User login
Early-Stage Hodgkin Lymphoma
INTRODUCTION
Hodgkin lymphoma, previously known as Hodgkin’s disease, is a B-cell malignancy with unique pathological and epidemiological features for which highly effective therapies exist. The disease is characterized by the presence of mononuclear and multinucleate giant cells called Hodgkin and Reed-Sternberg (HRS) cells.1
Hodgkin lymphoma is unique compared to other B-cell lymphomas because of the scarcity of the malignant cells in the tumor tissue. The HRS cells usually account for only 0.1% to 10% of the cells in the affected tissues, and the HRS cells induce accumulation of nonmalignant lymphocytes, macrophages, granulocytes, eosinophils, plasma cells, and histiocytes, which constitute more than 90% of tumor cellularity.2 Although the disease was first described by Sir Thomas Hodgkin in 1832, in part because of this unique histopathology, not until 1991 was it conclusively demonstrated that HRS cells are in fact monoclonal germinal center–derived B-cells. This article reviews management and frontline treatment options for limited-stage classical Hodgkin lymphoma and nodular lymphocyte predominant Hodgkin lymphoma. Treatment of advanced stage and relapsed/refractory Hodgkin lymphoma will be discussed in a separate article.
EPIDEMIOLOGY
Hodgkin lymphoma accounts for 0.5% of all malignancies and 11.7% of all lymphomas among adults in the United States.3 The incidence of Hodgkin lymphoma has been steadily increasing over the past 4 decades and was estimated to be 8260 cases in the United States in 2017, with a slight male predominance. Hodgkin lymphoma is expected to cause 1070 deaths in 2017, accounting for 0.2% of all cancer deaths.3 First-degree relatives of patients with Hodgkin lymphoma have a 3- to 9-fold increased risk of having the disease compared to the general population,4 and monozygotic twin siblings of Hodgkin lymphoma patients have a greatly increased risk for developing the disease—up to 100-fold—compared to normal cohorts.5 The incidence is highest among Caucasians, African Americans, and Hispanics, and lower in Asians and American Indians.3 Hodgkin lymphoma incidence shows a bimodal peak distribution, with 1 peak between the ages of 15 and 44 years, and another peak after age 65 years.6
ETIOLOGY/PATHOGENESIS
The cause of Hodgkin lymphoma is unknown. Epstein-Barr virus (EBV) infection is present in up to 40% of Hodgkin lymphoma cases, suggesting a role of this virus in the pathogenesis of some cases. The risk of EBV-positive Hodgkin lymphoma was found to be higher following an episode of infectious mononucleosis, while the risk of EBV-negative Hodgkin lymphoma remained unchanged.7 The incidence of Hodgkin lymphoma is 5 to 14 times higher in HIV-infected patients than in noninfected patients.8 It is not considered an AIDS-defining illness, but has become more frequent with the growth and aging of the HIV-positive population.9,10 Hodgkin lymphoma patients with HIV typically have CD4 lymphocyte counts greater than 200 cells/μL,11 with the incidence of Hodgkin lymphoma actually declining with lower CD4 lymphocyte counts.12 HIV-related Hodgkin lymphoma tends to have an aggressive course, with high rates of EBV positivity.13 The incidence of Hodgkin lymphoma is 1.8 times higher among smokers, and the risk appears to increase with duration of smoking.14,15
The cell of origin of Hodgkin lymphoma, while long suspected to be the HRS cell, remained unproven until the 1990s when micro-dissection and single-cell polymerase chain reaction techniques allowed for confirmation that the HRS cell was in fact a monoclonal germinal center derived B cell.16,17 These HRS cells lack immunoglobulin due to defective transcription regulation and not due to crippling mutations.18,19 The cellular infiltrate in Hodgkin lymphoma appears to play a decisive role in allowing the HRS cells to survive by providing an environment that suppresses cytotoxic immune responses as well as by providing cellular interactions and cytokines that support their growth and survival. The extensive inflammatory infiltrate in classical Hodgkin lymphoma is comprised of T helper 2 (Th2) and regulatory T cells and lacks T helper 1 (Th1) cells, CD8 cytotoxic T cells, and natural killer cells.20 The HRS cells escape apoptosis by several mechanisms which include latent EBV infection and constitutive nuclear factor (NF)-kB pathways, as well as other deregulated signaling pathways that promote survival, such as EBV nuclear antigen 1 (EBNA1) protein, EBV latent infection membrane protein 1 (LMP1), and LMP2.21,22
Genetic alterations in the 9p24 locus which encodes PD-L1/PD-L2 are nearly universally present in classical Hodgkin lymphoma and are now considered a disease-defining feature.23
PATHOLOGIC CLASSIFICATION
According to the 2008 World Health Organization (WHO) classification, Hodgkin lymphoma has 2 clearly defined entities: classical Hodgkin lymphoma (cHL), which accounts for approximately 95% cases, and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), which accounts for the remaining cases.24 These 2 entities differ in their clinical, pathological, and biological features, which in turn affect prognosis and treatment options. Classical Hodgkin lymphoma is characterized by a paucity of HRS cells surrounded by a background of mixed inflammatory infiltrate comprised of histiocytes, small lymphocytes, eosinophils, neutrophils, plasma cells, fibroblasts, and collagen. Depending on the particular combinations of these elements and the specific features of the neoplastic cells, cases can be subclassified into several cHL subtypes: the nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted types.25
The diagnosis of cHL is made based on a combination of morphology of HRS cells and the other cells infiltrating the tissue, combined with immunohistochemical staining. Because of the rare nature of the malignant (clonal) cell in Hodgkin lymphoma specimens, flow cytometry is generally of little value. The HRS cells in cHL are CD30-positive and CD45 negative in virtually all cases, and CD15-positive in 85% of cases.26 B-cell antigens are typically negative except for CD20, which is positive in about 20% cases.27
Nodular sclerosis Hodgkin lymphoma (NSHL) is the most common subtype of cHL, accounting for 65% to 75% of cases. It is common among young adults and tends to involve the mediastinal, supraclavicular, and cervical lymph nodes. NSHL is characterized by the presence of collagen bands that divide the lymphoid tissue into circumscribed nodules. This subtype usually presents as stage I or II disease, typically with neck and/or mediastinal disease, and evidence of EBV infection is present in approximately 10% to 40% of North American cases.7 Patients diagnosed with NSHL generally have a very good prognosis.
Mixed cellularity Hodgkin lymphoma (MCHL) constitutes about 20% to 25% of cHL cases. It affects a somewhat older population, with a median age at diagnosis of 38 years. The typical bimodal age distribution is not seen with MCHL. MCHL has a male predominance (70%), and is more frequent in HIV-infected patients (70% of whom also have EBV infection). Lymphoid tissues have classic HRS cells and significant inflammatory infiltrates. Approximately 50% of patients with MCHL present as stage III or IV with abdominal lymphadenopathy or splenic involvement, and B symptoms are frequent.24
Lymphocyte-rich Hodgkin lymphoma (LRHL) is uncommon, accounting for only 3% to 5% of cases of cHL.28 The disease usually presents at an older age and has a 2:1 male predominance. HRS cells are commonly seen and a large number of reactive lymphocytes are also present. Although on the basis of morphology and immunohistochemistry LRHL belongs to the cHL group, clinically it more closely resembles LPHL. Patients usually present at early stage and rarely have B symptoms. LRHL carries an excellent prognosis, with a greater than 90% PFS after 5 years.23,29
Lymphocyte-depleted Hodgkin lymphoma (LDHL) is the least common form of cHL, accounting for less than 5% of cases. Many cases previously placed in this category are now recognized as diffuse large B-cell lymphoma (DLBCL), anaplastic large-cell lymphoma (ALCL), or NSHL with lymphocyte depletion.30 HRS cells are frequently seen, but reactive inflammatory cells are relatively sparse. EBV infection is seen in up to 90% of cases, commonly associated with HIV-infected individuals. Advanced-stage and symptomatic disease are more common. Prognosis is slightly worse compared to other categories.
NLPHL accounts for approximately 5% of cases of Hodgkin lymphoma. It has a unimodal age distribution, with the peak incidence in the fourth decade, and male predilection of 3:1.28 NLPHL is characterized by large primary lymphoid follicles, with polytypic small B lymphocytes and extensive meshworks of follicular dendritic cells. The lymphocytic/histiocytic (L and H), or “popcorn,” cells scattered within the nodules differ from classic HRS cells, both in their morphology and in their biochemical profile, being frequently negative for CD15, CD30 and for the EBV genome, and usually positive for B-cell antigens such as CD20, suggesting that L and H cells may be immunoglobulin-synthesizing monoclonal B cells. CD45 is also typically positive in NLPHL, in distinction from cHL. NLPHL has an indolent course compared to cHL, and long-term survival is common.19,31 NLPHL commonly presents with limited-stage disease. NLPHL may eventually transform into a more aggressive lymphoma, such as diffuse large B-cell lymphoma (including centroblastic, immunoblastic, or T-cell/histiocyte–rich subtypes), at a rate of 4% to 12%. This can occur even 15 to 20 years after the initial diagnosis of NLPHL.32,33 In a recent large retrospective study of 222 patients with NLPHL, the rate of transformation to DLBCL was 7.6%, with a median time to transformation of 35 months. Overall survival was not adversely affected in patients undergoing transformation compared to those without transformation.34
PRESENTATION
Classical Hodgkin lymphoma usually presents with asymptomatic mediastinal or cervical lymphadenopathy. Half of patients present with stage I or stage II disease.35 A mediastinal mass is seen in most patients with NSHL, at times with bulky disease, with “bulky” defined as a mediastinal mass measuring one-third or more of the maximal thoracic diameter on chest x-ray, or 10 cm on computed tomography (CT) scan. Systemic symptoms, or "B" symptoms—fevers (> 38°C), drenching night sweats, and unexplained weight loss (> 10% of baseline body weight over the preceding 6 months or less)—are detected in approximately 25% of patients. Between 10% and 15% will have extranodal disease, most commonly involving lung, bone, and liver. NLPHL usually presents with limited-stage disease without B symptoms; it typically has a more indolent presentation and clinical course than cHL.
INITIAL EVALUATION AND STAGING
The initial workup includes a complete blood count (CBC), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), and chemistry studies to evaluate renal function and liver function. Fine-needle aspiration will usually fail to identify the infrequent HRS cells, and instead only reveal the reactive background of inflammatory cells. Generous (large gauge) core needle biopsies may provide diagnosis effectively in some cases, but in general, an excisional lymph node biopsy is preferred to ensure an accurate diagnosis and avoid the need for repeated biopsy procedures. In cases where an excisional biopsy would be difficult or risky, a core needle biopsy procedure is a reasonable first step, with the understanding that a subsequent surgical procedure may still be necessary.
Baseline imaging includes CT scans of the neck, chest, abdomen, and pelvis. Use of positron emission tomography (PET) scanning is now standard in the initial evaluation and assessment of treatment response in Hodgkin lymphoma.36 Due to the increased sensitivity of PET or PET/CT scan, additional lesions may be identified that were not seen on conventional CT scans. This will alter the staging, and potentially the treatment plan, in up to 25% to 30% of patients.37,38 PET/CT scan performed during initial evaluation also facilitates optimal interpretation of post-therapy PET/CT scans and is therefore strongly encouraged as a part of the initial staging evaluation.39
Recent studies have shown that bone marrow biopsy is not routinely needed in the initial staging of cHL. A study of 454 patients concluded that bone marrow biopsy would not have altered the stage in any stage I or II patients. It was further concluded that overall treatment strategy would not have been altered for any of the patients.40 Based on this study and others, it is now clear that FDG-PET has a high sensitivity, and when PET scan is negative (in the bone marrow and skeleton), a bone marrow biopsy provides little additional value. For patients with significant cytopenias, a bone marrow biopsy is reasonable. Such patients may benefit from a bilateral biopsy, which increases the probability of demonstrating bone marrow involvement by 16% to 33%.41,42 Techniques such as staging laparotomy and lymphangiography are now considered obsolete.
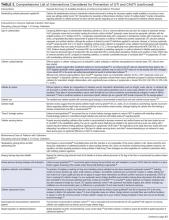
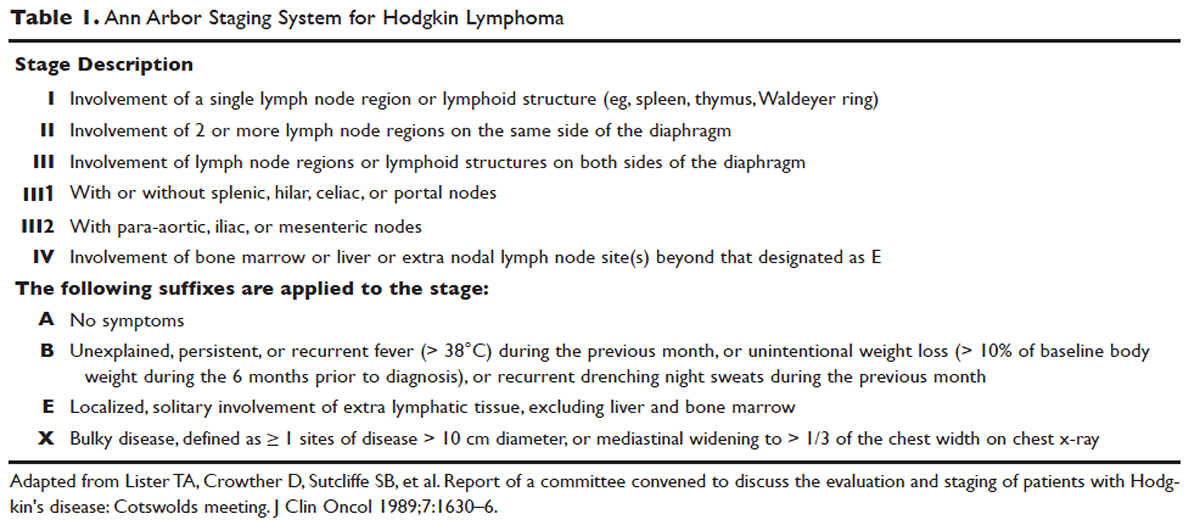
Hodgkin lymphoma is staged according to the Ann Arbor staging system (Table 1). The original Ann Arbor staging was published in 1971,43 and in 1989 the “Cotswold modifications” extended the definitions of stage IV disease and the suffix “X” was added to denote bulky disease.44 Both systems were developed for the staging of Hodgkin lymphoma, but are now used for staging non-Hodgkin lymphoma as well.
PROGNOSTIC FACTORS
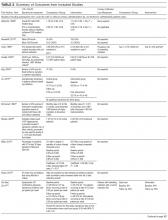
For the purposes of prognosis and selection of treatment, Hodgkin lymphoma is commonly classified into early-stage favorable, early-stage unfavorable, and advanced stage. Early-stage Hodgkin lymphoma refers to patients with Ann Arbor stage I or stage II disease. With early-stage Hodgkin lymphoma, the prognosis varies significantly based on factors such as the presence of B symptoms, elevated erythrocyte sedimentation rate ([ESR] > 50 mm/hr), number of nodal sites involved, older age, and a large mediastinal mass. For this reason, most clinical trials to evaluate treatment strategies for early-stage Hodgkin lymphoma are based on various combinations of these risk factors. The definition of early-stage unfavorable Hodgkin lymphoma varies across different clinical trial study groups, and it is important to understand the definition in interpreting the results of these trials (Table 2).45,46
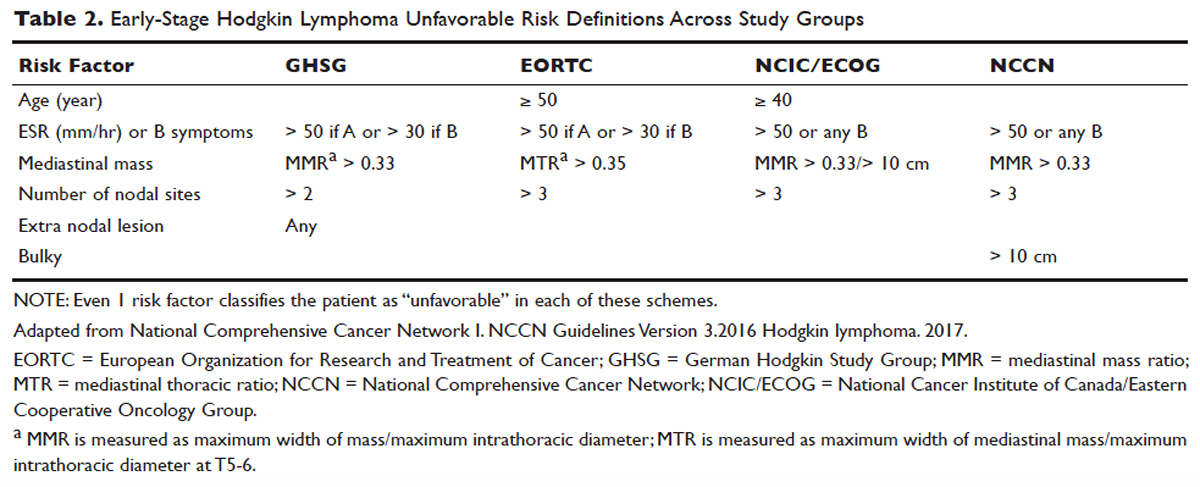
In the German Hodgkin Study Group (GHSG) trials, early-stage Hodgkin lymphoma is stratified into a high risk (“unfavorable”) group defined by any of the following: a large mediastinal mass (one third of maximum thoracic diameter), extra-nodal disease, 3 or more nodal areas, and an ESR of > 50 mm/hr in asymptomatic patients or > 30 mm/hr in patients with B symptoms. Low-risk (“favorable”) patients lack all of these factors.47 The European Organization for Research and Treatment of Cancer (EORTC) defines the unfavorable prognostic group as older than 50 years of age, large mediastinal adenopathy (maximum width on a chest radiograph of at least one third of the internal transverse diameter of the thorax at the level of T5 through T6 or any mass of ≥ 10 cm in the largest dimension), an ESR of 50 mm/hr and no B symptoms, or with an ESR of 30 mm/hr in those who have B symptoms, and/or 4 or more regions of involvement.48 The National Cancer Institute of Canada (NCIC) Clinical Trials Group and the Eastern Cooperative Oncology Group (ECOG) define high-risk groups as presence of B symptoms, bulky disease with a mediastinal mass width of at least one third of the maximum chest wall diameter, or any mass greater than 10 cm, and patients with intra-abdominal disease.49,50
Gene-expression profiling in Hodgkin lymphoma has identified a gene signature of tumor-associated macrophages that was able to identify patients with a higher risk for primary treatment failure. In an independent cohort of patients, an increased number of CD68-positive macrophages was correlated with inferior outcomes.51,52 Studies such as these underscore the importance of the tumor “microenvironment” (ie, the nonmalignant cells within a tumor) in determining the overall clinical behavior of a malignancy. While quantification of CD68-positive macrophages has potential to be applied to routine clinical practice, prospective data using CD68 as a tool for risk-adapted therapy is lacking.
Genetic alterations and amplifications in the 9p24.1 locus have recently been found to be a defining genetic feature of cHL. Amplification of 9p24.1 has been associated with unfavorable outcomes. Amplification of 9p24.1 (which includes the loci encoding the PD-L1 and PD-L2 genes) is more common in patients with advanced stage disease and is associated with shorter PFS.23
A recent study attempted to integrate several different prognostic factors in cHL patients who were treated with ABVD (adriamycin [doxorubicin], bleomycin, vinblastine, and dacarbazine) and underwent an interim PET (iPET) scan after 2 cycles of ABVD. Focusing on those with a negative iPET scan, it was found that expression of CD68 and PD-1 in microenvironment cells, and STAT1 negativity in HRS cells identified a subset of PET-2 negative patients with a 3-year PFS significantly lower than that of the remaining PET-2 negative population (64% versus 95%). The algorithm correctly predicted the response to treatment in more than half of the patients who had relapse or disease progression despite a negative PET-2 scan. It therefore appears feasible, using tissue biomarkers at diagnosis, to identify patients at increased risk for poor outcome, even if the iPET scan is negative.53
ROLE OF PET/CT IN ASSESSMENT OF RESPONSE TO THERAPY
PET/CT has been increasingly used for response assessment at various stages in lymphoma in recent years. Almost all types of lymphomas are fluorodeoxyglucose (FDG) avid; however, Hodgkin lymphoma is FDG avid in 97% to 100% of cases. In 2009, a 5-point scale was developed to score PET images with regard to treatment response, either partway through treatment (iPET) or at the end of therapy.54 It was recommended as the standard reporting tool at the First International Workshop on PET in Lymphoma in Deauville, France, in 2009, and is thus now referred to as the Deauville score. A score of 1 is given if there is no uptake, 2 if the uptake ≤ mediastinum, 3 if > mediastinum but ≤ liver, 4 if uptake moderately higher than liver, 5 if uptake is markedly higher than liver and/or new lesions. X designates new areas of uptake unlikely to be related to lymphoma. In most trials, a score of 1 or 2 is considered a complete response and a score of 4 or 5 is considered a treatment failure. A score of 3 is sometimes considered a complete response, depending on the study. The Deauville criteria have been widely used in newer clinical trials utilizing response-adapted treatment as defined by PET response. PET/CT is recommended for staging and restaging at the end of therapy, in clinical practice, and clinical trials. Interim PET/CT scan, while commonly performed in clinical practice, is only recommended if the results will alter therapy (eg, if that information will result in the clinician omitting radiation therapy [RT] or altering the chemotherapy plan).
Early studies of iPET showed that achieving PET negativity early in the course of treatment was strongly associated with PFS and overall survival.55 Subsequent studies confirmed the importance of achieving a negative iPET. As a result, considerable efforts have been put into designing response-adapted treatment approaches using iPET (see Treatment section), with some of these approaches now being listed in the National Comprehensive Cancer Network (NCCN) guidelines and being used in standard practice.
TREATMENT
EVOLUTION OF TREATMENT
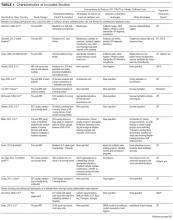
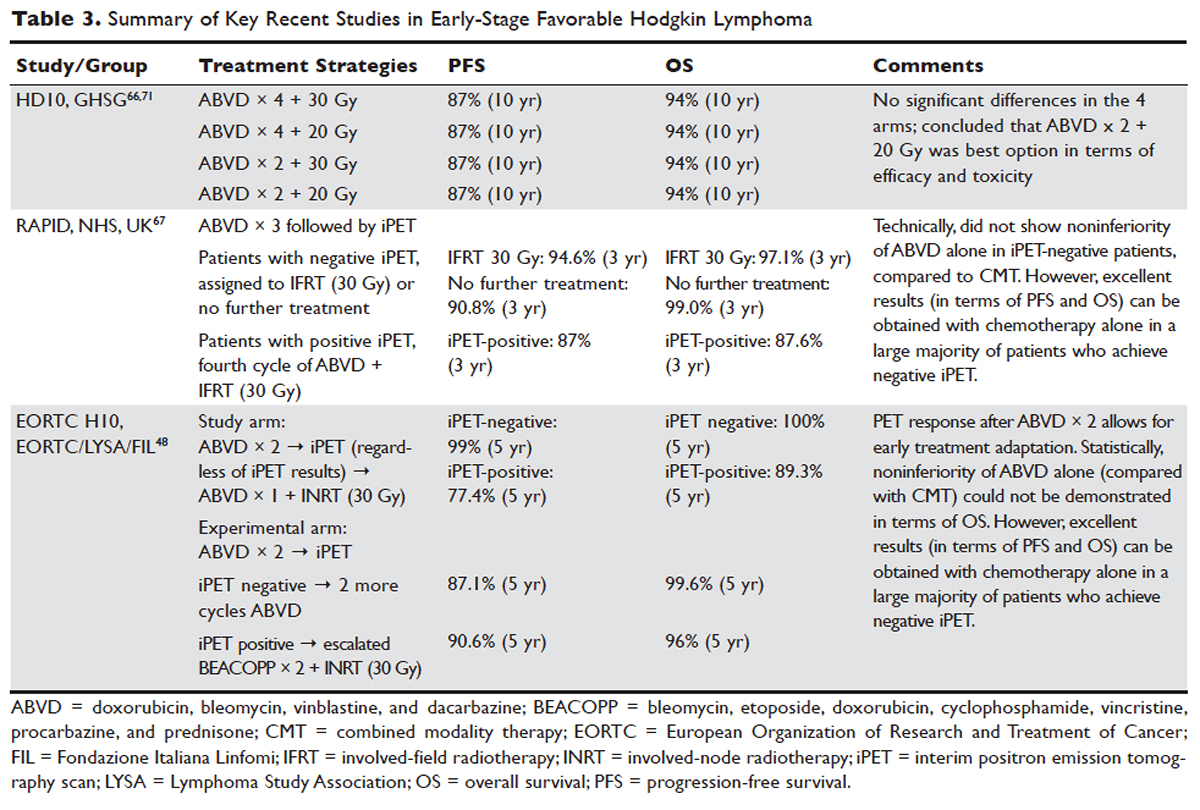
The treatment of Hodgkin lymphoma has evolved over the past century, starting with the discovery of RT as effective treatment in the early 20th century. Long-term survival of patients with Hodgkin lymphoma treated with involved-field radiation therapy (IFRT) was first reported in the 1960s.56,57 Outcomes improved further with the introduction of combined modality treatment (CMT) using chemotherapy and RT, with the overall 5-year relative survival for patients with Hodgkin lymphoma (all stages) treated in 2006–2012 being 85.4% to 87.3%.3 Since the majority of patients are now cured with modern therapy, treatment-related complications have become an important competing cause of mortality. Recent studies have therefore focused on maintaining efficacy while reducing toxicities, and refining the process of selecting patients who might benefit from more aggressive therapy. While RT was the first treatment modality shown to be curative for Hodgkin lymphoma,56,57 multiple subsequent studies showed that CMT is superior to RT alone in terms of relapse-free survival.58–63 In the GHSG H8-F trial, the estimated 5-year event-free survival and overall survival rates were significantly higher after 3 cycles of MOPP-ABV (mechlorethamine, vincristine, procarbazine, and prednisone combined with doxorubicin, bleomycin, and vinblastine) plus IFRT than after subtotal nodal radiotherapy alone. The 10-year overall survival estimates were 97% and 92%, respectively (P = 0.001).64 As a result, CMT replaced RT alone as the standard of care for limited-stage Hodgkin lymphoma. However, for elderly or infirm patients, or those with other comorbidities making them poor chemotherapy candidates, RT alone may be a very reasonable option.65 More recently, an increasing body of evidence has accumulated to support the use of chemotherapy alone in early stage cHL. This literature has consistently shown that omission of RT is associated with a modest increase in relapse, without a clear compromise in long-term overall survival. For some patients, the trade-off in terms of avoiding radiation (and the associated late effects) may be worth the small increase in relapse risk, since long-term survival does not appear to be substantially worse with chemo-therapy alone. Table 3 and Table 4 provide a summary of recent key studies which have defined treatment options for early-stage cHL.48,66–71

EARLY-STAGE NLPHL
NLPHL usually presents with limited-stage disease without B symptoms and has an indolent course with a slightly better prognosis compared to cHL.72 Due to the rarity of the disease, treatment guidelines are mostly based on retrospective analyses from single or multi-institution studies or subgroup analyses, often with relatively short follow-up. These studies must be interpreted with caution because of the possibility of inaccuracies in the pathologic diagnosis, small sample sizes, and selection bias. Treatment options for limited-stage NLPHL include observation, single-agent rituximab, IFRT (or involved-site radiation therapy [ISRT]) alone, or CMT.46
Historically, patients with limited-stage NLPHL have been treated with RT alone, with 80% to 85% PFS and 85% to 95% overall survival rates.73–75 Patients who relapse or progress after RT in general can successfully undergo salvage therapy.74 In one study, rates of PFS and overall survival were similar among patients who had limited-field, regional-field, or extended-field RT,75 indicating that IFRT is preferred. Studies comparing RT alone and CMT are limited. The GHSG HD7 trial included a subset of NLPHL patients, with a trend towards improved freedom from treatment failure (96% versus 83%) favoring CMT. This, however, did not translate into improved overall survival.47 A retrospective analysis of the British Columbia Cancer Agency database compared patients with limited-stage NLPHL treated with RT alone to patients who received 2 cycles of ABVD followed by RT. A significant improvement in PFS (91% versus 65%) and overall survival (93% versus 84%) was seen, favoring CMT.76
Chemotherapy alone is not recommended for limited-stage NLPHL since studies evaluating chemotherapy alone are quite limited and indicate relatively high rates of treatment failure. Given that the malignant cells in NLPHL are CD20-positive, single-agent rituximab has also been studied in this disease, including a study as frontline therapy in limited-stage patients. In this phase 2 trial in newly diagnosed patients with stage IA disease, an overall response rate (ORR) of 100% was seen, with an 85% complete response (CR) rate.77 At 3 years, overall survival was 100% and PFS was 81%, indicating that the responses with single-agent rituximab are less durable than those with RT.
Advani et al evaluated rituximab followed by observation versus rituximab (R) followed by maintenance rituximab (MR) for 2 years in 39 new or previously treated patients. At 4 weeks the ORR was 100% (with CR in 67%, and partial response in 33%). At a median follow up of 9.8 years for R alone, and 5 years for R+MR, median PFS was 3 and 5.6 years, respectively (P = 0.26). Estimated 5-yr PFS and overall survival in patients treated with R versus R+MR were 39.1% and 95.7% versus 58.9% and 85.7%, with Pvalues of 0.26 (PFS) and 0.38 (overall), respectively. Maintenance rituximab therefore appears to prolong remission, although the results did not quite reach statistical significance.78 Even though rituximab does not appear to be curative in NLPHL, it is a reasonable option for patients with early-stage NLPHL who are not good candidates for definitive RT. Whether combining rituximab with RT or CMT might further improve outcomes in early-stage NLPHL has not yet been determined.
In children, surgery alone may lead to long-term remission or possibly cure of limited-stage NLPHL. In a European multicenter retrospective study, 58 patients underwent surgery for limited-stage NLPHL. Among the 51 patients who achieved complete remission following surgery, 67% remained progression-free and 100% were alive at a median follow up of 43 months.79 In adults, there is no data to support surgical treatment alone for NLPHL. Finally, observation may be a reasonable option in elderly or infirm patients for whom NLPHL is unlikely to affect life expectancy. For younger patients, given the excellent outcome with modern therapy and the long-term risk of transformation of NLPHL into an aggressive non-Hodgkin lymphoma, observation is generally not recommended.
The NCCN recommends RT (ISRT or IFRT, 30–36 Gy) as the preferred treatment for stage IA and IIA non-bulky NLPHL. In patients with stage IA disease with complete excision of solitary nodule, observation may be appropriate. A course of chemotherapy with ISRT with or without rituximab is recommended for patients with stage IB or IIB disease, or patients with stage IA or IIA bulky disease.
FIRST-LINE TREATMENT OF LIMITED-STAGE CHL
Early-Stage Favorable cHL
There is lack of consensus regarding the ideal treatment approach for patients with early-stage favorable cHL. However, there are several excellent options available, with overall survival rates exceeding 90%. Most of these regimens involve CMT, although some chemotherapy-alone approaches have been evaluated as well. Concurrent with the demonstration of excellent long-term remission rates with CMT, it became apparent that the long-term survival and quality of life of these patients is determined in large part by the risk of serious (and potentially fatal) treatment-related toxicities. Such toxicities consist primarily of secondary malignancies and cardiovascular events, and can continue to cause significant morbidity and mortality even 2 to 3 decades after treatment.80–82 As a result, treatment decisions for these patients are complicated and require balancing efficacy against risk of late complications.
In the United States, until recently, CMT was generally considered the standard of care, with robust long-term data regarding efficacy. The most commonly used regimen has been ABVD for 2 to 4 cycles followed by IFRT. In some German studies, escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) has been used, but this is not a general standard of care in the United States for early-stage patients.
More recent data suggests that the rate of serious late complications in Hodgkin lymphoma patients is decreasing, likely due to less extensive radiation fields, lower radiation doses, and a movement away from the MOPP regimen to ABVD.83,84 For patients who meet the “favorable” criteria set forth in the GHSG HD10 trial (see Table 2), 2 cycles of ABVD followed by 20 Gy of IFRT is an attractive option, with efficacy preserved and a low anticipated rate of late effects.66 With this approach, and with long-term (10 years) follow up, all 4 arms had similar PFS (87%) and overall survival (94%), whether 2 or 4 cycles of ABVD were given. When the effects of 20-Gy and 30-Gy doses of RT were compared, there were also no significant differences in freedom from treatment failure or overall survival. Adverse events and acute toxic effects of treatment were most common in the patients who received 4 cycles of ABVD and 30 Gy of RT.66,71
In recent years, in an attempt to reduce late effects further, regimens consisting of chemotherapy alone have been investigated. In a study by Meyer et al, at 12 years the rate of overall survival was 94% among those receiving ABVD alone, as compared with 87% among those receiving subtotal nodal RT; the rates of freedom from disease progression were 87% and 92% in the 2 groups; and the rates of event-free survival were 85% and 80%, respectively.50 However, it is important to note that this study did not include a CMT arm for the early favorable patients, and did not utilize modern RT techniques. Nevertheless, this early study and others60 suggested that chemotherapy alone may be a reasonable option for some early-stage cHL patients, particularly for patients who are felt to be at increased risk for late toxicities from RT. As a result, additional studies have been conducted evaluating CMT versus chemotherapy alone for early-stage cHL. Many of these studies have incorporated interim PET/CT scan to develop a response-adapted approach to decide which patients are least likely to benefit from RT.
The HD-13 study was a follow-up study for HD-10, looking at deletion of bleomycin, dacarbazine, or both from the ABVD backbone. The ABD arm was closed early, because of an excess rate of treatment failure. Among the 1243 patents assigned to either the ABVD or AVD arm at 5 years of follow-up, there was 4.3% difference in PFS. This study was not able to demonstrate that 2 cycles of AVD was noninferior to 2 cycles of ABVD, each followed by 30 Gy IFRT, even though there was no difference in all 4 groups. It confirmed 2 cycles of ABVD as the preferred regimen in early favorable Hodgkin lymphoma, when CMT is the plan of care. However, for patients over age 60 to 65 years, or those with underlying cardiac or pulmonary comorbidities, bleomycin has significant risk of toxicity. In that setting, AVD is a safer option, with only a very modest decrease in 5-year PFS.
Based on the observation that iPET scan is highly predictive of outcome in Hodgkin lymphoma,55,85 several trials have employed the use of an iPET scan to guide therapy. It is hoped that such studies will lead to new PET-directed treatment algorithms in which patients who require more aggressive therapy (eg, with CMT, or escalated BEACOPP) can be identified, and the remaining patients can be safely treated less aggressively (eg, with chemotherapy alone).
In the EORTC H10 trial, performed to evaluate treatment adaptation on the basis of iPET scan results in stage I and II Hodgkin lymphoma, a control arm received standard combined modality treatment (3 or 4 cycles of ABVD with INRT) irrespective of PET scan results. In the experimental arm, patients with a negative PET scan after 2 cycles of ABVD continued with 1 or 2 cycles of ABVD and did not receive RT. The iPET-positive patients received either standard treatment with ABVD plus INRT or escalated BEACOPP plus INRT. The iPET-negative patients received either ABVD only or ABVD plus INRT. The final results of this study, published recently, showed that in the iPET-positive patients the 5-year PFS was improved from 77.4% with standard ABVD plus INRT to 90.6% with escalated BEACOPP plus INRT (P = 0.002). In iPET-negative patients, 5-year PFS in the favorable group was 99% versus 87.1% in favor of ABVD plus INRT. The H10 study suggested that PET results after 2 cycles of ABVD can be integrated into treatment planning, In iPET-negative patients, the study was technically not able to demonstrate the noninferiority of the ABVD only regimen, owing to a higher risk of relapse if INRT is omitted, particularly in the favorable group.48 However, this study does show that excellent outcomes can be obtained with omission of RT in patients with a negative iPET scan. This study provides a cautionary lesson though, in that the increase in relapse rate associated with omission of RT was more substantial (12%) for favorable versus unfavorable early-stage patients (2.5%), and this difference was only apparent after longer (5 years) follow-up. Despite this, chemotherapy alone is considered a reasonable treatment option, especially for patients felt to be at increased risk for late toxicities of RT or for patients who wish to avoid the risks of RT, with over 99% of patients alive at 5 years.
Similar results were shown in the RAPID trial, in which patients with limited-stage cHL underwent 3 cycles of ABVD followed by PET assessment.67 Patients with a negative PET (n = 426) were then randomized to RT (n = 209) versus no further therapy (n = 211). At a median of 60 months of follow-up, 3-year PFS was 94.6% in the RT group and 90.8% in the chemotherapy alone group. Similar to the H10 trial, it was concluded that chemotherapy alone was statistically inferior to CMT in terms of PFS. However, also similar to the H10 trial, the RAPID trial demonstrated that excellent results can be obtained in early-stage cHL patients with omission of RT, if iPET scan is negative after 3 cycles of ABVD, as there was no survival difference. These findings indicate that, when relapses occur as a result of omission of RT, such patients can be effectively treated later.
In the ongoing GHSG HD16 trial, patients with early-stage favorable cHL will be randomly assigned to a standard approach (ABVD × 2 cycles followed by 20-Gy IFRT) versus an experimental approach in which they receive ABVD for 2 cycles and then undergo PET scan. If the PET remains positive, they will receive 20-Gy IFRT. If the PET is negative, they will receive no further therapy. This trial could ultimately define ABVD for 2 cycles as a treatment option.
It is clear from these studies that omission of RT results in a somewhat higher rate of relapse but can be considered in selected patients. However, taking a less aggressive frontline approach may also be justified by the fact that, for those who do relapse, successful salvage therapies are available. Aggressive salvage therapy with autologous stem cell transplantation historically can cure approximately 50% of relapsed patients. With new and emerging therapies for relapsed disease, such as brentuximab vedotin and the PD-1 inhibitors (eg, nivolumab and pembrolizumab), the ability to cure relapsed patients may improve even more, further calling into question the practice of applying CMT uniformly for early-stage patients undergoing first-line therapy. Unfortunately, there is insufficient data from large randomized studies with long-term follow-up to fully address this issue currently, and there remains some controversy around this issue. NCCN recommends restaging PET/CT after 3 cycles of ABVD if a chemotherapy alone treatment modality is chosen. If the Deauville score is 1 or 2, either observation or 1 additional cycle of ABVD is recommended.46
Early-Stage Unfavorable cHL
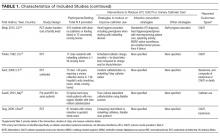
In the United States, historically early-stage unfavorable Hodgkin lymphoma has been treated with CMT, most commonly 4 to 6 cycles of ABVD followed by consolidative RT. With this approach one can expect a 5-year PFS of approximately 80% to 85%.58,64,86 The GHSG HD8 trial showed that RT volume size reduction from extended-field to involved-field after COPP + ABVD chemotherapy for 2 cycles produced similar results and less toxicity in patients with early-stage unfavorable cHL.86 The GHSG trial HD11 established ABVD for 4 cycles plus 30-Gy IFRT as a standard for early unfavorable Hodgkin lymphoma. The freedom from treatment failure at 5 years was 85.0%, and overall survival was 94.5%.68
In the HD14 study by the GHSG, patients with early unfavorable cHL were treated with 2 cycles of escalated BEACOPP followed by 2 cycles of ABVD, versus 4 cycles of ABVD. All patients then received 30 Gy of consolidative IFRT. A 5-year PFS of 95% was seen in the experimental arm, compared with 89% in the standard (ABVD) arm. As expected, this regimen was associated with more acute hematologic toxicity, and there was no difference between the 2 regimens with respect to overall survival or fertility.69 Given the lack of improved survival and increased toxicity, ABVD has remained the standard chemotherapy regimen for early unfavorable cHL in the United States. NCCN recommends a restaging PET scan after 2 cycles of ABVD and to continue with 2 to 4 cycles of ABVD or escalated BEACOPP with or without ISRT based on Deauville scores.
Another viable treatment option is the Stanford V regimen, a condensed, 12-week regimen that includes mechlorethamine, doxorubicin, vinblastine, prednisone, vincristine, etoposide, and bleomycin, followed by IFRT.87 In a randomized phase 3 trial conducted by ECOG (E2496), patients with stage I/II Hodgkin lymphoma with bulky mediastinal disease or advanced-stage disease were randomized to ABVD × 6 to 8 cycles versus Stanford V. RT was given (36 Gy) for those with bulky mediastinal disease or to sites of disease greater than 5 cm in the Stanford V arm. In a subset analysis focusing only on those with stage I/II bulky mediastinal disease, the 5-year failure free survival was 85% versus 79% and the 5-year overall survival was 96% versus 92% for the ABVD versus Stanford V arms, respectively. These differences were not statistically significant.70 While the Stanford V regimen has the advantages of a 12-week treatment duration and a lower cumulative amount of bleomycin and doxorubicin, the Stanford V arm had higher rates of grade 3 lymphopenia and grade 3 to 4 peripheral neuropathies. In addition, Stanford V requires that most patients undergo RT (to original sites of disease measuring 5 cm or more plus contiguous areas). As a result, the investigators concluded that ABVD × 4 cycles plus IFRT remains the standard of care for patients with early unfavorable Hodgkin lymphoma with bulky mediastinal disease.
Other regimens have been studied in hopes of reducing toxicity, including the EVE regimen (epirubicin, vinblastine, and etoposide). This regimen was compared to ABVD in early unfavorable Hodgkin lymphoma patients, with all patients undergoing the same RT program. No differences were observed between the ABVD and EVE arms in terms of complete remission rate and overall survival. However, patients who received EVE had a significantly worse outcome than those who received ABVD in terms of relapse-free survival and failure-free survival.88 EBVP (epirubicin, bleomycin, vinblastine, and prednisone) followed by IFRT was less efficacious compared with MOPP/ABV–type therapy.58
An area of active investigation is whether RT can be safely omitted in patients with early- stage unfavorable cHL. The EORTC H10 study showed that, for patients with a negative iPET scan (after 2 cycles), the 5-year PFS rates were 92.1% versus 89.6% for ABVD plus INRT versus ABVD alone, respectively. While this technically did not meet criteria for noninferiority of ABVD alone, this study demonstrated that, for those with negative iPET, ABVD × 6 cycles (without radiation) can result in long-term remission in a high proportion (89%) of patients. For iPET-positive patients, 2 cycles of escalated BEACOPP were given followed by 30 Gy of IFRT on the experimental arm. This resulted in a 5-year PFS of 90.6% versus 77.4%, suggesting this may be a preferred approach for early-stage unfavorable patients with a positive iPET.48 Even though the noninferiority of ABVD alone could not be established based on the statistical design of the study, the current NCCN guidelines recommend restaging after 2 cycles of ABVD for stage I or II unfavorable cHL and using that iPET as a guide, based on Deauville scores. For scores 1–3, ABVD × 2 cycles (total 4 cycles) plus ISRT or AVD × 4 (total 6) with or without ISRT is recommended. For a Deauville score of 4, escalated BEACOPP × 2 cycles or ABVD × 2 cycles (total 4) followed by ISRT is recommended. If the Deauville score is 5, further treatment decisions should be made based on repeat biopsy results. A follow up PET/CT is recommended for Deauville scores of 4 and 5 to confirm complete response.46
LATE EFFECTS AND THE EVOLUTION OF RADIATION THERAPY
The RT given in Hodgkin lymphoma has evolved considerably over the years, from extended field or subtotal nodal fields developed in the 1960s, to the more focused involved-field or even involved-site radiation commonly given now. This approach reduces radiation volumes, and it already is becoming evident that the relative risk of breast cancer among young females receiving mediastinal RT for Hodgkin lymphoma is declining.89 Cardiac dose is reduced significantly with IFRT compared to older radiation techniques as well. The extent of radiation may be reduced even further with involved-nodal/involved site or intensity-modulated approaches.90
With new RT techniques allowing for more focused therapy and lower doses of radiation, models predict that the rate of long-term complications will decline further.91,92 Furthermore, response-adapted (ie, PET-directed) approaches, as discussed in detail earlier in the article, are expected to increasingly allow for identification of patients who can safely avoid radiation entirely, which will hopefully lead to an even lower rate of late complications of therapy.
MONITORING FOR RELAPSE
A number of recent studies have shown that, for patients who achieve complete remission with first-line therapy, performing repeated scheduled surveillance imaging does not improve outcomes. In fact, most relapses are detected by the patient (due to symptoms or recurrence of lymph node enlargement). It is rare that a relapse would be detected by surveillance imaging alone. Furthermore, surveillance that includes routine imaging has not been associated with improved survival.93 As a result, it is now recommended that patients undergo regular follow-up with symptom review, physical exam, and basic laboratory studies. Imaging studies should be obtained as needed for patients who develop signs, symptoms, exam findings, or laboratory values concerning for relapse.
More important than scheduled surveillance imaging for relapse is monitoring for late effects of therapy. These fall into several broad categories such as cardiovascular disease (coronary disease, congestive heart failure, valvular disease, carotid artery disease), pulmonary disease, hypothyroidism, and secondary malignancies. Aggressive surveillance for breast cancer is especially warranted in female patients who underwent chest radiation.46
CONCLUSION
Hodgkin lymphoma is characterized pathologically by the presence of HRS cells accompanied by a polymorphous cellular infiltrate. It is a disease with a bimodal age distribution, several pathologic subtypes, and numerous treatment options. Overall, the prognosis for patients with early-stage disease is excellent, and although a majority of patients can now be cured, further studies are needed to optimize treatment such that short- and long-term treatment-related toxicities are minimized, without compromising disease control and cure.
- Küppers R, Rajewsky K, Zhao M, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A 1994;91:10962–6.
- Küppers R. The biology of Hodgkin›s lymphoma. Nat Rev Cancer 2009;9:15–27.
- National Cancer Institute. SEER cancer statistics review, 1975–2014. 2017. http://seer.cancer.gov/csr/1975_2013/. Accessed April 27, 2017.
- Haim N, Cohen Y, Robinson E. Malignant lymphoma in first-degree blood relatives. Cancer 1982;49:2197–200.
- Mack TM, Cozen W, Shibata DK, et al. Concordance for Hodgkin’s disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N Engl J Med 1995;332:413–8.
- Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 2010;116:3724–34.
- Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med 2003;349:1324–32.
- Hessol NA, Katz MH, Liu JY, et al. Increased incidence of Hodgkin disease in homosexual men with HIV infection. Ann Intern Med 1992;117:309–11.
- Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753–62.
- Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2009;27:884–90.
- Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr 2009;52:203–8.
- Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 2006;108:3786–91.
- Thompson LD, Fisher SI, Chu WS, et al. HIV-associated Hodgkin lymphoma: a clinicopathologic and immunophenotypic study of 45 cases. Am J Clin Pathol 2004;121:727–38.
- Briggs NC, Hall HI, Brann EA, et al. Cigarette smoking and risk of Hodgkin’s disease: a population-based case-control study. Am J Epidemiol 2002;156:1011–20.
- Castillo JJ, Dalia S, Shum H. Meta-analysis of the association between cigarette smoking and incidence of Hodgkin’s Lymphoma. J Clin Oncol 2011;29:3900–6.
- Kanzler H, Kuppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med 1996;184:1495–505.
- Stein H, Hummel M. Cellular origin and clonality of classic Hodgkin’s lymphoma: immunophenotypic and molecular studies. Semin Hematol 1999;36:233-41.
- Marafioti T, Hummel M, Foss HD, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000;95:1443–50.
- Marafioti T, Hummel M, Anagnostopoulos I, et al. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med 1997;337:453–8.
- van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin’s lymphoma. Am J Pathol 1999;154:1685–91.
- Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest 1997;100:2961–9.
- Luftig M, Yasui T, Soni V, et al. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc Natl Acad Sci U S A 2004;101:141–6.
- Roemer MGM, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016;34:2690–7.
- Swerdlow SH CE, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019–32.
- von Wasielewski R, Mengel M, Fischer R, et al. Classical Hodgkin’s disease. Clinical impact of the immunophenotype. Am J Pathol 1997;151:1123–30.
- Tzankov A, Krugmann J, Fend F, et al. Prognostic significance of CD20 expression in classical Hodgkin lymphoma: a clinicopathological study of 119 cases. Clin Cancer Res 2003;9:1381–6.
- Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol 1999;17:776–83.
- Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin’s Study Group trials. J Clin Oncol 2005;23:5739–45.
- Slack GW, Ferry JA, Hasserjian RP, et al. Lymphocyte depleted Hodgkin lymphoma: an evaluation with immunophenotyping and genetic analysis. Leuk Lymphoma 2009;50:937–43.
- Mason DY, Banks PM, Chan J, et al. Nodular lymphocyte predominance Hodgkin’s disease. A distinct clinicopathological entity. Am J Surg Pathol 1994;18:526–30.
- Rudiger T, Gascoyne RD, Jaffe ES, et al. Workshop on the relationship between nodular lymphocyte predominant Hodgkin’s lymphoma and T cell/histiocyte-rich B cell lymphoma. Ann Oncol 2002;13 Suppl 1:44–51.
- Sundeen JT, Cossman J, Jaffe ES. Lymphocyte predominant Hodgkin’s disease nodular subtype with coexistent “large cell lymphoma”. Histological progression or composite malignancy? Am J Surg Pathol 1988;12:599–606.
- Kenderian SS, Habermann TM, Macon WR, et al. Large B-cell transformation in nodular lymphocyte-predominant Hodgkin lymphoma: 40-year experience from a single institution. Blood. 2016;127:1960–6.
- Mauch PM, Kalish LA, Kadin M, et al. Patterns of presentation of Hodgkin disease. Implications for etiology and pathogenesis. Cancer 1993;71:2062–71.
- Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N J Engl Med 2006;354:496–507.
- Hutchings M, Loft A, Hansen M, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica 2006;91:482–9.
- Naumann R, Beuthien-Baumann B, Reiss A, et al. Substantial impact of FDG PET imaging on the therapy decision in patients with early-stage Hodgkin’s lymphoma. Br J Cancer 2004;90:620–5.
- Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571–8.
- El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 2012;30:4508–14.
- Wang J, Weiss LM, Chang KL, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer 2002;94:1522–31.
- Menon NC, Buchanan JG. Bilateral trephine bone marrow biopsies in Hodgkin’s and non-Hodgkin’s lymphoma. Pathology 1979;11:53–7.
- Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res 1971;31:1860–1.
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989;7:1630–6.
- Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med 2010;363:653–62.
- National Comprehensive Cancer Network I. NCCN Guidelines Version 3.2016 Hodgkin lymphoma. 2017.
- Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. J Clin Oncol 2007;25:3495–502.
- Andre MP, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 2017:Jco2016686394.
- Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol 2005;23:4634–42.
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 2012;366:399–408.
- Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 2010;362:875–85.
- Kamper P, Bendix K, Hamilton-Dutoit S, et al. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 2011;96:269–76.
- Agostinelli C, Gallamini A, Stracqualursi L, et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol 2016;3:e467–e79.
- Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymph 2009;50:1257–60.
- Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 2007;25:3746–52.
- Easson EC, Russell MH. Cure of Hodgkin’s Disease. Br Med J 1963;1(5347):1704–7.
- Kaplan HS. The radical radiotherapy of regionally localized Hodgkin’s disease. Radiology 1962;78:553–61.
- Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin’s lymphoma: long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol 2006;24:3128–35.
- Eghbali H, Raemaekers J, Carde P. The EORTC strategy in the treatment of Hodgkin’s lymphoma. Eur J Haematol Suppl 2005:135–40.
- Bloomfield CD PT, Glicksman AS, et al. Chemotherapy and combined modality therapy for Hodgkin’s disease: A progress report on cancer and leukemia group B studies. Cancer Treat Rep 1982;66:835–46.
- Pavlovsky S, Maschio M, Santarelli MT, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage I-II Hodgkin’s disease. J Natl Cancer Inst 1988;80:1466–73.
- Aviles A, Delgado S. A prospective clinical trial comparing chemotherapy, radiotherapy and combined therapy in the treatment of early stage Hodgkin’s disease with bulky disease. Clin Lab Haematol 1998;20:95–9.
- Herbst C, Rehan FA, Brillant C, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin’s lymphoma: a systematic review. Haematologica 2010;95:494–500.
- Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med 2007;357:1916–27.
- Landgren O, Axdorph U, Fears TR, et al. A population-based cohort study on early-stage Hodgkin lymphoma treated with radiotherapy alone: with special reference to older patients. Ann Oncol 2006;17:1290–5.
- Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med 2010;363:640–52.
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Eng J Med 2015;372:1598–607.
- Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 2010;28:4199–206.
- von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 2012;30:907–13.
- Advani RH, Hong F, Fisher RI, et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: a subset analysis of the North American Intergroup E2496 Trial. J Clin Oncol 2015;33:1936–42.
- Sasse S, Brockelmann PJ, Georgen H, et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: Updated analyses of the German Hodgkin Study Group HD7, HD8, HD10 and HD11 trials. J Clin Oncol 2017 Apr 18:JCO2016709410. doi: 10.1200/JCO.2016.70.9410. [Epub ahead of print]
- Nogova L, Reineke T, Brillant C, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma: a comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol 2008;26:434–9.
- Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma: a retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer 2005;104:1221–9.
- Chera BS, Olivier K, Morris CG, et al. Clinical presentation and outcomes of lymphocyte-predominant Hodgkin disease at the University of Florida. Am J Clin Oncol 2007;30:601–6.
- Chen RC, Chin MS, Ng AK, et al. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma: patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol 2010;28:136–41.
- Savage KJ, Skinnider B, Al-Mansour M, et al. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood 2011;118:4585–90.
- Eichenauer DA FM, Pluetschow A, et al. Phase 2 study of rituximab in newly diagnosed stage IA nodular lymphocytepredominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood 2011;118:4363–5.
- Advani RH, Horning SJ, Hoppe RT, et al. Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol 2014;32:912–8.
- Mauz-Korholz C, Gorde-Grosjean S, Hasenclever D, et al. Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma-experience from the European network group on pediatric Hodgkin lymphoma. Cancer 2007;110:179–85.
- Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol 2011;154:23–31.
- Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol 2011;29:1885–92.
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol 2003;21:3431–9.
- Girinsky T, van der Maazen R, Specht L, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol 2006;79:270–7.
- Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma: a question of field size. J Clin Oncol 2008;26:5170–4.
- Advani R, Maeda L, Lavori P, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin’s disease. J Clin Oncol 2007;25:3902–7.
- Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 2003;21:3601–8.
- Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease: mature results of a prospective clinical trial. J Clin Oncol 2002;20:630–7.
- Pavone V, Ricardi U, Luminari S, et al. ABVD plus radiotherapy versus EVE plus radiotherapy in unfavorable stage IA and IIA Hodgkin’s lymphoma: results from an Intergruppo Italiano Linfomi randomized study. Ann Oncol 2008;19:763–8.
- De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 2009;27:4239–46.
- Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:323–9.
- Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012;83:1232–7.
- Campbell BA, Hornby C, Cunninghame J, et al. Minimising critical organ irradiation in limited stage Hodgkin lymphoma: a dosimetric study of the benefit of involved node radiotherapy. Ann Oncol 2012;23:1259–66.
- Pingali SR, Jewell SE, Havlat L, et al. Limited utility of routine surveillance imaging for classical Hodgkin lymphoma patients in first complete remission. Cancer 2014;120:2122–9.
INTRODUCTION
Hodgkin lymphoma, previously known as Hodgkin’s disease, is a B-cell malignancy with unique pathological and epidemiological features for which highly effective therapies exist. The disease is characterized by the presence of mononuclear and multinucleate giant cells called Hodgkin and Reed-Sternberg (HRS) cells.1
Hodgkin lymphoma is unique compared to other B-cell lymphomas because of the scarcity of the malignant cells in the tumor tissue. The HRS cells usually account for only 0.1% to 10% of the cells in the affected tissues, and the HRS cells induce accumulation of nonmalignant lymphocytes, macrophages, granulocytes, eosinophils, plasma cells, and histiocytes, which constitute more than 90% of tumor cellularity.2 Although the disease was first described by Sir Thomas Hodgkin in 1832, in part because of this unique histopathology, not until 1991 was it conclusively demonstrated that HRS cells are in fact monoclonal germinal center–derived B-cells. This article reviews management and frontline treatment options for limited-stage classical Hodgkin lymphoma and nodular lymphocyte predominant Hodgkin lymphoma. Treatment of advanced stage and relapsed/refractory Hodgkin lymphoma will be discussed in a separate article.
EPIDEMIOLOGY
Hodgkin lymphoma accounts for 0.5% of all malignancies and 11.7% of all lymphomas among adults in the United States.3 The incidence of Hodgkin lymphoma has been steadily increasing over the past 4 decades and was estimated to be 8260 cases in the United States in 2017, with a slight male predominance. Hodgkin lymphoma is expected to cause 1070 deaths in 2017, accounting for 0.2% of all cancer deaths.3 First-degree relatives of patients with Hodgkin lymphoma have a 3- to 9-fold increased risk of having the disease compared to the general population,4 and monozygotic twin siblings of Hodgkin lymphoma patients have a greatly increased risk for developing the disease—up to 100-fold—compared to normal cohorts.5 The incidence is highest among Caucasians, African Americans, and Hispanics, and lower in Asians and American Indians.3 Hodgkin lymphoma incidence shows a bimodal peak distribution, with 1 peak between the ages of 15 and 44 years, and another peak after age 65 years.6
ETIOLOGY/PATHOGENESIS
The cause of Hodgkin lymphoma is unknown. Epstein-Barr virus (EBV) infection is present in up to 40% of Hodgkin lymphoma cases, suggesting a role of this virus in the pathogenesis of some cases. The risk of EBV-positive Hodgkin lymphoma was found to be higher following an episode of infectious mononucleosis, while the risk of EBV-negative Hodgkin lymphoma remained unchanged.7 The incidence of Hodgkin lymphoma is 5 to 14 times higher in HIV-infected patients than in noninfected patients.8 It is not considered an AIDS-defining illness, but has become more frequent with the growth and aging of the HIV-positive population.9,10 Hodgkin lymphoma patients with HIV typically have CD4 lymphocyte counts greater than 200 cells/μL,11 with the incidence of Hodgkin lymphoma actually declining with lower CD4 lymphocyte counts.12 HIV-related Hodgkin lymphoma tends to have an aggressive course, with high rates of EBV positivity.13 The incidence of Hodgkin lymphoma is 1.8 times higher among smokers, and the risk appears to increase with duration of smoking.14,15
The cell of origin of Hodgkin lymphoma, while long suspected to be the HRS cell, remained unproven until the 1990s when micro-dissection and single-cell polymerase chain reaction techniques allowed for confirmation that the HRS cell was in fact a monoclonal germinal center derived B cell.16,17 These HRS cells lack immunoglobulin due to defective transcription regulation and not due to crippling mutations.18,19 The cellular infiltrate in Hodgkin lymphoma appears to play a decisive role in allowing the HRS cells to survive by providing an environment that suppresses cytotoxic immune responses as well as by providing cellular interactions and cytokines that support their growth and survival. The extensive inflammatory infiltrate in classical Hodgkin lymphoma is comprised of T helper 2 (Th2) and regulatory T cells and lacks T helper 1 (Th1) cells, CD8 cytotoxic T cells, and natural killer cells.20 The HRS cells escape apoptosis by several mechanisms which include latent EBV infection and constitutive nuclear factor (NF)-kB pathways, as well as other deregulated signaling pathways that promote survival, such as EBV nuclear antigen 1 (EBNA1) protein, EBV latent infection membrane protein 1 (LMP1), and LMP2.21,22
Genetic alterations in the 9p24 locus which encodes PD-L1/PD-L2 are nearly universally present in classical Hodgkin lymphoma and are now considered a disease-defining feature.23
PATHOLOGIC CLASSIFICATION
According to the 2008 World Health Organization (WHO) classification, Hodgkin lymphoma has 2 clearly defined entities: classical Hodgkin lymphoma (cHL), which accounts for approximately 95% cases, and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), which accounts for the remaining cases.24 These 2 entities differ in their clinical, pathological, and biological features, which in turn affect prognosis and treatment options. Classical Hodgkin lymphoma is characterized by a paucity of HRS cells surrounded by a background of mixed inflammatory infiltrate comprised of histiocytes, small lymphocytes, eosinophils, neutrophils, plasma cells, fibroblasts, and collagen. Depending on the particular combinations of these elements and the specific features of the neoplastic cells, cases can be subclassified into several cHL subtypes: the nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted types.25
The diagnosis of cHL is made based on a combination of morphology of HRS cells and the other cells infiltrating the tissue, combined with immunohistochemical staining. Because of the rare nature of the malignant (clonal) cell in Hodgkin lymphoma specimens, flow cytometry is generally of little value. The HRS cells in cHL are CD30-positive and CD45 negative in virtually all cases, and CD15-positive in 85% of cases.26 B-cell antigens are typically negative except for CD20, which is positive in about 20% cases.27
Nodular sclerosis Hodgkin lymphoma (NSHL) is the most common subtype of cHL, accounting for 65% to 75% of cases. It is common among young adults and tends to involve the mediastinal, supraclavicular, and cervical lymph nodes. NSHL is characterized by the presence of collagen bands that divide the lymphoid tissue into circumscribed nodules. This subtype usually presents as stage I or II disease, typically with neck and/or mediastinal disease, and evidence of EBV infection is present in approximately 10% to 40% of North American cases.7 Patients diagnosed with NSHL generally have a very good prognosis.
Mixed cellularity Hodgkin lymphoma (MCHL) constitutes about 20% to 25% of cHL cases. It affects a somewhat older population, with a median age at diagnosis of 38 years. The typical bimodal age distribution is not seen with MCHL. MCHL has a male predominance (70%), and is more frequent in HIV-infected patients (70% of whom also have EBV infection). Lymphoid tissues have classic HRS cells and significant inflammatory infiltrates. Approximately 50% of patients with MCHL present as stage III or IV with abdominal lymphadenopathy or splenic involvement, and B symptoms are frequent.24
Lymphocyte-rich Hodgkin lymphoma (LRHL) is uncommon, accounting for only 3% to 5% of cases of cHL.28 The disease usually presents at an older age and has a 2:1 male predominance. HRS cells are commonly seen and a large number of reactive lymphocytes are also present. Although on the basis of morphology and immunohistochemistry LRHL belongs to the cHL group, clinically it more closely resembles LPHL. Patients usually present at early stage and rarely have B symptoms. LRHL carries an excellent prognosis, with a greater than 90% PFS after 5 years.23,29
Lymphocyte-depleted Hodgkin lymphoma (LDHL) is the least common form of cHL, accounting for less than 5% of cases. Many cases previously placed in this category are now recognized as diffuse large B-cell lymphoma (DLBCL), anaplastic large-cell lymphoma (ALCL), or NSHL with lymphocyte depletion.30 HRS cells are frequently seen, but reactive inflammatory cells are relatively sparse. EBV infection is seen in up to 90% of cases, commonly associated with HIV-infected individuals. Advanced-stage and symptomatic disease are more common. Prognosis is slightly worse compared to other categories.
NLPHL accounts for approximately 5% of cases of Hodgkin lymphoma. It has a unimodal age distribution, with the peak incidence in the fourth decade, and male predilection of 3:1.28 NLPHL is characterized by large primary lymphoid follicles, with polytypic small B lymphocytes and extensive meshworks of follicular dendritic cells. The lymphocytic/histiocytic (L and H), or “popcorn,” cells scattered within the nodules differ from classic HRS cells, both in their morphology and in their biochemical profile, being frequently negative for CD15, CD30 and for the EBV genome, and usually positive for B-cell antigens such as CD20, suggesting that L and H cells may be immunoglobulin-synthesizing monoclonal B cells. CD45 is also typically positive in NLPHL, in distinction from cHL. NLPHL has an indolent course compared to cHL, and long-term survival is common.19,31 NLPHL commonly presents with limited-stage disease. NLPHL may eventually transform into a more aggressive lymphoma, such as diffuse large B-cell lymphoma (including centroblastic, immunoblastic, or T-cell/histiocyte–rich subtypes), at a rate of 4% to 12%. This can occur even 15 to 20 years after the initial diagnosis of NLPHL.32,33 In a recent large retrospective study of 222 patients with NLPHL, the rate of transformation to DLBCL was 7.6%, with a median time to transformation of 35 months. Overall survival was not adversely affected in patients undergoing transformation compared to those without transformation.34
PRESENTATION
Classical Hodgkin lymphoma usually presents with asymptomatic mediastinal or cervical lymphadenopathy. Half of patients present with stage I or stage II disease.35 A mediastinal mass is seen in most patients with NSHL, at times with bulky disease, with “bulky” defined as a mediastinal mass measuring one-third or more of the maximal thoracic diameter on chest x-ray, or 10 cm on computed tomography (CT) scan. Systemic symptoms, or "B" symptoms—fevers (> 38°C), drenching night sweats, and unexplained weight loss (> 10% of baseline body weight over the preceding 6 months or less)—are detected in approximately 25% of patients. Between 10% and 15% will have extranodal disease, most commonly involving lung, bone, and liver. NLPHL usually presents with limited-stage disease without B symptoms; it typically has a more indolent presentation and clinical course than cHL.
INITIAL EVALUATION AND STAGING
The initial workup includes a complete blood count (CBC), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), and chemistry studies to evaluate renal function and liver function. Fine-needle aspiration will usually fail to identify the infrequent HRS cells, and instead only reveal the reactive background of inflammatory cells. Generous (large gauge) core needle biopsies may provide diagnosis effectively in some cases, but in general, an excisional lymph node biopsy is preferred to ensure an accurate diagnosis and avoid the need for repeated biopsy procedures. In cases where an excisional biopsy would be difficult or risky, a core needle biopsy procedure is a reasonable first step, with the understanding that a subsequent surgical procedure may still be necessary.
Baseline imaging includes CT scans of the neck, chest, abdomen, and pelvis. Use of positron emission tomography (PET) scanning is now standard in the initial evaluation and assessment of treatment response in Hodgkin lymphoma.36 Due to the increased sensitivity of PET or PET/CT scan, additional lesions may be identified that were not seen on conventional CT scans. This will alter the staging, and potentially the treatment plan, in up to 25% to 30% of patients.37,38 PET/CT scan performed during initial evaluation also facilitates optimal interpretation of post-therapy PET/CT scans and is therefore strongly encouraged as a part of the initial staging evaluation.39
Recent studies have shown that bone marrow biopsy is not routinely needed in the initial staging of cHL. A study of 454 patients concluded that bone marrow biopsy would not have altered the stage in any stage I or II patients. It was further concluded that overall treatment strategy would not have been altered for any of the patients.40 Based on this study and others, it is now clear that FDG-PET has a high sensitivity, and when PET scan is negative (in the bone marrow and skeleton), a bone marrow biopsy provides little additional value. For patients with significant cytopenias, a bone marrow biopsy is reasonable. Such patients may benefit from a bilateral biopsy, which increases the probability of demonstrating bone marrow involvement by 16% to 33%.41,42 Techniques such as staging laparotomy and lymphangiography are now considered obsolete.
Hodgkin lymphoma is staged according to the Ann Arbor staging system (Table 1). The original Ann Arbor staging was published in 1971,43 and in 1989 the “Cotswold modifications” extended the definitions of stage IV disease and the suffix “X” was added to denote bulky disease.44 Both systems were developed for the staging of Hodgkin lymphoma, but are now used for staging non-Hodgkin lymphoma as well.

PROGNOSTIC FACTORS
For the purposes of prognosis and selection of treatment, Hodgkin lymphoma is commonly classified into early-stage favorable, early-stage unfavorable, and advanced stage. Early-stage Hodgkin lymphoma refers to patients with Ann Arbor stage I or stage II disease. With early-stage Hodgkin lymphoma, the prognosis varies significantly based on factors such as the presence of B symptoms, elevated erythrocyte sedimentation rate ([ESR] > 50 mm/hr), number of nodal sites involved, older age, and a large mediastinal mass. For this reason, most clinical trials to evaluate treatment strategies for early-stage Hodgkin lymphoma are based on various combinations of these risk factors. The definition of early-stage unfavorable Hodgkin lymphoma varies across different clinical trial study groups, and it is important to understand the definition in interpreting the results of these trials (Table 2).45,46

In the German Hodgkin Study Group (GHSG) trials, early-stage Hodgkin lymphoma is stratified into a high risk (“unfavorable”) group defined by any of the following: a large mediastinal mass (one third of maximum thoracic diameter), extra-nodal disease, 3 or more nodal areas, and an ESR of > 50 mm/hr in asymptomatic patients or > 30 mm/hr in patients with B symptoms. Low-risk (“favorable”) patients lack all of these factors.47 The European Organization for Research and Treatment of Cancer (EORTC) defines the unfavorable prognostic group as older than 50 years of age, large mediastinal adenopathy (maximum width on a chest radiograph of at least one third of the internal transverse diameter of the thorax at the level of T5 through T6 or any mass of ≥ 10 cm in the largest dimension), an ESR of 50 mm/hr and no B symptoms, or with an ESR of 30 mm/hr in those who have B symptoms, and/or 4 or more regions of involvement.48 The National Cancer Institute of Canada (NCIC) Clinical Trials Group and the Eastern Cooperative Oncology Group (ECOG) define high-risk groups as presence of B symptoms, bulky disease with a mediastinal mass width of at least one third of the maximum chest wall diameter, or any mass greater than 10 cm, and patients with intra-abdominal disease.49,50
Gene-expression profiling in Hodgkin lymphoma has identified a gene signature of tumor-associated macrophages that was able to identify patients with a higher risk for primary treatment failure. In an independent cohort of patients, an increased number of CD68-positive macrophages was correlated with inferior outcomes.51,52 Studies such as these underscore the importance of the tumor “microenvironment” (ie, the nonmalignant cells within a tumor) in determining the overall clinical behavior of a malignancy. While quantification of CD68-positive macrophages has potential to be applied to routine clinical practice, prospective data using CD68 as a tool for risk-adapted therapy is lacking.
Genetic alterations and amplifications in the 9p24.1 locus have recently been found to be a defining genetic feature of cHL. Amplification of 9p24.1 has been associated with unfavorable outcomes. Amplification of 9p24.1 (which includes the loci encoding the PD-L1 and PD-L2 genes) is more common in patients with advanced stage disease and is associated with shorter PFS.23
A recent study attempted to integrate several different prognostic factors in cHL patients who were treated with ABVD (adriamycin [doxorubicin], bleomycin, vinblastine, and dacarbazine) and underwent an interim PET (iPET) scan after 2 cycles of ABVD. Focusing on those with a negative iPET scan, it was found that expression of CD68 and PD-1 in microenvironment cells, and STAT1 negativity in HRS cells identified a subset of PET-2 negative patients with a 3-year PFS significantly lower than that of the remaining PET-2 negative population (64% versus 95%). The algorithm correctly predicted the response to treatment in more than half of the patients who had relapse or disease progression despite a negative PET-2 scan. It therefore appears feasible, using tissue biomarkers at diagnosis, to identify patients at increased risk for poor outcome, even if the iPET scan is negative.53
ROLE OF PET/CT IN ASSESSMENT OF RESPONSE TO THERAPY
PET/CT has been increasingly used for response assessment at various stages in lymphoma in recent years. Almost all types of lymphomas are fluorodeoxyglucose (FDG) avid; however, Hodgkin lymphoma is FDG avid in 97% to 100% of cases. In 2009, a 5-point scale was developed to score PET images with regard to treatment response, either partway through treatment (iPET) or at the end of therapy.54 It was recommended as the standard reporting tool at the First International Workshop on PET in Lymphoma in Deauville, France, in 2009, and is thus now referred to as the Deauville score. A score of 1 is given if there is no uptake, 2 if the uptake ≤ mediastinum, 3 if > mediastinum but ≤ liver, 4 if uptake moderately higher than liver, 5 if uptake is markedly higher than liver and/or new lesions. X designates new areas of uptake unlikely to be related to lymphoma. In most trials, a score of 1 or 2 is considered a complete response and a score of 4 or 5 is considered a treatment failure. A score of 3 is sometimes considered a complete response, depending on the study. The Deauville criteria have been widely used in newer clinical trials utilizing response-adapted treatment as defined by PET response. PET/CT is recommended for staging and restaging at the end of therapy, in clinical practice, and clinical trials. Interim PET/CT scan, while commonly performed in clinical practice, is only recommended if the results will alter therapy (eg, if that information will result in the clinician omitting radiation therapy [RT] or altering the chemotherapy plan).
Early studies of iPET showed that achieving PET negativity early in the course of treatment was strongly associated with PFS and overall survival.55 Subsequent studies confirmed the importance of achieving a negative iPET. As a result, considerable efforts have been put into designing response-adapted treatment approaches using iPET (see Treatment section), with some of these approaches now being listed in the National Comprehensive Cancer Network (NCCN) guidelines and being used in standard practice.
TREATMENT
EVOLUTION OF TREATMENT
The treatment of Hodgkin lymphoma has evolved over the past century, starting with the discovery of RT as effective treatment in the early 20th century. Long-term survival of patients with Hodgkin lymphoma treated with involved-field radiation therapy (IFRT) was first reported in the 1960s.56,57 Outcomes improved further with the introduction of combined modality treatment (CMT) using chemotherapy and RT, with the overall 5-year relative survival for patients with Hodgkin lymphoma (all stages) treated in 2006–2012 being 85.4% to 87.3%.3 Since the majority of patients are now cured with modern therapy, treatment-related complications have become an important competing cause of mortality. Recent studies have therefore focused on maintaining efficacy while reducing toxicities, and refining the process of selecting patients who might benefit from more aggressive therapy. While RT was the first treatment modality shown to be curative for Hodgkin lymphoma,56,57 multiple subsequent studies showed that CMT is superior to RT alone in terms of relapse-free survival.58–63 In the GHSG H8-F trial, the estimated 5-year event-free survival and overall survival rates were significantly higher after 3 cycles of MOPP-ABV (mechlorethamine, vincristine, procarbazine, and prednisone combined with doxorubicin, bleomycin, and vinblastine) plus IFRT than after subtotal nodal radiotherapy alone. The 10-year overall survival estimates were 97% and 92%, respectively (P = 0.001).64 As a result, CMT replaced RT alone as the standard of care for limited-stage Hodgkin lymphoma. However, for elderly or infirm patients, or those with other comorbidities making them poor chemotherapy candidates, RT alone may be a very reasonable option.65 More recently, an increasing body of evidence has accumulated to support the use of chemotherapy alone in early stage cHL. This literature has consistently shown that omission of RT is associated with a modest increase in relapse, without a clear compromise in long-term overall survival. For some patients, the trade-off in terms of avoiding radiation (and the associated late effects) may be worth the small increase in relapse risk, since long-term survival does not appear to be substantially worse with chemo-therapy alone. Table 3 and Table 4 provide a summary of recent key studies which have defined treatment options for early-stage cHL.48,66–71


EARLY-STAGE NLPHL
NLPHL usually presents with limited-stage disease without B symptoms and has an indolent course with a slightly better prognosis compared to cHL.72 Due to the rarity of the disease, treatment guidelines are mostly based on retrospective analyses from single or multi-institution studies or subgroup analyses, often with relatively short follow-up. These studies must be interpreted with caution because of the possibility of inaccuracies in the pathologic diagnosis, small sample sizes, and selection bias. Treatment options for limited-stage NLPHL include observation, single-agent rituximab, IFRT (or involved-site radiation therapy [ISRT]) alone, or CMT.46
Historically, patients with limited-stage NLPHL have been treated with RT alone, with 80% to 85% PFS and 85% to 95% overall survival rates.73–75 Patients who relapse or progress after RT in general can successfully undergo salvage therapy.74 In one study, rates of PFS and overall survival were similar among patients who had limited-field, regional-field, or extended-field RT,75 indicating that IFRT is preferred. Studies comparing RT alone and CMT are limited. The GHSG HD7 trial included a subset of NLPHL patients, with a trend towards improved freedom from treatment failure (96% versus 83%) favoring CMT. This, however, did not translate into improved overall survival.47 A retrospective analysis of the British Columbia Cancer Agency database compared patients with limited-stage NLPHL treated with RT alone to patients who received 2 cycles of ABVD followed by RT. A significant improvement in PFS (91% versus 65%) and overall survival (93% versus 84%) was seen, favoring CMT.76
Chemotherapy alone is not recommended for limited-stage NLPHL since studies evaluating chemotherapy alone are quite limited and indicate relatively high rates of treatment failure. Given that the malignant cells in NLPHL are CD20-positive, single-agent rituximab has also been studied in this disease, including a study as frontline therapy in limited-stage patients. In this phase 2 trial in newly diagnosed patients with stage IA disease, an overall response rate (ORR) of 100% was seen, with an 85% complete response (CR) rate.77 At 3 years, overall survival was 100% and PFS was 81%, indicating that the responses with single-agent rituximab are less durable than those with RT.
Advani et al evaluated rituximab followed by observation versus rituximab (R) followed by maintenance rituximab (MR) for 2 years in 39 new or previously treated patients. At 4 weeks the ORR was 100% (with CR in 67%, and partial response in 33%). At a median follow up of 9.8 years for R alone, and 5 years for R+MR, median PFS was 3 and 5.6 years, respectively (P = 0.26). Estimated 5-yr PFS and overall survival in patients treated with R versus R+MR were 39.1% and 95.7% versus 58.9% and 85.7%, with Pvalues of 0.26 (PFS) and 0.38 (overall), respectively. Maintenance rituximab therefore appears to prolong remission, although the results did not quite reach statistical significance.78 Even though rituximab does not appear to be curative in NLPHL, it is a reasonable option for patients with early-stage NLPHL who are not good candidates for definitive RT. Whether combining rituximab with RT or CMT might further improve outcomes in early-stage NLPHL has not yet been determined.
In children, surgery alone may lead to long-term remission or possibly cure of limited-stage NLPHL. In a European multicenter retrospective study, 58 patients underwent surgery for limited-stage NLPHL. Among the 51 patients who achieved complete remission following surgery, 67% remained progression-free and 100% were alive at a median follow up of 43 months.79 In adults, there is no data to support surgical treatment alone for NLPHL. Finally, observation may be a reasonable option in elderly or infirm patients for whom NLPHL is unlikely to affect life expectancy. For younger patients, given the excellent outcome with modern therapy and the long-term risk of transformation of NLPHL into an aggressive non-Hodgkin lymphoma, observation is generally not recommended.
The NCCN recommends RT (ISRT or IFRT, 30–36 Gy) as the preferred treatment for stage IA and IIA non-bulky NLPHL. In patients with stage IA disease with complete excision of solitary nodule, observation may be appropriate. A course of chemotherapy with ISRT with or without rituximab is recommended for patients with stage IB or IIB disease, or patients with stage IA or IIA bulky disease.
FIRST-LINE TREATMENT OF LIMITED-STAGE CHL
Early-Stage Favorable cHL
There is lack of consensus regarding the ideal treatment approach for patients with early-stage favorable cHL. However, there are several excellent options available, with overall survival rates exceeding 90%. Most of these regimens involve CMT, although some chemotherapy-alone approaches have been evaluated as well. Concurrent with the demonstration of excellent long-term remission rates with CMT, it became apparent that the long-term survival and quality of life of these patients is determined in large part by the risk of serious (and potentially fatal) treatment-related toxicities. Such toxicities consist primarily of secondary malignancies and cardiovascular events, and can continue to cause significant morbidity and mortality even 2 to 3 decades after treatment.80–82 As a result, treatment decisions for these patients are complicated and require balancing efficacy against risk of late complications.
In the United States, until recently, CMT was generally considered the standard of care, with robust long-term data regarding efficacy. The most commonly used regimen has been ABVD for 2 to 4 cycles followed by IFRT. In some German studies, escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) has been used, but this is not a general standard of care in the United States for early-stage patients.
More recent data suggests that the rate of serious late complications in Hodgkin lymphoma patients is decreasing, likely due to less extensive radiation fields, lower radiation doses, and a movement away from the MOPP regimen to ABVD.83,84 For patients who meet the “favorable” criteria set forth in the GHSG HD10 trial (see Table 2), 2 cycles of ABVD followed by 20 Gy of IFRT is an attractive option, with efficacy preserved and a low anticipated rate of late effects.66 With this approach, and with long-term (10 years) follow up, all 4 arms had similar PFS (87%) and overall survival (94%), whether 2 or 4 cycles of ABVD were given. When the effects of 20-Gy and 30-Gy doses of RT were compared, there were also no significant differences in freedom from treatment failure or overall survival. Adverse events and acute toxic effects of treatment were most common in the patients who received 4 cycles of ABVD and 30 Gy of RT.66,71
In recent years, in an attempt to reduce late effects further, regimens consisting of chemotherapy alone have been investigated. In a study by Meyer et al, at 12 years the rate of overall survival was 94% among those receiving ABVD alone, as compared with 87% among those receiving subtotal nodal RT; the rates of freedom from disease progression were 87% and 92% in the 2 groups; and the rates of event-free survival were 85% and 80%, respectively.50 However, it is important to note that this study did not include a CMT arm for the early favorable patients, and did not utilize modern RT techniques. Nevertheless, this early study and others60 suggested that chemotherapy alone may be a reasonable option for some early-stage cHL patients, particularly for patients who are felt to be at increased risk for late toxicities from RT. As a result, additional studies have been conducted evaluating CMT versus chemotherapy alone for early-stage cHL. Many of these studies have incorporated interim PET/CT scan to develop a response-adapted approach to decide which patients are least likely to benefit from RT.
The HD-13 study was a follow-up study for HD-10, looking at deletion of bleomycin, dacarbazine, or both from the ABVD backbone. The ABD arm was closed early, because of an excess rate of treatment failure. Among the 1243 patents assigned to either the ABVD or AVD arm at 5 years of follow-up, there was 4.3% difference in PFS. This study was not able to demonstrate that 2 cycles of AVD was noninferior to 2 cycles of ABVD, each followed by 30 Gy IFRT, even though there was no difference in all 4 groups. It confirmed 2 cycles of ABVD as the preferred regimen in early favorable Hodgkin lymphoma, when CMT is the plan of care. However, for patients over age 60 to 65 years, or those with underlying cardiac or pulmonary comorbidities, bleomycin has significant risk of toxicity. In that setting, AVD is a safer option, with only a very modest decrease in 5-year PFS.
Based on the observation that iPET scan is highly predictive of outcome in Hodgkin lymphoma,55,85 several trials have employed the use of an iPET scan to guide therapy. It is hoped that such studies will lead to new PET-directed treatment algorithms in which patients who require more aggressive therapy (eg, with CMT, or escalated BEACOPP) can be identified, and the remaining patients can be safely treated less aggressively (eg, with chemotherapy alone).
In the EORTC H10 trial, performed to evaluate treatment adaptation on the basis of iPET scan results in stage I and II Hodgkin lymphoma, a control arm received standard combined modality treatment (3 or 4 cycles of ABVD with INRT) irrespective of PET scan results. In the experimental arm, patients with a negative PET scan after 2 cycles of ABVD continued with 1 or 2 cycles of ABVD and did not receive RT. The iPET-positive patients received either standard treatment with ABVD plus INRT or escalated BEACOPP plus INRT. The iPET-negative patients received either ABVD only or ABVD plus INRT. The final results of this study, published recently, showed that in the iPET-positive patients the 5-year PFS was improved from 77.4% with standard ABVD plus INRT to 90.6% with escalated BEACOPP plus INRT (P = 0.002). In iPET-negative patients, 5-year PFS in the favorable group was 99% versus 87.1% in favor of ABVD plus INRT. The H10 study suggested that PET results after 2 cycles of ABVD can be integrated into treatment planning, In iPET-negative patients, the study was technically not able to demonstrate the noninferiority of the ABVD only regimen, owing to a higher risk of relapse if INRT is omitted, particularly in the favorable group.48 However, this study does show that excellent outcomes can be obtained with omission of RT in patients with a negative iPET scan. This study provides a cautionary lesson though, in that the increase in relapse rate associated with omission of RT was more substantial (12%) for favorable versus unfavorable early-stage patients (2.5%), and this difference was only apparent after longer (5 years) follow-up. Despite this, chemotherapy alone is considered a reasonable treatment option, especially for patients felt to be at increased risk for late toxicities of RT or for patients who wish to avoid the risks of RT, with over 99% of patients alive at 5 years.
Similar results were shown in the RAPID trial, in which patients with limited-stage cHL underwent 3 cycles of ABVD followed by PET assessment.67 Patients with a negative PET (n = 426) were then randomized to RT (n = 209) versus no further therapy (n = 211). At a median of 60 months of follow-up, 3-year PFS was 94.6% in the RT group and 90.8% in the chemotherapy alone group. Similar to the H10 trial, it was concluded that chemotherapy alone was statistically inferior to CMT in terms of PFS. However, also similar to the H10 trial, the RAPID trial demonstrated that excellent results can be obtained in early-stage cHL patients with omission of RT, if iPET scan is negative after 3 cycles of ABVD, as there was no survival difference. These findings indicate that, when relapses occur as a result of omission of RT, such patients can be effectively treated later.
In the ongoing GHSG HD16 trial, patients with early-stage favorable cHL will be randomly assigned to a standard approach (ABVD × 2 cycles followed by 20-Gy IFRT) versus an experimental approach in which they receive ABVD for 2 cycles and then undergo PET scan. If the PET remains positive, they will receive 20-Gy IFRT. If the PET is negative, they will receive no further therapy. This trial could ultimately define ABVD for 2 cycles as a treatment option.
It is clear from these studies that omission of RT results in a somewhat higher rate of relapse but can be considered in selected patients. However, taking a less aggressive frontline approach may also be justified by the fact that, for those who do relapse, successful salvage therapies are available. Aggressive salvage therapy with autologous stem cell transplantation historically can cure approximately 50% of relapsed patients. With new and emerging therapies for relapsed disease, such as brentuximab vedotin and the PD-1 inhibitors (eg, nivolumab and pembrolizumab), the ability to cure relapsed patients may improve even more, further calling into question the practice of applying CMT uniformly for early-stage patients undergoing first-line therapy. Unfortunately, there is insufficient data from large randomized studies with long-term follow-up to fully address this issue currently, and there remains some controversy around this issue. NCCN recommends restaging PET/CT after 3 cycles of ABVD if a chemotherapy alone treatment modality is chosen. If the Deauville score is 1 or 2, either observation or 1 additional cycle of ABVD is recommended.46
Early-Stage Unfavorable cHL
In the United States, historically early-stage unfavorable Hodgkin lymphoma has been treated with CMT, most commonly 4 to 6 cycles of ABVD followed by consolidative RT. With this approach one can expect a 5-year PFS of approximately 80% to 85%.58,64,86 The GHSG HD8 trial showed that RT volume size reduction from extended-field to involved-field after COPP + ABVD chemotherapy for 2 cycles produced similar results and less toxicity in patients with early-stage unfavorable cHL.86 The GHSG trial HD11 established ABVD for 4 cycles plus 30-Gy IFRT as a standard for early unfavorable Hodgkin lymphoma. The freedom from treatment failure at 5 years was 85.0%, and overall survival was 94.5%.68
In the HD14 study by the GHSG, patients with early unfavorable cHL were treated with 2 cycles of escalated BEACOPP followed by 2 cycles of ABVD, versus 4 cycles of ABVD. All patients then received 30 Gy of consolidative IFRT. A 5-year PFS of 95% was seen in the experimental arm, compared with 89% in the standard (ABVD) arm. As expected, this regimen was associated with more acute hematologic toxicity, and there was no difference between the 2 regimens with respect to overall survival or fertility.69 Given the lack of improved survival and increased toxicity, ABVD has remained the standard chemotherapy regimen for early unfavorable cHL in the United States. NCCN recommends a restaging PET scan after 2 cycles of ABVD and to continue with 2 to 4 cycles of ABVD or escalated BEACOPP with or without ISRT based on Deauville scores.
Another viable treatment option is the Stanford V regimen, a condensed, 12-week regimen that includes mechlorethamine, doxorubicin, vinblastine, prednisone, vincristine, etoposide, and bleomycin, followed by IFRT.87 In a randomized phase 3 trial conducted by ECOG (E2496), patients with stage I/II Hodgkin lymphoma with bulky mediastinal disease or advanced-stage disease were randomized to ABVD × 6 to 8 cycles versus Stanford V. RT was given (36 Gy) for those with bulky mediastinal disease or to sites of disease greater than 5 cm in the Stanford V arm. In a subset analysis focusing only on those with stage I/II bulky mediastinal disease, the 5-year failure free survival was 85% versus 79% and the 5-year overall survival was 96% versus 92% for the ABVD versus Stanford V arms, respectively. These differences were not statistically significant.70 While the Stanford V regimen has the advantages of a 12-week treatment duration and a lower cumulative amount of bleomycin and doxorubicin, the Stanford V arm had higher rates of grade 3 lymphopenia and grade 3 to 4 peripheral neuropathies. In addition, Stanford V requires that most patients undergo RT (to original sites of disease measuring 5 cm or more plus contiguous areas). As a result, the investigators concluded that ABVD × 4 cycles plus IFRT remains the standard of care for patients with early unfavorable Hodgkin lymphoma with bulky mediastinal disease.
Other regimens have been studied in hopes of reducing toxicity, including the EVE regimen (epirubicin, vinblastine, and etoposide). This regimen was compared to ABVD in early unfavorable Hodgkin lymphoma patients, with all patients undergoing the same RT program. No differences were observed between the ABVD and EVE arms in terms of complete remission rate and overall survival. However, patients who received EVE had a significantly worse outcome than those who received ABVD in terms of relapse-free survival and failure-free survival.88 EBVP (epirubicin, bleomycin, vinblastine, and prednisone) followed by IFRT was less efficacious compared with MOPP/ABV–type therapy.58
An area of active investigation is whether RT can be safely omitted in patients with early- stage unfavorable cHL. The EORTC H10 study showed that, for patients with a negative iPET scan (after 2 cycles), the 5-year PFS rates were 92.1% versus 89.6% for ABVD plus INRT versus ABVD alone, respectively. While this technically did not meet criteria for noninferiority of ABVD alone, this study demonstrated that, for those with negative iPET, ABVD × 6 cycles (without radiation) can result in long-term remission in a high proportion (89%) of patients. For iPET-positive patients, 2 cycles of escalated BEACOPP were given followed by 30 Gy of IFRT on the experimental arm. This resulted in a 5-year PFS of 90.6% versus 77.4%, suggesting this may be a preferred approach for early-stage unfavorable patients with a positive iPET.48 Even though the noninferiority of ABVD alone could not be established based on the statistical design of the study, the current NCCN guidelines recommend restaging after 2 cycles of ABVD for stage I or II unfavorable cHL and using that iPET as a guide, based on Deauville scores. For scores 1–3, ABVD × 2 cycles (total 4 cycles) plus ISRT or AVD × 4 (total 6) with or without ISRT is recommended. For a Deauville score of 4, escalated BEACOPP × 2 cycles or ABVD × 2 cycles (total 4) followed by ISRT is recommended. If the Deauville score is 5, further treatment decisions should be made based on repeat biopsy results. A follow up PET/CT is recommended for Deauville scores of 4 and 5 to confirm complete response.46
LATE EFFECTS AND THE EVOLUTION OF RADIATION THERAPY
The RT given in Hodgkin lymphoma has evolved considerably over the years, from extended field or subtotal nodal fields developed in the 1960s, to the more focused involved-field or even involved-site radiation commonly given now. This approach reduces radiation volumes, and it already is becoming evident that the relative risk of breast cancer among young females receiving mediastinal RT for Hodgkin lymphoma is declining.89 Cardiac dose is reduced significantly with IFRT compared to older radiation techniques as well. The extent of radiation may be reduced even further with involved-nodal/involved site or intensity-modulated approaches.90
With new RT techniques allowing for more focused therapy and lower doses of radiation, models predict that the rate of long-term complications will decline further.91,92 Furthermore, response-adapted (ie, PET-directed) approaches, as discussed in detail earlier in the article, are expected to increasingly allow for identification of patients who can safely avoid radiation entirely, which will hopefully lead to an even lower rate of late complications of therapy.
MONITORING FOR RELAPSE
A number of recent studies have shown that, for patients who achieve complete remission with first-line therapy, performing repeated scheduled surveillance imaging does not improve outcomes. In fact, most relapses are detected by the patient (due to symptoms or recurrence of lymph node enlargement). It is rare that a relapse would be detected by surveillance imaging alone. Furthermore, surveillance that includes routine imaging has not been associated with improved survival.93 As a result, it is now recommended that patients undergo regular follow-up with symptom review, physical exam, and basic laboratory studies. Imaging studies should be obtained as needed for patients who develop signs, symptoms, exam findings, or laboratory values concerning for relapse.
More important than scheduled surveillance imaging for relapse is monitoring for late effects of therapy. These fall into several broad categories such as cardiovascular disease (coronary disease, congestive heart failure, valvular disease, carotid artery disease), pulmonary disease, hypothyroidism, and secondary malignancies. Aggressive surveillance for breast cancer is especially warranted in female patients who underwent chest radiation.46
CONCLUSION
Hodgkin lymphoma is characterized pathologically by the presence of HRS cells accompanied by a polymorphous cellular infiltrate. It is a disease with a bimodal age distribution, several pathologic subtypes, and numerous treatment options. Overall, the prognosis for patients with early-stage disease is excellent, and although a majority of patients can now be cured, further studies are needed to optimize treatment such that short- and long-term treatment-related toxicities are minimized, without compromising disease control and cure.
INTRODUCTION
Hodgkin lymphoma, previously known as Hodgkin’s disease, is a B-cell malignancy with unique pathological and epidemiological features for which highly effective therapies exist. The disease is characterized by the presence of mononuclear and multinucleate giant cells called Hodgkin and Reed-Sternberg (HRS) cells.1
Hodgkin lymphoma is unique compared to other B-cell lymphomas because of the scarcity of the malignant cells in the tumor tissue. The HRS cells usually account for only 0.1% to 10% of the cells in the affected tissues, and the HRS cells induce accumulation of nonmalignant lymphocytes, macrophages, granulocytes, eosinophils, plasma cells, and histiocytes, which constitute more than 90% of tumor cellularity.2 Although the disease was first described by Sir Thomas Hodgkin in 1832, in part because of this unique histopathology, not until 1991 was it conclusively demonstrated that HRS cells are in fact monoclonal germinal center–derived B-cells. This article reviews management and frontline treatment options for limited-stage classical Hodgkin lymphoma and nodular lymphocyte predominant Hodgkin lymphoma. Treatment of advanced stage and relapsed/refractory Hodgkin lymphoma will be discussed in a separate article.
EPIDEMIOLOGY
Hodgkin lymphoma accounts for 0.5% of all malignancies and 11.7% of all lymphomas among adults in the United States.3 The incidence of Hodgkin lymphoma has been steadily increasing over the past 4 decades and was estimated to be 8260 cases in the United States in 2017, with a slight male predominance. Hodgkin lymphoma is expected to cause 1070 deaths in 2017, accounting for 0.2% of all cancer deaths.3 First-degree relatives of patients with Hodgkin lymphoma have a 3- to 9-fold increased risk of having the disease compared to the general population,4 and monozygotic twin siblings of Hodgkin lymphoma patients have a greatly increased risk for developing the disease—up to 100-fold—compared to normal cohorts.5 The incidence is highest among Caucasians, African Americans, and Hispanics, and lower in Asians and American Indians.3 Hodgkin lymphoma incidence shows a bimodal peak distribution, with 1 peak between the ages of 15 and 44 years, and another peak after age 65 years.6
ETIOLOGY/PATHOGENESIS
The cause of Hodgkin lymphoma is unknown. Epstein-Barr virus (EBV) infection is present in up to 40% of Hodgkin lymphoma cases, suggesting a role of this virus in the pathogenesis of some cases. The risk of EBV-positive Hodgkin lymphoma was found to be higher following an episode of infectious mononucleosis, while the risk of EBV-negative Hodgkin lymphoma remained unchanged.7 The incidence of Hodgkin lymphoma is 5 to 14 times higher in HIV-infected patients than in noninfected patients.8 It is not considered an AIDS-defining illness, but has become more frequent with the growth and aging of the HIV-positive population.9,10 Hodgkin lymphoma patients with HIV typically have CD4 lymphocyte counts greater than 200 cells/μL,11 with the incidence of Hodgkin lymphoma actually declining with lower CD4 lymphocyte counts.12 HIV-related Hodgkin lymphoma tends to have an aggressive course, with high rates of EBV positivity.13 The incidence of Hodgkin lymphoma is 1.8 times higher among smokers, and the risk appears to increase with duration of smoking.14,15
The cell of origin of Hodgkin lymphoma, while long suspected to be the HRS cell, remained unproven until the 1990s when micro-dissection and single-cell polymerase chain reaction techniques allowed for confirmation that the HRS cell was in fact a monoclonal germinal center derived B cell.16,17 These HRS cells lack immunoglobulin due to defective transcription regulation and not due to crippling mutations.18,19 The cellular infiltrate in Hodgkin lymphoma appears to play a decisive role in allowing the HRS cells to survive by providing an environment that suppresses cytotoxic immune responses as well as by providing cellular interactions and cytokines that support their growth and survival. The extensive inflammatory infiltrate in classical Hodgkin lymphoma is comprised of T helper 2 (Th2) and regulatory T cells and lacks T helper 1 (Th1) cells, CD8 cytotoxic T cells, and natural killer cells.20 The HRS cells escape apoptosis by several mechanisms which include latent EBV infection and constitutive nuclear factor (NF)-kB pathways, as well as other deregulated signaling pathways that promote survival, such as EBV nuclear antigen 1 (EBNA1) protein, EBV latent infection membrane protein 1 (LMP1), and LMP2.21,22
Genetic alterations in the 9p24 locus which encodes PD-L1/PD-L2 are nearly universally present in classical Hodgkin lymphoma and are now considered a disease-defining feature.23
PATHOLOGIC CLASSIFICATION
According to the 2008 World Health Organization (WHO) classification, Hodgkin lymphoma has 2 clearly defined entities: classical Hodgkin lymphoma (cHL), which accounts for approximately 95% cases, and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), which accounts for the remaining cases.24 These 2 entities differ in their clinical, pathological, and biological features, which in turn affect prognosis and treatment options. Classical Hodgkin lymphoma is characterized by a paucity of HRS cells surrounded by a background of mixed inflammatory infiltrate comprised of histiocytes, small lymphocytes, eosinophils, neutrophils, plasma cells, fibroblasts, and collagen. Depending on the particular combinations of these elements and the specific features of the neoplastic cells, cases can be subclassified into several cHL subtypes: the nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted types.25
The diagnosis of cHL is made based on a combination of morphology of HRS cells and the other cells infiltrating the tissue, combined with immunohistochemical staining. Because of the rare nature of the malignant (clonal) cell in Hodgkin lymphoma specimens, flow cytometry is generally of little value. The HRS cells in cHL are CD30-positive and CD45 negative in virtually all cases, and CD15-positive in 85% of cases.26 B-cell antigens are typically negative except for CD20, which is positive in about 20% cases.27
Nodular sclerosis Hodgkin lymphoma (NSHL) is the most common subtype of cHL, accounting for 65% to 75% of cases. It is common among young adults and tends to involve the mediastinal, supraclavicular, and cervical lymph nodes. NSHL is characterized by the presence of collagen bands that divide the lymphoid tissue into circumscribed nodules. This subtype usually presents as stage I or II disease, typically with neck and/or mediastinal disease, and evidence of EBV infection is present in approximately 10% to 40% of North American cases.7 Patients diagnosed with NSHL generally have a very good prognosis.
Mixed cellularity Hodgkin lymphoma (MCHL) constitutes about 20% to 25% of cHL cases. It affects a somewhat older population, with a median age at diagnosis of 38 years. The typical bimodal age distribution is not seen with MCHL. MCHL has a male predominance (70%), and is more frequent in HIV-infected patients (70% of whom also have EBV infection). Lymphoid tissues have classic HRS cells and significant inflammatory infiltrates. Approximately 50% of patients with MCHL present as stage III or IV with abdominal lymphadenopathy or splenic involvement, and B symptoms are frequent.24
Lymphocyte-rich Hodgkin lymphoma (LRHL) is uncommon, accounting for only 3% to 5% of cases of cHL.28 The disease usually presents at an older age and has a 2:1 male predominance. HRS cells are commonly seen and a large number of reactive lymphocytes are also present. Although on the basis of morphology and immunohistochemistry LRHL belongs to the cHL group, clinically it more closely resembles LPHL. Patients usually present at early stage and rarely have B symptoms. LRHL carries an excellent prognosis, with a greater than 90% PFS after 5 years.23,29
Lymphocyte-depleted Hodgkin lymphoma (LDHL) is the least common form of cHL, accounting for less than 5% of cases. Many cases previously placed in this category are now recognized as diffuse large B-cell lymphoma (DLBCL), anaplastic large-cell lymphoma (ALCL), or NSHL with lymphocyte depletion.30 HRS cells are frequently seen, but reactive inflammatory cells are relatively sparse. EBV infection is seen in up to 90% of cases, commonly associated with HIV-infected individuals. Advanced-stage and symptomatic disease are more common. Prognosis is slightly worse compared to other categories.
NLPHL accounts for approximately 5% of cases of Hodgkin lymphoma. It has a unimodal age distribution, with the peak incidence in the fourth decade, and male predilection of 3:1.28 NLPHL is characterized by large primary lymphoid follicles, with polytypic small B lymphocytes and extensive meshworks of follicular dendritic cells. The lymphocytic/histiocytic (L and H), or “popcorn,” cells scattered within the nodules differ from classic HRS cells, both in their morphology and in their biochemical profile, being frequently negative for CD15, CD30 and for the EBV genome, and usually positive for B-cell antigens such as CD20, suggesting that L and H cells may be immunoglobulin-synthesizing monoclonal B cells. CD45 is also typically positive in NLPHL, in distinction from cHL. NLPHL has an indolent course compared to cHL, and long-term survival is common.19,31 NLPHL commonly presents with limited-stage disease. NLPHL may eventually transform into a more aggressive lymphoma, such as diffuse large B-cell lymphoma (including centroblastic, immunoblastic, or T-cell/histiocyte–rich subtypes), at a rate of 4% to 12%. This can occur even 15 to 20 years after the initial diagnosis of NLPHL.32,33 In a recent large retrospective study of 222 patients with NLPHL, the rate of transformation to DLBCL was 7.6%, with a median time to transformation of 35 months. Overall survival was not adversely affected in patients undergoing transformation compared to those without transformation.34
PRESENTATION
Classical Hodgkin lymphoma usually presents with asymptomatic mediastinal or cervical lymphadenopathy. Half of patients present with stage I or stage II disease.35 A mediastinal mass is seen in most patients with NSHL, at times with bulky disease, with “bulky” defined as a mediastinal mass measuring one-third or more of the maximal thoracic diameter on chest x-ray, or 10 cm on computed tomography (CT) scan. Systemic symptoms, or "B" symptoms—fevers (> 38°C), drenching night sweats, and unexplained weight loss (> 10% of baseline body weight over the preceding 6 months or less)—are detected in approximately 25% of patients. Between 10% and 15% will have extranodal disease, most commonly involving lung, bone, and liver. NLPHL usually presents with limited-stage disease without B symptoms; it typically has a more indolent presentation and clinical course than cHL.
INITIAL EVALUATION AND STAGING
The initial workup includes a complete blood count (CBC), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), and chemistry studies to evaluate renal function and liver function. Fine-needle aspiration will usually fail to identify the infrequent HRS cells, and instead only reveal the reactive background of inflammatory cells. Generous (large gauge) core needle biopsies may provide diagnosis effectively in some cases, but in general, an excisional lymph node biopsy is preferred to ensure an accurate diagnosis and avoid the need for repeated biopsy procedures. In cases where an excisional biopsy would be difficult or risky, a core needle biopsy procedure is a reasonable first step, with the understanding that a subsequent surgical procedure may still be necessary.
Baseline imaging includes CT scans of the neck, chest, abdomen, and pelvis. Use of positron emission tomography (PET) scanning is now standard in the initial evaluation and assessment of treatment response in Hodgkin lymphoma.36 Due to the increased sensitivity of PET or PET/CT scan, additional lesions may be identified that were not seen on conventional CT scans. This will alter the staging, and potentially the treatment plan, in up to 25% to 30% of patients.37,38 PET/CT scan performed during initial evaluation also facilitates optimal interpretation of post-therapy PET/CT scans and is therefore strongly encouraged as a part of the initial staging evaluation.39
Recent studies have shown that bone marrow biopsy is not routinely needed in the initial staging of cHL. A study of 454 patients concluded that bone marrow biopsy would not have altered the stage in any stage I or II patients. It was further concluded that overall treatment strategy would not have been altered for any of the patients.40 Based on this study and others, it is now clear that FDG-PET has a high sensitivity, and when PET scan is negative (in the bone marrow and skeleton), a bone marrow biopsy provides little additional value. For patients with significant cytopenias, a bone marrow biopsy is reasonable. Such patients may benefit from a bilateral biopsy, which increases the probability of demonstrating bone marrow involvement by 16% to 33%.41,42 Techniques such as staging laparotomy and lymphangiography are now considered obsolete.
Hodgkin lymphoma is staged according to the Ann Arbor staging system (Table 1). The original Ann Arbor staging was published in 1971,43 and in 1989 the “Cotswold modifications” extended the definitions of stage IV disease and the suffix “X” was added to denote bulky disease.44 Both systems were developed for the staging of Hodgkin lymphoma, but are now used for staging non-Hodgkin lymphoma as well.

PROGNOSTIC FACTORS
For the purposes of prognosis and selection of treatment, Hodgkin lymphoma is commonly classified into early-stage favorable, early-stage unfavorable, and advanced stage. Early-stage Hodgkin lymphoma refers to patients with Ann Arbor stage I or stage II disease. With early-stage Hodgkin lymphoma, the prognosis varies significantly based on factors such as the presence of B symptoms, elevated erythrocyte sedimentation rate ([ESR] > 50 mm/hr), number of nodal sites involved, older age, and a large mediastinal mass. For this reason, most clinical trials to evaluate treatment strategies for early-stage Hodgkin lymphoma are based on various combinations of these risk factors. The definition of early-stage unfavorable Hodgkin lymphoma varies across different clinical trial study groups, and it is important to understand the definition in interpreting the results of these trials (Table 2).45,46

In the German Hodgkin Study Group (GHSG) trials, early-stage Hodgkin lymphoma is stratified into a high risk (“unfavorable”) group defined by any of the following: a large mediastinal mass (one third of maximum thoracic diameter), extra-nodal disease, 3 or more nodal areas, and an ESR of > 50 mm/hr in asymptomatic patients or > 30 mm/hr in patients with B symptoms. Low-risk (“favorable”) patients lack all of these factors.47 The European Organization for Research and Treatment of Cancer (EORTC) defines the unfavorable prognostic group as older than 50 years of age, large mediastinal adenopathy (maximum width on a chest radiograph of at least one third of the internal transverse diameter of the thorax at the level of T5 through T6 or any mass of ≥ 10 cm in the largest dimension), an ESR of 50 mm/hr and no B symptoms, or with an ESR of 30 mm/hr in those who have B symptoms, and/or 4 or more regions of involvement.48 The National Cancer Institute of Canada (NCIC) Clinical Trials Group and the Eastern Cooperative Oncology Group (ECOG) define high-risk groups as presence of B symptoms, bulky disease with a mediastinal mass width of at least one third of the maximum chest wall diameter, or any mass greater than 10 cm, and patients with intra-abdominal disease.49,50
Gene-expression profiling in Hodgkin lymphoma has identified a gene signature of tumor-associated macrophages that was able to identify patients with a higher risk for primary treatment failure. In an independent cohort of patients, an increased number of CD68-positive macrophages was correlated with inferior outcomes.51,52 Studies such as these underscore the importance of the tumor “microenvironment” (ie, the nonmalignant cells within a tumor) in determining the overall clinical behavior of a malignancy. While quantification of CD68-positive macrophages has potential to be applied to routine clinical practice, prospective data using CD68 as a tool for risk-adapted therapy is lacking.
Genetic alterations and amplifications in the 9p24.1 locus have recently been found to be a defining genetic feature of cHL. Amplification of 9p24.1 has been associated with unfavorable outcomes. Amplification of 9p24.1 (which includes the loci encoding the PD-L1 and PD-L2 genes) is more common in patients with advanced stage disease and is associated with shorter PFS.23
A recent study attempted to integrate several different prognostic factors in cHL patients who were treated with ABVD (adriamycin [doxorubicin], bleomycin, vinblastine, and dacarbazine) and underwent an interim PET (iPET) scan after 2 cycles of ABVD. Focusing on those with a negative iPET scan, it was found that expression of CD68 and PD-1 in microenvironment cells, and STAT1 negativity in HRS cells identified a subset of PET-2 negative patients with a 3-year PFS significantly lower than that of the remaining PET-2 negative population (64% versus 95%). The algorithm correctly predicted the response to treatment in more than half of the patients who had relapse or disease progression despite a negative PET-2 scan. It therefore appears feasible, using tissue biomarkers at diagnosis, to identify patients at increased risk for poor outcome, even if the iPET scan is negative.53
ROLE OF PET/CT IN ASSESSMENT OF RESPONSE TO THERAPY
PET/CT has been increasingly used for response assessment at various stages in lymphoma in recent years. Almost all types of lymphomas are fluorodeoxyglucose (FDG) avid; however, Hodgkin lymphoma is FDG avid in 97% to 100% of cases. In 2009, a 5-point scale was developed to score PET images with regard to treatment response, either partway through treatment (iPET) or at the end of therapy.54 It was recommended as the standard reporting tool at the First International Workshop on PET in Lymphoma in Deauville, France, in 2009, and is thus now referred to as the Deauville score. A score of 1 is given if there is no uptake, 2 if the uptake ≤ mediastinum, 3 if > mediastinum but ≤ liver, 4 if uptake moderately higher than liver, 5 if uptake is markedly higher than liver and/or new lesions. X designates new areas of uptake unlikely to be related to lymphoma. In most trials, a score of 1 or 2 is considered a complete response and a score of 4 or 5 is considered a treatment failure. A score of 3 is sometimes considered a complete response, depending on the study. The Deauville criteria have been widely used in newer clinical trials utilizing response-adapted treatment as defined by PET response. PET/CT is recommended for staging and restaging at the end of therapy, in clinical practice, and clinical trials. Interim PET/CT scan, while commonly performed in clinical practice, is only recommended if the results will alter therapy (eg, if that information will result in the clinician omitting radiation therapy [RT] or altering the chemotherapy plan).
Early studies of iPET showed that achieving PET negativity early in the course of treatment was strongly associated with PFS and overall survival.55 Subsequent studies confirmed the importance of achieving a negative iPET. As a result, considerable efforts have been put into designing response-adapted treatment approaches using iPET (see Treatment section), with some of these approaches now being listed in the National Comprehensive Cancer Network (NCCN) guidelines and being used in standard practice.
TREATMENT
EVOLUTION OF TREATMENT
The treatment of Hodgkin lymphoma has evolved over the past century, starting with the discovery of RT as effective treatment in the early 20th century. Long-term survival of patients with Hodgkin lymphoma treated with involved-field radiation therapy (IFRT) was first reported in the 1960s.56,57 Outcomes improved further with the introduction of combined modality treatment (CMT) using chemotherapy and RT, with the overall 5-year relative survival for patients with Hodgkin lymphoma (all stages) treated in 2006–2012 being 85.4% to 87.3%.3 Since the majority of patients are now cured with modern therapy, treatment-related complications have become an important competing cause of mortality. Recent studies have therefore focused on maintaining efficacy while reducing toxicities, and refining the process of selecting patients who might benefit from more aggressive therapy. While RT was the first treatment modality shown to be curative for Hodgkin lymphoma,56,57 multiple subsequent studies showed that CMT is superior to RT alone in terms of relapse-free survival.58–63 In the GHSG H8-F trial, the estimated 5-year event-free survival and overall survival rates were significantly higher after 3 cycles of MOPP-ABV (mechlorethamine, vincristine, procarbazine, and prednisone combined with doxorubicin, bleomycin, and vinblastine) plus IFRT than after subtotal nodal radiotherapy alone. The 10-year overall survival estimates were 97% and 92%, respectively (P = 0.001).64 As a result, CMT replaced RT alone as the standard of care for limited-stage Hodgkin lymphoma. However, for elderly or infirm patients, or those with other comorbidities making them poor chemotherapy candidates, RT alone may be a very reasonable option.65 More recently, an increasing body of evidence has accumulated to support the use of chemotherapy alone in early stage cHL. This literature has consistently shown that omission of RT is associated with a modest increase in relapse, without a clear compromise in long-term overall survival. For some patients, the trade-off in terms of avoiding radiation (and the associated late effects) may be worth the small increase in relapse risk, since long-term survival does not appear to be substantially worse with chemo-therapy alone. Table 3 and Table 4 provide a summary of recent key studies which have defined treatment options for early-stage cHL.48,66–71


EARLY-STAGE NLPHL
NLPHL usually presents with limited-stage disease without B symptoms and has an indolent course with a slightly better prognosis compared to cHL.72 Due to the rarity of the disease, treatment guidelines are mostly based on retrospective analyses from single or multi-institution studies or subgroup analyses, often with relatively short follow-up. These studies must be interpreted with caution because of the possibility of inaccuracies in the pathologic diagnosis, small sample sizes, and selection bias. Treatment options for limited-stage NLPHL include observation, single-agent rituximab, IFRT (or involved-site radiation therapy [ISRT]) alone, or CMT.46
Historically, patients with limited-stage NLPHL have been treated with RT alone, with 80% to 85% PFS and 85% to 95% overall survival rates.73–75 Patients who relapse or progress after RT in general can successfully undergo salvage therapy.74 In one study, rates of PFS and overall survival were similar among patients who had limited-field, regional-field, or extended-field RT,75 indicating that IFRT is preferred. Studies comparing RT alone and CMT are limited. The GHSG HD7 trial included a subset of NLPHL patients, with a trend towards improved freedom from treatment failure (96% versus 83%) favoring CMT. This, however, did not translate into improved overall survival.47 A retrospective analysis of the British Columbia Cancer Agency database compared patients with limited-stage NLPHL treated with RT alone to patients who received 2 cycles of ABVD followed by RT. A significant improvement in PFS (91% versus 65%) and overall survival (93% versus 84%) was seen, favoring CMT.76
Chemotherapy alone is not recommended for limited-stage NLPHL since studies evaluating chemotherapy alone are quite limited and indicate relatively high rates of treatment failure. Given that the malignant cells in NLPHL are CD20-positive, single-agent rituximab has also been studied in this disease, including a study as frontline therapy in limited-stage patients. In this phase 2 trial in newly diagnosed patients with stage IA disease, an overall response rate (ORR) of 100% was seen, with an 85% complete response (CR) rate.77 At 3 years, overall survival was 100% and PFS was 81%, indicating that the responses with single-agent rituximab are less durable than those with RT.
Advani et al evaluated rituximab followed by observation versus rituximab (R) followed by maintenance rituximab (MR) for 2 years in 39 new or previously treated patients. At 4 weeks the ORR was 100% (with CR in 67%, and partial response in 33%). At a median follow up of 9.8 years for R alone, and 5 years for R+MR, median PFS was 3 and 5.6 years, respectively (P = 0.26). Estimated 5-yr PFS and overall survival in patients treated with R versus R+MR were 39.1% and 95.7% versus 58.9% and 85.7%, with Pvalues of 0.26 (PFS) and 0.38 (overall), respectively. Maintenance rituximab therefore appears to prolong remission, although the results did not quite reach statistical significance.78 Even though rituximab does not appear to be curative in NLPHL, it is a reasonable option for patients with early-stage NLPHL who are not good candidates for definitive RT. Whether combining rituximab with RT or CMT might further improve outcomes in early-stage NLPHL has not yet been determined.
In children, surgery alone may lead to long-term remission or possibly cure of limited-stage NLPHL. In a European multicenter retrospective study, 58 patients underwent surgery for limited-stage NLPHL. Among the 51 patients who achieved complete remission following surgery, 67% remained progression-free and 100% were alive at a median follow up of 43 months.79 In adults, there is no data to support surgical treatment alone for NLPHL. Finally, observation may be a reasonable option in elderly or infirm patients for whom NLPHL is unlikely to affect life expectancy. For younger patients, given the excellent outcome with modern therapy and the long-term risk of transformation of NLPHL into an aggressive non-Hodgkin lymphoma, observation is generally not recommended.
The NCCN recommends RT (ISRT or IFRT, 30–36 Gy) as the preferred treatment for stage IA and IIA non-bulky NLPHL. In patients with stage IA disease with complete excision of solitary nodule, observation may be appropriate. A course of chemotherapy with ISRT with or without rituximab is recommended for patients with stage IB or IIB disease, or patients with stage IA or IIA bulky disease.
FIRST-LINE TREATMENT OF LIMITED-STAGE CHL
Early-Stage Favorable cHL
There is lack of consensus regarding the ideal treatment approach for patients with early-stage favorable cHL. However, there are several excellent options available, with overall survival rates exceeding 90%. Most of these regimens involve CMT, although some chemotherapy-alone approaches have been evaluated as well. Concurrent with the demonstration of excellent long-term remission rates with CMT, it became apparent that the long-term survival and quality of life of these patients is determined in large part by the risk of serious (and potentially fatal) treatment-related toxicities. Such toxicities consist primarily of secondary malignancies and cardiovascular events, and can continue to cause significant morbidity and mortality even 2 to 3 decades after treatment.80–82 As a result, treatment decisions for these patients are complicated and require balancing efficacy against risk of late complications.
In the United States, until recently, CMT was generally considered the standard of care, with robust long-term data regarding efficacy. The most commonly used regimen has been ABVD for 2 to 4 cycles followed by IFRT. In some German studies, escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) has been used, but this is not a general standard of care in the United States for early-stage patients.
More recent data suggests that the rate of serious late complications in Hodgkin lymphoma patients is decreasing, likely due to less extensive radiation fields, lower radiation doses, and a movement away from the MOPP regimen to ABVD.83,84 For patients who meet the “favorable” criteria set forth in the GHSG HD10 trial (see Table 2), 2 cycles of ABVD followed by 20 Gy of IFRT is an attractive option, with efficacy preserved and a low anticipated rate of late effects.66 With this approach, and with long-term (10 years) follow up, all 4 arms had similar PFS (87%) and overall survival (94%), whether 2 or 4 cycles of ABVD were given. When the effects of 20-Gy and 30-Gy doses of RT were compared, there were also no significant differences in freedom from treatment failure or overall survival. Adverse events and acute toxic effects of treatment were most common in the patients who received 4 cycles of ABVD and 30 Gy of RT.66,71
In recent years, in an attempt to reduce late effects further, regimens consisting of chemotherapy alone have been investigated. In a study by Meyer et al, at 12 years the rate of overall survival was 94% among those receiving ABVD alone, as compared with 87% among those receiving subtotal nodal RT; the rates of freedom from disease progression were 87% and 92% in the 2 groups; and the rates of event-free survival were 85% and 80%, respectively.50 However, it is important to note that this study did not include a CMT arm for the early favorable patients, and did not utilize modern RT techniques. Nevertheless, this early study and others60 suggested that chemotherapy alone may be a reasonable option for some early-stage cHL patients, particularly for patients who are felt to be at increased risk for late toxicities from RT. As a result, additional studies have been conducted evaluating CMT versus chemotherapy alone for early-stage cHL. Many of these studies have incorporated interim PET/CT scan to develop a response-adapted approach to decide which patients are least likely to benefit from RT.
The HD-13 study was a follow-up study for HD-10, looking at deletion of bleomycin, dacarbazine, or both from the ABVD backbone. The ABD arm was closed early, because of an excess rate of treatment failure. Among the 1243 patents assigned to either the ABVD or AVD arm at 5 years of follow-up, there was 4.3% difference in PFS. This study was not able to demonstrate that 2 cycles of AVD was noninferior to 2 cycles of ABVD, each followed by 30 Gy IFRT, even though there was no difference in all 4 groups. It confirmed 2 cycles of ABVD as the preferred regimen in early favorable Hodgkin lymphoma, when CMT is the plan of care. However, for patients over age 60 to 65 years, or those with underlying cardiac or pulmonary comorbidities, bleomycin has significant risk of toxicity. In that setting, AVD is a safer option, with only a very modest decrease in 5-year PFS.
Based on the observation that iPET scan is highly predictive of outcome in Hodgkin lymphoma,55,85 several trials have employed the use of an iPET scan to guide therapy. It is hoped that such studies will lead to new PET-directed treatment algorithms in which patients who require more aggressive therapy (eg, with CMT, or escalated BEACOPP) can be identified, and the remaining patients can be safely treated less aggressively (eg, with chemotherapy alone).
In the EORTC H10 trial, performed to evaluate treatment adaptation on the basis of iPET scan results in stage I and II Hodgkin lymphoma, a control arm received standard combined modality treatment (3 or 4 cycles of ABVD with INRT) irrespective of PET scan results. In the experimental arm, patients with a negative PET scan after 2 cycles of ABVD continued with 1 or 2 cycles of ABVD and did not receive RT. The iPET-positive patients received either standard treatment with ABVD plus INRT or escalated BEACOPP plus INRT. The iPET-negative patients received either ABVD only or ABVD plus INRT. The final results of this study, published recently, showed that in the iPET-positive patients the 5-year PFS was improved from 77.4% with standard ABVD plus INRT to 90.6% with escalated BEACOPP plus INRT (P = 0.002). In iPET-negative patients, 5-year PFS in the favorable group was 99% versus 87.1% in favor of ABVD plus INRT. The H10 study suggested that PET results after 2 cycles of ABVD can be integrated into treatment planning, In iPET-negative patients, the study was technically not able to demonstrate the noninferiority of the ABVD only regimen, owing to a higher risk of relapse if INRT is omitted, particularly in the favorable group.48 However, this study does show that excellent outcomes can be obtained with omission of RT in patients with a negative iPET scan. This study provides a cautionary lesson though, in that the increase in relapse rate associated with omission of RT was more substantial (12%) for favorable versus unfavorable early-stage patients (2.5%), and this difference was only apparent after longer (5 years) follow-up. Despite this, chemotherapy alone is considered a reasonable treatment option, especially for patients felt to be at increased risk for late toxicities of RT or for patients who wish to avoid the risks of RT, with over 99% of patients alive at 5 years.
Similar results were shown in the RAPID trial, in which patients with limited-stage cHL underwent 3 cycles of ABVD followed by PET assessment.67 Patients with a negative PET (n = 426) were then randomized to RT (n = 209) versus no further therapy (n = 211). At a median of 60 months of follow-up, 3-year PFS was 94.6% in the RT group and 90.8% in the chemotherapy alone group. Similar to the H10 trial, it was concluded that chemotherapy alone was statistically inferior to CMT in terms of PFS. However, also similar to the H10 trial, the RAPID trial demonstrated that excellent results can be obtained in early-stage cHL patients with omission of RT, if iPET scan is negative after 3 cycles of ABVD, as there was no survival difference. These findings indicate that, when relapses occur as a result of omission of RT, such patients can be effectively treated later.
In the ongoing GHSG HD16 trial, patients with early-stage favorable cHL will be randomly assigned to a standard approach (ABVD × 2 cycles followed by 20-Gy IFRT) versus an experimental approach in which they receive ABVD for 2 cycles and then undergo PET scan. If the PET remains positive, they will receive 20-Gy IFRT. If the PET is negative, they will receive no further therapy. This trial could ultimately define ABVD for 2 cycles as a treatment option.
It is clear from these studies that omission of RT results in a somewhat higher rate of relapse but can be considered in selected patients. However, taking a less aggressive frontline approach may also be justified by the fact that, for those who do relapse, successful salvage therapies are available. Aggressive salvage therapy with autologous stem cell transplantation historically can cure approximately 50% of relapsed patients. With new and emerging therapies for relapsed disease, such as brentuximab vedotin and the PD-1 inhibitors (eg, nivolumab and pembrolizumab), the ability to cure relapsed patients may improve even more, further calling into question the practice of applying CMT uniformly for early-stage patients undergoing first-line therapy. Unfortunately, there is insufficient data from large randomized studies with long-term follow-up to fully address this issue currently, and there remains some controversy around this issue. NCCN recommends restaging PET/CT after 3 cycles of ABVD if a chemotherapy alone treatment modality is chosen. If the Deauville score is 1 or 2, either observation or 1 additional cycle of ABVD is recommended.46
Early-Stage Unfavorable cHL
In the United States, historically early-stage unfavorable Hodgkin lymphoma has been treated with CMT, most commonly 4 to 6 cycles of ABVD followed by consolidative RT. With this approach one can expect a 5-year PFS of approximately 80% to 85%.58,64,86 The GHSG HD8 trial showed that RT volume size reduction from extended-field to involved-field after COPP + ABVD chemotherapy for 2 cycles produced similar results and less toxicity in patients with early-stage unfavorable cHL.86 The GHSG trial HD11 established ABVD for 4 cycles plus 30-Gy IFRT as a standard for early unfavorable Hodgkin lymphoma. The freedom from treatment failure at 5 years was 85.0%, and overall survival was 94.5%.68
In the HD14 study by the GHSG, patients with early unfavorable cHL were treated with 2 cycles of escalated BEACOPP followed by 2 cycles of ABVD, versus 4 cycles of ABVD. All patients then received 30 Gy of consolidative IFRT. A 5-year PFS of 95% was seen in the experimental arm, compared with 89% in the standard (ABVD) arm. As expected, this regimen was associated with more acute hematologic toxicity, and there was no difference between the 2 regimens with respect to overall survival or fertility.69 Given the lack of improved survival and increased toxicity, ABVD has remained the standard chemotherapy regimen for early unfavorable cHL in the United States. NCCN recommends a restaging PET scan after 2 cycles of ABVD and to continue with 2 to 4 cycles of ABVD or escalated BEACOPP with or without ISRT based on Deauville scores.
Another viable treatment option is the Stanford V regimen, a condensed, 12-week regimen that includes mechlorethamine, doxorubicin, vinblastine, prednisone, vincristine, etoposide, and bleomycin, followed by IFRT.87 In a randomized phase 3 trial conducted by ECOG (E2496), patients with stage I/II Hodgkin lymphoma with bulky mediastinal disease or advanced-stage disease were randomized to ABVD × 6 to 8 cycles versus Stanford V. RT was given (36 Gy) for those with bulky mediastinal disease or to sites of disease greater than 5 cm in the Stanford V arm. In a subset analysis focusing only on those with stage I/II bulky mediastinal disease, the 5-year failure free survival was 85% versus 79% and the 5-year overall survival was 96% versus 92% for the ABVD versus Stanford V arms, respectively. These differences were not statistically significant.70 While the Stanford V regimen has the advantages of a 12-week treatment duration and a lower cumulative amount of bleomycin and doxorubicin, the Stanford V arm had higher rates of grade 3 lymphopenia and grade 3 to 4 peripheral neuropathies. In addition, Stanford V requires that most patients undergo RT (to original sites of disease measuring 5 cm or more plus contiguous areas). As a result, the investigators concluded that ABVD × 4 cycles plus IFRT remains the standard of care for patients with early unfavorable Hodgkin lymphoma with bulky mediastinal disease.
Other regimens have been studied in hopes of reducing toxicity, including the EVE regimen (epirubicin, vinblastine, and etoposide). This regimen was compared to ABVD in early unfavorable Hodgkin lymphoma patients, with all patients undergoing the same RT program. No differences were observed between the ABVD and EVE arms in terms of complete remission rate and overall survival. However, patients who received EVE had a significantly worse outcome than those who received ABVD in terms of relapse-free survival and failure-free survival.88 EBVP (epirubicin, bleomycin, vinblastine, and prednisone) followed by IFRT was less efficacious compared with MOPP/ABV–type therapy.58
An area of active investigation is whether RT can be safely omitted in patients with early- stage unfavorable cHL. The EORTC H10 study showed that, for patients with a negative iPET scan (after 2 cycles), the 5-year PFS rates were 92.1% versus 89.6% for ABVD plus INRT versus ABVD alone, respectively. While this technically did not meet criteria for noninferiority of ABVD alone, this study demonstrated that, for those with negative iPET, ABVD × 6 cycles (without radiation) can result in long-term remission in a high proportion (89%) of patients. For iPET-positive patients, 2 cycles of escalated BEACOPP were given followed by 30 Gy of IFRT on the experimental arm. This resulted in a 5-year PFS of 90.6% versus 77.4%, suggesting this may be a preferred approach for early-stage unfavorable patients with a positive iPET.48 Even though the noninferiority of ABVD alone could not be established based on the statistical design of the study, the current NCCN guidelines recommend restaging after 2 cycles of ABVD for stage I or II unfavorable cHL and using that iPET as a guide, based on Deauville scores. For scores 1–3, ABVD × 2 cycles (total 4 cycles) plus ISRT or AVD × 4 (total 6) with or without ISRT is recommended. For a Deauville score of 4, escalated BEACOPP × 2 cycles or ABVD × 2 cycles (total 4) followed by ISRT is recommended. If the Deauville score is 5, further treatment decisions should be made based on repeat biopsy results. A follow up PET/CT is recommended for Deauville scores of 4 and 5 to confirm complete response.46
LATE EFFECTS AND THE EVOLUTION OF RADIATION THERAPY
The RT given in Hodgkin lymphoma has evolved considerably over the years, from extended field or subtotal nodal fields developed in the 1960s, to the more focused involved-field or even involved-site radiation commonly given now. This approach reduces radiation volumes, and it already is becoming evident that the relative risk of breast cancer among young females receiving mediastinal RT for Hodgkin lymphoma is declining.89 Cardiac dose is reduced significantly with IFRT compared to older radiation techniques as well. The extent of radiation may be reduced even further with involved-nodal/involved site or intensity-modulated approaches.90
With new RT techniques allowing for more focused therapy and lower doses of radiation, models predict that the rate of long-term complications will decline further.91,92 Furthermore, response-adapted (ie, PET-directed) approaches, as discussed in detail earlier in the article, are expected to increasingly allow for identification of patients who can safely avoid radiation entirely, which will hopefully lead to an even lower rate of late complications of therapy.
MONITORING FOR RELAPSE
A number of recent studies have shown that, for patients who achieve complete remission with first-line therapy, performing repeated scheduled surveillance imaging does not improve outcomes. In fact, most relapses are detected by the patient (due to symptoms or recurrence of lymph node enlargement). It is rare that a relapse would be detected by surveillance imaging alone. Furthermore, surveillance that includes routine imaging has not been associated with improved survival.93 As a result, it is now recommended that patients undergo regular follow-up with symptom review, physical exam, and basic laboratory studies. Imaging studies should be obtained as needed for patients who develop signs, symptoms, exam findings, or laboratory values concerning for relapse.
More important than scheduled surveillance imaging for relapse is monitoring for late effects of therapy. These fall into several broad categories such as cardiovascular disease (coronary disease, congestive heart failure, valvular disease, carotid artery disease), pulmonary disease, hypothyroidism, and secondary malignancies. Aggressive surveillance for breast cancer is especially warranted in female patients who underwent chest radiation.46
CONCLUSION
Hodgkin lymphoma is characterized pathologically by the presence of HRS cells accompanied by a polymorphous cellular infiltrate. It is a disease with a bimodal age distribution, several pathologic subtypes, and numerous treatment options. Overall, the prognosis for patients with early-stage disease is excellent, and although a majority of patients can now be cured, further studies are needed to optimize treatment such that short- and long-term treatment-related toxicities are minimized, without compromising disease control and cure.
- Küppers R, Rajewsky K, Zhao M, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A 1994;91:10962–6.
- Küppers R. The biology of Hodgkin›s lymphoma. Nat Rev Cancer 2009;9:15–27.
- National Cancer Institute. SEER cancer statistics review, 1975–2014. 2017. http://seer.cancer.gov/csr/1975_2013/. Accessed April 27, 2017.
- Haim N, Cohen Y, Robinson E. Malignant lymphoma in first-degree blood relatives. Cancer 1982;49:2197–200.
- Mack TM, Cozen W, Shibata DK, et al. Concordance for Hodgkin’s disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N Engl J Med 1995;332:413–8.
- Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 2010;116:3724–34.
- Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med 2003;349:1324–32.
- Hessol NA, Katz MH, Liu JY, et al. Increased incidence of Hodgkin disease in homosexual men with HIV infection. Ann Intern Med 1992;117:309–11.
- Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753–62.
- Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2009;27:884–90.
- Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr 2009;52:203–8.
- Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 2006;108:3786–91.
- Thompson LD, Fisher SI, Chu WS, et al. HIV-associated Hodgkin lymphoma: a clinicopathologic and immunophenotypic study of 45 cases. Am J Clin Pathol 2004;121:727–38.
- Briggs NC, Hall HI, Brann EA, et al. Cigarette smoking and risk of Hodgkin’s disease: a population-based case-control study. Am J Epidemiol 2002;156:1011–20.
- Castillo JJ, Dalia S, Shum H. Meta-analysis of the association between cigarette smoking and incidence of Hodgkin’s Lymphoma. J Clin Oncol 2011;29:3900–6.
- Kanzler H, Kuppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med 1996;184:1495–505.
- Stein H, Hummel M. Cellular origin and clonality of classic Hodgkin’s lymphoma: immunophenotypic and molecular studies. Semin Hematol 1999;36:233-41.
- Marafioti T, Hummel M, Foss HD, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000;95:1443–50.
- Marafioti T, Hummel M, Anagnostopoulos I, et al. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med 1997;337:453–8.
- van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin’s lymphoma. Am J Pathol 1999;154:1685–91.
- Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest 1997;100:2961–9.
- Luftig M, Yasui T, Soni V, et al. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc Natl Acad Sci U S A 2004;101:141–6.
- Roemer MGM, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016;34:2690–7.
- Swerdlow SH CE, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019–32.
- von Wasielewski R, Mengel M, Fischer R, et al. Classical Hodgkin’s disease. Clinical impact of the immunophenotype. Am J Pathol 1997;151:1123–30.
- Tzankov A, Krugmann J, Fend F, et al. Prognostic significance of CD20 expression in classical Hodgkin lymphoma: a clinicopathological study of 119 cases. Clin Cancer Res 2003;9:1381–6.
- Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol 1999;17:776–83.
- Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin’s Study Group trials. J Clin Oncol 2005;23:5739–45.
- Slack GW, Ferry JA, Hasserjian RP, et al. Lymphocyte depleted Hodgkin lymphoma: an evaluation with immunophenotyping and genetic analysis. Leuk Lymphoma 2009;50:937–43.
- Mason DY, Banks PM, Chan J, et al. Nodular lymphocyte predominance Hodgkin’s disease. A distinct clinicopathological entity. Am J Surg Pathol 1994;18:526–30.
- Rudiger T, Gascoyne RD, Jaffe ES, et al. Workshop on the relationship between nodular lymphocyte predominant Hodgkin’s lymphoma and T cell/histiocyte-rich B cell lymphoma. Ann Oncol 2002;13 Suppl 1:44–51.
- Sundeen JT, Cossman J, Jaffe ES. Lymphocyte predominant Hodgkin’s disease nodular subtype with coexistent “large cell lymphoma”. Histological progression or composite malignancy? Am J Surg Pathol 1988;12:599–606.
- Kenderian SS, Habermann TM, Macon WR, et al. Large B-cell transformation in nodular lymphocyte-predominant Hodgkin lymphoma: 40-year experience from a single institution. Blood. 2016;127:1960–6.
- Mauch PM, Kalish LA, Kadin M, et al. Patterns of presentation of Hodgkin disease. Implications for etiology and pathogenesis. Cancer 1993;71:2062–71.
- Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N J Engl Med 2006;354:496–507.
- Hutchings M, Loft A, Hansen M, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica 2006;91:482–9.
- Naumann R, Beuthien-Baumann B, Reiss A, et al. Substantial impact of FDG PET imaging on the therapy decision in patients with early-stage Hodgkin’s lymphoma. Br J Cancer 2004;90:620–5.
- Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571–8.
- El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 2012;30:4508–14.
- Wang J, Weiss LM, Chang KL, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer 2002;94:1522–31.
- Menon NC, Buchanan JG. Bilateral trephine bone marrow biopsies in Hodgkin’s and non-Hodgkin’s lymphoma. Pathology 1979;11:53–7.
- Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res 1971;31:1860–1.
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989;7:1630–6.
- Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med 2010;363:653–62.
- National Comprehensive Cancer Network I. NCCN Guidelines Version 3.2016 Hodgkin lymphoma. 2017.
- Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. J Clin Oncol 2007;25:3495–502.
- Andre MP, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 2017:Jco2016686394.
- Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol 2005;23:4634–42.
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 2012;366:399–408.
- Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 2010;362:875–85.
- Kamper P, Bendix K, Hamilton-Dutoit S, et al. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 2011;96:269–76.
- Agostinelli C, Gallamini A, Stracqualursi L, et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol 2016;3:e467–e79.
- Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymph 2009;50:1257–60.
- Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 2007;25:3746–52.
- Easson EC, Russell MH. Cure of Hodgkin’s Disease. Br Med J 1963;1(5347):1704–7.
- Kaplan HS. The radical radiotherapy of regionally localized Hodgkin’s disease. Radiology 1962;78:553–61.
- Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin’s lymphoma: long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol 2006;24:3128–35.
- Eghbali H, Raemaekers J, Carde P. The EORTC strategy in the treatment of Hodgkin’s lymphoma. Eur J Haematol Suppl 2005:135–40.
- Bloomfield CD PT, Glicksman AS, et al. Chemotherapy and combined modality therapy for Hodgkin’s disease: A progress report on cancer and leukemia group B studies. Cancer Treat Rep 1982;66:835–46.
- Pavlovsky S, Maschio M, Santarelli MT, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage I-II Hodgkin’s disease. J Natl Cancer Inst 1988;80:1466–73.
- Aviles A, Delgado S. A prospective clinical trial comparing chemotherapy, radiotherapy and combined therapy in the treatment of early stage Hodgkin’s disease with bulky disease. Clin Lab Haematol 1998;20:95–9.
- Herbst C, Rehan FA, Brillant C, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin’s lymphoma: a systematic review. Haematologica 2010;95:494–500.
- Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med 2007;357:1916–27.
- Landgren O, Axdorph U, Fears TR, et al. A population-based cohort study on early-stage Hodgkin lymphoma treated with radiotherapy alone: with special reference to older patients. Ann Oncol 2006;17:1290–5.
- Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med 2010;363:640–52.
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Eng J Med 2015;372:1598–607.
- Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 2010;28:4199–206.
- von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 2012;30:907–13.
- Advani RH, Hong F, Fisher RI, et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: a subset analysis of the North American Intergroup E2496 Trial. J Clin Oncol 2015;33:1936–42.
- Sasse S, Brockelmann PJ, Georgen H, et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: Updated analyses of the German Hodgkin Study Group HD7, HD8, HD10 and HD11 trials. J Clin Oncol 2017 Apr 18:JCO2016709410. doi: 10.1200/JCO.2016.70.9410. [Epub ahead of print]
- Nogova L, Reineke T, Brillant C, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma: a comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol 2008;26:434–9.
- Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma: a retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer 2005;104:1221–9.
- Chera BS, Olivier K, Morris CG, et al. Clinical presentation and outcomes of lymphocyte-predominant Hodgkin disease at the University of Florida. Am J Clin Oncol 2007;30:601–6.
- Chen RC, Chin MS, Ng AK, et al. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma: patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol 2010;28:136–41.
- Savage KJ, Skinnider B, Al-Mansour M, et al. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood 2011;118:4585–90.
- Eichenauer DA FM, Pluetschow A, et al. Phase 2 study of rituximab in newly diagnosed stage IA nodular lymphocytepredominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood 2011;118:4363–5.
- Advani RH, Horning SJ, Hoppe RT, et al. Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol 2014;32:912–8.
- Mauz-Korholz C, Gorde-Grosjean S, Hasenclever D, et al. Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma-experience from the European network group on pediatric Hodgkin lymphoma. Cancer 2007;110:179–85.
- Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol 2011;154:23–31.
- Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol 2011;29:1885–92.
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol 2003;21:3431–9.
- Girinsky T, van der Maazen R, Specht L, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol 2006;79:270–7.
- Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma: a question of field size. J Clin Oncol 2008;26:5170–4.
- Advani R, Maeda L, Lavori P, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin’s disease. J Clin Oncol 2007;25:3902–7.
- Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 2003;21:3601–8.
- Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease: mature results of a prospective clinical trial. J Clin Oncol 2002;20:630–7.
- Pavone V, Ricardi U, Luminari S, et al. ABVD plus radiotherapy versus EVE plus radiotherapy in unfavorable stage IA and IIA Hodgkin’s lymphoma: results from an Intergruppo Italiano Linfomi randomized study. Ann Oncol 2008;19:763–8.
- De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 2009;27:4239–46.
- Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:323–9.
- Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012;83:1232–7.
- Campbell BA, Hornby C, Cunninghame J, et al. Minimising critical organ irradiation in limited stage Hodgkin lymphoma: a dosimetric study of the benefit of involved node radiotherapy. Ann Oncol 2012;23:1259–66.
- Pingali SR, Jewell SE, Havlat L, et al. Limited utility of routine surveillance imaging for classical Hodgkin lymphoma patients in first complete remission. Cancer 2014;120:2122–9.
- Küppers R, Rajewsky K, Zhao M, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A 1994;91:10962–6.
- Küppers R. The biology of Hodgkin›s lymphoma. Nat Rev Cancer 2009;9:15–27.
- National Cancer Institute. SEER cancer statistics review, 1975–2014. 2017. http://seer.cancer.gov/csr/1975_2013/. Accessed April 27, 2017.
- Haim N, Cohen Y, Robinson E. Malignant lymphoma in first-degree blood relatives. Cancer 1982;49:2197–200.
- Mack TM, Cozen W, Shibata DK, et al. Concordance for Hodgkin’s disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N Engl J Med 1995;332:413–8.
- Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 2010;116:3724–34.
- Hjalgrim H, Askling J, Rostgaard K, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med 2003;349:1324–32.
- Hessol NA, Katz MH, Liu JY, et al. Increased incidence of Hodgkin disease in homosexual men with HIV infection. Ann Intern Med 1992;117:309–11.
- Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103:753–62.
- Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol 2009;27:884–90.
- Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr 2009;52:203–8.
- Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 2006;108:3786–91.
- Thompson LD, Fisher SI, Chu WS, et al. HIV-associated Hodgkin lymphoma: a clinicopathologic and immunophenotypic study of 45 cases. Am J Clin Pathol 2004;121:727–38.
- Briggs NC, Hall HI, Brann EA, et al. Cigarette smoking and risk of Hodgkin’s disease: a population-based case-control study. Am J Epidemiol 2002;156:1011–20.
- Castillo JJ, Dalia S, Shum H. Meta-analysis of the association between cigarette smoking and incidence of Hodgkin’s Lymphoma. J Clin Oncol 2011;29:3900–6.
- Kanzler H, Kuppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med 1996;184:1495–505.
- Stein H, Hummel M. Cellular origin and clonality of classic Hodgkin’s lymphoma: immunophenotypic and molecular studies. Semin Hematol 1999;36:233-41.
- Marafioti T, Hummel M, Foss HD, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000;95:1443–50.
- Marafioti T, Hummel M, Anagnostopoulos I, et al. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med 1997;337:453–8.
- van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin’s lymphoma. Am J Pathol 1999;154:1685–91.
- Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest 1997;100:2961–9.
- Luftig M, Yasui T, Soni V, et al. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc Natl Acad Sci U S A 2004;101:141–6.
- Roemer MGM, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016;34:2690–7.
- Swerdlow SH CE, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
- Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019–32.
- von Wasielewski R, Mengel M, Fischer R, et al. Classical Hodgkin’s disease. Clinical impact of the immunophenotype. Am J Pathol 1997;151:1123–30.
- Tzankov A, Krugmann J, Fend F, et al. Prognostic significance of CD20 expression in classical Hodgkin lymphoma: a clinicopathological study of 119 cases. Clin Cancer Res 2003;9:1381–6.
- Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol 1999;17:776–83.
- Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin’s Study Group trials. J Clin Oncol 2005;23:5739–45.
- Slack GW, Ferry JA, Hasserjian RP, et al. Lymphocyte depleted Hodgkin lymphoma: an evaluation with immunophenotyping and genetic analysis. Leuk Lymphoma 2009;50:937–43.
- Mason DY, Banks PM, Chan J, et al. Nodular lymphocyte predominance Hodgkin’s disease. A distinct clinicopathological entity. Am J Surg Pathol 1994;18:526–30.
- Rudiger T, Gascoyne RD, Jaffe ES, et al. Workshop on the relationship between nodular lymphocyte predominant Hodgkin’s lymphoma and T cell/histiocyte-rich B cell lymphoma. Ann Oncol 2002;13 Suppl 1:44–51.
- Sundeen JT, Cossman J, Jaffe ES. Lymphocyte predominant Hodgkin’s disease nodular subtype with coexistent “large cell lymphoma”. Histological progression or composite malignancy? Am J Surg Pathol 1988;12:599–606.
- Kenderian SS, Habermann TM, Macon WR, et al. Large B-cell transformation in nodular lymphocyte-predominant Hodgkin lymphoma: 40-year experience from a single institution. Blood. 2016;127:1960–6.
- Mauch PM, Kalish LA, Kadin M, et al. Patterns of presentation of Hodgkin disease. Implications for etiology and pathogenesis. Cancer 1993;71:2062–71.
- Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N J Engl Med 2006;354:496–507.
- Hutchings M, Loft A, Hansen M, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica 2006;91:482–9.
- Naumann R, Beuthien-Baumann B, Reiss A, et al. Substantial impact of FDG PET imaging on the therapy decision in patients with early-stage Hodgkin’s lymphoma. Br J Cancer 2004;90:620–5.
- Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571–8.
- El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 2012;30:4508–14.
- Wang J, Weiss LM, Chang KL, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer 2002;94:1522–31.
- Menon NC, Buchanan JG. Bilateral trephine bone marrow biopsies in Hodgkin’s and non-Hodgkin’s lymphoma. Pathology 1979;11:53–7.
- Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res 1971;31:1860–1.
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989;7:1630–6.
- Armitage JO. Early-stage Hodgkin’s lymphoma. N Engl J Med 2010;363:653–62.
- National Comprehensive Cancer Network I. NCCN Guidelines Version 3.2016 Hodgkin lymphoma. 2017.
- Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. J Clin Oncol 2007;25:3495–502.
- Andre MP, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 2017:Jco2016686394.
- Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol 2005;23:4634–42.
- Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 2012;366:399–408.
- Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 2010;362:875–85.
- Kamper P, Bendix K, Hamilton-Dutoit S, et al. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 2011;96:269–76.
- Agostinelli C, Gallamini A, Stracqualursi L, et al. The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol 2016;3:e467–e79.
- Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymph 2009;50:1257–60.
- Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 2007;25:3746–52.
- Easson EC, Russell MH. Cure of Hodgkin’s Disease. Br Med J 1963;1(5347):1704–7.
- Kaplan HS. The radical radiotherapy of regionally localized Hodgkin’s disease. Radiology 1962;78:553–61.
- Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin’s lymphoma: long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol 2006;24:3128–35.
- Eghbali H, Raemaekers J, Carde P. The EORTC strategy in the treatment of Hodgkin’s lymphoma. Eur J Haematol Suppl 2005:135–40.
- Bloomfield CD PT, Glicksman AS, et al. Chemotherapy and combined modality therapy for Hodgkin’s disease: A progress report on cancer and leukemia group B studies. Cancer Treat Rep 1982;66:835–46.
- Pavlovsky S, Maschio M, Santarelli MT, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage I-II Hodgkin’s disease. J Natl Cancer Inst 1988;80:1466–73.
- Aviles A, Delgado S. A prospective clinical trial comparing chemotherapy, radiotherapy and combined therapy in the treatment of early stage Hodgkin’s disease with bulky disease. Clin Lab Haematol 1998;20:95–9.
- Herbst C, Rehan FA, Brillant C, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin’s lymphoma: a systematic review. Haematologica 2010;95:494–500.
- Ferme C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med 2007;357:1916–27.
- Landgren O, Axdorph U, Fears TR, et al. A population-based cohort study on early-stage Hodgkin lymphoma treated with radiotherapy alone: with special reference to older patients. Ann Oncol 2006;17:1290–5.
- Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med 2010;363:640–52.
- Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Eng J Med 2015;372:1598–607.
- Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 2010;28:4199–206.
- von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 2012;30:907–13.
- Advani RH, Hong F, Fisher RI, et al. Randomized phase III trial comparing ABVD plus radiotherapy with the Stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: a subset analysis of the North American Intergroup E2496 Trial. J Clin Oncol 2015;33:1936–42.
- Sasse S, Brockelmann PJ, Georgen H, et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: Updated analyses of the German Hodgkin Study Group HD7, HD8, HD10 and HD11 trials. J Clin Oncol 2017 Apr 18:JCO2016709410. doi: 10.1200/JCO.2016.70.9410. [Epub ahead of print]
- Nogova L, Reineke T, Brillant C, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma: a comprehensive analysis from the German Hodgkin Study Group. J Clin Oncol 2008;26:434–9.
- Wirth A, Yuen K, Barton M, et al. Long-term outcome after radiotherapy alone for lymphocyte-predominant Hodgkin lymphoma: a retrospective multicenter study of the Australasian Radiation Oncology Lymphoma Group. Cancer 2005;104:1221–9.
- Chera BS, Olivier K, Morris CG, et al. Clinical presentation and outcomes of lymphocyte-predominant Hodgkin disease at the University of Florida. Am J Clin Oncol 2007;30:601–6.
- Chen RC, Chin MS, Ng AK, et al. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma: patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol 2010;28:136–41.
- Savage KJ, Skinnider B, Al-Mansour M, et al. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood 2011;118:4585–90.
- Eichenauer DA FM, Pluetschow A, et al. Phase 2 study of rituximab in newly diagnosed stage IA nodular lymphocytepredominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood 2011;118:4363–5.
- Advani RH, Horning SJ, Hoppe RT, et al. Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol 2014;32:912–8.
- Mauz-Korholz C, Gorde-Grosjean S, Hasenclever D, et al. Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma-experience from the European network group on pediatric Hodgkin lymphoma. Cancer 2007;110:179–85.
- Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol 2011;154:23–31.
- Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol 2011;29:1885–92.
- Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol 2003;21:3431–9.
- Girinsky T, van der Maazen R, Specht L, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol 2006;79:270–7.
- Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma: a question of field size. J Clin Oncol 2008;26:5170–4.
- Advani R, Maeda L, Lavori P, et al. Impact of positive positron emission tomography on prediction of freedom from progression after Stanford V chemotherapy in Hodgkin’s disease. J Clin Oncol 2007;25:3902–7.
- Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 2003;21:3601–8.
- Horning SJ, Hoppe RT, Breslin S, et al. Stanford V and radiotherapy for locally extensive and advanced Hodgkin’s disease: mature results of a prospective clinical trial. J Clin Oncol 2002;20:630–7.
- Pavone V, Ricardi U, Luminari S, et al. ABVD plus radiotherapy versus EVE plus radiotherapy in unfavorable stage IA and IIA Hodgkin’s lymphoma: results from an Intergruppo Italiano Linfomi randomized study. Ann Oncol 2008;19:763–8.
- De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 2009;27:4239–46.
- Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2011;2011:323–9.
- Maraldo MV, Brodin NP, Vogelius IR, et al. Risk of developing cardiovascular disease after involved node radiotherapy versus mantle field for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2012;83:1232–7.
- Campbell BA, Hornby C, Cunninghame J, et al. Minimising critical organ irradiation in limited stage Hodgkin lymphoma: a dosimetric study of the benefit of involved node radiotherapy. Ann Oncol 2012;23:1259–66.
- Pingali SR, Jewell SE, Havlat L, et al. Limited utility of routine surveillance imaging for classical Hodgkin lymphoma patients in first complete remission. Cancer 2014;120:2122–9.
Systematic review of interventions to reduce urinary tract infection in nursing home residents
Given the limited number of geriatricians in the U.S., hospitalists commonly manage nursing home residents admitted for post-acute care.1-4 Urinary tract infection (UTI) is one of the most common infections in nursing homes, often leading to sepsis and readmission to acute care.5 Inappropriate use of antibiotics to treat asymptomatic bacteriuria is both common and hazardous to nursing home residents.6 Up to 10% of nursing home residents will have an indwelling urinary catheter at some point during their stay.7-9 Residents with indwelling urinary catheters are at increased risk for catheter-associated urinary tract infection (CAUTI) and bacteriuria, with an estimated 50% of catheterized residents developing symptomatic CAUTI.5 While urinary catheter prevalence is lower in nursing homes than in the acute care setting, duration of use is often prolonged.7,10 In a setting where utilization is low, but use is prolonged, interventions designed to reduce UTI in acutely ill patients11 may not be as helpful for preventing infection in nursing home residents.
Our objective was to review the available evidence to prevent UTIs in nursing home residents to inform both bedside care and research efforts. Two types of literature review and summary were performed. First, we conducted a systematic review of individual studies reporting outcomes of UTI, CAUTI, bacteriuria, or urinary catheter use after interventions for reducing catheter use, improving insertion and maintenance of catheters, and/or general infection prevention strategies (eg, improving hand hygiene, infection surveillance, contact precautions, standardizing UTI diagnosis, and antibiotic use). Second, we performed a narrative review to generate an overview of evidence and published recommendations in both acute care and nursing home settings to prevent UTI in catheterized and non-catheterized older adults, which is provided as a comprehensive reference table for clinicians and researchers choosing and refining interventions to reduce UTIs.
METHODS
The systematic review was performed according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis recommendations. The protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews, (CRD42013005787). The narrative review was performed using the articles obtained from the systematic search and a targeted literature review by topic for a comprehensive list of interventions, including other interventions summarized in published reviews and guidelines.
Eligibility Criteria Review
Study Design. To address the breadth and depth of literature available to inform interventions to prevent UTI in nursing homes, broad eligibility criteria were applied with the expectation of varied designs and outcomes. All included studies for the systematic review were published manuscripts reporting a comparison group. We included randomized controlled trials as well as nonrandomized trials (pretest/posttest, with or without concurrent or nonconcurrent controls), with any duration of postintervention follow-up. Observational and retrospective studies were excluded.
Participants. We were interested in interventions and outcomes reported for nursing homes, defined as facilities providing short-stay skilled nursing care and/or rehabilitation, as well as long-term care. We also included evidence derived from rehabilitation facilities and spinal cord injury programs focused on reducing CAUTI risk for chronically catheterized residents. We excluded long-term acute care hospitals, hospice, psychiatric/mental health facilities, pediatric, and community dwelling/outpatient settings.
Interventions. We included interventions involving urinary catheter use such as improving appropriate use, aseptic placement, maintenance care, and prompting removal of unnecessary catheters. We included infection prevention strategies with a particular interest in hand hygiene, barrier precautions, infection control strategies, infection surveillance, use of standardized infection definitions, and interventions to improve antibiotic use. We included single and multiple interventions.
Outcomes
1. Healthcare-associated urinary tract infection: UTI occurring after admission to a healthcare facility, not identified specifically as catheter-associated. We categorized UTI outcomes with as much detail as provided, such as whether the reported outcome included only noncatheter-associated UTIs, the time required after admission (eg, more than 2 days), and whether the UTIs were defined by only laboratory criteria, clinically diagnosed infections, symptomatic, or long-term care specific surveillance definitions.
2. Catheter-associated urinary tract infection: UTI occurring in patients during or immediately after use of a urinary catheter. We noted whether CAUTI was defined by laboratory criteria, clinical symptoms, provider diagnosis, or antimicrobial treatment for case identification. We were primarily interested in CAUTI developing after placing an indwelling urinary catheter, commonly known as a Foley, but also in CAUTI occurring with other catheter types such as intermittent straight catheters, external or “condom” catheters, and suprapubic catheters.
3. Bacteriuria: We included the laboratory-based definition of bacteriuria as an outcome to include studies that reduced asymptomatic bacteriuria.
4. Urinary catheter use measures: This includes measures such as urinary catheter utilization ratios (catheter-days/patient-days), prevalence of urinary catheter use, or percentage of catheters with an appropriate indication.
Study Characteristics for Inclusion. Our systematic search included published papers in the English language. We did not exclude studies based on the number of facilities included or eligible, residents/patients included (based on age, gender, catheter use or type, or antibiotic use), intervention details, study withdrawal, loss to follow-up, death, or duration of pre-intervention and postintervention phases.
Data Sources and Searches
The following data sources were searched: Ovid MEDLINE (1950 to June 22, 2015), Cochrane Library via Wiley (1960 to June 22, 2015), CINAHL (1981 to June 22, 2015), Web of Science (1926 to June 22, 2015), and Embase.com (1946 to June 22, 2015). Two major systematic search strategies were performed for this review (Figure). Systematic search 1 was designed broadly using all data sources described above to identify interventions aimed at reducing all UTI events (defined under “Outcomes” above) or urinary catheter use (all types), focusing on interventions evaluated in nursing homes. Systematic search 2 was conducted in Ovid MEDLINE to identify studies to reduce UTI events or urinary catheter use measures for patients with a history of long-term or chronic catheter use, including nursing homes and other post-acute care settings such as rehabilitation units or hospitals and spinal cord injury programs, which have large populations of patients with chronic catheter needs. To inform the completeness of the broader systematic searches, supplemental systematic search strategies were performed for specific topics including hydration (supplemental search 1), published work by nursing home researchers known to the authors (supplemental search 2), and contact precautions (supplemental search 3). Search 1 is available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005787. Full search strategies for search 2 and supplemental searches are available upon request.
Study Selection
One author performed an initial screen of all records retrieved by the systematic searches by title and abstract and applied the initial exclusions (eg, non-human, no outcomes of interest), identified duplicate records, and assigned potentially relevant studies into groups such as review articles, epidemiology, interventions, and articles requiring further text review before categorization (Figure). After initial screening, Dr. Meddings reviewed the records by title/abstract. Reference lists were reviewed for potential articles for inclusion. Full-text article review informed the selection of those for dual abstraction and quality scoring performed by 2 authors, with discrepancies resolved by a third author. We requested additional information from authors from whom our search had generated only an abstract or brief report, or when additional information such as pre-intervention data was needed.12-18
Data Extraction and Quality Assessment
Relevant data regarding study design, participants, inclusion/exclusion criteria, outcomes, and quality criteria were abstracted independently by 2 authors. Methodological quality scores were assigned using a modification of the Quality index checklist developed by Downs and Black appropriate for assessing both randomized and nonrandomized studies of healthcare interventions.19 We also reviewed study funding sources and other potential quality concerns.
Data Analysis
Due to large trial heterogeneity among these studies about interventions and outcomes reported, outcome data could not be combined into summary measures for meta-analysis to give overall estimates of treatment effects.
RESULTS
Systematic Search Results and Study Selection
As detailed in the study flow diagram (Figure), 5794 total records were retrieved by systematic search 1 (4697 studies), search 2 (909 studies), and supplemental searches (188 studies). Hand searching of reference lists of 41 reviews (including narrative and systematic reviews) yielded 77 additional studies for consideration. Twenty-nine records on interventions that were the focus of systematic reviews, including topics of cranberry use, catheter coatings, antimicrobial prophylaxis, washout/irrigation strategies, and sterile versus clean intermittent straight catheterization, were excluded from dual abstraction. Two records were excluded after team discussion of the dual-abstraction results, because 1 study did not meet criteria as an intervention study and 1 study’s setting was not applicable in nursing homes. A total of 20 records15,20-38 (in which 19 studies were described) were selected for final inclusion for detailed assessment and reporting for the systematic review.
Characteristics of Included Studies
Table 1 describes the 19 intervention studies in terms of design, participants, setting, and whether the study included specific categories of interventions expected to decrease UTI or catheter use. These studies included 8 randomized controlled trials (4 with cluster-randomization at the facility or unit level), 10 pre-post nonrandomized interventions, and 1 nonrandomized intervention with concurrent controls. Twelve studies included participants with or without catheters (ie, not limited to catheterized patients only) in nursing homes.15,20-31 Seven32-38 studies included catheterized patients only or settings with high expected catheterization rates; settings for these studies included spinal cord units (n=3), nursing homes (n=2), rehabilitation ward (n=1) and VA hospital (n=1), including acute care, nursing home, and rehabilitation units. Total quality scores for the studies ranged from 8 to 25 (median, 15), detailed in Supplemental Table 1.
As detailed in Table 1 and Supplemental Table 2, 7 studies22,24,26,31,32,35,36 involved single interventions and 12 studies15,20,21,23,25,27-30,33,34,37,38 included multiple interventions. Interventions to impact catheter use and care were evaluated in 13 studies, including appropriateness of use,21,25,29,30 improving catheter maintenance care,15,20,29,30 securement,15,29,30,32 prompting removal of unnecessary catheters,21,25,29,30 improving incontinence care,15,21,23,25 bladder scanners,37,38 catheter changes,35and comparing alternatives (condom catheter or intermittent straight catheter) to use of an indwelling catheter.36,38 None focused on improving aseptic insertion. General infection control practices studied included improving hand hygiene,20-22,29-31,33,34 improving antibiotic use,15,20,21,28,34 initiation of infection control programs,20,21,28 interventions to improve identification of UTIs/CAUTIs using infection symptom/sign criteria,15,20,21,34 infection surveillance as an intervention,28-30,33,34 and barrier precautions,33,34 including preemptive precautions for catheterized patients.34 Hydration was assessed in 3 studies.24-26
Outcomes of Included Studies
Table 2 describes the studies’ outcomes reported for UTI, CAUTI, or bacteriuria.15,20-38 The outcome definitions of UTI and CAUTI varied widely. Only 2 studies22,39 reported UTI outcomes using definitions specific for nursing home settings such as McGeer’s criteria40 a detailed review and comparison of published CAUTI definitions used clinically and for surveillance in nursing homes is provided in Supplemental Table 3. Two studies reported symptomatic CAUTIs per 1000 catheter-days.32,34 Another study22 reported symptomatic CAUTIs per 1000 resident-days. Three reported symptomatic CAUTIs as counts.35,38 Saint et al36 reported CAUTIs as part of a combined outcome (ie, bacteriuria, CAUTI, or death).
The 19 studies (Table 2) reported 12 UTI outcomes,15,20,21,23,25-31,33 9 CAUTI outcomes,15,22,32,34,35,38 4 bacteriuria outcomes,24,36,38 and 5 catheter use outcomes.21,29,30,37,38 Five studies showed CAUTI reduction15,22,32,34,35 (1 significantly34); 9 studies showed UTI reduction13,18,19,21,23-25,27,28,31 (none significantly); 2 studies showed bacteriuria reduction (none significantly). One study36 reported 2 composite outcomes including bacteriuria or CAUTI or death, with statistically significant improvement reported for 1 composite measure. Four studies reported catheter use, with all showing reduced catheter use in the intervention group; however, only 1 achieved statistically significant reduction.37
Synthesis of Systematic Review Results
Overall, many studies reported decreases in UTI, CAUTI, and urinary catheter use measures but without statistical significance, with many studies likely underpowered for our outcomes of interest. Often, the outcomes of interest in this systematic review were not the main outcome for which the study was designed and originally powered. The interventions studied included several currently implemented as part of CAUTI bundles in the acute care setting, such as improving catheter use, and care and infection control strategies. Other included interventions target common challenges specific to the nursing home setting such as removing indwelling catheters upon admission to the nursing home from an acute-care facility21,25 and applying interventions to address incontinence by either general strategies21,23,25,30,38 or the use of an incontinence specialist23 to provide individual treatment plans. The only intervention that demonstrated a statistically significant reduction in CAUTI in chronically catheterized patients employed a comprehensive program to improve antimicrobial use, hand hygiene (including hand hygiene and gloves for catheter care), and preemptive precautions for patients with devices, along with promotion of standardized CAUTI definitions and active multidrug resistant organism surveillance.34
Narrative Review Results
Table 3 includes a comprehensive list of potential interventions that have been considered for prevention of UTI or CAUTI (including those in acute care and nursing home settings), as summarized from this systematic review and prior narrative or systematic reviews.43-115
DISCUSSION
We performed a broad systematic review of strategies to decrease UTI, CAUTI, and urinary catheter use that may be helpful in nursing homes. While many studies reported decreased UTI, CAUTI, or urinary catheter use measures, few demonstrated statistically significant reductions perhaps because many were underpowered to assess statistical significance. Pooled analyses were not feasible to provide the expected impact of these interventions in the nursing home setting.
This review confirms that bundles of interventions for prevention of CAUTI have been implemented with some evidence of success in nursing home settings, with several components in common with those implemented in the acute care setting, such as hand hygiene and strategies to reduce and improve catheter use.41 Some studies focused on issues more common in nursing homes such as chronic catheterization and incontinence. A nursing home CAUTI bundle should be designed with the resources and challenges present in the nursing home environment in mind, and with recognition that, although the number of patients with catheters is less than in acute care, there will be more patients with chronic catheterization needs and incontinence.
Although catheter utilization in nursing homes is low, further reductions in catheter days and CAUTIs can be achieved. Catheter removal reminders and stop orders have demonstrated a greater than 50% reduction in CAUTIs in acute care settings;11 an example of a stop-order intervention in nursing homes is trial removal of indwelling catheters present at facility admission without clear urologic need present at the time of admission.25 Nursing home interventions to avoid catheter placement should include incontinence programs, discussion of alternatives to indwelling urinary catheters with patients, families, and frontline personnel, and urinary retention protocols. Programs to reduce CAUTI should include education to improve aseptic insertion, and to maintain awareness and proper care of catheters in place by regular assessment of catheter necessity, securement, hand hygiene, and preemptive barrier precautions for catheterized patients. Interventions that focus on improving appropriate use of urine tests and antibiotics to treat UTIs can also significantly affect the rates of reported symptomatic CAUTIs, with the potential to decrease unnecessary antibiotic use.20,21
The main limitation of this review is that many studies provided little information about their intervention and definition of outcomes. The strength of this review is the detailed and broad search strategy applied with generous inclusion of interventions and outcomes to highlight the available evidence and details of interventions that have been studied and implemented.
CONCLUSION
This review synthesizes the current state of evidence and proposes strategies to reduce UTIs in nursing homes. Interventions that motivate catheter avoidance and catheter removal to prevent CAUTI in acute care11 and nursing home settings are supported by the strongest available evidence, although the strength of that evidence is less in the nursing home setting. Limitations notwithstanding, interventions such as incontinence care planning and hydration programs can reduce UTI in this population and is important for overall wellbeing.
Acknowledgments
The authors appreciate the guidance that Vineet Chopra MD, MSc, provided regarding options for methodological quality assessment tools, and the assistance of Mary Rogers PhD, MS, in interpreting the published Downs and Black Quality Index items, which informed our modification of this tool for application in this study. The authors appreciate, also, the feedback provided by the Agency for Healthcare Research and Quality (AHRQ) Content and Materials Development Committee for the AHRQ Safety Program for Long-Term Care: Preventing CAUTI and other Healthcare-associated Infections.
Disclosures
Agency for Healthcare Research and Quality (AHRQ) contract #HHSA290201000025I provided funding for this study, which was developed in response to AHRQ Task Order #8 for ACTION II RFTO 26 CUSP for CAUTI in LTC. AHRQ developed the details of the task and provided comments on a draft report, which informed the report submitted to AHRQ in December 2013, used to inform the interventions for a national collaborative (http://www.hret.org/quality/projects/long-term-care-cauti.shtml). Dr. Meddings’s effort on this project was funded by concurrent effort from her AHRQ (K08 HS19767). Dr. Saint’s and Dr. Krein’s effort on this project was funded by concurrent effort from the Veterans Affairs National Center for Patient Safety, Ann Arbor Patient Safety Center of Inquiry. Dr. Meddings’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, and the VA Ann Arbor Patient Safety Center of Inquiry. Dr. Krein’s other research is funded by a VA Health Services Research and Development Award (RCS 11-222). Dr. Mody’s other research is funded by VA Healthcare System Geriatric Research Clinical Care Center (GRECC), NIA-Pepper Center, NIA (R01AG032298, R01AG041780, K24AG050685-01). Dr. Saint has received fees for serving on advisory boards for Doximity and Jvion. All other authors report no financial conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the U.S. Department of Veterans Affairs. These analyses were presented in part as a poster presentation at the ID Week Annual Meeting on October 10, 2014 in Philadelphia, PA.
1. Beresford L. Post-acute patient care: new frontier for hospitalists. The Hospitalist.
July 2015. http://www.the-hospitalist.org/hospitalist/article/122330/post-acute-patient-
care-new-frontier-hospitalists. Accessed March 31, 2017.
2. Butterfield S. Hospital medicine matures: Hospitalists and hospitalist groups move into post-acute care. 2012. Available at http://www.acphospitalist.org/archives/2012/10/coverstory.htm. Accessed April 6, 2016.
3. Pittman D. SNFs: New Turf for Hospitalists? 2013; Available at http://www.medpagetoday.com/HospitalBasedMedicine/Hospitalists/39401. Accessed April 6, 2016.
4. Society of Hospital Medicine. SHM and IPC Healthcare Develop First SHM Primer for Hospitalists in Skilled Nursing Facilities. 2015; Available at http://www.hospitalmedicine.org/Web/Media_Center/Press_Release/2015/SHM_and_IPC_Healthcare_Develop_First_SHM_Primer_for_Hospitalists_in_Skilled_Nursing_Facilities.aspx. Accessed April 6, 2016.
5. Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health. 2011;7(6):889-899. PubMed
6. Phillips CD, Adepoju O, Stone N, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012;12:73. PubMed
7. Rogers M, Mody L, Kaufman S, Fries B, McMahon L, Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Amer Geriatr Soc. 2008;56:854-861. PubMed
8. Castle N, Engberg JB, Wagner LM, Handler S. Resident and facility factors associated with the incidence of urinary tract infections identified in the nursing home minimum data set. J Appl Gerontol. 2015:doi: 10.1177/0733464815584666. PubMed
9. Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department of Veterans Affairs community living centers. Am J Infect Control. 2010;38(6):461-466. PubMed
10. Kunin CM, Chin QF, Chambers S. Morbidity and mortality associated with indwelling urinary catheters in elderly patients in a nursing home--confounding due to the presence of associated diseases. J Am Geriatr Soc. 1987;35(11):1001-1006. PubMed
11. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2013;23(4):277-289. PubMed
12. Abraham F, Abraham FP. A CAUTI bundle with a twist. Am J Infect Control. 2012;40(5):e79-e80.
13. Flynn ER, Zombolis K. Reducing hospital acquired indwelling urinary catheter-associated urinary tract infections through multidisciplinary team and shared governance practice model. Am J Infect Control. 2011;39(5):E28-E29.
14. Gokula MR, Gaspar P, Siram R. Implementation of an evidence based protocol to reduce use of indwelling urinary catheters in the long term care environment. J Am Med Dir Assoc. 2013;14(3):B23.
15. Brownhill K. Training in care homes to reduce avoidable harm. Nurs Times. 2013;109(43):20-22. PubMed
16. Galeon CP, Romero I. Implementing a performance improvement project in a multi-level teaching facility on reducing catheter associated urinary tract infections (CAUTI). Am J Infect Control. 2014:S130-S131.
17. Evans ME, Kralovic SM, Simbartl LA, et al. Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. Am J Infect Control. 2014;42(1):60-62. PubMed
18. Evans KA, Ligon R, Lipton C. Reduction of antibiotic starts for asymptomatic bacteriuria in skilled nursing facilities. J Am Geriatr Soc. 2015;63:S131.
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Ahlbrecht H, Shearen C, Degelau J, Guay DR. Team approach to infection prevention and control in the nursing home setting. Am J Infect Control. 1999;27(1):64-70. PubMed
21. Cools HJ, van der Meer JW. Infection control in a skilled nursing facility: a 6-year survey. J Hosp Infect. 1988;12(2):117-124. PubMed
22. Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30(4):226-233. PubMed
23. Klay M, Marfyak K. Use of a continence nurse specialist in an extended care facility. Urol Nurs. 2005;25(2):101-102. PubMed
24. Lin S. A pilot study: fluid intake and bacteriuria in nursing home residents in southern Taiwan. Nurs Res. 2013;62(1):66-72. PubMed
25. McConnell J. Preventing urinary tract infections. Geriatr Nurs. 1984;5(8):361-362. PubMed
26. Mentes JC, Culp K. Reducing hydration-linked events in nursing home residents. Clin Nurs Res. 2003;12(3):210-225; discussion 226-218. PubMed
27. Miller SC, Lepore M, Lima JC, Shield R, Tyler DA. Does the introduction of nursing home culture change practices improve quality? J Am Geriatr Soc. 2014;62(9):1675-1682. PubMed
28. Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Healthcare Infection. 2015;20(1):4-6.
29. van Gaal B, Schoonhoven L, Mintjes JAJ, Borm GF, Koopmans RTCM, van Achterberg T. The SAFE or SORRY? programme. Part II: Effect on preventive care. Int J Nurs Stud. 2011;48(9):1049-1057. PubMed
30. van Gaal BGI, Schoonhoven L, Mintjes JAJ, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part I: primary outcome of a cluster randomised trial. Int J Nurs Stud. 2011;48(9):1040-1048. PubMed
31. Yeung WK, Wilson Tam WS, Wong TW. Clustered randomized controlled trial of a hand hygiene intervention involving pocket-sized containers of alcohol-based hand rub for the control of infections in long-term care facilities. Infect Control Hosp Epidemiol. 2011;32(1):67-76. PubMed
32. Darouiche RO, Goetz L, Kaldis T, Cerra-Stewart C, AlSharif A, Priebe M. Impact of StatLock securing device on symptomatic catheter-related urinary tract infection: a prospective, randomized, multicenter clinical trial. Am J Infect Control. 2006;34(9):555-560. PubMed
33. Evans ME, Kralovic SM, Simbartl LA, et al. Prevention of methicillin-resistant Staphylococcus aureus infections in spinal cord injury units. Am J Infect Control. 2013;41(5):422-426. PubMed
34. Mody L, Krein S, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175:714-723. PubMed
35. Priefer BA, Duthie Jr EH, Gambert SR. Frequency of urinary catheter change and clinical urinary tract infection. Study in hospital-based, skilled nursing home. Urology. 1982;20(2):141-142. PubMed
36. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. PubMed
37. Suardi L, Cazzaniga M, Spinelli M, Tagliabue A. From intermittent catheterisation to time-volume dependent catheterisation in patients with spinal cord injuries, through the use of a portable, ultrasound instrument. Europa Medicophysica. 2001;37(2):111-114.
38. Tang MW, Kwok TC, Hui E, Woo J. Intermittent versus indwelling urinary catheterization in older female patients. Maturitas. 2006;53(3):274-281. PubMed
39. Cassel BG, Parkes V, Poon R, Rae H. Quality improvement best practices and long-term indwelling urinary catheters. Perspectives. 2008;32(1):13-17. PubMed
40. Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965-977. PubMed
41. Saint S, Greene MT, Krein SL, et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. New England Journal of Medicine. 2016;374(22):2111-2119. PubMed
42. McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1-7. PubMed
43. Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol. 2001;22(5):316-321. PubMed
44. Nicolle LE; SHEA Long-Term Care Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175. PubMed
45. Nicolle LE. Catheter-related urinary tract infection. Drug & Aging. 2005;22(8):627-639. PubMed
46. Cochran S. Care of the indwelling urinary catheter - Is it evidence based? J Wound Ostomy Cont Nurs. 2007;34(3):282-288. PubMed
47. Seiler WO, Stahelin HB. Practical management of catheter-associated UTIs. Geriatrics. 1988;43(8):43-50. PubMed
48. Stickler DJ, Chawla JC. The role of antiseptics in the management of patients with long-term indwelling bladder catheters. J Hosp Infect. 1987;10(3):219-228. PubMed
49. Gray M. Does the construction material affect outcomes in long-term catheterization? J Wound Ostomy Cont Nurs. 2006;33(2):116-121. PubMed
50. Trautner BW, Darouiche RO. Clinical review: prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;2002(25):277-283. PubMed
51. Maloney C. Estrogen & recurrent UTI in postmenopausal women. Am J Nurs. 2002;102(8):44-52. PubMed
52. Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(suppl 1):S74-S76. PubMed
53. Godfrey H. Older people, continence care and catheters: dilemmas and resolutions. Br J Nurs. 2008;17(9):S4-S11. PubMed
54. Godfrey H, Evans A. Management of long-term urethral catheters: minimizing complications. Br J Nurs. 2000;9(2):74-76. PubMed
55. Kunin CM. Chemoprophylaxis and suppressive therapy in the management of urinary tract infections. J Antimicrob Chemother. 1994;33(suppl A):51-62. PubMed
56. Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31(1):12-48. PubMed
57. Allan GM, Nicolle L. Cranberry for preventing urinary tract infection. Can Fam Physician. 2013;59(4):367. PubMed
58. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. PubMed
59. Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(13):988-996. PubMed
60. Moore KN, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev. 2007(4):CD006008. PubMed
61. Li L, Ye WQ, Ruan H, Yang BY, Zhang SQ. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2013;94(4):782-787. PubMed
62. Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2011(12):CD004375. PubMed
63. Gray M. What nursing interventions reduce the risk of symptomatic urinary tract infections in the patient with an indwelling catheter? J Wound Ostomy Cont Nurs. 2004;31(1):3-13. PubMed
64. Marschall J, Carpenter C, Fowler S, Trautner B. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147. PubMed
65. Sinclair L, Hagen S, Cross S. Washout policies in long-term indwelling urinary catheterization in adults: a short version Cochrane review. Neurourol Urodyn. 2011;30(7):1208-1212. PubMed
66. Hunter KF, Bharmal A, Moore KN. Long-term bladder drainage: suprapubic catheter versus other methods: a scoping review. Neurourol Urodyn. 2013;32(7):944-951. PubMed
67. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002;83(1):129-138. PubMed
68. Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev. 2012(8). PubMed
69. Jepson R, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;10(CD001321). PubMed
70. Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751-754. PubMed
71. Bianco L, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc. 2012;60(6):1180-1181. PubMed
72. Lin SC, Wang CC, Shih SC, Tjung JJ, Tsou MT, Lin CJ. Prevention of Asymptomatic Bacteriuria with Cranberries and Roselle Juice in Home-care Patients with Long-term Urinary Catheterization. Int J Gerontol. 2014;8(3):152-156.
73. Juthani-Mehta M, Perley L, Chen S, Dziura J, Gupta K. Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J Am Geriatr Soc. 2010;58(10):2028-2030. PubMed
74. Tully CL, Bastone P, Vaughan J, Ballentine L. Urinary tract infection prophylaxis with cranberry extract in the nursing home setting. J Am Geriatr Soc. 2004;52(4):S206-S206.
75. Woodward N. Use of cranberry extract for the prevention of UTIs in an at-risk population. 41st Annual Wound, Ostomy and Continence Nurses Annual Conference, St. Louis, Missouri, June 6-10, 2009. J Wound Ostomy Continence Nurs. 2009;36(3S):S62-S62.
76. Linsenmeyer TA, Harrison B, Oakley A, Kirshblum S, Stock JA, Millis SR. Evaluation of cranberry supplement for reduction of urinary tract infections in individuals with neurogenic bladders secondary to spinal cord injury. A prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med. 2004;27(1):29-34. PubMed
77. Waites KB, Canupp KC, Armstrong S, DeVivo MJ. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med. 2004;27(1):35-40. PubMed
78. Caljouw MAA, Van Den Hout WB, Putter H, Achterberg WP, Cools HJM, Gussekloo J. Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons. A double-blind randomized placebo-controlled trial in long-term care facilities. Eur Geriatr Med. 2013;4:S118-S119. PubMed
79. Hout WB, Caljouw MAA, Putter H, Cools HJM, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: economic evaluation with a randomized controlled trial. J Am Geriatr Soc. 2014;62(1):111-116. PubMed
80. Liu BA, McGeer A, McArthur MA, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. 2007;55(1):35-42. PubMed
81. Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072-1079. PubMed
82. Maloney C. Hormone replacement therapy in female nursing home residents with recurrent urinary tract infection. Ann Long-Term Care. 1998;6(3):77-82.
83. Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35(9):589-593. PubMed
84. Salamon L. Catheter-associated urinary tract infections: a nurse-sensitive indicator in an inpatient rehabilitation program. Rehabil Nurs. 2009;34(6):237-241. PubMed
85. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. PubMed
86. American Medical Directors Association (AMDA). Appropriate indications for use of a chronic indwelling catheter in the long-term care setting. Columbia, MD; excerpted from AMDA's Clinical Practice Guideline: Urinary Incontinence. 2005.
87. Rannikko S, Kyllastinen M, Granqvist B. Comparison of long-term indwelling catheters and bed-pads in the treatment of urinary incontinence in elderly patients. J Infect. 1986;12(3):221-227. PubMed
88. Carapeti E, Andrews S, Bentley P. Randomised study of sterile versus non-sterile urethral catheterization. Ann R. Coll Surg Engl. 1996;78(1):59-60. PubMed
89. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. PubMed
90. Olsen-Scribner RJ, Hayes C, Pottinger P. Sustaining reduction of catheter-associated urinary tract infection (CAUTI)-outcomes after two educational methods in a regional university-affiliated medical center. Am J Infect Control. 2014;1:S22.
91. Duffy LM, Cleary J, Ahern S, et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc. 1995;43(8):865-870. PubMed
92. Joseph C, Jacobson C, Strausbaugh L, Maxwell M, French M, Colling J. Sterile vs clean urinary catheterization. J Am Geriatr Soc. 1991;39(10):1042-1043. PubMed
93. Moore KN, Burt J, Voaklander DC. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clin Rehabili. 2006;20(6):461-468. PubMed
94. Moore KN, Kelm M, Sinclair O, Cadrain G. Bacteriuria in intermittent catheterization users: the effect of sterile versus clean reused catheters. Rehabil Nurs J. 1993;18(5):306-309. PubMed
95. Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005(3):CD004203. PubMed
96. Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc. 1987;35(12):1063-1070. PubMed
97. Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int. 2012;110(11 Pt C):E910-917. PubMed
98. Jahn P, Beutner K, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev. 2012(10):CD004997. PubMed
99. Pickard R, Lam T, Maclennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380(9857):1927-1935. PubMed
100. Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med. 1981;70(3):655-658. PubMed
101. Hagen S, Sinclair L, Cross S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2010(3). PubMed
102. Moore KN, Hunter KF, McGinnis R, et al. Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomized controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound Ostomy Continence Nurs. 2009;36(1):82-90. PubMed
103. Muncie HL Jr, Hoopes JM, Damron DJ, Tenney JH, Warren JW. Once-daily irrigation of long-term urethral catheters with normal saline. Lack of benefit. Arch Intern Med. 1989;149(2):441- PubMed
104. Ruwaldt MM. Irrigation of indwelling urinary catheters. Urology. 1983;21(2):127-129. PubMed
105. Palka MA. Evidenced based review of recommendations addressing the frequency of changing long-term indwelling urinary catheters in older adults. Geriatr Nurs. 2014;35(5):357-363. PubMed
106. Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11(3):609-622. PubMed
107. Fryklund B, Haeggman S, Burman LG. Transmission of urinary bacterial strains between patients with indwelling catheters--nursing in the same room and in separate rooms compared. J Hosp Infect. 1997;36(2):147-153. PubMed
108. Anderson RU. Non-sterile intermittent catheterization with antibiotic prophylaxis in the acute spinal cord injured male patient. J Urol. 1980;124(3):392-394. PubMed
109. Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol. 1980;123(3):364-366. PubMed
110. Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95(2):141-152. PubMed
111. Rutschmann OT, Zwahlen A. Use of norfloxacin for prevention of symptomatic urinary tract infection in chronically catheterized patients. Eur J Clin Microbiol Infect Dis. 1995;14(5):441-444. PubMed
112. Jewes LA, Gillespie WA, Leadbetter A, et al. Bacteriuria and bacteraemia in patients with long-term indwelling catheters--a domiciliary study. J Med Microbiol. 1988;26(1):61-65. PubMed
113. Warren JW, Damron D, Tenney JH, Hoopes JM, Deforge B, Muncie HL, Jr. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis. 1987;155(6):1151-1158. PubMed
114. Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47(7):565-569. PubMed
Given the limited number of geriatricians in the U.S., hospitalists commonly manage nursing home residents admitted for post-acute care.1-4 Urinary tract infection (UTI) is one of the most common infections in nursing homes, often leading to sepsis and readmission to acute care.5 Inappropriate use of antibiotics to treat asymptomatic bacteriuria is both common and hazardous to nursing home residents.6 Up to 10% of nursing home residents will have an indwelling urinary catheter at some point during their stay.7-9 Residents with indwelling urinary catheters are at increased risk for catheter-associated urinary tract infection (CAUTI) and bacteriuria, with an estimated 50% of catheterized residents developing symptomatic CAUTI.5 While urinary catheter prevalence is lower in nursing homes than in the acute care setting, duration of use is often prolonged.7,10 In a setting where utilization is low, but use is prolonged, interventions designed to reduce UTI in acutely ill patients11 may not be as helpful for preventing infection in nursing home residents.
Our objective was to review the available evidence to prevent UTIs in nursing home residents to inform both bedside care and research efforts. Two types of literature review and summary were performed. First, we conducted a systematic review of individual studies reporting outcomes of UTI, CAUTI, bacteriuria, or urinary catheter use after interventions for reducing catheter use, improving insertion and maintenance of catheters, and/or general infection prevention strategies (eg, improving hand hygiene, infection surveillance, contact precautions, standardizing UTI diagnosis, and antibiotic use). Second, we performed a narrative review to generate an overview of evidence and published recommendations in both acute care and nursing home settings to prevent UTI in catheterized and non-catheterized older adults, which is provided as a comprehensive reference table for clinicians and researchers choosing and refining interventions to reduce UTIs.
METHODS
The systematic review was performed according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis recommendations. The protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews, (CRD42013005787). The narrative review was performed using the articles obtained from the systematic search and a targeted literature review by topic for a comprehensive list of interventions, including other interventions summarized in published reviews and guidelines.
Eligibility Criteria Review
Study Design. To address the breadth and depth of literature available to inform interventions to prevent UTI in nursing homes, broad eligibility criteria were applied with the expectation of varied designs and outcomes. All included studies for the systematic review were published manuscripts reporting a comparison group. We included randomized controlled trials as well as nonrandomized trials (pretest/posttest, with or without concurrent or nonconcurrent controls), with any duration of postintervention follow-up. Observational and retrospective studies were excluded.
Participants. We were interested in interventions and outcomes reported for nursing homes, defined as facilities providing short-stay skilled nursing care and/or rehabilitation, as well as long-term care. We also included evidence derived from rehabilitation facilities and spinal cord injury programs focused on reducing CAUTI risk for chronically catheterized residents. We excluded long-term acute care hospitals, hospice, psychiatric/mental health facilities, pediatric, and community dwelling/outpatient settings.
Interventions. We included interventions involving urinary catheter use such as improving appropriate use, aseptic placement, maintenance care, and prompting removal of unnecessary catheters. We included infection prevention strategies with a particular interest in hand hygiene, barrier precautions, infection control strategies, infection surveillance, use of standardized infection definitions, and interventions to improve antibiotic use. We included single and multiple interventions.
Outcomes
1. Healthcare-associated urinary tract infection: UTI occurring after admission to a healthcare facility, not identified specifically as catheter-associated. We categorized UTI outcomes with as much detail as provided, such as whether the reported outcome included only noncatheter-associated UTIs, the time required after admission (eg, more than 2 days), and whether the UTIs were defined by only laboratory criteria, clinically diagnosed infections, symptomatic, or long-term care specific surveillance definitions.
2. Catheter-associated urinary tract infection: UTI occurring in patients during or immediately after use of a urinary catheter. We noted whether CAUTI was defined by laboratory criteria, clinical symptoms, provider diagnosis, or antimicrobial treatment for case identification. We were primarily interested in CAUTI developing after placing an indwelling urinary catheter, commonly known as a Foley, but also in CAUTI occurring with other catheter types such as intermittent straight catheters, external or “condom” catheters, and suprapubic catheters.
3. Bacteriuria: We included the laboratory-based definition of bacteriuria as an outcome to include studies that reduced asymptomatic bacteriuria.
4. Urinary catheter use measures: This includes measures such as urinary catheter utilization ratios (catheter-days/patient-days), prevalence of urinary catheter use, or percentage of catheters with an appropriate indication.
Study Characteristics for Inclusion. Our systematic search included published papers in the English language. We did not exclude studies based on the number of facilities included or eligible, residents/patients included (based on age, gender, catheter use or type, or antibiotic use), intervention details, study withdrawal, loss to follow-up, death, or duration of pre-intervention and postintervention phases.
Data Sources and Searches
The following data sources were searched: Ovid MEDLINE (1950 to June 22, 2015), Cochrane Library via Wiley (1960 to June 22, 2015), CINAHL (1981 to June 22, 2015), Web of Science (1926 to June 22, 2015), and Embase.com (1946 to June 22, 2015). Two major systematic search strategies were performed for this review (Figure). Systematic search 1 was designed broadly using all data sources described above to identify interventions aimed at reducing all UTI events (defined under “Outcomes” above) or urinary catheter use (all types), focusing on interventions evaluated in nursing homes. Systematic search 2 was conducted in Ovid MEDLINE to identify studies to reduce UTI events or urinary catheter use measures for patients with a history of long-term or chronic catheter use, including nursing homes and other post-acute care settings such as rehabilitation units or hospitals and spinal cord injury programs, which have large populations of patients with chronic catheter needs. To inform the completeness of the broader systematic searches, supplemental systematic search strategies were performed for specific topics including hydration (supplemental search 1), published work by nursing home researchers known to the authors (supplemental search 2), and contact precautions (supplemental search 3). Search 1 is available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005787. Full search strategies for search 2 and supplemental searches are available upon request.
Study Selection
One author performed an initial screen of all records retrieved by the systematic searches by title and abstract and applied the initial exclusions (eg, non-human, no outcomes of interest), identified duplicate records, and assigned potentially relevant studies into groups such as review articles, epidemiology, interventions, and articles requiring further text review before categorization (Figure). After initial screening, Dr. Meddings reviewed the records by title/abstract. Reference lists were reviewed for potential articles for inclusion. Full-text article review informed the selection of those for dual abstraction and quality scoring performed by 2 authors, with discrepancies resolved by a third author. We requested additional information from authors from whom our search had generated only an abstract or brief report, or when additional information such as pre-intervention data was needed.12-18
Data Extraction and Quality Assessment
Relevant data regarding study design, participants, inclusion/exclusion criteria, outcomes, and quality criteria were abstracted independently by 2 authors. Methodological quality scores were assigned using a modification of the Quality index checklist developed by Downs and Black appropriate for assessing both randomized and nonrandomized studies of healthcare interventions.19 We also reviewed study funding sources and other potential quality concerns.
Data Analysis
Due to large trial heterogeneity among these studies about interventions and outcomes reported, outcome data could not be combined into summary measures for meta-analysis to give overall estimates of treatment effects.
RESULTS
Systematic Search Results and Study Selection
As detailed in the study flow diagram (Figure), 5794 total records were retrieved by systematic search 1 (4697 studies), search 2 (909 studies), and supplemental searches (188 studies). Hand searching of reference lists of 41 reviews (including narrative and systematic reviews) yielded 77 additional studies for consideration. Twenty-nine records on interventions that were the focus of systematic reviews, including topics of cranberry use, catheter coatings, antimicrobial prophylaxis, washout/irrigation strategies, and sterile versus clean intermittent straight catheterization, were excluded from dual abstraction. Two records were excluded after team discussion of the dual-abstraction results, because 1 study did not meet criteria as an intervention study and 1 study’s setting was not applicable in nursing homes. A total of 20 records15,20-38 (in which 19 studies were described) were selected for final inclusion for detailed assessment and reporting for the systematic review.
Characteristics of Included Studies
Table 1 describes the 19 intervention studies in terms of design, participants, setting, and whether the study included specific categories of interventions expected to decrease UTI or catheter use. These studies included 8 randomized controlled trials (4 with cluster-randomization at the facility or unit level), 10 pre-post nonrandomized interventions, and 1 nonrandomized intervention with concurrent controls. Twelve studies included participants with or without catheters (ie, not limited to catheterized patients only) in nursing homes.15,20-31 Seven32-38 studies included catheterized patients only or settings with high expected catheterization rates; settings for these studies included spinal cord units (n=3), nursing homes (n=2), rehabilitation ward (n=1) and VA hospital (n=1), including acute care, nursing home, and rehabilitation units. Total quality scores for the studies ranged from 8 to 25 (median, 15), detailed in Supplemental Table 1.
As detailed in Table 1 and Supplemental Table 2, 7 studies22,24,26,31,32,35,36 involved single interventions and 12 studies15,20,21,23,25,27-30,33,34,37,38 included multiple interventions. Interventions to impact catheter use and care were evaluated in 13 studies, including appropriateness of use,21,25,29,30 improving catheter maintenance care,15,20,29,30 securement,15,29,30,32 prompting removal of unnecessary catheters,21,25,29,30 improving incontinence care,15,21,23,25 bladder scanners,37,38 catheter changes,35and comparing alternatives (condom catheter or intermittent straight catheter) to use of an indwelling catheter.36,38 None focused on improving aseptic insertion. General infection control practices studied included improving hand hygiene,20-22,29-31,33,34 improving antibiotic use,15,20,21,28,34 initiation of infection control programs,20,21,28 interventions to improve identification of UTIs/CAUTIs using infection symptom/sign criteria,15,20,21,34 infection surveillance as an intervention,28-30,33,34 and barrier precautions,33,34 including preemptive precautions for catheterized patients.34 Hydration was assessed in 3 studies.24-26
Outcomes of Included Studies
Table 2 describes the studies’ outcomes reported for UTI, CAUTI, or bacteriuria.15,20-38 The outcome definitions of UTI and CAUTI varied widely. Only 2 studies22,39 reported UTI outcomes using definitions specific for nursing home settings such as McGeer’s criteria40 a detailed review and comparison of published CAUTI definitions used clinically and for surveillance in nursing homes is provided in Supplemental Table 3. Two studies reported symptomatic CAUTIs per 1000 catheter-days.32,34 Another study22 reported symptomatic CAUTIs per 1000 resident-days. Three reported symptomatic CAUTIs as counts.35,38 Saint et al36 reported CAUTIs as part of a combined outcome (ie, bacteriuria, CAUTI, or death).
The 19 studies (Table 2) reported 12 UTI outcomes,15,20,21,23,25-31,33 9 CAUTI outcomes,15,22,32,34,35,38 4 bacteriuria outcomes,24,36,38 and 5 catheter use outcomes.21,29,30,37,38 Five studies showed CAUTI reduction15,22,32,34,35 (1 significantly34); 9 studies showed UTI reduction13,18,19,21,23-25,27,28,31 (none significantly); 2 studies showed bacteriuria reduction (none significantly). One study36 reported 2 composite outcomes including bacteriuria or CAUTI or death, with statistically significant improvement reported for 1 composite measure. Four studies reported catheter use, with all showing reduced catheter use in the intervention group; however, only 1 achieved statistically significant reduction.37
Synthesis of Systematic Review Results
Overall, many studies reported decreases in UTI, CAUTI, and urinary catheter use measures but without statistical significance, with many studies likely underpowered for our outcomes of interest. Often, the outcomes of interest in this systematic review were not the main outcome for which the study was designed and originally powered. The interventions studied included several currently implemented as part of CAUTI bundles in the acute care setting, such as improving catheter use, and care and infection control strategies. Other included interventions target common challenges specific to the nursing home setting such as removing indwelling catheters upon admission to the nursing home from an acute-care facility21,25 and applying interventions to address incontinence by either general strategies21,23,25,30,38 or the use of an incontinence specialist23 to provide individual treatment plans. The only intervention that demonstrated a statistically significant reduction in CAUTI in chronically catheterized patients employed a comprehensive program to improve antimicrobial use, hand hygiene (including hand hygiene and gloves for catheter care), and preemptive precautions for patients with devices, along with promotion of standardized CAUTI definitions and active multidrug resistant organism surveillance.34
Narrative Review Results
Table 3 includes a comprehensive list of potential interventions that have been considered for prevention of UTI or CAUTI (including those in acute care and nursing home settings), as summarized from this systematic review and prior narrative or systematic reviews.43-115
DISCUSSION
We performed a broad systematic review of strategies to decrease UTI, CAUTI, and urinary catheter use that may be helpful in nursing homes. While many studies reported decreased UTI, CAUTI, or urinary catheter use measures, few demonstrated statistically significant reductions perhaps because many were underpowered to assess statistical significance. Pooled analyses were not feasible to provide the expected impact of these interventions in the nursing home setting.
This review confirms that bundles of interventions for prevention of CAUTI have been implemented with some evidence of success in nursing home settings, with several components in common with those implemented in the acute care setting, such as hand hygiene and strategies to reduce and improve catheter use.41 Some studies focused on issues more common in nursing homes such as chronic catheterization and incontinence. A nursing home CAUTI bundle should be designed with the resources and challenges present in the nursing home environment in mind, and with recognition that, although the number of patients with catheters is less than in acute care, there will be more patients with chronic catheterization needs and incontinence.
Although catheter utilization in nursing homes is low, further reductions in catheter days and CAUTIs can be achieved. Catheter removal reminders and stop orders have demonstrated a greater than 50% reduction in CAUTIs in acute care settings;11 an example of a stop-order intervention in nursing homes is trial removal of indwelling catheters present at facility admission without clear urologic need present at the time of admission.25 Nursing home interventions to avoid catheter placement should include incontinence programs, discussion of alternatives to indwelling urinary catheters with patients, families, and frontline personnel, and urinary retention protocols. Programs to reduce CAUTI should include education to improve aseptic insertion, and to maintain awareness and proper care of catheters in place by regular assessment of catheter necessity, securement, hand hygiene, and preemptive barrier precautions for catheterized patients. Interventions that focus on improving appropriate use of urine tests and antibiotics to treat UTIs can also significantly affect the rates of reported symptomatic CAUTIs, with the potential to decrease unnecessary antibiotic use.20,21
The main limitation of this review is that many studies provided little information about their intervention and definition of outcomes. The strength of this review is the detailed and broad search strategy applied with generous inclusion of interventions and outcomes to highlight the available evidence and details of interventions that have been studied and implemented.
CONCLUSION
This review synthesizes the current state of evidence and proposes strategies to reduce UTIs in nursing homes. Interventions that motivate catheter avoidance and catheter removal to prevent CAUTI in acute care11 and nursing home settings are supported by the strongest available evidence, although the strength of that evidence is less in the nursing home setting. Limitations notwithstanding, interventions such as incontinence care planning and hydration programs can reduce UTI in this population and is important for overall wellbeing.
Acknowledgments
The authors appreciate the guidance that Vineet Chopra MD, MSc, provided regarding options for methodological quality assessment tools, and the assistance of Mary Rogers PhD, MS, in interpreting the published Downs and Black Quality Index items, which informed our modification of this tool for application in this study. The authors appreciate, also, the feedback provided by the Agency for Healthcare Research and Quality (AHRQ) Content and Materials Development Committee for the AHRQ Safety Program for Long-Term Care: Preventing CAUTI and other Healthcare-associated Infections.
Disclosures
Agency for Healthcare Research and Quality (AHRQ) contract #HHSA290201000025I provided funding for this study, which was developed in response to AHRQ Task Order #8 for ACTION II RFTO 26 CUSP for CAUTI in LTC. AHRQ developed the details of the task and provided comments on a draft report, which informed the report submitted to AHRQ in December 2013, used to inform the interventions for a national collaborative (http://www.hret.org/quality/projects/long-term-care-cauti.shtml). Dr. Meddings’s effort on this project was funded by concurrent effort from her AHRQ (K08 HS19767). Dr. Saint’s and Dr. Krein’s effort on this project was funded by concurrent effort from the Veterans Affairs National Center for Patient Safety, Ann Arbor Patient Safety Center of Inquiry. Dr. Meddings’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, and the VA Ann Arbor Patient Safety Center of Inquiry. Dr. Krein’s other research is funded by a VA Health Services Research and Development Award (RCS 11-222). Dr. Mody’s other research is funded by VA Healthcare System Geriatric Research Clinical Care Center (GRECC), NIA-Pepper Center, NIA (R01AG032298, R01AG041780, K24AG050685-01). Dr. Saint has received fees for serving on advisory boards for Doximity and Jvion. All other authors report no financial conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the U.S. Department of Veterans Affairs. These analyses were presented in part as a poster presentation at the ID Week Annual Meeting on October 10, 2014 in Philadelphia, PA.
Given the limited number of geriatricians in the U.S., hospitalists commonly manage nursing home residents admitted for post-acute care.1-4 Urinary tract infection (UTI) is one of the most common infections in nursing homes, often leading to sepsis and readmission to acute care.5 Inappropriate use of antibiotics to treat asymptomatic bacteriuria is both common and hazardous to nursing home residents.6 Up to 10% of nursing home residents will have an indwelling urinary catheter at some point during their stay.7-9 Residents with indwelling urinary catheters are at increased risk for catheter-associated urinary tract infection (CAUTI) and bacteriuria, with an estimated 50% of catheterized residents developing symptomatic CAUTI.5 While urinary catheter prevalence is lower in nursing homes than in the acute care setting, duration of use is often prolonged.7,10 In a setting where utilization is low, but use is prolonged, interventions designed to reduce UTI in acutely ill patients11 may not be as helpful for preventing infection in nursing home residents.
Our objective was to review the available evidence to prevent UTIs in nursing home residents to inform both bedside care and research efforts. Two types of literature review and summary were performed. First, we conducted a systematic review of individual studies reporting outcomes of UTI, CAUTI, bacteriuria, or urinary catheter use after interventions for reducing catheter use, improving insertion and maintenance of catheters, and/or general infection prevention strategies (eg, improving hand hygiene, infection surveillance, contact precautions, standardizing UTI diagnosis, and antibiotic use). Second, we performed a narrative review to generate an overview of evidence and published recommendations in both acute care and nursing home settings to prevent UTI in catheterized and non-catheterized older adults, which is provided as a comprehensive reference table for clinicians and researchers choosing and refining interventions to reduce UTIs.
METHODS
The systematic review was performed according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis recommendations. The protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews, (CRD42013005787). The narrative review was performed using the articles obtained from the systematic search and a targeted literature review by topic for a comprehensive list of interventions, including other interventions summarized in published reviews and guidelines.
Eligibility Criteria Review
Study Design. To address the breadth and depth of literature available to inform interventions to prevent UTI in nursing homes, broad eligibility criteria were applied with the expectation of varied designs and outcomes. All included studies for the systematic review were published manuscripts reporting a comparison group. We included randomized controlled trials as well as nonrandomized trials (pretest/posttest, with or without concurrent or nonconcurrent controls), with any duration of postintervention follow-up. Observational and retrospective studies were excluded.
Participants. We were interested in interventions and outcomes reported for nursing homes, defined as facilities providing short-stay skilled nursing care and/or rehabilitation, as well as long-term care. We also included evidence derived from rehabilitation facilities and spinal cord injury programs focused on reducing CAUTI risk for chronically catheterized residents. We excluded long-term acute care hospitals, hospice, psychiatric/mental health facilities, pediatric, and community dwelling/outpatient settings.
Interventions. We included interventions involving urinary catheter use such as improving appropriate use, aseptic placement, maintenance care, and prompting removal of unnecessary catheters. We included infection prevention strategies with a particular interest in hand hygiene, barrier precautions, infection control strategies, infection surveillance, use of standardized infection definitions, and interventions to improve antibiotic use. We included single and multiple interventions.
Outcomes
1. Healthcare-associated urinary tract infection: UTI occurring after admission to a healthcare facility, not identified specifically as catheter-associated. We categorized UTI outcomes with as much detail as provided, such as whether the reported outcome included only noncatheter-associated UTIs, the time required after admission (eg, more than 2 days), and whether the UTIs were defined by only laboratory criteria, clinically diagnosed infections, symptomatic, or long-term care specific surveillance definitions.
2. Catheter-associated urinary tract infection: UTI occurring in patients during or immediately after use of a urinary catheter. We noted whether CAUTI was defined by laboratory criteria, clinical symptoms, provider diagnosis, or antimicrobial treatment for case identification. We were primarily interested in CAUTI developing after placing an indwelling urinary catheter, commonly known as a Foley, but also in CAUTI occurring with other catheter types such as intermittent straight catheters, external or “condom” catheters, and suprapubic catheters.
3. Bacteriuria: We included the laboratory-based definition of bacteriuria as an outcome to include studies that reduced asymptomatic bacteriuria.
4. Urinary catheter use measures: This includes measures such as urinary catheter utilization ratios (catheter-days/patient-days), prevalence of urinary catheter use, or percentage of catheters with an appropriate indication.
Study Characteristics for Inclusion. Our systematic search included published papers in the English language. We did not exclude studies based on the number of facilities included or eligible, residents/patients included (based on age, gender, catheter use or type, or antibiotic use), intervention details, study withdrawal, loss to follow-up, death, or duration of pre-intervention and postintervention phases.
Data Sources and Searches
The following data sources were searched: Ovid MEDLINE (1950 to June 22, 2015), Cochrane Library via Wiley (1960 to June 22, 2015), CINAHL (1981 to June 22, 2015), Web of Science (1926 to June 22, 2015), and Embase.com (1946 to June 22, 2015). Two major systematic search strategies were performed for this review (Figure). Systematic search 1 was designed broadly using all data sources described above to identify interventions aimed at reducing all UTI events (defined under “Outcomes” above) or urinary catheter use (all types), focusing on interventions evaluated in nursing homes. Systematic search 2 was conducted in Ovid MEDLINE to identify studies to reduce UTI events or urinary catheter use measures for patients with a history of long-term or chronic catheter use, including nursing homes and other post-acute care settings such as rehabilitation units or hospitals and spinal cord injury programs, which have large populations of patients with chronic catheter needs. To inform the completeness of the broader systematic searches, supplemental systematic search strategies were performed for specific topics including hydration (supplemental search 1), published work by nursing home researchers known to the authors (supplemental search 2), and contact precautions (supplemental search 3). Search 1 is available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005787. Full search strategies for search 2 and supplemental searches are available upon request.
Study Selection
One author performed an initial screen of all records retrieved by the systematic searches by title and abstract and applied the initial exclusions (eg, non-human, no outcomes of interest), identified duplicate records, and assigned potentially relevant studies into groups such as review articles, epidemiology, interventions, and articles requiring further text review before categorization (Figure). After initial screening, Dr. Meddings reviewed the records by title/abstract. Reference lists were reviewed for potential articles for inclusion. Full-text article review informed the selection of those for dual abstraction and quality scoring performed by 2 authors, with discrepancies resolved by a third author. We requested additional information from authors from whom our search had generated only an abstract or brief report, or when additional information such as pre-intervention data was needed.12-18
Data Extraction and Quality Assessment
Relevant data regarding study design, participants, inclusion/exclusion criteria, outcomes, and quality criteria were abstracted independently by 2 authors. Methodological quality scores were assigned using a modification of the Quality index checklist developed by Downs and Black appropriate for assessing both randomized and nonrandomized studies of healthcare interventions.19 We also reviewed study funding sources and other potential quality concerns.
Data Analysis
Due to large trial heterogeneity among these studies about interventions and outcomes reported, outcome data could not be combined into summary measures for meta-analysis to give overall estimates of treatment effects.
RESULTS
Systematic Search Results and Study Selection
As detailed in the study flow diagram (Figure), 5794 total records were retrieved by systematic search 1 (4697 studies), search 2 (909 studies), and supplemental searches (188 studies). Hand searching of reference lists of 41 reviews (including narrative and systematic reviews) yielded 77 additional studies for consideration. Twenty-nine records on interventions that were the focus of systematic reviews, including topics of cranberry use, catheter coatings, antimicrobial prophylaxis, washout/irrigation strategies, and sterile versus clean intermittent straight catheterization, were excluded from dual abstraction. Two records were excluded after team discussion of the dual-abstraction results, because 1 study did not meet criteria as an intervention study and 1 study’s setting was not applicable in nursing homes. A total of 20 records15,20-38 (in which 19 studies were described) were selected for final inclusion for detailed assessment and reporting for the systematic review.
Characteristics of Included Studies
Table 1 describes the 19 intervention studies in terms of design, participants, setting, and whether the study included specific categories of interventions expected to decrease UTI or catheter use. These studies included 8 randomized controlled trials (4 with cluster-randomization at the facility or unit level), 10 pre-post nonrandomized interventions, and 1 nonrandomized intervention with concurrent controls. Twelve studies included participants with or without catheters (ie, not limited to catheterized patients only) in nursing homes.15,20-31 Seven32-38 studies included catheterized patients only or settings with high expected catheterization rates; settings for these studies included spinal cord units (n=3), nursing homes (n=2), rehabilitation ward (n=1) and VA hospital (n=1), including acute care, nursing home, and rehabilitation units. Total quality scores for the studies ranged from 8 to 25 (median, 15), detailed in Supplemental Table 1.
As detailed in Table 1 and Supplemental Table 2, 7 studies22,24,26,31,32,35,36 involved single interventions and 12 studies15,20,21,23,25,27-30,33,34,37,38 included multiple interventions. Interventions to impact catheter use and care were evaluated in 13 studies, including appropriateness of use,21,25,29,30 improving catheter maintenance care,15,20,29,30 securement,15,29,30,32 prompting removal of unnecessary catheters,21,25,29,30 improving incontinence care,15,21,23,25 bladder scanners,37,38 catheter changes,35and comparing alternatives (condom catheter or intermittent straight catheter) to use of an indwelling catheter.36,38 None focused on improving aseptic insertion. General infection control practices studied included improving hand hygiene,20-22,29-31,33,34 improving antibiotic use,15,20,21,28,34 initiation of infection control programs,20,21,28 interventions to improve identification of UTIs/CAUTIs using infection symptom/sign criteria,15,20,21,34 infection surveillance as an intervention,28-30,33,34 and barrier precautions,33,34 including preemptive precautions for catheterized patients.34 Hydration was assessed in 3 studies.24-26
Outcomes of Included Studies
Table 2 describes the studies’ outcomes reported for UTI, CAUTI, or bacteriuria.15,20-38 The outcome definitions of UTI and CAUTI varied widely. Only 2 studies22,39 reported UTI outcomes using definitions specific for nursing home settings such as McGeer’s criteria40 a detailed review and comparison of published CAUTI definitions used clinically and for surveillance in nursing homes is provided in Supplemental Table 3. Two studies reported symptomatic CAUTIs per 1000 catheter-days.32,34 Another study22 reported symptomatic CAUTIs per 1000 resident-days. Three reported symptomatic CAUTIs as counts.35,38 Saint et al36 reported CAUTIs as part of a combined outcome (ie, bacteriuria, CAUTI, or death).
The 19 studies (Table 2) reported 12 UTI outcomes,15,20,21,23,25-31,33 9 CAUTI outcomes,15,22,32,34,35,38 4 bacteriuria outcomes,24,36,38 and 5 catheter use outcomes.21,29,30,37,38 Five studies showed CAUTI reduction15,22,32,34,35 (1 significantly34); 9 studies showed UTI reduction13,18,19,21,23-25,27,28,31 (none significantly); 2 studies showed bacteriuria reduction (none significantly). One study36 reported 2 composite outcomes including bacteriuria or CAUTI or death, with statistically significant improvement reported for 1 composite measure. Four studies reported catheter use, with all showing reduced catheter use in the intervention group; however, only 1 achieved statistically significant reduction.37
Synthesis of Systematic Review Results
Overall, many studies reported decreases in UTI, CAUTI, and urinary catheter use measures but without statistical significance, with many studies likely underpowered for our outcomes of interest. Often, the outcomes of interest in this systematic review were not the main outcome for which the study was designed and originally powered. The interventions studied included several currently implemented as part of CAUTI bundles in the acute care setting, such as improving catheter use, and care and infection control strategies. Other included interventions target common challenges specific to the nursing home setting such as removing indwelling catheters upon admission to the nursing home from an acute-care facility21,25 and applying interventions to address incontinence by either general strategies21,23,25,30,38 or the use of an incontinence specialist23 to provide individual treatment plans. The only intervention that demonstrated a statistically significant reduction in CAUTI in chronically catheterized patients employed a comprehensive program to improve antimicrobial use, hand hygiene (including hand hygiene and gloves for catheter care), and preemptive precautions for patients with devices, along with promotion of standardized CAUTI definitions and active multidrug resistant organism surveillance.34
Narrative Review Results
Table 3 includes a comprehensive list of potential interventions that have been considered for prevention of UTI or CAUTI (including those in acute care and nursing home settings), as summarized from this systematic review and prior narrative or systematic reviews.43-115
DISCUSSION
We performed a broad systematic review of strategies to decrease UTI, CAUTI, and urinary catheter use that may be helpful in nursing homes. While many studies reported decreased UTI, CAUTI, or urinary catheter use measures, few demonstrated statistically significant reductions perhaps because many were underpowered to assess statistical significance. Pooled analyses were not feasible to provide the expected impact of these interventions in the nursing home setting.
This review confirms that bundles of interventions for prevention of CAUTI have been implemented with some evidence of success in nursing home settings, with several components in common with those implemented in the acute care setting, such as hand hygiene and strategies to reduce and improve catheter use.41 Some studies focused on issues more common in nursing homes such as chronic catheterization and incontinence. A nursing home CAUTI bundle should be designed with the resources and challenges present in the nursing home environment in mind, and with recognition that, although the number of patients with catheters is less than in acute care, there will be more patients with chronic catheterization needs and incontinence.
Although catheter utilization in nursing homes is low, further reductions in catheter days and CAUTIs can be achieved. Catheter removal reminders and stop orders have demonstrated a greater than 50% reduction in CAUTIs in acute care settings;11 an example of a stop-order intervention in nursing homes is trial removal of indwelling catheters present at facility admission without clear urologic need present at the time of admission.25 Nursing home interventions to avoid catheter placement should include incontinence programs, discussion of alternatives to indwelling urinary catheters with patients, families, and frontline personnel, and urinary retention protocols. Programs to reduce CAUTI should include education to improve aseptic insertion, and to maintain awareness and proper care of catheters in place by regular assessment of catheter necessity, securement, hand hygiene, and preemptive barrier precautions for catheterized patients. Interventions that focus on improving appropriate use of urine tests and antibiotics to treat UTIs can also significantly affect the rates of reported symptomatic CAUTIs, with the potential to decrease unnecessary antibiotic use.20,21
The main limitation of this review is that many studies provided little information about their intervention and definition of outcomes. The strength of this review is the detailed and broad search strategy applied with generous inclusion of interventions and outcomes to highlight the available evidence and details of interventions that have been studied and implemented.
CONCLUSION
This review synthesizes the current state of evidence and proposes strategies to reduce UTIs in nursing homes. Interventions that motivate catheter avoidance and catheter removal to prevent CAUTI in acute care11 and nursing home settings are supported by the strongest available evidence, although the strength of that evidence is less in the nursing home setting. Limitations notwithstanding, interventions such as incontinence care planning and hydration programs can reduce UTI in this population and is important for overall wellbeing.
Acknowledgments
The authors appreciate the guidance that Vineet Chopra MD, MSc, provided regarding options for methodological quality assessment tools, and the assistance of Mary Rogers PhD, MS, in interpreting the published Downs and Black Quality Index items, which informed our modification of this tool for application in this study. The authors appreciate, also, the feedback provided by the Agency for Healthcare Research and Quality (AHRQ) Content and Materials Development Committee for the AHRQ Safety Program for Long-Term Care: Preventing CAUTI and other Healthcare-associated Infections.
Disclosures
Agency for Healthcare Research and Quality (AHRQ) contract #HHSA290201000025I provided funding for this study, which was developed in response to AHRQ Task Order #8 for ACTION II RFTO 26 CUSP for CAUTI in LTC. AHRQ developed the details of the task and provided comments on a draft report, which informed the report submitted to AHRQ in December 2013, used to inform the interventions for a national collaborative (http://www.hret.org/quality/projects/long-term-care-cauti.shtml). Dr. Meddings’s effort on this project was funded by concurrent effort from her AHRQ (K08 HS19767). Dr. Saint’s and Dr. Krein’s effort on this project was funded by concurrent effort from the Veterans Affairs National Center for Patient Safety, Ann Arbor Patient Safety Center of Inquiry. Dr. Meddings’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, and the VA Ann Arbor Patient Safety Center of Inquiry. Dr. Krein’s other research is funded by a VA Health Services Research and Development Award (RCS 11-222). Dr. Mody’s other research is funded by VA Healthcare System Geriatric Research Clinical Care Center (GRECC), NIA-Pepper Center, NIA (R01AG032298, R01AG041780, K24AG050685-01). Dr. Saint has received fees for serving on advisory boards for Doximity and Jvion. All other authors report no financial conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the U.S. Department of Veterans Affairs. These analyses were presented in part as a poster presentation at the ID Week Annual Meeting on October 10, 2014 in Philadelphia, PA.
1. Beresford L. Post-acute patient care: new frontier for hospitalists. The Hospitalist.
July 2015. http://www.the-hospitalist.org/hospitalist/article/122330/post-acute-patient-
care-new-frontier-hospitalists. Accessed March 31, 2017.
2. Butterfield S. Hospital medicine matures: Hospitalists and hospitalist groups move into post-acute care. 2012. Available at http://www.acphospitalist.org/archives/2012/10/coverstory.htm. Accessed April 6, 2016.
3. Pittman D. SNFs: New Turf for Hospitalists? 2013; Available at http://www.medpagetoday.com/HospitalBasedMedicine/Hospitalists/39401. Accessed April 6, 2016.
4. Society of Hospital Medicine. SHM and IPC Healthcare Develop First SHM Primer for Hospitalists in Skilled Nursing Facilities. 2015; Available at http://www.hospitalmedicine.org/Web/Media_Center/Press_Release/2015/SHM_and_IPC_Healthcare_Develop_First_SHM_Primer_for_Hospitalists_in_Skilled_Nursing_Facilities.aspx. Accessed April 6, 2016.
5. Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health. 2011;7(6):889-899. PubMed
6. Phillips CD, Adepoju O, Stone N, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012;12:73. PubMed
7. Rogers M, Mody L, Kaufman S, Fries B, McMahon L, Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Amer Geriatr Soc. 2008;56:854-861. PubMed
8. Castle N, Engberg JB, Wagner LM, Handler S. Resident and facility factors associated with the incidence of urinary tract infections identified in the nursing home minimum data set. J Appl Gerontol. 2015:doi: 10.1177/0733464815584666. PubMed
9. Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department of Veterans Affairs community living centers. Am J Infect Control. 2010;38(6):461-466. PubMed
10. Kunin CM, Chin QF, Chambers S. Morbidity and mortality associated with indwelling urinary catheters in elderly patients in a nursing home--confounding due to the presence of associated diseases. J Am Geriatr Soc. 1987;35(11):1001-1006. PubMed
11. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2013;23(4):277-289. PubMed
12. Abraham F, Abraham FP. A CAUTI bundle with a twist. Am J Infect Control. 2012;40(5):e79-e80.
13. Flynn ER, Zombolis K. Reducing hospital acquired indwelling urinary catheter-associated urinary tract infections through multidisciplinary team and shared governance practice model. Am J Infect Control. 2011;39(5):E28-E29.
14. Gokula MR, Gaspar P, Siram R. Implementation of an evidence based protocol to reduce use of indwelling urinary catheters in the long term care environment. J Am Med Dir Assoc. 2013;14(3):B23.
15. Brownhill K. Training in care homes to reduce avoidable harm. Nurs Times. 2013;109(43):20-22. PubMed
16. Galeon CP, Romero I. Implementing a performance improvement project in a multi-level teaching facility on reducing catheter associated urinary tract infections (CAUTI). Am J Infect Control. 2014:S130-S131.
17. Evans ME, Kralovic SM, Simbartl LA, et al. Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. Am J Infect Control. 2014;42(1):60-62. PubMed
18. Evans KA, Ligon R, Lipton C. Reduction of antibiotic starts for asymptomatic bacteriuria in skilled nursing facilities. J Am Geriatr Soc. 2015;63:S131.
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Ahlbrecht H, Shearen C, Degelau J, Guay DR. Team approach to infection prevention and control in the nursing home setting. Am J Infect Control. 1999;27(1):64-70. PubMed
21. Cools HJ, van der Meer JW. Infection control in a skilled nursing facility: a 6-year survey. J Hosp Infect. 1988;12(2):117-124. PubMed
22. Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30(4):226-233. PubMed
23. Klay M, Marfyak K. Use of a continence nurse specialist in an extended care facility. Urol Nurs. 2005;25(2):101-102. PubMed
24. Lin S. A pilot study: fluid intake and bacteriuria in nursing home residents in southern Taiwan. Nurs Res. 2013;62(1):66-72. PubMed
25. McConnell J. Preventing urinary tract infections. Geriatr Nurs. 1984;5(8):361-362. PubMed
26. Mentes JC, Culp K. Reducing hydration-linked events in nursing home residents. Clin Nurs Res. 2003;12(3):210-225; discussion 226-218. PubMed
27. Miller SC, Lepore M, Lima JC, Shield R, Tyler DA. Does the introduction of nursing home culture change practices improve quality? J Am Geriatr Soc. 2014;62(9):1675-1682. PubMed
28. Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Healthcare Infection. 2015;20(1):4-6.
29. van Gaal B, Schoonhoven L, Mintjes JAJ, Borm GF, Koopmans RTCM, van Achterberg T. The SAFE or SORRY? programme. Part II: Effect on preventive care. Int J Nurs Stud. 2011;48(9):1049-1057. PubMed
30. van Gaal BGI, Schoonhoven L, Mintjes JAJ, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part I: primary outcome of a cluster randomised trial. Int J Nurs Stud. 2011;48(9):1040-1048. PubMed
31. Yeung WK, Wilson Tam WS, Wong TW. Clustered randomized controlled trial of a hand hygiene intervention involving pocket-sized containers of alcohol-based hand rub for the control of infections in long-term care facilities. Infect Control Hosp Epidemiol. 2011;32(1):67-76. PubMed
32. Darouiche RO, Goetz L, Kaldis T, Cerra-Stewart C, AlSharif A, Priebe M. Impact of StatLock securing device on symptomatic catheter-related urinary tract infection: a prospective, randomized, multicenter clinical trial. Am J Infect Control. 2006;34(9):555-560. PubMed
33. Evans ME, Kralovic SM, Simbartl LA, et al. Prevention of methicillin-resistant Staphylococcus aureus infections in spinal cord injury units. Am J Infect Control. 2013;41(5):422-426. PubMed
34. Mody L, Krein S, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175:714-723. PubMed
35. Priefer BA, Duthie Jr EH, Gambert SR. Frequency of urinary catheter change and clinical urinary tract infection. Study in hospital-based, skilled nursing home. Urology. 1982;20(2):141-142. PubMed
36. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. PubMed
37. Suardi L, Cazzaniga M, Spinelli M, Tagliabue A. From intermittent catheterisation to time-volume dependent catheterisation in patients with spinal cord injuries, through the use of a portable, ultrasound instrument. Europa Medicophysica. 2001;37(2):111-114.
38. Tang MW, Kwok TC, Hui E, Woo J. Intermittent versus indwelling urinary catheterization in older female patients. Maturitas. 2006;53(3):274-281. PubMed
39. Cassel BG, Parkes V, Poon R, Rae H. Quality improvement best practices and long-term indwelling urinary catheters. Perspectives. 2008;32(1):13-17. PubMed
40. Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965-977. PubMed
41. Saint S, Greene MT, Krein SL, et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. New England Journal of Medicine. 2016;374(22):2111-2119. PubMed
42. McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1-7. PubMed
43. Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol. 2001;22(5):316-321. PubMed
44. Nicolle LE; SHEA Long-Term Care Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175. PubMed
45. Nicolle LE. Catheter-related urinary tract infection. Drug & Aging. 2005;22(8):627-639. PubMed
46. Cochran S. Care of the indwelling urinary catheter - Is it evidence based? J Wound Ostomy Cont Nurs. 2007;34(3):282-288. PubMed
47. Seiler WO, Stahelin HB. Practical management of catheter-associated UTIs. Geriatrics. 1988;43(8):43-50. PubMed
48. Stickler DJ, Chawla JC. The role of antiseptics in the management of patients with long-term indwelling bladder catheters. J Hosp Infect. 1987;10(3):219-228. PubMed
49. Gray M. Does the construction material affect outcomes in long-term catheterization? J Wound Ostomy Cont Nurs. 2006;33(2):116-121. PubMed
50. Trautner BW, Darouiche RO. Clinical review: prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;2002(25):277-283. PubMed
51. Maloney C. Estrogen & recurrent UTI in postmenopausal women. Am J Nurs. 2002;102(8):44-52. PubMed
52. Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(suppl 1):S74-S76. PubMed
53. Godfrey H. Older people, continence care and catheters: dilemmas and resolutions. Br J Nurs. 2008;17(9):S4-S11. PubMed
54. Godfrey H, Evans A. Management of long-term urethral catheters: minimizing complications. Br J Nurs. 2000;9(2):74-76. PubMed
55. Kunin CM. Chemoprophylaxis and suppressive therapy in the management of urinary tract infections. J Antimicrob Chemother. 1994;33(suppl A):51-62. PubMed
56. Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31(1):12-48. PubMed
57. Allan GM, Nicolle L. Cranberry for preventing urinary tract infection. Can Fam Physician. 2013;59(4):367. PubMed
58. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. PubMed
59. Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(13):988-996. PubMed
60. Moore KN, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev. 2007(4):CD006008. PubMed
61. Li L, Ye WQ, Ruan H, Yang BY, Zhang SQ. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2013;94(4):782-787. PubMed
62. Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2011(12):CD004375. PubMed
63. Gray M. What nursing interventions reduce the risk of symptomatic urinary tract infections in the patient with an indwelling catheter? J Wound Ostomy Cont Nurs. 2004;31(1):3-13. PubMed
64. Marschall J, Carpenter C, Fowler S, Trautner B. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147. PubMed
65. Sinclair L, Hagen S, Cross S. Washout policies in long-term indwelling urinary catheterization in adults: a short version Cochrane review. Neurourol Urodyn. 2011;30(7):1208-1212. PubMed
66. Hunter KF, Bharmal A, Moore KN. Long-term bladder drainage: suprapubic catheter versus other methods: a scoping review. Neurourol Urodyn. 2013;32(7):944-951. PubMed
67. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002;83(1):129-138. PubMed
68. Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev. 2012(8). PubMed
69. Jepson R, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;10(CD001321). PubMed
70. Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751-754. PubMed
71. Bianco L, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc. 2012;60(6):1180-1181. PubMed
72. Lin SC, Wang CC, Shih SC, Tjung JJ, Tsou MT, Lin CJ. Prevention of Asymptomatic Bacteriuria with Cranberries and Roselle Juice in Home-care Patients with Long-term Urinary Catheterization. Int J Gerontol. 2014;8(3):152-156.
73. Juthani-Mehta M, Perley L, Chen S, Dziura J, Gupta K. Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J Am Geriatr Soc. 2010;58(10):2028-2030. PubMed
74. Tully CL, Bastone P, Vaughan J, Ballentine L. Urinary tract infection prophylaxis with cranberry extract in the nursing home setting. J Am Geriatr Soc. 2004;52(4):S206-S206.
75. Woodward N. Use of cranberry extract for the prevention of UTIs in an at-risk population. 41st Annual Wound, Ostomy and Continence Nurses Annual Conference, St. Louis, Missouri, June 6-10, 2009. J Wound Ostomy Continence Nurs. 2009;36(3S):S62-S62.
76. Linsenmeyer TA, Harrison B, Oakley A, Kirshblum S, Stock JA, Millis SR. Evaluation of cranberry supplement for reduction of urinary tract infections in individuals with neurogenic bladders secondary to spinal cord injury. A prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med. 2004;27(1):29-34. PubMed
77. Waites KB, Canupp KC, Armstrong S, DeVivo MJ. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med. 2004;27(1):35-40. PubMed
78. Caljouw MAA, Van Den Hout WB, Putter H, Achterberg WP, Cools HJM, Gussekloo J. Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons. A double-blind randomized placebo-controlled trial in long-term care facilities. Eur Geriatr Med. 2013;4:S118-S119. PubMed
79. Hout WB, Caljouw MAA, Putter H, Cools HJM, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: economic evaluation with a randomized controlled trial. J Am Geriatr Soc. 2014;62(1):111-116. PubMed
80. Liu BA, McGeer A, McArthur MA, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. 2007;55(1):35-42. PubMed
81. Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072-1079. PubMed
82. Maloney C. Hormone replacement therapy in female nursing home residents with recurrent urinary tract infection. Ann Long-Term Care. 1998;6(3):77-82.
83. Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35(9):589-593. PubMed
84. Salamon L. Catheter-associated urinary tract infections: a nurse-sensitive indicator in an inpatient rehabilitation program. Rehabil Nurs. 2009;34(6):237-241. PubMed
85. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. PubMed
86. American Medical Directors Association (AMDA). Appropriate indications for use of a chronic indwelling catheter in the long-term care setting. Columbia, MD; excerpted from AMDA's Clinical Practice Guideline: Urinary Incontinence. 2005.
87. Rannikko S, Kyllastinen M, Granqvist B. Comparison of long-term indwelling catheters and bed-pads in the treatment of urinary incontinence in elderly patients. J Infect. 1986;12(3):221-227. PubMed
88. Carapeti E, Andrews S, Bentley P. Randomised study of sterile versus non-sterile urethral catheterization. Ann R. Coll Surg Engl. 1996;78(1):59-60. PubMed
89. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. PubMed
90. Olsen-Scribner RJ, Hayes C, Pottinger P. Sustaining reduction of catheter-associated urinary tract infection (CAUTI)-outcomes after two educational methods in a regional university-affiliated medical center. Am J Infect Control. 2014;1:S22.
91. Duffy LM, Cleary J, Ahern S, et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc. 1995;43(8):865-870. PubMed
92. Joseph C, Jacobson C, Strausbaugh L, Maxwell M, French M, Colling J. Sterile vs clean urinary catheterization. J Am Geriatr Soc. 1991;39(10):1042-1043. PubMed
93. Moore KN, Burt J, Voaklander DC. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clin Rehabili. 2006;20(6):461-468. PubMed
94. Moore KN, Kelm M, Sinclair O, Cadrain G. Bacteriuria in intermittent catheterization users: the effect of sterile versus clean reused catheters. Rehabil Nurs J. 1993;18(5):306-309. PubMed
95. Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005(3):CD004203. PubMed
96. Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc. 1987;35(12):1063-1070. PubMed
97. Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int. 2012;110(11 Pt C):E910-917. PubMed
98. Jahn P, Beutner K, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev. 2012(10):CD004997. PubMed
99. Pickard R, Lam T, Maclennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380(9857):1927-1935. PubMed
100. Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med. 1981;70(3):655-658. PubMed
101. Hagen S, Sinclair L, Cross S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2010(3). PubMed
102. Moore KN, Hunter KF, McGinnis R, et al. Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomized controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound Ostomy Continence Nurs. 2009;36(1):82-90. PubMed
103. Muncie HL Jr, Hoopes JM, Damron DJ, Tenney JH, Warren JW. Once-daily irrigation of long-term urethral catheters with normal saline. Lack of benefit. Arch Intern Med. 1989;149(2):441- PubMed
104. Ruwaldt MM. Irrigation of indwelling urinary catheters. Urology. 1983;21(2):127-129. PubMed
105. Palka MA. Evidenced based review of recommendations addressing the frequency of changing long-term indwelling urinary catheters in older adults. Geriatr Nurs. 2014;35(5):357-363. PubMed
106. Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11(3):609-622. PubMed
107. Fryklund B, Haeggman S, Burman LG. Transmission of urinary bacterial strains between patients with indwelling catheters--nursing in the same room and in separate rooms compared. J Hosp Infect. 1997;36(2):147-153. PubMed
108. Anderson RU. Non-sterile intermittent catheterization with antibiotic prophylaxis in the acute spinal cord injured male patient. J Urol. 1980;124(3):392-394. PubMed
109. Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol. 1980;123(3):364-366. PubMed
110. Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95(2):141-152. PubMed
111. Rutschmann OT, Zwahlen A. Use of norfloxacin for prevention of symptomatic urinary tract infection in chronically catheterized patients. Eur J Clin Microbiol Infect Dis. 1995;14(5):441-444. PubMed
112. Jewes LA, Gillespie WA, Leadbetter A, et al. Bacteriuria and bacteraemia in patients with long-term indwelling catheters--a domiciliary study. J Med Microbiol. 1988;26(1):61-65. PubMed
113. Warren JW, Damron D, Tenney JH, Hoopes JM, Deforge B, Muncie HL, Jr. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis. 1987;155(6):1151-1158. PubMed
114. Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47(7):565-569. PubMed
1. Beresford L. Post-acute patient care: new frontier for hospitalists. The Hospitalist.
July 2015. http://www.the-hospitalist.org/hospitalist/article/122330/post-acute-patient-
care-new-frontier-hospitalists. Accessed March 31, 2017.
2. Butterfield S. Hospital medicine matures: Hospitalists and hospitalist groups move into post-acute care. 2012. Available at http://www.acphospitalist.org/archives/2012/10/coverstory.htm. Accessed April 6, 2016.
3. Pittman D. SNFs: New Turf for Hospitalists? 2013; Available at http://www.medpagetoday.com/HospitalBasedMedicine/Hospitalists/39401. Accessed April 6, 2016.
4. Society of Hospital Medicine. SHM and IPC Healthcare Develop First SHM Primer for Hospitalists in Skilled Nursing Facilities. 2015; Available at http://www.hospitalmedicine.org/Web/Media_Center/Press_Release/2015/SHM_and_IPC_Healthcare_Develop_First_SHM_Primer_for_Hospitalists_in_Skilled_Nursing_Facilities.aspx. Accessed April 6, 2016.
5. Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health. 2011;7(6):889-899. PubMed
6. Phillips CD, Adepoju O, Stone N, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012;12:73. PubMed
7. Rogers M, Mody L, Kaufman S, Fries B, McMahon L, Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Amer Geriatr Soc. 2008;56:854-861. PubMed
8. Castle N, Engberg JB, Wagner LM, Handler S. Resident and facility factors associated with the incidence of urinary tract infections identified in the nursing home minimum data set. J Appl Gerontol. 2015:doi: 10.1177/0733464815584666. PubMed
9. Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department of Veterans Affairs community living centers. Am J Infect Control. 2010;38(6):461-466. PubMed
10. Kunin CM, Chin QF, Chambers S. Morbidity and mortality associated with indwelling urinary catheters in elderly patients in a nursing home--confounding due to the presence of associated diseases. J Am Geriatr Soc. 1987;35(11):1001-1006. PubMed
11. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2013;23(4):277-289. PubMed
12. Abraham F, Abraham FP. A CAUTI bundle with a twist. Am J Infect Control. 2012;40(5):e79-e80.
13. Flynn ER, Zombolis K. Reducing hospital acquired indwelling urinary catheter-associated urinary tract infections through multidisciplinary team and shared governance practice model. Am J Infect Control. 2011;39(5):E28-E29.
14. Gokula MR, Gaspar P, Siram R. Implementation of an evidence based protocol to reduce use of indwelling urinary catheters in the long term care environment. J Am Med Dir Assoc. 2013;14(3):B23.
15. Brownhill K. Training in care homes to reduce avoidable harm. Nurs Times. 2013;109(43):20-22. PubMed
16. Galeon CP, Romero I. Implementing a performance improvement project in a multi-level teaching facility on reducing catheter associated urinary tract infections (CAUTI). Am J Infect Control. 2014:S130-S131.
17. Evans ME, Kralovic SM, Simbartl LA, et al. Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. Am J Infect Control. 2014;42(1):60-62. PubMed
18. Evans KA, Ligon R, Lipton C. Reduction of antibiotic starts for asymptomatic bacteriuria in skilled nursing facilities. J Am Geriatr Soc. 2015;63:S131.
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Ahlbrecht H, Shearen C, Degelau J, Guay DR. Team approach to infection prevention and control in the nursing home setting. Am J Infect Control. 1999;27(1):64-70. PubMed
21. Cools HJ, van der Meer JW. Infection control in a skilled nursing facility: a 6-year survey. J Hosp Infect. 1988;12(2):117-124. PubMed
22. Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30(4):226-233. PubMed
23. Klay M, Marfyak K. Use of a continence nurse specialist in an extended care facility. Urol Nurs. 2005;25(2):101-102. PubMed
24. Lin S. A pilot study: fluid intake and bacteriuria in nursing home residents in southern Taiwan. Nurs Res. 2013;62(1):66-72. PubMed
25. McConnell J. Preventing urinary tract infections. Geriatr Nurs. 1984;5(8):361-362. PubMed
26. Mentes JC, Culp K. Reducing hydration-linked events in nursing home residents. Clin Nurs Res. 2003;12(3):210-225; discussion 226-218. PubMed
27. Miller SC, Lepore M, Lima JC, Shield R, Tyler DA. Does the introduction of nursing home culture change practices improve quality? J Am Geriatr Soc. 2014;62(9):1675-1682. PubMed
28. Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Healthcare Infection. 2015;20(1):4-6.
29. van Gaal B, Schoonhoven L, Mintjes JAJ, Borm GF, Koopmans RTCM, van Achterberg T. The SAFE or SORRY? programme. Part II: Effect on preventive care. Int J Nurs Stud. 2011;48(9):1049-1057. PubMed
30. van Gaal BGI, Schoonhoven L, Mintjes JAJ, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part I: primary outcome of a cluster randomised trial. Int J Nurs Stud. 2011;48(9):1040-1048. PubMed
31. Yeung WK, Wilson Tam WS, Wong TW. Clustered randomized controlled trial of a hand hygiene intervention involving pocket-sized containers of alcohol-based hand rub for the control of infections in long-term care facilities. Infect Control Hosp Epidemiol. 2011;32(1):67-76. PubMed
32. Darouiche RO, Goetz L, Kaldis T, Cerra-Stewart C, AlSharif A, Priebe M. Impact of StatLock securing device on symptomatic catheter-related urinary tract infection: a prospective, randomized, multicenter clinical trial. Am J Infect Control. 2006;34(9):555-560. PubMed
33. Evans ME, Kralovic SM, Simbartl LA, et al. Prevention of methicillin-resistant Staphylococcus aureus infections in spinal cord injury units. Am J Infect Control. 2013;41(5):422-426. PubMed
34. Mody L, Krein S, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175:714-723. PubMed
35. Priefer BA, Duthie Jr EH, Gambert SR. Frequency of urinary catheter change and clinical urinary tract infection. Study in hospital-based, skilled nursing home. Urology. 1982;20(2):141-142. PubMed
36. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. PubMed
37. Suardi L, Cazzaniga M, Spinelli M, Tagliabue A. From intermittent catheterisation to time-volume dependent catheterisation in patients with spinal cord injuries, through the use of a portable, ultrasound instrument. Europa Medicophysica. 2001;37(2):111-114.
38. Tang MW, Kwok TC, Hui E, Woo J. Intermittent versus indwelling urinary catheterization in older female patients. Maturitas. 2006;53(3):274-281. PubMed
39. Cassel BG, Parkes V, Poon R, Rae H. Quality improvement best practices and long-term indwelling urinary catheters. Perspectives. 2008;32(1):13-17. PubMed
40. Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965-977. PubMed
41. Saint S, Greene MT, Krein SL, et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. New England Journal of Medicine. 2016;374(22):2111-2119. PubMed
42. McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1-7. PubMed
43. Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol. 2001;22(5):316-321. PubMed
44. Nicolle LE; SHEA Long-Term Care Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175. PubMed
45. Nicolle LE. Catheter-related urinary tract infection. Drug & Aging. 2005;22(8):627-639. PubMed
46. Cochran S. Care of the indwelling urinary catheter - Is it evidence based? J Wound Ostomy Cont Nurs. 2007;34(3):282-288. PubMed
47. Seiler WO, Stahelin HB. Practical management of catheter-associated UTIs. Geriatrics. 1988;43(8):43-50. PubMed
48. Stickler DJ, Chawla JC. The role of antiseptics in the management of patients with long-term indwelling bladder catheters. J Hosp Infect. 1987;10(3):219-228. PubMed
49. Gray M. Does the construction material affect outcomes in long-term catheterization? J Wound Ostomy Cont Nurs. 2006;33(2):116-121. PubMed
50. Trautner BW, Darouiche RO. Clinical review: prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;2002(25):277-283. PubMed
51. Maloney C. Estrogen & recurrent UTI in postmenopausal women. Am J Nurs. 2002;102(8):44-52. PubMed
52. Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(suppl 1):S74-S76. PubMed
53. Godfrey H. Older people, continence care and catheters: dilemmas and resolutions. Br J Nurs. 2008;17(9):S4-S11. PubMed
54. Godfrey H, Evans A. Management of long-term urethral catheters: minimizing complications. Br J Nurs. 2000;9(2):74-76. PubMed
55. Kunin CM. Chemoprophylaxis and suppressive therapy in the management of urinary tract infections. J Antimicrob Chemother. 1994;33(suppl A):51-62. PubMed
56. Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31(1):12-48. PubMed
57. Allan GM, Nicolle L. Cranberry for preventing urinary tract infection. Can Fam Physician. 2013;59(4):367. PubMed
58. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. PubMed
59. Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(13):988-996. PubMed
60. Moore KN, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev. 2007(4):CD006008. PubMed
61. Li L, Ye WQ, Ruan H, Yang BY, Zhang SQ. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2013;94(4):782-787. PubMed
62. Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2011(12):CD004375. PubMed
63. Gray M. What nursing interventions reduce the risk of symptomatic urinary tract infections in the patient with an indwelling catheter? J Wound Ostomy Cont Nurs. 2004;31(1):3-13. PubMed
64. Marschall J, Carpenter C, Fowler S, Trautner B. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147. PubMed
65. Sinclair L, Hagen S, Cross S. Washout policies in long-term indwelling urinary catheterization in adults: a short version Cochrane review. Neurourol Urodyn. 2011;30(7):1208-1212. PubMed
66. Hunter KF, Bharmal A, Moore KN. Long-term bladder drainage: suprapubic catheter versus other methods: a scoping review. Neurourol Urodyn. 2013;32(7):944-951. PubMed
67. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002;83(1):129-138. PubMed
68. Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev. 2012(8). PubMed
69. Jepson R, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;10(CD001321). PubMed
70. Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751-754. PubMed
71. Bianco L, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc. 2012;60(6):1180-1181. PubMed
72. Lin SC, Wang CC, Shih SC, Tjung JJ, Tsou MT, Lin CJ. Prevention of Asymptomatic Bacteriuria with Cranberries and Roselle Juice in Home-care Patients with Long-term Urinary Catheterization. Int J Gerontol. 2014;8(3):152-156.
73. Juthani-Mehta M, Perley L, Chen S, Dziura J, Gupta K. Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J Am Geriatr Soc. 2010;58(10):2028-2030. PubMed
74. Tully CL, Bastone P, Vaughan J, Ballentine L. Urinary tract infection prophylaxis with cranberry extract in the nursing home setting. J Am Geriatr Soc. 2004;52(4):S206-S206.
75. Woodward N. Use of cranberry extract for the prevention of UTIs in an at-risk population. 41st Annual Wound, Ostomy and Continence Nurses Annual Conference, St. Louis, Missouri, June 6-10, 2009. J Wound Ostomy Continence Nurs. 2009;36(3S):S62-S62.
76. Linsenmeyer TA, Harrison B, Oakley A, Kirshblum S, Stock JA, Millis SR. Evaluation of cranberry supplement for reduction of urinary tract infections in individuals with neurogenic bladders secondary to spinal cord injury. A prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med. 2004;27(1):29-34. PubMed
77. Waites KB, Canupp KC, Armstrong S, DeVivo MJ. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med. 2004;27(1):35-40. PubMed
78. Caljouw MAA, Van Den Hout WB, Putter H, Achterberg WP, Cools HJM, Gussekloo J. Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons. A double-blind randomized placebo-controlled trial in long-term care facilities. Eur Geriatr Med. 2013;4:S118-S119. PubMed
79. Hout WB, Caljouw MAA, Putter H, Cools HJM, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: economic evaluation with a randomized controlled trial. J Am Geriatr Soc. 2014;62(1):111-116. PubMed
80. Liu BA, McGeer A, McArthur MA, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. 2007;55(1):35-42. PubMed
81. Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072-1079. PubMed
82. Maloney C. Hormone replacement therapy in female nursing home residents with recurrent urinary tract infection. Ann Long-Term Care. 1998;6(3):77-82.
83. Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35(9):589-593. PubMed
84. Salamon L. Catheter-associated urinary tract infections: a nurse-sensitive indicator in an inpatient rehabilitation program. Rehabil Nurs. 2009;34(6):237-241. PubMed
85. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. PubMed
86. American Medical Directors Association (AMDA). Appropriate indications for use of a chronic indwelling catheter in the long-term care setting. Columbia, MD; excerpted from AMDA's Clinical Practice Guideline: Urinary Incontinence. 2005.
87. Rannikko S, Kyllastinen M, Granqvist B. Comparison of long-term indwelling catheters and bed-pads in the treatment of urinary incontinence in elderly patients. J Infect. 1986;12(3):221-227. PubMed
88. Carapeti E, Andrews S, Bentley P. Randomised study of sterile versus non-sterile urethral catheterization. Ann R. Coll Surg Engl. 1996;78(1):59-60. PubMed
89. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. PubMed
90. Olsen-Scribner RJ, Hayes C, Pottinger P. Sustaining reduction of catheter-associated urinary tract infection (CAUTI)-outcomes after two educational methods in a regional university-affiliated medical center. Am J Infect Control. 2014;1:S22.
91. Duffy LM, Cleary J, Ahern S, et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc. 1995;43(8):865-870. PubMed
92. Joseph C, Jacobson C, Strausbaugh L, Maxwell M, French M, Colling J. Sterile vs clean urinary catheterization. J Am Geriatr Soc. 1991;39(10):1042-1043. PubMed
93. Moore KN, Burt J, Voaklander DC. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clin Rehabili. 2006;20(6):461-468. PubMed
94. Moore KN, Kelm M, Sinclair O, Cadrain G. Bacteriuria in intermittent catheterization users: the effect of sterile versus clean reused catheters. Rehabil Nurs J. 1993;18(5):306-309. PubMed
95. Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005(3):CD004203. PubMed
96. Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc. 1987;35(12):1063-1070. PubMed
97. Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int. 2012;110(11 Pt C):E910-917. PubMed
98. Jahn P, Beutner K, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev. 2012(10):CD004997. PubMed
99. Pickard R, Lam T, Maclennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380(9857):1927-1935. PubMed
100. Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med. 1981;70(3):655-658. PubMed
101. Hagen S, Sinclair L, Cross S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2010(3). PubMed
102. Moore KN, Hunter KF, McGinnis R, et al. Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomized controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound Ostomy Continence Nurs. 2009;36(1):82-90. PubMed
103. Muncie HL Jr, Hoopes JM, Damron DJ, Tenney JH, Warren JW. Once-daily irrigation of long-term urethral catheters with normal saline. Lack of benefit. Arch Intern Med. 1989;149(2):441- PubMed
104. Ruwaldt MM. Irrigation of indwelling urinary catheters. Urology. 1983;21(2):127-129. PubMed
105. Palka MA. Evidenced based review of recommendations addressing the frequency of changing long-term indwelling urinary catheters in older adults. Geriatr Nurs. 2014;35(5):357-363. PubMed
106. Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11(3):609-622. PubMed
107. Fryklund B, Haeggman S, Burman LG. Transmission of urinary bacterial strains between patients with indwelling catheters--nursing in the same room and in separate rooms compared. J Hosp Infect. 1997;36(2):147-153. PubMed
108. Anderson RU. Non-sterile intermittent catheterization with antibiotic prophylaxis in the acute spinal cord injured male patient. J Urol. 1980;124(3):392-394. PubMed
109. Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol. 1980;123(3):364-366. PubMed
110. Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95(2):141-152. PubMed
111. Rutschmann OT, Zwahlen A. Use of norfloxacin for prevention of symptomatic urinary tract infection in chronically catheterized patients. Eur J Clin Microbiol Infect Dis. 1995;14(5):441-444. PubMed
112. Jewes LA, Gillespie WA, Leadbetter A, et al. Bacteriuria and bacteraemia in patients with long-term indwelling catheters--a domiciliary study. J Med Microbiol. 1988;26(1):61-65. PubMed
113. Warren JW, Damron D, Tenney JH, Hoopes JM, Deforge B, Muncie HL, Jr. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis. 1987;155(6):1151-1158. PubMed
114. Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47(7):565-569. PubMed
© 2017 Society of Hospital Medicine
Inpatient management of opioid use disorder: A review for hospitalists
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
© 2017 Society of Hospital Medicine
Acute pain management in hospitalized adult patients with opioid dependence: a narrative review and guide for clinicians
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
© 2017 Society of Hospital Medicine
Eliminating hepatitis in the United States: A road map
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
An ambitious new report by the National Academies of Sciences, Engineering, and Medicine lays out a detailed path by which some 90,000 deaths from hepatitis B and C infection could be prevented by 2030.
The National Academies, a group of nongovernmental advisory bodies that includes the former Institute of Medicine, said that “the tools to prevent these deaths” exist – namely vaccination to prevent new hepatitis B infections and antiviral drugs, including new oral medications that can cure chronic hepatitis C infections within months.
The authors of the 200-plus-page report, led by Brian Strom, MD, MPH, of Rutgers University in Newark, NJ, calculate that deaths from hepatitis B infection could be halved by 2030 if 90% of patients are diagnosed, if 90% of those diagnosed are connected to care, and if 80% of those for whom treatment is indicated receive it. Treating everyone with chronic hepatitis C would reduce new infections by 90% by 2030, while reducing related deaths by 65%, Dr. Strom and his colleagues estimate.
But the authors also concede that drastic changes to current health policy would be required to reach these goals. These include the adoption of “aggressive testing, diagnosis, treatment, and prevention methods, such as needle exchange.”
They propose that the federal government seek a unique licensing arrangement with one or more manufacturers to bring down the notoriously high cost of direct-acting drugs used in hepatitis C, as a way of raising treatment rates. Currently, fewer than half the patients on Medicaid who are eligible for hepatitis C treatment receive it, and fewer than 1% of prisoners, who have high rates of infection.
Dr. Joseph Lim, director of the viral hepatitis program at Yale University in New Haven. Conn., who was not involved in the National Academies report, called it helpful in the sense that “it casts a spotlight on something that those of us involved in the care of people with viral hepatitis have long known – which is that this is a national and global public health burden that has been under the radar and in the shadow of other important health priorities.”
Both hepatitis B and C increase the risk of liver cancer and are associated with significant morbidity and mortality. Though approximately 4 million people in the United States are estimated to be infected with chronic hepatitis B (1.3 million) or C (2.7 million), these diseases account for less than 1% of the research budget at the National Institutes of Health, the report said. This compares unfavorably to funding for HIV, which affects about 1 million Americans.
As the report states, the tools to radically reduce hepatitis B and C deaths already exist. However, Dr. Lim cautioned in an interview, “the public health infrastructure to address viral hepatitis has been woefully inadequate.” In the United States, he noted, most states receive federal funding for at most a single person in charge of viral hepatitis epidemiology. “The resources currently available are in no way adequate to achieve the very aggressive goals described in the report,” he said.
Even among people with a known diagnosis of hepatitis B or C, only some receive confirmatory testing, Dr. Lim said. And of those with confirmed infections, “only a fraction are linked to care from the diagnosing clinician to a provider with the capacity to assess the state of liver disease and determine whether antiviral therapy is warranted.” Finally, he said, “many patients continue to face barriers to curative therapy due to cost and restrictions by public and private payers.”
Among the recommendations contained in the report is that unrestricted, mass treatment of hepatitis C infections be undertaken – regardless of disease stage. Currently, direct-acting antiviral agents remain costly and are poorly covered, notably by Medicaid. The National Academies advise that the government rectify this by purchasing “a license or assignment to the patent on a direct-acting antiviral drug, and use it only in those market segments where the government pays for treatment and access is now limited, such as Medicaid and prisons.”
Dr. Lim called the licensing proposal “very novel and bold,” but noted that there is no precedent in the United States for diseases such as hepatitis C. “If it could be done it would be an incredible model of government-pharma partnership for the public health good, and have a very significant impact.”
Steven Flamm, MD, chief of the liver transplantation program at Northwestern University in Chicago, who like Dr. Lim was not involved in the creation of the report, said in an interview that it contained innovative ideas and helped underscore the fact that “hepatitis has been given short shrift. The NIH and other agencies do not devote time and energy to this particular medical issue for reasons that are not completely clear.”
But “the problem with these kinds of analyses,” he said, “is that carrying them out is harder than making the recommendations.”
Dr. Flamm echoed Dr. Lim’s concerns about the practicability of implementing some of the recommendations in what he considers a resource-deprived health care environment for viral hepatitis.
“Is elimination possible or can you take a big bite out of it? The answer to that question is yes. We now have agents that can treat chronic viral hepatitis well, which we didn’t have a few years ago.”
Still, he emphasized, having the tools is only one part of the picture. Hepatitis C diagnostic tests have been available since the early 1990s. Yet, Dr. Flamm pointed out, fewer than half of patients have been diagnosed. “If the new CDC screening guidelines gain traction, we will do better than that.”
Dr. Flamm said that he considered the report’s call for a unique government licensing agreement for hepatitis C drugs a tall order. The drugs are already heavily discounted by manufacturers in many cases, he said, yet remain unavailable to those in need of them. In Illinois, Dr. Flamm said, few Medicaid patients with confirmed hepatitis C are given the short-acting antivirals that have revolutionized treatment. “The vast majority have no access to the therapy at all,” he said.
One of the report’s strengths, he said, is in detailing innovative prevention strategies such as delivering and promoting hepatitis B vaccinations to adults through local pharmacies, after the model of influenza vaccinations, and also conducting needle exchanges through pharmacies for intravenous drug users, who are at high risk of contracting both hepatitis B and C.
“Many of these strategies are not very costly,” he said. “The problem is you run into moral platitudes – to eliminate hepatitis, we will have to overcome that,” Dr. Flamm said, something that cannot be taken for granted in the current political environment.
But even if the goals outlined in the report seem ambitious, its authors have done an important service in underscoring the burden of viral hepatitis and laying out how some barriers to prevention, diagnosis, and treatment might be broken, he said.
Viral hepatitis “is a big deal, and it does cost a tremendous amount of money,” he added. “Everybody focuses on the therapeutic cost, but nobody focuses on the costs, direct and indirect, of all the sick people that are out there.”
FROM THE NATIONAL ACADEMIES OF SCIENCES, ENGINEERING, AND MEDICINE
Life after breast, prostate, and colon cancer: Primary care’s role
In 2015, about 1.6 million Americans received a diagnosis of cancer.1 In 2012, when 13.7 million people were living with cancer in the United States, the estimated 5-year survival rate of all cancers was 66.5%.1 Today, breast, prostate, and colon cancers have 5-year survival rates of 89.4%, 98.9%, and 64.9%, respectively.
With this rising trend in survival, primary care physicians have been steadily assuming the long-term care of these patients. The phrase “cancer survivorship” was coined by the National Comprehensive Cancer Network to describe the experience of living with, through, and beyond a cancer diagnosis.2
As cancer becomes a chronic medical condition, the primary care physician assumes a vital role in the treatment of the unique and evolving needs of this patient population.
This article discusses the specific needs of patients surviving breast, prostate, and colon cancer with special focus on surveillance guidelines for recurrence, development of concomitant malignancies, assessment of psychosocial and physical effects, and disease conditions related to the treatment of cancer itself.
FOLLOW-UP CARE AND SURVEILLANCE
Follow-up care in patients being treated for cancer is vital to survivorship. It includes promoting healthy living, managing treatment side effects, and monitoring for long-term side effects and possible recurrence.
Patients previously treated for malignancy are more susceptible to second primary cancers, for an array of reasons including the effects of prior treatment, shared environmental exposures such as smoking, and genetic susceptibility.2 Therefore, it is important for the primary care physician to recognize signs and symptoms and to screen cancer survivors appropriately. The American Society of Clinical Oncology (ASCO) has developed evidence-based recommendations for follow-up care,3 which we review here.
Breast cancer
For breast cancer patients, history and physical examinations are recommended every 3 to 6 months for the first 3 years, every 6 to 12 months in years 4 and 5, and annually thereafter.3
A repeat mammogram should be performed 1 year after the initial mammogram that led to the diagnosis. If the patient underwent radiation therapy, a repeat mammogram of the affected breast should be done 6 months after completion of radiation. Finally, a mammogram should be done every 6 to 12 months thereafter.3 It is also recommended that patients perform a monthly breast self-examination, but this does not replace the annual mammogram.3
Women who are treated with tamoxifen should have an annual gynecologic assessment if they have a uterus and should be encouraged to discuss any abnormal vaginal bleeding with their physician, given the increased risk of uterine cancer.2
ASCO does not recommend routine use of complete blood cell counts, complete metabolic panels, bone scans, chest radiography, computed tomography, ultrasonography, positron-emission tomography, or tumor markers in patients who are asymptomatic.3
Prostate cancer
ASCO’s recommendations for patients recovering from prostate cancer include regular histories and physical examinations and general health promotion. Prostate-specific antigen (PSA) testing is recommended every 6 to 12 months for the first 5 years after treatment and annually thereafter. More frequent PSA testing may be required in men at higher risk of recurrence or in patients who may undergo additional treatment, including radiation and surgery.4 Higher risk of disease recurrence is thought to depend on disease-specific factors at the time of original diagnosis including pretreatment PSA, Gleason score, and tumor stage.4 This should be discussed between the oncologist and primary care physician.
In regard to digital rectal examinations, the oncologist and primary care physician should jointly determine the frequency of examination. The frequency of digital rectal examinations remains an area of controversy due to their low sensitivity for detecting recurrences. Digital rectal examinations may be omitted in patients with undetectable levels of PSA.4
In regard to second primary malignancies, prostate cancer survivors who have undergone pelvic radiation therapy have a slightly higher risk of bladder and colorectal cancer. The lifetime incidence of bladder cancer after pelvic radiation is 5% to 6%, compared with 2.4% in the general population.1,5 A prostate cancer survivor presenting with hematuria should be referred to a urologist for cystoscopy and evaluation of the upper urinary tract to rule out cancer.4
Similarly, patients presenting with rectal bleeding should be referred to a gastroenterologist and the treating radiation oncologist for complete evaluation. The risk of rectal cancer after pelvic radiation therapy increases to about the same level as in someone who has a first-degree relative with colorectal cancer.5 Therefore, it is recommended that patients undergo screening for colorectal cancer in conjunction with existing evidence-based guidelines. There is no evidence to suggest that increased intensity of screening improves overall or disease-specific survival.4
Colon cancer
In patients with resected colorectal cancer, continued surveillance is important to evaluate for recurrent cancer as well as for metachronous neoplasms. Consensus statements indicate that surveillance colonoscopy should be continued for those who have undergone surgical resection for stage I, II, or III colon or rectal cancers, and for those patients with stage IV who have undergone surgical resection with curative intent.6
Patients who undergo curative resection of rectal or colon cancer should have a colonoscopy 1 year after resection or 1 year after the colonoscopy was performed, to clear the colon of synchronous disease. Subsequently, if the colonoscopy done at 1 year is normal, the interval before the next colonoscopy is 3 years. If that colonoscopy is normal, the next colonoscopy is in 5 years. Time intervals may be shorter if there is evidence of hereditary nonpolyposis colorectal cancer or adenoma.
Patients who underwent low anterior resection of rectal cancer should undergo periodic examination of the rectum to evaluate for local recurrence. Although effectiveness is not proven, endoscopic ultrasonography or flexible sigmoidoscopy is suggested at 3- to 6-month intervals for the first 2 to 3 years after resection. This is independent of colonoscopy.6,7
Additionally, ASCO recommends a history and physical examination and carcinoembryonic antigen testing every 3 to 6 months for the first 5 years. Computed tomography of the chest, abdomen, and pelvis should be done annually for the first 3 years after the end of treatment.7
HEALTH PROMOTION
Maintaining a healthy body weight and a nutritionally balanced diet should be encouraged in all cancer survivors. Poor diet, lack of exercise, excessive alcohol consumption, and smoking reduce quality of life and increase the risk of cancer.2
Diet
Numerous studies have looked at dietary modifications and risk reduction, and although there is no consensus on specific dietary guidelines, there is consensus that diet modification to maintain normal body weight will improve overall quality of life.8 Patients should be encouraged to consume a well-balanced diet consisting mostly of fruits, vegetables, whole grains, and beans, and to limit consumption of animal protein.8
There is little evidence to support taking vitamins or other dietary supplements to prevent or control cancer or to prevent its recurrence. The primary care physician should assess supplement use on a regular basis during office visits.2
Exercise
Rest is an important component of the initial recovery process. Too much inactivity, however, leads to loss of physical conditioning and muscle strength. This in turn may negatively affect a patient’s ability to perform activities of daily living and may worsen fatigue associated with treatment.
Accordingly, the National Comprehensive Cancer Network recommends at least 150 minutes of moderate activity and up to 75 minutes of more rigorous activity divided throughout the week.2,8 The regimen should include endurance and muscle strength training, which aid in balance, bone, health, and functional status. The intensity of exercise should be increased in a stepwise fashion, taking into account individual capabilities and limitations.2
Cancer survivors may need specific exercise recommendations and supervised programs to ensure safety and limit long-term side effects of their treatment. For example:
Patients with neuropathy should have their stability, balance, and gait assessed before starting a new program. These patients may benefit from an aerobic exercise program that includes riding a stationary bike rather than running.
Patients with poor bone health should have their fracture risk assessed.
Those with an ostomy bag should empty the bag before physical activity. They should avoid contact sports and activities that increase intra-abdominal pressure.
Patients suffering from lymphedema should wear compression garments when engaging in physical activity. They should undergo baseline and periodic reevaluation of the lymphedema and initiate strength training in the affected body part only if the lymphedema is stable (ie, no need for therapy for 3 months, no recent infections requiring antibiotics, and no change in circumference > 10%).2
Mental health
Health promotion should focus not only on the physical health of the patient, but also on his or her emotional and psychological well-being. As they make the transition from cancer patient to survivor, many individuals may develop or have worsening depression and anxiety due to fear of recurrence. These feelings of powerlessness can linger for years after the initial treatment.
Patients should be screened regularly for signs and symptoms of depression by asking about their family and social support. Referral to support groups, psychologists, and psychiatrists may be warranted.9
MITIGATING CANCER-RELATED FATIGUE
Cancer-related fatigue involves a patient’s subjective sense of physical, emotional, and cognitive exhaustion related to cancer or treatment that is disproportionate to that expected from recent daily activity.2 Fatigue is a common complaint among survivors and can occur months to years after treatment ends.10
Patients should be screened for fatigue at regular intervals. The primary care physician should focus the history to include information regarding the onset, pattern, duration, associated factors, assessment of other treatable comorbidities, medications, psychological well-being, nutritional status, and pain level of each patient who complains of fatigue.
Laboratory tests for treatable causes of fatigue include:
- A complete blood cell count to evaluate for anemia
- A comprehensive metabolic panel to evaluate electrolytes, renal function, and hepatic function
- The thyroid-stimulating hormone level to evaluate thyroid function, particularly in breast cancer patients who have received radiation therapy.2
If no organic cause is uncovered, the focus should shift to lifestyle interventions as the treatment of choice. The treatment of fatigue in this situation is increased physical activity with the goals discussed above. Psychological intervention may be required with cognitive behavioral therapy, psychological and supportive therapies, education on sleep hygiene, and possible sleep restriction.
If alternative causes of fatigue are ruled out and physical and psychological support fails to reduce symptoms, the practitioner can consider a psychostimulant such as methylphenidate, but this should be used cautiously.2
SEXUAL DYSFUNCTION
Intimate relationships and sexuality are an important part of life and are affected by a variety of factors including physical health, psychological well-being, body image perception, and overall status of relationships. As a side effect of chemotherapy, cancer survivors may complain of erectile dysfunction, penile shortening, dyspareunia, vaginal dryness, decreased libido, anorgasmy, and changes in body image. These issues are frequently unaddressed, whether due to the physician’s discomfort in discussing the topic or to the patient’s embarrassment and reluctance to discuss the matter.11
Breast cancer patients may experience sexual dysfunction both during and after treatment due to a combination of systemic effects of treatment, changes in physical appearance leading to impaired body image, strains on partner relationships, and psychological sequelae of diagnosis and treatment of cancer.12 Manipulation and radiation to the breast affect sexual functioning by altering body contour and image. Additionally, chemotherapy can lead to early menopause and hormonal alterations due to endocrine therapies, which can negatively affect sexual organs.11
Studies have shown that treatment with tamoxifen causes less sexual dysfunction than do aromatase inhibitors. In the Arimidex, Tamoxifen, Alone or in Combination trial, therapy with anastrozole was associated with more vaginal dryness, dyspareunia, and decreased libido compared with tamoxifen.12
Treatment requires a comprehensive history, a physical examination, and a discussion of relationship satisfaction with the patient.
Vaginal dryness and dyspareunia can be treated with vaginal lubricants and moisturizers. Moisturizers are effective if used multiple times per week, whereas lubricants can be used on demand. Low-dose vaginal estrogen preparations can be used in select patients with severe vaginal dryness; the goal should be discussed. Hormone replacement therapy is contraindicated in breast cancer survivors due to the risk of recurrence.11
Prostate cancer survivors. Up to 70% of men felt their quality of life and sexual function were adversely affected after the diagnosis and treatment.13,14 Erectile dysfunction following radical prostatectomy and radiation therapy remains one of the leading causes of sexual dysfunction.14–15 Erectile dysfunction is multifactorial, with both psychogenic and organic causes. Comorbidities must be considered including hypertension, diabetes, hyperlipidemia, and smoking. Early penile rehabilitation is a proposed treatment strategy.14
First-line therapy for penile rehabilitation includes introduction of daily low-dose phosphodiesterase type 5 inhibitors as early as the time of catheter removal to within the first month after surgery. The need for daily dosing vs on-demand dosing has been controversial.15 Two large multicenter, double-blind studies had conflicting outcomes. The first reported that in men receiving nightly sildenafil (50 or 100 mg) after radical prostatectomy, 27% had increased return of spontaneous erectile function vs 4% with placebo16; the second study showed no difference between nightly and on-demand dosing.17 The consensus is that a phosphodiesterase type 5 inhibitor should be initiated early.
Second-line therapy includes intracavernosal injections and vacuum erection devices. Finally, a penile prosthesis implant can be offered to patients who have responded poorly to medical therapy.13
Colorectal cancer survivors suffer from sexually related problems similar to those stated above, including erectile dysfunction, ejaculation problems, dyspareunia, vaginal dryness, and decreased enjoyment.18 These patients should be provided treatments similar to those described above. In addition, they may suffer from body image issues, particularly those with a permanent ostomy.9
Sexual dysfunction is a multifaceted problem for patients. Encouraging couples to discuss sexual intimacy frequently helps to reveal and cope with the problems, whether physical or psychological. It is the primary care physician’s role to recognize any sexual concerns and refer to the appropriate specialist.
OSTEOPOROSIS
Osteoporosis is a metabolic bone disease characterized by low bone mineral density. As a result, bones become weak and fracture more easily from minor injuries.
Risk factors for osteoporosis include female sex, family history, advanced age, low body weight, low calcium and vitamin D levels, sedentary lifestyle, smoking, and low estrogen levels.19 Cancer treatment places patients at a greater risk for osteoporosis, particularly for those patients with chemotherapy-induced ovarian failure, those treated with aromatase inhibitors, men receiving androgen-deprivation therapy, and patients on glucocorticoid therapy. The morbidity and mortality associated with bone loss can be prevented with appropriate screening, lifestyle changes, and therapy.2
According to the National Osteoporosis Foundation Guideline for Preventing and Treating Osteoporosis, all men and postmenopausal women age 50 and older should be evaluated clinically for osteoporosis risk to determine the need for bone mineral density testing.2,19 The US Preventive Services Task Force recommends bone mineral density testing in all women age 65 and older, and for women 60 to 64 who are at high risk for bone loss. ASCO agrees, and further suggests bone mineral density screening for women with breast cancer who have risk factors such as positive family history, body weight less than 70 kg, and prior nontraumatic fracture, as well as for postmenopausal women of any age receiving aromatase inhibitors and for premenopausal women with therapy-induced ovarian failure.11
Androgen deprivation therapy is a mainstay of treatment in recurrent and metastatic prostate cancer. The effect is severe hypogonadism with reductions in serum testosterone levels. Androgen deprivation therapy accelerates bone turnover, decreases bone mineral density, and contributes to fracture risk. The National Comprehensive Cancer Network additionally suggests measuring bone mineral density at baseline for all men receiving androgen deprivation therapy or other medications associated with bone loss, repeating it 1 year after androgen deprivation therapy and then every 2 years, or as clinically indicated.20
The gold standard for measuring bone mineral density is dual-energy x-ray absorptiometry. The World Health Organization FRAX tool uses bone mineral density and several clinical factors to estimate the risk of fracture in the next 10 years, which can help guide therapy. Cancer patients with elevated fracture risk should be evaluated every 2 years. Counseling should be provided to address modifiable risk factors such as smoking, alcohol consumption, physical inactivity, and low calcium and vitamin D intake. Therapy should be strongly considered in patients with a bone mineral density below a T-score of –2.0. 2,19
Treatment begins with lifestyle modifications such as weight-bearing exercises to improve balance and muscle strength and to prevent falls, and adequate intake of calcium (≥ 1,200 mg daily) and vitamin D (800–1,000 IU daily) for adults age 50 and older. Treatment with bisphosphonates may be required.2,11,20
NEUROPATHY
Many chemotherapeutic agents can lead to neuropathy and can result in long-term disability in patients. Patients treated with taxane- and platinum-based chemotherapy are at particular risk.
Paclitaxel, used in the treatment of breast, ovarian, and lung cancer, can lead to distal neuropathy. This neuropathy commonly has a stocking-and-glove distribution and is primarily sensory; however, it may have motor and autonomic components. The neuropathy typically lessens when the medication is stopped, although in some patients it can persist and lead to long-term disability.
Treatment can include massage. Medications such as gabapentin and pregabalin can also be used, but randomized controlled trials do not support them, as they predominantly treat the tingling rather than the numbness.11
BLADDER AND BOWEL DYSFUNCTION
Urinary incontinence and dysfunction are frequent complications in prostate cancer survivors. Urinary function should be discussed regularly with patients, addressing quality of the urinary stream, difficulty emptying the bladder, timing, and incontinence.4 Urinary incontinence is frequently seen in postprostatectomy patients.
The cornerstone of treating urinary incontinence is determining the cause of the incontinence, whether it is stress or urge incontinence, or both.21 For those patients with urge incontinence alone, practitioners can address the problem with a combination of behavior modification, pelvic floor exercises, and anticholinergic medications such as oxybutynin. If the problem stems from difficulty initiating or a slow stream, physicians may consider alpha-blockers.4,21 If the incontinence is persistent, bothersome, and has components of stress incontinence, the patient should be referred to a urologist for urodynamic testing, cystoscopy, and surgical evaluation for possible placement of a male urethral sling or artificial urinary sphincter.21
Colorectal cancer survivors, particularly those who received radiation therapy, are at high risk of bowel dysfunction such as chronic diarrhea and stool incontinence. Patients should be educated about this possible side effect. Symptoms of bowel dysfunction can affect body image and interfere with social functioning and overall quality of life. Patients should be provided with coping tools such as antidiarrheal medication, stool bulking agents, changes in diet, and protective underwear.
CARDIOVASCULAR DISEASE
Evidence suggests that certain types of chemotherapy and radiation therapy increase the risk of cardiovascular disease. Prostate cancer survivors treated with androgen deprivation therapy, particularly those more than 75 years old, are at increased risk of cardiovascular disease and diabetes.22
It is recommended that men be screened with fasting plasma glucose at baseline and yearly thereafter while receiving androgen deprivation therapy. Lipid panel testing should be done 1 year from initiation of androgen deprivation therapy and then, if results are normal, every 5 years or as clinically warranted. The focus should be on primary prevention with emphasis on smoking cessation, treating hypertension per guidelines, lifestyle modifications, and treatment with aspirin and statins when clinically appropriate.20
Radiation therapy, chemotherapy, and endocrine therapy have all been suggested to lead to cardiotoxicity in breast cancer patients. Anthracycline-based chemotherapies have a well-recognized association with cardiomyopathy. Factors associated with increased risk of anthracycline-induced cardiomyopathy include older age, hypertension, pre-existing coronary artery disease, and previous mediastinal radiation.
Early detection of cardiomyopathy may lead to avoidance of irreversible cardiotoxicity, but there are currently no clear guidelines for cardiac screening in breast cancer survivors. If cardiomyopathy is detected, treatment should include beta-blockers and angiotensin-converting enzyme inhibitors as well as modification of other cardiovascular risk factors.11
A SURVIVORSHIP CARE PLAN
There is life beyond the diagnosis of cancer. As patients are living longer, with an estimated 5-year survival rate of 66.5% of all cancers in the United States, there must be a transition of care from the oncologist to the primary care physician.1 While the oncologist will remain involved in the initial years of follow-up care, these visits will go from twice a year to once a year, and eventually the patient will make a full transition to care by the primary care physician. The timing of this changeover varies from physician to physician, but the primary care physician is ultimately responsible for the follow-up.
A tool to ease this transition is a survivorship care plan. The goal of a survivorship care plan is to individualize a follow-up plan while keeping in mind the necessary surveillance as outlined. These care plans are created with the patient and oncologist and then brought to the primary care physician. While there is an abundance of literature regarding the creation and initiation of survivorship care plans, the success of these plans is uncertain. Ultimately, the goal of a survivorship care plan is to create open dialogue among the oncologist, the primary care physician, and the patient. This unique patient population requires close follow-up by a multidisciplinary team with the primary care physician serving as the steward.
- National Cancer Institute (NIH). Surveillance, Epidemiology, and End Results Program. SEER cancer stat fact sheets. http://seer.cancer.gov/statfacts. Accessed March 6, 2017.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines Survivorship: 2015. www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Accessed March 6, 2017.
- Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2013; 31:961–965.
- Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol 2015; 33:1078–1085.
- Sountoulides P, Koletsas N, Kikidakis D, Paschalidis K, Sofikitis N. Secondary malignancies following radiotherapy for prostate cancer. Ther Adv Urol 2010; 2:119–125.
- Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin 2006; 56:160–168.
- Meyerhardt JA, Mangu PB, Flynn PJ, et al; American Society of Clinical Oncology. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013; 31:4465–4470.
- Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer 2011; 105(suppl 1):S52–S73.
- Miller K, editor. Excellent Care for Cancer Survivors: A Guide to Fully Meet Their Needs in Medical Offices and in the Community (Praeger Series on Contemporary Health & Living). 1st ed. Santa Barbara, CA: Praeger; 2012.
- Stanton AL, Rowland JH, Ganz PA. Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am Psychol 2015; 70:159–174.
- Stan D, Loprinzi CL, Ruddy KJ. Breast cancer survivorship issues. Hematol Oncol Clin North Am 2013; 27:805–827.
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol 2004; 22:426–471.
- Chung E, Gillman M. Prostate cancer survivorship: a review of erectile dysfunction and penile rehabilitation after prostate cancer therapy. Med J Aust 2014; 200:582–585.
- Sherer BA, Levine LA. Current management of erectile dysfunction in prostate cancer survivors. Curr Opin Urol 2014; 24:401–416.
- Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med 2013; 10(suppl 1):102–111.
- Padma-Nathan H, McCullough AR, Levine LA, et al; Study Group. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res 2008; 20:479–486.
- Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol 2008; 54:924–931.
- Den Oudsten BL, Traa MJ, Thong MS, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: a population-based study. Eur J Cancer 2012; 48:3161–3170.
- National Osteoporosis Foundation (NOF). http://nof.org. Accessed March 3, 2017.
- Saylor PJ, Keating NL, Smith MR. Prostate cancer survivorship: prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med 2009; 24(suppl 2):S389–S394.
- Gupta S, Peterson AC. Stress urinary incontinence in the prostate cancer survivor. Curr Opin Urol 2014; 24:395–400.
- Morgans AK, Fan KH, Koyama T, et al. Influence of age on incident diabetes and cardiovascular disease in prostate cancer survivors receiving androgen deprivation therapy. J Urol 2015; 193:1226–1231.
In 2015, about 1.6 million Americans received a diagnosis of cancer.1 In 2012, when 13.7 million people were living with cancer in the United States, the estimated 5-year survival rate of all cancers was 66.5%.1 Today, breast, prostate, and colon cancers have 5-year survival rates of 89.4%, 98.9%, and 64.9%, respectively.
With this rising trend in survival, primary care physicians have been steadily assuming the long-term care of these patients. The phrase “cancer survivorship” was coined by the National Comprehensive Cancer Network to describe the experience of living with, through, and beyond a cancer diagnosis.2
As cancer becomes a chronic medical condition, the primary care physician assumes a vital role in the treatment of the unique and evolving needs of this patient population.
This article discusses the specific needs of patients surviving breast, prostate, and colon cancer with special focus on surveillance guidelines for recurrence, development of concomitant malignancies, assessment of psychosocial and physical effects, and disease conditions related to the treatment of cancer itself.
FOLLOW-UP CARE AND SURVEILLANCE
Follow-up care in patients being treated for cancer is vital to survivorship. It includes promoting healthy living, managing treatment side effects, and monitoring for long-term side effects and possible recurrence.
Patients previously treated for malignancy are more susceptible to second primary cancers, for an array of reasons including the effects of prior treatment, shared environmental exposures such as smoking, and genetic susceptibility.2 Therefore, it is important for the primary care physician to recognize signs and symptoms and to screen cancer survivors appropriately. The American Society of Clinical Oncology (ASCO) has developed evidence-based recommendations for follow-up care,3 which we review here.
Breast cancer
For breast cancer patients, history and physical examinations are recommended every 3 to 6 months for the first 3 years, every 6 to 12 months in years 4 and 5, and annually thereafter.3
A repeat mammogram should be performed 1 year after the initial mammogram that led to the diagnosis. If the patient underwent radiation therapy, a repeat mammogram of the affected breast should be done 6 months after completion of radiation. Finally, a mammogram should be done every 6 to 12 months thereafter.3 It is also recommended that patients perform a monthly breast self-examination, but this does not replace the annual mammogram.3
Women who are treated with tamoxifen should have an annual gynecologic assessment if they have a uterus and should be encouraged to discuss any abnormal vaginal bleeding with their physician, given the increased risk of uterine cancer.2
ASCO does not recommend routine use of complete blood cell counts, complete metabolic panels, bone scans, chest radiography, computed tomography, ultrasonography, positron-emission tomography, or tumor markers in patients who are asymptomatic.3
Prostate cancer
ASCO’s recommendations for patients recovering from prostate cancer include regular histories and physical examinations and general health promotion. Prostate-specific antigen (PSA) testing is recommended every 6 to 12 months for the first 5 years after treatment and annually thereafter. More frequent PSA testing may be required in men at higher risk of recurrence or in patients who may undergo additional treatment, including radiation and surgery.4 Higher risk of disease recurrence is thought to depend on disease-specific factors at the time of original diagnosis including pretreatment PSA, Gleason score, and tumor stage.4 This should be discussed between the oncologist and primary care physician.
In regard to digital rectal examinations, the oncologist and primary care physician should jointly determine the frequency of examination. The frequency of digital rectal examinations remains an area of controversy due to their low sensitivity for detecting recurrences. Digital rectal examinations may be omitted in patients with undetectable levels of PSA.4
In regard to second primary malignancies, prostate cancer survivors who have undergone pelvic radiation therapy have a slightly higher risk of bladder and colorectal cancer. The lifetime incidence of bladder cancer after pelvic radiation is 5% to 6%, compared with 2.4% in the general population.1,5 A prostate cancer survivor presenting with hematuria should be referred to a urologist for cystoscopy and evaluation of the upper urinary tract to rule out cancer.4
Similarly, patients presenting with rectal bleeding should be referred to a gastroenterologist and the treating radiation oncologist for complete evaluation. The risk of rectal cancer after pelvic radiation therapy increases to about the same level as in someone who has a first-degree relative with colorectal cancer.5 Therefore, it is recommended that patients undergo screening for colorectal cancer in conjunction with existing evidence-based guidelines. There is no evidence to suggest that increased intensity of screening improves overall or disease-specific survival.4
Colon cancer
In patients with resected colorectal cancer, continued surveillance is important to evaluate for recurrent cancer as well as for metachronous neoplasms. Consensus statements indicate that surveillance colonoscopy should be continued for those who have undergone surgical resection for stage I, II, or III colon or rectal cancers, and for those patients with stage IV who have undergone surgical resection with curative intent.6
Patients who undergo curative resection of rectal or colon cancer should have a colonoscopy 1 year after resection or 1 year after the colonoscopy was performed, to clear the colon of synchronous disease. Subsequently, if the colonoscopy done at 1 year is normal, the interval before the next colonoscopy is 3 years. If that colonoscopy is normal, the next colonoscopy is in 5 years. Time intervals may be shorter if there is evidence of hereditary nonpolyposis colorectal cancer or adenoma.
Patients who underwent low anterior resection of rectal cancer should undergo periodic examination of the rectum to evaluate for local recurrence. Although effectiveness is not proven, endoscopic ultrasonography or flexible sigmoidoscopy is suggested at 3- to 6-month intervals for the first 2 to 3 years after resection. This is independent of colonoscopy.6,7
Additionally, ASCO recommends a history and physical examination and carcinoembryonic antigen testing every 3 to 6 months for the first 5 years. Computed tomography of the chest, abdomen, and pelvis should be done annually for the first 3 years after the end of treatment.7
HEALTH PROMOTION
Maintaining a healthy body weight and a nutritionally balanced diet should be encouraged in all cancer survivors. Poor diet, lack of exercise, excessive alcohol consumption, and smoking reduce quality of life and increase the risk of cancer.2
Diet
Numerous studies have looked at dietary modifications and risk reduction, and although there is no consensus on specific dietary guidelines, there is consensus that diet modification to maintain normal body weight will improve overall quality of life.8 Patients should be encouraged to consume a well-balanced diet consisting mostly of fruits, vegetables, whole grains, and beans, and to limit consumption of animal protein.8
There is little evidence to support taking vitamins or other dietary supplements to prevent or control cancer or to prevent its recurrence. The primary care physician should assess supplement use on a regular basis during office visits.2
Exercise
Rest is an important component of the initial recovery process. Too much inactivity, however, leads to loss of physical conditioning and muscle strength. This in turn may negatively affect a patient’s ability to perform activities of daily living and may worsen fatigue associated with treatment.
Accordingly, the National Comprehensive Cancer Network recommends at least 150 minutes of moderate activity and up to 75 minutes of more rigorous activity divided throughout the week.2,8 The regimen should include endurance and muscle strength training, which aid in balance, bone, health, and functional status. The intensity of exercise should be increased in a stepwise fashion, taking into account individual capabilities and limitations.2
Cancer survivors may need specific exercise recommendations and supervised programs to ensure safety and limit long-term side effects of their treatment. For example:
Patients with neuropathy should have their stability, balance, and gait assessed before starting a new program. These patients may benefit from an aerobic exercise program that includes riding a stationary bike rather than running.
Patients with poor bone health should have their fracture risk assessed.
Those with an ostomy bag should empty the bag before physical activity. They should avoid contact sports and activities that increase intra-abdominal pressure.
Patients suffering from lymphedema should wear compression garments when engaging in physical activity. They should undergo baseline and periodic reevaluation of the lymphedema and initiate strength training in the affected body part only if the lymphedema is stable (ie, no need for therapy for 3 months, no recent infections requiring antibiotics, and no change in circumference > 10%).2
Mental health
Health promotion should focus not only on the physical health of the patient, but also on his or her emotional and psychological well-being. As they make the transition from cancer patient to survivor, many individuals may develop or have worsening depression and anxiety due to fear of recurrence. These feelings of powerlessness can linger for years after the initial treatment.
Patients should be screened regularly for signs and symptoms of depression by asking about their family and social support. Referral to support groups, psychologists, and psychiatrists may be warranted.9
MITIGATING CANCER-RELATED FATIGUE
Cancer-related fatigue involves a patient’s subjective sense of physical, emotional, and cognitive exhaustion related to cancer or treatment that is disproportionate to that expected from recent daily activity.2 Fatigue is a common complaint among survivors and can occur months to years after treatment ends.10
Patients should be screened for fatigue at regular intervals. The primary care physician should focus the history to include information regarding the onset, pattern, duration, associated factors, assessment of other treatable comorbidities, medications, psychological well-being, nutritional status, and pain level of each patient who complains of fatigue.
Laboratory tests for treatable causes of fatigue include:
- A complete blood cell count to evaluate for anemia
- A comprehensive metabolic panel to evaluate electrolytes, renal function, and hepatic function
- The thyroid-stimulating hormone level to evaluate thyroid function, particularly in breast cancer patients who have received radiation therapy.2
If no organic cause is uncovered, the focus should shift to lifestyle interventions as the treatment of choice. The treatment of fatigue in this situation is increased physical activity with the goals discussed above. Psychological intervention may be required with cognitive behavioral therapy, psychological and supportive therapies, education on sleep hygiene, and possible sleep restriction.
If alternative causes of fatigue are ruled out and physical and psychological support fails to reduce symptoms, the practitioner can consider a psychostimulant such as methylphenidate, but this should be used cautiously.2
SEXUAL DYSFUNCTION
Intimate relationships and sexuality are an important part of life and are affected by a variety of factors including physical health, psychological well-being, body image perception, and overall status of relationships. As a side effect of chemotherapy, cancer survivors may complain of erectile dysfunction, penile shortening, dyspareunia, vaginal dryness, decreased libido, anorgasmy, and changes in body image. These issues are frequently unaddressed, whether due to the physician’s discomfort in discussing the topic or to the patient’s embarrassment and reluctance to discuss the matter.11
Breast cancer patients may experience sexual dysfunction both during and after treatment due to a combination of systemic effects of treatment, changes in physical appearance leading to impaired body image, strains on partner relationships, and psychological sequelae of diagnosis and treatment of cancer.12 Manipulation and radiation to the breast affect sexual functioning by altering body contour and image. Additionally, chemotherapy can lead to early menopause and hormonal alterations due to endocrine therapies, which can negatively affect sexual organs.11
Studies have shown that treatment with tamoxifen causes less sexual dysfunction than do aromatase inhibitors. In the Arimidex, Tamoxifen, Alone or in Combination trial, therapy with anastrozole was associated with more vaginal dryness, dyspareunia, and decreased libido compared with tamoxifen.12
Treatment requires a comprehensive history, a physical examination, and a discussion of relationship satisfaction with the patient.
Vaginal dryness and dyspareunia can be treated with vaginal lubricants and moisturizers. Moisturizers are effective if used multiple times per week, whereas lubricants can be used on demand. Low-dose vaginal estrogen preparations can be used in select patients with severe vaginal dryness; the goal should be discussed. Hormone replacement therapy is contraindicated in breast cancer survivors due to the risk of recurrence.11
Prostate cancer survivors. Up to 70% of men felt their quality of life and sexual function were adversely affected after the diagnosis and treatment.13,14 Erectile dysfunction following radical prostatectomy and radiation therapy remains one of the leading causes of sexual dysfunction.14–15 Erectile dysfunction is multifactorial, with both psychogenic and organic causes. Comorbidities must be considered including hypertension, diabetes, hyperlipidemia, and smoking. Early penile rehabilitation is a proposed treatment strategy.14
First-line therapy for penile rehabilitation includes introduction of daily low-dose phosphodiesterase type 5 inhibitors as early as the time of catheter removal to within the first month after surgery. The need for daily dosing vs on-demand dosing has been controversial.15 Two large multicenter, double-blind studies had conflicting outcomes. The first reported that in men receiving nightly sildenafil (50 or 100 mg) after radical prostatectomy, 27% had increased return of spontaneous erectile function vs 4% with placebo16; the second study showed no difference between nightly and on-demand dosing.17 The consensus is that a phosphodiesterase type 5 inhibitor should be initiated early.
Second-line therapy includes intracavernosal injections and vacuum erection devices. Finally, a penile prosthesis implant can be offered to patients who have responded poorly to medical therapy.13
Colorectal cancer survivors suffer from sexually related problems similar to those stated above, including erectile dysfunction, ejaculation problems, dyspareunia, vaginal dryness, and decreased enjoyment.18 These patients should be provided treatments similar to those described above. In addition, they may suffer from body image issues, particularly those with a permanent ostomy.9
Sexual dysfunction is a multifaceted problem for patients. Encouraging couples to discuss sexual intimacy frequently helps to reveal and cope with the problems, whether physical or psychological. It is the primary care physician’s role to recognize any sexual concerns and refer to the appropriate specialist.
OSTEOPOROSIS
Osteoporosis is a metabolic bone disease characterized by low bone mineral density. As a result, bones become weak and fracture more easily from minor injuries.
Risk factors for osteoporosis include female sex, family history, advanced age, low body weight, low calcium and vitamin D levels, sedentary lifestyle, smoking, and low estrogen levels.19 Cancer treatment places patients at a greater risk for osteoporosis, particularly for those patients with chemotherapy-induced ovarian failure, those treated with aromatase inhibitors, men receiving androgen-deprivation therapy, and patients on glucocorticoid therapy. The morbidity and mortality associated with bone loss can be prevented with appropriate screening, lifestyle changes, and therapy.2
According to the National Osteoporosis Foundation Guideline for Preventing and Treating Osteoporosis, all men and postmenopausal women age 50 and older should be evaluated clinically for osteoporosis risk to determine the need for bone mineral density testing.2,19 The US Preventive Services Task Force recommends bone mineral density testing in all women age 65 and older, and for women 60 to 64 who are at high risk for bone loss. ASCO agrees, and further suggests bone mineral density screening for women with breast cancer who have risk factors such as positive family history, body weight less than 70 kg, and prior nontraumatic fracture, as well as for postmenopausal women of any age receiving aromatase inhibitors and for premenopausal women with therapy-induced ovarian failure.11
Androgen deprivation therapy is a mainstay of treatment in recurrent and metastatic prostate cancer. The effect is severe hypogonadism with reductions in serum testosterone levels. Androgen deprivation therapy accelerates bone turnover, decreases bone mineral density, and contributes to fracture risk. The National Comprehensive Cancer Network additionally suggests measuring bone mineral density at baseline for all men receiving androgen deprivation therapy or other medications associated with bone loss, repeating it 1 year after androgen deprivation therapy and then every 2 years, or as clinically indicated.20
The gold standard for measuring bone mineral density is dual-energy x-ray absorptiometry. The World Health Organization FRAX tool uses bone mineral density and several clinical factors to estimate the risk of fracture in the next 10 years, which can help guide therapy. Cancer patients with elevated fracture risk should be evaluated every 2 years. Counseling should be provided to address modifiable risk factors such as smoking, alcohol consumption, physical inactivity, and low calcium and vitamin D intake. Therapy should be strongly considered in patients with a bone mineral density below a T-score of –2.0. 2,19
Treatment begins with lifestyle modifications such as weight-bearing exercises to improve balance and muscle strength and to prevent falls, and adequate intake of calcium (≥ 1,200 mg daily) and vitamin D (800–1,000 IU daily) for adults age 50 and older. Treatment with bisphosphonates may be required.2,11,20
NEUROPATHY
Many chemotherapeutic agents can lead to neuropathy and can result in long-term disability in patients. Patients treated with taxane- and platinum-based chemotherapy are at particular risk.
Paclitaxel, used in the treatment of breast, ovarian, and lung cancer, can lead to distal neuropathy. This neuropathy commonly has a stocking-and-glove distribution and is primarily sensory; however, it may have motor and autonomic components. The neuropathy typically lessens when the medication is stopped, although in some patients it can persist and lead to long-term disability.
Treatment can include massage. Medications such as gabapentin and pregabalin can also be used, but randomized controlled trials do not support them, as they predominantly treat the tingling rather than the numbness.11
BLADDER AND BOWEL DYSFUNCTION
Urinary incontinence and dysfunction are frequent complications in prostate cancer survivors. Urinary function should be discussed regularly with patients, addressing quality of the urinary stream, difficulty emptying the bladder, timing, and incontinence.4 Urinary incontinence is frequently seen in postprostatectomy patients.
The cornerstone of treating urinary incontinence is determining the cause of the incontinence, whether it is stress or urge incontinence, or both.21 For those patients with urge incontinence alone, practitioners can address the problem with a combination of behavior modification, pelvic floor exercises, and anticholinergic medications such as oxybutynin. If the problem stems from difficulty initiating or a slow stream, physicians may consider alpha-blockers.4,21 If the incontinence is persistent, bothersome, and has components of stress incontinence, the patient should be referred to a urologist for urodynamic testing, cystoscopy, and surgical evaluation for possible placement of a male urethral sling or artificial urinary sphincter.21
Colorectal cancer survivors, particularly those who received radiation therapy, are at high risk of bowel dysfunction such as chronic diarrhea and stool incontinence. Patients should be educated about this possible side effect. Symptoms of bowel dysfunction can affect body image and interfere with social functioning and overall quality of life. Patients should be provided with coping tools such as antidiarrheal medication, stool bulking agents, changes in diet, and protective underwear.
CARDIOVASCULAR DISEASE
Evidence suggests that certain types of chemotherapy and radiation therapy increase the risk of cardiovascular disease. Prostate cancer survivors treated with androgen deprivation therapy, particularly those more than 75 years old, are at increased risk of cardiovascular disease and diabetes.22
It is recommended that men be screened with fasting plasma glucose at baseline and yearly thereafter while receiving androgen deprivation therapy. Lipid panel testing should be done 1 year from initiation of androgen deprivation therapy and then, if results are normal, every 5 years or as clinically warranted. The focus should be on primary prevention with emphasis on smoking cessation, treating hypertension per guidelines, lifestyle modifications, and treatment with aspirin and statins when clinically appropriate.20
Radiation therapy, chemotherapy, and endocrine therapy have all been suggested to lead to cardiotoxicity in breast cancer patients. Anthracycline-based chemotherapies have a well-recognized association with cardiomyopathy. Factors associated with increased risk of anthracycline-induced cardiomyopathy include older age, hypertension, pre-existing coronary artery disease, and previous mediastinal radiation.
Early detection of cardiomyopathy may lead to avoidance of irreversible cardiotoxicity, but there are currently no clear guidelines for cardiac screening in breast cancer survivors. If cardiomyopathy is detected, treatment should include beta-blockers and angiotensin-converting enzyme inhibitors as well as modification of other cardiovascular risk factors.11
A SURVIVORSHIP CARE PLAN
There is life beyond the diagnosis of cancer. As patients are living longer, with an estimated 5-year survival rate of 66.5% of all cancers in the United States, there must be a transition of care from the oncologist to the primary care physician.1 While the oncologist will remain involved in the initial years of follow-up care, these visits will go from twice a year to once a year, and eventually the patient will make a full transition to care by the primary care physician. The timing of this changeover varies from physician to physician, but the primary care physician is ultimately responsible for the follow-up.
A tool to ease this transition is a survivorship care plan. The goal of a survivorship care plan is to individualize a follow-up plan while keeping in mind the necessary surveillance as outlined. These care plans are created with the patient and oncologist and then brought to the primary care physician. While there is an abundance of literature regarding the creation and initiation of survivorship care plans, the success of these plans is uncertain. Ultimately, the goal of a survivorship care plan is to create open dialogue among the oncologist, the primary care physician, and the patient. This unique patient population requires close follow-up by a multidisciplinary team with the primary care physician serving as the steward.
In 2015, about 1.6 million Americans received a diagnosis of cancer.1 In 2012, when 13.7 million people were living with cancer in the United States, the estimated 5-year survival rate of all cancers was 66.5%.1 Today, breast, prostate, and colon cancers have 5-year survival rates of 89.4%, 98.9%, and 64.9%, respectively.
With this rising trend in survival, primary care physicians have been steadily assuming the long-term care of these patients. The phrase “cancer survivorship” was coined by the National Comprehensive Cancer Network to describe the experience of living with, through, and beyond a cancer diagnosis.2
As cancer becomes a chronic medical condition, the primary care physician assumes a vital role in the treatment of the unique and evolving needs of this patient population.
This article discusses the specific needs of patients surviving breast, prostate, and colon cancer with special focus on surveillance guidelines for recurrence, development of concomitant malignancies, assessment of psychosocial and physical effects, and disease conditions related to the treatment of cancer itself.
FOLLOW-UP CARE AND SURVEILLANCE
Follow-up care in patients being treated for cancer is vital to survivorship. It includes promoting healthy living, managing treatment side effects, and monitoring for long-term side effects and possible recurrence.
Patients previously treated for malignancy are more susceptible to second primary cancers, for an array of reasons including the effects of prior treatment, shared environmental exposures such as smoking, and genetic susceptibility.2 Therefore, it is important for the primary care physician to recognize signs and symptoms and to screen cancer survivors appropriately. The American Society of Clinical Oncology (ASCO) has developed evidence-based recommendations for follow-up care,3 which we review here.
Breast cancer
For breast cancer patients, history and physical examinations are recommended every 3 to 6 months for the first 3 years, every 6 to 12 months in years 4 and 5, and annually thereafter.3
A repeat mammogram should be performed 1 year after the initial mammogram that led to the diagnosis. If the patient underwent radiation therapy, a repeat mammogram of the affected breast should be done 6 months after completion of radiation. Finally, a mammogram should be done every 6 to 12 months thereafter.3 It is also recommended that patients perform a monthly breast self-examination, but this does not replace the annual mammogram.3
Women who are treated with tamoxifen should have an annual gynecologic assessment if they have a uterus and should be encouraged to discuss any abnormal vaginal bleeding with their physician, given the increased risk of uterine cancer.2
ASCO does not recommend routine use of complete blood cell counts, complete metabolic panels, bone scans, chest radiography, computed tomography, ultrasonography, positron-emission tomography, or tumor markers in patients who are asymptomatic.3
Prostate cancer
ASCO’s recommendations for patients recovering from prostate cancer include regular histories and physical examinations and general health promotion. Prostate-specific antigen (PSA) testing is recommended every 6 to 12 months for the first 5 years after treatment and annually thereafter. More frequent PSA testing may be required in men at higher risk of recurrence or in patients who may undergo additional treatment, including radiation and surgery.4 Higher risk of disease recurrence is thought to depend on disease-specific factors at the time of original diagnosis including pretreatment PSA, Gleason score, and tumor stage.4 This should be discussed between the oncologist and primary care physician.
In regard to digital rectal examinations, the oncologist and primary care physician should jointly determine the frequency of examination. The frequency of digital rectal examinations remains an area of controversy due to their low sensitivity for detecting recurrences. Digital rectal examinations may be omitted in patients with undetectable levels of PSA.4
In regard to second primary malignancies, prostate cancer survivors who have undergone pelvic radiation therapy have a slightly higher risk of bladder and colorectal cancer. The lifetime incidence of bladder cancer after pelvic radiation is 5% to 6%, compared with 2.4% in the general population.1,5 A prostate cancer survivor presenting with hematuria should be referred to a urologist for cystoscopy and evaluation of the upper urinary tract to rule out cancer.4
Similarly, patients presenting with rectal bleeding should be referred to a gastroenterologist and the treating radiation oncologist for complete evaluation. The risk of rectal cancer after pelvic radiation therapy increases to about the same level as in someone who has a first-degree relative with colorectal cancer.5 Therefore, it is recommended that patients undergo screening for colorectal cancer in conjunction with existing evidence-based guidelines. There is no evidence to suggest that increased intensity of screening improves overall or disease-specific survival.4
Colon cancer
In patients with resected colorectal cancer, continued surveillance is important to evaluate for recurrent cancer as well as for metachronous neoplasms. Consensus statements indicate that surveillance colonoscopy should be continued for those who have undergone surgical resection for stage I, II, or III colon or rectal cancers, and for those patients with stage IV who have undergone surgical resection with curative intent.6
Patients who undergo curative resection of rectal or colon cancer should have a colonoscopy 1 year after resection or 1 year after the colonoscopy was performed, to clear the colon of synchronous disease. Subsequently, if the colonoscopy done at 1 year is normal, the interval before the next colonoscopy is 3 years. If that colonoscopy is normal, the next colonoscopy is in 5 years. Time intervals may be shorter if there is evidence of hereditary nonpolyposis colorectal cancer or adenoma.
Patients who underwent low anterior resection of rectal cancer should undergo periodic examination of the rectum to evaluate for local recurrence. Although effectiveness is not proven, endoscopic ultrasonography or flexible sigmoidoscopy is suggested at 3- to 6-month intervals for the first 2 to 3 years after resection. This is independent of colonoscopy.6,7
Additionally, ASCO recommends a history and physical examination and carcinoembryonic antigen testing every 3 to 6 months for the first 5 years. Computed tomography of the chest, abdomen, and pelvis should be done annually for the first 3 years after the end of treatment.7
HEALTH PROMOTION
Maintaining a healthy body weight and a nutritionally balanced diet should be encouraged in all cancer survivors. Poor diet, lack of exercise, excessive alcohol consumption, and smoking reduce quality of life and increase the risk of cancer.2
Diet
Numerous studies have looked at dietary modifications and risk reduction, and although there is no consensus on specific dietary guidelines, there is consensus that diet modification to maintain normal body weight will improve overall quality of life.8 Patients should be encouraged to consume a well-balanced diet consisting mostly of fruits, vegetables, whole grains, and beans, and to limit consumption of animal protein.8
There is little evidence to support taking vitamins or other dietary supplements to prevent or control cancer or to prevent its recurrence. The primary care physician should assess supplement use on a regular basis during office visits.2
Exercise
Rest is an important component of the initial recovery process. Too much inactivity, however, leads to loss of physical conditioning and muscle strength. This in turn may negatively affect a patient’s ability to perform activities of daily living and may worsen fatigue associated with treatment.
Accordingly, the National Comprehensive Cancer Network recommends at least 150 minutes of moderate activity and up to 75 minutes of more rigorous activity divided throughout the week.2,8 The regimen should include endurance and muscle strength training, which aid in balance, bone, health, and functional status. The intensity of exercise should be increased in a stepwise fashion, taking into account individual capabilities and limitations.2
Cancer survivors may need specific exercise recommendations and supervised programs to ensure safety and limit long-term side effects of their treatment. For example:
Patients with neuropathy should have their stability, balance, and gait assessed before starting a new program. These patients may benefit from an aerobic exercise program that includes riding a stationary bike rather than running.
Patients with poor bone health should have their fracture risk assessed.
Those with an ostomy bag should empty the bag before physical activity. They should avoid contact sports and activities that increase intra-abdominal pressure.
Patients suffering from lymphedema should wear compression garments when engaging in physical activity. They should undergo baseline and periodic reevaluation of the lymphedema and initiate strength training in the affected body part only if the lymphedema is stable (ie, no need for therapy for 3 months, no recent infections requiring antibiotics, and no change in circumference > 10%).2
Mental health
Health promotion should focus not only on the physical health of the patient, but also on his or her emotional and psychological well-being. As they make the transition from cancer patient to survivor, many individuals may develop or have worsening depression and anxiety due to fear of recurrence. These feelings of powerlessness can linger for years after the initial treatment.
Patients should be screened regularly for signs and symptoms of depression by asking about their family and social support. Referral to support groups, psychologists, and psychiatrists may be warranted.9
MITIGATING CANCER-RELATED FATIGUE
Cancer-related fatigue involves a patient’s subjective sense of physical, emotional, and cognitive exhaustion related to cancer or treatment that is disproportionate to that expected from recent daily activity.2 Fatigue is a common complaint among survivors and can occur months to years after treatment ends.10
Patients should be screened for fatigue at regular intervals. The primary care physician should focus the history to include information regarding the onset, pattern, duration, associated factors, assessment of other treatable comorbidities, medications, psychological well-being, nutritional status, and pain level of each patient who complains of fatigue.
Laboratory tests for treatable causes of fatigue include:
- A complete blood cell count to evaluate for anemia
- A comprehensive metabolic panel to evaluate electrolytes, renal function, and hepatic function
- The thyroid-stimulating hormone level to evaluate thyroid function, particularly in breast cancer patients who have received radiation therapy.2
If no organic cause is uncovered, the focus should shift to lifestyle interventions as the treatment of choice. The treatment of fatigue in this situation is increased physical activity with the goals discussed above. Psychological intervention may be required with cognitive behavioral therapy, psychological and supportive therapies, education on sleep hygiene, and possible sleep restriction.
If alternative causes of fatigue are ruled out and physical and psychological support fails to reduce symptoms, the practitioner can consider a psychostimulant such as methylphenidate, but this should be used cautiously.2
SEXUAL DYSFUNCTION
Intimate relationships and sexuality are an important part of life and are affected by a variety of factors including physical health, psychological well-being, body image perception, and overall status of relationships. As a side effect of chemotherapy, cancer survivors may complain of erectile dysfunction, penile shortening, dyspareunia, vaginal dryness, decreased libido, anorgasmy, and changes in body image. These issues are frequently unaddressed, whether due to the physician’s discomfort in discussing the topic or to the patient’s embarrassment and reluctance to discuss the matter.11
Breast cancer patients may experience sexual dysfunction both during and after treatment due to a combination of systemic effects of treatment, changes in physical appearance leading to impaired body image, strains on partner relationships, and psychological sequelae of diagnosis and treatment of cancer.12 Manipulation and radiation to the breast affect sexual functioning by altering body contour and image. Additionally, chemotherapy can lead to early menopause and hormonal alterations due to endocrine therapies, which can negatively affect sexual organs.11
Studies have shown that treatment with tamoxifen causes less sexual dysfunction than do aromatase inhibitors. In the Arimidex, Tamoxifen, Alone or in Combination trial, therapy with anastrozole was associated with more vaginal dryness, dyspareunia, and decreased libido compared with tamoxifen.12
Treatment requires a comprehensive history, a physical examination, and a discussion of relationship satisfaction with the patient.
Vaginal dryness and dyspareunia can be treated with vaginal lubricants and moisturizers. Moisturizers are effective if used multiple times per week, whereas lubricants can be used on demand. Low-dose vaginal estrogen preparations can be used in select patients with severe vaginal dryness; the goal should be discussed. Hormone replacement therapy is contraindicated in breast cancer survivors due to the risk of recurrence.11
Prostate cancer survivors. Up to 70% of men felt their quality of life and sexual function were adversely affected after the diagnosis and treatment.13,14 Erectile dysfunction following radical prostatectomy and radiation therapy remains one of the leading causes of sexual dysfunction.14–15 Erectile dysfunction is multifactorial, with both psychogenic and organic causes. Comorbidities must be considered including hypertension, diabetes, hyperlipidemia, and smoking. Early penile rehabilitation is a proposed treatment strategy.14
First-line therapy for penile rehabilitation includes introduction of daily low-dose phosphodiesterase type 5 inhibitors as early as the time of catheter removal to within the first month after surgery. The need for daily dosing vs on-demand dosing has been controversial.15 Two large multicenter, double-blind studies had conflicting outcomes. The first reported that in men receiving nightly sildenafil (50 or 100 mg) after radical prostatectomy, 27% had increased return of spontaneous erectile function vs 4% with placebo16; the second study showed no difference between nightly and on-demand dosing.17 The consensus is that a phosphodiesterase type 5 inhibitor should be initiated early.
Second-line therapy includes intracavernosal injections and vacuum erection devices. Finally, a penile prosthesis implant can be offered to patients who have responded poorly to medical therapy.13
Colorectal cancer survivors suffer from sexually related problems similar to those stated above, including erectile dysfunction, ejaculation problems, dyspareunia, vaginal dryness, and decreased enjoyment.18 These patients should be provided treatments similar to those described above. In addition, they may suffer from body image issues, particularly those with a permanent ostomy.9
Sexual dysfunction is a multifaceted problem for patients. Encouraging couples to discuss sexual intimacy frequently helps to reveal and cope with the problems, whether physical or psychological. It is the primary care physician’s role to recognize any sexual concerns and refer to the appropriate specialist.
OSTEOPOROSIS
Osteoporosis is a metabolic bone disease characterized by low bone mineral density. As a result, bones become weak and fracture more easily from minor injuries.
Risk factors for osteoporosis include female sex, family history, advanced age, low body weight, low calcium and vitamin D levels, sedentary lifestyle, smoking, and low estrogen levels.19 Cancer treatment places patients at a greater risk for osteoporosis, particularly for those patients with chemotherapy-induced ovarian failure, those treated with aromatase inhibitors, men receiving androgen-deprivation therapy, and patients on glucocorticoid therapy. The morbidity and mortality associated with bone loss can be prevented with appropriate screening, lifestyle changes, and therapy.2
According to the National Osteoporosis Foundation Guideline for Preventing and Treating Osteoporosis, all men and postmenopausal women age 50 and older should be evaluated clinically for osteoporosis risk to determine the need for bone mineral density testing.2,19 The US Preventive Services Task Force recommends bone mineral density testing in all women age 65 and older, and for women 60 to 64 who are at high risk for bone loss. ASCO agrees, and further suggests bone mineral density screening for women with breast cancer who have risk factors such as positive family history, body weight less than 70 kg, and prior nontraumatic fracture, as well as for postmenopausal women of any age receiving aromatase inhibitors and for premenopausal women with therapy-induced ovarian failure.11
Androgen deprivation therapy is a mainstay of treatment in recurrent and metastatic prostate cancer. The effect is severe hypogonadism with reductions in serum testosterone levels. Androgen deprivation therapy accelerates bone turnover, decreases bone mineral density, and contributes to fracture risk. The National Comprehensive Cancer Network additionally suggests measuring bone mineral density at baseline for all men receiving androgen deprivation therapy or other medications associated with bone loss, repeating it 1 year after androgen deprivation therapy and then every 2 years, or as clinically indicated.20
The gold standard for measuring bone mineral density is dual-energy x-ray absorptiometry. The World Health Organization FRAX tool uses bone mineral density and several clinical factors to estimate the risk of fracture in the next 10 years, which can help guide therapy. Cancer patients with elevated fracture risk should be evaluated every 2 years. Counseling should be provided to address modifiable risk factors such as smoking, alcohol consumption, physical inactivity, and low calcium and vitamin D intake. Therapy should be strongly considered in patients with a bone mineral density below a T-score of –2.0. 2,19
Treatment begins with lifestyle modifications such as weight-bearing exercises to improve balance and muscle strength and to prevent falls, and adequate intake of calcium (≥ 1,200 mg daily) and vitamin D (800–1,000 IU daily) for adults age 50 and older. Treatment with bisphosphonates may be required.2,11,20
NEUROPATHY
Many chemotherapeutic agents can lead to neuropathy and can result in long-term disability in patients. Patients treated with taxane- and platinum-based chemotherapy are at particular risk.
Paclitaxel, used in the treatment of breast, ovarian, and lung cancer, can lead to distal neuropathy. This neuropathy commonly has a stocking-and-glove distribution and is primarily sensory; however, it may have motor and autonomic components. The neuropathy typically lessens when the medication is stopped, although in some patients it can persist and lead to long-term disability.
Treatment can include massage. Medications such as gabapentin and pregabalin can also be used, but randomized controlled trials do not support them, as they predominantly treat the tingling rather than the numbness.11
BLADDER AND BOWEL DYSFUNCTION
Urinary incontinence and dysfunction are frequent complications in prostate cancer survivors. Urinary function should be discussed regularly with patients, addressing quality of the urinary stream, difficulty emptying the bladder, timing, and incontinence.4 Urinary incontinence is frequently seen in postprostatectomy patients.
The cornerstone of treating urinary incontinence is determining the cause of the incontinence, whether it is stress or urge incontinence, or both.21 For those patients with urge incontinence alone, practitioners can address the problem with a combination of behavior modification, pelvic floor exercises, and anticholinergic medications such as oxybutynin. If the problem stems from difficulty initiating or a slow stream, physicians may consider alpha-blockers.4,21 If the incontinence is persistent, bothersome, and has components of stress incontinence, the patient should be referred to a urologist for urodynamic testing, cystoscopy, and surgical evaluation for possible placement of a male urethral sling or artificial urinary sphincter.21
Colorectal cancer survivors, particularly those who received radiation therapy, are at high risk of bowel dysfunction such as chronic diarrhea and stool incontinence. Patients should be educated about this possible side effect. Symptoms of bowel dysfunction can affect body image and interfere with social functioning and overall quality of life. Patients should be provided with coping tools such as antidiarrheal medication, stool bulking agents, changes in diet, and protective underwear.
CARDIOVASCULAR DISEASE
Evidence suggests that certain types of chemotherapy and radiation therapy increase the risk of cardiovascular disease. Prostate cancer survivors treated with androgen deprivation therapy, particularly those more than 75 years old, are at increased risk of cardiovascular disease and diabetes.22
It is recommended that men be screened with fasting plasma glucose at baseline and yearly thereafter while receiving androgen deprivation therapy. Lipid panel testing should be done 1 year from initiation of androgen deprivation therapy and then, if results are normal, every 5 years or as clinically warranted. The focus should be on primary prevention with emphasis on smoking cessation, treating hypertension per guidelines, lifestyle modifications, and treatment with aspirin and statins when clinically appropriate.20
Radiation therapy, chemotherapy, and endocrine therapy have all been suggested to lead to cardiotoxicity in breast cancer patients. Anthracycline-based chemotherapies have a well-recognized association with cardiomyopathy. Factors associated with increased risk of anthracycline-induced cardiomyopathy include older age, hypertension, pre-existing coronary artery disease, and previous mediastinal radiation.
Early detection of cardiomyopathy may lead to avoidance of irreversible cardiotoxicity, but there are currently no clear guidelines for cardiac screening in breast cancer survivors. If cardiomyopathy is detected, treatment should include beta-blockers and angiotensin-converting enzyme inhibitors as well as modification of other cardiovascular risk factors.11
A SURVIVORSHIP CARE PLAN
There is life beyond the diagnosis of cancer. As patients are living longer, with an estimated 5-year survival rate of 66.5% of all cancers in the United States, there must be a transition of care from the oncologist to the primary care physician.1 While the oncologist will remain involved in the initial years of follow-up care, these visits will go from twice a year to once a year, and eventually the patient will make a full transition to care by the primary care physician. The timing of this changeover varies from physician to physician, but the primary care physician is ultimately responsible for the follow-up.
A tool to ease this transition is a survivorship care plan. The goal of a survivorship care plan is to individualize a follow-up plan while keeping in mind the necessary surveillance as outlined. These care plans are created with the patient and oncologist and then brought to the primary care physician. While there is an abundance of literature regarding the creation and initiation of survivorship care plans, the success of these plans is uncertain. Ultimately, the goal of a survivorship care plan is to create open dialogue among the oncologist, the primary care physician, and the patient. This unique patient population requires close follow-up by a multidisciplinary team with the primary care physician serving as the steward.
- National Cancer Institute (NIH). Surveillance, Epidemiology, and End Results Program. SEER cancer stat fact sheets. http://seer.cancer.gov/statfacts. Accessed March 6, 2017.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines Survivorship: 2015. www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Accessed March 6, 2017.
- Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2013; 31:961–965.
- Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol 2015; 33:1078–1085.
- Sountoulides P, Koletsas N, Kikidakis D, Paschalidis K, Sofikitis N. Secondary malignancies following radiotherapy for prostate cancer. Ther Adv Urol 2010; 2:119–125.
- Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin 2006; 56:160–168.
- Meyerhardt JA, Mangu PB, Flynn PJ, et al; American Society of Clinical Oncology. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013; 31:4465–4470.
- Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer 2011; 105(suppl 1):S52–S73.
- Miller K, editor. Excellent Care for Cancer Survivors: A Guide to Fully Meet Their Needs in Medical Offices and in the Community (Praeger Series on Contemporary Health & Living). 1st ed. Santa Barbara, CA: Praeger; 2012.
- Stanton AL, Rowland JH, Ganz PA. Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am Psychol 2015; 70:159–174.
- Stan D, Loprinzi CL, Ruddy KJ. Breast cancer survivorship issues. Hematol Oncol Clin North Am 2013; 27:805–827.
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol 2004; 22:426–471.
- Chung E, Gillman M. Prostate cancer survivorship: a review of erectile dysfunction and penile rehabilitation after prostate cancer therapy. Med J Aust 2014; 200:582–585.
- Sherer BA, Levine LA. Current management of erectile dysfunction in prostate cancer survivors. Curr Opin Urol 2014; 24:401–416.
- Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med 2013; 10(suppl 1):102–111.
- Padma-Nathan H, McCullough AR, Levine LA, et al; Study Group. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res 2008; 20:479–486.
- Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol 2008; 54:924–931.
- Den Oudsten BL, Traa MJ, Thong MS, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: a population-based study. Eur J Cancer 2012; 48:3161–3170.
- National Osteoporosis Foundation (NOF). http://nof.org. Accessed March 3, 2017.
- Saylor PJ, Keating NL, Smith MR. Prostate cancer survivorship: prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med 2009; 24(suppl 2):S389–S394.
- Gupta S, Peterson AC. Stress urinary incontinence in the prostate cancer survivor. Curr Opin Urol 2014; 24:395–400.
- Morgans AK, Fan KH, Koyama T, et al. Influence of age on incident diabetes and cardiovascular disease in prostate cancer survivors receiving androgen deprivation therapy. J Urol 2015; 193:1226–1231.
- National Cancer Institute (NIH). Surveillance, Epidemiology, and End Results Program. SEER cancer stat fact sheets. http://seer.cancer.gov/statfacts. Accessed March 6, 2017.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines Survivorship: 2015. www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Accessed March 6, 2017.
- Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2013; 31:961–965.
- Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol 2015; 33:1078–1085.
- Sountoulides P, Koletsas N, Kikidakis D, Paschalidis K, Sofikitis N. Secondary malignancies following radiotherapy for prostate cancer. Ther Adv Urol 2010; 2:119–125.
- Rex DK, Kahi CJ, Levin B, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin 2006; 56:160–168.
- Meyerhardt JA, Mangu PB, Flynn PJ, et al; American Society of Clinical Oncology. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol 2013; 31:4465–4470.
- Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer 2011; 105(suppl 1):S52–S73.
- Miller K, editor. Excellent Care for Cancer Survivors: A Guide to Fully Meet Their Needs in Medical Offices and in the Community (Praeger Series on Contemporary Health & Living). 1st ed. Santa Barbara, CA: Praeger; 2012.
- Stanton AL, Rowland JH, Ganz PA. Life after diagnosis and treatment of cancer in adulthood: contributions from psychosocial oncology research. Am Psychol 2015; 70:159–174.
- Stan D, Loprinzi CL, Ruddy KJ. Breast cancer survivorship issues. Hematol Oncol Clin North Am 2013; 27:805–827.
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol 2004; 22:426–471.
- Chung E, Gillman M. Prostate cancer survivorship: a review of erectile dysfunction and penile rehabilitation after prostate cancer therapy. Med J Aust 2014; 200:582–585.
- Sherer BA, Levine LA. Current management of erectile dysfunction in prostate cancer survivors. Curr Opin Urol 2014; 24:401–416.
- Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med 2013; 10(suppl 1):102–111.
- Padma-Nathan H, McCullough AR, Levine LA, et al; Study Group. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res 2008; 20:479–486.
- Montorsi F, Brock G, Lee J, et al. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol 2008; 54:924–931.
- Den Oudsten BL, Traa MJ, Thong MS, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: a population-based study. Eur J Cancer 2012; 48:3161–3170.
- National Osteoporosis Foundation (NOF). http://nof.org. Accessed March 3, 2017.
- Saylor PJ, Keating NL, Smith MR. Prostate cancer survivorship: prevention and treatment of the adverse effects of androgen deprivation therapy. J Gen Intern Med 2009; 24(suppl 2):S389–S394.
- Gupta S, Peterson AC. Stress urinary incontinence in the prostate cancer survivor. Curr Opin Urol 2014; 24:395–400.
- Morgans AK, Fan KH, Koyama T, et al. Influence of age on incident diabetes and cardiovascular disease in prostate cancer survivors receiving androgen deprivation therapy. J Urol 2015; 193:1226–1231.
KEY POINTS
- The American Society of Clinical Oncology has developed evidence-based recommendations for follow-up care and surveillance for new and recurrent cancer in cancer survivors. In general, this surveillance should be more frequent in the first months and years after cancer treatment but can become less so as time goes on.
- Health promotion in cancer survivors involves the same advice regarding smoking cessation, diet, exercise, and mental health that all patients require.
- Depending on the type of cancer and treatment, long-term adverse effects include fatigue, sexual dysfunction, osteoporosis, neuropathy, bladder and bowel dysfunction, and cardiovascular disease.
- A survivorship care plan can be drawn up with input from the patient, oncologist, primary care physician, and other caregivers so that everyone can be clear as to what is going on.
Maternal asthma: Management strategies
The incidence of maternal asthma is rising. Based on US national health surveys, the prevalence of asthma during pregnancy is between 3.7% and 8.4%.1 It is the most common respiratory illness of pregnancy.2 Hence, clinicians need to know how asthma affects the mother and the fetus. Appropriate care of asthma during pregnancy is based on several management principles, as reviewed here, and is key to ensuring good outcomes for the mother and the baby.
EFFECT OF PREGNANCY ON ASTHMA CONTROL
Asthma control can vary in pregnancy. About a third of asthmatic women experience a worsening of asthma control with pregnancy, a third remain unchanged, and another third have improvement in asthma symptoms.3 The peak worsening of asthma tends to occur in the sixth month.4 Asthma control also tends to be better in the last month of pregnancy.3
The peak expiratory flow rate was noted to increase with each trimester in a small study of 43 women.5 The authors speculated that a rising progesterone level stimulates cyclic adenosine monophosphate to cause bronchodilation, thereby improving the expiratory flow rate and asthma control. Asthma control tends to follow the pattern experienced in the previous pregnancy: ie, if asthma worsened during the previous pregnancy, the same will be likely in the subsequent pregnancy.3
Two maternal factors that adversely affect asthma severity during pregnancy are the use of asthma medications contrary to guidelines such as those of the Global Initiative for Asthma (http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention) and inadquate control of asthma before becoming pregnant.6 Pregnancy can bring on stress, and stress is known to worsen asthma. In addition, when patients themselves were interviewed to elucidate the reasons for poor adherence to asthma medications during pregnancy, concerns about medication use, especially corticosteroids, stood out.7 A study based on prescription claims data showed that in the first trimester, there was a significant decline in asthma prescription medications (a 23% decline in inhaled corticosteroids, a 13% decline in short-acting bronchodilator agents, and a 54% decline in rescue corticosteroids).8 Lack of physician education about management of asthma in pregnancy and discomfort with prescribing to pregnant women also affect asthma control.
EFFECT OF ASTHMA ON MATERNAL AND FETAL OUTCOMES
Studies of the effects of asthma on fetal and maternal outcomes have yielded mixed and conflicting results.9 Adverse outcomes that have been shown to be associated with maternal asthma are listed in Table 1. Other studies have not demonstrated an association between asthma in pregnancy and maternal or fetal adverse events.9 Such discrepant findings are due to differences in study population characteristics that make comparisons difficult. A meta-analysis involving more than 1.6 million asthmatic women showed maternal asthma was associated with a 40% greater risk of low birth weight and preterm delivery, a 50% greater risk of preeclampsia, and a 20% greater risk of the baby being small for its gestational age.10
The association of maternal asthma and preterm birth may pose short-term and long-term health risks to the child associated with prematurity.9 Short-term risks with prematurity include infection, respiratory distress syndrome, brain injury, and necrotizing enterocolitis. Long-term risks include neurodevelopmental and behavioral sequelae. Furthermore, asthma exacerbations during pregnancy are associated with a twofold higher risk of low birth weight.11 The benefits of good adherence to asthma regimens during pregnancy outweigh the risks associated with frequent symptoms and exacerbations caused by untreated asthma.12
OUTPATIENT MANAGEMENT OF MATERNAL ASTHMA
Goals
In the 2004 update of the National Asthma Education and Prevention Program (NAEPP) Working Group Report on Managing Asthma During Pregnancy, goals focused mainly on adequate asthma control for maternal health and quality of life, as well as normal fetal maturation (Table 2),12 goals similar to those in nonpregnant asthmatic women.
Assessment and monitoring
Monthly physician visits during pregnancy are recommended for assessment of symptoms and pulmonary function. If symptoms are uncontrolled, therapy must be stepped up, and any trigger for exacerbation, such as gastroesophageal reflux disease (GERD), exposure, or rhinitis, must be treated and eliminated. NAEPP guidelines recommend baseline spirometry at the time of initial assessment.12 At follow-up visits, spirometry is preferred, but measurement of the peak expiratory flow rate usually suffices. Such objective data can help differentiate dyspnea from asthma and from dyspnea that usually accompanies the physiologic changes of pregnancy. In addition, patients should be advised to monitor for adequate fetal activity. If asthma is uncontrolled or poorly controlled, serial fetal ultrasonography should be considered from 32 weeks of gestation, as well as after recovery from an asthma exacerbation. Regular monitoring of the pregnant asthmatic patient by a multidisciplinary team can improve outcomes.13
Avoiding triggers
Patients should be advised to avoid asthma triggers such as pet dander, dust mites, pollen, smoke, mold, and perfumes, as this can decrease symptoms and allow for use of lower doses of medications.12 Additionally, smoking cessation must be strongly encouraged, not only to control maternal asthma, but also to prevent harm to the fetus.
MANAGEMENT OF SPECIFIC TRIGGERS
GERD
Reflux disease often worsens during pregnancy, and it can coexist with asthma and can also exacerbate it.14 Optimal control of GERD helps maintain adequate asthma control. For mild reflux symptoms, lifestyle modifications such as elevating the head of bed, avoiding eating too close to bedtime, and avoiding foods that cause heartburn may be adequate.15,16 If medications are needed, antacids (but not sodium bicarbonate, for fear of metabolic alkalosis) and sucralfate should be considered before using a histamine 2 receptor antagonist such as ranitidine. Proton pump inhibitors should be considered only if reflux symptoms are refractory to other therapies.
Allergic rhinitis
Intranasal corticosteroids are effective against allergic rhinitis in pregnancy (Table 3).12 Montelukast, a leukotriene receptor antagonist, can be used, but data to support its use for allergic rhinitis in pregnancy are limited.
Among antihistamines, second-generation drugs such as cetirizine or loratadine can be considered.12 Oral decongestants such as pseudoephedrine in early pregnancy are associated with a rare congenital fetal abnormality called gastroschisis, caused by vascular disruption.17 Hence, if a nasal decongestant is required in early pregnancy, a local therapy such as an intranasal corticosteroid, short-term oxymetazoline, or an external nasal dilator may be considered.12 These therapies must be combined with avoidance of allergens whenever possible.
Allergies
Diagnostic allergy and skin tests during pregnancy pose a risk of anaphylaxis and thus should be avoided. Instead, the focus should be on obtaining a thorough medical history about exposures and eliminating specific asthma triggers. It is also inadvisable to start allergen immunotherapy during pregnancy because of the risk of anaphylaxis and the effect of treatment on the mother and fetus.18 However, maintenance doses of allergen immunotherapy can be continued during pregnancy.18
Patient education
Because of concern about the risks of taking medications during pregnancy, many women with asthma stop using their inhalers during pregnancy, thus compromising asthma control.8,13 The physician and multidisciplinary team must use every opportunity to emphasize the importance of good asthma control during pregnancy. Inhaler technique should also be reviewed and, if defective, corrected. Again, trigger avoidance and tobacco cessation should be addressed.
Drugs
The NAEPP recommendations state that asthma therapy should be continued during pregnancy, as it is safer both for mother and fetus to avoid exacerbations and uncontrolled asthma.12 Despite this, 25% of primary care physicians instruct their patients to decrease or discontinue their inhaled corticosteroid during pregnancy.19 As with asthma in general, treatment should involve using the lowest dose of drugs that achieves adequate control of symptoms.
In 2015, the US Food and Drug Administration (FDA) amended the labeling rule for medications used in pregnancy and lactation. The previous risk categories A (safest), B, C, D, and X (highest risk) are in the process of being removed from labels for all human prescription drugs and biologic products, to be replaced with a summary of the risks of taking the drug during pregnancy and lactation, a discussion of the data supporting the use, and relevant information to help healthcare providers make prescribing decisions and counsel women about the use of drugs during pregnancy and lactation (www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm).
ROLES OF CONTROLLER THERAPY AND RESCUE THERAPY
Inhaled corticosteroids
Inhaled corticosteroids are the mainstay of asthma controller therapy during pregnancy. A meta-analysis of 16 studies showed no increased risk of congenital malformations, cesarean delivery, or stillbirth among mothers who used these agents during pregnancy.20 Because there are more safety data for budesonide, it is currently the preferred inhaled corticosteroid during pregnancy.9 However, if a patient’s asthma is controlled with a different corticosteroid before pregnancy, that agent may be continued during pregnancy, especially if it is thought that switching formulations could adversely affect asthma control.12 This is mainly because current data do not prove that other inhaled corticosteroids are unsafe.
Inhaled beta-agonists
Inhaled beta-agonists, both short-acting and long-acting, are used for rescue therapy. Albuterol is the preferred short-acting agent for rescue therapy in pregnant women with asthma.12 Meta-analysis has shown no increased risk of major or minor congenital malformations in pregnant patients who use bronchodilators.20 Long-acting beta-agonists typically are used as add-on therapy when asthma cannot be controlled by an inhaled corticosteroid. They should not be used without a controller medication (ie, an inhaled corticosteroid).
Guidelines for rescue therapy are similar to those for nonpregnant asthmatic patients. Although data are limited as to the gestational effects of long-acting beta-agonists (ie, formoterol, salmeterol), it can be assumed that the toxicologic and pharmacologic profiles are similar to those of the short-acting bronchodilators. Thus, the safety of albuterol can be extended potentially to the long-acting beta-agonists.12
Combining controller and rescue therapy
When asthma is not adequately controlled on inhaled corticosteroids, a long-acting beta-agonist can be added or the dose of corticosteroid can be increased. The 2004 NAEPP guidelines stated that based on available literature, there was no clear advantage of one option over the other.12 A study that compared the 2 approaches found no difference in rates of congenital malformations.21
Leukotriene receptor antagonists
There is little in the literature regarding the use of leukotriene receptor antagonists during pregnancy. However, animal safety data are reassuring,12 and human studies have not found a higher risk of major congenital malformations.22,23 Thus, they are an alternative for patients whose asthma has been well controlled on these agents before pregnancy. Montelukast and zafirlukast are in former FDA pregnancy risk factor category B (probably safe) (Table 3). However, 5-lipoxygenase inhibitors such as zileuton are contraindicated based on animal studies showing teratogenicity.24
Omalizumab
Omalizumab, a recombinant anti-immunoglobulin E antibody, can be used for allergic asthma not controlled with inhaled corticosteroids (Table 3). An analysis of the omalizumab pregnancy registry25 found no significant increase in the rate of major congenital malformations, prematurity, or babies small for gestational age in asthmatic women taking omalizumab 8 weeks before conception or during pregnancy vs pregnant asthmatic women not taking omalizumab. However, this drug carries a risk of anaphylaxis and so should not be started during pregnancy.25
Theophylline
Because of potential toxicity, use of theophylline during pregnancy requires careful monitoring to ensure the serum concentration remains between 5 and 12 µg/mL.12 Drug interactions are also common: for example, alcohol may increase the serum concentration of theophylline, and theophylline may increase the toxic effect of formoterol.
Systemic corticosteroids
Pregnant women with asthma that is not well controlled despite the therapies described above may require a daily oral corticosteroid such as prednisone to achieve adequate control. Oral steroids are also a mainstay of treatment of asthma exacerbation.
Although use of corticosteroids in the first trimester was associated with orofacial cleft in infants,12 these studies did not include many women with asthma. In 2011, a nationwide cohort study from Denmark showed no increase in the risk of orofacial cleft with the use of corticosteroids during pregnancy.26
Preeclampsia, low birth weight, and preterm delivery have been described with corticosteroid use in pregnancy. It is not known whether these problems were a result of corticosteroid use or were due to the uncontrolled nature of the underlying condition that led to the steroid use. Since the risk of uncontrolled asthma to mother and fetus outweighs the risk of systemic corticosteroids, these drugs are recommended when indicated for management of maternal asthma.12
ACUTE EXACERBATIONS REQUIRE AGGRESSIVE MANAGEMENT
Based on a systematic review, 20% of pregnant women with asthma require some intervention for an asthma exacerbation during pregnancy, and 5.8% are admitted to the hospital for an exacerbation.11 Exacerbations were associated with a higher risk of low birth weight compared with rates in women without asthma.
Exacerbations are more common late in the second trimester and are unlikely to occur during labor and delivery.2 The incidence of exacerbations increases with the severity of asthma, from 8% in mild asthma, to 47% in moderate asthma, to 65% in severe asthma.27 Risk factors for exacerbations include poor prenatal care, obesity, and lack of appropriate treatment with inhaled corticosteroids.2 The main triggers are viral respiratory infections and noncompliance with inhaled corticosteroid therapy.11
Asthma exacerbations during pregnancy should be managed aggressively (Table 4),12 as the risk to the fetus of hypoxia far outweighs any risk from asthma medications. Close collaboration between the primary care physician and the obstetrician allows closer monitoring of mother and fetus.
The goal oxygen saturation must be above 95%.12 Signs of acute respiratory failure in a pregnant patient include a partial pressure of arterial oxygen less than 70 mm Hg or a partial pressure of carbon dioxide greater than 35 mm Hg.
In a multicenter study comparing nonpregnant and pregnant women visiting the emergency room for asthma exacerbations,28 pregnant women were less likely to be prescribed systemic corticosteroids either in the emergency room or at the time of hospital discharge, and they were also more likely to describe an ongoing exacerbation at 2-week follow-up. However, a recent study showed a significant increase in systemic corticosteroid treatment in the emergency room (51% to 78% across the time periods, odds ratio 3.11, 95% confidence interval 1.27–7.60, P = .01). There was also an increase in steroid treatment at discharge (42% to 63%, odds ratio 2.49, 95% confidence interval 0.97–6.37, P = .054), though the increase was not statistically significant.29 Although emergency room care for pregnant asthmatic women has improved, this group concluded that further improvement is still warranted, as 1 in 3 women is discharged without corticosteroid treatment.
- Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol 2003; 13:317–324.
- Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011; 378:983–990.
- Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 1988; 81:509–517.
- Gluck JC, Gluck PA. The effect of pregnancy on the course of asthma. Immunol Allergy Clin North Am 2006; 26:63–80.
- Beckmann CA. Peak flow values by gestation in women with asthma. Clin Nurs Res 2008; 17:174–181.
- Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol 2010; 115:559–567.
- Lim AS, Stewart K, Abramson MJ, Ryan K, George J. Asthma during pregnancy: the experiences, concerns and views of pregnant women with asthma. J Asthma 2012; 49:474–479.
- Enriquez R, Wu P, Griffin MR, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol 2006; 195:149–153.
- Bain E, Pierides KL, Clifton VL, et al. Interventions for managing asthma in pregnancy. Cochrane Database Syst Rev 2014; 10:CD010660.
- Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011; 118:1314–1323.
- Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 2006; 61:169–176.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol 2005; 115:34–46.
- Lim AS, Stewart K, Abramson MJ, Walker SP, Smith CL, George J. Multidisciplinary Approach to Management of Maternal Asthma (MAMMA): a randomized controlled trial. Chest 2014; 145:1046–1054.
- Harding SM. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am 2005; 25:131–148.
- Ahmad S, Mokaddas E. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respir Med 2009; 103:1777–1790.
- van der Woude CJ, Metselaar HJ, Danese S. Management of gastrointestinal and liver diseases during pregnancy. Gut 2014; 63:1014–1023.
- Werler MM. Teratogen update: pseudoephedrine. Birth Defects Res A Clin Mol Teratol 2006; 76:445–452.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Lim AS, Stewart K, Abramson MJ, George J. Management of asthma in pregnant women by general practitioners: a cross sectional survey. BMC Fam Pract 2011; 12:121.
- Murphy VE, Wang G, Namazy JA, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013; 120:812–822.
- Eltonsy S, Forget A, Beauchesne MF, Blais L. Risk of congenital malformations for asthmatic pregnant women using a long-acting beta2-agonist and inhaled corticosteroid combination versus higher-dose inhaled corticosteroid monotherapy. J Allergy Clin Immunol 2015; 135:123–130.
- Nelsen LM, Shields KE, Cunningham ML, et al. Congenital malformations among infants born to women receiving montelukast, inhaled corticosteroids, and other asthma medications. J Allergy Clin Immunol 2012; 129:251–254.e1–e6.
- Sarkar M, Koren G, Kalra S, et al. Montelukast use during pregnancy: a multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol 2009; 65:1259–1264.
- Namazy JA, Schatz M. The safety of asthma medications during pregnancy: an update for clinicians. Ther Adv Respir Dis 2014; 8:103–110.
- Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135:407–412.
- Hviid A, Molgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ 2011; 183:796–804.
- Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol 2005; 106:1046–1054.
- Cydulka RK, Emerman CL, Schreiber D, Molander KH, Woodruff PG, Camargo CA Jr. Acute asthma among pregnant women presenting to the emergency department. Am J Respir Crit Care Med 1999; 160:887–892.
- Hasegawa K, Cydulka RK, Sullivan AF, et al. Improved management of acute asthma among pregnant women presenting to the ED. Chest 2015; 147:406–414.
The incidence of maternal asthma is rising. Based on US national health surveys, the prevalence of asthma during pregnancy is between 3.7% and 8.4%.1 It is the most common respiratory illness of pregnancy.2 Hence, clinicians need to know how asthma affects the mother and the fetus. Appropriate care of asthma during pregnancy is based on several management principles, as reviewed here, and is key to ensuring good outcomes for the mother and the baby.
EFFECT OF PREGNANCY ON ASTHMA CONTROL
Asthma control can vary in pregnancy. About a third of asthmatic women experience a worsening of asthma control with pregnancy, a third remain unchanged, and another third have improvement in asthma symptoms.3 The peak worsening of asthma tends to occur in the sixth month.4 Asthma control also tends to be better in the last month of pregnancy.3
The peak expiratory flow rate was noted to increase with each trimester in a small study of 43 women.5 The authors speculated that a rising progesterone level stimulates cyclic adenosine monophosphate to cause bronchodilation, thereby improving the expiratory flow rate and asthma control. Asthma control tends to follow the pattern experienced in the previous pregnancy: ie, if asthma worsened during the previous pregnancy, the same will be likely in the subsequent pregnancy.3
Two maternal factors that adversely affect asthma severity during pregnancy are the use of asthma medications contrary to guidelines such as those of the Global Initiative for Asthma (http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention) and inadquate control of asthma before becoming pregnant.6 Pregnancy can bring on stress, and stress is known to worsen asthma. In addition, when patients themselves were interviewed to elucidate the reasons for poor adherence to asthma medications during pregnancy, concerns about medication use, especially corticosteroids, stood out.7 A study based on prescription claims data showed that in the first trimester, there was a significant decline in asthma prescription medications (a 23% decline in inhaled corticosteroids, a 13% decline in short-acting bronchodilator agents, and a 54% decline in rescue corticosteroids).8 Lack of physician education about management of asthma in pregnancy and discomfort with prescribing to pregnant women also affect asthma control.
EFFECT OF ASTHMA ON MATERNAL AND FETAL OUTCOMES
Studies of the effects of asthma on fetal and maternal outcomes have yielded mixed and conflicting results.9 Adverse outcomes that have been shown to be associated with maternal asthma are listed in Table 1. Other studies have not demonstrated an association between asthma in pregnancy and maternal or fetal adverse events.9 Such discrepant findings are due to differences in study population characteristics that make comparisons difficult. A meta-analysis involving more than 1.6 million asthmatic women showed maternal asthma was associated with a 40% greater risk of low birth weight and preterm delivery, a 50% greater risk of preeclampsia, and a 20% greater risk of the baby being small for its gestational age.10
The association of maternal asthma and preterm birth may pose short-term and long-term health risks to the child associated with prematurity.9 Short-term risks with prematurity include infection, respiratory distress syndrome, brain injury, and necrotizing enterocolitis. Long-term risks include neurodevelopmental and behavioral sequelae. Furthermore, asthma exacerbations during pregnancy are associated with a twofold higher risk of low birth weight.11 The benefits of good adherence to asthma regimens during pregnancy outweigh the risks associated with frequent symptoms and exacerbations caused by untreated asthma.12
OUTPATIENT MANAGEMENT OF MATERNAL ASTHMA
Goals
In the 2004 update of the National Asthma Education and Prevention Program (NAEPP) Working Group Report on Managing Asthma During Pregnancy, goals focused mainly on adequate asthma control for maternal health and quality of life, as well as normal fetal maturation (Table 2),12 goals similar to those in nonpregnant asthmatic women.
Assessment and monitoring
Monthly physician visits during pregnancy are recommended for assessment of symptoms and pulmonary function. If symptoms are uncontrolled, therapy must be stepped up, and any trigger for exacerbation, such as gastroesophageal reflux disease (GERD), exposure, or rhinitis, must be treated and eliminated. NAEPP guidelines recommend baseline spirometry at the time of initial assessment.12 At follow-up visits, spirometry is preferred, but measurement of the peak expiratory flow rate usually suffices. Such objective data can help differentiate dyspnea from asthma and from dyspnea that usually accompanies the physiologic changes of pregnancy. In addition, patients should be advised to monitor for adequate fetal activity. If asthma is uncontrolled or poorly controlled, serial fetal ultrasonography should be considered from 32 weeks of gestation, as well as after recovery from an asthma exacerbation. Regular monitoring of the pregnant asthmatic patient by a multidisciplinary team can improve outcomes.13
Avoiding triggers
Patients should be advised to avoid asthma triggers such as pet dander, dust mites, pollen, smoke, mold, and perfumes, as this can decrease symptoms and allow for use of lower doses of medications.12 Additionally, smoking cessation must be strongly encouraged, not only to control maternal asthma, but also to prevent harm to the fetus.
MANAGEMENT OF SPECIFIC TRIGGERS
GERD
Reflux disease often worsens during pregnancy, and it can coexist with asthma and can also exacerbate it.14 Optimal control of GERD helps maintain adequate asthma control. For mild reflux symptoms, lifestyle modifications such as elevating the head of bed, avoiding eating too close to bedtime, and avoiding foods that cause heartburn may be adequate.15,16 If medications are needed, antacids (but not sodium bicarbonate, for fear of metabolic alkalosis) and sucralfate should be considered before using a histamine 2 receptor antagonist such as ranitidine. Proton pump inhibitors should be considered only if reflux symptoms are refractory to other therapies.
Allergic rhinitis
Intranasal corticosteroids are effective against allergic rhinitis in pregnancy (Table 3).12 Montelukast, a leukotriene receptor antagonist, can be used, but data to support its use for allergic rhinitis in pregnancy are limited.
Among antihistamines, second-generation drugs such as cetirizine or loratadine can be considered.12 Oral decongestants such as pseudoephedrine in early pregnancy are associated with a rare congenital fetal abnormality called gastroschisis, caused by vascular disruption.17 Hence, if a nasal decongestant is required in early pregnancy, a local therapy such as an intranasal corticosteroid, short-term oxymetazoline, or an external nasal dilator may be considered.12 These therapies must be combined with avoidance of allergens whenever possible.
Allergies
Diagnostic allergy and skin tests during pregnancy pose a risk of anaphylaxis and thus should be avoided. Instead, the focus should be on obtaining a thorough medical history about exposures and eliminating specific asthma triggers. It is also inadvisable to start allergen immunotherapy during pregnancy because of the risk of anaphylaxis and the effect of treatment on the mother and fetus.18 However, maintenance doses of allergen immunotherapy can be continued during pregnancy.18
Patient education
Because of concern about the risks of taking medications during pregnancy, many women with asthma stop using their inhalers during pregnancy, thus compromising asthma control.8,13 The physician and multidisciplinary team must use every opportunity to emphasize the importance of good asthma control during pregnancy. Inhaler technique should also be reviewed and, if defective, corrected. Again, trigger avoidance and tobacco cessation should be addressed.
Drugs
The NAEPP recommendations state that asthma therapy should be continued during pregnancy, as it is safer both for mother and fetus to avoid exacerbations and uncontrolled asthma.12 Despite this, 25% of primary care physicians instruct their patients to decrease or discontinue their inhaled corticosteroid during pregnancy.19 As with asthma in general, treatment should involve using the lowest dose of drugs that achieves adequate control of symptoms.
In 2015, the US Food and Drug Administration (FDA) amended the labeling rule for medications used in pregnancy and lactation. The previous risk categories A (safest), B, C, D, and X (highest risk) are in the process of being removed from labels for all human prescription drugs and biologic products, to be replaced with a summary of the risks of taking the drug during pregnancy and lactation, a discussion of the data supporting the use, and relevant information to help healthcare providers make prescribing decisions and counsel women about the use of drugs during pregnancy and lactation (www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm).
ROLES OF CONTROLLER THERAPY AND RESCUE THERAPY
Inhaled corticosteroids
Inhaled corticosteroids are the mainstay of asthma controller therapy during pregnancy. A meta-analysis of 16 studies showed no increased risk of congenital malformations, cesarean delivery, or stillbirth among mothers who used these agents during pregnancy.20 Because there are more safety data for budesonide, it is currently the preferred inhaled corticosteroid during pregnancy.9 However, if a patient’s asthma is controlled with a different corticosteroid before pregnancy, that agent may be continued during pregnancy, especially if it is thought that switching formulations could adversely affect asthma control.12 This is mainly because current data do not prove that other inhaled corticosteroids are unsafe.
Inhaled beta-agonists
Inhaled beta-agonists, both short-acting and long-acting, are used for rescue therapy. Albuterol is the preferred short-acting agent for rescue therapy in pregnant women with asthma.12 Meta-analysis has shown no increased risk of major or minor congenital malformations in pregnant patients who use bronchodilators.20 Long-acting beta-agonists typically are used as add-on therapy when asthma cannot be controlled by an inhaled corticosteroid. They should not be used without a controller medication (ie, an inhaled corticosteroid).
Guidelines for rescue therapy are similar to those for nonpregnant asthmatic patients. Although data are limited as to the gestational effects of long-acting beta-agonists (ie, formoterol, salmeterol), it can be assumed that the toxicologic and pharmacologic profiles are similar to those of the short-acting bronchodilators. Thus, the safety of albuterol can be extended potentially to the long-acting beta-agonists.12
Combining controller and rescue therapy
When asthma is not adequately controlled on inhaled corticosteroids, a long-acting beta-agonist can be added or the dose of corticosteroid can be increased. The 2004 NAEPP guidelines stated that based on available literature, there was no clear advantage of one option over the other.12 A study that compared the 2 approaches found no difference in rates of congenital malformations.21
Leukotriene receptor antagonists
There is little in the literature regarding the use of leukotriene receptor antagonists during pregnancy. However, animal safety data are reassuring,12 and human studies have not found a higher risk of major congenital malformations.22,23 Thus, they are an alternative for patients whose asthma has been well controlled on these agents before pregnancy. Montelukast and zafirlukast are in former FDA pregnancy risk factor category B (probably safe) (Table 3). However, 5-lipoxygenase inhibitors such as zileuton are contraindicated based on animal studies showing teratogenicity.24
Omalizumab
Omalizumab, a recombinant anti-immunoglobulin E antibody, can be used for allergic asthma not controlled with inhaled corticosteroids (Table 3). An analysis of the omalizumab pregnancy registry25 found no significant increase in the rate of major congenital malformations, prematurity, or babies small for gestational age in asthmatic women taking omalizumab 8 weeks before conception or during pregnancy vs pregnant asthmatic women not taking omalizumab. However, this drug carries a risk of anaphylaxis and so should not be started during pregnancy.25
Theophylline
Because of potential toxicity, use of theophylline during pregnancy requires careful monitoring to ensure the serum concentration remains between 5 and 12 µg/mL.12 Drug interactions are also common: for example, alcohol may increase the serum concentration of theophylline, and theophylline may increase the toxic effect of formoterol.
Systemic corticosteroids
Pregnant women with asthma that is not well controlled despite the therapies described above may require a daily oral corticosteroid such as prednisone to achieve adequate control. Oral steroids are also a mainstay of treatment of asthma exacerbation.
Although use of corticosteroids in the first trimester was associated with orofacial cleft in infants,12 these studies did not include many women with asthma. In 2011, a nationwide cohort study from Denmark showed no increase in the risk of orofacial cleft with the use of corticosteroids during pregnancy.26
Preeclampsia, low birth weight, and preterm delivery have been described with corticosteroid use in pregnancy. It is not known whether these problems were a result of corticosteroid use or were due to the uncontrolled nature of the underlying condition that led to the steroid use. Since the risk of uncontrolled asthma to mother and fetus outweighs the risk of systemic corticosteroids, these drugs are recommended when indicated for management of maternal asthma.12
ACUTE EXACERBATIONS REQUIRE AGGRESSIVE MANAGEMENT
Based on a systematic review, 20% of pregnant women with asthma require some intervention for an asthma exacerbation during pregnancy, and 5.8% are admitted to the hospital for an exacerbation.11 Exacerbations were associated with a higher risk of low birth weight compared with rates in women without asthma.
Exacerbations are more common late in the second trimester and are unlikely to occur during labor and delivery.2 The incidence of exacerbations increases with the severity of asthma, from 8% in mild asthma, to 47% in moderate asthma, to 65% in severe asthma.27 Risk factors for exacerbations include poor prenatal care, obesity, and lack of appropriate treatment with inhaled corticosteroids.2 The main triggers are viral respiratory infections and noncompliance with inhaled corticosteroid therapy.11
Asthma exacerbations during pregnancy should be managed aggressively (Table 4),12 as the risk to the fetus of hypoxia far outweighs any risk from asthma medications. Close collaboration between the primary care physician and the obstetrician allows closer monitoring of mother and fetus.
The goal oxygen saturation must be above 95%.12 Signs of acute respiratory failure in a pregnant patient include a partial pressure of arterial oxygen less than 70 mm Hg or a partial pressure of carbon dioxide greater than 35 mm Hg.
In a multicenter study comparing nonpregnant and pregnant women visiting the emergency room for asthma exacerbations,28 pregnant women were less likely to be prescribed systemic corticosteroids either in the emergency room or at the time of hospital discharge, and they were also more likely to describe an ongoing exacerbation at 2-week follow-up. However, a recent study showed a significant increase in systemic corticosteroid treatment in the emergency room (51% to 78% across the time periods, odds ratio 3.11, 95% confidence interval 1.27–7.60, P = .01). There was also an increase in steroid treatment at discharge (42% to 63%, odds ratio 2.49, 95% confidence interval 0.97–6.37, P = .054), though the increase was not statistically significant.29 Although emergency room care for pregnant asthmatic women has improved, this group concluded that further improvement is still warranted, as 1 in 3 women is discharged without corticosteroid treatment.
The incidence of maternal asthma is rising. Based on US national health surveys, the prevalence of asthma during pregnancy is between 3.7% and 8.4%.1 It is the most common respiratory illness of pregnancy.2 Hence, clinicians need to know how asthma affects the mother and the fetus. Appropriate care of asthma during pregnancy is based on several management principles, as reviewed here, and is key to ensuring good outcomes for the mother and the baby.
EFFECT OF PREGNANCY ON ASTHMA CONTROL
Asthma control can vary in pregnancy. About a third of asthmatic women experience a worsening of asthma control with pregnancy, a third remain unchanged, and another third have improvement in asthma symptoms.3 The peak worsening of asthma tends to occur in the sixth month.4 Asthma control also tends to be better in the last month of pregnancy.3
The peak expiratory flow rate was noted to increase with each trimester in a small study of 43 women.5 The authors speculated that a rising progesterone level stimulates cyclic adenosine monophosphate to cause bronchodilation, thereby improving the expiratory flow rate and asthma control. Asthma control tends to follow the pattern experienced in the previous pregnancy: ie, if asthma worsened during the previous pregnancy, the same will be likely in the subsequent pregnancy.3
Two maternal factors that adversely affect asthma severity during pregnancy are the use of asthma medications contrary to guidelines such as those of the Global Initiative for Asthma (http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention) and inadquate control of asthma before becoming pregnant.6 Pregnancy can bring on stress, and stress is known to worsen asthma. In addition, when patients themselves were interviewed to elucidate the reasons for poor adherence to asthma medications during pregnancy, concerns about medication use, especially corticosteroids, stood out.7 A study based on prescription claims data showed that in the first trimester, there was a significant decline in asthma prescription medications (a 23% decline in inhaled corticosteroids, a 13% decline in short-acting bronchodilator agents, and a 54% decline in rescue corticosteroids).8 Lack of physician education about management of asthma in pregnancy and discomfort with prescribing to pregnant women also affect asthma control.
EFFECT OF ASTHMA ON MATERNAL AND FETAL OUTCOMES
Studies of the effects of asthma on fetal and maternal outcomes have yielded mixed and conflicting results.9 Adverse outcomes that have been shown to be associated with maternal asthma are listed in Table 1. Other studies have not demonstrated an association between asthma in pregnancy and maternal or fetal adverse events.9 Such discrepant findings are due to differences in study population characteristics that make comparisons difficult. A meta-analysis involving more than 1.6 million asthmatic women showed maternal asthma was associated with a 40% greater risk of low birth weight and preterm delivery, a 50% greater risk of preeclampsia, and a 20% greater risk of the baby being small for its gestational age.10
The association of maternal asthma and preterm birth may pose short-term and long-term health risks to the child associated with prematurity.9 Short-term risks with prematurity include infection, respiratory distress syndrome, brain injury, and necrotizing enterocolitis. Long-term risks include neurodevelopmental and behavioral sequelae. Furthermore, asthma exacerbations during pregnancy are associated with a twofold higher risk of low birth weight.11 The benefits of good adherence to asthma regimens during pregnancy outweigh the risks associated with frequent symptoms and exacerbations caused by untreated asthma.12
OUTPATIENT MANAGEMENT OF MATERNAL ASTHMA
Goals
In the 2004 update of the National Asthma Education and Prevention Program (NAEPP) Working Group Report on Managing Asthma During Pregnancy, goals focused mainly on adequate asthma control for maternal health and quality of life, as well as normal fetal maturation (Table 2),12 goals similar to those in nonpregnant asthmatic women.
Assessment and monitoring
Monthly physician visits during pregnancy are recommended for assessment of symptoms and pulmonary function. If symptoms are uncontrolled, therapy must be stepped up, and any trigger for exacerbation, such as gastroesophageal reflux disease (GERD), exposure, or rhinitis, must be treated and eliminated. NAEPP guidelines recommend baseline spirometry at the time of initial assessment.12 At follow-up visits, spirometry is preferred, but measurement of the peak expiratory flow rate usually suffices. Such objective data can help differentiate dyspnea from asthma and from dyspnea that usually accompanies the physiologic changes of pregnancy. In addition, patients should be advised to monitor for adequate fetal activity. If asthma is uncontrolled or poorly controlled, serial fetal ultrasonography should be considered from 32 weeks of gestation, as well as after recovery from an asthma exacerbation. Regular monitoring of the pregnant asthmatic patient by a multidisciplinary team can improve outcomes.13
Avoiding triggers
Patients should be advised to avoid asthma triggers such as pet dander, dust mites, pollen, smoke, mold, and perfumes, as this can decrease symptoms and allow for use of lower doses of medications.12 Additionally, smoking cessation must be strongly encouraged, not only to control maternal asthma, but also to prevent harm to the fetus.
MANAGEMENT OF SPECIFIC TRIGGERS
GERD
Reflux disease often worsens during pregnancy, and it can coexist with asthma and can also exacerbate it.14 Optimal control of GERD helps maintain adequate asthma control. For mild reflux symptoms, lifestyle modifications such as elevating the head of bed, avoiding eating too close to bedtime, and avoiding foods that cause heartburn may be adequate.15,16 If medications are needed, antacids (but not sodium bicarbonate, for fear of metabolic alkalosis) and sucralfate should be considered before using a histamine 2 receptor antagonist such as ranitidine. Proton pump inhibitors should be considered only if reflux symptoms are refractory to other therapies.
Allergic rhinitis
Intranasal corticosteroids are effective against allergic rhinitis in pregnancy (Table 3).12 Montelukast, a leukotriene receptor antagonist, can be used, but data to support its use for allergic rhinitis in pregnancy are limited.
Among antihistamines, second-generation drugs such as cetirizine or loratadine can be considered.12 Oral decongestants such as pseudoephedrine in early pregnancy are associated with a rare congenital fetal abnormality called gastroschisis, caused by vascular disruption.17 Hence, if a nasal decongestant is required in early pregnancy, a local therapy such as an intranasal corticosteroid, short-term oxymetazoline, or an external nasal dilator may be considered.12 These therapies must be combined with avoidance of allergens whenever possible.
Allergies
Diagnostic allergy and skin tests during pregnancy pose a risk of anaphylaxis and thus should be avoided. Instead, the focus should be on obtaining a thorough medical history about exposures and eliminating specific asthma triggers. It is also inadvisable to start allergen immunotherapy during pregnancy because of the risk of anaphylaxis and the effect of treatment on the mother and fetus.18 However, maintenance doses of allergen immunotherapy can be continued during pregnancy.18
Patient education
Because of concern about the risks of taking medications during pregnancy, many women with asthma stop using their inhalers during pregnancy, thus compromising asthma control.8,13 The physician and multidisciplinary team must use every opportunity to emphasize the importance of good asthma control during pregnancy. Inhaler technique should also be reviewed and, if defective, corrected. Again, trigger avoidance and tobacco cessation should be addressed.
Drugs
The NAEPP recommendations state that asthma therapy should be continued during pregnancy, as it is safer both for mother and fetus to avoid exacerbations and uncontrolled asthma.12 Despite this, 25% of primary care physicians instruct their patients to decrease or discontinue their inhaled corticosteroid during pregnancy.19 As with asthma in general, treatment should involve using the lowest dose of drugs that achieves adequate control of symptoms.
In 2015, the US Food and Drug Administration (FDA) amended the labeling rule for medications used in pregnancy and lactation. The previous risk categories A (safest), B, C, D, and X (highest risk) are in the process of being removed from labels for all human prescription drugs and biologic products, to be replaced with a summary of the risks of taking the drug during pregnancy and lactation, a discussion of the data supporting the use, and relevant information to help healthcare providers make prescribing decisions and counsel women about the use of drugs during pregnancy and lactation (www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm).
ROLES OF CONTROLLER THERAPY AND RESCUE THERAPY
Inhaled corticosteroids
Inhaled corticosteroids are the mainstay of asthma controller therapy during pregnancy. A meta-analysis of 16 studies showed no increased risk of congenital malformations, cesarean delivery, or stillbirth among mothers who used these agents during pregnancy.20 Because there are more safety data for budesonide, it is currently the preferred inhaled corticosteroid during pregnancy.9 However, if a patient’s asthma is controlled with a different corticosteroid before pregnancy, that agent may be continued during pregnancy, especially if it is thought that switching formulations could adversely affect asthma control.12 This is mainly because current data do not prove that other inhaled corticosteroids are unsafe.
Inhaled beta-agonists
Inhaled beta-agonists, both short-acting and long-acting, are used for rescue therapy. Albuterol is the preferred short-acting agent for rescue therapy in pregnant women with asthma.12 Meta-analysis has shown no increased risk of major or minor congenital malformations in pregnant patients who use bronchodilators.20 Long-acting beta-agonists typically are used as add-on therapy when asthma cannot be controlled by an inhaled corticosteroid. They should not be used without a controller medication (ie, an inhaled corticosteroid).
Guidelines for rescue therapy are similar to those for nonpregnant asthmatic patients. Although data are limited as to the gestational effects of long-acting beta-agonists (ie, formoterol, salmeterol), it can be assumed that the toxicologic and pharmacologic profiles are similar to those of the short-acting bronchodilators. Thus, the safety of albuterol can be extended potentially to the long-acting beta-agonists.12
Combining controller and rescue therapy
When asthma is not adequately controlled on inhaled corticosteroids, a long-acting beta-agonist can be added or the dose of corticosteroid can be increased. The 2004 NAEPP guidelines stated that based on available literature, there was no clear advantage of one option over the other.12 A study that compared the 2 approaches found no difference in rates of congenital malformations.21
Leukotriene receptor antagonists
There is little in the literature regarding the use of leukotriene receptor antagonists during pregnancy. However, animal safety data are reassuring,12 and human studies have not found a higher risk of major congenital malformations.22,23 Thus, they are an alternative for patients whose asthma has been well controlled on these agents before pregnancy. Montelukast and zafirlukast are in former FDA pregnancy risk factor category B (probably safe) (Table 3). However, 5-lipoxygenase inhibitors such as zileuton are contraindicated based on animal studies showing teratogenicity.24
Omalizumab
Omalizumab, a recombinant anti-immunoglobulin E antibody, can be used for allergic asthma not controlled with inhaled corticosteroids (Table 3). An analysis of the omalizumab pregnancy registry25 found no significant increase in the rate of major congenital malformations, prematurity, or babies small for gestational age in asthmatic women taking omalizumab 8 weeks before conception or during pregnancy vs pregnant asthmatic women not taking omalizumab. However, this drug carries a risk of anaphylaxis and so should not be started during pregnancy.25
Theophylline
Because of potential toxicity, use of theophylline during pregnancy requires careful monitoring to ensure the serum concentration remains between 5 and 12 µg/mL.12 Drug interactions are also common: for example, alcohol may increase the serum concentration of theophylline, and theophylline may increase the toxic effect of formoterol.
Systemic corticosteroids
Pregnant women with asthma that is not well controlled despite the therapies described above may require a daily oral corticosteroid such as prednisone to achieve adequate control. Oral steroids are also a mainstay of treatment of asthma exacerbation.
Although use of corticosteroids in the first trimester was associated with orofacial cleft in infants,12 these studies did not include many women with asthma. In 2011, a nationwide cohort study from Denmark showed no increase in the risk of orofacial cleft with the use of corticosteroids during pregnancy.26
Preeclampsia, low birth weight, and preterm delivery have been described with corticosteroid use in pregnancy. It is not known whether these problems were a result of corticosteroid use or were due to the uncontrolled nature of the underlying condition that led to the steroid use. Since the risk of uncontrolled asthma to mother and fetus outweighs the risk of systemic corticosteroids, these drugs are recommended when indicated for management of maternal asthma.12
ACUTE EXACERBATIONS REQUIRE AGGRESSIVE MANAGEMENT
Based on a systematic review, 20% of pregnant women with asthma require some intervention for an asthma exacerbation during pregnancy, and 5.8% are admitted to the hospital for an exacerbation.11 Exacerbations were associated with a higher risk of low birth weight compared with rates in women without asthma.
Exacerbations are more common late in the second trimester and are unlikely to occur during labor and delivery.2 The incidence of exacerbations increases with the severity of asthma, from 8% in mild asthma, to 47% in moderate asthma, to 65% in severe asthma.27 Risk factors for exacerbations include poor prenatal care, obesity, and lack of appropriate treatment with inhaled corticosteroids.2 The main triggers are viral respiratory infections and noncompliance with inhaled corticosteroid therapy.11
Asthma exacerbations during pregnancy should be managed aggressively (Table 4),12 as the risk to the fetus of hypoxia far outweighs any risk from asthma medications. Close collaboration between the primary care physician and the obstetrician allows closer monitoring of mother and fetus.
The goal oxygen saturation must be above 95%.12 Signs of acute respiratory failure in a pregnant patient include a partial pressure of arterial oxygen less than 70 mm Hg or a partial pressure of carbon dioxide greater than 35 mm Hg.
In a multicenter study comparing nonpregnant and pregnant women visiting the emergency room for asthma exacerbations,28 pregnant women were less likely to be prescribed systemic corticosteroids either in the emergency room or at the time of hospital discharge, and they were also more likely to describe an ongoing exacerbation at 2-week follow-up. However, a recent study showed a significant increase in systemic corticosteroid treatment in the emergency room (51% to 78% across the time periods, odds ratio 3.11, 95% confidence interval 1.27–7.60, P = .01). There was also an increase in steroid treatment at discharge (42% to 63%, odds ratio 2.49, 95% confidence interval 0.97–6.37, P = .054), though the increase was not statistically significant.29 Although emergency room care for pregnant asthmatic women has improved, this group concluded that further improvement is still warranted, as 1 in 3 women is discharged without corticosteroid treatment.
- Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol 2003; 13:317–324.
- Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011; 378:983–990.
- Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 1988; 81:509–517.
- Gluck JC, Gluck PA. The effect of pregnancy on the course of asthma. Immunol Allergy Clin North Am 2006; 26:63–80.
- Beckmann CA. Peak flow values by gestation in women with asthma. Clin Nurs Res 2008; 17:174–181.
- Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol 2010; 115:559–567.
- Lim AS, Stewart K, Abramson MJ, Ryan K, George J. Asthma during pregnancy: the experiences, concerns and views of pregnant women with asthma. J Asthma 2012; 49:474–479.
- Enriquez R, Wu P, Griffin MR, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol 2006; 195:149–153.
- Bain E, Pierides KL, Clifton VL, et al. Interventions for managing asthma in pregnancy. Cochrane Database Syst Rev 2014; 10:CD010660.
- Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011; 118:1314–1323.
- Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 2006; 61:169–176.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol 2005; 115:34–46.
- Lim AS, Stewart K, Abramson MJ, Walker SP, Smith CL, George J. Multidisciplinary Approach to Management of Maternal Asthma (MAMMA): a randomized controlled trial. Chest 2014; 145:1046–1054.
- Harding SM. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am 2005; 25:131–148.
- Ahmad S, Mokaddas E. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respir Med 2009; 103:1777–1790.
- van der Woude CJ, Metselaar HJ, Danese S. Management of gastrointestinal and liver diseases during pregnancy. Gut 2014; 63:1014–1023.
- Werler MM. Teratogen update: pseudoephedrine. Birth Defects Res A Clin Mol Teratol 2006; 76:445–452.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Lim AS, Stewart K, Abramson MJ, George J. Management of asthma in pregnant women by general practitioners: a cross sectional survey. BMC Fam Pract 2011; 12:121.
- Murphy VE, Wang G, Namazy JA, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013; 120:812–822.
- Eltonsy S, Forget A, Beauchesne MF, Blais L. Risk of congenital malformations for asthmatic pregnant women using a long-acting beta2-agonist and inhaled corticosteroid combination versus higher-dose inhaled corticosteroid monotherapy. J Allergy Clin Immunol 2015; 135:123–130.
- Nelsen LM, Shields KE, Cunningham ML, et al. Congenital malformations among infants born to women receiving montelukast, inhaled corticosteroids, and other asthma medications. J Allergy Clin Immunol 2012; 129:251–254.e1–e6.
- Sarkar M, Koren G, Kalra S, et al. Montelukast use during pregnancy: a multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol 2009; 65:1259–1264.
- Namazy JA, Schatz M. The safety of asthma medications during pregnancy: an update for clinicians. Ther Adv Respir Dis 2014; 8:103–110.
- Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135:407–412.
- Hviid A, Molgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ 2011; 183:796–804.
- Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol 2005; 106:1046–1054.
- Cydulka RK, Emerman CL, Schreiber D, Molander KH, Woodruff PG, Camargo CA Jr. Acute asthma among pregnant women presenting to the emergency department. Am J Respir Crit Care Med 1999; 160:887–892.
- Hasegawa K, Cydulka RK, Sullivan AF, et al. Improved management of acute asthma among pregnant women presenting to the ED. Chest 2015; 147:406–414.
- Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol 2003; 13:317–324.
- Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011; 378:983–990.
- Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol 1988; 81:509–517.
- Gluck JC, Gluck PA. The effect of pregnancy on the course of asthma. Immunol Allergy Clin North Am 2006; 26:63–80.
- Beckmann CA. Peak flow values by gestation in women with asthma. Clin Nurs Res 2008; 17:174–181.
- Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol 2010; 115:559–567.
- Lim AS, Stewart K, Abramson MJ, Ryan K, George J. Asthma during pregnancy: the experiences, concerns and views of pregnant women with asthma. J Asthma 2012; 49:474–479.
- Enriquez R, Wu P, Griffin MR, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol 2006; 195:149–153.
- Bain E, Pierides KL, Clifton VL, et al. Interventions for managing asthma in pregnancy. Cochrane Database Syst Rev 2014; 10:CD010660.
- Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011; 118:1314–1323.
- Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax 2006; 61:169–176.
- National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Asthma and Pregnancy Working Group. NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment—2004 update. J Allergy Clin Immunol 2005; 115:34–46.
- Lim AS, Stewart K, Abramson MJ, Walker SP, Smith CL, George J. Multidisciplinary Approach to Management of Maternal Asthma (MAMMA): a randomized controlled trial. Chest 2014; 145:1046–1054.
- Harding SM. Gastroesophageal reflux: a potential asthma trigger. Immunol Allergy Clin North Am 2005; 25:131–148.
- Ahmad S, Mokaddas E. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respir Med 2009; 103:1777–1790.
- van der Woude CJ, Metselaar HJ, Danese S. Management of gastrointestinal and liver diseases during pregnancy. Gut 2014; 63:1014–1023.
- Werler MM. Teratogen update: pseudoephedrine. Birth Defects Res A Clin Mol Teratol 2006; 76:445–452.
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55.
- Lim AS, Stewart K, Abramson MJ, George J. Management of asthma in pregnant women by general practitioners: a cross sectional survey. BMC Fam Pract 2011; 12:121.
- Murphy VE, Wang G, Namazy JA, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013; 120:812–822.
- Eltonsy S, Forget A, Beauchesne MF, Blais L. Risk of congenital malformations for asthmatic pregnant women using a long-acting beta2-agonist and inhaled corticosteroid combination versus higher-dose inhaled corticosteroid monotherapy. J Allergy Clin Immunol 2015; 135:123–130.
- Nelsen LM, Shields KE, Cunningham ML, et al. Congenital malformations among infants born to women receiving montelukast, inhaled corticosteroids, and other asthma medications. J Allergy Clin Immunol 2012; 129:251–254.e1–e6.
- Sarkar M, Koren G, Kalra S, et al. Montelukast use during pregnancy: a multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol 2009; 65:1259–1264.
- Namazy JA, Schatz M. The safety of asthma medications during pregnancy: an update for clinicians. Ther Adv Respir Dis 2014; 8:103–110.
- Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015; 135:407–412.
- Hviid A, Molgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ 2011; 183:796–804.
- Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol 2005; 106:1046–1054.
- Cydulka RK, Emerman CL, Schreiber D, Molander KH, Woodruff PG, Camargo CA Jr. Acute asthma among pregnant women presenting to the emergency department. Am J Respir Crit Care Med 1999; 160:887–892.
- Hasegawa K, Cydulka RK, Sullivan AF, et al. Improved management of acute asthma among pregnant women presenting to the ED. Chest 2015; 147:406–414.
KEY POINTS
- The benefits of good adherence to asthma regimens during pregnancy outweigh the risks associated with the medications used.
- For treatment of reflux disease in pregnant women with asthma, antacids (but not sodium bicarbonate, for fear of metabolic alkalosis) and sucralfate should be considered before a histamine 2 receptor antagonist such as ranitidine. Proton pump inhibitors should be considered only if reflux symptoms are refractory to other therapies.
- Uncontrolled maternal asthma contributes to poor maternal and fetal outcomes. Management by a multi-disciplinary team, including internist, obstetrician, pharmacist, nurse, allergist, and pulmonologist, improves care and outcomes.
Treating Helicobacter pylori effectively while minimizing misuse of antibiotics
Helicobacter pylori infection is an infectious disease and should be treated like one, with due consideration of antibiotic resistance and stewardship.1–4
This was the consensus of the 2015 Kyoto H pylori conference,2 and it signaled a fundamental shift in thinking. Up to now, H pylori treatment has not been based on infectious disease principles, leading to suboptimal results and antibiotic resistance. In addition, the conference recommended that H pylori infection be treated whenever it is found unless there are compelling reasons not to.
Here we review current and possible future regimens for eradicating H pylori that we hope will be more effective and will lead to less resistance than in the past.
H PYLORI AS AN INFECTIOUS DISEASE
Not until the late 1980s was H pylori recognized as the cause of peptic ulcer disease, which until then accounted for hundreds of thousands of hospitalizations and more than 100,000 surgical procedures each year.5 Now, peptic ulcer disease is routinely treated by eradicating H pylori. In addition, the World Health Organization has recommended considering H pylori eradication to reduce the risk of gastric cancer,6 which causes 738,000 deaths worldwide per year.7
The problems of how to diagnose and treat H pylori infection were taken on by gastroenterologists, and not by specialists in infectious disease.1 Even now, almost all the major reviews and consensus statements on H pylori come from gastroenterologists and are published in gastroenterology journals.2,8,9
But infectious diseases differ from most gastrointestinal diseases. In gastrointestinal problems such as constipation or inflammatory bowel disease,10 the causes are generally unknown, and there is a large placebo response to therapy. In contrast, in infectious diseases, the cause is generally known, there is no placebo response, and treatment success depends on susceptibility of the organism. Failure of proven regimens is generally due to resistant organisms, poor adherence, or, in the case of H pylori, poorly designed regimens in terms of doses, frequency of administration, or duration of therapy.
The differences extend to clinical trials of treatment.3 In other infectious diseases, treatment is based on susceptibility. The usual comparative approach in infectious diseases is a noninferiority trial in which the new treatment is compared with standard care, ie, a regimen that reliably achieves nearly 100% cure rates. Not so with H pylori. Most trials of H pylori therapy compared regimens in populations with high but unknown prevalences of resistance and therefore are of limited or no help to the clinician in choosing the best regimen for an individual patient.3
Many thousands of H pylori-infected patients participated in clinical trials in which the results would have been predictable if the researchers had assessed susceptibility before giving the drugs.11–13 Worse, many patients were also randomized to receive regimens that the investigators knew provided poor cure rates in the population being studied. This knowledge was generally not shared with the patients. This approach was used to demonstrate that a new regimen was superior to an old one, even though the new one was already known to be less affected by resistance to the key element in the comparator.
Clinicians generally do not test for susceptibility when treating H pylori, one reason being that such testing is often unavailable.3 However, almost every hospital, clinic, and major laboratory in the world provides susceptibility testing for other common local pathogens. H pylori is easy to grow, and laboratories could test for susceptibility if we asked them to.
Current H pylori recommendations may also contribute to the global increase in antimicrobal resistance.
As discussed below, all recent guidelines have recommended 4-drug non-bismuth-containing concomitant therapy as first-line therapy. An infectious disease colleague described it as a “hope therapy” because the prescriber hoped that the infection would be susceptible to either metronidazole or clarithromycin. All who receive this combination receive an antibiotic they do not need. This is an expedient rather than a medically rational choice resulting from failure to deal with H pylori as an infectious disease.
H PYLORI THERAPIES
Conceptually, treating infectious disease is straightforward: one should prescribe antimicrobial drugs to which the organism is susceptible3 (Table 1). However, clinical success lies in the details, which include the doses, frequency of doses, duration of therapy, timing of doses in relation to meals, and use of adjuvants such as antisecretory drugs, antacids, and probiotics. A number of regimens reliably yield high cure rates—95% or higher—if the organism is susceptible and the patients are adherent.
The effectiveness of any regimen may vary depending on the population it is used in, due to polymorphisms in drug-metabolizing enzymes such as CYP2C19.
Sequential therapy is obsolete
Sequential therapy for H pylori infection consisted of amoxicillin plus a proton pump inhibitor for 7 days, followed by clarithromycin, tinidazole, or metronidazole plus a proton pump inhibitor for a further 7 days. This regimen should not be used any more because concomitant therapy will always be superior (see below).
Need for 14 days of therapy
H pylori occupies a number of different niches in the body ranging from gastric mucus (which is technically outside the body) to inside gastric epithelial cells. As a general rule, 14-day therapy provides the best results, in part because the longer duration helps kill the organisms that persist in different niches.14,15
In addition, proton pump inhibitors, which are part of all the currently recommended regimens, require 3 or more days to reach their full antisecretory effectiveness, which further limits the effectiveness of short-duration therapies.
Shorter regimens should be used only if they are proved to be as good as 14-day regimens and if both achieve 95% or greater cure rates with susceptible infections.
How to choose a therapy
Since rational infectious-disease therapy is based on susceptibility, one should start by considering the susceptibility pattern in the local population and, therefore, the likely susceptibility in the patient in front of us.
Unfortunately, we do not yet have local or regional susceptibility data on H pylori for most locales. Until those data are available, we must use the indirect information that is available, such as the patient’s history of antibiotic use.
Triple therapy should not be used empirically
Triple therapy (Table 1) consists of the combination of:
- Clarithromycin or metronidazole or a fluoroquinolone
- Amoxicillin
- A proton pump inhibitor.
However, prior use of a macrolide (eg, erythromycin, clarithromycin, or azithromycin), metronidazole, or a fluoroquinolone (eg, ciprofloxacin, levofloxacin) almost guarantees resistance to those drugs. In the United States, resistance to clarithromycin, metronidazole, levofloxacin, and related drugs is already widespread, and none should be used empirically in triple therapies. In contrast, amoxicillin, tetracycline, and furazolidone can often be used again, as resistance to them is rare even with prior use.
For example, 14 days of clarithromycin triple therapy (clarithromycin, amoxicillin, and a proton pump inhibitor) can be expected to cure more than 95% of patients who have susceptible infections and about 20% of those with resistant infections.16 This 20% is due to the proton pump inhibitor and amoxicillin, as the contribution to the cure rate from clarithromycin is close to zero.
If the prevalence of resistance to clarithromycin is 25%, the cure rate in the entire population will be a little more than 75%—97% in the 75% of the population with susceptible infections and 20% in patients who previously received clarithromycin (Figure 1).
If we know that our patient has an infection that is susceptible to clarithromycin, metronidazole, or levofloxacin, good results could be achieved with triple therapy that includes a proton pump inhibitor, for 14 days. Fluoroquinolones have a number of black-box warnings from the US Food and Drug Administration (www.fda.gov/Drugs/DrugSafety/ucm500143.htm) and should always be a last choice. However, in the United States, lacking definite data about susceptibility to clarithromycin, metronidazole, and levofloxacin, we should assume resistance is present and use a 4-drug regimen (eg, concomitant therapy or bismuth quadruple therapy).
Concomitant therapy is preferred
Concomitant therapy is the combination of:
- Amoxicillin
- Metronidazole
- Clarithromycin
- A proton pump inhibitor.
Functionally, this is a combination of clarithromycin and metronidazole triple therapies, given simultaneously.17 The premise is that even though the prevalence of metronidazole resistance in the United States is high (20%–40%), and so is the prevalence of clarithromycin resistance (about 20%), the prevalence of resistance to both drugs at the same time is expected to be low (eg, 0.4 × 0.2 = 0.08, or 8%) unless the drugs had previously been used together, as in some older regimens that contained both. Thus, the metronidazole will kill the clarithromycin-resistant but metronidazole-susceptible strains, and the clarithromycin will kill the clarithromycin-susceptible, metronidazole-resistant strains. Only with dual resistant strains will this regimen fail (with a 20% cure rate due to the proton pump inhibitor and amoxicillin and a population cure rate of slightly more than 90%).
The downside of this highly recommended therapy is that all who receive it are getting an antibiotic that they don’t need, which is, in a global sense, inappropriate. In other words, all those who are cured by clarithromycin also receive metronidazole, which plays no role in treatment success, and those cured by metronidazole receive unneeded clarithromycin (Figure 2). Had susceptibility testing been available, those with susceptible strains would have received appropriate triple therapies, and those with dual resistance would not have received either antibiotic.
Thus, while we recommend concomitant therapy as an empiric regimen in populations that do not have high levels of resistance to metronidazole or clarithromycin (as those would also have a high prevalence of dual resistance), one must be aware of the “dirty little secret” of inappropriate antibiotic use that accompanies it and some other H pylori therapies (eg, vonoprazan triple therapy in Japan).18–20
Bismuth quadruple therapy is an alternative
Bismuth quadruple therapy (Table 1) consists of:
- Bismuth
- Tetracycline
- Metronidazole
- A proton pump inhibitor.
This was the first truly effective regimen for H pylori. Its advantage is that it can partially or completely overcome metronidazole resistance.21,22 As such, it is potentially ideal, as it should be effective despite resistance to clarithromycin, metronidazole, or levofloxacin, and it can be used in patients allergic to penicillin.
The major downside is a high frequency of side effects, particularly abdominal pain, nausea, and vomiting, often resulting in poor adherence. Most regimens that contain antibiotics have side effects, but adherence seems to be more of a problem with bismuth quadruple therapy, probably because of the combination of the high doses of metronidazole and tetracycline.22 In our experience, this regimen can be effective if the physician takes the time to explain to the patient that side effects are common but treatment success depends on completing the full course of 14 days.
Another problem is that tetracycline has become difficult to obtain in many areas, and doxycycline cannot be substituted in those with metronidazole resistance. To date, it has been difficult or impossible to obtain the same excellent results with doxycycline as can be obtained with tetracycline. It is not clear why.21
To use bismuth quadruple therapy one must often use a name-brand product, Pylera. Pylera is packaged as a 10-day course, which is effective against metronidazole-susceptible infections. However, 14 days are generally required to achieve a high cure rate with metronidazole-resistant infections, which are the main indication for use of this product. Moreover, Pylera does not include a proton pump inhibitor, which must be prescribed separately.
In the United States, Pylera is expensive, costing $740 to $790 with a coupon for a 10-day supply and proportionally more for the required 14-day supply (www.goodrx.com/pylera?drug-name=pylera), whereas in Europe it costs less than 70 Euros ($73).21 If generic tetracycline is available, the US cost for 14 days of generic bismuth quadruple therapy is less than $50.
An alternate and simpler approach is to substitute amoxicillin for tetracycline.23 This regimen has been used successfully in China and was shown to be noninferior to the tetracycline-containing regimen in a head-to-head comparison.24
Recent studies have confirmed earlier Italian studies suggesting that twice-a-day bismuth and tetracycline is effective, which would further simplify therapy and possibly reduce side effects.21,23,24 These variations on bismuth quadruple therapy have not yet been optimized to where one can reliably achieve 95% or greater cure rates, and further studies are needed.
Why include more than 1 antibiotic?
The H pylori load in the stomach is typically large, which increases the odds that a subpopulation of resistant organisms is present. Resistance may be due to a relatively high rate of mutation in certain bacterial genes.25 This is particularly a problem with clarithromycin, metronidazole, and fluoroquinolones and is reflected in a high rate of resistance among patients for whom single-drug regimens have failed. These drugs are always given with a second antimicrobial to which H pylori rarely becomes resistant, such as amoxicillin or tetracycline.
Why include a proton pump inhibitor?
An antisecretory drug is needed to increase the gastric pH, which makes antimicrobial therapy more effective. It also decreases antibiotic washout from the stomach and likely protects and increases the gastric concentration of some antibiotics.
The activities of amoxicillin, fluoroquinolones, and to a lesser degree clarithromycin are pH-dependent. For example, keeping the gastric pH above 6.0 promotes H pylori replication,26,27 making it is more susceptible to amoxicillin (reviewed in detail by Dore et al21). A gastric pH of 6.0 or more is very difficult to achieve with proton pump inhibitors, and has been accomplished regularly only in people who metabolize these drugs slowly (“slow metabolizers”) who received both the proton pump inhibitor and amoxicillin every 6 hours for 14 days.21
With standard clarithromycin, metronidazole, or fluoroquinolone triple therapy, proton pump inhibitors appear to provide satisfactory cure rates when given for 14 days in standard doses. However, double doses (eg, 40 mg of omeprazole or an equivalent) may be slightly better, especially in the presence of resistance.
The cure rate reflects the sum of the 2 populations of organisms: the susceptible and the resistant. In triple therapy, increasing the gastric pH with a proton pump inhibitor makes the amoxicillin component of the regimen more effective against resistant organisms and thus increases the cure rate. For example, in Western countries, esomeprazole 40 mg (approximately equivalent to rabeprazole 40 mg, omeprazole or lansoprazole 60 mg, or pantoprazole 240 mg)28 given twice a day in a 14-day triple therapy regimen cures about 40% to 50% of resistant infections. This benefit will be evident in an improvement in cure rates in populations in which resistance has reduced the average cure rate. This is also why meta-analyses have shown better results with second-generation proton pump inhibitors and with longer duration of therapy.29,30
Generally, we recommend omeprazole 40 mg twice a day or an equivalent (Tables 1–3).
Would a potassium-competitive acid blocker be better than a proton pump inhibitor?
Vonoprazan is a potassium-competitive acid blocker. It does not require intermediate complex formation and is stable at low pH. It has a longer half-life than proton pump inhibitors, and its bioavailability is unaffected by food.31 It was recently approved in Japan for H pylori eradication in combination with clarithromycin or metronidazole plus amoxicillin.18
Vonoprazan is more effective than current proton pump inhibitors for keeping the gastric pH high. There are no published studies of vonoprazan dual therapy in Western countries, but given twice a day for 7 days along with twice-daily amoxicillin it cured only approximately 80% of clarithromycin-resistant strains. Further studies are needed to identify the optimum proton pump inhibitor or potassium-competitive acid blocker, dose, and duration.
Misuse of antibiotics
In triple therapy, the second antimicrobial drug (eg, amoxicillin) is given in part to prevent resistance from developing. It is not clear whether the combination is additive or synergistic, but until we can reliably maintain the intragastric pH above 6.0, which would increase the effectiveness of the amoxicillin component of the regimen, this practice cannot be considered as misuse of antibiotics.
In contrast, in the 4-drug nonbismuth combinations (concomitant, sequential, and hybrid therapies) and the new vonoprazan, clarithromycin, and metronidazole triple therapies, 1 of the antibiotics provides no benefit to some, often most, of the patients.18–20,32 This practice should end when susceptibility data become more widely available and when vonoprazan becomes available, so that we can deliver effective vonoprazan-amoxicillin dual therapy.
First-, second-, and third-line therapies
Many recommendations give advice in terms of first-, second-, and third-line therapies. In practice, a physician should have at least 2 first-line regimens (a first and a second choice). Both should be proven highly successful as empiric therapies in one’s patient population but differ in terms of primary antibiotics. This approach allows the clinician to tailor therapy depending on whether he or she suspects antibiotic resistance (eg, if the patient has taken clarithromycin before) or the patient is allergic or cannot take 1 or more drugs.
Two treatment failures with 2 different regimens known to be effective suggest poor compliance (a difficult patient) or a multiple-drug-resistant infection (a difficult infection). That patient would require salvage therapy (Table 2), which logically should be based on antimicrobial testing or, at a minimum, consultation with someone who frequently deals with this problem.
Test of cure
Monitoring the outcome of therapy (testing for cure) is essential, as it provides a reliable measure of the local effectiveness of particular therapies and also serves as an early warning of development of resistance in one’s patient population.14
Unless there are compelling reasons, testing for cure should use noninvasive testing with the urea breath test or stool antigen test. It is recommended that this be delayed at least 4 weeks to allow the organisms if still present to repopulate the stomach sufficiently for the tests to become positive. Because antibiotics, bismuth, and proton pump inhibitors reduce the bacterial load, they should be withheld at least 2 weeks before testing. Histamine-2 receptor antagonists can be substituted for proton pump inhibitors if antisecretory therapy is needed for symptoms, and continued up to the day before testing. The urea breath test should contain citric acid to overcome any residual pH effects. Physician groups should share their experience so as to alert the community about which therapies should likely be avoided.33
Salvage therapy
Salvage therapy is given after 2 or more treatment failures with different antibiotics. Ideally, the regimen should be based on the results of antimicrobial testing. Current regimens include rifabutin triple therapy, dual therapy (a protein pump inhibitor or vonoprazan and amoxicillin), or furazolidone quadruple therapy (Table 2).
Furazolidone is a synthetic nitrofuran derivative that is effective against many enteric organisms, including gram-negative bacteria and protozoa. It is not available in most Western countries but is available in many other parts of the world.34,35 It is also a monoamine oxidase inhibitor and thus interacts with many drugs and foods (eg, soy sauce, aged cheeses), leading to a relatively high rate of side effects such as fever, palpitations, and skin rash.
Rifabutin-containing regimens, generally, a proton pump inhibitor, amoxicillin 1 g, and rifabutin 150 mg, all twice a day (Table 3) provide average cure rates of less than 80% (typically in the mid-70% range).36 Borody et al37 reported greater than 95% success with a 12-day regimen consisting of rifabutin 150 mg once daily (half-dose), amoxicillin 1.5 g 3 times a day, and pantoprazole 80 mg (approximately equivalent to omeprazole 20 mg) 3 times a day. Ciccaglione et al,38 in a small study, used a 10-day quadruple regimen containing a proton pump inhibitor, amoxicillin, rifabutin, and bismuth (all twice a day), with high cure rates. The results of these studies are yet to be confirmed, and the optimal rifabutin-containing regimen remains to be determined.
PROBIOTICS
There is considerable interest in using probiotics to enhance the effectiveness of antimicrobial therapy for H pylori by increasing tolerability, reducing side effects, and therefore improving compliance.39,40
In a meta-analysis of 14 randomized trials (N = 1,671), when probiotics were added, pooled H pylori eradication rates were only slightly improved: 83.6% (95% CI 80.5%–86.7%) with probiotics and 74.8% (95% CI 71.1%–78.5%) without probiotics by intent-to-treat analysis.41
Another meta-analysis of probiotics suggested that those containing Saccharomyces boulardii, Lactobacillus, and Bifidobacterium significantly increased the eradication rate of triple therapy in populations with high rates of antimicrobial resistance and reduced the risk of overall H pylori therapy-related adverse effects, especially diarrhea.42,43
At present, we recommend that probiotics be considered only for patients who are likely not to comply with treatment (eg, those with irritable bowel syndrome or difficulty taking antibiotics), to try to take advantage of their ability to improve antibiotic tolerability.
- Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148:719–731.
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64:1353–1367.
- Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther 2016; 14:577–585.
- Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter 2016; 21(suppl 1):3–7.
- Grossman MI. Closing remarks. Gastroenterology 1978; 74:487–488.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 2012; 61:646–664.
- Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016; 151:51–69.
- IARC Helicobacter pylori Working Group. Volume 8. Helicobacter pylori eradication as a strategy for preventing gastric cancer. Lyon, France: International Agency for Research on Cancer, 2014.
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- Graham DY, Dore MP. Helicobacter pylori therapy demystified. Helicobacter 2011; 16:343–345.
- Gatta L, Vakil N, Leandro G, Di MF, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol 2009; 104:3069–3079.
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59:1143–1153.
- Graham DY. Helicobacter pylori eradication therapy research: ethical issues and description of results. Clin Gastroenterol Hepatol 2010; 8:1032–1036.
- Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5:321–331.
- Graham DY, Dore MP. Variability in the outcome of treatment of Helicobacter pylori infection: a critical analysis. In: Hunt RH, Tytgat GNJ, editors. Helicobacter pylori Basic Mechanisms to Clinical Cure 1998. Dordrecht, Netherlands: Kluwer Academic Publishers, 998:426–440.
- Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2015; 21:85–90.
- Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014; 12:177–186.
- Murakami K, Sakurai Y, Shiino M, Funao N, Nishmura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65:1439–1446.
- Graham DY. Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut 2016 Apr 7. pii: gutjnl-2016-311796. doi: 10.1136/gutjnl-2016-311796. [Epub ahead of print].
- Matsumoto H, Shiotani A, Katsumata R, et al. Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: the effect of clarithromycin resistance. Dig Dis Sci 2016; 61:3215–3220.
- Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65:870–878.
- Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. Gastroenterol Clin North Am 2015; 44:537–563.
- Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015; 64:1715–1720.
- Chen Q, Zhang X, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline for bismuth quadruple therapy. Am J Gastroenterol 2016; 111:1736–1742.
- Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 1998; 115:1272–1277.
- Marcus EA, Inatomi N, Nagami GT, et al. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 2012; 36:972–979.
- Sachs G, Shin JM, Munson K, et al. Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther 2000; 14:1383–1401.
- Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors: comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009; 65:19–31.
- Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev 2013;12:CD008337.
- McNicholl AG, Linares PM, Nyssen OP, et al. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 36:414–425.
- Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 2015; 6:e94.
- Graham DY, Laine L. The Toronto Helicobacter pylori consensus in context. Gastroenterology 2016; 151:9–12.
- Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med 2015; 3:9.
- Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013; 25:1134–1140.
- Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol 2012; 8:1–2.
- Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 35:209–221.
- Borody TJ, Pang G, Wettstein AR, et al. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment Pharmacol Ther 2006; 23:481–488.
- Ciccaglione AF, Tavani R, Grossi L, et al. Rifabutin containing triple therapy and rifabutin with bismuth containing quadruple therapy for third-line treatment of Helicobacter pylori infection: two pilot studies. Helicobacter 2016; 21:375–381.
- Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol 2015; 21:10644–10653.
- Zhang MM, Qian W, Qin YY, et al. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol 2015; 21:4345–4357.
- Tong JL, Ran ZH, Shen J, et al. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007; 25:155–168.
- Szajewska H, Setty M, Mrukowicz J, et al. Probiotics in gastrointestinal diseases in children: hard and not-so-hard evidence of efficacy. J Pediatr Gastroenterol Nutr 2006; 42:454–475.
- Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol 2013; 47:25–32.
Helicobacter pylori infection is an infectious disease and should be treated like one, with due consideration of antibiotic resistance and stewardship.1–4
This was the consensus of the 2015 Kyoto H pylori conference,2 and it signaled a fundamental shift in thinking. Up to now, H pylori treatment has not been based on infectious disease principles, leading to suboptimal results and antibiotic resistance. In addition, the conference recommended that H pylori infection be treated whenever it is found unless there are compelling reasons not to.
Here we review current and possible future regimens for eradicating H pylori that we hope will be more effective and will lead to less resistance than in the past.
H PYLORI AS AN INFECTIOUS DISEASE
Not until the late 1980s was H pylori recognized as the cause of peptic ulcer disease, which until then accounted for hundreds of thousands of hospitalizations and more than 100,000 surgical procedures each year.5 Now, peptic ulcer disease is routinely treated by eradicating H pylori. In addition, the World Health Organization has recommended considering H pylori eradication to reduce the risk of gastric cancer,6 which causes 738,000 deaths worldwide per year.7
The problems of how to diagnose and treat H pylori infection were taken on by gastroenterologists, and not by specialists in infectious disease.1 Even now, almost all the major reviews and consensus statements on H pylori come from gastroenterologists and are published in gastroenterology journals.2,8,9
But infectious diseases differ from most gastrointestinal diseases. In gastrointestinal problems such as constipation or inflammatory bowel disease,10 the causes are generally unknown, and there is a large placebo response to therapy. In contrast, in infectious diseases, the cause is generally known, there is no placebo response, and treatment success depends on susceptibility of the organism. Failure of proven regimens is generally due to resistant organisms, poor adherence, or, in the case of H pylori, poorly designed regimens in terms of doses, frequency of administration, or duration of therapy.
The differences extend to clinical trials of treatment.3 In other infectious diseases, treatment is based on susceptibility. The usual comparative approach in infectious diseases is a noninferiority trial in which the new treatment is compared with standard care, ie, a regimen that reliably achieves nearly 100% cure rates. Not so with H pylori. Most trials of H pylori therapy compared regimens in populations with high but unknown prevalences of resistance and therefore are of limited or no help to the clinician in choosing the best regimen for an individual patient.3
Many thousands of H pylori-infected patients participated in clinical trials in which the results would have been predictable if the researchers had assessed susceptibility before giving the drugs.11–13 Worse, many patients were also randomized to receive regimens that the investigators knew provided poor cure rates in the population being studied. This knowledge was generally not shared with the patients. This approach was used to demonstrate that a new regimen was superior to an old one, even though the new one was already known to be less affected by resistance to the key element in the comparator.
Clinicians generally do not test for susceptibility when treating H pylori, one reason being that such testing is often unavailable.3 However, almost every hospital, clinic, and major laboratory in the world provides susceptibility testing for other common local pathogens. H pylori is easy to grow, and laboratories could test for susceptibility if we asked them to.
Current H pylori recommendations may also contribute to the global increase in antimicrobal resistance.
As discussed below, all recent guidelines have recommended 4-drug non-bismuth-containing concomitant therapy as first-line therapy. An infectious disease colleague described it as a “hope therapy” because the prescriber hoped that the infection would be susceptible to either metronidazole or clarithromycin. All who receive this combination receive an antibiotic they do not need. This is an expedient rather than a medically rational choice resulting from failure to deal with H pylori as an infectious disease.
H PYLORI THERAPIES
Conceptually, treating infectious disease is straightforward: one should prescribe antimicrobial drugs to which the organism is susceptible3 (Table 1). However, clinical success lies in the details, which include the doses, frequency of doses, duration of therapy, timing of doses in relation to meals, and use of adjuvants such as antisecretory drugs, antacids, and probiotics. A number of regimens reliably yield high cure rates—95% or higher—if the organism is susceptible and the patients are adherent.
The effectiveness of any regimen may vary depending on the population it is used in, due to polymorphisms in drug-metabolizing enzymes such as CYP2C19.
Sequential therapy is obsolete
Sequential therapy for H pylori infection consisted of amoxicillin plus a proton pump inhibitor for 7 days, followed by clarithromycin, tinidazole, or metronidazole plus a proton pump inhibitor for a further 7 days. This regimen should not be used any more because concomitant therapy will always be superior (see below).
Need for 14 days of therapy
H pylori occupies a number of different niches in the body ranging from gastric mucus (which is technically outside the body) to inside gastric epithelial cells. As a general rule, 14-day therapy provides the best results, in part because the longer duration helps kill the organisms that persist in different niches.14,15
In addition, proton pump inhibitors, which are part of all the currently recommended regimens, require 3 or more days to reach their full antisecretory effectiveness, which further limits the effectiveness of short-duration therapies.
Shorter regimens should be used only if they are proved to be as good as 14-day regimens and if both achieve 95% or greater cure rates with susceptible infections.
How to choose a therapy
Since rational infectious-disease therapy is based on susceptibility, one should start by considering the susceptibility pattern in the local population and, therefore, the likely susceptibility in the patient in front of us.
Unfortunately, we do not yet have local or regional susceptibility data on H pylori for most locales. Until those data are available, we must use the indirect information that is available, such as the patient’s history of antibiotic use.
Triple therapy should not be used empirically
Triple therapy (Table 1) consists of the combination of:
- Clarithromycin or metronidazole or a fluoroquinolone
- Amoxicillin
- A proton pump inhibitor.
However, prior use of a macrolide (eg, erythromycin, clarithromycin, or azithromycin), metronidazole, or a fluoroquinolone (eg, ciprofloxacin, levofloxacin) almost guarantees resistance to those drugs. In the United States, resistance to clarithromycin, metronidazole, levofloxacin, and related drugs is already widespread, and none should be used empirically in triple therapies. In contrast, amoxicillin, tetracycline, and furazolidone can often be used again, as resistance to them is rare even with prior use.
For example, 14 days of clarithromycin triple therapy (clarithromycin, amoxicillin, and a proton pump inhibitor) can be expected to cure more than 95% of patients who have susceptible infections and about 20% of those with resistant infections.16 This 20% is due to the proton pump inhibitor and amoxicillin, as the contribution to the cure rate from clarithromycin is close to zero.
If the prevalence of resistance to clarithromycin is 25%, the cure rate in the entire population will be a little more than 75%—97% in the 75% of the population with susceptible infections and 20% in patients who previously received clarithromycin (Figure 1).
If we know that our patient has an infection that is susceptible to clarithromycin, metronidazole, or levofloxacin, good results could be achieved with triple therapy that includes a proton pump inhibitor, for 14 days. Fluoroquinolones have a number of black-box warnings from the US Food and Drug Administration (www.fda.gov/Drugs/DrugSafety/ucm500143.htm) and should always be a last choice. However, in the United States, lacking definite data about susceptibility to clarithromycin, metronidazole, and levofloxacin, we should assume resistance is present and use a 4-drug regimen (eg, concomitant therapy or bismuth quadruple therapy).
Concomitant therapy is preferred
Concomitant therapy is the combination of:
- Amoxicillin
- Metronidazole
- Clarithromycin
- A proton pump inhibitor.
Functionally, this is a combination of clarithromycin and metronidazole triple therapies, given simultaneously.17 The premise is that even though the prevalence of metronidazole resistance in the United States is high (20%–40%), and so is the prevalence of clarithromycin resistance (about 20%), the prevalence of resistance to both drugs at the same time is expected to be low (eg, 0.4 × 0.2 = 0.08, or 8%) unless the drugs had previously been used together, as in some older regimens that contained both. Thus, the metronidazole will kill the clarithromycin-resistant but metronidazole-susceptible strains, and the clarithromycin will kill the clarithromycin-susceptible, metronidazole-resistant strains. Only with dual resistant strains will this regimen fail (with a 20% cure rate due to the proton pump inhibitor and amoxicillin and a population cure rate of slightly more than 90%).
The downside of this highly recommended therapy is that all who receive it are getting an antibiotic that they don’t need, which is, in a global sense, inappropriate. In other words, all those who are cured by clarithromycin also receive metronidazole, which plays no role in treatment success, and those cured by metronidazole receive unneeded clarithromycin (Figure 2). Had susceptibility testing been available, those with susceptible strains would have received appropriate triple therapies, and those with dual resistance would not have received either antibiotic.
Thus, while we recommend concomitant therapy as an empiric regimen in populations that do not have high levels of resistance to metronidazole or clarithromycin (as those would also have a high prevalence of dual resistance), one must be aware of the “dirty little secret” of inappropriate antibiotic use that accompanies it and some other H pylori therapies (eg, vonoprazan triple therapy in Japan).18–20
Bismuth quadruple therapy is an alternative
Bismuth quadruple therapy (Table 1) consists of:
- Bismuth
- Tetracycline
- Metronidazole
- A proton pump inhibitor.
This was the first truly effective regimen for H pylori. Its advantage is that it can partially or completely overcome metronidazole resistance.21,22 As such, it is potentially ideal, as it should be effective despite resistance to clarithromycin, metronidazole, or levofloxacin, and it can be used in patients allergic to penicillin.
The major downside is a high frequency of side effects, particularly abdominal pain, nausea, and vomiting, often resulting in poor adherence. Most regimens that contain antibiotics have side effects, but adherence seems to be more of a problem with bismuth quadruple therapy, probably because of the combination of the high doses of metronidazole and tetracycline.22 In our experience, this regimen can be effective if the physician takes the time to explain to the patient that side effects are common but treatment success depends on completing the full course of 14 days.
Another problem is that tetracycline has become difficult to obtain in many areas, and doxycycline cannot be substituted in those with metronidazole resistance. To date, it has been difficult or impossible to obtain the same excellent results with doxycycline as can be obtained with tetracycline. It is not clear why.21
To use bismuth quadruple therapy one must often use a name-brand product, Pylera. Pylera is packaged as a 10-day course, which is effective against metronidazole-susceptible infections. However, 14 days are generally required to achieve a high cure rate with metronidazole-resistant infections, which are the main indication for use of this product. Moreover, Pylera does not include a proton pump inhibitor, which must be prescribed separately.
In the United States, Pylera is expensive, costing $740 to $790 with a coupon for a 10-day supply and proportionally more for the required 14-day supply (www.goodrx.com/pylera?drug-name=pylera), whereas in Europe it costs less than 70 Euros ($73).21 If generic tetracycline is available, the US cost for 14 days of generic bismuth quadruple therapy is less than $50.
An alternate and simpler approach is to substitute amoxicillin for tetracycline.23 This regimen has been used successfully in China and was shown to be noninferior to the tetracycline-containing regimen in a head-to-head comparison.24
Recent studies have confirmed earlier Italian studies suggesting that twice-a-day bismuth and tetracycline is effective, which would further simplify therapy and possibly reduce side effects.21,23,24 These variations on bismuth quadruple therapy have not yet been optimized to where one can reliably achieve 95% or greater cure rates, and further studies are needed.
Why include more than 1 antibiotic?
The H pylori load in the stomach is typically large, which increases the odds that a subpopulation of resistant organisms is present. Resistance may be due to a relatively high rate of mutation in certain bacterial genes.25 This is particularly a problem with clarithromycin, metronidazole, and fluoroquinolones and is reflected in a high rate of resistance among patients for whom single-drug regimens have failed. These drugs are always given with a second antimicrobial to which H pylori rarely becomes resistant, such as amoxicillin or tetracycline.
Why include a proton pump inhibitor?
An antisecretory drug is needed to increase the gastric pH, which makes antimicrobial therapy more effective. It also decreases antibiotic washout from the stomach and likely protects and increases the gastric concentration of some antibiotics.
The activities of amoxicillin, fluoroquinolones, and to a lesser degree clarithromycin are pH-dependent. For example, keeping the gastric pH above 6.0 promotes H pylori replication,26,27 making it is more susceptible to amoxicillin (reviewed in detail by Dore et al21). A gastric pH of 6.0 or more is very difficult to achieve with proton pump inhibitors, and has been accomplished regularly only in people who metabolize these drugs slowly (“slow metabolizers”) who received both the proton pump inhibitor and amoxicillin every 6 hours for 14 days.21
With standard clarithromycin, metronidazole, or fluoroquinolone triple therapy, proton pump inhibitors appear to provide satisfactory cure rates when given for 14 days in standard doses. However, double doses (eg, 40 mg of omeprazole or an equivalent) may be slightly better, especially in the presence of resistance.
The cure rate reflects the sum of the 2 populations of organisms: the susceptible and the resistant. In triple therapy, increasing the gastric pH with a proton pump inhibitor makes the amoxicillin component of the regimen more effective against resistant organisms and thus increases the cure rate. For example, in Western countries, esomeprazole 40 mg (approximately equivalent to rabeprazole 40 mg, omeprazole or lansoprazole 60 mg, or pantoprazole 240 mg)28 given twice a day in a 14-day triple therapy regimen cures about 40% to 50% of resistant infections. This benefit will be evident in an improvement in cure rates in populations in which resistance has reduced the average cure rate. This is also why meta-analyses have shown better results with second-generation proton pump inhibitors and with longer duration of therapy.29,30
Generally, we recommend omeprazole 40 mg twice a day or an equivalent (Tables 1–3).
Would a potassium-competitive acid blocker be better than a proton pump inhibitor?
Vonoprazan is a potassium-competitive acid blocker. It does not require intermediate complex formation and is stable at low pH. It has a longer half-life than proton pump inhibitors, and its bioavailability is unaffected by food.31 It was recently approved in Japan for H pylori eradication in combination with clarithromycin or metronidazole plus amoxicillin.18
Vonoprazan is more effective than current proton pump inhibitors for keeping the gastric pH high. There are no published studies of vonoprazan dual therapy in Western countries, but given twice a day for 7 days along with twice-daily amoxicillin it cured only approximately 80% of clarithromycin-resistant strains. Further studies are needed to identify the optimum proton pump inhibitor or potassium-competitive acid blocker, dose, and duration.
Misuse of antibiotics
In triple therapy, the second antimicrobial drug (eg, amoxicillin) is given in part to prevent resistance from developing. It is not clear whether the combination is additive or synergistic, but until we can reliably maintain the intragastric pH above 6.0, which would increase the effectiveness of the amoxicillin component of the regimen, this practice cannot be considered as misuse of antibiotics.
In contrast, in the 4-drug nonbismuth combinations (concomitant, sequential, and hybrid therapies) and the new vonoprazan, clarithromycin, and metronidazole triple therapies, 1 of the antibiotics provides no benefit to some, often most, of the patients.18–20,32 This practice should end when susceptibility data become more widely available and when vonoprazan becomes available, so that we can deliver effective vonoprazan-amoxicillin dual therapy.
First-, second-, and third-line therapies
Many recommendations give advice in terms of first-, second-, and third-line therapies. In practice, a physician should have at least 2 first-line regimens (a first and a second choice). Both should be proven highly successful as empiric therapies in one’s patient population but differ in terms of primary antibiotics. This approach allows the clinician to tailor therapy depending on whether he or she suspects antibiotic resistance (eg, if the patient has taken clarithromycin before) or the patient is allergic or cannot take 1 or more drugs.
Two treatment failures with 2 different regimens known to be effective suggest poor compliance (a difficult patient) or a multiple-drug-resistant infection (a difficult infection). That patient would require salvage therapy (Table 2), which logically should be based on antimicrobial testing or, at a minimum, consultation with someone who frequently deals with this problem.
Test of cure
Monitoring the outcome of therapy (testing for cure) is essential, as it provides a reliable measure of the local effectiveness of particular therapies and also serves as an early warning of development of resistance in one’s patient population.14
Unless there are compelling reasons, testing for cure should use noninvasive testing with the urea breath test or stool antigen test. It is recommended that this be delayed at least 4 weeks to allow the organisms if still present to repopulate the stomach sufficiently for the tests to become positive. Because antibiotics, bismuth, and proton pump inhibitors reduce the bacterial load, they should be withheld at least 2 weeks before testing. Histamine-2 receptor antagonists can be substituted for proton pump inhibitors if antisecretory therapy is needed for symptoms, and continued up to the day before testing. The urea breath test should contain citric acid to overcome any residual pH effects. Physician groups should share their experience so as to alert the community about which therapies should likely be avoided.33
Salvage therapy
Salvage therapy is given after 2 or more treatment failures with different antibiotics. Ideally, the regimen should be based on the results of antimicrobial testing. Current regimens include rifabutin triple therapy, dual therapy (a protein pump inhibitor or vonoprazan and amoxicillin), or furazolidone quadruple therapy (Table 2).
Furazolidone is a synthetic nitrofuran derivative that is effective against many enteric organisms, including gram-negative bacteria and protozoa. It is not available in most Western countries but is available in many other parts of the world.34,35 It is also a monoamine oxidase inhibitor and thus interacts with many drugs and foods (eg, soy sauce, aged cheeses), leading to a relatively high rate of side effects such as fever, palpitations, and skin rash.
Rifabutin-containing regimens, generally, a proton pump inhibitor, amoxicillin 1 g, and rifabutin 150 mg, all twice a day (Table 3) provide average cure rates of less than 80% (typically in the mid-70% range).36 Borody et al37 reported greater than 95% success with a 12-day regimen consisting of rifabutin 150 mg once daily (half-dose), amoxicillin 1.5 g 3 times a day, and pantoprazole 80 mg (approximately equivalent to omeprazole 20 mg) 3 times a day. Ciccaglione et al,38 in a small study, used a 10-day quadruple regimen containing a proton pump inhibitor, amoxicillin, rifabutin, and bismuth (all twice a day), with high cure rates. The results of these studies are yet to be confirmed, and the optimal rifabutin-containing regimen remains to be determined.
PROBIOTICS
There is considerable interest in using probiotics to enhance the effectiveness of antimicrobial therapy for H pylori by increasing tolerability, reducing side effects, and therefore improving compliance.39,40
In a meta-analysis of 14 randomized trials (N = 1,671), when probiotics were added, pooled H pylori eradication rates were only slightly improved: 83.6% (95% CI 80.5%–86.7%) with probiotics and 74.8% (95% CI 71.1%–78.5%) without probiotics by intent-to-treat analysis.41
Another meta-analysis of probiotics suggested that those containing Saccharomyces boulardii, Lactobacillus, and Bifidobacterium significantly increased the eradication rate of triple therapy in populations with high rates of antimicrobial resistance and reduced the risk of overall H pylori therapy-related adverse effects, especially diarrhea.42,43
At present, we recommend that probiotics be considered only for patients who are likely not to comply with treatment (eg, those with irritable bowel syndrome or difficulty taking antibiotics), to try to take advantage of their ability to improve antibiotic tolerability.
Helicobacter pylori infection is an infectious disease and should be treated like one, with due consideration of antibiotic resistance and stewardship.1–4
This was the consensus of the 2015 Kyoto H pylori conference,2 and it signaled a fundamental shift in thinking. Up to now, H pylori treatment has not been based on infectious disease principles, leading to suboptimal results and antibiotic resistance. In addition, the conference recommended that H pylori infection be treated whenever it is found unless there are compelling reasons not to.
Here we review current and possible future regimens for eradicating H pylori that we hope will be more effective and will lead to less resistance than in the past.
H PYLORI AS AN INFECTIOUS DISEASE
Not until the late 1980s was H pylori recognized as the cause of peptic ulcer disease, which until then accounted for hundreds of thousands of hospitalizations and more than 100,000 surgical procedures each year.5 Now, peptic ulcer disease is routinely treated by eradicating H pylori. In addition, the World Health Organization has recommended considering H pylori eradication to reduce the risk of gastric cancer,6 which causes 738,000 deaths worldwide per year.7
The problems of how to diagnose and treat H pylori infection were taken on by gastroenterologists, and not by specialists in infectious disease.1 Even now, almost all the major reviews and consensus statements on H pylori come from gastroenterologists and are published in gastroenterology journals.2,8,9
But infectious diseases differ from most gastrointestinal diseases. In gastrointestinal problems such as constipation or inflammatory bowel disease,10 the causes are generally unknown, and there is a large placebo response to therapy. In contrast, in infectious diseases, the cause is generally known, there is no placebo response, and treatment success depends on susceptibility of the organism. Failure of proven regimens is generally due to resistant organisms, poor adherence, or, in the case of H pylori, poorly designed regimens in terms of doses, frequency of administration, or duration of therapy.
The differences extend to clinical trials of treatment.3 In other infectious diseases, treatment is based on susceptibility. The usual comparative approach in infectious diseases is a noninferiority trial in which the new treatment is compared with standard care, ie, a regimen that reliably achieves nearly 100% cure rates. Not so with H pylori. Most trials of H pylori therapy compared regimens in populations with high but unknown prevalences of resistance and therefore are of limited or no help to the clinician in choosing the best regimen for an individual patient.3
Many thousands of H pylori-infected patients participated in clinical trials in which the results would have been predictable if the researchers had assessed susceptibility before giving the drugs.11–13 Worse, many patients were also randomized to receive regimens that the investigators knew provided poor cure rates in the population being studied. This knowledge was generally not shared with the patients. This approach was used to demonstrate that a new regimen was superior to an old one, even though the new one was already known to be less affected by resistance to the key element in the comparator.
Clinicians generally do not test for susceptibility when treating H pylori, one reason being that such testing is often unavailable.3 However, almost every hospital, clinic, and major laboratory in the world provides susceptibility testing for other common local pathogens. H pylori is easy to grow, and laboratories could test for susceptibility if we asked them to.
Current H pylori recommendations may also contribute to the global increase in antimicrobal resistance.
As discussed below, all recent guidelines have recommended 4-drug non-bismuth-containing concomitant therapy as first-line therapy. An infectious disease colleague described it as a “hope therapy” because the prescriber hoped that the infection would be susceptible to either metronidazole or clarithromycin. All who receive this combination receive an antibiotic they do not need. This is an expedient rather than a medically rational choice resulting from failure to deal with H pylori as an infectious disease.
H PYLORI THERAPIES
Conceptually, treating infectious disease is straightforward: one should prescribe antimicrobial drugs to which the organism is susceptible3 (Table 1). However, clinical success lies in the details, which include the doses, frequency of doses, duration of therapy, timing of doses in relation to meals, and use of adjuvants such as antisecretory drugs, antacids, and probiotics. A number of regimens reliably yield high cure rates—95% or higher—if the organism is susceptible and the patients are adherent.
The effectiveness of any regimen may vary depending on the population it is used in, due to polymorphisms in drug-metabolizing enzymes such as CYP2C19.
Sequential therapy is obsolete
Sequential therapy for H pylori infection consisted of amoxicillin plus a proton pump inhibitor for 7 days, followed by clarithromycin, tinidazole, or metronidazole plus a proton pump inhibitor for a further 7 days. This regimen should not be used any more because concomitant therapy will always be superior (see below).
Need for 14 days of therapy
H pylori occupies a number of different niches in the body ranging from gastric mucus (which is technically outside the body) to inside gastric epithelial cells. As a general rule, 14-day therapy provides the best results, in part because the longer duration helps kill the organisms that persist in different niches.14,15
In addition, proton pump inhibitors, which are part of all the currently recommended regimens, require 3 or more days to reach their full antisecretory effectiveness, which further limits the effectiveness of short-duration therapies.
Shorter regimens should be used only if they are proved to be as good as 14-day regimens and if both achieve 95% or greater cure rates with susceptible infections.
How to choose a therapy
Since rational infectious-disease therapy is based on susceptibility, one should start by considering the susceptibility pattern in the local population and, therefore, the likely susceptibility in the patient in front of us.
Unfortunately, we do not yet have local or regional susceptibility data on H pylori for most locales. Until those data are available, we must use the indirect information that is available, such as the patient’s history of antibiotic use.
Triple therapy should not be used empirically
Triple therapy (Table 1) consists of the combination of:
- Clarithromycin or metronidazole or a fluoroquinolone
- Amoxicillin
- A proton pump inhibitor.
However, prior use of a macrolide (eg, erythromycin, clarithromycin, or azithromycin), metronidazole, or a fluoroquinolone (eg, ciprofloxacin, levofloxacin) almost guarantees resistance to those drugs. In the United States, resistance to clarithromycin, metronidazole, levofloxacin, and related drugs is already widespread, and none should be used empirically in triple therapies. In contrast, amoxicillin, tetracycline, and furazolidone can often be used again, as resistance to them is rare even with prior use.
For example, 14 days of clarithromycin triple therapy (clarithromycin, amoxicillin, and a proton pump inhibitor) can be expected to cure more than 95% of patients who have susceptible infections and about 20% of those with resistant infections.16 This 20% is due to the proton pump inhibitor and amoxicillin, as the contribution to the cure rate from clarithromycin is close to zero.
If the prevalence of resistance to clarithromycin is 25%, the cure rate in the entire population will be a little more than 75%—97% in the 75% of the population with susceptible infections and 20% in patients who previously received clarithromycin (Figure 1).
If we know that our patient has an infection that is susceptible to clarithromycin, metronidazole, or levofloxacin, good results could be achieved with triple therapy that includes a proton pump inhibitor, for 14 days. Fluoroquinolones have a number of black-box warnings from the US Food and Drug Administration (www.fda.gov/Drugs/DrugSafety/ucm500143.htm) and should always be a last choice. However, in the United States, lacking definite data about susceptibility to clarithromycin, metronidazole, and levofloxacin, we should assume resistance is present and use a 4-drug regimen (eg, concomitant therapy or bismuth quadruple therapy).
Concomitant therapy is preferred
Concomitant therapy is the combination of:
- Amoxicillin
- Metronidazole
- Clarithromycin
- A proton pump inhibitor.
Functionally, this is a combination of clarithromycin and metronidazole triple therapies, given simultaneously.17 The premise is that even though the prevalence of metronidazole resistance in the United States is high (20%–40%), and so is the prevalence of clarithromycin resistance (about 20%), the prevalence of resistance to both drugs at the same time is expected to be low (eg, 0.4 × 0.2 = 0.08, or 8%) unless the drugs had previously been used together, as in some older regimens that contained both. Thus, the metronidazole will kill the clarithromycin-resistant but metronidazole-susceptible strains, and the clarithromycin will kill the clarithromycin-susceptible, metronidazole-resistant strains. Only with dual resistant strains will this regimen fail (with a 20% cure rate due to the proton pump inhibitor and amoxicillin and a population cure rate of slightly more than 90%).
The downside of this highly recommended therapy is that all who receive it are getting an antibiotic that they don’t need, which is, in a global sense, inappropriate. In other words, all those who are cured by clarithromycin also receive metronidazole, which plays no role in treatment success, and those cured by metronidazole receive unneeded clarithromycin (Figure 2). Had susceptibility testing been available, those with susceptible strains would have received appropriate triple therapies, and those with dual resistance would not have received either antibiotic.
Thus, while we recommend concomitant therapy as an empiric regimen in populations that do not have high levels of resistance to metronidazole or clarithromycin (as those would also have a high prevalence of dual resistance), one must be aware of the “dirty little secret” of inappropriate antibiotic use that accompanies it and some other H pylori therapies (eg, vonoprazan triple therapy in Japan).18–20
Bismuth quadruple therapy is an alternative
Bismuth quadruple therapy (Table 1) consists of:
- Bismuth
- Tetracycline
- Metronidazole
- A proton pump inhibitor.
This was the first truly effective regimen for H pylori. Its advantage is that it can partially or completely overcome metronidazole resistance.21,22 As such, it is potentially ideal, as it should be effective despite resistance to clarithromycin, metronidazole, or levofloxacin, and it can be used in patients allergic to penicillin.
The major downside is a high frequency of side effects, particularly abdominal pain, nausea, and vomiting, often resulting in poor adherence. Most regimens that contain antibiotics have side effects, but adherence seems to be more of a problem with bismuth quadruple therapy, probably because of the combination of the high doses of metronidazole and tetracycline.22 In our experience, this regimen can be effective if the physician takes the time to explain to the patient that side effects are common but treatment success depends on completing the full course of 14 days.
Another problem is that tetracycline has become difficult to obtain in many areas, and doxycycline cannot be substituted in those with metronidazole resistance. To date, it has been difficult or impossible to obtain the same excellent results with doxycycline as can be obtained with tetracycline. It is not clear why.21
To use bismuth quadruple therapy one must often use a name-brand product, Pylera. Pylera is packaged as a 10-day course, which is effective against metronidazole-susceptible infections. However, 14 days are generally required to achieve a high cure rate with metronidazole-resistant infections, which are the main indication for use of this product. Moreover, Pylera does not include a proton pump inhibitor, which must be prescribed separately.
In the United States, Pylera is expensive, costing $740 to $790 with a coupon for a 10-day supply and proportionally more for the required 14-day supply (www.goodrx.com/pylera?drug-name=pylera), whereas in Europe it costs less than 70 Euros ($73).21 If generic tetracycline is available, the US cost for 14 days of generic bismuth quadruple therapy is less than $50.
An alternate and simpler approach is to substitute amoxicillin for tetracycline.23 This regimen has been used successfully in China and was shown to be noninferior to the tetracycline-containing regimen in a head-to-head comparison.24
Recent studies have confirmed earlier Italian studies suggesting that twice-a-day bismuth and tetracycline is effective, which would further simplify therapy and possibly reduce side effects.21,23,24 These variations on bismuth quadruple therapy have not yet been optimized to where one can reliably achieve 95% or greater cure rates, and further studies are needed.
Why include more than 1 antibiotic?
The H pylori load in the stomach is typically large, which increases the odds that a subpopulation of resistant organisms is present. Resistance may be due to a relatively high rate of mutation in certain bacterial genes.25 This is particularly a problem with clarithromycin, metronidazole, and fluoroquinolones and is reflected in a high rate of resistance among patients for whom single-drug regimens have failed. These drugs are always given with a second antimicrobial to which H pylori rarely becomes resistant, such as amoxicillin or tetracycline.
Why include a proton pump inhibitor?
An antisecretory drug is needed to increase the gastric pH, which makes antimicrobial therapy more effective. It also decreases antibiotic washout from the stomach and likely protects and increases the gastric concentration of some antibiotics.
The activities of amoxicillin, fluoroquinolones, and to a lesser degree clarithromycin are pH-dependent. For example, keeping the gastric pH above 6.0 promotes H pylori replication,26,27 making it is more susceptible to amoxicillin (reviewed in detail by Dore et al21). A gastric pH of 6.0 or more is very difficult to achieve with proton pump inhibitors, and has been accomplished regularly only in people who metabolize these drugs slowly (“slow metabolizers”) who received both the proton pump inhibitor and amoxicillin every 6 hours for 14 days.21
With standard clarithromycin, metronidazole, or fluoroquinolone triple therapy, proton pump inhibitors appear to provide satisfactory cure rates when given for 14 days in standard doses. However, double doses (eg, 40 mg of omeprazole or an equivalent) may be slightly better, especially in the presence of resistance.
The cure rate reflects the sum of the 2 populations of organisms: the susceptible and the resistant. In triple therapy, increasing the gastric pH with a proton pump inhibitor makes the amoxicillin component of the regimen more effective against resistant organisms and thus increases the cure rate. For example, in Western countries, esomeprazole 40 mg (approximately equivalent to rabeprazole 40 mg, omeprazole or lansoprazole 60 mg, or pantoprazole 240 mg)28 given twice a day in a 14-day triple therapy regimen cures about 40% to 50% of resistant infections. This benefit will be evident in an improvement in cure rates in populations in which resistance has reduced the average cure rate. This is also why meta-analyses have shown better results with second-generation proton pump inhibitors and with longer duration of therapy.29,30
Generally, we recommend omeprazole 40 mg twice a day or an equivalent (Tables 1–3).
Would a potassium-competitive acid blocker be better than a proton pump inhibitor?
Vonoprazan is a potassium-competitive acid blocker. It does not require intermediate complex formation and is stable at low pH. It has a longer half-life than proton pump inhibitors, and its bioavailability is unaffected by food.31 It was recently approved in Japan for H pylori eradication in combination with clarithromycin or metronidazole plus amoxicillin.18
Vonoprazan is more effective than current proton pump inhibitors for keeping the gastric pH high. There are no published studies of vonoprazan dual therapy in Western countries, but given twice a day for 7 days along with twice-daily amoxicillin it cured only approximately 80% of clarithromycin-resistant strains. Further studies are needed to identify the optimum proton pump inhibitor or potassium-competitive acid blocker, dose, and duration.
Misuse of antibiotics
In triple therapy, the second antimicrobial drug (eg, amoxicillin) is given in part to prevent resistance from developing. It is not clear whether the combination is additive or synergistic, but until we can reliably maintain the intragastric pH above 6.0, which would increase the effectiveness of the amoxicillin component of the regimen, this practice cannot be considered as misuse of antibiotics.
In contrast, in the 4-drug nonbismuth combinations (concomitant, sequential, and hybrid therapies) and the new vonoprazan, clarithromycin, and metronidazole triple therapies, 1 of the antibiotics provides no benefit to some, often most, of the patients.18–20,32 This practice should end when susceptibility data become more widely available and when vonoprazan becomes available, so that we can deliver effective vonoprazan-amoxicillin dual therapy.
First-, second-, and third-line therapies
Many recommendations give advice in terms of first-, second-, and third-line therapies. In practice, a physician should have at least 2 first-line regimens (a first and a second choice). Both should be proven highly successful as empiric therapies in one’s patient population but differ in terms of primary antibiotics. This approach allows the clinician to tailor therapy depending on whether he or she suspects antibiotic resistance (eg, if the patient has taken clarithromycin before) or the patient is allergic or cannot take 1 or more drugs.
Two treatment failures with 2 different regimens known to be effective suggest poor compliance (a difficult patient) or a multiple-drug-resistant infection (a difficult infection). That patient would require salvage therapy (Table 2), which logically should be based on antimicrobial testing or, at a minimum, consultation with someone who frequently deals with this problem.
Test of cure
Monitoring the outcome of therapy (testing for cure) is essential, as it provides a reliable measure of the local effectiveness of particular therapies and also serves as an early warning of development of resistance in one’s patient population.14
Unless there are compelling reasons, testing for cure should use noninvasive testing with the urea breath test or stool antigen test. It is recommended that this be delayed at least 4 weeks to allow the organisms if still present to repopulate the stomach sufficiently for the tests to become positive. Because antibiotics, bismuth, and proton pump inhibitors reduce the bacterial load, they should be withheld at least 2 weeks before testing. Histamine-2 receptor antagonists can be substituted for proton pump inhibitors if antisecretory therapy is needed for symptoms, and continued up to the day before testing. The urea breath test should contain citric acid to overcome any residual pH effects. Physician groups should share their experience so as to alert the community about which therapies should likely be avoided.33
Salvage therapy
Salvage therapy is given after 2 or more treatment failures with different antibiotics. Ideally, the regimen should be based on the results of antimicrobial testing. Current regimens include rifabutin triple therapy, dual therapy (a protein pump inhibitor or vonoprazan and amoxicillin), or furazolidone quadruple therapy (Table 2).
Furazolidone is a synthetic nitrofuran derivative that is effective against many enteric organisms, including gram-negative bacteria and protozoa. It is not available in most Western countries but is available in many other parts of the world.34,35 It is also a monoamine oxidase inhibitor and thus interacts with many drugs and foods (eg, soy sauce, aged cheeses), leading to a relatively high rate of side effects such as fever, palpitations, and skin rash.
Rifabutin-containing regimens, generally, a proton pump inhibitor, amoxicillin 1 g, and rifabutin 150 mg, all twice a day (Table 3) provide average cure rates of less than 80% (typically in the mid-70% range).36 Borody et al37 reported greater than 95% success with a 12-day regimen consisting of rifabutin 150 mg once daily (half-dose), amoxicillin 1.5 g 3 times a day, and pantoprazole 80 mg (approximately equivalent to omeprazole 20 mg) 3 times a day. Ciccaglione et al,38 in a small study, used a 10-day quadruple regimen containing a proton pump inhibitor, amoxicillin, rifabutin, and bismuth (all twice a day), with high cure rates. The results of these studies are yet to be confirmed, and the optimal rifabutin-containing regimen remains to be determined.
PROBIOTICS
There is considerable interest in using probiotics to enhance the effectiveness of antimicrobial therapy for H pylori by increasing tolerability, reducing side effects, and therefore improving compliance.39,40
In a meta-analysis of 14 randomized trials (N = 1,671), when probiotics were added, pooled H pylori eradication rates were only slightly improved: 83.6% (95% CI 80.5%–86.7%) with probiotics and 74.8% (95% CI 71.1%–78.5%) without probiotics by intent-to-treat analysis.41
Another meta-analysis of probiotics suggested that those containing Saccharomyces boulardii, Lactobacillus, and Bifidobacterium significantly increased the eradication rate of triple therapy in populations with high rates of antimicrobial resistance and reduced the risk of overall H pylori therapy-related adverse effects, especially diarrhea.42,43
At present, we recommend that probiotics be considered only for patients who are likely not to comply with treatment (eg, those with irritable bowel syndrome or difficulty taking antibiotics), to try to take advantage of their ability to improve antibiotic tolerability.
- Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148:719–731.
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64:1353–1367.
- Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther 2016; 14:577–585.
- Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter 2016; 21(suppl 1):3–7.
- Grossman MI. Closing remarks. Gastroenterology 1978; 74:487–488.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 2012; 61:646–664.
- Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016; 151:51–69.
- IARC Helicobacter pylori Working Group. Volume 8. Helicobacter pylori eradication as a strategy for preventing gastric cancer. Lyon, France: International Agency for Research on Cancer, 2014.
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- Graham DY, Dore MP. Helicobacter pylori therapy demystified. Helicobacter 2011; 16:343–345.
- Gatta L, Vakil N, Leandro G, Di MF, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol 2009; 104:3069–3079.
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59:1143–1153.
- Graham DY. Helicobacter pylori eradication therapy research: ethical issues and description of results. Clin Gastroenterol Hepatol 2010; 8:1032–1036.
- Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5:321–331.
- Graham DY, Dore MP. Variability in the outcome of treatment of Helicobacter pylori infection: a critical analysis. In: Hunt RH, Tytgat GNJ, editors. Helicobacter pylori Basic Mechanisms to Clinical Cure 1998. Dordrecht, Netherlands: Kluwer Academic Publishers, 998:426–440.
- Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2015; 21:85–90.
- Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014; 12:177–186.
- Murakami K, Sakurai Y, Shiino M, Funao N, Nishmura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65:1439–1446.
- Graham DY. Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut 2016 Apr 7. pii: gutjnl-2016-311796. doi: 10.1136/gutjnl-2016-311796. [Epub ahead of print].
- Matsumoto H, Shiotani A, Katsumata R, et al. Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: the effect of clarithromycin resistance. Dig Dis Sci 2016; 61:3215–3220.
- Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65:870–878.
- Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. Gastroenterol Clin North Am 2015; 44:537–563.
- Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015; 64:1715–1720.
- Chen Q, Zhang X, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline for bismuth quadruple therapy. Am J Gastroenterol 2016; 111:1736–1742.
- Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 1998; 115:1272–1277.
- Marcus EA, Inatomi N, Nagami GT, et al. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 2012; 36:972–979.
- Sachs G, Shin JM, Munson K, et al. Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther 2000; 14:1383–1401.
- Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors: comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009; 65:19–31.
- Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev 2013;12:CD008337.
- McNicholl AG, Linares PM, Nyssen OP, et al. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 36:414–425.
- Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 2015; 6:e94.
- Graham DY, Laine L. The Toronto Helicobacter pylori consensus in context. Gastroenterology 2016; 151:9–12.
- Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med 2015; 3:9.
- Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013; 25:1134–1140.
- Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol 2012; 8:1–2.
- Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 35:209–221.
- Borody TJ, Pang G, Wettstein AR, et al. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment Pharmacol Ther 2006; 23:481–488.
- Ciccaglione AF, Tavani R, Grossi L, et al. Rifabutin containing triple therapy and rifabutin with bismuth containing quadruple therapy for third-line treatment of Helicobacter pylori infection: two pilot studies. Helicobacter 2016; 21:375–381.
- Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol 2015; 21:10644–10653.
- Zhang MM, Qian W, Qin YY, et al. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol 2015; 21:4345–4357.
- Tong JL, Ran ZH, Shen J, et al. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007; 25:155–168.
- Szajewska H, Setty M, Mrukowicz J, et al. Probiotics in gastrointestinal diseases in children: hard and not-so-hard evidence of efficacy. J Pediatr Gastroenterol Nutr 2006; 42:454–475.
- Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol 2013; 47:25–32.
- Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015; 148:719–731.
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64:1353–1367.
- Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther 2016; 14:577–585.
- Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter 2016; 21(suppl 1):3–7.
- Grossman MI. Closing remarks. Gastroenterology 1978; 74:487–488.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 2012; 61:646–664.
- Fallone CA, Chiba N, van Zanten SV, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016; 151:51–69.
- IARC Helicobacter pylori Working Group. Volume 8. Helicobacter pylori eradication as a strategy for preventing gastric cancer. Lyon, France: International Agency for Research on Cancer, 2014.
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- Graham DY, Dore MP. Helicobacter pylori therapy demystified. Helicobacter 2011; 16:343–345.
- Gatta L, Vakil N, Leandro G, Di MF, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol 2009; 104:3069–3079.
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59:1143–1153.
- Graham DY. Helicobacter pylori eradication therapy research: ethical issues and description of results. Clin Gastroenterol Hepatol 2010; 8:1032–1036.
- Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5:321–331.
- Graham DY, Dore MP. Variability in the outcome of treatment of Helicobacter pylori infection: a critical analysis. In: Hunt RH, Tytgat GNJ, editors. Helicobacter pylori Basic Mechanisms to Clinical Cure 1998. Dordrecht, Netherlands: Kluwer Academic Publishers, 998:426–440.
- Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2015; 21:85–90.
- Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014; 12:177–186.
- Murakami K, Sakurai Y, Shiino M, Funao N, Nishmura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65:1439–1446.
- Graham DY. Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut 2016 Apr 7. pii: gutjnl-2016-311796. doi: 10.1136/gutjnl-2016-311796. [Epub ahead of print].
- Matsumoto H, Shiotani A, Katsumata R, et al. Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: the effect of clarithromycin resistance. Dig Dis Sci 2016; 61:3215–3220.
- Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65:870–878.
- Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. Gastroenterol Clin North Am 2015; 44:537–563.
- Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015; 64:1715–1720.
- Chen Q, Zhang X, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline for bismuth quadruple therapy. Am J Gastroenterol 2016; 111:1736–1742.
- Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 1998; 115:1272–1277.
- Marcus EA, Inatomi N, Nagami GT, et al. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther 2012; 36:972–979.
- Sachs G, Shin JM, Munson K, et al. Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther 2000; 14:1383–1401.
- Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors: comparison of effects on intragastric pH. Eur J Clin Pharmacol 2009; 65:19–31.
- Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev 2013;12:CD008337.
- McNicholl AG, Linares PM, Nyssen OP, et al. Meta-analysis: esomeprazole or rabeprazole vs. first-generation pump inhibitors in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 36:414–425.
- Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 2015; 6:e94.
- Graham DY, Laine L. The Toronto Helicobacter pylori consensus in context. Gastroenterology 2016; 151:9–12.
- Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med 2015; 3:9.
- Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013; 25:1134–1140.
- Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol 2012; 8:1–2.
- Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther 2012; 35:209–221.
- Borody TJ, Pang G, Wettstein AR, et al. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment Pharmacol Ther 2006; 23:481–488.
- Ciccaglione AF, Tavani R, Grossi L, et al. Rifabutin containing triple therapy and rifabutin with bismuth containing quadruple therapy for third-line treatment of Helicobacter pylori infection: two pilot studies. Helicobacter 2016; 21:375–381.
- Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol 2015; 21:10644–10653.
- Zhang MM, Qian W, Qin YY, et al. Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J Gastroenterol 2015; 21:4345–4357.
- Tong JL, Ran ZH, Shen J, et al. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007; 25:155–168.
- Szajewska H, Setty M, Mrukowicz J, et al. Probiotics in gastrointestinal diseases in children: hard and not-so-hard evidence of efficacy. J Pediatr Gastroenterol Nutr 2006; 42:454–475.
- Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol 2013; 47:25–32.
KEY POINTS
- We recommend clinicians have 2 first-line options to accommodate prior antibiotic use or drug allergy.
- We recommend 4-drug combinations as first-line treatments, ie, either concomitant therapy or bismuth-containing quadruple therapy, to be taken for 14 days.
- Concomitant therapy consists of the combination of amoxicillin, metronidazole, clarithromycin, and a proton pump inhibitor.
- Bismuth quadruple therapy consists of the combination of bismuth, tetracycline, metronidazole, and a proton pump inhibitor.
- After 2 treatments have failed, therapy with different regimens should be based on susceptibility testing.
Hypoglycemia after gastric bypass: An emerging complication
Bariatric surgery, though beneficial, is associated with complications, one of which is post-gastric bypass hypoglycemia (PGBH).1 The mean time from gastric bypass to documented hypoglycemia is about 28 months.2
PGBH is probably more common than initially thought. In older reports, the prevalence was only 0.1% to 0.36%.1,3 In contrast, in a mail survey in 2015,4 one-third of bariatric surgery patients reported symptoms that raised the suspicion of hypoglycemia. Those with suspicious symptoms were more likely to have undergone Roux-en-Y surgery, to have had no preoperative diabetes, to have had a longer interval since surgery, and to be female. Restricting the suspicion of postprandial hypoglycemia to those who reported more serious symptoms, including needing third-party assistance, the prevalence was 11.6%.
Kefurt et al5 followed Roux-en-Y patients who wore a continuous glucose monitor for 86 months after surgery and found that 38% had hypoglycemia; however, symptoms of hypoglycemia were not discussed.
Thus, the exact prevalence is currently unknown. But as time goes by and more procedures are performed, the incidence will likely rise.
OBESITY IS ON THE RISE, AND SO IS WEIGHT-LOSS SURGERY
Obesity is rampant, and its prevalence continues to rise. In 2011–2012, more than two-thirds of adults in the United States were reported as obese.6 Complications of obesity such as cardiac disease, diabetes, and cancer lead to increased mortality risk.7 Obesity is difficult to reverse, as many people fail to lose weight with diet, exercise, and pharmacotherapy.
Given the difficulty of losing weight and the complications that arise from obesity, bariatric surgery has become increasingly popular. Not only do patients lose significantly more weight with bariatric surgery than with conventional measures, but surgery also reduces and often cures conditions associated with obesity.8
Nguyen et al9 reported that 671,959 patients underwent gastric bypass procedures in the United States from 2003 to 2008. In a registry maintained by the American Society for Metabolic and Bariatric Surgery10 from June 2007 to May 2009, the most common bariatric procedure in the United States was Roux-en-Y gastric bypass, followed by sleeve gastrectomy.
DIFFERENTIAL DIAGNOSIS AND DEFINITIONS
The differential diagnosis for hyperinsulinemic hypoglycemia after gastric bypass surgery includes exogenous and endogenous causes (Table 1). Exogenous causes include abuse of insulin secretagogues such as sulfonylureas or meglitinides and abuse of insulin, which may occur in patients with Munchausen syndrome, Munchausen syndrome by proxy, or malingering. Endogenous causes include insulinoma, early and late dumping syndromes, and PGBH.
When differentiating endogenous from exogenous hypoglycemia, insulin and C-peptide levels are useful (Table 2). The pancreas produces proinsulin, which is broken down into insulin and C-peptide. Since exogenous insulin does not have a C-peptide component, people abusing insulin have elevated insulin levels with a low C-peptide level.11 Insulin secretagogues cause endogenous insulin secretion, resulting in elevated levels of both insulin and C-peptide. Thus, a screen for these medications is necessary to determine this as the cause.
Differentiating endogenous causes of hypoglycemia
Differentiating the endogenous causes (insulinoma, early or late dumping syndrome, and PGBH) can be challenging, as all 3 have similar biochemical profiles (Table 2).
Insulinoma is a tumor of pancreatic beta cells that produces excessive amounts of insulin. Unlike dumping syndrome, which only occurs postprandially, insulinoma primarily causes fasting hypoglycemia, although postprandial hypoglycemia can occur less commonly. Insulinoma after Roux-en-Y is rare. Only 7 cases have been reported.12
Dumping syndrome is classified as either early or late.
Early dumping syndrome usually occurs within 20 minutes of eating. The rapid transit of carbohydrates into the small intestine results in a fluid shift and a sympathetic response characterized by tachycardia, nausea, and diarrhea. Hypoglycemia is not present. Early dumping syndrome usually arises during the first few months after surgery.13
Late dumping syndrome usually occurs 1 to 4 hours after ingestion of a carbohydrate load, with symptoms of diaphoresis, dizziness, and fatigue caused by hypoglycemia from an excessive insulin release in response to the carbohydrates.13 It does not tend to cause neuroglycopenic symptoms.14 We define late dumping syndrome as postprandial hypoglycemic symptoms that occur after eating simple sugars and that resolve with dietary changes alone.
Differentiating late dumping syndrome from PGBH is difficult, as the line between the 2 processes is blurred.13
PGBH is defined as postprandial hypoglycemia (although it can be fasting in severe cases), often with neuroglycopenic symptoms, that occurs despite adherence to an acceptable bariatric diet (outlined in Table 3). We categorize PGBH as mild, moderate, or severe. Mild PGBH resolves with dietary changes with or without an alpha-glucosidase inhibitor. Moderate PGBH does not respond to an alpha-glucosidase inhibitor and dietary changes, and alternative or additional medication or medications are needed for resolution. Severe PGBH does not respond to dietary or medical interventions, and patients experience persistent episodes of neuroglycopenia.
THE EXACT MECHANISM IS UNCERTAIN
Patients with PGBH have a significant postprandial rise in glucose (often with levels > 200 mg/dL), leading to a robust insulin response and a subsequent drop in blood glucose.15
The exact mechanisms causing hypoglycemia are unknown, but excessive release of the incretin hormones glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) are thought to contribute. GLP-1 is primarily secreted in the gut in response to nutrients, causing a glucose-dependent release of insulin and suppression of glucagon, as well as a delay in gastric emptying and motility. Salehi et al16 demonstrated excessive GLP-1 and insulin release after glucose administration in postbypass patients, with a more exaggerated response in those experiencing postprandial hypoglycemia.
Excessive incretin hormones may also contribute to pancreatic islet cell hyperplasia, leading to hyperinsulinism.17 Other proposed mechanisms of PGBH are the lack of a decrease in beta cell mass after gastric bypass, a postoperative increase in insulin sensitivity, a decrease in ghrelin (an insulin counterregulatory hormone), and an abnormal glucagon response.13,17
Pathologic changes vary widely
PGBH is a challenging diagnosis to make pathologically. On review of pancreatic tissue from 36 patients undergoing partial pancreatectomy for PGBH, the pancreatic islet cells of the PGBH group were larger and more irregular compared with controls.18,19 This histologic condition with islet-cell hypertrophy, hyperplasia, and other changes has been termed nesidioblastosis.11,14,20 However, the pancreatic tissue appears grossly normal. The histopathologic findings can vary greatly in individual cases and in one-third of cases the pancreatic changes can be minimal, so that “normal” and PGBH cells can be nearly impossible to distinguish from each other.21
DIAGNOSIS AND TREATMENT
We recommend a stepwise approach to evaluating and treating PGBH (Figures 1 and 2).
Step 1: Evaluate blood glucose and Whipple triad
The first step is a thorough history, including food consumption and timing of hypoglycemic symptoms. Give the patient a glucometer to take home, with instructions to check blood glucose levels when hypoglycemic symptoms occur. The patient should keep a log documenting time tested, food consumed, symptoms, and blood glucose data.
Hypoglycemic symptoms are categorized as autonomic and neuroglycopenic. Autonomic symptoms include anxiety, palpitations, tremulousness, and diaphoresis. Neuroglycopenic symptoms include confusion, falls, seizures, and loss of consciousness.12
There are degrees of hypoglycemia and hypoglycemic symptoms. Clinical hypoglycemia—a blood glucose level low enough to cause signs or symptoms—can be confirmed by the Whipple triad:
- Measured low blood glucose
- Symptoms of low blood glucose
- Relief of symptoms when low blood glucose is corrected.
Hypoglycemic symptoms can occur when the blood glucose level falls to less than 55 mg/dL in healthy people, but this cutoff can shift lower in someone who has recurrent hypoglycemia.
When the Whipple triad is documented, rule out nonhyperinsulinemic causes of hypoglycemia such as hypothyroidism, adrenal insufficiency, underlying organ dysfunction (ie, liver disease), and medications that cause hypoglycemia.
Step 2: Modify the diet
If postprandial hypoglycemia is occurring, the next step is dietary modification. Two studies showed that a low-carbohydrate diet prevented hypoglycemia; however, these diets contained nearly no carbohydrates (with meals consisting of eggs, sausage, cheese, and black coffee or tea).15,22
Instruct patients to never eat pure carbohydrates without fat or protein, as this can result in a more severe hypoglycemic response.22 In addition, foods with a high glycemic index (a measure of how a carbohydrate-containing food raises blood sugar) should be avoided, and a low glycemic index diet is recommended.23 High glycemic index foods include white bread, bagels, pretzels, and pineapple. Low glycemic index foods include 100% stone-ground whole wheat or pumpernickel bread, lima beans, butter beans, peas, legumes, lentils, and nonstarchy vegetables.
Our bariatric surgeons provide all postbariatric surgery patients with the dietary guidelines shown in Table 3.24 We also ask our patients with PGBH to limit carbohydrates to 15 to 30 g per meal and to limit added sugars to less than 4 g per meal, including regular and sugar alcohols (polyols). Snacks should contain only protein and fat. In severe cases, we further limit the diet to 15 g of carbohydrate per meal, with no added sugars.
The hypoglycemia occurring with PGBH is treated differently than the hypoglycemia that occurs in diabetic patients. Advise patients with PGBH to treat their hypoglycemic episodes with a simple sugar combined with a protein or fat (eg, a small handful of candy with a spoonful of peanut butter), as they will often have recurrent hypoglycemia if a simple sugar is used alone. If patients regain weight, ask them about frequent eating, which would be related to self-treatment of hypoglycemia.
Step 3: Start an alpha-glucosidase inhibitor
If postprandial hypoglycemia persists despite dietary modification, then start an alpha-glucosidase inhibitor such as acarbose. Acarbose inhibits carbohydrate absorption, resulting in a decreased insulin response; thus, it blunts the decline in postprandial blood glucose.
Unfortunately, gastrointestinal side effects such as flatulence, diarrhea, and abdominal pain occur in up to 20% of patients who take acarbose, often leading to its discontinuation.25 To minimize gastrointestinal side effects, we usually start with 25 mg of acarbose with 1 meal daily for 1 week, then increase the dosage weekly to 25 mg with the other 2 meals. If tolerated, acarbose can be increased to 50 to 100 mg with 3 meals daily.
Step 4: Obtain a mixed meal tolerance test or a provocation meal test
If dietary changes and an alpha-glucosidase inhibitor do not prevent postprandial hypoglycemia from recurring, then confirmation of PGBH is needed, using a mixed meal tolerance test or a provocation meal test.
In a mixed meal tolerance test, the meal consists of 55% carbohydrate, 30% fat, and 15% protein. Patients with hyperinsulinemic hypoglycemia have a rapid rise in blood glucose (> 200 mg/dL) with a robust insulin response that is often followed by hypoglycemia after ingesting a meal containing carbohydrates in this test. Insulin levels that remain elevated after the plasma glucose level falls to less than 55 mg/dL indicate hyperinsulinism.11
Nevertheless, a mixed meal tolerance test will not always induce hypoglycemia. In a study of 51 patients with PGBH, all wore a continuous glucose monitor, were instructed to follow their normal diet for 5 days, and then underwent a mixed meal tolerance test on day 6. The glucose monitor revealed hypoglycemia in 75% of patients, while the mixed meal tolerance test was positive in only 29%.5
Moreover, to date, there is no standardized mixed meal.5,15 This might also explain the difference in prevalence of hypoglycemia detected by this test.
Based on these conflicting findings, we recommend a provocation meal test—ie, the patient is given foods that have induced hypoglycemia earlier.
Of note, the Endocrine Society guidelines on hypoglycemia state that an oral glucose tolerance test should never be used to document postprandial hypoglycemia.26 Lev-Ran and Anderson27 found that an oral glucose tolerance test could be positive in at least 10% of normal people.
Step 5: Consider other pharmacotherapy
For moderate to severe PGBH in which dietary modification and acarbose have failed, additional medical therapy is the next step. Medical therapies include calcium channel blockers, somatostatin analogues (eg, octreotide), and diazoxide.
Calcium channel blockers inhibit insulin release from beta cells28 but at the risk of hypotension. Mordes and Alonso29 treated 6 PGBH patients with nifedipine or verapamil with or without acarbose, and symptoms resolved in 5 of the 6 patients.
When we treat PGBH, we often add a calcium channel blocker as the next step in therapy if the patient has hypertension or if the blood pressure can tolerate this. If the patient’s blood pressure is low, then avoiding calcium channel blocker therapy may be necessary. The next step would be octreotide and then diazoxide.
Somatostatin analogues such as octreotide inhibit GLP-1 and insulin release.30 The most common side effects of octreotide are diarrhea and abdominal pain. Bile stone formation can also occur, but this is not common.
Diazoxide opens adenosine triphosphate-sensitive potassium channels and reduces the opening of calcium channels, inhibiting insulin release and raising blood glucose. In a study of 6 Japanese patients with inoperable insulinoma, diazoxide was used to treat hypoglycemia.31 Unfortunately, the doses required to control the low blood sugars also led to adverse reactions, most of which involved edema secondary to volume overload and other heart failure symptoms. Diazoxide also commonly causes hypotension and hirsutism.
Step 6: 72-hour fast
A 72-hour fast is recommended in severe cases of PGBH in patients for whom dietary modification and the additional pharmacotherapy outlined in step 5 have failed. A 72-hour fast is always indicated in evaluating confirmed fasting hypoglycemia. People with insulinoma usually have fasting hypoglycemia, while patients with dumping syndrome do not. Patients with PGBH usually do not have fasting hypoglycemia, but they can in severe cases.11
For safety, this test should be done in the hospital. Baseline plasma levels of insulin, C-peptide, proinsulin, beta-hydroxybutyrate, and glucose should be obtained. The patient then fasts, consuming only noncaloric and noncaffeinated beverages for 72 hours. During this time, capillary glucose checks are performed every 6 hours. If the capillary glucose level falls below 55 mg/dL,11,26 then the baseline tests are redrawn along with a sulfonylurea screen. To reduce costs and unnecessary testing, the tests are not sent for laboratory processing unless the plasma glucose is less than 55 mg/dL.
When the plasma glucose is less than 55 mg/dL, insulin production should cease. Elevated insulin levels and insulin byproducts raise concern for hyperinsulinism. These values confirm hyperinsulinemic hypoglycemia26:
- Glucose < 55 mg/dL
- Insulin ≥ 3 µU/mL
- C-peptide ≥ 0.2 nmol/L
- Proinsulin ≥ 5.0 pmol/L.
After hypoglycemia is confirmed, 1 mg of glucagon is given intravenously, and plasma glucose levels are obtained at 10, 20, and 30 minutes.11,26 A rise in plasma glucose of at least 25 mg/dL after intravenous glucagon injection indicates hypoglycemia due to hyperinsulinemia. Two-thirds of patients with insulinoma experience hypoglycemia within the first 24 hours, and nearly all experience hypoglycemia within 48 hours.26
Step 7: Obtain pancreatic imaging
If fasting hypoglycemia is present and hyperinsulinemic hypoglycemia is confirmed during a 72-hour fast, then pancreatic imaging should be obtained to evaluate for an insulinoma. We also recommend pancreatic imaging to rule out insulinoma when severe PGBH has not responded to dietary modification or pharmacotherapy.
Imaging is not recommended in PGBH that has been successfully treated with dietary modification with or without pharmacotherapy.
Endoscopic ultrasonography alone has 80% to 92% sensitivity for localizing a pancreatic mass as small as 5 mm. However, when coupled with computed tomography or magnetic resonance imaging, the sensitivity increases to nearly 100%.12
Step 8: Selective arterial calcium stimulation test
If a patient is found to have hyperinsulinemic hypoglycemia during a 72-hour fast but pancreatic imaging is negative, then selective arterial calcium stimulation testing (SACST) and hepatic vein sampling should be performed. Also, for severe PGBH, in which hypoglycemia has persisted despite dietary modification and pharmacotherapy, SACST can be performed to evaluate for possible localization of hyperinsulinism in patients considering surgery. For mild and moderate cases of PGBH, in which the hypoglycemia has been successfully treated with dietary changes with or without pharmacotherapy, SACST is not necessary.
This test can localize the area of excess insulin production in the pancreas in patients with an insulinoma. Patients with severe PGBH usually have diffuse hyperinsulinism without localization on SACST.32,33
When SACST is performed, a sampling catheter is placed in the femoral vein. Calcium gluconate is injected into the major arteries of the pancreas (superior mesenteric, gastroduodenal, and splenic arteries). Calcium stimulates release of insulin from an insulinoma or hyperplastic beta cells. Resultant insulin levels are measured in the hepatic vein. If there is a greater than twofold increase in insulin release from 2 segments, then the test is considered positive.
Thompson et al34 documented that insulin release from insulinoma is almost 4 times higher than in diffuse nesidioblastosis. SACST has a sensitivity of 96% for detecting insulinomas.35
Step 9: Other alternatives and surgery
In patients with severe PGBH for whom dietary modification and all pharmacotherapy have failed and who continue to have debilitating neuroglycopenia, there are options before proceeding with surgery, the last resort in this condition.
Continuous glucose monitoring is helpful in many patients with severe PGBH. Many of them have hypoglycemia unawareness, and the monitor alerts them when their blood sugar is low. In addition, the monitor indicates when the blood sugar is dropping, so that intervention can occur before hypoglycemia occurs.
Unfortunately, insurance coverage for continuous monitors in this patient population is limited. We argue that insurance should cover the cost for these severe cases.
Pasireotide, a somatostatin analogue that is longer-acting than octreotide, is approved for use in Cushing disease and acromegaly and actually causes hyperglycemia. In a case report of a 50-year-old woman, pasireotide resulted in less hypoglycemia and higher glucagon levels then octreotide.36 Pasireotide is available from Novartis for compassionate use in patients with severe PGBH.
Glucocorticoids are another off-label option. However, in excess, they can lead to iatrogenic Cushing syndrome, which has its own complications. Prednisone and diazoxide have been used together to help prevent hypoglycemia in a patients with inoperable insulinoma.31
Tube feeding. Some researchers have studied altering nutrition access through surgical means. McLaughlin et al37 discussed a case of gastric tube insertion into the remnant stomach of a patient with PGBH, with resolution of hypoglycemic symptoms and hypoglycemia; however, this does not always provide complete resolution of symptoms.37,38 If gastric bypass reversal is being considered, a trial of solely remnant stomach tube feeds (with no oral intake) should be pursued first. If this ameliorates the hypoglycemia, then gastric bypass reversal may be of benefit.
Surgery is the last resort if all of the above treatments have failed and severe debilitating neuroglycopenia persists. However, surgery poses risks, and the success rate in correcting hypoglycemia is not ideal. Surgical options include Roux-en-Y reversal, gastric pouch resection, and pancreatic resection.
In a review by Mala,2 75 patients with documented PGBH underwent surgical therapy. Hypoglycemic symptoms resolved in 34 of 51 pancreatic resections, 13 of 17 Roux-en-Y reversals, and 9 of 11 gastric pouch resections. However, the follow-up period was short.
As noted above, we recommend calcium stimulation testing only for severe cases of PGBH when surgery is being considered to evaluate for possible localization of hyperinsulinism for which partial pancreatectomy would be of benefit. Since there is no localization in many PGBH cases and the success rates are slightly higher in gastric bypass reversal, bypass reversal is usually preferred over partial or complete pancreatectomy.2,32,33
POTENTIAL FUTURE THERAPIES
Given the elevated GLP-1 levels and robust insulin response to glucose observed in PGBH, blocking GLP-1 may provide clinical benefit. Salehi et al16 found that a GLP-1 antagonist prevented surges in GLP-1 and reduced hypoglycemic episodes in patients with PGBH. Unfortunately, the medication they used was given as a continuous infusion and is not currently available.
Conversely, a GLP-1 agonist showed benefit in a series of 5 cases of PGBH.39 In addition, an insulin receptor antibody is undergoing phase 2 trials and has been shown to reverse insulin-induced hypoglycemia in rodents and humans; it may be a novel therapy in the future for hyperinsulinemic hypoglycemia.40
MORE STUDY NEEDED
As the prevalence of obesity continues to rise and more people opt for bariatric surgery for weight loss, we will likely continue to see an increase in PGBH, since the onset of PGBH can be delayed for many years after surgery.28
Unfortunately, the disease process involved in PGBH is not well understood. For example, we do not know why GLP-1 elevations or a robust insulin response causing hypoglycemia occurs in some but not all gastric bypass patients. Study is needed to elucidate the pathophysiology to further understand why most patients have no hypoglycemia after gastric bypass, some have mild to moderate PGBH, and a small percentage have severe PGBH with debilitating neuroglycopenia unresponsive to dietary changes and medications.
- Sarwar H, Chapman WH 3rd, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg 2014; 24:1120–1124.
- Mala T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg Obes Relat Dis 2014; 10:1220–1225.
- Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia 2010; 53:2307–2311.
- Lee CJ, Clark JM, Schweitzer M, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring) 2015; 23:1079–1084.
- Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis 2015; 11:564–569.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014; 311:806–814.
- Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet 2016; 387:1947–1956.
- Hunter Mehaffey J, Turrentine FE, Miller MS, Schirmer BD, Hallowell PT. Roux-en-Y gastric bypass 10-year follow-up: the found population. Surg Obes Relat Dis 2016; 12:778–782.
- Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg 2011; 213:261–266.
- DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 2010; 6:347–355.
- Service FJ. Hypoglycemic disorders. N Engl J Med 1995; 332:1144–1152.
- Mulla CM, Storino A, Yee EU, et al. Insulinoma after bariatric surgery: diagnostic dilemma and therapeutic approaches. Obes Surg 2016; 26:874–881.
- Malik S, Mitchell JE, Steffen K, et al. Recognition and management of hyperinsulinemic hypoglycemia after bariatric surgery. Obes Res Clin Pract 2016; 10:1–14.
- Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353:249–254.
- Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis 2008; 4:492–499.
- Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014; 146:669–680.e2.
- Cummings DE. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med 2005; 353:300–302.
- Rumilla KM, Erickson LA, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis: histologic features and growth factor expression. Mod Pathol 2009; 22:239–245.
- Anlauf M, Wieben D, Perren A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. Am J Surg Pathol 2005; 29:524–533.
- Zumkeller W. Nesidioblastosis. Endocr Relat Cancer 1999; 6:421–428.
- Klöppel G, Anlauf M, Raffel A, Perren A, Knoefel WT. Adult diffuse nesidioblastosis: genetically or environmentally induced? Hum Pathol 2008; 39:3–8.
- Bantle JP, Ikramuddin S, Kellogg TA, Buchwald H. Hyperinsulinemic hypoglycemia developing late after gastric bypass. Obes Surg 2007; 17:592–594.
- Hirose S, Iwahashi Y, Seo A, Sumiyoshi M, Takahashi T, Tamori Y. Concurrent therapy with a low-carbohydrate diet and miglitol remarkably improved the postprandial blood glucose and insulin levels in a patient with reactive hypoglycemia due to late dumping syndrome. Intern Med 2016; 55:1137–1142.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9:159–191.
- Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 2009; 6:583–590.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Lev-Ran A, Anderson RW. The diagnosis of postprandial hypoglycemia. Diabetes 1981; 30:996–999.
- Szollosi A, Nenquin M, Henquin JC. Pharmacological stimulation and inhibition of insulin secretion in mouse islets lacking ATP-sensitive K+ channels. Br J Pharmacol 2010; 159:669–677.
- Mordes JP, Alonso LC. Evaluation, medical therapy, and course of adult persistent hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass surgery: a case series. Endocr Pract 2015; 21:237–246.
- Myint KS, Greenfield JR, Farooqi IS, Henning E, Holst JJ, Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Eur J Endocrinol 2012; 166:951–955.
- Komatsu Y, Nakamura A, Takihata M, et al. Safety and tolerability of diazoxide in Japanese patients with hyperinsulinemic hypoglycemia. Endocr J 2016; 63:311–314.
- Z’graggen K, Guweidhi A, Steffen R, et al. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg 2008; 18:981–988.
- Mathavan VK, Arregui M, Davis C, Singh K, Patel A, Meacham J. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc 2010; 24:2547–2555.
- Thompson SM, Vella A, Thompson GB, et al. Selective arterial calcium stimulation with hepatic venous sampling differentiates insulinoma from nesidioblastosis. J Clin Endocrinol Metab 2015; 100:4189–4197.
- Wiesli P, Brändle M, Schmid C, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol 2004; 15:1251–1256.
- de Heide LJ, Laskewitz AJ, Apers JA. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg Obes Relat Dis 2014; 10:e31–e33.
- McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 2010; 95:1851–1855.
- Rao BB, Click B, Eid G, Codario RA. Management of refractory noninsulinoma pancreatogenous hypoglycemia syndrome with gastric bypass reversal: a case report and review of the literature. Case Rep Endocrinol 2015; 2015:384526.
- Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol 2013; 169:885–889.
- Corbin JA, Bhaskar B, Goldfine ID, et al. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody: a potential new approach for the treatment of hyperinsulinemic hypoglycemia. MAbs 2014; 6:262–272.
Bariatric surgery, though beneficial, is associated with complications, one of which is post-gastric bypass hypoglycemia (PGBH).1 The mean time from gastric bypass to documented hypoglycemia is about 28 months.2
PGBH is probably more common than initially thought. In older reports, the prevalence was only 0.1% to 0.36%.1,3 In contrast, in a mail survey in 2015,4 one-third of bariatric surgery patients reported symptoms that raised the suspicion of hypoglycemia. Those with suspicious symptoms were more likely to have undergone Roux-en-Y surgery, to have had no preoperative diabetes, to have had a longer interval since surgery, and to be female. Restricting the suspicion of postprandial hypoglycemia to those who reported more serious symptoms, including needing third-party assistance, the prevalence was 11.6%.
Kefurt et al5 followed Roux-en-Y patients who wore a continuous glucose monitor for 86 months after surgery and found that 38% had hypoglycemia; however, symptoms of hypoglycemia were not discussed.
Thus, the exact prevalence is currently unknown. But as time goes by and more procedures are performed, the incidence will likely rise.
OBESITY IS ON THE RISE, AND SO IS WEIGHT-LOSS SURGERY
Obesity is rampant, and its prevalence continues to rise. In 2011–2012, more than two-thirds of adults in the United States were reported as obese.6 Complications of obesity such as cardiac disease, diabetes, and cancer lead to increased mortality risk.7 Obesity is difficult to reverse, as many people fail to lose weight with diet, exercise, and pharmacotherapy.
Given the difficulty of losing weight and the complications that arise from obesity, bariatric surgery has become increasingly popular. Not only do patients lose significantly more weight with bariatric surgery than with conventional measures, but surgery also reduces and often cures conditions associated with obesity.8
Nguyen et al9 reported that 671,959 patients underwent gastric bypass procedures in the United States from 2003 to 2008. In a registry maintained by the American Society for Metabolic and Bariatric Surgery10 from June 2007 to May 2009, the most common bariatric procedure in the United States was Roux-en-Y gastric bypass, followed by sleeve gastrectomy.
DIFFERENTIAL DIAGNOSIS AND DEFINITIONS
The differential diagnosis for hyperinsulinemic hypoglycemia after gastric bypass surgery includes exogenous and endogenous causes (Table 1). Exogenous causes include abuse of insulin secretagogues such as sulfonylureas or meglitinides and abuse of insulin, which may occur in patients with Munchausen syndrome, Munchausen syndrome by proxy, or malingering. Endogenous causes include insulinoma, early and late dumping syndromes, and PGBH.
When differentiating endogenous from exogenous hypoglycemia, insulin and C-peptide levels are useful (Table 2). The pancreas produces proinsulin, which is broken down into insulin and C-peptide. Since exogenous insulin does not have a C-peptide component, people abusing insulin have elevated insulin levels with a low C-peptide level.11 Insulin secretagogues cause endogenous insulin secretion, resulting in elevated levels of both insulin and C-peptide. Thus, a screen for these medications is necessary to determine this as the cause.
Differentiating endogenous causes of hypoglycemia
Differentiating the endogenous causes (insulinoma, early or late dumping syndrome, and PGBH) can be challenging, as all 3 have similar biochemical profiles (Table 2).
Insulinoma is a tumor of pancreatic beta cells that produces excessive amounts of insulin. Unlike dumping syndrome, which only occurs postprandially, insulinoma primarily causes fasting hypoglycemia, although postprandial hypoglycemia can occur less commonly. Insulinoma after Roux-en-Y is rare. Only 7 cases have been reported.12
Dumping syndrome is classified as either early or late.
Early dumping syndrome usually occurs within 20 minutes of eating. The rapid transit of carbohydrates into the small intestine results in a fluid shift and a sympathetic response characterized by tachycardia, nausea, and diarrhea. Hypoglycemia is not present. Early dumping syndrome usually arises during the first few months after surgery.13
Late dumping syndrome usually occurs 1 to 4 hours after ingestion of a carbohydrate load, with symptoms of diaphoresis, dizziness, and fatigue caused by hypoglycemia from an excessive insulin release in response to the carbohydrates.13 It does not tend to cause neuroglycopenic symptoms.14 We define late dumping syndrome as postprandial hypoglycemic symptoms that occur after eating simple sugars and that resolve with dietary changes alone.
Differentiating late dumping syndrome from PGBH is difficult, as the line between the 2 processes is blurred.13
PGBH is defined as postprandial hypoglycemia (although it can be fasting in severe cases), often with neuroglycopenic symptoms, that occurs despite adherence to an acceptable bariatric diet (outlined in Table 3). We categorize PGBH as mild, moderate, or severe. Mild PGBH resolves with dietary changes with or without an alpha-glucosidase inhibitor. Moderate PGBH does not respond to an alpha-glucosidase inhibitor and dietary changes, and alternative or additional medication or medications are needed for resolution. Severe PGBH does not respond to dietary or medical interventions, and patients experience persistent episodes of neuroglycopenia.
THE EXACT MECHANISM IS UNCERTAIN
Patients with PGBH have a significant postprandial rise in glucose (often with levels > 200 mg/dL), leading to a robust insulin response and a subsequent drop in blood glucose.15
The exact mechanisms causing hypoglycemia are unknown, but excessive release of the incretin hormones glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) are thought to contribute. GLP-1 is primarily secreted in the gut in response to nutrients, causing a glucose-dependent release of insulin and suppression of glucagon, as well as a delay in gastric emptying and motility. Salehi et al16 demonstrated excessive GLP-1 and insulin release after glucose administration in postbypass patients, with a more exaggerated response in those experiencing postprandial hypoglycemia.
Excessive incretin hormones may also contribute to pancreatic islet cell hyperplasia, leading to hyperinsulinism.17 Other proposed mechanisms of PGBH are the lack of a decrease in beta cell mass after gastric bypass, a postoperative increase in insulin sensitivity, a decrease in ghrelin (an insulin counterregulatory hormone), and an abnormal glucagon response.13,17
Pathologic changes vary widely
PGBH is a challenging diagnosis to make pathologically. On review of pancreatic tissue from 36 patients undergoing partial pancreatectomy for PGBH, the pancreatic islet cells of the PGBH group were larger and more irregular compared with controls.18,19 This histologic condition with islet-cell hypertrophy, hyperplasia, and other changes has been termed nesidioblastosis.11,14,20 However, the pancreatic tissue appears grossly normal. The histopathologic findings can vary greatly in individual cases and in one-third of cases the pancreatic changes can be minimal, so that “normal” and PGBH cells can be nearly impossible to distinguish from each other.21
DIAGNOSIS AND TREATMENT
We recommend a stepwise approach to evaluating and treating PGBH (Figures 1 and 2).
Step 1: Evaluate blood glucose and Whipple triad
The first step is a thorough history, including food consumption and timing of hypoglycemic symptoms. Give the patient a glucometer to take home, with instructions to check blood glucose levels when hypoglycemic symptoms occur. The patient should keep a log documenting time tested, food consumed, symptoms, and blood glucose data.
Hypoglycemic symptoms are categorized as autonomic and neuroglycopenic. Autonomic symptoms include anxiety, palpitations, tremulousness, and diaphoresis. Neuroglycopenic symptoms include confusion, falls, seizures, and loss of consciousness.12
There are degrees of hypoglycemia and hypoglycemic symptoms. Clinical hypoglycemia—a blood glucose level low enough to cause signs or symptoms—can be confirmed by the Whipple triad:
- Measured low blood glucose
- Symptoms of low blood glucose
- Relief of symptoms when low blood glucose is corrected.
Hypoglycemic symptoms can occur when the blood glucose level falls to less than 55 mg/dL in healthy people, but this cutoff can shift lower in someone who has recurrent hypoglycemia.
When the Whipple triad is documented, rule out nonhyperinsulinemic causes of hypoglycemia such as hypothyroidism, adrenal insufficiency, underlying organ dysfunction (ie, liver disease), and medications that cause hypoglycemia.
Step 2: Modify the diet
If postprandial hypoglycemia is occurring, the next step is dietary modification. Two studies showed that a low-carbohydrate diet prevented hypoglycemia; however, these diets contained nearly no carbohydrates (with meals consisting of eggs, sausage, cheese, and black coffee or tea).15,22
Instruct patients to never eat pure carbohydrates without fat or protein, as this can result in a more severe hypoglycemic response.22 In addition, foods with a high glycemic index (a measure of how a carbohydrate-containing food raises blood sugar) should be avoided, and a low glycemic index diet is recommended.23 High glycemic index foods include white bread, bagels, pretzels, and pineapple. Low glycemic index foods include 100% stone-ground whole wheat or pumpernickel bread, lima beans, butter beans, peas, legumes, lentils, and nonstarchy vegetables.
Our bariatric surgeons provide all postbariatric surgery patients with the dietary guidelines shown in Table 3.24 We also ask our patients with PGBH to limit carbohydrates to 15 to 30 g per meal and to limit added sugars to less than 4 g per meal, including regular and sugar alcohols (polyols). Snacks should contain only protein and fat. In severe cases, we further limit the diet to 15 g of carbohydrate per meal, with no added sugars.
The hypoglycemia occurring with PGBH is treated differently than the hypoglycemia that occurs in diabetic patients. Advise patients with PGBH to treat their hypoglycemic episodes with a simple sugar combined with a protein or fat (eg, a small handful of candy with a spoonful of peanut butter), as they will often have recurrent hypoglycemia if a simple sugar is used alone. If patients regain weight, ask them about frequent eating, which would be related to self-treatment of hypoglycemia.
Step 3: Start an alpha-glucosidase inhibitor
If postprandial hypoglycemia persists despite dietary modification, then start an alpha-glucosidase inhibitor such as acarbose. Acarbose inhibits carbohydrate absorption, resulting in a decreased insulin response; thus, it blunts the decline in postprandial blood glucose.
Unfortunately, gastrointestinal side effects such as flatulence, diarrhea, and abdominal pain occur in up to 20% of patients who take acarbose, often leading to its discontinuation.25 To minimize gastrointestinal side effects, we usually start with 25 mg of acarbose with 1 meal daily for 1 week, then increase the dosage weekly to 25 mg with the other 2 meals. If tolerated, acarbose can be increased to 50 to 100 mg with 3 meals daily.
Step 4: Obtain a mixed meal tolerance test or a provocation meal test
If dietary changes and an alpha-glucosidase inhibitor do not prevent postprandial hypoglycemia from recurring, then confirmation of PGBH is needed, using a mixed meal tolerance test or a provocation meal test.
In a mixed meal tolerance test, the meal consists of 55% carbohydrate, 30% fat, and 15% protein. Patients with hyperinsulinemic hypoglycemia have a rapid rise in blood glucose (> 200 mg/dL) with a robust insulin response that is often followed by hypoglycemia after ingesting a meal containing carbohydrates in this test. Insulin levels that remain elevated after the plasma glucose level falls to less than 55 mg/dL indicate hyperinsulinism.11
Nevertheless, a mixed meal tolerance test will not always induce hypoglycemia. In a study of 51 patients with PGBH, all wore a continuous glucose monitor, were instructed to follow their normal diet for 5 days, and then underwent a mixed meal tolerance test on day 6. The glucose monitor revealed hypoglycemia in 75% of patients, while the mixed meal tolerance test was positive in only 29%.5
Moreover, to date, there is no standardized mixed meal.5,15 This might also explain the difference in prevalence of hypoglycemia detected by this test.
Based on these conflicting findings, we recommend a provocation meal test—ie, the patient is given foods that have induced hypoglycemia earlier.
Of note, the Endocrine Society guidelines on hypoglycemia state that an oral glucose tolerance test should never be used to document postprandial hypoglycemia.26 Lev-Ran and Anderson27 found that an oral glucose tolerance test could be positive in at least 10% of normal people.
Step 5: Consider other pharmacotherapy
For moderate to severe PGBH in which dietary modification and acarbose have failed, additional medical therapy is the next step. Medical therapies include calcium channel blockers, somatostatin analogues (eg, octreotide), and diazoxide.
Calcium channel blockers inhibit insulin release from beta cells28 but at the risk of hypotension. Mordes and Alonso29 treated 6 PGBH patients with nifedipine or verapamil with or without acarbose, and symptoms resolved in 5 of the 6 patients.
When we treat PGBH, we often add a calcium channel blocker as the next step in therapy if the patient has hypertension or if the blood pressure can tolerate this. If the patient’s blood pressure is low, then avoiding calcium channel blocker therapy may be necessary. The next step would be octreotide and then diazoxide.
Somatostatin analogues such as octreotide inhibit GLP-1 and insulin release.30 The most common side effects of octreotide are diarrhea and abdominal pain. Bile stone formation can also occur, but this is not common.
Diazoxide opens adenosine triphosphate-sensitive potassium channels and reduces the opening of calcium channels, inhibiting insulin release and raising blood glucose. In a study of 6 Japanese patients with inoperable insulinoma, diazoxide was used to treat hypoglycemia.31 Unfortunately, the doses required to control the low blood sugars also led to adverse reactions, most of which involved edema secondary to volume overload and other heart failure symptoms. Diazoxide also commonly causes hypotension and hirsutism.
Step 6: 72-hour fast
A 72-hour fast is recommended in severe cases of PGBH in patients for whom dietary modification and the additional pharmacotherapy outlined in step 5 have failed. A 72-hour fast is always indicated in evaluating confirmed fasting hypoglycemia. People with insulinoma usually have fasting hypoglycemia, while patients with dumping syndrome do not. Patients with PGBH usually do not have fasting hypoglycemia, but they can in severe cases.11
For safety, this test should be done in the hospital. Baseline plasma levels of insulin, C-peptide, proinsulin, beta-hydroxybutyrate, and glucose should be obtained. The patient then fasts, consuming only noncaloric and noncaffeinated beverages for 72 hours. During this time, capillary glucose checks are performed every 6 hours. If the capillary glucose level falls below 55 mg/dL,11,26 then the baseline tests are redrawn along with a sulfonylurea screen. To reduce costs and unnecessary testing, the tests are not sent for laboratory processing unless the plasma glucose is less than 55 mg/dL.
When the plasma glucose is less than 55 mg/dL, insulin production should cease. Elevated insulin levels and insulin byproducts raise concern for hyperinsulinism. These values confirm hyperinsulinemic hypoglycemia26:
- Glucose < 55 mg/dL
- Insulin ≥ 3 µU/mL
- C-peptide ≥ 0.2 nmol/L
- Proinsulin ≥ 5.0 pmol/L.
After hypoglycemia is confirmed, 1 mg of glucagon is given intravenously, and plasma glucose levels are obtained at 10, 20, and 30 minutes.11,26 A rise in plasma glucose of at least 25 mg/dL after intravenous glucagon injection indicates hypoglycemia due to hyperinsulinemia. Two-thirds of patients with insulinoma experience hypoglycemia within the first 24 hours, and nearly all experience hypoglycemia within 48 hours.26
Step 7: Obtain pancreatic imaging
If fasting hypoglycemia is present and hyperinsulinemic hypoglycemia is confirmed during a 72-hour fast, then pancreatic imaging should be obtained to evaluate for an insulinoma. We also recommend pancreatic imaging to rule out insulinoma when severe PGBH has not responded to dietary modification or pharmacotherapy.
Imaging is not recommended in PGBH that has been successfully treated with dietary modification with or without pharmacotherapy.
Endoscopic ultrasonography alone has 80% to 92% sensitivity for localizing a pancreatic mass as small as 5 mm. However, when coupled with computed tomography or magnetic resonance imaging, the sensitivity increases to nearly 100%.12
Step 8: Selective arterial calcium stimulation test
If a patient is found to have hyperinsulinemic hypoglycemia during a 72-hour fast but pancreatic imaging is negative, then selective arterial calcium stimulation testing (SACST) and hepatic vein sampling should be performed. Also, for severe PGBH, in which hypoglycemia has persisted despite dietary modification and pharmacotherapy, SACST can be performed to evaluate for possible localization of hyperinsulinism in patients considering surgery. For mild and moderate cases of PGBH, in which the hypoglycemia has been successfully treated with dietary changes with or without pharmacotherapy, SACST is not necessary.
This test can localize the area of excess insulin production in the pancreas in patients with an insulinoma. Patients with severe PGBH usually have diffuse hyperinsulinism without localization on SACST.32,33
When SACST is performed, a sampling catheter is placed in the femoral vein. Calcium gluconate is injected into the major arteries of the pancreas (superior mesenteric, gastroduodenal, and splenic arteries). Calcium stimulates release of insulin from an insulinoma or hyperplastic beta cells. Resultant insulin levels are measured in the hepatic vein. If there is a greater than twofold increase in insulin release from 2 segments, then the test is considered positive.
Thompson et al34 documented that insulin release from insulinoma is almost 4 times higher than in diffuse nesidioblastosis. SACST has a sensitivity of 96% for detecting insulinomas.35
Step 9: Other alternatives and surgery
In patients with severe PGBH for whom dietary modification and all pharmacotherapy have failed and who continue to have debilitating neuroglycopenia, there are options before proceeding with surgery, the last resort in this condition.
Continuous glucose monitoring is helpful in many patients with severe PGBH. Many of them have hypoglycemia unawareness, and the monitor alerts them when their blood sugar is low. In addition, the monitor indicates when the blood sugar is dropping, so that intervention can occur before hypoglycemia occurs.
Unfortunately, insurance coverage for continuous monitors in this patient population is limited. We argue that insurance should cover the cost for these severe cases.
Pasireotide, a somatostatin analogue that is longer-acting than octreotide, is approved for use in Cushing disease and acromegaly and actually causes hyperglycemia. In a case report of a 50-year-old woman, pasireotide resulted in less hypoglycemia and higher glucagon levels then octreotide.36 Pasireotide is available from Novartis for compassionate use in patients with severe PGBH.
Glucocorticoids are another off-label option. However, in excess, they can lead to iatrogenic Cushing syndrome, which has its own complications. Prednisone and diazoxide have been used together to help prevent hypoglycemia in a patients with inoperable insulinoma.31
Tube feeding. Some researchers have studied altering nutrition access through surgical means. McLaughlin et al37 discussed a case of gastric tube insertion into the remnant stomach of a patient with PGBH, with resolution of hypoglycemic symptoms and hypoglycemia; however, this does not always provide complete resolution of symptoms.37,38 If gastric bypass reversal is being considered, a trial of solely remnant stomach tube feeds (with no oral intake) should be pursued first. If this ameliorates the hypoglycemia, then gastric bypass reversal may be of benefit.
Surgery is the last resort if all of the above treatments have failed and severe debilitating neuroglycopenia persists. However, surgery poses risks, and the success rate in correcting hypoglycemia is not ideal. Surgical options include Roux-en-Y reversal, gastric pouch resection, and pancreatic resection.
In a review by Mala,2 75 patients with documented PGBH underwent surgical therapy. Hypoglycemic symptoms resolved in 34 of 51 pancreatic resections, 13 of 17 Roux-en-Y reversals, and 9 of 11 gastric pouch resections. However, the follow-up period was short.
As noted above, we recommend calcium stimulation testing only for severe cases of PGBH when surgery is being considered to evaluate for possible localization of hyperinsulinism for which partial pancreatectomy would be of benefit. Since there is no localization in many PGBH cases and the success rates are slightly higher in gastric bypass reversal, bypass reversal is usually preferred over partial or complete pancreatectomy.2,32,33
POTENTIAL FUTURE THERAPIES
Given the elevated GLP-1 levels and robust insulin response to glucose observed in PGBH, blocking GLP-1 may provide clinical benefit. Salehi et al16 found that a GLP-1 antagonist prevented surges in GLP-1 and reduced hypoglycemic episodes in patients with PGBH. Unfortunately, the medication they used was given as a continuous infusion and is not currently available.
Conversely, a GLP-1 agonist showed benefit in a series of 5 cases of PGBH.39 In addition, an insulin receptor antibody is undergoing phase 2 trials and has been shown to reverse insulin-induced hypoglycemia in rodents and humans; it may be a novel therapy in the future for hyperinsulinemic hypoglycemia.40
MORE STUDY NEEDED
As the prevalence of obesity continues to rise and more people opt for bariatric surgery for weight loss, we will likely continue to see an increase in PGBH, since the onset of PGBH can be delayed for many years after surgery.28
Unfortunately, the disease process involved in PGBH is not well understood. For example, we do not know why GLP-1 elevations or a robust insulin response causing hypoglycemia occurs in some but not all gastric bypass patients. Study is needed to elucidate the pathophysiology to further understand why most patients have no hypoglycemia after gastric bypass, some have mild to moderate PGBH, and a small percentage have severe PGBH with debilitating neuroglycopenia unresponsive to dietary changes and medications.
Bariatric surgery, though beneficial, is associated with complications, one of which is post-gastric bypass hypoglycemia (PGBH).1 The mean time from gastric bypass to documented hypoglycemia is about 28 months.2
PGBH is probably more common than initially thought. In older reports, the prevalence was only 0.1% to 0.36%.1,3 In contrast, in a mail survey in 2015,4 one-third of bariatric surgery patients reported symptoms that raised the suspicion of hypoglycemia. Those with suspicious symptoms were more likely to have undergone Roux-en-Y surgery, to have had no preoperative diabetes, to have had a longer interval since surgery, and to be female. Restricting the suspicion of postprandial hypoglycemia to those who reported more serious symptoms, including needing third-party assistance, the prevalence was 11.6%.
Kefurt et al5 followed Roux-en-Y patients who wore a continuous glucose monitor for 86 months after surgery and found that 38% had hypoglycemia; however, symptoms of hypoglycemia were not discussed.
Thus, the exact prevalence is currently unknown. But as time goes by and more procedures are performed, the incidence will likely rise.
OBESITY IS ON THE RISE, AND SO IS WEIGHT-LOSS SURGERY
Obesity is rampant, and its prevalence continues to rise. In 2011–2012, more than two-thirds of adults in the United States were reported as obese.6 Complications of obesity such as cardiac disease, diabetes, and cancer lead to increased mortality risk.7 Obesity is difficult to reverse, as many people fail to lose weight with diet, exercise, and pharmacotherapy.
Given the difficulty of losing weight and the complications that arise from obesity, bariatric surgery has become increasingly popular. Not only do patients lose significantly more weight with bariatric surgery than with conventional measures, but surgery also reduces and often cures conditions associated with obesity.8
Nguyen et al9 reported that 671,959 patients underwent gastric bypass procedures in the United States from 2003 to 2008. In a registry maintained by the American Society for Metabolic and Bariatric Surgery10 from June 2007 to May 2009, the most common bariatric procedure in the United States was Roux-en-Y gastric bypass, followed by sleeve gastrectomy.
DIFFERENTIAL DIAGNOSIS AND DEFINITIONS
The differential diagnosis for hyperinsulinemic hypoglycemia after gastric bypass surgery includes exogenous and endogenous causes (Table 1). Exogenous causes include abuse of insulin secretagogues such as sulfonylureas or meglitinides and abuse of insulin, which may occur in patients with Munchausen syndrome, Munchausen syndrome by proxy, or malingering. Endogenous causes include insulinoma, early and late dumping syndromes, and PGBH.
When differentiating endogenous from exogenous hypoglycemia, insulin and C-peptide levels are useful (Table 2). The pancreas produces proinsulin, which is broken down into insulin and C-peptide. Since exogenous insulin does not have a C-peptide component, people abusing insulin have elevated insulin levels with a low C-peptide level.11 Insulin secretagogues cause endogenous insulin secretion, resulting in elevated levels of both insulin and C-peptide. Thus, a screen for these medications is necessary to determine this as the cause.
Differentiating endogenous causes of hypoglycemia
Differentiating the endogenous causes (insulinoma, early or late dumping syndrome, and PGBH) can be challenging, as all 3 have similar biochemical profiles (Table 2).
Insulinoma is a tumor of pancreatic beta cells that produces excessive amounts of insulin. Unlike dumping syndrome, which only occurs postprandially, insulinoma primarily causes fasting hypoglycemia, although postprandial hypoglycemia can occur less commonly. Insulinoma after Roux-en-Y is rare. Only 7 cases have been reported.12
Dumping syndrome is classified as either early or late.
Early dumping syndrome usually occurs within 20 minutes of eating. The rapid transit of carbohydrates into the small intestine results in a fluid shift and a sympathetic response characterized by tachycardia, nausea, and diarrhea. Hypoglycemia is not present. Early dumping syndrome usually arises during the first few months after surgery.13
Late dumping syndrome usually occurs 1 to 4 hours after ingestion of a carbohydrate load, with symptoms of diaphoresis, dizziness, and fatigue caused by hypoglycemia from an excessive insulin release in response to the carbohydrates.13 It does not tend to cause neuroglycopenic symptoms.14 We define late dumping syndrome as postprandial hypoglycemic symptoms that occur after eating simple sugars and that resolve with dietary changes alone.
Differentiating late dumping syndrome from PGBH is difficult, as the line between the 2 processes is blurred.13
PGBH is defined as postprandial hypoglycemia (although it can be fasting in severe cases), often with neuroglycopenic symptoms, that occurs despite adherence to an acceptable bariatric diet (outlined in Table 3). We categorize PGBH as mild, moderate, or severe. Mild PGBH resolves with dietary changes with or without an alpha-glucosidase inhibitor. Moderate PGBH does not respond to an alpha-glucosidase inhibitor and dietary changes, and alternative or additional medication or medications are needed for resolution. Severe PGBH does not respond to dietary or medical interventions, and patients experience persistent episodes of neuroglycopenia.
THE EXACT MECHANISM IS UNCERTAIN
Patients with PGBH have a significant postprandial rise in glucose (often with levels > 200 mg/dL), leading to a robust insulin response and a subsequent drop in blood glucose.15
The exact mechanisms causing hypoglycemia are unknown, but excessive release of the incretin hormones glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) are thought to contribute. GLP-1 is primarily secreted in the gut in response to nutrients, causing a glucose-dependent release of insulin and suppression of glucagon, as well as a delay in gastric emptying and motility. Salehi et al16 demonstrated excessive GLP-1 and insulin release after glucose administration in postbypass patients, with a more exaggerated response in those experiencing postprandial hypoglycemia.
Excessive incretin hormones may also contribute to pancreatic islet cell hyperplasia, leading to hyperinsulinism.17 Other proposed mechanisms of PGBH are the lack of a decrease in beta cell mass after gastric bypass, a postoperative increase in insulin sensitivity, a decrease in ghrelin (an insulin counterregulatory hormone), and an abnormal glucagon response.13,17
Pathologic changes vary widely
PGBH is a challenging diagnosis to make pathologically. On review of pancreatic tissue from 36 patients undergoing partial pancreatectomy for PGBH, the pancreatic islet cells of the PGBH group were larger and more irregular compared with controls.18,19 This histologic condition with islet-cell hypertrophy, hyperplasia, and other changes has been termed nesidioblastosis.11,14,20 However, the pancreatic tissue appears grossly normal. The histopathologic findings can vary greatly in individual cases and in one-third of cases the pancreatic changes can be minimal, so that “normal” and PGBH cells can be nearly impossible to distinguish from each other.21
DIAGNOSIS AND TREATMENT
We recommend a stepwise approach to evaluating and treating PGBH (Figures 1 and 2).
Step 1: Evaluate blood glucose and Whipple triad
The first step is a thorough history, including food consumption and timing of hypoglycemic symptoms. Give the patient a glucometer to take home, with instructions to check blood glucose levels when hypoglycemic symptoms occur. The patient should keep a log documenting time tested, food consumed, symptoms, and blood glucose data.
Hypoglycemic symptoms are categorized as autonomic and neuroglycopenic. Autonomic symptoms include anxiety, palpitations, tremulousness, and diaphoresis. Neuroglycopenic symptoms include confusion, falls, seizures, and loss of consciousness.12
There are degrees of hypoglycemia and hypoglycemic symptoms. Clinical hypoglycemia—a blood glucose level low enough to cause signs or symptoms—can be confirmed by the Whipple triad:
- Measured low blood glucose
- Symptoms of low blood glucose
- Relief of symptoms when low blood glucose is corrected.
Hypoglycemic symptoms can occur when the blood glucose level falls to less than 55 mg/dL in healthy people, but this cutoff can shift lower in someone who has recurrent hypoglycemia.
When the Whipple triad is documented, rule out nonhyperinsulinemic causes of hypoglycemia such as hypothyroidism, adrenal insufficiency, underlying organ dysfunction (ie, liver disease), and medications that cause hypoglycemia.
Step 2: Modify the diet
If postprandial hypoglycemia is occurring, the next step is dietary modification. Two studies showed that a low-carbohydrate diet prevented hypoglycemia; however, these diets contained nearly no carbohydrates (with meals consisting of eggs, sausage, cheese, and black coffee or tea).15,22
Instruct patients to never eat pure carbohydrates without fat or protein, as this can result in a more severe hypoglycemic response.22 In addition, foods with a high glycemic index (a measure of how a carbohydrate-containing food raises blood sugar) should be avoided, and a low glycemic index diet is recommended.23 High glycemic index foods include white bread, bagels, pretzels, and pineapple. Low glycemic index foods include 100% stone-ground whole wheat or pumpernickel bread, lima beans, butter beans, peas, legumes, lentils, and nonstarchy vegetables.
Our bariatric surgeons provide all postbariatric surgery patients with the dietary guidelines shown in Table 3.24 We also ask our patients with PGBH to limit carbohydrates to 15 to 30 g per meal and to limit added sugars to less than 4 g per meal, including regular and sugar alcohols (polyols). Snacks should contain only protein and fat. In severe cases, we further limit the diet to 15 g of carbohydrate per meal, with no added sugars.
The hypoglycemia occurring with PGBH is treated differently than the hypoglycemia that occurs in diabetic patients. Advise patients with PGBH to treat their hypoglycemic episodes with a simple sugar combined with a protein or fat (eg, a small handful of candy with a spoonful of peanut butter), as they will often have recurrent hypoglycemia if a simple sugar is used alone. If patients regain weight, ask them about frequent eating, which would be related to self-treatment of hypoglycemia.
Step 3: Start an alpha-glucosidase inhibitor
If postprandial hypoglycemia persists despite dietary modification, then start an alpha-glucosidase inhibitor such as acarbose. Acarbose inhibits carbohydrate absorption, resulting in a decreased insulin response; thus, it blunts the decline in postprandial blood glucose.
Unfortunately, gastrointestinal side effects such as flatulence, diarrhea, and abdominal pain occur in up to 20% of patients who take acarbose, often leading to its discontinuation.25 To minimize gastrointestinal side effects, we usually start with 25 mg of acarbose with 1 meal daily for 1 week, then increase the dosage weekly to 25 mg with the other 2 meals. If tolerated, acarbose can be increased to 50 to 100 mg with 3 meals daily.
Step 4: Obtain a mixed meal tolerance test or a provocation meal test
If dietary changes and an alpha-glucosidase inhibitor do not prevent postprandial hypoglycemia from recurring, then confirmation of PGBH is needed, using a mixed meal tolerance test or a provocation meal test.
In a mixed meal tolerance test, the meal consists of 55% carbohydrate, 30% fat, and 15% protein. Patients with hyperinsulinemic hypoglycemia have a rapid rise in blood glucose (> 200 mg/dL) with a robust insulin response that is often followed by hypoglycemia after ingesting a meal containing carbohydrates in this test. Insulin levels that remain elevated after the plasma glucose level falls to less than 55 mg/dL indicate hyperinsulinism.11
Nevertheless, a mixed meal tolerance test will not always induce hypoglycemia. In a study of 51 patients with PGBH, all wore a continuous glucose monitor, were instructed to follow their normal diet for 5 days, and then underwent a mixed meal tolerance test on day 6. The glucose monitor revealed hypoglycemia in 75% of patients, while the mixed meal tolerance test was positive in only 29%.5
Moreover, to date, there is no standardized mixed meal.5,15 This might also explain the difference in prevalence of hypoglycemia detected by this test.
Based on these conflicting findings, we recommend a provocation meal test—ie, the patient is given foods that have induced hypoglycemia earlier.
Of note, the Endocrine Society guidelines on hypoglycemia state that an oral glucose tolerance test should never be used to document postprandial hypoglycemia.26 Lev-Ran and Anderson27 found that an oral glucose tolerance test could be positive in at least 10% of normal people.
Step 5: Consider other pharmacotherapy
For moderate to severe PGBH in which dietary modification and acarbose have failed, additional medical therapy is the next step. Medical therapies include calcium channel blockers, somatostatin analogues (eg, octreotide), and diazoxide.
Calcium channel blockers inhibit insulin release from beta cells28 but at the risk of hypotension. Mordes and Alonso29 treated 6 PGBH patients with nifedipine or verapamil with or without acarbose, and symptoms resolved in 5 of the 6 patients.
When we treat PGBH, we often add a calcium channel blocker as the next step in therapy if the patient has hypertension or if the blood pressure can tolerate this. If the patient’s blood pressure is low, then avoiding calcium channel blocker therapy may be necessary. The next step would be octreotide and then diazoxide.
Somatostatin analogues such as octreotide inhibit GLP-1 and insulin release.30 The most common side effects of octreotide are diarrhea and abdominal pain. Bile stone formation can also occur, but this is not common.
Diazoxide opens adenosine triphosphate-sensitive potassium channels and reduces the opening of calcium channels, inhibiting insulin release and raising blood glucose. In a study of 6 Japanese patients with inoperable insulinoma, diazoxide was used to treat hypoglycemia.31 Unfortunately, the doses required to control the low blood sugars also led to adverse reactions, most of which involved edema secondary to volume overload and other heart failure symptoms. Diazoxide also commonly causes hypotension and hirsutism.
Step 6: 72-hour fast
A 72-hour fast is recommended in severe cases of PGBH in patients for whom dietary modification and the additional pharmacotherapy outlined in step 5 have failed. A 72-hour fast is always indicated in evaluating confirmed fasting hypoglycemia. People with insulinoma usually have fasting hypoglycemia, while patients with dumping syndrome do not. Patients with PGBH usually do not have fasting hypoglycemia, but they can in severe cases.11
For safety, this test should be done in the hospital. Baseline plasma levels of insulin, C-peptide, proinsulin, beta-hydroxybutyrate, and glucose should be obtained. The patient then fasts, consuming only noncaloric and noncaffeinated beverages for 72 hours. During this time, capillary glucose checks are performed every 6 hours. If the capillary glucose level falls below 55 mg/dL,11,26 then the baseline tests are redrawn along with a sulfonylurea screen. To reduce costs and unnecessary testing, the tests are not sent for laboratory processing unless the plasma glucose is less than 55 mg/dL.
When the plasma glucose is less than 55 mg/dL, insulin production should cease. Elevated insulin levels and insulin byproducts raise concern for hyperinsulinism. These values confirm hyperinsulinemic hypoglycemia26:
- Glucose < 55 mg/dL
- Insulin ≥ 3 µU/mL
- C-peptide ≥ 0.2 nmol/L
- Proinsulin ≥ 5.0 pmol/L.
After hypoglycemia is confirmed, 1 mg of glucagon is given intravenously, and plasma glucose levels are obtained at 10, 20, and 30 minutes.11,26 A rise in plasma glucose of at least 25 mg/dL after intravenous glucagon injection indicates hypoglycemia due to hyperinsulinemia. Two-thirds of patients with insulinoma experience hypoglycemia within the first 24 hours, and nearly all experience hypoglycemia within 48 hours.26
Step 7: Obtain pancreatic imaging
If fasting hypoglycemia is present and hyperinsulinemic hypoglycemia is confirmed during a 72-hour fast, then pancreatic imaging should be obtained to evaluate for an insulinoma. We also recommend pancreatic imaging to rule out insulinoma when severe PGBH has not responded to dietary modification or pharmacotherapy.
Imaging is not recommended in PGBH that has been successfully treated with dietary modification with or without pharmacotherapy.
Endoscopic ultrasonography alone has 80% to 92% sensitivity for localizing a pancreatic mass as small as 5 mm. However, when coupled with computed tomography or magnetic resonance imaging, the sensitivity increases to nearly 100%.12
Step 8: Selective arterial calcium stimulation test
If a patient is found to have hyperinsulinemic hypoglycemia during a 72-hour fast but pancreatic imaging is negative, then selective arterial calcium stimulation testing (SACST) and hepatic vein sampling should be performed. Also, for severe PGBH, in which hypoglycemia has persisted despite dietary modification and pharmacotherapy, SACST can be performed to evaluate for possible localization of hyperinsulinism in patients considering surgery. For mild and moderate cases of PGBH, in which the hypoglycemia has been successfully treated with dietary changes with or without pharmacotherapy, SACST is not necessary.
This test can localize the area of excess insulin production in the pancreas in patients with an insulinoma. Patients with severe PGBH usually have diffuse hyperinsulinism without localization on SACST.32,33
When SACST is performed, a sampling catheter is placed in the femoral vein. Calcium gluconate is injected into the major arteries of the pancreas (superior mesenteric, gastroduodenal, and splenic arteries). Calcium stimulates release of insulin from an insulinoma or hyperplastic beta cells. Resultant insulin levels are measured in the hepatic vein. If there is a greater than twofold increase in insulin release from 2 segments, then the test is considered positive.
Thompson et al34 documented that insulin release from insulinoma is almost 4 times higher than in diffuse nesidioblastosis. SACST has a sensitivity of 96% for detecting insulinomas.35
Step 9: Other alternatives and surgery
In patients with severe PGBH for whom dietary modification and all pharmacotherapy have failed and who continue to have debilitating neuroglycopenia, there are options before proceeding with surgery, the last resort in this condition.
Continuous glucose monitoring is helpful in many patients with severe PGBH. Many of them have hypoglycemia unawareness, and the monitor alerts them when their blood sugar is low. In addition, the monitor indicates when the blood sugar is dropping, so that intervention can occur before hypoglycemia occurs.
Unfortunately, insurance coverage for continuous monitors in this patient population is limited. We argue that insurance should cover the cost for these severe cases.
Pasireotide, a somatostatin analogue that is longer-acting than octreotide, is approved for use in Cushing disease and acromegaly and actually causes hyperglycemia. In a case report of a 50-year-old woman, pasireotide resulted in less hypoglycemia and higher glucagon levels then octreotide.36 Pasireotide is available from Novartis for compassionate use in patients with severe PGBH.
Glucocorticoids are another off-label option. However, in excess, they can lead to iatrogenic Cushing syndrome, which has its own complications. Prednisone and diazoxide have been used together to help prevent hypoglycemia in a patients with inoperable insulinoma.31
Tube feeding. Some researchers have studied altering nutrition access through surgical means. McLaughlin et al37 discussed a case of gastric tube insertion into the remnant stomach of a patient with PGBH, with resolution of hypoglycemic symptoms and hypoglycemia; however, this does not always provide complete resolution of symptoms.37,38 If gastric bypass reversal is being considered, a trial of solely remnant stomach tube feeds (with no oral intake) should be pursued first. If this ameliorates the hypoglycemia, then gastric bypass reversal may be of benefit.
Surgery is the last resort if all of the above treatments have failed and severe debilitating neuroglycopenia persists. However, surgery poses risks, and the success rate in correcting hypoglycemia is not ideal. Surgical options include Roux-en-Y reversal, gastric pouch resection, and pancreatic resection.
In a review by Mala,2 75 patients with documented PGBH underwent surgical therapy. Hypoglycemic symptoms resolved in 34 of 51 pancreatic resections, 13 of 17 Roux-en-Y reversals, and 9 of 11 gastric pouch resections. However, the follow-up period was short.
As noted above, we recommend calcium stimulation testing only for severe cases of PGBH when surgery is being considered to evaluate for possible localization of hyperinsulinism for which partial pancreatectomy would be of benefit. Since there is no localization in many PGBH cases and the success rates are slightly higher in gastric bypass reversal, bypass reversal is usually preferred over partial or complete pancreatectomy.2,32,33
POTENTIAL FUTURE THERAPIES
Given the elevated GLP-1 levels and robust insulin response to glucose observed in PGBH, blocking GLP-1 may provide clinical benefit. Salehi et al16 found that a GLP-1 antagonist prevented surges in GLP-1 and reduced hypoglycemic episodes in patients with PGBH. Unfortunately, the medication they used was given as a continuous infusion and is not currently available.
Conversely, a GLP-1 agonist showed benefit in a series of 5 cases of PGBH.39 In addition, an insulin receptor antibody is undergoing phase 2 trials and has been shown to reverse insulin-induced hypoglycemia in rodents and humans; it may be a novel therapy in the future for hyperinsulinemic hypoglycemia.40
MORE STUDY NEEDED
As the prevalence of obesity continues to rise and more people opt for bariatric surgery for weight loss, we will likely continue to see an increase in PGBH, since the onset of PGBH can be delayed for many years after surgery.28
Unfortunately, the disease process involved in PGBH is not well understood. For example, we do not know why GLP-1 elevations or a robust insulin response causing hypoglycemia occurs in some but not all gastric bypass patients. Study is needed to elucidate the pathophysiology to further understand why most patients have no hypoglycemia after gastric bypass, some have mild to moderate PGBH, and a small percentage have severe PGBH with debilitating neuroglycopenia unresponsive to dietary changes and medications.
- Sarwar H, Chapman WH 3rd, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg 2014; 24:1120–1124.
- Mala T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg Obes Relat Dis 2014; 10:1220–1225.
- Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia 2010; 53:2307–2311.
- Lee CJ, Clark JM, Schweitzer M, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring) 2015; 23:1079–1084.
- Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis 2015; 11:564–569.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014; 311:806–814.
- Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet 2016; 387:1947–1956.
- Hunter Mehaffey J, Turrentine FE, Miller MS, Schirmer BD, Hallowell PT. Roux-en-Y gastric bypass 10-year follow-up: the found population. Surg Obes Relat Dis 2016; 12:778–782.
- Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg 2011; 213:261–266.
- DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 2010; 6:347–355.
- Service FJ. Hypoglycemic disorders. N Engl J Med 1995; 332:1144–1152.
- Mulla CM, Storino A, Yee EU, et al. Insulinoma after bariatric surgery: diagnostic dilemma and therapeutic approaches. Obes Surg 2016; 26:874–881.
- Malik S, Mitchell JE, Steffen K, et al. Recognition and management of hyperinsulinemic hypoglycemia after bariatric surgery. Obes Res Clin Pract 2016; 10:1–14.
- Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353:249–254.
- Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis 2008; 4:492–499.
- Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014; 146:669–680.e2.
- Cummings DE. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med 2005; 353:300–302.
- Rumilla KM, Erickson LA, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis: histologic features and growth factor expression. Mod Pathol 2009; 22:239–245.
- Anlauf M, Wieben D, Perren A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. Am J Surg Pathol 2005; 29:524–533.
- Zumkeller W. Nesidioblastosis. Endocr Relat Cancer 1999; 6:421–428.
- Klöppel G, Anlauf M, Raffel A, Perren A, Knoefel WT. Adult diffuse nesidioblastosis: genetically or environmentally induced? Hum Pathol 2008; 39:3–8.
- Bantle JP, Ikramuddin S, Kellogg TA, Buchwald H. Hyperinsulinemic hypoglycemia developing late after gastric bypass. Obes Surg 2007; 17:592–594.
- Hirose S, Iwahashi Y, Seo A, Sumiyoshi M, Takahashi T, Tamori Y. Concurrent therapy with a low-carbohydrate diet and miglitol remarkably improved the postprandial blood glucose and insulin levels in a patient with reactive hypoglycemia due to late dumping syndrome. Intern Med 2016; 55:1137–1142.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9:159–191.
- Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 2009; 6:583–590.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Lev-Ran A, Anderson RW. The diagnosis of postprandial hypoglycemia. Diabetes 1981; 30:996–999.
- Szollosi A, Nenquin M, Henquin JC. Pharmacological stimulation and inhibition of insulin secretion in mouse islets lacking ATP-sensitive K+ channels. Br J Pharmacol 2010; 159:669–677.
- Mordes JP, Alonso LC. Evaluation, medical therapy, and course of adult persistent hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass surgery: a case series. Endocr Pract 2015; 21:237–246.
- Myint KS, Greenfield JR, Farooqi IS, Henning E, Holst JJ, Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Eur J Endocrinol 2012; 166:951–955.
- Komatsu Y, Nakamura A, Takihata M, et al. Safety and tolerability of diazoxide in Japanese patients with hyperinsulinemic hypoglycemia. Endocr J 2016; 63:311–314.
- Z’graggen K, Guweidhi A, Steffen R, et al. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg 2008; 18:981–988.
- Mathavan VK, Arregui M, Davis C, Singh K, Patel A, Meacham J. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc 2010; 24:2547–2555.
- Thompson SM, Vella A, Thompson GB, et al. Selective arterial calcium stimulation with hepatic venous sampling differentiates insulinoma from nesidioblastosis. J Clin Endocrinol Metab 2015; 100:4189–4197.
- Wiesli P, Brändle M, Schmid C, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol 2004; 15:1251–1256.
- de Heide LJ, Laskewitz AJ, Apers JA. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg Obes Relat Dis 2014; 10:e31–e33.
- McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 2010; 95:1851–1855.
- Rao BB, Click B, Eid G, Codario RA. Management of refractory noninsulinoma pancreatogenous hypoglycemia syndrome with gastric bypass reversal: a case report and review of the literature. Case Rep Endocrinol 2015; 2015:384526.
- Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol 2013; 169:885–889.
- Corbin JA, Bhaskar B, Goldfine ID, et al. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody: a potential new approach for the treatment of hyperinsulinemic hypoglycemia. MAbs 2014; 6:262–272.
- Sarwar H, Chapman WH 3rd, Pender JR, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes Surg 2014; 24:1120–1124.
- Mala T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg Obes Relat Dis 2014; 10:1220–1225.
- Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia 2010; 53:2307–2311.
- Lee CJ, Clark JM, Schweitzer M, et al. Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring) 2015; 23:1079–1084.
- Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis 2015; 11:564–569.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014; 311:806–814.
- Bray GA, Frühbeck G, Ryan DH, Wilding JPH. Management of obesity. Lancet 2016; 387:1947–1956.
- Hunter Mehaffey J, Turrentine FE, Miller MS, Schirmer BD, Hallowell PT. Roux-en-Y gastric bypass 10-year follow-up: the found population. Surg Obes Relat Dis 2016; 12:778–782.
- Nguyen NT, Masoomi H, Magno CP, Nguyen XM, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg 2011; 213:261–266.
- DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis 2010; 6:347–355.
- Service FJ. Hypoglycemic disorders. N Engl J Med 1995; 332:1144–1152.
- Mulla CM, Storino A, Yee EU, et al. Insulinoma after bariatric surgery: diagnostic dilemma and therapeutic approaches. Obes Surg 2016; 26:874–881.
- Malik S, Mitchell JE, Steffen K, et al. Recognition and management of hyperinsulinemic hypoglycemia after bariatric surgery. Obes Res Clin Pract 2016; 10:1–14.
- Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353:249–254.
- Kellogg TA, Bantle JP, Leslie DB, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis 2008; 4:492–499.
- Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014; 146:669–680.e2.
- Cummings DE. Gastric bypass and nesidioblastosis—too much of a good thing for islets? N Engl J Med 2005; 353:300–302.
- Rumilla KM, Erickson LA, Service FJ, et al. Hyperinsulinemic hypoglycemia with nesidioblastosis: histologic features and growth factor expression. Mod Pathol 2009; 22:239–245.
- Anlauf M, Wieben D, Perren A, et al. Persistent hyperinsulinemic hypoglycemia in 15 adults with diffuse nesidioblastosis: diagnostic criteria, incidence, and characterization of beta-cell changes. Am J Surg Pathol 2005; 29:524–533.
- Zumkeller W. Nesidioblastosis. Endocr Relat Cancer 1999; 6:421–428.
- Klöppel G, Anlauf M, Raffel A, Perren A, Knoefel WT. Adult diffuse nesidioblastosis: genetically or environmentally induced? Hum Pathol 2008; 39:3–8.
- Bantle JP, Ikramuddin S, Kellogg TA, Buchwald H. Hyperinsulinemic hypoglycemia developing late after gastric bypass. Obes Surg 2007; 17:592–594.
- Hirose S, Iwahashi Y, Seo A, Sumiyoshi M, Takahashi T, Tamori Y. Concurrent therapy with a low-carbohydrate diet and miglitol remarkably improved the postprandial blood glucose and insulin levels in a patient with reactive hypoglycemia due to late dumping syndrome. Intern Med 2016; 55:1137–1142.
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis 2013; 9:159–191.
- Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol 2009; 6:583–590.
- Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Lev-Ran A, Anderson RW. The diagnosis of postprandial hypoglycemia. Diabetes 1981; 30:996–999.
- Szollosi A, Nenquin M, Henquin JC. Pharmacological stimulation and inhibition of insulin secretion in mouse islets lacking ATP-sensitive K+ channels. Br J Pharmacol 2010; 159:669–677.
- Mordes JP, Alonso LC. Evaluation, medical therapy, and course of adult persistent hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass surgery: a case series. Endocr Pract 2015; 21:237–246.
- Myint KS, Greenfield JR, Farooqi IS, Henning E, Holst JJ, Finer N. Prolonged successful therapy for hyperinsulinaemic hypoglycaemia after gastric bypass: the pathophysiological role of GLP1 and its response to a somatostatin analogue. Eur J Endocrinol 2012; 166:951–955.
- Komatsu Y, Nakamura A, Takihata M, et al. Safety and tolerability of diazoxide in Japanese patients with hyperinsulinemic hypoglycemia. Endocr J 2016; 63:311–314.
- Z’graggen K, Guweidhi A, Steffen R, et al. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg 2008; 18:981–988.
- Mathavan VK, Arregui M, Davis C, Singh K, Patel A, Meacham J. Management of postgastric bypass noninsulinoma pancreatogenous hypoglycemia. Surg Endosc 2010; 24:2547–2555.
- Thompson SM, Vella A, Thompson GB, et al. Selective arterial calcium stimulation with hepatic venous sampling differentiates insulinoma from nesidioblastosis. J Clin Endocrinol Metab 2015; 100:4189–4197.
- Wiesli P, Brändle M, Schmid C, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol 2004; 15:1251–1256.
- de Heide LJ, Laskewitz AJ, Apers JA. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg Obes Relat Dis 2014; 10:e31–e33.
- McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 2010; 95:1851–1855.
- Rao BB, Click B, Eid G, Codario RA. Management of refractory noninsulinoma pancreatogenous hypoglycemia syndrome with gastric bypass reversal: a case report and review of the literature. Case Rep Endocrinol 2015; 2015:384526.
- Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. GLP1 analogs as treatment of postprandial hypoglycemia following gastric bypass surgery: a potential new indication? Eur J Endocrinol 2013; 169:885–889.
- Corbin JA, Bhaskar B, Goldfine ID, et al. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody: a potential new approach for the treatment of hyperinsulinemic hypoglycemia. MAbs 2014; 6:262–272.
KEY POINTS
- The differential diagnosis for endogenous causes of hyperinsulinemic hypoglycemia after gastric bypass surgery includes insulinoma, late dumping syndrome, and post-gastric bypass hypoglycemia (PGBH).
- The Whipple triad consists of measured low blood glucose, symptoms of low blood glucose, and reversal of symptoms when low blood glucose is corrected. If the triad is not present, then hypoglycemia is not causing the patient’s symptoms.
- PGBH should initially be treated with a high-protein, high-fiber, low-carbohydrate diet and then, if hypoglycemia persists, by medication (initially acarbose, then a calcium channel blocker and octreotide or diazoxide or both).
- PGBH ranges from mild, in which neuroglycopenia resolves with dietary changes with or without acarbose, to severe, in which neuroglycopenia persists despite dietary changes and multiple drugs.
- Gastric bypass reversal and pancreatic surgery are a last resort for patients with debilitating neuroglycopenia when dietary modification and drug therapy fail.