User login
In methamphetamine use disorder, consider off-label drugs
SAN DIEGO – Its toll is obscured by the opioid crisis, but methamphetamine use is on the rise in the United States. There are no approved treatments for methamphetamine use, but a psychiatrist told colleagues that several off-label medications might prove helpful.
However, the evidence supporting the use of these medications for patients taking methamphetamine is not robust, “and none are even close to [Food and Drug Administration] approval,” said Larissa J. Mooney, MD, of the University of California, Los Angeles, and the VA Greater Los Angeles Healthcare System. “But if I use something that’s approved for depression or might be helpful for anxiety symptoms, maybe it would also help reduce their likelihood of relapse in conjunction with an evidence-based behavioral program or treatment with a therapist.”
Dr. Mooney, who spoke at the annual Psych Congress, highlighted a federal report estimating that 0.4% of people aged 18-25 in 2017 used the drug within the past month, compared with 0.3% of those aged 26 and higher.
There were about 758,000 current adult users of methamphetamine in 2017, the report found.
Meanwhile, (Drug Alcohol Depend. 2018 Dec 1;193:14-20). And, Dr. Mooney said, deaths from stimulants are rising, even independent of opioid deaths.
Stimulant users typically have other psychiatric conditions, such as depression, anxiety, and concentration problems, Dr. Mooney said. In those cases, she said, treating those conditions might help with the substance use, too.
For methamphetamine use disorder, she highlighted some medications that might be helpful, although, again, she cautioned that evidence is not strong:
- Bupropion (Wellbutrin). Research suggests that this drug is more effective in patients with less severe methamphetamine use disorder, Dr. Mooney said. “It’s a more stimulating antidepressant, and can be helpful with concentration and attention.”
- Mirtazapine (Remeron). “I keep it in my list of options for some [who are] really anxious and not sleeping well,” she said. “It might be beneficial.”
- Naltrexone (ReVia, Depade, Vivitrol). “There are some early signs of efficacy,” she said, and a randomized, controlled trial is in progress.
- Methylphenidate (Ritalin, Concerta) and topiramate (Topamax). There’s “low-strength” evidence that the drugs can be helpful and lower use of methamphetamine, she said. However, methylphenidate is a stimulant. There’s controversy over the use of stimulants to treat patients with substance use disorders, Dr. Mooney said, and she tends to be conservative about their use in this population.
Why not use them to treat methamphetamine users in the same way that opioids such as methadone are used to treat opioid use addiction? “We don’t have an equivalent stimulant that works in the same way,” she said. “They don’t stay in the system for 24 hours. If you take a prescription stimulant, by the end of the day it wears off. It won’t stay in the same way as agonist treatments for opioid disorder.”
Even so, she said, “it makes sense that stimulants might be helpful.”
Dr. Mooney disclosed an advisory board relationship with Alkermes and grant/research support from the National Institute on Drug Abuse.
SAN DIEGO – Its toll is obscured by the opioid crisis, but methamphetamine use is on the rise in the United States. There are no approved treatments for methamphetamine use, but a psychiatrist told colleagues that several off-label medications might prove helpful.
However, the evidence supporting the use of these medications for patients taking methamphetamine is not robust, “and none are even close to [Food and Drug Administration] approval,” said Larissa J. Mooney, MD, of the University of California, Los Angeles, and the VA Greater Los Angeles Healthcare System. “But if I use something that’s approved for depression or might be helpful for anxiety symptoms, maybe it would also help reduce their likelihood of relapse in conjunction with an evidence-based behavioral program or treatment with a therapist.”
Dr. Mooney, who spoke at the annual Psych Congress, highlighted a federal report estimating that 0.4% of people aged 18-25 in 2017 used the drug within the past month, compared with 0.3% of those aged 26 and higher.
There were about 758,000 current adult users of methamphetamine in 2017, the report found.
Meanwhile, (Drug Alcohol Depend. 2018 Dec 1;193:14-20). And, Dr. Mooney said, deaths from stimulants are rising, even independent of opioid deaths.
Stimulant users typically have other psychiatric conditions, such as depression, anxiety, and concentration problems, Dr. Mooney said. In those cases, she said, treating those conditions might help with the substance use, too.
For methamphetamine use disorder, she highlighted some medications that might be helpful, although, again, she cautioned that evidence is not strong:
- Bupropion (Wellbutrin). Research suggests that this drug is more effective in patients with less severe methamphetamine use disorder, Dr. Mooney said. “It’s a more stimulating antidepressant, and can be helpful with concentration and attention.”
- Mirtazapine (Remeron). “I keep it in my list of options for some [who are] really anxious and not sleeping well,” she said. “It might be beneficial.”
- Naltrexone (ReVia, Depade, Vivitrol). “There are some early signs of efficacy,” she said, and a randomized, controlled trial is in progress.
- Methylphenidate (Ritalin, Concerta) and topiramate (Topamax). There’s “low-strength” evidence that the drugs can be helpful and lower use of methamphetamine, she said. However, methylphenidate is a stimulant. There’s controversy over the use of stimulants to treat patients with substance use disorders, Dr. Mooney said, and she tends to be conservative about their use in this population.
Why not use them to treat methamphetamine users in the same way that opioids such as methadone are used to treat opioid use addiction? “We don’t have an equivalent stimulant that works in the same way,” she said. “They don’t stay in the system for 24 hours. If you take a prescription stimulant, by the end of the day it wears off. It won’t stay in the same way as agonist treatments for opioid disorder.”
Even so, she said, “it makes sense that stimulants might be helpful.”
Dr. Mooney disclosed an advisory board relationship with Alkermes and grant/research support from the National Institute on Drug Abuse.
SAN DIEGO – Its toll is obscured by the opioid crisis, but methamphetamine use is on the rise in the United States. There are no approved treatments for methamphetamine use, but a psychiatrist told colleagues that several off-label medications might prove helpful.
However, the evidence supporting the use of these medications for patients taking methamphetamine is not robust, “and none are even close to [Food and Drug Administration] approval,” said Larissa J. Mooney, MD, of the University of California, Los Angeles, and the VA Greater Los Angeles Healthcare System. “But if I use something that’s approved for depression or might be helpful for anxiety symptoms, maybe it would also help reduce their likelihood of relapse in conjunction with an evidence-based behavioral program or treatment with a therapist.”
Dr. Mooney, who spoke at the annual Psych Congress, highlighted a federal report estimating that 0.4% of people aged 18-25 in 2017 used the drug within the past month, compared with 0.3% of those aged 26 and higher.
There were about 758,000 current adult users of methamphetamine in 2017, the report found.
Meanwhile, (Drug Alcohol Depend. 2018 Dec 1;193:14-20). And, Dr. Mooney said, deaths from stimulants are rising, even independent of opioid deaths.
Stimulant users typically have other psychiatric conditions, such as depression, anxiety, and concentration problems, Dr. Mooney said. In those cases, she said, treating those conditions might help with the substance use, too.
For methamphetamine use disorder, she highlighted some medications that might be helpful, although, again, she cautioned that evidence is not strong:
- Bupropion (Wellbutrin). Research suggests that this drug is more effective in patients with less severe methamphetamine use disorder, Dr. Mooney said. “It’s a more stimulating antidepressant, and can be helpful with concentration and attention.”
- Mirtazapine (Remeron). “I keep it in my list of options for some [who are] really anxious and not sleeping well,” she said. “It might be beneficial.”
- Naltrexone (ReVia, Depade, Vivitrol). “There are some early signs of efficacy,” she said, and a randomized, controlled trial is in progress.
- Methylphenidate (Ritalin, Concerta) and topiramate (Topamax). There’s “low-strength” evidence that the drugs can be helpful and lower use of methamphetamine, she said. However, methylphenidate is a stimulant. There’s controversy over the use of stimulants to treat patients with substance use disorders, Dr. Mooney said, and she tends to be conservative about their use in this population.
Why not use them to treat methamphetamine users in the same way that opioids such as methadone are used to treat opioid use addiction? “We don’t have an equivalent stimulant that works in the same way,” she said. “They don’t stay in the system for 24 hours. If you take a prescription stimulant, by the end of the day it wears off. It won’t stay in the same way as agonist treatments for opioid disorder.”
Even so, she said, “it makes sense that stimulants might be helpful.”
Dr. Mooney disclosed an advisory board relationship with Alkermes and grant/research support from the National Institute on Drug Abuse.
REPORTING FROM PSYCH CONGRESS 2019
Dismantling the opioid crisis
Dr. John Hickner’s editorial, “Doing our part to dismantle the opioid crisis” (J Fam Pract 2019;68:308) had important inaccuracies.
The Joint Commission, for which I serve as an executive vice president, did not “dub pain assessment the ‘fifth vital sign’. ” The concept of the fifth vital sign was developed by the American Pain Society in the 1990s.1 It gained national attention through a Veterans Health Administration initiative in 1999.2 And in 2001, the Joint Commission (then the Joint Commission on Accreditation of Healthcare Organizations or JCAHO) issued its Pain Standards.
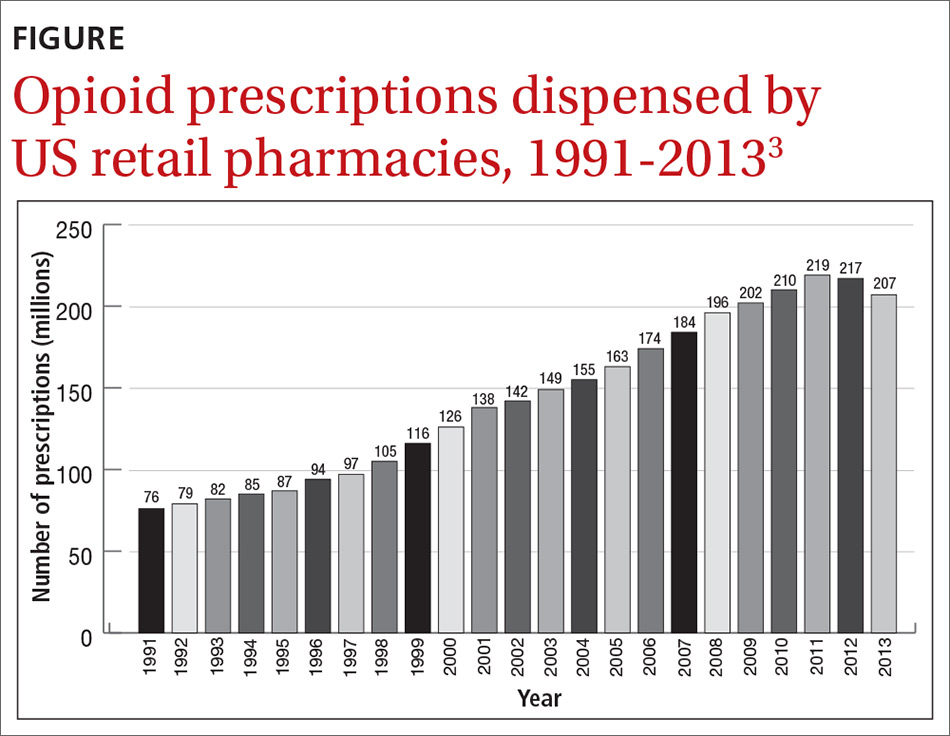
Dr. Hickner wrote that the push to assess for pain as the fifth vital sign was a central cause of the opioid epidemic; however, this is contrary to published data on the epidemic. Total opioid prescriptions had been steadily increasing in the United States for at least a decade before the Pain Standards went into effect in 2001 (FIGURE).3 Between 1991 and 1997, the number of prescriptions increased from 76 million to 97 million. The rate of increase from 1997 to 2011 appears to have been more rapid, which is likely due to the 1995 approval of the new sustained-release opioid OxyContin and the associated aggressive marketing campaigns to physicians.
Your readers should know that we, at the Joint Commission, are also “doing our part to dismantle the opioid crisis.” In 2016, we completely revised our Pain Standards, adding new criteria to help address the epidemic. Some adjustments include: requiring improved availability of nonpharmacologic therapy, encouraging engagement of patients in pain management plans, enhancing accessibility of Physician Drug Monitoring Program tools, and monitoring opioid prescribing.
David W. Baker, MD, FACP, executive vice president
The Joint Commission, Oakbrook Terrace, IL
1. American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Chronic Cancer Pain. 2nd ed. Skokie, Illinois: American Pain Society; 1989.
2. Department of Veteran’s Affairs. Pain: the fifth vital sign. www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed September 30, 2019.
3 National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. https://archives.drugabuse.gov/testimonies/2014/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Published May 14, 2014. Accessed September 30, 2019.
Dr. John Hickner’s editorial, “Doing our part to dismantle the opioid crisis” (J Fam Pract 2019;68:308) had important inaccuracies.
The Joint Commission, for which I serve as an executive vice president, did not “dub pain assessment the ‘fifth vital sign’. ” The concept of the fifth vital sign was developed by the American Pain Society in the 1990s.1 It gained national attention through a Veterans Health Administration initiative in 1999.2 And in 2001, the Joint Commission (then the Joint Commission on Accreditation of Healthcare Organizations or JCAHO) issued its Pain Standards.
Dr. Hickner wrote that the push to assess for pain as the fifth vital sign was a central cause of the opioid epidemic; however, this is contrary to published data on the epidemic. Total opioid prescriptions had been steadily increasing in the United States for at least a decade before the Pain Standards went into effect in 2001 (FIGURE).3 Between 1991 and 1997, the number of prescriptions increased from 76 million to 97 million. The rate of increase from 1997 to 2011 appears to have been more rapid, which is likely due to the 1995 approval of the new sustained-release opioid OxyContin and the associated aggressive marketing campaigns to physicians.

Your readers should know that we, at the Joint Commission, are also “doing our part to dismantle the opioid crisis.” In 2016, we completely revised our Pain Standards, adding new criteria to help address the epidemic. Some adjustments include: requiring improved availability of nonpharmacologic therapy, encouraging engagement of patients in pain management plans, enhancing accessibility of Physician Drug Monitoring Program tools, and monitoring opioid prescribing.
David W. Baker, MD, FACP, executive vice president
The Joint Commission, Oakbrook Terrace, IL
Dr. John Hickner’s editorial, “Doing our part to dismantle the opioid crisis” (J Fam Pract 2019;68:308) had important inaccuracies.
The Joint Commission, for which I serve as an executive vice president, did not “dub pain assessment the ‘fifth vital sign’. ” The concept of the fifth vital sign was developed by the American Pain Society in the 1990s.1 It gained national attention through a Veterans Health Administration initiative in 1999.2 And in 2001, the Joint Commission (then the Joint Commission on Accreditation of Healthcare Organizations or JCAHO) issued its Pain Standards.
Dr. Hickner wrote that the push to assess for pain as the fifth vital sign was a central cause of the opioid epidemic; however, this is contrary to published data on the epidemic. Total opioid prescriptions had been steadily increasing in the United States for at least a decade before the Pain Standards went into effect in 2001 (FIGURE).3 Between 1991 and 1997, the number of prescriptions increased from 76 million to 97 million. The rate of increase from 1997 to 2011 appears to have been more rapid, which is likely due to the 1995 approval of the new sustained-release opioid OxyContin and the associated aggressive marketing campaigns to physicians.

Your readers should know that we, at the Joint Commission, are also “doing our part to dismantle the opioid crisis.” In 2016, we completely revised our Pain Standards, adding new criteria to help address the epidemic. Some adjustments include: requiring improved availability of nonpharmacologic therapy, encouraging engagement of patients in pain management plans, enhancing accessibility of Physician Drug Monitoring Program tools, and monitoring opioid prescribing.
David W. Baker, MD, FACP, executive vice president
The Joint Commission, Oakbrook Terrace, IL
1. American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Chronic Cancer Pain. 2nd ed. Skokie, Illinois: American Pain Society; 1989.
2. Department of Veteran’s Affairs. Pain: the fifth vital sign. www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed September 30, 2019.
3 National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. https://archives.drugabuse.gov/testimonies/2014/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Published May 14, 2014. Accessed September 30, 2019.
1. American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Chronic Cancer Pain. 2nd ed. Skokie, Illinois: American Pain Society; 1989.
2. Department of Veteran’s Affairs. Pain: the fifth vital sign. www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed September 30, 2019.
3 National Institute on Drug Abuse. America’s addiction to opioids: heroin and prescription drug abuse. https://archives.drugabuse.gov/testimonies/2014/americas-addiction-to-opioids-heroin-prescription-drug-abuse. Published May 14, 2014. Accessed September 30, 2019.
SUDs are almost always comorbid with other disorders
SAN DIEGO – Substance use disorders rarely ride alone, a psychiatrist told colleagues, and it’s crucial to treat the accompanying mental illness that is almost always present.
“If you’re really depressed and you’re smoking marijuana, the smoking could have made it worse, but you were probably depressed before,” said Timothy E. Wilens, MD, of Harvard Medical School and Massachusetts General Hospital, both in Boston. Dr. Wilens spoke at the annual Psych Congress.
He pointed to numbers supporting the link between substance use and mental illness. He also offered several tips about treating substance use disorder (SUD).
In ADHD, consider the big picture. If a person has both ADHD and SUD, treat both if the level of substance abuse is lower. But focus on the SUD in more severe cases, he said, and realize that “most likely your treatment for ADHD isn’t going to work as well.”
The same goes for the anxiolytic buspirone (Buspar) in patients with depression and SUD.
Consider N-acetyl cysteine in cannabis use disorder. N-acetyl cysteine, a nutraceutical used as an asthma medication, has shown promise in trials as a treatment for cannabis use disorder, Dr. Wilens said. It helps patients avoid the temptation to smoke. “They won’t say they’ve lost all their cravings, but you’ll hear, ‘I just didn’t need to do it; I’m not smoking as much.’ If you hear that from your patients, you know it’s working. It’s a subtle effect, but it can help.”
Scamming’ drugs shouldn’t be your main worry. Substance use research suggests that users of pharmaceutical drugs for nonmedical uses rarely get them directly from practitioners (7%), but instead mainly get them through friends, Dr. Wilens said. “If you work with this population and treat ADHD or anxiety, you’re paranoid that everyone coming in wants to scam medicines. Be more concerned about oversupplying them with immediate-release medications and not [taking] them to task about keeping the medication safely stored.”
Interventions such as Alcoholics Anonymous are as “effective as any other treatment for substance abuse, and it’s not costly,” Dr. Wilens said. He added that the Rational Recovery program, an alternative to Alcoholics Anonymous, also seems to work well. The approaches to ending substance use differ in that Alcoholics Anonymous’s orientation is spiritual and Rational Recovery’s is cognitive.
Dr. Wilens reported various disclosures, including consulting relationships with Ironshore Pharmaceuticals, KemPharm, and Neurovance/Otsuka.
SAN DIEGO – Substance use disorders rarely ride alone, a psychiatrist told colleagues, and it’s crucial to treat the accompanying mental illness that is almost always present.
“If you’re really depressed and you’re smoking marijuana, the smoking could have made it worse, but you were probably depressed before,” said Timothy E. Wilens, MD, of Harvard Medical School and Massachusetts General Hospital, both in Boston. Dr. Wilens spoke at the annual Psych Congress.
He pointed to numbers supporting the link between substance use and mental illness. He also offered several tips about treating substance use disorder (SUD).
In ADHD, consider the big picture. If a person has both ADHD and SUD, treat both if the level of substance abuse is lower. But focus on the SUD in more severe cases, he said, and realize that “most likely your treatment for ADHD isn’t going to work as well.”
The same goes for the anxiolytic buspirone (Buspar) in patients with depression and SUD.
Consider N-acetyl cysteine in cannabis use disorder. N-acetyl cysteine, a nutraceutical used as an asthma medication, has shown promise in trials as a treatment for cannabis use disorder, Dr. Wilens said. It helps patients avoid the temptation to smoke. “They won’t say they’ve lost all their cravings, but you’ll hear, ‘I just didn’t need to do it; I’m not smoking as much.’ If you hear that from your patients, you know it’s working. It’s a subtle effect, but it can help.”
Scamming’ drugs shouldn’t be your main worry. Substance use research suggests that users of pharmaceutical drugs for nonmedical uses rarely get them directly from practitioners (7%), but instead mainly get them through friends, Dr. Wilens said. “If you work with this population and treat ADHD or anxiety, you’re paranoid that everyone coming in wants to scam medicines. Be more concerned about oversupplying them with immediate-release medications and not [taking] them to task about keeping the medication safely stored.”
Interventions such as Alcoholics Anonymous are as “effective as any other treatment for substance abuse, and it’s not costly,” Dr. Wilens said. He added that the Rational Recovery program, an alternative to Alcoholics Anonymous, also seems to work well. The approaches to ending substance use differ in that Alcoholics Anonymous’s orientation is spiritual and Rational Recovery’s is cognitive.
Dr. Wilens reported various disclosures, including consulting relationships with Ironshore Pharmaceuticals, KemPharm, and Neurovance/Otsuka.
SAN DIEGO – Substance use disorders rarely ride alone, a psychiatrist told colleagues, and it’s crucial to treat the accompanying mental illness that is almost always present.
“If you’re really depressed and you’re smoking marijuana, the smoking could have made it worse, but you were probably depressed before,” said Timothy E. Wilens, MD, of Harvard Medical School and Massachusetts General Hospital, both in Boston. Dr. Wilens spoke at the annual Psych Congress.
He pointed to numbers supporting the link between substance use and mental illness. He also offered several tips about treating substance use disorder (SUD).
In ADHD, consider the big picture. If a person has both ADHD and SUD, treat both if the level of substance abuse is lower. But focus on the SUD in more severe cases, he said, and realize that “most likely your treatment for ADHD isn’t going to work as well.”
The same goes for the anxiolytic buspirone (Buspar) in patients with depression and SUD.
Consider N-acetyl cysteine in cannabis use disorder. N-acetyl cysteine, a nutraceutical used as an asthma medication, has shown promise in trials as a treatment for cannabis use disorder, Dr. Wilens said. It helps patients avoid the temptation to smoke. “They won’t say they’ve lost all their cravings, but you’ll hear, ‘I just didn’t need to do it; I’m not smoking as much.’ If you hear that from your patients, you know it’s working. It’s a subtle effect, but it can help.”
Scamming’ drugs shouldn’t be your main worry. Substance use research suggests that users of pharmaceutical drugs for nonmedical uses rarely get them directly from practitioners (7%), but instead mainly get them through friends, Dr. Wilens said. “If you work with this population and treat ADHD or anxiety, you’re paranoid that everyone coming in wants to scam medicines. Be more concerned about oversupplying them with immediate-release medications and not [taking] them to task about keeping the medication safely stored.”
Interventions such as Alcoholics Anonymous are as “effective as any other treatment for substance abuse, and it’s not costly,” Dr. Wilens said. He added that the Rational Recovery program, an alternative to Alcoholics Anonymous, also seems to work well. The approaches to ending substance use differ in that Alcoholics Anonymous’s orientation is spiritual and Rational Recovery’s is cognitive.
Dr. Wilens reported various disclosures, including consulting relationships with Ironshore Pharmaceuticals, KemPharm, and Neurovance/Otsuka.
REPORTING FROM PSYCH CONGRESS 2019
How to use lofexidine for quick opioid withdrawal
SAN DIEGO – Lofexidine (Lucemyra), the new kid on the block in the United States for opioid withdrawal, can help patients get through the process in a few days, instead of a week or more, according to Thomas Kosten, MD, a psychiatry professor and director of the division of addictions at Baylor College of Medicine, Houston.
Lofexidine relieves symptom withdrawal and has significant advantages over clonidine, a similar drug, including easier dosing and no orthostatic hypertension.
In a video interview at the annual Psych Congress, Dr. Kosten went into the nuts and bolts of how to use lofexidine with buprenorphine and naltrexone – plus benzodiazepines when needed – to help people safely go through withdrawal and in just a few days.
Once chronic pain patients are off opioids, the next question is what to do for their pain. In a presentation before the interview, Dr. Kosten said he favors tricyclic antidepressants, especially desipramine because it has the fewest side effects. The effect size with tricyclic antidepressants is larger than with gabapentin and other options. They take a few weeks to kick in, however, so he’s thinking about a unique approach: using ketamine – either infusions or the new nasal spray esketamine (Spravato) – to tide people over in the meantime. It’s becoming well known that ketamine works amazingly fast for depression and suicidality, and there is emerging support that it might do the same for chronic pain. Dr. Kosten is a consultant for US Worldmeds, maker of lofexidine.
SAN DIEGO – Lofexidine (Lucemyra), the new kid on the block in the United States for opioid withdrawal, can help patients get through the process in a few days, instead of a week or more, according to Thomas Kosten, MD, a psychiatry professor and director of the division of addictions at Baylor College of Medicine, Houston.
Lofexidine relieves symptom withdrawal and has significant advantages over clonidine, a similar drug, including easier dosing and no orthostatic hypertension.
In a video interview at the annual Psych Congress, Dr. Kosten went into the nuts and bolts of how to use lofexidine with buprenorphine and naltrexone – plus benzodiazepines when needed – to help people safely go through withdrawal and in just a few days.
Once chronic pain patients are off opioids, the next question is what to do for their pain. In a presentation before the interview, Dr. Kosten said he favors tricyclic antidepressants, especially desipramine because it has the fewest side effects. The effect size with tricyclic antidepressants is larger than with gabapentin and other options. They take a few weeks to kick in, however, so he’s thinking about a unique approach: using ketamine – either infusions or the new nasal spray esketamine (Spravato) – to tide people over in the meantime. It’s becoming well known that ketamine works amazingly fast for depression and suicidality, and there is emerging support that it might do the same for chronic pain. Dr. Kosten is a consultant for US Worldmeds, maker of lofexidine.
SAN DIEGO – Lofexidine (Lucemyra), the new kid on the block in the United States for opioid withdrawal, can help patients get through the process in a few days, instead of a week or more, according to Thomas Kosten, MD, a psychiatry professor and director of the division of addictions at Baylor College of Medicine, Houston.
Lofexidine relieves symptom withdrawal and has significant advantages over clonidine, a similar drug, including easier dosing and no orthostatic hypertension.
In a video interview at the annual Psych Congress, Dr. Kosten went into the nuts and bolts of how to use lofexidine with buprenorphine and naltrexone – plus benzodiazepines when needed – to help people safely go through withdrawal and in just a few days.
Once chronic pain patients are off opioids, the next question is what to do for their pain. In a presentation before the interview, Dr. Kosten said he favors tricyclic antidepressants, especially desipramine because it has the fewest side effects. The effect size with tricyclic antidepressants is larger than with gabapentin and other options. They take a few weeks to kick in, however, so he’s thinking about a unique approach: using ketamine – either infusions or the new nasal spray esketamine (Spravato) – to tide people over in the meantime. It’s becoming well known that ketamine works amazingly fast for depression and suicidality, and there is emerging support that it might do the same for chronic pain. Dr. Kosten is a consultant for US Worldmeds, maker of lofexidine.
REPORTING FROM PSYCH CONGRESS 2019
Buprenorphine merits more attention for treatment of opioid use disorder
SAN DIEGO – Prescribing buprenorphine for the treatment of opioid use disorder requires strict discernment on the part of clinicians, Arwen Podesta, MD, said at the annual Psych Congress.
She encouraged clinicians to be prepared for a visit from the Drug Enforcement Administration, understand the unique properties of buprenorphine, and make sure that patients grasp the importance of sublingual administration.
Research shows that only 5% of physicians are allowed to prescribe buprenorphine – an opioid – by way of a DEA waiver, Dr. Podesta said. About half do not prescribe the drug. Barriers to prescribing buprenorphine include factors such as low reimbursement and untrained support staff, said Dr. Podesta, a board-certified psychiatrist who subspecializes in addiction medicine and practices in New Orleans.
But she noted that the Substance Abuse and Mental Health Services Administration has recommended that medication-assisted therapy (MAT) – methadone, buprenorphine, and naltrexone – be considered in all patients with opioid use disorder. The drugs are safe and effective when used correctly, the federal agency has said.
Remember, Dr. Podesta said, that “patients taking MAT are considered to be in recovery.” In the big picture, she added, “we have to improve access to care because we have so many people who don’t have access to treatment.”
Getting permission from the DEA to prescribe buprenorphine – a schedule III controlled substance – comes with a price, Dr. Podesta said. “We have special scrutiny from the DEA,” she said. They come in and want to see your records. It sounds very punitive, although it’s their jobs.”
The best approach is to document that you know what you’re doing, she said. “It’s your job to educate them about why you’re using buprenorphine and produce the records to show that.”
Being aware of buprenorphine’s unique properties is important, she said. The drug is safer on the overdose front than are other opioids, Dr. Podesta said, but it can be very dangerous in patients without opioid tolerance. According to the DEA, as an analgesic, buprenorphine is 20-30 times more potent than morphine. Also, like morphine, patients who take buprenorphine are likely to experience euphoria, papillary restriction, and respiratory depression and sedation.
The buprenorphine/naloxone formulation is preferred to treat opioid use disorder, she noted.
The reason that naloxone, which treats opioid overdoses, is part of the drug combo is because as an add-on, it reduces the risk that buprenorphine will be crushed and snorted for an opioid high, she said. Those who take the combo drug via that method could end up with sudden and nasty withdrawal symptoms.
When the drug combo is administered sublingually, the idea is that the “good stuff” (buprenorphine) is absorbed in the mouth, while the “bad stuff” (naloxone) is harmlessly absorbed in the gut, Dr. Podesta said. This happens because the drugs are absorbed differently.
But patients can mistakenly trigger symptoms of withdrawal if, for example, they put the combo drug on their tongue and then go to sleep. “That’s a peril,” she said, and it’s important to make sure patients know what to do – and what not to do.
Dr. Podesta emphasized the importance of choosing language related to patients with addictions carefully and respectfully.
“We have stigma,” she said. “We have been saying that patients are ‘dirty’ or ‘clean,’ and if they’re ‘clean,’ they’re the opposite of ‘dirty.’
She also suggested that clinicians drop the use of the word “contract” to describe treatment agreements between patients and clinicians. “Call it an ‘agreement,’ ” she said. “It seems more mutual and less punitive or risky for the patient to sign, especially when they’re in a precarious comfort zone.”
And consider that even the words “substance abuse” can be misleading, she said. “Many [patients] are taking the medications that the doctor prescribed and following instructions to the letter.”
Dr. Podesta disclosed consulting with Kaleo, Pear Therapeutics, and JayMac, and serving on the speakers bureau of Alkermes, Orexo, and US WorldMeds. She is the author of “Hooked: A Concise Guide to the Underlying Mechanics of Addiction and Treatment for Patients, Families, and Providers” (Dog Ear Publishing, 2016).
SAN DIEGO – Prescribing buprenorphine for the treatment of opioid use disorder requires strict discernment on the part of clinicians, Arwen Podesta, MD, said at the annual Psych Congress.
She encouraged clinicians to be prepared for a visit from the Drug Enforcement Administration, understand the unique properties of buprenorphine, and make sure that patients grasp the importance of sublingual administration.
Research shows that only 5% of physicians are allowed to prescribe buprenorphine – an opioid – by way of a DEA waiver, Dr. Podesta said. About half do not prescribe the drug. Barriers to prescribing buprenorphine include factors such as low reimbursement and untrained support staff, said Dr. Podesta, a board-certified psychiatrist who subspecializes in addiction medicine and practices in New Orleans.
But she noted that the Substance Abuse and Mental Health Services Administration has recommended that medication-assisted therapy (MAT) – methadone, buprenorphine, and naltrexone – be considered in all patients with opioid use disorder. The drugs are safe and effective when used correctly, the federal agency has said.
Remember, Dr. Podesta said, that “patients taking MAT are considered to be in recovery.” In the big picture, she added, “we have to improve access to care because we have so many people who don’t have access to treatment.”
Getting permission from the DEA to prescribe buprenorphine – a schedule III controlled substance – comes with a price, Dr. Podesta said. “We have special scrutiny from the DEA,” she said. They come in and want to see your records. It sounds very punitive, although it’s their jobs.”
The best approach is to document that you know what you’re doing, she said. “It’s your job to educate them about why you’re using buprenorphine and produce the records to show that.”
Being aware of buprenorphine’s unique properties is important, she said. The drug is safer on the overdose front than are other opioids, Dr. Podesta said, but it can be very dangerous in patients without opioid tolerance. According to the DEA, as an analgesic, buprenorphine is 20-30 times more potent than morphine. Also, like morphine, patients who take buprenorphine are likely to experience euphoria, papillary restriction, and respiratory depression and sedation.
The buprenorphine/naloxone formulation is preferred to treat opioid use disorder, she noted.
The reason that naloxone, which treats opioid overdoses, is part of the drug combo is because as an add-on, it reduces the risk that buprenorphine will be crushed and snorted for an opioid high, she said. Those who take the combo drug via that method could end up with sudden and nasty withdrawal symptoms.
When the drug combo is administered sublingually, the idea is that the “good stuff” (buprenorphine) is absorbed in the mouth, while the “bad stuff” (naloxone) is harmlessly absorbed in the gut, Dr. Podesta said. This happens because the drugs are absorbed differently.
But patients can mistakenly trigger symptoms of withdrawal if, for example, they put the combo drug on their tongue and then go to sleep. “That’s a peril,” she said, and it’s important to make sure patients know what to do – and what not to do.
Dr. Podesta emphasized the importance of choosing language related to patients with addictions carefully and respectfully.
“We have stigma,” she said. “We have been saying that patients are ‘dirty’ or ‘clean,’ and if they’re ‘clean,’ they’re the opposite of ‘dirty.’
She also suggested that clinicians drop the use of the word “contract” to describe treatment agreements between patients and clinicians. “Call it an ‘agreement,’ ” she said. “It seems more mutual and less punitive or risky for the patient to sign, especially when they’re in a precarious comfort zone.”
And consider that even the words “substance abuse” can be misleading, she said. “Many [patients] are taking the medications that the doctor prescribed and following instructions to the letter.”
Dr. Podesta disclosed consulting with Kaleo, Pear Therapeutics, and JayMac, and serving on the speakers bureau of Alkermes, Orexo, and US WorldMeds. She is the author of “Hooked: A Concise Guide to the Underlying Mechanics of Addiction and Treatment for Patients, Families, and Providers” (Dog Ear Publishing, 2016).
SAN DIEGO – Prescribing buprenorphine for the treatment of opioid use disorder requires strict discernment on the part of clinicians, Arwen Podesta, MD, said at the annual Psych Congress.
She encouraged clinicians to be prepared for a visit from the Drug Enforcement Administration, understand the unique properties of buprenorphine, and make sure that patients grasp the importance of sublingual administration.
Research shows that only 5% of physicians are allowed to prescribe buprenorphine – an opioid – by way of a DEA waiver, Dr. Podesta said. About half do not prescribe the drug. Barriers to prescribing buprenorphine include factors such as low reimbursement and untrained support staff, said Dr. Podesta, a board-certified psychiatrist who subspecializes in addiction medicine and practices in New Orleans.
But she noted that the Substance Abuse and Mental Health Services Administration has recommended that medication-assisted therapy (MAT) – methadone, buprenorphine, and naltrexone – be considered in all patients with opioid use disorder. The drugs are safe and effective when used correctly, the federal agency has said.
Remember, Dr. Podesta said, that “patients taking MAT are considered to be in recovery.” In the big picture, she added, “we have to improve access to care because we have so many people who don’t have access to treatment.”
Getting permission from the DEA to prescribe buprenorphine – a schedule III controlled substance – comes with a price, Dr. Podesta said. “We have special scrutiny from the DEA,” she said. They come in and want to see your records. It sounds very punitive, although it’s their jobs.”
The best approach is to document that you know what you’re doing, she said. “It’s your job to educate them about why you’re using buprenorphine and produce the records to show that.”
Being aware of buprenorphine’s unique properties is important, she said. The drug is safer on the overdose front than are other opioids, Dr. Podesta said, but it can be very dangerous in patients without opioid tolerance. According to the DEA, as an analgesic, buprenorphine is 20-30 times more potent than morphine. Also, like morphine, patients who take buprenorphine are likely to experience euphoria, papillary restriction, and respiratory depression and sedation.
The buprenorphine/naloxone formulation is preferred to treat opioid use disorder, she noted.
The reason that naloxone, which treats opioid overdoses, is part of the drug combo is because as an add-on, it reduces the risk that buprenorphine will be crushed and snorted for an opioid high, she said. Those who take the combo drug via that method could end up with sudden and nasty withdrawal symptoms.
When the drug combo is administered sublingually, the idea is that the “good stuff” (buprenorphine) is absorbed in the mouth, while the “bad stuff” (naloxone) is harmlessly absorbed in the gut, Dr. Podesta said. This happens because the drugs are absorbed differently.
But patients can mistakenly trigger symptoms of withdrawal if, for example, they put the combo drug on their tongue and then go to sleep. “That’s a peril,” she said, and it’s important to make sure patients know what to do – and what not to do.
Dr. Podesta emphasized the importance of choosing language related to patients with addictions carefully and respectfully.
“We have stigma,” she said. “We have been saying that patients are ‘dirty’ or ‘clean,’ and if they’re ‘clean,’ they’re the opposite of ‘dirty.’
She also suggested that clinicians drop the use of the word “contract” to describe treatment agreements between patients and clinicians. “Call it an ‘agreement,’ ” she said. “It seems more mutual and less punitive or risky for the patient to sign, especially when they’re in a precarious comfort zone.”
And consider that even the words “substance abuse” can be misleading, she said. “Many [patients] are taking the medications that the doctor prescribed and following instructions to the letter.”
Dr. Podesta disclosed consulting with Kaleo, Pear Therapeutics, and JayMac, and serving on the speakers bureau of Alkermes, Orexo, and US WorldMeds. She is the author of “Hooked: A Concise Guide to the Underlying Mechanics of Addiction and Treatment for Patients, Families, and Providers” (Dog Ear Publishing, 2016).
REPORTING FROM PSYCH CONGRESS 2019
Clinical Pharmacists Improve Patient Outcomes and Expand Access to Care
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
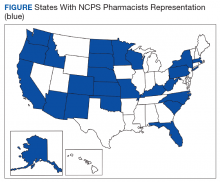
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
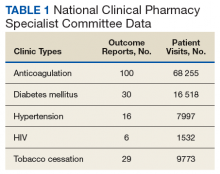
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
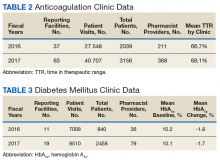
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
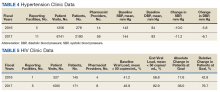
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
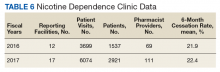
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
The US is in the midst of a chronic disease crisis. According to the latest published data available, 60% of Americans have at least 1 chronic condition, and 42% have ≥ 2 chronic conditions.1 Estimates by the Health Resources and Services Administration (HRSA) indicate a current shortfall of 13 800 primary care physicians and a projected escalation of that shortage to be between 14 800 and 49 300 physicians by the year 2030.2
The US Public Health Service (USPHS) has used pharmacists since 1930 to provide direct patient care to underserved and vulnerable populations. Clinical pharmacists currently serve in direct patient care roles within the Indian Health Service (IHS), Federal Bureau of Prisons (BOP), Immigration and Customs Enforcement (ICE), and the United States Coast Guard (USCG) in many states (Figure). These pharmacists play a vital role in improving access to care and delivering quality care by managing acute and chronic diseases in collaborative practice settings and pharmacist-managed clinics.
It has previously been reported that in the face of physician shortages and growing demand for primary health care providers, pharmacists are well-equipped and motivated to meet this demand.3 A review of the previous 2 years of outcomes reported by clinical pharmacists certified through the USPHS National Clinical Pharmacy Specialist (NCPS) Committee are presented to demonstrate the impact of pharmacists in advancing the health of the populations they serve and to showcase a model for ameliorating the ongoing physician shortage.
Background
The USPHS NCPS Committee serves to promote uniform competency among clinical pharmacists by establishing national standards for protocols, collaborative practice agreements (CPAs), credentialing and privileging of pharmacists, and by collecting, reviewing, and publishing health care outcomes. The committee, whose constituents include pharmacist and physician subject matter experts from across USPHS agencies, reviews applications and protocols and certifies pharmacists (civilian and uniformed) to recognize an advanced scope of practice in managing various diseases and optimizing medication therapy. NCPScertified pharmacists manage a wide spectrum of diseases, including coagulopathy, asthma, diabetes mellitus (DM), hepatitis C, HIV, hypertension, pain, seizure disorders, and tobacco use disorders.
Clinical pharmacists practicing chronic disease management establish a clinical service in collaboration with 1 or more physicians, physician assistants, or nurse practitioners. In this collaborative practice, the health care practitioner(s) refer patients to be managed by a pharmacist for specific medical needs, such as anticoagulation management, or for holistic medication- focused care (eg, cardiovascular risk reduction, DM management, HIV, hepatitis, or mental health). The pharmacist may order and interpret laboratory tests, check vital signs, perform a limited physical examination, and gather other pertinent information from the patient and the medical record in order to provide the best possible care to the patient.
Medications may be started, stopped, or adjusted, education is provided, and therapeutic lifestyle interventions may be recommended. The pharmacist-run clinic provides the patient more frequent interaction with a health care professional (pharmacist) and focused disease management. As a result, pharmacists increase access to care and allow the medical team to handle a larger panel of patients as the practitioner delegates specified diseases to the pharmacist- managed clinic(s). The number of NCPS-certified pharmacists grew 46% from 2012 (n = 230) to 2017 (n = 336), reflecting an evolution of pharmacists’ practice to better meet the need of patients across the nation.
Methods
The NCPS Committee requires NCPS pharmacists to report data annually from all patients referred for pharmacist management for specific diseases in which they have been certified. The data reflect the patient’s clinical outcome goal status at the time of referral as well as the same status at the end of the reporting period or on release from the pharmacist-run clinic. These data describe the impact prescribing pharmacists have on patients reaching clinical outcome goals acting as the team member specializing in the medication selection and dosing aspect of care.
These records were reviewed for the fiscal year (FY) periods of October 1, 2015 to September 30, 2016 (FY 2016) and October 1, 2016 to September 30, 2017 (FY 2017). A systematic review of submitted reports resulted in 181 reports that included all requested data points for the disease as published here for FYs 2016 and 2017. These include 66 reports from FY 2016 and 115 reports from FY 2017; they cover 76 BOP and IHS facilities located across 24 states. Table 1 shows the number of outcome reports collected from 104 075 patient visits in pharmacist-run clinics in FYs 2016 and 2017.
Results
The following tables represent the standardized outcomes collected by NCPS-certified pharmacists providing direct patient care. Patients on anticoagulants (eg, warfarin) require special monitoring and education for drug interactions and adverse effects. NCPS-certified pharmacists were able to achieve a mean patient time in therapeutic range (TTR) of 67.6% (regardless of indication) over the 2 years (calculated per each facility by Rosendaal method of linear interpolation then combined in a weighted average per visit). The TTR produced by NCPS-certified pharmacists are consistent with Chest Guidelines and Expert Panel Report suggesting that TTR should be between 65% and 70%.4 Table 2 shows data from 100 reports with 68 255 patient visits for anticoagulation management.
DM management can be complex and time-intensive. NCPS data indicate pharmacist intervention resulted in a mean decrease in hemoglobin A1c (HbA1c) of 1.8% from a baseline of 10.2% (decrease calculated per each facility then combined by weighted average per visit). Table 3 shows data from 30 reports with 16 518 patient visits for DM care.
In addition to diet and exercise, medication management plays a vital role in managing hypertension. Patients managed by an NCPS-certified pharmacist experienced a mean decrease in blood pressure from 144/83 to 133/77, putting them in goal for both systolic and diastolic ranges (decrease calculated per each facility then combined by weighted average per visit). Table 4 shows data from 16 reports and 7997 patient visits for treatment of hypertension.
HIV viral suppression is vital in order to best manage patients with HIV and reduce the risk of transmission. Pharmacistled clinics have shown a 32.9% absolute improvement in patients at goal (viral load < 50 copies/mL), from a mean baseline of 46.0% to a mean final assessment of 71.6% of patients at goal (combined by weighted average visits). Table 5 shows data from 6 reports covering 1532 patient encounters for management of HIV.
Nicotine dependence includes the use of cigarettes, cigars, pipe tobacco, chewing tobacco, and vaping products containing nicotine. NCPS-certified pharmacists have successfully helped patients improve their chance of quitting, with a 6-month quit rate of 22.2% (quit rate calculated per each facility then combined by weighted average by visits), which is higher than the national average of 9.4% as reported by the Centers for Disease and Control and Prevention. 5 Table 6 shows 29 reports covering 9773 patient visits for treatment of nicotine dependence.
Discussion
These data demonstrate the ability of advanced practice pharmacists in multiple locations within the federal sector to improve targeted clinical outcomes in patients with varying diseases. These results are strengthened by their varied origins as well as the improvements observed across the board. Limitations include the general lack of a comparable dataset, manual method of selfreporting by the individual facilities, and the relatively limited array of diseases reported. Although NCPS-certified pharmacists are currently providing care for patients with hepatitis C, asthma, seizure, pain and other diseases not reported here, there are insufficient data collected for FYs 2016 and 2017 to merit inclusion within this report.
Pharmacists are trusted, readily available medication experts. In a clinical role, NCPS-certified pharmacists have increased access to primary care services and demonstrated beneficial impact on important health outcomes as exhibited by the data reported above. Clinical pharmacy is a growing field, and NCPS has displayed continual growth in both the number of NCPS-certified pharmacists and the number of patient encounters performed by these providers. As more pharmacists in all settings collaborate with medical providers to offer high-quality clinical care, these providers will have more opportunity to delegate disease management. Continued reporting of clinical pharmacy outcomes is expected to increase confidence in pharmacists as primary care providers, increase utilization of pharmacy clinical services, and assist in easing the burden of primary care provider shortages across our nation.
Although these outcomes indicate demonstrable benefit in patient-centered outcomes, the need for ongoing assessment and continued improvement is not obviated. Future efforts may benefit from a comparison of alternative approaches to better facilitate the establishment of best practices. Alignment of clinical outcomes with the Centers for Medicare and Medicaid Services (CMS) Electronic Clinical Quality Measures, where applicable, also may prove beneficial by automating the reporting process and thereby decreasing the burden of reporting as well as providing an avenue for standard comparison across multiple populations. Clinical pharmacy interventions have positive outcomes based on the NCPS model, and the NCPS Committee invites other clinical settings to report outcomes data with which to compare.
Conclusion
The NCPS Committee has documented positive outcomes of clinical pharmacy intervention and anticipates growth of the pharmacy profession as additional states and health systems recognize the capacity of the pharmacist to provide high-quality, multidisciplinary patient care. Clinical pharmacists are prepared to address critical health care needs as the US continues to face a PCP shortage.2 The NCPS Committee challenges those participating in clinical pharmacy practice to report outcomes to amplify this body of evidence.
Acknowledgments
NCPS-certified pharmacists provided the outcomes detailed in this report. For document review and edits: Federal Bureau of Prison Publication Review Workgroup; RADM Ty Bingham, USPHS; CAPT Cindy Gunderson, USPHS; CAPT Kevin Brooks, USPHS.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
1. Buttorff C, Ruder T, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: Rand Corp; 2017.
2. Dall T, West T, Chakrabarti R, Reynolds R, Iacobucci W. The complexities of physician supply and demand: projections from 2016 to 2030, 2018 update. Association of American Medical Colleges. March 2018.
3. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General 2011. https://www .accp.com/docs/positions/misc/improving_patient_and _health_system_outcomes.pdf. Updated December 2011. Accessed September 11, 2019.
4. Lip G, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation. CHEST guideline and Expert Panel Report. Chest. 2018;154(5):1121-1201.
5. Babb S, Marlarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464.
Advancing Order Set Design
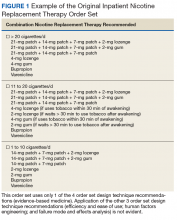
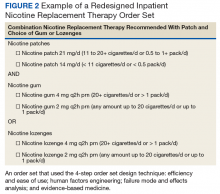
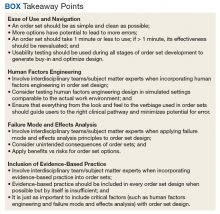
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
Long-term opioid use more common in hidradenitis suppurativa
, in a retrospective cohort study.
“These results suggest that periodic assessment of pain and screening for long-term opioid use may be warranted, particularly among patients who are older, who smoke tobacco, or who have depression and other medical comorbidities,” wrote the authors of the study (JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610).
Researchers led by Sarah Reddy, BA, of the Zucker School of Medicine at Hofstra/ Northwell, New Hyde Park, N.Y., used data from a health-care database that represents an estimated 17% of the U.S. population. They focused on opioid-naive adults who were in the database for at least 3 years from 2008-2018 and monitored whether they began opioid use and then maintained use for at least 1 year.
Nearly 829,000 patients were in the control group, and 22,277 were in the HS group. The mean age of those with HS was 41 years, 76% were women, and 59% were white.
Over 1 year, the crude incidence of long-term opioid use among HS patients who were opioid naive was 0.33%, compared with 0.14% of controls (P less than .001).
An analysis, adjusted for potential confounding factors, found that compared with controls, those with HS were more likely to develop long-term opioid use (odds ratio [OR], 1.53, 95% confidence interval, 1.20-1.95; P less than .001). In the adjusted analysis, long-term opioid use was increased among those in the HS group who had ever smoked tobacco (OR, 3.64, 95% CI, 2.06-6.41; P less than .001), compared with patients with HS who had never smoked; and those who had a history of depression (OR, 1.97, 95% CI, 1.21-3.19; P = .006), compared with HS patients who had not had depression.
The risk of long-term opioid use among those with HS increased by 2% with each additional year in age.
In addition, 5% of patients with HS and long-term opioid use were diagnosed with opioid use disorder over the study period. “Sex, race/ethnicity, disease duration, established dermatologic care, alcohol abuse, and nonopioid substance abuse were not associated with increased risk of long-term opioid use among patients with HS,” the authors wrote.
Emphasizing that these results “should not further stigmatize” people with HS, they said, “our hope is that the medical community, including dermatologists, will further embrace and engage in an integrated care plan that comprehensively supports the needs of patients with HS, including pain management.”
Future research, they added, “should include evaluating the association between disease severity and risk of opioid use, the role of disease-modifying therapies in reducing opioid use, and the development of effective and appropriate multimodal pain management strategies for HS.”
An educational grant to a study author from AbbVie partially funded the study. No other study funding was reported. Ms. Reddy had no disclosures; one author disclosed having received grants and personal fees from AbbVie and UCB during the study.
SOURCE: Reddy S et al. JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610.
, in a retrospective cohort study.
“These results suggest that periodic assessment of pain and screening for long-term opioid use may be warranted, particularly among patients who are older, who smoke tobacco, or who have depression and other medical comorbidities,” wrote the authors of the study (JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610).
Researchers led by Sarah Reddy, BA, of the Zucker School of Medicine at Hofstra/ Northwell, New Hyde Park, N.Y., used data from a health-care database that represents an estimated 17% of the U.S. population. They focused on opioid-naive adults who were in the database for at least 3 years from 2008-2018 and monitored whether they began opioid use and then maintained use for at least 1 year.
Nearly 829,000 patients were in the control group, and 22,277 were in the HS group. The mean age of those with HS was 41 years, 76% were women, and 59% were white.
Over 1 year, the crude incidence of long-term opioid use among HS patients who were opioid naive was 0.33%, compared with 0.14% of controls (P less than .001).
An analysis, adjusted for potential confounding factors, found that compared with controls, those with HS were more likely to develop long-term opioid use (odds ratio [OR], 1.53, 95% confidence interval, 1.20-1.95; P less than .001). In the adjusted analysis, long-term opioid use was increased among those in the HS group who had ever smoked tobacco (OR, 3.64, 95% CI, 2.06-6.41; P less than .001), compared with patients with HS who had never smoked; and those who had a history of depression (OR, 1.97, 95% CI, 1.21-3.19; P = .006), compared with HS patients who had not had depression.
The risk of long-term opioid use among those with HS increased by 2% with each additional year in age.
In addition, 5% of patients with HS and long-term opioid use were diagnosed with opioid use disorder over the study period. “Sex, race/ethnicity, disease duration, established dermatologic care, alcohol abuse, and nonopioid substance abuse were not associated with increased risk of long-term opioid use among patients with HS,” the authors wrote.
Emphasizing that these results “should not further stigmatize” people with HS, they said, “our hope is that the medical community, including dermatologists, will further embrace and engage in an integrated care plan that comprehensively supports the needs of patients with HS, including pain management.”
Future research, they added, “should include evaluating the association between disease severity and risk of opioid use, the role of disease-modifying therapies in reducing opioid use, and the development of effective and appropriate multimodal pain management strategies for HS.”
An educational grant to a study author from AbbVie partially funded the study. No other study funding was reported. Ms. Reddy had no disclosures; one author disclosed having received grants and personal fees from AbbVie and UCB during the study.
SOURCE: Reddy S et al. JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610.
, in a retrospective cohort study.
“These results suggest that periodic assessment of pain and screening for long-term opioid use may be warranted, particularly among patients who are older, who smoke tobacco, or who have depression and other medical comorbidities,” wrote the authors of the study (JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610).
Researchers led by Sarah Reddy, BA, of the Zucker School of Medicine at Hofstra/ Northwell, New Hyde Park, N.Y., used data from a health-care database that represents an estimated 17% of the U.S. population. They focused on opioid-naive adults who were in the database for at least 3 years from 2008-2018 and monitored whether they began opioid use and then maintained use for at least 1 year.
Nearly 829,000 patients were in the control group, and 22,277 were in the HS group. The mean age of those with HS was 41 years, 76% were women, and 59% were white.
Over 1 year, the crude incidence of long-term opioid use among HS patients who were opioid naive was 0.33%, compared with 0.14% of controls (P less than .001).
An analysis, adjusted for potential confounding factors, found that compared with controls, those with HS were more likely to develop long-term opioid use (odds ratio [OR], 1.53, 95% confidence interval, 1.20-1.95; P less than .001). In the adjusted analysis, long-term opioid use was increased among those in the HS group who had ever smoked tobacco (OR, 3.64, 95% CI, 2.06-6.41; P less than .001), compared with patients with HS who had never smoked; and those who had a history of depression (OR, 1.97, 95% CI, 1.21-3.19; P = .006), compared with HS patients who had not had depression.
The risk of long-term opioid use among those with HS increased by 2% with each additional year in age.
In addition, 5% of patients with HS and long-term opioid use were diagnosed with opioid use disorder over the study period. “Sex, race/ethnicity, disease duration, established dermatologic care, alcohol abuse, and nonopioid substance abuse were not associated with increased risk of long-term opioid use among patients with HS,” the authors wrote.
Emphasizing that these results “should not further stigmatize” people with HS, they said, “our hope is that the medical community, including dermatologists, will further embrace and engage in an integrated care plan that comprehensively supports the needs of patients with HS, including pain management.”
Future research, they added, “should include evaluating the association between disease severity and risk of opioid use, the role of disease-modifying therapies in reducing opioid use, and the development of effective and appropriate multimodal pain management strategies for HS.”
An educational grant to a study author from AbbVie partially funded the study. No other study funding was reported. Ms. Reddy had no disclosures; one author disclosed having received grants and personal fees from AbbVie and UCB during the study.
SOURCE: Reddy S et al. JAMA Dermatol. 2019 Sep 11. doi: 10.1001/jamadermatol.2019.2610.
FROM JAMA DERMATOLOGY
Assessing decisional capacity in patients with substance use disorders
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
Ms. B, age 31, is brought to the emergency department (ED) via ambulance after emergency medical technicians used naloxone nasal spray to revive her following an overdose on heroin. She reports daily IV heroin use for the last 4 years as well as frequent use of other illicit substances, including marijuana and alprazolam, for which she does not have
How can you determine if Ms. B has the capacity to make decisions regarding her care?
Decisional capacity is defined as a patient’s ability to use information about an illness and the proposed treatment options to make a choice that is congruent with one’s own values and preferences.1 Determining whether a patient has adequate capacity to make decisions regarding their care is an inherent aspect of all clinician-patient interactions.
Published reports have focused on the challenges clinicians face when assessing decisional capacity in patients with psychiatric and cognitive disorders. However, there is little evidence about assessing decisional capacity in patients with substance use disorders (SUDs), even though increasing numbers of patients with SUDs are presenting to EDs2 and being admitted as inpatients in general hospitals.3 In this article, I discuss:
- the biologic basis for impaired decision-making in patients with SUDs
- common substance use–related conditions that may impact a patient’s decisional capacity
- the clinical challenges and legal considerations clinicians face when assessing decisional capacity in patients with SUDs
- how to assess decisional capacity in such patients.
Decisional capacity vs competence
“Capacity” and “competence” are not the same. Decisional capacity, which refers to the ability to make decisions, is a clinical construct that is determined by clinicians and is generally used in the acute clinical setting. Because cognition is the main determinant of capacity, conditions or treatments that affect cognition can impair an individual’s decision-making capacity.1 Decisional capacity is not a global concept but a decision-specific one, subject to fluctuations depending on the time and the nature of the decision at hand. Therefore, requests for determination of decisional capacity in the clinical setting should be specific to an individual decision or set of decisions.
In contrast, competence is an enduring legal determination of incapacitation, typically made by a probate judge. It refers to the ability of an individual to perform actions needed to put decisions into effect. Decisional capacity as assessed by a clinician often serves as the basis for petitions submitted for the purpose of competency adjudication by the judicial system.
A biologic basis for impaired decision-making?
Jeste and Saks4 suggested that addiction itself is characterized by impaired decision-making because individuals keep using a substance despite experiencing recurrent physical, psychologic, or social problems caused or worsened by the substance. Several studies suggest there may be a biologic basis for impaired decision-making in these patients, even in the absence of severe psychiatric or cognitive disorders.
Continue to: Bechara and Damasio found...
Bechara and Damasio5 found that the decision-making impairment seen in some patients with SUDs was similar to that observed in patients who have lesions of the ventromedial prefrontal cortex. In both groups of patients, the impaired decision-making was characterized by a preference to opt for high immediate reward despite even higher future losses.
These deficits were also observed by Grant et al.6 In this study, patients with SUDs displayed markedly impaired performance on the Gambling Task, which examines decisions that result in long-term losses that exceed short-term gains. However, patients with SUDs performed similarly to controls on the Wisconsin Card Sorting Test, which evaluates the ability to form abstract concepts and to shift from established response sets.
MacDonald et al7 used a laboratory experiment and 2 field studies to test the hypothesis that alcohol affects attitudes and intentions toward drinking and driving. Their findings support the concept that alcohol intoxication decreases cognitive capacity such that people are more likely to attend to only the most salient cues.7
Whether the impairment documented in such studies is a contributing factor in addiction or is a result of addiction remains uncertain. While individuals with SUDs may have some level of impairment in decision-making in general, particularly in regard to their substance use, their decisional capacity on specific clinical decisions should be assessed carefully. In a study of 300 consecutive psychiatric consultations for decisional capacity at an urban hospital, Boettger et al8 found that 41% were related to SUDs. Of these, 37% were found to have impaired decisional capacity.
Impaired decision-making in patients with SUDs may specifically pertain to choices related to their addiction, including9:
- consent for addiction treatment
- consistency in maintaining a choice of recovery
- changing values regarding treatment over time
- capacity to participate in addiction research involving the use of addictive substances.
Continue to: It is important to recognize...
It is important to recognize that this impairment may not necessarily translate into altered decisional capacity regarding other health care decisions, such as consenting to surgery or other necessary medical interventions.9
Substance-related disorders that affect decisional capacity
Substance-related syndromes can affect mood, reality testing, and/or cognitive function, thereby directly impacting a patient’s decisional capacity. Substance-related syndromes can be divided into 2 categories: 1) disorders resulting from the direct effects of the substance, and 2) secondary disorders resulting from/or associated with substance use.
Disorders resulting from the direct effects of the substance
Temporary/reversible incapacitation
- Acute intoxication or intoxication delirium may be the most frequent type of temporary incapacitation. It can result from toxic levels of licit or illicit substances; alcohol is likely the most frequent offending agent. Although some individuals who are intoxicated may appear to be alert, oriented, and able to engage in lengthy conversations, the majority do not possess adequate decisional capacity.10
- Withdrawal delirium, associated with longstanding alcohol, sedative-hypnotic, or barbiturate dependence, is typically prolonged, but usually resolves, either spontaneously or with treatment. Although most deliria resolve once the underlying etiology is corrected, vulnerable individuals may experience irreversible cognitive impairment and permanent decisional incapacitation.11,12
- Severe substance-induced depressive disorders, especially if accompanied by frank psychotic symptoms or severe depressive distortions of reality, may result in decisional incapacity. Substance abuse treatment that incorporates multiple strategies, sometimes in conjunction with pharmacotherapy to manage depression, should lead to sufficient recovery and restoration of decisional capacity.
- Transient psychotic disorders such as those associated with the use of stimulants are often treatable. Patients may recover decisional capacity spontaneously or with treatment.
Permanent incapacitation
- Dementia is associated with substance use, particularly alcohol use.13 For a patient who develops dementia, no appreciable recovery can be expected, even with prolonged abstinence.
- Persistent amnestic disorders (eg, Korsakoff syndrome) resulting from undiagnosed or untreated severe thiamine deficiency (Wernicke’s encephalopathy). Although an isolated Korsakoff syndrome consists primarily of anterograde amnesia, these patients may experience additional cognitive impairment resulting from years of alcohol consumption or associated with other neurodegenerative processes, and therefore are sufficiently impaired and lack decisional capacity. Even in the absence of such concomitant cognitive deficits, a very severe anterograde amnestic disorder directly impacts a patient’s capacity to perform the necessary tasks required to give informed consent. The inability to consolidate information about new medical developments, treatments, and procedures, even when they are thoroughly explained by the medical team, can pose serious challenges. For example, a patient may protest to being taken to surgery because he/she does not recall signing a consent form the previous day.
- Enduring severe and treatment-refractory psychotic disorders associated with drug use, specifically stimulants, can result in permanent incapacitation similar to that seen in severe primary psychotic disorders (such as treatment-resistant schizophrenia).
- Hepatic encephalopathy may be seen in patients with advanced cirrhosis of the liver (due to hepatitis C resulting from IV drug use, and/or alcohol use). In late stages of cirrhosis, the confusional state patients experience may become severe and may no longer be reversible unless liver transplantation is available and successful. This would therefore constitute a basis for permanent decisional incapacitation.
- Human immunodeficiency virus encephalitis or dementia can result from IV drug use.
Continue to: Clinical challenges
Clinical challenges
In intensive care settings, where a patient with a SUD may be treated for acute life-threatening intoxication or severe withdrawal delirium, an assumption of decisional incapacitation often exists as a result of medical acuity and impaired mentation. In these situations, treatment usually proceeds with consent obtained from next-of-kin, a guardian, or an administrative (hospital) authority when other substitute decision makers are unavailable or unwilling. In such cases, psychiatric consultation can play a dual role in documenting the patient’s decisional capacity and also in contributing to the care of patients with SUDs.
It is critical to perform a cognitive evaluation and mental status examination in a medically compromised patient with an SUD. Unfortunately, serious cognitive disorders can often be concealed by a superficially jovial or verbally skilled patient, or by an uncooperative individual who refuses to engage in a thorough conversation with his/her clinicians. These scenarios present significant challenges and may result in missed opportunities for care or premature discharges. Negative countertransference by clinicians toward patients with SUDs may also promote poor outcomes. For difficult cases, legal and ethical consultations may help mitigate risk and guide management approaches (Box14).
Box
The legal system rarely views patients with substance use disorders (SUDs) as lacking decisional capacity in the absence of overt psychiatric or cognitive deficits. The penal system offers little if any mitigation of liability on account of addiction in civil or criminal cases. On the contrary, intoxication is an aggravating factor in such settings. Despite extensive literature that questions the “free will,” accountability, and responsibility of patients with SUDs, the legal system takes an “all-or-none” approach to this issue. It assumes free choice and accountability for patients with SUDs, except when a clear superimposed psychiatric or cognitive disorder (such as psychosis or dementia) exists. Rarely, some jurisdictions may allow for mental health commitments on account of severe and persistent addictive behaviors that clearly pose a risk to the individual or to society, implicitly recognizing that incapacitation can result from severe addiction. Nevertheless, a finding of imminent or impending dangerousness is generally required for such commitments to be justified.
In other situations, individual health care settings may resort to local hospital policies that allow impaired patients with SUDs with a clearly altered mental status to be detained for the purpose of completing medical treatment. Presumably, discharge would occur when the medical and psychiatric acuity has resolved (often under the umbrella of a “Medical Hold” policy). Jain et al14 suggested that although such commitment laws for patients with SUDs may be appealing to some people, especially family members, specific statutes and their implementation are highly variable; the deprivation of liberty raises ethical concerns; and outcome data are limited. Conversely, most states either do not have such legislation, or rarely enforce it.
How to assess decisional capacity
A direct conclusion of incapacity in an individual cannot be determined solely on the knowledge of the patient having a SUD-related clinical condition. (The possible exception to this may be a patient with severe dementia.)
- understand the decision at hand
- discuss its benefits and risks
- describe alternatives
- demonstrate an appreciation of the implications of treatment or lack thereof
- communicate a clear and consistent choice.
Continue to: While most clinicians...
While most clinicians rely on a psychiatric interview (with or without a cognitive examination) to make these determinations, several instruments have been developed to aid these evaluations, such as the MacArthur Competence Assessment Tool for Treatment (Mac-CAT-T).15 In patients with potentially reversible incapacitating conditions, serial examinations over time, especially re-evaluation when a patient has achieved and maintained sobriety, may be necessary and helpful.
The Table offers a guide to assessing decisional capacity in a patient with an SUD.
Who should conduct the assessment?
Mental health professionals—usually psychiatrists or psychologists—are consulted when there is uncertainty about a patient’s decisional capacity, and when a more thorough mental status examination is warranted to formulate an informed opinion.16 Unfortunately, this typically occurs only if a patient refuses treatment or demands to be discharged before treatment has been completed, or there is a high level of risk to the patient or others after discharge.
In acute settings, when a patient consents to treatment, a psychiatric consultation regarding decisional capacity is rarely requested. While it is often tempting for medical or surgical teams to proceed with an intervention in a cooperative patient who willingly signs a consent form without a formal assessment of his/her decisional capacity, doing so raises challenging ethical and legal questions in the event of an adverse outcome. It is therefore prudent to strongly recommend that medical and surgical colleagues obtain a psychiatric consultation when an individual’s decisional capacity is uncertain, especially when a patient is known to have a psychiatric or neurocognitive disorder, or exhibits evidence of recent mental status changes. In cases of potentially reversible impairment (eg, delirium, psychosis, or acute anxiety), targeted interventions may help restore capacity and allow treatment to proceed.
No jurisdictions mandate that the determination of decisional capacity should be made exclusively by a mental health professional. Any treating health care professional (usually the attending physician) can make a determination of decisional capacity in scenarios where there is no overt evidence the patient has a mental or cognitive disorder and the patient is communicating clear and reasoned choices, or when a patient is profoundly impaired and no meaningful communication can take place.
Continue to: CASE CONTINUED
CASE CONTINUED
The emergency physician requests a psychiatric consultation. You assess Ms. B’s decisional capacity using the Mac-CAT-T along with a standard psychiatric evaluation. Her score of 14 reflects that she is able to understand the risks associated with her opioid use, and although irritated by engaging in such a discussion, is capable of reasoning through the various medical and psychosocial aspects of her addiction, and shows moderate appreciation of the impact of her choices on her future and that of significant others. The psychiatric evaluation fails to elicit any substantial mood, anxiety, or psychotic disorders associated with/or resulting from her addiction, and her cognitive examination is within normal limits. She does not exhibit severe withdrawal and is not delirious on examination. Finally, she did not harbor thoughts of intentional harm to self or others and is not deemed imminently dangerous.
You document that in your opinion, despite Ms. B’s unfortunate choices and questionable judgment, she does have the capacity to make informed decisions regarding her care and could be released against medical advice if she so chooses, while providing her with information about available resources should she decide to seek rehabilitation in the future.
An increasingly common scenario
Decisional capacity assessment in patients with SUDs is an increasingly common reason for psychiatric consultations. Primary and secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. The same principles that guide the assessment of decisional capacity in patients with other psychiatric or cognitive disorders should be applied to compromised individuals with SUDs. In challenging cases, a skilled psychiatric evaluation that is supported by a thorough cognitive examination and, when required, complemented by a legal or ethical consultation, can help clinicians make safe and judicious decisions.
Bottom Line
Assessing the decisional capacity of a patient with a substance use disorder can be challenging. Primary or secondary conditions related to substance use can affect a patient’s decisional capacity on a temporary or permanent basis. A skilled psychiatric evaluation that includes a thorough cognitive examination and is complemented by legal or ethical consultation can help in making judicious decisions.
Related Resources
- Tan SY. Determining patients’ decisional capacity. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/137939/practice-management/determining-patients-decisional-capacity. Published May 10, 2017.
- Sorrentino R. Performing capacity evaluations: What’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Drug Brand Names
Alprazolam • Xanax
Naloxone nasal spray • Narcan
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
1. Karlawish K. Assessment of decision-making capacity in adults. UpToDate. https://www.uptodate.com/contents/assessment-of-decision-making-capacity-in-adults. Updated July 2019. Accessed August 19, 2019.
2. Owens PL, Mutter R, Stocks C. Mental health and substance abuse-related emergency department visits among adults, 2007. HCUP Statistical Brief #92. https://www.ncbi.nlm.nih.gov/books/NBK52659/pdf/Bookshelf_NBK52659.pdf. Published July 2010. Accessed August 19, 2019.
3. Smothers BA, Yahr HT. Alcohol use disorder and illicit drug use in admissions to general hospitals in the United States. Am J Addict. 2005;14(3):256-267.
4. Jeste DV, Saks E. Decisional capacity in mental illness and substance use disorders: empirical database and policy implications. Behav Sci Law. 2006;24(4):607-628.
5. Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40(10):1675-1689.
6. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38(8):1180-1187.
7. MacDonald TK, Zanna MP, Fong GT. Decision making in altered states: effects of alcohol on attitudes toward drinking and driving. J Pers Soc Psychol. 1995;68(6):973-985.
8. Boettger S, Bergman M, Jenewein J, et al. Assessment of decisional capacity: prevalence of medical illness and psychiatric comorbidities. Palliat Support Care. 2015;13(5):1275-1281.
9. Charland LC. Chapter 6: Decision-making capacity and responsibility in addiction. In: Poland J, Graham G. Addiction and responsibility. Cambridge, MA: MIT Press Scholarship Online; 2011:139-158.
10. Martel ML, Klein LR, Miner JR, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. 2018;36(1):18-23.
11. MacLullich AM, Beaglehole A, Hall RJ, et al. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21(1):30-42.
12. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316.
13. Rehm J, Hasan OSM, Black SE, et al. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11(1):1.
14. Jain A, Christopher P, Appelbaum PS. Civil commitment for opioid and other substance use disorders: does it work? Psychiatr Serv. 2018;69(4):374-376.
15. Grisso T, Appelbaum PS. Chapter 6: Using the MacArthur competence assessment tool – treatment. In: Grisso T, Appelbaum PS. Assessing competence to consent to treatment: a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998:101-126.
16. Hazelton LD, Sterns GL, Chisholm T. Decision-making capacity and alcohol abuse: clinical and ethical considerations in personal care choices. Gen Hosp Psychiatry. 2003;25(2):130-135.
Autism, pain, and the NMDA receptor
Ms. G, a 36-year-old woman, presented to the emergency department (ED) requesting a neurologic evaluation. She told clinicians she had “NMDA receptor encephalitis.”
Ms. G reported successful self-treatment of “life-long” body pain that was precipitated by multiple external stimuli (food, social encounters, interpersonal conflict, etc.). Through her own research, she had learned that both ketamine and magnesium could alter nociception in rats through N-methyl-
In the ED, Ms. G had a labile affect, pressured speech, and flight of ideas. She denied any history of psychiatric treatment, suicide attempts, or substance abuse. Ms. G’s family reported she had been unusually social, talkative, and impulsive. She was admitted to the inpatient psychiatric unit with a diagnosis of mania.
On psychiatric evaluation, Ms. G was grandiose, irritable, and perseverative about her aberrant symptoms. She felt she did not experience the world as other people did, but found relief from her chronic pain after taking Delsym. She was not taking other medications. Ms. G did not report a family history of bipolar disorder or psychosis. Her laboratory results, including a comprehensive metabolic panel, complete blood count, lipid panel, thyroid studies, urine drug screening, and urinalysis, were unremarkable. Her blood pressure was mildly elevated (141/82 mm Hg).
Ms. G’s eventual diagnosis was substance-induced mania (DXM). The DXM-containing cough syrup and magnesium were discontinued in the hospital. She was stabilized on lithium extended-release, 900 mg/d (blood level 0.8 mmol/L), and olanzapine, 10 mg/d at bedtime. However, after discharge, Ms. G resumed using Delsym, which resulted in 3 subsequent psychiatric hospitalizations for mania during the next year.
I first treated Ms. G as an outpatient after her second hospitalization. At that point, she was stable. Her mental status was calm and cooperative, and she had a linear thought process. At her baseline, in the absence of mania, she had a blunted affect. She understood that DXM caused her to have manic symptoms, but she continued to believe that Delsym and magnesium cured her physical suffering and social inhibition. I noticed Ms. G would use figurative language inappropriately. I later learned she had sensitivities to food textures and a specialized interest in electronics. Because of this, I suspected Ms. G was on the autism spectrum; she met several DSM-5 criteria for autism spectrum disorder (ASD), particularly deficits in social-emotional reciprocity, highly restricted interests, and hyperreactivity to sensory input.
Upon routine lab screening, Ms. G was found to have hypothyroidism, with a thyroid-stimulating hormone level of 6.67 mcIU/mL. This resolved after discontinuing lithium. Olanzapine caused adverse metabolic effects and also was discontinued. Ms. G remained euthymic without any mood-stabilizing medication, except during periods when she abused DXM, when she would again become manic. Eventually, her motivation to avoid hospitalization would promote her abstinence.
Continue to: Implications of NMDA receptor antagonism
Implications of NMDA receptor antagonism
The use of ketamine as an NMDA receptor antagonist for treating depression and other psychiatric illnesses has gained momentum. Esketamine, the S-enantiomer of racemic ketamine, is now available as an FDA-approved intranasal formulation for treatment-resistant depression. Ketamine stops afferent nociception to the brain and is used as an analgesic (at low concentrations) and anesthetic (at high concentrations).1
Dextromethorphan is abused as a recreational drug because at high doses it works similarly to both ketamine and phencyclidine. Individuals who abuse DXM can develop psychosis, motor/cognitive impairment, agitation, fevers, hypertension, tachycardia, and death.2 In patients with ASD, researchers have identified genetic variations of NMDA receptors that are linked to dysfunction of these receptors.3 In animal models, as well as in humans, researchers have found that suppression or excitation of the NMDA receptor can ameliorate ASD symptoms, including social withdrawal and repetitive behaviors.3
Many individuals with ASD suffer from sensory abnormalities, including a reduced sensitivity to pain or a crippling sensitivity to various stimuli. Patients with ASD may have difficulty describing these abnormalities, and as a result, they may be misdiagnosed. One case report described a 15-year-old girl diagnosed with social anxiety and chronic generalized pain when in social situations.4 Pediatric rheumatologists had diagnosed her with “amplified pain syndrome.” When she presented to a mental health clinic for a neurodevelopmental evaluation, she explained to clinicians how she simply “did not ‘get’ people; they are just empty shells” and subsequently was given a diagnosis of ASD.4
In psychiatric patients who have comorbid substance use disorders, it is vital for clinicians to not only detect the presence of substance misuse, but also to understand what drives the patient toward abuse. Ms. G’s case, with its combination of substance abuse and ASD, illustrates the importance of listening to our patients for more precise diagnostic formulations, which then shape our treatment recommendations.
1. Vadivelu N, Schermer E, Kodumudi V, et al. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
2. Martinak B, Bolis R, Black J, et al. Dextromethorphan in cough syrup: the poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Lee E, Choi S, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8-13.
4. Clarke C. Autism spectrum disorder and amplified pain. Case Rep Psychiatry. 2015;2015:930874. doi: 10.1155/2015/930874.
Ms. G, a 36-year-old woman, presented to the emergency department (ED) requesting a neurologic evaluation. She told clinicians she had “NMDA receptor encephalitis.”
Ms. G reported successful self-treatment of “life-long” body pain that was precipitated by multiple external stimuli (food, social encounters, interpersonal conflict, etc.). Through her own research, she had learned that both ketamine and magnesium could alter nociception in rats through N-methyl-
In the ED, Ms. G had a labile affect, pressured speech, and flight of ideas. She denied any history of psychiatric treatment, suicide attempts, or substance abuse. Ms. G’s family reported she had been unusually social, talkative, and impulsive. She was admitted to the inpatient psychiatric unit with a diagnosis of mania.
On psychiatric evaluation, Ms. G was grandiose, irritable, and perseverative about her aberrant symptoms. She felt she did not experience the world as other people did, but found relief from her chronic pain after taking Delsym. She was not taking other medications. Ms. G did not report a family history of bipolar disorder or psychosis. Her laboratory results, including a comprehensive metabolic panel, complete blood count, lipid panel, thyroid studies, urine drug screening, and urinalysis, were unremarkable. Her blood pressure was mildly elevated (141/82 mm Hg).
Ms. G’s eventual diagnosis was substance-induced mania (DXM). The DXM-containing cough syrup and magnesium were discontinued in the hospital. She was stabilized on lithium extended-release, 900 mg/d (blood level 0.8 mmol/L), and olanzapine, 10 mg/d at bedtime. However, after discharge, Ms. G resumed using Delsym, which resulted in 3 subsequent psychiatric hospitalizations for mania during the next year.
I first treated Ms. G as an outpatient after her second hospitalization. At that point, she was stable. Her mental status was calm and cooperative, and she had a linear thought process. At her baseline, in the absence of mania, she had a blunted affect. She understood that DXM caused her to have manic symptoms, but she continued to believe that Delsym and magnesium cured her physical suffering and social inhibition. I noticed Ms. G would use figurative language inappropriately. I later learned she had sensitivities to food textures and a specialized interest in electronics. Because of this, I suspected Ms. G was on the autism spectrum; she met several DSM-5 criteria for autism spectrum disorder (ASD), particularly deficits in social-emotional reciprocity, highly restricted interests, and hyperreactivity to sensory input.
Upon routine lab screening, Ms. G was found to have hypothyroidism, with a thyroid-stimulating hormone level of 6.67 mcIU/mL. This resolved after discontinuing lithium. Olanzapine caused adverse metabolic effects and also was discontinued. Ms. G remained euthymic without any mood-stabilizing medication, except during periods when she abused DXM, when she would again become manic. Eventually, her motivation to avoid hospitalization would promote her abstinence.
Continue to: Implications of NMDA receptor antagonism
Implications of NMDA receptor antagonism
The use of ketamine as an NMDA receptor antagonist for treating depression and other psychiatric illnesses has gained momentum. Esketamine, the S-enantiomer of racemic ketamine, is now available as an FDA-approved intranasal formulation for treatment-resistant depression. Ketamine stops afferent nociception to the brain and is used as an analgesic (at low concentrations) and anesthetic (at high concentrations).1
Dextromethorphan is abused as a recreational drug because at high doses it works similarly to both ketamine and phencyclidine. Individuals who abuse DXM can develop psychosis, motor/cognitive impairment, agitation, fevers, hypertension, tachycardia, and death.2 In patients with ASD, researchers have identified genetic variations of NMDA receptors that are linked to dysfunction of these receptors.3 In animal models, as well as in humans, researchers have found that suppression or excitation of the NMDA receptor can ameliorate ASD symptoms, including social withdrawal and repetitive behaviors.3
Many individuals with ASD suffer from sensory abnormalities, including a reduced sensitivity to pain or a crippling sensitivity to various stimuli. Patients with ASD may have difficulty describing these abnormalities, and as a result, they may be misdiagnosed. One case report described a 15-year-old girl diagnosed with social anxiety and chronic generalized pain when in social situations.4 Pediatric rheumatologists had diagnosed her with “amplified pain syndrome.” When she presented to a mental health clinic for a neurodevelopmental evaluation, she explained to clinicians how she simply “did not ‘get’ people; they are just empty shells” and subsequently was given a diagnosis of ASD.4
In psychiatric patients who have comorbid substance use disorders, it is vital for clinicians to not only detect the presence of substance misuse, but also to understand what drives the patient toward abuse. Ms. G’s case, with its combination of substance abuse and ASD, illustrates the importance of listening to our patients for more precise diagnostic formulations, which then shape our treatment recommendations.
Ms. G, a 36-year-old woman, presented to the emergency department (ED) requesting a neurologic evaluation. She told clinicians she had “NMDA receptor encephalitis.”
Ms. G reported successful self-treatment of “life-long” body pain that was precipitated by multiple external stimuli (food, social encounters, interpersonal conflict, etc.). Through her own research, she had learned that both ketamine and magnesium could alter nociception in rats through N-methyl-
In the ED, Ms. G had a labile affect, pressured speech, and flight of ideas. She denied any history of psychiatric treatment, suicide attempts, or substance abuse. Ms. G’s family reported she had been unusually social, talkative, and impulsive. She was admitted to the inpatient psychiatric unit with a diagnosis of mania.
On psychiatric evaluation, Ms. G was grandiose, irritable, and perseverative about her aberrant symptoms. She felt she did not experience the world as other people did, but found relief from her chronic pain after taking Delsym. She was not taking other medications. Ms. G did not report a family history of bipolar disorder or psychosis. Her laboratory results, including a comprehensive metabolic panel, complete blood count, lipid panel, thyroid studies, urine drug screening, and urinalysis, were unremarkable. Her blood pressure was mildly elevated (141/82 mm Hg).
Ms. G’s eventual diagnosis was substance-induced mania (DXM). The DXM-containing cough syrup and magnesium were discontinued in the hospital. She was stabilized on lithium extended-release, 900 mg/d (blood level 0.8 mmol/L), and olanzapine, 10 mg/d at bedtime. However, after discharge, Ms. G resumed using Delsym, which resulted in 3 subsequent psychiatric hospitalizations for mania during the next year.
I first treated Ms. G as an outpatient after her second hospitalization. At that point, she was stable. Her mental status was calm and cooperative, and she had a linear thought process. At her baseline, in the absence of mania, she had a blunted affect. She understood that DXM caused her to have manic symptoms, but she continued to believe that Delsym and magnesium cured her physical suffering and social inhibition. I noticed Ms. G would use figurative language inappropriately. I later learned she had sensitivities to food textures and a specialized interest in electronics. Because of this, I suspected Ms. G was on the autism spectrum; she met several DSM-5 criteria for autism spectrum disorder (ASD), particularly deficits in social-emotional reciprocity, highly restricted interests, and hyperreactivity to sensory input.
Upon routine lab screening, Ms. G was found to have hypothyroidism, with a thyroid-stimulating hormone level of 6.67 mcIU/mL. This resolved after discontinuing lithium. Olanzapine caused adverse metabolic effects and also was discontinued. Ms. G remained euthymic without any mood-stabilizing medication, except during periods when she abused DXM, when she would again become manic. Eventually, her motivation to avoid hospitalization would promote her abstinence.
Continue to: Implications of NMDA receptor antagonism
Implications of NMDA receptor antagonism
The use of ketamine as an NMDA receptor antagonist for treating depression and other psychiatric illnesses has gained momentum. Esketamine, the S-enantiomer of racemic ketamine, is now available as an FDA-approved intranasal formulation for treatment-resistant depression. Ketamine stops afferent nociception to the brain and is used as an analgesic (at low concentrations) and anesthetic (at high concentrations).1
Dextromethorphan is abused as a recreational drug because at high doses it works similarly to both ketamine and phencyclidine. Individuals who abuse DXM can develop psychosis, motor/cognitive impairment, agitation, fevers, hypertension, tachycardia, and death.2 In patients with ASD, researchers have identified genetic variations of NMDA receptors that are linked to dysfunction of these receptors.3 In animal models, as well as in humans, researchers have found that suppression or excitation of the NMDA receptor can ameliorate ASD symptoms, including social withdrawal and repetitive behaviors.3
Many individuals with ASD suffer from sensory abnormalities, including a reduced sensitivity to pain or a crippling sensitivity to various stimuli. Patients with ASD may have difficulty describing these abnormalities, and as a result, they may be misdiagnosed. One case report described a 15-year-old girl diagnosed with social anxiety and chronic generalized pain when in social situations.4 Pediatric rheumatologists had diagnosed her with “amplified pain syndrome.” When she presented to a mental health clinic for a neurodevelopmental evaluation, she explained to clinicians how she simply “did not ‘get’ people; they are just empty shells” and subsequently was given a diagnosis of ASD.4
In psychiatric patients who have comorbid substance use disorders, it is vital for clinicians to not only detect the presence of substance misuse, but also to understand what drives the patient toward abuse. Ms. G’s case, with its combination of substance abuse and ASD, illustrates the importance of listening to our patients for more precise diagnostic formulations, which then shape our treatment recommendations.
1. Vadivelu N, Schermer E, Kodumudi V, et al. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
2. Martinak B, Bolis R, Black J, et al. Dextromethorphan in cough syrup: the poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Lee E, Choi S, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8-13.
4. Clarke C. Autism spectrum disorder and amplified pain. Case Rep Psychiatry. 2015;2015:930874. doi: 10.1155/2015/930874.
1. Vadivelu N, Schermer E, Kodumudi V, et al. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
2. Martinak B, Bolis R, Black J, et al. Dextromethorphan in cough syrup: the poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Lee E, Choi S, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8-13.
4. Clarke C. Autism spectrum disorder and amplified pain. Case Rep Psychiatry. 2015;2015:930874. doi: 10.1155/2015/930874.