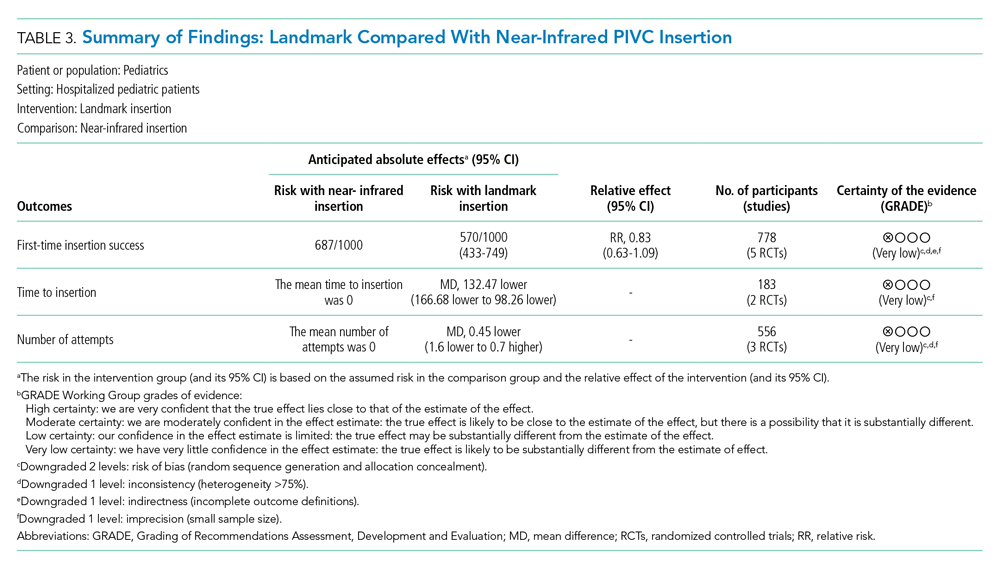
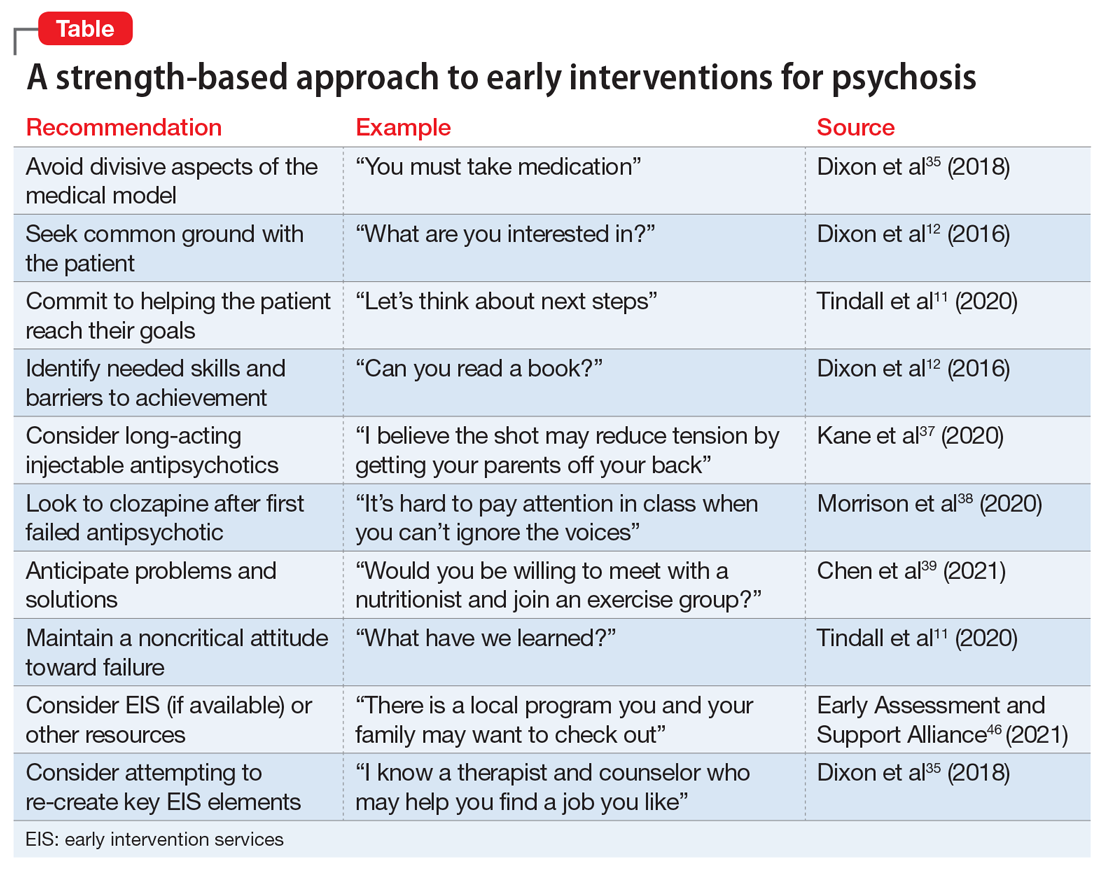
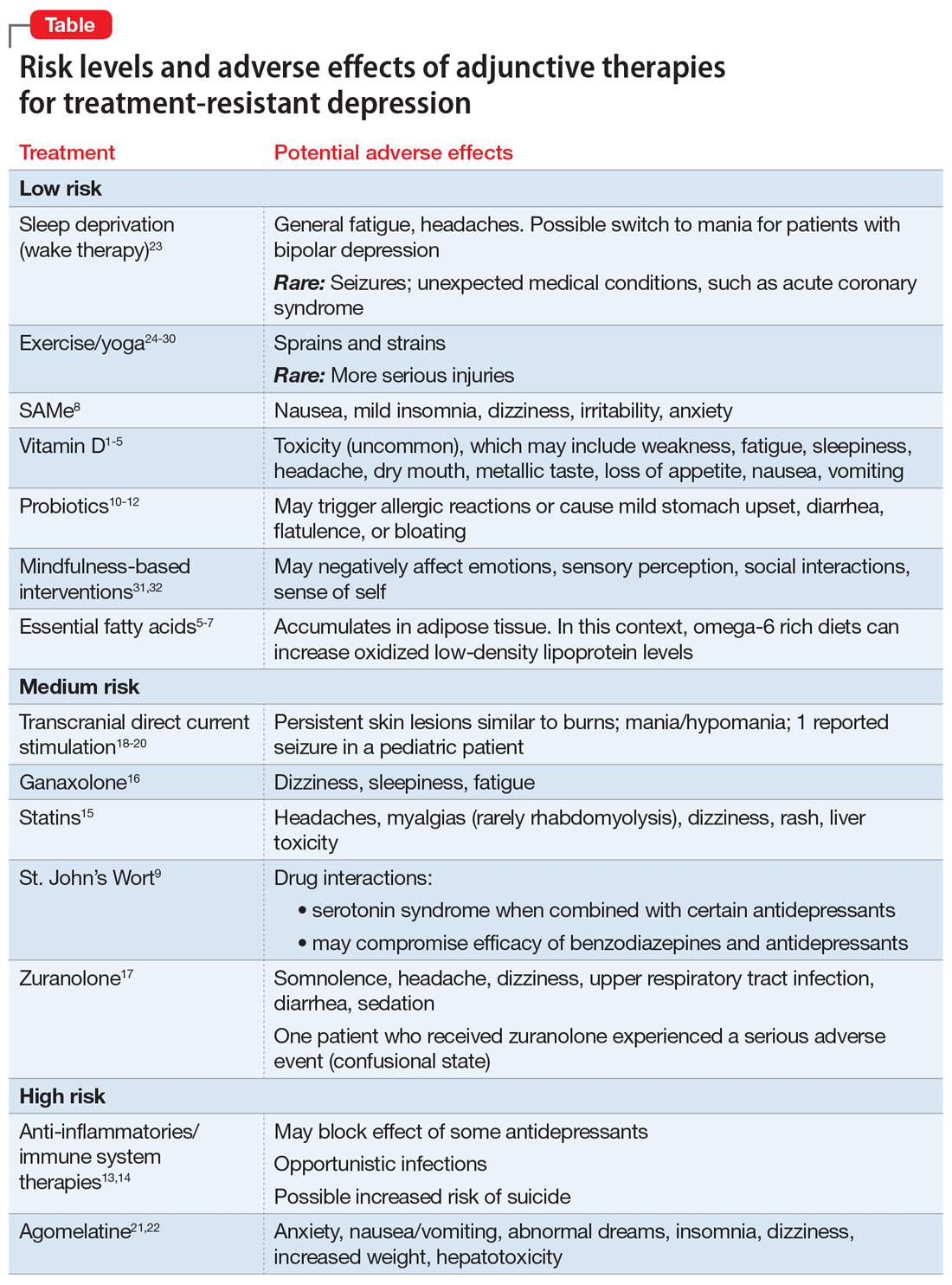
User login
Deprescribing in older adults: An overview
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
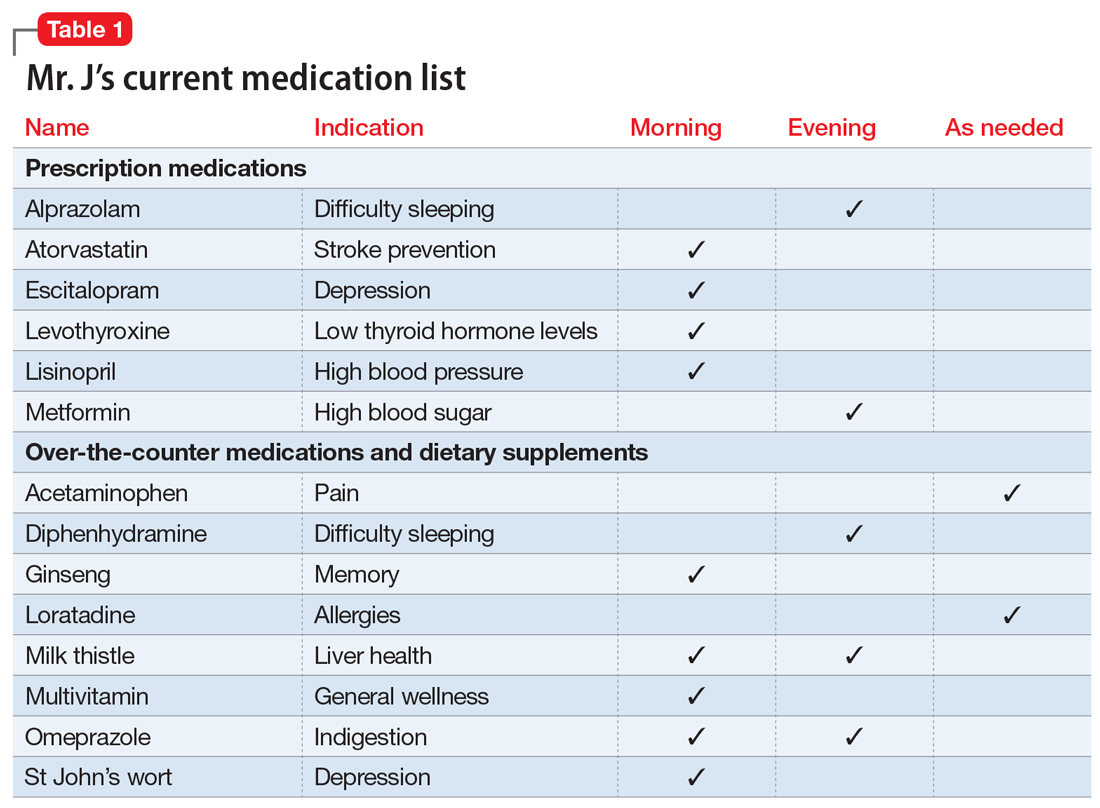
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
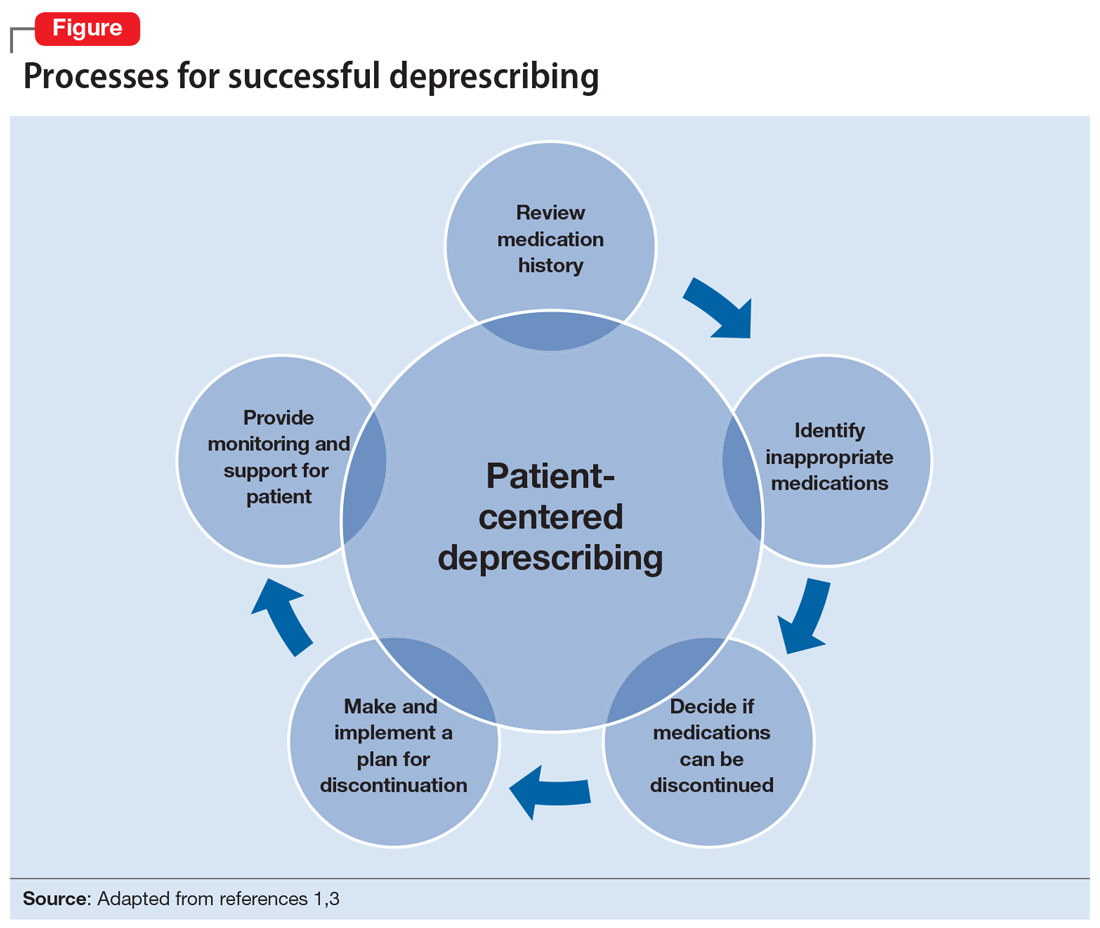
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
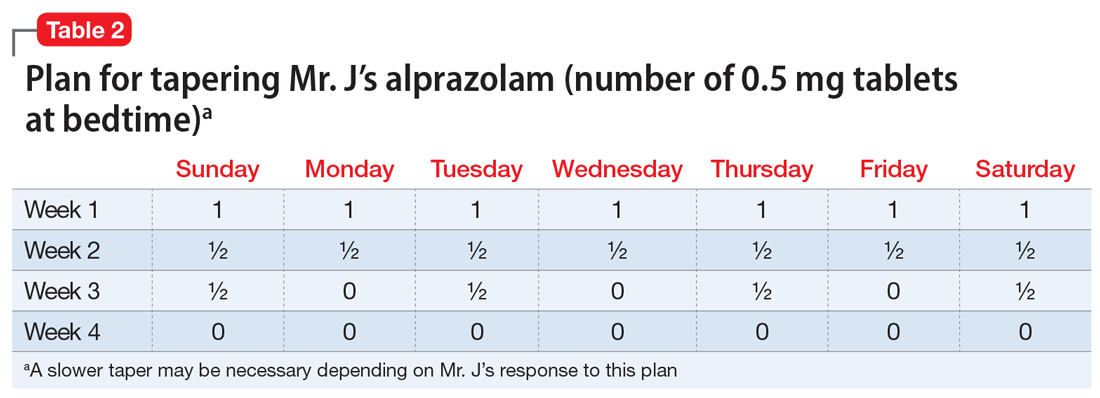
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
Mr. J, age 73, has a 25-year history of generalized anxiety disorder and major depressive disorder. His medical history includes hypertension, hyperlipidemia, type 2 diabetes mellitus, hypothyroidism, osteoarthritis, insomnia, and allergic rhinitis. His last laboratory test results indicate his hemoglobin A1c, thyroid-stimulating hormone, low-density lipoprotein, and blood pressure measurements are at goal. He believes his conditions are well controlled but cites concerns about taking multiple medications each day and being able to afford his medications.
You review the list of Mr. J’s current prescription medications, which include alprazolam 0.5 mg/d, atorvastatin 40 mg/d, escitalopram 10 mg/d, levothyroxine 0.125 mg/d, lisinopril 20 mg/d, and metformin XR 1,000 mg/d. Mr. J reports taking over-the-counter (OTC) acetaminophen as needed for pain, diphenhydramine for insomnia, loratadine as needed for allergic rhinitis, and omeprazole for 2 years for indigestion. After further questioning, he also reports taking ginseng, milk thistle, a multivitamin, and, based on a friend’s recommendation, St John’s Wort (Table 1).
Similar to Mr. J, many older adults take multiple medications to manage chronic health conditions and promote their overall health. On average, 30% of older adults take ≥5 medications.1 Among commonly prescribed medications for these patients, an estimated 1 in 5 of may be inappropriate.1 Older adults have high rates of polypharmacy (often defined as taking ≥5 medications1), age-related physiological changes, increased number of comorbidities, and frailty, all of which can increase the risk of medication-related adverse events.2 As a result, older patients’ medications should be regularly evaluated to determine if each medication is appropriate to continue or should be tapered or stopped.
Deprescribing, in which medications are tapered or discontinued using a patient-centered approach, should be considered when a patient is no longer receiving benefit from a medication, or when the harm may exceed the benefit.1,3
Several researchers1,3 and organizations have published detailed descriptions of and guidelines for the process of deprescribing (see Related Resources). Here we provide a brief overview of this process (Figure1,3). The first step is to assemble a list of all prescription and OTC medications, herbal products, vitamins, or nutritional supplements the patient is taking. It is important to specifically ask patients about their use of nonprescription products, because these products are infrequently documented in medical records.
The second step is to evaluate the indication, effectiveness, safety, and patient’s adherence to each medication while beginning to consider opportunities to limit treatment burden and the risk of harm from medications. Ideally, this assessment should involve a patient-centered conversation that considers the patient’s goals, preferences, and treatment values. Many resources can be used to evaluate which medications might be inappropriate for an older adult. Two examples are the American Geriatrics Society Beers Criteria5 and STOPP/START criteria.6 By looking at these resources, you could identify that (for example) anticholinergic medications should be avoided in older patients due to an increased risk of adverse effects, change in cognitive status, and falls.5,6 These resources can aid in identifying, prioritizing, and deprescribing potentially harmful and/or inappropriate medications.
The next step is to decide whether any medications should be discontinued. Whenever possible, include the patient in this conversation, as they may have strong feelings about their current medication regimen. When there are multiple medications that can be discontinued, consider which medication to stop first based on potential harm, patient resistance, and other factors.
Continue to: Subsequently, work with...
Subsequently, work with the patient to create a plan for stopping or lowering the dose or frequency of the medication. These changes should be individualized based on the patient’s preferences as well as the properties of the medication. For example, some medications can be immediately discontinued, while others (eg, benzodiazepines) may need to be slowly tapered. It is important to consider if the patient will need to switch to a safer medication, change their behaviors (eg, lifestyle changes), or engage in alternative treatments (such as cognitive-behavioral therapy for insomnia) when they stop their current medication. Take an active role in monitoring your patient during this process, and encourage them to reach out to you or to their primary clinician if they have concerns.
CASE CONTINUED
Mr. J is a candidate for deprescribing because he has expressed concerns about his current regimen, and because he is taking potentially unsafe medications. The 2 medications he’s taking that may cause the most harm are diphenhydramine and alprazolam, due to the risk of cognitive impairment and falls. Through a patient-centered conversation, Mr. J says he is willing to stop diphenhydramine immediately and taper off the alprazolam over the next month, with the support of a tapering chart (Table 2). You explain to him that a long tapering of alprazolam may be necessary. He is willing to try good sleep hygiene practices and will put off starting trazodone as an alternative to diphenhydramine until he sees if it will be necessary. You make a note to follow up with him in 1 week to assess his insomnia and adherence to the new treatment plan. You also teach Mr. J that some of his supplements may interact with his prescription medications, such as St John’s Wort with escitalopram (ie, risk of serotonin syndrome) and ginseng with metformin (ie, risk for hypoglycemia). He says he doesn’t take ginseng, milk thistle, or St John’s Wort regularly, and because he feels they do not offer any benefit, he will stop taking them. He says that at his next visit with his primary care physician, he will bring up the idea of stopping omeprazole.
Related Resources
- Deprescribing.org. Deprescribing guidelines and algorithms. https://deprescribing.org/resources/deprescribing-guidelines-algorithms/
- US Deprescribing Research Network. Resources for Clinicians. https://deprescribingresearch.org/resources-2/resources-for-clinicians/
Drug Brand Names
Alprazolam • Xanax
Atorvastatin • Lipitor
Escitalopram • Lexapro
Levothyroxine • Synthroid
Lisinopril • Zestril
Metformin XR • Glucophage XR
Trazodone • Desyrel
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
2. Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
3. Reeve E, Shakib S, Hendrix I, et al. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmcol. 2014;78(4):738-747.
4. Iyer S, Naganathan V, McLachlan AJ, et al. Medication withdrawal trials in people aged 65 years and older: a systematic review. Drugs Aging. 2008;25(12):1021-1031.
5. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
6. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218.
The woman who kept passing out
CASE An apparent code blue
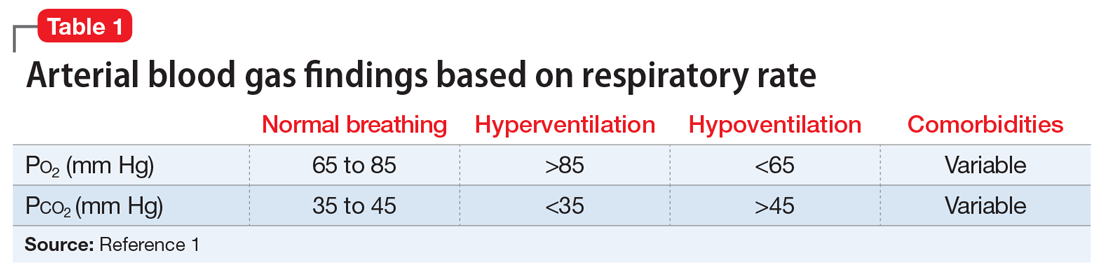
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
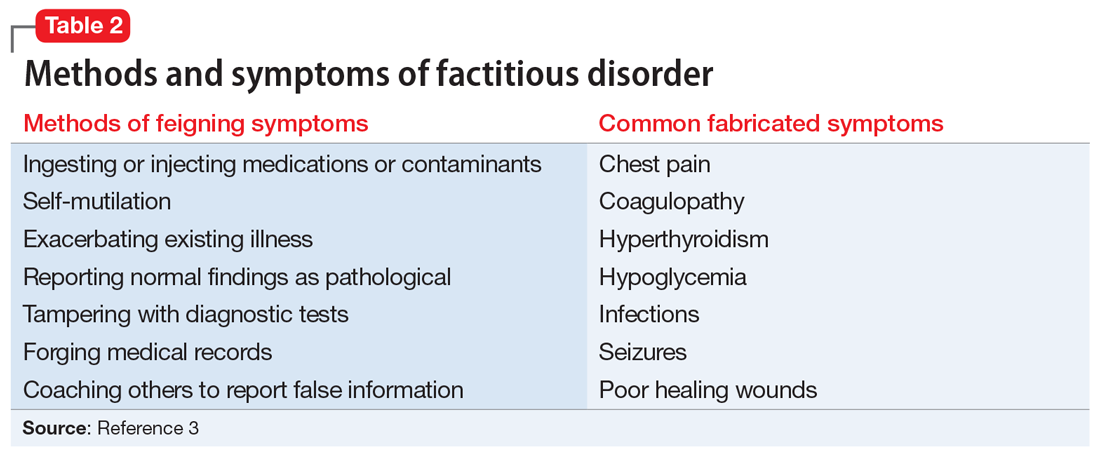
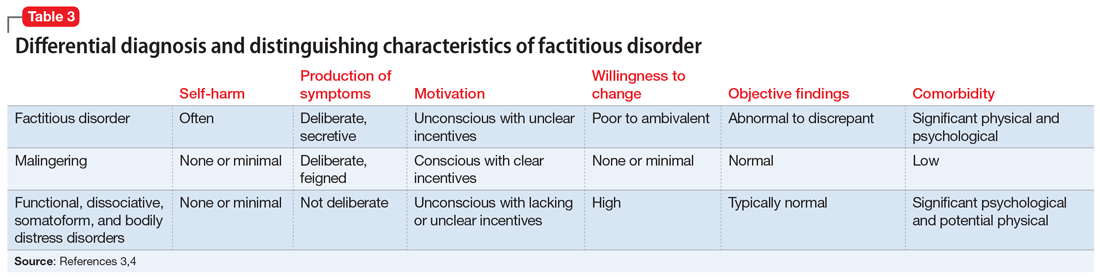
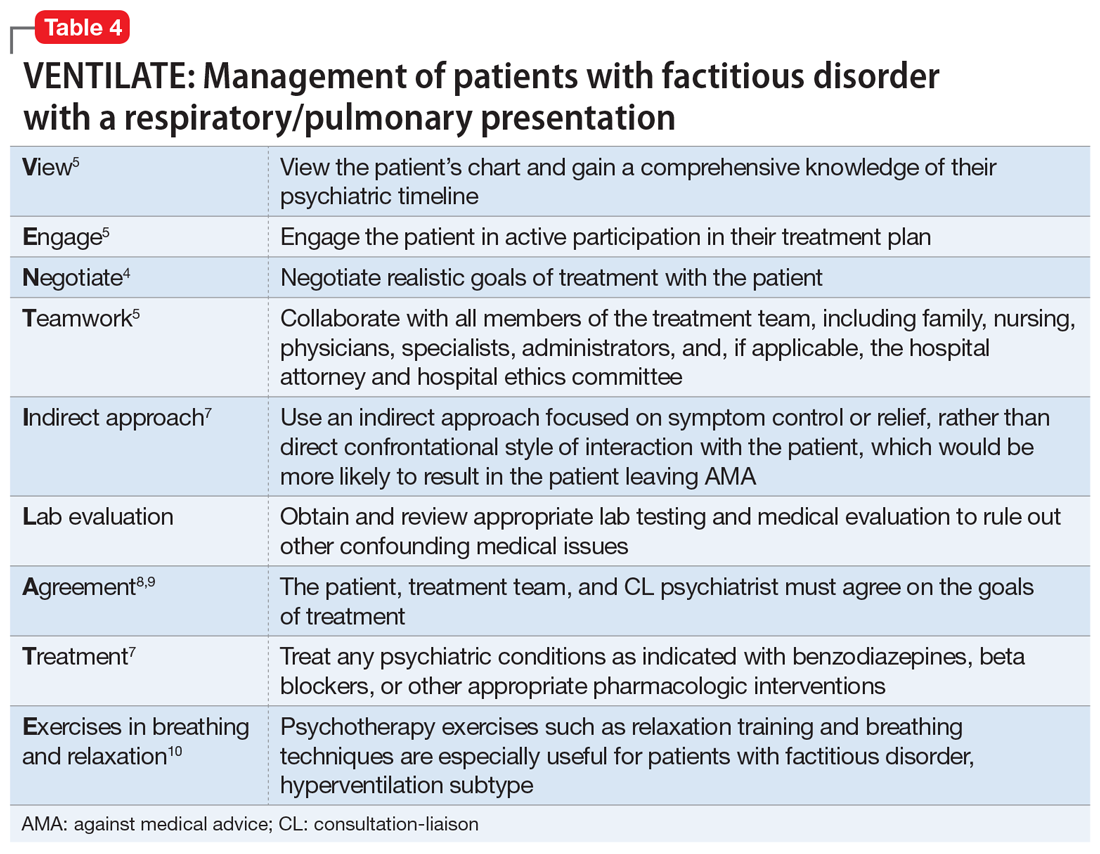
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
CASE An apparent code blue
Ms. B, age 44, has posttraumatic stress disorder (PTSD), bipolar disorder, and chronic obstructive pulmonary disease. She presents to the hospital for an outpatient orthopedic appointment. In the hospital cafeteria, she becomes unresponsive, and a code blue is called. Ms. B is admitted to the medicine intensive care unit (MICU), where she is sedated with propofol and intubated. The initial blood work for this supposed hypoxic event shows a Po2 of 336 mm Hg (reference range: 80 to 100 mm Hg; see Table 11). The MICU calls the psychiatric consultation-liaison (CL) team to evaluate this paradoxical finding.
HISTORY A pattern of similar symptoms
In the 12 months before her current hospital visit, Ms. B presented to the emergency department (ED) on 3 occasions. These were for a syncopal episode with shortness of breath and 2 incidences of passing out while receiving diagnostic testing. Each time, on Ms. B’s insistence, she was admitted and intubated. Once extubated, Ms. B left against medical advice (AMA) after a short period. She has an allergy list that includes more than 30 drugs spanning multiple drug classes, including antibiotics, contrast material, and some gamma aminobutyric acidergic medications. Notably, Ms. B is not allergic to benzodiazepines. She also has undergone more than 10 surgeries, including bariatric surgery, cholecystectomy, appendectomy, neurostimulator placement, and colon surgery.
EVALUATION Clues suggest a potential psychiatric diagnosis
When the CL team initially consults, Ms. B is intubated and sedated with dexmedetomidine, which limits the examination. She is able to better participate during interviews as she is weaned from sedation while in the MICU. A mental status exam reveals a woman who appears older than 44. She is oriented to person, place, time, and situation despite being mildly somnolent and having poor eye contact. Ms. B displays restricted affect, psychomotor retardation, and slowed speech. She denies suicidal or homicidal thoughts, intent, or plans; paranoia or other delusions; and any visual, auditory, somatic, or olfactory hallucinations. Her thought process is goal-directed and linear but with thought-blocking. Ms. B’s initial arterial blood gas (ABG) test is abnormal, showing she is acidotic with both hypercarbia and extreme hyperoxemia (pH 7.21 and P
[polldaddy:11104278]
The authors’ observations
Under normal code blue situations, patients are expected to have respiratory acidosis, with low Po2 levels and high Pco2 levels. However, Ms. B’s ABG revealed she had high Po2 levels and high Pco2levels. Her paradoxical findings of elevated Pco2 on the initial ABG were likely due to hyperventilation on pure oxygen in the context of her underlying chronic lung disease and respiratory fatigue.
The clinical team contacted Ms. B’s husband, who stated that during her prior hospitalizations, she had a history of physical aggression with staff when weaned off sedation. Additionally, he reported that 1 week before presenting to the ED, she had wanted to meet her dead father.
A review of Ms. B’s medical records revealed she had been prescribed alprazolam, 2 mg 3 times a day as needed, so she was prescribed scheduled lorazepam in addition to the Clinical Institute Withdrawal Assessment for Alcohol (CIWA) protocol to prevent benzodiazepine withdrawal. Ms. B had 2 prior long-term monitoring for epilepsy evaluations in our system for evaluation of seizure-like behavior. The first evaluation showed an episode of stiffening with tremulousness and eye closure for 20 to 25 minutes with no epileptiform discharge or other EEG changes. The second showed diffuse bihemispheric dysfunction consistent with toxic metabolic encephalopathies, but no epileptiform abnormality.
When hospital staff would collect arterial blood, Ms. B had periods when her eyes were closed, muscles flaccid, and she displayed an unresponsiveness to voice, touch, and noxious stimulation, including sternal rub. Opening her eyelids during these episodes revealed slow, wandering eye movements, but no nystagmus or fixed eye deviation. Vital signs and oxygenation were unchanged during these episodes. When this occurred, the phlebotomist would leave the room to notify the attending physician on call, but Ms. B would quickly return to her mildly impaired baseline. When the attending entered the room, Ms. B reported no memory of what happened during these episodes. At this point, the CL team begins to suspect that Ms. B may have factitious disorder.
Continue to: TREATMENT
TREATMENT Agitation, possibly due to benzo withdrawal
Ms. B is successfully weaned off sedation and transferred out of the MICU for continued CIWA protocol management on a different floor. However, she breaks free of her soft restraint, strips naked, and attempts to barricade her room to prevent staff from entering. Nursing staff administers haloperidol 4 mg to manage agitation.
[polldaddy:11104279]
The authors’ observations
To better match Ms. B’s prior alprazolam prescription, the treatment team increased her lorazepam dosage to a dose higher than her CIWA protocol. This allowed the team to manage her withdrawal, as they believed that benzodiazepine withdrawal was a major driving force behind her decision to leave AMA following prior hospitalizations. This enabled the CL team to coordinate care as Ms. B transitioned to outpatient management. The team suspected Ms. B may have factitious disorder, but did not discuss that specific diagnosis with the patient. However, they did talk through general treatment options with her.
Challenges of factitious disorder
DSM-5 classifies factitious disorder under Somatic Symptoms and Related Disorders, and describes it as “deceptive behavior in the absence of external incentives.”2 A prominent feature of factitious disorder is a persistent concern related to illness and identity causing significant distress and impairment.2 Patients with factitious disorder enact deceptive behavior such as intentionally falsifying medical and/or psychological symptoms, inducing illness to themselves, or exaggerated signs and symptoms.3 External motives and rewards are often unidentifiable but could result in a desire to receive care, an “adrenaline rush,” or a sense of control over health care personnel.3Table 23 outlines additional symptoms of factitious disorder. When evaluating a patient who may have factitious disorder, the differential diagnosis may include malingering, conversion disorder, somatic symptom disorder, delusional disorder somatic type, borderline personality disorder, and other impulse-control disorders (Table 33,4).
Consequences of factitious disorder include self-harm and a significant impact on health care costs related to excessive and inappropriate hospital admissions and treatments. Factitious disorder represents approximately 0.6% to 3% of referrals from general medicine and 0.02% to 0.9% of referrals from specialists.3
Patients may be treated at multiple hospitals, pharmacies, and medical institutions because of deceptive behaviors that lead to a lack of complete and accurate documentation and fragmentation in communication and care. Internet access may also play a role in enabling skillful and versatile feigning of symptoms. This is compounded with further complexity because many of these patients suffer from comorbid conditions.
Continue to: Management of self-imposed...
Management of self-imposed factitious disorder includes acute treatment in inpatient settings with multidisciplinary teams as well as in longer-term settings with ongoing medical and psychological support.5 The key to achieving positive outcomes in both settings is negotiation and agreement with the patient on their diagnosis and engagement in treatment.5 There is little evidence available to support the effectiveness of any particular management strategy for factitious disorder, specifically in the inpatient psychiatric setting. A primary reason for this paucity of data is that most patients are lost to follow-up after initiation of a treatment plan.6
Addressing factitious disorder with patients can be particularly difficult; it requires a thoughtful and balanced approach. Typical responses to confrontation of this deceptive behavior involve denial, leaving AMA, or potentially verbal and physical aggression.4 In a review of medical records, Krahn et al6 found that of 71 patients with factitious disorder who were confronted about their role in the illness, only 23% (n = 16) acknowledged factitious behavior. Confrontation can be conceptualized as direct or indirect. In direct confrontation, patients are directly told of their diagnosis. This frequently angers patients, because such confrontation can be interpreted as humiliating and can cause them to seek care from another clinician, leave the hospital AMA, or increase their self-destructive behavior.4 In contrast, indirect confrontation approaches the conversation with an explanatory view of the maladaptive behaviors, which may allow the patient to be more open to therapy.4 An example of this would be, “When some patients are very upset, they often do something to themselves to create illness as a way of seeking help. We believe that something such as this must be going on and we would like to help you focus on the true nature of your problem, which is emotional distress.” However, there is no evidence that either of these approaches is superior, or that a significant difference in outcomes exists between confrontational and nonconfrontational approaches.7
The treatment for factitious disorder most often initiated in inpatient settings and continued in outpatient care is psychotherapy, including cognitive-behavioral therapy, supportive psychotherapy, dialectical behavioral therapy, and short-term psychodynamic psychotherapy.4,8,9 There is, however, no evidence to support the efficacy of one form of psychotherapy over another, or even to establish the efficacy of treatment with psychotherapy compared to no psychotherapy. This is further complicated by some resources that suggest mood stabilizers, antipsychotics, or antidepressants as treatment options for psychiatric comorbidities in patients with factitious disorder; very little evidence supports these agents’ efficacy in treating the patient’s behaviors related to factitious disorder.7
No data are available to support a management strategy for patients with factitious disorder who have a respiratory/pulmonary presentation, such as Ms. B. Suggested treatment options for hyperventilation syndrome include relaxation therapy, breathing exercises, short-acting benzodiazepines, and beta-blockers; there is no evidence to support their efficacy, whether in the context of factitious disorder or another disorder.10 We suggest the acronym VENTILATE to guide the treating psychiatrist in managing a patient with factitious disorder with a respiratory/pulmonary presentation and hyperventilation (Table 44,5,7-10).
Bass et al5 suggest that regardless of the manifestation of a patient’s factitious disorder, for a CL psychiatrist, it is important to consult with the patient’s entire care team, hospital administrators, hospital and personal attorneys, and hospital ethics committee before making treatment decisions that deviate from usual medical practice.
Continue to: OUTCOME
OUTCOME Set up for success at home
Before Ms. B is discharged, her husband is contacted and amenable to removing all objects and medications that Ms. B could potentially use to cause self-harm at home. A follow-up with Ms. B’s psychiatric outpatient clinician is scheduled for the following week. By the end of her hospital stay, she denies any suicidal or homicidal ideation, delusions, or hallucinations. Ms. B is able to express multiple protective factors against the risk of self-harm, and engages in meaningful discussions on safety planning with her husband and the psychiatry team. This is the first time in more than 1 year that Ms. B does not leave the hospital AMA.
Bottom Line
Patients with factitious disorder may present with respiratory/pulmonary symptoms. There is limited data to support the efficacy of one approach over another for treating factitious disorder in an inpatient setting, but patient engagement and collaboration with the entire care team is critical to managing this difficult scenario.
Related Resources
- de Similien R, Lee BL, Hairston DR, et al. Sick, or faking it? Current Psychiatry. 2019;18(9):49-52.
Drug Brand Names
Alprazolam • Xanax
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
1. Castro D, Patil SM, Keenaghan M. Arterial Blood Gas. In: StatPearls. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK536919/
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
3. Yates GP, Feldman MD. Factitious disorder: a systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry. 2016;41:20-28.
4. Ford CV, Sonnier L, McCullumsmith C. Deception syndromes: factitious disorders and malingering. In: Levenson JL, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic Medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Assocation Publishing, Inc.; 2018:323-340.
5. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
6. Krahn LE, Li H, O’Connor MK. Patients who strive to be ill: factitious disorder with physical symptoms. Am J Psychiatry. 2003;160(6):1163-1168.
7. Eastwood S, Bisson JI. Management of factitious disorders: a systematic review. Psychother Psychosom. 2008;77(4):209-218.
8. Abbass A, Kisely S, Kroenke K. Short-term psychodynamic psychotherapy for somatic disorders. Systematic review and meta-analysis of clinical trials. Psychother Psychosom. 2009;78(5):265-274.
9. McDermott BE, Leamon MH, Feldman MD, et al. Factitious disorder and malingering. In: Hales RE, Yudofsky SC, Gabbard GO, eds. The American Psychiatric Publishing Textbook of Psychiatry. American Psychiatric Assocation Publishing, Inc.; 2008:643-664.
10. Jones M, Harvey A, Marston L, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev. 2013(5):CD009041.
How to ‘cybersecure’ your practice
The health care sector is not immune from cybersecurity attacks (malicious attempts to access or damage a computer or network system). Between October 2019 and October 2021, 857 data breaches were reported to the United States Department of Health and Human Services.1 The 3 main types of breaches reported were theft, hacking/IT incident, or unauthorized access/disclosure.1 Health care has become a common target due to the availability of valuable patient information (health, personal, and financial), the industry’s financial stability and resource capacity, and network susceptibility.2 The top 2 cybersecurity threats facing physician practices are:
- ransomware attacks, in which an external party uses a type of malicious software (malware) that prevents you from accessing your computer files, systems, or networks, and demands you pay a ransom for their return.
- employee-related threats, such as the theft or destruction of sensitive information by a disgruntled employee.3
The financial implications of health care–related cybersecurity threats coupled with exposure to potential litigation associated with breaches of confidentiality result in a need to “cybersecure” your practice.2 In this article, I outline steps to take to protect your practice against such threats. Although the recommendations I provide will increase your practice’s cybersecurity fortification, they are not exhaustive, and you may need to consult with an IT specialist to help protect your data and network.
Improve your network protection. A broadband internet connection is always operating, which makes it continuously susceptible to cybersecurity attacks. Install a firewall (a network security system that monitors and controls network traffic and permits or blocks traffic based on a defined set of rules) between your practice’s internal computer network and the internet.4 For maximum protection, enable all available firewall settings in your operating software.2 Prevent unauthorized access by ensuring that all network passwords are strong (ie, they include a combination of uppercase and lowercase letters, numbers, and symbols). Consider using different networks for online communication and for storing sensitive information.2 Create separate Wi-Fi networks for your practice and for your patients, and use unique passwords for each that are not easily guessed.4 If you or your employees use a virtual private network (VPN) to remotely access your practice’s network, ensure that all devices used to do so (cell phones, tablets, etc) are encrypted and secured with strong passwords.
Reduce employee-related threats. Not every employee in your practice will need to access to your patients’ clinical or financial data. Limiting employee access to sensitive clinical or financial data can reduce the risks of employee-related cybersecurity threats.3 In addition, restrict an employee’s ability to install software on computers and other devices that belong to your practice.2
Frequently incorporate cybersecurity training, such as teaching your employees about the risks of clicking on links and attachments in emails and how to identify phishing attacks (in which an individual sends a fraudulent communication that appears to come from a reputable source in order to trick the recipient into revealing financial information, system credentials, or other sensitive data).2,3 Use multifactor authentication to verify an employee’s login identity, and change passwords often. Reinforce these policies at staff meetings and educate new employees about this process.3 If you need to fire an employee, consider deploying cybersurveillance software to monitor the behavior of all employees before the employee is terminated.3 Once the employee has been terminated, change all logins and passwords.
1. U.S. Department of Health and Human Services. Office for Civil Rights. Breach portal: Notice to the Secretary of HHS breach of unsecured protected health information. Accessed December 26, 2021. https://ocrportal.hhs.gov/ocr/breach/breach_report.jsf
2. Umali G. How to safeguard your practice from cybersecurity threats. Psychiatric News. 2021;56(12):23.
3. Cryts A. Top two cybersecurity threats facing physician practices. Physicians Practice. March 13, 2020. Accessed December 26, 2021. https://www.physicianspractice.com/view/top-two-cybersecurity-threats-facing-physician-practices
4. American Medical Association. Protect your practice and patients from cybersecurity threats. 2017. Accessed December 26, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/network-security.pdf
The health care sector is not immune from cybersecurity attacks (malicious attempts to access or damage a computer or network system). Between October 2019 and October 2021, 857 data breaches were reported to the United States Department of Health and Human Services.1 The 3 main types of breaches reported were theft, hacking/IT incident, or unauthorized access/disclosure.1 Health care has become a common target due to the availability of valuable patient information (health, personal, and financial), the industry’s financial stability and resource capacity, and network susceptibility.2 The top 2 cybersecurity threats facing physician practices are:
- ransomware attacks, in which an external party uses a type of malicious software (malware) that prevents you from accessing your computer files, systems, or networks, and demands you pay a ransom for their return.
- employee-related threats, such as the theft or destruction of sensitive information by a disgruntled employee.3
The financial implications of health care–related cybersecurity threats coupled with exposure to potential litigation associated with breaches of confidentiality result in a need to “cybersecure” your practice.2 In this article, I outline steps to take to protect your practice against such threats. Although the recommendations I provide will increase your practice’s cybersecurity fortification, they are not exhaustive, and you may need to consult with an IT specialist to help protect your data and network.
Improve your network protection. A broadband internet connection is always operating, which makes it continuously susceptible to cybersecurity attacks. Install a firewall (a network security system that monitors and controls network traffic and permits or blocks traffic based on a defined set of rules) between your practice’s internal computer network and the internet.4 For maximum protection, enable all available firewall settings in your operating software.2 Prevent unauthorized access by ensuring that all network passwords are strong (ie, they include a combination of uppercase and lowercase letters, numbers, and symbols). Consider using different networks for online communication and for storing sensitive information.2 Create separate Wi-Fi networks for your practice and for your patients, and use unique passwords for each that are not easily guessed.4 If you or your employees use a virtual private network (VPN) to remotely access your practice’s network, ensure that all devices used to do so (cell phones, tablets, etc) are encrypted and secured with strong passwords.
Reduce employee-related threats. Not every employee in your practice will need to access to your patients’ clinical or financial data. Limiting employee access to sensitive clinical or financial data can reduce the risks of employee-related cybersecurity threats.3 In addition, restrict an employee’s ability to install software on computers and other devices that belong to your practice.2
Frequently incorporate cybersecurity training, such as teaching your employees about the risks of clicking on links and attachments in emails and how to identify phishing attacks (in which an individual sends a fraudulent communication that appears to come from a reputable source in order to trick the recipient into revealing financial information, system credentials, or other sensitive data).2,3 Use multifactor authentication to verify an employee’s login identity, and change passwords often. Reinforce these policies at staff meetings and educate new employees about this process.3 If you need to fire an employee, consider deploying cybersurveillance software to monitor the behavior of all employees before the employee is terminated.3 Once the employee has been terminated, change all logins and passwords.
The health care sector is not immune from cybersecurity attacks (malicious attempts to access or damage a computer or network system). Between October 2019 and October 2021, 857 data breaches were reported to the United States Department of Health and Human Services.1 The 3 main types of breaches reported were theft, hacking/IT incident, or unauthorized access/disclosure.1 Health care has become a common target due to the availability of valuable patient information (health, personal, and financial), the industry’s financial stability and resource capacity, and network susceptibility.2 The top 2 cybersecurity threats facing physician practices are:
- ransomware attacks, in which an external party uses a type of malicious software (malware) that prevents you from accessing your computer files, systems, or networks, and demands you pay a ransom for their return.
- employee-related threats, such as the theft or destruction of sensitive information by a disgruntled employee.3
The financial implications of health care–related cybersecurity threats coupled with exposure to potential litigation associated with breaches of confidentiality result in a need to “cybersecure” your practice.2 In this article, I outline steps to take to protect your practice against such threats. Although the recommendations I provide will increase your practice’s cybersecurity fortification, they are not exhaustive, and you may need to consult with an IT specialist to help protect your data and network.
Improve your network protection. A broadband internet connection is always operating, which makes it continuously susceptible to cybersecurity attacks. Install a firewall (a network security system that monitors and controls network traffic and permits or blocks traffic based on a defined set of rules) between your practice’s internal computer network and the internet.4 For maximum protection, enable all available firewall settings in your operating software.2 Prevent unauthorized access by ensuring that all network passwords are strong (ie, they include a combination of uppercase and lowercase letters, numbers, and symbols). Consider using different networks for online communication and for storing sensitive information.2 Create separate Wi-Fi networks for your practice and for your patients, and use unique passwords for each that are not easily guessed.4 If you or your employees use a virtual private network (VPN) to remotely access your practice’s network, ensure that all devices used to do so (cell phones, tablets, etc) are encrypted and secured with strong passwords.
Reduce employee-related threats. Not every employee in your practice will need to access to your patients’ clinical or financial data. Limiting employee access to sensitive clinical or financial data can reduce the risks of employee-related cybersecurity threats.3 In addition, restrict an employee’s ability to install software on computers and other devices that belong to your practice.2
Frequently incorporate cybersecurity training, such as teaching your employees about the risks of clicking on links and attachments in emails and how to identify phishing attacks (in which an individual sends a fraudulent communication that appears to come from a reputable source in order to trick the recipient into revealing financial information, system credentials, or other sensitive data).2,3 Use multifactor authentication to verify an employee’s login identity, and change passwords often. Reinforce these policies at staff meetings and educate new employees about this process.3 If you need to fire an employee, consider deploying cybersurveillance software to monitor the behavior of all employees before the employee is terminated.3 Once the employee has been terminated, change all logins and passwords.
1. U.S. Department of Health and Human Services. Office for Civil Rights. Breach portal: Notice to the Secretary of HHS breach of unsecured protected health information. Accessed December 26, 2021. https://ocrportal.hhs.gov/ocr/breach/breach_report.jsf
2. Umali G. How to safeguard your practice from cybersecurity threats. Psychiatric News. 2021;56(12):23.
3. Cryts A. Top two cybersecurity threats facing physician practices. Physicians Practice. March 13, 2020. Accessed December 26, 2021. https://www.physicianspractice.com/view/top-two-cybersecurity-threats-facing-physician-practices
4. American Medical Association. Protect your practice and patients from cybersecurity threats. 2017. Accessed December 26, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/network-security.pdf
1. U.S. Department of Health and Human Services. Office for Civil Rights. Breach portal: Notice to the Secretary of HHS breach of unsecured protected health information. Accessed December 26, 2021. https://ocrportal.hhs.gov/ocr/breach/breach_report.jsf
2. Umali G. How to safeguard your practice from cybersecurity threats. Psychiatric News. 2021;56(12):23.
3. Cryts A. Top two cybersecurity threats facing physician practices. Physicians Practice. March 13, 2020. Accessed December 26, 2021. https://www.physicianspractice.com/view/top-two-cybersecurity-threats-facing-physician-practices
4. American Medical Association. Protect your practice and patients from cybersecurity threats. 2017. Accessed December 26, 2021. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/network-security.pdf
Managing bipolar disorder in women who are pregnant
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
Psychiatrists who treat women of childbearing age should consider that those women may become pregnant, and that women with psychiatric illness are more likely to have unplanned pregnancies.1 Thus, thoughtful perinatal medication choices should begin before pregnancy. Pregnancy is a time of vulnerability to psychiatric illness for many reasons, including physiologic changes that can affect mental status; changes in medication efficacy; and numerous stressors, such as new responsibilities and limited sleep.1,2 For the treatment of pregnant—or potentially pregnant—patients, we recommend the following.
Do not panic! Knee-jerk medication changes in response to learning a patient is pregnant can lead to an exacerbation of psychiatric symptoms, as well as decrease trust in clinicians.2 Switching to a medication with a purportedly “safer” reproductive profile may worsen psychiatric illness, while also exposing the fetus to a medication of unknown benefit. 2
Recognize the risk of untreated or undertreated psychiatric illness, either of which has the potential to harm both the woman and her fetus. For example, a pregnant woman in a manic state may be more likely to engage in risky behaviors, such as drug use or risky sexual activity, which can lead to adverse fetal outcomes. They may also present with a higher risk of suicide. Compared to nonpregnant women, pregnant women for whom lithium was discontinued were equally likely to experience illness recurrence and significantly more likely to experience postpartum illness recurrence.3 In addition, the risk of recurrence was greater after rapid discontinuation compared with gradual discontinuation.3
Accurately communicate research findings. Pregnancy risk categories are no longer used. A nuanced interpretation of the potential adverse effects of a medication, such as malformations, impaired fetal growth, birth outcomes (such as preterm birth), and neurodevelopmental sequelae is necessary. Physicians must accurately convey information about risks to their patients, including both the absolute risk of an adverse event and the possible range of severity. For example, lithium use during pregnancy confers a higher relative risk of Ebstein’s anomaly (a cardiac defect).4 However, the absolute incidence of this risk remains low: 0.6% of lithium-exposed infants vs 0.18% among unexposed infants.4 Ebstein’s anomaly also varies significantly in severity—serious cases may require surgery, but less serious cases need only monitoring. A reliable database that compiles the latest evidence may help in staying abreast of the latest data.
Treat the psychiatric illness. Consider the optimal treatment for the psychiatric illness. Lithium remains the gold standard treatment for bipolar I disorder, regardless of reproductive status. Olanzapine and quetiapine are also commonly used and effective during pregnancy. This is an opportunity to conduct a detailed review of the patient’s previous medication regimens, including a review of medication trials and efficacy. Keep in mind that untreated bipolar disorder also carries an increased risk of adverse pregnancy outcomes.5
Consider pregnancy timing. Most organs form between weeks 3 to 8 of pregnancy. For example, if a medication potentially affects heart formation, but the patient is in the third trimester, explain to her that the heart has already been formed. Consider that medication may be required long-term and affect future pregnancies. Pregnant women require more frequent monitoring, because blood volume changes in pregnancy and postpartum can affect medication levels and efficacy. In addition, note whether a woman plans to breastfeed and be mindful of a medication’s profile in breastfeeding.
Ensure the patient can provide informed consent. Communicate your diagnostic formulation and treatment options. Consider involving the patient’s partner and/or support system in the discussion, if the patient consents. If a patient cannot provide informed consent, a surrogate decision-maker should be identified.6
Continue to: Collaborate with other clinicians
Collaborate with other clinicians, such as the patient’s OB/GYN and family medicine physician when possible. This will ensure that all clinicians are on the same page.
Plan for future pregnancies. Psychiatric medications can be long-term. Even patients who say they do not wish to become pregnant may someday become pregnant. Having discussions about medication choices, and their reproductive implications, prior to pregnancy allows patients to take an active role in their health.1,2
Consult a reproductive psychiatrist when indicated, and as early in the pregnancy as possible.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
1. Friedman SH. The ethics of treating depression in pregnancy. J Prim Health Care. 2015;7(1):81-83.
2. Friedman SH, Reed E. Treating psychosis in pregnant women: a measured approach. Current Psychiatry. 2021; 20(7):34-35.
3. Viguera AC, Nonacs R, Cohen LS, et al. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry. 2000;157(2):179-184.
4. Patorno E, Huybrechts KF, Bateman BT, et al. Lithium use in pregnancy and the risk of cardiac malformations. N Engl J Med. 2017;376(23):2245-2254.
5. Bodén R, Lundgren M, Brandt L, et al. Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ. 2012;345:e7085. doi:10.1136/bmj.e7085
6. Ross NE, Webster TG, Tastenhoye CA, et al. Reproductive decision-making capacity in women with psychiatric illness: a systematic review. J Acad Consult Liaison Psychiatry. 2022:63(1);61-70.
Loneliness: How psychiatry can help
Loneliness is distress that occurs when the quality or quantity of social relationships are less than desired.1 It is a symptom of many psychiatric disorders, and can lead to multiple negative health consequences, including depression, sleep deprivation, executive dysfunction, accelerated cognitive decline, and hypertension. Loneliness can increase the likelihood of immunocompromising conditions, including (but not limited to) stroke, anxiety, and depression, resulting in frequent emergency department visits and costly health expenses.2 Up to 80% of individuals younger than age 18 and 40% of adults older than age 65 report being lonely at least sometimes, with levels of loneliness gradually diminishing during middle age and then increasing in older adults.1 Loneliness is such a common and pervasive problem that in 2017, the government of the United Kingdom created a commission on loneliness and developed a Minister of Loneliness to find solutions to reduce it.3 In this article, I discuss the detrimental impact loneliness can have on our patients, and steps we can take to address it.
What contributes to loneliness?
Most people prefer the company of others, but some psychiatric disorders can cause individuals to become antisocial. For example, patients with schizoid personality disorder avoid social activities and interaction with others. Other patients may want to form bonds with others but their psychiatric disorder hinders this. For example, those with paranoia and social anxiety may avoid interacting with people due to their mistrust of others or their actions. Patients with substance use disorders can drive away those closest to them and lose familial bonds as a result of their behaviors. Patients with depression might not have the energy to pursue relationships and often have faulty cognitive patterns that lead them to believe they are unloved and unwanted.
Situational factors play a significant role in feelings of loneliness. Loss of a job or friends, ending a relationship, death of a loved one, or social isolation as experienced by COVID-19 or other illnesses can lead to loneliness. Social factors such as lack of income or transportation can make it difficult to attend or take part in social activities and events.
Some patients with dementia express feeling lonely, even after a visit from loved ones, because they forget the visit occurred. Nursing home residents often experience loneliness. Children may feel lonely after being subjected to bullying. College students, especially freshmen who are away from home for the first time, report significant levels of loneliness. Members of the LGBTQ+ community are often lonely due to familial rejection, prejudice, and religious beliefs. Anyone can experience loneliness, even married individuals if the marriage is unsatisfying.
What can psychiatry do to help?
Fortunately, psychiatric clinicians can play a large role in helping patients with loneliness.
Assessment. Ask the patient about the status of their present relationships and if they are feeling lonely. If yes, ask additional questions to identify possible causes. Are there conflicts that can be resolved? Is there abuse? What do they believe is the cause of their loneliness, and what might be the solution? How would their life be different if they weren’t lonely?
Treatment. When indicated, pharmacologic interventions might relieve symptoms that interfere with relationships and social interactions. For example, several types of antidepressants can improve mood and reduce anxiety, and selective serotonin reuptake inhibitors may relieve panic symptoms. Benzodiazepines and beta-blockers can reduce symptoms of social anxiety. Antipsychotics can reduce paranoia. Stimulants can aid patients with attention-deficit/hyperactivity disorder by improving their ability to interact with others.
Continue to: Psychotherapy and counseling...
Psychotherapy and counseling can specifically target loneliness. Solution-focused therapy, for example, involves solving the problem by deciding which actions the patient needs to take to relieve symptoms of loneliness. Dialectal behavior therapy can help patients with borderline and other personality disorders regulate their emotions and accept their feelings. Cognitive therapy and rational emotive therapy use various techniques to assist patients in changing their negative thought patterns. For example, a therapist might assign a patient to introduce themselves to a stranger or attend an event with others. The assignment is then discussed at the next session. Client-centered, psychodynamic, and behavior therapies also may be appropriate for a patient experiencing loneliness. Positive psychology can aid patients by helping them appreciate and not discount others in their lives. Meditation and mindfulness can motivate individuals to live in the present and enjoy those around them.
Referral and psychosocial support can be offered to direct patients to social services for help in improving their living circumstances. For example, a patient with an alcohol use disorder may benefit from a referral to a self-help organization such as Alcoholics Anonymous, where they can receive additional support and develop friendships. Other resources might offer patients the ability to discuss solutions, such as the benefits of owning a pet, attending a class, or volunteering opportunities, to combat loneliness.
Living a purposeful life is essential to engaging with others and avoiding isolation. Many people have turned to online support rooms, chat rooms, gaming, and social media to maintain relationships and meet others. Excessive computer use can be detrimental, however, if used in a manner that doesn’t involve interaction with others.
Regardless of the specific intervention, psychiatrists and other psychiatric clinicians can play a major role in reducing a patient’s loneliness. Simply by being present, you are showing the patient that at least one person in their life listens and cares.
1. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218-227.
2. Pimlott N. The ministry of loneliness. Can Fam Physician. 2018;64(3):166.
3. Leach N. The health consequences of loneliness. Causes and health consequences of being lonely. 2020. Accessed March 24, 2022. https://www.awpnow.com/main/2020/02/04/the-health-consequences-of-loneliness/
Loneliness is distress that occurs when the quality or quantity of social relationships are less than desired.1 It is a symptom of many psychiatric disorders, and can lead to multiple negative health consequences, including depression, sleep deprivation, executive dysfunction, accelerated cognitive decline, and hypertension. Loneliness can increase the likelihood of immunocompromising conditions, including (but not limited to) stroke, anxiety, and depression, resulting in frequent emergency department visits and costly health expenses.2 Up to 80% of individuals younger than age 18 and 40% of adults older than age 65 report being lonely at least sometimes, with levels of loneliness gradually diminishing during middle age and then increasing in older adults.1 Loneliness is such a common and pervasive problem that in 2017, the government of the United Kingdom created a commission on loneliness and developed a Minister of Loneliness to find solutions to reduce it.3 In this article, I discuss the detrimental impact loneliness can have on our patients, and steps we can take to address it.
What contributes to loneliness?
Most people prefer the company of others, but some psychiatric disorders can cause individuals to become antisocial. For example, patients with schizoid personality disorder avoid social activities and interaction with others. Other patients may want to form bonds with others but their psychiatric disorder hinders this. For example, those with paranoia and social anxiety may avoid interacting with people due to their mistrust of others or their actions. Patients with substance use disorders can drive away those closest to them and lose familial bonds as a result of their behaviors. Patients with depression might not have the energy to pursue relationships and often have faulty cognitive patterns that lead them to believe they are unloved and unwanted.
Situational factors play a significant role in feelings of loneliness. Loss of a job or friends, ending a relationship, death of a loved one, or social isolation as experienced by COVID-19 or other illnesses can lead to loneliness. Social factors such as lack of income or transportation can make it difficult to attend or take part in social activities and events.
Some patients with dementia express feeling lonely, even after a visit from loved ones, because they forget the visit occurred. Nursing home residents often experience loneliness. Children may feel lonely after being subjected to bullying. College students, especially freshmen who are away from home for the first time, report significant levels of loneliness. Members of the LGBTQ+ community are often lonely due to familial rejection, prejudice, and religious beliefs. Anyone can experience loneliness, even married individuals if the marriage is unsatisfying.
What can psychiatry do to help?
Fortunately, psychiatric clinicians can play a large role in helping patients with loneliness.
Assessment. Ask the patient about the status of their present relationships and if they are feeling lonely. If yes, ask additional questions to identify possible causes. Are there conflicts that can be resolved? Is there abuse? What do they believe is the cause of their loneliness, and what might be the solution? How would their life be different if they weren’t lonely?
Treatment. When indicated, pharmacologic interventions might relieve symptoms that interfere with relationships and social interactions. For example, several types of antidepressants can improve mood and reduce anxiety, and selective serotonin reuptake inhibitors may relieve panic symptoms. Benzodiazepines and beta-blockers can reduce symptoms of social anxiety. Antipsychotics can reduce paranoia. Stimulants can aid patients with attention-deficit/hyperactivity disorder by improving their ability to interact with others.
Continue to: Psychotherapy and counseling...
Psychotherapy and counseling can specifically target loneliness. Solution-focused therapy, for example, involves solving the problem by deciding which actions the patient needs to take to relieve symptoms of loneliness. Dialectal behavior therapy can help patients with borderline and other personality disorders regulate their emotions and accept their feelings. Cognitive therapy and rational emotive therapy use various techniques to assist patients in changing their negative thought patterns. For example, a therapist might assign a patient to introduce themselves to a stranger or attend an event with others. The assignment is then discussed at the next session. Client-centered, psychodynamic, and behavior therapies also may be appropriate for a patient experiencing loneliness. Positive psychology can aid patients by helping them appreciate and not discount others in their lives. Meditation and mindfulness can motivate individuals to live in the present and enjoy those around them.
Referral and psychosocial support can be offered to direct patients to social services for help in improving their living circumstances. For example, a patient with an alcohol use disorder may benefit from a referral to a self-help organization such as Alcoholics Anonymous, where they can receive additional support and develop friendships. Other resources might offer patients the ability to discuss solutions, such as the benefits of owning a pet, attending a class, or volunteering opportunities, to combat loneliness.
Living a purposeful life is essential to engaging with others and avoiding isolation. Many people have turned to online support rooms, chat rooms, gaming, and social media to maintain relationships and meet others. Excessive computer use can be detrimental, however, if used in a manner that doesn’t involve interaction with others.
Regardless of the specific intervention, psychiatrists and other psychiatric clinicians can play a major role in reducing a patient’s loneliness. Simply by being present, you are showing the patient that at least one person in their life listens and cares.
Loneliness is distress that occurs when the quality or quantity of social relationships are less than desired.1 It is a symptom of many psychiatric disorders, and can lead to multiple negative health consequences, including depression, sleep deprivation, executive dysfunction, accelerated cognitive decline, and hypertension. Loneliness can increase the likelihood of immunocompromising conditions, including (but not limited to) stroke, anxiety, and depression, resulting in frequent emergency department visits and costly health expenses.2 Up to 80% of individuals younger than age 18 and 40% of adults older than age 65 report being lonely at least sometimes, with levels of loneliness gradually diminishing during middle age and then increasing in older adults.1 Loneliness is such a common and pervasive problem that in 2017, the government of the United Kingdom created a commission on loneliness and developed a Minister of Loneliness to find solutions to reduce it.3 In this article, I discuss the detrimental impact loneliness can have on our patients, and steps we can take to address it.
What contributes to loneliness?
Most people prefer the company of others, but some psychiatric disorders can cause individuals to become antisocial. For example, patients with schizoid personality disorder avoid social activities and interaction with others. Other patients may want to form bonds with others but their psychiatric disorder hinders this. For example, those with paranoia and social anxiety may avoid interacting with people due to their mistrust of others or their actions. Patients with substance use disorders can drive away those closest to them and lose familial bonds as a result of their behaviors. Patients with depression might not have the energy to pursue relationships and often have faulty cognitive patterns that lead them to believe they are unloved and unwanted.
Situational factors play a significant role in feelings of loneliness. Loss of a job or friends, ending a relationship, death of a loved one, or social isolation as experienced by COVID-19 or other illnesses can lead to loneliness. Social factors such as lack of income or transportation can make it difficult to attend or take part in social activities and events.
Some patients with dementia express feeling lonely, even after a visit from loved ones, because they forget the visit occurred. Nursing home residents often experience loneliness. Children may feel lonely after being subjected to bullying. College students, especially freshmen who are away from home for the first time, report significant levels of loneliness. Members of the LGBTQ+ community are often lonely due to familial rejection, prejudice, and religious beliefs. Anyone can experience loneliness, even married individuals if the marriage is unsatisfying.
What can psychiatry do to help?
Fortunately, psychiatric clinicians can play a large role in helping patients with loneliness.
Assessment. Ask the patient about the status of their present relationships and if they are feeling lonely. If yes, ask additional questions to identify possible causes. Are there conflicts that can be resolved? Is there abuse? What do they believe is the cause of their loneliness, and what might be the solution? How would their life be different if they weren’t lonely?
Treatment. When indicated, pharmacologic interventions might relieve symptoms that interfere with relationships and social interactions. For example, several types of antidepressants can improve mood and reduce anxiety, and selective serotonin reuptake inhibitors may relieve panic symptoms. Benzodiazepines and beta-blockers can reduce symptoms of social anxiety. Antipsychotics can reduce paranoia. Stimulants can aid patients with attention-deficit/hyperactivity disorder by improving their ability to interact with others.
Continue to: Psychotherapy and counseling...
Psychotherapy and counseling can specifically target loneliness. Solution-focused therapy, for example, involves solving the problem by deciding which actions the patient needs to take to relieve symptoms of loneliness. Dialectal behavior therapy can help patients with borderline and other personality disorders regulate their emotions and accept their feelings. Cognitive therapy and rational emotive therapy use various techniques to assist patients in changing their negative thought patterns. For example, a therapist might assign a patient to introduce themselves to a stranger or attend an event with others. The assignment is then discussed at the next session. Client-centered, psychodynamic, and behavior therapies also may be appropriate for a patient experiencing loneliness. Positive psychology can aid patients by helping them appreciate and not discount others in their lives. Meditation and mindfulness can motivate individuals to live in the present and enjoy those around them.
Referral and psychosocial support can be offered to direct patients to social services for help in improving their living circumstances. For example, a patient with an alcohol use disorder may benefit from a referral to a self-help organization such as Alcoholics Anonymous, where they can receive additional support and develop friendships. Other resources might offer patients the ability to discuss solutions, such as the benefits of owning a pet, attending a class, or volunteering opportunities, to combat loneliness.
Living a purposeful life is essential to engaging with others and avoiding isolation. Many people have turned to online support rooms, chat rooms, gaming, and social media to maintain relationships and meet others. Excessive computer use can be detrimental, however, if used in a manner that doesn’t involve interaction with others.
Regardless of the specific intervention, psychiatrists and other psychiatric clinicians can play a major role in reducing a patient’s loneliness. Simply by being present, you are showing the patient that at least one person in their life listens and cares.
1. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218-227.
2. Pimlott N. The ministry of loneliness. Can Fam Physician. 2018;64(3):166.
3. Leach N. The health consequences of loneliness. Causes and health consequences of being lonely. 2020. Accessed March 24, 2022. https://www.awpnow.com/main/2020/02/04/the-health-consequences-of-loneliness/
1. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218-227.
2. Pimlott N. The ministry of loneliness. Can Fam Physician. 2018;64(3):166.
3. Leach N. The health consequences of loneliness. Causes and health consequences of being lonely. 2020. Accessed March 24, 2022. https://www.awpnow.com/main/2020/02/04/the-health-consequences-of-loneliness/
Techniques and Technologies to Improve Peripheral Intravenous Catheter Outcomes in Pediatric Patients: Systematic Review and Meta-Analysis
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.
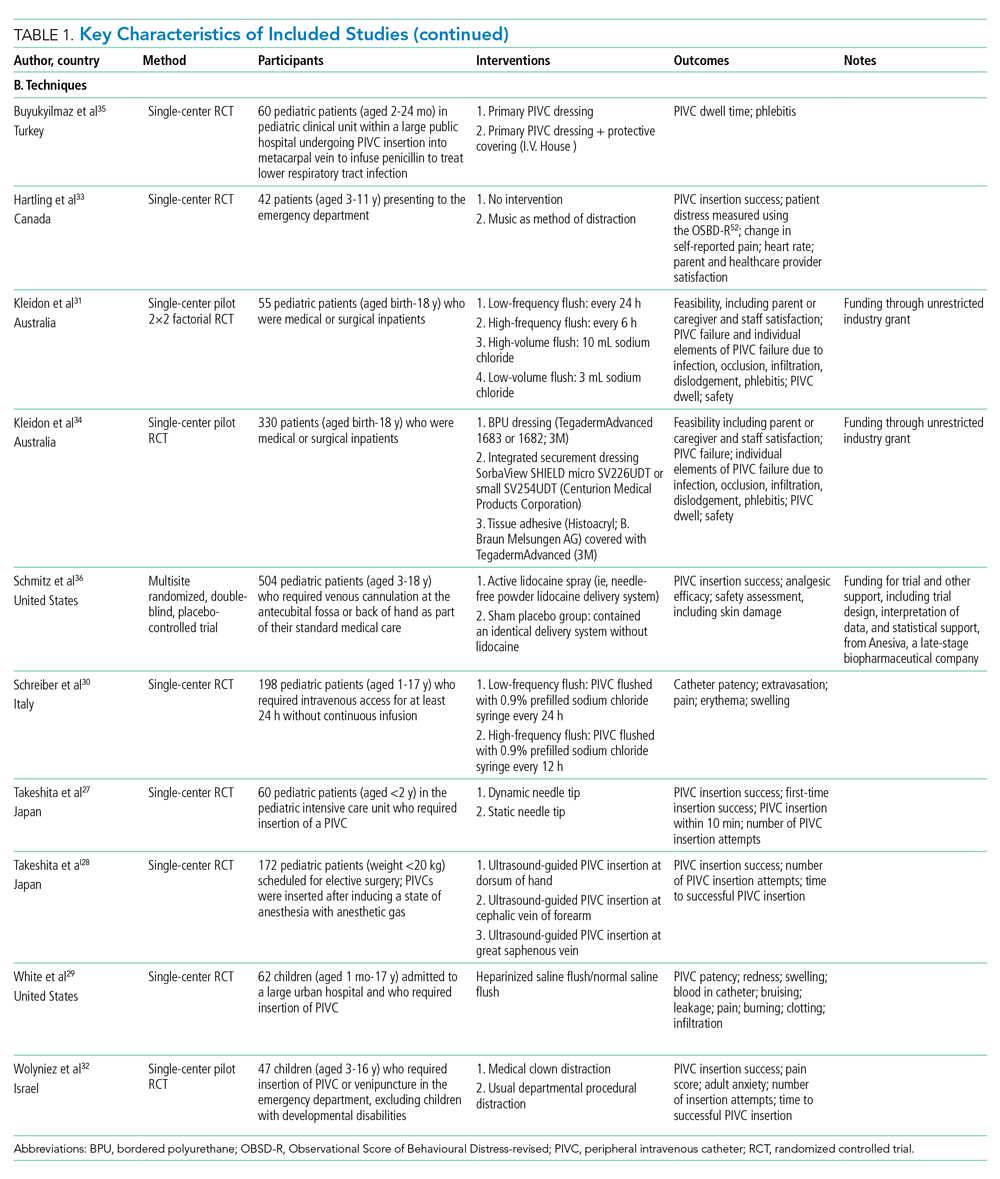
Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.

Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.

Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
© 2021 Society of Hospital Medicine
Evaluation and Medical Management of the Pediatric Patient With Orbital Cellulitis/Abscess: A Systematic Review
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.
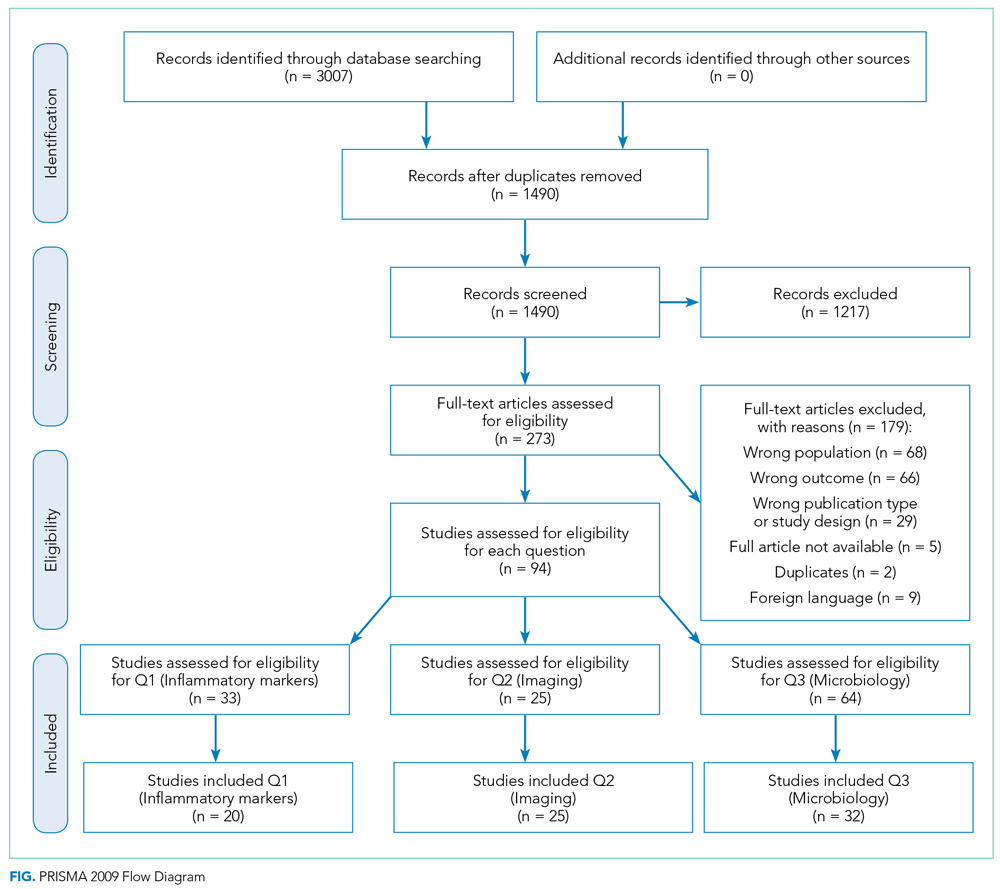
Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).
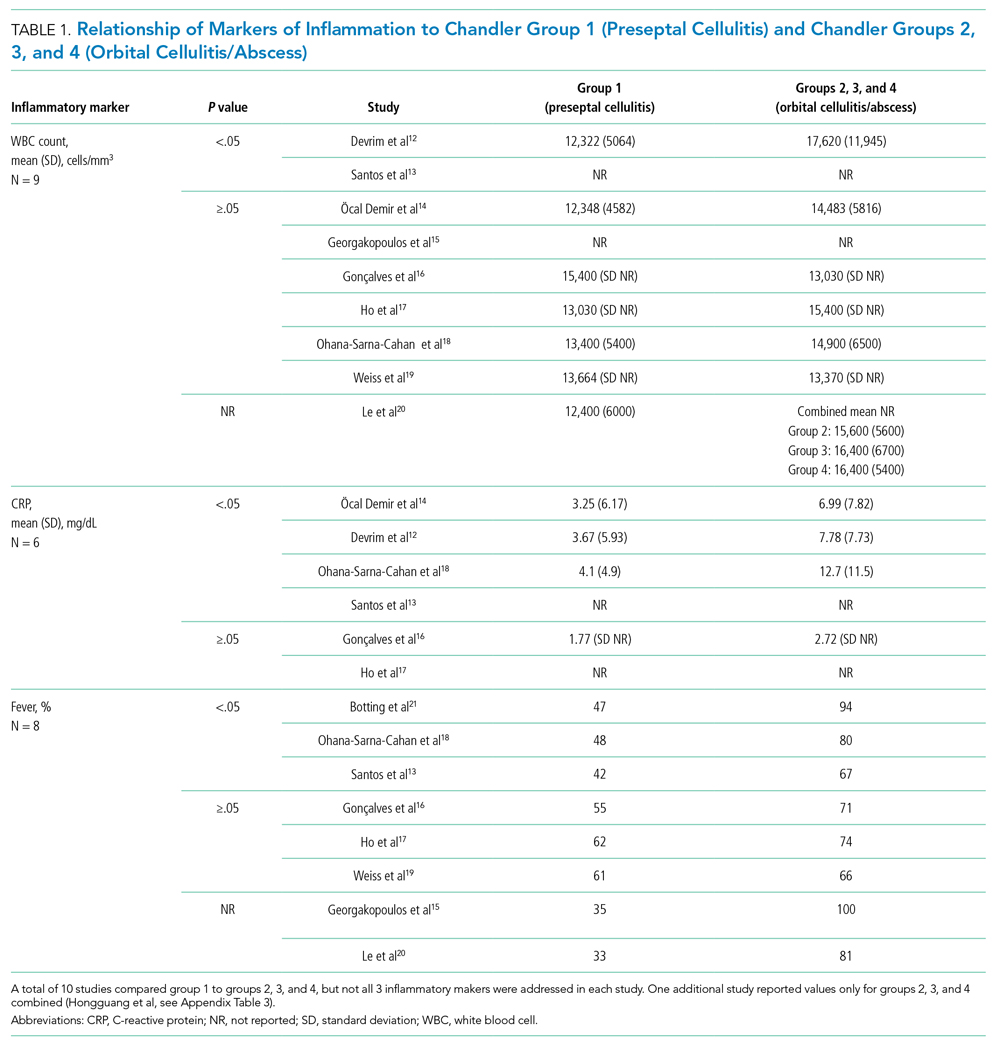
Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.

Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).

Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
Orbital cellulitis/abscess (OCA) is a potential complication of sinusitis. If not treated promptly, it can result in vision loss, intracranial infection, or cavernous sinus thrombosis.1,2 In 1970, Chandler et al3 classified orbital complications of acute sinusitis into five groups: inflammatory edema (group 1); orbital cellulitis (group 2); subperiosteal abscess (SPA) (group 3); orbital abscess (group 4); and cavernous sinus thrombosis (group 5). Group 1, or preseptal cellulitis, is significantly different from groups 2, 3, and 4, collectively referred to as OCA, which affect the actual orbital content.
Children with OCA are generally hospitalized so they can be treated with intravenous antibiotics. While orbital abscesses (group 4) are typically treated surgically, successful medical management has been reported for cases of orbital cellulitis and SPA (groups 2 and 3).4,5 No widely accepted guidelines exist for the evaluation and medical management of OCA, resulting in significant variation in care.6 The purpose of this systematic review is to summarize existing evidence guiding the medical management of OCA regarding laboratory testing, imaging, and microbiology. This review does not address surgical considerations.
METHODS
The review protocol has been registered in the PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp; identifier: CRD42020158463), and the review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7
Search Strategy
A systematic search of the literature was designed and conducted by a medical librarian (ES), with input from the research team (AB, SM). The search strategy included Medical Subject Headings (MeSH) terms and keywords related to orbital or subperiosteal cellulitis/abscess and children; see Appendix Table 1 for the complete search strategy. Searches were conducted in MEDLINE (Ovid), Web of Science Core Collection, Scopus, CINAHL (EBSCO), and Cochrane Central Register of Controlled Trials (CENTRAL) using advanced search techniques relative to each database. Searches were last conducted on February 9, 2021.
Eligibility Criteria
The study designs (retrospective and prospective) included in the search were limited to randomized clinical trials, cohort studies, case-control studies, and case series with participants <18 years of age. Case reports describing fewer than 5 patients and literature reviews were excluded. Studies including a combination of adult and pediatric patients were included if pediatric outcomes were reported separately. Only studies available in English were included.
Outcome Measures
The outcome measures were determined a priori based on three clinical questions:
- Q1. What is the role of inflammatory markers—white blood cell (WBC) count, C-reactive protein (CRP), and fever—in distinguishing between the following: preseptal cellulitis (group 1) and OCA (groups 2, 3, and 4); orbital cellulitis (group 2) and abscess (groups 3 and 4); and patients who do and do not require surgery?
- Q2. What is the role of imaging in the evaluation of OCA?
- Q3. What is the microbiology of OCA over the past 2 decades? What is the prevalence of methicillin-resistant Staphylococcus aureus (MRSA)?
Screening
Two review authors (AB, SM) performed both the title/abstract and full-text screen, independently applying the eligibility criteria. Disagreements were discussed, and conflicts were resolved with input from a third reviewer author (ES). Duplications were removed. When two studies had overlapping patient data, the study with fewer data points was excluded.
Data Extraction and Synthesis
All studies included after the full-text screen were divided based on the clinical question they answered (Q1, Q2, Q3 above). Some studies reported outcomes pertinent to more than one question. Two review authors were assigned to each clinical question. They independently reviewed each article and extracted the pertinent data into question-specific extraction sheets. Articles assigned to Q2 were reviewed by two pediatric neuroradiologists. For each study, the following details were extracted: authors, location, year, study type, study period, population, and number and ages of participants. Details that were question-specific included: (Q1) values and/or percentages for inflammatory markers; (Q2) reasons for imaging or type of imaging; and (Q3) participants managed surgically and culture results. The data were then synthesized in table and/or narrative format. For Q3, the organisms identified from intraoperative and blood cultures in each study were mathematically combined. When possible, prevalence was calculated using the number of patients with at least one pathogen recovered as the denominator. If this number was not available, the number of patients who underwent surgery was used as the denominator.
Quality Assessment
No randomized controlled trials were identified. More than 90% of the studies identified and included were retrospective descriptive studies. By the nature of the case series design, the study quality was felt to be poor, with high risk of bias. The Joanna Briggs Institute Critical Appraisal tools for systematic reviews were used to appraise each individual study included (Appendix Table 2).8 The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria were used in rating the quality of evidence for each question.9
RESULTS
A summary of the search strategy and study selection is provided in the Figure (PRISMA flow diagram). The initial search identified 3007 studies. After duplicates were removed and general eligibility criteria applied, 94 articles remained. Question-specific eligibility criteria, discussed in the following sections, were then applied, resulting in 63 articles included in the review.

Q1: Are Inflammatory Markers, Including Fever, WBC, and CRP, Useful in Distinguishing Preseptal Cellulitis (group 1) From OCA (Groups 2, 3, and 4); Orbital Cellulitis (group 2) From Abscess (Groups 3 and 4); or Identifying Patients Who Require Surgical Intervention?
Fever and elevation of the WBC count and CRP have been used to assess the severity of certain pediatric infections10,11 and therefore may be helpful in distinguishing severity of illness in OCA. Studies included in this section provided numerical values for at least one of the following: WBC count, CRP, or percentage of patients with fever for at least one type of orbital infection. Included studies had at least five patients per group.
Thirty-three articles were screened for the inflammatory marker section. Thirteen were excluded for the following reasons: no numbers reported for inflammatory markers (n = 6); group 1 and groups 2, 3, and 4 results combined (n = 6); fewer than five patients with orbital cellulitis included (n = 1). Twenty studies were included: 18 case series and 2 retrospective cohorts. Appendix Table 3 summarizes the data from studies included. Based on GRADE criteria, the body of evidence included in this section is of low quality.9
Distinguishing Between Preseptal and OCA
Eleven studies were included in this section (Table 1). WBC count was significantly higher in patients with groups 2, 3, and 4 than group 1 in two studies (Devrim et al,12P < .01; Santos et al,13P = .025). CRP was significantly higher in patients with groups 2, 3, and 4 than group 1 in four studies (Öcal Demir et al,14P = .02; Devrim et al,12P < .01; Ohana-Sarna-Cahan et al,18P < .001; Santos et al,13P < .001). Patients with groups 2, 3, and 4 had a significantly higher fever rate in three studies (Botting et al,21P < .001; Ohana-Sarna-Cahan et al,18P = .0001; Santos et al,13 P = .029).

Distinguishing Between Orbital Cellulitis and Abscess
Seven studies were included in this section (Appendix Table 3). One study showed significantly higher WBC count in group 3 than group 2 (P = .004), although results were reported as percentage of patients above a cutoff number calculated to distinguish between cellulitis and abscess (Appendix Table 3).22 CRP was not significantly different between group 2 and groups 3 and 4. One study found a significantly higher fever rate in patients with group 3 compared to patients with group 2 (P < .001).22
Identifying Patients Requiring Surgery
Six studies were included in this section (Appendix Table 3). One study found a significantly higher WBC count in patients treated surgically (Tabarino et al,24P < .05). Patients treated surgically had a significantly higher CRP in two studies (Cohen et al,25P = .02; Friling et al,26 P = .04). Fever was inconsistently reported in the studies, with some using mean presenting temperatures and some using rates of fever. One study found a significantly higher mean presenting temperature in patients treated surgically (P = .027), but the difference between the two groups was 0.7 °C.23
Summary
Most studies found no significant difference in WBC count, CRP, or fever between preseptal and OCA, cellulitis and abscess, or patients receiving medical and surgical interventions.
Q2: What Is the Role of Imaging in Evaluation of OCA?
Twenty-five articles were selected for the imaging section review. All the included studies were retrospective descriptive studies. Quantitative data extraction and analysis of these studies could not be performed because of their heterogeneous methodologies and lack of objective data. Therefore, the information gleaned from these studies is summarized in narrative format. Per GRADE criteria, the body of evidence included in this section is of low quality.
Who Needs Imaging?
Proptosis, ophthalmoplegia, decreased vision, and pain with eye movements are widely agreed-upon indications for imaging evaluation.21,27,28 Because of concern for radiation exposure in pediatric patients, some authors suggested that computed tomography (CT) should only be obtained if patients fail to respond to medical therapy or if surgery is being considered.17,29,30 However, Rudloe et al31 found that half of the patients with group 3 or higher disease on CT did not have proptosis, ophthalmoplegia, or pain with extraocular movement. In addition, evaluation of young children with acute periorbital swelling can be difficult, so a lower threshold for imaging is likely warranted in these younger patients.
What Type of Imaging Should Be Obtained?
The American College of Radiology 2018 Appropriateness Criteria (ACR criteria) for orbital imaging state that orbital CT is usually indicated for patients with suspected Chandler groups 2, 3, and 4 infections.32 CT with contrast is useful for evaluating the extent of orbital infection and size of the abscess and for delineating the adjacent osseous anatomy, which is essential for cases in which surgical intervention is planned.20,21,26,27,30,31,33,34 Distinguishing abscess from cellulitis on CT sometimes can be challenging; therefore, serial clinical examinations and, occasionally, surgical exploration may be required.35,36
Magnetic resonance imaging (MRI) is helpful for evaluating intracranial complications (eg, epidural abscess),27,37 but it is limited for evaluating the osseous components of the paranasal sinuses. Although one study suggested that rapid MRI is comparable to contrast CT for differentiating group 1 infections from groups 2, 3, and 4 infections, it provided limited assessment of other complications.38 With no definitive studies comparing CT with MRI for orbital infections, adherence to the ACR criteria is recommended.
Orbital ultrasound is limited by its small field of view and artifact produced by the surrounding bony interface, both of which can obscure posterior intraorbital pathologies.29,39,40 Plain radiographs are not helpful for evaluating OCA due to limited soft-tissue contrast.41
When Should Repeat Imaging Be Obtained?
Children with group 3 OCA have been successfully managed medically in a carefully monitored setting.42 Repeat CT imaging is sometimes useful in these patients, particularly if the clinical examination is difficult.42-44 However, improvement in CT findings may lag behind clinical improvement.39
Summary
Per ACR criteria, orbital CT with contrast is recommended to evaluate patients with suspected Chandler groups 2, 3, and 4 OCA. MRI is reserved for evaluating intracranial complications.
Q3: What Is the Microbiology of OCA? What Is the MRSA Prevalence?
Knowledge of the microbiology of OCA is essential for the appropriate selection of empiric antibiotics. Because fewer children with groups 2 and 3 OCA undergo surgery, intraoperative cultures often are not available to guide antibiotic selection.45 As a result, significant variation exists in antibiotic prescribing.6
Studies discussing the microbiology of OCA were included only if they were published in the past 2 decades (2000-2020) and were excluded if the study period was before 1990, as microbiology changes over time and new vaccines are introduced. To be included, the majority of cultures reported had to be intraoperative (orbital or sinus) specimens. Studies reporting only nasal, conjunctival, or other surface cultures were excluded. When studies included patients with group 1 OCA, only microbiology data for groups 2, 3, and 4 OCA were extracted. The pattern of resistance for S aureus was not always explicitly reported; however, when non-MRSA active antibiotics were used, methicillin-susceptible S aureus was assumed.
A total of 63 studies were screened for the microbiology section; 32 were excluded for the following reasons: published before 2000 or study period before 1990 (n = 18), reported surface cultures or culture site not clearly stated (n = 4), microbiology mixed between preseptal and orbital (n = 6), wrong study type (n = 2), and study group overlaps with a different article included (n = 2). Of the 32 studies included, 3 were prospective observational, 4 were retrospective cohort, and 25 were case series. Based on GRADE criteria, the body of evidence included in this section is of low quality.42
Appendix Table 4 summarizes the microbiologic data from the studies included. In the group of children that had a positive culture (orbital, sinus, or blood), the most commonly recovered organisms reported were S aureus (median, 22%; range, 0%-100%), Streptococcus anginosus group (median, 16%; range, 0%-100%), group A Streptococcus (median, 12%; range, 0%-80%), and Streptococcus pneumoniae (median, 8%; range, 0%-100%). Streptococcus as a group had a median prevalence of 57%, ranging from 0% to 100%. MRSA prevalence had a median of 3% (interquartile range [IQR], 0%-13%). Median prevalence of polymicrobial cultures was 20%, and median prevalence of anaerobic organisms was 14% (Table 2). Orbital and sinus cultures had the highest yield, with an average return of an organism of 72% (median, 75%; IQR, 64%-84%).

Microbiology was compared between studies completed in the United States and in other countries (Table 2). Based on median prevalence across studies, both S anginosus group and MRSA were more prevalent in the United States than internationally (28% vs 0% and 11% vs 0%, respectively). No clear trend in MRSA prevalence was evident over the 2 decades; however, the studies included were heterogeneous and did not have the power to detect such a trend.
Two reports suggest a difference of MRSA prevalence by patient age. Hsu et al46 found that three of eight MRSA infections were in infants age <1 year, which accounted for 50% (3/6) of infants included in the study. Miller et al47 reported MRSA in 4 of 9 (44%) infants with OCA. Age <1 year may be associated with increased frequency of MRSA infection in OCA.
Summary
Blood cultures have low yield. The most common organisms recovered from OCA are Streptococcus species (most commonly S anginosus group, group A Streptococcus, and pneumococcus) and S aureus. Polymicrobial infections including anaerobes are common. MRSA prevalence is low globally but varies significantly among geographic areas.
DISCUSSION
Our systematic review of the literature for the medical management of OCA revealed predominantly descriptive studies and only a limited number of comparison-based studies, likely reflecting the rarity of advanced forms of OCA. Given the lack of high-quality evidence and the level of heterogeneity among studies, the conclusions that can be drawn are limited.
Distinguishing between disease severity and OCA requiring surgical intervention remains challenging. Although studies in our review suggest a trend toward markers of inflammation (fever, elevated WBC count and CRP) being more common in more severe presentations, the results were mixed, and studies were low quality and underpowered to detect meaningful differences. For example, most studies do not define what constitutes a fever in their cohort. Our review suggests that markers of inflammation cannot be used to distinguish between Chandler groups or to identify patients requiring surgery. Of note, the presence of fever and elevated inflammatory markers may have influenced the decision to obtain imaging or to proceed to surgery, thereby also potentially biasing these clinical indicators toward predictors for more severe disease. Decisions regarding surgery should therefore be based on the entire clinical picture, including response to appropriate antibiotics.
We found a lack of high-quality evidence regarding the role of imaging in OCA, and the studies reviewed were heterogeneous. Recommendations for imaging therefore remain at the level of expert opinion (ACR criteria). CT imaging is the first-line modality for imaging in suspected OCA given the limitations of alternative imaging modalities, but the sensitivity and specificity of CT imaging remain unknown for diagnosis of orbital abscesses.
Our review of the published microbiology confirmed that Staphylococcus and Streptococcus species are the most common pathogens identified in OCA. Prevalence across the different studies varied greatly. Owing to the significant heterogeneity in studies, calculation of pooled prevalence was not possible. By using the number of positive cultures as our denominator (or total surgeries if number of positive cultures was unavailable), we likely overestimated the prevalence of S aureus. S aureus is generally recognized as a pyogenic pathogen, more likely to be associated with abscess formation.48 Therefore, culture results obtained predominantly from abscesses likely result in an overestimate of S aureus in OCA (groups 2, 3, and 4). Regardless, MRSA prevalence was generally low, both nationally and internationally. The MRSA results from the study by McKinley at el49 (Texas) was a notable outlier in the United States, with MRSA prevalence as high as 44% compared with the median prevalence of 3% (IQR, 0-13), highlighting the importance of local resistance patterns when choosing empiric antibiotics.
Limitations to the microbiology review included significant heterogeneity in both the types of cultures included and the reporting of results. Although we excluded studies that reported only surface culture results or did not specify culture type, we did include studies that had surface culture results combined with intraoperative culture results, making it impossible to separate the two. Since most of the cultures included in combined results reported organisms based on intraoperative cultures, we felt they provided valuable information that should be included. In most studies, blood cultures were not obtained in all participants, so the yield of blood cultures is likely an overestimate, as blood cultures are more likely to be obtained in higher-acuity patients.
CONCLUSION
Although the available evidence regarding the medical management of OCA remains low quality, certain limited conclusions can be drawn, as presented in this review. Further high-quality studies are needed to better inform the medical management of OCA.
Acknowledgment
The authors thank Dr Kyle Pronko for his help with data extraction for the imaging section.
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
1. Reynolds D.J, Kodsi SR, Rubin SE, Rodgers IR. Intracranial infection associated with preseptal and orbital cellulitis in the pediatric patient. J AAPOS. 2003;7(6):413-417. https://doi.org/10.1016/j.jaapos.2003.09.013
2. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology. 2007;114(2):345-354. https://doi.org/10.1016/j.ophtha.2006.07.059
3. Chandler JR, Langenbrunner DJ, Stevens ER. Pathogenesis of orbital complications in acute sinusitis. Laryngoscope. 1970;1414-1428. https://doi.org/10.1288/00005537-197009000-00007
4. Wong SJ, Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123-129. https://doi.org/10.1016/j.ijporl.2018.05.006
5. Liao JC, Harris GJ. Subperiosteal abscess of the orbit: evolving pathogens and the therapeutic protocol. Ophthalmology. 2015;122(3):639-647. https://doi.org/10.1016/j.ophtha.2014.09.009
6. Markham JL, Hall M, Bettenhausen JL, Myers AL, Puls HT, McColloh RJ. Variation in care and clinical outcomes in children hospitalized with orbital cellulitis. Hosp Pediatr. 2018;8(1):28-35. https://doi.org/10.1542/hpeds.2017-0040
7. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
8. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127-2133. https://doi.org/10.11124/JBISRIR-D-19-00099
9. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. https://doi.org/10.1016/j.jclinepi.2010.07.015
10. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc. 2018;7(4):323-334. https://doi.org/10.1093/jpids/piy046
11. Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25-36. https://doi.org/10.1159/000336629
12. Devrim I, Kanra G, Kara A, et al. Preseptal and orbital cellulitis: 15-year experience with sulbactam ampicillin treatment. Turk J Pediatr. 2008;50(3):214-218.
13. Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol. 2019;120:82-88. https://doi.org/10.1016/j.ijporl.2019.02.003
14. Öcal Demir S , Çagan E, Kepenekli Kadayifci E, et al. Clinical features and outcome of preseptal and orbital cellulitis in hospitalized children: four years experience. Medeni Med J. 2017;32(1):7-13. https://doi.org/10.5222/MMJ.2017.007
15. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbitaln cellulitis: a 10-year review of hospitalized children. Eur J Ophthalmol. 2010;20(6):1066-1072. https://doi.org/10.1177/112067211002000607
16. Gonçalves R, Menezes C, Machado R, Ribeiro I, Lemos JA. Periorbital cellulitis in children: analysis of outcome of intravenous antibiotic therapy. Orbit. 2016;34(4):175-180. https://doi.org/10.1080/01676830.2016.1176205
17. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2017;40(6):518-524.
18. Ohana-Sarna-Cahan L, Hurvitz N, Gross I, Cohen A, Hashavya S. Factors associated with increased risk of pediatric orbital cellulitis—who should be scanned? Pediatr Emerg Care. Published online ahead of print March 19, 2020. https://doi.org/10.1097/PEC.0000000000002083
19. Weiss A, Friendly D, Eglin K, Chang M, Gold B. Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology. 1983;90(3):195-203. https://doi.org/10.1016/s0161-6420(83)34573-5
20. Le TD, Liu ES, Adatia FA, Buncic JR Blaser S. The effect of adding orbital computed tomography findings to the Chandler criteria for classifying pediatric orbital cellulitis in predicting which patients will require surgical intervention. J AAPOS. 2014;18(3):271-277. https://doi.org/10.1016/j.jaapos.2014.01.015
21. Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol. 2008;72(3):377-383. https://doi.org/10.1016/j.ijporl.2007.11.013
22. Huang SF, Lee TJ, Lee YS, Chen CC, Chin SC, Wang NC. Acute rhinosinusitis-related orbital infection in pediatric patients: a retrospective analysis. Ann Otol Rhinol Laryngol. 2011;120(3):185-190. https://doi.org/10.1177/000348941112000307
23. Ryan JT, Preciado A, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907-911. https://doi.org/10.1016/j.otohns.2009.02.014
24. Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131-135. https://doi.org/10.1016/j.ijporl.2014.11.021
25. Cohen N, Erisson S, Anafy A, et al. Clinicians need to consider surgery when presented with some markers for severe paediatric orbital cellulitis. Acta Paediatr. 2020;109(6):1269-1270. https://doi.org/10.1111/apa.15125
26. Friling R, Garty BZ, Kornreich L, et al. Medical and surgical management of orbital cellulitis in children. Folia Med (Plovdiv). 2014;56(4):253-258. https://doi.org/10.1515/folmed-2015-0004
27. Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823-827. https://doi.org/10.1177/0194599811416559
28. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. https://doi.org/10.1259/bjr.20130503
29. Goodwin WJ Jr, Weinshall M, Chandler JR. The role of high resolution computerized tomography and standardized ultrasound in the evaluation of orbital cellulitis. Laryngoscope. 1982;92(7 pt 1):729-731.
30. Bilaniuk LT, Zimmerman RA. Computer‐assisted tomography: sinus lesions with orbital involvement. Head Neck Surg. 1980;2(4):293-301. https://doi.org/10.1002/hed.2890020407
31. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. https://doi.org/10.1542/peds.2009-1709
32. Kennedy TA, Corey AS, Policeni B, et al. ACR Appropriateness Criteria® orbits vision and visual loss. J Am Coll Radiol. 2018;15(5S):S116-S131. https://doi.org/10.1016/j.jacr.2018.03.023
33. De Silva M, Lam V, Broadfoot J. C.T. findings of orbital inflammation in children. Australas Radiol. 1987;31(3):241-245. https://doi.org/10.1111/j.1440-1673.1987.tb01822.x
34. Hirsch M, Lifshitz T. Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol. 1988;18(4):302-305. https://doi.org/10.1007/BF02388996
35. Andrews TM, Myer CM 3rd. The role of computed tomography in the diagnosis of subperiosteal abscess of the orbit. Clin Pediatr (Phila). 1992;31(1):37-43. https://doi.org/10.1177/000992289203100108
36. Clary RA, Cunningham MJ, Eavey RD. Orbital complications of acute sinusitis: comparison of computed tomography scan and surgical findings. Ann Otol Rhinol Laryngol. 1992;101(7):598-600. https://doi.org/10.1177/000348949210100710
37. Arjmand EM, LuskRP, Muntz HR. Pediatric sinusitis and subperiosteal orbital abscess formation: diagnosis and treatment. Otolaryngol Neck Surg. 1993;109(5):886.894. https://doi.org/10.1177/019459989310900518
38. Jain SF, Ishihara R, Wheelock L, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289.e1-289.e4. https://doi.org/10.1016/j.jaapos.2020.05.018
39. Harris GJ. Subperiosteal abscess of the orbit: computed tomography and the clinical course. Ophthal Plast Reconstr Surg. 1996;12:1-8. https://doi.org/10.1097/00002341-199603000-00001
40. Kaplan DM, Briscoe D, Gatot A, Niv A, Leiberman A, Fliss DM. The use of standardized orbital ultrasound in the diagnosis of sinus induced infections of the orbit in children: a preliminary report. Int J Pediatr Otorhinolaryngol. 1999;48(2):155-162. https://doi.org/10.1016/s0165-5876(99)00023-3
41. Towbin R, Han BK, Kaufman RA, Burke M. Postseptal cellulitis: CT in diagnosis and management. Radiology. 1986;158(3):735-737. https://doi.org/10.1148/radiology.158.3.3945747
42. Starkey CR, Steele RW. Medical management of orbital cellulitis. Pediatr Infect Dis J. 2001;20(10):1002-1005. https://doi.org/10.1097/00006454-200110000-00017
43. Brown CL, Graham SM, Griffin MC, et al. Pediatric medial subperiosteal orbital abscess: medical management where possible. Am J Rhinol. 2004;18(5):321-327.
44. Cossack MT, Herretes SP, Cham A, Sniegowski MC, Lyon DB. Radiographic course of medically managed pediatric orbital subperiosteal abscesses. J Pediatr Ophthalmol Strabismus. 2018;55(6):387-392. https://doi.org/10.3928/01913913-20180802-02
45. Zhao EE, Koochakzadeh S, Nguyen SA, et al. Orbital complications of acute bacterial rhinosinusitis in the pediatric population: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol. 2020;135:110078. https://doi.org/10.1016/j.ijporl.2020.110078
46. Hsu J, Treister AD, Ralay Ranaivo H, Rowley AH, Rahmani B. Microbiology of pediatric orbital cellulitis and trends in methicillin-resistant Staphylococcus aureus cases. Clin Pediatr (Phila). 2019;58(10):1056-1062. https://doi.org/10.1177/0009922819864587
47. Miller A, Castanes M, Yen M, Coats D, Yen K. Infantile orbital cellulitis. Ophthalmology. 2008;115(3):594. https://doi.org/10.1016/j.ophtha.2007.10.011
48. Dajani AS, Garcia RE, Wolinsky E. Etiology of cervical lymphadenitis in children. N Engl J Med. 1963;268:1329-1333. https://doi.org/10.1056/NEJM196306132682403
49. McKinley SH, Yen MT, Miller AM, Yen KG. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol. 2007;144(4):497-501. https://doi.org/10.1016/j.ajo.2007.04.049
© 2021 Society of Hospital Medicine
Early interventions for psychosis
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).
Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).
Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
Neuroscience research over the past half century has failed to significantly advance the treatment of severe mental illness.1,2 Hence, evidence that a longer duration of untreated psychosis (DUP) aggravates—and early intervention with medication and social supports ameliorates—the long-term adverse consequences of psychotic disorders generated a great deal of interest.3,4 This knowledge led to the development of diverse early intervention services worldwide aimed at this putative “critical window.” It raised the possibility that appropriate interventions could prevent the long-term disability that makes chronic psychosis one of the most debilitating disorders.5,6 However, even beyond the varied cultural and economic confounds, it is difficult to assess, compare, and optimize program effectiveness.7 Obstacles include paucity of sufficiently powered, well-designed randomized controlled trials (RCTs), the absence of diagnostic biomarkers or other prognostic indicators to better account for the inherent heterogeneity in the population and associated outcomes, and the absence of modifiable risk factors that can guide interventions and provide intermediate outcomes.4,8-10
To better appreciate these issues, it is important to distinguish whether a program is designed to prevent psychosis, or to mitigate the effects of psychosis. Two models include the:
- Prevention model, which focuses on young individuals who are not yet overtly psychotic but at high risk
- First-episode recovery model, which focuses on those who have experienced a first episode of psychosis (FEP) but have not yet developed a chronic disorder.
Both models share long-term goals and are hampered by many of the same issues summarized above. They both deviate markedly from the standard medical model by including psychosocial services designed to promote restoration of a self-defined trajectory to greater independence.11-14 The 2 differ, however, in the challenges they must overcome to produce their sample populations and establish effective interventions.10,15,16
In this article, we provide a succinct overview of these issues and a set of recommendations based on a “strength-based” approach. This approach focuses on finding common ground between patients, their support system, and the treatment team in the service of empowering patients to resume responsibility for transition to adulthood.
The prevention model
While most prevention initiatives in medicine rely on the growing ability to target specific pathophysiologic pathways,3 preventing psychosis relies on clinical evidence showing that DUP and early interventions predict a better course of severe mental illness.17 In contrast, initiatives such as normalizing neonatal neuronal pathways are more consistent with the strategy utilized in other fields but have yet to yield a pathophysiologic target for psychosis.3,18
Initial efforts to identify ‘at-risk’ individuals
The prevention model of psychosis is based on the ability to identify young individuals at high risk for developing a psychotic disorder (Figure). The first screening measures were focused on prodromal psychosis (eg, significant loss of function, family history, and “intermittent” and “attenuated” psychotic symptoms). When applied to referred (ie, pre-screened) samples, 30% to 40% of this group who met criteria transitioned to psychosis over the next 1 to 3 years despite antidepressant and psychosocial interventions.19 Comprising 8 academic medical centers, the North American Prodrome Longitudinal Study (NAPLS) produced similar results using the Structured Interview for Prodromal Syndromes (SIPS).17 Thus, 30% to 50% of pre-screened individuals referred by school counselors and mental health professionals met SIPS criteria, and 35% of these individuals transitioned to psychosis over 30 months. The validity of this measure was further supported by the fact that higher baseline levels of unusual thought content, suspicion/paranoia, social impairment, and substance abuse successfully distinguished approximately 80% of those who transitioned to psychosis. The results of this first generation of screening studies were exciting because they seemed to demonstrate that highly concentrated samples of young persons at high risk of developing psychosis could be identified, and that fine-tuning the screening criteria could produce even more enriched samples (ie, positive predictive power).
Initial interventions produced promising results
The development of effective screening measures led to reports of effective treatment interventions. These were largely applied in a clinical staging model that restricted antipsychotic medications to those who failed to improve after receiving potentially “less toxic” interventions (eg, omega-3 polyunsaturated fatty acids and other antioxidants; psychotherapy; cognitive-behavioral therapy [CBT]; family therapy).5 While study designs were typically quasi-experimental, the interventions appeared to dramatically diminish the transition to psychosis (ie, approximately 50%).
Continue to: The first generation...
The first generation of RCTs appeared to confirm these results, although sample sizes were small, and most study designs assessed only a single intervention. Initial meta-analyses of these data reported that both CBT and antipsychotics appeared to prevent approximately one-half of individuals from becoming psychotic at 12 months, and more than one-third at 2 to 4 years, compared with treatment as usual.20
While some researchers challenged the validity of these findings,21-23 the results generated tremendous international enthusiasm and calls for widespread implementation.6 The number of early intervention services (EIS) centers increased dramatically worldwide, and in 2014 the National Institute for Health and Care Excellence released standards for interventions to prevent transition to psychosis.24 These included close monitoring, CBT and family interventions, and avoiding antipsychotics when possible.24
Focusing on sensitivity over specificity
The first generation of studies generated by the prevention model relied on outreach programs or referrals, which produced small samples of carefully selected, pre-screened individuals (Figure, Pre-screened) who were then screened again to establish the high-risk sample.25 While approximately 33% of these individuals became psychotic, the screening process required a very efficient means of eliminating those not at high-risk (given the ultimate target population represented only approximately .5% of young people) (Figure). The pre-screening and screening processes in these first-generation studies were labor-intensive but could only identify approximately 5% of those individuals destined to become psychotic over the next 2 or 3 years. Thus, alternative methods to enhance sensitivity were needed to extend programming to the general population.
Second-generation pre-screening (Figure; Step 1). New pre-screening methods were identified that captured more individuals destined to become psychotic. For example, approximately 90% of this population were registered in health care organizations (eg, health maintenance organizations) and received a psychiatric diagnosis in the year prior to the onset of psychosis (true positives).8 These samples, however, contained a much higher percentage of persons not destined to become psychotic, and somehow the issue of specificity (decreasing false positives) was minimized.8,9 For example, pre-screened samples contained 20 to 50 individuals not destined to become psychotic for each one who did.26 Since screening measures could only eliminate approximately 20% of this group (Figure, Step 2, page 25), second-generation transition rates fell from 30% to 40% to 2% to 10%.27,28
Other pre-screening approaches were introduced, but they also focused on capturing more of those destined to become psychotic (sensitivity) than eliminating those who would not (specificity). For instance, Australia opened more than 100 “Headspace” community centers nationwide designed to promote engagement and self-esteem in youth experiencing anxiety; depression; stress; relationship, work, or school problems; or bullying.13 Most services were free and included mental health staff who screened for psychosis and provided a wide range of services in a destigmatized setting. These methods identified at least an additional 5% to 7% of individuals destined to become psychotic, but to our knowledge, no data have been published on whether they helped eliminate those who did not.
Continue to: Second-generation screening
Second-generation screening (Figure, Step 2). A second screening aims to retain those pre-screened individuals who will become psychotic (ie, minimizing false negatives) while further minimizing those who do not (ie, minimizing false positives). The addition of cognitive, neural (eg, structural MRI; neurophysiologic), and biochemical (eg, inflammatory immune and stress) markers to the risk calculators have produced a sensitivity close to 100%.8,9 Unfortunately, these studies downplayed specificity, which remained approximately 20%.8,9 Specificity is critical not just because of concerns about stigma (ie, labeling people as pre-psychotic when they are not) but also because of the adverse effects of antipsychotic medications and the effects on future program development (interventions are costly and labor-intensive). Also, diluting the pool with individuals not at risk makes it nearly impossible to identify effective interventions (ie, power).27,28
While some studies focused on increasing specificity (to approximately 75%), this leads to an unacceptable loss of sensitivity (from 90% to 60%),29 with 40% of pre-screened individuals who would become psychotic being eliminated from the study population. The addition of other biological markers (eg, salivary cortisol)30 and use of learning health systems may be able to enhance these numbers (initial reports of specificity = 87% and sensitivity = 85%).8,9 This is accomplished by integrating artificial and human intelligence measures of clinical (symptom and neurocognitive measures) and biological (eg, polygenetic risk scores; gray matter volume) variables.31 However, even if these results are replicated, more effective pre-screening measures will be required.
Identifying a suitable sample population for prevention program studies is clearly more complicated than for FEP studies, where one can usually identify many of those in the at-risk population by their first hospitalization for psychotic symptoms. The issues of false positives (eg, substance-induced psychosis) and negatives (eg, slow deterioration, prominent negative symptoms) are important concerns, but proportionately far less significant.
Prevention and FEP interventions
Once a study sample is constituted, 1 to 3 years of treatment interventions are initiated. Interventions for prevention programs typically include CBT directed at attenuated psychosis (eg, reframing or de-catastrophizing unusual thoughts and minimizing distress associated with unusual perceptions); case management to facilitate personal, educational, and vocational goals; and family therapy in single or multi-group formats to educate one’s support system about the risk state and to minimize adverse familial responses.14 Many programs also include supported education or employment services to promote reintegration in age-appropriate activities; group therapy focused on substance abuse and social skills training; cognitive remediation to ameliorate the cognitive dysfunction; and an array of pharmacologic interventions designed to delay or prevent transition to psychosis or to alleviate symptoms. While most interventions are similar, FEP programs have recently included peer support staff. This appears to instill hope in newly diagnosed patients, provide role models, and provide peer supporters an opportunity to use their experiences to help others and earn income.32
The breadth and depth of these services are critical because retention in the program is highly dependent on participant engagement, which in turn is highly dependent on whether the program can help individuals get what they want (eg, friends, employment, education, more autonomy, physical health). The setting and atmosphere of the treatment program and the willingness/ability of staff to meet participants in the community are also important elements.11,12 In this context, the Headspace community centers are having an impact far beyond Australia and may prove to be a particularly good model.13
Continue to: Assessing prevention and FEP interventions
Assessing prevention and FEP interventions
The second generation of studies of prevention programs has not confirmed, let alone extended, the earlier findings and meta-analyses. A 2020 report concluded CBT was still the most promising intervention; it was more effective than control treatments at 12 and 18 months, although not at 6, 24, or 48 months.33 This review included controlled, open-label, and naturalistic studies that assessed family therapy; omega-3 polyunsaturated fatty acids; integrated psychological therapy (a package of interventions that included family education, CBT, social skills training, and cognitive remediation); N-methyl-
While these disappointing findings are at least partly attributable to the methodological challenges described above and in the Figure, other factors may hinder establishing effective interventions. In contrast to FEP studies, those focused on prevention had a very ambitious agenda (eliminating psychosis) and tended to downplay more modest intermediate outcomes. These studies also tended to assess new ideas with small samples rather than pursue promising findings with larger multi-site studies focused on a group of interventions. The authors of a Cochrane review observed “There is the impression that in this whole area there is a triumph of hope over adversity. There is the repeated hope invested in another—often unique—study question and then a study of fewer than 100 participants are completed. This results in the set of comparisons reported here, all 9 of which are too underpowered to really highlight clear differences.”34 To use a baseball analogy, it seems that investigators are “swinging for the fence” when a few singles are what’s really needed.
From the outset, the goals of FEP studies were more modest, largely ignoring the task of developing consensus definitions of recovery that require following patients for up to 5 to 10 years. Instead, they use intermediate endpoints based on adapting treatments that already appeared effective in patients with chronic mental disorders.35 As a consequence, researchers examining FEP demonstrated clear, albeit limited, salutary effects using large multi-site trials and previously established outcome measures.3,10,36 For instance, the Recovery After an Initial Schizophrenia Episode-Early Treatment Program (RAISE-ETP) study was a 2-year, multi-site RCT (N = 404) funded by the National Institute of Mental Health (NIMH). The investigators reported improved indices of social function (eg, quality of life; education and work participation) and total ratings of psychopathology and depression compared with treatment as usual. Furthermore, they established that DUP predicted treatment response.35 The latter finding was underscored by improvement being limited to the 50% with <74 weeks DUP. Annual costs of the program per 1 standard deviation improvement in quality of life were approximately $1,000 for patients with <74 weeks DUP and $40,000 for those with >74 weeks DUP. Concurrent meta-analyses confirmed and extended these findings,16 showing higher remission rates; diminished relapses and hospital admissions; greater engagement in programming; greater involvement in work and school; improved quality of life; and other steps toward recovery. These studies were also able to establish a clear benefit of antipsychotic medications, particularly a high acceptance of long-acting injectable antipsychotic formulations, which promoted adherence and decreased some adverse events37; and early use of clozapine therapy, which improved remission rates and longer-term outcomes.38 Other findings underscored the need to anticipate and address new problems associated with effective antipsychotic therapy (eg, antipsychotic response correlates with weight gain, a particularly intolerable adverse event for this age group).39 Providing pre-emptive strategies such as exercise groups and nutritional education may be necessary to maintain adherence.
Limitations of FEP studies
The effect sizes in these FEP studies were small to medium on outcome measures tracking recovery and associated indicators (eg, global functioning, school/work participation, treatment engagement); the number needed to treat for each of these was >10. There is no clear evidence that recovery programs such as RAISE-ETP actually reduce longer-term disability. Most studies showed disability payments increased while clinical benefits tended to fade over time. In addition, by grouping interventions together, the studies made it difficult to identify effective vs ineffective treatments, let alone determine how best to personalize therapy for participants in future studies.
The next generation of FEP studies
While limited in scope, the results of the recent FEP studies justify a next generation of recovery interventions designed to address these shortcomings and optimize program outcomes.39 Most previous FEP studies were conducted in community mental health center settings, thus eliminating the need to transition services developed in academia into the “real world.” The next generation of NIMH studies will be primarily conducted in analogous settings under the Early Psychosis Intervention Network (EPINET).40 EPINET’s study design echoes that responsible for the stepwise successes in the late 20th century that produced cures for the deadliest childhood cancer, acute lymphoblastic leukemia (ALL). This disease was successfully treated by modifying diverse evidence-based practices without relying on pharmacologic or other major treatment breakthroughs. Despite this, the effort yielded successful personalized interventions that were not obtainable for other severe childhood conditions.40 EPINET hopes to automate much of these stepwise advances with a learning health system. This program relies on data routinely collected in clinical practice to drive the process of scientific discovery. Specifically, it determines the relationships between clinical features, biologic measures, treatment characteristics, and symptomatic and functional outcomes. EPINET aims to accelerate our understanding of biomarkers of psychosis risk and onset, as well as factors associated with recovery and cure. Dashboard displays of outcomes will allow for real-time comparisons within and across early intervention clinics. This in turn identifies performance gaps and drives continuous quality improvement.
Continue to: Barriers to optimizing program efficacy for both models
Barriers to optimizing program efficacy for both models
Unfortunately, there are stark differences between ALL and severe mental disorders that potentially jeopardize the achievement of these aims, despite the advances in data analytic abilities that drive the learning health system. Specifically, the heterogeneity of psychotic illnesses and the absence of reliable prognostic and modifiable risk markers (responsible for failed efforts to enhance treatment of serious mental illness over the last half century1,2,41) are unlikely to be resolved by a learning health system. These measures are vital to determine whether specific interventions are effective, particularly given the absence of a randomized control group in the EPINET/learning health system design. Fortunately, however, the National Institutes for Health has recently initiated the Accelerating Medicines Partnership–Schizophrenia (AMP-SCZ). This approach seeks “promising biological markers that can help identify those at risk of developing schizophrenia as early as possible, track the progression of symptoms and other outcomes and ultimately define targets for treatment development.”42 The Box1,4,9,10,36,41,43-45 describes some of the challenges involved in identifying biomarkers of severe mental illness.
Box
Biomarkers and modifiable risk factors4,9,10,41,43 are at the core of personalized medicine and its ultimate objective (ie, theragnostics). This is the ability to identify the correct intervention for a disorder based on a biomarker of the illness.10,36 The inability to identify biomarkers of severe mental illness is multifactorial but in part may be attributable to “looking in all the wrong places.”41 By focusing on neural processes that generate psychiatric symptomatology, investigators are assuming they can bridge the “mind gap”1 and specifically distinguish between pathological, compensatory, or collateral measures of poorly characterized limbic neural functions.41
It may be more productive to identify a pathological process within the limbic system that produces a medical condition as well as the mental disorder. If one can isolate the pathologic limbic circuit activity responsible for a medical condition, one may be able to reproduce this in animal models and determine whether analogous processes contribute to the core features of the mental illness. Characterization of the aberrant neural circuit in animal models also could yield targets for future therapies. For example, episodic water intoxication in a discrete subset of patients with schizophrenia44 appears to arise from a stress diathesis produced by anterior hippocampal pathology that disrupts regulation of antidiuretic hormone, oxytocin, and hypothalamic-pituitary-adrenal axis secretion. These patients also exhibit psychogenic polydipsia that may be a consequence of the same hippocampal pathology that disrupts ventral striatal and lateral hypothalamic circuits. These circuits, in turn, also modulate motivated behaviors and cognitive processes likely relevant to psychosis.45
A strength-based approach
The absence of sufficiently powered RCTs for prevention studies and the reliance on intermediate outcomes for FEP studies leaves unanswered whether such programs can effectively prevent chronic psychosis at a cost society is willing to pay. Still, substantial evidence indicates that outreach, long-acting injectable antipsychotics, early consideration of clozapine, family therapy, CBT for psychosis/attenuated psychosis, and services focused on competitive employment can preserve social and occupational functioning.16,34 Until these broader questions are more definitively addressed, it seems reasonable to apply what we have learned (Table11,12,35,37-39,46).
Simply avoiding the most divisive aspects of the medical model that inadvertently promote stigma and undercut self-confidence may help maintain patients’ willingness to learn how best to apply their strengths and manage their limitations.11 The progression to enduring psychotic features (eg, fixed delusions) may reflect ongoing social isolation and alienation. A strength-based approach seeks first to establish common goals (eg, school, work, friends, family support, housing, leaving home) and then works to empower the patient to successfully reach those goals.35 This typically involves giving them the opportunity to fail, avoiding criticism when they do, and focusing on these experiences as learning opportunities from which success can ultimately result.
It is difficult to offer all these services in a typical private practice setting. Instead, it may make more sense to use one of the hundreds of early intervention services programs in the United States.46 If a psychiatric clinician is dedicated to working with this population, it may also be possible to establish ongoing relationships with primary care physicians, family and CBT therapists, family support services (eg, National Alliance on Mental Illness), caseworkers and employment counselors. In essence, a psychiatrist may be able re-create a multidisciplinary effort by taking advantage of the expertise of these various professionals. The challenge is to create a consistent message for patients and families in the absence of regular meetings with the clinical team, although the recent reliance on and improved sophistication of virtual meetings may help. Psychiatrists often play a critical role even when the patient is not prescribed medication, partly because they are most comfortable handling the risks and may have the most comprehensive understanding of the issues at play. When medications are appropriate and patients with FEP are willing to take them, early consideration of long-acting injectable antipsychotics and clozapine may provide better stabilization and diminish the risk of earlier and more frequent relapses.
Bottom Line
Early interventions for psychosis include the prevention model and the first-episode recovery model. It is difficult to assess, compare, and optimize the effectiveness of such programs. Current evidence supports a ‘strength-based’ approach focused on finding common ground between patients, their support system, and the treatment team.
Related Resources
- Early Assessment and Support Alliance. National Early Psychosis Directory. https://easacommunity.org/nationaldirectory.php
- Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016 ;173(4):362-372
Drug Brand Name
Clozapine • Clozaril
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
1. Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm11. doi: 10.1126/scitranslmed.3003142
2. Harrington A. Mind fixers: psychiatry’s troubled search for the biology of mental illness. W.W. Norton & Company; 2019.
3. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485-515.
4. Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176(10):794-810.
5. McGorry PD, Nelson B, Nordentoft M, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70(9):1206-1212.
6. Csillag C, Nordentoft M, Mizuno M, et al. Early intervention in psychosis: From clinical intervention to health system implementation. Early Interv Psychiatry. 2018;12(4):757-764.
7. McGorry PD, Ratheesh A, O’Donoghue B. Early intervention—an implementation challenge for 21st century mental health care. JAMA Psychiatry. 2018;75(6):545-546.
8. Rosenheck R. Toward dissemination of secondary prevention for psychosis. Am J Psychiatry. 2018;175(5):393-394.
9. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765.
10. Oliver D, Reilly TJ, Baccaredda Boy O, et al. What causes the onset of psychosis in individuals at clinical high risk? A meta-analysis of risk and protective factors. Schizophr Bull. 2020;46(1):110-120.
11. Tindall R, Simmons M, Allott K, et al. Disengagement processes within an early intervention service for first-episode psychosis: a longitudinal, qualitative, multi-perspective study. Front Psychiatry. 2020;11:565-565.
12. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20.
13. Rickwood D, Paraskakis M, Quin D, et al. Australia’s innovation in youth mental health care: The headspace centre model. Early Interv Psychiatry. 2019;13(1):159-166.
14. Woodberry KA, Shapiro DI, Bryant C, et al. Progress and future directions in research on the psychosis prodrome: a review for clinicians. Harv Rev Psychiatry. 2016;24(2):87-103.
15. Gupta T, Mittal VA. Advances in clinical staging, early intervention, and the prevention of psychosis. F1000Res. 2019;8:F1000 Faculty Rev-2027. doi: 10.12688/f1000research.20346.1
16. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555-565.
17. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37.
18. Sommer IE, Bearden CE, van Dellen E, et al. Early interventions in risk groups for schizophrenia: what are we waiting for? NPJ Schizophr. 2016;2(1):16003-16003.
19. McGorry PD, Nelson B. Clinical high risk for psychosis—not seeing the trees for the wood. JAMA Psychiatry. 2020;77(7):559-560.
20. van der Gaag M, Smit F, Bechdolf A, et al. Preventing a first episode of psychosis: meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr Res. 2013;149(1):56-62.
21. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;(6):CD004718. doi: 10.1002/14651858.CD004718.pub3
22. Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28-29.
23. Amos AJ. Evidence that treatment prevents transition to psychosis in ultra-high-risk patients remains questionable. Schizophr Res. 2014;153(1):240.
24. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 1.3.7 How to deliver psychological interventions. Published February 12, 2014. Updated March 1, 2014. Accessed August 30, 2021. https://www.nice.org.uk/guidance/cg178/chapter/recommendations#how-to-deliver-psychological-interventions
25. Fusar-Poli P, Werbeloff N, Rutigliano G, et al. Transdiagnostic risk calculator for the automatic detection of individuals at risk and the prediction of psychosis: second replication in an independent National Health Service Trust. Schizophr Bull. 2019;45(3):562-570.
26. Fusar-Poli P, Oliver D, Spada G, et al. The case for improved transdiagnostic detection of first-episode psychosis: electronic health record cohort study. Schizophr Res. 2021;228:547-554.
27. Fusar-Poli P. Negative psychosis prevention trials. JAMA Psychiatry. 2017;74(6):651.
28. Cuijpers P, Smit F, Furukawa TA. Most at‐risk individuals will not develop a mental disorder: the limited predictive strength of risk factors. World Psychiatry. 2021;20(2):224-225.
29. Carrión RE, Cornblatt BA, Burton CZ, et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173(10):989-996.
30. Worthington MA, Walker EF, Addington J, et al. Incorporating cortisol into the NAPLS2 individualized risk calculator for prediction of psychosis. Schizophr Res. 2021;227:95-100.
31. Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209.
32. Simmons MB, Grace D, Fava NJ, et al. The experiences of youth mental health peer workers over time: a qualitative study with longitudinal analysis. Community Ment Health J. 2020;56(5):906-914.
33. Devoe DJ, Farris MS, Townes P, et al. Interventions and transition in youth at risk of psychosis: a systematic review and meta-analyses. J Clin Psychiatry. 2020;81(3):17r12053. doi: 10.4088/JCP.17r12053
34. Bosnjak Kuharic D, Kekin I, Hew J, et al. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11):CD012236
35. Dixon LB, Goldman HH, Srihari VH, et al. Transforming the treatment of schizophrenia in the United States: The RAISE Initiative. Annu Rev Clin Psychol. 2018;14:237-258.
36. Friedman-Yakoobian MS, Parrish EM, Eack SM, et al. Neurocognitive and social cognitive training for youth at clinical high risk (CHR) for psychosis: a randomized controlled feasibility trial. Schizophr Res. 2020;S0920-9964(20)30461-8. doi: 10.1016/j.schres.2020.09.005
37. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(12):1217-1224.
38. Morrison AP, Pyle M, Maughan D, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7(9):788-800.
39. Chen YQ, Li XR, Zhang L, et al. Therapeutic response is associated with antipsychotic-induced weight gain in drug-naive first-episode patients with schizophrenia: an 8-week prospective study. J Clin Psychiatry. 2021;82(3):20m13469. doi: 10.4088/JCP.20m13469
40. Insel TR. RAISE-ing our expectations for first-episode psychosis. Am J Psychiatry. 2016;173(4):311-312.
41. Tandon R, Goldman M. Overview of neurobiology. In: Janicak PG, Marder SR, Tandon R, et al, eds. Schizophrenia: recent advances in diagnosis and treatment. Springer; 2014:27-33.
42. National Institutes of Health. Accelerating Medicines Partnership. Schizophrenia. Accessed August 30, 2021. https://www.nih.gov/research-training/accelerating-medicines-partnership-amp/schizophrenia
43. Guloksuz S, van Os J. The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med. 2018;48(2):229-244.
44. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers. 2019;5(1):54.
45. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi: 10.1016/j.beem.2020.101469
46. Early Assessment and Support Alliance. National Early Psychosis Directory. Accessed August 30, 2021. https://easacommunity.org/national-directory.php
Nontraditional therapies for treatment-resistant depression: Part 2
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (
Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (
Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
When patients with major depressive disorder (MDD) do not achieve optimal outcomes after FDA-approved first-line treatments and standard adjunctive strategies, clinicians look for additional approaches to alleviate their patients’ symptoms. Recent research suggests that several “nontraditional” treatments used primarily as adjuncts to standard antidepressants have promise for treatment-resistant depression.
In Part 1 of this article (
Herbal/nutraceutical agents
This category encompasses a variety of commonly available “natural” options patients often ask about and at times self-prescribe. Examples evaluated in clinical trials include:
- vitamin D
- essential fatty acids (omega-3, omega-6)
- S-adenosyl-L-methionine (SAMe)
- hypericum perforatum (St. John’s Wort)
- probiotics.
Vitamin D deficiency has been linked to depression, possibly by lowering serotonin, norepinephrine, and dopamine concentrations.1-3
A meta-analysis of 3 prospective, observational studies (N = 8,815) found an elevated risk of affective disorders in patients with low vitamin D levels.4 In addition, a systematic review and meta-analysis supported a potential role for vitamin D supplementation for patients with treatment-resistant depresssion.5
Toxicity can occur at levels >100 ng/mL, and resulting adverse effects may include weakness, fatigue, sleepiness, headache, loss of appetite, dry mouth, metallic taste, nausea, and vomiting. This vitamin can be considered as an adjunct to standard antidepressants, particularly in patients with treatment-resistant depression who have low vitamin D levels, but regular monitoring is necessary to avoid toxicity.
Essential fatty acids. Protein receptors embedded in lipid membranes and their binding affinities are influenced by omega-3 and omega-6 polyunsaturated fatty acids. Thus, essential fatty acids may benefit depression by maintaining membrane integrity and fluidity, as well as via their anti-inflammatory activity.
Continue to: Although results from...
Although results from controlled trials are mixed, a systematic review and meta-analysis of adjunctive nutraceuticals supported a potential role for essential fatty acids, primarily eicosapentaenoic acid (EPA), by itself or in combination with docosahexaenoic acid (DHA), with total EPA >60%.5 A second meta-analysis of 26 studies (N = 2,160) that considered only essential fatty acids concluded that EPA ≥60% at ≤1 g/d could benefit depression.6 Furthermore, omega-3 fatty acids may be helpful as an add-on agent for postpartum depression.7
Be aware that a diet rich in omega-6 greatly increases oxidized low-density lipoprotein levels in adipose tissue, potentially posing a cardiac risk factor. Clinicians need to be aware that self-prescribed use of essential fatty acids is common, and to ask about and monitor their patients’ use of these agents.
S-adenosyl-L-methionine (SAMe) is an intracellular amino acid and methyl donor. Among other actions, it is involved in the biosynthesis of hormones and neurotransmitters. There is promising but limited preliminary evidence of its efficacy and safety as a monotherapy or for antidepressant augmentation.
- Five out of 6 earlier controlled studies reported SAMe IV (200 to 400 mg/d) or IM (45 to 50 mg/d) was more effective than placebo
- When the above studies were added to 14 subsequent studies for a meta-analysis, 12 of 19 RCTs reported that parenteral or oral SAMe was significantly more effective than placebo for depression (P < .05).
Overall, the safety and tolerability of SAMe are good. Common adverse effects include nausea, mild insomnia, dizziness, irritability, and anxiety. This is another compound widely available without a prescription and at times self-prescribed. It carries an acceptable risk/benefit balance, with decades of experience.
Hypericum perforatum (St. John’s Wort) is widely prescribed for depression in China and Europe, typically in doses ranging from 500 to 900 mg/d. Its mechanism of action in depression may relate to inhibition of serotonin, dopamine, and norepinephrine uptake from the synaptic cleft of these interconnecting neurotransmitter systems.
Continue to: A meta-analysis of 7 clinical trials...
A meta-analysis of 7 clinical trials (N = 3,808) comparing St. John’s Wort with various selective serotonin reuptake inhibitors (SSRIs) reported comparable rates of response (pooled relative risk .983, 95% CI .924 to 1.042; P < .001) and remission (pooled relative risk 1.013, 95% CI .892 to 1.134; P < .001).9 Further, there were significantly lower discontinuation/dropout rates (pooled odds ratio .587, 95% CI .478 to 0.697; P < .001) for St. John’s Wort compared with the SSRIs.
Existing evidence on the long-term efficacy and safety is limited (studies ranged from 4 to 12 weeks), as is evidence for patients with more severe depression or high suicidality.
Serious drug interactions include the potential for serotonin syndrome when St. John’s Wort is combined with certain antidepressants, compromised efficacy of benzodiazepines and standard antidepressants, and severe skin reactions to sun exposure. In addition, St. John’s Wort may not be safe to use during pregnancy or while breastfeeding. Because potential drug interactions can be serious and individuals often self-prescribe this agent, it is important to ask patients about their use of St. John’s Wort, and to be vigilant for such potential adverse interactions.
Probiotics. These agents produce neuroactive substances that act on the brain/gut axis. Preliminary evidence suggests that these “psychobiotics” confer mental health benefits.10-12 Relative to other approaches, their low-risk profile make them an attractive option for some patients.
Anti-inflammatory/immune system therapies
Inflammation is linked to various medical and brain disorders. For example, patients with depression often demonstrate increased levels of peripheral blood inflammatory biomarkers (such as C-reactive protein and interleukin-6 and -17) that are known to alter norepinephrine, neuroendocrine (eg, the hypothalamic-pituitary-adrenal axis), and microglia function in addition to neuroplasticity. Thus, targeting inflammation may facilitate the development of novel antidepressants. In addition, these agents may benefit depression associated with comorbid autoimmune disorders, such as psoriasis or rheumatoid arthritis. A systematic review and meta-analysis of 36 RCTs (N = 10,000) found 5 out of 6 anti-inflammatory agents improved depression.13,14 In general, reported disadvantages of anti-inflammatories/immunosuppressants include the potential to block the antidepressant effect of some agents, the risk of opportunistic infections, and an increased risk of suicide.
Continue to: Statins
Statins
In a meta-analysis of 3 randomized, double-blind trials, 3 statins (lovastatin, atorvastatin, and simvastatin) significantly improved depression scores when used as an adjunctive therapy to fluoxetine and citalopram, compared with adjunctive placebo (N = 165, P < .001).15
Specific adverse effects of statins include headaches, muscle pain (rarely rhabdomyolysis), dizziness, rash, and liver damage. Statins also have the potential for adverse interactions with other medications. Given the limited efficacy literature on statins for depression and the potential for serious adverse effects, these agents probably should be limited to patients with treatment-resistant depression for whom a statin is indicated for a comorbid medical disorder, such as hypercholesteremia.
Neurosteroids
Brexanolone is FDA-approved for the treatment of postpartum depression. It is an IV formulation of the neuroactive steroid hormone allopregnanolone (a metabolite of progesterone), which acts as a positive allosteric modulator of the GABA-A receptor. Unfortunately, the infusion needs to occur over a 60-hour period.
Ganaxolone is an oral analog formulation of allopregnanolone. In an uncontrolled, open-label pilot study, this medication was administered for 8 weeks as an adjunct to an adequately dosed antidepressant to 10 postmenopausal women with persistent MDD.16 Of the 9 women who completed the study, 4 (44%) improved significantly (P < .019) and the benefit was sustained for 2 additional weeks.16 Adverse effects of ganaxolone included dizziness in 60% of participants, and sleepiness and fatigue in all of them with twice-daily dosing. If the FDA approves ganaxolone, it would become an easier-to-administer option to brexanolone.
Zuranolone is an investigational agent being studied as a treatment for postpartum depression. In a double-blind RCT that evaluated 151 women with postpartum depression, those who took oral zuranolone, 30 mg daily at bedtime for 2 weeks, experienced significant reductions in Hamilton Depression Rating Scale-17 (HDRS-17) scores compared with placebo (P < .003).17 Improvement in core depression symptom ratings was seen as early as Day 3 and persisted through Day 45.
Continue to: The most common...
The most common (≥5%) treatment-emergent adverse effects were somnolence (15%), headache (9%), dizziness (8%), upper respiratory tract infection (8%), diarrhea (6%), and sedation (5%). Two patients experienced a serious adverse event: one who received zuranolone (confusional state) and one who received placebo (pancreatitis). One patient discontinued zuranolone due to adverse effects vs no discontinuations among those who received placebo. The risk of taking zuranolone while breastfeeding is not known.
Device-based strategies
In addition to FDA-cleared approaches (eg, electroconvulsive therapy [ECT], vagus nerve stimulation [VNS], transcranial magnetic stimulation [TMS]), other devices have also demonstrated promising results.
Transcranial direct current stimulation (tDCS) involves delivering weak electrical current to the cerebral cortex through small scalp electrodes to produce the following effects:
- anodal tDCS enhances cortical excitability
- cathodal tDCS reduces cortical excitability.
A typical protocol consists of delivering 1 to 2 mA over 20 minutes with scalp electrodes placed in different configurations based on the targeted symptom(s).
While tDCS has been evaluated as a treatment for various neuropsychiatric disorders, including bipolar depression, Parkinson’s disease, and schizophrenia, most trials have looked at its use for treating depression. Results have been promising but mixed. For example, 1 meta-analysis of 6 RCTs (comprising 96 active and 80 sham tDCS courses) reported that active tDCS was superior to a sham procedure (Hedges’ g = 0.743) for symptoms of depression.18 By contrast, another meta-analysis of 6 RCTs (N = 200) did not find a significant difference between active and sham tDCS for response and remission rates.19 More recently, a group of experts created an evidence-based guideline using a systematic review of the controlled trial literature. These authors concluded there is “probable efficacy for anodal tDCS of the left dorsolateral prefrontal cortex (DLPFC) (with right orbitofrontal cathode) in major depressive episodes without drug resistance but probable inefficacy for drug-resistant major depressive episodes.”20
Continue to: Adverse effects of tDCS...
Adverse effects of tDCS are typically mild but may include persistent skin lesions similar to burns; mania or hypomania; and one reported seizure in a pediatric patient.
Because various over-the-counter direct current stimulation devices are available for purchase at modest cost, clinicians should ask patients if they have been self-administering this treatment.
Chronotherapy strategies
Agomelatine combines serotonergic (5-HT2B and 5-HT2C antagonist) and melatonergic (MT1-MT2 agonist in the suprachiasmatic nucleus) actions that contribute to stabilization of circadian rhythms and subsequent improvement in sleep patterns. Agomelatine (n = 1,274) significantly lowered depression symptoms compared with placebo (n = 689) (standardized mean difference −0.26; P < 3.48×10-11), but the clinical relevance was questionable.21 A recent review of the literature and expert opinion suggest this agent may also have efficacy for anhedonia; however, in placebo-controlled, relapse prevention studies, its long-term efficacy was not consistent.22
Common adverse effects include anxiety; nausea, vomiting, and stomach pain; abnormal dreams and insomnia; dizziness; drowsiness and fatigue; and weight gain. Some reviewers have expressed concerns about agomelatine’s potential for hepatotoxicity and the need for repeated clinical laboratory tests. Although agomelatine is approved outside of the United States, limited efficacy data and the potential for serious adverse effects have precluded FDA approval of this agent.
Sleep deprivation as a treatment technique for depression has been developed over the past 50 years. With total sleep deprivation (TSD) over 1 cycle, patients stay awake for approximately 36 hours, from daytime until the next day’s evening. While 1 to 6 cycles can produce acute antidepressant effects, prompt relapse after sleep recovery is common.
Continue to: In a systematic review...
In a systematic review and meta-analysis of 7 studies that included a total of 311 patients with bipolar depression23:
- TSD plus medications resulted in a significant decrease in depressive symptoms at 1 week compared with medications alone
- higher response rates were maintained after 3 months with lithium.
Adverse effects commonly include general fatigue and headaches; possible switch into mania with bipolar depression; and rarely, seizures or other unexpected medical conditions (eg, acute coronary syndrome). Presently, this approach is limited to research laboratories with the appropriate sophistication to safely conduct such trials.
Other nontraditional strategies
Cardiovascular exercise, resistance training, mindfulness, and yoga have been shown to decrease severe depressive symptoms when used as adjuncts for patients with treatment-resistant depression, or as monotherapy to treat patients with milder depression.
Exercise. The significant benefits of exercise in various forms as treatment for mild to moderate depression are well described in the literature, but it is less clear if it is effective for treatment-resistant depression. A 2013 Cochrane report24 (39 studies with 2,326 participants total) and 2 meta-analyses undertaken in 2015 (Kvam et al25 included 23 studies with 977 participants, and Schuh et al26 included 25 trials with 1,487 participants) reported that various types of exercise ameliorate depression of differing subtypes and severity, with effect sizes ranging from small to large. Schuh et al26 found that publication bias underestimated effect size. Also, not surprisingly, separate analysis of only higher-quality trials decreased effect size.24-26 A meta-analysis that included tai chi and yoga in addition to aerobic exercise and strength training (25 trials with 2,083 participants) found low to moderate benefit for exercise and yoga.27 Finally, a meta-analysis by Cramer et al28 that included 12 RCTs (N = 619) supported the use of yoga plus controlled breathing techniques as an ancillary treatment for depression.
Two small exercise trials specifically evaluated patients with treatment-resistant depression.29,30 Mota-Pereira et al29 compared 22 participants who walked for 30 to 45 minutes, 5 days a week for 12 weeks in addition to pharmacotherapy with 11 patients who received pharmacotherapy only. Exercise improved all outcomes, including HDRS score (both compared to baseline and to the control group). Moreover, 26% of the exercise group went into remission. Pilu et al30 evaluated strength training as an adjunctive treatment. Participants received 1 hour of strength training twice weekly for 8 months (n = 10), or pharmacotherapy only (n = 20). The adjunct strength training group had a statistically significant (P < .0001) improvement in HDRS scores at the end of the 8 months, whereas the control group did not (P < .28).
Continue to: Adverse effects...
Adverse effects of exercise are typically limited to sprains or strains; rarely, participants experience serious injuries.
Mindfulness-based interventions involve purposely paying attention in the present moment to enhance self-understanding and decrease anxiety about the future and regrets about the past, both of which complicate depression. A meta-analysis of 12 RCTs (N = 578) found this approach significantly reduced depression severity when used as an adjunctive therapy.31 There may be risks if mindfulness-based interventions are practiced incorrectly. For example, some reports have linked mindfulness-based interventions to psychotic episodes, meditation addiction, and antisocial or asocial behavior.32
Bottom Line
Nonpharmacologic options for patients with treatment-resistant depression include herbal/nutraceuticals, anti-inflammatory/immune system therapies, and devices. While research suggests some of these approaches are promising, clinicians need to carefully consider potential adverse effects, some of which may be serious.
Related Resources
- Kaur M, Sanches M. Experimental therapeutics in treatmentresistant major depressive disorder. J Exp Pharmacol. 2021;13:181-196.
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
Drug Brand Names
Atorvastatin • Lipitor
Brexanolone • Zulresso
Citalopram • Celexa
Fluoxetine • Prozac
Lithium • Eskalith, Lithobid
Lovastatin • Altoprev, Mevacor
Minocycline • Dynacin, Minocin
Simvastatin • Flolipid, Zocor
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
1. Pittampalli S, Mekala HM, Upadhyayula, S, et al. Does vitamin D deficiency cause depression? Prim Care Companion CNS Disord. 2018;20(5):17l02263.
2. Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56-61.
3. Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80-92.
4. Anglin RE, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107.
5. Sarris J, Murphy J, Mischoulon D, et al. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry 2016;173(6);575-587.
6. Liao Y, Xie B, Zhang H, et al. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190.
7. Mocking RJT, Steijn K, Roos C, et al. Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J Clin Psychiatry. 2020;81(5):19r13106.
8. Sharma A, Gerbarg P, Bottiglieri T, et al; Work Group of the American Psychiatric Association Council on Research. S-Adenosylmethionine (SAMe) for neuropsychiatric disorders: a clinician-oriented review of research. J Clin Psychiatry. 2017;78(6):e656-e667.
9. Ng QX, Venkatanarayanan N, Ho CY. Clinical use of hypericum perforatum (St John’s wort) in depression: a meta-analysis. J Affect Disord 2017;210:211-221.
10. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483.
11. Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13-23.
12. Wallace CJK, Milev RV. The efficacy, safety, and tolerability of probiotics on depression: clinical results from an open-label pilot study. Front Psychiatry. 2021;12(132):618279.
13. Köhler-Forsberg O, N Lyndholm C, Hjorthøj C, et al. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139(5):404-419.
14. Jha MK. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J Clin Psychiatry. 2019;80(6)18ac12630.
15. Salagre E, Fernandes BS, Dodd S, et al. Statins for the treatment of depression: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord. 2016;200:235-242.
16. Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020;81(4):19m12887.
17. Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, et al. Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry. 2021;78(9):951-959.
18. Kalu UG, Sexton CE, Loo CK, et al. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 2012;42(9):1791-800.
19. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinical utility of transcranial direct current stimulation (tDCS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. J Psychiatr Res. 2013;47(1):1-7.
20. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92.
21. Singh SP, Singh V, Kar N. Efficacy of agomelatine in major depressive disorder: meta-analysis and appraisal. Int J Neuropsychopharmacol. 2012;15(3):417-428.
22. Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20(6):647-656.
23. Ramirez-Mahaluf JP, Rozas-Serri E, Ivanovic-Zuvic F, et al. Effectiveness of sleep deprivation in treating acute bipolar depression as augmentation strategy: a systematic review and meta-analysis. Front Psychiatry. 2020;11:70.
24. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;(9):CD004366.
25. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016;202:67-86.
26. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51.
27. Seshadri A, Adaji A, Orth SS, et al. Exercise, yoga, and tai chi for treatment of major depressive disorder in outpatient settings: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2020;23(1):20r02722.
28. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30(11):1068-1083.
29. Mota-Pereira J, Silverio J, Carvalho S, et al. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005-1011.
30. Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3:8.
31. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
32. Shonin E, Van Gordon W, Griffiths MD. Are there risks associated with using mindfulness for the treatment of psychopathology? Clinical Practice. 2014;11(4):389-392.
Intimate partner violence: Assessment in the era of telehealth
Intimate partner violence (IPV) includes “physical violence, sexual violence, stalking, and psychological aggression (including coercive tactics) by a current or former intimate partner.”1
Ensure a safe environment
At the onset of a telehealth appointment, ask the patient “Who is in the room with you?” If an adult or child age >2 years is present, do not assess for IPV because it may be unsafe for the patient to answer such questions. Encourage the patient to use privacy-enhancing strategies (eg, wearing headphones, going outside, calling from a vehicle). Be flexible; someone may not be able to discuss IPV during an appointment but might be able to at a different time, such as when their partner goes to work. For patients who disclose IPV, identify a word, phrase, or gesture to quickly communicate their partner’s presence or need for immediate help.2 While the “Signal for Help” (ie, thumb first tucked into the palm, then covered with fingers to form a fist) has been developed,3 it is not universally familiar; until then, establish specific communications and preferences with each patient. Include a plan for the patient to abruptly disconnect (eg, “You have the wrong number”) with a pre-determined method of follow-up.
Obtain informed consent
Before asking a patient about IPV, provide psychoeducation about the purpose, including its relationship to one’s health. Acknowledge reasons it may not be safe to provide and/or document answers, and describe limits of confidentiality and local mandated reporting requirements.
Standardize the assessment
Intimate partner violence assessment should be normalized (eg, “Because violence is common, I ask everyone about their relationships”), direct, and well-integrated. Know whether your site uses a specific IPV screening tool, such as the Relationship Health and Safety Screen (RHSS), which is used at the VA; if so, learn and practice asking the specific questions aloud until it feels routine and you can maintain eye contact throughout. Examples of other IPV assessment instruments include the Abuse Assessment Screen (AAS); Hurt, Insult, Threaten, and Scream (HITS), Partner Violence Screen (PVS), and Women Abuse Screening Tool (WAST).4 Pay attention to the populations in which a tool has been studied, any associated copyright fees, and gender-neutral and non-heteronormative language. Avoid asking leading questions (eg, “You’re not being hurt, are you?”) or using charged/interpretable terms (eg, “Is someone abusing you?”).
Document with intention
Use person-centered, recovery-oriented language (eg, someone who experiences or uses IPV) rather than stigmatizing language (eg, victim, batterer, abuser). Describe what happened using the individual’s own words and clearly identify the source of information, witnesses, and any weapons used. Choose nonpejorative language (ie, “states” instead of “claims”). Do not document details of the safety plan in the chart because doing so can compromise safety.
Provide resources and referrals
Regardless of whether a patient consents to screening/documentation or discloses IPV, you should offer universal education, resources, and referrals. Review national contacts (National Domestic Violence Hotline: 1-800-799-7233), community agencies (available through www.domesticshelters.org), and suggested safety apps such as myPlan (www.myplanapp.org), but do not send a patient electronic or physical materials without first confirming it is safe to do so. Assess the patient’s interest in legal steps (eg, obtaining a protection order, pressing charges) while recognizing and respecting valid concerns about law enforcement involvement, particularly among the Black community and Black transgender women. Provide options instead of instructions, which will empower patients to choose what is best for their situation, and support their decisions.
1. Breiding MJ, Chen J, Black MC. Intimate partner violence in the United States – 2010. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Published February 2014. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/cdc_nisvs_ipv_report_2013_v17_single_a.pdf
2. Evans ML, Lindauer JD, Farrell ME. A pandemic within a pandemic – intimate partner violence during Covid-19. N Engl J Med. 2020;383(24):2302-2304. doi:10.1056/NEJMp2024046
3. Canadian Women’s Foundation. Signal for help. 2020. Accessed January 12, 2021. https://canadianwomen.org/signal-for-help/
4. Basile KC, Hertz MF, Back SE. Intimate partner violence and sexual violence victimization assessment instruments for use in healthcare settings: Version 1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2007. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf
Intimate partner violence (IPV) includes “physical violence, sexual violence, stalking, and psychological aggression (including coercive tactics) by a current or former intimate partner.”1
Ensure a safe environment
At the onset of a telehealth appointment, ask the patient “Who is in the room with you?” If an adult or child age >2 years is present, do not assess for IPV because it may be unsafe for the patient to answer such questions. Encourage the patient to use privacy-enhancing strategies (eg, wearing headphones, going outside, calling from a vehicle). Be flexible; someone may not be able to discuss IPV during an appointment but might be able to at a different time, such as when their partner goes to work. For patients who disclose IPV, identify a word, phrase, or gesture to quickly communicate their partner’s presence or need for immediate help.2 While the “Signal for Help” (ie, thumb first tucked into the palm, then covered with fingers to form a fist) has been developed,3 it is not universally familiar; until then, establish specific communications and preferences with each patient. Include a plan for the patient to abruptly disconnect (eg, “You have the wrong number”) with a pre-determined method of follow-up.
Obtain informed consent
Before asking a patient about IPV, provide psychoeducation about the purpose, including its relationship to one’s health. Acknowledge reasons it may not be safe to provide and/or document answers, and describe limits of confidentiality and local mandated reporting requirements.
Standardize the assessment
Intimate partner violence assessment should be normalized (eg, “Because violence is common, I ask everyone about their relationships”), direct, and well-integrated. Know whether your site uses a specific IPV screening tool, such as the Relationship Health and Safety Screen (RHSS), which is used at the VA; if so, learn and practice asking the specific questions aloud until it feels routine and you can maintain eye contact throughout. Examples of other IPV assessment instruments include the Abuse Assessment Screen (AAS); Hurt, Insult, Threaten, and Scream (HITS), Partner Violence Screen (PVS), and Women Abuse Screening Tool (WAST).4 Pay attention to the populations in which a tool has been studied, any associated copyright fees, and gender-neutral and non-heteronormative language. Avoid asking leading questions (eg, “You’re not being hurt, are you?”) or using charged/interpretable terms (eg, “Is someone abusing you?”).
Document with intention
Use person-centered, recovery-oriented language (eg, someone who experiences or uses IPV) rather than stigmatizing language (eg, victim, batterer, abuser). Describe what happened using the individual’s own words and clearly identify the source of information, witnesses, and any weapons used. Choose nonpejorative language (ie, “states” instead of “claims”). Do not document details of the safety plan in the chart because doing so can compromise safety.
Provide resources and referrals
Regardless of whether a patient consents to screening/documentation or discloses IPV, you should offer universal education, resources, and referrals. Review national contacts (National Domestic Violence Hotline: 1-800-799-7233), community agencies (available through www.domesticshelters.org), and suggested safety apps such as myPlan (www.myplanapp.org), but do not send a patient electronic or physical materials without first confirming it is safe to do so. Assess the patient’s interest in legal steps (eg, obtaining a protection order, pressing charges) while recognizing and respecting valid concerns about law enforcement involvement, particularly among the Black community and Black transgender women. Provide options instead of instructions, which will empower patients to choose what is best for their situation, and support their decisions.
Intimate partner violence (IPV) includes “physical violence, sexual violence, stalking, and psychological aggression (including coercive tactics) by a current or former intimate partner.”1
Ensure a safe environment
At the onset of a telehealth appointment, ask the patient “Who is in the room with you?” If an adult or child age >2 years is present, do not assess for IPV because it may be unsafe for the patient to answer such questions. Encourage the patient to use privacy-enhancing strategies (eg, wearing headphones, going outside, calling from a vehicle). Be flexible; someone may not be able to discuss IPV during an appointment but might be able to at a different time, such as when their partner goes to work. For patients who disclose IPV, identify a word, phrase, or gesture to quickly communicate their partner’s presence or need for immediate help.2 While the “Signal for Help” (ie, thumb first tucked into the palm, then covered with fingers to form a fist) has been developed,3 it is not universally familiar; until then, establish specific communications and preferences with each patient. Include a plan for the patient to abruptly disconnect (eg, “You have the wrong number”) with a pre-determined method of follow-up.
Obtain informed consent
Before asking a patient about IPV, provide psychoeducation about the purpose, including its relationship to one’s health. Acknowledge reasons it may not be safe to provide and/or document answers, and describe limits of confidentiality and local mandated reporting requirements.
Standardize the assessment
Intimate partner violence assessment should be normalized (eg, “Because violence is common, I ask everyone about their relationships”), direct, and well-integrated. Know whether your site uses a specific IPV screening tool, such as the Relationship Health and Safety Screen (RHSS), which is used at the VA; if so, learn and practice asking the specific questions aloud until it feels routine and you can maintain eye contact throughout. Examples of other IPV assessment instruments include the Abuse Assessment Screen (AAS); Hurt, Insult, Threaten, and Scream (HITS), Partner Violence Screen (PVS), and Women Abuse Screening Tool (WAST).4 Pay attention to the populations in which a tool has been studied, any associated copyright fees, and gender-neutral and non-heteronormative language. Avoid asking leading questions (eg, “You’re not being hurt, are you?”) or using charged/interpretable terms (eg, “Is someone abusing you?”).
Document with intention
Use person-centered, recovery-oriented language (eg, someone who experiences or uses IPV) rather than stigmatizing language (eg, victim, batterer, abuser). Describe what happened using the individual’s own words and clearly identify the source of information, witnesses, and any weapons used. Choose nonpejorative language (ie, “states” instead of “claims”). Do not document details of the safety plan in the chart because doing so can compromise safety.
Provide resources and referrals
Regardless of whether a patient consents to screening/documentation or discloses IPV, you should offer universal education, resources, and referrals. Review national contacts (National Domestic Violence Hotline: 1-800-799-7233), community agencies (available through www.domesticshelters.org), and suggested safety apps such as myPlan (www.myplanapp.org), but do not send a patient electronic or physical materials without first confirming it is safe to do so. Assess the patient’s interest in legal steps (eg, obtaining a protection order, pressing charges) while recognizing and respecting valid concerns about law enforcement involvement, particularly among the Black community and Black transgender women. Provide options instead of instructions, which will empower patients to choose what is best for their situation, and support their decisions.
1. Breiding MJ, Chen J, Black MC. Intimate partner violence in the United States – 2010. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Published February 2014. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/cdc_nisvs_ipv_report_2013_v17_single_a.pdf
2. Evans ML, Lindauer JD, Farrell ME. A pandemic within a pandemic – intimate partner violence during Covid-19. N Engl J Med. 2020;383(24):2302-2304. doi:10.1056/NEJMp2024046
3. Canadian Women’s Foundation. Signal for help. 2020. Accessed January 12, 2021. https://canadianwomen.org/signal-for-help/
4. Basile KC, Hertz MF, Back SE. Intimate partner violence and sexual violence victimization assessment instruments for use in healthcare settings: Version 1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2007. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf
1. Breiding MJ, Chen J, Black MC. Intimate partner violence in the United States – 2010. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Published February 2014. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/cdc_nisvs_ipv_report_2013_v17_single_a.pdf
2. Evans ML, Lindauer JD, Farrell ME. A pandemic within a pandemic – intimate partner violence during Covid-19. N Engl J Med. 2020;383(24):2302-2304. doi:10.1056/NEJMp2024046
3. Canadian Women’s Foundation. Signal for help. 2020. Accessed January 12, 2021. https://canadianwomen.org/signal-for-help/
4. Basile KC, Hertz MF, Back SE. Intimate partner violence and sexual violence victimization assessment instruments for use in healthcare settings: Version 1. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2007. Accessed January 12, 2021. https://www.cdc.gov/violenceprevention/pdf/ipv/ipvandsvscreening.pdf