User login
VIDEO: Sofosbuvir with velpatasvir beat other HCV GT3 regimens
Regimens containing sofosbuvir and velpatasvir were most effective for treating both cirrhotic and noncirrhotic genotype 3 hepatitis C virus infection (HCV GT3), according to a meta-analysis reported in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.10.03).
“Our analyses indicated that ribavirin significantly increases SVR [sustained viral response] rates and should be considered, if tolerated,” added Floor A.C. Berden, MD, of Radboud University Medical Center, Nijmegen, the Netherlands, and her associates.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Direct-acting antiviral regimens successfully treat chronic HCV infection, but tend to perform suboptimally in HCV GT3, especially when patients are treatment experienced and have cirrhosis. Options for HCV GT3 infection include sofosbuvir combined with ribavirin, daclatasvir, or velpatasvir. But head-to-head trials of these regimens are lacking, and are unlikely to occur, in part because the Food and Drug Administration permits single-arm trials with historical controls as the comparator, the investigators said.
Therefore, they searched PubMed, Embase, and the Web of Science database through March 15, 2016, for randomized trials and real-world studies of at least one direct-acting antiviral agent in adults with chronic HCV GT3 infection. They also manually searched abstracts presented at the 2015 conferences of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. This work yielded 27 studies: 16 randomized controlled trials, 6 single-arm studies, and 5 observational cohort studies. The researchers used a Bayesian analysis based on Markov chain Monte Carlo methods.
For patients without cirrhosis, sofosbuvir and velpatasvir with ribavirin yielded the highest estimated likelihood of SVR (99%; 95% confidence interval, 98%-100%), followed by sofosbuvir and velpatasvir without ribavirin (97%; 95% CI, 95%-99%), sofosbuvir and daclatasvir with ribavirin (96%; 95% CI, 92%-98%), and sofosbuvir and peginterferon with ribavirin (95%; 95% CI, 91%-98%), all for 12 weeks, the investigators reported.
For patients with cirrhosis, the most effective regimen was sofosbuvir with velpatasvir for 24 weeks (estimated SVR, 96%; 95% CI, 92%-99%), followed by sofosbuvir and daclatasvir with ribavirin for 24 weeks (94%; 95% CI, 87%-98%), sofosbuvir and velpatasvir and ribavirin for 12 weeks (94%; 95% CI, 86%-98%). The estimated efficacy of sofosbuvir and velpatasvir held up in sensitivity analyses that honed in on studies with a low risk of bias, approved regimens, or those under regulatory evaluation, patients without decompensated cirrhosis, and patients without HIV coinfection.
Adding ribavirin to a direct-acting antiviral regimen improved the odds of SVR about 2.6-fold (95% CI, 1.3-4.7) among noncirrhotic patients and by about 4.5 times in cirrhotic patients (95% CI, 2.5-7.7), the investigators reported. “In clinical practice, choice of treatment may depend on several factors, such as availability and price of direct-acting antivirals, tolerance of ribavirin, risk of adverse events or drug-drug interactions, and the presence of resistance-associated substitutions,” they added. Nonetheless, these findings can help prioritize therapies for HCV GT3 infection in both clinical guidelines and practice, they emphasized.
Dr. Berden and four coinvestigators had no relevant financial disclosures. Senior author Joost Drenth, MD, PhD, disclosed serving on advisory boards and receiving research grants from several pharmaceutical companies.
The rapid development of direct-acting antiviral agents (DAAs) to treat hepatitis C has yielded many surprises and left some gaps in our knowledge.
One of the surprises was that genotype 3, previously considered “easier to treat,” proved quite resistant to the first generation of DAAs. One of the gaps in knowledge was a lack of randomized and head-to-head trials for current medications. One could argue that randomized trials have limited utility in a disease with essentially no spontaneous cures, and that head-to-head trials are pointless in a rapidly evolving field where regimens may be obsolete by the time the study is completed.
On the bright side, a hard endpoint like sustained virologic response (SVR) makes comparison between trials possible. The paper by Bergen et al. offers some guidance in closing the knowledge gap. Their meta-analysis using Bayesian Markov Chain Monte Carlo methods examined the effectiveness of currently available antiviral agents in 27 studies that focused entirely on genotype 3. All studies used antiviral agents that are currently available in the United States, and effectiveness was tested in both noncirrhotic and cirrhotic patients.
The evolution of antiviral therapy has been amazing. After decades of incremental gains, we entered an era of dizzying progress. Genotype 3 went from great news to bad news, and genotype 1 went from a scourge to a piece of cake.
Norman L. Sussman, MD, is associate professor of surgery, Baylor College of Medicine, Houston; director, Project ECHO. He has received speaking and consulting fees for AbbVie, BMS, Gilead, and Merck.
The rapid development of direct-acting antiviral agents (DAAs) to treat hepatitis C has yielded many surprises and left some gaps in our knowledge.
One of the surprises was that genotype 3, previously considered “easier to treat,” proved quite resistant to the first generation of DAAs. One of the gaps in knowledge was a lack of randomized and head-to-head trials for current medications. One could argue that randomized trials have limited utility in a disease with essentially no spontaneous cures, and that head-to-head trials are pointless in a rapidly evolving field where regimens may be obsolete by the time the study is completed.
On the bright side, a hard endpoint like sustained virologic response (SVR) makes comparison between trials possible. The paper by Bergen et al. offers some guidance in closing the knowledge gap. Their meta-analysis using Bayesian Markov Chain Monte Carlo methods examined the effectiveness of currently available antiviral agents in 27 studies that focused entirely on genotype 3. All studies used antiviral agents that are currently available in the United States, and effectiveness was tested in both noncirrhotic and cirrhotic patients.
The evolution of antiviral therapy has been amazing. After decades of incremental gains, we entered an era of dizzying progress. Genotype 3 went from great news to bad news, and genotype 1 went from a scourge to a piece of cake.
Norman L. Sussman, MD, is associate professor of surgery, Baylor College of Medicine, Houston; director, Project ECHO. He has received speaking and consulting fees for AbbVie, BMS, Gilead, and Merck.
The rapid development of direct-acting antiviral agents (DAAs) to treat hepatitis C has yielded many surprises and left some gaps in our knowledge.
One of the surprises was that genotype 3, previously considered “easier to treat,” proved quite resistant to the first generation of DAAs. One of the gaps in knowledge was a lack of randomized and head-to-head trials for current medications. One could argue that randomized trials have limited utility in a disease with essentially no spontaneous cures, and that head-to-head trials are pointless in a rapidly evolving field where regimens may be obsolete by the time the study is completed.
On the bright side, a hard endpoint like sustained virologic response (SVR) makes comparison between trials possible. The paper by Bergen et al. offers some guidance in closing the knowledge gap. Their meta-analysis using Bayesian Markov Chain Monte Carlo methods examined the effectiveness of currently available antiviral agents in 27 studies that focused entirely on genotype 3. All studies used antiviral agents that are currently available in the United States, and effectiveness was tested in both noncirrhotic and cirrhotic patients.
The evolution of antiviral therapy has been amazing. After decades of incremental gains, we entered an era of dizzying progress. Genotype 3 went from great news to bad news, and genotype 1 went from a scourge to a piece of cake.
Norman L. Sussman, MD, is associate professor of surgery, Baylor College of Medicine, Houston; director, Project ECHO. He has received speaking and consulting fees for AbbVie, BMS, Gilead, and Merck.
Regimens containing sofosbuvir and velpatasvir were most effective for treating both cirrhotic and noncirrhotic genotype 3 hepatitis C virus infection (HCV GT3), according to a meta-analysis reported in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.10.03).
“Our analyses indicated that ribavirin significantly increases SVR [sustained viral response] rates and should be considered, if tolerated,” added Floor A.C. Berden, MD, of Radboud University Medical Center, Nijmegen, the Netherlands, and her associates.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Direct-acting antiviral regimens successfully treat chronic HCV infection, but tend to perform suboptimally in HCV GT3, especially when patients are treatment experienced and have cirrhosis. Options for HCV GT3 infection include sofosbuvir combined with ribavirin, daclatasvir, or velpatasvir. But head-to-head trials of these regimens are lacking, and are unlikely to occur, in part because the Food and Drug Administration permits single-arm trials with historical controls as the comparator, the investigators said.
Therefore, they searched PubMed, Embase, and the Web of Science database through March 15, 2016, for randomized trials and real-world studies of at least one direct-acting antiviral agent in adults with chronic HCV GT3 infection. They also manually searched abstracts presented at the 2015 conferences of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. This work yielded 27 studies: 16 randomized controlled trials, 6 single-arm studies, and 5 observational cohort studies. The researchers used a Bayesian analysis based on Markov chain Monte Carlo methods.
For patients without cirrhosis, sofosbuvir and velpatasvir with ribavirin yielded the highest estimated likelihood of SVR (99%; 95% confidence interval, 98%-100%), followed by sofosbuvir and velpatasvir without ribavirin (97%; 95% CI, 95%-99%), sofosbuvir and daclatasvir with ribavirin (96%; 95% CI, 92%-98%), and sofosbuvir and peginterferon with ribavirin (95%; 95% CI, 91%-98%), all for 12 weeks, the investigators reported.
For patients with cirrhosis, the most effective regimen was sofosbuvir with velpatasvir for 24 weeks (estimated SVR, 96%; 95% CI, 92%-99%), followed by sofosbuvir and daclatasvir with ribavirin for 24 weeks (94%; 95% CI, 87%-98%), sofosbuvir and velpatasvir and ribavirin for 12 weeks (94%; 95% CI, 86%-98%). The estimated efficacy of sofosbuvir and velpatasvir held up in sensitivity analyses that honed in on studies with a low risk of bias, approved regimens, or those under regulatory evaluation, patients without decompensated cirrhosis, and patients without HIV coinfection.
Adding ribavirin to a direct-acting antiviral regimen improved the odds of SVR about 2.6-fold (95% CI, 1.3-4.7) among noncirrhotic patients and by about 4.5 times in cirrhotic patients (95% CI, 2.5-7.7), the investigators reported. “In clinical practice, choice of treatment may depend on several factors, such as availability and price of direct-acting antivirals, tolerance of ribavirin, risk of adverse events or drug-drug interactions, and the presence of resistance-associated substitutions,” they added. Nonetheless, these findings can help prioritize therapies for HCV GT3 infection in both clinical guidelines and practice, they emphasized.
Dr. Berden and four coinvestigators had no relevant financial disclosures. Senior author Joost Drenth, MD, PhD, disclosed serving on advisory boards and receiving research grants from several pharmaceutical companies.
Regimens containing sofosbuvir and velpatasvir were most effective for treating both cirrhotic and noncirrhotic genotype 3 hepatitis C virus infection (HCV GT3), according to a meta-analysis reported in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.10.03).
“Our analyses indicated that ribavirin significantly increases SVR [sustained viral response] rates and should be considered, if tolerated,” added Floor A.C. Berden, MD, of Radboud University Medical Center, Nijmegen, the Netherlands, and her associates.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
Direct-acting antiviral regimens successfully treat chronic HCV infection, but tend to perform suboptimally in HCV GT3, especially when patients are treatment experienced and have cirrhosis. Options for HCV GT3 infection include sofosbuvir combined with ribavirin, daclatasvir, or velpatasvir. But head-to-head trials of these regimens are lacking, and are unlikely to occur, in part because the Food and Drug Administration permits single-arm trials with historical controls as the comparator, the investigators said.
Therefore, they searched PubMed, Embase, and the Web of Science database through March 15, 2016, for randomized trials and real-world studies of at least one direct-acting antiviral agent in adults with chronic HCV GT3 infection. They also manually searched abstracts presented at the 2015 conferences of the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. This work yielded 27 studies: 16 randomized controlled trials, 6 single-arm studies, and 5 observational cohort studies. The researchers used a Bayesian analysis based on Markov chain Monte Carlo methods.
For patients without cirrhosis, sofosbuvir and velpatasvir with ribavirin yielded the highest estimated likelihood of SVR (99%; 95% confidence interval, 98%-100%), followed by sofosbuvir and velpatasvir without ribavirin (97%; 95% CI, 95%-99%), sofosbuvir and daclatasvir with ribavirin (96%; 95% CI, 92%-98%), and sofosbuvir and peginterferon with ribavirin (95%; 95% CI, 91%-98%), all for 12 weeks, the investigators reported.
For patients with cirrhosis, the most effective regimen was sofosbuvir with velpatasvir for 24 weeks (estimated SVR, 96%; 95% CI, 92%-99%), followed by sofosbuvir and daclatasvir with ribavirin for 24 weeks (94%; 95% CI, 87%-98%), sofosbuvir and velpatasvir and ribavirin for 12 weeks (94%; 95% CI, 86%-98%). The estimated efficacy of sofosbuvir and velpatasvir held up in sensitivity analyses that honed in on studies with a low risk of bias, approved regimens, or those under regulatory evaluation, patients without decompensated cirrhosis, and patients without HIV coinfection.
Adding ribavirin to a direct-acting antiviral regimen improved the odds of SVR about 2.6-fold (95% CI, 1.3-4.7) among noncirrhotic patients and by about 4.5 times in cirrhotic patients (95% CI, 2.5-7.7), the investigators reported. “In clinical practice, choice of treatment may depend on several factors, such as availability and price of direct-acting antivirals, tolerance of ribavirin, risk of adverse events or drug-drug interactions, and the presence of resistance-associated substitutions,” they added. Nonetheless, these findings can help prioritize therapies for HCV GT3 infection in both clinical guidelines and practice, they emphasized.
Dr. Berden and four coinvestigators had no relevant financial disclosures. Senior author Joost Drenth, MD, PhD, disclosed serving on advisory boards and receiving research grants from several pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point. Regimens containing sofosbuvir and velpatasvir were more effective than were other direct-acting antiviral combinations for treating genotype 3 hepatitis C virus infection, regardless of cirrhosis status.
Major finding: For patients without cirrhosis, sofosbuvir and velpatasvir with ribavirin for 12 weeks yielded the highest estimated likelihood of sustained viral response (99%). For patients with cirrhosis, the most effective regimen was sofosbuvir with velpatasvir for 24 weeks (estimated SVR, 96%).
Data source: A systematic review and meta-analysis of 27 studies: 16 randomized controlled trials, 6 single-arm studies, and 5 observational cohort studies.
Disclosures: Dr. Berden and four coinvestigators had no relevant financial disclosures. Senior author Joost Drenth, MD, PhD, disclosed serving on advisory boards and receiving research grants from several pharmaceutical companies.
VIDEO: Health law changes under new administration
WASHINGTON – A new president is taking office along with new staffers to lead the country’s top health care agencies.
In this video, Joyce Hall, chair of the American Bar Association Health Law Section, discusses what changes she foresees in health law issues under the new administration and what to expect from the leadership transition. Ms. Hall also speaks on potential alterations to the Medicare Access and CHIP Reauthorization Act of 2015 and whether the potential appointment of Rep. Tom Price (R-Ga.) as U.S. Department of Health and Human Services Secretary will be positive or negative for health care providers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
WASHINGTON – A new president is taking office along with new staffers to lead the country’s top health care agencies.
In this video, Joyce Hall, chair of the American Bar Association Health Law Section, discusses what changes she foresees in health law issues under the new administration and what to expect from the leadership transition. Ms. Hall also speaks on potential alterations to the Medicare Access and CHIP Reauthorization Act of 2015 and whether the potential appointment of Rep. Tom Price (R-Ga.) as U.S. Department of Health and Human Services Secretary will be positive or negative for health care providers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
WASHINGTON – A new president is taking office along with new staffers to lead the country’s top health care agencies.
In this video, Joyce Hall, chair of the American Bar Association Health Law Section, discusses what changes she foresees in health law issues under the new administration and what to expect from the leadership transition. Ms. Hall also speaks on potential alterations to the Medicare Access and CHIP Reauthorization Act of 2015 and whether the potential appointment of Rep. Tom Price (R-Ga.) as U.S. Department of Health and Human Services Secretary will be positive or negative for health care providers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
AT THE WASHINGTON HEALTH LAW SUMMIT
The Importance of Subclavian Angiography in the Evaluation of Chest Pain: Coronary-Subclavian Steal Syndrome
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
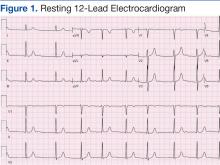
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
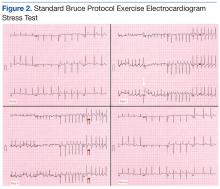
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
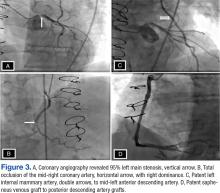
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
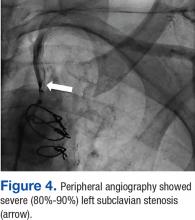
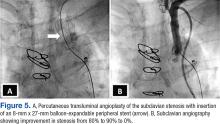
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
VIDEO: First multicenter trial of CAR T cells shows response in DLBCL
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
SAN DIEGO – Aggressive, refractory non-Hodgkin lymphomas responded to anti-CD19 chimeric antigen receptor T cells in ZUMA-1, the first multicenter trial of the cellular immunotherapy, based on early data reported at the annual meeting of the American Society of Hematology.
In an interim analysis of 51 patients with diffuse large B-cell lymphomas, 47% had complete remissions and 29% had partial remissions. But the remission rate declined to 33% complete remissions and 6% partial remissions after 3 months.
There have really been no new treatments in the last 20 years for patients with non-Hodgkin lymphoma that does not respond to chemotherapy or recurs after autologous stem cell transplant. With median overall survival of 6 months, and about 8% complete remissions with existing therapies, CAR T cells might be a solution for these patients, said ZUMA-1 investigator Sattva S. Neelapu, MD, of the University of Texas MD Anderson Cancer Center in Houston.
In our video interview at the meeting, Dr. Neelapu discussed initial results in the real-world setting of 22 participating centers, most of which had no previous experience with CAR T-cell therapy. With an efficient production and logistics plan, 91% of 110 patients were able to receive the investigational product, known as KTE-C19.
ZUMA-1 is funded by Kite, which makes KTE-C19, and the Leukemia & Lymphoma Society Therapy Acceleration Program. Dr. Neelapu receives research support from and is an advisor to Kite.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
VIDEO: Despite toxicities, ibrutinib is beneficial for treatment-resistant graft-vs.-host disease
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Key clinical point: Ibrutinib (420 mg) led to complete responses in one-third of patients with chronic, treatment-resistant graft-vs-host disease.
Major finding: No cardiotoxicities were observed, but 52% of patients had other serious adverse effects, such as sepsis, pyrexia, and pneumonia.
Data source: An open-label phase II study of 42 patients who developed chronic, treatment-resistant graft-vs.-host disease after undergoing allogeneic stem cell transplantation.
Disclosures: Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, reimbursement for travel and expenses, and research funding from Pharmacyclics.
VIDEO: HIV PrEP effective, but adoption lags among high-risk patients
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT IDWEEK 2016
VIDEO: New antisense inhibitor nets impressive reductions in lipids
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Treatment was associated with reductions in levels of triglycerides (–66%), ApoC-III (–68%), LDL cholesterol (–35%), total cholesterol (–36%), and non-HDL cholesterol (–40%), as well as levels of HDL cholesterol (–25%).
Data source: A phase I/IIa ascending-dose trial among 32 healthy adults with hypertriglyceridemia.
Disclosures: Dr. Tsimikas is an employee of and shareholder in Ionis Pharmaceuticals, which sponsored the trial.
VIDEO: ECG screen for cardiac disease in all youths is cost effective
NEW ORLEANS – Results of a first-of-its-kind nationwide U.K. cardiac disease screening program in the general population of teens and young adults indicate that incorporating a 12-lead ECG alongside history and physical examination not only increases the diagnostic yield for conditions predisposing to sudden cardiac death, but it’s actually cost saving, compared with screening by history and physical exam alone, Harshil Dhutia, MD, said at the American Heart Association scientific sessions.
Current practice in the United Kingdom and most other western countries, including the United States, is to screen for cardiac disease in the general population of young people by history and physical exam alone. Only those with symptoms or a positive family history on the initial screen go on to a more comprehensive evaluation including an ECG. The ECG isn’t part of the initial screen primarily because of cost concerns. However, those concerns are based upon conjecture. The diagnostic and financial implications of routine screening by 12-lead ECG in the general population of young people hadn’t been examined prior to the nationwide U.K. screening program – and the results of the U.K. project indicate it’s time for a change in policy, according to Dr. Dhutia, a cardiology research fellow at St. George’s University in London.
The screening project, known as the Cardiac Risk in the Young Program, included 26,900 subjects aged 14-35 years without known cardiovascular disease who responded to public service announcements and voluntarily presented for screening at schools, community centers, and health centers across the U.K. during 2011-2013. The mean age of the subjects was 19.4 years, roughly two-thirds were male, and 90% were white. All screening was performed by cardiologists who followed the AHA protocol for history-taking, performed the physical exam, and followed the European Society of Cardiology 2010 recommendations for ECG interpretation. Individuals with a positive screen were referred to their local hospital for further investigation. Participants were prospectively followed for 2 years.
“This was the first study of comprehensive cardiovascular screening of young individuals outside competitive sport,” Dr. Dhutia noted.
Among the key findings: 3.5% of subjects were deemed by their screening cardiologist to have an abnormal history and/or physical exam warranting further investigation, 8.1% had an abnormal 12-lead ECG, and 0.5% had both. Overall, 11.7% of subjects underwent echocardiography to confirm or refute a diagnosis of cardiac disease, 1.7% underwent Holter monitoring, 1.7% had an exercise stress test, and 0.9% underwent cardiac MRI.
At 2 years of follow-up, 87 individuals, or 0.3% of the overall study cohort, had been diagnosed with a serious cardiac disease predisposing to sudden cardiac death.
“The vast majority of these individuals were asymptomatic and diagnosed on the basis of an ECG abnormality,” Dr. Dhutia observed.
Indeed, 72 of the 87 patients with serious cardiac disease were diagnosed on the basis on their abnormal ECG. This tool proved particularly helpful in identifying individuals with cardiomyopathies or congenital accessory pathways, such as Wolff-Parkinson-White syndrome or long QT syndrome. At 2 years of follow-up, 42 of these 72 patients were on disease-modifying therapies beyond lifestyle interventions, most commonly ablation procedures or antiarrhythmic medication.
The history and physical exam was useful in identifying young people with channelopathies or Marfan syndrome. Roughly half of individuals identified by history and physical exam as having serious cardiac disease were on antiarrhythmic medication – or, less commonly, pacemaker therapy – at 2 years follow-up.
The overall cost per individual screened using history, physical exam, and ECG amounted to $110 on the basis of U.K. National Health Service rates. The cost per serious cardiac condition identified was $33,927. In contrast, the average cost per serious cardiac diagnosis under the current U.K. protocol of screening youth by history and physical exam only is 67% greater, at $56,597.
“Inclusion of ECG to history and physical examination is associated with a significantly improved diagnostic yield and superior economic profile. These findings have the potential to influence health care policy and certainly suggest that the National Health Service framework in the United Kingdom is counterintuitive and may warrant revision,” he concluded.
Session moderator Nisha Parikh, MD, of the University of California, San Francisco, asked if Dr. Dhutia and his coinvestigators had identified any characteristics associated with serious occult cardiac disease in adolescents and young adults that would permit a more refined, selective approach to screening.
Not in the general population of nearly 27,000 subjects in the U.K. study, he replied. Competitive athletes are a different story, though.
“In competitive athletes, certainly individuals who play stop/start sports such as basketball or football [soccer], appear to be at higher risk, especially those of Afro-Caribbean origin. The incidence of sudden cardiac death in Afro-Caribbean basketball players is 1 in 3,000,” according to Dr. Dhutia, who discussed his findings in a video interview.
He reported receiving a small research grant for his study from the Cardiac Risk in the Young Program, a U.K. charitable organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Results of a first-of-its-kind nationwide U.K. cardiac disease screening program in the general population of teens and young adults indicate that incorporating a 12-lead ECG alongside history and physical examination not only increases the diagnostic yield for conditions predisposing to sudden cardiac death, but it’s actually cost saving, compared with screening by history and physical exam alone, Harshil Dhutia, MD, said at the American Heart Association scientific sessions.
Current practice in the United Kingdom and most other western countries, including the United States, is to screen for cardiac disease in the general population of young people by history and physical exam alone. Only those with symptoms or a positive family history on the initial screen go on to a more comprehensive evaluation including an ECG. The ECG isn’t part of the initial screen primarily because of cost concerns. However, those concerns are based upon conjecture. The diagnostic and financial implications of routine screening by 12-lead ECG in the general population of young people hadn’t been examined prior to the nationwide U.K. screening program – and the results of the U.K. project indicate it’s time for a change in policy, according to Dr. Dhutia, a cardiology research fellow at St. George’s University in London.
The screening project, known as the Cardiac Risk in the Young Program, included 26,900 subjects aged 14-35 years without known cardiovascular disease who responded to public service announcements and voluntarily presented for screening at schools, community centers, and health centers across the U.K. during 2011-2013. The mean age of the subjects was 19.4 years, roughly two-thirds were male, and 90% were white. All screening was performed by cardiologists who followed the AHA protocol for history-taking, performed the physical exam, and followed the European Society of Cardiology 2010 recommendations for ECG interpretation. Individuals with a positive screen were referred to their local hospital for further investigation. Participants were prospectively followed for 2 years.
“This was the first study of comprehensive cardiovascular screening of young individuals outside competitive sport,” Dr. Dhutia noted.
Among the key findings: 3.5% of subjects were deemed by their screening cardiologist to have an abnormal history and/or physical exam warranting further investigation, 8.1% had an abnormal 12-lead ECG, and 0.5% had both. Overall, 11.7% of subjects underwent echocardiography to confirm or refute a diagnosis of cardiac disease, 1.7% underwent Holter monitoring, 1.7% had an exercise stress test, and 0.9% underwent cardiac MRI.
At 2 years of follow-up, 87 individuals, or 0.3% of the overall study cohort, had been diagnosed with a serious cardiac disease predisposing to sudden cardiac death.
“The vast majority of these individuals were asymptomatic and diagnosed on the basis of an ECG abnormality,” Dr. Dhutia observed.
Indeed, 72 of the 87 patients with serious cardiac disease were diagnosed on the basis on their abnormal ECG. This tool proved particularly helpful in identifying individuals with cardiomyopathies or congenital accessory pathways, such as Wolff-Parkinson-White syndrome or long QT syndrome. At 2 years of follow-up, 42 of these 72 patients were on disease-modifying therapies beyond lifestyle interventions, most commonly ablation procedures or antiarrhythmic medication.
The history and physical exam was useful in identifying young people with channelopathies or Marfan syndrome. Roughly half of individuals identified by history and physical exam as having serious cardiac disease were on antiarrhythmic medication – or, less commonly, pacemaker therapy – at 2 years follow-up.
The overall cost per individual screened using history, physical exam, and ECG amounted to $110 on the basis of U.K. National Health Service rates. The cost per serious cardiac condition identified was $33,927. In contrast, the average cost per serious cardiac diagnosis under the current U.K. protocol of screening youth by history and physical exam only is 67% greater, at $56,597.
“Inclusion of ECG to history and physical examination is associated with a significantly improved diagnostic yield and superior economic profile. These findings have the potential to influence health care policy and certainly suggest that the National Health Service framework in the United Kingdom is counterintuitive and may warrant revision,” he concluded.
Session moderator Nisha Parikh, MD, of the University of California, San Francisco, asked if Dr. Dhutia and his coinvestigators had identified any characteristics associated with serious occult cardiac disease in adolescents and young adults that would permit a more refined, selective approach to screening.
Not in the general population of nearly 27,000 subjects in the U.K. study, he replied. Competitive athletes are a different story, though.
“In competitive athletes, certainly individuals who play stop/start sports such as basketball or football [soccer], appear to be at higher risk, especially those of Afro-Caribbean origin. The incidence of sudden cardiac death in Afro-Caribbean basketball players is 1 in 3,000,” according to Dr. Dhutia, who discussed his findings in a video interview.
He reported receiving a small research grant for his study from the Cardiac Risk in the Young Program, a U.K. charitable organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Results of a first-of-its-kind nationwide U.K. cardiac disease screening program in the general population of teens and young adults indicate that incorporating a 12-lead ECG alongside history and physical examination not only increases the diagnostic yield for conditions predisposing to sudden cardiac death, but it’s actually cost saving, compared with screening by history and physical exam alone, Harshil Dhutia, MD, said at the American Heart Association scientific sessions.
Current practice in the United Kingdom and most other western countries, including the United States, is to screen for cardiac disease in the general population of young people by history and physical exam alone. Only those with symptoms or a positive family history on the initial screen go on to a more comprehensive evaluation including an ECG. The ECG isn’t part of the initial screen primarily because of cost concerns. However, those concerns are based upon conjecture. The diagnostic and financial implications of routine screening by 12-lead ECG in the general population of young people hadn’t been examined prior to the nationwide U.K. screening program – and the results of the U.K. project indicate it’s time for a change in policy, according to Dr. Dhutia, a cardiology research fellow at St. George’s University in London.
The screening project, known as the Cardiac Risk in the Young Program, included 26,900 subjects aged 14-35 years without known cardiovascular disease who responded to public service announcements and voluntarily presented for screening at schools, community centers, and health centers across the U.K. during 2011-2013. The mean age of the subjects was 19.4 years, roughly two-thirds were male, and 90% were white. All screening was performed by cardiologists who followed the AHA protocol for history-taking, performed the physical exam, and followed the European Society of Cardiology 2010 recommendations for ECG interpretation. Individuals with a positive screen were referred to their local hospital for further investigation. Participants were prospectively followed for 2 years.
“This was the first study of comprehensive cardiovascular screening of young individuals outside competitive sport,” Dr. Dhutia noted.
Among the key findings: 3.5% of subjects were deemed by their screening cardiologist to have an abnormal history and/or physical exam warranting further investigation, 8.1% had an abnormal 12-lead ECG, and 0.5% had both. Overall, 11.7% of subjects underwent echocardiography to confirm or refute a diagnosis of cardiac disease, 1.7% underwent Holter monitoring, 1.7% had an exercise stress test, and 0.9% underwent cardiac MRI.
At 2 years of follow-up, 87 individuals, or 0.3% of the overall study cohort, had been diagnosed with a serious cardiac disease predisposing to sudden cardiac death.
“The vast majority of these individuals were asymptomatic and diagnosed on the basis of an ECG abnormality,” Dr. Dhutia observed.
Indeed, 72 of the 87 patients with serious cardiac disease were diagnosed on the basis on their abnormal ECG. This tool proved particularly helpful in identifying individuals with cardiomyopathies or congenital accessory pathways, such as Wolff-Parkinson-White syndrome or long QT syndrome. At 2 years of follow-up, 42 of these 72 patients were on disease-modifying therapies beyond lifestyle interventions, most commonly ablation procedures or antiarrhythmic medication.
The history and physical exam was useful in identifying young people with channelopathies or Marfan syndrome. Roughly half of individuals identified by history and physical exam as having serious cardiac disease were on antiarrhythmic medication – or, less commonly, pacemaker therapy – at 2 years follow-up.
The overall cost per individual screened using history, physical exam, and ECG amounted to $110 on the basis of U.K. National Health Service rates. The cost per serious cardiac condition identified was $33,927. In contrast, the average cost per serious cardiac diagnosis under the current U.K. protocol of screening youth by history and physical exam only is 67% greater, at $56,597.
“Inclusion of ECG to history and physical examination is associated with a significantly improved diagnostic yield and superior economic profile. These findings have the potential to influence health care policy and certainly suggest that the National Health Service framework in the United Kingdom is counterintuitive and may warrant revision,” he concluded.
Session moderator Nisha Parikh, MD, of the University of California, San Francisco, asked if Dr. Dhutia and his coinvestigators had identified any characteristics associated with serious occult cardiac disease in adolescents and young adults that would permit a more refined, selective approach to screening.
Not in the general population of nearly 27,000 subjects in the U.K. study, he replied. Competitive athletes are a different story, though.
“In competitive athletes, certainly individuals who play stop/start sports such as basketball or football [soccer], appear to be at higher risk, especially those of Afro-Caribbean origin. The incidence of sudden cardiac death in Afro-Caribbean basketball players is 1 in 3,000,” according to Dr. Dhutia, who discussed his findings in a video interview.
He reported receiving a small research grant for his study from the Cardiac Risk in the Young Program, a U.K. charitable organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Key clinical point:
Major finding: The cost of identifying one case of serious cardiac disease predisposing to sudden cardiac death in young people was reduced by 40% when initial screening of the general population ages 14-35 years was conducted by history, physical examination, and 12-lead ECG, compared with no ECG.
Data source: A prospective observational study of nearly 27,000 young people ages 14-35 years in the general population across the United Kingdom who presented for cardiac screening conducted by cardiologists using history, physical examination, and a 12-lead ECG.
Disclosures: The study was funded by the Cardiac Risk in the Young Program, a U.K. charitable organization.