of this small amount that penetrated the skin, most stayed in the stratum corneum of guinea pigs (0.54%) and human cadavers (0.22%). The levels of acetyl hexapeptide-8 declined further with each layer of tape stripping removal. Epidermal levels of the peptide in tested skin were similar at 0.01%, and none of the peptide was found in the dermis.6 These results indicate negligible penetration by this highly touted peptide ingredient.
Some studies have shown that altering the formulation of acetyl hexapeptide-8 can enhance penetration. Hoppel et al. demonstrated that formulations of the peptide, especially in a water-oil-water (W/O/W emulsion [as compared with O/W and W/O emulsions] can increase penetration into the stratum corneum in porcine skin.7 Notably, this is still very superficial relative to the dermis and muscles. Irrespective of formulation, studies have shown that Argireline barely penetrates the stratum corneum, let alone the dermis. Therefore, I would give pause to attributing any clinical impact or benefit of Argireline to its neurotoxinlike effects measured in vitro.
Conclusion
Despite the growing popularity of this ingredient in cosmeceuticals and the praise it gets in media for acting as a topical neurotoxin, there are no rigorous clinical trials or data demonstrating its efficacy in suppressing dynamic facial wrinkles like BoNT does. Most importantly, without penetration into the stratum corneum and deeper layers of the skin, it seems unlikely that Argireline’s clinical benefit derives from a neurotoxiclike mechanism of action. It seems more likely that the Argireline-containing product enhances hydration or imparts some other quality to the skin surface. While there is certainly great appeal for a neurotoxinlike product without injections, I do not believe this ingredient will replace injections of BoNT in the foreseeable future, or at least until scientists can figure out how to enable these products to penetrate into the deeper layers of the skin.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Dr. Goldman has no relevant disclosures. Write to her at [email protected] or message her on Instagram @DrChloeGoldman.
References
1. Reddy BY et al. Exp Dermatol. 2012 Aug;21(8):569-75.
2. Blanes-Mira C et al. Int J Cosmet Sci. 2002 Oct;24(5):303-10.
3. Wang Y et al. J Cosmet Laser Ther. 2013 Aug;15(4):237-41.
4. Wang Y et al. J Cosmet Laser Ther. 2013;14(2):147-53.
5. Raikou V et al. J Cosmet Dermatol. 2017 Jun;16(2):271-8.
6. Kraeling ME et al. Cutan Ocul Toxicol. 2015 Mar;34(1):46-52.
7. Hoppel M et al. Eur J Pharm Sci. 2015 Feb 20;68:27-35.
Nodules on the Anterior Neck Following Poly-L-lactic Acid Injection
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).
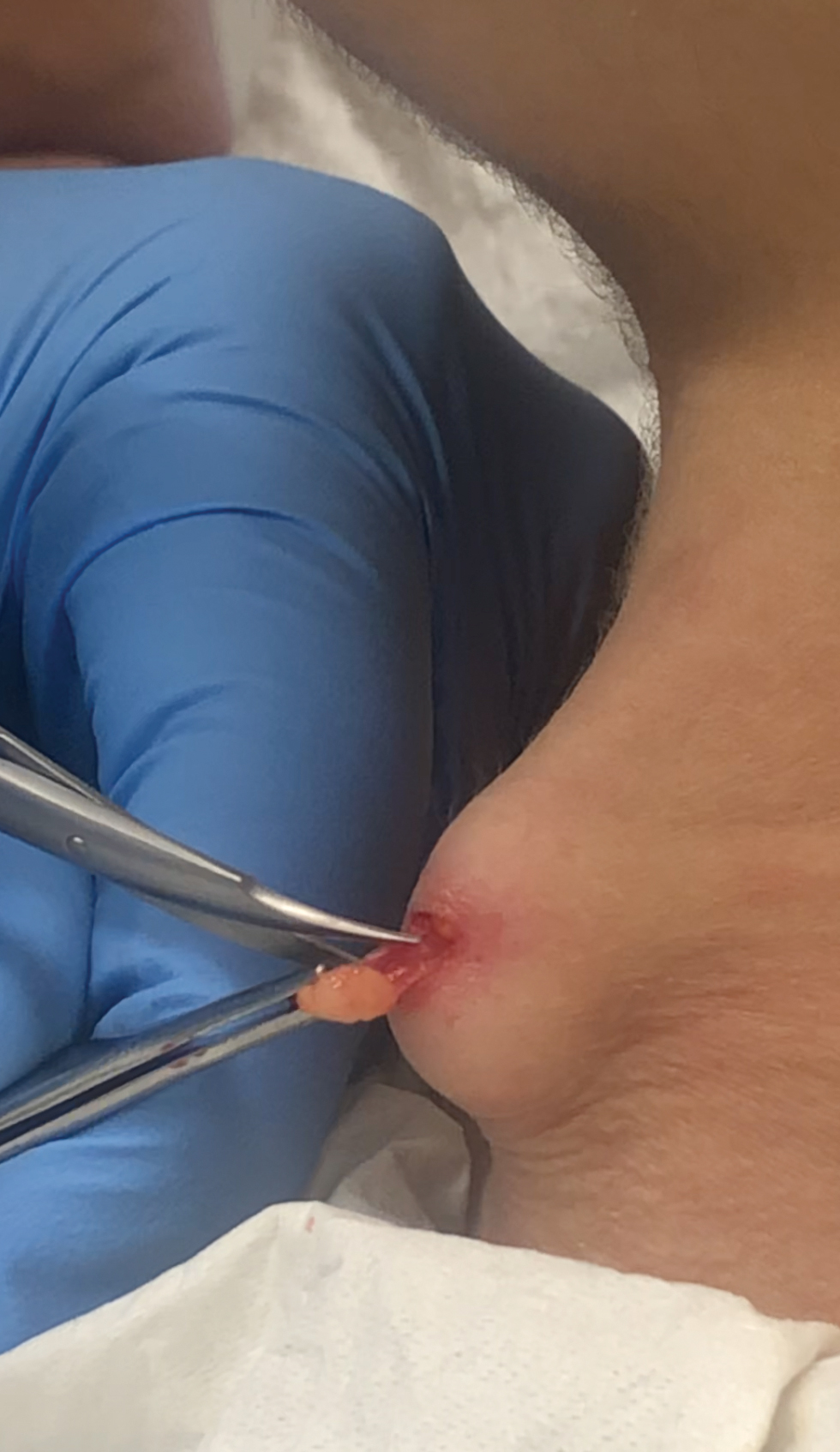
Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Practice Points
- Injecting poly-L-lactic acid (PLLA) into the anterior neck is an off-label procedure and may cause a higher incidence of nodule formation.
- Most nodules from PLLA can be treated with injections of 5-fluorouracil, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals.
- Treatment-resistant nodules may require surgical excision.
Understanding filler reversal with hyaluronidase
Hyaluronic acid is the most common filler used in the United States for cosmetic procedures. . However, there has been little research and there are no formal clinical guidelines on its use. Hyaluronidase is approved by the Food and Drug Administration for several indications, but its use in cosmetic procedures is off-label.
Hyaluronic acid filler complications can be local and transient or delayed and/or dangerous. Local reactions generally improve over time or respond to symptomatic care. But granulomatous reactions, misplaced injection, adverse aesthetic outcomes, and vascular occlusion are some of the detrimental outcomes that require immediate treatment, often using hyaluronidase, a naturally occurring enzyme that degrades hyaluronic acid.
Hyaluronic acid products vary in concentration, cross-linking, type of cross-linker used, and particle size, and therefore display different degradation patterns with hyaluronidase. The three hyaluronidase products available also vary in concentration, source, and enzyme activity. Hyaluronidase has a half-life of 2 minutes but has a duration of action of 24-48 hours depending on the product used.
In an interesting study by Casabona G et al., the dose and activity of five hyaluronidase products available worldwide were used to degrade five different fillers (Juvederm Volbella, Voluma, and Ultraplus; Belotero, and Belotero Balance) with various concentrations and cross-linking in human skin. The results showed that the Vycross products (Juvederm Voluma) are the least sensitive to hyaluronidase and require the greatest concentration of hyaluronidase and a longer time for dissolution requiring up to three times more hyaluronidase to degrade the same volume of other hyaluronic acid products.
In addition, the ovine hyaluronidase product marketed in the United States as Vitrase had the greatest activity against the range of hyaluronic acids used in the trial. Higher concentrations of hyaluronidase also could produce type-I hypersensitivity reactions and angioedema in susceptible patients as evidenced by eosinophilic tissue reactions at concentrations greater than 300 IU.
Hyaluronidase is stored at cool temperatures (35-46° F). It can be reconstituted with saline, water, or bacteriostatic saline for reducing injection site pain; however, it should not be mixed with local anesthetic. The volume of diluent used depends on the surface area treated and ranges from 1 mL to 10 mL. Smaller volumes are used for more concentrated local injection and larger volumes for more precise dosing.
For impending necrosis, hyaluronidase should be used within minutes to hours of blanching of the skin and the area should be flooded every 30 minutes until the tissue has reperfused. Depending on the type of filler used, the volume of injection varies and the area should continually be injected and tissue response observed. A high-dosed large-volume protocol allows the tissue perfusion to gradually infiltrate the vessel walls. Recommendations are 2 mL of bacteriostatic saline diluted with a vial of hyaluronidase. Retrobulbar injection of hyaluronidase within minutes of retinal artery occlusion in doses of 150-200 units in 2-4 mL of diluent into the inferolateral orbit by an experienced ophthalmologist or oculoplastic surgeon is recommended.
Although there is no consensus, there are various clinical studies using hyaluronidase dilutions varying between 5 and 30 units to break down 0.1mg/mL of hyaluronic acid for the reversal of facial hyaluronic acid fillers. In my clinical experience, the recommendation is that, apart from necrosis, the concentration used is titrated to clinical efficacy, which can also be done over multiple appointments every 48 hours until the desired outcome is achieved.
Complications from hyaluronidase injection include local tissue erythema, edema, pain, allergic reactions, and anaphylaxis. An intradermal patch test of 10-20 units of hyaluronidase in the forearm can be done in patients with a history of allergy to hyaluronidase, which, in people with sensitivity, results in a wheal within 30 minutes of injection. If a patient has a positive patch test, hyaluronidase cannot be used. In addition, a history of allergic reactions to bees may pose a heightened reaction to hyaluronidase and is a contraindication to use.
It is recommended that any practitioner using hyaluronic acid fillers keep 2-3 vials of hyaluronidase available at all times in the event of a vascular emergency. Stability, storage, and expiration dates should also be monitored closely.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub has no relevant disclosures.
References
Casabona G et al. Dermatol Surg. 2018 Nov;44 Suppl 1:S42-S50.
DeLorenzi C. Aesthet Surg J. 2017 Jul 1;37(7):814-25.
Juhász MLW et al. Dermatol Surg. 2017 Jun;43(6):841-7.
King M. J Clin Aesthet Dermatol. 2016 Nov; 9(11):E6–8.
Kim M et al. J Clin Aesthet Dermatol. 2018 Jun;11(6):E61-8.
Hyaluronic acid is the most common filler used in the United States for cosmetic procedures. . However, there has been little research and there are no formal clinical guidelines on its use. Hyaluronidase is approved by the Food and Drug Administration for several indications, but its use in cosmetic procedures is off-label.
Hyaluronic acid filler complications can be local and transient or delayed and/or dangerous. Local reactions generally improve over time or respond to symptomatic care. But granulomatous reactions, misplaced injection, adverse aesthetic outcomes, and vascular occlusion are some of the detrimental outcomes that require immediate treatment, often using hyaluronidase, a naturally occurring enzyme that degrades hyaluronic acid.
Hyaluronic acid products vary in concentration, cross-linking, type of cross-linker used, and particle size, and therefore display different degradation patterns with hyaluronidase. The three hyaluronidase products available also vary in concentration, source, and enzyme activity. Hyaluronidase has a half-life of 2 minutes but has a duration of action of 24-48 hours depending on the product used.
In an interesting study by Casabona G et al., the dose and activity of five hyaluronidase products available worldwide were used to degrade five different fillers (Juvederm Volbella, Voluma, and Ultraplus; Belotero, and Belotero Balance) with various concentrations and cross-linking in human skin. The results showed that the Vycross products (Juvederm Voluma) are the least sensitive to hyaluronidase and require the greatest concentration of hyaluronidase and a longer time for dissolution requiring up to three times more hyaluronidase to degrade the same volume of other hyaluronic acid products.
In addition, the ovine hyaluronidase product marketed in the United States as Vitrase had the greatest activity against the range of hyaluronic acids used in the trial. Higher concentrations of hyaluronidase also could produce type-I hypersensitivity reactions and angioedema in susceptible patients as evidenced by eosinophilic tissue reactions at concentrations greater than 300 IU.
Hyaluronidase is stored at cool temperatures (35-46° F). It can be reconstituted with saline, water, or bacteriostatic saline for reducing injection site pain; however, it should not be mixed with local anesthetic. The volume of diluent used depends on the surface area treated and ranges from 1 mL to 10 mL. Smaller volumes are used for more concentrated local injection and larger volumes for more precise dosing.
For impending necrosis, hyaluronidase should be used within minutes to hours of blanching of the skin and the area should be flooded every 30 minutes until the tissue has reperfused. Depending on the type of filler used, the volume of injection varies and the area should continually be injected and tissue response observed. A high-dosed large-volume protocol allows the tissue perfusion to gradually infiltrate the vessel walls. Recommendations are 2 mL of bacteriostatic saline diluted with a vial of hyaluronidase. Retrobulbar injection of hyaluronidase within minutes of retinal artery occlusion in doses of 150-200 units in 2-4 mL of diluent into the inferolateral orbit by an experienced ophthalmologist or oculoplastic surgeon is recommended.
Although there is no consensus, there are various clinical studies using hyaluronidase dilutions varying between 5 and 30 units to break down 0.1mg/mL of hyaluronic acid for the reversal of facial hyaluronic acid fillers. In my clinical experience, the recommendation is that, apart from necrosis, the concentration used is titrated to clinical efficacy, which can also be done over multiple appointments every 48 hours until the desired outcome is achieved.
Complications from hyaluronidase injection include local tissue erythema, edema, pain, allergic reactions, and anaphylaxis. An intradermal patch test of 10-20 units of hyaluronidase in the forearm can be done in patients with a history of allergy to hyaluronidase, which, in people with sensitivity, results in a wheal within 30 minutes of injection. If a patient has a positive patch test, hyaluronidase cannot be used. In addition, a history of allergic reactions to bees may pose a heightened reaction to hyaluronidase and is a contraindication to use.
It is recommended that any practitioner using hyaluronic acid fillers keep 2-3 vials of hyaluronidase available at all times in the event of a vascular emergency. Stability, storage, and expiration dates should also be monitored closely.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub has no relevant disclosures.
References
Casabona G et al. Dermatol Surg. 2018 Nov;44 Suppl 1:S42-S50.
DeLorenzi C. Aesthet Surg J. 2017 Jul 1;37(7):814-25.
Juhász MLW et al. Dermatol Surg. 2017 Jun;43(6):841-7.
King M. J Clin Aesthet Dermatol. 2016 Nov; 9(11):E6–8.
Kim M et al. J Clin Aesthet Dermatol. 2018 Jun;11(6):E61-8.
Hyaluronic acid is the most common filler used in the United States for cosmetic procedures. . However, there has been little research and there are no formal clinical guidelines on its use. Hyaluronidase is approved by the Food and Drug Administration for several indications, but its use in cosmetic procedures is off-label.
Hyaluronic acid filler complications can be local and transient or delayed and/or dangerous. Local reactions generally improve over time or respond to symptomatic care. But granulomatous reactions, misplaced injection, adverse aesthetic outcomes, and vascular occlusion are some of the detrimental outcomes that require immediate treatment, often using hyaluronidase, a naturally occurring enzyme that degrades hyaluronic acid.
Hyaluronic acid products vary in concentration, cross-linking, type of cross-linker used, and particle size, and therefore display different degradation patterns with hyaluronidase. The three hyaluronidase products available also vary in concentration, source, and enzyme activity. Hyaluronidase has a half-life of 2 minutes but has a duration of action of 24-48 hours depending on the product used.
In an interesting study by Casabona G et al., the dose and activity of five hyaluronidase products available worldwide were used to degrade five different fillers (Juvederm Volbella, Voluma, and Ultraplus; Belotero, and Belotero Balance) with various concentrations and cross-linking in human skin. The results showed that the Vycross products (Juvederm Voluma) are the least sensitive to hyaluronidase and require the greatest concentration of hyaluronidase and a longer time for dissolution requiring up to three times more hyaluronidase to degrade the same volume of other hyaluronic acid products.
In addition, the ovine hyaluronidase product marketed in the United States as Vitrase had the greatest activity against the range of hyaluronic acids used in the trial. Higher concentrations of hyaluronidase also could produce type-I hypersensitivity reactions and angioedema in susceptible patients as evidenced by eosinophilic tissue reactions at concentrations greater than 300 IU.
Hyaluronidase is stored at cool temperatures (35-46° F). It can be reconstituted with saline, water, or bacteriostatic saline for reducing injection site pain; however, it should not be mixed with local anesthetic. The volume of diluent used depends on the surface area treated and ranges from 1 mL to 10 mL. Smaller volumes are used for more concentrated local injection and larger volumes for more precise dosing.
For impending necrosis, hyaluronidase should be used within minutes to hours of blanching of the skin and the area should be flooded every 30 minutes until the tissue has reperfused. Depending on the type of filler used, the volume of injection varies and the area should continually be injected and tissue response observed. A high-dosed large-volume protocol allows the tissue perfusion to gradually infiltrate the vessel walls. Recommendations are 2 mL of bacteriostatic saline diluted with a vial of hyaluronidase. Retrobulbar injection of hyaluronidase within minutes of retinal artery occlusion in doses of 150-200 units in 2-4 mL of diluent into the inferolateral orbit by an experienced ophthalmologist or oculoplastic surgeon is recommended.
Although there is no consensus, there are various clinical studies using hyaluronidase dilutions varying between 5 and 30 units to break down 0.1mg/mL of hyaluronic acid for the reversal of facial hyaluronic acid fillers. In my clinical experience, the recommendation is that, apart from necrosis, the concentration used is titrated to clinical efficacy, which can also be done over multiple appointments every 48 hours until the desired outcome is achieved.
Complications from hyaluronidase injection include local tissue erythema, edema, pain, allergic reactions, and anaphylaxis. An intradermal patch test of 10-20 units of hyaluronidase in the forearm can be done in patients with a history of allergy to hyaluronidase, which, in people with sensitivity, results in a wheal within 30 minutes of injection. If a patient has a positive patch test, hyaluronidase cannot be used. In addition, a history of allergic reactions to bees may pose a heightened reaction to hyaluronidase and is a contraindication to use.
It is recommended that any practitioner using hyaluronic acid fillers keep 2-3 vials of hyaluronidase available at all times in the event of a vascular emergency. Stability, storage, and expiration dates should also be monitored closely.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to them at [email protected]. Dr. Talakoub has no relevant disclosures.
References
Casabona G et al. Dermatol Surg. 2018 Nov;44 Suppl 1:S42-S50.
DeLorenzi C. Aesthet Surg J. 2017 Jul 1;37(7):814-25.
Juhász MLW et al. Dermatol Surg. 2017 Jun;43(6):841-7.
King M. J Clin Aesthet Dermatol. 2016 Nov; 9(11):E6–8.
Kim M et al. J Clin Aesthet Dermatol. 2018 Jun;11(6):E61-8.
Online Information About Hydroquinone: An Assessment of Accuracy and Readability
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
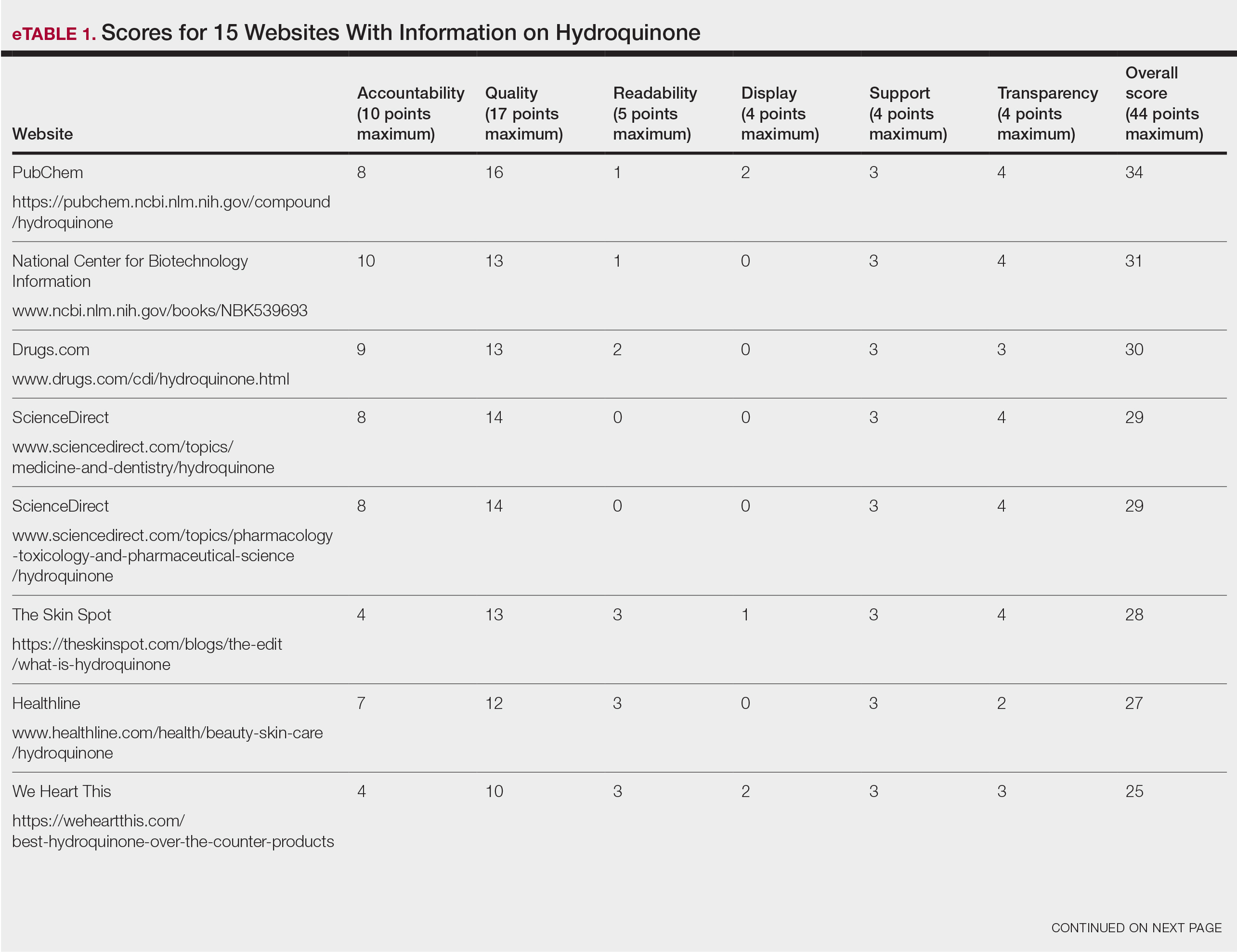
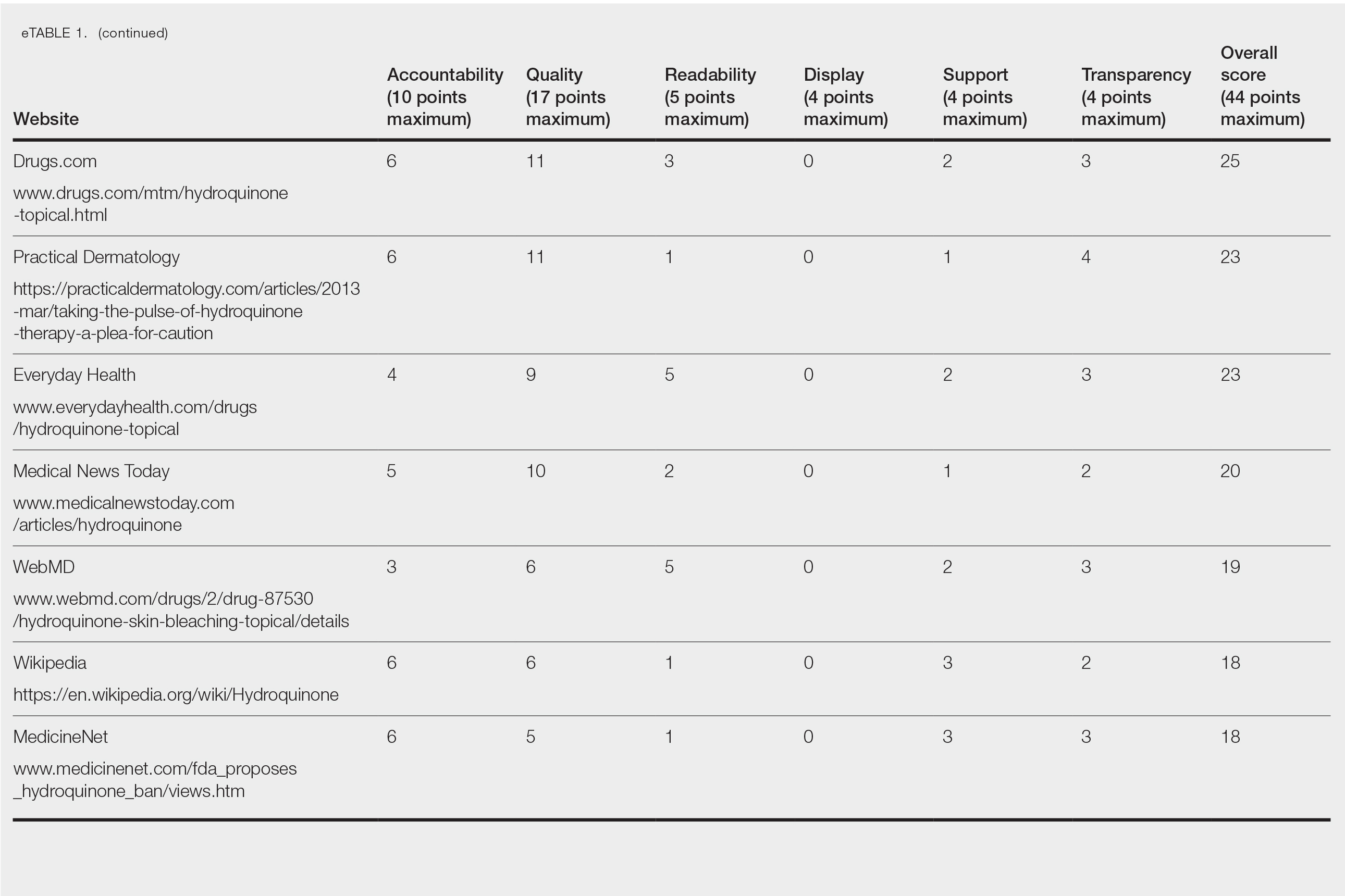
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
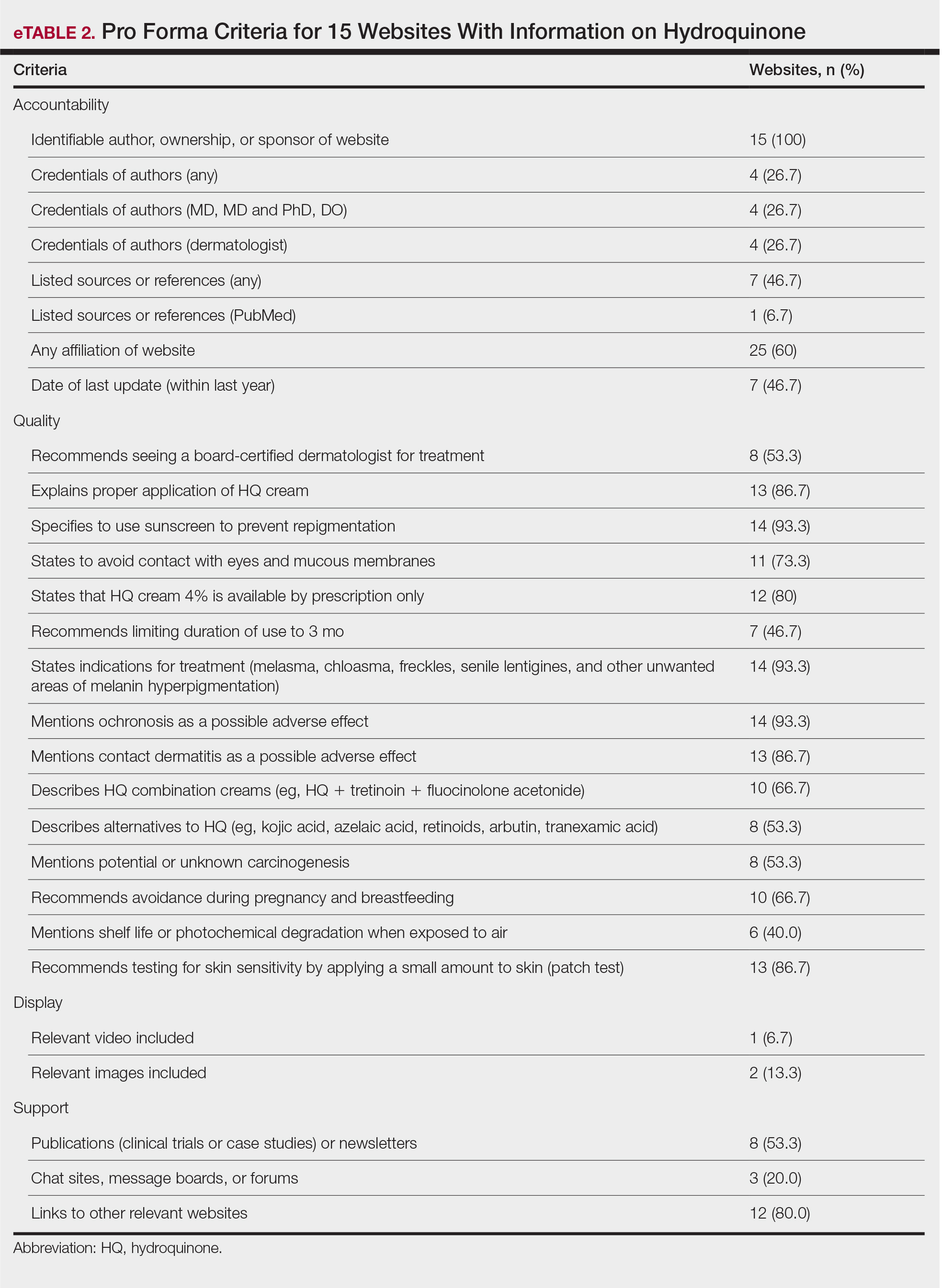
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
Practice Points
- Hydroquinone (HQ) 4% is US Food and Drug Administration (FDA) approved for skin hyperpigmentation including melasma.
- In September 2020, the FDA enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform, announcing that HQ is not classified as Category II/not generally recognized as safe and effective, thus prohibiting the distribution of OTC HQ products.
- Exogenous ochronosis is a potential side effect associated with HQ.
- There is a need for dermatologists to develop online patient education materials on HQ that are characterized by high-quality and up-to-date medical information.
FDA cautions against using OTC products to remove skin spots, moles
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Is benzophenone safe in skin care? Part 2: Environmental effects
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.