User login
Safe and effective bedside thoracentesis: A review of the evidence for practicing clinicians
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
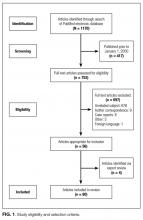
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
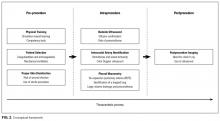
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
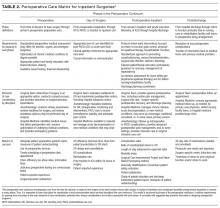
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.
PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
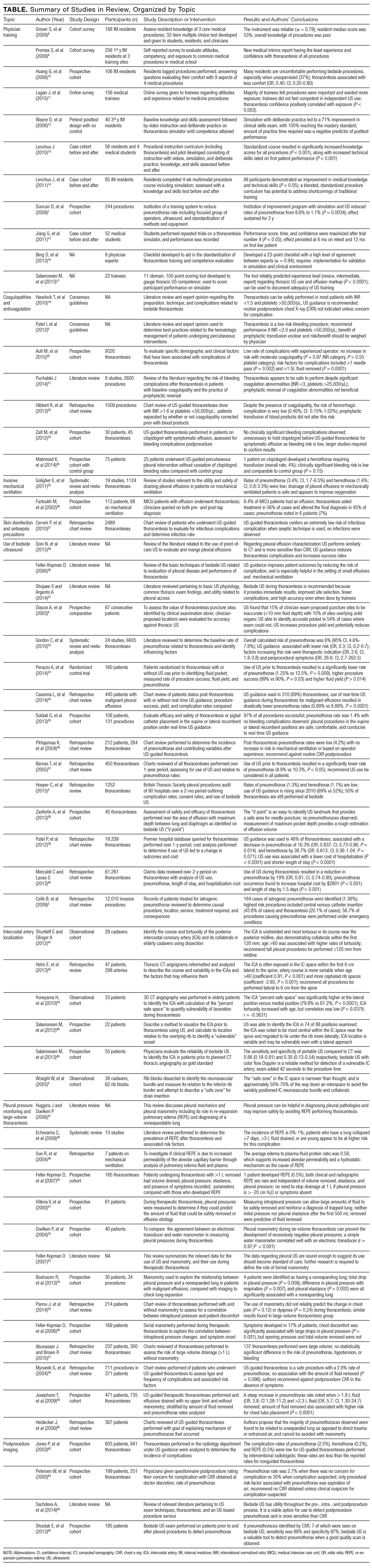
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
Pleural effusion can occur in myriad conditions including infection, heart failure, liver disease, and cancer.1 Consequently, physicians from many disciplines routinely encounter both inpatients and outpatients with this diagnosis. Often, evaluation and treatment require thoracentesis to obtain fluid for analysis or symptom relief.
Although historically performed at the bedside without imaging guidance or intraprocedural monitoring, thoracentesis performed in this fashion carries considerable risk of complications. In fact, it has 1 of the highest rates of iatrogenic pneumothorax among bedside procedures.2 However, recent advances in practice and adoption of newer technologies have helped to mitigate risks associated with this procedure. These advances are relevant because approximately 50% of thoracenteses are still performed at the bedside.3 In this review, we aim to identify the most recent key practices that enhance the safety and the effectiveness of thoracentesis for practicing clinicians.
METHODS
Information Sources and Search Strategy
With the assistance of a research librarian, we performed a systematic search of PubMed-indexed articles from January 1, 2000 to September 30, 2015. Articles were identified using search terms such as thoracentesis, pleural effusion, safety, medical error, adverse event, and ultrasound in combination with Boolean operators. Of note, as thoracentesis is indexed as a subgroup of paracentesis in PubMed, this term was also included to increase the sensitivity of the search. The full search strategy is available in the Appendix. Any references cited in this review outside of the date range of our search are provided only to give relevant background information or establish the origin of commonly performed practices.
Study Eligibility and Selection Criteria
Studies were included if they reported clinical aspects related to thoracentesis. We defined clinical aspects as those strategies that focused on operator training, procedural techniques, technology, management, or prevention of complications. Non-English language articles, animal studies, case reports, conference proceedings, and abstracts were excluded. As our intention was to focus on the contemporary advances related to thoracentesis performance, (eg, ultrasound [US]), our search was limited to studies published after the year 2000. Two authors, Drs. Schildhouse and Lai independently screened studies to determine inclusion, excluding studies with weak methodology, very small sample sizes, and those only tangentially related to our aim. Disagreements regarding study inclusion were resolved by consensus. Drs. Lai, Barsuk, and Mourad identified additional studies by hand review of reference lists and content experts (Figure 1).
Conceptual Framework
All selected articles were categorized by temporal relationship to thoracentesis as pre-, intra-, or postprocedure. Pre-procedural topics were those outcomes that had been identified and addressed before attempting thoracentesis, such as physician training or perceived risks of harm. Intraprocedural considerations included aspects such as use of bedside US, pleural manometry, and large-volume drainage. Finally, postprocedural factors were those related to evaluation after thoracentesis, such as follow-up imaging. This conceptual framework is outlined in Figure 2.
RESULTS
The PubMed search returned a total of 1170 manuscripts, of which 56 articles met inclusion criteria. Four additional articles were identified by experts and included in the study.4-7 Therefore, 60 articles were identified and included in this review. Study designs included cohort studies, case control studies, systematic reviews, meta-analyses, narrative reviews, consensus guidelines, and randomized controlled trials. A summary of all included articles by topic can be found in the Table.

PRE-PROCEDURAL CONSIDERATIONS
Physician Training
Studies indicate that graduate medical education may not adequately prepare clinicians to perform thoracentesis.8 In fact, residents have the least exposure and confidence in performing thoracentesis when compared to other bedside procedures.9,10 In 1 survey, 69% of medical trainees desired more exposure to procedures, and 98% felt that procedural skills were important to master.11 Not surprisingly, then, graduating internal medicine residents perform poorly when assessed on a thoracentesis simulator.12
Supplemental training outside of residency is useful to develop and maintain skills for thoracentesis, such as simulation with direct observation in a zero-risk environment. In 1 study, “simulation-based mastery learning” combined an educational video presentation with repeated, deliberate practice on a simulator until procedural competence was acquired, over two 2-hour sessions. In this study, 40 third-year medicine residents demonstrated a 71% improvement in clinical skills performance after course completion, with 93% achieving a passing score. The remaining 7% also achieved passing scores with extra practice time.12 Others have built upon the concept of simulation-based training. For instance, 2 studies suggest that use of a simulation-based curriculum improved both thoracentesis knowledge and performance skills in a 3-hour session.13,14 Similarly, 1 prospective study reported that a half-day thoracentesis workshop using simulation and 1:1 direct observation successfully lowered pneumothorax rates from 8.6% to 1.8% in a group of practicing clinicians. Notably, additional interventions including use of bedside US, limiting operators to a focused group, and standardization of equipment were also a part of this quality improvement initiative.7 Although repetition is required to gain proficiency when using a simulator, performance and confidence appear to plateau with only 4 simulator trials. In medical students, improvements derived through simulator-based teaching were sustained when retested 6 months following training.15
An instrument to ensure competency is necessary, given variability in procedural experience among both new graduates and practicing physicians,. Our search did not identify any clinically validated tools that adequately assessed thoracentesis performance. However, some have been proposed16 and 1 validated in a simulation environment.12 Regarding the incorporation of US for effusion markup, 1 validated tool used an 11-domain assessment covering knowledge of US machine manipulation, recognition of images with common pleural effusion characteristics, and performance of thoracic US with puncture-site marking on a simulator. When used on 22 participants, scores with the tool could reliably differentiate between novice, intermediate, and advanced groups (P < 0.0001).17
Patient Selection
Coagulopathies and Anticoagulation. Historically, the accepted cutoff for performing thoracentesis is an international normalized ratio (INR) less than 1.5 and a platelet count greater than 50,000/µL. McVay et al.18 first showed in 1991 that use of these cutoffs was associated with low rates of periprocedural bleeding, leading to endorsement in the British Thoracic Society (BTS) Pleural Disease Guideline 2010.19 Other recommendations include the 2012 Society for Interventional Radiology guidelines that endorse correction of an INR greater than 2, or platelets less than 50,000/µL, based almost exclusively on expert opinion.5
However, data suggest that thoracentesis may be safely performed outside these parameters. For instance, a prospective study of approximately 9000 thoracenteses over 12 years found that patients with an INR of 1.5-2.9 or platelets of 20,000 - 49,000/µL experienced rates of bleeding complications similar to those with normal values.20 Similarly, a 2014 review21 found that the overall risk of hemorrhage during thoracentesis in the setting of moderate coagulopathy (defined as an INR of 1.5 - 3 or platelets of 25,000-50,000/µL), was not increased. In 1 retrospective study of more than 1000 procedures, no differences in hemorrhagic events were noted in patients with bleeding diatheses that received prophylactic fresh frozen plasma or platelets vs. those who did not.22 Of note, included studies used a variety of criteria to define a hemorrhagic complication, which included: an isolated 2 g/dL or more decrement in hemoglobin, presence of bloody fluid on repeat tap with associated hemoglobin decrement, rapid re-accumulation of fluid with a hemoglobin decrement, or transfusion of 2 units or more of whole blood.
Whether it is safe to perform thoracentesis on patients taking antiplatelet therapy is less well understood. Although data are limited, a few small-scale studies23,24 suggest that hemorrhagic complications following thoracentesis in patients receiving clopidogrel are comparable to the general population. We found no compelling data regarding the safety of thoracentesis in the setting of direct oral anticoagulants, heparin, low-molecular weight heparin, or intravenous direct thrombin inhibitors. Current practice is to generally avoid thoracentesis while these therapeutic anticoagulants are used.
Invasive mechanical ventilation. Pleural effusion is common in patients in the intensive care unit, including those requiring mechanical ventilation.25 Thoracentesis in this population is clinically important: fluid analysis in 1 study was shown to aid the diagnosis in 45% of cases and changes in treatment in 33%.26 However, clinicians may be reluctant to perform thoracentesis on patients who require mechanical ventilation, given the perception of a greater risk of pneumothorax from positive pressure ventilation.
Despite this concern, a 2011 meta-analysis including 19 studies and more than 1100 patients revealed rates of pneumothorax and hemothorax comparable to nonventilated patients.25 Furthermore, a 2015 prospective study that examined thoracentesis in 1377 mechanically ventilated patients revealed no difference in complication rates as well.20 Therefore, evidence suggests that performance of thoracentesis in mechanically ventilated patients is not contraindicated.
Skin Disinfection and Antisepsis Precautions
The 2010 BTS guidelines list empyema and wound infection as possible complications of thoracentesis.19 However, no data regarding incidence are provided. Additionally, an alcohol-based skin cleanser (such as 2% chlorhexidine gluconate/70% isopropyl alcohol), along with sterile gloves, field, and dressing are suggested as precautionary measures.19 In 1 single-center registry of 2489 thoracenteses performed using alcohol or iodine-based antiseptic and sterile drapes, no postprocedure infections were identified.27 Of note, we did not find other studies (including case reports) that reported either incidence or rate of infectious complications such as wound infection and empyema. In an era of modern skin antiseptics that have effectively reduced complications such as catheter-related bloodstream infection,28 the incidence of this event is thus likely to be low.
INTRAPROCEDURAL CONSIDERATIONS
Use of Bedside Ultrasound
Portable US has particular advantages for evaluation of pleural effusion vs other imaging modalities. Compared with computerized tomography (CT), bedside US offers similar performance but is less costly, avoids both radiation exposure and need for patient transportation, and provides results instantaneously.29,30 Compared to chest x-ray (CXR), US is more sensitive at detecting the presence, volume, and characteristics of pleural fluid30,31 and can be up to 100% sensitive for effusions greater than 100 mL.29 Furthermore, whereas CXR typically requires 200 mL of fluid to be present for detection of an effusion, US can reliably detect as little as 20 mL of fluid.29 When US was used to confirm thoracentesis puncture sites in a study involving 30 physicians of varying experience and 67 consecutive patients, 15% of sites found by clinical exam were inaccurate (less than 10 mm fluid present), 10% were at high risk for organ puncture, and a suitable fluid pocket was found 54% of times when exam could not.4
A 2010 meta-analysis of 24 studies and 6605 thoracenteses estimated the overall rate of pneumothorax at 6%; however, procedures performed with US guidance were associated with a 70% reduced risk of this event (odds ratio, 0.30; 95% confidence interval, 0.20 - 0.70).32 In a 2014 randomized control trial of 160 patients that compared thoracentesis with US guidance for site marking vs no US use, 10 pneumothoraces occurred in the control group vs 1 in the US group (12.5% vs 1.25%, P = 0.009).33 Similarly, another retrospective review of 445 consecutive patients with malignant effusions revealed a pneumothorax rate of 0.97% using US in real time during needle insertion compared to 8.89% for unguided thoracenteses (P < 0.0001).34 Several other studies using US guidance for either site markup or in real time reported similar pneumothorax rates, ranging from 1.1% - 4.8%.35-37 However, it is unclear if real-time US specifically provides an additive effect vs site marking alone, as no studies directly comparing the 2 methods were found.
Benefits of US also include a higher rate of procedural success, with 1 study demonstrating a 99% success rate when using US vs. 90% without (P = 0.030).33 A larger volume of fluid removed has been observed with US use as well, and methods have been described using fluid-pocket depth to guide puncture site localization and maximize drainage.38 Finally, US use for thoracentesis has been associated with lower costs and length of stay.39,40
Intercostal Artery Localization
Although rare (incidence, 0.18%-2%20,21,39), the occurrence of hemothorax following thoracentesis is potentially catastrophic. This serious complication is often caused by laceration of the intercostal artery (ICA) or 1 of its branches during needle insertion.41
While risk of injury is theoretically reduced by needle insertion superior to the rib, studies using cadaver dissection and 3D angiography show significant tortuosity of the ICA.6,41-43 The degree of tortuosity is increased within 6 cm of the midline, in more cephalad rib spaces, and in the elderly (older than 60 years).41-43 Furthermore, 1 cadaveric study also demonstrated the presence of arterial collaterals branching off the ICA at multiple intercostal spaces, ranging between 8 cm and 11 cm from the midline.41 This anatomic variability may explain why some have observed low complication and hemothorax rates with an extreme lateral approach.35 Bedside US with color flow Doppler imaging has been used to identify the ICA, with 88% sensitivity compared to CT imaging while adding little to exam time.44,45 Of note, a 37% drop in the rate of hemothorax was observed in 1 study with routine US guidance alone.39
Pleural Pressure Monitoring and Large-Volume Thoracentesis
While normal intrapleural pressures are approximately -5 to -10 cm H2O,46 the presence of a pleural effusion creates a complex interaction between fluid, compressed lung, and chest wall that can increase these pressures.47 During drainage of an effusion, pleural pressures may rapidly drop, provoking re-expansion pulmonary edema (REPE). While rare (0 -1%), clinically-diagnosed REPE is a serious complication that can lead to rapid respiratory failure and death.20,48 REPE is postulated to be caused by increased capillary permeability resulting from inflammation, driven by rapid re-inflation of the lung when exposed to highly negative intrapleural pressures.47,49
Measurement of intrapleural pressure using a water manometer during thoracentesis may minimize REPE by terminating fluid drainage when intrapleural pressure begins to drop rapidly.50,51 A cutoff of -20 cm H2O has been cited repeatedly as safe since being suggested by Light in 1980, but this is based on animal models.50,52 In 1 prospective study of 185 thoracenteses in which manometry was performed, 15% of patients had intrapleural pressure drop to less than -20 cm H2O (at which point the procedure was terminated) but suffered no REPE.50
Manometry is valuable in the identification of an unexpandable or trapped lung when pleural pressures drop rapidly with only minimal fluid volume removal.47,53 Other findings correlated with an unexpandable lung include a negative opening pressure47 and large fluctuations in pressure during the respiratory cycle.54
While development of symptoms (eg, chest pain, cough, or dyspnea) is often used as a surrogate, the correlation between intrapleural pressure and patient symptoms is inconsistent and not a reliable proxy.55 One study found that 22% of patients with chest pain during thoracentesis had intrapleural pressures lower than -20 cm H2O compared with 8.6% of asymptomatic patients,56 but it is unclear if the association is causal.
Thoracentesis is often performed for symptomatic relief and removal of large fluid volume. However, it remains common to halt fluid removal after 1.5 L, a threshold endorsed by BTS.19 While some investigators have suggested that removal of 2 L or more of pleural fluid does not compromise safety,57,58 a 4- to 5-fold rise in the risk of pneumothorax was noted in 2 studies.20,59 when more than 1.5 L of fluid was removed. The majority of these may be related to pneumothorax ex vacuo, a condition in which fluid is drained from the chest, but the lung is unable to expand and fill the space (eg, “trapped lung”), resulting in a persistent pneumothorax. This condition generally does not require treatment.60 When manometry is employed at 200-mL intervals with termination at an intrapleural pressure of less than 20 mm H2O, drainage of 3 L or more has been reported with low rates of pneumothorax and very low rates of REPE.50,51 However, whether this is cause and effect is unknown because REPE is rare, and more work is needed to determine the role of manometry for its prevention.
POSTPROCEDURAL CONSIDERATIONS
Postprocedure Imaging
Performing an upright CXR following thoracentesis is a practice that remains routinely done by many practitioners to monitor for complications. Such imaging was also endorsed by the American Thoracic Society guidelines.61 However, more recent data question the utility of this practice. Multiple studies have confirmed that post-thoracentesis CXR is unnecessary unless clinical suspicion for pneumothorax or REPE is present.36,58,62,63 The BTS guidelines also advocate this approach.19 Interestingly, a potentially more effective way to screen for postprocedure complications is through bedside US, which has been shown to be more sensitive than CXR in detecting pneumothorax.64 In 1 study of 185 patients, bedside US demonstrated a sensitivity of 88% and a specificity of 97% for diagnosing pneumothorax in patients with adequate quality scans, with positive and negative likelihood ratios of 55 and 0.17, respectively.65
DISCUSSION
Thoracentesis remains a core procedural skill for hospitalists, critical care physicians, and emergency physicians. It is the foundational component when investigating and treating pleural effusions. When the most current training, techniques, and technology are used, data suggest this procedure is safe to perform at the bedside. Our review highlights these strategies and evaluates which aspects might be most applicable to clinical practice.
Our findings have several implications for those who perform this procedure. First, appropriate training is central to procedural safety, and both simulation and direct observation by procedural experts have been shown by multiple investigators to improve knowledge and skill. This training should integrate the use of US in performing a focused thoracic exam.
Second, recommendations regarding coagulopathy and a “safe cutoff” of an INR less than 1.5 or platelets greater than 50,000/µL had limited evidentiary support. Rather, multiple studies suggest no difference in bleeding risk following thoracentesis with an INR as high as 3.0 and platelets greater than 25,000/µL. Furthermore, prophylactic transfusion with fresh frozen plasma or platelets before thoracentesis did not alter bleeding risk and exposes patients to transfusion complications. Thus, routine use of this practice can no longer be recommended. Third, further research is needed to understand the bleeding risk for patients on antiplatelet medications, heparin products, and also direct oral anticoagulants, given the growing popularity in their use and the potential consequences of even temporary cessation. Regarding patients on mechanical ventilation, thoracentesis demonstrated no difference in complication rates vs. the general population, and its performance in this population is encouraged when clinically indicated.
Intraprocedural considerations include the use of bedside US. Due to multiple benefits including effusion characterization, puncture site localization, and significantly lower rates of pneumothorax, the standard of care should be to perform thoracentesis with US guidance. Both use of US to mark an effusion immediately prior to puncture or in real time during needle insertion demonstrated benefit; however, it is unclear if 1 method is superior because no direct comparison studies were found. Further work is needed to investigate this potential.
Our review suggests that the location and course of the ICA is variable, especially near the midline, in the elderly, and in higher intercostal spaces, leaving it vulnerable to laceration. We recommend physicians only attempt thoracentesis at least 6 cm lateral to the midline due to ICA tortuosity and, ideally, 12 cm lateral, to avoid the presence of collaterals. Although only 2 small-scale studies were found pertaining to the use of US in identifying the ICA, we encourage physicians to consider learning how to screen for its presence as a part of their routine thoracic US exam in the area underlying the planned puncture site.
Manometry is beneficial because it can diagnose a nonexpandable lung and allows for pleural pressure monitoring.52,53 A simple U-shaped manometer can be constructed from intravenous tubing included in most thoracentesis kits, which adds little to overall procedure time. While low rates of REPE have been observed when terminating thoracentesis if pressures drop below -20 cm H2O or chest pain develops, neither measure appears to have reliable predictive value, limiting clinical utility. Further work is required to determine if a “safe pressure cutoff” exists. In general, we recommend the use of manometry when a nonexpandable (trapped) lung is suspected, because large drops in intrapleural pressure, a negative opening pressure, and respiratory variation can help confirm the diagnosis and avoid pneumothorax ex vacuo or unnecessary procedures in the future. As this condition appears to be more common in the setting of larger effusions, use of manometry when large-volume thoracenteses are planned is also reasonable.
Postprocedurally, routine imaging after thoracentesis is not recommended unless there is objective concern for complication. When indicated, bedside US is better positioned for this role compared with CXR, because it is more sensitive in detecting pneumothorax, provides instantaneous results, and avoids radiation exposure.
Our review has limitations. First, we searched only for articles between defined time periods, restricted our search to a single database, and excluded non-English articles. This has the potential to introduce selection bias, as nonprimary articles that fall within our time restrictions may cite older studies that are outside our search range. To minimize this effect, we performed a critical review of all included studies, especially nonprimary articles. Second, despite the focus of our search strategy to identify any articles related to patient safety and adverse events, we cannot guarantee that all relevant articles for any particular complication or risk factor were captured given the lack of more specific search terms. Third, although we performed a systematic search of the literature, we did not perform a formal systematic review or formally grade included studies. As the goal of our review was to categorize and operationalize clinical aspects, this approach was necessary, and we acknowledge that the quality of studies is variable. Lastly, we aimed to generate clinical recommendations for physicians performing thoracentesis at the bedside; others reviewing this literature may find or emphasize different aspects relevant to practice outside this setting.
In conclusion, evaluation and treatment of pleural effusions with bedside thoracentesis is an important skill for physicians of many disciplines. The evidence presented in this review will help inform the process and ensure patient safety. Physicians should consider incorporating these recommendations into their practice.
The authors thank Whitney Townsend, MLIS, health sciences informationist, for assistance with serial literature searches.
Disclosure
Nothing to report.
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
1. Kasper DL. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw Hill Education; 2015.
2. Celik B, Sahin E, Nadir A, Kaptanoglu M. Iatrogenic pneumothorax: etiology, incidence and risk factors. Thorac Cardiovasc Surg. 2009;57(5):286-290. PubMed
3. Hooper CE, Welham SA, Maskell NA, Soc BT. Pleural procedures and patient safety: a national BTS audit of practice. Thorax. 2015;70(2):189-191. PubMed
4. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
5. Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727-736. PubMed
6. Wraight WM, Tweedie DJ, Parkin IG. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: a cadaveric study in respect of chest drain insertion. Clin Anat. 2005;18(5):346-349. PubMed
7. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
8. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents' knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
9. Promes SB, Chudgar SM, Grochowski CO, et al. Gaps in procedural experience and competency in medical school graduates. Acad Emerg Med. 2009;16 Suppl 2:S58-62. PubMed
10. Huang GC, Smith CC, Gordon CE, et al. Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures. Am J Med. 2006;119(1):71 e17-24. PubMed
11. Lagan J, Cutts L, Zaidi S, Benton I, Rylance J. Are we failing our trainees in providing opportunities to attain procedural confidence? Br J Hosp Med (Lond). 2015;76(2):105-108. PubMed
12. Wayne DB, Barsuk JH, O'Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
13. Lenchus JD. End of the "see one, do one, teach one" era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
14. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
15. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
16. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
17. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
18. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. PubMed
19. Havelock T, Teoh R, Laws D, Gleeson F, Group BTSPDG. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-76. PubMed
20. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
21. Puchalski J. Thoracentesis and the risks for bleeding: a new era. Curr Opin Pulm Med. 2014;20(4):377-384. PubMed
22. Hibbert RM, Atwell TD, Lekah A, et al. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest. 2013;144(2):456-463. PubMed
23. Zalt MB, Bechara RI, Parks C, Berkowitz DM. Effect of routine clopidogrel use on bleeding complications after ultrasound-guided thoracentesis. J Bronchology Interv Pulmonol. 2012;19(4):284-287. PubMed
24. Mahmood K, Shofer SL, Moser BK, Argento AC, Smathers EC, Wahidi MM. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann Am Thorac Soc. 2014;11(1):73-79. PubMed
25. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
26. Fartoukh M, Azoulay E, Galliot R, et al. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121(1):178-184. PubMed
27. Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195(4):846-850. PubMed
28. Mimoz O, Chopra V, Timsit JF. What's new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. 2016;42(11):1784-1786. PubMed
29. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
30. Feller-Kopman D. Ultrasound-guided thoracentesis. Chest. 2006;129(6):1709-1714. PubMed
31. Shojaee S, Argento AC. Ultrasound-guided pleural access. Semin Respir Crit Care Med. 2014;35(6):693-705. PubMed
32. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
33. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
34. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
35. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
36. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
37. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
38. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracenthesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
39. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
40. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
41. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol (Warsz). 2012;71(4):245-251. PubMed
42. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
43. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
44. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
45. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
46. Grippi MA. Fishman's pulmonary diseases and disorders. Fifth edition. ed. New York: McGraw-Hill Education; 2015.
47. Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27(2):229-240. PubMed
48. Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7(3):485-489. PubMed
49. Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30(10):1921-1926. PubMed
50. Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84(5):1656-1661. PubMed
51. Villena V, Lopez-Encuentra A, Pozo F, De-Pablo A, Martin-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1534-1538. PubMed
52. Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126(6):1764-1769. PubMed
53. Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13(4):312-318. PubMed
54. Boshuizen RC, Sinaasappel M, Vincent AD, Goldfinger V, Farag S, van den Heuvel MM. Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol. 2013;20(3):200-205. PubMed
55. Pannu J, DePew ZS, Mullon JJ, Daniels CE, Hagen CE, Maldonado F. Impact of pleural manometry on the development of chest discomfort during thoracentesis: a symptom-based study. J Bronchology Interv Pulmonol. 2014;21(4):306-313. PubMed
56. Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129(6):1556-1560. PubMed
57. Abunasser J, Brown R. Safety of large-volume thoracentesis. Conn Med. 2010;74(1):23-26. PubMed
58. Mynarek G, Brabrand K, Jakobsen JA, Kolbenstvedt A. Complications following ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(5):519-522. PubMed
59. Josephson T, Nordenskjold CA, Larsson J, Rosenberg LU, Kaijser M. Amount drained at ultrasound-guided thoracentesis and risk of pneumothorax. Acta Radiol. 2009;50(1):42-47. PubMed
60. Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130(4):1173-1184. PubMed
61. Sokolowski JW Jr, Burgher LW, Jones FL Jr, Patterson JR, Selecky PA. Guidelines for thoracentesis and needle biopsy of the pleura. This position paper of the American Thoracic Society was adopted by the ATS Board of Directors, June 1988. Am Rev Respir Dis. 1989;140(1):257-258. PubMed
62. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
63. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
64. Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: state of the art in 2013. Clin Chest Med. 2013;34(1):1-9. PubMed
65. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
© 2017 Society of Hospital Medicine
Hospital medicine and perioperative care: A framework for high-quality, high-value collaborative care
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
Of the 36 million US hospitalizations each year, 22% are surgical.1 Although less frequent than medical hospitalizations, surgical hospitalizations are more than twice as costly.2 Additionally, surgical hospitalizations are on average longer than medical hospitalizations.2 Given the increased scrutiny on cost and efficiency of care, attention has turned to optimizing perioperative care. Hospitalists are well positioned to provide specific expertise in the complex interdisciplinary medical management of surgical patients.
In recent decades, multiple models of hospitalist involvement in perioperative care have evolved across the United States.3-19 To consolidate knowledge and experience and to develop a framework for providing the best care for surgical patients, the Society of Hospital Medicine organized the Perioperative Care Work Group in 2015. This framework was designed for interdisciplinary collaboration in building and strengthening perioperative care programs.
METHODS
The Society of Hospital Medicine recognized hospital medicine programs’ need for guidance in developing collaborative care in perioperative medicine and appointed the Perioperative Care Work Group in May 2015. Work group members are perioperative medicine experts from US medical centers. They have extensive knowledge of the literature as well as administrative and clinical experience in a variety of perioperative care models.
Topic Development. Initial work was focused on reviewing and discussing multiple models of perioperative care and exploring the roles that hospital medicine physicians have within these models. Useful information was summarized to guide hospitals and physicians in designing, implementing, and expanding patient-centric perioperative medicine services with a focus on preoperative and postoperative care. A final document was created; it outlines system-level issues in perioperative care, organized by perioperative phases.
Initial Framework. Group members submitted written descriptions of key issues in each of 4 phases: (1) preoperative, (2) day of surgery, (3) postoperative inpatient, and (4) postdischarge. These descriptions were merged and reviewed by the content experts. Editing and discussion from the entire group were incorporated into the final matrix, which highlighted (1) perioperative phase definitions, (2) requirements for patients to move to next phase, (3) elements of care coordination typically provided by surgery, anesthesiology, and medicine disciplines, (4) concerns and risks particular to each phase, (5) unique considerations for each phase, (6) suggested metrics of success, and (7) key questions for determining the effectiveness of perioperative care in an institution. All members provided final evaluation and editing.
Final Approval. The Perioperative Care Matrix for Inpatient Surgeries (PCMIS) was presented to the board of the Society of Hospital Medicine in fall 2015 and was approved for use in centering and directing discussions regarding perioperative care.
Models of Care. The Perioperative Care Work Group surveyed examples of hospitalist engagement in perioperative care and synthesized these into synopses of existing models of care for the preoperative, day-of-surgery, postoperative-inpatient, and postdischarge phases.
RESULTS
Defining Key Concepts and Issues
Hospitalists have participated in a variety of perioperative roles for more than a decade. Roles include performing in-depth preoperative assessments, providing oversight to presurgical advanced practice provider assessments, providing inpatient comanagement and consultation both before and after surgery, and providing postdischarge follow-up within the surgical period for medical comorbidities.
Although a comprehensive look at the entire perioperative period is important, 4 specific phases were defined to guide this work (Figure). The phases identified were based on time relative to surgery, with unique considerations as to the overall perioperative period. Concerns and potential risks specific to each phase were considered (Table 1).
The PCMIS was constructed to provide a single coherent vision of key concepts in perioperative care (Table 2). Also identified were several key questions for determining the effectiveness of perioperative care within an institution (Table 3).
Models of Care
Multiple examples of hospitalist involvement were collected to inform the program development guidelines. The specifics noted among the reviewed practice models are described here.
Preoperative. In some centers, all patients scheduled for surgery are required to undergo evaluation at the institution’s preoperative clinic. At most others, referral to the preoperative clinic is at the discretion of the surgical specialists, who have been informed of the clinic’s available resources. Factors determining whether a patient has an in-person clinic visit, undergoes a telephone-based medical evaluation, or has a referral deferred to the primary care physician (PCP) include patient complexity and surgery-specific risk. Patients who have major medical comorbidities (eg, chronic lung or heart disease) or are undergoing higher risk procedures (eg, those lasting >1 hour, laparotomy) most often undergo a formal clinic evaluation. Often, even for a patient whose preoperative evaluation is completed by a PCP, the preoperative nursing staff will call before surgery to provide instructions and to confirm that preoperative planning is complete. Confirmation includes ensuring that the surgery consent and preoperative history and physical examination documents are in the medical record, and that all recommended tests have been performed. If deficiencies are found, surgical and preoperative clinic staff are notified.
During a typical preoperative clinic visit, nursing staff complete necessary regulatory documentation requirements and ensure that all items on the preoperative checklist are completed before day of surgery. Nurses or pharmacists perform complete medication reconciliation. For medical evaluation at institutions with a multidisciplinary preoperative clinic, patients are triaged according to comorbidity and procedure. These clinics often have anesthesiology and hospital medicine clinicians collaborating with interdisciplinary colleagues and with patients’ longitudinal care providers (eg, PCP, cardiologist). Hospitalists evaluate patients with comorbid medical diseases and address uncontrolled conditions and newly identified symptomatology. Additional testing is determined by evidence- and guideline-based standards. Patients receive preoperative education, including simple template-based medication management instructions. Perioperative clinicians follow up on test results, adjust therapy, and counsel patients to optimize health in preparation for surgery.
Patients who present to the hospital and require urgent surgical intervention are most often admitted to the surgical service, and hospital medicine provides timely consultation for preoperative recommendations. At some institutions, protocols may dictate that certain surgical patients (eg, elderly with hip fracture) are admitted to the hospital medicine service. In these scenarios, the hospitalist serves as the primary inpatient care provider and ensures preoperative medical optimization and coordination with the surgical service to expedite plans for surgery.
Day of Surgery. On the day of surgery, the surgical team verifies all patient demographic and clinical information, confirms that all necessary documentation is complete (eg, consents, history, physical examination), and marks the surgical site. The anesthesia team performs a focused review and examination while explaining the perioperative care plan to the patient. Most often, the preoperative history and physical examination, completed by a preoperative clinic provider or the patient’s PCP, is used by the anesthesiologist as the basis for clinical assessment. However, when information is incomplete or contradictory, surgery may be delayed for further record review and consultation.
Hospital medicine teams may be called to the pre-anesthesia holding area to evaluate acute medical problems (eg, hypertension, hyperglycemia, new-onset arrhythmia) or to give a second opinion in cases in which the anesthesiologist disagrees with the recommendations made by the provider who completed the preoperative evaluation. In either scenario, hospitalists must provide rapid service in close collaboration with anesthesiologists and surgeons. If a patient is found to be sufficiently optimized for surgery, the hospitalist clearly documents the evaluation and recommendation in the medical record. For a patient who requires further medical intervention before surgery, the hospitalist often coordinates the immediate disposition (eg, hospital admission or discharge home) and plans for optimization in the timeliest manner possible.
Occasionally, hospitalists are called to evaluate a patient in the postanesthesia care unit (PACU) for a new or chronic medical problem before the patient is transitioned to the next level of care. At most institutions, all PACU care is provided under the direction of anesthesiology, so it is imperative to collaborate with the patient’s anesthesiologist for all recommendations. When a patient is to be discharged home, the hospitalist coordinates outpatient follow-up plans for any medical issues to be addressed postoperatively. Hospitalists also apply their knowledge of the limitations of non–intensive care unit hospital care to decisions regarding appropriate triage of patients being admitted after surgery.
Postoperative Inpatient. Hospitalists provide a 24/7 model of care that deploys a staff physician for prompt assessment and management of medical problems in surgical patients. This care can be provided as part of the duties of a standard hospital medicine team or can be delivered by a dedicated perioperative medical consultation and comanagement service. In either situation, the type of medical care, comanagement or consultation, is determined at the outset. As consultants, hospitalists provide recommendations for medical care but do not write orders or take primary responsibility for management. Comanagement agreements are common, especially for orthopedic surgery and neurosurgery; these agreements delineate the specific circumstances and responsibilities of the hospitalist and surgical teams. Indications for comanagement, which may be identified during preoperative clinic evaluation or on admission, include uncontrolled or multiple medical comorbidities or the development of nonsurgical complications in the perioperative period. In the comanagement model, care of most medical issues is provided at the discretion of the hospitalist. Although this care includes order-writing privileges, management of analgesics, wounds, blood products, and antithrombotics is usually reserved for the surgical team, with the hospitalist only providing recommendations. In some circumstances, hospitalists may determine that the patient’s care requires consultation with other specialists. Although it is useful for the hospitalist to speak directly with other consultants and coordinate their recommendations, the surgical service should agree to the involvement of other services.
In addition to providing medical care throughout a patient’s hospitalization, the hospitalist consultant is crucial in the discharge process. During the admission, ideally in collaboration with a pharmacist, the hospitalist reviews the home medications and may change chronic medications. The hospitalist may also identify specific postdischarge needs of which the surgical team is not fully aware. These medical plans are incorporated through shared responsibility for discharge orders or through a reliable mechanism for ensuring the surgical team assumes responsibility. Final medication reconciliation at discharge, and a plan for prior and new medications, can be formulated with pharmacy assistance. Finally, the hospitalist is responsible for coordinating medically related hospital follow-up and handover back to the patient’s longitudinal care providers. The latter occurs through inclusion of medical care plans in the discharge summary completed by the surgical service and, in complex cases, through direct communication with the patient’s outpatient providers.
For some patients, medical problems eclipse surgical care as the primary focus of management. Collaborative discussion between the medical and surgical teams helps determine if it is more appropriate for the medical team to become the primary service, with the surgical team consulting. Such triage decisions should be jointly made by the attending physicians of the services rather than by intermediaries.
Postdischarge. Similar to their being used for medical problems after hospitalization, hospitalist-led postdischarge and extensivist clinics may be used for rapid follow-up of medical concerns in patients discharged after surgical admissions. A key benefit of this model is increased availability over what primary care clinics may be able to provide on short notice, particularly for patients who previously did not have a PCP. Additionally, the handover of specific follow-up items is more streamlined because the transition of care is between hospitalists from the same institution. Through the postdischarge clinic, hospitalists can provide care through either clinic visits or telephone-based follow-up. Once a patient’s immediate postoperative medical issues are fully stabilized, the patient can be transitioned to long-term primary care follow-up.
DISCUSSION
The United States is focused on sensible, high-value care. Perioperative care is burgeoning with opportunities for improvement, including reducing avoidable complications, developing systems for early recognition and treatment of complications, and streamlining processes to shorten length of stay and improve patient experience. The PCMIS provides the needed platform to catalyze detailed collaborative work between disciplines engaged in perioperative care.
As average age and level of medical comorbidity increase among surgical patients, hospitalists will increasingly be called on to assist in perioperative care. Hospitalists have long been involved in caring for medically complex surgical patients, through comanagement, consultation, and preoperative evaluations. As a provider group, hospitalists have comprehensive skills in quality and systems improvement, and in program development across hospital systems nationwide. Hospitalists have demonstrated their value by focusing on improving patient outcomes and enhancing patient engagement and experiences. Additionally, the perioperative period is fraught with multiple and complicated handoffs, a problem area for which hospital medicine has pioneered solutions and developed unique expertise. Hospital medicine is well prepared to provide skilled and proven leadership in the timely development, improvement, and expansion of perioperative care for this increasingly older and chronically ill population.
Hospitalists are established in multiple perioperative roles for high-risk surgical patients and have the opportunity to expand optimal patient-centric perioperative care systems working in close concert with surgeons and anesthesiologists. The basics of developing these systems include (1) assessing risk for medical complications, (2) planning for perioperative care, (3) developing programs aimed at risk reduction for preventable complications and early identification and intervention for unavoidable complications, and (4) guiding quality improvement efforts, including planning for frequent handoffs and transitions.
As a key partner in developing comprehensive programs in perioperative care, hospital medicine will continue to shape the future of hospital care for all patients. The PCMIS, as developed with support from the Society of Hospital Medicine, will aid efforts to achieve the best perioperative care models for our surgical patients.
Disclosures
Financial activities outside the submitted work: Drs. Pfeifer and Jaffer report payment for development of educational presentations; Dr. Grant reports payment for expert testimony pertaining to hospital medicine; Drs. Grant and Jaffer report royalties from publishing; Drs. Thompson, Pfiefer, Grant, Slawski, and Jaffer report travel expenses for speaking and serving on national committees; and Drs. Slawski and Jaffer serve on the board of the Society of Perioperative Assessment and Quality Improvement. The other authors have nothing to report.
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
1. Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 (Current Population Reports, P25-1143). Washington, DC: US Census Bureau; 2014. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf. Published March 2015. Accessed May 26, 2016.
2. Steiner C, Andrews R, Barrett M, Weiss A. HCUP Projections: Cost of Inpatient Discharges 2003 to 2013 (Rep 2013-01). Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.hcup-us.ahrq.gov/reports/projections/2013-01.pdf. Published December 11, 2013. Accessed May 26, 2016.
3. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. PubMed
4. Batsis JA, Phy MP, Melton LJ 3rd, et al. Effects of a hospitalist care model on mortality of elderly patients with hip fractures. J Hosp Med. 2007;2(4):219-225. PubMed
5. Carr AM, Irigoyen M, Wimmer RS, Arbeter AM. A pediatric residency experience with surgical co-management. Hosp Pediatr. 2013;3(2):144-148. PubMed
6. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10-15. PubMed
7. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma. 2006;20(3):172-178. PubMed
8. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349-1356. PubMed
9. Huddleston JM, Long KH, Naessens JM, et al; Hospitalist-Orthopedic Team Trial Investigators. Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. PubMed
10. Mendelson DA, Friedman SM. Principles of comanagement and the geriatric fracture center. Clin Geriatr Med. 2014;30(2):183-189. PubMed
11. Merli GJ. The hospitalist joins the surgical team. Ann Intern Med. 2004;141(1):67-69. PubMed
12. Phy MP, Vanness DJ, Melton LJ 3rd, et al. Effects of a hospitalist model on elderly patients with hip fracture. Arch Intern Med. 2005;165(7):796-801. PubMed
13. Pinzur MS, Gurza E, Kristopaitis T, et al. Hospitalist-orthopedic co-management of high-risk patients undergoing lower extremity reconstruction surgery. Orthopedics. 2009;32(7):495. PubMed
14. Rappaport DI, Adelizzi-Delany J, Rogers KJ, et al. Outcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgery. Hosp Pediatr. 2013;3(3):233-241. PubMed
15. Rappaport DI, Cerra S, Hossain J, Sharif I, Pressel DM. Pediatric hospitalist preoperative evaluation of children with neuromuscular scoliosis. J Hosp Med. 2013;8(12):684-688. PubMed
16. Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31. PubMed
17. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. PubMed
18. Simon TD, Eilert R, Dickinson LM, Kempe A, Benefield E, Berman S. Pediatric hospitalist comanagement of spinal fusion surgery patients. J Hosp Med. 2007;2(1):23-30. PubMed
19. Whinney C, Michota F. Surgical comanagement: a natural evolution of hospitalist practice. J Hosp Med. 2008;3(5):394-397. PubMed
© 2017 Society of Hospital Medicine
Psychosis in borderline personality disorder: How assessment and treatment differs from a psychotic disorder
Psychotic symptoms in patients with borderline personality disorder (BPD) are common, distressing to patients, and challenging to treat. Issues of comorbidities and misdiagnoses in BPD patients further complicate matters and could lead to iatrogenic harm. The dissociation that patients with BPD experience could be confused with psychosis and exacerbate treatment and diagnostic confusion. Furthermore, BPD patients with unstable identity and who are sensitive to rejection could present in a bizarre, disorganized, or agitated manner when under stress.
Although pitfalls occur when managing psychotic symptoms in patients with BPD, there are trends and clues to help clinicians navigate diagnostic and treatment challenges. This article will review the literature, propose how to distinguish psychotic symptoms in BPD from those in primary psychotic disorders such as schizophrenia, and explore reasonable treatment options.
The scope of the problem
The DSM-5 criteria for BPD states that “during periods of extreme stress, transient paranoid ideation or dissociative symptoms may occur.”1 The term “borderline” originated from the idea that symptoms bordered on the intersection of neurosis and psychosis.2 However, psychotic symptoms in BPD are more varied and frequent than what DSM-5 criteria suggests.
The prevalence of psychotic symptoms in patients with BPD has been estimated between 20% to 50%.3 There also is evidence of frequent auditory and visual hallucinations in patients with BPD, and a recent study using structured psychiatric interviews demonstrated that most BPD patients report at least 1 symptom of psychosis.4 Considering that psychiatric comorbidities are the rule rather than the exception in BPD, the presence of psychotic symptoms further complicates the diagnostic picture. Recognizing the symptoms of BPD is essential for understanding the course of the symptoms and predicting response to treatment.5
Treatment of BPD is strikingly different than that of a primary psychotic disorder. There is some evidence that low-dosage antipsychotics could ease mood instability and perceptual disturbances in patients with BPD.6 Antipsychotic dosages used to treat hallucinations and delusions in a primary psychotic disorder are unlikely to be as effective for a patient with BPD, and are associated with significant adverse effects. Furthermore, these adverse effects—such as weight gain, hyperlipidemia, and diabetes—could become new sources of distress. Clinicians also might miss an opportunity to engage a BPD patient in psychotherapy if the focus is on the anticipated effect of a medication. The mainstay treatment of BPD is an evidence-based psychotherapy, such as dialectical behavioral therapy, transference-focused psychotherapy, mentalization-based therapy, or good psychiatric management.7
CASE Hallucinations during times of stress
Ms. K, a 20-year-old single college student, presents to the psychiatric emergency room with worsening mood swings, anxiety, and hallucinations. Her mood swings are brief and intense, lasting minutes to hours. Anxiety often is triggered by feelings of emptiness and fear of abandonment. She describes herself as a “social chameleon” and notes that she changes how she behaves depending on who she spends time with.
She often hears the voice of her ex-boyfriend instructing her to kill herself and saying that she is a “terrible person.” Their relationship was intense, with many break-ups and reunions. She also reports feeling disconnected from herself at times as though she is being controlled by an outside entity. To relieve her emotional suffering, she cuts herself superficially. Although she has no family history of psychiatric illness, she fears that she may have schizophrenia.
Ms. K’s outpatient psychiatrist prescribes antipsychotics at escalating dosages over a few months (she now takes olanzapine, 40 mg/d, aripiprazole, 30 mg/d, clonazepam, 3 mg/d, and escitalopram, 30 mg/d), but the hallucinations remain. These symptoms worsen during stressful situations, and she notices that they almost are constant as she studies for final exams, prompting her psychiatrist to discuss a clozapine trial. Ms. K is not in psychotherapy, and recognizes that she does not deal with stress well. Despite her symptoms, she is organized in her thought process, has excellent grooming and hygiene, has many social connections, and performs well in school.
How does one approach a patient such as Ms. K?
A chief concern of hallucinations, particularly in a young adult at an age when psychotic disorders such as schizophrenia often emerge, can contribute to a diagnostic quandary. What evidence can guide the clinician? There are some key features to consider:
- Her “mood swings” are notable in their intensity and brevity, making a primary mood disorder with psychotic features less likely.
- Hallucinations are present in the absence of a prodromal period of functional decline or negative symptoms, making a primary psychotic disorder less likely.
- She does not have a family history of psychiatric illness, particularly a primary psychotic disorder.
- She maintains social connections, although her relationships are intense and tumultuous.
- Psychotic symptoms have not changed with higher dosages of antipsychotics.
- Complaints of feeling “disconnected from herself” and “empty” are common symptoms of BPD and necessitate further exploration.
- Psychotic symptoms are largely transient and stress-related, with an overwhelmingly negative tone.
- Techniques that individuals with schizophrenia use, such as distraction or trying to tune out voices, are not being employed. Instead, Ms. K attends to the voices and is anxiously focused on them.
- The relationship of her symptoms to interpersonal stress is key.
When evaluating a patient such as Ms. K, it is important to explore both the nature and timing of the psychotic symptoms and any other related psychiatric symptoms. This helps to determine a less ambiguous diagnosis and clearer treatment plan. Understanding the patient’s perspective about the psychotic symptoms also is useful to gauge the patient’s level of distress and her impression of what the symptoms mean.
Diagnostic considerations
BPD is characterized by a chaotic emotional climate with impulsivity and instability of self-image, affect, and relationships. Most BPD symptoms, including psychosis, often are exacerbated by the perception of abandonment or rejection and other interpersonal stressors.1 Both BPD and schizophrenia are estimated to affect at least 1% of the general population.8,9 Patients with BPD frequently meet criteria for comorbid mental illnesses, including major depressive disorder, substance use disorder, posttraumatic stress disorder, anxiety, and eating disorders.10 Because psychotic symptoms can present in some of these disorders, the context and time course of these symptoms are crucial to consider.
Misdiagnosis is common with BPD, and patients can receive the wrong treatment for years before BPD is considered, likely because of the stigma surrounding the diagnosis.5 One also must keep in mind that, although rare, a patient can have both BPD and a primary psychotic disorder.11 Although a patient with schizophrenia could be prone to social isolation because of delusions or paranoia, BPD patients are more apt to experience intense interpersonal relationships driven by the need to avoid abandonment. Manipulation, anger, and neediness in relationships with both peers and health care providers are common—stark contrasts to typical negative symptoms, blunted affect, and a lack of social drive characteristic of schizophrenia.12
Distinguishing between psychosis in BPD and a psychotic disorder
Studies have sought to explore the quality of psychotic symptoms in BPD vs primary psychotic disorders, which can be challenging to differentiate (Table 1). Some have found that transient symptoms, such as non-delusional paranoia, are more prevalent in BPD, and “true” psychotic symptoms that are long-lasting and bizarre are indicative of schizophrenia.13,14 Also, there is evidence that the lower levels of interpersonal functioning often found in BPD are predictive of psychotic symptoms in that disorder but not in schizophrenia.15

Auditory hallucinations in patients with BPD predominantly are negative and critical in tone.4 However, there is no consistent evidence that the quality of auditory hallucinations in BPD vs schizophrenia is different in any meaningful way.16 Because of the frequency of dissociative symptoms in BPD, it is likely that clinicians could misinterpret these symptoms to indicate disorganized behavior associated with a primary psychotic disorder. In one study, 50% of individuals with BPD experienced auditory hallucinations.11 Differentiating between “internal” or “external” voices did not help to clarify the diagnosis, and paranoid delusions occurred in less than one-third of patients with BPD, but in approximately two-third of those with a diagnosis of schizophrenia.
The McLean Study of Adult Development, a longitudinal study of BPD patients, found that the prevalence of psychotic symptoms diminished over time. It is unclear whether this was due to the spontaneus remission rate of BPD symptoms in general or because of effective treatment.13
Psychotic symptoms in BPD seem to react to stress and increase in intensity when patients are in crisis.17 Nonetheless, because of the prevalence of psychosis in BPD patients and the distress it causes, clinicians should be cautioned against using terms that imply that the symptoms are not “true” or “real.”3
Treatment recommendations
When considering pharmacologic management of psychotic symptoms in BPD, aim to limit antipsychotic medications to low dosages because of adverse effects and the limited evidence that escalating dosages—and especially using >1 antipsychotic concurrently—are more effective.18 Educate patients that in BPD medications are, at best, considered adjunctive treatments. Blaming psychotic symptoms on a purely biological process in BPD, not only is harmful because medications are unlikely to significantly or consistently help, but also because they can undermine patient autonomy and reinforce the need for an outside entity (ie, medication) to fix their problems.
When treatment is ineffective and symptoms do not improve, a patient with BPD likely will experience mounting distress. This, in turn, could exacerbate impulsive, suicidal, and self-injurious behaviors. Emphasize psychotherapy, particularly for those whose psychotic symptoms are transient, stress-related, and present during acute crises (Table 2). With evidence-based psychotherapy, BPD patients can become active participants in treatment, coupling developing insight with concrete skills and teachable principles. This leads to increased interpersonal effectiveness and resilience during times of stress. Challenging the patient’s psychotic symptoms as false or “made up” rarely is helpful and usually harmful, leading to the possible severance of the therapeutic alliance.3

Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) could look similar to those in primary psychotic disorders. Factors suggesting BPD include a pattern of worsening psychotic symptoms during stress, long-term symptom instability, lack of delusions, presence of dissociation, and nonresponse to antipsychotics. Although low-dosage antipsychotics could provide some relief of psychotic symptoms in a patient with BPD, they often are not consistently effective and frequently lead to adverse effects. Emphasize evidence-based psychotherapies.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern A. Borderline group of neuroses. The Psychoanalytic Quarterly. 1938;7:467-489.
3. Schroeder K, Fisher HL, Schäfer I, et al. Psychotic symptoms in patients with borderline personality disorder: prevalence and clinical management. Curr Opin Psychiatry. 2013;26(1):113-119.
4. Pearse LJ, Dibben C, Ziauddeen H, et al. A study of psychotic symptoms in borderline personality disorder. J Nerv Ment Dis. 2014;202(5):368-371.
5. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-
6. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29(5):461-467.
7. National Education Alliance for Borderline Personality Disorder. Treatments for BPD. http://www.borderlinepersonalitydisorder.com/what-is-bpd/treating-bpd. Accessed September 1, 2016.
8. Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85-94.
9. Lenzenweger MF, Lane MC, Loranger AW, et al. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553-564.
10. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155(12):1733-1739.
11. Kingdon DG, Ashcroft K, Bhandari B, et al. Schizophrenia and borderline personality disorder: similarities and differences in the experience of auditory hallucinations, paranoia, and childhood trauma. J Nerv Ment Dis. 2010;198(6):399-403.
12. Gunderson JG. Borderline personality disorder. Washington, DC: American Psychiatric Press; 1984.
13. Zanarini MC, Frankenburg FR, Wedig MM, et al. Cognitive experiences reported by patients with borderline personality disorder and Axis II comparison subjects: a 16-year prospective follow-up study. Am J Psychiatry. 2013;170(6):671-679.
14. Tschoeke S, Steinert T, Flammer E, et al. Similarities and differences in borderline personality disorder and schizophrenia with voice hearing. J Nerv Ment Dis. 2014;202(7):544-549.
15. Oliva F, Dalmotto M, Pirfo E, et al. A comparison of thought and perception disorders in borderline personality disorder and schizophrenia: psychotic experiences as a reaction to impaired social functioning. BMC Psychiatry. 2014;14:239.
16. Merrett Z, Rossell SL, Castle DJ, et al. Comparing the experience of voices in borderline personality disorder with the experience of voices in a psychotic disorder: a systematic review. Aust N Z J Psychiatry. 2016;50(7):640-648.
17. Glaser JP, Van Os J, Thewissen V, et al. Psychotic reactivity in borderline personality disorder. Acta Psychiatr Scand. 2010;121(2):125-134.
18. Rosenbluth M, Sinyor M. Off-label use of atypical antipsychotics in personality disorders. Expert Opin Pharmacother. 2012;13(11):1575-1585.
Psychotic symptoms in patients with borderline personality disorder (BPD) are common, distressing to patients, and challenging to treat. Issues of comorbidities and misdiagnoses in BPD patients further complicate matters and could lead to iatrogenic harm. The dissociation that patients with BPD experience could be confused with psychosis and exacerbate treatment and diagnostic confusion. Furthermore, BPD patients with unstable identity and who are sensitive to rejection could present in a bizarre, disorganized, or agitated manner when under stress.
Although pitfalls occur when managing psychotic symptoms in patients with BPD, there are trends and clues to help clinicians navigate diagnostic and treatment challenges. This article will review the literature, propose how to distinguish psychotic symptoms in BPD from those in primary psychotic disorders such as schizophrenia, and explore reasonable treatment options.
The scope of the problem
The DSM-5 criteria for BPD states that “during periods of extreme stress, transient paranoid ideation or dissociative symptoms may occur.”1 The term “borderline” originated from the idea that symptoms bordered on the intersection of neurosis and psychosis.2 However, psychotic symptoms in BPD are more varied and frequent than what DSM-5 criteria suggests.
The prevalence of psychotic symptoms in patients with BPD has been estimated between 20% to 50%.3 There also is evidence of frequent auditory and visual hallucinations in patients with BPD, and a recent study using structured psychiatric interviews demonstrated that most BPD patients report at least 1 symptom of psychosis.4 Considering that psychiatric comorbidities are the rule rather than the exception in BPD, the presence of psychotic symptoms further complicates the diagnostic picture. Recognizing the symptoms of BPD is essential for understanding the course of the symptoms and predicting response to treatment.5
Treatment of BPD is strikingly different than that of a primary psychotic disorder. There is some evidence that low-dosage antipsychotics could ease mood instability and perceptual disturbances in patients with BPD.6 Antipsychotic dosages used to treat hallucinations and delusions in a primary psychotic disorder are unlikely to be as effective for a patient with BPD, and are associated with significant adverse effects. Furthermore, these adverse effects—such as weight gain, hyperlipidemia, and diabetes—could become new sources of distress. Clinicians also might miss an opportunity to engage a BPD patient in psychotherapy if the focus is on the anticipated effect of a medication. The mainstay treatment of BPD is an evidence-based psychotherapy, such as dialectical behavioral therapy, transference-focused psychotherapy, mentalization-based therapy, or good psychiatric management.7
CASE Hallucinations during times of stress
Ms. K, a 20-year-old single college student, presents to the psychiatric emergency room with worsening mood swings, anxiety, and hallucinations. Her mood swings are brief and intense, lasting minutes to hours. Anxiety often is triggered by feelings of emptiness and fear of abandonment. She describes herself as a “social chameleon” and notes that she changes how she behaves depending on who she spends time with.
She often hears the voice of her ex-boyfriend instructing her to kill herself and saying that she is a “terrible person.” Their relationship was intense, with many break-ups and reunions. She also reports feeling disconnected from herself at times as though she is being controlled by an outside entity. To relieve her emotional suffering, she cuts herself superficially. Although she has no family history of psychiatric illness, she fears that she may have schizophrenia.
Ms. K’s outpatient psychiatrist prescribes antipsychotics at escalating dosages over a few months (she now takes olanzapine, 40 mg/d, aripiprazole, 30 mg/d, clonazepam, 3 mg/d, and escitalopram, 30 mg/d), but the hallucinations remain. These symptoms worsen during stressful situations, and she notices that they almost are constant as she studies for final exams, prompting her psychiatrist to discuss a clozapine trial. Ms. K is not in psychotherapy, and recognizes that she does not deal with stress well. Despite her symptoms, she is organized in her thought process, has excellent grooming and hygiene, has many social connections, and performs well in school.
How does one approach a patient such as Ms. K?
A chief concern of hallucinations, particularly in a young adult at an age when psychotic disorders such as schizophrenia often emerge, can contribute to a diagnostic quandary. What evidence can guide the clinician? There are some key features to consider:
- Her “mood swings” are notable in their intensity and brevity, making a primary mood disorder with psychotic features less likely.
- Hallucinations are present in the absence of a prodromal period of functional decline or negative symptoms, making a primary psychotic disorder less likely.
- She does not have a family history of psychiatric illness, particularly a primary psychotic disorder.
- She maintains social connections, although her relationships are intense and tumultuous.
- Psychotic symptoms have not changed with higher dosages of antipsychotics.
- Complaints of feeling “disconnected from herself” and “empty” are common symptoms of BPD and necessitate further exploration.
- Psychotic symptoms are largely transient and stress-related, with an overwhelmingly negative tone.
- Techniques that individuals with schizophrenia use, such as distraction or trying to tune out voices, are not being employed. Instead, Ms. K attends to the voices and is anxiously focused on them.
- The relationship of her symptoms to interpersonal stress is key.
When evaluating a patient such as Ms. K, it is important to explore both the nature and timing of the psychotic symptoms and any other related psychiatric symptoms. This helps to determine a less ambiguous diagnosis and clearer treatment plan. Understanding the patient’s perspective about the psychotic symptoms also is useful to gauge the patient’s level of distress and her impression of what the symptoms mean.
Diagnostic considerations
BPD is characterized by a chaotic emotional climate with impulsivity and instability of self-image, affect, and relationships. Most BPD symptoms, including psychosis, often are exacerbated by the perception of abandonment or rejection and other interpersonal stressors.1 Both BPD and schizophrenia are estimated to affect at least 1% of the general population.8,9 Patients with BPD frequently meet criteria for comorbid mental illnesses, including major depressive disorder, substance use disorder, posttraumatic stress disorder, anxiety, and eating disorders.10 Because psychotic symptoms can present in some of these disorders, the context and time course of these symptoms are crucial to consider.
Misdiagnosis is common with BPD, and patients can receive the wrong treatment for years before BPD is considered, likely because of the stigma surrounding the diagnosis.5 One also must keep in mind that, although rare, a patient can have both BPD and a primary psychotic disorder.11 Although a patient with schizophrenia could be prone to social isolation because of delusions or paranoia, BPD patients are more apt to experience intense interpersonal relationships driven by the need to avoid abandonment. Manipulation, anger, and neediness in relationships with both peers and health care providers are common—stark contrasts to typical negative symptoms, blunted affect, and a lack of social drive characteristic of schizophrenia.12
Distinguishing between psychosis in BPD and a psychotic disorder
Studies have sought to explore the quality of psychotic symptoms in BPD vs primary psychotic disorders, which can be challenging to differentiate (Table 1). Some have found that transient symptoms, such as non-delusional paranoia, are more prevalent in BPD, and “true” psychotic symptoms that are long-lasting and bizarre are indicative of schizophrenia.13,14 Also, there is evidence that the lower levels of interpersonal functioning often found in BPD are predictive of psychotic symptoms in that disorder but not in schizophrenia.15

Auditory hallucinations in patients with BPD predominantly are negative and critical in tone.4 However, there is no consistent evidence that the quality of auditory hallucinations in BPD vs schizophrenia is different in any meaningful way.16 Because of the frequency of dissociative symptoms in BPD, it is likely that clinicians could misinterpret these symptoms to indicate disorganized behavior associated with a primary psychotic disorder. In one study, 50% of individuals with BPD experienced auditory hallucinations.11 Differentiating between “internal” or “external” voices did not help to clarify the diagnosis, and paranoid delusions occurred in less than one-third of patients with BPD, but in approximately two-third of those with a diagnosis of schizophrenia.
The McLean Study of Adult Development, a longitudinal study of BPD patients, found that the prevalence of psychotic symptoms diminished over time. It is unclear whether this was due to the spontaneus remission rate of BPD symptoms in general or because of effective treatment.13
Psychotic symptoms in BPD seem to react to stress and increase in intensity when patients are in crisis.17 Nonetheless, because of the prevalence of psychosis in BPD patients and the distress it causes, clinicians should be cautioned against using terms that imply that the symptoms are not “true” or “real.”3
Treatment recommendations
When considering pharmacologic management of psychotic symptoms in BPD, aim to limit antipsychotic medications to low dosages because of adverse effects and the limited evidence that escalating dosages—and especially using >1 antipsychotic concurrently—are more effective.18 Educate patients that in BPD medications are, at best, considered adjunctive treatments. Blaming psychotic symptoms on a purely biological process in BPD, not only is harmful because medications are unlikely to significantly or consistently help, but also because they can undermine patient autonomy and reinforce the need for an outside entity (ie, medication) to fix their problems.
When treatment is ineffective and symptoms do not improve, a patient with BPD likely will experience mounting distress. This, in turn, could exacerbate impulsive, suicidal, and self-injurious behaviors. Emphasize psychotherapy, particularly for those whose psychotic symptoms are transient, stress-related, and present during acute crises (Table 2). With evidence-based psychotherapy, BPD patients can become active participants in treatment, coupling developing insight with concrete skills and teachable principles. This leads to increased interpersonal effectiveness and resilience during times of stress. Challenging the patient’s psychotic symptoms as false or “made up” rarely is helpful and usually harmful, leading to the possible severance of the therapeutic alliance.3

Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) could look similar to those in primary psychotic disorders. Factors suggesting BPD include a pattern of worsening psychotic symptoms during stress, long-term symptom instability, lack of delusions, presence of dissociation, and nonresponse to antipsychotics. Although low-dosage antipsychotics could provide some relief of psychotic symptoms in a patient with BPD, they often are not consistently effective and frequently lead to adverse effects. Emphasize evidence-based psychotherapies.
Psychotic symptoms in patients with borderline personality disorder (BPD) are common, distressing to patients, and challenging to treat. Issues of comorbidities and misdiagnoses in BPD patients further complicate matters and could lead to iatrogenic harm. The dissociation that patients with BPD experience could be confused with psychosis and exacerbate treatment and diagnostic confusion. Furthermore, BPD patients with unstable identity and who are sensitive to rejection could present in a bizarre, disorganized, or agitated manner when under stress.
Although pitfalls occur when managing psychotic symptoms in patients with BPD, there are trends and clues to help clinicians navigate diagnostic and treatment challenges. This article will review the literature, propose how to distinguish psychotic symptoms in BPD from those in primary psychotic disorders such as schizophrenia, and explore reasonable treatment options.
The scope of the problem
The DSM-5 criteria for BPD states that “during periods of extreme stress, transient paranoid ideation or dissociative symptoms may occur.”1 The term “borderline” originated from the idea that symptoms bordered on the intersection of neurosis and psychosis.2 However, psychotic symptoms in BPD are more varied and frequent than what DSM-5 criteria suggests.
The prevalence of psychotic symptoms in patients with BPD has been estimated between 20% to 50%.3 There also is evidence of frequent auditory and visual hallucinations in patients with BPD, and a recent study using structured psychiatric interviews demonstrated that most BPD patients report at least 1 symptom of psychosis.4 Considering that psychiatric comorbidities are the rule rather than the exception in BPD, the presence of psychotic symptoms further complicates the diagnostic picture. Recognizing the symptoms of BPD is essential for understanding the course of the symptoms and predicting response to treatment.5
Treatment of BPD is strikingly different than that of a primary psychotic disorder. There is some evidence that low-dosage antipsychotics could ease mood instability and perceptual disturbances in patients with BPD.6 Antipsychotic dosages used to treat hallucinations and delusions in a primary psychotic disorder are unlikely to be as effective for a patient with BPD, and are associated with significant adverse effects. Furthermore, these adverse effects—such as weight gain, hyperlipidemia, and diabetes—could become new sources of distress. Clinicians also might miss an opportunity to engage a BPD patient in psychotherapy if the focus is on the anticipated effect of a medication. The mainstay treatment of BPD is an evidence-based psychotherapy, such as dialectical behavioral therapy, transference-focused psychotherapy, mentalization-based therapy, or good psychiatric management.7
CASE Hallucinations during times of stress
Ms. K, a 20-year-old single college student, presents to the psychiatric emergency room with worsening mood swings, anxiety, and hallucinations. Her mood swings are brief and intense, lasting minutes to hours. Anxiety often is triggered by feelings of emptiness and fear of abandonment. She describes herself as a “social chameleon” and notes that she changes how she behaves depending on who she spends time with.
She often hears the voice of her ex-boyfriend instructing her to kill herself and saying that she is a “terrible person.” Their relationship was intense, with many break-ups and reunions. She also reports feeling disconnected from herself at times as though she is being controlled by an outside entity. To relieve her emotional suffering, she cuts herself superficially. Although she has no family history of psychiatric illness, she fears that she may have schizophrenia.
Ms. K’s outpatient psychiatrist prescribes antipsychotics at escalating dosages over a few months (she now takes olanzapine, 40 mg/d, aripiprazole, 30 mg/d, clonazepam, 3 mg/d, and escitalopram, 30 mg/d), but the hallucinations remain. These symptoms worsen during stressful situations, and she notices that they almost are constant as she studies for final exams, prompting her psychiatrist to discuss a clozapine trial. Ms. K is not in psychotherapy, and recognizes that she does not deal with stress well. Despite her symptoms, she is organized in her thought process, has excellent grooming and hygiene, has many social connections, and performs well in school.
How does one approach a patient such as Ms. K?
A chief concern of hallucinations, particularly in a young adult at an age when psychotic disorders such as schizophrenia often emerge, can contribute to a diagnostic quandary. What evidence can guide the clinician? There are some key features to consider:
- Her “mood swings” are notable in their intensity and brevity, making a primary mood disorder with psychotic features less likely.
- Hallucinations are present in the absence of a prodromal period of functional decline or negative symptoms, making a primary psychotic disorder less likely.
- She does not have a family history of psychiatric illness, particularly a primary psychotic disorder.
- She maintains social connections, although her relationships are intense and tumultuous.
- Psychotic symptoms have not changed with higher dosages of antipsychotics.
- Complaints of feeling “disconnected from herself” and “empty” are common symptoms of BPD and necessitate further exploration.
- Psychotic symptoms are largely transient and stress-related, with an overwhelmingly negative tone.
- Techniques that individuals with schizophrenia use, such as distraction or trying to tune out voices, are not being employed. Instead, Ms. K attends to the voices and is anxiously focused on them.
- The relationship of her symptoms to interpersonal stress is key.
When evaluating a patient such as Ms. K, it is important to explore both the nature and timing of the psychotic symptoms and any other related psychiatric symptoms. This helps to determine a less ambiguous diagnosis and clearer treatment plan. Understanding the patient’s perspective about the psychotic symptoms also is useful to gauge the patient’s level of distress and her impression of what the symptoms mean.
Diagnostic considerations
BPD is characterized by a chaotic emotional climate with impulsivity and instability of self-image, affect, and relationships. Most BPD symptoms, including psychosis, often are exacerbated by the perception of abandonment or rejection and other interpersonal stressors.1 Both BPD and schizophrenia are estimated to affect at least 1% of the general population.8,9 Patients with BPD frequently meet criteria for comorbid mental illnesses, including major depressive disorder, substance use disorder, posttraumatic stress disorder, anxiety, and eating disorders.10 Because psychotic symptoms can present in some of these disorders, the context and time course of these symptoms are crucial to consider.
Misdiagnosis is common with BPD, and patients can receive the wrong treatment for years before BPD is considered, likely because of the stigma surrounding the diagnosis.5 One also must keep in mind that, although rare, a patient can have both BPD and a primary psychotic disorder.11 Although a patient with schizophrenia could be prone to social isolation because of delusions or paranoia, BPD patients are more apt to experience intense interpersonal relationships driven by the need to avoid abandonment. Manipulation, anger, and neediness in relationships with both peers and health care providers are common—stark contrasts to typical negative symptoms, blunted affect, and a lack of social drive characteristic of schizophrenia.12
Distinguishing between psychosis in BPD and a psychotic disorder
Studies have sought to explore the quality of psychotic symptoms in BPD vs primary psychotic disorders, which can be challenging to differentiate (Table 1). Some have found that transient symptoms, such as non-delusional paranoia, are more prevalent in BPD, and “true” psychotic symptoms that are long-lasting and bizarre are indicative of schizophrenia.13,14 Also, there is evidence that the lower levels of interpersonal functioning often found in BPD are predictive of psychotic symptoms in that disorder but not in schizophrenia.15

Auditory hallucinations in patients with BPD predominantly are negative and critical in tone.4 However, there is no consistent evidence that the quality of auditory hallucinations in BPD vs schizophrenia is different in any meaningful way.16 Because of the frequency of dissociative symptoms in BPD, it is likely that clinicians could misinterpret these symptoms to indicate disorganized behavior associated with a primary psychotic disorder. In one study, 50% of individuals with BPD experienced auditory hallucinations.11 Differentiating between “internal” or “external” voices did not help to clarify the diagnosis, and paranoid delusions occurred in less than one-third of patients with BPD, but in approximately two-third of those with a diagnosis of schizophrenia.
The McLean Study of Adult Development, a longitudinal study of BPD patients, found that the prevalence of psychotic symptoms diminished over time. It is unclear whether this was due to the spontaneus remission rate of BPD symptoms in general or because of effective treatment.13
Psychotic symptoms in BPD seem to react to stress and increase in intensity when patients are in crisis.17 Nonetheless, because of the prevalence of psychosis in BPD patients and the distress it causes, clinicians should be cautioned against using terms that imply that the symptoms are not “true” or “real.”3
Treatment recommendations
When considering pharmacologic management of psychotic symptoms in BPD, aim to limit antipsychotic medications to low dosages because of adverse effects and the limited evidence that escalating dosages—and especially using >1 antipsychotic concurrently—are more effective.18 Educate patients that in BPD medications are, at best, considered adjunctive treatments. Blaming psychotic symptoms on a purely biological process in BPD, not only is harmful because medications are unlikely to significantly or consistently help, but also because they can undermine patient autonomy and reinforce the need for an outside entity (ie, medication) to fix their problems.
When treatment is ineffective and symptoms do not improve, a patient with BPD likely will experience mounting distress. This, in turn, could exacerbate impulsive, suicidal, and self-injurious behaviors. Emphasize psychotherapy, particularly for those whose psychotic symptoms are transient, stress-related, and present during acute crises (Table 2). With evidence-based psychotherapy, BPD patients can become active participants in treatment, coupling developing insight with concrete skills and teachable principles. This leads to increased interpersonal effectiveness and resilience during times of stress. Challenging the patient’s psychotic symptoms as false or “made up” rarely is helpful and usually harmful, leading to the possible severance of the therapeutic alliance.3

Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) could look similar to those in primary psychotic disorders. Factors suggesting BPD include a pattern of worsening psychotic symptoms during stress, long-term symptom instability, lack of delusions, presence of dissociation, and nonresponse to antipsychotics. Although low-dosage antipsychotics could provide some relief of psychotic symptoms in a patient with BPD, they often are not consistently effective and frequently lead to adverse effects. Emphasize evidence-based psychotherapies.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern A. Borderline group of neuroses. The Psychoanalytic Quarterly. 1938;7:467-489.
3. Schroeder K, Fisher HL, Schäfer I, et al. Psychotic symptoms in patients with borderline personality disorder: prevalence and clinical management. Curr Opin Psychiatry. 2013;26(1):113-119.
4. Pearse LJ, Dibben C, Ziauddeen H, et al. A study of psychotic symptoms in borderline personality disorder. J Nerv Ment Dis. 2014;202(5):368-371.
5. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-
6. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29(5):461-467.
7. National Education Alliance for Borderline Personality Disorder. Treatments for BPD. http://www.borderlinepersonalitydisorder.com/what-is-bpd/treating-bpd. Accessed September 1, 2016.
8. Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85-94.
9. Lenzenweger MF, Lane MC, Loranger AW, et al. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553-564.
10. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155(12):1733-1739.
11. Kingdon DG, Ashcroft K, Bhandari B, et al. Schizophrenia and borderline personality disorder: similarities and differences in the experience of auditory hallucinations, paranoia, and childhood trauma. J Nerv Ment Dis. 2010;198(6):399-403.
12. Gunderson JG. Borderline personality disorder. Washington, DC: American Psychiatric Press; 1984.
13. Zanarini MC, Frankenburg FR, Wedig MM, et al. Cognitive experiences reported by patients with borderline personality disorder and Axis II comparison subjects: a 16-year prospective follow-up study. Am J Psychiatry. 2013;170(6):671-679.
14. Tschoeke S, Steinert T, Flammer E, et al. Similarities and differences in borderline personality disorder and schizophrenia with voice hearing. J Nerv Ment Dis. 2014;202(7):544-549.
15. Oliva F, Dalmotto M, Pirfo E, et al. A comparison of thought and perception disorders in borderline personality disorder and schizophrenia: psychotic experiences as a reaction to impaired social functioning. BMC Psychiatry. 2014;14:239.
16. Merrett Z, Rossell SL, Castle DJ, et al. Comparing the experience of voices in borderline personality disorder with the experience of voices in a psychotic disorder: a systematic review. Aust N Z J Psychiatry. 2016;50(7):640-648.
17. Glaser JP, Van Os J, Thewissen V, et al. Psychotic reactivity in borderline personality disorder. Acta Psychiatr Scand. 2010;121(2):125-134.
18. Rosenbluth M, Sinyor M. Off-label use of atypical antipsychotics in personality disorders. Expert Opin Pharmacother. 2012;13(11):1575-1585.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern A. Borderline group of neuroses. The Psychoanalytic Quarterly. 1938;7:467-489.
3. Schroeder K, Fisher HL, Schäfer I, et al. Psychotic symptoms in patients with borderline personality disorder: prevalence and clinical management. Curr Opin Psychiatry. 2013;26(1):113-119.
4. Pearse LJ, Dibben C, Ziauddeen H, et al. A study of psychotic symptoms in borderline personality disorder. J Nerv Ment Dis. 2014;202(5):368-371.
5. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-
6. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29(5):461-467.
7. National Education Alliance for Borderline Personality Disorder. Treatments for BPD. http://www.borderlinepersonalitydisorder.com/what-is-bpd/treating-bpd. Accessed September 1, 2016.
8. Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85-94.
9. Lenzenweger MF, Lane MC, Loranger AW, et al. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553-564.
10. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155(12):1733-1739.
11. Kingdon DG, Ashcroft K, Bhandari B, et al. Schizophrenia and borderline personality disorder: similarities and differences in the experience of auditory hallucinations, paranoia, and childhood trauma. J Nerv Ment Dis. 2010;198(6):399-403.
12. Gunderson JG. Borderline personality disorder. Washington, DC: American Psychiatric Press; 1984.
13. Zanarini MC, Frankenburg FR, Wedig MM, et al. Cognitive experiences reported by patients with borderline personality disorder and Axis II comparison subjects: a 16-year prospective follow-up study. Am J Psychiatry. 2013;170(6):671-679.
14. Tschoeke S, Steinert T, Flammer E, et al. Similarities and differences in borderline personality disorder and schizophrenia with voice hearing. J Nerv Ment Dis. 2014;202(7):544-549.
15. Oliva F, Dalmotto M, Pirfo E, et al. A comparison of thought and perception disorders in borderline personality disorder and schizophrenia: psychotic experiences as a reaction to impaired social functioning. BMC Psychiatry. 2014;14:239.
16. Merrett Z, Rossell SL, Castle DJ, et al. Comparing the experience of voices in borderline personality disorder with the experience of voices in a psychotic disorder: a systematic review. Aust N Z J Psychiatry. 2016;50(7):640-648.
17. Glaser JP, Van Os J, Thewissen V, et al. Psychotic reactivity in borderline personality disorder. Acta Psychiatr Scand. 2010;121(2):125-134.
18. Rosenbluth M, Sinyor M. Off-label use of atypical antipsychotics in personality disorders. Expert Opin Pharmacother. 2012;13(11):1575-1585.
Evaluating and monitoring drug and alcohol use during child custody disputes
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.
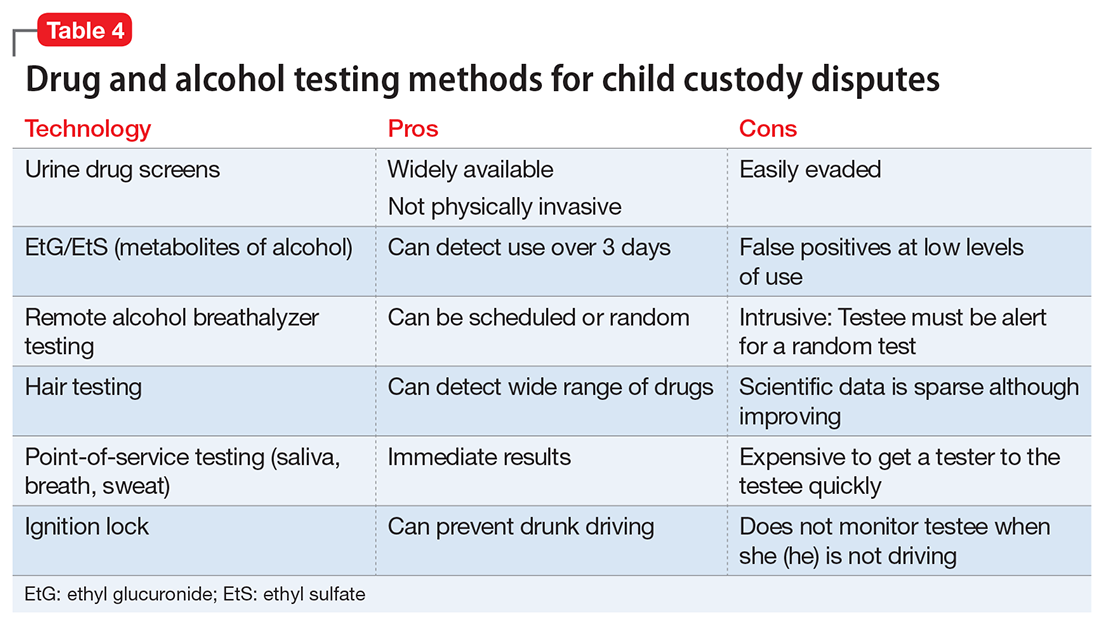
Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.

Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.

Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
USPSTF: No recommendation on screening for celiac disease
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
Even though the current evidence on the effectiveness of screening for celiac disease is scarce or absent, it remains reasonable for clinicians to have a low threshold for testing patients, especially in high-risk populations such as those with an affected family member or a related autoimmune disorder.
This is because most celiac disease is unrecognized, and patients can present with diverse symptoms rather than the classic triad of abdominal pain, diarrhea, and weight loss.
Rok Seon Choung, MD, and Joseph A. Murray, MD , are in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Murray reported ties to Alvine Pharmaceuticals, Alba Therapeutics, Celimmune, BioLineRx, and numerous others. Dr. Choung and Dr. Murray made these remarks in an editorial accompanying the USPSTF reports (JAMA. 2017 Mar 28;317:1221-3).
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
The current evidence is insufficient for the U.S. Preventive Services Task Force to recommend either for or against routine screening of asymptomatic people for celiac disease, according to a Recommendation Statement published online March 28 in JAMA.
The USPSTF is tasked with making recommendations regarding the effectiveness of specific preventive health care services for patients who have no related signs or symptoms. In this case, the group commissioned a systematic review of the literature on celiac disease from 1946 through June 2016, which became the basis for the evidence report informing their Recommendation Statement, said Kirsten Bibbins-Domingo, PhD, MD, chair of the USPSTF and lead author of the statement, and her associates.
However, only 4 studies out of the 3,036 that were examined addressed the question of screening adequately, and they offered few data of use. According to Roger Chou, MD, lead author of the Evidence Report, and his associates, no trials assessed the screening of asymptomatic children, adolescents, or adults for celiac disease with regard to morbidity, mortality, or quality of life. The evidence also was inadequate concerning targeted screening of at-risk individuals, and there were no studies of the effectiveness of treatment after screen-detected celiac disease was found.
There was some evidence supporting the diagnostic accuracy of the tissue transglutaminase-IgA test to detect celiac disease, but “little or no evidence ... to inform most of the key questions related to benefits and harms of screening for celiac disease in asymptomatic individuals,” said Dr. Chou, of the Pacific Northwest Evidence-Based Practice Center and Oregon Health & Science University, both in Portland, and his associates (JAMA. 2017 Mar 28. doi: 10.1001/jama.2016.10395).
Dr. Bibbins-Domingo noted that the American Academy of Family Physicians also has concluded that the evidence is insufficient to assess the balance of screening’s benefits and harms. In contrast, the American College of Gastroenterology recommends that screening be considered in asymptomatic people who have a first-degree relative with a confirmed diagnosis of celiac disease (JAMA. 2017 Mar 28. doi: 10.1001/jama.2017.1462).
In addition, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition recommends screening at age 3 years for asymptomatic children who have conditions associated with celiac disease, including type 1 diabetes, autoimmune thyroiditis, Down syndrome, Turner syndrome, William’s syndrome, or selective IgA deficiency, said Dr. Bobbins-Domingo, who is also professor of medicine at the University of California, San Francisco.
Copies of the Recommendation Statement, the Evidence Report, the authors’ disclosures, and other materials are available at www.uspreventiveservicestaskforce.org.
FROM JAMA
Key clinical point: The current evidence is insufficient for the USPSTF to recommend either for or against routine screening of asymptomatic people for celiac disease.
Major finding: Only 4 studies out of the 3,036 that were examined addressed the question of screening adequately.
Data source: An assessment of the benefits and harms of screening based on a review of four studies.
Disclosures: The USPSTF’s work is supported by the U.S. Agency for Healthcare Research and Quality. The authors’ financial disclosures are available at www.uspreventiveservicestaskforce.org.
Is your patient’s valproic acid dosage too low or high? Adjust it with this equation
Valproic acid (VPA) often is used to treat mania in bipolar disorder, and it has a therapeutic range of 50 to 125 µg/mL of total serum concentration.1 VPA binds highly to albumin, resulting in free drug concentrations (5 to 15 mg/L) that are responsible for its therapeutic effect.2 Monitoring total VPA levels in patients with hypoalbuminemia could reveal seemingly subtherapeutic VPA levels, which could lead to unnecessary dosage adjustments or drug toxicity. Hermida et al3 devised a correction equation to normalize total VPA serum concentrations <75 µg/mL in patients with hypoalbuminemia (Table 1, Box).
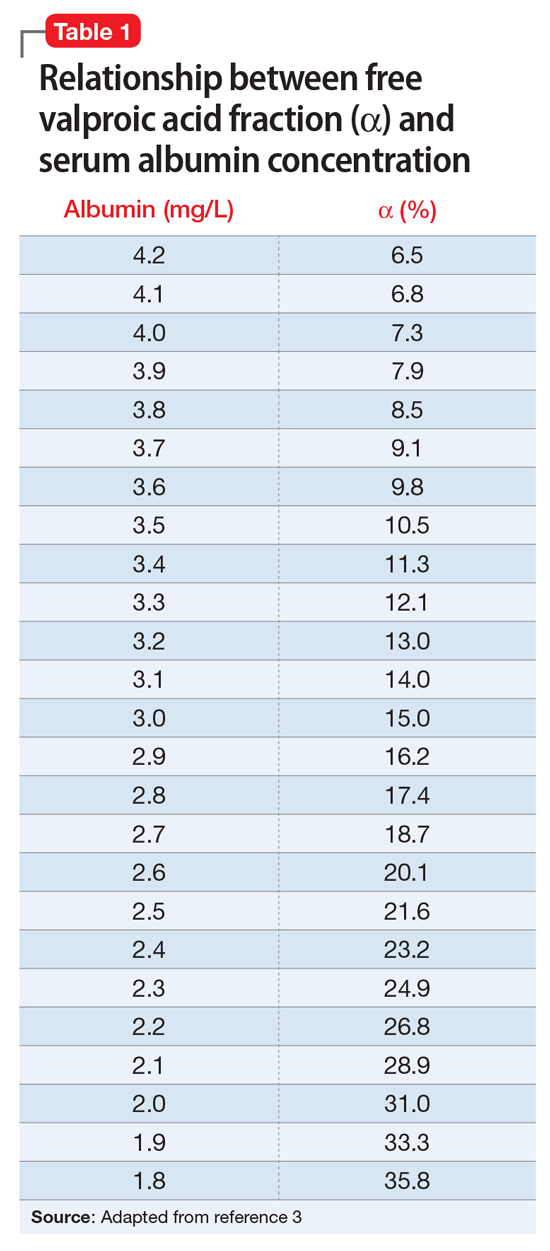
We present a case employing this equation in a patient
Case
Ms. T, age 75, is admitted to the hospital with delusional, paranoid, assaultive, and combative behavior. By applying Ms. T’s baseline lab findings (Table 2) to the equation, a normalized total VPA level and estimated free VPA level of 70 µg/mL and 7 µg/mL, respectively, can be approximated. These estimates fall within the therapeutic range and are validated by the measured free VPA level of 9 µg/mL.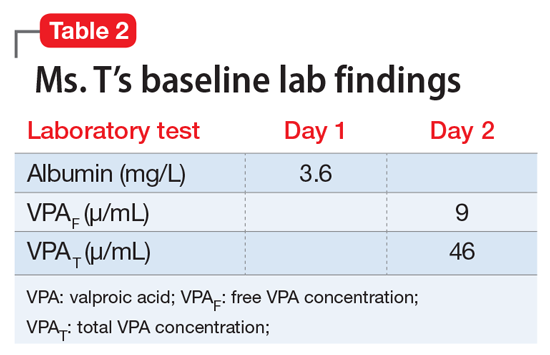
VPA serum levels should be assessed 2 to 4 days after initiation or dosage adjustments.1 Also, consider patient-specific goals and intended clinical effect when adjusting VPA dosage. In practice settings, where free VPA levels are not routinely monitored or are cost prohibitive, this equation can guide clinical decision-making.3
1. Depakote [divalproex sodium]. North Chicago, IL: AbbVie Inc; 2016.
2. DeVane CL. Pharmacokinetics, drug interactions, and tolerability of valproate. Psychopharmacol Bull. 2003;37(suppl 2):25-42.
3. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97(4):489-493.
Valproic acid (VPA) often is used to treat mania in bipolar disorder, and it has a therapeutic range of 50 to 125 µg/mL of total serum concentration.1 VPA binds highly to albumin, resulting in free drug concentrations (5 to 15 mg/L) that are responsible for its therapeutic effect.2 Monitoring total VPA levels in patients with hypoalbuminemia could reveal seemingly subtherapeutic VPA levels, which could lead to unnecessary dosage adjustments or drug toxicity. Hermida et al3 devised a correction equation to normalize total VPA serum concentrations <75 µg/mL in patients with hypoalbuminemia (Table 1, Box).

We present a case employing this equation in a patient
Case
Ms. T, age 75, is admitted to the hospital with delusional, paranoid, assaultive, and combative behavior. By applying Ms. T’s baseline lab findings (Table 2) to the equation, a normalized total VPA level and estimated free VPA level of 70 µg/mL and 7 µg/mL, respectively, can be approximated. These estimates fall within the therapeutic range and are validated by the measured free VPA level of 9 µg/mL.
VPA serum levels should be assessed 2 to 4 days after initiation or dosage adjustments.1 Also, consider patient-specific goals and intended clinical effect when adjusting VPA dosage. In practice settings, where free VPA levels are not routinely monitored or are cost prohibitive, this equation can guide clinical decision-making.3
Valproic acid (VPA) often is used to treat mania in bipolar disorder, and it has a therapeutic range of 50 to 125 µg/mL of total serum concentration.1 VPA binds highly to albumin, resulting in free drug concentrations (5 to 15 mg/L) that are responsible for its therapeutic effect.2 Monitoring total VPA levels in patients with hypoalbuminemia could reveal seemingly subtherapeutic VPA levels, which could lead to unnecessary dosage adjustments or drug toxicity. Hermida et al3 devised a correction equation to normalize total VPA serum concentrations <75 µg/mL in patients with hypoalbuminemia (Table 1, Box).

We present a case employing this equation in a patient
Case
Ms. T, age 75, is admitted to the hospital with delusional, paranoid, assaultive, and combative behavior. By applying Ms. T’s baseline lab findings (Table 2) to the equation, a normalized total VPA level and estimated free VPA level of 70 µg/mL and 7 µg/mL, respectively, can be approximated. These estimates fall within the therapeutic range and are validated by the measured free VPA level of 9 µg/mL.
VPA serum levels should be assessed 2 to 4 days after initiation or dosage adjustments.1 Also, consider patient-specific goals and intended clinical effect when adjusting VPA dosage. In practice settings, where free VPA levels are not routinely monitored or are cost prohibitive, this equation can guide clinical decision-making.3
1. Depakote [divalproex sodium]. North Chicago, IL: AbbVie Inc; 2016.
2. DeVane CL. Pharmacokinetics, drug interactions, and tolerability of valproate. Psychopharmacol Bull. 2003;37(suppl 2):25-42.
3. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97(4):489-493.
1. Depakote [divalproex sodium]. North Chicago, IL: AbbVie Inc; 2016.
2. DeVane CL. Pharmacokinetics, drug interactions, and tolerability of valproate. Psychopharmacol Bull. 2003;37(suppl 2):25-42.
3. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97(4):489-493.
Residual symptoms of schizophrenia: What are realistic treatment goals?
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8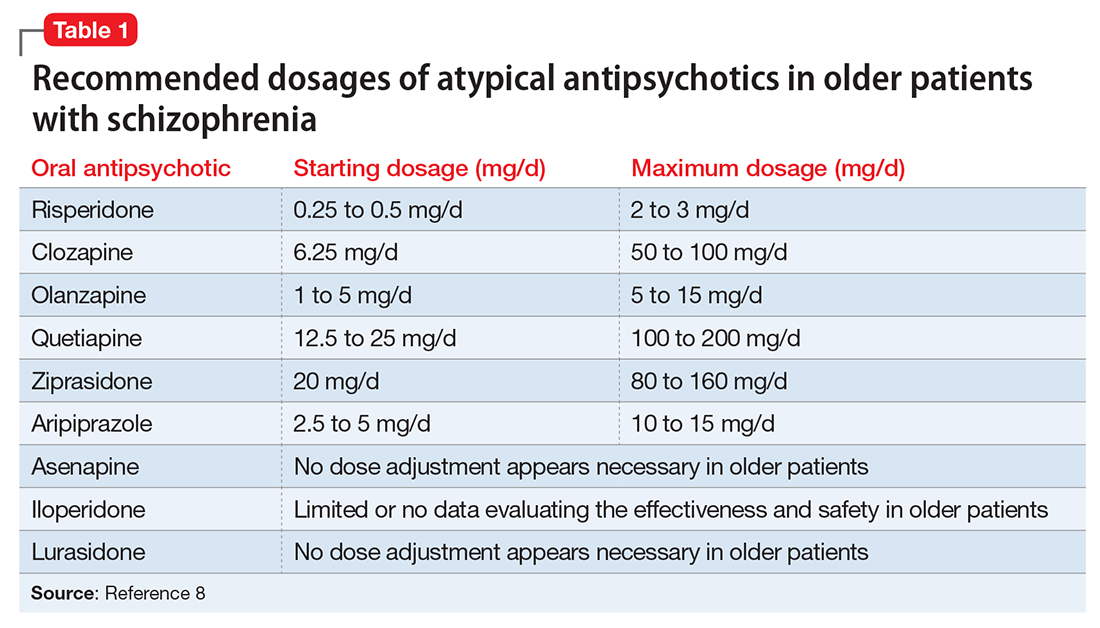
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30
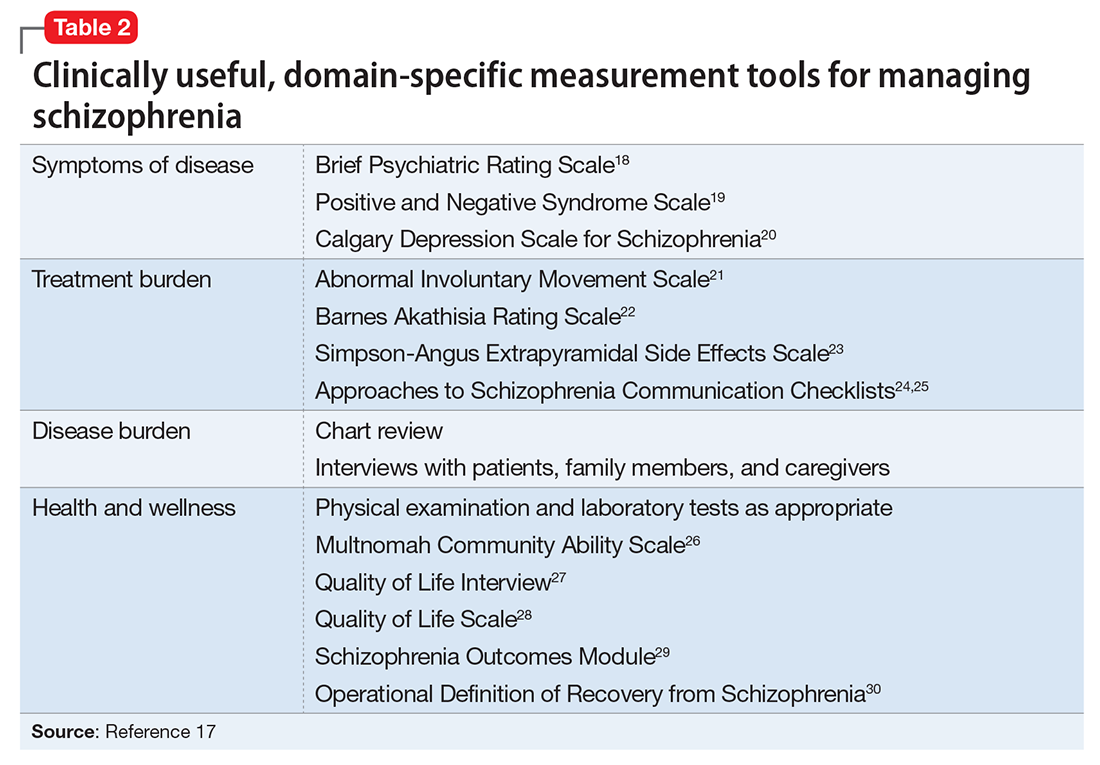
The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30

The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30

The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
Adult ADHD: Pharmacologic treatment in the DSM-5 era
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
Bedbugs: Helping your patient through an infestation
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
Bedbugs have been unwelcome bedfellows for humans for thousands of years. An increase in pyrethroid resistance, a ban on the insecticide dichloro-diphenyl-trichloroethane (DDT), increased international travel, and increased population density in large cities have led to an exponential rise in the incidence of bedbug infestations. Physicians are often at the forefront of bedbug infestation diagnosis.
Once the diagnosis is suggested, symptomatic treatment of the patient and extermination of the pests are essential, though time-consuming, costly, and often problematic. Measures to eliminate infestation and to prevent spread include identification of the pest, early detection, patient education, and professional eradication.
BEDBUGS: A BRIEF HISTORY
The term bedbug refers to the obligate parasitic arthropod Cimex lectularius (the common bedbug) and, less commonly, its tropical cousin C hemipterus. Bedbugs have coexisted with humans for centuries, dating back to the ancient Egyptians 3,500 years ago.1 Through the mid-20th century, about 30% of US households were infested with bedbugs.2 The introduction of pesticides during World War II markedly decreased the incidence, but with increased international travel, pesticide resistance, and the banning of certain pesticides in the last decade, bedbugs have reemerged worldwide.3
BIOLOGY
Bedbugs are red-brown, wingless, oval-shaped insects measuring 4 to 5 mm in length (Figure 1). They are hematophagous ectoparasites that preferentially feed on human blood, although they feed on some animals as well.2
Cimex lectularius dwells in temperate climates and C hemipterus in more tropical climates, but overlap and interbreeding are common. The usual life cycle is about 6 months, but some bugs live 12 months or longer. The female bedbug lays 5 to 8 eggs per week, or approximately 500 eggs in her lifetime, and each egg hatches in 5 to 10 days.4
These photophobic parasites do not live on their human hosts but rather simply visit for a meal. They cohabitate in dark locations, attacking human hosts when they are inactive or sleeping for long periods of time. Common living areas include mattress seams, box springs, bed linens and clothes, wallpaper seams, electrical outlets, and furniture seams (Table 1).5 The female bedbug lays her eggs in these secluded crevices, ensuring their safety until hatching. The dense nests of adult bedbugs, their eggs, and accumulated fecal matter allow for easy visual identification of infestation.5
Bedbugs typically feed between 1:00 am and 5:00 am. Though wingless, they successfully navigate towards their human host, attracted by emitted heat and carbon dioxide.2 Once attached to human skin, the bedbug bite releases enzymes and chemicals including nitrophorin and nitric oxide that facilitate bleeding; these substances are responsible for the resultant dermatitis. (Of note, bedbugs with experimentally excised salivary glands do not cause skin disease in humans.6) After feeding for 3 to 20 minutes, the length and weight of the arthropod can increase by 50% to 200%. A fully sated bedbug can survive for a year until its next meal.2,7 Even if an establishment, home, room, or article of clothing infested with bedbugs has been abandoned for several months, without proper eradication the item still represents a possible nidus for recurrent disease if used, inhabited, or worn again.
EPIDEMIOLOGY
From the earliest documented cases of Cimex in ancient Egyptian tombs to the mid-1900s, the cohabitation of humans and bedbugs was seen as inevitable. With the introduction of DDT 60 years ago, the bedbug population significantly decreased.8 Since DDT’s prohibition, coupled with increased travel and heightened resistance to over-the-counter insecticides, the bedbug population has reemerged exponentially.9,10
Infestations have been reported worldwide, on every continent, and in all 50 of the United States. In Australia, infestations have risen 4,500% in the last 10 to 15 years.11 In the United States, infestation occurs exclusively with C lectularius and the incidence is rising. Philadelphia and New York City are among the most bedbug-infested US cities. New York City experienced a 2,000% increase in bedbug complaints between 2004 and 2009.8
Bedbugs can be transmitted either through active migration of colonies from one area to another adjacent living area through wall spaces or ventilation, or through passive transportation in luggage, clothing, furniture, used mattresses, bookbags, and other personal items.1 Although infestation affects people of all socioeconomic classes and backgrounds, the likelihood increases in people who frequently travel and people who live in lower income neighborhoods with tightly packed apartments. Bedbug infestations are also common in refugee camps: 98% of the rooms in a refugee camp in Sierra Leone had bedbugs, and almost 90% of the residents had signs of bites.12 Unlike scabies, direct person-to-person, skin-to-skin transfer is rare.
CLINICAL FINDINGS
Bedbug bites are analogous, almost identical, to other arthropod bites: bites begin as pink macules that progress to papules (Figure 2), large plaques, or wheals (hives).13 Bites can arise minutes or even days after the initial assault. Some papules and plaques may have a central crust or erosion suggesting a bite.
Bites are typically intensely pruritic, and occasionally, hypersensitive victims can develop bullae, necrotic plaques, or even vasculitis. New papules and plaques form as older ones heal. Some patients may have fever and malaise.13 About 30% of patients may not have skin disease from bedbugs, making diagnosis in those individuals impossible.
The nonspecific nature of this presentation and the subsequent difficulty in prompt diagnosis can lead to a prolonged period of morbidity for the patient, as well as increasing the window of opportunity for the bedbugs to affect other surrounding individuals.
THE DIFFERENTIAL DIAGNOSIS IS BROAD
Commonly, bedbug bites have been misdiagnosed as drug eruptions, food allergies, dermatitis herpetiformis, staphylococcal or varicella infection, and scabies, as well as other arthropod bites.11 This broad differential diagnosis can often be narrowed by careful observation of the bite distribution. The clustering of bites in groups of 3, often in a linear pattern, sometimes overlying blood vessels, is known as the “breakfast, lunch, and dinner” sign (Figure 3), and this can help to guide the clinician toward the diagnosis of a bite as opposed to a diffuse urticarial response.2
If the characteristic clusters of bites are not present, distinguishing clinically between the various causes of pruritic urticarial lesions is difficult. Subtle clues that point towards bedbug bites can be that the rash appears to be most edematous in the morning and flattens throughout the day, as the bites occur typically during sleep.14 Likewise, the rash associated with bedbug bites has also been reported to last longer, to blanch less, and to be less responsive to steroid and antihistamine treatment than other urticarial rashes.14 If a skin biopsy specimen is available, histologic assessment can help to rule out similarly presenting conditions such as prodromal bullous pemphigoid, dermatitis herpetiformis, and urticarial dermatosis, even if it cannot provide a definitive answer as to the etiology.15
Bedbug bites vs other arthropod bites
Once a bite is suspected, differentiating between bedbug and other arthropod bites is the next challenge.
Once again, a detailed assessment of the location of the bites can yield valuable information. The waist, axillae, and uncovered parts of the body are the usual sites for bedbug bites.2 Likewise, inflammatory papules along the eyelid (the “eyelid sign”) are highly suggestive of a bedbug bite.16
The scant involvement of covered body areas, the lack of shallow burrows in the skin, and the lack of scabetic elements on skin scrapings exclude scabies as a diagnosis.
Skin biopsy is not helpful in differentiating arthropod bites, as the histologic findings are nonspecific. The key to a definitive diagnosis in these cases is identification of the suspected bug in characteristic locations. Patients should be encouraged to carefully inspect mattresses, floorboards, and other crevices for the small ovaloid bugs or the reddish-brown specks of heme and feces they typically leave behind on bed linens.15 A positive reported sighting of the bugs can lend credence to the diagnosis, whereas capture and laboratory assessment of a specimen is ideal.
BEDBUGS AS DISEASE VECTORS
Extracutaneous manifestations of bedbug assault are rare. Anaphylaxis to proteins in Cimex saliva may occur, as well as significant blood loss, even anemia, from extensive feeding.17 Bedbug infestations can exacerbate asthma, preexisting mental illness, anxiety, and insomnia.18 Since bedbugs extract blood from hosts, they have a putative ability to act as vectors of disease. Some 45 known pathogens have been isolated from the Cimex species including hepatitis B, human immunodeficiency virus (HIV), Trypanosoma cruzi, and methicillin-resistant Staphylococcus aureus. To date, however, there is no evidence to demonstrate transmission of pathogens to humans.5
TREATMENT AND ERADICATION
Treatment is mainly symptomatic—systemic antihistamines and topical corticosteroids to reduce pruritus and alleviate the dermatitis.2 Patients should be instructed to avoid scratching to prevent infection. Secondary bacterial infection can be treated with topical or systemic antibiotics. Rare cases of bite-induced asthma or anaphylaxis necessitate appropriate emergency treatment. Extermination of infestation is critical to therapy.
If bedbug infestation is suggested, mattresses, bedding, sleeping areas, and bed clothing should be inspected for insects, eggs, and fecal spotting. Adhesives or traps that emit heat or carbon dioxide can be used to capture the bedbugs. During widespread infestation, the arthropods release a pungent odor, which allows trained dogs to detect them with 95% to 98% accuracy.19
Eradication techniques
Once infestation is confirmed, patients should contact an exterminator who can confirm the presence of bedbugs. Typical eradication measures often require nonchemical control and chemical pesticides.
Professional exterminators have special equipment that can heat a room to 48 to 50°C (118–122°F). Heat sustained at this temperature for 90 minutes is sufficient to kill bedbugs.20
The infested area should be vacuumed daily, and vacuum bags and unwanted items should be sealed in plastic before discarding. Clothing, linens, and infested fabrics should be washed and dried in heat at 60°C (140°F) or greater.
Mattresses and furniture should be sealed in a special plastic that allows treatment with heat, steaming, or pesticides. Most professional pesticides contain pyrethroids, but resistance to these products is common, necessitating the use of multiple formulations to overcome resistance.8
Over-the-counter pesticides, almost exclusively pyrethroids, are variably effective and potentially hazardous to consumers.8 Patients must be advised to follow label directions to avoid adverse effects and toxicity.
Alternative chemical eradication methods to circumvent the problem of resistance include piperonyl butoxide, S-methoprene, boric acid, silicates (diatomaceous earth dust), and sulfuryl fluoride. Recent research has also posited the use of antiparasitic agents such as ivermectin and moxidectin in cases of resistant bedbug infestation, with promising results.21
All extermination products and techniques have variable risks, efficacies, and costs,8 and repeat inspections and retreatment are often required.
Prevention strategies include visual inspection of possibly infested rooms, with particular attention to mattress seams and crevices, placing luggage on a luggage rack away from the floor and bed, and careful examination of acquired second-hand items.7
Educating patients is the key to success
While all of the above eradication techniques are important curative strategies, the success of any treatment is contingent on appropriate patient education about the nature of the problem.
Resolving a bedbug infestation is notoriously difficult and requires meticulous adherence to hygiene and cleansing instructions throughout the household or institution for a sustained period of time. Information from sources such as the US Environmental Protection Agency (www.epa.gov) can empower patients to perform the necessary eradication protocols, and clinicians should routinely recommended them as part of a holistic treatment strategy.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
- Krause-Parello CA, Sciscione P. Bedbugs: an equal opportunist and cosmopolitan creature. J Sch Nurs 2009; 25:126–132.
- Sfeir M, Munoz-Price LS. Scabies and bedbugs in hospital outbreaks. Curr Infect Dis Rep 2014; 16:412.
- Romero A, Potter MF, Potter DA, Haynes KF. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 2007; 44:175–178.
- Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis 2011; 52:200–210.
- Doggett SL, Dwyer DE, Penas PF, Russell RC. Bed bugs: clinical relevance and control options. Clin Microbiol Rev 2012; 25:164–192.
- Goddard J, Edwards KT. Effects of bed bug saliva on human skin. JAMA Dermatol 2013; 149:372–373.
- Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA 2009; 301:1358–1366.
- Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 2012; 26:241–254.
- Saenz VL, Booth W, Schal C, Vargo EL. Genetic analysis of bed bug populations reveals small propagule size within individual infestations but high genetic diversity across infestations from the eastern United States. J Med Entomol 2012; 49:865–875.
- Jones SC, Bryant JL. Ineffectiveness of over-the-counter total-release foggers against the bed bug (Heteroptera: cimicidae). J Econ Entomol 2012; 105:957–963.
- Doggett SL, Russell R. Bed bugs—what the GP needs to know. Aust Fam Physician 2009; 38:880–884.
- Gbakima AA, Terry BC, Kanja F, Kortequee S, Dukuley I, Sahr F. High prevalence of bedbugs Cimex hemipterus and Cimex lectularis in camps for internally displaced persons in Freetown, Sierra Leone: a pilot humanitarian investigation. West Afr J Med 2002; 21:268–271.
- deShazo RD, Feldlaufer MF, Mihm MC Jr, Goddard J. Bullous reactions to bedbug bites reflect cutaneous vasculitis. Am J Med 2012; 125:688–694.
- Scarupa MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol 2006; 117:1508–1509.
- Thomas I, Kihiczak GG, Schwartz RA. Bedbug bites: a review. Int J Dermatol 2004; 43:430–433.
- Quach KA, Zaenglein AL. The eyelid sign: a clue to bed bug bites. Pediatr Dermatol 2014; 31:353–355.
- Paulke-Korinek M, Szell M, Laferl H, Auer H, Wenisch C. Bed bugs can cause severe anaemia in adults. Parasitol Res 2012; 110:2577–2579.
- Goddard J, de Shazo R. Psychological effects of bed bug attacks (Cimex lectularius L). Am J Med 2012; 125:101–103.
- Pfiester M, Koehler PG, Pereira RM. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J Econ Entomol 2008; 101:1389–1396.
- Kells SA, Goblirsch MJ. Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2011; 2:412–422.
- Sheele JM, Ridge GE. Toxicity and potential utility of ivermectin and moxidectin as xenointoxicants against the common bed bug Cimex lectularius L. Parasitol Res 2016; 115:3071–3081.
KEY POINTS
- The increase in pyrethroid resistance, the ban of DDT, the ease and frequency of travel, and the increased population density in large cities have led to an exponential rise in the incidence of bedbug infection.
- Once the diagnosis is suggested, patients deserve symptomatic treatment, and extermination of the pests becomes essential, though time-consuming, costly, and often problematic.
- Measures to eliminate infestation and prevent spread include early detection, identification of the pest, patient education, and professional eradication.