User login
A prescription for ‘deprescribing’: A case report
In 2016, Swapnil Gupta, MD, and John Daniel Cahill, MD, PhD, challenged the field of psychiatry to reexamine our prescribing patterns. They warned against our use of polypharmacy when not attached to improvement in functioning for our patients.1 They were concerned about the lack of evidence for those treatment regimens and for our diagnostic criteria. In their inspiring article, they described how psychiatrists might proceed in the process of “deprescribing” – which they define as a process of pharmacologic regimen optimization through reducing or ending medications for which “benefits no longer outweigh risks.”1
In my practice, I routinely confront medication regimens that I have never encountered in the literature. The evidence for two psychotropics is limited but certainly available, in particular adjunct treatment of antidepressants2 and mood stabilizers.3 The evidence supporting the use of more than two psychotropics, however, is quite sparse. Yet, patients often enter my office on more than five psychotropics. I am also confronted with poorly defined diagnostic labels – which present more as means to justify polypharmacy than a thorough review of the patient’s current state.
Dr. Gupta and Dr. Cahill recommend a series of steps aimed at attempting the deprescription of psychotropics. Those steps include timeliness, knowledge of the patient’s current regimen, discussion about the risk of prescriptions, discussion about deprescribing, choosing the right medications to stop, a plan for describing, and monitoring. In the case presented below, I used some of those steps in an effort to provide the best care for the patient. Key details of the case have been changed, including the name, to protect the patient’s confidentiality.
Overview of the case
Rosalie Bertin is a 54-year-old female who has been treated for depression by a variety of primary care physicians for the better part of the last 30 years. She had tried an array of antidepressants, including sertraline, citalopram, duloxetine, and mirtazapine, over that time. Each seemed to provide some benefit when reviewing the notes, but there is no mention of why she was continued on those medications despite the absence of continuing symptoms. Occasionally, Rosalie would present to her clinician tearful and endorsing sadness, though the record did not comment on reports of energy, concentration, sleep, appetite, and interest.
In 2014, Rosalie’s husband passed away from lung cancer. His death was fairly quick, and initially, Rosalie did not mention any significant emotional complaints. However, when visiting her primary care physician 4 months later, she was noted to experience auditory hallucinations. “Sometimes I hear my husband when I am alone in my home,” she said. Rosalie was referred to a psychiatrist with a diagnosis of “psychosis not otherwise specified.”
When discussing her condition with the psychiatrist, Rosalie mentioned experiencing low mood, and having diminished interest in engaging in activities. “I miss Marc when I go places; I used to do everything with him.” She reported hearing him often but only when at her home. He would say things like, “I miss you,” or ask her about her day. She was diagnosed with “major depressive disorder with psychotic features.” Risperidone was added to the escitalopram, buspirone, and gabapentin that had been started by her primary care physician.
After several months of psychotropic management, the dose of risperidone was titrated to 8 mg per day. Her mood symptoms were unchanged, but she now was complaining of poor concentration and memory. The psychiatrist performed a Mini-Mental State Examination (MMSE). It was noted that taking the MMSE engendered significant anxiety for the patient. Rosalie received a score suggesting mild cognitive impairment. She was started on donepezil for the memory complaint, quetiapine for the continued voices, and recommended for disability.
Once on short-term disability, the patient relocated to live closer to her mother in San Diego and subsequently contacted me about continuing psychiatric care.
Initial visit
Rosalie is a petite white woman, raised in the Midwest, who married her high school sweetheart, and subsequently became an administrative assistant. Rosalie and Marc were unable to have children. Marc was an engineer, and a longtime smoker. She describes their lives as simple – “few friends, few vacations, few problems, few regrets.” She states she misses her husband and often cries when thinking about him.
When asked about psychiatric diagnoses, she answered: “I have psychosis. … My doctor said maybe schizophrenia, but he is not sure yet.” She described schizophrenia as hearing voices. Rosalie also mentioned having memory problems: “They cannot tell if it is Alzheimer’s disease until I die and they look at my brain, but the medication should delay the progression.”
She reported no significant effect from her prior antidepressant trials: “I am not sure if or how they helped.” Rosalie could not explain the role of the medication. “I take medications as prescribed by my doctor,” she said.
When discussing her antipsychotics, she mentioned: “Those are strong medications; it is hard for me to stay awake with them.” She declared having had no changes in the voices while on the risperidone but said they went away since also being on the quetiapine: “I wonder if the combination of the two really fixed my brain imbalance.”
Assessment
I admit that I have a critical bias against the overuse of psychotropics, and this might have painted how I interpreted Rosalie’s story. Nonetheless, I was honest with her and told her of my concerns. I informed her that her diagnosis was not consistent with my understanding of mood and thought disorders. Her initial reports of depression neither met the DSM criteria for depression nor felt consistent with my conceptualization of the illness. She had retained appropriate functioning and seemed to be responding with the sadness expected when facing difficult challenges like grief.
Her subsequent reports of auditory hallucinations were not associated with delusions or forms of disorganization that I would expect in someone with a thought disorder. Furthermore, the context of the onset gave me the impression that this was part of her process of grief. Her poor result in the dementia screen was most surprising and inconsistent with my evaluation. I told her that I suspected that she was not suffering from Alzheimer’s but from being overmedicated and from anxiety at the time of the testing.
She was excited and hesitant about my report. She was surprised by the length of our visit and interested in hearing more from me. Strangely, I wished she had challenged my different approach. I think that I was hoping she would question my conceptualization, the way I hoped she would have done with her prior clinicians. Nonetheless, she agreed to make a plan with me.
Treatment plan
We decided to review each of her medications and discuss their benefits and risks over a couple of visits. She was most eager to discontinue the donepezil, which had caused diarrhea. She was concerned when I informed her of the potential side effects of antipsychotics. “My doctor asked me if I had any side effects at each visit; I answered that I felt nothing wrong; I had not realized that side effects could appear later.”
She was adamant about staying on buspirone, as she felt it helped her the most with her anxiety at social events. She voiced concern about discontinuing the antipsychotics despite being unsettled by my review of their risks. She asked that we taper them slowly.
In regard to receiving psychosocial support throughout this period of deprescribing, Rosalie declined weekly psychotherapy. She reported having a good social network in San Diego that she wanted to rely on.
Outcome
I often worry about consequences of stopping a medication, especially when I was not present at the time of its initiation. I agonize that the patient might relapse from my need to carry out my agenda on deprescribing. I try to remind myself that the evidence supports my decision making. The risks of psychotropics often are slow to show up, making the benefit of deprescribing less tangible. However, this case was straightforward.
Rosalie quickly improved. Tapering the antipsychotics was astonishing to her: “I can think clearly again.” Within 6 months, she was on buspirone only – though willing to discuss its discontinuation. She had a lead for a job and was hoping to return to work soon. Rosalie continued to miss her husband but had not heard him in some time. She has not had symptoms of psychosis or depression. Her cognition and mood were intact on my clinical assessment.
Discussion
Sadly and shockingly, cases like that of Rosalie are common. In my practice, I routinely see patients on multiple psychotropics – often on more than one antipsychotic. Their diagnoses are vague and dubious, and include diagnoses such as “unspecified psychosis” and “cognitive impairments.” Clinicians occasionally worry about relapse and promote a narrative that treatment must be not only long term but lifelong.4 There is some evidence for this perspective in a research context, but the clinical world also is filled with patients like Rosalie.
Her reports of auditory hallucinations were better explained by her grief than by a psychotic process.5 Her memory complaints were better explained by anxiety at the time of her testing while suffering from the side effects from her numerous psychotropics.6 Her depressive complaints were better explained by appropriate sadness in response to stressors. Several months later with fewer diagnoses and far fewer psychotropics, she is functioning better.
Take-home points
- Polypharmacy can lead to psychiatric symptoms and functional impairment.
- Patients often are unaware of the complete risks of psychotropics.
- Psychiatric symptoms are not always associated with a psychiatric disorder.
- Deprescribing can be performed safely and effectively.
- Deprescribing can be performed with the patient’s informed consent and agreement.
References
1. Psychiatr Serv. 2016 Aug 1;67(8):904-7.
2. Focus. 2016 Apr 13; doi: 10.1176/appi.focus.20150041.
3. Bipolar Disord. 2016 Dec;18(8):684-91.
4. Am J Psychiatry. 2017 Sep 1;174(9):840-9.
5. World Psychiatry. 2009 Jun;8(2):67-74.
6. Hosp Community Psychiatry. 1983 Sep;34(9):830-5.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019).
*This column was updated 1/11/2019.
In 2016, Swapnil Gupta, MD, and John Daniel Cahill, MD, PhD, challenged the field of psychiatry to reexamine our prescribing patterns. They warned against our use of polypharmacy when not attached to improvement in functioning for our patients.1 They were concerned about the lack of evidence for those treatment regimens and for our diagnostic criteria. In their inspiring article, they described how psychiatrists might proceed in the process of “deprescribing” – which they define as a process of pharmacologic regimen optimization through reducing or ending medications for which “benefits no longer outweigh risks.”1
In my practice, I routinely confront medication regimens that I have never encountered in the literature. The evidence for two psychotropics is limited but certainly available, in particular adjunct treatment of antidepressants2 and mood stabilizers.3 The evidence supporting the use of more than two psychotropics, however, is quite sparse. Yet, patients often enter my office on more than five psychotropics. I am also confronted with poorly defined diagnostic labels – which present more as means to justify polypharmacy than a thorough review of the patient’s current state.
Dr. Gupta and Dr. Cahill recommend a series of steps aimed at attempting the deprescription of psychotropics. Those steps include timeliness, knowledge of the patient’s current regimen, discussion about the risk of prescriptions, discussion about deprescribing, choosing the right medications to stop, a plan for describing, and monitoring. In the case presented below, I used some of those steps in an effort to provide the best care for the patient. Key details of the case have been changed, including the name, to protect the patient’s confidentiality.
Overview of the case
Rosalie Bertin is a 54-year-old female who has been treated for depression by a variety of primary care physicians for the better part of the last 30 years. She had tried an array of antidepressants, including sertraline, citalopram, duloxetine, and mirtazapine, over that time. Each seemed to provide some benefit when reviewing the notes, but there is no mention of why she was continued on those medications despite the absence of continuing symptoms. Occasionally, Rosalie would present to her clinician tearful and endorsing sadness, though the record did not comment on reports of energy, concentration, sleep, appetite, and interest.
In 2014, Rosalie’s husband passed away from lung cancer. His death was fairly quick, and initially, Rosalie did not mention any significant emotional complaints. However, when visiting her primary care physician 4 months later, she was noted to experience auditory hallucinations. “Sometimes I hear my husband when I am alone in my home,” she said. Rosalie was referred to a psychiatrist with a diagnosis of “psychosis not otherwise specified.”
When discussing her condition with the psychiatrist, Rosalie mentioned experiencing low mood, and having diminished interest in engaging in activities. “I miss Marc when I go places; I used to do everything with him.” She reported hearing him often but only when at her home. He would say things like, “I miss you,” or ask her about her day. She was diagnosed with “major depressive disorder with psychotic features.” Risperidone was added to the escitalopram, buspirone, and gabapentin that had been started by her primary care physician.
After several months of psychotropic management, the dose of risperidone was titrated to 8 mg per day. Her mood symptoms were unchanged, but she now was complaining of poor concentration and memory. The psychiatrist performed a Mini-Mental State Examination (MMSE). It was noted that taking the MMSE engendered significant anxiety for the patient. Rosalie received a score suggesting mild cognitive impairment. She was started on donepezil for the memory complaint, quetiapine for the continued voices, and recommended for disability.
Once on short-term disability, the patient relocated to live closer to her mother in San Diego and subsequently contacted me about continuing psychiatric care.
Initial visit
Rosalie is a petite white woman, raised in the Midwest, who married her high school sweetheart, and subsequently became an administrative assistant. Rosalie and Marc were unable to have children. Marc was an engineer, and a longtime smoker. She describes their lives as simple – “few friends, few vacations, few problems, few regrets.” She states she misses her husband and often cries when thinking about him.
When asked about psychiatric diagnoses, she answered: “I have psychosis. … My doctor said maybe schizophrenia, but he is not sure yet.” She described schizophrenia as hearing voices. Rosalie also mentioned having memory problems: “They cannot tell if it is Alzheimer’s disease until I die and they look at my brain, but the medication should delay the progression.”
She reported no significant effect from her prior antidepressant trials: “I am not sure if or how they helped.” Rosalie could not explain the role of the medication. “I take medications as prescribed by my doctor,” she said.
When discussing her antipsychotics, she mentioned: “Those are strong medications; it is hard for me to stay awake with them.” She declared having had no changes in the voices while on the risperidone but said they went away since also being on the quetiapine: “I wonder if the combination of the two really fixed my brain imbalance.”
Assessment
I admit that I have a critical bias against the overuse of psychotropics, and this might have painted how I interpreted Rosalie’s story. Nonetheless, I was honest with her and told her of my concerns. I informed her that her diagnosis was not consistent with my understanding of mood and thought disorders. Her initial reports of depression neither met the DSM criteria for depression nor felt consistent with my conceptualization of the illness. She had retained appropriate functioning and seemed to be responding with the sadness expected when facing difficult challenges like grief.
Her subsequent reports of auditory hallucinations were not associated with delusions or forms of disorganization that I would expect in someone with a thought disorder. Furthermore, the context of the onset gave me the impression that this was part of her process of grief. Her poor result in the dementia screen was most surprising and inconsistent with my evaluation. I told her that I suspected that she was not suffering from Alzheimer’s but from being overmedicated and from anxiety at the time of the testing.
She was excited and hesitant about my report. She was surprised by the length of our visit and interested in hearing more from me. Strangely, I wished she had challenged my different approach. I think that I was hoping she would question my conceptualization, the way I hoped she would have done with her prior clinicians. Nonetheless, she agreed to make a plan with me.
Treatment plan
We decided to review each of her medications and discuss their benefits and risks over a couple of visits. She was most eager to discontinue the donepezil, which had caused diarrhea. She was concerned when I informed her of the potential side effects of antipsychotics. “My doctor asked me if I had any side effects at each visit; I answered that I felt nothing wrong; I had not realized that side effects could appear later.”
She was adamant about staying on buspirone, as she felt it helped her the most with her anxiety at social events. She voiced concern about discontinuing the antipsychotics despite being unsettled by my review of their risks. She asked that we taper them slowly.
In regard to receiving psychosocial support throughout this period of deprescribing, Rosalie declined weekly psychotherapy. She reported having a good social network in San Diego that she wanted to rely on.
Outcome
I often worry about consequences of stopping a medication, especially when I was not present at the time of its initiation. I agonize that the patient might relapse from my need to carry out my agenda on deprescribing. I try to remind myself that the evidence supports my decision making. The risks of psychotropics often are slow to show up, making the benefit of deprescribing less tangible. However, this case was straightforward.
Rosalie quickly improved. Tapering the antipsychotics was astonishing to her: “I can think clearly again.” Within 6 months, she was on buspirone only – though willing to discuss its discontinuation. She had a lead for a job and was hoping to return to work soon. Rosalie continued to miss her husband but had not heard him in some time. She has not had symptoms of psychosis or depression. Her cognition and mood were intact on my clinical assessment.
Discussion
Sadly and shockingly, cases like that of Rosalie are common. In my practice, I routinely see patients on multiple psychotropics – often on more than one antipsychotic. Their diagnoses are vague and dubious, and include diagnoses such as “unspecified psychosis” and “cognitive impairments.” Clinicians occasionally worry about relapse and promote a narrative that treatment must be not only long term but lifelong.4 There is some evidence for this perspective in a research context, but the clinical world also is filled with patients like Rosalie.
Her reports of auditory hallucinations were better explained by her grief than by a psychotic process.5 Her memory complaints were better explained by anxiety at the time of her testing while suffering from the side effects from her numerous psychotropics.6 Her depressive complaints were better explained by appropriate sadness in response to stressors. Several months later with fewer diagnoses and far fewer psychotropics, she is functioning better.
Take-home points
- Polypharmacy can lead to psychiatric symptoms and functional impairment.
- Patients often are unaware of the complete risks of psychotropics.
- Psychiatric symptoms are not always associated with a psychiatric disorder.
- Deprescribing can be performed safely and effectively.
- Deprescribing can be performed with the patient’s informed consent and agreement.
References
1. Psychiatr Serv. 2016 Aug 1;67(8):904-7.
2. Focus. 2016 Apr 13; doi: 10.1176/appi.focus.20150041.
3. Bipolar Disord. 2016 Dec;18(8):684-91.
4. Am J Psychiatry. 2017 Sep 1;174(9):840-9.
5. World Psychiatry. 2009 Jun;8(2):67-74.
6. Hosp Community Psychiatry. 1983 Sep;34(9):830-5.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019).
*This column was updated 1/11/2019.
In 2016, Swapnil Gupta, MD, and John Daniel Cahill, MD, PhD, challenged the field of psychiatry to reexamine our prescribing patterns. They warned against our use of polypharmacy when not attached to improvement in functioning for our patients.1 They were concerned about the lack of evidence for those treatment regimens and for our diagnostic criteria. In their inspiring article, they described how psychiatrists might proceed in the process of “deprescribing” – which they define as a process of pharmacologic regimen optimization through reducing or ending medications for which “benefits no longer outweigh risks.”1
In my practice, I routinely confront medication regimens that I have never encountered in the literature. The evidence for two psychotropics is limited but certainly available, in particular adjunct treatment of antidepressants2 and mood stabilizers.3 The evidence supporting the use of more than two psychotropics, however, is quite sparse. Yet, patients often enter my office on more than five psychotropics. I am also confronted with poorly defined diagnostic labels – which present more as means to justify polypharmacy than a thorough review of the patient’s current state.
Dr. Gupta and Dr. Cahill recommend a series of steps aimed at attempting the deprescription of psychotropics. Those steps include timeliness, knowledge of the patient’s current regimen, discussion about the risk of prescriptions, discussion about deprescribing, choosing the right medications to stop, a plan for describing, and monitoring. In the case presented below, I used some of those steps in an effort to provide the best care for the patient. Key details of the case have been changed, including the name, to protect the patient’s confidentiality.
Overview of the case
Rosalie Bertin is a 54-year-old female who has been treated for depression by a variety of primary care physicians for the better part of the last 30 years. She had tried an array of antidepressants, including sertraline, citalopram, duloxetine, and mirtazapine, over that time. Each seemed to provide some benefit when reviewing the notes, but there is no mention of why she was continued on those medications despite the absence of continuing symptoms. Occasionally, Rosalie would present to her clinician tearful and endorsing sadness, though the record did not comment on reports of energy, concentration, sleep, appetite, and interest.
In 2014, Rosalie’s husband passed away from lung cancer. His death was fairly quick, and initially, Rosalie did not mention any significant emotional complaints. However, when visiting her primary care physician 4 months later, she was noted to experience auditory hallucinations. “Sometimes I hear my husband when I am alone in my home,” she said. Rosalie was referred to a psychiatrist with a diagnosis of “psychosis not otherwise specified.”
When discussing her condition with the psychiatrist, Rosalie mentioned experiencing low mood, and having diminished interest in engaging in activities. “I miss Marc when I go places; I used to do everything with him.” She reported hearing him often but only when at her home. He would say things like, “I miss you,” or ask her about her day. She was diagnosed with “major depressive disorder with psychotic features.” Risperidone was added to the escitalopram, buspirone, and gabapentin that had been started by her primary care physician.
After several months of psychotropic management, the dose of risperidone was titrated to 8 mg per day. Her mood symptoms were unchanged, but she now was complaining of poor concentration and memory. The psychiatrist performed a Mini-Mental State Examination (MMSE). It was noted that taking the MMSE engendered significant anxiety for the patient. Rosalie received a score suggesting mild cognitive impairment. She was started on donepezil for the memory complaint, quetiapine for the continued voices, and recommended for disability.
Once on short-term disability, the patient relocated to live closer to her mother in San Diego and subsequently contacted me about continuing psychiatric care.
Initial visit
Rosalie is a petite white woman, raised in the Midwest, who married her high school sweetheart, and subsequently became an administrative assistant. Rosalie and Marc were unable to have children. Marc was an engineer, and a longtime smoker. She describes their lives as simple – “few friends, few vacations, few problems, few regrets.” She states she misses her husband and often cries when thinking about him.
When asked about psychiatric diagnoses, she answered: “I have psychosis. … My doctor said maybe schizophrenia, but he is not sure yet.” She described schizophrenia as hearing voices. Rosalie also mentioned having memory problems: “They cannot tell if it is Alzheimer’s disease until I die and they look at my brain, but the medication should delay the progression.”
She reported no significant effect from her prior antidepressant trials: “I am not sure if or how they helped.” Rosalie could not explain the role of the medication. “I take medications as prescribed by my doctor,” she said.
When discussing her antipsychotics, she mentioned: “Those are strong medications; it is hard for me to stay awake with them.” She declared having had no changes in the voices while on the risperidone but said they went away since also being on the quetiapine: “I wonder if the combination of the two really fixed my brain imbalance.”
Assessment
I admit that I have a critical bias against the overuse of psychotropics, and this might have painted how I interpreted Rosalie’s story. Nonetheless, I was honest with her and told her of my concerns. I informed her that her diagnosis was not consistent with my understanding of mood and thought disorders. Her initial reports of depression neither met the DSM criteria for depression nor felt consistent with my conceptualization of the illness. She had retained appropriate functioning and seemed to be responding with the sadness expected when facing difficult challenges like grief.
Her subsequent reports of auditory hallucinations were not associated with delusions or forms of disorganization that I would expect in someone with a thought disorder. Furthermore, the context of the onset gave me the impression that this was part of her process of grief. Her poor result in the dementia screen was most surprising and inconsistent with my evaluation. I told her that I suspected that she was not suffering from Alzheimer’s but from being overmedicated and from anxiety at the time of the testing.
She was excited and hesitant about my report. She was surprised by the length of our visit and interested in hearing more from me. Strangely, I wished she had challenged my different approach. I think that I was hoping she would question my conceptualization, the way I hoped she would have done with her prior clinicians. Nonetheless, she agreed to make a plan with me.
Treatment plan
We decided to review each of her medications and discuss their benefits and risks over a couple of visits. She was most eager to discontinue the donepezil, which had caused diarrhea. She was concerned when I informed her of the potential side effects of antipsychotics. “My doctor asked me if I had any side effects at each visit; I answered that I felt nothing wrong; I had not realized that side effects could appear later.”
She was adamant about staying on buspirone, as she felt it helped her the most with her anxiety at social events. She voiced concern about discontinuing the antipsychotics despite being unsettled by my review of their risks. She asked that we taper them slowly.
In regard to receiving psychosocial support throughout this period of deprescribing, Rosalie declined weekly psychotherapy. She reported having a good social network in San Diego that she wanted to rely on.
Outcome
I often worry about consequences of stopping a medication, especially when I was not present at the time of its initiation. I agonize that the patient might relapse from my need to carry out my agenda on deprescribing. I try to remind myself that the evidence supports my decision making. The risks of psychotropics often are slow to show up, making the benefit of deprescribing less tangible. However, this case was straightforward.
Rosalie quickly improved. Tapering the antipsychotics was astonishing to her: “I can think clearly again.” Within 6 months, she was on buspirone only – though willing to discuss its discontinuation. She had a lead for a job and was hoping to return to work soon. Rosalie continued to miss her husband but had not heard him in some time. She has not had symptoms of psychosis or depression. Her cognition and mood were intact on my clinical assessment.
Discussion
Sadly and shockingly, cases like that of Rosalie are common. In my practice, I routinely see patients on multiple psychotropics – often on more than one antipsychotic. Their diagnoses are vague and dubious, and include diagnoses such as “unspecified psychosis” and “cognitive impairments.” Clinicians occasionally worry about relapse and promote a narrative that treatment must be not only long term but lifelong.4 There is some evidence for this perspective in a research context, but the clinical world also is filled with patients like Rosalie.
Her reports of auditory hallucinations were better explained by her grief than by a psychotic process.5 Her memory complaints were better explained by anxiety at the time of her testing while suffering from the side effects from her numerous psychotropics.6 Her depressive complaints were better explained by appropriate sadness in response to stressors. Several months later with fewer diagnoses and far fewer psychotropics, she is functioning better.
Take-home points
- Polypharmacy can lead to psychiatric symptoms and functional impairment.
- Patients often are unaware of the complete risks of psychotropics.
- Psychiatric symptoms are not always associated with a psychiatric disorder.
- Deprescribing can be performed safely and effectively.
- Deprescribing can be performed with the patient’s informed consent and agreement.
References
1. Psychiatr Serv. 2016 Aug 1;67(8):904-7.
2. Focus. 2016 Apr 13; doi: 10.1176/appi.focus.20150041.
3. Bipolar Disord. 2016 Dec;18(8):684-91.
4. Am J Psychiatry. 2017 Sep 1;174(9):840-9.
5. World Psychiatry. 2009 Jun;8(2):67-74.
6. Hosp Community Psychiatry. 1983 Sep;34(9):830-5.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019).
*This column was updated 1/11/2019.
Prenatal valproate and ADHD
Also today, one expert calls for better ways to preserve beta cell function in youth, synthetic opioids drive a spike in the number of fatal overdoses, and mothers may play a role in the link between depression in fathers and daughters.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, one expert calls for better ways to preserve beta cell function in youth, synthetic opioids drive a spike in the number of fatal overdoses, and mothers may play a role in the link between depression in fathers and daughters.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, one expert calls for better ways to preserve beta cell function in youth, synthetic opioids drive a spike in the number of fatal overdoses, and mothers may play a role in the link between depression in fathers and daughters.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Synthetic opioids drive spike in U.S. fatal drug overdoses
New federal statistics suggest that the opioid epidemic in the United States is evolving as physicians crack down on the use of prescription painkillers: Fatal drug overdose deaths rose by 12% from 2016 to 2017, boosted by a wave of fatalities linked to illicit synthetic opioids like fentanyl that are now linked to an estimated 60% of opioid-related deaths.
“Overall, the overdose epidemic continues to worsen, and it has grown increasingly complex by coinvolvement of prescription and illicit drugs,” Lawrence Scholl, PhD, MPH, and his associates at the Centers for Disease Control & Prevention wrote in the Morbidity and Mortality Weekly Report.
The new statistics provide more evidence that 2017 marked “a sharp increase in what has characterized as the third wave of the opioid epidemic,” said drug and health policy researcher Stephen Crystal, PhD, of Rutgers University, New Brunswick, N.J., in an interview. He was referring to a wave that experts believe started in 2013 amid a spike in U.S. overdose deaths from fentanyl and other synthetic opioids.
The new report analyzes fatal drug overdose data from 2013 to 2017. According to the findings, the total number of those overdoses rose to 70,237 in 2017, up from 63,632 in 2016. The highest drug overdose death rates in 2017 were in West Virginia, followed by Ohio, Pennsylvania, and the District of Columbia.
Some statistics did not change much from 2016 to 2017: About two-thirds of the drug overdose deaths were linked to opioids in both years, and the death rate of cases linked to prescription drugs and heroin remained steady. (Death rates in the report were age adjusted.)
However, the percentage of fatal overdose cases linked to synthetic opioids grew 45% from 2016 to 2017. Overall, 60% of opioid-related fatal overdoses in 2017 involved synthetic opioids.
The report identifies increases in several areas from 2016 to 2017. Opioid-related drug overdose deaths among black people rose by 25%, and an analysis of data from 34 states and the District of Columbia found the highest increases in death rates in North Carolina (29%), Ohio (19%), and Maine (19%).
In regard to deaths linked to synthetic opioids specifically, the highest death rates in 2017 were in West Virginia (37 per 100,000), Ohio (32 per 100,000), and New Hampshire (30 per 100,000).
“Part of what we’re seeing in these increased numbers are individuals who have pain, can’t get prescribed opioids, and turn to street drugs,” Dr. Crystal said, adding that “abruptly cutting patients off is not good, and leaving patients with a lot of untreated pain is not good. If people are going to be discontinued [from opioids] or have their doses reduced, the taper needs to be done very slowly and carefully.”
Synthetic opioids were not the only drugs that are driving up fatal overdoses, as the death rates of cases linked to cocaine and psychostimulants (such as methamphetamine) jumped by more than a third in 2017.
“The most important thing these numbers are telling me is that it’s becoming more and more attractive to drug dealers to put fentanyl in the heroin, cocaine, and other drugs they sell,” Dr. Crystal said. “When that happens, dependence on street drugs becomes much more deadly. It’s almost impossible to get the dose right. Every time you shoot up, you’re taking a chance that you’ll overdose.”
The report had limitations, including the fact that details about drug use were missing from 12% (2016) and 15% (2017) of death certificates in fatal overdose cases. By state, the percentages of those death certificates that included drug information ranged from as little as 55% to 99%.
There’s some possible positive news: The report points to preliminary data from 2018 suggesting that the number of annual drug overdose deaths may be leveling off – although it says more analysis is needed to confirm the trend.
Dr. Crystal, however, is not celebrating. “I don’t see this as a good news story, really,” he said, adding that there’s “a little too much of people patting themselves on the back” because they’re proud of cutbacks in opioid prescriptions.
“This doesn’t have to do with the huge number of people who got started with opioids years ago” and are now at risk of using street drugs, he said. “We haven’t engaged that population at the rate we need to. And flattening out at 70,000 drug overdoses a year is not a good news story.”
Dr. Crystal reported no relevant disclosures.
SOURCE: Scholl L et al. MMWR. 2019 Jan 4;67(5152):1419-27.
New federal statistics suggest that the opioid epidemic in the United States is evolving as physicians crack down on the use of prescription painkillers: Fatal drug overdose deaths rose by 12% from 2016 to 2017, boosted by a wave of fatalities linked to illicit synthetic opioids like fentanyl that are now linked to an estimated 60% of opioid-related deaths.
“Overall, the overdose epidemic continues to worsen, and it has grown increasingly complex by coinvolvement of prescription and illicit drugs,” Lawrence Scholl, PhD, MPH, and his associates at the Centers for Disease Control & Prevention wrote in the Morbidity and Mortality Weekly Report.
The new statistics provide more evidence that 2017 marked “a sharp increase in what has characterized as the third wave of the opioid epidemic,” said drug and health policy researcher Stephen Crystal, PhD, of Rutgers University, New Brunswick, N.J., in an interview. He was referring to a wave that experts believe started in 2013 amid a spike in U.S. overdose deaths from fentanyl and other synthetic opioids.
The new report analyzes fatal drug overdose data from 2013 to 2017. According to the findings, the total number of those overdoses rose to 70,237 in 2017, up from 63,632 in 2016. The highest drug overdose death rates in 2017 were in West Virginia, followed by Ohio, Pennsylvania, and the District of Columbia.
Some statistics did not change much from 2016 to 2017: About two-thirds of the drug overdose deaths were linked to opioids in both years, and the death rate of cases linked to prescription drugs and heroin remained steady. (Death rates in the report were age adjusted.)
However, the percentage of fatal overdose cases linked to synthetic opioids grew 45% from 2016 to 2017. Overall, 60% of opioid-related fatal overdoses in 2017 involved synthetic opioids.
The report identifies increases in several areas from 2016 to 2017. Opioid-related drug overdose deaths among black people rose by 25%, and an analysis of data from 34 states and the District of Columbia found the highest increases in death rates in North Carolina (29%), Ohio (19%), and Maine (19%).
In regard to deaths linked to synthetic opioids specifically, the highest death rates in 2017 were in West Virginia (37 per 100,000), Ohio (32 per 100,000), and New Hampshire (30 per 100,000).
“Part of what we’re seeing in these increased numbers are individuals who have pain, can’t get prescribed opioids, and turn to street drugs,” Dr. Crystal said, adding that “abruptly cutting patients off is not good, and leaving patients with a lot of untreated pain is not good. If people are going to be discontinued [from opioids] or have their doses reduced, the taper needs to be done very slowly and carefully.”
Synthetic opioids were not the only drugs that are driving up fatal overdoses, as the death rates of cases linked to cocaine and psychostimulants (such as methamphetamine) jumped by more than a third in 2017.
“The most important thing these numbers are telling me is that it’s becoming more and more attractive to drug dealers to put fentanyl in the heroin, cocaine, and other drugs they sell,” Dr. Crystal said. “When that happens, dependence on street drugs becomes much more deadly. It’s almost impossible to get the dose right. Every time you shoot up, you’re taking a chance that you’ll overdose.”
The report had limitations, including the fact that details about drug use were missing from 12% (2016) and 15% (2017) of death certificates in fatal overdose cases. By state, the percentages of those death certificates that included drug information ranged from as little as 55% to 99%.
There’s some possible positive news: The report points to preliminary data from 2018 suggesting that the number of annual drug overdose deaths may be leveling off – although it says more analysis is needed to confirm the trend.
Dr. Crystal, however, is not celebrating. “I don’t see this as a good news story, really,” he said, adding that there’s “a little too much of people patting themselves on the back” because they’re proud of cutbacks in opioid prescriptions.
“This doesn’t have to do with the huge number of people who got started with opioids years ago” and are now at risk of using street drugs, he said. “We haven’t engaged that population at the rate we need to. And flattening out at 70,000 drug overdoses a year is not a good news story.”
Dr. Crystal reported no relevant disclosures.
SOURCE: Scholl L et al. MMWR. 2019 Jan 4;67(5152):1419-27.
New federal statistics suggest that the opioid epidemic in the United States is evolving as physicians crack down on the use of prescription painkillers: Fatal drug overdose deaths rose by 12% from 2016 to 2017, boosted by a wave of fatalities linked to illicit synthetic opioids like fentanyl that are now linked to an estimated 60% of opioid-related deaths.
“Overall, the overdose epidemic continues to worsen, and it has grown increasingly complex by coinvolvement of prescription and illicit drugs,” Lawrence Scholl, PhD, MPH, and his associates at the Centers for Disease Control & Prevention wrote in the Morbidity and Mortality Weekly Report.
The new statistics provide more evidence that 2017 marked “a sharp increase in what has characterized as the third wave of the opioid epidemic,” said drug and health policy researcher Stephen Crystal, PhD, of Rutgers University, New Brunswick, N.J., in an interview. He was referring to a wave that experts believe started in 2013 amid a spike in U.S. overdose deaths from fentanyl and other synthetic opioids.
The new report analyzes fatal drug overdose data from 2013 to 2017. According to the findings, the total number of those overdoses rose to 70,237 in 2017, up from 63,632 in 2016. The highest drug overdose death rates in 2017 were in West Virginia, followed by Ohio, Pennsylvania, and the District of Columbia.
Some statistics did not change much from 2016 to 2017: About two-thirds of the drug overdose deaths were linked to opioids in both years, and the death rate of cases linked to prescription drugs and heroin remained steady. (Death rates in the report were age adjusted.)
However, the percentage of fatal overdose cases linked to synthetic opioids grew 45% from 2016 to 2017. Overall, 60% of opioid-related fatal overdoses in 2017 involved synthetic opioids.
The report identifies increases in several areas from 2016 to 2017. Opioid-related drug overdose deaths among black people rose by 25%, and an analysis of data from 34 states and the District of Columbia found the highest increases in death rates in North Carolina (29%), Ohio (19%), and Maine (19%).
In regard to deaths linked to synthetic opioids specifically, the highest death rates in 2017 were in West Virginia (37 per 100,000), Ohio (32 per 100,000), and New Hampshire (30 per 100,000).
“Part of what we’re seeing in these increased numbers are individuals who have pain, can’t get prescribed opioids, and turn to street drugs,” Dr. Crystal said, adding that “abruptly cutting patients off is not good, and leaving patients with a lot of untreated pain is not good. If people are going to be discontinued [from opioids] or have their doses reduced, the taper needs to be done very slowly and carefully.”
Synthetic opioids were not the only drugs that are driving up fatal overdoses, as the death rates of cases linked to cocaine and psychostimulants (such as methamphetamine) jumped by more than a third in 2017.
“The most important thing these numbers are telling me is that it’s becoming more and more attractive to drug dealers to put fentanyl in the heroin, cocaine, and other drugs they sell,” Dr. Crystal said. “When that happens, dependence on street drugs becomes much more deadly. It’s almost impossible to get the dose right. Every time you shoot up, you’re taking a chance that you’ll overdose.”
The report had limitations, including the fact that details about drug use were missing from 12% (2016) and 15% (2017) of death certificates in fatal overdose cases. By state, the percentages of those death certificates that included drug information ranged from as little as 55% to 99%.
There’s some possible positive news: The report points to preliminary data from 2018 suggesting that the number of annual drug overdose deaths may be leveling off – although it says more analysis is needed to confirm the trend.
Dr. Crystal, however, is not celebrating. “I don’t see this as a good news story, really,” he said, adding that there’s “a little too much of people patting themselves on the back” because they’re proud of cutbacks in opioid prescriptions.
“This doesn’t have to do with the huge number of people who got started with opioids years ago” and are now at risk of using street drugs, he said. “We haven’t engaged that population at the rate we need to. And flattening out at 70,000 drug overdoses a year is not a good news story.”
Dr. Crystal reported no relevant disclosures.
SOURCE: Scholl L et al. MMWR. 2019 Jan 4;67(5152):1419-27.
FROM MMWR
Liquid nicotine in e-cigarettes could prove more addictive; gratitude tied to less anxiety, depression
The image of inhaling the vapor from electronic cigarettes – vaping – is presented by some as an innocuous substitute to smoking traditional cigarettes. It is true that vaping might pose less danger than cigarettes and can wean people off smoking,
“Oh man, [withdrawal] was hell,” said Andrea “Nick” Tattanelli, a 39-year-old mortgage banker who reported engaging in vaping for more than 20 years, in a USA Today article. Mr. Tattanelli said quitting left him depressed.
Malissa M. Barbosa, DO, an addiction medicine specialist, wonders whether vaping is the best way to get patients to stop smoking. “The thing is, the studies aren’t fully available around vaping, and I’m very conservative. This is new, and I say, ‘Why aren’t we thinking of traditional means of quitting?’ ”
Vaping is more addictive than smoking traditional cigarettes “because the concentrated liquid form is more quickly metabolized,” said Dr. Barbosa, area medical director of CleanSlate Outpatient Addiction Medicine in Orlando.
And as the number of vapers grows, evidence is mounting that, rather than using it as a stepping stone to becoming nicotine-free, vaping is increasingly being used by adolescents as a form of delivering nicotine.
“We know how hard it is to quit smoking,” said Michael J. Blaha, MD, MPH, a cardiologist who serves as director of clinical research at the Ciccarone Center for the Prevention of Heart Disease at Johns Hopkins University, Baltimore. “[With vaping], we’re really dealing with much of the same problem. Early on, there were some reports vaping was less addictive, but that’s still something that can be debated.”
In the United States, vapers include nearly 4 million middle and high school students. Surgeon General Jerome M. Adams, MD, MPH, has suggested raising prices as a strategy aimed at curbing adolescent use.
Impact of gratitude on the brain
The beginning of a new year can be a time for reflection that can include a sense of gratitude for a relatively happy and secure life. And, according to an article at theconversation.com, the ability to have a sense of gratitude is good for well-being.
“Not only does gratitude go along with more optimism, less anxiety and depression, and greater goal attainment, but it’s also associated with fewer symptoms of illness and other physical benefits;” wrote Christina Karns, PhD, research associate in psychology at the University of Oregon, Portland.
A feeling of gratitude stimulates a part of the brain that controls the release of neurochemicals that confer pleasure. The benefits of gratitude aren’t just between the ears. Feeling gratitude can motivate people to pay it forward as altruistic behavior that helps others. Put another way, feeling good about life can trigger kindness.
Research by Dr. Karns and her colleagues also has demonstrated that this link between personal good feeling and altruism can be learned and accentuated. “So in terms of the brain’s reward response, it really can be true that giving is better than receiving,” wrote Dr. Karns, who also is affiliated with the Center for Brain Injury Research and Training at the university.
Imagine if the recipients of such goodwill, in turn, did some good for others, and they for others, and so on.
Did talk radio host save a life?
Talk radio can be filled with acrimony and argument – but it also can save lives. As reported in the Guardian, a show hosted by British TV and radio personality Iain Lee is different in that Mr. Lee sometimes connects with his audience by riffing on his own struggles with depression. A recent show extended the audience connection in a lifesaving way.
Mr. Lee received a call from a listener who reported overdosing on drugs with the intent of suicide. In hearing of that intent, Mr. Lee kept the caller on the line for 30 minutes. At one point, he responded: “Shut up, man, I know you want to die, brother, but I love you. I love you. You may want to die, but we can talk about that tomorrow.”
The response got through to the caller, who reportedly lay on the pavement outside a nightclub. Meanwhile, the call was being traced, and emergency medical personnel responded.
When Mr. Lee learned that the caller had been located and was still alive, he broke down on air. Later, he tweeted: “Tonight we took a call from a man who had taken an overdose … Long periods of silence where I thought he’d died. That was intense and upsetting. Thanks for your kind words. I really hope he makes it.”
A trip to Walmart can include therapy
A Walmart in Carrollton, Tex., is trying out a new service for customers: It is including an on-site mental health clinic. As reported by the Dallas Morning News, the idea is to make mental health care convenient and bring people who otherwise might forgo help through the clinic door.
“Twenty years ago, we would never imagine going to a retail location for a flu shot. You’d make an appointment with your primary care,” said Russell Petrella, chief executive of Beacon Health Options, which runs the in-store clinic. “The idea of bringing these services to places where consumers – potential patients – are more comfortable is getting more and more accepted.”
Initially, therapy was $25 for a 45-minute session with an individual or family. Prices will rise to $110 for an individual and $125 for a family early in this year. Lower prices are available for people who demonstrate a financial need.
The location for this trial run was deliberate. Texas has a disproportionately large number of residents without mental health care, ranking 49th in the nation, according to a 2018 report by Mental Health America.
Greg Hansch, public policy director of the National Alliance on Mental Illness in Texas, said he is encouraged by novel types of care like the Walmart clinic. He would like to see further integration of mental health care into schools, workplaces, and other retailers. “You remove some of that stigma if you can make services part of a person’s everyday routine,” he said.
Smartphones and the teenage brain
The explosion in smartphone use since 2012 has coincided with increased rates of depression in adolescents. Reduced sleep might be one reason. Teenagers in the United States routinely rack up 6 hours a day on social media, which includes texting and other online activities. “For teens in particular, it’s catnip,” said Jean M. Twenge, PhD, professor of psychology at San Diego State University and author of “I-Gen: Why Today’s Super-Connected Kids Are Growing Up Less Rebellious, More Tolerant, Less Happy – and Completely Unprepared for Adulthood” (Atria Books, 2017).
A smartphone is no substitute for face-to-face interactions, and offers little training in verbal communication and problem solving. A consequence of a smartphone-connected youth, according to Dr. Twenge, could be worsened mental health.
But there is some good news. Some teens are working to curb their smartphone use. Stopping the use of a smartphone as a relief for boredom, setting self-imposed time limits of phone use, and not succumbing to the wired world’s tendency to ratchet up anxiety are helpful strategies that can make smartphone use more productive.
The image of inhaling the vapor from electronic cigarettes – vaping – is presented by some as an innocuous substitute to smoking traditional cigarettes. It is true that vaping might pose less danger than cigarettes and can wean people off smoking,
“Oh man, [withdrawal] was hell,” said Andrea “Nick” Tattanelli, a 39-year-old mortgage banker who reported engaging in vaping for more than 20 years, in a USA Today article. Mr. Tattanelli said quitting left him depressed.
Malissa M. Barbosa, DO, an addiction medicine specialist, wonders whether vaping is the best way to get patients to stop smoking. “The thing is, the studies aren’t fully available around vaping, and I’m very conservative. This is new, and I say, ‘Why aren’t we thinking of traditional means of quitting?’ ”
Vaping is more addictive than smoking traditional cigarettes “because the concentrated liquid form is more quickly metabolized,” said Dr. Barbosa, area medical director of CleanSlate Outpatient Addiction Medicine in Orlando.
And as the number of vapers grows, evidence is mounting that, rather than using it as a stepping stone to becoming nicotine-free, vaping is increasingly being used by adolescents as a form of delivering nicotine.
“We know how hard it is to quit smoking,” said Michael J. Blaha, MD, MPH, a cardiologist who serves as director of clinical research at the Ciccarone Center for the Prevention of Heart Disease at Johns Hopkins University, Baltimore. “[With vaping], we’re really dealing with much of the same problem. Early on, there were some reports vaping was less addictive, but that’s still something that can be debated.”
In the United States, vapers include nearly 4 million middle and high school students. Surgeon General Jerome M. Adams, MD, MPH, has suggested raising prices as a strategy aimed at curbing adolescent use.
Impact of gratitude on the brain
The beginning of a new year can be a time for reflection that can include a sense of gratitude for a relatively happy and secure life. And, according to an article at theconversation.com, the ability to have a sense of gratitude is good for well-being.
“Not only does gratitude go along with more optimism, less anxiety and depression, and greater goal attainment, but it’s also associated with fewer symptoms of illness and other physical benefits;” wrote Christina Karns, PhD, research associate in psychology at the University of Oregon, Portland.
A feeling of gratitude stimulates a part of the brain that controls the release of neurochemicals that confer pleasure. The benefits of gratitude aren’t just between the ears. Feeling gratitude can motivate people to pay it forward as altruistic behavior that helps others. Put another way, feeling good about life can trigger kindness.
Research by Dr. Karns and her colleagues also has demonstrated that this link between personal good feeling and altruism can be learned and accentuated. “So in terms of the brain’s reward response, it really can be true that giving is better than receiving,” wrote Dr. Karns, who also is affiliated with the Center for Brain Injury Research and Training at the university.
Imagine if the recipients of such goodwill, in turn, did some good for others, and they for others, and so on.
Did talk radio host save a life?
Talk radio can be filled with acrimony and argument – but it also can save lives. As reported in the Guardian, a show hosted by British TV and radio personality Iain Lee is different in that Mr. Lee sometimes connects with his audience by riffing on his own struggles with depression. A recent show extended the audience connection in a lifesaving way.
Mr. Lee received a call from a listener who reported overdosing on drugs with the intent of suicide. In hearing of that intent, Mr. Lee kept the caller on the line for 30 minutes. At one point, he responded: “Shut up, man, I know you want to die, brother, but I love you. I love you. You may want to die, but we can talk about that tomorrow.”
The response got through to the caller, who reportedly lay on the pavement outside a nightclub. Meanwhile, the call was being traced, and emergency medical personnel responded.
When Mr. Lee learned that the caller had been located and was still alive, he broke down on air. Later, he tweeted: “Tonight we took a call from a man who had taken an overdose … Long periods of silence where I thought he’d died. That was intense and upsetting. Thanks for your kind words. I really hope he makes it.”
A trip to Walmart can include therapy
A Walmart in Carrollton, Tex., is trying out a new service for customers: It is including an on-site mental health clinic. As reported by the Dallas Morning News, the idea is to make mental health care convenient and bring people who otherwise might forgo help through the clinic door.
“Twenty years ago, we would never imagine going to a retail location for a flu shot. You’d make an appointment with your primary care,” said Russell Petrella, chief executive of Beacon Health Options, which runs the in-store clinic. “The idea of bringing these services to places where consumers – potential patients – are more comfortable is getting more and more accepted.”
Initially, therapy was $25 for a 45-minute session with an individual or family. Prices will rise to $110 for an individual and $125 for a family early in this year. Lower prices are available for people who demonstrate a financial need.
The location for this trial run was deliberate. Texas has a disproportionately large number of residents without mental health care, ranking 49th in the nation, according to a 2018 report by Mental Health America.
Greg Hansch, public policy director of the National Alliance on Mental Illness in Texas, said he is encouraged by novel types of care like the Walmart clinic. He would like to see further integration of mental health care into schools, workplaces, and other retailers. “You remove some of that stigma if you can make services part of a person’s everyday routine,” he said.
Smartphones and the teenage brain
The explosion in smartphone use since 2012 has coincided with increased rates of depression in adolescents. Reduced sleep might be one reason. Teenagers in the United States routinely rack up 6 hours a day on social media, which includes texting and other online activities. “For teens in particular, it’s catnip,” said Jean M. Twenge, PhD, professor of psychology at San Diego State University and author of “I-Gen: Why Today’s Super-Connected Kids Are Growing Up Less Rebellious, More Tolerant, Less Happy – and Completely Unprepared for Adulthood” (Atria Books, 2017).
A smartphone is no substitute for face-to-face interactions, and offers little training in verbal communication and problem solving. A consequence of a smartphone-connected youth, according to Dr. Twenge, could be worsened mental health.
But there is some good news. Some teens are working to curb their smartphone use. Stopping the use of a smartphone as a relief for boredom, setting self-imposed time limits of phone use, and not succumbing to the wired world’s tendency to ratchet up anxiety are helpful strategies that can make smartphone use more productive.
The image of inhaling the vapor from electronic cigarettes – vaping – is presented by some as an innocuous substitute to smoking traditional cigarettes. It is true that vaping might pose less danger than cigarettes and can wean people off smoking,
“Oh man, [withdrawal] was hell,” said Andrea “Nick” Tattanelli, a 39-year-old mortgage banker who reported engaging in vaping for more than 20 years, in a USA Today article. Mr. Tattanelli said quitting left him depressed.
Malissa M. Barbosa, DO, an addiction medicine specialist, wonders whether vaping is the best way to get patients to stop smoking. “The thing is, the studies aren’t fully available around vaping, and I’m very conservative. This is new, and I say, ‘Why aren’t we thinking of traditional means of quitting?’ ”
Vaping is more addictive than smoking traditional cigarettes “because the concentrated liquid form is more quickly metabolized,” said Dr. Barbosa, area medical director of CleanSlate Outpatient Addiction Medicine in Orlando.
And as the number of vapers grows, evidence is mounting that, rather than using it as a stepping stone to becoming nicotine-free, vaping is increasingly being used by adolescents as a form of delivering nicotine.
“We know how hard it is to quit smoking,” said Michael J. Blaha, MD, MPH, a cardiologist who serves as director of clinical research at the Ciccarone Center for the Prevention of Heart Disease at Johns Hopkins University, Baltimore. “[With vaping], we’re really dealing with much of the same problem. Early on, there were some reports vaping was less addictive, but that’s still something that can be debated.”
In the United States, vapers include nearly 4 million middle and high school students. Surgeon General Jerome M. Adams, MD, MPH, has suggested raising prices as a strategy aimed at curbing adolescent use.
Impact of gratitude on the brain
The beginning of a new year can be a time for reflection that can include a sense of gratitude for a relatively happy and secure life. And, according to an article at theconversation.com, the ability to have a sense of gratitude is good for well-being.
“Not only does gratitude go along with more optimism, less anxiety and depression, and greater goal attainment, but it’s also associated with fewer symptoms of illness and other physical benefits;” wrote Christina Karns, PhD, research associate in psychology at the University of Oregon, Portland.
A feeling of gratitude stimulates a part of the brain that controls the release of neurochemicals that confer pleasure. The benefits of gratitude aren’t just between the ears. Feeling gratitude can motivate people to pay it forward as altruistic behavior that helps others. Put another way, feeling good about life can trigger kindness.
Research by Dr. Karns and her colleagues also has demonstrated that this link between personal good feeling and altruism can be learned and accentuated. “So in terms of the brain’s reward response, it really can be true that giving is better than receiving,” wrote Dr. Karns, who also is affiliated with the Center for Brain Injury Research and Training at the university.
Imagine if the recipients of such goodwill, in turn, did some good for others, and they for others, and so on.
Did talk radio host save a life?
Talk radio can be filled with acrimony and argument – but it also can save lives. As reported in the Guardian, a show hosted by British TV and radio personality Iain Lee is different in that Mr. Lee sometimes connects with his audience by riffing on his own struggles with depression. A recent show extended the audience connection in a lifesaving way.
Mr. Lee received a call from a listener who reported overdosing on drugs with the intent of suicide. In hearing of that intent, Mr. Lee kept the caller on the line for 30 minutes. At one point, he responded: “Shut up, man, I know you want to die, brother, but I love you. I love you. You may want to die, but we can talk about that tomorrow.”
The response got through to the caller, who reportedly lay on the pavement outside a nightclub. Meanwhile, the call was being traced, and emergency medical personnel responded.
When Mr. Lee learned that the caller had been located and was still alive, he broke down on air. Later, he tweeted: “Tonight we took a call from a man who had taken an overdose … Long periods of silence where I thought he’d died. That was intense and upsetting. Thanks for your kind words. I really hope he makes it.”
A trip to Walmart can include therapy
A Walmart in Carrollton, Tex., is trying out a new service for customers: It is including an on-site mental health clinic. As reported by the Dallas Morning News, the idea is to make mental health care convenient and bring people who otherwise might forgo help through the clinic door.
“Twenty years ago, we would never imagine going to a retail location for a flu shot. You’d make an appointment with your primary care,” said Russell Petrella, chief executive of Beacon Health Options, which runs the in-store clinic. “The idea of bringing these services to places where consumers – potential patients – are more comfortable is getting more and more accepted.”
Initially, therapy was $25 for a 45-minute session with an individual or family. Prices will rise to $110 for an individual and $125 for a family early in this year. Lower prices are available for people who demonstrate a financial need.
The location for this trial run was deliberate. Texas has a disproportionately large number of residents without mental health care, ranking 49th in the nation, according to a 2018 report by Mental Health America.
Greg Hansch, public policy director of the National Alliance on Mental Illness in Texas, said he is encouraged by novel types of care like the Walmart clinic. He would like to see further integration of mental health care into schools, workplaces, and other retailers. “You remove some of that stigma if you can make services part of a person’s everyday routine,” he said.
Smartphones and the teenage brain
The explosion in smartphone use since 2012 has coincided with increased rates of depression in adolescents. Reduced sleep might be one reason. Teenagers in the United States routinely rack up 6 hours a day on social media, which includes texting and other online activities. “For teens in particular, it’s catnip,” said Jean M. Twenge, PhD, professor of psychology at San Diego State University and author of “I-Gen: Why Today’s Super-Connected Kids Are Growing Up Less Rebellious, More Tolerant, Less Happy – and Completely Unprepared for Adulthood” (Atria Books, 2017).
A smartphone is no substitute for face-to-face interactions, and offers little training in verbal communication and problem solving. A consequence of a smartphone-connected youth, according to Dr. Twenge, could be worsened mental health.
But there is some good news. Some teens are working to curb their smartphone use. Stopping the use of a smartphone as a relief for boredom, setting self-imposed time limits of phone use, and not succumbing to the wired world’s tendency to ratchet up anxiety are helpful strategies that can make smartphone use more productive.
The gift of misery
On the first day of my psychiatry clerkship, I sat at a table with another student, 2 residents, and our attending physician. This wasn’t my first clinical rotation, but it was my first formal exposure to psychiatry, and I was excited and a bit anxious because I was considering psychiatry as an area of specialty training for myself. I’d been assigned 1 patient that morning: a 42-year-old man admitted for alcohol withdrawal. Our team, the psychiatry consultation-liaison team, was asked to evaluate the patient’s depressed mood in the context of withdrawal. As I began to present the patient’s story, I spoke of how terrible this man’s life had been, and how depressed he had recently become; this depression, I said, was likely exacerbated by alcohol use, but he was dealing with his depression by drinking more. He now wanted to quit for good. My attending, whom I had just met, interrupted me: “Misery,” she said with an intense look, “is a gift to an addicted person.”
I have ruminated on those surprising words ever since, and in that time I have begun to understand something about misery through the eyes of my patients. Sick people often are miserable; physical ailments can wreck hopes and plans and suck the joy from seemingly everything. Individuals who are ill or in pain often are suffering psychologically as well as physically. This suffering has been especially apparent to me in patients withdrawing from addictive substances: alcohol, cocaine, heroin, nicotine. I have been begged, cursed, praised, thanked, and more based on my ability or inability to relieve someone’s suffering caused by the lack of a certain substance: Please, just one cigarette. Please, something for this pain. Please, something to drink. As a medical student, I did one of 2 things: stood there helpless, or promised I would do the best I could, knowing my resident or attending would likely tell them no.
Withdrawal from addictive substances is, unsurprisingly, not pleasant. Alcohol withdrawal is one of the few that can be fatal, due to its ability to cause autonomic instability and seizures. Withdrawing from alcohol is also unpleasant due to hallucinosis and tremors, on top of the very real cravings for the substance itself. My patient knew this; he had withdrawn from alcohol in the past. As he talked to me, though, it became clear he had finally decided this was the end. In the past, others encouraged him to stop drinking; this time he was doing it for himself. His life had become so dismal that he was willing to undergo the agony of withdrawal to be free from his addiction.
Was his suffering, then, his misery, a gift? As I came to know my attending better, I also came to understand what these jarring words meant to her. They were her version of the old adage: It’s only when you hit rock bottom that you can start climbing back out. It isn’t the misery of withdrawing, but the misery inflicted by the substance that might provide an unexpected opportunity to start fixing things. For my patient, this particular trip to the hospital—which happened to intersect in space and time with me, a third-year medical student keen to learn and to help—was rock bottom, and he knew it. His life had been destroyed by his addiction, and here, at this intersection, the destruction was so great that he was finally willing to make a change for the better.
It is counterintuitive to think of misery as a gift, but then again, this patient—and more broadly, all patients whose lives are tormented by addiction and substance abuse—are often on the receiving end of counterintuitive advice, and it is frequently the only way to enact lasting change. Consider, for example, Alcoholics’ Anonymous, which works for far more individuals than one might expect. It does not seem possible that a small group without formal training could keep people sober simply by talking openly about their struggles; yet every day throughout the world, it does just that.
Patients struggling with addiction—labeled as addicts and drug-seekers by most of the world—are often written off as “difficult patients.” Perhaps because of my inexperience, I didn’t see this man as difficult, or as just another case of alcohol withdrawal. Although it may often be easier to define someone by his or her disease, I believe in choosing to see the human underneath the label. To me, these patients are not difficult; they are broken and miserable, and they desperately need help. Knowing this, I am forced to consider just how bad things have gotten for them, and how hard it must be to make a change. Their brokenness may be an opportunity to start down a new path, but only if we extend that invitation. Such an invitation may be the first step to turning genuine misery into a gift.
When I’m asked why I have chosen psychiatry, willingly entering such a “difficult field,” I think about my experience on that consult service and this patient. I know that I’m still just beginning my journey, and that even more difficult moments and patients lie ahead. But difficulty depends on one’s perspective; certainly that patient, trying to free himself from addiction’s grasp, was “going through a difficult time.” This is of course a platitude; the word “misery” gets much closer to the truth. I usually answer with some variation of the following: Medicine, especially psychiatry, is about caring for those who need it most: hurting, vulnera
On the first day of my psychiatry clerkship, I sat at a table with another student, 2 residents, and our attending physician. This wasn’t my first clinical rotation, but it was my first formal exposure to psychiatry, and I was excited and a bit anxious because I was considering psychiatry as an area of specialty training for myself. I’d been assigned 1 patient that morning: a 42-year-old man admitted for alcohol withdrawal. Our team, the psychiatry consultation-liaison team, was asked to evaluate the patient’s depressed mood in the context of withdrawal. As I began to present the patient’s story, I spoke of how terrible this man’s life had been, and how depressed he had recently become; this depression, I said, was likely exacerbated by alcohol use, but he was dealing with his depression by drinking more. He now wanted to quit for good. My attending, whom I had just met, interrupted me: “Misery,” she said with an intense look, “is a gift to an addicted person.”
I have ruminated on those surprising words ever since, and in that time I have begun to understand something about misery through the eyes of my patients. Sick people often are miserable; physical ailments can wreck hopes and plans and suck the joy from seemingly everything. Individuals who are ill or in pain often are suffering psychologically as well as physically. This suffering has been especially apparent to me in patients withdrawing from addictive substances: alcohol, cocaine, heroin, nicotine. I have been begged, cursed, praised, thanked, and more based on my ability or inability to relieve someone’s suffering caused by the lack of a certain substance: Please, just one cigarette. Please, something for this pain. Please, something to drink. As a medical student, I did one of 2 things: stood there helpless, or promised I would do the best I could, knowing my resident or attending would likely tell them no.
Withdrawal from addictive substances is, unsurprisingly, not pleasant. Alcohol withdrawal is one of the few that can be fatal, due to its ability to cause autonomic instability and seizures. Withdrawing from alcohol is also unpleasant due to hallucinosis and tremors, on top of the very real cravings for the substance itself. My patient knew this; he had withdrawn from alcohol in the past. As he talked to me, though, it became clear he had finally decided this was the end. In the past, others encouraged him to stop drinking; this time he was doing it for himself. His life had become so dismal that he was willing to undergo the agony of withdrawal to be free from his addiction.
Was his suffering, then, his misery, a gift? As I came to know my attending better, I also came to understand what these jarring words meant to her. They were her version of the old adage: It’s only when you hit rock bottom that you can start climbing back out. It isn’t the misery of withdrawing, but the misery inflicted by the substance that might provide an unexpected opportunity to start fixing things. For my patient, this particular trip to the hospital—which happened to intersect in space and time with me, a third-year medical student keen to learn and to help—was rock bottom, and he knew it. His life had been destroyed by his addiction, and here, at this intersection, the destruction was so great that he was finally willing to make a change for the better.
It is counterintuitive to think of misery as a gift, but then again, this patient—and more broadly, all patients whose lives are tormented by addiction and substance abuse—are often on the receiving end of counterintuitive advice, and it is frequently the only way to enact lasting change. Consider, for example, Alcoholics’ Anonymous, which works for far more individuals than one might expect. It does not seem possible that a small group without formal training could keep people sober simply by talking openly about their struggles; yet every day throughout the world, it does just that.
Patients struggling with addiction—labeled as addicts and drug-seekers by most of the world—are often written off as “difficult patients.” Perhaps because of my inexperience, I didn’t see this man as difficult, or as just another case of alcohol withdrawal. Although it may often be easier to define someone by his or her disease, I believe in choosing to see the human underneath the label. To me, these patients are not difficult; they are broken and miserable, and they desperately need help. Knowing this, I am forced to consider just how bad things have gotten for them, and how hard it must be to make a change. Their brokenness may be an opportunity to start down a new path, but only if we extend that invitation. Such an invitation may be the first step to turning genuine misery into a gift.
When I’m asked why I have chosen psychiatry, willingly entering such a “difficult field,” I think about my experience on that consult service and this patient. I know that I’m still just beginning my journey, and that even more difficult moments and patients lie ahead. But difficulty depends on one’s perspective; certainly that patient, trying to free himself from addiction’s grasp, was “going through a difficult time.” This is of course a platitude; the word “misery” gets much closer to the truth. I usually answer with some variation of the following: Medicine, especially psychiatry, is about caring for those who need it most: hurting, vulnera
On the first day of my psychiatry clerkship, I sat at a table with another student, 2 residents, and our attending physician. This wasn’t my first clinical rotation, but it was my first formal exposure to psychiatry, and I was excited and a bit anxious because I was considering psychiatry as an area of specialty training for myself. I’d been assigned 1 patient that morning: a 42-year-old man admitted for alcohol withdrawal. Our team, the psychiatry consultation-liaison team, was asked to evaluate the patient’s depressed mood in the context of withdrawal. As I began to present the patient’s story, I spoke of how terrible this man’s life had been, and how depressed he had recently become; this depression, I said, was likely exacerbated by alcohol use, but he was dealing with his depression by drinking more. He now wanted to quit for good. My attending, whom I had just met, interrupted me: “Misery,” she said with an intense look, “is a gift to an addicted person.”
I have ruminated on those surprising words ever since, and in that time I have begun to understand something about misery through the eyes of my patients. Sick people often are miserable; physical ailments can wreck hopes and plans and suck the joy from seemingly everything. Individuals who are ill or in pain often are suffering psychologically as well as physically. This suffering has been especially apparent to me in patients withdrawing from addictive substances: alcohol, cocaine, heroin, nicotine. I have been begged, cursed, praised, thanked, and more based on my ability or inability to relieve someone’s suffering caused by the lack of a certain substance: Please, just one cigarette. Please, something for this pain. Please, something to drink. As a medical student, I did one of 2 things: stood there helpless, or promised I would do the best I could, knowing my resident or attending would likely tell them no.
Withdrawal from addictive substances is, unsurprisingly, not pleasant. Alcohol withdrawal is one of the few that can be fatal, due to its ability to cause autonomic instability and seizures. Withdrawing from alcohol is also unpleasant due to hallucinosis and tremors, on top of the very real cravings for the substance itself. My patient knew this; he had withdrawn from alcohol in the past. As he talked to me, though, it became clear he had finally decided this was the end. In the past, others encouraged him to stop drinking; this time he was doing it for himself. His life had become so dismal that he was willing to undergo the agony of withdrawal to be free from his addiction.
Was his suffering, then, his misery, a gift? As I came to know my attending better, I also came to understand what these jarring words meant to her. They were her version of the old adage: It’s only when you hit rock bottom that you can start climbing back out. It isn’t the misery of withdrawing, but the misery inflicted by the substance that might provide an unexpected opportunity to start fixing things. For my patient, this particular trip to the hospital—which happened to intersect in space and time with me, a third-year medical student keen to learn and to help—was rock bottom, and he knew it. His life had been destroyed by his addiction, and here, at this intersection, the destruction was so great that he was finally willing to make a change for the better.
It is counterintuitive to think of misery as a gift, but then again, this patient—and more broadly, all patients whose lives are tormented by addiction and substance abuse—are often on the receiving end of counterintuitive advice, and it is frequently the only way to enact lasting change. Consider, for example, Alcoholics’ Anonymous, which works for far more individuals than one might expect. It does not seem possible that a small group without formal training could keep people sober simply by talking openly about their struggles; yet every day throughout the world, it does just that.
Patients struggling with addiction—labeled as addicts and drug-seekers by most of the world—are often written off as “difficult patients.” Perhaps because of my inexperience, I didn’t see this man as difficult, or as just another case of alcohol withdrawal. Although it may often be easier to define someone by his or her disease, I believe in choosing to see the human underneath the label. To me, these patients are not difficult; they are broken and miserable, and they desperately need help. Knowing this, I am forced to consider just how bad things have gotten for them, and how hard it must be to make a change. Their brokenness may be an opportunity to start down a new path, but only if we extend that invitation. Such an invitation may be the first step to turning genuine misery into a gift.
When I’m asked why I have chosen psychiatry, willingly entering such a “difficult field,” I think about my experience on that consult service and this patient. I know that I’m still just beginning my journey, and that even more difficult moments and patients lie ahead. But difficulty depends on one’s perspective; certainly that patient, trying to free himself from addiction’s grasp, was “going through a difficult time.” This is of course a platitude; the word “misery” gets much closer to the truth. I usually answer with some variation of the following: Medicine, especially psychiatry, is about caring for those who need it most: hurting, vulnera
Motivational interviewing: The RULES, PACE, and OARS
CASE
Mr. C, a veteran in his 60s who has posttraumatic stress disorder (PTSD), presents to your clinic for a 45-minute follow-up visit. He has a remote history of depression and a 20-year history of substance use disorder (SUD); he uses heroin, at least 3 bags a day by insufflation. You review his response to his currently prescribed PTSD treatment regimen, ask if he is experiencing any adverse effects, and perform a mental status exam and a review of systems. You offer Mr. C detoxification and rehabilitation treatment for his heroin use, but he refuses. With 15 minutes left in the appointment, you consider conducting motivational interviewing (MI) to help him reconsider getting treatment for his SUD.
Even when delivered as a brief, one-time intervention, MI can be effective in getting patients to change their behavior.1 First created in part by psychologists William Miller, PhD, and Stephen Rollnick, PhD,2 MI is based on the premise that a patient’s ambivalence to change is normal and that all patients vary in their readiness to change. MI can be brief, and can be more helpful than providing only proscriptive advice, which sometimes can be counterproductitive.3
To effectively implement MI during a brief visit, it is helpful to keep in mind 3 mnemonics: RULE, PACE, and OARS.
RULE
RULE can be used to remember the core principles of MI.4 First, Resist the righting reflex, which means we should resist giving suggestions to our patients for their problems. While we may mean well, offering suggestions might actually make the patient less likely to make a positive change. Understand the patient’s motivation by being a curious listener and attempting to elicit the patient’s own underlying motivation for change. Listen with a patient-centered, empathic approach. Lastly, Empower the patient. He must understand that he is in control of his actions, and any change he desires will require him to take steps toward that change.
PACE
PACE is the “spirit” or mindset that clinicians should have when conducting MI.4,5 Always work in Partnership with the patient; this allows the patient and clinician to collaborate on the same level. While the physician is a clinical expert, the patient is an expert in prior efforts at trying to change his or her circumstances for the better. Make the therapeutic environment as positive as possible so that your patient will find it comfortable to discuss change. The patient should see the clinician as a guide who offers information about paths the patient may choose, not someone who decides the destination.5 While as physicians we must continue to educate our patients about the harms of behaviors such as excessive drinking or substance use, we recognize that ultimately the decision is the patient’s. Make every effort to draw from the patients’ goals and values, so that the patient, and not the clinician, can argue for why change is needed. This Acceptance helps foster an attitude that we are on the patient’s side and that his past choices in life do not negatively affect our perception of him. The patient should be accepted for who he is, and not met with disapproval over any personal decisions that he made.5 Exercise Compassion towards the patient’s struggles and experiences,5 and never be punitive. Make every attempt to have discussions that can be Evocative for the patient. Strong feelings and memories can be particularly salient to discuss, especially if they could help change the patient’s attitude towards maladaptive behaviors.
OARS
OARS can be used to help remember core skills of MI.5 These include asking Open-ended questions to get the patient to think before responding, providing frequent Affirmations of the patient’s positive traits, using Reflective listening techniques while your patient talks about his disorder, and providing succinct Summaries of the experiences expressed by your patient throughout the encounter to invite continued exploration of his behaviors.
Getting patients to talk about change
Use RULE, PACE, and OARS to elicit “change talk,”4 so that your patient makes his own arguments for change. Here ambivalence is good, in that an ambivalent patient may be open to discuss reasons for making changes. It is important to remember not to use the righting reflex to give suggestions to change.
Continue to: CASE...
CASE CONTINUED
You use the last 15 minutes of Mr. C’s visit to conduct MI and acknowledge his ambivalence to change. Mr. C reveals that his motivation for change centers on how he perceives himself as a disappointment to his daughter because of his continuous drug use. At the end of the encounter, Mr. C is in tears but has a renewed motivation to stop using heroin. He agrees to enter substance abuse treatment.
1. Dwommoh R, Sorsdahl K, Myers B, et al. Brief interventions to address substance use among patients presenting to emergency departments in resource poor settings: a cost-effectiveness analysis. Cost Eff Resour Alloc. 2018;16:24.
2. Rollnick S, Miller WR, Christopher CB. Motivational interviewing in health care: helping patients change behavior. New York, NY: The Guilford Press; 2008.
3. Bani-Yaghoub M, Elhomani A, Catley D. Effectiveness of motivational interviewing, health education and brief advice in a population of smokers who are not ready to quit. BMC Med Res Methodol. 2018;18:52.
4. Rosengren DB. Building motivational interviewing skills: a practitioner workbook. New York, NY: The Guilford Press; 2009:30-88.
5. Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd ed. New York, NY: The Guilford Press; 2012:37-243.
CASE
Mr. C, a veteran in his 60s who has posttraumatic stress disorder (PTSD), presents to your clinic for a 45-minute follow-up visit. He has a remote history of depression and a 20-year history of substance use disorder (SUD); he uses heroin, at least 3 bags a day by insufflation. You review his response to his currently prescribed PTSD treatment regimen, ask if he is experiencing any adverse effects, and perform a mental status exam and a review of systems. You offer Mr. C detoxification and rehabilitation treatment for his heroin use, but he refuses. With 15 minutes left in the appointment, you consider conducting motivational interviewing (MI) to help him reconsider getting treatment for his SUD.
Even when delivered as a brief, one-time intervention, MI can be effective in getting patients to change their behavior.1 First created in part by psychologists William Miller, PhD, and Stephen Rollnick, PhD,2 MI is based on the premise that a patient’s ambivalence to change is normal and that all patients vary in their readiness to change. MI can be brief, and can be more helpful than providing only proscriptive advice, which sometimes can be counterproductitive.3
To effectively implement MI during a brief visit, it is helpful to keep in mind 3 mnemonics: RULE, PACE, and OARS.
RULE
RULE can be used to remember the core principles of MI.4 First, Resist the righting reflex, which means we should resist giving suggestions to our patients for their problems. While we may mean well, offering suggestions might actually make the patient less likely to make a positive change. Understand the patient’s motivation by being a curious listener and attempting to elicit the patient’s own underlying motivation for change. Listen with a patient-centered, empathic approach. Lastly, Empower the patient. He must understand that he is in control of his actions, and any change he desires will require him to take steps toward that change.
PACE
PACE is the “spirit” or mindset that clinicians should have when conducting MI.4,5 Always work in Partnership with the patient; this allows the patient and clinician to collaborate on the same level. While the physician is a clinical expert, the patient is an expert in prior efforts at trying to change his or her circumstances for the better. Make the therapeutic environment as positive as possible so that your patient will find it comfortable to discuss change. The patient should see the clinician as a guide who offers information about paths the patient may choose, not someone who decides the destination.5 While as physicians we must continue to educate our patients about the harms of behaviors such as excessive drinking or substance use, we recognize that ultimately the decision is the patient’s. Make every effort to draw from the patients’ goals and values, so that the patient, and not the clinician, can argue for why change is needed. This Acceptance helps foster an attitude that we are on the patient’s side and that his past choices in life do not negatively affect our perception of him. The patient should be accepted for who he is, and not met with disapproval over any personal decisions that he made.5 Exercise Compassion towards the patient’s struggles and experiences,5 and never be punitive. Make every attempt to have discussions that can be Evocative for the patient. Strong feelings and memories can be particularly salient to discuss, especially if they could help change the patient’s attitude towards maladaptive behaviors.
OARS
OARS can be used to help remember core skills of MI.5 These include asking Open-ended questions to get the patient to think before responding, providing frequent Affirmations of the patient’s positive traits, using Reflective listening techniques while your patient talks about his disorder, and providing succinct Summaries of the experiences expressed by your patient throughout the encounter to invite continued exploration of his behaviors.
Getting patients to talk about change
Use RULE, PACE, and OARS to elicit “change talk,”4 so that your patient makes his own arguments for change. Here ambivalence is good, in that an ambivalent patient may be open to discuss reasons for making changes. It is important to remember not to use the righting reflex to give suggestions to change.
Continue to: CASE...
CASE CONTINUED
You use the last 15 minutes of Mr. C’s visit to conduct MI and acknowledge his ambivalence to change. Mr. C reveals that his motivation for change centers on how he perceives himself as a disappointment to his daughter because of his continuous drug use. At the end of the encounter, Mr. C is in tears but has a renewed motivation to stop using heroin. He agrees to enter substance abuse treatment.
CASE
Mr. C, a veteran in his 60s who has posttraumatic stress disorder (PTSD), presents to your clinic for a 45-minute follow-up visit. He has a remote history of depression and a 20-year history of substance use disorder (SUD); he uses heroin, at least 3 bags a day by insufflation. You review his response to his currently prescribed PTSD treatment regimen, ask if he is experiencing any adverse effects, and perform a mental status exam and a review of systems. You offer Mr. C detoxification and rehabilitation treatment for his heroin use, but he refuses. With 15 minutes left in the appointment, you consider conducting motivational interviewing (MI) to help him reconsider getting treatment for his SUD.
Even when delivered as a brief, one-time intervention, MI can be effective in getting patients to change their behavior.1 First created in part by psychologists William Miller, PhD, and Stephen Rollnick, PhD,2 MI is based on the premise that a patient’s ambivalence to change is normal and that all patients vary in their readiness to change. MI can be brief, and can be more helpful than providing only proscriptive advice, which sometimes can be counterproductitive.3
To effectively implement MI during a brief visit, it is helpful to keep in mind 3 mnemonics: RULE, PACE, and OARS.
RULE
RULE can be used to remember the core principles of MI.4 First, Resist the righting reflex, which means we should resist giving suggestions to our patients for their problems. While we may mean well, offering suggestions might actually make the patient less likely to make a positive change. Understand the patient’s motivation by being a curious listener and attempting to elicit the patient’s own underlying motivation for change. Listen with a patient-centered, empathic approach. Lastly, Empower the patient. He must understand that he is in control of his actions, and any change he desires will require him to take steps toward that change.
PACE
PACE is the “spirit” or mindset that clinicians should have when conducting MI.4,5 Always work in Partnership with the patient; this allows the patient and clinician to collaborate on the same level. While the physician is a clinical expert, the patient is an expert in prior efforts at trying to change his or her circumstances for the better. Make the therapeutic environment as positive as possible so that your patient will find it comfortable to discuss change. The patient should see the clinician as a guide who offers information about paths the patient may choose, not someone who decides the destination.5 While as physicians we must continue to educate our patients about the harms of behaviors such as excessive drinking or substance use, we recognize that ultimately the decision is the patient’s. Make every effort to draw from the patients’ goals and values, so that the patient, and not the clinician, can argue for why change is needed. This Acceptance helps foster an attitude that we are on the patient’s side and that his past choices in life do not negatively affect our perception of him. The patient should be accepted for who he is, and not met with disapproval over any personal decisions that he made.5 Exercise Compassion towards the patient’s struggles and experiences,5 and never be punitive. Make every attempt to have discussions that can be Evocative for the patient. Strong feelings and memories can be particularly salient to discuss, especially if they could help change the patient’s attitude towards maladaptive behaviors.
OARS
OARS can be used to help remember core skills of MI.5 These include asking Open-ended questions to get the patient to think before responding, providing frequent Affirmations of the patient’s positive traits, using Reflective listening techniques while your patient talks about his disorder, and providing succinct Summaries of the experiences expressed by your patient throughout the encounter to invite continued exploration of his behaviors.
Getting patients to talk about change
Use RULE, PACE, and OARS to elicit “change talk,”4 so that your patient makes his own arguments for change. Here ambivalence is good, in that an ambivalent patient may be open to discuss reasons for making changes. It is important to remember not to use the righting reflex to give suggestions to change.
Continue to: CASE...
CASE CONTINUED
You use the last 15 minutes of Mr. C’s visit to conduct MI and acknowledge his ambivalence to change. Mr. C reveals that his motivation for change centers on how he perceives himself as a disappointment to his daughter because of his continuous drug use. At the end of the encounter, Mr. C is in tears but has a renewed motivation to stop using heroin. He agrees to enter substance abuse treatment.
1. Dwommoh R, Sorsdahl K, Myers B, et al. Brief interventions to address substance use among patients presenting to emergency departments in resource poor settings: a cost-effectiveness analysis. Cost Eff Resour Alloc. 2018;16:24.
2. Rollnick S, Miller WR, Christopher CB. Motivational interviewing in health care: helping patients change behavior. New York, NY: The Guilford Press; 2008.
3. Bani-Yaghoub M, Elhomani A, Catley D. Effectiveness of motivational interviewing, health education and brief advice in a population of smokers who are not ready to quit. BMC Med Res Methodol. 2018;18:52.
4. Rosengren DB. Building motivational interviewing skills: a practitioner workbook. New York, NY: The Guilford Press; 2009:30-88.
5. Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd ed. New York, NY: The Guilford Press; 2012:37-243.
1. Dwommoh R, Sorsdahl K, Myers B, et al. Brief interventions to address substance use among patients presenting to emergency departments in resource poor settings: a cost-effectiveness analysis. Cost Eff Resour Alloc. 2018;16:24.
2. Rollnick S, Miller WR, Christopher CB. Motivational interviewing in health care: helping patients change behavior. New York, NY: The Guilford Press; 2008.
3. Bani-Yaghoub M, Elhomani A, Catley D. Effectiveness of motivational interviewing, health education and brief advice in a population of smokers who are not ready to quit. BMC Med Res Methodol. 2018;18:52.
4. Rosengren DB. Building motivational interviewing skills: a practitioner workbook. New York, NY: The Guilford Press; 2009:30-88.
5. Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd ed. New York, NY: The Guilford Press; 2012:37-243.
Injectable extended-release naltrexone for opioid dependence: 3 studies
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
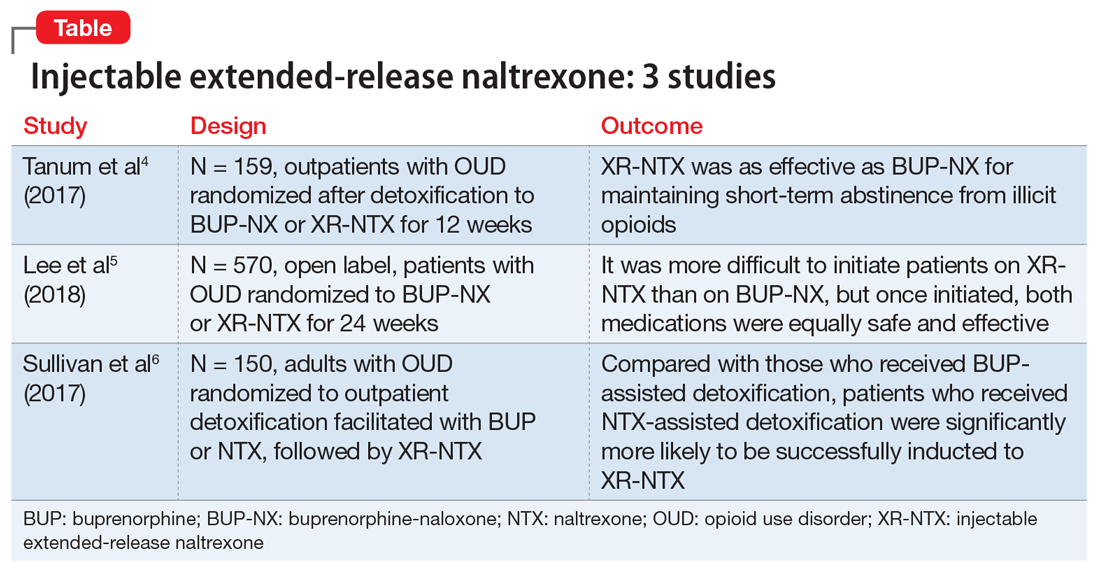
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).

1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).

1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Abuse of psychiatric medications: Not just stimulants and benzodiazepines
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15
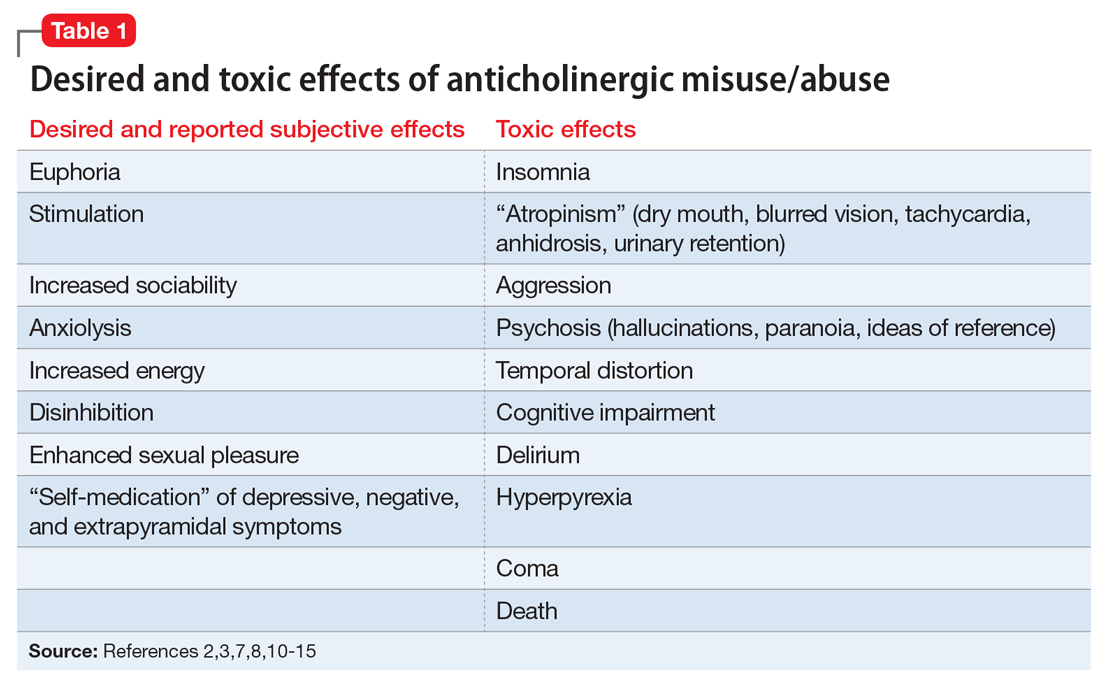
Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15

Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
While some classes of medications used to treat psychiatric disorders, such as stimulants and benzodiazepines, are well-recognized as controlled substances and drugs of abuse, clinicians may be less familiar with the potential misuse/abuse of other psychiatric medications. This article reviews the evidence related to the misuse/abuse of anticholinergics, antidepressants, antipsychotics, and gabapentinoids.
The terms “misuse,” “abuse,” and “addiction” are used variably in the literature without standardized definitions. For this review, “misuse/abuse (M/A)” will be used to collectively describe self-administration that is recreational or otherwise inconsistent with legal or medical guidelines, unless a specific distinction is made. Whether or not the medications reviewed are truly “addictive” will be briefly discussed for each drug class, but the focus will be on clinically relevant aspects of M/A, including:
- excessive self-administration
- self-administration by non-oral routes
- co-administration with other drugs of abuse
- malingering of psychiatric symptoms to obtain prescriptions
- diversion for sale to third parties
- toxicity from overdose.
Anticholinergic medications
The first case describing the deliberate M/A of an anticholinergic medication for its euphoric effects was published in 1960.Further reportsfollowed in Europe before the M/A potential of prescription anticholinergic medications among psychiatric patients with an overdose syndrome characterized by atropinism and toxic psychosis was more widely recognized in the United States in the 1970s. Most reported cases of M/A to date have occurred among patients with psychiatric illness because anticholinergic medications, including trihexyphenidyl, benztropine, biperiden, procyclidine, and orphenadrine, were commonly prescribed for the management of first-generation and high dopamine D2-affinity antipsychotic-induced extrapyramidal symptoms (EPS). For example, one study of 234 consecutively hospitalized patients with schizophrenia noted an anticholinergic M/A incidence of 6.5%.1
However, anticholinergic M/A is not limited to individuals with psychotic disorders. A UK study of 154 admissions to an inpatient unit specializing in behavioral disturbances found a 12-month trihexyphenidyl M/A incidence of 17%; the most common diagnosis among abusers was antisocial personality disorder.2 Anticholinergic M/A has also been reported among patients with a primary diagnosis of substance use disorders (SUDs)3 as well as more indiscriminately in prison settings,4 with some inmates exchanging trihexyphenidyl as currency and using it recreationally by crushing it into powder and smoking it with tobacco.5 Others have noted that abusers sometimes take anticholinergics with alcohol in order to “potentiate” the effects of each substance.6,7 Pullen et al8 described individuals with and without psychiatric illness who stole anticholinergic medications, purchased them from other patients, or bought them “on the street.” Malingering EPS in order to obtain anticholinergic medications has also been well documented.9 Clearly, anticholinergic M/A can occur in psychiatric and non-psychiatric populations, both within and outside of clinical settings. Although anticholinergic M/A appears to be less frequent in the United States now that second-generation antipsychotics (SGAs) are more frequently prescribed, M/A remains common in some settings outside of the United States.7
Among the various anticholinergic medications prescribed for EPS, trihexyphenidyl has been reported to have the greatest M/A potential, which has been attributed to its potency,10 its stimulating effects (whereas benztropine is more sedating),11 and its former popularity among prescribers.8 Marken et al11 published a review of 110 reports of M/A occurring in patients receiving anticholinergic medications as part of psychiatric treatment in which 69% of cases involved taking trihexyphenidyl 15 to 60 mg at a time (recommended dosing is 6 to 10 mg/d in divided doses).Most of these patients were prescribed anticholinergic medications for diagnostically appropriate reasons—only 7% were described as “true abusers” with no medical indication. Anticholinergic M/A was typically driven by a desire for euphoric and psychedelic/hallucinogenic effects, although in some cases, anticholinergic M/A was attributed to self-medication of EPS and depressive symptoms. These findings illustrate the blurred distinction between recreational use and perceived subjective benefit, and match those of a subsequent study of 50 psychiatric patients who reported anticholinergic M/A not only to “get high,” but to “decrease depression,” “increase energy,” and decrease antipsychotic adverse effects.12 Once again, trihexyphenidyl was the most frequently misused anticholinergic in this sample.
Table 12,3,7,8,10-15 outlines the subjective effects sought and experienced by anticholinergic abusers as well as potential toxic effects; there is the potential for overlap. Several authors have also described physiologic dependence with long-term trihexyphenidyl use, including tolerance and a withdrawal/abstinence syndrome.7,16 In addition, there have been several reports of coma13 and death in the setting of intended suicide by overdose of anticholinergic medications.14,15

Although anticholinergic M/A in the United States now appears to be less common, clinicians should remain aware of the M/A potential of anticholinergic medications prescribed for EPS. Management of M/A involves:
- detection
- reducing anticholinergic exposure by managing EPS with alternative strategies, such as switching or reducing the dose of the antipsychotic medication
- gradual tapering of anticholinergic medications to minimize withdrawal.11
Continue to: Antidepressants
Antidepressants
Haddad17 published a review of 21 English-language case reports from 1966 to 1998 describing antidepressant use in which individuals met DSM-IV criteria for substance dependence to the medication. An additional 14 cases of antidepressant M/A were excluded based on insufficient details to support a diagnosis of dependence. The 21 reported cases involved:
- tranylcypromine (a monoamine oxidase inhibitor [MAOI])
- amitriptyline (a tricyclic antidepressant [TCA])
- fluoxetine (a selective serotonin reuptake inhibitor [SSRI])
- amineptine (a TCA previously available in France but removed from the market in 1999 in part due to its abuse potential)
- nomifensine (a norepinephrine/dopamine reuptake inhibitor previously available in the United Kingdom but removed in 1986 due to hemolytic anemia).
In 95% of cases, the antidepressants were prescribed for treatment of an affective disorder but were abused for stimulant effects or the perceived ability to lift mood, cause euphoria or a “high,” or to improve functioning. Two-thirds of cases involved patients with preexisting substance misuse. Placing the case reports in the context of the millions of patients prescribed antidepressants during this period, Haddad concluded the “incidence of [antidepressant] addiction [is] so low as to be clinically irrelevant.”17
Despite this conclusion, Haddad singled out amineptine and tranylcypromine as antidepressants with some evidence of true addictive potential.17,18 A more recent case series described 14 patients who met DSM-IV criteria for substance abuse of tertiary amine TCAs (which have strong anticholinergic activity) and concluded that “misuse of [TCAs] is more common than generally appreciated.”19 In keeping with that claim, a study of 54 outpatients taking unspecified antidepressants found that up to 15% met DSM-III-R criteria for substance dependence (for the antidepressant) in the past year, although that rate was much lower than the rate of benzodiazepine dependence (47%) in a comparative sample.20 Finally, a comprehensive review by Evans and Sullivan21 found anecdotal reports published before 2014 that detailed misuse, abuse, and dependence with MAOIs, TCAs, fluoxetine, venlafaxine, bupropion, tianeptine, and amineptine. Taken together, existing evidence indicates that select individuals—typically those with other SUD comorbidity—sometimes misuse antidepressants in a way that suggests addiction.
Still, while it is well known that abrupt cessation of antidepressants can result in a discontinuation syndrome characterized by flu-like symptoms, nausea, and dizziness,22 physiologic withdrawal effects must be distinguished from historical definitions of substance “abuse” and the broader concept of psychological “addiction” or drug dependence18,23 now incorporated into the DSM-5 definition of SUDs.24 Indeed, although withdrawal symptoms were reported by more than half of those who took antidepressants and responded to a recent online survey,25 evidence to support the existence of significant antidepressant tolerance, craving, or compulsive use is lacking.17,18 Antidepressants as a class do not appear to be significantly rewarding or reinforcing and, on the contrary, discontinuation by patients is common in clinical practice.26 The popular claim that some individuals taking antidepressants “can’t quit”27 must also be disentangled from loss of therapeutic effects upon cessation.
Bupropion. A more convincing argument for antidepressant addiction can be made for bupropion, a weak norepinephrine and dopamine reuptake inhibitor with an otherwise unclear mechanism of action.28 In 2002, the first report of recreational bupropion M/A described a 13-year-old girl who took 2,400 mg orally (recommended maximum dose is 450 mg/d in divided doses) after being told it would give her “a better high than amphetamine.”29 This was followed in the same year by the first report of recreational M/A of bupropion via nasal insufflation (snorting), resulting in a seizure,30 and in 2013 by the first published case of M/A by IV self-administration.31
Continue to: The M/A potential of bupropion...
The M/A potential of bupropion, most commonly via intranasal administration, is now broadly recognized based on several case reports describing desired effects that include a euphoric high and a stimulating “buzz” similar to that of cocaine or methamphetamine but less intense.29-36 Among recreational users, bupropion tablets are referred to as “welbys,” “wellies,” “dubs,” or “barnies.”37 Media coverage of a 2013 outbreak of bupropion M/A in Toronto detailed administration by snorting, smoking, and injection, and described bupropion as “poor man’s cocaine.”38 Between 2003 and 2016, 2,232 cases of bupropion misuse/abuse/dependence adverse drug reactions were reported to the European Monitoring Agency.37 A review of intentional bupropion M/A reported to US Poison Control Centers between 2000 to 2013 found 975 such cases, with the yearly number tripling between 2000 and 2012.39 In this sample, nearly half (45%) of the users were age 13 to 19, and 76% of cases involved oral ingestion. In addition to bupropion M/A among younger people, individuals who misuse bupropion often include those with existing SUDs but limited access to illicit stimulants and those trying to evade detection by urine toxicology screening.33 For example, widespread use and diversion has been well documented within correctional settings, and as a result, many facilities have removed bupropion from their formularies.21,28,33,34,40
Beyond desired effects, the most common adverse events associated with bupropion M/A are listed in Table 2,28,30,32-34,36,39 along with their incidence based on cases brought to the attention of US Poison Control Centers.39 With relatively little evidence of a significant bupropion withdrawal syndrome,37 the argument in favor of modeling bupropion as a truly addictive drug is limited to anecdotal reports of cravings and compulsive self-administration35 and pro-dopaminergic activity (reuptake inhibition) that might provide a mechanism for potential rewarding and reinforcing effects.40 While early preclinical studies of bupropion failed to provide evidence of amphetamine-like abuse potential,41,42 non-oral administration in amounts well beyond therapeutic dosing could account for euphoric effects and a greater risk of psychological dependence and addiction.21,28,40
Bupropion also has an FDA indication as an aid to smoking cessation treatment, and the medication demonstrated early promise in the pharmacologic treatment of psychostimulant use disorders, with reported improvements in cravings and other SUD outcomes.43-45 However, subsequent randomized controlled trials (RCTs) failed to demonstrate a clear therapeutic role for bupropion in the treatment of cocaine46,47 and methamphetamine use disorders (although some secondary analyses suggest possible therapeutic effects among non-daily stimulant users who are able to maintain good adherence with bupropion).48-51 Given these overall discouraging results, the additive seizure risk of bupropion use with concomitant psychostimulant use, and the potential for M/A and diversion of bupropion (particularly among those with existing SUDs), the use of bupropion for the off-label treatment of stimulant use disorders is not advised.
Antipsychotics
As dopamine antagonists, antipsychotics are typically considered to have low potential for rewarding or reinforcing effects. Indeed, misuse of antipsychotics was a rarity in the first-generation era, with only a few published reports of haloperidol M/A within a small cluster of naïve young people who developed acute EPS,52 and a report of diversion in a prison with the “sadistic” intent of inflicting dystonic reactions on others.53 A more recent report described 2additional cases of M/A involving haloperidol and trifluoperazine.54 Some authors have described occasional drug-seeking behavior for low-potency D2 blockers such as chlorpromazine, presumably based on their M/A as anticholinergic medications.55
The potential for antipsychotic M/A has gained wider recognition since the advent of the SGAs. Three cases of prescription olanzapine M/A have been published to date. One involved a man who malingered manic symptoms to obtain olanzapine, taking ≥40 mg at a time (beyond his prescribed dose of 20 mg twice daily) to get a “buzz,” and combining it with alcohol and benzodiazepines for additive effects or to “come down” from cocaine.56 This patient noted that olanzapine was “a popular drug at parties” and was bought, sold, or traded among users, and occasionally administered intravenously. Two other cases described women who self-administered olanzapine, 40 to 50 mg/d, for euphoric and anxiolytic effects.57,58 James et al59 detailed a sample of 28 adults who reported “non-medical use” of olanzapine for anxiolytic effects, as a sleep aid, or to “escape from worries.”
Continue to: Quetiapine
Quetiapine. In contrast to some reports of olanzapine M/A in which the line between M/A and “self-medication” was blurred, quetiapine has become a more convincing example of clear recreational antipsychotic M/A. Since the first report of oral and intranasal quetiapine M/A in the Los Angeles County Jail published in 2004,55 subsequent cases have detailed other novel methods of recreational self-administration60-68 (Table 355,60-68), and additional reports have been published in non-English language journals.69,70 Collectively, these case reports have detailed that quetiapine is:
- misused for primary subjective effects as well as to mitigate the unpleasant effects of other drugs60,67
- referred to as “quell,”“Q,” “Susie-Q,” “squirrel,” and “baby heroin”55,71,72
- often obtained by malingering psychiatric symptoms55,61,63,65
- diverted/sold with “street value” both within and outside of psychiatric facilities and correctional settings.55,60-62,67,68,73
These anecdotal accounts of quetiapine M/A have since been corroborated on a larger scale based on several retrospective studies. Although early reports of quetiapine M/A occurring in correctional settings have resulted in formulary removal,71,74 quetiapine M/A is by no means limited to forensic populations and is especially common among those with comorbid SUDs. A survey of 74 patients enrolled in a Canadian methadone program reported that nearly 60% had misused quetiapine at some point.75 Among an Australian sample of 868 individuals with active IV drug abuse, 31% reported having misused quetiapine.76 Finally, within a small sample of patients with SUDs admitted to a detoxification unit in New York City, 17% reported M/A of SGAs.77 In this study, SGAs were often taken in conjunction with other drugs of abuse in order to “recover” from or “enhance” the effects of other drugs or to “experiment.” Quetiapine was by far the most frequently abused SGA, reported in 96% of the sample; the most frequently reported SGA/drug combinations were quetiapine/alcohol/opioids, quetiapine/cocaine, and quetiapine/opioids.
Looking more broadly at poison center data, reports to the US National Poison Data System (NPDS) from 2005 to 2011 included 3,116 cases of quetiapine abuse (37.5%, defined as intentional recreational use in order to obtain a “high”) or misuse (62.5%, defined as improper use or dosing for non-recreational purposes).78 A more recent analysis of NPDS reports from 2003 to 2013 found 2,118 cases of quetiapine abuse, representing 61% of all cases of reported SGA abuse.79 An analysis of the European Medicines Agency Adverse Drug Database yielded 18,112 reports of quetiapine misuse, abuse, dependence, and withdrawal for quetiapine (from 2005 to 2016) compared with 4,178 for olanzapine (from 2004 to 2016).80 These reports identified 368 fatalities associated with quetiapine.
The rate of quetiapine M/A appears to be increasing sharply. Reports of quetiapine M/A to poison centers in Australia increased nearly 7-fold from 2006 to 2016.81 Based on reports to the Drug Abuse Warning System, US emergency department visits for M/A of quetiapine increased from 19,195 in 2005 to 32,024 in 2011 (an average of 27,114 visits/year), with 75% of cases involving quetiapine taken in combination with other prescription drugs, alcohol, or illicit drugs.82 Consistent with poison center data, M/A was reported for other antipsychotics, but none nearly as frequently as for quetiapine.
With increasingly frequent quetiapine M/A, clinicians should be vigilant in monitoring for medical morbidity related to quetiapine and cumulative toxicity with other drugs. The most frequent adverse events associated with quetiapine M/A reported to US Poison Control Centers are presented in Table 4.78,79
Continue to: Unlike bupropion...
Unlike bupropion, quetiapine’s dopamine antagonism makes it unlikely to be a truly addictive drug, although this mechanism of action could mediate an increase in concurrent psychostimulant use.83 A few case reports have described a quetiapine discontinuation syndrome similar to that of antidepressants,60,65,84-88 but withdrawal symptoms suggestive of physiologic dependence may be mediated by non-dopaminergic effects through histamine and serotonin receptors.84,89 Evidence for quetiapine misuse being associated with craving and compulsive use is lacking, and true quetiapine addiction is probably rare.
Similar to bupropion, preliminary findings have suggested promise for quetiapine as a putative therapy for other SUDs.90-93 However, subsequent RCTs have failed to demonstrate a therapeutic effect for alcohol and cocaine use disorders.94-96 Given these negative results and the clear M/A potential of quetiapine, off-label use of quetiapine for the treatment of SUDs and psychiatric symptoms among those with SUDs must be considered judiciously, with an eye towards possible diversion and avoiding the substitution of one drug of abuse for another.
Gabapentinoids
In 1997, the first published case report of gabapentin M/A described a woman who self-administered her husband’s gabapentin to reduce cravings for and withdrawal from cocaine.97 The authors highlighted the possible therapeutic benefit of gabapentin in this regard rather than raising concerns about diversion and M/A. By 2004, however, reports of recreational gabapentin M/A emerged among inmates incarcerated within Florida correctional facilities who self-administered intranasal gabapentin to achieve a “high” that was “reminiscent of prior effects from intranasal ingestion of cocaine powder.”98 In 2007, a single case of gabapentin misuse up to 7,200 mg/d (recommended dosing is ≤3,600 mg/d) was reported, with documentation of both tolerance and withdrawal symptoms.99 As of 2017, a total of 36 cases of gabapentin M/A and 19 cases of pregabalin M/A have been published.100
In the past decade, anecdotal reports have given way to larger-scale epidemiologic data painting a clear picture of the now-widespread M/A of gabapentin and other gabapentinoids. For example, a study of online descriptions of gabapentin and pregabalin M/A from 2008 to 2010 documented:
- oral and IM use (gabapentin)
- IV and rectal (“plugging”) use (pregabalin)
- “parachuting” (emptying the contents of capsules for a larger dose) (pregabalin)
- euphoric, entactogenic, stimulant, calming/anxiolytic, and dissociative subjective effects (gabapentin/pregabalin)
- rapid development of tolerance to euphoric effects leading to self-administration of increasing doses (gabapentin/pregabalin)
- frequent co-administration with other drugs of abuse, including alcohol, benzodiazepines, cannabis, stimulants, opiates, hallucinogens, gamma-hydroxybutyrate, mephedrone, and Salvia divinorum (gabapentin/pregabalin)101
Several systematic reviews of both anecdotal reports and epidemiologic studies published in the past few years provide additional evidence of the above, such as:
- excessive dosing with self-administration
- intranasal and inhaled routes of administration
- diversion and “street value”
- greater M/A potential of pregabalin than gabapentin
- the presence of gabapentinoids in postmortem toxicology analyses, suggesting a role in overdose fatalities when combined with other drugs.100,102,103
Continue to: The European Medicine Agency's EudraVigilance database...
The European Medicine Agency’s EudraVigilance database included 4,301 reports of gabapentin misuse, abuse, or dependence, and 7,639 such reports for pregabalin, from 2006 to 2015 (rising sharply after 2012), with 86 gabapentin-related and 27 pregabalin-related fatalities.104 Data from the Drug Diversion Program of the Researched Abuse, Diversion, and Addiction-Related Surveillance System from 2002 to 2015 have likewise revealed that gabapentin diversion increased significantly in 2013.105
While the prevalence of gabapentinoid M/A is not known, rates appear to be significantly lower than for traditional drugs of abuse such as cannabis, cocaine, 3,4-methylenedioxymethamphetamine (MDMA), and opioids.106,107 However, gabapentin and pregabalin M/A appears to be increasingly common among individuals with SUDs and in particular among those with opioid use disorders (OUDs). For example, a 2015 report indicated that 15% of an adult cohort in Appalachian Kentucky with nonmedical use of diverted prescription opioids reported gabapentin M/A, an increase of nearly 3,000% since 2008.108 Based on data from a US insurance enrollment and claims database, researchers found that the rate of gabapentin overuse among those also overusing opioids was 12% compared with only 2% for those using gabapentin alone.109 It has also been reported that gabapentin is sometimes used as a “cutting agent” for heroin.110
Those who use gabapentinoids together with opioids report that gabapentin and pregabalin potentiate the euphoric effects of methadone111 and endorse specific beliefs that pregabalin increases both the desired effects of heroin as well as negative effects such as “blackouts,” loss of control, and risk of overdose.112 Indeed, sustained M/A of gabapentin and opioids together has been found to increase emergency department utilization, drug-related hospitalization, and respiratory depression.113 Based on a case-control study of opioid users in Canada, co-prescription of gabapentin and opioids was associated with a 50% increase in death from opioid-related causes compared with prescription of opioids alone.114
Case reports documenting tolerance, withdrawal, craving, and loss of control suggest a true addictive potential for gabapentinoids, but Bonnet and Sherbaum100 concluded that while there is robust evidence of abusers “liking” gabapentin and pregabalin (eg, reward), evidence of “wanting” them (eg, psychological dependence) in the absence of other SUDs has been limited to only a few anecdotal reports with pregabalin. Accordingly, the risk of true addiction to gabapentinoids by those without preexisting SUDs appears to be low. Nonetheless, the M/A potential of both gabapentin and pregabalin is clear and in the context of a nationwide opioid epidemic, the increased morbidity/mortality risk related to combined use of gabapentinoids and opioids is both striking and concerning. Consequently, the state of Kentucky recently recognized the M/A potential of gabapentin by designating it a Schedule V controlled substance (pregabalin is already a Schedule V drug according to the US Drug Enforcement Agency),103,113 and several other states now mandate the reporting of gabapentin prescriptions to prescription drug monitoring programs.115
Following a similar pattern to antidepressants and antipsychotics, a potential role for gabapentin in the treatment of cocaine use disorders was supported in preliminary studies,116-118 but not in subsequent RCTs.119-121 However, there is evidence from RCTs to support the use of gabapentin and pregabalin in the treatment of alcohol use disorders.122-124 Gabapentin was also found to significantly reduce cannabis use and withdrawal symptoms in patients compared with placebo in an RCT of individuals with cannabis use disorders.125 The perceived safety of gabapentinoids by clinicians, their subjective desirability by patients with SUDs, and efficacy data supporting a therapeutic role in SUDs must be balanced with recognition that approximately 80% of gabapentin prescriptions are written for off-label indications for which there is little supporting evidence,109 such as low back pain.126 Clinicians considering prescribing gabapentinoids to manage psychiatric symptoms, such as anxiety and insomnia, should carefully consider the risk of M/A and other potential morbidities, especially in the setting of SUDs and OUD in particular.
Continue to: Problematic, even if not addictive
Problematic, even if not addictive
It is sometimes claimed that “addiction” to psychiatric medications is not limited to stimulants and benzodiazepines.27,127 Although anticholinergics, antidepressants, antipsychotics, and gabapentinoids can be drugs of abuse, with some users reporting physiologic withdrawal upon discontinuation, there is only limited evidence that the M/A of these psychiatric medications is associated with the characteristic features of a more complete definition of “addiction,” which may include:
- inability to consistently abstain
- impairment in behavioral control
- diminished recognition of significant problems associated with use
- a dysfunctional emotional response to chronic use.128
Nonetheless, the literature documenting anticholinergic, antidepressant, antipsychotic, and gabapentinoid M/A includes several common features, including:
- initial reports among those with limited access to illicit drugs (eg, young people and incarcerated individuals) and subsequent spread to a wider population with more unconventional routes of administration
- use for recreational purposes and other subjective pseudo-therapeutic effects, often in combination with alcohol and illicit drugs
- greater M/A potential of certain medications within each of these drug classes (eg, trihexyphenidyl, bupropion, quetiapine)
- malingering psychiatric symptoms in order to obtain medications from prescribers and diversion for black market sale
- observations that medications might constitute therapy for SUDs that were not supported in subsequent RCTs (with the exception of gabapentin for alcohol and cannabis use disorders)
- increasing evidence of toxicity related to M/A, which suggests that prescription by clinicians has limited benefit and high risk for patients with SUDs.
Bottom Line
Some psychiatric medications are taken as drugs of abuse. Clinicians should be particularly aware of the misuse/abuse potential of anticholinergics, antidepressants, antipsychotics, and gabapentinoids, and use them cautiously, if at all, when treating patients with existing substance use disorders.
Related Resources
- Substance Abuse and Mental Health Services Administration. Prescription drug misuse and abuse. https://www.samhsa.gov/topics/prescription-drug-misuse-abuse.
- Substance Abuse and Mental Health Services Administration. Types of commonly misused or abused drugs. https://www.samhsa.gov/prescription-drug-misuse-abuse/types.
- National Institute on Drug Abuse. Misuse of prescription drugs. https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/summary.
- National Institute on Drug Abuse. New clinician screening tool available for substance use. https://www.drugabuse.gov/news-events/news-releases/2018/06/newclinician-screening-tool-available-substance-use.
Drug Brand Names
Amitriptyline • Elavil, Endep
Benztropine • Cogentin
Biperiden • Akineton
Bupropion • Wellbutrin, Zyban
Chlorpromazine • Thorzine
Fluoxetine • Prozac
Haloperidol • Haldol
Olanzapine • Zyprexa
Orphenadrine • Disipal, Norflex
Pregabalin • Lyrica, Lyrica CR
Procyclidine • Kemadrin
Quetiapine • Seroquel
Tianeptine • Coaxil, Stablon
Tranylcypromine • Parnate
Trifluoperazine • Stelazine
Trihexyphenidyl • Artane, Tremin
Venlafaxine • Effexor
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
1. Zemishlany Z, Aizenberg D, Weiner Z, et al. Trihexyphenidyl (Artane) abuse in schizophrenic patients. Int Clin Psychopharmacol. 1996;11(3):199-202.
2. Crawshaw JA, Mullen PE. A study of benzhexol abuse. Brit J Psychiatry. 1984;145:300-303.
3. Woody GE, O’Brien CP. Anticholinergic toxic psychosis in drug abusers treated with benztropine. Comp Psychiatry. 1974;15(5):439-442.
4. Lowry TP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
5. Rouchell AM, Dixon SP. Trihexyphenidyl abuse. Am J Psychiatry. 1977;134(11):1315.
6. Kaminer Y, Munitz H, Wijsenbeek H. Trihexyphenidyl (Artane) abuse: euphoriant and anxiolytic. Brit J Psychiatry. 1982;140(5):473-474.
7. Nappo SA, de Oliviera LG, Sanchez Zv, et al. Trihexyphenidyl (Artane): a Brazilian study of its abuse. Subst Use Misuse. 2005;40(4):473-482.
8. Pullen GP, Best NR, Macguire J. Anticholinergic drug abuse: a common problem? Brit Med J (Clin Res Ed). 1984;289(6445):612-613.
9. Rubinstein JS. Abuse of antiparkinsonian drugs: feigning of extrapyramidal symptoms to obtain trihexyphenidyl. JAMA. 1978;239(22):2365-2366.
10. Mohan D, Mohandas E, Dube S. Trihexyphenidyl abuse. Brit J Addiction. 1981:76(2);195-197.
11. Marken PA, Stoner SC, Bunker MT. Anticholinergic drug abuse and misuse. CNS Drugs. 1996;5(3):190-199.
12. Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatric Serv. 2000;51(7):928-929.
13. Goldstein MR, Kasper R. Hyperpyrexia and coma due to overdose of benztropine. South Med J. 1968;61(9):984.
14. Petkovi
15. McIntyre IM, Mallett P, Burton CG, et al. Acute benztropine intoxication and fatality. J Forensic Sci. 2014;59(6):1675-1678.
16. Dilsaver SC. Antimuscarinic agents as substances of abuse: A review. J Clin Psychopharmacol. 1988:8(1):14-22.
17. Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300-307.
18. Haddad PM. Do antidepressants cause dependence? Epidemiol Psichiatr Soc. 2005;14(2):58-62.
19. Shenouda R, Desan PH. Abuse of tricyclic antidepressant drugs: a case series. J Clin Psychopharmacol. 2013;33(3):440-442.
20. van Broekhoven F, Kan CC, Zitman FG. Dependence potential of antidepressants compared to benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(5):939-943.
21. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
22. Warner CH, Bobo W, Warner C, et al. Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449-456.
23. Lichtigfeld FJ, Gillman MA. Antidepressants are not drugs of abuse or dependence. Postgrad Med J. 1998;74(875):529-532.
24. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
25. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67-73.
26. Haddad P, Anderson I. Antidepressants aren’t addictive: clinicians have depended on them for years. J Psychopharmacol. 1999;13(3):291-292.
27. Carey B, Gebeloff R. Many people taking antidepressants discover they cannot quit. New York Times. https://www.nytimes.com/2018/04/07/health/antidepressants-withdrawal-prozac-cymbalta.html. Published April 7, 2018. Accessed December 11, 2018.
28. Kim D, Steinhart B. Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. CJEM. 2010;12(2):158-161.
29. McCormick J. Recreational bupropion in a teenager. Br J Clin Pharmacol. 2002;53(2):214.
30. Welsh C, Doyon S. Seizure induced by insufflation of bupropion. N Engl J Med. 2002; 347(2):951.
31. Baribeau D, Araki KF. Intravenous bupropion: A previously undocumented method of abuse of a commonly prescribed antidepressant agent. J Addict Med. 2013;7(3):216-217.
32. Hill SH, Sikand H, Lee J. A case report of seizure induced by bupropion nasal insufflation. Prim Care Companion J Clin Psych. 2007;9(1):67-69.
33. Yoon G, Westermeyer J. Intranasal bupropion abuse. Am J Addict. 2013;22(2):180.
34. Reeves RR, Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Psychopharmacol. 2013;33(4):584-585.
35. Oppek K, Koller G, Zwergal A, et al. Intravenous administration and abuse of bupropion: a case report and a review of the literature. J Addict Med. 2014;8(4):290-293.
36. Strike M, Hatcher S. Bupropion injection resulting in tissue necrosis and psychosis: previously undocumented complications of intravenous bupropion use disorder. J Addict Med. 2015;9(3):246-250.
37. Schifano F, Chiappini S. Is there a potential of misuse for venlafaxine and bupropion? Front Pharmacol. 2018;9:239.
38. Tryon J, Logan N. Antidepressant Wellbutrin becomes ‘poor man’s cocaine’ on Toronto streets. Global News. https://globalnews.ca/news/846576/antidepressant-wellbutrin-becomes-poor-mans-cocaine-on-toronto-streets/. Published September 18, 2013. Accessed December 11, 2018.
39. Stassinos GL, Klein-Schwartz W. Bupropion “abuse” reported to US Poison Centers. J Addict Med. 2016;10(5):357-362.
40. Hilliard WT, Barloon L, Farley P, et al. Bupropion diversion and misuse in the correctional facility. J Correct Health Care. 2013;19(3):211-217.
41. Griffith JD, Carranza J, Griffith C, et al. Bupropion clinical assay for amphetamine-like abuse potential. J Clin Psychiatry.1983;44(5 Pt 2):206-208.
42. Miller L, Griffith J. A comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacol (Berl). 1983;80(3):199-205.
43. Berigan TR, Russell ML. Treatment of methamphetamine cravings with bupropion: A case report. Prim Care Companion J Clin Psychiatry. 2001;3(6):267-268.
44. Tardieu T, Poirier Y, Micallef J, et al. Amphetamine-like stimulant cessation in an abusing patient treated with bupropion. Acta Psychiatr Scand. 2004;109(1):75-78.
45. Newton TF, Roache JD, De La Garza R, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced cravings. Neuropsychopharmacology. 2006;31(7):1537-1544.
46. Margolin A, Kosten TR, Avants SK, et al. A multicenter trial for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125-131.
47. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. J Addict Dis. 2008;27(1):13-23.
48. Anderson AL, Li S, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: a randomized, double-blind placebo-controlled trial. Drug Alcohol Depend. 2015;150:170-174.
49. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222-232.
50. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
51. Heinzerling KG, Swanson A, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109(11):1878-1886.
52. Doenecke AL, Heuerman RC. Treatment of haloperidol abuse with diphenhydramine. Am J Psychiatry. 1980;137(4):487-488.
53. Weddington WW, Leventhal BL. Sadistic abuse of haloperidol. Am J Psychiatry. 1982;139:132-133.
54. Basu D, Marudkar M, Khurana H. Abuse of neuroleptic drugs by psychiatric patients. Indian J Med Sci. 2000;54(2):59-62.
55. Pierre JM, Shnayder I, Wirshing DA, et al. Intranasal quetiapine abuse. Am J Psychiatry 2004;161(9):1718.
56. Reeves RR. Abuse of olanzapine by substance abusers. J Psychoactive Drugs. 2007;39(3):297-299.
57. Kumsar NA, Erol A. Olanzapine abuse. Subst Abus. 2013;34(1):73-74.
58. Lai C. Olanzapine abuse was relieved after switching to aripiprazole in a patient with psychotic depression. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34(7):1363-1364.
59. James PD, Fida AS, Konovalov P, et al. Non-medical use of olanzapine by people on methadone treatment. BJPsych Bull. 2016;40(6):314-317.
60. Reeves RR, Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J. 2007;100(8):834-836.
61. Murphy D, Bailey K, Stone M, et al. Addictive potential of quetiapine. Am J Psychiatry. 2008;165(7):918.
62. Paparrigopoulos T, Karaiskos D, Liappas J. Quetiapine: another drug with potential for misuse? J Clin Psychiatry. 2008;69(1):162-163.
63. Reeves RR, Burke RS. Abuse of the combination of gabapentin and quetiapine. Prim Care Companion CNS Disord. 2014;16(5): doi: 10.4088/PCC.14l01660.
64. Morin AK. Possible intranasal quetiapine misuse. Am J Health Syst Pharm. 2007;64(7):723-725.
65. Caniato RN, Gundabawady A, Baune BT, et al. Malingered psychotic symptoms and quetiapine abuse in a forensic setting. J Forens Psychiatr Psychol. 2009;20(6):928-935.
66. Hussain MZ, Waheed W, Hussain S. Intravenous quetiapine abuse. Am J Psychiatry. 2005; 162(9):1755-1756.
67. Waters BM, Joshi KG. Intravenous quetiapine-cocaine use (“Q-ball”). Am J Psychiatry. 2007;164(1):173-174.
68. Haridas A, Kushon D, Gurmu S, et al. Smoking quetiapine: a “Maq ball?” Prim Psychiatry. 2010;17:38-39.
69. Cubala WJ, Springer J. Quetiapine abuse and dependence in psychiatric patients: a systematic review of 25 case reports in the literature. J Subs Use. 2014;19(5):388-393.
70. Piróg-Balcerzak A, Habrat B, Mierzejewski P. Misuse and abuse of quetiapine [in Polish]. Psychiatr Pol. 2015;49(1):81-93.
71. Pinta ER, Taylor RE. Quetiapine addiction? Am J Psychiatry. 2007;164(1):174.
72. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Amer Acad Psychiatr Law. 2012;40(4):502-508.
73. Tarasoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164(2):350.
74. Reccoppa L. Less abuse potential with XR formulation of quetiapine. Am J Addiction. 2010;20(2):178.
75. McLarnon ME, Fulton HG, MacIsaac C, et al. Characteristics of quetiapine misuse among clients of a community-based methadone maintenance program. J Clin Psychopharmacol. 2012;32(5):721-723.
76. Reddel SE, Bruno R, Burns L, et al. Prevalence and associations of quetiapine fumarate misuse among an Australian national city sample of people who regularly inject drugs. Addiction. 2013;109(2):295-302.
77. Malekshahi T, Tioleco N, Ahmed N, et al. Misuse of atypical antipsychotics in conjunction with alcohol and other drugs of abuse. J Subs Abuse Treat. 2015;48(1):8-12.
78. Klein-Schwartz W, Schwartz EK, Anderson BD. Evaluation of quetiapine abuse and misuse reported to poison centers. J Addict Med. 2014;8(3):195-198.
79. Klein L, Bangh S, Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med. 2017;18(2):243-250.
80. Chiappini S, Schifano F. Is there a potential of misuse for quetiapine?: Literature review and analysis of the European Medicines Agency/European Medicines Agency Adverse Drug Reactions’ Database. J Clin Psychopharmacol. 2018;38(1):72-79.
81. Lee J, Pilgrim J, Gerostamoulos D, et al. Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend. 2018;187:95-99.
82. Mattson ME, Albright VA, Yoon J, et al. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: Results from the
83. Brutcher RE, Nader SH, Nader MA. Evaluation of the reinforcing effect of quetiapine, alone and in combination with cocaine, in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(2):244-250.
84. Kim DR, Staab JP. Quetiapine discontinuation syndrome. Am J Psychiatry. 2005;162(5):1020.
85. Thurstone CC, Alahi P. A possible case of quetiapine withdrawal syndrome. J Clin Psychiatry. 2000;61(8):602-603.
86. Kohen I, Kremen N. A case report of quetiapine withdrawal syndrome in a geriatric patient. World J Biol Psychiatry. 2009;10(4 pt 3):985-986.
87. Yargic I, Caferov C. Quetiapine dependence and withdrawal: a case report. Subst Abus. 2011;32(3):168-169.
88. Koch HJ. Severe quetiapine withdrawal syndrome with nausea and vomiting in a 65-year-old patient with psychotic depression. Therapie. 2015;70(6):537-538.
89. Fischer BA, Boggs DL. The role of antihistaminic effects in the misuse of quetiapine: a case report and review of the literature. Neurosci Biobehav Rev. 2010;34(4):555-558.
90. Longoria J, Brown ES, Perantie DC, et al. Quetiapine for alcohol use and craving in bipolar disorder. J Clin Psychopharmacol. 2004;24(1):101-102.
91. Monnelly EP, Ciraulo DA, Knapp C, et al. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24(5):532-535.
92. Kennedy A, Wood AE, Saxon AJ, et al. Quetiapine for the treatment of cocaine dependence: an open-label trial. J Clin Psychopharmacol. 2008;28(2):221-224.
93. Mariani JJ, Pavlicova M, Mamczur A, et al. Open-label pilot study of quetiapine treatment for cannabis dependence. Am J Drug Alcohol Abuse. 2014;40(4):280-284.
94. Guardia J, Roncero C, Galan J, et al. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36(3):265-269.
95. Litten RZ, Fertig JB, Falk DE, et al; NCIG 001 Study Group. A double-blind, placebo-controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy-drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(3):406-416.
96. Tapp A, Wood AE, Kennedy A, et al. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18-24.
97. Markowitz JS, Finkenbine R, Myrick H, et al. Gabapentin abuse in a cocaine user: Implications for treatment. J Clin Psychopharmacol. 1997;17(5):423-424.
98. Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13(3):321-323.
99. Victorri-Vigneau C, Guelais M, Jolliet P. Abuse, dependency and withdrawal with gabapentin: a first case report. Pharmacopsychiatry. 2007;40(1):43-44.
100. Bonnet U, Sherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185-1215.
101. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118-122.
102. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426.
103. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160-1174.
104. Chiappini S, Shifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs. 2016;30(7):647-654.
105. Buttram ME, Kurtz SP, Dart R, et al. Law enforcement-derived data on gabapentin diversion and misuse, 2002-2015: diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083-1086.
106. Kapil V, Green JL, Le Lait M, et al. Misuse of the y-aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2013;78(1):190-191.
107. Peckham AM, Fairman KA, Sclar DA. Prevalence of gabapentin abuse: comparison with agents with known abuse potential in a commercially insured US population. Clin Drug Invest. 2017;37(8):763-773.
108. Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488.
109. Peckham AM, Evoy KE, Covvey JR, et al. Predictors of gabapentin overuse with or without concomitant opioids in a commercially insured U.S. population. Pharmacotherapy. 2018;38(4):436-443.
110. Smith BH, Higgins C, Baldacchino A, et al. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):401-407.
111. Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res. 2014;20(3):115-118.
112. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580-1589.
113. Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213-228.
114. Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e10022396. doi: 10.1371/journal.pmed.1002396.
115. Peckham AM, Fairman K, Sclar DA. Policies to mitigate nonmedical use of prescription medications: how should emerging evidence of gabapentin misuse be addressed? Exp Opin Drug Saf. 2018;17(5):519-523.
116. Raby WN. Gabapentin for cocaine cravings. Am J Psychiatry. 2000;157(12):2058-2059.
117. Myrick H, Henderson S, Brady KT, et al. Gabapentin in the treatment of cocaine dependence: a case series. J CLin Psychiatry. 2001;62(1):19-23.
118. Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65(1):84-86.
119. Hart CL, Ward AS, Collins ED, et al. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73(3):279-287.
120. Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alc Depend. 2006;81(3):267-274.
121. Hart CL, Haney M, Collins ED, et al. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86(2-3):274-277.
122. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
123. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
124. Martinotti G, Di Nicola M, Tedeschi D, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24(9):1367-1374.
125. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychpharmacology. 2012;27(7):1689-1698.
126. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793.
127. Cartwright C, Gibson K, Read J, et al. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407.
128. American Society of Addiction Medicine. Public policy statement: definition of addiction. https://www.asam.org/docs/default-source/public-policy-statements/1definition_of_addiction_long_4-11.pdf?sfvrsn=a8f64512_4. Published August 15, 2011. Accessed July 23, 2018.
With hemp now legal, FDA reaffirms rules for cannabis compounds
The newly enacted Agriculture Improvement Act of 2018 legalizes hemp production and use, but the Food and Drug Administration’s regulation of cannabis and cannabis-derived products remains unchanged.
The act (H.R. 2) revamps federal authorities’ regulatory approach to hemp production. The law removes hemp from the Controlled Substances Act, which means it is no longer an illegal substance. Hemp is now defined as cannabis and derivatives of cannabis that have extremely low concentrations (less than 0.3%, on a dry weight basis) of the psychoactive compound delta-9-tetrahydrocannabinol (THC).
Despite hemp’s new legal status, FDA Commissioner Scott Gottlieb, MD, said the FDA’s regulation of cannabis and cannabis-derived products remains the same.
“In short, we treat products containing cannabis or cannabis-derived compounds as we do any other FDA-regulated products,” FDA Commissioner Scott Gottlieb, MD, said in a Dec. 20 statement published on the FDA website. That means “they’re subject to the same authorities and requirements as FDA-regulated products containing any other substance.”
The regulation of those products will be the same regardless of the source of the substance, including from plants classified as hemp. The FDA will require cannabis and cannabis-derived products to undergo testing similar to other drug products, given the concern regarding medical claims made about those products.
“Cannabis and cannabis-derived products claiming in their marketing and promotional materials that they’re intended for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases (such as cancer, Alzheimer’s disease, psychiatric disorders and diabetes) are considered new drugs or new animal drugs,” Dr. Gottlieb explained. And they “must go through the FDA drug approval process for human or animal use before they are marketed in the United States.”
Selling unapproved products with unsubstantiated claims is a “violation of the law.”
In addition, “it’s unlawful under the [Food, Drug & Cosmetics Act] to introduce food containing added CBD [cannabidiol] or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp derived,” Dr. Gottlieb noted. That’s because both CBD and THC are active ingredients in FDA-approved drugs.
Commissioner Gottlieb also noted that “pathways remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement.” The FDA announced plans for a future meeting to discuss the lawful marketing of hemp-derived foods that do not contain CBD or THC.
The newly enacted Agriculture Improvement Act of 2018 legalizes hemp production and use, but the Food and Drug Administration’s regulation of cannabis and cannabis-derived products remains unchanged.
The act (H.R. 2) revamps federal authorities’ regulatory approach to hemp production. The law removes hemp from the Controlled Substances Act, which means it is no longer an illegal substance. Hemp is now defined as cannabis and derivatives of cannabis that have extremely low concentrations (less than 0.3%, on a dry weight basis) of the psychoactive compound delta-9-tetrahydrocannabinol (THC).
Despite hemp’s new legal status, FDA Commissioner Scott Gottlieb, MD, said the FDA’s regulation of cannabis and cannabis-derived products remains the same.
“In short, we treat products containing cannabis or cannabis-derived compounds as we do any other FDA-regulated products,” FDA Commissioner Scott Gottlieb, MD, said in a Dec. 20 statement published on the FDA website. That means “they’re subject to the same authorities and requirements as FDA-regulated products containing any other substance.”
The regulation of those products will be the same regardless of the source of the substance, including from plants classified as hemp. The FDA will require cannabis and cannabis-derived products to undergo testing similar to other drug products, given the concern regarding medical claims made about those products.
“Cannabis and cannabis-derived products claiming in their marketing and promotional materials that they’re intended for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases (such as cancer, Alzheimer’s disease, psychiatric disorders and diabetes) are considered new drugs or new animal drugs,” Dr. Gottlieb explained. And they “must go through the FDA drug approval process for human or animal use before they are marketed in the United States.”
Selling unapproved products with unsubstantiated claims is a “violation of the law.”
In addition, “it’s unlawful under the [Food, Drug & Cosmetics Act] to introduce food containing added CBD [cannabidiol] or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp derived,” Dr. Gottlieb noted. That’s because both CBD and THC are active ingredients in FDA-approved drugs.
Commissioner Gottlieb also noted that “pathways remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement.” The FDA announced plans for a future meeting to discuss the lawful marketing of hemp-derived foods that do not contain CBD or THC.
The newly enacted Agriculture Improvement Act of 2018 legalizes hemp production and use, but the Food and Drug Administration’s regulation of cannabis and cannabis-derived products remains unchanged.
The act (H.R. 2) revamps federal authorities’ regulatory approach to hemp production. The law removes hemp from the Controlled Substances Act, which means it is no longer an illegal substance. Hemp is now defined as cannabis and derivatives of cannabis that have extremely low concentrations (less than 0.3%, on a dry weight basis) of the psychoactive compound delta-9-tetrahydrocannabinol (THC).
Despite hemp’s new legal status, FDA Commissioner Scott Gottlieb, MD, said the FDA’s regulation of cannabis and cannabis-derived products remains the same.
“In short, we treat products containing cannabis or cannabis-derived compounds as we do any other FDA-regulated products,” FDA Commissioner Scott Gottlieb, MD, said in a Dec. 20 statement published on the FDA website. That means “they’re subject to the same authorities and requirements as FDA-regulated products containing any other substance.”
The regulation of those products will be the same regardless of the source of the substance, including from plants classified as hemp. The FDA will require cannabis and cannabis-derived products to undergo testing similar to other drug products, given the concern regarding medical claims made about those products.
“Cannabis and cannabis-derived products claiming in their marketing and promotional materials that they’re intended for use in the diagnosis, cure, mitigation, treatment, or prevention of diseases (such as cancer, Alzheimer’s disease, psychiatric disorders and diabetes) are considered new drugs or new animal drugs,” Dr. Gottlieb explained. And they “must go through the FDA drug approval process for human or animal use before they are marketed in the United States.”
Selling unapproved products with unsubstantiated claims is a “violation of the law.”
In addition, “it’s unlawful under the [Food, Drug & Cosmetics Act] to introduce food containing added CBD [cannabidiol] or THC into interstate commerce, or to market CBD or THC products as, or in, dietary supplements, regardless of whether the substances are hemp derived,” Dr. Gottlieb noted. That’s because both CBD and THC are active ingredients in FDA-approved drugs.
Commissioner Gottlieb also noted that “pathways remain available for the FDA to consider whether there are circumstances in which certain cannabis-derived compounds might be permitted in a food or dietary supplement.” The FDA announced plans for a future meeting to discuss the lawful marketing of hemp-derived foods that do not contain CBD or THC.
Best of Psychopharmacology: Stimulants, ketamine, benzodiazapines
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify