User login
Adolescent alcohol, opioid misuse linked to risky behaviors
Binge drinking and misuse of opioids led to risky behavior during adolescence, two studies from the journal Pediatrics highlighted. And the binge drinking in high school may predict risky driving behaviors up to 4 years after high school.
Federico E. Vaca, MD, of the developmental neurocognitive driving simulation research center at Yale University, New Haven, Conn., and colleagues examined the associations between risky driving behaviors and binge drinking of 2,785 adolescents in the nationally representative, longitudinal NEXT Generation Health Study. The researchers studied the effects of binge drinking on driving while impaired (DWI), riding with an impaired driver (RWI), blackouts, extreme binge drinking, and risky driving.
The adolescents were studied across seven waves, with Wave 1 beginning in the 2009-2010 school year (10th grade; mean age, 16 years), and data extended up to 4 years after high school. Of all adolescents enrolled, 91% completed Wave 1, 88% completed Wave 2, 86% completed Wave 3 (12th grade), 78% completed Wave 4, 79% completed Wave 5, 84% completed Wave 6, and 83% completed Wave 7 (4 years after leaving high school) of the study.
High school binge drinking predicts later risky behavior
About one-quarter of adolescents reported binge drinking in Waves 1-3, with an incidence of 27% in Wave 1, 24% in Wave 2, and 27% in Wave 3. Adolescents who reported binge drinking in Wave 3 had a higher likelihood of DWI in subsequent waves, with nearly six times higher odds in Wave 5 and more than twice as likely in Wave 7, researchers said. Binge drinking in Wave 3 also was associated with greater than four times higher odds of RWI in Wave 4, and more than two and a half times higher odds of RWI in Wave 7. Among adolescents who reported binge drinking across 3 years in high school, there was a higher likelihood of extreme binge drinking in Wave 7, and higher likelihood of risky driving after graduating.
Impact of parental knowledge of drinking
in some waves. Father monitoring knowledge of drinking in Waves 1-3 lowered the odds of DWI by 30% in Wave 5 and 20% in Wave 6, while also lowering the odds of RWI in Wave 4 and Wave 7 by 20%.
Mother knowledge of drinking in Waves 1-3 was associated with 60% lower odds of DWI in Wave 4, but did not lower odds in any wave for RWI.
Overall, parental support for not drinking lowered odds for DWI by 40% in Waves 4 and 5, and by 30% in Wave 7 while also lowering odds of RWI in Wave 4 by 20%.
The results are consistent with other studies examining risky driving behavior and binge drinking in adolescent populations, but researchers noted that “to an important but limited extent, parental practices while the teenager is in high school may protect against DWI, RWI, and blackouts as adolescents move into early adulthood.”
“Our findings are relevant to prevention programs that seek to incorporate alcohol screening with intentional inquiry about binge drinking. Moreover, our results may be instructive to programs that seek to leverage facets of parental practices to reduce health-risk contexts for youth,” Dr. Vaca and colleagues concluded. “Such prevention activities coupled with strengthening of policies and practices reducing adolescents’ access to alcohol could reduce later major alcohol-related health-risk behaviors and their consequences.”
Opioid misuse and risky behavior
In a second study, Devika Bhatia, MD, of the University of Colorado at Denver, Aurora, and colleagues examined opioid misuse in a nationally-representative sample of 14,765 adolescents from the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Surveillance Survey. The researchers measured opioid misuse by categorizing adolescents into groups based on whether they had ever misused prescription opioids and whether they had engaged in risky driving behavior, violent behavior, risky sexual behavior, had a history of substance abuse, or attempted suicide.
Dr. Bhatia and colleagues found 14% of adolescents in the study reported misusing opioids, with an overrepresentation of 17-year-old and 18-year-old participants reporting opioid misuse (P less than .0001). there were no statistically significant difference between those who misused opioids and those who did not in terms of race, ethnicity, or sex.
Those adolescents who reported misusing opioids were 2.8 times more likely to not use a seatbelt; were 2.8 times more likely to have RWI; were 5.8 times more likely to have DWI; or 2.3 times more likely to have texted or emailed while driving. In each of these cases, P was less than .0001.
Adolescents who misused opioids also had significantly increased odds of engaging in risky sexual behaviors such as having sex before 13 years (3.9 times); having sex with four or more partners (4.8 times); using substances before sex (3.6 times); and not using a condom before sex (2.0 times). In each of these cases, P was less than .0001.
Additionally, adolescents in this category were between 5.4 times and 22.3 times more likely to use other substances (P less than .0001 for 10 variables); 4.9 times more likely to have attempted suicide (P less than .0001); or more likely to have engaged in violent behavior such as getting into physical fights (4.0 times), carrying a weapon (3.4 times) or a gun (5.1 times) within the last 30 days. In the four latter cases, P was less than .0001.
“With the ongoing opioid epidemic, pediatricians and child psychiatrists are likely to be more attuned to opioid misuse in their patients,” Dr. Bhatia and colleagues concluded. “If youth are screening positive for opioid misuse, pediatricians, nurses, social workers, child psychiatrists, and other providers assessing adolescents may have a new, broad range of other risky behaviors for which to screen regardless of the direction of the association.”
Substance use screening for treating substance use disorder traditionally has been is provided by a specialist, Jessica A. Kulak, PhD, MPH, said in an interview. “However, integration of care services may help to change societal norms around problematic substance use – both by decreasing stigma associated with substance use, as well as increasing clinicians’ preparedness, knowledge, and confidence in preventing and intervening on adolescents’ substance experimentation and use.” She recommended that clinicians in primary care improve their training by using the Substance Abuse and Mental Health Services Administration’s Screening, Brief Intervention, and Referral to Treatment program, which is available as a free online course.
Confidentiality is important in adolescent health, said Dr. Kulak, who is an assistant professor in the department of health, nutrition, and dietetics at State University of New York at Buffalo. “When discussing sensitive topics, such as binge drinking and opioid misuse, adolescents may fear that these or other risky activities may be disclosed to parents or law enforcement officials. Therefore, adolescent health providers should be aware of local, state, and federal laws pertaining to the confidentiality of minors.”
She added, “adolescents are often susceptible to others’ influences, so having open communication and support from a trusted adult – be it a parent or clinician – may also be protective against risky behaviors.”
The study by Vaca et al. was funded by the National Institutes of Health with support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; the National Institute on Alcohol Abuse and Alcoholism; the National Institute on Drug Abuse; and the Maternal and Child Health Bureau of the Health Resources and Services Administration. The study by Bhatia et al. had no external funding. The authors from both studies reported no relevant financial disclosures. Dr. Kulak said she had no financial disclosures or other conflicts of interest.
SOURCE: Vaca FE et al. Pediatrics. 2020; doi: 10.1542/peds.2018-4095. Bhatia D et al. Pediatrics. 2020; doi: 10.1542/peds.2019-2470.
These newly published reports indicate the high prevalence of risky behaviors and their associations – cross-sectionally and longitudinally – with major threats to adolescent health – so asking about alcohol use, opioid misuse, and associated health risks is truly “in the lane” of clinicians, school professionals, and parents who see and care about adolescents.
At this point, I think it’s incontrovertible that clinicians should screen adolescents to learn about their physical, emotional, and behavioral health. And they should seek opportunities for professional training, skills development, and expansion of their professional networks so they are able to address – individually or collaboratively via referrals – the behavioral and psychosocial health risks of their patients.
The good news is that there is growing awareness of the importance of using validated screening tools to identify patient behavioral health risks – including those pertaining to adolescent and young adult alcohol use and opioid misuse. “Best practice” dictates that screening approaches rely on asking questions using structured tools; intuition and “just winging it” are not effective or reliable for identifying patient behavior. Forward-looking clinics and practices could be asking patients to report about health behaviors in the waiting room (on a computer tablet, for example), or even remotely (using a secure app or data collection tool) in advance of a visit. Asking should be periodic – since behaviors can change fairly rapidly among young people. The benefit is that patient-reported information can be processed in advance to cue clinician follow-up and intervention. And youth tend to share more about their behaviors when they are asked electronically, rather than face to face. Intelligent screens can provide near real-time estimation of risk – to support in-office brief intervention tailored to the risk level of a young person or to trigger follow-up.
These studies indicate that binge alcohol use and misuse of prescription opioids among adolescents are real, pervasive, and deserving of our considered attention. There is no magic bullet. However busy clinicians may have a significant role to play in identifying and addressing these problems.
Elissa Weitzman, ScD, MSc, is an associate professor of pediatrics at Harvard Medical School, Boston, and an associate scientist based in adolescent/young adult medicine and the computational health informatics program at Boston Children’s Hospital. She was asked to comment on the articles by Vaca et al. and Bhatia et al. Dr. Weitzman said she had no relevant financial disclosures.
These newly published reports indicate the high prevalence of risky behaviors and their associations – cross-sectionally and longitudinally – with major threats to adolescent health – so asking about alcohol use, opioid misuse, and associated health risks is truly “in the lane” of clinicians, school professionals, and parents who see and care about adolescents.
At this point, I think it’s incontrovertible that clinicians should screen adolescents to learn about their physical, emotional, and behavioral health. And they should seek opportunities for professional training, skills development, and expansion of their professional networks so they are able to address – individually or collaboratively via referrals – the behavioral and psychosocial health risks of their patients.
The good news is that there is growing awareness of the importance of using validated screening tools to identify patient behavioral health risks – including those pertaining to adolescent and young adult alcohol use and opioid misuse. “Best practice” dictates that screening approaches rely on asking questions using structured tools; intuition and “just winging it” are not effective or reliable for identifying patient behavior. Forward-looking clinics and practices could be asking patients to report about health behaviors in the waiting room (on a computer tablet, for example), or even remotely (using a secure app or data collection tool) in advance of a visit. Asking should be periodic – since behaviors can change fairly rapidly among young people. The benefit is that patient-reported information can be processed in advance to cue clinician follow-up and intervention. And youth tend to share more about their behaviors when they are asked electronically, rather than face to face. Intelligent screens can provide near real-time estimation of risk – to support in-office brief intervention tailored to the risk level of a young person or to trigger follow-up.
These studies indicate that binge alcohol use and misuse of prescription opioids among adolescents are real, pervasive, and deserving of our considered attention. There is no magic bullet. However busy clinicians may have a significant role to play in identifying and addressing these problems.
Elissa Weitzman, ScD, MSc, is an associate professor of pediatrics at Harvard Medical School, Boston, and an associate scientist based in adolescent/young adult medicine and the computational health informatics program at Boston Children’s Hospital. She was asked to comment on the articles by Vaca et al. and Bhatia et al. Dr. Weitzman said she had no relevant financial disclosures.
These newly published reports indicate the high prevalence of risky behaviors and their associations – cross-sectionally and longitudinally – with major threats to adolescent health – so asking about alcohol use, opioid misuse, and associated health risks is truly “in the lane” of clinicians, school professionals, and parents who see and care about adolescents.
At this point, I think it’s incontrovertible that clinicians should screen adolescents to learn about their physical, emotional, and behavioral health. And they should seek opportunities for professional training, skills development, and expansion of their professional networks so they are able to address – individually or collaboratively via referrals – the behavioral and psychosocial health risks of their patients.
The good news is that there is growing awareness of the importance of using validated screening tools to identify patient behavioral health risks – including those pertaining to adolescent and young adult alcohol use and opioid misuse. “Best practice” dictates that screening approaches rely on asking questions using structured tools; intuition and “just winging it” are not effective or reliable for identifying patient behavior. Forward-looking clinics and practices could be asking patients to report about health behaviors in the waiting room (on a computer tablet, for example), or even remotely (using a secure app or data collection tool) in advance of a visit. Asking should be periodic – since behaviors can change fairly rapidly among young people. The benefit is that patient-reported information can be processed in advance to cue clinician follow-up and intervention. And youth tend to share more about their behaviors when they are asked electronically, rather than face to face. Intelligent screens can provide near real-time estimation of risk – to support in-office brief intervention tailored to the risk level of a young person or to trigger follow-up.
These studies indicate that binge alcohol use and misuse of prescription opioids among adolescents are real, pervasive, and deserving of our considered attention. There is no magic bullet. However busy clinicians may have a significant role to play in identifying and addressing these problems.
Elissa Weitzman, ScD, MSc, is an associate professor of pediatrics at Harvard Medical School, Boston, and an associate scientist based in adolescent/young adult medicine and the computational health informatics program at Boston Children’s Hospital. She was asked to comment on the articles by Vaca et al. and Bhatia et al. Dr. Weitzman said she had no relevant financial disclosures.
Binge drinking and misuse of opioids led to risky behavior during adolescence, two studies from the journal Pediatrics highlighted. And the binge drinking in high school may predict risky driving behaviors up to 4 years after high school.
Federico E. Vaca, MD, of the developmental neurocognitive driving simulation research center at Yale University, New Haven, Conn., and colleagues examined the associations between risky driving behaviors and binge drinking of 2,785 adolescents in the nationally representative, longitudinal NEXT Generation Health Study. The researchers studied the effects of binge drinking on driving while impaired (DWI), riding with an impaired driver (RWI), blackouts, extreme binge drinking, and risky driving.
The adolescents were studied across seven waves, with Wave 1 beginning in the 2009-2010 school year (10th grade; mean age, 16 years), and data extended up to 4 years after high school. Of all adolescents enrolled, 91% completed Wave 1, 88% completed Wave 2, 86% completed Wave 3 (12th grade), 78% completed Wave 4, 79% completed Wave 5, 84% completed Wave 6, and 83% completed Wave 7 (4 years after leaving high school) of the study.
High school binge drinking predicts later risky behavior
About one-quarter of adolescents reported binge drinking in Waves 1-3, with an incidence of 27% in Wave 1, 24% in Wave 2, and 27% in Wave 3. Adolescents who reported binge drinking in Wave 3 had a higher likelihood of DWI in subsequent waves, with nearly six times higher odds in Wave 5 and more than twice as likely in Wave 7, researchers said. Binge drinking in Wave 3 also was associated with greater than four times higher odds of RWI in Wave 4, and more than two and a half times higher odds of RWI in Wave 7. Among adolescents who reported binge drinking across 3 years in high school, there was a higher likelihood of extreme binge drinking in Wave 7, and higher likelihood of risky driving after graduating.
Impact of parental knowledge of drinking
in some waves. Father monitoring knowledge of drinking in Waves 1-3 lowered the odds of DWI by 30% in Wave 5 and 20% in Wave 6, while also lowering the odds of RWI in Wave 4 and Wave 7 by 20%.
Mother knowledge of drinking in Waves 1-3 was associated with 60% lower odds of DWI in Wave 4, but did not lower odds in any wave for RWI.
Overall, parental support for not drinking lowered odds for DWI by 40% in Waves 4 and 5, and by 30% in Wave 7 while also lowering odds of RWI in Wave 4 by 20%.
The results are consistent with other studies examining risky driving behavior and binge drinking in adolescent populations, but researchers noted that “to an important but limited extent, parental practices while the teenager is in high school may protect against DWI, RWI, and blackouts as adolescents move into early adulthood.”
“Our findings are relevant to prevention programs that seek to incorporate alcohol screening with intentional inquiry about binge drinking. Moreover, our results may be instructive to programs that seek to leverage facets of parental practices to reduce health-risk contexts for youth,” Dr. Vaca and colleagues concluded. “Such prevention activities coupled with strengthening of policies and practices reducing adolescents’ access to alcohol could reduce later major alcohol-related health-risk behaviors and their consequences.”
Opioid misuse and risky behavior
In a second study, Devika Bhatia, MD, of the University of Colorado at Denver, Aurora, and colleagues examined opioid misuse in a nationally-representative sample of 14,765 adolescents from the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Surveillance Survey. The researchers measured opioid misuse by categorizing adolescents into groups based on whether they had ever misused prescription opioids and whether they had engaged in risky driving behavior, violent behavior, risky sexual behavior, had a history of substance abuse, or attempted suicide.
Dr. Bhatia and colleagues found 14% of adolescents in the study reported misusing opioids, with an overrepresentation of 17-year-old and 18-year-old participants reporting opioid misuse (P less than .0001). there were no statistically significant difference between those who misused opioids and those who did not in terms of race, ethnicity, or sex.
Those adolescents who reported misusing opioids were 2.8 times more likely to not use a seatbelt; were 2.8 times more likely to have RWI; were 5.8 times more likely to have DWI; or 2.3 times more likely to have texted or emailed while driving. In each of these cases, P was less than .0001.
Adolescents who misused opioids also had significantly increased odds of engaging in risky sexual behaviors such as having sex before 13 years (3.9 times); having sex with four or more partners (4.8 times); using substances before sex (3.6 times); and not using a condom before sex (2.0 times). In each of these cases, P was less than .0001.
Additionally, adolescents in this category were between 5.4 times and 22.3 times more likely to use other substances (P less than .0001 for 10 variables); 4.9 times more likely to have attempted suicide (P less than .0001); or more likely to have engaged in violent behavior such as getting into physical fights (4.0 times), carrying a weapon (3.4 times) or a gun (5.1 times) within the last 30 days. In the four latter cases, P was less than .0001.
“With the ongoing opioid epidemic, pediatricians and child psychiatrists are likely to be more attuned to opioid misuse in their patients,” Dr. Bhatia and colleagues concluded. “If youth are screening positive for opioid misuse, pediatricians, nurses, social workers, child psychiatrists, and other providers assessing adolescents may have a new, broad range of other risky behaviors for which to screen regardless of the direction of the association.”
Substance use screening for treating substance use disorder traditionally has been is provided by a specialist, Jessica A. Kulak, PhD, MPH, said in an interview. “However, integration of care services may help to change societal norms around problematic substance use – both by decreasing stigma associated with substance use, as well as increasing clinicians’ preparedness, knowledge, and confidence in preventing and intervening on adolescents’ substance experimentation and use.” She recommended that clinicians in primary care improve their training by using the Substance Abuse and Mental Health Services Administration’s Screening, Brief Intervention, and Referral to Treatment program, which is available as a free online course.
Confidentiality is important in adolescent health, said Dr. Kulak, who is an assistant professor in the department of health, nutrition, and dietetics at State University of New York at Buffalo. “When discussing sensitive topics, such as binge drinking and opioid misuse, adolescents may fear that these or other risky activities may be disclosed to parents or law enforcement officials. Therefore, adolescent health providers should be aware of local, state, and federal laws pertaining to the confidentiality of minors.”
She added, “adolescents are often susceptible to others’ influences, so having open communication and support from a trusted adult – be it a parent or clinician – may also be protective against risky behaviors.”
The study by Vaca et al. was funded by the National Institutes of Health with support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; the National Institute on Alcohol Abuse and Alcoholism; the National Institute on Drug Abuse; and the Maternal and Child Health Bureau of the Health Resources and Services Administration. The study by Bhatia et al. had no external funding. The authors from both studies reported no relevant financial disclosures. Dr. Kulak said she had no financial disclosures or other conflicts of interest.
SOURCE: Vaca FE et al. Pediatrics. 2020; doi: 10.1542/peds.2018-4095. Bhatia D et al. Pediatrics. 2020; doi: 10.1542/peds.2019-2470.
Binge drinking and misuse of opioids led to risky behavior during adolescence, two studies from the journal Pediatrics highlighted. And the binge drinking in high school may predict risky driving behaviors up to 4 years after high school.
Federico E. Vaca, MD, of the developmental neurocognitive driving simulation research center at Yale University, New Haven, Conn., and colleagues examined the associations between risky driving behaviors and binge drinking of 2,785 adolescents in the nationally representative, longitudinal NEXT Generation Health Study. The researchers studied the effects of binge drinking on driving while impaired (DWI), riding with an impaired driver (RWI), blackouts, extreme binge drinking, and risky driving.
The adolescents were studied across seven waves, with Wave 1 beginning in the 2009-2010 school year (10th grade; mean age, 16 years), and data extended up to 4 years after high school. Of all adolescents enrolled, 91% completed Wave 1, 88% completed Wave 2, 86% completed Wave 3 (12th grade), 78% completed Wave 4, 79% completed Wave 5, 84% completed Wave 6, and 83% completed Wave 7 (4 years after leaving high school) of the study.
High school binge drinking predicts later risky behavior
About one-quarter of adolescents reported binge drinking in Waves 1-3, with an incidence of 27% in Wave 1, 24% in Wave 2, and 27% in Wave 3. Adolescents who reported binge drinking in Wave 3 had a higher likelihood of DWI in subsequent waves, with nearly six times higher odds in Wave 5 and more than twice as likely in Wave 7, researchers said. Binge drinking in Wave 3 also was associated with greater than four times higher odds of RWI in Wave 4, and more than two and a half times higher odds of RWI in Wave 7. Among adolescents who reported binge drinking across 3 years in high school, there was a higher likelihood of extreme binge drinking in Wave 7, and higher likelihood of risky driving after graduating.
Impact of parental knowledge of drinking
in some waves. Father monitoring knowledge of drinking in Waves 1-3 lowered the odds of DWI by 30% in Wave 5 and 20% in Wave 6, while also lowering the odds of RWI in Wave 4 and Wave 7 by 20%.
Mother knowledge of drinking in Waves 1-3 was associated with 60% lower odds of DWI in Wave 4, but did not lower odds in any wave for RWI.
Overall, parental support for not drinking lowered odds for DWI by 40% in Waves 4 and 5, and by 30% in Wave 7 while also lowering odds of RWI in Wave 4 by 20%.
The results are consistent with other studies examining risky driving behavior and binge drinking in adolescent populations, but researchers noted that “to an important but limited extent, parental practices while the teenager is in high school may protect against DWI, RWI, and blackouts as adolescents move into early adulthood.”
“Our findings are relevant to prevention programs that seek to incorporate alcohol screening with intentional inquiry about binge drinking. Moreover, our results may be instructive to programs that seek to leverage facets of parental practices to reduce health-risk contexts for youth,” Dr. Vaca and colleagues concluded. “Such prevention activities coupled with strengthening of policies and practices reducing adolescents’ access to alcohol could reduce later major alcohol-related health-risk behaviors and their consequences.”
Opioid misuse and risky behavior
In a second study, Devika Bhatia, MD, of the University of Colorado at Denver, Aurora, and colleagues examined opioid misuse in a nationally-representative sample of 14,765 adolescents from the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Surveillance Survey. The researchers measured opioid misuse by categorizing adolescents into groups based on whether they had ever misused prescription opioids and whether they had engaged in risky driving behavior, violent behavior, risky sexual behavior, had a history of substance abuse, or attempted suicide.
Dr. Bhatia and colleagues found 14% of adolescents in the study reported misusing opioids, with an overrepresentation of 17-year-old and 18-year-old participants reporting opioid misuse (P less than .0001). there were no statistically significant difference between those who misused opioids and those who did not in terms of race, ethnicity, or sex.
Those adolescents who reported misusing opioids were 2.8 times more likely to not use a seatbelt; were 2.8 times more likely to have RWI; were 5.8 times more likely to have DWI; or 2.3 times more likely to have texted or emailed while driving. In each of these cases, P was less than .0001.
Adolescents who misused opioids also had significantly increased odds of engaging in risky sexual behaviors such as having sex before 13 years (3.9 times); having sex with four or more partners (4.8 times); using substances before sex (3.6 times); and not using a condom before sex (2.0 times). In each of these cases, P was less than .0001.
Additionally, adolescents in this category were between 5.4 times and 22.3 times more likely to use other substances (P less than .0001 for 10 variables); 4.9 times more likely to have attempted suicide (P less than .0001); or more likely to have engaged in violent behavior such as getting into physical fights (4.0 times), carrying a weapon (3.4 times) or a gun (5.1 times) within the last 30 days. In the four latter cases, P was less than .0001.
“With the ongoing opioid epidemic, pediatricians and child psychiatrists are likely to be more attuned to opioid misuse in their patients,” Dr. Bhatia and colleagues concluded. “If youth are screening positive for opioid misuse, pediatricians, nurses, social workers, child psychiatrists, and other providers assessing adolescents may have a new, broad range of other risky behaviors for which to screen regardless of the direction of the association.”
Substance use screening for treating substance use disorder traditionally has been is provided by a specialist, Jessica A. Kulak, PhD, MPH, said in an interview. “However, integration of care services may help to change societal norms around problematic substance use – both by decreasing stigma associated with substance use, as well as increasing clinicians’ preparedness, knowledge, and confidence in preventing and intervening on adolescents’ substance experimentation and use.” She recommended that clinicians in primary care improve their training by using the Substance Abuse and Mental Health Services Administration’s Screening, Brief Intervention, and Referral to Treatment program, which is available as a free online course.
Confidentiality is important in adolescent health, said Dr. Kulak, who is an assistant professor in the department of health, nutrition, and dietetics at State University of New York at Buffalo. “When discussing sensitive topics, such as binge drinking and opioid misuse, adolescents may fear that these or other risky activities may be disclosed to parents or law enforcement officials. Therefore, adolescent health providers should be aware of local, state, and federal laws pertaining to the confidentiality of minors.”
She added, “adolescents are often susceptible to others’ influences, so having open communication and support from a trusted adult – be it a parent or clinician – may also be protective against risky behaviors.”
The study by Vaca et al. was funded by the National Institutes of Health with support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute; the National Institute on Alcohol Abuse and Alcoholism; the National Institute on Drug Abuse; and the Maternal and Child Health Bureau of the Health Resources and Services Administration. The study by Bhatia et al. had no external funding. The authors from both studies reported no relevant financial disclosures. Dr. Kulak said she had no financial disclosures or other conflicts of interest.
SOURCE: Vaca FE et al. Pediatrics. 2020; doi: 10.1542/peds.2018-4095. Bhatia D et al. Pediatrics. 2020; doi: 10.1542/peds.2019-2470.
FROM PEDIATRICS
FDA targets flavored cartridge-based e-cigarettes, but says it is not a ‘ban’
but states it is not a “ban.”
On Jan. 2, the agency issued enforcement guidance alerting companies that manufacture, distribute, and sell unauthorized flavored cartridge-based e-cigarettes within the next 30 days will risk FDA enforcement action.
FDA has had the authority to require premarket authorization of all e-cigarettes and other electronic nicotine delivery systems (ENDS) since August 2016, but thus far has exercised enforcement discretion regarding the need for premarket authorization for these types of products.
“By prioritizing enforcement against the products that are most widely used by children, our action today seeks to strike the right public health balance by maintaining e-cigarettes as a potential off-ramp for adults using combustible tobacco while ensuring these products don’t provide an on-ramp to nicotine addiction for our youth,” Department of Health & Human Services Secretary Alex Azar said in a statement.
The action comes in the wake of more than 2,500 vaping-related injuries being reported, including more than 50 deaths associated with vaping reported by the Centers for Disease Control and Prevention (although many are related to the use of tetrahydrocannabinol [THC] within vaping products) and a continued rise in youth use of e-cigarettes noted in government surveys.
The agency noted in a Jan. 2 statement announcing the enforcement action that, to date, no ENDS products have received a premarket authorization, “meaning that all ENDS products currently on the market are considered illegally marketed and are subject to enforcement, at any time, in the FDA’s discretion.”
FDA said it is prioritizing enforcement in 30 days against:
- Any flavored, cartridge-based ENDS product, other than those with a tobacco or menthol flavoring.
- All other ENDS products for which manufacturers are failing to take adequate measures to prevent access by minors.
- Any ENDS product that is targeted to minors or is likely to promote use by minors.
In the last category, this might include labeling or advertising resembling “kid-friendly food and drinks such as juice boxes or kid-friendly cereal; products marketed directly to minors by promoting ease of concealing the product or disguising it as another product; and products marketed with characters designed to appeal to youth,” according to the FDA statement.
As of May 12, FDA also will prioritize enforcement against any ENDS product for which the manufacturer has not submitted a premarket application. The agency will continue to exercise enforcement discretion for up to 1 year on these products if an application has been submitted, pending the review of that application.
“By not prioritizing enforcement against other flavored ENDS products in the same way as flavored cartridge-based ENDS products, the FDA has attempted to balance the public health concerns related to youth use of ENDS products with consideration regarding addicted adult cigarette smokers who may try to use ENDS products to transition away from combustible tobacco products,” the agency stated, adding that cartridge-based ENDS products are most commonly used among youth.
The FDA statement noted that the enforcement priorities outlined in the guidance document were not a “ban” on flavored or cartridge-based ENDS, noting the agency “has already accepted and begun review of several premarket applications for flavored ENDS products through the pathway that Congress established in the Tobacco Control Act. ... If a company can demonstrate to the FDA that a specific product meets the applicable standard set forth by Congress, including considering how the marketing of the product may affect youth initiation and use, then the FDA could authorize that product for sale.”
“Coupled with the recently signed legislation increasing the minimum age of sale of tobacco to 21, we believe this policy balances the urgency with which we must address the public health threat of youth use of e-cigarette products with the potential role that e-cigarettes may play in helping adult smokers transition completely away from combustible tobacco to a potentially less risky form of nicotine delivery,” FDA Commissioner Stephen Hahn, MD, said in a statement. “While we expect that responsible members of industry will comply with premarket requirements, we’re ready to take action against any unauthorized e-cigarette products as outlined in our priorities. We’ll also closely monitor the use rates of all e-cigarette products and take additional steps to address youth use as necessary.”
The American Medical Association criticized the action as not going far enough, even though it was a step in the right direction.
“The AMA is disappointed that menthol flavors, one of the most popular, will still be allowed, and that flavored e-liquids will remain on the market, leaving young people with easy access to alternative flavored e-cigarette products,” AMA President Patrice A. Harris, MD, said in a statement. “If we are serious about tackling this epidemic and keeping these harmful products out of the hands of young people, a total ban on all flavored e-cigarettes, in all forms and at all locations, is prudent and urgently needed. We are pleased the administration committed today to closely monitoring the situation and trends in e-cigarette use among young people, and to taking further action if needed.”
but states it is not a “ban.”
On Jan. 2, the agency issued enforcement guidance alerting companies that manufacture, distribute, and sell unauthorized flavored cartridge-based e-cigarettes within the next 30 days will risk FDA enforcement action.
FDA has had the authority to require premarket authorization of all e-cigarettes and other electronic nicotine delivery systems (ENDS) since August 2016, but thus far has exercised enforcement discretion regarding the need for premarket authorization for these types of products.
“By prioritizing enforcement against the products that are most widely used by children, our action today seeks to strike the right public health balance by maintaining e-cigarettes as a potential off-ramp for adults using combustible tobacco while ensuring these products don’t provide an on-ramp to nicotine addiction for our youth,” Department of Health & Human Services Secretary Alex Azar said in a statement.
The action comes in the wake of more than 2,500 vaping-related injuries being reported, including more than 50 deaths associated with vaping reported by the Centers for Disease Control and Prevention (although many are related to the use of tetrahydrocannabinol [THC] within vaping products) and a continued rise in youth use of e-cigarettes noted in government surveys.
The agency noted in a Jan. 2 statement announcing the enforcement action that, to date, no ENDS products have received a premarket authorization, “meaning that all ENDS products currently on the market are considered illegally marketed and are subject to enforcement, at any time, in the FDA’s discretion.”
FDA said it is prioritizing enforcement in 30 days against:
- Any flavored, cartridge-based ENDS product, other than those with a tobacco or menthol flavoring.
- All other ENDS products for which manufacturers are failing to take adequate measures to prevent access by minors.
- Any ENDS product that is targeted to minors or is likely to promote use by minors.
In the last category, this might include labeling or advertising resembling “kid-friendly food and drinks such as juice boxes or kid-friendly cereal; products marketed directly to minors by promoting ease of concealing the product or disguising it as another product; and products marketed with characters designed to appeal to youth,” according to the FDA statement.
As of May 12, FDA also will prioritize enforcement against any ENDS product for which the manufacturer has not submitted a premarket application. The agency will continue to exercise enforcement discretion for up to 1 year on these products if an application has been submitted, pending the review of that application.
“By not prioritizing enforcement against other flavored ENDS products in the same way as flavored cartridge-based ENDS products, the FDA has attempted to balance the public health concerns related to youth use of ENDS products with consideration regarding addicted adult cigarette smokers who may try to use ENDS products to transition away from combustible tobacco products,” the agency stated, adding that cartridge-based ENDS products are most commonly used among youth.
The FDA statement noted that the enforcement priorities outlined in the guidance document were not a “ban” on flavored or cartridge-based ENDS, noting the agency “has already accepted and begun review of several premarket applications for flavored ENDS products through the pathway that Congress established in the Tobacco Control Act. ... If a company can demonstrate to the FDA that a specific product meets the applicable standard set forth by Congress, including considering how the marketing of the product may affect youth initiation and use, then the FDA could authorize that product for sale.”
“Coupled with the recently signed legislation increasing the minimum age of sale of tobacco to 21, we believe this policy balances the urgency with which we must address the public health threat of youth use of e-cigarette products with the potential role that e-cigarettes may play in helping adult smokers transition completely away from combustible tobacco to a potentially less risky form of nicotine delivery,” FDA Commissioner Stephen Hahn, MD, said in a statement. “While we expect that responsible members of industry will comply with premarket requirements, we’re ready to take action against any unauthorized e-cigarette products as outlined in our priorities. We’ll also closely monitor the use rates of all e-cigarette products and take additional steps to address youth use as necessary.”
The American Medical Association criticized the action as not going far enough, even though it was a step in the right direction.
“The AMA is disappointed that menthol flavors, one of the most popular, will still be allowed, and that flavored e-liquids will remain on the market, leaving young people with easy access to alternative flavored e-cigarette products,” AMA President Patrice A. Harris, MD, said in a statement. “If we are serious about tackling this epidemic and keeping these harmful products out of the hands of young people, a total ban on all flavored e-cigarettes, in all forms and at all locations, is prudent and urgently needed. We are pleased the administration committed today to closely monitoring the situation and trends in e-cigarette use among young people, and to taking further action if needed.”
but states it is not a “ban.”
On Jan. 2, the agency issued enforcement guidance alerting companies that manufacture, distribute, and sell unauthorized flavored cartridge-based e-cigarettes within the next 30 days will risk FDA enforcement action.
FDA has had the authority to require premarket authorization of all e-cigarettes and other electronic nicotine delivery systems (ENDS) since August 2016, but thus far has exercised enforcement discretion regarding the need for premarket authorization for these types of products.
“By prioritizing enforcement against the products that are most widely used by children, our action today seeks to strike the right public health balance by maintaining e-cigarettes as a potential off-ramp for adults using combustible tobacco while ensuring these products don’t provide an on-ramp to nicotine addiction for our youth,” Department of Health & Human Services Secretary Alex Azar said in a statement.
The action comes in the wake of more than 2,500 vaping-related injuries being reported, including more than 50 deaths associated with vaping reported by the Centers for Disease Control and Prevention (although many are related to the use of tetrahydrocannabinol [THC] within vaping products) and a continued rise in youth use of e-cigarettes noted in government surveys.
The agency noted in a Jan. 2 statement announcing the enforcement action that, to date, no ENDS products have received a premarket authorization, “meaning that all ENDS products currently on the market are considered illegally marketed and are subject to enforcement, at any time, in the FDA’s discretion.”
FDA said it is prioritizing enforcement in 30 days against:
- Any flavored, cartridge-based ENDS product, other than those with a tobacco or menthol flavoring.
- All other ENDS products for which manufacturers are failing to take adequate measures to prevent access by minors.
- Any ENDS product that is targeted to minors or is likely to promote use by minors.
In the last category, this might include labeling or advertising resembling “kid-friendly food and drinks such as juice boxes or kid-friendly cereal; products marketed directly to minors by promoting ease of concealing the product or disguising it as another product; and products marketed with characters designed to appeal to youth,” according to the FDA statement.
As of May 12, FDA also will prioritize enforcement against any ENDS product for which the manufacturer has not submitted a premarket application. The agency will continue to exercise enforcement discretion for up to 1 year on these products if an application has been submitted, pending the review of that application.
“By not prioritizing enforcement against other flavored ENDS products in the same way as flavored cartridge-based ENDS products, the FDA has attempted to balance the public health concerns related to youth use of ENDS products with consideration regarding addicted adult cigarette smokers who may try to use ENDS products to transition away from combustible tobacco products,” the agency stated, adding that cartridge-based ENDS products are most commonly used among youth.
The FDA statement noted that the enforcement priorities outlined in the guidance document were not a “ban” on flavored or cartridge-based ENDS, noting the agency “has already accepted and begun review of several premarket applications for flavored ENDS products through the pathway that Congress established in the Tobacco Control Act. ... If a company can demonstrate to the FDA that a specific product meets the applicable standard set forth by Congress, including considering how the marketing of the product may affect youth initiation and use, then the FDA could authorize that product for sale.”
“Coupled with the recently signed legislation increasing the minimum age of sale of tobacco to 21, we believe this policy balances the urgency with which we must address the public health threat of youth use of e-cigarette products with the potential role that e-cigarettes may play in helping adult smokers transition completely away from combustible tobacco to a potentially less risky form of nicotine delivery,” FDA Commissioner Stephen Hahn, MD, said in a statement. “While we expect that responsible members of industry will comply with premarket requirements, we’re ready to take action against any unauthorized e-cigarette products as outlined in our priorities. We’ll also closely monitor the use rates of all e-cigarette products and take additional steps to address youth use as necessary.”
The American Medical Association criticized the action as not going far enough, even though it was a step in the right direction.
“The AMA is disappointed that menthol flavors, one of the most popular, will still be allowed, and that flavored e-liquids will remain on the market, leaving young people with easy access to alternative flavored e-cigarette products,” AMA President Patrice A. Harris, MD, said in a statement. “If we are serious about tackling this epidemic and keeping these harmful products out of the hands of young people, a total ban on all flavored e-cigarettes, in all forms and at all locations, is prudent and urgently needed. We are pleased the administration committed today to closely monitoring the situation and trends in e-cigarette use among young people, and to taking further action if needed.”
Lofexidine: An option for treating opioid withdrawal
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
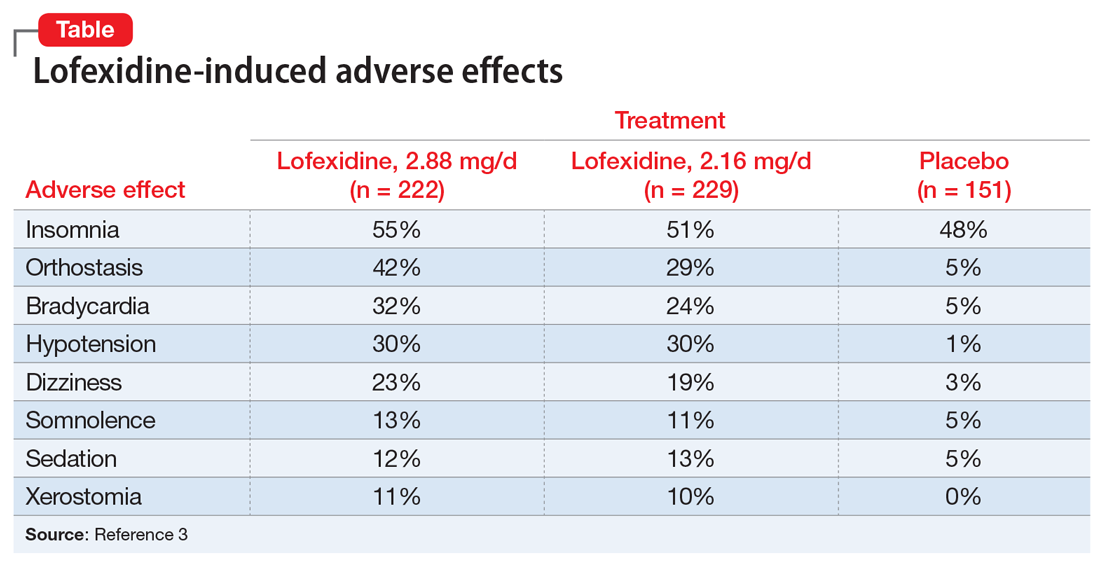
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Dual e-cigarette and combustible tobacco use compound respiratory disease risk
according to recent longitudinal analysis published in the American Journal of Preventive Medicine.
E-cigarettes have been promoted as a safer alternative to combustible tobacco, and until recently, there has been little and conflicting evidence by which to test this hypothesis. This study conducted by Dharma N. Bhatta, PhD, and Stanton A. Glantz, PhD, of the Center for Tobacco Control Research and Education at the University of California, San Francisco, is one of the first longitudinal examinations of e-cigarette use and controlling for combustible tobacco use.
Dr. Bhatta and Dr. Glantz performed a multivariable, logistic regression analysis of adults enrolled in the nationally representative, population-based, longitudinal Population Assessment of Tobacco and Health study. The researchers analyzed the tobacco use of adults in the study in three waves, following them through wave 1 (September 2013 to December 2014), wave 2 (October 2014 to October 2015), and wave 3 (October 2015 to October 2016), analyzing the data between 2018 and 2019. Overall, wave 1 began with 32,320 participants, and 15.1% of adults reported respiratory disease at baseline.
Lung or respiratory disease was assessed by asking participants whether they had been told by a health professional that they had chronic obstructive pulmonary disease, chronic bronchitis, emphysema, or asthma. The researchers defined e-cigarette and combustible tobacco use as participants who never, currently, or formerly used e-cigarettes or smoked combustible tobacco. Participants who indicated they used e-cigarettes or combustible tobacco frequently or infrequently were placed in the current-user group, while past users were those participants who said they used to, but no longer use e-cigarettes or combustible tobacco.
The results showed former e-cigarette use (adjusted odds ratio, 1.34; 95% confidence interval, 1.23-1.46) and current e-cigarette use (aOR, 1.32; 95% CI, 1.17-1.49) were associated with an increased risk of having incident respiratory disease.
The data showed a not unexpected statistically significant association between former combustible tobacco use (aOR, 1.29; 95% CI, 1.14-1.47) as well as current combustible tobacco use (aOR, 1.61; 95% CI, 1.42-1.82) and incident respiratory disease risk.
There was a statistically significant association between respiratory disease and former or current e-cigarette use for adults who did not have respiratory disease at baseline, after adjusting for factors such as current combustible tobacco use, clinical variables, and demographic differences. Participants in wave 1 who reported former (aOR, 1.31; 95% CI, 1.07-1.60) or current e-cigarette use (aOR, 1.29; 95% CI, 1.03-1.61) had a significantly higher risk of developing incident respiratory disease in subsequent waves. There was also a statistically significant association between use of combustible tobacco and subsequent respiratory disease in later waves of the study (aOR, 2.56; 95% CI, 1.92-3.41), which the researchers noted was independent of the usual risks associated with combustible tobacco.
The investigators also looked at the link between dual use of e-cigarettes and combustible tobacco and respiratory disease risk. “The much more common pattern is dual use, in which an e-cigarette user continues to smoke combusted tobacco products at the same time (93.7% of e-cigarette users at wave 2 and 91.2% at wave 3 also used combustible tobacco; 73.3% of e-cigarette users at wave 2 and 64.9% at wave 3 also smoked cigarettes),” they wrote.
The odds of developing respiratory disease for participants who used both e-cigarettes and combustible tobacco were 3.30, compared with a participant who never used e-cigarettes, with similar results seen when comparing e-cigarettes and cigarettes.
“Although switching from combustible tobacco, including cigarettes, to e-cigarettes theoretically could reduce the risk of developing respiratory disease, current evidence indicates a high prevalence of dual use, which is associated with in-creased risk beyond combustible tobacco use,” the investigators wrote.
Harold J. Farber, MD, FCCP, professor of pediatrics in the pulmonary section at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, said in an interview that the increased respiratory risk among dual users, who are likely using e-cigarettes and combustible tobacco together as a way to quit smoking, is particularly concerning.
“There is substantial reason to be concerned about efficacy of electronic cigarette products. Real-world observational studies have shown that, on average, tobacco smokers who use electronic cigarettes are less likely to stop smoking than those who do not use electronic cigarettes,” he said. “People who have stopped tobacco smoking but use electronic cigarettes are more likely to relapse to tobacco smoking than those who do not use electronic cigarettes.”
Dr. Farber noted that there are other Food and Drug Administration–approved medications for treating tobacco addiction. In addition, the World Health Organization, American Medical Association, Centers for Disease Control and Prevention, and FDA have all advised that e-cigarettes should not be used as smoking cessation aids, he said, especially in light of current outbreak of life-threatening e-cigarette and vaping lung injuries currently being investigated by the CDC and FDA.
“These study results suggest that the CDC reports of e-cigarette, or vaping, product use–associated lung injury are likely to be just the tip of the iceberg,” he said. “Although the CDC has identified vitamin E acetate–containing products as an important culprit, it is unlikely to be the only one. There are many substances in the emissions of e-cigarettes that have known irritant and/or toxic effects on the airways.”
Dr. Bhatta and Dr. Glantz acknowledged several limitations in their analysis, including the possibility of recall bias, not distinguishing between nondaily and daily e-cigarette or combustible tobacco use, and combining respiratory conditions together to achieve adequate power. The study shows an association, but the mechanism by which e-cigarettes may contribute to the development of lung disease remains under investigation.
This study was supported by grants from the National Institute on Drug Abuse; the National Cancer Institute; the FDA Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center Global Cancer Program. Dr. Bhatta and Dr. Glantz reported no relevant conflicts of interest.
SOURCE: Bhatta DN, Glantz SA. Am J Prev Med. 2019 Dec 16. doi: 10.1016/j.amepre.2019.07.028.
according to recent longitudinal analysis published in the American Journal of Preventive Medicine.
E-cigarettes have been promoted as a safer alternative to combustible tobacco, and until recently, there has been little and conflicting evidence by which to test this hypothesis. This study conducted by Dharma N. Bhatta, PhD, and Stanton A. Glantz, PhD, of the Center for Tobacco Control Research and Education at the University of California, San Francisco, is one of the first longitudinal examinations of e-cigarette use and controlling for combustible tobacco use.
Dr. Bhatta and Dr. Glantz performed a multivariable, logistic regression analysis of adults enrolled in the nationally representative, population-based, longitudinal Population Assessment of Tobacco and Health study. The researchers analyzed the tobacco use of adults in the study in three waves, following them through wave 1 (September 2013 to December 2014), wave 2 (October 2014 to October 2015), and wave 3 (October 2015 to October 2016), analyzing the data between 2018 and 2019. Overall, wave 1 began with 32,320 participants, and 15.1% of adults reported respiratory disease at baseline.
Lung or respiratory disease was assessed by asking participants whether they had been told by a health professional that they had chronic obstructive pulmonary disease, chronic bronchitis, emphysema, or asthma. The researchers defined e-cigarette and combustible tobacco use as participants who never, currently, or formerly used e-cigarettes or smoked combustible tobacco. Participants who indicated they used e-cigarettes or combustible tobacco frequently or infrequently were placed in the current-user group, while past users were those participants who said they used to, but no longer use e-cigarettes or combustible tobacco.
The results showed former e-cigarette use (adjusted odds ratio, 1.34; 95% confidence interval, 1.23-1.46) and current e-cigarette use (aOR, 1.32; 95% CI, 1.17-1.49) were associated with an increased risk of having incident respiratory disease.
The data showed a not unexpected statistically significant association between former combustible tobacco use (aOR, 1.29; 95% CI, 1.14-1.47) as well as current combustible tobacco use (aOR, 1.61; 95% CI, 1.42-1.82) and incident respiratory disease risk.
There was a statistically significant association between respiratory disease and former or current e-cigarette use for adults who did not have respiratory disease at baseline, after adjusting for factors such as current combustible tobacco use, clinical variables, and demographic differences. Participants in wave 1 who reported former (aOR, 1.31; 95% CI, 1.07-1.60) or current e-cigarette use (aOR, 1.29; 95% CI, 1.03-1.61) had a significantly higher risk of developing incident respiratory disease in subsequent waves. There was also a statistically significant association between use of combustible tobacco and subsequent respiratory disease in later waves of the study (aOR, 2.56; 95% CI, 1.92-3.41), which the researchers noted was independent of the usual risks associated with combustible tobacco.
The investigators also looked at the link between dual use of e-cigarettes and combustible tobacco and respiratory disease risk. “The much more common pattern is dual use, in which an e-cigarette user continues to smoke combusted tobacco products at the same time (93.7% of e-cigarette users at wave 2 and 91.2% at wave 3 also used combustible tobacco; 73.3% of e-cigarette users at wave 2 and 64.9% at wave 3 also smoked cigarettes),” they wrote.
The odds of developing respiratory disease for participants who used both e-cigarettes and combustible tobacco were 3.30, compared with a participant who never used e-cigarettes, with similar results seen when comparing e-cigarettes and cigarettes.
“Although switching from combustible tobacco, including cigarettes, to e-cigarettes theoretically could reduce the risk of developing respiratory disease, current evidence indicates a high prevalence of dual use, which is associated with in-creased risk beyond combustible tobacco use,” the investigators wrote.
Harold J. Farber, MD, FCCP, professor of pediatrics in the pulmonary section at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, said in an interview that the increased respiratory risk among dual users, who are likely using e-cigarettes and combustible tobacco together as a way to quit smoking, is particularly concerning.
“There is substantial reason to be concerned about efficacy of electronic cigarette products. Real-world observational studies have shown that, on average, tobacco smokers who use electronic cigarettes are less likely to stop smoking than those who do not use electronic cigarettes,” he said. “People who have stopped tobacco smoking but use electronic cigarettes are more likely to relapse to tobacco smoking than those who do not use electronic cigarettes.”
Dr. Farber noted that there are other Food and Drug Administration–approved medications for treating tobacco addiction. In addition, the World Health Organization, American Medical Association, Centers for Disease Control and Prevention, and FDA have all advised that e-cigarettes should not be used as smoking cessation aids, he said, especially in light of current outbreak of life-threatening e-cigarette and vaping lung injuries currently being investigated by the CDC and FDA.
“These study results suggest that the CDC reports of e-cigarette, or vaping, product use–associated lung injury are likely to be just the tip of the iceberg,” he said. “Although the CDC has identified vitamin E acetate–containing products as an important culprit, it is unlikely to be the only one. There are many substances in the emissions of e-cigarettes that have known irritant and/or toxic effects on the airways.”
Dr. Bhatta and Dr. Glantz acknowledged several limitations in their analysis, including the possibility of recall bias, not distinguishing between nondaily and daily e-cigarette or combustible tobacco use, and combining respiratory conditions together to achieve adequate power. The study shows an association, but the mechanism by which e-cigarettes may contribute to the development of lung disease remains under investigation.
This study was supported by grants from the National Institute on Drug Abuse; the National Cancer Institute; the FDA Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center Global Cancer Program. Dr. Bhatta and Dr. Glantz reported no relevant conflicts of interest.
SOURCE: Bhatta DN, Glantz SA. Am J Prev Med. 2019 Dec 16. doi: 10.1016/j.amepre.2019.07.028.
according to recent longitudinal analysis published in the American Journal of Preventive Medicine.
E-cigarettes have been promoted as a safer alternative to combustible tobacco, and until recently, there has been little and conflicting evidence by which to test this hypothesis. This study conducted by Dharma N. Bhatta, PhD, and Stanton A. Glantz, PhD, of the Center for Tobacco Control Research and Education at the University of California, San Francisco, is one of the first longitudinal examinations of e-cigarette use and controlling for combustible tobacco use.
Dr. Bhatta and Dr. Glantz performed a multivariable, logistic regression analysis of adults enrolled in the nationally representative, population-based, longitudinal Population Assessment of Tobacco and Health study. The researchers analyzed the tobacco use of adults in the study in three waves, following them through wave 1 (September 2013 to December 2014), wave 2 (October 2014 to October 2015), and wave 3 (October 2015 to October 2016), analyzing the data between 2018 and 2019. Overall, wave 1 began with 32,320 participants, and 15.1% of adults reported respiratory disease at baseline.
Lung or respiratory disease was assessed by asking participants whether they had been told by a health professional that they had chronic obstructive pulmonary disease, chronic bronchitis, emphysema, or asthma. The researchers defined e-cigarette and combustible tobacco use as participants who never, currently, or formerly used e-cigarettes or smoked combustible tobacco. Participants who indicated they used e-cigarettes or combustible tobacco frequently or infrequently were placed in the current-user group, while past users were those participants who said they used to, but no longer use e-cigarettes or combustible tobacco.
The results showed former e-cigarette use (adjusted odds ratio, 1.34; 95% confidence interval, 1.23-1.46) and current e-cigarette use (aOR, 1.32; 95% CI, 1.17-1.49) were associated with an increased risk of having incident respiratory disease.
The data showed a not unexpected statistically significant association between former combustible tobacco use (aOR, 1.29; 95% CI, 1.14-1.47) as well as current combustible tobacco use (aOR, 1.61; 95% CI, 1.42-1.82) and incident respiratory disease risk.
There was a statistically significant association between respiratory disease and former or current e-cigarette use for adults who did not have respiratory disease at baseline, after adjusting for factors such as current combustible tobacco use, clinical variables, and demographic differences. Participants in wave 1 who reported former (aOR, 1.31; 95% CI, 1.07-1.60) or current e-cigarette use (aOR, 1.29; 95% CI, 1.03-1.61) had a significantly higher risk of developing incident respiratory disease in subsequent waves. There was also a statistically significant association between use of combustible tobacco and subsequent respiratory disease in later waves of the study (aOR, 2.56; 95% CI, 1.92-3.41), which the researchers noted was independent of the usual risks associated with combustible tobacco.
The investigators also looked at the link between dual use of e-cigarettes and combustible tobacco and respiratory disease risk. “The much more common pattern is dual use, in which an e-cigarette user continues to smoke combusted tobacco products at the same time (93.7% of e-cigarette users at wave 2 and 91.2% at wave 3 also used combustible tobacco; 73.3% of e-cigarette users at wave 2 and 64.9% at wave 3 also smoked cigarettes),” they wrote.
The odds of developing respiratory disease for participants who used both e-cigarettes and combustible tobacco were 3.30, compared with a participant who never used e-cigarettes, with similar results seen when comparing e-cigarettes and cigarettes.
“Although switching from combustible tobacco, including cigarettes, to e-cigarettes theoretically could reduce the risk of developing respiratory disease, current evidence indicates a high prevalence of dual use, which is associated with in-creased risk beyond combustible tobacco use,” the investigators wrote.
Harold J. Farber, MD, FCCP, professor of pediatrics in the pulmonary section at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, said in an interview that the increased respiratory risk among dual users, who are likely using e-cigarettes and combustible tobacco together as a way to quit smoking, is particularly concerning.
“There is substantial reason to be concerned about efficacy of electronic cigarette products. Real-world observational studies have shown that, on average, tobacco smokers who use electronic cigarettes are less likely to stop smoking than those who do not use electronic cigarettes,” he said. “People who have stopped tobacco smoking but use electronic cigarettes are more likely to relapse to tobacco smoking than those who do not use electronic cigarettes.”
Dr. Farber noted that there are other Food and Drug Administration–approved medications for treating tobacco addiction. In addition, the World Health Organization, American Medical Association, Centers for Disease Control and Prevention, and FDA have all advised that e-cigarettes should not be used as smoking cessation aids, he said, especially in light of current outbreak of life-threatening e-cigarette and vaping lung injuries currently being investigated by the CDC and FDA.
“These study results suggest that the CDC reports of e-cigarette, or vaping, product use–associated lung injury are likely to be just the tip of the iceberg,” he said. “Although the CDC has identified vitamin E acetate–containing products as an important culprit, it is unlikely to be the only one. There are many substances in the emissions of e-cigarettes that have known irritant and/or toxic effects on the airways.”
Dr. Bhatta and Dr. Glantz acknowledged several limitations in their analysis, including the possibility of recall bias, not distinguishing between nondaily and daily e-cigarette or combustible tobacco use, and combining respiratory conditions together to achieve adequate power. The study shows an association, but the mechanism by which e-cigarettes may contribute to the development of lung disease remains under investigation.
This study was supported by grants from the National Institute on Drug Abuse; the National Cancer Institute; the FDA Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center Global Cancer Program. Dr. Bhatta and Dr. Glantz reported no relevant conflicts of interest.
SOURCE: Bhatta DN, Glantz SA. Am J Prev Med. 2019 Dec 16. doi: 10.1016/j.amepre.2019.07.028.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Vaping marijuana gaining traction among U.S. teens
Monitoring the Future survey asked about daily vaping this year for first time
Vaping has expanded as a popular method of drug delivery for U.S. teenagers, and one in five students in grades 10 and 12 reported vaping marijuana in the past year, according to results of the 2019 Monitoring the Future survey conducted by the National Institute on Drug Abuse (NIDA).
This year’s findings, announced Dec. 18, continue to illustrate “a clear shift in the pattern of drug taking among teenagers,” said NIDA Director Nora D. Volkow, MD, in a teleconference held to review the results.
Use of alcohol and drugs – including opioids and stimulants – continues to decline among teens, but vaping continues its significant rise, with a surge in marijuana vaping this year.
The increase in past-month marijuana vaping among 12th graders, from 7.5% in 2018 to 14% in 2019, represents the second-largest 1-year jump tracked for any substance in the survey’s history, Dr. Volkow said. The largest jump was the increase in past-month nicotine vaping among 12th-graders from 2017-2018.
“It is very unfortunate that we are seeing the steep rise in the use of vaping devices” because the devices deliver drugs in very high concentration, Dr. Volkow said. The growing popularity of vaping “threatens to undo years of progress protecting the health of adolescents in the U.S.,” Dr. Volkow said in a statement. The Monitoring the Future survey began including vaping questions in 2017.
Monitoring the Future is a national tool to assess drug and alcohol use and related attitudes among adolescent students across the United States. This year’s self-reported survey included 42,531 in grades 8, 10, and 12 from 396 public and private schools.
Nicotine vaping increased from 2018 to 2019 across all three grades; past-month nicotine use equated to 1 in 4, 1 in 5, and 1 in 10 (26%, 20%, and 10%) among 12th, 10th, and 8th graders, respectively, according to the survey. Daily nicotine vaping, measured for the first time this year because of public health concerns, was approximately 12% for 12th graders, 7% for 10th graders, and 2% for 8th graders. Daily marijuana vaping, also measured for the first time this year, was approximately 4%, 3%, and 1% among 12th, 10th, and 8th graders, respectively. Additional findings on the rise of vaping by U.S. teenagers were released Dec. 17 in a research letter published online in JAMA (doi: 10.1001/jama.2019.20185).
Meanwhile, positive trends in this year’s survey included a reduction in the misuse of prescription drugs, including OxyContin, Vicodin, and Adderall, and in the use of traditional cigarettes and other tobacco products, as well as alcohol, noted Richard A. Miech, PhD, MPH, of the University of Michigan, Ann Arbor, principal investigator for Monitoring the Future. However, the challenge of preventing and reducing vaping in teens remains “a whole new uncharted territory,” in part because the design of the vaping devices facilitates discreet use at home and at school, he said.
Physicians and parents have important roles to play in screening for vaping among teens, Dr. Volkow said in a question and answer session. Health care clinicians, including pediatricians and family physicians, “are in a unique position to communicate with their young patients” by educating them about the dangers of vaping, encouraging them to stop if they have started using these devices, and referring them for further treatment if they are showing signs of addiction, she said.
Monitoring the Future was funded by NIDA. The researchers had no disclosures.
Monitoring the Future survey asked about daily vaping this year for first time
Monitoring the Future survey asked about daily vaping this year for first time
Vaping has expanded as a popular method of drug delivery for U.S. teenagers, and one in five students in grades 10 and 12 reported vaping marijuana in the past year, according to results of the 2019 Monitoring the Future survey conducted by the National Institute on Drug Abuse (NIDA).
This year’s findings, announced Dec. 18, continue to illustrate “a clear shift in the pattern of drug taking among teenagers,” said NIDA Director Nora D. Volkow, MD, in a teleconference held to review the results.
Use of alcohol and drugs – including opioids and stimulants – continues to decline among teens, but vaping continues its significant rise, with a surge in marijuana vaping this year.
The increase in past-month marijuana vaping among 12th graders, from 7.5% in 2018 to 14% in 2019, represents the second-largest 1-year jump tracked for any substance in the survey’s history, Dr. Volkow said. The largest jump was the increase in past-month nicotine vaping among 12th-graders from 2017-2018.
“It is very unfortunate that we are seeing the steep rise in the use of vaping devices” because the devices deliver drugs in very high concentration, Dr. Volkow said. The growing popularity of vaping “threatens to undo years of progress protecting the health of adolescents in the U.S.,” Dr. Volkow said in a statement. The Monitoring the Future survey began including vaping questions in 2017.
Monitoring the Future is a national tool to assess drug and alcohol use and related attitudes among adolescent students across the United States. This year’s self-reported survey included 42,531 in grades 8, 10, and 12 from 396 public and private schools.
Nicotine vaping increased from 2018 to 2019 across all three grades; past-month nicotine use equated to 1 in 4, 1 in 5, and 1 in 10 (26%, 20%, and 10%) among 12th, 10th, and 8th graders, respectively, according to the survey. Daily nicotine vaping, measured for the first time this year because of public health concerns, was approximately 12% for 12th graders, 7% for 10th graders, and 2% for 8th graders. Daily marijuana vaping, also measured for the first time this year, was approximately 4%, 3%, and 1% among 12th, 10th, and 8th graders, respectively. Additional findings on the rise of vaping by U.S. teenagers were released Dec. 17 in a research letter published online in JAMA (doi: 10.1001/jama.2019.20185).
Meanwhile, positive trends in this year’s survey included a reduction in the misuse of prescription drugs, including OxyContin, Vicodin, and Adderall, and in the use of traditional cigarettes and other tobacco products, as well as alcohol, noted Richard A. Miech, PhD, MPH, of the University of Michigan, Ann Arbor, principal investigator for Monitoring the Future. However, the challenge of preventing and reducing vaping in teens remains “a whole new uncharted territory,” in part because the design of the vaping devices facilitates discreet use at home and at school, he said.
Physicians and parents have important roles to play in screening for vaping among teens, Dr. Volkow said in a question and answer session. Health care clinicians, including pediatricians and family physicians, “are in a unique position to communicate with their young patients” by educating them about the dangers of vaping, encouraging them to stop if they have started using these devices, and referring them for further treatment if they are showing signs of addiction, she said.
Monitoring the Future was funded by NIDA. The researchers had no disclosures.
Vaping has expanded as a popular method of drug delivery for U.S. teenagers, and one in five students in grades 10 and 12 reported vaping marijuana in the past year, according to results of the 2019 Monitoring the Future survey conducted by the National Institute on Drug Abuse (NIDA).
This year’s findings, announced Dec. 18, continue to illustrate “a clear shift in the pattern of drug taking among teenagers,” said NIDA Director Nora D. Volkow, MD, in a teleconference held to review the results.
Use of alcohol and drugs – including opioids and stimulants – continues to decline among teens, but vaping continues its significant rise, with a surge in marijuana vaping this year.
The increase in past-month marijuana vaping among 12th graders, from 7.5% in 2018 to 14% in 2019, represents the second-largest 1-year jump tracked for any substance in the survey’s history, Dr. Volkow said. The largest jump was the increase in past-month nicotine vaping among 12th-graders from 2017-2018.
“It is very unfortunate that we are seeing the steep rise in the use of vaping devices” because the devices deliver drugs in very high concentration, Dr. Volkow said. The growing popularity of vaping “threatens to undo years of progress protecting the health of adolescents in the U.S.,” Dr. Volkow said in a statement. The Monitoring the Future survey began including vaping questions in 2017.
Monitoring the Future is a national tool to assess drug and alcohol use and related attitudes among adolescent students across the United States. This year’s self-reported survey included 42,531 in grades 8, 10, and 12 from 396 public and private schools.
Nicotine vaping increased from 2018 to 2019 across all three grades; past-month nicotine use equated to 1 in 4, 1 in 5, and 1 in 10 (26%, 20%, and 10%) among 12th, 10th, and 8th graders, respectively, according to the survey. Daily nicotine vaping, measured for the first time this year because of public health concerns, was approximately 12% for 12th graders, 7% for 10th graders, and 2% for 8th graders. Daily marijuana vaping, also measured for the first time this year, was approximately 4%, 3%, and 1% among 12th, 10th, and 8th graders, respectively. Additional findings on the rise of vaping by U.S. teenagers were released Dec. 17 in a research letter published online in JAMA (doi: 10.1001/jama.2019.20185).
Meanwhile, positive trends in this year’s survey included a reduction in the misuse of prescription drugs, including OxyContin, Vicodin, and Adderall, and in the use of traditional cigarettes and other tobacco products, as well as alcohol, noted Richard A. Miech, PhD, MPH, of the University of Michigan, Ann Arbor, principal investigator for Monitoring the Future. However, the challenge of preventing and reducing vaping in teens remains “a whole new uncharted territory,” in part because the design of the vaping devices facilitates discreet use at home and at school, he said.
Physicians and parents have important roles to play in screening for vaping among teens, Dr. Volkow said in a question and answer session. Health care clinicians, including pediatricians and family physicians, “are in a unique position to communicate with their young patients” by educating them about the dangers of vaping, encouraging them to stop if they have started using these devices, and referring them for further treatment if they are showing signs of addiction, she said.
Monitoring the Future was funded by NIDA. The researchers had no disclosures.
The vaping problem
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
The first time I was sure I was witnessing someone vaping occurred when I saw an alarming cloud of smoke billowing from driver’s side window of the car in front of me. My initial concern was that vehicle was on fire. But none of the other drivers around me seemed concerned and as I pulled up next to the car I could see the driver ostentatiously inhaling deeply in preparation for releasing another monstrous cloud of vapor.
However, you probably have learned, as have I, that most vaping is done furtively. In fact, the pocketability of vaping devices is part of their appeal to teenagers. Hiding a lit cigarette in one’s pocket is something even the most risk-loving adolescent usually won’t attempt. I suspect that regardless of what is in the vapor, the high one can get by putting one over on the school administration by vaping in the school restroom or in the middle of history class is a temptation that many teenagers can’t resist.
Listening to educators, substance abuse counselors, and police officers who have first hand knowledge,
Part of the problem seems to be that vaping was flying under the radar and expanding rapidly long before educators, parents, and I fear physicians woke up to the severity and magnitude of the problem. And now everybody is playing catchup.
Of course the initial, and as yet unconfirmed, notion that e-cigarettes might provide a viable strategy for tobacco withdrawal has added confusion to the mix. It turns out that vaping can provide many orders of magnitude more nicotine in a small volume than cigarettes, which creates an outsized addiction potential for those more vulnerable users – even with a very short history of use. My experts tell me that this level of addiction has forced them to consider strategies and dosages far beyond those they are accustomed to using with patients whose addiction stems from standard cigarette use.
The recent discovery of lung damage related to vaping provided a brief glimmer of hope that fear would turn the tide in the vaping epidemic. But unfortunately the Centers for Disease Control and Prevention did its job too well. Although maybe it was a bit late to uncover the condition, the agency acted quickly to chase down the epidemiology and eventually the chemical responsible for the pulmonary injury. My local experts tell me that, while the cause of the lung damage was still a mystery, they noticed a decline in vaping generated by the fear of this unknown killer. Young people were reporting that they were rethinking their vaping usage. However, once the chemical culprit was identified, their clients felt that they could safely vape again as long as they were more careful in choosing the source of liquid in their devices.
Not surprisingly, the current administration has been providing mixed messages about how it will address vaping. There always will be the argument that if you ban a substance, it will be driven underground and become more difficult to manage. However, in the case of vaping, its appeal and risk to young people and the apparent ineffectiveness of local efforts to control it demand a firm unwavering response at the federal level.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Emergency physicians not yet embracing buprenorphine for opioid users
SAN DIEGO – Emergency physicians can be persuaded to follow a recommended strategy to prescribe buprenorphine to patients with opioid addictions and to refer them to follow-up care, Kathryn F. Hawk, MD, said at the annual meeting of the American Academy of Addiction Psychiatry.
“People are willing to change their practices and evolve as long as they have the support to do so,” Dr. Hawk, assistant professor of emergency medicine at Yale University, New Haven, Conn., said at the meeting.
Dr. Hawk highlighted a landmark 2015 study led by Yale colleagues that compared three strategies to treating patients with opioid use disorder in the emergency department. Researchers randomly assigned 329 patients to 1) referral to treatment; 2) brief intervention and facilitated referral to community-based treatment services; and 3) emergency department-initiated treatment with buprenorphine/naloxone (Suboxone) plus referral to primary care for 10-week follow-up.
At 30 days, 78% of patients in the third group were in addiction treatment vs. 37% in the first group and 45% in the second group. (P less than .001). However, the percentage of patients in the groups who had negative urine screens for opioids were not statistically different (JAMA. 2015. Apr 28;313[16]:1636-44).
Both the American College of Emergency Physicians (ACEP) and the American College of Medical Toxicology have endorsed the use of buprenorphine in the ED “as a bridge to long-term addiction treatment,” said Dr. Hawk, who also is affiliated with Yale New Haven Hospital.
Emergency department physicians, however, have been reluctant to start prescribing buprenorphine and get more deeply involved in referrals to care, said E. Jennifer Edelman, MD, associate professor of general internal medicine at Yale. She described the results of a 2017-2019 survey of 268 medical professionals at urban emergency departments in Seattle, Cincinnati, New York City, and Baltimore. Only 20% of the survey respondents said they were “ready” to initiate the buprenorphine treatment protocol.
Researchers also held focus groups with 74 clinicians who offered insight into their hesitation. “That’s not something that we’re even really taught in medical school and certainly not in our training as emergency physicians,” one faculty member said. “It is this detox black box across the street, and that’s how it is in many places.”
Another faculty member expressed regret about the current system: “I feel like this is particularly vulnerable patient population [and] we’re just saying, ‘Here’s a sheet. Call some numbers. Good luck.’ That’s the way it feels when I discharge these folks.” And a resident said: “We can’t provide all of that care up front. It’s just too time-consuming, and there are other patients to see.”
But not all of the findings were grim.
Dr. Edelman said.
According to her, strategies aimed at boosting the Suboxone approach include establishing protocols, and providing leadership support and resources. Addiction psychiatrists also can be helpful, she said.
“Let’s think about partnering together to bridge that gap,” she said. One idea: Invite emergency physicians to observe a treatment initiation.
“Showing how you counsel patients to start medication at home would be really a wonderful way to facilitate practices in the emergency department,” she said.
Another idea, she said, is to “give them feedback on their patients.” If an emergency physician refers a patient and they walk in the door, “let them know how they did. That’s going to be really, really powerful.”
ACEP and the American Society of Addiction Medicine have created a tool aimed at helping facilitate the use of buprenorphine and naloxone in the emergency department.
Dr. Hawk and Dr. Edelman reported no relevant disclosures.
SAN DIEGO – Emergency physicians can be persuaded to follow a recommended strategy to prescribe buprenorphine to patients with opioid addictions and to refer them to follow-up care, Kathryn F. Hawk, MD, said at the annual meeting of the American Academy of Addiction Psychiatry.
“People are willing to change their practices and evolve as long as they have the support to do so,” Dr. Hawk, assistant professor of emergency medicine at Yale University, New Haven, Conn., said at the meeting.
Dr. Hawk highlighted a landmark 2015 study led by Yale colleagues that compared three strategies to treating patients with opioid use disorder in the emergency department. Researchers randomly assigned 329 patients to 1) referral to treatment; 2) brief intervention and facilitated referral to community-based treatment services; and 3) emergency department-initiated treatment with buprenorphine/naloxone (Suboxone) plus referral to primary care for 10-week follow-up.
At 30 days, 78% of patients in the third group were in addiction treatment vs. 37% in the first group and 45% in the second group. (P less than .001). However, the percentage of patients in the groups who had negative urine screens for opioids were not statistically different (JAMA. 2015. Apr 28;313[16]:1636-44).
Both the American College of Emergency Physicians (ACEP) and the American College of Medical Toxicology have endorsed the use of buprenorphine in the ED “as a bridge to long-term addiction treatment,” said Dr. Hawk, who also is affiliated with Yale New Haven Hospital.
Emergency department physicians, however, have been reluctant to start prescribing buprenorphine and get more deeply involved in referrals to care, said E. Jennifer Edelman, MD, associate professor of general internal medicine at Yale. She described the results of a 2017-2019 survey of 268 medical professionals at urban emergency departments in Seattle, Cincinnati, New York City, and Baltimore. Only 20% of the survey respondents said they were “ready” to initiate the buprenorphine treatment protocol.
Researchers also held focus groups with 74 clinicians who offered insight into their hesitation. “That’s not something that we’re even really taught in medical school and certainly not in our training as emergency physicians,” one faculty member said. “It is this detox black box across the street, and that’s how it is in many places.”
Another faculty member expressed regret about the current system: “I feel like this is particularly vulnerable patient population [and] we’re just saying, ‘Here’s a sheet. Call some numbers. Good luck.’ That’s the way it feels when I discharge these folks.” And a resident said: “We can’t provide all of that care up front. It’s just too time-consuming, and there are other patients to see.”
But not all of the findings were grim.
Dr. Edelman said.
According to her, strategies aimed at boosting the Suboxone approach include establishing protocols, and providing leadership support and resources. Addiction psychiatrists also can be helpful, she said.
“Let’s think about partnering together to bridge that gap,” she said. One idea: Invite emergency physicians to observe a treatment initiation.
“Showing how you counsel patients to start medication at home would be really a wonderful way to facilitate practices in the emergency department,” she said.
Another idea, she said, is to “give them feedback on their patients.” If an emergency physician refers a patient and they walk in the door, “let them know how they did. That’s going to be really, really powerful.”
ACEP and the American Society of Addiction Medicine have created a tool aimed at helping facilitate the use of buprenorphine and naloxone in the emergency department.
Dr. Hawk and Dr. Edelman reported no relevant disclosures.
SAN DIEGO – Emergency physicians can be persuaded to follow a recommended strategy to prescribe buprenorphine to patients with opioid addictions and to refer them to follow-up care, Kathryn F. Hawk, MD, said at the annual meeting of the American Academy of Addiction Psychiatry.
“People are willing to change their practices and evolve as long as they have the support to do so,” Dr. Hawk, assistant professor of emergency medicine at Yale University, New Haven, Conn., said at the meeting.
Dr. Hawk highlighted a landmark 2015 study led by Yale colleagues that compared three strategies to treating patients with opioid use disorder in the emergency department. Researchers randomly assigned 329 patients to 1) referral to treatment; 2) brief intervention and facilitated referral to community-based treatment services; and 3) emergency department-initiated treatment with buprenorphine/naloxone (Suboxone) plus referral to primary care for 10-week follow-up.
At 30 days, 78% of patients in the third group were in addiction treatment vs. 37% in the first group and 45% in the second group. (P less than .001). However, the percentage of patients in the groups who had negative urine screens for opioids were not statistically different (JAMA. 2015. Apr 28;313[16]:1636-44).
Both the American College of Emergency Physicians (ACEP) and the American College of Medical Toxicology have endorsed the use of buprenorphine in the ED “as a bridge to long-term addiction treatment,” said Dr. Hawk, who also is affiliated with Yale New Haven Hospital.
Emergency department physicians, however, have been reluctant to start prescribing buprenorphine and get more deeply involved in referrals to care, said E. Jennifer Edelman, MD, associate professor of general internal medicine at Yale. She described the results of a 2017-2019 survey of 268 medical professionals at urban emergency departments in Seattle, Cincinnati, New York City, and Baltimore. Only 20% of the survey respondents said they were “ready” to initiate the buprenorphine treatment protocol.
Researchers also held focus groups with 74 clinicians who offered insight into their hesitation. “That’s not something that we’re even really taught in medical school and certainly not in our training as emergency physicians,” one faculty member said. “It is this detox black box across the street, and that’s how it is in many places.”
Another faculty member expressed regret about the current system: “I feel like this is particularly vulnerable patient population [and] we’re just saying, ‘Here’s a sheet. Call some numbers. Good luck.’ That’s the way it feels when I discharge these folks.” And a resident said: “We can’t provide all of that care up front. It’s just too time-consuming, and there are other patients to see.”
But not all of the findings were grim.
Dr. Edelman said.
According to her, strategies aimed at boosting the Suboxone approach include establishing protocols, and providing leadership support and resources. Addiction psychiatrists also can be helpful, she said.
“Let’s think about partnering together to bridge that gap,” she said. One idea: Invite emergency physicians to observe a treatment initiation.
“Showing how you counsel patients to start medication at home would be really a wonderful way to facilitate practices in the emergency department,” she said.
Another idea, she said, is to “give them feedback on their patients.” If an emergency physician refers a patient and they walk in the door, “let them know how they did. That’s going to be really, really powerful.”
ACEP and the American Society of Addiction Medicine have created a tool aimed at helping facilitate the use of buprenorphine and naloxone in the emergency department.
Dr. Hawk and Dr. Edelman reported no relevant disclosures.
REPORTING FROM AAAP 2019
In addiction, abusive partners can wreak havoc
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
Gender-based violence could be driver of opioid epidemic, expert suggests
Gender-based violence could be driver of opioid epidemic, expert suggests
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
SAN DIEGO – Many factors drive addiction. But clinicians often fail to address the important role played by abusive intimate partners, a psychiatrist told colleagues at the annual meeting of the American Academy of Addiction Psychiatry.
Violence is not the only source of harm, said Carole Warshaw, MD, as abusers also turn to sabotage, gaslighting, and manipulation – especially when substance users seek help.
“Abusive partners deliberately engage in behaviors designed to undermine their partner’s sanity or sobriety,” said Dr. Warshaw, director of the National Center on Domestic Violence, Trauma & Mental Health in Chicago, in a presentation at the meeting. “We’ve talked a lot about drivers of the opioid epidemic, including pharmaceutical industry greed and disorders of despair. But nobody’s been really talking about gender-based violence as a potential driver of the opioid epidemic, including intimate-partner violence, trafficking, and commercial sex exploitation.”
Dr. Warshaw highlighted the findings of a 2014 study that examined the survey responses of 2,546 adult women (54% white, 19% black, 19% Hispanic) who called the National Domestic Violence Hotline. The study, led by Dr. Warshaw, only included women who had experienced domestic violence and were not in immediate crisis.
The women answered questions about abusive partners, and their responses were often emotional, Dr. Warshaw said. “People would say: ‘No one asked me this before,’ and they’d be in tears. It was just very moving for people to start thinking about this.”
Gaslighting, sabotage, and accusations of mental illness were common. More than 85% of respondents said their current or ex-partner had called them “crazy,” and 74% agreed that “your partner or ex-partner has ... deliberately done things to make you feel like you are going crazy or losing your mind.”
Strategies of abusive partners include sabotaging and discrediting their partners’ attempts at recovery, Dr. Warshaw said. Half of callers agreed that a partner or ex-partner “tried to prevent or discourage you from getting ... help or taking medication you were prescribed for your feelings.”
About 92% of callers who said they’d tried to get help in recent years “reported that their partner or ex-partner had threatened to report their alcohol or other drug use to authorities to keep them from getting something they wanted or needed,” the study found.
All of the abuse can create a kind of addiction feedback loop, she said. “Research has consistently documented that abuse by an intimate partner increases a person’s risk for developing a range of health and mental health conditions – including depression, PTSD, anxiety – that are risk factors for opioid and substance use.”
The toolkit, she said, provides insight into how to integrate questions about abusive partners into your practice and how to partner with domestic violence programs.
Dr. Warshaw reported no relevant disclosures.
REPORTING FROM AAAP 2019
Addiction specialists: Cannabis policies should go up in smoke
SAN DIEGO – Addiction specialists have a message for American policymakers who are rushing to create laws to allow the use of medical and recreational marijuana: You’re doing it wrong, but we know how you can do it right.
“We can have spirited debates on these policies, recreational, medical decriminalization, etc. But we can’t argue how we’ve done a poor job implementing these policies in the United States,” psychiatrist Kevin P. Hill, MD, of Harvard Medical School, Boston, said in a symposium about cannabis policy at the annual meeting of the American Academy of Addiction Psychiatry.
The AAAP is proposing a “model state law” regarding cannabis. Among other things, the proposal urges states to:
- Ban recreational use of cannabis until the age of 21, and perhaps even until 25.
- Not denote psychiatric indications such as posttraumatic stress disorder, anxiety, and depression as qualifying conditions for the use of medical marijuana.
- Educate the public about potential harms of cannabis.
- Provide state-level regulation that includes funding of high-grade analytic equipment to test cannabis.
- Maintain a public registry that reports annually on adverse outcomes.
Research suggests that marijuana use has spiked in recent years, Dr. Hill said. Meanwhile, states have dramatically broadened the legality of marijuana. According to the National Conference of State Legislatures, 33 states and the District of Columbia allow the medical use of marijuana. Of those, 11 states and the District of Columbia also allow the adult use of recreational marijuana. Several other states allow access to cannabidiol (CBD)/low-THC products in some cases (www.ncsl.org/research/health/state-medical-marijuana-laws.aspx).
The problem, Dr. Hill said, is that there’s “a big gap between what the science says and what the laws are saying, unfortunately. So we’re in this precarious spot.”
He pointed to his own 2015 review of cannabinoid studies that found high-quality evidence for an effect for just three conditions – chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis. The study notes that Food and Drug Administration–approved cannabinoids are also available to treat nausea and vomiting linked to chemotherapy and to boost appetite in patients with wasting disease. (JAMA. 2015 Jun 23-30;313(24):2474-83).
However, states have listed dozens of conditions – 53 overall – as qualifying conditions for the use of medical marijuana, Dr. Hill said. And, he said, “the reality is that a lot of people who are using medical cannabis don’t have any of these conditions,” he said.
Researchers at the symposium focused on the use of cannabis as a treatment for addiction and other psychiatric illnesses.
Four states have legalized the use of cannabis in patients with opioid use disorder, said cannabis researcher Ziva D. Cooper, PhD, of the University of California, Los Angeles, who spoke at the symposium. But can cannabis actually reduce opioid use? Preliminary clinical data suggest THC could reduce opioid use, Dr. Cooper said, while population and state-level research is mixed.
What about other mental health disorders? Posttraumatic stress disorder is commonly listed as a qualifying condition for medical marijuana use in state laws. And some states, like California, give physicians wide leeway in recommending marijuana use for patients with conditions that aren’t listed in the law.
However, symposium speaker and psychiatrist Frances R. Levin, MD, of New York State Psychiatric Institute, pointed to a 2019 review that suggests “there is scarce evidence to suggest that cannabinoids improve depressive disorders and symptoms, anxiety disorders, attention-deficit hyperactivity disorder, Tourette syndrome, posttraumatic stress disorder, or psychosis” (Lancet Psychiatry. 2019 Dec;6[12]:995-1010).
What now? The AAAP hopes lawmakers will pay attention to its proposed model state law, which will be published soon in the association’s journal, the American Journal on Addictions.
SAN DIEGO – Addiction specialists have a message for American policymakers who are rushing to create laws to allow the use of medical and recreational marijuana: You’re doing it wrong, but we know how you can do it right.
“We can have spirited debates on these policies, recreational, medical decriminalization, etc. But we can’t argue how we’ve done a poor job implementing these policies in the United States,” psychiatrist Kevin P. Hill, MD, of Harvard Medical School, Boston, said in a symposium about cannabis policy at the annual meeting of the American Academy of Addiction Psychiatry.
The AAAP is proposing a “model state law” regarding cannabis. Among other things, the proposal urges states to:
- Ban recreational use of cannabis until the age of 21, and perhaps even until 25.
- Not denote psychiatric indications such as posttraumatic stress disorder, anxiety, and depression as qualifying conditions for the use of medical marijuana.
- Educate the public about potential harms of cannabis.
- Provide state-level regulation that includes funding of high-grade analytic equipment to test cannabis.
- Maintain a public registry that reports annually on adverse outcomes.
Research suggests that marijuana use has spiked in recent years, Dr. Hill said. Meanwhile, states have dramatically broadened the legality of marijuana. According to the National Conference of State Legislatures, 33 states and the District of Columbia allow the medical use of marijuana. Of those, 11 states and the District of Columbia also allow the adult use of recreational marijuana. Several other states allow access to cannabidiol (CBD)/low-THC products in some cases (www.ncsl.org/research/health/state-medical-marijuana-laws.aspx).
The problem, Dr. Hill said, is that there’s “a big gap between what the science says and what the laws are saying, unfortunately. So we’re in this precarious spot.”
He pointed to his own 2015 review of cannabinoid studies that found high-quality evidence for an effect for just three conditions – chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis. The study notes that Food and Drug Administration–approved cannabinoids are also available to treat nausea and vomiting linked to chemotherapy and to boost appetite in patients with wasting disease. (JAMA. 2015 Jun 23-30;313(24):2474-83).
However, states have listed dozens of conditions – 53 overall – as qualifying conditions for the use of medical marijuana, Dr. Hill said. And, he said, “the reality is that a lot of people who are using medical cannabis don’t have any of these conditions,” he said.
Researchers at the symposium focused on the use of cannabis as a treatment for addiction and other psychiatric illnesses.
Four states have legalized the use of cannabis in patients with opioid use disorder, said cannabis researcher Ziva D. Cooper, PhD, of the University of California, Los Angeles, who spoke at the symposium. But can cannabis actually reduce opioid use? Preliminary clinical data suggest THC could reduce opioid use, Dr. Cooper said, while population and state-level research is mixed.
What about other mental health disorders? Posttraumatic stress disorder is commonly listed as a qualifying condition for medical marijuana use in state laws. And some states, like California, give physicians wide leeway in recommending marijuana use for patients with conditions that aren’t listed in the law.
However, symposium speaker and psychiatrist Frances R. Levin, MD, of New York State Psychiatric Institute, pointed to a 2019 review that suggests “there is scarce evidence to suggest that cannabinoids improve depressive disorders and symptoms, anxiety disorders, attention-deficit hyperactivity disorder, Tourette syndrome, posttraumatic stress disorder, or psychosis” (Lancet Psychiatry. 2019 Dec;6[12]:995-1010).
What now? The AAAP hopes lawmakers will pay attention to its proposed model state law, which will be published soon in the association’s journal, the American Journal on Addictions.
SAN DIEGO – Addiction specialists have a message for American policymakers who are rushing to create laws to allow the use of medical and recreational marijuana: You’re doing it wrong, but we know how you can do it right.
“We can have spirited debates on these policies, recreational, medical decriminalization, etc. But we can’t argue how we’ve done a poor job implementing these policies in the United States,” psychiatrist Kevin P. Hill, MD, of Harvard Medical School, Boston, said in a symposium about cannabis policy at the annual meeting of the American Academy of Addiction Psychiatry.
The AAAP is proposing a “model state law” regarding cannabis. Among other things, the proposal urges states to:
- Ban recreational use of cannabis until the age of 21, and perhaps even until 25.
- Not denote psychiatric indications such as posttraumatic stress disorder, anxiety, and depression as qualifying conditions for the use of medical marijuana.
- Educate the public about potential harms of cannabis.
- Provide state-level regulation that includes funding of high-grade analytic equipment to test cannabis.
- Maintain a public registry that reports annually on adverse outcomes.
Research suggests that marijuana use has spiked in recent years, Dr. Hill said. Meanwhile, states have dramatically broadened the legality of marijuana. According to the National Conference of State Legislatures, 33 states and the District of Columbia allow the medical use of marijuana. Of those, 11 states and the District of Columbia also allow the adult use of recreational marijuana. Several other states allow access to cannabidiol (CBD)/low-THC products in some cases (www.ncsl.org/research/health/state-medical-marijuana-laws.aspx).
The problem, Dr. Hill said, is that there’s “a big gap between what the science says and what the laws are saying, unfortunately. So we’re in this precarious spot.”
He pointed to his own 2015 review of cannabinoid studies that found high-quality evidence for an effect for just three conditions – chronic pain, neuropathic pain, and spasticity associated with multiple sclerosis. The study notes that Food and Drug Administration–approved cannabinoids are also available to treat nausea and vomiting linked to chemotherapy and to boost appetite in patients with wasting disease. (JAMA. 2015 Jun 23-30;313(24):2474-83).
However, states have listed dozens of conditions – 53 overall – as qualifying conditions for the use of medical marijuana, Dr. Hill said. And, he said, “the reality is that a lot of people who are using medical cannabis don’t have any of these conditions,” he said.
Researchers at the symposium focused on the use of cannabis as a treatment for addiction and other psychiatric illnesses.
Four states have legalized the use of cannabis in patients with opioid use disorder, said cannabis researcher Ziva D. Cooper, PhD, of the University of California, Los Angeles, who spoke at the symposium. But can cannabis actually reduce opioid use? Preliminary clinical data suggest THC could reduce opioid use, Dr. Cooper said, while population and state-level research is mixed.
What about other mental health disorders? Posttraumatic stress disorder is commonly listed as a qualifying condition for medical marijuana use in state laws. And some states, like California, give physicians wide leeway in recommending marijuana use for patients with conditions that aren’t listed in the law.
However, symposium speaker and psychiatrist Frances R. Levin, MD, of New York State Psychiatric Institute, pointed to a 2019 review that suggests “there is scarce evidence to suggest that cannabinoids improve depressive disorders and symptoms, anxiety disorders, attention-deficit hyperactivity disorder, Tourette syndrome, posttraumatic stress disorder, or psychosis” (Lancet Psychiatry. 2019 Dec;6[12]:995-1010).
What now? The AAAP hopes lawmakers will pay attention to its proposed model state law, which will be published soon in the association’s journal, the American Journal on Addictions.
REPORTING FROM AAAP 2019
Does using e-cigarettes increase cigarette smoking in adolescents?
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
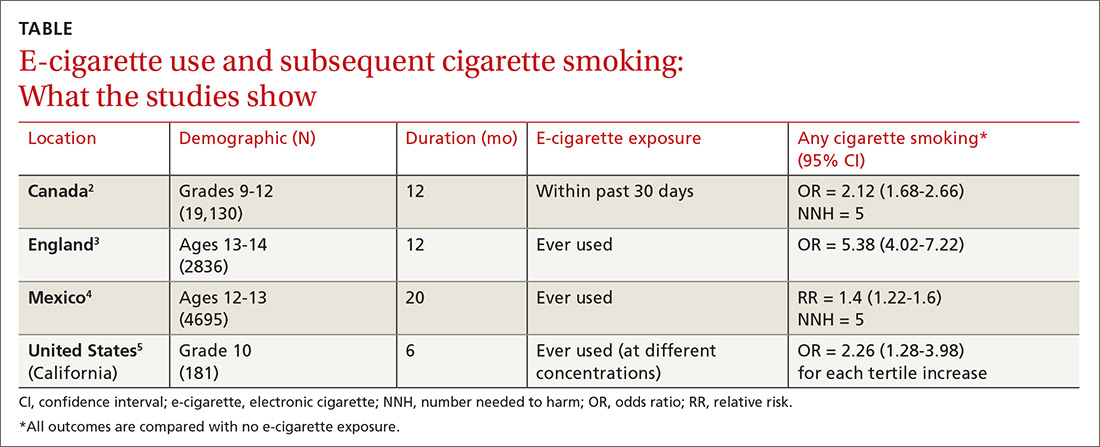
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5

RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5

RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE-BASED ANSWER:
Probably. Electronic cigarette (e-cigarette) use by adolescents is associated with a 2- to 4-fold increase in cigarette smoking over the next year (strength of recommendation: A, meta-analysis and subsequent prospective cohort studies).

















