User login
Cannabis and prenatal care
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Counseling on cannabis use in pregnancy
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
Recognizing and treating ketamine abuse
The N-methyl-
Physicians need to be aware, however, that many patients use illicit ketamine, either for recreational purposes or as self-treatment to control depressive symptoms. To help clinicians identify the signs of ketamine abuse, we discuss the adverse effects of illicit use, and suggest treatment approaches.
Ad
Ketamine can be consumed in various ways; snorting it in a powder form is a preferred route for recreational use.2 The primary disadvantage of oral use is that it increases the likelihood of nausea and vomiting.2
While ketamine is generally safe in a supervised clinical setting, approximately 2.5 million individuals use various illicit forms of ketamine—which is known as Special K and by other names—in recreational settings (eg, dance clubs) where it might be used with other substances.3 Alcohol, in particular, compounds the sedative effects of ketamine and can lead to death by overdose.
At a subanesthetic dose, ketamine can induce dissociative and/or transcendental states that are particularly attractive to those intrigued by mystical experiences, pronounced changes in perception, or euphoria.4 High doses of ketamine—relative to a commonly used recreational dose—can produce a unique “K-hole” state in which a user is unable to control his/her body and could lose consciousness.5 A K-hole state may trigger a cycle of delirium that warrants immediate clinical attention.3
Researchers have postulated that NMDA antagonism may negatively impact memory consolidation.3,6 Even more troubling is the potential for systemic injuries because illicit ketamine use may contribute to ulcerative cystitis, severely disturbed kidney function (eg, hydronephrosis), or epigastric pain.3 Chronic abuse tends to result in more systemic sequelae, affecting the bladder, kidneys, and heart. Adverse effects that require emergent care include blood in urine, changes in vision (eg, nystagmus), chest discomfort, labored breathing, agitation, seizures, and/or altered consciousness.6
Treating ketamine abuse
Treatment should be tailored to the patient’s symptoms. If the patient presents with “K-bladder” (ie, ketamine bladder syndrome), he/she may need surgical intervention or a cystectomy.4,7 Therapeutic management of K-bladder entails recognizing bladder symptoms that are specific to ketamine use, such as interstitial or ulcerative cystitis and lower urinary tract symptoms.7 Clinicians should monitor patients for increased voiding episodes during the day, voiding urgency, or a general sense of bladder fullness. Patients with K-bladder also may complain of suprapubic pain or blood in the urine.7
Continue to: Consider referring patients to...
Consider referring patients to an individualized, ketamine-specific rehabilitation program that is modeled after other substance-specific rehabilitation programs. It is critical to address withdrawal symptoms (eg, anorexia, fatigue, tremors, chills, tachycardia, nightmares, etc.). Patients undergoing ketamine withdrawal may develop anxiety and depression, with or without suicidal ideation, that might persist during a 4- to 5-day withdrawal period.8
‘Self-medicating’ ketamine users
Clinicians need to be particularly vigilant for situations in which a patient has used ketamine in an attempt to control his/her depressive symptoms. Some researchers have described ketamine as a revolutionary drug for TRD, and it is reasonable to suspect that some patients with depressive symptoms may have consulted Internet sources to learn how to self-medicate using ketamine. Patients who have consumed smaller doses of ketamine recreationally may have developed a tolerance in which the receptors are no longer responsive to the effects at that dose, and therefore might not respond when given ketamine in a clinical setting. Proper history taking and patient education are essential for these users, and clinicians may need to develop a personalized therapeutic plan for ketamine administration. If, on the other hand, a patient has a history of chronic ketamine use (perhaps at high doses), depression may occur secondary to this type of ketamine abuse. For such patients, clinicians should explore alternative treatment modalities, such as transcranial magnetic stimulation.
1. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
2. Davis K. What are the uses of ketamine? Medical News Today. https://www.medicalnewstoday.com/articles/302663.php. Updated October 12, 2017. Published October 11, 2019.
3. Chaverneff F. Ketamine: mechanisms of action, uses in pain medicine, and side effects. Clinical Pain Advisor. https://www.clinicalpainadvisor.com/home/conference-highlights/painweek-2018/ketamine-mechanisms-of-action-uses-in-pain-medicine-and-side-effects/. Published 2018. Accessed October 11, 2019.
4. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865-872.
5. Orhurhu VJ, Claus LE, Cohen SP. Ketamine toxicity. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541087. Updated April 11, 2019. Accessed October 18, 2019.
6. Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia Critical Care & Pain. 20071;7(2):59-63.
7. Logan K. Addressing ketamine bladder syndrome. Nursing Times. https://www.nursingtimes.net/clinical-archive/medicine-management/addressing-ketamine-bladder-syndrome-19-06-2011/. Published June 19, 2011. Accessed October 11, 2019.
8. Lin PC, Lane HY, Lin CH. Spontaneous remission of ketamine withdrawal-related depression. Clin Neuropharmacol. 2016;39(1):51-52.
The N-methyl-
Physicians need to be aware, however, that many patients use illicit ketamine, either for recreational purposes or as self-treatment to control depressive symptoms. To help clinicians identify the signs of ketamine abuse, we discuss the adverse effects of illicit use, and suggest treatment approaches.
Ad
Ketamine can be consumed in various ways; snorting it in a powder form is a preferred route for recreational use.2 The primary disadvantage of oral use is that it increases the likelihood of nausea and vomiting.2
While ketamine is generally safe in a supervised clinical setting, approximately 2.5 million individuals use various illicit forms of ketamine—which is known as Special K and by other names—in recreational settings (eg, dance clubs) where it might be used with other substances.3 Alcohol, in particular, compounds the sedative effects of ketamine and can lead to death by overdose.
At a subanesthetic dose, ketamine can induce dissociative and/or transcendental states that are particularly attractive to those intrigued by mystical experiences, pronounced changes in perception, or euphoria.4 High doses of ketamine—relative to a commonly used recreational dose—can produce a unique “K-hole” state in which a user is unable to control his/her body and could lose consciousness.5 A K-hole state may trigger a cycle of delirium that warrants immediate clinical attention.3
Researchers have postulated that NMDA antagonism may negatively impact memory consolidation.3,6 Even more troubling is the potential for systemic injuries because illicit ketamine use may contribute to ulcerative cystitis, severely disturbed kidney function (eg, hydronephrosis), or epigastric pain.3 Chronic abuse tends to result in more systemic sequelae, affecting the bladder, kidneys, and heart. Adverse effects that require emergent care include blood in urine, changes in vision (eg, nystagmus), chest discomfort, labored breathing, agitation, seizures, and/or altered consciousness.6
Treating ketamine abuse
Treatment should be tailored to the patient’s symptoms. If the patient presents with “K-bladder” (ie, ketamine bladder syndrome), he/she may need surgical intervention or a cystectomy.4,7 Therapeutic management of K-bladder entails recognizing bladder symptoms that are specific to ketamine use, such as interstitial or ulcerative cystitis and lower urinary tract symptoms.7 Clinicians should monitor patients for increased voiding episodes during the day, voiding urgency, or a general sense of bladder fullness. Patients with K-bladder also may complain of suprapubic pain or blood in the urine.7
Continue to: Consider referring patients to...
Consider referring patients to an individualized, ketamine-specific rehabilitation program that is modeled after other substance-specific rehabilitation programs. It is critical to address withdrawal symptoms (eg, anorexia, fatigue, tremors, chills, tachycardia, nightmares, etc.). Patients undergoing ketamine withdrawal may develop anxiety and depression, with or without suicidal ideation, that might persist during a 4- to 5-day withdrawal period.8
‘Self-medicating’ ketamine users
Clinicians need to be particularly vigilant for situations in which a patient has used ketamine in an attempt to control his/her depressive symptoms. Some researchers have described ketamine as a revolutionary drug for TRD, and it is reasonable to suspect that some patients with depressive symptoms may have consulted Internet sources to learn how to self-medicate using ketamine. Patients who have consumed smaller doses of ketamine recreationally may have developed a tolerance in which the receptors are no longer responsive to the effects at that dose, and therefore might not respond when given ketamine in a clinical setting. Proper history taking and patient education are essential for these users, and clinicians may need to develop a personalized therapeutic plan for ketamine administration. If, on the other hand, a patient has a history of chronic ketamine use (perhaps at high doses), depression may occur secondary to this type of ketamine abuse. For such patients, clinicians should explore alternative treatment modalities, such as transcranial magnetic stimulation.
The N-methyl-
Physicians need to be aware, however, that many patients use illicit ketamine, either for recreational purposes or as self-treatment to control depressive symptoms. To help clinicians identify the signs of ketamine abuse, we discuss the adverse effects of illicit use, and suggest treatment approaches.
Ad
Ketamine can be consumed in various ways; snorting it in a powder form is a preferred route for recreational use.2 The primary disadvantage of oral use is that it increases the likelihood of nausea and vomiting.2
While ketamine is generally safe in a supervised clinical setting, approximately 2.5 million individuals use various illicit forms of ketamine—which is known as Special K and by other names—in recreational settings (eg, dance clubs) where it might be used with other substances.3 Alcohol, in particular, compounds the sedative effects of ketamine and can lead to death by overdose.
At a subanesthetic dose, ketamine can induce dissociative and/or transcendental states that are particularly attractive to those intrigued by mystical experiences, pronounced changes in perception, or euphoria.4 High doses of ketamine—relative to a commonly used recreational dose—can produce a unique “K-hole” state in which a user is unable to control his/her body and could lose consciousness.5 A K-hole state may trigger a cycle of delirium that warrants immediate clinical attention.3
Researchers have postulated that NMDA antagonism may negatively impact memory consolidation.3,6 Even more troubling is the potential for systemic injuries because illicit ketamine use may contribute to ulcerative cystitis, severely disturbed kidney function (eg, hydronephrosis), or epigastric pain.3 Chronic abuse tends to result in more systemic sequelae, affecting the bladder, kidneys, and heart. Adverse effects that require emergent care include blood in urine, changes in vision (eg, nystagmus), chest discomfort, labored breathing, agitation, seizures, and/or altered consciousness.6
Treating ketamine abuse
Treatment should be tailored to the patient’s symptoms. If the patient presents with “K-bladder” (ie, ketamine bladder syndrome), he/she may need surgical intervention or a cystectomy.4,7 Therapeutic management of K-bladder entails recognizing bladder symptoms that are specific to ketamine use, such as interstitial or ulcerative cystitis and lower urinary tract symptoms.7 Clinicians should monitor patients for increased voiding episodes during the day, voiding urgency, or a general sense of bladder fullness. Patients with K-bladder also may complain of suprapubic pain or blood in the urine.7
Continue to: Consider referring patients to...
Consider referring patients to an individualized, ketamine-specific rehabilitation program that is modeled after other substance-specific rehabilitation programs. It is critical to address withdrawal symptoms (eg, anorexia, fatigue, tremors, chills, tachycardia, nightmares, etc.). Patients undergoing ketamine withdrawal may develop anxiety and depression, with or without suicidal ideation, that might persist during a 4- to 5-day withdrawal period.8
‘Self-medicating’ ketamine users
Clinicians need to be particularly vigilant for situations in which a patient has used ketamine in an attempt to control his/her depressive symptoms. Some researchers have described ketamine as a revolutionary drug for TRD, and it is reasonable to suspect that some patients with depressive symptoms may have consulted Internet sources to learn how to self-medicate using ketamine. Patients who have consumed smaller doses of ketamine recreationally may have developed a tolerance in which the receptors are no longer responsive to the effects at that dose, and therefore might not respond when given ketamine in a clinical setting. Proper history taking and patient education are essential for these users, and clinicians may need to develop a personalized therapeutic plan for ketamine administration. If, on the other hand, a patient has a history of chronic ketamine use (perhaps at high doses), depression may occur secondary to this type of ketamine abuse. For such patients, clinicians should explore alternative treatment modalities, such as transcranial magnetic stimulation.
1. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
2. Davis K. What are the uses of ketamine? Medical News Today. https://www.medicalnewstoday.com/articles/302663.php. Updated October 12, 2017. Published October 11, 2019.
3. Chaverneff F. Ketamine: mechanisms of action, uses in pain medicine, and side effects. Clinical Pain Advisor. https://www.clinicalpainadvisor.com/home/conference-highlights/painweek-2018/ketamine-mechanisms-of-action-uses-in-pain-medicine-and-side-effects/. Published 2018. Accessed October 11, 2019.
4. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865-872.
5. Orhurhu VJ, Claus LE, Cohen SP. Ketamine toxicity. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541087. Updated April 11, 2019. Accessed October 18, 2019.
6. Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia Critical Care & Pain. 20071;7(2):59-63.
7. Logan K. Addressing ketamine bladder syndrome. Nursing Times. https://www.nursingtimes.net/clinical-archive/medicine-management/addressing-ketamine-bladder-syndrome-19-06-2011/. Published June 19, 2011. Accessed October 11, 2019.
8. Lin PC, Lane HY, Lin CH. Spontaneous remission of ketamine withdrawal-related depression. Clin Neuropharmacol. 2016;39(1):51-52.
1. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
2. Davis K. What are the uses of ketamine? Medical News Today. https://www.medicalnewstoday.com/articles/302663.php. Updated October 12, 2017. Published October 11, 2019.
3. Chaverneff F. Ketamine: mechanisms of action, uses in pain medicine, and side effects. Clinical Pain Advisor. https://www.clinicalpainadvisor.com/home/conference-highlights/painweek-2018/ketamine-mechanisms-of-action-uses-in-pain-medicine-and-side-effects/. Published 2018. Accessed October 11, 2019.
4. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865-872.
5. Orhurhu VJ, Claus LE, Cohen SP. Ketamine toxicity. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541087. Updated April 11, 2019. Accessed October 18, 2019.
6. Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia Critical Care & Pain. 20071;7(2):59-63.
7. Logan K. Addressing ketamine bladder syndrome. Nursing Times. https://www.nursingtimes.net/clinical-archive/medicine-management/addressing-ketamine-bladder-syndrome-19-06-2011/. Published June 19, 2011. Accessed October 11, 2019.
8. Lin PC, Lane HY, Lin CH. Spontaneous remission of ketamine withdrawal-related depression. Clin Neuropharmacol. 2016;39(1):51-52.
CBD: What physicians need to know about it
Cannabidiol is a derivative of marijuana that is sold everywhere from medical marijuana stores to health food markets to gas stations. While this chemical is derived from marijuana plants, it can be sold in many states as a supplement and is largely unregulated. The ubiquity of cannabidiol (CBD) and its potential benefits means that doctors need to be able to counsel patients about what we know, what we don’t, and how to use it safely. For conditions such as chronic pain and addiction, where we have few safe and effective alternatives, CBD may be reasonable to recommend.
To find out what physicians need to know about CBD, Elisabeth Poorman, MD, a general internist at a University of Washington neighborhood clinic in Kent and member of the editorial advisory board of Internal Medicine News, interviewed Peter Grinspoon, MD, who provides free consultation to primary care patients on the benefits and risks of using various forms of cannabis, including CBD. Dr. Grinspoon is an internist at Massachusetts General Hospital Chelsea Healthcare Center and is an instructor at Harvard Medical School, Boston. He has contributed to the Harvard Health Blog on the topic of medical marijuana, delivered grand rounds on cannabis at Massachusetts General Hospital, and lectured at the American College of Physicians. Dr. Grinspoon is also medical director for Galenas, a medical marijuana company.
Dr. Grinspoon is the son of Lester Grinspoon, MD, associate professor emeritus of psychiatry at Harvard Medical School, who researched the medicinal legitimacy of marijuana prohibition and has authored books on the medical benefits of marijuana.
and his knowledge of CBD’s efficacy for various medical conditions. Below are excerpts from that conversation.
Dr. Poorman: How do you explain the difference between THC and CBD to patients?
Dr. Grinspoon: Cannabis contains at least a hundred different chemicals called cannabinoids, of which tetrahydrocannabinol (THC) and CBD are the most prevalent. THC is the one that gets you high and can be used recreationally and medically. The CBD molecule is not intoxicating, and people use it for a variety of medical purposes, most commonly to treat anxiety, insomnia, and pain.
Dr. Poorman: There are a lot of gaps in what we now about CBD’s potential benefits. Why don’t we know more?
Dr. Grinspoon: CBD has no abuse liability according to the World Health Organization, but because it is a cannabinoid, it is still technically a schedule I substance under the Controlled Substances Act, and that makes it difficult to study.
Dr. Poorman: What kinds of conditions can CBD treat?
Dr. Grinspoon: In anxiety, the enthusiasm has outpaced the science; there’s no question about that. And most of the studies have done in animals. That said, some studies have shown that CBD helps treat components of anxiety, like public speaking. Unlike THC, it is nonintoxicating and non–habit forming. But we don’t have the wealth of randomized controlled trials that we have for official psychiatric medications.
CBD’s benefits have been most extensively studied in pediatric epilepsy. The one Food and Drug Administration–approved drug derived from cannabis is Epidiolex, used to treat rare forms of childhood epilepsy. There is some evidence that as an adjunct, it can be used for glioblastoma multiforme in patients receiving other appropriate therapy. There is also some preliminary evidence that it can be used for addiction, including to opioids, cannabis, tobacco, and stimulants.
Most of the evidence for using CBD in chronic pain comes from animal studies, including a study published in the European Journal of Pain in 2016. Among my patients to whom I have suggested CBD for chronic pain, a few have noticed great benefit, a few have noticed some benefit, and a lot have noticed no benefit. For those who have said they noticed benefit it is unclear whether that benefit was just the placebo effect.
In insomnia, I usually have them take CBD under the tongue half an hour time before bedtime, or if it’s an edible, an hour before bedtime. I start with a lower dose and slowly try higher doses. I also encourage them to do the other sleep hygiene things, like no screens, increasing exercise, and decreasing caffeine. It seems that CBD helps them fall asleep, though it’s hard to know if it’s the CBD or the fact that they have started taking something, and have simultaneously made various lifestyle changes.
Dr. Poorman: Can CBD interfere with your normal sleep architecture, the way benzodiazepines and Benadryl can?
Dr. Grinspoon: We know that THC affects your sleep architecture and affects what percentage of REM sleep you have. But I don’t know if the effects of CBD on sleep architecture have been studied.
Dr. Poorman: What harms do you counsel patients about when discussing CBD?
Dr. Grinspoon: There are four main harms. The first is the price. It’s overpriced, and the doses are very low. In most animal studies, the doses are about 20 milligrams per kilogram of weight. And you go to the market, and it’s like a dollar for a hundredth of that.
Number two is that it’s not regulated; it’s a supplement. A few years ago, the government tested a bunch of samples of CBD, and some didn’t actually contain CBD, some didn’t have the right amount; and worse, some contained THC that had not been disclosed in the packaging. So you can’t just go to a roadside gas station and assume that if you buy CBD, it’s actually that. You want a place that has a certificate of assurance. Make sure third-party testing was done, including testing for pesticides and other heavy metals.
The third thing is drug interactions. It affects the body like grapefruit and inhibits the cytochrome P450 system. The medications doctors should be most concerned about are blood thinners like Coumadin. And if you’re on blood thinners, you definitely want to tell your doctor that you are on CBD and he or she might want to check your blood levels more frequently than they usually do.
The fourth concern is liver inflammation. In the childhood epilepsy studies, a bump in some liver enzymes was seen, although I haven’t heard of any clinically significant cases of chemical hepatitis related to CBD. But if someone has liver disease you want to keep an eye on their liver enzymes.
Dr. Poorman: What methods of ingestion do you recommend or not recommend?
Dr. Grinspoon: It’s basically trial and error, but I usually recommend oral form. If people feel comfortable taking a gummy bear, or a pill, I’m not particular about that. If the product being taken contains less than 0.3% THC, it won’t get you high.
The topical form probably works better for treating chronic pain if it contains some THC, suggests a review article published in the Cleveland Clinic Journal of Medicine. Topical THC is nonintoxicating, unless you managed to sit in a bathtub for 8 hours after applying it.
I don’t recommend smoking CBD, and right now, I don’t recommend vaping anything.
If people have severe pain, like moderately severe arthritis, CBD may not be enough, whereas medical cannabis with THC could help, a report suggests.
Dr. Poorman: Do you ever encourage patients to stop using CBD products?
Dr. Grinspoon: I work in a low-income area, and my patients don’t have a ton of disposable income. If it’s not working, I worry about the expense.
Dr. Poorman: The CBD industry is growing quickly. What changes are you seeing in what products are out there, and what changes would you like to see?
Dr. Grinspoon: CBD is being put in everything, and it’s comical. On the one hand, you can say if people want to waste their money on a CBD emitting pillowcase, that’s fine. On the other hand, you can say that certainly seems like misleading advertising, because a CBD emitting pillowcase isn’t going to help you sleep any better.
I think the purported benefits are far beyond what we can say scientifically. We do know that CBD has anti-inflammatory characteristics. But that doesn’t mean that putting CBD in all skin products is good for your skin. It’s bad for your pocketbook, though. I would like there to be less of a gap between the claims and the science.
Dr. Elisabeth Poorman has no conflicts to disclose.
Cannabidiol is a derivative of marijuana that is sold everywhere from medical marijuana stores to health food markets to gas stations. While this chemical is derived from marijuana plants, it can be sold in many states as a supplement and is largely unregulated. The ubiquity of cannabidiol (CBD) and its potential benefits means that doctors need to be able to counsel patients about what we know, what we don’t, and how to use it safely. For conditions such as chronic pain and addiction, where we have few safe and effective alternatives, CBD may be reasonable to recommend.
To find out what physicians need to know about CBD, Elisabeth Poorman, MD, a general internist at a University of Washington neighborhood clinic in Kent and member of the editorial advisory board of Internal Medicine News, interviewed Peter Grinspoon, MD, who provides free consultation to primary care patients on the benefits and risks of using various forms of cannabis, including CBD. Dr. Grinspoon is an internist at Massachusetts General Hospital Chelsea Healthcare Center and is an instructor at Harvard Medical School, Boston. He has contributed to the Harvard Health Blog on the topic of medical marijuana, delivered grand rounds on cannabis at Massachusetts General Hospital, and lectured at the American College of Physicians. Dr. Grinspoon is also medical director for Galenas, a medical marijuana company.
Dr. Grinspoon is the son of Lester Grinspoon, MD, associate professor emeritus of psychiatry at Harvard Medical School, who researched the medicinal legitimacy of marijuana prohibition and has authored books on the medical benefits of marijuana.
and his knowledge of CBD’s efficacy for various medical conditions. Below are excerpts from that conversation.
Dr. Poorman: How do you explain the difference between THC and CBD to patients?
Dr. Grinspoon: Cannabis contains at least a hundred different chemicals called cannabinoids, of which tetrahydrocannabinol (THC) and CBD are the most prevalent. THC is the one that gets you high and can be used recreationally and medically. The CBD molecule is not intoxicating, and people use it for a variety of medical purposes, most commonly to treat anxiety, insomnia, and pain.
Dr. Poorman: There are a lot of gaps in what we now about CBD’s potential benefits. Why don’t we know more?
Dr. Grinspoon: CBD has no abuse liability according to the World Health Organization, but because it is a cannabinoid, it is still technically a schedule I substance under the Controlled Substances Act, and that makes it difficult to study.
Dr. Poorman: What kinds of conditions can CBD treat?
Dr. Grinspoon: In anxiety, the enthusiasm has outpaced the science; there’s no question about that. And most of the studies have done in animals. That said, some studies have shown that CBD helps treat components of anxiety, like public speaking. Unlike THC, it is nonintoxicating and non–habit forming. But we don’t have the wealth of randomized controlled trials that we have for official psychiatric medications.
CBD’s benefits have been most extensively studied in pediatric epilepsy. The one Food and Drug Administration–approved drug derived from cannabis is Epidiolex, used to treat rare forms of childhood epilepsy. There is some evidence that as an adjunct, it can be used for glioblastoma multiforme in patients receiving other appropriate therapy. There is also some preliminary evidence that it can be used for addiction, including to opioids, cannabis, tobacco, and stimulants.
Most of the evidence for using CBD in chronic pain comes from animal studies, including a study published in the European Journal of Pain in 2016. Among my patients to whom I have suggested CBD for chronic pain, a few have noticed great benefit, a few have noticed some benefit, and a lot have noticed no benefit. For those who have said they noticed benefit it is unclear whether that benefit was just the placebo effect.
In insomnia, I usually have them take CBD under the tongue half an hour time before bedtime, or if it’s an edible, an hour before bedtime. I start with a lower dose and slowly try higher doses. I also encourage them to do the other sleep hygiene things, like no screens, increasing exercise, and decreasing caffeine. It seems that CBD helps them fall asleep, though it’s hard to know if it’s the CBD or the fact that they have started taking something, and have simultaneously made various lifestyle changes.
Dr. Poorman: Can CBD interfere with your normal sleep architecture, the way benzodiazepines and Benadryl can?
Dr. Grinspoon: We know that THC affects your sleep architecture and affects what percentage of REM sleep you have. But I don’t know if the effects of CBD on sleep architecture have been studied.
Dr. Poorman: What harms do you counsel patients about when discussing CBD?
Dr. Grinspoon: There are four main harms. The first is the price. It’s overpriced, and the doses are very low. In most animal studies, the doses are about 20 milligrams per kilogram of weight. And you go to the market, and it’s like a dollar for a hundredth of that.
Number two is that it’s not regulated; it’s a supplement. A few years ago, the government tested a bunch of samples of CBD, and some didn’t actually contain CBD, some didn’t have the right amount; and worse, some contained THC that had not been disclosed in the packaging. So you can’t just go to a roadside gas station and assume that if you buy CBD, it’s actually that. You want a place that has a certificate of assurance. Make sure third-party testing was done, including testing for pesticides and other heavy metals.
The third thing is drug interactions. It affects the body like grapefruit and inhibits the cytochrome P450 system. The medications doctors should be most concerned about are blood thinners like Coumadin. And if you’re on blood thinners, you definitely want to tell your doctor that you are on CBD and he or she might want to check your blood levels more frequently than they usually do.
The fourth concern is liver inflammation. In the childhood epilepsy studies, a bump in some liver enzymes was seen, although I haven’t heard of any clinically significant cases of chemical hepatitis related to CBD. But if someone has liver disease you want to keep an eye on their liver enzymes.
Dr. Poorman: What methods of ingestion do you recommend or not recommend?
Dr. Grinspoon: It’s basically trial and error, but I usually recommend oral form. If people feel comfortable taking a gummy bear, or a pill, I’m not particular about that. If the product being taken contains less than 0.3% THC, it won’t get you high.
The topical form probably works better for treating chronic pain if it contains some THC, suggests a review article published in the Cleveland Clinic Journal of Medicine. Topical THC is nonintoxicating, unless you managed to sit in a bathtub for 8 hours after applying it.
I don’t recommend smoking CBD, and right now, I don’t recommend vaping anything.
If people have severe pain, like moderately severe arthritis, CBD may not be enough, whereas medical cannabis with THC could help, a report suggests.
Dr. Poorman: Do you ever encourage patients to stop using CBD products?
Dr. Grinspoon: I work in a low-income area, and my patients don’t have a ton of disposable income. If it’s not working, I worry about the expense.
Dr. Poorman: The CBD industry is growing quickly. What changes are you seeing in what products are out there, and what changes would you like to see?
Dr. Grinspoon: CBD is being put in everything, and it’s comical. On the one hand, you can say if people want to waste their money on a CBD emitting pillowcase, that’s fine. On the other hand, you can say that certainly seems like misleading advertising, because a CBD emitting pillowcase isn’t going to help you sleep any better.
I think the purported benefits are far beyond what we can say scientifically. We do know that CBD has anti-inflammatory characteristics. But that doesn’t mean that putting CBD in all skin products is good for your skin. It’s bad for your pocketbook, though. I would like there to be less of a gap between the claims and the science.
Dr. Elisabeth Poorman has no conflicts to disclose.
Cannabidiol is a derivative of marijuana that is sold everywhere from medical marijuana stores to health food markets to gas stations. While this chemical is derived from marijuana plants, it can be sold in many states as a supplement and is largely unregulated. The ubiquity of cannabidiol (CBD) and its potential benefits means that doctors need to be able to counsel patients about what we know, what we don’t, and how to use it safely. For conditions such as chronic pain and addiction, where we have few safe and effective alternatives, CBD may be reasonable to recommend.
To find out what physicians need to know about CBD, Elisabeth Poorman, MD, a general internist at a University of Washington neighborhood clinic in Kent and member of the editorial advisory board of Internal Medicine News, interviewed Peter Grinspoon, MD, who provides free consultation to primary care patients on the benefits and risks of using various forms of cannabis, including CBD. Dr. Grinspoon is an internist at Massachusetts General Hospital Chelsea Healthcare Center and is an instructor at Harvard Medical School, Boston. He has contributed to the Harvard Health Blog on the topic of medical marijuana, delivered grand rounds on cannabis at Massachusetts General Hospital, and lectured at the American College of Physicians. Dr. Grinspoon is also medical director for Galenas, a medical marijuana company.
Dr. Grinspoon is the son of Lester Grinspoon, MD, associate professor emeritus of psychiatry at Harvard Medical School, who researched the medicinal legitimacy of marijuana prohibition and has authored books on the medical benefits of marijuana.
and his knowledge of CBD’s efficacy for various medical conditions. Below are excerpts from that conversation.
Dr. Poorman: How do you explain the difference between THC and CBD to patients?
Dr. Grinspoon: Cannabis contains at least a hundred different chemicals called cannabinoids, of which tetrahydrocannabinol (THC) and CBD are the most prevalent. THC is the one that gets you high and can be used recreationally and medically. The CBD molecule is not intoxicating, and people use it for a variety of medical purposes, most commonly to treat anxiety, insomnia, and pain.
Dr. Poorman: There are a lot of gaps in what we now about CBD’s potential benefits. Why don’t we know more?
Dr. Grinspoon: CBD has no abuse liability according to the World Health Organization, but because it is a cannabinoid, it is still technically a schedule I substance under the Controlled Substances Act, and that makes it difficult to study.
Dr. Poorman: What kinds of conditions can CBD treat?
Dr. Grinspoon: In anxiety, the enthusiasm has outpaced the science; there’s no question about that. And most of the studies have done in animals. That said, some studies have shown that CBD helps treat components of anxiety, like public speaking. Unlike THC, it is nonintoxicating and non–habit forming. But we don’t have the wealth of randomized controlled trials that we have for official psychiatric medications.
CBD’s benefits have been most extensively studied in pediatric epilepsy. The one Food and Drug Administration–approved drug derived from cannabis is Epidiolex, used to treat rare forms of childhood epilepsy. There is some evidence that as an adjunct, it can be used for glioblastoma multiforme in patients receiving other appropriate therapy. There is also some preliminary evidence that it can be used for addiction, including to opioids, cannabis, tobacco, and stimulants.
Most of the evidence for using CBD in chronic pain comes from animal studies, including a study published in the European Journal of Pain in 2016. Among my patients to whom I have suggested CBD for chronic pain, a few have noticed great benefit, a few have noticed some benefit, and a lot have noticed no benefit. For those who have said they noticed benefit it is unclear whether that benefit was just the placebo effect.
In insomnia, I usually have them take CBD under the tongue half an hour time before bedtime, or if it’s an edible, an hour before bedtime. I start with a lower dose and slowly try higher doses. I also encourage them to do the other sleep hygiene things, like no screens, increasing exercise, and decreasing caffeine. It seems that CBD helps them fall asleep, though it’s hard to know if it’s the CBD or the fact that they have started taking something, and have simultaneously made various lifestyle changes.
Dr. Poorman: Can CBD interfere with your normal sleep architecture, the way benzodiazepines and Benadryl can?
Dr. Grinspoon: We know that THC affects your sleep architecture and affects what percentage of REM sleep you have. But I don’t know if the effects of CBD on sleep architecture have been studied.
Dr. Poorman: What harms do you counsel patients about when discussing CBD?
Dr. Grinspoon: There are four main harms. The first is the price. It’s overpriced, and the doses are very low. In most animal studies, the doses are about 20 milligrams per kilogram of weight. And you go to the market, and it’s like a dollar for a hundredth of that.
Number two is that it’s not regulated; it’s a supplement. A few years ago, the government tested a bunch of samples of CBD, and some didn’t actually contain CBD, some didn’t have the right amount; and worse, some contained THC that had not been disclosed in the packaging. So you can’t just go to a roadside gas station and assume that if you buy CBD, it’s actually that. You want a place that has a certificate of assurance. Make sure third-party testing was done, including testing for pesticides and other heavy metals.
The third thing is drug interactions. It affects the body like grapefruit and inhibits the cytochrome P450 system. The medications doctors should be most concerned about are blood thinners like Coumadin. And if you’re on blood thinners, you definitely want to tell your doctor that you are on CBD and he or she might want to check your blood levels more frequently than they usually do.
The fourth concern is liver inflammation. In the childhood epilepsy studies, a bump in some liver enzymes was seen, although I haven’t heard of any clinically significant cases of chemical hepatitis related to CBD. But if someone has liver disease you want to keep an eye on their liver enzymes.
Dr. Poorman: What methods of ingestion do you recommend or not recommend?
Dr. Grinspoon: It’s basically trial and error, but I usually recommend oral form. If people feel comfortable taking a gummy bear, or a pill, I’m not particular about that. If the product being taken contains less than 0.3% THC, it won’t get you high.
The topical form probably works better for treating chronic pain if it contains some THC, suggests a review article published in the Cleveland Clinic Journal of Medicine. Topical THC is nonintoxicating, unless you managed to sit in a bathtub for 8 hours after applying it.
I don’t recommend smoking CBD, and right now, I don’t recommend vaping anything.
If people have severe pain, like moderately severe arthritis, CBD may not be enough, whereas medical cannabis with THC could help, a report suggests.
Dr. Poorman: Do you ever encourage patients to stop using CBD products?
Dr. Grinspoon: I work in a low-income area, and my patients don’t have a ton of disposable income. If it’s not working, I worry about the expense.
Dr. Poorman: The CBD industry is growing quickly. What changes are you seeing in what products are out there, and what changes would you like to see?
Dr. Grinspoon: CBD is being put in everything, and it’s comical. On the one hand, you can say if people want to waste their money on a CBD emitting pillowcase, that’s fine. On the other hand, you can say that certainly seems like misleading advertising, because a CBD emitting pillowcase isn’t going to help you sleep any better.
I think the purported benefits are far beyond what we can say scientifically. We do know that CBD has anti-inflammatory characteristics. But that doesn’t mean that putting CBD in all skin products is good for your skin. It’s bad for your pocketbook, though. I would like there to be less of a gap between the claims and the science.
Dr. Elisabeth Poorman has no conflicts to disclose.
Preconception marijuana use by male partner raises spontaneous abortion risk
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
REPORTING FROM ASRM 2019
Clinical interventions for global drug use need updating
Public health approach requires greater emphasis on harms, benefits of substance use.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Public health approach requires greater emphasis on harms, benefits of substance use.
Public health approach requires greater emphasis on harms, benefits of substance use.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Strategies aimed at reducing drug-related harm should be informed by evidence, and recognize the contribution of social and economic factors to drug use, report the authors of a series of four papers published in The Lancet.
Louisa Degenhardt, PhD, and coauthors wrote in the first paper that, although the availability and use of drugs have been transformed over recent decades – including the emergence of hundreds of new psychoactive substances – professional and public policy has not yet adapted to those new realities (Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9).
, in a way that you don’t see in other areas of public health,” Dr. Degenhardt, of the National Drug and Alcohol Research Centre at the University of New South Wales in Sydney, said in an interview. “There has been an increasing level of awareness of issues but also level of recognition that we need to have hard evidence to work out the best ways to respond.”
The paper by Dr. Degenhardt and coauthors addressed the issue of opioid use and dependence around the world, citing evidence that in 2017, 40.5 million people were dependent on opioids and 109,500 deaths were attributable to opioid overdose. An effective treatment exists in the form of opioid agonists methadone and buprenorphine, both of which are recognized as World Health Organization essential medicines.
While the best evidence for positive outcomes from opioid agonist treatment is in people using illicit opioids such as heroin, there is also evidence for their effectiveness in people with pharmaceutical opioid dependence. A study in Kentucky suggested that scaling up the use and retention of opioid agonist treatment, including in prison, could prevent 57% of overdose deaths among injecting drug users.
“Despite strong evidence for the effectiveness of a range of interventions to improve the health and well-being of people who are dependent on opioids, coverage is low, even in high-income countries,” the authors wrote. They also called for international efforts to eliminate marketing strategies that have contributed to the increase in opioid prescription and harms in North America.
The second paper examined the public health implications of legalizing cannabis for medicinal and recreational use (Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1). Cannabis has been considered an illicit drug for more than 50 years but recently has been decriminalized or legalized in many parts of the world in recognition of the lower levels of harm, compared with other illicit substances.
Cannabis is used to treat a range of medical conditions, including muscle spasticity in multiple sclerosis. It also is used to treat pain, nausea, and vomiting in palliative care, and to reduce seizures in epilepsy. However, the authors noted that the evidence for many medical applications was absent, and that weakly regulated medical cannabis programs in some U.S. states were blurring the boundaries between medicinal and nonmedicinal use.
They also wrote that the public health effects of legalization could not be assessed, because legalization had happened only in the last 5 years.
“A major determinant of the public health effect of cannabis legalization will be the effect that it has on alcohol use,” they wrote. “The substitution of cannabis for alcohol would produce substantial public health gains, but any increase in the combined use of alcohol and cannabis could increase harm.”
The authors also looked at the effect of use of stimulants such as cocaine and amphetamines. While their use is associated with higher mortality, increased incidence of HIV and hepatitis C infection, poor mental health, and increased risk of cardiovascular events, no effective pharmacotherapies are available, and psychosocial interventions such as cognitive-behavioral therapy have only a weak effect.
“Many governments rely on punitive responses, such as involuntary detention in drug centers, despite the absence of evidence for their effectiveness and their potential to increase harm,” the authors wrote. “Substantial research investment is needed to develop more effective, innovative, and impactful prevention and treatment” (Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5).
They focused on interventions to prevent the transmission of blood-borne and sexually transmitted infections – such as the provision of safe injecting equipment, condoms or pre-exposure prophylaxis against HIV – and improve treatment of these, and interventions to prevent and treat overdose, injury, and other harms.
The final paper in the series explored new psychoactive substances, such as synthetic cannabinoids, stimulants, hallucinogens, and dissociative and depressant substances (Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7).
There really needs to be massive change in systems in terms of the way monitoring occurs and the speed with which new drugs are identified, Dr. Degenhardt said in the interview. She also said the risks that are identified need to be communicated more effectively.
“At the moment, the way that drug surveillance works in most countries, things come and then particular drugs may spread in use, cause massive harm, and all of our systems of detecting and responding are not fit to detect those things in a timely way and disseminate information to reduce those risks.”
The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
SOURCES: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
FROM THE LANCET
Key clinical point: People with drug use disorders around the world need evidence-based and nonjudgmental clinical care.
Major finding: Many interventions aimed at reducing the harm of illicit drug use are not informed by evidence.
Study details: Series of four papers reviewing the evidence on cannabinoids, opioids, new psychoactive substances, and stimulants.
Disclosures: The papers were supported by European Monitoring Centre on Drugs and Drug Addiction, and the Australian National Drug and Alcohol Research Centre. The authors declared support from a range of institutions and funding bodies, and several also declared unrelated grants, funding, and other support from the pharmaceutical sector.
Sources: Degenhardt L et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32229-9; Hall W et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)31789-1; Farrell M et al. Lancet. 2019 Oct 23. doi: 10.1016/S0140-6736(19)32230-5; and Peacock A et al. Lancet 2019 Oct 23. doi: 10.1016/S0140-6736(19)32231-7.
Treating comorbid ADHD-SUD presents challenges
Consider medications that might improve both conditions
SAN DIEGO – Research suggests that as many as 23% of patients with substance use disorder (SUD) also have ADHD, adding an extra layer of complexity to a difficult-to-treat condition. What to do?
“Treating the ADHD can be useful in reducing the severity of symptoms without worsening the substance use disorder. It shouldn’t be avoided,” said psychiatrist Larissa J. Mooney, MD, of the University of California, Los Angeles, and the Veterans Affairs Greater Los Angeles Healthcare System, in a presentation at the annual Psych Congress.
When ADHD is on board, “it’s a more complicated and challenging clinical course,” Dr. Mooney said. The duo of disorders is linked to higher rates of polysubstance abuse and other psychiatric conditions, such as anxiety, bipolar disorder, posttraumatic stress, and antisocial/borderline conditions (Eur Addict Res. 2018;24[1]:43-51).
“These individuals typically have more difficulty with [drug] abstinence, more health consequences, and reduced quality of life, and social and professional consequences,” she said. “Some studies have suggested that they may not respond to lower doses of medication for attention-deficit/hyperactivity disorder and may require doses in the higher range.”
Research has hinted that several drugs that might prove helpful in these patients by improving both conditions, Dr. Mooney said. These include up to 180 mg/day of methylphenidate (Ritalin), 60- and 80-mg doses of mixed amphetamine salts/extended release, atomoxetine (Strattera), and bupropion.
In regard to bupropion, she said, “I find it to be a good choice in my substance use disorder patients for their depression and concentration problems. I have a greater number of individuals at 450 milligrams per day and the XL formulation.”
“We need more research to show if this is helpful,” she said. “It’s a reasonable choice in terms of weighing pros and cons, because it’s not [a controlled substance].”
Still, some of those medications are stimulants, Dr. Mooney said, and their use in patients with SUD is controversial. There are concerns about misuse and diversion.
“We want to have some flexibility,” she said, but it’s important to think about risks and priorities. In certain cases, ADHD may be a secondary concern.
“Some patients have a severe substance use disorder that keeps landing them in the emergency room or causing them to be hospitalized,” she said. “I’m more worried about that than the impairment function from ADHD.”
If you do consider stimulants, she said, longer-acting formulations can be less risky because there’s less potential for diversion. “Also, think about their treatment plan: Is their functioning improving? Are they or showing up for appointments? These are factors that will say: ‘Oh, I’m on the right path with this medication.’ ”
Behavioral treatment also can be helpful in these patients, she said, although “some may not be willing or motivated to put in the time that it takes to do the behavioral work.”
Dr. Mooney disclosed an advisory board relationship with Alkermes and grant/research support from the National Institute on Drug Abuse.
Consider medications that might improve both conditions
Consider medications that might improve both conditions
SAN DIEGO – Research suggests that as many as 23% of patients with substance use disorder (SUD) also have ADHD, adding an extra layer of complexity to a difficult-to-treat condition. What to do?
“Treating the ADHD can be useful in reducing the severity of symptoms without worsening the substance use disorder. It shouldn’t be avoided,” said psychiatrist Larissa J. Mooney, MD, of the University of California, Los Angeles, and the Veterans Affairs Greater Los Angeles Healthcare System, in a presentation at the annual Psych Congress.
When ADHD is on board, “it’s a more complicated and challenging clinical course,” Dr. Mooney said. The duo of disorders is linked to higher rates of polysubstance abuse and other psychiatric conditions, such as anxiety, bipolar disorder, posttraumatic stress, and antisocial/borderline conditions (Eur Addict Res. 2018;24[1]:43-51).
“These individuals typically have more difficulty with [drug] abstinence, more health consequences, and reduced quality of life, and social and professional consequences,” she said. “Some studies have suggested that they may not respond to lower doses of medication for attention-deficit/hyperactivity disorder and may require doses in the higher range.”
Research has hinted that several drugs that might prove helpful in these patients by improving both conditions, Dr. Mooney said. These include up to 180 mg/day of methylphenidate (Ritalin), 60- and 80-mg doses of mixed amphetamine salts/extended release, atomoxetine (Strattera), and bupropion.
In regard to bupropion, she said, “I find it to be a good choice in my substance use disorder patients for their depression and concentration problems. I have a greater number of individuals at 450 milligrams per day and the XL formulation.”
“We need more research to show if this is helpful,” she said. “It’s a reasonable choice in terms of weighing pros and cons, because it’s not [a controlled substance].”
Still, some of those medications are stimulants, Dr. Mooney said, and their use in patients with SUD is controversial. There are concerns about misuse and diversion.
“We want to have some flexibility,” she said, but it’s important to think about risks and priorities. In certain cases, ADHD may be a secondary concern.
“Some patients have a severe substance use disorder that keeps landing them in the emergency room or causing them to be hospitalized,” she said. “I’m more worried about that than the impairment function from ADHD.”
If you do consider stimulants, she said, longer-acting formulations can be less risky because there’s less potential for diversion. “Also, think about their treatment plan: Is their functioning improving? Are they or showing up for appointments? These are factors that will say: ‘Oh, I’m on the right path with this medication.’ ”
Behavioral treatment also can be helpful in these patients, she said, although “some may not be willing or motivated to put in the time that it takes to do the behavioral work.”
Dr. Mooney disclosed an advisory board relationship with Alkermes and grant/research support from the National Institute on Drug Abuse.
SAN DIEGO – Research suggests that as many as 23% of patients with substance use disorder (SUD) also have ADHD, adding an extra layer of complexity to a difficult-to-treat condition. What to do?
“Treating the ADHD can be useful in reducing the severity of symptoms without worsening the substance use disorder. It shouldn’t be avoided,” said psychiatrist Larissa J. Mooney, MD, of the University of California, Los Angeles, and the Veterans Affairs Greater Los Angeles Healthcare System, in a presentation at the annual Psych Congress.
When ADHD is on board, “it’s a more complicated and challenging clinical course,” Dr. Mooney said. The duo of disorders is linked to higher rates of polysubstance abuse and other psychiatric conditions, such as anxiety, bipolar disorder, posttraumatic stress, and antisocial/borderline conditions (Eur Addict Res. 2018;24[1]:43-51).
“These individuals typically have more difficulty with [drug] abstinence, more health consequences, and reduced quality of life, and social and professional consequences,” she said. “Some studies have suggested that they may not respond to lower doses of medication for attention-deficit/hyperactivity disorder and may require doses in the higher range.”
Research has hinted that several drugs that might prove helpful in these patients by improving both conditions, Dr. Mooney said. These include up to 180 mg/day of methylphenidate (Ritalin), 60- and 80-mg doses of mixed amphetamine salts/extended release, atomoxetine (Strattera), and bupropion.
In regard to bupropion, she said, “I find it to be a good choice in my substance use disorder patients for their depression and concentration problems. I have a greater number of individuals at 450 milligrams per day and the XL formulation.”
“We need more research to show if this is helpful,” she said. “It’s a reasonable choice in terms of weighing pros and cons, because it’s not [a controlled substance].”
Still, some of those medications are stimulants, Dr. Mooney said, and their use in patients with SUD is controversial. There are concerns about misuse and diversion.
“We want to have some flexibility,” she said, but it’s important to think about risks and priorities. In certain cases, ADHD may be a secondary concern.
“Some patients have a severe substance use disorder that keeps landing them in the emergency room or causing them to be hospitalized,” she said. “I’m more worried about that than the impairment function from ADHD.”
If you do consider stimulants, she said, longer-acting formulations can be less risky because there’s less potential for diversion. “Also, think about their treatment plan: Is their functioning improving? Are they or showing up for appointments? These are factors that will say: ‘Oh, I’m on the right path with this medication.’ ”
Behavioral treatment also can be helpful in these patients, she said, although “some may not be willing or motivated to put in the time that it takes to do the behavioral work.”
Dr. Mooney disclosed an advisory board relationship with Alkermes and grant/research support from the National Institute on Drug Abuse.
EXPERT ANALYSIS FROM PSYCH CONGRESS 2019
Food addiction is pervasive among psychiatric patients
COPENHAGEN – Food addiction is threefold more prevalent among individuals with clinically diagnosed mental disorders than in the general population, according to a report from the Food Addiction Denmark (FADK) project.
This finding provides support for the hypothesis that food addiction is a key link in the chain connecting psychiatric disorders to increased risk of obesity, which in turn contributes to the substantially shorter life expectancy of psychiatric patients, Christina Horsager, MD, a cofounder of the project, said at the annual congress of the European College of Neuropsychopharmacology.
The FADK project is designed to fill in major gaps in the understanding of food addiction. The project included a 2018 Danish nationwide questionnaire survey of 1,394 individuals with various mental disorders and 1,699 others from the general population. The questionnaire included the Yale Food Addiction Scale Version 2.0 (Psychol Addict Behav. 2016 Feb;30[1]:113-21), which was used to identify affected individuals, as well as psychopathology rating scales, explained Dr. Horsager, of the child and adolescent psychiatry department at Aalborg (Denmark) University Hospital.
The prevalence of food addiction was 9% in the general population and 26.5% in individuals with mental disorders. The highest prevalence was, not surprisingly, in individuals with a DSM-5 diagnosis of an eating disorder. The rate was 30% in individuals with a DSM-5 personality disorder, 28% in those with a mood disorder, 17% with autism and other pervasive developmental disorders, just under 12% with a psychoactive substance use disorder, and 16% among patients with ADHD and other behavioral disorders.
But then again, the medications for ADHD tend to suppress appetite.
Obesity was significantly more prevalent among survey respondents who met criteria for food addiction, by a margin of 44.7% to 33.4%.
Food addiction is not an official DSM disorder. In fact, it’s a highly controversial construct: Some behavioral scientists think it has the classic hallmarks of a bona fide eating or substance use disorder; others don’t. Dr. Horsager highlighted the first systematic review of the evidence regarding food addiction, in which the University of Florida, Gainesville, authors concluded: “Overall, findings support food addiction as a unique construct consistent with criteria for other substance use disorder diagnoses. ... Though both behavioral and substance-related factors are implicated in the addictive process, symptoms appear to better fit criteria for substance use disorder than behavioral addiction” (Nutrients. 2018 Apr 12;10[4]:477. doi: 10.3390/nu10040477).
Food addiction is characterized by a compulsion to overeat calorie-dense, highly processed, super-palatable, sugar- and fat-laden foods. In this era of an ongoing global obesity epidemic, the public has become enthralled with the concept; a recent Google search of the term “food addiction” coughed up 288 million results.
The Food Addiction Denmark project findings warrant prospective studies examining whether treatment of food addiction might improve the prognosis of patients with mental disorders, according to Dr. Horsager.
She reported having no financial conflicts regarding her presentation.
COPENHAGEN – Food addiction is threefold more prevalent among individuals with clinically diagnosed mental disorders than in the general population, according to a report from the Food Addiction Denmark (FADK) project.
This finding provides support for the hypothesis that food addiction is a key link in the chain connecting psychiatric disorders to increased risk of obesity, which in turn contributes to the substantially shorter life expectancy of psychiatric patients, Christina Horsager, MD, a cofounder of the project, said at the annual congress of the European College of Neuropsychopharmacology.
The FADK project is designed to fill in major gaps in the understanding of food addiction. The project included a 2018 Danish nationwide questionnaire survey of 1,394 individuals with various mental disorders and 1,699 others from the general population. The questionnaire included the Yale Food Addiction Scale Version 2.0 (Psychol Addict Behav. 2016 Feb;30[1]:113-21), which was used to identify affected individuals, as well as psychopathology rating scales, explained Dr. Horsager, of the child and adolescent psychiatry department at Aalborg (Denmark) University Hospital.
The prevalence of food addiction was 9% in the general population and 26.5% in individuals with mental disorders. The highest prevalence was, not surprisingly, in individuals with a DSM-5 diagnosis of an eating disorder. The rate was 30% in individuals with a DSM-5 personality disorder, 28% in those with a mood disorder, 17% with autism and other pervasive developmental disorders, just under 12% with a psychoactive substance use disorder, and 16% among patients with ADHD and other behavioral disorders.
But then again, the medications for ADHD tend to suppress appetite.
Obesity was significantly more prevalent among survey respondents who met criteria for food addiction, by a margin of 44.7% to 33.4%.
Food addiction is not an official DSM disorder. In fact, it’s a highly controversial construct: Some behavioral scientists think it has the classic hallmarks of a bona fide eating or substance use disorder; others don’t. Dr. Horsager highlighted the first systematic review of the evidence regarding food addiction, in which the University of Florida, Gainesville, authors concluded: “Overall, findings support food addiction as a unique construct consistent with criteria for other substance use disorder diagnoses. ... Though both behavioral and substance-related factors are implicated in the addictive process, symptoms appear to better fit criteria for substance use disorder than behavioral addiction” (Nutrients. 2018 Apr 12;10[4]:477. doi: 10.3390/nu10040477).
Food addiction is characterized by a compulsion to overeat calorie-dense, highly processed, super-palatable, sugar- and fat-laden foods. In this era of an ongoing global obesity epidemic, the public has become enthralled with the concept; a recent Google search of the term “food addiction” coughed up 288 million results.
The Food Addiction Denmark project findings warrant prospective studies examining whether treatment of food addiction might improve the prognosis of patients with mental disorders, according to Dr. Horsager.
She reported having no financial conflicts regarding her presentation.
COPENHAGEN – Food addiction is threefold more prevalent among individuals with clinically diagnosed mental disorders than in the general population, according to a report from the Food Addiction Denmark (FADK) project.
This finding provides support for the hypothesis that food addiction is a key link in the chain connecting psychiatric disorders to increased risk of obesity, which in turn contributes to the substantially shorter life expectancy of psychiatric patients, Christina Horsager, MD, a cofounder of the project, said at the annual congress of the European College of Neuropsychopharmacology.
The FADK project is designed to fill in major gaps in the understanding of food addiction. The project included a 2018 Danish nationwide questionnaire survey of 1,394 individuals with various mental disorders and 1,699 others from the general population. The questionnaire included the Yale Food Addiction Scale Version 2.0 (Psychol Addict Behav. 2016 Feb;30[1]:113-21), which was used to identify affected individuals, as well as psychopathology rating scales, explained Dr. Horsager, of the child and adolescent psychiatry department at Aalborg (Denmark) University Hospital.
The prevalence of food addiction was 9% in the general population and 26.5% in individuals with mental disorders. The highest prevalence was, not surprisingly, in individuals with a DSM-5 diagnosis of an eating disorder. The rate was 30% in individuals with a DSM-5 personality disorder, 28% in those with a mood disorder, 17% with autism and other pervasive developmental disorders, just under 12% with a psychoactive substance use disorder, and 16% among patients with ADHD and other behavioral disorders.
But then again, the medications for ADHD tend to suppress appetite.
Obesity was significantly more prevalent among survey respondents who met criteria for food addiction, by a margin of 44.7% to 33.4%.
Food addiction is not an official DSM disorder. In fact, it’s a highly controversial construct: Some behavioral scientists think it has the classic hallmarks of a bona fide eating or substance use disorder; others don’t. Dr. Horsager highlighted the first systematic review of the evidence regarding food addiction, in which the University of Florida, Gainesville, authors concluded: “Overall, findings support food addiction as a unique construct consistent with criteria for other substance use disorder diagnoses. ... Though both behavioral and substance-related factors are implicated in the addictive process, symptoms appear to better fit criteria for substance use disorder than behavioral addiction” (Nutrients. 2018 Apr 12;10[4]:477. doi: 10.3390/nu10040477).
Food addiction is characterized by a compulsion to overeat calorie-dense, highly processed, super-palatable, sugar- and fat-laden foods. In this era of an ongoing global obesity epidemic, the public has become enthralled with the concept; a recent Google search of the term “food addiction” coughed up 288 million results.
The Food Addiction Denmark project findings warrant prospective studies examining whether treatment of food addiction might improve the prognosis of patients with mental disorders, according to Dr. Horsager.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM ECNP 2019
A cigarette in one hand and a Fitbit on the other
A cardiologist friend of mine told me a story about one of his patients. The man had recently been in to see him for an office visit. He had quite a scare needing two stents after an episode of prolonged chest pain and, during the office visit, apparently had said that he had “found religion” and was going to change his ways. He showed off the Fitbit that he had gotten and shared his excitement about using a new app to track his diet on his smart phone. His blood pressure was a little elevated, so my friend added a third antihypertensive in an effort to get his blood pressure under control. He referred the patient back to his primary care physician to address his elevated hemoglobin A1c.
My friend saw the patient again a couple of weeks later – this time at the mall. As he was driving through the parking lot, he noticed his patient sitting on a bench outside the entrance. He also noticed a cigarette in his patient’s right hand and saw the Fitbit still on his wrist. Now, it’s not that there is anything wrong with wearing a Fitbit, but …
My friend is an incredibly respectful person, and very nice. He decided not to say hello and risk embarrassing his patient, so he walked to a different door far from the bench and went inside. Nonetheless, the image bothered him. It bothered him enough to repeat the story to me 2 weeks later. It bothers me too.
The other day I was talking to a healthy young nurse with whom I work. She has been trying to get into shape, and her goal is to get to the gym 5 days a week after work. She read on a popular website that she should use a heart rate monitor to keep track of her training and that, if her heart rate is too slow, she should run faster and, if her heart rate is too fast, she should slow down. She was discouraged the other day, however, because her watch indicated that her pulse was going up to 170 while she was running hard, and she had heard that could be dangerous for her heart.
When she doesn’t push hard, though, she told me that her heart rate often plateaus at about 110, sometimes 115. She has been finding it difficult to achieve her calculated target heart rate of 120-160 beats per minute. She is frustrated and was going to skip her workout that evening. I explained to her that she should stop checking her pulse and just run – if she felt she was running too slow she could run faster.
With everything that we have learned about science and technology, the reality is that we are still people, with all our weaknesses and strengths. We often set goals with ambivalence, then rush forward hoping that a technological solution will move us in the direction we think we want to move. Unfortunately, owning a Fitbit will not make us more fit, and checking our pulse every five minutes while working out will not lead to a better exercise session. With the availability of so much technology for tracking our daily exercise, vital signs, and various other measures of health, we need to be more careful than ever to determine specifically what it is that we are trying to accomplish with the use of our technology.
When it comes to good health, it is the fundamentals that matter, and achieving the fundamentals requires being mindful and making repeated efforts to master them. For almost all adults, the most important habits to develop are still related to diet and exercise. Consuming the right diet and exercising adequately requires that the correct choices be made each and every day, all day long. Technology can help but will not do it for us. We need to be thoughtful about how we use technology and explicit about how we expect it to help. After a reasonable amount of time, we should evaluate to see if it is working for us. If it is, then we should continue to use it. If it is not, then we should stop using it or make a different change, like performing a new type of exercise.
Our goal should be to have intelligent empathic integration of technological and behavioral techniques to achieve an optimal health outcome. Putting running shoes by the bed at night is a great thing to do to encourage us to run in the morning. Choosing motivational music can help us get the energy and enthusiasm to go for that run (our favorites include the Rocky theme song and “I Didn’t Come this Far to Only Come this Far”). A visual reminder over the refrigerator can “nudge” us to make good choices as we open the door.
For those who want to learn more about how to integrate behavioral management into their advice for patients we highly recommend reading “Switch: How to Change Things When Change Is Hard” by Chip Heath and “Nudge: Improving Decisions About Health, Wealth, and Happiness” by Richard Thaler. We have always been, and remain, excited about the promise of technology to help us accomplish our goals. That said, we told the nurse to stop checking her pulse, to put on some music, and to appreciate the leaves on the trees this autumn while she was running. As for the gentleman outside the mall, well …
We are interested in your thoughts. Please email us at [email protected].
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A cardiologist friend of mine told me a story about one of his patients. The man had recently been in to see him for an office visit. He had quite a scare needing two stents after an episode of prolonged chest pain and, during the office visit, apparently had said that he had “found religion” and was going to change his ways. He showed off the Fitbit that he had gotten and shared his excitement about using a new app to track his diet on his smart phone. His blood pressure was a little elevated, so my friend added a third antihypertensive in an effort to get his blood pressure under control. He referred the patient back to his primary care physician to address his elevated hemoglobin A1c.
My friend saw the patient again a couple of weeks later – this time at the mall. As he was driving through the parking lot, he noticed his patient sitting on a bench outside the entrance. He also noticed a cigarette in his patient’s right hand and saw the Fitbit still on his wrist. Now, it’s not that there is anything wrong with wearing a Fitbit, but …
My friend is an incredibly respectful person, and very nice. He decided not to say hello and risk embarrassing his patient, so he walked to a different door far from the bench and went inside. Nonetheless, the image bothered him. It bothered him enough to repeat the story to me 2 weeks later. It bothers me too.
The other day I was talking to a healthy young nurse with whom I work. She has been trying to get into shape, and her goal is to get to the gym 5 days a week after work. She read on a popular website that she should use a heart rate monitor to keep track of her training and that, if her heart rate is too slow, she should run faster and, if her heart rate is too fast, she should slow down. She was discouraged the other day, however, because her watch indicated that her pulse was going up to 170 while she was running hard, and she had heard that could be dangerous for her heart.
When she doesn’t push hard, though, she told me that her heart rate often plateaus at about 110, sometimes 115. She has been finding it difficult to achieve her calculated target heart rate of 120-160 beats per minute. She is frustrated and was going to skip her workout that evening. I explained to her that she should stop checking her pulse and just run – if she felt she was running too slow she could run faster.
With everything that we have learned about science and technology, the reality is that we are still people, with all our weaknesses and strengths. We often set goals with ambivalence, then rush forward hoping that a technological solution will move us in the direction we think we want to move. Unfortunately, owning a Fitbit will not make us more fit, and checking our pulse every five minutes while working out will not lead to a better exercise session. With the availability of so much technology for tracking our daily exercise, vital signs, and various other measures of health, we need to be more careful than ever to determine specifically what it is that we are trying to accomplish with the use of our technology.
When it comes to good health, it is the fundamentals that matter, and achieving the fundamentals requires being mindful and making repeated efforts to master them. For almost all adults, the most important habits to develop are still related to diet and exercise. Consuming the right diet and exercising adequately requires that the correct choices be made each and every day, all day long. Technology can help but will not do it for us. We need to be thoughtful about how we use technology and explicit about how we expect it to help. After a reasonable amount of time, we should evaluate to see if it is working for us. If it is, then we should continue to use it. If it is not, then we should stop using it or make a different change, like performing a new type of exercise.
Our goal should be to have intelligent empathic integration of technological and behavioral techniques to achieve an optimal health outcome. Putting running shoes by the bed at night is a great thing to do to encourage us to run in the morning. Choosing motivational music can help us get the energy and enthusiasm to go for that run (our favorites include the Rocky theme song and “I Didn’t Come this Far to Only Come this Far”). A visual reminder over the refrigerator can “nudge” us to make good choices as we open the door.
For those who want to learn more about how to integrate behavioral management into their advice for patients we highly recommend reading “Switch: How to Change Things When Change Is Hard” by Chip Heath and “Nudge: Improving Decisions About Health, Wealth, and Happiness” by Richard Thaler. We have always been, and remain, excited about the promise of technology to help us accomplish our goals. That said, we told the nurse to stop checking her pulse, to put on some music, and to appreciate the leaves on the trees this autumn while she was running. As for the gentleman outside the mall, well …
We are interested in your thoughts. Please email us at [email protected].
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A cardiologist friend of mine told me a story about one of his patients. The man had recently been in to see him for an office visit. He had quite a scare needing two stents after an episode of prolonged chest pain and, during the office visit, apparently had said that he had “found religion” and was going to change his ways. He showed off the Fitbit that he had gotten and shared his excitement about using a new app to track his diet on his smart phone. His blood pressure was a little elevated, so my friend added a third antihypertensive in an effort to get his blood pressure under control. He referred the patient back to his primary care physician to address his elevated hemoglobin A1c.
My friend saw the patient again a couple of weeks later – this time at the mall. As he was driving through the parking lot, he noticed his patient sitting on a bench outside the entrance. He also noticed a cigarette in his patient’s right hand and saw the Fitbit still on his wrist. Now, it’s not that there is anything wrong with wearing a Fitbit, but …
My friend is an incredibly respectful person, and very nice. He decided not to say hello and risk embarrassing his patient, so he walked to a different door far from the bench and went inside. Nonetheless, the image bothered him. It bothered him enough to repeat the story to me 2 weeks later. It bothers me too.
The other day I was talking to a healthy young nurse with whom I work. She has been trying to get into shape, and her goal is to get to the gym 5 days a week after work. She read on a popular website that she should use a heart rate monitor to keep track of her training and that, if her heart rate is too slow, she should run faster and, if her heart rate is too fast, she should slow down. She was discouraged the other day, however, because her watch indicated that her pulse was going up to 170 while she was running hard, and she had heard that could be dangerous for her heart.
When she doesn’t push hard, though, she told me that her heart rate often plateaus at about 110, sometimes 115. She has been finding it difficult to achieve her calculated target heart rate of 120-160 beats per minute. She is frustrated and was going to skip her workout that evening. I explained to her that she should stop checking her pulse and just run – if she felt she was running too slow she could run faster.
With everything that we have learned about science and technology, the reality is that we are still people, with all our weaknesses and strengths. We often set goals with ambivalence, then rush forward hoping that a technological solution will move us in the direction we think we want to move. Unfortunately, owning a Fitbit will not make us more fit, and checking our pulse every five minutes while working out will not lead to a better exercise session. With the availability of so much technology for tracking our daily exercise, vital signs, and various other measures of health, we need to be more careful than ever to determine specifically what it is that we are trying to accomplish with the use of our technology.
When it comes to good health, it is the fundamentals that matter, and achieving the fundamentals requires being mindful and making repeated efforts to master them. For almost all adults, the most important habits to develop are still related to diet and exercise. Consuming the right diet and exercising adequately requires that the correct choices be made each and every day, all day long. Technology can help but will not do it for us. We need to be thoughtful about how we use technology and explicit about how we expect it to help. After a reasonable amount of time, we should evaluate to see if it is working for us. If it is, then we should continue to use it. If it is not, then we should stop using it or make a different change, like performing a new type of exercise.
Our goal should be to have intelligent empathic integration of technological and behavioral techniques to achieve an optimal health outcome. Putting running shoes by the bed at night is a great thing to do to encourage us to run in the morning. Choosing motivational music can help us get the energy and enthusiasm to go for that run (our favorites include the Rocky theme song and “I Didn’t Come this Far to Only Come this Far”). A visual reminder over the refrigerator can “nudge” us to make good choices as we open the door.
For those who want to learn more about how to integrate behavioral management into their advice for patients we highly recommend reading “Switch: How to Change Things When Change Is Hard” by Chip Heath and “Nudge: Improving Decisions About Health, Wealth, and Happiness” by Richard Thaler. We have always been, and remain, excited about the promise of technology to help us accomplish our goals. That said, we told the nurse to stop checking her pulse, to put on some music, and to appreciate the leaves on the trees this autumn while she was running. As for the gentleman outside the mall, well …
We are interested in your thoughts. Please email us at [email protected].
Dr. Notte is a family physician and associate chief medical information officer for Abington (Pa.) Jefferson Health. Follow him on Twitter @doctornotte. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Drug crisis continues to evolve beyond opioids
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.
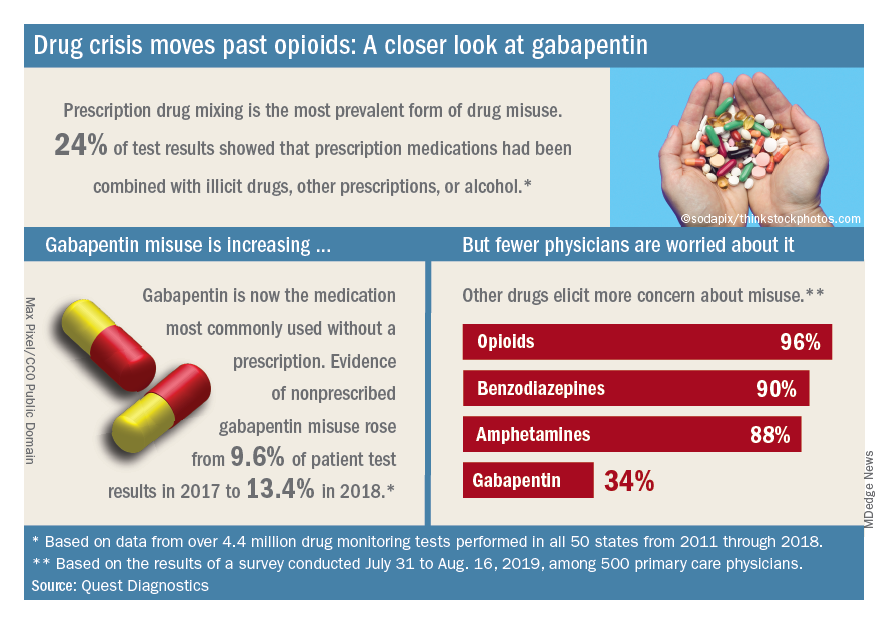
“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.












