User login
Acute cardiorenal syndrome: Mechanisms and clinical implications
As the heart goes, so go the kidneys—and vice versa. Cardiac and renal function are intricately interdependent, and failure of either organ causes injury to the other in a vicious circle of worsening function.1
Here, we discuss acute cardiorenal syndrome, ie, acute exacerbation of heart failure leading to acute kidney injury, a common cause of hospitalization and admission to the intensive care unit. We examine its clinical definition, pathophysiology, hemodynamic derangements, clues that help in diagnosing it, and its treatment.
A GROUP OF LINKED DISORDERS
Two types of acute cardiac dysfunction
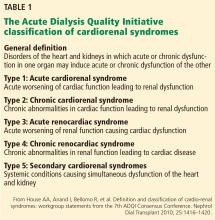
Although these definitions offer a good general description, further clarification of the nature of organ dysfunction is needed. Acute renal dysfunction can be unambiguously defined using the AKIN (Acute Kidney Injury Network) and RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) classifications.3 Acute cardiac dysfunction, on the other hand, is an ambiguous term that encompasses 2 clinically and pathophysiologically distinct conditions: cardiogenic shock and acute heart failure.
Cardiogenic shock is characterized by a catastrophic compromise of cardiac pump function leading to global hypoperfusion severe enough to cause systemic organ damage.4 The cardiac index at which organs start to fail varies in different cases, but a value of less than 1.8 L/min/m2 is typically used to define cardiogenic shock.4
Acute heart failure, on the other hand, is defined as gradually or rapidly worsening signs and symptoms of congestive heart failure due to worsening pulmonary or systemic congestion.5 Hypervolemia is the hallmark of acute heart failure, whereas patients with cardiogenic shock may be hypervolemic, normovolemic, or hypovolemic. Although cardiac output may be mildly reduced in some cases of acute heart failure, systemic perfusion is enough to maintain organ function.
These two conditions cause renal injury by distinct mechanisms and have entirely different therapeutic implications. As we discuss later, reduced renal perfusion due to renal venous congestion is now believed to be the major hemodynamic mechanism of renal injury in acute heart failure. On the other hand, in cardiogenic shock, renal perfusion is reduced due to a critical decline of cardiac pump function.
The ideal definition of acute cardiorenal syndrome should describe a distinct pathophysiology of the syndrome and offer distinct therapeutic options that counteract it. Hence, we propose that renal injury from cardiogenic shock should not be included in its definition, an approach that has been adopted in some of the recent reviews as well.6 Our discussion of acute cardiorenal syndrome is restricted to renal injury caused by acute heart failure.
PATHOPHYSIOLOGY OF ACUTE CARDIORENAL SYNDROME
Multiple mechanisms have been implicated in the pathophysiology of cardiorenal syndrome.7,8
Sympathetic hyperactivity is a compensatory mechanism in heart failure and may be aggravated if cardiac output is further reduced. Its effects include constriction of afferent and efferent arterioles, causing reduced renal perfusion and increased tubular sodium and water reabsorption.7
The renin-angiotensin-aldosterone system is activated in patients with stable congestive heart failure and may be further stimulated in a state of reduced renal perfusion, which is a hallmark of acute cardiorenal syndrome. Its activation can cause further salt and water retention.
However, direct hemodynamic mechanisms likely play the most important role and have obvious diagnostic and therapeutic implications.
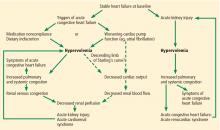
Elevated venous pressure, not reduced cardiac output, drives kidney injury
The classic view was that renal dysfunction in acute heart failure is caused by reduced renal blood flow due to failing cardiac pump function. Cardiac output may be reduced in acute heart failure for various reasons, such as atrial fibrillation, myocardial infarction, or other processes, but reduced cardiac output has a minimal role, if any, in the pathogenesis of renal injury in acute heart failure.
As evidence of this, acute heart failure is not always associated with reduced cardiac output.5 Even if the cardiac index (cardiac output divided by body surface area) is mildly reduced, renal blood flow is largely unaffected, thanks to effective renal autoregulatory mechanisms. Not until the mean arterial pressure falls below 70 mm Hg do these mechanisms fail and renal blood flow starts to drop.9 Hence, unless cardiac performance is compromised enough to cause cardiogenic shock, renal blood flow usually does not change significantly with mild reduction in cardiac output.
Hanberg et al10 performed a post hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheter Effectiveness (ESCAPE) trial, in which 525 patients with advanced heart failure underwent pulmonary artery catheterization to measure their cardiac index. The authors found no association between the cardiac index and renal function in these patients.
How venous congestion impairs the kidney
In view of the current clinical evidence, the focus has shifted to renal venous congestion. According to Poiseuille’s law, blood flow through the kidneys depends on the pressure gradient—high pressure on the arterial side, low pressure on the venous side.8 Increased renal venous pressure causes reduced renal perfusion pressure, thereby affecting renal perfusion. This is now recognized as an important hemodynamic mechanism of acute cardiorenal syndrome.
Renal congestion can also affect renal function through indirect mechanisms. For example, it can cause renal interstitial edema that may then increase the intratubular pressure, thereby reducing the transglomerular pressure gradient.11
Firth et al,14 in experiments in animals, found that increasing the renal venous pressure above 18.75 mm Hg significantly reduced the glomerular filtration rate, which completely resolved when renal venous pressure was restored to basal levels.
Mullens et al,15 in a study of 145 patients admitted with acute heart failure, reported that 58 (40%) developed acute kidney injury. Pulmonary artery catheterization revealed that elevated central venous pressure, rather than reduced cardiac index, was the primary hemodynamic factor driving renal dysfunction.
DIAGNOSIS AND CLINICAL ASSESSMENT
Patients with acute cardiorenal syndrome present with clinical features of pulmonary or systemic congestion (or both) and acute kidney injury.
Elevated left-sided pressures are usually but not always associated with elevated right-sided pressures. In a study of 1,000 patients with advanced heart failure, a pulmonary capillary wedge pressure of 22 mm Hg or higher had a positive predictive value of 88% for a right atrial pressure of 10 mm Hg or higher.16 Hence, the clinical presentation may vary depending on the location (pulmonary, systemic, or both) and degree of congestion.
Symptoms of pulmonary congestion include worsening exertional dyspnea and orthopnea; bilateral crackles may be heard on physical examination if pulmonary edema is present.
Systemic congestion can cause significant peripheral edema and weight gain. Jugular venous distention may be noted. Oliguria may be present due to renal dysfunction; patients on maintenance diuretic therapy often note its lack of efficacy.
Signs of acute heart failure
Wang et al,17 in a meta-analysis of 22 studies, concluded that the features that most strongly suggested acute heart failure were:
- History of paroxysmal nocturnal dyspnea
- A third heart sound
- Evidence of pulmonary venous congestion on chest radiography.
Features that most strongly suggested the patient did not have acute heart failure were:
- Absence of exertional dyspnea
- Absence of rales
- Absence of radiographic evidence of cardiomegaly.
Patients may present without some of these classic clinical features, and the diagnosis of acute heart failure may be challenging. For example, even if left-sided pressures are very high, pulmonary edema may be absent because of pulmonary vascular remodeling in chronic heart failure.18 Pulmonary artery catheterization reveals elevated cardiac filling pressures and can be used to guide therapy, but clinical evidence argues against its routine use.19
Urine electrolytes (fractional excretion of sodium < 1% and fractional excretion of urea < 35%) often suggest a prerenal form of acute kidney injury, since the hemodynamic derangements in acute cardiorenal syndrome reduce renal perfusion.
Biomarkers of cell-cycle arrest such as urine insulinlike growth factor-binding protein 7 and tissue inhibitor of metalloproteinase 2 have recently been shown to identify patients with acute heart failure at risk of developing acute cardiorenal syndrome.20
Acute cardiorenal syndrome vs renal injury due to hypovolemia
The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Patients with stable heart failure usually have mild hypervolemia at baseline, but they can become hypovolemic due to overaggressive diuretic therapy, severe diarrhea, or other causes.
Although the fluid status of patients in these 2 conditions is opposite, they can be difficult to distinguish. In both conditions, urine electrolytes suggest a prerenal acute kidney injury. A history of recent fluid losses or diuretic overuse may help identify hypovolemia. If available, analysis of the recent trend in weight can be vital in making the right diagnosis.
Misdiagnosis of acute cardiorenal syndrome as hypovolemia-induced acute kidney injury can be catastrophic. If volume depletion is erroneously judged to be the cause of acute kidney injury, fluid administration can further worsen both cardiac and renal function. This can perpetuate the vicious circle that is already in play. Lack of renal recovery may invite further fluid administration.
TREATMENT
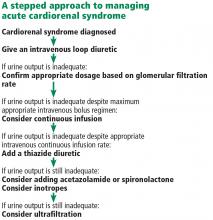
Fluid removal with diuresis or ultrafiltration is the cornerstone of treatment. Other treatments such as inotropes are reserved for patients with resistant disease.
Diuretics
The goal of therapy in acute cardiorenal syndrome is to achieve aggressive diuresis, typically using intravenous diuretics. Loop diuretics are the most potent class of diuretics and are the first-line drugs for this purpose. Other classes of diuretics can be used in conjunction with loop diuretics; however, using them by themselves is neither effective nor recommended.
Resistance to diuretics at usual doses is common in patients with acute cardiorenal syndrome. Several mechanisms contribute to diuretic resistance in these patients.21
Oral bioavailability of diuretics may be reduced due to intestinal edema.
Diuretic pharmacokinetics are significantly deranged in cardiorenal syndrome. All diuretics except mineralocorticoid antagonists (ie, spironolactone and eplerenone) act on targets on the luminal side of renal tubules, but are highly protein-bound and are hence not filtered at the glomerulus. Loop diuretics, thiazides, and carbonic anhydrase inhibitors are secreted in the proximal convoluted tubule via the organic anion transporter,22 whereas epithelial sodium channel inhibitors (amiloride and triamterene) are secreted via the organic cation transporter 2.23 In renal dysfunction, various uremic toxins accumulate in the body and compete with diuretics for secretion into the proximal convoluted tubule via these transporters.24
Finally, activation of the sympathetic nervous system and renin-angiotensin-aldosterone system leads to increased tubular sodium and water retention, thereby also blunting the diuretic response.
Diuretic dosage. In patients whose creatinine clearance is less than 15 mL/min, only 10% to 20% as much loop diuretic is secreted into the renal tubule as in normal individuals.25 This effect warrants dose adjustment of diuretics during uremia.
Continuous infusion or bolus? Continuous infusion of loop diuretics is another strategy to optimize drug delivery. Compared with bolus therapy, continuous infusion provides more sustained and uniform drug delivery and prevents postdiuretic sodium retention.
The Diuretic Optimization Strategies Evaluation (DOSE) trial compared the efficacy and safety of continuous vs bolus furosemide therapy in 308 patients admitted with acute decompensated heart failure.26 There was no difference in symptom control or net fluid loss at 72 hours in either group. Other studies have shown more diuresis with continuous infusion than with a similarly dosed bolus regimen.27 However, definitive clinical evidence is lacking at this point to support routine use of continuous loop diuretic therapy.
Combination diuretic therapy. Sequential nephron blockade with combination diuretic therapy is an important therapeutic strategy against diuretic resistance. Notably, urine output-guided diuretic therapy has been shown to be superior to standard diuretic therapy.28 Such therapeutic protocols may employ combination diuretic therapy as a next step when the desired diuretic response is not obtained with high doses of loop diuretic monotherapy.
The desired diuretic response depends on the clinical situation. For example, in patients with severe congestion, we would like the net fluid output to be at least 2 to 3 L more than the fluid intake after the first 24 hours. Sometimes, patients in the intensive care unit are on several essential drug infusions, so that their net intake amounts to 1 to 2 L. In these patients, the desired urine output would be even more than in patients not on these drug infusions.
Loop diuretics block sodium reabsorption at the thick ascending loop of Henle. This disrupts the countercurrent exchange mechanism and reduces renal medullary interstitial osmolarity; these effects prevent water reabsorption. However, the unresorbed sodium can be taken up by the sodium-chloride cotransporter and the epithelial sodium channel in the distal nephron, thereby blunting the diuretic effect. This is the rationale for combining loop diuretics with thiazides or potassium-sparing diuretics.
Similarly, carbonic anhydrase inhibitors (eg, acetazolamide) reduce sodium reabsorption from the proximal convoluted tubule, but most of this sodium is then reabsorbed distally. Hence, the combination of a loop diuretic and acetazolamide can also have a synergistic diuretic effect.
The most popular combination is a loop diuretic plus a thiazide, although no large-scale placebo-controlled trials have been performed.29 Metolazone (a thiazidelike diuretic) is typically used due to its low cost and availability.30 Metolazone has also been shown to block sodium reabsorption at the proximal tubule, which may contribute to its synergistic effect. Chlorothiazide is available in an intravenous formulation and has a faster onset of action than metolazone. However, studies have failed to detect any benefit of one over the other.31
The potential benefit of combining a loop diuretic with acetazolamide is a lower tendency to develop metabolic alkalosis, a potential side effect of loop diuretics and thiazides. Although data are limited, a recent study showed that adding acetazolamide to bumetanide led to significantly increased natriuresis.32
In the Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, adding spironolactone in high doses to usual therapy was not found to cause any significant change in N-terminal pro-B-type natriuretic peptide level or net urine output.33
Ultrafiltration
Venovenous ultrafiltration (or aquapheresis) employs an extracorporeal circuit, similar to the one used in hemodialysis, which removes iso-osmolar fluid at a fixed rate.34 Newer ultrafiltration systems are more portable, can be used with peripheral venous access, and require minimal nursing supervision.35
Although ultrafiltration seems an attractive alternative to diuresis in acute heart failure, studies have been inconclusive. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial compared ultrafiltration and diuresis in 188 patients with acute heart failure and acute cardiorenal syndrome.36 Diuresis, performed according to an algorithm, was found to be superior to ultrafiltration in terms of a bivariate end point of change in weight and change in serum creatinine level at 96 hours. However, the level of cystatin C is thought to be a more accurate indicator of renal function, and the change in cystatin C level from baseline did not differ between the two treatment groups. Also, the ultrafiltration rate was 200 mL per hour, which, some argue, may have been excessive and may have caused intravascular depletion.
Although the ideal rate of fluid removal is unknown, it should be individualized and adjusted based on the patient’s renal function, volume status, and hemodynamic status. The initial rate should be based on the degree of fluid overload and the anticipated plasma refill rate from the interstitial fluid.37 For example, a malnourished patient may have low serum oncotic pressure and hence have low plasma refill upon ultrafiltration. Disturbance of this delicate balance between the rates of ultrafiltration and plasma refill may lead to intravascular volume contraction.
In summary, although ultrafiltration is a valuable alternative to diuretics in resistant cases, its use as a primary decongestive therapy cannot be endorsed in view of the current data.
Inotropes
Inotropes such as dobutamine and milrinone are typically used in cases of cardiogenic shock to maintain organ perfusion. There is a physiologic rationale to using inotropes in acute cardiorenal syndrome as well, especially when the aforementioned strategies fail to overcome diuretic resistance.7
Inotropes increase cardiac output, improve renal blood flow, improve right ventricular output, and thereby relieve systemic congestion. These hemodynamic effects may improve renal perfusion and response to diuretics. However, clinical evidence to support this is lacking.
The Renal Optimization Strategies Evaluation (ROSE) trial enrolled 360 patients with acute heart failure and renal dysfunction. Adding dopamine in a low dose (2 μg/kg/min) to diuretic therapy had no significant effect on 72-hour cumulative urine output or renal function as measured by cystatin C levels.38 However, acute kidney injury was not identified in this trial, and the renal function of many of these patients may have been at its baseline when they were admitted. In other words, this trial did not necessarily include patients with acute kidney injury along with acute heart failure. Hence, it did not necessarily include patients with acute cardiorenal syndrome.
Vasodilators
Vasodilators such as nitroglycerin, sodium nitroprusside, and hydralazine are commonly used in patients with acute heart failure, although the clinical evidence supporting their use is weak.
Physiologically, arterial dilation reduces afterload and can help relieve pulmonary congestion, and venodilation increases capacitance and reduces preload. In theory, venodilators such as nitroglycerin can relieve renal venous congestion in patients with acute cardiorenal syndrome, thereby improving renal perfusion.
However, the use of vasodilators is often limited by their adverse effects, the most important being hypotension. This is especially relevant in light of recent data identifying reduction in blood pressure during treatment of acute heart failure as an independent risk factor for worsening renal function.39,40 It is important to note that in these studies, changes in cardiac index did not affect the propensity for developing worsening renal function. The precise mechanism of this finding is unclear but it is plausible that systemic vasodilation redistributes the cardiac output to nonrenal tissues, thereby overriding the renal autoregulatory mechanisms that are normally employed in low output states.
Preventive strategies
Various strategies can be used to prevent acute cardiorenal syndrome. An optimal outpatient diuretic regimen to avoid hypervolemia is essential. Patients with advanced congestive heart failure should be followed up closely in dedicated heart failure clinics until their diuretic regimen is optimized. Patients should be advised to check their weight on a regular basis and seek medical advice if they notice an increase in their weight or a reduction in their urine output.
TAKE-HOME POINTS
- A robust clinical definition of cardiorenal syndrome is lacking. Hence, recognition of this condition can be challenging.
- Volume overload is central to its pathogenesis, and accurate assessment of volume status is critical.
- Renal venous congestion is the major mechanism of type 1 cardiorenal syndrome.
- Misdiagnosis can have devastating consequences, as it may lead to an opposite therapeutic approach.
- Fluid removal by various strategies is the mainstay of treatment.
- Temporary inotropic support should be saved for the last resort.
- Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Cleve Clin J Med 2006; 73:485–491.
- House AA, Anand I, Bellomo R, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Chang CH, Lin CY, Tian YC, et al. Acute kidney injury classification: comparison of AKIN and RIFLE criteria. Shock 2010; 33:247-252.
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008; 117:686–697.
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009; 53:557–573.
- ter Maaten JM, Valente MA, Damman K, et al. Diuretic response in acute heart failure—pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015; 12:184–192.
- Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 2013; 9:99–111.
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121:2592–2600.
- Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Afsar B, Ortiz A, Covic A, et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9:39–47.
- Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013; 62:485–495.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1988; 1:1033–1035.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18:1126–1132.
- Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 2004; 125:669–682.
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294:1625–1633.
- Schanz M, Shi J , Wasser C , Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 2017; doi.org/10.1002/clc.22683.
- Bowman BN, Nawarskas JJ, Anderson JR. Treating diuretic resistance: an overview. Cardiol Rev 2016; 24:256–260.
- Uwai Y, Saito H, Hashimoto Y, Inui KI. Interaction and transport of thiazide diuretics, loop diuretics, and acetazolamide via rat renal organic anion transporter rOAT1. J Pharmacol Exp Ther 2000; 295:261–265.
- Hacker K, Maas R, Kornhuber J, et al. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One 2015; 10:e0136451.
- Schophuizen CM, Wilmer MJ, Jansen J, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch 2013; 465:1701–1714.
- Brater DC. Diuretic therapy. N Engl J Med 1998; 339:387–395.
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail 2010; 16:188–193.
- Grodin JL, Stevens SR, de Las Fuentes L, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail 2016; 22:26–32.
- Ng TM, Konopka E, Hyderi AF, et al. Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther 2013; 18:345–353.
- Sica DA. Metolazone and its role in edema management. Congest Heart Fail 2003; 9:100–105.
- Moranville MP, Choi S, Hogg J, Anderson AS, Rich JD. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015; 33:42–49.
- Verbrugge FH, Dupont M, Bertrand PB, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 2015; 70:265–373.
- Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017 Jul 12. doi: 10.1001/jamacardio.2017.2198. [Epub ahead of print]
- Pourafshar N, Karimi A, Kazory A. Extracorporeal ultrafiltration therapy for acute decompensated heart failure. Expert Rev Cardiovasc Ther 2016; 14:5–13.
- Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail 2003; 9:227–231.
- Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Kazory A. Cardiorenal syndrome: ultrafiltration therapy for heart failure—trials and tribulations. Clin J Am Soc Nephrol 2013; 8:1816–1828.
- Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Testani JM, Coca SG, McCauley BD, et al. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011; 13:877–884.
- Dupont M, Mullens W, Finucan M, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013; 15:433–440.
As the heart goes, so go the kidneys—and vice versa. Cardiac and renal function are intricately interdependent, and failure of either organ causes injury to the other in a vicious circle of worsening function.1
Here, we discuss acute cardiorenal syndrome, ie, acute exacerbation of heart failure leading to acute kidney injury, a common cause of hospitalization and admission to the intensive care unit. We examine its clinical definition, pathophysiology, hemodynamic derangements, clues that help in diagnosing it, and its treatment.
A GROUP OF LINKED DISORDERS
Two types of acute cardiac dysfunction
Although these definitions offer a good general description, further clarification of the nature of organ dysfunction is needed. Acute renal dysfunction can be unambiguously defined using the AKIN (Acute Kidney Injury Network) and RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) classifications.3 Acute cardiac dysfunction, on the other hand, is an ambiguous term that encompasses 2 clinically and pathophysiologically distinct conditions: cardiogenic shock and acute heart failure.
Cardiogenic shock is characterized by a catastrophic compromise of cardiac pump function leading to global hypoperfusion severe enough to cause systemic organ damage.4 The cardiac index at which organs start to fail varies in different cases, but a value of less than 1.8 L/min/m2 is typically used to define cardiogenic shock.4
Acute heart failure, on the other hand, is defined as gradually or rapidly worsening signs and symptoms of congestive heart failure due to worsening pulmonary or systemic congestion.5 Hypervolemia is the hallmark of acute heart failure, whereas patients with cardiogenic shock may be hypervolemic, normovolemic, or hypovolemic. Although cardiac output may be mildly reduced in some cases of acute heart failure, systemic perfusion is enough to maintain organ function.
These two conditions cause renal injury by distinct mechanisms and have entirely different therapeutic implications. As we discuss later, reduced renal perfusion due to renal venous congestion is now believed to be the major hemodynamic mechanism of renal injury in acute heart failure. On the other hand, in cardiogenic shock, renal perfusion is reduced due to a critical decline of cardiac pump function.
The ideal definition of acute cardiorenal syndrome should describe a distinct pathophysiology of the syndrome and offer distinct therapeutic options that counteract it. Hence, we propose that renal injury from cardiogenic shock should not be included in its definition, an approach that has been adopted in some of the recent reviews as well.6 Our discussion of acute cardiorenal syndrome is restricted to renal injury caused by acute heart failure.
PATHOPHYSIOLOGY OF ACUTE CARDIORENAL SYNDROME
Multiple mechanisms have been implicated in the pathophysiology of cardiorenal syndrome.7,8
Sympathetic hyperactivity is a compensatory mechanism in heart failure and may be aggravated if cardiac output is further reduced. Its effects include constriction of afferent and efferent arterioles, causing reduced renal perfusion and increased tubular sodium and water reabsorption.7
The renin-angiotensin-aldosterone system is activated in patients with stable congestive heart failure and may be further stimulated in a state of reduced renal perfusion, which is a hallmark of acute cardiorenal syndrome. Its activation can cause further salt and water retention.
However, direct hemodynamic mechanisms likely play the most important role and have obvious diagnostic and therapeutic implications.
Elevated venous pressure, not reduced cardiac output, drives kidney injury
The classic view was that renal dysfunction in acute heart failure is caused by reduced renal blood flow due to failing cardiac pump function. Cardiac output may be reduced in acute heart failure for various reasons, such as atrial fibrillation, myocardial infarction, or other processes, but reduced cardiac output has a minimal role, if any, in the pathogenesis of renal injury in acute heart failure.
As evidence of this, acute heart failure is not always associated with reduced cardiac output.5 Even if the cardiac index (cardiac output divided by body surface area) is mildly reduced, renal blood flow is largely unaffected, thanks to effective renal autoregulatory mechanisms. Not until the mean arterial pressure falls below 70 mm Hg do these mechanisms fail and renal blood flow starts to drop.9 Hence, unless cardiac performance is compromised enough to cause cardiogenic shock, renal blood flow usually does not change significantly with mild reduction in cardiac output.
Hanberg et al10 performed a post hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheter Effectiveness (ESCAPE) trial, in which 525 patients with advanced heart failure underwent pulmonary artery catheterization to measure their cardiac index. The authors found no association between the cardiac index and renal function in these patients.
How venous congestion impairs the kidney
In view of the current clinical evidence, the focus has shifted to renal venous congestion. According to Poiseuille’s law, blood flow through the kidneys depends on the pressure gradient—high pressure on the arterial side, low pressure on the venous side.8 Increased renal venous pressure causes reduced renal perfusion pressure, thereby affecting renal perfusion. This is now recognized as an important hemodynamic mechanism of acute cardiorenal syndrome.
Renal congestion can also affect renal function through indirect mechanisms. For example, it can cause renal interstitial edema that may then increase the intratubular pressure, thereby reducing the transglomerular pressure gradient.11
Firth et al,14 in experiments in animals, found that increasing the renal venous pressure above 18.75 mm Hg significantly reduced the glomerular filtration rate, which completely resolved when renal venous pressure was restored to basal levels.
Mullens et al,15 in a study of 145 patients admitted with acute heart failure, reported that 58 (40%) developed acute kidney injury. Pulmonary artery catheterization revealed that elevated central venous pressure, rather than reduced cardiac index, was the primary hemodynamic factor driving renal dysfunction.
DIAGNOSIS AND CLINICAL ASSESSMENT
Patients with acute cardiorenal syndrome present with clinical features of pulmonary or systemic congestion (or both) and acute kidney injury.
Elevated left-sided pressures are usually but not always associated with elevated right-sided pressures. In a study of 1,000 patients with advanced heart failure, a pulmonary capillary wedge pressure of 22 mm Hg or higher had a positive predictive value of 88% for a right atrial pressure of 10 mm Hg or higher.16 Hence, the clinical presentation may vary depending on the location (pulmonary, systemic, or both) and degree of congestion.
Symptoms of pulmonary congestion include worsening exertional dyspnea and orthopnea; bilateral crackles may be heard on physical examination if pulmonary edema is present.
Systemic congestion can cause significant peripheral edema and weight gain. Jugular venous distention may be noted. Oliguria may be present due to renal dysfunction; patients on maintenance diuretic therapy often note its lack of efficacy.
Signs of acute heart failure
Wang et al,17 in a meta-analysis of 22 studies, concluded that the features that most strongly suggested acute heart failure were:
- History of paroxysmal nocturnal dyspnea
- A third heart sound
- Evidence of pulmonary venous congestion on chest radiography.
Features that most strongly suggested the patient did not have acute heart failure were:
- Absence of exertional dyspnea
- Absence of rales
- Absence of radiographic evidence of cardiomegaly.
Patients may present without some of these classic clinical features, and the diagnosis of acute heart failure may be challenging. For example, even if left-sided pressures are very high, pulmonary edema may be absent because of pulmonary vascular remodeling in chronic heart failure.18 Pulmonary artery catheterization reveals elevated cardiac filling pressures and can be used to guide therapy, but clinical evidence argues against its routine use.19
Urine electrolytes (fractional excretion of sodium < 1% and fractional excretion of urea < 35%) often suggest a prerenal form of acute kidney injury, since the hemodynamic derangements in acute cardiorenal syndrome reduce renal perfusion.
Biomarkers of cell-cycle arrest such as urine insulinlike growth factor-binding protein 7 and tissue inhibitor of metalloproteinase 2 have recently been shown to identify patients with acute heart failure at risk of developing acute cardiorenal syndrome.20
Acute cardiorenal syndrome vs renal injury due to hypovolemia
The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Patients with stable heart failure usually have mild hypervolemia at baseline, but they can become hypovolemic due to overaggressive diuretic therapy, severe diarrhea, or other causes.
Although the fluid status of patients in these 2 conditions is opposite, they can be difficult to distinguish. In both conditions, urine electrolytes suggest a prerenal acute kidney injury. A history of recent fluid losses or diuretic overuse may help identify hypovolemia. If available, analysis of the recent trend in weight can be vital in making the right diagnosis.
Misdiagnosis of acute cardiorenal syndrome as hypovolemia-induced acute kidney injury can be catastrophic. If volume depletion is erroneously judged to be the cause of acute kidney injury, fluid administration can further worsen both cardiac and renal function. This can perpetuate the vicious circle that is already in play. Lack of renal recovery may invite further fluid administration.
TREATMENT
Fluid removal with diuresis or ultrafiltration is the cornerstone of treatment. Other treatments such as inotropes are reserved for patients with resistant disease.
Diuretics
The goal of therapy in acute cardiorenal syndrome is to achieve aggressive diuresis, typically using intravenous diuretics. Loop diuretics are the most potent class of diuretics and are the first-line drugs for this purpose. Other classes of diuretics can be used in conjunction with loop diuretics; however, using them by themselves is neither effective nor recommended.
Resistance to diuretics at usual doses is common in patients with acute cardiorenal syndrome. Several mechanisms contribute to diuretic resistance in these patients.21
Oral bioavailability of diuretics may be reduced due to intestinal edema.
Diuretic pharmacokinetics are significantly deranged in cardiorenal syndrome. All diuretics except mineralocorticoid antagonists (ie, spironolactone and eplerenone) act on targets on the luminal side of renal tubules, but are highly protein-bound and are hence not filtered at the glomerulus. Loop diuretics, thiazides, and carbonic anhydrase inhibitors are secreted in the proximal convoluted tubule via the organic anion transporter,22 whereas epithelial sodium channel inhibitors (amiloride and triamterene) are secreted via the organic cation transporter 2.23 In renal dysfunction, various uremic toxins accumulate in the body and compete with diuretics for secretion into the proximal convoluted tubule via these transporters.24
Finally, activation of the sympathetic nervous system and renin-angiotensin-aldosterone system leads to increased tubular sodium and water retention, thereby also blunting the diuretic response.
Diuretic dosage. In patients whose creatinine clearance is less than 15 mL/min, only 10% to 20% as much loop diuretic is secreted into the renal tubule as in normal individuals.25 This effect warrants dose adjustment of diuretics during uremia.
Continuous infusion or bolus? Continuous infusion of loop diuretics is another strategy to optimize drug delivery. Compared with bolus therapy, continuous infusion provides more sustained and uniform drug delivery and prevents postdiuretic sodium retention.
The Diuretic Optimization Strategies Evaluation (DOSE) trial compared the efficacy and safety of continuous vs bolus furosemide therapy in 308 patients admitted with acute decompensated heart failure.26 There was no difference in symptom control or net fluid loss at 72 hours in either group. Other studies have shown more diuresis with continuous infusion than with a similarly dosed bolus regimen.27 However, definitive clinical evidence is lacking at this point to support routine use of continuous loop diuretic therapy.
Combination diuretic therapy. Sequential nephron blockade with combination diuretic therapy is an important therapeutic strategy against diuretic resistance. Notably, urine output-guided diuretic therapy has been shown to be superior to standard diuretic therapy.28 Such therapeutic protocols may employ combination diuretic therapy as a next step when the desired diuretic response is not obtained with high doses of loop diuretic monotherapy.
The desired diuretic response depends on the clinical situation. For example, in patients with severe congestion, we would like the net fluid output to be at least 2 to 3 L more than the fluid intake after the first 24 hours. Sometimes, patients in the intensive care unit are on several essential drug infusions, so that their net intake amounts to 1 to 2 L. In these patients, the desired urine output would be even more than in patients not on these drug infusions.
Loop diuretics block sodium reabsorption at the thick ascending loop of Henle. This disrupts the countercurrent exchange mechanism and reduces renal medullary interstitial osmolarity; these effects prevent water reabsorption. However, the unresorbed sodium can be taken up by the sodium-chloride cotransporter and the epithelial sodium channel in the distal nephron, thereby blunting the diuretic effect. This is the rationale for combining loop diuretics with thiazides or potassium-sparing diuretics.
Similarly, carbonic anhydrase inhibitors (eg, acetazolamide) reduce sodium reabsorption from the proximal convoluted tubule, but most of this sodium is then reabsorbed distally. Hence, the combination of a loop diuretic and acetazolamide can also have a synergistic diuretic effect.
The most popular combination is a loop diuretic plus a thiazide, although no large-scale placebo-controlled trials have been performed.29 Metolazone (a thiazidelike diuretic) is typically used due to its low cost and availability.30 Metolazone has also been shown to block sodium reabsorption at the proximal tubule, which may contribute to its synergistic effect. Chlorothiazide is available in an intravenous formulation and has a faster onset of action than metolazone. However, studies have failed to detect any benefit of one over the other.31
The potential benefit of combining a loop diuretic with acetazolamide is a lower tendency to develop metabolic alkalosis, a potential side effect of loop diuretics and thiazides. Although data are limited, a recent study showed that adding acetazolamide to bumetanide led to significantly increased natriuresis.32
In the Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, adding spironolactone in high doses to usual therapy was not found to cause any significant change in N-terminal pro-B-type natriuretic peptide level or net urine output.33
Ultrafiltration
Venovenous ultrafiltration (or aquapheresis) employs an extracorporeal circuit, similar to the one used in hemodialysis, which removes iso-osmolar fluid at a fixed rate.34 Newer ultrafiltration systems are more portable, can be used with peripheral venous access, and require minimal nursing supervision.35
Although ultrafiltration seems an attractive alternative to diuresis in acute heart failure, studies have been inconclusive. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial compared ultrafiltration and diuresis in 188 patients with acute heart failure and acute cardiorenal syndrome.36 Diuresis, performed according to an algorithm, was found to be superior to ultrafiltration in terms of a bivariate end point of change in weight and change in serum creatinine level at 96 hours. However, the level of cystatin C is thought to be a more accurate indicator of renal function, and the change in cystatin C level from baseline did not differ between the two treatment groups. Also, the ultrafiltration rate was 200 mL per hour, which, some argue, may have been excessive and may have caused intravascular depletion.
Although the ideal rate of fluid removal is unknown, it should be individualized and adjusted based on the patient’s renal function, volume status, and hemodynamic status. The initial rate should be based on the degree of fluid overload and the anticipated plasma refill rate from the interstitial fluid.37 For example, a malnourished patient may have low serum oncotic pressure and hence have low plasma refill upon ultrafiltration. Disturbance of this delicate balance between the rates of ultrafiltration and plasma refill may lead to intravascular volume contraction.
In summary, although ultrafiltration is a valuable alternative to diuretics in resistant cases, its use as a primary decongestive therapy cannot be endorsed in view of the current data.
Inotropes
Inotropes such as dobutamine and milrinone are typically used in cases of cardiogenic shock to maintain organ perfusion. There is a physiologic rationale to using inotropes in acute cardiorenal syndrome as well, especially when the aforementioned strategies fail to overcome diuretic resistance.7
Inotropes increase cardiac output, improve renal blood flow, improve right ventricular output, and thereby relieve systemic congestion. These hemodynamic effects may improve renal perfusion and response to diuretics. However, clinical evidence to support this is lacking.
The Renal Optimization Strategies Evaluation (ROSE) trial enrolled 360 patients with acute heart failure and renal dysfunction. Adding dopamine in a low dose (2 μg/kg/min) to diuretic therapy had no significant effect on 72-hour cumulative urine output or renal function as measured by cystatin C levels.38 However, acute kidney injury was not identified in this trial, and the renal function of many of these patients may have been at its baseline when they were admitted. In other words, this trial did not necessarily include patients with acute kidney injury along with acute heart failure. Hence, it did not necessarily include patients with acute cardiorenal syndrome.
Vasodilators
Vasodilators such as nitroglycerin, sodium nitroprusside, and hydralazine are commonly used in patients with acute heart failure, although the clinical evidence supporting their use is weak.
Physiologically, arterial dilation reduces afterload and can help relieve pulmonary congestion, and venodilation increases capacitance and reduces preload. In theory, venodilators such as nitroglycerin can relieve renal venous congestion in patients with acute cardiorenal syndrome, thereby improving renal perfusion.
However, the use of vasodilators is often limited by their adverse effects, the most important being hypotension. This is especially relevant in light of recent data identifying reduction in blood pressure during treatment of acute heart failure as an independent risk factor for worsening renal function.39,40 It is important to note that in these studies, changes in cardiac index did not affect the propensity for developing worsening renal function. The precise mechanism of this finding is unclear but it is plausible that systemic vasodilation redistributes the cardiac output to nonrenal tissues, thereby overriding the renal autoregulatory mechanisms that are normally employed in low output states.
Preventive strategies
Various strategies can be used to prevent acute cardiorenal syndrome. An optimal outpatient diuretic regimen to avoid hypervolemia is essential. Patients with advanced congestive heart failure should be followed up closely in dedicated heart failure clinics until their diuretic regimen is optimized. Patients should be advised to check their weight on a regular basis and seek medical advice if they notice an increase in their weight or a reduction in their urine output.
TAKE-HOME POINTS
- A robust clinical definition of cardiorenal syndrome is lacking. Hence, recognition of this condition can be challenging.
- Volume overload is central to its pathogenesis, and accurate assessment of volume status is critical.
- Renal venous congestion is the major mechanism of type 1 cardiorenal syndrome.
- Misdiagnosis can have devastating consequences, as it may lead to an opposite therapeutic approach.
- Fluid removal by various strategies is the mainstay of treatment.
- Temporary inotropic support should be saved for the last resort.
As the heart goes, so go the kidneys—and vice versa. Cardiac and renal function are intricately interdependent, and failure of either organ causes injury to the other in a vicious circle of worsening function.1
Here, we discuss acute cardiorenal syndrome, ie, acute exacerbation of heart failure leading to acute kidney injury, a common cause of hospitalization and admission to the intensive care unit. We examine its clinical definition, pathophysiology, hemodynamic derangements, clues that help in diagnosing it, and its treatment.
A GROUP OF LINKED DISORDERS
Two types of acute cardiac dysfunction
Although these definitions offer a good general description, further clarification of the nature of organ dysfunction is needed. Acute renal dysfunction can be unambiguously defined using the AKIN (Acute Kidney Injury Network) and RIFLE (risk, injury, failure, loss of kidney function, and end-stage kidney disease) classifications.3 Acute cardiac dysfunction, on the other hand, is an ambiguous term that encompasses 2 clinically and pathophysiologically distinct conditions: cardiogenic shock and acute heart failure.
Cardiogenic shock is characterized by a catastrophic compromise of cardiac pump function leading to global hypoperfusion severe enough to cause systemic organ damage.4 The cardiac index at which organs start to fail varies in different cases, but a value of less than 1.8 L/min/m2 is typically used to define cardiogenic shock.4
Acute heart failure, on the other hand, is defined as gradually or rapidly worsening signs and symptoms of congestive heart failure due to worsening pulmonary or systemic congestion.5 Hypervolemia is the hallmark of acute heart failure, whereas patients with cardiogenic shock may be hypervolemic, normovolemic, or hypovolemic. Although cardiac output may be mildly reduced in some cases of acute heart failure, systemic perfusion is enough to maintain organ function.
These two conditions cause renal injury by distinct mechanisms and have entirely different therapeutic implications. As we discuss later, reduced renal perfusion due to renal venous congestion is now believed to be the major hemodynamic mechanism of renal injury in acute heart failure. On the other hand, in cardiogenic shock, renal perfusion is reduced due to a critical decline of cardiac pump function.
The ideal definition of acute cardiorenal syndrome should describe a distinct pathophysiology of the syndrome and offer distinct therapeutic options that counteract it. Hence, we propose that renal injury from cardiogenic shock should not be included in its definition, an approach that has been adopted in some of the recent reviews as well.6 Our discussion of acute cardiorenal syndrome is restricted to renal injury caused by acute heart failure.
PATHOPHYSIOLOGY OF ACUTE CARDIORENAL SYNDROME
Multiple mechanisms have been implicated in the pathophysiology of cardiorenal syndrome.7,8
Sympathetic hyperactivity is a compensatory mechanism in heart failure and may be aggravated if cardiac output is further reduced. Its effects include constriction of afferent and efferent arterioles, causing reduced renal perfusion and increased tubular sodium and water reabsorption.7
The renin-angiotensin-aldosterone system is activated in patients with stable congestive heart failure and may be further stimulated in a state of reduced renal perfusion, which is a hallmark of acute cardiorenal syndrome. Its activation can cause further salt and water retention.
However, direct hemodynamic mechanisms likely play the most important role and have obvious diagnostic and therapeutic implications.
Elevated venous pressure, not reduced cardiac output, drives kidney injury
The classic view was that renal dysfunction in acute heart failure is caused by reduced renal blood flow due to failing cardiac pump function. Cardiac output may be reduced in acute heart failure for various reasons, such as atrial fibrillation, myocardial infarction, or other processes, but reduced cardiac output has a minimal role, if any, in the pathogenesis of renal injury in acute heart failure.
As evidence of this, acute heart failure is not always associated with reduced cardiac output.5 Even if the cardiac index (cardiac output divided by body surface area) is mildly reduced, renal blood flow is largely unaffected, thanks to effective renal autoregulatory mechanisms. Not until the mean arterial pressure falls below 70 mm Hg do these mechanisms fail and renal blood flow starts to drop.9 Hence, unless cardiac performance is compromised enough to cause cardiogenic shock, renal blood flow usually does not change significantly with mild reduction in cardiac output.
Hanberg et al10 performed a post hoc analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheter Effectiveness (ESCAPE) trial, in which 525 patients with advanced heart failure underwent pulmonary artery catheterization to measure their cardiac index. The authors found no association between the cardiac index and renal function in these patients.
How venous congestion impairs the kidney
In view of the current clinical evidence, the focus has shifted to renal venous congestion. According to Poiseuille’s law, blood flow through the kidneys depends on the pressure gradient—high pressure on the arterial side, low pressure on the venous side.8 Increased renal venous pressure causes reduced renal perfusion pressure, thereby affecting renal perfusion. This is now recognized as an important hemodynamic mechanism of acute cardiorenal syndrome.
Renal congestion can also affect renal function through indirect mechanisms. For example, it can cause renal interstitial edema that may then increase the intratubular pressure, thereby reducing the transglomerular pressure gradient.11
Firth et al,14 in experiments in animals, found that increasing the renal venous pressure above 18.75 mm Hg significantly reduced the glomerular filtration rate, which completely resolved when renal venous pressure was restored to basal levels.
Mullens et al,15 in a study of 145 patients admitted with acute heart failure, reported that 58 (40%) developed acute kidney injury. Pulmonary artery catheterization revealed that elevated central venous pressure, rather than reduced cardiac index, was the primary hemodynamic factor driving renal dysfunction.
DIAGNOSIS AND CLINICAL ASSESSMENT
Patients with acute cardiorenal syndrome present with clinical features of pulmonary or systemic congestion (or both) and acute kidney injury.
Elevated left-sided pressures are usually but not always associated with elevated right-sided pressures. In a study of 1,000 patients with advanced heart failure, a pulmonary capillary wedge pressure of 22 mm Hg or higher had a positive predictive value of 88% for a right atrial pressure of 10 mm Hg or higher.16 Hence, the clinical presentation may vary depending on the location (pulmonary, systemic, or both) and degree of congestion.
Symptoms of pulmonary congestion include worsening exertional dyspnea and orthopnea; bilateral crackles may be heard on physical examination if pulmonary edema is present.
Systemic congestion can cause significant peripheral edema and weight gain. Jugular venous distention may be noted. Oliguria may be present due to renal dysfunction; patients on maintenance diuretic therapy often note its lack of efficacy.
Signs of acute heart failure
Wang et al,17 in a meta-analysis of 22 studies, concluded that the features that most strongly suggested acute heart failure were:
- History of paroxysmal nocturnal dyspnea
- A third heart sound
- Evidence of pulmonary venous congestion on chest radiography.
Features that most strongly suggested the patient did not have acute heart failure were:
- Absence of exertional dyspnea
- Absence of rales
- Absence of radiographic evidence of cardiomegaly.
Patients may present without some of these classic clinical features, and the diagnosis of acute heart failure may be challenging. For example, even if left-sided pressures are very high, pulmonary edema may be absent because of pulmonary vascular remodeling in chronic heart failure.18 Pulmonary artery catheterization reveals elevated cardiac filling pressures and can be used to guide therapy, but clinical evidence argues against its routine use.19
Urine electrolytes (fractional excretion of sodium < 1% and fractional excretion of urea < 35%) often suggest a prerenal form of acute kidney injury, since the hemodynamic derangements in acute cardiorenal syndrome reduce renal perfusion.
Biomarkers of cell-cycle arrest such as urine insulinlike growth factor-binding protein 7 and tissue inhibitor of metalloproteinase 2 have recently been shown to identify patients with acute heart failure at risk of developing acute cardiorenal syndrome.20
Acute cardiorenal syndrome vs renal injury due to hypovolemia
The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Patients with stable heart failure usually have mild hypervolemia at baseline, but they can become hypovolemic due to overaggressive diuretic therapy, severe diarrhea, or other causes.
Although the fluid status of patients in these 2 conditions is opposite, they can be difficult to distinguish. In both conditions, urine electrolytes suggest a prerenal acute kidney injury. A history of recent fluid losses or diuretic overuse may help identify hypovolemia. If available, analysis of the recent trend in weight can be vital in making the right diagnosis.
Misdiagnosis of acute cardiorenal syndrome as hypovolemia-induced acute kidney injury can be catastrophic. If volume depletion is erroneously judged to be the cause of acute kidney injury, fluid administration can further worsen both cardiac and renal function. This can perpetuate the vicious circle that is already in play. Lack of renal recovery may invite further fluid administration.
TREATMENT
Fluid removal with diuresis or ultrafiltration is the cornerstone of treatment. Other treatments such as inotropes are reserved for patients with resistant disease.
Diuretics
The goal of therapy in acute cardiorenal syndrome is to achieve aggressive diuresis, typically using intravenous diuretics. Loop diuretics are the most potent class of diuretics and are the first-line drugs for this purpose. Other classes of diuretics can be used in conjunction with loop diuretics; however, using them by themselves is neither effective nor recommended.
Resistance to diuretics at usual doses is common in patients with acute cardiorenal syndrome. Several mechanisms contribute to diuretic resistance in these patients.21
Oral bioavailability of diuretics may be reduced due to intestinal edema.
Diuretic pharmacokinetics are significantly deranged in cardiorenal syndrome. All diuretics except mineralocorticoid antagonists (ie, spironolactone and eplerenone) act on targets on the luminal side of renal tubules, but are highly protein-bound and are hence not filtered at the glomerulus. Loop diuretics, thiazides, and carbonic anhydrase inhibitors are secreted in the proximal convoluted tubule via the organic anion transporter,22 whereas epithelial sodium channel inhibitors (amiloride and triamterene) are secreted via the organic cation transporter 2.23 In renal dysfunction, various uremic toxins accumulate in the body and compete with diuretics for secretion into the proximal convoluted tubule via these transporters.24
Finally, activation of the sympathetic nervous system and renin-angiotensin-aldosterone system leads to increased tubular sodium and water retention, thereby also blunting the diuretic response.
Diuretic dosage. In patients whose creatinine clearance is less than 15 mL/min, only 10% to 20% as much loop diuretic is secreted into the renal tubule as in normal individuals.25 This effect warrants dose adjustment of diuretics during uremia.
Continuous infusion or bolus? Continuous infusion of loop diuretics is another strategy to optimize drug delivery. Compared with bolus therapy, continuous infusion provides more sustained and uniform drug delivery and prevents postdiuretic sodium retention.
The Diuretic Optimization Strategies Evaluation (DOSE) trial compared the efficacy and safety of continuous vs bolus furosemide therapy in 308 patients admitted with acute decompensated heart failure.26 There was no difference in symptom control or net fluid loss at 72 hours in either group. Other studies have shown more diuresis with continuous infusion than with a similarly dosed bolus regimen.27 However, definitive clinical evidence is lacking at this point to support routine use of continuous loop diuretic therapy.
Combination diuretic therapy. Sequential nephron blockade with combination diuretic therapy is an important therapeutic strategy against diuretic resistance. Notably, urine output-guided diuretic therapy has been shown to be superior to standard diuretic therapy.28 Such therapeutic protocols may employ combination diuretic therapy as a next step when the desired diuretic response is not obtained with high doses of loop diuretic monotherapy.
The desired diuretic response depends on the clinical situation. For example, in patients with severe congestion, we would like the net fluid output to be at least 2 to 3 L more than the fluid intake after the first 24 hours. Sometimes, patients in the intensive care unit are on several essential drug infusions, so that their net intake amounts to 1 to 2 L. In these patients, the desired urine output would be even more than in patients not on these drug infusions.
Loop diuretics block sodium reabsorption at the thick ascending loop of Henle. This disrupts the countercurrent exchange mechanism and reduces renal medullary interstitial osmolarity; these effects prevent water reabsorption. However, the unresorbed sodium can be taken up by the sodium-chloride cotransporter and the epithelial sodium channel in the distal nephron, thereby blunting the diuretic effect. This is the rationale for combining loop diuretics with thiazides or potassium-sparing diuretics.
Similarly, carbonic anhydrase inhibitors (eg, acetazolamide) reduce sodium reabsorption from the proximal convoluted tubule, but most of this sodium is then reabsorbed distally. Hence, the combination of a loop diuretic and acetazolamide can also have a synergistic diuretic effect.
The most popular combination is a loop diuretic plus a thiazide, although no large-scale placebo-controlled trials have been performed.29 Metolazone (a thiazidelike diuretic) is typically used due to its low cost and availability.30 Metolazone has also been shown to block sodium reabsorption at the proximal tubule, which may contribute to its synergistic effect. Chlorothiazide is available in an intravenous formulation and has a faster onset of action than metolazone. However, studies have failed to detect any benefit of one over the other.31
The potential benefit of combining a loop diuretic with acetazolamide is a lower tendency to develop metabolic alkalosis, a potential side effect of loop diuretics and thiazides. Although data are limited, a recent study showed that adding acetazolamide to bumetanide led to significantly increased natriuresis.32
In the Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial, adding spironolactone in high doses to usual therapy was not found to cause any significant change in N-terminal pro-B-type natriuretic peptide level or net urine output.33
Ultrafiltration
Venovenous ultrafiltration (or aquapheresis) employs an extracorporeal circuit, similar to the one used in hemodialysis, which removes iso-osmolar fluid at a fixed rate.34 Newer ultrafiltration systems are more portable, can be used with peripheral venous access, and require minimal nursing supervision.35
Although ultrafiltration seems an attractive alternative to diuresis in acute heart failure, studies have been inconclusive. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial compared ultrafiltration and diuresis in 188 patients with acute heart failure and acute cardiorenal syndrome.36 Diuresis, performed according to an algorithm, was found to be superior to ultrafiltration in terms of a bivariate end point of change in weight and change in serum creatinine level at 96 hours. However, the level of cystatin C is thought to be a more accurate indicator of renal function, and the change in cystatin C level from baseline did not differ between the two treatment groups. Also, the ultrafiltration rate was 200 mL per hour, which, some argue, may have been excessive and may have caused intravascular depletion.
Although the ideal rate of fluid removal is unknown, it should be individualized and adjusted based on the patient’s renal function, volume status, and hemodynamic status. The initial rate should be based on the degree of fluid overload and the anticipated plasma refill rate from the interstitial fluid.37 For example, a malnourished patient may have low serum oncotic pressure and hence have low plasma refill upon ultrafiltration. Disturbance of this delicate balance between the rates of ultrafiltration and plasma refill may lead to intravascular volume contraction.
In summary, although ultrafiltration is a valuable alternative to diuretics in resistant cases, its use as a primary decongestive therapy cannot be endorsed in view of the current data.
Inotropes
Inotropes such as dobutamine and milrinone are typically used in cases of cardiogenic shock to maintain organ perfusion. There is a physiologic rationale to using inotropes in acute cardiorenal syndrome as well, especially when the aforementioned strategies fail to overcome diuretic resistance.7
Inotropes increase cardiac output, improve renal blood flow, improve right ventricular output, and thereby relieve systemic congestion. These hemodynamic effects may improve renal perfusion and response to diuretics. However, clinical evidence to support this is lacking.
The Renal Optimization Strategies Evaluation (ROSE) trial enrolled 360 patients with acute heart failure and renal dysfunction. Adding dopamine in a low dose (2 μg/kg/min) to diuretic therapy had no significant effect on 72-hour cumulative urine output or renal function as measured by cystatin C levels.38 However, acute kidney injury was not identified in this trial, and the renal function of many of these patients may have been at its baseline when they were admitted. In other words, this trial did not necessarily include patients with acute kidney injury along with acute heart failure. Hence, it did not necessarily include patients with acute cardiorenal syndrome.
Vasodilators
Vasodilators such as nitroglycerin, sodium nitroprusside, and hydralazine are commonly used in patients with acute heart failure, although the clinical evidence supporting their use is weak.
Physiologically, arterial dilation reduces afterload and can help relieve pulmonary congestion, and venodilation increases capacitance and reduces preload. In theory, venodilators such as nitroglycerin can relieve renal venous congestion in patients with acute cardiorenal syndrome, thereby improving renal perfusion.
However, the use of vasodilators is often limited by their adverse effects, the most important being hypotension. This is especially relevant in light of recent data identifying reduction in blood pressure during treatment of acute heart failure as an independent risk factor for worsening renal function.39,40 It is important to note that in these studies, changes in cardiac index did not affect the propensity for developing worsening renal function. The precise mechanism of this finding is unclear but it is plausible that systemic vasodilation redistributes the cardiac output to nonrenal tissues, thereby overriding the renal autoregulatory mechanisms that are normally employed in low output states.
Preventive strategies
Various strategies can be used to prevent acute cardiorenal syndrome. An optimal outpatient diuretic regimen to avoid hypervolemia is essential. Patients with advanced congestive heart failure should be followed up closely in dedicated heart failure clinics until their diuretic regimen is optimized. Patients should be advised to check their weight on a regular basis and seek medical advice if they notice an increase in their weight or a reduction in their urine output.
TAKE-HOME POINTS
- A robust clinical definition of cardiorenal syndrome is lacking. Hence, recognition of this condition can be challenging.
- Volume overload is central to its pathogenesis, and accurate assessment of volume status is critical.
- Renal venous congestion is the major mechanism of type 1 cardiorenal syndrome.
- Misdiagnosis can have devastating consequences, as it may lead to an opposite therapeutic approach.
- Fluid removal by various strategies is the mainstay of treatment.
- Temporary inotropic support should be saved for the last resort.
- Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Cleve Clin J Med 2006; 73:485–491.
- House AA, Anand I, Bellomo R, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Chang CH, Lin CY, Tian YC, et al. Acute kidney injury classification: comparison of AKIN and RIFLE criteria. Shock 2010; 33:247-252.
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008; 117:686–697.
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009; 53:557–573.
- ter Maaten JM, Valente MA, Damman K, et al. Diuretic response in acute heart failure—pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015; 12:184–192.
- Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 2013; 9:99–111.
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121:2592–2600.
- Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Afsar B, Ortiz A, Covic A, et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9:39–47.
- Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013; 62:485–495.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1988; 1:1033–1035.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18:1126–1132.
- Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 2004; 125:669–682.
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294:1625–1633.
- Schanz M, Shi J , Wasser C , Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 2017; doi.org/10.1002/clc.22683.
- Bowman BN, Nawarskas JJ, Anderson JR. Treating diuretic resistance: an overview. Cardiol Rev 2016; 24:256–260.
- Uwai Y, Saito H, Hashimoto Y, Inui KI. Interaction and transport of thiazide diuretics, loop diuretics, and acetazolamide via rat renal organic anion transporter rOAT1. J Pharmacol Exp Ther 2000; 295:261–265.
- Hacker K, Maas R, Kornhuber J, et al. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One 2015; 10:e0136451.
- Schophuizen CM, Wilmer MJ, Jansen J, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch 2013; 465:1701–1714.
- Brater DC. Diuretic therapy. N Engl J Med 1998; 339:387–395.
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail 2010; 16:188–193.
- Grodin JL, Stevens SR, de Las Fuentes L, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail 2016; 22:26–32.
- Ng TM, Konopka E, Hyderi AF, et al. Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther 2013; 18:345–353.
- Sica DA. Metolazone and its role in edema management. Congest Heart Fail 2003; 9:100–105.
- Moranville MP, Choi S, Hogg J, Anderson AS, Rich JD. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015; 33:42–49.
- Verbrugge FH, Dupont M, Bertrand PB, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 2015; 70:265–373.
- Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017 Jul 12. doi: 10.1001/jamacardio.2017.2198. [Epub ahead of print]
- Pourafshar N, Karimi A, Kazory A. Extracorporeal ultrafiltration therapy for acute decompensated heart failure. Expert Rev Cardiovasc Ther 2016; 14:5–13.
- Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail 2003; 9:227–231.
- Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Kazory A. Cardiorenal syndrome: ultrafiltration therapy for heart failure—trials and tribulations. Clin J Am Soc Nephrol 2013; 8:1816–1828.
- Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Testani JM, Coca SG, McCauley BD, et al. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011; 13:877–884.
- Dupont M, Mullens W, Finucan M, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013; 15:433–440.
- Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Cleve Clin J Med 2006; 73:485–491.
- House AA, Anand I, Bellomo R, et al. Definition and classification of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25:1416–1420.
- Chang CH, Lin CY, Tian YC, et al. Acute kidney injury classification: comparison of AKIN and RIFLE criteria. Shock 2010; 33:247-252.
- Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation 2008; 117:686–697.
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009; 53:557–573.
- ter Maaten JM, Valente MA, Damman K, et al. Diuretic response in acute heart failure—pathophysiology, evaluation, and therapy. Nat Rev Cardiol 2015; 12:184–192.
- Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 2013; 9:99–111.
- Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010; 121:2592–2600.
- Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol 2014; 12:845–858.
- Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 2016; 67:2199–2208.
- Afsar B, Ortiz A, Covic A, et al. Focus on renal congestion in heart failure. Clin Kidney J 2016; 9:39–47.
- Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013; 62:485–495.
- Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 2008; 51:300–306.
- Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1988; 1:1033–1035.
- Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596.
- Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant 1999; 18:1126–1132.
- Wang CS, FitzGerald JM, Schulzer M, et al. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005; 294:1944–1956.
- Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest 2004; 125:669–682.
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005; 294:1625–1633.
- Schanz M, Shi J , Wasser C , Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol 2017; doi.org/10.1002/clc.22683.
- Bowman BN, Nawarskas JJ, Anderson JR. Treating diuretic resistance: an overview. Cardiol Rev 2016; 24:256–260.
- Uwai Y, Saito H, Hashimoto Y, Inui KI. Interaction and transport of thiazide diuretics, loop diuretics, and acetazolamide via rat renal organic anion transporter rOAT1. J Pharmacol Exp Ther 2000; 295:261–265.
- Hacker K, Maas R, Kornhuber J, et al. Substrate-dependent inhibition of the human organic cation transporter OCT2: a comparison of metformin with experimental substrates. PLoS One 2015; 10:e0136451.
- Schophuizen CM, Wilmer MJ, Jansen J, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflugers Arch 2013; 465:1701–1714.
- Brater DC. Diuretic therapy. N Engl J Med 1998; 339:387–395.
- Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail 2010; 16:188–193.
- Grodin JL, Stevens SR, de Las Fuentes L, et al. Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail 2016; 22:26–32.
- Ng TM, Konopka E, Hyderi AF, et al. Comparison of bumetanide- and metolazone-based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther 2013; 18:345–353.
- Sica DA. Metolazone and its role in edema management. Congest Heart Fail 2003; 9:100–105.
- Moranville MP, Choi S, Hogg J, Anderson AS, Rich JD. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther 2015; 33:42–49.
- Verbrugge FH, Dupont M, Bertrand PB, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 2015; 70:265–373.
- Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol 2017 Jul 12. doi: 10.1001/jamacardio.2017.2198. [Epub ahead of print]
- Pourafshar N, Karimi A, Kazory A. Extracorporeal ultrafiltration therapy for acute decompensated heart failure. Expert Rev Cardiovasc Ther 2016; 14:5–13.
- Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail 2003; 9:227–231.
- Jaski BE, Ha J, Denys BG, Lamba S, Trupp RJ, Abraham WT. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Kazory A. Cardiorenal syndrome: ultrafiltration therapy for heart failure—trials and tribulations. Clin J Am Soc Nephrol 2013; 8:1816–1828.
- Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310:2533–2543.
- Testani JM, Coca SG, McCauley BD, et al. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail 2011; 13:877–884.
- Dupont M, Mullens W, Finucan M, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail 2013; 15:433–440.
KEY POINTS
- Acute cardiorenal syndrome is the acute worsening of renal function due to acute decompensated heart failure.
- The most important mechanism of acute cardiorenal syndrome is now believed to be systemic congestion leading to increased renal venous pressure, which in turn reduces renal perfusion.
- The major alternative in the differential diagnosis of acute cardiorenal syndrome is renal injury due to hypovolemia. Differentiating the 2 may be challenging if signs of systemic and pulmonary congestion are not obvious.
- Diuretic resistance is common in acute cardiorenal syndrome but may be overcome by using higher doses of diuretics and combinations of diuretics that block reabsorption at different segments of the renal tubules.
Which test for CAD should be used in patients with left bundle branch block?
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
KEY POINTS
- Although current guidelines recommend exercise stress echocardiography, it cannot reliably detect significant obstructive CAD in patients who have left bundle branch block at rest.
- CT angiography is the first-line imaging test for these patients if they are under age 65. For those 65 and older, the first-line test is either pharmacologic stress nuclear myocardial perfusion imaging with coronary vasodilators or dobutamine stress echocardiography.
- For patients who cannot tolerate CT contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography can be alternatives.
Alzheimer dementia: Starting, stopping drug therapy
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
Cholinesterase inhibitors
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
NMDA receptor antagonist
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Combination therapy
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
Cholinesterase inhibitors
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
NMDA receptor antagonist
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Off-label use to treat behavioral problems
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19 Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
Combination therapy
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease. Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013; 80:1778–1783.
- Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994; 271:992–998.
- Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004; 363:2105–2115.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008; 148:379–397.
- Lanctot KL, Hermann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 2003; 169:557–564.
- Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008; 148:370–378.
- Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003; 289:210–216.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331:321–327.
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1:CD005593.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139.
- Mitchell SL. Advanced dementia. N Engl J Med 2015; 372:2533–2540.
- Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson’s disease dementia and cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2012; 3:CD006504.
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer’s disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291:317–324.
- Reuters Staff. Pfizer ends research for new Alzheimer’s, Parkinson’s drugs. January 7, 2018. https://www.reuters.com/article/us-pfizer-alzheimers/pfizer-ends-research-for-new-alzheimers-parkinsons-drugs-idUSKBN1EW0TN. Accessed February 2, 2018.
- Aerosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005 Jul 20;(3):CD003154.
- Rossom R, Adityanjee, Dysken M. Efficacy and tolerability of memantine in the treatment of dementia. Am J Geriatr Pharmacother 2004; 2:303–312.
- Wang J, Yu JT, Wang HF, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2015; 86:101–109.
- McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036.
- Cummings JL, Schneider E, Tariot PN, Graham SM, Memantine MEM-MD-02 Study Group. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology 2006; 67:57–63.
- Wilcock GK, Ballard CG, Cooper JA, Loft H. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69:341–348.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006; 2:CD003154.
- Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003; 348:1333–1341.
- Howard R, McShane R, Lindesay J, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012; 366:893–903.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- O’Regan J, Lanctot KL, Mazereeuw G, Herrmann N. Cholinesterase inhibitor discontinuation in patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry 2015; 76:e1424–e1431.
- Herrmann N, O’Reagan J, Ruthirahukhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc 2016; 17:142–174.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacological management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schwab W, Messinger-Rapport B, Franco K. Psychiatric symptoms of dementia: treatable, but no silver bullet. Cleve Clin J Med 2009; 76:167–174.
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effects of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314:1242–1254.
- Centers for Medicare and Medicaid Services. Dementia care in nursing homes: clarification to Appendix P State Operations Manual (SOM) and Appendix PP in the SOM for F309—quality of care and F329—unnecessary drugs. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-35.pdf. Accessed February 1, 2018.
KEY POINTS
- In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease; by 2050, the prevalence is expected to be 13.8 million.
- Cognitive enhancers (cholinesterase inhibitors and an N-methyl-d-aspartate receptor antagonist) have shown modest efficacy in preserving cognitive function.
- When evaluating therapy with a cognitive enhancer, practitioners need to consider the potential adverse effects, especially gastrointestinal effects with cholinesterase inhibitors.
- Discontinuation should be considered when the dementia reaches the advanced stage and the initial intended purpose of these drugs is no longer achievable.
Primary care management of chronic pelvic pain in women
Chronic pelvic pain is a common clinical problem in women, as prevalent in primary care as asthma or back pain.1,2 It is often associated with lost work days and decreased productivity, increased healthcare spending, mood disorders, and negative effects on personal relationships.1–3
While specialty care referral may eventually be indicated, primary care doctors can take steps to diagnose and effectively manage the condition.
COMPREHENSIVE MANAGEMENT LED BY PRIMARY CARE
Chronic pelvic pain is defined as pain in the lower abdomen persisting for 3 to 6 months and of sufficient severity to require medical care or cause a functional disability.3 It is often detrimental to a woman’s personal life and overall health, making a comprehensive assessment and multidisciplinary approach to management especially important.
The ideal care-delivery model is the patient-centered medical home, whereby a primary care physician coordinates comprehensive care with the help of an interdisciplinary team.4,5 For complex cases, referral may be needed to other specialties (eg, obstetrics and gynecology, pain medicine) to help manage care.
TARGETED EVALUATION
Chronic pelvic pain often coexists with other systemic pain syndromes or psychiatric conditions common in primary care. Table 1 lists common causes and associated findings.
Detailed history is critical
The history is of utmost importance. Clinicians should query patients about the characteristics of the pain as well as their medical and surgical history. Particular attention should be given to obtaining a complete gynecologic history, including pregnancy, delivery complications, dyspareunia, sexual assault, and trauma. A detailed review of systems should focus on the reproductive, gastroenterologic, musculoskeletal, urologic, and neuropsychiatric systems.
As with many pain syndromes, allowing the patient to “tell her story” helps to establish rapport and obtain a more complete assessment. Chronic pelvic pain has been associated with physical or sexual abuse as a child or adult, so is essential to foster the doctor-patient relationship and create a safe and open space for disclosure.3,6 It is important to screen women for safety at home as well as for satisfaction or dissatisfaction with their relationships with their spouse or partner and family.
Physical examination
The physical examination should be directed by the history but should always include abdominal and pelvic examinations. These should be conducted slowly and gently, assessing for areas of tenderness, masses, and other abnormalities. Clinicians should aim to pinpoint the exact anatomic locations of tenderness if possible. Ongoing dialogue facilitates this process by inquiring about pain at each point of the examination.
The pelvic examination should begin with visual inspection for redness, discharge, lesions, fissures, excoriations, and other abnormalities. A moistened cotton swab may be used to evaluate the vulva and vestibule for localized tenderness. The manual portion of the pelvic examination should begin with a single digit, noting any introital tenderness or spasm. Next, the levator ani muscles should be directly palpated for tone and tenderness. The pelvic floor should be evaluated with attention to tenderness of the bladder or musculoskeletal structures (Figure 1). A bimanual examination assessing uterine size and tenderness, nodularity, or a fixed, immobile uterus should be conducted.
Diagnostic workup
Because the differential diagnosis of chronic pelvic pain is broad, the diagnostic workup and testing should be based on findings of the history and physical examination. In general, extensive laboratory testing is of limited use for evaluating women with chronic pelvic pain.3,7
Urinalysis should be obtained for symptoms suggesting bladder involvement such as interstitial cystitis.
Pelvic ultrasonography can help identify pelvic masses palpated during the physical examination, but routine use of imaging is not recommended.3,7 If pelvic congestion syndrome is suspected, starting with pelvic ultrasonography is reasonable before incurring the risk or cost of computed tomography or magnetic resonance imaging.8
GENERAL TREATMENT
Medical therapy
The main goals of medical therapy are to improve function and quality of life while minimizing adverse effects. General treatments include the following:
Analgesics. Nonsteroidal anti-inflammatory drugs and acetaminophen may provide pain relief, although there is weak evidence for their efficacy in treating chronic pelvic pain.9
Neuropathic agents. One of several available neuropathic agents commonly used in the treatment of chronic pain can be tried on patients who fail to respond to analgesics. Tricyclic antidepressants such as amitriptyline and imipramine decrease pain, reduce symptoms of depression, and improve sleep.10 The results of a small randomized controlled trial suggest that gabapentin is more effective than amitriptyline for reducing chronic pelvic pain.11,12 Published guidelines currently list both amitriptyline and gabapentin as first-line agents; nortriptyline and pregabalin are considered acceptable initial alternatives.9
Venlafaxine and duloxetine may help chronic pelvic pain, although specific evidence is lacking. Duloxetine may be an appropriate choice for women with chronic pelvic pain who also experience depression and urinary stress incontinence.9
Opioids. Opioid therapy should be considered only when all other reasonable therapies have failed.10 Patients may develop tolerance or dependence, as well as opioid-induced adverse effects such as hyperalgesia.9,10 Guidelines recommend that primary care providers consult with a pain management specialist before prescribing opioids, and that patients be thoroughly counseled about the risks and side effects.9
Nerve block and neuromodulation. There is weak evidence for the use of these modalities for treating chronic pelvic pain.9 If used, they should be part of a broader treatment plan and should be performed by providers who specialize in management of chronic pain.
DISEASE-SPECIFIC TREATMENT
Endometriosis: Hormonal therapy
Pelvic pain that significantly fluctuates with the menstrual cycle may be caused by endometriosis, the most common gynecologic cause of chronic pelvic pain. Women with cyclic chronic pelvic pain should be empirically treated with hormonal therapy for at least 3 to 6 months before diagnostic laparoscopy is performed.13
Oral contraceptives, gonadotropin-releasing hormone (GnRH) analogues, progestogens, and danazol have proven efficacy, although side-effect profiles differ significantly. In a comparative trial, patients treated with GnRH analogues had more improvement in pain scores compared with those treated with oral contraceptives, but they experienced a significant decrease in bone mineral density.11 The effects on bone mineral density associated with GnRH analogue therapy can be mitigated by “add-back” low-dose hormonal therapy (norethindrone, low-dose estrogen, or a combination of estrogen and progesterone), which may also provide symptomatic relief for associated hot flashes and vaginal symptoms.11
Interstitial cystitis often accompanies endometriosis
Recognizing that chronic pelvic pain may have more than one cause is important when developing a comprehensive care plan. Interstitial cystitis coexists with endometriosis in up to 60% of patients.14 Initial treatment is pentosan polysulfate sodium, an oral treatment approved by the US Food and Drug Administration for interstitial cystitis that works by restoring the protective glycosaminoglycan layer in the bladder.14,15 Amitriptyline may also be used to treat interstitial cystitis-associated nocturia.
Myofascial pain: Neuromuscular blockers
According to a recent systematic review of therapies for chronic pelvic pain, patients with symptoms related to myofascial pain may benefit from neuromuscular blockade.12 One randomized controlled trial of the effectiveness of botulinum toxin A vs saline for the treatment of chronic pelvic pain secondary to pelvic floor spasm found that after 6 months of observation, women who received botulinum toxin had significantly lower pain scores than those who received saline.12
Pelvic congestion syndrome: Multiple options
Pelvic congestion syndrome may be treated with hormonal, radiologic, or surgical therapy.16 A randomized controlled trial involving patients with chronic pelvic pain secondary to pelvic congestion demonstrated that treatment with medroxyprogesterone acetate or a GnRH agonist (goserelin) improved pelvic symptoms.17
A Cochrane review of nonsurgical interventions for chronic pelvic pain included women with a diagnosis of pelvic congestion syndrome or adhesions. It found that patients treated with medroxyprogesterone acetate were more likely to have 50% pain reduction lasting up to 9 months compared with patients taking placebo.12 In comparative studies, GnRH analogues were more effective in relieving pelvic pain than progestogen therapy.
Radiologic embolization therapy is as effective as hysterectomy for the relief of chronic pelvic pain related to pelvic congestion syndrome, and it can be performed in the outpatient setting.
Irritable bowel syndrome: Try dietary changes
Symptoms of chronic pelvic pain that are associated with changes in stool consistency and frequency suggest irritable bowel syndrome. Symptoms may improve with dietary changes and fiber supplementation. Antispasmodic agents are frequently used but their anticholinergic effects may worsen constipation.14
PELVIC PHYSICAL THERAPY
Pelvic physical therapy targets the musculoskeletal components of bowel, bladder, and sexual function to restore strength, flexibility, balance, and coordination to the pelvic floor and surrounding lumbopelvic muscles. Patients with dyspareunia, pain with activity, or a significant musculoskeletal abnormality (eg, vaginismus or point tenderness on examination) are particularly good candidates for this therapy. It is done by a physical therapist with special training in techniques to manipulate the pelvic floor to address pelvic pain.
Educating the patient
Informing the patient before the initial physical therapy visit is essential for success. Referring clinicians should emphasize to patients that treatment response can help to guide further physician intervention. Patients should be counseled that pelvic physical therapy includes a pelvic examination and an expectation to participate in a home program. Although noticeable improvement takes time, encouragement provided by the entire team, including medical providers, can help a patient maintain her care plan.
Therapists typically see a patient once a week for 8 to 12 visits initially. Insurance usually covers pelvic physical therapy through the same policy as routine physical therapy.
During the initial evaluation, the patient receives an external and internal pelvic examination assessing muscle length, strength, and coordination of the back, hip, and internal pelvic floor. Internal evaluation can be done vaginally or rectally, with one gloved finger, without the need for speculum or stirrups. Biofeedback and surface electromyography (using either perianal or internal electrode placement) are used to evaluate muscle activity and to assist the patient in developing appropriate motor control during strengthening or relaxation.18
Up-training (or strengthening) aims to improve pelvic floor endurance. It can improve pelvic instability and symptoms of heaviness and discomfort from prolapse. Patients learn to appropriately utilize the pelvic floor in isolation. If a patient is too weak to contract on her own, neuromuscular electrical stimulation is used with an internal electrode to provide an assisted contraction.
Down-training (or relaxation) focuses on reducing tone in overactive pelvic muscles. It can improve symptoms of chronic pelvic pain, sexual pain, vulvodynia, and pudendal neuralgias. Patients are made aware of chronic holding patterns that lead to excess tone in the pelvic floor and learn how to release them through stretching, cardiovascular activity, meditation, and manual release of the involved muscle groups internally and externally. Internal musculature can be manipulated by a therapist in clinic or by the patient’s trained partner; the patient can also reach necessary areas with a vaginal dilator.
Functional coordination of the pelvic floor is needed for comfortable vaginal penetration and defecation. Training with biofeedback improves a patient’s ability to relax and open the pelvic floor.18 Vaginal dilators with surface electromyography are used to treat vaginismus to eliminate reflexive pelvic floor spasm during penetration. Perineal and vaginal compliance can be improved through manual release techniques with hands or vaginal dilators to restore normal mobility of tissues. This can reduce pain from postsurgical changes, postpartum sequelae, atrophic vaginal changes, shortened muscles from chronic holding, and adhesions.
PSYCHOSOCIAL INTERVENTIONS
Pelvic pain is not only a biomedical difficulty; psychosocial factors can contribute to and be affected by pelvic pain. Patients with pelvic pain often experience lower quality of life, higher rates of anxiety and depression, and increased stress compared with others.19,20 People with pain also have more relationship stress, and patients’ partners often experience emotional distress, isolation, and feelings of powerlessness in the relationship.21
Psychosocial interventions, provided along with biomedical treatment, can help to reduce pain, anxiety, and depression and improve relational well-being.22,23 In addition to attending to pain-related symptoms, comprehensive care involves recognizing and treating coexisting anxiety, depression, stress, and relationship conflict. Interventions for these difficulties are many, and a comprehensive list of interventions is beyond the focus of this section.19
Cognitive behavioral therapy
Cognitive behavioral therapy is based on the idea that maladaptive cognitions can lead to problematic behaviors and emotional distress.24 Interventions are carried out by a provider with specialized training in its use (eg, therapist, pain psychologist, psychiatrist).
Meta-analyses of studies that investigated the efficacy of cognitive behavioral therapy for chronic pain found consistent small to medium improvement in pain-related symptoms.24 Studies that used cognitive behavioral therapy for pelvic pain found reduced overall pain severity and pain during intercourse, increased sexual satisfaction, enhanced sexual function, and less-exaggerated responses to pain.25–27
Although cognitive behavioral therapy and mindfulness-based interventions produce positive outcomes, research on these interventions typically includes treatment carried out over a span of weeks. Common barriers to such care include lack of patient motivation, financial limitations, transportation problems, and time constraints.
The following psychosocial interventions have been chosen because they can be delivered in a short amount of time and integrated into a patient’s medical care by a medical or behavioral health provider. Because of the brevity and simplicity of these interventions, more patients with pelvic pain can receive psychosocial care as part of their usual medical encounters.
Behavioral activation
People experiencing depressive symptoms tend to isolate themselves and stop participating in activities they enjoy, including spending time with family and friends. Behavioral activation interventions that address such isolating behaviors have been shown to be effective in improving depressive symptoms.28–30
A simple, brief intervention can be administered during routine medical care,28 involving the following steps:
- Determine activities that the patient might implement that would decrease depressive symptoms. Questions such as, “When do you feel less depressed?” or “What brings you some happiness in your life?” can generate possible activities.
- Ask the patient to identify people in her life who have been supportive and with whom she could engage.
- Create with the patient a list of possible activities and social interactions that may enhance well-being.
- Make a schedule for participating in activities, possibly with rewards for completing them. Patients should be encouraged to follow the prescribed schedule of activities rather than make decisions based on mood or other factors.
Relaxation strategies
Relaxation can help patients reduce stress and anxiety, and can also help reduce pain.31–33
Diaphragmatic or “belly breathing” is a deep-breathing technique in which participants are asked to take in air through the nose and fully fill the lungs and lower belly. This technique allows the body to take in more oxygen, helping to lower blood pressure and slow the heartbeat. In addition to physiologic benefits, concentrating on deep breathing can help slow down or stop intrusive thoughts and distressing physical sensations.34
Progressive muscle relaxation involves the systematic tensing and relaxing of each large muscle group in the body.35 The goal is to eliminate physical and emotional stress through focusing on the sensations of tension and relaxation.
Scripts and audio and video resources for belly breathing and progressive muscle relaxation can be found on the Internet. The techniques can be taught during the medical appointment or offered as resources for home practice.
Couple-based care
Targeting couples is more effective for improving well-being than focusing solely on a patient’s psychosocial difficulties, so each of the above interventions may be more effective if tailored to include the patient’s partner.36 If the partner is with the patient during medical visits or is included in long-term psychosocial treatment, he or she can be directly involved in learning and practicing interventions with the patient. If the partner is not present, the patient can be asked to practice newly learned well-being-enhancing strategies with her partner outside the appointment time. Couples therapy can improve psychosocial well-being for both partners.
Setting goals
- Zondervan KT, Yudkin PL, Vessey MP, et al. The community prevalence of chronic pelvic pain in women and associated illness behavior. Br J Gen Pract 2001; 51:541–547.
- Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol 1996; 81:321–327.
- Howard FM. Chronic pelvic pain. Obstet Gynecol 2003; 101:594–611.
- AHRQ PCMH Resource Center. Transforming the organization and delivery of primary care. www.pcmh.ahrq.gov/. Accessed February 2, 2018.
- Pryzbylkowski P, Ashburn MA. The pain medical home: a patient-centered medical home model of care for patients with chronic pain. Anesthesiol Clin 2015; 33:785–793.
- Jamieson DJ, Steege JF. The association of sexual abuse with pelvic pain complaints in a primary care population. Am J Obstet Gynecol 1997; 177:1408–1412.
- Gambone JC, Mittman BS, Munro MG, Scialli AR, Winkel CA; Chronic Pelvic Pain/Endometriosis Working Group. Consensus statement for the management of chronic pelvic pain and endometriosis: proceedings of an expert-panel consensus process. Fertil Steril 2002; 78:961–972.
- Ganeshan A, Upponi S, Hon LQ, Uthappa MC, Warakaulle DR, Uberoi R. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol 2007; 30:1105–1111.
- Engeler D, Baranowski AP, Elneil S, et al; European Association of Urology. Guidelines on chronic pelvic pain. http://uroweb.org/wp-content/uploads/EAU-Guidelines-Chronic-Pelvic-Pain-2015.pdf. Accessed February 5, 2018.
- Vercellini P, Vigano P, Somigliana E, Abbiati A, Barbara G, Fedele L. Medical, surgical and alternative treatments for chronic pelvic pain in women: a descriptive review. Gynecol Endocrinol 2009; 25:208–221.
- Rafique S, DeCherney AH. Medical management of endometriosis. Clin Obstet Gynecol 2017; 60:485–496.
- Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev 2014; 3:CD008797.
- Royal College of Obstetricians and Gynecologists. The initial management of chronic pelvic pain, Green-top guideline No.41. www.rcog.org.uk/globalassets/documents/guidelines/gtg_41.pdf. Accessed February 2, 2018.
- Shin JH, Howard FM. Management of chronic pelvic pain. Curr Pain Headache Rep 2011; 15:377–385.
- Nelson P, Apte G, Justiz R, Brismee JM, Dedrick G, Sizer PS. Chronic female pelvic pain—Part 2: differential diagnosis and management. Pain Pract 2012; 12:111–141.
- Holloran-Schwartz MB. Surgical evaluation and treatment of the patient with chronic pelvic pain. Obstet Gynecol Clin North Am 2014; 41:357–369.
- Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod 2001; 16:931–939.
- Arnouk A, De E, Rehfuss A, Cappadocia C, Dickson S, Lian F. Physical, complementary, and alternative medicine in the treatment of pelvic floor disorders. Curr Urol Rep 2017; 18:47.
- Faccin F, Barbara G, Saita E, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol 2015; 36:135–141.
- Naliboff BD, Stephens AJ, Afari N, et al; MAPP Research Network. Widespread psychosocial difficulties in men and women with urologic chronic pelvic pain syndromes: case-control findings from the multidisciplinary approach to the study of chronic pelvic pain research network. Urology 2015; 85:1319–1327.
- West C, Usher K, Foster K, Stewart L. Chronic pain and the family: the experience of the partners of people living with chronic pain. J Clin Nurs 2012; 21:3352–3360.
- Khatri P, Mays K. Brief interventions in primary care. www.integration.samhsa.gov/Brief_Intervention_in_PC,_pdf.pdf. Accessed February 2, 2018.
- Roy-Byrne P, Veitengruber JP, Bystritsky A, et al. Brief intervention for anxiety in primary care patients. J Am Board Fam Med 2009; 22:175–186.
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012; 36:427–440.
- Masheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S. A randomized clinical trial for women with vulvodynia: cognitive-behavioral therapy vs. supportive psychotherapy. Pain 2009; 141:31–40.
- ter Kuile MM, Weijenborg PT. A cognitive-behavioral group program for women with vulvar vestibulitis syndrome (VVS): factors associated with treatment success. J Sex Marital Ther 2006; 32:199–213.
- Bergeron S, Khalifé S, Glazer HI, Binik YM. Surgical and behavioral treatments for vestibulodynia: two-and-one-half year follow-up and predictors of outcome. Obstet Gynecol 2008; 111:159–166.
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev 2007; 27:318–326.
- Mazzucchelli T, Kane R, Rees C. Behavioral activation treatments for depression in adults: a meta-analysis and review. Clin Psychol Sci Practice 2009; 16:383–411.
- Riebe G, Fan MY, Unützer J, Vannoy S. Activity scheduling as a core component of effective care management for late-life depression. Int J Geriatr Psychiatry 2012; 27:1298–1304.
- Chen YF, Huang XY, Chien CH, Cheng JF. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care 2017; 53:329–336.
- Klainin-Yobas P, Oo WN, Yew PYS, Lau Y. Effects of relaxation interventions on depression and anxiety among older adults: a systematic review. Aging Ment Health 2015; 19:1043–1055.
- Finlay KA, Rogers J. Maximizing self-care through familiarity: the role of practice effects in enhancing music listening and progressive muscle relaxation for pain management. Psychology of Music 2015; 43:511–529.
- Harvard Health Publications; Harvard Medical School. Relaxation techniques: breath control helps quell errant stress response. www.health.harvard.edu/mind-and-mood/relaxation-techniques-breath-control-helps-quell-errant-stress-response. Accessed February 2, 2018.
- Bernstein DA, Borkovec TD. Progressive relaxation training: a manual for the helping professions. Champaign, IL: Research Press; 1973.
- Whisman MA, Baucom DH. Intimate relationships and psychopathology. Clin Child Fam Psychol Rev 2012; 15:4–13.
Chronic pelvic pain is a common clinical problem in women, as prevalent in primary care as asthma or back pain.1,2 It is often associated with lost work days and decreased productivity, increased healthcare spending, mood disorders, and negative effects on personal relationships.1–3
While specialty care referral may eventually be indicated, primary care doctors can take steps to diagnose and effectively manage the condition.
COMPREHENSIVE MANAGEMENT LED BY PRIMARY CARE
Chronic pelvic pain is defined as pain in the lower abdomen persisting for 3 to 6 months and of sufficient severity to require medical care or cause a functional disability.3 It is often detrimental to a woman’s personal life and overall health, making a comprehensive assessment and multidisciplinary approach to management especially important.
The ideal care-delivery model is the patient-centered medical home, whereby a primary care physician coordinates comprehensive care with the help of an interdisciplinary team.4,5 For complex cases, referral may be needed to other specialties (eg, obstetrics and gynecology, pain medicine) to help manage care.
TARGETED EVALUATION
Chronic pelvic pain often coexists with other systemic pain syndromes or psychiatric conditions common in primary care. Table 1 lists common causes and associated findings.
Detailed history is critical
The history is of utmost importance. Clinicians should query patients about the characteristics of the pain as well as their medical and surgical history. Particular attention should be given to obtaining a complete gynecologic history, including pregnancy, delivery complications, dyspareunia, sexual assault, and trauma. A detailed review of systems should focus on the reproductive, gastroenterologic, musculoskeletal, urologic, and neuropsychiatric systems.
As with many pain syndromes, allowing the patient to “tell her story” helps to establish rapport and obtain a more complete assessment. Chronic pelvic pain has been associated with physical or sexual abuse as a child or adult, so is essential to foster the doctor-patient relationship and create a safe and open space for disclosure.3,6 It is important to screen women for safety at home as well as for satisfaction or dissatisfaction with their relationships with their spouse or partner and family.
Physical examination
The physical examination should be directed by the history but should always include abdominal and pelvic examinations. These should be conducted slowly and gently, assessing for areas of tenderness, masses, and other abnormalities. Clinicians should aim to pinpoint the exact anatomic locations of tenderness if possible. Ongoing dialogue facilitates this process by inquiring about pain at each point of the examination.
The pelvic examination should begin with visual inspection for redness, discharge, lesions, fissures, excoriations, and other abnormalities. A moistened cotton swab may be used to evaluate the vulva and vestibule for localized tenderness. The manual portion of the pelvic examination should begin with a single digit, noting any introital tenderness or spasm. Next, the levator ani muscles should be directly palpated for tone and tenderness. The pelvic floor should be evaluated with attention to tenderness of the bladder or musculoskeletal structures (Figure 1). A bimanual examination assessing uterine size and tenderness, nodularity, or a fixed, immobile uterus should be conducted.
Diagnostic workup
Because the differential diagnosis of chronic pelvic pain is broad, the diagnostic workup and testing should be based on findings of the history and physical examination. In general, extensive laboratory testing is of limited use for evaluating women with chronic pelvic pain.3,7
Urinalysis should be obtained for symptoms suggesting bladder involvement such as interstitial cystitis.
Pelvic ultrasonography can help identify pelvic masses palpated during the physical examination, but routine use of imaging is not recommended.3,7 If pelvic congestion syndrome is suspected, starting with pelvic ultrasonography is reasonable before incurring the risk or cost of computed tomography or magnetic resonance imaging.8
GENERAL TREATMENT
Medical therapy
The main goals of medical therapy are to improve function and quality of life while minimizing adverse effects. General treatments include the following:
Analgesics. Nonsteroidal anti-inflammatory drugs and acetaminophen may provide pain relief, although there is weak evidence for their efficacy in treating chronic pelvic pain.9
Neuropathic agents. One of several available neuropathic agents commonly used in the treatment of chronic pain can be tried on patients who fail to respond to analgesics. Tricyclic antidepressants such as amitriptyline and imipramine decrease pain, reduce symptoms of depression, and improve sleep.10 The results of a small randomized controlled trial suggest that gabapentin is more effective than amitriptyline for reducing chronic pelvic pain.11,12 Published guidelines currently list both amitriptyline and gabapentin as first-line agents; nortriptyline and pregabalin are considered acceptable initial alternatives.9
Venlafaxine and duloxetine may help chronic pelvic pain, although specific evidence is lacking. Duloxetine may be an appropriate choice for women with chronic pelvic pain who also experience depression and urinary stress incontinence.9
Opioids. Opioid therapy should be considered only when all other reasonable therapies have failed.10 Patients may develop tolerance or dependence, as well as opioid-induced adverse effects such as hyperalgesia.9,10 Guidelines recommend that primary care providers consult with a pain management specialist before prescribing opioids, and that patients be thoroughly counseled about the risks and side effects.9
Nerve block and neuromodulation. There is weak evidence for the use of these modalities for treating chronic pelvic pain.9 If used, they should be part of a broader treatment plan and should be performed by providers who specialize in management of chronic pain.
DISEASE-SPECIFIC TREATMENT
Endometriosis: Hormonal therapy
Pelvic pain that significantly fluctuates with the menstrual cycle may be caused by endometriosis, the most common gynecologic cause of chronic pelvic pain. Women with cyclic chronic pelvic pain should be empirically treated with hormonal therapy for at least 3 to 6 months before diagnostic laparoscopy is performed.13
Oral contraceptives, gonadotropin-releasing hormone (GnRH) analogues, progestogens, and danazol have proven efficacy, although side-effect profiles differ significantly. In a comparative trial, patients treated with GnRH analogues had more improvement in pain scores compared with those treated with oral contraceptives, but they experienced a significant decrease in bone mineral density.11 The effects on bone mineral density associated with GnRH analogue therapy can be mitigated by “add-back” low-dose hormonal therapy (norethindrone, low-dose estrogen, or a combination of estrogen and progesterone), which may also provide symptomatic relief for associated hot flashes and vaginal symptoms.11
Interstitial cystitis often accompanies endometriosis
Recognizing that chronic pelvic pain may have more than one cause is important when developing a comprehensive care plan. Interstitial cystitis coexists with endometriosis in up to 60% of patients.14 Initial treatment is pentosan polysulfate sodium, an oral treatment approved by the US Food and Drug Administration for interstitial cystitis that works by restoring the protective glycosaminoglycan layer in the bladder.14,15 Amitriptyline may also be used to treat interstitial cystitis-associated nocturia.
Myofascial pain: Neuromuscular blockers
According to a recent systematic review of therapies for chronic pelvic pain, patients with symptoms related to myofascial pain may benefit from neuromuscular blockade.12 One randomized controlled trial of the effectiveness of botulinum toxin A vs saline for the treatment of chronic pelvic pain secondary to pelvic floor spasm found that after 6 months of observation, women who received botulinum toxin had significantly lower pain scores than those who received saline.12
Pelvic congestion syndrome: Multiple options
Pelvic congestion syndrome may be treated with hormonal, radiologic, or surgical therapy.16 A randomized controlled trial involving patients with chronic pelvic pain secondary to pelvic congestion demonstrated that treatment with medroxyprogesterone acetate or a GnRH agonist (goserelin) improved pelvic symptoms.17
A Cochrane review of nonsurgical interventions for chronic pelvic pain included women with a diagnosis of pelvic congestion syndrome or adhesions. It found that patients treated with medroxyprogesterone acetate were more likely to have 50% pain reduction lasting up to 9 months compared with patients taking placebo.12 In comparative studies, GnRH analogues were more effective in relieving pelvic pain than progestogen therapy.
Radiologic embolization therapy is as effective as hysterectomy for the relief of chronic pelvic pain related to pelvic congestion syndrome, and it can be performed in the outpatient setting.
Irritable bowel syndrome: Try dietary changes
Symptoms of chronic pelvic pain that are associated with changes in stool consistency and frequency suggest irritable bowel syndrome. Symptoms may improve with dietary changes and fiber supplementation. Antispasmodic agents are frequently used but their anticholinergic effects may worsen constipation.14
PELVIC PHYSICAL THERAPY
Pelvic physical therapy targets the musculoskeletal components of bowel, bladder, and sexual function to restore strength, flexibility, balance, and coordination to the pelvic floor and surrounding lumbopelvic muscles. Patients with dyspareunia, pain with activity, or a significant musculoskeletal abnormality (eg, vaginismus or point tenderness on examination) are particularly good candidates for this therapy. It is done by a physical therapist with special training in techniques to manipulate the pelvic floor to address pelvic pain.
Educating the patient
Informing the patient before the initial physical therapy visit is essential for success. Referring clinicians should emphasize to patients that treatment response can help to guide further physician intervention. Patients should be counseled that pelvic physical therapy includes a pelvic examination and an expectation to participate in a home program. Although noticeable improvement takes time, encouragement provided by the entire team, including medical providers, can help a patient maintain her care plan.
Therapists typically see a patient once a week for 8 to 12 visits initially. Insurance usually covers pelvic physical therapy through the same policy as routine physical therapy.
During the initial evaluation, the patient receives an external and internal pelvic examination assessing muscle length, strength, and coordination of the back, hip, and internal pelvic floor. Internal evaluation can be done vaginally or rectally, with one gloved finger, without the need for speculum or stirrups. Biofeedback and surface electromyography (using either perianal or internal electrode placement) are used to evaluate muscle activity and to assist the patient in developing appropriate motor control during strengthening or relaxation.18
Up-training (or strengthening) aims to improve pelvic floor endurance. It can improve pelvic instability and symptoms of heaviness and discomfort from prolapse. Patients learn to appropriately utilize the pelvic floor in isolation. If a patient is too weak to contract on her own, neuromuscular electrical stimulation is used with an internal electrode to provide an assisted contraction.
Down-training (or relaxation) focuses on reducing tone in overactive pelvic muscles. It can improve symptoms of chronic pelvic pain, sexual pain, vulvodynia, and pudendal neuralgias. Patients are made aware of chronic holding patterns that lead to excess tone in the pelvic floor and learn how to release them through stretching, cardiovascular activity, meditation, and manual release of the involved muscle groups internally and externally. Internal musculature can be manipulated by a therapist in clinic or by the patient’s trained partner; the patient can also reach necessary areas with a vaginal dilator.
Functional coordination of the pelvic floor is needed for comfortable vaginal penetration and defecation. Training with biofeedback improves a patient’s ability to relax and open the pelvic floor.18 Vaginal dilators with surface electromyography are used to treat vaginismus to eliminate reflexive pelvic floor spasm during penetration. Perineal and vaginal compliance can be improved through manual release techniques with hands or vaginal dilators to restore normal mobility of tissues. This can reduce pain from postsurgical changes, postpartum sequelae, atrophic vaginal changes, shortened muscles from chronic holding, and adhesions.
PSYCHOSOCIAL INTERVENTIONS
Pelvic pain is not only a biomedical difficulty; psychosocial factors can contribute to and be affected by pelvic pain. Patients with pelvic pain often experience lower quality of life, higher rates of anxiety and depression, and increased stress compared with others.19,20 People with pain also have more relationship stress, and patients’ partners often experience emotional distress, isolation, and feelings of powerlessness in the relationship.21
Psychosocial interventions, provided along with biomedical treatment, can help to reduce pain, anxiety, and depression and improve relational well-being.22,23 In addition to attending to pain-related symptoms, comprehensive care involves recognizing and treating coexisting anxiety, depression, stress, and relationship conflict. Interventions for these difficulties are many, and a comprehensive list of interventions is beyond the focus of this section.19
Cognitive behavioral therapy
Cognitive behavioral therapy is based on the idea that maladaptive cognitions can lead to problematic behaviors and emotional distress.24 Interventions are carried out by a provider with specialized training in its use (eg, therapist, pain psychologist, psychiatrist).
Meta-analyses of studies that investigated the efficacy of cognitive behavioral therapy for chronic pain found consistent small to medium improvement in pain-related symptoms.24 Studies that used cognitive behavioral therapy for pelvic pain found reduced overall pain severity and pain during intercourse, increased sexual satisfaction, enhanced sexual function, and less-exaggerated responses to pain.25–27
Although cognitive behavioral therapy and mindfulness-based interventions produce positive outcomes, research on these interventions typically includes treatment carried out over a span of weeks. Common barriers to such care include lack of patient motivation, financial limitations, transportation problems, and time constraints.
The following psychosocial interventions have been chosen because they can be delivered in a short amount of time and integrated into a patient’s medical care by a medical or behavioral health provider. Because of the brevity and simplicity of these interventions, more patients with pelvic pain can receive psychosocial care as part of their usual medical encounters.
Behavioral activation
People experiencing depressive symptoms tend to isolate themselves and stop participating in activities they enjoy, including spending time with family and friends. Behavioral activation interventions that address such isolating behaviors have been shown to be effective in improving depressive symptoms.28–30
A simple, brief intervention can be administered during routine medical care,28 involving the following steps:
- Determine activities that the patient might implement that would decrease depressive symptoms. Questions such as, “When do you feel less depressed?” or “What brings you some happiness in your life?” can generate possible activities.
- Ask the patient to identify people in her life who have been supportive and with whom she could engage.
- Create with the patient a list of possible activities and social interactions that may enhance well-being.
- Make a schedule for participating in activities, possibly with rewards for completing them. Patients should be encouraged to follow the prescribed schedule of activities rather than make decisions based on mood or other factors.
Relaxation strategies
Relaxation can help patients reduce stress and anxiety, and can also help reduce pain.31–33
Diaphragmatic or “belly breathing” is a deep-breathing technique in which participants are asked to take in air through the nose and fully fill the lungs and lower belly. This technique allows the body to take in more oxygen, helping to lower blood pressure and slow the heartbeat. In addition to physiologic benefits, concentrating on deep breathing can help slow down or stop intrusive thoughts and distressing physical sensations.34
Progressive muscle relaxation involves the systematic tensing and relaxing of each large muscle group in the body.35 The goal is to eliminate physical and emotional stress through focusing on the sensations of tension and relaxation.
Scripts and audio and video resources for belly breathing and progressive muscle relaxation can be found on the Internet. The techniques can be taught during the medical appointment or offered as resources for home practice.
Couple-based care
Targeting couples is more effective for improving well-being than focusing solely on a patient’s psychosocial difficulties, so each of the above interventions may be more effective if tailored to include the patient’s partner.36 If the partner is with the patient during medical visits or is included in long-term psychosocial treatment, he or she can be directly involved in learning and practicing interventions with the patient. If the partner is not present, the patient can be asked to practice newly learned well-being-enhancing strategies with her partner outside the appointment time. Couples therapy can improve psychosocial well-being for both partners.
Setting goals
Chronic pelvic pain is a common clinical problem in women, as prevalent in primary care as asthma or back pain.1,2 It is often associated with lost work days and decreased productivity, increased healthcare spending, mood disorders, and negative effects on personal relationships.1–3
While specialty care referral may eventually be indicated, primary care doctors can take steps to diagnose and effectively manage the condition.
COMPREHENSIVE MANAGEMENT LED BY PRIMARY CARE
Chronic pelvic pain is defined as pain in the lower abdomen persisting for 3 to 6 months and of sufficient severity to require medical care or cause a functional disability.3 It is often detrimental to a woman’s personal life and overall health, making a comprehensive assessment and multidisciplinary approach to management especially important.
The ideal care-delivery model is the patient-centered medical home, whereby a primary care physician coordinates comprehensive care with the help of an interdisciplinary team.4,5 For complex cases, referral may be needed to other specialties (eg, obstetrics and gynecology, pain medicine) to help manage care.
TARGETED EVALUATION
Chronic pelvic pain often coexists with other systemic pain syndromes or psychiatric conditions common in primary care. Table 1 lists common causes and associated findings.
Detailed history is critical
The history is of utmost importance. Clinicians should query patients about the characteristics of the pain as well as their medical and surgical history. Particular attention should be given to obtaining a complete gynecologic history, including pregnancy, delivery complications, dyspareunia, sexual assault, and trauma. A detailed review of systems should focus on the reproductive, gastroenterologic, musculoskeletal, urologic, and neuropsychiatric systems.
As with many pain syndromes, allowing the patient to “tell her story” helps to establish rapport and obtain a more complete assessment. Chronic pelvic pain has been associated with physical or sexual abuse as a child or adult, so is essential to foster the doctor-patient relationship and create a safe and open space for disclosure.3,6 It is important to screen women for safety at home as well as for satisfaction or dissatisfaction with their relationships with their spouse or partner and family.
Physical examination
The physical examination should be directed by the history but should always include abdominal and pelvic examinations. These should be conducted slowly and gently, assessing for areas of tenderness, masses, and other abnormalities. Clinicians should aim to pinpoint the exact anatomic locations of tenderness if possible. Ongoing dialogue facilitates this process by inquiring about pain at each point of the examination.
The pelvic examination should begin with visual inspection for redness, discharge, lesions, fissures, excoriations, and other abnormalities. A moistened cotton swab may be used to evaluate the vulva and vestibule for localized tenderness. The manual portion of the pelvic examination should begin with a single digit, noting any introital tenderness or spasm. Next, the levator ani muscles should be directly palpated for tone and tenderness. The pelvic floor should be evaluated with attention to tenderness of the bladder or musculoskeletal structures (Figure 1). A bimanual examination assessing uterine size and tenderness, nodularity, or a fixed, immobile uterus should be conducted.
Diagnostic workup
Because the differential diagnosis of chronic pelvic pain is broad, the diagnostic workup and testing should be based on findings of the history and physical examination. In general, extensive laboratory testing is of limited use for evaluating women with chronic pelvic pain.3,7
Urinalysis should be obtained for symptoms suggesting bladder involvement such as interstitial cystitis.
Pelvic ultrasonography can help identify pelvic masses palpated during the physical examination, but routine use of imaging is not recommended.3,7 If pelvic congestion syndrome is suspected, starting with pelvic ultrasonography is reasonable before incurring the risk or cost of computed tomography or magnetic resonance imaging.8
GENERAL TREATMENT
Medical therapy
The main goals of medical therapy are to improve function and quality of life while minimizing adverse effects. General treatments include the following:
Analgesics. Nonsteroidal anti-inflammatory drugs and acetaminophen may provide pain relief, although there is weak evidence for their efficacy in treating chronic pelvic pain.9
Neuropathic agents. One of several available neuropathic agents commonly used in the treatment of chronic pain can be tried on patients who fail to respond to analgesics. Tricyclic antidepressants such as amitriptyline and imipramine decrease pain, reduce symptoms of depression, and improve sleep.10 The results of a small randomized controlled trial suggest that gabapentin is more effective than amitriptyline for reducing chronic pelvic pain.11,12 Published guidelines currently list both amitriptyline and gabapentin as first-line agents; nortriptyline and pregabalin are considered acceptable initial alternatives.9
Venlafaxine and duloxetine may help chronic pelvic pain, although specific evidence is lacking. Duloxetine may be an appropriate choice for women with chronic pelvic pain who also experience depression and urinary stress incontinence.9
Opioids. Opioid therapy should be considered only when all other reasonable therapies have failed.10 Patients may develop tolerance or dependence, as well as opioid-induced adverse effects such as hyperalgesia.9,10 Guidelines recommend that primary care providers consult with a pain management specialist before prescribing opioids, and that patients be thoroughly counseled about the risks and side effects.9
Nerve block and neuromodulation. There is weak evidence for the use of these modalities for treating chronic pelvic pain.9 If used, they should be part of a broader treatment plan and should be performed by providers who specialize in management of chronic pain.
DISEASE-SPECIFIC TREATMENT
Endometriosis: Hormonal therapy
Pelvic pain that significantly fluctuates with the menstrual cycle may be caused by endometriosis, the most common gynecologic cause of chronic pelvic pain. Women with cyclic chronic pelvic pain should be empirically treated with hormonal therapy for at least 3 to 6 months before diagnostic laparoscopy is performed.13
Oral contraceptives, gonadotropin-releasing hormone (GnRH) analogues, progestogens, and danazol have proven efficacy, although side-effect profiles differ significantly. In a comparative trial, patients treated with GnRH analogues had more improvement in pain scores compared with those treated with oral contraceptives, but they experienced a significant decrease in bone mineral density.11 The effects on bone mineral density associated with GnRH analogue therapy can be mitigated by “add-back” low-dose hormonal therapy (norethindrone, low-dose estrogen, or a combination of estrogen and progesterone), which may also provide symptomatic relief for associated hot flashes and vaginal symptoms.11
Interstitial cystitis often accompanies endometriosis
Recognizing that chronic pelvic pain may have more than one cause is important when developing a comprehensive care plan. Interstitial cystitis coexists with endometriosis in up to 60% of patients.14 Initial treatment is pentosan polysulfate sodium, an oral treatment approved by the US Food and Drug Administration for interstitial cystitis that works by restoring the protective glycosaminoglycan layer in the bladder.14,15 Amitriptyline may also be used to treat interstitial cystitis-associated nocturia.
Myofascial pain: Neuromuscular blockers
According to a recent systematic review of therapies for chronic pelvic pain, patients with symptoms related to myofascial pain may benefit from neuromuscular blockade.12 One randomized controlled trial of the effectiveness of botulinum toxin A vs saline for the treatment of chronic pelvic pain secondary to pelvic floor spasm found that after 6 months of observation, women who received botulinum toxin had significantly lower pain scores than those who received saline.12
Pelvic congestion syndrome: Multiple options
Pelvic congestion syndrome may be treated with hormonal, radiologic, or surgical therapy.16 A randomized controlled trial involving patients with chronic pelvic pain secondary to pelvic congestion demonstrated that treatment with medroxyprogesterone acetate or a GnRH agonist (goserelin) improved pelvic symptoms.17
A Cochrane review of nonsurgical interventions for chronic pelvic pain included women with a diagnosis of pelvic congestion syndrome or adhesions. It found that patients treated with medroxyprogesterone acetate were more likely to have 50% pain reduction lasting up to 9 months compared with patients taking placebo.12 In comparative studies, GnRH analogues were more effective in relieving pelvic pain than progestogen therapy.
Radiologic embolization therapy is as effective as hysterectomy for the relief of chronic pelvic pain related to pelvic congestion syndrome, and it can be performed in the outpatient setting.
Irritable bowel syndrome: Try dietary changes
Symptoms of chronic pelvic pain that are associated with changes in stool consistency and frequency suggest irritable bowel syndrome. Symptoms may improve with dietary changes and fiber supplementation. Antispasmodic agents are frequently used but their anticholinergic effects may worsen constipation.14
PELVIC PHYSICAL THERAPY
Pelvic physical therapy targets the musculoskeletal components of bowel, bladder, and sexual function to restore strength, flexibility, balance, and coordination to the pelvic floor and surrounding lumbopelvic muscles. Patients with dyspareunia, pain with activity, or a significant musculoskeletal abnormality (eg, vaginismus or point tenderness on examination) are particularly good candidates for this therapy. It is done by a physical therapist with special training in techniques to manipulate the pelvic floor to address pelvic pain.
Educating the patient
Informing the patient before the initial physical therapy visit is essential for success. Referring clinicians should emphasize to patients that treatment response can help to guide further physician intervention. Patients should be counseled that pelvic physical therapy includes a pelvic examination and an expectation to participate in a home program. Although noticeable improvement takes time, encouragement provided by the entire team, including medical providers, can help a patient maintain her care plan.
Therapists typically see a patient once a week for 8 to 12 visits initially. Insurance usually covers pelvic physical therapy through the same policy as routine physical therapy.
During the initial evaluation, the patient receives an external and internal pelvic examination assessing muscle length, strength, and coordination of the back, hip, and internal pelvic floor. Internal evaluation can be done vaginally or rectally, with one gloved finger, without the need for speculum or stirrups. Biofeedback and surface electromyography (using either perianal or internal electrode placement) are used to evaluate muscle activity and to assist the patient in developing appropriate motor control during strengthening or relaxation.18
Up-training (or strengthening) aims to improve pelvic floor endurance. It can improve pelvic instability and symptoms of heaviness and discomfort from prolapse. Patients learn to appropriately utilize the pelvic floor in isolation. If a patient is too weak to contract on her own, neuromuscular electrical stimulation is used with an internal electrode to provide an assisted contraction.
Down-training (or relaxation) focuses on reducing tone in overactive pelvic muscles. It can improve symptoms of chronic pelvic pain, sexual pain, vulvodynia, and pudendal neuralgias. Patients are made aware of chronic holding patterns that lead to excess tone in the pelvic floor and learn how to release them through stretching, cardiovascular activity, meditation, and manual release of the involved muscle groups internally and externally. Internal musculature can be manipulated by a therapist in clinic or by the patient’s trained partner; the patient can also reach necessary areas with a vaginal dilator.
Functional coordination of the pelvic floor is needed for comfortable vaginal penetration and defecation. Training with biofeedback improves a patient’s ability to relax and open the pelvic floor.18 Vaginal dilators with surface electromyography are used to treat vaginismus to eliminate reflexive pelvic floor spasm during penetration. Perineal and vaginal compliance can be improved through manual release techniques with hands or vaginal dilators to restore normal mobility of tissues. This can reduce pain from postsurgical changes, postpartum sequelae, atrophic vaginal changes, shortened muscles from chronic holding, and adhesions.
PSYCHOSOCIAL INTERVENTIONS
Pelvic pain is not only a biomedical difficulty; psychosocial factors can contribute to and be affected by pelvic pain. Patients with pelvic pain often experience lower quality of life, higher rates of anxiety and depression, and increased stress compared with others.19,20 People with pain also have more relationship stress, and patients’ partners often experience emotional distress, isolation, and feelings of powerlessness in the relationship.21
Psychosocial interventions, provided along with biomedical treatment, can help to reduce pain, anxiety, and depression and improve relational well-being.22,23 In addition to attending to pain-related symptoms, comprehensive care involves recognizing and treating coexisting anxiety, depression, stress, and relationship conflict. Interventions for these difficulties are many, and a comprehensive list of interventions is beyond the focus of this section.19
Cognitive behavioral therapy
Cognitive behavioral therapy is based on the idea that maladaptive cognitions can lead to problematic behaviors and emotional distress.24 Interventions are carried out by a provider with specialized training in its use (eg, therapist, pain psychologist, psychiatrist).
Meta-analyses of studies that investigated the efficacy of cognitive behavioral therapy for chronic pain found consistent small to medium improvement in pain-related symptoms.24 Studies that used cognitive behavioral therapy for pelvic pain found reduced overall pain severity and pain during intercourse, increased sexual satisfaction, enhanced sexual function, and less-exaggerated responses to pain.25–27
Although cognitive behavioral therapy and mindfulness-based interventions produce positive outcomes, research on these interventions typically includes treatment carried out over a span of weeks. Common barriers to such care include lack of patient motivation, financial limitations, transportation problems, and time constraints.
The following psychosocial interventions have been chosen because they can be delivered in a short amount of time and integrated into a patient’s medical care by a medical or behavioral health provider. Because of the brevity and simplicity of these interventions, more patients with pelvic pain can receive psychosocial care as part of their usual medical encounters.
Behavioral activation
People experiencing depressive symptoms tend to isolate themselves and stop participating in activities they enjoy, including spending time with family and friends. Behavioral activation interventions that address such isolating behaviors have been shown to be effective in improving depressive symptoms.28–30
A simple, brief intervention can be administered during routine medical care,28 involving the following steps:
- Determine activities that the patient might implement that would decrease depressive symptoms. Questions such as, “When do you feel less depressed?” or “What brings you some happiness in your life?” can generate possible activities.
- Ask the patient to identify people in her life who have been supportive and with whom she could engage.
- Create with the patient a list of possible activities and social interactions that may enhance well-being.
- Make a schedule for participating in activities, possibly with rewards for completing them. Patients should be encouraged to follow the prescribed schedule of activities rather than make decisions based on mood or other factors.
Relaxation strategies
Relaxation can help patients reduce stress and anxiety, and can also help reduce pain.31–33
Diaphragmatic or “belly breathing” is a deep-breathing technique in which participants are asked to take in air through the nose and fully fill the lungs and lower belly. This technique allows the body to take in more oxygen, helping to lower blood pressure and slow the heartbeat. In addition to physiologic benefits, concentrating on deep breathing can help slow down or stop intrusive thoughts and distressing physical sensations.34
Progressive muscle relaxation involves the systematic tensing and relaxing of each large muscle group in the body.35 The goal is to eliminate physical and emotional stress through focusing on the sensations of tension and relaxation.
Scripts and audio and video resources for belly breathing and progressive muscle relaxation can be found on the Internet. The techniques can be taught during the medical appointment or offered as resources for home practice.
Couple-based care
Targeting couples is more effective for improving well-being than focusing solely on a patient’s psychosocial difficulties, so each of the above interventions may be more effective if tailored to include the patient’s partner.36 If the partner is with the patient during medical visits or is included in long-term psychosocial treatment, he or she can be directly involved in learning and practicing interventions with the patient. If the partner is not present, the patient can be asked to practice newly learned well-being-enhancing strategies with her partner outside the appointment time. Couples therapy can improve psychosocial well-being for both partners.
Setting goals
- Zondervan KT, Yudkin PL, Vessey MP, et al. The community prevalence of chronic pelvic pain in women and associated illness behavior. Br J Gen Pract 2001; 51:541–547.
- Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol 1996; 81:321–327.
- Howard FM. Chronic pelvic pain. Obstet Gynecol 2003; 101:594–611.
- AHRQ PCMH Resource Center. Transforming the organization and delivery of primary care. www.pcmh.ahrq.gov/. Accessed February 2, 2018.
- Pryzbylkowski P, Ashburn MA. The pain medical home: a patient-centered medical home model of care for patients with chronic pain. Anesthesiol Clin 2015; 33:785–793.
- Jamieson DJ, Steege JF. The association of sexual abuse with pelvic pain complaints in a primary care population. Am J Obstet Gynecol 1997; 177:1408–1412.
- Gambone JC, Mittman BS, Munro MG, Scialli AR, Winkel CA; Chronic Pelvic Pain/Endometriosis Working Group. Consensus statement for the management of chronic pelvic pain and endometriosis: proceedings of an expert-panel consensus process. Fertil Steril 2002; 78:961–972.
- Ganeshan A, Upponi S, Hon LQ, Uthappa MC, Warakaulle DR, Uberoi R. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol 2007; 30:1105–1111.
- Engeler D, Baranowski AP, Elneil S, et al; European Association of Urology. Guidelines on chronic pelvic pain. http://uroweb.org/wp-content/uploads/EAU-Guidelines-Chronic-Pelvic-Pain-2015.pdf. Accessed February 5, 2018.
- Vercellini P, Vigano P, Somigliana E, Abbiati A, Barbara G, Fedele L. Medical, surgical and alternative treatments for chronic pelvic pain in women: a descriptive review. Gynecol Endocrinol 2009; 25:208–221.
- Rafique S, DeCherney AH. Medical management of endometriosis. Clin Obstet Gynecol 2017; 60:485–496.
- Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev 2014; 3:CD008797.
- Royal College of Obstetricians and Gynecologists. The initial management of chronic pelvic pain, Green-top guideline No.41. www.rcog.org.uk/globalassets/documents/guidelines/gtg_41.pdf. Accessed February 2, 2018.
- Shin JH, Howard FM. Management of chronic pelvic pain. Curr Pain Headache Rep 2011; 15:377–385.
- Nelson P, Apte G, Justiz R, Brismee JM, Dedrick G, Sizer PS. Chronic female pelvic pain—Part 2: differential diagnosis and management. Pain Pract 2012; 12:111–141.
- Holloran-Schwartz MB. Surgical evaluation and treatment of the patient with chronic pelvic pain. Obstet Gynecol Clin North Am 2014; 41:357–369.
- Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod 2001; 16:931–939.
- Arnouk A, De E, Rehfuss A, Cappadocia C, Dickson S, Lian F. Physical, complementary, and alternative medicine in the treatment of pelvic floor disorders. Curr Urol Rep 2017; 18:47.
- Faccin F, Barbara G, Saita E, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol 2015; 36:135–141.
- Naliboff BD, Stephens AJ, Afari N, et al; MAPP Research Network. Widespread psychosocial difficulties in men and women with urologic chronic pelvic pain syndromes: case-control findings from the multidisciplinary approach to the study of chronic pelvic pain research network. Urology 2015; 85:1319–1327.
- West C, Usher K, Foster K, Stewart L. Chronic pain and the family: the experience of the partners of people living with chronic pain. J Clin Nurs 2012; 21:3352–3360.
- Khatri P, Mays K. Brief interventions in primary care. www.integration.samhsa.gov/Brief_Intervention_in_PC,_pdf.pdf. Accessed February 2, 2018.
- Roy-Byrne P, Veitengruber JP, Bystritsky A, et al. Brief intervention for anxiety in primary care patients. J Am Board Fam Med 2009; 22:175–186.
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012; 36:427–440.
- Masheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S. A randomized clinical trial for women with vulvodynia: cognitive-behavioral therapy vs. supportive psychotherapy. Pain 2009; 141:31–40.
- ter Kuile MM, Weijenborg PT. A cognitive-behavioral group program for women with vulvar vestibulitis syndrome (VVS): factors associated with treatment success. J Sex Marital Ther 2006; 32:199–213.
- Bergeron S, Khalifé S, Glazer HI, Binik YM. Surgical and behavioral treatments for vestibulodynia: two-and-one-half year follow-up and predictors of outcome. Obstet Gynecol 2008; 111:159–166.
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev 2007; 27:318–326.
- Mazzucchelli T, Kane R, Rees C. Behavioral activation treatments for depression in adults: a meta-analysis and review. Clin Psychol Sci Practice 2009; 16:383–411.
- Riebe G, Fan MY, Unützer J, Vannoy S. Activity scheduling as a core component of effective care management for late-life depression. Int J Geriatr Psychiatry 2012; 27:1298–1304.
- Chen YF, Huang XY, Chien CH, Cheng JF. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care 2017; 53:329–336.
- Klainin-Yobas P, Oo WN, Yew PYS, Lau Y. Effects of relaxation interventions on depression and anxiety among older adults: a systematic review. Aging Ment Health 2015; 19:1043–1055.
- Finlay KA, Rogers J. Maximizing self-care through familiarity: the role of practice effects in enhancing music listening and progressive muscle relaxation for pain management. Psychology of Music 2015; 43:511–529.
- Harvard Health Publications; Harvard Medical School. Relaxation techniques: breath control helps quell errant stress response. www.health.harvard.edu/mind-and-mood/relaxation-techniques-breath-control-helps-quell-errant-stress-response. Accessed February 2, 2018.
- Bernstein DA, Borkovec TD. Progressive relaxation training: a manual for the helping professions. Champaign, IL: Research Press; 1973.
- Whisman MA, Baucom DH. Intimate relationships and psychopathology. Clin Child Fam Psychol Rev 2012; 15:4–13.
- Zondervan KT, Yudkin PL, Vessey MP, et al. The community prevalence of chronic pelvic pain in women and associated illness behavior. Br J Gen Pract 2001; 51:541–547.
- Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol 1996; 81:321–327.
- Howard FM. Chronic pelvic pain. Obstet Gynecol 2003; 101:594–611.
- AHRQ PCMH Resource Center. Transforming the organization and delivery of primary care. www.pcmh.ahrq.gov/. Accessed February 2, 2018.
- Pryzbylkowski P, Ashburn MA. The pain medical home: a patient-centered medical home model of care for patients with chronic pain. Anesthesiol Clin 2015; 33:785–793.
- Jamieson DJ, Steege JF. The association of sexual abuse with pelvic pain complaints in a primary care population. Am J Obstet Gynecol 1997; 177:1408–1412.
- Gambone JC, Mittman BS, Munro MG, Scialli AR, Winkel CA; Chronic Pelvic Pain/Endometriosis Working Group. Consensus statement for the management of chronic pelvic pain and endometriosis: proceedings of an expert-panel consensus process. Fertil Steril 2002; 78:961–972.
- Ganeshan A, Upponi S, Hon LQ, Uthappa MC, Warakaulle DR, Uberoi R. Chronic pelvic pain due to pelvic congestion syndrome: the role of diagnostic and interventional radiology. Cardiovasc Intervent Radiol 2007; 30:1105–1111.
- Engeler D, Baranowski AP, Elneil S, et al; European Association of Urology. Guidelines on chronic pelvic pain. http://uroweb.org/wp-content/uploads/EAU-Guidelines-Chronic-Pelvic-Pain-2015.pdf. Accessed February 5, 2018.
- Vercellini P, Vigano P, Somigliana E, Abbiati A, Barbara G, Fedele L. Medical, surgical and alternative treatments for chronic pelvic pain in women: a descriptive review. Gynecol Endocrinol 2009; 25:208–221.
- Rafique S, DeCherney AH. Medical management of endometriosis. Clin Obstet Gynecol 2017; 60:485–496.
- Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev 2014; 3:CD008797.
- Royal College of Obstetricians and Gynecologists. The initial management of chronic pelvic pain, Green-top guideline No.41. www.rcog.org.uk/globalassets/documents/guidelines/gtg_41.pdf. Accessed February 2, 2018.
- Shin JH, Howard FM. Management of chronic pelvic pain. Curr Pain Headache Rep 2011; 15:377–385.
- Nelson P, Apte G, Justiz R, Brismee JM, Dedrick G, Sizer PS. Chronic female pelvic pain—Part 2: differential diagnosis and management. Pain Pract 2012; 12:111–141.
- Holloran-Schwartz MB. Surgical evaluation and treatment of the patient with chronic pelvic pain. Obstet Gynecol Clin North Am 2014; 41:357–369.
- Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod 2001; 16:931–939.
- Arnouk A, De E, Rehfuss A, Cappadocia C, Dickson S, Lian F. Physical, complementary, and alternative medicine in the treatment of pelvic floor disorders. Curr Urol Rep 2017; 18:47.
- Faccin F, Barbara G, Saita E, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol 2015; 36:135–141.
- Naliboff BD, Stephens AJ, Afari N, et al; MAPP Research Network. Widespread psychosocial difficulties in men and women with urologic chronic pelvic pain syndromes: case-control findings from the multidisciplinary approach to the study of chronic pelvic pain research network. Urology 2015; 85:1319–1327.
- West C, Usher K, Foster K, Stewart L. Chronic pain and the family: the experience of the partners of people living with chronic pain. J Clin Nurs 2012; 21:3352–3360.
- Khatri P, Mays K. Brief interventions in primary care. www.integration.samhsa.gov/Brief_Intervention_in_PC,_pdf.pdf. Accessed February 2, 2018.
- Roy-Byrne P, Veitengruber JP, Bystritsky A, et al. Brief intervention for anxiety in primary care patients. J Am Board Fam Med 2009; 22:175–186.
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012; 36:427–440.
- Masheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S. A randomized clinical trial for women with vulvodynia: cognitive-behavioral therapy vs. supportive psychotherapy. Pain 2009; 141:31–40.
- ter Kuile MM, Weijenborg PT. A cognitive-behavioral group program for women with vulvar vestibulitis syndrome (VVS): factors associated with treatment success. J Sex Marital Ther 2006; 32:199–213.
- Bergeron S, Khalifé S, Glazer HI, Binik YM. Surgical and behavioral treatments for vestibulodynia: two-and-one-half year follow-up and predictors of outcome. Obstet Gynecol 2008; 111:159–166.
- Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev 2007; 27:318–326.
- Mazzucchelli T, Kane R, Rees C. Behavioral activation treatments for depression in adults: a meta-analysis and review. Clin Psychol Sci Practice 2009; 16:383–411.
- Riebe G, Fan MY, Unützer J, Vannoy S. Activity scheduling as a core component of effective care management for late-life depression. Int J Geriatr Psychiatry 2012; 27:1298–1304.
- Chen YF, Huang XY, Chien CH, Cheng JF. The effectiveness of diaphragmatic breathing relaxation training for reducing anxiety. Perspect Psychiatr Care 2017; 53:329–336.
- Klainin-Yobas P, Oo WN, Yew PYS, Lau Y. Effects of relaxation interventions on depression and anxiety among older adults: a systematic review. Aging Ment Health 2015; 19:1043–1055.
- Finlay KA, Rogers J. Maximizing self-care through familiarity: the role of practice effects in enhancing music listening and progressive muscle relaxation for pain management. Psychology of Music 2015; 43:511–529.
- Harvard Health Publications; Harvard Medical School. Relaxation techniques: breath control helps quell errant stress response. www.health.harvard.edu/mind-and-mood/relaxation-techniques-breath-control-helps-quell-errant-stress-response. Accessed February 2, 2018.
- Bernstein DA, Borkovec TD. Progressive relaxation training: a manual for the helping professions. Champaign, IL: Research Press; 1973.
- Whisman MA, Baucom DH. Intimate relationships and psychopathology. Clin Child Fam Psychol Rev 2012; 15:4–13.
KEY POINTS
- Diagnosing and managing chronic pelvic pain may be difficult, but patients are often best served when their primary care provider directs a team-based approach to their care.
- A detailed history, thorough abdominal and pelvic examinations, and targeted testing facilitate the diagnosis.
- As in other chronic pain syndromes, the goals of therapy should be incremental and meaningful improvements in pain, function, and overall well-being.
Substance abuse among older adults: A growing problem
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.
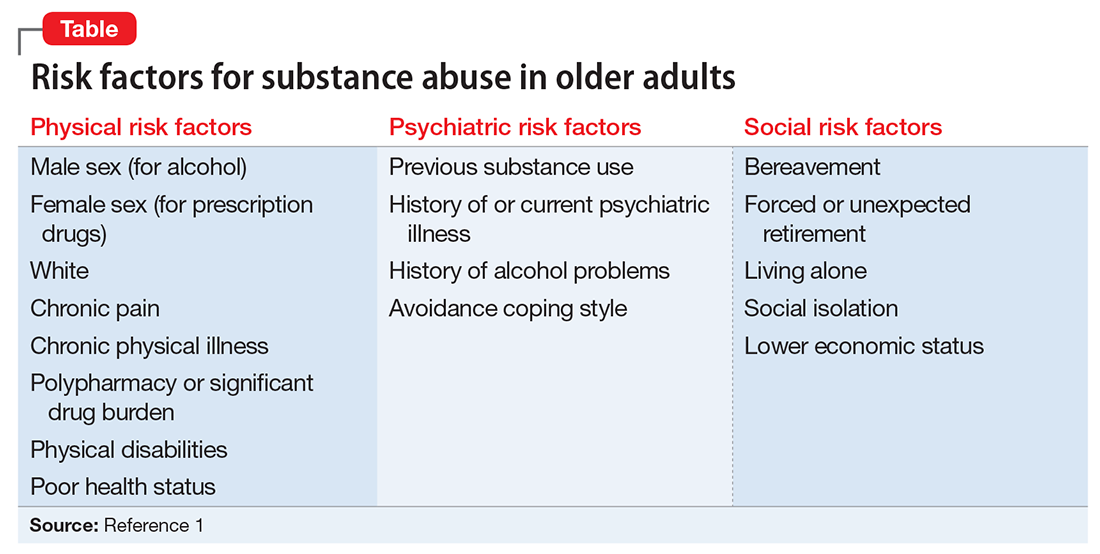
Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.

Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.

Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
Mental health apps: What to tell patients
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.
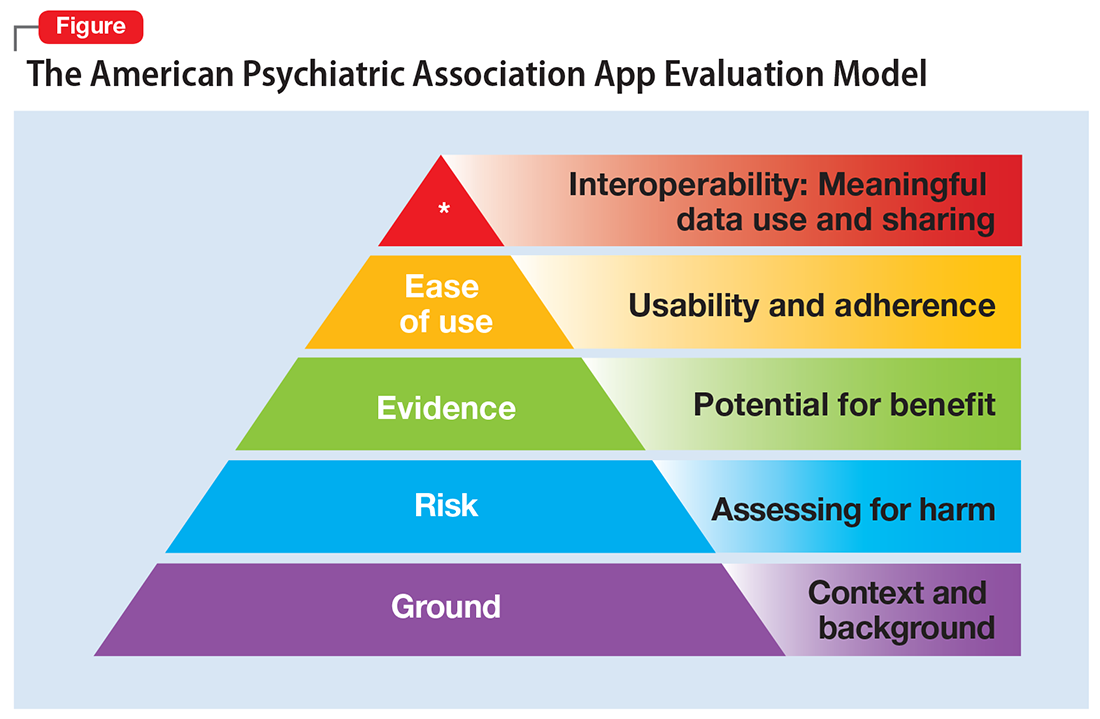
Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.

Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
Have your patients asked you about smartphone apps? If they haven’t yet, they may soon, as interest in apps for mental health continues to expand. There are now >10,000 mental health–related smartphone apps.1 The rapid rise of these apps is partly due to their potential to transform a patient’s smartphone into a monitoring and therapeutic platform, capable of capturing mental health symptoms in real time and delivering on-the-go therapy. Setting aside questions about the potential of mobile health, 2 urgent questions remain for the busy psychiatrist in clinical practice: What is the current evidence base for mental health apps, and what should you tell your patients about them?
For most apps, evidence of efficacy is limited
While the evidence base for mental health smartphone apps continues to expand, for many of these apps, there is no evidence of effectiveness. The growing consensus is that most commercially available apps are not evidence-based and some are even dangerous. For example, researchers who examined >700 mindfulness apps on the iTunes and Google Play stores found that only 4% provided acceptable mindfulness training and education.2 Another study of 58 apps that claimed to offer sobriety assessments found that none had ever been formally evaluated.3 Evidence-based reviews of suicide prevention apps have identified potentially harmful apps,4 and studies evaluating apps for bipolar disorder5 and depression6 have yielded similar results—few have any evidence supporting their use, and some offer dangerous and harmful advice. For example, researchers found that one app for bipolar disorder advised patients who are experiencing a manic episode to drink alcohol.5 Currently, the vast majority of commercially available apps are not appropriate for clinical care. This finding is not unique to mental health; similar findings have been reported for apps for cancer.7 The bottom line is that the apps that your patients are finding, and perhaps already using, may not be useful or effective.
However, early studies have demonstrated efficacy of some apps for several conditions, including schizophrenia,8 depression,9 anxiety disorders,10 and suicidal ideation.11 Although many of the apps evaluated in these studies are not available to the public, or still require large-scale assessment before they are ready for mainstream clinical care, this research demonstrates that mental health apps can help improve treatment outcomes. As this research develops, a wave of evidence-based and effective mental health apps may be available in the near future.
Although it is unknown how many patients are presently using mental health apps, there is strong anecdotal evidence that an increasing number of patients who use these apps and other forms of digital technology are finding some benefits. In many cases, patients may actually be ahead of the research. For example, one study that conducted an online survey of patients with schizophrenia noted that some patients are using their smartphones to play music to help block auditory hallucinations.12
Why online reviews are of limited use
As this evidence continues to mature, and with an ever-growing number of mental health apps available on commercial marketplaces, busy psychiatrists need to navigate this complex space. Even psychiatrists who decide to not use apps as part of care still need to be knowledgeable about them, because patients are likely to ask about the benefits of using apps, and they will expect an informed response. How would you reply if your patient asked you about a new mood-tracking app he or she recently heard about? On what would you base your recommendation and opinion?
Reading online app reviews for guidance is not a good solution. A recent study found little relationship between the star ratings of health apps and the quality of those apps,13 which suggests that a 5-star rating on the app store is of limited use.
Unlike medications whose ingredients do not change over time, or manualized psychotherapies that use specific protocols, mental health apps are dynamic and constantly changing.14 Think of how often the apps on your smartphone update. Thus, the version of a mental health app that your patient downloads today may be very different from the version that received a favorable user review last month. And just as there is no single medication or therapy that is ideal for every patient, neither is there a single “best” app for all patients with the same disorder. Picking an app is a personal decision that cannot be made based on a single score or numeric rating. Furthermore, the validity of app rating systems is unclear. One study found a wide variation in the interrater reliability of measures used to evaluate apps from sources that included PsyberGuide, the Anxiety and Depression Association of America, and the research literature. Quality measures such as effectiveness, ease of use, and performance had relatively poor interrater reliability.15 This means that, for example, an app that one patient finds “easy to use” may be difficult to use for another. Thus, providing patients with suggestions based on an app’s ratings may result in providing information that sounds useful, but often is misleading.
A model for evaluating apps
One possible solution is a risk-based and personalized assessment approach to evaluating mental health apps. Although it does not offer scoring or recommendations of specific apps, the American Psychiatric Association (APA) App Evaluation Model (Figure) provides a framework to guide discussion and informed decision-making about apps. (The authors of this article helped create this model, but receive no compensation for that volunteer work.) The pyramid shape reflects the hierarchical nature of the model. To begin the process, start at the base of the pyramid and work upward.

Ground. First, consider the context of the app by determining basic facts, such as who made it, how much it costs, and its technology requirements. This ground layer establishes the credibility of the app’s creator by questioning his or her reputation, ability to update the app, and funding sources. Understanding the app’s business model also will help you determine whether the app will stand the test of time: Will it continue to exist next month or next year, or will a lack of reliable funding lead the vendor to abandon it?
Risk. The next layer assesses the risk, privacy, and security features of the app. Many mental health apps actively aim to avoid falling under the jurisdiction of U.S. federal health care privacy rules, such as the Health Insurance Portability and Accountability Act of 1996, so there is no guarantee that sensitive data supplied to an app will be protected. The true cost of a “free” app often is your patient’s personal mental health information, which the app’s developer may accumulate and sell for profit. Thus, it is wise to check the privacy policy to learn where your patient’s data goes. Furthermore, patients and psychiatrists must be vigilant that malware-infected apps can be uploaded to the app store, which can further compromise privacy.16 You may be surprised to learn that many apps lack a privacy policy, which means there are no protections for personal information or safeguards against the misuse of mental health data.17 Checking that an app at least promises to digitally protect mental health data through encryption and secure storage also is a good step.
The goal of considering these factors is not to create a score, but rather to be aware of them and consider them in the context of the specific app, patient, and clinical situation. Doing so helps determine whether the app meets the appropriate risk, privacy, and security standards for your patient.
Evidence. The next layer of the evaluation framework is evidence. The goal is to seek an app with clinical evidence of effectiveness. Simply put, if a patient is going to use an app, he should use one that works. An app without formal evidence may be effective, but it is important to make sure the patient is aware that these claims have not been verified. Many apps claim that they offer cognitive-behavioral therapy or mindfulness therapy, but few deliver on such claims.18 It is wise to try an app before recommending it to a patient to ensure that it does what it claims it does, and does not offer dangerous or harmful recommendations.
Ease of use. Across all health apps, there is growing recognition that most downloaded apps are never used. Patient engagement with mental health apps appears to rapidly decline over the first week of use.19 There also is emerging evidence that many apps are not user-friendly. A recent study of several common mood-tracking apps found that patients with depression had difficulty entering and accessing their data.20 Because many psychiatric disorders are chronic or last at least several months, it is especially important to consider how engaging and usable the app will be for your patient. Usability varies from patient to patient, so it is best to check directly with your patient regarding his comfort with apps and mobile technology. Offering check-ins and support to help patients keep on track with apps may be critical for successful outcomes.
Interoperability. The final layer of the model is data sharing and interoperability. It is important to determine if the data collected or generated by the app are available to you, the patient, the treatment team, and others involved in the patient’s care. As mental health treatment moves toward integrated care, apps that fragment care (by not sharing information) impede care. Check if the app can share data with an electronic medical record, or if there is a plan to review and act on data from the app as part of your patient’s treatment plan.
More information about the APA App Evaluation Model, including additional factors to consider within each layer, is available from the APA for free at https://www.psychiatry.org/psychiatrists/practice/mental-health-apps/app-evaluation-model. For a sample of factors to consider when evaluating a mental health app, see the Table.
A reasonable strategy
Although the APA App Evaluation Model does not endorse any particular app, it can help guide more informed decision-making. As the evidence on mental health apps continues to evolve, it will become easier to make definitive statements on what constitutes a useful app. For now, the best strategy when discussing mental health apps with patients is to combine the use of this model with your clinical judgment.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
1. Torous J, Roberts LW. Needed innovation in digital health and smartphone applications for mental health: transparency and trust. JAMA Psychiatry. 2017;74(5):437-438.
2. Mani M, Kavanagh DJ, Hides L, et al. Review and evaluation of mindfulness-based iPhone apps. JMIR Mhealth Uhealth. 2015;3(3):e82. doi: 10.2196/mhealth.4328.
3. Wilson H, Stoyanov SR, Gandabhai S, et al. The quality and accuracy of mobile apps to prevent driving after drinking alcohol. JMIR Mhealth Uhealth. 2016;4(3):e98. doi: 10.2196/mhealth.5961.
4. Larsen ME, Nicholas J, Christensen H. A systematic assessment of smartphone tools for suicide prevention. PLoS One. 2016;11(4):e0152285. doi: 10.1371/journal.pone.0152285.
5. Nicholas J, Larsen ME, Proudfoot J, et al. Mobile apps for bipolar disorder: a systematic review of features and content quality. J Med Internet Res. 2015;17(8):e198. doi: 10.2196/jmir.4581.
6. Shen N, Levitan MJ, Johnson A, et al. Finding a depression app: a review and content analysis of the depression app marketplace. JMIR Mhealth Uhealth. 2015;3(1):e16. doi: 10.2196/mhealth.3713.
7. Davis SW, Oakley-Girvan I. Achieving value in mobile health applications for cancer survivors. J Cancer Surviv. 2017;11(4):498-504.
8. Ben-Zeev D, Brenner CJ, Begale M, et al. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244-1253.
9. Mohr DC, Tomasino KN, Lattie EG, et al. IntelliCare: an eclectic, skills-based app suite for the treatment of depression and anxiety. J Med Internet Res. 2017;19(1):e10. doi: 10.2196/jmir.6645.
10. Tighe J, Shand F, Ridani R, et al. Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open. 2017;7(1):e013518. doi: 10.1136/bmjopen-2016-013518.
11. Firth J, Torous J, Nicholas J, et al. Can smartphone mental health interventions reduce symptoms of anxiety? A meta-analysis of randomized controlled trials. J Affect Disord. 2017;218:15-22.
12. Gay K, Torous J, Joseph A, et al. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Mental Health. 2016;3(2):e15. doi: 10.2196/mental.5379.
13. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high-need, high-cost populations, but gaps remain. Health Aff (Millwood). 2016;35(12):2310-2318.
14. Larsen ME, Nicholas J, Christensen H. Quantifying app store dynamics: longitudinal tracking of mental health apps. JMIR Mhealth Uhealth. 2016;4(3):e96. doi: 10.2196/mhealth.6020.
15. Powell AC, Torous J, Chan S, et al. Interrater reliability of mHealth app rating measures: analysis of top depression and smoking cessation apps. JMIR Mhealth Uhealth. 2016;4(1):e15. doi: 10.2196/mhealth.5176.
16. Ducklin P. Apple’s XcodeGhost malware still in the machine…. https://nakedsecurity.sophos.com/2015/11/09/apples-xcodeghost-malware-still-in-the-machine. Published November 9, 2015. Accessed May 11, 2017.
17. Rosenfeld L, Torous J, Vahia IV. Data security and privacy in apps for dementia: an analysis of existing privacy policies. Am J Geriatr Psychiatry. 2017;25(8):873-877.
18. Torous J, Levin ME, Ahern DK, et al. Cognitive behavioral mobile applications: clinical studies, marketplace overview, and research agenda. Cogn Behav Pract. 2017;24(2):215-225.
19. Owen JE, Jaworski BK, Kuhn E, et al. mHealth in the wild: using novel data to examine the reach, use, and impact of PTSD coach. JMIR Ment Health. 2015;2(1):e7. doi: 10.2196/mental.3935.
20. Sarkar U, Gourley GI, Lyles CR, et al. Usability of commercially available mobile applications for diverse patients. J Gen Intern Med. 2016;31(12):1417-1426.
Neuromodulatory options for treatment-resistant depression
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.
Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6
Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.

Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6

Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
The emergence of treatment-resistant depression (TRD) poses a great clinical and public health challenge. There is no clear consensus on criteria to define TRD. The criteria range from failure to respond to 4 weeks of a single antidepressant to failure to respond to a single trial of electroconvulsive therapy (ECT).1
Neuromodulatory treatments for depression involve electrical stimulation of the brain through invasive or noninvasive methods. In this article, we discuss criteria for defining TRD, and compare the advantages and disadvantages of 4 neuromodulatory treatment options—ECT, vagus nerve stimulation (VNS), repetitive transcranial magnetic stimulation (rTMS), and deep brain stimulation (DBS)—for patients with depression who fail to respond to appropriate pharmacologic interventions (Table 1). Most of the studies we discuss selected patients who had severe depression and had not responded to numerous treatment trials.

Defining treatment resistance
Thase and Rush2 suggested progressive stages for categorizing TRD, ranging from Stage I (failure of at least 1 adequate trial of antidepressants) to Stage V (failure of adequate treatment with 2 selective serotonin reuptake inhibitors [SSRIs], a tricyclic antidepressant, a monoamine oxidase inhibitor, and a course of bilateral ECT). The Massachusetts General Hospital Staging Model suggested a quantitative scale to help characterize the degree of treatment resistance in which a higher score corresponds to a higher level of resistance.3 For every failed 6-week trial with adequate dose of an antidepressant, the patient is given a score of 1. The patient receives an extra .5 point for failure to respond to optimization of the dosage and augmentation with another medication. The patient also is given 3 points for failure to respond to ECT. Souery et al4,5 proposed a model in which they defined TRD as a failure to respond after ≥1 adequate antidepressant trials of ≥12 weeks.
Treatment resistance often is the result of inadequate treatment of depressive symptoms. Inadequate treatment includes an inadequate dose of antidepressants and/or an inadequate duration of treatment. Treatment of depression also is often complicated by medical (cardiovascular, neurologic, endocrine disorders) and psychiatric (substance abuse disorders, personality disorders) comorbidities (Table 2). Patients with such comorbidities are at increased risk of mortality, and have lower response rates and increased morbidity.6

Electroconvulsive therapy
ECT involves the application of electric current to induce a self-limiting seizure. It affects multiple brain functions to produce its antidepressant effects. Patients with depression have a reduced concentration of γ-aminobutyric acid (GABA) in their plasma, CSF, and cortex. ECT increases GABAergic transmission in cortical circuits as demonstrated by increased levels of GABA in the occipital cortex, which may be responsible for ECT’s antidepressant effects.7 Sensitization of the 5-HT1A receptors and increased dopamine receptor binding in the striatum also have been associated with the antidepressant action of ECT.8 The antidepressant effects of ECT also can be attributed to increased neuroplasticity, as evidenced by increased neurotrophic factors and cell proliferation in animal models.9 Dysfunction of the HPA axis has long been associated with depressive disorders; ECT improves this dysfunction, as evidenced by normalization of the dexamethasone suppression test in patients who receive ECT.7
The results of neuroimaging studies exploring the effects of ECT vary widely based on the specific neuroimaging method, population, and statistical methods used to assess the changes. Some of the most consistent findings include reduced glucose metabolism in the frontal brain regions; reduced glucose metabolism in the hippocampus and medial temporal lobes; and reduction in functional connectivity in the anterior cingulate, parietal, medical frontal, and dorsolateral prefrontal cortex (DLPFC).10
Randomized control trials (RCTs) have established the superiority of ECT over pharmacotherapy and sham ECT. Compared with other neuromodulatory treatments, ECT has higher remission rates. On average, the remission rate among patients receiving ECT whose depression did not respond to pharmacotherapy is approximately 48%; this increases to 64.9% among patients who previously had responded to a medication.11
Some earlier trials found bilateral ECT to be more effective than unilateral ECT.12 Recent studies suggest that high-dose unilateral ECT (6 times the seizure threshold) is as effective as bilateral ECT.13 Studies have shown no significant differences in efficacy or treatment outcomes between twice- and thrice-weekly ECT regimens. Some studies suggest that twice-weekly ECT may be associated with a lower risk of short-term cognitive impairment compared with thrice-weekly ECT.14
In highly refractory cases, the effects of ECT can be augmented by using pre-treatment strategies such as hyperventilation, which may increase the duration of the seizure, and remifentanil, which helps reduce the anticonvulsant effect of agents used for anesthesia.15 Advanced age, psychotic features, resistance to pharmacotherapy, and comorbid personality disorders predict poor response to ECT.16
Adverse effects. Concerns about cognitive deficits secondary to ECT may curtail its use. Retrograde and anterograde amnesia are the most common deficits observed acutely after ECT.12 Other commonly affected cognitive functions include processing speed, attention/working memory, verbal and visual episodic memory, spatial problem solving, and executive functioning. The specific patterns of these deficits (in terms of duration and severity) vary between studies. In general, high-dose, thrice-weekly ECT and bilateral ECT are associated with greater cognitive deficits, whereas twice-weekly ECT and unilateral ECT are associated with a lower risk of cognitive adverse effects.12 A recent meta-analysis by Semkovska and McLoughlin17 found that most cognitive deficits seen after ECT are limited to the first 3 days after treatment. The authors of this meta-analysis concluded that these impairments improve over time and approach baseline 2 weeks after treatment. In fact, some of these impairments (processing speed, working memory, anterograde memory, and some aspects of executive function) improved beyond baseline after 15 days of treatment.17 The need for anesthesia and associated potential adverse effects also are a cause of concern with ECT.
Combining ECT with medication. Several patient-specific factors, including medication regimen and comorbid medical conditions, need to be considered before using ECT in combination with pharmacotherapy. Although most antipsychotics are safe to use with ECT, concomitant use of agents with higher antihistaminic properties may increase the risk of delirium. The risk of delirium also is increased with the use of anticonvulsants and mood stabilizers (eg, lithium) because these agents increase the seizure threshold. The potential for drug interactions may affect the choice of the anesthetic agents. Also, SSRIs and serotonin-norepinephrine reuptake inhibitors can increase the duration of induced seizures.18
Vagus nerve stimulation
VNS, in which an implanted device stimulates the vagus nerve with electrical impulses, initially was used to reduce the frequency of seizures in patients with epilepsy and treatment-resistant partial onset seizures.19 VNS was FDA-approved for TRD in July 2005.20 One VNS system, the NCP System, consists of an implantable, multi-programmable generator, known as a pulse generator, that is subcutaneously placed in the anterior chest wall during an outpatient surgical procedure. Separate bipolar nerve-stimulating electrodes are surgically wrapped around the left cervical vagus nerve, and then connected to the generator via a tunneling procedure. A telemetric wand is subsequently linked to a portable computer and used to adjust stimulation parameters.21,22
Support for using VNS for TRD came from a multitude of investigations and observations. Harden et al23 and Elger et al24 prospectively evaluated epileptic patients with standard depression symptom severity rating scales. They found that VNS was associated with statistically significant improvements in mood that were not related to reductions in seizures.23,24
The mechanism of action of VNS is not clear. Earlier researchers had found evidence that VNS affected brain regions associated with norepinephrine25 and serotonin systems26; both of these neurotransmitters have been implicated in the pathophysiology of depression. Positron emission tomography studies conducted during VNS treatment of epilepsy showed metabolic changes in cortical and subcortical areas of the brain, including the amygdala, hippocampus, and cingulate gyrus, all structures implicated in the pathophysiology of mood disorders.27
Most studies conducted to evaluate the efficacy of VNS have been observational, looking at depression ratings before and after treatment with VNS. The short-term studies measured the difference in depression rating scales at baseline and after 10 weeks of treatment. In most of these studies, treatment with VNS resulted in a statistically significant drop in depression rating scales scores, such as on the Hamilton Depression Rating Scale (HAM-D). Based on the study design and number of study participants, response rates have varied from 13%28 to 40%,29 whereas remission rates have varied from 15.3%30 to 28%.31 More than one-half of the reduction in symptoms occurred after 6 weeks of treatment.30 In longer-term follow-up studies, the antidepressant effect generally was sustained over time. Response rates remained essentially unchanged, but the remission rates increased to approximately 29%.29 Only 1 RCT has compared patients with controls; it found no significant differences in the response or remission rates between active VNS and sham VNS.32 In this study, all patients had VNS implanted, but in the control group, the VNS was never turned on.32 In a meta-analysis conducted by Martin and Martín-Sánchez,33 31.8% (95% confidence interval [CI], 23.2% to 41.8%; P < .001) of patients treated with VNS had a significant reduction in HAM-D scores. The response rate in patients with TRD ranged from 27% to 37% and the remission rate was approximately 13%. In studies that followed patients over longer periods, both the remission and response rates increased over time.34
Recent evidence suggests that the effectiveness of VNS may depend on the stimulation level. A multi-center double-blind study randomized patients to receive either a low (0.25 mA current, 130-millisecond pulse width), medium (0.5e1.0 mA, 250 millisecond), or high (1.25e1.5 mA, 250 millisecond) dose of VNS.35 Although all dose levels were associated with improvement in symptoms, a statistically significant durability in response was associated with the medium- and high-dose treatments.
Adverse effects. VNS has no major adverse effects on cognitive functioning, and some studies have found improvement in executive functioning that corresponded to improvement in depressive symptoms.30 VNS also may result in improved sleep patterns as evidenced by EEG changes.31 The most commonly reported adverse effects include pain in the incision site, hoarseness of voice, throat pain, and neck pain.36
Repetitive transcranial magnetic stimulation
rTMS is a noninvasive technique that uses high-intensity magnetic impulses to stimulate cortical neurons. A magnetic field is produced when current passes through a coil, which in turn causes electrical stimulation in the cortical neurons that results in transient changes in the excitability of the cortical neurons.37 Although many stimulation parameters exist for TMS, high-frequency stimulation to the left prefrontal cortex (HFL-rTMS) and low-frequency stimulation to the right prefrontal cortex (LFR-rTMS) have been shown most efficacious for treating depression.38 High-frequency (5 Hz to 20 Hz) stimulation using rTMS increases cortical neuron excitability, whereas low-frequency (approximately 1 Hz) is associated with reduced cortical neuron excitability.39 The choice of targeting the DLPFC stems from a large body of functional neuroimaging studies that have shown reduction in activity/blood flow in the left DLPFC and abnormal activity/blood flow in the right DLPFC.40
There is no dearth of RCTs evaluating the efficacy of rTMS vs sham rTMS (where no magnetic stimulation was provided). In a meta-analysis of 8 RCTs, low-frequency rTMS applied to the right DLPFC was associated with a remission rate of approximately 34.6%, compared with a 9.7% remission rate with sham rTMS.41 A response rate of approximately 38.2% was observed with HFL-rTMS, compared with a response rate of 15.1% for sham rTMS.41
Gaynes et al42 conducted a meta-analysis to determine the efficacy of rTMS in TRD. They found that for patients with TRD, rTMs produced a response rate of 29% and a remission rate of 30%. In long-term, naturalistic, observational studies, the response rates and remission rates were much higher (58% and 37.1%, respectively).43 Over a 1-year follow-up, almost two-thirds of patients continued to meet criteria for response to treatment.44 Trials comparing HFL-rTMS and LFR-rTMS have found no significant differences in efficacy.45
Advanced age, psychotic symptoms, and a longer duration of the current depressive episode predict poor response to rTMS. Also, imaging studies have shown that a lower metabolism in cerebellar, temporal, anterior cingulate, and occipital parts of the brain correlate with better response to HFL-rTMS.46,47
Adverse effects. The major adverse effect associated with rTMS is the risk of inducing seizures, which is more commonly associated with high-frequency rTMS. Other common adverse effects include headache, facial muscle twitching, and tinnitus.37
Deep brain stimulation
DBS is an invasive stereotactic surgical procedure. It involves unilateral or bilateral placement of electrodes at neuroanatomical locations to deliver continuous stimulation from a subcutaneously implanted pulse generator.48 In the past, destructive surgical procedures were used to treat intractable depression. Surgeries such as anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leucotomy have been shown to effectively reduce depressive symptoms.49 The advantages of DBS over destructive procedures include the fact that DBS is reversible and that the stimulation levels can easily be adjusted, and the treatment can easily be stopped or restarted.
There is no consensus on the optimal anatomic locations for the electrode implantation in DBS. Electrodes have been implanted in the subcallosal cingulate gyrus, inferior thalamic peduncle, ventral capsule/ventral striatum, superolateral branch of the medial forebrain bundle (MFB), and nucleus accumbens.
The choice of anatomic locations stems from the large body of neuroimaging literature characterizing functional changes associated with acute depression and response to treatment. The electrode placement targets “nodes” that form an integral part of the affected neural circuits that are responsible for regulating depressive symptoms.50 Increased metabolic activity and blood flow to the subgenual cingulate gyrus and reduction in the blood flow to the DLPFC and the striatum have been associated with active depressed states. Response to antidepressant treatment has been associated with reversal of these findings.51 Functional magnetic resonance imaging studies have consistently shown increased activity in the amygdala in response to negative stimuli among patients with depression.
Regardless of the site of electrode placement, studies have reported symptomatic improvement among patients with depression who are treated with DBS. In 2 case reports, the electrode was implanted in the inferior thalamic peduncle.52,53 Each study had 1 participant, and each patient remitted.52,53
Placement of the electrodes in the nucleus accumbens resulted in a response rate of 45% in 1 study,54 whereas in a different study, all patients reported improvement in anhedonia.55 A response rate of 71% and a remission rate of 35% were observed in a study in which the electrode was implanted in the ventral capsule/ventral striatum area.56
Berlim et al57 published a systematic review and exploratory meta-analysis of studies in which the electrode had been implanted in the subgenual cingulate cortex. At 12 months, the response rate was 39.9% (95% CI, 28.4% to 52.8%), and 26.3% (95% CI, 13% to 45.9%) of patients achieved remission. The most significant drop in depression scores was observed 3 to 6 months after the surgery. No significant change in scores was observed between 6 to 12 months after surgery.57
The MFB, specifically the superolateral branch, is emerging as an exciting new target for electrode placement in DBS. Schlaepfer et al58 studied the effects of electrodes implanted bilaterally in the superolateral branch of the MFB. They observed an almost 50% reduction in symptoms by Day 7, and at the last follow-up visit (12 to 33 weeks) 4 of the 6 patients had achieved remission.58 In a recent systematic review, Gálvez et al59 found most studies had high response/remission rates without any significant adverse effects. In a recent study of DBS targeting the MFB, 3 of 4 patients had a >50% reduction in Montgomery-Åsberg Depression Rating Scale scores at the end of first week. Although 1 patient withdrew, 2 of the other 3 patients continued to report a >80% reduction in depressive symptoms, even at Week 26.60
Accurate localization of target areas (white matter tracts) and subsequent electrode placement might be an important factor governing treatment response. Riva-Posse et al61 found that clinical response was seen when the electrodes stimulated 3 specific white matter bundles. Interestingly, nonresponders were converted to responders simply by changing the position of the electrodes to include these white matter tracts.61
Adverse effects. The most common adverse effects noted during studies of DBS include pain at the site of implantation and wound infection. Other adverse effects include lead fracture, transient dysphagia, and other hardware-related problems.49
Sorting out the evidence
In the absence of head-to-head trials, it is difficult to establish a hierarchal algorithm for use of the 4 neuromodulatory treatments discussed in the article. If we were to base our decision solely on the current literature, ECT by far has the most evidence and highest remission rates.11 We can reduce the risk of cognitive deficits by using twice-weekly instead of thrice-weekly ECT, or by using unilateral instead of bilateral ECT.12 Another strategy for reducing adverse effects associated with long-term maintenance ECT is by using it in combination with VNS. ECT and VNS can be used safely concomitantly; ECT can be used to treat acutely worsening depression, and VNS for maintaining the antidepressant effect.62
Aside from ECT, rTMS is the only other treatment that has evidence from RCTs. Although the remission rates are not as high as ECT, its preferable adverse effects profile, noninvasive nature, and comparative low cost (compared with surgical procedures) make it a favorable choice. The Canadian Network for Mood and Anxiety Treatment guidelines suggest rTMS as the first-line treatment for patients who do not respond to pharmacologic treatments.63 ECT can be considered second-line treatment unless the patient has acute suicidal ideation, catatonia, psychotic features, greater treatment resistance, or physical deterioration, in which case ECT should be tried before TMS.63
Among the invasive options, VNS has more evidence and is FDA-approved for TRD. However, DBS has shown great promise in early studies, with remission rates as high as 35%.56 DBS has the advantage of being reversible, and the amount of stimulation can be adjusted easily. Despite early promise, more research is needed before DBS can be widely used in clinical settings.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
3. Petersen T, Papakostas GI, Posternak MA, et al. Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol. 2005;25(4):336-341.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Souery D, Amsterdam J, de Montigny C, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1-2):83-91.
6. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol. Psychiatry. 2003;54(3):177-180.
7. Sanacora G, Mason GF, Rothman DL, et al. Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160(3):577-579.
8. Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219(1):20-26.
9. Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27(18):4894-4901.
10. Abbott CC, Gallegos P, Rediske N et al. A review of longitudinal electroconvulsive therapy: neuroimaging investigations. J Geriatr Psychiatry Neurol. 2014;27(1):33-46.
11. Heijnen WT, Birkenhäger TK, Wierdsma AI, et al. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616-619.
12. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
13. Semkovska M, Landau S, Dunne R et al. Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): a pragmatic, randomized, non-inferiority trial. Am J Psychiatry. 2016;173(4):408-417.
14. Charlson F, Siskind D, Doi SA, et al. ECT efficacy and treatment course: a systematic review and meta-analysis of twice vs thrice weekly schedules. J Affect Disord. 2012;138(1-2):1-8.
15. Loo CK, Kaill A, Paton P, et al. The difficult-to-treat electroconvulsive therapy patient—strategies for augmenting outcomes. J Affect Disord. 2010;124(3):219-227.
16. de Vreede IM, Burger H, van Vliet IM. Prediction of response to ECT with routinely collected data in major depression. J Affect Disord. 2005;86(2-3):323-327.
17. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
18. Baghai TC, Marcuse A, Brosch M, et al. The influence of concomitant antidepressant medication on safety, tolerability and clinical effectiveness of electroconvulsive therapy. World J Biol Psychiatry. 2006;7(2):82-90.
19. Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616-626.
20. Nemeroff CB, Mayberg HS, Krahl SE, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345-1355.
21. Matthews K, Eljamel MS. Vagus nerve stimulation and refractory depression: please can you switch me on doctor? Br J Psychiatry. 2003;183:181-183.
22. George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83.
23. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99.
24. Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203-210.
25. Krahl SE, Clark KB, Smith DC, et al. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709-714.
26. Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221-227.
27. Henry TR, Bakay RA, Votaw JR, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39(9):983-990.
28. O’Keane V, Dinan TG, Scott L, et al. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58(12):963-968.
29. Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47(4):276-286.
30. Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25(5):713-728.
31. Armitage R, Husain M, Hoffmann R, et al. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54(5):475-482.
32. Rush AJ, Marangell LB, Sackeim HA, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347-354.
33. Martin JL, Martín-Sánchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry. 2012;27(3):147-155.
34. Shah A, Carreno FR, Frazer A. Therapeutic modalities for treatment resistant depression: focus on vagal nerve stimulation and ketamine. Clin Psychopharmacol Neurosci. 2014;12(2):83-93.
35. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631-640.
36. Daban C, Martinez-Aran A, Cruz N, et al. Safety and efficacy of vagus nerve stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008;110(1-2):1-15.
37. Eitan R, Lerer B. Nonpharmacological, somatic treatments of depression: electroconvulsive therapy and novel brain stimulation modalities. Dialogues Clin Neurosci. 2006;8(2):241-258.
38. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621-631.
39. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584-2596.
40. Fitzgerald PB, Oxley TJ, Laird AR, et al. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148(1):33-45.
41. Berlim MT, Van den Eynde F, Daskalakis ZJ. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543-551.
42. Gaynes BN, Lloyd SW, Lux L, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression. J Clin Psychiatry. 2014;75(5):477-489; quiz 489.
43. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
44. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder. J Clin Psychiatry. 2014;75(12):1394-1401.
45. Fitzgerald PB, Hoy K, Daskalakis ZJ, et al. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229-234.
46. Dumas R, Padovani R, Richieri R, et al. Repetitive transcranial magnetic stimulation in major depression: response factor [in French]. Encephale. 2012;38(4):360-368.
47. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int. J. Neuropsychopharmacol. 2006;9(6):641-654.
48. Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502-510.
49. Taghva AS, Malone DA, Rezai AR. Deep brain stimulation for treatment-resistant depression. World Neurosurg. 2013;80(3-4):S27.e17-S27.e24.
50. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
51. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
52. Jiménez F, Velasco F, Salín-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir Suppl. 2007;97(pt 2):393-398.
53. Jiménez F, Velasco F, Salin-Pascual R, et al. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57(3):585-593; discussion 585-593.
54. Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110-116.
55. Schlaepfer TE, Bewernick BH, Kayser S, et al. Deep brain stimulation of the human reward system for major depression—rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39(6):1303-1314.
56. Malone DA Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65(4):267-275.
57. Berlim MT, McGirr A, Van den Eynde F, et al. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
58. Schlaepfer TE, Bewernick BH, Kayser S, et al. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73(12):1204-1212.
59. Gálvez JF, Keser Z, Mwangi B, et al. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Prog Neuropsychopharmacol Biol Psychiatry. 2015;58:59-70.
60. Fenoy AJ, Schulz P, Selvaraj. Deep brain stimulation of the medial forebrain bundle: distinctive responses in resistant depression. J Affect Disord. 2016;203:143-151.
61. Riva-Posse P, Choi KS, Holtzheimer PE, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76(12):963-969.
62. Burke MJ, Husain MM. Concomitant use of vagus nerve stimulation and electroconvulsive therapy for treatment-resistant depression. J ECT. 2006;22(3):218-222.
63. Milev R V, Giacobbe P, Kennedy SH, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561-575.
Admission to an inpatient psychiatry unit or a medical unit? Consider 3 Ms and 3 Ps
Hospital psychiatrists often are asked whether a patient with comorbid medical and psychiatric illnesses should be admitted to an inpatient psychiatry unit or to a medical unit. Psychiatric units vary widely in their capacity to manage patients’ medical conditions. Medical comorbidity also is associated with longer psychiatric hospitalizations.1 The decision of where to admit may be particularly challenging when presented with a patient with delirium, which often mimics primary psychiatric illnesses such as depression but will not resolve without treatment of the underlying illness. While diagnosis and treatment of delirium typically occur in the hospital setting, 1 study found that approximately 15% of 199 psychiatric inpatients were delirious and that these patients had hospital stays that were approximately 62% longer than those without delirium.2
When you need to determine whether a patient should be admitted to an inpatient psychiatry unit with a medical consult or vice versa, consider the following 3 Ms and 3 Ps.
Medications. Can medications, including those that are given intravenously or require serum monitoring, be administered on the psychiatric unit? Can the medical unit administer involuntary psychotropics?
Mobility. Does the patient require assistance with mobility? Does the patient pose a fall risk? A physical therapy consult may be helpful.
Monitoring. Suicide risk is the most common indication for patient sitters.3 Would a patient sitter be needed for the patient? On the other hand, can the psychiatry unit manage telemetry, frequent vital signs, or infectious disease precautions?
People. Would the patient benefit from the therapeutic milieu and specialized staff of an inpatient psychiatry unit?
Prognosis. What ongoing medical and psychiatric management is required? What are the medical and psychiatric prognoses?
Placement. To where will the patient be transferred after hospitalization? How does admission to inpatient psychiatry vs medical impact the ultimate disposition?
Help the treatment team make the decision
Determining the ideal patient placement often evokes strong feelings among treatment teams. Psychiatrists can help facilitate the conversation by asking the questions outlined above, and by keeping in mind, “What is best for this patient?”
1. Rodrigues-Silva N, Ribeiro L. Impact of medical comorbidity in psychiatric inpatient length of stay. J Ment Health. 2017:1-5 [epub ahead of print].
2. Ritchie J, Steiner W, Abrahamowicz M. Incidence of and risk factors for delirium among psychiatric inpatients. Psychiatr Serv. 1996;47(7):727-730.
3. Solimine S, Takeshita J, Goebert D, et al. Characteristics of patients with constant observers. Psychosomatics. 2018;59(1):67-74.
Hospital psychiatrists often are asked whether a patient with comorbid medical and psychiatric illnesses should be admitted to an inpatient psychiatry unit or to a medical unit. Psychiatric units vary widely in their capacity to manage patients’ medical conditions. Medical comorbidity also is associated with longer psychiatric hospitalizations.1 The decision of where to admit may be particularly challenging when presented with a patient with delirium, which often mimics primary psychiatric illnesses such as depression but will not resolve without treatment of the underlying illness. While diagnosis and treatment of delirium typically occur in the hospital setting, 1 study found that approximately 15% of 199 psychiatric inpatients were delirious and that these patients had hospital stays that were approximately 62% longer than those without delirium.2
When you need to determine whether a patient should be admitted to an inpatient psychiatry unit with a medical consult or vice versa, consider the following 3 Ms and 3 Ps.
Medications. Can medications, including those that are given intravenously or require serum monitoring, be administered on the psychiatric unit? Can the medical unit administer involuntary psychotropics?
Mobility. Does the patient require assistance with mobility? Does the patient pose a fall risk? A physical therapy consult may be helpful.
Monitoring. Suicide risk is the most common indication for patient sitters.3 Would a patient sitter be needed for the patient? On the other hand, can the psychiatry unit manage telemetry, frequent vital signs, or infectious disease precautions?
People. Would the patient benefit from the therapeutic milieu and specialized staff of an inpatient psychiatry unit?
Prognosis. What ongoing medical and psychiatric management is required? What are the medical and psychiatric prognoses?
Placement. To where will the patient be transferred after hospitalization? How does admission to inpatient psychiatry vs medical impact the ultimate disposition?
Help the treatment team make the decision
Determining the ideal patient placement often evokes strong feelings among treatment teams. Psychiatrists can help facilitate the conversation by asking the questions outlined above, and by keeping in mind, “What is best for this patient?”
Hospital psychiatrists often are asked whether a patient with comorbid medical and psychiatric illnesses should be admitted to an inpatient psychiatry unit or to a medical unit. Psychiatric units vary widely in their capacity to manage patients’ medical conditions. Medical comorbidity also is associated with longer psychiatric hospitalizations.1 The decision of where to admit may be particularly challenging when presented with a patient with delirium, which often mimics primary psychiatric illnesses such as depression but will not resolve without treatment of the underlying illness. While diagnosis and treatment of delirium typically occur in the hospital setting, 1 study found that approximately 15% of 199 psychiatric inpatients were delirious and that these patients had hospital stays that were approximately 62% longer than those without delirium.2
When you need to determine whether a patient should be admitted to an inpatient psychiatry unit with a medical consult or vice versa, consider the following 3 Ms and 3 Ps.
Medications. Can medications, including those that are given intravenously or require serum monitoring, be administered on the psychiatric unit? Can the medical unit administer involuntary psychotropics?
Mobility. Does the patient require assistance with mobility? Does the patient pose a fall risk? A physical therapy consult may be helpful.
Monitoring. Suicide risk is the most common indication for patient sitters.3 Would a patient sitter be needed for the patient? On the other hand, can the psychiatry unit manage telemetry, frequent vital signs, or infectious disease precautions?
People. Would the patient benefit from the therapeutic milieu and specialized staff of an inpatient psychiatry unit?
Prognosis. What ongoing medical and psychiatric management is required? What are the medical and psychiatric prognoses?
Placement. To where will the patient be transferred after hospitalization? How does admission to inpatient psychiatry vs medical impact the ultimate disposition?
Help the treatment team make the decision
Determining the ideal patient placement often evokes strong feelings among treatment teams. Psychiatrists can help facilitate the conversation by asking the questions outlined above, and by keeping in mind, “What is best for this patient?”
1. Rodrigues-Silva N, Ribeiro L. Impact of medical comorbidity in psychiatric inpatient length of stay. J Ment Health. 2017:1-5 [epub ahead of print].
2. Ritchie J, Steiner W, Abrahamowicz M. Incidence of and risk factors for delirium among psychiatric inpatients. Psychiatr Serv. 1996;47(7):727-730.
3. Solimine S, Takeshita J, Goebert D, et al. Characteristics of patients with constant observers. Psychosomatics. 2018;59(1):67-74.
1. Rodrigues-Silva N, Ribeiro L. Impact of medical comorbidity in psychiatric inpatient length of stay. J Ment Health. 2017:1-5 [epub ahead of print].
2. Ritchie J, Steiner W, Abrahamowicz M. Incidence of and risk factors for delirium among psychiatric inpatients. Psychiatr Serv. 1996;47(7):727-730.
3. Solimine S, Takeshita J, Goebert D, et al. Characteristics of patients with constant observers. Psychosomatics. 2018;59(1):67-74.
How to handle unsolicited e-mails
The ubiquitous use of e-mail has opened the proverbial “Pandora’s box” of access to psychiatrists. Our e-mail addresses are readily available online via search engines or on hospital Web sites. E-mail has become a convenient method of communicating with patients; however, it also has resulted in a proliferation of unsolicited e-mails sent to physicians from people they don’t know seeking professional advice.1 If you publish medical literature or make media appearances, you may be contacted by such individuals requesting your expertise.
Unsolicited e-mails present psychiatrists with ethical and legal quandaries that force them to consider how they can balance the human reflex to offer assistance against the potential ramifications of replying. These conundrums include:
- whether the sender is an actual person, and whether he or she is asking for advice
- the risks of replying vs not replying
- the possibility that there is a plausible crisis or danger to the sender or others
- the potential for establishing a doctor–patient relationship by replying
- the legal liability that might be incurred by replying.2
Take preemptive measures
There is guidance on how to e-mail your patients and respond to solicited e-mails, but there is a dearth of literature on how to respond to unsolicited e-mails. Anecdotal reports and limited literature suggest several possible measures you could take for managing unsolicited e-mails:
- Establish a policy of never opening unsolicited e-mails
- Create a strict junk-mail filter to prevent unsolicited e-mails from being delivered to your inbox
- Set up an automatic reply stating that unwanted or unsolicited e-mails will not be read and/or that no reply will be provided
- Read unsolicited e-mails, but immediately delete them without replying
- Acknowledge the sender in a reply, but state that you are unable to assist and decline further contact
- Send a generic reply clarifying that you are unable to provide medical assistance, and encourage the sender to seek help locally.2
Despite the urge to help, consider the consequences
In addition to taking up valuable time, unsolicited e-mails create legal and ethical predicaments that could subject you to legal liability if you choose to reply. Even though your intentions may be altruistic and you want to be helpfu
1. D’Alessandro DM, D’Alessandro MP, Colbert S. A proposed solution for addressing the challenge of patient cries for help through an analysis of unsolicited electronic email. Pediatrics. 2000;105(6):E74.
2. Friedman SH, Appel JM, Ash P, et al. Unsolicited e-mails to forensic psychiatrists. J Am Acad Psychiatry Law. 2016;44(4):470-478.
The ubiquitous use of e-mail has opened the proverbial “Pandora’s box” of access to psychiatrists. Our e-mail addresses are readily available online via search engines or on hospital Web sites. E-mail has become a convenient method of communicating with patients; however, it also has resulted in a proliferation of unsolicited e-mails sent to physicians from people they don’t know seeking professional advice.1 If you publish medical literature or make media appearances, you may be contacted by such individuals requesting your expertise.
Unsolicited e-mails present psychiatrists with ethical and legal quandaries that force them to consider how they can balance the human reflex to offer assistance against the potential ramifications of replying. These conundrums include:
- whether the sender is an actual person, and whether he or she is asking for advice
- the risks of replying vs not replying
- the possibility that there is a plausible crisis or danger to the sender or others
- the potential for establishing a doctor–patient relationship by replying
- the legal liability that might be incurred by replying.2
Take preemptive measures
There is guidance on how to e-mail your patients and respond to solicited e-mails, but there is a dearth of literature on how to respond to unsolicited e-mails. Anecdotal reports and limited literature suggest several possible measures you could take for managing unsolicited e-mails:
- Establish a policy of never opening unsolicited e-mails
- Create a strict junk-mail filter to prevent unsolicited e-mails from being delivered to your inbox
- Set up an automatic reply stating that unwanted or unsolicited e-mails will not be read and/or that no reply will be provided
- Read unsolicited e-mails, but immediately delete them without replying
- Acknowledge the sender in a reply, but state that you are unable to assist and decline further contact
- Send a generic reply clarifying that you are unable to provide medical assistance, and encourage the sender to seek help locally.2
Despite the urge to help, consider the consequences
In addition to taking up valuable time, unsolicited e-mails create legal and ethical predicaments that could subject you to legal liability if you choose to reply. Even though your intentions may be altruistic and you want to be helpfu
The ubiquitous use of e-mail has opened the proverbial “Pandora’s box” of access to psychiatrists. Our e-mail addresses are readily available online via search engines or on hospital Web sites. E-mail has become a convenient method of communicating with patients; however, it also has resulted in a proliferation of unsolicited e-mails sent to physicians from people they don’t know seeking professional advice.1 If you publish medical literature or make media appearances, you may be contacted by such individuals requesting your expertise.
Unsolicited e-mails present psychiatrists with ethical and legal quandaries that force them to consider how they can balance the human reflex to offer assistance against the potential ramifications of replying. These conundrums include:
- whether the sender is an actual person, and whether he or she is asking for advice
- the risks of replying vs not replying
- the possibility that there is a plausible crisis or danger to the sender or others
- the potential for establishing a doctor–patient relationship by replying
- the legal liability that might be incurred by replying.2
Take preemptive measures
There is guidance on how to e-mail your patients and respond to solicited e-mails, but there is a dearth of literature on how to respond to unsolicited e-mails. Anecdotal reports and limited literature suggest several possible measures you could take for managing unsolicited e-mails:
- Establish a policy of never opening unsolicited e-mails
- Create a strict junk-mail filter to prevent unsolicited e-mails from being delivered to your inbox
- Set up an automatic reply stating that unwanted or unsolicited e-mails will not be read and/or that no reply will be provided
- Read unsolicited e-mails, but immediately delete them without replying
- Acknowledge the sender in a reply, but state that you are unable to assist and decline further contact
- Send a generic reply clarifying that you are unable to provide medical assistance, and encourage the sender to seek help locally.2
Despite the urge to help, consider the consequences
In addition to taking up valuable time, unsolicited e-mails create legal and ethical predicaments that could subject you to legal liability if you choose to reply. Even though your intentions may be altruistic and you want to be helpfu
1. D’Alessandro DM, D’Alessandro MP, Colbert S. A proposed solution for addressing the challenge of patient cries for help through an analysis of unsolicited electronic email. Pediatrics. 2000;105(6):E74.
2. Friedman SH, Appel JM, Ash P, et al. Unsolicited e-mails to forensic psychiatrists. J Am Acad Psychiatry Law. 2016;44(4):470-478.
1. D’Alessandro DM, D’Alessandro MP, Colbert S. A proposed solution for addressing the challenge of patient cries for help through an analysis of unsolicited electronic email. Pediatrics. 2000;105(6):E74.
2. Friedman SH, Appel JM, Ash P, et al. Unsolicited e-mails to forensic psychiatrists. J Am Acad Psychiatry Law. 2016;44(4):470-478.