User login
Soft Tissue Sarcoma: Diagnosis and Treatment
Introduction
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
Epidemiology and Classification
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
Clinical Evaluation
› Case Presentation
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (
• What is the typical presentation for sarcomas?
A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
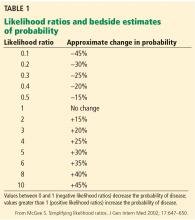
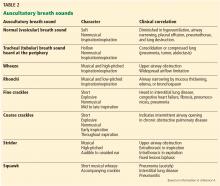
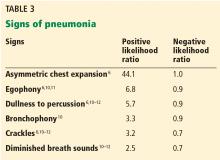
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”
Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
Management
› Case Continued
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
Surgery
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
› Case Continued
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
Radiation THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
› Case Continued
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
Chemotherapy
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study.35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
› Case Continued
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dysp-nea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
› Case Continued
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51 A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
› Case Continued
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
Conclusion
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma. TSJ
CORRESPONDENCE
Ashley Pariser, MD, Resident, Department of Medicine, Northwestern University Feinberg School of Medicine Chicago, IL. Accepted for publication Jan/Feb 2017; Hosp Phys; Vol. 12, Part1
References
1. American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
2. National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
3. Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
4. Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4: 252–66.
5. Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
6. Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
7. Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
8. Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
9. Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.

10. Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
11. Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
13. Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
14. Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
15. Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
16. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
17. Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
18. Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
19. Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
20. Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
21. Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
22. Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
23. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
24. Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
25. Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
26. Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
27. Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
28. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
29. Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
30. Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
31. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
32. Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
33. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
34. Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
35. Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
36. Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
37. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
38. Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.

39. Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
40. Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
41. Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
42. Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
43. Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
44. Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
45. Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
46. Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
47. Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
48. Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
49. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
50. Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
51. Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
52. Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
53. Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
54. Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
55. Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
56. Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
57. Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
58. Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
59. Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
Introduction
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
Epidemiology and Classification
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
Clinical Evaluation
› Case Presentation
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (
• What is the typical presentation for sarcomas?
A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”
Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
Management
› Case Continued
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
Surgery
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
› Case Continued
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
Radiation THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
› Case Continued
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
Chemotherapy
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study.35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
› Case Continued
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dysp-nea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
› Case Continued
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51 A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
› Case Continued
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
Conclusion
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma. TSJ
CORRESPONDENCE
Ashley Pariser, MD, Resident, Department of Medicine, Northwestern University Feinberg School of Medicine Chicago, IL. Accepted for publication Jan/Feb 2017; Hosp Phys; Vol. 12, Part1
Introduction
Soft tissue sarcomas (STSs) are rare adult tumors, with 3.4 new cases per 100,000 persons or 12,310 expected new cases in 2016.1 Sarcomas are a heterogeneous collection of tumors that affect fat, muscle, nerve, nerve sheath, vascular, and connective tissues. There are more than 50 histological subtypes that comprise this diverse category of tumors. Treatment varies by stage, with limb-sparing surgery representing the mainstay of curative-intent treatment. Radiation and chemotherapy may also be considered depending on the size, grade, and location of the tumor. Survival rates have been stagnant until recently, with a disease-specific survival hovering around 65%.1 Given the complexity of these cases, all patients ideally should be evaluated and treated by a multidisciplinary team at an institution with extensive experience treating STS.2
Epidemiology and Classification
The most common STS subtypes are gastrointestinal stromal tumor (GIST), undifferentiate pleomorphic sarcoma (previously referred to as malignant fibrous histiocytoma), liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, and unclassified sarcoma.3 Liposarcoma is one of the most common subtypes, comprising 20% of all STSs; it is subdivided into well-differentiated/dedifferentiated liposarcomas, myxoid/round cell liposarcomas, and pleomorphic liposarcomas. Well-differentiated liposarcomas tend to occur in the retroperitoneum and limbs, while both myxoid and round cell as well as pleomorphic liposarcomas more commonly originate on the limbs. Histology varies based on subtype and ranges from mature-appearing adipocytes and fibroblasts to undifferentiated cells with minimal lipogenic differentiation.4
Leiomyosarcomas are smooth muscle tumors and are usually located in the retroperitoneum, but have also been associated with peripheral soft tissue and vasculature. Typical histology ranges from well-defined areas of spindle-shaped cells to poorly differentiated anaplastic spindle cells.5,6 Synovial sarcomas are a distinct type of STS that can show epithelial differentiation and account for 5% of adult STSs. The extremities are the most common presenting location (90%).7
Rhabdomyosarcomas are skeletal muscle tumors and are further subdivided into embryonal, alveolar, and pleomorphic subtypes. Embryonal histology ranges from primitive mesenchymal-appearing cells to highly differentiated muscle cells. Alveolar rhabdomyosarcoma has the worst prognosis of the subtypes and consists of round cells with high nuclear-to-chromatin ratios that form “glandular-like” or “alveolar” spaces.8 Pleomorphic rhabdomyosarcomas are composed of rhabdomyoblasts that can affect many different locations, but most commonly present on the lower extremities.9
Malignant peripheral nerve sheath tumor (MPNST) comprises 5% to 10% of all STSs. These tumors are associated with neurofibromatosis type 1 (NF-1), with 25% to 50% of tumors occurring in NF-1 patients. Additionally, most patients have a truncating lesion in the NF1 gene on chromosome 17.10 Anghileri et al in their single institution analysis of 205 patients with MPNSTs found the 2 most common presenting sites were the trunk and extremities. Histologically, these tumors have dense fascicles of spindle cells.10
GISTs are the most common STS of the gastrointestinal (GI) tract. Previously, GISTs were classified as smooth muscle tumors and were not accounted for in the literature as a separate entity distinct from leiomyomas, leiomyoblastomas, and leiomyosarcomas.11 GISTs are found throughout the GI tract: the most common sites are the stomach (60%) and small intestine (30%). Less common sites include duodenum (4%–5%), esophagus (1%), rectum (1%–2%), and appendix (< 0.2%).12 GISTs can be spindle cell, epithelioid, or mesenchymal tumors. Immunohistochemically, GISTs are KIT (CD117) positive. Other cell markers that are also commonly positive include CD34 (60%–70%) and smooth muscle actin (SMA) (25%).11 The majority of GISTs (80%) have an activating c-KIT gene mutation. The most common mutation site is exon 11, with less common c-KIT gene mutations also occurring at exon 9 or 13. Not all GISTs have KIT mutations. The second most common mutation is the PDGFRA mutation (5%–10% of GISTs).2 A minority of GISTs are negative for both KIT and PDGFRA mutations. These tumors were previously called wild-type, but as the majority have either a succinate dehydrogenase (SDH) loss of function or loss of SDHB protein expression, they are now referred to as SDH-deficient GISTs.2 GISTs vary in aggressiveness from incidental to aggressive. Typically, small intestine and rectal GISTs are more aggressive than gastric GISTs. Both size and mitotic rate help to predict the metastatic potential of the tumor. Tumors less than 2 cm in size and having a mitotic rate of less than 5 per 50 high-power fields (hpf) have the lowest risk of metastases, while tumors greater than 5 cm and with more than 5 mitoses per 50 hpf have the highest rates of metastases.12
Angiosarcomas are rare tumors comprising 4% of all STSs. Although they can occur in any site, the majority are cutaneous and occur most frequently in the head and neck regions. These tumors are either of vascular or lymphatic origin and are comprised of abnormal, pleomorphic, malignant endothelial cells. The most useful immunohistochemical markers include von Willebrand factor, CD31, and Ulex europaeus agglutinin 1. The majority of these tumors occur sporadically; however, radiation exposure, chronic lymphedema, and certain toxins including vinyl chloride and thorium dioxide are known risk factors.13
Undifferentiated sarcomas have no specific features and typically consist of primitive mesenchymal cells.
Clinical Evaluation
› Case Presentation
Initial Presentation and History
A 55-year-old man presents to his primary care physician with a painless mass in his anterior thigh. The mass has been present for the past 3 months and he believes that it is enlarging. The patient has a history of well-controlled hypertension and hyperlipidemia. His medications include atorvastatin and hydrochlorothiazide. He has no known drug allergies. Family history is notable for diabetes and hypertension. He drinks 4 to 5 alcoholic drinks a week and he is a former smoker. He quit smoking in his 30s and only smoked intermittently prior to quitting. He denies any illicit drug use. He works as a high school principal. Currently, he feels well. His review of systems is otherwise noncontributory.
Physical Examination
On physical exam, he is afebrile with a blood pressure of 132/75 mm Hg, respiratory rate of 10 breaths/min, and oxygen saturation of 99% on room air. He is a well appearing, overweight male. His head and neck exam is unremarkable. Lung exam reveals clear breath sounds, and cardiac exam reveals a regular rate and rhythm. His abdomen is obese, soft, and without hepatosplenomegaly. There is a large, fixed mass on the anterior lateral aspect of his right thigh. He has no appreciable lymphadenopathy. His neurological exam is unremarkable.
• What are risk factors for sarcoma?
There are few known risk factors for sarcoma. Established risks factors include prior radiation therapy, chronic lymphedema, viruses, and genetic cancer syndromes including Li-Fraumeni syndrome, hereditary retinoblastoma, and NF-1. Other environmental exposures include phenoxyacetic acids and chlorophenols.14 The majority of cases are sporadic, with only a minority of patients having one of these known risk factors.15 Up to one third of sarcomas have a specific translocation and are driven by fusion oncogenes (
• What is the typical presentation for sarcomas?
A painless mass is the most typical presenting symptom. Size at presentation varies based on location, with extremity and head and neck locations typically presenting at smaller sizes than retroperitoneal tumors.14 Patients may experience pain and numbness as the mass enlarges and impinges on surrounding structures including nerves and vasculature. The vast majority of patients are without systemic symptoms.
• How is sarcoma staged?
The American Joint Committee on Cancer (AJCC) staging system is the most widely used staging system in the United States. The latest AJCC manual was updated in 2010 to include a 3-tiered grading system where the tumor is classified according to tumor size, lymph node involvement, metastases, and grade at time of diagnosis (Table 2 and Table 3). Additionally, tumor depth in relation to deep fascia is also taken into account, with superficial tumors being assigned a designation of “a” and deep tumors a designation of “b.”
Previously, 2 of the most widely used grading systems were the National Cancer Institute (NCI) and French Federation of Cancer Centers Sarcoma Group (FNCLCC) systems, both 3-tier grading systems. The main components that determine the NCI grade are the tumor’s histologic type and location and the amount of tumor necrosis. The FNCLCC system evaluation focuses on tumor differentiation, mitotic rate, and amount of tumor necrosis. A study that compared the NCI and FNCLCC grading systems found that FNCLCC was a better predictor of mortality and distant metastasis.16 Previously, the AJCC was a 4-tier grading system, but the 2010 version was updated to the 3-tier FNCLCC grading system. Additionally, the AJCC system has reclassified single lymph node disease as stage III as it confers better survival than metastatic disease.17 It is important that pathology be evaluated by a sarcoma specialist as disagreements with regard to histologic subtype and grade are common.18,19
• What are the most important prognostic factors?
Prognostic factors include grade, size, and presence of metastases at presentation. Best survival is associated with low-grade, small tumors with no metastases at time of diagnosis.14
• What imaging should be considered?
Imaging should be undertaken to help differentiate between benign and malignant lesions. Ideally, it should be undertaken before a biopsy is planned as the imaging can be used to plan biopsy as well as provide invaluable prognostic information. There are several imaging modalities that should be considered during the preliminary work-up and staging of STSs. Conventional imaging includes magnetic resonance imaging (MRI) of the original tumor site; computed tomography (CT) to evaluate for pulmonary metastases and, depending on location, liver metastases; and in the case of small, low-grade tumors, chest radiography. MRI is considered the test of choice for soft tissue masses and can help delineate benign masses such as hematomas, lipomas, and hemangiomas from sarcomas.20 It is difficult to compare the accuracy of positron emission tomography (PET)/CT to CT and MRI because most studies have evaluated PET/CT in parallel with CT and MRI.21 Tateishi et al compared the accuracy of conventional imaging, PET/CT, and PET/CT combined with conventional imaging at determining the TNM staging for 117 patients. They found that conventional imaging correctly classified 77% of patients, PET alone correctly classified 70%, PET/CT correctly classified 83%, and PET/CT combined with conventional imaging correctly staged 87%.22
• Which subtypes are most likely to metastasize?
Although the vast majority of sarcomas spread hematogenously, 3 have a propensity to spread lymphogenously: epithelioid sarcoma, rhabdomyosarcoma, and clear-cell sarcoma. Additionally, certain subtypes are more likely to metastasize: leiomyosarcomas, synovial sarcomas, neurogenic sarcomas, rhabdomyosarcomas, and epithelioid sarcomas.23 Sarcomas metastasize to the lungs more frequently than to the liver. The metastatic pattern is defined primarily by sarcoma subtype and site of primary tumor. Sarcomas rarely metastasize to the brain (~1%).
Management
› Case Continued
The patient undergoes an ultrasound to better visualize the mass. Given the heterogeneous character of the mass, he is referred for an MRI to evaluate the mass and a CT scan of the chest, abdomen, and pelvis to evaluate for distant metastases. MRI reveals a 5.1 cm × 4.6 cm heterogeneous mass invading the superficial fascia of the rectus femoris muscle. No suspicious lymph nodes or other masses are identified on imaging. The patient next undergoes an image-guided core needle biopsy. Pathology from that procedure is consistent with a stage III, T2bNxMx, grade 3, dedifferentiated liposarcoma.
• What is the best management approach for this patient?
Surgery
Surgery is the mainstay of treatment for STS. Patients with the best prognosis are those who undergo complete resection with negative surgical margins.24,25 Goal tumor-free margin is 1 to 3 cm.26 Complete resection confers the best long-term survival. Both local and metastatic recurrence is higher in patients with incomplete resection and positive margins.24,25 In a study that analyzed 2084 localized primary STSs, patients with negative margins had a local recurrence rate of 15% versus a rate of 28% in patients with positive margins. This translated into higher 5-year local recurrence-free survival for patients with negative surgical margins (82%) compared to patients with positive margins (65%).27 Another study similarly found that patients with negative margins at referral to their institution who underwent postoperative radiation had high local control rates of 93% (95% confidence interval [CI] 87% to 97%) at 5, 10, and 15 years.26 Although radiation improves local control, neither preoperative or postoperative radiation has been shown to improve progression-free or overall survival.28 Other factors that are associated with risk of recurrence are tumor location, history of previous recurrence, age of patient, histopathology, tumor grade, and tumor size. Approximately 40% to 50% of patients with high-grade tumors (defined as size > 5 cm, deep location, and high grade) will develop distant metastases.29
Zagars et al found that positive or uncertain resection margin had a relative risk of local recurrence of 2.0 (95% CI 1.3 to 3.1; P = 0.002), and presentation with locally recurrent disease (vs new tumor) had a relative risk of local recurrence of 2.0 (95% CI 1.2 to 3.4; P = 0.013).26 Patients with STS of head and neck and deep trunk have higher recurrence rates than those with superficial trunk and extremity STS. A single-institution retrospective review demonstrated that patients with completely resectable retroperitoneal sarcomas have longer median survival (103 months) compared to patients with incompletely resected abdominal sarcomas (18 months).25Rosenberg and colleagues compared amputation to limb-sparing surgery and radiation.24 Their prospective analysis of 65 patients found no difference in disease-free and overall survival between the 2 treatment groups.The limb-sparing treatment group had higher rates of local recurrence, which was highly correlated with positive surgical margins on pathology.24 Evidence from this and similar studies has resulted in radical amputations being replaced by conservative limb-sparing procedures and radiation therapy. In those found to have positive margins, re-resection is an option for some. Patients who undergo re-resection have higher local control rates than patients with positive margins who do not undergo re-resection. The 5-year control rate for patients who undergo re-resection is 85% (95% CI 80% to 89%) compared to 78% (95% CI 71% to 83%) for those who do not undergo re-resection. Similarly, patients who undergo re-resection have lower rates of metastases at 5, 10, and 15 years as well as higher 5-, 10-, and 15-year disease-free survival rates.26
› Case Continued
The patient is referred for limb-sparing surgery after presentation at a multidisciplinary tumor board. Prior to undergoing resection of the tumor, he is also referred to radiation-oncology to discuss the risks and benefits of combination radiotherapy and surgery as opposed to surgical resection alone.
• What is the evidence for radiation therapy?
Radiation THERAPY
Radiation therapy is used in the preoperative, intraoperative, and postoperative settings to reduce the risk of local recurrence. There are several options for radiation, including external beam radiation therapy (EBRT), intraoperative radiation, and brachytherapy. A newer strategy, intensity-modulated radiation therapy (IMRT), utilizes 3-dimensional modeling to reduce radiation dosages. Overall there are no differences in overall survival or local recurrence rates between preoperative and postoperative radiation in STS.28
The rationale behind preoperative radiation is that it reduces seeding of tumor cells, especially at the time of surgery.30 Additionally, for EBRT, preoperative radiation has smaller field sizes and lower radiation doses. It can also help to reduce the size of the tumor prior to resection. Intraoperative radiation is often paired with preoperative radiation as a boost dose given only to the area of residual tumor.
Suit et al reviewed patients treated at a single institution with limb-sparing surgery and different radiation strategies. Local control rates between preoperative and postoperative radiation groups were not statistically significant. Local recurrence was linked to grade and size of the tumor in both groups. The authors did note, however, that the preoperative radiation group tended to have larger tumor sizes at baseline compared to the patients who received postoperative radiation.30 A study that compared 190 patients who received preoperative and postoperative EBRT or brachytherapy (primary end point was wound complications, and local control was a secondary end point) showed a trend towards greater local control with preoperative radiation; however, the preoperative radiation group had significantly more wound complications compared to the postoperative radiation group.31
Yang et al found that postoperative EBRT decreases rates of local recurrence compared to surgery alone in high-grade extremity sarcomas.32 However, there were no differences in rates of distant metastases and overall survival between the 2 treatment groups. Similarly, in patients with low-grade sarcoma, there were fewer local recurrences in those who received EBRT and surgery as compared to surgery alone.32 Another study that evaluated 164 patients who received either adjuvant brachytherapy or no further therapy after complete resection found that brachytherapy reduced local recurrence in high-grade sarcomas. No difference in local recurrence rates was found in patients with low-grade sarcomas, nor was a significant difference found in the rates of distant metastases and overall survival between the 2 treatment groups.33 With regards to IMRT, a single institution cohort experience with 41 patients who received IMRT following limb-sparing surgery had similar local control rates when compared to historical controls.34
› Case Continued
After discussion of the risks and benefits of radiation therapy, the patient opts for preoperative radiation prior to resection of his liposarcoma. He receives 50 Gy of EBRT prior to undergoing resection. Resection results in R1 margin consistent with microscopic disease. He receives 16 Gy of EBRT as a boost after recovery from his resection.2
• What is the evidence for neoadjuvant and adjuvant chemotherapy for stage I tumors?
Chemotherapy
Localized Sarcoma
For localized sarcoma, limb-sparing resection with or without radiation forms the backbone of treatment. Studies have evaluated chemotherapy in both the neoadjuvant and adjuvant settings, with the vast majority of studies evaluating doxorubicin-based chemotherapy regimens in the adjuvant settings. Due to the rare nature of sarcomas, most studies are not sufficiently powered to detect significant benefit from chemotherapy. Several trials evaluating chemotherapy regimens in the neoadjuvant and adjuvant settings needed to be terminated prematurely due to inadequate enrollment into the study.35,36
For stage IA (T1a-Tb, N0, M0, low grade) tumors, no additional therapy is recommended after limb-sparing surgery with appropriate surgical margins. For stage IB (T2a-2b, N0, M0, low grade) tumors with insufficient margins, re-resection and radiation therapy should be considered, while for stage IIA (T1a-1b, N0, M0, G2-3) tumors preoperative or postoperative radiation therapy is recommended.2 Studies have not found benefit of adjuvant chemotherapy in these low-grade, stage I tumors in terms of progression-free survival and overall survival.37
• At what stage should chemotherapy be considered?
For stage IIb and stage III tumors, surgery and radiation therapy again form the backbone of therapy; however, neoadjuvant and adjuvant chemotherapy are also recommended as considerations. Anthracycline-based chemotherapy with either single-agent doxorubicin or doxorubicin and ifosfamide in combination are considered first-line chemotherapy agents in locally advanced STS.2,29,37
Evidence regarding the efficacy of both neoadjuvant and adjuvant chemotherapy regimens in the setting of locally advanced high-grade STS has been mixed. The Sarcoma Meta-analysis Collaboration evaluated 14 trials of doxorubicin-based adjuvant chemotherapy and found a trend towards overall survival in the treatment groups that received chemotherapy.37 All trials included in the meta-analysis compared patients with localized resectable soft-tissue sarcomas who were randomized to either adjuvant chemotherapy or no adjuvant chemotherapy after limb-sparing surgery with or without radiation therapy. None of the individual trials showed a significant benefit, and all trials had large confidence intervals; however, the meta-analysis showed significant benefit in the chemotherapy treatment groups with regard to local recurrence, distant recurrence, and progression-free survival. No significant difference in overall survival was found.37 Pervais et al updated the Sarcoma Meta-analysis Collaboration’s 1997 meta-analysis with the inclusion of 4 new trials that evaluated doxorubicin combined with ifosfamide and found that both patients who received doxorubicin-based regimens or doxorubicin with ifosfamide had significant decreases in distant and overall recurrences. Only the trials that utilized doxorubicin and ifosfamide had an improved overall survival that was statistically significant (hazard ratio 0.56 [95% CI 0.36 to 0.85]; P = 0.01).29 Although no significant heterogeneity was found among the trials included in either meta-analysis, a variety of sarcomas were included in each clinical trial evaluated. Given the extremely small number of each sarcoma subtype present in each trial, subgroup analysis is difficult and prone to inaccuracies. As a result, it is not known if certain histological subtypes are more or less responsive to chemotherapy.37–39
One randomized controlled trial evaluated neoadjuvant chemotherapy in high-risk sarcomas defined as tumors greater than 8 cm or grade II/III tumors. This study evaluated doxorubicin and ifosfamide and found no significant difference in disease-free and overall survival in the neoadjuvant therapy group compared to the control group.35 There remains controversy in the literature with regards to adjuvant chemotherapy. Many oncologists offer adjuvant chemotherapy to patients with certain stage III subtypes. Examples of subtypes that may be offered adjuvant therapy include myxoid liposarcomas, synovial sarcomas, and leiomyosarcomas.2 With regards to how many cycles of chemotherapy should be considered, a noninferiority study compared 3 cycles of epirubicin and ifosfamide to 5 cycles of epirubicin and ifosfamide in patients with high-risk locally advanced adult STSs. Three cycles of preoperative epirubicin and ifosfamide was found to be noninferior to 5 cycles with regards to overall survival.38
• What is this patient’s risk for recurrence?
The patient is at intermediate risk for recurrence. Numerous studies have demonstrated that tumor size, grade, and location are the most important factors to determine risk of recurrence, with larger size, higher grades, and deeper locations being associated with higher risk of recurrence. In an analysis of 1041 patients with STS of the extremities, high grade was the most important risk factor for distant metastases.39 The highest risk of recurrence is within the first 2 years. Given that the patient’s initial tumor was located in the extremity, he is more likely to have a distant metastasis as his site of recurrence; individuals with retroperitoneal tumors and visceral tumors are more likely to recur locally.40 For STSs of the extremity, distant metastases determine overall survival, whereas patients with retroperitoneal sarcomas can die from complications of local metastases.41 Once a patient develops distant metastases, the most important prognostic factor is the size of the tumor, with tumors larger than 10 cm having a relative risk of 1.5 (95% CI 1.0 to 2.0).39
• What are the recommendations for surveillance?
Surveillance recommendations are based on the stage of the sarcoma. Stage I tumors are the least likely to recur either locally or distally. As a result, it is recommended that stage I tumors be followed with history and physical exam every 3 to 6 months for the first 2 to 3 years, and then annually after the first 2 to 3 years. Chest x-rays should be considered every 6 to 12 months.2 For stage II–IV tumors, history and physical exam is recommended every 3 to 6 months for the first 2 to 3 years. Chest and distant metastases imaging should also be performed every 3 to 6 months during this time frame. For the next 2 years, history and physical exam and imaging are recommended every 6 months. After the first 4 to 5 years, annual follow-up is recommended.2
A study that followed 141 patients with primary extremity STSs for a median interval of 49 months found that high-grade tumors were most likely to recur during the first 2 years, with 20% of their patients recurring locally and 40% recurring distally. Chest x-rays performed during surveillance follow-up found distant lung metastases in 36 asymptomatic patients and had a positive predictive value of 92%, a negative predictive value of 97%, and a quality-adjusted life-year of $30,000.40,41 No laboratory testing was found to aid in detection of recurrence.
› Case Continued
The patient does well for 1 year. With physical therapy, he regains most of the strength and coordination of the lower extremity. He is followed every 3 months with chest x-rays and a MRI of the thigh for the first year. On his fourth follow-up clinic visit, he describes increased dysp-nea on exertion over the previous few weeks and is found to have multiple lung metastases in both lungs on chest x-ray. He undergoes further evaluation for metastases and is not found to have any other metastatic lesions. Bronchoscopy and biopsy of 1 of the lung nodules confirms recurrent dedifferentiated liposarcoma.
• Should this patient undergo metastectomy?
An analysis of 3149 patients with STS treated at Memorial Sloan-Kettering who developed lung metastases found that patients with pulmonary metastases have survival rates of 25%. The most important prognostic factor for survival was complete resection of all metastases.42 For stage IV disease, surgery is used only in certain instances. In instances where tumor is more localized or limited, removal of metastases or metastectomy can play a role in management.2
› Case Continued
Because the patient’s metastases are limited to the lungs, he is referred for metastectomy. He undergoes wedge resection for definitive diagnosis but it is not possible to completely resect all of the metastases. He is thus referred to a medical oncologist to discuss his treatment options.
• What are treatment options for unresectable or metastatic disease?
Metastatic Disease
Unlike local and locally advanced disease, chemotherapy forms the backbone of treatment in stage IV disease. Doxorubicin and olaratumab or doxorubicin and ifosfamide in combination are considered first line in metastatic disease. Response rates for single-agent doxorubicin range from 16% to 27%, while phase 2 and phase 3 studies of doxorubicin and ifosfamide have found response rates ranging from 18% to 36%.43 In addition, the effectiveness of doxorubicin and ifosfamide phase 2 and 3 trials varied. Edmonson et al found a tumor regression rate of 34% for doxorubicin and ifosfamide as compared to 20% for doxorubicin alone.44 In comparison, Santoro et al found a response rate of 21.3% for doxorubicin alone and 25.2% for doxorubicin and ifosfamide.45 Neither study found increased survival benefit for doxorubicin and ifosfamide when compared to doxorubicin alone. In a Cochrane review evaluating randomized trials that compared doxorubicin and combination chemotherapy regimens, response rates varied from 14% for doxorubicin in combination with streptomycin to 34% for doxorubicin and ifosfamide. Most trials did not show a significant benefit for combination therapies when compared to doxorubicin alone.43 Mean survival with doxorubicin or doxorubicin and ifosfamide is 12 months. High rates of recurrence highlight the need for additional chemotherapy regimens.
The newest approved agent is olaratumab, a monoclonal antibody that binds platelet-derived growth factor receptor alpha and prevents receptor activation. A phase 1-b and phase 2 trial evaluated patients with locally advanced and metastatic STS and randomly assigned them to either olaratumab and doxorubicin or doxorubicin alone.46 Progression-free survival for olaratumab/doxorubicin was 6.6 months (95% CI 4.1 to 8.3) compared to 4.1 months (95% CI 2.8 to 5.4) for doxorubicin alone. The objective response rate was 18.2% (95% CI 9.8 to 29.6) for olaratumab/doxorubicin compared to 7.5% (95% CI 2.5 to 6.6) for doxorubicin alone. Furthermore, the median overall survival for olaratumab plus doxorubicin was 26.5 months (95% CI 20.9 to 31.7) compared to 14.7 months for doxorubicin alone (95% CI 5.5 to 26.0). Impressively, this improved response was notable across histological types. Furthermore, patients who had previously been treated with more than 1 regimen and those who were treatment naïve had similar response rates.46
• What are second-line treatment options?
Doxorubicin has been used in combination with several other agents including dacarbazine (DTIC) as well as DTIC and ifosfamide (MAID). Borden et al evaluated patients with metastatic STS and randomly assigned the patients to either doxorubicin or doxorubicin and DTIC. Combination therapy demonstrated better tumor response than doxorubicin alone: 30% complete or partial response for combination therapy and 18% for doxorubicin alone.47 However, Omura et al found similar rates of efficacy between doxorubicin and combination doxorubicin and DTIC in women with recurrent or nonresectable uterine sarcomas.48 MAID has never been directly compared in a randomized trial to doxorubicin alone. In a study that compared MAID to doxorubicin and DTIC (AD) in patients with unresectable or metastatic sarcomas, MAID had superior response rates (32% versus 17%), but there was no difference with regards to overall survival (mean survival of 12.5 months).49
Several additional regimens have undergone evaluation in metastatic and recurrent STSs. Gemcitabine has been used both as a single agent and as part of combination therapy in many studies. Studies with gemcitabine in combination with either docetaxel or DTIC have been the most efficacious. In a phase 2 trial, patients with metastatic STS were randomly assigned to either gemcitabine alone or gemcitabine and docetaxel. Combination therapy had a higher response rate (16% versus 8%) and longer overall survival (17.9 months versus 11.5 months) than gemcitabine alone.50 Furthermore, a phase 2 trial of gemcitabine and docetaxel in patients with unresectable leiomyosarcoma showed an overall response rate of 56%, with 3 complete and 15 partial responses among the 34 patients enrolled in the study.51 A phase 2 trial randomly assigned patients with unresectable or metastatic STS to either DTIC or combination gemcitabine and DTIC.52 Gemcitabine-DTIC had a superior progression-free survival at 3 months (56% [95% CI 43% to 69%]) as compared to DTIC alone (37% [95% CI 23.5% to 50%]). Furthermore, mean progression-free survival and overall survival were improved in the gemcitabine-DTIC group (4.2 months and 16.8 months) as compared to the DTIC group (2.0 months and 8.2 months).52 DTIC has a single-agent response rate of 16%, but has been shown to be particularly effective in the setting of leiomyosarcomas.49
• Does response to treatment regimens differ by histologic subtype?
The majority of STS trials include many different histologic subtypes. Given the rarity of sarcomas as a whole, many trials have had difficulty recruiting adequate numbers of patients to have sufficient power to definitely determine if the treatment under investigation has clinical benefit. Furthermore, the patients recruited have been heterogeneous with regard to subtype. Many older studies hypothesized that the efficacy of chemotherapeutic agents vary based on histologic subtype; however, for most subtypes the number of individuals included in those trials was too low to evaluate efficacy based on subtype.
Some exceptions exist, however. For example, both gemcitabine-DTIC and gemcitabine-docetaxel have been found to be particularly effective in the treatment of leiomyosarcomas.50,52 Additionally, a retrospective study found a 51% overall response rate for patients with myxoid liposarcomas treated with trabectedin.53 Studies of patients with angiosarcoma treated with paclitaxel have demonstrated response rates of 43% and 53%.54,55
• What are the newest approved and investigational agents?
A recently approved agent is trabectedin, a tris tetrahydroisoquinoline alkaloid isolated from ascidians that binds to the minor groove of DNA and causes disruptions in the cell cycle. Samuels et al reported data from a single-arm, open-label expanded access trial that evaluated patients with advanced metastatic sarcomas.56 In this study, patients with liposarcomas and leiomyosarcomas had an objective response rate of 6.9% (95% CI 4.8 to 9.6) as compared to a rate of 5.9% (95% CI 4.4 to 7.8) for all assessable patients. Median survival was 11.9 months for all patients, with improved median survivals for liposarcoma and leiomyosarcomas of 16.2 months (95% CI 14.1 to 19.5) compared to 8.4 months (95% CI 7.1 to 10.7 months) for other subtypes.56
Schöffski et al evaluated eribulin, a chemotherapeutic agent that affects microtubule dynamics, in a phase 2 trial of patients with progressive or high-grade STS with progression on previous chemotherapy. They found a median progression-free survival of 2.6 months (95% CI 1.7 to 6.2) for adipocytic sarcoma, 2.9 months (95% CI 2.4 to 4.6) for leiomyosarcoma, 2.6 months (95% CI 2.3 to 4.3) for synovial sarcoma, and 2.1 months (95% CI 1.4 to 2.9) for other sarcomas.57
Van der Graaf and colleagues randomly assigned patients with metastatic nonadipocytic STS to pazopanib or placebo in a phase 3 trial. Pazopanib is a small-molecule endothelial growth factor inhibitor with activity against vascular endothelial growth factors 1, 2, and 3 as well as platelet-derived growth factors. Median progression-free survival was 4.6 months (95% CI 3.7 to 4.8) with pazopanib compared to 1.6 months (95% CI 0.9 to 1.8) with placebo.58 Adipocytic sarcomas (liposarcomas) were excluded from the trial because phase 2 trials had found a lower rate of progression-free survival (26%) for them compared to other subtypes.
• What are the most common toxicities associated with the approved and investigational chemotherapeutic agents?
Toxicities were seen with each of the regimens studied and were common in the randomized trials, with higher rates of toxicities in the combination chemotherapy regimens. The most common toxicities are myelosuppression, nausea, and vomiting. In the doxorubicin trials, the most common toxicities were myelosuppression, nausea, and vomiting.44
Ifosfamide both as an individual agent and in combination with doxorubicin has higher rates and higher grades of toxicity than doxorubicin alone. Myelosuppression is the most common toxicity associated with ifosfamide, and the most commonly affected cell line is leukocytes.44 Combination doxorubicin and ifosfamide also had high rates of nausea and vomiting (95%) and alopecia (100%).35Neutropenia is the most common toxicity associated with gemcitabine and dacarbazine, while their most common nonhematologic toxicities are fatigue and nausea.52,59 Trabectedin’s most common toxicities are nausea (29%), neutropenia (24%), and fatigue (23%). It has also been shown to cause increased alkaline phosphatase (20%) and alanine aminotransferase (19%) levels.56 In a phase 2 study of eribulin, 50% of patients had neutropenia, and other toxicities included fatigue, alopecia, nausea, sensory neuropathy, and thrombocytopenia.57 Pazopanib is generally well tolerated; the most common toxicities are fatigue (65%), diarrhea (58%), nausea (54%), and hypertension (41%).58 Higher rates of neutropenia, mucositis, nausea, vomiting, diarrhea, and transfusion reactions were seen with olaratumab and doxorubicin compared to doxorubicin alone in phase 1b and 2 studies.46
› Case Continued
Given his poor prognosis with unresectable metastatic undifferentiated liposarcoma, the patient considers a clinical trial prior to undergoing combined therapy with doxorubicin and ifosfamide. He tolerates therapy well with stable disease at 6 months.
Conclusion
STSs are a heterogeneous collection of rare tumors. Low-grade, localized tumors have the best prognosis, and patients who undergo complete resection have the best long-term survival. Due to the rarity of STSs, trials often have limited enrollment, and little progress has been made with regards to treatment and survival rates for metastatic and unresectable disease. All patients should be evaluated and treated at specialized sarcoma centers. This case highlights the need for continued research and clinical trials to improve overall survival of patients with sarcoma. TSJ
CORRESPONDENCE
Ashley Pariser, MD, Resident, Department of Medicine, Northwestern University Feinberg School of Medicine Chicago, IL. Accepted for publication Jan/Feb 2017; Hosp Phys; Vol. 12, Part1
References
1. American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
2. National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
3. Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
4. Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4: 252–66.
5. Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
6. Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
7. Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
8. Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
9. Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.

10. Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
11. Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
13. Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
14. Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
15. Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
16. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
17. Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
18. Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
19. Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
20. Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
21. Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
22. Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
23. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
24. Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
25. Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
26. Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
27. Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
28. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
29. Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
30. Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
31. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
32. Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
33. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
34. Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
35. Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
36. Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
37. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
38. Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.

39. Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
40. Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
41. Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
42. Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
43. Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
44. Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
45. Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
46. Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
47. Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
48. Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
49. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
50. Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
51. Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
52. Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
53. Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
54. Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
55. Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
56. Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
57. Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
58. Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
59. Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
References
1. American Cancer Society. Cancer facts and figures 2016. American Cancer Society Web site. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed December 20, 2016.
2. National Comprehensive Cancer Network. NCCN clinical guidelines in oncology: soft tissue sarcoma. 2016
3. Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001;91:1914–26.
4. Dei Tos A. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol 2000;4: 252–66.
5. Wile AG, Evans HL, Romsdahl MM. Leiomyosarcoma of soft tissue: a clinicopathologic study. Cancer 1981;48:1022–32.
6. Hashimoto H, Daimaru Y, Tsuneyoshi M, Enjoji M. Leiomyosarcoma of the external soft tissues. A clinicopathologic, immunohistochemical, and electron microscopic study. Cancer 1986;57:2077–88
7. Fisher C. Synovial sarcoma. Ann Diagn Pathol 1998;2:401–21.
8. Newton WA Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer 1995;76:1073–85.
9. Furlong MA. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595–603.

10. Anghileri M, Miceli R, Fiore M. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–74.
11. Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archive 2001;438:1–12.
12. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83.
13. Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983–91.
14. Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54:94–109.
15. Penel N, Grosjean J, Robin YM, et al. Frequency of certain established risk factors in soft tissue sarcomas in adults: a prospective descriptive study of 658 cases. Sarcoma 2008;2008:459386.
16. Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997;15:350–62.
17. Maki RG, Moraco N, Antonescu CR, et al. Toward better soft tissue sarcoma staging: building on American joint committee on cancer staging systems versions 6 and 7. Ann Surg Oncol 2013;20:3377–83.
18. Shiraki M, Enterline HT, Brooks JJ, et al. Pathologic analysis of advanced adult soft tissue sarcomas, bone sarcomas, and mesotheliomas. The Eastern Cooperative Oncology Group (ECOG) experience. Cancer 1989;64:484–90.
19. Presant CA, Russell WO, Alexander RW, Fu YS. Soft-tissue and bone sarcoma histopathology peer review: The frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. J Clin Oncol 1986; 4:1658–61.
20. Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol 1990;155:817–24.
21. Ioannidis JP, Lau J. 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 2003;44:717–24.
22. Tateishi U, Yamaguchi U, Seki K, et al. Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 2007;245:839–47.
23. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer 2003;97:2530–43
24. Rosenberg S, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982;196:305–14.
25. Lewis J, Leung D, Woodruff J, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;288:355–65.
26. Zagars GK, Ballo MT, Pisters PW, et al. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
27. Stojadinovic A, Leung DH, Hoos A. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tisusse sarcomas. Ann Surg 2002;235:424–34.
28. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
29. Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81.
30. Suit HD, Mankin HJ, Wood WC, Proppe KH. Preoperative, intraoperative, and postoperative radiation in the treatment of primary soft tissue sarcoma. Cancer 1985;55:2659–67
31. O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 2002;359:2235–41.
32. Yang J, Chang A, Baker A, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16:197–203.
33. Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68.
34. Alektiar KM, Brennan MF, Healey JH, Singer S. Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 2008;26:3440–5.
35. Gortzak E, Azzarelli A, Buesa J, et al. A randomized phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer 2001;37:1096–1103.
36. Fakhari N, Ebm C, Kostler WJ, et al. Intensified adjuvant IFADIC chemotherapy in combination with radiotherapy versus radiotherapy alone for soft tissue sarcoma: long-term follow-up of a prospective randomized feasibility trial. Wein Klin Wochenschr 2010;122:614–9.
37. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet 1997;350:1647–54.
38. Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–56.

39. Pisters PW, Leung DH, Woodruff J. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996;14:1679–89.
40. Whooley B, Gibbs J, Mooney M. Primary Extremity Sarcoma: What is the Appropriate Follow-up? Annals of Surg Oncol 2000; 7: 9-14.
41. Whooley BP, Mooney MN, Gibbs JF, Graybill WG. Effective follow-up strategies in soft tissue sarcoma. Sem Surg Oncol 1999;17:83–87.
42. Billingsley KG, Burt ME, Jara E, et al. Pulmonary metastases from soft tissue sarcoma: analysis of patterns of diseases and postmetastasis survival. Ann Surg 1999;229:602–10.
43. Bramwell VH, Anderson D, Charette ML; Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev 2003;(3):CD003293.
44. Edmonson J, Ryan L, Blum R. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993;11:1269–75.
45. Santoro A, Tursz T, Mouridsen H. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–45.
46. Tap WD, Jones RL, Van Tine B, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–97.
47. Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol 1987;5:840–50.
48. Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer 1983;52:626–32.
49. Antman K, Crowley J, Balcerzak SP, et al. An intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomas. J Clin Oncol 1993;11:1276–85.
50. Maki R, Wathen K, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007; 25: 2755–63.
51. Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol 2002;12:2824–31.
52. Garcia-del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol 2011;29:2528–33.
53. Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;7:595–602.
54. Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330–6.
55. Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve outcome. Ann Oncol 2012;23:517–23.
56. Samuels BL, Chawla S, Patel S, et al. Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 2013;24:1703–9.
57. Schöffski P, Ray-Coquard IL, Cioffi A, et al. Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histolical subtypes. Lancet 2011;11:1045–52.
58. Van der Graaf W, Blay JY, Chawla S, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86.
59. Dileo P, Morgan JA, Zahrieh D, et al. Gemcitabine and vinorelbine combination chemotherapy for patients with advanced soft tissue sarcomas: results of a phase II trial. Cancer 2007;109:1863–9.
Diagnosis and treatment of hyperkalemia
Hyperkalemia is common in patients with cardiovascular disease. Its consequences can be severe and life-threatening, and its management and prevention require a multidisciplinary approach that entails reducing intake of high-potassium foods, adjusting medications that cause hyperkalemia, and adding medications that reduce the plasma potassium concentration. With this approach, patients at high risk can receive the cardiorenal benefits of drugs that block the renin-angiotensin-aldosterone system without developing hyperkalemia.
98% OF POTASSIUM IS INSIDE CELLS
The body of a typical 70-kg man contains about 3,500 mmol of potassium, 98% of which is in the intracellular space; the remaining 2% is in the extracellular space. This large intracellular-to-extracellular gradient determines the cell voltage and explains why disorders in plasma potassium give rise to manifestations in excitable tissues such as the heart and nervous system.
The most important determinants of potassium distribution between the intracellular and extracellular space are insulin and beta-adrenergic receptor stimulation.
Maintenance of total-body potassium content is primarily the job of the kidneys, with a small contribution by the gastrointestinal tract.1,2 Hyperkalemia is most commonly encountered in patients with decreased kidney function.
The normal kidney can secrete a large amount of potassium, making hyperkalemia uncommon in the absence of kidney disease. This large capacity may have evolved to handle the diet of Paleolithic humans, which contained 4 times as much potassium as contemporary diets.3,4 With the onset of agriculture, dietary intake of potassium has progressively declined while sodium intake has risen. A popular theory suggests this mismatch between the modern diet and the nutritional requirements encoded in the human genome during evolution may contribute to chronic diseases such as hypertension, stroke, kidney stones, and bone disease.5
MANY POTENTIAL CAUSES OF HYPERKALEMIA
Causes of hyperkalemia are outlined in Table 1. Shifting of potassium from the cells to the extracellular space is a cause of transient hyperkalemia, while chronic hyperkalemia indicates an impairment in renal potassium secretion. The following discussion is a guide to the approach to the hyperkalemic patient.
Is the patient’s hyperkalemia really pseudohyperkalemia?
Pseudohyperkalemia, an artifact of measurement, occurs due to mechanical release of potassium from cells during phlebotomy or specimen processing.6 This diagnosis is made when the serum potassium concentration exceeds the plasma potassium concentration by more than 0.5 mmol/L, and should be considered when hyperkalemia occurs in the absence of a clinical risk factor. Fist-clenching, application of a tight-fitting tourniquet, or use of small-bore needles during phlebotomy can all cause pseudohyperkalemia.
Mechanism of pseudohyperkalemia. Since serum is the liquid part of blood remaining after coagulation, release of potassium from cells injured during the process of coagulation raises the potassium level in the serum. Plasma is the cell-free part of blood that has been treated with anticoagulants; it has no cells that can be injured and release potassium. Thus, the serum potassium level will be higher than that in the plasma.
Reverse pseudohyperkalemia, in contrast, occurs when the plasma potassium level is falsely elevated but the serum value is normal. This situation has been described in hematologic disorders characterized by pronounced leukocytosis in which malignant cells are prone to lysis with minimal mechanical stress due to increased fragility or altered sodium-potassium ATPase pump activity.7 This phenomenon is unusual but occurs because the cells are so fragile.
A spurious increase in plasma potassium concentration along with a low plasma calcium concentration raises the possibility of calcium chelation and release of potassium in a sample tube contaminated with the anticoagulant ethylenediaminetetraacetic acid.
Is there increased potassium intake?
Increased potassium intake is a potential cause of hyperkalemia in patients with decreased kidney function or adrenal disease.
Foods naturally rich in potassium include bananas (a medium-sized banana contains 451 mg or 12 mmol of potassium) and potatoes (844 mg or 22 mmol in a large baked potato with skin). Other potassium-rich foods are melons, citrus juice, and avocados. Less-obvious food sources include raw coconut juice (potassium concentration 44.3 mmol/L) and noni juice (56 mmol/L).
Salt substitutes, recommended to hypertensive patients with chronic kidney disease, can be a hidden source of dietary potassium.
Clay ingestion is a potential cause of dyskalemia. White clay consumption causes hypokalemia due to potassium binding in the gastrointestinal tract. Red clay or river bed clay, on the other hand, is enriched in potassium (100 mmol of potassium in 100 g of clay) and can cause life-threatening hyperkalemia in patients with chronic kidney disease.8
Eating burnt match heads. Some individuals chew and ingest burnt match heads, a condition called cautopyreiophagia. In one reported case,9 this activity contributed an additional 80 mmol of daily potassium intake in a dialysis patient, resulting in a plasma potassium concentration of 8 mmol/L.
Is the hyperkalemia the result of a cellular shift?
Acute hyperkalemia can be the result of redistribution of cellular potassium. Shifting of as little as 2% of the body’s potassium from the intracellular to the extracellular space can double the plasma potassium concentration.
Tissue injury. Hyperkalemia frequently occurs in diseases that cause tissue injury such as rhabdomyolysis, trauma, massive hemolysis, and tumor lysis.
Insulin deficiency. Insulin and catecholamines are major regulators of potassium distribution within the body. After a meal, release of insulin not only regulates the plasma glucose concentration, it also causes potassium to move into cells until the kidneys have had sufficient time to excrete the dietary potassium load and reestablish total-body potassium content.
Exercise, beta-blockers. During exercise, potassium is released from skeletal muscle cells and accumulates in the interstitial compartment, where it exerts a vasodilatory effect. The simultaneous increase in circulating catecholamines regulates this release by promoting cell potassium uptake through beta-adrenergic receptor stimulation.
Metabolic acidosis can facilitate exit (ie, shift) of potassium from cells, but this effect depends on the type of acidosis. Hyperchloremic normal anion gap acidosis (mineral acidosis) most commonly causes this effect due to the relative impermeability of the cell membrane to the chloride anion. As hydrogen ions move into the cell due to accumulation of ammonium chloride or hydrogen chloride, electrical neutrality is maintained by potassium exit.
In contrast, organic acidosis (due to lactic, beta-hydroxybutyric, or methylmalonic acid) tends not to cause a potassium shift, since most organic anions readily cross the cell membrane along with hydrogen. Lactic acidosis is often associated with potassium shift, but this effect is due to loss of cell integrity as a result of cell ischemia. The hyperkalemia typically present on admission in patients with diabetic ketoacidosis is the result of insulin deficiency and hypertonicity and not the underlying organic acidosis.10
Hypertonic states can cause hyperkalemia due to cell shift. For example, hyperglycemia, as in diabetic ketoacidosis, pulls water from the intracellular into the extracellular compartment, thereby concentrating intracellular potassium and creating a more favorable gradient for potassium efflux through membrane channels. This same effect can occur in neurosurgical patients given large amounts of hypertonic mannitol. Repetitive doses of immunoglobulin can lead to extracellular accumulation of sorbitol, maltose, or sucrose, since these sugars are added to the preparations to prevent immunoglobulin aggregation.11
Is a disturbance in renal potassium excretion present?
Sustained hyperkalemia is more commonly associated with decreases in renal potassium excretion than with a cellular shift. In most instances the clinician can distinguish between cell shift and impaired renal excretion based on the available clinical data.
The transtubular potassium gradient has been used to determine whether there is a disturbance in renal potassium excretion and to assess renal potassium handling.12
This calculation is based on the assumption that only water is reabsorbed past the cortical collecting duct, and not solutes. It has fallen out of favor since we have found this assumption to be incorrect; a large amount of urea is reabsorbed daily in the downstream medullary collecting duct as a result of intrarenal recycling of urea.
The one situation in which the transtubular potassium gradient may be of use is determining whether hyperkalemia is a result of low aldosterone levels as opposed to aldosterone resistance. One can compare the transtubular potassium gradient before and after a physiologic dose (0.05 mg) of 9-alpha fludrocortisone. An increase of more than 6 over a 4-hour period favors aldosterone deficiency, whereas smaller changes would indicate aldosterone resistance.
24-hour potassium excretion, spot urine potassium-creatinine ratio. A better way to assess renal potassium handling is to measure the amount of potassium in a 24-hour urine collection or determine a spot urine potassium-creatinine ratio. A 24-hour urinary potassium excretion of less than 15 mmol or a potassium-creatinine ratio less than 1 suggests an extrarenal cause of hypokalemia. A ratio greater than 20 would be an appropriate renal response to hyperkalemia.
One or more of 3 abnormalities should be considered in the hyperkalemic patient with impaired renal excretion of potassium:
- Decreased distal delivery of sodium
- Mineralocorticoid deficiency
- Abnormal cortical collecting tubule function.13
Decreased distal delivery of sodium
Under normal circumstances, potassium is freely filtered across the glomerulus and then mostly reabsorbed in the proximal tubule and thick ascending limb. Potassium secretion begins in the distal convoluted tubule and increases in magnitude into the collecting duct. Tubular secretion is the component of potassium handling that varies and is regulated according to physiologic needs.
In acute kidney injury, the rapid decline in glomerular filtration rate and reduction in functioning nephron mass lead to decreased distal potassium secretion.
Hyperkalemia is a frequent problem when oliguria is present, since the reduction in distal delivery of sodium and water further impairs potassium secretion. Patients with oliguric acute kidney injury are more likely to have a more severe underlying disease state, and therefore tissue breakdown and catabolism further increase the risk of hyperkalemia.
In contrast, in nonoliguric patients, the renal injury tends to be less severe, and enough sodium and water are usually delivered distally to prevent hyperkalemia.
In chronic kidney disease, nephron dropout and reduction in collecting tubule mass also lead to a global decline in distal potassium secretion. However, this is countered by an increased capacity of the remaining individual nephrons for potassium secretion. High flow, increased distal sodium delivery, and increased activity and number of sodium-potassium ATPase pumps in the remaining nephrons account for this increased secretory capacity.14 As renal function declines over time, colonic potassium secretion progressively increases.15
These adaptive changes help to keep the plasma potassium concentration within the normal range until the glomerular filtration rate falls to less than 10 or 15 mL/min. Development of hyperkalemia with more modest reductions in the glomerular filtration rate suggest decreased mineralocorticoid activity or a specific lesion of the tubule.
Mineralocorticoid deficiency
Aldosterone deficiency can occur alone or in combination with decreased cortisol levels. Destruction of the adrenal glands is suggested when both hormones are reduced. Enzyme defects in cortisol metabolism can result in either isolated deficiency of aldosterone or adrenogenital syndromes associated with decreased mineralocorticoid activity.
Heparin administration leads to a reversible defect in adrenal synthesis of aldosterone. Drugs that block the stimulatory effect of angiotensin II on the zona glomerulosa cells of the adrenal gland will lower aldosterone.
Renin-angiotensin-aldosterone system blockers. Angiotensin-converting enzyme inhibitors block the formation of angiotensin II, whereas angiotensin II receptor blockers prevent angiotensin II from binding to its adrenal receptor. The direct renin inhibitor aliskiren lowers angiotensin II levels by blocking the enzymatic activity of renin and lowers the circulating levels of both angiotensin I and II.16
The syndrome of hyporeninemic hypoaldosteronism is a common cause of hyperkalemia in patients who have a glomerular filtration rate between 40 and 60 mL/min. Diabetic nephropathy and interstitial renal disease are the most common clinical entities associated with this syndrome.10 Other causes include analgesic nephropathy, urinary tract obstruction, sickle cell disease, systemic lupus erythematosus, and amyloidosis.
Nonsteroidal anti-inflammatory drugs can cause hyperkalemia by suppressing renin release and reducing delivery of sodium to the distal nephron.18
Calcineurin inhibitors impair potassium secretion by suppressing renin release and by direct tubular effects.19
Beta-blockers. Beta-1 and to a lesser extent beta-2 receptor blockade can also result in a hyporeninemic state.
Distal tubular defect
Hyperkalemia can result from interstitial renal diseases that specifically affect the distal nephron. In this setting, the glomerular filtration rate is only mildly reduced, and circulating aldosterone levels are normal.
Renal transplant, lupus erythematosus, amyloidosis, urinary obstruction, and sickle cell disease are conditions in which an impairment in renin release may coexist with a defect in tubular secretion.
Potassium-sparing diuretics impair the ability of the cortical collecting tubule to secrete potassium. Specifically, amiloride and triamterene inhibit sodium reabsorption mediated by the epithelial sodium channel located on the apical membrane of the principal cell. This effect abolishes the lumen’s negative potential and thereby removes a driving force for potassium secretion.
Trimethoprim and pentamidine cause similar effects.
Spironolactone and eplerenone compete with aldosterone at the level of the mineralocorticoid receptor and can result in hyperkalemia.
Drospirenone, a non-testosterone-derived progestin contained in certain oral contraceptives, possesses mineralocorticoid-blocking effects similar to those of spironolactone.
The plasma potassium level should be monitored when these drugs are prescribed in patients receiving potassium supplements, renin-angiotensin-aldosterone system blockers, or nonsteroidal anti-inflammatory drugs.20
CLINICAL FEATURES OF HYPERKALEMIA
Neuromuscular manifestations of hyperkalemia include paresthesias and fasciculations in the arms and legs. Severe elevation in potassium can give rise to an ascending paralysis with eventual flaccid quadriplegia. Typically, the trunk, head, and respiratory muscles are spared, and respiratory failure is rare.
Cardiac signs
Hyperkalemia has depolarizing effects on the heart that are manifested by changes in the electrocardiogram (Figure 2). The progressive changes of hyperkalemia are classically listed as:
- Peaked T waves that are tall, narrow, and symmetrical and can occasionally be confused with the hyperacute T-wave change associated with an ST-segment elevation myocardial infarction.21 However, in the latter condition, the T waves tend to be more broad-based and asymmetric in shape.
- ST-segment depression
- Widening of the PR interval
- Widening of the QRS interval
- Loss of the P wave
- A sine-wave pattern—an ominous development and a harbinger of impending ventricular fibrillation and asystole.
The plasma potassium concentration often correlates poorly with cardiac manifestations. In a retrospective review, only 16 of 90 cases met strict criteria for electrocardiographic changes reflective of hyperkalemia (defined as new peaked and symmetric T waves that resolved on follow-up).22 In 13 of these cases, the electrocardiogram was interpreted as showing no T-wave changes even when read by a cardiologist. In addition, electrocardiographic criteria for hyperkalemia were noted in only 1 of 14 patients who manifested arrhythmias or cardiac arrest attributed to increased plasma potassium concentration.
TREATMENT OF ACUTE HYPERKALEMIA
The treatment of hyperkalemia depends on the magnitude of increase in the plasma potassium concentration and the presence or absence of electrocardiographic changes or neuromuscular symptoms.23 Acute treatment is indicated for marked electrocardiographic changes and severe muscle weakness.
Intravenous calcium rapidly normalizes membrane excitability by antagonizing the potassium-induced decrease in membrane excitability but does not alter the plasma potassium concentration.
Insulin lowers the plasma potassium concentration by promoting its entry into cells. To avoid hypoglycemia, 10 units of short-acting insulin should be accompanied by a 50-g infusion of glucose, increased to 60 g if 20 units of insulin are given.24
Beta-2 receptor agonists produce a similar effect. The shift of potassium into cells with insulin and beta-2-adrenergic receptor stimulation is brought about by increases in sodium-potassium ATPase pump activity, primarily in skeletal muscle cells.
Sodium bicarbonate, in the absence of acidosis, lowers the plasma potassium concentration only slightly. It should be reserved for hyperkalemic patients who have coexisting metabolic acidosis after the patient has received insulin and glucose, an adrenergic agent, and calcium.
These acute treatments need to be followed by therapies designed to lower the total body potassium content such as diuretics, potassium-binding drugs, and dialysis.
TREATMENT OF CHRONIC HYPERKALEMIA
Review medications. Once the diagnosis of hyperkalemia has been made, the initial approach should be to review the patient’s medications and make every effort to discontinue drugs that can impair renal potassium excretion.16 Patients should be asked about their use of over-the-counter nonsteroidal anti-inflammatory drugs and herbal remedies, since herbs may be a hidden source of dietary potassium.
Dietary counseling. Patients should be instructed to reduce their dietary intake of potassium and to avoid salt substitutes that contain potassium.
Diuretic therapy is beneficial in minimizing hyperkalemia in patients with chronic kidney disease. Thiazide and loop diuretics enhance renal potassium excretion by increasing flow and delivery of sodium to the collecting duct. Thiazide diuretics are effective when the estimated glomerular filtration rate is greater than 30 mL/min, while loop diuretics should be used in patients with more severe renal insufficiency (Table 2).
Sodium bicarbonate is an effective agent to minimize increases in the plasma potassium concentration in patients with chronic kidney disease and metabolic acidosis. This drug increases renal potassium excretion by increasing distal sodium delivery and shifts potassium into cells as the acidosis is corrected. The likelihood of developing volume overload as a complication of sodium bicarbonate administration can be minimized with effective diuretic therapy.
Avoiding hyperkalemia if renin-angiotensin-aldosterone system blockers are needed
Renin-angiotensin-aldosterone system blockers can be problematic, as these drugs cause hyperkalemia, often in the very patients who derive the greatest cardiovascular benefit from them.16 A number of steps can reduce the risk of hyperkalemia and allow these drugs to be used.
The initial dose should be low and the plasma potassium should be measured within 1 to 2 weeks after drug initiation. If the potassium level is normal, the dose can be titrated upwards with remeasurement of the plasma potassium after each dose titration. If the plasma potassium concentration rises to 5.5 mmol/L, in some cases lowering the dose will reduce the potassium concentration and allow the patient to remain on the drug.
In patients at risk of hyperkalemia, angiotensin II receptor blockers and direct renin inhibitors should be used with the same caution as angiotensin-converting enzyme inhibitors.
If the plasma potassium concentration exceeds 5.5 mmol/L despite the above precautions, one can consider using a potassium-binding drug (see below) before deciding to avoid renin-angiotensin-aldosterone system blockers.
Sodium polystyrene sulfonate binds potassium in the gastrointestinal tract in exchange for sodium and has been used to manage hyperkalemia. This drug is most commonly given along with sorbitol as a therapy for acute hyperkalemia. Although the drug is widely used, most of the potassium-lowering effect is due to an increase in stool volume caused by sorbitol.25,26 In addition, long-term use is poorly tolerated, and the drug has been linked to gastrointestinal toxicity in rare cases.
Patiromer and sodium zirconium cyclosilicate are two new potassium-binding drugs that have been shown to be effective in reducing plasma potassium concentration in the setting of ongoing use of renin-angiotensin-aldosterone system blockers.
Patiromer is a nonabsorbed polymer approved for clinical use to treat hyperkalemia. The drug binds potassium in exchange for calcium in the gastrointestinal tract, predominantly in the colon, and lowers the plasma potassium concentration in a dose-dependent manner, with the greatest reduction in those with higher starting values.27,28
Patiromer effectively controlled plasma potassium concentrations in a 1-year randomized trial in high-risk patients on renin-angiotensin-aldosterone system blockers.29 The main adverse events in clinical trials have been constipation and hypomagnesemia, which required magnesium replacement in a small number of patients, but overall, the drug is well tolerated.
Sodium zirconium cyclosilicate is a nonabsorbed microporous compound that binds potassium in exchange for sodium throughout the gastrointestinal tract. It has been found effective in lowering plasma potassium concentration in a dose-dependent fashion in high-risk patients, most of whom were receiving renin-angiotensin-aldosterone system blockers.30–32 Adverse events were generally comparable to those with placebo in clinical trials; however, edema occurred more frequently when higher doses were used. This drug is not yet approved for clinical use.
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ 2016; 40:480–490.
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10:1050–1060.
- Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312:283–289.
- Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol 2006; 26:447–453.
- Palmer BF, Clegg DJ. Achieving the benefits of a high potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc 2016; 91:496–508.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013; 38:50–57.
- Mansoor S, Holtzman N, Emadi A. Reverse pseudohyperkalemia: an important clinical entity in chronic lymphocytic leukemia. Case Rep Hematol 2015; 2015:930379.
- Gelfand M, Zarate A, Knepshield J. Geophagia. A cause of life-threatening hyperkalemia in patients with chronic renal failure. JAMA 1975; 234:738–740.
- Abu-Hamdan D, Sondheimer J, Mahajan S. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med 1985; 79:517–519.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Daphnis E, Stylianou K, Alexandrakis M, et al. Acute renal failure, translocational hyponatremia and hyperkalemia following intravenous immunoglobulin therapy. Nephron Clin Pract 2007; 106:c143–c148.
- Choi M, Ziyadeh F. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J Am Soc Nephrol 2008; 19:424–426.
- Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis 2010; 56:387–393.
- Stanton BA. Renal potassium transport: morphological and functional adaptations. Am J Physiol 1989; 257:R989–R997.
- Hayes CP Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 1967; 80:207–216.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351:585–592.
- Palmer BF. Renal dysfunction complicating treatment of hypertension. N Engl J Med 2002; 347:1256–1261.
- Palmer BF. Renal complications associated with use of nonsteroidal anti-inflammatory agents. J Investig Med 1995; 43:516–533.
- Hoorn E, Walsh S, McCormick J, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 2011; 17:1304–1309.
- Bird ST, Pepe SR, Etminan M, Liu X, Brophy JM, Delaney JA. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol 2011; 11:23.
- Wang K. Images in clinical medicine. “Pseudoinfarction” pattern due to hyperkalemia. N Engl J Med 2004; 351:593.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol 2008; 3:324–330.
- Weisberg LS. Management of severe hyperkalemia. Crit Care Med 2008; 36:3246–3251.
- Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One 2016; 11:e0154963.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21:733–735.
- Emmett M, Hootkins RE, Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology 1995; 108:752–760.
- Bushinsky DA, Spiegel DM, Gross C, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11:1769–1776.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372:211–221.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia. The HARMONIZE randomized clinical trial. JAMA 2014; 312:2223–2233.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372:222–231.
- Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015; 17:1050–1056.
Hyperkalemia is common in patients with cardiovascular disease. Its consequences can be severe and life-threatening, and its management and prevention require a multidisciplinary approach that entails reducing intake of high-potassium foods, adjusting medications that cause hyperkalemia, and adding medications that reduce the plasma potassium concentration. With this approach, patients at high risk can receive the cardiorenal benefits of drugs that block the renin-angiotensin-aldosterone system without developing hyperkalemia.
98% OF POTASSIUM IS INSIDE CELLS
The body of a typical 70-kg man contains about 3,500 mmol of potassium, 98% of which is in the intracellular space; the remaining 2% is in the extracellular space. This large intracellular-to-extracellular gradient determines the cell voltage and explains why disorders in plasma potassium give rise to manifestations in excitable tissues such as the heart and nervous system.
The most important determinants of potassium distribution between the intracellular and extracellular space are insulin and beta-adrenergic receptor stimulation.
Maintenance of total-body potassium content is primarily the job of the kidneys, with a small contribution by the gastrointestinal tract.1,2 Hyperkalemia is most commonly encountered in patients with decreased kidney function.
The normal kidney can secrete a large amount of potassium, making hyperkalemia uncommon in the absence of kidney disease. This large capacity may have evolved to handle the diet of Paleolithic humans, which contained 4 times as much potassium as contemporary diets.3,4 With the onset of agriculture, dietary intake of potassium has progressively declined while sodium intake has risen. A popular theory suggests this mismatch between the modern diet and the nutritional requirements encoded in the human genome during evolution may contribute to chronic diseases such as hypertension, stroke, kidney stones, and bone disease.5
MANY POTENTIAL CAUSES OF HYPERKALEMIA
Causes of hyperkalemia are outlined in Table 1. Shifting of potassium from the cells to the extracellular space is a cause of transient hyperkalemia, while chronic hyperkalemia indicates an impairment in renal potassium secretion. The following discussion is a guide to the approach to the hyperkalemic patient.
Is the patient’s hyperkalemia really pseudohyperkalemia?
Pseudohyperkalemia, an artifact of measurement, occurs due to mechanical release of potassium from cells during phlebotomy or specimen processing.6 This diagnosis is made when the serum potassium concentration exceeds the plasma potassium concentration by more than 0.5 mmol/L, and should be considered when hyperkalemia occurs in the absence of a clinical risk factor. Fist-clenching, application of a tight-fitting tourniquet, or use of small-bore needles during phlebotomy can all cause pseudohyperkalemia.
Mechanism of pseudohyperkalemia. Since serum is the liquid part of blood remaining after coagulation, release of potassium from cells injured during the process of coagulation raises the potassium level in the serum. Plasma is the cell-free part of blood that has been treated with anticoagulants; it has no cells that can be injured and release potassium. Thus, the serum potassium level will be higher than that in the plasma.
Reverse pseudohyperkalemia, in contrast, occurs when the plasma potassium level is falsely elevated but the serum value is normal. This situation has been described in hematologic disorders characterized by pronounced leukocytosis in which malignant cells are prone to lysis with minimal mechanical stress due to increased fragility or altered sodium-potassium ATPase pump activity.7 This phenomenon is unusual but occurs because the cells are so fragile.
A spurious increase in plasma potassium concentration along with a low plasma calcium concentration raises the possibility of calcium chelation and release of potassium in a sample tube contaminated with the anticoagulant ethylenediaminetetraacetic acid.
Is there increased potassium intake?
Increased potassium intake is a potential cause of hyperkalemia in patients with decreased kidney function or adrenal disease.
Foods naturally rich in potassium include bananas (a medium-sized banana contains 451 mg or 12 mmol of potassium) and potatoes (844 mg or 22 mmol in a large baked potato with skin). Other potassium-rich foods are melons, citrus juice, and avocados. Less-obvious food sources include raw coconut juice (potassium concentration 44.3 mmol/L) and noni juice (56 mmol/L).
Salt substitutes, recommended to hypertensive patients with chronic kidney disease, can be a hidden source of dietary potassium.
Clay ingestion is a potential cause of dyskalemia. White clay consumption causes hypokalemia due to potassium binding in the gastrointestinal tract. Red clay or river bed clay, on the other hand, is enriched in potassium (100 mmol of potassium in 100 g of clay) and can cause life-threatening hyperkalemia in patients with chronic kidney disease.8
Eating burnt match heads. Some individuals chew and ingest burnt match heads, a condition called cautopyreiophagia. In one reported case,9 this activity contributed an additional 80 mmol of daily potassium intake in a dialysis patient, resulting in a plasma potassium concentration of 8 mmol/L.
Is the hyperkalemia the result of a cellular shift?
Acute hyperkalemia can be the result of redistribution of cellular potassium. Shifting of as little as 2% of the body’s potassium from the intracellular to the extracellular space can double the plasma potassium concentration.
Tissue injury. Hyperkalemia frequently occurs in diseases that cause tissue injury such as rhabdomyolysis, trauma, massive hemolysis, and tumor lysis.
Insulin deficiency. Insulin and catecholamines are major regulators of potassium distribution within the body. After a meal, release of insulin not only regulates the plasma glucose concentration, it also causes potassium to move into cells until the kidneys have had sufficient time to excrete the dietary potassium load and reestablish total-body potassium content.
Exercise, beta-blockers. During exercise, potassium is released from skeletal muscle cells and accumulates in the interstitial compartment, where it exerts a vasodilatory effect. The simultaneous increase in circulating catecholamines regulates this release by promoting cell potassium uptake through beta-adrenergic receptor stimulation.
Metabolic acidosis can facilitate exit (ie, shift) of potassium from cells, but this effect depends on the type of acidosis. Hyperchloremic normal anion gap acidosis (mineral acidosis) most commonly causes this effect due to the relative impermeability of the cell membrane to the chloride anion. As hydrogen ions move into the cell due to accumulation of ammonium chloride or hydrogen chloride, electrical neutrality is maintained by potassium exit.
In contrast, organic acidosis (due to lactic, beta-hydroxybutyric, or methylmalonic acid) tends not to cause a potassium shift, since most organic anions readily cross the cell membrane along with hydrogen. Lactic acidosis is often associated with potassium shift, but this effect is due to loss of cell integrity as a result of cell ischemia. The hyperkalemia typically present on admission in patients with diabetic ketoacidosis is the result of insulin deficiency and hypertonicity and not the underlying organic acidosis.10
Hypertonic states can cause hyperkalemia due to cell shift. For example, hyperglycemia, as in diabetic ketoacidosis, pulls water from the intracellular into the extracellular compartment, thereby concentrating intracellular potassium and creating a more favorable gradient for potassium efflux through membrane channels. This same effect can occur in neurosurgical patients given large amounts of hypertonic mannitol. Repetitive doses of immunoglobulin can lead to extracellular accumulation of sorbitol, maltose, or sucrose, since these sugars are added to the preparations to prevent immunoglobulin aggregation.11
Is a disturbance in renal potassium excretion present?
Sustained hyperkalemia is more commonly associated with decreases in renal potassium excretion than with a cellular shift. In most instances the clinician can distinguish between cell shift and impaired renal excretion based on the available clinical data.
The transtubular potassium gradient has been used to determine whether there is a disturbance in renal potassium excretion and to assess renal potassium handling.12
This calculation is based on the assumption that only water is reabsorbed past the cortical collecting duct, and not solutes. It has fallen out of favor since we have found this assumption to be incorrect; a large amount of urea is reabsorbed daily in the downstream medullary collecting duct as a result of intrarenal recycling of urea.
The one situation in which the transtubular potassium gradient may be of use is determining whether hyperkalemia is a result of low aldosterone levels as opposed to aldosterone resistance. One can compare the transtubular potassium gradient before and after a physiologic dose (0.05 mg) of 9-alpha fludrocortisone. An increase of more than 6 over a 4-hour period favors aldosterone deficiency, whereas smaller changes would indicate aldosterone resistance.
24-hour potassium excretion, spot urine potassium-creatinine ratio. A better way to assess renal potassium handling is to measure the amount of potassium in a 24-hour urine collection or determine a spot urine potassium-creatinine ratio. A 24-hour urinary potassium excretion of less than 15 mmol or a potassium-creatinine ratio less than 1 suggests an extrarenal cause of hypokalemia. A ratio greater than 20 would be an appropriate renal response to hyperkalemia.
One or more of 3 abnormalities should be considered in the hyperkalemic patient with impaired renal excretion of potassium:
- Decreased distal delivery of sodium
- Mineralocorticoid deficiency
- Abnormal cortical collecting tubule function.13
Decreased distal delivery of sodium
Under normal circumstances, potassium is freely filtered across the glomerulus and then mostly reabsorbed in the proximal tubule and thick ascending limb. Potassium secretion begins in the distal convoluted tubule and increases in magnitude into the collecting duct. Tubular secretion is the component of potassium handling that varies and is regulated according to physiologic needs.
In acute kidney injury, the rapid decline in glomerular filtration rate and reduction in functioning nephron mass lead to decreased distal potassium secretion.
Hyperkalemia is a frequent problem when oliguria is present, since the reduction in distal delivery of sodium and water further impairs potassium secretion. Patients with oliguric acute kidney injury are more likely to have a more severe underlying disease state, and therefore tissue breakdown and catabolism further increase the risk of hyperkalemia.
In contrast, in nonoliguric patients, the renal injury tends to be less severe, and enough sodium and water are usually delivered distally to prevent hyperkalemia.
In chronic kidney disease, nephron dropout and reduction in collecting tubule mass also lead to a global decline in distal potassium secretion. However, this is countered by an increased capacity of the remaining individual nephrons for potassium secretion. High flow, increased distal sodium delivery, and increased activity and number of sodium-potassium ATPase pumps in the remaining nephrons account for this increased secretory capacity.14 As renal function declines over time, colonic potassium secretion progressively increases.15
These adaptive changes help to keep the plasma potassium concentration within the normal range until the glomerular filtration rate falls to less than 10 or 15 mL/min. Development of hyperkalemia with more modest reductions in the glomerular filtration rate suggest decreased mineralocorticoid activity or a specific lesion of the tubule.
Mineralocorticoid deficiency
Aldosterone deficiency can occur alone or in combination with decreased cortisol levels. Destruction of the adrenal glands is suggested when both hormones are reduced. Enzyme defects in cortisol metabolism can result in either isolated deficiency of aldosterone or adrenogenital syndromes associated with decreased mineralocorticoid activity.
Heparin administration leads to a reversible defect in adrenal synthesis of aldosterone. Drugs that block the stimulatory effect of angiotensin II on the zona glomerulosa cells of the adrenal gland will lower aldosterone.
Renin-angiotensin-aldosterone system blockers. Angiotensin-converting enzyme inhibitors block the formation of angiotensin II, whereas angiotensin II receptor blockers prevent angiotensin II from binding to its adrenal receptor. The direct renin inhibitor aliskiren lowers angiotensin II levels by blocking the enzymatic activity of renin and lowers the circulating levels of both angiotensin I and II.16
The syndrome of hyporeninemic hypoaldosteronism is a common cause of hyperkalemia in patients who have a glomerular filtration rate between 40 and 60 mL/min. Diabetic nephropathy and interstitial renal disease are the most common clinical entities associated with this syndrome.10 Other causes include analgesic nephropathy, urinary tract obstruction, sickle cell disease, systemic lupus erythematosus, and amyloidosis.
Nonsteroidal anti-inflammatory drugs can cause hyperkalemia by suppressing renin release and reducing delivery of sodium to the distal nephron.18
Calcineurin inhibitors impair potassium secretion by suppressing renin release and by direct tubular effects.19
Beta-blockers. Beta-1 and to a lesser extent beta-2 receptor blockade can also result in a hyporeninemic state.
Distal tubular defect
Hyperkalemia can result from interstitial renal diseases that specifically affect the distal nephron. In this setting, the glomerular filtration rate is only mildly reduced, and circulating aldosterone levels are normal.
Renal transplant, lupus erythematosus, amyloidosis, urinary obstruction, and sickle cell disease are conditions in which an impairment in renin release may coexist with a defect in tubular secretion.
Potassium-sparing diuretics impair the ability of the cortical collecting tubule to secrete potassium. Specifically, amiloride and triamterene inhibit sodium reabsorption mediated by the epithelial sodium channel located on the apical membrane of the principal cell. This effect abolishes the lumen’s negative potential and thereby removes a driving force for potassium secretion.
Trimethoprim and pentamidine cause similar effects.
Spironolactone and eplerenone compete with aldosterone at the level of the mineralocorticoid receptor and can result in hyperkalemia.
Drospirenone, a non-testosterone-derived progestin contained in certain oral contraceptives, possesses mineralocorticoid-blocking effects similar to those of spironolactone.
The plasma potassium level should be monitored when these drugs are prescribed in patients receiving potassium supplements, renin-angiotensin-aldosterone system blockers, or nonsteroidal anti-inflammatory drugs.20
CLINICAL FEATURES OF HYPERKALEMIA
Neuromuscular manifestations of hyperkalemia include paresthesias and fasciculations in the arms and legs. Severe elevation in potassium can give rise to an ascending paralysis with eventual flaccid quadriplegia. Typically, the trunk, head, and respiratory muscles are spared, and respiratory failure is rare.
Cardiac signs
Hyperkalemia has depolarizing effects on the heart that are manifested by changes in the electrocardiogram (Figure 2). The progressive changes of hyperkalemia are classically listed as:
- Peaked T waves that are tall, narrow, and symmetrical and can occasionally be confused with the hyperacute T-wave change associated with an ST-segment elevation myocardial infarction.21 However, in the latter condition, the T waves tend to be more broad-based and asymmetric in shape.
- ST-segment depression
- Widening of the PR interval
- Widening of the QRS interval
- Loss of the P wave
- A sine-wave pattern—an ominous development and a harbinger of impending ventricular fibrillation and asystole.
The plasma potassium concentration often correlates poorly with cardiac manifestations. In a retrospective review, only 16 of 90 cases met strict criteria for electrocardiographic changes reflective of hyperkalemia (defined as new peaked and symmetric T waves that resolved on follow-up).22 In 13 of these cases, the electrocardiogram was interpreted as showing no T-wave changes even when read by a cardiologist. In addition, electrocardiographic criteria for hyperkalemia were noted in only 1 of 14 patients who manifested arrhythmias or cardiac arrest attributed to increased plasma potassium concentration.
TREATMENT OF ACUTE HYPERKALEMIA
The treatment of hyperkalemia depends on the magnitude of increase in the plasma potassium concentration and the presence or absence of electrocardiographic changes or neuromuscular symptoms.23 Acute treatment is indicated for marked electrocardiographic changes and severe muscle weakness.
Intravenous calcium rapidly normalizes membrane excitability by antagonizing the potassium-induced decrease in membrane excitability but does not alter the plasma potassium concentration.
Insulin lowers the plasma potassium concentration by promoting its entry into cells. To avoid hypoglycemia, 10 units of short-acting insulin should be accompanied by a 50-g infusion of glucose, increased to 60 g if 20 units of insulin are given.24
Beta-2 receptor agonists produce a similar effect. The shift of potassium into cells with insulin and beta-2-adrenergic receptor stimulation is brought about by increases in sodium-potassium ATPase pump activity, primarily in skeletal muscle cells.
Sodium bicarbonate, in the absence of acidosis, lowers the plasma potassium concentration only slightly. It should be reserved for hyperkalemic patients who have coexisting metabolic acidosis after the patient has received insulin and glucose, an adrenergic agent, and calcium.
These acute treatments need to be followed by therapies designed to lower the total body potassium content such as diuretics, potassium-binding drugs, and dialysis.
TREATMENT OF CHRONIC HYPERKALEMIA
Review medications. Once the diagnosis of hyperkalemia has been made, the initial approach should be to review the patient’s medications and make every effort to discontinue drugs that can impair renal potassium excretion.16 Patients should be asked about their use of over-the-counter nonsteroidal anti-inflammatory drugs and herbal remedies, since herbs may be a hidden source of dietary potassium.
Dietary counseling. Patients should be instructed to reduce their dietary intake of potassium and to avoid salt substitutes that contain potassium.
Diuretic therapy is beneficial in minimizing hyperkalemia in patients with chronic kidney disease. Thiazide and loop diuretics enhance renal potassium excretion by increasing flow and delivery of sodium to the collecting duct. Thiazide diuretics are effective when the estimated glomerular filtration rate is greater than 30 mL/min, while loop diuretics should be used in patients with more severe renal insufficiency (Table 2).
Sodium bicarbonate is an effective agent to minimize increases in the plasma potassium concentration in patients with chronic kidney disease and metabolic acidosis. This drug increases renal potassium excretion by increasing distal sodium delivery and shifts potassium into cells as the acidosis is corrected. The likelihood of developing volume overload as a complication of sodium bicarbonate administration can be minimized with effective diuretic therapy.
Avoiding hyperkalemia if renin-angiotensin-aldosterone system blockers are needed
Renin-angiotensin-aldosterone system blockers can be problematic, as these drugs cause hyperkalemia, often in the very patients who derive the greatest cardiovascular benefit from them.16 A number of steps can reduce the risk of hyperkalemia and allow these drugs to be used.
The initial dose should be low and the plasma potassium should be measured within 1 to 2 weeks after drug initiation. If the potassium level is normal, the dose can be titrated upwards with remeasurement of the plasma potassium after each dose titration. If the plasma potassium concentration rises to 5.5 mmol/L, in some cases lowering the dose will reduce the potassium concentration and allow the patient to remain on the drug.
In patients at risk of hyperkalemia, angiotensin II receptor blockers and direct renin inhibitors should be used with the same caution as angiotensin-converting enzyme inhibitors.
If the plasma potassium concentration exceeds 5.5 mmol/L despite the above precautions, one can consider using a potassium-binding drug (see below) before deciding to avoid renin-angiotensin-aldosterone system blockers.
Sodium polystyrene sulfonate binds potassium in the gastrointestinal tract in exchange for sodium and has been used to manage hyperkalemia. This drug is most commonly given along with sorbitol as a therapy for acute hyperkalemia. Although the drug is widely used, most of the potassium-lowering effect is due to an increase in stool volume caused by sorbitol.25,26 In addition, long-term use is poorly tolerated, and the drug has been linked to gastrointestinal toxicity in rare cases.
Patiromer and sodium zirconium cyclosilicate are two new potassium-binding drugs that have been shown to be effective in reducing plasma potassium concentration in the setting of ongoing use of renin-angiotensin-aldosterone system blockers.
Patiromer is a nonabsorbed polymer approved for clinical use to treat hyperkalemia. The drug binds potassium in exchange for calcium in the gastrointestinal tract, predominantly in the colon, and lowers the plasma potassium concentration in a dose-dependent manner, with the greatest reduction in those with higher starting values.27,28
Patiromer effectively controlled plasma potassium concentrations in a 1-year randomized trial in high-risk patients on renin-angiotensin-aldosterone system blockers.29 The main adverse events in clinical trials have been constipation and hypomagnesemia, which required magnesium replacement in a small number of patients, but overall, the drug is well tolerated.
Sodium zirconium cyclosilicate is a nonabsorbed microporous compound that binds potassium in exchange for sodium throughout the gastrointestinal tract. It has been found effective in lowering plasma potassium concentration in a dose-dependent fashion in high-risk patients, most of whom were receiving renin-angiotensin-aldosterone system blockers.30–32 Adverse events were generally comparable to those with placebo in clinical trials; however, edema occurred more frequently when higher doses were used. This drug is not yet approved for clinical use.
Hyperkalemia is common in patients with cardiovascular disease. Its consequences can be severe and life-threatening, and its management and prevention require a multidisciplinary approach that entails reducing intake of high-potassium foods, adjusting medications that cause hyperkalemia, and adding medications that reduce the plasma potassium concentration. With this approach, patients at high risk can receive the cardiorenal benefits of drugs that block the renin-angiotensin-aldosterone system without developing hyperkalemia.
98% OF POTASSIUM IS INSIDE CELLS
The body of a typical 70-kg man contains about 3,500 mmol of potassium, 98% of which is in the intracellular space; the remaining 2% is in the extracellular space. This large intracellular-to-extracellular gradient determines the cell voltage and explains why disorders in plasma potassium give rise to manifestations in excitable tissues such as the heart and nervous system.
The most important determinants of potassium distribution between the intracellular and extracellular space are insulin and beta-adrenergic receptor stimulation.
Maintenance of total-body potassium content is primarily the job of the kidneys, with a small contribution by the gastrointestinal tract.1,2 Hyperkalemia is most commonly encountered in patients with decreased kidney function.
The normal kidney can secrete a large amount of potassium, making hyperkalemia uncommon in the absence of kidney disease. This large capacity may have evolved to handle the diet of Paleolithic humans, which contained 4 times as much potassium as contemporary diets.3,4 With the onset of agriculture, dietary intake of potassium has progressively declined while sodium intake has risen. A popular theory suggests this mismatch between the modern diet and the nutritional requirements encoded in the human genome during evolution may contribute to chronic diseases such as hypertension, stroke, kidney stones, and bone disease.5
MANY POTENTIAL CAUSES OF HYPERKALEMIA
Causes of hyperkalemia are outlined in Table 1. Shifting of potassium from the cells to the extracellular space is a cause of transient hyperkalemia, while chronic hyperkalemia indicates an impairment in renal potassium secretion. The following discussion is a guide to the approach to the hyperkalemic patient.
Is the patient’s hyperkalemia really pseudohyperkalemia?
Pseudohyperkalemia, an artifact of measurement, occurs due to mechanical release of potassium from cells during phlebotomy or specimen processing.6 This diagnosis is made when the serum potassium concentration exceeds the plasma potassium concentration by more than 0.5 mmol/L, and should be considered when hyperkalemia occurs in the absence of a clinical risk factor. Fist-clenching, application of a tight-fitting tourniquet, or use of small-bore needles during phlebotomy can all cause pseudohyperkalemia.
Mechanism of pseudohyperkalemia. Since serum is the liquid part of blood remaining after coagulation, release of potassium from cells injured during the process of coagulation raises the potassium level in the serum. Plasma is the cell-free part of blood that has been treated with anticoagulants; it has no cells that can be injured and release potassium. Thus, the serum potassium level will be higher than that in the plasma.
Reverse pseudohyperkalemia, in contrast, occurs when the plasma potassium level is falsely elevated but the serum value is normal. This situation has been described in hematologic disorders characterized by pronounced leukocytosis in which malignant cells are prone to lysis with minimal mechanical stress due to increased fragility or altered sodium-potassium ATPase pump activity.7 This phenomenon is unusual but occurs because the cells are so fragile.
A spurious increase in plasma potassium concentration along with a low plasma calcium concentration raises the possibility of calcium chelation and release of potassium in a sample tube contaminated with the anticoagulant ethylenediaminetetraacetic acid.
Is there increased potassium intake?
Increased potassium intake is a potential cause of hyperkalemia in patients with decreased kidney function or adrenal disease.
Foods naturally rich in potassium include bananas (a medium-sized banana contains 451 mg or 12 mmol of potassium) and potatoes (844 mg or 22 mmol in a large baked potato with skin). Other potassium-rich foods are melons, citrus juice, and avocados. Less-obvious food sources include raw coconut juice (potassium concentration 44.3 mmol/L) and noni juice (56 mmol/L).
Salt substitutes, recommended to hypertensive patients with chronic kidney disease, can be a hidden source of dietary potassium.
Clay ingestion is a potential cause of dyskalemia. White clay consumption causes hypokalemia due to potassium binding in the gastrointestinal tract. Red clay or river bed clay, on the other hand, is enriched in potassium (100 mmol of potassium in 100 g of clay) and can cause life-threatening hyperkalemia in patients with chronic kidney disease.8
Eating burnt match heads. Some individuals chew and ingest burnt match heads, a condition called cautopyreiophagia. In one reported case,9 this activity contributed an additional 80 mmol of daily potassium intake in a dialysis patient, resulting in a plasma potassium concentration of 8 mmol/L.
Is the hyperkalemia the result of a cellular shift?
Acute hyperkalemia can be the result of redistribution of cellular potassium. Shifting of as little as 2% of the body’s potassium from the intracellular to the extracellular space can double the plasma potassium concentration.
Tissue injury. Hyperkalemia frequently occurs in diseases that cause tissue injury such as rhabdomyolysis, trauma, massive hemolysis, and tumor lysis.
Insulin deficiency. Insulin and catecholamines are major regulators of potassium distribution within the body. After a meal, release of insulin not only regulates the plasma glucose concentration, it also causes potassium to move into cells until the kidneys have had sufficient time to excrete the dietary potassium load and reestablish total-body potassium content.
Exercise, beta-blockers. During exercise, potassium is released from skeletal muscle cells and accumulates in the interstitial compartment, where it exerts a vasodilatory effect. The simultaneous increase in circulating catecholamines regulates this release by promoting cell potassium uptake through beta-adrenergic receptor stimulation.
Metabolic acidosis can facilitate exit (ie, shift) of potassium from cells, but this effect depends on the type of acidosis. Hyperchloremic normal anion gap acidosis (mineral acidosis) most commonly causes this effect due to the relative impermeability of the cell membrane to the chloride anion. As hydrogen ions move into the cell due to accumulation of ammonium chloride or hydrogen chloride, electrical neutrality is maintained by potassium exit.
In contrast, organic acidosis (due to lactic, beta-hydroxybutyric, or methylmalonic acid) tends not to cause a potassium shift, since most organic anions readily cross the cell membrane along with hydrogen. Lactic acidosis is often associated with potassium shift, but this effect is due to loss of cell integrity as a result of cell ischemia. The hyperkalemia typically present on admission in patients with diabetic ketoacidosis is the result of insulin deficiency and hypertonicity and not the underlying organic acidosis.10
Hypertonic states can cause hyperkalemia due to cell shift. For example, hyperglycemia, as in diabetic ketoacidosis, pulls water from the intracellular into the extracellular compartment, thereby concentrating intracellular potassium and creating a more favorable gradient for potassium efflux through membrane channels. This same effect can occur in neurosurgical patients given large amounts of hypertonic mannitol. Repetitive doses of immunoglobulin can lead to extracellular accumulation of sorbitol, maltose, or sucrose, since these sugars are added to the preparations to prevent immunoglobulin aggregation.11
Is a disturbance in renal potassium excretion present?
Sustained hyperkalemia is more commonly associated with decreases in renal potassium excretion than with a cellular shift. In most instances the clinician can distinguish between cell shift and impaired renal excretion based on the available clinical data.
The transtubular potassium gradient has been used to determine whether there is a disturbance in renal potassium excretion and to assess renal potassium handling.12
This calculation is based on the assumption that only water is reabsorbed past the cortical collecting duct, and not solutes. It has fallen out of favor since we have found this assumption to be incorrect; a large amount of urea is reabsorbed daily in the downstream medullary collecting duct as a result of intrarenal recycling of urea.
The one situation in which the transtubular potassium gradient may be of use is determining whether hyperkalemia is a result of low aldosterone levels as opposed to aldosterone resistance. One can compare the transtubular potassium gradient before and after a physiologic dose (0.05 mg) of 9-alpha fludrocortisone. An increase of more than 6 over a 4-hour period favors aldosterone deficiency, whereas smaller changes would indicate aldosterone resistance.
24-hour potassium excretion, spot urine potassium-creatinine ratio. A better way to assess renal potassium handling is to measure the amount of potassium in a 24-hour urine collection or determine a spot urine potassium-creatinine ratio. A 24-hour urinary potassium excretion of less than 15 mmol or a potassium-creatinine ratio less than 1 suggests an extrarenal cause of hypokalemia. A ratio greater than 20 would be an appropriate renal response to hyperkalemia.
One or more of 3 abnormalities should be considered in the hyperkalemic patient with impaired renal excretion of potassium:
- Decreased distal delivery of sodium
- Mineralocorticoid deficiency
- Abnormal cortical collecting tubule function.13
Decreased distal delivery of sodium
Under normal circumstances, potassium is freely filtered across the glomerulus and then mostly reabsorbed in the proximal tubule and thick ascending limb. Potassium secretion begins in the distal convoluted tubule and increases in magnitude into the collecting duct. Tubular secretion is the component of potassium handling that varies and is regulated according to physiologic needs.
In acute kidney injury, the rapid decline in glomerular filtration rate and reduction in functioning nephron mass lead to decreased distal potassium secretion.
Hyperkalemia is a frequent problem when oliguria is present, since the reduction in distal delivery of sodium and water further impairs potassium secretion. Patients with oliguric acute kidney injury are more likely to have a more severe underlying disease state, and therefore tissue breakdown and catabolism further increase the risk of hyperkalemia.
In contrast, in nonoliguric patients, the renal injury tends to be less severe, and enough sodium and water are usually delivered distally to prevent hyperkalemia.
In chronic kidney disease, nephron dropout and reduction in collecting tubule mass also lead to a global decline in distal potassium secretion. However, this is countered by an increased capacity of the remaining individual nephrons for potassium secretion. High flow, increased distal sodium delivery, and increased activity and number of sodium-potassium ATPase pumps in the remaining nephrons account for this increased secretory capacity.14 As renal function declines over time, colonic potassium secretion progressively increases.15
These adaptive changes help to keep the plasma potassium concentration within the normal range until the glomerular filtration rate falls to less than 10 or 15 mL/min. Development of hyperkalemia with more modest reductions in the glomerular filtration rate suggest decreased mineralocorticoid activity or a specific lesion of the tubule.
Mineralocorticoid deficiency
Aldosterone deficiency can occur alone or in combination with decreased cortisol levels. Destruction of the adrenal glands is suggested when both hormones are reduced. Enzyme defects in cortisol metabolism can result in either isolated deficiency of aldosterone or adrenogenital syndromes associated with decreased mineralocorticoid activity.
Heparin administration leads to a reversible defect in adrenal synthesis of aldosterone. Drugs that block the stimulatory effect of angiotensin II on the zona glomerulosa cells of the adrenal gland will lower aldosterone.
Renin-angiotensin-aldosterone system blockers. Angiotensin-converting enzyme inhibitors block the formation of angiotensin II, whereas angiotensin II receptor blockers prevent angiotensin II from binding to its adrenal receptor. The direct renin inhibitor aliskiren lowers angiotensin II levels by blocking the enzymatic activity of renin and lowers the circulating levels of both angiotensin I and II.16
The syndrome of hyporeninemic hypoaldosteronism is a common cause of hyperkalemia in patients who have a glomerular filtration rate between 40 and 60 mL/min. Diabetic nephropathy and interstitial renal disease are the most common clinical entities associated with this syndrome.10 Other causes include analgesic nephropathy, urinary tract obstruction, sickle cell disease, systemic lupus erythematosus, and amyloidosis.
Nonsteroidal anti-inflammatory drugs can cause hyperkalemia by suppressing renin release and reducing delivery of sodium to the distal nephron.18
Calcineurin inhibitors impair potassium secretion by suppressing renin release and by direct tubular effects.19
Beta-blockers. Beta-1 and to a lesser extent beta-2 receptor blockade can also result in a hyporeninemic state.
Distal tubular defect
Hyperkalemia can result from interstitial renal diseases that specifically affect the distal nephron. In this setting, the glomerular filtration rate is only mildly reduced, and circulating aldosterone levels are normal.
Renal transplant, lupus erythematosus, amyloidosis, urinary obstruction, and sickle cell disease are conditions in which an impairment in renin release may coexist with a defect in tubular secretion.
Potassium-sparing diuretics impair the ability of the cortical collecting tubule to secrete potassium. Specifically, amiloride and triamterene inhibit sodium reabsorption mediated by the epithelial sodium channel located on the apical membrane of the principal cell. This effect abolishes the lumen’s negative potential and thereby removes a driving force for potassium secretion.
Trimethoprim and pentamidine cause similar effects.
Spironolactone and eplerenone compete with aldosterone at the level of the mineralocorticoid receptor and can result in hyperkalemia.
Drospirenone, a non-testosterone-derived progestin contained in certain oral contraceptives, possesses mineralocorticoid-blocking effects similar to those of spironolactone.
The plasma potassium level should be monitored when these drugs are prescribed in patients receiving potassium supplements, renin-angiotensin-aldosterone system blockers, or nonsteroidal anti-inflammatory drugs.20
CLINICAL FEATURES OF HYPERKALEMIA
Neuromuscular manifestations of hyperkalemia include paresthesias and fasciculations in the arms and legs. Severe elevation in potassium can give rise to an ascending paralysis with eventual flaccid quadriplegia. Typically, the trunk, head, and respiratory muscles are spared, and respiratory failure is rare.
Cardiac signs
Hyperkalemia has depolarizing effects on the heart that are manifested by changes in the electrocardiogram (Figure 2). The progressive changes of hyperkalemia are classically listed as:
- Peaked T waves that are tall, narrow, and symmetrical and can occasionally be confused with the hyperacute T-wave change associated with an ST-segment elevation myocardial infarction.21 However, in the latter condition, the T waves tend to be more broad-based and asymmetric in shape.
- ST-segment depression
- Widening of the PR interval
- Widening of the QRS interval
- Loss of the P wave
- A sine-wave pattern—an ominous development and a harbinger of impending ventricular fibrillation and asystole.
The plasma potassium concentration often correlates poorly with cardiac manifestations. In a retrospective review, only 16 of 90 cases met strict criteria for electrocardiographic changes reflective of hyperkalemia (defined as new peaked and symmetric T waves that resolved on follow-up).22 In 13 of these cases, the electrocardiogram was interpreted as showing no T-wave changes even when read by a cardiologist. In addition, electrocardiographic criteria for hyperkalemia were noted in only 1 of 14 patients who manifested arrhythmias or cardiac arrest attributed to increased plasma potassium concentration.
TREATMENT OF ACUTE HYPERKALEMIA
The treatment of hyperkalemia depends on the magnitude of increase in the plasma potassium concentration and the presence or absence of electrocardiographic changes or neuromuscular symptoms.23 Acute treatment is indicated for marked electrocardiographic changes and severe muscle weakness.
Intravenous calcium rapidly normalizes membrane excitability by antagonizing the potassium-induced decrease in membrane excitability but does not alter the plasma potassium concentration.
Insulin lowers the plasma potassium concentration by promoting its entry into cells. To avoid hypoglycemia, 10 units of short-acting insulin should be accompanied by a 50-g infusion of glucose, increased to 60 g if 20 units of insulin are given.24
Beta-2 receptor agonists produce a similar effect. The shift of potassium into cells with insulin and beta-2-adrenergic receptor stimulation is brought about by increases in sodium-potassium ATPase pump activity, primarily in skeletal muscle cells.
Sodium bicarbonate, in the absence of acidosis, lowers the plasma potassium concentration only slightly. It should be reserved for hyperkalemic patients who have coexisting metabolic acidosis after the patient has received insulin and glucose, an adrenergic agent, and calcium.
These acute treatments need to be followed by therapies designed to lower the total body potassium content such as diuretics, potassium-binding drugs, and dialysis.
TREATMENT OF CHRONIC HYPERKALEMIA
Review medications. Once the diagnosis of hyperkalemia has been made, the initial approach should be to review the patient’s medications and make every effort to discontinue drugs that can impair renal potassium excretion.16 Patients should be asked about their use of over-the-counter nonsteroidal anti-inflammatory drugs and herbal remedies, since herbs may be a hidden source of dietary potassium.
Dietary counseling. Patients should be instructed to reduce their dietary intake of potassium and to avoid salt substitutes that contain potassium.
Diuretic therapy is beneficial in minimizing hyperkalemia in patients with chronic kidney disease. Thiazide and loop diuretics enhance renal potassium excretion by increasing flow and delivery of sodium to the collecting duct. Thiazide diuretics are effective when the estimated glomerular filtration rate is greater than 30 mL/min, while loop diuretics should be used in patients with more severe renal insufficiency (Table 2).
Sodium bicarbonate is an effective agent to minimize increases in the plasma potassium concentration in patients with chronic kidney disease and metabolic acidosis. This drug increases renal potassium excretion by increasing distal sodium delivery and shifts potassium into cells as the acidosis is corrected. The likelihood of developing volume overload as a complication of sodium bicarbonate administration can be minimized with effective diuretic therapy.
Avoiding hyperkalemia if renin-angiotensin-aldosterone system blockers are needed
Renin-angiotensin-aldosterone system blockers can be problematic, as these drugs cause hyperkalemia, often in the very patients who derive the greatest cardiovascular benefit from them.16 A number of steps can reduce the risk of hyperkalemia and allow these drugs to be used.
The initial dose should be low and the plasma potassium should be measured within 1 to 2 weeks after drug initiation. If the potassium level is normal, the dose can be titrated upwards with remeasurement of the plasma potassium after each dose titration. If the plasma potassium concentration rises to 5.5 mmol/L, in some cases lowering the dose will reduce the potassium concentration and allow the patient to remain on the drug.
In patients at risk of hyperkalemia, angiotensin II receptor blockers and direct renin inhibitors should be used with the same caution as angiotensin-converting enzyme inhibitors.
If the plasma potassium concentration exceeds 5.5 mmol/L despite the above precautions, one can consider using a potassium-binding drug (see below) before deciding to avoid renin-angiotensin-aldosterone system blockers.
Sodium polystyrene sulfonate binds potassium in the gastrointestinal tract in exchange for sodium and has been used to manage hyperkalemia. This drug is most commonly given along with sorbitol as a therapy for acute hyperkalemia. Although the drug is widely used, most of the potassium-lowering effect is due to an increase in stool volume caused by sorbitol.25,26 In addition, long-term use is poorly tolerated, and the drug has been linked to gastrointestinal toxicity in rare cases.
Patiromer and sodium zirconium cyclosilicate are two new potassium-binding drugs that have been shown to be effective in reducing plasma potassium concentration in the setting of ongoing use of renin-angiotensin-aldosterone system blockers.
Patiromer is a nonabsorbed polymer approved for clinical use to treat hyperkalemia. The drug binds potassium in exchange for calcium in the gastrointestinal tract, predominantly in the colon, and lowers the plasma potassium concentration in a dose-dependent manner, with the greatest reduction in those with higher starting values.27,28
Patiromer effectively controlled plasma potassium concentrations in a 1-year randomized trial in high-risk patients on renin-angiotensin-aldosterone system blockers.29 The main adverse events in clinical trials have been constipation and hypomagnesemia, which required magnesium replacement in a small number of patients, but overall, the drug is well tolerated.
Sodium zirconium cyclosilicate is a nonabsorbed microporous compound that binds potassium in exchange for sodium throughout the gastrointestinal tract. It has been found effective in lowering plasma potassium concentration in a dose-dependent fashion in high-risk patients, most of whom were receiving renin-angiotensin-aldosterone system blockers.30–32 Adverse events were generally comparable to those with placebo in clinical trials; however, edema occurred more frequently when higher doses were used. This drug is not yet approved for clinical use.
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ 2016; 40:480–490.
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10:1050–1060.
- Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312:283–289.
- Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol 2006; 26:447–453.
- Palmer BF, Clegg DJ. Achieving the benefits of a high potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc 2016; 91:496–508.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013; 38:50–57.
- Mansoor S, Holtzman N, Emadi A. Reverse pseudohyperkalemia: an important clinical entity in chronic lymphocytic leukemia. Case Rep Hematol 2015; 2015:930379.
- Gelfand M, Zarate A, Knepshield J. Geophagia. A cause of life-threatening hyperkalemia in patients with chronic renal failure. JAMA 1975; 234:738–740.
- Abu-Hamdan D, Sondheimer J, Mahajan S. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med 1985; 79:517–519.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Daphnis E, Stylianou K, Alexandrakis M, et al. Acute renal failure, translocational hyponatremia and hyperkalemia following intravenous immunoglobulin therapy. Nephron Clin Pract 2007; 106:c143–c148.
- Choi M, Ziyadeh F. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J Am Soc Nephrol 2008; 19:424–426.
- Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis 2010; 56:387–393.
- Stanton BA. Renal potassium transport: morphological and functional adaptations. Am J Physiol 1989; 257:R989–R997.
- Hayes CP Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 1967; 80:207–216.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351:585–592.
- Palmer BF. Renal dysfunction complicating treatment of hypertension. N Engl J Med 2002; 347:1256–1261.
- Palmer BF. Renal complications associated with use of nonsteroidal anti-inflammatory agents. J Investig Med 1995; 43:516–533.
- Hoorn E, Walsh S, McCormick J, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 2011; 17:1304–1309.
- Bird ST, Pepe SR, Etminan M, Liu X, Brophy JM, Delaney JA. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol 2011; 11:23.
- Wang K. Images in clinical medicine. “Pseudoinfarction” pattern due to hyperkalemia. N Engl J Med 2004; 351:593.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol 2008; 3:324–330.
- Weisberg LS. Management of severe hyperkalemia. Crit Care Med 2008; 36:3246–3251.
- Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One 2016; 11:e0154963.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21:733–735.
- Emmett M, Hootkins RE, Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology 1995; 108:752–760.
- Bushinsky DA, Spiegel DM, Gross C, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11:1769–1776.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372:211–221.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia. The HARMONIZE randomized clinical trial. JAMA 2014; 312:2223–2233.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372:222–231.
- Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015; 17:1050–1056.
- Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ 2016; 40:480–490.
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10:1050–1060.
- Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med 1985; 312:283–289.
- Sebastian A, Frassetto LA, Sellmeyer DE, Morris RC Jr. The evolution-informed optimal dietary potassium intake of human beings greatly exceeds current and recommended intakes. Semin Nephrol 2006; 26:447–453.
- Palmer BF, Clegg DJ. Achieving the benefits of a high potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc 2016; 91:496–508.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013; 38:50–57.
- Mansoor S, Holtzman N, Emadi A. Reverse pseudohyperkalemia: an important clinical entity in chronic lymphocytic leukemia. Case Rep Hematol 2015; 2015:930379.
- Gelfand M, Zarate A, Knepshield J. Geophagia. A cause of life-threatening hyperkalemia in patients with chronic renal failure. JAMA 1975; 234:738–740.
- Abu-Hamdan D, Sondheimer J, Mahajan S. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med 1985; 79:517–519.
- Palmer BF, Clegg DJ. Electrolyte and acid-base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373:548–559.
- Daphnis E, Stylianou K, Alexandrakis M, et al. Acute renal failure, translocational hyponatremia and hyperkalemia following intravenous immunoglobulin therapy. Nephron Clin Pract 2007; 106:c143–c148.
- Choi M, Ziyadeh F. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J Am Soc Nephrol 2008; 19:424–426.
- Palmer BF. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis 2010; 56:387–393.
- Stanton BA. Renal potassium transport: morphological and functional adaptations. Am J Physiol 1989; 257:R989–R997.
- Hayes CP Jr, McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians 1967; 80:207–216.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004; 351:585–592.
- Palmer BF. Renal dysfunction complicating treatment of hypertension. N Engl J Med 2002; 347:1256–1261.
- Palmer BF. Renal complications associated with use of nonsteroidal anti-inflammatory agents. J Investig Med 1995; 43:516–533.
- Hoorn E, Walsh S, McCormick J, et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 2011; 17:1304–1309.
- Bird ST, Pepe SR, Etminan M, Liu X, Brophy JM, Delaney JA. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol 2011; 11:23.
- Wang K. Images in clinical medicine. “Pseudoinfarction” pattern due to hyperkalemia. N Engl J Med 2004; 351:593.
- Montague BT, Ouellette JR, Buller GK. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol 2008; 3:324–330.
- Weisberg LS. Management of severe hyperkalemia. Crit Care Med 2008; 36:3246–3251.
- Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One 2016; 11:e0154963.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21:733–735.
- Emmett M, Hootkins RE, Fine KD, Santa Ana CA, Porter JL, Fordtran JS. Effect of three laxatives and a cation exchange resin on fecal sodium and potassium excretion. Gastroenterology 1995; 108:752–760.
- Bushinsky DA, Spiegel DM, Gross C, et al. Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 2016; 11:1769–1776.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372:211–221.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 2015; 314:151–161.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia. The HARMONIZE randomized clinical trial. JAMA 2014; 312:2223–2233.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372:222–231.
- Anker SD, Kosiborod M, Zannad F, et al. Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 2015; 17:1050–1056.
KEY POINTS
- Exclude pseudohyperkalemia in patients who have a normal electrocardiogram and no risk factors for the development of hyperkalemia.
- Decreased distal delivery of sodium, reduced mineralocorticoid levels or activity, and a distal tubular defect are causes of impaired renal potassium secretion.
- Medical conditions and medications that alter the renin-angiotensin-aldosterone system can give rise to hyperkalemia.
Diagnostic value of the physical examination in patients with dyspnea
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
Laennec’s stethoscope has survived more than 200 years, much longer than some of his contemporaries predicted. But will it survive the challenge of bedside ultrasonography and other technologic advances?
The physical examination, with its roots extending at least as far back as Hippocrates, may be at a crossroads as the mainstay of diagnosis. Physical signs can be subjective and lack sensitivity and specificity. Modern imaging and laboratory studies may already be more trusted.
If the physical examination is to survive, it must be accurate, reproducible, and efficient. Needed is a simple, evidence-based approach to the physical examination that enhances its diagnostic accuracy while maintaining bedside efficiency.
Here, we analyze the accuracy of the physical signs that are most effective in the clinical diagnosis of 4 common cardiopulmonary conditions that often present with dyspnea: pneumonia, pleural effusion, chronic obstructive pulmonary disease (COPD), and congestive heart failure.
LIKELIHOOD RATIOS
To grasp the significance of physical findings, it is necessary to understand the concept of likelihood ratios, which are widely accepted measures of the accuracy of a test or clinical finding.1,2 The positive likelihood ratio is the probability of a disease being present when the test is positive or the clinical finding is present, while the negative likelihood ratio is the probability that the disease is present when the test is negative or the clinical finding is absent. They are calculated as follows1:
Positive likelihood ratio = sensitivity / (1 – specificity)
Negative likelihood ratio = (1 – sensitivity) / specificity
Table 1 shows how the likelihood ratio of a test changes the posttest probability that a condition is present or absent, according to an analysis by McGee.2
STANDARDIZED TERMINOLOGY
PNEUMONIA
Pneumonia is a common disease, with more than 2 million cases annually in the United States. It is most often diagnosed by standard chest radiography, although computed tomography can identify it earlier and with higher sensitivity and specificity.5 The amount of published data on physical examination findings in pneumonia is surprisingly small.
Asymmetry in chest expansion: Specific, reproducible, but not sensitive
The physical finding with the highest positive likelihood ratio for diagnosing pneumonia is asymmetry in chest expansion.6,7
In a 1984 study of 1,819 patients presenting to an emergency department with acute cough, Diehr et al6 evaluated several physical signs of pneumonia. Asymmetric chest expansion had a specificity and positive predictive value of 100%, but its sensitivity was only 4.3%. Thus, it is not a good screening test, but it is a good diagnostic or confirmatory test. From these numbers, Metlay et al8 calculated that the positive likelihood ratio was infinity and the negative likelihood ratio was 0.96.
McGee,7 on the other hand, calculated the positive likelihood ratio of asymmetric chest expansion at 44.1. McGee also found chest expansion to be a highly reproducible finding, with an interobserver agreement kappa score of 0.85.7 (A kappa score of 1.0 would indicate perfect interobserver agreement.) Interestingly, chest radiographs interpreted for pulmonary infiltrates have an interobserver kappa score of only 0.38.7 Further studies of this physical sign could shed more light upon this area of uncertainty.
Other signs of pneumonia
None of the other physical signs studied for the diagnosis of pneumonia has as high a positive likelihood ratio as asymmetric chest expansion.6–12
Egophony is a high-pitched or nasal quality of the patient’s voice heard on auscultation over lung tissue that is consolidated or fibrosed, due to enhanced transmission of high-frequency sound across fluid. It is often described as the “E-to-A change.” Although listening for egophony is widely done and easy to do, we calculate that this sign has a positive likelihood ratio of only 6.8 based on pooled data from 3 trials with a total of 3,245 patients.6,10,11
Faring less favorably, in descending order of diagnostic accuracy, are:
Percussion dullness (positive likelihood ratio 5.7 based on 4 studies with 3,653 patients)6,10–12
Bronchophony or bronchial breath sounds (positive likelihood ratio 3.3 based on 1,118 patients)10
Crackles have long been taught as a common physical finding in pneumonia. Bohadana et al pointed out that “crackle” can be defined acoustically but does not suggest any means or site of generation.4 Pooled data from 4 studies in 3,647 patients6,10–12 result in a positive likelihood ratio for crackles in the diagnosis of pneumonia of only 3.2.
Diminished breath sounds (positive likelihood ratio 2.5 based on 3 studies with 1,828 patients).10–12
Consider pneumonia signs in combination
These physical examination maneuvers are time-honored and part of the rite of training for medical students and residents. As we have shown, they are not extremely helpful as individual tests in diagnosing pneumonia; however, they may be useful when used in combination as a clinical prediction rule or diagnostic algorithm. These rules often have higher diagnostic accuracy but drawbacks of taking more time and not being easily reproducible.
PLEURAL EFFUSION
Pleural effusion commonly occurs in patients with congestive heart failure, pneumonia, and malignancies. The following are signs of effusion.
Dullness to percussion had a positive likelihood ratio of 5.7 from pooled data from 3 studies analyzed by Wong et al.13
Asymmetric chest expansion, in a study by Kalantri et al,14 had a positive likelihood ratio of 8.1 and a negative likelihood ratio of 0.29, the latter making it a reasonably good test to help rule out a pleural effusion.
Negative signs. Since a pleural effusion is an abnormal fluid collection in the pleural space and not the lung parenchyma, one would not expect it to cause loud breath sounds, adventitious sounds, or vocal resonance. Since these 3 findings emanate from the lung, their absence would be expected to support the presence of a pleural effusion.
Tactile fremitus, also known as vocal fremitus, is the vibration felt on the chest wall while the patient is speaking. Traditionally, the patient says “ninety-nine” as the examiner feels for asymmetry in vibration. A consolidation such as pneumonia increases the vibration, while fluid in a pleural effusion diminishes it.
DIAGNOSTIC ALGORITHM FOR PNEUMONIA OR PLEURAL EFFUSION
Patients presenting with cough or dyspnea will most likely be evaluated for pneumonia and pleural effusion, among other diagnoses. We propose the following physical examination strategy in this setting.
First, evaluate the patient for asymmetric chest expansion. The positive likelihood ratio for this sign is excellent for pneumonia (44.1) and moderate for pleural effusion (8.1); therefore, both conditions are possible with a positive test.
Second, percuss the chest. Dullness to percussion has a low positive likelihood ratio for pneumonia but a moderate one for pleural effusion.13 The absence of this sign is only modest in excluding a pleural effusion (negative likelihood ratio 0.31 in pooled data analyzed by Wong et al).13
Third, auscultate the chest to elicit normal, diminished, or adventitious breath sounds. Diminished breath sounds may be noted in both conditions, but vocal resonance (egophony or bronchophony) and tactile fremitus should not be present directly over a pleural effusion. Either vocal resonance or tactile fremitus in a patient with asymmetric chest expansion would strongly support the diagnosis of pneumonia.
Figure 2 summarizes our proposed diagnostic algorithm for pneumonia and pleural effusion.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD imposes a heavy burden on public health worldwide in terms of cost and mortality. It is the third leading cause of death in the United States, after heart disease and cancer.15
Spirometry remains the gold standard for diagnosis. The Global Initiative for Chronic Obstructive Lung Disease standard for diagnosing COPD was the better of 2 spirometry test results, showing a forced expiratory volume in 1 second (FEV1) and FEV1/forced vital capacity ratio less than 70%.16
Unfortunately, there is little evidence that physical signs aid in the early diagnosis of COPD, as physical signs of airflow limitation may not manifest until lung function is substantially impaired.17,18
Early inspiratory crackles had a positive likelihood ratio of 14.6 based on 2 small studies.19,20
Percussion dullness over the left sternal border in the fifth intercostal space should be present in the normal situation and is known as cardiac dullness. Absent cardiac dullness had a positive likelihood ratio of 16 and a negative likelihood ratio of 0.8 for diagnosing COPD in a study in 92 patients with a history of smoking or self-reported COPD.21 The kappa score was 0.49, signifying moderate interobserver agreement.
A combined strategy using the history and physical examination may have the highest diagnostic accuracy. Many of these combinations are too cumbersome for practical clinical use. However, 1 of them is based on only 3 questions21:
- Has the patient smoked for more than 70-pack years?
- Has the patient been previously diagnosed with chronic bronchitis or emphysema?
- Are breath sounds diminished in intensity?
Answering yes to 2 of these questions gives a positive likelihood ratio of a diagnosis of COPD of 33.5.
Early detection of COPD may improve outcomes and lower healthcare costs and thus would be clinically useful. Unfortunately, a diagnostic approach using the history and physical in the early diagnosis of COPD remains uncertain at this time.
CONGESTIVE HEART FAILURE
The clinical presentation of acute congestive heart failure has much in common with pneumonia, pleural effusion, and COPD.
Echocardiography, the gold standard for diagnosis, is costly and may not be immediately available for most patients evaluated for cardiorespiratory complaints. The American College of Cardiology reports the cost of standard echocardiography to be between $1,000 and $2,000.22 A physical examination approach in the assessment of dyspnea can be very useful.
Height of jugular venous distention approximates central venous pressure
Assessing the central venous pressure by estimating the vertical height of distention of the right internal or external jugular vein is validated and easily reproducible.23,24 The use of the external jugular vein is supported by correlation with catheter-measured central venous pressure in critically ill patients.25,26 The central venous pressure reflects the right atrial pressure, and in the absence of tricuspid stenosis, the right ventricular end-diastolic pressure. An elevation in central venous pressure can be seen in patients with congestive heart failure, pulmonary hypertension, and pulmonary valve stenosis.
The right side is preferred due to its anatomically direct route to the heart. In contrast, the left internal jugular vein crosses the mediastinum and can be compressed by the aorta, causing a false elevation.
In summary, an elevated jugular venous pressure on examination is a good test to rule in an elevated central venous pressure, and its absence is a good sign in ruling out an elevated central venous pressure. When using jugular venous pressure specifically for the diagnosis of congestive heart failure with reduced ejection fraction (ie, ejection fraction < 50%), the positive likelihood ratio is 6.3 based on 3 studies.25–27
Heart failure with preserved ejection fraction has not been well studied for physical examination. The Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve)28 looked only at the sensitivity of elevated jugular venous pressure in 4,128 patients, which was 8%. Specificity was not reported.
The abdominojugular reflux
Another way to gauge the jugular venous pressure is to examine the neck veins while firmly pressing on the mid-abdomen for 10 to 15 seconds to look for the abdominojugular reflux, also known as the hepatojugular reflux. An increase in the jugular venous pressure of 3 cm from baseline constitutes a positive abdominojugular reflux. It has a positive likelihood ratio of 8.0 and a negative likelihood ratio of 0.3 for the diagnosis of congestive heart failure by the assessment of end-diastolic pressure of the left ventricle (Table 5).29–31
The abdominojugular reflux is a much more reliable test than examination of neck veins for jugular venous pressure. The interobserver agreement for examining neck veins has a wide range of kappa scores (0.08–0.81), whereas the abdominojugular reflux has a very high kappa score of 0.92.7 Interestingly, chest radiography showing interstitial edema has a kappa of 0.83.7
Displaced apical impulse
An evaluation of the apical impulse of the heart is also a very good and quick test in the examination of patients suspected of having congestive heart failure. An abnormal finding is defined by an apical impulse displaced laterally (to the left of the midclavicular line).
Using data from several studies,32–35 a displaced apical impulse has a positive likelihood ratio of 10.3. The absence of this finding, however, is not very good for ruling out congestive heart failure, with a negative likelihood ratio of 0.7. Interobserver agreement is moderate to excellent (kappa score 0.43–0.86).7
A third heart sound
Auscultation to assess the third heart sound is much more difficult. A systematic review found that likelihood ratios vary widely and confidence intervals are wide.36 Interobserver agreement also varies widely (kappa scores –0.17 to 0.84).7 In a primary care study,37 a third heart sound had a very low sensitivity (4.3%) but a specificity of 99.8%.
Therefore, we are uncertain about a conclusion for this physical finding based on the concern for wide ranges in likelihood ratio and poor interobserver reliability.
PHYSICAL EXAMINATION STILL HAS A FUTURE
The physical examination has a long and distinguished place in the history of medicine. Technologic advances have changed the manner in which clinicians practice the art of healing. Modern technology in US healthcare has become a double-edged sword, with many benefits as well as detriments.3 Reproducibility and accuracy are paramount for the physical examination to remain a core component of medical diagnosis. Advances in the diagnostic accuracy of laboratory and imaging studies challenge the importance of the physical examination. However, we firmly believe that the traditional techniques have stood the test of time and have a future in the clinical practice of medicine.
Acknowledgments: The authors thank Ruby Marr, MD, Mohammed Nabhan, MD, Rajiv Doddamani, MD, and Sohaib Galani, MD, for their important contributions to this article, which included research assistance and editorial advice.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
- Lang TA, Secic M. Chapter 10. Determining the presence or absence of disease. Reporting the characteristics of diagnostic tests. In: Lang TC, Secic M. How to Report Statistics in Medicine. Annotated Guidelines for Authors, Editors, and Reviewers. Philadelphia, PA, American College of Physicians, 1997:147–169.
- McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; 17:647–650.
- Mikami R, Murao M, Cugell DW, et al. International symposium on lung sounds. Synopsis of proceedings. Chest 1987; 92:342–345.
- Bohadana A, Izbicki G, Kraman SS. Fundamentals of lung auscultation. N Engl J Med 2014; 370:744–751.
- Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol 2004; 14:256–271.
- Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984; 37:215–225.
- McGee S. Evidence-Based Physical Diagnosis. 4th ed. Philadelphia, PA: Elsevier; 2017.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Melbye H, Straume B, Aasebo U, Brox J. The diagnosis of adult pneumonia in general practice. The diagnostic value of history, physical examination and some blood tests. Scand J Prim Health Care 1988; 6:111–117.
- Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990; 113:664–670.
- Gennis P, Gallagher J, Falvo C, Baker S, Than W. Clinical criteria for the detection of pneumonia in adults: guidelines for ordering chest roentgenograms in the emergency department. J Emerg Med 1989; 7:263–268.
- Melbye H, Straume B, Aasebo U, Dale K. Diagnosis of pneumonia in adults in general practice. Relative importance of typical symptoms and abnormal chest signs evaluated against a radiographic reference standard. Scand J Prim Health Care 1992; 10:226–233.
- Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA 2009; 301:309–317.
- Kalantri S, Joshi R, Lokhande T, et al. Accuracy and reliability of physical signs in the diagnosis of pleural effusion. Respir Med 2007; 101:431–438.
- Heron M. Deaths: leading causes for 2014. National Vital Statistics Reports 2016; 65(5) June 30, 2016. www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_05.pdf. Accessed October 20, 2017.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention. http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf. Accessed November 13, 2017.
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364:613–620.
- Oshaug K, Halvorsen PA, Melbye H. Should chest examination be reinstated in the early diagnosis of chronic obstructive pulmonary disease? Int J Chron Obstruct Pulmon Dis 2013; 8:369–377.
- Bettencourt PE, Del Bono EA, Spiegelman D, Hertzmark E, Murphy RL Jr. Clinical utility of chest auscultation in common pulmonary diseases. Am J Respir Crit Care Med 1994; 150:1291–1297.
- Nath AR, Capel LH. Inspiratory crackles and mechanical events of breathing. Thorax 1974; 29:695–698.
- Badgett RG, Tanaka DJ, Hunt DK, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993; 94:188–196.
- ABIM Foundation. Choosing wisely. Echocardiograms for heart valve disease. www.choosingwisely.org. Accessed November 13, 2017.
- Davison R, Cannon R. Estimation of central venous pressure by examination of jugular veins. Am Heart J 1974; 87:279–282.
- Ducas J, Magder S, McGregor M. Validity of the hepatojugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983; 52:1299–1303.
- Vinayak AG, Levitt J, Gehlbach B, Pohlman AS, Hall JB, Kress JP. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med 2006; 166:2132–2137.
- Sankoff J, Zidulka A. Non-invasive method for the rapid assessment of central venous pressure: description and validation by a single examiner. West J Emerg Med 2008; 9:201–205.
- Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? QJM 1997; 90:335–339.
- Kristensen SL, Mogensen UM, Jhund PS, et al. Clinical and echocardiographic characeristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction. A report from the Irbesartan in Heart Failure with Preserved Ejection Fraction Trial (I-Preserve). Circulation 2017; https://doi.org/10.1161/CIRCULATIONAHA.116.024593. Accessed November 1, 2017.
- Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. J Am Coll Cardiol 1993; 22:968–974.
- Sochowski RA, Dubbin JD, Naqvi SZ. Clinical and hemodynamic assessment of the hepatojugular reflux. Am J Cardiol 1990; 66:1002–1006.
- Ewy GA. The abdominojugular test: technique and hemodynamic correlates. Ann Intern Med 1988; 109:456–460.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculography and right heart catheterization. Eur Heart J 1989; 10:1017–1028.
- Fahey T, Jeyaseelan S, McCowan C, et al. Diagnosis of left ventricular systolic dysfunction (LVSD): development and validation of a clinical prediction rule in primary care. Fam Pract 2007; 24:628–635.
- Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol 1989; 63:1301–1307.
- Mattleman SJ, Hakki AH, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardiol 1983; 1:417–420.
- Madhok V, Falk G, Rogers A, Struthers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in the diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. BMC Fam Pract 2008; 9:56.
- Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 2011; 124:2865–2873.
KEY POINTS
- Asymmetrical chest expansion, diminished breath sounds, egophony, bronchophony, and tactile fremitus can be used in combination to accurately diagnose pneumonia and pleural effusion.
- No physical sign performs with a high degree of accuracy for diagnosing early-stage chronic obstructive pulmonary disease.
- Inspiratory crackles, diminished breath sounds, and cardiac dullness have high diagnostic value for advanced obstructive airway disease.
- Congestive heart failure can be diagnosed at the bedside by examining the jugular veins and palpating the point of maximal intensity.
Palliative and supportive interventions to improve patient-reported outcomes in rural residents with cancer
People in rural areas have increased rates of advanced cancer and mortality compared with those who live in more affluent and urban areas.1,2 Indeed, a recent report from the Center for Disease Control found that rural residents have higher mortality rates from 5 leading causes of death, including cancer, compared with their urban counterparts.1 Significant challenges facing rural residents are due largely to not having easy access to cancer care and supportive care services.3 In addition, living in a rural area is associated with: a lower socioeconomic status, inadequate health insurance coverage, and less flexible employment that in turn decreases the ability to obtain the full range of supportive oncology services.4 The closest available specialists may be several hours away. Individuals may be unwilling or unable to travel hundreds of miles or more to see a specialist.3 Traveling places financial burdens on patients because of the cost of traveling and loss of work, which can compound the stress and fatigue associated with cancer treatment. People living in rural areas also may have less social support in commuting between their place of living and hospitals.5
Background
Typically, the primary goals of treatment for individuals with advanced cancer are to control the spread of the disease; maintain important patient-reported outcomes (PROs) such as physical, mental, and psychosocial function; and optimize quality of life (QoL). Health-related QoL (ie, the physical and mental health perceptions) are increasingly being used to assess effectiveness of cancer treatment.6 Palliative care and supportive oncology focus on managing physical, social, psychological, and spiritual needs of patients and have been recommended by the American Society of Clinical Oncology to be integrated into standard oncology care.7
People living in rural areas are less likely to get their care within a single health system. Often, their care is divided across multiple facilities and providers, which increases the chances of miscommunication between providers and can lead to inferior clinical outcomes and decreased patient QoL.8 There is a growing body of research describing the impact of palliative care on people with advanced cancer. Specifically, palliative care has been shown to reduce symptoms, improve QoL, and increase survival.9-11 Differences have been observed in the palliative care needs between people with cancer living in urban and suburban areas.12 It is likely that palliative care needs as well as the impact of palliative care services for people with advanced cancer in rural areas differs from those of their urban and suburban counterparts. Despite the known differences in access to care and impact of cancer between rural and nonrural residents, the impact of palliative care on people with advanced cancer living in rural areas has not been well described in the literature.
The purpose of this systematic review is to examine effect of palliative care and supportive oncology interventions on QoL in people with advanced cancer living in rural areas.
Methods
This systematic review was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Eligibility criteria
To achieve the objective of a systemic review of studies describing supportive oncology and palliative care interventions in rural communities articles had to meet 4 inclusion criteria:
All research methods were eligible, including mixed-methods and program evaluations, as long as the article met the 4 inclusion criteria. Review articles were ineligible for inclusion as only original research was considered.
Search process
Search terms were developed by the research team with consultation from a medical librarian. Four main search terms were developed and included: palliative care, supportive oncology, rural, and cancer. Synonyms and terms closely related to the main terms were included in the search using the OR command. Examples of closely related search terms include: Palliative care: palliative; Rural: remote; Cancer: neoplasms (Table).
We systematically searched PyschINFO, PubMed, CINHAL, and Scopus for articles that had been published during 1991-2016 and written in English. Databases were chosen to reflect the different subfields that encompass palliative care and supportive oncology: PyschINFO to capture the psychological perspective, CINHAL to capture the nursing perspective, and PubMed to capture the medical perspective. Finally, Scopus was searched to ensure that articles not indexed by the other databases would be included. The search was limited to the past 25 years to capture the most up-to-date literature.
Selection process
In accordance with PRISMA guidelines, articles underwent an initial screening and an eligibility screening for inclusion in the final review.13 After duplicates were removed, 2 research team members reviewed all abstracts to screen for initial eligibility. Articles that successfully passed the screening process were reviewed in full by 4 research team members. Each member made an independent inclusion decision based on the stated inclusion criteria. Disagreements across team members were resolved through discussion and consensus.
Analysis
The articles that met the inclusion criteria were heterogeneous in design and analytic approach. The set of manuscripts identified, therefore, did not meet the statistical assumptions for meta-analytic data analysis. The analytic plan for this review consisted of sorting the results described in the identified articles into meaningful categories, identifying cross cutting themes, and presenting the results of these themes in narrative forms.
Results
Study selection
The search strategy resulted in 886 articles across the 4 databases. The breakdown for each database is as follows: PsychINFO (n = 286), PubMed (n = 194), CINHAL (n = 334), Scopus (n = 72). After duplicates were removed, 864 articles were left and were initially screened resulting in 844 articles being excluded. The remaining 20 articles were reviewed and 12 articles failed to meet the inclusion criteria. Reasons for exclusion included: the population was not rural; no advanced cancer in sample; intervention was not specifically palliative care or supportive oncology. Nine articles representing 8 projects (one project published 2 manuscripts included in this review) were included in the final review (Figure).
After reviewing the articles, 2 clear themes arose: PROs, and overall impact of rural palliative care for people and society. The PRO theme included articles that provided information on how an intervention or program improved the personal lives experience of rural cancer patients. PROs, such as decreased symptomology, were often reported. The “overall impact of rural palliative care for people and society” theme included articles that provided information on how an intervention or program improved the lives of rural people and society as a larger group. An example would include results indicating how a program increased access to supportive oncology care in a rural area.
Study characteristics
Nine publications, describing 8 projects were included in this review (Table). These projects were conducted in Canada (n = 3)14-16 Australia (n = 1)17 and the United States (n = 5).18-22 All of the the projects used a quantitative approach for the analysis, except 1 that used mixed-methods.16 The studies designs were: 4 feasibilities/pilot studies, 1 randomized control trials (RCT), and 3 program evaluations.
A total of 807 patients participated across the 9 articles. Participants’ age ranged from 20 to 88 years. The average ages for participants ranged from 50.4 to 70.7 years. Overall, there were slightly more men (55%) than women (45%) when all the demographic data were combined across the 9 articles; however, 2 articles exclusively had women as part of the sample.17,20 The cancer types that participants had included: gastrointestinal, genitourinary, breast, lung, brain, kidney, and hematological. Finally, the articles had inconsistent reporting of race/ethnicity data with only 4 studies reporting this information; of the 4 studies, 91% of participants self-identified as white.
The projects targeted multiple PROs, including physical symptoms and psychosocial issues (ie, stress management, grief, mood, emotional distress, coping, self-efficacy, dignity, joy, affection) domains. Publications dates ranged from 1996 to 2013. The sample sizes ranged from 8 to 322; 11.7%-100% of the study population had advanced cancer, and 20%-100% were living in rural area. The duration of the clinical intervention described was 30-120 minutes. The modes of delivery for the palliative intervention were videoconference/videophone (n = 3), telephone/teleconference (n = 3), and in person (n = 2). The interventions were delivered by nurses, psychiatrists, and social workers. In 5 of these studies, participants received palliative care on an individual basis and 2 studies delivered their intervention through groups. The individual basis studies focused on physical aspects of care and the group studies focused on emotional aspects of care.
Patient-reported outcomes
Cancer and its treatments are often associated with physical and emotional sequelae that can have a significant impact on patients and therefore PROs. The interventions reviewed in this article often reported data on the reduction of the physical and/or emotional symptom burden of cancer as well as overall QoL.
Reduction in physical symptoms. Three articles included physical symptoms as an outcome measure. Of those, 2 were pilot or feasibility studies, and 1 was a randomized control trial. Common physical symptoms included: shortness of breath, pain, fatigue, nausea, and appetite change. Across the articles, the Edmonton Symptom Assessment Scale (ESAS), a 10-item inventory of common cancer symptoms, was frequently used to measure of symptom scores in these interventions.14,15,19 The ESAS is an empirically validated measure that is used in palliative care research and clinical practice. Individuals are asked to rank 10 common symptoms on an ascending scale from 1 to 10 (0, the symptom is absent; 10, worst possible severity).23
The findings from these 3 research studies were encouraging. In a large randomized control trial of a supportive education program, researchers reported decreased physical symptom intensity after the intervention, however the change did not reach statistical significance.18 Similar findings were reported in a videoconferencing and a home health program to improve access of palliative and supportive oncology health care.14,15 Physical symptoms that had decreasing trends were pain, tiredness, and appetite, however, trends for shortness of breath found increasing severity.14,15 Although these trends were observed, it is important to note that scores on the ESAS did not reach statistical significance for physical symptoms in any of these studies.
Reduction in emotional symptom reduction. In addition to reducing physical symptoms, researchers also sought to understand the impact of programs on the emotional symptoms of cancer including: anxiety, depression, negative affect, and posttraumatic stress disorder (PTSD). Five articles included emotional symptoms as an outcome measure. Four were pilot or feasibility studies, and 1 was a randomized control trial.
Results across studies indicated an observable decrease in the severity of anxiety and depression for those exposed to an intervention program.14,15,18,19 Again, although trends were found, the results were not statistically significant. Only Watanabe and colleagues14 reported a statistically significant a decrease in anxiety in participants after the implementation of a rural palliative care videoconference consultation program. One report indicated that data on depression severity was collected but was not analyzed because of a small sample size.21
O’Brien and colleagues17 also collected data on negative affect and found that participants who participated in a supportive-expressive therapy group had a reduction in the negative affect as measured by the Derogatis Affects Balance Scale (ABS). Other researchers found no change in emotional distress.15
Finally, Collie and colleagues20 also measured the impact of a videoconference support group of PTSD symptomology for people with breast cancer in rural areas. Their results indicated a statistically significant decrease on the PTSD Checklist-Specific after intervention. Analysis of the data also found a medium effect size. Participants in the intervention group spoke about how participation in the support group allowed them to be generative and share information about breast cancer as well as build an emotional bond with other women with cancer.
Overall quality of life and well-being. Researchers have also looked into impact of intervention on overall QoL. Two articles included QoL or Well-being as an outcome measure. One was a pilot study and 1 was a randomized control trial.
Bakitas and colleagues18,19 found that those enrolled in the intervention arm of their study had higher QoL scores on the Functional Assessment of Cancer Therapy-General (FACT-G) compared with those in the control arm. These results were also found in an analysis of data from participants who subsequently died during the intervention. Improvements in overall well-being were also found by O’Brien and colleagues17 using ABS. They reported that a post hoc comparison of participants’ total positive affect score was significantly higher at the 12-month follow-up. In addition, the authors also noted qualitative improvements in well-being, including increased effort to be at the support group and the low attrition rates.
Overall impact of rural palliative care on individuals and society. In addition to reducing physical and emotional symptoms in patients, several of the articles also addressed other measures of the overall impact of the intervention or program on society as a whole. The authors evaluated patient satisfaction and quality of life, access to health care services, and financial impact on individuals and society at large.
Satisfaction with intervention. In 2 of the articles, individuals or their family members reported to be satisfied with the intervention14,20 and said they would recommend it to others as well.20 Both of those studies used teleconferencing to provide access to the intervention to people in rural communities.
Increasing access to the health care services and quality of care. Four of the articles evaluated the impact of intervention on patient’s access to the health care services.14,16,20,22 Specifically, after the interventions individuals had increased access to palliative care information in rural areas where it had previously been unavailable20 as well as actual delivery of clinical care in their home community, thus eliminating the need to travel to urban areas.14,20,22 This increase of access to health care services in rural area had significant effect on time and distance spent traveling. In 1 study, the amount of saving in terms of distance was 471.13 km and time in, 7.96 hours, for each visit.14
In addition, the quality of overall cancer care in rural area was increased. In an early clinical program, to increase access of palliative care in rural communities, the authors reported an increase in the breast conservation from 20% at the start of the program to 70% 2 years after the program was implemented.22 Breast conservation is not a typical outcome for palliative care studies, but the authors highlighted this practice change because of the improved QoL that is associated with the use of breast conservation therapies. In the same study, the authors reported an increased use of curative therapies for other cancers such as lymphoma as well as an increase use of pain management medication.
Financial impact. Two articles described the financial impact of cancer care costs on the patient and society.14,22 In a study by Watanabe and colleagues in Canada,14 the amount of savings after the intervention in terms of travel expenses was C$192.71 for each visit because patients had previously had to travel from their rural communities to urban tertiary hospitals to receive palliative care. For some patients in that study, the amount of saving for expenses was as high as C$500 a visit. In addition, some individuals were not able to travel and would not have received anything if the intervention had not been available remotely.14 In a study by Smith and colleagues in the United States, there was a 62% decrease in the cost to society for each patient, from US$10,233 to US$3,862.22 The factors contributing to that reduction included increasing outpatient services, engaging nurses and primary care providers instead of specialists, and the lower costs of living in rural areas. In addition, the rural hospitals saw an increase in revenue and profits because of higher admission rates ($500,00 for each hospital annually).22
Discussion
The articles identified in this review provide some evidence of the potential impact that palliative and supportive oncology interventions could have on PROs for rural residents with advanced cancer. Noteworthy results were seen for impact on reducing physical and emotional symptoms, increasing overall QoL and well-being, increasing satisfaction and access to palliative care, and reducing the overall cost of palliative care for individuals and society.14-18,20-22
Although statistical significance was not observed for most of the symptom assessment, trends toward improved symptom reports were observed. A likely explanation for this finding, is the small sample size or inadequate design to evaluate symptoms as an outcome measure. Three studies were pilot or feasibility projects15,20,21 that were not powered to detect the impact of the intervention on symptoms. In contrast, QoL stands out as an outcome that was positively affected by palliative care interventions. Further research is needed to determine if there are important mediating and moderating factors that contribute to improve QoL that are specific to rural residents. Significant outcomes were also reported for participant satisfaction with the interventions, the increase in access to services, and the decrease in costs.
Although there were not enough studies to determine the efficacy of these interventions, these results suggest that palliative and supportive interventions can have an impact on important patient-reported outcomes, such as symptoms and quality of life, and on health care system outcomes, such as cost. Evidence supporting the extent of the effectiveness of palliative care on various PROs in rural people is limited. None of the studies in this review evaluated the different aspects of palliative care specifically in rural residents.
It is interesting to note that all but one of the interventions used a telehealth approach to deliver the intervention. Telehealth interventions seem to be feasible, acceptable to people in rural areas, and show preliminary evidence that they can have an impact on PROs.
Limitations of this review include only inclusion of publications in English. In addition, some studies in this review include populations that were not exclusively rural residents, which makes it difficult for generalization.
Conclusion
Palliative and supportive interventions may improve various PROs in people with advanced cancer living in rural areas. Technologies that support remote access to people in rural areas, such as teleconferencing and videoconferencing, seem particularly promising delivery modalities with their potential to increase access to palliative and supportive interventions in underserved communities. Large-scale studies that are powered to test the impact of palliative care and support oncology interventions on PROs and other aspects of quality care among rural residents with advanced cancer are needed.
The authors thank Jennifer DeBerg, Health Science Librarian at the University of Iowa for her assistance in developing the literature search strategies.
1. Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas – United States, 1999-2014 [published correction at https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a11.htm]. MMWR Surveillance Summaries [serial online]. https://www.cdc.gov/mmwr/volumes/66/ss/ss6601a1.htm?s_cid=ss6601a1_w. Published January 13, 2017. Accessed January 20, 2017.
2. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US Cancer Mortality: Part I – All cancers and lung cancer and Part II – Colorectal, prostate, breast, and cervical cancers. https://www.hindawi.com/journals/jce/2011/107497/. Published 2011. Accessed April 28, 2017.
3. Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633-640.
4. Weaver KE, Geiger AM, Lu L, Case LD. Rural‐urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050-1057.
5. Fuchsia Howard A, Smillie K, Turnbull K, et al. Access to medical and supportive care for rural and remote cancer survivors in northern British Columbia. J Rural Health. 2014;30(3):311-321.
6. Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol. 2007;25(32):5082-5086.
7. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887.
8. Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24(4):390-399.
9. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
10. McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med. 2015;18(11):962-969.
11. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
12. Regn R, Robinson W, Robinson WR. Differences in palliative care needs among cancer survivors in an inner city academic facility versus a suburban community facility. J Clin Oncol. 2015;33(29_suppl):61.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;339:b2535.
14. Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer. 2013;21(4):1201-1207.
15. Howell D, Marshall D, Brazil K, et al. A shared care model pilot for palliative home care in a rural area: impact on symptoms, distress, and place of death. J Pain Symptom Manage. 2011;42(1):60-75.
16. Stern A, Valaitis R, Weir R, Jadad AR. Use of home telehealth in palliative cancer care: a case study. J Telemed Telecare. 2012;18(5):297-300.
17. O’Brien M, Harris J, King R, O’Brien T. Supportive-expressive group therapy for women with metastatic breast cancer: Improving access for Australian women through use of teleconference. Counselling Psychother Res. 2008;8(1):28-35.
18. Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Supportive Care. 2009;7(1):75-86.
19. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
20. Collie K, Kreshka MA, Ferrier S, et al. Videoconferencing for delivery of breast cancer support groups to women living in rural communities: a pilot study. Psychooncology. 200
21. Passik SD, Kirsh KL, Leibee S, et al. A feasibility study of dignity psychotherapy delivered via telemedicine. Palliat Support Care. 2004;2(2):149-155.
22. Smith TJ, Desch CE, Grasso MA, et al. The Rural Cancer Outreach Program: clinical and financial analysis of palliative and curative care for an underserved population. Cancer Treat Rev. 1996;22(Suppl A):97-101.
23. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
People in rural areas have increased rates of advanced cancer and mortality compared with those who live in more affluent and urban areas.1,2 Indeed, a recent report from the Center for Disease Control found that rural residents have higher mortality rates from 5 leading causes of death, including cancer, compared with their urban counterparts.1 Significant challenges facing rural residents are due largely to not having easy access to cancer care and supportive care services.3 In addition, living in a rural area is associated with: a lower socioeconomic status, inadequate health insurance coverage, and less flexible employment that in turn decreases the ability to obtain the full range of supportive oncology services.4 The closest available specialists may be several hours away. Individuals may be unwilling or unable to travel hundreds of miles or more to see a specialist.3 Traveling places financial burdens on patients because of the cost of traveling and loss of work, which can compound the stress and fatigue associated with cancer treatment. People living in rural areas also may have less social support in commuting between their place of living and hospitals.5
Background
Typically, the primary goals of treatment for individuals with advanced cancer are to control the spread of the disease; maintain important patient-reported outcomes (PROs) such as physical, mental, and psychosocial function; and optimize quality of life (QoL). Health-related QoL (ie, the physical and mental health perceptions) are increasingly being used to assess effectiveness of cancer treatment.6 Palliative care and supportive oncology focus on managing physical, social, psychological, and spiritual needs of patients and have been recommended by the American Society of Clinical Oncology to be integrated into standard oncology care.7
People living in rural areas are less likely to get their care within a single health system. Often, their care is divided across multiple facilities and providers, which increases the chances of miscommunication between providers and can lead to inferior clinical outcomes and decreased patient QoL.8 There is a growing body of research describing the impact of palliative care on people with advanced cancer. Specifically, palliative care has been shown to reduce symptoms, improve QoL, and increase survival.9-11 Differences have been observed in the palliative care needs between people with cancer living in urban and suburban areas.12 It is likely that palliative care needs as well as the impact of palliative care services for people with advanced cancer in rural areas differs from those of their urban and suburban counterparts. Despite the known differences in access to care and impact of cancer between rural and nonrural residents, the impact of palliative care on people with advanced cancer living in rural areas has not been well described in the literature.
The purpose of this systematic review is to examine effect of palliative care and supportive oncology interventions on QoL in people with advanced cancer living in rural areas.
Methods
This systematic review was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Eligibility criteria
To achieve the objective of a systemic review of studies describing supportive oncology and palliative care interventions in rural communities articles had to meet 4 inclusion criteria:
All research methods were eligible, including mixed-methods and program evaluations, as long as the article met the 4 inclusion criteria. Review articles were ineligible for inclusion as only original research was considered.
Search process
Search terms were developed by the research team with consultation from a medical librarian. Four main search terms were developed and included: palliative care, supportive oncology, rural, and cancer. Synonyms and terms closely related to the main terms were included in the search using the OR command. Examples of closely related search terms include: Palliative care: palliative; Rural: remote; Cancer: neoplasms (Table).
We systematically searched PyschINFO, PubMed, CINHAL, and Scopus for articles that had been published during 1991-2016 and written in English. Databases were chosen to reflect the different subfields that encompass palliative care and supportive oncology: PyschINFO to capture the psychological perspective, CINHAL to capture the nursing perspective, and PubMed to capture the medical perspective. Finally, Scopus was searched to ensure that articles not indexed by the other databases would be included. The search was limited to the past 25 years to capture the most up-to-date literature.
Selection process
In accordance with PRISMA guidelines, articles underwent an initial screening and an eligibility screening for inclusion in the final review.13 After duplicates were removed, 2 research team members reviewed all abstracts to screen for initial eligibility. Articles that successfully passed the screening process were reviewed in full by 4 research team members. Each member made an independent inclusion decision based on the stated inclusion criteria. Disagreements across team members were resolved through discussion and consensus.
Analysis
The articles that met the inclusion criteria were heterogeneous in design and analytic approach. The set of manuscripts identified, therefore, did not meet the statistical assumptions for meta-analytic data analysis. The analytic plan for this review consisted of sorting the results described in the identified articles into meaningful categories, identifying cross cutting themes, and presenting the results of these themes in narrative forms.
Results
Study selection
The search strategy resulted in 886 articles across the 4 databases. The breakdown for each database is as follows: PsychINFO (n = 286), PubMed (n = 194), CINHAL (n = 334), Scopus (n = 72). After duplicates were removed, 864 articles were left and were initially screened resulting in 844 articles being excluded. The remaining 20 articles were reviewed and 12 articles failed to meet the inclusion criteria. Reasons for exclusion included: the population was not rural; no advanced cancer in sample; intervention was not specifically palliative care or supportive oncology. Nine articles representing 8 projects (one project published 2 manuscripts included in this review) were included in the final review (Figure).
After reviewing the articles, 2 clear themes arose: PROs, and overall impact of rural palliative care for people and society. The PRO theme included articles that provided information on how an intervention or program improved the personal lives experience of rural cancer patients. PROs, such as decreased symptomology, were often reported. The “overall impact of rural palliative care for people and society” theme included articles that provided information on how an intervention or program improved the lives of rural people and society as a larger group. An example would include results indicating how a program increased access to supportive oncology care in a rural area.
Study characteristics
Nine publications, describing 8 projects were included in this review (Table). These projects were conducted in Canada (n = 3)14-16 Australia (n = 1)17 and the United States (n = 5).18-22 All of the the projects used a quantitative approach for the analysis, except 1 that used mixed-methods.16 The studies designs were: 4 feasibilities/pilot studies, 1 randomized control trials (RCT), and 3 program evaluations.
A total of 807 patients participated across the 9 articles. Participants’ age ranged from 20 to 88 years. The average ages for participants ranged from 50.4 to 70.7 years. Overall, there were slightly more men (55%) than women (45%) when all the demographic data were combined across the 9 articles; however, 2 articles exclusively had women as part of the sample.17,20 The cancer types that participants had included: gastrointestinal, genitourinary, breast, lung, brain, kidney, and hematological. Finally, the articles had inconsistent reporting of race/ethnicity data with only 4 studies reporting this information; of the 4 studies, 91% of participants self-identified as white.
The projects targeted multiple PROs, including physical symptoms and psychosocial issues (ie, stress management, grief, mood, emotional distress, coping, self-efficacy, dignity, joy, affection) domains. Publications dates ranged from 1996 to 2013. The sample sizes ranged from 8 to 322; 11.7%-100% of the study population had advanced cancer, and 20%-100% were living in rural area. The duration of the clinical intervention described was 30-120 minutes. The modes of delivery for the palliative intervention were videoconference/videophone (n = 3), telephone/teleconference (n = 3), and in person (n = 2). The interventions were delivered by nurses, psychiatrists, and social workers. In 5 of these studies, participants received palliative care on an individual basis and 2 studies delivered their intervention through groups. The individual basis studies focused on physical aspects of care and the group studies focused on emotional aspects of care.
Patient-reported outcomes
Cancer and its treatments are often associated with physical and emotional sequelae that can have a significant impact on patients and therefore PROs. The interventions reviewed in this article often reported data on the reduction of the physical and/or emotional symptom burden of cancer as well as overall QoL.
Reduction in physical symptoms. Three articles included physical symptoms as an outcome measure. Of those, 2 were pilot or feasibility studies, and 1 was a randomized control trial. Common physical symptoms included: shortness of breath, pain, fatigue, nausea, and appetite change. Across the articles, the Edmonton Symptom Assessment Scale (ESAS), a 10-item inventory of common cancer symptoms, was frequently used to measure of symptom scores in these interventions.14,15,19 The ESAS is an empirically validated measure that is used in palliative care research and clinical practice. Individuals are asked to rank 10 common symptoms on an ascending scale from 1 to 10 (0, the symptom is absent; 10, worst possible severity).23
The findings from these 3 research studies were encouraging. In a large randomized control trial of a supportive education program, researchers reported decreased physical symptom intensity after the intervention, however the change did not reach statistical significance.18 Similar findings were reported in a videoconferencing and a home health program to improve access of palliative and supportive oncology health care.14,15 Physical symptoms that had decreasing trends were pain, tiredness, and appetite, however, trends for shortness of breath found increasing severity.14,15 Although these trends were observed, it is important to note that scores on the ESAS did not reach statistical significance for physical symptoms in any of these studies.
Reduction in emotional symptom reduction. In addition to reducing physical symptoms, researchers also sought to understand the impact of programs on the emotional symptoms of cancer including: anxiety, depression, negative affect, and posttraumatic stress disorder (PTSD). Five articles included emotional symptoms as an outcome measure. Four were pilot or feasibility studies, and 1 was a randomized control trial.
Results across studies indicated an observable decrease in the severity of anxiety and depression for those exposed to an intervention program.14,15,18,19 Again, although trends were found, the results were not statistically significant. Only Watanabe and colleagues14 reported a statistically significant a decrease in anxiety in participants after the implementation of a rural palliative care videoconference consultation program. One report indicated that data on depression severity was collected but was not analyzed because of a small sample size.21
O’Brien and colleagues17 also collected data on negative affect and found that participants who participated in a supportive-expressive therapy group had a reduction in the negative affect as measured by the Derogatis Affects Balance Scale (ABS). Other researchers found no change in emotional distress.15
Finally, Collie and colleagues20 also measured the impact of a videoconference support group of PTSD symptomology for people with breast cancer in rural areas. Their results indicated a statistically significant decrease on the PTSD Checklist-Specific after intervention. Analysis of the data also found a medium effect size. Participants in the intervention group spoke about how participation in the support group allowed them to be generative and share information about breast cancer as well as build an emotional bond with other women with cancer.
Overall quality of life and well-being. Researchers have also looked into impact of intervention on overall QoL. Two articles included QoL or Well-being as an outcome measure. One was a pilot study and 1 was a randomized control trial.
Bakitas and colleagues18,19 found that those enrolled in the intervention arm of their study had higher QoL scores on the Functional Assessment of Cancer Therapy-General (FACT-G) compared with those in the control arm. These results were also found in an analysis of data from participants who subsequently died during the intervention. Improvements in overall well-being were also found by O’Brien and colleagues17 using ABS. They reported that a post hoc comparison of participants’ total positive affect score was significantly higher at the 12-month follow-up. In addition, the authors also noted qualitative improvements in well-being, including increased effort to be at the support group and the low attrition rates.
Overall impact of rural palliative care on individuals and society. In addition to reducing physical and emotional symptoms in patients, several of the articles also addressed other measures of the overall impact of the intervention or program on society as a whole. The authors evaluated patient satisfaction and quality of life, access to health care services, and financial impact on individuals and society at large.
Satisfaction with intervention. In 2 of the articles, individuals or their family members reported to be satisfied with the intervention14,20 and said they would recommend it to others as well.20 Both of those studies used teleconferencing to provide access to the intervention to people in rural communities.
Increasing access to the health care services and quality of care. Four of the articles evaluated the impact of intervention on patient’s access to the health care services.14,16,20,22 Specifically, after the interventions individuals had increased access to palliative care information in rural areas where it had previously been unavailable20 as well as actual delivery of clinical care in their home community, thus eliminating the need to travel to urban areas.14,20,22 This increase of access to health care services in rural area had significant effect on time and distance spent traveling. In 1 study, the amount of saving in terms of distance was 471.13 km and time in, 7.96 hours, for each visit.14
In addition, the quality of overall cancer care in rural area was increased. In an early clinical program, to increase access of palliative care in rural communities, the authors reported an increase in the breast conservation from 20% at the start of the program to 70% 2 years after the program was implemented.22 Breast conservation is not a typical outcome for palliative care studies, but the authors highlighted this practice change because of the improved QoL that is associated with the use of breast conservation therapies. In the same study, the authors reported an increased use of curative therapies for other cancers such as lymphoma as well as an increase use of pain management medication.
Financial impact. Two articles described the financial impact of cancer care costs on the patient and society.14,22 In a study by Watanabe and colleagues in Canada,14 the amount of savings after the intervention in terms of travel expenses was C$192.71 for each visit because patients had previously had to travel from their rural communities to urban tertiary hospitals to receive palliative care. For some patients in that study, the amount of saving for expenses was as high as C$500 a visit. In addition, some individuals were not able to travel and would not have received anything if the intervention had not been available remotely.14 In a study by Smith and colleagues in the United States, there was a 62% decrease in the cost to society for each patient, from US$10,233 to US$3,862.22 The factors contributing to that reduction included increasing outpatient services, engaging nurses and primary care providers instead of specialists, and the lower costs of living in rural areas. In addition, the rural hospitals saw an increase in revenue and profits because of higher admission rates ($500,00 for each hospital annually).22
Discussion
The articles identified in this review provide some evidence of the potential impact that palliative and supportive oncology interventions could have on PROs for rural residents with advanced cancer. Noteworthy results were seen for impact on reducing physical and emotional symptoms, increasing overall QoL and well-being, increasing satisfaction and access to palliative care, and reducing the overall cost of palliative care for individuals and society.14-18,20-22
Although statistical significance was not observed for most of the symptom assessment, trends toward improved symptom reports were observed. A likely explanation for this finding, is the small sample size or inadequate design to evaluate symptoms as an outcome measure. Three studies were pilot or feasibility projects15,20,21 that were not powered to detect the impact of the intervention on symptoms. In contrast, QoL stands out as an outcome that was positively affected by palliative care interventions. Further research is needed to determine if there are important mediating and moderating factors that contribute to improve QoL that are specific to rural residents. Significant outcomes were also reported for participant satisfaction with the interventions, the increase in access to services, and the decrease in costs.
Although there were not enough studies to determine the efficacy of these interventions, these results suggest that palliative and supportive interventions can have an impact on important patient-reported outcomes, such as symptoms and quality of life, and on health care system outcomes, such as cost. Evidence supporting the extent of the effectiveness of palliative care on various PROs in rural people is limited. None of the studies in this review evaluated the different aspects of palliative care specifically in rural residents.
It is interesting to note that all but one of the interventions used a telehealth approach to deliver the intervention. Telehealth interventions seem to be feasible, acceptable to people in rural areas, and show preliminary evidence that they can have an impact on PROs.
Limitations of this review include only inclusion of publications in English. In addition, some studies in this review include populations that were not exclusively rural residents, which makes it difficult for generalization.
Conclusion
Palliative and supportive interventions may improve various PROs in people with advanced cancer living in rural areas. Technologies that support remote access to people in rural areas, such as teleconferencing and videoconferencing, seem particularly promising delivery modalities with their potential to increase access to palliative and supportive interventions in underserved communities. Large-scale studies that are powered to test the impact of palliative care and support oncology interventions on PROs and other aspects of quality care among rural residents with advanced cancer are needed.
The authors thank Jennifer DeBerg, Health Science Librarian at the University of Iowa for her assistance in developing the literature search strategies.
People in rural areas have increased rates of advanced cancer and mortality compared with those who live in more affluent and urban areas.1,2 Indeed, a recent report from the Center for Disease Control found that rural residents have higher mortality rates from 5 leading causes of death, including cancer, compared with their urban counterparts.1 Significant challenges facing rural residents are due largely to not having easy access to cancer care and supportive care services.3 In addition, living in a rural area is associated with: a lower socioeconomic status, inadequate health insurance coverage, and less flexible employment that in turn decreases the ability to obtain the full range of supportive oncology services.4 The closest available specialists may be several hours away. Individuals may be unwilling or unable to travel hundreds of miles or more to see a specialist.3 Traveling places financial burdens on patients because of the cost of traveling and loss of work, which can compound the stress and fatigue associated with cancer treatment. People living in rural areas also may have less social support in commuting between their place of living and hospitals.5
Background
Typically, the primary goals of treatment for individuals with advanced cancer are to control the spread of the disease; maintain important patient-reported outcomes (PROs) such as physical, mental, and psychosocial function; and optimize quality of life (QoL). Health-related QoL (ie, the physical and mental health perceptions) are increasingly being used to assess effectiveness of cancer treatment.6 Palliative care and supportive oncology focus on managing physical, social, psychological, and spiritual needs of patients and have been recommended by the American Society of Clinical Oncology to be integrated into standard oncology care.7
People living in rural areas are less likely to get their care within a single health system. Often, their care is divided across multiple facilities and providers, which increases the chances of miscommunication between providers and can lead to inferior clinical outcomes and decreased patient QoL.8 There is a growing body of research describing the impact of palliative care on people with advanced cancer. Specifically, palliative care has been shown to reduce symptoms, improve QoL, and increase survival.9-11 Differences have been observed in the palliative care needs between people with cancer living in urban and suburban areas.12 It is likely that palliative care needs as well as the impact of palliative care services for people with advanced cancer in rural areas differs from those of their urban and suburban counterparts. Despite the known differences in access to care and impact of cancer between rural and nonrural residents, the impact of palliative care on people with advanced cancer living in rural areas has not been well described in the literature.
The purpose of this systematic review is to examine effect of palliative care and supportive oncology interventions on QoL in people with advanced cancer living in rural areas.
Methods
This systematic review was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13
Eligibility criteria
To achieve the objective of a systemic review of studies describing supportive oncology and palliative care interventions in rural communities articles had to meet 4 inclusion criteria:
All research methods were eligible, including mixed-methods and program evaluations, as long as the article met the 4 inclusion criteria. Review articles were ineligible for inclusion as only original research was considered.
Search process
Search terms were developed by the research team with consultation from a medical librarian. Four main search terms were developed and included: palliative care, supportive oncology, rural, and cancer. Synonyms and terms closely related to the main terms were included in the search using the OR command. Examples of closely related search terms include: Palliative care: palliative; Rural: remote; Cancer: neoplasms (Table).
We systematically searched PyschINFO, PubMed, CINHAL, and Scopus for articles that had been published during 1991-2016 and written in English. Databases were chosen to reflect the different subfields that encompass palliative care and supportive oncology: PyschINFO to capture the psychological perspective, CINHAL to capture the nursing perspective, and PubMed to capture the medical perspective. Finally, Scopus was searched to ensure that articles not indexed by the other databases would be included. The search was limited to the past 25 years to capture the most up-to-date literature.
Selection process
In accordance with PRISMA guidelines, articles underwent an initial screening and an eligibility screening for inclusion in the final review.13 After duplicates were removed, 2 research team members reviewed all abstracts to screen for initial eligibility. Articles that successfully passed the screening process were reviewed in full by 4 research team members. Each member made an independent inclusion decision based on the stated inclusion criteria. Disagreements across team members were resolved through discussion and consensus.
Analysis
The articles that met the inclusion criteria were heterogeneous in design and analytic approach. The set of manuscripts identified, therefore, did not meet the statistical assumptions for meta-analytic data analysis. The analytic plan for this review consisted of sorting the results described in the identified articles into meaningful categories, identifying cross cutting themes, and presenting the results of these themes in narrative forms.
Results
Study selection
The search strategy resulted in 886 articles across the 4 databases. The breakdown for each database is as follows: PsychINFO (n = 286), PubMed (n = 194), CINHAL (n = 334), Scopus (n = 72). After duplicates were removed, 864 articles were left and were initially screened resulting in 844 articles being excluded. The remaining 20 articles were reviewed and 12 articles failed to meet the inclusion criteria. Reasons for exclusion included: the population was not rural; no advanced cancer in sample; intervention was not specifically palliative care or supportive oncology. Nine articles representing 8 projects (one project published 2 manuscripts included in this review) were included in the final review (Figure).
After reviewing the articles, 2 clear themes arose: PROs, and overall impact of rural palliative care for people and society. The PRO theme included articles that provided information on how an intervention or program improved the personal lives experience of rural cancer patients. PROs, such as decreased symptomology, were often reported. The “overall impact of rural palliative care for people and society” theme included articles that provided information on how an intervention or program improved the lives of rural people and society as a larger group. An example would include results indicating how a program increased access to supportive oncology care in a rural area.
Study characteristics
Nine publications, describing 8 projects were included in this review (Table). These projects were conducted in Canada (n = 3)14-16 Australia (n = 1)17 and the United States (n = 5).18-22 All of the the projects used a quantitative approach for the analysis, except 1 that used mixed-methods.16 The studies designs were: 4 feasibilities/pilot studies, 1 randomized control trials (RCT), and 3 program evaluations.
A total of 807 patients participated across the 9 articles. Participants’ age ranged from 20 to 88 years. The average ages for participants ranged from 50.4 to 70.7 years. Overall, there were slightly more men (55%) than women (45%) when all the demographic data were combined across the 9 articles; however, 2 articles exclusively had women as part of the sample.17,20 The cancer types that participants had included: gastrointestinal, genitourinary, breast, lung, brain, kidney, and hematological. Finally, the articles had inconsistent reporting of race/ethnicity data with only 4 studies reporting this information; of the 4 studies, 91% of participants self-identified as white.
The projects targeted multiple PROs, including physical symptoms and psychosocial issues (ie, stress management, grief, mood, emotional distress, coping, self-efficacy, dignity, joy, affection) domains. Publications dates ranged from 1996 to 2013. The sample sizes ranged from 8 to 322; 11.7%-100% of the study population had advanced cancer, and 20%-100% were living in rural area. The duration of the clinical intervention described was 30-120 minutes. The modes of delivery for the palliative intervention were videoconference/videophone (n = 3), telephone/teleconference (n = 3), and in person (n = 2). The interventions were delivered by nurses, psychiatrists, and social workers. In 5 of these studies, participants received palliative care on an individual basis and 2 studies delivered their intervention through groups. The individual basis studies focused on physical aspects of care and the group studies focused on emotional aspects of care.
Patient-reported outcomes
Cancer and its treatments are often associated with physical and emotional sequelae that can have a significant impact on patients and therefore PROs. The interventions reviewed in this article often reported data on the reduction of the physical and/or emotional symptom burden of cancer as well as overall QoL.
Reduction in physical symptoms. Three articles included physical symptoms as an outcome measure. Of those, 2 were pilot or feasibility studies, and 1 was a randomized control trial. Common physical symptoms included: shortness of breath, pain, fatigue, nausea, and appetite change. Across the articles, the Edmonton Symptom Assessment Scale (ESAS), a 10-item inventory of common cancer symptoms, was frequently used to measure of symptom scores in these interventions.14,15,19 The ESAS is an empirically validated measure that is used in palliative care research and clinical practice. Individuals are asked to rank 10 common symptoms on an ascending scale from 1 to 10 (0, the symptom is absent; 10, worst possible severity).23
The findings from these 3 research studies were encouraging. In a large randomized control trial of a supportive education program, researchers reported decreased physical symptom intensity after the intervention, however the change did not reach statistical significance.18 Similar findings were reported in a videoconferencing and a home health program to improve access of palliative and supportive oncology health care.14,15 Physical symptoms that had decreasing trends were pain, tiredness, and appetite, however, trends for shortness of breath found increasing severity.14,15 Although these trends were observed, it is important to note that scores on the ESAS did not reach statistical significance for physical symptoms in any of these studies.
Reduction in emotional symptom reduction. In addition to reducing physical symptoms, researchers also sought to understand the impact of programs on the emotional symptoms of cancer including: anxiety, depression, negative affect, and posttraumatic stress disorder (PTSD). Five articles included emotional symptoms as an outcome measure. Four were pilot or feasibility studies, and 1 was a randomized control trial.
Results across studies indicated an observable decrease in the severity of anxiety and depression for those exposed to an intervention program.14,15,18,19 Again, although trends were found, the results were not statistically significant. Only Watanabe and colleagues14 reported a statistically significant a decrease in anxiety in participants after the implementation of a rural palliative care videoconference consultation program. One report indicated that data on depression severity was collected but was not analyzed because of a small sample size.21
O’Brien and colleagues17 also collected data on negative affect and found that participants who participated in a supportive-expressive therapy group had a reduction in the negative affect as measured by the Derogatis Affects Balance Scale (ABS). Other researchers found no change in emotional distress.15
Finally, Collie and colleagues20 also measured the impact of a videoconference support group of PTSD symptomology for people with breast cancer in rural areas. Their results indicated a statistically significant decrease on the PTSD Checklist-Specific after intervention. Analysis of the data also found a medium effect size. Participants in the intervention group spoke about how participation in the support group allowed them to be generative and share information about breast cancer as well as build an emotional bond with other women with cancer.
Overall quality of life and well-being. Researchers have also looked into impact of intervention on overall QoL. Two articles included QoL or Well-being as an outcome measure. One was a pilot study and 1 was a randomized control trial.
Bakitas and colleagues18,19 found that those enrolled in the intervention arm of their study had higher QoL scores on the Functional Assessment of Cancer Therapy-General (FACT-G) compared with those in the control arm. These results were also found in an analysis of data from participants who subsequently died during the intervention. Improvements in overall well-being were also found by O’Brien and colleagues17 using ABS. They reported that a post hoc comparison of participants’ total positive affect score was significantly higher at the 12-month follow-up. In addition, the authors also noted qualitative improvements in well-being, including increased effort to be at the support group and the low attrition rates.
Overall impact of rural palliative care on individuals and society. In addition to reducing physical and emotional symptoms in patients, several of the articles also addressed other measures of the overall impact of the intervention or program on society as a whole. The authors evaluated patient satisfaction and quality of life, access to health care services, and financial impact on individuals and society at large.
Satisfaction with intervention. In 2 of the articles, individuals or their family members reported to be satisfied with the intervention14,20 and said they would recommend it to others as well.20 Both of those studies used teleconferencing to provide access to the intervention to people in rural communities.
Increasing access to the health care services and quality of care. Four of the articles evaluated the impact of intervention on patient’s access to the health care services.14,16,20,22 Specifically, after the interventions individuals had increased access to palliative care information in rural areas where it had previously been unavailable20 as well as actual delivery of clinical care in their home community, thus eliminating the need to travel to urban areas.14,20,22 This increase of access to health care services in rural area had significant effect on time and distance spent traveling. In 1 study, the amount of saving in terms of distance was 471.13 km and time in, 7.96 hours, for each visit.14
In addition, the quality of overall cancer care in rural area was increased. In an early clinical program, to increase access of palliative care in rural communities, the authors reported an increase in the breast conservation from 20% at the start of the program to 70% 2 years after the program was implemented.22 Breast conservation is not a typical outcome for palliative care studies, but the authors highlighted this practice change because of the improved QoL that is associated with the use of breast conservation therapies. In the same study, the authors reported an increased use of curative therapies for other cancers such as lymphoma as well as an increase use of pain management medication.
Financial impact. Two articles described the financial impact of cancer care costs on the patient and society.14,22 In a study by Watanabe and colleagues in Canada,14 the amount of savings after the intervention in terms of travel expenses was C$192.71 for each visit because patients had previously had to travel from their rural communities to urban tertiary hospitals to receive palliative care. For some patients in that study, the amount of saving for expenses was as high as C$500 a visit. In addition, some individuals were not able to travel and would not have received anything if the intervention had not been available remotely.14 In a study by Smith and colleagues in the United States, there was a 62% decrease in the cost to society for each patient, from US$10,233 to US$3,862.22 The factors contributing to that reduction included increasing outpatient services, engaging nurses and primary care providers instead of specialists, and the lower costs of living in rural areas. In addition, the rural hospitals saw an increase in revenue and profits because of higher admission rates ($500,00 for each hospital annually).22
Discussion
The articles identified in this review provide some evidence of the potential impact that palliative and supportive oncology interventions could have on PROs for rural residents with advanced cancer. Noteworthy results were seen for impact on reducing physical and emotional symptoms, increasing overall QoL and well-being, increasing satisfaction and access to palliative care, and reducing the overall cost of palliative care for individuals and society.14-18,20-22
Although statistical significance was not observed for most of the symptom assessment, trends toward improved symptom reports were observed. A likely explanation for this finding, is the small sample size or inadequate design to evaluate symptoms as an outcome measure. Three studies were pilot or feasibility projects15,20,21 that were not powered to detect the impact of the intervention on symptoms. In contrast, QoL stands out as an outcome that was positively affected by palliative care interventions. Further research is needed to determine if there are important mediating and moderating factors that contribute to improve QoL that are specific to rural residents. Significant outcomes were also reported for participant satisfaction with the interventions, the increase in access to services, and the decrease in costs.
Although there were not enough studies to determine the efficacy of these interventions, these results suggest that palliative and supportive interventions can have an impact on important patient-reported outcomes, such as symptoms and quality of life, and on health care system outcomes, such as cost. Evidence supporting the extent of the effectiveness of palliative care on various PROs in rural people is limited. None of the studies in this review evaluated the different aspects of palliative care specifically in rural residents.
It is interesting to note that all but one of the interventions used a telehealth approach to deliver the intervention. Telehealth interventions seem to be feasible, acceptable to people in rural areas, and show preliminary evidence that they can have an impact on PROs.
Limitations of this review include only inclusion of publications in English. In addition, some studies in this review include populations that were not exclusively rural residents, which makes it difficult for generalization.
Conclusion
Palliative and supportive interventions may improve various PROs in people with advanced cancer living in rural areas. Technologies that support remote access to people in rural areas, such as teleconferencing and videoconferencing, seem particularly promising delivery modalities with their potential to increase access to palliative and supportive interventions in underserved communities. Large-scale studies that are powered to test the impact of palliative care and support oncology interventions on PROs and other aspects of quality care among rural residents with advanced cancer are needed.
The authors thank Jennifer DeBerg, Health Science Librarian at the University of Iowa for her assistance in developing the literature search strategies.
1. Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas – United States, 1999-2014 [published correction at https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a11.htm]. MMWR Surveillance Summaries [serial online]. https://www.cdc.gov/mmwr/volumes/66/ss/ss6601a1.htm?s_cid=ss6601a1_w. Published January 13, 2017. Accessed January 20, 2017.
2. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US Cancer Mortality: Part I – All cancers and lung cancer and Part II – Colorectal, prostate, breast, and cervical cancers. https://www.hindawi.com/journals/jce/2011/107497/. Published 2011. Accessed April 28, 2017.
3. Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633-640.
4. Weaver KE, Geiger AM, Lu L, Case LD. Rural‐urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050-1057.
5. Fuchsia Howard A, Smillie K, Turnbull K, et al. Access to medical and supportive care for rural and remote cancer survivors in northern British Columbia. J Rural Health. 2014;30(3):311-321.
6. Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol. 2007;25(32):5082-5086.
7. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887.
8. Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24(4):390-399.
9. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
10. McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med. 2015;18(11):962-969.
11. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
12. Regn R, Robinson W, Robinson WR. Differences in palliative care needs among cancer survivors in an inner city academic facility versus a suburban community facility. J Clin Oncol. 2015;33(29_suppl):61.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;339:b2535.
14. Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer. 2013;21(4):1201-1207.
15. Howell D, Marshall D, Brazil K, et al. A shared care model pilot for palliative home care in a rural area: impact on symptoms, distress, and place of death. J Pain Symptom Manage. 2011;42(1):60-75.
16. Stern A, Valaitis R, Weir R, Jadad AR. Use of home telehealth in palliative cancer care: a case study. J Telemed Telecare. 2012;18(5):297-300.
17. O’Brien M, Harris J, King R, O’Brien T. Supportive-expressive group therapy for women with metastatic breast cancer: Improving access for Australian women through use of teleconference. Counselling Psychother Res. 2008;8(1):28-35.
18. Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Supportive Care. 2009;7(1):75-86.
19. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
20. Collie K, Kreshka MA, Ferrier S, et al. Videoconferencing for delivery of breast cancer support groups to women living in rural communities: a pilot study. Psychooncology. 200
21. Passik SD, Kirsh KL, Leibee S, et al. A feasibility study of dignity psychotherapy delivered via telemedicine. Palliat Support Care. 2004;2(2):149-155.
22. Smith TJ, Desch CE, Grasso MA, et al. The Rural Cancer Outreach Program: clinical and financial analysis of palliative and curative care for an underserved population. Cancer Treat Rev. 1996;22(Suppl A):97-101.
23. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
1. Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas – United States, 1999-2014 [published correction at https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a11.htm]. MMWR Surveillance Summaries [serial online]. https://www.cdc.gov/mmwr/volumes/66/ss/ss6601a1.htm?s_cid=ss6601a1_w. Published January 13, 2017. Accessed January 20, 2017.
2. Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US Cancer Mortality: Part I – All cancers and lung cancer and Part II – Colorectal, prostate, breast, and cervical cancers. https://www.hindawi.com/journals/jce/2011/107497/. Published 2011. Accessed April 28, 2017.
3. Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of rural cancer care in the United States. Oncology (Williston Park). 2015;29(9):633-640.
4. Weaver KE, Geiger AM, Lu L, Case LD. Rural‐urban disparities in health status among US cancer survivors. Cancer. 2013;119(5):1050-1057.
5. Fuchsia Howard A, Smillie K, Turnbull K, et al. Access to medical and supportive care for rural and remote cancer survivors in northern British Columbia. J Rural Health. 2014;30(3):311-321.
6. Bottomley A, Aaronson NK. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol. 2007;25(32):5082-5086.
7. Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30(8):880-887.
8. Baldwin LM, Cai Y, Larson EH, et al. Access to cancer services for rural colorectal cancer patients. J Rural Health. 2008;24(4):390-399.
9. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
10. McCorkle R, Jeon S, Ercolano E, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med. 2015;18(11):962-969.
11. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
12. Regn R, Robinson W, Robinson WR. Differences in palliative care needs among cancer survivors in an inner city academic facility versus a suburban community facility. J Clin Oncol. 2015;33(29_suppl):61.
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;339:b2535.
14. Watanabe SM, Fairchild A, Pituskin E, Borgersen P, Hanson J, Fassbender K. Improving access to specialist multidisciplinary palliative care consultation for rural cancer patients by videoconferencing: report of a pilot project. Support Care Cancer. 2013;21(4):1201-1207.
15. Howell D, Marshall D, Brazil K, et al. A shared care model pilot for palliative home care in a rural area: impact on symptoms, distress, and place of death. J Pain Symptom Manage. 2011;42(1):60-75.
16. Stern A, Valaitis R, Weir R, Jadad AR. Use of home telehealth in palliative cancer care: a case study. J Telemed Telecare. 2012;18(5):297-300.
17. O’Brien M, Harris J, King R, O’Brien T. Supportive-expressive group therapy for women with metastatic breast cancer: Improving access for Australian women through use of teleconference. Counselling Psychother Res. 2008;8(1):28-35.
18. Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: baseline findings, methodological challenges, and solutions. Palliat Supportive Care. 2009;7(1):75-86.
19. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
20. Collie K, Kreshka MA, Ferrier S, et al. Videoconferencing for delivery of breast cancer support groups to women living in rural communities: a pilot study. Psychooncology. 200
21. Passik SD, Kirsh KL, Leibee S, et al. A feasibility study of dignity psychotherapy delivered via telemedicine. Palliat Support Care. 2004;2(2):149-155.
22. Smith TJ, Desch CE, Grasso MA, et al. The Rural Cancer Outreach Program: clinical and financial analysis of palliative and curative care for an underserved population. Cancer Treat Rev. 1996;22(Suppl A):97-101.
23. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
The SDM 3 Circle Model: A Literature Synthesis and Adaptation for Shared Decision Making in the Hospital
Evolving models of medical care emphasize the importance of shared decision-making (SDM) on practical and ethical grounds.1-3 SDM is a cognitive, emotional, and relational process in which provider and patient collaborate in a decision after discussing the options, evidence, and potential benefits and harms, while considering the patient’s values, preferences, and circumstances.4 Categories of decisions include information gathering, pharmacotherapy, therapeutic procedures, consultations and referrals, counseling and precautions (eg, behavior modification, goals of care, end-of-life care), and care transitions (eg, transfer or discharge to home).5 Decisions span the continuum of urgency and may be anticipatory or reactive.6 The patient’s environment7,8 and the provider-patient relationship9 have been explicitly incorporated into the ideal SDM process.
SDM has been conceptually and empirically linked with evidence-based practice,1 although the relationship between SDM and clinical outcomes is less clear.10,11 SDM is desired by patients12 and may bolster patient satisfaction, trust, and adherence.13,14 Limited evidence suggests SDM could reduce inappropriate treatments and testing,15 decrease adverse events,16 and promote greater patient safety,17-19 but more well-designed studies are needed.
Provider, patient, and contextual factors influence the extent to which SDM occurs. Providers commonly cite time constraints and perceived lack of applicability to certain clinical scenarios or settings.19 Providers may also lack training and competency in SDM skills.2 Patients may be reluctant to disagree with their provider or fear being mislabeled as “difficult.”20 When faced with high stakes or emotionally charged decisions, patients’ surrogates may prefer to have the provider serve as the sole decision-maker.21 Contextually, there may be limited evidence, high clinical stake, or a number of equally beneficial (or harmful) options.22,23
Current SDM models guide clinicians in determining when and how to engage in SDM, yet models vary widely. For example, Elwyn’s model emphasizes the ethical imperative for SDM and outlines 3 SDM steps: introduce choice, describe options, and help patients explore preferences and make decisions.3 Using a multimodal review and clinician-driven feedback, Legaré’s “IP-SDM” (Interprofessional Shared Decision Making) model illustrates the roles of the interprofessional team and emphasizes the influence of environmental factors on decision-making.24 Recent systematic reviews of SDM models have attempted to identify common elements, language, and processes.2,25,26
This study reviews leading SDM models to construct a more environmentally and contextually sensitive model that is appropriate for the hospital setting. Although developed with hospital medicine in mind, a synthesized model that attends to environmental and systems context, provider/team factors, patient factors, and disease/medical variables is highly relevant in any setting where SDM occurs.
METHODS
We constructed a model that is appropriate for SDM across the care continuum through the following 3-part, iterative group process: (1) a comprehensive literature review of existing SDM models, (2) synthesis and inductive development of a new draft model, and (3) modification of the new model using feedback from SDM experts.
Narrative Literature Review
We performed a structured, comprehensive literature review 29 to compare and contrast existing SDM models and frameworks. Leading models and key concepts were first identified using 2 systematic reviews 25,26 and a comprehensive review.2 In order to extend the search to 2016 and include any overlooked articles, a PubMed search was performed using the terms “shared decision-making” or “medical decision-making” AND “model” or “theory” or “framework” for English-language articles from inception to 2016. The search was repeated using Google Scholar to verify results and obtain the number of citations per article as a proxy for impact and saturation. In order to minimize possible search error or selection bias, reference lists in high-impact publications were hand searched to identify additional articles. All abstracts were manually reviewed by 2 independent authors for relevance and later inclusion in our group iterative process. A priori inclusion criteria were limited to provider-patient SDM (ie, not clinical reasoning or making decisions in general) and complete descriptions of a conceptual model or framework. Additional publications suggested by experts (eg, perspective pieces or terminology summaries) were also reviewed.
Model Development and Expert Review
The draft model and a standardized set of questions (supplementary Appendix A) were then emailed to all first and last authors of the reviewed studies (Table 2). Expert responses were compiled, coded, and analyzed independently by 3 coauthors. Inductive coding techniques and a constant comparative approach were used to code the qualitative data.32 Preliminary findings were shared among the 3 reviewers and discussed until consensus was reached on emerging themes and implications for the new SDM model and multistep SDM pathway. A master list of suggested revisions was shared with the larger authorship team and the model was refined accordingly.
RESULTS
Two previously published systematic reviews25,26 identified 494 articles, 161 conceptual definitions of SDM, and over 30 separate key concepts. The additional PubMed search garnered 1957 publications (with many overlapping from the systematic reviews). A manual search of the systematic reviews and PubMed abstracts identified 16 unique and complete decision-making models for further review. Hand searches of their citations yielded an additional 6 models for a total of 22 models.3,4,13,23,33-51 The majority of excluded articles described specific decision aids and small clinical studies, focused on only one step of the decision-making process, or were not otherwise relevant. The first (SR) and senior authors (JS) reviewed the 22 models for SDM relevance, generalizability, and content saturation, yielding a final sample of 9 SDM models. A subsequent Google Scholar search did not identify any new SDM models but 2 SDM theory papers1,52 and 2 commentaries53,54 were selected based on influence (ie, number of citations), expert recommendation, or coverage of a novel aspect of SDM. A total of 15 studies (9 SDM models + 6 reviews; Table 2) were used by our development team to create a synthesized SDM model. A 10th SDM model55 and 3 additional descriptive and normative studies8,56,57 were later added based on expert feedback and incorporated into our final SDM 3 Circle Model.
Expert Feedback
Twenty-one of 27 (78%) SDM expert authors responded to our e-mail request for feedback. The majority (62%) agreed with the basic elements of the model, including the environmental frame and the 3 domains. Some respondents viewed SDM as strictly a process between patient and provider independent of the disease, leading to refinement of the medical context category. Several experts emphasized the importance of SDM “set-up,” which includes the elicitation of patient preferences in how decisions are made and the extent of patient and/or surrogate involvement.
Several respondents identified time constraints (N = 2), acuity of disease (N = 3), and presence of multiple teams (N = 6) to be the significant factors distinguishing inpatient from outpatient SDM. For some experts, “team” referred to the interprofessional care team, whereas others referred to it as the collaboration among attending physicians and trainees. Experts noted that although the intensity and frequency of inpatient interactions could promote SDM, higher patient acuity and the urgency of decisions could negatively influence SDM and/or the patient’s ability to participate. Similarly, the presence of other team members may either impede or promote SDM by either contributing to miscommunication or bringing well-trained SDM experts to the bedside. Financial impact on patients and resource constraints were also noted as relevant. All of these elements have been incorporated into the final SDM 3 Circle Model and multistep SDM Pathway (Supplemental Appendix A and B).
The SDM 3 Circle Model
The SDM 3 Circle Model comprises 3 categories of SDM barriers and facilitators that intersect within the environmental frame of an inpatient ward or other setting: (1) provider/team, (2) patient/family, and (3) medical context. A Venn diagram visually represents the conceptual overlaps and distinctions among these categories that are all affected by the environment in which they occur (Supplemental Appendix A).
The patient/family circle mirrors prior SDM models that address the role of patient preferences in making decisions,3,4,12 with the explicit addition of the roles of families and surrogates as either decision-makers or influencers. This circle includes personal characteristics, such as cognitions (eg, beliefs, attitudes), emotions (eg, anxiety, hope), behaviors (eg, adherence, assertiveness), illness history (ie, subjective experience and understanding of one’s own medical history), and related social features (eg, culture, education, literacy, social supports).
Patient factors are not static over time or context. They occur within an environmental setting and are likely to be influenced by concurrent provider and medical variables (the second and third circles). Disease exacerbation leading to hospitalization or transfer to a subacute facility could dramatically shift the calculus a patient uses to determine preferences or activate dormant family dynamics. Strong provider-patient rapport (the overlap of patient and provider factors) may influence the development of trust and subsequent decisions.9 The type of disease or symptom presentation (circle 3–medical context) may further influence patient factors due to stigma, perceived vulnerability, or assumed prognosis.
The provider/team circle includes both individual and team-based factors falling into similar categories as the patient/family domain, such as cognitions, behavior, and social features; however, these factors include both personal (eg, the provider’s personal history, values, and beliefs) and professional (eg, past medical training, decision-making style, past experiences treating a disease) characteristics. Decisions may involve an interprofessional team representing a broad range of personalities and professional values. Decisions and decision-making processes may change over time as team composition changes, as level of provider expertise varies, or as environmental, patient, or disease/illness factors influence providers and teams.
Medical context includes factors related to the disease and the potential ways to evaluate or manage it. Examples of disease factors include acuity, symptoms, course, and prognosis. Most obviously, disease factors will influence the content of risk-benefit discussions but may also affect the SDM process through disease stigma or cultural assumptions about etiology. Disease evaluation factors include the psychometrics of a diagnostic screen, invasive and noninvasive testing, or a range of different preventive or therapeutic interventions. Treatment variables include the available options, costs, and risk of complications. Medical context variables evolve as evidence-based medicine and biomedical knowledge increase and new treatment options emerge.
Each of the 3 circles operates within the same environmental frame, such as an inpatient medicine ward, which itself operates within a hospital and the broader healthcare system. This frame exerts overt and subtle influences on providers, patients, and even the medical context. Features of the environmental frame include culture (eg, values, preferences, social norms), university versus community setting, incentives, formularies, quality improvement campaigns, regulations, and technology use.
The dynamic interactivity of the environmental frame and the 3 circles inform the process of SDM and highlight key differences that may occur between care settings. Certain features may predominate in different situations, but all will influence and be influenced by features of other circles during the course of SDM.
Application of the SDM 3 Circle Model
Although the SDM process is similar across clinical settings, its operationalization varies in important ways for hospital decision-making. In some situations, patients may defer all decisions to their providers or decisions may be considered with multiple providers concurrently. In the hospital, SDM may not be possible, such as in emergency surgery for an obtunded patient or when the patient and surrogate are not available or able to participate in the decision. Therefore, providers may bypass the steps of information sharing and discussion of the decision (big arrow in the Figure and supplemental
DISCUSSION
The SDM 3 Circle Model provides a concise, ecologically valid, contextually sensitive representation of SDM that synthesizes and extends beyond recent SDM models.3,7,40 Each circle represents the forces that influence SDM across settings. Although the multistep SDM pathway occurs similarly in outpatient and inpatient settings, how each step is operationalized and how each “circle” exerts its influence may differ and warrants further consideration throughout the SDM process. For example, hospitalized patients may have greater stress and anxiety, have more family involvement, be more motivated to adhere to treatment, and may be under greater financial and social pressures. Unlike outpatient primary care, patients are less likely to have an existing relationship with their inpatient providers, potentially compromising patient confidence in the provider, and necessitating expeditious trust building.
The SDM 3 Circle Model captures “setting” in both the broader environmental frame and within the provider/team category of variables. The frame also captures health system and broader community variables that may influence the practicality of some medical decisions. Within this essential frame, all 3 categories of patient, provider, and medical context are included as part of the SDM process. A better understanding of their interplay may be of great value for clinicians, researchers, administrators, and policy makers who wish to further study and promote SDM. Both the SDM 3 Circle Model and its accompanying pathway (Figures 1 and 2) highlight opportunities for intervention and research, and may drive quality improvement initiatives to improve clinical outcomes.
Limitations
We did not perform a new systematic review, potentially omitting lesser-known publications. We mitigated this risk by using recent systematic reviews, searching multiple databases, hand searching citation lists, and making inquiries to SDM experts. Our selection of models used as a foundation for the synthesized model was based on consensus, which included an element of subjective, clinical judgment. Our SDM expert sample was small and limited to authors of the papers we reviewed, potentially restricting the range of viewpoints received. Lastly, the SDM 3 Circle Model highlights key concept areas rather than all possible factors that influence SDM.
CONCLUSIONS
We present a peer-reviewed, literature-based SDM model capable of accounting for the unique circumstances and challenges of SDM in the hospital. The SDM 3 Circle Model identifies the primary categories of variables thought to influence SDM, places them in a shared environmental frame, and visually represents their interactive nature. A multistep representation of the SDM process further illustrates how the unique features and challenges of hospitalization might exert influence at various points as patients and providers reach a shared decision. As the interrelationships of patient and provider/team, medical context, and the environmental frame in which they occur are better understood, more effective and targeted interventions to promote SDM can be developed and evaluated.
Acknowledgments
The authors would like to thank Evans Whitaker for his assistance with the literature review and the Patient Engagement Project volunteers for their support and assistance with data collection.
Disclosure
Financial support for this study was provided entirely by a grant from NIH/NCCIH (grant #R25 AT006573, awarded to Dr. Jason Satterfield). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Stephanie Rennke, MD, Patrick Yuan, BA, Brad Monash, MD, Rebecca Blankenburg, MD, MPH, Ian Chua, MD, Stephanie Harman, MD, Debbie S. Sakai, MD, Joan F. Hilton, DSc, MPH., and Jason Satterfield, PhD.
1. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295-1296. doi:10.1001/jama.2014.10186. PubMed
2. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
3. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
4. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651-661. PubMed
5. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. What is a medical decision? A units. Am J Respir Crit Care Med. 2011;183(7):915-921. doi:10.1164/rccm.201008-1214OC. PubMed
22. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
23. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
24. Légaré F, Bekker H, Desroches S, et al. How can continuing professional development better promote shared decision-making? Perspectives from an international collaboration. Implement Sci. 2011;6:68. doi:10.1186/1748-5908-6-68. PubMed
25. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301-312. doi:10.1016/j.pec.2005.06.010. PubMed
26. Moumjid N, Gafni A, Brémond A, Carrère MO. Shared decision making in the medical taxonomy based on physician statements in hospital encounters: a qualitative study. BMJ Open. 2016;6(2):e010098. doi:10.1136/bmjopen-2015-010098. PubMed
6. Fowler FJ, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood). 2011;30(4):699-706. doi:10.1377/hlthaff.2011.0003. PubMed
7. Weiner SJ, Kelly B, Ashley N, et al. Content coding for contextualization of care: evaluating physician performance at patient-centered decision making. Med Decis Making. 2014;34(1):97-106. doi:10.1177/0272989X13493146. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91(2):176-179. doi:10.1016/j.pec.2013.01.006 PubMed
10. Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Making. 2016;36(4):427-452. doi:10.1177/0272989X15613530. PubMed
11. Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295-301. doi:10.1016/j.pec.2014.07.017. PubMed
12. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18. doi:10.1016/j.pec.2011.02.004. PubMed
13. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
14. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219-226. doi:10.1159/000126073. PubMed
15. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
16. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. doi:10.1093/intqhc/mzr002. PubMed
17. Mohammed K, Nolan MB, Rajjo T, et al. Creating a Patient-Centered Health Care Delivery System: A Systematic Review of Health Care Quality From the Patient Perspective. Am J Med Qual. 2014;31(1):12-21. doi:10.1177/1062860614545124. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. doi:10.1136/bmjqs-2012-001769. PubMed
19. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526-535. doi:10.1016/j.pec.2008.07.018. PubMed
20. Frosch DL, May SG, Rendle KAS, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled “difficult” among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. doi:10.1377/hlthaff.2011.0576. PubMed
21. Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care encounter: are we all talking about the same thing? Med Decis Making. 2007;27(5):539-546. doi:10.1177/0272989X07306779. PubMed
27. Hallström I, Elander G. Decision-making during hospitalization: parents’ and children’s involvement. J Clin Nurs. 2004;13(3):367-375. PubMed
28. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. Temporal characteristics of decisions in hospital encounters: a threshold for shared decision making? A qualitative study. Patient Educ Couns. 2014;97(2):216-222. doi:10.1016/j.pec.2014.08.005. PubMed
29. Baumeister RF, Leary MR. Writing narrative literature reviews. Rev Gen Psychol. 1997;1(3):311.
30. Moody DL. Theoretical and practical issues in evaluating the quality of conceptual models: current state and future directions. Data Knowl Eng. 2005;55(3):243-276. doi:10.1016/j.datak.2004.12.005.
31. McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351-377. PubMed
32. Basics of Qualitative Research | SAGE Publications Inc. https://us.sagepub.com/en-us/nam/basics-of-qualitative-research/book235578. Accessed on September 13, 2016. PubMed
33. 2013;2(4):421-433. doi:10.2217/cer.13.46.J Comp Eff Res33. Halley MC, Rendle KA, Frosch DL. A conceptual model of the multiple stages of communication necessary to support patient-centered care. PubMed
34. 2012;87(1):54-61. doi:10.1016/j.pec.2011.07.027.Patient Educ Couns34. Torke AM, Petronio S, Sachs GA, Helft PR, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. PubMed
35. 2009;15(6):1142-1151. doi:10.1111/j.1365-2753.2009.01315.x.J Eval Clin Pract35. Falzer PR, Garman MD. A conditional model of evidence-based decision making: Model of evidence-based decision making. PubMed
36. 2012;8(4):161-164. doi:10.1097/PTS.0b013e318267c56e.J Patient Saf36. Holzmueller CG, Wu AW, Pronovost PJ. A framework for encouraging patient engagement in medical decision making. PubMed
37. 2014;97(2):158-164. doi:10.1016/j.pec.2014.07.027.Patient Educ Couns37. Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: a model for patient care. PubMed
38. 2002;35(5-6):313-321. doi:10.1016/S1532-0464(03)00037-6.J Biomed Inform38. Ruland CM, Bakken S. Developing, implementing, and evaluating decision support systems for shared decision making in patient care: a conceptual model and case illustration. PubMed
39. 1999;319(7212):764.BMJ39. Shepperd S, Charnock D, Gann B. Helping patients access high quality health information. PubMed
40. 2011;25(1):18-25. doi:10.3109/13561820.2010.490502.J Interprof Care40. Légaré F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. PubMed
41. 2015;25(1):141-152. doi:10.1007/s10926-014-9532-7.J Occup Rehabil41. Coutu MF, Légaré F, Durand MJ, et al. Operationalizing a Shared Decision Making Model for Work Rehabilitation Programs: A Consensus Process. PubMed
42. 2013;13:231.BMC Health Serv Res42. Hölzel LP, Kriston L, Härter M. Patient preference for involvement, experienced involvement, decisional conflict, and satisfaction with physician: a structural equation model test. PubMed
43. 2008;134(4):835-843. doi:10.1378/chest.08-0235.Chest43. Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. PubMed
44. 2013;8:29-36. doi:10.4137/IMI.S12783.Integr Med Insights44. Brooks AT, Silverman L, Wallen G. Shared Decision Making: A Fundamental Tenet in a Conceptual Framework of Integrative Healthcare Delivery. PubMed
45. 2013;33(1):37-47. doi:10.1177/0272989X12458159.Med Decis Making45. Müller-Engelmann M, Donner-Banzhoff N, Keller H, et al. When decisions should be shared: a study of social norms in medical decision making using a factorial survey approach. PubMed
46. 2007;101(4):205-211.Z Arztl Fortbild Qualitatssich46. Mccaffery KJ, Shepherd HL, Trevena L, et al. Shared decision-making in Australia. PubMed
47. 2014;20(2):311-318. doi:10.1007/s12028-013-9922-2.Neurocrit Care
47. Rubin MA. The Collaborative Autonomy Model of Medical Decision-Making. 48. 2013;70(1 Suppl):141S-158S. doi:10.1177/1077558712461952.Med Care Res Rev PubMed
48. McCullough LB. The professional medical ethics model of decision making under conditions of clinical uncertainty. PubMed
49. 2009;87(2):368–390.Milbank Q49. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. PubMed
50. 2015;25(3):276-282. doi:10.1016/j.whi.2015.02.002.Womens Health Issues50. Moore JE, Titler MG, Kane Low L, Dalton VK, Sampselle CM. Transforming Patient-Centered Care: Development of the Evidence Informed Decision Making through Engagement Model. PubMed
51. 1997;44(5):681-692.Soc Sci Med51. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). PubMed
52. 2010;80(2):164-172. doi:10.1016/j.pec.2009.10.015.Patient Educ Couns52. Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. PubMed
53. 2013;70(1 Suppl):94S-112S. doi:10.1177/1077558712459216.Med Care Res Rev53. Epstein RM, Gramling RE. What is shared in shared decision making? Complex decisions when the evidence is unclear. PubMed
54. 2010;304(8):903-904. doi:10.1001/jama.2010.1208.JAMA54. Kon AA. The shared decision-making continuum. PubMed
55. 2008;30(3):429-444. doi:10.1111/j.1467-9566.2007.01064.x.Sociol Health Illn55. Rapley T. Distributed decision making: the anatomy of decisions-in-action. PubMed
56. 1997;12(6):339-345.J Gen Intern Med56. Braddock CH 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. PubMed
57. 1999;282(24):2313-2320.JAMA57. Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. PubMed
58. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056.Soc Sci Med58. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups.
2006;9(4):321-332. doi:10.1111/j.1369-7625.2006.00404.x.Health Expect. PubMed
59. Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. PubMed
60. 2011;17(4):554-564. doi: 10.1111/j.1365-2753.2010.01515.x.J Eval Clin Pract60. Légaré F, Stacey D, Gagnon S, et al. Validating a conceptual model for an interprofessional approach to shared decision making: a mixed methods study. PubMed
Evolving models of medical care emphasize the importance of shared decision-making (SDM) on practical and ethical grounds.1-3 SDM is a cognitive, emotional, and relational process in which provider and patient collaborate in a decision after discussing the options, evidence, and potential benefits and harms, while considering the patient’s values, preferences, and circumstances.4 Categories of decisions include information gathering, pharmacotherapy, therapeutic procedures, consultations and referrals, counseling and precautions (eg, behavior modification, goals of care, end-of-life care), and care transitions (eg, transfer or discharge to home).5 Decisions span the continuum of urgency and may be anticipatory or reactive.6 The patient’s environment7,8 and the provider-patient relationship9 have been explicitly incorporated into the ideal SDM process.
SDM has been conceptually and empirically linked with evidence-based practice,1 although the relationship between SDM and clinical outcomes is less clear.10,11 SDM is desired by patients12 and may bolster patient satisfaction, trust, and adherence.13,14 Limited evidence suggests SDM could reduce inappropriate treatments and testing,15 decrease adverse events,16 and promote greater patient safety,17-19 but more well-designed studies are needed.
Provider, patient, and contextual factors influence the extent to which SDM occurs. Providers commonly cite time constraints and perceived lack of applicability to certain clinical scenarios or settings.19 Providers may also lack training and competency in SDM skills.2 Patients may be reluctant to disagree with their provider or fear being mislabeled as “difficult.”20 When faced with high stakes or emotionally charged decisions, patients’ surrogates may prefer to have the provider serve as the sole decision-maker.21 Contextually, there may be limited evidence, high clinical stake, or a number of equally beneficial (or harmful) options.22,23
Current SDM models guide clinicians in determining when and how to engage in SDM, yet models vary widely. For example, Elwyn’s model emphasizes the ethical imperative for SDM and outlines 3 SDM steps: introduce choice, describe options, and help patients explore preferences and make decisions.3 Using a multimodal review and clinician-driven feedback, Legaré’s “IP-SDM” (Interprofessional Shared Decision Making) model illustrates the roles of the interprofessional team and emphasizes the influence of environmental factors on decision-making.24 Recent systematic reviews of SDM models have attempted to identify common elements, language, and processes.2,25,26
This study reviews leading SDM models to construct a more environmentally and contextually sensitive model that is appropriate for the hospital setting. Although developed with hospital medicine in mind, a synthesized model that attends to environmental and systems context, provider/team factors, patient factors, and disease/medical variables is highly relevant in any setting where SDM occurs.
METHODS
We constructed a model that is appropriate for SDM across the care continuum through the following 3-part, iterative group process: (1) a comprehensive literature review of existing SDM models, (2) synthesis and inductive development of a new draft model, and (3) modification of the new model using feedback from SDM experts.
Narrative Literature Review
We performed a structured, comprehensive literature review 29 to compare and contrast existing SDM models and frameworks. Leading models and key concepts were first identified using 2 systematic reviews 25,26 and a comprehensive review.2 In order to extend the search to 2016 and include any overlooked articles, a PubMed search was performed using the terms “shared decision-making” or “medical decision-making” AND “model” or “theory” or “framework” for English-language articles from inception to 2016. The search was repeated using Google Scholar to verify results and obtain the number of citations per article as a proxy for impact and saturation. In order to minimize possible search error or selection bias, reference lists in high-impact publications were hand searched to identify additional articles. All abstracts were manually reviewed by 2 independent authors for relevance and later inclusion in our group iterative process. A priori inclusion criteria were limited to provider-patient SDM (ie, not clinical reasoning or making decisions in general) and complete descriptions of a conceptual model or framework. Additional publications suggested by experts (eg, perspective pieces or terminology summaries) were also reviewed.
Model Development and Expert Review
The draft model and a standardized set of questions (supplementary Appendix A) were then emailed to all first and last authors of the reviewed studies (Table 2). Expert responses were compiled, coded, and analyzed independently by 3 coauthors. Inductive coding techniques and a constant comparative approach were used to code the qualitative data.32 Preliminary findings were shared among the 3 reviewers and discussed until consensus was reached on emerging themes and implications for the new SDM model and multistep SDM pathway. A master list of suggested revisions was shared with the larger authorship team and the model was refined accordingly.
RESULTS
Two previously published systematic reviews25,26 identified 494 articles, 161 conceptual definitions of SDM, and over 30 separate key concepts. The additional PubMed search garnered 1957 publications (with many overlapping from the systematic reviews). A manual search of the systematic reviews and PubMed abstracts identified 16 unique and complete decision-making models for further review. Hand searches of their citations yielded an additional 6 models for a total of 22 models.3,4,13,23,33-51 The majority of excluded articles described specific decision aids and small clinical studies, focused on only one step of the decision-making process, or were not otherwise relevant. The first (SR) and senior authors (JS) reviewed the 22 models for SDM relevance, generalizability, and content saturation, yielding a final sample of 9 SDM models. A subsequent Google Scholar search did not identify any new SDM models but 2 SDM theory papers1,52 and 2 commentaries53,54 were selected based on influence (ie, number of citations), expert recommendation, or coverage of a novel aspect of SDM. A total of 15 studies (9 SDM models + 6 reviews; Table 2) were used by our development team to create a synthesized SDM model. A 10th SDM model55 and 3 additional descriptive and normative studies8,56,57 were later added based on expert feedback and incorporated into our final SDM 3 Circle Model.
Expert Feedback
Twenty-one of 27 (78%) SDM expert authors responded to our e-mail request for feedback. The majority (62%) agreed with the basic elements of the model, including the environmental frame and the 3 domains. Some respondents viewed SDM as strictly a process between patient and provider independent of the disease, leading to refinement of the medical context category. Several experts emphasized the importance of SDM “set-up,” which includes the elicitation of patient preferences in how decisions are made and the extent of patient and/or surrogate involvement.
Several respondents identified time constraints (N = 2), acuity of disease (N = 3), and presence of multiple teams (N = 6) to be the significant factors distinguishing inpatient from outpatient SDM. For some experts, “team” referred to the interprofessional care team, whereas others referred to it as the collaboration among attending physicians and trainees. Experts noted that although the intensity and frequency of inpatient interactions could promote SDM, higher patient acuity and the urgency of decisions could negatively influence SDM and/or the patient’s ability to participate. Similarly, the presence of other team members may either impede or promote SDM by either contributing to miscommunication or bringing well-trained SDM experts to the bedside. Financial impact on patients and resource constraints were also noted as relevant. All of these elements have been incorporated into the final SDM 3 Circle Model and multistep SDM Pathway (Supplemental Appendix A and B).
The SDM 3 Circle Model
The SDM 3 Circle Model comprises 3 categories of SDM barriers and facilitators that intersect within the environmental frame of an inpatient ward or other setting: (1) provider/team, (2) patient/family, and (3) medical context. A Venn diagram visually represents the conceptual overlaps and distinctions among these categories that are all affected by the environment in which they occur (Supplemental Appendix A).
The patient/family circle mirrors prior SDM models that address the role of patient preferences in making decisions,3,4,12 with the explicit addition of the roles of families and surrogates as either decision-makers or influencers. This circle includes personal characteristics, such as cognitions (eg, beliefs, attitudes), emotions (eg, anxiety, hope), behaviors (eg, adherence, assertiveness), illness history (ie, subjective experience and understanding of one’s own medical history), and related social features (eg, culture, education, literacy, social supports).
Patient factors are not static over time or context. They occur within an environmental setting and are likely to be influenced by concurrent provider and medical variables (the second and third circles). Disease exacerbation leading to hospitalization or transfer to a subacute facility could dramatically shift the calculus a patient uses to determine preferences or activate dormant family dynamics. Strong provider-patient rapport (the overlap of patient and provider factors) may influence the development of trust and subsequent decisions.9 The type of disease or symptom presentation (circle 3–medical context) may further influence patient factors due to stigma, perceived vulnerability, or assumed prognosis.
The provider/team circle includes both individual and team-based factors falling into similar categories as the patient/family domain, such as cognitions, behavior, and social features; however, these factors include both personal (eg, the provider’s personal history, values, and beliefs) and professional (eg, past medical training, decision-making style, past experiences treating a disease) characteristics. Decisions may involve an interprofessional team representing a broad range of personalities and professional values. Decisions and decision-making processes may change over time as team composition changes, as level of provider expertise varies, or as environmental, patient, or disease/illness factors influence providers and teams.
Medical context includes factors related to the disease and the potential ways to evaluate or manage it. Examples of disease factors include acuity, symptoms, course, and prognosis. Most obviously, disease factors will influence the content of risk-benefit discussions but may also affect the SDM process through disease stigma or cultural assumptions about etiology. Disease evaluation factors include the psychometrics of a diagnostic screen, invasive and noninvasive testing, or a range of different preventive or therapeutic interventions. Treatment variables include the available options, costs, and risk of complications. Medical context variables evolve as evidence-based medicine and biomedical knowledge increase and new treatment options emerge.
Each of the 3 circles operates within the same environmental frame, such as an inpatient medicine ward, which itself operates within a hospital and the broader healthcare system. This frame exerts overt and subtle influences on providers, patients, and even the medical context. Features of the environmental frame include culture (eg, values, preferences, social norms), university versus community setting, incentives, formularies, quality improvement campaigns, regulations, and technology use.
The dynamic interactivity of the environmental frame and the 3 circles inform the process of SDM and highlight key differences that may occur between care settings. Certain features may predominate in different situations, but all will influence and be influenced by features of other circles during the course of SDM.
Application of the SDM 3 Circle Model
Although the SDM process is similar across clinical settings, its operationalization varies in important ways for hospital decision-making. In some situations, patients may defer all decisions to their providers or decisions may be considered with multiple providers concurrently. In the hospital, SDM may not be possible, such as in emergency surgery for an obtunded patient or when the patient and surrogate are not available or able to participate in the decision. Therefore, providers may bypass the steps of information sharing and discussion of the decision (big arrow in the Figure and supplemental
DISCUSSION
The SDM 3 Circle Model provides a concise, ecologically valid, contextually sensitive representation of SDM that synthesizes and extends beyond recent SDM models.3,7,40 Each circle represents the forces that influence SDM across settings. Although the multistep SDM pathway occurs similarly in outpatient and inpatient settings, how each step is operationalized and how each “circle” exerts its influence may differ and warrants further consideration throughout the SDM process. For example, hospitalized patients may have greater stress and anxiety, have more family involvement, be more motivated to adhere to treatment, and may be under greater financial and social pressures. Unlike outpatient primary care, patients are less likely to have an existing relationship with their inpatient providers, potentially compromising patient confidence in the provider, and necessitating expeditious trust building.
The SDM 3 Circle Model captures “setting” in both the broader environmental frame and within the provider/team category of variables. The frame also captures health system and broader community variables that may influence the practicality of some medical decisions. Within this essential frame, all 3 categories of patient, provider, and medical context are included as part of the SDM process. A better understanding of their interplay may be of great value for clinicians, researchers, administrators, and policy makers who wish to further study and promote SDM. Both the SDM 3 Circle Model and its accompanying pathway (Figures 1 and 2) highlight opportunities for intervention and research, and may drive quality improvement initiatives to improve clinical outcomes.
Limitations
We did not perform a new systematic review, potentially omitting lesser-known publications. We mitigated this risk by using recent systematic reviews, searching multiple databases, hand searching citation lists, and making inquiries to SDM experts. Our selection of models used as a foundation for the synthesized model was based on consensus, which included an element of subjective, clinical judgment. Our SDM expert sample was small and limited to authors of the papers we reviewed, potentially restricting the range of viewpoints received. Lastly, the SDM 3 Circle Model highlights key concept areas rather than all possible factors that influence SDM.
CONCLUSIONS
We present a peer-reviewed, literature-based SDM model capable of accounting for the unique circumstances and challenges of SDM in the hospital. The SDM 3 Circle Model identifies the primary categories of variables thought to influence SDM, places them in a shared environmental frame, and visually represents their interactive nature. A multistep representation of the SDM process further illustrates how the unique features and challenges of hospitalization might exert influence at various points as patients and providers reach a shared decision. As the interrelationships of patient and provider/team, medical context, and the environmental frame in which they occur are better understood, more effective and targeted interventions to promote SDM can be developed and evaluated.
Acknowledgments
The authors would like to thank Evans Whitaker for his assistance with the literature review and the Patient Engagement Project volunteers for their support and assistance with data collection.
Disclosure
Financial support for this study was provided entirely by a grant from NIH/NCCIH (grant #R25 AT006573, awarded to Dr. Jason Satterfield). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Stephanie Rennke, MD, Patrick Yuan, BA, Brad Monash, MD, Rebecca Blankenburg, MD, MPH, Ian Chua, MD, Stephanie Harman, MD, Debbie S. Sakai, MD, Joan F. Hilton, DSc, MPH., and Jason Satterfield, PhD.
Evolving models of medical care emphasize the importance of shared decision-making (SDM) on practical and ethical grounds.1-3 SDM is a cognitive, emotional, and relational process in which provider and patient collaborate in a decision after discussing the options, evidence, and potential benefits and harms, while considering the patient’s values, preferences, and circumstances.4 Categories of decisions include information gathering, pharmacotherapy, therapeutic procedures, consultations and referrals, counseling and precautions (eg, behavior modification, goals of care, end-of-life care), and care transitions (eg, transfer or discharge to home).5 Decisions span the continuum of urgency and may be anticipatory or reactive.6 The patient’s environment7,8 and the provider-patient relationship9 have been explicitly incorporated into the ideal SDM process.
SDM has been conceptually and empirically linked with evidence-based practice,1 although the relationship between SDM and clinical outcomes is less clear.10,11 SDM is desired by patients12 and may bolster patient satisfaction, trust, and adherence.13,14 Limited evidence suggests SDM could reduce inappropriate treatments and testing,15 decrease adverse events,16 and promote greater patient safety,17-19 but more well-designed studies are needed.
Provider, patient, and contextual factors influence the extent to which SDM occurs. Providers commonly cite time constraints and perceived lack of applicability to certain clinical scenarios or settings.19 Providers may also lack training and competency in SDM skills.2 Patients may be reluctant to disagree with their provider or fear being mislabeled as “difficult.”20 When faced with high stakes or emotionally charged decisions, patients’ surrogates may prefer to have the provider serve as the sole decision-maker.21 Contextually, there may be limited evidence, high clinical stake, or a number of equally beneficial (or harmful) options.22,23
Current SDM models guide clinicians in determining when and how to engage in SDM, yet models vary widely. For example, Elwyn’s model emphasizes the ethical imperative for SDM and outlines 3 SDM steps: introduce choice, describe options, and help patients explore preferences and make decisions.3 Using a multimodal review and clinician-driven feedback, Legaré’s “IP-SDM” (Interprofessional Shared Decision Making) model illustrates the roles of the interprofessional team and emphasizes the influence of environmental factors on decision-making.24 Recent systematic reviews of SDM models have attempted to identify common elements, language, and processes.2,25,26
This study reviews leading SDM models to construct a more environmentally and contextually sensitive model that is appropriate for the hospital setting. Although developed with hospital medicine in mind, a synthesized model that attends to environmental and systems context, provider/team factors, patient factors, and disease/medical variables is highly relevant in any setting where SDM occurs.
METHODS
We constructed a model that is appropriate for SDM across the care continuum through the following 3-part, iterative group process: (1) a comprehensive literature review of existing SDM models, (2) synthesis and inductive development of a new draft model, and (3) modification of the new model using feedback from SDM experts.
Narrative Literature Review
We performed a structured, comprehensive literature review 29 to compare and contrast existing SDM models and frameworks. Leading models and key concepts were first identified using 2 systematic reviews 25,26 and a comprehensive review.2 In order to extend the search to 2016 and include any overlooked articles, a PubMed search was performed using the terms “shared decision-making” or “medical decision-making” AND “model” or “theory” or “framework” for English-language articles from inception to 2016. The search was repeated using Google Scholar to verify results and obtain the number of citations per article as a proxy for impact and saturation. In order to minimize possible search error or selection bias, reference lists in high-impact publications were hand searched to identify additional articles. All abstracts were manually reviewed by 2 independent authors for relevance and later inclusion in our group iterative process. A priori inclusion criteria were limited to provider-patient SDM (ie, not clinical reasoning or making decisions in general) and complete descriptions of a conceptual model or framework. Additional publications suggested by experts (eg, perspective pieces or terminology summaries) were also reviewed.
Model Development and Expert Review
The draft model and a standardized set of questions (supplementary Appendix A) were then emailed to all first and last authors of the reviewed studies (Table 2). Expert responses were compiled, coded, and analyzed independently by 3 coauthors. Inductive coding techniques and a constant comparative approach were used to code the qualitative data.32 Preliminary findings were shared among the 3 reviewers and discussed until consensus was reached on emerging themes and implications for the new SDM model and multistep SDM pathway. A master list of suggested revisions was shared with the larger authorship team and the model was refined accordingly.
RESULTS
Two previously published systematic reviews25,26 identified 494 articles, 161 conceptual definitions of SDM, and over 30 separate key concepts. The additional PubMed search garnered 1957 publications (with many overlapping from the systematic reviews). A manual search of the systematic reviews and PubMed abstracts identified 16 unique and complete decision-making models for further review. Hand searches of their citations yielded an additional 6 models for a total of 22 models.3,4,13,23,33-51 The majority of excluded articles described specific decision aids and small clinical studies, focused on only one step of the decision-making process, or were not otherwise relevant. The first (SR) and senior authors (JS) reviewed the 22 models for SDM relevance, generalizability, and content saturation, yielding a final sample of 9 SDM models. A subsequent Google Scholar search did not identify any new SDM models but 2 SDM theory papers1,52 and 2 commentaries53,54 were selected based on influence (ie, number of citations), expert recommendation, or coverage of a novel aspect of SDM. A total of 15 studies (9 SDM models + 6 reviews; Table 2) were used by our development team to create a synthesized SDM model. A 10th SDM model55 and 3 additional descriptive and normative studies8,56,57 were later added based on expert feedback and incorporated into our final SDM 3 Circle Model.
Expert Feedback
Twenty-one of 27 (78%) SDM expert authors responded to our e-mail request for feedback. The majority (62%) agreed with the basic elements of the model, including the environmental frame and the 3 domains. Some respondents viewed SDM as strictly a process between patient and provider independent of the disease, leading to refinement of the medical context category. Several experts emphasized the importance of SDM “set-up,” which includes the elicitation of patient preferences in how decisions are made and the extent of patient and/or surrogate involvement.
Several respondents identified time constraints (N = 2), acuity of disease (N = 3), and presence of multiple teams (N = 6) to be the significant factors distinguishing inpatient from outpatient SDM. For some experts, “team” referred to the interprofessional care team, whereas others referred to it as the collaboration among attending physicians and trainees. Experts noted that although the intensity and frequency of inpatient interactions could promote SDM, higher patient acuity and the urgency of decisions could negatively influence SDM and/or the patient’s ability to participate. Similarly, the presence of other team members may either impede or promote SDM by either contributing to miscommunication or bringing well-trained SDM experts to the bedside. Financial impact on patients and resource constraints were also noted as relevant. All of these elements have been incorporated into the final SDM 3 Circle Model and multistep SDM Pathway (Supplemental Appendix A and B).
The SDM 3 Circle Model
The SDM 3 Circle Model comprises 3 categories of SDM barriers and facilitators that intersect within the environmental frame of an inpatient ward or other setting: (1) provider/team, (2) patient/family, and (3) medical context. A Venn diagram visually represents the conceptual overlaps and distinctions among these categories that are all affected by the environment in which they occur (Supplemental Appendix A).
The patient/family circle mirrors prior SDM models that address the role of patient preferences in making decisions,3,4,12 with the explicit addition of the roles of families and surrogates as either decision-makers or influencers. This circle includes personal characteristics, such as cognitions (eg, beliefs, attitudes), emotions (eg, anxiety, hope), behaviors (eg, adherence, assertiveness), illness history (ie, subjective experience and understanding of one’s own medical history), and related social features (eg, culture, education, literacy, social supports).
Patient factors are not static over time or context. They occur within an environmental setting and are likely to be influenced by concurrent provider and medical variables (the second and third circles). Disease exacerbation leading to hospitalization or transfer to a subacute facility could dramatically shift the calculus a patient uses to determine preferences or activate dormant family dynamics. Strong provider-patient rapport (the overlap of patient and provider factors) may influence the development of trust and subsequent decisions.9 The type of disease or symptom presentation (circle 3–medical context) may further influence patient factors due to stigma, perceived vulnerability, or assumed prognosis.
The provider/team circle includes both individual and team-based factors falling into similar categories as the patient/family domain, such as cognitions, behavior, and social features; however, these factors include both personal (eg, the provider’s personal history, values, and beliefs) and professional (eg, past medical training, decision-making style, past experiences treating a disease) characteristics. Decisions may involve an interprofessional team representing a broad range of personalities and professional values. Decisions and decision-making processes may change over time as team composition changes, as level of provider expertise varies, or as environmental, patient, or disease/illness factors influence providers and teams.
Medical context includes factors related to the disease and the potential ways to evaluate or manage it. Examples of disease factors include acuity, symptoms, course, and prognosis. Most obviously, disease factors will influence the content of risk-benefit discussions but may also affect the SDM process through disease stigma or cultural assumptions about etiology. Disease evaluation factors include the psychometrics of a diagnostic screen, invasive and noninvasive testing, or a range of different preventive or therapeutic interventions. Treatment variables include the available options, costs, and risk of complications. Medical context variables evolve as evidence-based medicine and biomedical knowledge increase and new treatment options emerge.
Each of the 3 circles operates within the same environmental frame, such as an inpatient medicine ward, which itself operates within a hospital and the broader healthcare system. This frame exerts overt and subtle influences on providers, patients, and even the medical context. Features of the environmental frame include culture (eg, values, preferences, social norms), university versus community setting, incentives, formularies, quality improvement campaigns, regulations, and technology use.
The dynamic interactivity of the environmental frame and the 3 circles inform the process of SDM and highlight key differences that may occur between care settings. Certain features may predominate in different situations, but all will influence and be influenced by features of other circles during the course of SDM.
Application of the SDM 3 Circle Model
Although the SDM process is similar across clinical settings, its operationalization varies in important ways for hospital decision-making. In some situations, patients may defer all decisions to their providers or decisions may be considered with multiple providers concurrently. In the hospital, SDM may not be possible, such as in emergency surgery for an obtunded patient or when the patient and surrogate are not available or able to participate in the decision. Therefore, providers may bypass the steps of information sharing and discussion of the decision (big arrow in the Figure and supplemental
DISCUSSION
The SDM 3 Circle Model provides a concise, ecologically valid, contextually sensitive representation of SDM that synthesizes and extends beyond recent SDM models.3,7,40 Each circle represents the forces that influence SDM across settings. Although the multistep SDM pathway occurs similarly in outpatient and inpatient settings, how each step is operationalized and how each “circle” exerts its influence may differ and warrants further consideration throughout the SDM process. For example, hospitalized patients may have greater stress and anxiety, have more family involvement, be more motivated to adhere to treatment, and may be under greater financial and social pressures. Unlike outpatient primary care, patients are less likely to have an existing relationship with their inpatient providers, potentially compromising patient confidence in the provider, and necessitating expeditious trust building.
The SDM 3 Circle Model captures “setting” in both the broader environmental frame and within the provider/team category of variables. The frame also captures health system and broader community variables that may influence the practicality of some medical decisions. Within this essential frame, all 3 categories of patient, provider, and medical context are included as part of the SDM process. A better understanding of their interplay may be of great value for clinicians, researchers, administrators, and policy makers who wish to further study and promote SDM. Both the SDM 3 Circle Model and its accompanying pathway (Figures 1 and 2) highlight opportunities for intervention and research, and may drive quality improvement initiatives to improve clinical outcomes.
Limitations
We did not perform a new systematic review, potentially omitting lesser-known publications. We mitigated this risk by using recent systematic reviews, searching multiple databases, hand searching citation lists, and making inquiries to SDM experts. Our selection of models used as a foundation for the synthesized model was based on consensus, which included an element of subjective, clinical judgment. Our SDM expert sample was small and limited to authors of the papers we reviewed, potentially restricting the range of viewpoints received. Lastly, the SDM 3 Circle Model highlights key concept areas rather than all possible factors that influence SDM.
CONCLUSIONS
We present a peer-reviewed, literature-based SDM model capable of accounting for the unique circumstances and challenges of SDM in the hospital. The SDM 3 Circle Model identifies the primary categories of variables thought to influence SDM, places them in a shared environmental frame, and visually represents their interactive nature. A multistep representation of the SDM process further illustrates how the unique features and challenges of hospitalization might exert influence at various points as patients and providers reach a shared decision. As the interrelationships of patient and provider/team, medical context, and the environmental frame in which they occur are better understood, more effective and targeted interventions to promote SDM can be developed and evaluated.
Acknowledgments
The authors would like to thank Evans Whitaker for his assistance with the literature review and the Patient Engagement Project volunteers for their support and assistance with data collection.
Disclosure
Financial support for this study was provided entirely by a grant from NIH/NCCIH (grant #R25 AT006573, awarded to Dr. Jason Satterfield). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors are employed by the sponsor: Stephanie Rennke, MD, Patrick Yuan, BA, Brad Monash, MD, Rebecca Blankenburg, MD, MPH, Ian Chua, MD, Stephanie Harman, MD, Debbie S. Sakai, MD, Joan F. Hilton, DSc, MPH., and Jason Satterfield, PhD.
1. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295-1296. doi:10.1001/jama.2014.10186. PubMed
2. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
3. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
4. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651-661. PubMed
5. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. What is a medical decision? A units. Am J Respir Crit Care Med. 2011;183(7):915-921. doi:10.1164/rccm.201008-1214OC. PubMed
22. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
23. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
24. Légaré F, Bekker H, Desroches S, et al. How can continuing professional development better promote shared decision-making? Perspectives from an international collaboration. Implement Sci. 2011;6:68. doi:10.1186/1748-5908-6-68. PubMed
25. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301-312. doi:10.1016/j.pec.2005.06.010. PubMed
26. Moumjid N, Gafni A, Brémond A, Carrère MO. Shared decision making in the medical taxonomy based on physician statements in hospital encounters: a qualitative study. BMJ Open. 2016;6(2):e010098. doi:10.1136/bmjopen-2015-010098. PubMed
6. Fowler FJ, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood). 2011;30(4):699-706. doi:10.1377/hlthaff.2011.0003. PubMed
7. Weiner SJ, Kelly B, Ashley N, et al. Content coding for contextualization of care: evaluating physician performance at patient-centered decision making. Med Decis Making. 2014;34(1):97-106. doi:10.1177/0272989X13493146. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91(2):176-179. doi:10.1016/j.pec.2013.01.006 PubMed
10. Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Making. 2016;36(4):427-452. doi:10.1177/0272989X15613530. PubMed
11. Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295-301. doi:10.1016/j.pec.2014.07.017. PubMed
12. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18. doi:10.1016/j.pec.2011.02.004. PubMed
13. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
14. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219-226. doi:10.1159/000126073. PubMed
15. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
16. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. doi:10.1093/intqhc/mzr002. PubMed
17. Mohammed K, Nolan MB, Rajjo T, et al. Creating a Patient-Centered Health Care Delivery System: A Systematic Review of Health Care Quality From the Patient Perspective. Am J Med Qual. 2014;31(1):12-21. doi:10.1177/1062860614545124. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. doi:10.1136/bmjqs-2012-001769. PubMed
19. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526-535. doi:10.1016/j.pec.2008.07.018. PubMed
20. Frosch DL, May SG, Rendle KAS, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled “difficult” among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. doi:10.1377/hlthaff.2011.0576. PubMed
21. Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care encounter: are we all talking about the same thing? Med Decis Making. 2007;27(5):539-546. doi:10.1177/0272989X07306779. PubMed
27. Hallström I, Elander G. Decision-making during hospitalization: parents’ and children’s involvement. J Clin Nurs. 2004;13(3):367-375. PubMed
28. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. Temporal characteristics of decisions in hospital encounters: a threshold for shared decision making? A qualitative study. Patient Educ Couns. 2014;97(2):216-222. doi:10.1016/j.pec.2014.08.005. PubMed
29. Baumeister RF, Leary MR. Writing narrative literature reviews. Rev Gen Psychol. 1997;1(3):311.
30. Moody DL. Theoretical and practical issues in evaluating the quality of conceptual models: current state and future directions. Data Knowl Eng. 2005;55(3):243-276. doi:10.1016/j.datak.2004.12.005.
31. McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351-377. PubMed
32. Basics of Qualitative Research | SAGE Publications Inc. https://us.sagepub.com/en-us/nam/basics-of-qualitative-research/book235578. Accessed on September 13, 2016. PubMed
33. 2013;2(4):421-433. doi:10.2217/cer.13.46.J Comp Eff Res33. Halley MC, Rendle KA, Frosch DL. A conceptual model of the multiple stages of communication necessary to support patient-centered care. PubMed
34. 2012;87(1):54-61. doi:10.1016/j.pec.2011.07.027.Patient Educ Couns34. Torke AM, Petronio S, Sachs GA, Helft PR, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. PubMed
35. 2009;15(6):1142-1151. doi:10.1111/j.1365-2753.2009.01315.x.J Eval Clin Pract35. Falzer PR, Garman MD. A conditional model of evidence-based decision making: Model of evidence-based decision making. PubMed
36. 2012;8(4):161-164. doi:10.1097/PTS.0b013e318267c56e.J Patient Saf36. Holzmueller CG, Wu AW, Pronovost PJ. A framework for encouraging patient engagement in medical decision making. PubMed
37. 2014;97(2):158-164. doi:10.1016/j.pec.2014.07.027.Patient Educ Couns37. Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: a model for patient care. PubMed
38. 2002;35(5-6):313-321. doi:10.1016/S1532-0464(03)00037-6.J Biomed Inform38. Ruland CM, Bakken S. Developing, implementing, and evaluating decision support systems for shared decision making in patient care: a conceptual model and case illustration. PubMed
39. 1999;319(7212):764.BMJ39. Shepperd S, Charnock D, Gann B. Helping patients access high quality health information. PubMed
40. 2011;25(1):18-25. doi:10.3109/13561820.2010.490502.J Interprof Care40. Légaré F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. PubMed
41. 2015;25(1):141-152. doi:10.1007/s10926-014-9532-7.J Occup Rehabil41. Coutu MF, Légaré F, Durand MJ, et al. Operationalizing a Shared Decision Making Model for Work Rehabilitation Programs: A Consensus Process. PubMed
42. 2013;13:231.BMC Health Serv Res42. Hölzel LP, Kriston L, Härter M. Patient preference for involvement, experienced involvement, decisional conflict, and satisfaction with physician: a structural equation model test. PubMed
43. 2008;134(4):835-843. doi:10.1378/chest.08-0235.Chest43. Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. PubMed
44. 2013;8:29-36. doi:10.4137/IMI.S12783.Integr Med Insights44. Brooks AT, Silverman L, Wallen G. Shared Decision Making: A Fundamental Tenet in a Conceptual Framework of Integrative Healthcare Delivery. PubMed
45. 2013;33(1):37-47. doi:10.1177/0272989X12458159.Med Decis Making45. Müller-Engelmann M, Donner-Banzhoff N, Keller H, et al. When decisions should be shared: a study of social norms in medical decision making using a factorial survey approach. PubMed
46. 2007;101(4):205-211.Z Arztl Fortbild Qualitatssich46. Mccaffery KJ, Shepherd HL, Trevena L, et al. Shared decision-making in Australia. PubMed
47. 2014;20(2):311-318. doi:10.1007/s12028-013-9922-2.Neurocrit Care
47. Rubin MA. The Collaborative Autonomy Model of Medical Decision-Making. 48. 2013;70(1 Suppl):141S-158S. doi:10.1177/1077558712461952.Med Care Res Rev PubMed
48. McCullough LB. The professional medical ethics model of decision making under conditions of clinical uncertainty. PubMed
49. 2009;87(2):368–390.Milbank Q49. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. PubMed
50. 2015;25(3):276-282. doi:10.1016/j.whi.2015.02.002.Womens Health Issues50. Moore JE, Titler MG, Kane Low L, Dalton VK, Sampselle CM. Transforming Patient-Centered Care: Development of the Evidence Informed Decision Making through Engagement Model. PubMed
51. 1997;44(5):681-692.Soc Sci Med51. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). PubMed
52. 2010;80(2):164-172. doi:10.1016/j.pec.2009.10.015.Patient Educ Couns52. Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. PubMed
53. 2013;70(1 Suppl):94S-112S. doi:10.1177/1077558712459216.Med Care Res Rev53. Epstein RM, Gramling RE. What is shared in shared decision making? Complex decisions when the evidence is unclear. PubMed
54. 2010;304(8):903-904. doi:10.1001/jama.2010.1208.JAMA54. Kon AA. The shared decision-making continuum. PubMed
55. 2008;30(3):429-444. doi:10.1111/j.1467-9566.2007.01064.x.Sociol Health Illn55. Rapley T. Distributed decision making: the anatomy of decisions-in-action. PubMed
56. 1997;12(6):339-345.J Gen Intern Med56. Braddock CH 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. PubMed
57. 1999;282(24):2313-2320.JAMA57. Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. PubMed
58. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056.Soc Sci Med58. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups.
2006;9(4):321-332. doi:10.1111/j.1369-7625.2006.00404.x.Health Expect. PubMed
59. Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. PubMed
60. 2011;17(4):554-564. doi: 10.1111/j.1365-2753.2010.01515.x.J Eval Clin Pract60. Légaré F, Stacey D, Gagnon S, et al. Validating a conceptual model for an interprofessional approach to shared decision making: a mixed methods study. PubMed
1. Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295-1296. doi:10.1001/jama.2014.10186. PubMed
2. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172-1179. doi:10.1016/j.pec.2015.06.022. PubMed
3. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361-1367. doi:10.1007/s11606-012-2077-6. PubMed
4. Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651-661. PubMed
5. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. What is a medical decision? A units. Am J Respir Crit Care Med. 2011;183(7):915-921. doi:10.1164/rccm.201008-1214OC. PubMed
22. Müller-Engelmann M, Keller H, Donner-Banzhoff N, Krones T. Shared decision making in medicine: The influence of situational treatment factors. Patient Educ Couns. 2011;82(2):240-246. doi:10.1016/j.pec.2010.04.028. PubMed
23. Whitney SN. A New Model of Medical Decisions: Exploring the Limits of Shared Decision Making. Med Decis Making. 2003;23(4):275-280. doi:10.1177/0272989X03256006. PubMed
24. Légaré F, Bekker H, Desroches S, et al. How can continuing professional development better promote shared decision-making? Perspectives from an international collaboration. Implement Sci. 2011;6:68. doi:10.1186/1748-5908-6-68. PubMed
25. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301-312. doi:10.1016/j.pec.2005.06.010. PubMed
26. Moumjid N, Gafni A, Brémond A, Carrère MO. Shared decision making in the medical taxonomy based on physician statements in hospital encounters: a qualitative study. BMJ Open. 2016;6(2):e010098. doi:10.1136/bmjopen-2015-010098. PubMed
6. Fowler FJ, Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood). 2011;30(4):699-706. doi:10.1377/hlthaff.2011.0003. PubMed
7. Weiner SJ, Kelly B, Ashley N, et al. Content coding for contextualization of care: evaluating physician performance at patient-centered decision making. Med Decis Making. 2014;34(1):97-106. doi:10.1177/0272989X13493146. PubMed
8. Weiner SJ, Schwartz A, Sharma G, et al. Patient-centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573-579. doi:10.7326/0003-4819-158-8-201304160-00001. PubMed
9. Matthias MS, Salyers MP, Frankel RM. Re-thinking shared decision-making: context matters. Patient Educ Couns. 2013;91(2):176-179. doi:10.1016/j.pec.2013.01.006 PubMed
10. Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Making. 2016;36(4):427-452. doi:10.1177/0272989X15613530. PubMed
11. Shay LA, Lafata JE. Understanding patient perceptions of shared decision making. Patient Educ Couns. 2014;96(3):295-301. doi:10.1016/j.pec.2014.07.017. PubMed
12. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18. doi:10.1016/j.pec.2011.02.004. PubMed
13. Butterworth JE, Campbell JL. Older patients and their GPs: shared decision making in enhancing trust. Br J Gen Pract. 2014;64(628):e709-e718. doi:10.3399/bjgp14X682297. PubMed
14. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77(4):219-226. doi:10.1159/000126073. PubMed
15. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi:10.1002/14651858.CD001431.pub4. PubMed
16. Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269-277. doi:10.1093/intqhc/mzr002. PubMed
17. Mohammed K, Nolan MB, Rajjo T, et al. Creating a Patient-Centered Health Care Delivery System: A Systematic Review of Health Care Quality From the Patient Perspective. Am J Med Qual. 2014;31(1):12-21. doi:10.1177/1062860614545124. PubMed
18. Berger Z, Flickinger TE, Pfoh E, Martinez KA, Dy SM. Promoting engagement by patients and families to reduce adverse events in acute care settings: a systematic review. BMJ Qual Saf. 2014;23(7):548-555. doi:10.1136/bmjqs-2012-001769. PubMed
19. Légaré F, Ratté S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73(3):526-535. doi:10.1016/j.pec.2008.07.018. PubMed
20. Frosch DL, May SG, Rendle KAS, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled “difficult” among key obstacles to shared decision making. Health Aff (Millwood). 2012;31(5):1030-1038. doi:10.1377/hlthaff.2011.0576. PubMed
21. Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care encounter: are we all talking about the same thing? Med Decis Making. 2007;27(5):539-546. doi:10.1177/0272989X07306779. PubMed
27. Hallström I, Elander G. Decision-making during hospitalization: parents’ and children’s involvement. J Clin Nurs. 2004;13(3):367-375. PubMed
28. Ofstad EH, Frich JC, Schei E, Frankel RM, Gulbrandsen P. Temporal characteristics of decisions in hospital encounters: a threshold for shared decision making? A qualitative study. Patient Educ Couns. 2014;97(2):216-222. doi:10.1016/j.pec.2014.08.005. PubMed
29. Baumeister RF, Leary MR. Writing narrative literature reviews. Rev Gen Psychol. 1997;1(3):311.
30. Moody DL. Theoretical and practical issues in evaluating the quality of conceptual models: current state and future directions. Data Knowl Eng. 2005;55(3):243-276. doi:10.1016/j.datak.2004.12.005.
31. McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15(4):351-377. PubMed
32. Basics of Qualitative Research | SAGE Publications Inc. https://us.sagepub.com/en-us/nam/basics-of-qualitative-research/book235578. Accessed on September 13, 2016. PubMed
33. 2013;2(4):421-433. doi:10.2217/cer.13.46.J Comp Eff Res33. Halley MC, Rendle KA, Frosch DL. A conceptual model of the multiple stages of communication necessary to support patient-centered care. PubMed
34. 2012;87(1):54-61. doi:10.1016/j.pec.2011.07.027.Patient Educ Couns34. Torke AM, Petronio S, Sachs GA, Helft PR, Purnell C. A conceptual model of the role of communication in surrogate decision making for hospitalized adults. PubMed
35. 2009;15(6):1142-1151. doi:10.1111/j.1365-2753.2009.01315.x.J Eval Clin Pract35. Falzer PR, Garman MD. A conditional model of evidence-based decision making: Model of evidence-based decision making. PubMed
36. 2012;8(4):161-164. doi:10.1097/PTS.0b013e318267c56e.J Patient Saf36. Holzmueller CG, Wu AW, Pronovost PJ. A framework for encouraging patient engagement in medical decision making. PubMed
37. 2014;97(2):158-164. doi:10.1016/j.pec.2014.07.027.Patient Educ Couns37. Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: a model for patient care. PubMed
38. 2002;35(5-6):313-321. doi:10.1016/S1532-0464(03)00037-6.J Biomed Inform38. Ruland CM, Bakken S. Developing, implementing, and evaluating decision support systems for shared decision making in patient care: a conceptual model and case illustration. PubMed
39. 1999;319(7212):764.BMJ39. Shepperd S, Charnock D, Gann B. Helping patients access high quality health information. PubMed
40. 2011;25(1):18-25. doi:10.3109/13561820.2010.490502.J Interprof Care40. Légaré F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. PubMed
41. 2015;25(1):141-152. doi:10.1007/s10926-014-9532-7.J Occup Rehabil41. Coutu MF, Légaré F, Durand MJ, et al. Operationalizing a Shared Decision Making Model for Work Rehabilitation Programs: A Consensus Process. PubMed
42. 2013;13:231.BMC Health Serv Res42. Hölzel LP, Kriston L, Härter M. Patient preference for involvement, experienced involvement, decisional conflict, and satisfaction with physician: a structural equation model test. PubMed
43. 2008;134(4):835-843. doi:10.1378/chest.08-0235.Chest43. Curtis JR, White DB. Practical guidance for evidence-based ICU family conferences. PubMed
44. 2013;8:29-36. doi:10.4137/IMI.S12783.Integr Med Insights44. Brooks AT, Silverman L, Wallen G. Shared Decision Making: A Fundamental Tenet in a Conceptual Framework of Integrative Healthcare Delivery. PubMed
45. 2013;33(1):37-47. doi:10.1177/0272989X12458159.Med Decis Making45. Müller-Engelmann M, Donner-Banzhoff N, Keller H, et al. When decisions should be shared: a study of social norms in medical decision making using a factorial survey approach. PubMed
46. 2007;101(4):205-211.Z Arztl Fortbild Qualitatssich46. Mccaffery KJ, Shepherd HL, Trevena L, et al. Shared decision-making in Australia. PubMed
47. 2014;20(2):311-318. doi:10.1007/s12028-013-9922-2.Neurocrit Care
47. Rubin MA. The Collaborative Autonomy Model of Medical Decision-Making. 48. 2013;70(1 Suppl):141S-158S. doi:10.1177/1077558712461952.Med Care Res Rev PubMed
48. McCullough LB. The professional medical ethics model of decision making under conditions of clinical uncertainty. PubMed
49. 2009;87(2):368–390.Milbank Q49. Satterfield JM, Spring B, Brownson RC, et al. Toward a Transdisciplinary Model of Evidence-Based Practice. PubMed
50. 2015;25(3):276-282. doi:10.1016/j.whi.2015.02.002.Womens Health Issues50. Moore JE, Titler MG, Kane Low L, Dalton VK, Sampselle CM. Transforming Patient-Centered Care: Development of the Evidence Informed Decision Making through Engagement Model. PubMed
51. 1997;44(5):681-692.Soc Sci Med51. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). PubMed
52. 2010;80(2):164-172. doi:10.1016/j.pec.2009.10.015.Patient Educ Couns52. Stacey D, Légaré F, Pouliot S, Kryworuchko J, Dunn S. Shared decision making models to inform an interprofessional perspective on decision making: a theory analysis. PubMed
53. 2013;70(1 Suppl):94S-112S. doi:10.1177/1077558712459216.Med Care Res Rev53. Epstein RM, Gramling RE. What is shared in shared decision making? Complex decisions when the evidence is unclear. PubMed
54. 2010;304(8):903-904. doi:10.1001/jama.2010.1208.JAMA54. Kon AA. The shared decision-making continuum. PubMed
55. 2008;30(3):429-444. doi:10.1111/j.1467-9566.2007.01064.x.Sociol Health Illn55. Rapley T. Distributed decision making: the anatomy of decisions-in-action. PubMed
56. 1997;12(6):339-345.J Gen Intern Med56. Braddock CH 3rd, Fihn SD, Levinson W, Jonsen AR, Pearlman RA. How doctors and patients discuss routine clinical decisions. Informed decision making in the outpatient setting. PubMed
57. 1999;282(24):2313-2320.JAMA57. Braddock CH 3rd, Edwards KA, Hasenberg NM, Laidley TL, Levinson W. Informed decision making in outpatient practice: time to get back to basics. PubMed
58. 2009;69(12):1805-1812. doi:10.1016/j.socscimed.2009.09.056.Soc Sci Med58. Smith SK, Dixon A, Trevena L, Nutbeam D, McCaffery KJ. Exploring patient involvement in healthcare decision making across different education and functional health literacy groups.
2006;9(4):321-332. doi:10.1111/j.1369-7625.2006.00404.x.Health Expect. PubMed
59. Towle A, Godolphin W, Grams G, Lamarre A. Putting informed and shared decision making into practice. PubMed
60. 2011;17(4):554-564. doi: 10.1111/j.1365-2753.2010.01515.x.J Eval Clin Pract60. Légaré F, Stacey D, Gagnon S, et al. Validating a conceptual model for an interprofessional approach to shared decision making: a mixed methods study. PubMed
© 2017 Society of Hospital Medicine
Inpatient Management of Diabetic Foot Infections: A Review of the Guidelines for Hospitalists
Diabetic foot infection (DFI) is a common result of diabetes and represents the most frequent complication requiring hospitalization and lower extremity amputation.1,2 Hospital discharges related to diabetic lower extremity ulcers increased from 72,000 in 1988 to 113,000 in 2007,3 and admissions related to infection rose 30% between 2005 and 2010.2 Ulceration and amputation are associated with a 40% to 50% 5-year mortality rate.4,5
Aggressive risk-factor management and interprofessional care can significantly reduce major amputations and mortality.6-13 Consistent and high-quality care for patients admitted with DFI is essential for optimizing outcomes; however, management varies widely, and critical assessment and prevention measures are often not employed by providers.14 This review synthesizes recommendations from existing guidelines to provide an overview of the best practices for the diagnosis, management, and discharge of DFI in the hospital setting (Supplementary Table 1, Supplementary Figure).
DETECTION AND STAGING OF INFECTION
The first step in the management of a DFI is a careful assessment of the presence and depth of infection.15 The Infectious Diseases Society of America (IDSA) guidelines recommend using at least 2 signs of classic inflammation (erythema, warmth, swelling, tenderness, or pain) or purulent drainage to diagnose soft tissue infection.1,15,16 Patients with ischemia may present atypically, with nonpurulent secretions, friable or discolored granulation tissue, undermining of wound edges, and foul odor. 1,15,16 Additional risk factors for DFI include ulceration for more than 30 days, recurrent foot ulcers, a traumatic foot wound, severe peripheral arterial disease (PAD) in the affected limb (ankle brachial index [ABI] <0.4), prior lower extremity amputation, loss of protective sensation, end-stage renal disease, and a history of walking barefoot.15,17,18
CRITERIA FOR HOSPITALIZATION
In practice, the decision to admit is based on clinical and systems-based drivers (Supplementary Table 2). The IDSA and IWGDF guidelines recommend hospitalization for patients with severe (PEDIS grade 4) infection, moderate (PEDIS grade 3) infection with certain complications (eg, severe PAD or lack of home support), an inability to comply with required outpatient treatment, lack of improvement with outpatient therapy, or presence of metabolic or hemodynamic instability.1,15 Clinicians must also consider the need for surgical debridement or complex antibiotic choices due to allergies and comorbidities. Hospitalists may also consider admission in cases in which outpatient follow-up cannot be easily arranged (eg, uninsured patients).
Outpatient management may be appropriate for patients with mild infections who are willing to be reassessed within 72 hours, or sooner if the infection worsens.23 For patients with moderate infections (eg, osteomyelitis without systemic signs of infection), access to an outpatient interprofessional DFI care team can potentially decrease the need for admission.
DIAGNOSIS OF OSTEOMYELITIS
Clinical features that raise suspicion for osteomyelitis include ulceration for at least 6 weeks with appropriate wound care and offloading, wound extension to the bone or joint, exposed bone, ulcers larger than 2 cm2, previous history of a wound, multiple wounds, and appearance of a sausage digit.15
The gold standard for diagnosis of osteomyelitis is a bone biopsy with histology. In the absence of histology, physicians rely on physical examination, inflammatory markers, and imaging to make the diagnosis. The presence of visible, chronically exposed bone within a forefoot ulcer is diagnostic. The accuracy of a probe to bone test depends on the pretest probability of osteomyelitis. Sensitivity and specificity range from 60% to 87% and from 85% to 91%, respectively.24 For patients with a single forefoot ulcer and PEDIS grade 2 or 3 infection, considering both ulcer depth and serum inflammatory markers (ulcer depth greater than 3 mm, or C-reactive protein greater than 3.2 mg/dL; ulcer depth greater than 3 mm, or erythrocyte sedimentation rate greater than 60 mm/h) increases sensitivity to 100%, although the specificity is relatively low (55% and 60%, respectively).25 When the diagnosis remains uncertain by physical examination, imaging is necessary for further evaluation.
ROLE OF IMAGING
All patients with DFI should have plain radiographs to look for foot deformities, soft tissue gas, foreign bodies, and osteomyelitis. If plain radiographs show classic evidence of osteomyelitis, (ie, cortical erosion, periosteal reaction, mixed lucency, and sclerosis in the absence of neuro-osteoarthropathy), advanced imaging is not necessary. However, these changes may not appear on plain films for up to 1 month after infection onset.15,26
The purpose of advanced imaging in the inpatient management of DFI is to detect conditions not obvious by physical examination or by plain radiographs that would alter surgical management (ie, deep abscess or necrotic bone) or antibiotic duration (ie, osteomyelitis or tenosynovitis).15 Magnetic resonance imaging (MRI) is the diagnostic modality of choice when the wound does not probe to bone and the diagnosis remains uncertain27 due to its accuracy and availability.1,15 However, MRI cannot always distinguish between infection and neuro-osteoarthropathy, especially in patients who have infection superimposed on a Charcot foot, have had recent surgical intervention, or have osteosynthesis material at the infection site.24 If MRI is contraindicated, guidelines vary on the next recommended test. The IDSA and the Society for Vascular Surgery recommend a labeled white blood cell scan combined with a bone scan, whereas the IWGDF recommends a labeled leukocyte scan, a single photon emission computed tomography (SPECT/CT), or a fluorodeoxyglucose positron emission tomography (FDG PET) scan.1,15,19 A recent comparison of a labeled white blood cell SPECT/CT versus MRI (using histology as the gold standard) reported that SPECT/CT had a similar sensitivity (89% versus 87%, respectively) and specificity (35% versus 37%, respectively) to MRI.28 In practice, physicians should consider which studies are readily available and confidently interpreted by radiologists at their institution.
ASSESSMENT OF ULCER ETIOLOGY
After infection is diagnosed and staged, clinicians should determine the underlying derangement in order to prevent recurrence after discharge. Common derangements leading to ulceration in diabetics include PAD, neuropathy, muscular tension, altered foot mechanics, trauma, or a combination of the above.1,15,29-31 All patients with DFI should undergo pedal perfusion assessment by an ABI, ankle and pedal Doppler arterial waveforms, and either toe brachial index (TBI) or transcutaneous oxygen pressure.1,15,19 In cases of suspected calcification, TBI is a more reliable measure of ischemia compared with the ABI.16,19 For patients with signs and symptoms of ischemia and an abnormal ABI or TBI measurement (ABI <0.9 and TBI <0.7), a nonurgent consultation with a vascular surgeon is recommended, while patients with severe ischemia (ABI <0.4) usually require urgent revascularization.15,32
A sensory examination with a Semmes-Weinstein monofilament should be conducted to identify patients with loss of protective sensation who may benefit from offloading devices and custom orthotics.15 Foot anatomy and mechanics as well as potential Achilles tendon contractures should be evaluated by a foot specialist such as a podiatrist, orthotist, orthopedist, or vascular surgeon, especially if debridement or amputation is being contemplated.
OBTAINING CULTURES
After diagnosing the infection clinically, appropriately obtained cultures are essential to guide therapy in all except mild cases with no prior antibiotic exposure or MRSA risk.1,15 Guidelines strongly recommend that specimens be obtained by biopsy or curettage from deep tissue at the base of the ulcer after the wound has been cleansed and debrided and prior to initiating antibiotics.1,15,33 Aspiration of purulent secretions using a sterile needle and syringe is another acceptable culturing method.15 While convenient, swab cultures are prone to both false-positive and false-negative results.34 Repeat cultures are only needed for patients who are not responding to treatment or for surveillance of resistant organisms.1
In cases of osteomyelitis, bone specimens should be sent for culture and histology either during surgical debridement or a bone biopsy. At the time of debridement, cultures and pathology should be sent from the proximal (clean) bone margin in order to document whether there is residual osteomyelitis postdebridement.35 For patients not planned for debridement, a bone biopsy is recommended if the diagnosis of osteomyelitis is unclear, response to empiric therapy is poor, broad-spectrum antibiotics are being considered, or the infection is in the midfoot or hindfoot.1,15,19 Results from soft tissue or sinus tract specimens should not be used to guide antibiotic selection in osteomyelitis, as several studies suggest that they do not correlate with bone culture results; one retrospective review found a mere 22.5% correlation between wound swabs and bone biopsy.1,36 A 2-week antibiotic-free period prior to biopsy is recommended in order to minimize the risk of false-negative results but must be balanced with the risk of worsening infection.1,15 If possible, the biopsy should be performed through uninfected tissue under fluoroscopy or CT guidance, with 2 to 3 cores obtained for culture and histology.1,15
INTERPROFESSIONAL INPATIENT CARE
A growing number of health systems have created inpatient and/or outpatient interprofessional diabetic foot care teams, and several studies demonstrated an association between these teams and a reduction in major amputations.7-11,13 The goal of the inpatient team is to rapidly triage patients with moderate to severe infections, expedite surgical interventions and culture collection, establish an effective treatment plan, and ensure adherence postdischarge to optimize outcomes. The common core of most teams includes podiatry, endocrinology, wound care, and vascular surgery, but team composition may vary based on the availability of local specialists with interest and expertise in DFI.9,10,33
The division of consultation between podiatry and orthopedic surgery is highly dependent upon individual practice patterns and hospital structure. In general, forefoot ulcers may be managed by podiatry or orthopedic surgery, while severe Charcot deformities are most often treated by orthopedic surgeons. Wound care nurses are often integral to successful wound healing, collaborating across specialties and serving as a weekly or biweekly point of contact for patients.
Early involvement of Infectious Disease (ID) specialists can be useful for guiding antibiotic choices and facilitating follow-up. ID should be involved with patients who require long-term antibiotic therapy (ie, cases of deep-tissue infection that are not completely amputated or debrided), have failed outpatient or empiric therapy, have antibiotic allergies or drug-resistant pathogens, or are being considered for outpatient parenteral antibiotic therapy.
ANTIBIOTIC THERAPY
The duration of antibiotic treatment for DFI is based on the severity of infection and response to treatment (Supplementary Table 3). Treatment should continue until the signs and symptoms of infection resolve, but there is no strong evidence to support treatment through complete healing. Healing will usually occur in 1 to 2 weeks for mild infections and in 2 to 3 weeks for moderate or severe infections.
Traditional management of diabetic foot osteomyelitis has relied almost exclusively on resection of all infected bone. However, data have emerged over the last 10 years to support initial medical management of select patients. Further research regarding the optimal treatment regimen and duration is ongoing, with 1 recent, randomized control trial comparing 6 versus 12 weeks of antibiotics for patients treated medically for osteomyelitis finding no difference in remission rates.1,41 Patients managed surgically for osteomyelitis are often treated parenterally for at least 4 weeks, but this practice is not based on strong evidence, and guidelines suggest most patients could be switched to highly bioavailable oral agents after a shorter course of intravenous therapy.1,15 Guidelines recommend 2 to 5 days of antibiotics after complete resection of infected bone and soft tissue (Supplementary Table 3). If the infected soft tissue remains, 1 to 3 weeks of therapy is usually sufficient, while 4 to 6 weeks is often needed if there is residually infected but viable bone.15
SURGICAL MANAGEMENT
In patients with osteomyelitis, the decision between medical and surgical management is complex. Absolute indications for surgical resection include systemic toxicity with associated tissue infection, an open or infected joint space, and patients with prosthetic heart valves.27 However, the need for surgery is unclear beyond these absolute indications, and approximately two-thirds of osteomyelitis cases may be arrested or cured with antibiotic therapy alone.1 A prospective randomized comparative trial of patients with diabetic foot osteomyelitis found that patients treated with 90 days of antibiotics had similar healing rates, times to healing, and short-term complications as compared with those who underwent conservative bone resection.44 While further research is needed to determine which types of patients with osteomyelitis may be successfully treated without surgery, the IWGDF, the IDSA, and osteomyelitis experts have offered guidance on this decision (Table 2).1,15,27 If resection is necessary, hospitalists should request at least 4 specimens to help guide postoperative antibiotic therapy (1 sample for histology and 1 for microbiology, at both the grossly abnormal bone and the bone margin), as negative margin cultures predict a lower relapse risk for infection.1,35
CRITERIA FOR DISCHARGE
Guidelines suggest that patients be clinically stable before discharge, complete any urgent surgery, achieve acceptable glycemic control, and be presented with a comprehensive outpatient plan, including antibiotic therapy, offloading, wound care instructions, and outpatient follow-up (Supplementary Table 4). Physicians must consider patient and family preferences, expected adherence to therapy, availability of home support, and payer and cost issues when creating the discharge plan.15
INTERPROFESSIONAL OUTPATIENT CARE
An effective outpatient care team is critical to ensure wound healing and infection resolution. Efforts should be made to discharge patients to a comprehensive outpatient interprofessional foot care team, with a plan that includes professional foot care, patient education, and adequate footwear.48 Team composition varies but often includes representatives from vascular surgery, podiatry, orthotics, wound care, endocrinology, orthopedics, physical therapy and rehabilitation, infectious disease, and dermatology.11-13
CONCLUSION
DFIs are a common cause of morbidity in patients with diabetes and result in significant costs to the US healthcare system. Hospitalized patients with a DFI require appropriate classification of wound severity and assessment of vascular status, protective sensation, and potential osteomyelitis. Inpatient management of these patients includes obtaining necessary cultures, choosing an antibiotic regimen based on infection severity and the likely causative agent, and evaluating the need for surgical intervention. Prior to discharge, providers should determine a comprehensive follow-up plan and ensure patient engagement. Finally, interprofessional management has been shown to improve outcomes in DFI and should be adopted in both the inpatient and outpatient settings.
Disclosure
The authors report no conflicts of interest.
1. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32 Suppl 1:45-74. PubMed
2. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149-158. PubMed
3. Number (in thousands) of hospital discharges with peripheral arterial disease (PAD), ulcer/inflammation/infection (ULCER), or neuropathy as first-listed diagnosis and diabetes as any-listed diagnosis United States, 1988-2007. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/statistics/hosplea/diabetes_complications/fig1_number.htm. Updated 2014. Accessed September 23, 2016.
4. Wilbek TE, Jansen RB, Jørgensen B, Svendsen OL. The diabetic foot in a multidisciplinary team setting. Number of amputations below ankle level and mortality. Exp Clin Endocrinol Diabetes. 2016;124(9):535-540. PubMed
5. Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int Wound J. 2016;13(5):892-903. PubMed
6. Young MJ, McCardle JE, Randall LE, Barclay JI. Improved survival of diabetic foot ulcer patients 1995-2008: Possible impact of aggressive cardiovascular risk management. Diabetes Care. 2008;31(11):2143-2147. PubMed
7. Troisi N, Baggiore C, Landini G, Michelagnoli S. How daily practice changed in an urban area after establishing a multidisciplinary diabetic foot program. J Diabetes. 2016;8(4):594-595. PubMed
8. Wang C, Mai L, Yang C, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team in patient with diabetes foot ulcer. BMC Endocr Disord. 2016;16(1):38. PubMed
9. Rubio JA, Aragón-Sánchez J, Jiménez S, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team for the diabetic foot. Int J Low Extrem Wounds. 2014;13(1):22-26. PubMed
10. Yesil S, Akinci B, Bayraktar F, et al. Reduction of major amputations after starting a multidisciplinary diabetic foot care team: Single centre experience from Turkey. Exp Clin Endocrinol Diabetes. 2009;117(7):345-349. PubMed
11. Dargis V, Pantelejeva O, Jonushaite A, Vileikyte L, Boulton AJ. Benefits of a multidisciplinary approach in the management of recurrent diabetic foot ulceration in Lithuania: A prospective study. Diabetes Care. 1999;22(9):1428-1431. PubMed
12. Driver VR, Goodman RA, Fabbi M, French MA, Andersen CA. The impact of a podiatric lead limb preservation team on disease outcomes and risk prediction in the diabetic lower extremity: a retrospective cohort study. J Am Podiatr Med Assoc. 2010;100(4):235-241. PubMed
13. Hamonet J, Verdié-Kessler C, Daviet JC, et al. Evaluation of a multidisciplinary consultation of diabetic foot. Ann Phys Rehabil Med. 2010;53(5):306-318. PubMed
14. Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: Results of the Eurodiale study, a prospective cohort study. Diabet Med. 2008;25(6):700-707. PubMed
15. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173. PubMed
16. Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr. 2016;11(2):149-156. PubMed
17. Hill MN, Feldman HI, Hilton SC, Holechek MJ, Ylitalo M, Benedict GW. Risk of foot complications in long-term diabetic patients with and without ESRD: A preliminary study. ANNA J. 1996;23(4):381-386; discussion 387-388. PubMed
18. Mohler ER, III. Peripheral arterial disease: Identification and implications. Arch Intern Med. 2003;163(19):2306-2314. PubMed
19. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2 Suppl):3S-21S. PubMed
20. Noor S, Zubair M, Ahmad J. Diabetic foot ulcer--A review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192-199. PubMed
21. Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int. 2013;34(3):351-358. PubMed
22. Wukich DK, Hobizal KB, Raspovic KM, Rosario BL. SIRS is valid in discriminating between severe and moderate diabetic foot infections. Diabetes Care. 2013;36(11):3706-3711. PubMed
23. Grigoropoulou P, Eleftheriadou I, Jude EB, Tentolouris N. Diabetic foot infections: An update in diagnosis and management. Curr Diab Rep. 2017;17(1):3. PubMed
24. Glaudemans AW, Uçkay I, Lipsky BA. Challenges in diagnosing infection in the diabetic foot. Diabet Med. 2015;32(6):748-759. PubMed
25. Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg. 2009;48(1):39-46. PubMed
26. Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39 Suppl 2:S115-S122. PubMed
27. Allahabadi S, Haroun KB, Musher DM, Lipsky BA, Barshes NR. Consensus on surgical aspects of managing osteomyelitis in the diabetic foot. Diabet Foot Ankle. 2016;7:30079. PubMed
28. La Fontaine J, Bhavan K, Lam K, et al. Comparison between Tc-99m WBC SPECT/CT and MRI for the diagnosis of biopsy-proven diabetic foot osteomyelitis. Wounds. 2016;28(8):271-278. PubMed
29. Bembi V, Singh S, Singh P, Aneja GK, Arya TV, Arora R. Prevalence of peripheral arterial disease in a cohort of diabetic patients. South Med J. 2006;99(6):564-569. PubMed
30. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921-929. PubMed
31. Hinchliffe RJ, Andros G, Apelqvist J, et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab Res Rev. 2012;28 Suppl 1:179-217. PubMed
32. Brownrigg JR, Apelqvist J, Bakker K, Schaper NC, Hinchliffe RJ. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg. 2013;45(6):673-681. PubMed
33. 2015;13(2):115-122.Ann Fam Med49. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. PubMed
34. 2016;32 Suppl 1:16-24.Diabetes Metab Res Rev48. Bus SA, van Netten JJ, Lavery LA, et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. PubMed
35. 2003;85-A(8):1436-1445.J Bone Joint Surg Am47. Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. PubMed
36. 2015;21(2):77-85.Foot Ankle Surg46. Cychosz CC, Phisitkul P, Belatti DA, Glazebrook MA, DiGiovanni CW. Gastrocnemius recession for foot and ankle conditions in adults: Evidence-based recommendations. PubMed
37. 2016;32 Suppl 1:25-36.Diabetes Metab Res Rev45. Bus SA, Armstrong DG, van Deursen RW, et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. PubMed
38. 2014;37(3):789-795.Diabetes Care44. Lázaro-Martínez JL, Aragón-Sánchez J, García-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: A randomized comparative trial. PubMed
39. 1996;183(1):61-64.J Am Coll Surg43. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. PubMed
40. 2002;10(6):354-359.Wound Repair Regen42. Saap LJ, Falanga V. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. PubMed
41. 2015;38(2):302-307.Diabetes Care41. Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: A multicenter open-label controlled randomized study. PubMed
42. 2014;35(10):1229-1235.Infect Control Hosp Epidemiol40. Schultz L, Lowe TJ, Srinivasan A, Neilson D, Pugliese G. Economic impact of redundant antimicrobial therapy in US hospitals. PubMed
43. 2015;31(4):395-401.Diabetes Metab Res Rev39. Lipsky BA, Cannon CM, Ramani A, et al. Ceftaroline fosamil for treatment of diabetic foot infections: the CAPTURE study experience. PubMed
2011;55(9):4154-4160.Antimicrob Agents Chemother.38. Richter SS, Heilmann KP, Dohrn CL, et al. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. PubMed
44. 2011;52(3):e18-e55.Clin Infect Dis37. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. PubMed
45. 2006;42(1):57-62.Clin Infect Dis36. Senneville E, Melliez H, Beltrand E, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: Concordance with ulcer swab cultures. PubMed
46. 2012;51(6):749-752.J Foot Ankle Surg35. Atway S, Nerone VS, Springer KD, Woodruff DM. Rate of residual osteomyelitis after partial foot amputation in diabetic patients: A standardized method for evaluating bone margins with intraoperative culture. PubMed
47. 2010;5(7):415-420.J Hosp Med34. Chakraborti C, Le C, Yanofsky A. Sensitivity of superficial cultures in lower extremity wounds. PubMed
48. 2013;36(9):2862-2871.Diabetes Care33. Wukich DK, Armstrong DG, Attinger CE, et al. Inpatient management of diabetic foot disorders: A clinical guide. PubMed
Diabetic foot infection (DFI) is a common result of diabetes and represents the most frequent complication requiring hospitalization and lower extremity amputation.1,2 Hospital discharges related to diabetic lower extremity ulcers increased from 72,000 in 1988 to 113,000 in 2007,3 and admissions related to infection rose 30% between 2005 and 2010.2 Ulceration and amputation are associated with a 40% to 50% 5-year mortality rate.4,5
Aggressive risk-factor management and interprofessional care can significantly reduce major amputations and mortality.6-13 Consistent and high-quality care for patients admitted with DFI is essential for optimizing outcomes; however, management varies widely, and critical assessment and prevention measures are often not employed by providers.14 This review synthesizes recommendations from existing guidelines to provide an overview of the best practices for the diagnosis, management, and discharge of DFI in the hospital setting (Supplementary Table 1, Supplementary Figure).
DETECTION AND STAGING OF INFECTION
The first step in the management of a DFI is a careful assessment of the presence and depth of infection.15 The Infectious Diseases Society of America (IDSA) guidelines recommend using at least 2 signs of classic inflammation (erythema, warmth, swelling, tenderness, or pain) or purulent drainage to diagnose soft tissue infection.1,15,16 Patients with ischemia may present atypically, with nonpurulent secretions, friable or discolored granulation tissue, undermining of wound edges, and foul odor. 1,15,16 Additional risk factors for DFI include ulceration for more than 30 days, recurrent foot ulcers, a traumatic foot wound, severe peripheral arterial disease (PAD) in the affected limb (ankle brachial index [ABI] <0.4), prior lower extremity amputation, loss of protective sensation, end-stage renal disease, and a history of walking barefoot.15,17,18
CRITERIA FOR HOSPITALIZATION
In practice, the decision to admit is based on clinical and systems-based drivers (Supplementary Table 2). The IDSA and IWGDF guidelines recommend hospitalization for patients with severe (PEDIS grade 4) infection, moderate (PEDIS grade 3) infection with certain complications (eg, severe PAD or lack of home support), an inability to comply with required outpatient treatment, lack of improvement with outpatient therapy, or presence of metabolic or hemodynamic instability.1,15 Clinicians must also consider the need for surgical debridement or complex antibiotic choices due to allergies and comorbidities. Hospitalists may also consider admission in cases in which outpatient follow-up cannot be easily arranged (eg, uninsured patients).
Outpatient management may be appropriate for patients with mild infections who are willing to be reassessed within 72 hours, or sooner if the infection worsens.23 For patients with moderate infections (eg, osteomyelitis without systemic signs of infection), access to an outpatient interprofessional DFI care team can potentially decrease the need for admission.
DIAGNOSIS OF OSTEOMYELITIS
Clinical features that raise suspicion for osteomyelitis include ulceration for at least 6 weeks with appropriate wound care and offloading, wound extension to the bone or joint, exposed bone, ulcers larger than 2 cm2, previous history of a wound, multiple wounds, and appearance of a sausage digit.15
The gold standard for diagnosis of osteomyelitis is a bone biopsy with histology. In the absence of histology, physicians rely on physical examination, inflammatory markers, and imaging to make the diagnosis. The presence of visible, chronically exposed bone within a forefoot ulcer is diagnostic. The accuracy of a probe to bone test depends on the pretest probability of osteomyelitis. Sensitivity and specificity range from 60% to 87% and from 85% to 91%, respectively.24 For patients with a single forefoot ulcer and PEDIS grade 2 or 3 infection, considering both ulcer depth and serum inflammatory markers (ulcer depth greater than 3 mm, or C-reactive protein greater than 3.2 mg/dL; ulcer depth greater than 3 mm, or erythrocyte sedimentation rate greater than 60 mm/h) increases sensitivity to 100%, although the specificity is relatively low (55% and 60%, respectively).25 When the diagnosis remains uncertain by physical examination, imaging is necessary for further evaluation.
ROLE OF IMAGING
All patients with DFI should have plain radiographs to look for foot deformities, soft tissue gas, foreign bodies, and osteomyelitis. If plain radiographs show classic evidence of osteomyelitis, (ie, cortical erosion, periosteal reaction, mixed lucency, and sclerosis in the absence of neuro-osteoarthropathy), advanced imaging is not necessary. However, these changes may not appear on plain films for up to 1 month after infection onset.15,26
The purpose of advanced imaging in the inpatient management of DFI is to detect conditions not obvious by physical examination or by plain radiographs that would alter surgical management (ie, deep abscess or necrotic bone) or antibiotic duration (ie, osteomyelitis or tenosynovitis).15 Magnetic resonance imaging (MRI) is the diagnostic modality of choice when the wound does not probe to bone and the diagnosis remains uncertain27 due to its accuracy and availability.1,15 However, MRI cannot always distinguish between infection and neuro-osteoarthropathy, especially in patients who have infection superimposed on a Charcot foot, have had recent surgical intervention, or have osteosynthesis material at the infection site.24 If MRI is contraindicated, guidelines vary on the next recommended test. The IDSA and the Society for Vascular Surgery recommend a labeled white blood cell scan combined with a bone scan, whereas the IWGDF recommends a labeled leukocyte scan, a single photon emission computed tomography (SPECT/CT), or a fluorodeoxyglucose positron emission tomography (FDG PET) scan.1,15,19 A recent comparison of a labeled white blood cell SPECT/CT versus MRI (using histology as the gold standard) reported that SPECT/CT had a similar sensitivity (89% versus 87%, respectively) and specificity (35% versus 37%, respectively) to MRI.28 In practice, physicians should consider which studies are readily available and confidently interpreted by radiologists at their institution.
ASSESSMENT OF ULCER ETIOLOGY
After infection is diagnosed and staged, clinicians should determine the underlying derangement in order to prevent recurrence after discharge. Common derangements leading to ulceration in diabetics include PAD, neuropathy, muscular tension, altered foot mechanics, trauma, or a combination of the above.1,15,29-31 All patients with DFI should undergo pedal perfusion assessment by an ABI, ankle and pedal Doppler arterial waveforms, and either toe brachial index (TBI) or transcutaneous oxygen pressure.1,15,19 In cases of suspected calcification, TBI is a more reliable measure of ischemia compared with the ABI.16,19 For patients with signs and symptoms of ischemia and an abnormal ABI or TBI measurement (ABI <0.9 and TBI <0.7), a nonurgent consultation with a vascular surgeon is recommended, while patients with severe ischemia (ABI <0.4) usually require urgent revascularization.15,32
A sensory examination with a Semmes-Weinstein monofilament should be conducted to identify patients with loss of protective sensation who may benefit from offloading devices and custom orthotics.15 Foot anatomy and mechanics as well as potential Achilles tendon contractures should be evaluated by a foot specialist such as a podiatrist, orthotist, orthopedist, or vascular surgeon, especially if debridement or amputation is being contemplated.
OBTAINING CULTURES
After diagnosing the infection clinically, appropriately obtained cultures are essential to guide therapy in all except mild cases with no prior antibiotic exposure or MRSA risk.1,15 Guidelines strongly recommend that specimens be obtained by biopsy or curettage from deep tissue at the base of the ulcer after the wound has been cleansed and debrided and prior to initiating antibiotics.1,15,33 Aspiration of purulent secretions using a sterile needle and syringe is another acceptable culturing method.15 While convenient, swab cultures are prone to both false-positive and false-negative results.34 Repeat cultures are only needed for patients who are not responding to treatment or for surveillance of resistant organisms.1
In cases of osteomyelitis, bone specimens should be sent for culture and histology either during surgical debridement or a bone biopsy. At the time of debridement, cultures and pathology should be sent from the proximal (clean) bone margin in order to document whether there is residual osteomyelitis postdebridement.35 For patients not planned for debridement, a bone biopsy is recommended if the diagnosis of osteomyelitis is unclear, response to empiric therapy is poor, broad-spectrum antibiotics are being considered, or the infection is in the midfoot or hindfoot.1,15,19 Results from soft tissue or sinus tract specimens should not be used to guide antibiotic selection in osteomyelitis, as several studies suggest that they do not correlate with bone culture results; one retrospective review found a mere 22.5% correlation between wound swabs and bone biopsy.1,36 A 2-week antibiotic-free period prior to biopsy is recommended in order to minimize the risk of false-negative results but must be balanced with the risk of worsening infection.1,15 If possible, the biopsy should be performed through uninfected tissue under fluoroscopy or CT guidance, with 2 to 3 cores obtained for culture and histology.1,15
INTERPROFESSIONAL INPATIENT CARE
A growing number of health systems have created inpatient and/or outpatient interprofessional diabetic foot care teams, and several studies demonstrated an association between these teams and a reduction in major amputations.7-11,13 The goal of the inpatient team is to rapidly triage patients with moderate to severe infections, expedite surgical interventions and culture collection, establish an effective treatment plan, and ensure adherence postdischarge to optimize outcomes. The common core of most teams includes podiatry, endocrinology, wound care, and vascular surgery, but team composition may vary based on the availability of local specialists with interest and expertise in DFI.9,10,33
The division of consultation between podiatry and orthopedic surgery is highly dependent upon individual practice patterns and hospital structure. In general, forefoot ulcers may be managed by podiatry or orthopedic surgery, while severe Charcot deformities are most often treated by orthopedic surgeons. Wound care nurses are often integral to successful wound healing, collaborating across specialties and serving as a weekly or biweekly point of contact for patients.
Early involvement of Infectious Disease (ID) specialists can be useful for guiding antibiotic choices and facilitating follow-up. ID should be involved with patients who require long-term antibiotic therapy (ie, cases of deep-tissue infection that are not completely amputated or debrided), have failed outpatient or empiric therapy, have antibiotic allergies or drug-resistant pathogens, or are being considered for outpatient parenteral antibiotic therapy.
ANTIBIOTIC THERAPY
The duration of antibiotic treatment for DFI is based on the severity of infection and response to treatment (Supplementary Table 3). Treatment should continue until the signs and symptoms of infection resolve, but there is no strong evidence to support treatment through complete healing. Healing will usually occur in 1 to 2 weeks for mild infections and in 2 to 3 weeks for moderate or severe infections.
Traditional management of diabetic foot osteomyelitis has relied almost exclusively on resection of all infected bone. However, data have emerged over the last 10 years to support initial medical management of select patients. Further research regarding the optimal treatment regimen and duration is ongoing, with 1 recent, randomized control trial comparing 6 versus 12 weeks of antibiotics for patients treated medically for osteomyelitis finding no difference in remission rates.1,41 Patients managed surgically for osteomyelitis are often treated parenterally for at least 4 weeks, but this practice is not based on strong evidence, and guidelines suggest most patients could be switched to highly bioavailable oral agents after a shorter course of intravenous therapy.1,15 Guidelines recommend 2 to 5 days of antibiotics after complete resection of infected bone and soft tissue (Supplementary Table 3). If the infected soft tissue remains, 1 to 3 weeks of therapy is usually sufficient, while 4 to 6 weeks is often needed if there is residually infected but viable bone.15
SURGICAL MANAGEMENT
In patients with osteomyelitis, the decision between medical and surgical management is complex. Absolute indications for surgical resection include systemic toxicity with associated tissue infection, an open or infected joint space, and patients with prosthetic heart valves.27 However, the need for surgery is unclear beyond these absolute indications, and approximately two-thirds of osteomyelitis cases may be arrested or cured with antibiotic therapy alone.1 A prospective randomized comparative trial of patients with diabetic foot osteomyelitis found that patients treated with 90 days of antibiotics had similar healing rates, times to healing, and short-term complications as compared with those who underwent conservative bone resection.44 While further research is needed to determine which types of patients with osteomyelitis may be successfully treated without surgery, the IWGDF, the IDSA, and osteomyelitis experts have offered guidance on this decision (Table 2).1,15,27 If resection is necessary, hospitalists should request at least 4 specimens to help guide postoperative antibiotic therapy (1 sample for histology and 1 for microbiology, at both the grossly abnormal bone and the bone margin), as negative margin cultures predict a lower relapse risk for infection.1,35
CRITERIA FOR DISCHARGE
Guidelines suggest that patients be clinically stable before discharge, complete any urgent surgery, achieve acceptable glycemic control, and be presented with a comprehensive outpatient plan, including antibiotic therapy, offloading, wound care instructions, and outpatient follow-up (Supplementary Table 4). Physicians must consider patient and family preferences, expected adherence to therapy, availability of home support, and payer and cost issues when creating the discharge plan.15
INTERPROFESSIONAL OUTPATIENT CARE
An effective outpatient care team is critical to ensure wound healing and infection resolution. Efforts should be made to discharge patients to a comprehensive outpatient interprofessional foot care team, with a plan that includes professional foot care, patient education, and adequate footwear.48 Team composition varies but often includes representatives from vascular surgery, podiatry, orthotics, wound care, endocrinology, orthopedics, physical therapy and rehabilitation, infectious disease, and dermatology.11-13
CONCLUSION
DFIs are a common cause of morbidity in patients with diabetes and result in significant costs to the US healthcare system. Hospitalized patients with a DFI require appropriate classification of wound severity and assessment of vascular status, protective sensation, and potential osteomyelitis. Inpatient management of these patients includes obtaining necessary cultures, choosing an antibiotic regimen based on infection severity and the likely causative agent, and evaluating the need for surgical intervention. Prior to discharge, providers should determine a comprehensive follow-up plan and ensure patient engagement. Finally, interprofessional management has been shown to improve outcomes in DFI and should be adopted in both the inpatient and outpatient settings.
Disclosure
The authors report no conflicts of interest.
Diabetic foot infection (DFI) is a common result of diabetes and represents the most frequent complication requiring hospitalization and lower extremity amputation.1,2 Hospital discharges related to diabetic lower extremity ulcers increased from 72,000 in 1988 to 113,000 in 2007,3 and admissions related to infection rose 30% between 2005 and 2010.2 Ulceration and amputation are associated with a 40% to 50% 5-year mortality rate.4,5
Aggressive risk-factor management and interprofessional care can significantly reduce major amputations and mortality.6-13 Consistent and high-quality care for patients admitted with DFI is essential for optimizing outcomes; however, management varies widely, and critical assessment and prevention measures are often not employed by providers.14 This review synthesizes recommendations from existing guidelines to provide an overview of the best practices for the diagnosis, management, and discharge of DFI in the hospital setting (Supplementary Table 1, Supplementary Figure).
DETECTION AND STAGING OF INFECTION
The first step in the management of a DFI is a careful assessment of the presence and depth of infection.15 The Infectious Diseases Society of America (IDSA) guidelines recommend using at least 2 signs of classic inflammation (erythema, warmth, swelling, tenderness, or pain) or purulent drainage to diagnose soft tissue infection.1,15,16 Patients with ischemia may present atypically, with nonpurulent secretions, friable or discolored granulation tissue, undermining of wound edges, and foul odor. 1,15,16 Additional risk factors for DFI include ulceration for more than 30 days, recurrent foot ulcers, a traumatic foot wound, severe peripheral arterial disease (PAD) in the affected limb (ankle brachial index [ABI] <0.4), prior lower extremity amputation, loss of protective sensation, end-stage renal disease, and a history of walking barefoot.15,17,18
CRITERIA FOR HOSPITALIZATION
In practice, the decision to admit is based on clinical and systems-based drivers (Supplementary Table 2). The IDSA and IWGDF guidelines recommend hospitalization for patients with severe (PEDIS grade 4) infection, moderate (PEDIS grade 3) infection with certain complications (eg, severe PAD or lack of home support), an inability to comply with required outpatient treatment, lack of improvement with outpatient therapy, or presence of metabolic or hemodynamic instability.1,15 Clinicians must also consider the need for surgical debridement or complex antibiotic choices due to allergies and comorbidities. Hospitalists may also consider admission in cases in which outpatient follow-up cannot be easily arranged (eg, uninsured patients).
Outpatient management may be appropriate for patients with mild infections who are willing to be reassessed within 72 hours, or sooner if the infection worsens.23 For patients with moderate infections (eg, osteomyelitis without systemic signs of infection), access to an outpatient interprofessional DFI care team can potentially decrease the need for admission.
DIAGNOSIS OF OSTEOMYELITIS
Clinical features that raise suspicion for osteomyelitis include ulceration for at least 6 weeks with appropriate wound care and offloading, wound extension to the bone or joint, exposed bone, ulcers larger than 2 cm2, previous history of a wound, multiple wounds, and appearance of a sausage digit.15
The gold standard for diagnosis of osteomyelitis is a bone biopsy with histology. In the absence of histology, physicians rely on physical examination, inflammatory markers, and imaging to make the diagnosis. The presence of visible, chronically exposed bone within a forefoot ulcer is diagnostic. The accuracy of a probe to bone test depends on the pretest probability of osteomyelitis. Sensitivity and specificity range from 60% to 87% and from 85% to 91%, respectively.24 For patients with a single forefoot ulcer and PEDIS grade 2 or 3 infection, considering both ulcer depth and serum inflammatory markers (ulcer depth greater than 3 mm, or C-reactive protein greater than 3.2 mg/dL; ulcer depth greater than 3 mm, or erythrocyte sedimentation rate greater than 60 mm/h) increases sensitivity to 100%, although the specificity is relatively low (55% and 60%, respectively).25 When the diagnosis remains uncertain by physical examination, imaging is necessary for further evaluation.
ROLE OF IMAGING
All patients with DFI should have plain radiographs to look for foot deformities, soft tissue gas, foreign bodies, and osteomyelitis. If plain radiographs show classic evidence of osteomyelitis, (ie, cortical erosion, periosteal reaction, mixed lucency, and sclerosis in the absence of neuro-osteoarthropathy), advanced imaging is not necessary. However, these changes may not appear on plain films for up to 1 month after infection onset.15,26
The purpose of advanced imaging in the inpatient management of DFI is to detect conditions not obvious by physical examination or by plain radiographs that would alter surgical management (ie, deep abscess or necrotic bone) or antibiotic duration (ie, osteomyelitis or tenosynovitis).15 Magnetic resonance imaging (MRI) is the diagnostic modality of choice when the wound does not probe to bone and the diagnosis remains uncertain27 due to its accuracy and availability.1,15 However, MRI cannot always distinguish between infection and neuro-osteoarthropathy, especially in patients who have infection superimposed on a Charcot foot, have had recent surgical intervention, or have osteosynthesis material at the infection site.24 If MRI is contraindicated, guidelines vary on the next recommended test. The IDSA and the Society for Vascular Surgery recommend a labeled white blood cell scan combined with a bone scan, whereas the IWGDF recommends a labeled leukocyte scan, a single photon emission computed tomography (SPECT/CT), or a fluorodeoxyglucose positron emission tomography (FDG PET) scan.1,15,19 A recent comparison of a labeled white blood cell SPECT/CT versus MRI (using histology as the gold standard) reported that SPECT/CT had a similar sensitivity (89% versus 87%, respectively) and specificity (35% versus 37%, respectively) to MRI.28 In practice, physicians should consider which studies are readily available and confidently interpreted by radiologists at their institution.
ASSESSMENT OF ULCER ETIOLOGY
After infection is diagnosed and staged, clinicians should determine the underlying derangement in order to prevent recurrence after discharge. Common derangements leading to ulceration in diabetics include PAD, neuropathy, muscular tension, altered foot mechanics, trauma, or a combination of the above.1,15,29-31 All patients with DFI should undergo pedal perfusion assessment by an ABI, ankle and pedal Doppler arterial waveforms, and either toe brachial index (TBI) or transcutaneous oxygen pressure.1,15,19 In cases of suspected calcification, TBI is a more reliable measure of ischemia compared with the ABI.16,19 For patients with signs and symptoms of ischemia and an abnormal ABI or TBI measurement (ABI <0.9 and TBI <0.7), a nonurgent consultation with a vascular surgeon is recommended, while patients with severe ischemia (ABI <0.4) usually require urgent revascularization.15,32
A sensory examination with a Semmes-Weinstein monofilament should be conducted to identify patients with loss of protective sensation who may benefit from offloading devices and custom orthotics.15 Foot anatomy and mechanics as well as potential Achilles tendon contractures should be evaluated by a foot specialist such as a podiatrist, orthotist, orthopedist, or vascular surgeon, especially if debridement or amputation is being contemplated.
OBTAINING CULTURES
After diagnosing the infection clinically, appropriately obtained cultures are essential to guide therapy in all except mild cases with no prior antibiotic exposure or MRSA risk.1,15 Guidelines strongly recommend that specimens be obtained by biopsy or curettage from deep tissue at the base of the ulcer after the wound has been cleansed and debrided and prior to initiating antibiotics.1,15,33 Aspiration of purulent secretions using a sterile needle and syringe is another acceptable culturing method.15 While convenient, swab cultures are prone to both false-positive and false-negative results.34 Repeat cultures are only needed for patients who are not responding to treatment or for surveillance of resistant organisms.1
In cases of osteomyelitis, bone specimens should be sent for culture and histology either during surgical debridement or a bone biopsy. At the time of debridement, cultures and pathology should be sent from the proximal (clean) bone margin in order to document whether there is residual osteomyelitis postdebridement.35 For patients not planned for debridement, a bone biopsy is recommended if the diagnosis of osteomyelitis is unclear, response to empiric therapy is poor, broad-spectrum antibiotics are being considered, or the infection is in the midfoot or hindfoot.1,15,19 Results from soft tissue or sinus tract specimens should not be used to guide antibiotic selection in osteomyelitis, as several studies suggest that they do not correlate with bone culture results; one retrospective review found a mere 22.5% correlation between wound swabs and bone biopsy.1,36 A 2-week antibiotic-free period prior to biopsy is recommended in order to minimize the risk of false-negative results but must be balanced with the risk of worsening infection.1,15 If possible, the biopsy should be performed through uninfected tissue under fluoroscopy or CT guidance, with 2 to 3 cores obtained for culture and histology.1,15
INTERPROFESSIONAL INPATIENT CARE
A growing number of health systems have created inpatient and/or outpatient interprofessional diabetic foot care teams, and several studies demonstrated an association between these teams and a reduction in major amputations.7-11,13 The goal of the inpatient team is to rapidly triage patients with moderate to severe infections, expedite surgical interventions and culture collection, establish an effective treatment plan, and ensure adherence postdischarge to optimize outcomes. The common core of most teams includes podiatry, endocrinology, wound care, and vascular surgery, but team composition may vary based on the availability of local specialists with interest and expertise in DFI.9,10,33
The division of consultation between podiatry and orthopedic surgery is highly dependent upon individual practice patterns and hospital structure. In general, forefoot ulcers may be managed by podiatry or orthopedic surgery, while severe Charcot deformities are most often treated by orthopedic surgeons. Wound care nurses are often integral to successful wound healing, collaborating across specialties and serving as a weekly or biweekly point of contact for patients.
Early involvement of Infectious Disease (ID) specialists can be useful for guiding antibiotic choices and facilitating follow-up. ID should be involved with patients who require long-term antibiotic therapy (ie, cases of deep-tissue infection that are not completely amputated or debrided), have failed outpatient or empiric therapy, have antibiotic allergies or drug-resistant pathogens, or are being considered for outpatient parenteral antibiotic therapy.
ANTIBIOTIC THERAPY
The duration of antibiotic treatment for DFI is based on the severity of infection and response to treatment (Supplementary Table 3). Treatment should continue until the signs and symptoms of infection resolve, but there is no strong evidence to support treatment through complete healing. Healing will usually occur in 1 to 2 weeks for mild infections and in 2 to 3 weeks for moderate or severe infections.
Traditional management of diabetic foot osteomyelitis has relied almost exclusively on resection of all infected bone. However, data have emerged over the last 10 years to support initial medical management of select patients. Further research regarding the optimal treatment regimen and duration is ongoing, with 1 recent, randomized control trial comparing 6 versus 12 weeks of antibiotics for patients treated medically for osteomyelitis finding no difference in remission rates.1,41 Patients managed surgically for osteomyelitis are often treated parenterally for at least 4 weeks, but this practice is not based on strong evidence, and guidelines suggest most patients could be switched to highly bioavailable oral agents after a shorter course of intravenous therapy.1,15 Guidelines recommend 2 to 5 days of antibiotics after complete resection of infected bone and soft tissue (Supplementary Table 3). If the infected soft tissue remains, 1 to 3 weeks of therapy is usually sufficient, while 4 to 6 weeks is often needed if there is residually infected but viable bone.15
SURGICAL MANAGEMENT
In patients with osteomyelitis, the decision between medical and surgical management is complex. Absolute indications for surgical resection include systemic toxicity with associated tissue infection, an open or infected joint space, and patients with prosthetic heart valves.27 However, the need for surgery is unclear beyond these absolute indications, and approximately two-thirds of osteomyelitis cases may be arrested or cured with antibiotic therapy alone.1 A prospective randomized comparative trial of patients with diabetic foot osteomyelitis found that patients treated with 90 days of antibiotics had similar healing rates, times to healing, and short-term complications as compared with those who underwent conservative bone resection.44 While further research is needed to determine which types of patients with osteomyelitis may be successfully treated without surgery, the IWGDF, the IDSA, and osteomyelitis experts have offered guidance on this decision (Table 2).1,15,27 If resection is necessary, hospitalists should request at least 4 specimens to help guide postoperative antibiotic therapy (1 sample for histology and 1 for microbiology, at both the grossly abnormal bone and the bone margin), as negative margin cultures predict a lower relapse risk for infection.1,35
CRITERIA FOR DISCHARGE
Guidelines suggest that patients be clinically stable before discharge, complete any urgent surgery, achieve acceptable glycemic control, and be presented with a comprehensive outpatient plan, including antibiotic therapy, offloading, wound care instructions, and outpatient follow-up (Supplementary Table 4). Physicians must consider patient and family preferences, expected adherence to therapy, availability of home support, and payer and cost issues when creating the discharge plan.15
INTERPROFESSIONAL OUTPATIENT CARE
An effective outpatient care team is critical to ensure wound healing and infection resolution. Efforts should be made to discharge patients to a comprehensive outpatient interprofessional foot care team, with a plan that includes professional foot care, patient education, and adequate footwear.48 Team composition varies but often includes representatives from vascular surgery, podiatry, orthotics, wound care, endocrinology, orthopedics, physical therapy and rehabilitation, infectious disease, and dermatology.11-13
CONCLUSION
DFIs are a common cause of morbidity in patients with diabetes and result in significant costs to the US healthcare system. Hospitalized patients with a DFI require appropriate classification of wound severity and assessment of vascular status, protective sensation, and potential osteomyelitis. Inpatient management of these patients includes obtaining necessary cultures, choosing an antibiotic regimen based on infection severity and the likely causative agent, and evaluating the need for surgical intervention. Prior to discharge, providers should determine a comprehensive follow-up plan and ensure patient engagement. Finally, interprofessional management has been shown to improve outcomes in DFI and should be adopted in both the inpatient and outpatient settings.
Disclosure
The authors report no conflicts of interest.
1. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32 Suppl 1:45-74. PubMed
2. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149-158. PubMed
3. Number (in thousands) of hospital discharges with peripheral arterial disease (PAD), ulcer/inflammation/infection (ULCER), or neuropathy as first-listed diagnosis and diabetes as any-listed diagnosis United States, 1988-2007. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/statistics/hosplea/diabetes_complications/fig1_number.htm. Updated 2014. Accessed September 23, 2016.
4. Wilbek TE, Jansen RB, Jørgensen B, Svendsen OL. The diabetic foot in a multidisciplinary team setting. Number of amputations below ankle level and mortality. Exp Clin Endocrinol Diabetes. 2016;124(9):535-540. PubMed
5. Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int Wound J. 2016;13(5):892-903. PubMed
6. Young MJ, McCardle JE, Randall LE, Barclay JI. Improved survival of diabetic foot ulcer patients 1995-2008: Possible impact of aggressive cardiovascular risk management. Diabetes Care. 2008;31(11):2143-2147. PubMed
7. Troisi N, Baggiore C, Landini G, Michelagnoli S. How daily practice changed in an urban area after establishing a multidisciplinary diabetic foot program. J Diabetes. 2016;8(4):594-595. PubMed
8. Wang C, Mai L, Yang C, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team in patient with diabetes foot ulcer. BMC Endocr Disord. 2016;16(1):38. PubMed
9. Rubio JA, Aragón-Sánchez J, Jiménez S, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team for the diabetic foot. Int J Low Extrem Wounds. 2014;13(1):22-26. PubMed
10. Yesil S, Akinci B, Bayraktar F, et al. Reduction of major amputations after starting a multidisciplinary diabetic foot care team: Single centre experience from Turkey. Exp Clin Endocrinol Diabetes. 2009;117(7):345-349. PubMed
11. Dargis V, Pantelejeva O, Jonushaite A, Vileikyte L, Boulton AJ. Benefits of a multidisciplinary approach in the management of recurrent diabetic foot ulceration in Lithuania: A prospective study. Diabetes Care. 1999;22(9):1428-1431. PubMed
12. Driver VR, Goodman RA, Fabbi M, French MA, Andersen CA. The impact of a podiatric lead limb preservation team on disease outcomes and risk prediction in the diabetic lower extremity: a retrospective cohort study. J Am Podiatr Med Assoc. 2010;100(4):235-241. PubMed
13. Hamonet J, Verdié-Kessler C, Daviet JC, et al. Evaluation of a multidisciplinary consultation of diabetic foot. Ann Phys Rehabil Med. 2010;53(5):306-318. PubMed
14. Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: Results of the Eurodiale study, a prospective cohort study. Diabet Med. 2008;25(6):700-707. PubMed
15. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173. PubMed
16. Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr. 2016;11(2):149-156. PubMed
17. Hill MN, Feldman HI, Hilton SC, Holechek MJ, Ylitalo M, Benedict GW. Risk of foot complications in long-term diabetic patients with and without ESRD: A preliminary study. ANNA J. 1996;23(4):381-386; discussion 387-388. PubMed
18. Mohler ER, III. Peripheral arterial disease: Identification and implications. Arch Intern Med. 2003;163(19):2306-2314. PubMed
19. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2 Suppl):3S-21S. PubMed
20. Noor S, Zubair M, Ahmad J. Diabetic foot ulcer--A review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192-199. PubMed
21. Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int. 2013;34(3):351-358. PubMed
22. Wukich DK, Hobizal KB, Raspovic KM, Rosario BL. SIRS is valid in discriminating between severe and moderate diabetic foot infections. Diabetes Care. 2013;36(11):3706-3711. PubMed
23. Grigoropoulou P, Eleftheriadou I, Jude EB, Tentolouris N. Diabetic foot infections: An update in diagnosis and management. Curr Diab Rep. 2017;17(1):3. PubMed
24. Glaudemans AW, Uçkay I, Lipsky BA. Challenges in diagnosing infection in the diabetic foot. Diabet Med. 2015;32(6):748-759. PubMed
25. Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg. 2009;48(1):39-46. PubMed
26. Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39 Suppl 2:S115-S122. PubMed
27. Allahabadi S, Haroun KB, Musher DM, Lipsky BA, Barshes NR. Consensus on surgical aspects of managing osteomyelitis in the diabetic foot. Diabet Foot Ankle. 2016;7:30079. PubMed
28. La Fontaine J, Bhavan K, Lam K, et al. Comparison between Tc-99m WBC SPECT/CT and MRI for the diagnosis of biopsy-proven diabetic foot osteomyelitis. Wounds. 2016;28(8):271-278. PubMed
29. Bembi V, Singh S, Singh P, Aneja GK, Arya TV, Arora R. Prevalence of peripheral arterial disease in a cohort of diabetic patients. South Med J. 2006;99(6):564-569. PubMed
30. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921-929. PubMed
31. Hinchliffe RJ, Andros G, Apelqvist J, et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab Res Rev. 2012;28 Suppl 1:179-217. PubMed
32. Brownrigg JR, Apelqvist J, Bakker K, Schaper NC, Hinchliffe RJ. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg. 2013;45(6):673-681. PubMed
33. 2015;13(2):115-122.Ann Fam Med49. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. PubMed
34. 2016;32 Suppl 1:16-24.Diabetes Metab Res Rev48. Bus SA, van Netten JJ, Lavery LA, et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. PubMed
35. 2003;85-A(8):1436-1445.J Bone Joint Surg Am47. Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. PubMed
36. 2015;21(2):77-85.Foot Ankle Surg46. Cychosz CC, Phisitkul P, Belatti DA, Glazebrook MA, DiGiovanni CW. Gastrocnemius recession for foot and ankle conditions in adults: Evidence-based recommendations. PubMed
37. 2016;32 Suppl 1:25-36.Diabetes Metab Res Rev45. Bus SA, Armstrong DG, van Deursen RW, et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. PubMed
38. 2014;37(3):789-795.Diabetes Care44. Lázaro-Martínez JL, Aragón-Sánchez J, García-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: A randomized comparative trial. PubMed
39. 1996;183(1):61-64.J Am Coll Surg43. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. PubMed
40. 2002;10(6):354-359.Wound Repair Regen42. Saap LJ, Falanga V. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. PubMed
41. 2015;38(2):302-307.Diabetes Care41. Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: A multicenter open-label controlled randomized study. PubMed
42. 2014;35(10):1229-1235.Infect Control Hosp Epidemiol40. Schultz L, Lowe TJ, Srinivasan A, Neilson D, Pugliese G. Economic impact of redundant antimicrobial therapy in US hospitals. PubMed
43. 2015;31(4):395-401.Diabetes Metab Res Rev39. Lipsky BA, Cannon CM, Ramani A, et al. Ceftaroline fosamil for treatment of diabetic foot infections: the CAPTURE study experience. PubMed
2011;55(9):4154-4160.Antimicrob Agents Chemother.38. Richter SS, Heilmann KP, Dohrn CL, et al. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. PubMed
44. 2011;52(3):e18-e55.Clin Infect Dis37. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. PubMed
45. 2006;42(1):57-62.Clin Infect Dis36. Senneville E, Melliez H, Beltrand E, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: Concordance with ulcer swab cultures. PubMed
46. 2012;51(6):749-752.J Foot Ankle Surg35. Atway S, Nerone VS, Springer KD, Woodruff DM. Rate of residual osteomyelitis after partial foot amputation in diabetic patients: A standardized method for evaluating bone margins with intraoperative culture. PubMed
47. 2010;5(7):415-420.J Hosp Med34. Chakraborti C, Le C, Yanofsky A. Sensitivity of superficial cultures in lower extremity wounds. PubMed
48. 2013;36(9):2862-2871.Diabetes Care33. Wukich DK, Armstrong DG, Attinger CE, et al. Inpatient management of diabetic foot disorders: A clinical guide. PubMed
1. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32 Suppl 1:45-74. PubMed
2. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149-158. PubMed
3. Number (in thousands) of hospital discharges with peripheral arterial disease (PAD), ulcer/inflammation/infection (ULCER), or neuropathy as first-listed diagnosis and diabetes as any-listed diagnosis United States, 1988-2007. Centers for Disease Control and Prevention website. http://www.cdc.gov/diabetes/statistics/hosplea/diabetes_complications/fig1_number.htm. Updated 2014. Accessed September 23, 2016.
4. Wilbek TE, Jansen RB, Jørgensen B, Svendsen OL. The diabetic foot in a multidisciplinary team setting. Number of amputations below ankle level and mortality. Exp Clin Endocrinol Diabetes. 2016;124(9):535-540. PubMed
5. Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int Wound J. 2016;13(5):892-903. PubMed
6. Young MJ, McCardle JE, Randall LE, Barclay JI. Improved survival of diabetic foot ulcer patients 1995-2008: Possible impact of aggressive cardiovascular risk management. Diabetes Care. 2008;31(11):2143-2147. PubMed
7. Troisi N, Baggiore C, Landini G, Michelagnoli S. How daily practice changed in an urban area after establishing a multidisciplinary diabetic foot program. J Diabetes. 2016;8(4):594-595. PubMed
8. Wang C, Mai L, Yang C, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team in patient with diabetes foot ulcer. BMC Endocr Disord. 2016;16(1):38. PubMed
9. Rubio JA, Aragón-Sánchez J, Jiménez S, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team for the diabetic foot. Int J Low Extrem Wounds. 2014;13(1):22-26. PubMed
10. Yesil S, Akinci B, Bayraktar F, et al. Reduction of major amputations after starting a multidisciplinary diabetic foot care team: Single centre experience from Turkey. Exp Clin Endocrinol Diabetes. 2009;117(7):345-349. PubMed
11. Dargis V, Pantelejeva O, Jonushaite A, Vileikyte L, Boulton AJ. Benefits of a multidisciplinary approach in the management of recurrent diabetic foot ulceration in Lithuania: A prospective study. Diabetes Care. 1999;22(9):1428-1431. PubMed
12. Driver VR, Goodman RA, Fabbi M, French MA, Andersen CA. The impact of a podiatric lead limb preservation team on disease outcomes and risk prediction in the diabetic lower extremity: a retrospective cohort study. J Am Podiatr Med Assoc. 2010;100(4):235-241. PubMed
13. Hamonet J, Verdié-Kessler C, Daviet JC, et al. Evaluation of a multidisciplinary consultation of diabetic foot. Ann Phys Rehabil Med. 2010;53(5):306-318. PubMed
14. Prompers L, Huijberts M, Apelqvist J, et al. Delivery of care to diabetic patients with foot ulcers in daily practice: Results of the Eurodiale study, a prospective cohort study. Diabet Med. 2008;25(6):700-707. PubMed
15. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132-e173. PubMed
16. Noor S, Khan RU, Ahmad J. Understanding diabetic foot infection and its management. Diabetes Metab Syndr. 2016;11(2):149-156. PubMed
17. Hill MN, Feldman HI, Hilton SC, Holechek MJ, Ylitalo M, Benedict GW. Risk of foot complications in long-term diabetic patients with and without ESRD: A preliminary study. ANNA J. 1996;23(4):381-386; discussion 387-388. PubMed
18. Mohler ER, III. Peripheral arterial disease: Identification and implications. Arch Intern Med. 2003;163(19):2306-2314. PubMed
19. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2 Suppl):3S-21S. PubMed
20. Noor S, Zubair M, Ahmad J. Diabetic foot ulcer--A review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9(3):192-199. PubMed
21. Wukich DK, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int. 2013;34(3):351-358. PubMed
22. Wukich DK, Hobizal KB, Raspovic KM, Rosario BL. SIRS is valid in discriminating between severe and moderate diabetic foot infections. Diabetes Care. 2013;36(11):3706-3711. PubMed
23. Grigoropoulou P, Eleftheriadou I, Jude EB, Tentolouris N. Diabetic foot infections: An update in diagnosis and management. Curr Diab Rep. 2017;17(1):3. PubMed
24. Glaudemans AW, Uçkay I, Lipsky BA. Challenges in diagnosing infection in the diabetic foot. Diabet Med. 2015;32(6):748-759. PubMed
25. Fleischer AE, Didyk AA, Woods JB, Burns SE, Wrobel JS, Armstrong DG. Combined clinical and laboratory testing improves diagnostic accuracy for osteomyelitis in the diabetic foot. J Foot Ankle Surg. 2009;48(1):39-46. PubMed
26. Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39 Suppl 2:S115-S122. PubMed
27. Allahabadi S, Haroun KB, Musher DM, Lipsky BA, Barshes NR. Consensus on surgical aspects of managing osteomyelitis in the diabetic foot. Diabet Foot Ankle. 2016;7:30079. PubMed
28. La Fontaine J, Bhavan K, Lam K, et al. Comparison between Tc-99m WBC SPECT/CT and MRI for the diagnosis of biopsy-proven diabetic foot osteomyelitis. Wounds. 2016;28(8):271-278. PubMed
29. Bembi V, Singh S, Singh P, Aneja GK, Arya TV, Arora R. Prevalence of peripheral arterial disease in a cohort of diabetic patients. South Med J. 2006;99(6):564-569. PubMed
30. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921-929. PubMed
31. Hinchliffe RJ, Andros G, Apelqvist J, et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab Res Rev. 2012;28 Suppl 1:179-217. PubMed
32. Brownrigg JR, Apelqvist J, Bakker K, Schaper NC, Hinchliffe RJ. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg. 2013;45(6):673-681. PubMed
33. 2015;13(2):115-122.Ann Fam Med49. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. PubMed
34. 2016;32 Suppl 1:16-24.Diabetes Metab Res Rev48. Bus SA, van Netten JJ, Lavery LA, et al. IWGDF guidance on the prevention of foot ulcers in at-risk patients with diabetes. PubMed
35. 2003;85-A(8):1436-1445.J Bone Joint Surg Am47. Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. PubMed
36. 2015;21(2):77-85.Foot Ankle Surg46. Cychosz CC, Phisitkul P, Belatti DA, Glazebrook MA, DiGiovanni CW. Gastrocnemius recession for foot and ankle conditions in adults: Evidence-based recommendations. PubMed
37. 2016;32 Suppl 1:25-36.Diabetes Metab Res Rev45. Bus SA, Armstrong DG, van Deursen RW, et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. PubMed
38. 2014;37(3):789-795.Diabetes Care44. Lázaro-Martínez JL, Aragón-Sánchez J, García-Morales E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: A randomized comparative trial. PubMed
39. 1996;183(1):61-64.J Am Coll Surg43. Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. PubMed
40. 2002;10(6):354-359.Wound Repair Regen42. Saap LJ, Falanga V. Debridement performance index and its correlation with complete closure of diabetic foot ulcers. PubMed
41. 2015;38(2):302-307.Diabetes Care41. Tone A, Nguyen S, Devemy F, et al. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: A multicenter open-label controlled randomized study. PubMed
42. 2014;35(10):1229-1235.Infect Control Hosp Epidemiol40. Schultz L, Lowe TJ, Srinivasan A, Neilson D, Pugliese G. Economic impact of redundant antimicrobial therapy in US hospitals. PubMed
43. 2015;31(4):395-401.Diabetes Metab Res Rev39. Lipsky BA, Cannon CM, Ramani A, et al. Ceftaroline fosamil for treatment of diabetic foot infections: the CAPTURE study experience. PubMed
2011;55(9):4154-4160.Antimicrob Agents Chemother.38. Richter SS, Heilmann KP, Dohrn CL, et al. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. PubMed
44. 2011;52(3):e18-e55.Clin Infect Dis37. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. PubMed
45. 2006;42(1):57-62.Clin Infect Dis36. Senneville E, Melliez H, Beltrand E, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: Concordance with ulcer swab cultures. PubMed
46. 2012;51(6):749-752.J Foot Ankle Surg35. Atway S, Nerone VS, Springer KD, Woodruff DM. Rate of residual osteomyelitis after partial foot amputation in diabetic patients: A standardized method for evaluating bone margins with intraoperative culture. PubMed
47. 2010;5(7):415-420.J Hosp Med34. Chakraborti C, Le C, Yanofsky A. Sensitivity of superficial cultures in lower extremity wounds. PubMed
48. 2013;36(9):2862-2871.Diabetes Care33. Wukich DK, Armstrong DG, Attinger CE, et al. Inpatient management of diabetic foot disorders: A clinical guide. PubMed
© 2017 Society of Hospital Medicine
A concise guide to monoamine oxidase inhibitors
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.
Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.
Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.
Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.
Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
Despite an abundance of evidenced-based literature supporting monoamine oxidase inhibitors (MAOIs) as an effective treatment for depression, use of these agents has decreased drastically in the past 3 decades. A lack of industry support and the ease of use of other agents are contributing factors, but the biggest impediments to routine use of MAOIs are unfamiliarity with their efficacy advantages and concerns about adverse effects, particularly the risk of hypertensive crises and serotonin syndrome. Many misconceptions regarding these medications are based on outdated data and studies that are no longer reliable.
The goal of this 2-part review is to provide clinicians with updated information regarding MAOIs. Part 1 provides a brief description of:
- the pharmacology of nonselective irreversible MAOIs
- the mechanism by which tyramine induces hypertension
- sources of clinically significant tyramine exposure
- what to tell patients about dietary restrictions and MAOIs.
Part 2 of this guide will cover the risk of serotonin syndrome when MAOIs are combined with inhibitors of serotonin reuptake, how to initiate MAOI therapy, and augmenting MAOIs with other agents.
The pharmacology of MAOIs
First used clinically in the 1950s to treat tuberculosis, MAOIs have a long and interesting history (see the Box “A brief history of monoamine oxidase inhibitors”). Table 11 lists MAOIs currently available in the United States, including the MAO-B–specific agent rasagiline, which is used for Parkinson’s disease.
Manipulation of the monoamines serotonin, norepinephrine, and dopamine is fundamental to managing major depressive disorder (MDD), yet only nonselective MAOIs directly promote neurotransmission of all 3 by inhibiting MAO-A and MAO-B.2 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study demonstrated that <50% of MDD patients achieve remission in monotherapy trials of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, mirtazapine, or bupropion, necessitating consideration of antidepressant combinations, augmentation options, and eventually irreversible, nonselective MAOIs such as phenelzine, tranylcypromine, or isocarboxazid.3,4 Nonselective MAOIs thus offer a therapeutic opportunity for patients who do not respond to single or dual-mechanism strategies; moreover, nonselective MAOIs have compelling effectiveness data for other conditions, including panic disorder and social phobia.5 Although MAOIs are among the most effective pharmacologic agents for MDD,6 they are underutilized because of an inadequate understanding of risk mechanisms and resultant fear of catastrophic outcomes. Because of the difficulties encountered in achieving clinical remission for MDD, the nonselective MAOIs deserve a second look.
Differentiation of MAO-A from MAO-B. It is essential to understand the mechanism of action of MAOIs, specifically the impact of MAO-A inhibition. Although the enzyme MAO was known in the 1950s, it wasn’t until 1968 that Johnston7 postulated the existence of >1 form. In 1971, Goridis and Neff8 used clorgyline to examine the deamination rate by MAO of tyramine and norepinephrine. They found that tyramine appeared to be a substrate of both MAO isoforms, but only 1 of the MAO types was sensitive to the inhibitory effects of clorgyline. They also discerned that norepinephrine was only a substrate for MAO-A, and that this form of MAO was sensitive to clorgyline inhibition. Thus, the forms of MAO were characterized by their preferred substrates (Table 29,10), and then later by their tissue distribution. Phenylethylamine is a naturally occurring compound found in foods, such as chocolate, and has an in vitro pharmacology similar to amphetamine but with 1 important difference: it has a short half-life of 5 to 10 minutes after oral ingestion, and therefore no appreciable CNS impact.
Within the CNS, norepinephrine and dopamine neurons possess both MAO forms, with the MAO-A content greater than MAO-B. Serotonergic neurons only contain MAO-B.11 Outside of the CNS, MAO-A predominates, with only platelets and lymphocytes possessing MAO-B activity.11 The overall relative tissue proportions of MAO-A to MAO-B activity are: brain, 25% MAO-A, 75% MAO-B; liver, 50% MAO-A, 50% MAO-B; intestine, 80% MAO-A, 20% MAO-B; and peripheral adrenergic neurons, 90% MAO-A, 10% MAO-B.
Because of its specificity for serotonin and norepinephrine, CNS MAO-A inhibition is necessary for antidepressant effects. MAO-B inhibition by itself does not appear to raise CNS dopamine levels unless exogenous dopamine is supplied.11 All MAOIs used in the United States to treat depression are irreversible, nonselective inhibitors of MAO-A and MAO-B.
Selegiline in oral form generates low plasma levels and primarily inhibits MAO-B. The transdermal form of selegiline achieves significantly greater systemic exposure, and at these higher plasma levels selegiline is a nonselective, irreversible MAOI effective for MDD (Figure 112). Administering selegiline systemically via a transdermal patch avoids clinically significant MAOI effects in the gut, so no dietary warnings exist for the lowest dose (6 mg/24 hours), although there are warnings for the higher dosages (9 mg/24 hours and 12 mg/24 hours).
Differentiation of MAOIs by chemical class. The earliest MAOI, iproniazid, was a hydrazine derivative and exhibited hepatotoxicity,13 as did certain other hydrazine MAOIs. This lead to a search for safer hydrazine and nonhydrazine alternatives. Isocarboxazid and phenelzine are the 2 hydrazine MAOIs available in the United States, while tranylcypromine and selegiline transdermal are nonhydrazines (Figure 2).
What distinguishes the nonhydrazine medication selegiline is that its metabolism generates L-amphetamine metabolites (Figure 314). This property was thought to be shared by other nonhydrazines, but recent studies indicate than neither tranylcypromine15 nor the MAO-B–selective rasagiline possess amphetamine metabolites.16 Unlike the dextro isomers, L-amphetamine structures do not inhibit dopamine reuptake or cause euphoria, but can cause stimulation (eg, sleep disturbance) by inhibiting norepinephrine reuptake, and also by interacting with the trace amine-associated receptor 1 (TAAR1), an intracellular receptor expressed within the presynaptic terminal of monoamine neurons. Activation of TAAR1 by tyramine is an important part of the hypertensive effects related to excessive tyramine exposure.17 (The importance of TAAR1 and the interaction with tyramine is discussed in the next section.) Importantly, patients taking selegiline must be warned that certain drug screens may not discriminate between levo and dextro isomers of amphetamines, and that the use of selegiline should be disclosed prior to drug testing procedures.
MAOIs and tyramine: Dietary requirements
Clinicians who are familiar with MAOIs recognize that there are dietary restrictions to minimize patients’ exposure to tyramine. As most clinicians know, significant tyramine ingestion may cause an increase in blood pressure (BP) in patients taking an MAOI, but many overestimate the prevalence of foods high in tyramine content since the original reports emerged in the early 1960s.18 In a recent monograph, one of the leading experts on MAOIs, Professor Ken Gillman, stated:
Very few foods now contain problematically high tyramine levels, that is a result of great changes in international food production methods and hygiene regulations. Cheese is the only food that, in the past, has been associated with documented fatalities resulting from hypertension. Nowadays most cheeses are quite safe, and even ‘matured’ cheeses are usually safe in healthy-sized portions. The variability of sensitivity to tyramine between individuals, and the sometimes unpredictable amount of tyramine content in foods, means a little knowledge and care are still required.19
What is tyramine? Tyramine is a biogenic amine that is virtually absent in fresh animal protein sources but is enriched after decay or fermentation.20 Modern food processing and handling methods have significantly limited the tyramine content in processed foods, with the exception of certain cheeses and sauces, as discussed below. Moreover, modern assaying techniques using high-performance liquid chromatography have generated extremely accurate assessments of the tyramine content of specific foods.21 Data published prior to 2000 are not reliable, because many of these publications employed outdated methods.17
When ingested, tyramine is metabolized by gut MAO-A, with doses up to 400 mg causing no known effects, although most people rarely ingest >25 mg during a meal.22 In addition to being a substrate for MAO-A, tyramine is also a substrate for the dopamine transporter, norepinephrine transporter (NET), the vesicular monoamine transporter 2, and TAAR1.23 Tyramine enters the cell via NET, where it interacts with TAAR1, a G protein-coupled receptor that is responsive to trace amines, such as tyramine, as well as amphetamines.20 The agonist properties at TAAR1 are the presumed site of action for the BP effects of tyramine, because binding results in potent release of norepinephrine.20,24 When tyramine is supplied to an animal in which MAO-A is inhibited, the decreased peripheral catabolism of tyramine results in markedly increased norepinephrine release by peripheral adrenergic neurons. Moreover, the absence of MAO-A activity in those neurons prevents any norepinephrine breakdown, resulting in robust synaptic norepinephrine delivery and peripheral effects.
All orally administered irreversible MAOIs potently inhibit gut and systemic MAO-A, and are susceptible to the impact of significant tyramine ingestion. The exception is selegiline transdermal (Figure 112), as appreciable gut MAO-A inhibition does not occur until doses >6 mg/24 hours are reached.22 No significant pressor response was seen in participants taking selegiline transdermal, 6 mg/24 hours for 13 days, who consumed a meal that provided 400 mg of tyramine.22 Conversely, for oral agents that produce gut MAO-A inhibition, tyramine doses as low as 8 to 10 mg (when administered as tyramine capsules) may increase systolic pressure by 30 mm Hg.25 The dietary warnings do not apply to rasagiline, which is a selective MAO-B inhibitor, although rasagiline may have an impact on resting BP; the prescribing information for rasagiline includes warnings about hypotension and hypertension.26
What to tell patients about tyramine. Although administering pure tyramine capsules can induce a measurable change in systolic BP, when ingested as food, tyramine doses <50 mg are unlikely to cause an increase in BP sufficient to warrant clinical intervention, although some individuals can be sensitive to 10 to 25 mg.19 When discussing with patients safety issues related to diet, there are a few important concepts to remember19:
- In an era when the tyramine content of foods was much higher (1960 to 1964) and MAOI users received no dietary guidance, only 14 deaths were reported among an estimated 1.5 million patients who took MAOIs.
- MAOIs do not raise BP, and their use is associated with orthostasis in some patients.
- Routine exercise or other vigorous activities (eg, weightlifting) can raise systolic pressure well above 200 mm Hg, and routine baseline systolic pressures, ranging from 180 to 220 mm Hg, do not increase the risk of subarachnoid hemorrhage.
- Hospital evaluation is needed only if a substantial amount of tyramine is ingested (eg, estimated ≥100 mg), and self-monitoring shows a systolic BP ≥220 mm Hg over a prolonged period (eg, 2 hours). Ingestion of 100 mg of tyramine would almost certainly have to be intentional, as it would require one to consume 3.5 oz of the most highly tyramine-laden cheeses.
Emphasize to patients that only a small number of highly aged cheeses, foods, and sauces contain high quantities of tyramine, and that even these foods can be enjoyed in small amounts. All patients who are prescribed an MAOI also should purchase a portable BP cuff for those rare instances when a dietary indiscretion may have occurred and the person experiences a headache within 1 to 2 hours after tyramine ingestion. Most reactions are self-limited and resolve over 2 to 4 hours.
Patients who ingest ≥100 mg of tyramine should be evaluated by a physician. Under no circumstances should a patient be given a prescription for nifedipine or other medications that can abruptly lower BP, because this may result in complications, including myocardial infarction.27,28 Counsel patients to remain calm. Some clinicians endorse the use of low doses of benzodiazepines (the equivalent of alprazolam 0.5 mg) to facilitate this, because anxiety elevates BP. A recent emergency room study of patients with an initial systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg without end organ damage demonstrated that alprazolam, 0.5 mg, was as effective as captopril, 25 mg, in lowering BP.29
Also, tell patients that if a food is unfamiliar and highly aged or fermented, they should avoid it until they can further inquire about it. In a review, Gillman19 provides the tyramine content of an exhaustive list of cheeses, aged meats, and sauces (see Related Resources). For other products, patients often can obtain information directly from the manufacturer. In many parts of the world, assays for tyramine content are required as a demonstration of adequate product safety procedures. Even the most highly aged cheeses with a tyramine content of 1,000 g/kg can be enjoyed in small amounts (<1 oz), and most products would require heroic intake to achieve clinically significant tyramine ingestion (Table 319).
Improved education can clarify the risks
Medications such as lithium, clozapine, and MAOIs have a proven record of efficacy, yet often are underused due to fears engendered by lack of systematic training. A recent initiative in New York thus aimed to increase rates of
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
1. Panisset M, Chen JJ, Rhyee SH, et al. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250-1258.
2. López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15(14):1563-1586.
3. Nierenberg AA, Fava M, Trivedi MH, et al. A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1519-1530; quiz 1665.
4. Trivedi MH, Fava M, Wisniewski SR, et al; STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. New Engl J Med. 2006;354(12):1243-1252.
5. Bandelow B, Zohar J, Hollander E, et al; World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Posttraumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders. World J Biol Psychiatry. 2002;3(4):171-199.
6. Shulman KI, Herrmann N, Walker SE. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789-797.
7. Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285-1297.
8. Goridis C, Neff NH. Monoamine oxidase in sympathetic nerves: a transmitter specific enzyme type. Br J Pharmacol. 1971;43(4):814-818.
9. Geha RM, Rebrin I, Chen K, et al. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276(13):9877-9882.
10. O’Carroll AM, Fowler CJ, Phillips JP, et al. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(3):198-202.
11. Stahl SM, Felker A. Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr. 2008;13(10):855-780.
12. Mawhinney M, Cole D, Azzaro AJ. Daily transdermal administration of selegiline to guinea-pigs preferentially inhibits monoamine oxidase activity in brain when compared with intestinal and hepatic tissues. J Pharm Pharmacol. 2003;55(1):27-34.
13. Maille F, Duvoux C, Cherqui D, et al. Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France? [in French]. Gastroenterol Clin Biol. 1999;23(10):1083-1085.
14. Salonen JS, Nyman L, Boobis AR, et al. Comparative studies on the cytochrome p450-associated metabolism and interaction potential of selegiline between human liver-derived in vitro systems. Drug Metab Dispos. 2003;31(9):1093-1102.
15. Iwersen S, Schmoldt A. One fatal and one nonfatal intoxication with tranylcypromine. Absence of amphetamines as metabolites. J Anal Toxicol. 1996;20(5):301-304.
16. Müller T, Hoffmann JA, Dimpfel W, et al. Switch from selegiline to rasagiline is beneficial in patients with Parkinson’s disease. J Neural Transm (Vienna). 2013;120(5):761-765.
17. Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg Med Chem. 2011;19(23):7044-7048.
18. Blackwell B. Hypertensive crisis due to monoamine-oxidase inhibitors. Lancet. 1963;2(7313):849-850.
19. Gillman PK. Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. PsychoTropical Commentaries. 2016;16(6):1-97.
20. Pei Y, Asif-Malik A, Canales JJ. Trace amines and the trace amine-associated receptor 1: pharmacology, neurochemistry, and clinical implications. Front Neurosci. 2016;10:148.
21. Fiechter G, Sivec G, Mayer HK. Application of UHPLC for the simultaneous analysis of free amino acids and biogenic amines in ripened acid-curd cheeses. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;927:191-200.
22. Blob LF, Sharoky M, Campbell BJ, et al. Effects of a tyramine-enriched meal on blood pressure response in healthy male volunteers treated with selegiline transdermal system 6 mg/24 hour. CNS Spectr. 2007;12(1):25-34.
23. Partilla JS, Dempsey AG, Nagpal AS, et al. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319(1):237-246.
24. Borowsky B, Adham N, Jones KA, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci U S A. 2001;98(16):8966-8971.
25. Azzaro AJ, Vandenberg CM, Blob LF, et al. Tyramine pressor sensitivity during treatment with the selegiline transdermal system 6 mg/24 h in healthy subjects. J Clin Pharmacol. 2006;46(8):933-944.
26. Azilect [package insert]. Overland Park, KS: Teva Neuroscience, Inc.; 2014.
27. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
28. Burton TJ, Wilkinson IB. The dangers of immediate-release nifedipine in the emergency treatment of hypertension. J Hum Hypertens. 2008;22(4):301-302.
29. Yilmaz S, Pekdemir M, Tural U, et al. Comparison of alprazolam versus captopril in high blood pressure: a randomized controlled trial. Blood Press. 2011;20(4):239-243.
30. Carruthers J, Radigan M, Erlich MD, et al. An initiative to improve clozapine prescribing in New York State. Psychiatr Serv. 2016;67(4):369-371.
Prescribing antipsychotics in geriatric patients: Focus on dementia
According to the U.S. Department of Health and Human Services, in 2007, 88% of 1.4 million Medicare claims for
Because of the aging population and widespread prescription of antipsychotics to older patients, clinicians need information on the relative risks of using these medications in this population. In the United States, all antipsychotics carry a FDA “black-box” warning of the increased risk of death in older adults with dementia. In addition, the risk of death is increased when prescribing antipsychotics to older adults with other conditions, such as Parkinson’s disease,6 and other safety and tolerability concerns, including falls and fractures, sedation, metabolic abnormalities, and extrapyramidal effects, are highly relevant to geriatric patients.
This 3-part review summarizes findings and recommendations on prescribing antipsychotics to older individuals with schizophrenia, bipolar disorder, depression, and dementia. This third and final installment:
- briefly summarizes the major studies and analyses relevant to prescribing antipsychotics to older patients with dementia
- provides a summative opinion on safety and tolerability issues in these older patients
- highlights the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
Behavioral and psychological symptoms of dementia (BPSD) include agitation, delusional beliefs, repetitive questioning, hallucinations, aggression, wandering, and various socially inappropriate behaviors.7 These occur almost universally in all types and stages of dementia.7 BPSD are among the most complex, stressful, and costly aspects of dementia care, and lead to a myriad of poor health outcomes, including excess morbidity, mortality, hospital stays, and early nursing home placement.8-11 Because BPSD usually occur across all types and stages of dementia,7,12-16 the prevalence of BPSD mirrors the overall prevalence of dementia.
Although all expert organizations, including the American Psychiatric Association,17 recommend nonpharmacologic strategies as first-line treatment for BPSD, for the most part, these recommendations have not been translated into standard clinical management or routine care.18 Because of a perceived lack of other options, the current mainstay of treatment is the off-label use of psychotropics such as antipsychotics. Of all the agents currently used for BPSD, SGAs have the strongest evidence base, although benefits are modest at best (standardized effect size 0.13 to 0.16).19,20 In terms of individual SGAs, only risperidone is indicated for aggression in Canada and in Europe (not in the United States);
Clinical Trials
Adverse effects. A meta-analysis of RCTs of SGAs found that, compared with placebo, SGAs have increased rates of several adverse effects. These include somnolence (17% drug vs 7% placebo;
In the 42-site Clinical Antipsychotic Trials of Intervention Effectiveness Alzheimer’s disease RCT, 421 outpatients with Alzheimer’s disease and BPSD were randomized to an SGA (risperidone,
In the 2005 FDA black-box warning, pneumonia and cardiac adverse effects were cited as primary causes of death for patients with dementia taking SGAs. A subsequent observational study confirmed that use of either FGAs or SGAs in geriatric patients was associated with an increased risk of pneumonia, in a dose-dependent manner.27 Although there is limited data on cardiac adverse effects in older adults, especially those with dementia taking antipsychotics,28 1 observational study of nursing home residents29 found that those taking FGAs had a significantly higher risk of hospitalization for ventricular arrhythmia or cardiac arrest compared with those who were not taking FGAs. In contrast, there was no increased risk with SGAs.
Mortality.
In 2005, the FDA announced that based on a reanalysis of 17 placebo-controlled trials (many of which were unpublished) that SGAs were associated with a 1.7-fold increase in mortality compared with placebo.30 As a result, the FDA issued a black-box warning for using SGAs in patients with dementia. The overall OR in a published meta-analysis of mortality with SGAs was 1.54 (1.06 to 2.23; z = 2.28; P = .02), with pooled events of 3.5% mortality vs 2.3% (drug vs placebo).21 This meta-analysis21 also included ad hoc analyses of haloperidol; using combined data from 2 contrasts of haloperidol (with risperidone and quetiapine; 243 patients receiving haloperidol and 239 receiving placebo) they also found 15 deaths (6.2%) with haloperidol and 9 (3.8%) with placebo, resulting in an OR of 1.68.
Other clinical data
Observational studies. Most observational studies have confirmed concerns regarding increased mortality in patients with BPSD who take antipsychotics, with FGAs having a higher risk than SGAs18,31 and SGAs having a higher risk compared with most other psychotropics.32 Three studies that found no increase in mortality with antipsychotics in patients with dementia had methodological issues, including examining prevalence as opposed to new users,33,34 not controlling for exposure,10,33,34 power issues,10,34 not controlling for other psychiatric medications,10 and varying lengths of follow-up.10 An FDA black-box warning for FGAs was announced in 200830 based on 2 observational studies that showed an increased risk of mortality in older adults taking FGAs vs SGAs.35,36
In terms of specific SGAs, Kales et al37 examined the mortality risk associated with individual antipsychotics using various methods to control for confounding. Among a national sample of >33,000 older veterans with dementia newly started on haloperidol, risperidone, olanzapine, quetiapine, or
Most recently, a retrospective case-control study (90,786 patients age ≥65 with dementia) examined the number needed to harm (NNH; ie, number of patients needed to receive treatment that would result in 1 death) over 180 days following initiation of an FGA or SGA.38 This study found the following NNHs: haloperidol, 26 (95% CI, 15 to 99); risperidone, 27 (95% CI, 19 to 46); olanzapine, 40 (95% CI, 21 to 312); and quetiapine, 50 (95% CI, 30 to 150).38 These results are congruent with a review of observational studies that found the highest risk of mortality was associated with haloperidol and
Patterns of antipsychotic use in older dementia patients
There are high rates of antipsychotic use in patients with dementia. Before the FDA issued the black-box warning, the Aging Demographics and Memory study found that the rate of antipsychotic use in community (outpatient) older adults with dementia was approximately 19% between 2002 and 2004 in a representative sample of 307 older adults.39 Another study examining trends in community antipsychotic use in the U.S. Department of Veterans Affairs (VA) found that in the 1990s, SGA use was increasing; approximately 18% of outpatients with dementia were taking these agents.40 Use of SGAs began to decline in 2003, ahead of the 2005 black-box warning, in tandem with other advisories (eg, diabetes, metabolic syndrome,41 and stroke risk).42,43 Olanzapine and risperidone showed declining rates between 2003 and 2005, whereas quetiapine use significantly increased during this period. All 3 SGAs declined after the black-box warning. However, by the end of 2007, the use of SGAs had leveled off to approximately 12% of VA patients with dementia. A recent U.S. Government Accountability Office (GAO) report found that in 2012, 14% of older adult Medicare Part D enrollees with dementia living in the community were prescribed an antipsychotic.44
Use in nursing home residents. Because BPSD are one of the main reasons people with dementia are placed in nursing homes, it is not surprising that rates of antipsychotic use are higher in these settings than in the community. Prior to the black-box warning, studies found that 24% to 32% of nursing home residents were treated with antipsychotics.45-47 A study examining VA nursing homes (n = 133 facilities, n = 3,692 veterans) found that approximately 26% of residents were prescribed antipsychotics in 2004 to 2005.48 The Center for Medicare and Medicaid Services (CMS) National Partnership to Improve Dementia Care in Nursing Homes has appeared to lower antipsychotic medication use in nursing homes; the rate decreased from 24% in long-stay nursing home residents nationwide in 2011 to 19% by the end of 2014. Specific to dementia, a 2010 CMS report49 indicated that approximately 40% of nursing home residents with cognitive impairment and behavioral issues, without psychosis, received antipsychotics. The GAO data indicated that approximately 33% of older Medicare Part D enrollees with dementia who spent >100 days in a nursing home were prescribed an antipsychotic in 2012.44 A recent Canadian study using drug claims data found that overall psychotropic use in patients with dementia remains high, finding that three-fourths of all patients with dementia in long-term care are given at least 1 psychotropic, and up to one-third are prescribed SGAs.50 European data similarly show that antipsychotics continue to be prescribed to up to one-third of long-term care residents with dementia, with 7 out of 10 receiving an SGA.1

Conclusions

The Table provides a summary of the evidence regarding the use of antipsychotics in patients with dementia. Expert consensus is that among BPSD, aggression and psychosis are the primary indications for using antipsychotics.51 Based on multiple RCTs and meta-analyses, the evidence for using SGAs to treat these symptoms is moderate at best. However, in real-world practice settings, SGAs are widely used for symptoms, such as wandering, inappropriate behaviors, resistance to care, etc., for which there is no evidence for efficacy other than sedation. Furthermore, even when there is a potential for benefit, this must be balanced against the risk of adverse effects, including somnolence, worsened cognition, extrapyramidal symptoms, stroke, and mortality.
Clinicians who care for older adults with BPSD should strive to increase the use of first-line nonpharmacologic strategies, by using structured approaches such as DICE (Describe, Investigate, Create, Evaluate) described in the Box.51 Antipsychotics should be reserved for situations in which nonpharmacologic approaches are unsuccessful, or there is concern for serious or imminent risk to the patient or others.
In the future, observational studies using biomarkers, such as neuroimaging markers, of brain health in older patients taking antipsychotics for various durations may give us a better understanding of long-term antipsychotic safety and tolerability and the monitoring required to assess long-term burden of specific antipsychotics in real-world samples.52 However, because of various biases, observational data may not provide answers to all questions,53 and a major challenge is that the number of published RCTs specific to geriatric patients is not growing substantially. Pharmacotherapy evidence is not keeping up with demographic trends. Key developments in RCTs will be the inclusion of biomarkers via neuroimaging, drug serum or brain levels, and genetic profiling. Because of the modest findings of benefits of antipsychotics in dementia and safety concerns addressing brain health in preclinical or early stages, identification of effective non-drug interventions and identifying true disease-modifying agents will be the next challenges of dementia research.
1. Foebel AD, Liperoti R, Onder G, et al; SHELTER Study Investigators. Use of antipsychotic drugs among residents with dementia in European long-term care facilities: results from the SHELTER study. J Am Med Dir Assoc. 2014;15(12):911-917.
2. Foebel A, Ballokova A, Wellens NI, et al. A retrospective, longitudinal study of factors associated with new antipsychotic medication use among recently admitted long-term care residents. BMC Geriatr. 2015;15:128.
3. Parsons C, Johnston S, Mathie E, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging. 2012;29(2):143-155.
4. Vidal X, Agustí A, Vallano A, et al; Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ project. Elderly patients treated with psychotropic medicines admitted to hospital: associated characteristics and inappropriate use. Eur J Clin Pharmacol. 2016;72(6):755-764.
5. Caron L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280-3297.
6. Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with parkinson disease. JAMA Neurol. 2016;73(5):535-541.
7. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532-539.
8. Kales HC, Chen P, Blow FC, et al. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13(6):441-449.
9. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
10. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
11. Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135-1143.
12. Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):1026-1035.
13. Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177.
14. Finkel SI, Burns A. Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update-introduction. International Psychogeriatrics. 2000;12:9-12.
15. Lyketsos CG. Neuropsychiatric symptoms (behavioral and psychological symptoms of dementia) and the development of dementia treatments. Int Psychogeriatr. 2007;19(3):409-420.
16. Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145-1152.
17. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
18. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369.
19. Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553-563.
20. Yury CA, Fisher JE. Meta-analysis of the effectiveness of atypical antipsychotics for the treatment of behavioural problems in persons with dementia. Psychother Psychosom. 2007;76(4):213-218.
21. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191-210.
22. Ballard CG, Waite J. The effectiveness of atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006:1:CD003476.
23. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
24. Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006395. doi: 10.1101/cshperspect.a006395.
25. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
26. Trifirò G, Sultana J, Spina E. Are the safety profiles of antipsychotic drugs used in dementia the same? An updated review of observational studies. Drug Saf. 2014;37(7):501-520.
27. Trifirò G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152(7):418-425, W139-W140.
28. Sultana J, Trifirò G. Drug safety warnings: a message in a bottle. Analysis. 2008;179:438-446.
29. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090-1096.
30. U.S. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafety information forpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed October 20, 2017.
31. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335-2341.
32. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
33. Simoni-Wastila L, Ryder PT, Qian J, et al. Association of antipsychotic use with hospital events and mortality among medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry. 2009;17(5):417-427.
34. Raivio MM, Laurila JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15(5):416-424.
35. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
36. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
37. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
38. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445.
39. Rhee Y, Csernansky JG, Emanuel LL, et al. Psychotropic medication burden and factors associated with antipsychotic use: an analysis of a population-based sample of community-dwelling older persons with dementia. J Am Geriatr Soc. 2011;59(11):2100-2107.
40. Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999-2007. Arch Gen Psychiatry. 2011;68(2):190-197.
41. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
42. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134-143.
43. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269-1270.
44. United States Government Accountability Office. Antipsychotic drug use: HHS has initiatives to reduce use among older adults in nursing homes, but should expand efforts to other settings. http://www.gao.gov/assets/670/668221.pdf. Published January 2015. Accessed October 20, 2017.
45. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89-95.
46. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24(10):1110-1118.
47. Kamble P, Chen H, Sherer J, et al. Antipsychotic drug use among elderly nursing home residents in the United States. Am J Geriatr Pharmacother. 2008;6(4):187-197.
48. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
49. Bonner A. Improving dementia care and reducing unnecessary use of antipsychotic medications in nursing homes. Center for Medicare and Medicaid Services. http://ltcombudsman.org/uploads/files/support/alice-bonner-slides.pdf. Published April 28, 2013. Accessed October 20, 2017.
50. Vasudev A, Shariff SZ, Liu K, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. Am J Geriatr Psychiatry. 2015;23(12):1259-1269.
51. Kales HC, Gitlin LN, Lyketsos CG, et al; Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
52. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615.
53. Mulsant BH. Challenges of the treatment of neuropsychiatric symptoms associated with dementia. Am J Geriatr Psychiatry. 2014;22(4):317-320.
According to the U.S. Department of Health and Human Services, in 2007, 88% of 1.4 million Medicare claims for
Because of the aging population and widespread prescription of antipsychotics to older patients, clinicians need information on the relative risks of using these medications in this population. In the United States, all antipsychotics carry a FDA “black-box” warning of the increased risk of death in older adults with dementia. In addition, the risk of death is increased when prescribing antipsychotics to older adults with other conditions, such as Parkinson’s disease,6 and other safety and tolerability concerns, including falls and fractures, sedation, metabolic abnormalities, and extrapyramidal effects, are highly relevant to geriatric patients.
This 3-part review summarizes findings and recommendations on prescribing antipsychotics to older individuals with schizophrenia, bipolar disorder, depression, and dementia. This third and final installment:
- briefly summarizes the major studies and analyses relevant to prescribing antipsychotics to older patients with dementia
- provides a summative opinion on safety and tolerability issues in these older patients
- highlights the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
Behavioral and psychological symptoms of dementia (BPSD) include agitation, delusional beliefs, repetitive questioning, hallucinations, aggression, wandering, and various socially inappropriate behaviors.7 These occur almost universally in all types and stages of dementia.7 BPSD are among the most complex, stressful, and costly aspects of dementia care, and lead to a myriad of poor health outcomes, including excess morbidity, mortality, hospital stays, and early nursing home placement.8-11 Because BPSD usually occur across all types and stages of dementia,7,12-16 the prevalence of BPSD mirrors the overall prevalence of dementia.
Although all expert organizations, including the American Psychiatric Association,17 recommend nonpharmacologic strategies as first-line treatment for BPSD, for the most part, these recommendations have not been translated into standard clinical management or routine care.18 Because of a perceived lack of other options, the current mainstay of treatment is the off-label use of psychotropics such as antipsychotics. Of all the agents currently used for BPSD, SGAs have the strongest evidence base, although benefits are modest at best (standardized effect size 0.13 to 0.16).19,20 In terms of individual SGAs, only risperidone is indicated for aggression in Canada and in Europe (not in the United States);
Clinical Trials
Adverse effects. A meta-analysis of RCTs of SGAs found that, compared with placebo, SGAs have increased rates of several adverse effects. These include somnolence (17% drug vs 7% placebo;
In the 42-site Clinical Antipsychotic Trials of Intervention Effectiveness Alzheimer’s disease RCT, 421 outpatients with Alzheimer’s disease and BPSD were randomized to an SGA (risperidone,
In the 2005 FDA black-box warning, pneumonia and cardiac adverse effects were cited as primary causes of death for patients with dementia taking SGAs. A subsequent observational study confirmed that use of either FGAs or SGAs in geriatric patients was associated with an increased risk of pneumonia, in a dose-dependent manner.27 Although there is limited data on cardiac adverse effects in older adults, especially those with dementia taking antipsychotics,28 1 observational study of nursing home residents29 found that those taking FGAs had a significantly higher risk of hospitalization for ventricular arrhythmia or cardiac arrest compared with those who were not taking FGAs. In contrast, there was no increased risk with SGAs.
Mortality.
In 2005, the FDA announced that based on a reanalysis of 17 placebo-controlled trials (many of which were unpublished) that SGAs were associated with a 1.7-fold increase in mortality compared with placebo.30 As a result, the FDA issued a black-box warning for using SGAs in patients with dementia. The overall OR in a published meta-analysis of mortality with SGAs was 1.54 (1.06 to 2.23; z = 2.28; P = .02), with pooled events of 3.5% mortality vs 2.3% (drug vs placebo).21 This meta-analysis21 also included ad hoc analyses of haloperidol; using combined data from 2 contrasts of haloperidol (with risperidone and quetiapine; 243 patients receiving haloperidol and 239 receiving placebo) they also found 15 deaths (6.2%) with haloperidol and 9 (3.8%) with placebo, resulting in an OR of 1.68.
Other clinical data
Observational studies. Most observational studies have confirmed concerns regarding increased mortality in patients with BPSD who take antipsychotics, with FGAs having a higher risk than SGAs18,31 and SGAs having a higher risk compared with most other psychotropics.32 Three studies that found no increase in mortality with antipsychotics in patients with dementia had methodological issues, including examining prevalence as opposed to new users,33,34 not controlling for exposure,10,33,34 power issues,10,34 not controlling for other psychiatric medications,10 and varying lengths of follow-up.10 An FDA black-box warning for FGAs was announced in 200830 based on 2 observational studies that showed an increased risk of mortality in older adults taking FGAs vs SGAs.35,36
In terms of specific SGAs, Kales et al37 examined the mortality risk associated with individual antipsychotics using various methods to control for confounding. Among a national sample of >33,000 older veterans with dementia newly started on haloperidol, risperidone, olanzapine, quetiapine, or
Most recently, a retrospective case-control study (90,786 patients age ≥65 with dementia) examined the number needed to harm (NNH; ie, number of patients needed to receive treatment that would result in 1 death) over 180 days following initiation of an FGA or SGA.38 This study found the following NNHs: haloperidol, 26 (95% CI, 15 to 99); risperidone, 27 (95% CI, 19 to 46); olanzapine, 40 (95% CI, 21 to 312); and quetiapine, 50 (95% CI, 30 to 150).38 These results are congruent with a review of observational studies that found the highest risk of mortality was associated with haloperidol and
Patterns of antipsychotic use in older dementia patients
There are high rates of antipsychotic use in patients with dementia. Before the FDA issued the black-box warning, the Aging Demographics and Memory study found that the rate of antipsychotic use in community (outpatient) older adults with dementia was approximately 19% between 2002 and 2004 in a representative sample of 307 older adults.39 Another study examining trends in community antipsychotic use in the U.S. Department of Veterans Affairs (VA) found that in the 1990s, SGA use was increasing; approximately 18% of outpatients with dementia were taking these agents.40 Use of SGAs began to decline in 2003, ahead of the 2005 black-box warning, in tandem with other advisories (eg, diabetes, metabolic syndrome,41 and stroke risk).42,43 Olanzapine and risperidone showed declining rates between 2003 and 2005, whereas quetiapine use significantly increased during this period. All 3 SGAs declined after the black-box warning. However, by the end of 2007, the use of SGAs had leveled off to approximately 12% of VA patients with dementia. A recent U.S. Government Accountability Office (GAO) report found that in 2012, 14% of older adult Medicare Part D enrollees with dementia living in the community were prescribed an antipsychotic.44
Use in nursing home residents. Because BPSD are one of the main reasons people with dementia are placed in nursing homes, it is not surprising that rates of antipsychotic use are higher in these settings than in the community. Prior to the black-box warning, studies found that 24% to 32% of nursing home residents were treated with antipsychotics.45-47 A study examining VA nursing homes (n = 133 facilities, n = 3,692 veterans) found that approximately 26% of residents were prescribed antipsychotics in 2004 to 2005.48 The Center for Medicare and Medicaid Services (CMS) National Partnership to Improve Dementia Care in Nursing Homes has appeared to lower antipsychotic medication use in nursing homes; the rate decreased from 24% in long-stay nursing home residents nationwide in 2011 to 19% by the end of 2014. Specific to dementia, a 2010 CMS report49 indicated that approximately 40% of nursing home residents with cognitive impairment and behavioral issues, without psychosis, received antipsychotics. The GAO data indicated that approximately 33% of older Medicare Part D enrollees with dementia who spent >100 days in a nursing home were prescribed an antipsychotic in 2012.44 A recent Canadian study using drug claims data found that overall psychotropic use in patients with dementia remains high, finding that three-fourths of all patients with dementia in long-term care are given at least 1 psychotropic, and up to one-third are prescribed SGAs.50 European data similarly show that antipsychotics continue to be prescribed to up to one-third of long-term care residents with dementia, with 7 out of 10 receiving an SGA.1

Conclusions

The Table provides a summary of the evidence regarding the use of antipsychotics in patients with dementia. Expert consensus is that among BPSD, aggression and psychosis are the primary indications for using antipsychotics.51 Based on multiple RCTs and meta-analyses, the evidence for using SGAs to treat these symptoms is moderate at best. However, in real-world practice settings, SGAs are widely used for symptoms, such as wandering, inappropriate behaviors, resistance to care, etc., for which there is no evidence for efficacy other than sedation. Furthermore, even when there is a potential for benefit, this must be balanced against the risk of adverse effects, including somnolence, worsened cognition, extrapyramidal symptoms, stroke, and mortality.
Clinicians who care for older adults with BPSD should strive to increase the use of first-line nonpharmacologic strategies, by using structured approaches such as DICE (Describe, Investigate, Create, Evaluate) described in the Box.51 Antipsychotics should be reserved for situations in which nonpharmacologic approaches are unsuccessful, or there is concern for serious or imminent risk to the patient or others.
In the future, observational studies using biomarkers, such as neuroimaging markers, of brain health in older patients taking antipsychotics for various durations may give us a better understanding of long-term antipsychotic safety and tolerability and the monitoring required to assess long-term burden of specific antipsychotics in real-world samples.52 However, because of various biases, observational data may not provide answers to all questions,53 and a major challenge is that the number of published RCTs specific to geriatric patients is not growing substantially. Pharmacotherapy evidence is not keeping up with demographic trends. Key developments in RCTs will be the inclusion of biomarkers via neuroimaging, drug serum or brain levels, and genetic profiling. Because of the modest findings of benefits of antipsychotics in dementia and safety concerns addressing brain health in preclinical or early stages, identification of effective non-drug interventions and identifying true disease-modifying agents will be the next challenges of dementia research.
According to the U.S. Department of Health and Human Services, in 2007, 88% of 1.4 million Medicare claims for
Because of the aging population and widespread prescription of antipsychotics to older patients, clinicians need information on the relative risks of using these medications in this population. In the United States, all antipsychotics carry a FDA “black-box” warning of the increased risk of death in older adults with dementia. In addition, the risk of death is increased when prescribing antipsychotics to older adults with other conditions, such as Parkinson’s disease,6 and other safety and tolerability concerns, including falls and fractures, sedation, metabolic abnormalities, and extrapyramidal effects, are highly relevant to geriatric patients.
This 3-part review summarizes findings and recommendations on prescribing antipsychotics to older individuals with schizophrenia, bipolar disorder, depression, and dementia. This third and final installment:
- briefly summarizes the major studies and analyses relevant to prescribing antipsychotics to older patients with dementia
- provides a summative opinion on safety and tolerability issues in these older patients
- highlights the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
Behavioral and psychological symptoms of dementia (BPSD) include agitation, delusional beliefs, repetitive questioning, hallucinations, aggression, wandering, and various socially inappropriate behaviors.7 These occur almost universally in all types and stages of dementia.7 BPSD are among the most complex, stressful, and costly aspects of dementia care, and lead to a myriad of poor health outcomes, including excess morbidity, mortality, hospital stays, and early nursing home placement.8-11 Because BPSD usually occur across all types and stages of dementia,7,12-16 the prevalence of BPSD mirrors the overall prevalence of dementia.
Although all expert organizations, including the American Psychiatric Association,17 recommend nonpharmacologic strategies as first-line treatment for BPSD, for the most part, these recommendations have not been translated into standard clinical management or routine care.18 Because of a perceived lack of other options, the current mainstay of treatment is the off-label use of psychotropics such as antipsychotics. Of all the agents currently used for BPSD, SGAs have the strongest evidence base, although benefits are modest at best (standardized effect size 0.13 to 0.16).19,20 In terms of individual SGAs, only risperidone is indicated for aggression in Canada and in Europe (not in the United States);
Clinical Trials
Adverse effects. A meta-analysis of RCTs of SGAs found that, compared with placebo, SGAs have increased rates of several adverse effects. These include somnolence (17% drug vs 7% placebo;
In the 42-site Clinical Antipsychotic Trials of Intervention Effectiveness Alzheimer’s disease RCT, 421 outpatients with Alzheimer’s disease and BPSD were randomized to an SGA (risperidone,
In the 2005 FDA black-box warning, pneumonia and cardiac adverse effects were cited as primary causes of death for patients with dementia taking SGAs. A subsequent observational study confirmed that use of either FGAs or SGAs in geriatric patients was associated with an increased risk of pneumonia, in a dose-dependent manner.27 Although there is limited data on cardiac adverse effects in older adults, especially those with dementia taking antipsychotics,28 1 observational study of nursing home residents29 found that those taking FGAs had a significantly higher risk of hospitalization for ventricular arrhythmia or cardiac arrest compared with those who were not taking FGAs. In contrast, there was no increased risk with SGAs.
Mortality.
In 2005, the FDA announced that based on a reanalysis of 17 placebo-controlled trials (many of which were unpublished) that SGAs were associated with a 1.7-fold increase in mortality compared with placebo.30 As a result, the FDA issued a black-box warning for using SGAs in patients with dementia. The overall OR in a published meta-analysis of mortality with SGAs was 1.54 (1.06 to 2.23; z = 2.28; P = .02), with pooled events of 3.5% mortality vs 2.3% (drug vs placebo).21 This meta-analysis21 also included ad hoc analyses of haloperidol; using combined data from 2 contrasts of haloperidol (with risperidone and quetiapine; 243 patients receiving haloperidol and 239 receiving placebo) they also found 15 deaths (6.2%) with haloperidol and 9 (3.8%) with placebo, resulting in an OR of 1.68.
Other clinical data
Observational studies. Most observational studies have confirmed concerns regarding increased mortality in patients with BPSD who take antipsychotics, with FGAs having a higher risk than SGAs18,31 and SGAs having a higher risk compared with most other psychotropics.32 Three studies that found no increase in mortality with antipsychotics in patients with dementia had methodological issues, including examining prevalence as opposed to new users,33,34 not controlling for exposure,10,33,34 power issues,10,34 not controlling for other psychiatric medications,10 and varying lengths of follow-up.10 An FDA black-box warning for FGAs was announced in 200830 based on 2 observational studies that showed an increased risk of mortality in older adults taking FGAs vs SGAs.35,36
In terms of specific SGAs, Kales et al37 examined the mortality risk associated with individual antipsychotics using various methods to control for confounding. Among a national sample of >33,000 older veterans with dementia newly started on haloperidol, risperidone, olanzapine, quetiapine, or
Most recently, a retrospective case-control study (90,786 patients age ≥65 with dementia) examined the number needed to harm (NNH; ie, number of patients needed to receive treatment that would result in 1 death) over 180 days following initiation of an FGA or SGA.38 This study found the following NNHs: haloperidol, 26 (95% CI, 15 to 99); risperidone, 27 (95% CI, 19 to 46); olanzapine, 40 (95% CI, 21 to 312); and quetiapine, 50 (95% CI, 30 to 150).38 These results are congruent with a review of observational studies that found the highest risk of mortality was associated with haloperidol and
Patterns of antipsychotic use in older dementia patients
There are high rates of antipsychotic use in patients with dementia. Before the FDA issued the black-box warning, the Aging Demographics and Memory study found that the rate of antipsychotic use in community (outpatient) older adults with dementia was approximately 19% between 2002 and 2004 in a representative sample of 307 older adults.39 Another study examining trends in community antipsychotic use in the U.S. Department of Veterans Affairs (VA) found that in the 1990s, SGA use was increasing; approximately 18% of outpatients with dementia were taking these agents.40 Use of SGAs began to decline in 2003, ahead of the 2005 black-box warning, in tandem with other advisories (eg, diabetes, metabolic syndrome,41 and stroke risk).42,43 Olanzapine and risperidone showed declining rates between 2003 and 2005, whereas quetiapine use significantly increased during this period. All 3 SGAs declined after the black-box warning. However, by the end of 2007, the use of SGAs had leveled off to approximately 12% of VA patients with dementia. A recent U.S. Government Accountability Office (GAO) report found that in 2012, 14% of older adult Medicare Part D enrollees with dementia living in the community were prescribed an antipsychotic.44
Use in nursing home residents. Because BPSD are one of the main reasons people with dementia are placed in nursing homes, it is not surprising that rates of antipsychotic use are higher in these settings than in the community. Prior to the black-box warning, studies found that 24% to 32% of nursing home residents were treated with antipsychotics.45-47 A study examining VA nursing homes (n = 133 facilities, n = 3,692 veterans) found that approximately 26% of residents were prescribed antipsychotics in 2004 to 2005.48 The Center for Medicare and Medicaid Services (CMS) National Partnership to Improve Dementia Care in Nursing Homes has appeared to lower antipsychotic medication use in nursing homes; the rate decreased from 24% in long-stay nursing home residents nationwide in 2011 to 19% by the end of 2014. Specific to dementia, a 2010 CMS report49 indicated that approximately 40% of nursing home residents with cognitive impairment and behavioral issues, without psychosis, received antipsychotics. The GAO data indicated that approximately 33% of older Medicare Part D enrollees with dementia who spent >100 days in a nursing home were prescribed an antipsychotic in 2012.44 A recent Canadian study using drug claims data found that overall psychotropic use in patients with dementia remains high, finding that three-fourths of all patients with dementia in long-term care are given at least 1 psychotropic, and up to one-third are prescribed SGAs.50 European data similarly show that antipsychotics continue to be prescribed to up to one-third of long-term care residents with dementia, with 7 out of 10 receiving an SGA.1

Conclusions

The Table provides a summary of the evidence regarding the use of antipsychotics in patients with dementia. Expert consensus is that among BPSD, aggression and psychosis are the primary indications for using antipsychotics.51 Based on multiple RCTs and meta-analyses, the evidence for using SGAs to treat these symptoms is moderate at best. However, in real-world practice settings, SGAs are widely used for symptoms, such as wandering, inappropriate behaviors, resistance to care, etc., for which there is no evidence for efficacy other than sedation. Furthermore, even when there is a potential for benefit, this must be balanced against the risk of adverse effects, including somnolence, worsened cognition, extrapyramidal symptoms, stroke, and mortality.
Clinicians who care for older adults with BPSD should strive to increase the use of first-line nonpharmacologic strategies, by using structured approaches such as DICE (Describe, Investigate, Create, Evaluate) described in the Box.51 Antipsychotics should be reserved for situations in which nonpharmacologic approaches are unsuccessful, or there is concern for serious or imminent risk to the patient or others.
In the future, observational studies using biomarkers, such as neuroimaging markers, of brain health in older patients taking antipsychotics for various durations may give us a better understanding of long-term antipsychotic safety and tolerability and the monitoring required to assess long-term burden of specific antipsychotics in real-world samples.52 However, because of various biases, observational data may not provide answers to all questions,53 and a major challenge is that the number of published RCTs specific to geriatric patients is not growing substantially. Pharmacotherapy evidence is not keeping up with demographic trends. Key developments in RCTs will be the inclusion of biomarkers via neuroimaging, drug serum or brain levels, and genetic profiling. Because of the modest findings of benefits of antipsychotics in dementia and safety concerns addressing brain health in preclinical or early stages, identification of effective non-drug interventions and identifying true disease-modifying agents will be the next challenges of dementia research.
1. Foebel AD, Liperoti R, Onder G, et al; SHELTER Study Investigators. Use of antipsychotic drugs among residents with dementia in European long-term care facilities: results from the SHELTER study. J Am Med Dir Assoc. 2014;15(12):911-917.
2. Foebel A, Ballokova A, Wellens NI, et al. A retrospective, longitudinal study of factors associated with new antipsychotic medication use among recently admitted long-term care residents. BMC Geriatr. 2015;15:128.
3. Parsons C, Johnston S, Mathie E, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging. 2012;29(2):143-155.
4. Vidal X, Agustí A, Vallano A, et al; Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ project. Elderly patients treated with psychotropic medicines admitted to hospital: associated characteristics and inappropriate use. Eur J Clin Pharmacol. 2016;72(6):755-764.
5. Caron L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280-3297.
6. Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with parkinson disease. JAMA Neurol. 2016;73(5):535-541.
7. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532-539.
8. Kales HC, Chen P, Blow FC, et al. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13(6):441-449.
9. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
10. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
11. Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135-1143.
12. Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):1026-1035.
13. Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177.
14. Finkel SI, Burns A. Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update-introduction. International Psychogeriatrics. 2000;12:9-12.
15. Lyketsos CG. Neuropsychiatric symptoms (behavioral and psychological symptoms of dementia) and the development of dementia treatments. Int Psychogeriatr. 2007;19(3):409-420.
16. Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145-1152.
17. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
18. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369.
19. Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553-563.
20. Yury CA, Fisher JE. Meta-analysis of the effectiveness of atypical antipsychotics for the treatment of behavioural problems in persons with dementia. Psychother Psychosom. 2007;76(4):213-218.
21. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191-210.
22. Ballard CG, Waite J. The effectiveness of atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006:1:CD003476.
23. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
24. Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006395. doi: 10.1101/cshperspect.a006395.
25. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
26. Trifirò G, Sultana J, Spina E. Are the safety profiles of antipsychotic drugs used in dementia the same? An updated review of observational studies. Drug Saf. 2014;37(7):501-520.
27. Trifirò G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152(7):418-425, W139-W140.
28. Sultana J, Trifirò G. Drug safety warnings: a message in a bottle. Analysis. 2008;179:438-446.
29. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090-1096.
30. U.S. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafety information forpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed October 20, 2017.
31. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335-2341.
32. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
33. Simoni-Wastila L, Ryder PT, Qian J, et al. Association of antipsychotic use with hospital events and mortality among medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry. 2009;17(5):417-427.
34. Raivio MM, Laurila JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15(5):416-424.
35. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
36. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
37. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
38. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445.
39. Rhee Y, Csernansky JG, Emanuel LL, et al. Psychotropic medication burden and factors associated with antipsychotic use: an analysis of a population-based sample of community-dwelling older persons with dementia. J Am Geriatr Soc. 2011;59(11):2100-2107.
40. Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999-2007. Arch Gen Psychiatry. 2011;68(2):190-197.
41. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
42. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134-143.
43. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269-1270.
44. United States Government Accountability Office. Antipsychotic drug use: HHS has initiatives to reduce use among older adults in nursing homes, but should expand efforts to other settings. http://www.gao.gov/assets/670/668221.pdf. Published January 2015. Accessed October 20, 2017.
45. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89-95.
46. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24(10):1110-1118.
47. Kamble P, Chen H, Sherer J, et al. Antipsychotic drug use among elderly nursing home residents in the United States. Am J Geriatr Pharmacother. 2008;6(4):187-197.
48. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
49. Bonner A. Improving dementia care and reducing unnecessary use of antipsychotic medications in nursing homes. Center for Medicare and Medicaid Services. http://ltcombudsman.org/uploads/files/support/alice-bonner-slides.pdf. Published April 28, 2013. Accessed October 20, 2017.
50. Vasudev A, Shariff SZ, Liu K, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. Am J Geriatr Psychiatry. 2015;23(12):1259-1269.
51. Kales HC, Gitlin LN, Lyketsos CG, et al; Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
52. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615.
53. Mulsant BH. Challenges of the treatment of neuropsychiatric symptoms associated with dementia. Am J Geriatr Psychiatry. 2014;22(4):317-320.
1. Foebel AD, Liperoti R, Onder G, et al; SHELTER Study Investigators. Use of antipsychotic drugs among residents with dementia in European long-term care facilities: results from the SHELTER study. J Am Med Dir Assoc. 2014;15(12):911-917.
2. Foebel A, Ballokova A, Wellens NI, et al. A retrospective, longitudinal study of factors associated with new antipsychotic medication use among recently admitted long-term care residents. BMC Geriatr. 2015;15:128.
3. Parsons C, Johnston S, Mathie E, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging. 2012;29(2):143-155.
4. Vidal X, Agustí A, Vallano A, et al; Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ project. Elderly patients treated with psychotropic medicines admitted to hospital: associated characteristics and inappropriate use. Eur J Clin Pharmacol. 2016;72(6):755-764.
5. Caron L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280-3297.
6. Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with parkinson disease. JAMA Neurol. 2016;73(5):535-541.
7. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532-539.
8. Kales HC, Chen P, Blow FC, et al. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13(6):441-449.
9. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
10. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
11. Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135-1143.
12. Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):1026-1035.
13. Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177.
14. Finkel SI, Burns A. Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update-introduction. International Psychogeriatrics. 2000;12:9-12.
15. Lyketsos CG. Neuropsychiatric symptoms (behavioral and psychological symptoms of dementia) and the development of dementia treatments. Int Psychogeriatr. 2007;19(3):409-420.
16. Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145-1152.
17. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
18. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369.
19. Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553-563.
20. Yury CA, Fisher JE. Meta-analysis of the effectiveness of atypical antipsychotics for the treatment of behavioural problems in persons with dementia. Psychother Psychosom. 2007;76(4):213-218.
21. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191-210.
22. Ballard CG, Waite J. The effectiveness of atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006:1:CD003476.
23. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
24. Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006395. doi: 10.1101/cshperspect.a006395.
25. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
26. Trifirò G, Sultana J, Spina E. Are the safety profiles of antipsychotic drugs used in dementia the same? An updated review of observational studies. Drug Saf. 2014;37(7):501-520.
27. Trifirò G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152(7):418-425, W139-W140.
28. Sultana J, Trifirò G. Drug safety warnings: a message in a bottle. Analysis. 2008;179:438-446.
29. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090-1096.
30. U.S. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafety information forpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed October 20, 2017.
31. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335-2341.
32. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
33. Simoni-Wastila L, Ryder PT, Qian J, et al. Association of antipsychotic use with hospital events and mortality among medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry. 2009;17(5):417-427.
34. Raivio MM, Laurila JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15(5):416-424.
35. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
36. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
37. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
38. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445.
39. Rhee Y, Csernansky JG, Emanuel LL, et al. Psychotropic medication burden and factors associated with antipsychotic use: an analysis of a population-based sample of community-dwelling older persons with dementia. J Am Geriatr Soc. 2011;59(11):2100-2107.
40. Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999-2007. Arch Gen Psychiatry. 2011;68(2):190-197.
41. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
42. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134-143.
43. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269-1270.
44. United States Government Accountability Office. Antipsychotic drug use: HHS has initiatives to reduce use among older adults in nursing homes, but should expand efforts to other settings. http://www.gao.gov/assets/670/668221.pdf. Published January 2015. Accessed October 20, 2017.
45. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89-95.
46. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24(10):1110-1118.
47. Kamble P, Chen H, Sherer J, et al. Antipsychotic drug use among elderly nursing home residents in the United States. Am J Geriatr Pharmacother. 2008;6(4):187-197.
48. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
49. Bonner A. Improving dementia care and reducing unnecessary use of antipsychotic medications in nursing homes. Center for Medicare and Medicaid Services. http://ltcombudsman.org/uploads/files/support/alice-bonner-slides.pdf. Published April 28, 2013. Accessed October 20, 2017.
50. Vasudev A, Shariff SZ, Liu K, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. Am J Geriatr Psychiatry. 2015;23(12):1259-1269.
51. Kales HC, Gitlin LN, Lyketsos CG, et al; Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
52. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615.
53. Mulsant BH. Challenges of the treatment of neuropsychiatric symptoms associated with dementia. Am J Geriatr Psychiatry. 2014;22(4):317-320.
Sexting: What are the clinical and legal implications?
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5
Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).
How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5
Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).
How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
Sexting includes sending sexually explicit (or sexually suggestive) text messages and photos, usually by cell phone. This article focuses on sexts involving photos. Cell phones are almost ubiquitous among American teens, and with technological advances, sexts are getting easier to send. Sexting may occur to initiate a relationship or sustain one. Some teenagers are coerced into sexting. Many people do not realize the potential long-term consequences of sexting—particularly because of the impulsive nature of sexting and the belief that the behavior is harmless.
Media attention has recently focused on teens who face legal charges related to sexting. Sexting photos may be considered child pornography—even though the teens made it themselves. There are also social consequences to sexting. Photos meant to be private are sometimes forwarded to others. Cyberbullying is not uncommon with teen sexting, and suicides after experiencing this behavior have been reported.
Sexting may be a form of modern flirtation, but in some cases, it may be a marker of other risk behaviors, such as substance abuse. Psychiatrists must be aware of the frequency and meaning of this potentially dangerous behavior. Clinicians should feel comfortable asking their patients about it and provide education and counseling.
CASE
Private photos get shared
K, age 14, a freshman with no psychiatric history, is referred to you by her school psychologist for evaluation of suicidal ideation. K reports depressed mood, poor sleep, inattention, loss of appetite, anhedonia, and feelings of guilt for the past month. She recently ended a relationship with her boyfriend of 1 year after she learned that he had shared with his friends naked photos of her that she had sent him. The school administration learned of the photos when a student posted them on one of the school computers.
K’s boyfriend, age 16, was suspended after the school learned that he had shared the photos without K’s consent. K, who is a good student, missed several days of school, including cheerleading practice; previously she had never missed a day of school.
On evaluation, K is tearful, stating that her life is “over.” She says that her ex-boyfriend’s friends are harassing her, calling her “slut” and making sexual comments. She also feels guilty, because she learned that the police interviewed her ex-boyfriend in connection with posting her photos on the Internet. In a text, he said he “might get charged with child pornography.” On further questioning, K confides that she had naked photos of her ex-boyfriend on her phone. She admits to sharing the pictures with her best friend, because she was “angry and wanted to get back” at her ex-boyfriend. She also reports a several-month history of sexting with her ex-boyfriend. K deleted the photos and texts after learning that her ex-boyfriend “was in trouble with the police.”
K has no prior sexual experience. She dated 1 boy her age prior to her ex-boyfriend. She had never been evaluated by a mental health clinician. She is dysphoric and reports feeling “hopeless … Unless this can be erased, I can’t go back to school.”
Sexting: What is the extent of the problem?
The true prevalence of sexting is difficult to ascertain, because different studies have used different definitions and methodologies. However, the rates are far from negligible. Sexting rates increase with age, over the teen years.1-3 Among American minors, 2.5% to 28% of middle school and high school students report that they have sent a sext (Table 11-9). Studies of American young adults (age ≥18) and university students have found 30% to 60% have sent sexts, and >40% have received a sext.4,5
Many people receive sexts—including individuals who are not the intended recipient. In 1 study, although most teens intended to share sexts only with their boyfriend/girlfriend, 25% to 33% had received sext photos intended for other people.6 In another recent study, 25% of teens had forwarded a sext that they received.7 Moreover, 12% of teenage boys and 5% of teenage girls had sent a sexually explicit photo that they took of another teen to a third person.7 Forwarding sexts can add exponentially to the psychosocial risks of the photographed teenager.
Who sexts? Current research indicates that the likelihood of sexting is related to age, personality, and social situation. Teens are approaching the peak age of their sex drive, and often are curious and feel invincible. Teens are more impulsive than adults. When it takes less than a minute to send a sext, irreversible poor choices can be made quickly. Teens who send sexts often engage in more text messaging than other teens.7
Teens may use sexting to initiate or sustain a relationship. Sexts also may be sent because of coercion. More than one-half of girls cited pressure to sext from a boy.6 Temple et al3 found that more than one-half of their study sample had been asked to send a sext. Girls were more likely than boys to be asked to send a sext; most were troubled by this.
One study that assessed knowledge of potential legal consequences of sexting found that many teens who sent sexts were aware of the potential consequences.7 Regarding personality traits, sexting among undergraduates was predicted by neuroticism and low agreeableness.10 Conversely, sending text messages with sexually suggestive writing was predicted by extraversion and problematic cell phone use.
Comorbidities. There are mixed findings about whether sexting is simply a modern dating strategy or a marker of other risk behaviors; age may play an important discriminating role. Sexual activity appears to be correlated with sexting. According to Temple and Choi,11 “Sexting fits within the context of adolescent sexual development and may be a viable indicator of adolescent sexual activity.”11
Some authors have suggested that sexting is a contemporary risk behavior that is likely to correlate with other risk behaviors. Among young teens—seventh graders who were referred to a risk prevention trial because of behavioral/emotional difficulties—those who sexted were more likely to engage in early sexual behaviors.8 These younger at-risk teens also had less understanding of their emotions and greater difficulty in regulating their emotions.
Among the general population of high school students, teens who sext are more likely to be sexually active.3 High school girls who engaged in sexting were noted to engage in other risk behaviors, including having multiple partners in the past year and using alcohol or drugs before sex.3 Teens who had sent a sext were more likely to be sexually active 1 year later than teens who had not.11Studies of sexting among university students also have had mixed findings. One study found that among undergraduates, sexting was associated with substance use and other risk behaviors.9 Another young adult study found sexting was not related to sexual risk or psychological well-being.4
Legal issues affect psychiatrists as well as patients
As a psychiatrist evaluating K, what are your duties as a mandated reporter? Psychiatrists are legally required to report suspected maltreatment or abuse of children.12 The circumstances under which psychiatrists may have a mandate to report include when a psychiatrist:
- evaluates a child and suspects abuse
- suspects child abuse based on an adult patient’s report
- learns from a third party that a child may have been/is being abused.
Psychiatrists usually are not mandated to report other types of potentially criminal behavior. As such, reporting sexting might be considered a breach of confidentiality. Psychiatrists should be familiar with the specific reporting guidelines for the jurisdiction in which they practice. Psychiatrists who work with individuals who commit crimes should focus on changing the potentially dangerous behaviors rather than reporting them.
Does the transmission of naked photos of a minor in a sexual pose or act constitute child pornography or another criminal offense? The legal answer varies, but the role of the psychiatrist does not. Psychiatrists should educate their patients about potentially dangerous behaviors.
With regards to the legal consequences, some states classify underage sexting photos as child pornography. Others have less rigid definitions of child pornography and take into account the age of the participants and their intent. Such jurisdictions point out that sexting naked photos among adolescents is “age appropriate.” Some have enacted specific sexting laws to address the transmission of obscene material to a child through the Internet. In some jurisdictions, sexting laws are categorized to refer to behavior of individuals under or over age 18. The term “revenge porn” is used to refer to nonconsensual pornography with its dissemination motivated by spite.13 Some states have defined specific revenge porn laws to address the behavior. Currently, 20 states have sexting laws and 26 states have revenge porn laws.14 Twenty states address a minor age <18 sending the photo, while only 18 address the recipient. The law in this area can be complex and detailed, taking into account the age of the sender, the intentions of the sender, and the nature of the relationship between the sender and the recipient and the behavior of the recipient.
Laws regarding sexting vary greatly. Sexting may be a misdemeanor or a felony, depending on the state, the specific behavior, and the frequency. In the United States, 11 state laws include a diversion remedy—an option to pursue the case outside of the criminal juvenile system; 10 laws require counseling or another informal sanction; 11 states laws have the potential for misdemeanor punishment; and 4 state laws have the potential for felony punishment.14 Depending on the criminal charge, the perpetrator may have to register as a sex offender. For example, in some jurisdictions, a conviction for possession of child pornography requires sex offender registration. Thirty-eight states include juvenile sex offenders in their sex offender registries. Other states require juveniles to register if they are age ≥15 years or have been tried as an adult.15
The frequency of police involvement in sexting cases also greatly varies. A national study examining the characteristics of youth sexting cases revealed that law enforcement agencies handled approximately 3,477 cases of youth-produced sexual photos in 2008 and 2009.16 Situations that involved an adult or a minor engaged in malicious, nonconsensual, or abusive behavior comprised two-thirds of cases. Arrests occurred in 62% of the adult-involved cases and 36% of the aggravated youth-only cases. Arrests occurred in only 18% of investigated non-aggravated youth-only cases. Table 2 describes recent American sexting legal cases and their outcomes.
In K’s case, depending on the jurisdiction, K or her ex-boyfriend may be subject to arrest for child pornography, revenge pornography, or sexting.
Potential social and psychiatric consequences
What are the social and psychiatric ramifications for K? Aside from potential legal consequences of sexting, K is experiencing psychological and social consequences. She has developed depressive symptoms and suicidal ideation. Her ex-boyfriend’s dissemination of her nude photos on the school computer could be interpreted as cyberbullying. (The National Center for Missing and Exploited Children defines cyberbullying as “bullying through the use of technology or electronic devices such as telephones, cell phones, computers, and the Internet.”17 All 50 states have enacted laws against bullying; 48 states have electronic harassment in their bullying laws; and 22 states have laws specifically referencing “cyberbullying.”)
Her depressive symptoms developed in response to her feelings of guilt and shame related to sexting as well as the subsequent peer harassment. She is refusing to return to school because of her concerns about bullying. A careful inquiry into suicidality should be part of the evaluation when sexting has led to psychiatric symptoms. Several cases of sexting and cyberbullying have ended in suicide (Table 3).
How to ask patients about sexting
To screen patients for sexting, clinicians need to develop a new skill set, which at first may be uncomfortable. However, the questions to ask are not all that different from other questions about adolescent and young adult sexuality. The importance of patients seeing that we as physicians are comfortable with the topic and approachable about their sexual health cannot be overemphasized. When discussing sexting with patients, it is essential to:
- explain that you are asking questions about their sexual health because they are important to overall health
- engage patients in discussion in a nonthreatening and nonjudgmental way
- develop rapport so patients feel comfortable disclosing behavior that may be embarrassing
- listen to their stories and build a context for understanding their experiences. As you listen, ask questions when needed to help move the story along.
Sometimes when asking about topics that are uncomfortable, clinicians revert from open-ended to closed-ended questions, but when asking about a patient’s sexual life, it is especially important to be open-ended and ask questions in a nonjudgmental way. Contextualizing sexual questions by (for example) asking them while discussing the teen’s relationships will make them seem more natural.18 To best understand, inquire explicitly about specific behaviors, but do so without appearing voyeuristic.18
Sexting may precede sexual intercourse. Keep in mind that a patient may report that she (he) is not sexually active but still may be involved in sexting. Therefore, discuss sexting even if your patient reports not being sexually active. By understanding the prevalence of sexting among teens, you can ask questions in a normalizing way. Clinicians can inquire about sexting while discussing relationships and dating or online risk behaviors.
Also consider whether any of your patient’s sexual behaviors, including sexting, are the result of coercion: “Some of my patients tell me they feel pressured or coerced into having sex. Have you ever felt this way?”19 and “Have you ever been picked on or bullied? Is that still a problem?” are suggested safety screening questions about bullying,18 and one can also ask about specific cyberbullying behaviors.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.
1. Mitchell KJ, Finkelhor D, Jones LM, et al. Prevalence and characteristics of youth sexting: a national study. Pediatrics. 2012;129(1):13-20.
2. Lenhart A. Teens and sexting. The Pew Research Center. http://www.pewinternet.org/2009/12/15/teens-and-sexting. Published December 15, 2009. Accessed October 31, 2017.
3. Temple JR, Paul JA, van den Berg P, et al. Teen sexting and its association with sexual behaviors. Arch Pediatr Adolesc Med. 2012;166(9):828-833.
4. Gordon-Messer D, Bauermeister JA, Grodzinski A, et al. Sexting among young adults. J Adolesc Health. 2013;52(3):301-306.
5. Henderson L. Sexting and sexual relationships among teens and young adults. McNair Scholars Research Journal. 2011;7(1):31-39.
6. The National Campaign to Prevent Teen and Unplanned Pregnancy. Sex and tech: results from a survey of teens and young adults. https://thenationalcampaign.org/sites/default/files/resource-primary-download/sex_and_tech_summary.pdf. Published December 2008. Accessed October 31, 2017.
7. Strassberg DS, McKinnon RK, Sustaíta MA, et al. Sexting by high school students: an exploratory and descriptive study. Arch Sex Behav. 2013;42(1):15-21.
8. Houck CD, Barker D, Rizzo C, et al. Sexting and sexual behavior in at-risk adolescents. Pediatrics. 2014;133(2):e276-e282.
9. Benotsch EG, Snipes DJ, Martin AM, et al. Sexting, substance use, and sexual risk behavior in young adults. J Adolesc Health. 2013;52(3):307-313.
10. Delevi R, Weisskirch RS. Personality factors as predictors of sexting. Comput Human Behav. 2013;29(6):2589-2594.
11. Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134(5):1287-1292.
12. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psych Clin North Am. 2016;39(4):691-700.
13. Citron DK, Franks MA. Criminalizing revenge porn. Wake Forest Law Review. 2014;49:345-391.
14. Hinduja S, Patchin JW. State cyberbullying laws: a brief review of state cyberbullying laws and policies. Cyberbullying Research Center. https://cyberbullying.org/Bullying-and-Cyberbullying-Laws.pdf. Updated 2016. Accessed October 31, 2017.
15. Beitsch R. States slowly scale back juvenile sex offender registries. The Pew Charitable Trusts. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2015/11/19/states-slowly-scale-back-juvenile-sex-offender-registries. Published November 19, 2015. Accessed October 31, 2017.
16. Wolak J, Finkelhor D, Mitchell KJ. How often are teens arrested for sexting? Data from a national sample of police cases. Pediatrics. 2012;129(1):4-12.
17. The Campus School at Boston College. Bullying prevention policy. https://www.bc.edu/bc-web/schools/lsoe/sites/campus-school/who-we-are/policies-and-procedures/bullying-prevention-policy.html. Accessed October 31, 2017.
18. Goldenring JM, Rosen DS. Getting into adolescent heads: an essential update. Contemporary Pediatrics. 2004;21(1):64.
19. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: the psychosocial interview for adolescents updated for a new century fueled by media. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/content/tags/adolescent-medicine/heeadsss-30-psychosocial-interview-adolesce?page=full. Published January 1, 2014. Accessed October 31, 2017.