User login
Texas launches website in fight against opioid abuse; Gen Z’ers report more mental health problems
Officials in Texas see their new “Dose of Reality” website as a tool that might help address the opioid crisis in their state.
Dose of Reality, an initiative of the state attorney general, the Texas Department of State Health Services, and Texas Health and Human Services, offers for download material on opioids. People also can learn about risk factors of opioid abuse and how to safely store the medications. Drug disposal sites statewide also are included, according to an article published by the Dallas Morning News.
“The misuse of prescription opioids costs lives and devastates Texas families in every corner of our state. Dose of Reality is a one-stop shop of information on the opioid epidemic in Texas. [It] will pull back the curtain on opioids, educate Texans and save, hopefully, many lives,” Texas Attorney General Ken Paxton reportedly said at a press conference announcing the website launch.
Of the 42,249 deaths tied to opioid overdoses reported nationwide by the National Institute on Drug Abuse in 2016, 1,375 of those deaths reportedly occurred in Texas. According to Mr. Paxton, deceptive marketing and promotion by pharmaceutical companies have been part of the problem.
Generation Z and mental health
Gen Z’ers – young people born from the mid-1990s to the early 2000s – are the most likely age group to report mental health problems, according to a report from the American Psychological Association.
The findings from the group’s 12th annual Stress in America survey of 3,458 Americans aged 18 years or older and 300 teens aged 15-17 years showed that issues such as sexual harassment and gun violence are significant stressors for Gen Z. America’s youngest adults are most likely of all generations to report poor mental health, and Gen Z also is significantly more likely to seek professional help for mental health issues, the study authors wrote.
Adolescents and young adults aged 15-21 years are more concerned than are other generations about the state of the United States, and overall, 71% of the Gen Z’ers are more positive about the country’s future. About 60% had gotten politically involved in the past year.
But that optimism did not extend to Gen Z’ers of color. “For around 4 in 10 Gen Zs of color, personal debt [41%] and housing instability (40%) are significant sources of stress, while 3 in 10 white Gen Zs [30%] say the same about personal debt and less than one-quarter [24%] of this demographic cite housing instability,” the authors wrote.
“Solutions” center in the works
A new facility to be built in a Denver neighborhood will enable offenders with mental health issues to receive treatment instead of incarceration. Once up and running, the facility, dubbed a “solutions” or “stabilization” center, will be a go-to option for police officers who have picked up someone judged to be in the throes of a mental health crisis, instead of a trip to the police station and booking, the Denver Post reported.
People referred to the center will be eligible to stay for up to 5 days and referrals will be available for continued counseling. Walk-ins will not be admitted.
“In my heart, I’m committed to making this an addition to the neighborhood that will make the neighborhood a safer place and not a more difficult place,” said Jay Flynn, a vice president of the Mental Health Center of Denver, which helped spearhead the initiative.
Not everyone is on board. Residents near the center site have voiced their concern about neighborhood safety. “It’s not that we don’t understand the needs of homelessness in our community,” said one resident at a community meeting held to discuss the center. “The fact is that our community is extremely stressed and we need to preserve a safe environment.”
The center is scheduled to open in 2020.
Is masculinity really toxic?
A new ad by Gillette raises questions about what it means to be male. The ad initially presents a more traditional view of men as boors, bullies, and sexual oppressors, then morphs into a call for a sea change to males with empathy, compassion, and a need to help. The ad came a few months after the American Psychological Association issued new practice guidelines for boys and men, in which traditional masculinity ideology was conceptualized as limiting.
Those developments prompted an op-ed piece in the Los Angeles Times that considered whether masculinity really is toxic.
“Some of the angry responses to the [Gillette] ad were over the top, and yet the detractors have a point. Take the way the ad exhorts men to start doing and saying ‘the right thing,’ and then continues, ‘Some already are. But some is not enough.’ This suggests decent men are a minority while brutes are the norm,” wrote Cathy Young, a contributing editor at Reason magazine.
“What’s more, some of the ‘toxic’ behavior shown is pretty innocuous, such as teenage boys ogling bikini-clad babes on television. (Should we shame girls who drool over cute male pop stars?) The ad also blurs the line between fighting and roughhousing, implicitly condemning the physical play styles more common among boys,” she wrote.
Meanwhile, the psychologists pointed out that, in light of many factors, including higher death rates in the United States for boys and men – compared with those of girls and women – understanding “how boys and men experience masculinity is an important cultural competency.”
Dementia and an aging workforce
As the American workforce continues to age, employers are having tough conversations about dementia and other cognitive issues, according an article from the Associated Press.
“And it’s not just managing missed deadlines,” Sarah Wood, director of global work-life services at an organization called Workplace Options, said in the piece. “If this person has been a dependable employee for 40 years and is now missing meetings, they’ll be beating themselves up over this.”
According to the Bureau of Labor Statistics, the number of U.S. workers aged 65-74 years was expected to skyrocket by 55% between 2014 and 2024.
Those aged 65 years and older are more likely to face dementia diagnoses. Because of the Americans with Disabilities Act, employers with dementia – including Alzheimer’s – are protected, “depending on the employee’s position and level of impairment,” according to the article.
Employers can accommodate employees by taking steps such as writing instructions rather than communicating verbally and reassigning employees who operate heavy machines to desk work, according to David K. Fram, director of the Americans with Disabilities Act equal opportunity services at the National Employment Law Institute. But employees must be able to do the “essential functions of the job,” he said.
Officials in Texas see their new “Dose of Reality” website as a tool that might help address the opioid crisis in their state.
Dose of Reality, an initiative of the state attorney general, the Texas Department of State Health Services, and Texas Health and Human Services, offers for download material on opioids. People also can learn about risk factors of opioid abuse and how to safely store the medications. Drug disposal sites statewide also are included, according to an article published by the Dallas Morning News.
“The misuse of prescription opioids costs lives and devastates Texas families in every corner of our state. Dose of Reality is a one-stop shop of information on the opioid epidemic in Texas. [It] will pull back the curtain on opioids, educate Texans and save, hopefully, many lives,” Texas Attorney General Ken Paxton reportedly said at a press conference announcing the website launch.
Of the 42,249 deaths tied to opioid overdoses reported nationwide by the National Institute on Drug Abuse in 2016, 1,375 of those deaths reportedly occurred in Texas. According to Mr. Paxton, deceptive marketing and promotion by pharmaceutical companies have been part of the problem.
Generation Z and mental health
Gen Z’ers – young people born from the mid-1990s to the early 2000s – are the most likely age group to report mental health problems, according to a report from the American Psychological Association.
The findings from the group’s 12th annual Stress in America survey of 3,458 Americans aged 18 years or older and 300 teens aged 15-17 years showed that issues such as sexual harassment and gun violence are significant stressors for Gen Z. America’s youngest adults are most likely of all generations to report poor mental health, and Gen Z also is significantly more likely to seek professional help for mental health issues, the study authors wrote.
Adolescents and young adults aged 15-21 years are more concerned than are other generations about the state of the United States, and overall, 71% of the Gen Z’ers are more positive about the country’s future. About 60% had gotten politically involved in the past year.
But that optimism did not extend to Gen Z’ers of color. “For around 4 in 10 Gen Zs of color, personal debt [41%] and housing instability (40%) are significant sources of stress, while 3 in 10 white Gen Zs [30%] say the same about personal debt and less than one-quarter [24%] of this demographic cite housing instability,” the authors wrote.
“Solutions” center in the works
A new facility to be built in a Denver neighborhood will enable offenders with mental health issues to receive treatment instead of incarceration. Once up and running, the facility, dubbed a “solutions” or “stabilization” center, will be a go-to option for police officers who have picked up someone judged to be in the throes of a mental health crisis, instead of a trip to the police station and booking, the Denver Post reported.
People referred to the center will be eligible to stay for up to 5 days and referrals will be available for continued counseling. Walk-ins will not be admitted.
“In my heart, I’m committed to making this an addition to the neighborhood that will make the neighborhood a safer place and not a more difficult place,” said Jay Flynn, a vice president of the Mental Health Center of Denver, which helped spearhead the initiative.
Not everyone is on board. Residents near the center site have voiced their concern about neighborhood safety. “It’s not that we don’t understand the needs of homelessness in our community,” said one resident at a community meeting held to discuss the center. “The fact is that our community is extremely stressed and we need to preserve a safe environment.”
The center is scheduled to open in 2020.
Is masculinity really toxic?
A new ad by Gillette raises questions about what it means to be male. The ad initially presents a more traditional view of men as boors, bullies, and sexual oppressors, then morphs into a call for a sea change to males with empathy, compassion, and a need to help. The ad came a few months after the American Psychological Association issued new practice guidelines for boys and men, in which traditional masculinity ideology was conceptualized as limiting.
Those developments prompted an op-ed piece in the Los Angeles Times that considered whether masculinity really is toxic.
“Some of the angry responses to the [Gillette] ad were over the top, and yet the detractors have a point. Take the way the ad exhorts men to start doing and saying ‘the right thing,’ and then continues, ‘Some already are. But some is not enough.’ This suggests decent men are a minority while brutes are the norm,” wrote Cathy Young, a contributing editor at Reason magazine.
“What’s more, some of the ‘toxic’ behavior shown is pretty innocuous, such as teenage boys ogling bikini-clad babes on television. (Should we shame girls who drool over cute male pop stars?) The ad also blurs the line between fighting and roughhousing, implicitly condemning the physical play styles more common among boys,” she wrote.
Meanwhile, the psychologists pointed out that, in light of many factors, including higher death rates in the United States for boys and men – compared with those of girls and women – understanding “how boys and men experience masculinity is an important cultural competency.”
Dementia and an aging workforce
As the American workforce continues to age, employers are having tough conversations about dementia and other cognitive issues, according an article from the Associated Press.
“And it’s not just managing missed deadlines,” Sarah Wood, director of global work-life services at an organization called Workplace Options, said in the piece. “If this person has been a dependable employee for 40 years and is now missing meetings, they’ll be beating themselves up over this.”
According to the Bureau of Labor Statistics, the number of U.S. workers aged 65-74 years was expected to skyrocket by 55% between 2014 and 2024.
Those aged 65 years and older are more likely to face dementia diagnoses. Because of the Americans with Disabilities Act, employers with dementia – including Alzheimer’s – are protected, “depending on the employee’s position and level of impairment,” according to the article.
Employers can accommodate employees by taking steps such as writing instructions rather than communicating verbally and reassigning employees who operate heavy machines to desk work, according to David K. Fram, director of the Americans with Disabilities Act equal opportunity services at the National Employment Law Institute. But employees must be able to do the “essential functions of the job,” he said.
Officials in Texas see their new “Dose of Reality” website as a tool that might help address the opioid crisis in their state.
Dose of Reality, an initiative of the state attorney general, the Texas Department of State Health Services, and Texas Health and Human Services, offers for download material on opioids. People also can learn about risk factors of opioid abuse and how to safely store the medications. Drug disposal sites statewide also are included, according to an article published by the Dallas Morning News.
“The misuse of prescription opioids costs lives and devastates Texas families in every corner of our state. Dose of Reality is a one-stop shop of information on the opioid epidemic in Texas. [It] will pull back the curtain on opioids, educate Texans and save, hopefully, many lives,” Texas Attorney General Ken Paxton reportedly said at a press conference announcing the website launch.
Of the 42,249 deaths tied to opioid overdoses reported nationwide by the National Institute on Drug Abuse in 2016, 1,375 of those deaths reportedly occurred in Texas. According to Mr. Paxton, deceptive marketing and promotion by pharmaceutical companies have been part of the problem.
Generation Z and mental health
Gen Z’ers – young people born from the mid-1990s to the early 2000s – are the most likely age group to report mental health problems, according to a report from the American Psychological Association.
The findings from the group’s 12th annual Stress in America survey of 3,458 Americans aged 18 years or older and 300 teens aged 15-17 years showed that issues such as sexual harassment and gun violence are significant stressors for Gen Z. America’s youngest adults are most likely of all generations to report poor mental health, and Gen Z also is significantly more likely to seek professional help for mental health issues, the study authors wrote.
Adolescents and young adults aged 15-21 years are more concerned than are other generations about the state of the United States, and overall, 71% of the Gen Z’ers are more positive about the country’s future. About 60% had gotten politically involved in the past year.
But that optimism did not extend to Gen Z’ers of color. “For around 4 in 10 Gen Zs of color, personal debt [41%] and housing instability (40%) are significant sources of stress, while 3 in 10 white Gen Zs [30%] say the same about personal debt and less than one-quarter [24%] of this demographic cite housing instability,” the authors wrote.
“Solutions” center in the works
A new facility to be built in a Denver neighborhood will enable offenders with mental health issues to receive treatment instead of incarceration. Once up and running, the facility, dubbed a “solutions” or “stabilization” center, will be a go-to option for police officers who have picked up someone judged to be in the throes of a mental health crisis, instead of a trip to the police station and booking, the Denver Post reported.
People referred to the center will be eligible to stay for up to 5 days and referrals will be available for continued counseling. Walk-ins will not be admitted.
“In my heart, I’m committed to making this an addition to the neighborhood that will make the neighborhood a safer place and not a more difficult place,” said Jay Flynn, a vice president of the Mental Health Center of Denver, which helped spearhead the initiative.
Not everyone is on board. Residents near the center site have voiced their concern about neighborhood safety. “It’s not that we don’t understand the needs of homelessness in our community,” said one resident at a community meeting held to discuss the center. “The fact is that our community is extremely stressed and we need to preserve a safe environment.”
The center is scheduled to open in 2020.
Is masculinity really toxic?
A new ad by Gillette raises questions about what it means to be male. The ad initially presents a more traditional view of men as boors, bullies, and sexual oppressors, then morphs into a call for a sea change to males with empathy, compassion, and a need to help. The ad came a few months after the American Psychological Association issued new practice guidelines for boys and men, in which traditional masculinity ideology was conceptualized as limiting.
Those developments prompted an op-ed piece in the Los Angeles Times that considered whether masculinity really is toxic.
“Some of the angry responses to the [Gillette] ad were over the top, and yet the detractors have a point. Take the way the ad exhorts men to start doing and saying ‘the right thing,’ and then continues, ‘Some already are. But some is not enough.’ This suggests decent men are a minority while brutes are the norm,” wrote Cathy Young, a contributing editor at Reason magazine.
“What’s more, some of the ‘toxic’ behavior shown is pretty innocuous, such as teenage boys ogling bikini-clad babes on television. (Should we shame girls who drool over cute male pop stars?) The ad also blurs the line between fighting and roughhousing, implicitly condemning the physical play styles more common among boys,” she wrote.
Meanwhile, the psychologists pointed out that, in light of many factors, including higher death rates in the United States for boys and men – compared with those of girls and women – understanding “how boys and men experience masculinity is an important cultural competency.”
Dementia and an aging workforce
As the American workforce continues to age, employers are having tough conversations about dementia and other cognitive issues, according an article from the Associated Press.
“And it’s not just managing missed deadlines,” Sarah Wood, director of global work-life services at an organization called Workplace Options, said in the piece. “If this person has been a dependable employee for 40 years and is now missing meetings, they’ll be beating themselves up over this.”
According to the Bureau of Labor Statistics, the number of U.S. workers aged 65-74 years was expected to skyrocket by 55% between 2014 and 2024.
Those aged 65 years and older are more likely to face dementia diagnoses. Because of the Americans with Disabilities Act, employers with dementia – including Alzheimer’s – are protected, “depending on the employee’s position and level of impairment,” according to the article.
Employers can accommodate employees by taking steps such as writing instructions rather than communicating verbally and reassigning employees who operate heavy machines to desk work, according to David K. Fram, director of the Americans with Disabilities Act equal opportunity services at the National Employment Law Institute. But employees must be able to do the “essential functions of the job,” he said.
We must counsel against heat-not-burn cigarettes
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
Tobacco companies are marketing a new version of cigarettes dubbed heat-not-burn (HNB) cigarettes.1,2 Offered as a “modified-risk tobacco product,” HNB cigarettes utilize a lithium battery-powered heating element and are available all over the world.1,2 Like conventional smokes, they contain tobacco, but deliver nicotine by heating leaves at 350° C rather than burning them at 600° C.1-3 Heating the tobacco produces an inhalable aerosol with tobacco flavor and nicotine, without smoke. These HNB cigarettes are also different from e-cigarettes that aerosolize a liquid.
Tobacco companies contend that HNB cigarettes are safer than smoking tobacco.1 Consumers inhale a heated tobacco aerosol that reportedly contains less nicotine and fewer toxicities; yet, HNB are not independently substantiated as being healthier, nor proven safe.1-5 Thermal decomposition, rather than combustion, may afford a less dangerous nicotine consumption; however, HNB aerosols deliver many of the same dangerous compounds as traditional cigarettes, including carbon monoxide, tar, and aromatic hydrocarbons.2-6 Despite possible harm reduction in the short-run, long-term safety remains unconfirmed.
Safety in passive environmental inhalations is not established.2 HNB cigarettes are contraindicated during pregnancy and/or lactation. Nicotine is provided in addictive quantities, enough to foster continued dependence. Exposure to HNB products can promote longer-term usage or lead to smoking traditional tobacco cigarettes. There is also an increased risk to non-smokers of exposure to HNB aerosols. Additionally, lithium batteries have been known to burn or explode. HNB devices may even lead to privacy concerns due micro-controller chips contained within that harvest information. These chips could inform manufacturers about device usage.7
Tobacco is a global health hazard and smoking is the number one preventable cause of disease.1,5,8 Global smoking prevalence is nearing 19%.9 There are concerns about dual use, rather than HNB cigarettes alone as a substitute for conventional smoking. The ultimate hope is to abstain from all tobacco and nicotine. Although HNB inhalations contain fewer toxic chemicals than by smoking, evidence regarding mitigation of tobacco-related diseases is inconclusive.10
Physicians have an obligation to minimize tobacco and nicotine-related hazards. Ongoing research and clinical exposure might better document the health impact of HNB cigarettes. Until the risks and benefits of HNB cigarettes are confirmed, health care professionals would be wise to counsel against their use.
Diksha Mohanty, MD; Steven Lippmann, MD
Louisville, Ky
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
1. Combustible cigarettes kill millions a year. Can Big Tobacco save them? The Economist Web site. https://www.economist.com/business/2017/12/19/combustible-cigarettes-kill-millions-a-year-can-big-tobacco-save-them. Accessed November 9, 2018.
2. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-not-burn tobacco cigarettes: smoke by any other name. JAMA Intern Med. 2017;177:1050-1052.
3. Caputi TL. Industry watch: heat-not-burn tobacco products are about to reach their boiling point. Tob Control. 2016;26:609-610.
4. Jenssen BP, Walley SC, McGrath-Morrow SA. Heat-not-burn tobacco products: Tobacco industry claims no substitute for science. Pediatrics. 2018;141:e20172383.
5. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112:8-17.
6. Bekki K, Inaba Y, Uchiyama S, et al. Comparison of chemicals in mainstream smoke in heat-not-burn tobacco and combustion cigarettes. J UOEH, 2017;39:201-207.
7. Lasseter T, Wilson D, Wilson T, et al. Philip Morris device knows a lot about your smoking habit. Reuters. https://www.reuters.com/investigates/special-report/tobacco-iqos-device. Accessed November 9, 2018.
8. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality — beyond established causes. New Engl J Med. 2015;372:631-640.
9. World Health Organization. WHO global report on trends in tobacco smoking 2000-2025 - First edition. http://www.who.int/tobacco/publications/surveillance/reportontrendstobaccosmoking/en/index4.html. Accessed November 9, 2018.
10. U.S. Food & Drug Administration. CTPConnect—September 2017. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm576895.htm. Updated June 14, 2018. Accessed Nov ember 9, 2018.
Alcohol use disorder: How best to screen and intervene
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
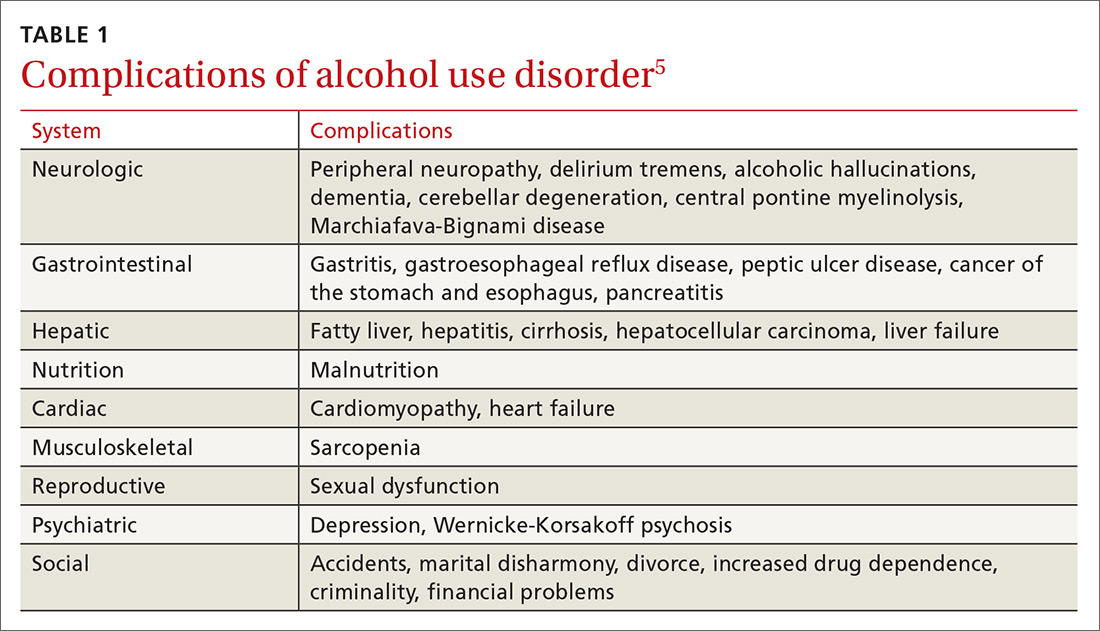
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6
Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
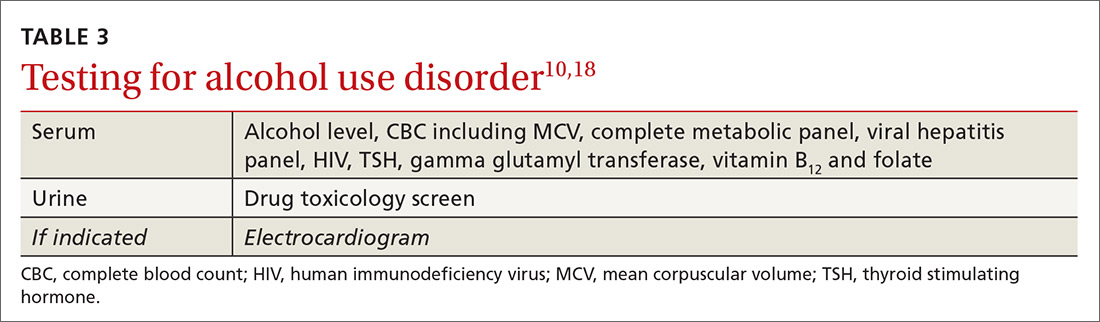
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18
What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
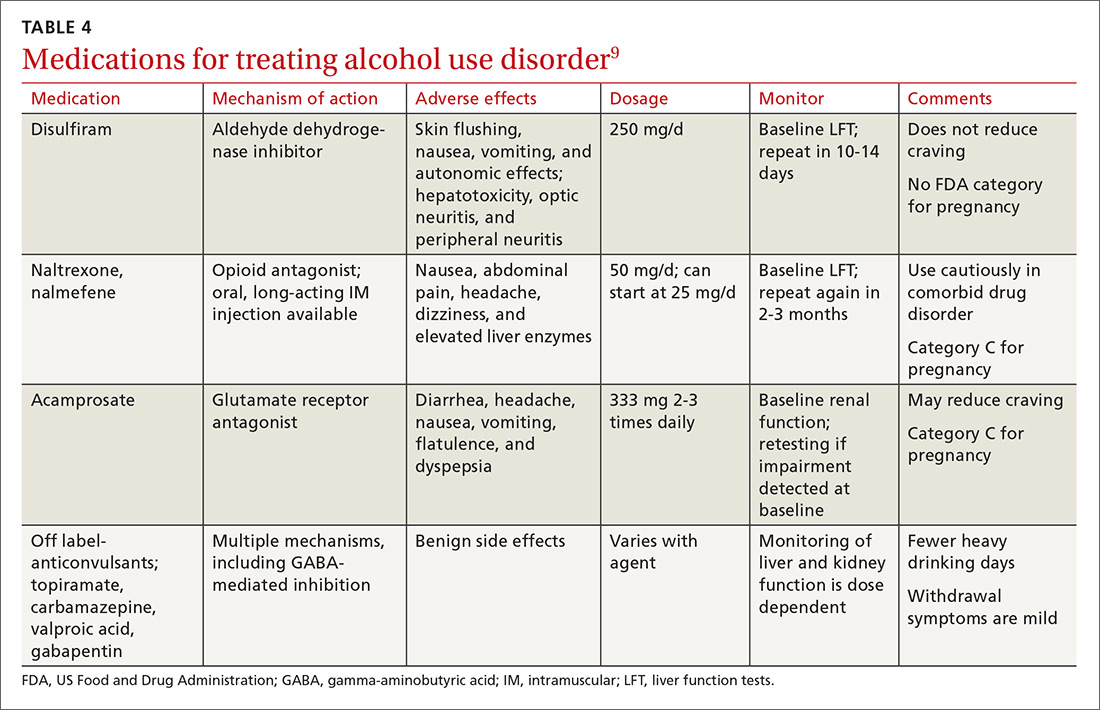
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24
Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
THE CASE
Ms. E, a 42-year-old woman, visited her new physician for a physical exam. When asked about alcohol intake, she reported that she drank 3 to 4 beers after work and sometimes 5 to 8 beers a day on the weekends. Occasionally, she exceeded those amounts, but she didn’t feel guilty about her drinking. She was often late to work and said her relationship with her boyfriend was strained. A review of systems was positive for fatigue, poor concentration, abdominal pain, and weight gain. Her body mass index was 41, pulse 100 beats/min, blood pressure 125/75 mm Hg, and she was afebrile. Her physical exam was otherwise within normal limits.
How would you proceed with this patient?
Alcohol use disorder (AUD) is a common and often untreated condition that is increasingly prevalent in the United States.1 The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) characterizes AUD as a combination of signs and symptoms typifying alcohol abuse and dependence (discussed in a bit).2
Data from the 2015 National Survey on Drug Use and Health (NSDUH) showed 15.7 million Americans with AUD, affecting 6.2% of the population ages 18 years or older and 2.5% of adolescents ages 12 to 17 years.3
Alcohol use and AUD account for an estimated 3.8% of all global deaths and 4.6% of global disability-adjusted life years.4 AUD adversely affects several systems (TABLE 15), and patients with AUD are sicker and more likely to die younger than those without AUD.4 In the United States, prevalence of AUD has increased in recent years among women, older adults, racial minorities, and individuals with a low education level.6

Screening for AUD is reasonable and straightforward, although diagnosis and treatment of AUD in primary care settings may be challenging due to competing clinical priorities; lack of training, resources, and support; and skepticism about the efficacy of behavioral and pharmacologic treatments.7,8 However, family physicians are in an excellent position to diagnose and help address the complex biopsychosocial needs of patients with AUD, often in collaboration with colleagues and community organizations.
Signs and symptoms of AUD
In clinical practice, at least 2 of the following 11 behaviors or symptoms are required to diagnose AUD2:
- consuming larger amounts of alcohol over a longer period than intended
- persistent desire or unsuccessful efforts to cut down or control alcohol use
- making a significant effort to obtain, use, or recover from alcohol
In moderate-to-severe cases:
- cravings or urges to use alcohol
- recurrent failure to fulfill major work, school, or social obligations
- continued alcohol use despite recurrent social and interpersonal problems
- giving up social, occupational, and recreational activities due to alcohol
- using alcohol in physically dangerous situations
- continued alcohol use despite having physical or psychological problems
- tolerance to alcohol’s effects
- withdrawal symptoms.
Continue to: Patients meet criteria for mild AUD severity if...
Patients meet criteria for mild AUD severity if they exhibit 2 or 3 symptoms, moderate AUD with 4 or 5 symptoms, and severe AUD if there are 6 or more symptoms.2
Those who meet criteria for AUD and are able to stop using alcohol are deemed to be in early remission if the criteria have gone unfulfilled for at least 3 months and less than 12 months. Patients are considered to be in sustained remission if they have not met criteria for AUD at any time during a period of 12 months or longer.
How to detect AUD
Several clues in a patient’s history can suggest AUD (TABLE 29,10). Most imbibers are unaware of the dangers and may consider themselves merely “social drinkers.” Binge drinking may be an early indicator of vulnerability to AUD and should be assessed as part of a thorough clinical evaluation.11 The US Preventive Services Task Force (USPSTF) recommends (Grade B) that clinicians screen adults ages 18 years or older for alcohol misuse.12

Studies demonstrate that both genetic and environmental factors play important roles in the development of AUD.13 A family history of excessive alcohol use increases the risk of AUD. Comorbidity of AUD and other mental health conditions is extremely common. For example, high rates of association between major depressive disorder and AUD have been observed.14
Tools to use in screening and diagnosing AUD
Screening for AUD during an office visit can be done fairly quickly. While 96% of primary care physicians screen for alcohol misuse in some way, only 38% use 1 of the 3 tools recommended by the USPSTF15—the Alcohol Use Disorders Identification Test (AUDIT), the abbreviated AUDIT-C, or the National Institute on Alcohol Abuse and Alcoholism (NIAAA) single question screen—which detect the full spectrum of alcohol misuse in adults.12 Although the commonly used CAGE questionnaire is one of the most studied self-report tools, it has lower sensitivity at a lower level of alcohol intake.16
Continue to: The NIAAA single-question screen asks...
The NIAAA single-question screen asks how many times in the past year the patient had ≥4 drinks (women) or ≥5 drinks (men) in a day.15 The sensitivity and specificity of single-question screening are 82% to 87% and 61% to 79%, respectively, and the test has been validated in several different settings.12 The AUDIT screening tool, freely available from the World Health Organization, is a 10-item questionnaire that probes an individual’s alcohol intake, alcohol dependence, and adverse consequences of alcohol use. Administration of the AUDIT typically requires only 2 minutes. AUDIT-C17 is an abbreviated version of the AUDIT questionnaire that asks 3 consumption questions to screen for AUD.
It was found that AUDIT scores in the range of 8 to 15 indicate a medium-level alcohol problem, whereas a score of ≥16 indicates a high-level alcohol problem. The AUDIT-C is scored from 0 to 12, with ≥4 indicating a problem in men and ≥3
THE CASE
The physician had used the NIAAA single- question screen to determine that Ms. E drank more than 4 beers per day during social events and weekends, which occurred 2 to 3 times per month over the past year. She lives alone and said that she’d been seeing less and less of her boyfriend lately. Her score on the Patient Health Questionnaire (PHQ), which screens for depression, was 11, indicating moderate impairment. Her response on the CAGE questionnaire was negative for a problem with alcohol. However, her AUDIT score was 17, indicating a high-level alcohol problem. Based on these findings, her physician expressed concern that her alcohol use might be contributing to her symptoms and difficulties.
Although she did not have a history of increasing usage per day, a persistent desire to cut down, significant effort to obtain alcohol, or cravings, she was having work troubles and continued to drink even though it was straining relationships, promoting weight gain, and causing abdominal pain.
The physician asked her to schedule a return visit and ordered several blood studies. He also offered to connect her with a colleague with whom he collaborated who could speak with her about possible alcohol use disorders and depression.
Continue to: Selecting blood work in screening for AUD
Selecting blood work in screening for AUD
Lab tests used to measure hepatic injury due to alcohol include gamma-glutamyl-transferase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and macrocytic volume, although the indices of hepatic damage have low specificity. Elevated serum ethanol levels can reveal recent alcohol use, and vitamin deficiencies and other abnormalities can be used to differentiate other causes of hepatic inflammation and co-existing health issues (TABLE 310,18). A number of as-yet-unvalidated biomarkers are being studied to assist in screening, diagnosing, and treating AUD.18

What treatment approaches work for AUD?
Family physicians can efficiently and productively address AUD by using alcohol screening and brief intervention, which have been shown to reduce risky drinking. Reimbursement for this service is covered by such CPT codes as 99408, 99409, or H0049, or with other evaluation and management (E/M) codes by using modifier 25.
Treatment of AUD varies and should be customized to each patient’s needs, readiness, preferences, and resources. Individual and group counseling approaches can be effective, and medications are available for inpatient and outpatient settings. Psychotherapy options include brief interventions, 12-step programs (eg, Alcoholics Anonymous—https://www.aa.org/pages/en_US/find-aa-resources),motivational enhancement therapy, and cognitive behavioral therapy. Although it is beyond the scope of this article to describe these options in detail, resources are available for those who wish to learn more.19-21
Psychopharmacologic management includes US Food and Drug Administration (FDA)-approved medications such as disulfiram, naltrexone, and acamprosate, and off-label uses of other medications (TABLE 49). Not enough empiric evidence is available to judge the effectiveness of these medications in adolescents, and the FDA has not approved them for such use. Evidence from meta-analyses comparing naltrexone and acamprosate have shown naltrexone to be more efficacious in reducing heavy drinking and cravings, while acamprosate is effective in promoting abstinence.22,23 Naltrexone combined with behavioral intervention reduces the heavy drinking days and percentage of abstinence days.24

Current guideline recommendations from the American Psychiatric Association25 include:
- Naltrexone and acamprosate are recommended to treat patients with moderate-to-severe AUD in specific circumstances (eg, when nonpharmacologic approaches have failed to produce an effect or when patients prefer to use one of these medications).
- Topiramate and gabapentin are also suggested as medications for patients with moderate-to-severe AUD, but typically after first trying naltrexone and acamprosate.
- Disulfiram generally should not be used as first-line treatment. It produces physical reactions (eg, flushing) if alcohol is consumed within 12 to 24 hours of medication use.
Continue to: THE CASE
THE CASE
Ms. E was open to the idea of decreasing her alcohol use and agreed that she was depressed. Her lab tests at follow-up were normal other than an elevated AST/ALT of 90/80 U/L. S
She continued to get counseling for her AUD and for her comorbid depression in addition to taking a selective serotonin reuptake inhibitor. She is now in early remission for her alcohol use.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Department of Family Medicine, Metro Health Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; [email protected].
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
1. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
2. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington DC; 2013.
3. HHS. Results from the 2015 National Survey on Drug Use and Health: summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed November 27, 2018.
4. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223-2233.
5. Chase V, Neild R, Sadler CW, et al. The medical complications of alcohol use: understanding mechanisms to improve management. Drug Alcohol Rev. 2005;24:253-265.
6. Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923.
7. Williams EC, Achtmeyer CE, Young JP, et al. Barriers to and facilitators of alcohol use disorder pharmacotherapy in primary care: a qualitative study in five VA clinics. J Gen Intern Med. 2018;33:258-267.
8. Zhang DX, Li ST, Lee QK, et al. Systematic review of guidelines on managing patients with harmful use of alcohol in primary healthcare settings. Alcohol Alcohol. 2017;52:595-609.
9. Wackernah RC, Minnick MJ, Clapp P. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil. 2014;5:1-12.
10. Kattimani S, Bharadwaj B. Clinical management of alcohol withdrawal: a systematic review. Ind Psychiatry J. 2013;22:100-108.
11. Gowin JL, Sloan ME, Stangl BL, et al. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry. 2017;174:1094-1101.
12. Moyer VA; Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210-218.
13. Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: a behavior-genetic perspective. J Stud Alcohol. 1985;46:329-356.
14. Brière FN, Rohde P, Seeley JR, et al. Comorbidity between major depression and alcohol use disorder from adolescence to adulthood [published online ahead of print, October 22, 2013]. Compr Psychiatry. 2014;55:526-533. doi: 10.1016/j.comppsych.2013.10.007.
15. Tan CH, Hungerford DW, Denny CH, et al. Screening for alcohol misuse: practices among U.S. primary care providers, DocStyles 2016. Am J Prev Med. 2018;54:173-180.
16. Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30-39.
17. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789-1795.
18. Nanau RM, Neuman MG. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339-1385.
19. Raddock M, Martukovich R, Berko E, et al. 7 tools to help patients adopt healthier behaviors. J Fam Pract. 2015;64:97-103.
20. AHRQ. Whitlock EP, Green CA, Polen MR, et al. Behavioral Counseling Interventions in Primary Care to Reduce Risky/Harmful Alcohol Use. 2004. https://www.ncbi.nlm.nih.gov/books/NBK42863/. Accessed November 17, 2018.
21. Miller WR, Baca C, Compton WM, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292-302.
22. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275-293.
23. Rosner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11-23.
24. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003-2017.
25. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry. 2018;175:86-90.
Buprenorphine for NAS shows promise in reducing length of stay
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
FROM JAMA PEDIATRICS
Key clinical point: A larger study comparing buprenorphine and morphine is needed to confirm study findings.
Major finding: Although morphine and phenobarbital are prescribed most frequently in the United States, they were found to be the least effective treatments available.
Study details: Systematic review and network meta-analysis.
Disclosures: The authors had no financial relationships relevant to this article to disclose.
Source: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
FDA labeling templates smooth way for OTC naloxone
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Family estrangement: Would mutual respect make a difference?
Families are the bedrock of the lives of many, but some people opt to separate permanently from family members. A recent episode of the NPR program “1A” explored why families sever ties.
For journalist Harriet Brown, author of “Shadow Daughter: A Memoir of Estrangement,” (Da Capo Press, 2018), the decision to end her relationship with her mother came after she blamed Ms. Brown in “a blistering email” for the relapsed anorexia of Ms. Brown’s daughter.
“I was done with her,” Ms. Brown said on the program. “I told her I was done. That was it. And I never talked to her again. I think for both of us it felt final in some way.”
She said a lot of shame and stigma comes from having a bad relationship with a parent. “I really wanted to make it clear that ... sometimes walking away is really the best thing to do.”
Kristina M. Scharp, PhD, who has studied the estrangement phenomenon, said no national data exist on family estrangement. “About 12% of mothers and research would suggest even more fathers would report being estranged from one of their children,” said Dr. Scharp, who is with the University of Washington, Seattle. “It’s fairly common.”
Estrangement might be more common today because times have changed, said Joshua Coleman, PhD, author of “When Parents Hurt: Compassionate Strategies When you and Your Grown Child Don’t get Along” (HarperCollins, 2008). “Today’s adult children don’t view their relationships with their parents the way their folks did with their parents … the principles of obligation, duty, and respect that baby boomers and generations before them had for their elders aren’t necessarily there anymore,” Dr. Coleman said in a previous interview with the Chicago Tribune.
For her part, Ms. Brown said, the relationship with her mother – who is now deceased – might have been healed with respect. she said. “Honestly, it was that basic with my mother.”
Phelps gets mental health advocacy award
In the pool, Michael Phelps was golden, with 28 Olympics medals, 23 of them gold, hanging around his neck by the end of his swimming career. But the release from the water to real life after the 2016 Summer Olympics left no outlet for troubles that had dogged him for years. Drunk driving convictions and a ban from competition during his competitive years had failed to stop his downward spiral of depression. His thoughts turned to suicide.
But his story has a bright ending. With his realization that he had hit rock bottom, and with the help of his wife and therapists, he accepted his depression and learned to live with the reality that his life is, for the most part, pretty good.
His openness about his struggles with depression has been influential. The latest recognition came in early January, when he received the Morton E. Ruderman Award in Inclusion from the Ruderman Family Foundation. As reported in the Boston Globe, Mr. Phelps was recognized for his advocacy for people with disabilities and “his own journey with mental health.”
“I do like who I am and I’m comfortable with who I am. I couldn’t say that a few years ago. So I’m in a very good place and just living life one day at a time,” Mr. Phelps remarked in an interview with CNN last year.
Mr. Phelps is now a paid spokesperson for TalkSpace, an online therapy company.
“I’d like to make a difference. I’d like to be able to save a life if I can. You know, for me that’s more important than winning a gold medal. The stuff that I’m doing now is very exciting. It’s hard, it’s challenging but it’s fun for me. That’s what drives me to get out of bed every morning,” he said.
Offenders with mental illness get a break
A law included as part of budget legislation that was signed by then-Gov. Jerry Brown of California in June 2018 has offered people with mental health troubles who have been charged with a crime the opportunity for treatment instead of jail time.
As reported in the Los Angeles Times, the law provides judges with the discretion to order offenders into treatment rather than sentencing them. Success in treatment can lead to charges being dropped.
The law has been praised by some mental health advocates but panned by law enforcement officials and prosecutors with a harder view of criminal justice. The opposition stems largely from a December 2018 ruling by the 4th District Court of Appeal that the law could be applied to retroactively address the case of a man imprisoned for 29 years in 2017 for multiple felony charges that included domestic violence and assault.
The law does not extend to those charged with murder, manslaughter, rape, and child sexual abuse. To date, citing mental illness in seeking diversion of sentencing has not proven successful in most cases.
Schools get mental health allocation
The Orleans Parish School Board, which serves all of New Orleans, will allocate $1.3 million to the Center for Resilience, a local mental health day treatment program, beginning this year. The new program will expand mental health help to children in grades 9-12, according to a report in the Times-Picayune.
The funding will enable the center to expand an existing program that helps students with behavioral issues. Such help is not available in the traditional school system. By offsetting part of the price tag for the mental health care, the initiative “[helps to make] this critical service more available for our students most in need,” said Dominique Ellis, a spokesperson for the school board. “Mental health day treatment programs like the Center for Resilience typically cost between the ranges of $80,000-$100,000 per student to operate effectively.”
The development brings New Orleans level with money spent on similar programs in other school boards nationwide. It’s a service that is sorely needed. According to statistics supplied by the Center for Resilience, 60% of New Orleans children suffer from PTSD and are 4.5 times more likely to suffer from hyperactivity, aggression, and social withdrawal than similarly aged children elsewhere in the United States.
Bill addressing opioid crisis a “no-brainer”
Legislation put forward in the current session of the Texas Legislature would require pharmacists to identify all prescription opioids with a red cap and a hard-to-miss warning of the addictive risk of the medications. In addition, pharmacists would have to explain the risk in person to those receiving the medications and get signed acknowledgment of the conversation before dispensing the drugs.
As described in an article in the Austin American-Stateman, nearly 3,000 people in Texas died from drug overdoses in 2017. The deaths tied to opioid overdoses are not certain, but other data from 2015 suggest that one-third is a reasonable estimate.
“Losing a loved one to an opioid overdose is a tragedy that far too many Texas families experience,” said Rep. Shawn Thierry last month, when she introduced the three bills. “These distinctive red caps will serve as a clear notice to Texans that opioids are unlike milder forms of prescription pain relievers and have life-altering risks that must be considered before taking them.”
Warning labels on everything from food to tobacco products, and including them on prescription opioids is a “no-brainer,” Ms. Thierry said. “The more we can educate our residents the less likely they will be to misuse these medications.”
A report issued in November 2018 chronicled the drug crisis in Texas and offered recommendations. Many of the 100 recommendations involved the absence of treatment resources in the state.
Families are the bedrock of the lives of many, but some people opt to separate permanently from family members. A recent episode of the NPR program “1A” explored why families sever ties.
For journalist Harriet Brown, author of “Shadow Daughter: A Memoir of Estrangement,” (Da Capo Press, 2018), the decision to end her relationship with her mother came after she blamed Ms. Brown in “a blistering email” for the relapsed anorexia of Ms. Brown’s daughter.
“I was done with her,” Ms. Brown said on the program. “I told her I was done. That was it. And I never talked to her again. I think for both of us it felt final in some way.”
She said a lot of shame and stigma comes from having a bad relationship with a parent. “I really wanted to make it clear that ... sometimes walking away is really the best thing to do.”
Kristina M. Scharp, PhD, who has studied the estrangement phenomenon, said no national data exist on family estrangement. “About 12% of mothers and research would suggest even more fathers would report being estranged from one of their children,” said Dr. Scharp, who is with the University of Washington, Seattle. “It’s fairly common.”
Estrangement might be more common today because times have changed, said Joshua Coleman, PhD, author of “When Parents Hurt: Compassionate Strategies When you and Your Grown Child Don’t get Along” (HarperCollins, 2008). “Today’s adult children don’t view their relationships with their parents the way their folks did with their parents … the principles of obligation, duty, and respect that baby boomers and generations before them had for their elders aren’t necessarily there anymore,” Dr. Coleman said in a previous interview with the Chicago Tribune.
For her part, Ms. Brown said, the relationship with her mother – who is now deceased – might have been healed with respect. she said. “Honestly, it was that basic with my mother.”
Phelps gets mental health advocacy award
In the pool, Michael Phelps was golden, with 28 Olympics medals, 23 of them gold, hanging around his neck by the end of his swimming career. But the release from the water to real life after the 2016 Summer Olympics left no outlet for troubles that had dogged him for years. Drunk driving convictions and a ban from competition during his competitive years had failed to stop his downward spiral of depression. His thoughts turned to suicide.
But his story has a bright ending. With his realization that he had hit rock bottom, and with the help of his wife and therapists, he accepted his depression and learned to live with the reality that his life is, for the most part, pretty good.
His openness about his struggles with depression has been influential. The latest recognition came in early January, when he received the Morton E. Ruderman Award in Inclusion from the Ruderman Family Foundation. As reported in the Boston Globe, Mr. Phelps was recognized for his advocacy for people with disabilities and “his own journey with mental health.”
“I do like who I am and I’m comfortable with who I am. I couldn’t say that a few years ago. So I’m in a very good place and just living life one day at a time,” Mr. Phelps remarked in an interview with CNN last year.
Mr. Phelps is now a paid spokesperson for TalkSpace, an online therapy company.
“I’d like to make a difference. I’d like to be able to save a life if I can. You know, for me that’s more important than winning a gold medal. The stuff that I’m doing now is very exciting. It’s hard, it’s challenging but it’s fun for me. That’s what drives me to get out of bed every morning,” he said.
Offenders with mental illness get a break
A law included as part of budget legislation that was signed by then-Gov. Jerry Brown of California in June 2018 has offered people with mental health troubles who have been charged with a crime the opportunity for treatment instead of jail time.
As reported in the Los Angeles Times, the law provides judges with the discretion to order offenders into treatment rather than sentencing them. Success in treatment can lead to charges being dropped.
The law has been praised by some mental health advocates but panned by law enforcement officials and prosecutors with a harder view of criminal justice. The opposition stems largely from a December 2018 ruling by the 4th District Court of Appeal that the law could be applied to retroactively address the case of a man imprisoned for 29 years in 2017 for multiple felony charges that included domestic violence and assault.
The law does not extend to those charged with murder, manslaughter, rape, and child sexual abuse. To date, citing mental illness in seeking diversion of sentencing has not proven successful in most cases.
Schools get mental health allocation
The Orleans Parish School Board, which serves all of New Orleans, will allocate $1.3 million to the Center for Resilience, a local mental health day treatment program, beginning this year. The new program will expand mental health help to children in grades 9-12, according to a report in the Times-Picayune.
The funding will enable the center to expand an existing program that helps students with behavioral issues. Such help is not available in the traditional school system. By offsetting part of the price tag for the mental health care, the initiative “[helps to make] this critical service more available for our students most in need,” said Dominique Ellis, a spokesperson for the school board. “Mental health day treatment programs like the Center for Resilience typically cost between the ranges of $80,000-$100,000 per student to operate effectively.”
The development brings New Orleans level with money spent on similar programs in other school boards nationwide. It’s a service that is sorely needed. According to statistics supplied by the Center for Resilience, 60% of New Orleans children suffer from PTSD and are 4.5 times more likely to suffer from hyperactivity, aggression, and social withdrawal than similarly aged children elsewhere in the United States.
Bill addressing opioid crisis a “no-brainer”
Legislation put forward in the current session of the Texas Legislature would require pharmacists to identify all prescription opioids with a red cap and a hard-to-miss warning of the addictive risk of the medications. In addition, pharmacists would have to explain the risk in person to those receiving the medications and get signed acknowledgment of the conversation before dispensing the drugs.
As described in an article in the Austin American-Stateman, nearly 3,000 people in Texas died from drug overdoses in 2017. The deaths tied to opioid overdoses are not certain, but other data from 2015 suggest that one-third is a reasonable estimate.
“Losing a loved one to an opioid overdose is a tragedy that far too many Texas families experience,” said Rep. Shawn Thierry last month, when she introduced the three bills. “These distinctive red caps will serve as a clear notice to Texans that opioids are unlike milder forms of prescription pain relievers and have life-altering risks that must be considered before taking them.”
Warning labels on everything from food to tobacco products, and including them on prescription opioids is a “no-brainer,” Ms. Thierry said. “The more we can educate our residents the less likely they will be to misuse these medications.”
A report issued in November 2018 chronicled the drug crisis in Texas and offered recommendations. Many of the 100 recommendations involved the absence of treatment resources in the state.
Families are the bedrock of the lives of many, but some people opt to separate permanently from family members. A recent episode of the NPR program “1A” explored why families sever ties.
For journalist Harriet Brown, author of “Shadow Daughter: A Memoir of Estrangement,” (Da Capo Press, 2018), the decision to end her relationship with her mother came after she blamed Ms. Brown in “a blistering email” for the relapsed anorexia of Ms. Brown’s daughter.
“I was done with her,” Ms. Brown said on the program. “I told her I was done. That was it. And I never talked to her again. I think for both of us it felt final in some way.”
She said a lot of shame and stigma comes from having a bad relationship with a parent. “I really wanted to make it clear that ... sometimes walking away is really the best thing to do.”
Kristina M. Scharp, PhD, who has studied the estrangement phenomenon, said no national data exist on family estrangement. “About 12% of mothers and research would suggest even more fathers would report being estranged from one of their children,” said Dr. Scharp, who is with the University of Washington, Seattle. “It’s fairly common.”
Estrangement might be more common today because times have changed, said Joshua Coleman, PhD, author of “When Parents Hurt: Compassionate Strategies When you and Your Grown Child Don’t get Along” (HarperCollins, 2008). “Today’s adult children don’t view their relationships with their parents the way their folks did with their parents … the principles of obligation, duty, and respect that baby boomers and generations before them had for their elders aren’t necessarily there anymore,” Dr. Coleman said in a previous interview with the Chicago Tribune.
For her part, Ms. Brown said, the relationship with her mother – who is now deceased – might have been healed with respect. she said. “Honestly, it was that basic with my mother.”
Phelps gets mental health advocacy award
In the pool, Michael Phelps was golden, with 28 Olympics medals, 23 of them gold, hanging around his neck by the end of his swimming career. But the release from the water to real life after the 2016 Summer Olympics left no outlet for troubles that had dogged him for years. Drunk driving convictions and a ban from competition during his competitive years had failed to stop his downward spiral of depression. His thoughts turned to suicide.
But his story has a bright ending. With his realization that he had hit rock bottom, and with the help of his wife and therapists, he accepted his depression and learned to live with the reality that his life is, for the most part, pretty good.
His openness about his struggles with depression has been influential. The latest recognition came in early January, when he received the Morton E. Ruderman Award in Inclusion from the Ruderman Family Foundation. As reported in the Boston Globe, Mr. Phelps was recognized for his advocacy for people with disabilities and “his own journey with mental health.”
“I do like who I am and I’m comfortable with who I am. I couldn’t say that a few years ago. So I’m in a very good place and just living life one day at a time,” Mr. Phelps remarked in an interview with CNN last year.
Mr. Phelps is now a paid spokesperson for TalkSpace, an online therapy company.
“I’d like to make a difference. I’d like to be able to save a life if I can. You know, for me that’s more important than winning a gold medal. The stuff that I’m doing now is very exciting. It’s hard, it’s challenging but it’s fun for me. That’s what drives me to get out of bed every morning,” he said.
Offenders with mental illness get a break
A law included as part of budget legislation that was signed by then-Gov. Jerry Brown of California in June 2018 has offered people with mental health troubles who have been charged with a crime the opportunity for treatment instead of jail time.
As reported in the Los Angeles Times, the law provides judges with the discretion to order offenders into treatment rather than sentencing them. Success in treatment can lead to charges being dropped.
The law has been praised by some mental health advocates but panned by law enforcement officials and prosecutors with a harder view of criminal justice. The opposition stems largely from a December 2018 ruling by the 4th District Court of Appeal that the law could be applied to retroactively address the case of a man imprisoned for 29 years in 2017 for multiple felony charges that included domestic violence and assault.
The law does not extend to those charged with murder, manslaughter, rape, and child sexual abuse. To date, citing mental illness in seeking diversion of sentencing has not proven successful in most cases.
Schools get mental health allocation
The Orleans Parish School Board, which serves all of New Orleans, will allocate $1.3 million to the Center for Resilience, a local mental health day treatment program, beginning this year. The new program will expand mental health help to children in grades 9-12, according to a report in the Times-Picayune.
The funding will enable the center to expand an existing program that helps students with behavioral issues. Such help is not available in the traditional school system. By offsetting part of the price tag for the mental health care, the initiative “[helps to make] this critical service more available for our students most in need,” said Dominique Ellis, a spokesperson for the school board. “Mental health day treatment programs like the Center for Resilience typically cost between the ranges of $80,000-$100,000 per student to operate effectively.”
The development brings New Orleans level with money spent on similar programs in other school boards nationwide. It’s a service that is sorely needed. According to statistics supplied by the Center for Resilience, 60% of New Orleans children suffer from PTSD and are 4.5 times more likely to suffer from hyperactivity, aggression, and social withdrawal than similarly aged children elsewhere in the United States.
Bill addressing opioid crisis a “no-brainer”
Legislation put forward in the current session of the Texas Legislature would require pharmacists to identify all prescription opioids with a red cap and a hard-to-miss warning of the addictive risk of the medications. In addition, pharmacists would have to explain the risk in person to those receiving the medications and get signed acknowledgment of the conversation before dispensing the drugs.
As described in an article in the Austin American-Stateman, nearly 3,000 people in Texas died from drug overdoses in 2017. The deaths tied to opioid overdoses are not certain, but other data from 2015 suggest that one-third is a reasonable estimate.
“Losing a loved one to an opioid overdose is a tragedy that far too many Texas families experience,” said Rep. Shawn Thierry last month, when she introduced the three bills. “These distinctive red caps will serve as a clear notice to Texans that opioids are unlike milder forms of prescription pain relievers and have life-altering risks that must be considered before taking them.”
Warning labels on everything from food to tobacco products, and including them on prescription opioids is a “no-brainer,” Ms. Thierry said. “The more we can educate our residents the less likely they will be to misuse these medications.”
A report issued in November 2018 chronicled the drug crisis in Texas and offered recommendations. Many of the 100 recommendations involved the absence of treatment resources in the state.
App aims to detect respiratory failure in opioid overdoses
A new smartphone app under development seeks to detect the first moments of an overdose-related respiratory crisis and summon help before it’s too late.
“We’re hoping a device that most people carry around could be transformed into technology that could save your life in an overdose,” said anesthesiologist Jacob (Jake) E. Sunshine, MD, an assistant professor with the University of Washington, Seattle, and coauthor of a study about the app’s development.
The ultimate goal is “to provide a harm reduction system that can automatically connect naloxone-equipped friends and family or emergency medical services to help prevent fatal overdose events,” Rajalakshmi Nandakumar, and her associates wrote in the study, published in Science Translational Medicine.
An estimated 70,000 people in the United States died from drug overdoses in 2017, according to a 2018 data brief from the Centers for Disease Control and Prevention. On an age-adjusted basis, the overdose death rate in 2017 was more than three times higher than in 1999.
The app, which builds on previous work aimed at detecting disordered breathing in sleep apnea, uses a “short-range active sonar system” to detect respiration in a person within the distance of about 3 feet. The approach is similar to the echolocation strategy used by a dolphin or bat, Dr. Sunshine said, and relies on sending out an audio tone that humans cannot hear.
The app’s microphone detects an “audio reflection” of the tone after it bounces off a nearby person’s body and then analyzes it to calculate the distance to the person’s chest. “We’re able to use those distances to measure when someone is taking a breath, and when they’re not taking a breath,” said Dr. Sunshine, who conceptualized the study.
If a disordered breathing pattern is detected, the app is designed to send a text message with a GPS-pinpointed location to a prespecified contact, Dr. Sunshine said. Or the app could be set to call 911.
In the study, the investigators tested the app’s algorithm at a supervised injection facility – a space designed to allow users to inject illicit drugs safely – in Vancouver. They tested the app on 94 drug users as they injected themselves; half of the users “experienced clinically important respiratory depression,” and two needed to be treated by clinic staff for overdose. Both users survived, reported Ms. Nandakumar, a PhD candidate at the University of Washington, Seattle; Shyamnath Gollakota, PhD, an associate professor at the university; and Dr. Sunshine.
(95% confidence interval, 86.0%-99.5%) with 97.7% specificity (95% confidence interval, 88.2%-99.9%). However, the app was less adept at identifying respiratory depression (respiratory rate equal to or less than 7 breaths per minute): The investigators reported 87.2% sensitivity (95% CI, 74.2%-95.1%) and 89.3% specificity (95% CI, 76.9%-96.4%).
Ms. Nandakumar and her associates also tested the app’s algorithm on patients undergoing anesthesia. It correctly detected disordered breathing in 19 of 20 patients.
It’s not clear how the app would work in environments full of breathing people and, potentially, breathing animals such as pets. And the app has clear limitations. Since it needs to be able to bounce audio signals off a user’s chest, it will not work if a phone is in a pocket or if a user is face down, turns around, or wanders off.
However, the app can detect sudden changes in motion, Dr. Sunshine said, and investigators are developing a way to require users to check in with the app in certain situations that might signal trouble.
“For harm reduction interventions to be efficacious, further studies with participant feedback and human factor testing are needed to ensure that the system meets the needs, values, and preferences of people who use opioids, in addition to establishing the system’s safety vis-à-vis its potential to encourage moral hazard,” the investigators wrote in the article.
The next steps are to refine the app’s user interface and figure out how to connect it to the 911 emergency-response system, Dr. Sunshine said. Meanwhile, researchers have created a company to develop the product. “We’re going to do additional development through that entity and seek [Food and Drug Administration] approval,” Dr. Sunshine said. The investigators do not plan to charge users for the product, which can be used on iPhones and Androids, he said.
The study was funded by the Foundation for Anesthesia Education and Research, the National Science Foundation, and the University of Washington’s Alcohol and Drug Abuse Institute. Dr. Sunshine, Ms. Nandakumar, and Dr. Gollakota are inventors on a provisional patient application related to the project, and all have equity stakes in a company that is developing the technology. Dr. Gollakota is a paid consultant to Jeeva Wireless and Edus Health.
SOURCE: Nandakumar R et al. Sci Transl Med. 2019 Jan 9;11(474). doi: 10.1126/scitranslmed.aau8914.
A new smartphone app under development seeks to detect the first moments of an overdose-related respiratory crisis and summon help before it’s too late.
“We’re hoping a device that most people carry around could be transformed into technology that could save your life in an overdose,” said anesthesiologist Jacob (Jake) E. Sunshine, MD, an assistant professor with the University of Washington, Seattle, and coauthor of a study about the app’s development.
The ultimate goal is “to provide a harm reduction system that can automatically connect naloxone-equipped friends and family or emergency medical services to help prevent fatal overdose events,” Rajalakshmi Nandakumar, and her associates wrote in the study, published in Science Translational Medicine.
An estimated 70,000 people in the United States died from drug overdoses in 2017, according to a 2018 data brief from the Centers for Disease Control and Prevention. On an age-adjusted basis, the overdose death rate in 2017 was more than three times higher than in 1999.
The app, which builds on previous work aimed at detecting disordered breathing in sleep apnea, uses a “short-range active sonar system” to detect respiration in a person within the distance of about 3 feet. The approach is similar to the echolocation strategy used by a dolphin or bat, Dr. Sunshine said, and relies on sending out an audio tone that humans cannot hear.
The app’s microphone detects an “audio reflection” of the tone after it bounces off a nearby person’s body and then analyzes it to calculate the distance to the person’s chest. “We’re able to use those distances to measure when someone is taking a breath, and when they’re not taking a breath,” said Dr. Sunshine, who conceptualized the study.
If a disordered breathing pattern is detected, the app is designed to send a text message with a GPS-pinpointed location to a prespecified contact, Dr. Sunshine said. Or the app could be set to call 911.
In the study, the investigators tested the app’s algorithm at a supervised injection facility – a space designed to allow users to inject illicit drugs safely – in Vancouver. They tested the app on 94 drug users as they injected themselves; half of the users “experienced clinically important respiratory depression,” and two needed to be treated by clinic staff for overdose. Both users survived, reported Ms. Nandakumar, a PhD candidate at the University of Washington, Seattle; Shyamnath Gollakota, PhD, an associate professor at the university; and Dr. Sunshine.
(95% confidence interval, 86.0%-99.5%) with 97.7% specificity (95% confidence interval, 88.2%-99.9%). However, the app was less adept at identifying respiratory depression (respiratory rate equal to or less than 7 breaths per minute): The investigators reported 87.2% sensitivity (95% CI, 74.2%-95.1%) and 89.3% specificity (95% CI, 76.9%-96.4%).
Ms. Nandakumar and her associates also tested the app’s algorithm on patients undergoing anesthesia. It correctly detected disordered breathing in 19 of 20 patients.
It’s not clear how the app would work in environments full of breathing people and, potentially, breathing animals such as pets. And the app has clear limitations. Since it needs to be able to bounce audio signals off a user’s chest, it will not work if a phone is in a pocket or if a user is face down, turns around, or wanders off.
However, the app can detect sudden changes in motion, Dr. Sunshine said, and investigators are developing a way to require users to check in with the app in certain situations that might signal trouble.
“For harm reduction interventions to be efficacious, further studies with participant feedback and human factor testing are needed to ensure that the system meets the needs, values, and preferences of people who use opioids, in addition to establishing the system’s safety vis-à-vis its potential to encourage moral hazard,” the investigators wrote in the article.
The next steps are to refine the app’s user interface and figure out how to connect it to the 911 emergency-response system, Dr. Sunshine said. Meanwhile, researchers have created a company to develop the product. “We’re going to do additional development through that entity and seek [Food and Drug Administration] approval,” Dr. Sunshine said. The investigators do not plan to charge users for the product, which can be used on iPhones and Androids, he said.
The study was funded by the Foundation for Anesthesia Education and Research, the National Science Foundation, and the University of Washington’s Alcohol and Drug Abuse Institute. Dr. Sunshine, Ms. Nandakumar, and Dr. Gollakota are inventors on a provisional patient application related to the project, and all have equity stakes in a company that is developing the technology. Dr. Gollakota is a paid consultant to Jeeva Wireless and Edus Health.
SOURCE: Nandakumar R et al. Sci Transl Med. 2019 Jan 9;11(474). doi: 10.1126/scitranslmed.aau8914.
A new smartphone app under development seeks to detect the first moments of an overdose-related respiratory crisis and summon help before it’s too late.
“We’re hoping a device that most people carry around could be transformed into technology that could save your life in an overdose,” said anesthesiologist Jacob (Jake) E. Sunshine, MD, an assistant professor with the University of Washington, Seattle, and coauthor of a study about the app’s development.
The ultimate goal is “to provide a harm reduction system that can automatically connect naloxone-equipped friends and family or emergency medical services to help prevent fatal overdose events,” Rajalakshmi Nandakumar, and her associates wrote in the study, published in Science Translational Medicine.
An estimated 70,000 people in the United States died from drug overdoses in 2017, according to a 2018 data brief from the Centers for Disease Control and Prevention. On an age-adjusted basis, the overdose death rate in 2017 was more than three times higher than in 1999.
The app, which builds on previous work aimed at detecting disordered breathing in sleep apnea, uses a “short-range active sonar system” to detect respiration in a person within the distance of about 3 feet. The approach is similar to the echolocation strategy used by a dolphin or bat, Dr. Sunshine said, and relies on sending out an audio tone that humans cannot hear.
The app’s microphone detects an “audio reflection” of the tone after it bounces off a nearby person’s body and then analyzes it to calculate the distance to the person’s chest. “We’re able to use those distances to measure when someone is taking a breath, and when they’re not taking a breath,” said Dr. Sunshine, who conceptualized the study.
If a disordered breathing pattern is detected, the app is designed to send a text message with a GPS-pinpointed location to a prespecified contact, Dr. Sunshine said. Or the app could be set to call 911.
In the study, the investigators tested the app’s algorithm at a supervised injection facility – a space designed to allow users to inject illicit drugs safely – in Vancouver. They tested the app on 94 drug users as they injected themselves; half of the users “experienced clinically important respiratory depression,” and two needed to be treated by clinic staff for overdose. Both users survived, reported Ms. Nandakumar, a PhD candidate at the University of Washington, Seattle; Shyamnath Gollakota, PhD, an associate professor at the university; and Dr. Sunshine.
(95% confidence interval, 86.0%-99.5%) with 97.7% specificity (95% confidence interval, 88.2%-99.9%). However, the app was less adept at identifying respiratory depression (respiratory rate equal to or less than 7 breaths per minute): The investigators reported 87.2% sensitivity (95% CI, 74.2%-95.1%) and 89.3% specificity (95% CI, 76.9%-96.4%).
Ms. Nandakumar and her associates also tested the app’s algorithm on patients undergoing anesthesia. It correctly detected disordered breathing in 19 of 20 patients.
It’s not clear how the app would work in environments full of breathing people and, potentially, breathing animals such as pets. And the app has clear limitations. Since it needs to be able to bounce audio signals off a user’s chest, it will not work if a phone is in a pocket or if a user is face down, turns around, or wanders off.
However, the app can detect sudden changes in motion, Dr. Sunshine said, and investigators are developing a way to require users to check in with the app in certain situations that might signal trouble.
“For harm reduction interventions to be efficacious, further studies with participant feedback and human factor testing are needed to ensure that the system meets the needs, values, and preferences of people who use opioids, in addition to establishing the system’s safety vis-à-vis its potential to encourage moral hazard,” the investigators wrote in the article.
The next steps are to refine the app’s user interface and figure out how to connect it to the 911 emergency-response system, Dr. Sunshine said. Meanwhile, researchers have created a company to develop the product. “We’re going to do additional development through that entity and seek [Food and Drug Administration] approval,” Dr. Sunshine said. The investigators do not plan to charge users for the product, which can be used on iPhones and Androids, he said.
The study was funded by the Foundation for Anesthesia Education and Research, the National Science Foundation, and the University of Washington’s Alcohol and Drug Abuse Institute. Dr. Sunshine, Ms. Nandakumar, and Dr. Gollakota are inventors on a provisional patient application related to the project, and all have equity stakes in a company that is developing the technology. Dr. Gollakota is a paid consultant to Jeeva Wireless and Edus Health.
SOURCE: Nandakumar R et al. Sci Transl Med. 2019 Jan 9;11(474). doi: 10.1126/scitranslmed.aau8914.
Getting the Right Numbers for Native American Drug-Overdose Deaths
More Native Americans have died of a drug overdose than members of any other racial or ethnic group in the US—which as a whole has seen drug overdose deaths triple since 1999. But little is known about the regional impact of opioids in tribal and urban American Indian/Alaska Native (AI/AN) communities, according to Indian Health Service (IHS) researchers from Portland, Oregon. They examined death records from the Washington State Center for Health Statistics to identify trends and disparities in drug, opioid-involved, and heroin-involved overdose deaths for AI/AN and non-Hispanic whites in Washington.
AI/AN and whites had similar overdose-related death rates during 1999 and 2001, but then the deaths began to multiply faster among Native Americans: Between 2013 and 2015, Native Americans were dying at nearly 3 times the rate of drug and opioid-related overdose. Heroin-related deaths were 4 times higher.
During 2013-2015, 184 AI/AN people died of a drug overdose in Washington; 126 from opioids. Most of the deaths were in urban communities (both Native American and white). Men were nearly twice as likely as women to die of overdose. Adults aged 25 to 54 years had the highest rates. Age-specific drug overdose mortality rates among AI/AN were almost twice those among whites.
The death rates are disturbing enough, but the researchers also found that death certificates that were not corrected for misclassification of AI/AN race underestimated the mortality rates among AI/AN by about 40%. For example, the uncorrected data showed 28.7 drug-overdose deaths in Washington. The corrected number was 40.9. In contrast, the uncorrected number for whites was 15.7, vs 15.1.
Even before correction for misclassification, AI/AN in Washington had higher drug-opioid-involved, and heroin-involved overdose mortality rates than did whites in Washington and AI/AN in the US.
The researchers note that misclassification of AI/AN in public health data can obscure the prevalence of disease and result in suppression of health statistics because of small numbers. That, in turn, can affect the ability of state and federal programs to direct resources needed for a “robust public health response to this epidemic.”
More Native Americans have died of a drug overdose than members of any other racial or ethnic group in the US—which as a whole has seen drug overdose deaths triple since 1999. But little is known about the regional impact of opioids in tribal and urban American Indian/Alaska Native (AI/AN) communities, according to Indian Health Service (IHS) researchers from Portland, Oregon. They examined death records from the Washington State Center for Health Statistics to identify trends and disparities in drug, opioid-involved, and heroin-involved overdose deaths for AI/AN and non-Hispanic whites in Washington.
AI/AN and whites had similar overdose-related death rates during 1999 and 2001, but then the deaths began to multiply faster among Native Americans: Between 2013 and 2015, Native Americans were dying at nearly 3 times the rate of drug and opioid-related overdose. Heroin-related deaths were 4 times higher.
During 2013-2015, 184 AI/AN people died of a drug overdose in Washington; 126 from opioids. Most of the deaths were in urban communities (both Native American and white). Men were nearly twice as likely as women to die of overdose. Adults aged 25 to 54 years had the highest rates. Age-specific drug overdose mortality rates among AI/AN were almost twice those among whites.
The death rates are disturbing enough, but the researchers also found that death certificates that were not corrected for misclassification of AI/AN race underestimated the mortality rates among AI/AN by about 40%. For example, the uncorrected data showed 28.7 drug-overdose deaths in Washington. The corrected number was 40.9. In contrast, the uncorrected number for whites was 15.7, vs 15.1.
Even before correction for misclassification, AI/AN in Washington had higher drug-opioid-involved, and heroin-involved overdose mortality rates than did whites in Washington and AI/AN in the US.
The researchers note that misclassification of AI/AN in public health data can obscure the prevalence of disease and result in suppression of health statistics because of small numbers. That, in turn, can affect the ability of state and federal programs to direct resources needed for a “robust public health response to this epidemic.”
More Native Americans have died of a drug overdose than members of any other racial or ethnic group in the US—which as a whole has seen drug overdose deaths triple since 1999. But little is known about the regional impact of opioids in tribal and urban American Indian/Alaska Native (AI/AN) communities, according to Indian Health Service (IHS) researchers from Portland, Oregon. They examined death records from the Washington State Center for Health Statistics to identify trends and disparities in drug, opioid-involved, and heroin-involved overdose deaths for AI/AN and non-Hispanic whites in Washington.
AI/AN and whites had similar overdose-related death rates during 1999 and 2001, but then the deaths began to multiply faster among Native Americans: Between 2013 and 2015, Native Americans were dying at nearly 3 times the rate of drug and opioid-related overdose. Heroin-related deaths were 4 times higher.
During 2013-2015, 184 AI/AN people died of a drug overdose in Washington; 126 from opioids. Most of the deaths were in urban communities (both Native American and white). Men were nearly twice as likely as women to die of overdose. Adults aged 25 to 54 years had the highest rates. Age-specific drug overdose mortality rates among AI/AN were almost twice those among whites.
The death rates are disturbing enough, but the researchers also found that death certificates that were not corrected for misclassification of AI/AN race underestimated the mortality rates among AI/AN by about 40%. For example, the uncorrected data showed 28.7 drug-overdose deaths in Washington. The corrected number was 40.9. In contrast, the uncorrected number for whites was 15.7, vs 15.1.
Even before correction for misclassification, AI/AN in Washington had higher drug-opioid-involved, and heroin-involved overdose mortality rates than did whites in Washington and AI/AN in the US.
The researchers note that misclassification of AI/AN in public health data can obscure the prevalence of disease and result in suppression of health statistics because of small numbers. That, in turn, can affect the ability of state and federal programs to direct resources needed for a “robust public health response to this epidemic.”
Meth’s resurgence spotlights lack of meds to combat the addiction
In 2016, news reports warned the public of an opioid epidemic gripping the nation.
But Madeline Vaughn, then a lead clinical intake coordinator at the Houston-based addiction treatment organization Council on Recovery, sensed something different was going on with the patients she checked in from the street.
Their behavior, marked by twitchy suspicion, a poor memory, and the feeling that someone was following them, signaled that the people coming through the center’s doors were increasingly hooked on a different drug: methamphetamine.
“When you’re in the boots on the ground,” Ms. Vaughn said, “what you see may surprise you, because it’s not in the headlines.”
In the time since, it’s become increasingly clear that, even as the opioid epidemic continues, the toll of methamphetamine use, also known as meth or crystal meth, is on the rise, too.
The rate of overdose deaths involving the stimulant more than tripled from 2011 to 2016, the Centers for Disease Control and Prevention reported.
But unlike the opioid epidemic – for which medications exist to help combat addiction – medical providers have few such tools to help methamphetamine users survive and recover. A drug such as naloxone, which can reverse an opioid overdose, does not exist for meth. And there are no drugs approved by the Food and Drug Administration that can treat a meth addiction.
“We’re realizing that we don’t have everything we might wish we had to address these different kinds of drugs,” said Margaret Jarvis, MD, a psychiatrist and distinguished fellow for the American Society of Addiction Medicine.
Meth revs up the human body, causing euphoria, elevated blood pressure, and energy that enables users to go for days without sleeping or eating. In some cases, long-term use alters the user’s brain and causes psychotic symptoms that can take up to one year after the person has stopped using it to dissipate.
Overdosing can trigger heart attacks, strokes, and seizures, which can make pinpointing the drug’s involvement difficult.
Meth users also tend to abuse other substances, which complicates first responders’ efforts to treat a patient in the event of an overdose, said David Persse, MD, EMS physician director for Houston. With multiple drugs in a patient’s system, overdose symptoms may not neatly fit under the description for one substance.
“If we had five or six miracle drugs,” Dr. Persse said, to use immediately on the scene of the overdose, “it’s still gonna be difficult to know which one that patient needs.”
Research is underway to develop a medication that helps those with methamphetamine addiction overcome their condition. The National Institute on Drug Abuse Clinical Trials Network is testing a combination of naltrexone, a medication typically used to treat opioid and alcohol use disorders, and an antidepressant called bupropion.
And a team from the Universities of Kentucky and Arkansas created a molecule called lobeline that shows promise in blocking meth’s effects in the brain.
For now, though, existing treatments, such as the Matrix Model, a drug counseling technique, and contingency management, which offers patients incentives to stay away from drugs, are key options for what appears to be a meth resurgence, said Dr. Jarvis.
Illegal drugs never disappear from the street, she said. Their popularity waxes and wanes with demand. And as the demand for methamphetamine use increases, the gaps in treatment become more apparent.
Dr. Persse said he hasn’t seen a rise in the number of calls related to methamphetamine overdoses in his area. However, the death toll in Texas from meth now exceeds that of heroin.
Provisional death counts for 2017 showed methamphetamine claimed 813 lives in the Lone Star State. By comparison, 591 people died because of heroin.
The Drug Enforcement Administration reported that the price of meth is the lowest the agency has seen in years. It is increasingly available in the eastern region of the United States. Primary suppliers are Mexican drug cartels. And the meth on the streets is now more than 90% pure.
“The new methods [of making methamphetamine] have really altered the potency,” said Jane Maxwell, PhD, research professor at the University of Texas at Austin’s social work school. “So the meth we’re looking at today is much more potent than it was 10 years ago.”
For Ms. Vaughn, who works as an outpatient therapist and treatment coordinator, these variables are a regular part of her daily challenge. So until the research arms her with something new, her go-to strategy is to use the available tools to tackle her patients’ methamphetamine addiction in layers.
She starts with writing assignments, then coping skills until they are capable of unpacking their trauma. Addiction is rarely the sole demon patients wrestle with, Ms. Vaughn said.
“Substance use is often a symptom for what’s really going on with someone,” she said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
In 2016, news reports warned the public of an opioid epidemic gripping the nation.
But Madeline Vaughn, then a lead clinical intake coordinator at the Houston-based addiction treatment organization Council on Recovery, sensed something different was going on with the patients she checked in from the street.
Their behavior, marked by twitchy suspicion, a poor memory, and the feeling that someone was following them, signaled that the people coming through the center’s doors were increasingly hooked on a different drug: methamphetamine.
“When you’re in the boots on the ground,” Ms. Vaughn said, “what you see may surprise you, because it’s not in the headlines.”
In the time since, it’s become increasingly clear that, even as the opioid epidemic continues, the toll of methamphetamine use, also known as meth or crystal meth, is on the rise, too.
The rate of overdose deaths involving the stimulant more than tripled from 2011 to 2016, the Centers for Disease Control and Prevention reported.
But unlike the opioid epidemic – for which medications exist to help combat addiction – medical providers have few such tools to help methamphetamine users survive and recover. A drug such as naloxone, which can reverse an opioid overdose, does not exist for meth. And there are no drugs approved by the Food and Drug Administration that can treat a meth addiction.
“We’re realizing that we don’t have everything we might wish we had to address these different kinds of drugs,” said Margaret Jarvis, MD, a psychiatrist and distinguished fellow for the American Society of Addiction Medicine.
Meth revs up the human body, causing euphoria, elevated blood pressure, and energy that enables users to go for days without sleeping or eating. In some cases, long-term use alters the user’s brain and causes psychotic symptoms that can take up to one year after the person has stopped using it to dissipate.
Overdosing can trigger heart attacks, strokes, and seizures, which can make pinpointing the drug’s involvement difficult.
Meth users also tend to abuse other substances, which complicates first responders’ efforts to treat a patient in the event of an overdose, said David Persse, MD, EMS physician director for Houston. With multiple drugs in a patient’s system, overdose symptoms may not neatly fit under the description for one substance.
“If we had five or six miracle drugs,” Dr. Persse said, to use immediately on the scene of the overdose, “it’s still gonna be difficult to know which one that patient needs.”
Research is underway to develop a medication that helps those with methamphetamine addiction overcome their condition. The National Institute on Drug Abuse Clinical Trials Network is testing a combination of naltrexone, a medication typically used to treat opioid and alcohol use disorders, and an antidepressant called bupropion.
And a team from the Universities of Kentucky and Arkansas created a molecule called lobeline that shows promise in blocking meth’s effects in the brain.
For now, though, existing treatments, such as the Matrix Model, a drug counseling technique, and contingency management, which offers patients incentives to stay away from drugs, are key options for what appears to be a meth resurgence, said Dr. Jarvis.
Illegal drugs never disappear from the street, she said. Their popularity waxes and wanes with demand. And as the demand for methamphetamine use increases, the gaps in treatment become more apparent.
Dr. Persse said he hasn’t seen a rise in the number of calls related to methamphetamine overdoses in his area. However, the death toll in Texas from meth now exceeds that of heroin.
Provisional death counts for 2017 showed methamphetamine claimed 813 lives in the Lone Star State. By comparison, 591 people died because of heroin.
The Drug Enforcement Administration reported that the price of meth is the lowest the agency has seen in years. It is increasingly available in the eastern region of the United States. Primary suppliers are Mexican drug cartels. And the meth on the streets is now more than 90% pure.
“The new methods [of making methamphetamine] have really altered the potency,” said Jane Maxwell, PhD, research professor at the University of Texas at Austin’s social work school. “So the meth we’re looking at today is much more potent than it was 10 years ago.”
For Ms. Vaughn, who works as an outpatient therapist and treatment coordinator, these variables are a regular part of her daily challenge. So until the research arms her with something new, her go-to strategy is to use the available tools to tackle her patients’ methamphetamine addiction in layers.
She starts with writing assignments, then coping skills until they are capable of unpacking their trauma. Addiction is rarely the sole demon patients wrestle with, Ms. Vaughn said.
“Substance use is often a symptom for what’s really going on with someone,” she said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
In 2016, news reports warned the public of an opioid epidemic gripping the nation.
But Madeline Vaughn, then a lead clinical intake coordinator at the Houston-based addiction treatment organization Council on Recovery, sensed something different was going on with the patients she checked in from the street.
Their behavior, marked by twitchy suspicion, a poor memory, and the feeling that someone was following them, signaled that the people coming through the center’s doors were increasingly hooked on a different drug: methamphetamine.
“When you’re in the boots on the ground,” Ms. Vaughn said, “what you see may surprise you, because it’s not in the headlines.”
In the time since, it’s become increasingly clear that, even as the opioid epidemic continues, the toll of methamphetamine use, also known as meth or crystal meth, is on the rise, too.
The rate of overdose deaths involving the stimulant more than tripled from 2011 to 2016, the Centers for Disease Control and Prevention reported.
But unlike the opioid epidemic – for which medications exist to help combat addiction – medical providers have few such tools to help methamphetamine users survive and recover. A drug such as naloxone, which can reverse an opioid overdose, does not exist for meth. And there are no drugs approved by the Food and Drug Administration that can treat a meth addiction.
“We’re realizing that we don’t have everything we might wish we had to address these different kinds of drugs,” said Margaret Jarvis, MD, a psychiatrist and distinguished fellow for the American Society of Addiction Medicine.
Meth revs up the human body, causing euphoria, elevated blood pressure, and energy that enables users to go for days without sleeping or eating. In some cases, long-term use alters the user’s brain and causes psychotic symptoms that can take up to one year after the person has stopped using it to dissipate.
Overdosing can trigger heart attacks, strokes, and seizures, which can make pinpointing the drug’s involvement difficult.
Meth users also tend to abuse other substances, which complicates first responders’ efforts to treat a patient in the event of an overdose, said David Persse, MD, EMS physician director for Houston. With multiple drugs in a patient’s system, overdose symptoms may not neatly fit under the description for one substance.
“If we had five or six miracle drugs,” Dr. Persse said, to use immediately on the scene of the overdose, “it’s still gonna be difficult to know which one that patient needs.”
Research is underway to develop a medication that helps those with methamphetamine addiction overcome their condition. The National Institute on Drug Abuse Clinical Trials Network is testing a combination of naltrexone, a medication typically used to treat opioid and alcohol use disorders, and an antidepressant called bupropion.
And a team from the Universities of Kentucky and Arkansas created a molecule called lobeline that shows promise in blocking meth’s effects in the brain.
For now, though, existing treatments, such as the Matrix Model, a drug counseling technique, and contingency management, which offers patients incentives to stay away from drugs, are key options for what appears to be a meth resurgence, said Dr. Jarvis.
Illegal drugs never disappear from the street, she said. Their popularity waxes and wanes with demand. And as the demand for methamphetamine use increases, the gaps in treatment become more apparent.
Dr. Persse said he hasn’t seen a rise in the number of calls related to methamphetamine overdoses in his area. However, the death toll in Texas from meth now exceeds that of heroin.
Provisional death counts for 2017 showed methamphetamine claimed 813 lives in the Lone Star State. By comparison, 591 people died because of heroin.
The Drug Enforcement Administration reported that the price of meth is the lowest the agency has seen in years. It is increasingly available in the eastern region of the United States. Primary suppliers are Mexican drug cartels. And the meth on the streets is now more than 90% pure.
“The new methods [of making methamphetamine] have really altered the potency,” said Jane Maxwell, PhD, research professor at the University of Texas at Austin’s social work school. “So the meth we’re looking at today is much more potent than it was 10 years ago.”
For Ms. Vaughn, who works as an outpatient therapist and treatment coordinator, these variables are a regular part of her daily challenge. So until the research arms her with something new, her go-to strategy is to use the available tools to tackle her patients’ methamphetamine addiction in layers.
She starts with writing assignments, then coping skills until they are capable of unpacking their trauma. Addiction is rarely the sole demon patients wrestle with, Ms. Vaughn said.
“Substance use is often a symptom for what’s really going on with someone,” she said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Opioid crisis offers poignant lessons for public health
Populations and circumstances matter
As a medical student in New York City in the mid-1980s, I did several of my clinical clerkships at Memorial Sloan Kettering Cancer Center. One night during my general surgery rotation, there was a young woman with anal cancer who complained of pain.
My resident did not want to give her more medication and I asked why. After all, this was a cancer center with progressive ideas about pain management, this patient was suffering, and she was ill enough to be hospitalized.
The resident responded to my inquiry: “She doesn’t have a terminal condition and she has an addictive personality.” It seemed to me a draconian (and perhaps sexist) response in a hospital where patient-controlled analgesia was becoming routine and, as an aspiring psychiatrist, I didn’t quite trust the surgical resident’s evaluation of the patient’s personality or his ability to predict if she might become addicted to opiates.
This encounter happened about 6 years after Jane Porter and Hershel Jink, MD, had published a letter titled, “Addiction Rare in Patients Treated with Narcotics” in the New England Journal of Medicine (1980 Jan 10;302:123) with the following finding: “... of 11,800 patients given narcotic painkillers while in hospital, only four developed an addiction to those drugs.” This fragment of a sentence, published as a one-paragraph letter and not as a full, peer-reviewed study, was in the process of changing how all of American medicine responded to pain.
In his book, “Dreamland: The True Tale of America’s Opiate Epidemic” (Bloomsbury Press, 2015), journalist Sam Quinones was quick to point out that these findings were made at a time when doctors, like my surgical resident, were hesitant to use opiates for fear of addiction. Their use was limited to cancer patients, postoperative patients, and those suffering from an acute injury. This finding that prescribed opiates did not cause addiction was true in these hospitalized patients, at a time when pills were doled out with caution for short-term use, and their use for chronic pain had not yet been tested.
Nearly 40 years later, we know that the answer to that national experiment did not work out so well: A proportion of patients given long-term, sometimes high-dose, opiates for chronic pain do sometimes become addicted. Some chronic pain patients received narcotics at “pill mills,” and some went on to use heroin obtained illegally. Furthermore, the widespread use of these medicines made them more readily available to those looking for something besides pain relief.
I would like to suggest that the opioid epidemic is not solely the fault of the medical community: We had drug addiction long before we had the Porter and Jink paragraph and not all addiction starts with a prescription pad. Still, the lesson for public health is a poignant one: Populations and circumstances matter. Be careful with generalizations.
Still, we see these generalizations all the time. I am sometimes surprised at how many people have “the answer.” Whether it’s more widespread availability of Narcan, medication-assisted treatment (MAT), safe injection sites, 12-step programs, or “Just Say No,” every method has its proponents. I always wonder when I see public health officials propose safe injection sites as something that would surely save thousands of lives, citing data out of cities such as Vancouver, as well as in Europe, and Australia, if results in those places would transfer to my city – Baltimore – where drug addiction, violence, and poverty are rampant. Perhaps they would, and I would love to see Baltimore try anything that might work. But I hope cities that do set up such sites will follow the numbers and halt any program that does not offer robust results.
I wonder, as well, why, with the clear success of MAT strategies in reducing mortality, we don’t experiment with ways of making these methods more accessible. Might Suboxone work if doctors could prescribe it as easily as they can prescribe oxycodone, with no 8-hour course or DEA waiver? Might methadone both work and be more acceptable to patients if given in a way that didn’t require daily travel to a clinic for administration? With such a deadly pervasive epidemic, I wonder also about our focus on treating addiction, when it seems we should have a parallel focus on understanding and addressing the factors that cause addiction. Medical prescribing is but one avenue to addiction, yet we have no understanding as to why some people become addicted when others do not. Shouldn’t we be able to prevent addiction? From Richard Nixon’s “war on drugs” to Donald Trump’s physical border wall, there are many answers, but few solutions.
There are other public health issues that suffer from the same generalizations. In psychiatry, advocacy groups tout involuntary outpatient treatment as a successful way of getting treatment to vulnerable individuals who will not willingly negotiate their own care. While a pilot study at Bellevue showed no benefit to mandated care, a follow-up study showed that mandated treatment was effective at reducing hospital days. While outpatient commitment studies look at rates of hospitalization, incarceration, and quality-of-life measures, mandated treatment is often cited as a means to prevent all forms of violence, including mass shootings, while there is no evidence to support these ideas. Still, 47 states and the District of Columbia now have outpatient commitment laws.
Does involuntary care benefit those with substance use disorders? In Massachusetts, Section 35 allows for civil commitment for drug treatment, and many of the treatment facilities are run by the Department of Corrections. It would be good to know if these measures worked. So far, it looks like opioid deaths in Massachusetts have stabilized, while the overdose death rate continues to rise in other states. Whether this is a result of Section 35 or other measures is unknown.
I’m not against innovation, and desperate situations call for creative responses. We need to be careful that our responses are measured and these experiments are contained while ascertaining what really does work and what does not cause unintended harms. Will a concrete wall stem the flow of illegal heroin? I imagine a new world of drones making drug drops.
Sometimes our innovative best guesses don’t work, and sometimes they do. Despite easy access to antidepressant medications, a national suicide hotline, increased numbers of mental health professionals, and anti-stigma/awareness campaigns, suicide rates continue to rise. Efforts to end smoking, however, have been quite successful, as have measures to get Americans to buckle their seat belts, and these measures have decreased mortality rates. The recommendation for healthy women to take hormone therapy is a good example: It was an innovative recommendation to help cardiac and orthopedic outcomes, yet studies that were run alongside these recommendations were quick to show an unintended increased risk of breast and uterine cancer.
I don’t know what happened to the young woman on my surgical rotation. If the decision were mine, I would have given her more pain medication, even now, but I don’t know if that would have been the right thing to do., and to look carefully at our outcomes in a variety of populations and circumstances.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
Populations and circumstances matter
Populations and circumstances matter
As a medical student in New York City in the mid-1980s, I did several of my clinical clerkships at Memorial Sloan Kettering Cancer Center. One night during my general surgery rotation, there was a young woman with anal cancer who complained of pain.
My resident did not want to give her more medication and I asked why. After all, this was a cancer center with progressive ideas about pain management, this patient was suffering, and she was ill enough to be hospitalized.
The resident responded to my inquiry: “She doesn’t have a terminal condition and she has an addictive personality.” It seemed to me a draconian (and perhaps sexist) response in a hospital where patient-controlled analgesia was becoming routine and, as an aspiring psychiatrist, I didn’t quite trust the surgical resident’s evaluation of the patient’s personality or his ability to predict if she might become addicted to opiates.
This encounter happened about 6 years after Jane Porter and Hershel Jink, MD, had published a letter titled, “Addiction Rare in Patients Treated with Narcotics” in the New England Journal of Medicine (1980 Jan 10;302:123) with the following finding: “... of 11,800 patients given narcotic painkillers while in hospital, only four developed an addiction to those drugs.” This fragment of a sentence, published as a one-paragraph letter and not as a full, peer-reviewed study, was in the process of changing how all of American medicine responded to pain.
In his book, “Dreamland: The True Tale of America’s Opiate Epidemic” (Bloomsbury Press, 2015), journalist Sam Quinones was quick to point out that these findings were made at a time when doctors, like my surgical resident, were hesitant to use opiates for fear of addiction. Their use was limited to cancer patients, postoperative patients, and those suffering from an acute injury. This finding that prescribed opiates did not cause addiction was true in these hospitalized patients, at a time when pills were doled out with caution for short-term use, and their use for chronic pain had not yet been tested.
Nearly 40 years later, we know that the answer to that national experiment did not work out so well: A proportion of patients given long-term, sometimes high-dose, opiates for chronic pain do sometimes become addicted. Some chronic pain patients received narcotics at “pill mills,” and some went on to use heroin obtained illegally. Furthermore, the widespread use of these medicines made them more readily available to those looking for something besides pain relief.
I would like to suggest that the opioid epidemic is not solely the fault of the medical community: We had drug addiction long before we had the Porter and Jink paragraph and not all addiction starts with a prescription pad. Still, the lesson for public health is a poignant one: Populations and circumstances matter. Be careful with generalizations.
Still, we see these generalizations all the time. I am sometimes surprised at how many people have “the answer.” Whether it’s more widespread availability of Narcan, medication-assisted treatment (MAT), safe injection sites, 12-step programs, or “Just Say No,” every method has its proponents. I always wonder when I see public health officials propose safe injection sites as something that would surely save thousands of lives, citing data out of cities such as Vancouver, as well as in Europe, and Australia, if results in those places would transfer to my city – Baltimore – where drug addiction, violence, and poverty are rampant. Perhaps they would, and I would love to see Baltimore try anything that might work. But I hope cities that do set up such sites will follow the numbers and halt any program that does not offer robust results.
I wonder, as well, why, with the clear success of MAT strategies in reducing mortality, we don’t experiment with ways of making these methods more accessible. Might Suboxone work if doctors could prescribe it as easily as they can prescribe oxycodone, with no 8-hour course or DEA waiver? Might methadone both work and be more acceptable to patients if given in a way that didn’t require daily travel to a clinic for administration? With such a deadly pervasive epidemic, I wonder also about our focus on treating addiction, when it seems we should have a parallel focus on understanding and addressing the factors that cause addiction. Medical prescribing is but one avenue to addiction, yet we have no understanding as to why some people become addicted when others do not. Shouldn’t we be able to prevent addiction? From Richard Nixon’s “war on drugs” to Donald Trump’s physical border wall, there are many answers, but few solutions.
There are other public health issues that suffer from the same generalizations. In psychiatry, advocacy groups tout involuntary outpatient treatment as a successful way of getting treatment to vulnerable individuals who will not willingly negotiate their own care. While a pilot study at Bellevue showed no benefit to mandated care, a follow-up study showed that mandated treatment was effective at reducing hospital days. While outpatient commitment studies look at rates of hospitalization, incarceration, and quality-of-life measures, mandated treatment is often cited as a means to prevent all forms of violence, including mass shootings, while there is no evidence to support these ideas. Still, 47 states and the District of Columbia now have outpatient commitment laws.
Does involuntary care benefit those with substance use disorders? In Massachusetts, Section 35 allows for civil commitment for drug treatment, and many of the treatment facilities are run by the Department of Corrections. It would be good to know if these measures worked. So far, it looks like opioid deaths in Massachusetts have stabilized, while the overdose death rate continues to rise in other states. Whether this is a result of Section 35 or other measures is unknown.
I’m not against innovation, and desperate situations call for creative responses. We need to be careful that our responses are measured and these experiments are contained while ascertaining what really does work and what does not cause unintended harms. Will a concrete wall stem the flow of illegal heroin? I imagine a new world of drones making drug drops.
Sometimes our innovative best guesses don’t work, and sometimes they do. Despite easy access to antidepressant medications, a national suicide hotline, increased numbers of mental health professionals, and anti-stigma/awareness campaigns, suicide rates continue to rise. Efforts to end smoking, however, have been quite successful, as have measures to get Americans to buckle their seat belts, and these measures have decreased mortality rates. The recommendation for healthy women to take hormone therapy is a good example: It was an innovative recommendation to help cardiac and orthopedic outcomes, yet studies that were run alongside these recommendations were quick to show an unintended increased risk of breast and uterine cancer.
I don’t know what happened to the young woman on my surgical rotation. If the decision were mine, I would have given her more pain medication, even now, but I don’t know if that would have been the right thing to do., and to look carefully at our outcomes in a variety of populations and circumstances.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
As a medical student in New York City in the mid-1980s, I did several of my clinical clerkships at Memorial Sloan Kettering Cancer Center. One night during my general surgery rotation, there was a young woman with anal cancer who complained of pain.
My resident did not want to give her more medication and I asked why. After all, this was a cancer center with progressive ideas about pain management, this patient was suffering, and she was ill enough to be hospitalized.
The resident responded to my inquiry: “She doesn’t have a terminal condition and she has an addictive personality.” It seemed to me a draconian (and perhaps sexist) response in a hospital where patient-controlled analgesia was becoming routine and, as an aspiring psychiatrist, I didn’t quite trust the surgical resident’s evaluation of the patient’s personality or his ability to predict if she might become addicted to opiates.
This encounter happened about 6 years after Jane Porter and Hershel Jink, MD, had published a letter titled, “Addiction Rare in Patients Treated with Narcotics” in the New England Journal of Medicine (1980 Jan 10;302:123) with the following finding: “... of 11,800 patients given narcotic painkillers while in hospital, only four developed an addiction to those drugs.” This fragment of a sentence, published as a one-paragraph letter and not as a full, peer-reviewed study, was in the process of changing how all of American medicine responded to pain.
In his book, “Dreamland: The True Tale of America’s Opiate Epidemic” (Bloomsbury Press, 2015), journalist Sam Quinones was quick to point out that these findings were made at a time when doctors, like my surgical resident, were hesitant to use opiates for fear of addiction. Their use was limited to cancer patients, postoperative patients, and those suffering from an acute injury. This finding that prescribed opiates did not cause addiction was true in these hospitalized patients, at a time when pills were doled out with caution for short-term use, and their use for chronic pain had not yet been tested.
Nearly 40 years later, we know that the answer to that national experiment did not work out so well: A proportion of patients given long-term, sometimes high-dose, opiates for chronic pain do sometimes become addicted. Some chronic pain patients received narcotics at “pill mills,” and some went on to use heroin obtained illegally. Furthermore, the widespread use of these medicines made them more readily available to those looking for something besides pain relief.
I would like to suggest that the opioid epidemic is not solely the fault of the medical community: We had drug addiction long before we had the Porter and Jink paragraph and not all addiction starts with a prescription pad. Still, the lesson for public health is a poignant one: Populations and circumstances matter. Be careful with generalizations.
Still, we see these generalizations all the time. I am sometimes surprised at how many people have “the answer.” Whether it’s more widespread availability of Narcan, medication-assisted treatment (MAT), safe injection sites, 12-step programs, or “Just Say No,” every method has its proponents. I always wonder when I see public health officials propose safe injection sites as something that would surely save thousands of lives, citing data out of cities such as Vancouver, as well as in Europe, and Australia, if results in those places would transfer to my city – Baltimore – where drug addiction, violence, and poverty are rampant. Perhaps they would, and I would love to see Baltimore try anything that might work. But I hope cities that do set up such sites will follow the numbers and halt any program that does not offer robust results.
I wonder, as well, why, with the clear success of MAT strategies in reducing mortality, we don’t experiment with ways of making these methods more accessible. Might Suboxone work if doctors could prescribe it as easily as they can prescribe oxycodone, with no 8-hour course or DEA waiver? Might methadone both work and be more acceptable to patients if given in a way that didn’t require daily travel to a clinic for administration? With such a deadly pervasive epidemic, I wonder also about our focus on treating addiction, when it seems we should have a parallel focus on understanding and addressing the factors that cause addiction. Medical prescribing is but one avenue to addiction, yet we have no understanding as to why some people become addicted when others do not. Shouldn’t we be able to prevent addiction? From Richard Nixon’s “war on drugs” to Donald Trump’s physical border wall, there are many answers, but few solutions.
There are other public health issues that suffer from the same generalizations. In psychiatry, advocacy groups tout involuntary outpatient treatment as a successful way of getting treatment to vulnerable individuals who will not willingly negotiate their own care. While a pilot study at Bellevue showed no benefit to mandated care, a follow-up study showed that mandated treatment was effective at reducing hospital days. While outpatient commitment studies look at rates of hospitalization, incarceration, and quality-of-life measures, mandated treatment is often cited as a means to prevent all forms of violence, including mass shootings, while there is no evidence to support these ideas. Still, 47 states and the District of Columbia now have outpatient commitment laws.
Does involuntary care benefit those with substance use disorders? In Massachusetts, Section 35 allows for civil commitment for drug treatment, and many of the treatment facilities are run by the Department of Corrections. It would be good to know if these measures worked. So far, it looks like opioid deaths in Massachusetts have stabilized, while the overdose death rate continues to rise in other states. Whether this is a result of Section 35 or other measures is unknown.
I’m not against innovation, and desperate situations call for creative responses. We need to be careful that our responses are measured and these experiments are contained while ascertaining what really does work and what does not cause unintended harms. Will a concrete wall stem the flow of illegal heroin? I imagine a new world of drones making drug drops.
Sometimes our innovative best guesses don’t work, and sometimes they do. Despite easy access to antidepressant medications, a national suicide hotline, increased numbers of mental health professionals, and anti-stigma/awareness campaigns, suicide rates continue to rise. Efforts to end smoking, however, have been quite successful, as have measures to get Americans to buckle their seat belts, and these measures have decreased mortality rates. The recommendation for healthy women to take hormone therapy is a good example: It was an innovative recommendation to help cardiac and orthopedic outcomes, yet studies that were run alongside these recommendations were quick to show an unintended increased risk of breast and uterine cancer.
I don’t know what happened to the young woman on my surgical rotation. If the decision were mine, I would have given her more pain medication, even now, but I don’t know if that would have been the right thing to do., and to look carefully at our outcomes in a variety of populations and circumstances.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.