User login
6 Brief exercises for introducing mindfulness
Mindfulness is actively being aware of one’s inner and outer environments in the present moment. Core mindfulness skills include observation, description, participation, a nonjudgmental approach, focusing on 1 thing at a time, and effectiveness.1
Psychotherapeutic interventions based on each of these skills have been developed to instill a mindful state in psychiatric patients. Evidence suggests these interventions can be helpful when treating borderline personality disorder, somatization, pain, depression, and anxiety, among other conditions.2
Elements of mindfulness can be integrated into brief interventions. The following 6 simple, practical exercises can be used to help patients develop these skills.
Observation involves noticing internal and external experiences, including thoughts and sensations, without applying words or labels. Guide your patient through the following exercise:
Focus your attention on the ground beneath your feet, feeling the pressure, temperature, and texture of this sensation. Do the same with your seat, your breath, and the sounds, sights, and smells of the room. Be aware of your thoughts and watch them come and go like fish in a fishbowl.
Description entails assigning purely descriptive words to one’s observations. To help your patient develop this skill, ask him (her) to describe the sensations he (she) observed in the previous exercise.
Participation entails immersive engagement in an activity. Ask your patient to listen to a song he has never heard before, and then play it again and dance or sing along. Instruct him to engage wholly, conscious of each step or note, without being judgmental or self-conscious. If he feels embarrassed or self-critical, tell him to observe these thoughts and emotions, put them aside, and return to the activity.
A nonjudgmental approach consists of separating out the facts and recognizing emotional responses without clinging to them. To practice this skill, ask your patient to play a song that he likes and one that he dislikes. The patient should listen to each, observing and describing the way they sound without judgment. Tell the patient that if judgmental words or phrases, such as “beautiful,” “ugly,” “I love…,” or “I hate…,” appear as thoughts, he should observe them, put them aside, and then return to nonjudgmental description and observation.
Focusing on 1 thing at a time means dedicating complete attention to a single task, activity, or thought. Give your patient a short paragraph or poem to read. Instruct
Effectiveness involves focusing on what works to attain one’s goals. For this exercise, set up a task for your patient by placing several items in a location that is neither immediately obvious nor readily accessible without an intermediate step. Instruct your patient to obtain these objects. Then guide them as follows:
What do you have to do to get them? Ask permission? Borrow a key? Recruit assistance? Determine the location? Brainstorm ways to obtain the items, and then complete the task.
1. Linehan MM. DBT skills training manual. 2nd ed. New York, NY: The Guilford Press; 2015.
2. Gotink RA, Chu P, Busschbach JJ, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. doi: 10.1371/journal.pone.0124344.
Mindfulness is actively being aware of one’s inner and outer environments in the present moment. Core mindfulness skills include observation, description, participation, a nonjudgmental approach, focusing on 1 thing at a time, and effectiveness.1
Psychotherapeutic interventions based on each of these skills have been developed to instill a mindful state in psychiatric patients. Evidence suggests these interventions can be helpful when treating borderline personality disorder, somatization, pain, depression, and anxiety, among other conditions.2
Elements of mindfulness can be integrated into brief interventions. The following 6 simple, practical exercises can be used to help patients develop these skills.
Observation involves noticing internal and external experiences, including thoughts and sensations, without applying words or labels. Guide your patient through the following exercise:
Focus your attention on the ground beneath your feet, feeling the pressure, temperature, and texture of this sensation. Do the same with your seat, your breath, and the sounds, sights, and smells of the room. Be aware of your thoughts and watch them come and go like fish in a fishbowl.
Description entails assigning purely descriptive words to one’s observations. To help your patient develop this skill, ask him (her) to describe the sensations he (she) observed in the previous exercise.
Participation entails immersive engagement in an activity. Ask your patient to listen to a song he has never heard before, and then play it again and dance or sing along. Instruct him to engage wholly, conscious of each step or note, without being judgmental or self-conscious. If he feels embarrassed or self-critical, tell him to observe these thoughts and emotions, put them aside, and return to the activity.
A nonjudgmental approach consists of separating out the facts and recognizing emotional responses without clinging to them. To practice this skill, ask your patient to play a song that he likes and one that he dislikes. The patient should listen to each, observing and describing the way they sound without judgment. Tell the patient that if judgmental words or phrases, such as “beautiful,” “ugly,” “I love…,” or “I hate…,” appear as thoughts, he should observe them, put them aside, and then return to nonjudgmental description and observation.
Focusing on 1 thing at a time means dedicating complete attention to a single task, activity, or thought. Give your patient a short paragraph or poem to read. Instruct
Effectiveness involves focusing on what works to attain one’s goals. For this exercise, set up a task for your patient by placing several items in a location that is neither immediately obvious nor readily accessible without an intermediate step. Instruct your patient to obtain these objects. Then guide them as follows:
What do you have to do to get them? Ask permission? Borrow a key? Recruit assistance? Determine the location? Brainstorm ways to obtain the items, and then complete the task.
Mindfulness is actively being aware of one’s inner and outer environments in the present moment. Core mindfulness skills include observation, description, participation, a nonjudgmental approach, focusing on 1 thing at a time, and effectiveness.1
Psychotherapeutic interventions based on each of these skills have been developed to instill a mindful state in psychiatric patients. Evidence suggests these interventions can be helpful when treating borderline personality disorder, somatization, pain, depression, and anxiety, among other conditions.2
Elements of mindfulness can be integrated into brief interventions. The following 6 simple, practical exercises can be used to help patients develop these skills.
Observation involves noticing internal and external experiences, including thoughts and sensations, without applying words or labels. Guide your patient through the following exercise:
Focus your attention on the ground beneath your feet, feeling the pressure, temperature, and texture of this sensation. Do the same with your seat, your breath, and the sounds, sights, and smells of the room. Be aware of your thoughts and watch them come and go like fish in a fishbowl.
Description entails assigning purely descriptive words to one’s observations. To help your patient develop this skill, ask him (her) to describe the sensations he (she) observed in the previous exercise.
Participation entails immersive engagement in an activity. Ask your patient to listen to a song he has never heard before, and then play it again and dance or sing along. Instruct him to engage wholly, conscious of each step or note, without being judgmental or self-conscious. If he feels embarrassed or self-critical, tell him to observe these thoughts and emotions, put them aside, and return to the activity.
A nonjudgmental approach consists of separating out the facts and recognizing emotional responses without clinging to them. To practice this skill, ask your patient to play a song that he likes and one that he dislikes. The patient should listen to each, observing and describing the way they sound without judgment. Tell the patient that if judgmental words or phrases, such as “beautiful,” “ugly,” “I love…,” or “I hate…,” appear as thoughts, he should observe them, put them aside, and then return to nonjudgmental description and observation.
Focusing on 1 thing at a time means dedicating complete attention to a single task, activity, or thought. Give your patient a short paragraph or poem to read. Instruct
Effectiveness involves focusing on what works to attain one’s goals. For this exercise, set up a task for your patient by placing several items in a location that is neither immediately obvious nor readily accessible without an intermediate step. Instruct your patient to obtain these objects. Then guide them as follows:
What do you have to do to get them? Ask permission? Borrow a key? Recruit assistance? Determine the location? Brainstorm ways to obtain the items, and then complete the task.
1. Linehan MM. DBT skills training manual. 2nd ed. New York, NY: The Guilford Press; 2015.
2. Gotink RA, Chu P, Busschbach JJ, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. doi: 10.1371/journal.pone.0124344.
1. Linehan MM. DBT skills training manual. 2nd ed. New York, NY: The Guilford Press; 2015.
2. Gotink RA, Chu P, Busschbach JJ, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. doi: 10.1371/journal.pone.0124344.
Caring for Muslim patients: Understanding cultural and religious factors
Patients who are Muslim—followers of the religion of Islam—struggle with a political climate that has demonized them and the continued fallout of terrorist attacks perpetrated by individuals who identify themselves as Muslim. These patients may experience low self-esteem, bullying, depression, anxiety, or posttraumatic stress disorder.1 Some have expressed feeling judged, labeled, attacked, and subjected to discrimination. Islamophobia and a spike in hate crimes have further marginalized this already vulnerable population.2 Thus, understanding your Muslim patients is the first step to treating their mental illness.
How Muslim culture might affect care
Muslims are not a monolithic group; they vary widely in their religious adherence, cultural background, and acculturation. Some are American-born, including a significant African American Muslim population. Others are children of immigrants or have recently immigrated, including many who came to the United States because of the ongoing war in Syria. Many can trace their heritage to >50 predominantly Muslim countries. Many Muslim patients want to find a balance between their religious and American identities.
As clinicians, we should not make assumptions based on outward appearances or our preconceived notions of our patients, especially when it comes to gender roles. Our job is to ask how highly personal, individualized decisions, such as a woman’s choice to wear a hijab as an expression of her faith and a symbol of modesty, factor into our patients’ day-to-day lives. Doing so can help build the therapeutic alliance and improve the accuracy of the diagnosis and the appropriateness of treatment.
Mental health clinicians are well aware of the dangers of the social stigma that their patients may experience.3 These dangers are no different when it comes to Muslim patients, who often may face “double discrimination” for their religion and for having a mental illness. They may seek support from religious leaders, family, and friends before seeing a mental health provider. Some may view their mental illness as a weakness of faith, a punishment by God, or an affliction caused by a supernatural spirit, and therefore may feel that following religious doctrine will resolve their psychological distress.4 They may need additional encouragement to see a therapist or take psychotropics, and they may prefer specific treatments that reflect their cultural values, such as supplements.
Because some Muslim patients may be more comfortable presenting their psychological concerns as somatic symptoms, they may first seek care from a primary care physician. Some patients may not be open or comfortable enough to address sensitive issues, such as substance use. Providing psychoeducation, comparing mental illness with medical illness, and emphasizing doctor–patient confidentiality may help these patients overcome the stigma that can act as a barrier to care.
Provide culturally competent care
Resources are available to help us provide the best possible care to our patients from various cultures and religions, including Muslim patients. A good starting point is the DSM-5’s Cultural Formulation Interview, which is a set of 16 questions psychiatrists can use to determine the impact of culture on a patient’s clinical presentation and care.5 Other resources include the American Psychiatric Association’s Assessment of Cultural Factors and the American Academy of Child and Adolescent Psychiatry’s Practice Parameter for Cultural Competence.6
When treating Muslim patients, remember to:
- Ask about what roles their culture and religion play
- Understand their explanation of their symptoms
- Work to overcome any stigma patients may perceive related to having a psychiatric disorder
- Engage your team to identify cultural and religious factors
- Connect to community resources, such as the patient’s family and friends.
1. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
2. Nadal KL, Griffin KE, Hamit S, et al. Subtle and overt forms of Islamophobia: microaggressions toward Muslim Americans. J Muslim Mental Health. 2012;6(2):15-37.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. J Muslim Mental Health. 2013;7(1):17-32.
4. Haque A. Religion and mental health: the case of American Muslims. J Relig Health. 2004;43(1):45-58.
5. American Psychiatric Association. Cultural formulation interview. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:750-757.
6. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for cultural competence in child and adolescent psychiatric practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
Patients who are Muslim—followers of the religion of Islam—struggle with a political climate that has demonized them and the continued fallout of terrorist attacks perpetrated by individuals who identify themselves as Muslim. These patients may experience low self-esteem, bullying, depression, anxiety, or posttraumatic stress disorder.1 Some have expressed feeling judged, labeled, attacked, and subjected to discrimination. Islamophobia and a spike in hate crimes have further marginalized this already vulnerable population.2 Thus, understanding your Muslim patients is the first step to treating their mental illness.
How Muslim culture might affect care
Muslims are not a monolithic group; they vary widely in their religious adherence, cultural background, and acculturation. Some are American-born, including a significant African American Muslim population. Others are children of immigrants or have recently immigrated, including many who came to the United States because of the ongoing war in Syria. Many can trace their heritage to >50 predominantly Muslim countries. Many Muslim patients want to find a balance between their religious and American identities.
As clinicians, we should not make assumptions based on outward appearances or our preconceived notions of our patients, especially when it comes to gender roles. Our job is to ask how highly personal, individualized decisions, such as a woman’s choice to wear a hijab as an expression of her faith and a symbol of modesty, factor into our patients’ day-to-day lives. Doing so can help build the therapeutic alliance and improve the accuracy of the diagnosis and the appropriateness of treatment.
Mental health clinicians are well aware of the dangers of the social stigma that their patients may experience.3 These dangers are no different when it comes to Muslim patients, who often may face “double discrimination” for their religion and for having a mental illness. They may seek support from religious leaders, family, and friends before seeing a mental health provider. Some may view their mental illness as a weakness of faith, a punishment by God, or an affliction caused by a supernatural spirit, and therefore may feel that following religious doctrine will resolve their psychological distress.4 They may need additional encouragement to see a therapist or take psychotropics, and they may prefer specific treatments that reflect their cultural values, such as supplements.
Because some Muslim patients may be more comfortable presenting their psychological concerns as somatic symptoms, they may first seek care from a primary care physician. Some patients may not be open or comfortable enough to address sensitive issues, such as substance use. Providing psychoeducation, comparing mental illness with medical illness, and emphasizing doctor–patient confidentiality may help these patients overcome the stigma that can act as a barrier to care.
Provide culturally competent care
Resources are available to help us provide the best possible care to our patients from various cultures and religions, including Muslim patients. A good starting point is the DSM-5’s Cultural Formulation Interview, which is a set of 16 questions psychiatrists can use to determine the impact of culture on a patient’s clinical presentation and care.5 Other resources include the American Psychiatric Association’s Assessment of Cultural Factors and the American Academy of Child and Adolescent Psychiatry’s Practice Parameter for Cultural Competence.6
When treating Muslim patients, remember to:
- Ask about what roles their culture and religion play
- Understand their explanation of their symptoms
- Work to overcome any stigma patients may perceive related to having a psychiatric disorder
- Engage your team to identify cultural and religious factors
- Connect to community resources, such as the patient’s family and friends.
Patients who are Muslim—followers of the religion of Islam—struggle with a political climate that has demonized them and the continued fallout of terrorist attacks perpetrated by individuals who identify themselves as Muslim. These patients may experience low self-esteem, bullying, depression, anxiety, or posttraumatic stress disorder.1 Some have expressed feeling judged, labeled, attacked, and subjected to discrimination. Islamophobia and a spike in hate crimes have further marginalized this already vulnerable population.2 Thus, understanding your Muslim patients is the first step to treating their mental illness.
How Muslim culture might affect care
Muslims are not a monolithic group; they vary widely in their religious adherence, cultural background, and acculturation. Some are American-born, including a significant African American Muslim population. Others are children of immigrants or have recently immigrated, including many who came to the United States because of the ongoing war in Syria. Many can trace their heritage to >50 predominantly Muslim countries. Many Muslim patients want to find a balance between their religious and American identities.
As clinicians, we should not make assumptions based on outward appearances or our preconceived notions of our patients, especially when it comes to gender roles. Our job is to ask how highly personal, individualized decisions, such as a woman’s choice to wear a hijab as an expression of her faith and a symbol of modesty, factor into our patients’ day-to-day lives. Doing so can help build the therapeutic alliance and improve the accuracy of the diagnosis and the appropriateness of treatment.
Mental health clinicians are well aware of the dangers of the social stigma that their patients may experience.3 These dangers are no different when it comes to Muslim patients, who often may face “double discrimination” for their religion and for having a mental illness. They may seek support from religious leaders, family, and friends before seeing a mental health provider. Some may view their mental illness as a weakness of faith, a punishment by God, or an affliction caused by a supernatural spirit, and therefore may feel that following religious doctrine will resolve their psychological distress.4 They may need additional encouragement to see a therapist or take psychotropics, and they may prefer specific treatments that reflect their cultural values, such as supplements.
Because some Muslim patients may be more comfortable presenting their psychological concerns as somatic symptoms, they may first seek care from a primary care physician. Some patients may not be open or comfortable enough to address sensitive issues, such as substance use. Providing psychoeducation, comparing mental illness with medical illness, and emphasizing doctor–patient confidentiality may help these patients overcome the stigma that can act as a barrier to care.
Provide culturally competent care
Resources are available to help us provide the best possible care to our patients from various cultures and religions, including Muslim patients. A good starting point is the DSM-5’s Cultural Formulation Interview, which is a set of 16 questions psychiatrists can use to determine the impact of culture on a patient’s clinical presentation and care.5 Other resources include the American Psychiatric Association’s Assessment of Cultural Factors and the American Academy of Child and Adolescent Psychiatry’s Practice Parameter for Cultural Competence.6
When treating Muslim patients, remember to:
- Ask about what roles their culture and religion play
- Understand their explanation of their symptoms
- Work to overcome any stigma patients may perceive related to having a psychiatric disorder
- Engage your team to identify cultural and religious factors
- Connect to community resources, such as the patient’s family and friends.
1. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
2. Nadal KL, Griffin KE, Hamit S, et al. Subtle and overt forms of Islamophobia: microaggressions toward Muslim Americans. J Muslim Mental Health. 2012;6(2):15-37.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. J Muslim Mental Health. 2013;7(1):17-32.
4. Haque A. Religion and mental health: the case of American Muslims. J Relig Health. 2004;43(1):45-58.
5. American Psychiatric Association. Cultural formulation interview. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:750-757.
6. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for cultural competence in child and adolescent psychiatric practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
1. Basit A, Hamid M. Mental health issues of Muslim Americans. J IMA. 2010;42(3):106-110.
2. Nadal KL, Griffin KE, Hamit S, et al. Subtle and overt forms of Islamophobia: microaggressions toward Muslim Americans. J Muslim Mental Health. 2012;6(2):15-37.
3. Ciftci A, Jones N, Corrigan PW. Mental health stigma in the Muslim community. J Muslim Mental Health. 2013;7(1):17-32.
4. Haque A. Religion and mental health: the case of American Muslims. J Relig Health. 2004;43(1):45-58.
5. American Psychiatric Association. Cultural formulation interview. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:750-757.
6. Pumariega AJ, Rothe E, Mian A, et al; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for cultural competence in child and adolescent psychiatric practice. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1101-1115.
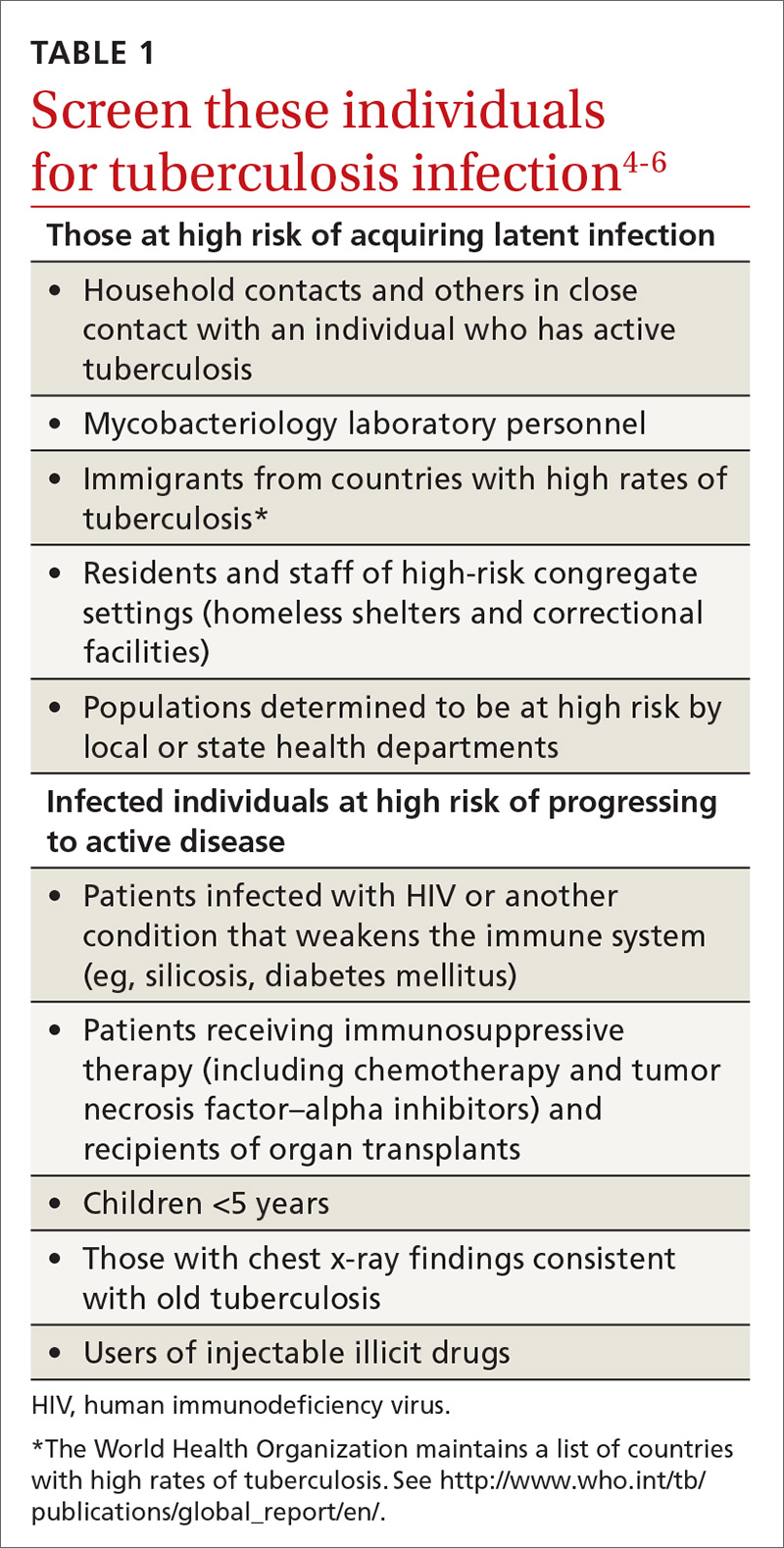
Screening for tuberculosis: Updated recommendations
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
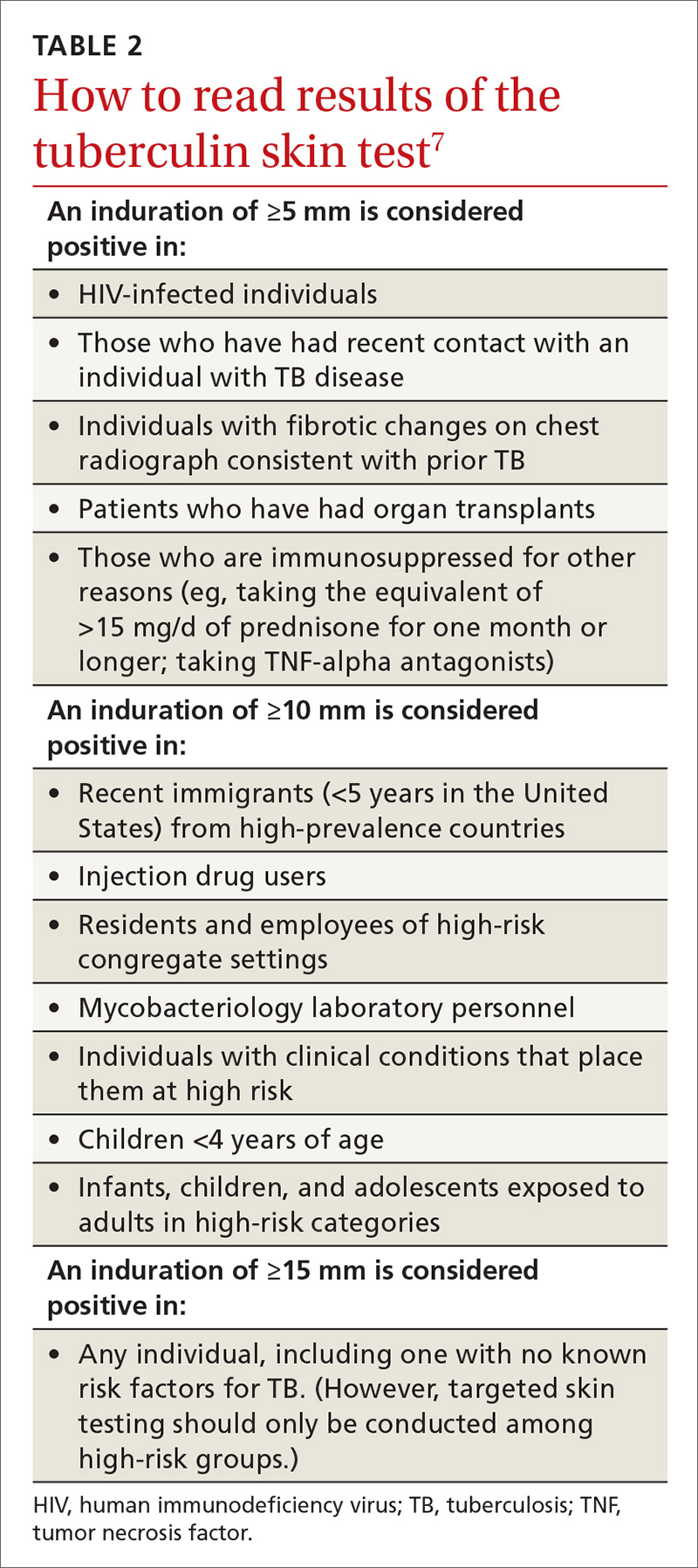
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
From The Journal of Family Practice | 2017;66(12):755-757.
A compounded, nonbenzodiazepine option for treating acute anxiety
Treating short-term or situational anxiety or anxiety attacks with benzodiazepines carries the risk of withdrawal and dependence. Other options include various antidepressants and buspirone. Although such medications decrease overall anxiety and can prevent anxiety from building, they are not effective for breakthrough anxiety. Other mainstays are antihistamines, antipsychotics, or newer antiepileptics such as gabapentin and pregabalin, but none of these have strong clinical literature support regarding their effectiveness for treating anxiety disorders.
PanX compounded medications are dual drug combinations of a beta blocker plus an antiemetic antimuscarinic agent.1 They are designed and patented for as-needed treatment of anxiety disorders without using any controlled substances. Compounded medications are not FDA-approved, but are commercially available and subject to Section 503A of the Federal Food, Drug, and Cosmetics Act of 2013.2
In PanX medications, the beta blocker is intended to address the sympathetic cardiovascular symptoms of anxiety. Beta adrenergic receptor antagonists have been prescribed off-label for decades to treat social anxiety disorder, including performance anxiety. At least 7 beta blockers—atenolol, propranolol, pindolol, timolol, nadolol, betaxolol, and oxprenolol—have been reported to have anxiolytic effects, although these are limited to cardiovascular symptoms of anxiety.1
However, there is a need to augment the limited effects of the beta blocker with another agent, such as an antimuscarinic agent, which is intended for parasympathetic noncardiovascular and CNS symptoms of anxiety. Scopolamine is a preferred antimuscarinic because it has been known for over a century to exhibit anxiolytic effects.3 Scopolamine’s mechanism of action is antagonism of acetylcholine binding to the M1 and/or M2 muscarinic receptors.4
We present a case of a patient who needed a nonbenzodiazepine treatment for acute anxiety. She received a compounded PanX combination of the beta-1 selective beta blocker atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, as needed for acute anxiety.
Case report
Acute anxiety, benzodiazepine abuse
Ms. L, age 30, with a family history of depression and anxiety, has had anxiety, depression, and posttraumatic stress disorder since she was in her mid-20s. She is evaluated in a 30-day rehabilitation program for alprazolam abuse. She is detoxed from alprazolam and stabilized with lurasidone, 60 mg once in the morning, gabapentin, 1,200 mg 4 times a day, and quetiapine, 125 mg as needed for sleep.
Ms. L improves significantly and is transferred to an intensive outpatient program. While there, she experiences increased periods of anxiety related to ruminative thoughts about relationship, occupational, and living stressors. She requests a medication for breakthrough anxiety and recognizes that, because of her history, a benzodiazepine is not medically indicated.
Ms. L signs a consent to a physician-sponsored trial of a PanX medication consisting of orally disintegrating tablets of atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, (in a polyglycol troche base plus mannitol, silica gel, and Steviol glycosides), which is prepared by a compounding pharmacy. Over 6 days, she takes the PanX combination 3 times. Immediately before she takes the medication, her symptoms are intense anxiety, nervousness, and agitation; feelings of panic; increased heart rate and palpitations; and shortness of breath. Ms. L says these symptoms developed approximately 20 minutes before she took the PanX combination. Approximately 30 minutes after taking the medication, she describes having a complete resolution of these symptoms that lasted for 4 hours. She says the medication “calmed [her] down” and had a “Klonopin or benzo-like effect.” She notes that her heart rate slowed quickly, followed by her breathing, and that she also was “more focused.” No information regarding her heart rate or blood pressure when she experienced the symptoms or after treatment is available. She denies experiencing dry mouth, dizziness, fatigue, sleepiness, blurred vision, or confusion.
Targets for future research
This case provides some preliminary clinical evidence of a rapid anxiolytic effect from a novel medication—a beta blocker plus scopolamine combination—that was beneficial in a situation where it may be likely that a benzodiazepine would have been utilized. This is our first case report documenting a trial of any PanX combination (ie, a combination of any beta blocker with any antimuscarinic agent) regarding anxiolytic efficacy and timing, tolerability, and adverse effects. With recognition that this is a report of 1 patient who took the medication 3 times, there is much that is not known.
Additional clinical studies are needed to evaluate the efficacy, tolerability, and adverse effects associated with using a beta blocker/antiemetic antimuscarinic combination to treat acute anxiety. Medication interactions also need to be considered. Whether this combination medication would be best for treating breakthrough anxiety or other acute anxiety episodes, and/or used as a regularly dosed medication is unknown. With documented risks of long-term benzodiazepine use, other novel therapeutics, such as the atenolol/scopolamine combination, may be welcome in treating acute anxiety.
1. Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: a review. Mental Health Fam Med. 2015;11(1):89-99.
2. U.S. Food and Drug Administration. Guidance, compliance and regulatory information: compounding. Section 503A of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/ucm376733.htm. Updated December 12, 2013. Accessed October 25, 2017.
3. Houde A. Scopolamine: a physiological and clinical study. The Am J Clin Med. 1906;13:365-367.
4. Witkin JM, Overshiner C, Li X, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351(2):448-456.
Treating short-term or situational anxiety or anxiety attacks with benzodiazepines carries the risk of withdrawal and dependence. Other options include various antidepressants and buspirone. Although such medications decrease overall anxiety and can prevent anxiety from building, they are not effective for breakthrough anxiety. Other mainstays are antihistamines, antipsychotics, or newer antiepileptics such as gabapentin and pregabalin, but none of these have strong clinical literature support regarding their effectiveness for treating anxiety disorders.
PanX compounded medications are dual drug combinations of a beta blocker plus an antiemetic antimuscarinic agent.1 They are designed and patented for as-needed treatment of anxiety disorders without using any controlled substances. Compounded medications are not FDA-approved, but are commercially available and subject to Section 503A of the Federal Food, Drug, and Cosmetics Act of 2013.2
In PanX medications, the beta blocker is intended to address the sympathetic cardiovascular symptoms of anxiety. Beta adrenergic receptor antagonists have been prescribed off-label for decades to treat social anxiety disorder, including performance anxiety. At least 7 beta blockers—atenolol, propranolol, pindolol, timolol, nadolol, betaxolol, and oxprenolol—have been reported to have anxiolytic effects, although these are limited to cardiovascular symptoms of anxiety.1
However, there is a need to augment the limited effects of the beta blocker with another agent, such as an antimuscarinic agent, which is intended for parasympathetic noncardiovascular and CNS symptoms of anxiety. Scopolamine is a preferred antimuscarinic because it has been known for over a century to exhibit anxiolytic effects.3 Scopolamine’s mechanism of action is antagonism of acetylcholine binding to the M1 and/or M2 muscarinic receptors.4
We present a case of a patient who needed a nonbenzodiazepine treatment for acute anxiety. She received a compounded PanX combination of the beta-1 selective beta blocker atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, as needed for acute anxiety.
Case report
Acute anxiety, benzodiazepine abuse
Ms. L, age 30, with a family history of depression and anxiety, has had anxiety, depression, and posttraumatic stress disorder since she was in her mid-20s. She is evaluated in a 30-day rehabilitation program for alprazolam abuse. She is detoxed from alprazolam and stabilized with lurasidone, 60 mg once in the morning, gabapentin, 1,200 mg 4 times a day, and quetiapine, 125 mg as needed for sleep.
Ms. L improves significantly and is transferred to an intensive outpatient program. While there, she experiences increased periods of anxiety related to ruminative thoughts about relationship, occupational, and living stressors. She requests a medication for breakthrough anxiety and recognizes that, because of her history, a benzodiazepine is not medically indicated.
Ms. L signs a consent to a physician-sponsored trial of a PanX medication consisting of orally disintegrating tablets of atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, (in a polyglycol troche base plus mannitol, silica gel, and Steviol glycosides), which is prepared by a compounding pharmacy. Over 6 days, she takes the PanX combination 3 times. Immediately before she takes the medication, her symptoms are intense anxiety, nervousness, and agitation; feelings of panic; increased heart rate and palpitations; and shortness of breath. Ms. L says these symptoms developed approximately 20 minutes before she took the PanX combination. Approximately 30 minutes after taking the medication, she describes having a complete resolution of these symptoms that lasted for 4 hours. She says the medication “calmed [her] down” and had a “Klonopin or benzo-like effect.” She notes that her heart rate slowed quickly, followed by her breathing, and that she also was “more focused.” No information regarding her heart rate or blood pressure when she experienced the symptoms or after treatment is available. She denies experiencing dry mouth, dizziness, fatigue, sleepiness, blurred vision, or confusion.
Targets for future research
This case provides some preliminary clinical evidence of a rapid anxiolytic effect from a novel medication—a beta blocker plus scopolamine combination—that was beneficial in a situation where it may be likely that a benzodiazepine would have been utilized. This is our first case report documenting a trial of any PanX combination (ie, a combination of any beta blocker with any antimuscarinic agent) regarding anxiolytic efficacy and timing, tolerability, and adverse effects. With recognition that this is a report of 1 patient who took the medication 3 times, there is much that is not known.
Additional clinical studies are needed to evaluate the efficacy, tolerability, and adverse effects associated with using a beta blocker/antiemetic antimuscarinic combination to treat acute anxiety. Medication interactions also need to be considered. Whether this combination medication would be best for treating breakthrough anxiety or other acute anxiety episodes, and/or used as a regularly dosed medication is unknown. With documented risks of long-term benzodiazepine use, other novel therapeutics, such as the atenolol/scopolamine combination, may be welcome in treating acute anxiety.
Treating short-term or situational anxiety or anxiety attacks with benzodiazepines carries the risk of withdrawal and dependence. Other options include various antidepressants and buspirone. Although such medications decrease overall anxiety and can prevent anxiety from building, they are not effective for breakthrough anxiety. Other mainstays are antihistamines, antipsychotics, or newer antiepileptics such as gabapentin and pregabalin, but none of these have strong clinical literature support regarding their effectiveness for treating anxiety disorders.
PanX compounded medications are dual drug combinations of a beta blocker plus an antiemetic antimuscarinic agent.1 They are designed and patented for as-needed treatment of anxiety disorders without using any controlled substances. Compounded medications are not FDA-approved, but are commercially available and subject to Section 503A of the Federal Food, Drug, and Cosmetics Act of 2013.2
In PanX medications, the beta blocker is intended to address the sympathetic cardiovascular symptoms of anxiety. Beta adrenergic receptor antagonists have been prescribed off-label for decades to treat social anxiety disorder, including performance anxiety. At least 7 beta blockers—atenolol, propranolol, pindolol, timolol, nadolol, betaxolol, and oxprenolol—have been reported to have anxiolytic effects, although these are limited to cardiovascular symptoms of anxiety.1
However, there is a need to augment the limited effects of the beta blocker with another agent, such as an antimuscarinic agent, which is intended for parasympathetic noncardiovascular and CNS symptoms of anxiety. Scopolamine is a preferred antimuscarinic because it has been known for over a century to exhibit anxiolytic effects.3 Scopolamine’s mechanism of action is antagonism of acetylcholine binding to the M1 and/or M2 muscarinic receptors.4
We present a case of a patient who needed a nonbenzodiazepine treatment for acute anxiety. She received a compounded PanX combination of the beta-1 selective beta blocker atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, as needed for acute anxiety.
Case report
Acute anxiety, benzodiazepine abuse
Ms. L, age 30, with a family history of depression and anxiety, has had anxiety, depression, and posttraumatic stress disorder since she was in her mid-20s. She is evaluated in a 30-day rehabilitation program for alprazolam abuse. She is detoxed from alprazolam and stabilized with lurasidone, 60 mg once in the morning, gabapentin, 1,200 mg 4 times a day, and quetiapine, 125 mg as needed for sleep.
Ms. L improves significantly and is transferred to an intensive outpatient program. While there, she experiences increased periods of anxiety related to ruminative thoughts about relationship, occupational, and living stressors. She requests a medication for breakthrough anxiety and recognizes that, because of her history, a benzodiazepine is not medically indicated.
Ms. L signs a consent to a physician-sponsored trial of a PanX medication consisting of orally disintegrating tablets of atenolol, 25 mg, plus scopolamine hydrobromide, 0.2 mg, (in a polyglycol troche base plus mannitol, silica gel, and Steviol glycosides), which is prepared by a compounding pharmacy. Over 6 days, she takes the PanX combination 3 times. Immediately before she takes the medication, her symptoms are intense anxiety, nervousness, and agitation; feelings of panic; increased heart rate and palpitations; and shortness of breath. Ms. L says these symptoms developed approximately 20 minutes before she took the PanX combination. Approximately 30 minutes after taking the medication, she describes having a complete resolution of these symptoms that lasted for 4 hours. She says the medication “calmed [her] down” and had a “Klonopin or benzo-like effect.” She notes that her heart rate slowed quickly, followed by her breathing, and that she also was “more focused.” No information regarding her heart rate or blood pressure when she experienced the symptoms or after treatment is available. She denies experiencing dry mouth, dizziness, fatigue, sleepiness, blurred vision, or confusion.
Targets for future research
This case provides some preliminary clinical evidence of a rapid anxiolytic effect from a novel medication—a beta blocker plus scopolamine combination—that was beneficial in a situation where it may be likely that a benzodiazepine would have been utilized. This is our first case report documenting a trial of any PanX combination (ie, a combination of any beta blocker with any antimuscarinic agent) regarding anxiolytic efficacy and timing, tolerability, and adverse effects. With recognition that this is a report of 1 patient who took the medication 3 times, there is much that is not known.
Additional clinical studies are needed to evaluate the efficacy, tolerability, and adverse effects associated with using a beta blocker/antiemetic antimuscarinic combination to treat acute anxiety. Medication interactions also need to be considered. Whether this combination medication would be best for treating breakthrough anxiety or other acute anxiety episodes, and/or used as a regularly dosed medication is unknown. With documented risks of long-term benzodiazepine use, other novel therapeutics, such as the atenolol/scopolamine combination, may be welcome in treating acute anxiety.
1. Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: a review. Mental Health Fam Med. 2015;11(1):89-99.
2. U.S. Food and Drug Administration. Guidance, compliance and regulatory information: compounding. Section 503A of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/ucm376733.htm. Updated December 12, 2013. Accessed October 25, 2017.
3. Houde A. Scopolamine: a physiological and clinical study. The Am J Clin Med. 1906;13:365-367.
4. Witkin JM, Overshiner C, Li X, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351(2):448-456.
1. Dooley TP. Treating anxiety with either beta blockers or antiemetic antimuscarinic drugs: a review. Mental Health Fam Med. 2015;11(1):89-99.
2. U.S. Food and Drug Administration. Guidance, compliance and regulatory information: compounding. Section 503A of the Federal Food, Drug, and Cosmetic Act. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/pharmacycompounding/ucm376733.htm. Updated December 12, 2013. Accessed October 25, 2017.
3. Houde A. Scopolamine: a physiological and clinical study. The Am J Clin Med. 1906;13:365-367.
4. Witkin JM, Overshiner C, Li X, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014;351(2):448-456.
Proposed In-Training Electrocardiogram Interpretation Competencies for Undergraduate and Postgraduate Trainees
The 12-lead electrocardiogram (ECG) remains one of the most widely used and readily available diagnostic tests in modern medicine.1 Reflecting the electrical behavior of the heart, this point-of-care diagnostic test is used in almost every area of medicine for diagnosis, prognostication, and selection of appropriate treatment. The ECG is sometimes the only and most efficient way of detecting life-threatening conditions, thus allowing a timely delivery of emergency care.2 However, the practical power of the 12-lead ECG relies on the ability of the clinician to interpret this test correctly.
For decades, ECG interpretation has been a core component of undergraduate and postgraduate medical training.3-5 Unfortunately, numerous studies have demonstrated alarming rates of inaccuracy and variability in interpreting ECGs among trainees at all levels of education.4,6,7 Senior medical students have been repeatedly shown to miss 26% to 62% of acute myocardial infarctions (MI).6,8-10 Another recent study involving internal medicine residents demonstrated that only half of the straightforward common ECGs were interpreted correctly, while 26% of trainees missed an acute MI and 56% missed ventricular tachycardia (VT).11 Even cardiology subspecialty fellows demonstrated poor performance, missing up to 26% of ST-elevation MIs on ECGs that had multiple findings.12 Inaccurate interpretations of ECGs can lead to inappropriate management decisions, adverse patient outcomes, unnecessary additional testing, and even preventable deaths.4,13-15
Several guidelines have emphasized the importance of teaching trainees 12-lead ECG interpretation and have recognized the value of assessments in ensuring that learners acquire the necessary competencies.16-19 Similarly, there have been many calls for more rigorous and structured curricula for ECG interpretation throughout undergraduate and postgraduate medical education.11,16 However, we still lack a thoughtful guideline outlining the specific competencies that medical trainees should attain. This includes medical students, nurses working in hospital and in out-of-hospital settings, and residents of different specialties, including emergency medicine, cardiology, and electrophysiology (EP) fellows.
Setting goals and objectives for target learners is recognized to be the initial step and a core prerequisite for effective curriculum development.20 In this publication, we summarize the objectives from previously published trainee assessments and propose reasonably attainable ECG interpretation competencies for both graduating medical students and residents at the end of their postgraduate training. This document is being endorsed by researchers and educators of 2 international societies dedicated to the study of electrical heart diseases: the International Society of Electrocardiology (ISE) and the International Society of Holter and Noninvasive Electrocardiology (ISHNE).
METHODS
Current Competencies in Literature
We performed a systematic search to identify ECG competencies that are currently mentioned in the literature. Information was retrieved from MEDLINE (1946-2016) and EMBASE (1947-2016) by using the following MeSH terms: electrocardiogram, electrocardiography, electrocardiogram interpretation, electrocardiogram competency, medical school, medical student, undergraduate medicine, undergraduate medical education, residency education, internship, and residency. Our search was limited to English-language articles that studied physician trainees. The references of the full-length articles were examined for additional citations. The search revealed a total of 65 publications involving medical students and 120 publications involving residents. Abstracts of publications were then assessed for relevance, and the methods of the remaining articles were scrutinized for references to specific ECG interpretation objectives. This strategy narrowed the search to 9 and 14 articles involving medical students and residents, respectively. Studies were not graded for quality because the purpose of the search was to identify the specific ECG competencies that authors expected trainees to obtain. Almost all the articles proposed teaching tools and specific objectives that were defined by the investigators arbitrarily and assessed the trainee’s ability to interpret ECGs (summarized in supplementary Table).
Defining ECG Interpretation Competencies
The initial draft of proposed ECG interpretation competencies was developed at Queen’s University in Ontario, Canada. A list of ECG patterns and diagnoses previously mentioned in literature was used as a starting point. From there, each item was refined and organized into 4 main categories (see Figures 1 and 2).
Class A “Common electrocardiographic emergencies” represent patterns that are frequently seen in hospitals, in which accurate interpretation of the ECG within minutes is essential for delivering care that is potentially lifesaving to the patient (eg, ST-elevation MI).
Class B “Common nonemergency patterns” represent ECG findings that are encountered daily in patients who are not acutely ill, which may impact their care in the appropriate clinical context (eg, left ventricular hypertrophy).
Class C “Uncommon electrocardiographic emergencies” represent ECG findings that are not encountered on a daily basis but can be potentially lifesaving if recognized (eg ventricular preexcitation).
Class D “Uncommon nonemergency patterns” represent findings that are uncommon but may diagnostically contribute to patient care in a clinically appropriate setting (eg, right atrial abnormality).
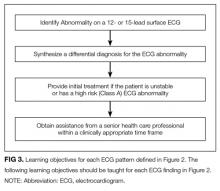
ECG interpretation patterns were then assigned to medical students and residents based on the specific goals of training. At the time of graduation, medical students should develop the foundation for learning ECG interpretation in residency training, provide ECG interpretation and initial management for electrocardiographic emergencies, and obtain assistance from a more senior medical professional within a clinically appropriate time frame. The training goal for a resident is to develop ECG interpretation competencies for safe independent clinical practice (Figure 1).
The final segregated ECG interpretation competencies were distributed to members of ISE and ISHNE for input, modifications, and revisions. The proposed list of competencies went through several revisions until a consensus was reached.
RESULTS
The final distribution of ECG patterns is illustrated in Figure 2. (Figure 3 defines the learning objectives for each ECG pattern defined in Figure 2.) Here, we provide a rationale for
Class A: Common Electrocardiographic Emergencies
This group contains ECG findings that require recognition within minutes to deliver potentially lifesaving care. For this reason, undergraduate medical education programs should prioritize mastering class A conditions to minimize the risk of misdiagnosis and late recognition.
Class A patterns include ST elevation MI (STEMI) and localization of territory to ensure ST-segment elevations are seen in contiguous leads.29,30 Students should learn the criteria for STEMI as per the “Universal Definition of Myocardial Infarction” and be aware of early signs of STEMI that may be seen prior to ST-segment changes, such as hyper-acute T-waves (increased amplitude and symmetrical).30
Asystole, wide complex tachycardias, and ventricular fibrillation (VF) are all crucial ECG patterns that must be identified to deliver advanced cardiac life support (ACLS) care as per the 2010 AHA Guidelines for cardiopulmonary resuscitation and emergency cardio care.31 Of note, students should understand the differential diagnosis of wide complex tachycardias and should be able to suspect VF in clinically appropriate scenarios. We included the category “unstable/symptomatic supraventricular tachycardia” to represent rapid rhythms that are supraventricular in origin, which either produce symptoms or cause impairment of vital organ function.31 In emergency situations, it may not be crucial to correctly identify the specific supraventricular rhythm to deliver ACLS care; hence, the specific supraventricular tachycardia diagnoses were included in Class B.
Finally, we believe that medical students should be able to recognize long QT, hypo/hyperkalemia, and distinguish types of atrioventricular (AV) block. Distinguishing types of AV block is important because both third degree AV block and second degree AV block Mobitz II can be life threatening and require further investigation or emergency treatment in an inpatient setting.32 Prompt recognition of long QT is crucial because it can be associated with ventricular tachyarrhythmias. This includes a polymorphic pattern characterized by the twisting of QRS peaks around the baseline (torsades des pointes), which can eventually lead to VF.
Class B: Common Nonemergency Patterns
Class B patterns represent common findings that are seen on a daily basis that may impact patient care in a clinically appropriate context. Diagnoses in this section were divided into “tachycardia syndromes,” “bradycardia syndromes,” “conduction abnormalities,” “ischemia,” and “other.”
Undergraduate trainees should become proficient in identifying the cause of bradycardia and distinguishing types of AV blocks. Similarly, they should also have an approach to differentiate tachycardia syndromes.33,34 These skills are required to correctly manage patients in both inpatient and outpatient settings. They should be taught in undergraduate programs and reinforced in postgraduate training.
Common findings, such as bundle branch blocks, left anterior fascicular block, premature ventricular/atrial complexes, electronic pacemakers, and left ventricular hypertrophy, are essential to the daily interpretation of ECGs. Junior learners should be proficient in recognizing these patterns. Findings consistent with pericarditis are not uncommon and can be very helpful to guide the clinician to the diagnosis. Notable exceptions from the medical student competency list include detection of lead misplacement, common artifacts, nonspecific intraventricular conduction delay, interatrial block, and benign early repolarization. These findings require a deeper understanding of electrocardiography and would be more appropriate for senior learners.
Class C: Uncommon Electrocardiographic Emergencies
Class C findings represent uncommon conditions that, if recognized, can prevent serious adverse patient outcomes. These include preexcitation, STEMI with preexisting left bundle branch block sinus pauses, Brugada pattern, hypothermia, effects of toxic drugs, ventricular aneurysm, and right ventricular hypertrophy. The recognition of these patterns is crucial to avoid severe adverse patient outcomes, and independent practicing physicians should be aware of these findings. However, given that a high proportion of senior medical students miss common electrocardiographic emergencies, undergraduate medical education programs should instead focus resources on ensuring medical students are proficient in identifying class A and class B conditions.6,8-10 Postgraduate programs should ensure that postgraduate trainees can identify these potentially life-threatening conditions (see section “How to Teach Electrocardiology”).
Class D: Uncommon and Nonemergency Patterns
Class D findings represent less common findings that are not seen every day and do not require urgent medical attention. These include right atrial abnormality, left posterior fascicular block, low atrial rhythms, and electrolyte abnormalities that exclude potassium. Notably, electrolyte abnormalities are important to identify; however, typically, treatment is guided by the lab results.35 Overall, postgraduate trainees should certainly be aware of these findings, but medical student training should instead focus on learning the framework and correctly identifying class A and class B ECG patterns.
HOW TO TEACH ELECTROCARDIOLOGY
Teaching ECG Interpretation Strategies
No clear teaching approaches to ECG interpretation have been described in the literature, and no recommendations on knowledge translation have been formally explored. A possible educational approach to the teaching of electrocardiology could involve several methods for helping students with ECG interpretation:36
1. Pattern recognition: The ECG, at its most immediate level, is a graphic image, and recognition of images is essentially recognition of patterns. These patterns can only be learned through repeated visualization of examples with a written or verbal explanation. Repeated visualization over time will help avoid “erosion” of knowledge. Examples of learning tools include periodic in-person ECG rounds, well-illustrated books or atlases, and online tools with good quality ECGs and explanations. These learning opportunities are strongly reinforced by collecting cases from the clinical encounters of the trainee that illustrate the aforementioned patterns. Some of these patterns can be found in guidelines, such as the one published by the AHA and ACC.29
2. Application of published criteria: Guidelines, review papers, and books offer diagnostic criteria for many entities, such as chamber enlargement, bundle branch blocks, and abnormal Q waves. Learning these criteria and applying them to the analysis of ECGs is a commonly used learning strategy.
3. Inductive-deductive reasoning: This strategy requires a deeper understanding of the pathophysiology behind ECG patterns. It requires ECGs to be interpreted in a certain clinical context, and the goal of ECG interpretation is to answer a clinical question that is used to guide patient care. This strategy typically employs the use of algorithms to lead the interpreter to the correct diagnosis, and mastery of this skill grows from ongoing clinical experience. Examples of the “inductive-deductive reasoning” are localizing an accessory AV pathway, the differential diagnosis of narrow or wide complex tachycardias, and identifying the site of coronary artery occlusion in a patient with a STEMI.
4. Ladder diagrams: Ladder diagrams have been used for over 100 years to graphically illustrate the mechanism of arrhythmias. They can be incredibly useful to help learners visualize impulse conduction in reentry mechanisms as well as other abnormal rhythms. However, there are some rhythms that are difficult to illustrate on ladder diagrams.37
5. Peer and near-peer teaching: Peer teaching occurs when learners prepare and deliver teaching material to learners of a similar training level. The expectation to deliver a teaching session encourages students to learn and organize information in thoughtful ways. It builds strong teamwork skills and has been shown to positively affect all involved learners.38-40
Each ECG interpretation strategy has its advantages, and we recommend that students be exposed to all available approaches if teaching resources are available.
Teaching Delivery Format
Each of the above teaching strategies can be delivered to students in various ways. The following teaching formats have been previously documented in the literature:
1. Classroom-based teaching: This is a traditional learning format that takes place in a large- or small-group classroom. Typically, these sessions are led by a single instructor, and they are focused on the direct sharing of information and group discussion.41
2. Electronic practice tools: Numerous electronic tools have been developed with the purpose of providing deliberate practice to master ECG interpretation. Some of these tools employ active learner engagement, while others provide a bank of ECGs for self-directed passive learning.42-46
3. Video lectures: Short video lectures have been created to facilitate self-directed lecture based learning. These lectures are hosted on a variety of web-based platforms, including YouTube and Vimeo.47
4. Traditional and electronic books: Numerous traditional textbooks have been published on ECG interpretation and are designed to facilitate independent learning. Some textbooks directly deliver teaching material, while others contain sets of ECGs to allow for repetitive practice. More recently, iBooks incorporating self-assessment tools have been used to assist ECG teaching.34 The advantage of these tools is that they can also be used to supplement in-person classes.
5. Games: A unique ECG interpretation learning strategy consists of using puzzles and games to learn ECGs. This is meant to improve student engagement and interest in learning ECG interpretation.48
Given that there is currently a lack of evidence-based data to support 1 instructional format over another, we do not favor any particular one. This decision should be left to instructors and individual learners based on their preference and available resources. Further studies would be helpful to determine the effectiveness of various methods in teaching ECG interpretation and to identify any additional specific factors that facilitate learning.
Evaluation Strategies
1. Longitudinal ongoing feedback: This form of feedback universally takes place in all training programs and focuses on direct observation and point-of-care feedback by a senior healthcare professional during clinical practice. Typically, the feedback is informal and is centered around specific case presentations.
2. Formative testing: This assessment strategy is aimed at monitoring the learning of trainees and providing them with appropriate feedback. Tutors and teachers can use this data to individualize instruction and fill any training gaps that individuals and the class may have. Students themselves can use this information to encourage additional study to ensure they acquire required skills. Examples of formative testing are low-stakes in-training exams and asking audience questions during a workshop or lecture.49
3. Summative testing: Summative assessments are created to measure the level of proficiency developed by a learner and compare it against some standard or benchmark. This form of assessment establishes the extent to which educational objectives have been met. The most common example is an end-of-term examination.
Online ECG examination has been successfully used to provide methods of testing. They are easy to distribute, highly convenient for learners, and allow the display of high-quality graphics. They can also be graded electronically, thereby minimizing the resources required to administer and grade exams.36,50
We recommend using a combination of assessment formats to ensure the optimal evaluation of learner skill and to focus learning on areas of weakness. Summative assessments are highly valuable to ensure learners acquired the necessary ECG interpretation competencies. Remediation strategies should be available to provide additional practice to learners who do not meet competencies expected at their level of training.
DISCUSSION
The Need for ECG Interpretation Competencies and Milestones
Since the introduction of ECG in the late 1800s, there continues to be a significant variation in ECG interpretation skills among trainees and medical professionals.4,6-12 Concerns continue to exist about the rate of missed diagnoses involving critical ECGs, leading to inappropriate patient management decisions. Despite the obvious need, teaching ECG interpretation is given little emphasis in medical education, and the curriculum remains quite disorganized. In this position paper, we call for a more structured ECG interpretation curriculum in medical education and hope to assist this process by assigning ECG patterns to 2 milestones in training: graduating medical students and first year postgraduate medical residents.
Defining competencies would help medical education programs to focus resources on teaching clinically important conditions for the appropriate level of training. We divide ECG findings into 4 categories (classes A to D), and we place emphasis on learning electrocardiographic emergencies early in training and spending less time on ECG findings that are unlikely to change patient management.
The goal is to ensure 100% recognition of class A (electrocardiographic emergencies) by the end of medical school. To ensure each medical education program fulfils this goal, a structured curriculum including a summative assessment is required.
Methods of Teaching
Various instructional mediums have been successfully implemented to teach ECG interpretation competencies, including lectures, puzzles, web-based programs, iBooks, and YouTube.34-41-44,47,48.51-53 A survey of clerkship directors in internal medicine revealed that 75% of clerkship programs teach ECG interpretation in a classroom lecture-based setting, 44% use teaching rounds, and only 17% utilize online/web-based instruction.3 Canadian family medicine programs have a relatively equal distribution between classroom-based, computer-based, and bedside teaching.5
In comparing the efficacy of instructional styles, several small comparative studies favor an electronic teaching format because of the enhanced learner interaction and visual learning, but there does not appear to be a consistently proven large advantage of 1 teaching format over another.43,48,51,54 The overall theme emerging from this literature is the importance of repetition and active engagement in ECG interpretation, which appear to be more important than 1 particular strategy.22 Computer-based training appears to deliver these 2 qualities, unlike the traditional lecture-style passive learning model. The concept of repetition and engagement is also well supported in medical education literature outside ECG interpretation.55,56
Given these data, we recommend that each medical education program select teaching methods based on their available resources, as long as adequate teaching time is allotted to ensure that trainees acquire the competencies defined in this publication.
Assessment Methods
It appears that the larger factor in determining ECG interpretation performance is not the learning format, but the form of assessment. Two studies have demonstrated that summative assessment substantially improves ECG interpretation performance when compared with formative assessment; in fact, this effect was so large that it overshadowed any small difference in teaching formats.57,58 This concept aligns with medical education literature, which acknowledges that assessment drives learning by raising the stakes, thereby boosting student effort and encouraging learning to an effect much larger than can be generated by any particular learning style.57,59 Nevertheless, well-designed formative assessment can focus students on effective learning by identifying gaps and important information.60 Only 33% of Canadian family medicine residency programs and 71% of American clerkship programs have formal assessment of ECG interpretation skills.3,5 There is no doubt that assessment, both formative and summative, should be implemented in all undergraduate and postgraduate medical training programs. Online assessment methods have the advantage of delivering high-quality images and a variety of question formats; hence, their use should be encouraged.36,50,61-63
Teaching Personnel and Timing of Training
Who should teach ECG interpretation and when should this teaching take place? ECG interpretation in training programs is typically taught by attending physicians in each respective field. However, given that there is a large ECG interpretation error rate by noncardiologist physicians, we advise that ECG training content be created with input from own-specialty attending physicians and cardiologists.4 This teaching should take place early in medical school at the time medical students learn pathophysiology of the heart and should continue throughout training. Longitudinal training is preferred to block-based training because of improved resident satisfaction, but medical education literature did not reveal a difference in student performance with either strategy.64-66
CONCLUSIONS
Despite its immense clinical value, there continues to be a lack of a comprehensive ECG interpretation curriculum in medical education programs. The goal of this position paper is to encourage the development of organized curricula in undergraduate and postgraduate medical education programs, and to ensure the acquisition of level-appropriate ECG interpretation skills while maintaining patient safety. We assist this process by grouping ECG findings into 4 classes (A to D) based on the frequency of encounter and emergent nature and by assigning them to each level of training. Methods of teaching ECG interpretation are less important and can be selected based on the available resources of each education program and student preference; however, online learning is encouraged. We also recommend that summative trainee evaluation methods be implemented in all programs to ensure that appropriate competencies are acquired and to further encourage self-directed learning. Resources should be allocated to ensure that every trainee is reaching their training milestones and should ensure that no electrocardiographic emergency (class A condition) is ever missed by a trainee. We hope that these guidelines will inform medical education systems and help prevent adverse patient outcomes caused by the misinterpretation of this valuable clinical diagnostic tool.
Disclosure
On behalf of all authors, the corresponding author states that there is no conflict of interest. This manuscript did not utilize any sources of funding.
1. Baranchuk A, Chiale PA, Green M, Caldwell JC. Editorial: surface electrocardiogram remains alive in the XXI century. Curr Cardiol Rev. 2014;10(3):173-174. http://www.ncbi.nlm.nih.gov/pubmed/24856069. Accessed January 4, 2017. PubMed
2. Fisch C. Evolution of the clinical electrocardiogram. J Am Coll Cardiol. 1989;14(5):1127-1138. doi:10.1016/0735-1097(89)90407-5. PubMed
3. O’Brien KE, Cannarozzi ML, Torre DM, Mechaber AJ, Durning SJ. Training and assessment of ECG interpretation skills: results from the 2005 CDIM survey. Teach Learn Med. 2005;21(2):111-115. doi:10.1080/10401330902791255. PubMed
4. Salerno SM, Alguire PC, Waxman HS. Competency in Interpretation of 12-Lead Electrocardiograms: A Summary and Appraisal of Published Evidence. Ann Intern Med. 2003;138(9):751-760. doi:10.1016/S1062-1458(03)00283-6. PubMed
5. Paul B, Baranchuk A. Electrocardiography teaching in Canadian family medicine residency programs: A national survey. Fam Med. 2011;43(4):267-271. http://www.ncbi.nlm.nih.gov/pubmed/21500000. Accessed January 4, 2017. PubMed
6. Jablonover RS, Lundberg E, Zhang Y, Stagnaro-Green A. Competency in electrocardiogram interpretation among graduating medical students. Teach Learn Med. 2014;26(3):279-284. doi:10.1080/10401334.2014.918882. PubMed
7. Elnicki DM, van Londen J, Hemmer PA, Fagan M, Wong R. US and Canadian internal medicine clerkship directors’ opinions about teaching procedural and interpretive skills to medical students. Acad Med. 2004;79(11):1108-1113. http://www.ncbi.nlm.nih.gov/pubmed/15504782. Accessed January 31, 2017. PubMed
8. Shams M, Sullivan A, Abudureyimu S, et al. Optimizing Electrocardiogram Interpretation and Catheterization Laboratory Activation in St-Segment Elevation Myocardial Infarction: a Teaching Module for Medical Students. J Am Coll Cardiol. 2016;67(13):643. doi:10.1016/S0735-1097(16)30644-1.
9. Grum CM, Gruppen LD, Woolliscroft JO. The influence of vignettes on EKG interpretation by third-year students. Acad Med. 1993;68:S61-S63. PubMed
10. Little B, Ho KJ, Scott L. Electrocardiogram and rhythm strip interpretation by final year medical students. Ulster Med J. 2001;70(2):108-110. PubMed
11. Eslava D, Dhillon S, Berger J, Homel P, Bergmann S. Interpretation of electrocardiograms by first-year residents: the need for change. J Electrocardiol. 2009;42(6):693-697. doi:10.1016/j.jelectrocard.2009.07.020. PubMed
12. Sibbald M, Davies EG, Dorian P, Yu EHC. Electrocardiographic Interpretation Skills of Cardiology Residents: Are They Competent? Can J Cardiol. 2014;30(12):1721-1724. doi:10.1016/j.cjca.2014.08.026. PubMed
13. Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224. Accessed January 4, 2017. PubMed
14. Todd KH, Hoffman JR, Morgan MT. Effect of cardiologist ECG review on emergency department practice. Ann Emerg Med. 1996;27(1):16-21. Accessed January 4, 2017. PubMed
15. Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and Minor ECG Abnormalities in Asymptomatic Women and Risk of Cardiovascular Events and Mortality. JAMA. 2007;297(9):978. doi:10.1001/jama.297.9.978. PubMed
16. Salerno SM, Alguire PC, Waxman HS. Training and Competency Evaluation for Interpretation of 12-Lead Electrocardiograms: Recommendations from the American College of Physicians. Ann Intern Med. 2003;138(9):747-750. doi:10.7326/0003-4819-138-9-200305060-00012. PubMed
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Cardiovascular Disease (Internal Medicine); 2016. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/152_interventional_cardiology_2017-07-01.pdf. Accessed January 4, 2017.
18. American Board of Internal Medicine. Policies and Procedures For Certification; 2016. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed January 4, 2017.
19. Kadish AH, Buxton AE, Kennedy HL, et al. ACC/AHA Clinical Competence Statement on Electrocardiography and Ambulatory Electrocardiography. J Am Coll Cardiol. 2001;38(7):3169-3178. PubMed
20. Kern D, Thomas PA, Hughes MT, editors. Curriculum Development for Medical Education: A Six-Step Approach. 2nd edition. Baltimore: The Johns Hopkins University Press; 2009.
21. De Fer T, Fazio S, Goroll A. Core Medicine Clerkship: Curriculum Guide V3.0. Alliance for Academic Internal Medicine; 2006. http://www.im.org/p/cm/ld/fid=385. Accessed January 12, 2017.
22. Hatala RM, Brooks LR, Norman GR. Practice makes perfect: The critical role of mixed practice in the acquisition of ECG interpretation skills. Adv Heal Sci Educ. 2003;8(1):17-26. doi:10.1023/A:1022687404380. PubMed
23. Bayes de Luna A. ECGs For Beginners. Barcelona: Wiley Blackwell; 2014.
24. O’Keefe J, Hammill S, Freed M, Pogwizd S. The Complete Guide to ECGs. Third edition. Kansas City: Physicians’ Press - Jones and Bartlett Publishers; 2008.
25. Khan G. Rapid ECG Interpretation. Third edition. Ottawa: Humana Press (Springer Science); 2008.
26. Garcia T. 12-Lead ECG: The Art of Interpretation. Second edition. Burlington: Jones & Bartlett Learning; 2015.
27. Olson CW, Warner RA, Wagner GS, Selvester RH. A dynamic three-dimensional display of ventricular excitation and the generation of the vector and electrocardiogram. J Electrocardiol. 2001;34 Suppl:7-15. doi:10.1054/jelc.2001.29793. PubMed
28. Olson CW, Lange D, Chan JK, et al. 3D Heart: A new visual training method for Electrocardiographic Analysis. J Electrocardiol. 2007;40(5):1-7. doi:10.1016/j.jelectrocard.2007.04.001. PubMed
29. Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):1003-1011. doi:10.1016/j.jacc.2008.12.016. PubMed
30. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525-2538. doi:10.1093/eurheartj/ehm355. PubMed
31. Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3). doi:10.1161/CIRCULATIONAHA.110.970988. PubMed
32. Barold SS, Hayes DL. Second-Degree Atrioventricular Block: A Reappraisal. Mayo Clin Proc. 2001;76(1):44-57. doi:10.4065/76.1.44. PubMed
 33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
34. Nadeau-Routhier C, Baranchuk A. Electrocardiography in Practice: What to Do? 1st ed. Kingston: Apple Inc. iBook; 2015.
35. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2):153-160. doi:10.1016/j.jemermed.2004.04.006. PubMed
36. Quinn KL, Crystal E, Lashevsky I, Arouny B, Baranchuk A. Validation of a Novel Digital Tool in Automatic Scoring of an Online ECG Examination at an International Cardiology Meeting. Ann Noninvasive Electrocardiol. 2016;21(4):376-381. doi:10.1111/anec.12311. PubMed
37. Johnson NP, Denes P. The Ladder Diagram (A 100+ Year History). Am J Cardiol. 2008;101(12):1801-1804. doi:10.1016/j.amjcard.2008.02.085. PubMed
38. Bulte C, Betts A, Garner K, Durning S. Student teaching: views of student near-peer teachers and learners. Med Teach. 2007;29(0):583-590. doi:10.1080/01421590701583824. PubMed
39. Nestojko JF, Bui DC, Kornell N, Ligon Bjork E. Expecting to teach enhances learning and organization of knowledge in free recall of text passages. Mem Cogn. 2014;42:1038-1048. doi:10.3758/s13421-014-0416-z. PubMed
40. Bené KL, Bergus G. When learners become teachers: A review of peer teaching in medical student education. Fam Med. 2014;46(10):783-787. doi:10.4300/JGME-D-13-00426. PubMed
41. Lucas J, McKay S, Baxley E. EKG arrhythmia recognition: a third-year clerkship teaching experience. Fam Med. 2003;35(3):163-164. Accessed January 31, 2017. PubMed
42. DeBonis K, Blair TR, Payne ST, Wigan K, Kim S. Viability of a Web-Based Module for Teaching Electrocardiogram Reading Skills to Psychiatry Residents: Learning Outcomes and Trainee Interest. Acad Psychiatry. 2015;39(6):645-648. doi:10.1007/s40596-014-0249-x. PubMed
43. Chudgar SM, Engle DL, Grochowski COC, Gagliardi JP. Teaching crucial skills: An electrocardiogram teaching module for medical students. J Electrocardiol. 2016;49(4):490-495. doi:10.1016/j.jelectrocard.2016.03.021. PubMed
44. Nathanson LA, Safran C, McClennen S, Goldberger AL. ECG Wave-Maven: a self-assessment program for students and clinicians. Proc AMIA Symp. 2001:488-492. Accessed January 31, 2017. PubMed
45. Farré J, Wellens H. ECGcorner (Online). ECGcorner. http://www.ecgcorner.org. Published 2017. Accessed February 15, 2017.
46. Waechter J. Teaching Medicine (Online). https://www.teachingmedicine.com/ Accessed Feb 15, 2017.
47. Akgun T, Karabay CY, Kocabay G, et al. Learning electrocardiogram on YouTube: How useful is it? J Electrocardiol. 2014;47(1):113-117. doi:10.1016/j.jelectrocard.2013.09.004. PubMed
48. Rubinstein J, Dhoble A, Ferenchick G. Puzzle based teaching versus traditional instruction in electrocardiogram interpretation for medical students – a pilot study. BMC Med Educ. 2009;9(1):4. doi:10.1186/1472-6920-9-4. PubMed
49. Black P, Wiliam D. Assessment and Classroom Learning. Assess Educ. 1998;5(1):7-73. doi:10.1080/0969595980050102.
50. Quinn KL, Baranchuk A. Feasibility of a novel digital tool in automatic scoring of an online ECG examination. Int J Cardiol. 2015;185:88-89. doi:10.1016/j.ijcard.2015.03.135. PubMed
51. Nilsson M, Bolinder G, Held C, et al. Evaluation of a web-based ECG-interpretation programme for undergraduate medical students. BMC Med Educ. 2008;8(1):25. doi:10.1186/1
52. Lessard Y, Sinteff J-P, Siregar P, et al. An ECG analysis interactive training system for understanding arrhythmias. Stud Health Technol Inform. 2009;150:931-935. Accessed January 31, 2017. PubMed
53. Zakowski, Dean Keller L. An effective ECG curriculum for third-year medical students in a community-based clerkship. Med Teach. 2000;22(4):354-358. doi:10.1080/014215900409447.
54. Mahler SA, Wolcott CJ, Swoboda TK, Wang H, Arnold TC. Techniques for teaching electrocardiogram interpretation: Self-directed learning is less effective than a workshop or lecture. Med Educ. 2011;45(4):347-353. doi:10.1111/j.1365-2923.2010.03891.x. PubMed
55. Biggs J. What the Student Does: Teaching for enhanced learning. High Educ Res Dev. 1999;18(1):57-75.
56. Ericsson KA. Deliberate practice and acquisition of expert performance: A general overview. Acad Emerg Med. 2008;15(11):988-994. doi:10.1111/j.1553-2712.2008.00227.x. PubMed
57. Raupach T, Hanneforth N, Anders S, Pukrop T, Th J Ten Cate O, Harendza S. Impact of teaching and assessment format on electrocardiogram interpretation skills. Med Educ. 2010;44(7):731-740. doi:10.1111/j.1365-2923.2010.03687.x. PubMed
58. Raupach T, Brown J, Anders S, Hasenfuss G, Harendza S. Summative assessments are more powerful drivers of student learning than resource intensive teaching formats. BMC Med. 2013;11:61. doi:10.1186/1741-7015-11-61. PubMed
59. Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests imporves ong-term retention. Psychol Sci. 2006;17(3):249-255. doi:10.1111/j.1467-9280.2006.01693.x. PubMed
60. Ferris HA, O’ Flynn D. Assessment in Medical Education; What Are We Trying to Achieve? Int J High Educ. 2015;4(2):139-144. doi:10.5430/ijhe.v4n2p139.
61. Hartman ND, Wheaton NB, Williamson K, Quattromani EN, Branzetti JB, Aldeen AZ. A Novel Tool for Assessment of Emergency Medicine Resident Skill in Determining Diagnosis and Management for Emergent Electrocardiograms: A Multicenter Study. J Emerg Med. 2016;51(6):697-704. doi:10.1016/j.jemermed.2016.06.054. PubMed
62. Pines JM, Perina DG, Brady WJ. Electrocardiogram interpretation training and competency assessment in emergency medicine residency programs. Acad Emerg Med. 2004;11(9):982-984. doi:10.1197/j.aem.2004.03.023. PubMed
63. Demircan A, Bildik F, Ergin M. Electrocardiography interpretation training in emergency medicine : methods, resources, competency assessment, and national standardization. Signa Vitae. 2015;10(1):38-52.
64. Ferrell BG, Camp DL. Comparing a Four-Week Block Clerkship to a Twelve-Week Longitudinal Experience in Family Medicine. In: Scherpbier AJJA, van der Vleuten CPM, Rethans JJ, and van der Steeg AFW, editors. Advances in Medical Education. Dordrecht: Springer Netherlands; 1997:744-746. doi:10.1007/978-94-011-4886-3_226.
65. Marinović D, Hren D, Sambunjak D, et al. Transition from longitudinal to block structure of preclinical courses: outcomes and experiences. Croat Med J. 2009;50(5):492-506. doi:10.3325/cmj.2009.50.492. PubMed
66. Melo J, Kaneshiro B, Kellett L, Hiraoka M. The impact of a longitudinal curriculum on medical student obstetrics and gynecology clinical training. Hawaii J Med Public Health. 2014;73(5):144-147. Accessed January 31, 2017. PubMed
The 12-lead electrocardiogram (ECG) remains one of the most widely used and readily available diagnostic tests in modern medicine.1 Reflecting the electrical behavior of the heart, this point-of-care diagnostic test is used in almost every area of medicine for diagnosis, prognostication, and selection of appropriate treatment. The ECG is sometimes the only and most efficient way of detecting life-threatening conditions, thus allowing a timely delivery of emergency care.2 However, the practical power of the 12-lead ECG relies on the ability of the clinician to interpret this test correctly.
For decades, ECG interpretation has been a core component of undergraduate and postgraduate medical training.3-5 Unfortunately, numerous studies have demonstrated alarming rates of inaccuracy and variability in interpreting ECGs among trainees at all levels of education.4,6,7 Senior medical students have been repeatedly shown to miss 26% to 62% of acute myocardial infarctions (MI).6,8-10 Another recent study involving internal medicine residents demonstrated that only half of the straightforward common ECGs were interpreted correctly, while 26% of trainees missed an acute MI and 56% missed ventricular tachycardia (VT).11 Even cardiology subspecialty fellows demonstrated poor performance, missing up to 26% of ST-elevation MIs on ECGs that had multiple findings.12 Inaccurate interpretations of ECGs can lead to inappropriate management decisions, adverse patient outcomes, unnecessary additional testing, and even preventable deaths.4,13-15
Several guidelines have emphasized the importance of teaching trainees 12-lead ECG interpretation and have recognized the value of assessments in ensuring that learners acquire the necessary competencies.16-19 Similarly, there have been many calls for more rigorous and structured curricula for ECG interpretation throughout undergraduate and postgraduate medical education.11,16 However, we still lack a thoughtful guideline outlining the specific competencies that medical trainees should attain. This includes medical students, nurses working in hospital and in out-of-hospital settings, and residents of different specialties, including emergency medicine, cardiology, and electrophysiology (EP) fellows.
Setting goals and objectives for target learners is recognized to be the initial step and a core prerequisite for effective curriculum development.20 In this publication, we summarize the objectives from previously published trainee assessments and propose reasonably attainable ECG interpretation competencies for both graduating medical students and residents at the end of their postgraduate training. This document is being endorsed by researchers and educators of 2 international societies dedicated to the study of electrical heart diseases: the International Society of Electrocardiology (ISE) and the International Society of Holter and Noninvasive Electrocardiology (ISHNE).
METHODS
Current Competencies in Literature
We performed a systematic search to identify ECG competencies that are currently mentioned in the literature. Information was retrieved from MEDLINE (1946-2016) and EMBASE (1947-2016) by using the following MeSH terms: electrocardiogram, electrocardiography, electrocardiogram interpretation, electrocardiogram competency, medical school, medical student, undergraduate medicine, undergraduate medical education, residency education, internship, and residency. Our search was limited to English-language articles that studied physician trainees. The references of the full-length articles were examined for additional citations. The search revealed a total of 65 publications involving medical students and 120 publications involving residents. Abstracts of publications were then assessed for relevance, and the methods of the remaining articles were scrutinized for references to specific ECG interpretation objectives. This strategy narrowed the search to 9 and 14 articles involving medical students and residents, respectively. Studies were not graded for quality because the purpose of the search was to identify the specific ECG competencies that authors expected trainees to obtain. Almost all the articles proposed teaching tools and specific objectives that were defined by the investigators arbitrarily and assessed the trainee’s ability to interpret ECGs (summarized in supplementary Table).
Defining ECG Interpretation Competencies
The initial draft of proposed ECG interpretation competencies was developed at Queen’s University in Ontario, Canada. A list of ECG patterns and diagnoses previously mentioned in literature was used as a starting point. From there, each item was refined and organized into 4 main categories (see Figures 1 and 2).
Class A “Common electrocardiographic emergencies” represent patterns that are frequently seen in hospitals, in which accurate interpretation of the ECG within minutes is essential for delivering care that is potentially lifesaving to the patient (eg, ST-elevation MI).
Class B “Common nonemergency patterns” represent ECG findings that are encountered daily in patients who are not acutely ill, which may impact their care in the appropriate clinical context (eg, left ventricular hypertrophy).
Class C “Uncommon electrocardiographic emergencies” represent ECG findings that are not encountered on a daily basis but can be potentially lifesaving if recognized (eg ventricular preexcitation).
Class D “Uncommon nonemergency patterns” represent findings that are uncommon but may diagnostically contribute to patient care in a clinically appropriate setting (eg, right atrial abnormality).
ECG interpretation patterns were then assigned to medical students and residents based on the specific goals of training. At the time of graduation, medical students should develop the foundation for learning ECG interpretation in residency training, provide ECG interpretation and initial management for electrocardiographic emergencies, and obtain assistance from a more senior medical professional within a clinically appropriate time frame. The training goal for a resident is to develop ECG interpretation competencies for safe independent clinical practice (Figure 1).
The final segregated ECG interpretation competencies were distributed to members of ISE and ISHNE for input, modifications, and revisions. The proposed list of competencies went through several revisions until a consensus was reached.
RESULTS
The final distribution of ECG patterns is illustrated in Figure 2. (Figure 3 defines the learning objectives for each ECG pattern defined in Figure 2.) Here, we provide a rationale for
Class A: Common Electrocardiographic Emergencies
This group contains ECG findings that require recognition within minutes to deliver potentially lifesaving care. For this reason, undergraduate medical education programs should prioritize mastering class A conditions to minimize the risk of misdiagnosis and late recognition.
Class A patterns include ST elevation MI (STEMI) and localization of territory to ensure ST-segment elevations are seen in contiguous leads.29,30 Students should learn the criteria for STEMI as per the “Universal Definition of Myocardial Infarction” and be aware of early signs of STEMI that may be seen prior to ST-segment changes, such as hyper-acute T-waves (increased amplitude and symmetrical).30
Asystole, wide complex tachycardias, and ventricular fibrillation (VF) are all crucial ECG patterns that must be identified to deliver advanced cardiac life support (ACLS) care as per the 2010 AHA Guidelines for cardiopulmonary resuscitation and emergency cardio care.31 Of note, students should understand the differential diagnosis of wide complex tachycardias and should be able to suspect VF in clinically appropriate scenarios. We included the category “unstable/symptomatic supraventricular tachycardia” to represent rapid rhythms that are supraventricular in origin, which either produce symptoms or cause impairment of vital organ function.31 In emergency situations, it may not be crucial to correctly identify the specific supraventricular rhythm to deliver ACLS care; hence, the specific supraventricular tachycardia diagnoses were included in Class B.
Finally, we believe that medical students should be able to recognize long QT, hypo/hyperkalemia, and distinguish types of atrioventricular (AV) block. Distinguishing types of AV block is important because both third degree AV block and second degree AV block Mobitz II can be life threatening and require further investigation or emergency treatment in an inpatient setting.32 Prompt recognition of long QT is crucial because it can be associated with ventricular tachyarrhythmias. This includes a polymorphic pattern characterized by the twisting of QRS peaks around the baseline (torsades des pointes), which can eventually lead to VF.
Class B: Common Nonemergency Patterns
Class B patterns represent common findings that are seen on a daily basis that may impact patient care in a clinically appropriate context. Diagnoses in this section were divided into “tachycardia syndromes,” “bradycardia syndromes,” “conduction abnormalities,” “ischemia,” and “other.”
Undergraduate trainees should become proficient in identifying the cause of bradycardia and distinguishing types of AV blocks. Similarly, they should also have an approach to differentiate tachycardia syndromes.33,34 These skills are required to correctly manage patients in both inpatient and outpatient settings. They should be taught in undergraduate programs and reinforced in postgraduate training.
Common findings, such as bundle branch blocks, left anterior fascicular block, premature ventricular/atrial complexes, electronic pacemakers, and left ventricular hypertrophy, are essential to the daily interpretation of ECGs. Junior learners should be proficient in recognizing these patterns. Findings consistent with pericarditis are not uncommon and can be very helpful to guide the clinician to the diagnosis. Notable exceptions from the medical student competency list include detection of lead misplacement, common artifacts, nonspecific intraventricular conduction delay, interatrial block, and benign early repolarization. These findings require a deeper understanding of electrocardiography and would be more appropriate for senior learners.
Class C: Uncommon Electrocardiographic Emergencies
Class C findings represent uncommon conditions that, if recognized, can prevent serious adverse patient outcomes. These include preexcitation, STEMI with preexisting left bundle branch block sinus pauses, Brugada pattern, hypothermia, effects of toxic drugs, ventricular aneurysm, and right ventricular hypertrophy. The recognition of these patterns is crucial to avoid severe adverse patient outcomes, and independent practicing physicians should be aware of these findings. However, given that a high proportion of senior medical students miss common electrocardiographic emergencies, undergraduate medical education programs should instead focus resources on ensuring medical students are proficient in identifying class A and class B conditions.6,8-10 Postgraduate programs should ensure that postgraduate trainees can identify these potentially life-threatening conditions (see section “How to Teach Electrocardiology”).
Class D: Uncommon and Nonemergency Patterns
Class D findings represent less common findings that are not seen every day and do not require urgent medical attention. These include right atrial abnormality, left posterior fascicular block, low atrial rhythms, and electrolyte abnormalities that exclude potassium. Notably, electrolyte abnormalities are important to identify; however, typically, treatment is guided by the lab results.35 Overall, postgraduate trainees should certainly be aware of these findings, but medical student training should instead focus on learning the framework and correctly identifying class A and class B ECG patterns.
HOW TO TEACH ELECTROCARDIOLOGY
Teaching ECG Interpretation Strategies
No clear teaching approaches to ECG interpretation have been described in the literature, and no recommendations on knowledge translation have been formally explored. A possible educational approach to the teaching of electrocardiology could involve several methods for helping students with ECG interpretation:36
1. Pattern recognition: The ECG, at its most immediate level, is a graphic image, and recognition of images is essentially recognition of patterns. These patterns can only be learned through repeated visualization of examples with a written or verbal explanation. Repeated visualization over time will help avoid “erosion” of knowledge. Examples of learning tools include periodic in-person ECG rounds, well-illustrated books or atlases, and online tools with good quality ECGs and explanations. These learning opportunities are strongly reinforced by collecting cases from the clinical encounters of the trainee that illustrate the aforementioned patterns. Some of these patterns can be found in guidelines, such as the one published by the AHA and ACC.29
2. Application of published criteria: Guidelines, review papers, and books offer diagnostic criteria for many entities, such as chamber enlargement, bundle branch blocks, and abnormal Q waves. Learning these criteria and applying them to the analysis of ECGs is a commonly used learning strategy.
3. Inductive-deductive reasoning: This strategy requires a deeper understanding of the pathophysiology behind ECG patterns. It requires ECGs to be interpreted in a certain clinical context, and the goal of ECG interpretation is to answer a clinical question that is used to guide patient care. This strategy typically employs the use of algorithms to lead the interpreter to the correct diagnosis, and mastery of this skill grows from ongoing clinical experience. Examples of the “inductive-deductive reasoning” are localizing an accessory AV pathway, the differential diagnosis of narrow or wide complex tachycardias, and identifying the site of coronary artery occlusion in a patient with a STEMI.
4. Ladder diagrams: Ladder diagrams have been used for over 100 years to graphically illustrate the mechanism of arrhythmias. They can be incredibly useful to help learners visualize impulse conduction in reentry mechanisms as well as other abnormal rhythms. However, there are some rhythms that are difficult to illustrate on ladder diagrams.37
5. Peer and near-peer teaching: Peer teaching occurs when learners prepare and deliver teaching material to learners of a similar training level. The expectation to deliver a teaching session encourages students to learn and organize information in thoughtful ways. It builds strong teamwork skills and has been shown to positively affect all involved learners.38-40
Each ECG interpretation strategy has its advantages, and we recommend that students be exposed to all available approaches if teaching resources are available.
Teaching Delivery Format
Each of the above teaching strategies can be delivered to students in various ways. The following teaching formats have been previously documented in the literature:
1. Classroom-based teaching: This is a traditional learning format that takes place in a large- or small-group classroom. Typically, these sessions are led by a single instructor, and they are focused on the direct sharing of information and group discussion.41
2. Electronic practice tools: Numerous electronic tools have been developed with the purpose of providing deliberate practice to master ECG interpretation. Some of these tools employ active learner engagement, while others provide a bank of ECGs for self-directed passive learning.42-46
3. Video lectures: Short video lectures have been created to facilitate self-directed lecture based learning. These lectures are hosted on a variety of web-based platforms, including YouTube and Vimeo.47
4. Traditional and electronic books: Numerous traditional textbooks have been published on ECG interpretation and are designed to facilitate independent learning. Some textbooks directly deliver teaching material, while others contain sets of ECGs to allow for repetitive practice. More recently, iBooks incorporating self-assessment tools have been used to assist ECG teaching.34 The advantage of these tools is that they can also be used to supplement in-person classes.
5. Games: A unique ECG interpretation learning strategy consists of using puzzles and games to learn ECGs. This is meant to improve student engagement and interest in learning ECG interpretation.48
Given that there is currently a lack of evidence-based data to support 1 instructional format over another, we do not favor any particular one. This decision should be left to instructors and individual learners based on their preference and available resources. Further studies would be helpful to determine the effectiveness of various methods in teaching ECG interpretation and to identify any additional specific factors that facilitate learning.
Evaluation Strategies
1. Longitudinal ongoing feedback: This form of feedback universally takes place in all training programs and focuses on direct observation and point-of-care feedback by a senior healthcare professional during clinical practice. Typically, the feedback is informal and is centered around specific case presentations.
2. Formative testing: This assessment strategy is aimed at monitoring the learning of trainees and providing them with appropriate feedback. Tutors and teachers can use this data to individualize instruction and fill any training gaps that individuals and the class may have. Students themselves can use this information to encourage additional study to ensure they acquire required skills. Examples of formative testing are low-stakes in-training exams and asking audience questions during a workshop or lecture.49
3. Summative testing: Summative assessments are created to measure the level of proficiency developed by a learner and compare it against some standard or benchmark. This form of assessment establishes the extent to which educational objectives have been met. The most common example is an end-of-term examination.
Online ECG examination has been successfully used to provide methods of testing. They are easy to distribute, highly convenient for learners, and allow the display of high-quality graphics. They can also be graded electronically, thereby minimizing the resources required to administer and grade exams.36,50
We recommend using a combination of assessment formats to ensure the optimal evaluation of learner skill and to focus learning on areas of weakness. Summative assessments are highly valuable to ensure learners acquired the necessary ECG interpretation competencies. Remediation strategies should be available to provide additional practice to learners who do not meet competencies expected at their level of training.
DISCUSSION
The Need for ECG Interpretation Competencies and Milestones
Since the introduction of ECG in the late 1800s, there continues to be a significant variation in ECG interpretation skills among trainees and medical professionals.4,6-12 Concerns continue to exist about the rate of missed diagnoses involving critical ECGs, leading to inappropriate patient management decisions. Despite the obvious need, teaching ECG interpretation is given little emphasis in medical education, and the curriculum remains quite disorganized. In this position paper, we call for a more structured ECG interpretation curriculum in medical education and hope to assist this process by assigning ECG patterns to 2 milestones in training: graduating medical students and first year postgraduate medical residents.
Defining competencies would help medical education programs to focus resources on teaching clinically important conditions for the appropriate level of training. We divide ECG findings into 4 categories (classes A to D), and we place emphasis on learning electrocardiographic emergencies early in training and spending less time on ECG findings that are unlikely to change patient management.
The goal is to ensure 100% recognition of class A (electrocardiographic emergencies) by the end of medical school. To ensure each medical education program fulfils this goal, a structured curriculum including a summative assessment is required.
Methods of Teaching
Various instructional mediums have been successfully implemented to teach ECG interpretation competencies, including lectures, puzzles, web-based programs, iBooks, and YouTube.34-41-44,47,48.51-53 A survey of clerkship directors in internal medicine revealed that 75% of clerkship programs teach ECG interpretation in a classroom lecture-based setting, 44% use teaching rounds, and only 17% utilize online/web-based instruction.3 Canadian family medicine programs have a relatively equal distribution between classroom-based, computer-based, and bedside teaching.5
In comparing the efficacy of instructional styles, several small comparative studies favor an electronic teaching format because of the enhanced learner interaction and visual learning, but there does not appear to be a consistently proven large advantage of 1 teaching format over another.43,48,51,54 The overall theme emerging from this literature is the importance of repetition and active engagement in ECG interpretation, which appear to be more important than 1 particular strategy.22 Computer-based training appears to deliver these 2 qualities, unlike the traditional lecture-style passive learning model. The concept of repetition and engagement is also well supported in medical education literature outside ECG interpretation.55,56
Given these data, we recommend that each medical education program select teaching methods based on their available resources, as long as adequate teaching time is allotted to ensure that trainees acquire the competencies defined in this publication.
Assessment Methods
It appears that the larger factor in determining ECG interpretation performance is not the learning format, but the form of assessment. Two studies have demonstrated that summative assessment substantially improves ECG interpretation performance when compared with formative assessment; in fact, this effect was so large that it overshadowed any small difference in teaching formats.57,58 This concept aligns with medical education literature, which acknowledges that assessment drives learning by raising the stakes, thereby boosting student effort and encouraging learning to an effect much larger than can be generated by any particular learning style.57,59 Nevertheless, well-designed formative assessment can focus students on effective learning by identifying gaps and important information.60 Only 33% of Canadian family medicine residency programs and 71% of American clerkship programs have formal assessment of ECG interpretation skills.3,5 There is no doubt that assessment, both formative and summative, should be implemented in all undergraduate and postgraduate medical training programs. Online assessment methods have the advantage of delivering high-quality images and a variety of question formats; hence, their use should be encouraged.36,50,61-63
Teaching Personnel and Timing of Training
Who should teach ECG interpretation and when should this teaching take place? ECG interpretation in training programs is typically taught by attending physicians in each respective field. However, given that there is a large ECG interpretation error rate by noncardiologist physicians, we advise that ECG training content be created with input from own-specialty attending physicians and cardiologists.4 This teaching should take place early in medical school at the time medical students learn pathophysiology of the heart and should continue throughout training. Longitudinal training is preferred to block-based training because of improved resident satisfaction, but medical education literature did not reveal a difference in student performance with either strategy.64-66
CONCLUSIONS
Despite its immense clinical value, there continues to be a lack of a comprehensive ECG interpretation curriculum in medical education programs. The goal of this position paper is to encourage the development of organized curricula in undergraduate and postgraduate medical education programs, and to ensure the acquisition of level-appropriate ECG interpretation skills while maintaining patient safety. We assist this process by grouping ECG findings into 4 classes (A to D) based on the frequency of encounter and emergent nature and by assigning them to each level of training. Methods of teaching ECG interpretation are less important and can be selected based on the available resources of each education program and student preference; however, online learning is encouraged. We also recommend that summative trainee evaluation methods be implemented in all programs to ensure that appropriate competencies are acquired and to further encourage self-directed learning. Resources should be allocated to ensure that every trainee is reaching their training milestones and should ensure that no electrocardiographic emergency (class A condition) is ever missed by a trainee. We hope that these guidelines will inform medical education systems and help prevent adverse patient outcomes caused by the misinterpretation of this valuable clinical diagnostic tool.
Disclosure
On behalf of all authors, the corresponding author states that there is no conflict of interest. This manuscript did not utilize any sources of funding.
The 12-lead electrocardiogram (ECG) remains one of the most widely used and readily available diagnostic tests in modern medicine.1 Reflecting the electrical behavior of the heart, this point-of-care diagnostic test is used in almost every area of medicine for diagnosis, prognostication, and selection of appropriate treatment. The ECG is sometimes the only and most efficient way of detecting life-threatening conditions, thus allowing a timely delivery of emergency care.2 However, the practical power of the 12-lead ECG relies on the ability of the clinician to interpret this test correctly.
For decades, ECG interpretation has been a core component of undergraduate and postgraduate medical training.3-5 Unfortunately, numerous studies have demonstrated alarming rates of inaccuracy and variability in interpreting ECGs among trainees at all levels of education.4,6,7 Senior medical students have been repeatedly shown to miss 26% to 62% of acute myocardial infarctions (MI).6,8-10 Another recent study involving internal medicine residents demonstrated that only half of the straightforward common ECGs were interpreted correctly, while 26% of trainees missed an acute MI and 56% missed ventricular tachycardia (VT).11 Even cardiology subspecialty fellows demonstrated poor performance, missing up to 26% of ST-elevation MIs on ECGs that had multiple findings.12 Inaccurate interpretations of ECGs can lead to inappropriate management decisions, adverse patient outcomes, unnecessary additional testing, and even preventable deaths.4,13-15
Several guidelines have emphasized the importance of teaching trainees 12-lead ECG interpretation and have recognized the value of assessments in ensuring that learners acquire the necessary competencies.16-19 Similarly, there have been many calls for more rigorous and structured curricula for ECG interpretation throughout undergraduate and postgraduate medical education.11,16 However, we still lack a thoughtful guideline outlining the specific competencies that medical trainees should attain. This includes medical students, nurses working in hospital and in out-of-hospital settings, and residents of different specialties, including emergency medicine, cardiology, and electrophysiology (EP) fellows.
Setting goals and objectives for target learners is recognized to be the initial step and a core prerequisite for effective curriculum development.20 In this publication, we summarize the objectives from previously published trainee assessments and propose reasonably attainable ECG interpretation competencies for both graduating medical students and residents at the end of their postgraduate training. This document is being endorsed by researchers and educators of 2 international societies dedicated to the study of electrical heart diseases: the International Society of Electrocardiology (ISE) and the International Society of Holter and Noninvasive Electrocardiology (ISHNE).
METHODS
Current Competencies in Literature
We performed a systematic search to identify ECG competencies that are currently mentioned in the literature. Information was retrieved from MEDLINE (1946-2016) and EMBASE (1947-2016) by using the following MeSH terms: electrocardiogram, electrocardiography, electrocardiogram interpretation, electrocardiogram competency, medical school, medical student, undergraduate medicine, undergraduate medical education, residency education, internship, and residency. Our search was limited to English-language articles that studied physician trainees. The references of the full-length articles were examined for additional citations. The search revealed a total of 65 publications involving medical students and 120 publications involving residents. Abstracts of publications were then assessed for relevance, and the methods of the remaining articles were scrutinized for references to specific ECG interpretation objectives. This strategy narrowed the search to 9 and 14 articles involving medical students and residents, respectively. Studies were not graded for quality because the purpose of the search was to identify the specific ECG competencies that authors expected trainees to obtain. Almost all the articles proposed teaching tools and specific objectives that were defined by the investigators arbitrarily and assessed the trainee’s ability to interpret ECGs (summarized in supplementary Table).
Defining ECG Interpretation Competencies
The initial draft of proposed ECG interpretation competencies was developed at Queen’s University in Ontario, Canada. A list of ECG patterns and diagnoses previously mentioned in literature was used as a starting point. From there, each item was refined and organized into 4 main categories (see Figures 1 and 2).
Class A “Common electrocardiographic emergencies” represent patterns that are frequently seen in hospitals, in which accurate interpretation of the ECG within minutes is essential for delivering care that is potentially lifesaving to the patient (eg, ST-elevation MI).
Class B “Common nonemergency patterns” represent ECG findings that are encountered daily in patients who are not acutely ill, which may impact their care in the appropriate clinical context (eg, left ventricular hypertrophy).
Class C “Uncommon electrocardiographic emergencies” represent ECG findings that are not encountered on a daily basis but can be potentially lifesaving if recognized (eg ventricular preexcitation).
Class D “Uncommon nonemergency patterns” represent findings that are uncommon but may diagnostically contribute to patient care in a clinically appropriate setting (eg, right atrial abnormality).
ECG interpretation patterns were then assigned to medical students and residents based on the specific goals of training. At the time of graduation, medical students should develop the foundation for learning ECG interpretation in residency training, provide ECG interpretation and initial management for electrocardiographic emergencies, and obtain assistance from a more senior medical professional within a clinically appropriate time frame. The training goal for a resident is to develop ECG interpretation competencies for safe independent clinical practice (Figure 1).
The final segregated ECG interpretation competencies were distributed to members of ISE and ISHNE for input, modifications, and revisions. The proposed list of competencies went through several revisions until a consensus was reached.
RESULTS
The final distribution of ECG patterns is illustrated in Figure 2. (Figure 3 defines the learning objectives for each ECG pattern defined in Figure 2.) Here, we provide a rationale for
Class A: Common Electrocardiographic Emergencies
This group contains ECG findings that require recognition within minutes to deliver potentially lifesaving care. For this reason, undergraduate medical education programs should prioritize mastering class A conditions to minimize the risk of misdiagnosis and late recognition.
Class A patterns include ST elevation MI (STEMI) and localization of territory to ensure ST-segment elevations are seen in contiguous leads.29,30 Students should learn the criteria for STEMI as per the “Universal Definition of Myocardial Infarction” and be aware of early signs of STEMI that may be seen prior to ST-segment changes, such as hyper-acute T-waves (increased amplitude and symmetrical).30
Asystole, wide complex tachycardias, and ventricular fibrillation (VF) are all crucial ECG patterns that must be identified to deliver advanced cardiac life support (ACLS) care as per the 2010 AHA Guidelines for cardiopulmonary resuscitation and emergency cardio care.31 Of note, students should understand the differential diagnosis of wide complex tachycardias and should be able to suspect VF in clinically appropriate scenarios. We included the category “unstable/symptomatic supraventricular tachycardia” to represent rapid rhythms that are supraventricular in origin, which either produce symptoms or cause impairment of vital organ function.31 In emergency situations, it may not be crucial to correctly identify the specific supraventricular rhythm to deliver ACLS care; hence, the specific supraventricular tachycardia diagnoses were included in Class B.
Finally, we believe that medical students should be able to recognize long QT, hypo/hyperkalemia, and distinguish types of atrioventricular (AV) block. Distinguishing types of AV block is important because both third degree AV block and second degree AV block Mobitz II can be life threatening and require further investigation or emergency treatment in an inpatient setting.32 Prompt recognition of long QT is crucial because it can be associated with ventricular tachyarrhythmias. This includes a polymorphic pattern characterized by the twisting of QRS peaks around the baseline (torsades des pointes), which can eventually lead to VF.
Class B: Common Nonemergency Patterns
Class B patterns represent common findings that are seen on a daily basis that may impact patient care in a clinically appropriate context. Diagnoses in this section were divided into “tachycardia syndromes,” “bradycardia syndromes,” “conduction abnormalities,” “ischemia,” and “other.”
Undergraduate trainees should become proficient in identifying the cause of bradycardia and distinguishing types of AV blocks. Similarly, they should also have an approach to differentiate tachycardia syndromes.33,34 These skills are required to correctly manage patients in both inpatient and outpatient settings. They should be taught in undergraduate programs and reinforced in postgraduate training.
Common findings, such as bundle branch blocks, left anterior fascicular block, premature ventricular/atrial complexes, electronic pacemakers, and left ventricular hypertrophy, are essential to the daily interpretation of ECGs. Junior learners should be proficient in recognizing these patterns. Findings consistent with pericarditis are not uncommon and can be very helpful to guide the clinician to the diagnosis. Notable exceptions from the medical student competency list include detection of lead misplacement, common artifacts, nonspecific intraventricular conduction delay, interatrial block, and benign early repolarization. These findings require a deeper understanding of electrocardiography and would be more appropriate for senior learners.
Class C: Uncommon Electrocardiographic Emergencies
Class C findings represent uncommon conditions that, if recognized, can prevent serious adverse patient outcomes. These include preexcitation, STEMI with preexisting left bundle branch block sinus pauses, Brugada pattern, hypothermia, effects of toxic drugs, ventricular aneurysm, and right ventricular hypertrophy. The recognition of these patterns is crucial to avoid severe adverse patient outcomes, and independent practicing physicians should be aware of these findings. However, given that a high proportion of senior medical students miss common electrocardiographic emergencies, undergraduate medical education programs should instead focus resources on ensuring medical students are proficient in identifying class A and class B conditions.6,8-10 Postgraduate programs should ensure that postgraduate trainees can identify these potentially life-threatening conditions (see section “How to Teach Electrocardiology”).
Class D: Uncommon and Nonemergency Patterns
Class D findings represent less common findings that are not seen every day and do not require urgent medical attention. These include right atrial abnormality, left posterior fascicular block, low atrial rhythms, and electrolyte abnormalities that exclude potassium. Notably, electrolyte abnormalities are important to identify; however, typically, treatment is guided by the lab results.35 Overall, postgraduate trainees should certainly be aware of these findings, but medical student training should instead focus on learning the framework and correctly identifying class A and class B ECG patterns.
HOW TO TEACH ELECTROCARDIOLOGY
Teaching ECG Interpretation Strategies
No clear teaching approaches to ECG interpretation have been described in the literature, and no recommendations on knowledge translation have been formally explored. A possible educational approach to the teaching of electrocardiology could involve several methods for helping students with ECG interpretation:36
1. Pattern recognition: The ECG, at its most immediate level, is a graphic image, and recognition of images is essentially recognition of patterns. These patterns can only be learned through repeated visualization of examples with a written or verbal explanation. Repeated visualization over time will help avoid “erosion” of knowledge. Examples of learning tools include periodic in-person ECG rounds, well-illustrated books or atlases, and online tools with good quality ECGs and explanations. These learning opportunities are strongly reinforced by collecting cases from the clinical encounters of the trainee that illustrate the aforementioned patterns. Some of these patterns can be found in guidelines, such as the one published by the AHA and ACC.29
2. Application of published criteria: Guidelines, review papers, and books offer diagnostic criteria for many entities, such as chamber enlargement, bundle branch blocks, and abnormal Q waves. Learning these criteria and applying them to the analysis of ECGs is a commonly used learning strategy.
3. Inductive-deductive reasoning: This strategy requires a deeper understanding of the pathophysiology behind ECG patterns. It requires ECGs to be interpreted in a certain clinical context, and the goal of ECG interpretation is to answer a clinical question that is used to guide patient care. This strategy typically employs the use of algorithms to lead the interpreter to the correct diagnosis, and mastery of this skill grows from ongoing clinical experience. Examples of the “inductive-deductive reasoning” are localizing an accessory AV pathway, the differential diagnosis of narrow or wide complex tachycardias, and identifying the site of coronary artery occlusion in a patient with a STEMI.
4. Ladder diagrams: Ladder diagrams have been used for over 100 years to graphically illustrate the mechanism of arrhythmias. They can be incredibly useful to help learners visualize impulse conduction in reentry mechanisms as well as other abnormal rhythms. However, there are some rhythms that are difficult to illustrate on ladder diagrams.37
5. Peer and near-peer teaching: Peer teaching occurs when learners prepare and deliver teaching material to learners of a similar training level. The expectation to deliver a teaching session encourages students to learn and organize information in thoughtful ways. It builds strong teamwork skills and has been shown to positively affect all involved learners.38-40
Each ECG interpretation strategy has its advantages, and we recommend that students be exposed to all available approaches if teaching resources are available.
Teaching Delivery Format
Each of the above teaching strategies can be delivered to students in various ways. The following teaching formats have been previously documented in the literature:
1. Classroom-based teaching: This is a traditional learning format that takes place in a large- or small-group classroom. Typically, these sessions are led by a single instructor, and they are focused on the direct sharing of information and group discussion.41
2. Electronic practice tools: Numerous electronic tools have been developed with the purpose of providing deliberate practice to master ECG interpretation. Some of these tools employ active learner engagement, while others provide a bank of ECGs for self-directed passive learning.42-46
3. Video lectures: Short video lectures have been created to facilitate self-directed lecture based learning. These lectures are hosted on a variety of web-based platforms, including YouTube and Vimeo.47
4. Traditional and electronic books: Numerous traditional textbooks have been published on ECG interpretation and are designed to facilitate independent learning. Some textbooks directly deliver teaching material, while others contain sets of ECGs to allow for repetitive practice. More recently, iBooks incorporating self-assessment tools have been used to assist ECG teaching.34 The advantage of these tools is that they can also be used to supplement in-person classes.
5. Games: A unique ECG interpretation learning strategy consists of using puzzles and games to learn ECGs. This is meant to improve student engagement and interest in learning ECG interpretation.48
Given that there is currently a lack of evidence-based data to support 1 instructional format over another, we do not favor any particular one. This decision should be left to instructors and individual learners based on their preference and available resources. Further studies would be helpful to determine the effectiveness of various methods in teaching ECG interpretation and to identify any additional specific factors that facilitate learning.
Evaluation Strategies
1. Longitudinal ongoing feedback: This form of feedback universally takes place in all training programs and focuses on direct observation and point-of-care feedback by a senior healthcare professional during clinical practice. Typically, the feedback is informal and is centered around specific case presentations.
2. Formative testing: This assessment strategy is aimed at monitoring the learning of trainees and providing them with appropriate feedback. Tutors and teachers can use this data to individualize instruction and fill any training gaps that individuals and the class may have. Students themselves can use this information to encourage additional study to ensure they acquire required skills. Examples of formative testing are low-stakes in-training exams and asking audience questions during a workshop or lecture.49
3. Summative testing: Summative assessments are created to measure the level of proficiency developed by a learner and compare it against some standard or benchmark. This form of assessment establishes the extent to which educational objectives have been met. The most common example is an end-of-term examination.
Online ECG examination has been successfully used to provide methods of testing. They are easy to distribute, highly convenient for learners, and allow the display of high-quality graphics. They can also be graded electronically, thereby minimizing the resources required to administer and grade exams.36,50
We recommend using a combination of assessment formats to ensure the optimal evaluation of learner skill and to focus learning on areas of weakness. Summative assessments are highly valuable to ensure learners acquired the necessary ECG interpretation competencies. Remediation strategies should be available to provide additional practice to learners who do not meet competencies expected at their level of training.
DISCUSSION
The Need for ECG Interpretation Competencies and Milestones
Since the introduction of ECG in the late 1800s, there continues to be a significant variation in ECG interpretation skills among trainees and medical professionals.4,6-12 Concerns continue to exist about the rate of missed diagnoses involving critical ECGs, leading to inappropriate patient management decisions. Despite the obvious need, teaching ECG interpretation is given little emphasis in medical education, and the curriculum remains quite disorganized. In this position paper, we call for a more structured ECG interpretation curriculum in medical education and hope to assist this process by assigning ECG patterns to 2 milestones in training: graduating medical students and first year postgraduate medical residents.
Defining competencies would help medical education programs to focus resources on teaching clinically important conditions for the appropriate level of training. We divide ECG findings into 4 categories (classes A to D), and we place emphasis on learning electrocardiographic emergencies early in training and spending less time on ECG findings that are unlikely to change patient management.
The goal is to ensure 100% recognition of class A (electrocardiographic emergencies) by the end of medical school. To ensure each medical education program fulfils this goal, a structured curriculum including a summative assessment is required.
Methods of Teaching
Various instructional mediums have been successfully implemented to teach ECG interpretation competencies, including lectures, puzzles, web-based programs, iBooks, and YouTube.34-41-44,47,48.51-53 A survey of clerkship directors in internal medicine revealed that 75% of clerkship programs teach ECG interpretation in a classroom lecture-based setting, 44% use teaching rounds, and only 17% utilize online/web-based instruction.3 Canadian family medicine programs have a relatively equal distribution between classroom-based, computer-based, and bedside teaching.5
In comparing the efficacy of instructional styles, several small comparative studies favor an electronic teaching format because of the enhanced learner interaction and visual learning, but there does not appear to be a consistently proven large advantage of 1 teaching format over another.43,48,51,54 The overall theme emerging from this literature is the importance of repetition and active engagement in ECG interpretation, which appear to be more important than 1 particular strategy.22 Computer-based training appears to deliver these 2 qualities, unlike the traditional lecture-style passive learning model. The concept of repetition and engagement is also well supported in medical education literature outside ECG interpretation.55,56
Given these data, we recommend that each medical education program select teaching methods based on their available resources, as long as adequate teaching time is allotted to ensure that trainees acquire the competencies defined in this publication.
Assessment Methods
It appears that the larger factor in determining ECG interpretation performance is not the learning format, but the form of assessment. Two studies have demonstrated that summative assessment substantially improves ECG interpretation performance when compared with formative assessment; in fact, this effect was so large that it overshadowed any small difference in teaching formats.57,58 This concept aligns with medical education literature, which acknowledges that assessment drives learning by raising the stakes, thereby boosting student effort and encouraging learning to an effect much larger than can be generated by any particular learning style.57,59 Nevertheless, well-designed formative assessment can focus students on effective learning by identifying gaps and important information.60 Only 33% of Canadian family medicine residency programs and 71% of American clerkship programs have formal assessment of ECG interpretation skills.3,5 There is no doubt that assessment, both formative and summative, should be implemented in all undergraduate and postgraduate medical training programs. Online assessment methods have the advantage of delivering high-quality images and a variety of question formats; hence, their use should be encouraged.36,50,61-63
Teaching Personnel and Timing of Training
Who should teach ECG interpretation and when should this teaching take place? ECG interpretation in training programs is typically taught by attending physicians in each respective field. However, given that there is a large ECG interpretation error rate by noncardiologist physicians, we advise that ECG training content be created with input from own-specialty attending physicians and cardiologists.4 This teaching should take place early in medical school at the time medical students learn pathophysiology of the heart and should continue throughout training. Longitudinal training is preferred to block-based training because of improved resident satisfaction, but medical education literature did not reveal a difference in student performance with either strategy.64-66
CONCLUSIONS
Despite its immense clinical value, there continues to be a lack of a comprehensive ECG interpretation curriculum in medical education programs. The goal of this position paper is to encourage the development of organized curricula in undergraduate and postgraduate medical education programs, and to ensure the acquisition of level-appropriate ECG interpretation skills while maintaining patient safety. We assist this process by grouping ECG findings into 4 classes (A to D) based on the frequency of encounter and emergent nature and by assigning them to each level of training. Methods of teaching ECG interpretation are less important and can be selected based on the available resources of each education program and student preference; however, online learning is encouraged. We also recommend that summative trainee evaluation methods be implemented in all programs to ensure that appropriate competencies are acquired and to further encourage self-directed learning. Resources should be allocated to ensure that every trainee is reaching their training milestones and should ensure that no electrocardiographic emergency (class A condition) is ever missed by a trainee. We hope that these guidelines will inform medical education systems and help prevent adverse patient outcomes caused by the misinterpretation of this valuable clinical diagnostic tool.
Disclosure
On behalf of all authors, the corresponding author states that there is no conflict of interest. This manuscript did not utilize any sources of funding.
1. Baranchuk A, Chiale PA, Green M, Caldwell JC. Editorial: surface electrocardiogram remains alive in the XXI century. Curr Cardiol Rev. 2014;10(3):173-174. http://www.ncbi.nlm.nih.gov/pubmed/24856069. Accessed January 4, 2017. PubMed
2. Fisch C. Evolution of the clinical electrocardiogram. J Am Coll Cardiol. 1989;14(5):1127-1138. doi:10.1016/0735-1097(89)90407-5. PubMed
3. O’Brien KE, Cannarozzi ML, Torre DM, Mechaber AJ, Durning SJ. Training and assessment of ECG interpretation skills: results from the 2005 CDIM survey. Teach Learn Med. 2005;21(2):111-115. doi:10.1080/10401330902791255. PubMed
4. Salerno SM, Alguire PC, Waxman HS. Competency in Interpretation of 12-Lead Electrocardiograms: A Summary and Appraisal of Published Evidence. Ann Intern Med. 2003;138(9):751-760. doi:10.1016/S1062-1458(03)00283-6. PubMed
5. Paul B, Baranchuk A. Electrocardiography teaching in Canadian family medicine residency programs: A national survey. Fam Med. 2011;43(4):267-271. http://www.ncbi.nlm.nih.gov/pubmed/21500000. Accessed January 4, 2017. PubMed
6. Jablonover RS, Lundberg E, Zhang Y, Stagnaro-Green A. Competency in electrocardiogram interpretation among graduating medical students. Teach Learn Med. 2014;26(3):279-284. doi:10.1080/10401334.2014.918882. PubMed
7. Elnicki DM, van Londen J, Hemmer PA, Fagan M, Wong R. US and Canadian internal medicine clerkship directors’ opinions about teaching procedural and interpretive skills to medical students. Acad Med. 2004;79(11):1108-1113. http://www.ncbi.nlm.nih.gov/pubmed/15504782. Accessed January 31, 2017. PubMed
8. Shams M, Sullivan A, Abudureyimu S, et al. Optimizing Electrocardiogram Interpretation and Catheterization Laboratory Activation in St-Segment Elevation Myocardial Infarction: a Teaching Module for Medical Students. J Am Coll Cardiol. 2016;67(13):643. doi:10.1016/S0735-1097(16)30644-1.
9. Grum CM, Gruppen LD, Woolliscroft JO. The influence of vignettes on EKG interpretation by third-year students. Acad Med. 1993;68:S61-S63. PubMed
10. Little B, Ho KJ, Scott L. Electrocardiogram and rhythm strip interpretation by final year medical students. Ulster Med J. 2001;70(2):108-110. PubMed
11. Eslava D, Dhillon S, Berger J, Homel P, Bergmann S. Interpretation of electrocardiograms by first-year residents: the need for change. J Electrocardiol. 2009;42(6):693-697. doi:10.1016/j.jelectrocard.2009.07.020. PubMed
12. Sibbald M, Davies EG, Dorian P, Yu EHC. Electrocardiographic Interpretation Skills of Cardiology Residents: Are They Competent? Can J Cardiol. 2014;30(12):1721-1724. doi:10.1016/j.cjca.2014.08.026. PubMed
13. Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224. Accessed January 4, 2017. PubMed
14. Todd KH, Hoffman JR, Morgan MT. Effect of cardiologist ECG review on emergency department practice. Ann Emerg Med. 1996;27(1):16-21. Accessed January 4, 2017. PubMed
15. Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and Minor ECG Abnormalities in Asymptomatic Women and Risk of Cardiovascular Events and Mortality. JAMA. 2007;297(9):978. doi:10.1001/jama.297.9.978. PubMed
16. Salerno SM, Alguire PC, Waxman HS. Training and Competency Evaluation for Interpretation of 12-Lead Electrocardiograms: Recommendations from the American College of Physicians. Ann Intern Med. 2003;138(9):747-750. doi:10.7326/0003-4819-138-9-200305060-00012. PubMed
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Cardiovascular Disease (Internal Medicine); 2016. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/152_interventional_cardiology_2017-07-01.pdf. Accessed January 4, 2017.
18. American Board of Internal Medicine. Policies and Procedures For Certification; 2016. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed January 4, 2017.
19. Kadish AH, Buxton AE, Kennedy HL, et al. ACC/AHA Clinical Competence Statement on Electrocardiography and Ambulatory Electrocardiography. J Am Coll Cardiol. 2001;38(7):3169-3178. PubMed
20. Kern D, Thomas PA, Hughes MT, editors. Curriculum Development for Medical Education: A Six-Step Approach. 2nd edition. Baltimore: The Johns Hopkins University Press; 2009.
21. De Fer T, Fazio S, Goroll A. Core Medicine Clerkship: Curriculum Guide V3.0. Alliance for Academic Internal Medicine; 2006. http://www.im.org/p/cm/ld/fid=385. Accessed January 12, 2017.
22. Hatala RM, Brooks LR, Norman GR. Practice makes perfect: The critical role of mixed practice in the acquisition of ECG interpretation skills. Adv Heal Sci Educ. 2003;8(1):17-26. doi:10.1023/A:1022687404380. PubMed
23. Bayes de Luna A. ECGs For Beginners. Barcelona: Wiley Blackwell; 2014.
24. O’Keefe J, Hammill S, Freed M, Pogwizd S. The Complete Guide to ECGs. Third edition. Kansas City: Physicians’ Press - Jones and Bartlett Publishers; 2008.
25. Khan G. Rapid ECG Interpretation. Third edition. Ottawa: Humana Press (Springer Science); 2008.
26. Garcia T. 12-Lead ECG: The Art of Interpretation. Second edition. Burlington: Jones & Bartlett Learning; 2015.
27. Olson CW, Warner RA, Wagner GS, Selvester RH. A dynamic three-dimensional display of ventricular excitation and the generation of the vector and electrocardiogram. J Electrocardiol. 2001;34 Suppl:7-15. doi:10.1054/jelc.2001.29793. PubMed
28. Olson CW, Lange D, Chan JK, et al. 3D Heart: A new visual training method for Electrocardiographic Analysis. J Electrocardiol. 2007;40(5):1-7. doi:10.1016/j.jelectrocard.2007.04.001. PubMed
29. Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):1003-1011. doi:10.1016/j.jacc.2008.12.016. PubMed
30. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525-2538. doi:10.1093/eurheartj/ehm355. PubMed
31. Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3). doi:10.1161/CIRCULATIONAHA.110.970988. PubMed
32. Barold SS, Hayes DL. Second-Degree Atrioventricular Block: A Reappraisal. Mayo Clin Proc. 2001;76(1):44-57. doi:10.4065/76.1.44. PubMed
 33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
34. Nadeau-Routhier C, Baranchuk A. Electrocardiography in Practice: What to Do? 1st ed. Kingston: Apple Inc. iBook; 2015.
35. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2):153-160. doi:10.1016/j.jemermed.2004.04.006. PubMed
36. Quinn KL, Crystal E, Lashevsky I, Arouny B, Baranchuk A. Validation of a Novel Digital Tool in Automatic Scoring of an Online ECG Examination at an International Cardiology Meeting. Ann Noninvasive Electrocardiol. 2016;21(4):376-381. doi:10.1111/anec.12311. PubMed
37. Johnson NP, Denes P. The Ladder Diagram (A 100+ Year History). Am J Cardiol. 2008;101(12):1801-1804. doi:10.1016/j.amjcard.2008.02.085. PubMed
38. Bulte C, Betts A, Garner K, Durning S. Student teaching: views of student near-peer teachers and learners. Med Teach. 2007;29(0):583-590. doi:10.1080/01421590701583824. PubMed
39. Nestojko JF, Bui DC, Kornell N, Ligon Bjork E. Expecting to teach enhances learning and organization of knowledge in free recall of text passages. Mem Cogn. 2014;42:1038-1048. doi:10.3758/s13421-014-0416-z. PubMed
40. Bené KL, Bergus G. When learners become teachers: A review of peer teaching in medical student education. Fam Med. 2014;46(10):783-787. doi:10.4300/JGME-D-13-00426. PubMed
41. Lucas J, McKay S, Baxley E. EKG arrhythmia recognition: a third-year clerkship teaching experience. Fam Med. 2003;35(3):163-164. Accessed January 31, 2017. PubMed
42. DeBonis K, Blair TR, Payne ST, Wigan K, Kim S. Viability of a Web-Based Module for Teaching Electrocardiogram Reading Skills to Psychiatry Residents: Learning Outcomes and Trainee Interest. Acad Psychiatry. 2015;39(6):645-648. doi:10.1007/s40596-014-0249-x. PubMed
43. Chudgar SM, Engle DL, Grochowski COC, Gagliardi JP. Teaching crucial skills: An electrocardiogram teaching module for medical students. J Electrocardiol. 2016;49(4):490-495. doi:10.1016/j.jelectrocard.2016.03.021. PubMed
44. Nathanson LA, Safran C, McClennen S, Goldberger AL. ECG Wave-Maven: a self-assessment program for students and clinicians. Proc AMIA Symp. 2001:488-492. Accessed January 31, 2017. PubMed
45. Farré J, Wellens H. ECGcorner (Online). ECGcorner. http://www.ecgcorner.org. Published 2017. Accessed February 15, 2017.
46. Waechter J. Teaching Medicine (Online). https://www.teachingmedicine.com/ Accessed Feb 15, 2017.
47. Akgun T, Karabay CY, Kocabay G, et al. Learning electrocardiogram on YouTube: How useful is it? J Electrocardiol. 2014;47(1):113-117. doi:10.1016/j.jelectrocard.2013.09.004. PubMed
48. Rubinstein J, Dhoble A, Ferenchick G. Puzzle based teaching versus traditional instruction in electrocardiogram interpretation for medical students – a pilot study. BMC Med Educ. 2009;9(1):4. doi:10.1186/1472-6920-9-4. PubMed
49. Black P, Wiliam D. Assessment and Classroom Learning. Assess Educ. 1998;5(1):7-73. doi:10.1080/0969595980050102.
50. Quinn KL, Baranchuk A. Feasibility of a novel digital tool in automatic scoring of an online ECG examination. Int J Cardiol. 2015;185:88-89. doi:10.1016/j.ijcard.2015.03.135. PubMed
51. Nilsson M, Bolinder G, Held C, et al. Evaluation of a web-based ECG-interpretation programme for undergraduate medical students. BMC Med Educ. 2008;8(1):25. doi:10.1186/1
52. Lessard Y, Sinteff J-P, Siregar P, et al. An ECG analysis interactive training system for understanding arrhythmias. Stud Health Technol Inform. 2009;150:931-935. Accessed January 31, 2017. PubMed
53. Zakowski, Dean Keller L. An effective ECG curriculum for third-year medical students in a community-based clerkship. Med Teach. 2000;22(4):354-358. doi:10.1080/014215900409447.
54. Mahler SA, Wolcott CJ, Swoboda TK, Wang H, Arnold TC. Techniques for teaching electrocardiogram interpretation: Self-directed learning is less effective than a workshop or lecture. Med Educ. 2011;45(4):347-353. doi:10.1111/j.1365-2923.2010.03891.x. PubMed
55. Biggs J. What the Student Does: Teaching for enhanced learning. High Educ Res Dev. 1999;18(1):57-75.
56. Ericsson KA. Deliberate practice and acquisition of expert performance: A general overview. Acad Emerg Med. 2008;15(11):988-994. doi:10.1111/j.1553-2712.2008.00227.x. PubMed
57. Raupach T, Hanneforth N, Anders S, Pukrop T, Th J Ten Cate O, Harendza S. Impact of teaching and assessment format on electrocardiogram interpretation skills. Med Educ. 2010;44(7):731-740. doi:10.1111/j.1365-2923.2010.03687.x. PubMed
58. Raupach T, Brown J, Anders S, Hasenfuss G, Harendza S. Summative assessments are more powerful drivers of student learning than resource intensive teaching formats. BMC Med. 2013;11:61. doi:10.1186/1741-7015-11-61. PubMed
59. Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests imporves ong-term retention. Psychol Sci. 2006;17(3):249-255. doi:10.1111/j.1467-9280.2006.01693.x. PubMed
60. Ferris HA, O’ Flynn D. Assessment in Medical Education; What Are We Trying to Achieve? Int J High Educ. 2015;4(2):139-144. doi:10.5430/ijhe.v4n2p139.
61. Hartman ND, Wheaton NB, Williamson K, Quattromani EN, Branzetti JB, Aldeen AZ. A Novel Tool for Assessment of Emergency Medicine Resident Skill in Determining Diagnosis and Management for Emergent Electrocardiograms: A Multicenter Study. J Emerg Med. 2016;51(6):697-704. doi:10.1016/j.jemermed.2016.06.054. PubMed
62. Pines JM, Perina DG, Brady WJ. Electrocardiogram interpretation training and competency assessment in emergency medicine residency programs. Acad Emerg Med. 2004;11(9):982-984. doi:10.1197/j.aem.2004.03.023. PubMed
63. Demircan A, Bildik F, Ergin M. Electrocardiography interpretation training in emergency medicine : methods, resources, competency assessment, and national standardization. Signa Vitae. 2015;10(1):38-52.
64. Ferrell BG, Camp DL. Comparing a Four-Week Block Clerkship to a Twelve-Week Longitudinal Experience in Family Medicine. In: Scherpbier AJJA, van der Vleuten CPM, Rethans JJ, and van der Steeg AFW, editors. Advances in Medical Education. Dordrecht: Springer Netherlands; 1997:744-746. doi:10.1007/978-94-011-4886-3_226.
65. Marinović D, Hren D, Sambunjak D, et al. Transition from longitudinal to block structure of preclinical courses: outcomes and experiences. Croat Med J. 2009;50(5):492-506. doi:10.3325/cmj.2009.50.492. PubMed
66. Melo J, Kaneshiro B, Kellett L, Hiraoka M. The impact of a longitudinal curriculum on medical student obstetrics and gynecology clinical training. Hawaii J Med Public Health. 2014;73(5):144-147. Accessed January 31, 2017. PubMed
1. Baranchuk A, Chiale PA, Green M, Caldwell JC. Editorial: surface electrocardiogram remains alive in the XXI century. Curr Cardiol Rev. 2014;10(3):173-174. http://www.ncbi.nlm.nih.gov/pubmed/24856069. Accessed January 4, 2017. PubMed
2. Fisch C. Evolution of the clinical electrocardiogram. J Am Coll Cardiol. 1989;14(5):1127-1138. doi:10.1016/0735-1097(89)90407-5. PubMed
3. O’Brien KE, Cannarozzi ML, Torre DM, Mechaber AJ, Durning SJ. Training and assessment of ECG interpretation skills: results from the 2005 CDIM survey. Teach Learn Med. 2005;21(2):111-115. doi:10.1080/10401330902791255. PubMed
4. Salerno SM, Alguire PC, Waxman HS. Competency in Interpretation of 12-Lead Electrocardiograms: A Summary and Appraisal of Published Evidence. Ann Intern Med. 2003;138(9):751-760. doi:10.1016/S1062-1458(03)00283-6. PubMed
5. Paul B, Baranchuk A. Electrocardiography teaching in Canadian family medicine residency programs: A national survey. Fam Med. 2011;43(4):267-271. http://www.ncbi.nlm.nih.gov/pubmed/21500000. Accessed January 4, 2017. PubMed
6. Jablonover RS, Lundberg E, Zhang Y, Stagnaro-Green A. Competency in electrocardiogram interpretation among graduating medical students. Teach Learn Med. 2014;26(3):279-284. doi:10.1080/10401334.2014.918882. PubMed
7. Elnicki DM, van Londen J, Hemmer PA, Fagan M, Wong R. US and Canadian internal medicine clerkship directors’ opinions about teaching procedural and interpretive skills to medical students. Acad Med. 2004;79(11):1108-1113. http://www.ncbi.nlm.nih.gov/pubmed/15504782. Accessed January 31, 2017. PubMed
8. Shams M, Sullivan A, Abudureyimu S, et al. Optimizing Electrocardiogram Interpretation and Catheterization Laboratory Activation in St-Segment Elevation Myocardial Infarction: a Teaching Module for Medical Students. J Am Coll Cardiol. 2016;67(13):643. doi:10.1016/S0735-1097(16)30644-1.
9. Grum CM, Gruppen LD, Woolliscroft JO. The influence of vignettes on EKG interpretation by third-year students. Acad Med. 1993;68:S61-S63. PubMed
10. Little B, Ho KJ, Scott L. Electrocardiogram and rhythm strip interpretation by final year medical students. Ulster Med J. 2001;70(2):108-110. PubMed
11. Eslava D, Dhillon S, Berger J, Homel P, Bergmann S. Interpretation of electrocardiograms by first-year residents: the need for change. J Electrocardiol. 2009;42(6):693-697. doi:10.1016/j.jelectrocard.2009.07.020. PubMed
12. Sibbald M, Davies EG, Dorian P, Yu EHC. Electrocardiographic Interpretation Skills of Cardiology Residents: Are They Competent? Can J Cardiol. 2014;30(12):1721-1724. doi:10.1016/j.cjca.2014.08.026. PubMed
13. Lee TH, Rouan GW, Weisberg MC, et al. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room. Am J Cardiol. 1987;60(4):219-224. Accessed January 4, 2017. PubMed
14. Todd KH, Hoffman JR, Morgan MT. Effect of cardiologist ECG review on emergency department practice. Ann Emerg Med. 1996;27(1):16-21. Accessed January 4, 2017. PubMed
15. Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and Minor ECG Abnormalities in Asymptomatic Women and Risk of Cardiovascular Events and Mortality. JAMA. 2007;297(9):978. doi:10.1001/jama.297.9.978. PubMed
16. Salerno SM, Alguire PC, Waxman HS. Training and Competency Evaluation for Interpretation of 12-Lead Electrocardiograms: Recommendations from the American College of Physicians. Ann Intern Med. 2003;138(9):747-750. doi:10.7326/0003-4819-138-9-200305060-00012. PubMed
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Cardiovascular Disease (Internal Medicine); 2016. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/152_interventional_cardiology_2017-07-01.pdf. Accessed January 4, 2017.
18. American Board of Internal Medicine. Policies and Procedures For Certification; 2016. http://www.abim.org/~/media/ABIM Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf. Accessed January 4, 2017.
19. Kadish AH, Buxton AE, Kennedy HL, et al. ACC/AHA Clinical Competence Statement on Electrocardiography and Ambulatory Electrocardiography. J Am Coll Cardiol. 2001;38(7):3169-3178. PubMed
20. Kern D, Thomas PA, Hughes MT, editors. Curriculum Development for Medical Education: A Six-Step Approach. 2nd edition. Baltimore: The Johns Hopkins University Press; 2009.
21. De Fer T, Fazio S, Goroll A. Core Medicine Clerkship: Curriculum Guide V3.0. Alliance for Academic Internal Medicine; 2006. http://www.im.org/p/cm/ld/fid=385. Accessed January 12, 2017.
22. Hatala RM, Brooks LR, Norman GR. Practice makes perfect: The critical role of mixed practice in the acquisition of ECG interpretation skills. Adv Heal Sci Educ. 2003;8(1):17-26. doi:10.1023/A:1022687404380. PubMed
23. Bayes de Luna A. ECGs For Beginners. Barcelona: Wiley Blackwell; 2014.
24. O’Keefe J, Hammill S, Freed M, Pogwizd S. The Complete Guide to ECGs. Third edition. Kansas City: Physicians’ Press - Jones and Bartlett Publishers; 2008.
25. Khan G. Rapid ECG Interpretation. Third edition. Ottawa: Humana Press (Springer Science); 2008.
26. Garcia T. 12-Lead ECG: The Art of Interpretation. Second edition. Burlington: Jones & Bartlett Learning; 2015.
27. Olson CW, Warner RA, Wagner GS, Selvester RH. A dynamic three-dimensional display of ventricular excitation and the generation of the vector and electrocardiogram. J Electrocardiol. 2001;34 Suppl:7-15. doi:10.1054/jelc.2001.29793. PubMed
28. Olson CW, Lange D, Chan JK, et al. 3D Heart: A new visual training method for Electrocardiographic Analysis. J Electrocardiol. 2007;40(5):1-7. doi:10.1016/j.jelectrocard.2007.04.001. PubMed
29. Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):1003-1011. doi:10.1016/j.jacc.2008.12.016. PubMed
30. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28(20):2525-2538. doi:10.1093/eurheartj/ehm355. PubMed
31. Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3). doi:10.1161/CIRCULATIONAHA.110.970988. PubMed
32. Barold SS, Hayes DL. Second-Degree Atrioventricular Block: A Reappraisal. Mayo Clin Proc. 2001;76(1):44-57. doi:10.4065/76.1.44. PubMed
 33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
33. Borloz MP, Mark DG, Pines JM, Brady WJ. Electrocardiographic differential diagnosis of narrow QRS complex tachycardia: an ED-oriented algorithmic approach. Am J Emerg Med. 2010;28(3):378-381. doi:10.1016/j.ajem.2008.12.019. PubMed
34. Nadeau-Routhier C, Baranchuk A. Electrocardiography in Practice: What to Do? 1st ed. Kingston: Apple Inc. iBook; 2015.
35. Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27(2):153-160. doi:10.1016/j.jemermed.2004.04.006. PubMed
36. Quinn KL, Crystal E, Lashevsky I, Arouny B, Baranchuk A. Validation of a Novel Digital Tool in Automatic Scoring of an Online ECG Examination at an International Cardiology Meeting. Ann Noninvasive Electrocardiol. 2016;21(4):376-381. doi:10.1111/anec.12311. PubMed
37. Johnson NP, Denes P. The Ladder Diagram (A 100+ Year History). Am J Cardiol. 2008;101(12):1801-1804. doi:10.1016/j.amjcard.2008.02.085. PubMed
38. Bulte C, Betts A, Garner K, Durning S. Student teaching: views of student near-peer teachers and learners. Med Teach. 2007;29(0):583-590. doi:10.1080/01421590701583824. PubMed
39. Nestojko JF, Bui DC, Kornell N, Ligon Bjork E. Expecting to teach enhances learning and organization of knowledge in free recall of text passages. Mem Cogn. 2014;42:1038-1048. doi:10.3758/s13421-014-0416-z. PubMed
40. Bené KL, Bergus G. When learners become teachers: A review of peer teaching in medical student education. Fam Med. 2014;46(10):783-787. doi:10.4300/JGME-D-13-00426. PubMed
41. Lucas J, McKay S, Baxley E. EKG arrhythmia recognition: a third-year clerkship teaching experience. Fam Med. 2003;35(3):163-164. Accessed January 31, 2017. PubMed
42. DeBonis K, Blair TR, Payne ST, Wigan K, Kim S. Viability of a Web-Based Module for Teaching Electrocardiogram Reading Skills to Psychiatry Residents: Learning Outcomes and Trainee Interest. Acad Psychiatry. 2015;39(6):645-648. doi:10.1007/s40596-014-0249-x. PubMed
43. Chudgar SM, Engle DL, Grochowski COC, Gagliardi JP. Teaching crucial skills: An electrocardiogram teaching module for medical students. J Electrocardiol. 2016;49(4):490-495. doi:10.1016/j.jelectrocard.2016.03.021. PubMed
44. Nathanson LA, Safran C, McClennen S, Goldberger AL. ECG Wave-Maven: a self-assessment program for students and clinicians. Proc AMIA Symp. 2001:488-492. Accessed January 31, 2017. PubMed
45. Farré J, Wellens H. ECGcorner (Online). ECGcorner. http://www.ecgcorner.org. Published 2017. Accessed February 15, 2017.
46. Waechter J. Teaching Medicine (Online). https://www.teachingmedicine.com/ Accessed Feb 15, 2017.
47. Akgun T, Karabay CY, Kocabay G, et al. Learning electrocardiogram on YouTube: How useful is it? J Electrocardiol. 2014;47(1):113-117. doi:10.1016/j.jelectrocard.2013.09.004. PubMed
48. Rubinstein J, Dhoble A, Ferenchick G. Puzzle based teaching versus traditional instruction in electrocardiogram interpretation for medical students – a pilot study. BMC Med Educ. 2009;9(1):4. doi:10.1186/1472-6920-9-4. PubMed
49. Black P, Wiliam D. Assessment and Classroom Learning. Assess Educ. 1998;5(1):7-73. doi:10.1080/0969595980050102.
50. Quinn KL, Baranchuk A. Feasibility of a novel digital tool in automatic scoring of an online ECG examination. Int J Cardiol. 2015;185:88-89. doi:10.1016/j.ijcard.2015.03.135. PubMed
51. Nilsson M, Bolinder G, Held C, et al. Evaluation of a web-based ECG-interpretation programme for undergraduate medical students. BMC Med Educ. 2008;8(1):25. doi:10.1186/1
52. Lessard Y, Sinteff J-P, Siregar P, et al. An ECG analysis interactive training system for understanding arrhythmias. Stud Health Technol Inform. 2009;150:931-935. Accessed January 31, 2017. PubMed
53. Zakowski, Dean Keller L. An effective ECG curriculum for third-year medical students in a community-based clerkship. Med Teach. 2000;22(4):354-358. doi:10.1080/014215900409447.
54. Mahler SA, Wolcott CJ, Swoboda TK, Wang H, Arnold TC. Techniques for teaching electrocardiogram interpretation: Self-directed learning is less effective than a workshop or lecture. Med Educ. 2011;45(4):347-353. doi:10.1111/j.1365-2923.2010.03891.x. PubMed
55. Biggs J. What the Student Does: Teaching for enhanced learning. High Educ Res Dev. 1999;18(1):57-75.
56. Ericsson KA. Deliberate practice and acquisition of expert performance: A general overview. Acad Emerg Med. 2008;15(11):988-994. doi:10.1111/j.1553-2712.2008.00227.x. PubMed
57. Raupach T, Hanneforth N, Anders S, Pukrop T, Th J Ten Cate O, Harendza S. Impact of teaching and assessment format on electrocardiogram interpretation skills. Med Educ. 2010;44(7):731-740. doi:10.1111/j.1365-2923.2010.03687.x. PubMed
58. Raupach T, Brown J, Anders S, Hasenfuss G, Harendza S. Summative assessments are more powerful drivers of student learning than resource intensive teaching formats. BMC Med. 2013;11:61. doi:10.1186/1741-7015-11-61. PubMed
59. Roediger HL, Karpicke JD. Test-enhanced learning: Taking memory tests imporves ong-term retention. Psychol Sci. 2006;17(3):249-255. doi:10.1111/j.1467-9280.2006.01693.x. PubMed
60. Ferris HA, O’ Flynn D. Assessment in Medical Education; What Are We Trying to Achieve? Int J High Educ. 2015;4(2):139-144. doi:10.5430/ijhe.v4n2p139.
61. Hartman ND, Wheaton NB, Williamson K, Quattromani EN, Branzetti JB, Aldeen AZ. A Novel Tool for Assessment of Emergency Medicine Resident Skill in Determining Diagnosis and Management for Emergent Electrocardiograms: A Multicenter Study. J Emerg Med. 2016;51(6):697-704. doi:10.1016/j.jemermed.2016.06.054. PubMed
62. Pines JM, Perina DG, Brady WJ. Electrocardiogram interpretation training and competency assessment in emergency medicine residency programs. Acad Emerg Med. 2004;11(9):982-984. doi:10.1197/j.aem.2004.03.023. PubMed
63. Demircan A, Bildik F, Ergin M. Electrocardiography interpretation training in emergency medicine : methods, resources, competency assessment, and national standardization. Signa Vitae. 2015;10(1):38-52.
64. Ferrell BG, Camp DL. Comparing a Four-Week Block Clerkship to a Twelve-Week Longitudinal Experience in Family Medicine. In: Scherpbier AJJA, van der Vleuten CPM, Rethans JJ, and van der Steeg AFW, editors. Advances in Medical Education. Dordrecht: Springer Netherlands; 1997:744-746. doi:10.1007/978-94-011-4886-3_226.
65. Marinović D, Hren D, Sambunjak D, et al. Transition from longitudinal to block structure of preclinical courses: outcomes and experiences. Croat Med J. 2009;50(5):492-506. doi:10.3325/cmj.2009.50.492. PubMed
66. Melo J, Kaneshiro B, Kellett L, Hiraoka M. The impact of a longitudinal curriculum on medical student obstetrics and gynecology clinical training. Hawaii J Med Public Health. 2014;73(5):144-147. Accessed January 31, 2017. PubMed
© 2018 Society of Hospital Medicine
Review of Strategies to Reduce Central Line-Associated Bloodstream Infection (CLABSI) and Catheter-Associated Urinary Tract Infection (CAUTI) in Adult ICUs
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
© 2018 Society of Hospital Medicine
Fuller Road, Ann Arbor, MI 48105; Telephone: 734-845-3460; Fax: 734-845-3290, E-mail: [email protected]
2017 Update in perioperative medicine: 6 questions answered
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
Perioperative care is increasingly complex, and the rapid evolution of literature in this field makes it a challenge for clinicians to stay up-to-date. To help meet this challenge, we used a systematic approach to identify appropriate articles in the medical literature and then, by consensus, to develop a list of 6 clinical questions based on their novelty and potential to change perioperative medical practice:
- How should we screen for cardiac risk in patients undergoing noncardiac surgery?
- What is the appropriate timing for surgery after coronary intervention?
- Can we use statin therapy to reduce perioperative cardiac risk?
- How should we manage sleep apnea risk perioperatively?
- Which patients with atrial fibrillation should receive perioperative bridging anticoagulation?
- Is frailty screening beneficial for elderly patients before noncardiac surgery?
The summaries in this article are a composite of perioperative medicine updates presented at the Perioperative Medicine Summit and the annual meetings of the Society for General Internal Medicine and the Society of Hospital Medicine. “Perioperative care is complex and changing”1–10 (page 864) offers a brief overview.
HOW TO SCREEN FOR CARDIAC RISK BEFORE NONCARDIAC SURGERY
Perioperative cardiac risk can be estimated by clinical risk indexes (based on history, physical examination, common blood tests, and electrocardiography), cardiac biomarkers (natriuretic peptide or troponin levels), and noninvasive cardiac tests.

American and European guidelines
In 2014, the American College of Cardiology/American Heart Association2 and the European Society of Cardiology11 published guidelines on perioperative cardiovascular evaluation and management. They recommended several tools to calculate the risk of postoperative cardiac complications but did not specify a preference. These tools include:
- The Revised Cardiac Risk Index (RCRI)12 (www.mdcalc.com/revised-cardiac-risk-index-pre-operative-risk), which has been externally validated in multiple studies and is the most widely used
- The American College of Surgeons surgical risk calculator13 (www.riskcalculator.facs.org), derived from the National Surgery Quality Improvement Program (NSQIP) database
- The myocardial infarction or cardiac arrest (MICA) calculator14 (www.surgicalriskcalculator.com/miorcardiacarrest), also derived from the NSQIP database.
2017 Canadian guidelines differ
In 2017, the Canadian Cardiovascular Society published its own guidelines on perioperative risk assessment and management.1 These differ from the American and European guidelines on several points.
RCRI recommended. The Canadian guidelines suggested using the RCRI over the other risk predictors, which despite superior discrimination lacked external validation (conditional recommendation; low-quality evidence). Additionally, the Canadians believed that the NSQIP risk indexes underestimated cardiac risk because patients did not undergo routine biomarker screening.
Biomarker measurement. The Canadian guidelines went a step further in their algorithm (Figure 1) and recommended measuring N-terminal-pro B-type natriuretic peptide (NT-proBNP) or BNP preoperatively to improve risk prediction in 3 groups (strong recommendation; moderate-quality evidence):
- Patients ages 65 and older
- Patients ages 45 to 64 with significant cardiovascular disease
- Patients with an RCRI score of 1 or more.
This differs from the American guidelines, which did not recommend measuring preoperative biomarkers but did acknowledge that they may provide incremental value. The American College of Cardiology/American Heart Association authors felt that there were no data to suggest that targeting these biomarkers for treatment and intervention would reduce postoperative risk. The European guidelines did not recommend routinely using biomarkers, but stated that they may be considered in high-risk patients (who have a functional capacity ≤ 4 metabolic equivalents or an RCRI score > 1 undergoing vascular surgery, or > 2 undergoing nonvascular surgery).
Stress testing deemphasized. The Canadian guidelines recommended biomarker testing rather than noninvasive tests to enhance risk assessment based on cost, potential delays in surgery, and absence of evidence of an overall absolute net improvement in risk reclassification. This contrasts with the American and European guidelines and algorithms, which recommended pharmacologic stress testing in patients at elevated risk with poor functional capacity undergoing intermediate- to high-risk surgery if the results would change how they are managed.
Postoperative monitoring. The Canadian guidelines recommended that if patients have an NT-proBNP level higher than 300 mg/L or a BNP level higher than 92 mg/L, they should receive postoperative monitoring with electrocardiography in the postanesthesia care unit and daily troponin measurements for 48 to 72 hours. The American guidelines recommended postoperative electrocardiography and troponin measurement only for patients suspected of having myocardial ischemia, and the European guidelines said postoperative biomarkers may be considered in patients at high risk.
Physician judgment needed
While guidelines and risk calculators are potentially helpful in risk assessment, the lack of consensus and the conflicting recommendations force the physician to weigh the evidence and make individual decisions based on his or her interpretation of the data.
Until there are studies directly comparing the various risk calculators, physicians will most likely use the RCRI, which is simple and has been externally validated, in conjunction with the American guidelines.
At this time, it is unclear how biomarkers should be used—preoperatively, postoperatively, or both—because there are no studies demonstrating that management strategies based on the results lead to better outcomes. We do not believe that biomarker testing will be accepted in lieu of stress testing by our surgery, anesthesiology, or cardiology colleagues, but going forward, it will probably be used more frequently postoperatively, particularly in patients at moderate to high risk.
WHAT IS THE APPROPRIATE TIMING FOR SURGERY AFTER PCI?
A 2014 American College of Cardiology/American Heart Association guideline recommended delaying noncardiac surgery for 1 month after percutaneous coronary intervention (PCI) with bare-metal stents and 1 year after PCI with drug-eluting stents.15 The guideline suggested that surgery may be performed 6 months after drug-eluting stent placement if the risks of delaying surgery outweigh the risk of thrombosis.15
The primary rationale behind these timeframes was to provide dual antiplatelet therapy for a minimally acceptable duration before temporary interruption for a procedure. These recommendations were influenced largely by observational studies of first-generation devices, which are no longer used. Studies of newer-generation stents have suggested that the risk of stent thrombosis reaches a plateau considerably earlier than 6 to 12 months after PCI.
2016 Revised guideline on dual antiplatelet therapy
Although not separately delineated in the recommendations, risk factors for stent thrombosis that should influence the decision include smoking, multivessel coronary artery disease, and suboptimally controlled diabetes mellitus or hyperlipidemia.17 The presence of such stent thrombosis risk factors should be factored into the decision about proceeding with surgery within 3 to 6 months after drug-eluting stent placement.
Holcomb et al: Higher postoperative risk after PCI for myocardial infarction
Another important consideration is the indication for which PCI was performed. In a recent study, Holcomb et al16 found an association between postoperative major adverse cardiac events and PCI for myocardial infarction (MI) that was independent of stent type.
Compared with patients who underwent PCI not associated with acute coronary syndrome, the odds ratios and 95% confidence intervals (CIs) for major adverse cardiac events in those who underwent PCI for MI were:
- 5.25 (4.08–6.75) in the first 3 months
- 2.45 (1.80–3.35) in months 3 to 6
- 2.50 (1.90–3.28) in months 6 to 12.
In absolute terms, patients with stenting performed for an MI had an incidence of major adverse cardiac events of:
- 22.2% in the first 3 months
- 9.4% in months 3 to 6
- 5.8% in months 6 to 12
- 4.4% in months 12 to 24.
The perioperative risks were reduced after 12 months but still remained greater in patients whose PCI was performed for MI rather than another indication.16
The authors of this study suggested delaying noncardiac surgery for up to 6 months after PCI for MI, regardless of stent type.16
A careful, individualized approach
Optimal timing of noncardiac surgery PCI requires a careful, individualized approach and should always be coordinated with the patient’s cardiologist, surgeon, and anesthesiologist.3,15 For most patients, surgery should be delayed for 30 days after bare-metal stent placement and 6 months after drug-eluting stent placement.3 However, for those with greater surgical need and less thrombotic risk, noncardiac surgery can be considered 3 to 6 months after drug-eluting stent placement.3
Additional discussion of the prolonged increased risk of postoperative major adverse cardiac events is warranted in patients whose PCI was performed for MI, in whom delaying noncardiac surgery for up to 6 months (irrespective of stent type) should be considered.16
CAN WE USE STATINS TO REDUCE PERIOPERATIVE RISK?
Current recommendations from the American College of Cardiology/American Heart Association support continuing statins in the perioperative period, but the evidence supporting starting statins in this period has yet to be fully determined. In 2013, a Cochrane review18 found insufficient evidence to conclude that statins reduced perioperative adverse cardiac events, though several large studies were excluded due to controversial methods and data.
In contrast, the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) study,4 a multicenter, prospective, cohort-matched study of approximately 7,200 patients, found a lower risk of a composite primary outcome of all-cause mortality, myocardial injury after noncardiac surgery, or stroke at 30 days for patients exposed to statin therapy (relative risk [RR] 0.83, 95% CI 0.73–0.95, P = .007).4
London et al retrospective study: 30-day mortality rate is lower with statins
In 2017, London et al5 published the results of a very large retrospective, observational cohort study of approximately 96,000 elective or emergency surgery patients in Department of Veterans Affairs hospitals. The patients were propensity-matched and evaluated for exposure to statins on the day of or the day after surgery, for a total of approximately 48,000 pairs.
The primary outcome was death at 30 days, and statin exposure was associated with a significant reduction (RR 0.82; 95% CI 0.75–0.89; P < .001). Significant risk reductions were demonstrated in nearly all secondary end points as well, except for stroke or coma and thrombosis (pulmonary embolism, deep vein thrombosis, or graft failure). Overall, the number needed to treat to prevent any complication was 67. Statin therapy did not show significant harm, though on subgroup analysis, those who received high-intensity statin therapy had a slightly higher risk of renal injury (odds ratio 1.18, 95% CI 1.02–1.37, P = .03). Also on subgroup analysis, after propensity matching, patients on long-term moderate- or high-intensity statin therapy for 6 to 12 months before surgery had a small risk reduction for many of the outcomes, including death.
The authors also noted that only 62% of the patients who were prescribed statins as outpatients received them in the hospital, which suggests that improvement is necessary in educating perioperative physicians about the benefits and widespread support for continuing statins perioperatively.5
LOAD trial: No benefit from starting statins
Both London et al5 and the VISION investigators4 called for a large randomized controlled trial of perioperative statin initiation. The Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) trial attempted to answer this call.6
This trial randomized 648 statin-naïve Brazilian patients at high risk of perioperative cardiac events to receive either atorvastatin or placebo before surgery and then continuously for another 7 days. The primary outcomes were the rates of death, nonfatal myocardial injury after noncardiac surgery, and cerebrovascular accident at 30 days.6
The investigators found no significant difference in outcomes between the two groups and estimated that the sample size would need to be approximately 7,000 patients to demonstrate a significant benefit. Nonetheless, this trial established that a prospective perioperative statin trial is feasible.
When to continue or start statins
Although we cannot recommend starting statins for all perioperative patients, perioperative statins clearly can carry significant benefit and should be continued in all patients who have been taking them. It is also likely beneficial to initiate statins in those patients who would otherwise warrant therapy based on the American College of Cardiology/American Heart Association Pooled Cohort Equations Risk calculator.19
HOW SHOULD WE MANAGE SLEEP APNEA RISK PERIOPERATIVELY?
From 20% to 30% of US men and 10% to 15% of US women have obstructive sleep apnea, and many are undiagnosed. Obstructive sleep apnea increases the risk of perioperative respiratory failure, unplanned reintubation, unplanned transfer to the intensive care unit, and death.20 Sentinel events (unexpected respiratory arrest after surgery on general surgical wards) have prompted the development of guidelines that aim to identify patients with previously undiagnosed obstructive sleep apnea before surgery and to develop approaches to reduce perioperative morbidity and mortality.
Kaw et al: Beware obesity hypoventilation syndrome
A 2016 study suggested that patients with obstructive sleep apnea and obesity hypoventilation syndrome may be at particularly high risk of perioperative complications.21
Kaw et al21 queried a database of patients with obstructive sleep apnea undergoing elective noncardiac surgery at Cleveland Clinic. All patients (N = 519) had obstructive sleep apnea confirmed by polysomnography, and a body mass index greater than 30 kg/m2. The authors considered a patient to have obesity hypoventilation syndrome (n = 194) if he or she also had hypercapnia (Paco2 ≥ 45 mm Hg) on at least 2 occasions before or after surgery.
In an adjusted analysis, the odds ratios and 95% CIs for adverse outcomes in patients with obesity hypoventilation syndrome were:
- 10.9 (3.7–32.3) for respiratory failure
- 5.4 (1.9–15.7) for heart failure
- 10.9 (3.7–32.3) for intensive care unit transfer.
The absolute increases in risk in the presence of obesity hypoventilation syndrome were:
- 19% (21% vs 2%) for respiratory failure
- 8% (8% vs 0) for heart failure
- 15% (21% vs 6%) for intensive care unit transfer.
There was no difference in rates of perioperative mortality.21
The authors proposed an algorithm to identify patients with possible obesity hypoventilation syndrome before surgery that included prior sleep study results, STOP-BANG score (Table 2),22 and serum bicarbonate level.
Important limitations of the study were that most patients with obesity hypoventilation syndrome were undiagnosed at the time of surgery. Still, the study does offer a tool to potentially identify patients at high risk for perioperative morbidity due to obesity hypoventilation syndrome. Clinicians could then choose to cancel nonessential surgery, propose a lower-risk alternative procedure, or maximize the use of strategies known to reduce perioperative risk for patients with obstructive sleep apnea in general.
Two guidelines on obstructive sleep apnea
Two professional societies have issued guidelines aiming to improve detection of previously undiagnosed obstructive sleep apnea and perioperative outcomes in patients known to have it or suspected of having it:
- The American Society of Anesthesiologists in 201423
- The Society of Anesthesia and Sleep Medicine in 2016.7
Both guidelines recommend that each institution develop a local protocol to screen patients for possible obstructive sleep apnea before elective surgery. The American Society of Anesthesiologists does not recommend any particular tool, but does recommend taking a history and performing a focused examination that includes evaluation of the airway, nasopharyngeal characteristics, neck circumference, and tonsil and tongue size. The Society of Anesthesia and Sleep Medicine recommends using a validated tool such as the STOP-BANG score to estimate the risk of obstructive sleep apnea.
If this screening suggests that a patient has obstructive sleep apnea, should surgery be delayed until a formal sleep study can be done? Or should the patient be treated empirically as if he or she has obstructive sleep apnea? Both professional societies recommend shared decision-making with the patient in this situation, with the Society of Anesthesia and Sleep Medicine recommending additional cardiopulmonary evaluation for patients with hypoventilation, severe pulmonary hypertension, or resting hypoxemia.
Both recommend using continuous positive airway pressure (CPAP) after surgery in patients with known obstructive sleep apnea, although there is not enough evidence to determine if empiric CPAP for screening-positive patients (without polysomnography-diagnosed obstructive sleep apnea) is beneficial. The Society of Anesthesia and Sleep Medicine advises that it is safe to proceed to surgery if obstructive sleep apnea is suspected as long as monitoring and risk-reduction strategies are implemented after surgery to reduce complication rates.
During surgery, the American Society of Anesthesiologists advises peripheral nerve blocks when appropriate, general anesthesia with a secure airway rather than deep sedation, capnography when using moderate sedation, awake extubation, and full reversal of neuromuscular blockade before extubation. After surgery, they recommend reducing opioid use, minimizing postoperative sedatives, supplemental oxygen, and continuous pulse oximetry. The Society of Anesthesia and Sleep Medicine guideline addresses preoperative assessment and therefore makes no recommendations regarding postoperative care.
In conclusion, use of pertinent findings from the history and physical examination and a validated obstructive sleep apnea screening tool such as STOP-BANG before surgery are recommended, with joint decision-making as to proceeding with surgery with empiric CPAP vs a formal sleep study for patients who screen as high risk. The Society of Anesthesia and Sleep Medicine recommends further cardiopulmonary evaluation if there is evidence of hypoventilation, hypoxemia, or pulmonary hypertension in addition to likely obstructive sleep apnea.
WHICH ATRIAL FIBRILLATION PATIENTS NEED BRIDGING ANTICOAGULATION?
When patients receiving anticoagulation need surgery, we need to carefully assess the risks of thromboembolism without anticoagulation vs bleeding with anticoagulation.
Historically, we tended to worry more about thromboembolism24; however, recent studies have revealed a significant risk of bleeding when long-term anticoagulant therapy is bridged (ie, interrupted and replaced with a shorter-acting agent in the perioperative period), with minimal to no decrease in thromboembolic events.25–27
American College of Cardiology guideline
In 2017, the American College of Cardiology8 published a guideline on periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. The guideline includes a series of decision algorithms on whether and when to interrupt anticoagulation, whether and how to provide bridging anticoagulation, and how to restart postprocedural anticoagulation.
When deciding whether to interrupt anticoagulation, we need to consider the risk of bleeding posed both by patient-specific factors and by the type of surgery. Bridging anticoagulation is not indicated when direct oral anticoagulants (eg, dabigatran, apixaban, edoxaban, rivaroxaban) are interrupted for procedures.
Unlike an earlier guideline statement by the American College of Chest Physicians,24 this consensus statement emphasizes using the CHA2DS2-VASc score as a predictor of thromboembolic events rather than the CHADS2 core.
Table 3 summarizes the key points in the guidance statement about which patients should receive periprocedural bridging anticoagulation.
As evidence continues to evolve in this complicated area of perioperative medicine, it will remain important to continue to create patient management plans that take individual patient and procedural risks into account.
IS FRAILTY SCREENING BENEFICIAL BEFORE NONCARDIAC SURGERY?
Frailty, defined as a composite score of a patient’s age and comorbidities, has great potential to become an obligatory factor in perioperative risk assessment. However, it remains difficult to incorporate frailty scoring into clinical practice due to variations among scoring systems,28 uncertain outcome data, and the imprecise role of socioeconomic factors. In particular, the effect of frailty on perioperative mortality over longer periods of time is uncertain.
McIsaac et al: Higher risk in frail patients
McIsaac and colleagues at the University of Ottawa used a frailty scoring system developed at Johns Hopkins University to evaluate the effect of frailty on all-cause postoperative mortality in approximately 202,000 patients over a 10-year period.9 Although this scoring system is proprietary, it is based on factors such as malnutrition, dementia, impaired vision, decubitus ulcers, urinary incontinence, weight loss, poverty, barriers to access of care, difficulty in walking, and falls.
After adjusting for the procedure risk, patient age, sex, and neighborhood income quintile, the 1-year mortality risk was significantly higher in the frail group (absolute risk 13.6% vs 4.8%; adjusted hazard ratio 2.23; 95% CI 2.08–2.40). The risk of death in the first 3 days was much higher in frail than in nonfrail patients (hazard ratio 35.58; 95% CI 29.78–40.1), but the hazard ratio decreased to approximately 2.4 by day 90.
The authors emphasize that the elevated risk for frail patients warrants particular perioperative planning, though it is not yet clear what frailty-specific interventions should be performed. Further study is needed into the benefit of “prehabilitation” (ie, exercise training to “build up” a patient before surgery) for perioperative risk reduction.
Hall et al: Better care for frail patients
Hall et al10 instituted a quality improvement initiative for perioperative care of patients at the Omaha Veterans Affairs Hospital. Frail patients were identified using the Risk Analysis Index, a 14-question screening tool previously developed and validated over several years using Veterans Administration databases.29 Questions in the Risk Analysis Index cover living situation, any diagnosis of cancer, ability to perform activities of daily living, and others.
To maximize compliance, a Risk Analysis Index score was required to schedule a surgery. Patients with high scores underwent further review by a designated team of physicians who initiated informal and formal consultations with anesthesiologists, critical care physicians, surgeons, and palliative care providers, with the goals of minimizing risk, clarifying patient goals or resuscitation wishes, and developing comprehensive perioperative planning.10
Approximately 9,100 patients were included in the cohort. The authors demonstrated a significant improvement in mortality for frail patients at 30, 180, and 365 days, but noted an improvement in postoperative mortality for the nonfrail patients as well, perhaps due to increased focus on geriatric patient care. In particular, the mortality rate at 365 days dropped from 34.5% to 11.7% for frail patients who underwent this intervention.
While this quality improvement initiative was unable to examine how surgical rates changed in frail patients, it is highly likely that very high-risk patients opted out of surgery or had their surgical plan change, though the authors point out that the overall surgical volume at the institution did not change significantly. As well, it remains unclear which particular interventions may have had the most effect in improving survival, as the perioperative plans were individualized and continually adjusted throughout the study period.
Nonetheless, this article highlights how higher vigilance, individualized planning and appreciation of the high risks of frail patients is associated with improved patient survival postoperatively. Although frailty screening is still in its early stages and further work is needed, it is likely that performing frailty screening in elderly patients and utilizing interdisciplinary collaboration for comprehensive management of frail patients can improve their postoperative course.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:2373–2405.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016; 134:e123–e155.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- London MJ, Schwartz GG, Hur K, Henderson WG. Association of perioperative statin use with mortality and morbidity after major noncardiac surgery. JAMA Intern Med 2017; 177:231–242.
- Berwanger O, de Barros E Silva PG, Barbosa RR, et al. Atorvastatin for high-risk statin-naïve patients undergoing noncardiac surgery: the Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) randomized trial. Am Heart J 2017; 184:88–96.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- Doherty JU, Gluckman TJ, Hucker W, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017; 69:871–898.
- McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg 2016; 151:538–545.
- Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg 2017; 152:233–240.
- Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35:2383–2431.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, Cohen ME. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Holcomb CN, Hollis RH, Graham LA, et al. Association of coronary stent indication with postoperative outcomes following noncardiac surgery. JAMA Surg 2016; 151:462–469.
- Lemesle G, Tricot O, Meurice T, et al. Incident myocardial infarction and very late stent thrombosis in outpatients with stable coronary artery disease. J Am Coll Cardiol 2017; 69:2149–2156.
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev 2013; 7:CD009971.
- Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:2935–2959.
- Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest 2012; 141:436–441.
- Kaw R, Bhateja P, Mar HP, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149:84–91.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Gross JB, Apfelbaum JL, Caplan RA, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology 2014; 120:268–286.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2 suppl):e326S–e350S.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630–1639.
- Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med 2015; 175:1163–1168.
- Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013; 61:1537–1551.
- Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg 2017; 152:175–182.
KEY POINTS
- Noncardiac surgery after drug-eluting stent placement can be considered after 3 to 6 months for those with greater surgical need and lower risk of stent thrombosis.
- Perioperative statin use continues to show benefits with minimal risk in large cohort studies, but significant randomized controlled trial data are lacking.
- Patients should be screened for obstructive sleep apnea before surgery, and further cardiopulmonary testing should be performed if the patient has evidence of significant sequelae from obstructive sleep apnea.
- For patients with atrial fibrillation on vitamin K antagonists, bridging can be considered for those with a CHA2DS2-VASc score of 5 or 6 and a history of stroke, transient ischemic attack, or systemic thromboembolism. Direct oral anticoagulation should not be bridged.
- Frailty carries significant perioperative mortality risk; systems-based changes to minimize these patients’ risks can be beneficial and warrant further study.
ADHD: Overdiagnosed and overtreated, or misdiagnosed and mistreated?
Pharmacotherapy and behavioral therapy are currently used with success in treating attention-deficit/hyperactivity disorder (ADHD) in children, adolescents, and adults. Ongoing changes in healthcare require physicians to improve the quality of care, reduce costs of treatment, and manage their patients’ health, not just their illnesses. Behavioral and pharmacologic studies provide us with an opportunity to maximize treatment of ADHD and adapt it to the needs of individuals.
This article identifies common problems in treating ADHD, discusses limits of care in pharmacotherapy and behavioral intervention, and offers practical recommendations for treating ADHD in the changing world of healthcare.
A CHANGING MEDICAL CLIMATE
The Affordable Care Act of 2010 sought to transform medical care in the United States from procedures to performance, from acute episodes of illness to integrated care across the lifespan, and from inefficient care to efficient and affordable care with measurable outcomes. At the time of this writing, nobody knows whether the Affordable Care Act will survive, but these are still good goals. Because ADHD is the most common behavioral disorder of childhood, value-based care is essential.1
ADHD ON THE RISE—WHY?
The prevalence of ADHD increased 42% from 2003 to 2011,2 with increases in nearly all demographic groups in the United States regardless of race, sex, and socioeconomic status. More than 1 in 10 school-age children (11%) in the United States now meet the criteria for the diagnosis of ADHD; among adolescents, 1 in 5 high school boys and 1 in 11 high school girls meet the criteria.2
Rates vary among states, from a low of 4.2% for children ages 4 to 17 in Nevada to a high of 14.6% in Arkansas.3 Worldwide estimates of ADHD prevalence range from 2.2% to 17.8%,4 with the most recent meta-analysis for North America and Europe indicating a 7.2% worldwide prevalence in people age 18 and younger.5
Such data have sparked criticism, with some saying that ADHD is overdiagnosed, others saying it is underdiagnosed, and most agreeing that it is misdiagnosed.
Changing definitions of ADHD may have had a small effect on the increase in prevalence,6 but the change is more likely a result of heightened awareness and recognition of symptoms. Even so, guidelines for diagnosing ADHD are still not rigorously applied, contributing to misdiagnosis. For example, in a study of 50 pediatric practices, only half of clinicians said they followed diagnostic guidelines to determine symptom criteria from at least 2 sources and across 2 settings, yet nearly all (93%) reported immediately prescribing medications for treatment.7
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,8 requires evidence of a persistent pattern of inattention or hyperactivity/impulsivity, or both, with a severity that interferes with developmental functioning in 2 or more settings; was present before age 12; and cannot be accounted for by another behavioral health disorder such as depression, anxiety, or trauma. The diagnosis should document the presence of at least 6 of 9 symptoms of inattention (or 5 symptoms for teens age 17 or older), or at least 6 of 9 symptoms of hyperactive/impulsive behavior (5 symptoms for teens age 17 and older). Symptoms are best documented when reported by at least 2 observers.
COSTS OF ADHD
ADHD is expensive to society. National yearly healthcare costs have ranged from $143 billion to $266 billion,9 with over half this amount assumed directly by families.10 Even in previous decades when prevalence rates hovered around 5%, the cost of workday loss in the United States was high for adult patients and for parents of young children with ADHD needing to take time off from work for doctors’ visits.11 Projections across 10 countries indicated that adults with ADHD lost more workdays than did workers without ADHD.12
There is also a trend toward visits that are more expensive. Between 2000 and 2010, the number of visits for ADHD to psychiatrists rose from 24% to 36%, while the number of less-costly visits to pediatricians decreased from 54% to 47%.13
Thus, over the past 15 years, symptoms of ADHD have become more readily recognized, prevalence rates in the population have increased significantly, and associated costs have increased dramatically, with costs extending beyond individual impairment to a loss of productivity at the workplace. And treatment, typically with drugs, has been used without sufficient application of current diagnostic criteria. What impact does this have on the practicing physician?
DRUG TREATMENT: GOLD STANDARD OR NATIONAL DISASTER?
Stimulants are considered the standard of medical care for the symptoms of ADHD, according to the 2011 practice guidelines of the American Academy of Pediatrics.14 They are efficacious and cost-effective when optimal dosing is achieved, since the patient usually manages treatment independently, requiring minimal physician input in the months and years after successful titration.
For these reasons, the use of stimulants to treat ADHD has increased dramatically in the last decade. According to the National Survey of Children’s Health, as a result of an increase in parent-reported ADHD, more US children were receiving medical treatment for the disorder in 2011 than in any previous year reported, and the prevalence of pharmacotherapy in children ages 14 to 17 increased 28% over the 4 years from 2007 to 2011.2
Dr. Keith Conners, an early advocate for recognition of ADHD, has called the staggering increase in the rates of diagnosis and drug treatment a “national disaster of dangerous proportions.”15 Nevertheless, many children and families have benefited in a cost-effective manner.
STRATEGIES FOR TITRATION
Physicians typically rely on 4 strategies to titrate stimulants,16 presented below in order of increasing complexity.
Prescribe-and-wait
Often, physicians write a prescription and direct the parent to call back or visit the office to relay the child’s response after a specified period, typically 1 week to 1 month.
This method is convenient in a busy practice and is informative to the physician in a general way. The drawback to this method is that it seldom results in optimal treatment. If the parent does not call back, the physician may assume the treatment was successful without being certain.
Dose-to-improvement
In this approach, the physician monitors titration more closely and increases the dose until a positive response is achieved, after which the dose is maintained. This method reduces symptoms but does not ensure optimal treatment, as there still may be room for improvement.
Forced-dose titration
This method is often used in clinical trials. The dose is ramped up until side effects occur and is then reduced until the side effects go away.
This method often results in optimal dosing, as a forced dose yields a greater reduction in symptoms. But it requires close monitoring by the physician, with multiple reports from parents and teachers after each dose increase to determine whether benefit at the higher dose outweighs the side effects and whether side effects can be managed.
Blinded placebo trial
Also often used in research, this method typically requires a research pharmacy to prepare capsules of stimulant medicine in low, moderate, high, and placebo doses.17 All doses are blinded and given over 4 weeks in a forced-dose titration—a placebo capsule with 3 active medication doses in escalating order, which is typical of outpatient pediatric practice. Placebo capsules are randomly assigned to 1 of the 4 weeks, and behavior is monitored over the 7 days of administration by teachers and parents.
This strategy has benefits similar to those of forced-dose titration, and it further delineates medicine response—both side effects and behavior change—by adding a no-medicine placebo condition. It is a systematic, monitored “experiment” for parents who are wary or distrustful of ADHD pharmacotherapy, and it has notable benefits.18 It is also useful for teenagers who are reluctant to use medicine to treat symptoms. It arrives at optimal treatment in a timely manner, usually about 4 to 5 weeks.
On the other hand, this approach requires diligence from families, teachers, and caregivers during the initiation phase, and it requires consistent engagement of the physician team.
Some pediatricians designate a caregiver to monitor titration with the parent; with each new weekly dose, the caregiver reports the child’s progress to the physician.
ENSURING ADHERENCE
Essential to effective stimulant treatment for ADHD is not whether the medicine works (it does),19 but whether the patient continues to use it.
In treatment studies and pharmacy database analyses, rates of inconsistent use or discontinuation of medication (both considered nonadherence) were 13.2% to 64% within the first year,20 and more than 95% of teenagers discontinue pharmacotherapy before age 21.21
Clinician engagement at the onset of stimulant titration is instrumental to treatment adherence.22,23 When pharmacotherapy is loosely monitored during initiation, adherence is highly inconsistent. Some physicians wait as long as 72 days after first prescribing a medication to contact the patient or family,7 and most children with ADHD who discontinue their medications do so within the first year.24
FACTORS THAT INHIBIT ADHERENCE
What factors inhibit adherence to successful pharmacotherapy for ADHD?
Treatment nonadherence is often associated with a parent’s perception that the medication is not working.25 Physicians can often overcome this perception by speaking with the parent, conveying that at the start of treatment titrating to the optimal dose takes time, and that it does not mean “something is wrong.” But without physician contact, parents do not have the occasion to discuss side effects and benefits and tend not to voice fears such as whether the medicine will affect the child’s physical development or result in drug abuse later in life.26
At the beginning of treatment, a child may become too focused, alarming the parent. This overfocused effect is often misunderstood and does not always persist. In addition, when a child better manages his or her own behavior, the contrast to previous behavior may look like something is wrong, when instead the child’s behavior is actually normalizing. Medicine-induced anxiety—in the child or, by association, in the parent—may be misunderstood, and subsequently the parent just stops the child’s treatment rather than seek physician guidance.
Nonadherence is also more prevalent with immediate-release than with extended-release formulations.27,28
Problems can be summarized as follows7:
- Systematic physician observation of response to stimulant titration is often missing at the onset of treatment
- “Best dose” is inconsistently achieved
- Patient adherence to treatment is inconsistently monitored.
The long-term consequences of nonadherence to therapy for ADHD have not been sufficiently examined,20 but some groups, especially adolescents, show problematic outcomes when treatment is not applied. For example, in one longitudinal study, substance use disorder was significantly higher in youths with ADHD who were never treated with medicine than in “neurotypical” youths and those with ADHD who were treated pharmacologically.29
BEHAVIORAL INTERVENTION
Although opinions vary as to the advantages of drug therapy vs behavioral intervention in ADHD, there is evidence that a combined approach is best.30–33 Pharmacotherapy works inside the skin to reduce symptoms of inattention and overactivity, and behavioral therapy works outside the skin to teach new skills.
A report from the US Centers for Disease Control and Prevention in 2016 stated that behavioral therapy should be the first treatment for young children with ADHD (ages 2 to 5), but noted that only 40% to 50% of young children with ADHD receive psychological services.36 At the same time, the use of pharmacotherapy has increased tremendously.
Beginning treatment with behavioral therapy rather than medicine has been found to be more cost-effective over time. For children ages 4 to 5, behavioral therapy is recommended as the first line by the clinical practice guidelines of the American Academy of Pediatrics.14 Beginning treatment with behavioral intervention has been shown to produce better outcomes overall than beginning with medication and indicates that lower doses may be used compared with pharmacotherapy that is not preceded by behavioral therapy.37 Findings also indicate that starting with behavioral therapy increases the cost-effectiveness of treatment for children with ADHD.38
Behavioral intervention has modest advantages over medicine for non-ADHD symptoms,42 as the practice satisfies the adage “pills don’t teach skills.”26 One advantage is that caregivers take an active role in managing child compliance, social interactions, and classroom deportment, as opposed to the relatively passive role of prescribing medicine only. Parents and teachers form collaborative partnerships to increase consistency and extend the reach of change. In the National Institute of Mental Health multimodal treatment study, the only children whose behavior normalized were those who used medicine and whose caregivers gave up negative, harsh, inconsistent, and ineffective discipline43; that is, parents changed their own behavior.
Parent training is important, as parents must often manage their children’s behavior on their own the best they can, with little coaching and assistance. Primary care physicians may often refer parents to established local programs for training, and ongoing coaching can ensure that skills acquired in such training programs continue to be systematically applied. Pharmacotherapy is focused almost solely on reducing symptoms, but reducing symptoms does not necessarily lead to improved functioning. A multimodal approach helps individuals adapt to demanding settings, achieve personal goals, and contribute to social relationships. Outcomes depend on teaching what to do as well as reducing what not to do. Behavioral therapy44 shaped by peers, caregivers, teachers, and other factors can be effectively remediate the difficulties of children with ADHD.
The disadvantages of behavioral therapy are that it is not readily available, adds initial cost to treatment, and requires parents to invest more time at the beginning of intervention. But behavioral therapy reduces costs over time, enhances ADHD pharmacotherapy, often reduces the need for higher dosing, reduces visits to the doctor’s office, maintains behavior improvement and symptom reduction in the long term, and significantly increases quality of care.42
A RECOMMENDED ADHD CARE PATH
How do we increase quality of care, reduce costs, and improve value of care for patients with ADHD? The treatment of ADHD as a chronic condition is collaborative. Several practices may be combined in a quality care path.
Follow up more frequently at the start of drug treatment
Physicians may give more frequent attention to the process of pharmacotherapy at the start of treatment. Pharmacotherapy is typically introduced by the prescribe-and-wait method, which often produces less than optimal dosing, limited treatment adherence, and inconsistent outcomes.45,46 Though the cost of giving a prescription is low, the cost for unsustained treatment is high, and this undermines the usefulness of medical therapy. The simple solution is systematic titration through frequent contact between the prescribing physician and the parents in the first few weeks of pharmacotherapy. Subsequent ongoing monitoring of adherence in the first year is likely to reduce costs over time.47
Achieve optimal dosing
Pharmacotherapy should be applied with a plan in mind to produce evidence that optimal dosing has been achieved, ie, improvement is consistently observed in school and home.48
If side effects occur, parents and physician must determine whether they outweigh the benefits. If the benefits outweigh the side effects, then the physician and parents should maintain treatment and manage side effects accordingly. If the side effects outweigh the benefits, the titration process should continue with different dosing or delivery until optimal dosing is achieved or until the physician determines that pharmacotherapy is no longer appropriate.
Though different procedures to measure optimal dosing are available, medication effectiveness can be determined in 7-day-per-dose exposure during a period when the child’s schedule is consistent. A consistent schedule is important, as medicine effects are difficult to determine during loosely defined schedules such as during school vacations or holidays. Involving multiple observers is important as well. Teachers, for example, are rarely consulted during titration49 though they are excellent observers and are with the child daily when medication is most effective.
Integrate behavioral therapy
Given the evidence that behavioral intervention enhances drug therapy,50 behavioral therapy should be integrated with drug therapy to create an inclusive context for change. Behavioral therapy is delivered in a variety of ways including individual and group parent training, home management consultation, daily school report cards, behavioral coaching, classroom behavior management, and peer interventions. Behavioral intervention enhances stimulant effectiveness51 to improve compliance, on-task behavior, academic performance, social relationships and family functioning.52
Behavioral therapy is now generally included in health insurance coverage. In addition, many clinics now offer shared medical appointments that combine close monitoring of drug therapy with behavioral coaching to small groups of parents in order to manage symptoms of ADHD at a minimal cost.
Measure outcomes
Measuring outcomes of ADHD treatment over time improves care. The primary care physician may use electronic medical record data management to track a patient’s progress related to ADHD features. The Clinical Global Improvement scale is a 7-point assessment that is easily done by parents and the physician at well visits and is ubiquitous in ADHD clinical trials.53 Change over time indicates when to suggest changes in treatment.
Finally, clinicians can demonstrate that appropriate, comprehensive care does not simply relieve ADHD symptoms, but also promotes quality of life. Healthcare providers can guide parents to improve existing abilities in children rather than leave parents with the notion that something is wrong with their child.
For example, research suggests that some patients with ADHD show enhanced creativity54,55; cognitive profiles with abilities in logical thinking, reasoning, and common sense56; and the capacity for intense focus in areas of interest.57 Some authors have even speculated that historical figures such as Thomas Edison and Albert Einstein would have been diagnosed with ADHD by today’s standards.58
MEETING THE DEMANDS OF AFFORDABLE CARE
Many children and youth diagnosed with ADHD still receive no or insufficient pharmacotherapy and behavioral therapy. More than one-third of children reported by their parents as not receiving treatment were also reported to have moderate or severe ADHD.59,60
At the same time, though more children today are being prescribed pharmacotherapy when ADHD is diagnosed, physician involvement is often limited during titration,7 and treatment usually consists of reducing symptoms without increasing adaptive behaviors with behavioral therapy.45 In addition, even though ADHD symptoms initially improve with pharmacotherapy, improvement is not sustained because of poor adherence.
The healthcare costs of ADHD are high because impairment extends beyond the patient to disrupt family life and even the workplace, as parents take time off to manage children. Because of uncertain costs of quality treatment, the best-practice treatment option for ADHD—ie, combined behavioral therapy and medicine—is increasingly accessible but still not as widely accessible as medication treatment. The value of care improves slowly while the number of patients continues to increase. However, caregivers have the opportunity to add value to the treatment of ADHD.
When we improve medication management, improve adherence to treatment, combine behavioral therapy and pharmacotherapy, consistently measure outcomes, and recognize positive traits of ADHD in our patients, we may turn the demands of affordable care into a breakthrough for many who live with the condition.
Acknowledgment: The authors wish to thank Ralph D’Alessio, BA, for his services in reference review and for his conscientious participation in the Cleveland Clinic Medication Monitoring Clinic, ADHD Center for Evaluation and Treatment.
- Rostain A, Jensen PS, Connor DF, Miesle LM, Faraone SV. Toward quality care in ADHD: defining the goals of treatment. J Atten Disord 2015; 19:99–117.
- Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry 2014; 53:34–46.e2.
- Visser SN, Blumberg SJ, Danielson ML, Bitsko RH, Kogan MD. State-based and demographic variation in parent-reported medication rates for attention-deficit/hyperactivity disorder, 2007–2008. Prev Chronic Dis 2013; 10:E09.
- Skounti M, Philalithis A, Galanakis E. Variations in prevalence of attention deficit hyperactivity disorder worldwide. Eur J Pediatr 2007; 166:117–123.
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 2015; 135:e994–1001.
- McKeown RE, Holbrook JR, Danielson ML, Cuffe SP, Wolraich ML, Visser SN. The impact of case definition on attention-deficit/hyperactivity disorder prevalence estimates in community-based samples of school-aged children. J Am Acad Child Adolesc Psychiatry 2015; 54:53–61.
- Epstein JN, Kelleher KJ, Baum R, et al. Variability in ADHD care in community-based pediatrics. Pediatrics 2014; 134:1136–1143.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington VA: American Psychiatric Association Publishing, 2013.
- Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 2012; 51:990–1002.e2.
- Abright AR. Estimating the costs of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:987–989.
- Birnbaum HG, Kessler RC, Lowe SW, et al. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Curr Med Res Opin 2005; 21:195–206.
- de Graaf R, Kessler RC, Fayyad J, et al. The prevalence and effects of adult attention-deficit/hyperactivity disorder (ADHD) on the performance of workers: results from the WHO World Mental Health Survey Initiative. Occup Environ Med 2008; 65:835–842.
- Garfield CF, Dorsey ER, Zhu S, et al. Trends in attention deficit hyperactivity disorder ambulatory diagnosis and medical treatment in the United States, 2000–2010. Acad Pediatr 2012; 12:110–116.
- Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011; 128:1007–1022.
- Schwarz A. The selling of attention deficit disorder. New York Times December 14, 2013:A1.
- Manos MJ, Tom-Revzon C, Bukstein OG, Crismon ML. Changes and challenges: managing ADHD in a fast-paced world. J Manag Care Pharm 2007; 13(suppl B):S2–S16.
- Rapport MD, Denney C. Titrating methylphenidate in children with attention-deficit/hyperactivity disorder: is body mass predictive of clinical response? J Am Acad Child Adolesc Psychiatry 1997; 36:523–530.
- Sandler A, Glesne C, Geller G. Children’s and parents’ perspectives on open-label use of placebos in the treatment of ADHD. Child Care Health Dev 2008; 34:111–120.
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry 2010; 19:353–364.
- Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med 2010; 122:184–191.
- McCarthy S, Asherson P, Coghill D, et al. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry 2009; 194:273–277.
- Bussing R, Narwaney KJ, Winterstein AG, et al. Pharmacotherapy for incident attention-deficit/hyperactivity disorder: practice patterns and quality metrics. Curr Med Res Opin 2014; 30:1687–1699.
- O’Callaghan P. Adherence to stimulants in adult ADHD. Atten Defic Hyperact Disord 2014; 6:111–120.
- Toomey SL, Sox CM, Rusinak D, Finkelstein JA. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila) 2012; 51:763–769.
- Bussing R, Koro-Ljungberg M, Noguchi K, Mason D, Mayerson G, Garvan CW. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med 2012; 74:92–100.
- Schoenfelder EN, Sasser T. Skills versus pills: psychosocial treatments for ADHD in childhood and adolescence. Pediatr Ann 2016; 45:e367–e372.
- López FA, Leroux JR. Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges. Atten Defic Hyperact Disord 2013; 5:249–265.
- Atzori P, Usala T, Carucci S, Danjou F, Zuddas A. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol 2009; 19:673–681.
- Yule AM, Martelon M, Faraone SV, Carrellas N, Wilens TE, Bierderman J. Examining the association between attention deficit hyperactivity disorder and substance use disorders: a familial risk analysis. J Psychiatr Res 2017; 85:49–55.
- Hauk L. AAP releases guideline on diagnosis, evaluation, and treatment of ADHD. Am Fam Physician 2013; 87:61–62.
- Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health collaborative multimodal treatment study of children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997; 54:865–870.
- Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996; 35:1304–1313.
- Richters JE, Arnold LE, Jensen PS, et al. NIMH collaborative multisite multimodal treatment study of children with ADHD: I. Background and rationale. J Am Acad Child Adolesc Psychiatry 1995; 34:987–1000.
- Manos MJ, Caserta DA, Short EJ, et al. Evaluation of the duration of action and comparative effectiveness of lisdexamfetamine dimesylate and behavioral treatment in youth with ADHD in a quasi-naturalistic setting. J Atten Disord 2015; 19:578–590.
- Evans SW, Owens JS, Bunford N. Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol 2014; 43:527–551.
- Visser SN, Danielson ML, Wolraich ML, et al. Vital signs: national and state-specific patterns of attention deficit/hyperactivity disorder treatment among insured children aged 2–5 years—United States, 2008-2014. MMWR Morb Mortal Wkly Rep 2016; 65:443–450.
- Pelham WE Jr, Fabiano GA, Waxmonsky JG, et al. Treatment sequencing for childhood ADHD: a multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol 2016; 45:396–415.
- Page TF, Pelham WE 3rd, Fabiano GA, et al. Comparative cost analysis of sequential, adaptive, behavioral, pharmacological, and combined treatments for childhood ADHD. J Clin Child Adolesc Psychol 2016; 45:416–427.
- Fabiano GA, Schatz NK, Pelham WE Jr. Summer treatment programs for youth with ADHD. Child Adolesc Psychiatr Clin N Am 2014; 23:757–773.
- Pelham WE, Burrows-MacLean L, Gnagy EM, et al. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. J Abnorm Child Psychol 2014; 42:1019–1031.
- Fabiano GA, Pelham WE Jr, Gnagy EM, et al. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Rev 2007; 36:195–216.
- Reeves G, Anthony B. Multimodal treatments versus pharmacotherapy alone in children with psychiatric disorders: implications of access, effectiveness, and contextual treatment. Paediatr Drugs 2009; 11:165–169.
- Hinshaw SP. Moderators and mediators of treatment outcome for youth with ADHD: understanding for whom and how interventions work. J Pediatr Psychol 2007; 32:664–675.
- Hayes SC, Villatte M, Levin M, Hildebrandt M. Open, aware, and active: contextual approaches as an emerging trend in the behavioral and cognitive therapies. Annu Rev Clin Psychol 2011; 7:141–168.
- Epstein JN, Langberg JM, Lichtenstein PK, et al. Attention-deficit/hyperactivity disorder outcomes for children treated in community-based pediatric settings. Arch Pediatr Adolesc Med 2010; 164:160–165.
- Manos MJ. Pharmacologic treatment of ADHD: road conditions in driving patients to successful outcomes. Medscape J Med 2008; 10:5.
- Braun S, Russo L, Zeidler J, Linder R, Hodgkins P. Descriptive comparison of drug treatment-persistent, -nonpersistent, and nondrug treatment patients with newly diagnosed attention deficit/hyperactivity disorder in Germany. Clin Ther 2013; 35:673–685.
- Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2006; 45:642–657.
- Pelham WE Jr, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J Clin Child Adolesc Psychol 2005; 34:449–476.
- Fabiano GA, Pelham WE Jr, Coles EK, Gnagy EM, Chronis-Tuscano A, O’Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev 2009; 29:129–140.
- Pelham WE Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol 2008; 37:184–214.
- Knight LA, Rooney M, Chronis-Tuscano A. Psychosocial treatments for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep 2008; 10:412–418.
- Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Marchant BK. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 2007; 68:93–101.
- Healey D, Rucklidge JJ. An investigation into the relationship among ADHD symptomatology, creativity, and neuropsychological functioning in children. Child Neuropsychol 2006; 12:421–438.
- Abraham A, Windmann S, Siefen R, Daum I, Güntürkün O. Creative thinking in adolescents with attention deficit hyperactivity disorder (ADHD). Child Neuropsychol 2006; 12:111–123.
- Ek U, Fernell E, Westerlund J, Holmberg K, Olsson PO, Gillberg C. Cognitive strengths and deficits in schoolchildren with ADHD. Acta Paediatr 2007; 96:756–761.
- Ozel-Kizil ET, Kokurcan A, Aksoy UM, et al. Hyperfocusing as a dimension of adult attention deficit hyperactivity disorder. Res Dev Disabil 2016; 59:351–358.
- Hartmann T. ADD Success Stories: A Guide to Fulfillment for Families With Attention Deficit Disorder. Nevada City, CA: Underwood Books, 1995.
- Visser SN, Danielson ML, Bitsko RH, Perou R, Blumberg SJ. Convergent validity of parent-reported attention-deficit/hyperactivity disorder diagnosis: a cross-study comparison. JAMA Pediatr 2013; 167:674–675.
- Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics 2007; 119(suppl 1):S99–S106.
Pharmacotherapy and behavioral therapy are currently used with success in treating attention-deficit/hyperactivity disorder (ADHD) in children, adolescents, and adults. Ongoing changes in healthcare require physicians to improve the quality of care, reduce costs of treatment, and manage their patients’ health, not just their illnesses. Behavioral and pharmacologic studies provide us with an opportunity to maximize treatment of ADHD and adapt it to the needs of individuals.
This article identifies common problems in treating ADHD, discusses limits of care in pharmacotherapy and behavioral intervention, and offers practical recommendations for treating ADHD in the changing world of healthcare.
A CHANGING MEDICAL CLIMATE
The Affordable Care Act of 2010 sought to transform medical care in the United States from procedures to performance, from acute episodes of illness to integrated care across the lifespan, and from inefficient care to efficient and affordable care with measurable outcomes. At the time of this writing, nobody knows whether the Affordable Care Act will survive, but these are still good goals. Because ADHD is the most common behavioral disorder of childhood, value-based care is essential.1
ADHD ON THE RISE—WHY?
The prevalence of ADHD increased 42% from 2003 to 2011,2 with increases in nearly all demographic groups in the United States regardless of race, sex, and socioeconomic status. More than 1 in 10 school-age children (11%) in the United States now meet the criteria for the diagnosis of ADHD; among adolescents, 1 in 5 high school boys and 1 in 11 high school girls meet the criteria.2
Rates vary among states, from a low of 4.2% for children ages 4 to 17 in Nevada to a high of 14.6% in Arkansas.3 Worldwide estimates of ADHD prevalence range from 2.2% to 17.8%,4 with the most recent meta-analysis for North America and Europe indicating a 7.2% worldwide prevalence in people age 18 and younger.5
Such data have sparked criticism, with some saying that ADHD is overdiagnosed, others saying it is underdiagnosed, and most agreeing that it is misdiagnosed.
Changing definitions of ADHD may have had a small effect on the increase in prevalence,6 but the change is more likely a result of heightened awareness and recognition of symptoms. Even so, guidelines for diagnosing ADHD are still not rigorously applied, contributing to misdiagnosis. For example, in a study of 50 pediatric practices, only half of clinicians said they followed diagnostic guidelines to determine symptom criteria from at least 2 sources and across 2 settings, yet nearly all (93%) reported immediately prescribing medications for treatment.7
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,8 requires evidence of a persistent pattern of inattention or hyperactivity/impulsivity, or both, with a severity that interferes with developmental functioning in 2 or more settings; was present before age 12; and cannot be accounted for by another behavioral health disorder such as depression, anxiety, or trauma. The diagnosis should document the presence of at least 6 of 9 symptoms of inattention (or 5 symptoms for teens age 17 or older), or at least 6 of 9 symptoms of hyperactive/impulsive behavior (5 symptoms for teens age 17 and older). Symptoms are best documented when reported by at least 2 observers.
COSTS OF ADHD
ADHD is expensive to society. National yearly healthcare costs have ranged from $143 billion to $266 billion,9 with over half this amount assumed directly by families.10 Even in previous decades when prevalence rates hovered around 5%, the cost of workday loss in the United States was high for adult patients and for parents of young children with ADHD needing to take time off from work for doctors’ visits.11 Projections across 10 countries indicated that adults with ADHD lost more workdays than did workers without ADHD.12
There is also a trend toward visits that are more expensive. Between 2000 and 2010, the number of visits for ADHD to psychiatrists rose from 24% to 36%, while the number of less-costly visits to pediatricians decreased from 54% to 47%.13
Thus, over the past 15 years, symptoms of ADHD have become more readily recognized, prevalence rates in the population have increased significantly, and associated costs have increased dramatically, with costs extending beyond individual impairment to a loss of productivity at the workplace. And treatment, typically with drugs, has been used without sufficient application of current diagnostic criteria. What impact does this have on the practicing physician?
DRUG TREATMENT: GOLD STANDARD OR NATIONAL DISASTER?
Stimulants are considered the standard of medical care for the symptoms of ADHD, according to the 2011 practice guidelines of the American Academy of Pediatrics.14 They are efficacious and cost-effective when optimal dosing is achieved, since the patient usually manages treatment independently, requiring minimal physician input in the months and years after successful titration.
For these reasons, the use of stimulants to treat ADHD has increased dramatically in the last decade. According to the National Survey of Children’s Health, as a result of an increase in parent-reported ADHD, more US children were receiving medical treatment for the disorder in 2011 than in any previous year reported, and the prevalence of pharmacotherapy in children ages 14 to 17 increased 28% over the 4 years from 2007 to 2011.2
Dr. Keith Conners, an early advocate for recognition of ADHD, has called the staggering increase in the rates of diagnosis and drug treatment a “national disaster of dangerous proportions.”15 Nevertheless, many children and families have benefited in a cost-effective manner.
STRATEGIES FOR TITRATION
Physicians typically rely on 4 strategies to titrate stimulants,16 presented below in order of increasing complexity.
Prescribe-and-wait
Often, physicians write a prescription and direct the parent to call back or visit the office to relay the child’s response after a specified period, typically 1 week to 1 month.
This method is convenient in a busy practice and is informative to the physician in a general way. The drawback to this method is that it seldom results in optimal treatment. If the parent does not call back, the physician may assume the treatment was successful without being certain.
Dose-to-improvement
In this approach, the physician monitors titration more closely and increases the dose until a positive response is achieved, after which the dose is maintained. This method reduces symptoms but does not ensure optimal treatment, as there still may be room for improvement.
Forced-dose titration
This method is often used in clinical trials. The dose is ramped up until side effects occur and is then reduced until the side effects go away.
This method often results in optimal dosing, as a forced dose yields a greater reduction in symptoms. But it requires close monitoring by the physician, with multiple reports from parents and teachers after each dose increase to determine whether benefit at the higher dose outweighs the side effects and whether side effects can be managed.
Blinded placebo trial
Also often used in research, this method typically requires a research pharmacy to prepare capsules of stimulant medicine in low, moderate, high, and placebo doses.17 All doses are blinded and given over 4 weeks in a forced-dose titration—a placebo capsule with 3 active medication doses in escalating order, which is typical of outpatient pediatric practice. Placebo capsules are randomly assigned to 1 of the 4 weeks, and behavior is monitored over the 7 days of administration by teachers and parents.
This strategy has benefits similar to those of forced-dose titration, and it further delineates medicine response—both side effects and behavior change—by adding a no-medicine placebo condition. It is a systematic, monitored “experiment” for parents who are wary or distrustful of ADHD pharmacotherapy, and it has notable benefits.18 It is also useful for teenagers who are reluctant to use medicine to treat symptoms. It arrives at optimal treatment in a timely manner, usually about 4 to 5 weeks.
On the other hand, this approach requires diligence from families, teachers, and caregivers during the initiation phase, and it requires consistent engagement of the physician team.
Some pediatricians designate a caregiver to monitor titration with the parent; with each new weekly dose, the caregiver reports the child’s progress to the physician.
ENSURING ADHERENCE
Essential to effective stimulant treatment for ADHD is not whether the medicine works (it does),19 but whether the patient continues to use it.
In treatment studies and pharmacy database analyses, rates of inconsistent use or discontinuation of medication (both considered nonadherence) were 13.2% to 64% within the first year,20 and more than 95% of teenagers discontinue pharmacotherapy before age 21.21
Clinician engagement at the onset of stimulant titration is instrumental to treatment adherence.22,23 When pharmacotherapy is loosely monitored during initiation, adherence is highly inconsistent. Some physicians wait as long as 72 days after first prescribing a medication to contact the patient or family,7 and most children with ADHD who discontinue their medications do so within the first year.24
FACTORS THAT INHIBIT ADHERENCE
What factors inhibit adherence to successful pharmacotherapy for ADHD?
Treatment nonadherence is often associated with a parent’s perception that the medication is not working.25 Physicians can often overcome this perception by speaking with the parent, conveying that at the start of treatment titrating to the optimal dose takes time, and that it does not mean “something is wrong.” But without physician contact, parents do not have the occasion to discuss side effects and benefits and tend not to voice fears such as whether the medicine will affect the child’s physical development or result in drug abuse later in life.26
At the beginning of treatment, a child may become too focused, alarming the parent. This overfocused effect is often misunderstood and does not always persist. In addition, when a child better manages his or her own behavior, the contrast to previous behavior may look like something is wrong, when instead the child’s behavior is actually normalizing. Medicine-induced anxiety—in the child or, by association, in the parent—may be misunderstood, and subsequently the parent just stops the child’s treatment rather than seek physician guidance.
Nonadherence is also more prevalent with immediate-release than with extended-release formulations.27,28
Problems can be summarized as follows7:
- Systematic physician observation of response to stimulant titration is often missing at the onset of treatment
- “Best dose” is inconsistently achieved
- Patient adherence to treatment is inconsistently monitored.
The long-term consequences of nonadherence to therapy for ADHD have not been sufficiently examined,20 but some groups, especially adolescents, show problematic outcomes when treatment is not applied. For example, in one longitudinal study, substance use disorder was significantly higher in youths with ADHD who were never treated with medicine than in “neurotypical” youths and those with ADHD who were treated pharmacologically.29
BEHAVIORAL INTERVENTION
Although opinions vary as to the advantages of drug therapy vs behavioral intervention in ADHD, there is evidence that a combined approach is best.30–33 Pharmacotherapy works inside the skin to reduce symptoms of inattention and overactivity, and behavioral therapy works outside the skin to teach new skills.
A report from the US Centers for Disease Control and Prevention in 2016 stated that behavioral therapy should be the first treatment for young children with ADHD (ages 2 to 5), but noted that only 40% to 50% of young children with ADHD receive psychological services.36 At the same time, the use of pharmacotherapy has increased tremendously.
Beginning treatment with behavioral therapy rather than medicine has been found to be more cost-effective over time. For children ages 4 to 5, behavioral therapy is recommended as the first line by the clinical practice guidelines of the American Academy of Pediatrics.14 Beginning treatment with behavioral intervention has been shown to produce better outcomes overall than beginning with medication and indicates that lower doses may be used compared with pharmacotherapy that is not preceded by behavioral therapy.37 Findings also indicate that starting with behavioral therapy increases the cost-effectiveness of treatment for children with ADHD.38
Behavioral intervention has modest advantages over medicine for non-ADHD symptoms,42 as the practice satisfies the adage “pills don’t teach skills.”26 One advantage is that caregivers take an active role in managing child compliance, social interactions, and classroom deportment, as opposed to the relatively passive role of prescribing medicine only. Parents and teachers form collaborative partnerships to increase consistency and extend the reach of change. In the National Institute of Mental Health multimodal treatment study, the only children whose behavior normalized were those who used medicine and whose caregivers gave up negative, harsh, inconsistent, and ineffective discipline43; that is, parents changed their own behavior.
Parent training is important, as parents must often manage their children’s behavior on their own the best they can, with little coaching and assistance. Primary care physicians may often refer parents to established local programs for training, and ongoing coaching can ensure that skills acquired in such training programs continue to be systematically applied. Pharmacotherapy is focused almost solely on reducing symptoms, but reducing symptoms does not necessarily lead to improved functioning. A multimodal approach helps individuals adapt to demanding settings, achieve personal goals, and contribute to social relationships. Outcomes depend on teaching what to do as well as reducing what not to do. Behavioral therapy44 shaped by peers, caregivers, teachers, and other factors can be effectively remediate the difficulties of children with ADHD.
The disadvantages of behavioral therapy are that it is not readily available, adds initial cost to treatment, and requires parents to invest more time at the beginning of intervention. But behavioral therapy reduces costs over time, enhances ADHD pharmacotherapy, often reduces the need for higher dosing, reduces visits to the doctor’s office, maintains behavior improvement and symptom reduction in the long term, and significantly increases quality of care.42
A RECOMMENDED ADHD CARE PATH
How do we increase quality of care, reduce costs, and improve value of care for patients with ADHD? The treatment of ADHD as a chronic condition is collaborative. Several practices may be combined in a quality care path.
Follow up more frequently at the start of drug treatment
Physicians may give more frequent attention to the process of pharmacotherapy at the start of treatment. Pharmacotherapy is typically introduced by the prescribe-and-wait method, which often produces less than optimal dosing, limited treatment adherence, and inconsistent outcomes.45,46 Though the cost of giving a prescription is low, the cost for unsustained treatment is high, and this undermines the usefulness of medical therapy. The simple solution is systematic titration through frequent contact between the prescribing physician and the parents in the first few weeks of pharmacotherapy. Subsequent ongoing monitoring of adherence in the first year is likely to reduce costs over time.47
Achieve optimal dosing
Pharmacotherapy should be applied with a plan in mind to produce evidence that optimal dosing has been achieved, ie, improvement is consistently observed in school and home.48
If side effects occur, parents and physician must determine whether they outweigh the benefits. If the benefits outweigh the side effects, then the physician and parents should maintain treatment and manage side effects accordingly. If the side effects outweigh the benefits, the titration process should continue with different dosing or delivery until optimal dosing is achieved or until the physician determines that pharmacotherapy is no longer appropriate.
Though different procedures to measure optimal dosing are available, medication effectiveness can be determined in 7-day-per-dose exposure during a period when the child’s schedule is consistent. A consistent schedule is important, as medicine effects are difficult to determine during loosely defined schedules such as during school vacations or holidays. Involving multiple observers is important as well. Teachers, for example, are rarely consulted during titration49 though they are excellent observers and are with the child daily when medication is most effective.
Integrate behavioral therapy
Given the evidence that behavioral intervention enhances drug therapy,50 behavioral therapy should be integrated with drug therapy to create an inclusive context for change. Behavioral therapy is delivered in a variety of ways including individual and group parent training, home management consultation, daily school report cards, behavioral coaching, classroom behavior management, and peer interventions. Behavioral intervention enhances stimulant effectiveness51 to improve compliance, on-task behavior, academic performance, social relationships and family functioning.52
Behavioral therapy is now generally included in health insurance coverage. In addition, many clinics now offer shared medical appointments that combine close monitoring of drug therapy with behavioral coaching to small groups of parents in order to manage symptoms of ADHD at a minimal cost.
Measure outcomes
Measuring outcomes of ADHD treatment over time improves care. The primary care physician may use electronic medical record data management to track a patient’s progress related to ADHD features. The Clinical Global Improvement scale is a 7-point assessment that is easily done by parents and the physician at well visits and is ubiquitous in ADHD clinical trials.53 Change over time indicates when to suggest changes in treatment.
Finally, clinicians can demonstrate that appropriate, comprehensive care does not simply relieve ADHD symptoms, but also promotes quality of life. Healthcare providers can guide parents to improve existing abilities in children rather than leave parents with the notion that something is wrong with their child.
For example, research suggests that some patients with ADHD show enhanced creativity54,55; cognitive profiles with abilities in logical thinking, reasoning, and common sense56; and the capacity for intense focus in areas of interest.57 Some authors have even speculated that historical figures such as Thomas Edison and Albert Einstein would have been diagnosed with ADHD by today’s standards.58
MEETING THE DEMANDS OF AFFORDABLE CARE
Many children and youth diagnosed with ADHD still receive no or insufficient pharmacotherapy and behavioral therapy. More than one-third of children reported by their parents as not receiving treatment were also reported to have moderate or severe ADHD.59,60
At the same time, though more children today are being prescribed pharmacotherapy when ADHD is diagnosed, physician involvement is often limited during titration,7 and treatment usually consists of reducing symptoms without increasing adaptive behaviors with behavioral therapy.45 In addition, even though ADHD symptoms initially improve with pharmacotherapy, improvement is not sustained because of poor adherence.
The healthcare costs of ADHD are high because impairment extends beyond the patient to disrupt family life and even the workplace, as parents take time off to manage children. Because of uncertain costs of quality treatment, the best-practice treatment option for ADHD—ie, combined behavioral therapy and medicine—is increasingly accessible but still not as widely accessible as medication treatment. The value of care improves slowly while the number of patients continues to increase. However, caregivers have the opportunity to add value to the treatment of ADHD.
When we improve medication management, improve adherence to treatment, combine behavioral therapy and pharmacotherapy, consistently measure outcomes, and recognize positive traits of ADHD in our patients, we may turn the demands of affordable care into a breakthrough for many who live with the condition.
Acknowledgment: The authors wish to thank Ralph D’Alessio, BA, for his services in reference review and for his conscientious participation in the Cleveland Clinic Medication Monitoring Clinic, ADHD Center for Evaluation and Treatment.
Pharmacotherapy and behavioral therapy are currently used with success in treating attention-deficit/hyperactivity disorder (ADHD) in children, adolescents, and adults. Ongoing changes in healthcare require physicians to improve the quality of care, reduce costs of treatment, and manage their patients’ health, not just their illnesses. Behavioral and pharmacologic studies provide us with an opportunity to maximize treatment of ADHD and adapt it to the needs of individuals.
This article identifies common problems in treating ADHD, discusses limits of care in pharmacotherapy and behavioral intervention, and offers practical recommendations for treating ADHD in the changing world of healthcare.
A CHANGING MEDICAL CLIMATE
The Affordable Care Act of 2010 sought to transform medical care in the United States from procedures to performance, from acute episodes of illness to integrated care across the lifespan, and from inefficient care to efficient and affordable care with measurable outcomes. At the time of this writing, nobody knows whether the Affordable Care Act will survive, but these are still good goals. Because ADHD is the most common behavioral disorder of childhood, value-based care is essential.1
ADHD ON THE RISE—WHY?
The prevalence of ADHD increased 42% from 2003 to 2011,2 with increases in nearly all demographic groups in the United States regardless of race, sex, and socioeconomic status. More than 1 in 10 school-age children (11%) in the United States now meet the criteria for the diagnosis of ADHD; among adolescents, 1 in 5 high school boys and 1 in 11 high school girls meet the criteria.2
Rates vary among states, from a low of 4.2% for children ages 4 to 17 in Nevada to a high of 14.6% in Arkansas.3 Worldwide estimates of ADHD prevalence range from 2.2% to 17.8%,4 with the most recent meta-analysis for North America and Europe indicating a 7.2% worldwide prevalence in people age 18 and younger.5
Such data have sparked criticism, with some saying that ADHD is overdiagnosed, others saying it is underdiagnosed, and most agreeing that it is misdiagnosed.
Changing definitions of ADHD may have had a small effect on the increase in prevalence,6 but the change is more likely a result of heightened awareness and recognition of symptoms. Even so, guidelines for diagnosing ADHD are still not rigorously applied, contributing to misdiagnosis. For example, in a study of 50 pediatric practices, only half of clinicians said they followed diagnostic guidelines to determine symptom criteria from at least 2 sources and across 2 settings, yet nearly all (93%) reported immediately prescribing medications for treatment.7
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,8 requires evidence of a persistent pattern of inattention or hyperactivity/impulsivity, or both, with a severity that interferes with developmental functioning in 2 or more settings; was present before age 12; and cannot be accounted for by another behavioral health disorder such as depression, anxiety, or trauma. The diagnosis should document the presence of at least 6 of 9 symptoms of inattention (or 5 symptoms for teens age 17 or older), or at least 6 of 9 symptoms of hyperactive/impulsive behavior (5 symptoms for teens age 17 and older). Symptoms are best documented when reported by at least 2 observers.
COSTS OF ADHD
ADHD is expensive to society. National yearly healthcare costs have ranged from $143 billion to $266 billion,9 with over half this amount assumed directly by families.10 Even in previous decades when prevalence rates hovered around 5%, the cost of workday loss in the United States was high for adult patients and for parents of young children with ADHD needing to take time off from work for doctors’ visits.11 Projections across 10 countries indicated that adults with ADHD lost more workdays than did workers without ADHD.12
There is also a trend toward visits that are more expensive. Between 2000 and 2010, the number of visits for ADHD to psychiatrists rose from 24% to 36%, while the number of less-costly visits to pediatricians decreased from 54% to 47%.13
Thus, over the past 15 years, symptoms of ADHD have become more readily recognized, prevalence rates in the population have increased significantly, and associated costs have increased dramatically, with costs extending beyond individual impairment to a loss of productivity at the workplace. And treatment, typically with drugs, has been used without sufficient application of current diagnostic criteria. What impact does this have on the practicing physician?
DRUG TREATMENT: GOLD STANDARD OR NATIONAL DISASTER?
Stimulants are considered the standard of medical care for the symptoms of ADHD, according to the 2011 practice guidelines of the American Academy of Pediatrics.14 They are efficacious and cost-effective when optimal dosing is achieved, since the patient usually manages treatment independently, requiring minimal physician input in the months and years after successful titration.
For these reasons, the use of stimulants to treat ADHD has increased dramatically in the last decade. According to the National Survey of Children’s Health, as a result of an increase in parent-reported ADHD, more US children were receiving medical treatment for the disorder in 2011 than in any previous year reported, and the prevalence of pharmacotherapy in children ages 14 to 17 increased 28% over the 4 years from 2007 to 2011.2
Dr. Keith Conners, an early advocate for recognition of ADHD, has called the staggering increase in the rates of diagnosis and drug treatment a “national disaster of dangerous proportions.”15 Nevertheless, many children and families have benefited in a cost-effective manner.
STRATEGIES FOR TITRATION
Physicians typically rely on 4 strategies to titrate stimulants,16 presented below in order of increasing complexity.
Prescribe-and-wait
Often, physicians write a prescription and direct the parent to call back or visit the office to relay the child’s response after a specified period, typically 1 week to 1 month.
This method is convenient in a busy practice and is informative to the physician in a general way. The drawback to this method is that it seldom results in optimal treatment. If the parent does not call back, the physician may assume the treatment was successful without being certain.
Dose-to-improvement
In this approach, the physician monitors titration more closely and increases the dose until a positive response is achieved, after which the dose is maintained. This method reduces symptoms but does not ensure optimal treatment, as there still may be room for improvement.
Forced-dose titration
This method is often used in clinical trials. The dose is ramped up until side effects occur and is then reduced until the side effects go away.
This method often results in optimal dosing, as a forced dose yields a greater reduction in symptoms. But it requires close monitoring by the physician, with multiple reports from parents and teachers after each dose increase to determine whether benefit at the higher dose outweighs the side effects and whether side effects can be managed.
Blinded placebo trial
Also often used in research, this method typically requires a research pharmacy to prepare capsules of stimulant medicine in low, moderate, high, and placebo doses.17 All doses are blinded and given over 4 weeks in a forced-dose titration—a placebo capsule with 3 active medication doses in escalating order, which is typical of outpatient pediatric practice. Placebo capsules are randomly assigned to 1 of the 4 weeks, and behavior is monitored over the 7 days of administration by teachers and parents.
This strategy has benefits similar to those of forced-dose titration, and it further delineates medicine response—both side effects and behavior change—by adding a no-medicine placebo condition. It is a systematic, monitored “experiment” for parents who are wary or distrustful of ADHD pharmacotherapy, and it has notable benefits.18 It is also useful for teenagers who are reluctant to use medicine to treat symptoms. It arrives at optimal treatment in a timely manner, usually about 4 to 5 weeks.
On the other hand, this approach requires diligence from families, teachers, and caregivers during the initiation phase, and it requires consistent engagement of the physician team.
Some pediatricians designate a caregiver to monitor titration with the parent; with each new weekly dose, the caregiver reports the child’s progress to the physician.
ENSURING ADHERENCE
Essential to effective stimulant treatment for ADHD is not whether the medicine works (it does),19 but whether the patient continues to use it.
In treatment studies and pharmacy database analyses, rates of inconsistent use or discontinuation of medication (both considered nonadherence) were 13.2% to 64% within the first year,20 and more than 95% of teenagers discontinue pharmacotherapy before age 21.21
Clinician engagement at the onset of stimulant titration is instrumental to treatment adherence.22,23 When pharmacotherapy is loosely monitored during initiation, adherence is highly inconsistent. Some physicians wait as long as 72 days after first prescribing a medication to contact the patient or family,7 and most children with ADHD who discontinue their medications do so within the first year.24
FACTORS THAT INHIBIT ADHERENCE
What factors inhibit adherence to successful pharmacotherapy for ADHD?
Treatment nonadherence is often associated with a parent’s perception that the medication is not working.25 Physicians can often overcome this perception by speaking with the parent, conveying that at the start of treatment titrating to the optimal dose takes time, and that it does not mean “something is wrong.” But without physician contact, parents do not have the occasion to discuss side effects and benefits and tend not to voice fears such as whether the medicine will affect the child’s physical development or result in drug abuse later in life.26
At the beginning of treatment, a child may become too focused, alarming the parent. This overfocused effect is often misunderstood and does not always persist. In addition, when a child better manages his or her own behavior, the contrast to previous behavior may look like something is wrong, when instead the child’s behavior is actually normalizing. Medicine-induced anxiety—in the child or, by association, in the parent—may be misunderstood, and subsequently the parent just stops the child’s treatment rather than seek physician guidance.
Nonadherence is also more prevalent with immediate-release than with extended-release formulations.27,28
Problems can be summarized as follows7:
- Systematic physician observation of response to stimulant titration is often missing at the onset of treatment
- “Best dose” is inconsistently achieved
- Patient adherence to treatment is inconsistently monitored.
The long-term consequences of nonadherence to therapy for ADHD have not been sufficiently examined,20 but some groups, especially adolescents, show problematic outcomes when treatment is not applied. For example, in one longitudinal study, substance use disorder was significantly higher in youths with ADHD who were never treated with medicine than in “neurotypical” youths and those with ADHD who were treated pharmacologically.29
BEHAVIORAL INTERVENTION
Although opinions vary as to the advantages of drug therapy vs behavioral intervention in ADHD, there is evidence that a combined approach is best.30–33 Pharmacotherapy works inside the skin to reduce symptoms of inattention and overactivity, and behavioral therapy works outside the skin to teach new skills.
A report from the US Centers for Disease Control and Prevention in 2016 stated that behavioral therapy should be the first treatment for young children with ADHD (ages 2 to 5), but noted that only 40% to 50% of young children with ADHD receive psychological services.36 At the same time, the use of pharmacotherapy has increased tremendously.
Beginning treatment with behavioral therapy rather than medicine has been found to be more cost-effective over time. For children ages 4 to 5, behavioral therapy is recommended as the first line by the clinical practice guidelines of the American Academy of Pediatrics.14 Beginning treatment with behavioral intervention has been shown to produce better outcomes overall than beginning with medication and indicates that lower doses may be used compared with pharmacotherapy that is not preceded by behavioral therapy.37 Findings also indicate that starting with behavioral therapy increases the cost-effectiveness of treatment for children with ADHD.38
Behavioral intervention has modest advantages over medicine for non-ADHD symptoms,42 as the practice satisfies the adage “pills don’t teach skills.”26 One advantage is that caregivers take an active role in managing child compliance, social interactions, and classroom deportment, as opposed to the relatively passive role of prescribing medicine only. Parents and teachers form collaborative partnerships to increase consistency and extend the reach of change. In the National Institute of Mental Health multimodal treatment study, the only children whose behavior normalized were those who used medicine and whose caregivers gave up negative, harsh, inconsistent, and ineffective discipline43; that is, parents changed their own behavior.
Parent training is important, as parents must often manage their children’s behavior on their own the best they can, with little coaching and assistance. Primary care physicians may often refer parents to established local programs for training, and ongoing coaching can ensure that skills acquired in such training programs continue to be systematically applied. Pharmacotherapy is focused almost solely on reducing symptoms, but reducing symptoms does not necessarily lead to improved functioning. A multimodal approach helps individuals adapt to demanding settings, achieve personal goals, and contribute to social relationships. Outcomes depend on teaching what to do as well as reducing what not to do. Behavioral therapy44 shaped by peers, caregivers, teachers, and other factors can be effectively remediate the difficulties of children with ADHD.
The disadvantages of behavioral therapy are that it is not readily available, adds initial cost to treatment, and requires parents to invest more time at the beginning of intervention. But behavioral therapy reduces costs over time, enhances ADHD pharmacotherapy, often reduces the need for higher dosing, reduces visits to the doctor’s office, maintains behavior improvement and symptom reduction in the long term, and significantly increases quality of care.42
A RECOMMENDED ADHD CARE PATH
How do we increase quality of care, reduce costs, and improve value of care for patients with ADHD? The treatment of ADHD as a chronic condition is collaborative. Several practices may be combined in a quality care path.
Follow up more frequently at the start of drug treatment
Physicians may give more frequent attention to the process of pharmacotherapy at the start of treatment. Pharmacotherapy is typically introduced by the prescribe-and-wait method, which often produces less than optimal dosing, limited treatment adherence, and inconsistent outcomes.45,46 Though the cost of giving a prescription is low, the cost for unsustained treatment is high, and this undermines the usefulness of medical therapy. The simple solution is systematic titration through frequent contact between the prescribing physician and the parents in the first few weeks of pharmacotherapy. Subsequent ongoing monitoring of adherence in the first year is likely to reduce costs over time.47
Achieve optimal dosing
Pharmacotherapy should be applied with a plan in mind to produce evidence that optimal dosing has been achieved, ie, improvement is consistently observed in school and home.48
If side effects occur, parents and physician must determine whether they outweigh the benefits. If the benefits outweigh the side effects, then the physician and parents should maintain treatment and manage side effects accordingly. If the side effects outweigh the benefits, the titration process should continue with different dosing or delivery until optimal dosing is achieved or until the physician determines that pharmacotherapy is no longer appropriate.
Though different procedures to measure optimal dosing are available, medication effectiveness can be determined in 7-day-per-dose exposure during a period when the child’s schedule is consistent. A consistent schedule is important, as medicine effects are difficult to determine during loosely defined schedules such as during school vacations or holidays. Involving multiple observers is important as well. Teachers, for example, are rarely consulted during titration49 though they are excellent observers and are with the child daily when medication is most effective.
Integrate behavioral therapy
Given the evidence that behavioral intervention enhances drug therapy,50 behavioral therapy should be integrated with drug therapy to create an inclusive context for change. Behavioral therapy is delivered in a variety of ways including individual and group parent training, home management consultation, daily school report cards, behavioral coaching, classroom behavior management, and peer interventions. Behavioral intervention enhances stimulant effectiveness51 to improve compliance, on-task behavior, academic performance, social relationships and family functioning.52
Behavioral therapy is now generally included in health insurance coverage. In addition, many clinics now offer shared medical appointments that combine close monitoring of drug therapy with behavioral coaching to small groups of parents in order to manage symptoms of ADHD at a minimal cost.
Measure outcomes
Measuring outcomes of ADHD treatment over time improves care. The primary care physician may use electronic medical record data management to track a patient’s progress related to ADHD features. The Clinical Global Improvement scale is a 7-point assessment that is easily done by parents and the physician at well visits and is ubiquitous in ADHD clinical trials.53 Change over time indicates when to suggest changes in treatment.
Finally, clinicians can demonstrate that appropriate, comprehensive care does not simply relieve ADHD symptoms, but also promotes quality of life. Healthcare providers can guide parents to improve existing abilities in children rather than leave parents with the notion that something is wrong with their child.
For example, research suggests that some patients with ADHD show enhanced creativity54,55; cognitive profiles with abilities in logical thinking, reasoning, and common sense56; and the capacity for intense focus in areas of interest.57 Some authors have even speculated that historical figures such as Thomas Edison and Albert Einstein would have been diagnosed with ADHD by today’s standards.58
MEETING THE DEMANDS OF AFFORDABLE CARE
Many children and youth diagnosed with ADHD still receive no or insufficient pharmacotherapy and behavioral therapy. More than one-third of children reported by their parents as not receiving treatment were also reported to have moderate or severe ADHD.59,60
At the same time, though more children today are being prescribed pharmacotherapy when ADHD is diagnosed, physician involvement is often limited during titration,7 and treatment usually consists of reducing symptoms without increasing adaptive behaviors with behavioral therapy.45 In addition, even though ADHD symptoms initially improve with pharmacotherapy, improvement is not sustained because of poor adherence.
The healthcare costs of ADHD are high because impairment extends beyond the patient to disrupt family life and even the workplace, as parents take time off to manage children. Because of uncertain costs of quality treatment, the best-practice treatment option for ADHD—ie, combined behavioral therapy and medicine—is increasingly accessible but still not as widely accessible as medication treatment. The value of care improves slowly while the number of patients continues to increase. However, caregivers have the opportunity to add value to the treatment of ADHD.
When we improve medication management, improve adherence to treatment, combine behavioral therapy and pharmacotherapy, consistently measure outcomes, and recognize positive traits of ADHD in our patients, we may turn the demands of affordable care into a breakthrough for many who live with the condition.
Acknowledgment: The authors wish to thank Ralph D’Alessio, BA, for his services in reference review and for his conscientious participation in the Cleveland Clinic Medication Monitoring Clinic, ADHD Center for Evaluation and Treatment.
- Rostain A, Jensen PS, Connor DF, Miesle LM, Faraone SV. Toward quality care in ADHD: defining the goals of treatment. J Atten Disord 2015; 19:99–117.
- Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry 2014; 53:34–46.e2.
- Visser SN, Blumberg SJ, Danielson ML, Bitsko RH, Kogan MD. State-based and demographic variation in parent-reported medication rates for attention-deficit/hyperactivity disorder, 2007–2008. Prev Chronic Dis 2013; 10:E09.
- Skounti M, Philalithis A, Galanakis E. Variations in prevalence of attention deficit hyperactivity disorder worldwide. Eur J Pediatr 2007; 166:117–123.
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 2015; 135:e994–1001.
- McKeown RE, Holbrook JR, Danielson ML, Cuffe SP, Wolraich ML, Visser SN. The impact of case definition on attention-deficit/hyperactivity disorder prevalence estimates in community-based samples of school-aged children. J Am Acad Child Adolesc Psychiatry 2015; 54:53–61.
- Epstein JN, Kelleher KJ, Baum R, et al. Variability in ADHD care in community-based pediatrics. Pediatrics 2014; 134:1136–1143.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington VA: American Psychiatric Association Publishing, 2013.
- Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 2012; 51:990–1002.e2.
- Abright AR. Estimating the costs of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:987–989.
- Birnbaum HG, Kessler RC, Lowe SW, et al. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Curr Med Res Opin 2005; 21:195–206.
- de Graaf R, Kessler RC, Fayyad J, et al. The prevalence and effects of adult attention-deficit/hyperactivity disorder (ADHD) on the performance of workers: results from the WHO World Mental Health Survey Initiative. Occup Environ Med 2008; 65:835–842.
- Garfield CF, Dorsey ER, Zhu S, et al. Trends in attention deficit hyperactivity disorder ambulatory diagnosis and medical treatment in the United States, 2000–2010. Acad Pediatr 2012; 12:110–116.
- Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011; 128:1007–1022.
- Schwarz A. The selling of attention deficit disorder. New York Times December 14, 2013:A1.
- Manos MJ, Tom-Revzon C, Bukstein OG, Crismon ML. Changes and challenges: managing ADHD in a fast-paced world. J Manag Care Pharm 2007; 13(suppl B):S2–S16.
- Rapport MD, Denney C. Titrating methylphenidate in children with attention-deficit/hyperactivity disorder: is body mass predictive of clinical response? J Am Acad Child Adolesc Psychiatry 1997; 36:523–530.
- Sandler A, Glesne C, Geller G. Children’s and parents’ perspectives on open-label use of placebos in the treatment of ADHD. Child Care Health Dev 2008; 34:111–120.
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry 2010; 19:353–364.
- Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med 2010; 122:184–191.
- McCarthy S, Asherson P, Coghill D, et al. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry 2009; 194:273–277.
- Bussing R, Narwaney KJ, Winterstein AG, et al. Pharmacotherapy for incident attention-deficit/hyperactivity disorder: practice patterns and quality metrics. Curr Med Res Opin 2014; 30:1687–1699.
- O’Callaghan P. Adherence to stimulants in adult ADHD. Atten Defic Hyperact Disord 2014; 6:111–120.
- Toomey SL, Sox CM, Rusinak D, Finkelstein JA. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila) 2012; 51:763–769.
- Bussing R, Koro-Ljungberg M, Noguchi K, Mason D, Mayerson G, Garvan CW. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med 2012; 74:92–100.
- Schoenfelder EN, Sasser T. Skills versus pills: psychosocial treatments for ADHD in childhood and adolescence. Pediatr Ann 2016; 45:e367–e372.
- López FA, Leroux JR. Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges. Atten Defic Hyperact Disord 2013; 5:249–265.
- Atzori P, Usala T, Carucci S, Danjou F, Zuddas A. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol 2009; 19:673–681.
- Yule AM, Martelon M, Faraone SV, Carrellas N, Wilens TE, Bierderman J. Examining the association between attention deficit hyperactivity disorder and substance use disorders: a familial risk analysis. J Psychiatr Res 2017; 85:49–55.
- Hauk L. AAP releases guideline on diagnosis, evaluation, and treatment of ADHD. Am Fam Physician 2013; 87:61–62.
- Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health collaborative multimodal treatment study of children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997; 54:865–870.
- Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996; 35:1304–1313.
- Richters JE, Arnold LE, Jensen PS, et al. NIMH collaborative multisite multimodal treatment study of children with ADHD: I. Background and rationale. J Am Acad Child Adolesc Psychiatry 1995; 34:987–1000.
- Manos MJ, Caserta DA, Short EJ, et al. Evaluation of the duration of action and comparative effectiveness of lisdexamfetamine dimesylate and behavioral treatment in youth with ADHD in a quasi-naturalistic setting. J Atten Disord 2015; 19:578–590.
- Evans SW, Owens JS, Bunford N. Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol 2014; 43:527–551.
- Visser SN, Danielson ML, Wolraich ML, et al. Vital signs: national and state-specific patterns of attention deficit/hyperactivity disorder treatment among insured children aged 2–5 years—United States, 2008-2014. MMWR Morb Mortal Wkly Rep 2016; 65:443–450.
- Pelham WE Jr, Fabiano GA, Waxmonsky JG, et al. Treatment sequencing for childhood ADHD: a multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol 2016; 45:396–415.
- Page TF, Pelham WE 3rd, Fabiano GA, et al. Comparative cost analysis of sequential, adaptive, behavioral, pharmacological, and combined treatments for childhood ADHD. J Clin Child Adolesc Psychol 2016; 45:416–427.
- Fabiano GA, Schatz NK, Pelham WE Jr. Summer treatment programs for youth with ADHD. Child Adolesc Psychiatr Clin N Am 2014; 23:757–773.
- Pelham WE, Burrows-MacLean L, Gnagy EM, et al. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. J Abnorm Child Psychol 2014; 42:1019–1031.
- Fabiano GA, Pelham WE Jr, Gnagy EM, et al. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Rev 2007; 36:195–216.
- Reeves G, Anthony B. Multimodal treatments versus pharmacotherapy alone in children with psychiatric disorders: implications of access, effectiveness, and contextual treatment. Paediatr Drugs 2009; 11:165–169.
- Hinshaw SP. Moderators and mediators of treatment outcome for youth with ADHD: understanding for whom and how interventions work. J Pediatr Psychol 2007; 32:664–675.
- Hayes SC, Villatte M, Levin M, Hildebrandt M. Open, aware, and active: contextual approaches as an emerging trend in the behavioral and cognitive therapies. Annu Rev Clin Psychol 2011; 7:141–168.
- Epstein JN, Langberg JM, Lichtenstein PK, et al. Attention-deficit/hyperactivity disorder outcomes for children treated in community-based pediatric settings. Arch Pediatr Adolesc Med 2010; 164:160–165.
- Manos MJ. Pharmacologic treatment of ADHD: road conditions in driving patients to successful outcomes. Medscape J Med 2008; 10:5.
- Braun S, Russo L, Zeidler J, Linder R, Hodgkins P. Descriptive comparison of drug treatment-persistent, -nonpersistent, and nondrug treatment patients with newly diagnosed attention deficit/hyperactivity disorder in Germany. Clin Ther 2013; 35:673–685.
- Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2006; 45:642–657.
- Pelham WE Jr, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J Clin Child Adolesc Psychol 2005; 34:449–476.
- Fabiano GA, Pelham WE Jr, Coles EK, Gnagy EM, Chronis-Tuscano A, O’Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev 2009; 29:129–140.
- Pelham WE Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol 2008; 37:184–214.
- Knight LA, Rooney M, Chronis-Tuscano A. Psychosocial treatments for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep 2008; 10:412–418.
- Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Marchant BK. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 2007; 68:93–101.
- Healey D, Rucklidge JJ. An investigation into the relationship among ADHD symptomatology, creativity, and neuropsychological functioning in children. Child Neuropsychol 2006; 12:421–438.
- Abraham A, Windmann S, Siefen R, Daum I, Güntürkün O. Creative thinking in adolescents with attention deficit hyperactivity disorder (ADHD). Child Neuropsychol 2006; 12:111–123.
- Ek U, Fernell E, Westerlund J, Holmberg K, Olsson PO, Gillberg C. Cognitive strengths and deficits in schoolchildren with ADHD. Acta Paediatr 2007; 96:756–761.
- Ozel-Kizil ET, Kokurcan A, Aksoy UM, et al. Hyperfocusing as a dimension of adult attention deficit hyperactivity disorder. Res Dev Disabil 2016; 59:351–358.
- Hartmann T. ADD Success Stories: A Guide to Fulfillment for Families With Attention Deficit Disorder. Nevada City, CA: Underwood Books, 1995.
- Visser SN, Danielson ML, Bitsko RH, Perou R, Blumberg SJ. Convergent validity of parent-reported attention-deficit/hyperactivity disorder diagnosis: a cross-study comparison. JAMA Pediatr 2013; 167:674–675.
- Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics 2007; 119(suppl 1):S99–S106.
- Rostain A, Jensen PS, Connor DF, Miesle LM, Faraone SV. Toward quality care in ADHD: defining the goals of treatment. J Atten Disord 2015; 19:99–117.
- Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry 2014; 53:34–46.e2.
- Visser SN, Blumberg SJ, Danielson ML, Bitsko RH, Kogan MD. State-based and demographic variation in parent-reported medication rates for attention-deficit/hyperactivity disorder, 2007–2008. Prev Chronic Dis 2013; 10:E09.
- Skounti M, Philalithis A, Galanakis E. Variations in prevalence of attention deficit hyperactivity disorder worldwide. Eur J Pediatr 2007; 166:117–123.
- Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 2015; 135:e994–1001.
- McKeown RE, Holbrook JR, Danielson ML, Cuffe SP, Wolraich ML, Visser SN. The impact of case definition on attention-deficit/hyperactivity disorder prevalence estimates in community-based samples of school-aged children. J Am Acad Child Adolesc Psychiatry 2015; 54:53–61.
- Epstein JN, Kelleher KJ, Baum R, et al. Variability in ADHD care in community-based pediatrics. Pediatrics 2014; 134:1136–1143.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington VA: American Psychiatric Association Publishing, 2013.
- Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 2012; 51:990–1002.e2.
- Abright AR. Estimating the costs of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:987–989.
- Birnbaum HG, Kessler RC, Lowe SW, et al. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Curr Med Res Opin 2005; 21:195–206.
- de Graaf R, Kessler RC, Fayyad J, et al. The prevalence and effects of adult attention-deficit/hyperactivity disorder (ADHD) on the performance of workers: results from the WHO World Mental Health Survey Initiative. Occup Environ Med 2008; 65:835–842.
- Garfield CF, Dorsey ER, Zhu S, et al. Trends in attention deficit hyperactivity disorder ambulatory diagnosis and medical treatment in the United States, 2000–2010. Acad Pediatr 2012; 12:110–116.
- Subcommittee on Attention-Deficit/Hyperactivity Disorder, Steering Committee on Quality Improvement and Management, Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011; 128:1007–1022.
- Schwarz A. The selling of attention deficit disorder. New York Times December 14, 2013:A1.
- Manos MJ, Tom-Revzon C, Bukstein OG, Crismon ML. Changes and challenges: managing ADHD in a fast-paced world. J Manag Care Pharm 2007; 13(suppl B):S2–S16.
- Rapport MD, Denney C. Titrating methylphenidate in children with attention-deficit/hyperactivity disorder: is body mass predictive of clinical response? J Am Acad Child Adolesc Psychiatry 1997; 36:523–530.
- Sandler A, Glesne C, Geller G. Children’s and parents’ perspectives on open-label use of placebos in the treatment of ADHD. Child Care Health Dev 2008; 34:111–120.
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry 2010; 19:353–364.
- Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med 2010; 122:184–191.
- McCarthy S, Asherson P, Coghill D, et al. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry 2009; 194:273–277.
- Bussing R, Narwaney KJ, Winterstein AG, et al. Pharmacotherapy for incident attention-deficit/hyperactivity disorder: practice patterns and quality metrics. Curr Med Res Opin 2014; 30:1687–1699.
- O’Callaghan P. Adherence to stimulants in adult ADHD. Atten Defic Hyperact Disord 2014; 6:111–120.
- Toomey SL, Sox CM, Rusinak D, Finkelstein JA. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila) 2012; 51:763–769.
- Bussing R, Koro-Ljungberg M, Noguchi K, Mason D, Mayerson G, Garvan CW. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med 2012; 74:92–100.
- Schoenfelder EN, Sasser T. Skills versus pills: psychosocial treatments for ADHD in childhood and adolescence. Pediatr Ann 2016; 45:e367–e372.
- López FA, Leroux JR. Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges. Atten Defic Hyperact Disord 2013; 5:249–265.
- Atzori P, Usala T, Carucci S, Danjou F, Zuddas A. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol 2009; 19:673–681.
- Yule AM, Martelon M, Faraone SV, Carrellas N, Wilens TE, Bierderman J. Examining the association between attention deficit hyperactivity disorder and substance use disorders: a familial risk analysis. J Psychiatr Res 2017; 85:49–55.
- Hauk L. AAP releases guideline on diagnosis, evaluation, and treatment of ADHD. Am Fam Physician 2013; 87:61–62.
- Arnold LE, Abikoff HB, Cantwell DP, et al. National Institute of Mental Health collaborative multimodal treatment study of children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry 1997; 54:865–870.
- Greenhill LL, Abikoff HB, Arnold LE, et al. Medication treatment strategies in the MTA study: relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 1996; 35:1304–1313.
- Richters JE, Arnold LE, Jensen PS, et al. NIMH collaborative multisite multimodal treatment study of children with ADHD: I. Background and rationale. J Am Acad Child Adolesc Psychiatry 1995; 34:987–1000.
- Manos MJ, Caserta DA, Short EJ, et al. Evaluation of the duration of action and comparative effectiveness of lisdexamfetamine dimesylate and behavioral treatment in youth with ADHD in a quasi-naturalistic setting. J Atten Disord 2015; 19:578–590.
- Evans SW, Owens JS, Bunford N. Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol 2014; 43:527–551.
- Visser SN, Danielson ML, Wolraich ML, et al. Vital signs: national and state-specific patterns of attention deficit/hyperactivity disorder treatment among insured children aged 2–5 years—United States, 2008-2014. MMWR Morb Mortal Wkly Rep 2016; 65:443–450.
- Pelham WE Jr, Fabiano GA, Waxmonsky JG, et al. Treatment sequencing for childhood ADHD: a multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol 2016; 45:396–415.
- Page TF, Pelham WE 3rd, Fabiano GA, et al. Comparative cost analysis of sequential, adaptive, behavioral, pharmacological, and combined treatments for childhood ADHD. J Clin Child Adolesc Psychol 2016; 45:416–427.
- Fabiano GA, Schatz NK, Pelham WE Jr. Summer treatment programs for youth with ADHD. Child Adolesc Psychiatr Clin N Am 2014; 23:757–773.
- Pelham WE, Burrows-MacLean L, Gnagy EM, et al. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. J Abnorm Child Psychol 2014; 42:1019–1031.
- Fabiano GA, Pelham WE Jr, Gnagy EM, et al. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Rev 2007; 36:195–216.
- Reeves G, Anthony B. Multimodal treatments versus pharmacotherapy alone in children with psychiatric disorders: implications of access, effectiveness, and contextual treatment. Paediatr Drugs 2009; 11:165–169.
- Hinshaw SP. Moderators and mediators of treatment outcome for youth with ADHD: understanding for whom and how interventions work. J Pediatr Psychol 2007; 32:664–675.
- Hayes SC, Villatte M, Levin M, Hildebrandt M. Open, aware, and active: contextual approaches as an emerging trend in the behavioral and cognitive therapies. Annu Rev Clin Psychol 2011; 7:141–168.
- Epstein JN, Langberg JM, Lichtenstein PK, et al. Attention-deficit/hyperactivity disorder outcomes for children treated in community-based pediatric settings. Arch Pediatr Adolesc Med 2010; 164:160–165.
- Manos MJ. Pharmacologic treatment of ADHD: road conditions in driving patients to successful outcomes. Medscape J Med 2008; 10:5.
- Braun S, Russo L, Zeidler J, Linder R, Hodgkins P. Descriptive comparison of drug treatment-persistent, -nonpersistent, and nondrug treatment patients with newly diagnosed attention deficit/hyperactivity disorder in Germany. Clin Ther 2013; 35:673–685.
- Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2006; 45:642–657.
- Pelham WE Jr, Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J Clin Child Adolesc Psychol 2005; 34:449–476.
- Fabiano GA, Pelham WE Jr, Coles EK, Gnagy EM, Chronis-Tuscano A, O’Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev 2009; 29:129–140.
- Pelham WE Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol 2008; 37:184–214.
- Knight LA, Rooney M, Chronis-Tuscano A. Psychosocial treatments for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep 2008; 10:412–418.
- Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Marchant BK. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry 2007; 68:93–101.
- Healey D, Rucklidge JJ. An investigation into the relationship among ADHD symptomatology, creativity, and neuropsychological functioning in children. Child Neuropsychol 2006; 12:421–438.
- Abraham A, Windmann S, Siefen R, Daum I, Güntürkün O. Creative thinking in adolescents with attention deficit hyperactivity disorder (ADHD). Child Neuropsychol 2006; 12:111–123.
- Ek U, Fernell E, Westerlund J, Holmberg K, Olsson PO, Gillberg C. Cognitive strengths and deficits in schoolchildren with ADHD. Acta Paediatr 2007; 96:756–761.
- Ozel-Kizil ET, Kokurcan A, Aksoy UM, et al. Hyperfocusing as a dimension of adult attention deficit hyperactivity disorder. Res Dev Disabil 2016; 59:351–358.
- Hartmann T. ADD Success Stories: A Guide to Fulfillment for Families With Attention Deficit Disorder. Nevada City, CA: Underwood Books, 1995.
- Visser SN, Danielson ML, Bitsko RH, Perou R, Blumberg SJ. Convergent validity of parent-reported attention-deficit/hyperactivity disorder diagnosis: a cross-study comparison. JAMA Pediatr 2013; 167:674–675.
- Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics 2007; 119(suppl 1):S99–S106.
KEY POINTS
- Despite concerns about overdiagnosis and overtreatment, many children and youth diagnosed with ADHD still receive no treatment or insufficient treatment.
- Today, more children are prescribed drug therapy when ADHD is diagnosed, but the initial titration of medication is often done without sufficient physician supervision.
- ADHD symptoms improve with drug therapy, but improvement is inconsistently sustained due to poor treatment adherence.
- Drug therapy and behavioral therapy work together. Outcomes can be determined by measuring both improved behaviors and reduced symptoms.
Methamphetamine-induced psychosis: Who says all drug use is reversible?
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4
Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4
Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
Use of methamphetamine, an N-methyl analog of amphetamine, is a serious public health problem; throughout the world an estimated 35.7 million people use the drug recreationally.1 Methamphetamine is easy to obtain because it is cheap to produce and can be synthesized anywhere. In the United States, methamphetamine is commonly manufactured in small-scale laboratories using relatively inexpensive, legally available ingredients. Large-scale manufacturing in clandestine laboratories also contributes to methamphetamine abuse. The drug, known as meth, crystal meth, ice, and other names, is available as a powder, tablet, or crystalline salt, and is used by various routes of administration (Table).
Although FDA-approved for treating attention-deficit/hyperactivity disorder, methamphetamine is taken recreationally for its euphoric effects; however, it also produces anhedonia, paranoia, and a host of cognitive deficits and other adverse effects.
Methamphetamine causes psychiatric diseases that resemble naturally occurring illnesses but are more difficult to treat. Dependence occurs over a period of escalating use (Figure). Long-term exposure to the drug has been shown to cause severe neurotoxic and neuropathological effects with consequent disturbances in several cognitive domains.4
Despite advances in understanding the basic neurobiology of methamphetamine-induced effects on the brain, much remains to be done to translate this knowledge to treating patients and the complications that result from chronic abuse of this stimulant. In this review, we:
- provide a brief synopsis of the clinical presentation of patients who use methamphetamine
- describe some of the complications of methamphetamine abuse/dependence, focusing on methamphetamine-induced psychosis
- suggest ways to approach the treatment of these patients, including those with methamphetamine-induced psychosis.
Acute effects of methamphetamine use
Psychiatric symptoms. Patients under the influence of methamphetamine may present with clinical symptoms that mimic psychiatric disorders. For example, the drug can cause marked euphoria, hyperactivity, and disturbed speech patterns, thus mimicking a manic state. Patients also may present with anxiety, agitation, and irritability or aggressiveness. Although an individual may take methamphetamine for sexual enhancement, the drug can cause hypersexuality, which often is associated with unintended and unsafe sexual activities. These signs and symptoms are exacerbated during drug binges that can last for days, during which time large quantities of the drug are consumed.
Methamphetamine users may become preoccupied with their own thought patterns, and their actions can become compulsive and nonsensical. For example, a patient may become obsessed with an object of no specific value in his (her) environment, such as a doorknob or a cloud. Patients also may become suspicious of their friends and family members or think that police officers are after them. Less commonly, a patient also may suffer from poverty of speech, psychomotor retardation, and diminished social engagement similar to that reported in some patients with schizophrenia with deficit syndrome. Usually, acute symptoms will last 4 to 7 days after drug cessation, and then resolve completely with protracted abstinence from the drug.
Neurologic signs of methamphetamine use include hemorrhagic strokes in young people without any evidence of previous neurologic impairments. Studies have documented similarities between methamphetamine-induced neurotoxicity and traumatic brain injury.5 Postmortem studies have reported the presence of arteriovenous malformation in some patients with hemorrhagic strokes.
Hyperthermia is a dangerous acute effect of methamphetamine use. High body temperatures can cause both peripheral and central abnormalities, including muscular and cardiovascular dysfunction, renal failure secondary to rhabdomyolysis, heat stroke, and other heat-induced malignant syndromes. Some of the central dysfunctions may be related to heat-induced production of free radicals in various brain regions. There are no pharmacologic treatments for methamphetamine-induced thermal dysregulation.6 Therefore, clinicians need to focus on reducing body temperature by using cooling fans or cold water baths. Efforts should be made to avoid overhydrating patients because of the risk of developing the syndrome of inappropriate antidiuretic hormone secretion.
Chronic methamphetamine abuse
Psychosis is a long-term complication of chronic abuse of the drug.7 Although psychosis has been a reported complication of methamphetamine use since the 1950s,8 most of the subsequent literature is from Japan, where methamphetamine use was highly prevalent after World War II.9,10 The prevalence of methamphetamine-induced psychosis in methamphetamine-dependent patients varies from 13% (in the United States11) to 50% (in Asia12). This difference might be related to variability in the purity of methamphetamine used in different locations.
Methamphetamine users may experience a pre-psychotic state that consists of ideas of reference and delusional moods. This is followed by a psychotic state that includes hallucinations and delusions. The time it takes to develop these symptoms can vary from a few months up to >20 years after starting to use methamphetamine.10,13 Psychosis can occur in patients who do not have a history of psychiatric illness.10
The clinical presentation of methamphetamine-induced psychosis includes delusions of reference and persecutions.8-10 Paranoid delusions may be accompanied by violent behavior. Some patients may present with grandiose or jealousy delusions. Patients may experience auditory, tactile, or visual hallucinations. They may exhibit mania and logorrheic verbal outputs, symptoms consistent with a diagnosis of methamphetamine-induced mood disorder with manic features. Patients who use large daily doses of the drug also may report that there are ants or other parasites crawling under their skin (eg, formication, “meth mites”) and might present with infected excoriations of their skin as a result of attempting to remove insects. This is clinically important because penicillin-resistant bacteria are common in patients who use methamphetamine, and strains tend to be virulent.
Psychotic symptoms can last from a few days to several weeks after stopping methamphetamine use, although methamphetamine-induced psychosis can persist after long periods of abstinence.14 Psychotic symptoms may recur with re-exposure to the drug9 or repeated stressful life events.15 Patients with recurrent psychosis in the absence of a drug trigger appear to have high levels of peripheral norepinephrine.15 Patients with psychosis caused by long-term methamphetamine use will not necessarily show signs of sympathomimetic dysfunction because they may not have any methamphetamine in the body when they first present for clinical evaluation. Importantly, patients with methamphetamine-induced psychosis have been reported to have poor outcomes at follow-up.16 They have an increased risk of suicide, recurrent drug-induced psychosis, and comorbid alcohol abuse.16
Doses required to induce psychosis vary from patient to patient and may depend on the patient’s genetic background and/or environmental conditions. Methamphetamine can increase the severity of many psychiatric symptoms17 and may expedite the development of schizophrenia in first-degree relatives of patients with schizophrenia.18
The diagnosis of methamphetamine-induced psychosis should focus on differentiating it from schizophrenia. Wang et al19 found similar patterns of delusions in patients with schizophrenia and those with methamphetamine-induced psychosis. However, compared with patients with schizophrenia, patients with methamphetamine-induced psychosis have a higher prevalence of visual and tactile hallucinations, and less disorganization, blunted affect, and motor retardation. Some patients may present with depression and suicidal ideation; these features may be more prominent during withdrawal, but also may be obvious during periods of active use.16
Although these clinical features may be helpful initially, more comparative neurobiologic investigations are needed to identify potential biologic differences between schizophrenia and methamphetamine-induced psychosis because these differences will impact therapeutic approaches to these diverse population groups.
Neurologic complications. Chronic methamphetamine users may develop various neurologic disorders.20 They may present with stereotypies involving finger movements or repeated rubbing of mouth or face, orofacial dyskinesia, and choreoathetoid movements reminiscent of classical neurologic disorders. These movement disorders can persist after cessation of methamphetamine use. In some cases, these movement abnormalities may respond to dopamine receptor antagonists such as haloperidol.
Neuropsychological findings. Chronic methamphetamine users show mild signs of cognitive decline that affects a broad range of neuropsychological functions.21-23 There are deficits in several cognitive processes that are dependent on the function of frontostriatal and limbic circuits.24-26 Specifically, episodic memory, executive functions, complex information processing speed, and psychomotor functions all have been reported to be negatively impacted.
Methamphetamine use often results in psychiatric distress that impacts users’ interpersonal relationships.27 Additionally, impulsivity may exacerbate their psychosocial difficulties and promote maintenance of drug-seeking behaviors.28 Cognitive deficits lead to poor health outcomes, high-risk behaviors, employment difficulties, and repeated relapse.29,30
Partial recovery of neuropsychological functioning and improvement in affective distress can be achieved after sustained abstinence from methamphetamine, but recovery may not be complete. Because cognitive dysfunction can influence treatment outcomes, clinicians need to be fully aware of the cognitive status of those patients, and a thorough neuropsychological evaluation is necessary before initiating treatment.
Treatment
Methamphetamine abuse. Because patients who abuse methamphetamine are at high risk of developing psychosis, neurologic complications, and neuropsychological disorders, initiating treatment early in the course of their addiction is of paramount importance. Treatment of methamphetamine addiction is complicated by the fact that these patients have a high prevalence of comorbid psychiatric disorders, which clinicians need to keep in mind when selecting therapeutic interventions.
There are no FDA-approved agents for treating methamphetamine abuse.31 Several drugs have been tried with varying degrees of success, including bupropion, modafinil, and naltrexone. A study of modafinil found no clinically significant effects for treating methamphetamine abuse; however, only approximately one-half of participants in this study took modafinil as instructed.32 Certain selective serotonin reuptake inhibitors, including fluoxetine and paroxetine, have not been shown to be effective in treating these patients. Naltrexone may be a reasonable medication to consider because of the high prevalence of comorbid alcohol abuse among methamphetamine users.
Other treatments for methamphetamine addiction consist of behavioral interventions such as cognitive-behavioral therapy. Clinical experience has shown that the risk of relapse depends on how long the patient has been abstinent prior to entering a treatment program, the presence of attention and memory deficits, and findings of poor decision-making on neuropsychological tests.
The presence of cognitive abnormalities has been reported to impact methamphetamine abusers’ response to treatment.33 These findings suggest the need to develop approaches that might improve cognition in patients who are undergoing treatment for methamphetamine abuse. The monoaminergic agent modafinil and similar drugs need to be evaluated in large populations to increase the possibility of identifying characteristics of patients who might respond to cognitive enhancement.34
Methamphetamine-induced psychosis. First-generation antipsychotics, such as haloperidol or fluphenazine, need to be used sparingly in patients with methamphetamine-induced psychosis because of the risk of developing extrapyramidal symptoms (EPS) and because these patients are prone to develop motor complications as a result of methamphetamine abuse. Second-generation antipsychotics, such as risperidone and olanzapine, may be more appropriate because of the lower risks of EPS.35 The presence of high norepinephrine levels in some patients with recurrent methamphetamine psychosis suggests that drugs that block norepinephrine receptors, such as prazosin or propranolol, might be of therapeutic benefit if they are shown to be effective in controlled clinical trials.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.
1. United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication, Sales No. E.16.XI.7. http://www.unodc.org/wdr2016. Published 2016. Accessed September 28, 2017.
2. Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379-407.
3. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760-773.
4. Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127(1):91-107.
5. Gold MS, Kobeissy FH, Wang KK, et al. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118-127.
6. Gold MS, Graham NA, Kobeissy FH, et al. Speed, cocaine, and other psychostimulants death rates. Am J Cardiol. 2007;100(7):1184.
7. Shelly J, Uhlmann A, Sinclair H, et al. First-rank symptoms in methamphetamine psychosis and schizophrenia. Psychopathology. 2016;49(6):429-435.
8. Connell PH. Amphetamine psychosis. In: Connell PH. Maudsley monographs. No. 5. London, United Kingdom: Oxford Press; 1958:5.
9. Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654(1):160-170.
10. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci. 2004;1025(1):279-287.
11. Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, et al; Methamphetamine Treatment Project Corporate Authors. Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev. 2010;29(1):12-20.
12. Sulaiman AH, Said MA, Habil MH, et al. The risk and associated factors of methamphetamine psychosis in methamphetamine-dependent patients in Malaysia. Compr Psychiatry. 2014;55(suppl 1):S89-S94.
13. Fasihpour B, Molavi S, Shariat SV. Clinical features of inpatients with methamphetamine-induced psychosis. J Ment Health. 2013;22(4):341-349.
14. Akiyama K, Saito A, Shimoda K. Chronic methamphetamine psychosis after long-term abstinence in Japanese incarcerated patients. Am J Addict. 2011;20(3):240-249.
15. Yui K, Goto K, Ikemoto S, et al. Methamphetamine psychosis: spontaneous recurrence of paranoid-hallucinatory states and monoamine neurotransmitter function. J Clin Psychopharmacol. 1997;17(1):34-43.
16. Kittirattanapaiboon P, Mahatnirunkul S, Booncharoen H, et al. Long-term outcomes in methamphetamine psychosis patients after first hospitalisation. Drug Alcohol Rev. 2010;29(4):456-461.
17. McKetin R, Dawe S, Burns RA, et al. The profile of psychiatric symptoms exacerbated by methamphetamine use. Drug Alcohol Depend. 2016;161:104-109.
18. Li H, Lu Q, Xiao E, et al. Methamphetamine enhances the development of schizophrenia in first-degree relatives of patients with schizophrenia. Can J Psychiatry. 2014;59(2):107-113.
19. Wang LJ, Lin SK, Chen YC, et al. Differences in clinical features of methamphetamine users with persistent psychosis and patients with schizophrenia. Psychopathology. 2016;49(2):108-115.
20. Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Psychiatr Clin North Am. 2013;36(2):261-275.
21. Simon SL, Domier C, Carnell J, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9(3):222-231.
22. Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53-63.
23. Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology (Berl). 2009;203(3):609-616.
24. Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383-393.
25. Nestor LJ, Ghahremani DG, Monterosso J, et al. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287-295.
26. Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297.
27. Cretzmeyer M, Sarrazin MV, Huber DL, et al. Treatment of methamphetamine abuse: research findings and clinical directions. J Subst Abuse Treat. 2003;24(3):267-277.
28. Semple SJ, Zians J, Grant I, et al. Impulsivity and methamphetamine use. J Subst Abuse Treat. 2005;29(2):85-93.
29. Hester R, Lee N, Pennay A, et al. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489-497.
30. Weber E, Blackstone K, Iudicello JE, et al; Translational Methamphetamine AIDS Research Center (TMARC) Group. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125(1-2):146-153.
31. Ballester J, Valentine G, Sofuoglu M. Pharmacological treatments for methamphetamine addiction: current status and future directions. Expert Rev Clin Pharmacol. 2017;10(3):305-314.
32. Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135-141.
33. Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry. 2016;6:189.
34. Loland CJ, Mereu M, Okunola OM, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5):405-413.
35. Farnia V, Shakeri J, Tatari F, et al. Randomized controlled trial of aripiprazole versus risperidone for the treatment of amphetamine-induced psychosis. Am J Drug Alcohol Abuse. 2014;40(1):10-15.