User login
First death from severe lung illness associated with vaping reported in Illinois
The first death to occur in a patient with severe lung illness associated with e-cigarette product use has been reported in Illinois, officials announced at a Centers for Disease Control and Prevention telebriefing.
The cause for the mysterious lung illnesses has not been determined, but an infectious disease does not appear to be implicated. As of yesterday, 193 potential cases have been identified in 22 states since June 28.
No specific product has been implicated in all cases, and it is unclear if there is a common cause or if these are several diseases with a similar presentation.
Wisconsin and Illinois have asked the CDC to directly assist them in their investigations of cases. Other states are handling their own investigations. Further information is available from the CDC at cdc.gov/e-cigarettes.
There have been 22 cases of the illness in Illinois and an additional 12 individuals are being evaluated as possible cases, according to Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist, Illinois Department of Public Health.
Illinois is working with the CDC and the Food and Drug Administration to investigate devices that affected patients have used. No specific product has been implicated across all cases; all patients have reported vaping in recent months Several patients in Illinois have reported using tetrahydrocannabinol (THC) product oils, but Dr. Layden reiterated the investigations are reliant on information reported by affected patients only.
Mitch Zeller, JD, director, Center for Tobacco Products at the FDA, said product samples from a number of states are being evaluated to determine their contents. The FDA is examining samples sent and trying to identify product contents.
The cases reported to date have been in adults aged 17-38 years and have occurred primarily men. The investigation is in a relatively early stage and is working with incomplete case reports. These will become standardized to include more specific information, such as the name of the product, where it was purchased, and whether it was used as intended or whether other products were added, he said.
As e-cigarettes are not a new product, it’s possible that cases of this illness has been occurring but that the link was not recognized, and the cases were neither captured nor reported, said Brian King, PhD, MPH, deputy director, Research Translation, Office on Smoking and Health, CDC. He noted that e-cigarettes may contain “a variety of constituents that could be problematic in terms of pulmonary illness,” such as ingredients in certain flavorings and ultrafine particulates.
The agencies are now trying to harmonize reporting across all states so cases can be evaluated in a more standardized way. Information on standardized reporting on a national level will be issued in the next few days, according to the CDC.
The CDC notified U.S. health care systems and clinicians about the illnesses and what to watch for via a Clinician Outreach and Communication Activity Clinical Action Message.
In general, patients have reported a gradual onset of symptoms including shortness of breath or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
The first death to occur in a patient with severe lung illness associated with e-cigarette product use has been reported in Illinois, officials announced at a Centers for Disease Control and Prevention telebriefing.
The cause for the mysterious lung illnesses has not been determined, but an infectious disease does not appear to be implicated. As of yesterday, 193 potential cases have been identified in 22 states since June 28.
No specific product has been implicated in all cases, and it is unclear if there is a common cause or if these are several diseases with a similar presentation.
Wisconsin and Illinois have asked the CDC to directly assist them in their investigations of cases. Other states are handling their own investigations. Further information is available from the CDC at cdc.gov/e-cigarettes.
There have been 22 cases of the illness in Illinois and an additional 12 individuals are being evaluated as possible cases, according to Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist, Illinois Department of Public Health.
Illinois is working with the CDC and the Food and Drug Administration to investigate devices that affected patients have used. No specific product has been implicated across all cases; all patients have reported vaping in recent months Several patients in Illinois have reported using tetrahydrocannabinol (THC) product oils, but Dr. Layden reiterated the investigations are reliant on information reported by affected patients only.
Mitch Zeller, JD, director, Center for Tobacco Products at the FDA, said product samples from a number of states are being evaluated to determine their contents. The FDA is examining samples sent and trying to identify product contents.
The cases reported to date have been in adults aged 17-38 years and have occurred primarily men. The investigation is in a relatively early stage and is working with incomplete case reports. These will become standardized to include more specific information, such as the name of the product, where it was purchased, and whether it was used as intended or whether other products were added, he said.
As e-cigarettes are not a new product, it’s possible that cases of this illness has been occurring but that the link was not recognized, and the cases were neither captured nor reported, said Brian King, PhD, MPH, deputy director, Research Translation, Office on Smoking and Health, CDC. He noted that e-cigarettes may contain “a variety of constituents that could be problematic in terms of pulmonary illness,” such as ingredients in certain flavorings and ultrafine particulates.
The agencies are now trying to harmonize reporting across all states so cases can be evaluated in a more standardized way. Information on standardized reporting on a national level will be issued in the next few days, according to the CDC.
The CDC notified U.S. health care systems and clinicians about the illnesses and what to watch for via a Clinician Outreach and Communication Activity Clinical Action Message.
In general, patients have reported a gradual onset of symptoms including shortness of breath or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
The first death to occur in a patient with severe lung illness associated with e-cigarette product use has been reported in Illinois, officials announced at a Centers for Disease Control and Prevention telebriefing.
The cause for the mysterious lung illnesses has not been determined, but an infectious disease does not appear to be implicated. As of yesterday, 193 potential cases have been identified in 22 states since June 28.
No specific product has been implicated in all cases, and it is unclear if there is a common cause or if these are several diseases with a similar presentation.
Wisconsin and Illinois have asked the CDC to directly assist them in their investigations of cases. Other states are handling their own investigations. Further information is available from the CDC at cdc.gov/e-cigarettes.
There have been 22 cases of the illness in Illinois and an additional 12 individuals are being evaluated as possible cases, according to Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist, Illinois Department of Public Health.
Illinois is working with the CDC and the Food and Drug Administration to investigate devices that affected patients have used. No specific product has been implicated across all cases; all patients have reported vaping in recent months Several patients in Illinois have reported using tetrahydrocannabinol (THC) product oils, but Dr. Layden reiterated the investigations are reliant on information reported by affected patients only.
Mitch Zeller, JD, director, Center for Tobacco Products at the FDA, said product samples from a number of states are being evaluated to determine their contents. The FDA is examining samples sent and trying to identify product contents.
The cases reported to date have been in adults aged 17-38 years and have occurred primarily men. The investigation is in a relatively early stage and is working with incomplete case reports. These will become standardized to include more specific information, such as the name of the product, where it was purchased, and whether it was used as intended or whether other products were added, he said.
As e-cigarettes are not a new product, it’s possible that cases of this illness has been occurring but that the link was not recognized, and the cases were neither captured nor reported, said Brian King, PhD, MPH, deputy director, Research Translation, Office on Smoking and Health, CDC. He noted that e-cigarettes may contain “a variety of constituents that could be problematic in terms of pulmonary illness,” such as ingredients in certain flavorings and ultrafine particulates.
The agencies are now trying to harmonize reporting across all states so cases can be evaluated in a more standardized way. Information on standardized reporting on a national level will be issued in the next few days, according to the CDC.
The CDC notified U.S. health care systems and clinicians about the illnesses and what to watch for via a Clinician Outreach and Communication Activity Clinical Action Message.
In general, patients have reported a gradual onset of symptoms including shortness of breath or chest pain that increased over days or weeks before hospital admission. Gastrointestinal symptoms including vomiting, diarrhea, and fatigue have been reported by some.
Opioid Epidemic
FDA takes another swing at updating cigarette pack warnings
illustrating the harms of smoking, but this could be subjected to legal challenge.
Several years ago, tobacco companies filed a lawsuit, which ultimately shut down a similar proposal.
The warnings focus on lesser-known complications – including diabetes, cataracts, gangrene, stroke, bladder cancer, erectile dysfunction, and obstructive pulmonary disease – and would take up the top half of the front and back of cigarette packs, and at least the top 20% of print advertisements. Each pack and ad would be required to carry 1 of the 13 proposed warnings, according to the announcement.
The approach would be similar to, but not as aggressive as Canada’s. For years, cigarettes packs sold in Canada have included disturbing photographs of diseased lungs, rotted teeth, and dying patients. The lasting impact of such imagery has been demonstrated in the literature (for example, Am J Prev Med. 2007 Mar;32[3]:202-9).
The new proposal is the FDA’s second attempt to enact something comparable in the United States, after being directed to do so by the Tobacco Control Act of 2009.
The first effort to add strong, illustrated warnings to cigarette packs was widely backed by medical groups, but challenged in the courts by R.J. Reynolds and other tobacco companies, and blocked on appeal in 2012 as an abridgment of commercial free speech. The federal government dropped the case in 2013.
The American Lung Association and other public health groups subsequently sued the FDA in 2016 to enact the Tobacco Act mandate. Subsequently, a federal judge ordered the agency to publish a new rule by August 2019, and issue a final rule in March 2020.
This time around, the FDA “took the necessary time to get these new proposed warnings right ... based on – and within the limits of – both science and the law,” the agency said. The new images, though graphic, are less disturbing than those used in Canada and the agency’s previous proposals, which included an apparent corpse with a sternotomy. The 1-800-Quit-Now cessation hotline number, which was a sticking point in the 2012 ruling, has also been dropped.
When asked about the new efforts, R.J. Reynolds spokesperson Kaelan Hollon said, “We are carefully reviewing FDA’s latest proposal for graphic warnings on cigarettes. We firmly support public awareness of the harms of smoking cigarettes, but the manner in which those messages are delivered to the public cannot run afoul of the First Amendment protections that apply to all speakers, including cigarette manufacturers.”
Warnings on U.S. cigarettes haven’t changed since 1984, when the risks of lung cancer, heart disease, emphysema, and pregnancy complications were added to the side of cigarette packs. With time, the FDA said the surgeon general’s warnings have become “virtually invisible” to consumers.
The American Lung Association, American Academy of Pediatrics, and other plaintiffs in the 2016 suit called the new proposal a “dramatic improvement” over the current situation and “long overdue” in a joint statement on Aug. 15.
Although rates have declined substantially in recent decades, about 34.3 million U.S. adults and almost 1.4 million teenagers still smoke. The habit kills about a half million Americans every year, at a health cost of more than $300 billion, the FDA said.
Comments on the proposed rule are being accepted through Oct. 15. The agency is open to suggestions for alternative text and images.
illustrating the harms of smoking, but this could be subjected to legal challenge.
Several years ago, tobacco companies filed a lawsuit, which ultimately shut down a similar proposal.
The warnings focus on lesser-known complications – including diabetes, cataracts, gangrene, stroke, bladder cancer, erectile dysfunction, and obstructive pulmonary disease – and would take up the top half of the front and back of cigarette packs, and at least the top 20% of print advertisements. Each pack and ad would be required to carry 1 of the 13 proposed warnings, according to the announcement.
The approach would be similar to, but not as aggressive as Canada’s. For years, cigarettes packs sold in Canada have included disturbing photographs of diseased lungs, rotted teeth, and dying patients. The lasting impact of such imagery has been demonstrated in the literature (for example, Am J Prev Med. 2007 Mar;32[3]:202-9).
The new proposal is the FDA’s second attempt to enact something comparable in the United States, after being directed to do so by the Tobacco Control Act of 2009.
The first effort to add strong, illustrated warnings to cigarette packs was widely backed by medical groups, but challenged in the courts by R.J. Reynolds and other tobacco companies, and blocked on appeal in 2012 as an abridgment of commercial free speech. The federal government dropped the case in 2013.
The American Lung Association and other public health groups subsequently sued the FDA in 2016 to enact the Tobacco Act mandate. Subsequently, a federal judge ordered the agency to publish a new rule by August 2019, and issue a final rule in March 2020.
This time around, the FDA “took the necessary time to get these new proposed warnings right ... based on – and within the limits of – both science and the law,” the agency said. The new images, though graphic, are less disturbing than those used in Canada and the agency’s previous proposals, which included an apparent corpse with a sternotomy. The 1-800-Quit-Now cessation hotline number, which was a sticking point in the 2012 ruling, has also been dropped.
When asked about the new efforts, R.J. Reynolds spokesperson Kaelan Hollon said, “We are carefully reviewing FDA’s latest proposal for graphic warnings on cigarettes. We firmly support public awareness of the harms of smoking cigarettes, but the manner in which those messages are delivered to the public cannot run afoul of the First Amendment protections that apply to all speakers, including cigarette manufacturers.”
Warnings on U.S. cigarettes haven’t changed since 1984, when the risks of lung cancer, heart disease, emphysema, and pregnancy complications were added to the side of cigarette packs. With time, the FDA said the surgeon general’s warnings have become “virtually invisible” to consumers.
The American Lung Association, American Academy of Pediatrics, and other plaintiffs in the 2016 suit called the new proposal a “dramatic improvement” over the current situation and “long overdue” in a joint statement on Aug. 15.
Although rates have declined substantially in recent decades, about 34.3 million U.S. adults and almost 1.4 million teenagers still smoke. The habit kills about a half million Americans every year, at a health cost of more than $300 billion, the FDA said.
Comments on the proposed rule are being accepted through Oct. 15. The agency is open to suggestions for alternative text and images.
illustrating the harms of smoking, but this could be subjected to legal challenge.
Several years ago, tobacco companies filed a lawsuit, which ultimately shut down a similar proposal.
The warnings focus on lesser-known complications – including diabetes, cataracts, gangrene, stroke, bladder cancer, erectile dysfunction, and obstructive pulmonary disease – and would take up the top half of the front and back of cigarette packs, and at least the top 20% of print advertisements. Each pack and ad would be required to carry 1 of the 13 proposed warnings, according to the announcement.
The approach would be similar to, but not as aggressive as Canada’s. For years, cigarettes packs sold in Canada have included disturbing photographs of diseased lungs, rotted teeth, and dying patients. The lasting impact of such imagery has been demonstrated in the literature (for example, Am J Prev Med. 2007 Mar;32[3]:202-9).
The new proposal is the FDA’s second attempt to enact something comparable in the United States, after being directed to do so by the Tobacco Control Act of 2009.
The first effort to add strong, illustrated warnings to cigarette packs was widely backed by medical groups, but challenged in the courts by R.J. Reynolds and other tobacco companies, and blocked on appeal in 2012 as an abridgment of commercial free speech. The federal government dropped the case in 2013.
The American Lung Association and other public health groups subsequently sued the FDA in 2016 to enact the Tobacco Act mandate. Subsequently, a federal judge ordered the agency to publish a new rule by August 2019, and issue a final rule in March 2020.
This time around, the FDA “took the necessary time to get these new proposed warnings right ... based on – and within the limits of – both science and the law,” the agency said. The new images, though graphic, are less disturbing than those used in Canada and the agency’s previous proposals, which included an apparent corpse with a sternotomy. The 1-800-Quit-Now cessation hotline number, which was a sticking point in the 2012 ruling, has also been dropped.
When asked about the new efforts, R.J. Reynolds spokesperson Kaelan Hollon said, “We are carefully reviewing FDA’s latest proposal for graphic warnings on cigarettes. We firmly support public awareness of the harms of smoking cigarettes, but the manner in which those messages are delivered to the public cannot run afoul of the First Amendment protections that apply to all speakers, including cigarette manufacturers.”
Warnings on U.S. cigarettes haven’t changed since 1984, when the risks of lung cancer, heart disease, emphysema, and pregnancy complications were added to the side of cigarette packs. With time, the FDA said the surgeon general’s warnings have become “virtually invisible” to consumers.
The American Lung Association, American Academy of Pediatrics, and other plaintiffs in the 2016 suit called the new proposal a “dramatic improvement” over the current situation and “long overdue” in a joint statement on Aug. 15.
Although rates have declined substantially in recent decades, about 34.3 million U.S. adults and almost 1.4 million teenagers still smoke. The habit kills about a half million Americans every year, at a health cost of more than $300 billion, the FDA said.
Comments on the proposed rule are being accepted through Oct. 15. The agency is open to suggestions for alternative text and images.
USPSTF draft guidance calls for drug use screening
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
FROM THE USPSTF
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
HCV-infected people who inject drugs also have substantial alcohol use
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
FROM ADDICTIVE BEHAVIORS
Urine drug tests: How to make the most of them
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
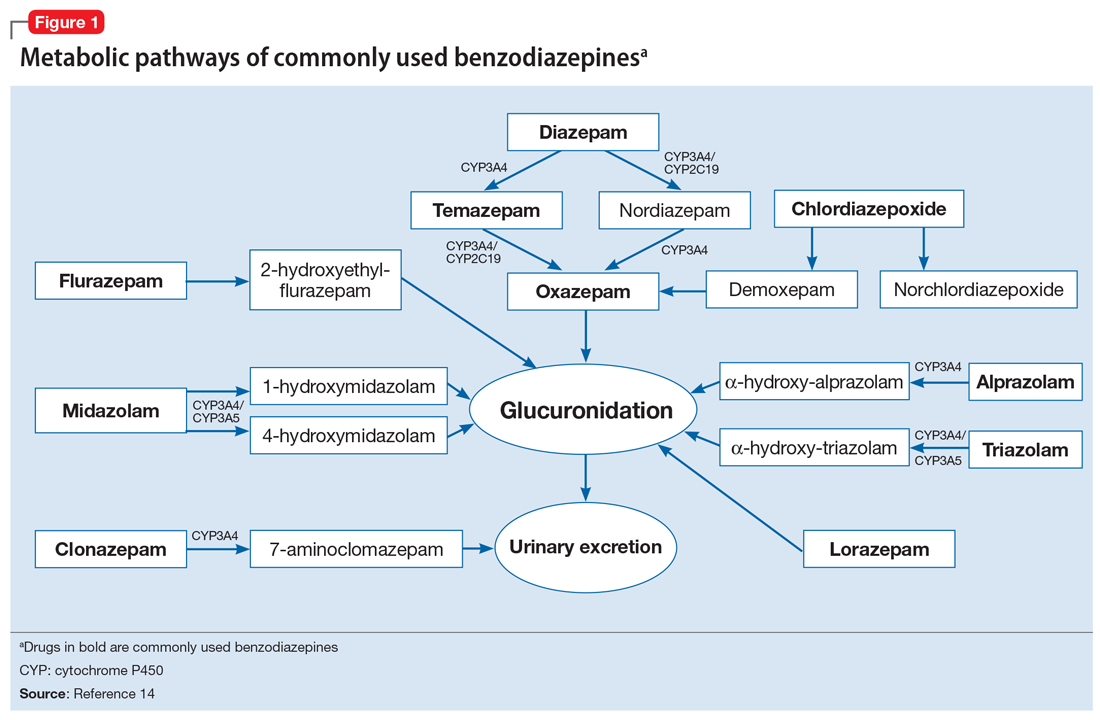
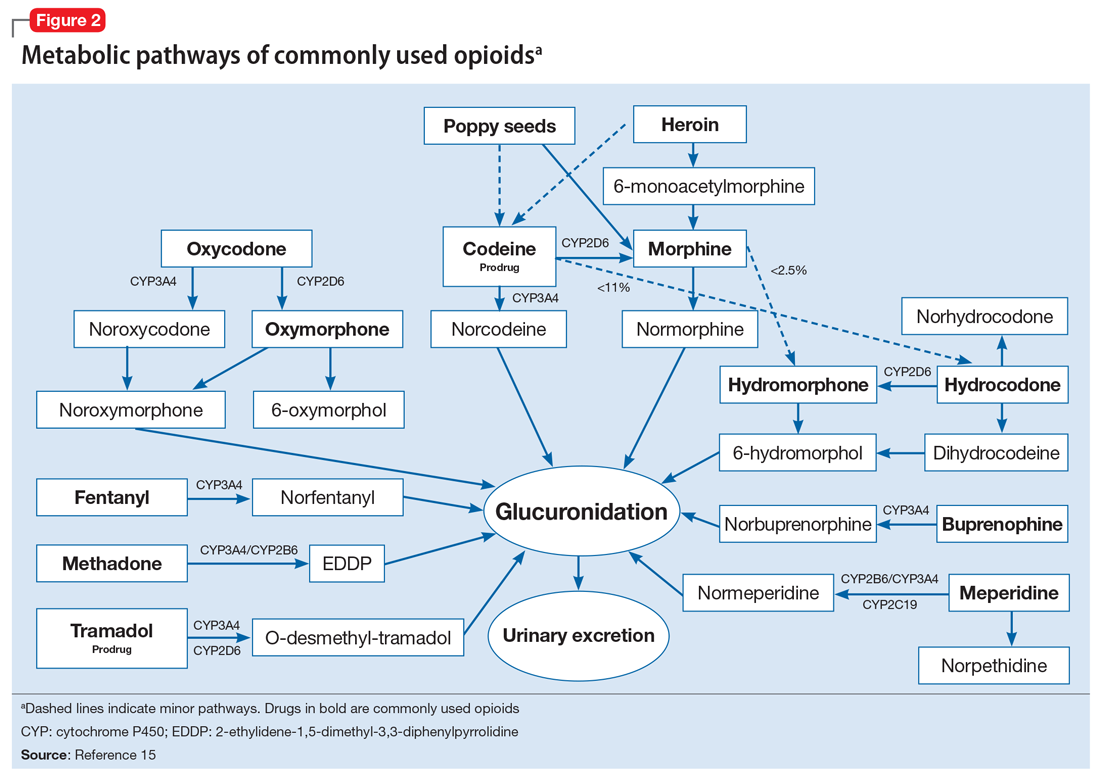
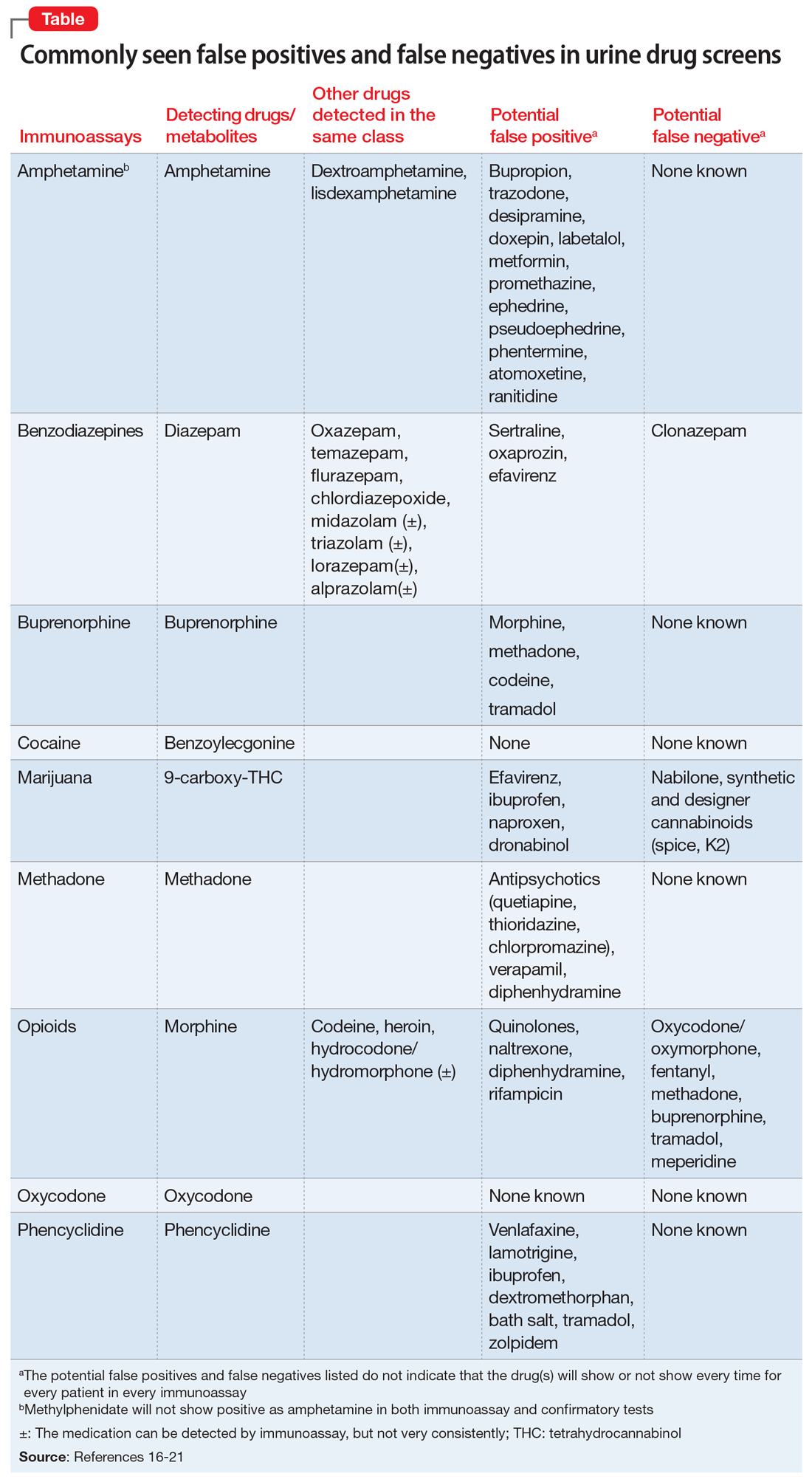
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.
Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.
Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.
Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).
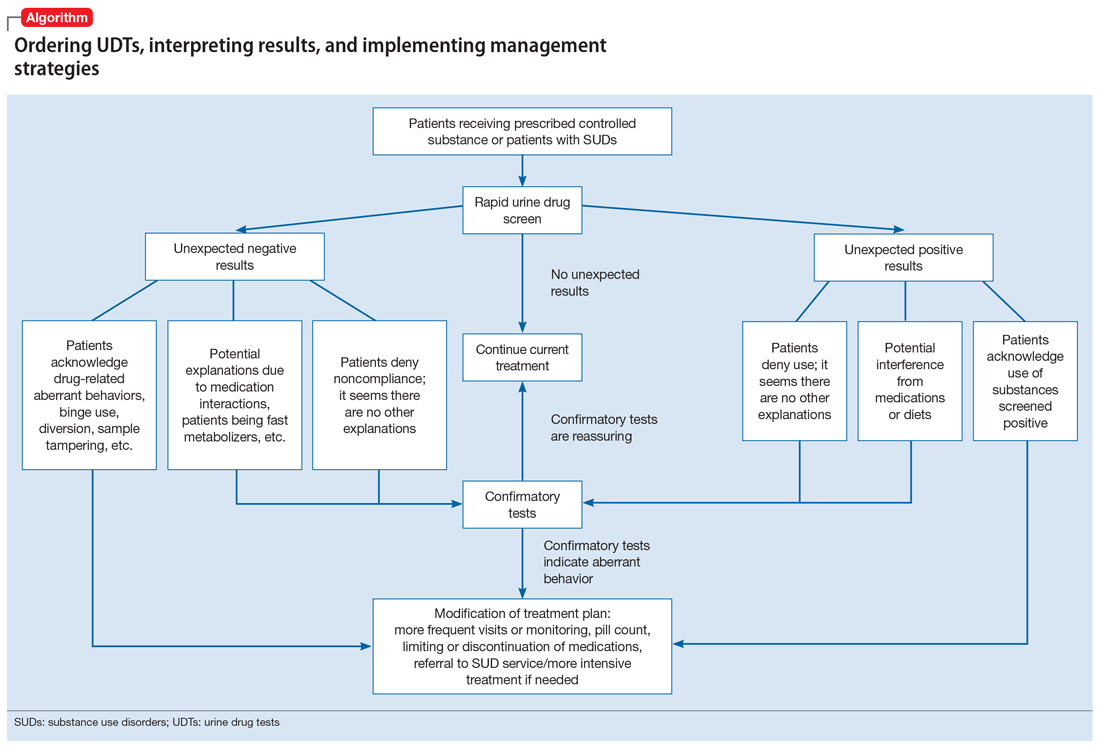
Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.

Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.

Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.

Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).

Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
Urine drug tests (UDTs) are useful clinical tools for assessing and monitoring the risk of misuse, abuse, and diversion when prescribing controlled substances, or for monitoring abstinence in patients with substance use disorders (SUDs). However, UDTs have been underutilized, and have been used without systematic documentation of reasons and results.1,2 In addition, many clinicians may lack the knowledge needed to effectively interpret test results.3,4 Although the reported use of UDTs is much higher among clinicians who are members of American Society of Addiction Medicine (ASAM), there is still a need for improved education.5
The appropriate use of UDTs strengthens the therapeutic relationship and promotes healthy behaviors and patients’ recovery. On the other hand, incorrect interpretation of test results may lead to missing potential aberrant behaviors, or inappropriate consequences for patients, such as discontinuing necessary medications or discharging them from care secondary to a perceived violation of a treatment contract due to unexpected positive or negative drug screening results.6 In this article, we review the basic concepts of UDTs and provide an algorithm to determine when to order these tests, how to interpret the results, and how to modify treatment accordingly.
Urine drug tests 101
Urine drug tests include rapid urine drug screening (UDS) and confirmatory tests. Urine drug screenings are usually based on various types of immunoassays. They are fast, sensitive, and cost-effective. Because immunoassa
Urine drug tests based on mass spectrometry, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography/mass spectrometry (LC/MS) are gold standards to confirm toxicology results. They are highly sensitive and specific, with accurate quantitative measurement. However, they are more expensive than UDS and usually need to be sent to a laboratory with capacity to perform GC/MS or LC/MS, with a turnaround time of up to 1 week.8 In clinical practice, we usually start with UDS tests and order confirmatory tests when needed.
When to order UDTs in outpatient psychiatry
On December 12, 2013, the ASAM released a white paper that suggests the use of drug testing as a primary prevention, diagnostic, and monitoring tool in the management of addiction or drug misuse and its application in a wide variety of medical settings.9 Many clinicians use treatment contracts when prescribing controlled substances as a part of a risk-mitigation strategy, and these contracts often include the use of UDTs. Urine drug tests provide objective evidence to support or negate self-report, because many people may underreport their use.10 The literature has shown significant “abnormal” urine test results, ranging from 9% to 53%, in patients receiving chronic opioid therapy.2,11
The CDC and the American Academy of Pain Medicine recommend UDS before initiating any controlled substance for pain therapy.12,13 They also suggest random drug testing at least once or twice a year for low-risk patients, and more frequent screening for high-risk patients, such as those with a history of addiction.12,13 For example, for patients with opioid use disorder who participate in a methadone program, weekly UDTs are mandated for the first 90 days, and at least 8 UDTs a year are required after that.
However, UDTs carry significant stigma due to their association with SUDs. Talking with patients from the start of treatment helps to reduce this stigma, and makes it easier to have further discussions when patients have unexpected results during treatment. For example, clinicians can explain to patients that monitoring UDTs when prescribing controlled substances is similar to monitoring thyroid function with lithium use because treatment with a controlled substance carries an inherent risk of misuse, abuse, and diversion. For patients with SUDs, clinicians can explain that using UDTs to monitor their abstinence is similar to monitoring HbA1c for glucose control in patients with diabetes.
Continue to: Factors that can affect UDT results
Factors that can affect UDT results
In addition to knowing when to order UDT, it is critical to know how to interpret the results of UDS and follow up with confirmatory tests when needed. Other than the limitations of the tests, the following factors could contribute to unexpected UDT results:
- the drug itself, including its half-life, metabolic pathways, and potential interactions with other medications
- how patients take their medications, including dose, frequency, and pattern of drug use
- all the medications that patients are taking, including prescription, over-the-counter, and herbal and supplemental preparations
- when the last dose of a prescribed controlled substance was taken. Always ask when the patient’s last dose was taken before you consider ordering a UDT.
To help better understand UDT results, Figure 114 and Figure 215 demonstrate metabolic pathways of commonly used benzodiazepines and opioids, respectively. There are several comprehensive reviews on commonly seen false positives and negatives for each drug or each class of drugs in immunoassays.16-21 Confirmatory tests are usually very accurate. However, chiral analysis is needed to differentiate enantiomers, such as methamphetamine (active R-enantiomer) and selegiline, which is metabolized into L-methamphetamine (inactive S-enantiomer).22 In addition, detection of tetrahydrocannabivarin (THCV), an ingredient of the cannabis plant, via GC/MS can be used to distinguish between consumption of dronabinol and natural cannabis products.23 The Table16-21 summarizes the prototype agents, other detectable agents in the same class, and false positives and negatives in immunoassays.

Interpreting UDT results and management strategies
Our Algorithm outlines how to interpret UDT results, and management strategies to consider based on whether the results are as expected or unexpected, with a few key caveats as described below.

Expected results
If there are no concerns based on the patient’s clinical presentation or collateral information, simply continue the current treatment. However, for patients taking medications that are undetectable by UDS (for example, regular use of clonazepam or oxycodone), consider ordering confirmatory tests at least once to ensure compliance, even when UDS results are negative.

Unexpected positive results, including the presence of illicit drugs and/or unprescribed licit drugs
Drug misuse, abuse, or dependence. The first step is to talk with the patient, who may acknowledge drug misuse, abuse, or dependence. Next, consider modifying the treatment plan; this may include more frequent monitoring and visits, limiting or discontinuing prescribed controlled substances, or referring the patient to inpatient or outpatient SUD treatment, as appropriate.
Continue to: Interference from medications or diet
Interference from medications or diets. One example of a positive opioid screening result due to interference from diet is the consumption of foods that contain poppy seeds. Because of this potential interference, the cutoff value for a positive opioid immunoassay in workplace drug testing was increased from 300 to 2,000 ug/L.24 Educating patients regarding medication and lifestyle choices can help them avoid any interference with drug monitoring. Confirmatory tests can be ordered at the clinician’s discretion. The same principle applies to medication choice when prescribing. For example, a patient taking bupropion may experience a false positive result on a UDS for amphetamines, and a different antidepressant might be a better choice (Box 1).
Box 1
A patient with methamphetamine use disorder asked his psychiatrist for a letter to his probation officer because his recent urine drug screening (UDS) was positive for amphetamine. At a previous visit, the patient had been started on bupropion for depression and methamphetamine use disorder. After his most recent positive UDS, the patient stopped taking bupropion because he was aware that bupropion could cause a false-positive result on amphetamine screening. However, the psychiatrist could not confirm the results of the UDS, because he did not have the original sample for confirmatory testing. In this case, starting the patient on bupropion may not have been the best option without contacting the patient’s probation officer to discuss a good strategy for distinguishing true vs false-positive UDS results.
Urine sample tampering. Consider the possibility that urine samples could be substituted, especially when there are signs or indications of tampering, such as a positive pregnancy test for a male patient, or the presence of multiple prescription medications not prescribed to the patient. If there is high suspicion of urine sample tampering, consider observed urine sample collection.
When to order confirmatory tests for unexpected positive results.
Order a confirmatory test if a patient adamantly denies taking the substance(s) for which he/she has screened positive, and there’s no other explanation for the positive result. Continue the patient’s current treatment if the confirmatory test is negative. However, if the confirmatory test is positive, then modify the treatment plan (Algorithm).

Special circumstances.
A positive opioid screen in a patient who has been prescribed a synthetic or semisynthetic opioid indicates the patient is likely using opioids other than the one he/she has been prescribed. Similarly, clonazepam is expected to be negative in a benzodiazepine immunoassay. If such testing is positive, consider the possibility that the patient is taking other benzodiazepines, such as diazepam. The results of UDTs can also be complicated by common metabolites in the same class of drugs. For example, the presence of hydromorphone for patients taking hydrocodone does not necessarily indicate the use of hydromorphone, because hydromorphone is a metabolite of hydrocodone (Figure 215).
Unexpected negative results
Prescribed medications exist in low concentration that are below the UDS detection threshold. This unexpected UDS result could occur if patients:
- take their medications less often than prescribed (because of financial difficulties or the patient feels better and does not think he/she needs it, etc.)
- hydrate too much (intentionally or unintentionally), are pregnant, or are fast metabolizers (Box 2)
- take other medications that increase the metabolism of the prescribed medication.
Box 2
A patient with opioid use disorder kept requesting a higher dose of methadone due to poorly controlled cravings. Even after he was observed taking methadone by the clinic staff, he was negative for methadone in immunoassay screening, and had a very low level of methadone based on liquid chromatography/mass spectrometry. Pharmacogenetic testing revealed that the patient was a cytochrome P450 2B6 ultra-rapid metabolizer; 2B6 is a primary metabolic enzyme for methadone. He also had a high concentration of 2-ethylidene- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), the primary metabolite of methadone, which was consistent with increased methadone metabolism.
Continue to: Further inquiry will...
Further inquiry will clarify these concerns. Clinicians should educate patients and manage accordingly. Confirmatory tests may be ordered upon clinicians’ discretion.
Urine sample tampering. Dilution or substitution of urine samples may lead to unexpected negative results. Usually, the urine sample will have abnormal parameters, including temperature, pH, specific gravity, urine creatinine level, or detection of adulterants. If needed, consider observed urine sample collection. Jaffee et al25 reviewed tampering methods in urine drug testing.
Diversion or binge use of medications. If patients adamantly deny diverting or binge using their medication, order confirmatory tests. If the confirmatory test also is negative, modify the treatment plan accordingly, and consider the following options:
- adjust the medication dosage or frequency
- discontinue the medication
- conduct pill counts for more definitive evidence of diversion or misuse, especially if discontinuation may lead to potential harm (for example, for patients prescribed buprenorphine for opioid use disorder).
When to order confirmatory tests for unexpected negative results.
Because confirmatory tests also measure drug concentrations, clinicians sometimes order serial confirmatory testing to monitor lipophilic drugs after a patient reports discontinuation, such as in the case of a patient using marijuana, ketamine, or alprazolam. The level of a lipophilic drug, such as these 3, should continue to decline if the patient has discontinued using it. However, because the drug level is affected by how concentrated the urine samples are, it is necessary to compare the ratios of drug levels over urine creatinine levels.26 Another use for confirmatory-quantitative testing is to detect “urine spiking,”27,28 when a patient adds an unconsumed drug to his/her urine sample to produce a positive result without actually taking the drug (Box 3).
Box 3
On a confirmatory urine drug test, a patient taking buprenorphine/naloxone had a very high level of buprenorphine, but almost no norbuprenorphine (a metabolite of buprenorphine). After further discussion with the clinician, the patient admitted that he had dipped his buprenorphine/naltrexone pill in his urine sample (“spiking”) to disguise the fact that he stopped taking buprenorphine/naloxone several days ago in an effort to get high from taking opioids.
When to consult lab specialists
Because many clinicians may find it challenging to stay abreast of all of the factors necessary to properly interpret UDT results, consulting with qualified laboratory professionals is appropriate when needed. For example, a patient was prescribed codeine, and his UDTs showed morphine as anticipated; however, the prescribing clinician suspected that the patient was also using heroin. In this case, consultation with a specialist may be warranted to look for 6-mono-acetylemorphine (6-MAM, a unique heroin metabolite) and/or the ratio of morphine to codeine.
Continue to: In summary...
In summary, UDTs are important tools to use in general psychiatry practice, especially when prescribing controlled substances. To use UDTs effectively, it is essential to possess knowledge of drug metabolism and the limitations of these tests. All immunoassay results should be considered as presumptive, and confirmatory tests are often needed for making treatment decisions. Many clinicians are unlikely to possess all the knowledge needed to correctly interpret UDTs, and in some cases, communication with qualified laboratory professionals may be necessary. In addition, the patient’s history and clinical presentation, collateral information, and data from prescription drug monitoring programs are all important factors to consider.
The cost of UDTs, variable insurance coverage, and a lack of on-site laboratory services can be deterrents to implementing UDTs as recommended. These factors vary significantly across regions, facilities, and insurance providers (see Related Resources). If faced with these issues and you expect to often need UDTs in your practice, consider using point-of-care UDTs as an alternative to improve access, convenience, and possibly cost.
Bottom Line
Urine drug tests (UDTs) should be standard clinical practice when prescribing controlled substances and treating patients with substance use disorders in the outpatient setting. Clinicians need to be knowledgeable about the limitations of UDTs, drug metabolism, and relevant patient history to interpret UDTs proficiently for optimal patient care. Consult laboratory specialists when needed to help interpret the results.
Related Resources
- Islam FA, Choudhry Z. Urine drug screens: Not just for job applicants. Current Psychiatry. 2018;17(12):43-44.
- HealthCare.gov. Health benefits & coverage: Mental health & substance abuse coverage. www.healthcare.gov/coverage/mental-health-substance-abuse-coverage/.
Drug Brand Names
Alprazolam • Xanax
Amphetamine • Adderall
Atomoxetine • Strattera
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone, Zubsolv
Bupropion • Wellbutrin, Zyban
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clonazepam • Klonopin
Desipramine • Norpramin
Dextroamphetamine • Dexedrine, ProCentra
Diazepam • Valium
Doxepin • Silenor
Dronabinol • Marinol
Efavirenz • Sustiva
Ephedrine • Akovaz
Fentanyl • Actiq, Duragesic
Flurazepam • Dalmane
Hydrocodone • Hysingla, Zohydro ER
Hydromorphone • Dilaudid, Exalgo
Labetalol • Normodyne, Trandate
Lamotrigine • Lamictal
Lisdexamfetamine • Vyvanse
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Meperidine • Demerol
Metformin • Fortamet, Glucophage
Methadone • Dolophine, Methadose
Methylphenidate • Ritalin
Midazolam • Versed
Morphine • Kadian, MorphaBond
Nabilone • Cesamet
Naltrexone • Vivitrol
Oxaprozin • Daypro
Oxazepam • Serax
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Adipex-P, Ionamin
Promethazine • Phenergan
Quetiapine • Seroquel
Ranitidine • Zantac
Rifampicin • Rifadin
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Temazepam • Restoril
Thioridazine • Mellaril
Tramadol • Conzip, Ultram
Trazodone • Desyrel
Triazolam • Halcion
Venlafaxine • Effexor
Verapamil • Calan, Verelan
Zolpidem • Ambien
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
1. Passik SD, Schreiber J, Kirsh KL, et al. A chart review of the ordering and documentation of urine toxicology screens in a cancer center: do they influence patient management? J Pain Symptom Manag. 2000;19(1):40-44.
2. Arthur JA, Edwards T, Lu Z, et al. Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer. 2016;122(23):3732-3739.
3. Suzuki JM, Garayalde SM, Dodoo MM, et al. Psychiatry residents’ and fellows’ confidence and knowledge in interpreting urine drug testing results related to opioids. Subst Abus. 2018;39(4):518-521.
4. Reisfield GM, Bertholf R, Barkin RL, et al. Urine drug test interpretation: what do physicians know? J Opioid Manag. 2007;3(2):80-86.
5. Kirsh KL, Baxter LE, Rzetelny A, et al. A survey of ASAM members’ knowledge, attitudes, and practices in urine drug testing. J Addict Med. 2015;9(5):399-404.
6. Morasco BJ, Krebs EE, Adams MH, et al. Clinician response to aberrant urine drug test results of patients prescribed opioid therapy for chronic pain. Clin J Pain. 2019;35(1):1-6.
7. Liu RH. Comparison of common immunoassay kits for effective application in workplace drug urinalysis. Forensic Sci Rev. 1994;6(1):19-57.
8. Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62(1):92-98.
9. American Society of Addiction Medicine. Resources: ASAM releases white paper on drug testing. https://www.asam.org/resources/publications/magazine/read/article/2013/12/16/asam-releases-white-paper-on-drug-testing. Published December 16, 2019. Accessed June 25, 2019.
10. Fishbain DA, Cutler RB, Rosomoff HL, et al. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15(3):184-191.
11. Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain. 2007;23(2):173-179.
12. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645.
13. Chou R. 2009 clinical guidelines from the American Pain Society and the American Academy of Pain medicine on the use of chronic opioid therapy in chronic noncancer pain: what are the key messages for clinical practice? Pol Arch Med Wewn. 2009;119(7-8):469-477.
14. Mihic SJ, Harris RA. Hypnotics and sedatives. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York, NY: McGrawHill Medical; 2017:343-344.
15. DePriest AZ, Puet BL, Holt AC, et al. Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev. 2015;27(2):115-145.
16. Tenore PL. Advanced urine toxicology testing. J Addict Dis. 2010;29(4):436-448.
17. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
18. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
19. Moeller KE, Kissack JC, Atayee RS, et al. Clinical interpretation of urine drug tests: what clinicians need to know about urine drug screens. Mayo Clin Proc. 2017;92(5):774-796.
20. Nelson ZJ, Stellpflug SJ, Engebretsen KM. What can a urine drug screening immunoassay really tell us? J Pharm Pract. 2016;29(5):516-526.
21. Reisfield GM, Goldberger BA, Bertholf RL. ‘False-positive’ and ‘false-negative’ test results in clinical urine drug testing. Bioanalysis. 2009;1(5):937-952.
22. Poklis A, Moore KA. Response of EMIT amphetamine immunoassays to urinary desoxyephedrine following Vicks inhaler use. Ther Drug Monit. 1995;17(1):89-94.
23. ElSohly MA, Feng S, Murphy TP, et al. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J Anal Toxicol. 2001;25(6):476-480.
24. Selavka CM. Poppy seed ingestion as a contributing factor to opiate-positive urinalysis results: the pacific perspective. J Forensic Sci. 1991;36(3):685-696.
25. Jaffee WB, Trucco E, Levy S, et al. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
26. Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9-tetrahydrocannabinol: a delta9-thccooh to creatinine ratio study. J Anal Toxicol. 1999;23(6):531-534.
27. Holt SR, Donroe JH, Cavallo DA, et al. Addressing discordant quantitative urine buprenorphine and norbuprenorphine levels: case examples in opioid use disorder. Drug Alcohol Depend. 2018;186:171-174.
28. Accurso AJ, Lee JD, McNeely J. High prevalence of urine tampering in an office-based opioid treatment practice detected by evaluating the norbuprenorphine to buprenorphine ratio. J Subst Abuse Treat. 2017;83:62-67.
The benefits of a standardized approach to opioid prescribing
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.
We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.
There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).
DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.
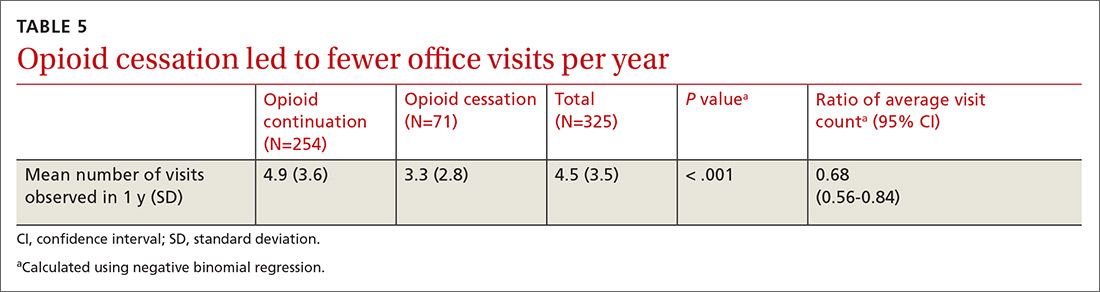
We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.
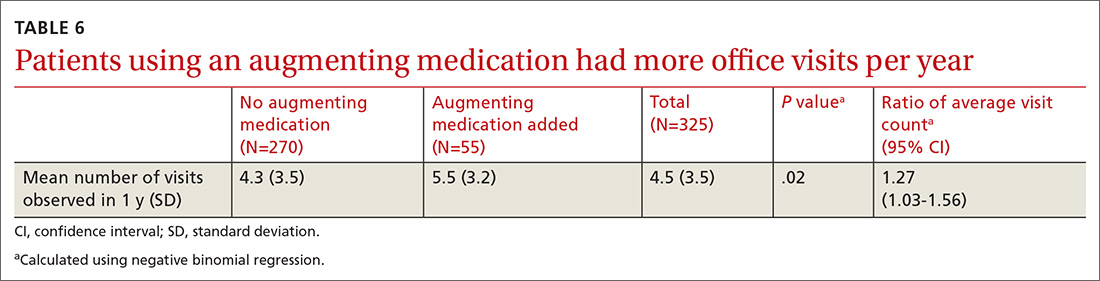
There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.

We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.

There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).

DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.

We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.

There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
ABSTRACT
Purpose The “opioid epidemic” in the United States has received increasing attention over the past few years. Most drug overdose deaths involve an opioid, and prescription opioid deaths have quadrupled since 1999. We sought to improve patient safety and adhere to clinical guidelines by standardizing opioid prescribing in our practice.
Methods We implemented a standardized approach to opioid prescribing based on Arizona Department of Health Services guidelines. All of our providers received instruction on Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP) database and were encouraged to use it online. Our goal was for patients to have quarterly office visits, complete random urine drug screens, and sign a controlled substance agreement (CSA). The CSA acknowledged their understanding of the risks and benefits of opioid therapy as well as our updated prescribing policies.
Results Three-hundred fifty-eight of our practice’s patients were receiving chronic opioid therapy. All providers enrolled in AZCSPMP and used it for patient care. We increased rates of signed CSAs from 4.5% to 43.6%, and urine drug screening from 0.8% to 20.1%. For 325 patients remaining in the practice after our interventions, a postintervention chart review demonstrated a statistically significant discontinuation of opioid therapy (71/325, 21.8%; 95% confidence interval, 17.4%-26.7%).
Conclusion Implementation of a standardized opioid prescribing process resulted in discontinuation of therapy for some patients. Rates increased for signed CSAs and completed random urine drug screening. Future process interventions may improve patient and provider adherence. All primary care physicians should examine their prescribing processes to enhance the safety of opioid therapy.
[polldaddy:10370177]
The US opioid epidemic has received increased attention both nationally and at the state level over the past 2 years. This attention is warranted given the significant societal burden of opioid misuse, abuse, and overdose. Most drug overdose deaths (> 6/10) involve an opioid.1 Deaths from prescription opioids have quadrupled since 1999 in the United States.2 Arizona, the state in which we practice, ranked sixth highest in the nation for drug overdose deaths and had the fifth highest opioid prescribing rate in 2011.3 In response to the growing epidemic, the Centers for Disease Control and Prevention (CDC) released guidelines in 2016 for prescribing and monitoring opioids for chronic pain.4
Chronic nonterminal pain (CNTP) remains a significant cause of human suffering and is more prevalent in the United States than cancer, diabetes, and heart disease combined.5 The increased use of opioids since 1999 to ease CNTP has not reduced Americans’ reports of pain overall.6,7 Given the growing opioid epidemic and disease burden of CNTP, we embarked on a quality improvement (QI) project to safely prescribe and refill opioid medications in the Department of Family Medicine at the Mayo Clinic Arizona.
METHODS
This project received an exemption from internal review board evaluation as a QI intervention. We used a team-based approach to address standardization of opioid prescribing and monitoring within our practice. The team included physicians (MD/DO), nurses (LPN/RN), and allied health staff (MA), operations and administrative personnel, and information technology (IT) support. We did not involve patients in the initial design of our project. With future quality efforts in this area, we plan to involve patients in design processes.
Continue to: We began by identifying...
We began by identifying the scope of the problem, establishing criteria to search the electronic medical record (EMR) and identify appropriate patients. Chronic pain is often defined as pain lasting more than 3 months. Chronic opioid therapy (COT) has been defined as opioid use lasting longer than 3 months.8 Working with our IT colleagues, we defined COT patients as those with 3 or more prescriptions for opioids in the past year or those who received ≥ 30 pills a month (ie, patients who received 180 pills with 2 prescriptions written for the year). This definition gave us the ability to query our EMR to determine which patients were on COT, and we prepared lists of patients by primary care provider (FIGURE). Providers reviewed the lists to ensure these individuals were in fact on COT for CNTP. The number of patients identified after EMR query and provider review was 358, comprising 2.6% of 14,000 empaneled patients.

We based our interventions on the Arizona Department of Health Services 2014 opioid prescribing guidelines.3 The Arizona guidelines used existing national and state opioid prescribing guidelines along with clinical practice guidelines. Our study began prior to the 2016 CDC guidelines, so they were not used in this study. Our practice guidelines recommended that all 23 of our providers (MDs, DOs, and NPs) sign up for Arizona’s Controlled Substance Prescription Monitoring Program (AZCSPMP). We asked each patient to sign a controlled substance agreement (CSA), acknowledging their awareness of our proposed processes and the discussion of opioid therapy. Patients were expected to have face-to-face visits with providers at least quarterly and to complete a random urine drug screen at least annually. Patients were not incentivized to complete the process. We placed reminder calls for appointments just as we do for regular appointments.
Providers were asked to complete the Opioid Risk Tool9 with the patient at the initial visit, discuss the risks, benefits, and alternatives of long-term use of opioid medication, and review the 6 As (analgesia, activity, aberrant drug related behavior, adverse effects, affect, and adjunctive treatments). On the day before each patient visit, providers were reminded by a note in the EMR schedule to check AZCSPMP. Initial appointment times would be 30 minutes and follow-up appointments would be scheduled for 15 minutes if only addressing COT.
The QI project was introduced at an all-staff meeting in October 2015 that included providers, allied health staff, front desk personnel, and administrative staff, with the goal of beginning our COT process in November. We mailed letters to COT patients describing our new guidelines and asking them to call to schedule an appointment. If patients on COT came into the office for an alternate appointment and had not yet been seen for a COT visit, providers were encouraged to complete the COT process at that time.
We created a standard order set in the EMR for initial and follow-up visits and for the urine drug screen. We also added an interactive form to the EMR allowing providers to electronically complete the Opioid Risk Tool, and to confirm CSA completion and AZCSPMP review. We developed a database that would query the EMR for patient office visit frequency, CSA completion, and urine drug screen collection. We also placed paper copies of forms in exam rooms with a laminated instruction sheet reviewing the process steps and the 6 As.
Continue to: Soft rollout was...
Soft rollout was November 1, 2015, to assist in working through the process before full rollout. We asked providers to complete the full process on at least 1 patient during this period. This run-through would help ensure that allied health staff who room the patients would have the CSA and Opioid Risk Tool already in the chart before the visit. Full rollout was January 2, 2016. Every 2 to 4 weeks after the full rollout, regular email reminders were sent to providers about the project process and allowed for any feedback about issues that arose.
We provided regular updates and discussed the process at department meetings monthly. Quarterly data were reviewed and discussed for the first year of implementation. Providers and staff completed a chart review for each COT patient at project completion, to determine whether opioids had been decreased (in dosage) or discontinued, a nonopioid medicine had been initiated to augment pain control, or whether patients had died or left the practice.
Statistical analysis
We summarized binary data as counts and proportions and compared them using the chi square test. We summarized discrete data by their mean and standard deviation. To analyze binary variables measured repeatedly in time, we used the logistic generalized estimating equation (GEE) with an autoregressive (AR-1) correlation structure. We computed 95% confidence intervals (CIs)for odds ratios using the empirical or “sandwich” standard error estimates. For discrete variables representing counts, we used the negative binomial regression model.
For count data, a Poisson model is typically used; in our case the variance was considerably larger than the mean, exceeding the Poisson-model requirement that they not be significantly different if not exactly the same. This implies that the data are “over dispersed” or more variable than a Poisson model is thought to be able to model accurately. We therefore used a negative binomial model, which is regarded as the better model in this situation. The 95% CIs for the estimate resulting from the negative binomial regression model were computed using the profile-likelihood.10 All GEEs were clustered on patients (n = 358). We used SAS version 9.3 (Cary, NC) for all analyses.

RESULTS
All providers enrolled for AZCSPMP. CSA completion increased from 16 (4.5%) at baseline to 156 (43.6%) after intervention (P < .001). Patients completed a urine drug screen more frequently as well, from 3 (0.8%) to 72 (20.1%) (P < .001) (TABLES 1 and 2). No statistically significant change was noted in the frequency of office visits.

Continue to: We excluded 33 patients...
We excluded 33 patients from the post-intervention chart review (TABLE 3). Twenty-seven had left the practice and 6 had died, leaving 325 patients included in the post-intervention chart review.

There was a statistically significant association between patients who discontinued opioids and those who neglected to sign a CSA (P < .001) (TABLE 4). We tested for associations between office visit frequency and process step completion. There was a nonsignificant trend between increased frequency of office visits and opioid dose reduction. Patients who stopped opioids had fewer office visits (TABLE 5), while patients who had initiated a medication to augment pain relief had more frequent office visits (TABLE 6).

DISCUSSION
Our interventions to improve the quality of our COT processes were moderately successful. We achieved statistically significant increases in our rates of CSA completion and in urine drug screening. However, these increases were not as clinically impactful as we had hoped. Improvements in both patient and provider adherence are needed. We plan to engage allied health staff more fully to assist with adherence and thereby improve quality. This study was not intended to obtain patient-oriented outcomes, such as decreased pain and improved function. The study was designed to improve patient safety and to standardize a process for prescribing and monitoring patients on COT. In the future we plan to look at patient outcomes and expand our focus to patients on high-dose opioids and those on combination therapy with benzodiazepines.

We believe the most impactful process steps were our letters sent to COT patients describing our updated, standardized prescribing process, and the ensuing provider-patient discussion to review the risks, benefits, and alternatives to opioid therapy. This frank discussion of treatment options resulted in more than 1 in 5 patients electing to discontinue COT.

There was an association between opioid discontinuation and patients not signing the CSA. This may have been due to patients deciding to discontinue opioids at the initiation review with providers after they received their letter. Therefore, signing the agreement was no longer necessary.
Continue to: We noted that some patients...
We noted that some patients elected to begin a new, nonopioid medication intended to augment their pain relief. However, they did not decrease their use of opioid medicines. We did not collect pain rating scale scores to determine whether the addition of augmenting medicines provided a reduction in pain perception.
Close monitoring of COT patients with frequent office visits may have had an impact on their care. We noted an association between more frequent visits and initiation of pain augmentation medicines. There was also a nonsignificant trend between office visit frequency and dose reduction. These are topics we may re-examine in our practice over time. There was no change in office visit frequency with our intervention, likely a result of these patients having frequent office visits for multiple comorbid medical conditions at baseline.
Evidence of similar benefits in primary care practices that standardized their opioid prescribing guidelines for patients on COT11 illustrates the importance of such a process for ensuring patient safety and decreasing opioid dosage and use.
Limitations to our project are that we did not measure functional changes and quality-of-life scores for patients. We also did not note the opioid dosages for individuals who chose to stop using opioids.
Looking forward. Based on our experience, patient notification with discussion of COT risks, benefits, and alternatives, as well as implementation of a process to monitor COT, appear to be related to patients’ decisions to discontinue COT. Our new standard process did show QI in the process steps but remained suboptimal to our expectations of clinical impact. More frequent office visits may impact patient decisions to reduce opioid dose and to add an augmenting pain medication. We plan to increase the involvement and responsibilities of our allied health staff in our processes to improve rates of adherence and the overall quality of how we manage patients on chronic opioid therapy.
CORRESPONDENCE
David Patchett, DO, Mayo Clinic, 13400 East Shea Blvd, Scottsdale, AZ 85259; [email protected]
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths — United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445-1452.
2. CDC. Opioid data analysis and resources. https://www.cdc.gov/drugoverdose/data/analysis.html. Published December 19, 2018. Accessed May 27, 2019.
3. Arizona Department of Health Services. Arizona opioid prescribing guidelines. https://www.azdhs.gov/documents/audiences/clinicians/clinical-guidelines-recommendations/prescribing-guidelines/az-opiod-prescribing-guidelines.pdf. Published November 2014. Accessed May 27, 2019.
4. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
5. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
6. Chang H, Daubresse M, Kruszewski S, et al. Prevalence and treatment of pain in EDs in the United States, 2000 to 2010. Am J Emerg Med. 2014;32:421-431.
7. Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000 - 2010. Med Care. 2013;51:870-878.
8. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162:276-286.
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
10. Hilbe JM. Negative Binomial Regression. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Liebschutz JM, Xuan Z, Shanahan CW, et al. Improving adherence to long-term opioid therapy guidelines to reduce opioid misuse in primary care: a cluster-randomized clinical trial. JAMA Intern Med. 2017;177:1265-1272.
Doing our part to dismantle the opioid crisis
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
Is it time to taper that opioid? (And how best to do it)
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
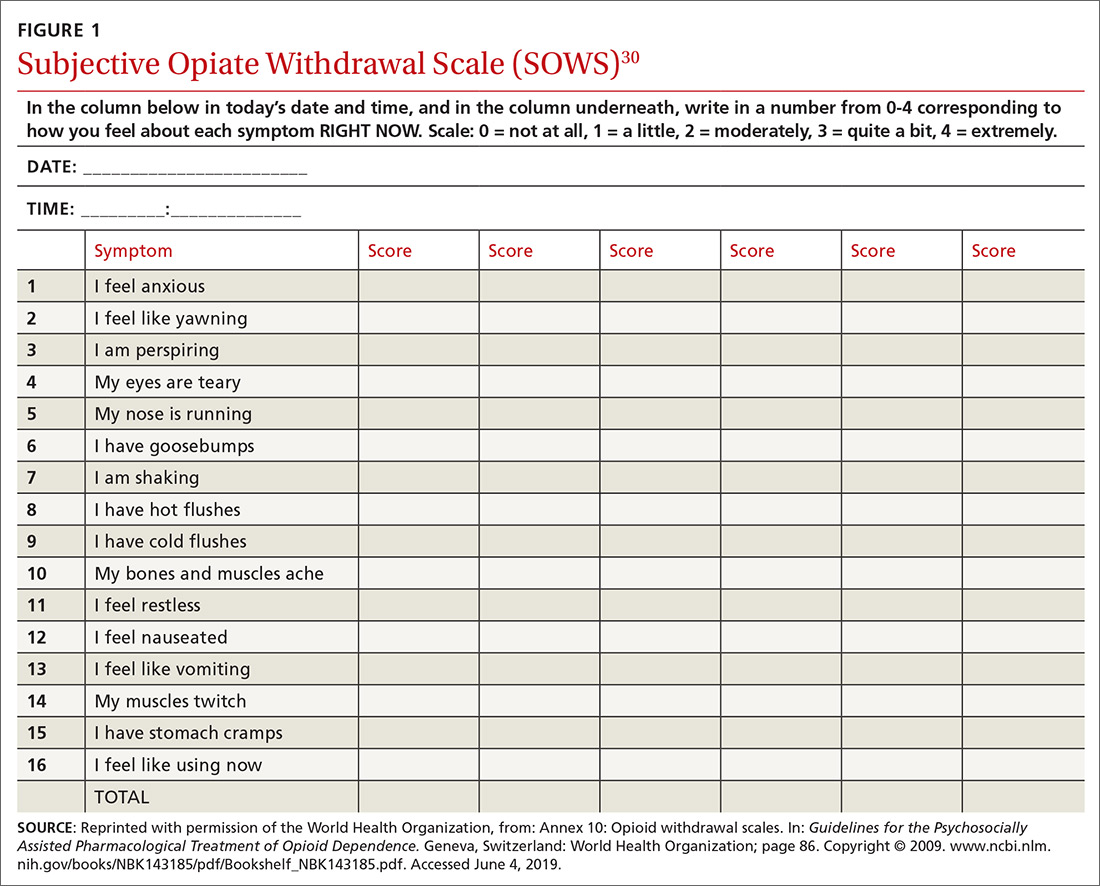
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
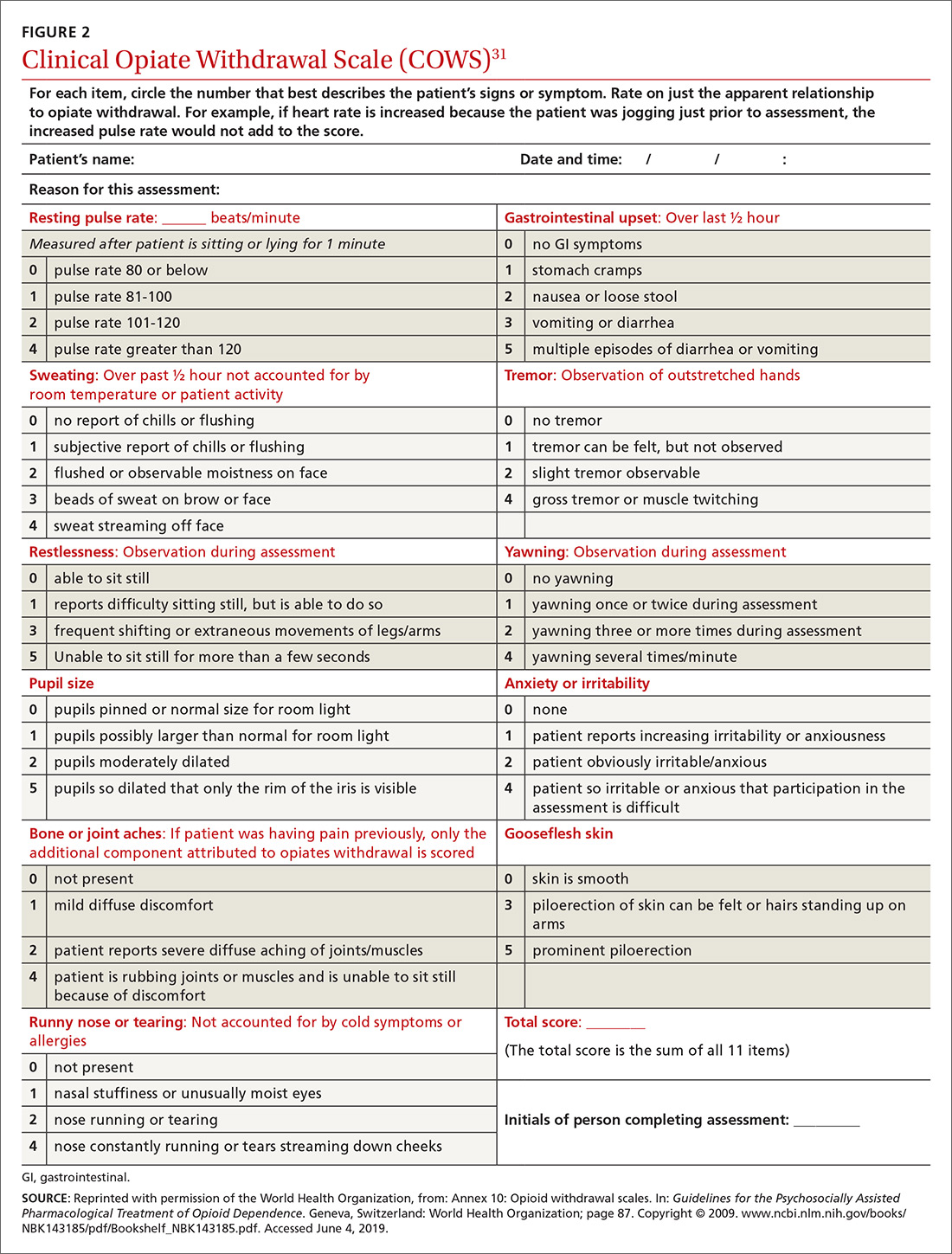
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
PRACTICE RECOMMENDATIONS
› Continue opioid therapy only when it has brought clinically meaningful improvement in pain and function and when the benefits outweigh adverse events or risks. C
› Review the selected opioid tapering plan in detail with the patient and provide close follow-up monitoring of ongoing or emerging risks. C
› Be vigilant: Enacting an opioid-tapering plan can unmask opioid use disorder, which can cause the patient to seek alternative forms of opioids, including illicit, potentially lethal fentanyl analogues. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series