User login
Managing ‘difficult’ patient encounters
“I did not like those patients… They made me angry and I found myself irritated to experience them as they seemed so distant from myself and from all that is human. This is an astonishing intolerance which brands me a poor psychiatrist.”
Sigmund Freud, Letter to István Hollós (1928)
While Freud was referring to psychotic patients,1 his evident frustration shows that difficult and challenging patients have vexed even the best of us. All physicians and other clinicians will experience patient encounters that lead to anger or frustration, or even challenge their sense of equanimity and professional identity. In short, difficult and challenging patient interactions are unavoidable, regardless of the physician’s discipline.2-5 At times, physicians might struggle with demanding, unpleasant, ungrateful, and possibly dangerous patients, while sometimes the struggle is with the patient’s family members. No physician is immune to the problem, which makes it crucial to learn to anticipate and manage difficult patient interactions, skills which are generally not taught in medical schools or residency programs.
One prospective study of clinic patients found that up to 15% of patient encounters are deemed “difficult.”6 Common scenarios include patients (or their relatives) who seek certain tests after researching symptoms online, threats of legal or social media action in response to feeling that the physician is not listening to them, demands for a second opinion after disagreeing with the physician’s diagnosis, and mistrust of doctors after presenting with symptoms and not receiving a diagnosis. It is also common to care for patients who focus on negative outcomes or fail to adhere to treatment recommendations. These encounters can make physicians feel stressed out, disrespected, abused, or even fearful if threatened. Some physicians may come to feel they are trapped in a hostile work environment with little support from their supervisors or administrators. Patients often have a complaint office or department to turn to, but there is no equivalent for physicians, who are expected to soldier on regardless.
This article highlights a model that describes poor physician-patient encounters, factors contributing to these issues, how to manage these difficult interactions, and what to do if the relationship cannot be remediated.
Describing the ‘difficult’ patient
In a landmark 1978 paper, Groves7 provided one of the first descriptions of “difficult” patients. His colorful observations continue to provide useful insights. Groves emphasized that most medical texts ignore the issue of difficult patients and provide little or no guidance—which is still true 43 years later. He observed that physicians cannot avoid occasional negative feelings toward some patients. Further, Groves suggested that countertransference is often at the root of hateful reactions, a process he defines as “conscious or unconscious unbidden and unwanted hostile or sexual feelings toward the patient.”7Table 17 outlines how Groves divided “hateful” patients into several categories, and how physicians might respond to such patients.
A model for understanding difficult patient encounters
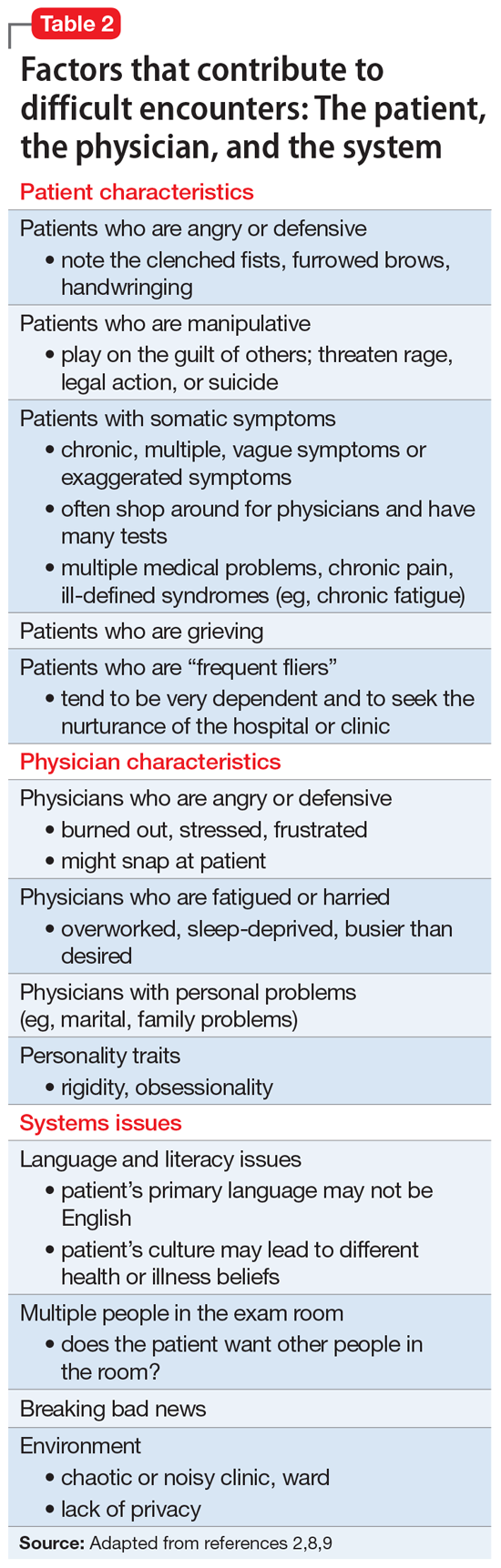
Adams and Murray2 created a model to help explain interactions with difficult or challenging patients that consists of 3 elements: the patient, the physician, and the system (ie, situation or environment). Hull and Broquet8 and Hardavella et al9 later adapted the model and described its components (Table 22,8,9).
Continue to: When considering...
When considering difficult interactions, it is important to be aware that all 3 components could interact, or merely 1 or 2 could come into play, but all should be explored as possible contributing factors.
Patient factors
The patient’s role in initiating or maintaining a problematic interaction should be explored. While some physicians are tempted to conclude that a personality disorder underlies difficult interactions, research shows a more complex picture. First, not all difficult patients have a psychiatric disorder, let alone a personality disorder. Jackson and Kroenke6 reported that among 74 difficult patients in an ambulatory clinic, 29% had a depressive disorder or anxiety disorder, with 11% experiencing 2 or more disorders. Major depressive disorder was present in 8.4% patients, other depressive disorders in 17.4%, panic disorder in 1.4%, and other anxiety disorders in 14.2%.6 These researchers found that difficult patient interactions were associated with the presence of a psychiatric disorder, especially depressive or anxiety disorders, and multiple physical symptoms.
Importantly, difficult patients are not unique to psychiatry, and are found in all medical disciplines and every type of practice situation. Some problematic patients have a substance use disorder, and their difficulty might stem from intoxication, withdrawal, or drug-seeking behaviors. Psychotic disorders can be the source of difficult interactions, typically resulting from the patient’s symptoms (ie, hallucinations, delusions, or bizarre behavior). Physicians tend to be forgiving toward these patients because they understand the extent of the individual’s illness. The same is true for a patient with dementia, who might be disruptive and loud, yet clearly is not in control of their behavior.
Koekkoek et al5 reviewed 94 articles that focused on difficult patients seen in mental health settings. Most patients were male (60% to 68%), and most were age 26 to 32 years. Diagnoses of psychotic disorders and personality disorders were the most frequent, while mood and other disorders were less common. In 1 of the studies reviewed, 6% of psychiatric inpatients were considered difficult. Koekkoek et al5 proposed that there are 3 groups of difficult patients:
- care avoiders: patients with psychosis who lack insight
- care seekers: patients who are chronically ill who have trouble maintaining a steady relationship with their caregivers
- care claimers: patients who do not require long-term care, but need housing, medication, or a “declaration of incompetence.”
Physician factors
Physicians are frequent contributors to bad interactions with their patients.2,7,8 They can become angry or defensive because of burnout, stress, or frustration, which might lead them to snap or otherwise respond inappropriately to their patients. Many physicians are overworked, sleep-deprived, or busier than they would prefer. Personal problems can be preoccupying and contribute to a physician being ill-tempered or distracted (eg, marital or family problems). Some physicians are simply poor communicators and might not understand the need to adapt their communication style to their patient, instead using medical jargon the patient does not understand. Ideally, physicians should modify their language to suit the patient’s level of education, degree of medical sophistication, and cultural background.
Continue to: A physician's personality traits...
A physician’s personality traits could clash with those of the patient, particularly if the physician is especially rigid or obsessional. Rather than “going with the flow,” the overly rigid physician might become impatient with patients who fail to understand diagnostic assessments or treatment recommendations. Inefficient physicians might not be able to keep up with the daily schedule, which could fuel impatience and perhaps even lead them to think that the patient is taking too much of their valuable time. Some might not know how to convey empathy, for example when giving bad news (“The tests show you have cancer…”). Others fail to make consistent eye contact with patients without understanding its importance to communication, a problem made worse by the use of electronic medical record systems (EMRs).
Systems issues
Systems issues also contribute to suboptimal physician-patient interactions, and some issues can be attributed to administrative problems. Examples of systems issues include:
- when a patient has difficulty making an appointment and is forced to listen to a confusing menu of choices
- a busy clinic that can only offer a patient an appointment 6 months away
- crowded or noisy waiting rooms
- language barriers for patients whose primary langage is not English. Not having access to an interpreter can exacerbate their frustration
- the use of EMRs is a growing threat to positive physician-patient interactions, yet their influence is often ignored. Widely disliked by physicians,10 EMRs are required in all but the smallest independent practice settings. Many busy physicians focus their attention on the computer, giving the patient the impression that the physician is not listening to them. Many patients conclude that they are less important than the process.
The consequences of difficult interactions
Following a bad interaction, dissatisfied patients are more likely to leave the clinic or hospital and ignore medical advice. These patients might then show up in crowded emergency departments, which may lead to poor use of health care resources. For physicians, challenging situations sap their emotional energy, cause demoralization, and interfere with their sense of job fulfillment. In extreme cases, such feelings might lead the physician to dislike and even avoid the patient.
How to manage challenging situations
Taking the following steps can help physicians work through challenging situations with their patients.
Diagnose the problem. First, recognize the difficult situation, analyze it, and identify how the patient, the physician, and the system are contributing to a bad physician-patient interaction. Diagnosing the interactional difficulty should precede the diagnosis and management of the patient’s disease. Physicians should acknowledge their own contribution through their attitude or actions. Finally, determine if there are system issues that are contributing to the problem, or if it is the clinic or inpatient setting itself (eg, noisy inpatient unit).
Continue to: Maintain your cool
Maintain your cool. With any difficult interaction, a physician’s first obligation is to remain calm and professional, while modeling appropriate behavior. If the patient is angry or emotionally intense, talking over them or interrupting them only makes the situation worse. Try to see the interaction from the patient’s perspective. Both parties should work together to find a common ground.
Collaborate, respect boundaries, and empathize. One study of a group of 100 family physicians found that having the following 3 skills were essential to successfully managing situations with difficult patients11,12:
- the ability to collaborate (vs opposition)
- the appropriate use of power (vs misuse of power, or violation of boundaries by either party)
- the ability to empathize, which for most physicians involves understanding and validating the patient’s subjective experiences.
Although a description of the many facets of empathy (cognitive, affective, motivational) is beyond the scope of this article, it is worth pointing out that a patient’s positive perception of their physician’s empathy improves not only patient satisfaction but health outcomes.13 The Box describes a difficult patient whose actions changed through the collaboration and empathy of his treatment team.
Box 1
Mr. L, a 60-year-old veteran, is admitted to an inpatient unit following a suicide attempt that was prompted by eviction from his apartment. Mr. L is physically disabled and has difficulty walking without assistance. His main concern is his homelessness, and he insists that the inpatient team find a suitable “Americans with Disabilities (ADA)-compliant apartment” that he can afford on his $800 monthly income. He implies that he will kill himself if the team fails in that task. He makes it clear that his problems are the team’s problems. He is prescribed an antidepressant, and both his mood and reported suicidal ideations gradually resolve.
The team’s social worker finds an opening at a well-run veterans home, but Mr. L rejects it because he doesn’t want to “give up his independence.” The social worker finds a small apartment in a nearby community that is ADA-compliant, but Mr. L complains that it is small. He asks the resident psychiatrist, “Where will I put all my things?” The next day, after insulting the attending psychiatrist for failing to find an adequate apartment, Mr. L says from under the bedsheet: “How come none of you ever help me?”
Mr. L presents a challenge to the entire team. At times, he is rude, demanding, and entitled. The team recognizes that although he had served in the military with distinction, he is now alone after having divorced many years earlier, and nearly friendless because of his increasing disability. The team surmises that Mr. L lashes out due to frustration and feelings of powerlessness.
Resolving this conflict involves treating Mr. L with respect and listening without judgment. No one ever confronts him or argues with him. The team psychologist meets with him to help him work through his many losses. Closer to discharge, he is enrolled in several post-hospitalization programs to keep him connected with other veterans. At discharge, the hospital arranges for his belongings that had been in storage to be delivered to his new home. He is pleasant and social with his peers, and although he is still concerned about the size of the apartment, he thanks the team members for their care.
Verbalize the difficulty. It is important to openly discuss the problem. For example, “We both have very different views about how your symptoms should be investigated, and that’s causing some difficulty between us. Do you agree?” This approach names the “elephant in the room” and avoids casting blame. It also creates a sense of shared ownership by externalizing the problem from both the patient and physician. Verbalizing the difficulty can help build trust and pave the way to working together toward a common solution.
Consider other explanations for the patient’s behavior. For example, anger directed at a physician could be due to anxiety about an unrelated matter, such as the patient’s recent job loss or impending divorce. Psychiatrists might understand this behavior better as displacement, which is considered a maladaptive defense mechanism. It is important to listen to the patient and offer empathy, which will help the patient feel supported and build a rapport that can help to resolve the encounter.
Continue to: When helping patients...
When helping patients with multiple issues, which is a common scenario, the physician might start by asking, “What would you like to address today?”14 Keep a list of the issues so you do not forget the patient’s concerns, and then ask: “What do you think is going on?” Give patients time to verbalize their concerns. Physicians should:
- validate concerns: “I understand where you’re coming from.”
- offer empathy: “I can see how difficult this has been for you.”
- reframe: “Let me make sure I hear you correctly.”
- refocus: “Let’s agree on what we need to do at this visit.”
Find common ground. When the patient and physician have different ideas on diagnosis or treatment, finding common ground is another way to resolve a difficult encounter. Difficulties arise when there appears to be little common ground, which often results from unrealistic expectations. Patients might be seen as “demanding” or “manipulative”’ if they push for a diagnosis or treatment the doctor is not comfortable with. As soon as there is some overlap and common ground, the difficulty rapidly subsides.
Set clear boundaries and limits. Physicians should set limits on what patient behavior might “cross the line.” A “behavioral contract” (or “treatment contract”) can help by setting explicit expectations. For example, showing up late for appointments or inappropriately seeking drugs of abuse (eg, opioids, benzodiazepines) might be identified as violations of the contract. Once the contract is set, the patient should be asked to restate key components. Clarify any confusion or barriers to compliance and define clear expectations. The patient should be informed of potential consequences of contract violations, including termination.
Staff members involved in the patient’s care should agree with the terms of any behavioral contract, and should receive a copy of it. Patients should have “buy in,” meaning that they have had an opportunity to provide input to the contract and have agreed to its elements. Both the physician and patient should sign the document.
When all else fails
When there is a breakdown in rapport that makes it difficult or impossible to continue offering treatment, consider termination. This could be due to threatening or abusive patient behavior, sexual advances, repeated no-shows, treatment noncompliance that jeopardizes patient safety, refusal to follow the treatment plan, or violating the terms of a behavioral contract. In some settings, it might be the failure to pay bills.
Continue to: If a patient is unable to...
If a patient is unable to follow the contract, the physician should explore possible extenuating circumstances. The physician should seek to remedy the problem and involve other team members if possible (eg, case manager, nurse), advising a patient about behaviors that could lead to termination.
If the problem is irremediable, notify the patient in writing, give them time to find another physician, and facilitate the transfer of care.15 Take steps to prevent the patient from running out of any medications associated with withdrawal or discontinuation syndromes (eg, selective serotonin reuptake inhibitors, benzodiazepines) during the care transition. While there is no requirement regarding the amount of time allowed, at least 30 days is typical.
Bottom Line
Difficult patient interactions are common and unavoidable. Physicians should acknowledge and recognize contributing factors in such encounters—including their own role. When handling such situations, physicians should remain calm and model appropriate behavior. Improving communication, offering empathy, and validating the patient’s concerns can help resolve factors that contribute to poor patient interactions. If efforts to remediate the physician-patient relationship fail, termination may be necessary.
Related Resources
- Koekkoek B, Berno van Meijel CNS, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57(6):795-802.
- Pereira MR, Figueiredo AF. Challenging patient-doctor interactions in psychiatry – difficult patient syndrome. European Psychiatry. 2017;41(supplement):S719. doi. org/10.1016/j.eurpsy.2017.01.1297
1. Dupont J. Ferenczi’s madness. Contemp Psychoanal. 1988;24(2):250-261.
2. Adams J, Murray R. The difficult diagnosis: the general approach to the difficult patient. Emerg Med Clin North Am. 1998;16(4):689-700.
3. Davies M. Managing challenging interactions with patients. BMJ. 2013;347:f4673. doi: https://doi.org/10.1136/bmj.f4673
4. Chou C. Dealing with the “difficult” patient. Wisc Med J. 2004;103:35-38.
5. Koekkoek B, Berno van Meijel CNS, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57(6):795-802.
6. Jackson JL, Kroenke K. Difficult patient encounter in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med 1999;159(10):1069-1075.
7. Groves JE. Taking care of the hateful patient. N Eng J Med. 1978;298:883-887.
8. Hull S, Broquet K. How to manage difficult encounters. Fam Prac Manag. 2007;14(6):30-34.
9. Hardavella G, Aamli-Gaagnat A, Frille A, et al. Top tips to deal with challenging situations: doctor patient interactions. Breathe. 2017;13(2):129-135.
10. Black DW, Balon R. Editorial: electronic medical records (EMRs) and the psychiatrist shortage. Ann Clin Psychiatry. 2018;30(4):257-259.
11. Elder N, Ricer R, Tobias B. How respected family physicians manage difficult patient encounters. J Am Board Fam Med. 2006;19(6):533-541.
12. Campbell RJ. Campbell’s Psychiatric Dictionary. 8th Edition. Oxford University Press; 2004:219-220.
13. Decety J, Fotopoulou A. Why empathy has a beneficial impact on others in medicine: unifying theories. Front Behav Neurosci. 2014;8:457. https://doi.org/10.3389/fnbeh.2014.00457
14. Klugman B. The difficult patient. Accessed May 24, 2021. https://www.umassmed.edu/globalassets/office-of-continuing-medical-education/pdfs/cme-primary-care-days/e2-the-difficult-patient.pdf
15. Mossman D, Farrell HM, Gilday E. ‘Firing’ a patient: may psychiatrists unilaterally terminate care? Current Psychiatry. 2010;9(12):18-29.
“I did not like those patients… They made me angry and I found myself irritated to experience them as they seemed so distant from myself and from all that is human. This is an astonishing intolerance which brands me a poor psychiatrist.”
Sigmund Freud, Letter to István Hollós (1928)
While Freud was referring to psychotic patients,1 his evident frustration shows that difficult and challenging patients have vexed even the best of us. All physicians and other clinicians will experience patient encounters that lead to anger or frustration, or even challenge their sense of equanimity and professional identity. In short, difficult and challenging patient interactions are unavoidable, regardless of the physician’s discipline.2-5 At times, physicians might struggle with demanding, unpleasant, ungrateful, and possibly dangerous patients, while sometimes the struggle is with the patient’s family members. No physician is immune to the problem, which makes it crucial to learn to anticipate and manage difficult patient interactions, skills which are generally not taught in medical schools or residency programs.
One prospective study of clinic patients found that up to 15% of patient encounters are deemed “difficult.”6 Common scenarios include patients (or their relatives) who seek certain tests after researching symptoms online, threats of legal or social media action in response to feeling that the physician is not listening to them, demands for a second opinion after disagreeing with the physician’s diagnosis, and mistrust of doctors after presenting with symptoms and not receiving a diagnosis. It is also common to care for patients who focus on negative outcomes or fail to adhere to treatment recommendations. These encounters can make physicians feel stressed out, disrespected, abused, or even fearful if threatened. Some physicians may come to feel they are trapped in a hostile work environment with little support from their supervisors or administrators. Patients often have a complaint office or department to turn to, but there is no equivalent for physicians, who are expected to soldier on regardless.
This article highlights a model that describes poor physician-patient encounters, factors contributing to these issues, how to manage these difficult interactions, and what to do if the relationship cannot be remediated.
Describing the ‘difficult’ patient
In a landmark 1978 paper, Groves7 provided one of the first descriptions of “difficult” patients. His colorful observations continue to provide useful insights. Groves emphasized that most medical texts ignore the issue of difficult patients and provide little or no guidance—which is still true 43 years later. He observed that physicians cannot avoid occasional negative feelings toward some patients. Further, Groves suggested that countertransference is often at the root of hateful reactions, a process he defines as “conscious or unconscious unbidden and unwanted hostile or sexual feelings toward the patient.”7Table 17 outlines how Groves divided “hateful” patients into several categories, and how physicians might respond to such patients.
A model for understanding difficult patient encounters
Adams and Murray2 created a model to help explain interactions with difficult or challenging patients that consists of 3 elements: the patient, the physician, and the system (ie, situation or environment). Hull and Broquet8 and Hardavella et al9 later adapted the model and described its components (Table 22,8,9).
Continue to: When considering...
When considering difficult interactions, it is important to be aware that all 3 components could interact, or merely 1 or 2 could come into play, but all should be explored as possible contributing factors.
Patient factors
The patient’s role in initiating or maintaining a problematic interaction should be explored. While some physicians are tempted to conclude that a personality disorder underlies difficult interactions, research shows a more complex picture. First, not all difficult patients have a psychiatric disorder, let alone a personality disorder. Jackson and Kroenke6 reported that among 74 difficult patients in an ambulatory clinic, 29% had a depressive disorder or anxiety disorder, with 11% experiencing 2 or more disorders. Major depressive disorder was present in 8.4% patients, other depressive disorders in 17.4%, panic disorder in 1.4%, and other anxiety disorders in 14.2%.6 These researchers found that difficult patient interactions were associated with the presence of a psychiatric disorder, especially depressive or anxiety disorders, and multiple physical symptoms.
Importantly, difficult patients are not unique to psychiatry, and are found in all medical disciplines and every type of practice situation. Some problematic patients have a substance use disorder, and their difficulty might stem from intoxication, withdrawal, or drug-seeking behaviors. Psychotic disorders can be the source of difficult interactions, typically resulting from the patient’s symptoms (ie, hallucinations, delusions, or bizarre behavior). Physicians tend to be forgiving toward these patients because they understand the extent of the individual’s illness. The same is true for a patient with dementia, who might be disruptive and loud, yet clearly is not in control of their behavior.
Koekkoek et al5 reviewed 94 articles that focused on difficult patients seen in mental health settings. Most patients were male (60% to 68%), and most were age 26 to 32 years. Diagnoses of psychotic disorders and personality disorders were the most frequent, while mood and other disorders were less common. In 1 of the studies reviewed, 6% of psychiatric inpatients were considered difficult. Koekkoek et al5 proposed that there are 3 groups of difficult patients:
- care avoiders: patients with psychosis who lack insight
- care seekers: patients who are chronically ill who have trouble maintaining a steady relationship with their caregivers
- care claimers: patients who do not require long-term care, but need housing, medication, or a “declaration of incompetence.”
Physician factors
Physicians are frequent contributors to bad interactions with their patients.2,7,8 They can become angry or defensive because of burnout, stress, or frustration, which might lead them to snap or otherwise respond inappropriately to their patients. Many physicians are overworked, sleep-deprived, or busier than they would prefer. Personal problems can be preoccupying and contribute to a physician being ill-tempered or distracted (eg, marital or family problems). Some physicians are simply poor communicators and might not understand the need to adapt their communication style to their patient, instead using medical jargon the patient does not understand. Ideally, physicians should modify their language to suit the patient’s level of education, degree of medical sophistication, and cultural background.
Continue to: A physician's personality traits...
A physician’s personality traits could clash with those of the patient, particularly if the physician is especially rigid or obsessional. Rather than “going with the flow,” the overly rigid physician might become impatient with patients who fail to understand diagnostic assessments or treatment recommendations. Inefficient physicians might not be able to keep up with the daily schedule, which could fuel impatience and perhaps even lead them to think that the patient is taking too much of their valuable time. Some might not know how to convey empathy, for example when giving bad news (“The tests show you have cancer…”). Others fail to make consistent eye contact with patients without understanding its importance to communication, a problem made worse by the use of electronic medical record systems (EMRs).
Systems issues
Systems issues also contribute to suboptimal physician-patient interactions, and some issues can be attributed to administrative problems. Examples of systems issues include:
- when a patient has difficulty making an appointment and is forced to listen to a confusing menu of choices
- a busy clinic that can only offer a patient an appointment 6 months away
- crowded or noisy waiting rooms
- language barriers for patients whose primary langage is not English. Not having access to an interpreter can exacerbate their frustration
- the use of EMRs is a growing threat to positive physician-patient interactions, yet their influence is often ignored. Widely disliked by physicians,10 EMRs are required in all but the smallest independent practice settings. Many busy physicians focus their attention on the computer, giving the patient the impression that the physician is not listening to them. Many patients conclude that they are less important than the process.
The consequences of difficult interactions
Following a bad interaction, dissatisfied patients are more likely to leave the clinic or hospital and ignore medical advice. These patients might then show up in crowded emergency departments, which may lead to poor use of health care resources. For physicians, challenging situations sap their emotional energy, cause demoralization, and interfere with their sense of job fulfillment. In extreme cases, such feelings might lead the physician to dislike and even avoid the patient.
How to manage challenging situations
Taking the following steps can help physicians work through challenging situations with their patients.
Diagnose the problem. First, recognize the difficult situation, analyze it, and identify how the patient, the physician, and the system are contributing to a bad physician-patient interaction. Diagnosing the interactional difficulty should precede the diagnosis and management of the patient’s disease. Physicians should acknowledge their own contribution through their attitude or actions. Finally, determine if there are system issues that are contributing to the problem, or if it is the clinic or inpatient setting itself (eg, noisy inpatient unit).
Continue to: Maintain your cool
Maintain your cool. With any difficult interaction, a physician’s first obligation is to remain calm and professional, while modeling appropriate behavior. If the patient is angry or emotionally intense, talking over them or interrupting them only makes the situation worse. Try to see the interaction from the patient’s perspective. Both parties should work together to find a common ground.
Collaborate, respect boundaries, and empathize. One study of a group of 100 family physicians found that having the following 3 skills were essential to successfully managing situations with difficult patients11,12:
- the ability to collaborate (vs opposition)
- the appropriate use of power (vs misuse of power, or violation of boundaries by either party)
- the ability to empathize, which for most physicians involves understanding and validating the patient’s subjective experiences.
Although a description of the many facets of empathy (cognitive, affective, motivational) is beyond the scope of this article, it is worth pointing out that a patient’s positive perception of their physician’s empathy improves not only patient satisfaction but health outcomes.13 The Box describes a difficult patient whose actions changed through the collaboration and empathy of his treatment team.
Box 1
Mr. L, a 60-year-old veteran, is admitted to an inpatient unit following a suicide attempt that was prompted by eviction from his apartment. Mr. L is physically disabled and has difficulty walking without assistance. His main concern is his homelessness, and he insists that the inpatient team find a suitable “Americans with Disabilities (ADA)-compliant apartment” that he can afford on his $800 monthly income. He implies that he will kill himself if the team fails in that task. He makes it clear that his problems are the team’s problems. He is prescribed an antidepressant, and both his mood and reported suicidal ideations gradually resolve.
The team’s social worker finds an opening at a well-run veterans home, but Mr. L rejects it because he doesn’t want to “give up his independence.” The social worker finds a small apartment in a nearby community that is ADA-compliant, but Mr. L complains that it is small. He asks the resident psychiatrist, “Where will I put all my things?” The next day, after insulting the attending psychiatrist for failing to find an adequate apartment, Mr. L says from under the bedsheet: “How come none of you ever help me?”
Mr. L presents a challenge to the entire team. At times, he is rude, demanding, and entitled. The team recognizes that although he had served in the military with distinction, he is now alone after having divorced many years earlier, and nearly friendless because of his increasing disability. The team surmises that Mr. L lashes out due to frustration and feelings of powerlessness.
Resolving this conflict involves treating Mr. L with respect and listening without judgment. No one ever confronts him or argues with him. The team psychologist meets with him to help him work through his many losses. Closer to discharge, he is enrolled in several post-hospitalization programs to keep him connected with other veterans. At discharge, the hospital arranges for his belongings that had been in storage to be delivered to his new home. He is pleasant and social with his peers, and although he is still concerned about the size of the apartment, he thanks the team members for their care.
Verbalize the difficulty. It is important to openly discuss the problem. For example, “We both have very different views about how your symptoms should be investigated, and that’s causing some difficulty between us. Do you agree?” This approach names the “elephant in the room” and avoids casting blame. It also creates a sense of shared ownership by externalizing the problem from both the patient and physician. Verbalizing the difficulty can help build trust and pave the way to working together toward a common solution.
Consider other explanations for the patient’s behavior. For example, anger directed at a physician could be due to anxiety about an unrelated matter, such as the patient’s recent job loss or impending divorce. Psychiatrists might understand this behavior better as displacement, which is considered a maladaptive defense mechanism. It is important to listen to the patient and offer empathy, which will help the patient feel supported and build a rapport that can help to resolve the encounter.
Continue to: When helping patients...
When helping patients with multiple issues, which is a common scenario, the physician might start by asking, “What would you like to address today?”14 Keep a list of the issues so you do not forget the patient’s concerns, and then ask: “What do you think is going on?” Give patients time to verbalize their concerns. Physicians should:
- validate concerns: “I understand where you’re coming from.”
- offer empathy: “I can see how difficult this has been for you.”
- reframe: “Let me make sure I hear you correctly.”
- refocus: “Let’s agree on what we need to do at this visit.”
Find common ground. When the patient and physician have different ideas on diagnosis or treatment, finding common ground is another way to resolve a difficult encounter. Difficulties arise when there appears to be little common ground, which often results from unrealistic expectations. Patients might be seen as “demanding” or “manipulative”’ if they push for a diagnosis or treatment the doctor is not comfortable with. As soon as there is some overlap and common ground, the difficulty rapidly subsides.
Set clear boundaries and limits. Physicians should set limits on what patient behavior might “cross the line.” A “behavioral contract” (or “treatment contract”) can help by setting explicit expectations. For example, showing up late for appointments or inappropriately seeking drugs of abuse (eg, opioids, benzodiazepines) might be identified as violations of the contract. Once the contract is set, the patient should be asked to restate key components. Clarify any confusion or barriers to compliance and define clear expectations. The patient should be informed of potential consequences of contract violations, including termination.
Staff members involved in the patient’s care should agree with the terms of any behavioral contract, and should receive a copy of it. Patients should have “buy in,” meaning that they have had an opportunity to provide input to the contract and have agreed to its elements. Both the physician and patient should sign the document.
When all else fails
When there is a breakdown in rapport that makes it difficult or impossible to continue offering treatment, consider termination. This could be due to threatening or abusive patient behavior, sexual advances, repeated no-shows, treatment noncompliance that jeopardizes patient safety, refusal to follow the treatment plan, or violating the terms of a behavioral contract. In some settings, it might be the failure to pay bills.
Continue to: If a patient is unable to...
If a patient is unable to follow the contract, the physician should explore possible extenuating circumstances. The physician should seek to remedy the problem and involve other team members if possible (eg, case manager, nurse), advising a patient about behaviors that could lead to termination.
If the problem is irremediable, notify the patient in writing, give them time to find another physician, and facilitate the transfer of care.15 Take steps to prevent the patient from running out of any medications associated with withdrawal or discontinuation syndromes (eg, selective serotonin reuptake inhibitors, benzodiazepines) during the care transition. While there is no requirement regarding the amount of time allowed, at least 30 days is typical.
Bottom Line
Difficult patient interactions are common and unavoidable. Physicians should acknowledge and recognize contributing factors in such encounters—including their own role. When handling such situations, physicians should remain calm and model appropriate behavior. Improving communication, offering empathy, and validating the patient’s concerns can help resolve factors that contribute to poor patient interactions. If efforts to remediate the physician-patient relationship fail, termination may be necessary.
Related Resources
- Koekkoek B, Berno van Meijel CNS, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57(6):795-802.
- Pereira MR, Figueiredo AF. Challenging patient-doctor interactions in psychiatry – difficult patient syndrome. European Psychiatry. 2017;41(supplement):S719. doi. org/10.1016/j.eurpsy.2017.01.1297
“I did not like those patients… They made me angry and I found myself irritated to experience them as they seemed so distant from myself and from all that is human. This is an astonishing intolerance which brands me a poor psychiatrist.”
Sigmund Freud, Letter to István Hollós (1928)
While Freud was referring to psychotic patients,1 his evident frustration shows that difficult and challenging patients have vexed even the best of us. All physicians and other clinicians will experience patient encounters that lead to anger or frustration, or even challenge their sense of equanimity and professional identity. In short, difficult and challenging patient interactions are unavoidable, regardless of the physician’s discipline.2-5 At times, physicians might struggle with demanding, unpleasant, ungrateful, and possibly dangerous patients, while sometimes the struggle is with the patient’s family members. No physician is immune to the problem, which makes it crucial to learn to anticipate and manage difficult patient interactions, skills which are generally not taught in medical schools or residency programs.
One prospective study of clinic patients found that up to 15% of patient encounters are deemed “difficult.”6 Common scenarios include patients (or their relatives) who seek certain tests after researching symptoms online, threats of legal or social media action in response to feeling that the physician is not listening to them, demands for a second opinion after disagreeing with the physician’s diagnosis, and mistrust of doctors after presenting with symptoms and not receiving a diagnosis. It is also common to care for patients who focus on negative outcomes or fail to adhere to treatment recommendations. These encounters can make physicians feel stressed out, disrespected, abused, or even fearful if threatened. Some physicians may come to feel they are trapped in a hostile work environment with little support from their supervisors or administrators. Patients often have a complaint office or department to turn to, but there is no equivalent for physicians, who are expected to soldier on regardless.
This article highlights a model that describes poor physician-patient encounters, factors contributing to these issues, how to manage these difficult interactions, and what to do if the relationship cannot be remediated.
Describing the ‘difficult’ patient
In a landmark 1978 paper, Groves7 provided one of the first descriptions of “difficult” patients. His colorful observations continue to provide useful insights. Groves emphasized that most medical texts ignore the issue of difficult patients and provide little or no guidance—which is still true 43 years later. He observed that physicians cannot avoid occasional negative feelings toward some patients. Further, Groves suggested that countertransference is often at the root of hateful reactions, a process he defines as “conscious or unconscious unbidden and unwanted hostile or sexual feelings toward the patient.”7Table 17 outlines how Groves divided “hateful” patients into several categories, and how physicians might respond to such patients.
A model for understanding difficult patient encounters
Adams and Murray2 created a model to help explain interactions with difficult or challenging patients that consists of 3 elements: the patient, the physician, and the system (ie, situation or environment). Hull and Broquet8 and Hardavella et al9 later adapted the model and described its components (Table 22,8,9).
Continue to: When considering...
When considering difficult interactions, it is important to be aware that all 3 components could interact, or merely 1 or 2 could come into play, but all should be explored as possible contributing factors.
Patient factors
The patient’s role in initiating or maintaining a problematic interaction should be explored. While some physicians are tempted to conclude that a personality disorder underlies difficult interactions, research shows a more complex picture. First, not all difficult patients have a psychiatric disorder, let alone a personality disorder. Jackson and Kroenke6 reported that among 74 difficult patients in an ambulatory clinic, 29% had a depressive disorder or anxiety disorder, with 11% experiencing 2 or more disorders. Major depressive disorder was present in 8.4% patients, other depressive disorders in 17.4%, panic disorder in 1.4%, and other anxiety disorders in 14.2%.6 These researchers found that difficult patient interactions were associated with the presence of a psychiatric disorder, especially depressive or anxiety disorders, and multiple physical symptoms.
Importantly, difficult patients are not unique to psychiatry, and are found in all medical disciplines and every type of practice situation. Some problematic patients have a substance use disorder, and their difficulty might stem from intoxication, withdrawal, or drug-seeking behaviors. Psychotic disorders can be the source of difficult interactions, typically resulting from the patient’s symptoms (ie, hallucinations, delusions, or bizarre behavior). Physicians tend to be forgiving toward these patients because they understand the extent of the individual’s illness. The same is true for a patient with dementia, who might be disruptive and loud, yet clearly is not in control of their behavior.
Koekkoek et al5 reviewed 94 articles that focused on difficult patients seen in mental health settings. Most patients were male (60% to 68%), and most were age 26 to 32 years. Diagnoses of psychotic disorders and personality disorders were the most frequent, while mood and other disorders were less common. In 1 of the studies reviewed, 6% of psychiatric inpatients were considered difficult. Koekkoek et al5 proposed that there are 3 groups of difficult patients:
- care avoiders: patients with psychosis who lack insight
- care seekers: patients who are chronically ill who have trouble maintaining a steady relationship with their caregivers
- care claimers: patients who do not require long-term care, but need housing, medication, or a “declaration of incompetence.”
Physician factors
Physicians are frequent contributors to bad interactions with their patients.2,7,8 They can become angry or defensive because of burnout, stress, or frustration, which might lead them to snap or otherwise respond inappropriately to their patients. Many physicians are overworked, sleep-deprived, or busier than they would prefer. Personal problems can be preoccupying and contribute to a physician being ill-tempered or distracted (eg, marital or family problems). Some physicians are simply poor communicators and might not understand the need to adapt their communication style to their patient, instead using medical jargon the patient does not understand. Ideally, physicians should modify their language to suit the patient’s level of education, degree of medical sophistication, and cultural background.
Continue to: A physician's personality traits...
A physician’s personality traits could clash with those of the patient, particularly if the physician is especially rigid or obsessional. Rather than “going with the flow,” the overly rigid physician might become impatient with patients who fail to understand diagnostic assessments or treatment recommendations. Inefficient physicians might not be able to keep up with the daily schedule, which could fuel impatience and perhaps even lead them to think that the patient is taking too much of their valuable time. Some might not know how to convey empathy, for example when giving bad news (“The tests show you have cancer…”). Others fail to make consistent eye contact with patients without understanding its importance to communication, a problem made worse by the use of electronic medical record systems (EMRs).
Systems issues
Systems issues also contribute to suboptimal physician-patient interactions, and some issues can be attributed to administrative problems. Examples of systems issues include:
- when a patient has difficulty making an appointment and is forced to listen to a confusing menu of choices
- a busy clinic that can only offer a patient an appointment 6 months away
- crowded or noisy waiting rooms
- language barriers for patients whose primary langage is not English. Not having access to an interpreter can exacerbate their frustration
- the use of EMRs is a growing threat to positive physician-patient interactions, yet their influence is often ignored. Widely disliked by physicians,10 EMRs are required in all but the smallest independent practice settings. Many busy physicians focus their attention on the computer, giving the patient the impression that the physician is not listening to them. Many patients conclude that they are less important than the process.
The consequences of difficult interactions
Following a bad interaction, dissatisfied patients are more likely to leave the clinic or hospital and ignore medical advice. These patients might then show up in crowded emergency departments, which may lead to poor use of health care resources. For physicians, challenging situations sap their emotional energy, cause demoralization, and interfere with their sense of job fulfillment. In extreme cases, such feelings might lead the physician to dislike and even avoid the patient.
How to manage challenging situations
Taking the following steps can help physicians work through challenging situations with their patients.
Diagnose the problem. First, recognize the difficult situation, analyze it, and identify how the patient, the physician, and the system are contributing to a bad physician-patient interaction. Diagnosing the interactional difficulty should precede the diagnosis and management of the patient’s disease. Physicians should acknowledge their own contribution through their attitude or actions. Finally, determine if there are system issues that are contributing to the problem, or if it is the clinic or inpatient setting itself (eg, noisy inpatient unit).
Continue to: Maintain your cool
Maintain your cool. With any difficult interaction, a physician’s first obligation is to remain calm and professional, while modeling appropriate behavior. If the patient is angry or emotionally intense, talking over them or interrupting them only makes the situation worse. Try to see the interaction from the patient’s perspective. Both parties should work together to find a common ground.
Collaborate, respect boundaries, and empathize. One study of a group of 100 family physicians found that having the following 3 skills were essential to successfully managing situations with difficult patients11,12:
- the ability to collaborate (vs opposition)
- the appropriate use of power (vs misuse of power, or violation of boundaries by either party)
- the ability to empathize, which for most physicians involves understanding and validating the patient’s subjective experiences.
Although a description of the many facets of empathy (cognitive, affective, motivational) is beyond the scope of this article, it is worth pointing out that a patient’s positive perception of their physician’s empathy improves not only patient satisfaction but health outcomes.13 The Box describes a difficult patient whose actions changed through the collaboration and empathy of his treatment team.
Box 1
Mr. L, a 60-year-old veteran, is admitted to an inpatient unit following a suicide attempt that was prompted by eviction from his apartment. Mr. L is physically disabled and has difficulty walking without assistance. His main concern is his homelessness, and he insists that the inpatient team find a suitable “Americans with Disabilities (ADA)-compliant apartment” that he can afford on his $800 monthly income. He implies that he will kill himself if the team fails in that task. He makes it clear that his problems are the team’s problems. He is prescribed an antidepressant, and both his mood and reported suicidal ideations gradually resolve.
The team’s social worker finds an opening at a well-run veterans home, but Mr. L rejects it because he doesn’t want to “give up his independence.” The social worker finds a small apartment in a nearby community that is ADA-compliant, but Mr. L complains that it is small. He asks the resident psychiatrist, “Where will I put all my things?” The next day, after insulting the attending psychiatrist for failing to find an adequate apartment, Mr. L says from under the bedsheet: “How come none of you ever help me?”
Mr. L presents a challenge to the entire team. At times, he is rude, demanding, and entitled. The team recognizes that although he had served in the military with distinction, he is now alone after having divorced many years earlier, and nearly friendless because of his increasing disability. The team surmises that Mr. L lashes out due to frustration and feelings of powerlessness.
Resolving this conflict involves treating Mr. L with respect and listening without judgment. No one ever confronts him or argues with him. The team psychologist meets with him to help him work through his many losses. Closer to discharge, he is enrolled in several post-hospitalization programs to keep him connected with other veterans. At discharge, the hospital arranges for his belongings that had been in storage to be delivered to his new home. He is pleasant and social with his peers, and although he is still concerned about the size of the apartment, he thanks the team members for their care.
Verbalize the difficulty. It is important to openly discuss the problem. For example, “We both have very different views about how your symptoms should be investigated, and that’s causing some difficulty between us. Do you agree?” This approach names the “elephant in the room” and avoids casting blame. It also creates a sense of shared ownership by externalizing the problem from both the patient and physician. Verbalizing the difficulty can help build trust and pave the way to working together toward a common solution.
Consider other explanations for the patient’s behavior. For example, anger directed at a physician could be due to anxiety about an unrelated matter, such as the patient’s recent job loss or impending divorce. Psychiatrists might understand this behavior better as displacement, which is considered a maladaptive defense mechanism. It is important to listen to the patient and offer empathy, which will help the patient feel supported and build a rapport that can help to resolve the encounter.
Continue to: When helping patients...
When helping patients with multiple issues, which is a common scenario, the physician might start by asking, “What would you like to address today?”14 Keep a list of the issues so you do not forget the patient’s concerns, and then ask: “What do you think is going on?” Give patients time to verbalize their concerns. Physicians should:
- validate concerns: “I understand where you’re coming from.”
- offer empathy: “I can see how difficult this has been for you.”
- reframe: “Let me make sure I hear you correctly.”
- refocus: “Let’s agree on what we need to do at this visit.”
Find common ground. When the patient and physician have different ideas on diagnosis or treatment, finding common ground is another way to resolve a difficult encounter. Difficulties arise when there appears to be little common ground, which often results from unrealistic expectations. Patients might be seen as “demanding” or “manipulative”’ if they push for a diagnosis or treatment the doctor is not comfortable with. As soon as there is some overlap and common ground, the difficulty rapidly subsides.
Set clear boundaries and limits. Physicians should set limits on what patient behavior might “cross the line.” A “behavioral contract” (or “treatment contract”) can help by setting explicit expectations. For example, showing up late for appointments or inappropriately seeking drugs of abuse (eg, opioids, benzodiazepines) might be identified as violations of the contract. Once the contract is set, the patient should be asked to restate key components. Clarify any confusion or barriers to compliance and define clear expectations. The patient should be informed of potential consequences of contract violations, including termination.
Staff members involved in the patient’s care should agree with the terms of any behavioral contract, and should receive a copy of it. Patients should have “buy in,” meaning that they have had an opportunity to provide input to the contract and have agreed to its elements. Both the physician and patient should sign the document.
When all else fails
When there is a breakdown in rapport that makes it difficult or impossible to continue offering treatment, consider termination. This could be due to threatening or abusive patient behavior, sexual advances, repeated no-shows, treatment noncompliance that jeopardizes patient safety, refusal to follow the treatment plan, or violating the terms of a behavioral contract. In some settings, it might be the failure to pay bills.
Continue to: If a patient is unable to...
If a patient is unable to follow the contract, the physician should explore possible extenuating circumstances. The physician should seek to remedy the problem and involve other team members if possible (eg, case manager, nurse), advising a patient about behaviors that could lead to termination.
If the problem is irremediable, notify the patient in writing, give them time to find another physician, and facilitate the transfer of care.15 Take steps to prevent the patient from running out of any medications associated with withdrawal or discontinuation syndromes (eg, selective serotonin reuptake inhibitors, benzodiazepines) during the care transition. While there is no requirement regarding the amount of time allowed, at least 30 days is typical.
Bottom Line
Difficult patient interactions are common and unavoidable. Physicians should acknowledge and recognize contributing factors in such encounters—including their own role. When handling such situations, physicians should remain calm and model appropriate behavior. Improving communication, offering empathy, and validating the patient’s concerns can help resolve factors that contribute to poor patient interactions. If efforts to remediate the physician-patient relationship fail, termination may be necessary.
Related Resources
- Koekkoek B, Berno van Meijel CNS, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57(6):795-802.
- Pereira MR, Figueiredo AF. Challenging patient-doctor interactions in psychiatry – difficult patient syndrome. European Psychiatry. 2017;41(supplement):S719. doi. org/10.1016/j.eurpsy.2017.01.1297
1. Dupont J. Ferenczi’s madness. Contemp Psychoanal. 1988;24(2):250-261.
2. Adams J, Murray R. The difficult diagnosis: the general approach to the difficult patient. Emerg Med Clin North Am. 1998;16(4):689-700.
3. Davies M. Managing challenging interactions with patients. BMJ. 2013;347:f4673. doi: https://doi.org/10.1136/bmj.f4673
4. Chou C. Dealing with the “difficult” patient. Wisc Med J. 2004;103:35-38.
5. Koekkoek B, Berno van Meijel CNS, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57(6):795-802.
6. Jackson JL, Kroenke K. Difficult patient encounter in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med 1999;159(10):1069-1075.
7. Groves JE. Taking care of the hateful patient. N Eng J Med. 1978;298:883-887.
8. Hull S, Broquet K. How to manage difficult encounters. Fam Prac Manag. 2007;14(6):30-34.
9. Hardavella G, Aamli-Gaagnat A, Frille A, et al. Top tips to deal with challenging situations: doctor patient interactions. Breathe. 2017;13(2):129-135.
10. Black DW, Balon R. Editorial: electronic medical records (EMRs) and the psychiatrist shortage. Ann Clin Psychiatry. 2018;30(4):257-259.
11. Elder N, Ricer R, Tobias B. How respected family physicians manage difficult patient encounters. J Am Board Fam Med. 2006;19(6):533-541.
12. Campbell RJ. Campbell’s Psychiatric Dictionary. 8th Edition. Oxford University Press; 2004:219-220.
13. Decety J, Fotopoulou A. Why empathy has a beneficial impact on others in medicine: unifying theories. Front Behav Neurosci. 2014;8:457. https://doi.org/10.3389/fnbeh.2014.00457
14. Klugman B. The difficult patient. Accessed May 24, 2021. https://www.umassmed.edu/globalassets/office-of-continuing-medical-education/pdfs/cme-primary-care-days/e2-the-difficult-patient.pdf
15. Mossman D, Farrell HM, Gilday E. ‘Firing’ a patient: may psychiatrists unilaterally terminate care? Current Psychiatry. 2010;9(12):18-29.
1. Dupont J. Ferenczi’s madness. Contemp Psychoanal. 1988;24(2):250-261.
2. Adams J, Murray R. The difficult diagnosis: the general approach to the difficult patient. Emerg Med Clin North Am. 1998;16(4):689-700.
3. Davies M. Managing challenging interactions with patients. BMJ. 2013;347:f4673. doi: https://doi.org/10.1136/bmj.f4673
4. Chou C. Dealing with the “difficult” patient. Wisc Med J. 2004;103:35-38.
5. Koekkoek B, Berno van Meijel CNS, Hutschemaekers G. “Difficult patients” in mental health care: a review. Psychiatr Serv. 2006;57(6):795-802.
6. Jackson JL, Kroenke K. Difficult patient encounter in the ambulatory clinic: clinical predictors and outcomes. Arch Intern Med 1999;159(10):1069-1075.
7. Groves JE. Taking care of the hateful patient. N Eng J Med. 1978;298:883-887.
8. Hull S, Broquet K. How to manage difficult encounters. Fam Prac Manag. 2007;14(6):30-34.
9. Hardavella G, Aamli-Gaagnat A, Frille A, et al. Top tips to deal with challenging situations: doctor patient interactions. Breathe. 2017;13(2):129-135.
10. Black DW, Balon R. Editorial: electronic medical records (EMRs) and the psychiatrist shortage. Ann Clin Psychiatry. 2018;30(4):257-259.
11. Elder N, Ricer R, Tobias B. How respected family physicians manage difficult patient encounters. J Am Board Fam Med. 2006;19(6):533-541.
12. Campbell RJ. Campbell’s Psychiatric Dictionary. 8th Edition. Oxford University Press; 2004:219-220.
13. Decety J, Fotopoulou A. Why empathy has a beneficial impact on others in medicine: unifying theories. Front Behav Neurosci. 2014;8:457. https://doi.org/10.3389/fnbeh.2014.00457
14. Klugman B. The difficult patient. Accessed May 24, 2021. https://www.umassmed.edu/globalassets/office-of-continuing-medical-education/pdfs/cme-primary-care-days/e2-the-difficult-patient.pdf
15. Mossman D, Farrell HM, Gilday E. ‘Firing’ a patient: may psychiatrists unilaterally terminate care? Current Psychiatry. 2010;9(12):18-29.
Minor-attracted persons: A neglected population
Approximately 1 in 5 Americans report childhood sexual abuse.1 While 50% to 65% of child sexual abuse occurs in the absence of pedophilic interests and is thought to be driven by additional factors such as the availability of an appropriate sexual partner,2,3 a substantial portion of childhood sexual abuse is perpetrated by individuals with pedophilia.
However, many individuals with pedophilic interests never have sexual contact with a child or the penal system. This non-offending pedophile group reports a greater prevalence of psychiatric symptoms compared with the general population, but given the intense stigmatization of their preferences, they are largely psychiatrically underrecognized and underserved. This article focuses on the unique psychiatric needs of this neglected population. By understanding and addressing the treatment needs of these patients, psychiatrists and other mental health clinicians can serve a pivotal role in decreasing stigma, promoting wellness, and preventing sexual abuse.
Understanding the terminology
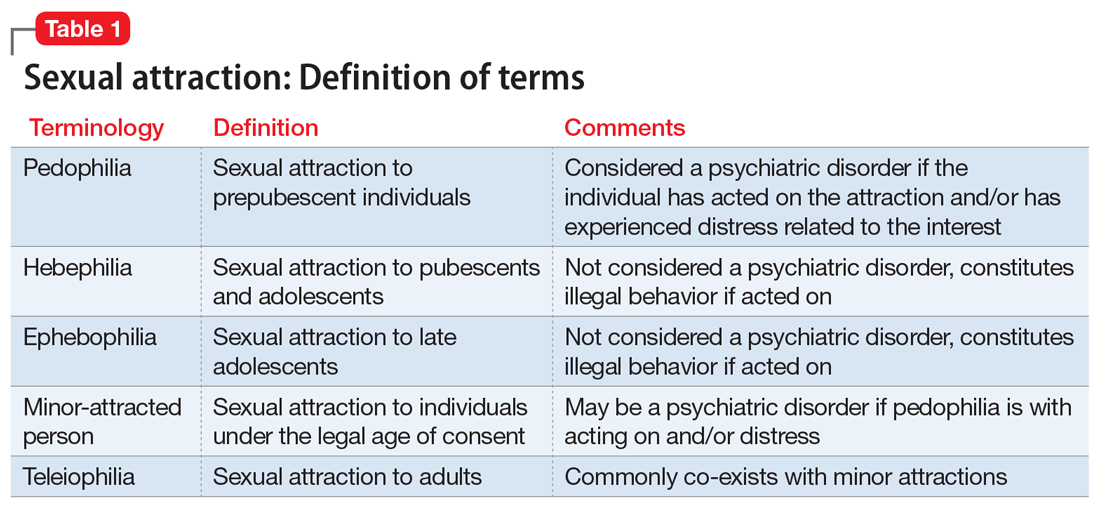
DSM-5 defines paraphilia as “any intense and persistent sexual interest other than sexual interest in genital stimulation or preparatory fondling with phenotypically normal, physiologically mature, consenting human partners.”4 The addition of the word “disorder” to the paraphilias was introduced in DSM-5 to distinguish between paraphilias that are not of clinical concern and paraphilic disorders that cause distress or impairment to the individual, or whereby satisfaction entails personal harm or risk of harm to others. As outlined in DSM-5, pedophilic disorder refers to at least 6 months of recurrent, intense sexually arousing fantasies, sexual urges, or behaviors involving sexual activity with a prepubescent child.4 The individual has either acted on these sexual urges, or the sexual urges or fantasies cause marked distress or interpersonal difficulty. Lastly, the individual must be at least age 16 years and at least 5 years older than the child. Sexual attraction to peri- or postpubescent minors is not considered a psychiatric disorder, but is illegal.
Coined by B4U-ACT (www.b4uact.org), the term minor-attracted person (MAP) refers to individuals with sexual attraction to individuals who are minors or below the legal age of consent. MAP is an umbrella term that includes sexual attraction to prepubescent individuals but also includes sexual attraction to peri- and postpubescent individuals (Table 1). A MAP may or may not meet criteria for pedophilia or pedophilic disorder, based on the age of their sexual interest and whether they have experienced distress or acted on the attraction. Although many individuals with minor attraction identify with the term MAP, not all do. The term has been critiqued for being too inclusive and conflating pedophilia with minor attractions.
It is important to keep in mind that the terms pedophilia and minor attraction are not synonymous with childhood sexual abuser or “child molester” because neither term specifies whether the individual has had sexual contact with a child or legal consequences. The terms offending/non-offending and acting/non-acting are used to specify the presence of sexual contact with a child, and do not convey any clinical information.
Prevalence data
The true prevalence of pedophilia and/or attraction to minors is unknown, and estimates vary considerably. In some studies, 1% to 4% of the general population were thought to have persistent attraction to prepubescent children.5,6 In a community sample of 8,718 German men, 4.1% reported sexual fantasies involving prepubescent children, 3.2% reported sexual offending against prepubescent children, and 0.1% reported a pedophilic sexual preference.5 In a study of 367 adult German men surveyed from the community, 15.5% reported fantasies (9.5% daydream and 6.0% masturbation fantasies) involving prepubescent children.7
Stigmatization of minor-attracted persons
Stigmatization is the process of forming negative evaluations of an individual or groups of people based on limited characteristics.8,9 MAPs are a highly stigmatized group. This stigmatization can be profound, regardless of whether the MAP has had sexual contact with a child. A public survey of nearly 1,000 individuals showed that 39% believed that non-acting MAPs should be incarcerated, and 14% believed that they would be “better off dead.”10 Societal misconceptions of minor attraction are pervasive and include10:
- MAP sexual orientation is a choice
- MAPs cannot resist their sexual urges
- all MAPs have offended, or inevitably will
- MAPs will not respond to therapy
- MAPs are fundamentally predatory and immoral.
Continue to: In addition to...
In addition to societal stigma, internalized stigma among MAPs has been documented. Lievesley et al9 found that MAPs who engaged in suppression of unwanted thought strategies had higher levels of shame and guilt, low levels of hope, and a propensity to actively avoid children. Similarly, Grady et al11 surveyed 293 MAPs and found prominent themes of viewing themselves as “bad.”
Psychiatric presentations include suicidal ideation
Many MAPs, including non-acting MAPs, internalize this societal stigma, which contributes to a significant mental health burden.12 A survey of 342 MAP actors and 223 MAP non-actors revealed that one-third of both groups reported chronic suicidal ideation.13 In addition, online surveys conducted by B4U-ACT and Virtuous Pedophiles (www.virped.org)—both internet-based organizations dedicated to supporting non-acting MAPs—have provided similar results. In a 2011 B4U-ACT survey, nearly one-half of participants reported suicidal ideation due to their minor attraction, 32% had planned suicide attempts, and 13% had non-fatal suicide attempts. Notably, the age group with the most prevalent suicidal ideation was age 14 to 16 years,14 which makes minor attraction a prominent risk factor for suicidal ideation among patients seen by child psychiatrists.
A 2019 thematic analysis of 5,210 posts on the Virtuous Pedophiles website showed high rates of addiction, anxiety, depression, self-harm, self-hatred, and suicidal thoughts and behaviors among MAPs.2 The majority of posts regarding substance use described such use as a means of dissociation. One post read, “…There are days I cannot bear to be sober … I … drink myself into a coma.” Anxiety themes regarding the ability to have a meaningful relationship with an age-appropriate partner and concerns about being “outed” followed by public persecution were prominent. Posts regarding self-injurious and suicidal behavior were common: “I want to kill myself so badly … I have to mutilate myself as punishment for my attractions. I wish myself dead. I don’t want to be attracted to children; I despise myself for fantasizing about them.”2
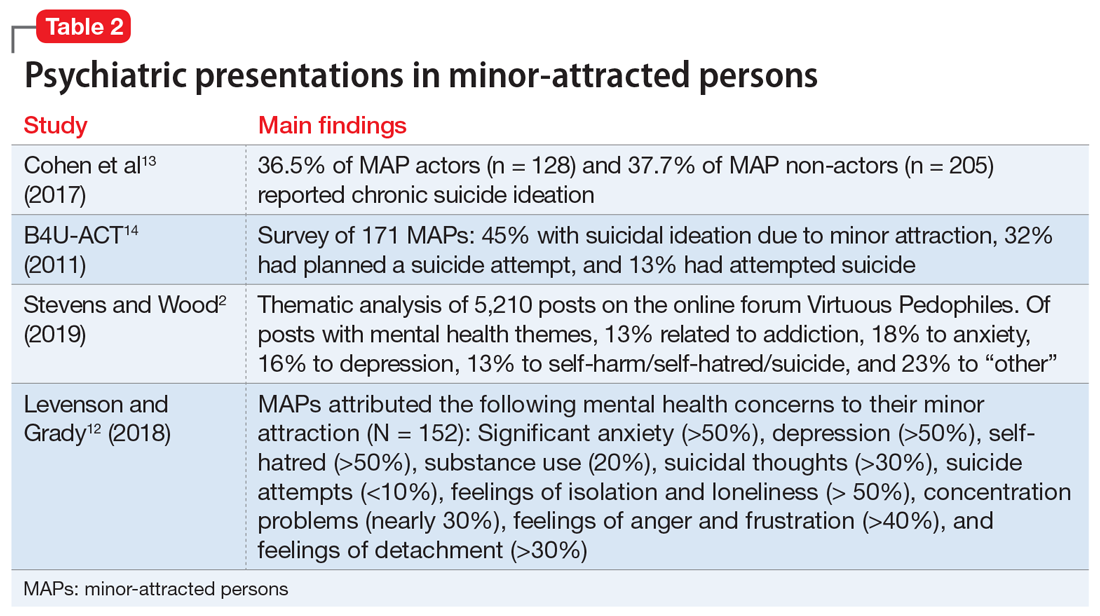
A study that analyzed a survey of 152 MAPs sampled from websites such as Virtuous Pedophiles and others showed >50% of respondents had strong feelings of isolation and loneliness, nearly 30% had extreme difficulty with concentration, >40% had significant anger and frustration, and >30% were struggling with feelings of detachment.12 Notably, the respondents attributed these difficulties to their minor attraction.12 Table 22,12-14 summarizes the findings of studies evaluating psychiatric symptoms in MAPs.
Consider OCD, hypersexuality
It is important to be aware that an attraction to minors may be a symptom of obsessive-compulsive disorder (OCD) or hypersexuality.15 Pedophilia-themed OCD (POCD) is a manifestation of OCD in which the individual experiences shame, fear, and excessive worry related to sexual attraction to children. Typically, individuals with POCD experience sexual thoughts of children as ego-dystonic, whereas MAPs experience such thoughts as ego-syntonic and arousing.15 However, much like individuals with POCD, MAPs also experience sexual thoughts of minors as distressing. Initial presentations of POCD may be confused with MAPs or pedophilia because of the overlap of symptoms such as anxiety, shame, distress, or suicidal ideation related to the idea of child sexual interests. The distinguishing feature of POCD is the absence of sexual arousal to children.
Continue to: Clinical presentations of...
Clinical presentations of hypersexuality may include sexual arousal to children. These individuals are distinguished from MAPs or those with pedophilia because they lack a preferred or sustained sexual interest in this group. On the contrary, individuals with hypersexuality present with a diversity of sexual interests explained by their high libido. Some individuals, however, may meet criteria for both hypersexuality and pedophilia. These individuals may pose a higher risk of sexual offending due to the presence of a heightened sexual drive and pedophilic interests, and thereby may require more intensive treatment, such as biologic treatment.
Focus on individualized treatment needs
Understanding the treatment needs of MAPs means understanding the goals of the individual MAP. Improving self-esteem, decreasing social isolation, and managing stigma are common treatment goals among MAPs.16 Levenson and Grady12 found that most MAPs identified treatment goals unrelated to sexual interests, such as addressing depression, anxiety, and low self-esteem. A smaller percentage identified sexual frustration related to the absence of healthy sexual outlets. Because many MAPs identify common psychiatric treatment needs, most clinicians should be equipped to foster a nonjudgmental therapeutic alliance to treat these patients. Effective treatment outcomes occur when comorbid psychiatric illnesses are treated as well as addressing the internal stigmatization that many MAPs experience.
Specialized treatment may be indicated for individuals who request treatment specific to sexual interests. This may include safety planning, including developing support systems to decrease the risk around children. For MAPs who have been unsuccessful at managing their sexual interests, pharmacotherapy may be an option. To date, research on pharmacotherapy for pedophilia is largely limited to studies of sexual offenders. Testosterone-lowering medications such as gonadotropin-releasing hormone (GnRH) analogue treatment constitutes the most effective treatment for patients who are not helped by conventional psychotherapeutic interventions.17 Other psychotropic medications, such as selective serotonin reuptake inhibitors or naltrexone, have not demonstrated efficacy outside of case reports.17
Addressing barriers to care
MAPs have a strong desire but significant hesitation when seeking mental health treatment.13,18 Nearly half (47%) of the 154 MAP respondents in the Levenson and Grady12 survey had never told anyone about their minor attraction. MAPs are understandably hesitant to disclose these thoughts and feelings due to fear of public exposure and intense stigmatization, as well as potential punitive and legal consequences.18,19 One post from the 2011 B4U-ACT online survey read, “Parents will disown you; teachers will report you; friends will abandon you … people in my situation can’t discuss this without serious risk of persecution and/or harassment.”14 In this survey, 78% of respondents feared a negative reaction by the professional, 78% feared being reported to law enforcement, and 68% feared being reported to family, an employer, or the community.14 This hesitancy due to fear of being exposed even extended to accessing self-help books, informational websites, and online forums, even though these sources are strongly desired and perceived as helpful.20
Even if MAPs were to decide to seek help, the lack of specific training and experience among psychiatrists make them unlikely to find it in the medical field.21 Furthermore, MAPs who desire help often worry it will be inadequate and they will be misunderstood by their clinicians.22 According to the Levenson and Grady survey,12 when asked what they would like most from therapy, most MAPs said they would want the treatment to focus on depression, anxiety, and low self-esteem rather than on sexual interest. In the B4U-ACT survey,14 many respondents identified the need for treatment of issues surrounding their sexual attraction, such as assistance in learning how to live in society with the attraction, dealing with society’s negative response to the attraction, and improving their self-concept in the presence of the extreme shame associated with the attraction. However, many MAPs find that clinicians tend to focus on protecting society from them, rather than on offering general psychiatric treatment or treatment focused on improving their well-being.18 This inability to locate appropriate services is known to exacerbate depression, suicidality, fear, anxiety, hopelessness, and substance abuse among MAPs.18 There is also evidence that individuals with minor attraction who are in a negative affective state are more likely to act on their attractions.23
Continue to: An ethical responsibility
An ethical responsibility. Physicians have a long-recognized responsibility to participate in activities to protect and promote the health of the public. The American Medical Association Code of Medical Ethics includes “justice,” or treating patients fairly and equitably.24 This includes patients who have pedophilic interests. Unfortunately, the stigma associated with individuals who have sexual attraction to children is pervasive in our society, including among medical professionals. The first consideration in treating MAPs is to overcome the stigmatization within our field, to remember that as physicians we took an oath to provide treatment fairly, equitably, and in accordance with the patient’s rights and entitlement.24 This includes listening to MAPs’ treatment needs. Not all MAPs want or need treatment related to their sexual interest. As is the case with all patients, listening to the individual’s chief complaint is paramount. If a patient’s treatment needs are beyond the clinician’s expertise, the patient should be referred to another clinician.
Mandated reporting. MAPs may not engage in psychiatric treatment for fear of being reported to authorities as a result of mandated reporting laws. Although the circumstances under which mandated reporting may be required vary by jurisdiction, they generally include situations in which the health care professional has reasonable cause to believe that a child is suffering from abuse or neglect. A patient’s report of sexual urges and fantasies to have sexual contact with minors is not sufficient for mandated reporting. While professionals vary in their interpretation of mandated reporting laws, sexual thoughts alone do not meet the threshold for mandated reporting. Mandated reporting duties should be discussed when first meeting a patient with minor attraction. For clinicians who are uneasy about such distinctions, either supervision or not working with such patients is the solution.
The importance of providing competent and individualized treatment to MAPs is 2-fold. First, individuals who are experiencing psychiatric symptoms deserve to have access treatment. Second, providing psychiatric treatment to individuals with minor attractions is a step toward preventing child sexual abuse. The Prevention Project Dunkelfeld in Germany used public service announcements to advertise confidential treatment for individuals who had sexual interest in children.25 Many of the participants were interested in mental health treatment unrelated to their sexual interests. Such projects may help us understand the best way to meet the treatment needs of minor-attracted individuals, as well as reduce child sexual abuse. As psychiatrists, we can stop making the problem worse by withholding psychiatric treatment from an important population.
Resources for MAPs and clinicians
Currently, resources for MAPs and clinicians are limited. MAPs can communicate and find support among other MAPs in online forums (see Related Resources). These websites provide online peer support groups and guides for seeking therapy. Information for mental health professionals, including available literature, research projects, clinicians who provide specialized treatment, and a monthly “dialog on therapy” can be found on the B4U-ACT and the Global Prevention Project websites. However, beyond the DSM-5 definitions, psychiatric education and training on this topic is almost entirely lacking.
In light of the information discussed in this article, several important issues remain, including how psychiatrists can best reach this population, and how they can work toward decreasing stigma so they can provide meaningful care. The solutions start with education. Educating psychiatrists about this important population can decrease stigma and facilitate appropriate, compassionate care to these patients, with the result of improving the mental health of people with minor attraction and decreasing the incidence of child sexual abuse.
Continue to: Bottom Line
Bottom Line
Minor-attracted persons report a high prevalence of general psychiatric symptoms that often go untreated due to a lack of willing clinicians with appropriate expertise. Providing psychiatric treatment to these patients can improve their mental health and possibly decrease the incidence of individuals who act on their attractions.
Related Resources
- B4U-ACT. www.b4uact.org • The Global Prevention Project. http://theglobalprevention project.org
- Virtuous Pedophiles. www.virped.org
Drug Brand Names
Naltrexone • ReVia
1. Briere J, Elliott D. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27(10):1205-1222. doi: 10.1016/j.chiabu.2003.09.008
2. Stevens E, Wood J. “I despise myself for thinking about them.” A thematic analysis of the mental health implications and employed coping mechanisms of self-reported non-offending minor attracted persons. J Child Sex Abus. 2019;28(8):968-989. doi: 10.1080/10538712.2019.1657539
3. Sorrentino R. Normal human sexuality and sexual and gender identity disorders: paraphilias. In: Sadock BJ, Sadock VA, Ruis P, eds. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9th ed. Wolters Kluwer; 2012:2093-2094.
4. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:685-705.
5. Dombert B, Schmidt AF, Banse R, et al. How common is men’s self-reported sexual interest in prepubescent children? J Sex Res. 2016;53(2):214-23. doi: 10.1080/00224499.2015.1020108
6. Seto MC. Pedophilia and sexual offending against children: theory, assessment, and intervention. 2nd ed. American Psychological Association; 2018.
7. Ahlers CJ, Schaefer GA, Mundt IA, et al. How unusual are the contents of paraphilias? Paraphilia-associated sexual arousal patterns in a community-based sample of men. J Sex Med. 2011;8(5):1362-1370. doi: 10.1111/j.1743-6109.2009.01597.x
8. Corrigan PW, Roe D, Tsang HWH. Challenging the public stigma of mental illness: lessons for therapists and advocates. Wiley Blackwell; 2011:55-114.
9. Lievesley R, Harper CA, Elliott H. The internalization of social stigma among minor-attracted persons: implications for treatment. Arch Sex Behav. 2020;49(4):1291-1304. doi: 10.1007/s10508-019-01569-x
10. Jahnke S, Imhoff R, Hoyer J. Stigmatization of people with pedophilia: two comparative surveys. Arch Sex Behav. 2015;44(1):21-34. doi: 10.1007/s10508-014-0312-4
11. Grady MD, Levenson JS, Mesias G, et al. “‘I can’t talk about that”: Stigma and fear as barriers to preventative services for minor-attracted persons. Stigma and Health. 2019;4(4):400-410. doi: 10.1037/sah0000154
12. Levenson JS, Grady MD. Preventing sexual abuse: perspectives of minor-attracted persons about seeking help. Sex Abuse. 2019;31(8):991-1013. doi: 10.1177/1079063218797713
13. Cohen L, Ndukwe N, Yaseen Z, et al. Comparison of self-identified minor-attracted persons who have and have not successfully refrained from sexual activity with children. J Sex Marital Ther. 2018;44(3):217-230. doi: 10.1080/0092623X.2017.1377129
14. B4U-ACT. Awareness of sexuality in youth, suicidality, and seeking care. 2011. Accessed June 4, 2021. www.b4uact.org/research/survey-results/spring-2011-survey
15. Bruce SL, Ching THW, Williams MT. Pedophilia-themed obsessive-compulsive disorder: assessment, differential diagnosis, and treatment with exposure and response prevention. Arch Sex Behav. 2018;47(2):389-402. doi: 10.1007/s10508-017-1031-4
16. Levenson JS, Grady MD, Morin JW. Beyond the “ick factor”: counseling non-offending persons with pedophilia. Clinical Social Work Journal. 2020;48:380-388. doi: 10.007/s10615-019-00712-4
1 7. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490. doi: 10.1080/15622975.2020.1744723
18. B4U-ACT. Principles and perspectives of practice. 2017. Accessed June 4, 2021. www.b4uact.org/about-us/principles-and-perspectives-of-practice/
19. McPhail IV, Stephens S, Heasman A. Legal and ethical issues in treating clients with pedohebephilic interests. Canadian Psychology/Psychologie Canadienne. 2018;59(4):369-381. doi:10.1037/cap0000157
20. Levenson JS, Willis GM, Vicencio CP. Obstacles to help-seeking for sexual offenders: implications for prevention of sexual abuse. J Child Sex Abus. 2017;26(2):99-120. doi: 10.1080/10538712.2016.1276116
21. Sorrentino R. DSM-5 and paraphilias: what psychiatrists need to know. Psychiatric Times. November 28, 2016. Accessed June 4, 2021. https://www.psychiatrictimes.com/view/dsm-5-and-paraphilias-what-psychiatrists-need-know
22. Cantor JM, McPhail IV. Non-offending pedophiles. Current Sexual Health Reports. 2016;8:121-128. doi:10.1007/s11930-016-0076-z
23. Ward T, Louden K, Hudson SM, et al. A descriptive model of the offense chain for child molesters. Journal of Interpersonal Violence. 1995;10(4):452-472. doi:10.1177/088626095010004005
24. American Medical Association. AMA Code of Medical Ethics. 2016. Accessed June 4, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/principles-of-medical-ethics.pdf
25. Beier KM, Grundmann D, Kuhle LF, et al. The German Dunkelfeld project: a pilot study to prevent child sexual abuse and the use of child abusive images. J Sex Med. 2015;12(2):529-42. doi: 10.1111/jsm.12785
Approximately 1 in 5 Americans report childhood sexual abuse.1 While 50% to 65% of child sexual abuse occurs in the absence of pedophilic interests and is thought to be driven by additional factors such as the availability of an appropriate sexual partner,2,3 a substantial portion of childhood sexual abuse is perpetrated by individuals with pedophilia.
However, many individuals with pedophilic interests never have sexual contact with a child or the penal system. This non-offending pedophile group reports a greater prevalence of psychiatric symptoms compared with the general population, but given the intense stigmatization of their preferences, they are largely psychiatrically underrecognized and underserved. This article focuses on the unique psychiatric needs of this neglected population. By understanding and addressing the treatment needs of these patients, psychiatrists and other mental health clinicians can serve a pivotal role in decreasing stigma, promoting wellness, and preventing sexual abuse.
Understanding the terminology
DSM-5 defines paraphilia as “any intense and persistent sexual interest other than sexual interest in genital stimulation or preparatory fondling with phenotypically normal, physiologically mature, consenting human partners.”4 The addition of the word “disorder” to the paraphilias was introduced in DSM-5 to distinguish between paraphilias that are not of clinical concern and paraphilic disorders that cause distress or impairment to the individual, or whereby satisfaction entails personal harm or risk of harm to others. As outlined in DSM-5, pedophilic disorder refers to at least 6 months of recurrent, intense sexually arousing fantasies, sexual urges, or behaviors involving sexual activity with a prepubescent child.4 The individual has either acted on these sexual urges, or the sexual urges or fantasies cause marked distress or interpersonal difficulty. Lastly, the individual must be at least age 16 years and at least 5 years older than the child. Sexual attraction to peri- or postpubescent minors is not considered a psychiatric disorder, but is illegal.
Coined by B4U-ACT (www.b4uact.org), the term minor-attracted person (MAP) refers to individuals with sexual attraction to individuals who are minors or below the legal age of consent. MAP is an umbrella term that includes sexual attraction to prepubescent individuals but also includes sexual attraction to peri- and postpubescent individuals (Table 1). A MAP may or may not meet criteria for pedophilia or pedophilic disorder, based on the age of their sexual interest and whether they have experienced distress or acted on the attraction. Although many individuals with minor attraction identify with the term MAP, not all do. The term has been critiqued for being too inclusive and conflating pedophilia with minor attractions.
It is important to keep in mind that the terms pedophilia and minor attraction are not synonymous with childhood sexual abuser or “child molester” because neither term specifies whether the individual has had sexual contact with a child or legal consequences. The terms offending/non-offending and acting/non-acting are used to specify the presence of sexual contact with a child, and do not convey any clinical information.
Prevalence data
The true prevalence of pedophilia and/or attraction to minors is unknown, and estimates vary considerably. In some studies, 1% to 4% of the general population were thought to have persistent attraction to prepubescent children.5,6 In a community sample of 8,718 German men, 4.1% reported sexual fantasies involving prepubescent children, 3.2% reported sexual offending against prepubescent children, and 0.1% reported a pedophilic sexual preference.5 In a study of 367 adult German men surveyed from the community, 15.5% reported fantasies (9.5% daydream and 6.0% masturbation fantasies) involving prepubescent children.7
Stigmatization of minor-attracted persons
Stigmatization is the process of forming negative evaluations of an individual or groups of people based on limited characteristics.8,9 MAPs are a highly stigmatized group. This stigmatization can be profound, regardless of whether the MAP has had sexual contact with a child. A public survey of nearly 1,000 individuals showed that 39% believed that non-acting MAPs should be incarcerated, and 14% believed that they would be “better off dead.”10 Societal misconceptions of minor attraction are pervasive and include10:
- MAP sexual orientation is a choice
- MAPs cannot resist their sexual urges
- all MAPs have offended, or inevitably will
- MAPs will not respond to therapy
- MAPs are fundamentally predatory and immoral.
Continue to: In addition to...
In addition to societal stigma, internalized stigma among MAPs has been documented. Lievesley et al9 found that MAPs who engaged in suppression of unwanted thought strategies had higher levels of shame and guilt, low levels of hope, and a propensity to actively avoid children. Similarly, Grady et al11 surveyed 293 MAPs and found prominent themes of viewing themselves as “bad.”
Psychiatric presentations include suicidal ideation
Many MAPs, including non-acting MAPs, internalize this societal stigma, which contributes to a significant mental health burden.12 A survey of 342 MAP actors and 223 MAP non-actors revealed that one-third of both groups reported chronic suicidal ideation.13 In addition, online surveys conducted by B4U-ACT and Virtuous Pedophiles (www.virped.org)—both internet-based organizations dedicated to supporting non-acting MAPs—have provided similar results. In a 2011 B4U-ACT survey, nearly one-half of participants reported suicidal ideation due to their minor attraction, 32% had planned suicide attempts, and 13% had non-fatal suicide attempts. Notably, the age group with the most prevalent suicidal ideation was age 14 to 16 years,14 which makes minor attraction a prominent risk factor for suicidal ideation among patients seen by child psychiatrists.
A 2019 thematic analysis of 5,210 posts on the Virtuous Pedophiles website showed high rates of addiction, anxiety, depression, self-harm, self-hatred, and suicidal thoughts and behaviors among MAPs.2 The majority of posts regarding substance use described such use as a means of dissociation. One post read, “…There are days I cannot bear to be sober … I … drink myself into a coma.” Anxiety themes regarding the ability to have a meaningful relationship with an age-appropriate partner and concerns about being “outed” followed by public persecution were prominent. Posts regarding self-injurious and suicidal behavior were common: “I want to kill myself so badly … I have to mutilate myself as punishment for my attractions. I wish myself dead. I don’t want to be attracted to children; I despise myself for fantasizing about them.”2
A study that analyzed a survey of 152 MAPs sampled from websites such as Virtuous Pedophiles and others showed >50% of respondents had strong feelings of isolation and loneliness, nearly 30% had extreme difficulty with concentration, >40% had significant anger and frustration, and >30% were struggling with feelings of detachment.12 Notably, the respondents attributed these difficulties to their minor attraction.12 Table 22,12-14 summarizes the findings of studies evaluating psychiatric symptoms in MAPs.
Consider OCD, hypersexuality
It is important to be aware that an attraction to minors may be a symptom of obsessive-compulsive disorder (OCD) or hypersexuality.15 Pedophilia-themed OCD (POCD) is a manifestation of OCD in which the individual experiences shame, fear, and excessive worry related to sexual attraction to children. Typically, individuals with POCD experience sexual thoughts of children as ego-dystonic, whereas MAPs experience such thoughts as ego-syntonic and arousing.15 However, much like individuals with POCD, MAPs also experience sexual thoughts of minors as distressing. Initial presentations of POCD may be confused with MAPs or pedophilia because of the overlap of symptoms such as anxiety, shame, distress, or suicidal ideation related to the idea of child sexual interests. The distinguishing feature of POCD is the absence of sexual arousal to children.
Continue to: Clinical presentations of...
Clinical presentations of hypersexuality may include sexual arousal to children. These individuals are distinguished from MAPs or those with pedophilia because they lack a preferred or sustained sexual interest in this group. On the contrary, individuals with hypersexuality present with a diversity of sexual interests explained by their high libido. Some individuals, however, may meet criteria for both hypersexuality and pedophilia. These individuals may pose a higher risk of sexual offending due to the presence of a heightened sexual drive and pedophilic interests, and thereby may require more intensive treatment, such as biologic treatment.
Focus on individualized treatment needs
Understanding the treatment needs of MAPs means understanding the goals of the individual MAP. Improving self-esteem, decreasing social isolation, and managing stigma are common treatment goals among MAPs.16 Levenson and Grady12 found that most MAPs identified treatment goals unrelated to sexual interests, such as addressing depression, anxiety, and low self-esteem. A smaller percentage identified sexual frustration related to the absence of healthy sexual outlets. Because many MAPs identify common psychiatric treatment needs, most clinicians should be equipped to foster a nonjudgmental therapeutic alliance to treat these patients. Effective treatment outcomes occur when comorbid psychiatric illnesses are treated as well as addressing the internal stigmatization that many MAPs experience.
Specialized treatment may be indicated for individuals who request treatment specific to sexual interests. This may include safety planning, including developing support systems to decrease the risk around children. For MAPs who have been unsuccessful at managing their sexual interests, pharmacotherapy may be an option. To date, research on pharmacotherapy for pedophilia is largely limited to studies of sexual offenders. Testosterone-lowering medications such as gonadotropin-releasing hormone (GnRH) analogue treatment constitutes the most effective treatment for patients who are not helped by conventional psychotherapeutic interventions.17 Other psychotropic medications, such as selective serotonin reuptake inhibitors or naltrexone, have not demonstrated efficacy outside of case reports.17
Addressing barriers to care
MAPs have a strong desire but significant hesitation when seeking mental health treatment.13,18 Nearly half (47%) of the 154 MAP respondents in the Levenson and Grady12 survey had never told anyone about their minor attraction. MAPs are understandably hesitant to disclose these thoughts and feelings due to fear of public exposure and intense stigmatization, as well as potential punitive and legal consequences.18,19 One post from the 2011 B4U-ACT online survey read, “Parents will disown you; teachers will report you; friends will abandon you … people in my situation can’t discuss this without serious risk of persecution and/or harassment.”14 In this survey, 78% of respondents feared a negative reaction by the professional, 78% feared being reported to law enforcement, and 68% feared being reported to family, an employer, or the community.14 This hesitancy due to fear of being exposed even extended to accessing self-help books, informational websites, and online forums, even though these sources are strongly desired and perceived as helpful.20
Even if MAPs were to decide to seek help, the lack of specific training and experience among psychiatrists make them unlikely to find it in the medical field.21 Furthermore, MAPs who desire help often worry it will be inadequate and they will be misunderstood by their clinicians.22 According to the Levenson and Grady survey,12 when asked what they would like most from therapy, most MAPs said they would want the treatment to focus on depression, anxiety, and low self-esteem rather than on sexual interest. In the B4U-ACT survey,14 many respondents identified the need for treatment of issues surrounding their sexual attraction, such as assistance in learning how to live in society with the attraction, dealing with society’s negative response to the attraction, and improving their self-concept in the presence of the extreme shame associated with the attraction. However, many MAPs find that clinicians tend to focus on protecting society from them, rather than on offering general psychiatric treatment or treatment focused on improving their well-being.18 This inability to locate appropriate services is known to exacerbate depression, suicidality, fear, anxiety, hopelessness, and substance abuse among MAPs.18 There is also evidence that individuals with minor attraction who are in a negative affective state are more likely to act on their attractions.23
Continue to: An ethical responsibility
An ethical responsibility. Physicians have a long-recognized responsibility to participate in activities to protect and promote the health of the public. The American Medical Association Code of Medical Ethics includes “justice,” or treating patients fairly and equitably.24 This includes patients who have pedophilic interests. Unfortunately, the stigma associated with individuals who have sexual attraction to children is pervasive in our society, including among medical professionals. The first consideration in treating MAPs is to overcome the stigmatization within our field, to remember that as physicians we took an oath to provide treatment fairly, equitably, and in accordance with the patient’s rights and entitlement.24 This includes listening to MAPs’ treatment needs. Not all MAPs want or need treatment related to their sexual interest. As is the case with all patients, listening to the individual’s chief complaint is paramount. If a patient’s treatment needs are beyond the clinician’s expertise, the patient should be referred to another clinician.
Mandated reporting. MAPs may not engage in psychiatric treatment for fear of being reported to authorities as a result of mandated reporting laws. Although the circumstances under which mandated reporting may be required vary by jurisdiction, they generally include situations in which the health care professional has reasonable cause to believe that a child is suffering from abuse or neglect. A patient’s report of sexual urges and fantasies to have sexual contact with minors is not sufficient for mandated reporting. While professionals vary in their interpretation of mandated reporting laws, sexual thoughts alone do not meet the threshold for mandated reporting. Mandated reporting duties should be discussed when first meeting a patient with minor attraction. For clinicians who are uneasy about such distinctions, either supervision or not working with such patients is the solution.
The importance of providing competent and individualized treatment to MAPs is 2-fold. First, individuals who are experiencing psychiatric symptoms deserve to have access treatment. Second, providing psychiatric treatment to individuals with minor attractions is a step toward preventing child sexual abuse. The Prevention Project Dunkelfeld in Germany used public service announcements to advertise confidential treatment for individuals who had sexual interest in children.25 Many of the participants were interested in mental health treatment unrelated to their sexual interests. Such projects may help us understand the best way to meet the treatment needs of minor-attracted individuals, as well as reduce child sexual abuse. As psychiatrists, we can stop making the problem worse by withholding psychiatric treatment from an important population.
Resources for MAPs and clinicians
Currently, resources for MAPs and clinicians are limited. MAPs can communicate and find support among other MAPs in online forums (see Related Resources). These websites provide online peer support groups and guides for seeking therapy. Information for mental health professionals, including available literature, research projects, clinicians who provide specialized treatment, and a monthly “dialog on therapy” can be found on the B4U-ACT and the Global Prevention Project websites. However, beyond the DSM-5 definitions, psychiatric education and training on this topic is almost entirely lacking.
In light of the information discussed in this article, several important issues remain, including how psychiatrists can best reach this population, and how they can work toward decreasing stigma so they can provide meaningful care. The solutions start with education. Educating psychiatrists about this important population can decrease stigma and facilitate appropriate, compassionate care to these patients, with the result of improving the mental health of people with minor attraction and decreasing the incidence of child sexual abuse.
Continue to: Bottom Line
Bottom Line
Minor-attracted persons report a high prevalence of general psychiatric symptoms that often go untreated due to a lack of willing clinicians with appropriate expertise. Providing psychiatric treatment to these patients can improve their mental health and possibly decrease the incidence of individuals who act on their attractions.
Related Resources
- B4U-ACT. www.b4uact.org • The Global Prevention Project. http://theglobalprevention project.org
- Virtuous Pedophiles. www.virped.org
Drug Brand Names
Naltrexone • ReVia
Approximately 1 in 5 Americans report childhood sexual abuse.1 While 50% to 65% of child sexual abuse occurs in the absence of pedophilic interests and is thought to be driven by additional factors such as the availability of an appropriate sexual partner,2,3 a substantial portion of childhood sexual abuse is perpetrated by individuals with pedophilia.
However, many individuals with pedophilic interests never have sexual contact with a child or the penal system. This non-offending pedophile group reports a greater prevalence of psychiatric symptoms compared with the general population, but given the intense stigmatization of their preferences, they are largely psychiatrically underrecognized and underserved. This article focuses on the unique psychiatric needs of this neglected population. By understanding and addressing the treatment needs of these patients, psychiatrists and other mental health clinicians can serve a pivotal role in decreasing stigma, promoting wellness, and preventing sexual abuse.
Understanding the terminology
DSM-5 defines paraphilia as “any intense and persistent sexual interest other than sexual interest in genital stimulation or preparatory fondling with phenotypically normal, physiologically mature, consenting human partners.”4 The addition of the word “disorder” to the paraphilias was introduced in DSM-5 to distinguish between paraphilias that are not of clinical concern and paraphilic disorders that cause distress or impairment to the individual, or whereby satisfaction entails personal harm or risk of harm to others. As outlined in DSM-5, pedophilic disorder refers to at least 6 months of recurrent, intense sexually arousing fantasies, sexual urges, or behaviors involving sexual activity with a prepubescent child.4 The individual has either acted on these sexual urges, or the sexual urges or fantasies cause marked distress or interpersonal difficulty. Lastly, the individual must be at least age 16 years and at least 5 years older than the child. Sexual attraction to peri- or postpubescent minors is not considered a psychiatric disorder, but is illegal.
Coined by B4U-ACT (www.b4uact.org), the term minor-attracted person (MAP) refers to individuals with sexual attraction to individuals who are minors or below the legal age of consent. MAP is an umbrella term that includes sexual attraction to prepubescent individuals but also includes sexual attraction to peri- and postpubescent individuals (Table 1). A MAP may or may not meet criteria for pedophilia or pedophilic disorder, based on the age of their sexual interest and whether they have experienced distress or acted on the attraction. Although many individuals with minor attraction identify with the term MAP, not all do. The term has been critiqued for being too inclusive and conflating pedophilia with minor attractions.
It is important to keep in mind that the terms pedophilia and minor attraction are not synonymous with childhood sexual abuser or “child molester” because neither term specifies whether the individual has had sexual contact with a child or legal consequences. The terms offending/non-offending and acting/non-acting are used to specify the presence of sexual contact with a child, and do not convey any clinical information.
Prevalence data
The true prevalence of pedophilia and/or attraction to minors is unknown, and estimates vary considerably. In some studies, 1% to 4% of the general population were thought to have persistent attraction to prepubescent children.5,6 In a community sample of 8,718 German men, 4.1% reported sexual fantasies involving prepubescent children, 3.2% reported sexual offending against prepubescent children, and 0.1% reported a pedophilic sexual preference.5 In a study of 367 adult German men surveyed from the community, 15.5% reported fantasies (9.5% daydream and 6.0% masturbation fantasies) involving prepubescent children.7
Stigmatization of minor-attracted persons
Stigmatization is the process of forming negative evaluations of an individual or groups of people based on limited characteristics.8,9 MAPs are a highly stigmatized group. This stigmatization can be profound, regardless of whether the MAP has had sexual contact with a child. A public survey of nearly 1,000 individuals showed that 39% believed that non-acting MAPs should be incarcerated, and 14% believed that they would be “better off dead.”10 Societal misconceptions of minor attraction are pervasive and include10:
- MAP sexual orientation is a choice
- MAPs cannot resist their sexual urges
- all MAPs have offended, or inevitably will
- MAPs will not respond to therapy
- MAPs are fundamentally predatory and immoral.
Continue to: In addition to...
In addition to societal stigma, internalized stigma among MAPs has been documented. Lievesley et al9 found that MAPs who engaged in suppression of unwanted thought strategies had higher levels of shame and guilt, low levels of hope, and a propensity to actively avoid children. Similarly, Grady et al11 surveyed 293 MAPs and found prominent themes of viewing themselves as “bad.”
Psychiatric presentations include suicidal ideation
Many MAPs, including non-acting MAPs, internalize this societal stigma, which contributes to a significant mental health burden.12 A survey of 342 MAP actors and 223 MAP non-actors revealed that one-third of both groups reported chronic suicidal ideation.13 In addition, online surveys conducted by B4U-ACT and Virtuous Pedophiles (www.virped.org)—both internet-based organizations dedicated to supporting non-acting MAPs—have provided similar results. In a 2011 B4U-ACT survey, nearly one-half of participants reported suicidal ideation due to their minor attraction, 32% had planned suicide attempts, and 13% had non-fatal suicide attempts. Notably, the age group with the most prevalent suicidal ideation was age 14 to 16 years,14 which makes minor attraction a prominent risk factor for suicidal ideation among patients seen by child psychiatrists.
A 2019 thematic analysis of 5,210 posts on the Virtuous Pedophiles website showed high rates of addiction, anxiety, depression, self-harm, self-hatred, and suicidal thoughts and behaviors among MAPs.2 The majority of posts regarding substance use described such use as a means of dissociation. One post read, “…There are days I cannot bear to be sober … I … drink myself into a coma.” Anxiety themes regarding the ability to have a meaningful relationship with an age-appropriate partner and concerns about being “outed” followed by public persecution were prominent. Posts regarding self-injurious and suicidal behavior were common: “I want to kill myself so badly … I have to mutilate myself as punishment for my attractions. I wish myself dead. I don’t want to be attracted to children; I despise myself for fantasizing about them.”2
A study that analyzed a survey of 152 MAPs sampled from websites such as Virtuous Pedophiles and others showed >50% of respondents had strong feelings of isolation and loneliness, nearly 30% had extreme difficulty with concentration, >40% had significant anger and frustration, and >30% were struggling with feelings of detachment.12 Notably, the respondents attributed these difficulties to their minor attraction.12 Table 22,12-14 summarizes the findings of studies evaluating psychiatric symptoms in MAPs.
Consider OCD, hypersexuality
It is important to be aware that an attraction to minors may be a symptom of obsessive-compulsive disorder (OCD) or hypersexuality.15 Pedophilia-themed OCD (POCD) is a manifestation of OCD in which the individual experiences shame, fear, and excessive worry related to sexual attraction to children. Typically, individuals with POCD experience sexual thoughts of children as ego-dystonic, whereas MAPs experience such thoughts as ego-syntonic and arousing.15 However, much like individuals with POCD, MAPs also experience sexual thoughts of minors as distressing. Initial presentations of POCD may be confused with MAPs or pedophilia because of the overlap of symptoms such as anxiety, shame, distress, or suicidal ideation related to the idea of child sexual interests. The distinguishing feature of POCD is the absence of sexual arousal to children.
Continue to: Clinical presentations of...
Clinical presentations of hypersexuality may include sexual arousal to children. These individuals are distinguished from MAPs or those with pedophilia because they lack a preferred or sustained sexual interest in this group. On the contrary, individuals with hypersexuality present with a diversity of sexual interests explained by their high libido. Some individuals, however, may meet criteria for both hypersexuality and pedophilia. These individuals may pose a higher risk of sexual offending due to the presence of a heightened sexual drive and pedophilic interests, and thereby may require more intensive treatment, such as biologic treatment.
Focus on individualized treatment needs
Understanding the treatment needs of MAPs means understanding the goals of the individual MAP. Improving self-esteem, decreasing social isolation, and managing stigma are common treatment goals among MAPs.16 Levenson and Grady12 found that most MAPs identified treatment goals unrelated to sexual interests, such as addressing depression, anxiety, and low self-esteem. A smaller percentage identified sexual frustration related to the absence of healthy sexual outlets. Because many MAPs identify common psychiatric treatment needs, most clinicians should be equipped to foster a nonjudgmental therapeutic alliance to treat these patients. Effective treatment outcomes occur when comorbid psychiatric illnesses are treated as well as addressing the internal stigmatization that many MAPs experience.
Specialized treatment may be indicated for individuals who request treatment specific to sexual interests. This may include safety planning, including developing support systems to decrease the risk around children. For MAPs who have been unsuccessful at managing their sexual interests, pharmacotherapy may be an option. To date, research on pharmacotherapy for pedophilia is largely limited to studies of sexual offenders. Testosterone-lowering medications such as gonadotropin-releasing hormone (GnRH) analogue treatment constitutes the most effective treatment for patients who are not helped by conventional psychotherapeutic interventions.17 Other psychotropic medications, such as selective serotonin reuptake inhibitors or naltrexone, have not demonstrated efficacy outside of case reports.17
Addressing barriers to care
MAPs have a strong desire but significant hesitation when seeking mental health treatment.13,18 Nearly half (47%) of the 154 MAP respondents in the Levenson and Grady12 survey had never told anyone about their minor attraction. MAPs are understandably hesitant to disclose these thoughts and feelings due to fear of public exposure and intense stigmatization, as well as potential punitive and legal consequences.18,19 One post from the 2011 B4U-ACT online survey read, “Parents will disown you; teachers will report you; friends will abandon you … people in my situation can’t discuss this without serious risk of persecution and/or harassment.”14 In this survey, 78% of respondents feared a negative reaction by the professional, 78% feared being reported to law enforcement, and 68% feared being reported to family, an employer, or the community.14 This hesitancy due to fear of being exposed even extended to accessing self-help books, informational websites, and online forums, even though these sources are strongly desired and perceived as helpful.20
Even if MAPs were to decide to seek help, the lack of specific training and experience among psychiatrists make them unlikely to find it in the medical field.21 Furthermore, MAPs who desire help often worry it will be inadequate and they will be misunderstood by their clinicians.22 According to the Levenson and Grady survey,12 when asked what they would like most from therapy, most MAPs said they would want the treatment to focus on depression, anxiety, and low self-esteem rather than on sexual interest. In the B4U-ACT survey,14 many respondents identified the need for treatment of issues surrounding their sexual attraction, such as assistance in learning how to live in society with the attraction, dealing with society’s negative response to the attraction, and improving their self-concept in the presence of the extreme shame associated with the attraction. However, many MAPs find that clinicians tend to focus on protecting society from them, rather than on offering general psychiatric treatment or treatment focused on improving their well-being.18 This inability to locate appropriate services is known to exacerbate depression, suicidality, fear, anxiety, hopelessness, and substance abuse among MAPs.18 There is also evidence that individuals with minor attraction who are in a negative affective state are more likely to act on their attractions.23
Continue to: An ethical responsibility
An ethical responsibility. Physicians have a long-recognized responsibility to participate in activities to protect and promote the health of the public. The American Medical Association Code of Medical Ethics includes “justice,” or treating patients fairly and equitably.24 This includes patients who have pedophilic interests. Unfortunately, the stigma associated with individuals who have sexual attraction to children is pervasive in our society, including among medical professionals. The first consideration in treating MAPs is to overcome the stigmatization within our field, to remember that as physicians we took an oath to provide treatment fairly, equitably, and in accordance with the patient’s rights and entitlement.24 This includes listening to MAPs’ treatment needs. Not all MAPs want or need treatment related to their sexual interest. As is the case with all patients, listening to the individual’s chief complaint is paramount. If a patient’s treatment needs are beyond the clinician’s expertise, the patient should be referred to another clinician.
Mandated reporting. MAPs may not engage in psychiatric treatment for fear of being reported to authorities as a result of mandated reporting laws. Although the circumstances under which mandated reporting may be required vary by jurisdiction, they generally include situations in which the health care professional has reasonable cause to believe that a child is suffering from abuse or neglect. A patient’s report of sexual urges and fantasies to have sexual contact with minors is not sufficient for mandated reporting. While professionals vary in their interpretation of mandated reporting laws, sexual thoughts alone do not meet the threshold for mandated reporting. Mandated reporting duties should be discussed when first meeting a patient with minor attraction. For clinicians who are uneasy about such distinctions, either supervision or not working with such patients is the solution.
The importance of providing competent and individualized treatment to MAPs is 2-fold. First, individuals who are experiencing psychiatric symptoms deserve to have access treatment. Second, providing psychiatric treatment to individuals with minor attractions is a step toward preventing child sexual abuse. The Prevention Project Dunkelfeld in Germany used public service announcements to advertise confidential treatment for individuals who had sexual interest in children.25 Many of the participants were interested in mental health treatment unrelated to their sexual interests. Such projects may help us understand the best way to meet the treatment needs of minor-attracted individuals, as well as reduce child sexual abuse. As psychiatrists, we can stop making the problem worse by withholding psychiatric treatment from an important population.
Resources for MAPs and clinicians
Currently, resources for MAPs and clinicians are limited. MAPs can communicate and find support among other MAPs in online forums (see Related Resources). These websites provide online peer support groups and guides for seeking therapy. Information for mental health professionals, including available literature, research projects, clinicians who provide specialized treatment, and a monthly “dialog on therapy” can be found on the B4U-ACT and the Global Prevention Project websites. However, beyond the DSM-5 definitions, psychiatric education and training on this topic is almost entirely lacking.
In light of the information discussed in this article, several important issues remain, including how psychiatrists can best reach this population, and how they can work toward decreasing stigma so they can provide meaningful care. The solutions start with education. Educating psychiatrists about this important population can decrease stigma and facilitate appropriate, compassionate care to these patients, with the result of improving the mental health of people with minor attraction and decreasing the incidence of child sexual abuse.
Continue to: Bottom Line
Bottom Line
Minor-attracted persons report a high prevalence of general psychiatric symptoms that often go untreated due to a lack of willing clinicians with appropriate expertise. Providing psychiatric treatment to these patients can improve their mental health and possibly decrease the incidence of individuals who act on their attractions.
Related Resources
- B4U-ACT. www.b4uact.org • The Global Prevention Project. http://theglobalprevention project.org
- Virtuous Pedophiles. www.virped.org
Drug Brand Names
Naltrexone • ReVia
1. Briere J, Elliott D. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27(10):1205-1222. doi: 10.1016/j.chiabu.2003.09.008
2. Stevens E, Wood J. “I despise myself for thinking about them.” A thematic analysis of the mental health implications and employed coping mechanisms of self-reported non-offending minor attracted persons. J Child Sex Abus. 2019;28(8):968-989. doi: 10.1080/10538712.2019.1657539
3. Sorrentino R. Normal human sexuality and sexual and gender identity disorders: paraphilias. In: Sadock BJ, Sadock VA, Ruis P, eds. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9th ed. Wolters Kluwer; 2012:2093-2094.
4. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:685-705.
5. Dombert B, Schmidt AF, Banse R, et al. How common is men’s self-reported sexual interest in prepubescent children? J Sex Res. 2016;53(2):214-23. doi: 10.1080/00224499.2015.1020108
6. Seto MC. Pedophilia and sexual offending against children: theory, assessment, and intervention. 2nd ed. American Psychological Association; 2018.
7. Ahlers CJ, Schaefer GA, Mundt IA, et al. How unusual are the contents of paraphilias? Paraphilia-associated sexual arousal patterns in a community-based sample of men. J Sex Med. 2011;8(5):1362-1370. doi: 10.1111/j.1743-6109.2009.01597.x
8. Corrigan PW, Roe D, Tsang HWH. Challenging the public stigma of mental illness: lessons for therapists and advocates. Wiley Blackwell; 2011:55-114.
9. Lievesley R, Harper CA, Elliott H. The internalization of social stigma among minor-attracted persons: implications for treatment. Arch Sex Behav. 2020;49(4):1291-1304. doi: 10.1007/s10508-019-01569-x
10. Jahnke S, Imhoff R, Hoyer J. Stigmatization of people with pedophilia: two comparative surveys. Arch Sex Behav. 2015;44(1):21-34. doi: 10.1007/s10508-014-0312-4
11. Grady MD, Levenson JS, Mesias G, et al. “‘I can’t talk about that”: Stigma and fear as barriers to preventative services for minor-attracted persons. Stigma and Health. 2019;4(4):400-410. doi: 10.1037/sah0000154
12. Levenson JS, Grady MD. Preventing sexual abuse: perspectives of minor-attracted persons about seeking help. Sex Abuse. 2019;31(8):991-1013. doi: 10.1177/1079063218797713
13. Cohen L, Ndukwe N, Yaseen Z, et al. Comparison of self-identified minor-attracted persons who have and have not successfully refrained from sexual activity with children. J Sex Marital Ther. 2018;44(3):217-230. doi: 10.1080/0092623X.2017.1377129
14. B4U-ACT. Awareness of sexuality in youth, suicidality, and seeking care. 2011. Accessed June 4, 2021. www.b4uact.org/research/survey-results/spring-2011-survey
15. Bruce SL, Ching THW, Williams MT. Pedophilia-themed obsessive-compulsive disorder: assessment, differential diagnosis, and treatment with exposure and response prevention. Arch Sex Behav. 2018;47(2):389-402. doi: 10.1007/s10508-017-1031-4
16. Levenson JS, Grady MD, Morin JW. Beyond the “ick factor”: counseling non-offending persons with pedophilia. Clinical Social Work Journal. 2020;48:380-388. doi: 10.007/s10615-019-00712-4
1 7. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490. doi: 10.1080/15622975.2020.1744723
18. B4U-ACT. Principles and perspectives of practice. 2017. Accessed June 4, 2021. www.b4uact.org/about-us/principles-and-perspectives-of-practice/
19. McPhail IV, Stephens S, Heasman A. Legal and ethical issues in treating clients with pedohebephilic interests. Canadian Psychology/Psychologie Canadienne. 2018;59(4):369-381. doi:10.1037/cap0000157
20. Levenson JS, Willis GM, Vicencio CP. Obstacles to help-seeking for sexual offenders: implications for prevention of sexual abuse. J Child Sex Abus. 2017;26(2):99-120. doi: 10.1080/10538712.2016.1276116
21. Sorrentino R. DSM-5 and paraphilias: what psychiatrists need to know. Psychiatric Times. November 28, 2016. Accessed June 4, 2021. https://www.psychiatrictimes.com/view/dsm-5-and-paraphilias-what-psychiatrists-need-know
22. Cantor JM, McPhail IV. Non-offending pedophiles. Current Sexual Health Reports. 2016;8:121-128. doi:10.1007/s11930-016-0076-z
23. Ward T, Louden K, Hudson SM, et al. A descriptive model of the offense chain for child molesters. Journal of Interpersonal Violence. 1995;10(4):452-472. doi:10.1177/088626095010004005
24. American Medical Association. AMA Code of Medical Ethics. 2016. Accessed June 4, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/principles-of-medical-ethics.pdf
25. Beier KM, Grundmann D, Kuhle LF, et al. The German Dunkelfeld project: a pilot study to prevent child sexual abuse and the use of child abusive images. J Sex Med. 2015;12(2):529-42. doi: 10.1111/jsm.12785
1. Briere J, Elliott D. Prevalence and psychological sequelae of self-reported childhood physical and sexual abuse in a general population sample of men and women. Child Abuse Negl. 2003;27(10):1205-1222. doi: 10.1016/j.chiabu.2003.09.008
2. Stevens E, Wood J. “I despise myself for thinking about them.” A thematic analysis of the mental health implications and employed coping mechanisms of self-reported non-offending minor attracted persons. J Child Sex Abus. 2019;28(8):968-989. doi: 10.1080/10538712.2019.1657539
3. Sorrentino R. Normal human sexuality and sexual and gender identity disorders: paraphilias. In: Sadock BJ, Sadock VA, Ruis P, eds. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9th ed. Wolters Kluwer; 2012:2093-2094.
4. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013:685-705.
5. Dombert B, Schmidt AF, Banse R, et al. How common is men’s self-reported sexual interest in prepubescent children? J Sex Res. 2016;53(2):214-23. doi: 10.1080/00224499.2015.1020108
6. Seto MC. Pedophilia and sexual offending against children: theory, assessment, and intervention. 2nd ed. American Psychological Association; 2018.
7. Ahlers CJ, Schaefer GA, Mundt IA, et al. How unusual are the contents of paraphilias? Paraphilia-associated sexual arousal patterns in a community-based sample of men. J Sex Med. 2011;8(5):1362-1370. doi: 10.1111/j.1743-6109.2009.01597.x
8. Corrigan PW, Roe D, Tsang HWH. Challenging the public stigma of mental illness: lessons for therapists and advocates. Wiley Blackwell; 2011:55-114.
9. Lievesley R, Harper CA, Elliott H. The internalization of social stigma among minor-attracted persons: implications for treatment. Arch Sex Behav. 2020;49(4):1291-1304. doi: 10.1007/s10508-019-01569-x
10. Jahnke S, Imhoff R, Hoyer J. Stigmatization of people with pedophilia: two comparative surveys. Arch Sex Behav. 2015;44(1):21-34. doi: 10.1007/s10508-014-0312-4
11. Grady MD, Levenson JS, Mesias G, et al. “‘I can’t talk about that”: Stigma and fear as barriers to preventative services for minor-attracted persons. Stigma and Health. 2019;4(4):400-410. doi: 10.1037/sah0000154
12. Levenson JS, Grady MD. Preventing sexual abuse: perspectives of minor-attracted persons about seeking help. Sex Abuse. 2019;31(8):991-1013. doi: 10.1177/1079063218797713
13. Cohen L, Ndukwe N, Yaseen Z, et al. Comparison of self-identified minor-attracted persons who have and have not successfully refrained from sexual activity with children. J Sex Marital Ther. 2018;44(3):217-230. doi: 10.1080/0092623X.2017.1377129
14. B4U-ACT. Awareness of sexuality in youth, suicidality, and seeking care. 2011. Accessed June 4, 2021. www.b4uact.org/research/survey-results/spring-2011-survey
15. Bruce SL, Ching THW, Williams MT. Pedophilia-themed obsessive-compulsive disorder: assessment, differential diagnosis, and treatment with exposure and response prevention. Arch Sex Behav. 2018;47(2):389-402. doi: 10.1007/s10508-017-1031-4
16. Levenson JS, Grady MD, Morin JW. Beyond the “ick factor”: counseling non-offending persons with pedophilia. Clinical Social Work Journal. 2020;48:380-388. doi: 10.007/s10615-019-00712-4
1 7. Thibaut F, Cosyns P, Fedoroff JP, et al; WFSBP Task Force on Paraphilias. The World Federation of Societies of Biological Psychiatry (WFSBP) 2020 guidelines for the pharmacological treatment of paraphilic disorders. World J Biol Psychiatry. 2020;21(6):412-490. doi: 10.1080/15622975.2020.1744723
18. B4U-ACT. Principles and perspectives of practice. 2017. Accessed June 4, 2021. www.b4uact.org/about-us/principles-and-perspectives-of-practice/
19. McPhail IV, Stephens S, Heasman A. Legal and ethical issues in treating clients with pedohebephilic interests. Canadian Psychology/Psychologie Canadienne. 2018;59(4):369-381. doi:10.1037/cap0000157
20. Levenson JS, Willis GM, Vicencio CP. Obstacles to help-seeking for sexual offenders: implications for prevention of sexual abuse. J Child Sex Abus. 2017;26(2):99-120. doi: 10.1080/10538712.2016.1276116
21. Sorrentino R. DSM-5 and paraphilias: what psychiatrists need to know. Psychiatric Times. November 28, 2016. Accessed June 4, 2021. https://www.psychiatrictimes.com/view/dsm-5-and-paraphilias-what-psychiatrists-need-know
22. Cantor JM, McPhail IV. Non-offending pedophiles. Current Sexual Health Reports. 2016;8:121-128. doi:10.1007/s11930-016-0076-z
23. Ward T, Louden K, Hudson SM, et al. A descriptive model of the offense chain for child molesters. Journal of Interpersonal Violence. 1995;10(4):452-472. doi:10.1177/088626095010004005
24. American Medical Association. AMA Code of Medical Ethics. 2016. Accessed June 4, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/principles-of-medical-ethics.pdf
25. Beier KM, Grundmann D, Kuhle LF, et al. The German Dunkelfeld project: a pilot study to prevent child sexual abuse and the use of child abusive images. J Sex Med. 2015;12(2):529-42. doi: 10.1111/jsm.12785
Improving nonverbal communication during telepsychiatry sessions
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
Treating psychosis in pregnant women: A measured approach
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
Recommending esketamine? 4 factors to consider
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
Algorithms for Prediction of Clinical Deterioration on the General Wards: A Scoping Review
The early identification of clinical deterioration among adult hospitalized patients remains a challenge.1 Delayed identification is associated with increased morbidity and mortality, unplanned intensive care unit (ICU) admissions, prolonged hospitalization, and higher costs.2,3 Earlier detection of deterioration using predictive algorithms of vital sign monitoring might avoid these negative outcomes.4 In this scoping review, we summarize current algorithms and their evidence.
Vital signs provide the backbone for detecting clinical deterioration. Early warning scores (EWS) and outreach protocols were developed to bring structure to the assessment of vital signs. Most EWS claim to predict clinical end points such as unplanned ICU admission up to 24 hours in advance.5,6 Reviews of EWS showed a positive trend toward reduced length of stay and mortality. However, conclusions about general efficacy could not be generated because of case heterogeneity and methodologic shortcomings.4,7 Continuous automated vital sign monitoring of patients on the general ward can now be accomplished with wearable devices.8 The first reports on continuous monitoring showed earlier detection of deterioration but not improved clinical end points.4,9 Since then, different reports on continuous monitoring have shown positive effects but concluded that unprocessed monitoring data per se falls short of generating actionable alarms.4,10,11
Predictive algorithms, which often use artificial intelligence (AI), are increasingly employed to recognize complex patterns or abnormalities and support predictions of events in big data sets.12,13 Especially when combined with continuous vital sign monitoring, predictive algorithms have the potential to expedite detection of clinical deterioration and improve patient outcomes. Predictive algorithms using vital signs in the ICU have shown promising results.14 The impact of predictive algorithms on the general wards, however, is unclear.
The aims of our scoping review were to explore the extent and range of and evidence for predictive vital signs–based algorithms on the adult general ward; to describe the variety of these algorithms; and to categorize effects, facilitators, and barriers of their implementation.15
MATERIALS AND METHODS
We performed a scoping review to create a summary of the current state of research. We used the five-step method of Levac and followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses Extension for Scoping Reviews guidelines (Appendix 1).16,17
PubMed, Embase, and CINAHL databases were searched for English-language articles written between January 1, 2010, and November 20, 2020. We developed the search queries with an experienced information scientist, and we used database-specific terms and strategies for input, clinical outcome, method, predictive capability, and population (Appendix 2). Additionally, we searched the references of the selected articles, as well as publications citing these articles.
All studies identified were screened by title and abstract by two researchers (RP and YE). The selected studies were read in their entirety and checked for eligibility using the following inclusion criteria: automated algorithm; vital signs-based; real-time prediction; of clinical deterioration; in an adult, general ward population. In cases where there were successive publications with the same algorithm and population, we selected the most recent study.
For screening and selection, we used the Rayyan QCRI online tool (Qatar Computing Research Institute) and Endnote X9 (Clarivate Analytics). We extracted information using a data extraction form and organized it into descriptive characteristics of the selected studies (Table 1): an input data table showing number of admissions, intermittent or continuous measurements, vital signs measured, laboratory results (Appendix Table 1), a table summarizing study designs and settings (Appendix Table 2), and a prediction performance table (Table 2). We report characteristics of the populations and algorithms, prediction specifications such as area under the receiver operating curve (AUROC), and predictive values. Predictive values are affected by prevalence, which may differ among populations. To compare the algorithms, we calculated an indexed positive predictive value (PPV) and a number needed to evaluate (NNE) using a weighted average prevalence of clinical deterioration of 3.0%.

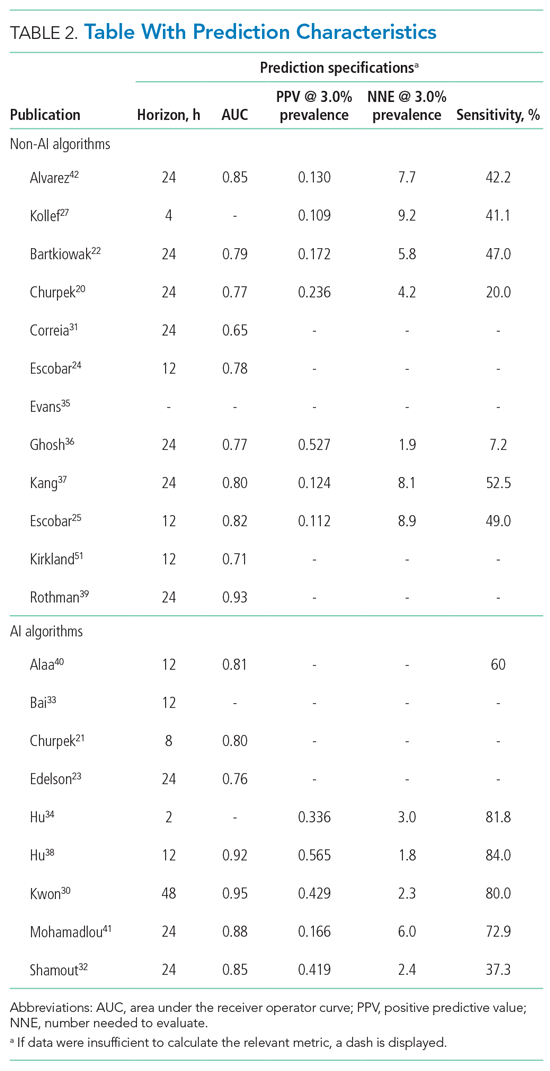
We defined clinical deterioration as end points, including rapid response team activation, cardiopulmonary resuscitation, transfer to an ICU, or death.
Effects, facilitators, and barriers were identified and categorized using ATLAS.ti 8 software (ATLAS.ti) and evaluated by three researchers (RP, MK, and THvdB). These were categorized using the adapted frameworks of Gagnon et al18 for the barriers and facilitators and of Donabedian19 for the effects (Appendix 3).
The Gagnon et al framework was adapted by changing two of four domains—that is, “Individual” was changed to “Professional” and “Human” to “Physiology.” The domains of “Technology” and “Organization” remained unchanged. The Donabedian domains of “Outcome,” “Process,” and “Structure” also remained unchanged (Table 3).
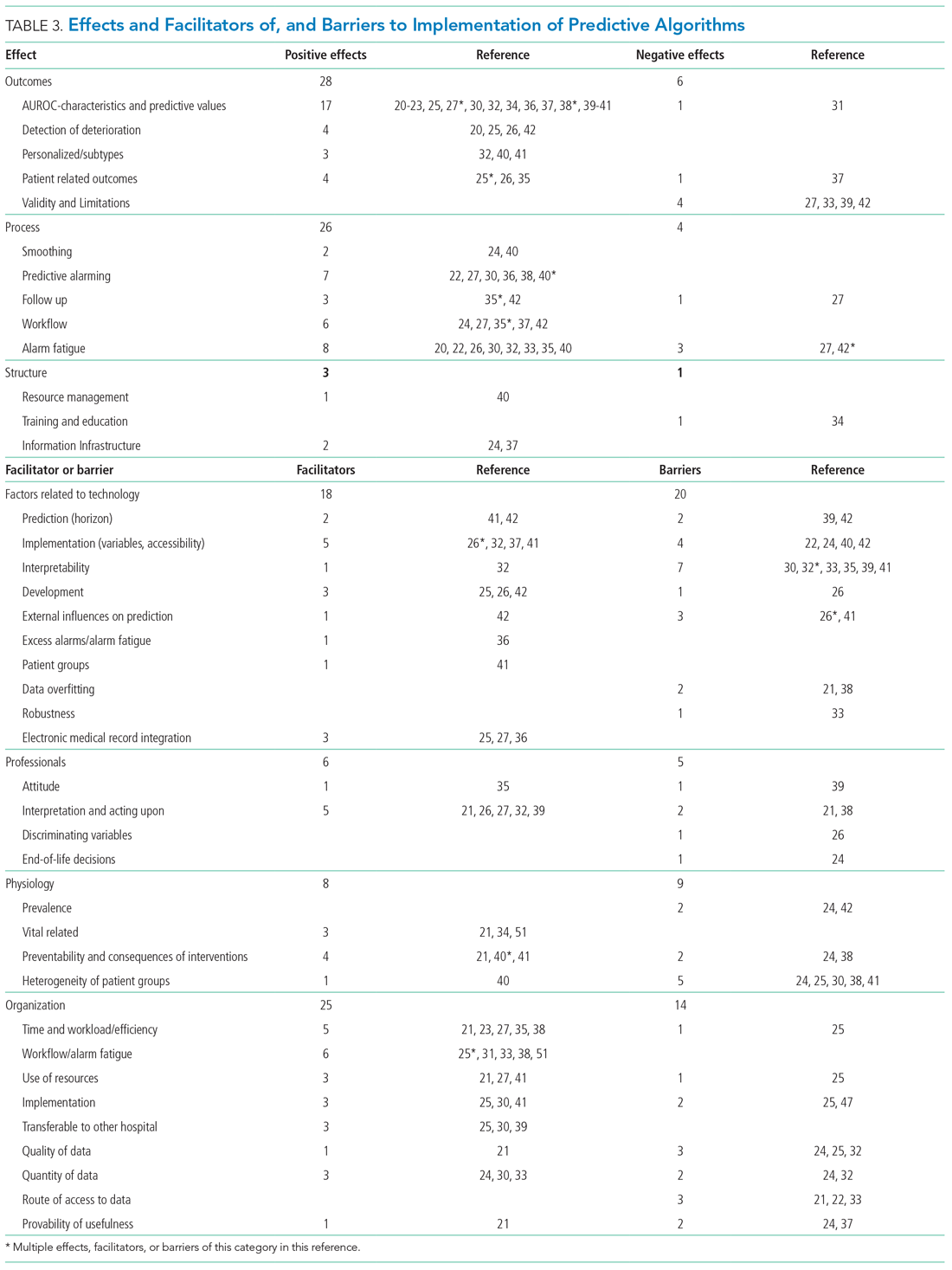
We divided the studies into two groups: studies on predictive algorithms with and without AI when reporting on characteristics and performance. For the secondary aim of exploring implementation impact, we reported facilitators and barriers in a narrative way, highlighting the most frequent and notable findings.
RESULTS
As shown in the Figure, we found 1741 publications, of which we read the full-text of 109. There were 1632 publications that did not meet the inclusion criteria. The publications by Churpek et al,20,21 Bartkiowak et al,22 Edelson et al,23 Escobar et al,24,25 and Kipnis et al26 reported on the same algorithms or databases but had significantly different approaches. For multiple publications using the same algorithm and population, the most recent was named with inclusion of the earlier findings.20,21,27-29 The resulting 21 papers are included in this review.
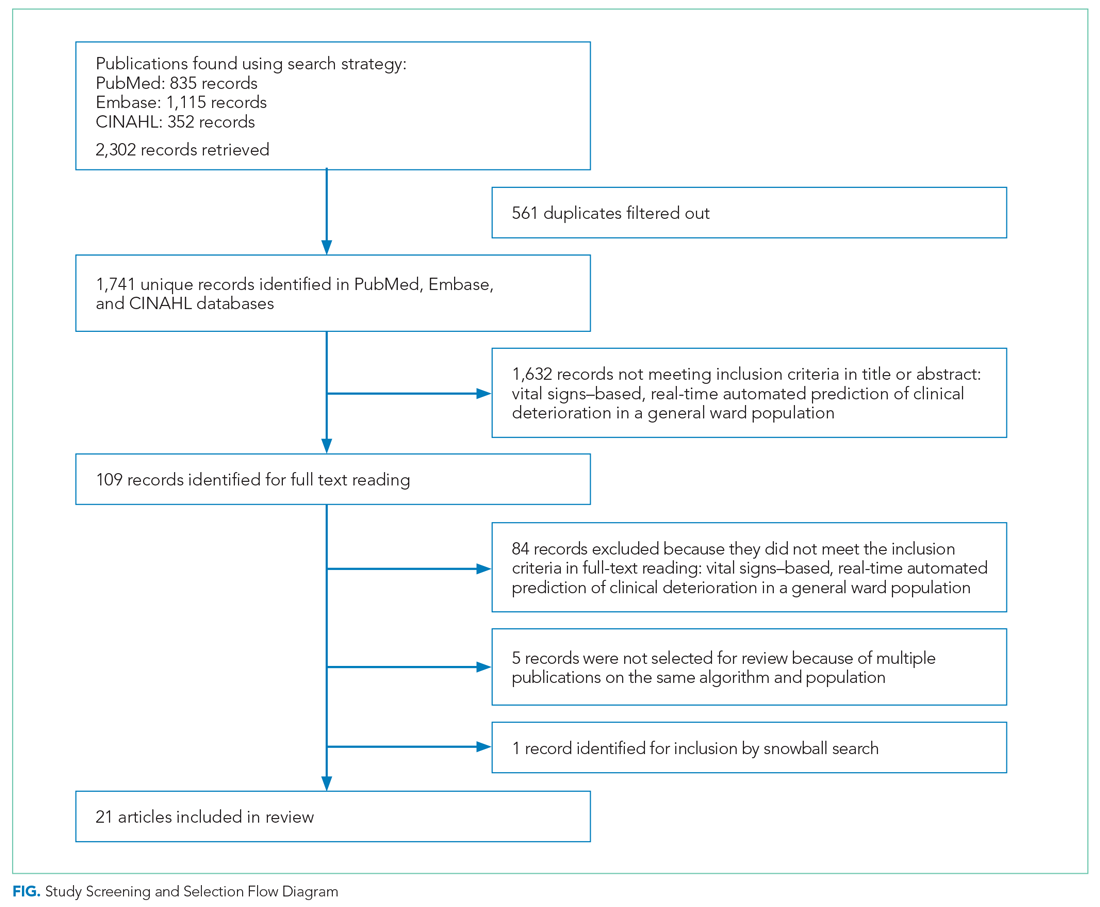
Descriptive characteristics of the studies are summarized in Table 1. Nineteen of the publications were full papers and two were conference abstracts. Most of the studies (n = 18) were from the United States; there was one study from South Korea,30 one study from Portugal,31 and one study from the United Kingdom.32 In 15 of the studies, there was a strict focus on general or specific wards; 6 studies also included the ICU and/or emergency departments.
Two of the studies were clinical trials, 2 were prospective observational studies, and 17 were retrospective studies. Five studies reported on an active predictive model during admission. Of these, 3 reported that the model was clinically implemented, using the predictions in their clinical workflow. None of the implemented studies used AI.
All input variables are presented in Appendix Table 1.
The non-AI algorithm prediction horizons ranged from 4 to 24 hours, with a median of 24 hours (interquartile range [IQR], 12-24 hours). The AI algorithms ranged from 2 to 48 hours and had a median horizon of 14 hours (IQR, 12-24 hours).
We found three studies reporting patient outcomes. The most recent of these was a large multicenter implementation study by Escobar et al25 that included an extensive follow-up response. This study reported a significantly decreased 30-day mortality in the intervention cohort. A smaller randomized controlled trial reported no significant differences in patient outcomes with earlier warning alarms.27 A third study reported more appropriate rapid response team deployment and decreased mortality in a subgroup analysis.35
Effects, Facilitators, and Barriers
As shown in the Appendix Figure and further detailed in Table 3, the described effects were predominantly positive—57 positive effects vs 11 negative effects. These positive effects sorted primarily into the outcome and process domains.
All of the studies that compared their proposed model with one of various warning systems (eg, EWS, National Early Warning Score [NEWS], Modified Early Warning Score [MEWS]) showed superior performance (based on AUROC and reported predictive values). In 17 studies, the authors reported their model as more useful or superior to the EWS.20-23,26-28,34,36-41 Four studies reported real-time detection of deterioration before regular EWS,20,26,42 and three studies reported positive effects on patient-related outcomes.26,35 Four negative effects were noted on the controllability, validity, and potential limitations.27,42
Of the 38 remarks in the Technology domain, difficulty with implementation in daily practice was a commonly cited barrier.22,24,40,42 Difficulties included creating real-time data feeds out of the EMR, though there were mentions of some successful examples.25,27,36 Difficulty in the interpretability of AI was also considered a potential barrier.30,32,33,35,39,41 There were remarks as to the applicability of the prolonged prediction horizon because of the associated decoupling from the clinical view.39,42
Conservative attitudes toward new technologies and inadequate knowledge were mentioned as barriers.39 Repeated remarks were made on the difficulty of interpreting and responding to a predicted escalation, as the clinical pattern might not be recognizable at such an early stage. On the other hand, it is expected that less invasive countermeasures would be adequate to avert further escalation. Earlier recognition of possible escalations also raised potential ethical questions, such as when to discuss palliative care.24
The heterogeneity of the general ward population and the relatively low prevalence of deterioration were mentioned as barriers.24,30,38,41 There were also concerns that not all escalations are preventable and that some patient outcomes may not be modifiable.24,38
Many investigators expected reductions in false alarms and associated alarm fatigue (reflected as higher PPVs). Furthermore, they expected workflow to improve and workload to decrease.21,23,27,31,33,35,38,41 Despite the capacity of modern EMRs to store large amounts of patient data, some investigators felt improvements to real-time access, data quality and validity, and data density are needed to ensure valid associated predictions.21,22,24,32,37
DISCUSSION
As the complexity and comorbidity of hospitalized adults grow, predicting clinical deterioration is becoming more important. With an ever-increasing amount of available
There are several important limitations across these studies. In a clinical setting, these models would function as a screening test. Almost all studies report an AUROC; however, sensitivity and PPV or NNE (defined as 1/PPV) may be more useful than AUROC when predicting low-frequency events with high-potential clinical impact.44 Assessing the NNE is especially relevant because of its relation to alarm fatigue and responsiveness of clinicians.43 Alarm fatigue and lack of adequate response to alarms were repeatedly cited as potential barriers for application of automated scores.
Although the results of our scoping review are promising, there are limited data on clinical outcomes using these algorithms. Only three of five algorithms were used to guide clinical decision-making.25,27,35 Kollef et al27 showed shorter hospitalizations and Evans et al35 found decreased mortality rates in a multimorbid subgroup. Escobar et al25 found an overall and consistent decrease in mortality in a large, heterogenic population of inpatients across 21 hospitals. While Escobar et al’s findings provide strong evidence that predictive algorithms and structured follow-up on alarms can improve patient outcomes, it recognizes that not all facilities will have the resources to implement them.25 Dedicated round-the-clock follow-up of alarms has yet to be proven feasible for smaller institutions, and leaner solutions must be explored. The example set by Escobar et al25 should be translated into various settings to prove its reproducibility and to substantiate the clinical impact of predictive models and structured follow-up.
According to expert opinion, the use of high-frequency or continuous monitoring at low-acuity wards and AI algorithms to detect trends and patterns will reduce failure-to-rescue rates.4,9,43 However, most studies in our review focused on periodic spot-checked vital signs, and none of the AI algorithms were implemented in clinical care (Appendix Table 1
STRENGTHS AND LIMITATIONS
We performed a comprehensive review of the current literature using a clear and reproducible methodology to minimize the risk of missing relevant publications. The identified research is mainly limited to large US centers and consists of mostly retrospective studies. Heterogeneity among inputs, endpoints, time horizons, and evaluation metrics make comparisons challenging. Comments on facilitators, barriers, and effects were limited.
RECOMMENDATIONS FOR FUTURE RESEARCH
Artificial intelligence and the use of continuous monitoring hold great promise in creating optimal predictive algorithms. Future studies should directly compare AI- and non-AI-based algorithms using continuous monitoring to determine predictive accuracy, feasibility, costs, and outcomes. A consensus on endpoint definitions, input variables, methodology, and reporting is needed to enhance reproducibility, comparability, and generalizability of future research.
CONCLUSION
- van Galen LS, Struik PW, Driesen BEJM, et al. Delayed recognition of deterioration of patients in general wards is mostly caused by human related monitoring failures: a root cause analysis of unplanned ICU admissions. PLoS One. 2016;11(8):e0161393. https://doi.org/10.1371/journal. pone.0161393
- Mardini L, Lipes J, Jayaraman D. Adverse outcomes associated with delayed intensive care consultation in medical and surgical inpatients. J Crit Care. 2012;27(6):688-693. https://doi.org/10.1016/j.jcrc.2012.04.011
- Young MP, Gooder VJ, McBride K, James B, Fisher ES. Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18(2):77-83. https://doi.org/10.1046/ j.1525-1497.2003.20441.x
- Khanna AK, Hoppe P, Saugel B. Automated continuous noninvasive ward monitoring: future directions and challenges. Crit Care. 2019;23(1):194. https://doi.org/10.1186/s13054-019-2485-7
- Ludikhuize J, Hamming A, de Jonge E, Fikkers BG. Rapid response systems in The Netherlands. Jt Comm J Qual Patient Saf. 2011;37(3):138-197. https:// doi.org/10.1016/s1553-7250(11)37017-1
- Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. https://doi.org/10.1097/01.ccm.0000254826.10520.87
- Alam N, Hobbelink EL, van Tienhoven AJ, van de Ven PM, Jansma EP, Nanayakkara PWB. The impact of the use of the Early Warning Score (EWS) on patient outcomes: a systematic review. Resuscitation. 2014;85(5):587-594. https://doi.org/10.1016/j.resuscitation.2014.01.013
- Weenk M, Koeneman M, van de Belt TH, Engelen LJLPG, van Goor H, Bredie SJH. Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation. 2019;136:47-53. https://doi.org/10.1016/j.resuscitation.2019.01.017
- Cardona-Morrell M, Prgomet M, Turner RM, Nicholson M, Hillman K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta-analysis. Int J Clin Pract. 2016;70(10):806-824. https://doi.org/10.1111/ijcp.12846
- Brown H, Terrence J, Vasquez P, Bates DW, Zimlichman E. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med. 2014;127(3):226-232. https://doi.org/10.1016/j.amjmed.2013.12.004
- Mestrom E, De Bie A, van de Steeg M, Driessen M, Atallah L, Bezemer R. Implementation of an automated early warning scoring system in a E8 Journal of Hospital Medicine® Published Online June 2021 An Official Publication of the Society of Hospital Medicine Peelen et al | Predicting Deterioration: A Scoping Review surgical ward: practical use and effects on patient outcomes. PLoS One. 2019;14(5):e0213402. https://doi.org/10.1371/journal.pone.0213402
- Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. https://doi.org/10.1136/ svn-2017-000101
- Iwashyna TJ, Liu V. What’s so different about big data? A primer for clinicians trained to think epidemiologically. Ann Am Thorac Soc. 2014;11(7):1130- 1135. https://doi.org/10.1513/annalsats.201405-185as
- Jalali A, Bender D, Rehman M, Nadkanri V, Nataraj C. Advanced analytics for outcome prediction in intensive care units. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:2520-2524. https://doi.org/10.1109/embc.2016.7591243
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. https://doi.org/10.1186/s12874-018-0611-x
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19-32. https://doi.org/10.1080/13645 57032000119616
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMAScR): checklist and explanation. Ann Intern Med. 2018;169(7):467- 473. https://doi.org/10.7326/m18-0850
- Gagnon MP, Desmartis M, Gagnon J, et al. Framework for user involvement in health technology assessment at the local level: views of health managers, user representatives, and clinicians. Int J Technol Assess Health Care. 2015;31(1-2):68-77. https://doi.org/10.1017/s0266462315000070
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. https://doi.org/10.1001/jama.260.12.1743
- Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649-655. https://doi.org/10.1164/rccm.201406-1022oc
- Churpek MM, Yuen TC, Winslow C, Meltzer DO, Kattan MW, Edelson DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368-374. https://doi.org/10.1097/ccm.0000000000001571
- Bartkowiak B, Snyder AM, Benjamin A, et al. Validating the electronic cardiac arrest risk triage (eCART) score for risk stratification of surgical inpatients in the postoperative setting: retrospective cohort study. Ann Surg. 2019;269(6):1059-1063. https://doi.org/10.1097/sla.0000000000002665
- Edelson DP, Carey K, Winslow CJ, Churpek MM. Less is more: detecting clinical deterioration in the hospital with machine learning using only age, heart rate and respiratory rate. Abstract presented at: American Thoracic Society International Conference; May 22, 2018; San Diego, California. Am J Resp Crit Care Med. 2018;197:A4444.
- Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. https:// doi.org/10.1002/jhm.1929
- Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/nejmsa2001090
- Kipnis P, Turk BJ, Wulf DA, et al. Development and validation of an electronic medical record-based alert score for detection of inpatient deterioration outside the ICU. J Biomed Inform. 2016;64:10-19. https://doi.org/10.1016/j. jbi.2016.09.013
- Kollef MH, Chen Y, Heard K, et al. A randomized trial of real-time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424-429. https://doi.org/10.1002/jhm.2193
- Hackmann G, Chen M, Chipara O, et al. Toward a two-tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511-519.
- Bailey TC, Chen Y, Mao Y, Lu, C, Hackmann G, Micek ST. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8(5):236-242. https://doi.org/10.1002/jhm.2009
- Kwon JM, Lee Y, Lee Y, Lee S, Park J. An algorithm based on deep learning for predicting in-hospital cardiac arrest. J Am Heart Assoc. 2018;7(13):e008678. https://doi.org/10.1161/jaha.118.008678
- Correia S, Gomes A, Shahriari S, Almeida JP, Severo M, Azevedo A. Performance of the early warning system vital to predict unanticipated higher-level of care admission and in-hospital death of ward patients. Value Health. 2018;21(S3):S360. https://doi.org/10.1016/j.jval.2018.09.2152
- Shamout FE, Zhu T, Sharma P, Watkinson PJ, Clifton DA. Deep interpretable early warning system for the detection of clinical deterioration. IEEE J Biomed Health Inform. 2020;24(2):437-446. https://doi.org/10.1109/ jbhi.2019.2937803
- Bai Y, Do DH, Harris PRE, et al. Integrating monitor alarms with laboratory test results to enhance patient deterioration prediction. J Biomed Inform. 2015;53:81-92. https://doi.org/10.1016/j.jbi.2014.09.006
- Hu X, Sapo M, Nenov V, et al. Predictive combinations of monitor alarms preceding in-hospital code blue events. J Biomed Inform. 2012;45(5):913-921. https://doi.org/10.1016/j.jbi.2012.03.001
- Evans RS, Kuttler KG, Simpson KJ, et al. Automated detection of physiologic deterioration in hospitalized patients. J Am Med Inform Assoc. 2015;22(2):350-360. https://doi.org/10.1136/amiajnl-2014-002816
- Ghosh E, Eshelman L, Yang L, Carlson E, Lord B. Early deterioration indicator: data-driven approach to detecting deterioration in general ward. Resuscitation. 2018;122:99-105. https://doi.org/10.1016/j.resuscitation. 2017.10.026
- Kang MA, Churpek MM, Zadravecz FJ, Adhikari R, Twu NM, Edelson DP: Real-time risk prediction on the wards: a feasibility study. Crit Care Med. 2016;44(8):1468-1473. https://doi.org/10.1097/ccm.0000000000001716
- Hu SB, Wong DJL, Correa A, Li N, Deng JC. Prediction of clinical deterioration in hospitalized adult patients with hematologic malignancies using a neural network model. PLoS One. 2016;11(8):e0161401. https://doi. org/10.1371/journal.pone.0161401
- Rothman MJ, Rothman SI, Beals J 4th. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848. https://doi.org/10.1016/j. jbi.2013.06.011
- Alaa AM, Yoon J, Hu S, van der Schaar M. Personalized risk scoring for critical care prognosis using mixtures of Gaussian processes. IEEE Trans Biomed Eng. 2018;65(1):207-218. https://doi.org/10.1109/tbme.2017.2698602
- Mohamadlou H, Panchavati S, Calvert J, et al. Multicenter validation of a machine-learning algorithm for 48-h all-cause mortality prediction. Health Informatics J. 2020;26(3):1912-1925. https://doi.org/10.1177/1460458219894494
- Alvarez CA, Clark CA, Zhang S, et al. Predicting out of intensive care unit cardiopulmonary arrest or death using electronic medical record data. BMC Med Inform Decis Mak. 2013;13:28. https://doi.org/10.1186/1472-6947-13-28
- Vincent JL, Einav S, Pearse R, et al. Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol. 2018;35(5):325-333. https://doi.org/10.1097/eja.0000000000000798
- Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
- Weenk M, Bredie SJ, Koeneman M, Hesselink G, van Goor H, van de Belt TH. Continuous monitoring of the vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res. 2020;22(6):e15471. https://doi.org/10.2196/15471
- Wellner B, Grand J, Canzone E, et al. Predicting unplanned transfers to the intensive care unit: a machine learning approach leveraging diverse clinical elements. JMIR Med Inform. 2017;5(4):e45. https://doi.org/10.2196/medinform.8680
- Elliott M, Baird J. Pulse oximetry and the enduring neglect of respiratory rate assessment: a commentary on patient surveillance. Br J Nurs. 2019;28(19):1256-1259. https://doi.org/10.12968/bjon.2019.28.19.1256
- Blackwell JN, Keim-Malpass J, Clark MT, et al. Early detection of in-patient deterioration: one prediction model does not fit all. Crit Care Explor. 2020;2(5):e0116. https://doi.org/10.1097/cce.0000000000000116
- Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. https://doi.org/10.1038/sdata.2016.35
- Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. https://doi. org/10.1370/afm.1713
- Kirkland LL, Malinchoc M, O’Byrne M, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142 https://doi.org/10.1177/1062860612450459
The early identification of clinical deterioration among adult hospitalized patients remains a challenge.1 Delayed identification is associated with increased morbidity and mortality, unplanned intensive care unit (ICU) admissions, prolonged hospitalization, and higher costs.2,3 Earlier detection of deterioration using predictive algorithms of vital sign monitoring might avoid these negative outcomes.4 In this scoping review, we summarize current algorithms and their evidence.
Vital signs provide the backbone for detecting clinical deterioration. Early warning scores (EWS) and outreach protocols were developed to bring structure to the assessment of vital signs. Most EWS claim to predict clinical end points such as unplanned ICU admission up to 24 hours in advance.5,6 Reviews of EWS showed a positive trend toward reduced length of stay and mortality. However, conclusions about general efficacy could not be generated because of case heterogeneity and methodologic shortcomings.4,7 Continuous automated vital sign monitoring of patients on the general ward can now be accomplished with wearable devices.8 The first reports on continuous monitoring showed earlier detection of deterioration but not improved clinical end points.4,9 Since then, different reports on continuous monitoring have shown positive effects but concluded that unprocessed monitoring data per se falls short of generating actionable alarms.4,10,11
Predictive algorithms, which often use artificial intelligence (AI), are increasingly employed to recognize complex patterns or abnormalities and support predictions of events in big data sets.12,13 Especially when combined with continuous vital sign monitoring, predictive algorithms have the potential to expedite detection of clinical deterioration and improve patient outcomes. Predictive algorithms using vital signs in the ICU have shown promising results.14 The impact of predictive algorithms on the general wards, however, is unclear.
The aims of our scoping review were to explore the extent and range of and evidence for predictive vital signs–based algorithms on the adult general ward; to describe the variety of these algorithms; and to categorize effects, facilitators, and barriers of their implementation.15
MATERIALS AND METHODS
We performed a scoping review to create a summary of the current state of research. We used the five-step method of Levac and followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses Extension for Scoping Reviews guidelines (Appendix 1).16,17
PubMed, Embase, and CINAHL databases were searched for English-language articles written between January 1, 2010, and November 20, 2020. We developed the search queries with an experienced information scientist, and we used database-specific terms and strategies for input, clinical outcome, method, predictive capability, and population (Appendix 2). Additionally, we searched the references of the selected articles, as well as publications citing these articles.
All studies identified were screened by title and abstract by two researchers (RP and YE). The selected studies were read in their entirety and checked for eligibility using the following inclusion criteria: automated algorithm; vital signs-based; real-time prediction; of clinical deterioration; in an adult, general ward population. In cases where there were successive publications with the same algorithm and population, we selected the most recent study.
For screening and selection, we used the Rayyan QCRI online tool (Qatar Computing Research Institute) and Endnote X9 (Clarivate Analytics). We extracted information using a data extraction form and organized it into descriptive characteristics of the selected studies (Table 1): an input data table showing number of admissions, intermittent or continuous measurements, vital signs measured, laboratory results (Appendix Table 1), a table summarizing study designs and settings (Appendix Table 2), and a prediction performance table (Table 2). We report characteristics of the populations and algorithms, prediction specifications such as area under the receiver operating curve (AUROC), and predictive values. Predictive values are affected by prevalence, which may differ among populations. To compare the algorithms, we calculated an indexed positive predictive value (PPV) and a number needed to evaluate (NNE) using a weighted average prevalence of clinical deterioration of 3.0%.


We defined clinical deterioration as end points, including rapid response team activation, cardiopulmonary resuscitation, transfer to an ICU, or death.
Effects, facilitators, and barriers were identified and categorized using ATLAS.ti 8 software (ATLAS.ti) and evaluated by three researchers (RP, MK, and THvdB). These were categorized using the adapted frameworks of Gagnon et al18 for the barriers and facilitators and of Donabedian19 for the effects (Appendix 3).
The Gagnon et al framework was adapted by changing two of four domains—that is, “Individual” was changed to “Professional” and “Human” to “Physiology.” The domains of “Technology” and “Organization” remained unchanged. The Donabedian domains of “Outcome,” “Process,” and “Structure” also remained unchanged (Table 3).

We divided the studies into two groups: studies on predictive algorithms with and without AI when reporting on characteristics and performance. For the secondary aim of exploring implementation impact, we reported facilitators and barriers in a narrative way, highlighting the most frequent and notable findings.
RESULTS
As shown in the Figure, we found 1741 publications, of which we read the full-text of 109. There were 1632 publications that did not meet the inclusion criteria. The publications by Churpek et al,20,21 Bartkiowak et al,22 Edelson et al,23 Escobar et al,24,25 and Kipnis et al26 reported on the same algorithms or databases but had significantly different approaches. For multiple publications using the same algorithm and population, the most recent was named with inclusion of the earlier findings.20,21,27-29 The resulting 21 papers are included in this review.

Descriptive characteristics of the studies are summarized in Table 1. Nineteen of the publications were full papers and two were conference abstracts. Most of the studies (n = 18) were from the United States; there was one study from South Korea,30 one study from Portugal,31 and one study from the United Kingdom.32 In 15 of the studies, there was a strict focus on general or specific wards; 6 studies also included the ICU and/or emergency departments.
Two of the studies were clinical trials, 2 were prospective observational studies, and 17 were retrospective studies. Five studies reported on an active predictive model during admission. Of these, 3 reported that the model was clinically implemented, using the predictions in their clinical workflow. None of the implemented studies used AI.
All input variables are presented in Appendix Table 1.
The non-AI algorithm prediction horizons ranged from 4 to 24 hours, with a median of 24 hours (interquartile range [IQR], 12-24 hours). The AI algorithms ranged from 2 to 48 hours and had a median horizon of 14 hours (IQR, 12-24 hours).
We found three studies reporting patient outcomes. The most recent of these was a large multicenter implementation study by Escobar et al25 that included an extensive follow-up response. This study reported a significantly decreased 30-day mortality in the intervention cohort. A smaller randomized controlled trial reported no significant differences in patient outcomes with earlier warning alarms.27 A third study reported more appropriate rapid response team deployment and decreased mortality in a subgroup analysis.35
Effects, Facilitators, and Barriers
As shown in the Appendix Figure and further detailed in Table 3, the described effects were predominantly positive—57 positive effects vs 11 negative effects. These positive effects sorted primarily into the outcome and process domains.
All of the studies that compared their proposed model with one of various warning systems (eg, EWS, National Early Warning Score [NEWS], Modified Early Warning Score [MEWS]) showed superior performance (based on AUROC and reported predictive values). In 17 studies, the authors reported their model as more useful or superior to the EWS.20-23,26-28,34,36-41 Four studies reported real-time detection of deterioration before regular EWS,20,26,42 and three studies reported positive effects on patient-related outcomes.26,35 Four negative effects were noted on the controllability, validity, and potential limitations.27,42
Of the 38 remarks in the Technology domain, difficulty with implementation in daily practice was a commonly cited barrier.22,24,40,42 Difficulties included creating real-time data feeds out of the EMR, though there were mentions of some successful examples.25,27,36 Difficulty in the interpretability of AI was also considered a potential barrier.30,32,33,35,39,41 There were remarks as to the applicability of the prolonged prediction horizon because of the associated decoupling from the clinical view.39,42
Conservative attitudes toward new technologies and inadequate knowledge were mentioned as barriers.39 Repeated remarks were made on the difficulty of interpreting and responding to a predicted escalation, as the clinical pattern might not be recognizable at such an early stage. On the other hand, it is expected that less invasive countermeasures would be adequate to avert further escalation. Earlier recognition of possible escalations also raised potential ethical questions, such as when to discuss palliative care.24
The heterogeneity of the general ward population and the relatively low prevalence of deterioration were mentioned as barriers.24,30,38,41 There were also concerns that not all escalations are preventable and that some patient outcomes may not be modifiable.24,38
Many investigators expected reductions in false alarms and associated alarm fatigue (reflected as higher PPVs). Furthermore, they expected workflow to improve and workload to decrease.21,23,27,31,33,35,38,41 Despite the capacity of modern EMRs to store large amounts of patient data, some investigators felt improvements to real-time access, data quality and validity, and data density are needed to ensure valid associated predictions.21,22,24,32,37
DISCUSSION
As the complexity and comorbidity of hospitalized adults grow, predicting clinical deterioration is becoming more important. With an ever-increasing amount of available
There are several important limitations across these studies. In a clinical setting, these models would function as a screening test. Almost all studies report an AUROC; however, sensitivity and PPV or NNE (defined as 1/PPV) may be more useful than AUROC when predicting low-frequency events with high-potential clinical impact.44 Assessing the NNE is especially relevant because of its relation to alarm fatigue and responsiveness of clinicians.43 Alarm fatigue and lack of adequate response to alarms were repeatedly cited as potential barriers for application of automated scores.
Although the results of our scoping review are promising, there are limited data on clinical outcomes using these algorithms. Only three of five algorithms were used to guide clinical decision-making.25,27,35 Kollef et al27 showed shorter hospitalizations and Evans et al35 found decreased mortality rates in a multimorbid subgroup. Escobar et al25 found an overall and consistent decrease in mortality in a large, heterogenic population of inpatients across 21 hospitals. While Escobar et al’s findings provide strong evidence that predictive algorithms and structured follow-up on alarms can improve patient outcomes, it recognizes that not all facilities will have the resources to implement them.25 Dedicated round-the-clock follow-up of alarms has yet to be proven feasible for smaller institutions, and leaner solutions must be explored. The example set by Escobar et al25 should be translated into various settings to prove its reproducibility and to substantiate the clinical impact of predictive models and structured follow-up.
According to expert opinion, the use of high-frequency or continuous monitoring at low-acuity wards and AI algorithms to detect trends and patterns will reduce failure-to-rescue rates.4,9,43 However, most studies in our review focused on periodic spot-checked vital signs, and none of the AI algorithms were implemented in clinical care (Appendix Table 1
STRENGTHS AND LIMITATIONS
We performed a comprehensive review of the current literature using a clear and reproducible methodology to minimize the risk of missing relevant publications. The identified research is mainly limited to large US centers and consists of mostly retrospective studies. Heterogeneity among inputs, endpoints, time horizons, and evaluation metrics make comparisons challenging. Comments on facilitators, barriers, and effects were limited.
RECOMMENDATIONS FOR FUTURE RESEARCH
Artificial intelligence and the use of continuous monitoring hold great promise in creating optimal predictive algorithms. Future studies should directly compare AI- and non-AI-based algorithms using continuous monitoring to determine predictive accuracy, feasibility, costs, and outcomes. A consensus on endpoint definitions, input variables, methodology, and reporting is needed to enhance reproducibility, comparability, and generalizability of future research.
CONCLUSION
The early identification of clinical deterioration among adult hospitalized patients remains a challenge.1 Delayed identification is associated with increased morbidity and mortality, unplanned intensive care unit (ICU) admissions, prolonged hospitalization, and higher costs.2,3 Earlier detection of deterioration using predictive algorithms of vital sign monitoring might avoid these negative outcomes.4 In this scoping review, we summarize current algorithms and their evidence.
Vital signs provide the backbone for detecting clinical deterioration. Early warning scores (EWS) and outreach protocols were developed to bring structure to the assessment of vital signs. Most EWS claim to predict clinical end points such as unplanned ICU admission up to 24 hours in advance.5,6 Reviews of EWS showed a positive trend toward reduced length of stay and mortality. However, conclusions about general efficacy could not be generated because of case heterogeneity and methodologic shortcomings.4,7 Continuous automated vital sign monitoring of patients on the general ward can now be accomplished with wearable devices.8 The first reports on continuous monitoring showed earlier detection of deterioration but not improved clinical end points.4,9 Since then, different reports on continuous monitoring have shown positive effects but concluded that unprocessed monitoring data per se falls short of generating actionable alarms.4,10,11
Predictive algorithms, which often use artificial intelligence (AI), are increasingly employed to recognize complex patterns or abnormalities and support predictions of events in big data sets.12,13 Especially when combined with continuous vital sign monitoring, predictive algorithms have the potential to expedite detection of clinical deterioration and improve patient outcomes. Predictive algorithms using vital signs in the ICU have shown promising results.14 The impact of predictive algorithms on the general wards, however, is unclear.
The aims of our scoping review were to explore the extent and range of and evidence for predictive vital signs–based algorithms on the adult general ward; to describe the variety of these algorithms; and to categorize effects, facilitators, and barriers of their implementation.15
MATERIALS AND METHODS
We performed a scoping review to create a summary of the current state of research. We used the five-step method of Levac and followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses Extension for Scoping Reviews guidelines (Appendix 1).16,17
PubMed, Embase, and CINAHL databases were searched for English-language articles written between January 1, 2010, and November 20, 2020. We developed the search queries with an experienced information scientist, and we used database-specific terms and strategies for input, clinical outcome, method, predictive capability, and population (Appendix 2). Additionally, we searched the references of the selected articles, as well as publications citing these articles.
All studies identified were screened by title and abstract by two researchers (RP and YE). The selected studies were read in their entirety and checked for eligibility using the following inclusion criteria: automated algorithm; vital signs-based; real-time prediction; of clinical deterioration; in an adult, general ward population. In cases where there were successive publications with the same algorithm and population, we selected the most recent study.
For screening and selection, we used the Rayyan QCRI online tool (Qatar Computing Research Institute) and Endnote X9 (Clarivate Analytics). We extracted information using a data extraction form and organized it into descriptive characteristics of the selected studies (Table 1): an input data table showing number of admissions, intermittent or continuous measurements, vital signs measured, laboratory results (Appendix Table 1), a table summarizing study designs and settings (Appendix Table 2), and a prediction performance table (Table 2). We report characteristics of the populations and algorithms, prediction specifications such as area under the receiver operating curve (AUROC), and predictive values. Predictive values are affected by prevalence, which may differ among populations. To compare the algorithms, we calculated an indexed positive predictive value (PPV) and a number needed to evaluate (NNE) using a weighted average prevalence of clinical deterioration of 3.0%.


We defined clinical deterioration as end points, including rapid response team activation, cardiopulmonary resuscitation, transfer to an ICU, or death.
Effects, facilitators, and barriers were identified and categorized using ATLAS.ti 8 software (ATLAS.ti) and evaluated by three researchers (RP, MK, and THvdB). These were categorized using the adapted frameworks of Gagnon et al18 for the barriers and facilitators and of Donabedian19 for the effects (Appendix 3).
The Gagnon et al framework was adapted by changing two of four domains—that is, “Individual” was changed to “Professional” and “Human” to “Physiology.” The domains of “Technology” and “Organization” remained unchanged. The Donabedian domains of “Outcome,” “Process,” and “Structure” also remained unchanged (Table 3).

We divided the studies into two groups: studies on predictive algorithms with and without AI when reporting on characteristics and performance. For the secondary aim of exploring implementation impact, we reported facilitators and barriers in a narrative way, highlighting the most frequent and notable findings.
RESULTS
As shown in the Figure, we found 1741 publications, of which we read the full-text of 109. There were 1632 publications that did not meet the inclusion criteria. The publications by Churpek et al,20,21 Bartkiowak et al,22 Edelson et al,23 Escobar et al,24,25 and Kipnis et al26 reported on the same algorithms or databases but had significantly different approaches. For multiple publications using the same algorithm and population, the most recent was named with inclusion of the earlier findings.20,21,27-29 The resulting 21 papers are included in this review.

Descriptive characteristics of the studies are summarized in Table 1. Nineteen of the publications were full papers and two were conference abstracts. Most of the studies (n = 18) were from the United States; there was one study from South Korea,30 one study from Portugal,31 and one study from the United Kingdom.32 In 15 of the studies, there was a strict focus on general or specific wards; 6 studies also included the ICU and/or emergency departments.
Two of the studies were clinical trials, 2 were prospective observational studies, and 17 were retrospective studies. Five studies reported on an active predictive model during admission. Of these, 3 reported that the model was clinically implemented, using the predictions in their clinical workflow. None of the implemented studies used AI.
All input variables are presented in Appendix Table 1.
The non-AI algorithm prediction horizons ranged from 4 to 24 hours, with a median of 24 hours (interquartile range [IQR], 12-24 hours). The AI algorithms ranged from 2 to 48 hours and had a median horizon of 14 hours (IQR, 12-24 hours).
We found three studies reporting patient outcomes. The most recent of these was a large multicenter implementation study by Escobar et al25 that included an extensive follow-up response. This study reported a significantly decreased 30-day mortality in the intervention cohort. A smaller randomized controlled trial reported no significant differences in patient outcomes with earlier warning alarms.27 A third study reported more appropriate rapid response team deployment and decreased mortality in a subgroup analysis.35
Effects, Facilitators, and Barriers
As shown in the Appendix Figure and further detailed in Table 3, the described effects were predominantly positive—57 positive effects vs 11 negative effects. These positive effects sorted primarily into the outcome and process domains.
All of the studies that compared their proposed model with one of various warning systems (eg, EWS, National Early Warning Score [NEWS], Modified Early Warning Score [MEWS]) showed superior performance (based on AUROC and reported predictive values). In 17 studies, the authors reported their model as more useful or superior to the EWS.20-23,26-28,34,36-41 Four studies reported real-time detection of deterioration before regular EWS,20,26,42 and three studies reported positive effects on patient-related outcomes.26,35 Four negative effects were noted on the controllability, validity, and potential limitations.27,42
Of the 38 remarks in the Technology domain, difficulty with implementation in daily practice was a commonly cited barrier.22,24,40,42 Difficulties included creating real-time data feeds out of the EMR, though there were mentions of some successful examples.25,27,36 Difficulty in the interpretability of AI was also considered a potential barrier.30,32,33,35,39,41 There were remarks as to the applicability of the prolonged prediction horizon because of the associated decoupling from the clinical view.39,42
Conservative attitudes toward new technologies and inadequate knowledge were mentioned as barriers.39 Repeated remarks were made on the difficulty of interpreting and responding to a predicted escalation, as the clinical pattern might not be recognizable at such an early stage. On the other hand, it is expected that less invasive countermeasures would be adequate to avert further escalation. Earlier recognition of possible escalations also raised potential ethical questions, such as when to discuss palliative care.24
The heterogeneity of the general ward population and the relatively low prevalence of deterioration were mentioned as barriers.24,30,38,41 There were also concerns that not all escalations are preventable and that some patient outcomes may not be modifiable.24,38
Many investigators expected reductions in false alarms and associated alarm fatigue (reflected as higher PPVs). Furthermore, they expected workflow to improve and workload to decrease.21,23,27,31,33,35,38,41 Despite the capacity of modern EMRs to store large amounts of patient data, some investigators felt improvements to real-time access, data quality and validity, and data density are needed to ensure valid associated predictions.21,22,24,32,37
DISCUSSION
As the complexity and comorbidity of hospitalized adults grow, predicting clinical deterioration is becoming more important. With an ever-increasing amount of available
There are several important limitations across these studies. In a clinical setting, these models would function as a screening test. Almost all studies report an AUROC; however, sensitivity and PPV or NNE (defined as 1/PPV) may be more useful than AUROC when predicting low-frequency events with high-potential clinical impact.44 Assessing the NNE is especially relevant because of its relation to alarm fatigue and responsiveness of clinicians.43 Alarm fatigue and lack of adequate response to alarms were repeatedly cited as potential barriers for application of automated scores.
Although the results of our scoping review are promising, there are limited data on clinical outcomes using these algorithms. Only three of five algorithms were used to guide clinical decision-making.25,27,35 Kollef et al27 showed shorter hospitalizations and Evans et al35 found decreased mortality rates in a multimorbid subgroup. Escobar et al25 found an overall and consistent decrease in mortality in a large, heterogenic population of inpatients across 21 hospitals. While Escobar et al’s findings provide strong evidence that predictive algorithms and structured follow-up on alarms can improve patient outcomes, it recognizes that not all facilities will have the resources to implement them.25 Dedicated round-the-clock follow-up of alarms has yet to be proven feasible for smaller institutions, and leaner solutions must be explored. The example set by Escobar et al25 should be translated into various settings to prove its reproducibility and to substantiate the clinical impact of predictive models and structured follow-up.
According to expert opinion, the use of high-frequency or continuous monitoring at low-acuity wards and AI algorithms to detect trends and patterns will reduce failure-to-rescue rates.4,9,43 However, most studies in our review focused on periodic spot-checked vital signs, and none of the AI algorithms were implemented in clinical care (Appendix Table 1
STRENGTHS AND LIMITATIONS
We performed a comprehensive review of the current literature using a clear and reproducible methodology to minimize the risk of missing relevant publications. The identified research is mainly limited to large US centers and consists of mostly retrospective studies. Heterogeneity among inputs, endpoints, time horizons, and evaluation metrics make comparisons challenging. Comments on facilitators, barriers, and effects were limited.
RECOMMENDATIONS FOR FUTURE RESEARCH
Artificial intelligence and the use of continuous monitoring hold great promise in creating optimal predictive algorithms. Future studies should directly compare AI- and non-AI-based algorithms using continuous monitoring to determine predictive accuracy, feasibility, costs, and outcomes. A consensus on endpoint definitions, input variables, methodology, and reporting is needed to enhance reproducibility, comparability, and generalizability of future research.
CONCLUSION
- van Galen LS, Struik PW, Driesen BEJM, et al. Delayed recognition of deterioration of patients in general wards is mostly caused by human related monitoring failures: a root cause analysis of unplanned ICU admissions. PLoS One. 2016;11(8):e0161393. https://doi.org/10.1371/journal. pone.0161393
- Mardini L, Lipes J, Jayaraman D. Adverse outcomes associated with delayed intensive care consultation in medical and surgical inpatients. J Crit Care. 2012;27(6):688-693. https://doi.org/10.1016/j.jcrc.2012.04.011
- Young MP, Gooder VJ, McBride K, James B, Fisher ES. Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18(2):77-83. https://doi.org/10.1046/ j.1525-1497.2003.20441.x
- Khanna AK, Hoppe P, Saugel B. Automated continuous noninvasive ward monitoring: future directions and challenges. Crit Care. 2019;23(1):194. https://doi.org/10.1186/s13054-019-2485-7
- Ludikhuize J, Hamming A, de Jonge E, Fikkers BG. Rapid response systems in The Netherlands. Jt Comm J Qual Patient Saf. 2011;37(3):138-197. https:// doi.org/10.1016/s1553-7250(11)37017-1
- Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. https://doi.org/10.1097/01.ccm.0000254826.10520.87
- Alam N, Hobbelink EL, van Tienhoven AJ, van de Ven PM, Jansma EP, Nanayakkara PWB. The impact of the use of the Early Warning Score (EWS) on patient outcomes: a systematic review. Resuscitation. 2014;85(5):587-594. https://doi.org/10.1016/j.resuscitation.2014.01.013
- Weenk M, Koeneman M, van de Belt TH, Engelen LJLPG, van Goor H, Bredie SJH. Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation. 2019;136:47-53. https://doi.org/10.1016/j.resuscitation.2019.01.017
- Cardona-Morrell M, Prgomet M, Turner RM, Nicholson M, Hillman K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta-analysis. Int J Clin Pract. 2016;70(10):806-824. https://doi.org/10.1111/ijcp.12846
- Brown H, Terrence J, Vasquez P, Bates DW, Zimlichman E. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med. 2014;127(3):226-232. https://doi.org/10.1016/j.amjmed.2013.12.004
- Mestrom E, De Bie A, van de Steeg M, Driessen M, Atallah L, Bezemer R. Implementation of an automated early warning scoring system in a E8 Journal of Hospital Medicine® Published Online June 2021 An Official Publication of the Society of Hospital Medicine Peelen et al | Predicting Deterioration: A Scoping Review surgical ward: practical use and effects on patient outcomes. PLoS One. 2019;14(5):e0213402. https://doi.org/10.1371/journal.pone.0213402
- Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. https://doi.org/10.1136/ svn-2017-000101
- Iwashyna TJ, Liu V. What’s so different about big data? A primer for clinicians trained to think epidemiologically. Ann Am Thorac Soc. 2014;11(7):1130- 1135. https://doi.org/10.1513/annalsats.201405-185as
- Jalali A, Bender D, Rehman M, Nadkanri V, Nataraj C. Advanced analytics for outcome prediction in intensive care units. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:2520-2524. https://doi.org/10.1109/embc.2016.7591243
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. https://doi.org/10.1186/s12874-018-0611-x
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19-32. https://doi.org/10.1080/13645 57032000119616
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMAScR): checklist and explanation. Ann Intern Med. 2018;169(7):467- 473. https://doi.org/10.7326/m18-0850
- Gagnon MP, Desmartis M, Gagnon J, et al. Framework for user involvement in health technology assessment at the local level: views of health managers, user representatives, and clinicians. Int J Technol Assess Health Care. 2015;31(1-2):68-77. https://doi.org/10.1017/s0266462315000070
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. https://doi.org/10.1001/jama.260.12.1743
- Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649-655. https://doi.org/10.1164/rccm.201406-1022oc
- Churpek MM, Yuen TC, Winslow C, Meltzer DO, Kattan MW, Edelson DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368-374. https://doi.org/10.1097/ccm.0000000000001571
- Bartkowiak B, Snyder AM, Benjamin A, et al. Validating the electronic cardiac arrest risk triage (eCART) score for risk stratification of surgical inpatients in the postoperative setting: retrospective cohort study. Ann Surg. 2019;269(6):1059-1063. https://doi.org/10.1097/sla.0000000000002665
- Edelson DP, Carey K, Winslow CJ, Churpek MM. Less is more: detecting clinical deterioration in the hospital with machine learning using only age, heart rate and respiratory rate. Abstract presented at: American Thoracic Society International Conference; May 22, 2018; San Diego, California. Am J Resp Crit Care Med. 2018;197:A4444.
- Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. https:// doi.org/10.1002/jhm.1929
- Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/nejmsa2001090
- Kipnis P, Turk BJ, Wulf DA, et al. Development and validation of an electronic medical record-based alert score for detection of inpatient deterioration outside the ICU. J Biomed Inform. 2016;64:10-19. https://doi.org/10.1016/j. jbi.2016.09.013
- Kollef MH, Chen Y, Heard K, et al. A randomized trial of real-time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424-429. https://doi.org/10.1002/jhm.2193
- Hackmann G, Chen M, Chipara O, et al. Toward a two-tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511-519.
- Bailey TC, Chen Y, Mao Y, Lu, C, Hackmann G, Micek ST. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8(5):236-242. https://doi.org/10.1002/jhm.2009
- Kwon JM, Lee Y, Lee Y, Lee S, Park J. An algorithm based on deep learning for predicting in-hospital cardiac arrest. J Am Heart Assoc. 2018;7(13):e008678. https://doi.org/10.1161/jaha.118.008678
- Correia S, Gomes A, Shahriari S, Almeida JP, Severo M, Azevedo A. Performance of the early warning system vital to predict unanticipated higher-level of care admission and in-hospital death of ward patients. Value Health. 2018;21(S3):S360. https://doi.org/10.1016/j.jval.2018.09.2152
- Shamout FE, Zhu T, Sharma P, Watkinson PJ, Clifton DA. Deep interpretable early warning system for the detection of clinical deterioration. IEEE J Biomed Health Inform. 2020;24(2):437-446. https://doi.org/10.1109/ jbhi.2019.2937803
- Bai Y, Do DH, Harris PRE, et al. Integrating monitor alarms with laboratory test results to enhance patient deterioration prediction. J Biomed Inform. 2015;53:81-92. https://doi.org/10.1016/j.jbi.2014.09.006
- Hu X, Sapo M, Nenov V, et al. Predictive combinations of monitor alarms preceding in-hospital code blue events. J Biomed Inform. 2012;45(5):913-921. https://doi.org/10.1016/j.jbi.2012.03.001
- Evans RS, Kuttler KG, Simpson KJ, et al. Automated detection of physiologic deterioration in hospitalized patients. J Am Med Inform Assoc. 2015;22(2):350-360. https://doi.org/10.1136/amiajnl-2014-002816
- Ghosh E, Eshelman L, Yang L, Carlson E, Lord B. Early deterioration indicator: data-driven approach to detecting deterioration in general ward. Resuscitation. 2018;122:99-105. https://doi.org/10.1016/j.resuscitation. 2017.10.026
- Kang MA, Churpek MM, Zadravecz FJ, Adhikari R, Twu NM, Edelson DP: Real-time risk prediction on the wards: a feasibility study. Crit Care Med. 2016;44(8):1468-1473. https://doi.org/10.1097/ccm.0000000000001716
- Hu SB, Wong DJL, Correa A, Li N, Deng JC. Prediction of clinical deterioration in hospitalized adult patients with hematologic malignancies using a neural network model. PLoS One. 2016;11(8):e0161401. https://doi. org/10.1371/journal.pone.0161401
- Rothman MJ, Rothman SI, Beals J 4th. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848. https://doi.org/10.1016/j. jbi.2013.06.011
- Alaa AM, Yoon J, Hu S, van der Schaar M. Personalized risk scoring for critical care prognosis using mixtures of Gaussian processes. IEEE Trans Biomed Eng. 2018;65(1):207-218. https://doi.org/10.1109/tbme.2017.2698602
- Mohamadlou H, Panchavati S, Calvert J, et al. Multicenter validation of a machine-learning algorithm for 48-h all-cause mortality prediction. Health Informatics J. 2020;26(3):1912-1925. https://doi.org/10.1177/1460458219894494
- Alvarez CA, Clark CA, Zhang S, et al. Predicting out of intensive care unit cardiopulmonary arrest or death using electronic medical record data. BMC Med Inform Decis Mak. 2013;13:28. https://doi.org/10.1186/1472-6947-13-28
- Vincent JL, Einav S, Pearse R, et al. Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol. 2018;35(5):325-333. https://doi.org/10.1097/eja.0000000000000798
- Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
- Weenk M, Bredie SJ, Koeneman M, Hesselink G, van Goor H, van de Belt TH. Continuous monitoring of the vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res. 2020;22(6):e15471. https://doi.org/10.2196/15471
- Wellner B, Grand J, Canzone E, et al. Predicting unplanned transfers to the intensive care unit: a machine learning approach leveraging diverse clinical elements. JMIR Med Inform. 2017;5(4):e45. https://doi.org/10.2196/medinform.8680
- Elliott M, Baird J. Pulse oximetry and the enduring neglect of respiratory rate assessment: a commentary on patient surveillance. Br J Nurs. 2019;28(19):1256-1259. https://doi.org/10.12968/bjon.2019.28.19.1256
- Blackwell JN, Keim-Malpass J, Clark MT, et al. Early detection of in-patient deterioration: one prediction model does not fit all. Crit Care Explor. 2020;2(5):e0116. https://doi.org/10.1097/cce.0000000000000116
- Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. https://doi.org/10.1038/sdata.2016.35
- Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. https://doi. org/10.1370/afm.1713
- Kirkland LL, Malinchoc M, O’Byrne M, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142 https://doi.org/10.1177/1062860612450459
- van Galen LS, Struik PW, Driesen BEJM, et al. Delayed recognition of deterioration of patients in general wards is mostly caused by human related monitoring failures: a root cause analysis of unplanned ICU admissions. PLoS One. 2016;11(8):e0161393. https://doi.org/10.1371/journal. pone.0161393
- Mardini L, Lipes J, Jayaraman D. Adverse outcomes associated with delayed intensive care consultation in medical and surgical inpatients. J Crit Care. 2012;27(6):688-693. https://doi.org/10.1016/j.jcrc.2012.04.011
- Young MP, Gooder VJ, McBride K, James B, Fisher ES. Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18(2):77-83. https://doi.org/10.1046/ j.1525-1497.2003.20441.x
- Khanna AK, Hoppe P, Saugel B. Automated continuous noninvasive ward monitoring: future directions and challenges. Crit Care. 2019;23(1):194. https://doi.org/10.1186/s13054-019-2485-7
- Ludikhuize J, Hamming A, de Jonge E, Fikkers BG. Rapid response systems in The Netherlands. Jt Comm J Qual Patient Saf. 2011;37(3):138-197. https:// doi.org/10.1016/s1553-7250(11)37017-1
- Cuthbertson BH, Boroujerdi M, McKie L, Aucott L, Prescott G. Can physiological variables and early warning scoring systems allow early recognition of the deteriorating surgical patient? Crit Care Med. 2007;35(2):402-409. https://doi.org/10.1097/01.ccm.0000254826.10520.87
- Alam N, Hobbelink EL, van Tienhoven AJ, van de Ven PM, Jansma EP, Nanayakkara PWB. The impact of the use of the Early Warning Score (EWS) on patient outcomes: a systematic review. Resuscitation. 2014;85(5):587-594. https://doi.org/10.1016/j.resuscitation.2014.01.013
- Weenk M, Koeneman M, van de Belt TH, Engelen LJLPG, van Goor H, Bredie SJH. Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation. 2019;136:47-53. https://doi.org/10.1016/j.resuscitation.2019.01.017
- Cardona-Morrell M, Prgomet M, Turner RM, Nicholson M, Hillman K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta-analysis. Int J Clin Pract. 2016;70(10):806-824. https://doi.org/10.1111/ijcp.12846
- Brown H, Terrence J, Vasquez P, Bates DW, Zimlichman E. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med. 2014;127(3):226-232. https://doi.org/10.1016/j.amjmed.2013.12.004
- Mestrom E, De Bie A, van de Steeg M, Driessen M, Atallah L, Bezemer R. Implementation of an automated early warning scoring system in a E8 Journal of Hospital Medicine® Published Online June 2021 An Official Publication of the Society of Hospital Medicine Peelen et al | Predicting Deterioration: A Scoping Review surgical ward: practical use and effects on patient outcomes. PLoS One. 2019;14(5):e0213402. https://doi.org/10.1371/journal.pone.0213402
- Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. https://doi.org/10.1136/ svn-2017-000101
- Iwashyna TJ, Liu V. What’s so different about big data? A primer for clinicians trained to think epidemiologically. Ann Am Thorac Soc. 2014;11(7):1130- 1135. https://doi.org/10.1513/annalsats.201405-185as
- Jalali A, Bender D, Rehman M, Nadkanri V, Nataraj C. Advanced analytics for outcome prediction in intensive care units. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:2520-2524. https://doi.org/10.1109/embc.2016.7591243
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. https://doi.org/10.1186/s12874-018-0611-x
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19-32. https://doi.org/10.1080/13645 57032000119616
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMAScR): checklist and explanation. Ann Intern Med. 2018;169(7):467- 473. https://doi.org/10.7326/m18-0850
- Gagnon MP, Desmartis M, Gagnon J, et al. Framework for user involvement in health technology assessment at the local level: views of health managers, user representatives, and clinicians. Int J Technol Assess Health Care. 2015;31(1-2):68-77. https://doi.org/10.1017/s0266462315000070
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. https://doi.org/10.1001/jama.260.12.1743
- Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649-655. https://doi.org/10.1164/rccm.201406-1022oc
- Churpek MM, Yuen TC, Winslow C, Meltzer DO, Kattan MW, Edelson DP. Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med. 2016;44(2):368-374. https://doi.org/10.1097/ccm.0000000000001571
- Bartkowiak B, Snyder AM, Benjamin A, et al. Validating the electronic cardiac arrest risk triage (eCART) score for risk stratification of surgical inpatients in the postoperative setting: retrospective cohort study. Ann Surg. 2019;269(6):1059-1063. https://doi.org/10.1097/sla.0000000000002665
- Edelson DP, Carey K, Winslow CJ, Churpek MM. Less is more: detecting clinical deterioration in the hospital with machine learning using only age, heart rate and respiratory rate. Abstract presented at: American Thoracic Society International Conference; May 22, 2018; San Diego, California. Am J Resp Crit Care Med. 2018;197:A4444.
- Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7(5):388-395. https:// doi.org/10.1002/jhm.1929
- Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/nejmsa2001090
- Kipnis P, Turk BJ, Wulf DA, et al. Development and validation of an electronic medical record-based alert score for detection of inpatient deterioration outside the ICU. J Biomed Inform. 2016;64:10-19. https://doi.org/10.1016/j. jbi.2016.09.013
- Kollef MH, Chen Y, Heard K, et al. A randomized trial of real-time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014;9(7):424-429. https://doi.org/10.1002/jhm.2193
- Hackmann G, Chen M, Chipara O, et al. Toward a two-tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511-519.
- Bailey TC, Chen Y, Mao Y, Lu, C, Hackmann G, Micek ST. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medical wards. J Hosp Med. 2013;8(5):236-242. https://doi.org/10.1002/jhm.2009
- Kwon JM, Lee Y, Lee Y, Lee S, Park J. An algorithm based on deep learning for predicting in-hospital cardiac arrest. J Am Heart Assoc. 2018;7(13):e008678. https://doi.org/10.1161/jaha.118.008678
- Correia S, Gomes A, Shahriari S, Almeida JP, Severo M, Azevedo A. Performance of the early warning system vital to predict unanticipated higher-level of care admission and in-hospital death of ward patients. Value Health. 2018;21(S3):S360. https://doi.org/10.1016/j.jval.2018.09.2152
- Shamout FE, Zhu T, Sharma P, Watkinson PJ, Clifton DA. Deep interpretable early warning system for the detection of clinical deterioration. IEEE J Biomed Health Inform. 2020;24(2):437-446. https://doi.org/10.1109/ jbhi.2019.2937803
- Bai Y, Do DH, Harris PRE, et al. Integrating monitor alarms with laboratory test results to enhance patient deterioration prediction. J Biomed Inform. 2015;53:81-92. https://doi.org/10.1016/j.jbi.2014.09.006
- Hu X, Sapo M, Nenov V, et al. Predictive combinations of monitor alarms preceding in-hospital code blue events. J Biomed Inform. 2012;45(5):913-921. https://doi.org/10.1016/j.jbi.2012.03.001
- Evans RS, Kuttler KG, Simpson KJ, et al. Automated detection of physiologic deterioration in hospitalized patients. J Am Med Inform Assoc. 2015;22(2):350-360. https://doi.org/10.1136/amiajnl-2014-002816
- Ghosh E, Eshelman L, Yang L, Carlson E, Lord B. Early deterioration indicator: data-driven approach to detecting deterioration in general ward. Resuscitation. 2018;122:99-105. https://doi.org/10.1016/j.resuscitation. 2017.10.026
- Kang MA, Churpek MM, Zadravecz FJ, Adhikari R, Twu NM, Edelson DP: Real-time risk prediction on the wards: a feasibility study. Crit Care Med. 2016;44(8):1468-1473. https://doi.org/10.1097/ccm.0000000000001716
- Hu SB, Wong DJL, Correa A, Li N, Deng JC. Prediction of clinical deterioration in hospitalized adult patients with hematologic malignancies using a neural network model. PLoS One. 2016;11(8):e0161401. https://doi. org/10.1371/journal.pone.0161401
- Rothman MJ, Rothman SI, Beals J 4th. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848. https://doi.org/10.1016/j. jbi.2013.06.011
- Alaa AM, Yoon J, Hu S, van der Schaar M. Personalized risk scoring for critical care prognosis using mixtures of Gaussian processes. IEEE Trans Biomed Eng. 2018;65(1):207-218. https://doi.org/10.1109/tbme.2017.2698602
- Mohamadlou H, Panchavati S, Calvert J, et al. Multicenter validation of a machine-learning algorithm for 48-h all-cause mortality prediction. Health Informatics J. 2020;26(3):1912-1925. https://doi.org/10.1177/1460458219894494
- Alvarez CA, Clark CA, Zhang S, et al. Predicting out of intensive care unit cardiopulmonary arrest or death using electronic medical record data. BMC Med Inform Decis Mak. 2013;13:28. https://doi.org/10.1186/1472-6947-13-28
- Vincent JL, Einav S, Pearse R, et al. Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol. 2018;35(5):325-333. https://doi.org/10.1097/eja.0000000000000798
- Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
- Weenk M, Bredie SJ, Koeneman M, Hesselink G, van Goor H, van de Belt TH. Continuous monitoring of the vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res. 2020;22(6):e15471. https://doi.org/10.2196/15471
- Wellner B, Grand J, Canzone E, et al. Predicting unplanned transfers to the intensive care unit: a machine learning approach leveraging diverse clinical elements. JMIR Med Inform. 2017;5(4):e45. https://doi.org/10.2196/medinform.8680
- Elliott M, Baird J. Pulse oximetry and the enduring neglect of respiratory rate assessment: a commentary on patient surveillance. Br J Nurs. 2019;28(19):1256-1259. https://doi.org/10.12968/bjon.2019.28.19.1256
- Blackwell JN, Keim-Malpass J, Clark MT, et al. Early detection of in-patient deterioration: one prediction model does not fit all. Crit Care Explor. 2020;2(5):e0116. https://doi.org/10.1097/cce.0000000000000116
- Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. https://doi.org/10.1038/sdata.2016.35
- Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. https://doi. org/10.1370/afm.1713
- Kirkland LL, Malinchoc M, O’Byrne M, et al. A clinical deterioration prediction tool for internal medicine patients. Am J Med Qual. 2013;28(2):135-142 https://doi.org/10.1177/1062860612450459
Reducing Overuse of Proton Pump Inhibitors for Stress Ulcer Prophylaxis and Nonvariceal Gastrointestinal Bleeding in the Hospital: A Narrative Review and Implementation Guide
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).
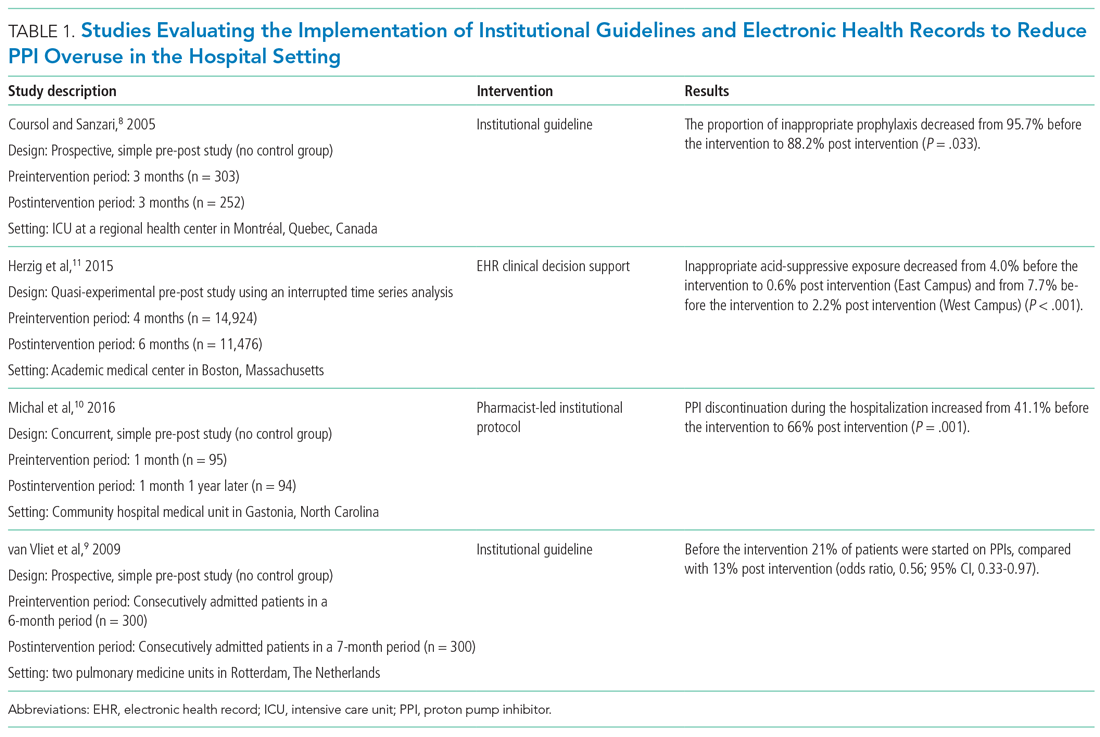
Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.
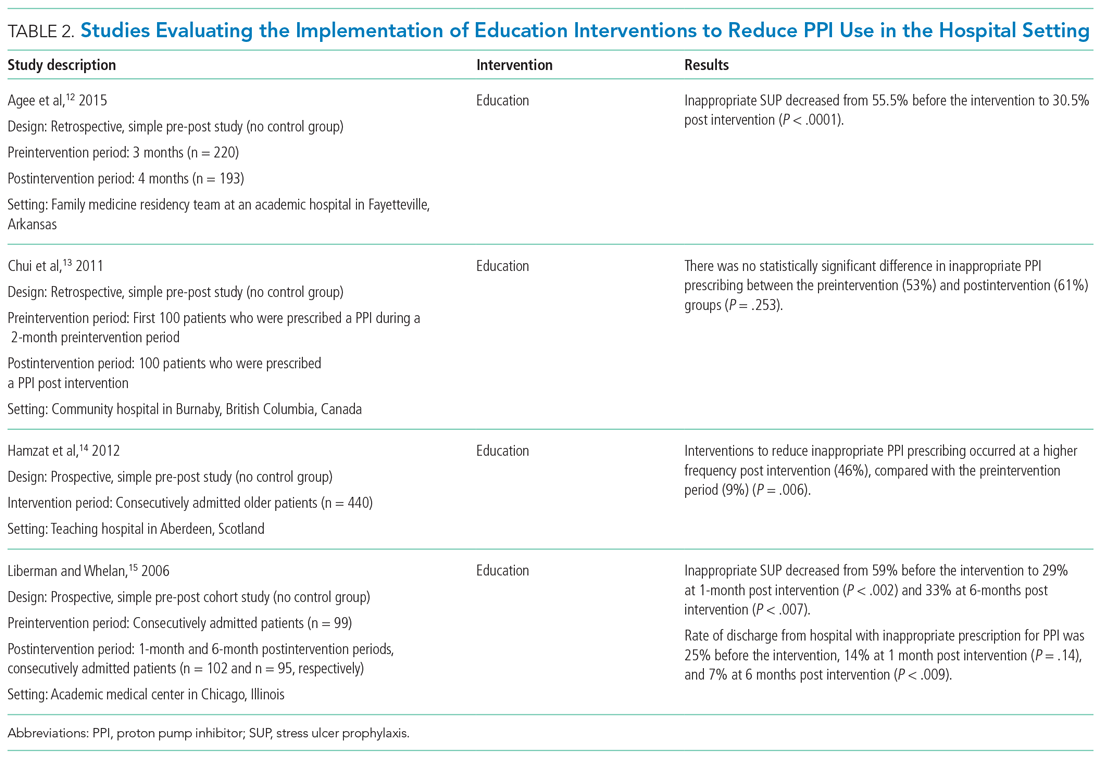
Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.
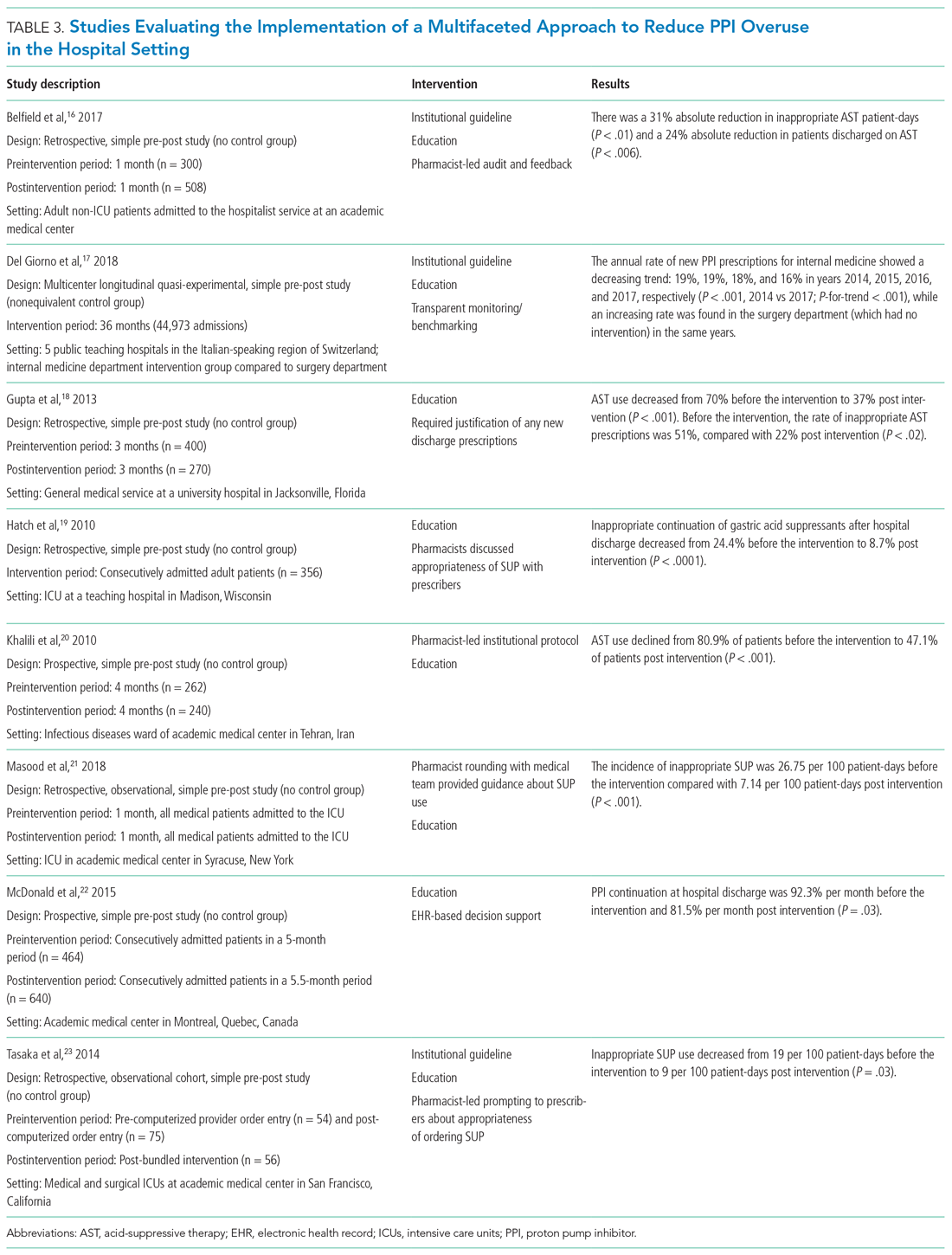
Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
© 2021 Society of Hospital Medicine
4 tips for working with caregivers of children with somatic disorders
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
Pharmacogenetic testing: Navigating through the confusion
Mr. J, age 30, a Black man with major depressive disorder (MDD), has been your patient for the past year. At the time of his diagnosis, Mr. J received sertraline, 100 mg/d, but had little to no improvement. During the past year, he received trials of citalopram and paroxetine, but they were not effective for his recurrent depressive symptoms and/or resulted in significant adverse effects.
During a recent visit, Mr. J asks you about “the genetic tests that help determine which medications will work.” He mentions that his brother had this testing done and that it had “worked for him,” but offers no other details. You research the different testing panels to see which test you might use. After a brief online review, you identify at least 4 different products, and are not sure which test—if any—you should consider.
During the last few years, there has been a rise in commercial pharmacogenetic testing options, including tests available to clinicians at academic medical centers as well as direct-to-consumer testing (Table). Clinician and patient interest regarding pharmacogenetic testing in practice is often followed by the question, “Which test is best?” Although this is a logical question, providing an answer is multifactorial.1-3 Because none of the currently available tests have been compared in head-to-head clinical trials, it is nearly impossible to identify the “best” test.
In this article, we focus on the evidence-based principles that clinicians should consider when adopting pharmacogenetic testing in their practice. We discuss which genes are of most interest when prescribing psychotropic medications, the value of decision support tools, cost considerations, and patient education regarding this type of testing.
Which genes and variants should be tested?
The genes relevant to medication treatment outcomes can be broadly classified into those with pharmacokinetic vs pharmacodynamic effects. Pharmacogenes, such as those coding for the drug-metabolizing enzymes cytochrome P450 (CYP) 1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and UDP-glucuronosyltransferase (UGT)2B1, may alter the rate at which medications are metabolized, thus varying the serum drug concentration across patients. Variants that impact the function of these enzymes are considered pharmacokinetic. Up to 40% of the variance in patients’ response to antidepressants may be due to variations in the pharmacokinetic genes.4 Alternatively, pharmacodynamic pharmacogenes impact drug action and therefore may affect the degree of receptor activation at a given drug concentration, overall drug efficacy, and/or the occurrence of medication sensitivity. These pharmacogenes may include:
- brain-derived neurotrophic factor (BDNF)
- catechol-O-methyltransferase (COMT)
- human leukocyte antigens A (HLA-A)
- serotonin receptor subtype 2 (HTR2)
- serotonin receptor subtype 2C (HTR2C)
- opioid receptor mu 1 (OPRM1)
- solute carrier family 6 member 4 (SLC6A4).
In articles previously published in
Currently, there is no standardization among commercial pharmacogenetic tests on:
- which genes to test
- which variants specific to a gene need to be included
- how the genetic data is translated to phenotype
- how the phenotype is translated to a treatment recommendation.
Continue to: Due to these factors...
Due to these factors, the FDA has advised clinicians to consult the dosing recommendations provided in a medication’s package insert for information regarding how genetic information should be used in making treatment decisions.2
The value of decision support tools
Researchers have assessed how various manufacturers’ decision support tools (DSTs) (ie, the reports the commercial testing companies send to the clinician who orders the test) agree on genotypes, predicted phenotypes, and medication recommendations.4 Overall, this research found varying levels of disagreement in the medication recommendations of the testing panels they studied, which indicates that not all tests are equivalent or interchangeable.4 Of the actionable recommendations for antidepressants, 16% were conflicting; the recommendations for fluoxetine and imipramine were most frequently in disagreement.4 Similarly, 20% of the actionable antipsychotic advice was conflicting, with the recommendations for aripiprazole and clozapine most frequently in disagreement.4 Researchers also reported a situation in which 4 testing panels agreed on the patient’s phenotyping status for CYP2C19, but the dosing recommendations provided for the CYP2C19 substrate, amitriptyline, differed.4 Thus, it is understandable why DSTs can result in confusion, and why clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Additionally, while the genes included on these panels vary, these testing panels also may not evaluate the same variants within a specific gene. These differences may impact the patient’s reported phenotypes and medication recommendations across DSTs. For example, the FDA has recommended HLA gene testing prior to prescribing carbamazepine. However, few of the available tests may include the HLA-B*15:02 variant, which has been associated with carbamazepine-induced severe cutaneous reactions in patients of Asian descent, and fewer may include the HLA-A*31:01 variant, for which testing is recommended prior to prescribing carbamazepine in patients of Caucasian descent.4 Additionally, some of the CYP enzymes—such as CYP2D6*17 and CYP2C19*3 variants, which may be more common in certain populations of patients who are members of ethnic or racial minority groups—may not be consistently included in the various panels. Thus, before deciding on a specific test, clinicians should understand which gene variants are relevant to their patients with regard to race and ethnicity, and key variants for specific medications. Clinicians should refer to FDA guidance and the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines to determine the appropriate interpretations of genetic test results.1,2
Despite the disagreement in recommendations from the various testing companies, DSTs are useful and have been shown to facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. A recently published meta-analysis of randomized controlled trials (RCTs) of pharmacogenetic testing found that DSTs improved symptom remission among individuals with MDD by 70%.5 This suggests that pharmacogenetic-guided DSTs may provide superior treatment compared with treatment for DSTs were not used. However, the RCTs in this meta-analysis only included patients who had previously failed an antidepressant trial.5 Therefore, it is currently unknown at what point in care DSTs should be used, and whether they would be more beneficial if they are used when starting a new therapy, or after several trials have failed.
Consider the cost
The cost and availability of pharmacogenetic testing can be an issue when making treatment decisions, and such testing may not be covered by a patient’s insurance plan. Recently, the Centers for Medicare & Medicaid Services announced that Medicare would cover FDA-approved genomic tests that encompass broad gene panels if the evidence supports their use. Similarly, commercial insurers such as UnitedHealthcare have begun to cover some pharmacogenetic tests.6 Medicare or Medicaid plans cover some testing panels’ costs and patients do not incur any out-of-pocket costs; however, some private insurance companies require patients to pay at least a portion of the cost, and many companies offer financial assistance for patients based on income and other factors. Although financial coverage for testing has improved, patients may still face out-of-pocket costs; therefore, clinicians may need to weigh the benefits of pharmacogenetic testing vs its cost.7 Clinicians should also determine what timeline best suits their patient’s financial and clinical needs, and test accordingly.
Continue to: Patient education is critical
Patient education is critical
Although the benefits of using pharmacogenetic testing information when making certain treatment decisions is promising, it is important for both patients and clinicians to understand that test results do not always change therapy. A study on the impact of pharmacogenetic testing on clinical outcomes of patients with MDD found that 79% of patients were already prescribed medications that aligned with recommendations.8 Therefore, switching medications based on the test results of a patient who is doing well clinically is not recommended. However, DSTs may help with clinical decisions for ambiguous cases. For example, if a patient has a genotype and/or phenotype that aligns with medication recommendations, the DST might not be able to identify a better medication to use, but may be able to recommend dosing guidance to improve the tolerability of the patient’s current therapy.6 It is also important to understand that the results of such testing may have a broader use beyond the initial reason for obtaining testing, such as when prescribing a common blood thinner such as warfarin or clopidogrel. However, for many of the pharmacodynamic genes that are included in these panels, their use beyond the treatment of depression may be limited because outcome studies for pharmacodynamic pharmacogenes may vary based on psychiatric diagnosis. Regardless, it may be beneficial to securely save and store patient test results in a standardized place within the medical record for future use.
CASE CONTINUED
You work with Mr. J to help him understand the benefits and limitations associated with pharmacogenetic testing. Assuming Mr. J is comfortable with the costs of obtaining testing, you contact the testing companies you identified to determine the specific pharmacogene variants included on each of these panels, and which would be the most appropriate given his race. If the decision is made to order the testing, provide Mr. J with a copy of his testing report so that he can use this information should he need any additional pharmacotherapy in the future, and also maintain a copy in his patient records using a standardized location for easy future access. If Mr. J is not comfortable with the costs associated with the testing, find out which medication his brother is currently receiving for treatment; this information may help identify a treatment plan for Mr. J.
Impact on practice
As psychiatry continues to gain experience in using pharmacogenetic testing and DSTs to help guide treatments for depression and other disorders, clinicians need to learn about these tools and how to use an evidence-based approach to best implement them in their practice. Many academic medical centers have developed continuing education programs or consult services to help with this.9,10 Just as the choice of which medication to use may be based partly on clinician experience, so too may be which pharmacogenetic test to use.
Bottom Line
Pharmacogenetic tests have not been examined in head-to-head clinical trials, which makes it nearly impossible to identify which test is best to use. Although the testing companies’ decision support tools (DSTs) often disagree in their recommendations, research has shown that using DSTs can facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. Clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Related Resources
- PGx Gene-specific information tables. www.pharmgkb.org/page/pgxGeneRef
- Clinical Pharmacogenetics Implementation Consortium. https://cpicpgx.org/guidelines/
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Fluoxetine • Prozac
Imipramine • Tofranil
Paroxetine • Paxil
Sertraline • Zoloft
Warfarin • Coumadin, Jantoven
1. Ellingrod, VL. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
2. Ellingrod VL. Pharmacogenomics testing: what the FDA says. Current Psychiatry. 2019;18(4):29-33.
3. Ramsey LB. Pharmacogenetic testing in children: what to test and how to use it. Current Psychiatry. 2018;17(9):30-36.
4. Bousman CA, Dunlop BW. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. The Pharmacogenomics Journal. 2018;18(5):613-622. doi:10.1038/s41397-018-0027-3
5. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1). doi:10.2217/pgs-2018-0142
6. Nicholson WT, Formea CM, Matey ET, et al. Considerations when applying pharmacogenomics to your practice. Mayo Clin Proc. 2021;96(1);218-230. doi:10.1016/j.mayocp.2020.03.011
7. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Human Genomics. 2019;13(1). doi:10.1186/s40246-019-0229-z
8. Greden JF, Parikh S, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111;59-67. doi:10.1016/j.jpsychires.2019.01.003
9. Haga SB. Integrating pharmacogenetic testing into primary care. Expert Review of Precision Medicine and Drug Development. 2017;2(6):327-336. doi:10.1080/23808993.2017.1398046
10. Ward KM, Taubman DS, Pasternak AL, et al. Teaching psychiatric pharmacogenomics effectively: evaluation of a novel interprofessional online course. J Am Coll Clin Pharm. 2021; 4:176-183.
Mr. J, age 30, a Black man with major depressive disorder (MDD), has been your patient for the past year. At the time of his diagnosis, Mr. J received sertraline, 100 mg/d, but had little to no improvement. During the past year, he received trials of citalopram and paroxetine, but they were not effective for his recurrent depressive symptoms and/or resulted in significant adverse effects.
During a recent visit, Mr. J asks you about “the genetic tests that help determine which medications will work.” He mentions that his brother had this testing done and that it had “worked for him,” but offers no other details. You research the different testing panels to see which test you might use. After a brief online review, you identify at least 4 different products, and are not sure which test—if any—you should consider.
During the last few years, there has been a rise in commercial pharmacogenetic testing options, including tests available to clinicians at academic medical centers as well as direct-to-consumer testing (Table). Clinician and patient interest regarding pharmacogenetic testing in practice is often followed by the question, “Which test is best?” Although this is a logical question, providing an answer is multifactorial.1-3 Because none of the currently available tests have been compared in head-to-head clinical trials, it is nearly impossible to identify the “best” test.
In this article, we focus on the evidence-based principles that clinicians should consider when adopting pharmacogenetic testing in their practice. We discuss which genes are of most interest when prescribing psychotropic medications, the value of decision support tools, cost considerations, and patient education regarding this type of testing.
Which genes and variants should be tested?
The genes relevant to medication treatment outcomes can be broadly classified into those with pharmacokinetic vs pharmacodynamic effects. Pharmacogenes, such as those coding for the drug-metabolizing enzymes cytochrome P450 (CYP) 1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and UDP-glucuronosyltransferase (UGT)2B1, may alter the rate at which medications are metabolized, thus varying the serum drug concentration across patients. Variants that impact the function of these enzymes are considered pharmacokinetic. Up to 40% of the variance in patients’ response to antidepressants may be due to variations in the pharmacokinetic genes.4 Alternatively, pharmacodynamic pharmacogenes impact drug action and therefore may affect the degree of receptor activation at a given drug concentration, overall drug efficacy, and/or the occurrence of medication sensitivity. These pharmacogenes may include:
- brain-derived neurotrophic factor (BDNF)
- catechol-O-methyltransferase (COMT)
- human leukocyte antigens A (HLA-A)
- serotonin receptor subtype 2 (HTR2)
- serotonin receptor subtype 2C (HTR2C)
- opioid receptor mu 1 (OPRM1)
- solute carrier family 6 member 4 (SLC6A4).
In articles previously published in
Currently, there is no standardization among commercial pharmacogenetic tests on:
- which genes to test
- which variants specific to a gene need to be included
- how the genetic data is translated to phenotype
- how the phenotype is translated to a treatment recommendation.
Continue to: Due to these factors...
Due to these factors, the FDA has advised clinicians to consult the dosing recommendations provided in a medication’s package insert for information regarding how genetic information should be used in making treatment decisions.2
The value of decision support tools
Researchers have assessed how various manufacturers’ decision support tools (DSTs) (ie, the reports the commercial testing companies send to the clinician who orders the test) agree on genotypes, predicted phenotypes, and medication recommendations.4 Overall, this research found varying levels of disagreement in the medication recommendations of the testing panels they studied, which indicates that not all tests are equivalent or interchangeable.4 Of the actionable recommendations for antidepressants, 16% were conflicting; the recommendations for fluoxetine and imipramine were most frequently in disagreement.4 Similarly, 20% of the actionable antipsychotic advice was conflicting, with the recommendations for aripiprazole and clozapine most frequently in disagreement.4 Researchers also reported a situation in which 4 testing panels agreed on the patient’s phenotyping status for CYP2C19, but the dosing recommendations provided for the CYP2C19 substrate, amitriptyline, differed.4 Thus, it is understandable why DSTs can result in confusion, and why clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Additionally, while the genes included on these panels vary, these testing panels also may not evaluate the same variants within a specific gene. These differences may impact the patient’s reported phenotypes and medication recommendations across DSTs. For example, the FDA has recommended HLA gene testing prior to prescribing carbamazepine. However, few of the available tests may include the HLA-B*15:02 variant, which has been associated with carbamazepine-induced severe cutaneous reactions in patients of Asian descent, and fewer may include the HLA-A*31:01 variant, for which testing is recommended prior to prescribing carbamazepine in patients of Caucasian descent.4 Additionally, some of the CYP enzymes—such as CYP2D6*17 and CYP2C19*3 variants, which may be more common in certain populations of patients who are members of ethnic or racial minority groups—may not be consistently included in the various panels. Thus, before deciding on a specific test, clinicians should understand which gene variants are relevant to their patients with regard to race and ethnicity, and key variants for specific medications. Clinicians should refer to FDA guidance and the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines to determine the appropriate interpretations of genetic test results.1,2
Despite the disagreement in recommendations from the various testing companies, DSTs are useful and have been shown to facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. A recently published meta-analysis of randomized controlled trials (RCTs) of pharmacogenetic testing found that DSTs improved symptom remission among individuals with MDD by 70%.5 This suggests that pharmacogenetic-guided DSTs may provide superior treatment compared with treatment for DSTs were not used. However, the RCTs in this meta-analysis only included patients who had previously failed an antidepressant trial.5 Therefore, it is currently unknown at what point in care DSTs should be used, and whether they would be more beneficial if they are used when starting a new therapy, or after several trials have failed.
Consider the cost
The cost and availability of pharmacogenetic testing can be an issue when making treatment decisions, and such testing may not be covered by a patient’s insurance plan. Recently, the Centers for Medicare & Medicaid Services announced that Medicare would cover FDA-approved genomic tests that encompass broad gene panels if the evidence supports their use. Similarly, commercial insurers such as UnitedHealthcare have begun to cover some pharmacogenetic tests.6 Medicare or Medicaid plans cover some testing panels’ costs and patients do not incur any out-of-pocket costs; however, some private insurance companies require patients to pay at least a portion of the cost, and many companies offer financial assistance for patients based on income and other factors. Although financial coverage for testing has improved, patients may still face out-of-pocket costs; therefore, clinicians may need to weigh the benefits of pharmacogenetic testing vs its cost.7 Clinicians should also determine what timeline best suits their patient’s financial and clinical needs, and test accordingly.
Continue to: Patient education is critical
Patient education is critical
Although the benefits of using pharmacogenetic testing information when making certain treatment decisions is promising, it is important for both patients and clinicians to understand that test results do not always change therapy. A study on the impact of pharmacogenetic testing on clinical outcomes of patients with MDD found that 79% of patients were already prescribed medications that aligned with recommendations.8 Therefore, switching medications based on the test results of a patient who is doing well clinically is not recommended. However, DSTs may help with clinical decisions for ambiguous cases. For example, if a patient has a genotype and/or phenotype that aligns with medication recommendations, the DST might not be able to identify a better medication to use, but may be able to recommend dosing guidance to improve the tolerability of the patient’s current therapy.6 It is also important to understand that the results of such testing may have a broader use beyond the initial reason for obtaining testing, such as when prescribing a common blood thinner such as warfarin or clopidogrel. However, for many of the pharmacodynamic genes that are included in these panels, their use beyond the treatment of depression may be limited because outcome studies for pharmacodynamic pharmacogenes may vary based on psychiatric diagnosis. Regardless, it may be beneficial to securely save and store patient test results in a standardized place within the medical record for future use.
CASE CONTINUED
You work with Mr. J to help him understand the benefits and limitations associated with pharmacogenetic testing. Assuming Mr. J is comfortable with the costs of obtaining testing, you contact the testing companies you identified to determine the specific pharmacogene variants included on each of these panels, and which would be the most appropriate given his race. If the decision is made to order the testing, provide Mr. J with a copy of his testing report so that he can use this information should he need any additional pharmacotherapy in the future, and also maintain a copy in his patient records using a standardized location for easy future access. If Mr. J is not comfortable with the costs associated with the testing, find out which medication his brother is currently receiving for treatment; this information may help identify a treatment plan for Mr. J.
Impact on practice
As psychiatry continues to gain experience in using pharmacogenetic testing and DSTs to help guide treatments for depression and other disorders, clinicians need to learn about these tools and how to use an evidence-based approach to best implement them in their practice. Many academic medical centers have developed continuing education programs or consult services to help with this.9,10 Just as the choice of which medication to use may be based partly on clinician experience, so too may be which pharmacogenetic test to use.
Bottom Line
Pharmacogenetic tests have not been examined in head-to-head clinical trials, which makes it nearly impossible to identify which test is best to use. Although the testing companies’ decision support tools (DSTs) often disagree in their recommendations, research has shown that using DSTs can facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. Clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Related Resources
- PGx Gene-specific information tables. www.pharmgkb.org/page/pgxGeneRef
- Clinical Pharmacogenetics Implementation Consortium. https://cpicpgx.org/guidelines/
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Fluoxetine • Prozac
Imipramine • Tofranil
Paroxetine • Paxil
Sertraline • Zoloft
Warfarin • Coumadin, Jantoven
Mr. J, age 30, a Black man with major depressive disorder (MDD), has been your patient for the past year. At the time of his diagnosis, Mr. J received sertraline, 100 mg/d, but had little to no improvement. During the past year, he received trials of citalopram and paroxetine, but they were not effective for his recurrent depressive symptoms and/or resulted in significant adverse effects.
During a recent visit, Mr. J asks you about “the genetic tests that help determine which medications will work.” He mentions that his brother had this testing done and that it had “worked for him,” but offers no other details. You research the different testing panels to see which test you might use. After a brief online review, you identify at least 4 different products, and are not sure which test—if any—you should consider.
During the last few years, there has been a rise in commercial pharmacogenetic testing options, including tests available to clinicians at academic medical centers as well as direct-to-consumer testing (Table). Clinician and patient interest regarding pharmacogenetic testing in practice is often followed by the question, “Which test is best?” Although this is a logical question, providing an answer is multifactorial.1-3 Because none of the currently available tests have been compared in head-to-head clinical trials, it is nearly impossible to identify the “best” test.
In this article, we focus on the evidence-based principles that clinicians should consider when adopting pharmacogenetic testing in their practice. We discuss which genes are of most interest when prescribing psychotropic medications, the value of decision support tools, cost considerations, and patient education regarding this type of testing.
Which genes and variants should be tested?
The genes relevant to medication treatment outcomes can be broadly classified into those with pharmacokinetic vs pharmacodynamic effects. Pharmacogenes, such as those coding for the drug-metabolizing enzymes cytochrome P450 (CYP) 1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and UDP-glucuronosyltransferase (UGT)2B1, may alter the rate at which medications are metabolized, thus varying the serum drug concentration across patients. Variants that impact the function of these enzymes are considered pharmacokinetic. Up to 40% of the variance in patients’ response to antidepressants may be due to variations in the pharmacokinetic genes.4 Alternatively, pharmacodynamic pharmacogenes impact drug action and therefore may affect the degree of receptor activation at a given drug concentration, overall drug efficacy, and/or the occurrence of medication sensitivity. These pharmacogenes may include:
- brain-derived neurotrophic factor (BDNF)
- catechol-O-methyltransferase (COMT)
- human leukocyte antigens A (HLA-A)
- serotonin receptor subtype 2 (HTR2)
- serotonin receptor subtype 2C (HTR2C)
- opioid receptor mu 1 (OPRM1)
- solute carrier family 6 member 4 (SLC6A4).
In articles previously published in
Currently, there is no standardization among commercial pharmacogenetic tests on:
- which genes to test
- which variants specific to a gene need to be included
- how the genetic data is translated to phenotype
- how the phenotype is translated to a treatment recommendation.
Continue to: Due to these factors...
Due to these factors, the FDA has advised clinicians to consult the dosing recommendations provided in a medication’s package insert for information regarding how genetic information should be used in making treatment decisions.2
The value of decision support tools
Researchers have assessed how various manufacturers’ decision support tools (DSTs) (ie, the reports the commercial testing companies send to the clinician who orders the test) agree on genotypes, predicted phenotypes, and medication recommendations.4 Overall, this research found varying levels of disagreement in the medication recommendations of the testing panels they studied, which indicates that not all tests are equivalent or interchangeable.4 Of the actionable recommendations for antidepressants, 16% were conflicting; the recommendations for fluoxetine and imipramine were most frequently in disagreement.4 Similarly, 20% of the actionable antipsychotic advice was conflicting, with the recommendations for aripiprazole and clozapine most frequently in disagreement.4 Researchers also reported a situation in which 4 testing panels agreed on the patient’s phenotyping status for CYP2C19, but the dosing recommendations provided for the CYP2C19 substrate, amitriptyline, differed.4 Thus, it is understandable why DSTs can result in confusion, and why clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Additionally, while the genes included on these panels vary, these testing panels also may not evaluate the same variants within a specific gene. These differences may impact the patient’s reported phenotypes and medication recommendations across DSTs. For example, the FDA has recommended HLA gene testing prior to prescribing carbamazepine. However, few of the available tests may include the HLA-B*15:02 variant, which has been associated with carbamazepine-induced severe cutaneous reactions in patients of Asian descent, and fewer may include the HLA-A*31:01 variant, for which testing is recommended prior to prescribing carbamazepine in patients of Caucasian descent.4 Additionally, some of the CYP enzymes—such as CYP2D6*17 and CYP2C19*3 variants, which may be more common in certain populations of patients who are members of ethnic or racial minority groups—may not be consistently included in the various panels. Thus, before deciding on a specific test, clinicians should understand which gene variants are relevant to their patients with regard to race and ethnicity, and key variants for specific medications. Clinicians should refer to FDA guidance and the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines to determine the appropriate interpretations of genetic test results.1,2
Despite the disagreement in recommendations from the various testing companies, DSTs are useful and have been shown to facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. A recently published meta-analysis of randomized controlled trials (RCTs) of pharmacogenetic testing found that DSTs improved symptom remission among individuals with MDD by 70%.5 This suggests that pharmacogenetic-guided DSTs may provide superior treatment compared with treatment for DSTs were not used. However, the RCTs in this meta-analysis only included patients who had previously failed an antidepressant trial.5 Therefore, it is currently unknown at what point in care DSTs should be used, and whether they would be more beneficial if they are used when starting a new therapy, or after several trials have failed.
Consider the cost
The cost and availability of pharmacogenetic testing can be an issue when making treatment decisions, and such testing may not be covered by a patient’s insurance plan. Recently, the Centers for Medicare & Medicaid Services announced that Medicare would cover FDA-approved genomic tests that encompass broad gene panels if the evidence supports their use. Similarly, commercial insurers such as UnitedHealthcare have begun to cover some pharmacogenetic tests.6 Medicare or Medicaid plans cover some testing panels’ costs and patients do not incur any out-of-pocket costs; however, some private insurance companies require patients to pay at least a portion of the cost, and many companies offer financial assistance for patients based on income and other factors. Although financial coverage for testing has improved, patients may still face out-of-pocket costs; therefore, clinicians may need to weigh the benefits of pharmacogenetic testing vs its cost.7 Clinicians should also determine what timeline best suits their patient’s financial and clinical needs, and test accordingly.
Continue to: Patient education is critical
Patient education is critical
Although the benefits of using pharmacogenetic testing information when making certain treatment decisions is promising, it is important for both patients and clinicians to understand that test results do not always change therapy. A study on the impact of pharmacogenetic testing on clinical outcomes of patients with MDD found that 79% of patients were already prescribed medications that aligned with recommendations.8 Therefore, switching medications based on the test results of a patient who is doing well clinically is not recommended. However, DSTs may help with clinical decisions for ambiguous cases. For example, if a patient has a genotype and/or phenotype that aligns with medication recommendations, the DST might not be able to identify a better medication to use, but may be able to recommend dosing guidance to improve the tolerability of the patient’s current therapy.6 It is also important to understand that the results of such testing may have a broader use beyond the initial reason for obtaining testing, such as when prescribing a common blood thinner such as warfarin or clopidogrel. However, for many of the pharmacodynamic genes that are included in these panels, their use beyond the treatment of depression may be limited because outcome studies for pharmacodynamic pharmacogenes may vary based on psychiatric diagnosis. Regardless, it may be beneficial to securely save and store patient test results in a standardized place within the medical record for future use.
CASE CONTINUED
You work with Mr. J to help him understand the benefits and limitations associated with pharmacogenetic testing. Assuming Mr. J is comfortable with the costs of obtaining testing, you contact the testing companies you identified to determine the specific pharmacogene variants included on each of these panels, and which would be the most appropriate given his race. If the decision is made to order the testing, provide Mr. J with a copy of his testing report so that he can use this information should he need any additional pharmacotherapy in the future, and also maintain a copy in his patient records using a standardized location for easy future access. If Mr. J is not comfortable with the costs associated with the testing, find out which medication his brother is currently receiving for treatment; this information may help identify a treatment plan for Mr. J.
Impact on practice
As psychiatry continues to gain experience in using pharmacogenetic testing and DSTs to help guide treatments for depression and other disorders, clinicians need to learn about these tools and how to use an evidence-based approach to best implement them in their practice. Many academic medical centers have developed continuing education programs or consult services to help with this.9,10 Just as the choice of which medication to use may be based partly on clinician experience, so too may be which pharmacogenetic test to use.
Bottom Line
Pharmacogenetic tests have not been examined in head-to-head clinical trials, which makes it nearly impossible to identify which test is best to use. Although the testing companies’ decision support tools (DSTs) often disagree in their recommendations, research has shown that using DSTs can facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. Clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Related Resources
- PGx Gene-specific information tables. www.pharmgkb.org/page/pgxGeneRef
- Clinical Pharmacogenetics Implementation Consortium. https://cpicpgx.org/guidelines/
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Fluoxetine • Prozac
Imipramine • Tofranil
Paroxetine • Paxil
Sertraline • Zoloft
Warfarin • Coumadin, Jantoven
1. Ellingrod, VL. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
2. Ellingrod VL. Pharmacogenomics testing: what the FDA says. Current Psychiatry. 2019;18(4):29-33.
3. Ramsey LB. Pharmacogenetic testing in children: what to test and how to use it. Current Psychiatry. 2018;17(9):30-36.
4. Bousman CA, Dunlop BW. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. The Pharmacogenomics Journal. 2018;18(5):613-622. doi:10.1038/s41397-018-0027-3
5. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1). doi:10.2217/pgs-2018-0142
6. Nicholson WT, Formea CM, Matey ET, et al. Considerations when applying pharmacogenomics to your practice. Mayo Clin Proc. 2021;96(1);218-230. doi:10.1016/j.mayocp.2020.03.011
7. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Human Genomics. 2019;13(1). doi:10.1186/s40246-019-0229-z
8. Greden JF, Parikh S, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111;59-67. doi:10.1016/j.jpsychires.2019.01.003
9. Haga SB. Integrating pharmacogenetic testing into primary care. Expert Review of Precision Medicine and Drug Development. 2017;2(6):327-336. doi:10.1080/23808993.2017.1398046
10. Ward KM, Taubman DS, Pasternak AL, et al. Teaching psychiatric pharmacogenomics effectively: evaluation of a novel interprofessional online course. J Am Coll Clin Pharm. 2021; 4:176-183.
1. Ellingrod, VL. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
2. Ellingrod VL. Pharmacogenomics testing: what the FDA says. Current Psychiatry. 2019;18(4):29-33.
3. Ramsey LB. Pharmacogenetic testing in children: what to test and how to use it. Current Psychiatry. 2018;17(9):30-36.
4. Bousman CA, Dunlop BW. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. The Pharmacogenomics Journal. 2018;18(5):613-622. doi:10.1038/s41397-018-0027-3
5. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1). doi:10.2217/pgs-2018-0142
6. Nicholson WT, Formea CM, Matey ET, et al. Considerations when applying pharmacogenomics to your practice. Mayo Clin Proc. 2021;96(1);218-230. doi:10.1016/j.mayocp.2020.03.011
7. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Human Genomics. 2019;13(1). doi:10.1186/s40246-019-0229-z
8. Greden JF, Parikh S, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111;59-67. doi:10.1016/j.jpsychires.2019.01.003
9. Haga SB. Integrating pharmacogenetic testing into primary care. Expert Review of Precision Medicine and Drug Development. 2017;2(6):327-336. doi:10.1080/23808993.2017.1398046
10. Ward KM, Taubman DS, Pasternak AL, et al. Teaching psychiatric pharmacogenomics effectively: evaluation of a novel interprofessional online course. J Am Coll Clin Pharm. 2021; 4:176-183.
How COVID-19 affects peripartum women’s mental health
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290