User login
Treating major depressive disorder after limited response to an initial agent
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
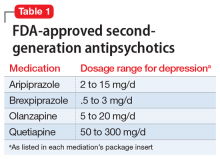
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
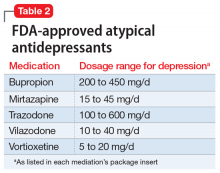
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
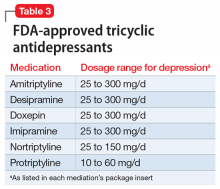
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
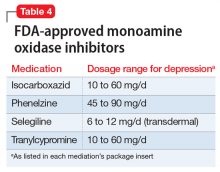
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly used first-line agents for treating major depressive disorder. Less than one-half of patients with major depressive disorder experience remission after 1 acute trial of an antidepressant.1 After optimization of an initial agent’s dose and duration, potential next steps include switching agents or augmentation. Augmentation strategies may lead to clinical improvement but carry the risks of polypharmacy, including increased risk of adverse effects and drug interactions. Clinicians can consider the following evidence-based options for a patient with a limited response to an initial SSRI or SNRI.
Second-generation antipsychotics, when used as augmentation agents to treat a patient with major depressive disorder, can lead to an approximately 10% improvement in remission rate compared with placebo.2 Aripiprazole, brexpiprazole, olanzapine (in combination with fluoxetine only), and quetiapine are FDA-approved as adjunctive therapies with an antidepressant (Table 1). Second-generation antipsychotics should be started at lower doses than those used for schizophrenia, and these agents have an increased risk of metabolic adverse effects as well as extrapyramidal symptoms.
Atypical antidepressants are those that are not classified as an SSRI, SNRI, tricyclic antidepressant (TCA), or monoamine oxidase inhibitor (MAOI). These include bupropion, mirtazapine, trazodone, vilazodone, and vortioxetine (Table 2). Bupropion is a dopamine and norepinephrine reuptake inhibitor. When used for augmentation in clinical studies, it led to a 30% remission rate.3 Mirtazapine is an alpha-2 antagonist that can be used as monotherapy or in combination with another antidepressant.4 Trazodone is an antidepressant with activity at histamine and alpha-1-adrenergic receptors that is often used off-label for insomnia. Trazodone can be used safely and effectively in combination with other agents for treatment-resistant depression.5 Vilazodone is a 5-HT1A partial agonist, and vortioxetine is a 5-HT1A agonist and 5-HT3 antagonist; both are FDA-approved as alternative agents for monotherapy for major depressive disorder. Choosing among these agents for switching or augmenting can be guided by patient preference, adverse effect profile, and targeting specific symptoms, such as using mirtazapine to address poor sleep and appetite.
Lithium augmentation has been frequently investigated in placebo-controlled, double-blind studies. A meta-analysis showed that patients receiving lithium augmentation with a serum level of ≥0.5 mEq/L were >3 times more likely to respond than those receiving placebo.6 When lithium is used to treat bipolar disorder, the therapeutic serum range for lithium is 0.8 to 1.2 mEq/L, with an increased risk of adverse effects (including toxicity) at higher levels.7
Triiodothyronine (T3) augmentation of antidepressants led to remission in approximately 1 in 4 patients who had not achieved remission or who were intolerant to an initial treatment with citalopram and a second switch or augmentation trial.8 In this study, the mean dose of T3 was 45.2 µg/d, with an average length of treatment of 9 weeks.
Tricyclic antidepressants are another option when considering switching agents (Table 3). TCAs are additionally effective for comorbid pain conditions.9 When TCAs are used in combination with SSRIs, drug interactions may occur that increase TCA plasma levels. There is also an increased risk of serotonin syndrome when used with serotonergic agents, though an SSRI/ TCA combination may be appropriate for a patient with treatment-resistant depression.10 Additionally, TCAs carry unique risks of cardiovascular effects, including cardiac arrhythmias. A meta-analysis comparing fluoxetine, paroxetine, and sertraline to TCAs (amitriptyline, clomipramine, desipramine, doxepin, imipramine, and nortriptyline) concluded that both classes had similar efficacy in treating depression, though the drop-out rate was significantly higher among patients receiving TCAs.11
Buspirone is approved for generalized anxiety disorder. In studies where buspirone was used as an augmentation agent for major depressive disorder at a mean daily dose of 40.9 mg divided into 2 doses, it led to a remission rate >30%.3
Continue to: Monoamine oxidase inhibitors
Monoamine oxidase inhibitors should typically be avoided in initial or early treatment of depression due to tolerability issues, drug interactions, and dietary restrictions to avoid hypertensive crisis. MAOIs are generally not recommended to be used with SSRIs, SNRIs, or TCAs, and typically require a “washout” period from other antidepressants (Table 4). One review found that MAOI treatment had advantage over TCA treatment for patients with early-stage treatment-resistant depression, though this advantage decreased as the number of failed antidepressant trials increased.12 One MAOI, selegiline, is available in a transdermal patch, and the 6-mg patch does not require dietary restriction.
Esketamine (intranasal) is FDA-approved for treatment-resistant depression (failure of response after at least 2 antidepressant trials with adequate dose and duration) in conjunction with an oral antidepressant. In clinical studies, a significant response was noted after 1 week of treatment.13 Esketamine requires an induction period of twice-weekly doses of 56 or 84 mg, with maintenance doses every 1 to 2 weeks. Each dosage administration requires monitoring for at least 2 hours by a health care professional at a certified treatment center. Esketamine’s indication was recently expanded to include treatment of patients with major depressive disorder with suicidal ideation or behavior.
Stimulants such as amphetamines, methylphenidate, or modafinil have been effective in open studies for augmentation in depression.14 However, no stimulant is FDA-approved for the treatment of depression. In addition to other adverse effects, these medications are controlled substances and carry risk of misuse, and their use may not be appropriate for all patients.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
1. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
2. Kato M, Chang CM. Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs. 2013;27 Suppl 1:S11-S19.
3. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
4. Carpenter LL, Jocic Z, Hall JM, et al. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
5. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201-210.
6. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol. 1999;19(5):427-434.
7. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
8. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
9. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;17(4):CD005454.
10. Taylor D. Selective serotonin reuptake inhibitors and tricyclic antidepressants in combination. Interactions and therapeutic uses. Br J Psychiatry. 1995;167(5):575-580.
11. Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? Comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6(1):10-18.
12. Kim T, Xu C, Amsterdam JD. Relative effectiveness of tricyclic antidepressant versus monoamine oxidase inhibitor monotherapy for treatment-resistant depression. J Affect Disord. 2019;250:199-203.
13. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139-148.
14. DeBattista C. Augmentation and combination strategies for depression. J Psychopharmacol. 2006;20(3 Suppl):11-18.
Nontraditional therapies for treatment-resistant depression
Presently, FDA-approved first-line treatments and standard adjunctive strategies (eg, lithium, thyroid supplementation, stimulants, second-generation antipsychotics) for major depressive disorder (MDD) often produce less-than-desired outcomes while carrying a potentially substantial safety and tolerability burden. The lack of clinically useful and individual-based biomarkers (eg, genetic, neurophysiological, imaging) is a major obstacle to enhancing treatment efficacy and/or decreasing associated adverse effects (AEs). While the discovery of such tools is being aggressively pursued and ultimately will facilitate a more precision-based choice of therapy, empirical strategies remain our primary approach.
In controlled trials, several nontraditional treatments used primarily as adjuncts to standard antidepressants have shown promise. These include “repurposed” (off-label) medications, herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
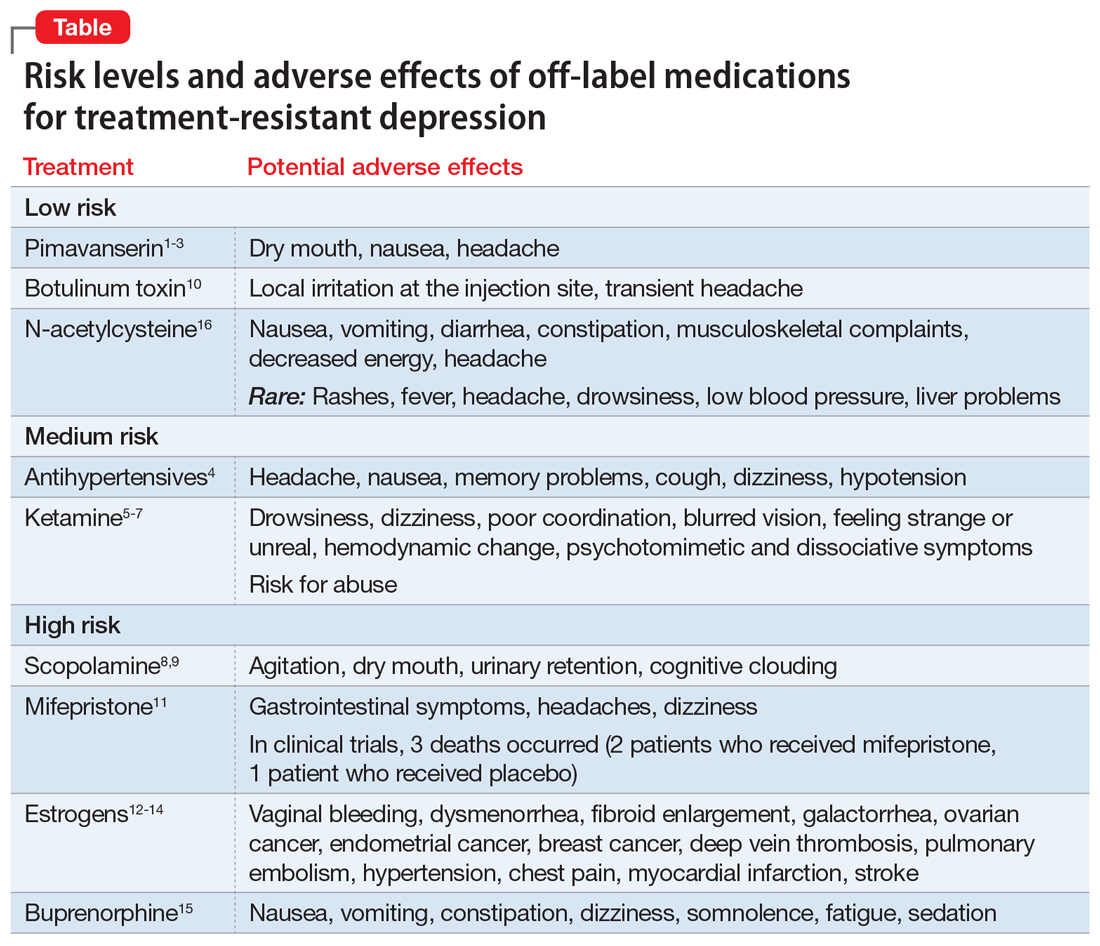
Importantly, some nontraditional treatments also demonstrate AEs (Table1-16). With a careful consideration of the risk/benefit balance, this article reviews some of the better-studied treatment options for patients with treatment-resistant depression (TRD). In Part 1, we will examine off-label medications. In Part 2, we will review other nontraditional approaches to TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
We believe this review will help clinicians who need to formulate a different approach after their patient with depression is not helped by traditional first-, second-, and third-line treatments. The potential options discussed in Part 1 of this article are categorized based on their putative mechanism of action (MOA) for depression.
Serotonergic and noradrenergic strategies
Pimavanserin is FDA-approved for treatment of Parkinson’s psychosis. Its potential MOA as an adjunctive strategy for MDD may involve 5-HT2A antagonist and inverse agonist receptor activity, as well as lesser effects at the 5-HT2Creceptor.
A 2-stage, 5-week randomized controlled trial (RCT) (CLARITY; N = 207) found adjunctive pimavanserin (34 mg/d) produced a robust antidepressant effect vs placebo in patients whose depression did not respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).1 Furthermore, a secondary analysis of the data suggested that pimavanserin also improved sleepiness (P < .0003) and daily functioning (P < .014) at Week 5.2
Unfortunately, two 6-week, Phase III RCTs (CLARITY-2 and -3; N = 298) did not find a statistically significant difference between active treatment and placebo. This was based on change in the primary outcome measure (Hamilton Depression Rating Scale-17 score) when adjunctive pimavanserin (34 mg/d) was added to an SSRI or SNRI in patients with TRD.3 There was, however, a significant difference favoring active treatment over placebo based on the Clinical Global Impression–Severity score.
Continue to: In these trials...
In these trials, pimavanserin was generally well-tolerated. The most common AEs were dry mouth, nausea, and headache. Pimavanserin has minimal activity at norepinephrine, dopamine, histamine, or acetylcholine receptors, thus avoiding AEs associated with these receptor interactions.
Given the mixed efficacy results of existing trials, further studies are needed to clarify this agent’s overall risk/benefit in the context of TRD.
Antihypertensive medications
Emerging data suggest that some beta-adrenergic blockers, angiotensin-inhibiting agents, and calcium antagonists are associated with a decreased incidence of depression. A large 2020 study (N = 3,747,190) used population-based Danish registries (2005 to 2015) to evaluate if any of the 41 most commonly prescribed antihypertensive medications were associated with the diagnosis of depressive disorder or use of antidepressants.4 These researchers found that enalapril, ramipril, amlodipine, propranolol, atenolol, bisoprolol, carvedilol (P < .001), and verapamil (P < .004) were strongly associated with a decreased risk of depression.4
Adverse effects across these different classes of antihypertensives are well characterized, can be substantial, and commonly are related to their impact on cardiovascular function (eg, hypotension). Clinically, these agents may be potential adjuncts for patients with TRD who need antihypertensive therapy. Their use and the choice of specific agent should only be determined in consultation with the patient’s primary care physician (PCP) or appropriate specialist.
Glutamatergic strategies
Ketamine is a dissociative anesthetic and analgesic. Its MOA for treating depression appears to occur primarily through antagonist activity at the N-methyl-
Continue to: Many published studies...
Many published studies and reviews have described ketamine’s role for treating MDD. Several studies have reported that low-dose (0.5 mg/kg) IV ketamine infusions can rapidly attenuate severe episodes of MDD as well as associated suicidality. For example, a meta-analysis of 9 RCTs (N = 368) comparing ketamine to placebo for acute treatment of unipolar and bipolar depression reported superior therapeutic effects with active treatment at 24 hours, 72 hours, and 7 days.6 The response and remission rates for ketamine were 52% and 21% at 24 hours; 48% and 24% at 72 hours; and 40% and 26% at 7 days, respectively.6
The most commonly reported AEs during the 4 hours after ketamine infusion included7:
- drowsiness, dizziness, poor coordination
- blurred vision, feeling strange or unreal
- hemodynamic changes (approximately 33%)
- small but significant (P < .05) increases in psychotomimetic and dissociative symptoms.
Because some individuals use ketamine recreationally, this agent also carries the risk of abuse.
Research is ongoing on strategies for long-term maintenance ketamine treatment, and the results of both short- and long-term trials will require careful scrutiny to better assess this agent’s safety and tolerability. Clinicians should first consider esketamine—the S-enantiomer of ketamine—because an intranasal formulation of this agent is FDA-approved for treating patients with TRD or MDD with suicidality when administered in a Risk Evaluation and Mitigation Strategy–certified setting.
Cholinergic strategies
Scopolamine is a potent muscarinic receptor antagonist used to prevent nausea and vomiting caused by motion sickness or medications used during surgery. Its use for MDD is based on the theory that muscarinic receptors may be hypersensitive in mood disorders.
Continue to: Several double-blind RCTs...
Several double-blind RCTs of patients with unipolar or bipolar depression that used 3 pulsed IV infusions (4.0 mcg/kg) over 15 minutes found a rapid, robust antidepressant effect with scopolamine vs placebo.8,9 The oral formulation might also be effective, but would not have a rapid onset.
Common adverse effects of scopolamine include agitation, dry mouth, urinary retention, and cognitive clouding. Given scopolamine’s substantial AE profile, it should be considered only for patients with TRD who could also benefit from the oral formulation for the medical indications noted above, should generally be avoided in older patients, and should be prescribed in consultation with the patient’s PCP.
Botulinum toxin. This neurotoxin inhibits acetylcholine release. It is used to treat disorders characterized by abnormal muscular contraction, such as strabismus, blepharospasm, and chronic pain syndromes. Its MOA for depression may involve its paralytic effects after injection into the glabella forehead muscle (based on the facial feedback hypothesis), as well as modulation of neurotransmitters implicated in the pathophysiology of depression.
In several small trials, injectable botulinum toxin type A (BTA) (29 units) demonstrated antidepressant effects. A recent review that considered 6 trials (N = 235; 4 of the 6 studies were RCTs, 3 of which were rated as high quality) concluded that BTA may be a promising treatment for MDD.10 Limitations of this review included lack of a priori hypotheses, small sample sizes, gender bias, and difficulty in blinding.
In clinical trials, the most common AEs included local irritation at the injection site and transient headache. This agent’s relatively mild AE profile and possible overlap when used for some of the medical indications noted above opens its potential use as an adjunct in patients with comorbid TRD.
Continue to: Endocrine strategies
Endocrine strategies
Mifepristone (RU486). This anti-glucocorticoid receptor antagonist is used as an abortifacient. Based on the theory that hyperactivity of the hypothalamic-pituitary-adrenal axis is implicated in the pathophysiology of MDD with psychotic features (psychotic depression), this agent has been studied as a treatment for this indication.
An analysis of 5 double-blind RCTs (N = 1,460) found that 7 days of mifepristone, 1,200 mg/d, was superior to placebo (P < .004) in reducing psychotic symptoms of depression.11 Plasma concentrations ≥1,600 ng/mL may be required to maximize benefit.11
Overall, this agent demonstrated a good safety profile in clinical trials, with treatment-emergent AEs reported in 556 (66.7%) patients who received mifepristone vs 386 (61.6%) patients who received placebo.11 Common AEs included gastrointestinal (GI) symptoms, headache, and dizziness. However, 3 deaths occurred: 2 patients who received mifepristone and 1 patient who received placebo. Given this potential for a fatal outcome, clinicians should first consider prescribing an adjunctive antipsychotic agent or electroconvulsive therapy.
Estrogens. These hormones are important for sexual and reproductive development and are used to treat various sexual/reproductive disorders, primarily in women. Their role in treating depression is based on the observation that perimenopause is accompanied by an increased risk of new and recurrent depression coincident with declining ovarian function.
Evidence supports the antidepressant efficacy of transdermal estradiol plus progesterone for perimenopausal depression, but not for postmenopausal depression.12-14 However, estrogens carry significant risks that must be carefully considered in relationship to their potential benefits. These risks include:
- vaginal bleeding, dysmenorrhea
- fibroid enlargement
- galactorrhea
- ovarian cancer, endometrial cancer, breast cancer
- deep vein thrombosis, pulmonary embolism
- hypertension, chest pain, myocardial infarction, stroke.
Continue to: The use of estrogens...
The use of estrogens as an adjunctive therapy for women with treatment-resistant perimenopausal depression should only be undertaken when standard strategies have failed, and in consultation with an endocrine specialist who can monitor for potentially serious AEs.
Opioid medications
Buprenorphine is used to treat opioid use disorder (OUD) as well as acute and chronic pain. The opioid system is involved in the regulation of mood and may be an appropriate target for novel antidepressants. The use of buprenorphine in combination with samidorphan (a preferential mu-opioid receptor antagonist) has shown initial promise for TRD while minimizing abuse potential.
Although earlier results were mixed, a pooled analysis of 2 recent large RCTs (N = 760) of patients with MDD who had not responded to antidepressants reported greater reduction in Montgomery-Åsberg Depression Rating Scale scores from baseline for active treatment (buprenorphine/samidorphan; 2 mg/2 mg) vs placebo at multiple timepoints, including end of treatment (-1.8; P < .010).15
The most common AEs included nausea, constipation, dizziness, vomiting, somnolence, fatigue, and sedation. There was minimal evidence of abuse, dependence, or opioid withdrawal. Due to the opioid crisis in the United States, the resulting relaxation of regulations regarding prescribing buprenorphine, and the high rates of depression among patients with OUD, buprenorphine/samidorphan, which is an investigational agent that is not FDA-approved, may be particularly helpful for patients with OUD who also experience comorbid TRD.
Antioxidant agents
N-acetylcysteine (NAC) is an amino acid that can treat acetaminophen toxicity and moderate hepatic damage by increasing glutathione levels. Glutathione is also the primary antioxidant in the CNS. NAC may protect against oxidative stress, chelate heavy metals, reduce inflammation, protect against mitochondrial dysfunction, inhibit apoptosis, and enhance neurogenesis, all potential pathophysiological processes that may contribute to depression.16
Continue to: A systematic review...
A systematic review and meta-analysis of 5 RCTs (N = 574) considered patients with various depression diagnoses who were randomized to adjunctive NAC, 1,000 mg twice a day, or placebo. Over 12 to 24 weeks, there was a significantly greater improvement in mood symptoms and functionality with NAC vs placebo.16
Overall, NAC was well-tolerated. The most common AEs were GI symptoms, musculoskeletal complaints, decreased energy, and headache. While NAC has been touted as a potential adjunct therapy for several psychiatric disorders, including TRD, the evidence for benefit remains limited. Given its favorable AE profile, however, and over-the-counter availability, it remains an option for select patients. It is important to ask patients if they are already taking NAC.
Options beyond off-label medications
There are a multitude of options available for addressing TRD. Many FDA-approved medications are repurposed and prescribed off-label for other indications when the risk/benefit balance is favorable. In Part 1 of this article, we reviewed several off-label medications that have supportive controlled data for treating TRD. In Part 2, we will review other nontraditional therapies for TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Bottom Line
Off-label medications that may offer benefit for patients with treatment-resistant depression (TRD) include pimavanserin, antihypertensive agents, ketamine, scopolamine, botulinum toxin, mifepristone, estrogens, buprenorphine, and N-acetylcysteine. Although some evidence supports use of these agents as adjuncts for TRD, an individualized risk/benefit analysis is required.
Related Resource
- Joshi KG, Frierson RL. Off-label prescribing: How to limit your liability. Current Psychiatry. 2020;19(9):12,39.
Drug Brand Names
Amlodipine • Katerzia, Norvasc
Atenolol • Tenormin
Bisoprolol • Zebeta
Buprenorphine • Sublocade, Subutex
Carvedilol • Coreg
Enalapril • Vasotec
Esketamine • Spravato
Estradiol transdermal • Estraderm
Ketamine • Ketalar
Mifepristone • Mifeprex
Pimavanserin • Nuplazid
Progesterone • Prometrium
Propranolol • Inderal
Ramipril • Altace
Verapamil • Calan, Verelan
1. Fava M, Dirks B, Freeman M, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80(6):19m12928.
2. Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2020;82(1):20m13425.
3. ACADIA Pharmaceuticals announces top-line results from the Phase 3 CLARITY study evaluating pimavanserin for the adjunctive treatment of major depressive disorder. News release. Acadia Pharmaceuticals Inc. Published July 20, 2020. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-top-line-results-phase-3-0
4. Kessing LV, Rytgaard HC, Ekstrom CT, et al. Antihypertensive drugs and risk of depression: a nationwide population-based study. Hypertension. 2020;76(4):1263-1279.
5. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
6. Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859-2867.
7. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
8. Hasselmann, H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
9. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
10. Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20(5): 18r02298.
11. Block TS, Kushner H, Kalin N, et al. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry. 2018;84(1):46-54.
12. Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32(8):539-549.
13. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
14. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149-157.
15. Fava M, Thase ME, Trivedi MH, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25(7):1580-1591.
16. Fernandes BS, Dean OM, Dodd S, et al. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77(4):e457-466.
Presently, FDA-approved first-line treatments and standard adjunctive strategies (eg, lithium, thyroid supplementation, stimulants, second-generation antipsychotics) for major depressive disorder (MDD) often produce less-than-desired outcomes while carrying a potentially substantial safety and tolerability burden. The lack of clinically useful and individual-based biomarkers (eg, genetic, neurophysiological, imaging) is a major obstacle to enhancing treatment efficacy and/or decreasing associated adverse effects (AEs). While the discovery of such tools is being aggressively pursued and ultimately will facilitate a more precision-based choice of therapy, empirical strategies remain our primary approach.
In controlled trials, several nontraditional treatments used primarily as adjuncts to standard antidepressants have shown promise. These include “repurposed” (off-label) medications, herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Importantly, some nontraditional treatments also demonstrate AEs (Table1-16). With a careful consideration of the risk/benefit balance, this article reviews some of the better-studied treatment options for patients with treatment-resistant depression (TRD). In Part 1, we will examine off-label medications. In Part 2, we will review other nontraditional approaches to TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.

We believe this review will help clinicians who need to formulate a different approach after their patient with depression is not helped by traditional first-, second-, and third-line treatments. The potential options discussed in Part 1 of this article are categorized based on their putative mechanism of action (MOA) for depression.
Serotonergic and noradrenergic strategies
Pimavanserin is FDA-approved for treatment of Parkinson’s psychosis. Its potential MOA as an adjunctive strategy for MDD may involve 5-HT2A antagonist and inverse agonist receptor activity, as well as lesser effects at the 5-HT2Creceptor.
A 2-stage, 5-week randomized controlled trial (RCT) (CLARITY; N = 207) found adjunctive pimavanserin (34 mg/d) produced a robust antidepressant effect vs placebo in patients whose depression did not respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).1 Furthermore, a secondary analysis of the data suggested that pimavanserin also improved sleepiness (P < .0003) and daily functioning (P < .014) at Week 5.2
Unfortunately, two 6-week, Phase III RCTs (CLARITY-2 and -3; N = 298) did not find a statistically significant difference between active treatment and placebo. This was based on change in the primary outcome measure (Hamilton Depression Rating Scale-17 score) when adjunctive pimavanserin (34 mg/d) was added to an SSRI or SNRI in patients with TRD.3 There was, however, a significant difference favoring active treatment over placebo based on the Clinical Global Impression–Severity score.
Continue to: In these trials...
In these trials, pimavanserin was generally well-tolerated. The most common AEs were dry mouth, nausea, and headache. Pimavanserin has minimal activity at norepinephrine, dopamine, histamine, or acetylcholine receptors, thus avoiding AEs associated with these receptor interactions.
Given the mixed efficacy results of existing trials, further studies are needed to clarify this agent’s overall risk/benefit in the context of TRD.
Antihypertensive medications
Emerging data suggest that some beta-adrenergic blockers, angiotensin-inhibiting agents, and calcium antagonists are associated with a decreased incidence of depression. A large 2020 study (N = 3,747,190) used population-based Danish registries (2005 to 2015) to evaluate if any of the 41 most commonly prescribed antihypertensive medications were associated with the diagnosis of depressive disorder or use of antidepressants.4 These researchers found that enalapril, ramipril, amlodipine, propranolol, atenolol, bisoprolol, carvedilol (P < .001), and verapamil (P < .004) were strongly associated with a decreased risk of depression.4
Adverse effects across these different classes of antihypertensives are well characterized, can be substantial, and commonly are related to their impact on cardiovascular function (eg, hypotension). Clinically, these agents may be potential adjuncts for patients with TRD who need antihypertensive therapy. Their use and the choice of specific agent should only be determined in consultation with the patient’s primary care physician (PCP) or appropriate specialist.
Glutamatergic strategies
Ketamine is a dissociative anesthetic and analgesic. Its MOA for treating depression appears to occur primarily through antagonist activity at the N-methyl-
Continue to: Many published studies...
Many published studies and reviews have described ketamine’s role for treating MDD. Several studies have reported that low-dose (0.5 mg/kg) IV ketamine infusions can rapidly attenuate severe episodes of MDD as well as associated suicidality. For example, a meta-analysis of 9 RCTs (N = 368) comparing ketamine to placebo for acute treatment of unipolar and bipolar depression reported superior therapeutic effects with active treatment at 24 hours, 72 hours, and 7 days.6 The response and remission rates for ketamine were 52% and 21% at 24 hours; 48% and 24% at 72 hours; and 40% and 26% at 7 days, respectively.6
The most commonly reported AEs during the 4 hours after ketamine infusion included7:
- drowsiness, dizziness, poor coordination
- blurred vision, feeling strange or unreal
- hemodynamic changes (approximately 33%)
- small but significant (P < .05) increases in psychotomimetic and dissociative symptoms.
Because some individuals use ketamine recreationally, this agent also carries the risk of abuse.
Research is ongoing on strategies for long-term maintenance ketamine treatment, and the results of both short- and long-term trials will require careful scrutiny to better assess this agent’s safety and tolerability. Clinicians should first consider esketamine—the S-enantiomer of ketamine—because an intranasal formulation of this agent is FDA-approved for treating patients with TRD or MDD with suicidality when administered in a Risk Evaluation and Mitigation Strategy–certified setting.
Cholinergic strategies
Scopolamine is a potent muscarinic receptor antagonist used to prevent nausea and vomiting caused by motion sickness or medications used during surgery. Its use for MDD is based on the theory that muscarinic receptors may be hypersensitive in mood disorders.
Continue to: Several double-blind RCTs...
Several double-blind RCTs of patients with unipolar or bipolar depression that used 3 pulsed IV infusions (4.0 mcg/kg) over 15 minutes found a rapid, robust antidepressant effect with scopolamine vs placebo.8,9 The oral formulation might also be effective, but would not have a rapid onset.
Common adverse effects of scopolamine include agitation, dry mouth, urinary retention, and cognitive clouding. Given scopolamine’s substantial AE profile, it should be considered only for patients with TRD who could also benefit from the oral formulation for the medical indications noted above, should generally be avoided in older patients, and should be prescribed in consultation with the patient’s PCP.
Botulinum toxin. This neurotoxin inhibits acetylcholine release. It is used to treat disorders characterized by abnormal muscular contraction, such as strabismus, blepharospasm, and chronic pain syndromes. Its MOA for depression may involve its paralytic effects after injection into the glabella forehead muscle (based on the facial feedback hypothesis), as well as modulation of neurotransmitters implicated in the pathophysiology of depression.
In several small trials, injectable botulinum toxin type A (BTA) (29 units) demonstrated antidepressant effects. A recent review that considered 6 trials (N = 235; 4 of the 6 studies were RCTs, 3 of which were rated as high quality) concluded that BTA may be a promising treatment for MDD.10 Limitations of this review included lack of a priori hypotheses, small sample sizes, gender bias, and difficulty in blinding.
In clinical trials, the most common AEs included local irritation at the injection site and transient headache. This agent’s relatively mild AE profile and possible overlap when used for some of the medical indications noted above opens its potential use as an adjunct in patients with comorbid TRD.
Continue to: Endocrine strategies
Endocrine strategies
Mifepristone (RU486). This anti-glucocorticoid receptor antagonist is used as an abortifacient. Based on the theory that hyperactivity of the hypothalamic-pituitary-adrenal axis is implicated in the pathophysiology of MDD with psychotic features (psychotic depression), this agent has been studied as a treatment for this indication.
An analysis of 5 double-blind RCTs (N = 1,460) found that 7 days of mifepristone, 1,200 mg/d, was superior to placebo (P < .004) in reducing psychotic symptoms of depression.11 Plasma concentrations ≥1,600 ng/mL may be required to maximize benefit.11
Overall, this agent demonstrated a good safety profile in clinical trials, with treatment-emergent AEs reported in 556 (66.7%) patients who received mifepristone vs 386 (61.6%) patients who received placebo.11 Common AEs included gastrointestinal (GI) symptoms, headache, and dizziness. However, 3 deaths occurred: 2 patients who received mifepristone and 1 patient who received placebo. Given this potential for a fatal outcome, clinicians should first consider prescribing an adjunctive antipsychotic agent or electroconvulsive therapy.
Estrogens. These hormones are important for sexual and reproductive development and are used to treat various sexual/reproductive disorders, primarily in women. Their role in treating depression is based on the observation that perimenopause is accompanied by an increased risk of new and recurrent depression coincident with declining ovarian function.
Evidence supports the antidepressant efficacy of transdermal estradiol plus progesterone for perimenopausal depression, but not for postmenopausal depression.12-14 However, estrogens carry significant risks that must be carefully considered in relationship to their potential benefits. These risks include:
- vaginal bleeding, dysmenorrhea
- fibroid enlargement
- galactorrhea
- ovarian cancer, endometrial cancer, breast cancer
- deep vein thrombosis, pulmonary embolism
- hypertension, chest pain, myocardial infarction, stroke.
Continue to: The use of estrogens...
The use of estrogens as an adjunctive therapy for women with treatment-resistant perimenopausal depression should only be undertaken when standard strategies have failed, and in consultation with an endocrine specialist who can monitor for potentially serious AEs.
Opioid medications
Buprenorphine is used to treat opioid use disorder (OUD) as well as acute and chronic pain. The opioid system is involved in the regulation of mood and may be an appropriate target for novel antidepressants. The use of buprenorphine in combination with samidorphan (a preferential mu-opioid receptor antagonist) has shown initial promise for TRD while minimizing abuse potential.
Although earlier results were mixed, a pooled analysis of 2 recent large RCTs (N = 760) of patients with MDD who had not responded to antidepressants reported greater reduction in Montgomery-Åsberg Depression Rating Scale scores from baseline for active treatment (buprenorphine/samidorphan; 2 mg/2 mg) vs placebo at multiple timepoints, including end of treatment (-1.8; P < .010).15
The most common AEs included nausea, constipation, dizziness, vomiting, somnolence, fatigue, and sedation. There was minimal evidence of abuse, dependence, or opioid withdrawal. Due to the opioid crisis in the United States, the resulting relaxation of regulations regarding prescribing buprenorphine, and the high rates of depression among patients with OUD, buprenorphine/samidorphan, which is an investigational agent that is not FDA-approved, may be particularly helpful for patients with OUD who also experience comorbid TRD.
Antioxidant agents
N-acetylcysteine (NAC) is an amino acid that can treat acetaminophen toxicity and moderate hepatic damage by increasing glutathione levels. Glutathione is also the primary antioxidant in the CNS. NAC may protect against oxidative stress, chelate heavy metals, reduce inflammation, protect against mitochondrial dysfunction, inhibit apoptosis, and enhance neurogenesis, all potential pathophysiological processes that may contribute to depression.16
Continue to: A systematic review...
A systematic review and meta-analysis of 5 RCTs (N = 574) considered patients with various depression diagnoses who were randomized to adjunctive NAC, 1,000 mg twice a day, or placebo. Over 12 to 24 weeks, there was a significantly greater improvement in mood symptoms and functionality with NAC vs placebo.16
Overall, NAC was well-tolerated. The most common AEs were GI symptoms, musculoskeletal complaints, decreased energy, and headache. While NAC has been touted as a potential adjunct therapy for several psychiatric disorders, including TRD, the evidence for benefit remains limited. Given its favorable AE profile, however, and over-the-counter availability, it remains an option for select patients. It is important to ask patients if they are already taking NAC.
Options beyond off-label medications
There are a multitude of options available for addressing TRD. Many FDA-approved medications are repurposed and prescribed off-label for other indications when the risk/benefit balance is favorable. In Part 1 of this article, we reviewed several off-label medications that have supportive controlled data for treating TRD. In Part 2, we will review other nontraditional therapies for TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Bottom Line
Off-label medications that may offer benefit for patients with treatment-resistant depression (TRD) include pimavanserin, antihypertensive agents, ketamine, scopolamine, botulinum toxin, mifepristone, estrogens, buprenorphine, and N-acetylcysteine. Although some evidence supports use of these agents as adjuncts for TRD, an individualized risk/benefit analysis is required.
Related Resource
- Joshi KG, Frierson RL. Off-label prescribing: How to limit your liability. Current Psychiatry. 2020;19(9):12,39.
Drug Brand Names
Amlodipine • Katerzia, Norvasc
Atenolol • Tenormin
Bisoprolol • Zebeta
Buprenorphine • Sublocade, Subutex
Carvedilol • Coreg
Enalapril • Vasotec
Esketamine • Spravato
Estradiol transdermal • Estraderm
Ketamine • Ketalar
Mifepristone • Mifeprex
Pimavanserin • Nuplazid
Progesterone • Prometrium
Propranolol • Inderal
Ramipril • Altace
Verapamil • Calan, Verelan
Presently, FDA-approved first-line treatments and standard adjunctive strategies (eg, lithium, thyroid supplementation, stimulants, second-generation antipsychotics) for major depressive disorder (MDD) often produce less-than-desired outcomes while carrying a potentially substantial safety and tolerability burden. The lack of clinically useful and individual-based biomarkers (eg, genetic, neurophysiological, imaging) is a major obstacle to enhancing treatment efficacy and/or decreasing associated adverse effects (AEs). While the discovery of such tools is being aggressively pursued and ultimately will facilitate a more precision-based choice of therapy, empirical strategies remain our primary approach.
In controlled trials, several nontraditional treatments used primarily as adjuncts to standard antidepressants have shown promise. These include “repurposed” (off-label) medications, herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Importantly, some nontraditional treatments also demonstrate AEs (Table1-16). With a careful consideration of the risk/benefit balance, this article reviews some of the better-studied treatment options for patients with treatment-resistant depression (TRD). In Part 1, we will examine off-label medications. In Part 2, we will review other nontraditional approaches to TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.

We believe this review will help clinicians who need to formulate a different approach after their patient with depression is not helped by traditional first-, second-, and third-line treatments. The potential options discussed in Part 1 of this article are categorized based on their putative mechanism of action (MOA) for depression.
Serotonergic and noradrenergic strategies
Pimavanserin is FDA-approved for treatment of Parkinson’s psychosis. Its potential MOA as an adjunctive strategy for MDD may involve 5-HT2A antagonist and inverse agonist receptor activity, as well as lesser effects at the 5-HT2Creceptor.
A 2-stage, 5-week randomized controlled trial (RCT) (CLARITY; N = 207) found adjunctive pimavanserin (34 mg/d) produced a robust antidepressant effect vs placebo in patients whose depression did not respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).1 Furthermore, a secondary analysis of the data suggested that pimavanserin also improved sleepiness (P < .0003) and daily functioning (P < .014) at Week 5.2
Unfortunately, two 6-week, Phase III RCTs (CLARITY-2 and -3; N = 298) did not find a statistically significant difference between active treatment and placebo. This was based on change in the primary outcome measure (Hamilton Depression Rating Scale-17 score) when adjunctive pimavanserin (34 mg/d) was added to an SSRI or SNRI in patients with TRD.3 There was, however, a significant difference favoring active treatment over placebo based on the Clinical Global Impression–Severity score.
Continue to: In these trials...
In these trials, pimavanserin was generally well-tolerated. The most common AEs were dry mouth, nausea, and headache. Pimavanserin has minimal activity at norepinephrine, dopamine, histamine, or acetylcholine receptors, thus avoiding AEs associated with these receptor interactions.
Given the mixed efficacy results of existing trials, further studies are needed to clarify this agent’s overall risk/benefit in the context of TRD.
Antihypertensive medications
Emerging data suggest that some beta-adrenergic blockers, angiotensin-inhibiting agents, and calcium antagonists are associated with a decreased incidence of depression. A large 2020 study (N = 3,747,190) used population-based Danish registries (2005 to 2015) to evaluate if any of the 41 most commonly prescribed antihypertensive medications were associated with the diagnosis of depressive disorder or use of antidepressants.4 These researchers found that enalapril, ramipril, amlodipine, propranolol, atenolol, bisoprolol, carvedilol (P < .001), and verapamil (P < .004) were strongly associated with a decreased risk of depression.4
Adverse effects across these different classes of antihypertensives are well characterized, can be substantial, and commonly are related to their impact on cardiovascular function (eg, hypotension). Clinically, these agents may be potential adjuncts for patients with TRD who need antihypertensive therapy. Their use and the choice of specific agent should only be determined in consultation with the patient’s primary care physician (PCP) or appropriate specialist.
Glutamatergic strategies
Ketamine is a dissociative anesthetic and analgesic. Its MOA for treating depression appears to occur primarily through antagonist activity at the N-methyl-
Continue to: Many published studies...
Many published studies and reviews have described ketamine’s role for treating MDD. Several studies have reported that low-dose (0.5 mg/kg) IV ketamine infusions can rapidly attenuate severe episodes of MDD as well as associated suicidality. For example, a meta-analysis of 9 RCTs (N = 368) comparing ketamine to placebo for acute treatment of unipolar and bipolar depression reported superior therapeutic effects with active treatment at 24 hours, 72 hours, and 7 days.6 The response and remission rates for ketamine were 52% and 21% at 24 hours; 48% and 24% at 72 hours; and 40% and 26% at 7 days, respectively.6
The most commonly reported AEs during the 4 hours after ketamine infusion included7:
- drowsiness, dizziness, poor coordination
- blurred vision, feeling strange or unreal
- hemodynamic changes (approximately 33%)
- small but significant (P < .05) increases in psychotomimetic and dissociative symptoms.
Because some individuals use ketamine recreationally, this agent also carries the risk of abuse.
Research is ongoing on strategies for long-term maintenance ketamine treatment, and the results of both short- and long-term trials will require careful scrutiny to better assess this agent’s safety and tolerability. Clinicians should first consider esketamine—the S-enantiomer of ketamine—because an intranasal formulation of this agent is FDA-approved for treating patients with TRD or MDD with suicidality when administered in a Risk Evaluation and Mitigation Strategy–certified setting.
Cholinergic strategies
Scopolamine is a potent muscarinic receptor antagonist used to prevent nausea and vomiting caused by motion sickness or medications used during surgery. Its use for MDD is based on the theory that muscarinic receptors may be hypersensitive in mood disorders.
Continue to: Several double-blind RCTs...
Several double-blind RCTs of patients with unipolar or bipolar depression that used 3 pulsed IV infusions (4.0 mcg/kg) over 15 minutes found a rapid, robust antidepressant effect with scopolamine vs placebo.8,9 The oral formulation might also be effective, but would not have a rapid onset.
Common adverse effects of scopolamine include agitation, dry mouth, urinary retention, and cognitive clouding. Given scopolamine’s substantial AE profile, it should be considered only for patients with TRD who could also benefit from the oral formulation for the medical indications noted above, should generally be avoided in older patients, and should be prescribed in consultation with the patient’s PCP.
Botulinum toxin. This neurotoxin inhibits acetylcholine release. It is used to treat disorders characterized by abnormal muscular contraction, such as strabismus, blepharospasm, and chronic pain syndromes. Its MOA for depression may involve its paralytic effects after injection into the glabella forehead muscle (based on the facial feedback hypothesis), as well as modulation of neurotransmitters implicated in the pathophysiology of depression.
In several small trials, injectable botulinum toxin type A (BTA) (29 units) demonstrated antidepressant effects. A recent review that considered 6 trials (N = 235; 4 of the 6 studies were RCTs, 3 of which were rated as high quality) concluded that BTA may be a promising treatment for MDD.10 Limitations of this review included lack of a priori hypotheses, small sample sizes, gender bias, and difficulty in blinding.
In clinical trials, the most common AEs included local irritation at the injection site and transient headache. This agent’s relatively mild AE profile and possible overlap when used for some of the medical indications noted above opens its potential use as an adjunct in patients with comorbid TRD.
Continue to: Endocrine strategies
Endocrine strategies
Mifepristone (RU486). This anti-glucocorticoid receptor antagonist is used as an abortifacient. Based on the theory that hyperactivity of the hypothalamic-pituitary-adrenal axis is implicated in the pathophysiology of MDD with psychotic features (psychotic depression), this agent has been studied as a treatment for this indication.
An analysis of 5 double-blind RCTs (N = 1,460) found that 7 days of mifepristone, 1,200 mg/d, was superior to placebo (P < .004) in reducing psychotic symptoms of depression.11 Plasma concentrations ≥1,600 ng/mL may be required to maximize benefit.11
Overall, this agent demonstrated a good safety profile in clinical trials, with treatment-emergent AEs reported in 556 (66.7%) patients who received mifepristone vs 386 (61.6%) patients who received placebo.11 Common AEs included gastrointestinal (GI) symptoms, headache, and dizziness. However, 3 deaths occurred: 2 patients who received mifepristone and 1 patient who received placebo. Given this potential for a fatal outcome, clinicians should first consider prescribing an adjunctive antipsychotic agent or electroconvulsive therapy.
Estrogens. These hormones are important for sexual and reproductive development and are used to treat various sexual/reproductive disorders, primarily in women. Their role in treating depression is based on the observation that perimenopause is accompanied by an increased risk of new and recurrent depression coincident with declining ovarian function.
Evidence supports the antidepressant efficacy of transdermal estradiol plus progesterone for perimenopausal depression, but not for postmenopausal depression.12-14 However, estrogens carry significant risks that must be carefully considered in relationship to their potential benefits. These risks include:
- vaginal bleeding, dysmenorrhea
- fibroid enlargement
- galactorrhea
- ovarian cancer, endometrial cancer, breast cancer
- deep vein thrombosis, pulmonary embolism
- hypertension, chest pain, myocardial infarction, stroke.
Continue to: The use of estrogens...
The use of estrogens as an adjunctive therapy for women with treatment-resistant perimenopausal depression should only be undertaken when standard strategies have failed, and in consultation with an endocrine specialist who can monitor for potentially serious AEs.
Opioid medications
Buprenorphine is used to treat opioid use disorder (OUD) as well as acute and chronic pain. The opioid system is involved in the regulation of mood and may be an appropriate target for novel antidepressants. The use of buprenorphine in combination with samidorphan (a preferential mu-opioid receptor antagonist) has shown initial promise for TRD while minimizing abuse potential.
Although earlier results were mixed, a pooled analysis of 2 recent large RCTs (N = 760) of patients with MDD who had not responded to antidepressants reported greater reduction in Montgomery-Åsberg Depression Rating Scale scores from baseline for active treatment (buprenorphine/samidorphan; 2 mg/2 mg) vs placebo at multiple timepoints, including end of treatment (-1.8; P < .010).15
The most common AEs included nausea, constipation, dizziness, vomiting, somnolence, fatigue, and sedation. There was minimal evidence of abuse, dependence, or opioid withdrawal. Due to the opioid crisis in the United States, the resulting relaxation of regulations regarding prescribing buprenorphine, and the high rates of depression among patients with OUD, buprenorphine/samidorphan, which is an investigational agent that is not FDA-approved, may be particularly helpful for patients with OUD who also experience comorbid TRD.
Antioxidant agents
N-acetylcysteine (NAC) is an amino acid that can treat acetaminophen toxicity and moderate hepatic damage by increasing glutathione levels. Glutathione is also the primary antioxidant in the CNS. NAC may protect against oxidative stress, chelate heavy metals, reduce inflammation, protect against mitochondrial dysfunction, inhibit apoptosis, and enhance neurogenesis, all potential pathophysiological processes that may contribute to depression.16
Continue to: A systematic review...
A systematic review and meta-analysis of 5 RCTs (N = 574) considered patients with various depression diagnoses who were randomized to adjunctive NAC, 1,000 mg twice a day, or placebo. Over 12 to 24 weeks, there was a significantly greater improvement in mood symptoms and functionality with NAC vs placebo.16
Overall, NAC was well-tolerated. The most common AEs were GI symptoms, musculoskeletal complaints, decreased energy, and headache. While NAC has been touted as a potential adjunct therapy for several psychiatric disorders, including TRD, the evidence for benefit remains limited. Given its favorable AE profile, however, and over-the-counter availability, it remains an option for select patients. It is important to ask patients if they are already taking NAC.
Options beyond off-label medications
There are a multitude of options available for addressing TRD. Many FDA-approved medications are repurposed and prescribed off-label for other indications when the risk/benefit balance is favorable. In Part 1 of this article, we reviewed several off-label medications that have supportive controlled data for treating TRD. In Part 2, we will review other nontraditional therapies for TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
Bottom Line
Off-label medications that may offer benefit for patients with treatment-resistant depression (TRD) include pimavanserin, antihypertensive agents, ketamine, scopolamine, botulinum toxin, mifepristone, estrogens, buprenorphine, and N-acetylcysteine. Although some evidence supports use of these agents as adjuncts for TRD, an individualized risk/benefit analysis is required.
Related Resource
- Joshi KG, Frierson RL. Off-label prescribing: How to limit your liability. Current Psychiatry. 2020;19(9):12,39.
Drug Brand Names
Amlodipine • Katerzia, Norvasc
Atenolol • Tenormin
Bisoprolol • Zebeta
Buprenorphine • Sublocade, Subutex
Carvedilol • Coreg
Enalapril • Vasotec
Esketamine • Spravato
Estradiol transdermal • Estraderm
Ketamine • Ketalar
Mifepristone • Mifeprex
Pimavanserin • Nuplazid
Progesterone • Prometrium
Propranolol • Inderal
Ramipril • Altace
Verapamil • Calan, Verelan
1. Fava M, Dirks B, Freeman M, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80(6):19m12928.
2. Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2020;82(1):20m13425.
3. ACADIA Pharmaceuticals announces top-line results from the Phase 3 CLARITY study evaluating pimavanserin for the adjunctive treatment of major depressive disorder. News release. Acadia Pharmaceuticals Inc. Published July 20, 2020. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-top-line-results-phase-3-0
4. Kessing LV, Rytgaard HC, Ekstrom CT, et al. Antihypertensive drugs and risk of depression: a nationwide population-based study. Hypertension. 2020;76(4):1263-1279.
5. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
6. Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859-2867.
7. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
8. Hasselmann, H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
9. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
10. Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20(5): 18r02298.
11. Block TS, Kushner H, Kalin N, et al. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry. 2018;84(1):46-54.
12. Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32(8):539-549.
13. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
14. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149-157.
15. Fava M, Thase ME, Trivedi MH, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25(7):1580-1591.
16. Fernandes BS, Dean OM, Dodd S, et al. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77(4):e457-466.
1. Fava M, Dirks B, Freeman M, et al. A phase 2, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin in patients with major depressive disorder and an inadequate response to therapy (CLARITY). J Clin Psychiatry. 2019;80(6):19m12928.
2. Jha MK, Fava M, Freeman MP, et al. Effect of adjunctive pimavanserin on sleep/wakefulness in patients with major depressive disorder: secondary analysis from CLARITY. J Clin Psychiatry. 2020;82(1):20m13425.
3. ACADIA Pharmaceuticals announces top-line results from the Phase 3 CLARITY study evaluating pimavanserin for the adjunctive treatment of major depressive disorder. News release. Acadia Pharmaceuticals Inc. Published July 20, 2020. https://ir.acadia-pharm.com/news-releases/news-release-details/acadia-pharmaceuticals-announces-top-line-results-phase-3-0
4. Kessing LV, Rytgaard HC, Ekstrom CT, et al. Antihypertensive drugs and risk of depression: a nationwide population-based study. Hypertension. 2020;76(4):1263-1279.
5. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
6. Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859-2867.
7. Wan LB, Levitch CF, Perez AM, et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76(3):247-252.
8. Hasselmann, H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
9. Drevets WC, Zarate CA Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156-1163.
10. Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20(5): 18r02298.
11. Block TS, Kushner H, Kalin N, et al. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry. 2018;84(1):46-54.
12. Rubinow DR, Johnson SL, Schmidt PJ, et al. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015;32(8):539-549.
13. Schmidt PJ, Ben Dor R, Martinez PE, et al. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry. 2015;72(7):714-726.
14. Gordon JL, Rubinow DR, Eisenlohr-Moul TA, et al. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):149-157.
15. Fava M, Thase ME, Trivedi MH, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25(7):1580-1591.
16. Fernandes BS, Dean OM, Dodd S, et al. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77(4):e457-466.
Confidentiality and privilege: What you don’t know can hurt you
Mrs. W, age 35, presents to your clinic seeking treatment for anxiety and depression. She has no psychiatric history but reports feeling sad, overwhelmed, and stressed. Mrs. W has been married for 10 years, has 2 young children, and is currently pregnant. She recently discovered that her husband has been having an affair. Mrs. W tells you that she feels her marriage is unsalvageable and would like to ask her husband for a divorce, but worries that he will “put up a fight” and demand full custody of their children. When you ask why, she states that her husband is “pretty narcissistic” and tends to become combative when criticized or threatened, such as a recent discussion they had about his affair that ended with him concluding that if she were “sexier and more confident” he would not have cheated on her.
As Mrs. W is talking, you recall a conversation you recently overheard at a continuing medical education event. Two clinicians were discussing how their records had been subpoenaed in a child custody case, even though the patient’s mental health was not contested. You realize that Mrs. W’s situation may also fit under this exception to confidentiality or privilege. You wonder if you should have disclosed this possibility to her at the outset of your session and wonder what you should say now, because she is clearly in distress and in need of psychiatric treatment. On the other hand, you want her to be fully informed of the potential repercussions if she continues with treatment.
Confidentiality and privilege allow our patients to disclose sensitive details in a safe space. The psychiatrist’s duty is to keep the patient’s information confidential, except in limited circumstances. The patient’s privilege is their right to prevent someone in a special confidential relationship from testifying against them or releasing their private records. In certain instances, a patient may waive or be forced to waive privilege, and a psychiatrist may be compelled to testify or release treatment records to a court. This article reviews exceptions to confidentiality and privilege, focusing specifically on a little-known exception to privilege that arises in divorce and child custody cases. We discuss relevant legislation and provide recommendations for psychiatrists to better understand how to discuss these legal realities with patients who are or may go through a divorce or child custody case.
Understanding confidentiality and privilege
Confidentiality and privilege are related but distinct concepts. Confidentiality relates to the overall trusting relationship created between 2 parties, such as a physician and their patient, and the duty on the part of the trusted individual to keep information private. Privilege refers to a person’s specific legal right to prevent someone in that confidential, trusting relationship from testifying against them in court or releasing confidential records. Privilege is owned by the patient and must be asserted or waived by the patient in legal proceedings. The concepts of confidentiality and privilege are crucial in creating an open, candid therapeutic environment. Many courts, including the US Supreme Court,1 have recognized the importance of confidentiality and privilege in establishing a positive therapeutic relationship between a psychotherapist and a patient. Without confidentiality and privilege, patients would be less likely to share sensitive yet clinically important information.

Commonly encountered exceptions to confidentiality (Table 12) and privilege (Table 2) exist in medical practice. Psychiatrists should discuss these exceptions with patients at the outset of clinical treatment. A little-known exception to privilege that may compel a psychiatrist to disclose confidential records can occur in child custody proceedings. In certain states, the mere filing of a child custody claim constitutes an exception to physician-patient privilege. In these states, the parent filing for divorce and custody may automatically waive privilege and thus compel disclosure of psychiatric records, even if their mental health is not in question. The following recent Ohio Supreme Court case illustrates this exception.

Friedenberg v Friedenberg (2020)
Friedenberg v Friedenberg3 addressed the issue of privilege and release of mental health treatment records in custody disputes. Belinda Torres Friedenberg and Keith Friedenberg were married with 4 minor children. Mrs. Friedenberg filed for divorce in 2016, requesting custody of the children and spousal support. In response, Mr. Friedenberg also filed a complaint seeking custody. Mr. Friedenberg subpoenaed mental health treatment records for Mrs. Friedenberg, who responded by filing a request to prevent the release of these records given physician-patient privilege. Mr. Friedenberg argued that Mrs. Friedenberg had placed her physical and mental health at issue when she filed for divorce and custody. At no point did Mr. Friedenberg allege that Mrs. Friedenberg’s mental health made her an unfit parent. The court agreed with Mr. Friedenberg and compelled disclosure of Mrs. Friedenberg’s psychiatric records, stating it is “hard to imagine a scenario where the mental health records of a parent would not be relevant to issues around custody and the best interests of the children.” The judge reviewed Mrs. Friedenberg’s psychiatric records privately and released records deemed relevant to the custody proceedings. On appeal, the Ohio Supreme Court agreed with this approach, holding that a parent’s mental fitness is always an issue in child custody cases, even if not asserted by either party. The court further held that unnecessary disclosure of sensitive information was prevented by the judge’s private review of records before deciding which records to release to the opposing spouse.
Waiver of physician-patient privilege
Waiver of physician-patient privilege in custody and divorce proceedings varies by state (Table 33-6). The Friedenberg decision highlights the most restrictive approach, where the mere filing of a divorce and child custody request automatically waives privilege. Some states, such as Indiana,4 follow a similar scheme to Ohio. Other states are silent on this issue or explicitly prohibit a waiver of privilege, asserting that custody disputes alone do not trigger disclosure without additional justifications, such as aberrant parental behaviors or other historical information concerning for abuse, neglect, or lack of parental fitness, or if a parent places their mental health at issue.7

Continue to: Once privilege is waived...
Once privilege is waived, the next step is to determine who should examine psychiatric records, deem relevance, and disclose sensitive information to the court and the opposing party. A judge may make this determination, as in the Friedenberg case. Alternatively, an independent psychiatric examiner may be appointed by the court to examine one or both parties; to obtain collateral information, including psychiatric records; and to submit a report to the court with medicolegal opinions regarding parental fitness. For example, in Maryland,6 the mere filing of a custody suit does not waive privilege. If a parent’s mental health is questioned, the judge may order an independent psychiatric examination to determine the parent’s fitness, thus balancing the best interests of the child with the parent’s right to physician-patient privilege.
The problem with automatic waivers
The foundation of the physician-patient relationship is trust and confidentiality. While this holds true for every specialty, perhaps it is even more important in psychiatry, where our patients routinely disclose sensitive, personal information in hopes of healing. Patients may not be aware of exceptions to confidentiality, or only be aware of the most well-known exceptions, such as the clinician’s duty to report abuse, or to warn a third party about risk of harm by a patient. Furthermore, clinicians and patients alike may not be aware of less-common exceptions to privilege, such as those that may occur in custody proceedings. This is critically important in light of the high number of patients who are or may be seeking divorce and custody of their children.
As psychiatric clinicians, it is highly likely that we will see patients going through divorce and custody proceedings. In 2018, there were 2.24 marriages for every divorce,8 and in 2014, 27% of all American children were living with a custodial parent, with the other parent living elsewhere.9 Divorce can be a profoundly stressful time; thus, it would be expected that many individuals going through divorce would seek psychiatric treatment and support.
Our concern is that the Friedenberg approach, which results in automatic disclosure of sensitive mental health information when a party files for divorce and custody, could deter patients from seeking psychiatric treatment, especially those anticipating divorce. Importantly, because women are nearly twice as likely as men to experience depression and anxiety10 and are more likely to seek treatment, this approach could disproportionately impact them.11 In general, an automatic waiver policy may create an additional obstacle for individuals who are already reticent to seek treatment.
How to handle these situations
As a psychiatrist, you should be familiar with your state’s laws regarding exceptions to patient-physician privilege, and should discuss exceptions at the outset of treatment. However, you will need to weigh the potential negative impacts of this information on the therapeutic relationship, including possible early termination. Furthermore, this information may impact a patient’s willingness to disclose all relevant information to mental health treatment if there is concern for later court disclosure. How should you balance these concerns? First, encourage patients to ask questions and raise concerns about confidentiality and privilege.12 In addition, you may direct the patient to other resources, such as a family law attorney, if they have questions about how certain information may be used in a legal proceeding.
Continue to: Second, you should be...
Second, you should be transparent regarding documentation of psychiatric visits. While documentation must meet ethical, legal, and billing requirements, you should take care to include only relevant information needed to make a diagnosis and provide indicated treatment while maintaining a neutral tone and avoiding medical jargon.13 For instance, we frequently use the term “denied” in medical documentation, as in “Mr. X denied cough, sore throat, fever or chills.” However, in psychiatric notes, if a patient “denied alcohol use,” the colloquial interpretation of this word could imply a tone of distrust toward the patient. A more sensitive way to document this might be: “When screened, reported no alcohol use.” If a patient divulges information and then asks you to omit this from their chart, but you do not feel comfortable doing so, explain what and why you must include the information in the chart.14
Third, if you receive a subpoena or other document requesting privileged information, first contact the patient and inform them of the request, and then seek legal consultation through your employer or malpractice insurer.15 Not all subpoenas are valid and enforceable, so it is important for an attorney to examine the subpoena; in addition, the patient’s attorney may choose to challenge the subpoena and limit the disclosure of privileged information.
Finally, inform legislatures and courts about the potential harm of automatic waivers in custody proceedings. A judge’s examination of the psychiatric records, as in Friedenberg, is not an adequate safeguard. A judge is not a trained mental health professional and may deem “relevant” information to be nearly everything: a history of abuse, remote drug or alcohol use, disclosure of a past crime, or financial troubles. We advocate for courts to follow the Maryland model, where a spouse does not automatically waive privilege if filing for divorce or custody. If mental health becomes an issue in a case, then the court may seek an independent psychiatric examination. The independent examiner will have access to patient records but will be in a better position to determine which details are relevant in determining diagnosis and parental fitness, and to render an opinion to the court.
CASE CONTINUED
You inform Mrs. W about a possible exception to privilege in divorce and custody cases. She decides to first talk with a family law attorney before proceeding with treatment. You defer your diagnosis and wait to see if she wants to proceed with treatment. Unfortunately, she does not return to your office.
Bottom Line
Some states limit the confidentiality and privilege of parents who are in psychiatric treatment and also involved in divorce and child custody cases. Psychiatrists should be mindful of these exceptions, and discuss them with patients at the onset of treatment.
Related Resources
- Legal Information Institute. Child custody: an overview. www.law.cornell.edu/wex/child_custody
- Melton GB, Petrila J, Poythress NG, et al. Child custody in divorce. In: Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. Guilford Press; 2018:530-533.
1. Jaffee v Redmond, 518 US 1 (1996).
2. Tarasoff v Regents of the University of California, 118 Cal Rptr 129 (Cal 1974); modified by Tarasoff v Regents of the Univ. of Cal., 551 P.2d 334 (Cal 1976).
3. Friedenberg v Friedenberg, No. 2019-0416 (Ohio 2020).
4. Owen v Owen, 563 NE 2d 605 (Ind 1991).
5. People ex. Rel. Hickox v Hickox, 410 NY S 2d 81 (NY App Div 1978).
6. Laznovsky v Laznovsky, 745 A 2d 1054 (Md 2000).
7. Eykel I, Miskel E. The mental health privilege in divorce and custody cases. Journal of the American Academy of Matrimonial Lawyers. 2012;25(2):453-476.
8. Center for Disease Control and Prevention. FastStats: Family life. Marriage and divorce. Published May 2020. Accessed July 29, 2021. www.cdc.gov/nchs/fastats/marriage-divorce.htm
9. The United States Census Bureau. Current population reports: custodial mothers and fathers and their child support: 2013. Published January 2016. Accessed July 29, 2021. https://www.census.gov/content/dam/Census/library/publications/2016/demo/P60-255.pdf
10. World Health Organization. Gender and mental health. Published June 2002. Accessed August 2, 2021. https://www.who.int/gender/other_health/genderMH.pdf
11. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):629-640.
12. Younggren J, Harris E. Can you keep a secret? Confidentiality in psychotherapy. J Clin Psychol. 2008;64(5):589-600.
13. The Committee on Psychiatry and Law. Confidentiality and privilege communication in the practice of psychiatry. Report no. 45. Group for the Advancement of Psychiatry; 1960.
14. Wiger D. Ethical considerations in documentation. In: Wiger D. The psychotherapy documentation primer. 3rd ed. Wiley; 2013:35-45.
15. Stansbury CD. Accessibility to a parent’s psychotherapy records in custody disputes: how can the competing interests be balanced? Behav Sci Law. 2010;28(4):522-541.
Mrs. W, age 35, presents to your clinic seeking treatment for anxiety and depression. She has no psychiatric history but reports feeling sad, overwhelmed, and stressed. Mrs. W has been married for 10 years, has 2 young children, and is currently pregnant. She recently discovered that her husband has been having an affair. Mrs. W tells you that she feels her marriage is unsalvageable and would like to ask her husband for a divorce, but worries that he will “put up a fight” and demand full custody of their children. When you ask why, she states that her husband is “pretty narcissistic” and tends to become combative when criticized or threatened, such as a recent discussion they had about his affair that ended with him concluding that if she were “sexier and more confident” he would not have cheated on her.
As Mrs. W is talking, you recall a conversation you recently overheard at a continuing medical education event. Two clinicians were discussing how their records had been subpoenaed in a child custody case, even though the patient’s mental health was not contested. You realize that Mrs. W’s situation may also fit under this exception to confidentiality or privilege. You wonder if you should have disclosed this possibility to her at the outset of your session and wonder what you should say now, because she is clearly in distress and in need of psychiatric treatment. On the other hand, you want her to be fully informed of the potential repercussions if she continues with treatment.
Confidentiality and privilege allow our patients to disclose sensitive details in a safe space. The psychiatrist’s duty is to keep the patient’s information confidential, except in limited circumstances. The patient’s privilege is their right to prevent someone in a special confidential relationship from testifying against them or releasing their private records. In certain instances, a patient may waive or be forced to waive privilege, and a psychiatrist may be compelled to testify or release treatment records to a court. This article reviews exceptions to confidentiality and privilege, focusing specifically on a little-known exception to privilege that arises in divorce and child custody cases. We discuss relevant legislation and provide recommendations for psychiatrists to better understand how to discuss these legal realities with patients who are or may go through a divorce or child custody case.
Understanding confidentiality and privilege
Confidentiality and privilege are related but distinct concepts. Confidentiality relates to the overall trusting relationship created between 2 parties, such as a physician and their patient, and the duty on the part of the trusted individual to keep information private. Privilege refers to a person’s specific legal right to prevent someone in that confidential, trusting relationship from testifying against them in court or releasing confidential records. Privilege is owned by the patient and must be asserted or waived by the patient in legal proceedings. The concepts of confidentiality and privilege are crucial in creating an open, candid therapeutic environment. Many courts, including the US Supreme Court,1 have recognized the importance of confidentiality and privilege in establishing a positive therapeutic relationship between a psychotherapist and a patient. Without confidentiality and privilege, patients would be less likely to share sensitive yet clinically important information.

Commonly encountered exceptions to confidentiality (Table 12) and privilege (Table 2) exist in medical practice. Psychiatrists should discuss these exceptions with patients at the outset of clinical treatment. A little-known exception to privilege that may compel a psychiatrist to disclose confidential records can occur in child custody proceedings. In certain states, the mere filing of a child custody claim constitutes an exception to physician-patient privilege. In these states, the parent filing for divorce and custody may automatically waive privilege and thus compel disclosure of psychiatric records, even if their mental health is not in question. The following recent Ohio Supreme Court case illustrates this exception.

Friedenberg v Friedenberg (2020)
Friedenberg v Friedenberg3 addressed the issue of privilege and release of mental health treatment records in custody disputes. Belinda Torres Friedenberg and Keith Friedenberg were married with 4 minor children. Mrs. Friedenberg filed for divorce in 2016, requesting custody of the children and spousal support. In response, Mr. Friedenberg also filed a complaint seeking custody. Mr. Friedenberg subpoenaed mental health treatment records for Mrs. Friedenberg, who responded by filing a request to prevent the release of these records given physician-patient privilege. Mr. Friedenberg argued that Mrs. Friedenberg had placed her physical and mental health at issue when she filed for divorce and custody. At no point did Mr. Friedenberg allege that Mrs. Friedenberg’s mental health made her an unfit parent. The court agreed with Mr. Friedenberg and compelled disclosure of Mrs. Friedenberg’s psychiatric records, stating it is “hard to imagine a scenario where the mental health records of a parent would not be relevant to issues around custody and the best interests of the children.” The judge reviewed Mrs. Friedenberg’s psychiatric records privately and released records deemed relevant to the custody proceedings. On appeal, the Ohio Supreme Court agreed with this approach, holding that a parent’s mental fitness is always an issue in child custody cases, even if not asserted by either party. The court further held that unnecessary disclosure of sensitive information was prevented by the judge’s private review of records before deciding which records to release to the opposing spouse.
Waiver of physician-patient privilege
Waiver of physician-patient privilege in custody and divorce proceedings varies by state (Table 33-6). The Friedenberg decision highlights the most restrictive approach, where the mere filing of a divorce and child custody request automatically waives privilege. Some states, such as Indiana,4 follow a similar scheme to Ohio. Other states are silent on this issue or explicitly prohibit a waiver of privilege, asserting that custody disputes alone do not trigger disclosure without additional justifications, such as aberrant parental behaviors or other historical information concerning for abuse, neglect, or lack of parental fitness, or if a parent places their mental health at issue.7

Continue to: Once privilege is waived...
Once privilege is waived, the next step is to determine who should examine psychiatric records, deem relevance, and disclose sensitive information to the court and the opposing party. A judge may make this determination, as in the Friedenberg case. Alternatively, an independent psychiatric examiner may be appointed by the court to examine one or both parties; to obtain collateral information, including psychiatric records; and to submit a report to the court with medicolegal opinions regarding parental fitness. For example, in Maryland,6 the mere filing of a custody suit does not waive privilege. If a parent’s mental health is questioned, the judge may order an independent psychiatric examination to determine the parent’s fitness, thus balancing the best interests of the child with the parent’s right to physician-patient privilege.
The problem with automatic waivers
The foundation of the physician-patient relationship is trust and confidentiality. While this holds true for every specialty, perhaps it is even more important in psychiatry, where our patients routinely disclose sensitive, personal information in hopes of healing. Patients may not be aware of exceptions to confidentiality, or only be aware of the most well-known exceptions, such as the clinician’s duty to report abuse, or to warn a third party about risk of harm by a patient. Furthermore, clinicians and patients alike may not be aware of less-common exceptions to privilege, such as those that may occur in custody proceedings. This is critically important in light of the high number of patients who are or may be seeking divorce and custody of their children.
As psychiatric clinicians, it is highly likely that we will see patients going through divorce and custody proceedings. In 2018, there were 2.24 marriages for every divorce,8 and in 2014, 27% of all American children were living with a custodial parent, with the other parent living elsewhere.9 Divorce can be a profoundly stressful time; thus, it would be expected that many individuals going through divorce would seek psychiatric treatment and support.
Our concern is that the Friedenberg approach, which results in automatic disclosure of sensitive mental health information when a party files for divorce and custody, could deter patients from seeking psychiatric treatment, especially those anticipating divorce. Importantly, because women are nearly twice as likely as men to experience depression and anxiety10 and are more likely to seek treatment, this approach could disproportionately impact them.11 In general, an automatic waiver policy may create an additional obstacle for individuals who are already reticent to seek treatment.
How to handle these situations
As a psychiatrist, you should be familiar with your state’s laws regarding exceptions to patient-physician privilege, and should discuss exceptions at the outset of treatment. However, you will need to weigh the potential negative impacts of this information on the therapeutic relationship, including possible early termination. Furthermore, this information may impact a patient’s willingness to disclose all relevant information to mental health treatment if there is concern for later court disclosure. How should you balance these concerns? First, encourage patients to ask questions and raise concerns about confidentiality and privilege.12 In addition, you may direct the patient to other resources, such as a family law attorney, if they have questions about how certain information may be used in a legal proceeding.
Continue to: Second, you should be...
Second, you should be transparent regarding documentation of psychiatric visits. While documentation must meet ethical, legal, and billing requirements, you should take care to include only relevant information needed to make a diagnosis and provide indicated treatment while maintaining a neutral tone and avoiding medical jargon.13 For instance, we frequently use the term “denied” in medical documentation, as in “Mr. X denied cough, sore throat, fever or chills.” However, in psychiatric notes, if a patient “denied alcohol use,” the colloquial interpretation of this word could imply a tone of distrust toward the patient. A more sensitive way to document this might be: “When screened, reported no alcohol use.” If a patient divulges information and then asks you to omit this from their chart, but you do not feel comfortable doing so, explain what and why you must include the information in the chart.14
Third, if you receive a subpoena or other document requesting privileged information, first contact the patient and inform them of the request, and then seek legal consultation through your employer or malpractice insurer.15 Not all subpoenas are valid and enforceable, so it is important for an attorney to examine the subpoena; in addition, the patient’s attorney may choose to challenge the subpoena and limit the disclosure of privileged information.
Finally, inform legislatures and courts about the potential harm of automatic waivers in custody proceedings. A judge’s examination of the psychiatric records, as in Friedenberg, is not an adequate safeguard. A judge is not a trained mental health professional and may deem “relevant” information to be nearly everything: a history of abuse, remote drug or alcohol use, disclosure of a past crime, or financial troubles. We advocate for courts to follow the Maryland model, where a spouse does not automatically waive privilege if filing for divorce or custody. If mental health becomes an issue in a case, then the court may seek an independent psychiatric examination. The independent examiner will have access to patient records but will be in a better position to determine which details are relevant in determining diagnosis and parental fitness, and to render an opinion to the court.
CASE CONTINUED
You inform Mrs. W about a possible exception to privilege in divorce and custody cases. She decides to first talk with a family law attorney before proceeding with treatment. You defer your diagnosis and wait to see if she wants to proceed with treatment. Unfortunately, she does not return to your office.
Bottom Line
Some states limit the confidentiality and privilege of parents who are in psychiatric treatment and also involved in divorce and child custody cases. Psychiatrists should be mindful of these exceptions, and discuss them with patients at the onset of treatment.
Related Resources
- Legal Information Institute. Child custody: an overview. www.law.cornell.edu/wex/child_custody
- Melton GB, Petrila J, Poythress NG, et al. Child custody in divorce. In: Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. Guilford Press; 2018:530-533.
Mrs. W, age 35, presents to your clinic seeking treatment for anxiety and depression. She has no psychiatric history but reports feeling sad, overwhelmed, and stressed. Mrs. W has been married for 10 years, has 2 young children, and is currently pregnant. She recently discovered that her husband has been having an affair. Mrs. W tells you that she feels her marriage is unsalvageable and would like to ask her husband for a divorce, but worries that he will “put up a fight” and demand full custody of their children. When you ask why, she states that her husband is “pretty narcissistic” and tends to become combative when criticized or threatened, such as a recent discussion they had about his affair that ended with him concluding that if she were “sexier and more confident” he would not have cheated on her.
As Mrs. W is talking, you recall a conversation you recently overheard at a continuing medical education event. Two clinicians were discussing how their records had been subpoenaed in a child custody case, even though the patient’s mental health was not contested. You realize that Mrs. W’s situation may also fit under this exception to confidentiality or privilege. You wonder if you should have disclosed this possibility to her at the outset of your session and wonder what you should say now, because she is clearly in distress and in need of psychiatric treatment. On the other hand, you want her to be fully informed of the potential repercussions if she continues with treatment.
Confidentiality and privilege allow our patients to disclose sensitive details in a safe space. The psychiatrist’s duty is to keep the patient’s information confidential, except in limited circumstances. The patient’s privilege is their right to prevent someone in a special confidential relationship from testifying against them or releasing their private records. In certain instances, a patient may waive or be forced to waive privilege, and a psychiatrist may be compelled to testify or release treatment records to a court. This article reviews exceptions to confidentiality and privilege, focusing specifically on a little-known exception to privilege that arises in divorce and child custody cases. We discuss relevant legislation and provide recommendations for psychiatrists to better understand how to discuss these legal realities with patients who are or may go through a divorce or child custody case.
Understanding confidentiality and privilege
Confidentiality and privilege are related but distinct concepts. Confidentiality relates to the overall trusting relationship created between 2 parties, such as a physician and their patient, and the duty on the part of the trusted individual to keep information private. Privilege refers to a person’s specific legal right to prevent someone in that confidential, trusting relationship from testifying against them in court or releasing confidential records. Privilege is owned by the patient and must be asserted or waived by the patient in legal proceedings. The concepts of confidentiality and privilege are crucial in creating an open, candid therapeutic environment. Many courts, including the US Supreme Court,1 have recognized the importance of confidentiality and privilege in establishing a positive therapeutic relationship between a psychotherapist and a patient. Without confidentiality and privilege, patients would be less likely to share sensitive yet clinically important information.

Commonly encountered exceptions to confidentiality (Table 12) and privilege (Table 2) exist in medical practice. Psychiatrists should discuss these exceptions with patients at the outset of clinical treatment. A little-known exception to privilege that may compel a psychiatrist to disclose confidential records can occur in child custody proceedings. In certain states, the mere filing of a child custody claim constitutes an exception to physician-patient privilege. In these states, the parent filing for divorce and custody may automatically waive privilege and thus compel disclosure of psychiatric records, even if their mental health is not in question. The following recent Ohio Supreme Court case illustrates this exception.

Friedenberg v Friedenberg (2020)
Friedenberg v Friedenberg3 addressed the issue of privilege and release of mental health treatment records in custody disputes. Belinda Torres Friedenberg and Keith Friedenberg were married with 4 minor children. Mrs. Friedenberg filed for divorce in 2016, requesting custody of the children and spousal support. In response, Mr. Friedenberg also filed a complaint seeking custody. Mr. Friedenberg subpoenaed mental health treatment records for Mrs. Friedenberg, who responded by filing a request to prevent the release of these records given physician-patient privilege. Mr. Friedenberg argued that Mrs. Friedenberg had placed her physical and mental health at issue when she filed for divorce and custody. At no point did Mr. Friedenberg allege that Mrs. Friedenberg’s mental health made her an unfit parent. The court agreed with Mr. Friedenberg and compelled disclosure of Mrs. Friedenberg’s psychiatric records, stating it is “hard to imagine a scenario where the mental health records of a parent would not be relevant to issues around custody and the best interests of the children.” The judge reviewed Mrs. Friedenberg’s psychiatric records privately and released records deemed relevant to the custody proceedings. On appeal, the Ohio Supreme Court agreed with this approach, holding that a parent’s mental fitness is always an issue in child custody cases, even if not asserted by either party. The court further held that unnecessary disclosure of sensitive information was prevented by the judge’s private review of records before deciding which records to release to the opposing spouse.
Waiver of physician-patient privilege
Waiver of physician-patient privilege in custody and divorce proceedings varies by state (Table 33-6). The Friedenberg decision highlights the most restrictive approach, where the mere filing of a divorce and child custody request automatically waives privilege. Some states, such as Indiana,4 follow a similar scheme to Ohio. Other states are silent on this issue or explicitly prohibit a waiver of privilege, asserting that custody disputes alone do not trigger disclosure without additional justifications, such as aberrant parental behaviors or other historical information concerning for abuse, neglect, or lack of parental fitness, or if a parent places their mental health at issue.7

Continue to: Once privilege is waived...
Once privilege is waived, the next step is to determine who should examine psychiatric records, deem relevance, and disclose sensitive information to the court and the opposing party. A judge may make this determination, as in the Friedenberg case. Alternatively, an independent psychiatric examiner may be appointed by the court to examine one or both parties; to obtain collateral information, including psychiatric records; and to submit a report to the court with medicolegal opinions regarding parental fitness. For example, in Maryland,6 the mere filing of a custody suit does not waive privilege. If a parent’s mental health is questioned, the judge may order an independent psychiatric examination to determine the parent’s fitness, thus balancing the best interests of the child with the parent’s right to physician-patient privilege.
The problem with automatic waivers
The foundation of the physician-patient relationship is trust and confidentiality. While this holds true for every specialty, perhaps it is even more important in psychiatry, where our patients routinely disclose sensitive, personal information in hopes of healing. Patients may not be aware of exceptions to confidentiality, or only be aware of the most well-known exceptions, such as the clinician’s duty to report abuse, or to warn a third party about risk of harm by a patient. Furthermore, clinicians and patients alike may not be aware of less-common exceptions to privilege, such as those that may occur in custody proceedings. This is critically important in light of the high number of patients who are or may be seeking divorce and custody of their children.
As psychiatric clinicians, it is highly likely that we will see patients going through divorce and custody proceedings. In 2018, there were 2.24 marriages for every divorce,8 and in 2014, 27% of all American children were living with a custodial parent, with the other parent living elsewhere.9 Divorce can be a profoundly stressful time; thus, it would be expected that many individuals going through divorce would seek psychiatric treatment and support.
Our concern is that the Friedenberg approach, which results in automatic disclosure of sensitive mental health information when a party files for divorce and custody, could deter patients from seeking psychiatric treatment, especially those anticipating divorce. Importantly, because women are nearly twice as likely as men to experience depression and anxiety10 and are more likely to seek treatment, this approach could disproportionately impact them.11 In general, an automatic waiver policy may create an additional obstacle for individuals who are already reticent to seek treatment.
How to handle these situations
As a psychiatrist, you should be familiar with your state’s laws regarding exceptions to patient-physician privilege, and should discuss exceptions at the outset of treatment. However, you will need to weigh the potential negative impacts of this information on the therapeutic relationship, including possible early termination. Furthermore, this information may impact a patient’s willingness to disclose all relevant information to mental health treatment if there is concern for later court disclosure. How should you balance these concerns? First, encourage patients to ask questions and raise concerns about confidentiality and privilege.12 In addition, you may direct the patient to other resources, such as a family law attorney, if they have questions about how certain information may be used in a legal proceeding.
Continue to: Second, you should be...
Second, you should be transparent regarding documentation of psychiatric visits. While documentation must meet ethical, legal, and billing requirements, you should take care to include only relevant information needed to make a diagnosis and provide indicated treatment while maintaining a neutral tone and avoiding medical jargon.13 For instance, we frequently use the term “denied” in medical documentation, as in “Mr. X denied cough, sore throat, fever or chills.” However, in psychiatric notes, if a patient “denied alcohol use,” the colloquial interpretation of this word could imply a tone of distrust toward the patient. A more sensitive way to document this might be: “When screened, reported no alcohol use.” If a patient divulges information and then asks you to omit this from their chart, but you do not feel comfortable doing so, explain what and why you must include the information in the chart.14
Third, if you receive a subpoena or other document requesting privileged information, first contact the patient and inform them of the request, and then seek legal consultation through your employer or malpractice insurer.15 Not all subpoenas are valid and enforceable, so it is important for an attorney to examine the subpoena; in addition, the patient’s attorney may choose to challenge the subpoena and limit the disclosure of privileged information.
Finally, inform legislatures and courts about the potential harm of automatic waivers in custody proceedings. A judge’s examination of the psychiatric records, as in Friedenberg, is not an adequate safeguard. A judge is not a trained mental health professional and may deem “relevant” information to be nearly everything: a history of abuse, remote drug or alcohol use, disclosure of a past crime, or financial troubles. We advocate for courts to follow the Maryland model, where a spouse does not automatically waive privilege if filing for divorce or custody. If mental health becomes an issue in a case, then the court may seek an independent psychiatric examination. The independent examiner will have access to patient records but will be in a better position to determine which details are relevant in determining diagnosis and parental fitness, and to render an opinion to the court.
CASE CONTINUED
You inform Mrs. W about a possible exception to privilege in divorce and custody cases. She decides to first talk with a family law attorney before proceeding with treatment. You defer your diagnosis and wait to see if she wants to proceed with treatment. Unfortunately, she does not return to your office.
Bottom Line
Some states limit the confidentiality and privilege of parents who are in psychiatric treatment and also involved in divorce and child custody cases. Psychiatrists should be mindful of these exceptions, and discuss them with patients at the onset of treatment.
Related Resources
- Legal Information Institute. Child custody: an overview. www.law.cornell.edu/wex/child_custody
- Melton GB, Petrila J, Poythress NG, et al. Child custody in divorce. In: Melton GB, Petrila J, Poythress NG, et al. Psychological evaluations for the courts. Guilford Press; 2018:530-533.
1. Jaffee v Redmond, 518 US 1 (1996).
2. Tarasoff v Regents of the University of California, 118 Cal Rptr 129 (Cal 1974); modified by Tarasoff v Regents of the Univ. of Cal., 551 P.2d 334 (Cal 1976).
3. Friedenberg v Friedenberg, No. 2019-0416 (Ohio 2020).
4. Owen v Owen, 563 NE 2d 605 (Ind 1991).
5. People ex. Rel. Hickox v Hickox, 410 NY S 2d 81 (NY App Div 1978).
6. Laznovsky v Laznovsky, 745 A 2d 1054 (Md 2000).
7. Eykel I, Miskel E. The mental health privilege in divorce and custody cases. Journal of the American Academy of Matrimonial Lawyers. 2012;25(2):453-476.
8. Center for Disease Control and Prevention. FastStats: Family life. Marriage and divorce. Published May 2020. Accessed July 29, 2021. www.cdc.gov/nchs/fastats/marriage-divorce.htm
9. The United States Census Bureau. Current population reports: custodial mothers and fathers and their child support: 2013. Published January 2016. Accessed July 29, 2021. https://www.census.gov/content/dam/Census/library/publications/2016/demo/P60-255.pdf
10. World Health Organization. Gender and mental health. Published June 2002. Accessed August 2, 2021. https://www.who.int/gender/other_health/genderMH.pdf
11. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):629-640.
12. Younggren J, Harris E. Can you keep a secret? Confidentiality in psychotherapy. J Clin Psychol. 2008;64(5):589-600.
13. The Committee on Psychiatry and Law. Confidentiality and privilege communication in the practice of psychiatry. Report no. 45. Group for the Advancement of Psychiatry; 1960.
14. Wiger D. Ethical considerations in documentation. In: Wiger D. The psychotherapy documentation primer. 3rd ed. Wiley; 2013:35-45.
15. Stansbury CD. Accessibility to a parent’s psychotherapy records in custody disputes: how can the competing interests be balanced? Behav Sci Law. 2010;28(4):522-541.
1. Jaffee v Redmond, 518 US 1 (1996).
2. Tarasoff v Regents of the University of California, 118 Cal Rptr 129 (Cal 1974); modified by Tarasoff v Regents of the Univ. of Cal., 551 P.2d 334 (Cal 1976).
3. Friedenberg v Friedenberg, No. 2019-0416 (Ohio 2020).
4. Owen v Owen, 563 NE 2d 605 (Ind 1991).
5. People ex. Rel. Hickox v Hickox, 410 NY S 2d 81 (NY App Div 1978).
6. Laznovsky v Laznovsky, 745 A 2d 1054 (Md 2000).
7. Eykel I, Miskel E. The mental health privilege in divorce and custody cases. Journal of the American Academy of Matrimonial Lawyers. 2012;25(2):453-476.
8. Center for Disease Control and Prevention. FastStats: Family life. Marriage and divorce. Published May 2020. Accessed July 29, 2021. www.cdc.gov/nchs/fastats/marriage-divorce.htm
9. The United States Census Bureau. Current population reports: custodial mothers and fathers and their child support: 2013. Published January 2016. Accessed July 29, 2021. https://www.census.gov/content/dam/Census/library/publications/2016/demo/P60-255.pdf
10. World Health Organization. Gender and mental health. Published June 2002. Accessed August 2, 2021. https://www.who.int/gender/other_health/genderMH.pdf
11. Wang PS, Lane M, Olfson M, et al. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):629-640.
12. Younggren J, Harris E. Can you keep a secret? Confidentiality in psychotherapy. J Clin Psychol. 2008;64(5):589-600.
13. The Committee on Psychiatry and Law. Confidentiality and privilege communication in the practice of psychiatry. Report no. 45. Group for the Advancement of Psychiatry; 1960.
14. Wiger D. Ethical considerations in documentation. In: Wiger D. The psychotherapy documentation primer. 3rd ed. Wiley; 2013:35-45.
15. Stansbury CD. Accessibility to a parent’s psychotherapy records in custody disputes: how can the competing interests be balanced? Behav Sci Law. 2010;28(4):522-541.
Psychological/neuropsychological testing: When to refer for reexamination
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.
Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.

Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.

Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
Conspiracy theory or delusion? 3 questions to tell them apart
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
Workplace violence: Enhance your safety in outpatient settings
In the health care setting, workplace violence directed by patients against clinicians or other staff (eg, verbal or physical assaults) is common.1-3 Factors that contribute to violent incidents within mental health settings include communication problems, substance use, patients’ noncompliance with medications, procedural failures (administrative and legal), and a lack of resources.4
Being verbally or physically assaulted, stalked, or threatened by a patient is a reality for mental health professionals, especially in outpatient settings with limited resources and a lack of onsite security.5 Addressing the concerns outlined in this article can enhance your safety in outpatient settings. These steps should be customized for your practice with the possible assistance of legal counsel, risk management, and/or law enforcement.5
Plans and policies to mitigate the risk of violence. Assess for hazards within and around the workplace.5 Learn to assess your patient’s violence risk level in pre-screening interviews before their first appointment. Create a violence prevention and response plan, which may involve calling law enforcement if you fear for your safety or the safety of others.5 The confidentiality clauses of the Health Insurance Portability and Accountability Act make an exception to allow for disclosure to prevent or reduce a serious and substantial threat to the health or safety of an individual or society (you should limit your disclosure to pertinent nonclinical information).5,6 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors as well as policies and procedures to terminate care of patients who display these concerning behaviors.5 These plans and policies should include informing patients that neither violence nor threats of any kind will be tolerated. Frequently review these plans and policies with clinic personnel; these documents should be easily accessible to everyone (eg, posted on a board).
Communication and education. Keep open lines of communication with all clinic personnel, and encourage them to promptly report incidents and any concerning patient behaviors. Frequently check in with them about any safety concerns they have, and encourage them to suggest ways to reduce risks.7 Include discussions about safety during clinic meetings. Educate clinic personnel about the nonverbal warning signs of behavior escalation, and provide de-escalation and response training.5 Hold simulation drills so clinic personnel can become more familiar with the violence prevention and response plan.
Office safety. Install a security barrier between the waiting room and office spaces so that patients cannot easily barge into the office spaces. Ensure access to the office areas is restricted to clinic personnel using access card readers, electronic locks, locks with deadbolts, etc.5 Escort patients within the office and ensure that individuals who are not associated with the clinic are not permitted to enter any area of the office alone.5 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.5 Post signs stating that concealed weapons are not allowed on the premises. Install panic buttons in each office, at the reception desk, and other areas (eg, restrooms).5 Develop a code word or phrase that will allow front desk staff to know that you are in trouble when they call your office. Have a designated room in which staff can gather and lock themselves if they are not able to escape.5 Provide law enforcement with floor plans of the clinic to help expedite their response.2
Personal safety. During patient visits, position yourself so you can exit a room quickly if needed, and avoid having your back to the exit.5,7 Ensure the patient is not blocking the exit. Avoid wearing attire that can be used as a weapon against you, such as a tie or necklace, or can impede your escape, such as high heels.7 Avoid wearing valuable accessories that can be damaged or destroyed during a “take down.”7 Wear an audible alarm.5 Avoid posting personal information that is publicly accessible (eg, in the office or online) and may reveal your habits.5 Insist upon a “buddy system” in which no one works alone, including outside normal business hours, or goes to their car alone.5
1. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
2. Workplace violence: issues in response. Rugala EA, Issacs AR (eds). Critical Incident Response Group, National Center for Analysis of Violent Crime, FBI Academy. 2003. Accessed November 27, 2020. https://www.fbi.gov/file-repository/stats-services-publications-workplace-violence-workplace-violence/view
3. Velani KH. 2019 Healthcare Crime Survey. International Association for Healthcare Security and Safety – Foundation (IAHSS – Foundation). Accessed November 27, 2020. https://iahssf.org/crime-surveys/2019-healthcare-crime-survey/3/
4. O’Rourke M, Wrigley C, Hammond S. Violence within mental health services: how to enhance risk management. Risk Manag Healthc Policy. 2018;11:159-167.
5. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
6. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
7. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
In the health care setting, workplace violence directed by patients against clinicians or other staff (eg, verbal or physical assaults) is common.1-3 Factors that contribute to violent incidents within mental health settings include communication problems, substance use, patients’ noncompliance with medications, procedural failures (administrative and legal), and a lack of resources.4
Being verbally or physically assaulted, stalked, or threatened by a patient is a reality for mental health professionals, especially in outpatient settings with limited resources and a lack of onsite security.5 Addressing the concerns outlined in this article can enhance your safety in outpatient settings. These steps should be customized for your practice with the possible assistance of legal counsel, risk management, and/or law enforcement.5
Plans and policies to mitigate the risk of violence. Assess for hazards within and around the workplace.5 Learn to assess your patient’s violence risk level in pre-screening interviews before their first appointment. Create a violence prevention and response plan, which may involve calling law enforcement if you fear for your safety or the safety of others.5 The confidentiality clauses of the Health Insurance Portability and Accountability Act make an exception to allow for disclosure to prevent or reduce a serious and substantial threat to the health or safety of an individual or society (you should limit your disclosure to pertinent nonclinical information).5,6 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors as well as policies and procedures to terminate care of patients who display these concerning behaviors.5 These plans and policies should include informing patients that neither violence nor threats of any kind will be tolerated. Frequently review these plans and policies with clinic personnel; these documents should be easily accessible to everyone (eg, posted on a board).
Communication and education. Keep open lines of communication with all clinic personnel, and encourage them to promptly report incidents and any concerning patient behaviors. Frequently check in with them about any safety concerns they have, and encourage them to suggest ways to reduce risks.7 Include discussions about safety during clinic meetings. Educate clinic personnel about the nonverbal warning signs of behavior escalation, and provide de-escalation and response training.5 Hold simulation drills so clinic personnel can become more familiar with the violence prevention and response plan.
Office safety. Install a security barrier between the waiting room and office spaces so that patients cannot easily barge into the office spaces. Ensure access to the office areas is restricted to clinic personnel using access card readers, electronic locks, locks with deadbolts, etc.5 Escort patients within the office and ensure that individuals who are not associated with the clinic are not permitted to enter any area of the office alone.5 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.5 Post signs stating that concealed weapons are not allowed on the premises. Install panic buttons in each office, at the reception desk, and other areas (eg, restrooms).5 Develop a code word or phrase that will allow front desk staff to know that you are in trouble when they call your office. Have a designated room in which staff can gather and lock themselves if they are not able to escape.5 Provide law enforcement with floor plans of the clinic to help expedite their response.2
Personal safety. During patient visits, position yourself so you can exit a room quickly if needed, and avoid having your back to the exit.5,7 Ensure the patient is not blocking the exit. Avoid wearing attire that can be used as a weapon against you, such as a tie or necklace, or can impede your escape, such as high heels.7 Avoid wearing valuable accessories that can be damaged or destroyed during a “take down.”7 Wear an audible alarm.5 Avoid posting personal information that is publicly accessible (eg, in the office or online) and may reveal your habits.5 Insist upon a “buddy system” in which no one works alone, including outside normal business hours, or goes to their car alone.5
In the health care setting, workplace violence directed by patients against clinicians or other staff (eg, verbal or physical assaults) is common.1-3 Factors that contribute to violent incidents within mental health settings include communication problems, substance use, patients’ noncompliance with medications, procedural failures (administrative and legal), and a lack of resources.4
Being verbally or physically assaulted, stalked, or threatened by a patient is a reality for mental health professionals, especially in outpatient settings with limited resources and a lack of onsite security.5 Addressing the concerns outlined in this article can enhance your safety in outpatient settings. These steps should be customized for your practice with the possible assistance of legal counsel, risk management, and/or law enforcement.5
Plans and policies to mitigate the risk of violence. Assess for hazards within and around the workplace.5 Learn to assess your patient’s violence risk level in pre-screening interviews before their first appointment. Create a violence prevention and response plan, which may involve calling law enforcement if you fear for your safety or the safety of others.5 The confidentiality clauses of the Health Insurance Portability and Accountability Act make an exception to allow for disclosure to prevent or reduce a serious and substantial threat to the health or safety of an individual or society (you should limit your disclosure to pertinent nonclinical information).5,6 Develop policies and procedures to identify, communicate, track, and document patients’ concerning behaviors as well as policies and procedures to terminate care of patients who display these concerning behaviors.5 These plans and policies should include informing patients that neither violence nor threats of any kind will be tolerated. Frequently review these plans and policies with clinic personnel; these documents should be easily accessible to everyone (eg, posted on a board).
Communication and education. Keep open lines of communication with all clinic personnel, and encourage them to promptly report incidents and any concerning patient behaviors. Frequently check in with them about any safety concerns they have, and encourage them to suggest ways to reduce risks.7 Include discussions about safety during clinic meetings. Educate clinic personnel about the nonverbal warning signs of behavior escalation, and provide de-escalation and response training.5 Hold simulation drills so clinic personnel can become more familiar with the violence prevention and response plan.
Office safety. Install a security barrier between the waiting room and office spaces so that patients cannot easily barge into the office spaces. Ensure access to the office areas is restricted to clinic personnel using access card readers, electronic locks, locks with deadbolts, etc.5 Escort patients within the office and ensure that individuals who are not associated with the clinic are not permitted to enter any area of the office alone.5 Install video surveillance cameras at entrances, exits, and other strategic locations and post signs signaling their presence.5 Post signs stating that concealed weapons are not allowed on the premises. Install panic buttons in each office, at the reception desk, and other areas (eg, restrooms).5 Develop a code word or phrase that will allow front desk staff to know that you are in trouble when they call your office. Have a designated room in which staff can gather and lock themselves if they are not able to escape.5 Provide law enforcement with floor plans of the clinic to help expedite their response.2
Personal safety. During patient visits, position yourself so you can exit a room quickly if needed, and avoid having your back to the exit.5,7 Ensure the patient is not blocking the exit. Avoid wearing attire that can be used as a weapon against you, such as a tie or necklace, or can impede your escape, such as high heels.7 Avoid wearing valuable accessories that can be damaged or destroyed during a “take down.”7 Wear an audible alarm.5 Avoid posting personal information that is publicly accessible (eg, in the office or online) and may reveal your habits.5 Insist upon a “buddy system” in which no one works alone, including outside normal business hours, or goes to their car alone.5
1. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
2. Workplace violence: issues in response. Rugala EA, Issacs AR (eds). Critical Incident Response Group, National Center for Analysis of Violent Crime, FBI Academy. 2003. Accessed November 27, 2020. https://www.fbi.gov/file-repository/stats-services-publications-workplace-violence-workplace-violence/view
3. Velani KH. 2019 Healthcare Crime Survey. International Association for Healthcare Security and Safety – Foundation (IAHSS – Foundation). Accessed November 27, 2020. https://iahssf.org/crime-surveys/2019-healthcare-crime-survey/3/
4. O’Rourke M, Wrigley C, Hammond S. Violence within mental health services: how to enhance risk management. Risk Manag Healthc Policy. 2018;11:159-167.
5. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
6. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
7. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
1. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374(17):1661-1669.
2. Workplace violence: issues in response. Rugala EA, Issacs AR (eds). Critical Incident Response Group, National Center for Analysis of Violent Crime, FBI Academy. 2003. Accessed November 27, 2020. https://www.fbi.gov/file-repository/stats-services-publications-workplace-violence-workplace-violence/view
3. Velani KH. 2019 Healthcare Crime Survey. International Association for Healthcare Security and Safety – Foundation (IAHSS – Foundation). Accessed November 27, 2020. https://iahssf.org/crime-surveys/2019-healthcare-crime-survey/3/
4. O’Rourke M, Wrigley C, Hammond S. Violence within mental health services: how to enhance risk management. Risk Manag Healthc Policy. 2018;11:159-167.
5. Neal D. Seven actions to ensure safety in psychiatric office settings. Psychiatric News. 2020;55(7):15.
6. Health Insurance Portability and Accountability Act of 1996. Public Law No. 104–191, 110 Stat. 1936 (1996).
7. Xiong GL, Newman WJ. Take CAUTION in emergency and inpatient psychiatric settings. Current Psychiatry. 2013;12(7):9-10.
Writing letters for transgender patients undergoing medical transition
Transgender and nonconforming people are estimated to make up 0.3% to 1.4% of the population, and these estimates are likely undercounts.1 Knowingly or unknowingly, psychiatric and mental health clinicians are caring for transgender patients, and need to become familiar with ways to provide proper clinical care for this often-marginalized population. Being knowledgeable about the requirements for letter writing for patients who are transgender and desire to transition medically is one way that we can assist and affirm these individuals.
The World Professional Association for Transgender Health (WPATH) publishes standards of care (SOC) that discuss the role of mental health professionals during a patient’s gender transition.2 The initial mental health evaluation should establish if gender dysphoria exists, and not just assume that it does. It is also important to assess whether the patient has had past negative experiences in the treatment setting or a history of trauma, and to evaluate for stressors in social life. Some transgender people may present to mental health professionals solely for the purpose of pursuing gender-related services, and others do not. The transgender person may or may not choose to undergo hormone replacement therapy or surgical transition.
Within the United States, a small percentage of clinicians use the informed consent model and, instead of requiring letters for medical intervention, will conduct an assessment to determine if the patient can provide informed consent about the procedures. But because the WPATH SOC are considered the primary standards and insurance companies will not cover the surgeries without these letters, most surgeons will not accept a patient without this documentation.3
Letters for hormone therapy and upper body surgery
Adults need 1 letter of recommendation from a qualified mental health professional, and the following WPATH criteria must be met: 1) persistent, well-documented gender dysphoria, 2) capacity to make a fully informed decision to consent for treatment, 3) age of majority, and 4) if significant medical or mental health conditions are present, they must be reasonably well-controlled.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; an explanation that the criteria for hormone therapy have been met/clinical rationale (gender dysphoria, capacity to consent, age of majority, that other mental health conditions are reasonably well-controlled); a statement that informed consent had been obtained; and a statement that the referring clinician is available for coordination of care.
Letters for lower body surgery
WPATH recommends letters from 2 mental health clinicians who evaluated the patient. In addition to the criteria set for hormone therapy described above, the SOC recommend 12 months of continuous living in the gender role that is congruent with a patient’s gender identity before genital surgery. It is also suggested that the patient undergoes 12 months of hormone therapy before hysterectomy/oophorectomy in transgender men or before orchiectomy in transgender women.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; criteria for surgery/clinical rationale (gender dysphoria, capacity to consent, age of majority, other health concerns are well-controlled, hormone therapy, real-life experience), informed consent; and availability for coordination of care.
Transgender individuals need clinicians who can provide competent, sensitive health care, and gender affirmation can enhance psychological health.
1. Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390-400.
2. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th version. Published 2012. Accessed July 14, 2021. https://www.wpath.org/publications/soc
3. Budge SL, Dickey LM. Barriers, challenges, and decision-making in the letter writing process for gender transition. Psychiatr Clin N Am. 2016;40(1):65-78.
4. Coleman E, Bocking W, Botzer M, et al. Standards of care for the health of transsexual, transgender and gender-nonconforming people. Version 7. International Journal of Transgenderism. 2011;13:165-232.
Transgender and nonconforming people are estimated to make up 0.3% to 1.4% of the population, and these estimates are likely undercounts.1 Knowingly or unknowingly, psychiatric and mental health clinicians are caring for transgender patients, and need to become familiar with ways to provide proper clinical care for this often-marginalized population. Being knowledgeable about the requirements for letter writing for patients who are transgender and desire to transition medically is one way that we can assist and affirm these individuals.
The World Professional Association for Transgender Health (WPATH) publishes standards of care (SOC) that discuss the role of mental health professionals during a patient’s gender transition.2 The initial mental health evaluation should establish if gender dysphoria exists, and not just assume that it does. It is also important to assess whether the patient has had past negative experiences in the treatment setting or a history of trauma, and to evaluate for stressors in social life. Some transgender people may present to mental health professionals solely for the purpose of pursuing gender-related services, and others do not. The transgender person may or may not choose to undergo hormone replacement therapy or surgical transition.
Within the United States, a small percentage of clinicians use the informed consent model and, instead of requiring letters for medical intervention, will conduct an assessment to determine if the patient can provide informed consent about the procedures. But because the WPATH SOC are considered the primary standards and insurance companies will not cover the surgeries without these letters, most surgeons will not accept a patient without this documentation.3
Letters for hormone therapy and upper body surgery
Adults need 1 letter of recommendation from a qualified mental health professional, and the following WPATH criteria must be met: 1) persistent, well-documented gender dysphoria, 2) capacity to make a fully informed decision to consent for treatment, 3) age of majority, and 4) if significant medical or mental health conditions are present, they must be reasonably well-controlled.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; an explanation that the criteria for hormone therapy have been met/clinical rationale (gender dysphoria, capacity to consent, age of majority, that other mental health conditions are reasonably well-controlled); a statement that informed consent had been obtained; and a statement that the referring clinician is available for coordination of care.
Letters for lower body surgery
WPATH recommends letters from 2 mental health clinicians who evaluated the patient. In addition to the criteria set for hormone therapy described above, the SOC recommend 12 months of continuous living in the gender role that is congruent with a patient’s gender identity before genital surgery. It is also suggested that the patient undergoes 12 months of hormone therapy before hysterectomy/oophorectomy in transgender men or before orchiectomy in transgender women.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; criteria for surgery/clinical rationale (gender dysphoria, capacity to consent, age of majority, other health concerns are well-controlled, hormone therapy, real-life experience), informed consent; and availability for coordination of care.
Transgender individuals need clinicians who can provide competent, sensitive health care, and gender affirmation can enhance psychological health.
Transgender and nonconforming people are estimated to make up 0.3% to 1.4% of the population, and these estimates are likely undercounts.1 Knowingly or unknowingly, psychiatric and mental health clinicians are caring for transgender patients, and need to become familiar with ways to provide proper clinical care for this often-marginalized population. Being knowledgeable about the requirements for letter writing for patients who are transgender and desire to transition medically is one way that we can assist and affirm these individuals.
The World Professional Association for Transgender Health (WPATH) publishes standards of care (SOC) that discuss the role of mental health professionals during a patient’s gender transition.2 The initial mental health evaluation should establish if gender dysphoria exists, and not just assume that it does. It is also important to assess whether the patient has had past negative experiences in the treatment setting or a history of trauma, and to evaluate for stressors in social life. Some transgender people may present to mental health professionals solely for the purpose of pursuing gender-related services, and others do not. The transgender person may or may not choose to undergo hormone replacement therapy or surgical transition.
Within the United States, a small percentage of clinicians use the informed consent model and, instead of requiring letters for medical intervention, will conduct an assessment to determine if the patient can provide informed consent about the procedures. But because the WPATH SOC are considered the primary standards and insurance companies will not cover the surgeries without these letters, most surgeons will not accept a patient without this documentation.3
Letters for hormone therapy and upper body surgery
Adults need 1 letter of recommendation from a qualified mental health professional, and the following WPATH criteria must be met: 1) persistent, well-documented gender dysphoria, 2) capacity to make a fully informed decision to consent for treatment, 3) age of majority, and 4) if significant medical or mental health conditions are present, they must be reasonably well-controlled.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; an explanation that the criteria for hormone therapy have been met/clinical rationale (gender dysphoria, capacity to consent, age of majority, that other mental health conditions are reasonably well-controlled); a statement that informed consent had been obtained; and a statement that the referring clinician is available for coordination of care.
Letters for lower body surgery
WPATH recommends letters from 2 mental health clinicians who evaluated the patient. In addition to the criteria set for hormone therapy described above, the SOC recommend 12 months of continuous living in the gender role that is congruent with a patient’s gender identity before genital surgery. It is also suggested that the patient undergoes 12 months of hormone therapy before hysterectomy/oophorectomy in transgender men or before orchiectomy in transgender women.4
The letter should contain identifying characteristics; diagnoses and psychosocial assessment; duration of clinical relationship; type of evaluation or therapy; criteria for surgery/clinical rationale (gender dysphoria, capacity to consent, age of majority, other health concerns are well-controlled, hormone therapy, real-life experience), informed consent; and availability for coordination of care.
Transgender individuals need clinicians who can provide competent, sensitive health care, and gender affirmation can enhance psychological health.
1. Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390-400.
2. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th version. Published 2012. Accessed July 14, 2021. https://www.wpath.org/publications/soc
3. Budge SL, Dickey LM. Barriers, challenges, and decision-making in the letter writing process for gender transition. Psychiatr Clin N Am. 2016;40(1):65-78.
4. Coleman E, Bocking W, Botzer M, et al. Standards of care for the health of transsexual, transgender and gender-nonconforming people. Version 7. International Journal of Transgenderism. 2011;13:165-232.
1. Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390-400.
2. World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people. 7th version. Published 2012. Accessed July 14, 2021. https://www.wpath.org/publications/soc
3. Budge SL, Dickey LM. Barriers, challenges, and decision-making in the letter writing process for gender transition. Psychiatr Clin N Am. 2016;40(1):65-78.
4. Coleman E, Bocking W, Botzer M, et al. Standards of care for the health of transsexual, transgender and gender-nonconforming people. Version 7. International Journal of Transgenderism. 2011;13:165-232.
Building a better work/life balance
Physician burnout is a common and serious problem. In a 2017 survey of >14,000 US physicians across 27 specialties, 42% reported burnout,1 which typically is defined as a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment.2
Creating a focused, yet comfortable professional life is essential for preventing burnout. For our patients’ sake and for our own personal fulfillment, there is much we can do to maintain a healthy professional and home life balance. This article describes the factors that contribute to physician burnout, and outlines steps you can take to improve your work/life balance.
The multifactorial roots of stress
Many physicians frequently blend their professional and personal lives. Most are absorbed in their practices, which leaves limited time for family interactions, daily life, or wellness.
Work hours are often long, and schedules are filled with obligations. In recent years, changes to medical practice have resulted in many additional responsibilities for physicians, such as administrative tasks and adapting to electronic health care, particularly to the use of electronic health records (EHRs). This has escalated workload and worries while diminishing patient interaction, creating more distant clinical relationships and providing less financial remuneration. Monetary pressures to see more patients limit the quality of care. Overload often forces physicians to stay at work late and/or labor excessive hours at home with less family interaction. The introduction of EHRs escalated this trend, while detracting from a healthy family, personal, and professional life. Addressing cumbersome documentation requirements while striving to maintain contact with patients is frustrating.3
Physician job dissatisfaction has worsened over time. Burnout escalates errors, diminishes patient rapport and safety, and produces suboptimal outcomes, all resulting in declining professional satisfaction.
Improving your work/life balance
The following strategies can help you make changes to better balance your professional and personal lives.
Assess priorities and goals. Before taking steps to achieve an optimal work-home balance, first review medical, spousal, and parental expectations. Social support is key.
Continue to: Identify stressors
Identify stressors. Use self-report questionnaires and collegial discussions to assess for the presence and/or severity of burnout. Prevention and/or intervention at personal and organizational levels can positively impact physician well-being.4
Focus on self-care. Prioritize your personal health care, sleep hygiene, exercise routines, quality of diet, and recreational activities. Do not self-prescribe medications, and avoid excessive alcohol use.5
Make changes to your practice. In your office, make efforts to maximize social connectiveness. Consider assigning routine tasks to other staff members. Upgrading your typing skills, employing medical records scribes, and/or using voice recording systems can reduce your workload.5
Advocate for better legislation. Both through professional medical organizations and at government levels, work to modify regulations that require physicians to spend their time on nonclinical tasks. This might include advocating to simplify EHRs and insurance company reimbursement requirements to decrease paperwork and reduce barriers to prescribing. Stress management seminars, which typically are offered at state and national conferences, can foster interpersonal and professional competencies throughout one’s medical career.6 Medical licensure boards should make efforts to reduce the stigma of reporting mental health issues; they should assure confidentiality protection and help for those who seek assistance.5
1. Peckham C. Medscape psychiatrist lifestyle report: race and ethnicity, bias and burnout. Medscape. Published January 11, 2017. Accessed July 12, 2021. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1
2. Agency for Healthcare Research and Quality. Physician burnout. Published July 2017. Accessed July 13, 2021. https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
3. Lippmann S. Can shrinks “shrink” the electronic health record? Internet and Psychiatry. December 19, 2019. Accessed on August 15, 2020. https://www.internetandpsychiatry.com/wp/editorials/can-shrinks-shrink-the-electronic-health-record/
4. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388(10057):2272-2281.
5. Mohanty D, Prabhu A, Lippmann S. Physician burnout: signs and solutions. J Fam Pract. 2019;68(8):442-446.
6. McCue JD, Sachs CL. A stress management workshop improves residents’ coping skills. Arch Intern Med. 1991;151(11):2273-2277.
Physician burnout is a common and serious problem. In a 2017 survey of >14,000 US physicians across 27 specialties, 42% reported burnout,1 which typically is defined as a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment.2
Creating a focused, yet comfortable professional life is essential for preventing burnout. For our patients’ sake and for our own personal fulfillment, there is much we can do to maintain a healthy professional and home life balance. This article describes the factors that contribute to physician burnout, and outlines steps you can take to improve your work/life balance.
The multifactorial roots of stress
Many physicians frequently blend their professional and personal lives. Most are absorbed in their practices, which leaves limited time for family interactions, daily life, or wellness.
Work hours are often long, and schedules are filled with obligations. In recent years, changes to medical practice have resulted in many additional responsibilities for physicians, such as administrative tasks and adapting to electronic health care, particularly to the use of electronic health records (EHRs). This has escalated workload and worries while diminishing patient interaction, creating more distant clinical relationships and providing less financial remuneration. Monetary pressures to see more patients limit the quality of care. Overload often forces physicians to stay at work late and/or labor excessive hours at home with less family interaction. The introduction of EHRs escalated this trend, while detracting from a healthy family, personal, and professional life. Addressing cumbersome documentation requirements while striving to maintain contact with patients is frustrating.3
Physician job dissatisfaction has worsened over time. Burnout escalates errors, diminishes patient rapport and safety, and produces suboptimal outcomes, all resulting in declining professional satisfaction.
Improving your work/life balance
The following strategies can help you make changes to better balance your professional and personal lives.
Assess priorities and goals. Before taking steps to achieve an optimal work-home balance, first review medical, spousal, and parental expectations. Social support is key.
Continue to: Identify stressors
Identify stressors. Use self-report questionnaires and collegial discussions to assess for the presence and/or severity of burnout. Prevention and/or intervention at personal and organizational levels can positively impact physician well-being.4
Focus on self-care. Prioritize your personal health care, sleep hygiene, exercise routines, quality of diet, and recreational activities. Do not self-prescribe medications, and avoid excessive alcohol use.5
Make changes to your practice. In your office, make efforts to maximize social connectiveness. Consider assigning routine tasks to other staff members. Upgrading your typing skills, employing medical records scribes, and/or using voice recording systems can reduce your workload.5
Advocate for better legislation. Both through professional medical organizations and at government levels, work to modify regulations that require physicians to spend their time on nonclinical tasks. This might include advocating to simplify EHRs and insurance company reimbursement requirements to decrease paperwork and reduce barriers to prescribing. Stress management seminars, which typically are offered at state and national conferences, can foster interpersonal and professional competencies throughout one’s medical career.6 Medical licensure boards should make efforts to reduce the stigma of reporting mental health issues; they should assure confidentiality protection and help for those who seek assistance.5
Physician burnout is a common and serious problem. In a 2017 survey of >14,000 US physicians across 27 specialties, 42% reported burnout,1 which typically is defined as a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment.2
Creating a focused, yet comfortable professional life is essential for preventing burnout. For our patients’ sake and for our own personal fulfillment, there is much we can do to maintain a healthy professional and home life balance. This article describes the factors that contribute to physician burnout, and outlines steps you can take to improve your work/life balance.
The multifactorial roots of stress
Many physicians frequently blend their professional and personal lives. Most are absorbed in their practices, which leaves limited time for family interactions, daily life, or wellness.
Work hours are often long, and schedules are filled with obligations. In recent years, changes to medical practice have resulted in many additional responsibilities for physicians, such as administrative tasks and adapting to electronic health care, particularly to the use of electronic health records (EHRs). This has escalated workload and worries while diminishing patient interaction, creating more distant clinical relationships and providing less financial remuneration. Monetary pressures to see more patients limit the quality of care. Overload often forces physicians to stay at work late and/or labor excessive hours at home with less family interaction. The introduction of EHRs escalated this trend, while detracting from a healthy family, personal, and professional life. Addressing cumbersome documentation requirements while striving to maintain contact with patients is frustrating.3
Physician job dissatisfaction has worsened over time. Burnout escalates errors, diminishes patient rapport and safety, and produces suboptimal outcomes, all resulting in declining professional satisfaction.
Improving your work/life balance
The following strategies can help you make changes to better balance your professional and personal lives.
Assess priorities and goals. Before taking steps to achieve an optimal work-home balance, first review medical, spousal, and parental expectations. Social support is key.
Continue to: Identify stressors
Identify stressors. Use self-report questionnaires and collegial discussions to assess for the presence and/or severity of burnout. Prevention and/or intervention at personal and organizational levels can positively impact physician well-being.4
Focus on self-care. Prioritize your personal health care, sleep hygiene, exercise routines, quality of diet, and recreational activities. Do not self-prescribe medications, and avoid excessive alcohol use.5
Make changes to your practice. In your office, make efforts to maximize social connectiveness. Consider assigning routine tasks to other staff members. Upgrading your typing skills, employing medical records scribes, and/or using voice recording systems can reduce your workload.5
Advocate for better legislation. Both through professional medical organizations and at government levels, work to modify regulations that require physicians to spend their time on nonclinical tasks. This might include advocating to simplify EHRs and insurance company reimbursement requirements to decrease paperwork and reduce barriers to prescribing. Stress management seminars, which typically are offered at state and national conferences, can foster interpersonal and professional competencies throughout one’s medical career.6 Medical licensure boards should make efforts to reduce the stigma of reporting mental health issues; they should assure confidentiality protection and help for those who seek assistance.5
1. Peckham C. Medscape psychiatrist lifestyle report: race and ethnicity, bias and burnout. Medscape. Published January 11, 2017. Accessed July 12, 2021. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1
2. Agency for Healthcare Research and Quality. Physician burnout. Published July 2017. Accessed July 13, 2021. https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
3. Lippmann S. Can shrinks “shrink” the electronic health record? Internet and Psychiatry. December 19, 2019. Accessed on August 15, 2020. https://www.internetandpsychiatry.com/wp/editorials/can-shrinks-shrink-the-electronic-health-record/
4. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388(10057):2272-2281.
5. Mohanty D, Prabhu A, Lippmann S. Physician burnout: signs and solutions. J Fam Pract. 2019;68(8):442-446.
6. McCue JD, Sachs CL. A stress management workshop improves residents’ coping skills. Arch Intern Med. 1991;151(11):2273-2277.
1. Peckham C. Medscape psychiatrist lifestyle report: race and ethnicity, bias and burnout. Medscape. Published January 11, 2017. Accessed July 12, 2021. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1
2. Agency for Healthcare Research and Quality. Physician burnout. Published July 2017. Accessed July 13, 2021. https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
3. Lippmann S. Can shrinks “shrink” the electronic health record? Internet and Psychiatry. December 19, 2019. Accessed on August 15, 2020. https://www.internetandpsychiatry.com/wp/editorials/can-shrinks-shrink-the-electronic-health-record/
4. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388(10057):2272-2281.
5. Mohanty D, Prabhu A, Lippmann S. Physician burnout: signs and solutions. J Fam Pract. 2019;68(8):442-446.
6. McCue JD, Sachs CL. A stress management workshop improves residents’ coping skills. Arch Intern Med. 1991;151(11):2273-2277.
Becoming vaccine ambassadors: A new role for psychiatrists
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15
Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41
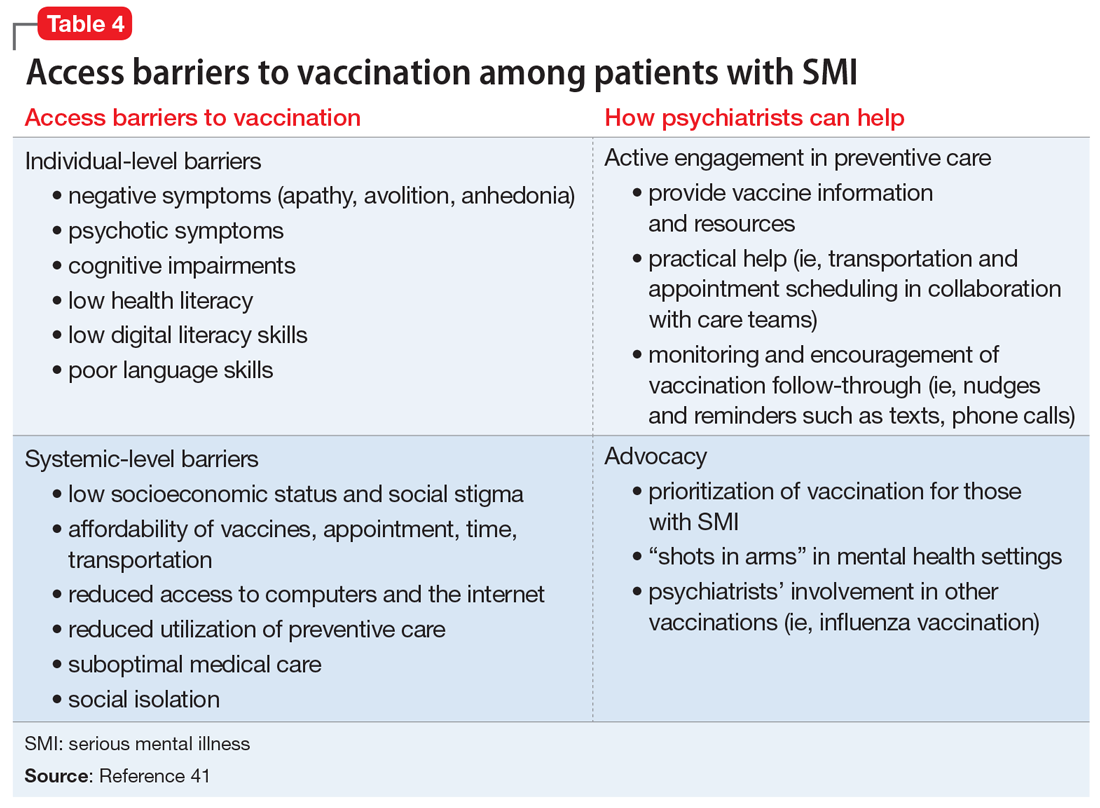
Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.
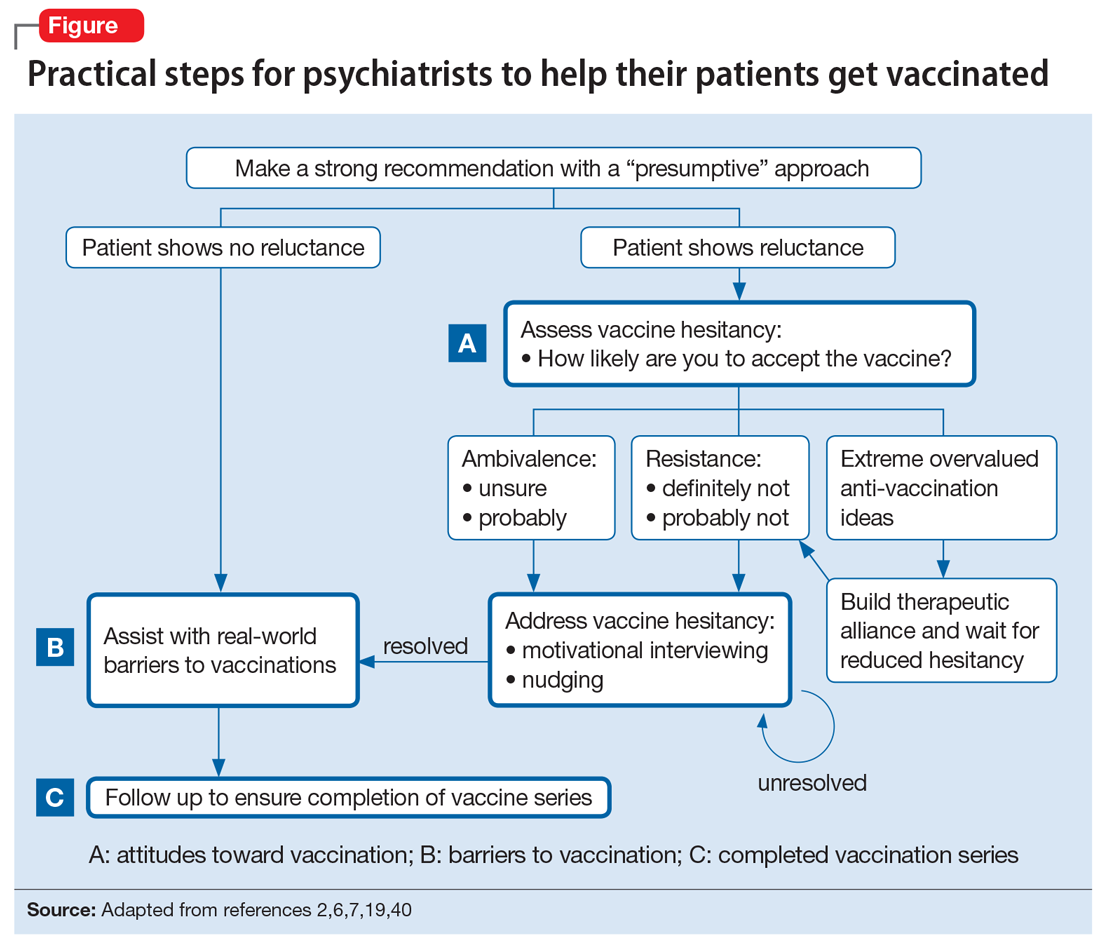
Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15

Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41

Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.

Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15

Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41

Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.

Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
Risk of Intestinal Necrosis With Sodium Polystyrene Sulfonate: A Systematic Review and Meta-analysis
Sodium polystyrene sulfonate (SPS) was first approved in the United States in 1958 and is a commonly prescribed medication for hyperkalemia.1 SPS works by exchanging potassium for sodium in the colonic lumen, thereby promoting potassium loss in the stool. However, reports of severe gastrointestinal side effects, particularly intestinal necrosis, have been persistent since the 1970s,2 leading some authors to recommend against the use of SPS.3,4 In 2009, the US Food and Drug Administration (FDA) warned against concomitant sorbitol administration, which was implicated in some studies.4,5 The concern about gastrointestinal side effects has also led to the development and FDA approval of two new cation-exchange resins for treatment of hyperkalemia.6 A prior systematic review of the literature found 30 separate case reports or case series including a total of 58 patients who were treated with SPS and developed severe gastrointestinal side effects.7 Because the included studies were all case reports or case series and therefore did not include comparison groups, it could not be determined whether SPS had a causal role in gastrointestinal side effects, and the authors could only conclude that there was a “possible” association. In contrast to case reports, several large cohort studies have been published more recently and report the risk of severe gastrointestinal adverse events associated with SPS compared with controls.8-10 While some studies found an increased risk, others have not. Given this uncertainty, we undertook a systematic review of studies that report the incidence of severe gastrointestinal side effects with SPS compared with controls.
METHODS
Data Sources and Search Strategy
A systematic search of the literature was conducted by a medical librarian using the Cochrane Library, Embase, Medline, Google Scholar, PubMed, Scopus, and Web of Science Core Collection databases to find relevant articles published from database inception to October 4, 2020. The search was peer reviewed by a second medical librarian using Peer Review of Electronic Search Strategies (PRESS).11 Databases were searched using a combination of controlled vocabulary and free-text terms for “SPS” and “bowel necrosis.” Details of the full search strategy are listed in Appendix A. References from all databases were imported into an EndNote X9 library, duplicates removed, and then uploaded into Coviden
Data Extraction and Quality Assessment
We used a standardized form to extract data, which included author, year, country, study design, setting, number of patients, SPS formulation, dosing, exposure, sorbitol content, outcomes of intestinal necrosis and the composite severe gastrointestinal adverse events, and the duration of time from SPS exposure to outcome occurrence. Two reviewers (JLH and AER) independently assessed the methodological quality of included studies using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies13 and the Revised Cochrane risk of bias (RoB 2) tool for randomized controlled trials (RCTs).14 Additionally, two reviewers (JLH and CGG) graded overall strength of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.15 Disagreement was resolved by consensus.
Data Synthesis and Analysis
The proportion of patients with intestinal necrosis was compared using random effects meta-analysis using the restricted maximum likelihood method.16 For the two studies that reported hazard ratios (HRs), meta-analysis was performed after log transformation of the HRs and CIs. One study that performed survival analysis presented data for both the duration of the study (up to 11 years) and up to 1 year after exposure.9 We used the data up to 1 year after exposure because we believed later events were more likely to be due to chance than exposure to SPS. For studies with zero events, we used the treat ment-arm continuity correction, which has been reported to be preferable to the standard fixed-correction factor.17 We also performed two sensitivity analyses, including omitting the studies with zero events and performing meta-analysis using risk difference. The prevalence of intestinal ischemia was pooled using the DerSimonian and Laird18 random effects model with Freeman-Tukey19 double arcsine transformation. Heterogeneity was estimated using the I² statistic. I² values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.20 Meta-regression and tests for small-study effects were not performed because of the small number of included studies.21 In addition to random effects meta-analysis, we calculated the 90% predicted interval for future studies for the pooled effect of intestinal ischemia.22 Statistical analysis was performed using meta and metaprop commands in Stata/IC, version 16.1 (StataCorp).
RESULTS
Selected Studies
The electronic search yielded 806 unique articles, of which 791 were excluded based on title and abstract, leaving 15 articles for full-text review (Appendix B). Appendix C describes the nine studies that were excluded, including the reason for exclusion. Table 1 describes the characteristics of the six studies that met study inclusion criteria. Studies were published between 1992 and 2020. Three studies were from Canada,10,24,25 two from the United States,8,23 and one from Sweden.9 Three studies occurred in an outpatient setting,9,10,25 and three were described as inpatient studies.8,23,24 SPS preparations included sorbitol in three studies,8,23,24 were not specified in one study,10 and were not included in two studies.9,25 SPS dosing varied widely, with median doses of 15 to 30 g in three studies,9,24,25 45 to 50 g in two studies,8,23 and unspecified in one study.10 Duration of exposure typically ranged from 1 to 7 days but was not consistently described. For example, two of the studies did not report duration of exposure,8,10 and a third study reported a single dispensation of 450 g in 41% of patients, with the remaining 59% averaging three dispensations within the first year.9 Sample size ranged from 33 to 123,391 patients. Most patients were male, and mean ages ranged from 44 to 78 years. Two studies limited participation to those with chronic kidney disease (CKD) with glomerular filtration rate (GFR) <4024 or CKD stage 4 or 5 or dialysis.9 Two studies specifically limited participation to patients with potassium levels of 5.0 to 5.9 mmol/L.24,25 All six studies reported outcomes for intestinal necrosis, and four reported composite outcomes for major adverse gastrointestinal events.9,10,24,25
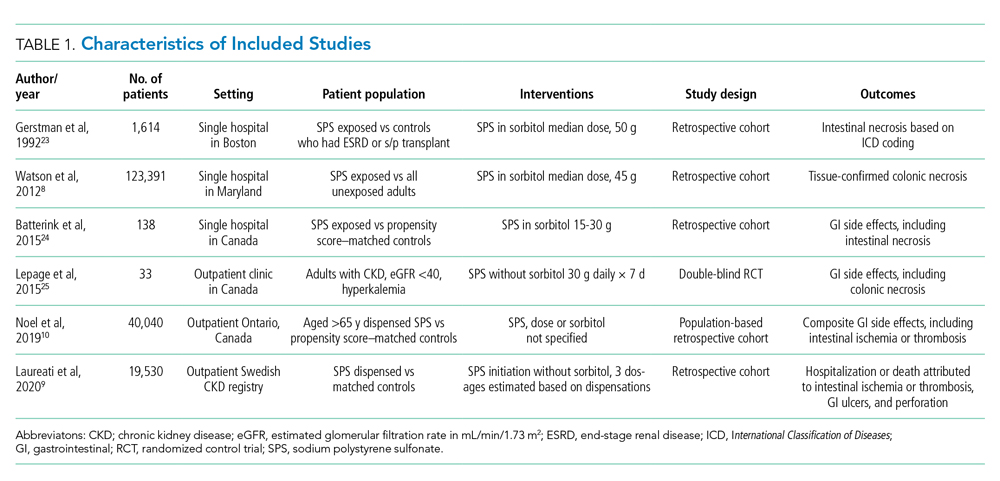
Table 2 describes the assessment of risk of bias using the ROBINS-I tool for the five retrospective observational studies and the RoB 2 tool for the one RCT.13,14 Three studies were rated as having serious risk of bias, with the remainder having a moderate risk of bias or some concerns. Two studies were judged as having a serious risk of bias because of potential confounding.8,23 To be judged low or moderate risk, studies needed to measure and control for potential risk factors for intestinal ischemia, such as age, diabetes, vascular disease, and heart failure.26,27 One study also had serious risk of bias for selective reporting because the published abstract of the study used a different analysis and had contradictory results from the published study.9,28 An additional area of risk of bias that did not fit into the ROBINS-I tool is that the two studies that used survival analysis chose durations for the outcome that were longer than would be expected for adverse events from SPS to be evident. One study chose 30 days and the other up to a maximum of 11 years from the time of exposure.9,10
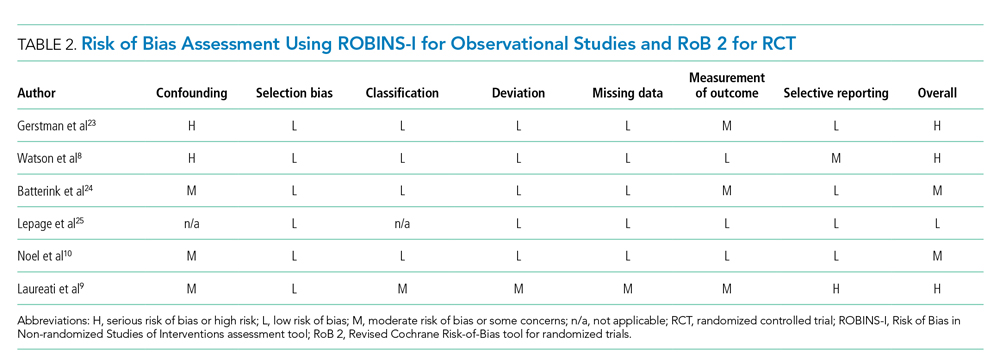
Quantitative Outcomes
Six studies including 26,716 patients treated with SPS and controls reported the proportion of patients who developed intestinal necrosis. The Figure shows the individual study and pooled results for intestinal necrosis. The prevalence of intestinal ischemia in patients treated with SPS was 0.1% (95% CI, 0.03%-0.17%). The pooled odds ratio (OR) of intestinal necrosis was 1.43 (95% CI, 0.39-5.20). The 90% predicted interval for future studies was 0.08 to 26.6. Two studies reported rates of intestinal necrosis using survival analysis. The pooled HR from these studies was 2.00 (95% CI, 0.45-8.78). Two studies performed survival analysis for a composite outcome of severe gastrointestinal adverse events. The pooled HR for these two studies was 1.46 (95% CI, 1.01-2.11).
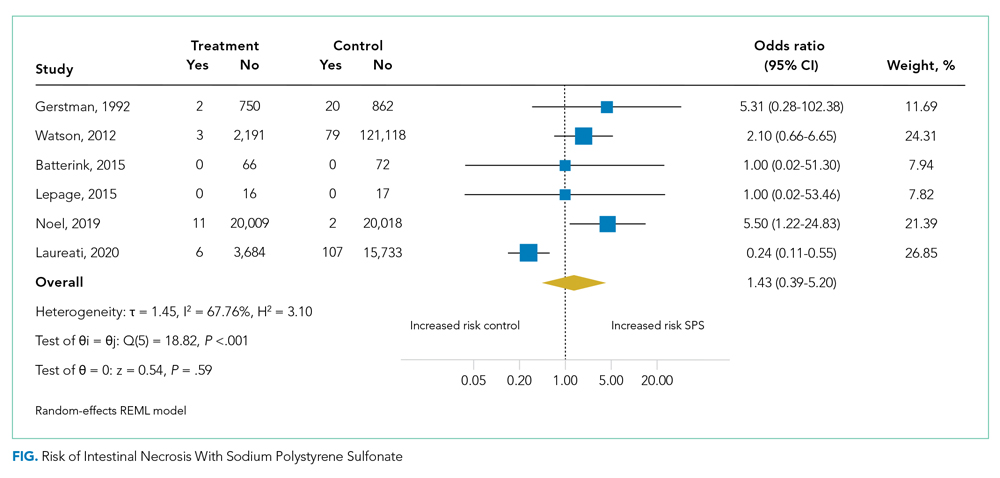
For the meta-analysis of intestinal necrosis, we found moderate-high statistical significance (Q = 18.82; P < .01; I² = 67.8%). Sensitivity analysis removing each study did not affect heterogeneity, with the exception of removing the study by Laureati et al,9 which resolved the heterogeneity (Q = 1.7, P = .8, I² = 0%). The pooled effect for intestinal necrosis also became statistically significant after removing Laureati et al (OR, 2.87; 95% CI, 1.24-6.63).9 We also performed two subgroup analyses, including studies that involved the concomitant use of sorbitol8,23,24 compared with studies that did not9,25 and subgroup analysis removing studies with zero events. Studies that included sorbitol found higher rates of intestinal necrosis (OR, 2.26; 95% CI, 0.80-6.38; I² = 0%) compared with studies that did not include sorbitol (OR, 0.25; 95% CI, 0.11-0.57; I² = 0%; test of group difference, P < .01). Removing the three studies with zero events resulted in a similar overall effect (OR, 1.30; 95% CI, 0.21-8.19). Finally, a meta-analysis using risk difference instead of ORs found a non–statistically significant difference in rate of intestinal necrosis favoring the control group (risk difference, −0.00033; 95% CI, −0.0022 to 0.0015; I² = 84.6%).
Table 3 summarizes our review findings and presents overall strength of evidence. Overall strength of evidence was found to be very low. Per GRADE criteria,15,29 strength of evidence for observational studies starts at low and may then be modified by the presence of bias, inconsistency, indirectness, imprecision, effect size, and direction of confounding. In the case of the three meta-analyses in the present study, risk of bias was serious for more than half of the study weights. Strength of evidence was also downrated for imprecision because of the low number of events and resultant wide CIs.

DISCUSSION
In total, we found six studies that reported rates of intestinal necrosis or severe gastrointestinal adverse events with SPS use compared with controls. The pooled rate of intestinal necrosis was not significantly higher for patients exposed to SPS when analyzed either as the proportion of patients with events or as HRs. The pooled rate for a composite outcome of severe gastrointestinal side effects was significantly higher (HR, 1.46; 95% CI, 1.01-2.11). The overall strength of evidence for the association of SPS with either intestinal necrosis or the composite outcome was found to be very low because of risk of bias and imprecision.
In some ways, our results emphasize the difficulty of showing a causal link between a medication and a possible rare adverse event. The first included study to assess the risk of intestinal necrosis after exposure to SPS compared with controls found only two events in the SPS group and no events in the control arm.23 Two additional studies that we found were small and did not report any events in either arm.24,25 The first large study to assess the risk of intestinal ischemia included more than 2,000 patients treated with SPS and more than 100,000 controls but found no difference in risk.8 The next large study did find increased risk of both intestinal necrosis (incidence rate, 6.82 per 1,000 person-years compared with 1.22 per 1,000 person-years for controls) and a composite outcome (incidence rate, 22.97 per 1,000 person-years compared with 11.01 per 1000 person-years for controls), but in the time to event analysis included events up to 30 days after treatment with SPS.10 A prior review of case reports of SPS and intestinal necrosis found a median of 2 days between SPS treatment and symptom onset.7 It is unlikely the authors would have had sufficient events to meaningfully compare rates if they limited the analysis to events within 7 days of SPS treatment, but events after a week of exposure are unlikely to be due to SPS. The final study to assess the association of SPS with intestinal necrosis actually found higher rates of intestinal necrosis in the control group when analyzed as proportions with events but reported a higher rate of a composite outcome of severe gastrointestinal adverse events that included nine separate International Classification of Diseases codes occurring up to 11 years after SPS exposure.9 This study was limited by evidence of selective reporting and was funded by the manufacturers of an alternative cation-exchange medication.
Based on our review of the literature, it is unclear if SPS does cause intestinal ischemia. The pooled results for intestinal ischemia analyzed as a proportion with events or with survival analysis did not find a statistically significantly increased risk. Because most o
A cost analysis of SPS vs potential alternatives such as patiromer for patients on chronic RAAS-I with a history of hyperkalemia or CKD published by Little et al26 concluded that SPS remained the cost-effective option when colonic necrosis incidence is 19.9% or less, and our systematic review reveals an incidence of 0.1% (95% CI, 0.03-0.17%). The incremental cost-effectiveness ratio was an astronomical $26,088,369 per quality-adjusted life-year gained, per Little’s analysis.
Limitations of our review are the heterogeneity of studies, which varied regarding inpatient or outpatient setting, formulations such as dosing, frequency, whether sorbitol was used, and interval from exposure to outcome measurement, which ranged from 7 days to 1 year. On sensitivity analysis, statistical heterogeneity was resolved by removing the study by Laureati et al.9 This study was notably different from the others because it included events occurring up to 1 year after exposure to SPS, which may have resulted in any true effect being diluted by later events unrelated to SPS. We did not exclude this study post hoc because this would result in bias; however, because the overall result becomes statistically significant without this study, our overall conclusion should be interpreted with caution.30 It is possible that future well-conducted studies may still find an effect of SPS on intestinal necrosis. Similarly, the finding that studies with SPS coformulated with sorbitol had statistically significantly increased risk of intestinal necrosis compared with studies without sorbitol should be interpreted with caution because the study by Laureati et al9 was included in the studies without sorbitol.
CONCLUSIONS
Based on our r
This work was presented at the Society of General Internal Medicine and Society of Hospital Medicine 2021 annual conferences.
1. Labriola L, Jadoul M. Sodium polystyrene sulfonate: still news after 60 years on the market. Nephrol Dial Transplant. 2020;35(9):1455-1458. https://doi.org/10.1093/ndt/gfaa004
2. Arvanitakis C, Malek G, Uehling D, Morrissey JF. Colonic complications after renal transplantation. Gastroenterology. 1973;64(4):533-538.
3. Parks M, Grady D. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med. 2019;179(8):1023-1024. https://doi.org/10.1001/jamainternmed.2019.1291
4. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733-735. https://doi.org/10.1681/ASN.2010010079
5. Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101(3):267-272.
6. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546-554. https://doi.org/10.1016/j.kint.2015.11.018
7. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e269-24. https://doi.org/10.1016/j.amjmed.2012.08.016
8. Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409-416. https://doi.org/10.1053/j.ajkd.2012.04.023
9. Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518-1526. https://doi.org/10.1093/ndt/gfz150
10. Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025-1033. https://doi.org/10.1001/jamainternmed.2019.0631
11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
14. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
15. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. https://doi.org/10.1016/j.jclinepi.2010.04.026
16. Raudenbush SW. Analyzing effect sizes: random-effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russel Sage Foundation; 2009:295-316.
17. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. https://doi.org/10.1002/sim.1761
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. https://doi.org/10.1016/0197-2456(86)90046-2
19. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607-611. https://doi.org/10.1214/aoms/1177729756
20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. https://doi.org/10.1136/bmj.327.7414.557
21. Higgins JPT, Chandler TJ, Cumptson M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook
22. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. Jan 2009;172(1):137-159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
23. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159-161. https://doi.org/10.1016/s0272-6386(12)80544-0
24. Batterink J, Lin J, Au-Yeung SHM, Cessford T. Effectiveness of sodium polystyrene sulfonate for short-term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68(4):296-303. https://doi.org/10.4212/cjhp.v68i4.1469
25. Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136-2142. https://doi.org/10.2215/CJN.03640415
26. Little DJ, Nee R, Abbott KC, Watson MA, Yuan CM. Cost-utility analysis of sodium polystyrene sulfonate vs. potential alternatives for chronic hyperkalemia. Clin Nephrol. 2014;81(4):259-268. https://doi.org/10.5414/cn108103
27. Cubiella Fernández J, Núñez Calvo L, González Vázquez E, et al. Risk factors associated with the development of ischemic colitis. World J Gastroenterol. 2010;16(36):4564-4569. https://doi.org/10.3748/wjg.v16.i36.4564
28. Laureati P, Evans M, Trevisan M, et al. Sodium polystyrene sulfonate, practice patterns and associated adverse event risk; a nationwide analysis from the Swedish Renal Register [abstract]. Nephroly Dial Transplant. 2019;34(suppl 1):i94. https://doi.org/10.1093/ndt/gfz106.FP151
29. Santesso N, Carrasco-Labra A, Langendam M, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28-39. https://doi.org/10.1016/j.jclinepi.2015.12.006
30. Deeks JJ HJ, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane, 2020. www.training.cochrane.org/handbook
Sodium polystyrene sulfonate (SPS) was first approved in the United States in 1958 and is a commonly prescribed medication for hyperkalemia.1 SPS works by exchanging potassium for sodium in the colonic lumen, thereby promoting potassium loss in the stool. However, reports of severe gastrointestinal side effects, particularly intestinal necrosis, have been persistent since the 1970s,2 leading some authors to recommend against the use of SPS.3,4 In 2009, the US Food and Drug Administration (FDA) warned against concomitant sorbitol administration, which was implicated in some studies.4,5 The concern about gastrointestinal side effects has also led to the development and FDA approval of two new cation-exchange resins for treatment of hyperkalemia.6 A prior systematic review of the literature found 30 separate case reports or case series including a total of 58 patients who were treated with SPS and developed severe gastrointestinal side effects.7 Because the included studies were all case reports or case series and therefore did not include comparison groups, it could not be determined whether SPS had a causal role in gastrointestinal side effects, and the authors could only conclude that there was a “possible” association. In contrast to case reports, several large cohort studies have been published more recently and report the risk of severe gastrointestinal adverse events associated with SPS compared with controls.8-10 While some studies found an increased risk, others have not. Given this uncertainty, we undertook a systematic review of studies that report the incidence of severe gastrointestinal side effects with SPS compared with controls.
METHODS
Data Sources and Search Strategy
A systematic search of the literature was conducted by a medical librarian using the Cochrane Library, Embase, Medline, Google Scholar, PubMed, Scopus, and Web of Science Core Collection databases to find relevant articles published from database inception to October 4, 2020. The search was peer reviewed by a second medical librarian using Peer Review of Electronic Search Strategies (PRESS).11 Databases were searched using a combination of controlled vocabulary and free-text terms for “SPS” and “bowel necrosis.” Details of the full search strategy are listed in Appendix A. References from all databases were imported into an EndNote X9 library, duplicates removed, and then uploaded into Coviden
Data Extraction and Quality Assessment
We used a standardized form to extract data, which included author, year, country, study design, setting, number of patients, SPS formulation, dosing, exposure, sorbitol content, outcomes of intestinal necrosis and the composite severe gastrointestinal adverse events, and the duration of time from SPS exposure to outcome occurrence. Two reviewers (JLH and AER) independently assessed the methodological quality of included studies using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies13 and the Revised Cochrane risk of bias (RoB 2) tool for randomized controlled trials (RCTs).14 Additionally, two reviewers (JLH and CGG) graded overall strength of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.15 Disagreement was resolved by consensus.
Data Synthesis and Analysis
The proportion of patients with intestinal necrosis was compared using random effects meta-analysis using the restricted maximum likelihood method.16 For the two studies that reported hazard ratios (HRs), meta-analysis was performed after log transformation of the HRs and CIs. One study that performed survival analysis presented data for both the duration of the study (up to 11 years) and up to 1 year after exposure.9 We used the data up to 1 year after exposure because we believed later events were more likely to be due to chance than exposure to SPS. For studies with zero events, we used the treat ment-arm continuity correction, which has been reported to be preferable to the standard fixed-correction factor.17 We also performed two sensitivity analyses, including omitting the studies with zero events and performing meta-analysis using risk difference. The prevalence of intestinal ischemia was pooled using the DerSimonian and Laird18 random effects model with Freeman-Tukey19 double arcsine transformation. Heterogeneity was estimated using the I² statistic. I² values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.20 Meta-regression and tests for small-study effects were not performed because of the small number of included studies.21 In addition to random effects meta-analysis, we calculated the 90% predicted interval for future studies for the pooled effect of intestinal ischemia.22 Statistical analysis was performed using meta and metaprop commands in Stata/IC, version 16.1 (StataCorp).
RESULTS
Selected Studies
The electronic search yielded 806 unique articles, of which 791 were excluded based on title and abstract, leaving 15 articles for full-text review (Appendix B). Appendix C describes the nine studies that were excluded, including the reason for exclusion. Table 1 describes the characteristics of the six studies that met study inclusion criteria. Studies were published between 1992 and 2020. Three studies were from Canada,10,24,25 two from the United States,8,23 and one from Sweden.9 Three studies occurred in an outpatient setting,9,10,25 and three were described as inpatient studies.8,23,24 SPS preparations included sorbitol in three studies,8,23,24 were not specified in one study,10 and were not included in two studies.9,25 SPS dosing varied widely, with median doses of 15 to 30 g in three studies,9,24,25 45 to 50 g in two studies,8,23 and unspecified in one study.10 Duration of exposure typically ranged from 1 to 7 days but was not consistently described. For example, two of the studies did not report duration of exposure,8,10 and a third study reported a single dispensation of 450 g in 41% of patients, with the remaining 59% averaging three dispensations within the first year.9 Sample size ranged from 33 to 123,391 patients. Most patients were male, and mean ages ranged from 44 to 78 years. Two studies limited participation to those with chronic kidney disease (CKD) with glomerular filtration rate (GFR) <4024 or CKD stage 4 or 5 or dialysis.9 Two studies specifically limited participation to patients with potassium levels of 5.0 to 5.9 mmol/L.24,25 All six studies reported outcomes for intestinal necrosis, and four reported composite outcomes for major adverse gastrointestinal events.9,10,24,25

Table 2 describes the assessment of risk of bias using the ROBINS-I tool for the five retrospective observational studies and the RoB 2 tool for the one RCT.13,14 Three studies were rated as having serious risk of bias, with the remainder having a moderate risk of bias or some concerns. Two studies were judged as having a serious risk of bias because of potential confounding.8,23 To be judged low or moderate risk, studies needed to measure and control for potential risk factors for intestinal ischemia, such as age, diabetes, vascular disease, and heart failure.26,27 One study also had serious risk of bias for selective reporting because the published abstract of the study used a different analysis and had contradictory results from the published study.9,28 An additional area of risk of bias that did not fit into the ROBINS-I tool is that the two studies that used survival analysis chose durations for the outcome that were longer than would be expected for adverse events from SPS to be evident. One study chose 30 days and the other up to a maximum of 11 years from the time of exposure.9,10

Quantitative Outcomes
Six studies including 26,716 patients treated with SPS and controls reported the proportion of patients who developed intestinal necrosis. The Figure shows the individual study and pooled results for intestinal necrosis. The prevalence of intestinal ischemia in patients treated with SPS was 0.1% (95% CI, 0.03%-0.17%). The pooled odds ratio (OR) of intestinal necrosis was 1.43 (95% CI, 0.39-5.20). The 90% predicted interval for future studies was 0.08 to 26.6. Two studies reported rates of intestinal necrosis using survival analysis. The pooled HR from these studies was 2.00 (95% CI, 0.45-8.78). Two studies performed survival analysis for a composite outcome of severe gastrointestinal adverse events. The pooled HR for these two studies was 1.46 (95% CI, 1.01-2.11).

For the meta-analysis of intestinal necrosis, we found moderate-high statistical significance (Q = 18.82; P < .01; I² = 67.8%). Sensitivity analysis removing each study did not affect heterogeneity, with the exception of removing the study by Laureati et al,9 which resolved the heterogeneity (Q = 1.7, P = .8, I² = 0%). The pooled effect for intestinal necrosis also became statistically significant after removing Laureati et al (OR, 2.87; 95% CI, 1.24-6.63).9 We also performed two subgroup analyses, including studies that involved the concomitant use of sorbitol8,23,24 compared with studies that did not9,25 and subgroup analysis removing studies with zero events. Studies that included sorbitol found higher rates of intestinal necrosis (OR, 2.26; 95% CI, 0.80-6.38; I² = 0%) compared with studies that did not include sorbitol (OR, 0.25; 95% CI, 0.11-0.57; I² = 0%; test of group difference, P < .01). Removing the three studies with zero events resulted in a similar overall effect (OR, 1.30; 95% CI, 0.21-8.19). Finally, a meta-analysis using risk difference instead of ORs found a non–statistically significant difference in rate of intestinal necrosis favoring the control group (risk difference, −0.00033; 95% CI, −0.0022 to 0.0015; I² = 84.6%).
Table 3 summarizes our review findings and presents overall strength of evidence. Overall strength of evidence was found to be very low. Per GRADE criteria,15,29 strength of evidence for observational studies starts at low and may then be modified by the presence of bias, inconsistency, indirectness, imprecision, effect size, and direction of confounding. In the case of the three meta-analyses in the present study, risk of bias was serious for more than half of the study weights. Strength of evidence was also downrated for imprecision because of the low number of events and resultant wide CIs.

DISCUSSION
In total, we found six studies that reported rates of intestinal necrosis or severe gastrointestinal adverse events with SPS use compared with controls. The pooled rate of intestinal necrosis was not significantly higher for patients exposed to SPS when analyzed either as the proportion of patients with events or as HRs. The pooled rate for a composite outcome of severe gastrointestinal side effects was significantly higher (HR, 1.46; 95% CI, 1.01-2.11). The overall strength of evidence for the association of SPS with either intestinal necrosis or the composite outcome was found to be very low because of risk of bias and imprecision.
In some ways, our results emphasize the difficulty of showing a causal link between a medication and a possible rare adverse event. The first included study to assess the risk of intestinal necrosis after exposure to SPS compared with controls found only two events in the SPS group and no events in the control arm.23 Two additional studies that we found were small and did not report any events in either arm.24,25 The first large study to assess the risk of intestinal ischemia included more than 2,000 patients treated with SPS and more than 100,000 controls but found no difference in risk.8 The next large study did find increased risk of both intestinal necrosis (incidence rate, 6.82 per 1,000 person-years compared with 1.22 per 1,000 person-years for controls) and a composite outcome (incidence rate, 22.97 per 1,000 person-years compared with 11.01 per 1000 person-years for controls), but in the time to event analysis included events up to 30 days after treatment with SPS.10 A prior review of case reports of SPS and intestinal necrosis found a median of 2 days between SPS treatment and symptom onset.7 It is unlikely the authors would have had sufficient events to meaningfully compare rates if they limited the analysis to events within 7 days of SPS treatment, but events after a week of exposure are unlikely to be due to SPS. The final study to assess the association of SPS with intestinal necrosis actually found higher rates of intestinal necrosis in the control group when analyzed as proportions with events but reported a higher rate of a composite outcome of severe gastrointestinal adverse events that included nine separate International Classification of Diseases codes occurring up to 11 years after SPS exposure.9 This study was limited by evidence of selective reporting and was funded by the manufacturers of an alternative cation-exchange medication.
Based on our review of the literature, it is unclear if SPS does cause intestinal ischemia. The pooled results for intestinal ischemia analyzed as a proportion with events or with survival analysis did not find a statistically significantly increased risk. Because most o
A cost analysis of SPS vs potential alternatives such as patiromer for patients on chronic RAAS-I with a history of hyperkalemia or CKD published by Little et al26 concluded that SPS remained the cost-effective option when colonic necrosis incidence is 19.9% or less, and our systematic review reveals an incidence of 0.1% (95% CI, 0.03-0.17%). The incremental cost-effectiveness ratio was an astronomical $26,088,369 per quality-adjusted life-year gained, per Little’s analysis.
Limitations of our review are the heterogeneity of studies, which varied regarding inpatient or outpatient setting, formulations such as dosing, frequency, whether sorbitol was used, and interval from exposure to outcome measurement, which ranged from 7 days to 1 year. On sensitivity analysis, statistical heterogeneity was resolved by removing the study by Laureati et al.9 This study was notably different from the others because it included events occurring up to 1 year after exposure to SPS, which may have resulted in any true effect being diluted by later events unrelated to SPS. We did not exclude this study post hoc because this would result in bias; however, because the overall result becomes statistically significant without this study, our overall conclusion should be interpreted with caution.30 It is possible that future well-conducted studies may still find an effect of SPS on intestinal necrosis. Similarly, the finding that studies with SPS coformulated with sorbitol had statistically significantly increased risk of intestinal necrosis compared with studies without sorbitol should be interpreted with caution because the study by Laureati et al9 was included in the studies without sorbitol.
CONCLUSIONS
Based on our r
This work was presented at the Society of General Internal Medicine and Society of Hospital Medicine 2021 annual conferences.
Sodium polystyrene sulfonate (SPS) was first approved in the United States in 1958 and is a commonly prescribed medication for hyperkalemia.1 SPS works by exchanging potassium for sodium in the colonic lumen, thereby promoting potassium loss in the stool. However, reports of severe gastrointestinal side effects, particularly intestinal necrosis, have been persistent since the 1970s,2 leading some authors to recommend against the use of SPS.3,4 In 2009, the US Food and Drug Administration (FDA) warned against concomitant sorbitol administration, which was implicated in some studies.4,5 The concern about gastrointestinal side effects has also led to the development and FDA approval of two new cation-exchange resins for treatment of hyperkalemia.6 A prior systematic review of the literature found 30 separate case reports or case series including a total of 58 patients who were treated with SPS and developed severe gastrointestinal side effects.7 Because the included studies were all case reports or case series and therefore did not include comparison groups, it could not be determined whether SPS had a causal role in gastrointestinal side effects, and the authors could only conclude that there was a “possible” association. In contrast to case reports, several large cohort studies have been published more recently and report the risk of severe gastrointestinal adverse events associated with SPS compared with controls.8-10 While some studies found an increased risk, others have not. Given this uncertainty, we undertook a systematic review of studies that report the incidence of severe gastrointestinal side effects with SPS compared with controls.
METHODS
Data Sources and Search Strategy
A systematic search of the literature was conducted by a medical librarian using the Cochrane Library, Embase, Medline, Google Scholar, PubMed, Scopus, and Web of Science Core Collection databases to find relevant articles published from database inception to October 4, 2020. The search was peer reviewed by a second medical librarian using Peer Review of Electronic Search Strategies (PRESS).11 Databases were searched using a combination of controlled vocabulary and free-text terms for “SPS” and “bowel necrosis.” Details of the full search strategy are listed in Appendix A. References from all databases were imported into an EndNote X9 library, duplicates removed, and then uploaded into Coviden
Data Extraction and Quality Assessment
We used a standardized form to extract data, which included author, year, country, study design, setting, number of patients, SPS formulation, dosing, exposure, sorbitol content, outcomes of intestinal necrosis and the composite severe gastrointestinal adverse events, and the duration of time from SPS exposure to outcome occurrence. Two reviewers (JLH and AER) independently assessed the methodological quality of included studies using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies13 and the Revised Cochrane risk of bias (RoB 2) tool for randomized controlled trials (RCTs).14 Additionally, two reviewers (JLH and CGG) graded overall strength of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.15 Disagreement was resolved by consensus.
Data Synthesis and Analysis
The proportion of patients with intestinal necrosis was compared using random effects meta-analysis using the restricted maximum likelihood method.16 For the two studies that reported hazard ratios (HRs), meta-analysis was performed after log transformation of the HRs and CIs. One study that performed survival analysis presented data for both the duration of the study (up to 11 years) and up to 1 year after exposure.9 We used the data up to 1 year after exposure because we believed later events were more likely to be due to chance than exposure to SPS. For studies with zero events, we used the treat ment-arm continuity correction, which has been reported to be preferable to the standard fixed-correction factor.17 We also performed two sensitivity analyses, including omitting the studies with zero events and performing meta-analysis using risk difference. The prevalence of intestinal ischemia was pooled using the DerSimonian and Laird18 random effects model with Freeman-Tukey19 double arcsine transformation. Heterogeneity was estimated using the I² statistic. I² values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.20 Meta-regression and tests for small-study effects were not performed because of the small number of included studies.21 In addition to random effects meta-analysis, we calculated the 90% predicted interval for future studies for the pooled effect of intestinal ischemia.22 Statistical analysis was performed using meta and metaprop commands in Stata/IC, version 16.1 (StataCorp).
RESULTS
Selected Studies
The electronic search yielded 806 unique articles, of which 791 were excluded based on title and abstract, leaving 15 articles for full-text review (Appendix B). Appendix C describes the nine studies that were excluded, including the reason for exclusion. Table 1 describes the characteristics of the six studies that met study inclusion criteria. Studies were published between 1992 and 2020. Three studies were from Canada,10,24,25 two from the United States,8,23 and one from Sweden.9 Three studies occurred in an outpatient setting,9,10,25 and three were described as inpatient studies.8,23,24 SPS preparations included sorbitol in three studies,8,23,24 were not specified in one study,10 and were not included in two studies.9,25 SPS dosing varied widely, with median doses of 15 to 30 g in three studies,9,24,25 45 to 50 g in two studies,8,23 and unspecified in one study.10 Duration of exposure typically ranged from 1 to 7 days but was not consistently described. For example, two of the studies did not report duration of exposure,8,10 and a third study reported a single dispensation of 450 g in 41% of patients, with the remaining 59% averaging three dispensations within the first year.9 Sample size ranged from 33 to 123,391 patients. Most patients were male, and mean ages ranged from 44 to 78 years. Two studies limited participation to those with chronic kidney disease (CKD) with glomerular filtration rate (GFR) <4024 or CKD stage 4 or 5 or dialysis.9 Two studies specifically limited participation to patients with potassium levels of 5.0 to 5.9 mmol/L.24,25 All six studies reported outcomes for intestinal necrosis, and four reported composite outcomes for major adverse gastrointestinal events.9,10,24,25

Table 2 describes the assessment of risk of bias using the ROBINS-I tool for the five retrospective observational studies and the RoB 2 tool for the one RCT.13,14 Three studies were rated as having serious risk of bias, with the remainder having a moderate risk of bias or some concerns. Two studies were judged as having a serious risk of bias because of potential confounding.8,23 To be judged low or moderate risk, studies needed to measure and control for potential risk factors for intestinal ischemia, such as age, diabetes, vascular disease, and heart failure.26,27 One study also had serious risk of bias for selective reporting because the published abstract of the study used a different analysis and had contradictory results from the published study.9,28 An additional area of risk of bias that did not fit into the ROBINS-I tool is that the two studies that used survival analysis chose durations for the outcome that were longer than would be expected for adverse events from SPS to be evident. One study chose 30 days and the other up to a maximum of 11 years from the time of exposure.9,10

Quantitative Outcomes
Six studies including 26,716 patients treated with SPS and controls reported the proportion of patients who developed intestinal necrosis. The Figure shows the individual study and pooled results for intestinal necrosis. The prevalence of intestinal ischemia in patients treated with SPS was 0.1% (95% CI, 0.03%-0.17%). The pooled odds ratio (OR) of intestinal necrosis was 1.43 (95% CI, 0.39-5.20). The 90% predicted interval for future studies was 0.08 to 26.6. Two studies reported rates of intestinal necrosis using survival analysis. The pooled HR from these studies was 2.00 (95% CI, 0.45-8.78). Two studies performed survival analysis for a composite outcome of severe gastrointestinal adverse events. The pooled HR for these two studies was 1.46 (95% CI, 1.01-2.11).

For the meta-analysis of intestinal necrosis, we found moderate-high statistical significance (Q = 18.82; P < .01; I² = 67.8%). Sensitivity analysis removing each study did not affect heterogeneity, with the exception of removing the study by Laureati et al,9 which resolved the heterogeneity (Q = 1.7, P = .8, I² = 0%). The pooled effect for intestinal necrosis also became statistically significant after removing Laureati et al (OR, 2.87; 95% CI, 1.24-6.63).9 We also performed two subgroup analyses, including studies that involved the concomitant use of sorbitol8,23,24 compared with studies that did not9,25 and subgroup analysis removing studies with zero events. Studies that included sorbitol found higher rates of intestinal necrosis (OR, 2.26; 95% CI, 0.80-6.38; I² = 0%) compared with studies that did not include sorbitol (OR, 0.25; 95% CI, 0.11-0.57; I² = 0%; test of group difference, P < .01). Removing the three studies with zero events resulted in a similar overall effect (OR, 1.30; 95% CI, 0.21-8.19). Finally, a meta-analysis using risk difference instead of ORs found a non–statistically significant difference in rate of intestinal necrosis favoring the control group (risk difference, −0.00033; 95% CI, −0.0022 to 0.0015; I² = 84.6%).
Table 3 summarizes our review findings and presents overall strength of evidence. Overall strength of evidence was found to be very low. Per GRADE criteria,15,29 strength of evidence for observational studies starts at low and may then be modified by the presence of bias, inconsistency, indirectness, imprecision, effect size, and direction of confounding. In the case of the three meta-analyses in the present study, risk of bias was serious for more than half of the study weights. Strength of evidence was also downrated for imprecision because of the low number of events and resultant wide CIs.

DISCUSSION
In total, we found six studies that reported rates of intestinal necrosis or severe gastrointestinal adverse events with SPS use compared with controls. The pooled rate of intestinal necrosis was not significantly higher for patients exposed to SPS when analyzed either as the proportion of patients with events or as HRs. The pooled rate for a composite outcome of severe gastrointestinal side effects was significantly higher (HR, 1.46; 95% CI, 1.01-2.11). The overall strength of evidence for the association of SPS with either intestinal necrosis or the composite outcome was found to be very low because of risk of bias and imprecision.
In some ways, our results emphasize the difficulty of showing a causal link between a medication and a possible rare adverse event. The first included study to assess the risk of intestinal necrosis after exposure to SPS compared with controls found only two events in the SPS group and no events in the control arm.23 Two additional studies that we found were small and did not report any events in either arm.24,25 The first large study to assess the risk of intestinal ischemia included more than 2,000 patients treated with SPS and more than 100,000 controls but found no difference in risk.8 The next large study did find increased risk of both intestinal necrosis (incidence rate, 6.82 per 1,000 person-years compared with 1.22 per 1,000 person-years for controls) and a composite outcome (incidence rate, 22.97 per 1,000 person-years compared with 11.01 per 1000 person-years for controls), but in the time to event analysis included events up to 30 days after treatment with SPS.10 A prior review of case reports of SPS and intestinal necrosis found a median of 2 days between SPS treatment and symptom onset.7 It is unlikely the authors would have had sufficient events to meaningfully compare rates if they limited the analysis to events within 7 days of SPS treatment, but events after a week of exposure are unlikely to be due to SPS. The final study to assess the association of SPS with intestinal necrosis actually found higher rates of intestinal necrosis in the control group when analyzed as proportions with events but reported a higher rate of a composite outcome of severe gastrointestinal adverse events that included nine separate International Classification of Diseases codes occurring up to 11 years after SPS exposure.9 This study was limited by evidence of selective reporting and was funded by the manufacturers of an alternative cation-exchange medication.
Based on our review of the literature, it is unclear if SPS does cause intestinal ischemia. The pooled results for intestinal ischemia analyzed as a proportion with events or with survival analysis did not find a statistically significantly increased risk. Because most o
A cost analysis of SPS vs potential alternatives such as patiromer for patients on chronic RAAS-I with a history of hyperkalemia or CKD published by Little et al26 concluded that SPS remained the cost-effective option when colonic necrosis incidence is 19.9% or less, and our systematic review reveals an incidence of 0.1% (95% CI, 0.03-0.17%). The incremental cost-effectiveness ratio was an astronomical $26,088,369 per quality-adjusted life-year gained, per Little’s analysis.
Limitations of our review are the heterogeneity of studies, which varied regarding inpatient or outpatient setting, formulations such as dosing, frequency, whether sorbitol was used, and interval from exposure to outcome measurement, which ranged from 7 days to 1 year. On sensitivity analysis, statistical heterogeneity was resolved by removing the study by Laureati et al.9 This study was notably different from the others because it included events occurring up to 1 year after exposure to SPS, which may have resulted in any true effect being diluted by later events unrelated to SPS. We did not exclude this study post hoc because this would result in bias; however, because the overall result becomes statistically significant without this study, our overall conclusion should be interpreted with caution.30 It is possible that future well-conducted studies may still find an effect of SPS on intestinal necrosis. Similarly, the finding that studies with SPS coformulated with sorbitol had statistically significantly increased risk of intestinal necrosis compared with studies without sorbitol should be interpreted with caution because the study by Laureati et al9 was included in the studies without sorbitol.
CONCLUSIONS
Based on our r
This work was presented at the Society of General Internal Medicine and Society of Hospital Medicine 2021 annual conferences.
1. Labriola L, Jadoul M. Sodium polystyrene sulfonate: still news after 60 years on the market. Nephrol Dial Transplant. 2020;35(9):1455-1458. https://doi.org/10.1093/ndt/gfaa004
2. Arvanitakis C, Malek G, Uehling D, Morrissey JF. Colonic complications after renal transplantation. Gastroenterology. 1973;64(4):533-538.
3. Parks M, Grady D. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med. 2019;179(8):1023-1024. https://doi.org/10.1001/jamainternmed.2019.1291
4. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733-735. https://doi.org/10.1681/ASN.2010010079
5. Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101(3):267-272.
6. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546-554. https://doi.org/10.1016/j.kint.2015.11.018
7. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e269-24. https://doi.org/10.1016/j.amjmed.2012.08.016
8. Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409-416. https://doi.org/10.1053/j.ajkd.2012.04.023
9. Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518-1526. https://doi.org/10.1093/ndt/gfz150
10. Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025-1033. https://doi.org/10.1001/jamainternmed.2019.0631
11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
14. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
15. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. https://doi.org/10.1016/j.jclinepi.2010.04.026
16. Raudenbush SW. Analyzing effect sizes: random-effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russel Sage Foundation; 2009:295-316.
17. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. https://doi.org/10.1002/sim.1761
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. https://doi.org/10.1016/0197-2456(86)90046-2
19. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607-611. https://doi.org/10.1214/aoms/1177729756
20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. https://doi.org/10.1136/bmj.327.7414.557
21. Higgins JPT, Chandler TJ, Cumptson M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook
22. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. Jan 2009;172(1):137-159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
23. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159-161. https://doi.org/10.1016/s0272-6386(12)80544-0
24. Batterink J, Lin J, Au-Yeung SHM, Cessford T. Effectiveness of sodium polystyrene sulfonate for short-term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68(4):296-303. https://doi.org/10.4212/cjhp.v68i4.1469
25. Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136-2142. https://doi.org/10.2215/CJN.03640415
26. Little DJ, Nee R, Abbott KC, Watson MA, Yuan CM. Cost-utility analysis of sodium polystyrene sulfonate vs. potential alternatives for chronic hyperkalemia. Clin Nephrol. 2014;81(4):259-268. https://doi.org/10.5414/cn108103
27. Cubiella Fernández J, Núñez Calvo L, González Vázquez E, et al. Risk factors associated with the development of ischemic colitis. World J Gastroenterol. 2010;16(36):4564-4569. https://doi.org/10.3748/wjg.v16.i36.4564
28. Laureati P, Evans M, Trevisan M, et al. Sodium polystyrene sulfonate, practice patterns and associated adverse event risk; a nationwide analysis from the Swedish Renal Register [abstract]. Nephroly Dial Transplant. 2019;34(suppl 1):i94. https://doi.org/10.1093/ndt/gfz106.FP151
29. Santesso N, Carrasco-Labra A, Langendam M, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28-39. https://doi.org/10.1016/j.jclinepi.2015.12.006
30. Deeks JJ HJ, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane, 2020. www.training.cochrane.org/handbook
1. Labriola L, Jadoul M. Sodium polystyrene sulfonate: still news after 60 years on the market. Nephrol Dial Transplant. 2020;35(9):1455-1458. https://doi.org/10.1093/ndt/gfaa004
2. Arvanitakis C, Malek G, Uehling D, Morrissey JF. Colonic complications after renal transplantation. Gastroenterology. 1973;64(4):533-538.
3. Parks M, Grady D. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med. 2019;179(8):1023-1024. https://doi.org/10.1001/jamainternmed.2019.1291
4. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733-735. https://doi.org/10.1681/ASN.2010010079
5. Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101(3):267-272.
6. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546-554. https://doi.org/10.1016/j.kint.2015.11.018
7. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e269-24. https://doi.org/10.1016/j.amjmed.2012.08.016
8. Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409-416. https://doi.org/10.1053/j.ajkd.2012.04.023
9. Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518-1526. https://doi.org/10.1093/ndt/gfz150
10. Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025-1033. https://doi.org/10.1001/jamainternmed.2019.0631
11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
14. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
15. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. https://doi.org/10.1016/j.jclinepi.2010.04.026
16. Raudenbush SW. Analyzing effect sizes: random-effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russel Sage Foundation; 2009:295-316.
17. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. https://doi.org/10.1002/sim.1761
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. https://doi.org/10.1016/0197-2456(86)90046-2
19. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607-611. https://doi.org/10.1214/aoms/1177729756
20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. https://doi.org/10.1136/bmj.327.7414.557
21. Higgins JPT, Chandler TJ, Cumptson M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook
22. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. Jan 2009;172(1):137-159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
23. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159-161. https://doi.org/10.1016/s0272-6386(12)80544-0
24. Batterink J, Lin J, Au-Yeung SHM, Cessford T. Effectiveness of sodium polystyrene sulfonate for short-term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68(4):296-303. https://doi.org/10.4212/cjhp.v68i4.1469
25. Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136-2142. https://doi.org/10.2215/CJN.03640415
26. Little DJ, Nee R, Abbott KC, Watson MA, Yuan CM. Cost-utility analysis of sodium polystyrene sulfonate vs. potential alternatives for chronic hyperkalemia. Clin Nephrol. 2014;81(4):259-268. https://doi.org/10.5414/cn108103
27. Cubiella Fernández J, Núñez Calvo L, González Vázquez E, et al. Risk factors associated with the development of ischemic colitis. World J Gastroenterol. 2010;16(36):4564-4569. https://doi.org/10.3748/wjg.v16.i36.4564
28. Laureati P, Evans M, Trevisan M, et al. Sodium polystyrene sulfonate, practice patterns and associated adverse event risk; a nationwide analysis from the Swedish Renal Register [abstract]. Nephroly Dial Transplant. 2019;34(suppl 1):i94. https://doi.org/10.1093/ndt/gfz106.FP151
29. Santesso N, Carrasco-Labra A, Langendam M, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28-39. https://doi.org/10.1016/j.jclinepi.2015.12.006
30. Deeks JJ HJ, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane, 2020. www.training.cochrane.org/handbook
© 2021 Society of Hospital Medicine