User login
Fish Oil and Osteoarthritis: Current Evidence
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
First-line treatments for osteoarthritis (OA) are targeted at the inflammatory reaction that occurs after breakdown of articular cartilage through regular use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, or surgical intervention. Associated activity restrictions and chronic pain have spurred a search for alternative treatments, commonly daily supplements such as glucosamine, chondroitin, and fish oil, to name a select few of the innumerable products reported to benefit patients with OA.
Background
Fish oil is 1 of the 2 most popular supplements among patients with OA. However, its effectiveness and precise benefit are still debated,1,2 and there is confusion about the definition of the product, the nature of investigations into its effectiveness, and the standardization of research unique to OA. Most fish oil research relates to patients with rheumatoid arthritis (RA). The anti-inflammatory benefits seen in patients with RA are generally applied to characterize fish oils as anti-inflammatory agents with a logical benefit in reducing OA symptoms. However, there is a dearth of independent and focused clinical results justifying that assumption. Further, lack of federal regulation of the supplement industry hinders conducting generalizable studies regarding medical benefit in a regulated and verified dose and form.3
The benefits of fish oil in RA treatment are well supported and accepted. In patients with RA, daily fish oil supplementation has been shown to reduce use of other medications and improve pain scores reported by both physicians and patients.4-10 The clinical efficacy of fish oil use in RA has been determined to be “reasonably strong,” with multiple studies confirming suppression of inflammatory cytokines in vitro and in vivo.11,12 The mechanism by which the inflammatory processes are augmented by fish oil supplementation suggests potential benefit to patients with OA, though review articles as recent as 2011 have concluded that research in that capacity is not sufficient to warrant recommendation.13,14
Most studies of OA-specific use of fish oils have been conducted in in vitro models. Treatment of bovine chondrocytes with omega-3 fatty acids causes reductions in inflammatory markers induced by interleukin 1, one of several proinflammatory cytokines that induce inflammation in OA at the gene and plasma levels, and these reductions have been reproduced.15-17 Although a preventive benefit was found in a study of pig medial collateral ligament fibroblasts, findings of later studies have been inconsistent.18 It also appears that fish oils may alter lipid composition in membranes, favoring incorporation of anti-inflammatory precursor n-3 fatty acids over proinflammatory precursor n-6 fatty acids in these model systems.19,20
Animal in vivo models have also been used to describe the effects of fish oil supplementation on OA. Assessment of dogs with OA before and after supplementation with the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) revealed improvement in clinical signs observed by owners, improvement in weight-bearing measured by veterinary clinicians, and decreased use of NSAIDs.21-24
Fish oil studies using osteoarthritic cartilage samples harvested during surgical procedures have demonstrated results consistent with other model systems described thus far. They have demonstrated a dose-dependent decrease in induced inflammatory destruction of tissue associated with fish oil supplementation. In addition, finding a lack of cellular toxicity, they have validated the safety of supplements.25,26 Proposed but unproven mechanisms for the anti-inflammatory actions of EPA and DHA include competition with n-6 fatty acids; presence of resolvins (anti-inflammatory molecules derived from EPA and DHA); presence of n-3 products that compete with proinflammatory molecules for receptors; reduction in gene expression of cytokines, cyclo-oxygenase 2, and degrading proteinases; interference in the signaling pathways of inflammation; and reduction in lymphocyte proliferation.26,27
Reduction in the n-6/n-3 ratio has been correlated with reduced inflammatory conditions such as OA, stemming from the epidemiologic evidence that higher n-3 intake in Eastern diets and lower intake of n-6 result in a lower incidence of these diseases.18,28,29 Studies have found sufficient evidence to suggest that this ratio has a role in OA, though not sufficient to recommend supplement use over diet modification.19 One study demonstrated an ability to favorably alter bone marrow lipid composition with n-3 fatty acid supplementation.10
The evidence leads to a conclusion of anti-inflammatory benefits from fish oils in these abstracted models. The multitude of basic science studies conducted on the anti-inflammatory properties of omega-3 fatty acids, only briefly reviewed here, supports the potential benefits colloquially ascribed to fish oil in the treatment of OA yet also implies the need for human clinical trials to address these properties clinically.
We reviewed the literature to address claims that fish oil supplementation can prevent or decrease severity of OA. We hypothesized there would be insufficient clinical studies to justify recommending supplementation to patients. Of note, the degree of heterogeneity in the evidence precluded performing a meta-analysis with any statistical validity.
Literature Review
In the PubMed database, we targeted the subject of fish oils and OA by using search terms that included omega-3, DHA, EPA, and alpha-linolenic acid. The MedLine and Google Scholar databases were searched as well. Results were limited to those reported in English and involving human subjects and clinical trials; results were excluded if they primarily involved patients with RA. Studies cited or mentioned in articles found through the PubMed search were evaluated according to the criteria mentioned, such that all relevant articles available at time of search are thought to be included, and these articles represent a reasonable presentation of the available evidence.
Findings
Our search revealed 6 clinical trials in which omega-3–containing supplements were used in the treatment of human OA with differing endpoints. We reviewed these trials in detail. One study, which used alteration of bone marrow lipids as an endpoint, was included for completeness of the evaluation of the relevant evidence.20 In addition, the study by Wang and colleagues,30 who assessed patients without clinical evidence of OA for development of bone marrow lesions, was reviewed. This study was deemed relevant to examine the process by which n-3 fatty acids alter knee structure, as subsequent risk of OA has not been elucidated, and effects on bone marrow lesions may indeed have a direct impact on the OA process. Results of the trials that were identified were varied between no significant difference in OA symptoms between treatment and control groups, implied benefits, and substantial benefits.
The first clinical study of omega-3 supplementation in OA treatment was conducted in 1992.31 The study compared 10 g of cod liver oil (containing 786 mg of EPA) with 10 g of olive oil, both taken daily over 24 weeks by 86 patients with OA. Effects were assessed by NSAID use (recorded in patient diary) and pain score (evaluated by clinician) every 4 weeks. The trial found no significant difference in effects between the oils.
Wang and colleagues30 used a food questionnaire to measure the n-3 intake of 293 healthy adults and quantified their bone marrow lesions after 10 years in an effort to describe how n-3 intake correlates with development of OA or pre-OA lesions. Higher intake of n-6 fatty acids was positively associated with presence of bone marrow lesions; n-3 intake had no association.
In a study of 84 patients who had joint replacement, Pritchett20 evaluated lipid alterations resulting from a regimen of 3 g of fish oil containing 11% DHA daily for a 6-month trial period, measuring lipids before and after the trial period. Pritchett20 found a 20% increase in long-chain fatty acids and a corresponding decrease in saturated fatty acids, as measured in bone marrow.
The supplement Phytalgic (Phythea Laboratories), which is advertised for OA, includes n-3 fatty acids, n-6 fatty acids, extract from Urtica dioica (the common nettle), zinc, and vitamin E. In a study by Jacquet and colleagues,32 this supplement was given 3 times daily over 3 separate 4-week periods to 81 patients with knee or hip OA. Measuring NSAID use with patient diaries and assessing pain with the WOMAC (Western Ontario and McMaster Universities) Osteoarthritis Index every 4 weeks for 12 weeks, the authors found a significant decrease in NSAID use and, according to WOMAC results, a more than 50% reduction in pain and stiffness, and improved function.
One study compared the effects of glucosamine with and without omega-3 fatty acids in 182 patients with knee or hip OA.33 Each day, patients took 500 mg of glucosamine plus 3 capsules each containing either 444 mg of omega-3 fatty acids or 444 mg of an oil mixture. Pain was assessed with visual analog scale and the WOMAC scale 3 times over the 26-week study. More than 90% reductions in morning stiffness and pain were found for the combination of fish oil and glucosamine.
The Multicenter Osteoarthritis Study (MOST), published in February 2012, demonstrated that plasma levels of n-3 and n-6 polyunsaturated fatty acids (PUFAs) may be related to knee structural findings.34 This study confirmed that dietary modification of n-3 and n-6 PUFAs altered plasma concentration predictably. Higher DHA intake was associated with less evidence of OA on patellofemoral cartilage, though no association was found on tibiofemoral cartilage.34
Discussion
The lack of human clinical trials detailing the effects of fish oil supplementation in patients with OA is arguably the most significant hindrance to fish oil being routinely recommended. Since 1992, only 6 studies have addressed this topic, and their endpoints and results were inconsistent. These interventional trials had their limitations, including short duration, insufficient dosage, inappropriate n-3 choice, dietary interactions, genotype, and medication interactions.18 The present review is limited as well, by the quantity of evidence on the topic and by the focus (of the majority of the studies) on short-term alterations in pain and mobility instead of on disease-modifying potential. Short-term evaluation is unlikely to capture such an effect, which may require long-term supplementation to become evident.
The results of the study by Stammers and colleagues31 must be examined critically, as the likelihood of detection bias is high. Highly subjective assessments of effect, lack of standardized NSAID treatments, and limitations in patient numbers and disease severity raise concerns about validity. In addition, confounding variables (eg, medication interactions, alternative treatments, olive oil use) undermine the design. It is therefore difficult to interpret the results of this trial.
The study by Wang and colleagues30 did not involve supplementation, and intake was assessed only with food frequency questionnaires. It is therefore difficult to apply their results or findings to this review. In addition, the authors did not obtain baseline magnetic resonance imaging for comparison with that obtained at study completion—that is, they did not address any subclinical disease before dietary recording.
Pritchett20 acknowledged study limitations of small sample size and use of 1 subject as both patient and control. Although the study seemed to demonstrate that omega-3 supplementation augmented the lipid profile of joints, it did not directly demonstrate improvement in or prevention of OA. Identification of bone marrow lesions is not definitive proof of OA but an alteration that may correlate with development. The logical supposition is that altering the local environment may alter development of disease within that environment, though this is not proven.
An article reviewing the Phytalgic study highlighted the suspect nature of its results—claims that the supplement is 76% more effective than gold-standard corticosteroid injection.35 Also highlighted were lack of confirmed mechanism, questionable control, detection bias caused by aftertaste, and the high attrition rate in the placebo group. It is difficult to apply these results to fish oil supplementation, as Phytalgic contains other potentially confounding substances.
Of note, the findings of MOST were observational; n-3 and n-6 levels were not altered or supplemented. Altered disease process was demonstrated in patellofemoral cartilage but not in tibiofemoral cartilage in the same patient. The inconsistencies may be explained by the observational nature of the study and the lack of supplementation that would have produced a more significant increase in n-3 PUFA levels and thus more uniform conclusions, if in fact n-3 PUFAs were the significant factor in the altered cartilage structure. Although supportive of a preventive or disease-altering benefit, the results do not speak to supplementation.
Perhaps the most convincing evidence supporting fish oil for OA comes from a 2009 study by Gruenwald and colleagues.33 However, this 2-supplement study addressing synergy was financed by Seven Seas, a company with industry ties. The study was not placebo-controlled and was registered only after completion. The authors omitted baseline values, apparently did not correct for baseline in the statistical analysis, and did not report the distribution of results. The implication is that the results were overstated, or that, at minimum, the supporting data were not reported. Nevertheless, this study demonstrated benefits consistent with the animal and human laboratory studies. However, research is needed to repeat and validate these results, elucidate the mechanism of action, and quantify the benefit unique to fish oil.
Conclusion
Despite the overwhelming popularity of fish oil supplements and the assumption of benefit for patients with arthritis, there appears to be insufficient clinical evidence to justify use of fish oils in the treatment or prevention of OA. Possible efficacy in laboratory and animal studies has yet to be sufficiently observed and verified in clinical trials. Although it is impossible to refute the promise of these agents as beneficial adjuncts to anti-inflammatory regimens, there remains a need for significant, well-designed clinical trials to evaluate the efficacy, safety, and clinical parameters of omega-3 fatty acids in a standardized form before they can in good faith be recommended to patients with OA.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.
1. Jordan KM, Sawyer S, Coakley HE, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology. 2003;43(3):381-384.
2. Vista ES, Lau CS. What about supplements for osteoarthritis? A critical and evidenced-based review. Int J Rheum Dis. 2011;14(2):152-158.
3. European Food Safety Authority Panel on Biological Hazards (BIOHAZ). Scientific opinion on fish oil for human consumption. Food hygiene, including rancidity. EFSA J. 2010;8(10):1874.
4. Berbert AA, Kondo CR, Almendra CL, Matsuo T, Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21(2):131-136.
5. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 suppl):1505S-1519S.
6. Calder PC, Zurier RB. Polyunsaturated fatty acids and rheumatoid arthritis. Curr Opin Clin Nutr Metab Care. 2001;4(2):115-121.
7. Kremer JM. Effects of modulation of inflammatory and immune parameters in patients with rheumatic and inflammatory disease receiving dietary supplementation of n-3 and n-6 fatty acids. Lipids. 1996;31(suppl):S243-S247.
8. Kremer JM, Jubiz W, Michalek A, et al. Fish oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann Intern Med. 1987:106(4):497-503.
9. Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810-820.
10. Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22(10):687-691.
11. Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129(1-2):210-223.
12. van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49(2):76-80.
13. Rosenbaum CC, O’Mathúna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16(2):32-40.
14. Sanghi D, Avasthi S, Srivastava RN, Singh A. Nutritional factors and osteoarthritis: a review article. Internet J Med Update. 2009;4(1).
15. Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
16. Curtis CL, Rees SG, Cramp J, et al. Effects of fatty acids on cartilage metabolism. Proc Nutr Soc. 2002;61(3):381-389.
17. Zainal Z, Longman AJ, Hurst S, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis Cartilage. 2009;17(7):896-905.
18. Hankenson KD, Watkins BA, Schoenlein IA, Allen KG, Turek JJ. Omega-3 fatty acids enhance ligament fibroblast collagen formation in association with changes in interleukin-6 production. Proc Soc Exp Biol Med. 2000;223(1):88-95.
19. Melanson KJ. Diet, nutrition and osteoarthritis. Am J Lifestyle Med. 2007;1(4):260-263.
20. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res. 2007;(456):233-237.
21. Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(1):67-73.
22. Roush JK, Dodd CE, Fritsch DA, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. 2010;236(1):59-66.
23. Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236(5):535-539.
24. Fritsch DA, Allen TA, Dodd CE, et al. Dose-titration effects of fish oil in osteoarthritic dogs. J Vet Intern Med. 2010;24(5):1020-1026.
25. Curtis CL, Rees SG, Little CB, et al. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46(6):1544-1553.
26. Shen CL, Dunn DM, Henry JH, Li Y, Watkins BA. Decreased production of inflammatory mediators in human osteoarthritic chondrocytes by conjugated linoleic acids. Lipids. 2004;39(2):161-166.
27. Hurst S, Zainal Z, Caterson B, Hughes CE, Harwood JL. Dietary fatty acids and arthritis. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):315-318.
28. Cleland LG, Hill CL, James MJ. Diet and arthritis. Baillieres Clin Rheumatol. 1995;9(4):771-785.
29. Maresz K, Meus K, Porwolik B. Krill oil: background and benefits. Int Sci Health Found. 2010;1-11.
30. Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579-583.
31. Stammers T, Sibbald B, Freeling P. Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. 1992;51(1):128-129.
32. Jacquet A, Girodet PO, Pariente A, Forest K, Mallet L, Moore N. Phytalgic, a food supplement, vs placebo in patients with osteoarthritis of the knee or hip: a randomised double-blind placebo-controlled clinical trial. Arthritis Res Ther. 2009;11(6):R192.
33. Gruenwald J, Petzold E, Busch R, Petzold HP, Graubaum HJ. Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv Ther. 2009;26(9):858-871.
34. Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis Cartilage. 2012;20(5):382-387.
35. Christensen R, Bliddal H. Is Phytalgic® a goldmine for osteoarthritis patients or is there something fishy about this neutraceutical? A summary of findings and risk-of-bias assessment. Arthritis Res Ther. 2010;12(1):105.
The Effect of Arthroscopic Rotator Interval Closure on Glenohumeral Volume
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
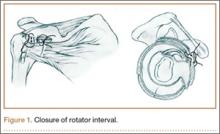
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
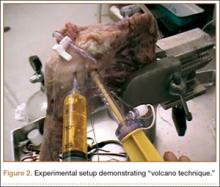
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
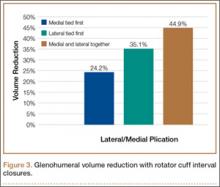
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
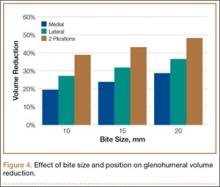
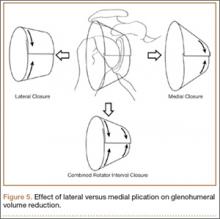
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
Since Neer described the rotator interval in 1970, its closure, often used in conjunction with capsulorrhaphy, has become an important surgical technique in managing shoulder instability.1-11 Numerous studies have sought to define the function of the rotator interval.1-3,6-20 The etiology of lesions of the rotator interval has been debated, and there is evidence that such lesions may be in part congenital.21 Increased rotator interval depth and width, along with increased size of the distended inferior and posteroinferior joint capsule on magnetic resonance arthrography, have been reported in cases of multidirectional shoulder instability.22 However, confusion remains about the role of the rotator interval in shoulder instability and about the effect its closure has on shoulder function. No one knows the degree of volume reduction that results from closure of the rotator interval and whether medial and lateral sutures differ in the volume reduction achieved.
Cadaveric studies have shown that the rotator interval has an important role in shoulder motion.6,13-16,19,20,23 Harryman and colleagues13 found that sectioning the coracohumeral ligament (CHL) increased shoulder range of motion (ROM), and medial-to-lateral closure of the rotator interval restricted motion in all planes. Most notably, interval closure limited inferior translation in the adducted shoulder, posterior translation in the flexed adducted shoulder, and external rotation in the neutral position. Subsequent studies,17,18 using rotator interval closure combined with thermal capsulorrhaphy, confirmed the results reported by Harryman and colleagues.13
More recent cadaveric studies using superior-to-inferior rotator interval closures have shown a decrease in anterior translation but not posterior translation.14-16,19-21 A superior-to-inferior interval closure technique limited external rotation less than a medial-to-lateral closure did.13-16,19-21 The majority of arthroscopically described rotator interval closures involve a superior-to-inferior technique and use 2 or 3 sutures.1,3,9-11
Plausinis and colleagues15 examined the effects of an isolated medial, an isolated lateral, and a medial combined with a lateral closure of the rotator interval. They noted that all 3 methods limited anterior translation and motion by means of 6° flexion and 10° external rotation; however, there was no statistical difference between methods. They also found that occasionally the medial interval closure resulted in massive loss of external rotation. Earlier, Jost and colleagues14 noted that a medial rotator interval could cause this massive loss by tethering the CHL, resulting in a medial-to-lateral imbrication of the CHL.
Arthroscopic rotator interval closure has clinically demonstrated an additive effect on shoulder stability. The recurrence rate was lower for arthroscopic Bankart repair combined with arthroscopic rotator interval closure (8%) than for arthroscopic Bankart repair alone (13%).24 In addition, time to recurrent dislocation was longer (42 vs 13 months) for the group that underwent the combination of Bankart repair and rotator interval closure. Regarding the concern about loss of motion after arthroscopic rotator interval closure, Chiang and colleagues25 recently noted no significant loss of motion 5 years after arthroscopic Bankart repair with rotator interval closure.
What effect rotator interval closure has on intra-articular glenohumeral volume (GHV) remains unknown. Using a cadaveric model, Yamamoto and colleagues20 showed that decreasing GHV can increase the responsiveness of the glenohumeral joint to the intra-articular pressure. Thus, reducing the volume can improve stability in vitro by increasing the magnitude of negative pressure stabilizing the glenohumeral joint.
We conducted a study to quantify the effects of arthroscopic rotator interval closure on capsular volume and to determine whether medial and lateral interval closures resulted in different degrees of volume reduction. Our hypothesis was that shoulder volume would be significantly reduced by closing the rotator interval.
Materials and Methods
Previous studies have not specifically evaluated GHV after rotator interval closure. Our power analysis was performed with data from a study by Karas and colleagues,26 who evaluated GHV after capsular plication. To detect a capsular volume reduction of 20% per stitch, with a 2-sided 5% significance level and a power of 80%, we needed a sample size of 5 specimens per group.
After receiving institutional review board approval for this study, we obtained 10 cadaveric shoulders (5 matched pairs). Exclusion criteria included arthroscopic evaluation revealing a full-thickness rotator cuff tear or significant osteoarthritis. Two shoulders had full-thickness cuff tears, leaving 8 shoulders to be tested; 6 of these were matched pairs. The shoulders were from 1 man (matched pair) and 4 women (2 matched pairs). Age ranged from 38 to 70 years (mean, 59.6 years). Differences in material properties between the specimens were accounted for by using primarily matched pairs.
The 2 study groups consisted of 4 shoulders each. After specimens were thawed, the skin, subcutaneous tissues, and periscapular muscles were removed from the shoulder. Only the capsule, biceps, and rotator cuff remained. For measurement purposes, the shoulders were mounted in a vice clamp in a beach-chair orientation. We placed a total of 2 portals with fully threaded 8.25-mm cannulas (Arthrex, Naples, Florida). A standard posterior portal was placed in the soft spot. A low anterior portal was then placed just superior to the subscapularis tendon. For arthroscopic examination and instrumentation in a saline environment, the shoulders were rotated into the lateral decubitus position, with suspension in 30° abduction and 20° forward flexion, by a rope attached to a pin in the distal shaft of the humerus.
In both groups, medial and lateral stitches with No. 2 FiberWire (Arthrex) were used to close the interval. The medial interval closure stitch was placed more than 10 mm away from the glenoid to prevent unpredictable CHL tethering; the lateral closure stitch was placed 10 mm lateral to the medial stitch (Figure 1).14 All sutures were placed intra-articularly under direct arthroscopic visualization, similar to the methods described in the literature.1,3,9-11 Sutures were passed through the superior glenohumeral ligament (SGHL) and through the upper subscapularis using a suture shuttle (SutureLasso; Arthrex) and Penetrator II Suture Retriever (Arthrex). The upper subscapularis was incorporated because of the unpredictable nature of the middle glenohumeral ligament (MGHL). Both rotator interval sutures were placed before tying either. In the medial group, the medial stitch was tied first, using alternating half-hitches, followed by the lateral stitch. In the lateral group, the lateral stitch was tied first, followed by the medial stitch. GHV was measured at baseline and after tying each stitch. Dr. Ponce instrumented all shoulders.
Modifying a beach-chair technique described by Miller and colleagues,27 we used a viscous fatty-acid sulfate solution, liquid soap, to measure GHV.27-29 A small slit in line with the fibers was made in the supraspinatus tendon just lateral to the musculotendinous junction. A 3-way stop-cock was placed into the joint though this defect. A 20-mL syringe with a 16-gauge needle was used to inject the soap. The needle was inserted into the rotator cuff interval, and the viscous solution was injected in 5-mL increments until there was active extravasation through the supraspinatus cannula (Figure 2). This technique, the “volcano method,” marked the maximum capacity of the joint. The joint was then copiously irrigated with normal saline and suctioned until all normal saline was evacuated. Dr. Rosenzweig took 2 measurements on each shoulder, and their mean was used for analysis.
The baseline measurement was taken with the 2 working cannulas in the shoulder joint. Measurements were obtained with cannulas to simulate normal clinical conditions. Subsequent measurements were done with the cannulas in place and inserted up to the same thread each time so as not to change the volume. The capsule and the rotator cuff were then dissected from the humerus so the size of the capsulolabral plication could be directly evaluated. Methylene blue was used to mark the capsular suture holes before removing the sutures. With use of a caliper, the size of the plication bite was measured (in millimeters).
Statistical Analysis
The primary outcome was percent reduction in GHV as a function of number of plications and size of plication. When only the first plication was tightened, the effect of position (medial or lateral) was also of interest. Percent volume reduction was calculated as (original – new) / original × 100. SAS 8.02 (SAS Institute, Cary, North Carolina) was used to fit a repeated random-intercept regression model for each outcome. This technique properly accounts for the paired nature of the specimens and the repeated measures (baseline plus 2 plications). Model fit was assessed by the method of difference in log likelihood.
Results
In the medial group, GHV was reduced by a mean of 24.2% with a single medial stitch; in the lateral group, GHV was reduced by a mean of 35.1% (Figure 3). The difference was significant (P < .02). In the medial group, when a second lateral stitch was used, GHV was reduced by another 18.7%; in the lateral group, when a medial stitch was added, GHV was reduced by another 11.4%. Final GHV for the medial and lateral groups was 42.9% and 46.5%, respectively. There was no statistical difference in final GHV, regardless of which stitch was placed first. When the 2 groups were combined, GHV was reduced by 44.9% with use of medial and lateral rotator interval closure stitches.
Mean amount of tissue purchased, or “bite size,” was 18 mm with a lateral suture and 15 mm with a medial suture (P < .05). In addition, an increase in bite size to GHV reduction was essentially linear, where an increase in bite size of 1 mm reduced GHV by about 1% (Figure 4).
Discussion
Although there have been numerous clinical series and biomechanical studies focused on isolated rotator interval closure (or its use as an adjunct) in shoulder stabilization, the precise function of the rotator interval remains poorly understood.1-3,6-11,19 Consequently, the in vivo effects of interval closure are unknown.
Initial studies proposed that rotator interval closure limited inferior and posterior translation.30 More recent studies have demonstrated that rotator interval closure confers little effect on posterior instability but increases anterior stability in cadaveric models.15,16 Clinical series have provided evidence that rotator interval closure can increase anterior stability.1,3,7,9,12 In a series of isolated rotator interval closures for multidirectional instability, Field and colleagues12 found that preoperative anterior and inferior symptoms predominated over posterior symptoms. Isolated closure of the rotator interval resulted in 100% excellent results with no cases of recurrent instability. Moon and colleagues31 reported that arthroscopic rotator interval closure with or without inferior capsular plication in multidirectional instability and predominant symptomatic inferior instability has shown benefit by improving function and stability. Other clinical reports of rotator interval closure in conjunction with arthroscopic Bankart repair have suggested it has an additive effect on anterior shoulder stability without limiting motion.24,25
In our study, arthroscopic closure of the rotator interval with 2 superior-to-inferior stitches reduced intracapsular volume by 45%. Even though open capsular shifts use different surgical techniques, similar technique volume reduction studies have reported reductions between 34% and 54% with open shifts.27,30 It is unknown if the stability resulting from decreased GHV is primarily from increasing intra-articular pressures or from restricting ROM, or from a combination of both. In shoulders with multidirectional instability, the joint volume may be increased, the joint capsule may be enlarged, or the glenohumeral ligaments may be lax and thin.4,6,32,33 Yamamoto and colleagues19 stated that intra-articular pressure is determined by 3 factors: load, joint volume, and material properties of the capsule. Load is a constant; joint volume and material properties can be changed.19 In our study, material properties were controlled by using a majority of matched specimens. Regardless of the stabilizing mechanism, our study results demonstrated that arthroscopic rotator interval closure may be a powerful tool in reducing shoulder volume, a consistent principle of surgical techniques used in reestablishing shoulder stability.19,20
When a single rotator interval closure stitch was used, volume reduction with a lateral stitch was superior to that with a medial stitch. This finding is logical, as anatomically the dimensions of the rotator interval are larger laterally as the CHL fans out to insert on the greater and lesser tuberosities.14 This finding has also been reported in open capsular shifts for multidirectional instability, with a lateral humeral shift having a larger volume reduction than a medial glenoid shift.27 Miller and colleagues27 used the image of a cone, with its larger opening facing the humerus and narrower side facing the glenoid, to illustrate this difference in open capsular shifts.
Our study also showed a larger volume reduction with 2 rotator interval closure stitches than with a single interval stitch. As ROM testing has not shown a difference between results with 1 and 2 sutures, we recommend a minimum of 2 sutures for arthroscopic rotator interval closure.15 If a single plication stitch is preferred, a lateral stitch (vs a medial stitch) can be used for a significantly larger reduction in shoulder volume. We think this is because of a larger amount of capsule being purchased with lateral closure (Figure 5). However, if a medial stitch is used, it is important to not place it too near the glenoid to avoid CHL tethering and subsequent excessive loss of external rotation.15
This study had several weaknesses. First, it was a cadaveric study, and use of specimens not known to have instability or specific rotator interval injury may make generalization to a clinical situation difficult. Second, although our power analysis called for 5 shoulders in each group, full-thickness rotator cuff tears rendered 2 shoulders unusable. This reduced our sample sizes and potentially decreased the power of the study, though the data demonstrated statistically significant differences. Third, we did not compare the effects of an open medial-to-lateral imbrication of the rotator interval on intracapsular volume with the effects of our arthroscopic method. We also did not assess our specimens’ ROM, effects of interval closure stitches on shoulder stability, or glenohumeral contact surface pressures, as these factors have already been studied.13-19 Instead, we focused on the effects of rotator interval closure on intracapsular volume, which had not been quantified until now. The clinical significance of such a volume reduction is unknown, especially with respect to influence on ROM, but the degree of volume reduction was larger than with previously reported arthroscopic instability repairs and smaller than with open capsular shifts, demonstrating that it may be a powerful tool in restoring stability in an unstable shoulder.26-30,34 Fourth, the role of isolated rotator interval closure is poorly defined, as only 1 clinical series of isolated rotator interval closure has been reported thus far.12 It has been far more common for rotator interval closure to be used with Bankart repair or capsulorrhaphy.1-3,7-9
In a cadaveric study by Provencher and colleagues,16 open rotator interval closure with medial-to-lateral imbrication of the interval altered shoulder kinematics differently from what occurred with arthroscopic closure of the MGHL to the SGHL, resulting in superior-to-inferior shift. Comparing the 2 methods may therefore be inappropriate. Currently we reserve rotator interval closure for infrequent cases of revision instability and cases in which glenoid bone loss is marginal (5%-15%) and there is a willingness to potentially sacrifice ROM to restore stability and avoid an open stabilization procedure. Continued investigation into the clinical role of rotator interval closure in shoulder stability is needed. We should identify the pathology in a patient with instability and use this technique as an adjuvant to other stabilization procedures.
Conclusion
Arthroscopic rotator interval closure with 2 plication stitches is a powerful tool in reducing the intracapsular volume of the shoulder. If a single plication stitch is preferred, a lateral rotator interval closure stitch (vs a medial stitch) can be used for a larger reduction in shoulder volume.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
1. Creighton RA, Romeo AA, Brown FM, Hayden JK, Verma NN. Revision arthroscopic shoulder instability repair. Arthroscopy. 2007;23(7):703-709.
2. Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg Am. 2000;82(7):991-1003.
3. Gartsman GM, Taverna E, Hammerman SM. Arthroscopic rotator interval repair in glenohumeral instability: description of an operative technique. Arthroscopy. 1999;15(3):330-332.
4. Neer CS 2nd, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder: a preliminary report. J Bone Joint Surg Am. 1980;62(6):897-908.
5. Neer CS 2nd. Displaced proximal humerus fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
6. Nobuhara K, Ikeda H. Rotator interval lesion. Clin Orthop. 1987;(223):44-50.
7. Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. J Bone Joint Surg Am. 1984;66(2):159-168.
8. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63(6):863-872.
9. Stokes DA, Savoie FH, Field LD. Arthroscopic repair of anterior glenohumeral instability and rotator interval lesions. Orthop Clin North Am. 2003;34(4):529-539.
10. Taverna E, Sansone V, Battistella F. Arthroscopic rotator interval repair: the three-step all-inside technique. Arthroscopy. 2004;20 Suppl 2:105-109.
11. Treacy SH, Field LD, Savoie FH. Rotator interval capsule closure: an arthroscopic technique. Arthroscopy. 1997;13(1):103-106.
12. Field LD, Warren RF, O’Brien SJ, Altcheck DW, Wickiewicz TL. Isolated closure of rotator interval defects for shoulder instability. Am J Sports Med. 1995;23(5):557-563.
13. Harryman DT 2nd, Sidles JA, Harris SL, Matsen FA 3rd. The role of the rotator interval capsule in passive motion and stability of the shoulder. J Bone Joint Surg Am. 1992;74(1):53-66.
14. Jost B, Koch PP, Gerber C. Anatomy and functional aspects of the rotator interval. J Shoulder Elbow Surg. 2000;9(4):336-341.
15. Plausinis D, Bravman JT, Heywood C, Kummer FJ, Kwon YM, Jazrawi LM. Arthroscopic rotator interval closure: effect of sutures on glenohumeral motion and anterior-posterior translation. Am J Sports Med. 2006;34(10):1656-1661.
16. Provencher MT, Mologne TS, Hongo M, Zhao K, Tasto JP, An KN. Arthroscopic versus open rotator interval closure: biomechanical evaluation of stability and motion. Arthroscopy. 2007;23(6):583-592.
17. Selecky MT, Tibone JE, Yang BY, et al. Glenohumeral joint translation after thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139-143.
18. Wolf R, Zheng N, Iero J, Weichel D. The effects of thermal capsulorrhaphy and rotator interval closure on multidirectional laxity in the glenohumeral joint: a cadaveric biomechanical study. Arthroscopy. 2004;20(10):1044-1049.
19. Yamamoto N, Itoi E, Tuoheti Y, et al. Effect of rotator interval closure on glenohumeral stability and motion: a cadaveric study. J Shoulder Elbow Surg. 2006;15(6):750-758.
20. Yamamoto N, Itoi E, Tuoheti Y, et al. The effect of the inferior capsular shift on shoulder intra-articular pressure: a cadaveric study. Am J Sports Med. 2006;34(6):939-944.
21. Cole BJ, Rodeo SA, O’Brien SJ, et al. The anatomy and histology of the rotator interval capsule of the shoulder. Clin Orthop. 2001;(390):129-137.
22. Lee HJ, Kim NR, Moon SG, Ko SM, Park JY. Multidirectional instability of the shoulder: rotator interval dimension and capsular laxity evaluation using MR arthrography. Skeletal Radiol. 2013;42(2):231-238.
23. Warner JP, Deng X, Warren RF, Torzilli PA, O’Brien SJ. Superoinferior translation in intact and vented glenohumeral joint. J Shoulder Elbow Surg. 1993;2(2):99-105.
24. Chechik O, Maman E, Dolkart O, Khashan M, Shabtai L, Mozes G. Arthroscopic rotator interval closure in shoulder instability repair: a retrospective study. J Shoulder Elbow Surg. 2010;19(7):1056-1062.
25. Chiang, E, Wang J, Wang S, et al. Arthroscopic posteroinferior capsular plication and rotator interval closure after Bankart repair in patients with traumatic anterior glenohumeral instability—a minimum follow-up of 5 years. Injury. 2010;41(10):1075-1078.
26. Karas SG, Creighton RA, DeMorat GJ. Glenohumeral volume reduction in arthroscopic shoulder reconstruction: a cadaveric analysis of suture plication and thermal capsulorrhaphy. Arthroscopy. 2004;20(2):179-184.
27. Miller MD, Larsen KM, Luke T, Leis HT, Plancher KD. Anterior capsular shift volume reduction: an in vitro comparison of 3 techniques. J Shoulder Elbow Surg. 2003;12(4):350-354.
28. Luke TA, Rovner AD, Karas SG, Hawkins RJ, Plancher KD. Volumetric change in the shoulder capsule after open inferior capsular shift versus arthroscopic thermal capsular shrinkage: a cadaveric model. J Shoulder Elbow Surg. 2004;13(2):146-149.
29. Ponce BA, Rosenzweig SD, Thompson KJ, Tokish J. Sequential volume reduction with capsular plications: relationship between cumulative size of plications and volumetric reduction for multidirectional instability of the shoulder. Am J Sports Med. 2011;39(3):526-531.
30. Lubowitz J, Bartolozzi A, Rubenstein D, et al. How much does inferior capsular shift reduce shoulder volume? Clin Orthop. 1996;(328):86-90.
31. Moon YL, Singh H, Yang H, Chul LK. Arthroscopic rotator interval closure by purse string suture for symptomatic inferior shoulder instability. Orthopedics. 2011;34(4).
32. Jerosch J, Castro WH. Shoulder instability in Ehlers-Danlos syndrome: an indication for surgical treatment? Acta Orthop Belg. 1990;56(2):451-453.
33. Schenk TJ, Brems JJ. Multidirectional instability of the shoulder: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 1998;6(1):65-72.
34. Cohen SB, Wiley W, Goradia VK, Pearson S, Miller MD. Anterior capsulorrhaphy: an in vitro comparison of volume reduction. Arthroscopic plication versus open capsular shift. Arthroscopy. 2005;21(6):659-664.
Technique for Lumbar Pedicle Subtraction Osteotomy for Sagittal Plane Deformity in Revision
Pedicle subtraction osteotomies (PSOs) have been used in the treatment of multiple spinal conditions involving a fixed sagittal imbalance, such as degenerative scoliosis, idiopathic scoliosis, posttraumatic deformities, iatrogenic flatback syndrome, and ankylosing spondylitis. The procedure was first described by Thomasen1 for the treatment of ankylosing spondylitis. More recently, multiple centers have reported the expanded use and good success of PSO in the treatment of fixed sagittal imbalance of other etiologies.2,3 According to Bridwell and colleagues,2 lumbar lordosis can be increased 34.1°, and sagittal plumb line can be improved 13.5 cm.
PSO is a complex, extensive surgery most often performed in the revision setting. Multiple authors have described the technique for PSO.4,5 There are significant technical challenges and many complications, including neurologic deficits, pseudarthrosis of adjacent levels, and wound infections.6 Short-term challenges include a large loss of blood, 2.4 L on average, according to Bridwell and colleagues.6 Time of closure of the osteotomy gap is a crucial point in the surgery. Blood loss, often large, slows only after the gap is closed and stabilized.
In this article, we describe a technique in which an additional rod or pedicle screw construct is used at the periosteotomy levels to close the osteotomy gap during PSO and simplify subsequent instrumentation. In addition, we report our experience with the procedure.
Materials and Methods
Seventeen consecutive patients (mean age, 58 years; range, 12-81 years) with fixed sagittal imbalance were treated with lumbar PSO. The indication in all cases was flatback syndrome after previous spinal surgery. Mean follow-up was 13 months. Mean number of prior surgeries was 3. Thirteen PSOs were performed at L3, and 4 were performed at L2.
Radiographic data were collected from before surgery, in the immediate postoperative period, and at final follow-up. All the radiographs were standing films. Established radiographic parameters were measured: thoracic kyphosis from T5 to T12, lumbar lordosis from L1 to S1, PSO angle (1 level above to 1 level below osteotomy level), sagittal plumb line (from center of C7 body to posterosuperior aspect of S1 body), and coronal plumb line (from center of C7 body to center of S1 body).2
Good clinical outcomes in the treatment of spinal disorders require careful attention to the alignment of the spine in the sagittal plane.7,8 When evaluating the preoperative radiographs, we measured and documented pelvic parameters. Figure 1A shows how pelvic incidence was determined. We measured this as the angle between a line drawn from the center of the S1 endplate to the center of the femoral head and the perpendicular off the S1 endplate. Figure 1B shows pelvic tilt as determined by the angle between a line drawn from the center of S1 to the femoral head and a vertical line originating from the center of the femoral head. Figure 1C shows the sacral slope, which we measured as the angle between a line drawn parallel to the endplate of S1 and its intersection with a horizontal line.
Surgical Technique
The overall surgical technique for PSO has been well described.4,5 Here we describe the “outrigger” modification to osteotomy closure (Figures 2, 3).
Most of our 17 cases were revisions. In these cases, new fixation points are first established. All fixation points that will be needed for the final fusion are placed. If a pedicle above or below the osteotomy level is not suitable for a screw, it can be skipped.
Wide decompression of the involved level is performed from pedicle to pedicle, ensuring that the nerve roots are completely decompressed. The dissection is then continued around the lateral wall of the vertebral body. While the neural elements are protected with gentle retraction, the pedicle and a portion of the posterior aspect of the vertebral body are removed with a combination of a rongeur and reverse-angle curettes. Resection of the vertebral body can be facilitated by attaching a short rod to the pedicle screws on either side of the osteotomy level and using it to provide gentle distraction.
Once sufficient bone has been removed to close the osteotomy, short rods are placed in the pedicle screws in the level above and the level below the osteotomy site. These rods are attached with offset connectors that allow the rods to be placed lateral to the screws. Before the surgical procedure is started, the patient is positioned on 2 sets of posts separated by the break in the table. The break in the table allows flexion to accommodate the preoperative kyphosis and allows hyperextension to help close the osteotomy site. Now, with the osteotomy site ready for closure, the table is gradually positioned in extension along with a combination of posterior pressure and compression between the pedicle screws above and below the osteotomy. Once the osteotomy is adequately compressed, the short rods are tightened, holding the osteotomy in good position. With the osteotomy held by the short rods and table positioning, decompression of the neural elements is confirmed and hemostasis obtained.
Final instrumentation is then performed with long rods that can bypass the osteotomized levels, allowing for simpler contouring. If desired, a cross connector can be placed between the long rod of the fusion construct and the short rod holding the osteotomy. The rest of the fusion procedure is completed in standard fashion with at least 1 subfascial drain.
Results
Our 17 patients’ results are summarized in the Table. Mean sagittal plumb line improved from 17.7 cm (range, 5.9 to 29 cm) before surgery to 4.5 cm (range, –0.2 to 12.9 cm) after surgery, for a mean improvement of 13.2 cm. At final follow-up, mean sagittal plumb line was 5.1 cm (range, –1.4 to 10.2 cm).
Mean lumbar lordosis improved from 10° (range, –14° to 34°) before surgery to 49° (range, 36° to 63°) after surgery, for a mean improvement of 39°. Mean PSO angle improved from 3° (range, –36° to 23°) before surgery to 41° (range, 25° to 65°) after surgery, for a mean improvement of 38°. At final follow-up, mean lumbar lordosis remained at 47° (range, 26° to 64°), and mean PSO angle was 39° (range, 24° to 59°).
Mean thoracic kyphosis improved from 18° (range, –8° to 52°) before surgery to 30° (range, 3° to 58°) after surgery, for a mean improvement of 12°. At final follow-up, mean thoracic kyphosis was 31° (range, 2° to 57°).
Fourteen patients did not have complications during the study period. Of the 3 patients with complications, 1 had an early infection, treated effectively with irrigation and débridement and intravenous antibiotics; 1 had a late deep infection, treated with multiple débridements, hardware removal, and, eventually, suppressive antibiotics; and 1 had cauda equina syndrome (caused by extensive scar tissue on the dura, which buckled with restoration of lordosis leading to cord compression), treated with duraplasty, which resulted in full neurologic recovery.
Discussion
In the present series of patients, the described technique for facilitating PSO for correction of sagittal imbalance was effective, and complications were similar to those previously reported.
The benefit of the outrigger construct is that it allows controlled compression of the osteotomy site and can be left in place at time of final instrumentation, locking in compression and correction. Other techniques involve removing the temporary rod and replacing it with final instrumentation4,5—an extra step that complicates instrumentation of the additional levels of the fusion construct and possibly adds pedicle screw stress and contributes to loosening when the new rod is reduced to the pedicle screw. The final long rod construct can bypass the osteotomy levels and allow for simpler instrumentation.
Mean age was 58 years in this series versus 52.4 years in the series reported by Bridwell and colleagues.2 Given the higher mean age of our patients, though no objective measures of bone quality were available, this technique is likely applicable to patients with poor bone quality.
The complications we have reported are in line with those reported in previous series, and maintenance of radiographic parameters at final follow-up indicates that this osteotomy technique allows for solid fusion constructs.
The outrigger technique for controlling PSO closure is an effective method that simplifies instrumentation during a complex revision case.
1. Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop. 1985;(194):142-152.
2. Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85(3):454-463.
3. Berven SH, Deviren V, Smith JA, Emami A, Hu SS, Bradford DS. Management of fixed sagittal plane deformity: results of the transpedicular wedge resection osteotomy. Spine. 2001;26(18):2036-2043.
4. Bridwell KH, Lewis SJ, Rinella A, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. Surgical technique. J Bone Joint Surg Am. 2004;86(suppl 1):44-50.
5. Wang MY, Berven SH. Lumbar pedicle subtraction osteotomy. Neurosurgery. 2007;60(2 suppl 1):ONS140-ONS146.
6. Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine. 2003;28(18):2093-2101.
7. Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87(2):260-267.
8. Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine. 2009;34(17):1828-1833.
Pedicle subtraction osteotomies (PSOs) have been used in the treatment of multiple spinal conditions involving a fixed sagittal imbalance, such as degenerative scoliosis, idiopathic scoliosis, posttraumatic deformities, iatrogenic flatback syndrome, and ankylosing spondylitis. The procedure was first described by Thomasen1 for the treatment of ankylosing spondylitis. More recently, multiple centers have reported the expanded use and good success of PSO in the treatment of fixed sagittal imbalance of other etiologies.2,3 According to Bridwell and colleagues,2 lumbar lordosis can be increased 34.1°, and sagittal plumb line can be improved 13.5 cm.
PSO is a complex, extensive surgery most often performed in the revision setting. Multiple authors have described the technique for PSO.4,5 There are significant technical challenges and many complications, including neurologic deficits, pseudarthrosis of adjacent levels, and wound infections.6 Short-term challenges include a large loss of blood, 2.4 L on average, according to Bridwell and colleagues.6 Time of closure of the osteotomy gap is a crucial point in the surgery. Blood loss, often large, slows only after the gap is closed and stabilized.
In this article, we describe a technique in which an additional rod or pedicle screw construct is used at the periosteotomy levels to close the osteotomy gap during PSO and simplify subsequent instrumentation. In addition, we report our experience with the procedure.
Materials and Methods
Seventeen consecutive patients (mean age, 58 years; range, 12-81 years) with fixed sagittal imbalance were treated with lumbar PSO. The indication in all cases was flatback syndrome after previous spinal surgery. Mean follow-up was 13 months. Mean number of prior surgeries was 3. Thirteen PSOs were performed at L3, and 4 were performed at L2.
Radiographic data were collected from before surgery, in the immediate postoperative period, and at final follow-up. All the radiographs were standing films. Established radiographic parameters were measured: thoracic kyphosis from T5 to T12, lumbar lordosis from L1 to S1, PSO angle (1 level above to 1 level below osteotomy level), sagittal plumb line (from center of C7 body to posterosuperior aspect of S1 body), and coronal plumb line (from center of C7 body to center of S1 body).2
Good clinical outcomes in the treatment of spinal disorders require careful attention to the alignment of the spine in the sagittal plane.7,8 When evaluating the preoperative radiographs, we measured and documented pelvic parameters. Figure 1A shows how pelvic incidence was determined. We measured this as the angle between a line drawn from the center of the S1 endplate to the center of the femoral head and the perpendicular off the S1 endplate. Figure 1B shows pelvic tilt as determined by the angle between a line drawn from the center of S1 to the femoral head and a vertical line originating from the center of the femoral head. Figure 1C shows the sacral slope, which we measured as the angle between a line drawn parallel to the endplate of S1 and its intersection with a horizontal line.
Surgical Technique
The overall surgical technique for PSO has been well described.4,5 Here we describe the “outrigger” modification to osteotomy closure (Figures 2, 3).
Most of our 17 cases were revisions. In these cases, new fixation points are first established. All fixation points that will be needed for the final fusion are placed. If a pedicle above or below the osteotomy level is not suitable for a screw, it can be skipped.
Wide decompression of the involved level is performed from pedicle to pedicle, ensuring that the nerve roots are completely decompressed. The dissection is then continued around the lateral wall of the vertebral body. While the neural elements are protected with gentle retraction, the pedicle and a portion of the posterior aspect of the vertebral body are removed with a combination of a rongeur and reverse-angle curettes. Resection of the vertebral body can be facilitated by attaching a short rod to the pedicle screws on either side of the osteotomy level and using it to provide gentle distraction.
Once sufficient bone has been removed to close the osteotomy, short rods are placed in the pedicle screws in the level above and the level below the osteotomy site. These rods are attached with offset connectors that allow the rods to be placed lateral to the screws. Before the surgical procedure is started, the patient is positioned on 2 sets of posts separated by the break in the table. The break in the table allows flexion to accommodate the preoperative kyphosis and allows hyperextension to help close the osteotomy site. Now, with the osteotomy site ready for closure, the table is gradually positioned in extension along with a combination of posterior pressure and compression between the pedicle screws above and below the osteotomy. Once the osteotomy is adequately compressed, the short rods are tightened, holding the osteotomy in good position. With the osteotomy held by the short rods and table positioning, decompression of the neural elements is confirmed and hemostasis obtained.
Final instrumentation is then performed with long rods that can bypass the osteotomized levels, allowing for simpler contouring. If desired, a cross connector can be placed between the long rod of the fusion construct and the short rod holding the osteotomy. The rest of the fusion procedure is completed in standard fashion with at least 1 subfascial drain.
Results
Our 17 patients’ results are summarized in the Table. Mean sagittal plumb line improved from 17.7 cm (range, 5.9 to 29 cm) before surgery to 4.5 cm (range, –0.2 to 12.9 cm) after surgery, for a mean improvement of 13.2 cm. At final follow-up, mean sagittal plumb line was 5.1 cm (range, –1.4 to 10.2 cm).
Mean lumbar lordosis improved from 10° (range, –14° to 34°) before surgery to 49° (range, 36° to 63°) after surgery, for a mean improvement of 39°. Mean PSO angle improved from 3° (range, –36° to 23°) before surgery to 41° (range, 25° to 65°) after surgery, for a mean improvement of 38°. At final follow-up, mean lumbar lordosis remained at 47° (range, 26° to 64°), and mean PSO angle was 39° (range, 24° to 59°).
Mean thoracic kyphosis improved from 18° (range, –8° to 52°) before surgery to 30° (range, 3° to 58°) after surgery, for a mean improvement of 12°. At final follow-up, mean thoracic kyphosis was 31° (range, 2° to 57°).
Fourteen patients did not have complications during the study period. Of the 3 patients with complications, 1 had an early infection, treated effectively with irrigation and débridement and intravenous antibiotics; 1 had a late deep infection, treated with multiple débridements, hardware removal, and, eventually, suppressive antibiotics; and 1 had cauda equina syndrome (caused by extensive scar tissue on the dura, which buckled with restoration of lordosis leading to cord compression), treated with duraplasty, which resulted in full neurologic recovery.
Discussion
In the present series of patients, the described technique for facilitating PSO for correction of sagittal imbalance was effective, and complications were similar to those previously reported.
The benefit of the outrigger construct is that it allows controlled compression of the osteotomy site and can be left in place at time of final instrumentation, locking in compression and correction. Other techniques involve removing the temporary rod and replacing it with final instrumentation4,5—an extra step that complicates instrumentation of the additional levels of the fusion construct and possibly adds pedicle screw stress and contributes to loosening when the new rod is reduced to the pedicle screw. The final long rod construct can bypass the osteotomy levels and allow for simpler instrumentation.
Mean age was 58 years in this series versus 52.4 years in the series reported by Bridwell and colleagues.2 Given the higher mean age of our patients, though no objective measures of bone quality were available, this technique is likely applicable to patients with poor bone quality.
The complications we have reported are in line with those reported in previous series, and maintenance of radiographic parameters at final follow-up indicates that this osteotomy technique allows for solid fusion constructs.
The outrigger technique for controlling PSO closure is an effective method that simplifies instrumentation during a complex revision case.
Pedicle subtraction osteotomies (PSOs) have been used in the treatment of multiple spinal conditions involving a fixed sagittal imbalance, such as degenerative scoliosis, idiopathic scoliosis, posttraumatic deformities, iatrogenic flatback syndrome, and ankylosing spondylitis. The procedure was first described by Thomasen1 for the treatment of ankylosing spondylitis. More recently, multiple centers have reported the expanded use and good success of PSO in the treatment of fixed sagittal imbalance of other etiologies.2,3 According to Bridwell and colleagues,2 lumbar lordosis can be increased 34.1°, and sagittal plumb line can be improved 13.5 cm.
PSO is a complex, extensive surgery most often performed in the revision setting. Multiple authors have described the technique for PSO.4,5 There are significant technical challenges and many complications, including neurologic deficits, pseudarthrosis of adjacent levels, and wound infections.6 Short-term challenges include a large loss of blood, 2.4 L on average, according to Bridwell and colleagues.6 Time of closure of the osteotomy gap is a crucial point in the surgery. Blood loss, often large, slows only after the gap is closed and stabilized.
In this article, we describe a technique in which an additional rod or pedicle screw construct is used at the periosteotomy levels to close the osteotomy gap during PSO and simplify subsequent instrumentation. In addition, we report our experience with the procedure.
Materials and Methods
Seventeen consecutive patients (mean age, 58 years; range, 12-81 years) with fixed sagittal imbalance were treated with lumbar PSO. The indication in all cases was flatback syndrome after previous spinal surgery. Mean follow-up was 13 months. Mean number of prior surgeries was 3. Thirteen PSOs were performed at L3, and 4 were performed at L2.
Radiographic data were collected from before surgery, in the immediate postoperative period, and at final follow-up. All the radiographs were standing films. Established radiographic parameters were measured: thoracic kyphosis from T5 to T12, lumbar lordosis from L1 to S1, PSO angle (1 level above to 1 level below osteotomy level), sagittal plumb line (from center of C7 body to posterosuperior aspect of S1 body), and coronal plumb line (from center of C7 body to center of S1 body).2
Good clinical outcomes in the treatment of spinal disorders require careful attention to the alignment of the spine in the sagittal plane.7,8 When evaluating the preoperative radiographs, we measured and documented pelvic parameters. Figure 1A shows how pelvic incidence was determined. We measured this as the angle between a line drawn from the center of the S1 endplate to the center of the femoral head and the perpendicular off the S1 endplate. Figure 1B shows pelvic tilt as determined by the angle between a line drawn from the center of S1 to the femoral head and a vertical line originating from the center of the femoral head. Figure 1C shows the sacral slope, which we measured as the angle between a line drawn parallel to the endplate of S1 and its intersection with a horizontal line.
Surgical Technique
The overall surgical technique for PSO has been well described.4,5 Here we describe the “outrigger” modification to osteotomy closure (Figures 2, 3).
Most of our 17 cases were revisions. In these cases, new fixation points are first established. All fixation points that will be needed for the final fusion are placed. If a pedicle above or below the osteotomy level is not suitable for a screw, it can be skipped.
Wide decompression of the involved level is performed from pedicle to pedicle, ensuring that the nerve roots are completely decompressed. The dissection is then continued around the lateral wall of the vertebral body. While the neural elements are protected with gentle retraction, the pedicle and a portion of the posterior aspect of the vertebral body are removed with a combination of a rongeur and reverse-angle curettes. Resection of the vertebral body can be facilitated by attaching a short rod to the pedicle screws on either side of the osteotomy level and using it to provide gentle distraction.
Once sufficient bone has been removed to close the osteotomy, short rods are placed in the pedicle screws in the level above and the level below the osteotomy site. These rods are attached with offset connectors that allow the rods to be placed lateral to the screws. Before the surgical procedure is started, the patient is positioned on 2 sets of posts separated by the break in the table. The break in the table allows flexion to accommodate the preoperative kyphosis and allows hyperextension to help close the osteotomy site. Now, with the osteotomy site ready for closure, the table is gradually positioned in extension along with a combination of posterior pressure and compression between the pedicle screws above and below the osteotomy. Once the osteotomy is adequately compressed, the short rods are tightened, holding the osteotomy in good position. With the osteotomy held by the short rods and table positioning, decompression of the neural elements is confirmed and hemostasis obtained.
Final instrumentation is then performed with long rods that can bypass the osteotomized levels, allowing for simpler contouring. If desired, a cross connector can be placed between the long rod of the fusion construct and the short rod holding the osteotomy. The rest of the fusion procedure is completed in standard fashion with at least 1 subfascial drain.
Results
Our 17 patients’ results are summarized in the Table. Mean sagittal plumb line improved from 17.7 cm (range, 5.9 to 29 cm) before surgery to 4.5 cm (range, –0.2 to 12.9 cm) after surgery, for a mean improvement of 13.2 cm. At final follow-up, mean sagittal plumb line was 5.1 cm (range, –1.4 to 10.2 cm).
Mean lumbar lordosis improved from 10° (range, –14° to 34°) before surgery to 49° (range, 36° to 63°) after surgery, for a mean improvement of 39°. Mean PSO angle improved from 3° (range, –36° to 23°) before surgery to 41° (range, 25° to 65°) after surgery, for a mean improvement of 38°. At final follow-up, mean lumbar lordosis remained at 47° (range, 26° to 64°), and mean PSO angle was 39° (range, 24° to 59°).
Mean thoracic kyphosis improved from 18° (range, –8° to 52°) before surgery to 30° (range, 3° to 58°) after surgery, for a mean improvement of 12°. At final follow-up, mean thoracic kyphosis was 31° (range, 2° to 57°).
Fourteen patients did not have complications during the study period. Of the 3 patients with complications, 1 had an early infection, treated effectively with irrigation and débridement and intravenous antibiotics; 1 had a late deep infection, treated with multiple débridements, hardware removal, and, eventually, suppressive antibiotics; and 1 had cauda equina syndrome (caused by extensive scar tissue on the dura, which buckled with restoration of lordosis leading to cord compression), treated with duraplasty, which resulted in full neurologic recovery.
Discussion
In the present series of patients, the described technique for facilitating PSO for correction of sagittal imbalance was effective, and complications were similar to those previously reported.
The benefit of the outrigger construct is that it allows controlled compression of the osteotomy site and can be left in place at time of final instrumentation, locking in compression and correction. Other techniques involve removing the temporary rod and replacing it with final instrumentation4,5—an extra step that complicates instrumentation of the additional levels of the fusion construct and possibly adds pedicle screw stress and contributes to loosening when the new rod is reduced to the pedicle screw. The final long rod construct can bypass the osteotomy levels and allow for simpler instrumentation.
Mean age was 58 years in this series versus 52.4 years in the series reported by Bridwell and colleagues.2 Given the higher mean age of our patients, though no objective measures of bone quality were available, this technique is likely applicable to patients with poor bone quality.
The complications we have reported are in line with those reported in previous series, and maintenance of radiographic parameters at final follow-up indicates that this osteotomy technique allows for solid fusion constructs.
The outrigger technique for controlling PSO closure is an effective method that simplifies instrumentation during a complex revision case.
1. Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop. 1985;(194):142-152.
2. Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85(3):454-463.
3. Berven SH, Deviren V, Smith JA, Emami A, Hu SS, Bradford DS. Management of fixed sagittal plane deformity: results of the transpedicular wedge resection osteotomy. Spine. 2001;26(18):2036-2043.
4. Bridwell KH, Lewis SJ, Rinella A, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. Surgical technique. J Bone Joint Surg Am. 2004;86(suppl 1):44-50.
5. Wang MY, Berven SH. Lumbar pedicle subtraction osteotomy. Neurosurgery. 2007;60(2 suppl 1):ONS140-ONS146.
6. Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine. 2003;28(18):2093-2101.
7. Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87(2):260-267.
8. Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine. 2009;34(17):1828-1833.
1. Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop. 1985;(194):142-152.
2. Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surg Am. 2003;85(3):454-463.
3. Berven SH, Deviren V, Smith JA, Emami A, Hu SS, Bradford DS. Management of fixed sagittal plane deformity: results of the transpedicular wedge resection osteotomy. Spine. 2001;26(18):2036-2043.
4. Bridwell KH, Lewis SJ, Rinella A, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. Surgical technique. J Bone Joint Surg Am. 2004;86(suppl 1):44-50.
5. Wang MY, Berven SH. Lumbar pedicle subtraction osteotomy. Neurosurgery. 2007;60(2 suppl 1):ONS140-ONS146.
6. Bridwell KH, Lewis SJ, Edwards C, et al. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine. 2003;28(18):2093-2101.
7. Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignment and balance of the spine in asymptomatic subjects. J Bone Joint Surg Am. 2005;87(2):260-267.
8. Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine. 2009;34(17):1828-1833.
Targeting a New Safe Zone: A Step in the Development of Patient-Specific Component Positioning for Total Hip Arthroplasty
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
Postoperative dislocation remains a common complication of primary total hip arthroplasties (THAs), affecting less than 1% to more than 10% in reported series.1,2 In large datasets for modern implants, the incidence of dislocation is 2% to 4%.3,4 Given that more than 200,000 THAs are performed in the United States each year,5 these low percentages represent a large number of patients. The multiplex patient variables that affect THA stability include age, sex, body mass index (BMI), and comorbid conditions.6-8 Surgical approach, restoration of leg length and femoral offset, femoral head size, and component positioning are also important surgical factors that can increase or decrease the incidence of dislocation.3,8,9 In particular, appropriate acetabular component orientation is crucial; surgeons can control this factor and thereby limit the occurrence of dislocation.10 Furthermore, acetabular malpositioning can increase the risk of liner fractures and accelerate bearing-surface wear.11-14
To minimize the risk of postoperative dislocation, surgeons traditionally have targeted the Lewinnek safe zone, with its mean (SD) inclination of 40° (10°) and mean (SD) anteversion of 15° (10°), for acetabular component orientation.15 However, the applicability of this target zone to preventing hip instability using modern implant designs, components, and surgical techniques remains unknown. Achieving acetabular orientation based on maximizing range of motion (ROM) before impingement may be optimal, with anteversion from 20° to 30° and inclination from 40° to 45°.16,17 Furthermore, mean (SD) native acetabular anteversion ranges from 21.3° (6.2°) for men to 24.6° (6.6°) for women.18 Placing THA acetabular components near the native range for anteversion may best provide impingement-free ROM and thus optimize THA stability,16,19 but this has not been proved in a clinical study.
Early dislocation is typically classified as occurring within 6 months after surgery,9 with almost 80% of dislocations occurring within 3 months after surgery.10 Surgeon-specific factors, such as acetabular component positioning, are thought to have a predominant effect on dislocations in the early postoperative period.10 Computer-assisted surgery (CAS), such as imageless navigation, is more accurate than conventional methods for acetabular component placement,20-23 but the clinical relevance of improving accuracy for acetabular component placement has not been shown with respect to altering patient outcomes.23
We conducted a study in a large single-surgeon patient cohort to determine the incidence of early postoperative dislocation with target anteversion increased to 25°, approximating mean native acetabular anteversion.16,19 In addition, we sought to determine the accuracy of imageless navigation in achieving target acetabular component placement.
Materials and Methods
After obtaining institutional review board approval for this retrospective clinical study, we reviewed 671 consecutive cases of primary THA performed by a single surgeon using an imageless CAS system (AchieveCAS; Smith & Nephew, Memphis, Tennessee) between July 2006 and October 2012. THAs were excluded if a metal-on-metal bearing surface was used, if an adequate 6-week postoperative supine anteroposterior (AP) pelvis radiograph was unavailable, or if 6-month clinical follow-up findings were not available (Figure 1). The quality of AP radiographs was deemed poor if they were not centered on the symphysis pubis and if the sacrococcygeal joint was not centered over the symphysis pubis. After exclusion criteria were applied, 553 arthroplasties (479 patients) with a mean (SD) follow-up of 2.4 (1.4) years remained. Perioperative demographic data and component sizes are listed in Table 1.
During surgery, the anterior pelvic plane, defined by the anterior-superior iliac spines and pubic tubercle, was registered with the CAS system with the patient in the supine position. THA was performed with the patient in the lateral decubitus position using a posterolateral technique. For all patients, the surgeon used a hemispherical acetabular component (R3 Acetabular System; Smith & Nephew); bearings that were either metal on highly cross-linked polyethylene (XLPE) or Oxinium (Smith & Nephew) on XLPE; and neutral XLPE acetabular inserts. The goals for acetabular inclination and anteversion were 40° and 25°, respectively, with ±10° each for the target zone. The CAS system was used to adjust target anteversion for sagittal pelvic tilt.24 Uncemented femoral components were used for all patients, and the goal for femoral component anteversion was 15°. Transosseous repair of the posterior capsule and short external rotators was performed after component implantation.25
On each 6-week postoperative radiograph, acetabular orientation was measured with Ein-Bild-Röntgen-Analyse (EBRA; University of Innsbruck, Austria) software, which provides a validated method for measuring acetabular inclination and anteversion on supine AP pelvis radiographs.10,26 Pelvic boundaries were delineated with grid lines defining pelvic position. Reference points around the projections of the prosthetic femoral head, the hemispherical cup, and the rim of the cup were marked (Figure 2). EBRA calculated radiographic inclination and anteversion of the acetabular component based on the spatial position of the cup center in relation to the plane of the radiograph and the pelvic position.26
Charts were reviewed to identify patients with early postoperative dislocations, as well as dislocation timing, recurrence, and other characteristics. We defined early dislocation as instability occurring within 6 months after surgery. Revision surgery for instability was also identified.
For the statistical analysis, orientation error was defined as the absolute value of the difference between target orientation (40° inclination, 25° anteversion) and radiographic measurements. Repeated-measures multiple regression with the generalized estimating equations approach was used to identify baseline patient characteristics (age, sex, BMI, primary diagnosis, laterality) associated with component positioning outside of our targeted ranges for inclination and anteversion. Fisher exact tests were used to examine the relationship between dislocation and component placement in either the Lewinnek safe zone or our targeted zone. All tests were 2-sided with a significance level of .05. All analyses were performed with SAS for Windows 9.3 (SAS Institute, Cary, North Carolina).
Results
Mean (SD) acetabular inclination was 42.2° (4.9°) (range, 27.6°-65.0°), with a mean (SD) orientation error of 4.2° (3.4°) (Figure 3A). Mean (SD) anteversion was 23.9° (6.5°) (range, 6.2°-48.0°), with a mean (SD) orientation error of 5.2° (4.1°) (Figure 3B). Components were placed outside the Lewinnek safe zone for inclination or anteversion in 46.5% of cases and outside the target zone in 17.7% of cases (Figure 4). Variation in acetabular anteversion alone accounted for 67.3% of target zone outliers (Table 2). Only 0.9% of components were placed outside the target ranges for both inclination and anteversion.
Regression analysis was performed separately for inclination and anteversion to determine the risk factors for placing the acetabular component outside the target orientation ranges. Only higher BMI was associated with malposition with respect to inclination (hazard ratio [HR], 1.059; 95% confidence interval [CI], 1.011-1.111; P = .017). Of obese patients with inclination outside the target range, 90.9% had an inclination angle of more than 50°. Associations between inclination outside the target range and age (P = .769), sex (P = .217), preoperative diagnosis (P > .99), and laterality (P = .106) were statistically insignificant. Only female sex was associated with position of the acetabular component outside the target range for anteversion (HR, 1.871; 95% CI, 1.061-3.299; P = .030). Of female patients with anteversion outside the target range, 70.0% had anteversion of less than 15°. Associations between anteversion outside the target range and age (P = .762), BMI (P = .583), preoperative diagnosis (P > .99), and laterality (P = .235) were statistically insignificant.
Six THAs (1.1%) in 6 patients experienced dislocation within 6 months after surgery (Table 3); mean (SD) time of dislocation was 58.3 (13.8) days after surgery. There was no relationship between dislocation incidence and component placement in the Lewinnek zone (P = .224) or our target zone (P = .287). Of the dislocation cases, 50% involved female patients, and 50% involved right hips. Mean (SD) age of these patients was 53.3 (7.6) years. Mean (SD) BMI was 25.4 (0.9) kg/m2. Osteoarthritis was the primary diagnosis for all patients with early dislocation; 32- or 36-mm femoral heads were used in these cases. Two patients had acetabular components placed outside of our target zone. One patient, who had abnormal pelvic obliquity and sagittal tilt from scoliosis (Figures 5A, 5B), had an acetabular component placed outside both the target zone and the Lewinnek safe zone. Mean (SD) acetabular inclination was 39.8° (3.6°), and mean (SD) anteversion was 21.8° (7.3°) (Figure 5C). Two dislocations resulted from trauma, 1 dislocation was related to hyperlaxity, 1 patient had cerebral palsy, and 1 patient had no evident predisposing risk factors. Three patients (0.54%) had multiple episodes of instability requiring revision during the follow-up period.
Discussion
To our knowledge, this study represents the largest cohort of primary THAs performed with an imageless navigation system. Our results showed that increasing targeted acetabular anteversion to 25° using a posterolateral surgical approach and modern implants resulted in a 1.1% incidence of early dislocation and a 0.54% incidence of recurrent instability requiring reoperation. Of the patients with a dislocation, only 1 did not experience trauma and did not have a risk factor for dislocation. Only 1 patient with a dislocation had acetabular components positioned outside both the target zone and the Lewinnek safe zone. The acetabular component was placed within the target zone in 82.3% of cases in which the imageless navigation system was used. In our cohort, BMI was the only risk factor for placement of the acetabular component outside our target range for inclination, and sex was associated with components outside the target range for anteversion.
Early dislocation after THA is often related to improper implant orientation, inadequate restoration of offset and myofascial tension, and decreased femoral head–neck ratio.8 Although dislocation rates in the literature vary widely,1,2 Medicare data suggest that the rate for the first 6 months after surgery can be as high as 4.21%.3,4 Although use of femoral heads with a diameter of 32 mm or larger may decrease this rate to 2.14%,3 accurate acetabular component orientation helps prevent postoperative dislocation.10 Using an imageless navigation system to target 25° of anteversion and 40° of inclination resulted in an early-dislocation rate about 49% less than the rate in a Medicare population treated with similar, modern implants.3
Callanan and colleagues11 found that freehand techniques were inaccurate for acetabular positioning in up to 50% of cases, and several studies have demonstrated that imageless navigation systems were more accurate than conventional guides.20,21,27-29 Higher BMI has been implicated as a risk factor for acetabular malpositioning in several studies of the accuracy of freehand techniques11 and imageless navigation techniques.23,30 Soft-tissue impediment to the component insertion handle poses a risk of increased inclination and inadequate anteversion, regardless of method used (conventional, CAS). When the acetabular component is placed freehand in obese patients, it is difficult to judge the position of the pelvis on the operating room table. For imageless navigation, a larger amount of adipose tissue over bony landmarks may limit the accuracy of anterior pelvic plane registration.30 Sex typically is not cited as a risk factor for inaccurate acetabular component positioning. We speculate that omitted-variable bias may explain the observed association between female sex and anteversion. For example, changes in postoperative pelvic tilt alter apparent anteversion on plain radiographs,31-34 but preoperative and postoperative sagittal pelvic tilt was not recorded in this study.
The proper position of the acetabular component has been debated.15,16,35,36 Although it is generally agreed that inclination of 40° ± 10° balances ROM, stability, and bearing-surface wear,12,13,15,16 proposed targets for anteversion vary widely, from 0° to 40°.35,36 Patel and colleagues16 formulated computer models based on cadaveric specimens to determine that THA impingement was minimized when the acetabular component was placed to match the native anteversion of the acetabulum.In their study model, 20° of anteversion paralleled native acetabular orientation. Tohtz and colleagues18 reviewed computed tomography scans of 144 female hips and 192 male hips and found that mean (SD) anteversion was 24.6° (6.6°) for women and 21.3° (6.2°) for men. Whether native anatomy is a valid reference for acetabular anteversion is controversial,19 and definitive recommendations for target anteversion cannot be made, as the effect of acetabular anteversion on the wear of various bearing materials is unknown.14 Yet, as with inclination, ideal anteversion is likely a compromise between maximizing impingement-free ROM and minimizing wear.
The present study had several limitations. A single-surgeon patient series was reviewed retrospectively, and there was no control group. We determined the incidence only of early dislocation, and 5.3% of THAs that were not metal-on-metal were either lost to follow-up or had inadequate radiographs. However, of the patients excluded for inadequate radiographs, none had an early dislocation. The effects of our surgical techniques on long-term outcomes, bearing wear, and dislocation are unknown. We were not able to comment on the direction of dislocation for any of the 6 patients with early dislocation, as all dislocations were reduced at facilities other than our hospital. Therefore, we cannot determine whether increasing acetabular anteversion resulted in a larger number of anterior versus posterior dislocations.15
We did not use CAS to place any of the femoral components. Therefore, we could not accurately target combined anteversion, defined as the sum of acetabular and femoral version, which may be an important determinant of THA stability.28 Although restoration of femoral offset and leg length is important in preventing THA dislocation,8 the CAS techniques used did not influence these parameters, and they were not measured.
As an imageless navigation system was used, there were no preoperative axial images, which could have been used to assess native acetabular orientation. This limited our assessment with respect to matching each patient’s natural anteversion. Imageless navigation, which references only the anterior pelvic plane, may not be reliable in patients with excessive sagittal pelvic tilt.37 Furthermore, changes in the functional position of the pelvis from supine to sitting to standing were not accounted for, and changes in sagittal tilt between these positions can be significant.38 Changes in sagittal pelvic tilt affect measurement of acetabular anteversion on plain radiographs, with anterior tilt reducing apparent anteversion and posterior tilt increasing it.32,34 Although postoperative computed tomography is the gold standard for assessing acetabular component orientation, EBRA significantly reduces errors of measurement on plain radiographs.10 Some variability in measured anteversion may be explained by our surgical technique. In particular, if the cup was uncovered anteriorly, additional anteversion was usually accepted during surgery to minimize anterior impingement and limit the risk of iliopsoas tendonitis.16,39
Our study results suggested that increasing target acetabular anteversion to 25° may reduce the incidence of early postoperative instability relative to rates reported in the literature. Despite the higher accuracy of component placement with an imageless navigation system, dislocations occurred in patients with acetabular components positioned in our target zone and in the historical safe zone. These dislocations support the notion that there likely is no absolute safe range for acetabular component positioning, as THA stability depends on many factors. Ideal targets for implant orientation for acetabulum and femur may be patient-specific.16,19 Investigators should prospectively evaluate patient-specific THA component positioning and determine its effect on postoperative dislocation and bearing-surface wear. As specific implant targets are further defined, tools that are more precise and accurate than conventional techniques will be needed to achieve goal component positioning. Our study results confirmed that imageless navigation is an accurate method for achieving acetabular orientation targets.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
1. Kwon MS, Kuskowski M, Mulhall KJ, Macaulay W, Brown TE, Saleh KJ. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop. 2006;(447):34-38.
2. Sierra RJ, Raposo JM, Trousdale RT, Cabanela ME. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop. 2005;(441):262-267.
3. Malkani AL, Ong KL, Lau E, Kurtz SM, Justice BJ, Manley MT. Early- and late-term dislocation risk after primary hip arthroplasty in the Medicare population. J Arthroplasty. 2010;25(6 suppl):21-25.
4. Berry DJ, von Knoch M, Schleck CD, Harmsen WS. Effect of femoral head diameter and operative approach on risk of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87(11):2456-2463.
5. Nho SJ, Kymes SM, Callaghan JJ, Felson DT. The burden of hip osteoarthritis in the United States: epidemiologic and economic considerations. J Am Acad Orthop Surg. 2013;21(suppl 1):S1-S6.
6. Sadr Azodi O, Adami J, Lindstrom D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. 2008;79(1):141-147.
7. Conroy JL, Whitehouse SL, Graves SE, Pratt NL, Ryan P, Crawford RW. Risk factors for revision for early dislocation in total hip arthroplasty. J Arthroplasty. 2008;23(6):867-872.
8. Morrey BF. Difficult complications after hip joint replacement. Dislocation. Clin Orthop. 1997;(344):179-187.
9. Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg. 2012;132(7):1031-1036.
10. Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87(6):762-769.
11. Callanan MC, Jarrett B, Bragdon CR, et al. The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop. 2011;469(2):319-329.
12. Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34(1):19-26.
13. Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259-2265.
14. Esposito CI, Walter WL, Roques A, et al. Wear in alumina-on-alumina ceramic total hip replacements: a retrieval analysis of edge loading. J Bone Joint Surg Br. 2012;94(7):901-907.
15. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
16. Patel AB, Wagle RR, Usrey MM, Thompson MT, Incavo SJ, Noble PC. Guidelines for implant placement to minimize impingement during activities of daily living after total hip arthroplasty. J Arthroplasty. 2010;25(8):1275-1281.e1.
17. Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22(4):815-821.
18. Tohtz SW, Sassy D, Matziolis G, Preininger B, Perka C, Hasart O. CT evaluation of native acetabular orientation and localization: sex-specific data comparison on 336 hip joints. Technol Health Care. 2010;18(2):129-136.
19. Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95(22):e172.
20. Nogler M, Kessler O, Prassl A, et al. Reduced variability of acetabular cup positioning with use of an imageless navigation system. Clin Orthop. 2004;(426):159-163.
21. Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51-56.
22. Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop. 2004;(426):174-179.
23. Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29(4):786-791.
24. Babisch JW, Layher F, Amiot LP. The rationale for tilt-adjusted acetabular cup navigation. J Bone Joint Surg Am. 2008;90(2):357-365.
25. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop. 1998;(355):224-228.
26. Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech. 1995;28(10):1225-1236.
27. Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89(3):494-499.
28. Dorr LD, Malik A, Wan Z, Long WT, Harris M. Precision and bias of imageless computer navigation and surgeon estimates for acetabular component position. Clin Orthop. 2007;(465):92-99.
29. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15-21.
30. Hohmann E, Bryant A, Tetsworth K. Anterior pelvic soft tissue thickness influences acetabular cup positioning with imageless navigation. J Arthroplasty. 2012;27(6):945-952.
31. Nguyen AD, Shultz SJ. Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007;37(7):389-398.
32. Malik A, Wan Z, Jaramaz B, Bowman G, Dorr LD. A validation model for measurement of acetabular component position. J Arthroplasty. 2010;25(5):812-819.
33. Tannast M, Murphy SB, Langlotz F, Anderson SE, Siebenrock KA. Estimation of pelvic tilt on anteroposterior X-rays—a comparison of six parameters. Skeletal Radiol. 2006;35(3):149-155.
34. Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield Award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop. 2009;467(1):43-49.
35. McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop. 1990;(261):159-170.
36. Kummer FJ, Shah S, Iyer S, DiCesare PE. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-513.
37. Lin F, Lim D, Wixson RL, Milos S, Hendrix RW, Makhsous M. Limitations of imageless computer-assisted navigation for total hip arthroplasty. J Arthroplasty. 2011;26(4):596-605.
38. Lazennec JY, Riwan A, Gravez F, et al. Hip spine relationships: application to total hip arthroplasty. Hip Int. 2007;17(suppl 5):S91-S104.
39. Trousdale RT, Cabanela ME, Berry DJ. Anterior iliopsoas impingement after total hip arthroplasty. J Arthroplasty. 1995;10(4):546-549.
Computer Navigation and Robotics for Total Knee Arthroplasty
Total knee arthroplasty (TKA) is a good surgical option to relieve pain and improve function in patients with osteoarthritis. The goal of surgery is to achieve a well-aligned prosthesis with well-balanced ligaments in order to minimize wear and improve implant survival. Overall, 82% to 89% of patients are satisfied with their outcomes after TKA, with good 10- to 15-year implant survivorship; however, there is still a subset of patients that are unsatisfied. In many cases, patient dissatisfaction is attributed to improper component alignment.1-3 Over the past decade, computer navigation and robotics have been introduced to control surgical variables so as to gain greater consistency in implant placement and postoperative component alignment.
Computer-assisted navigation tools were introduced not only to improve implant alignment but, more importantly, to optimize clinical outcomes. Most studies have demonstrated that the use of navigation is associated with fewer radiographic outliers after TKA.4 Various studies have compared radiographic results of navigated TKA with results of TKA using standard instrumentation.4-7 While long-term studies are necessary, short-term follow-up has shown that computer-assisted TKA can improve alignment, especially in patients with severe deformity.8-10 Currently, there is no definitive consensus that computer-assisted TKA leads to significantly better component alignment or postoperative outcomes due to the fact that many studies are limited by study design or small cohorts. However, the currently published articles support better component alignment and clinical outcomes with computer-assisted TKA. While some argue that the use of computer-assisted surgery is dependent on the user’s experience, computer-assisted surgery can assist less-experienced surgeons to reliably achieve good midterm outcomes with a low complication rate.8,11 Various studies have looked at computer-assisted TKA at midterm follow-up, with no significant differences in clinical outcome between navigated and traditional techniques. However, long-term studies showing the benefits of computer navigation are beginning to emerge. For example, de Steiger and colleagues12 recently found that computer-assisted TKA reduced the overall revision rate for loosening after TKA in patients less than 65 years of age.
While surgical navigation helps improve implant planning, robotic tools have emerged as a tool to help refine surgical execution. Coupled with surgical navigation tools, robotic control of surgical gestures may further enhance precision in implant placement and/or enable novel implant design features. At present, robotic techniques are increasingly used in unicompartmental knee arthroplasty (UKA) and TKA.13 Studies have demonstrated that the robotic tool is 3 times more accurate with 3 times less variability than conventional techniques in UKA.14 The utility of robotic tools for TKA remains unclear. Robotic-driven automatic cutting guides have been shown to reduce time and improve accuracy compared with navigation guides in femoral TKA cutting procedures in a cadaveric model.15 However, robotic-enabled TKA procedures are poorly described at present, and the clinical implications of their proposed improved precision remain unclear.
Computer navigation and robotic tools in TKA hold the promise of enhanced control of surgical variables that influence clinical outcome. The variables that may be impacted by these advanced tools include implant positioning, lower limb alignment, soft-tissue balance, and, potentially, implant design and fixation. At present, these tools have primarily been shown to improve lower limb alignment in TKA. The clinical impact of the enhanced control of this single surgical variable (lower limb alignment) has been muted in short-term and midterm studies. Future studies should be directed at understanding which surgical variable, or combination of variables, it is most essential to precisely control so as to positively impact clinical outcomes. ◾
1. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
3. Emmerson KP, Morgan CG, Pinder IM. Survivorship analysis of the Kinematic Stabilizer total knee replacement: a 10- to 14-year follow-up. J Bone Joint Surg Br. 1996;78(3):441-445.
4. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomized study. J Arthroplasty. 2014;29(12):2373-2377.
5. Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomized study. J Bone Joint Surg Br. 2003;85(6):830-835.
6. Hoffart HE, Langenstein E, Vasak N. A prospective study comparing the functional outcome of computer-assisted and conventional total knee replacement. J Bone Joint Surg Br. 2012;94(2):194-199.
7. Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplasty. 2014;29(9):1795-1802.
8. Huang TW, Peng KT, Huang KC, Lee MS, Hsu RW. Differences in component and limb alignment between computer-assisted and conventional surgery total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):2954-2961.
9. Lee CY, Lin SJ, Kuo LT, et al. The benefits of computer-assisted total knee arthroplasty on coronal alignment with marked femoral bowing in Asian patients. J Orthop Surg Res. 2014;9:122.
10. Hernandez-Vaquero D, Noriega-Fernandez A, Fernandez-Carreira JM, Fernandez-Simon JM, Llorens de los Rios J. Computer-assisted surgery improves rotational positioning of the femoral component but not the tibial component in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3127-3134.
11. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial [published online ahead of print March 14, 2015]. J Arthroplasty.
12. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
13. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
14. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
15. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
Total knee arthroplasty (TKA) is a good surgical option to relieve pain and improve function in patients with osteoarthritis. The goal of surgery is to achieve a well-aligned prosthesis with well-balanced ligaments in order to minimize wear and improve implant survival. Overall, 82% to 89% of patients are satisfied with their outcomes after TKA, with good 10- to 15-year implant survivorship; however, there is still a subset of patients that are unsatisfied. In many cases, patient dissatisfaction is attributed to improper component alignment.1-3 Over the past decade, computer navigation and robotics have been introduced to control surgical variables so as to gain greater consistency in implant placement and postoperative component alignment.
Computer-assisted navigation tools were introduced not only to improve implant alignment but, more importantly, to optimize clinical outcomes. Most studies have demonstrated that the use of navigation is associated with fewer radiographic outliers after TKA.4 Various studies have compared radiographic results of navigated TKA with results of TKA using standard instrumentation.4-7 While long-term studies are necessary, short-term follow-up has shown that computer-assisted TKA can improve alignment, especially in patients with severe deformity.8-10 Currently, there is no definitive consensus that computer-assisted TKA leads to significantly better component alignment or postoperative outcomes due to the fact that many studies are limited by study design or small cohorts. However, the currently published articles support better component alignment and clinical outcomes with computer-assisted TKA. While some argue that the use of computer-assisted surgery is dependent on the user’s experience, computer-assisted surgery can assist less-experienced surgeons to reliably achieve good midterm outcomes with a low complication rate.8,11 Various studies have looked at computer-assisted TKA at midterm follow-up, with no significant differences in clinical outcome between navigated and traditional techniques. However, long-term studies showing the benefits of computer navigation are beginning to emerge. For example, de Steiger and colleagues12 recently found that computer-assisted TKA reduced the overall revision rate for loosening after TKA in patients less than 65 years of age.
While surgical navigation helps improve implant planning, robotic tools have emerged as a tool to help refine surgical execution. Coupled with surgical navigation tools, robotic control of surgical gestures may further enhance precision in implant placement and/or enable novel implant design features. At present, robotic techniques are increasingly used in unicompartmental knee arthroplasty (UKA) and TKA.13 Studies have demonstrated that the robotic tool is 3 times more accurate with 3 times less variability than conventional techniques in UKA.14 The utility of robotic tools for TKA remains unclear. Robotic-driven automatic cutting guides have been shown to reduce time and improve accuracy compared with navigation guides in femoral TKA cutting procedures in a cadaveric model.15 However, robotic-enabled TKA procedures are poorly described at present, and the clinical implications of their proposed improved precision remain unclear.
Computer navigation and robotic tools in TKA hold the promise of enhanced control of surgical variables that influence clinical outcome. The variables that may be impacted by these advanced tools include implant positioning, lower limb alignment, soft-tissue balance, and, potentially, implant design and fixation. At present, these tools have primarily been shown to improve lower limb alignment in TKA. The clinical impact of the enhanced control of this single surgical variable (lower limb alignment) has been muted in short-term and midterm studies. Future studies should be directed at understanding which surgical variable, or combination of variables, it is most essential to precisely control so as to positively impact clinical outcomes. ◾
Total knee arthroplasty (TKA) is a good surgical option to relieve pain and improve function in patients with osteoarthritis. The goal of surgery is to achieve a well-aligned prosthesis with well-balanced ligaments in order to minimize wear and improve implant survival. Overall, 82% to 89% of patients are satisfied with their outcomes after TKA, with good 10- to 15-year implant survivorship; however, there is still a subset of patients that are unsatisfied. In many cases, patient dissatisfaction is attributed to improper component alignment.1-3 Over the past decade, computer navigation and robotics have been introduced to control surgical variables so as to gain greater consistency in implant placement and postoperative component alignment.
Computer-assisted navigation tools were introduced not only to improve implant alignment but, more importantly, to optimize clinical outcomes. Most studies have demonstrated that the use of navigation is associated with fewer radiographic outliers after TKA.4 Various studies have compared radiographic results of navigated TKA with results of TKA using standard instrumentation.4-7 While long-term studies are necessary, short-term follow-up has shown that computer-assisted TKA can improve alignment, especially in patients with severe deformity.8-10 Currently, there is no definitive consensus that computer-assisted TKA leads to significantly better component alignment or postoperative outcomes due to the fact that many studies are limited by study design or small cohorts. However, the currently published articles support better component alignment and clinical outcomes with computer-assisted TKA. While some argue that the use of computer-assisted surgery is dependent on the user’s experience, computer-assisted surgery can assist less-experienced surgeons to reliably achieve good midterm outcomes with a low complication rate.8,11 Various studies have looked at computer-assisted TKA at midterm follow-up, with no significant differences in clinical outcome between navigated and traditional techniques. However, long-term studies showing the benefits of computer navigation are beginning to emerge. For example, de Steiger and colleagues12 recently found that computer-assisted TKA reduced the overall revision rate for loosening after TKA in patients less than 65 years of age.
While surgical navigation helps improve implant planning, robotic tools have emerged as a tool to help refine surgical execution. Coupled with surgical navigation tools, robotic control of surgical gestures may further enhance precision in implant placement and/or enable novel implant design features. At present, robotic techniques are increasingly used in unicompartmental knee arthroplasty (UKA) and TKA.13 Studies have demonstrated that the robotic tool is 3 times more accurate with 3 times less variability than conventional techniques in UKA.14 The utility of robotic tools for TKA remains unclear. Robotic-driven automatic cutting guides have been shown to reduce time and improve accuracy compared with navigation guides in femoral TKA cutting procedures in a cadaveric model.15 However, robotic-enabled TKA procedures are poorly described at present, and the clinical implications of their proposed improved precision remain unclear.
Computer navigation and robotic tools in TKA hold the promise of enhanced control of surgical variables that influence clinical outcome. The variables that may be impacted by these advanced tools include implant positioning, lower limb alignment, soft-tissue balance, and, potentially, implant design and fixation. At present, these tools have primarily been shown to improve lower limb alignment in TKA. The clinical impact of the enhanced control of this single surgical variable (lower limb alignment) has been muted in short-term and midterm studies. Future studies should be directed at understanding which surgical variable, or combination of variables, it is most essential to precisely control so as to positively impact clinical outcomes. ◾
1. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
3. Emmerson KP, Morgan CG, Pinder IM. Survivorship analysis of the Kinematic Stabilizer total knee replacement: a 10- to 14-year follow-up. J Bone Joint Surg Br. 1996;78(3):441-445.
4. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomized study. J Arthroplasty. 2014;29(12):2373-2377.
5. Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomized study. J Bone Joint Surg Br. 2003;85(6):830-835.
6. Hoffart HE, Langenstein E, Vasak N. A prospective study comparing the functional outcome of computer-assisted and conventional total knee replacement. J Bone Joint Surg Br. 2012;94(2):194-199.
7. Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplasty. 2014;29(9):1795-1802.
8. Huang TW, Peng KT, Huang KC, Lee MS, Hsu RW. Differences in component and limb alignment between computer-assisted and conventional surgery total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):2954-2961.
9. Lee CY, Lin SJ, Kuo LT, et al. The benefits of computer-assisted total knee arthroplasty on coronal alignment with marked femoral bowing in Asian patients. J Orthop Surg Res. 2014;9:122.
10. Hernandez-Vaquero D, Noriega-Fernandez A, Fernandez-Carreira JM, Fernandez-Simon JM, Llorens de los Rios J. Computer-assisted surgery improves rotational positioning of the femoral component but not the tibial component in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3127-3134.
11. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial [published online ahead of print March 14, 2015]. J Arthroplasty.
12. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
13. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
14. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
15. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
1. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;(404):7-13.
3. Emmerson KP, Morgan CG, Pinder IM. Survivorship analysis of the Kinematic Stabilizer total knee replacement: a 10- to 14-year follow-up. J Bone Joint Surg Br. 1996;78(3):441-445.
4. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomized study. J Arthroplasty. 2014;29(12):2373-2377.
5. Sparmann M, Wolke B, Czupalla H, Banzer D, Zink A. Positioning of total knee arthroplasty with and without navigation support. A prospective, randomized study. J Bone Joint Surg Br. 2003;85(6):830-835.
6. Hoffart HE, Langenstein E, Vasak N. A prospective study comparing the functional outcome of computer-assisted and conventional total knee replacement. J Bone Joint Surg Br. 2012;94(2):194-199.
7. Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplasty. 2014;29(9):1795-1802.
8. Huang TW, Peng KT, Huang KC, Lee MS, Hsu RW. Differences in component and limb alignment between computer-assisted and conventional surgery total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):2954-2961.
9. Lee CY, Lin SJ, Kuo LT, et al. The benefits of computer-assisted total knee arthroplasty on coronal alignment with marked femoral bowing in Asian patients. J Orthop Surg Res. 2014;9:122.
10. Hernandez-Vaquero D, Noriega-Fernandez A, Fernandez-Carreira JM, Fernandez-Simon JM, Llorens de los Rios J. Computer-assisted surgery improves rotational positioning of the femoral component but not the tibial component in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3127-3134.
11. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial [published online ahead of print March 14, 2015]. J Arthroplasty.
12. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
13. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
14. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
15. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
Assessment of Scapular Morphology and Surgical Technique as Predictors of Notching in Reverse Shoulder Arthroplasty
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
Reverse shoulder arthroplasty (RSA) is a treatment option for a spectrum of diseases in shoulders with rotator cuff deficiency. There are distinct morphologic changes in the scapular and glenoid anatomy in patients with chronic rotator cuff tears.1 A muscular imbalance that occurs in the joint as a result of rotator cuff deficiency leads to morphologic changes that eliminate the compressive forces that hold the humeral head against the glenoid.2 RSA effectively stabilizes the glenohumeral joint in shoulders with deficient rotator cuffs.3,4 In early work, Grammont proposed that the glenosphere center of rotation should be medialized (concentric to the central axis of the metaglene or baseplate) and lowered.5 Although the medialized center of rotation in Grammont prostheses decreases shear forces and improves the deltoid lever arm, it also tends to result in mechanical impingement between the superomedial aspect of the humeral polyethylene insert and the scapular neck—so-called inferior scapular notching.6-9
Notching, which has been reported in 50% to 96% of patients who receive a Delta III prosthesis, typically appears within the first few months after surgery but may be seen as late as 14 months after surgery.5,10-12 Postmortem studies have shown that notching corresponds with erosion of the inferior pole of the glenoid and scapular neck, thought to be caused by the polyethylene cup of the implant.13 Although some studies have found that notching stabilizes after 1 year, others have shown notching progressing for up to 4 years after surgery.11,12,14 The clinical relevance of notching continues to be controversial, but notching has been associated with poorer clinical outcomes, polyethylene wear, and local osteolysis. Component loosening has also been reported with notching of grade 3 or more.8,10 Ultimately, there is concern that scapular notching could progress, ultimately leading to late glenoid loosening and potentially catastrophic failure.
Scapular anatomy has become an area of increased focus in rotator cuff disorders and in effects on RSA biomechanics.9 Recent reports have described important scapular morphology variations that suggest more individualized adjustments are needed during RSA.9,15 In addition, some investigators have reported that development of notching appears to depend on the height and inclination of the implanted glenoid component, where an inferior position of the glenosphere leads to less impingement and better range of motion.8,16 Simovitch and colleagues8 found the angle between the glenosphere and scapular neck and the craniocaudal position of the glenosphere to be highly correlated with inferior notching. They combined these 2 parameters into a predictive algorithm that provides a guideline (notching index, <35) for prevention of notching.
We conducted a study to evaluate the scapular notching index as a predictive tool and to consider other factors that may be associated with scapular notching occurring with use of Grammont reverse replacement systems. We hypothesized that patients with a notching index of less than 35 would not develop notching and that patients with an index of more than 35 would have increased incidence and severity of notching.
Materials and Methods
Patients treated with RSA for painful cuff tear arthropathy or irreparable rotator cuff tear with pseudoparesis (inability to actively elevate shoulder >90° in presence of free passive anterior elevation) were included in this retrospective review. All patients were treated between 2006 and 2010 by 1 of 2 established senior shoulder subspecialty surgeons. Patients treated with a Delta (DePuy Orthopaedics, Warsaw, Indiana) or an Aequalis (Tornier, Edina, Minnesota) reverse shoulder implant were included in the study. A standard polyethylene liner was used for all patients. These prostheses have the same neck shaft angle, 155º, as they have similar geometric designs, both based on the Grammont design—semiconstrained inverted with a fixed, lowered, medialized center of rotation. Standard instrumentation was used for all procedures. Patients were excluded if any nonstandard techniques or components were used (constrained or high-mobility liner, glenoid bone grafting). Patients who underwent revision for a previous reverse total arthroplasty, a total shoulder arthroplasty, or a hemiarthroplasty, or for treatment of acute fracture, posttraumatic deformity, or posttraumatic arthritis, were also excluded from our analyses. Minimum follow-up for study inclusion was 24 months.
All procedures were performed with the patient in the semi-beach-chair position and with use of a deltopectoral approach. The glenoid was prepared such that minimal reaming was needed to preserve the subchondral plate. The glenoid baseplate was positioned in the recommended inferior position to minimize notching and optimize functional outcomes.13 After surgery, all patients were managed with a simple soft immobilizer with or without a pillow with the arm at the patient’s side in internal rotation. Immediate passive mobilization was begun under the direction of physical therapists. Passive and active-assisted exercises were continued with gradual progression to independent activities of daily living at 6 weeks. Clinical evaluations were performed before and after surgery by the operating surgeon or independent research nurse. Active forward flexion, passive external rotation, strength, and visual analog scale (VAS) pain scores were reviewed and recorded. Case-specific complications were also reviewed.
Preoperative and postoperative anteroposterior radiographs were evaluated by 2 independent observers (attending surgeon, junior resident). Per standard technique, each radiograph was positioned horizontal to the scapular plane. Of the 91 patients, 66 had preoperative shoulder radiographs of acceptable quality, with complete visualization of scapular morphology. Radiographs were reviewed to measure the scapular neck angle (SNA), inferior scapular notching, prosthesis–scapular neck angle (PSNA), and peg glenoid rim distance (PGRD) (Figure 1). For the 66 patients with acceptable preoperative radiographs, SNA was determined by subtracting preoperative SNA from postoperative PSNA. Postoperative anteroposterior radiographs were used to classify degree of inferior scapular notching based on the Nerot grading scale (0-4). In addition, glenosphere overhang and glenosphere inclination were measured on postoperative radiographs.
The 91 shoulders were sorted into 2 groups based on degree of scapular notching: group 1, Nerot grade 0 (no inferior notching) and grade 1, and group 2, Nerot grades 2, 3, and 4. Group 1 had 37 patients with a size 36 glenosphere, 3 patients with size 38, and 8 patients with size 42; group 2 had 34 patients with a size 36 glenosphere, 1 patient with size 38, and 8 patients with size 42. All measurements were normalized to account for differences in glenosphere size. Groups 1 and 2 were compared on each radiographic parameter (inferior scapular notching, PSNA, PGRD, SNA).
Notching indexes were calculated ([PSNA × 0.13] + PGRD) and compared with the suggested index of 35.8 Simovitch and colleagues8 demonstrated that a notching index of more than 35 had 91% sensitivity and 88% specificity in predicting inferior notching, whereas a notching index of 35 or less avoided inferior notching 91% of the time. In this study, notching index was calculated for each patient, and then the mean values of groups 1 and 2 were compared (Table 1).
The effect of scapular notching and other individual radiographic parameters on outcomes was also evaluated with respect to forward flexion, external rotation, VAS pain score, complications, and external rotation lag sign. Mann-Whitney U test was used to test these variables; Spearman rank test was performed to determine correlation between each variable and scapular notching; logistic regression was used to explore the relationship of variables (PGRD, PSNA) as predictors of Nerot degree of inferior scapular notching, and postoperative complications; and independent-samples t test was used to determine group differences for each variable. For each investigation, the level of significance was set at P < .05. A biostatistician performed all statistical analyses using SPSS Version 19 (IBM, Armonk, New York).
Results
Our study cohort consisted of 91 shoulders. Mean follow-up was 41.8 months (range, 24.0-80.8 months). Seventy-five (82%) of the 91 shoulders developed scapular notching. Mean (SD) SNA on preoperative radiographs, used to assess preoperative scapular morphology, was 103.9° (14.5°). For all 91 shoulders, mean (SD) PSNA was 125.6° (16°), and mean (SD) PGRD was 16 (5.4) mm (Table 1). Inclination measurements were available for 86 patients. Mean (SD) inclination from 90° was 2.5° (10.3°) (range, 21°-30°). Mean (SD) SNA (postoperative PSNA minus preoperative SNA) for the 66 patients with acceptable preoperative radiographs was 24.3° (21.3°) (Table 1). Forty-eight of the 91 shoulders were placed in scapular notching group 1 (16 grade-0 shoulders, 32 grade-1 shoulders); the other 43 shoulders were placed in group 2 (33 grade-2 shoulders, 9 grade-3 shoulders, 1 grade-4 shoulder). Mean follow-up was 40 months for group 1 and 43 months for group 2.
There were no significant differences between groups 1 and 2 in SNA (102.8° vs 105.4°; P = .3), PGRD (15.4 vs 16.8 mm; P = . 47), or PSNA (125.8° vs 125.4°; P = .82) (Table 1). In addition, groups 1 and 2 had no significant differences (P > .05) in glenoid overhang and glenosphere inclination (other possible factors influencing notching).
Mean (SD) notching index was 31.8 (4.4) for group 1 and 33.1 (7.2) for group 2. These values were not significantly different (P = .29) (Table 1, Figure 2).8 Each was below the recommended threshold of 35 for prevention of notching (Table 1, Figure 2).
To try to understand why mean scapular notching index was low for both groups, we examined the contributing factors individually. Our cohort’s mean PGRD of 16.1 mm (15.4 and 16.8 mm for groups 1 and 2, respectively) was significantly lower than the cohort mean reported by Simovitch and colleagues8 (Table 2). Given that PGRD is more strongly weighted in the originally described notching index ([PSNA × 0.13] + PGRD),8 it was the primary driver for our notching index results, even though on average our results demonstrated a PSNA higher than that found by Simovitch and colleagues8 (Table 2; Figures 3, 4). Analyzing PGRD and PSNA together, we found no relationship between these variables and increased severity of inferior notching (Figure 5).
Regarding the effects of notching severity on outcomes in our study cohort, there were no significant differences between groups 1 and 2 in postoperative function, including forward flexion (123° vs 112.4°; P = .11), external rotation (18.8° vs 16.7°; P = .76), positive lag sign (P = .2), and VAS pain scores (1.2 vs 2.1; P = .15). There were also no significant differences between groups in the rate of complications (P = .92). Regression analysis determined that PSNA, PGRD, glenosphere inclination, glenosphere overhang, and implant manufacturer were not significant predictors of complications.
Discussion
RSA has provided good pain relief and restored function in patients with irreparable rotator cuff disease associated with arthritis.5,12,17,18 Scapular notching is a complex, multifactorial process. Nevertheless, surgeons remain cautious about the implications of inferior scapular notching, which is being reported by a significant number of patients. Our cohort’s high incidence of scapular notching (82%) in the early postoperative period clearly highlights the importance of predictive models, such as the notching index.8 Although concerns about consequences of notching have been expressed, notching severity did not affect outcomes or increase complications in this cohort.5,8,11,12,17-19
We conducted this study to examine use of a predictive tool for scapular notching, the notching index, in a large cohort of patients who underwent primary RSA. This index combines 2 well-established factors that contribute to notching—craniocaudal position and PSNA—into a predictive formula based on statistical analyses performed in a prospective cohort study.4,5,8,12,18 In their clinical study, Simovitch and colleagues8 found that both craniocaudal position and PSNA were tightly coupled with inferior scapular notching, and they developed a notching index that accounts for this relationship. We hypothesized that patients with a notching index of less than the recommended 35 would not develop notching and that patients with a notching index of more than 35 would have increased incidence and severity of notching. With our cohort, the recommended index of 35 was not an appropriate threshold predictive of notching. Furthermore, the 35 threshold applied to our cohort had 89% sensitivity and 21% specificity in predicting notching. Although the sensitivity is high, and correctly predicted true instances of notching, the low specificity compromises the precision of the notching formula ([PSNA × 0.13] + PGRD).
From the formula, it can be inferred that higher PSNA values can be compensated for by decreasing PGRD and inferiorizing the glenosphere. However, this recommendation appears limited based on increasing PSNA values, as in our cohort. The previously described notching formula cannot be universally applied to all patients treated with RSA because of the complexity of this relationship and patient-specific anatomy.
We assessed other possible anatomical and surgical factors, specific to scapular morphology, that could contribute to scapular notching. In other studies, reaming that produced an inferior tilt of the glenoid increased the likelihood of inferior notching.8,20,21 Furthermore, we expected less inferior glenoid overhang and smaller glenosphere would predispose patients to more notching.8,12,19 In our cohort, notching grade was not correlated with inferior tilt, glenoid overhang, or glenosphere size, which may be attributed to minimal variability in glenosphere size and a small range of glenosphere overhang.
There were limitations to this study. We examined only 2 types of RSA systems, and they had very similar Grammont designs. Other RSA designs might not have similar shortcomings with respect to inferior notching. In addition, we examined patient cases at a single time point and did not evaluate the effect of notching over time.
Overall, our results suggest that PGRD and PSNA have little effect on development of higher grade notching, particularly with use of Grammont prostheses. With newer surgical techniques, the recommendation is for inferior craniocaudal placement of the glenosphere, but this may not prevent notching with some types of patient-specific scapular morphology. Clearer surgical guidelines and techniques may help delineate the contribution of each parameter causing inferior scapular notching. Surgeons must weigh the evidence to determine how to correct patient-specific glenoid pathology and orient the glenosphere. Recent studies on bony increased-offset reverse shoulder arthroplasty (bio-RSA) techniques or newer prosthetic designs that considerably alter PSNA and the center of rotation may prevent inferior notching and provide a promising alternative to Grammont designs. Ultimately, longer follow-up is also needed to understand the clinical relevance of increased scapular notching.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
1. Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27(1):7-10.
2. Inman VT, Saunders JB, Abbott LC. Observations of the function of the shoulder joint. 1944. Clin Orthop. 1996;(330):3-12.
3. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
4. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
5. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
6. Kowalsky MS, Galatz LM, Shia DS, Steger-May K, Keener JD. The relationship between scapular notching and reverse shoulder arthroplasty prosthesis design. J Shoulder Elbow Surg. 2012;21(10):1430-1441.
7. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925-935.
8. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588-600.
9. Torrens C, Corrales M, Gonzalez G, Solano A, Caceres E. Morphology of the scapula relative to the reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):146-150.
10. McFarland EG, Sanguanjit P, Tasaki A, Keyurapan E, Fishman EK, Fayad LM. The reverse shoulder prosthesis: a review of imaging features and complications. Skeletal Radiol. 2006;35(7):488-496.
11. Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long-term results (>5 years). In: Walch G, Boileau P, Molé D, eds. Shoulder Prosthesis: Two to Ten Years Follow-Up. Montpellier, France: Sauramps Medical; 2001:253-259.
12. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
13. Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187-1191.
14. Grassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. J Orthop Surg (Hong Kong). 2009;17(2):151-156.
15. Lévigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop. 2011;469(9):2512-2520.
16. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524-528.
17. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
18. Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219-225.
19. Kempton LB, Balasubramaniam M, Ankerson E, Wiater JM. A radiographic analysis of the effects of glenosphere position on scapular notching following reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(6):968-974.
20. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 suppl):S9-S12.
21. Roche CP, Diep P, Hamilton M, et al. Impact of inferior glenoid tilt, humeral retroversion, bone grafting, and design parameters on muscle length and deltoid wrapping in reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(4):284-293.
Percutaneous Fixation of Hypertrophic Nonunion of the Inferior Pubic Ramus: A Report of Two Cases and Surgical Technique
Fractures of the superior and inferior pelvic rami are common in pelvic ring injuries.1 These fractures are routinely treated successfully without surgery.2 When the pelvic ring is injured, and ramus fracture or fractures represent a point of instability, surgical fixation can be performed to impart stability and reduce discomfort.3 Patients with pubic ramus fracture(s) have overall greater long-term morbidity and mortality.4 Operative stabilization of the superior pubic ramus can be achieved with open reduction and internal fixation, external fixation, and percutaneous medullary screw fixation.5-8 Inferior ramus fractures are seldom treated directly and acutely with operative reduction and fixation, as the mechanical advantage inferior ramus fixation provides is unknown.
Persistent nonunion of the pelvic ring can cause pain and disability and make reconstruction increasingly difficult.9 Open and percutaneous fixation techniques have been used to address symptomatic nonunions of the superior pubic ramus.9,10 There is limited evidence supporting surgical fixation of the inferior ramus. Open surgical fixation for symptomatic nonunions of the inferior ramus has been described.11,12 The inferior ramus has an osseous fixation pathway (OFP) amenable to percutaneous screw placement.13 Placement of a percutaneous screw in the inferior ramus requires use of preoperative computed tomography (CT) and is technically demanding. Surgeons must understand use of intraoperative fluoroscopy to ensure that the screw is contained within bone and crosses the intended zone of nonunion.
In this article, we report 2 cases of adults with symptomatic hypertrophic nonunions of the inferior ramus, treated with percutaneous screw fixation. Both patients presented with focal groin pain and activity limitations. Each had concurrent ipsilateral hypertrophic nonunions of the superior ramus, treated with percutaneous antegrade intramedullary stabilization. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 45-year-old woman fell from a horse about 8 months before presenting to the orthopedic outpatient clinic. Pelvic radiographs obtained after the fall were negative for fracture, but subsequent pelvic magnetic resonance imaging led to the diagnoses of minimally displaced left superior and inferior pubic ramus fractures and associated right-sided sacral ala fracture. The patient was treated with protected weight-bearing according to symptoms, but increasing activity-related pain and discomfort in the left groin persisted for months after injury. These symptoms were treated with analgesic medication, physical therapy, and chiropractic manipulation. Repeat imaging showed hypertrophic nonunions of the left superior and inferior pubic rami (Figure 1). Findings of the serologic testing performed for infection and metabolic deficiencies were normal at that time. The patient was referred for surgical consultation.
On evaluation, she reported constant pain in the left groin with ambulation. Specifically, squatting, pushing and pulling activities were extremely uncomfortable. She had been unable to return to work either full-time or part-time. On physical examination, she walked with an antalgic gait with a decreased stance phase of the left lower extremity. She had tenderness to palpation medial to the hip joint without evidence of hernia or lymphadenopathy. The pelvis was stable to manual compression testing.
Pelvic CT showed the nonunion site and the osteology of the inferior and superior pubic ramus of the pelvis, as well as minimal displacement and good alignment of the rami.
The patient was placed supine on a flat radiolucent table (Mizuho OSI, Union City, California). Preoperative cephalosporin antibiotics were administered. After induction of general anesthesia, the lumbosacral spine was elevated under 2 folded blankets. Arms were abducted to allow for pelvic imaging, and all bony prominences were padded. A urinary catheter was inserted aseptically to decompress the bladder. The entire abdomen and bilateral flanks were shaved, prepared, and draped in usual sterile fashion. A partially threaded cannulated screw was placed using a percutaneous antegrade technique to address the hypertrophic superior ramus nonunion.
A C-arm fluoroscopy unit (Ziehm, Orlando, Florida) was positioned on the injured side. The surgeon stood on the contralateral side. A pelvic OOOCI (outlet obturator oblique combinative image) of the symphysis pubis was obtained. This view defined the medial and lateral extents of the inferior ramus. A 0.062-in smooth Kirschner wire was used to percutaneously locate an ideal starting point on the cranial aspect of the contralateral superior pubic ramus. The starting point was adjusted on this view until an ideal intended trajectory into the contralateral (affected) inferior pubic ramus was visualized (Figure 2A).
The C-arm beam was then oriented to an “excessive” pelvic inlet view tangential to the posterior cortical surface of the affected inferior pubic ramus (Figure 2B). The tip of the wire was then adjusted to position and aim it slightly anterior to the posterior cortical surface of the affected inferior ramus. The wire was advanced into the bone about 1 cm, and the location and direction of the wire were reconfirmed as accurate.
A vertical skin incision was then made around the wire, and the 4.5-mm cannulated drill was placed over the wire. A soft-tissue protective drill sleeve and oscillating technique were used to protect the soft-tissue anatomy. The trajectory of the drill was again confirmed on pelvic OOOCI and advanced into the bone. The intended path of the drill was from the cranial-medial symphyseal cortex of the contralateral superior ramus, through the symphysis pubis obliquely, and then into the medullary canal of the affected inferior ramus. Frequent biplanar fluoroscopic imaging followed this progression of the drill to the nonunion site. The cannulated drill was then removed and exchanged for a calibrated extra-long 2.5-mm drill bit, placed through the soft-tissue drill sleeve and into the glide hole created by the 4.5-mm cannulated drill. The C-arm unit ensured accurate positioning of the 2.5-mm drill on both pelvic OOOCI and “excessive” inlet view before advancement (Figures 3A, 3B). The 2.5-mm drill was advanced caudally, laterally, and anteriorly in the ramus, past the nonunion site, and then was stopped before it exited the cortex of the ischial tuberosity (Figures 4A, 4B).
The depth of the drill bit was assessed with a known-length protective drill sleeve and calibrated drill. Alternatively, depth can be assessed with another same-length calibrated drill bit positioned adjacent to the inserted drill bit. A fully threaded, blunt-tipped 4.5-mm cortical screw was then placed through the glide hole. Both fluoroscopic views were used to confirm that the screw followed the same trajectory as the drill. Finally, the screw was again checked on biplanar fluoroscopy to confirm it had remained in the OFP of the inferior ramus (Figures 5A, 5B).
Postoperative pelvic CT confirmed position and length of the screws. The patient was allowed weight-of-limb weight-bearing on her affected side after surgery. She was discharged the first day after surgery and allowed use of oral analgesics. Six weeks after surgery, pelvic radiographs showed partial healing, and she reported symptom relief. Resistive strengthening exercises were instituted, and progressive weight-bearing proceeded to full weight-bearing over the next 6 weeks. The patient reported almost complete relief of pain by 3 months, and she was able to return to work and daily activities without medication. Radiographs showed consolidation of the fractures. She was essentially symptom-free 17 months after surgery (Figure 6).
Case 2
An obese 51-year-old woman presented to the orthopedic clinic with a 6-month history of left groin pain that worsened with ambulation. She did not recall a specific injury but acknowledged a history of previous falls. Past medical history was significant for ulcerative colitis/irritable bowel syndrome and degenerative disease in the lumbar spine and right ankle. Previous pelvic radiographs showed no evidence of fracture or abnormality, but radiographs obtained before evaluation in the clinic showed hypertrophic nonunion of the left superior and inferior pubic ramus.
The patient had pain deep in the left groin with weight-bearing. On physical examination, she denied pain with log roll of the left hip or resisted straight leg raise. The pelvis was stable to manual compression. There was no sign of hernia or lymphadenopathy in the region of the left groin.
The patient had obtained a technetium-99 nuclear medicine scan of the pelvis in addition to standard preoperative CT of the nonunion area. The nuclear medicine scan showed uptake in the area of the superior and inferior ramus, and CT confirmed presence of a superior and inferior ramus that would accommodate a medullary screw.
The patient was taken to the operating room, where percutaneous fixation of the left superior and inferior ramus was performed (as described above). The patient was discharged on postoperative day 2 and followed the same weight-bearing protocol that the first patient used.
At 6 weeks, the patient returned to clinic with improved comfort. At 3 months, she denied left groin pain and was limited in activity only by preexisting arthrosis in the left ankle and lumbar spine. She was using a walker only for long distances and was symptom-free 13 months after surgery.
Discussion
Acute surgical fixation of the inferior ramus is seldom performed. The anatomical location of the inferior ramus and the lack of defined criteria for fixation often leave the inferior ramus ignored, unreduced, and without stabilization. In the setting of symptomatic nonunion, open stabilization has been used.11,12 Plate fixation after open débridement of an inferior ramus nonunion requires more extensive dissection and may increase the risk for perioperative infection and hardware prominence compared with an intramedullary implant.14 If plate prominence becomes symptomatic, the plate must be removed in a second surgical procedure. Percutaneous medullary screw fixation avoids the risks of surgical soft-tissue dissection and placement of a surface implant on the bone and reduces the need for a second surgical procedure to remove bothersome hardware. Percutaneous pelvic fixation has been well described and shown to provide stability to the pelvis. It can also be used to treat hypertrophic nonunions of the pelvis when mechanical stability is required for healing.13
In the cases reported here, inferior ramus stabilization was combined with intramedullary fixation of the superior ramus. As each patient had deep groin pain that could not be localized to either ramus, both rami were stabilized after close assessment on preoperative CT. Solitary fixation of the superior ramus may or may not provide stability sufficient for inferior ramus union and should be performed when the OFP of the inferior ramus is unavailable.
The anatomy of the inferior ramus must be carefully reviewed before surgery, as it is seldom encountered in open and percutaneous orthopedic pelvic surgery. The inferior ramus extends from the symphysis pubis to the ischial tuberosity. The ramus is wider medially and thinner laterally near the obturator foramen. The anterior surface of the ramus is flat and concave, whereas the posterior surface is flat and convex. The anatomy of the inferior ramus varies somewhat, and any distortion (eg, fracture, nonunion) of the OFP can render it incapable of accommodating screw fixation.13
Percutaneous placement of a medullary screw in the inferior ramus requires an understanding of the fluoroscopy required. Challenges, including body habitus and unique osseous anatomy, must be recognized. Soft tissues must be protected with a drill sleeve during preparation of the screw pathway, and care must be taken to avoid placing the screw beyond the cortex of the ischial tuberosity. A prominent screw tip can irritate the patient in the hamstrings or while sitting.
Intramedullary screw fixation of the inferior ramus is a technically demanding surgical procedure. Meticulous evaluation of preoperative radiographic studies must accompany strict attention to surgical detail. A misplaced or malpositioned drill bit or screw can injure surrounding neurovascular structures. A screw that does not cross the fracture or is not in the OFP of the inferior ramus will be ineffective and
potentially dangerous.
Conclusion
We have presented a technique for percutaneous screw placement in the inferior ramus. This technique requires an understanding of the anatomy of the inferior ramus and of the intraoperative fluoroscopy required for screw placement. We have used this technique to successfully treat symptomatic hypertrophic nonunions of the inferior ramus that require skeletal stability for healing.
1. Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami. Epidemiology and five-year survival. J Bone Joint Surg Br. 2001;83(8):1141-1144.
2. Matta JM, Dickson KF, Markovich GD. Surgical treatment of pelvic nonunions and malunions. Clin Orthop. 1996;(329):199-206.
3. Barei DP, Shafer BL, Beingessner DM, Gardner MJ, Nork SE, Routt ML. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68(4):949-953.
4. van Dijk WA, Poeze M, van Helden SH, Brink PR, Verbruggen JP. Ten-year mortality among hospitalised patients with fractures of the pubic rami. Injury. 2010;41(4):411-414.
5. Simonian PT, Routt ML Jr, Harrington RM, Tencer AF. Internal fixation of the unstable anterior pelvic ring: a biomechanical comparison of standard plating techniques and the retrograde medullary superior pubic ramus screw. J Orthop Trauma. 1994;8(6):476-482.
6. Routt ML Jr, Simonian PT, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(1):35-44.
7. Matta JM. Indications for anterior fixation of pelvic fractures. Clin Orthop. 1996;(329):88-96.
8. Routt ML Jr, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop. 2000;(375):15-29.
9. Gautier E, Rommens PM, Matta JM. Late reconstruction after pelvic ring injuries. Injury. 1996;27(suppl 2):B39-B46.
10. Altman GT, Altman DT, Routt ML Jr. Symptomatic hypertrophic pubic ramus nonunion treated with a retrograde medullary screw. J Orthop Trauma. 2000;14(8):582-585.
11. Archdeacon MT, Kuhlman G, Kazemi N. Fellow’s Corner: grand rounds from the University of Cincinnati Medical Center—painful superior and inferior pubic rami nonunion. J Orthop Trauma. 2010;24(11):e109-e112.
12. Schofer M, Illian C, Fuchs-Winkelmann S, Kortmann HR. Pseudoarthrosis of anterior pelvic ring fracture [in German]. Unfallchirurg. 2008;111(4):264, 266-267.
13. Bishop JA, Routt ML Jr. Osseous fixation pathways in pelvic and acetabular fracture surgery: osteology, radiology, and clinical applications. J Trauma Acute Care Surg. 2012;72(6):1502-1509.
14. Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8(5):285-291.
Fractures of the superior and inferior pelvic rami are common in pelvic ring injuries.1 These fractures are routinely treated successfully without surgery.2 When the pelvic ring is injured, and ramus fracture or fractures represent a point of instability, surgical fixation can be performed to impart stability and reduce discomfort.3 Patients with pubic ramus fracture(s) have overall greater long-term morbidity and mortality.4 Operative stabilization of the superior pubic ramus can be achieved with open reduction and internal fixation, external fixation, and percutaneous medullary screw fixation.5-8 Inferior ramus fractures are seldom treated directly and acutely with operative reduction and fixation, as the mechanical advantage inferior ramus fixation provides is unknown.
Persistent nonunion of the pelvic ring can cause pain and disability and make reconstruction increasingly difficult.9 Open and percutaneous fixation techniques have been used to address symptomatic nonunions of the superior pubic ramus.9,10 There is limited evidence supporting surgical fixation of the inferior ramus. Open surgical fixation for symptomatic nonunions of the inferior ramus has been described.11,12 The inferior ramus has an osseous fixation pathway (OFP) amenable to percutaneous screw placement.13 Placement of a percutaneous screw in the inferior ramus requires use of preoperative computed tomography (CT) and is technically demanding. Surgeons must understand use of intraoperative fluoroscopy to ensure that the screw is contained within bone and crosses the intended zone of nonunion.
In this article, we report 2 cases of adults with symptomatic hypertrophic nonunions of the inferior ramus, treated with percutaneous screw fixation. Both patients presented with focal groin pain and activity limitations. Each had concurrent ipsilateral hypertrophic nonunions of the superior ramus, treated with percutaneous antegrade intramedullary stabilization. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 45-year-old woman fell from a horse about 8 months before presenting to the orthopedic outpatient clinic. Pelvic radiographs obtained after the fall were negative for fracture, but subsequent pelvic magnetic resonance imaging led to the diagnoses of minimally displaced left superior and inferior pubic ramus fractures and associated right-sided sacral ala fracture. The patient was treated with protected weight-bearing according to symptoms, but increasing activity-related pain and discomfort in the left groin persisted for months after injury. These symptoms were treated with analgesic medication, physical therapy, and chiropractic manipulation. Repeat imaging showed hypertrophic nonunions of the left superior and inferior pubic rami (Figure 1). Findings of the serologic testing performed for infection and metabolic deficiencies were normal at that time. The patient was referred for surgical consultation.
On evaluation, she reported constant pain in the left groin with ambulation. Specifically, squatting, pushing and pulling activities were extremely uncomfortable. She had been unable to return to work either full-time or part-time. On physical examination, she walked with an antalgic gait with a decreased stance phase of the left lower extremity. She had tenderness to palpation medial to the hip joint without evidence of hernia or lymphadenopathy. The pelvis was stable to manual compression testing.
Pelvic CT showed the nonunion site and the osteology of the inferior and superior pubic ramus of the pelvis, as well as minimal displacement and good alignment of the rami.
The patient was placed supine on a flat radiolucent table (Mizuho OSI, Union City, California). Preoperative cephalosporin antibiotics were administered. After induction of general anesthesia, the lumbosacral spine was elevated under 2 folded blankets. Arms were abducted to allow for pelvic imaging, and all bony prominences were padded. A urinary catheter was inserted aseptically to decompress the bladder. The entire abdomen and bilateral flanks were shaved, prepared, and draped in usual sterile fashion. A partially threaded cannulated screw was placed using a percutaneous antegrade technique to address the hypertrophic superior ramus nonunion.
A C-arm fluoroscopy unit (Ziehm, Orlando, Florida) was positioned on the injured side. The surgeon stood on the contralateral side. A pelvic OOOCI (outlet obturator oblique combinative image) of the symphysis pubis was obtained. This view defined the medial and lateral extents of the inferior ramus. A 0.062-in smooth Kirschner wire was used to percutaneously locate an ideal starting point on the cranial aspect of the contralateral superior pubic ramus. The starting point was adjusted on this view until an ideal intended trajectory into the contralateral (affected) inferior pubic ramus was visualized (Figure 2A).
The C-arm beam was then oriented to an “excessive” pelvic inlet view tangential to the posterior cortical surface of the affected inferior pubic ramus (Figure 2B). The tip of the wire was then adjusted to position and aim it slightly anterior to the posterior cortical surface of the affected inferior ramus. The wire was advanced into the bone about 1 cm, and the location and direction of the wire were reconfirmed as accurate.
A vertical skin incision was then made around the wire, and the 4.5-mm cannulated drill was placed over the wire. A soft-tissue protective drill sleeve and oscillating technique were used to protect the soft-tissue anatomy. The trajectory of the drill was again confirmed on pelvic OOOCI and advanced into the bone. The intended path of the drill was from the cranial-medial symphyseal cortex of the contralateral superior ramus, through the symphysis pubis obliquely, and then into the medullary canal of the affected inferior ramus. Frequent biplanar fluoroscopic imaging followed this progression of the drill to the nonunion site. The cannulated drill was then removed and exchanged for a calibrated extra-long 2.5-mm drill bit, placed through the soft-tissue drill sleeve and into the glide hole created by the 4.5-mm cannulated drill. The C-arm unit ensured accurate positioning of the 2.5-mm drill on both pelvic OOOCI and “excessive” inlet view before advancement (Figures 3A, 3B). The 2.5-mm drill was advanced caudally, laterally, and anteriorly in the ramus, past the nonunion site, and then was stopped before it exited the cortex of the ischial tuberosity (Figures 4A, 4B).
The depth of the drill bit was assessed with a known-length protective drill sleeve and calibrated drill. Alternatively, depth can be assessed with another same-length calibrated drill bit positioned adjacent to the inserted drill bit. A fully threaded, blunt-tipped 4.5-mm cortical screw was then placed through the glide hole. Both fluoroscopic views were used to confirm that the screw followed the same trajectory as the drill. Finally, the screw was again checked on biplanar fluoroscopy to confirm it had remained in the OFP of the inferior ramus (Figures 5A, 5B).
Postoperative pelvic CT confirmed position and length of the screws. The patient was allowed weight-of-limb weight-bearing on her affected side after surgery. She was discharged the first day after surgery and allowed use of oral analgesics. Six weeks after surgery, pelvic radiographs showed partial healing, and she reported symptom relief. Resistive strengthening exercises were instituted, and progressive weight-bearing proceeded to full weight-bearing over the next 6 weeks. The patient reported almost complete relief of pain by 3 months, and she was able to return to work and daily activities without medication. Radiographs showed consolidation of the fractures. She was essentially symptom-free 17 months after surgery (Figure 6).
Case 2
An obese 51-year-old woman presented to the orthopedic clinic with a 6-month history of left groin pain that worsened with ambulation. She did not recall a specific injury but acknowledged a history of previous falls. Past medical history was significant for ulcerative colitis/irritable bowel syndrome and degenerative disease in the lumbar spine and right ankle. Previous pelvic radiographs showed no evidence of fracture or abnormality, but radiographs obtained before evaluation in the clinic showed hypertrophic nonunion of the left superior and inferior pubic ramus.
The patient had pain deep in the left groin with weight-bearing. On physical examination, she denied pain with log roll of the left hip or resisted straight leg raise. The pelvis was stable to manual compression. There was no sign of hernia or lymphadenopathy in the region of the left groin.
The patient had obtained a technetium-99 nuclear medicine scan of the pelvis in addition to standard preoperative CT of the nonunion area. The nuclear medicine scan showed uptake in the area of the superior and inferior ramus, and CT confirmed presence of a superior and inferior ramus that would accommodate a medullary screw.
The patient was taken to the operating room, where percutaneous fixation of the left superior and inferior ramus was performed (as described above). The patient was discharged on postoperative day 2 and followed the same weight-bearing protocol that the first patient used.
At 6 weeks, the patient returned to clinic with improved comfort. At 3 months, she denied left groin pain and was limited in activity only by preexisting arthrosis in the left ankle and lumbar spine. She was using a walker only for long distances and was symptom-free 13 months after surgery.
Discussion
Acute surgical fixation of the inferior ramus is seldom performed. The anatomical location of the inferior ramus and the lack of defined criteria for fixation often leave the inferior ramus ignored, unreduced, and without stabilization. In the setting of symptomatic nonunion, open stabilization has been used.11,12 Plate fixation after open débridement of an inferior ramus nonunion requires more extensive dissection and may increase the risk for perioperative infection and hardware prominence compared with an intramedullary implant.14 If plate prominence becomes symptomatic, the plate must be removed in a second surgical procedure. Percutaneous medullary screw fixation avoids the risks of surgical soft-tissue dissection and placement of a surface implant on the bone and reduces the need for a second surgical procedure to remove bothersome hardware. Percutaneous pelvic fixation has been well described and shown to provide stability to the pelvis. It can also be used to treat hypertrophic nonunions of the pelvis when mechanical stability is required for healing.13
In the cases reported here, inferior ramus stabilization was combined with intramedullary fixation of the superior ramus. As each patient had deep groin pain that could not be localized to either ramus, both rami were stabilized after close assessment on preoperative CT. Solitary fixation of the superior ramus may or may not provide stability sufficient for inferior ramus union and should be performed when the OFP of the inferior ramus is unavailable.
The anatomy of the inferior ramus must be carefully reviewed before surgery, as it is seldom encountered in open and percutaneous orthopedic pelvic surgery. The inferior ramus extends from the symphysis pubis to the ischial tuberosity. The ramus is wider medially and thinner laterally near the obturator foramen. The anterior surface of the ramus is flat and concave, whereas the posterior surface is flat and convex. The anatomy of the inferior ramus varies somewhat, and any distortion (eg, fracture, nonunion) of the OFP can render it incapable of accommodating screw fixation.13
Percutaneous placement of a medullary screw in the inferior ramus requires an understanding of the fluoroscopy required. Challenges, including body habitus and unique osseous anatomy, must be recognized. Soft tissues must be protected with a drill sleeve during preparation of the screw pathway, and care must be taken to avoid placing the screw beyond the cortex of the ischial tuberosity. A prominent screw tip can irritate the patient in the hamstrings or while sitting.
Intramedullary screw fixation of the inferior ramus is a technically demanding surgical procedure. Meticulous evaluation of preoperative radiographic studies must accompany strict attention to surgical detail. A misplaced or malpositioned drill bit or screw can injure surrounding neurovascular structures. A screw that does not cross the fracture or is not in the OFP of the inferior ramus will be ineffective and
potentially dangerous.
Conclusion
We have presented a technique for percutaneous screw placement in the inferior ramus. This technique requires an understanding of the anatomy of the inferior ramus and of the intraoperative fluoroscopy required for screw placement. We have used this technique to successfully treat symptomatic hypertrophic nonunions of the inferior ramus that require skeletal stability for healing.
Fractures of the superior and inferior pelvic rami are common in pelvic ring injuries.1 These fractures are routinely treated successfully without surgery.2 When the pelvic ring is injured, and ramus fracture or fractures represent a point of instability, surgical fixation can be performed to impart stability and reduce discomfort.3 Patients with pubic ramus fracture(s) have overall greater long-term morbidity and mortality.4 Operative stabilization of the superior pubic ramus can be achieved with open reduction and internal fixation, external fixation, and percutaneous medullary screw fixation.5-8 Inferior ramus fractures are seldom treated directly and acutely with operative reduction and fixation, as the mechanical advantage inferior ramus fixation provides is unknown.
Persistent nonunion of the pelvic ring can cause pain and disability and make reconstruction increasingly difficult.9 Open and percutaneous fixation techniques have been used to address symptomatic nonunions of the superior pubic ramus.9,10 There is limited evidence supporting surgical fixation of the inferior ramus. Open surgical fixation for symptomatic nonunions of the inferior ramus has been described.11,12 The inferior ramus has an osseous fixation pathway (OFP) amenable to percutaneous screw placement.13 Placement of a percutaneous screw in the inferior ramus requires use of preoperative computed tomography (CT) and is technically demanding. Surgeons must understand use of intraoperative fluoroscopy to ensure that the screw is contained within bone and crosses the intended zone of nonunion.
In this article, we report 2 cases of adults with symptomatic hypertrophic nonunions of the inferior ramus, treated with percutaneous screw fixation. Both patients presented with focal groin pain and activity limitations. Each had concurrent ipsilateral hypertrophic nonunions of the superior ramus, treated with percutaneous antegrade intramedullary stabilization. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 45-year-old woman fell from a horse about 8 months before presenting to the orthopedic outpatient clinic. Pelvic radiographs obtained after the fall were negative for fracture, but subsequent pelvic magnetic resonance imaging led to the diagnoses of minimally displaced left superior and inferior pubic ramus fractures and associated right-sided sacral ala fracture. The patient was treated with protected weight-bearing according to symptoms, but increasing activity-related pain and discomfort in the left groin persisted for months after injury. These symptoms were treated with analgesic medication, physical therapy, and chiropractic manipulation. Repeat imaging showed hypertrophic nonunions of the left superior and inferior pubic rami (Figure 1). Findings of the serologic testing performed for infection and metabolic deficiencies were normal at that time. The patient was referred for surgical consultation.
On evaluation, she reported constant pain in the left groin with ambulation. Specifically, squatting, pushing and pulling activities were extremely uncomfortable. She had been unable to return to work either full-time or part-time. On physical examination, she walked with an antalgic gait with a decreased stance phase of the left lower extremity. She had tenderness to palpation medial to the hip joint without evidence of hernia or lymphadenopathy. The pelvis was stable to manual compression testing.
Pelvic CT showed the nonunion site and the osteology of the inferior and superior pubic ramus of the pelvis, as well as minimal displacement and good alignment of the rami.
The patient was placed supine on a flat radiolucent table (Mizuho OSI, Union City, California). Preoperative cephalosporin antibiotics were administered. After induction of general anesthesia, the lumbosacral spine was elevated under 2 folded blankets. Arms were abducted to allow for pelvic imaging, and all bony prominences were padded. A urinary catheter was inserted aseptically to decompress the bladder. The entire abdomen and bilateral flanks were shaved, prepared, and draped in usual sterile fashion. A partially threaded cannulated screw was placed using a percutaneous antegrade technique to address the hypertrophic superior ramus nonunion.
A C-arm fluoroscopy unit (Ziehm, Orlando, Florida) was positioned on the injured side. The surgeon stood on the contralateral side. A pelvic OOOCI (outlet obturator oblique combinative image) of the symphysis pubis was obtained. This view defined the medial and lateral extents of the inferior ramus. A 0.062-in smooth Kirschner wire was used to percutaneously locate an ideal starting point on the cranial aspect of the contralateral superior pubic ramus. The starting point was adjusted on this view until an ideal intended trajectory into the contralateral (affected) inferior pubic ramus was visualized (Figure 2A).
The C-arm beam was then oriented to an “excessive” pelvic inlet view tangential to the posterior cortical surface of the affected inferior pubic ramus (Figure 2B). The tip of the wire was then adjusted to position and aim it slightly anterior to the posterior cortical surface of the affected inferior ramus. The wire was advanced into the bone about 1 cm, and the location and direction of the wire were reconfirmed as accurate.
A vertical skin incision was then made around the wire, and the 4.5-mm cannulated drill was placed over the wire. A soft-tissue protective drill sleeve and oscillating technique were used to protect the soft-tissue anatomy. The trajectory of the drill was again confirmed on pelvic OOOCI and advanced into the bone. The intended path of the drill was from the cranial-medial symphyseal cortex of the contralateral superior ramus, through the symphysis pubis obliquely, and then into the medullary canal of the affected inferior ramus. Frequent biplanar fluoroscopic imaging followed this progression of the drill to the nonunion site. The cannulated drill was then removed and exchanged for a calibrated extra-long 2.5-mm drill bit, placed through the soft-tissue drill sleeve and into the glide hole created by the 4.5-mm cannulated drill. The C-arm unit ensured accurate positioning of the 2.5-mm drill on both pelvic OOOCI and “excessive” inlet view before advancement (Figures 3A, 3B). The 2.5-mm drill was advanced caudally, laterally, and anteriorly in the ramus, past the nonunion site, and then was stopped before it exited the cortex of the ischial tuberosity (Figures 4A, 4B).
The depth of the drill bit was assessed with a known-length protective drill sleeve and calibrated drill. Alternatively, depth can be assessed with another same-length calibrated drill bit positioned adjacent to the inserted drill bit. A fully threaded, blunt-tipped 4.5-mm cortical screw was then placed through the glide hole. Both fluoroscopic views were used to confirm that the screw followed the same trajectory as the drill. Finally, the screw was again checked on biplanar fluoroscopy to confirm it had remained in the OFP of the inferior ramus (Figures 5A, 5B).
Postoperative pelvic CT confirmed position and length of the screws. The patient was allowed weight-of-limb weight-bearing on her affected side after surgery. She was discharged the first day after surgery and allowed use of oral analgesics. Six weeks after surgery, pelvic radiographs showed partial healing, and she reported symptom relief. Resistive strengthening exercises were instituted, and progressive weight-bearing proceeded to full weight-bearing over the next 6 weeks. The patient reported almost complete relief of pain by 3 months, and she was able to return to work and daily activities without medication. Radiographs showed consolidation of the fractures. She was essentially symptom-free 17 months after surgery (Figure 6).
Case 2
An obese 51-year-old woman presented to the orthopedic clinic with a 6-month history of left groin pain that worsened with ambulation. She did not recall a specific injury but acknowledged a history of previous falls. Past medical history was significant for ulcerative colitis/irritable bowel syndrome and degenerative disease in the lumbar spine and right ankle. Previous pelvic radiographs showed no evidence of fracture or abnormality, but radiographs obtained before evaluation in the clinic showed hypertrophic nonunion of the left superior and inferior pubic ramus.
The patient had pain deep in the left groin with weight-bearing. On physical examination, she denied pain with log roll of the left hip or resisted straight leg raise. The pelvis was stable to manual compression. There was no sign of hernia or lymphadenopathy in the region of the left groin.
The patient had obtained a technetium-99 nuclear medicine scan of the pelvis in addition to standard preoperative CT of the nonunion area. The nuclear medicine scan showed uptake in the area of the superior and inferior ramus, and CT confirmed presence of a superior and inferior ramus that would accommodate a medullary screw.
The patient was taken to the operating room, where percutaneous fixation of the left superior and inferior ramus was performed (as described above). The patient was discharged on postoperative day 2 and followed the same weight-bearing protocol that the first patient used.
At 6 weeks, the patient returned to clinic with improved comfort. At 3 months, she denied left groin pain and was limited in activity only by preexisting arthrosis in the left ankle and lumbar spine. She was using a walker only for long distances and was symptom-free 13 months after surgery.
Discussion
Acute surgical fixation of the inferior ramus is seldom performed. The anatomical location of the inferior ramus and the lack of defined criteria for fixation often leave the inferior ramus ignored, unreduced, and without stabilization. In the setting of symptomatic nonunion, open stabilization has been used.11,12 Plate fixation after open débridement of an inferior ramus nonunion requires more extensive dissection and may increase the risk for perioperative infection and hardware prominence compared with an intramedullary implant.14 If plate prominence becomes symptomatic, the plate must be removed in a second surgical procedure. Percutaneous medullary screw fixation avoids the risks of surgical soft-tissue dissection and placement of a surface implant on the bone and reduces the need for a second surgical procedure to remove bothersome hardware. Percutaneous pelvic fixation has been well described and shown to provide stability to the pelvis. It can also be used to treat hypertrophic nonunions of the pelvis when mechanical stability is required for healing.13
In the cases reported here, inferior ramus stabilization was combined with intramedullary fixation of the superior ramus. As each patient had deep groin pain that could not be localized to either ramus, both rami were stabilized after close assessment on preoperative CT. Solitary fixation of the superior ramus may or may not provide stability sufficient for inferior ramus union and should be performed when the OFP of the inferior ramus is unavailable.
The anatomy of the inferior ramus must be carefully reviewed before surgery, as it is seldom encountered in open and percutaneous orthopedic pelvic surgery. The inferior ramus extends from the symphysis pubis to the ischial tuberosity. The ramus is wider medially and thinner laterally near the obturator foramen. The anterior surface of the ramus is flat and concave, whereas the posterior surface is flat and convex. The anatomy of the inferior ramus varies somewhat, and any distortion (eg, fracture, nonunion) of the OFP can render it incapable of accommodating screw fixation.13
Percutaneous placement of a medullary screw in the inferior ramus requires an understanding of the fluoroscopy required. Challenges, including body habitus and unique osseous anatomy, must be recognized. Soft tissues must be protected with a drill sleeve during preparation of the screw pathway, and care must be taken to avoid placing the screw beyond the cortex of the ischial tuberosity. A prominent screw tip can irritate the patient in the hamstrings or while sitting.
Intramedullary screw fixation of the inferior ramus is a technically demanding surgical procedure. Meticulous evaluation of preoperative radiographic studies must accompany strict attention to surgical detail. A misplaced or malpositioned drill bit or screw can injure surrounding neurovascular structures. A screw that does not cross the fracture or is not in the OFP of the inferior ramus will be ineffective and
potentially dangerous.
Conclusion
We have presented a technique for percutaneous screw placement in the inferior ramus. This technique requires an understanding of the anatomy of the inferior ramus and of the intraoperative fluoroscopy required for screw placement. We have used this technique to successfully treat symptomatic hypertrophic nonunions of the inferior ramus that require skeletal stability for healing.
1. Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami. Epidemiology and five-year survival. J Bone Joint Surg Br. 2001;83(8):1141-1144.
2. Matta JM, Dickson KF, Markovich GD. Surgical treatment of pelvic nonunions and malunions. Clin Orthop. 1996;(329):199-206.
3. Barei DP, Shafer BL, Beingessner DM, Gardner MJ, Nork SE, Routt ML. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68(4):949-953.
4. van Dijk WA, Poeze M, van Helden SH, Brink PR, Verbruggen JP. Ten-year mortality among hospitalised patients with fractures of the pubic rami. Injury. 2010;41(4):411-414.
5. Simonian PT, Routt ML Jr, Harrington RM, Tencer AF. Internal fixation of the unstable anterior pelvic ring: a biomechanical comparison of standard plating techniques and the retrograde medullary superior pubic ramus screw. J Orthop Trauma. 1994;8(6):476-482.
6. Routt ML Jr, Simonian PT, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(1):35-44.
7. Matta JM. Indications for anterior fixation of pelvic fractures. Clin Orthop. 1996;(329):88-96.
8. Routt ML Jr, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop. 2000;(375):15-29.
9. Gautier E, Rommens PM, Matta JM. Late reconstruction after pelvic ring injuries. Injury. 1996;27(suppl 2):B39-B46.
10. Altman GT, Altman DT, Routt ML Jr. Symptomatic hypertrophic pubic ramus nonunion treated with a retrograde medullary screw. J Orthop Trauma. 2000;14(8):582-585.
11. Archdeacon MT, Kuhlman G, Kazemi N. Fellow’s Corner: grand rounds from the University of Cincinnati Medical Center—painful superior and inferior pubic rami nonunion. J Orthop Trauma. 2010;24(11):e109-e112.
12. Schofer M, Illian C, Fuchs-Winkelmann S, Kortmann HR. Pseudoarthrosis of anterior pelvic ring fracture [in German]. Unfallchirurg. 2008;111(4):264, 266-267.
13. Bishop JA, Routt ML Jr. Osseous fixation pathways in pelvic and acetabular fracture surgery: osteology, radiology, and clinical applications. J Trauma Acute Care Surg. 2012;72(6):1502-1509.
14. Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8(5):285-291.
1. Hill RM, Robinson CM, Keating JF. Fractures of the pubic rami. Epidemiology and five-year survival. J Bone Joint Surg Br. 2001;83(8):1141-1144.
2. Matta JM, Dickson KF, Markovich GD. Surgical treatment of pelvic nonunions and malunions. Clin Orthop. 1996;(329):199-206.
3. Barei DP, Shafer BL, Beingessner DM, Gardner MJ, Nork SE, Routt ML. The impact of open reduction internal fixation on acute pain management in unstable pelvic ring injuries. J Trauma. 2010;68(4):949-953.
4. van Dijk WA, Poeze M, van Helden SH, Brink PR, Verbruggen JP. Ten-year mortality among hospitalised patients with fractures of the pubic rami. Injury. 2010;41(4):411-414.
5. Simonian PT, Routt ML Jr, Harrington RM, Tencer AF. Internal fixation of the unstable anterior pelvic ring: a biomechanical comparison of standard plating techniques and the retrograde medullary superior pubic ramus screw. J Orthop Trauma. 1994;8(6):476-482.
6. Routt ML Jr, Simonian PT, Grujic L. The retrograde medullary superior pubic ramus screw for the treatment of anterior pelvic ring disruptions: a new technique. J Orthop Trauma. 1995;9(1):35-44.
7. Matta JM. Indications for anterior fixation of pelvic fractures. Clin Orthop. 1996;(329):88-96.
8. Routt ML Jr, Nork SE, Mills WJ. Percutaneous fixation of pelvic ring disruptions. Clin Orthop. 2000;(375):15-29.
9. Gautier E, Rommens PM, Matta JM. Late reconstruction after pelvic ring injuries. Injury. 1996;27(suppl 2):B39-B46.
10. Altman GT, Altman DT, Routt ML Jr. Symptomatic hypertrophic pubic ramus nonunion treated with a retrograde medullary screw. J Orthop Trauma. 2000;14(8):582-585.
11. Archdeacon MT, Kuhlman G, Kazemi N. Fellow’s Corner: grand rounds from the University of Cincinnati Medical Center—painful superior and inferior pubic rami nonunion. J Orthop Trauma. 2010;24(11):e109-e112.
12. Schofer M, Illian C, Fuchs-Winkelmann S, Kortmann HR. Pseudoarthrosis of anterior pelvic ring fracture [in German]. Unfallchirurg. 2008;111(4):264, 266-267.
13. Bishop JA, Routt ML Jr. Osseous fixation pathways in pelvic and acetabular fracture surgery: osteology, radiology, and clinical applications. J Trauma Acute Care Surg. 2012;72(6):1502-1509.
14. Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8(5):285-291.
Retrograde Reamer/Irrigator/Aspirator Technique for Autologous Bone Graft Harvesting With the Patient in the Prone Position
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
The Reamer/Irrigator/Aspirator (RIA) system (Synthes, West Chester, Pennsylvania) has become a powerful tool for harvesting autologous bone graft from the intramedullary canal of the long bones of the lower extremity for the treatment of osseous defects, nonunions, and joint fusions.1,2 The RIA system provides satisfactory quality and quantity of bone graft (range, 40-90 mL)3-5 with osteogenic properties that rival those harvested from the iliac crest.6,7 Minimal donor-site morbidity and mortality have been reported in association with the RIA technique compared with iliac crest bone graft harvest.8
The RIA technique for the femur—with the antegrade approach and the supine position,8 with the antegrade approach and the prone position,9 and with the retrograde approach and the supine position4—has been described in the literature. To our knowledge, however, the RIA technique for the femur with the retrograde approach and the prone position has not been described. Antegrade harvesting uses the trochanteric entry point, and retrograde harvesting uses an entry at the intercondylar notch just anterior to the posterior cruciate ligament. In this article, we detail the technique for RIA harvesting of the femur with the patient in the prone position. Patient positioning is based on the diagnosis and the proposed procedure.
Advantages of a retrograde starting point include a more concentric trajectory (vs that of an antegrade starting point) and more efficient canal pressure reduction, which might decrease the risk of intraoperative fat embolization.10 This technique offers a more efficient solution to any procedure that requires the prone position, and it avoids the need to reposition, reprepare, or redrape the extremity. It is also very useful in treating obese patients.
After obtaining institutional review board (IRB) approval, we retrospectively reviewed patient files. Because the study was retrospective, the IRB waived the requirement for informed consent. The patients described here provided written informed consent for print and electronic publication of these case reports.
Surgical Technique
The patient is placed in a prone position on a radiolucent table with a bump under the thigh to allow access to the knee joint with full extension of the hip (Figures 1, 2A, 2B). The knee is then flexed to gain access to the intercondylar notch.
The anatomical axis of the femur is identified in the coronal and sagittal planes with the help of an image intensifier. Frequent intraoperative fluoroscopic imaging is required to prevent eccentric reaming and guide-wire movement from causing iatrogenic fractures and perforations, respectively.8 A 2-mm Steinmann pin is used to identify the point of entry into the femoral canal, which is located just above the posterior cruciate ligament insertion in the intercondylar notch, and care is taken not to ream this structure. A minimally invasive incision of about 15 mm is centered on this pin using a patellar tendon–splitting approach.
An 8-mm cannulated anterior cruciate ligament reamer is passed over the pin to enlarge the opening at the entry point, and a 2.5-mm ball-tipped guide wire is positioned in the femur. The image intensifier is used to confirm positioning of the guide in the trochanteric region and centered in the intramedullary canal. A radiolucent diving board facilitates fluoroscopic imaging.
The diameter (12.5 or 16.5 mm) of the reaming head is selected after the intramedullary guide is placed in the femoral canal. The isthmus of the femur is then identified radiographically, and a radiopaque ruler with increments in millimeters is used to measure the canal diameter (Figures 3A, 3B). Because the femoral canal is an ellipsoid, the canal diameter usually is much larger anteroposteriorly than laterally.8 We prefer to use a reaming head that overlaps the inner cortical diameter by 1 mm on each side. An alternative method includes measuring the outer diameter of the narrowest portion of the bone and using a reamer head no more than 45% of the outer diameter at the isthmus.8
The RIA system is prepared on the back table by attaching the reaming head to the irrigation and suction systems. As the reamer head enters the intramedullary canal, an approach–withdraw–pause technique is used to slowly advance the reamer through the femur. It is crucial to use the image intensifier to guide reaming in order to avoid overdrilling the anterior cortex and prevent eccentric reaming of the canal, which more commonly occurs in patients with large anterior femoral bows.11 When the collection filter becomes full, reaming is stopped. The bone graft in the filter is emptied into a specimen cup for measurement and storage until subsequent use (Figure 4). Suctioning is suspended when reaming is stopped because substantial blood loss can occur with prolonged suction and aspiration.12 When repeat reaming is required, care is taken not to overream the cortices, thereby avoiding the risk of iatrogenic fracture.10,12
The knee joint is irrigated to remove any intramedullary debris. Typically there is no debris, as it is captured by the RIA. The wound is closed in 2 layers. Dressing with Ace bandage (3M, St. Paul, Minnesota) is placed around the knee for comfort. Weight-bearing status is determined by the index procedure.
Case Reports
Case 1
A 68-year-old female smoker presented to our facility with right ankle pain after recent ankle arthrodesis for pilon fracture nonunion. Almost 3 years earlier, the patient sustained a Gustilo-Anderson type II open pilon fracture in a motorcycle accident. She underwent antibiotic therapy, irrigation and débridement of the fracture site, and external fixation before definitive treatment with repeat irrigation and débridement and open reduction and internal fixation of the tibial plafond. About 6 months after surgery, she presented to her surgeon with a draining abscess over the anteromedial surgical incision. Multiple débridement procedures were performed, the implant was removed, the ankle was stabilized with a bridging external fixator, and culture-specific antibiotic therapy was administered. Intraoperative cultures confirmed methicillin-resistant Staphylococcus aureus. Vancomycin was administered intravenously for 6 weeks. Once C-reactive protein level and erythrocyte sedimentation rate returned to normal, repeat débridement with a rectus abdominis free flap and ankle fusion were performed.
When the patient presented to our clinic, we saw atrophic nonunion of the ankle fusion on radiographs. Smoking cessation was encouraged but not required before surgery. The patient returned to the operating suite for tibiotalocalcaneal fusion with a retrograde intramedullary nail. With the patient in the prone position, retrograde femoral RIA reaming was performed to harvest 30 mL of autologous bone. After resection of the nonunion site using a trans-Achilles approach and insertion of the intramedullary nail, the autologous bone graft was mixed with recombinant human bone morphogenetic protein 2 (BMP-2), and the mixture was introduced into the fusion site. At final follow-up, 18 months after surgery, the patient was clinically asymptomatic and radiographically healed—without further intervention and despite continued smoking. She did not report any knee pain from the harvest site.
Case 2
A 59-year-old noncompliant woman with diabetes and Charcot neuropathy sustained a trimalleolar ankle fracture-dislocation that was initially treated with ankle and hindfoot arthrodesis. The postoperative course was uneventful, and she was discharged home. Less than a week later, she presented to the emergency department with a midshaft tibial fracture just proximal to the ankle and hindfoot fusion nail. She subsequently had the device removed and a long arthrodesis rod inserted to span the fracture site up to the proximal tibial metadiaphysis. About 9 months later, she returned to our office complaining of ankle pain. No signs of infection were clinically evident. Radiographs showed nonunion of the ankle and subtalar joint. Findings of the initial bone biopsy and pathologic examination were negative for infection. The patient returned to the operating room 4 weeks later for revision ankle fusion. With the patient in the prone position, autologous bone (~30 mL) was harvested using retrograde femoral RIA reaming. The nonunion site was resected, and a mixture of autologous bone graft and BMP-2 was applied. Through a posterior approach, an anterior ankle arthrodesis locking plate was applied to the posterior aspect of the calcaneus and tibia. The patient was kept non-weight-bearing for 3 months and progressed in weight-bearing for another 4 to 6 weeks. Ambulatory status was restored about 4 months after surgery. No harvest-site knee pain was reported.
Discussion
Given its osteogenic, osteoconductive, and osteoinductive properties, autologous cancellous bone graft is the gold standard for reconstruction and fusion procedures in foot and ankle surgery.13 Bone graft can be obtained from many potential donor sites, but the most common is the iliac crest.2 However, many comorbidities, such as residual donor-site pain, neurovascular injuries, infection, and increased surgical time, have been reported in the literature.14,15 The RIA system was initially developed for simultaneous reaming and aspiration to reduce intramedullary pressure, heat generation, operating time, and the systemic effects of reaming, such as the embolic phenomenon.16-22 The single-pass reamer has provided a minimally invasive strategy for procuring voluminous amounts of autologous cancellous bone from the intramedullary canal of lower extremity long bones. Schmidmaier and colleagues3 recently quantified the measurements of several growth factors, such as insulinlike growth factor 1, transforming growth factor β 1, and BMP-2—proving that RIA-derived aspirates have amounts comparable to if not larger than those of iliac crest autologous bone graft. Pratt and colleagues23 provided insight into the possibility of induction of mesenchymal stem cells using the previously unwanted supernatant reamings after filtration. Recently, the RIA technique of autologous tibial and hindfoot bone graft harvest was described for use in ankle or tibiotalocalcaneal arthrodesis.2 Although this technique is a useful surgical option, tibia size remains a limiting factor. Kovar and Wozasek24 reported harvesting significantly more bone graft in the femur than in the tibia. A tibia that cannot accommodate the 12-mm (smallest) reamer head in the RIA system would be a contraindication. In addition, concerns about the association between tibial stress fractures and reaming of the entire tibial canal and concerns about the overall donor-site morbidity of the tibial shaft remain.
Conclusion
With its retrograde approach and prone positioning, this RIA technique is an effective and efficient solution for harvesting autologous femoral bone graft. Although we have described its use in ankle and hindfoot arthrodesis, this technique can be applied to any prone-position surgical procedure, including spine surgery.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
1. Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation. An indication for the “reamer-irrigator-aspirator-” (RIA-)technique [in German]. Unfallchirurg. 2008;111(6):469-472.
2. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94(1):75-79.
3. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Lehman AA, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer-irrigator-aspirator in tibiotalocalcaneal arthrodesis: surgical technique and case series. Foot Ankle Int. 2012;33(12):1133-1138.
6. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007;81(2):437-442.
7. Sagi HC, Young ML, Gerstenfeld L, Einhorn TA, Tornetta P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a reamer/irrigator/aspirator) and the iliac crest of the same patient. J Bone Joint Surg Am. 2012;94(23):2128-2135.
8. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466(12):2973-2980.
9. Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 suppl 1):E179.
10. Van Gorp CC, Falk JV, Kmiec SJ Jr, Siston RA. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop. 2009;467(3):805-809.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6(2):100-107.
13. Whitehouse MR, Lankester BJ, Winson IG, Hepple S. Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int. 2006;27(11):913-916.
14. Scharfenberger A, Weber T. RIA for bone graft harvest: applications for grafting large segmental defects in the tibia and femur. Presented at: 21st Annual Meeting of the Orthopaedic Trauma Association; 2005; Ottawa, Canada.
15. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;(329):300-309.
16. Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359-366.
17. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192-197.
18. Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935-940.
19. Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37(suppl 4):S39-S49.
20. Pape HC, Dwenger A, Grotz M, et al. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8(4):300-309.
21. Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515-2522.
22. Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186-1192.
23. Pratt DJ, Papagiannopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18(3):177-179.
24. Kovar FM, Wozasek GE. Bone graft harvesting using the RIA (reamer irrigation aspirator) system—a quantitative assessment. Wien Klin Wochenschr. 2011;123(9-10):285-290.
Revision Anterior Cruciate Ligament Reconstruction With Bone–Patellar Tendon–Bone Allograft and Extra-Articular Iliotibial Band Tenodesis
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
Primary anterior cruciate ligament (ACL) reconstruction has satisfactory outcomes in 75% to 97% of patients.1-3 Despite this high success rate, the number of revision ACL reconstructions has risen4 and is likely underreported.5 Recurrent instability occurs if the reconstructed ligament fails to provide adequate anterior and rotational knee stability. Causes of graft failure include repeat trauma, early return to high-demand activity, poor operative technique (including poor graft placement), failure to address concomitant pathology, and perioperative complications (eg, infection, stiffness).4 In addition, most patients who have revision ACL reconstruction received autograft tissue in the initial surgery, and allograft is thus not uncommon in revision ACL surgery. Allograft tissue has longer incorporation times6 and increased incidence of recurrent postoperative instability when compared with autograft tissue.7 Extra-articular tenodesis may thus be used to provide additional stability to the revision allograft tissue while it incorporates.
In this article, we describe our use of an extra-articular iliotibial band (ITB) tenodesis as an augmentative procedure in patients undergoing revision ACL reconstruction with bone–patellar tendon–bone (BPTB) allograft.
Surgical Technique
After induction of anesthesia and careful positioning, the patient is prepared and draped in the usual sterile fashion. Standard anteromedial, anterolateral, and superolateral outflow portals are established, and diagnostic arthroscopy is performed to inspect the cruciate ligaments, menisci, and articular cartilage (Figure 1). Peripheral meniscal tears should be repaired (Figure 2), and central or inner tears should be débrided to a stable rim. If meniscal repair is performed, sutures should be tied at the end of the case. Unstable articular cartilage defects should also be débrided. An 8- to 12-cm lateral hockey-stick incision is then made from the Gerdy tubercle to the inferior edge of the lateral femoral epicondyle in preparation for the ITB tenodesis (Figure 1). The lateral collateral ligament (LCL), the lateral head of the gastrocnemius, and the ITB are identified. The peroneal nerve should be significantly distal to the working field.
Remnants of the previous ACL graft are débrided, and, if necessary, a modified notchplasty is performed. A position for the new femoral tunnel is located and is confirmed with intraoperative fluoroscopy. This tunnel is established with compaction drill bits and dilated to the appropriate diameter through the anteromedial portal with the knee in 120° of flexion.
BPTB allograft is prepared first by cutting its central third to the desired diameter (Figure 3). The bone-plug ends are prepared with compaction pliers. Two 2.0-mm drill holes are made in each of the allograft bone plugs, and a No. 5 Ti-Cron suture (Covidien, New Haven, Connecticut) is placed through each of the holes. We typically use 2 sutures on each bone plug.
A tibial tunnel is then established with an ACL drill guide under arthroscopic visualization and intraoperative fluoroscopy for confirmation of correct pin placement. We use Kirschner wires (with parallel pin guides as needed), compaction drills, and dilators to create a well-positioned tunnel of the appropriate diameter. The allograft is then passed through the tibia and femur in retrograde fashion. We secure the femoral side with an AO (Arbeitsgemeinschaft für Osteosynthesefragen) 4.5-mm bicortical screw and washer. Our tibial fixation is secured after the ITB tenodesis. The knee is then cycled a dozen times.
In preparation for the ITB tenodesis, we lengthen our previously made incision by about 4 cm proximally along the posterior aspect of the ITB. The central portion of the ITB is then outlined at the Gerdy tubercle and split with a No. 10 blade. This generally leaves an approximately 12- to 14-mm strip of ITB centrally (Figure 4). This portion should be gently lifted from the underlying tissue attachments distally at the insertion on the Gerdy tubercle. The interval between the LCL and lateral capsule of the knee is identified, and a No. 2 Ti-Cron whip-stitch is thrown through the free end of the ITB graft (Figure 5). The anterior aspect of the femoral tunnel is at the distal aspect of the lateral femoral condyle, and the posterior aspect is at the juncture of the proximal LCL and the lateral head of the gastrocnemius. The cortices of these landmarks should be perforated with a drill, and a curved instrument should be used to create a bone tunnel at this location (Figure 6). The tibial tunnel is just posterior and distal to the Gerdy tubercle and should be created in similar fashion. The graft is then passed underneath the LCL (Figure 7), through the proximal tunnel that has been created on the lateral femoral condyle, and then back down through the LCL and back onto itself after exiting the tibial tunnel (Figure 8). With the knee at 30° of flexion, the ITB graft is tensioned and sutured down to intact ITB fascia just proximal to the tibial tunnel orifice (Figure 9). We check knee range of motion (ROM) and then perform a Lachman test to assess changes in knee stability. The pivot shift examination is omitted to avoid placing excessive stress on the tenodesis. The tibial side of the patellar tendon allograft is then tensioned and secured over an AO 4.5-mm bicortical screw with washer with the knee in full extension. The screw is then tightened at 30° of knee flexion.
The ITB fascia is closed to the lateral femoral epicondyle with a running heavy suture, and all incisions are then irrigated and closed (Figures 10, 11). Standard sterile surgical dressing, Cryo/Cuff (Aircast, Vista, California), and brace are applied with the knee locked at 20°. Patients are generally discharged home the same day and followed up in clinic 1 week after surgery.
Complications
The peroneal nerve must be identified and protected during the open lateral procedure. In addition, the need for the extra lateral incision poses a slightly higher risk for infection compared with the traditional arthroscopic revision ACL procedure. Last, the additional tunnels required for the tenodesis can increase the theoretical potential for distal femur fracture and ACL graft fixation failure on the femoral side.
Postoperative Management
The operative knee is kept in extension in a brace locked at 20° for week 1 after surgery. Isometric quadriceps exercises are started immediately after surgery. Flexion to 90° is allowed starting week 2 after surgery, when the patient begins supervised active/passive flexion and progressive ROM exercises. In most cases, full ROM should be achieved by 6 to 8 weeks after surgery. Patients are progressed in their weight-bearing status by about 25% of their body weight per week, and use of crutches should be discontinued by week 4 after surgery. The brace should be discontinued by week 6 after surgery, when use of stationary bicycle and closed chain exercises begin. The patient may begin jogging when the operative leg regains 80% of contralateral quadriceps strength via Cybex strength testing. Functional drills begin in month 6, but patients should be counseled against returning to sport any earlier than 9 months after surgery.
Discussion
Achieving a successful outcome in revision ACL surgery (vs primary ACL surgery) is a significant challenge. Any of numerous factors can make the revision surgery more challenging, including existing poorly placed tunnels, tunnel expansion, lack of ideal graft choice, loss of secondary stabilizers, and deviations of the weight-bearing axis. Therefore, outcomes of revision surgery tend to be more moderate than outcomes of primary procedures.4,8-12
Revision ACL reconstruction techniques are varied and can involve use of autograft or allograft tissue as well as extra-articular augmentation techniques. Diamantopoulos and colleagues8 reported the outcomes of revision ACL reconstruction using bone–tendon–bone, hamstring, or quadriceps autografts in 107 patients. The majority of patients had improved outcome measures (mean Lysholm score improved from 51.5 to 88.5) and side-to-side laxity measurements. However, only 36.4% returned to preinjury activity level. Similarly, Noyes and Barber-Westin9 reported the outcomes of revision ACL reconstruction using quadriceps tendon–patellar bone autograft in 21 patients. Although there was significant improvement in terms of symptoms and activity level, 4 of the 21 knees were graded abnormal or severely abnormal on the IKDC (International Knee Documentation Committee) ligament rating. In a systematic review, pooled results of revision ACL reconstructions reiterated the above results.10 Eight hundred sixty-three patients from 21 studies were included in the analysis, which found significantly worse subjective outcomes than for primary procedures and a dramatically higher failure rate for the re-reconstructed ACL.
Several authors have directly compared primary cohorts with revision cohorts. Ahn and colleagues11 compared the outcomes of 59 revision ACL reconstructions with those of 117 primary reconstructions at a single institution. Although statistical comparison of stability between primary and revision ACL reconstructions showed no difference, revision reconstructions fared more poorly in terms of quality of life and return to activity compared with primary reconstructions. In a large cohort study of the Danish registry, revisions were found to have worse subjective outcomes than primary reconstructions as well.12 The study also found that the rerupture risk was significantly higher (relative risk, 2.05) when allograft was used.
Given the inferior results of revision surgery, our technique is recommended to augment the stability of reconstructed knees in the setting of revision ACL reconstruction. Adding the extra-articular procedure may augment the revised graft and protect it from excessive stress.13 A cadaver study compared double-bundle ACL reconstruction with single-bundle hamstring reconstruction plus extra-articular lateral tenodesis and found improved internal rotation control at 30° of flexion in the latter.14 Using contralateral 4-strand hamstring autograft in combination with an extra-articular lateral augment can have encouraging outcomes. Ferretti and colleagues15 reported an average Lysholm score of 95 in 12 patients who underwent this revision procedure and good anterior-to-posterior stability in 11 of the 12 patients. Trojani and colleagues16 reported on a cohort of 163 patients who underwent ACL revision surgery over a 10-year period. The authors found that 80% of patients with a lateral extra-articular tenodesis performed to augment their revision reconstruction had a negative pivot shift at long-term follow-up—versus only 63% of patients who underwent isolated revision ACL reconstruction. This finding was statistically significant, but the authors did not find any differences in IKDC scores between groups. These results support the initial biomechanical findings of Engebretsen and colleagues,17 who found that adding a lateral tenodesis decreased the forces on the reconstructed graft by 15%.
Conclusion
This technique allows for protection of the intra-articular allograft ligament reconstruction with improved rotational control that may potentially allow for improved subjective outcomes and protect against graft failure. Given the common pitfalls with stability in revision ACL surgery with allograft, this lateral extra-articular procedure can be an important structural augmentation in this challenging clinical issue in knee surgery.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
1. Bach BR Jr. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19(suppl 1):14-29.
2. Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661-681.
3. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone–tendon–bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
4. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
5. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622-627.
6. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
7. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-S66.
8. Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851-860.
9. Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon–patellar bone autograft. Am J Sports Med. 2006;34(4):553-564.
10. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
11. Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36(10):1889-1895.
12. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551-1557.
13. Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88(11):2373-2379.
14. Monaco E, Labianca L, Conteduca F, De Carli A, Ferretti A. Double bundle or single bundle plus extraarticular tenodesis in ACL reconstruction? A CAOS study. Knee Surg Sports Traumatol Arthrosc. 2007;15(10):1168-1174.
15. Ferretti A, Monaco E, Caperna L, Palma T, Conteduca F. Revision ACL reconstruction using contralateral hamstrings. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):690-695.
16. Trojani C, Beaufils P, Burdin G, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1565-1570.
17. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169-176.
Google Glass Distal Biceps Repair
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.
To read the authors' full article "5 Points on Using Wearable Technology to Record Surgical Videos," click here.