User login
A patient-centered approach to tapering opioids
Many Americans who are treated with prescription opioid analgesics would be better off with less opioid or none at all. To that end, published opioid prescribing guidelines do provide guidance on the mechanics of tapering patients off opioids1-4—but they have a major flaw: They do not adequately account for the fact that people who have a diagnosis of chronic pain are a heterogeneous group and require diagnosis-specific treatment planning. A patient-centered approach to opioid tapers must account for the reality that many people who are given a prescription for an opioid to treat pain have significant mental health conditions—for which opioids act as a psychotropic agent. An opioid taper must therefore address psychological trauma, in particular.5 (See “Tapering and harm-reduction strategies have failed.”6-14)
SIDEBAR
Tapering and harm-reduction strategies have failed
Efforts to address the rising number of overdose events that involve opioids began in earnest in 2010. In a 2011 Government Accountability Office report to Congress, the Drug Enforcement Agency reported that “the number of regulatory investigations (of medical providers who prescribed opioids) tripled between fiscal years 2009- 2010.”6
How has it gone since 2010? High-dosage prescribing of opioids has fallen by 48% since 2011, yet the decline has not reduced overdose events of any kind.7,8 Just the opposite: The 19,000 overdose deaths recorded in 2010 involving any opioid increased to 49,068 by 2017, the National Institute on Drug Abuse reports.9 The increase in opioid overdose deaths is fueled by a recent 9-fold increase in consumption of the synthetic opioid fentanyl: “The rate of drug overdose deaths involving synthetic opioids other than methadone … increased on average by 8% per year from 1999 through 2013 and by 71% per year from 2013 through 2017.”10
These and other statistics document only a modest rise in deaths that involve prescription opioids: from 15,000 in 2010 to 19,000 in 2016.9,10 Since 2010, the crisis of opioid overdose deaths burns hotter, and the pattern of opioid use has shifted from prescription drugs to much deadlier illicit drugs, such as heroin.
Interventions have not been successful overall. Results of research focused on the impact of opioid tapering and harm-reduction strategies implemented this decade are likewise discouraging. In 2018, the US Department of Veterans Affairs reported that opioid discontinuation was not associated with a reduction in overdose but was associated with an increase in suicide.11,12 Von Korff and colleagues, in a 2017 report, concluded that “Long-term implementation of opioid dose and risk reduction initiatives [in Washington state] was not associated with lower rates of prescription opioid use disorder among prevalent [chronic opioid therapy] patients.”13
Evidence suggests that efforts to address the opioid crisis of the past decade have had an effect that is the opposite of what was intended. The federal government recognized this in April 2019 in a Drug Safety Communication: “The US Food and Drug Administration (FDA) has received reports of serious harm in patients who are physically dependent on opioid pain medicines suddenly having these medicines discontinued or the dose rapidly decreased. These include serious withdrawal symptoms, uncontrolled pain, psychological distress, and suicide.”14
In this article, we present an evidence-based consensus approach to opioid tapering for your practice that is informed by a broader understanding of why patients take prescription opioids and why they, occasionally, switch to illicit drugs when their prescription is tapered. This consensus approach is based on the experience of the authors, members of the pain faculty of Project ECHO (Extension for Community Healthcare Outcomes) of the ECHO Institute, a worldwide initiative that uses adult learning techniques and interactive video technology to connect community providers with specialists at centers of excellence in regular real-time collaborative sessions. We are variously experts in pain medicine, primary care, psychology, addiction medicine, pharmacy, behavioral health therapy, occupational medicine, and Chinese medicine.
Why Americans obtain prescription opioids
There are 4 principal reasons why patients obtain prescription opioids, beyond indicated analgesic uses:
1. Patients seek the antianxiety and antidepressant effects of opioids. Multiple converging lines of evidence suggest that antianxiety and antidepressant effects of opioids are a significant reason that patients in the United States persist in requesting prescriptions for opioids:
- In our experience with more than 500 primary care telemedicine case presentations, at least 50% of patients say that the main effect of opioids prescribed for them is “it makes me feel calm” or “more relaxed.”
- In a 2007 survey of 91,823 US residents older than 18 years, nonmedical use of opioids was statistically associated with panic, social anxiety, and depressive symptoms.15
- Ten years later, Von Korff and colleagues found that more than half of opioid prescriptions written in the United States were for the small percentage of patients who have a diagnosis of serious anxiety or depression.13
- In 2016, Yovell and colleagues reported that ultra-low-dosage buprenorphine markedly reduced suicidal ideation over 4 weeks in 62 patients with varied levels of depression.16
There is also mechanistic evidence that the antianxiety and antidepressant effects of opioids are significant reasons Americans persist in requesting prescription opioids. The literature suggests that opioid receptors play a role in mood regulation, including alleviation of depression and anxiety; recent research suggests that oxycodone might be a unique mood-altering drug compared to other common prescription opioids because of its ability to affect mood through the δ opioid receptor.17-20
It should not be a surprise that Americans often turn to opioids to address posttraumatic stress disorder (PTSD), anxiety, and depression. A recent study of the state of the US mental health system concluded that mental health services in the United States are inadequate—despite evidence that > 50% of Americans seek, or consider seeking, treatment for mental health problems for themselves or others.21
2. Patients experience pain unrelated to tissue damage. Rather, they are in pain “for psychological reasons.”22 In 2016, Davis and Vanderah wrote: “We theorize that a functional change in the [central nervous system] can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents).” They connect this change to central sensitization and a reduced pain-perception threshold,23 and strongly suspect that many patients with chronic pain have undiagnosed and untreated psychological trauma that has changed the way their central nervous system processes sensory stimuli. The authors call this “trauma-induced hyperalgesia.”
Continue to: Psychological trauma...
Psychological trauma is uniquely capable of producing hyperalgesia, compared to anxiety or depression. In a study of veterans, Defrin and colleagues demonstrated hyperalgesia in patients who had a diagnosis of PTSD but not in controls group who had an anxiety disorder only.24
To support successful opioid tapering, trauma-induced hyperalgesia, when present, must be addressed. Treatment of what the International Association for the Study of Pain calls “pain due to psychological factors”22 requires specific trauma therapy. However, our experience validates what researchers have to say about access to treatment of psychological trauma in the United States: “…[C]linical research has identified certain psychological interventions that effectively ameliorate the symptoms of PTSD. But most people struggling with PTSD don’t receive those treatments.”25
We have no doubt that this is due, in part, to underdiagnosis of psychological trauma, even in mental health clinics. According to Miele and colleagues, “PTSD remains largely undiagnosed and undertreated in mental health outpatients, even in teaching hospitals, with diagnosis rates as low as 4% while published prevalence is between 7% and 50% in this population.”26
3. Patients suffer from opioid use disorder (OUD) and complain of pain to obtain opioids by prescription. For patients with OUD, their use is out of control; they devote increasing mental and physical resources to obtaining, using, and recovering from substances; and they continue to use despite adverse consequences.27 The prevalence of OUD in primary care clinics varies strikingly by the location of clinics. In Washington state, the prevalence of moderate and severe OUD in a large population of patients who had been prescribed opioids through primary care clinics was recently determined to be between 21.5% and 23.9%.13
4. Patients are obtaining opioid prescriptions for people other than themselves. While this is a reason that patients obtain opioid prescriptions, it is not necessarily common. Statistics show that the likelihood of a prescription being diverted intentionally is low: Dart and colleagues found that diversion has become uncommon in the general population.28
Continue to: Why we taper opioid analgesics
Why we taper opioid analgesics
Reasons for an opioid taper include concern that the patient has, or will develop, an OUD; will experience accidental or intentional overdose; might be diverting opioids; is not benefiting from opioid therapy for pain; or is experiencing severe adverse effects. A patient who has nociceptive pain and might have opioid-induced hyperalgesia will require a much different opioid taper plan than a patient with untreated PTSD or a patient with severe OUD.
Misunderstanding can lead to inappropriate tapering
We often encounter primary care providers who believe that a large percentage of patients on chronic opioid therapy inevitably develop OUD. This is a common reason for initiating opioid taper. Most patients on a chronic opioid do become physically dependent, but only a small percentage of patients develop psychological dependence (ie, addiction or OUD).29
Physical dependence is “a state of adaptation that is manifested by a drug class–specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.”30 Symptoms of opioid withdrawal include muscle aches; abdominal cramping; increased lacrimation, rhinorrhea, and perspiration; diarrhea; agitation and anxiety; insomnia; and piloerection. Opioid withdrawal symptoms are caused by physical dependence, not by addiction. They can be mitigated by tapering slowly and instituting adjuvant medications, such as clonidine, to attenuate symptoms.
Psychological dependence, or addiction (that is, OUD, as described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition27), comprises primarily 3 behavioral criteria:
- Loss of control of the medication, with compulsive use
- Continued use despite adverse consequences of using opioids, such as arrest for driving under the influence and deterioration of social, family, or work performance
- Obsession or preoccupation with obtaining and using the substance. In properly selected chronic opioid therapy patients, there is evidence that new-onset OUD is not as common as has been thought. A recent study of the risk for opioid addiction after use of an opioid for ≥ 90 days for chronic noncancer pain found that the absolute rate of de novo OUD among patients treated for 90 days was 0.72%.29 A systematic review by Fishbain and colleagues of 24 studies of opioid-exposed patients found a risk of 3.27% overall—0.19% for patients who did not have a history of abuse or addiction.31 As Director of the National Institute on Drug Abuse Norma Volkow, MD, wrote in 2016: “Addiction occurs in only a small percentage of people who are exposed to opioids—even among those with preexisting vulnerabilities.”32
Assessment should focus on why the patient is taking an opioid
A strong case can be made that less opioid is better for many of the people for whom these medications are prescribed for chronic noncancer pain. However, a one-size-fits-all dosage reduction and addiction-focused approach to opioid tapering has not worked: The assessment and treatment paradigm must change, in our view.
Continue to: During assessment...
During assessment, we must adopt the means to identify the reason that a patient is using a prescription opioid. It is of particular importance that we identify patients using opioids for their psychotropic properties, particularly when the goal is to cope with the effects of psychological trauma. The subsequent treatment protocol will then need to include time for effective, evidence-based behavioral health treatment of anxiety, PTSD, or depression. If opioids are serving primarily as psychotropic medication, an attempt to taper before establishing effective behavioral health treatment might lead the patient to pursue illegal means of procuring opioid medication.
We acknowledge that primary care physicians are not reimbursed for trauma screening and that evidence-based intensive trauma treatment is generally unavailable in the United States. Both of these shortcomings must be corrected if we want to stem the opioid crisis.
If diversion is suspected and there is evidence that the patient is not currently taking prescribed opioids (eg, a negative urine drug screen), discontinuing the opioid prescription is the immediate next step for the sake of public safety.
SIDEBAR
2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain
#1 Should I provide the patient with a prescription for an opioid for a few days, while I await more information?a
Yes. Writing a prescription is a reasonable decision if all of the following apply:
- You do not have significant suspicion of diversion (based on a clinical interview).
- You do not suspect an active addiction disorder, based on the score of the 10-question Drug Abuse Screening Test (DAST-10) and on a clinical interview. (DAST-10 is available at: https://cde.drugabuse.gov/instrument/e9053390-ee9c-9140-e040-bb89ad433d69.)
- The patient is likely to experience withdrawal symptoms if you don’t provide the medication immediately.
- The patient’s pain and function are likely to be impaired if you do not provide the medication.
- The patient does not display altered mental status during the visit (eg, drowsy, slurred speech).
No. If writing a prescription for an opioid for a few days does not seem to be a reasonable decision because the criteria above are not met, but withdrawal symptoms are likely, you can prescribe medication to mitigate symptoms or refer the patient for treatment of withdrawal.
#2 I’ve decided to provide the patient with a prescription for an opioid. For how many days should I write it?
The usual practice, for a patient whose case is familiar to you, is to prescribe a 1-month supply.
However, if any 1 of the following criteria is met, prescribing a 1-month supply is unsafe under most circumstances:
- An unstable social or living environment places the patient at risk by possessing a supply of opioids (based on a clinical interview).
- You suspect an unstable or severe behavioral health condition or a mental health diagnosis (based on a clinical interview or on the patient record from outside your practice).
- The patient scores as “high risk” on the Opioid Risk Tool (ORT; www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf), Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R; www.ncbi.nlm.nih.gov/pmc/articles/PMC4706778/), or a similar opioid risk assessment tool.
When 1 or more of these exclusionary criteria are met, you have 3 options:
- Prescribe an opioid for a brief duration and see the patient often.
- Do not prescribe an opioid; instead, refer the patient as necessary for treatment of withdrawal.
- Refer the patient for treatment of the underlying behavioral health condition.
a Additional information might include findings from consultants you’ve engaged regarding the patient’s diagnosis; a response to your call from a past prescriber; urine drug screen results; and results of a prescription monitoring program check.
Considering a taper? Take this 5-step approach
Once it appears that tapering an opioid is indicated, we propose that you take the following steps:
- Establish whether it is safe to continue prescribing (follow the route provided in “2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain”); if continuing it is not safe, take steps to protect the patient and the community
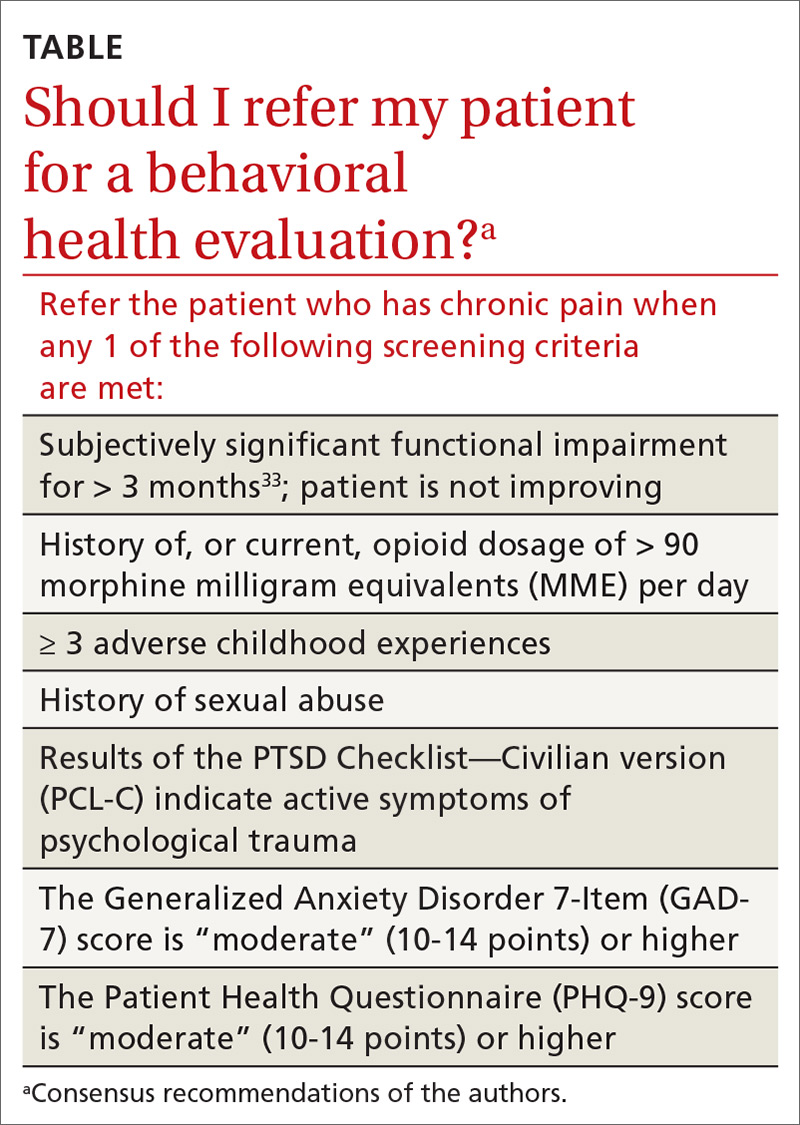
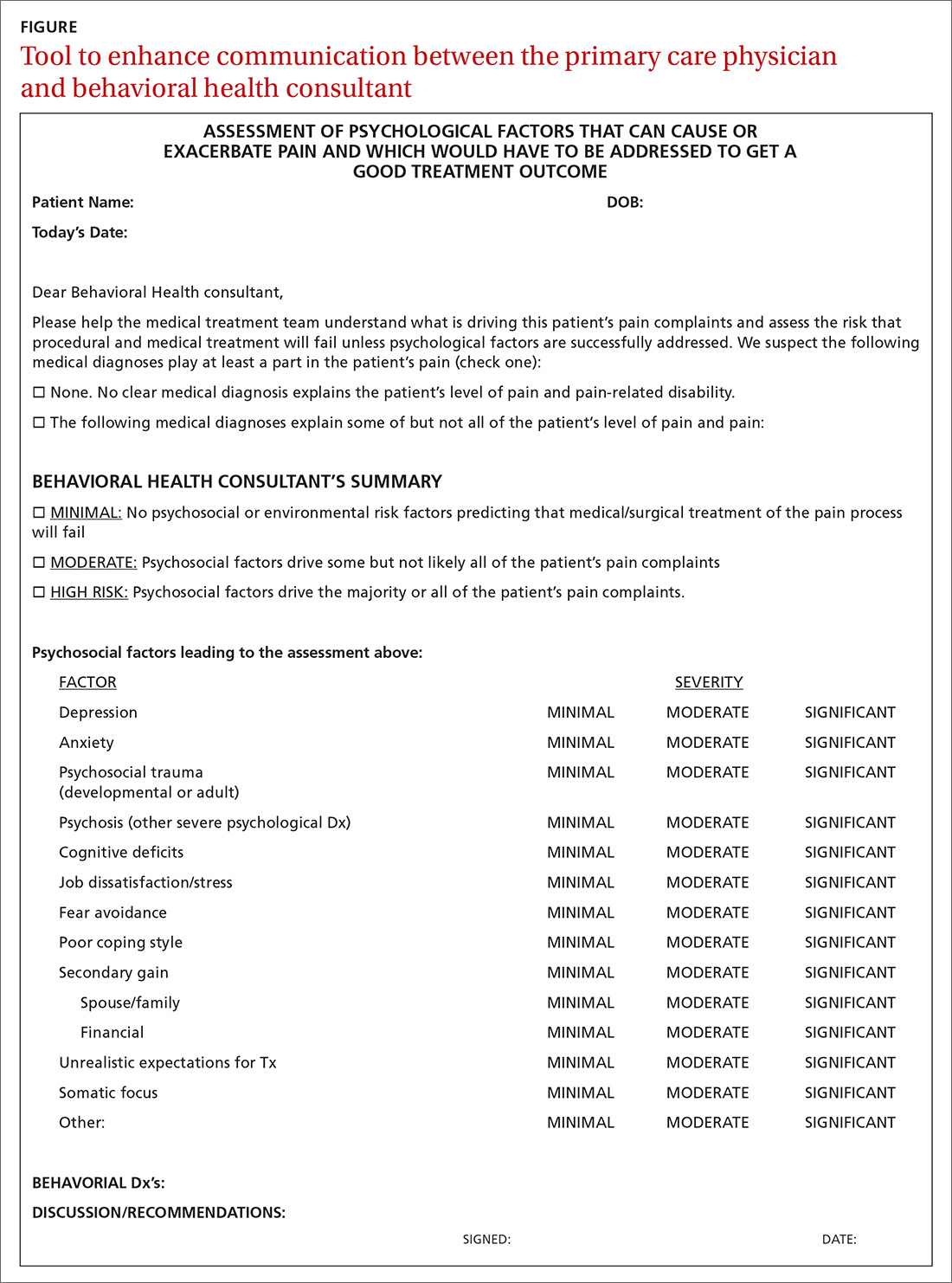
- Determine whether assessment by a trauma-informed behavioral health expert is needed, assuming that, in your judgment, it is safe to continue the opioid (TABLE33). When behavioral health assessment is needed, you need 3 questions answered by that assessment: (1) Are psychological factors present that might put the patient at risk during an opioid taper? (2) What are those factors? (3) What needs to done about them before the taper is started? Recalling that psychological trauma often is not assessed by behavioral health colleagues, it is necessary to provide the behavioral health provider with a specific request to assess trauma burden, and state the physical diagnoses that are causing pain or provide a clear statement that no such diagnoses can be made. (See the FIGURE, which we developed in conjunction with behavioral health colleagues to help the consultant understand what the primary care physician needs from a behavioral health assessment.)
- Obtain consultation from a physical therapist, pain medicine specialist, and, if possible, an alternative or complementary medicine provider to determine what nonpharmacotherapeutic modalities can be instituted to treat pain before tapering the opioid.
- Initiate the Screening, Brief Intervention and Referral to Treatment (SBIRT) approach if OUD is suspected (www.samhsa.gov/sbirt).34 This motivational interviewing tool identifies patients with a substance use disorder, severity of use, and appropriate level of treatment. (If OUD is suspected during assessment, next steps are to stop prescribing and implement harm-reduction strategies, such as primary care level medically assisted treatment [MAT] with buprenorphine, followed by expert behavioral health-centered addiction treatment.)
- Experiment with dosage reduction according to published guidance, if (1) psychological factors are absent or have been adequately addressed, according to the behavioral health consultant, and (2) nonpharmacotherapeutic strategies are in place.8-11
Shifting to a patient-centered approach
The timing and choice of opioid tapers, in relation to harm reduction and intervention targeting the root cause of a patient’s complaint of pain, have not been adequately explored. In our practice, we’ve shifted from an addiction-centered, dosage-centered approach to opioid taper to a patient-centered approach35 that emphasizes behavioral-medical integration—an approach that we broadly endorse. Such an approach (1) is based on a clear understanding of why the patient is taking opioid pain medication, (2) engages medical and complementary or alternative medicine specialists, (3) addresses underdiagnosis of psychological trauma, and (4) requires a quantum leap in access to trauma-specific behavioral health treatment resources. 36
Continue to: To underscore the case...
To underscore the case for shifting to a patient-centered approach35 we present sample cases in “How a patient-centered approach to tapering opioids looks in practice.”
SIDEBAR
How a patient-centered approach to tapering opioids looks in practice
Five hypothetical cases illustrate what might happen when a practice shifts from an addiction-centered, dosage-centered approach to one that places the individual at the center of care.
CASE #1: Brett F
Mr. F appears to use medication responsibly; benefits functionally from an opioid; has tolerable adverse effects; does not have significant psychosocial risk factors (based on the score of the Opioid Risk Tool [ORT] or the Screener and Opioid Assessment for Patients with Pain–Revised [SOAPP-R]); and is engaged in effective self-management. Most of Mr. F’s pain is thought to have a nociceptive or neuropathic source.
Mr F could reasonably contemplate continuing current opioid treatment.
Action: If the daily morphine milligram equivalent (MME) dosage is high, Mr. F should be referred to a pain medicine specialist. We recommend a periodic (at least annually) empiric trial of dosage reduction to see whether he is indeed best served by the current dosage.
CASE #2: Brett F (version 2.0)
Envision Mr. F having the same profile in all respects except that he is not engaged in effective self-management.
Optimal treatment of chronic pain often requires supplemental modalities beyond opioids.
Action: Physical therapy; an individualized, ongoing exercise regimen; interventional procedures; weight loss (if the patient is obese); smoking cessation; and improving coping skills for anxiety and depression without pharmacotherapy might not only temporarily alleviate the pain but, over time, improve Mr. F’s physical condition.
If Mr. F is not willing to do more than take the prescribed opioids, nothing is likely to change: Over time, his condition is likely to deteriorate. A patient like Mr. F can be harmed if opioids continue to be prescribed for him long-term.
Further action: If Mr. F won’t engage in broadening the approach to treating his pain, the opioid medication should be tapered, in his long-term best interest. A carrot-and-stick approach can facilitate Mr. F’s involvement in his care.
CASE #3: Clark S
Mr. S has a significant psychosocial component driving his pain: depression.a
Prescribing opioids without addressing the root cause of trauma is not in the patient’s best interest.
Action: Because of Mr. S’s depression, refer him to a behavioral health provider. If you determine that he is emotionally stable, wait until he is engaged in trauma treatment to begin the taper. If he appears unstable (eg, crying in the office, recent psychological stressors, recent impulsive behaviors, poor insight) consider (1) urgent behavioral health referral and (2) prescribing only enough opioid medication (ie, at close intervals) to prevent withdrawal and panic. Consider whether a psychotropic medication might be of benefit (eg, a serotonin–norepinephrine reuptake inhibitor or selective serotonin reuptake inhibitor).
Further action: Harm-reduction steps, such as close monitoring and, perhaps, a change to a buprenorphine product, is indicated, especially when the patient is overwhelmed by recent psychosocial stressors. Harm-reduction treatment is available through Medication-Assisted Therapy (MAT) programs; however, patients often run into difficulty obtaining access to these programs because regulations and laws restrict MAT to patients who have a diagnosis of opioid use disorder (OUD) and because some health plans and pharmacy benefit managers require prior authorization.
CASE #4: Gloria B
Ms. B isn’t managing her medications responsibly—although you don’t suspect OUD.
When a patient has shown the inability to manage opioid medication responsibly, you should delve into the reason to determine your next step.
Action: Evaluate Ms. B for a cognitive disorder or a thought disorder. Alternatively, as in the case of Mr. S, a psychosocial component might underlie her pain; in that case, the same recommendations can be made for her. In addition, you can propose that she identify a responsible person to dispense her medication.
CASE #5: Nicole L
You suspect that Ms. L, who is taking opioid medication to alleviate pain, also has a substance use disorder.
Action: Implement harm-reduction early for Ms. L: Obtain addiction medicine consultation and implement behavioral health strategies for addiction treatment.
A key characteristic of a substance use disorder is loss of control over use of the substance. A patient like Ms. L—who is in pain and who has an active OUD—cannot be expected to manage her opioid use responsibly.
Further action: We recommend that Ms. L be referred to an addiction specialist for MAT. Evidence of the harmreduction benefit of MAT is sufficient to strongly recommend it. Continue any other treatment modalities for pain that Ms. L has been using, such as non-opioid medication, physical therapy, alternative treatments, and behavioral therapy, or begin such treatments as appropriate.
a Depression is not the only psychosocial component that can underlie pain. Others include anxiety, posttraumatic stress disorder, and grief.
An eye toward the future. To inform future approaches to opioid tapering, more resources need to be deployed to
- support screening and risk stratification for PTSD, anxiety, and related disorders at the primary care level,
- continue the effort to identify and treat OUD,
- develop best-practice responses to screening, and
- make harm-reduction strategies that are now reserved for patients with OUD available to those who don't have OUD.
We urge that research be pursued into best practices for chronic pain interventions that target psychological trauma, anxiety, and depression.
CORRESPONDENCE
Bennet Davis MD, 2092 East Calle de Dulcinea, Tucson, AZ 85718; [email protected].
1. Centers for Disease Control and Prevention. Pocket guide: tapering for chronic pain. https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf. Accessed November 25, 2019.
2. Kral LA, Jackson K, Uritsky TJ. A practical guide to tapering opioids. Ment Health Clin. 2015;5:102-108.
3. Murphy L, Babaei-Rad R, Buna D, et al. Guidance on opioid tapering in the context of chronic pain: evidence, practical advice and frequently asked questions. Can Pharm J (Ott). 2018;151:114-120.
4. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2005;90:828-842.
5. Davis M. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:407-417.
6. US Government Accountability Office. Report to Congressional Requestors. Prescription drug control: DEA has enhanced efforts to combat diversion, but could better assess and report program results. August 2011. www.gao.gov/assets/520/511464.pdf. Accessed November 25, 2019.
7. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Annual surveillance report of drug-related risks and outcomes. United States, 2017. www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf. Accessed November 25, 2019.
8. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016, NCHS Data Brief No. 294. December 21, 2017. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/products/databriefs/db294.htm. Accessed November 25, 2019.
9. Overdose death rates. Bethesda, MD: National Institute on Drug Abuse. January 2019. www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed November 25, 2019.
10. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No. 329. November 2018. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/data/databriefs/db329-h.pdf . Accessed November 25, 2019.
11. Manhapra A, Kertesz S, Oliva A, et al. VA data about Rx opioids and overdose and suicide: clinical implications. Presented at the 2018 National Rx Drug Abuse and Heroin Summit, Atlanta Georgia, April 4, 2018.
12. Demidenko M, Dobscha SK, Morasco BJ, et al. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35.
13. Von Korff M, Walker RL, Saunders K, et al. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. Int J Drug Policy. 2017;46:90-98.
14. United States Food and Drug Administration. FDA Drug Safety Communication: FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. April 9, 2019. www.fda.gov/Drugs/DrugSafety/ucm635038.htm. Accessed November 25, 2019.
15. Becker W, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38-47.
16. Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173:491-498.
17. Pradhan AA, Befort K, Nozaki C, et al. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581-590.
18. Sugiyama A, Yamada M, Saitoh A, et al. Administration of a delta opioid receptor agonist KNT-127 to the basolateral amygdala has robust anxiolytic-like effects in rats. Psychopharmacology (Berl). 2018;235:2947-2955.
19. Richards EM, Mathews DC, Luckenbaugh DA, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl). 2016;233:1119-1130.
20. Yang PP, Yeh GC, Yeh TK, et al. Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res. 2016;111:867-876.
21. America’s mental health 2018. Stamford, CT: Cohen Veterans Network. October 10, 2018. https://www.cohenveteransnetwork.org/wp-content/uploads/2018/10/Research-Summary-10-10-2018.pdf. Accessed November 25, 2019.
22. Classification of Chronic Pain, Second Edition (Revised). Washington, DC: International Association for the Study of Pain. Updated 2012. www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673. Accessed November 25, 2019.
23. Davis B, Vanderah TW. A new paradigm for pain? J Fam Pract. 2016 65:598-605.
24. Defrin R, Ginzburg K, Solomon Z, et al. Quantitative testing of pain perception in subjects with PTSD—implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450-459.
25. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Science Public Interest. 2013;14:65-111.
26. Miele D, O’Brien EJ. Underdiagnosis of posttraumatic stress disorder in at risk youth. J Trauma Stress. 2010;23:591-598.
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Publishing; 2013:541.
28. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241-248.
29. Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425-426.
30. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain. 2001. www.naabt.org/documents/APS_consensus_document.pdf. Accessed November 25, 2019.
31. Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444-459.
32. Volkow ND, McClellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253-1263.
33. Treede RD, Rief W, Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003-1007.
34. Screening, brief intervention, and referral to treatment (SBIRT). Rockville, MD: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/sbirt. Accessed November 25, 2019.
35. Schneider JP, Davis B. How well do you know your patient? Pract Pain Manag. 2017;17(2). www.practicalpainmanagement.com/resources/practice-management/how-well-do-you-know-your-patient. Accessed November 25, 2019.
36. Schneider JP. A patient-centered approach to the opioid overdose crisis. J Miss State Med Assoc. 2018;59:232-233.
Many Americans who are treated with prescription opioid analgesics would be better off with less opioid or none at all. To that end, published opioid prescribing guidelines do provide guidance on the mechanics of tapering patients off opioids1-4—but they have a major flaw: They do not adequately account for the fact that people who have a diagnosis of chronic pain are a heterogeneous group and require diagnosis-specific treatment planning. A patient-centered approach to opioid tapers must account for the reality that many people who are given a prescription for an opioid to treat pain have significant mental health conditions—for which opioids act as a psychotropic agent. An opioid taper must therefore address psychological trauma, in particular.5 (See “Tapering and harm-reduction strategies have failed.”6-14)
SIDEBAR
Tapering and harm-reduction strategies have failed
Efforts to address the rising number of overdose events that involve opioids began in earnest in 2010. In a 2011 Government Accountability Office report to Congress, the Drug Enforcement Agency reported that “the number of regulatory investigations (of medical providers who prescribed opioids) tripled between fiscal years 2009- 2010.”6
How has it gone since 2010? High-dosage prescribing of opioids has fallen by 48% since 2011, yet the decline has not reduced overdose events of any kind.7,8 Just the opposite: The 19,000 overdose deaths recorded in 2010 involving any opioid increased to 49,068 by 2017, the National Institute on Drug Abuse reports.9 The increase in opioid overdose deaths is fueled by a recent 9-fold increase in consumption of the synthetic opioid fentanyl: “The rate of drug overdose deaths involving synthetic opioids other than methadone … increased on average by 8% per year from 1999 through 2013 and by 71% per year from 2013 through 2017.”10
These and other statistics document only a modest rise in deaths that involve prescription opioids: from 15,000 in 2010 to 19,000 in 2016.9,10 Since 2010, the crisis of opioid overdose deaths burns hotter, and the pattern of opioid use has shifted from prescription drugs to much deadlier illicit drugs, such as heroin.
Interventions have not been successful overall. Results of research focused on the impact of opioid tapering and harm-reduction strategies implemented this decade are likewise discouraging. In 2018, the US Department of Veterans Affairs reported that opioid discontinuation was not associated with a reduction in overdose but was associated with an increase in suicide.11,12 Von Korff and colleagues, in a 2017 report, concluded that “Long-term implementation of opioid dose and risk reduction initiatives [in Washington state] was not associated with lower rates of prescription opioid use disorder among prevalent [chronic opioid therapy] patients.”13
Evidence suggests that efforts to address the opioid crisis of the past decade have had an effect that is the opposite of what was intended. The federal government recognized this in April 2019 in a Drug Safety Communication: “The US Food and Drug Administration (FDA) has received reports of serious harm in patients who are physically dependent on opioid pain medicines suddenly having these medicines discontinued or the dose rapidly decreased. These include serious withdrawal symptoms, uncontrolled pain, psychological distress, and suicide.”14
In this article, we present an evidence-based consensus approach to opioid tapering for your practice that is informed by a broader understanding of why patients take prescription opioids and why they, occasionally, switch to illicit drugs when their prescription is tapered. This consensus approach is based on the experience of the authors, members of the pain faculty of Project ECHO (Extension for Community Healthcare Outcomes) of the ECHO Institute, a worldwide initiative that uses adult learning techniques and interactive video technology to connect community providers with specialists at centers of excellence in regular real-time collaborative sessions. We are variously experts in pain medicine, primary care, psychology, addiction medicine, pharmacy, behavioral health therapy, occupational medicine, and Chinese medicine.
Why Americans obtain prescription opioids
There are 4 principal reasons why patients obtain prescription opioids, beyond indicated analgesic uses:
1. Patients seek the antianxiety and antidepressant effects of opioids. Multiple converging lines of evidence suggest that antianxiety and antidepressant effects of opioids are a significant reason that patients in the United States persist in requesting prescriptions for opioids:
- In our experience with more than 500 primary care telemedicine case presentations, at least 50% of patients say that the main effect of opioids prescribed for them is “it makes me feel calm” or “more relaxed.”
- In a 2007 survey of 91,823 US residents older than 18 years, nonmedical use of opioids was statistically associated with panic, social anxiety, and depressive symptoms.15
- Ten years later, Von Korff and colleagues found that more than half of opioid prescriptions written in the United States were for the small percentage of patients who have a diagnosis of serious anxiety or depression.13
- In 2016, Yovell and colleagues reported that ultra-low-dosage buprenorphine markedly reduced suicidal ideation over 4 weeks in 62 patients with varied levels of depression.16
There is also mechanistic evidence that the antianxiety and antidepressant effects of opioids are significant reasons Americans persist in requesting prescription opioids. The literature suggests that opioid receptors play a role in mood regulation, including alleviation of depression and anxiety; recent research suggests that oxycodone might be a unique mood-altering drug compared to other common prescription opioids because of its ability to affect mood through the δ opioid receptor.17-20
It should not be a surprise that Americans often turn to opioids to address posttraumatic stress disorder (PTSD), anxiety, and depression. A recent study of the state of the US mental health system concluded that mental health services in the United States are inadequate—despite evidence that > 50% of Americans seek, or consider seeking, treatment for mental health problems for themselves or others.21
2. Patients experience pain unrelated to tissue damage. Rather, they are in pain “for psychological reasons.”22 In 2016, Davis and Vanderah wrote: “We theorize that a functional change in the [central nervous system] can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents).” They connect this change to central sensitization and a reduced pain-perception threshold,23 and strongly suspect that many patients with chronic pain have undiagnosed and untreated psychological trauma that has changed the way their central nervous system processes sensory stimuli. The authors call this “trauma-induced hyperalgesia.”
Continue to: Psychological trauma...
Psychological trauma is uniquely capable of producing hyperalgesia, compared to anxiety or depression. In a study of veterans, Defrin and colleagues demonstrated hyperalgesia in patients who had a diagnosis of PTSD but not in controls group who had an anxiety disorder only.24
To support successful opioid tapering, trauma-induced hyperalgesia, when present, must be addressed. Treatment of what the International Association for the Study of Pain calls “pain due to psychological factors”22 requires specific trauma therapy. However, our experience validates what researchers have to say about access to treatment of psychological trauma in the United States: “…[C]linical research has identified certain psychological interventions that effectively ameliorate the symptoms of PTSD. But most people struggling with PTSD don’t receive those treatments.”25
We have no doubt that this is due, in part, to underdiagnosis of psychological trauma, even in mental health clinics. According to Miele and colleagues, “PTSD remains largely undiagnosed and undertreated in mental health outpatients, even in teaching hospitals, with diagnosis rates as low as 4% while published prevalence is between 7% and 50% in this population.”26
3. Patients suffer from opioid use disorder (OUD) and complain of pain to obtain opioids by prescription. For patients with OUD, their use is out of control; they devote increasing mental and physical resources to obtaining, using, and recovering from substances; and they continue to use despite adverse consequences.27 The prevalence of OUD in primary care clinics varies strikingly by the location of clinics. In Washington state, the prevalence of moderate and severe OUD in a large population of patients who had been prescribed opioids through primary care clinics was recently determined to be between 21.5% and 23.9%.13
4. Patients are obtaining opioid prescriptions for people other than themselves. While this is a reason that patients obtain opioid prescriptions, it is not necessarily common. Statistics show that the likelihood of a prescription being diverted intentionally is low: Dart and colleagues found that diversion has become uncommon in the general population.28
Continue to: Why we taper opioid analgesics
Why we taper opioid analgesics
Reasons for an opioid taper include concern that the patient has, or will develop, an OUD; will experience accidental or intentional overdose; might be diverting opioids; is not benefiting from opioid therapy for pain; or is experiencing severe adverse effects. A patient who has nociceptive pain and might have opioid-induced hyperalgesia will require a much different opioid taper plan than a patient with untreated PTSD or a patient with severe OUD.
Misunderstanding can lead to inappropriate tapering
We often encounter primary care providers who believe that a large percentage of patients on chronic opioid therapy inevitably develop OUD. This is a common reason for initiating opioid taper. Most patients on a chronic opioid do become physically dependent, but only a small percentage of patients develop psychological dependence (ie, addiction or OUD).29
Physical dependence is “a state of adaptation that is manifested by a drug class–specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.”30 Symptoms of opioid withdrawal include muscle aches; abdominal cramping; increased lacrimation, rhinorrhea, and perspiration; diarrhea; agitation and anxiety; insomnia; and piloerection. Opioid withdrawal symptoms are caused by physical dependence, not by addiction. They can be mitigated by tapering slowly and instituting adjuvant medications, such as clonidine, to attenuate symptoms.
Psychological dependence, or addiction (that is, OUD, as described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition27), comprises primarily 3 behavioral criteria:
- Loss of control of the medication, with compulsive use
- Continued use despite adverse consequences of using opioids, such as arrest for driving under the influence and deterioration of social, family, or work performance
- Obsession or preoccupation with obtaining and using the substance. In properly selected chronic opioid therapy patients, there is evidence that new-onset OUD is not as common as has been thought. A recent study of the risk for opioid addiction after use of an opioid for ≥ 90 days for chronic noncancer pain found that the absolute rate of de novo OUD among patients treated for 90 days was 0.72%.29 A systematic review by Fishbain and colleagues of 24 studies of opioid-exposed patients found a risk of 3.27% overall—0.19% for patients who did not have a history of abuse or addiction.31 As Director of the National Institute on Drug Abuse Norma Volkow, MD, wrote in 2016: “Addiction occurs in only a small percentage of people who are exposed to opioids—even among those with preexisting vulnerabilities.”32
Assessment should focus on why the patient is taking an opioid
A strong case can be made that less opioid is better for many of the people for whom these medications are prescribed for chronic noncancer pain. However, a one-size-fits-all dosage reduction and addiction-focused approach to opioid tapering has not worked: The assessment and treatment paradigm must change, in our view.
Continue to: During assessment...
During assessment, we must adopt the means to identify the reason that a patient is using a prescription opioid. It is of particular importance that we identify patients using opioids for their psychotropic properties, particularly when the goal is to cope with the effects of psychological trauma. The subsequent treatment protocol will then need to include time for effective, evidence-based behavioral health treatment of anxiety, PTSD, or depression. If opioids are serving primarily as psychotropic medication, an attempt to taper before establishing effective behavioral health treatment might lead the patient to pursue illegal means of procuring opioid medication.
We acknowledge that primary care physicians are not reimbursed for trauma screening and that evidence-based intensive trauma treatment is generally unavailable in the United States. Both of these shortcomings must be corrected if we want to stem the opioid crisis.
If diversion is suspected and there is evidence that the patient is not currently taking prescribed opioids (eg, a negative urine drug screen), discontinuing the opioid prescription is the immediate next step for the sake of public safety.
SIDEBAR
2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain
#1 Should I provide the patient with a prescription for an opioid for a few days, while I await more information?a
Yes. Writing a prescription is a reasonable decision if all of the following apply:
- You do not have significant suspicion of diversion (based on a clinical interview).
- You do not suspect an active addiction disorder, based on the score of the 10-question Drug Abuse Screening Test (DAST-10) and on a clinical interview. (DAST-10 is available at: https://cde.drugabuse.gov/instrument/e9053390-ee9c-9140-e040-bb89ad433d69.)
- The patient is likely to experience withdrawal symptoms if you don’t provide the medication immediately.
- The patient’s pain and function are likely to be impaired if you do not provide the medication.
- The patient does not display altered mental status during the visit (eg, drowsy, slurred speech).
No. If writing a prescription for an opioid for a few days does not seem to be a reasonable decision because the criteria above are not met, but withdrawal symptoms are likely, you can prescribe medication to mitigate symptoms or refer the patient for treatment of withdrawal.
#2 I’ve decided to provide the patient with a prescription for an opioid. For how many days should I write it?
The usual practice, for a patient whose case is familiar to you, is to prescribe a 1-month supply.
However, if any 1 of the following criteria is met, prescribing a 1-month supply is unsafe under most circumstances:
- An unstable social or living environment places the patient at risk by possessing a supply of opioids (based on a clinical interview).
- You suspect an unstable or severe behavioral health condition or a mental health diagnosis (based on a clinical interview or on the patient record from outside your practice).
- The patient scores as “high risk” on the Opioid Risk Tool (ORT; www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf), Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R; www.ncbi.nlm.nih.gov/pmc/articles/PMC4706778/), or a similar opioid risk assessment tool.
When 1 or more of these exclusionary criteria are met, you have 3 options:
- Prescribe an opioid for a brief duration and see the patient often.
- Do not prescribe an opioid; instead, refer the patient as necessary for treatment of withdrawal.
- Refer the patient for treatment of the underlying behavioral health condition.
a Additional information might include findings from consultants you’ve engaged regarding the patient’s diagnosis; a response to your call from a past prescriber; urine drug screen results; and results of a prescription monitoring program check.

Considering a taper? Take this 5-step approach
Once it appears that tapering an opioid is indicated, we propose that you take the following steps:
- Establish whether it is safe to continue prescribing (follow the route provided in “2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain”); if continuing it is not safe, take steps to protect the patient and the community
- Determine whether assessment by a trauma-informed behavioral health expert is needed, assuming that, in your judgment, it is safe to continue the opioid (TABLE33). When behavioral health assessment is needed, you need 3 questions answered by that assessment: (1) Are psychological factors present that might put the patient at risk during an opioid taper? (2) What are those factors? (3) What needs to done about them before the taper is started? Recalling that psychological trauma often is not assessed by behavioral health colleagues, it is necessary to provide the behavioral health provider with a specific request to assess trauma burden, and state the physical diagnoses that are causing pain or provide a clear statement that no such diagnoses can be made. (See the FIGURE, which we developed in conjunction with behavioral health colleagues to help the consultant understand what the primary care physician needs from a behavioral health assessment.)
- Obtain consultation from a physical therapist, pain medicine specialist, and, if possible, an alternative or complementary medicine provider to determine what nonpharmacotherapeutic modalities can be instituted to treat pain before tapering the opioid.
- Initiate the Screening, Brief Intervention and Referral to Treatment (SBIRT) approach if OUD is suspected (www.samhsa.gov/sbirt).34 This motivational interviewing tool identifies patients with a substance use disorder, severity of use, and appropriate level of treatment. (If OUD is suspected during assessment, next steps are to stop prescribing and implement harm-reduction strategies, such as primary care level medically assisted treatment [MAT] with buprenorphine, followed by expert behavioral health-centered addiction treatment.)
- Experiment with dosage reduction according to published guidance, if (1) psychological factors are absent or have been adequately addressed, according to the behavioral health consultant, and (2) nonpharmacotherapeutic strategies are in place.8-11

Shifting to a patient-centered approach
The timing and choice of opioid tapers, in relation to harm reduction and intervention targeting the root cause of a patient’s complaint of pain, have not been adequately explored. In our practice, we’ve shifted from an addiction-centered, dosage-centered approach to opioid taper to a patient-centered approach35 that emphasizes behavioral-medical integration—an approach that we broadly endorse. Such an approach (1) is based on a clear understanding of why the patient is taking opioid pain medication, (2) engages medical and complementary or alternative medicine specialists, (3) addresses underdiagnosis of psychological trauma, and (4) requires a quantum leap in access to trauma-specific behavioral health treatment resources. 36
Continue to: To underscore the case...
To underscore the case for shifting to a patient-centered approach35 we present sample cases in “How a patient-centered approach to tapering opioids looks in practice.”
SIDEBAR
How a patient-centered approach to tapering opioids looks in practice
Five hypothetical cases illustrate what might happen when a practice shifts from an addiction-centered, dosage-centered approach to one that places the individual at the center of care.
CASE #1: Brett F
Mr. F appears to use medication responsibly; benefits functionally from an opioid; has tolerable adverse effects; does not have significant psychosocial risk factors (based on the score of the Opioid Risk Tool [ORT] or the Screener and Opioid Assessment for Patients with Pain–Revised [SOAPP-R]); and is engaged in effective self-management. Most of Mr. F’s pain is thought to have a nociceptive or neuropathic source.
Mr F could reasonably contemplate continuing current opioid treatment.
Action: If the daily morphine milligram equivalent (MME) dosage is high, Mr. F should be referred to a pain medicine specialist. We recommend a periodic (at least annually) empiric trial of dosage reduction to see whether he is indeed best served by the current dosage.
CASE #2: Brett F (version 2.0)
Envision Mr. F having the same profile in all respects except that he is not engaged in effective self-management.
Optimal treatment of chronic pain often requires supplemental modalities beyond opioids.
Action: Physical therapy; an individualized, ongoing exercise regimen; interventional procedures; weight loss (if the patient is obese); smoking cessation; and improving coping skills for anxiety and depression without pharmacotherapy might not only temporarily alleviate the pain but, over time, improve Mr. F’s physical condition.
If Mr. F is not willing to do more than take the prescribed opioids, nothing is likely to change: Over time, his condition is likely to deteriorate. A patient like Mr. F can be harmed if opioids continue to be prescribed for him long-term.
Further action: If Mr. F won’t engage in broadening the approach to treating his pain, the opioid medication should be tapered, in his long-term best interest. A carrot-and-stick approach can facilitate Mr. F’s involvement in his care.
CASE #3: Clark S
Mr. S has a significant psychosocial component driving his pain: depression.a
Prescribing opioids without addressing the root cause of trauma is not in the patient’s best interest.
Action: Because of Mr. S’s depression, refer him to a behavioral health provider. If you determine that he is emotionally stable, wait until he is engaged in trauma treatment to begin the taper. If he appears unstable (eg, crying in the office, recent psychological stressors, recent impulsive behaviors, poor insight) consider (1) urgent behavioral health referral and (2) prescribing only enough opioid medication (ie, at close intervals) to prevent withdrawal and panic. Consider whether a psychotropic medication might be of benefit (eg, a serotonin–norepinephrine reuptake inhibitor or selective serotonin reuptake inhibitor).
Further action: Harm-reduction steps, such as close monitoring and, perhaps, a change to a buprenorphine product, is indicated, especially when the patient is overwhelmed by recent psychosocial stressors. Harm-reduction treatment is available through Medication-Assisted Therapy (MAT) programs; however, patients often run into difficulty obtaining access to these programs because regulations and laws restrict MAT to patients who have a diagnosis of opioid use disorder (OUD) and because some health plans and pharmacy benefit managers require prior authorization.
CASE #4: Gloria B
Ms. B isn’t managing her medications responsibly—although you don’t suspect OUD.
When a patient has shown the inability to manage opioid medication responsibly, you should delve into the reason to determine your next step.
Action: Evaluate Ms. B for a cognitive disorder or a thought disorder. Alternatively, as in the case of Mr. S, a psychosocial component might underlie her pain; in that case, the same recommendations can be made for her. In addition, you can propose that she identify a responsible person to dispense her medication.
CASE #5: Nicole L
You suspect that Ms. L, who is taking opioid medication to alleviate pain, also has a substance use disorder.
Action: Implement harm-reduction early for Ms. L: Obtain addiction medicine consultation and implement behavioral health strategies for addiction treatment.
A key characteristic of a substance use disorder is loss of control over use of the substance. A patient like Ms. L—who is in pain and who has an active OUD—cannot be expected to manage her opioid use responsibly.
Further action: We recommend that Ms. L be referred to an addiction specialist for MAT. Evidence of the harmreduction benefit of MAT is sufficient to strongly recommend it. Continue any other treatment modalities for pain that Ms. L has been using, such as non-opioid medication, physical therapy, alternative treatments, and behavioral therapy, or begin such treatments as appropriate.
a Depression is not the only psychosocial component that can underlie pain. Others include anxiety, posttraumatic stress disorder, and grief.
An eye toward the future. To inform future approaches to opioid tapering, more resources need to be deployed to
- support screening and risk stratification for PTSD, anxiety, and related disorders at the primary care level,
- continue the effort to identify and treat OUD,
- develop best-practice responses to screening, and
- make harm-reduction strategies that are now reserved for patients with OUD available to those who don't have OUD.
We urge that research be pursued into best practices for chronic pain interventions that target psychological trauma, anxiety, and depression.
CORRESPONDENCE
Bennet Davis MD, 2092 East Calle de Dulcinea, Tucson, AZ 85718; [email protected].
Many Americans who are treated with prescription opioid analgesics would be better off with less opioid or none at all. To that end, published opioid prescribing guidelines do provide guidance on the mechanics of tapering patients off opioids1-4—but they have a major flaw: They do not adequately account for the fact that people who have a diagnosis of chronic pain are a heterogeneous group and require diagnosis-specific treatment planning. A patient-centered approach to opioid tapers must account for the reality that many people who are given a prescription for an opioid to treat pain have significant mental health conditions—for which opioids act as a psychotropic agent. An opioid taper must therefore address psychological trauma, in particular.5 (See “Tapering and harm-reduction strategies have failed.”6-14)
SIDEBAR
Tapering and harm-reduction strategies have failed
Efforts to address the rising number of overdose events that involve opioids began in earnest in 2010. In a 2011 Government Accountability Office report to Congress, the Drug Enforcement Agency reported that “the number of regulatory investigations (of medical providers who prescribed opioids) tripled between fiscal years 2009- 2010.”6
How has it gone since 2010? High-dosage prescribing of opioids has fallen by 48% since 2011, yet the decline has not reduced overdose events of any kind.7,8 Just the opposite: The 19,000 overdose deaths recorded in 2010 involving any opioid increased to 49,068 by 2017, the National Institute on Drug Abuse reports.9 The increase in opioid overdose deaths is fueled by a recent 9-fold increase in consumption of the synthetic opioid fentanyl: “The rate of drug overdose deaths involving synthetic opioids other than methadone … increased on average by 8% per year from 1999 through 2013 and by 71% per year from 2013 through 2017.”10
These and other statistics document only a modest rise in deaths that involve prescription opioids: from 15,000 in 2010 to 19,000 in 2016.9,10 Since 2010, the crisis of opioid overdose deaths burns hotter, and the pattern of opioid use has shifted from prescription drugs to much deadlier illicit drugs, such as heroin.
Interventions have not been successful overall. Results of research focused on the impact of opioid tapering and harm-reduction strategies implemented this decade are likewise discouraging. In 2018, the US Department of Veterans Affairs reported that opioid discontinuation was not associated with a reduction in overdose but was associated with an increase in suicide.11,12 Von Korff and colleagues, in a 2017 report, concluded that “Long-term implementation of opioid dose and risk reduction initiatives [in Washington state] was not associated with lower rates of prescription opioid use disorder among prevalent [chronic opioid therapy] patients.”13
Evidence suggests that efforts to address the opioid crisis of the past decade have had an effect that is the opposite of what was intended. The federal government recognized this in April 2019 in a Drug Safety Communication: “The US Food and Drug Administration (FDA) has received reports of serious harm in patients who are physically dependent on opioid pain medicines suddenly having these medicines discontinued or the dose rapidly decreased. These include serious withdrawal symptoms, uncontrolled pain, psychological distress, and suicide.”14
In this article, we present an evidence-based consensus approach to opioid tapering for your practice that is informed by a broader understanding of why patients take prescription opioids and why they, occasionally, switch to illicit drugs when their prescription is tapered. This consensus approach is based on the experience of the authors, members of the pain faculty of Project ECHO (Extension for Community Healthcare Outcomes) of the ECHO Institute, a worldwide initiative that uses adult learning techniques and interactive video technology to connect community providers with specialists at centers of excellence in regular real-time collaborative sessions. We are variously experts in pain medicine, primary care, psychology, addiction medicine, pharmacy, behavioral health therapy, occupational medicine, and Chinese medicine.
Why Americans obtain prescription opioids
There are 4 principal reasons why patients obtain prescription opioids, beyond indicated analgesic uses:
1. Patients seek the antianxiety and antidepressant effects of opioids. Multiple converging lines of evidence suggest that antianxiety and antidepressant effects of opioids are a significant reason that patients in the United States persist in requesting prescriptions for opioids:
- In our experience with more than 500 primary care telemedicine case presentations, at least 50% of patients say that the main effect of opioids prescribed for them is “it makes me feel calm” or “more relaxed.”
- In a 2007 survey of 91,823 US residents older than 18 years, nonmedical use of opioids was statistically associated with panic, social anxiety, and depressive symptoms.15
- Ten years later, Von Korff and colleagues found that more than half of opioid prescriptions written in the United States were for the small percentage of patients who have a diagnosis of serious anxiety or depression.13
- In 2016, Yovell and colleagues reported that ultra-low-dosage buprenorphine markedly reduced suicidal ideation over 4 weeks in 62 patients with varied levels of depression.16
There is also mechanistic evidence that the antianxiety and antidepressant effects of opioids are significant reasons Americans persist in requesting prescription opioids. The literature suggests that opioid receptors play a role in mood regulation, including alleviation of depression and anxiety; recent research suggests that oxycodone might be a unique mood-altering drug compared to other common prescription opioids because of its ability to affect mood through the δ opioid receptor.17-20
It should not be a surprise that Americans often turn to opioids to address posttraumatic stress disorder (PTSD), anxiety, and depression. A recent study of the state of the US mental health system concluded that mental health services in the United States are inadequate—despite evidence that > 50% of Americans seek, or consider seeking, treatment for mental health problems for themselves or others.21
2. Patients experience pain unrelated to tissue damage. Rather, they are in pain “for psychological reasons.”22 In 2016, Davis and Vanderah wrote: “We theorize that a functional change in the [central nervous system] can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents).” They connect this change to central sensitization and a reduced pain-perception threshold,23 and strongly suspect that many patients with chronic pain have undiagnosed and untreated psychological trauma that has changed the way their central nervous system processes sensory stimuli. The authors call this “trauma-induced hyperalgesia.”
Continue to: Psychological trauma...
Psychological trauma is uniquely capable of producing hyperalgesia, compared to anxiety or depression. In a study of veterans, Defrin and colleagues demonstrated hyperalgesia in patients who had a diagnosis of PTSD but not in controls group who had an anxiety disorder only.24
To support successful opioid tapering, trauma-induced hyperalgesia, when present, must be addressed. Treatment of what the International Association for the Study of Pain calls “pain due to psychological factors”22 requires specific trauma therapy. However, our experience validates what researchers have to say about access to treatment of psychological trauma in the United States: “…[C]linical research has identified certain psychological interventions that effectively ameliorate the symptoms of PTSD. But most people struggling with PTSD don’t receive those treatments.”25
We have no doubt that this is due, in part, to underdiagnosis of psychological trauma, even in mental health clinics. According to Miele and colleagues, “PTSD remains largely undiagnosed and undertreated in mental health outpatients, even in teaching hospitals, with diagnosis rates as low as 4% while published prevalence is between 7% and 50% in this population.”26
3. Patients suffer from opioid use disorder (OUD) and complain of pain to obtain opioids by prescription. For patients with OUD, their use is out of control; they devote increasing mental and physical resources to obtaining, using, and recovering from substances; and they continue to use despite adverse consequences.27 The prevalence of OUD in primary care clinics varies strikingly by the location of clinics. In Washington state, the prevalence of moderate and severe OUD in a large population of patients who had been prescribed opioids through primary care clinics was recently determined to be between 21.5% and 23.9%.13
4. Patients are obtaining opioid prescriptions for people other than themselves. While this is a reason that patients obtain opioid prescriptions, it is not necessarily common. Statistics show that the likelihood of a prescription being diverted intentionally is low: Dart and colleagues found that diversion has become uncommon in the general population.28
Continue to: Why we taper opioid analgesics
Why we taper opioid analgesics
Reasons for an opioid taper include concern that the patient has, or will develop, an OUD; will experience accidental or intentional overdose; might be diverting opioids; is not benefiting from opioid therapy for pain; or is experiencing severe adverse effects. A patient who has nociceptive pain and might have opioid-induced hyperalgesia will require a much different opioid taper plan than a patient with untreated PTSD or a patient with severe OUD.
Misunderstanding can lead to inappropriate tapering
We often encounter primary care providers who believe that a large percentage of patients on chronic opioid therapy inevitably develop OUD. This is a common reason for initiating opioid taper. Most patients on a chronic opioid do become physically dependent, but only a small percentage of patients develop psychological dependence (ie, addiction or OUD).29
Physical dependence is “a state of adaptation that is manifested by a drug class–specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.”30 Symptoms of opioid withdrawal include muscle aches; abdominal cramping; increased lacrimation, rhinorrhea, and perspiration; diarrhea; agitation and anxiety; insomnia; and piloerection. Opioid withdrawal symptoms are caused by physical dependence, not by addiction. They can be mitigated by tapering slowly and instituting adjuvant medications, such as clonidine, to attenuate symptoms.
Psychological dependence, or addiction (that is, OUD, as described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition27), comprises primarily 3 behavioral criteria:
- Loss of control of the medication, with compulsive use
- Continued use despite adverse consequences of using opioids, such as arrest for driving under the influence and deterioration of social, family, or work performance
- Obsession or preoccupation with obtaining and using the substance. In properly selected chronic opioid therapy patients, there is evidence that new-onset OUD is not as common as has been thought. A recent study of the risk for opioid addiction after use of an opioid for ≥ 90 days for chronic noncancer pain found that the absolute rate of de novo OUD among patients treated for 90 days was 0.72%.29 A systematic review by Fishbain and colleagues of 24 studies of opioid-exposed patients found a risk of 3.27% overall—0.19% for patients who did not have a history of abuse or addiction.31 As Director of the National Institute on Drug Abuse Norma Volkow, MD, wrote in 2016: “Addiction occurs in only a small percentage of people who are exposed to opioids—even among those with preexisting vulnerabilities.”32
Assessment should focus on why the patient is taking an opioid
A strong case can be made that less opioid is better for many of the people for whom these medications are prescribed for chronic noncancer pain. However, a one-size-fits-all dosage reduction and addiction-focused approach to opioid tapering has not worked: The assessment and treatment paradigm must change, in our view.
Continue to: During assessment...
During assessment, we must adopt the means to identify the reason that a patient is using a prescription opioid. It is of particular importance that we identify patients using opioids for their psychotropic properties, particularly when the goal is to cope with the effects of psychological trauma. The subsequent treatment protocol will then need to include time for effective, evidence-based behavioral health treatment of anxiety, PTSD, or depression. If opioids are serving primarily as psychotropic medication, an attempt to taper before establishing effective behavioral health treatment might lead the patient to pursue illegal means of procuring opioid medication.
We acknowledge that primary care physicians are not reimbursed for trauma screening and that evidence-based intensive trauma treatment is generally unavailable in the United States. Both of these shortcomings must be corrected if we want to stem the opioid crisis.
If diversion is suspected and there is evidence that the patient is not currently taking prescribed opioids (eg, a negative urine drug screen), discontinuing the opioid prescription is the immediate next step for the sake of public safety.
SIDEBAR
2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain
#1 Should I provide the patient with a prescription for an opioid for a few days, while I await more information?a
Yes. Writing a prescription is a reasonable decision if all of the following apply:
- You do not have significant suspicion of diversion (based on a clinical interview).
- You do not suspect an active addiction disorder, based on the score of the 10-question Drug Abuse Screening Test (DAST-10) and on a clinical interview. (DAST-10 is available at: https://cde.drugabuse.gov/instrument/e9053390-ee9c-9140-e040-bb89ad433d69.)
- The patient is likely to experience withdrawal symptoms if you don’t provide the medication immediately.
- The patient’s pain and function are likely to be impaired if you do not provide the medication.
- The patient does not display altered mental status during the visit (eg, drowsy, slurred speech).
No. If writing a prescription for an opioid for a few days does not seem to be a reasonable decision because the criteria above are not met, but withdrawal symptoms are likely, you can prescribe medication to mitigate symptoms or refer the patient for treatment of withdrawal.
#2 I’ve decided to provide the patient with a prescription for an opioid. For how many days should I write it?
The usual practice, for a patient whose case is familiar to you, is to prescribe a 1-month supply.
However, if any 1 of the following criteria is met, prescribing a 1-month supply is unsafe under most circumstances:
- An unstable social or living environment places the patient at risk by possessing a supply of opioids (based on a clinical interview).
- You suspect an unstable or severe behavioral health condition or a mental health diagnosis (based on a clinical interview or on the patient record from outside your practice).
- The patient scores as “high risk” on the Opioid Risk Tool (ORT; www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf), Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R; www.ncbi.nlm.nih.gov/pmc/articles/PMC4706778/), or a similar opioid risk assessment tool.
When 1 or more of these exclusionary criteria are met, you have 3 options:
- Prescribe an opioid for a brief duration and see the patient often.
- Do not prescribe an opioid; instead, refer the patient as necessary for treatment of withdrawal.
- Refer the patient for treatment of the underlying behavioral health condition.
a Additional information might include findings from consultants you’ve engaged regarding the patient’s diagnosis; a response to your call from a past prescriber; urine drug screen results; and results of a prescription monitoring program check.

Considering a taper? Take this 5-step approach
Once it appears that tapering an opioid is indicated, we propose that you take the following steps:
- Establish whether it is safe to continue prescribing (follow the route provided in “2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain”); if continuing it is not safe, take steps to protect the patient and the community
- Determine whether assessment by a trauma-informed behavioral health expert is needed, assuming that, in your judgment, it is safe to continue the opioid (TABLE33). When behavioral health assessment is needed, you need 3 questions answered by that assessment: (1) Are psychological factors present that might put the patient at risk during an opioid taper? (2) What are those factors? (3) What needs to done about them before the taper is started? Recalling that psychological trauma often is not assessed by behavioral health colleagues, it is necessary to provide the behavioral health provider with a specific request to assess trauma burden, and state the physical diagnoses that are causing pain or provide a clear statement that no such diagnoses can be made. (See the FIGURE, which we developed in conjunction with behavioral health colleagues to help the consultant understand what the primary care physician needs from a behavioral health assessment.)
- Obtain consultation from a physical therapist, pain medicine specialist, and, if possible, an alternative or complementary medicine provider to determine what nonpharmacotherapeutic modalities can be instituted to treat pain before tapering the opioid.
- Initiate the Screening, Brief Intervention and Referral to Treatment (SBIRT) approach if OUD is suspected (www.samhsa.gov/sbirt).34 This motivational interviewing tool identifies patients with a substance use disorder, severity of use, and appropriate level of treatment. (If OUD is suspected during assessment, next steps are to stop prescribing and implement harm-reduction strategies, such as primary care level medically assisted treatment [MAT] with buprenorphine, followed by expert behavioral health-centered addiction treatment.)
- Experiment with dosage reduction according to published guidance, if (1) psychological factors are absent or have been adequately addressed, according to the behavioral health consultant, and (2) nonpharmacotherapeutic strategies are in place.8-11

Shifting to a patient-centered approach
The timing and choice of opioid tapers, in relation to harm reduction and intervention targeting the root cause of a patient’s complaint of pain, have not been adequately explored. In our practice, we’ve shifted from an addiction-centered, dosage-centered approach to opioid taper to a patient-centered approach35 that emphasizes behavioral-medical integration—an approach that we broadly endorse. Such an approach (1) is based on a clear understanding of why the patient is taking opioid pain medication, (2) engages medical and complementary or alternative medicine specialists, (3) addresses underdiagnosis of psychological trauma, and (4) requires a quantum leap in access to trauma-specific behavioral health treatment resources. 36
Continue to: To underscore the case...
To underscore the case for shifting to a patient-centered approach35 we present sample cases in “How a patient-centered approach to tapering opioids looks in practice.”
SIDEBAR
How a patient-centered approach to tapering opioids looks in practice
Five hypothetical cases illustrate what might happen when a practice shifts from an addiction-centered, dosage-centered approach to one that places the individual at the center of care.
CASE #1: Brett F
Mr. F appears to use medication responsibly; benefits functionally from an opioid; has tolerable adverse effects; does not have significant psychosocial risk factors (based on the score of the Opioid Risk Tool [ORT] or the Screener and Opioid Assessment for Patients with Pain–Revised [SOAPP-R]); and is engaged in effective self-management. Most of Mr. F’s pain is thought to have a nociceptive or neuropathic source.
Mr F could reasonably contemplate continuing current opioid treatment.
Action: If the daily morphine milligram equivalent (MME) dosage is high, Mr. F should be referred to a pain medicine specialist. We recommend a periodic (at least annually) empiric trial of dosage reduction to see whether he is indeed best served by the current dosage.
CASE #2: Brett F (version 2.0)
Envision Mr. F having the same profile in all respects except that he is not engaged in effective self-management.
Optimal treatment of chronic pain often requires supplemental modalities beyond opioids.
Action: Physical therapy; an individualized, ongoing exercise regimen; interventional procedures; weight loss (if the patient is obese); smoking cessation; and improving coping skills for anxiety and depression without pharmacotherapy might not only temporarily alleviate the pain but, over time, improve Mr. F’s physical condition.
If Mr. F is not willing to do more than take the prescribed opioids, nothing is likely to change: Over time, his condition is likely to deteriorate. A patient like Mr. F can be harmed if opioids continue to be prescribed for him long-term.
Further action: If Mr. F won’t engage in broadening the approach to treating his pain, the opioid medication should be tapered, in his long-term best interest. A carrot-and-stick approach can facilitate Mr. F’s involvement in his care.
CASE #3: Clark S
Mr. S has a significant psychosocial component driving his pain: depression.a
Prescribing opioids without addressing the root cause of trauma is not in the patient’s best interest.
Action: Because of Mr. S’s depression, refer him to a behavioral health provider. If you determine that he is emotionally stable, wait until he is engaged in trauma treatment to begin the taper. If he appears unstable (eg, crying in the office, recent psychological stressors, recent impulsive behaviors, poor insight) consider (1) urgent behavioral health referral and (2) prescribing only enough opioid medication (ie, at close intervals) to prevent withdrawal and panic. Consider whether a psychotropic medication might be of benefit (eg, a serotonin–norepinephrine reuptake inhibitor or selective serotonin reuptake inhibitor).
Further action: Harm-reduction steps, such as close monitoring and, perhaps, a change to a buprenorphine product, is indicated, especially when the patient is overwhelmed by recent psychosocial stressors. Harm-reduction treatment is available through Medication-Assisted Therapy (MAT) programs; however, patients often run into difficulty obtaining access to these programs because regulations and laws restrict MAT to patients who have a diagnosis of opioid use disorder (OUD) and because some health plans and pharmacy benefit managers require prior authorization.
CASE #4: Gloria B
Ms. B isn’t managing her medications responsibly—although you don’t suspect OUD.
When a patient has shown the inability to manage opioid medication responsibly, you should delve into the reason to determine your next step.
Action: Evaluate Ms. B for a cognitive disorder or a thought disorder. Alternatively, as in the case of Mr. S, a psychosocial component might underlie her pain; in that case, the same recommendations can be made for her. In addition, you can propose that she identify a responsible person to dispense her medication.
CASE #5: Nicole L
You suspect that Ms. L, who is taking opioid medication to alleviate pain, also has a substance use disorder.
Action: Implement harm-reduction early for Ms. L: Obtain addiction medicine consultation and implement behavioral health strategies for addiction treatment.
A key characteristic of a substance use disorder is loss of control over use of the substance. A patient like Ms. L—who is in pain and who has an active OUD—cannot be expected to manage her opioid use responsibly.
Further action: We recommend that Ms. L be referred to an addiction specialist for MAT. Evidence of the harmreduction benefit of MAT is sufficient to strongly recommend it. Continue any other treatment modalities for pain that Ms. L has been using, such as non-opioid medication, physical therapy, alternative treatments, and behavioral therapy, or begin such treatments as appropriate.
a Depression is not the only psychosocial component that can underlie pain. Others include anxiety, posttraumatic stress disorder, and grief.
An eye toward the future. To inform future approaches to opioid tapering, more resources need to be deployed to
- support screening and risk stratification for PTSD, anxiety, and related disorders at the primary care level,
- continue the effort to identify and treat OUD,
- develop best-practice responses to screening, and
- make harm-reduction strategies that are now reserved for patients with OUD available to those who don't have OUD.
We urge that research be pursued into best practices for chronic pain interventions that target psychological trauma, anxiety, and depression.
CORRESPONDENCE
Bennet Davis MD, 2092 East Calle de Dulcinea, Tucson, AZ 85718; [email protected].
1. Centers for Disease Control and Prevention. Pocket guide: tapering for chronic pain. https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf. Accessed November 25, 2019.
2. Kral LA, Jackson K, Uritsky TJ. A practical guide to tapering opioids. Ment Health Clin. 2015;5:102-108.
3. Murphy L, Babaei-Rad R, Buna D, et al. Guidance on opioid tapering in the context of chronic pain: evidence, practical advice and frequently asked questions. Can Pharm J (Ott). 2018;151:114-120.
4. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2005;90:828-842.
5. Davis M. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:407-417.
6. US Government Accountability Office. Report to Congressional Requestors. Prescription drug control: DEA has enhanced efforts to combat diversion, but could better assess and report program results. August 2011. www.gao.gov/assets/520/511464.pdf. Accessed November 25, 2019.
7. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Annual surveillance report of drug-related risks and outcomes. United States, 2017. www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf. Accessed November 25, 2019.
8. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016, NCHS Data Brief No. 294. December 21, 2017. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/products/databriefs/db294.htm. Accessed November 25, 2019.
9. Overdose death rates. Bethesda, MD: National Institute on Drug Abuse. January 2019. www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed November 25, 2019.
10. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No. 329. November 2018. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/data/databriefs/db329-h.pdf . Accessed November 25, 2019.
11. Manhapra A, Kertesz S, Oliva A, et al. VA data about Rx opioids and overdose and suicide: clinical implications. Presented at the 2018 National Rx Drug Abuse and Heroin Summit, Atlanta Georgia, April 4, 2018.
12. Demidenko M, Dobscha SK, Morasco BJ, et al. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35.
13. Von Korff M, Walker RL, Saunders K, et al. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. Int J Drug Policy. 2017;46:90-98.
14. United States Food and Drug Administration. FDA Drug Safety Communication: FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. April 9, 2019. www.fda.gov/Drugs/DrugSafety/ucm635038.htm. Accessed November 25, 2019.
15. Becker W, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38-47.
16. Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173:491-498.
17. Pradhan AA, Befort K, Nozaki C, et al. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581-590.
18. Sugiyama A, Yamada M, Saitoh A, et al. Administration of a delta opioid receptor agonist KNT-127 to the basolateral amygdala has robust anxiolytic-like effects in rats. Psychopharmacology (Berl). 2018;235:2947-2955.
19. Richards EM, Mathews DC, Luckenbaugh DA, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl). 2016;233:1119-1130.
20. Yang PP, Yeh GC, Yeh TK, et al. Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res. 2016;111:867-876.
21. America’s mental health 2018. Stamford, CT: Cohen Veterans Network. October 10, 2018. https://www.cohenveteransnetwork.org/wp-content/uploads/2018/10/Research-Summary-10-10-2018.pdf. Accessed November 25, 2019.
22. Classification of Chronic Pain, Second Edition (Revised). Washington, DC: International Association for the Study of Pain. Updated 2012. www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673. Accessed November 25, 2019.
23. Davis B, Vanderah TW. A new paradigm for pain? J Fam Pract. 2016 65:598-605.
24. Defrin R, Ginzburg K, Solomon Z, et al. Quantitative testing of pain perception in subjects with PTSD—implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450-459.
25. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Science Public Interest. 2013;14:65-111.
26. Miele D, O’Brien EJ. Underdiagnosis of posttraumatic stress disorder in at risk youth. J Trauma Stress. 2010;23:591-598.
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Publishing; 2013:541.
28. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241-248.
29. Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425-426.
30. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain. 2001. www.naabt.org/documents/APS_consensus_document.pdf. Accessed November 25, 2019.
31. Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444-459.
32. Volkow ND, McClellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253-1263.
33. Treede RD, Rief W, Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003-1007.
34. Screening, brief intervention, and referral to treatment (SBIRT). Rockville, MD: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/sbirt. Accessed November 25, 2019.
35. Schneider JP, Davis B. How well do you know your patient? Pract Pain Manag. 2017;17(2). www.practicalpainmanagement.com/resources/practice-management/how-well-do-you-know-your-patient. Accessed November 25, 2019.
36. Schneider JP. A patient-centered approach to the opioid overdose crisis. J Miss State Med Assoc. 2018;59:232-233.
1. Centers for Disease Control and Prevention. Pocket guide: tapering for chronic pain. https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf. Accessed November 25, 2019.
2. Kral LA, Jackson K, Uritsky TJ. A practical guide to tapering opioids. Ment Health Clin. 2015;5:102-108.
3. Murphy L, Babaei-Rad R, Buna D, et al. Guidance on opioid tapering in the context of chronic pain: evidence, practical advice and frequently asked questions. Can Pharm J (Ott). 2018;151:114-120.
4. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2005;90:828-842.
5. Davis M. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:407-417.
6. US Government Accountability Office. Report to Congressional Requestors. Prescription drug control: DEA has enhanced efforts to combat diversion, but could better assess and report program results. August 2011. www.gao.gov/assets/520/511464.pdf. Accessed November 25, 2019.
7. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Annual surveillance report of drug-related risks and outcomes. United States, 2017. www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf. Accessed November 25, 2019.
8. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016, NCHS Data Brief No. 294. December 21, 2017. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/products/databriefs/db294.htm. Accessed November 25, 2019.
9. Overdose death rates. Bethesda, MD: National Institute on Drug Abuse. January 2019. www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed November 25, 2019.
10. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No. 329. November 2018. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/data/databriefs/db329-h.pdf . Accessed November 25, 2019.
11. Manhapra A, Kertesz S, Oliva A, et al. VA data about Rx opioids and overdose and suicide: clinical implications. Presented at the 2018 National Rx Drug Abuse and Heroin Summit, Atlanta Georgia, April 4, 2018.
12. Demidenko M, Dobscha SK, Morasco BJ, et al. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35.
13. Von Korff M, Walker RL, Saunders K, et al. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. Int J Drug Policy. 2017;46:90-98.
14. United States Food and Drug Administration. FDA Drug Safety Communication: FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. April 9, 2019. www.fda.gov/Drugs/DrugSafety/ucm635038.htm. Accessed November 25, 2019.
15. Becker W, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38-47.
16. Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173:491-498.
17. Pradhan AA, Befort K, Nozaki C, et al. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581-590.
18. Sugiyama A, Yamada M, Saitoh A, et al. Administration of a delta opioid receptor agonist KNT-127 to the basolateral amygdala has robust anxiolytic-like effects in rats. Psychopharmacology (Berl). 2018;235:2947-2955.
19. Richards EM, Mathews DC, Luckenbaugh DA, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl). 2016;233:1119-1130.
20. Yang PP, Yeh GC, Yeh TK, et al. Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res. 2016;111:867-876.
21. America’s mental health 2018. Stamford, CT: Cohen Veterans Network. October 10, 2018. https://www.cohenveteransnetwork.org/wp-content/uploads/2018/10/Research-Summary-10-10-2018.pdf. Accessed November 25, 2019.
22. Classification of Chronic Pain, Second Edition (Revised). Washington, DC: International Association for the Study of Pain. Updated 2012. www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673. Accessed November 25, 2019.
23. Davis B, Vanderah TW. A new paradigm for pain? J Fam Pract. 2016 65:598-605.
24. Defrin R, Ginzburg K, Solomon Z, et al. Quantitative testing of pain perception in subjects with PTSD—implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450-459.
25. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Science Public Interest. 2013;14:65-111.
26. Miele D, O’Brien EJ. Underdiagnosis of posttraumatic stress disorder in at risk youth. J Trauma Stress. 2010;23:591-598.
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Publishing; 2013:541.
28. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241-248.
29. Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425-426.
30. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain. 2001. www.naabt.org/documents/APS_consensus_document.pdf. Accessed November 25, 2019.
31. Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444-459.
32. Volkow ND, McClellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253-1263.
33. Treede RD, Rief W, Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003-1007.
34. Screening, brief intervention, and referral to treatment (SBIRT). Rockville, MD: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/sbirt. Accessed November 25, 2019.
35. Schneider JP, Davis B. How well do you know your patient? Pract Pain Manag. 2017;17(2). www.practicalpainmanagement.com/resources/practice-management/how-well-do-you-know-your-patient. Accessed November 25, 2019.
36. Schneider JP. A patient-centered approach to the opioid overdose crisis. J Miss State Med Assoc. 2018;59:232-233.
PRACTICE RECOMMENDATIONS
› Screen for developmental and adult trauma, for current trauma symptoms, and for opioid use disorder before tapering an opioid. B
› Refer the patient for in-depth behavioral health evaluation when screening identifies risk of behavioral problems, to identify psychological, behavioral, emotional, cognitive, and social factors pertinent to the prevention, treatment, or management of physical health problems, such as chronic pain. A
› Refer the patient for addiction medicine treatment, either within your practice or to an outside consultant, when screening for opioid use disorder indicates that the patient is at risk. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Feigning alcohol withdrawal symptoms can render the CIWA-Ar scale useless
The Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) scale is a well-established protocol that attempts to measure the degree of alcohol and benzodiazepine withdrawal. The CIWA-Ar scale measures 10 domains and indexes the severity of withdrawal on a scale from 0 to 67; scores >8 are generally considered to be indicative of at least mild-to-moderate withdrawal, and scores >20 represent significant withdrawal.1 Despite its common use in many medical settings, the CIWA-Ar scale has been impugned as a less-than-reliable index of true alcohol withdrawal2 and has the potential for misuse among ordering physicians.3 In this case report, I describe a malingering patient who intentionally and successfully feigned symptoms of alcohol withdrawal, which demonstrates that the purposeful reproduction of symptoms measured by the CIWA-Ar scale can render the protocol clinically useless.
CASE REPORT
Mr. G, a 63-year-old African-American man, was admitted to the general medical floor with a chief complaint of alcohol withdrawal. He had a history of alcohol use disorder, severe, and unspecified depression. He said he had been drinking a gallon of wine plus “a fifth” of vodka every day for the past 1.5 months. More than 1 year ago, he had been admitted for alcohol withdrawal with subsequent delirium tremens, but he denied having any other psychiatric history.
In the emergency department, Mr. G was given IV lorazepam, 6 mg total, for alcohol withdrawal. He was reported to be “scoring” on the CIWA-Ar scale with apparently uncontrollable tremulousness, visual hallucinations, and confusion. His vitals were within normal limits, his mean corpuscular volume and lipase level were within normal limits, and the rest of his presentation was largely unremarkable.
Once admitted to the general medical floor, he continued to receive benzodiazepines for what was documented as severe alcohol withdrawal. When clinical staff were not in the room, the patient was observed to be resting comfortably without tremulousness. When the patient was seen by the psychiatry consultation service, he produced full body tremulousness with marked shoulder and hip thrusting. His account of how much he had been drinking contradicted the amount he reported to other teams in the hospital. When the consulting psychiatrist appeared unimpressed by his full body jerking, the patient abruptly pointed to the corner of the room and yelled “What is that?” when nothing was there. When the primary medical team suggested to the patient that his vitals were within normal limits and he did not appear to be in true alcohol withdrawal, the patient escalated the degree of his full body jerking.
Over the next few days, the patient routinely would tell clinical staff “I’m having DTs.” He also specifically requested lorazepam. After consultation, the medical and psychiatry teams determined the patient was feigning symptoms of alcohol withdrawal. The lorazepam was discontinued, and the patient was discharged home with outpatient psychiatric follow-up.
Limitations of the CIWA-Ar scale
The CIWA-Ar scale is intended to guide the need for medications, such as benzodiazepines, to help mitigate symptoms of alcohol withdrawal. Symptom-triggered benzodiazepine treatment has been shown to be superior to fixed-schedule dosing.4 However, symptom-triggered treatment is problematic in the setting of feigned symptoms.
When psychiatrists and nurses calculate a CIWA-Ar score, they rely on both subjective accounts of a patient’s withdrawal severity as well as objective signs, such as vitals and a physical examination. Many of the elements included in the CIWA-Ar scale can be easily feigned (Table). Feigned alcohol withdrawal may fall into 2 categories: (1) the false reporting of subjective symptoms, and (2) the false portrayal of objective signs.
Continue to: The false reporting...
The false reporting of subjective symptoms can include the reported presence of nausea or vomiting, anxiety, tactile hallucinations, auditory hallucinations, headache or head fullness, and visual hallucinations. The false portrayal of objective signs can include the feigning of tremulousness, agitation, and confusion (eg, incorrectly answering orienting questions). In both categories, the simple presence of these signs or symptoms, whether falsely reported or falsely portrayed, would cause the patient to “score” on the CIWA-Ar scale.
Thus, the need to effectively rule out feigned symptoms is essential because inappropriate dosing of benzodiazepines can be dangerous, costly, and utilize limited hospital resources that could otherwise be diverted to a patient with a true medical or psychiatric illness. In these instances, it is crucial to pay close attention to vital signs because these are more reliable indices of withdrawal. A patient’s ability to purposefully feign symptoms of alcohol withdrawal highlights the limitations of the CIWA-Ar scale as a validated measure of alcohol withdrawal, and renders it effectively useless in the setting of either malingering or factitious disorder.
Resnick5 describes malingering as either pure malingering, partial malingering, or false imputation. Pure malingering refers to the feigning of a nonexistent disorder or illness. Partial malingering refers to the exaggeration of symptoms that are present, but to a lesser degree. False imputation refers to the attribution of symptoms from a separate disorder to one the patient knows is unrelated (eg, attributing chronic low back pain from a prior sports injury to a recent motor vehicle accident). In Mr. G’s case, he had multiple prior admissions for true, non-feigned alcohol withdrawal with subsequent delirium tremens. His knowledge of the signs and symptoms of alcohol withdrawal therefore helped him make calculated efforts to manipulate clinical staff in his quest to obtain benzodiazepines. Whether this was pure or partial malingering remained unclear because Mr. G’s true level of withdrawal could not be adequately assessed.
Potentially serious consequences
The CIWA-Ar scale is among the most widely used scales to determine the level of alcohol withdrawal and need for subsequent benzodiazepine treatment. However, its effective use is limited because it relies on subjective symptoms and objective signs that can be easily feigned or manipulated. In the setting of malingering or factitious disorder, when a patient is feigning symptoms of alcohol withdrawal, the CIWA-Ar scale may be rendered clinically useless. This can lead to dangerous iatrogenic adverse effects, lengthy and nontherapeutic hospital stays, and an increasing financial burden on health care systems.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knight E, Lappalainen L. Clinical Institute Withdrawal Assessment for Alcohol–Revised might be an unreliable tool in the management of alcohol withdrawal. Can Fam Physician. 2017;63(9):691-695.
3. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
4. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121.
5. Resnick PJ. The detection of malingered mental illness. Behav Sci Law. 1984;2(1):20-38.
The Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) scale is a well-established protocol that attempts to measure the degree of alcohol and benzodiazepine withdrawal. The CIWA-Ar scale measures 10 domains and indexes the severity of withdrawal on a scale from 0 to 67; scores >8 are generally considered to be indicative of at least mild-to-moderate withdrawal, and scores >20 represent significant withdrawal.1 Despite its common use in many medical settings, the CIWA-Ar scale has been impugned as a less-than-reliable index of true alcohol withdrawal2 and has the potential for misuse among ordering physicians.3 In this case report, I describe a malingering patient who intentionally and successfully feigned symptoms of alcohol withdrawal, which demonstrates that the purposeful reproduction of symptoms measured by the CIWA-Ar scale can render the protocol clinically useless.
CASE REPORT
Mr. G, a 63-year-old African-American man, was admitted to the general medical floor with a chief complaint of alcohol withdrawal. He had a history of alcohol use disorder, severe, and unspecified depression. He said he had been drinking a gallon of wine plus “a fifth” of vodka every day for the past 1.5 months. More than 1 year ago, he had been admitted for alcohol withdrawal with subsequent delirium tremens, but he denied having any other psychiatric history.
In the emergency department, Mr. G was given IV lorazepam, 6 mg total, for alcohol withdrawal. He was reported to be “scoring” on the CIWA-Ar scale with apparently uncontrollable tremulousness, visual hallucinations, and confusion. His vitals were within normal limits, his mean corpuscular volume and lipase level were within normal limits, and the rest of his presentation was largely unremarkable.
Once admitted to the general medical floor, he continued to receive benzodiazepines for what was documented as severe alcohol withdrawal. When clinical staff were not in the room, the patient was observed to be resting comfortably without tremulousness. When the patient was seen by the psychiatry consultation service, he produced full body tremulousness with marked shoulder and hip thrusting. His account of how much he had been drinking contradicted the amount he reported to other teams in the hospital. When the consulting psychiatrist appeared unimpressed by his full body jerking, the patient abruptly pointed to the corner of the room and yelled “What is that?” when nothing was there. When the primary medical team suggested to the patient that his vitals were within normal limits and he did not appear to be in true alcohol withdrawal, the patient escalated the degree of his full body jerking.
Over the next few days, the patient routinely would tell clinical staff “I’m having DTs.” He also specifically requested lorazepam. After consultation, the medical and psychiatry teams determined the patient was feigning symptoms of alcohol withdrawal. The lorazepam was discontinued, and the patient was discharged home with outpatient psychiatric follow-up.
Limitations of the CIWA-Ar scale
The CIWA-Ar scale is intended to guide the need for medications, such as benzodiazepines, to help mitigate symptoms of alcohol withdrawal. Symptom-triggered benzodiazepine treatment has been shown to be superior to fixed-schedule dosing.4 However, symptom-triggered treatment is problematic in the setting of feigned symptoms.
When psychiatrists and nurses calculate a CIWA-Ar score, they rely on both subjective accounts of a patient’s withdrawal severity as well as objective signs, such as vitals and a physical examination. Many of the elements included in the CIWA-Ar scale can be easily feigned (Table). Feigned alcohol withdrawal may fall into 2 categories: (1) the false reporting of subjective symptoms, and (2) the false portrayal of objective signs.
Continue to: The false reporting...
The false reporting of subjective symptoms can include the reported presence of nausea or vomiting, anxiety, tactile hallucinations, auditory hallucinations, headache or head fullness, and visual hallucinations. The false portrayal of objective signs can include the feigning of tremulousness, agitation, and confusion (eg, incorrectly answering orienting questions). In both categories, the simple presence of these signs or symptoms, whether falsely reported or falsely portrayed, would cause the patient to “score” on the CIWA-Ar scale.
Thus, the need to effectively rule out feigned symptoms is essential because inappropriate dosing of benzodiazepines can be dangerous, costly, and utilize limited hospital resources that could otherwise be diverted to a patient with a true medical or psychiatric illness. In these instances, it is crucial to pay close attention to vital signs because these are more reliable indices of withdrawal. A patient’s ability to purposefully feign symptoms of alcohol withdrawal highlights the limitations of the CIWA-Ar scale as a validated measure of alcohol withdrawal, and renders it effectively useless in the setting of either malingering or factitious disorder.
Resnick5 describes malingering as either pure malingering, partial malingering, or false imputation. Pure malingering refers to the feigning of a nonexistent disorder or illness. Partial malingering refers to the exaggeration of symptoms that are present, but to a lesser degree. False imputation refers to the attribution of symptoms from a separate disorder to one the patient knows is unrelated (eg, attributing chronic low back pain from a prior sports injury to a recent motor vehicle accident). In Mr. G’s case, he had multiple prior admissions for true, non-feigned alcohol withdrawal with subsequent delirium tremens. His knowledge of the signs and symptoms of alcohol withdrawal therefore helped him make calculated efforts to manipulate clinical staff in his quest to obtain benzodiazepines. Whether this was pure or partial malingering remained unclear because Mr. G’s true level of withdrawal could not be adequately assessed.
Potentially serious consequences
The CIWA-Ar scale is among the most widely used scales to determine the level of alcohol withdrawal and need for subsequent benzodiazepine treatment. However, its effective use is limited because it relies on subjective symptoms and objective signs that can be easily feigned or manipulated. In the setting of malingering or factitious disorder, when a patient is feigning symptoms of alcohol withdrawal, the CIWA-Ar scale may be rendered clinically useless. This can lead to dangerous iatrogenic adverse effects, lengthy and nontherapeutic hospital stays, and an increasing financial burden on health care systems.
The Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) scale is a well-established protocol that attempts to measure the degree of alcohol and benzodiazepine withdrawal. The CIWA-Ar scale measures 10 domains and indexes the severity of withdrawal on a scale from 0 to 67; scores >8 are generally considered to be indicative of at least mild-to-moderate withdrawal, and scores >20 represent significant withdrawal.1 Despite its common use in many medical settings, the CIWA-Ar scale has been impugned as a less-than-reliable index of true alcohol withdrawal2 and has the potential for misuse among ordering physicians.3 In this case report, I describe a malingering patient who intentionally and successfully feigned symptoms of alcohol withdrawal, which demonstrates that the purposeful reproduction of symptoms measured by the CIWA-Ar scale can render the protocol clinically useless.
CASE REPORT
Mr. G, a 63-year-old African-American man, was admitted to the general medical floor with a chief complaint of alcohol withdrawal. He had a history of alcohol use disorder, severe, and unspecified depression. He said he had been drinking a gallon of wine plus “a fifth” of vodka every day for the past 1.5 months. More than 1 year ago, he had been admitted for alcohol withdrawal with subsequent delirium tremens, but he denied having any other psychiatric history.
In the emergency department, Mr. G was given IV lorazepam, 6 mg total, for alcohol withdrawal. He was reported to be “scoring” on the CIWA-Ar scale with apparently uncontrollable tremulousness, visual hallucinations, and confusion. His vitals were within normal limits, his mean corpuscular volume and lipase level were within normal limits, and the rest of his presentation was largely unremarkable.
Once admitted to the general medical floor, he continued to receive benzodiazepines for what was documented as severe alcohol withdrawal. When clinical staff were not in the room, the patient was observed to be resting comfortably without tremulousness. When the patient was seen by the psychiatry consultation service, he produced full body tremulousness with marked shoulder and hip thrusting. His account of how much he had been drinking contradicted the amount he reported to other teams in the hospital. When the consulting psychiatrist appeared unimpressed by his full body jerking, the patient abruptly pointed to the corner of the room and yelled “What is that?” when nothing was there. When the primary medical team suggested to the patient that his vitals were within normal limits and he did not appear to be in true alcohol withdrawal, the patient escalated the degree of his full body jerking.
Over the next few days, the patient routinely would tell clinical staff “I’m having DTs.” He also specifically requested lorazepam. After consultation, the medical and psychiatry teams determined the patient was feigning symptoms of alcohol withdrawal. The lorazepam was discontinued, and the patient was discharged home with outpatient psychiatric follow-up.
Limitations of the CIWA-Ar scale
The CIWA-Ar scale is intended to guide the need for medications, such as benzodiazepines, to help mitigate symptoms of alcohol withdrawal. Symptom-triggered benzodiazepine treatment has been shown to be superior to fixed-schedule dosing.4 However, symptom-triggered treatment is problematic in the setting of feigned symptoms.
When psychiatrists and nurses calculate a CIWA-Ar score, they rely on both subjective accounts of a patient’s withdrawal severity as well as objective signs, such as vitals and a physical examination. Many of the elements included in the CIWA-Ar scale can be easily feigned (Table). Feigned alcohol withdrawal may fall into 2 categories: (1) the false reporting of subjective symptoms, and (2) the false portrayal of objective signs.
Continue to: The false reporting...
The false reporting of subjective symptoms can include the reported presence of nausea or vomiting, anxiety, tactile hallucinations, auditory hallucinations, headache or head fullness, and visual hallucinations. The false portrayal of objective signs can include the feigning of tremulousness, agitation, and confusion (eg, incorrectly answering orienting questions). In both categories, the simple presence of these signs or symptoms, whether falsely reported or falsely portrayed, would cause the patient to “score” on the CIWA-Ar scale.
Thus, the need to effectively rule out feigned symptoms is essential because inappropriate dosing of benzodiazepines can be dangerous, costly, and utilize limited hospital resources that could otherwise be diverted to a patient with a true medical or psychiatric illness. In these instances, it is crucial to pay close attention to vital signs because these are more reliable indices of withdrawal. A patient’s ability to purposefully feign symptoms of alcohol withdrawal highlights the limitations of the CIWA-Ar scale as a validated measure of alcohol withdrawal, and renders it effectively useless in the setting of either malingering or factitious disorder.
Resnick5 describes malingering as either pure malingering, partial malingering, or false imputation. Pure malingering refers to the feigning of a nonexistent disorder or illness. Partial malingering refers to the exaggeration of symptoms that are present, but to a lesser degree. False imputation refers to the attribution of symptoms from a separate disorder to one the patient knows is unrelated (eg, attributing chronic low back pain from a prior sports injury to a recent motor vehicle accident). In Mr. G’s case, he had multiple prior admissions for true, non-feigned alcohol withdrawal with subsequent delirium tremens. His knowledge of the signs and symptoms of alcohol withdrawal therefore helped him make calculated efforts to manipulate clinical staff in his quest to obtain benzodiazepines. Whether this was pure or partial malingering remained unclear because Mr. G’s true level of withdrawal could not be adequately assessed.
Potentially serious consequences
The CIWA-Ar scale is among the most widely used scales to determine the level of alcohol withdrawal and need for subsequent benzodiazepine treatment. However, its effective use is limited because it relies on subjective symptoms and objective signs that can be easily feigned or manipulated. In the setting of malingering or factitious disorder, when a patient is feigning symptoms of alcohol withdrawal, the CIWA-Ar scale may be rendered clinically useless. This can lead to dangerous iatrogenic adverse effects, lengthy and nontherapeutic hospital stays, and an increasing financial burden on health care systems.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knight E, Lappalainen L. Clinical Institute Withdrawal Assessment for Alcohol–Revised might be an unreliable tool in the management of alcohol withdrawal. Can Fam Physician. 2017;63(9):691-695.
3. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
4. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121.
5. Resnick PJ. The detection of malingered mental illness. Behav Sci Law. 1984;2(1):20-38.
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Knight E, Lappalainen L. Clinical Institute Withdrawal Assessment for Alcohol–Revised might be an unreliable tool in the management of alcohol withdrawal. Can Fam Physician. 2017;63(9):691-695.
3. Hecksel KA, Bostwick JM, Jaeger TM, et al. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc. 2008;83(3):274-279.
4. Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med. 2002;162(10):1117-1121.
5. Resnick PJ. The detection of malingered mental illness. Behav Sci Law. 1984;2(1):20-38.
Support for medical marijuana transcends political affiliation
There is not much common ground between Republicans and Democrats these days, but both sides strongly supported the use of medical marijuana in a recent survey by the American Society of Clinical Oncology.
Overall support of medical marijuana among all 4,001 respondents was higher (84%) for use among cancer patients, but 76% also supported its use for any medical reason, according to data from the survey conducted for ASCO by the Harris Poll.
The differences in support between Republicans and Democrats were significant, but both parties were over 80% for marijuana use by cancer patients and over 70% for use for any medical reason. In both cases, the independents in between mirrored the overall population, with support at 84% and 76%, respectively, ASCO said.
Support for medical marijuana also was consistent based on the respondents’ cancer experience. For use by cancer patients, those who were current or previous patients were at 84%, caregivers (those providing unpaid care to an immediate family member or loved one with cancer) and other family members/loved ones were both at 87%, and those with no cancer experiences were at 82%, the survey results showed.
Use of marijuana for any medical reason was supported by 72% of current/previous patients, 79% of family members and loved ones, 80% of caregivers, and 74% of those with no cancer experience, ASCO reported.
In a question asked only of current or previous patients, 62% said that they are/were open to use of marijuana to alleviate cancer-related pain, nausea, or other symptoms, and 60% said that they wished they had more information about the benefits of medical marijuana use, according to the results of the survey, which was conducted online from July 9 to Aug. 10, 2019.
There is not much common ground between Republicans and Democrats these days, but both sides strongly supported the use of medical marijuana in a recent survey by the American Society of Clinical Oncology.

Overall support of medical marijuana among all 4,001 respondents was higher (84%) for use among cancer patients, but 76% also supported its use for any medical reason, according to data from the survey conducted for ASCO by the Harris Poll.
The differences in support between Republicans and Democrats were significant, but both parties were over 80% for marijuana use by cancer patients and over 70% for use for any medical reason. In both cases, the independents in between mirrored the overall population, with support at 84% and 76%, respectively, ASCO said.
Support for medical marijuana also was consistent based on the respondents’ cancer experience. For use by cancer patients, those who were current or previous patients were at 84%, caregivers (those providing unpaid care to an immediate family member or loved one with cancer) and other family members/loved ones were both at 87%, and those with no cancer experiences were at 82%, the survey results showed.
Use of marijuana for any medical reason was supported by 72% of current/previous patients, 79% of family members and loved ones, 80% of caregivers, and 74% of those with no cancer experience, ASCO reported.
In a question asked only of current or previous patients, 62% said that they are/were open to use of marijuana to alleviate cancer-related pain, nausea, or other symptoms, and 60% said that they wished they had more information about the benefits of medical marijuana use, according to the results of the survey, which was conducted online from July 9 to Aug. 10, 2019.
There is not much common ground between Republicans and Democrats these days, but both sides strongly supported the use of medical marijuana in a recent survey by the American Society of Clinical Oncology.

Overall support of medical marijuana among all 4,001 respondents was higher (84%) for use among cancer patients, but 76% also supported its use for any medical reason, according to data from the survey conducted for ASCO by the Harris Poll.
The differences in support between Republicans and Democrats were significant, but both parties were over 80% for marijuana use by cancer patients and over 70% for use for any medical reason. In both cases, the independents in between mirrored the overall population, with support at 84% and 76%, respectively, ASCO said.
Support for medical marijuana also was consistent based on the respondents’ cancer experience. For use by cancer patients, those who were current or previous patients were at 84%, caregivers (those providing unpaid care to an immediate family member or loved one with cancer) and other family members/loved ones were both at 87%, and those with no cancer experiences were at 82%, the survey results showed.
Use of marijuana for any medical reason was supported by 72% of current/previous patients, 79% of family members and loved ones, 80% of caregivers, and 74% of those with no cancer experience, ASCO reported.
In a question asked only of current or previous patients, 62% said that they are/were open to use of marijuana to alleviate cancer-related pain, nausea, or other symptoms, and 60% said that they wished they had more information about the benefits of medical marijuana use, according to the results of the survey, which was conducted online from July 9 to Aug. 10, 2019.
Vaping-linked lung injury: 2,172 cases, 42 deaths
The Centers for Disease Control and Prevention has from 49 states (all except Alaska), the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Forty-two deaths have been confirmed in 24 states and the District of Columbia, the CDC reported.
Laboratory test results of bronchoalveolar lavage fluid samples from 29 patients submitted to CDC from 10 states found vitamin E acetate in all of the samples. This is the first time a chemical of concern has been found in biologic samples from patients with EVALI. These findings provide direct evidence of vitamin E acetate at the primary site of injury within the lungs.
Tetrahydrocannabinol (THC) was identified in 82% of the samples and nicotine was identified in 62% of the samples. Testing continues for other chemicals including plant oils, petroleum distillates like mineral oil, medium-chain triglycerides oil, and terpenes, which are compounds commonly found in or added to THC products. None of these chemicals has been detected in the bronchoalveolar lavage fluid samples tested.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
The Centers for Disease Control and Prevention has from 49 states (all except Alaska), the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Forty-two deaths have been confirmed in 24 states and the District of Columbia, the CDC reported.
Laboratory test results of bronchoalveolar lavage fluid samples from 29 patients submitted to CDC from 10 states found vitamin E acetate in all of the samples. This is the first time a chemical of concern has been found in biologic samples from patients with EVALI. These findings provide direct evidence of vitamin E acetate at the primary site of injury within the lungs.
Tetrahydrocannabinol (THC) was identified in 82% of the samples and nicotine was identified in 62% of the samples. Testing continues for other chemicals including plant oils, petroleum distillates like mineral oil, medium-chain triglycerides oil, and terpenes, which are compounds commonly found in or added to THC products. None of these chemicals has been detected in the bronchoalveolar lavage fluid samples tested.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
The Centers for Disease Control and Prevention has from 49 states (all except Alaska), the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Forty-two deaths have been confirmed in 24 states and the District of Columbia, the CDC reported.
Laboratory test results of bronchoalveolar lavage fluid samples from 29 patients submitted to CDC from 10 states found vitamin E acetate in all of the samples. This is the first time a chemical of concern has been found in biologic samples from patients with EVALI. These findings provide direct evidence of vitamin E acetate at the primary site of injury within the lungs.
Tetrahydrocannabinol (THC) was identified in 82% of the samples and nicotine was identified in 62% of the samples. Testing continues for other chemicals including plant oils, petroleum distillates like mineral oil, medium-chain triglycerides oil, and terpenes, which are compounds commonly found in or added to THC products. None of these chemicals has been detected in the bronchoalveolar lavage fluid samples tested.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
REPORTING FROM CDC
Pediatricians uniquely qualified to treat adolescents with opioid use disorder
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
NEW ORLEANS –
“One of the real benefits of treatment in primary care is that it removes the stigma so that these patients aren’t isolated into addiction clinics; they’re being treated by providers that they know well and that their family knows well,” Dr. Reynolds, a pediatrician who practices in Wareham, Mass., said at the annual meeting of the American Academy of Pediatrics. “That feels a lot better to them, and I think it makes a statement in the community that these people don’t need to be isolated. Anything we can do to reduce the stigma of opioid use disorder is important. We in primary care are well suited to manage chronic disease over the continuum.”
In 2016, the AAP released a policy statement advocating for pediatricians to consider providing medication-assisted treatment to patients with OUD (Pediatrics. 2016;138[3]e20161893). The statement cited results from a nationally representative sample of 345 addiction treatment programs serving adolescents and adults. It found that fewer than 50% of those programs used medication-assisted treatment (J Addict Med. 2011;5[1]:21-7). “When they looked at patients who actually had opioid dependence, the numbers were even lower,” said Dr. Reynolds, who was not involved with the study. “In fact, 34% of opioid-dependent patients received medication-assisted treatment. When they stratified it by age, the younger you were, the less likely you were to be treated. Only 11.5% of youth under 18 are actually being treated. We know that youth with opioid use disorders have very bad health outcomes over their lifetime. The fact that such few patients receive what is considered to be a gold-standard treatment is really alarming.”
Dr. Reynolds acknowledged that many perceived barriers exist to providing treatment of OUD in pediatric primary care, including the fact that patients with addiction are not easy to treat. “They can be manipulative and can make you feel both sad for them and angry at them within the same visit,” he said. “They also have complex needs. For many of these patients, it’s not just that they use opiates; they have medical problems and psychological diagnoses, and oftentimes they have social issues such as being in foster care. They also may have issues with their parents, employer, or their school, so there are many needs that need to be juggled. That can be overwhelming.”
However, he said that such patients “are actually in our wheelhouse, because as primary care physicians we’re used to coordinating care. These are the perfect patients to have a medical home. We manage chronic disease over the continuum of care. This is a chronic disease, and we have to help patients.”
Another perceived barrier for treating adolescents with OUD relates to reimbursement. While most patients with OUD have insurance, Dr. Reynolds finds that the requirement for prior authorizations can result in delay of treatment and poses an unnecessary burden on care providers. “It’s an administrative task that either the physician or the office staff has to take care of,” he said. “Interestingly, reimbursement ranks as a low concern in studies of buprenorphine providers. That tells me that this is not a major hurdle.”
Pediatricians also cite a lack of knowledge as a reason they’re leery of providing OUD treatment in their office. “They wonder: ‘How do I do this? What’s the right way to do it? Are there best practices?’ ” Dr. Reynolds said. “There’s a feeling that it must be dangerous, the idea that if I don’t do it right I’m going to hurt somebody. The reality is, buprenorphine is no more dangerous than any of the other opiates. Technically, because it’s a partial agonist, it’s probably less dangerous than some of the opiates that we prescribe. It’s no more dangerous than prescribing amitriptyline for chronic pain.”
One key resource, the Providers Clinical Support System (www.pcssnow.org), provides resources for clinicians and family members, education and training, and access to mentoring. Another resource, the American Society of Addiction Medicine (www.asam.org), includes clinical practice guidelines, online courses and training on the treatment of OUD, and sample consent and opioid-withdrawal forms. Dr. Reynolds characterized learning how to treat patients with OUD as no different than learning step therapy for asthma. “Once you look into it, you realize that there’s no sort of magic behind this,” he said. “It’s something that any of us can do. Staff can be trained. There are modules to train your staff into the protocols. Learn the knowledge and put it into action. Have the confidence and the knowledge.”
The Drug Addiction and Treatment Act of 2000 set up the waiver process by which physicians can obtain a waiver from the Drug Enforcement Agency after completing an 8-hour CME course on substance abuse disorder and buprenorphine prescribing. To receive a waiver to practice opioid dependency treatment with approved buprenorphine medications, a clinician must notify the SAMHSA Center for Substance Abuse Treatment of their intent to practice this form of medication-assisted treatment.
Dr. Reynolds acknowledged that not every practice is equipped to provide psychosocial support for complex patients with OUD. “When I first started this in 2017, I wanted to make sure that my patients were in some form of counseling,” he said. “However, the medical literature shows that you can treat OUD without counseling, and some of those patients will be fine, too. There have been reports that just going to Narcotics Anonymous meetings weekly has been shown to improve the effectiveness of medication-assisted treatment.”
For clinicians concerned about having backup when they face challenging cases, data shows that having more than one waivered provider in a practice is associated with completing waiver training. “This makes sense,” Dr. Reynolds said. “We like to be able to discuss our cases with colleagues, but a lot of us don’t want to be on call 365 days a year for our patients. Shared responsibility makes it easier. Access to specialty telemedicine consult has also been identified as a facilitator to physicians prescribing medical-assisted therapy.”
He concluded his presentation by noting that increasing numbers of OUD patients are initiating buprenorphine treatment in the ED. “That takes advantage of the fact that most of these patients present to the emergency room after receiving Narcan for an overdose,” Dr. Reynolds said. “In the emergency room, they’re counseled and instructed on how to start buprenorphine, they’re given the first dose, and they’re told to go home and avoid using any other opiates for 24 hours, start the buprenorphine, and follow up with their primary care doctor or an addiction medicine specialist in 3 days. In my community, this is what our local emergency department is doing for adult patients, except they’re not referring back to primary care. They’re referring to a hospital-based addiction medicine specialist. This is a way to increase access and get people started on buprenorphine treatment.”
Dr. Reynolds reported having no financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Opioid reduction works after minimally invasive gynecologic surgery
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
VANCOUVER – Two new randomized trials demonstrate that pain following minimally invasive gynecologic surgery can be successfully managed using reduced opioid prescriptions.
In each case, patients were randomized to receive higher or lower numbers of oxycodone tablets. In both trials, the lower amount was five 5-mg oxycodone tablets. The work should reassure surgeons who wish to change their prescribing patterns, but may worry about patient dissatisfaction, at least in the context of prolapse repair and benign minor gynecologic laparoscopy, which were the focus of the two studies.
The ob.gyn. literature cites rates of 4%-6% of persistent opioid use after surgery on opioid-naive patients, and that’s a risk that needs to be addressed. “If we look at this as a risk factor of our surgical process, this is much higher than any other risk in patients undergoing surgery, and it’s not something we routinely talk to patients about,” Kari Plewniak, MD, an ob.gyn. at Montefiore Medical Center, New York, said during her presentation on pain control during benign gynecologic laparoscopy at the meeting sponsored by AAGL.
The trials provide some welcome guidance. “They provide pretty concrete guidelines with strong evidence of safety, so this is really helpful,” said Sean Dowdy, MD, chair of gynecologic oncology at Mayo Clinic in Rochester, Minn., while speaking as a discussant for the presentations.
Emily Davidson, MD, and associates at the Cleveland Clinic conducted a single-institution, noninferiority trial of standard- versus reduced-prescription opioids in 116 women undergoing prolapse repair. Half were randomized to receive 28 tablets of 5 mg oxycodone (routine arm) and half were prescribed just 5 tablets (reduced arm). All patients also received multimodal pain therapy featuring acetaminophen and ibuprofen. The mean age of patients was 62 years, 91% were white, and 84% were post menopausal. The most common surgery was hysterectomy combined with native tissue repair (60.2%), followed by vaginal colpopexy (15.3%), hysteropexy (15.3%), and sacrocolpopexy (9.3%).
At their postsurgical visit, patients were asked about their satisfaction with their postoperative pain management; 93% in the reduced arm reported that they were very satisfied or somewhat satisfied, as did 93% in the routine arm, which met the standard for noninferiority with a 15% margin. About 15% of patients in the reduced arm used more opioids than originally prescribed, compared with 2% of patients in the routine arm (P less than .01). The reduced arm had an average of 4 unused opioid tablets, compared with 26 in the routine arm. On average, the reduced arm used one tablet, compared with three in the routine arm (P = .03).
The researchers suggested that clinicians should consider prescribing 5-10 tablets for most patients, and all patients should receive multimodal pain management.
The noninferiority nature of the design was welcome, according to Dr. Dowdy. “I think we need to do more noninferiority trial designs because it allows us to make more observations about other parts of the value equation, so if we have two interventions that are equivalent, we can pick the one that has the best patient experience and the lowest cost, so it simplifies a lot of our management.”
The other study, conducted at Montefiore Medical Center, set out to see if a similar regimen of 5 5-mg oxycodone tablets, combined with acetaminophen and ibuprofen, could adequately manage postoperative pain after minor benign gynecologic laparoscopy (excluding hysterectomy), compared with a 10-tablet regimen. All patients received 25 tablets of 600 mg ibuprofen (1 tablet every 6 hours or as needed), plus 50 tablets of 250 mg acetaminophen (1-2 tablets every 6 hours or as needed).
The median number of opioid tablets taken was 2.0 in the 5-tablet group and 2.5 in the 10-tablet group; 32% and 28% took no tablets, and 68% and 65% took three or fewer tablets in the respective groups. The median number of leftover opioid tablets was 3 in the 5-tablet group and 8 in the 10-tablet group, reported Dr. Plewniak.
The studies are a good first step, but more is needed, according to Dr. Dowdy. It’s important to begin looking at more-challenging patient groups, such as those who are not opioid naive, as well as patients taking buprenorphine. “That creates some unique challenges with postoperative pain management,” he said.
Dr. Dowdy, Dr. Davidson, and Dr. Plewniak have no relevant financial disclosures.*
* This article was updated 11/27/2019.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Kratom: Botanical with opiate-like effects increasingly blamed for liver injury
BOSTON – Kratom, a botanical product with opioid-like activity, is increasingly responsible for cases of liver injury in the United States, according to investigators.
Kratom-associated liver damage involves a mixed pattern of hepatocellular and cholestatic injury that typically occurs after about 2-6 weeks of use, reported lead author Victor J. Navarro, MD, division head of gastroenterology at Einstein Healthcare Network in Philadelphia, and colleagues.
“I think it’s important for clinicians to have heightened awareness of the abuse potential [of kratom], because it is an opioid agonist and [because of] its capacity to cause liver injury,” Dr. Navarro said.
Kratom acts as a stimulant at low doses, while higher doses have sedating and narcotic properties. These effects are attributed to several alkaloids found in kratom’s source plant, Mitragyna speciose, of which mitragynine, a suspected opioid agonist, is most common.
Presenting at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Navarro cited figures from the National Poison Data System that suggest an upward trend in kratom usage in the United States, from very little use in 2011 to 1 exposure per million people in 2014 and more recently to slightly more than 2.5 exposures per million people in 2017, predominantly among individuals aged 20 years and older. According to the Centers for Disease Control and Prevention, more than 90 kratom-associated deaths occurred between July 2016 and December 2017. Because of growing concerns, the Food and Drug Administration has issued multiple public warnings about kratom, ranging from products contaminated with Salmonella and heavy metals, to adverse effects such as seizures and liver toxicity.
The present study aimed to characterize kratom-associated liver injury through a case series analysis. First, the investigators reviewed 404 cases of herbal and dietary supplement-associated liver injury from the Drug-Induced Liver Injury Network prospective study. They found 11 suspected cases of kratom-related liver injury, with an upward trend in recent years. At this time, seven of the cases have been adjudicated by an expert panel and confirmed to be highly likely or probably associated with kratom.
Of these seven cases, all patients were hospitalized, although all recovered without need for liver transplant. Patients presented after a median of 15 days of kratom use, with a 28-day symptom latency period. However, Dr. Navarro noted that some cases presented after just 5 days of use. The most common presenting symptom was itching (86%), followed by jaundice (71%), abdominal pain (71%), nausea (57%), and fever (43%). Blood work revealed a mixed hepatocellular and cholestatic pattern. Median peak ALT was 362 U/L, peak alkaline phosphatase was 294 U/L, and peak total bilirubin was 20.1 mg/dL. Despite these changes, patients did not have significant liver dysfunction, such as coagulopathy.
Following this clinical characterization, Dr. Navarro reviewed existing toxicity data. Rat studies suggest that kratom is safe at doses between 1-10 mg/kg, while toxicity occurs after prolonged exposure to more than 100 mg/kg. A cross-sectional human study reported that kratom was safe at doses up to 75 mg/day. However, in the present case series, some patients presented after ingesting as little as 0.66 mg/day, and Dr. Navarro pointed out wide variations in product concentrations of mitragynine.
“Certainly, we need more human toxicity studies to determine what a safe dose really is, because this product is not going away,” Dr. Navarro said.
The investigators disclosed relationships with Gilead, Bristol-Myers Squibb, Sanofi, and others.
SOURCE: Navarro VJ et al. The Liver Meeting 2019, Abstract 212.
BOSTON – Kratom, a botanical product with opioid-like activity, is increasingly responsible for cases of liver injury in the United States, according to investigators.
Kratom-associated liver damage involves a mixed pattern of hepatocellular and cholestatic injury that typically occurs after about 2-6 weeks of use, reported lead author Victor J. Navarro, MD, division head of gastroenterology at Einstein Healthcare Network in Philadelphia, and colleagues.
“I think it’s important for clinicians to have heightened awareness of the abuse potential [of kratom], because it is an opioid agonist and [because of] its capacity to cause liver injury,” Dr. Navarro said.
Kratom acts as a stimulant at low doses, while higher doses have sedating and narcotic properties. These effects are attributed to several alkaloids found in kratom’s source plant, Mitragyna speciose, of which mitragynine, a suspected opioid agonist, is most common.
Presenting at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Navarro cited figures from the National Poison Data System that suggest an upward trend in kratom usage in the United States, from very little use in 2011 to 1 exposure per million people in 2014 and more recently to slightly more than 2.5 exposures per million people in 2017, predominantly among individuals aged 20 years and older. According to the Centers for Disease Control and Prevention, more than 90 kratom-associated deaths occurred between July 2016 and December 2017. Because of growing concerns, the Food and Drug Administration has issued multiple public warnings about kratom, ranging from products contaminated with Salmonella and heavy metals, to adverse effects such as seizures and liver toxicity.
The present study aimed to characterize kratom-associated liver injury through a case series analysis. First, the investigators reviewed 404 cases of herbal and dietary supplement-associated liver injury from the Drug-Induced Liver Injury Network prospective study. They found 11 suspected cases of kratom-related liver injury, with an upward trend in recent years. At this time, seven of the cases have been adjudicated by an expert panel and confirmed to be highly likely or probably associated with kratom.
Of these seven cases, all patients were hospitalized, although all recovered without need for liver transplant. Patients presented after a median of 15 days of kratom use, with a 28-day symptom latency period. However, Dr. Navarro noted that some cases presented after just 5 days of use. The most common presenting symptom was itching (86%), followed by jaundice (71%), abdominal pain (71%), nausea (57%), and fever (43%). Blood work revealed a mixed hepatocellular and cholestatic pattern. Median peak ALT was 362 U/L, peak alkaline phosphatase was 294 U/L, and peak total bilirubin was 20.1 mg/dL. Despite these changes, patients did not have significant liver dysfunction, such as coagulopathy.
Following this clinical characterization, Dr. Navarro reviewed existing toxicity data. Rat studies suggest that kratom is safe at doses between 1-10 mg/kg, while toxicity occurs after prolonged exposure to more than 100 mg/kg. A cross-sectional human study reported that kratom was safe at doses up to 75 mg/day. However, in the present case series, some patients presented after ingesting as little as 0.66 mg/day, and Dr. Navarro pointed out wide variations in product concentrations of mitragynine.
“Certainly, we need more human toxicity studies to determine what a safe dose really is, because this product is not going away,” Dr. Navarro said.
The investigators disclosed relationships with Gilead, Bristol-Myers Squibb, Sanofi, and others.
SOURCE: Navarro VJ et al. The Liver Meeting 2019, Abstract 212.
BOSTON – Kratom, a botanical product with opioid-like activity, is increasingly responsible for cases of liver injury in the United States, according to investigators.
Kratom-associated liver damage involves a mixed pattern of hepatocellular and cholestatic injury that typically occurs after about 2-6 weeks of use, reported lead author Victor J. Navarro, MD, division head of gastroenterology at Einstein Healthcare Network in Philadelphia, and colleagues.
“I think it’s important for clinicians to have heightened awareness of the abuse potential [of kratom], because it is an opioid agonist and [because of] its capacity to cause liver injury,” Dr. Navarro said.
Kratom acts as a stimulant at low doses, while higher doses have sedating and narcotic properties. These effects are attributed to several alkaloids found in kratom’s source plant, Mitragyna speciose, of which mitragynine, a suspected opioid agonist, is most common.
Presenting at the annual meeting of the American Association for the Study of Liver Diseases, Dr. Navarro cited figures from the National Poison Data System that suggest an upward trend in kratom usage in the United States, from very little use in 2011 to 1 exposure per million people in 2014 and more recently to slightly more than 2.5 exposures per million people in 2017, predominantly among individuals aged 20 years and older. According to the Centers for Disease Control and Prevention, more than 90 kratom-associated deaths occurred between July 2016 and December 2017. Because of growing concerns, the Food and Drug Administration has issued multiple public warnings about kratom, ranging from products contaminated with Salmonella and heavy metals, to adverse effects such as seizures and liver toxicity.
The present study aimed to characterize kratom-associated liver injury through a case series analysis. First, the investigators reviewed 404 cases of herbal and dietary supplement-associated liver injury from the Drug-Induced Liver Injury Network prospective study. They found 11 suspected cases of kratom-related liver injury, with an upward trend in recent years. At this time, seven of the cases have been adjudicated by an expert panel and confirmed to be highly likely or probably associated with kratom.
Of these seven cases, all patients were hospitalized, although all recovered without need for liver transplant. Patients presented after a median of 15 days of kratom use, with a 28-day symptom latency period. However, Dr. Navarro noted that some cases presented after just 5 days of use. The most common presenting symptom was itching (86%), followed by jaundice (71%), abdominal pain (71%), nausea (57%), and fever (43%). Blood work revealed a mixed hepatocellular and cholestatic pattern. Median peak ALT was 362 U/L, peak alkaline phosphatase was 294 U/L, and peak total bilirubin was 20.1 mg/dL. Despite these changes, patients did not have significant liver dysfunction, such as coagulopathy.
Following this clinical characterization, Dr. Navarro reviewed existing toxicity data. Rat studies suggest that kratom is safe at doses between 1-10 mg/kg, while toxicity occurs after prolonged exposure to more than 100 mg/kg. A cross-sectional human study reported that kratom was safe at doses up to 75 mg/day. However, in the present case series, some patients presented after ingesting as little as 0.66 mg/day, and Dr. Navarro pointed out wide variations in product concentrations of mitragynine.
“Certainly, we need more human toxicity studies to determine what a safe dose really is, because this product is not going away,” Dr. Navarro said.
The investigators disclosed relationships with Gilead, Bristol-Myers Squibb, Sanofi, and others.
SOURCE: Navarro VJ et al. The Liver Meeting 2019, Abstract 212.
REPORTING FROM THE LIVER MEETING 2019
Fentanyl-related deaths show strong regional pattern
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.
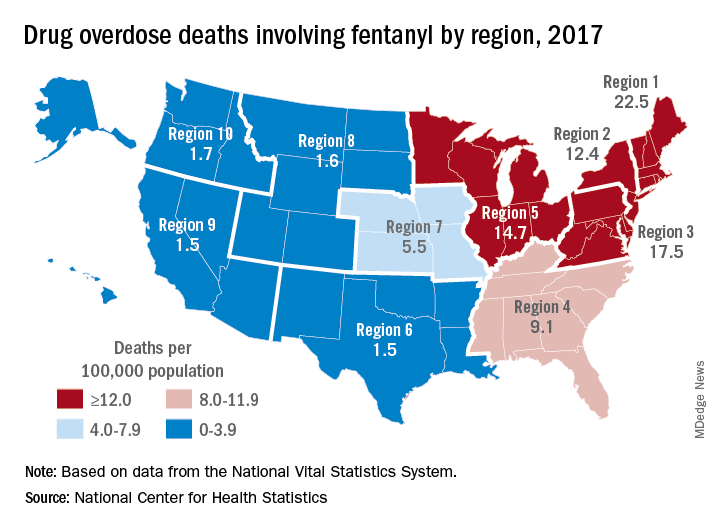
Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.

Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Fentanyl was involved in more overdose deaths than any other drug in 2017, and the death rate in New England was 15 times higher than in regions of the Midwest and West, according to the National Center for Health Statistics.

Nationally, fentanyl was involved in 39% of all drug overdose deaths and had an age-adjusted death rate of 8.7/100,000 standard population in 2017. In 2016, when fentanyl also was the most involved drug in the United States, the corresponding figures were 29% and 5.9/100,000, the agency said in a recent report.
Fentanyl was the most involved drug in overdose deaths for 6 of the country’s 10 public health regions in 2017, with a clear pattern of decreasing use from east to west. The highest death rate (22.5/100,000) occurred in Region 1 (New England) and the lowest rates (1.5/100,000) came in Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) and Region 9 (Arizona, California, Hawaii, and Nevada), the researchers said.
A somewhat similar pattern was seen for heroin, which was second nationally on the list of drugs most frequently involved in overdose deaths (23%), except that New England was somewhat below three other regions in the East and upper Midwest. The highest heroin death rate (8.6/100,000) was seen in Region 2 (New Jersey and New York) and the lowest (2.2) occurred in Region 9, they said, based on data from the National Vital Statistics System’s mortality files.
The fentanyl pattern was even more closely repeated with cocaine, third in involvement nationally at 21% of overdose deaths in 2017. The high in overdose deaths (9.5/100,000) came in Region 1 again, and the low in Region 9 (1.3), along with Region 7 (Iowa, Kansas, Missouri, and Nebraska) and Region 10 (Alaska, Idaho, Oregon, and Washington), the report showed.
The regional pattern of overdose deaths for methamphetamine, which was fourth nationally in involvement (13.3%), basically reversed the other three drugs: highest in the West and lowest in the Northeast. Region 9 had the highest death rate (5.2/100,000) and Region 2 the lowest (0.4), with Region 1 just ahead at 0.6.
Student vapers make mint the most popular Juul flavor
according to data from the 2019 Monitoring the Future study.
Almost half (47.1%) of the 12th graders who had used Juul e-cigarettes in the past 30 days reported that mint was the flavor they most often used, compared with 23.8% for mango and 8.6% for fruit, which is a combination of flavors, Adam M. Leventhal, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
Mint was also the flavor most often used by 10th graders (43.5%), with mango again second at 27.3%, and fruit third at 10.8%. Eighth-grade students switched mango (33.5%) and mint (29.2%) but had fruit third again at 16.0%, the investigators reported, based on data for 1,739 respondents to the Monitoring the Future survey who had used a vaping product within the past 30 days.
Juul has suspended sales of four – mango, fruit, creme, and cucumber – of its original eight flavors, Dr. Leventhal and associates noted, and e-cigarette flavors other than tobacco, menthol, and mint have been prohibited by some local municipalities.
“The current findings raise uncertainty whether regulations or sales suspensions that exempt mint flavors are optimal strategies for reducing youth e-cigarette use,” they wrote.
As this article was being written, the Wall Street Journal had just reported that the Food and Drug Administration will ban mint and all other e-cigarette flavors except tobacco and menthol.
SOURCE: Leventhal AM et al. JAMA. 2019 Nov 5. doi: 10.1001/jama.2019.17968.
according to data from the 2019 Monitoring the Future study.

Almost half (47.1%) of the 12th graders who had used Juul e-cigarettes in the past 30 days reported that mint was the flavor they most often used, compared with 23.8% for mango and 8.6% for fruit, which is a combination of flavors, Adam M. Leventhal, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
Mint was also the flavor most often used by 10th graders (43.5%), with mango again second at 27.3%, and fruit third at 10.8%. Eighth-grade students switched mango (33.5%) and mint (29.2%) but had fruit third again at 16.0%, the investigators reported, based on data for 1,739 respondents to the Monitoring the Future survey who had used a vaping product within the past 30 days.
Juul has suspended sales of four – mango, fruit, creme, and cucumber – of its original eight flavors, Dr. Leventhal and associates noted, and e-cigarette flavors other than tobacco, menthol, and mint have been prohibited by some local municipalities.
“The current findings raise uncertainty whether regulations or sales suspensions that exempt mint flavors are optimal strategies for reducing youth e-cigarette use,” they wrote.
As this article was being written, the Wall Street Journal had just reported that the Food and Drug Administration will ban mint and all other e-cigarette flavors except tobacco and menthol.
SOURCE: Leventhal AM et al. JAMA. 2019 Nov 5. doi: 10.1001/jama.2019.17968.
according to data from the 2019 Monitoring the Future study.

Almost half (47.1%) of the 12th graders who had used Juul e-cigarettes in the past 30 days reported that mint was the flavor they most often used, compared with 23.8% for mango and 8.6% for fruit, which is a combination of flavors, Adam M. Leventhal, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
Mint was also the flavor most often used by 10th graders (43.5%), with mango again second at 27.3%, and fruit third at 10.8%. Eighth-grade students switched mango (33.5%) and mint (29.2%) but had fruit third again at 16.0%, the investigators reported, based on data for 1,739 respondents to the Monitoring the Future survey who had used a vaping product within the past 30 days.
Juul has suspended sales of four – mango, fruit, creme, and cucumber – of its original eight flavors, Dr. Leventhal and associates noted, and e-cigarette flavors other than tobacco, menthol, and mint have been prohibited by some local municipalities.
“The current findings raise uncertainty whether regulations or sales suspensions that exempt mint flavors are optimal strategies for reducing youth e-cigarette use,” they wrote.
As this article was being written, the Wall Street Journal had just reported that the Food and Drug Administration will ban mint and all other e-cigarette flavors except tobacco and menthol.
SOURCE: Leventhal AM et al. JAMA. 2019 Nov 5. doi: 10.1001/jama.2019.17968.
FROM JAMA
Ask about vaping and e-cigarette use
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.