User login
Get patients vaccinated: Avoid unwelcome international travel souvenirs
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.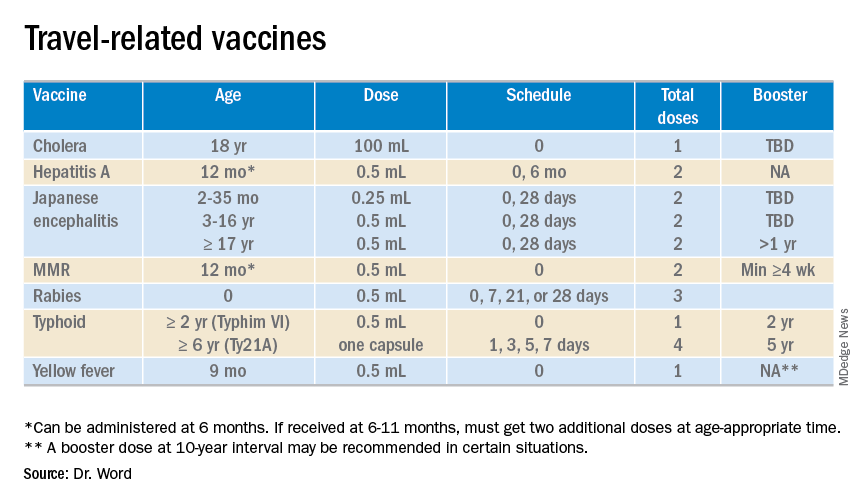
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
Summer officially began June 21, 2019, but many of your patients already may have departed or will soon be headed to international destinations. Reasons for travel are as variable as their destinations and include but are not limited to family vacations, mission trips, study abroad, parental job relocation, and visiting friends and relatives. The majority of the trips are planned at least 3 months in advance; however, for many travelers and their parents, they suddenly get an aha moment and realize there is/are specific vaccines required to obtain a visa or entry to their final destination. Unfortunately, too much emphasis is focused on required vaccines. The well-informed traveler knows that they may be exposed to multiple diseases and many are vaccine preventable.
The accompanying table lists vaccines traditionally considered to be travel vaccines. Several require multiple doses administered over 21-28 days to provide protection. Others such as cholera and yellow fever must be completed at least 10 days prior to departure to be effective. Typhoid has two formulations: The oral and injectable typhoid vaccines should be completed 1 and 2 weeks, respectively, prior to travel. Several vaccines have age limitations. Routine immunization of all infants against hepatitis A was recommended in 2006. Depending on your region, there may be adolescents who have not been immunized. Fortunately, hepatitis A vaccine works immediately.
One of the challenges you face is identifying someone in your area that provides travel medicine advice and immunizations to children and adolescents. Most children and teens travel with their parents, but today many adolescents travel independently with organized groups. Most of the vaccines listed are not routinely administered at your office, yet you most likely will be the first call a parent makes seeking travel advice.
Let me tell you about a few vaccines in particular.
Japanese encephalitis
This is most common cause of encephalitis in Asia and parts of the western Pacific. Risk generally is limited to rural agricultural areas where the causative virus is transmitted by a mosquito. Fatality rates are 20%-30%. Among survivors, 30%-50% have significant neurologic, cognitive, and psychiatric sequelae. Candidates for this vaccine are long-term travelers and short-term travelers with extensive outdoor rural activities.
Meningococcal conjugate vaccines (MCV4)
All travelers to the Hajj Pilgrimage (Aug. 9-14, 2019) and/or Umrah must show proof of immunization. Vaccine must be received at least 10 days prior to and no greater than 5 years prior to arrival to Saudi Arabia. Conjugate vaccine must clearly be documented for validity of 5 years. For all health entry requirements, go to www.moh.gov.sa/en/hajj/pages/healthregulations.aspx.
Measles
The Advisory Committee on Immunization Practices recommends all infants 6-11 months old receive one dose of MMR prior to international travel regardless of the destination. This should be followed by two additional countable doses. All persons at least 12 months of age and born after 1956 should receive two doses of MMR at least 28 days apart prior to international travel.
Rabies
Rabies is a viral disease endemic in more than 150 countries with approximately 60,000 fatal cases worldwide each year. Asia and Africa are the areas with the highest risk of exposure, and dogs are the principal hosts. Human rabies is almost always fatal once symptoms develop. Preexposure vaccine is recommended for persons with prolonged and/or remote travel to countries where rabies immunoglobulin is unavailable and the occurrence of animal rabies is high. Post exposure vaccination on days 0 and 3 still would be required.*
Typhoid
A bacterial infection caused by Salmonella enterica serotype Typhi and Paratyphi manifests with fever, headache, abdominal pain, diarrhea, or constipation. When bacteremia occurs, it usually is referred to as enteric fever. It is acquired by consumption of food/water contaminated with human feces. Highest risk areas include Africa, Southern Asia, and Southeast Asia
Yellow fever
Risk is limited to sub-Saharan Africa and the tropical areas of South America. It is transmitted by the bite of an infected mosquito. The vaccine is required for entry into at least 16 countries. In a country where yellow fever is present, persons transiting through for more than 12 hours to reach their final destination may actually cause a change in the entry requirements for the destination country. For example, travel from the United States to Tanzania requires no yellow fever vaccine while travel from the United States to Nairobi (more than 12 hours) to Tanzania requires yellow fever vaccine for entry into Tanzania. Travel sequence and duration is extremely important. Check the Centers for Disease Control and Prevention yellow fever site and/or the consulate for the most up-to-date yellow fever vaccine requirements.
YF-Vax (yellow fever vaccine) produced by Sanofi Pasteur in the United States currently is unavailable. The company is building a new facility, and vaccine will not be available for the remainder of 2019. To assure vaccine for U.S. travelers, Stamaril, a yellow fever vaccine produced by Sanofi Pasteur in France has been made available at more than 250 sites nationwide. Because Stamaril is offered at a limited number of locations, persons in need of vaccine should not delay seeking it. Because of increased demand related to summer travel, travelers in some areas have reported delays of several weeks in scheduling an appointment. To locate a Stamaril site in your area, go to wwwnc.cdc.gov/travel/page/search-for-stamaril-clinics.
There are several other diseases transmitted by mosquitoes and ticks including malaria, dengue, Zika and rickettsial diseases. Vigilant use of mosquito repellents is a must. Prophylactic medication is available for only malaria and should be initiated prior to exposure. Frequency and duration depends on the medication selected.
So how do you assist your patients?
Once you’ve identified a travel medicine facility in your area, encourage them to seek pretravel advice 4-6 weeks prior to international travel and make sure their routine immunizations are up to date. Generally, this is not an issue. One challenge is the early administration of MMR. While most practitioners know that early administration for international travel has been recommended for years, many office staff are accustomed to administration at only the 12 month and 4 year visit. When parents call requesting immunization, they often are informed that is it unnecessary and the appointment denied. This is a challenge, especially when coordination of administration of another live vaccine, such as yellow fever, is planned. Familiarizing all members of the health care team with current vaccine recommendations is critical.
For country-specific information, up-to-date travel alerts, and to locate a travel medicine clinic, visit www.cdc.gov/travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Email her at [email protected].
*This article was updated 6/18/2019.
FACTS Consortium calls for research on preventing pediatric firearm injuries
The 26 research agenda items from FACTS, which span across the broad topic areas of epidemiology, surveillance, and risk and protective factors; primary, secondary, and cross-cutting protective factors; policy-related issues; and data-enhancement priorities, were published in JAMA Pediatrics.
“Firearms are the second leading cause of death among children and adolescents aged 1 to 18 years in the United States and responsible for more than 2,570 deaths and nearly 12,000 nonfatal injuries requiring emergency department treatment in 2017,” said Rebecca Cunningham, MD, of the University of Michigan, Ann Arbor, and colleagues who are members of the FACTS Consortium.
“Pediatric firearm injuries result from a range of causes, including the unintentional discharge of a firearm, self-inflicted wounds, or the escalation of interpersonal violence,” they continued. “Nearly 265 million firearms are in civilian hands in the United States, and a 44% increase in pediatric firearm mortality rate has been documented during the past 5 years.”
The FACTS Consortium defined an agenda “to serve as a guide for future research efforts to decrease pediatric death and injury.”
Some of the research agenda items include the following:
- Understanding epidemiologic trends and how demographic factors are associated with fatal and nonfatal outcomes.
- The long-term cost associated with pediatric firearm outcomes.
- The effectiveness of health care–focused primary prevention strategies for children, adolescents, and their families to reduce firearm outcomes.
- Examination of health care–based interventions for children and adolescents who have experienced or witnessed a firearm injury to prevent (or reduce) subsequent firearm outcomes including firearm injury recidivism and mental health, socioemotional, and educational outcomes.
“It is an excellent list, and I think it was very thoughtful to come out with a research agenda for firearm safety for kids,” Marlene Melzer-Lange, MD, chair of the American Academy of Pediatrics subcommittee on violence prevention, said in an interview. “The thing that’s been lacking nationally has been funding for firearm injury prevention, both for children and adults, and to have something focused specifically on children – because children have the biggest chance of living the longest – is really important.”
She described the research agenda as being “comprehensive” and didn’t see anything that stood out as missing from the list, although the big issue that remains is getting funding for the research now that an agenda has been outlined.
And getting that is not going to be easy.
“I think it’s going to take some political will of the people and particularly of our legislators ... to allow the [Centers for Disease Control and Prevention] to provide funding” said Dr. Melzer-Lange, an attending emergency department physician at Children’s Hospital of Wisconsin, Milwaukee, adding that AAP has long been asking for such funding. “It’s going [to come down to] the legislators [having] enough guts to actually put the funding in and not be afraid of external groups that might ask them not to do that.”
She remains optimistic and hopeful that the legislators are going to look at it and will eventually say that this is an crisis and will take more action beyond just talking about it.
Dr. Melzer-Lange also emphasized that this is about firearm safety and not firearm control, especially for children and teenagers.
All authors reported receiving grants from National Institutes of Health/National Institute of Child Health and Human Development.
SOURCE: Cunningham RM et al. JAMA Pediatrics. 2019. doi: 10.1001/jamapediatrics.2019.1494.
The 26 research agenda items from FACTS, which span across the broad topic areas of epidemiology, surveillance, and risk and protective factors; primary, secondary, and cross-cutting protective factors; policy-related issues; and data-enhancement priorities, were published in JAMA Pediatrics.
“Firearms are the second leading cause of death among children and adolescents aged 1 to 18 years in the United States and responsible for more than 2,570 deaths and nearly 12,000 nonfatal injuries requiring emergency department treatment in 2017,” said Rebecca Cunningham, MD, of the University of Michigan, Ann Arbor, and colleagues who are members of the FACTS Consortium.
“Pediatric firearm injuries result from a range of causes, including the unintentional discharge of a firearm, self-inflicted wounds, or the escalation of interpersonal violence,” they continued. “Nearly 265 million firearms are in civilian hands in the United States, and a 44% increase in pediatric firearm mortality rate has been documented during the past 5 years.”
The FACTS Consortium defined an agenda “to serve as a guide for future research efforts to decrease pediatric death and injury.”
Some of the research agenda items include the following:
- Understanding epidemiologic trends and how demographic factors are associated with fatal and nonfatal outcomes.
- The long-term cost associated with pediatric firearm outcomes.
- The effectiveness of health care–focused primary prevention strategies for children, adolescents, and their families to reduce firearm outcomes.
- Examination of health care–based interventions for children and adolescents who have experienced or witnessed a firearm injury to prevent (or reduce) subsequent firearm outcomes including firearm injury recidivism and mental health, socioemotional, and educational outcomes.
“It is an excellent list, and I think it was very thoughtful to come out with a research agenda for firearm safety for kids,” Marlene Melzer-Lange, MD, chair of the American Academy of Pediatrics subcommittee on violence prevention, said in an interview. “The thing that’s been lacking nationally has been funding for firearm injury prevention, both for children and adults, and to have something focused specifically on children – because children have the biggest chance of living the longest – is really important.”
She described the research agenda as being “comprehensive” and didn’t see anything that stood out as missing from the list, although the big issue that remains is getting funding for the research now that an agenda has been outlined.
And getting that is not going to be easy.
“I think it’s going to take some political will of the people and particularly of our legislators ... to allow the [Centers for Disease Control and Prevention] to provide funding” said Dr. Melzer-Lange, an attending emergency department physician at Children’s Hospital of Wisconsin, Milwaukee, adding that AAP has long been asking for such funding. “It’s going [to come down to] the legislators [having] enough guts to actually put the funding in and not be afraid of external groups that might ask them not to do that.”
She remains optimistic and hopeful that the legislators are going to look at it and will eventually say that this is an crisis and will take more action beyond just talking about it.
Dr. Melzer-Lange also emphasized that this is about firearm safety and not firearm control, especially for children and teenagers.
All authors reported receiving grants from National Institutes of Health/National Institute of Child Health and Human Development.
SOURCE: Cunningham RM et al. JAMA Pediatrics. 2019. doi: 10.1001/jamapediatrics.2019.1494.
The 26 research agenda items from FACTS, which span across the broad topic areas of epidemiology, surveillance, and risk and protective factors; primary, secondary, and cross-cutting protective factors; policy-related issues; and data-enhancement priorities, were published in JAMA Pediatrics.
“Firearms are the second leading cause of death among children and adolescents aged 1 to 18 years in the United States and responsible for more than 2,570 deaths and nearly 12,000 nonfatal injuries requiring emergency department treatment in 2017,” said Rebecca Cunningham, MD, of the University of Michigan, Ann Arbor, and colleagues who are members of the FACTS Consortium.
“Pediatric firearm injuries result from a range of causes, including the unintentional discharge of a firearm, self-inflicted wounds, or the escalation of interpersonal violence,” they continued. “Nearly 265 million firearms are in civilian hands in the United States, and a 44% increase in pediatric firearm mortality rate has been documented during the past 5 years.”
The FACTS Consortium defined an agenda “to serve as a guide for future research efforts to decrease pediatric death and injury.”
Some of the research agenda items include the following:
- Understanding epidemiologic trends and how demographic factors are associated with fatal and nonfatal outcomes.
- The long-term cost associated with pediatric firearm outcomes.
- The effectiveness of health care–focused primary prevention strategies for children, adolescents, and their families to reduce firearm outcomes.
- Examination of health care–based interventions for children and adolescents who have experienced or witnessed a firearm injury to prevent (or reduce) subsequent firearm outcomes including firearm injury recidivism and mental health, socioemotional, and educational outcomes.
“It is an excellent list, and I think it was very thoughtful to come out with a research agenda for firearm safety for kids,” Marlene Melzer-Lange, MD, chair of the American Academy of Pediatrics subcommittee on violence prevention, said in an interview. “The thing that’s been lacking nationally has been funding for firearm injury prevention, both for children and adults, and to have something focused specifically on children – because children have the biggest chance of living the longest – is really important.”
She described the research agenda as being “comprehensive” and didn’t see anything that stood out as missing from the list, although the big issue that remains is getting funding for the research now that an agenda has been outlined.
And getting that is not going to be easy.
“I think it’s going to take some political will of the people and particularly of our legislators ... to allow the [Centers for Disease Control and Prevention] to provide funding” said Dr. Melzer-Lange, an attending emergency department physician at Children’s Hospital of Wisconsin, Milwaukee, adding that AAP has long been asking for such funding. “It’s going [to come down to] the legislators [having] enough guts to actually put the funding in and not be afraid of external groups that might ask them not to do that.”
She remains optimistic and hopeful that the legislators are going to look at it and will eventually say that this is an crisis and will take more action beyond just talking about it.
Dr. Melzer-Lange also emphasized that this is about firearm safety and not firearm control, especially for children and teenagers.
All authors reported receiving grants from National Institutes of Health/National Institute of Child Health and Human Development.
SOURCE: Cunningham RM et al. JAMA Pediatrics. 2019. doi: 10.1001/jamapediatrics.2019.1494.
FROM JAMA PEDIATRICS
In MS, the challenges for women are unique
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
SEATTLE – Mitzi Joi Williams, MD.
About three in four people with MS are female – about 750,000 in the United States. And the risk and incidence may be highest in African American women.
In a presentation about the unique needs of women with MS, Dr. Williams, an assistant professor of internal medicine at the Morehouse School of Medicine in Atlanta, offered these tips at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Pay attention to sexual dysfunction
Patients with MS often are ashamed to talk about sexual dysfunction, Dr. Williams said, but it is on many minds. “If I have a program on intimacy in MS, people are out the door.”
She urged colleagues to understand that MS can affect sexuality through three routes: primary, secondary, and tertiary dysfunction.
In primary sexual dysfunction, brain and spinal lesions directly related to MS can cause problems such as lack of sensation or abnormal sensations, decreased libido, vaginal dryness, and difficult orgasm.
Secondary sexual dysfunction refers to problems caused by symptoms of MS such as fatigue, which can worsen as the day progresses and affect nighttime intimacy, she said. Bladder dysfunction is another sensitive area in sexuality, with patients – especially women – “concerned that they will lose control of their bladder or they have already lost control.”
Cognitive dysfunction also can disrupt sexual function. “It is important to focus, and certain things cannot happen if you do not. If you are not able to focus and concentrate, it can affect interest,” Dr. Williams said.
Additionally, medications can improve some symptoms while making others worse. For example, a drug may relieve spasticity but boost fatigue. “We have to walk this tightrope,” she said. “But if we are not asking our patients, they may not volunteer this information.”
Finally, she said, MS can spark tertiary sexual dysfunction – poor body image, depression, anxiety, and disruptive changes in familial roles. For example, one partner may become a caregiver, and “it is hard to go from caregiving to sexy time.”
“It is something we have to acknowledge and find ways to deal with,” Dr. Williams said.
To address these issues, she pointed to strategies for symptomatic relief and disease-modifying therapy (DMT) and pinpointed several treatment options.
- Fatigue – stimulants, diet, exercise.
- Spasticity – muscle relaxants, exercise.
- Bladder dysfunction – fluid restriction, medication.
- Paresthesia – antidepressants, anticonvulsants.
- Numbness – vibrators, devices to increase stimulation.
Sexual therapy, couples therapy, and pelvic floor physical therapy also can be helpful.
Be aware of special needs during prepregnancy and pregnancy
“MS itself does not have a lot of effects on fertility, pregnancy, or pregnancy outcomes,” Dr. Williams said. However, “medications cause concern about how we manage pregnancy and fertility.”
In vitro fertilization may increase the risk of relapse, she added, and patients on dimethyl fumarate who experience vomiting or diarrhea may not be able to properly absorb oral contraceptives.
Women with MS may not need to go off DMT when they are trying to conceive, she said. “If patients have very aggressive disease, they may need to be on DMT through conception, through the first trimester, and even the entire pregnancy to prevent long-term disability.”
What about pregnancy itself? “An MS diagnosis alone does not mean that a pregnancy is high risk,” she said. “There are not necessarily additional tests and ultrasounds that are recommended for our patients based on MS diagnosis alone.”
Treatment discontinuation may be warranted during pregnancy, when MS generally improves. However, some MS symptoms – fatigue, bladder dysfunction, and balance – may increase. Corticosteroids can be appropriate if relapses occur during pregnancy.
Menopause and MS symptoms may overlap
Symptoms such as hot flashes, mood changes, sleep disturbance, bladder dysfunction, and decreased energy may be signs of MS, or they could indicate menopause, Dr. Williams said. “Sometimes patients come in and they are getting worse, and we look into it and discover they are premenopausal.”
A decline in estrogen during menopause may worsen MS symptoms, she added, and hormone therapy may be appropriate. A phase 2 study found a benefit in menopausal patients with MS for estriol in conjunction with a DMT, but more studies are needed.
Dr. Williams reported no relevant financial disclosures.
EXPERT ANALYSIS FROM CMSC 2019
How to have ‘the talk’ with vaccine skeptics
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
LJUBLJANA, SLOVENIA – An effective strategy in helping vaccine skeptics to come around to accepting immunizations for their children is to pivot the conversation away from vaccine safety and focus instead on the disease itself and its potential consequences, Saad B. Omer, MBBS, PhD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
“Why do we cede ground by focusing too much on the vaccine itself? I call it the disease salience approach,” said Dr. Omer, professor of global health, epidemiology, and pediatrics at Emory University in Atlanta.
It’s a strategy guided by developments in social psychology, persuasion theory, and communication theory. But if applied incorrectly, the disease salience approach can backfire, causing behavioral paralysis and an inability to act, he cautioned.
Dr. Omer explained that it’s a matter of framing.
“Always include a solution to promote self-efficacy and response-efficacy. After you inform parents of disease risks, provide them with actions they can take. Now readdress the vaccine, pointing out that this is the single best way to protect yourself and your baby,” he said. “The lesson is that since vaccines are a social norm, reframe nonvaccination as an active act, rather than vaccination as an active act.”
Don’t attempt to wow parents with statistics on how vaccine complication rates are dwarfed by the disease risk if left unvaccinated, he advised. Studies have shown that‘s generally not effective. What actually works is to provide narratives of disease severity.
“We are excellent linguists, but really, really poor statisticians,” Dr. Omer observed.
Is it ethical to talk to parents about disease risks to influence their behavior? Absolutely, in his view.
“We’re not selling toothpaste. We are in the business of life-saving vaccines. And I would submit that if it’s done correctly it’s entirely ethical to talk about the disease, and sometimes even the severe risks of the disease, instead of the vaccine,” said Dr. Omer.
If parents cite a myth about vaccines, it’s necessary to address it head on without lingering on it. But debunking a myth is tricky because people tend to remember negative information they received earlier.
“If you’re going to debunk a myth, clearly label it as a myth in the headline as you introduce it. State why it’s not true. Replace the myth with the best alternative explanation. Think of it like a blank space where the myth used to reside. That space needs to be filled with an alternative explanation or the myth will come back,” Dr. Omer said.
He is a coauthor of a book titled, ‘The Clinician’s Vaccine Safety Resource Guide: Optimizing Prevention of Vaccine-Preventable Diseases Across the Lifespan.’
EXPERT ANALYSIS FROM ESPID 2019
Judge bars contraceptive mandate from being enforced
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
In a June 5, 2019, opinion, U.S. District Judge Reed O’Connor granted a permanent injunction on the contraceptive mandate, ruling that both the mandate and the accommodation process violate the Religious Freedom Restoration Act. The injunction applies to all individuals and employers – regardless of size or nonprofit status – that oppose contraceptive coverage based on religious beliefs.
In his ruling, Judge O’Connor said the contraceptive mandate substantially burdens the plaintiffs’ religious exercise.
“The point of the contraceptive mandate is to ensure all ACA-compliant insurance plans include cost-free coverage of all FDA [Food and Drug Administration]-approved contraceptive methods [and] the point of the individual mandate is to ensure individuals purchase ACA-compliant insurance plans,” Judge O’Conner wrote. “The result? The individual plaintiffs are forced out of either the health insurance market or their religious exercise. And by choosing to adhere to their religious beliefs, not only are the individual plaintiffs excluded from the insurance market, they are forced to violate federal law. That the contraceptive mandate systematically discriminates against the individual class by blocking members’ entrance into the marketplace – due to religious exercise – is a substantial burden of the highest order.”
The case, DeOtte v. Azar, started with an October 2018 legal challenge by several Texas residents and a business over having to comply with the Affordable Care Act mandate. The plaintiffs argued the requirement violates their religious freedom, and that the court should strike it down as unconstitutional. The current Justice Department has largely chosen not to defend the case, agreeing that forcing people and employers with religious objections to comply with the contraceptive mandate violates the Religious Freedom Restoration Act. In 2018, the department issued new rules expanding exemptions to the ACA’s contraceptive mandate on moral or religious grounds.
Legal challenges against the expanded exemptions continue through the courts. Judges in California and Pennsylvania have temporarily banned the rules from taking effect. Analysts say the final answer on the contraceptive mandate could come from the U.S. Supreme Court.
USPSTF reaffirms HIV screening recommendations
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
According to the task force, screening is recommended for all patients aged 15-65 years. Screening also is recommended for adolescents and older adults at increased risk for acquiring HIV infection and for all pregnant patients, including those in labor whose HIV status is unknown (JAMA. 2019. doi: 10.1001/jama.2019.6587).
Patients who are considered at increased risk for acquiring HIV include the following: Men who have sex with men, those who inject drugs, those who have receptive sex without a condom, those with at least one partner whose HIV status is positive or unknown, those who have transactional sex, and those who request testing for sexually transmitted infection, including HIV. All recommendations are A-level, meaning the task force recommends the service,with high certainty that the net benefit is substantial.
In a systematic review created for the task force, Roger Chou, MD, of Oregon Health & Science University, Portland, and colleagues found there continued to be no studies that examined the benefits and harms of HIV screening for HIV infections, compared with no screening, but new evidence found beginning antiretroviral therapy (ART) for patients with CD4 cell counts greater than 500/mm3 who are otherwise asymptomatic was associated with a reduced risk of mortality, compared with waiting for ART in cases of CD4 cell counts less than 350/mm3 (JAMA. 2019. doi: 10.1001/jama.2019.2592).
A second systematic review of pregnant patients by Shelley S. Selph, MD, also of Oregon Health & Science University, Portland, and colleagues found no studies examining the effectiveness of prenatal screening on mother-to-child HIV transmission, but combination ART was significantly effective at reducing transmission between mother and child, while ART that includes a boosted protease inhibitor may result in preterm delivery (JAMA. 2019. doi: 10.1001/jama.2019.2593).
Although no studies have been conducted that compare the benefits of screening with not screening for HIV, the task force concluded with “high certainty” that early HIV detection and treatment has “substantial benefits.”
“Clinicians can make a real difference toward reducing the burden of HIV in the United States,” Douglas K. Owens, MD, task force chairman, said in a statement. “HIV screening and HIV prevention work to reduce new HIV infections and ultimately save lives.”
The USPSTF is a voluntary, independent body, with operations supported by the U.S. Agency for Healthcare Research and Quality. Task force members received travel reimbursement and an honorarium for attending meetings. Dr. Owens reports financial disclosures with relation to HIV infection screening, preexposure prophylaxis for HIV prevention, and hepatitis C screening. Other task force members reported no relevant conflicts of interest.
SOURCE: JAMA. 2019. doi: 10.1001/jama.2019.6587.
FROM JAMA
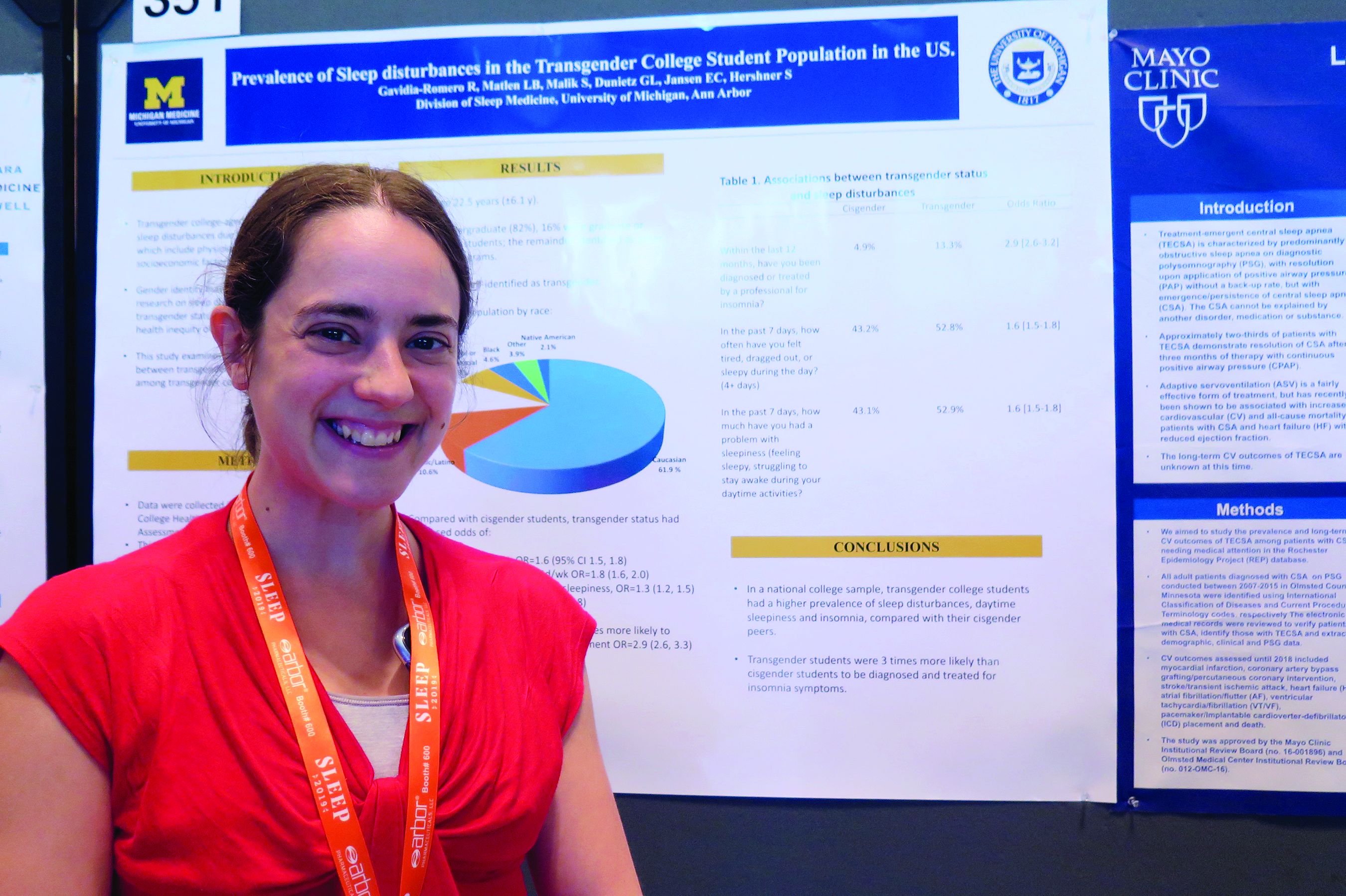
Insomnia common among transgender college students
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
SAN ANTONIO – Compared with their cisgender counterparts, , results from a large national population-based survey showed.
“That was a stronger association than we expected,” one of the study’s researchers, Lisa B. Matlen, MD, said during an interview at the annual meeting of the Associated Professional Sleep Societies.
According to Dr. Matlen, a fellow in the division of sleep medicine at the University of Michigan, Ann Arbor, the transgender population is “extremely understudied” when it comes to research on sleep disturbances. In an effort to examine the prevalence of sleep disturbances and the association between transgender identity and sleep disturbances among transgender college students in the United States, she and her colleagues drew from the 2016 and 2017 American College Health Association National College Health Assessment II, a confidential, voluntary, electronically administered survey of college and university students. In all, 224,233 students were polled, and the researchers analyzed their responses to questions about gender identity, sleep symptoms, and diagnoses.
The mean age of the respondents was 23 years, and most (82%) were undergraduate students. Of the 224,233 students, 3,471 (1.6%) self-identified as transgender. More than half of the transgender population (61.9%) was white, 10.6% were Hispanic/Latino, 10.5% were Asian or Pacific Islander, 6.3% were biracial or multiracial, 4.6% were black, and the rest were from other ethnicities. Compared with cisgender students, transgender students had increased odds of sleep disturbances (odds ratio, 1.6), not feeling well rested on 4 or more days per week (OR, 1.8), going to bed early on 3 or more days per week due to sleepiness (OR, 1.3), and having insomnia 3 or more days per week (OR, 1.7). In addition, transgender students were nearly three times more likely to have an insomnia diagnosis and treatment, compared with their cisgender counterparts (OR, 2.9).
Dr. Matlen acknowledged certain limitations of the study, including the fact that it drew from a population-based sample and that the survey was based on self-reported information. The study’s first author was Ronald R. Gavidia Romero, MD. The researchers reported having no financial disclosures.
REPORTING FROM SLEEP 2019
United States now over 1,000 measles cases this year
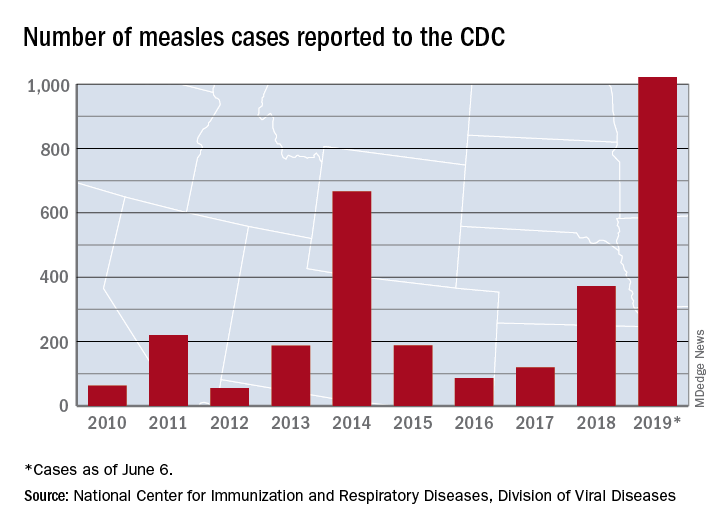
The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.
Postpartum LARC uptake increased with separate payment
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
The introduction of separate payment for the immediate postpartum implantation of long-acting reversible contraception was associated with increased use and a slow-down in the number of short-interval births in patients covered by South Carolina’s Medicaid program.
Immediate postpartum long-acting reversible contraception (IPP-LARC) is recommended to reduce the incidence of short pregnancy intervals – pregnancies within 6-24 months of each other. The global payment for hospital labor and delivery, however, may act as a disincentive to providing IPP-LARC, according to Maria W. Steenland of Brown University, Providence, R.I., and co-authors.
They looked at inpatient Medicaid claims data for 242,825 childbirth hospitalizations in South Carolina from 2010-2017; during that time the state Medicaid program began to provide an additional payment for IPP-LARC.
At the start of the study, just 0.07% of women received an IPP-LARC. After the change in reimbursement policy in March 2012, there was a steady 0.07 percentage point monthly increase in their use in adults and 0.1 percentage point increase per month in adolescents. In December 2017, 5.65% of adults and 10.48% of adolescents received an IPP-LARC (JAMA. 2019; doi: 10.1001/jama.2019.6854).
There was a corresponding, significant change in the trend of short-interval births among adolescents. Before the policy change, adolescent short-interval births had been increasing, but by March 2016 – 4 years after the payment change – the adolescent short-interval birth rate was 5.28 percentage points lower than what was expected had the increasing trend continued.
There was no significant change in the trend for short-interval births among adults.
“These findings suggest that IPP-LARC reimbursement could increase immediate postpartum contraceptive options and help adolescents avoid short-interval births,” the authors wrote, noting that as of February 2018, 36 other states’ Medicaid programs had began separately reimbursing for IPP-LARC.
They also raised the possibility that there may have been confounding due to other events that occurred at the same time as the policy changes.
The study was supported by the Eric M. Mindich Research Fund and one author was supported by National Institutes of Health. No conflicts of interest were declared.
SOURCE: Steenland M et al. JAMA 2019, DOI:10.1001/jama.2019.6854.
FROM JAMA
Diverse vaginal microbiome may signal risk for preterm birth
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
in an analysis of approximately 12,000 samples, according to a study published in Nature Medicine.
Preterm births, defined as less than 37 weeks’ gestation, remain the second most common cause of neonatal death worldwide, but few strategies exist to prevent and predict preterm birth (PTB) wrote Jennifer M. Fettweis, MD, of Virginia Commonwealth University, Richmond, and her colleagues. In the United States, women of African ancestry are at significantly greater risk for PTB.
A highly diverse vaginal microbiome is thought to be associated with an increased risk of inflammation, infection, and PTB, “however, many asymptomatic healthy women have diverse vaginal microbiota,” the researchers said.
To identify vaginal microbiota distinct to women who experienced PTB, the researchers analyzed data from the Multi-Omic Microbiome Study: Pregnancy Initiative (MOMS-PI), part of the National Institutes of Health–sponsored Integrative Human Microbiome Project. The MOMS-PI study included 12,039 samples of vaginal flora from 597 pregnancies; the analysis included 45 singleton pregnancies that met the criteria for spontaneous PTB (23-36 weeks, 6 days of gestation) and 90 case-matched full-term singleton pregnancies (greater than or equal to 39 weeks). Approximately 78% of the women were of African descent in both groups, and their average age was 26 years in both groups.
Overall, the diversity of the vaginal microbiome was greater among women who experienced PTB, compared with term birth (TB). Women who experienced PTB had less Lactobacillus crispatus, but more bacterial vaginosis–associated bacterium-1 (BVAB1), Prevotella cluster 2, and Sneathia amnii, compared with TB women.
Of note, vaginal cytokine data showed that proinflammatory cytokines, which may be associated with the induction of labor, may be prompted by inflammation in the vaginal microbiome, Dr. Fettweis and her associates said. “We observed that vaginal IP-10/CXCL10 levels were inversely correlated with BVAB1 in PTB, inversely correlated with L. crispatus in TB, and positively correlated with L. iners in TB, suggesting complex host-microbiome interactions in pregnancy,” they said.
“Further studies are needed to determine whether the signatures of PTB reported in the present study replicate in other cohorts of women of African ancestry, to examine whether the observed differences in vaginal microbiome composition between women of different ancestries has a direct causal link to the ethnic and racial disparities in PTB rates, and to establish whether population-specific microbial markers can be ultimately integrated into a generalizable spectrum of vaginal microbiome states linked to the risk for PTB,” Dr. Fettweis and her associates said.
In a companion study also published in Nature Medicine, Myrna G. Serrano, MD, also of Virginia Commonwealth University, and her colleagues as part of the MOMS-PI initially determined that vaginal microbiome profiles varied between 613 pregnant and 1,969 nonpregnant women in that “pregnant women had significantly higher prevalence of the four most common Lactobacillus vagitypes (L. crispatus, L. iners, L. gasseri, and L. jensenii) and a commensurately lower prevalence of vagitypes dominated by other taxa.” The primary driver of the differences was L. iners.
They then compared vaginal microbiome data from 300 pregnant and 300 nonpregnant case-matched women of African, Hispanic, or European ancestry, as well as 90 pregnant women (49 of African ancestry and 41 of European) ancestry.
In the subset of 300 pregnant and 300 nonpregnant women, the vaginal microbiome of the pregnant women overall became more dominated by Lactobacillus early in pregnancy. Further stratification by race showed that pregnant women of African and Hispanic ancestry had significantly higher levels of four types of Lactobacillus than their nonpregnant counterparts, but no significant difference was seen between pregnant and nonpregnant women of European ancestry.
“It appears that changes occurring during pregnancy may render the reproductive tracts of women of all racial backgrounds more hospitable to taxa of Lactobacillus and less favorable for Gardnerella vaginalis and other taxa associated with BV [bacterial vaginosis] and dysbiosis,” the researchers said.
“Interestingly, BVAB1, which has been associated with dysbiotic vaginal conditions and risk of PTB, and which is present as a major vagitype largely in women of African ancestry, is not noticeably decreased in prevalence in pregnancy,” Dr. Serrano and her associates said. “Thus, BVAB1, for reasons yet to be determined, is apparently resistant to factors sculpting the microbiome in pregnant women, possibly explaining in part the enhanced risk for PTB experienced by women of African ancestry.”
In a look at the 49 pregnant women of African ancestry and 41 of European ancestry, those of African ancestry had “significantly lower representation of the L. crispatus, L. gasseri and L. jensenii vagitypes, and higher representation of L. iners and BVAB1 vagitypes. Variability in women of African ancestry was driven by BVAB1 and L. iners, whereas variability in women of non-African ancestry was driven by L. crispatus and L. iners. Again, pregnancy had no significant effect on prevalence of the BVAB1 vagitype. Prevalence of Lactobacillus-dominated profiles in women of African ancestry was lower in the first than in later trimesters, whereas women of European ancestry had a higher prevalence of Lactobacillus vagitypes throughout pregnancy.”
The presence of vaginal microbiome profiles associated with adverse pregnancy outcomes highlights the need for further studies that take advantage of this information, Dr. Serrano and her associates said. “That the vaginal microbiomes known to confer higher risk of poor health and adverse outcomes of pregnancy are more highly associated with women of African and Hispanic ancestry, but that pregnancy tends to drive these microbiomes toward more favorable microbiota, suggests that an external intervention that favors this trend might be beneficial for these populations,” they concluded. “What remains is to verify the most favorable microbiome and the most effective strategy for intervention.”
Dr. Fettweis had no financial conflicts to disclose; two coauthors are full-time employees at Pacific Biosciences. Dr. Serrano and her coauthors had no relevant financial disclosures. Dr. Serrano’s study received grants from the National Institutes of Health and other sources, as well as support from the Common Fund, the National Center for Complementary and Integrative Health, the Office of Research on Women’s Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases.
SOURCES: Fettweis J et al. Nature Medicine 2019 May 29. doi: 10.1038/s41591-019-0450-2; Serrano M et al. Nature Medicine. 2019 May 29. doi: 10.1038/s41591-019-0465-8.
FROM NATURE MEDICINE