User login
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
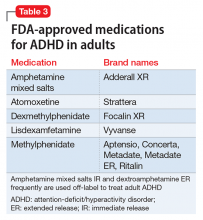
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.