User login
Treatment in prison systems might lead to drop in overdose deaths
Incarceration versus treatment takes center stage in a new analysis of U.S. data from researchers in the United Kingdom.
The researchers performed an observational study looking at rates of incarceration, income, and drug-related deaths from 1983 to 2014 in the United States. They found a strong association between incarceration rates and drug-related deaths. Also, a very strong association was found between lower household income and drug-related deaths. Strikingly, in the counties with the highest incarceration rates, there was a 50% higher rate of drug deaths, reported Elias Nosrati, PhD, and associates (Lancet Public Health. 2019 Jul 3;4:e326-33). It is clearer every day that our opioid epidemic was in part wrought by a zealous push to change protocols on treating chronic pain. The epidemic also appears tied to well-meaning but overprescribing doctors and allegedly unscrupulous pharmaceutical companies and distributors. What we are learning through this most recent study is that another factor tied to the opioid and overdose epidemic could be incarceration.
According to the study, an increase in crime rates combined with sentencing reforms led the number of people incarcerated in state and federal prisons to soar from less than 200,000 in 1970 to almost 1 million in 1995. Furthermore, Dr. Nosrati and associates wrote, “Incarceration is directly associated with stigma, discrimination, poor mental health, and chronic economic hardship, all of which are linked to drug use disorders.”
Treatment for drug addiction in prison systems is rare, as is adequate mental health treatment. However, treatment for this population would likely help reduce drug overdose deaths and improve the quality of life for people who are incarcerated and their families. In the Philadelphia prison system, for example, treatment for inmates is available for opioid addiction, both with methadone and now more recently with buprenorphine (Suboxone). The Philadelphia Department of Prisons also provides cognitive-behavioral therapy. In Florida, Chapter 397 of the Florida statutes – known as the Marchman Act – provides for the involuntary (and voluntary) treatment of individuals with substance abuse problems.
The court systems in South Florida have a robust drug-diversion program, aimed at directing people facing incarceration for drug offenses into treatment instead. North Carolina has studied this issue specifically and found through a model simulation that diverting 10% of drug-abusing offenders out of incarceration into treatment would save $4.8 billion in legal costs for North Carolina counties and state legal systems. Diverting 40% of individuals would close to triple that savings.
There are striking data from programs treating individuals who are leveraged into treatment in order to maintain professional licenses. These such individuals, many of whom are physicians, airline pilots, and nurses, have a rate of sobriety of 90% or greater after 5 years. This data show that
In addition to the potential reduction in morbidity and mortality as well as the financial savings, why is treatment important? Because of societal costs. When parents or family members are put in jail for a drug charge or other charge, they leave behind a community, family, and very often children who are affected economically, emotionally, and socially. Those children in particular have higher risks of depression and PTSD. Diverting an offender into treatment or treating an incarcerated person for drug and mental health problems can change the life of a child or family member, and ultimately can change society.
Dr. Jorandby is chief medical officer of Lakeview Health in Jacksonville, Fla. She trained in addiction psychiatry at Yale University, New Haven, Conn.
Incarceration versus treatment takes center stage in a new analysis of U.S. data from researchers in the United Kingdom.
The researchers performed an observational study looking at rates of incarceration, income, and drug-related deaths from 1983 to 2014 in the United States. They found a strong association between incarceration rates and drug-related deaths. Also, a very strong association was found between lower household income and drug-related deaths. Strikingly, in the counties with the highest incarceration rates, there was a 50% higher rate of drug deaths, reported Elias Nosrati, PhD, and associates (Lancet Public Health. 2019 Jul 3;4:e326-33). It is clearer every day that our opioid epidemic was in part wrought by a zealous push to change protocols on treating chronic pain. The epidemic also appears tied to well-meaning but overprescribing doctors and allegedly unscrupulous pharmaceutical companies and distributors. What we are learning through this most recent study is that another factor tied to the opioid and overdose epidemic could be incarceration.
According to the study, an increase in crime rates combined with sentencing reforms led the number of people incarcerated in state and federal prisons to soar from less than 200,000 in 1970 to almost 1 million in 1995. Furthermore, Dr. Nosrati and associates wrote, “Incarceration is directly associated with stigma, discrimination, poor mental health, and chronic economic hardship, all of which are linked to drug use disorders.”
Treatment for drug addiction in prison systems is rare, as is adequate mental health treatment. However, treatment for this population would likely help reduce drug overdose deaths and improve the quality of life for people who are incarcerated and their families. In the Philadelphia prison system, for example, treatment for inmates is available for opioid addiction, both with methadone and now more recently with buprenorphine (Suboxone). The Philadelphia Department of Prisons also provides cognitive-behavioral therapy. In Florida, Chapter 397 of the Florida statutes – known as the Marchman Act – provides for the involuntary (and voluntary) treatment of individuals with substance abuse problems.
The court systems in South Florida have a robust drug-diversion program, aimed at directing people facing incarceration for drug offenses into treatment instead. North Carolina has studied this issue specifically and found through a model simulation that diverting 10% of drug-abusing offenders out of incarceration into treatment would save $4.8 billion in legal costs for North Carolina counties and state legal systems. Diverting 40% of individuals would close to triple that savings.
There are striking data from programs treating individuals who are leveraged into treatment in order to maintain professional licenses. These such individuals, many of whom are physicians, airline pilots, and nurses, have a rate of sobriety of 90% or greater after 5 years. This data show that
In addition to the potential reduction in morbidity and mortality as well as the financial savings, why is treatment important? Because of societal costs. When parents or family members are put in jail for a drug charge or other charge, they leave behind a community, family, and very often children who are affected economically, emotionally, and socially. Those children in particular have higher risks of depression and PTSD. Diverting an offender into treatment or treating an incarcerated person for drug and mental health problems can change the life of a child or family member, and ultimately can change society.
Dr. Jorandby is chief medical officer of Lakeview Health in Jacksonville, Fla. She trained in addiction psychiatry at Yale University, New Haven, Conn.
Incarceration versus treatment takes center stage in a new analysis of U.S. data from researchers in the United Kingdom.
The researchers performed an observational study looking at rates of incarceration, income, and drug-related deaths from 1983 to 2014 in the United States. They found a strong association between incarceration rates and drug-related deaths. Also, a very strong association was found between lower household income and drug-related deaths. Strikingly, in the counties with the highest incarceration rates, there was a 50% higher rate of drug deaths, reported Elias Nosrati, PhD, and associates (Lancet Public Health. 2019 Jul 3;4:e326-33). It is clearer every day that our opioid epidemic was in part wrought by a zealous push to change protocols on treating chronic pain. The epidemic also appears tied to well-meaning but overprescribing doctors and allegedly unscrupulous pharmaceutical companies and distributors. What we are learning through this most recent study is that another factor tied to the opioid and overdose epidemic could be incarceration.
According to the study, an increase in crime rates combined with sentencing reforms led the number of people incarcerated in state and federal prisons to soar from less than 200,000 in 1970 to almost 1 million in 1995. Furthermore, Dr. Nosrati and associates wrote, “Incarceration is directly associated with stigma, discrimination, poor mental health, and chronic economic hardship, all of which are linked to drug use disorders.”
Treatment for drug addiction in prison systems is rare, as is adequate mental health treatment. However, treatment for this population would likely help reduce drug overdose deaths and improve the quality of life for people who are incarcerated and their families. In the Philadelphia prison system, for example, treatment for inmates is available for opioid addiction, both with methadone and now more recently with buprenorphine (Suboxone). The Philadelphia Department of Prisons also provides cognitive-behavioral therapy. In Florida, Chapter 397 of the Florida statutes – known as the Marchman Act – provides for the involuntary (and voluntary) treatment of individuals with substance abuse problems.
The court systems in South Florida have a robust drug-diversion program, aimed at directing people facing incarceration for drug offenses into treatment instead. North Carolina has studied this issue specifically and found through a model simulation that diverting 10% of drug-abusing offenders out of incarceration into treatment would save $4.8 billion in legal costs for North Carolina counties and state legal systems. Diverting 40% of individuals would close to triple that savings.
There are striking data from programs treating individuals who are leveraged into treatment in order to maintain professional licenses. These such individuals, many of whom are physicians, airline pilots, and nurses, have a rate of sobriety of 90% or greater after 5 years. This data show that
In addition to the potential reduction in morbidity and mortality as well as the financial savings, why is treatment important? Because of societal costs. When parents or family members are put in jail for a drug charge or other charge, they leave behind a community, family, and very often children who are affected economically, emotionally, and socially. Those children in particular have higher risks of depression and PTSD. Diverting an offender into treatment or treating an incarcerated person for drug and mental health problems can change the life of a child or family member, and ultimately can change society.
Dr. Jorandby is chief medical officer of Lakeview Health in Jacksonville, Fla. She trained in addiction psychiatry at Yale University, New Haven, Conn.
Poverty, incarceration may drive deaths from drug use
High rates of both incarceration and reduced household income are significantly associated with drug-related deaths in the United States, based a regression analysis of several decades of data.
“More than half a million drug-related deaths have occurred in the USA in the past three and half decades, however, no studies have investigated the association between these deaths and the expansion of the incarcerated population,” wrote Elias Nosrati, PhD, of the University of Oxford (England) and colleagues.
The researchers reviewed previously unavailable data on jail and prison incarceration at the county level from the nonprofit Vera Institute of Justice in New York, as well as mortality data from the U.S. National Vital Statistics System. The analysis was published in the Lancet Public Health.
After adjustment for multiple confounding variables, each standard deviation in admission rates to local jails (an average of 7,018 per 100,000 population) was associated with a significant 1.5% increase in drug-related deaths, and each standard deviation in admission rates to state prisons (an average of 254.6 per 100,000 population) was associated with a significant 2.6% increase in drug-related deaths, reported Dr. Nosrati and colleagues.
“On average, the researchers wrote. In addition, each standard-deviation decrease in median household income was associated with a 12.8% increase in drug-related deaths within counties.
The findings were limited by several factors, including the observational nature of the study, the potential skewing of results because of missing data from some counties, and the inability to examine support for individuals released from jail or prison, the researchers noted.
However, the results suggest that, “when coupled with economic hardship, the operations of the prison and jail systems constitute an upstream determinant of despair, whereby regular exposures to neighborhood violence, unstable social and family relationships, and psychosocial stress trigger destructive behaviours,” they wrote.
In an accompanying comment, James LePage, PhD, wrote that current laws regarding trespassing, loitering, and vagrancy “unfairly criminalize individuals of low economic status and homeless individuals” by increasing their likelihood of interaction with the legal system and thus increasing the incarceration rate in this population.
“Future studies should focus on racial and ethnic biases in arrests and sentencing, and the subsequent effect on drug-related mortality,” wrote Dr. LePage of the VA North Texas Health Care System in Dallas.
Neither the researchers in the main study nor Dr. LePage had financial conflicts to disclose.
SOURCE: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
High rates of both incarceration and reduced household income are significantly associated with drug-related deaths in the United States, based a regression analysis of several decades of data.
“More than half a million drug-related deaths have occurred in the USA in the past three and half decades, however, no studies have investigated the association between these deaths and the expansion of the incarcerated population,” wrote Elias Nosrati, PhD, of the University of Oxford (England) and colleagues.
The researchers reviewed previously unavailable data on jail and prison incarceration at the county level from the nonprofit Vera Institute of Justice in New York, as well as mortality data from the U.S. National Vital Statistics System. The analysis was published in the Lancet Public Health.
After adjustment for multiple confounding variables, each standard deviation in admission rates to local jails (an average of 7,018 per 100,000 population) was associated with a significant 1.5% increase in drug-related deaths, and each standard deviation in admission rates to state prisons (an average of 254.6 per 100,000 population) was associated with a significant 2.6% increase in drug-related deaths, reported Dr. Nosrati and colleagues.
“On average, the researchers wrote. In addition, each standard-deviation decrease in median household income was associated with a 12.8% increase in drug-related deaths within counties.
The findings were limited by several factors, including the observational nature of the study, the potential skewing of results because of missing data from some counties, and the inability to examine support for individuals released from jail or prison, the researchers noted.
However, the results suggest that, “when coupled with economic hardship, the operations of the prison and jail systems constitute an upstream determinant of despair, whereby regular exposures to neighborhood violence, unstable social and family relationships, and psychosocial stress trigger destructive behaviours,” they wrote.
In an accompanying comment, James LePage, PhD, wrote that current laws regarding trespassing, loitering, and vagrancy “unfairly criminalize individuals of low economic status and homeless individuals” by increasing their likelihood of interaction with the legal system and thus increasing the incarceration rate in this population.
“Future studies should focus on racial and ethnic biases in arrests and sentencing, and the subsequent effect on drug-related mortality,” wrote Dr. LePage of the VA North Texas Health Care System in Dallas.
Neither the researchers in the main study nor Dr. LePage had financial conflicts to disclose.
SOURCE: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
High rates of both incarceration and reduced household income are significantly associated with drug-related deaths in the United States, based a regression analysis of several decades of data.
“More than half a million drug-related deaths have occurred in the USA in the past three and half decades, however, no studies have investigated the association between these deaths and the expansion of the incarcerated population,” wrote Elias Nosrati, PhD, of the University of Oxford (England) and colleagues.
The researchers reviewed previously unavailable data on jail and prison incarceration at the county level from the nonprofit Vera Institute of Justice in New York, as well as mortality data from the U.S. National Vital Statistics System. The analysis was published in the Lancet Public Health.
After adjustment for multiple confounding variables, each standard deviation in admission rates to local jails (an average of 7,018 per 100,000 population) was associated with a significant 1.5% increase in drug-related deaths, and each standard deviation in admission rates to state prisons (an average of 254.6 per 100,000 population) was associated with a significant 2.6% increase in drug-related deaths, reported Dr. Nosrati and colleagues.
“On average, the researchers wrote. In addition, each standard-deviation decrease in median household income was associated with a 12.8% increase in drug-related deaths within counties.
The findings were limited by several factors, including the observational nature of the study, the potential skewing of results because of missing data from some counties, and the inability to examine support for individuals released from jail or prison, the researchers noted.
However, the results suggest that, “when coupled with economic hardship, the operations of the prison and jail systems constitute an upstream determinant of despair, whereby regular exposures to neighborhood violence, unstable social and family relationships, and psychosocial stress trigger destructive behaviours,” they wrote.
In an accompanying comment, James LePage, PhD, wrote that current laws regarding trespassing, loitering, and vagrancy “unfairly criminalize individuals of low economic status and homeless individuals” by increasing their likelihood of interaction with the legal system and thus increasing the incarceration rate in this population.
“Future studies should focus on racial and ethnic biases in arrests and sentencing, and the subsequent effect on drug-related mortality,” wrote Dr. LePage of the VA North Texas Health Care System in Dallas.
Neither the researchers in the main study nor Dr. LePage had financial conflicts to disclose.
SOURCE: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
FROM THE LANCET PUBLIC HEALTH
Key clinical point: Reduced household income and increased incarceration are significantly associated with drug-related deaths in the U.S. population.
Major finding: High incarceration rates are associated with an increase in drug-related deaths of more than 50% at the county level.
Study details: The data come from a regression analysis of data from multiple institutions, including the U.S. National Vital Statistics System and the Institute for Health Metrics and Evaluation, as well as incarceration data from the Vera Institute of Justice for 2,640 U.S. counties from 1983 to 2014.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Nosrati E et al. Lancet Public Health. 2019 Jul 3;4:e326-33.
Improved Patient Outcomes and Reduced Wait Times: Transforming a VA Outpatient Substance Use Disorder Program
Substance use disorders (SUDs) are an increasing public health concern in the US. The 2015 National Survey on Drug Use and Health indicated that 27 million people (8% of the US population) reported current use of recreational drugs or misuse of alcohol or prescription medications.1 The 2013 National Survey on Drug Use and Health indicated that 1.5 million veterans (roughly 6.6%) met the criteria for a SUD.2 More than 50% of patients awaiting entry into a SUD treatment program will never achieve admission due, in part, to long wait times.3-5
National attention has been focused on increasing veteran access to quality treatment based on evidence-based practices (EBPs). Several national legislative measures and treatment protocols have been implemented: the Uniform Mental Health Services in US Department of Veterans Affairs (VA) medical centers and clinics; Veterans Access, Choice, and Accountability Act (2014); Cognitive Behavioral Therapy for Substance Use Disorders (CBT-SUD) Training Program; and the Psychotropic Drug Safety Initiative (PDSI).6-8 Consistent with these directives and in line with American Society of Addiction Medicine (ASAM) and Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines for medication-assisted therapies (MAT),the James A. Haley Veterans’ Hospital (JAHVH) Mental Health and Behavioral Sciences Service (MH&BSS) Substance Use Disorders Service (SUDS) in Tampa, Florida, implemented an evidence-based, treatment-on-demand model of care.9-11
Meeting SUD Treatment Needs
What does the new supervisor of a clinical program do when a 24-employee outpatient VA Alcohol and Drug Addiction Treatment Program (ADATP) has an average 33-day wait time for treatment with 54% of patients lost to care between initial evaluation and admission?12 Patients lacked consistent access to SUD pharmacotherapy. The national VA clinical performance indicators were substandard and there are no additional resources available to apply to the program.
At JAHVH the program supervisor enlisted hospital leadership to support program redesign. The redesign sought to improve operational efficiency and eliminate patient wait time; adopt national standards for assessment and treatment developed by ASAM; implement strictly evidence-based psychotherapeutic treatments; educate program psychiatrists about evidence-based psychopharmacologic treatments and hold them accountable for patient adherence; streamline documentation templates; free clinical providers from nonclinical tasks; create an inpatient addiction consult team to diagnose and treat chronic hospitalized patients with SUDs; ensure continuity of care; and, standardize consistent, objective measures of patient response to treatment to track the program’s effectiveness.
In this article, the authors provide an explanation of the clinical, theoretical foundation and the practical steps taken to design and implement this transformation. They then describe the lessons learned, hoping that their process will serve as a model for those in similar situations.
Program Redesign
July 1, 2015, a new program supervisor was hired and began a 2-month evaluation and analysis of the program with input from leadership, staff, and hospital/community stakeholders. September 1, the monthlong process of developing the redesign began. On September 30 the plan was presented to, and approved by, MH&BSS leadership. October was spent preparing for change with an implementation date of November 2 selected. On November 2, 2015, the complete redesign was implemented.
Needs Assessment
A needs assessment yielded improvement opportunities in program structure (levels of care); clinical content; staff and resource allocation, including clinical workflow and management systems. Staff identified philosophical and practical variance in the program, often leading to confusion for patients and clinicians and potentially resulting in disparate quality care and patient outcomes. Recommendations for addressing these needs included incorporating ASAM guidelines for assignment to clinically appropriate levels of care, implementation of consistent EBPs for SUD and comorbid conditions,9 and emphasis on staff training and development to champion evidence-based program philosophy and service delivery.
The assessment determined that the average waitlist time was 33 days, and patients were required to abstain from substance or alcohol use prior to admission to the Intensive Outpatient Program. If a waitlisted patient relapsed, she or he was removed from the waitlist and denied admission. A study conducted at JAHVH reported that 54% of waitlisted patients in this clinic (prior to November 2, 2015) never were admitted to the program.12 Access to care was considered a significant issue.
Program Implementation
September was spent developing a comprehensive redesign of the SUD clinic. The vision included incorporating all ASAM levels of care; creating an evidence-based, treatment-on-demand model of care; and, securing the support of MH&BSS leadership team, staff, and patients for the redesign. The supervisory clinician interviewed staff both individually and as a group. Clinicians were provided extensive training on EBP for SUDs, including psychotherapies, psychosocial treatments, and psychopharmacologic interventions. A journal club was started with staff-generated topics that offered articles sharing current research, EBPs, and psychotherapeutic techniques, continuing education on substances, and management of coexisting diagnoses. Clinicians increased the frequency of SUD in-service trainings. Psychiatrists provided several Grand Rounds to the MH&BSS service. All counselors were assigned to 1 of the program’s 3 clinical psychologists for individual weekly clinical supervision.
By providing all staff with current, evidence-based, clinically relevant treatment information and emphasizing its relationship to successful patient outcomes, program leadership energized staff support. Staff were encouraged to perform at the top of their scope of practice and engage in training and consultation. Each staff member was delegated a role in the process to inspire buy-in.
Preparation for the Shift
October was spent preparing for a seamless, one-day implementation of proposed changes, including implementation of updated clinical policies, procedures, and document templates (rewritten to include only clinically appropriate information required by VA policy or the Joint Commission); streamlined staff schedules; and utilization of staff-developed and research/policy-driven EBP handbook. Finally, the Brief Addiction Monitor (BAM) was selected as objective criteria to consistently assess patient progress in treatment, and staff were instructed to use this measure at regular intervals and for all levels of care.
Emphasis was placed on ongoing fortification of staff and patient support for the reorganization. For example, the Addiction Severity Index, though not required by policy, was historically used, adding 90 minutes to the evaluation and admission session. Staff agreed to remove this measure to improve clinician availability. Staff were also empowered to rename the redesigned program, and chose Substance Use Disorders Service (SUDS).
Process Changes
To achieve same-day access to clinical care, program leadership created a daily morning orientation group. Patients are scheduled or may attend as a walk-in. The orientation’s purpose is to explain what services are available and to offer each patient an opportunity for immediate evaluation and treatment. Staff schedules were modified to provide patient evaluation appointment slots immediately following orientation. The number of immediate evaluation slots was initially assessed by analyzing the demand for treatment over the previous 6 months, determining the daily mean, and setting the number of slots to accommodate 3 standard deviations above the daily mean. If a patient in a daily orientation group expresses a willingness to engage in treatment, he or she is immediately evaluated by a counselor during a 90-minute session and seen by a psychiatrist to determine whether pharmacologic treatment would be appropriate. If needed, the medication is prescribed that day. The primary purpose of the patient’s initial clinical evaluation is to determine the most appropriate level of care based on ASAM criteria. Also available were 90-minute afternoon evaluation appointments with psychiatrists for patients who walk into the clinic after the morning orientation group had ended.
Prior to the redesign, clinic psychiatrists were minimally prescribing evidence-based pharmacotherapy for sobriety support. At the time of redesign, only 8% of patients diagnosed with opioid use disorders (OUDs) were prescribed buprenorphine/naloxone or naltrexone. Just 1.9% of patients diagnosed with alcohol use disorder (AUD) were prescribed naltrexone or acamprosate. With the redesign, access to these medications has significantly expanded.
All templates were redesigned to ensure consistent documentation. This change decreased the overall provider task burden, and explicitly supported the use of ASAM multidimensional criteria and the Brief Addiction Monitor (BAM) to identify a pretreatment baseline score and track each patient’s clinical progress.13 Evidence-based written curricula were standardized for individual and group psychotherapies to reduce provider and programmatic variation.
The redesign creates distinct levels of care based on ASAM criteria, including harm reduction, ambulatory detoxification, outpatient group and individual psychotherapy, an evidence-based Intensive Outpatient Program (IOP), and aftercare. Application of the ASAM standards has allowed clinicians to make accurate placement decisions that best meet individual patient needs and to serve as effective stewards even with limited treatment and financial resources. Although JAHVH does not have a residential SUD program, procedures were developed to refer veterans to community-based residential treatment programs when appropriate.
Group Therapies
With the redesign, SUDS was no longer exclusively a 12-step program; however, it still supported and recognized the value of this approach for some patients. A psychologist periodically audits group sessions to prevent drift from that group’s curriculum. Counselors are assigned to weekly hour-long clinical supervision sessions with a psychologist to review patient care and reinforce the application of evidence-based individualized treatment.
After reviewing empirical literature and VA directives, CBT-SUD was adopted. It encompasses individual and group interventions, such as motivational interviewing (MI), contingency management (CM), and medication-assisted therapies as primary therapeutic treatment modalities, all of which have demonstrated efficacy as measured by length of sobriety postintervention.9,14,15
Clinical Staff Improvements
Staff were reorganized into 3 interdisciplinary treatment teams. A weekly team meeting is scheduled to coordinate care and discuss the treatment of complex patients. Clinical staff focus has shifted from case-management to diagnosis and treatment; now patients are referred to their primary care team’s social worker for case management services. Allowing clinical staff to focus solely on the diagnosis and clinical treatment of SUDs has significantly enhanced productivity and morale.
Staff receive training in the newly adopted interventions during brief monthly refresher courses provided by inhouse psychologists. Additional training includes participation in local and national SUD teleconferences and onsite meetings with experts in harm reduction and motivational interventions. During the transition, clinicians were encouraged to attend staff resiliency training. Continuing education was available to the SUDS psychiatrists and all inpatient and outpatient psychiatrists at JAHVH. Recently, this educational initiative was expanded to include all primary care and inpatient internal medicine physicians.
Implementation
On November 2, 2015, all planned programmatic changes were simultaneously implemented. On that day, clinician and patient schedules changed, the new EBP curriculum was administered, the use of streamlined documentation procedures began, and daily orientation groups followed by same-day evaluations were initiated.
The pretreatment sobriety requirement was eliminated as a barrier to care, and the program began to use a harm-reduction treatment track as recommended by ASAM guidelines. Patients with urgent or emergent medical or psychiatric problems were immediately assessed by SUDS health care providers and treated in the clinic or transported to the emergency department. Previously unavailable, patient access to ambulatory detoxification was initiated. The prescription of buprenorphine/naloxone for the treatment of OUD treatments increased from 1 prescriber to all 3.
Three months after program reorganization, the leadership reviewed overall workflow, conducted patient satisfaction surveys, and evaluated facility use and productivity. To address patient needs and facilitate optimal use of space, the number of same-day evaluation slots was reduced while the number of individual therapy slots was increased.
Staff meet in workgroups to discuss EBPs and further refine content with feedback from the supervisory clinician and team psychologists who routinely audit group therapy sessions. Staff report ongoing benefit from weekly supervision with a clinical psychologist. An inpatient addiction consultation team that uses existing manpower and resources has been developed.
Program Goals and Outcomes
The SUDS program serves more patients at multiple levels of standardized care with 2 fewer full-time positions. One counselor and one advanced practice registered nurse were reallocated to different programs within the JAHVH VA mental health clinic. Following a review of all program clinic profiles in the VA’s Computerized Patient Record System (CPRS) for utilization, accuracy, and necessity, and allowing for accurate program data capture, the transition resulted in a reduction of distinct clinics from 114 to 67 (-58.7%). In fiscal year 2018, review of CPRS yielded 19,786 total visits (3,645 unique visits).
Eliminate Patient Wait Tme
Patient wait time, as measured in CPRS from date of initial evaluation to date of treatment was reduced from an average of 33 days to 0 within 2 weeks of program implementation. A review of CPRS data also indicated that preadmission attrition dropped from 54% to < 1%; all patients desiring treatment are assigned a counselor and treatment is initiated the same day.
Adopt ASAM Criteria
After the redesign, patients have received more appropriate care based on individualized treatment plans. Due to the implementation of a fluid and supportive model, patients can move through levels of care as clinical need dictates rather than failing treatment and having to reengage. Staff receive ongoing education on the use of ASAM. Evaluation and treatment plan templates now reflect assignment to level of care rationale using ASAM guidelines.
Use of Evidence-Based Psychotherapeutic Treatments
More consistent, coordinated, and effective psychotherapies have improved patient care. The program’s previous issues with patients receiving conflicting treatment guidance from different providers has been resolved. Duplicate and ineffective treatments, including multiple readmissions to the IOP level of care, overemphasis of abstinence-based modalities for patients in active use, and referrals to inpatient SUD care under the assumption that “higher level of care is better” have ceased through staff education, leadership support, and appropriate staffing and communication. Review of patient advocate complaints tracked by and resolved by the service demonstrated an 80% decrease in patient advocate complaints regarding SUD clinic services.
Implement Evidence-Based Psychopharmacologic Treatments
The pharmacotherapy education initiative yielded tangible benefits and is likely a significant contributor to the program’s improved clinical outcomes. Prescription of pharmacotherapy for patients with OUD has risen from 8% to 25.1% in eligible patients. Appropriate medication prescription for the treatment of AUD has risen from 1.9% to 9.8% in eligible patients. These data are reflected in the VA Pharmaceutical Drug Safety Initiative (PDSI) dashboard.
Streamline Documentation
Significantly reducing the charting burden was likely a significant contributor to increased provider productivity and improved patient outcomes. Regular meetings between SUDS leadership and clinical informatics ensure that standardized note templates meet hospital policy and gather all necessary accreditation information.
Improve Employee Morale
Increased staff morale is indicated by a noticeable reduction in employee sick days; a decrease of > 20% (over the same time period the previous year), per the VA electronic timekeeping system, during the first 6 months following the November 2 program implementation.
SUDS Inpatient Addiction Consult Team
In January of 2017, SUDS began an inpatient medicine consultation service to offer evaluation, pharmacotherapy, and supportive counseling to patients diagnosed with SUDs who had been admitted to inpatient medical and surgical services. This team includes existing SUDS staff members reallocated to the inpatient service, is led by a SUDS psychiatrist, and includes 3 multidisciplinary clinicians with extensive training in assessment, diagnosis, and treatment planning of SUDs and comorbid conditions. Prior to implementation, the SUDS inpatient addiction consult team met with hospital leadership and attending physicians for inpatient medicine and psychiatry physicians.
To access the SUDS inpatient addiction consult team, physicians request a consult. Patients are offered an evaluation and are assigned to a level of care with orders for outpatient appointments with a counselor and psychiatrist within 7 days of hospital discharge. Medication-assisted treatment for chronic SUDs is implemented while patients remain admitted to the inpatient medical service. In fiscal year 2018, the SUDS inpatient addiction consult team performed 1,428 inpatient evaluations.
Consistent Treatment Outcome Measures
The BAM is a clinical tool designed to measure patient outcomes in substance use disorders.13 Its 17-item scale measures substance use risk factors that may lead to relapse, and protective factors that are recovery-oriented behaviors that help prevent relapse. It demonstrates sensitivity to change and has excellent test-retest reliability. The BAM has been in use in the addictions treatment program since 2011 but was previously administered only after admission to the IOP and again after a 30- to 90-day follow-up period. Since the program redesign, all SUDS patients are administered the BAM at their initial evaluation and at each individual appointment thereafter. The initial BAM assessment encompasses the previous 30 days; this 30-day version is also used for monthly follow-ups. For BAM assessments that occur within 30 days from the time of the last evaluation, a 7-day version is used. Prior to the redesign, about 24% of patients received a follow-up 30-day BAM assessment.12 Per CPRS review of veterans participating in continued treatment, the rate rose to 100% 3 months after the redesign.
When program staff compared preredesign and postredesign BAM data, they detected significant clinical differences. Data demonstrate a 22.2% improvement in protective factors, including patient confidence in their ability to remain abstinent; engaging in self-help activities, such as attending Alcoholics Anonymous meetings; engaging in organized spiritual activities; going to school, working, or volunteering; securing a regular income; and time spent with friends or family who are supportive of recovery.
The data also show a marked reduction in substance use at follow-up points in treatment and a corresponding decrease in risk factors. One item of the BAM assesses patient level of satisfaction with their treatment. Since the redesign, patients report that they are “considerably” satisfied with their SUD treatment.
Currently, program staff are conducting a review of BAM scores by level of care to further parse the impact of various treatments and best target patient need using measurement-based care and EBP, such as contingency management, which provides small monetary incentives when patients maintain clean urine drug screens.16 In addition, the program plans to achieve more uniformity in BAM assessment intervals at all levels of care, and possibly also integrate BAM data into SUD group therapies. Correlation of the BAM scores to other metrics, such as readmission to inpatient medicine, relapse, urine drug screen, or critical laboratory values, will provide additional insight into impact of programmatic changes.
Discussion
Feedback from other clinics and services within the hospital has been very positive. Some providers have reported that they appreciate the ease and availability of access to SUDS. Additionally, patients engaged in treatment prior to the redesign have been contacted for an updated evaluation and assignment to a counselor and appropriate level of care. From the staff’s perspective, the shift to immediate access to care has allowed a more streamlined process with fewer hurdles for patient admission. Staff report that they now feel empowered to meet the needs of veterans in a comprehensive, same-day fashion.
The success of our redesign was contingent on internal and external sta
The successful implementation of these changes has revealed several important elements regarding patient care. The first lesson was that improving access and integrating best practices is possible without additional resources, outside monies, or disruption to patient services. With the support of MH&BSS leadership, the program streamlined existing processes and used both staff and clinic resources more efficiently.
The second lesson involved the importance of continually reviewing and revising standard operating procedures to match the needs of the current patient population. Policies and procedures that once were viewed as potential barriers to change have been replaced with a more flexible approach and willingness to evolve.
As a result, far fewer patients have been lost to treatment. The time and resources that staff historically dedicated to nonclinical patient care are now redirected to immediate service provision. This increase in operational efficiency and treatment efficacy has resulted in a boost to staff morale, even during a time of immense change and increased productivity. Program staff are now able to personally witness the significant changes in their patients’ lives and feel a sense of pride at being a member of a hard-working team that provides the highest quality of substance use treatment. This is critical to job satisfaction and meets the VA mission to provide timely, effective, and evidence-based treatments to patients.
Conclusion
JAHVH strives to continue to provide the highest quality of SUD treatment available. Future directions aim to streamline clinic operations by constantly monitoring and reviewing workloads, while also considering patient feedback. A continuous review of EBP is part of our clinic’s culture. Program leadership endeavors to promote an open environment where providers can share their triumphs and frustrations and foster a team approach to problem solving. Further plans include expanding the range of treatment levels offered by developing a residential SUD treatment facility.
1. Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health: Summary of the Effects of the 2015 NSDUH Questionnaire Redesign: Implications for Data Users. https://www.samhsa.gov/data/sites/default/files/NSDUH-TrendBreak-2015.pdf. Published June 2016. Accessed June 12, 2019.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan PDSKL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96(8):1149-1160.
4. Hser Y, Maglione M, Polinsky ML, Anglin MD. Predicting treatment entry among treatment-seeking drug abusers. J Subst Abuse Treatment. 1997;15(3):213-220.
5. Stark MJ, Campbell BK, Brinkerhoff CV. “Hello, may we help you?” A study of attrition prevention at the time of the first phone contact with substance-abusing clients. Am J Drug Alcohol Abuse. 1990;16:67-76.
6. US Department of Veterans Affairs. Uniform Mental Health Services in VA Medical Centers and Clinics. VHA Handbook 1160.01. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762. Updated November 2015. Accessed December 12, 2017.
7. Veterans Access, Choice and Accountability Act of 2014, 2 USC § 933.
8. DeMarce JM, Gnys M, Raffa SD, Karlin, BE. Cognitive Behavioral Therapy for Substance Use Disorders Among Veterans: Therapist Manual. Washington, DC: US Department of Veterans Affairs; 2014.
9. Mee-Lee D, Shulman GD, Fishman MJ, Gastfriend DR, Miller MM, eds. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions. 3rd ed. Carson City, NV: The Change Companies; 2013.
10. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
11. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for Opioid Use Disorder – Full Document. HHS Publication No. (SMA) 18-5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
12. Winn JL, Shealy SE, Kropp GJ, Felkins-Dohm D, Gonzales-Nolas C, Francis E. Housing assistance and case management: Improving access to substance use disorder treatment for homeless veterans. Psychological Serv. 2013;10(2):233-240.
13. Cacciola JS, Alterman AI, DePhilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treatment. 2013;44(3):256-263.
14. McHugh RK, Hearon BA, Otto MW. Cognitive-behavioral therapy for substance use disorders, Psychiatr Clinics North Am. 2010;33:511–525.
15. Karlin, BE, Cross, G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs health care system. Am Psychol. 2014;69:19-33.
16. DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes, Drug Alcohol Dependence. 2018;185:367-373.
Substance use disorders (SUDs) are an increasing public health concern in the US. The 2015 National Survey on Drug Use and Health indicated that 27 million people (8% of the US population) reported current use of recreational drugs or misuse of alcohol or prescription medications.1 The 2013 National Survey on Drug Use and Health indicated that 1.5 million veterans (roughly 6.6%) met the criteria for a SUD.2 More than 50% of patients awaiting entry into a SUD treatment program will never achieve admission due, in part, to long wait times.3-5
National attention has been focused on increasing veteran access to quality treatment based on evidence-based practices (EBPs). Several national legislative measures and treatment protocols have been implemented: the Uniform Mental Health Services in US Department of Veterans Affairs (VA) medical centers and clinics; Veterans Access, Choice, and Accountability Act (2014); Cognitive Behavioral Therapy for Substance Use Disorders (CBT-SUD) Training Program; and the Psychotropic Drug Safety Initiative (PDSI).6-8 Consistent with these directives and in line with American Society of Addiction Medicine (ASAM) and Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines for medication-assisted therapies (MAT),the James A. Haley Veterans’ Hospital (JAHVH) Mental Health and Behavioral Sciences Service (MH&BSS) Substance Use Disorders Service (SUDS) in Tampa, Florida, implemented an evidence-based, treatment-on-demand model of care.9-11
Meeting SUD Treatment Needs
What does the new supervisor of a clinical program do when a 24-employee outpatient VA Alcohol and Drug Addiction Treatment Program (ADATP) has an average 33-day wait time for treatment with 54% of patients lost to care between initial evaluation and admission?12 Patients lacked consistent access to SUD pharmacotherapy. The national VA clinical performance indicators were substandard and there are no additional resources available to apply to the program.
At JAHVH the program supervisor enlisted hospital leadership to support program redesign. The redesign sought to improve operational efficiency and eliminate patient wait time; adopt national standards for assessment and treatment developed by ASAM; implement strictly evidence-based psychotherapeutic treatments; educate program psychiatrists about evidence-based psychopharmacologic treatments and hold them accountable for patient adherence; streamline documentation templates; free clinical providers from nonclinical tasks; create an inpatient addiction consult team to diagnose and treat chronic hospitalized patients with SUDs; ensure continuity of care; and, standardize consistent, objective measures of patient response to treatment to track the program’s effectiveness.
In this article, the authors provide an explanation of the clinical, theoretical foundation and the practical steps taken to design and implement this transformation. They then describe the lessons learned, hoping that their process will serve as a model for those in similar situations.
Program Redesign
July 1, 2015, a new program supervisor was hired and began a 2-month evaluation and analysis of the program with input from leadership, staff, and hospital/community stakeholders. September 1, the monthlong process of developing the redesign began. On September 30 the plan was presented to, and approved by, MH&BSS leadership. October was spent preparing for change with an implementation date of November 2 selected. On November 2, 2015, the complete redesign was implemented.
Needs Assessment
A needs assessment yielded improvement opportunities in program structure (levels of care); clinical content; staff and resource allocation, including clinical workflow and management systems. Staff identified philosophical and practical variance in the program, often leading to confusion for patients and clinicians and potentially resulting in disparate quality care and patient outcomes. Recommendations for addressing these needs included incorporating ASAM guidelines for assignment to clinically appropriate levels of care, implementation of consistent EBPs for SUD and comorbid conditions,9 and emphasis on staff training and development to champion evidence-based program philosophy and service delivery.
The assessment determined that the average waitlist time was 33 days, and patients were required to abstain from substance or alcohol use prior to admission to the Intensive Outpatient Program. If a waitlisted patient relapsed, she or he was removed from the waitlist and denied admission. A study conducted at JAHVH reported that 54% of waitlisted patients in this clinic (prior to November 2, 2015) never were admitted to the program.12 Access to care was considered a significant issue.
Program Implementation
September was spent developing a comprehensive redesign of the SUD clinic. The vision included incorporating all ASAM levels of care; creating an evidence-based, treatment-on-demand model of care; and, securing the support of MH&BSS leadership team, staff, and patients for the redesign. The supervisory clinician interviewed staff both individually and as a group. Clinicians were provided extensive training on EBP for SUDs, including psychotherapies, psychosocial treatments, and psychopharmacologic interventions. A journal club was started with staff-generated topics that offered articles sharing current research, EBPs, and psychotherapeutic techniques, continuing education on substances, and management of coexisting diagnoses. Clinicians increased the frequency of SUD in-service trainings. Psychiatrists provided several Grand Rounds to the MH&BSS service. All counselors were assigned to 1 of the program’s 3 clinical psychologists for individual weekly clinical supervision.
By providing all staff with current, evidence-based, clinically relevant treatment information and emphasizing its relationship to successful patient outcomes, program leadership energized staff support. Staff were encouraged to perform at the top of their scope of practice and engage in training and consultation. Each staff member was delegated a role in the process to inspire buy-in.
Preparation for the Shift
October was spent preparing for a seamless, one-day implementation of proposed changes, including implementation of updated clinical policies, procedures, and document templates (rewritten to include only clinically appropriate information required by VA policy or the Joint Commission); streamlined staff schedules; and utilization of staff-developed and research/policy-driven EBP handbook. Finally, the Brief Addiction Monitor (BAM) was selected as objective criteria to consistently assess patient progress in treatment, and staff were instructed to use this measure at regular intervals and for all levels of care.
Emphasis was placed on ongoing fortification of staff and patient support for the reorganization. For example, the Addiction Severity Index, though not required by policy, was historically used, adding 90 minutes to the evaluation and admission session. Staff agreed to remove this measure to improve clinician availability. Staff were also empowered to rename the redesigned program, and chose Substance Use Disorders Service (SUDS).
Process Changes
To achieve same-day access to clinical care, program leadership created a daily morning orientation group. Patients are scheduled or may attend as a walk-in. The orientation’s purpose is to explain what services are available and to offer each patient an opportunity for immediate evaluation and treatment. Staff schedules were modified to provide patient evaluation appointment slots immediately following orientation. The number of immediate evaluation slots was initially assessed by analyzing the demand for treatment over the previous 6 months, determining the daily mean, and setting the number of slots to accommodate 3 standard deviations above the daily mean. If a patient in a daily orientation group expresses a willingness to engage in treatment, he or she is immediately evaluated by a counselor during a 90-minute session and seen by a psychiatrist to determine whether pharmacologic treatment would be appropriate. If needed, the medication is prescribed that day. The primary purpose of the patient’s initial clinical evaluation is to determine the most appropriate level of care based on ASAM criteria. Also available were 90-minute afternoon evaluation appointments with psychiatrists for patients who walk into the clinic after the morning orientation group had ended.
Prior to the redesign, clinic psychiatrists were minimally prescribing evidence-based pharmacotherapy for sobriety support. At the time of redesign, only 8% of patients diagnosed with opioid use disorders (OUDs) were prescribed buprenorphine/naloxone or naltrexone. Just 1.9% of patients diagnosed with alcohol use disorder (AUD) were prescribed naltrexone or acamprosate. With the redesign, access to these medications has significantly expanded.
All templates were redesigned to ensure consistent documentation. This change decreased the overall provider task burden, and explicitly supported the use of ASAM multidimensional criteria and the Brief Addiction Monitor (BAM) to identify a pretreatment baseline score and track each patient’s clinical progress.13 Evidence-based written curricula were standardized for individual and group psychotherapies to reduce provider and programmatic variation.
The redesign creates distinct levels of care based on ASAM criteria, including harm reduction, ambulatory detoxification, outpatient group and individual psychotherapy, an evidence-based Intensive Outpatient Program (IOP), and aftercare. Application of the ASAM standards has allowed clinicians to make accurate placement decisions that best meet individual patient needs and to serve as effective stewards even with limited treatment and financial resources. Although JAHVH does not have a residential SUD program, procedures were developed to refer veterans to community-based residential treatment programs when appropriate.
Group Therapies
With the redesign, SUDS was no longer exclusively a 12-step program; however, it still supported and recognized the value of this approach for some patients. A psychologist periodically audits group sessions to prevent drift from that group’s curriculum. Counselors are assigned to weekly hour-long clinical supervision sessions with a psychologist to review patient care and reinforce the application of evidence-based individualized treatment.
After reviewing empirical literature and VA directives, CBT-SUD was adopted. It encompasses individual and group interventions, such as motivational interviewing (MI), contingency management (CM), and medication-assisted therapies as primary therapeutic treatment modalities, all of which have demonstrated efficacy as measured by length of sobriety postintervention.9,14,15
Clinical Staff Improvements
Staff were reorganized into 3 interdisciplinary treatment teams. A weekly team meeting is scheduled to coordinate care and discuss the treatment of complex patients. Clinical staff focus has shifted from case-management to diagnosis and treatment; now patients are referred to their primary care team’s social worker for case management services. Allowing clinical staff to focus solely on the diagnosis and clinical treatment of SUDs has significantly enhanced productivity and morale.
Staff receive training in the newly adopted interventions during brief monthly refresher courses provided by inhouse psychologists. Additional training includes participation in local and national SUD teleconferences and onsite meetings with experts in harm reduction and motivational interventions. During the transition, clinicians were encouraged to attend staff resiliency training. Continuing education was available to the SUDS psychiatrists and all inpatient and outpatient psychiatrists at JAHVH. Recently, this educational initiative was expanded to include all primary care and inpatient internal medicine physicians.
Implementation
On November 2, 2015, all planned programmatic changes were simultaneously implemented. On that day, clinician and patient schedules changed, the new EBP curriculum was administered, the use of streamlined documentation procedures began, and daily orientation groups followed by same-day evaluations were initiated.
The pretreatment sobriety requirement was eliminated as a barrier to care, and the program began to use a harm-reduction treatment track as recommended by ASAM guidelines. Patients with urgent or emergent medical or psychiatric problems were immediately assessed by SUDS health care providers and treated in the clinic or transported to the emergency department. Previously unavailable, patient access to ambulatory detoxification was initiated. The prescription of buprenorphine/naloxone for the treatment of OUD treatments increased from 1 prescriber to all 3.
Three months after program reorganization, the leadership reviewed overall workflow, conducted patient satisfaction surveys, and evaluated facility use and productivity. To address patient needs and facilitate optimal use of space, the number of same-day evaluation slots was reduced while the number of individual therapy slots was increased.
Staff meet in workgroups to discuss EBPs and further refine content with feedback from the supervisory clinician and team psychologists who routinely audit group therapy sessions. Staff report ongoing benefit from weekly supervision with a clinical psychologist. An inpatient addiction consultation team that uses existing manpower and resources has been developed.
Program Goals and Outcomes
The SUDS program serves more patients at multiple levels of standardized care with 2 fewer full-time positions. One counselor and one advanced practice registered nurse were reallocated to different programs within the JAHVH VA mental health clinic. Following a review of all program clinic profiles in the VA’s Computerized Patient Record System (CPRS) for utilization, accuracy, and necessity, and allowing for accurate program data capture, the transition resulted in a reduction of distinct clinics from 114 to 67 (-58.7%). In fiscal year 2018, review of CPRS yielded 19,786 total visits (3,645 unique visits).
Eliminate Patient Wait Tme
Patient wait time, as measured in CPRS from date of initial evaluation to date of treatment was reduced from an average of 33 days to 0 within 2 weeks of program implementation. A review of CPRS data also indicated that preadmission attrition dropped from 54% to < 1%; all patients desiring treatment are assigned a counselor and treatment is initiated the same day.
Adopt ASAM Criteria
After the redesign, patients have received more appropriate care based on individualized treatment plans. Due to the implementation of a fluid and supportive model, patients can move through levels of care as clinical need dictates rather than failing treatment and having to reengage. Staff receive ongoing education on the use of ASAM. Evaluation and treatment plan templates now reflect assignment to level of care rationale using ASAM guidelines.
Use of Evidence-Based Psychotherapeutic Treatments
More consistent, coordinated, and effective psychotherapies have improved patient care. The program’s previous issues with patients receiving conflicting treatment guidance from different providers has been resolved. Duplicate and ineffective treatments, including multiple readmissions to the IOP level of care, overemphasis of abstinence-based modalities for patients in active use, and referrals to inpatient SUD care under the assumption that “higher level of care is better” have ceased through staff education, leadership support, and appropriate staffing and communication. Review of patient advocate complaints tracked by and resolved by the service demonstrated an 80% decrease in patient advocate complaints regarding SUD clinic services.
Implement Evidence-Based Psychopharmacologic Treatments
The pharmacotherapy education initiative yielded tangible benefits and is likely a significant contributor to the program’s improved clinical outcomes. Prescription of pharmacotherapy for patients with OUD has risen from 8% to 25.1% in eligible patients. Appropriate medication prescription for the treatment of AUD has risen from 1.9% to 9.8% in eligible patients. These data are reflected in the VA Pharmaceutical Drug Safety Initiative (PDSI) dashboard.
Streamline Documentation
Significantly reducing the charting burden was likely a significant contributor to increased provider productivity and improved patient outcomes. Regular meetings between SUDS leadership and clinical informatics ensure that standardized note templates meet hospital policy and gather all necessary accreditation information.
Improve Employee Morale
Increased staff morale is indicated by a noticeable reduction in employee sick days; a decrease of > 20% (over the same time period the previous year), per the VA electronic timekeeping system, during the first 6 months following the November 2 program implementation.
SUDS Inpatient Addiction Consult Team
In January of 2017, SUDS began an inpatient medicine consultation service to offer evaluation, pharmacotherapy, and supportive counseling to patients diagnosed with SUDs who had been admitted to inpatient medical and surgical services. This team includes existing SUDS staff members reallocated to the inpatient service, is led by a SUDS psychiatrist, and includes 3 multidisciplinary clinicians with extensive training in assessment, diagnosis, and treatment planning of SUDs and comorbid conditions. Prior to implementation, the SUDS inpatient addiction consult team met with hospital leadership and attending physicians for inpatient medicine and psychiatry physicians.
To access the SUDS inpatient addiction consult team, physicians request a consult. Patients are offered an evaluation and are assigned to a level of care with orders for outpatient appointments with a counselor and psychiatrist within 7 days of hospital discharge. Medication-assisted treatment for chronic SUDs is implemented while patients remain admitted to the inpatient medical service. In fiscal year 2018, the SUDS inpatient addiction consult team performed 1,428 inpatient evaluations.
Consistent Treatment Outcome Measures
The BAM is a clinical tool designed to measure patient outcomes in substance use disorders.13 Its 17-item scale measures substance use risk factors that may lead to relapse, and protective factors that are recovery-oriented behaviors that help prevent relapse. It demonstrates sensitivity to change and has excellent test-retest reliability. The BAM has been in use in the addictions treatment program since 2011 but was previously administered only after admission to the IOP and again after a 30- to 90-day follow-up period. Since the program redesign, all SUDS patients are administered the BAM at their initial evaluation and at each individual appointment thereafter. The initial BAM assessment encompasses the previous 30 days; this 30-day version is also used for monthly follow-ups. For BAM assessments that occur within 30 days from the time of the last evaluation, a 7-day version is used. Prior to the redesign, about 24% of patients received a follow-up 30-day BAM assessment.12 Per CPRS review of veterans participating in continued treatment, the rate rose to 100% 3 months after the redesign.
When program staff compared preredesign and postredesign BAM data, they detected significant clinical differences. Data demonstrate a 22.2% improvement in protective factors, including patient confidence in their ability to remain abstinent; engaging in self-help activities, such as attending Alcoholics Anonymous meetings; engaging in organized spiritual activities; going to school, working, or volunteering; securing a regular income; and time spent with friends or family who are supportive of recovery.
The data also show a marked reduction in substance use at follow-up points in treatment and a corresponding decrease in risk factors. One item of the BAM assesses patient level of satisfaction with their treatment. Since the redesign, patients report that they are “considerably” satisfied with their SUD treatment.
Currently, program staff are conducting a review of BAM scores by level of care to further parse the impact of various treatments and best target patient need using measurement-based care and EBP, such as contingency management, which provides small monetary incentives when patients maintain clean urine drug screens.16 In addition, the program plans to achieve more uniformity in BAM assessment intervals at all levels of care, and possibly also integrate BAM data into SUD group therapies. Correlation of the BAM scores to other metrics, such as readmission to inpatient medicine, relapse, urine drug screen, or critical laboratory values, will provide additional insight into impact of programmatic changes.
Discussion
Feedback from other clinics and services within the hospital has been very positive. Some providers have reported that they appreciate the ease and availability of access to SUDS. Additionally, patients engaged in treatment prior to the redesign have been contacted for an updated evaluation and assignment to a counselor and appropriate level of care. From the staff’s perspective, the shift to immediate access to care has allowed a more streamlined process with fewer hurdles for patient admission. Staff report that they now feel empowered to meet the needs of veterans in a comprehensive, same-day fashion.
The success of our redesign was contingent on internal and external sta
The successful implementation of these changes has revealed several important elements regarding patient care. The first lesson was that improving access and integrating best practices is possible without additional resources, outside monies, or disruption to patient services. With the support of MH&BSS leadership, the program streamlined existing processes and used both staff and clinic resources more efficiently.
The second lesson involved the importance of continually reviewing and revising standard operating procedures to match the needs of the current patient population. Policies and procedures that once were viewed as potential barriers to change have been replaced with a more flexible approach and willingness to evolve.
As a result, far fewer patients have been lost to treatment. The time and resources that staff historically dedicated to nonclinical patient care are now redirected to immediate service provision. This increase in operational efficiency and treatment efficacy has resulted in a boost to staff morale, even during a time of immense change and increased productivity. Program staff are now able to personally witness the significant changes in their patients’ lives and feel a sense of pride at being a member of a hard-working team that provides the highest quality of substance use treatment. This is critical to job satisfaction and meets the VA mission to provide timely, effective, and evidence-based treatments to patients.
Conclusion
JAHVH strives to continue to provide the highest quality of SUD treatment available. Future directions aim to streamline clinic operations by constantly monitoring and reviewing workloads, while also considering patient feedback. A continuous review of EBP is part of our clinic’s culture. Program leadership endeavors to promote an open environment where providers can share their triumphs and frustrations and foster a team approach to problem solving. Further plans include expanding the range of treatment levels offered by developing a residential SUD treatment facility.
Substance use disorders (SUDs) are an increasing public health concern in the US. The 2015 National Survey on Drug Use and Health indicated that 27 million people (8% of the US population) reported current use of recreational drugs or misuse of alcohol or prescription medications.1 The 2013 National Survey on Drug Use and Health indicated that 1.5 million veterans (roughly 6.6%) met the criteria for a SUD.2 More than 50% of patients awaiting entry into a SUD treatment program will never achieve admission due, in part, to long wait times.3-5
National attention has been focused on increasing veteran access to quality treatment based on evidence-based practices (EBPs). Several national legislative measures and treatment protocols have been implemented: the Uniform Mental Health Services in US Department of Veterans Affairs (VA) medical centers and clinics; Veterans Access, Choice, and Accountability Act (2014); Cognitive Behavioral Therapy for Substance Use Disorders (CBT-SUD) Training Program; and the Psychotropic Drug Safety Initiative (PDSI).6-8 Consistent with these directives and in line with American Society of Addiction Medicine (ASAM) and Substance Abuse and Mental Health Services Administration (SAMHSA) guidelines for medication-assisted therapies (MAT),the James A. Haley Veterans’ Hospital (JAHVH) Mental Health and Behavioral Sciences Service (MH&BSS) Substance Use Disorders Service (SUDS) in Tampa, Florida, implemented an evidence-based, treatment-on-demand model of care.9-11
Meeting SUD Treatment Needs
What does the new supervisor of a clinical program do when a 24-employee outpatient VA Alcohol and Drug Addiction Treatment Program (ADATP) has an average 33-day wait time for treatment with 54% of patients lost to care between initial evaluation and admission?12 Patients lacked consistent access to SUD pharmacotherapy. The national VA clinical performance indicators were substandard and there are no additional resources available to apply to the program.
At JAHVH the program supervisor enlisted hospital leadership to support program redesign. The redesign sought to improve operational efficiency and eliminate patient wait time; adopt national standards for assessment and treatment developed by ASAM; implement strictly evidence-based psychotherapeutic treatments; educate program psychiatrists about evidence-based psychopharmacologic treatments and hold them accountable for patient adherence; streamline documentation templates; free clinical providers from nonclinical tasks; create an inpatient addiction consult team to diagnose and treat chronic hospitalized patients with SUDs; ensure continuity of care; and, standardize consistent, objective measures of patient response to treatment to track the program’s effectiveness.
In this article, the authors provide an explanation of the clinical, theoretical foundation and the practical steps taken to design and implement this transformation. They then describe the lessons learned, hoping that their process will serve as a model for those in similar situations.
Program Redesign
July 1, 2015, a new program supervisor was hired and began a 2-month evaluation and analysis of the program with input from leadership, staff, and hospital/community stakeholders. September 1, the monthlong process of developing the redesign began. On September 30 the plan was presented to, and approved by, MH&BSS leadership. October was spent preparing for change with an implementation date of November 2 selected. On November 2, 2015, the complete redesign was implemented.
Needs Assessment
A needs assessment yielded improvement opportunities in program structure (levels of care); clinical content; staff and resource allocation, including clinical workflow and management systems. Staff identified philosophical and practical variance in the program, often leading to confusion for patients and clinicians and potentially resulting in disparate quality care and patient outcomes. Recommendations for addressing these needs included incorporating ASAM guidelines for assignment to clinically appropriate levels of care, implementation of consistent EBPs for SUD and comorbid conditions,9 and emphasis on staff training and development to champion evidence-based program philosophy and service delivery.
The assessment determined that the average waitlist time was 33 days, and patients were required to abstain from substance or alcohol use prior to admission to the Intensive Outpatient Program. If a waitlisted patient relapsed, she or he was removed from the waitlist and denied admission. A study conducted at JAHVH reported that 54% of waitlisted patients in this clinic (prior to November 2, 2015) never were admitted to the program.12 Access to care was considered a significant issue.
Program Implementation
September was spent developing a comprehensive redesign of the SUD clinic. The vision included incorporating all ASAM levels of care; creating an evidence-based, treatment-on-demand model of care; and, securing the support of MH&BSS leadership team, staff, and patients for the redesign. The supervisory clinician interviewed staff both individually and as a group. Clinicians were provided extensive training on EBP for SUDs, including psychotherapies, psychosocial treatments, and psychopharmacologic interventions. A journal club was started with staff-generated topics that offered articles sharing current research, EBPs, and psychotherapeutic techniques, continuing education on substances, and management of coexisting diagnoses. Clinicians increased the frequency of SUD in-service trainings. Psychiatrists provided several Grand Rounds to the MH&BSS service. All counselors were assigned to 1 of the program’s 3 clinical psychologists for individual weekly clinical supervision.
By providing all staff with current, evidence-based, clinically relevant treatment information and emphasizing its relationship to successful patient outcomes, program leadership energized staff support. Staff were encouraged to perform at the top of their scope of practice and engage in training and consultation. Each staff member was delegated a role in the process to inspire buy-in.
Preparation for the Shift
October was spent preparing for a seamless, one-day implementation of proposed changes, including implementation of updated clinical policies, procedures, and document templates (rewritten to include only clinically appropriate information required by VA policy or the Joint Commission); streamlined staff schedules; and utilization of staff-developed and research/policy-driven EBP handbook. Finally, the Brief Addiction Monitor (BAM) was selected as objective criteria to consistently assess patient progress in treatment, and staff were instructed to use this measure at regular intervals and for all levels of care.
Emphasis was placed on ongoing fortification of staff and patient support for the reorganization. For example, the Addiction Severity Index, though not required by policy, was historically used, adding 90 minutes to the evaluation and admission session. Staff agreed to remove this measure to improve clinician availability. Staff were also empowered to rename the redesigned program, and chose Substance Use Disorders Service (SUDS).
Process Changes
To achieve same-day access to clinical care, program leadership created a daily morning orientation group. Patients are scheduled or may attend as a walk-in. The orientation’s purpose is to explain what services are available and to offer each patient an opportunity for immediate evaluation and treatment. Staff schedules were modified to provide patient evaluation appointment slots immediately following orientation. The number of immediate evaluation slots was initially assessed by analyzing the demand for treatment over the previous 6 months, determining the daily mean, and setting the number of slots to accommodate 3 standard deviations above the daily mean. If a patient in a daily orientation group expresses a willingness to engage in treatment, he or she is immediately evaluated by a counselor during a 90-minute session and seen by a psychiatrist to determine whether pharmacologic treatment would be appropriate. If needed, the medication is prescribed that day. The primary purpose of the patient’s initial clinical evaluation is to determine the most appropriate level of care based on ASAM criteria. Also available were 90-minute afternoon evaluation appointments with psychiatrists for patients who walk into the clinic after the morning orientation group had ended.
Prior to the redesign, clinic psychiatrists were minimally prescribing evidence-based pharmacotherapy for sobriety support. At the time of redesign, only 8% of patients diagnosed with opioid use disorders (OUDs) were prescribed buprenorphine/naloxone or naltrexone. Just 1.9% of patients diagnosed with alcohol use disorder (AUD) were prescribed naltrexone or acamprosate. With the redesign, access to these medications has significantly expanded.
All templates were redesigned to ensure consistent documentation. This change decreased the overall provider task burden, and explicitly supported the use of ASAM multidimensional criteria and the Brief Addiction Monitor (BAM) to identify a pretreatment baseline score and track each patient’s clinical progress.13 Evidence-based written curricula were standardized for individual and group psychotherapies to reduce provider and programmatic variation.
The redesign creates distinct levels of care based on ASAM criteria, including harm reduction, ambulatory detoxification, outpatient group and individual psychotherapy, an evidence-based Intensive Outpatient Program (IOP), and aftercare. Application of the ASAM standards has allowed clinicians to make accurate placement decisions that best meet individual patient needs and to serve as effective stewards even with limited treatment and financial resources. Although JAHVH does not have a residential SUD program, procedures were developed to refer veterans to community-based residential treatment programs when appropriate.
Group Therapies
With the redesign, SUDS was no longer exclusively a 12-step program; however, it still supported and recognized the value of this approach for some patients. A psychologist periodically audits group sessions to prevent drift from that group’s curriculum. Counselors are assigned to weekly hour-long clinical supervision sessions with a psychologist to review patient care and reinforce the application of evidence-based individualized treatment.
After reviewing empirical literature and VA directives, CBT-SUD was adopted. It encompasses individual and group interventions, such as motivational interviewing (MI), contingency management (CM), and medication-assisted therapies as primary therapeutic treatment modalities, all of which have demonstrated efficacy as measured by length of sobriety postintervention.9,14,15
Clinical Staff Improvements
Staff were reorganized into 3 interdisciplinary treatment teams. A weekly team meeting is scheduled to coordinate care and discuss the treatment of complex patients. Clinical staff focus has shifted from case-management to diagnosis and treatment; now patients are referred to their primary care team’s social worker for case management services. Allowing clinical staff to focus solely on the diagnosis and clinical treatment of SUDs has significantly enhanced productivity and morale.
Staff receive training in the newly adopted interventions during brief monthly refresher courses provided by inhouse psychologists. Additional training includes participation in local and national SUD teleconferences and onsite meetings with experts in harm reduction and motivational interventions. During the transition, clinicians were encouraged to attend staff resiliency training. Continuing education was available to the SUDS psychiatrists and all inpatient and outpatient psychiatrists at JAHVH. Recently, this educational initiative was expanded to include all primary care and inpatient internal medicine physicians.
Implementation
On November 2, 2015, all planned programmatic changes were simultaneously implemented. On that day, clinician and patient schedules changed, the new EBP curriculum was administered, the use of streamlined documentation procedures began, and daily orientation groups followed by same-day evaluations were initiated.
The pretreatment sobriety requirement was eliminated as a barrier to care, and the program began to use a harm-reduction treatment track as recommended by ASAM guidelines. Patients with urgent or emergent medical or psychiatric problems were immediately assessed by SUDS health care providers and treated in the clinic or transported to the emergency department. Previously unavailable, patient access to ambulatory detoxification was initiated. The prescription of buprenorphine/naloxone for the treatment of OUD treatments increased from 1 prescriber to all 3.
Three months after program reorganization, the leadership reviewed overall workflow, conducted patient satisfaction surveys, and evaluated facility use and productivity. To address patient needs and facilitate optimal use of space, the number of same-day evaluation slots was reduced while the number of individual therapy slots was increased.
Staff meet in workgroups to discuss EBPs and further refine content with feedback from the supervisory clinician and team psychologists who routinely audit group therapy sessions. Staff report ongoing benefit from weekly supervision with a clinical psychologist. An inpatient addiction consultation team that uses existing manpower and resources has been developed.
Program Goals and Outcomes
The SUDS program serves more patients at multiple levels of standardized care with 2 fewer full-time positions. One counselor and one advanced practice registered nurse were reallocated to different programs within the JAHVH VA mental health clinic. Following a review of all program clinic profiles in the VA’s Computerized Patient Record System (CPRS) for utilization, accuracy, and necessity, and allowing for accurate program data capture, the transition resulted in a reduction of distinct clinics from 114 to 67 (-58.7%). In fiscal year 2018, review of CPRS yielded 19,786 total visits (3,645 unique visits).
Eliminate Patient Wait Tme
Patient wait time, as measured in CPRS from date of initial evaluation to date of treatment was reduced from an average of 33 days to 0 within 2 weeks of program implementation. A review of CPRS data also indicated that preadmission attrition dropped from 54% to < 1%; all patients desiring treatment are assigned a counselor and treatment is initiated the same day.
Adopt ASAM Criteria
After the redesign, patients have received more appropriate care based on individualized treatment plans. Due to the implementation of a fluid and supportive model, patients can move through levels of care as clinical need dictates rather than failing treatment and having to reengage. Staff receive ongoing education on the use of ASAM. Evaluation and treatment plan templates now reflect assignment to level of care rationale using ASAM guidelines.
Use of Evidence-Based Psychotherapeutic Treatments
More consistent, coordinated, and effective psychotherapies have improved patient care. The program’s previous issues with patients receiving conflicting treatment guidance from different providers has been resolved. Duplicate and ineffective treatments, including multiple readmissions to the IOP level of care, overemphasis of abstinence-based modalities for patients in active use, and referrals to inpatient SUD care under the assumption that “higher level of care is better” have ceased through staff education, leadership support, and appropriate staffing and communication. Review of patient advocate complaints tracked by and resolved by the service demonstrated an 80% decrease in patient advocate complaints regarding SUD clinic services.
Implement Evidence-Based Psychopharmacologic Treatments
The pharmacotherapy education initiative yielded tangible benefits and is likely a significant contributor to the program’s improved clinical outcomes. Prescription of pharmacotherapy for patients with OUD has risen from 8% to 25.1% in eligible patients. Appropriate medication prescription for the treatment of AUD has risen from 1.9% to 9.8% in eligible patients. These data are reflected in the VA Pharmaceutical Drug Safety Initiative (PDSI) dashboard.
Streamline Documentation
Significantly reducing the charting burden was likely a significant contributor to increased provider productivity and improved patient outcomes. Regular meetings between SUDS leadership and clinical informatics ensure that standardized note templates meet hospital policy and gather all necessary accreditation information.
Improve Employee Morale
Increased staff morale is indicated by a noticeable reduction in employee sick days; a decrease of > 20% (over the same time period the previous year), per the VA electronic timekeeping system, during the first 6 months following the November 2 program implementation.
SUDS Inpatient Addiction Consult Team
In January of 2017, SUDS began an inpatient medicine consultation service to offer evaluation, pharmacotherapy, and supportive counseling to patients diagnosed with SUDs who had been admitted to inpatient medical and surgical services. This team includes existing SUDS staff members reallocated to the inpatient service, is led by a SUDS psychiatrist, and includes 3 multidisciplinary clinicians with extensive training in assessment, diagnosis, and treatment planning of SUDs and comorbid conditions. Prior to implementation, the SUDS inpatient addiction consult team met with hospital leadership and attending physicians for inpatient medicine and psychiatry physicians.
To access the SUDS inpatient addiction consult team, physicians request a consult. Patients are offered an evaluation and are assigned to a level of care with orders for outpatient appointments with a counselor and psychiatrist within 7 days of hospital discharge. Medication-assisted treatment for chronic SUDs is implemented while patients remain admitted to the inpatient medical service. In fiscal year 2018, the SUDS inpatient addiction consult team performed 1,428 inpatient evaluations.
Consistent Treatment Outcome Measures
The BAM is a clinical tool designed to measure patient outcomes in substance use disorders.13 Its 17-item scale measures substance use risk factors that may lead to relapse, and protective factors that are recovery-oriented behaviors that help prevent relapse. It demonstrates sensitivity to change and has excellent test-retest reliability. The BAM has been in use in the addictions treatment program since 2011 but was previously administered only after admission to the IOP and again after a 30- to 90-day follow-up period. Since the program redesign, all SUDS patients are administered the BAM at their initial evaluation and at each individual appointment thereafter. The initial BAM assessment encompasses the previous 30 days; this 30-day version is also used for monthly follow-ups. For BAM assessments that occur within 30 days from the time of the last evaluation, a 7-day version is used. Prior to the redesign, about 24% of patients received a follow-up 30-day BAM assessment.12 Per CPRS review of veterans participating in continued treatment, the rate rose to 100% 3 months after the redesign.
When program staff compared preredesign and postredesign BAM data, they detected significant clinical differences. Data demonstrate a 22.2% improvement in protective factors, including patient confidence in their ability to remain abstinent; engaging in self-help activities, such as attending Alcoholics Anonymous meetings; engaging in organized spiritual activities; going to school, working, or volunteering; securing a regular income; and time spent with friends or family who are supportive of recovery.
The data also show a marked reduction in substance use at follow-up points in treatment and a corresponding decrease in risk factors. One item of the BAM assesses patient level of satisfaction with their treatment. Since the redesign, patients report that they are “considerably” satisfied with their SUD treatment.
Currently, program staff are conducting a review of BAM scores by level of care to further parse the impact of various treatments and best target patient need using measurement-based care and EBP, such as contingency management, which provides small monetary incentives when patients maintain clean urine drug screens.16 In addition, the program plans to achieve more uniformity in BAM assessment intervals at all levels of care, and possibly also integrate BAM data into SUD group therapies. Correlation of the BAM scores to other metrics, such as readmission to inpatient medicine, relapse, urine drug screen, or critical laboratory values, will provide additional insight into impact of programmatic changes.
Discussion
Feedback from other clinics and services within the hospital has been very positive. Some providers have reported that they appreciate the ease and availability of access to SUDS. Additionally, patients engaged in treatment prior to the redesign have been contacted for an updated evaluation and assignment to a counselor and appropriate level of care. From the staff’s perspective, the shift to immediate access to care has allowed a more streamlined process with fewer hurdles for patient admission. Staff report that they now feel empowered to meet the needs of veterans in a comprehensive, same-day fashion.
The success of our redesign was contingent on internal and external sta
The successful implementation of these changes has revealed several important elements regarding patient care. The first lesson was that improving access and integrating best practices is possible without additional resources, outside monies, or disruption to patient services. With the support of MH&BSS leadership, the program streamlined existing processes and used both staff and clinic resources more efficiently.
The second lesson involved the importance of continually reviewing and revising standard operating procedures to match the needs of the current patient population. Policies and procedures that once were viewed as potential barriers to change have been replaced with a more flexible approach and willingness to evolve.
As a result, far fewer patients have been lost to treatment. The time and resources that staff historically dedicated to nonclinical patient care are now redirected to immediate service provision. This increase in operational efficiency and treatment efficacy has resulted in a boost to staff morale, even during a time of immense change and increased productivity. Program staff are now able to personally witness the significant changes in their patients’ lives and feel a sense of pride at being a member of a hard-working team that provides the highest quality of substance use treatment. This is critical to job satisfaction and meets the VA mission to provide timely, effective, and evidence-based treatments to patients.
Conclusion
JAHVH strives to continue to provide the highest quality of SUD treatment available. Future directions aim to streamline clinic operations by constantly monitoring and reviewing workloads, while also considering patient feedback. A continuous review of EBP is part of our clinic’s culture. Program leadership endeavors to promote an open environment where providers can share their triumphs and frustrations and foster a team approach to problem solving. Further plans include expanding the range of treatment levels offered by developing a residential SUD treatment facility.
1. Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health: Summary of the Effects of the 2015 NSDUH Questionnaire Redesign: Implications for Data Users. https://www.samhsa.gov/data/sites/default/files/NSDUH-TrendBreak-2015.pdf. Published June 2016. Accessed June 12, 2019.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan PDSKL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96(8):1149-1160.
4. Hser Y, Maglione M, Polinsky ML, Anglin MD. Predicting treatment entry among treatment-seeking drug abusers. J Subst Abuse Treatment. 1997;15(3):213-220.
5. Stark MJ, Campbell BK, Brinkerhoff CV. “Hello, may we help you?” A study of attrition prevention at the time of the first phone contact with substance-abusing clients. Am J Drug Alcohol Abuse. 1990;16:67-76.
6. US Department of Veterans Affairs. Uniform Mental Health Services in VA Medical Centers and Clinics. VHA Handbook 1160.01. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762. Updated November 2015. Accessed December 12, 2017.
7. Veterans Access, Choice and Accountability Act of 2014, 2 USC § 933.
8. DeMarce JM, Gnys M, Raffa SD, Karlin, BE. Cognitive Behavioral Therapy for Substance Use Disorders Among Veterans: Therapist Manual. Washington, DC: US Department of Veterans Affairs; 2014.
9. Mee-Lee D, Shulman GD, Fishman MJ, Gastfriend DR, Miller MM, eds. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions. 3rd ed. Carson City, NV: The Change Companies; 2013.
10. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
11. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for Opioid Use Disorder – Full Document. HHS Publication No. (SMA) 18-5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
12. Winn JL, Shealy SE, Kropp GJ, Felkins-Dohm D, Gonzales-Nolas C, Francis E. Housing assistance and case management: Improving access to substance use disorder treatment for homeless veterans. Psychological Serv. 2013;10(2):233-240.
13. Cacciola JS, Alterman AI, DePhilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treatment. 2013;44(3):256-263.
14. McHugh RK, Hearon BA, Otto MW. Cognitive-behavioral therapy for substance use disorders, Psychiatr Clinics North Am. 2010;33:511–525.
15. Karlin, BE, Cross, G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs health care system. Am Psychol. 2014;69:19-33.
16. DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes, Drug Alcohol Dependence. 2018;185:367-373.
1. Substance Abuse and Mental Health Services Administration. 2015 National Survey on Drug Use and Health: Summary of the Effects of the 2015 NSDUH Questionnaire Redesign: Implications for Data Users. https://www.samhsa.gov/data/sites/default/files/NSDUH-TrendBreak-2015.pdf. Published June 2016. Accessed June 12, 2019.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Donovan DM, Rosengren DB, Downey L, Cox GB, Sloan PDSKL. Attrition prevention with individuals awaiting publicly funded drug treatment. Addiction. 2001;96(8):1149-1160.
4. Hser Y, Maglione M, Polinsky ML, Anglin MD. Predicting treatment entry among treatment-seeking drug abusers. J Subst Abuse Treatment. 1997;15(3):213-220.
5. Stark MJ, Campbell BK, Brinkerhoff CV. “Hello, may we help you?” A study of attrition prevention at the time of the first phone contact with substance-abusing clients. Am J Drug Alcohol Abuse. 1990;16:67-76.
6. US Department of Veterans Affairs. Uniform Mental Health Services in VA Medical Centers and Clinics. VHA Handbook 1160.01. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762. Updated November 2015. Accessed December 12, 2017.
7. Veterans Access, Choice and Accountability Act of 2014, 2 USC § 933.
8. DeMarce JM, Gnys M, Raffa SD, Karlin, BE. Cognitive Behavioral Therapy for Substance Use Disorders Among Veterans: Therapist Manual. Washington, DC: US Department of Veterans Affairs; 2014.
9. Mee-Lee D, Shulman GD, Fishman MJ, Gastfriend DR, Miller MM, eds. The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions. 3rd ed. Carson City, NV: The Change Companies; 2013.
10. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the Treatment of Alcohol Use Disorder: A Brief Guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
11. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for Opioid Use Disorder – Full Document. HHS Publication No. (SMA) 18-5063FULLDOC. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
12. Winn JL, Shealy SE, Kropp GJ, Felkins-Dohm D, Gonzales-Nolas C, Francis E. Housing assistance and case management: Improving access to substance use disorder treatment for homeless veterans. Psychological Serv. 2013;10(2):233-240.
13. Cacciola JS, Alterman AI, DePhilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treatment. 2013;44(3):256-263.
14. McHugh RK, Hearon BA, Otto MW. Cognitive-behavioral therapy for substance use disorders, Psychiatr Clinics North Am. 2010;33:511–525.
15. Karlin, BE, Cross, G. From the laboratory to the therapy room: national dissemination and implementation of evidence-based psychotherapies in the U.S. Department of Veterans Affairs health care system. Am Psychol. 2014;69:19-33.
16. DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of contingency management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes, Drug Alcohol Dependence. 2018;185:367-373.
Data sharing to third parties prevalent in depression, smoking cessation apps
“Mechanisms that potentially enable a small number of dominant online service providers to link information about the use of mental health apps, without either user consent or awareness, appear to be prevalent,” Kit Huckvale, MB ChB, MSc, PhD, of Black Dog Institute at the University of New South Wales Sydney in Randwick, New South Wales, Australia, and colleagues wrote in their study. “Mismatches between declared privacy policies and observed behavior highlight the continuing need for innovation around trust and transparency for health apps.” The study was published in JAMA Network Open.
Dr. Huckvale and colleagues examined the top 36 depression and smoking cessation apps for Android and iOS in the United States accessed in January 2018; Of the apps downloaded, 15 apps were Android-only, 14 apps were iOS-only, and 7 apps were available on both platforms. The apps were assessed over a series of two sessions while network traffic was captured during use, which allowed researchers to determine what personal information was in each data transmission and where the information was going.
There were 25 apps with a privacy policy (69%), 22 of 25 apps (88%) described how that app primarily collected data, and only 16 of 25 apps (64%) provided information on secondary uses of data. Despite 23 of 25 apps (92%) addressing “the possibility of transmission of data to any third party,” 33 of 36 apps overall (92%) transmitted data to third parties. The two most common entities that received third-party data for marketing, advertising, or analytic purposes were Google and Facebook (29 of 36 apps; 81%). However, 12 of 28 apps (43%) that sent data to Google and 6 of 12 apps (50%) that sent data to Facebook disclosed that they would share data with those companies.
The type of data sent to Google and Facebook consisted of a strong identifier to the device or a username (9 of 33 apps; 27%), or a weak identifier in the form of an advertising identifier or a pseudonymous profile that can link users to their behavior on the app and on other products and platforms (26 of 33 apps; 79%).
“As smartphones continue to gain capabilities to collect new forms of personal, biometric, and health information, it is imperative for the health care community to respond with new methods and processes to review apps and ensure they remain safe and protect personal health information,” the researchers concluded.
One of the investigators, Mark E. Larsen, DPhil, reported receiving grants from National Health and Medical Research Council. The other authors reported no relevant conflicts of interest.
SOURCE: Huckvale K et al. JAMA Netw Open. 2019. doi: 10.1001/jamanetworkopen.2019.2542.
“Mechanisms that potentially enable a small number of dominant online service providers to link information about the use of mental health apps, without either user consent or awareness, appear to be prevalent,” Kit Huckvale, MB ChB, MSc, PhD, of Black Dog Institute at the University of New South Wales Sydney in Randwick, New South Wales, Australia, and colleagues wrote in their study. “Mismatches between declared privacy policies and observed behavior highlight the continuing need for innovation around trust and transparency for health apps.” The study was published in JAMA Network Open.
Dr. Huckvale and colleagues examined the top 36 depression and smoking cessation apps for Android and iOS in the United States accessed in January 2018; Of the apps downloaded, 15 apps were Android-only, 14 apps were iOS-only, and 7 apps were available on both platforms. The apps were assessed over a series of two sessions while network traffic was captured during use, which allowed researchers to determine what personal information was in each data transmission and where the information was going.
There were 25 apps with a privacy policy (69%), 22 of 25 apps (88%) described how that app primarily collected data, and only 16 of 25 apps (64%) provided information on secondary uses of data. Despite 23 of 25 apps (92%) addressing “the possibility of transmission of data to any third party,” 33 of 36 apps overall (92%) transmitted data to third parties. The two most common entities that received third-party data for marketing, advertising, or analytic purposes were Google and Facebook (29 of 36 apps; 81%). However, 12 of 28 apps (43%) that sent data to Google and 6 of 12 apps (50%) that sent data to Facebook disclosed that they would share data with those companies.
The type of data sent to Google and Facebook consisted of a strong identifier to the device or a username (9 of 33 apps; 27%), or a weak identifier in the form of an advertising identifier or a pseudonymous profile that can link users to their behavior on the app and on other products and platforms (26 of 33 apps; 79%).
“As smartphones continue to gain capabilities to collect new forms of personal, biometric, and health information, it is imperative for the health care community to respond with new methods and processes to review apps and ensure they remain safe and protect personal health information,” the researchers concluded.
One of the investigators, Mark E. Larsen, DPhil, reported receiving grants from National Health and Medical Research Council. The other authors reported no relevant conflicts of interest.
SOURCE: Huckvale K et al. JAMA Netw Open. 2019. doi: 10.1001/jamanetworkopen.2019.2542.
“Mechanisms that potentially enable a small number of dominant online service providers to link information about the use of mental health apps, without either user consent or awareness, appear to be prevalent,” Kit Huckvale, MB ChB, MSc, PhD, of Black Dog Institute at the University of New South Wales Sydney in Randwick, New South Wales, Australia, and colleagues wrote in their study. “Mismatches between declared privacy policies and observed behavior highlight the continuing need for innovation around trust and transparency for health apps.” The study was published in JAMA Network Open.
Dr. Huckvale and colleagues examined the top 36 depression and smoking cessation apps for Android and iOS in the United States accessed in January 2018; Of the apps downloaded, 15 apps were Android-only, 14 apps were iOS-only, and 7 apps were available on both platforms. The apps were assessed over a series of two sessions while network traffic was captured during use, which allowed researchers to determine what personal information was in each data transmission and where the information was going.
There were 25 apps with a privacy policy (69%), 22 of 25 apps (88%) described how that app primarily collected data, and only 16 of 25 apps (64%) provided information on secondary uses of data. Despite 23 of 25 apps (92%) addressing “the possibility of transmission of data to any third party,” 33 of 36 apps overall (92%) transmitted data to third parties. The two most common entities that received third-party data for marketing, advertising, or analytic purposes were Google and Facebook (29 of 36 apps; 81%). However, 12 of 28 apps (43%) that sent data to Google and 6 of 12 apps (50%) that sent data to Facebook disclosed that they would share data with those companies.
The type of data sent to Google and Facebook consisted of a strong identifier to the device or a username (9 of 33 apps; 27%), or a weak identifier in the form of an advertising identifier or a pseudonymous profile that can link users to their behavior on the app and on other products and platforms (26 of 33 apps; 79%).
“As smartphones continue to gain capabilities to collect new forms of personal, biometric, and health information, it is imperative for the health care community to respond with new methods and processes to review apps and ensure they remain safe and protect personal health information,” the researchers concluded.
One of the investigators, Mark E. Larsen, DPhil, reported receiving grants from National Health and Medical Research Council. The other authors reported no relevant conflicts of interest.
SOURCE: Huckvale K et al. JAMA Netw Open. 2019. doi: 10.1001/jamanetworkopen.2019.2542.
FROM JAMA NETWORK OPEN
IHS Announces Requirements to Increase Access to OUD Treatment
Native American communities have experienced the largest increase in drug overdose deaths of all racial/ethnic groups in the US. Between 1999 and 2015, drug overdose deaths rose > 500%. To help ensure that American Indians and Alaska Natives (AI/AN) get the treatment they need, the Indian Health Service (IHS) has released Special General Memorandum 2019-01: Assuring Access to Medication Assisted Treatment for Opioid Use Disorder. It requires all IHS federal facilities to:
- Identify opioid use disorder (OUD) treatment resources in their local areas;
- Create an action plan, no later than Dec. 11, 2019; and
- Provide or coordinate patient access to medication-assisted treatment (MAT), specifically increasing access to culturally appropriate prevention, treatment, and recovery support services.
MAT is a comprehensive evidence-based approach that combines pharmacologic interventions with substance abuse counseling and culturally sensitive social support.
The IHS has recently taken other steps to further facilitate MAT access in tribal communities. For example, it has added 3 FDA-approved medications to the National Core Formulary: buprenorphine, buprenorphine/naloxone, and injectable naltrexone, all of which relieve withdrawal symptoms and psychological cravings, supporting adherence to treatment and reducing illicit opioid use.
In addition, the IHS has published the Internet Eligible Controlled Substance Provider Designation Policy. This policy, established in 2018, is designed to increase access to treatment for AI/AN who live in rural or remote areas, where it can be difficult to access a provider with the necessary training and Drug Enforcement Administration approval to prescribe buprenorphine in an outpatient or office-based setting. Once approved, IHS, tribal, and urban Indian organization health care providers can prescribe controlled substances for MAT through telemedicine.
In 2018, the IHS also launched a new website (www.IHS.gov/opioids) to share information about opioids with patients, health care providers, tribal leaders, tribal and urban program administrators, and other community members. The site includes information on approaches to prevent opioid abuse, pain management, recovery tools, and funding opportunities.
Native American communities have experienced the largest increase in drug overdose deaths of all racial/ethnic groups in the US. Between 1999 and 2015, drug overdose deaths rose > 500%. To help ensure that American Indians and Alaska Natives (AI/AN) get the treatment they need, the Indian Health Service (IHS) has released Special General Memorandum 2019-01: Assuring Access to Medication Assisted Treatment for Opioid Use Disorder. It requires all IHS federal facilities to:
- Identify opioid use disorder (OUD) treatment resources in their local areas;
- Create an action plan, no later than Dec. 11, 2019; and
- Provide or coordinate patient access to medication-assisted treatment (MAT), specifically increasing access to culturally appropriate prevention, treatment, and recovery support services.
MAT is a comprehensive evidence-based approach that combines pharmacologic interventions with substance abuse counseling and culturally sensitive social support.
The IHS has recently taken other steps to further facilitate MAT access in tribal communities. For example, it has added 3 FDA-approved medications to the National Core Formulary: buprenorphine, buprenorphine/naloxone, and injectable naltrexone, all of which relieve withdrawal symptoms and psychological cravings, supporting adherence to treatment and reducing illicit opioid use.
In addition, the IHS has published the Internet Eligible Controlled Substance Provider Designation Policy. This policy, established in 2018, is designed to increase access to treatment for AI/AN who live in rural or remote areas, where it can be difficult to access a provider with the necessary training and Drug Enforcement Administration approval to prescribe buprenorphine in an outpatient or office-based setting. Once approved, IHS, tribal, and urban Indian organization health care providers can prescribe controlled substances for MAT through telemedicine.
In 2018, the IHS also launched a new website (www.IHS.gov/opioids) to share information about opioids with patients, health care providers, tribal leaders, tribal and urban program administrators, and other community members. The site includes information on approaches to prevent opioid abuse, pain management, recovery tools, and funding opportunities.
Native American communities have experienced the largest increase in drug overdose deaths of all racial/ethnic groups in the US. Between 1999 and 2015, drug overdose deaths rose > 500%. To help ensure that American Indians and Alaska Natives (AI/AN) get the treatment they need, the Indian Health Service (IHS) has released Special General Memorandum 2019-01: Assuring Access to Medication Assisted Treatment for Opioid Use Disorder. It requires all IHS federal facilities to:
- Identify opioid use disorder (OUD) treatment resources in their local areas;
- Create an action plan, no later than Dec. 11, 2019; and
- Provide or coordinate patient access to medication-assisted treatment (MAT), specifically increasing access to culturally appropriate prevention, treatment, and recovery support services.
MAT is a comprehensive evidence-based approach that combines pharmacologic interventions with substance abuse counseling and culturally sensitive social support.
The IHS has recently taken other steps to further facilitate MAT access in tribal communities. For example, it has added 3 FDA-approved medications to the National Core Formulary: buprenorphine, buprenorphine/naloxone, and injectable naltrexone, all of which relieve withdrawal symptoms and psychological cravings, supporting adherence to treatment and reducing illicit opioid use.
In addition, the IHS has published the Internet Eligible Controlled Substance Provider Designation Policy. This policy, established in 2018, is designed to increase access to treatment for AI/AN who live in rural or remote areas, where it can be difficult to access a provider with the necessary training and Drug Enforcement Administration approval to prescribe buprenorphine in an outpatient or office-based setting. Once approved, IHS, tribal, and urban Indian organization health care providers can prescribe controlled substances for MAT through telemedicine.
In 2018, the IHS also launched a new website (www.IHS.gov/opioids) to share information about opioids with patients, health care providers, tribal leaders, tribal and urban program administrators, and other community members. The site includes information on approaches to prevent opioid abuse, pain management, recovery tools, and funding opportunities.
Co-use of opioids, methamphetamine on rise in rural Oregon
Survey shows simultaneous use climbed from 19% to 34% between 2011 and 2017
SAN ANTONIO – A perceived low risk of using methamphetamine and a belief that methamphetamine helps with opioid addiction are both driving increasing levels of concurrent methamphetamine and opioid use in rural Oregon, according to recent qualitative research.
Use of methamphetamine by those who use opioids increased from 19% to 34% between 2011 and 2017, Gillian Leichtling, research manager at HealthInsight Oregon, said at the annual meeting of the College on Problems of Drug Dependence.
The highest prevalence of simultaneous use is in the western states, where 63% of opioid users also use methamphetamine, she said. Hospitalizations and overdoses related to methamphetamine have likewise increased, particularly in rural communities.
To better understand the motivations and implications of this trend, Ms. Leichtling and her colleagues conducted a survey from March 2018 to April 2019 of adults who had nonmedically used/injected opioids or methamphetamine in the past month. All participants lived in Lane or Douglas counties in southwestern Oregon, where half the land is controlled by the U.S. Forest Service and Bureau of Land Management, and opioid overdose rates surpass that of the state average. Additional 60-minute semistructured qualitative interviews were conducted in summer 2018.
Among the 144 surveyed, 78% had used an opioid in the past month, nearly all of whom (96%) had also used methamphetamine in the past month. The interviewees included adults fairly evenly spread across ages, but most (94%) were white.
The main themes that emerged from the interviews involved the perceived benefits and consequences of those who used both opioids and methamphetamine, and the environmental circumstances that supported methamphetamine use, Ms. Leichtling explained.
Most people interviewed had their first experience with methamphetamine early in life, typically in early or mid-adolescence, she said. Two respondents, for example, first began using at 8 and 12 years old, the former learning from a preteen neighbor.
Methamphetamine’s wide availability and low cost also increased its use. In addition, methamphetamine use carries less stigma than heroin use, participants told the researchers. One person who noted the popularity of methamphetamine added: “You get treated really badly if you’re a heroin addict.”
In addition to less stigma, many of the perceived benefits of methamphetamine use related to opioids: Participants said methamphetamine “relieves opioid withdrawal, helps reduce opioid use, enhances functioning, and combines well with opioids” for a pleasurable effect, Ms. Leichtling said. Some also perceived methamphetamine as a way to reverse opioid overdose.
“I’m getting out of [the buprenorphine] program; they’re titrating me down rapidly, and so I’ve been sick for a week,” one respondent told researchers. “I’ve been doing so much more meth just to try to deflect the pain ... they’re too hard to come down from. It’s just you can’t do it without another drug ... especially if you have a job or responsibilities or kids,” they told researchers.
Another woman said she and her mother were able to come off heroin by using methamphetamine instead, and a yet another said she and her ex-boyfriend used methamphetamine to stop using opioids.
Several respondents also mentioned using methamphetamine to help them go to work, effectively put in long days, and then care for their families when they get home.
Ms. Leichtling described three main implications of the findings for interventions in rural areas. One was the need at the community level for greater access to medication-assisted treatment (MAT) of opioid use disorder to reduce the use of methamphetamine to taper opioid use or withdrawal.
Next, clinicians need to provide tailored treatment for the co-use of opioids and methamphetamine, and educate patients on alternatives to being dropped from medication-assisted opioid use disorder treatment. Finally, individual users need education on overdose that addresses the misconceptions and risks related to methamphetamine risk, Ms. Leichtling said.
Since the survey and interviews came only from two rural Oregon counties, the findings might not be generalizable, Ms. Leichtling said, and their study did not explore social determinants of health that might be at work.
The National Institute on Drug Abuse funded the research. The authors had no conflicts of interest.
Survey shows simultaneous use climbed from 19% to 34% between 2011 and 2017
Survey shows simultaneous use climbed from 19% to 34% between 2011 and 2017
SAN ANTONIO – A perceived low risk of using methamphetamine and a belief that methamphetamine helps with opioid addiction are both driving increasing levels of concurrent methamphetamine and opioid use in rural Oregon, according to recent qualitative research.
Use of methamphetamine by those who use opioids increased from 19% to 34% between 2011 and 2017, Gillian Leichtling, research manager at HealthInsight Oregon, said at the annual meeting of the College on Problems of Drug Dependence.
The highest prevalence of simultaneous use is in the western states, where 63% of opioid users also use methamphetamine, she said. Hospitalizations and overdoses related to methamphetamine have likewise increased, particularly in rural communities.
To better understand the motivations and implications of this trend, Ms. Leichtling and her colleagues conducted a survey from March 2018 to April 2019 of adults who had nonmedically used/injected opioids or methamphetamine in the past month. All participants lived in Lane or Douglas counties in southwestern Oregon, where half the land is controlled by the U.S. Forest Service and Bureau of Land Management, and opioid overdose rates surpass that of the state average. Additional 60-minute semistructured qualitative interviews were conducted in summer 2018.
Among the 144 surveyed, 78% had used an opioid in the past month, nearly all of whom (96%) had also used methamphetamine in the past month. The interviewees included adults fairly evenly spread across ages, but most (94%) were white.
The main themes that emerged from the interviews involved the perceived benefits and consequences of those who used both opioids and methamphetamine, and the environmental circumstances that supported methamphetamine use, Ms. Leichtling explained.
Most people interviewed had their first experience with methamphetamine early in life, typically in early or mid-adolescence, she said. Two respondents, for example, first began using at 8 and 12 years old, the former learning from a preteen neighbor.
Methamphetamine’s wide availability and low cost also increased its use. In addition, methamphetamine use carries less stigma than heroin use, participants told the researchers. One person who noted the popularity of methamphetamine added: “You get treated really badly if you’re a heroin addict.”
In addition to less stigma, many of the perceived benefits of methamphetamine use related to opioids: Participants said methamphetamine “relieves opioid withdrawal, helps reduce opioid use, enhances functioning, and combines well with opioids” for a pleasurable effect, Ms. Leichtling said. Some also perceived methamphetamine as a way to reverse opioid overdose.
“I’m getting out of [the buprenorphine] program; they’re titrating me down rapidly, and so I’ve been sick for a week,” one respondent told researchers. “I’ve been doing so much more meth just to try to deflect the pain ... they’re too hard to come down from. It’s just you can’t do it without another drug ... especially if you have a job or responsibilities or kids,” they told researchers.
Another woman said she and her mother were able to come off heroin by using methamphetamine instead, and a yet another said she and her ex-boyfriend used methamphetamine to stop using opioids.
Several respondents also mentioned using methamphetamine to help them go to work, effectively put in long days, and then care for their families when they get home.
Ms. Leichtling described three main implications of the findings for interventions in rural areas. One was the need at the community level for greater access to medication-assisted treatment (MAT) of opioid use disorder to reduce the use of methamphetamine to taper opioid use or withdrawal.
Next, clinicians need to provide tailored treatment for the co-use of opioids and methamphetamine, and educate patients on alternatives to being dropped from medication-assisted opioid use disorder treatment. Finally, individual users need education on overdose that addresses the misconceptions and risks related to methamphetamine risk, Ms. Leichtling said.
Since the survey and interviews came only from two rural Oregon counties, the findings might not be generalizable, Ms. Leichtling said, and their study did not explore social determinants of health that might be at work.
The National Institute on Drug Abuse funded the research. The authors had no conflicts of interest.
SAN ANTONIO – A perceived low risk of using methamphetamine and a belief that methamphetamine helps with opioid addiction are both driving increasing levels of concurrent methamphetamine and opioid use in rural Oregon, according to recent qualitative research.
Use of methamphetamine by those who use opioids increased from 19% to 34% between 2011 and 2017, Gillian Leichtling, research manager at HealthInsight Oregon, said at the annual meeting of the College on Problems of Drug Dependence.
The highest prevalence of simultaneous use is in the western states, where 63% of opioid users also use methamphetamine, she said. Hospitalizations and overdoses related to methamphetamine have likewise increased, particularly in rural communities.
To better understand the motivations and implications of this trend, Ms. Leichtling and her colleagues conducted a survey from March 2018 to April 2019 of adults who had nonmedically used/injected opioids or methamphetamine in the past month. All participants lived in Lane or Douglas counties in southwestern Oregon, where half the land is controlled by the U.S. Forest Service and Bureau of Land Management, and opioid overdose rates surpass that of the state average. Additional 60-minute semistructured qualitative interviews were conducted in summer 2018.
Among the 144 surveyed, 78% had used an opioid in the past month, nearly all of whom (96%) had also used methamphetamine in the past month. The interviewees included adults fairly evenly spread across ages, but most (94%) were white.
The main themes that emerged from the interviews involved the perceived benefits and consequences of those who used both opioids and methamphetamine, and the environmental circumstances that supported methamphetamine use, Ms. Leichtling explained.
Most people interviewed had their first experience with methamphetamine early in life, typically in early or mid-adolescence, she said. Two respondents, for example, first began using at 8 and 12 years old, the former learning from a preteen neighbor.
Methamphetamine’s wide availability and low cost also increased its use. In addition, methamphetamine use carries less stigma than heroin use, participants told the researchers. One person who noted the popularity of methamphetamine added: “You get treated really badly if you’re a heroin addict.”
In addition to less stigma, many of the perceived benefits of methamphetamine use related to opioids: Participants said methamphetamine “relieves opioid withdrawal, helps reduce opioid use, enhances functioning, and combines well with opioids” for a pleasurable effect, Ms. Leichtling said. Some also perceived methamphetamine as a way to reverse opioid overdose.
“I’m getting out of [the buprenorphine] program; they’re titrating me down rapidly, and so I’ve been sick for a week,” one respondent told researchers. “I’ve been doing so much more meth just to try to deflect the pain ... they’re too hard to come down from. It’s just you can’t do it without another drug ... especially if you have a job or responsibilities or kids,” they told researchers.
Another woman said she and her mother were able to come off heroin by using methamphetamine instead, and a yet another said she and her ex-boyfriend used methamphetamine to stop using opioids.
Several respondents also mentioned using methamphetamine to help them go to work, effectively put in long days, and then care for their families when they get home.
Ms. Leichtling described three main implications of the findings for interventions in rural areas. One was the need at the community level for greater access to medication-assisted treatment (MAT) of opioid use disorder to reduce the use of methamphetamine to taper opioid use or withdrawal.
Next, clinicians need to provide tailored treatment for the co-use of opioids and methamphetamine, and educate patients on alternatives to being dropped from medication-assisted opioid use disorder treatment. Finally, individual users need education on overdose that addresses the misconceptions and risks related to methamphetamine risk, Ms. Leichtling said.
Since the survey and interviews came only from two rural Oregon counties, the findings might not be generalizable, Ms. Leichtling said, and their study did not explore social determinants of health that might be at work.
The National Institute on Drug Abuse funded the research. The authors had no conflicts of interest.
REPORTING FROM CPDD 2019
Parent education improves quick disposal of children’s unused prescription opioids
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
REPORTING FROM CPDD 2019
Medical cannabis laws appear no longer tied to drop in opioid overdose mortality
Correlations do not hold when analysis is expanded to 2017
Contrary to previous research indicating that medical cannabis laws reduced opioid overdose mortality, the association between these two has reversed, with opioid overdose mortality increased in states with comprehensive medical cannabis laws, according to Chelsea L. Shover, PhD, and associates.
The original research by Marcus A. Bachhuber, MD, and associates showed that the introduction of state medical cannabis laws was associated with a 24.8% reduction in opioid overdose deaths per 100,000 population between 1999 and 2010. In contrast, the new research – which looked at a longer time period than the original research did – found that the association between state medical cannabis laws and opioid overdose mortality reversed direction, from –21% to +23%.
“We find it unlikely that medical cannabis – used by about 2.5% of the U.S. population – has exerted large conflicting effects on opioid overdose mortality,” wrote Dr. Shover, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and associates. “A more plausible interpretation is that this association is spurious.” Their study was published in the Proceedings of the National Academy of Sciences.
To conduct their analysis, Dr. Shover and associates extended the timeline reviewed by Dr. Bachhuber and associates to 2017. During 2010-2017, 32 states enacted medical cannabis laws, including 17 allowing only medical cannabis with low levels of tetrahydrocannabinol (THC), and 8 legalized recreational marijuana. In the expanded timeline during 1999-2017, states possessing a comprehensive medical marijuana law saw an increase in opioid overdose mortality of 28.2%. Meanwhile, states with recreational marijuana laws saw a decrease of 14.7% in opioid overdose mortality, and states with low-THC medical cannabis laws saw a decrease of 7.1%. However, the investigators noted that those values had wide confidence intervals, which indicates “compatibility with large range of true associations.”
Corporate actors with deep pockets have substantial ability to promote congenial results, and suffering people are desperate for effective solutions. Cannabinoids have demonstrated therapeutic benefits, but reducing population-level opioid overdose mortality does not appear to be among them,” Dr. Shover and associates noted.
Dr. Shover reported receiving support from National Institute on Drug Abuse and the Wu Tsai Neurosciences Institute. Another coauthor received support from the Veterans Health Administration, Wu Tsai Neurosciences Institute, and the Esther Ting Memorial Professorship at Stanford.
SOURCE: Shover CL et al. Proc Natl Acad Sci U S A. 2019 Jun 10. doi: 10.1073/pnas.1903434116.
Correlations do not hold when analysis is expanded to 2017
Correlations do not hold when analysis is expanded to 2017
Contrary to previous research indicating that medical cannabis laws reduced opioid overdose mortality, the association between these two has reversed, with opioid overdose mortality increased in states with comprehensive medical cannabis laws, according to Chelsea L. Shover, PhD, and associates.
The original research by Marcus A. Bachhuber, MD, and associates showed that the introduction of state medical cannabis laws was associated with a 24.8% reduction in opioid overdose deaths per 100,000 population between 1999 and 2010. In contrast, the new research – which looked at a longer time period than the original research did – found that the association between state medical cannabis laws and opioid overdose mortality reversed direction, from –21% to +23%.
“We find it unlikely that medical cannabis – used by about 2.5% of the U.S. population – has exerted large conflicting effects on opioid overdose mortality,” wrote Dr. Shover, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and associates. “A more plausible interpretation is that this association is spurious.” Their study was published in the Proceedings of the National Academy of Sciences.
To conduct their analysis, Dr. Shover and associates extended the timeline reviewed by Dr. Bachhuber and associates to 2017. During 2010-2017, 32 states enacted medical cannabis laws, including 17 allowing only medical cannabis with low levels of tetrahydrocannabinol (THC), and 8 legalized recreational marijuana. In the expanded timeline during 1999-2017, states possessing a comprehensive medical marijuana law saw an increase in opioid overdose mortality of 28.2%. Meanwhile, states with recreational marijuana laws saw a decrease of 14.7% in opioid overdose mortality, and states with low-THC medical cannabis laws saw a decrease of 7.1%. However, the investigators noted that those values had wide confidence intervals, which indicates “compatibility with large range of true associations.”
Corporate actors with deep pockets have substantial ability to promote congenial results, and suffering people are desperate for effective solutions. Cannabinoids have demonstrated therapeutic benefits, but reducing population-level opioid overdose mortality does not appear to be among them,” Dr. Shover and associates noted.
Dr. Shover reported receiving support from National Institute on Drug Abuse and the Wu Tsai Neurosciences Institute. Another coauthor received support from the Veterans Health Administration, Wu Tsai Neurosciences Institute, and the Esther Ting Memorial Professorship at Stanford.
SOURCE: Shover CL et al. Proc Natl Acad Sci U S A. 2019 Jun 10. doi: 10.1073/pnas.1903434116.
Contrary to previous research indicating that medical cannabis laws reduced opioid overdose mortality, the association between these two has reversed, with opioid overdose mortality increased in states with comprehensive medical cannabis laws, according to Chelsea L. Shover, PhD, and associates.
The original research by Marcus A. Bachhuber, MD, and associates showed that the introduction of state medical cannabis laws was associated with a 24.8% reduction in opioid overdose deaths per 100,000 population between 1999 and 2010. In contrast, the new research – which looked at a longer time period than the original research did – found that the association between state medical cannabis laws and opioid overdose mortality reversed direction, from –21% to +23%.
“We find it unlikely that medical cannabis – used by about 2.5% of the U.S. population – has exerted large conflicting effects on opioid overdose mortality,” wrote Dr. Shover, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and associates. “A more plausible interpretation is that this association is spurious.” Their study was published in the Proceedings of the National Academy of Sciences.
To conduct their analysis, Dr. Shover and associates extended the timeline reviewed by Dr. Bachhuber and associates to 2017. During 2010-2017, 32 states enacted medical cannabis laws, including 17 allowing only medical cannabis with low levels of tetrahydrocannabinol (THC), and 8 legalized recreational marijuana. In the expanded timeline during 1999-2017, states possessing a comprehensive medical marijuana law saw an increase in opioid overdose mortality of 28.2%. Meanwhile, states with recreational marijuana laws saw a decrease of 14.7% in opioid overdose mortality, and states with low-THC medical cannabis laws saw a decrease of 7.1%. However, the investigators noted that those values had wide confidence intervals, which indicates “compatibility with large range of true associations.”
Corporate actors with deep pockets have substantial ability to promote congenial results, and suffering people are desperate for effective solutions. Cannabinoids have demonstrated therapeutic benefits, but reducing population-level opioid overdose mortality does not appear to be among them,” Dr. Shover and associates noted.
Dr. Shover reported receiving support from National Institute on Drug Abuse and the Wu Tsai Neurosciences Institute. Another coauthor received support from the Veterans Health Administration, Wu Tsai Neurosciences Institute, and the Esther Ting Memorial Professorship at Stanford.
SOURCE: Shover CL et al. Proc Natl Acad Sci U S A. 2019 Jun 10. doi: 10.1073/pnas.1903434116.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Vaping among teens increased significantly from 2017 to 2018
according to data from national cross-sectional surveys.
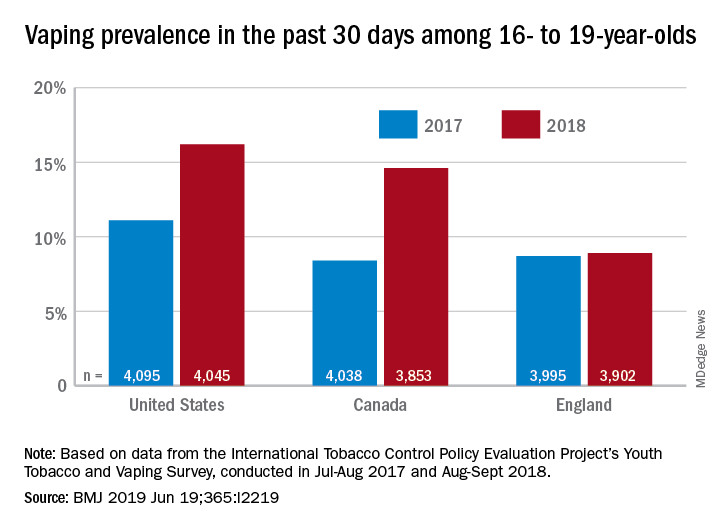
The prevalence of vaping in the past 30 days rose from 11% to 16% in the United States and from 8% to 14.6% in Canada, while use in England showed an nonsignificant increase of 8.7% to 8.9%, David Hammond, PhD, of the University of Waterloo (Canada) and associates said in the BMJ.
Embedded in those U.S. and Canadian increases is the recent evolution of the vaping market brought about by “the growth of JUUL e-cigarettes and similar products [that use] benzoic acid and nicotine salt technology to deliver higher concentrations of nicotine than conventional e-cigarettes,” they explained.
In England, the JUUL system is limited to less than half the nicotine concentration, at 20 mg/mL, compared with more than 50 mg/mL in the United States and Canada, and it was not available at all types of retail outlets at the time of the surveys. That situation changed in March 2019, when the company expanded to convenience stores, the investigators noted.
In the United States, JUUL was the second-most popular product among past–30-day vapers who had a usual brand in 2017, with 9% reporting use. In 2018, JUUL was the most popular brand and use was up to 28%. In Canada, the brand was not among the top five in 2017, but was third in 2018 at 10% in those who reported vaping in the past 30 days. The leading Canadian brand in 2018 was Smok, which released a nicotine-salt version in March of 2018, Dr. Hammond and associates reported.
“Before 2018, there was relatively little evidence of regular vaping among adolescents that might be indicative of nicotine addiction; however, the emergence of JUUL and nicotine salt–based products might signal a change,” they wrote.
The International Tobacco Control Policy Evaluation Project’s Youth Tobacco and Vaping Survey was conducted online in each country in two waves – July to August 2017 and August to September 2018 – with a sample size of approximately 12,000 for each.
The study was funded by the U.S. National Institutes of Health. Dr. Hammond is supported by a Canadian Institutes of Health Research–Public Health Agency of Canada applied public health research chair. The investigators said that they had no other financial disclosures to report, but several have served as paid witnesses in legal challenges against tobacco companies.
SOURCE: Hammond D et al. BMJ 2019 Jun 19. doi: 10.1136/bmj.l2219.
according to data from national cross-sectional surveys.

The prevalence of vaping in the past 30 days rose from 11% to 16% in the United States and from 8% to 14.6% in Canada, while use in England showed an nonsignificant increase of 8.7% to 8.9%, David Hammond, PhD, of the University of Waterloo (Canada) and associates said in the BMJ.
Embedded in those U.S. and Canadian increases is the recent evolution of the vaping market brought about by “the growth of JUUL e-cigarettes and similar products [that use] benzoic acid and nicotine salt technology to deliver higher concentrations of nicotine than conventional e-cigarettes,” they explained.
In England, the JUUL system is limited to less than half the nicotine concentration, at 20 mg/mL, compared with more than 50 mg/mL in the United States and Canada, and it was not available at all types of retail outlets at the time of the surveys. That situation changed in March 2019, when the company expanded to convenience stores, the investigators noted.
In the United States, JUUL was the second-most popular product among past–30-day vapers who had a usual brand in 2017, with 9% reporting use. In 2018, JUUL was the most popular brand and use was up to 28%. In Canada, the brand was not among the top five in 2017, but was third in 2018 at 10% in those who reported vaping in the past 30 days. The leading Canadian brand in 2018 was Smok, which released a nicotine-salt version in March of 2018, Dr. Hammond and associates reported.
“Before 2018, there was relatively little evidence of regular vaping among adolescents that might be indicative of nicotine addiction; however, the emergence of JUUL and nicotine salt–based products might signal a change,” they wrote.
The International Tobacco Control Policy Evaluation Project’s Youth Tobacco and Vaping Survey was conducted online in each country in two waves – July to August 2017 and August to September 2018 – with a sample size of approximately 12,000 for each.
The study was funded by the U.S. National Institutes of Health. Dr. Hammond is supported by a Canadian Institutes of Health Research–Public Health Agency of Canada applied public health research chair. The investigators said that they had no other financial disclosures to report, but several have served as paid witnesses in legal challenges against tobacco companies.
SOURCE: Hammond D et al. BMJ 2019 Jun 19. doi: 10.1136/bmj.l2219.
according to data from national cross-sectional surveys.

The prevalence of vaping in the past 30 days rose from 11% to 16% in the United States and from 8% to 14.6% in Canada, while use in England showed an nonsignificant increase of 8.7% to 8.9%, David Hammond, PhD, of the University of Waterloo (Canada) and associates said in the BMJ.
Embedded in those U.S. and Canadian increases is the recent evolution of the vaping market brought about by “the growth of JUUL e-cigarettes and similar products [that use] benzoic acid and nicotine salt technology to deliver higher concentrations of nicotine than conventional e-cigarettes,” they explained.
In England, the JUUL system is limited to less than half the nicotine concentration, at 20 mg/mL, compared with more than 50 mg/mL in the United States and Canada, and it was not available at all types of retail outlets at the time of the surveys. That situation changed in March 2019, when the company expanded to convenience stores, the investigators noted.
In the United States, JUUL was the second-most popular product among past–30-day vapers who had a usual brand in 2017, with 9% reporting use. In 2018, JUUL was the most popular brand and use was up to 28%. In Canada, the brand was not among the top five in 2017, but was third in 2018 at 10% in those who reported vaping in the past 30 days. The leading Canadian brand in 2018 was Smok, which released a nicotine-salt version in March of 2018, Dr. Hammond and associates reported.
“Before 2018, there was relatively little evidence of regular vaping among adolescents that might be indicative of nicotine addiction; however, the emergence of JUUL and nicotine salt–based products might signal a change,” they wrote.
The International Tobacco Control Policy Evaluation Project’s Youth Tobacco and Vaping Survey was conducted online in each country in two waves – July to August 2017 and August to September 2018 – with a sample size of approximately 12,000 for each.
The study was funded by the U.S. National Institutes of Health. Dr. Hammond is supported by a Canadian Institutes of Health Research–Public Health Agency of Canada applied public health research chair. The investigators said that they had no other financial disclosures to report, but several have served as paid witnesses in legal challenges against tobacco companies.
SOURCE: Hammond D et al. BMJ 2019 Jun 19. doi: 10.1136/bmj.l2219.
FROM THE BMJ
Key clinical point: Recent increases in vaping prevalence among teens “might be indicative of nicotine addiction.”
Major finding: Vaping prevalence increased from 11% to 16% in the United States and from 8% to 14.6% in Canada.
Study details: Two waves of a national, cross-sectional survey that included approximately 12,000 respondents each.
Disclosures: The study was funded by the U.S. National Institutes of Health. Dr. Hammond is supported by a Canadian Institutes of Health Research–Public Health Agency of Canada applied public health research chair. The investigators said that they had no other financial disclosures to report, but several have served as paid witnesses in legal challenges against tobacco companies.
Source: Hammond D et al. BMJ. 2019 Jun 19. doi: 10.1136/bmj.l2219.
Nicotine replacement therapy beats varenicline for smokers with OUD
SAN ANTONIO – People who smoke and have opioid use disorder have a lower likelihood of drug use several months after initiating smoking cessation treatment if they are treated with nicotine replacement therapy rather than varenicline, new research suggests.
“Differences were not due to the pretreatment differences in drug use, which were covaried,” wrote Damaris J. Rohsenow, PhD, and colleagues at Brown University’s Center for Alcohol and Addiction Studies, Providence, R.I. “Results suggest it may be preferable to offer smokers with opioid use disorder [nicotine replacement therapy] rather than varenicline, given their lower adherence and more illicit drug use days during follow-up when given varenicline compared to [nicotine replacement therapy].”
They shared their research poster at the annual meeting of the College on Problems of Drug Dependence.
About 80%-90% of patients with OUD smoke, and those patients have a particularly difficult time with smoking cessation partly because of nonadherence to cessation medications, the authors noted. Smoking increases the risk of relapse from any substance use disorder, and pain – frequently comorbid with smoking – contributes to opioid use, they added.
Though smoking treatment has been shown not to increase drug or alcohol use, varenicline and nicotine replacement therapy have different effects on a4b2 nicotinic acetylcholinergic receptors (nAChRs). The authors noted that nicotine offers greater pain inhibition via full agonist effects across multiple nAChRs, whereas varenicline has only a partial agonist effect on a single nAChR.
“Smokers may receive more rewarding dopamine effects from the full nicotine agonist,” they wrote. The researchers therefore aimed to compare responses to nicotine replacement therapy and varenicline among smokers with and without OUD.
Ninety patients without OUD and 47 patients with it were randomly assigned to receive transdermal nicotine replacement therapy with placebo capsules or varenicline capsules with a placebo patch for 12 weeks with 3- and 6-month follow-ups. At baseline, those with OUD were significantly more likely to be white and slightly younger and have twice as many drug use days than those without the disorder.
Differences also existed between those with and without OUD for comorbid alcohol use disorder (55% vs. 81%), marijuana use disorder (32% vs. 19%) and cocaine use disorder (70% vs. 55%).
Those without OUD had slightly greater medication adherence, but with only borderline significance just among those taking varenicline. Loss to follow-up, meanwhile, was significantly greater for those with OUD in both treatment groups.
Those patients had 16.5 drug use days at 4-6 months’ follow-up, compared with 0.13 days among those with OUD using nicotine replacement therapy (P less than .026). Among those without OUD, nicotine replacement therapy patients had 5 drug use days, and varenicline patients had 2.5 drug use days.
“Given interactions between nicotine and the opioid system and given that [nicotine replacement therapy] binds to more types of nAChRs than varenicline does, it is possible that [nicotine replacement therapy] dampens desire to use opiates compared to varenicline by stimulating more nAChRs,” the authors wrote. “Increasing nicotine dose may be better for smokers with opioid use disorder,” they added, though they noted the small size of the study and the need for replication with larger populations.
The research was funded by the National Institute on Drug Abuse and the Department of Veterans Affairs. The authors reported no disclosures.
SAN ANTONIO – People who smoke and have opioid use disorder have a lower likelihood of drug use several months after initiating smoking cessation treatment if they are treated with nicotine replacement therapy rather than varenicline, new research suggests.
“Differences were not due to the pretreatment differences in drug use, which were covaried,” wrote Damaris J. Rohsenow, PhD, and colleagues at Brown University’s Center for Alcohol and Addiction Studies, Providence, R.I. “Results suggest it may be preferable to offer smokers with opioid use disorder [nicotine replacement therapy] rather than varenicline, given their lower adherence and more illicit drug use days during follow-up when given varenicline compared to [nicotine replacement therapy].”
They shared their research poster at the annual meeting of the College on Problems of Drug Dependence.
About 80%-90% of patients with OUD smoke, and those patients have a particularly difficult time with smoking cessation partly because of nonadherence to cessation medications, the authors noted. Smoking increases the risk of relapse from any substance use disorder, and pain – frequently comorbid with smoking – contributes to opioid use, they added.
Though smoking treatment has been shown not to increase drug or alcohol use, varenicline and nicotine replacement therapy have different effects on a4b2 nicotinic acetylcholinergic receptors (nAChRs). The authors noted that nicotine offers greater pain inhibition via full agonist effects across multiple nAChRs, whereas varenicline has only a partial agonist effect on a single nAChR.
“Smokers may receive more rewarding dopamine effects from the full nicotine agonist,” they wrote. The researchers therefore aimed to compare responses to nicotine replacement therapy and varenicline among smokers with and without OUD.
Ninety patients without OUD and 47 patients with it were randomly assigned to receive transdermal nicotine replacement therapy with placebo capsules or varenicline capsules with a placebo patch for 12 weeks with 3- and 6-month follow-ups. At baseline, those with OUD were significantly more likely to be white and slightly younger and have twice as many drug use days than those without the disorder.
Differences also existed between those with and without OUD for comorbid alcohol use disorder (55% vs. 81%), marijuana use disorder (32% vs. 19%) and cocaine use disorder (70% vs. 55%).
Those without OUD had slightly greater medication adherence, but with only borderline significance just among those taking varenicline. Loss to follow-up, meanwhile, was significantly greater for those with OUD in both treatment groups.
Those patients had 16.5 drug use days at 4-6 months’ follow-up, compared with 0.13 days among those with OUD using nicotine replacement therapy (P less than .026). Among those without OUD, nicotine replacement therapy patients had 5 drug use days, and varenicline patients had 2.5 drug use days.
“Given interactions between nicotine and the opioid system and given that [nicotine replacement therapy] binds to more types of nAChRs than varenicline does, it is possible that [nicotine replacement therapy] dampens desire to use opiates compared to varenicline by stimulating more nAChRs,” the authors wrote. “Increasing nicotine dose may be better for smokers with opioid use disorder,” they added, though they noted the small size of the study and the need for replication with larger populations.
The research was funded by the National Institute on Drug Abuse and the Department of Veterans Affairs. The authors reported no disclosures.
SAN ANTONIO – People who smoke and have opioid use disorder have a lower likelihood of drug use several months after initiating smoking cessation treatment if they are treated with nicotine replacement therapy rather than varenicline, new research suggests.
“Differences were not due to the pretreatment differences in drug use, which were covaried,” wrote Damaris J. Rohsenow, PhD, and colleagues at Brown University’s Center for Alcohol and Addiction Studies, Providence, R.I. “Results suggest it may be preferable to offer smokers with opioid use disorder [nicotine replacement therapy] rather than varenicline, given their lower adherence and more illicit drug use days during follow-up when given varenicline compared to [nicotine replacement therapy].”
They shared their research poster at the annual meeting of the College on Problems of Drug Dependence.
About 80%-90% of patients with OUD smoke, and those patients have a particularly difficult time with smoking cessation partly because of nonadherence to cessation medications, the authors noted. Smoking increases the risk of relapse from any substance use disorder, and pain – frequently comorbid with smoking – contributes to opioid use, they added.
Though smoking treatment has been shown not to increase drug or alcohol use, varenicline and nicotine replacement therapy have different effects on a4b2 nicotinic acetylcholinergic receptors (nAChRs). The authors noted that nicotine offers greater pain inhibition via full agonist effects across multiple nAChRs, whereas varenicline has only a partial agonist effect on a single nAChR.
“Smokers may receive more rewarding dopamine effects from the full nicotine agonist,” they wrote. The researchers therefore aimed to compare responses to nicotine replacement therapy and varenicline among smokers with and without OUD.
Ninety patients without OUD and 47 patients with it were randomly assigned to receive transdermal nicotine replacement therapy with placebo capsules or varenicline capsules with a placebo patch for 12 weeks with 3- and 6-month follow-ups. At baseline, those with OUD were significantly more likely to be white and slightly younger and have twice as many drug use days than those without the disorder.
Differences also existed between those with and without OUD for comorbid alcohol use disorder (55% vs. 81%), marijuana use disorder (32% vs. 19%) and cocaine use disorder (70% vs. 55%).
Those without OUD had slightly greater medication adherence, but with only borderline significance just among those taking varenicline. Loss to follow-up, meanwhile, was significantly greater for those with OUD in both treatment groups.
Those patients had 16.5 drug use days at 4-6 months’ follow-up, compared with 0.13 days among those with OUD using nicotine replacement therapy (P less than .026). Among those without OUD, nicotine replacement therapy patients had 5 drug use days, and varenicline patients had 2.5 drug use days.
“Given interactions between nicotine and the opioid system and given that [nicotine replacement therapy] binds to more types of nAChRs than varenicline does, it is possible that [nicotine replacement therapy] dampens desire to use opiates compared to varenicline by stimulating more nAChRs,” the authors wrote. “Increasing nicotine dose may be better for smokers with opioid use disorder,” they added, though they noted the small size of the study and the need for replication with larger populations.
The research was funded by the National Institute on Drug Abuse and the Department of Veterans Affairs. The authors reported no disclosures.
REPORTING FROM CPDD 2019



