User login
Substance abuse among older adults: A growing problem
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
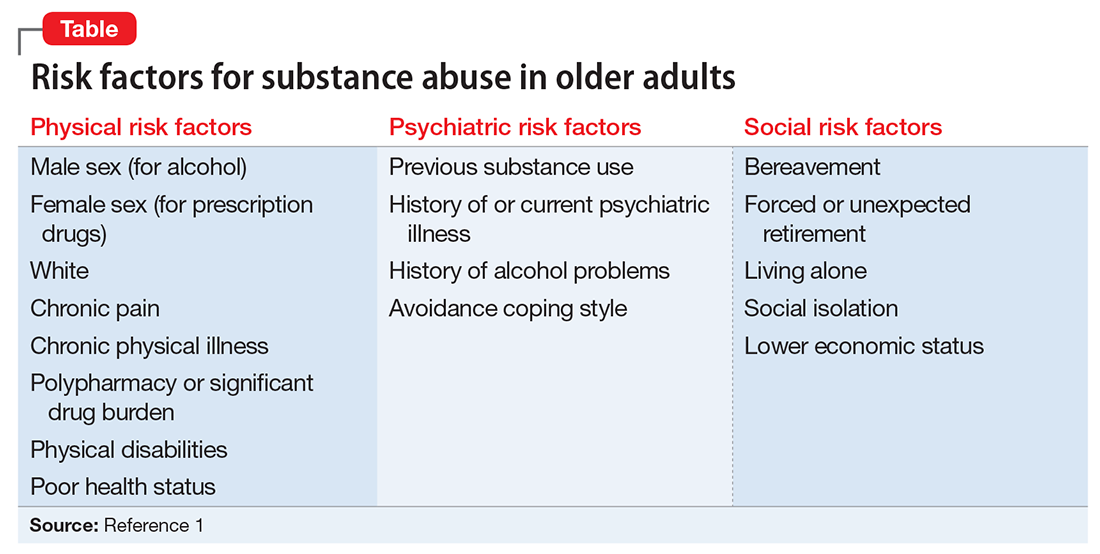
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.
Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.

Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
Baby Boomers—a term used to refer to individuals born in the United States between 1946 and 1964—are now approaching old age. Surprisingly, these older adults are using illicit substances in a pattern not seen in prior generations of older adults, including developing substance use disorders (SUDs) at increasingly higher rates; in previous generations, the prevalence of such disorders typically lowered with advancing age.
This article discusses how to recognize and treat SUDs in older adults. Alcohol is the most commonly used substance among older adults,1 and there is a largebody of literature describing the identification and treatment of alcohol-related disorders in these patients. Therefore, this article will instead focus on older adults’ use of illicit substances, including marijuana, cocaine, and heroin.
Epidemiology
Prior clinical data regarding substance abuse in older adults focused on alcohol, prescription drugs, nicotine, and caffeine.2 In the past, compared with younger adults, older adults had lower rates of alcohol and other illicit drug use.3,4 Baby Boomers appear to be defying this trend.
A 2013 Substance Abuse and Mental Health Services Administration survey found that the percentage of adults ages 50 to 64 who used illicit substances increased from 2.7% in 2002 to 6.0% in 2013.5 Specifically, during that time, past-month illicit substance use increased from 3.4% to 7.9% among those ages 50 to 54, from 1.9% to 5.7% among those ages 55 to 59, and from 2.5% to 3.9% among those ages 60 to 64.5
More recently, a 2014 study of geriatric patients found that of the 1,302 patients age ≥65 admitted to a Level 1 trauma center, 48.3% had a positive urine drug screen.6 Someresearchers have estimated that 5.7 million older adults will require treatment for a substance use disorder in 2020, which is roughly double the 2.8 million who had an SUD in 2002 to 2006.7
Risk factors and patterns of substance abuse
Individual, social, and familial factors can contribute to substance use and abuse in late life. The Table1 outlines some of the potential risk factors for older adults associated with the use of illicit substances. Substance abuse among older adults can be divided into 2 broad categories: early onset (starting before age 50) and late onset (starting after age 50).8 While data are limited, in general, early-onset use is a more common pattern; late-onset use represents an estimated <10% of substance use among older adults. The factors that lead some adults to continue substance use in late life, or to begin substance use later in life, have not been thoroughly evaluated.

Although older adults may abuse a wide variety of illicit substances, here we describe their use of marijuana, cocaine, and heroin.
Marijuana use has changed substantially in the last decade. While marijuana is illegal under federal law, as of November 2017, 29 states had legalized marijuana for medicinal purposes and 7 states and the District of Columbia had legalized it for recreational use. The increased legal and social acceptance of marijuana has led to new businesses and methods of use beyond smoking. New types of marijuana products include edible substances, tinctures, and oils that can be vaporized and inhaled.
In addition to euphoria and relaxation, the effects of marijuana use include increased latency time and decreased ability to respond to stimuli.2 Nonpsychiatric effects of marijuana include shallow breathing, weakened immune system, and increasing cardiac workload.2 The latter effect is especially important for older adults, many of whom may have preexisting cardiac illness and may be more likely to experience an adverse cardiac event as a result of marijuana use.2 Older adults who begin to use marijuana in late life may do so not primarily as a social activity, but more likely to experience the drug’s potentially beneficial effects on pain or appetite.2 For more on theuse of marijuana for these reasons, see “Medical marijuana: Do the benefits outweigh the risks?” in
Cocaine. Although cocaine is a CNS stimulant that causes a short-lived euphoria, its adverse effects impact many body systems.9 Myocardial infarction (MI) secondary to coronary artery vasospasms, stroke (hemorrhagic and ischemic), seizures, psychosis, aortic dissection, and acute renal injury are some of the most severe complications. Acute MI is the most frequent and severe cardiovascular complication seen among abusers.10 Cocaine use can cause dizziness, restlessness, headache, mydriasis, and anxiety.
In a pilot study, Kalapatapu et al11 compared the effects of cocaine abuse in younger vs older users. They found that older users had similar patterns of cocaine abuse in terms of the amount of cocaine used and frequency of use.11 They also found that specific cognitive functions, including psychomotor speed, attention, and short-term memory, are particularly sensitive to the combined effects of aging and cocaine abuse.11
Heroin is an opioid and a CNS depressant. Common effects include slowed heart rate, decreased blood pressure, and decreased respiration rate. Chronic heroin users show an overall decrease in immune system functioning12; this deficit might be particularly pronounced in an older person whose immune system functioning has already begun to decline as a result of aging. In recent years, as is the case with younger substance users, prescription opioids have replaced heroin as the opioid of choice among older users. However, for some early-onset heroin users, the use of this particular drug becomes well entrenched and unlikely to change, even in late life. Each year of heroin use increases the likelihood of continued use the next year by approximately 3%.2 Some research suggests that older heroin users do not decrease their use over time, and face many of the same risks as younger users, including poorer physical and mental health, severe physical disability, and mortality.13
Challenges to recognizing the problem
There are no screening protocols in the clinical setting that are designed specifically for detecting illicit substance abuse among older adults. Furthermore, diagnosis can be easily overlooked because the signs and symptoms of illicit substance use can be mistaken for other illnesses. To complicate matters further, older adults often do not disclose their substance use, understate it, or even try to explain away their symptoms.1 Many older adults live alone, which may increase their risk of receiving no treatment.14
Older adults generally experience reduced tolerance to the effects of illicit substances because of age-related physiologic changes, such as decreases in renal functioning, motor functioning, and cardiac output; altered liver metabolism of certain drugs; and elevated blood glucose levels.15 As a result, symptoms of illicit substance use could be mistaken for dementia or other forms of cognitive impairment.1,16
Although not designed specifically for older adults, an evidence-based screening instrument, such as the CAGE Questionnaire Adapted to Include Drugs, may be helpful in identifying substance abuse in these patients. Urine and/or serum drug screening, along with obtaining a comprehensive history from a trustworthy source, is useful for diagnosis.
Pharmacologic treatments
Research evaluating the use of medication for treating substance abuse specifically in older adults is extremely limited; studies have focused primarily on younger patients or mixed-age populations. Treatments that have been shown to be effective for younger patients may or may not be effective for older adults.
Marijuana. There are no FDA-approved treatments for marijuana abuse. An open-label study found that N-acetylcysteine, 1,200 mg twice a day, resulted in a significant reduction in marijuana craving as measured by the 12-item version of the Marijuana Craving Questionnaire.17 In a double-blinded placebo-controlled study, adolescents who were dependent on marijuana who received N-acetylcysteine, 1,200 mg twice a day, were more than twice likely to stop marijuana use compared with those who received placebo.18 Some researchers have proposed that N-acetylcysteine may prevent continued use of marijuana via glutamate modulation in the nucleus accumbens. Animal models have demonstrated that chronic drug self-administration downregulates the cystine-glutamate exchanger in the nucleus accumbens, and that N-acetylcysteine upregulates this exchanger, which reduces reinstatement of drug seeking.Further studies are needed to verify this speculation.
Cocaine. There are no FDA-approved treatments for cocaine abuse. No specific treatment approach has been found to be consistently effective.
A potential “cocaine vaccine” called TA-CD, which is made from succinyl norcocaine conjugated to cholera toxin, is being evaluated. An initial study had promising results, finding a significant reduction in cocaine use among those who received TA-CD.19 A later double-blinded placebo-controlled study only partially replicated the efficacy found in the initial study.20
Currently, other cocaine treatments are also being investigated. An enzyme to rapidly metabolize cocaine is being evaluated.21 So far, none of these treatments have targeted older adults, and there may be age-specific issues to consider if these approaches eventually receive FDA approval.
Heroin. Several FDA-approved medications are available for treating dependency to heroin and other opioids, including naltrexone, buprenorphine, and methadone, but none have been studied specifically in older adults. Some studies of transdermal buprenorphine for treating chronic pain in older adults have concluded that this formulation may offer advantages for older patients.22,23 Compared with oral or sublingual buprenorphine, the transdermal formulation avoids the first-pass effect in the liver, thus greatly increasing bioavailability of the drug; avoids renal metabolism; and offers greater tolerability in patients with mild to moderate hepatic impairment.22,23 However, transdermal buprenorphine has been approved only for the treatment of pain. These beneficial aspects of transdermal buprenorphine may be applicable to older opioid users, but no age-specific studies of buprenorphine for treating opioid abuse have been conducted.
Nonpharmacologic treatments
The same psychotherapeutic treatments used to treat younger patients with SUDs may be appropriate for older adults. Older patients may experience feelings of isolation and shame related to needing treatment for substance abuse. These factors in treatment of older patients often are overcome by group psychotherapy. Self-help programs, such as Narcotics Anonymous or Alcoholics Anonymous, and group therapy also may be options.
On the other hand, individual psychotherapy, such as cognitive-behavioral therapy (CBT), interpersonal therapy, and psychodynamic therapy, can provide a private and confidential environment for older adults who are less social.24
The highly structured nature of CBT may be well suited to older adults who have memory difficulties.1 A study of 110 older veterans with substance abuse problems found evidence for the effectiveness of group CBT among these patients.25 All but 8 participants in this study were age ≥65. The intervention consisted of 16 weekly group sessions that began with analysis of substance use behavior to determine high-risk situations for use, followed by a series of modules to teach skills for coping with social pressure, being at home and alone, feelings of depression and loneliness, anxiety and tension, anger and frustration, cues for substance use, and other factors. Approximately 44% (49 of 110) completed treatment (≥13 sessions). Approximately 55% of those who completed the treatment were abstinent at 6-month follow-up.25
Don’t assume your older patient is not using illicit substances
It is a myth that older adults do not use and abuse illicit substances. Illicit drug use among older adults is increasing. Older adults with SUDs may not present with the same symptoms as their younger counterparts, and thus it may be difficult to identify the problem. Maintain a high index of suspicion regarding the use of illicit substances in these patients.
Treatment options are generally limited and health care settings offer few interventions designed specifically for older adults. In general, proper identification of SUDs and targeted treatment can highly improve outcomes.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
1. Kuerbis A, Sacco P, Blazer DG, et al. Substance abuse among older adults. Clin Geriatr Med. 2014;30(3):629-654.
2. Taylor MH, Grossberg GT. (2012). The growing problem of illicit substance abuse in the elderly: a review. Prim Care Companion CNS Disord. 2012;14(4):PCC.11r01320. doi: 10.4088/PCC.11r01320.
3. Cummings SM, Bride B, Rawlings-Shaw AM. Alcohol abuse treatment for older adults: a review of recent empirical research. J Evid Based Soc Work. 2006;3(1):79-99.
4. Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: summary of national findings, NSDUH Series H-46, HHS Publication No (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
5. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
6. Ekeh AP, Parikh P, Walusimbi MS, et al. The prevalence of positive drug and alcohol screens in elderly trauma patients. Subst Abus. 2014;35(1):51-55.
7. Wu LT, Blazer DG. Illicit and nonmedical drug use among older adults: a review. J Aging Health. 2011;23(3):481-504.
8. Roe B, Beynon C, Pickering L, et al. Experiences of drug use and ageing: health, quality of life, relationship and service implications. J Adv Nurs. 2010;66(9):1968-1979.
9. Zimmerman JL. Cocaine intoxication. Crit Care Clin. 2012;28(4):517-526.
10. Weber JE, Chudnofsky CR, Boczar M, et al. Cocaine-associated chest pain: how common is myocardial infarction? Acad Emerg Med. 2000;7(8):873-877.
11. Kalapatapu RK, Vadhan NP, Rubin E, et al. A pilot study of neurocognitive function in older and younger cocaine abusers and controls. Am J Addict. 2011;20(3):228-239.
12. Edelman EJ, Cheng DM, Krupitsky EM, et al. Heroin use and HIV disease progression: results from a pilot study of a Russian cohort. AIDS Behav. 2015;19(6):1089-1097.
13. Darke S, Mills KL, Ross J, et al. The ageing heroin user: career length, clinical profile and outcomes across 36 months. Drug Alcohol Rev. 2009;28(3):243-249.
14. West LA, Cole S, Goodkind D, et al. U.S. Census Bureau, P23-212. 65+ in the United States: 2010. Washington, DC: United States Census Bureau; 2014.
15. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;135(6):434-440.
16. Ruiz P, Strain EC, Langrod JG. The substance abuse handbook. Philadelphia, PA: Wolters Kluwer Health; 2007.
17. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
18. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
19. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116-1123
20. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42-47.
21. Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Ther. 2004;310(3):1046-1052.
22. Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clin Interv Aging. 2008;3(3):421-430.
23. Al-Tawil N, Odar-Cederlöf I, Berggren AC, et al. Pharmacokinetics of transdermal buprenorphine patch in the elderly. Eur J Clin Pharmacol. 2013;69(2):143-149.
24. Schultz SK, Arndt S, Liesveld J. Locations of facilities with special programs for older substance abuse clients in the US. Int J Geriatr Psychiatry. 2003;18(9):839-843.
25. Schonfeld L, Dupree LW, Dickson-Fuhrman E, et al. Cognitive-behavioral treatment of older veterans with substance abuse problems. J Geriatr Psychiatry Neurol. 2000;13(3):124-129.
The puzzling self-poisoner
CASE: Unusual suicide attempt
After a friend calls 911, Ms. M, age 20, is brought to an emergency room (ER) complaining of severe leg and abdominal pain. The ER physician finds she is bleeding from her vagina and nose and has severe ecchymosis anemia. After Ms. M is admitted, clinicians discover these conditions are secondary to a suicide attempt—she ingested 15 to 16 pellets of rat poison daily for approximately 2 months.
While hospitalized, Ms. M is treated with several transfusions of fresh frozen plasma, packed red blood cells, and phytonadione (vitamin K). A consultation-liaison psychiatrist diagnoses bipolar disorder and starts Ms. M on lamotrigine, 25 mg once daily. (The justification for this diagnosis was not documented.) After physicians judge her to be medically stable, Ms. M is involuntarily committed to a short-term psychiatric care facility. Her vital signs and coagulation values are stable.
At the psychiatric facility, our team determines that her symptoms and history are consistent with major depressive disorder, recurrent. For 5 months, Ms. M had depressed mood for most of the day, diminished interest in activities, and feelings of worthlessness. She also experienced weight loss—10 lbs in 2 months—with decreased appetite and low energy for most of the day. She denies past symptoms of mania or psychosis. She says she does not know why she was diagnosed with bipolar disorder. She admits to multiple previous suicide attempts via hanging and ingesting cleaning fluid or rat poison.
We place Ms. M on suicide precautions and prescribe escitalopram, 10 mg/d, in addition to lamotrigine, 50 mg once daily. We continue lamotrigine despite a lack of documentation for Ms. M’s bipolar diagnosis because evidence suggests the drug may be an effective augmentation to antidepressants in patients with treatment-resistant depression.1
The author’s observations
Any patient transferred from a medical floor to a psychiatric inpatient unit should have documentation that clarifies any need for further medical treatment. Ms. M’s physicians told us that she was medically stable and should require little if any further treatment for her ingestion of rat poison.
TREATMENT: Coagulation concerns
We request a medical consult to monitor possible complications from the rat poison. The physician advises that rat poison essentially is the same as the anticoagulant warfarin and its effects should steadily decrease over time because its half-life is 20 to 60 hours. However, for safety reasons, we closely follow Ms. M’s coagulation values and order daily vitamin K injections, 5 mg SC.
Further medical investigation shows no evidence of complications, but Ms. M continues to request medication for pain in her left leg. The physician prescribes acetaminophen, 650 mg every 6 hours as needed for pain, which the patient takes at almost every opportunity, often 4 times a day. The physician does not choose a nonsteroidal anti-inflammatory drug (NSAID) for pain to avoid the possibility of gastrointestinal (GI) irritation that could lead to bleeding.
In the psychiatric facility, the patient’s international normalized ratio (INR) is found to be rising, indicating a lack of clotting and a risk of uncontrolled bleeding. The physician states that given the half-life of warfarin, Ms. M’s INR should be decreasing. Liver function testing does not show that liver dysfunction is contributing to the increasing INR.
Because we assume the vitamin K the patient received has been absorbed, we hypothesize that Ms. M has continued to surreptitiously ingest rat poison or another anticoagulant, which she denies. We search Ms. M and her room. She is placed on 1-to-1 observation 24 hours a day. Ms. M’s visitors also are searched, and visits are observed. We find no evidence of an anticoagulant agent.
Ms. M’s INR continues to rise. We search the facility to rule out the possibility that the patient had hidden a supply of anticoagulant outside her room. The search finds nothing. At this point we consider performing an abdominal x-ray to rule out the possibility that Ms. M may have a supply of medication hidden in her gastrointestinal tract.
The author’s observations
Patients hiding and using contraband is a common problem in involuntary inpatient units. It seemed that Ms. M was secretly ingesting rat poison. Her history showed she was determined to end her life, and she ingested rat poison daily for months. However, because an exhaustive search for contraband and 1-to-1 observation yielded no positive results, the evidence did not support this theory. Some team members thought we were not searching hard enough. I decided we needed to pursue other theories.
I was skeptical that escitalopram could be contributing to Ms. M’s rising anticoagulation values. Selective serotonin reuptake inhibitors have antiplatelet effects, but platelet function does not affect INR to the degree we were observing.
‘Superwarfarins’
Physicians had advised us that Ms. M’s INR should decrease under the assumption that rat poison is for all practical purposes the same as warfarin, but we had not investigated distinctions between the 2 compounds. A literature search revealed that several rat poisons are not simply warfarin repackaged as a pesticide. Most are “superwarfarins”—chemicals similar to warfarin but more potent and with a much longer half-life.2 Case report data suggest the plasma half-life of these chemicals is 20 to 62 days.3
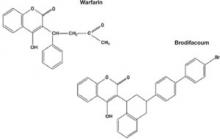
Most commercial rat poisons are made of brodifacoum, which has a chemical structure similar to warfarin but with an additional long polycyclic hydrocarbon side chain (Figure 1). The potency of brodifacoum compared with warfarin is approximately 100 to 1.4-6 The chemical is highly lipophilic and can stay in the body for an extended period.4-6 Lab tests can measure serum brodifacoum levels.3
After Ms. M identifies the brand name of the rat poison she ingested, we contact the American Association of Poison Control Centers and verify the agent she used was brodifacoum. This explains why her INR was not decreasing—but does not explain the increase.
A drug interaction? Because Ms. M’s liver function is within normal limits, the next theory to investigate is if brodifacoum is interacting with any medications she is taking. I could not find any medical journal articles, programs, or Web sites describing brodifacoum’s interactions with medications. After all, brodifacoum is a pesticide, not a medication.
I considered that because brodifacoum and warfarin have a similar structure and function, they may interact with medications in a similar manner. After another literature search, only acetaminophen had evidence of interaction with warfarin that could explain her rising INR.
Documentation of interactions between warfarin and acetaminophen are sparse. In one case, a 74-year-old man receiving warfarin for atrial fibrillation experienced an abrupt increase in INR after taking acetaminophen.7 In a double-blind, placebo controlled, randomized trial of patients taking warfarin, INR rose rapidly after the start of acetaminophen and was significantly increased within 1 week compared with patients receiving placebo.8
Figure 1 Chemical structures of warfarin and rat poison
Most commercial rat poisons are made of brodifacoum, which is chemically similar to warfarin but has an additional long polycyclic hydrocarbon side chain.
FOLLOW-UP: Analgesic substitution
We suggest to the physician that Ms. M’s INR may be increasing because of an interaction between brodifacoum and acetaminophen, which she took several times a day. On day 8 of Ms. M’s hospitalization, the physician discontinues acetaminophen and prescribes ibuprofen, 400 mg tid as needed for pain, and pantoprazole, 40 mg/d, to prevent GI bleeding from possible irritation caused by ibuprofen. The team continues to monitor Ms. M’s coagulation values.
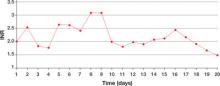
Within a day of discontinuing acetaminophen, Ms. M’s INR decreased as expected (Figure 2). The rest of her medication regimen is continued, and her INR levels continued to decrease.
One-to-one observation is discontinued. However, because of the patient’s continued determination to end her life and no significant improvement in her depression, Ms. M is considered a danger to herself and involuntary inpatient hospitalization is continued.
Figure 2 Ms. M’s INR values during hospitalization
The patient’s INR values began to rise mysteriously after she was transferred to the inpatient psychiatric unit. Acetaminophen was discontinued on day 8, and within a day her INR values began to drop.
INR: International normalized ratio
The author’s observations
Poisoning is a common method of attempting suicide, patients may use substances that clinicians rarely encounter. For most toxic, nonmedication substances, data on interactions with medications are sparse. if you suspect your patient has ingested a toxic substance with which the treatment team has little experience, contact the American Association of Poison Control Centers at 800-222-1222.
Suspect superwarfarin poisoning in suicidal patients with coagulopathy, prolonged prothrombin time, and elevated INR that does not respond to large amounts of vitamin K.9,10 These patients are at high risk of successfully completing suicide because of superwarfarins’ long half-life and daily maintenance required to keep coagulation levels within a safe range for at least several weeks.
The most serious complication these patients face is intracranial hemorrhage, which occurs in 2% of patients with excessive warfarin-based coagulation and is associated with a 77% mortality rate.11 GI bleeding occurs in 67% of these patients.2
Also take into account medical conditions—such as hypertension or hepatic disease—and medication side effects that can increase bleeding risk. When treating pain in these patients, consider avoiding acetaminophen but be aware of the risks of NSAIDs, such as gastritis or GI bleeding.
Related resource
- The American Association of Poison Control Centers. 800-222-1222; www.aapcc.org.
Drug brand names
- Escitalopram • Lexapro
- Lamotrigine • Lamictal
- Pantoprazole • Protonix
- Warfarin • Coumadin
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sharma V, Khan M, Corpse C. Role of lamotrigine in the management of treatment-resistant bipolar II depression: a chart review. J Affect Disord Epub 2008 Mar 1.
2. Su M, Hoffman R. Anticoagulants. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s toxicologic emergencies. 8th ed. New York, NY: McGraw-Hill Medical Publishing; 2006:891-4.
3. Chua JD, Friedenberg WR. Superwarfarin poisoning. Anaesth Intensive Care 1997;25:707-9.
4. Leck JB, Park BK. A comparative study of the effect of warfarin and brodifacoum on the relationship between vitamin K1 metabolism and clotting factor activity in warfarin susceptible and warfarin resistant rats. Biochem Pharmacol 1981;30:123-9.
5. Lund M. Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg 1981;87:101-7.
6. Park BK, Scott AK, Wilson AC, et al. Plasma disposition of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol 1982;31:3535-639.
7. Gebauer MG, Nyfort-Hansen K, Henschke PJ, Gallus AS. Warfarin and acetaminophen interaction. Pharmacotherapy 2003;23(1):109-12.
8. Mahe I, Bertrand N, Drouet L, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica 2006;91(12):1621-7.
9. Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet 2005;365:552-3.
10. Scully M. Warfarin therapy: rat poison and the prevention of thrombosis. Biochemist 2002;24:15-7.
11. Mathiesen T, Benediktsdottir K, Johnsson H, Lindqvist M. Intracranial traumatic and nontraumatic haemorrhagic complications of warfarin treatment. Acta Neurol Scan 1995;91:208-14.
CASE: Unusual suicide attempt
After a friend calls 911, Ms. M, age 20, is brought to an emergency room (ER) complaining of severe leg and abdominal pain. The ER physician finds she is bleeding from her vagina and nose and has severe ecchymosis anemia. After Ms. M is admitted, clinicians discover these conditions are secondary to a suicide attempt—she ingested 15 to 16 pellets of rat poison daily for approximately 2 months.
While hospitalized, Ms. M is treated with several transfusions of fresh frozen plasma, packed red blood cells, and phytonadione (vitamin K). A consultation-liaison psychiatrist diagnoses bipolar disorder and starts Ms. M on lamotrigine, 25 mg once daily. (The justification for this diagnosis was not documented.) After physicians judge her to be medically stable, Ms. M is involuntarily committed to a short-term psychiatric care facility. Her vital signs and coagulation values are stable.
At the psychiatric facility, our team determines that her symptoms and history are consistent with major depressive disorder, recurrent. For 5 months, Ms. M had depressed mood for most of the day, diminished interest in activities, and feelings of worthlessness. She also experienced weight loss—10 lbs in 2 months—with decreased appetite and low energy for most of the day. She denies past symptoms of mania or psychosis. She says she does not know why she was diagnosed with bipolar disorder. She admits to multiple previous suicide attempts via hanging and ingesting cleaning fluid or rat poison.
We place Ms. M on suicide precautions and prescribe escitalopram, 10 mg/d, in addition to lamotrigine, 50 mg once daily. We continue lamotrigine despite a lack of documentation for Ms. M’s bipolar diagnosis because evidence suggests the drug may be an effective augmentation to antidepressants in patients with treatment-resistant depression.1
The author’s observations
Any patient transferred from a medical floor to a psychiatric inpatient unit should have documentation that clarifies any need for further medical treatment. Ms. M’s physicians told us that she was medically stable and should require little if any further treatment for her ingestion of rat poison.
TREATMENT: Coagulation concerns
We request a medical consult to monitor possible complications from the rat poison. The physician advises that rat poison essentially is the same as the anticoagulant warfarin and its effects should steadily decrease over time because its half-life is 20 to 60 hours. However, for safety reasons, we closely follow Ms. M’s coagulation values and order daily vitamin K injections, 5 mg SC.
Further medical investigation shows no evidence of complications, but Ms. M continues to request medication for pain in her left leg. The physician prescribes acetaminophen, 650 mg every 6 hours as needed for pain, which the patient takes at almost every opportunity, often 4 times a day. The physician does not choose a nonsteroidal anti-inflammatory drug (NSAID) for pain to avoid the possibility of gastrointestinal (GI) irritation that could lead to bleeding.
In the psychiatric facility, the patient’s international normalized ratio (INR) is found to be rising, indicating a lack of clotting and a risk of uncontrolled bleeding. The physician states that given the half-life of warfarin, Ms. M’s INR should be decreasing. Liver function testing does not show that liver dysfunction is contributing to the increasing INR.
Because we assume the vitamin K the patient received has been absorbed, we hypothesize that Ms. M has continued to surreptitiously ingest rat poison or another anticoagulant, which she denies. We search Ms. M and her room. She is placed on 1-to-1 observation 24 hours a day. Ms. M’s visitors also are searched, and visits are observed. We find no evidence of an anticoagulant agent.
Ms. M’s INR continues to rise. We search the facility to rule out the possibility that the patient had hidden a supply of anticoagulant outside her room. The search finds nothing. At this point we consider performing an abdominal x-ray to rule out the possibility that Ms. M may have a supply of medication hidden in her gastrointestinal tract.
The author’s observations
Patients hiding and using contraband is a common problem in involuntary inpatient units. It seemed that Ms. M was secretly ingesting rat poison. Her history showed she was determined to end her life, and she ingested rat poison daily for months. However, because an exhaustive search for contraband and 1-to-1 observation yielded no positive results, the evidence did not support this theory. Some team members thought we were not searching hard enough. I decided we needed to pursue other theories.
I was skeptical that escitalopram could be contributing to Ms. M’s rising anticoagulation values. Selective serotonin reuptake inhibitors have antiplatelet effects, but platelet function does not affect INR to the degree we were observing.
‘Superwarfarins’
Physicians had advised us that Ms. M’s INR should decrease under the assumption that rat poison is for all practical purposes the same as warfarin, but we had not investigated distinctions between the 2 compounds. A literature search revealed that several rat poisons are not simply warfarin repackaged as a pesticide. Most are “superwarfarins”—chemicals similar to warfarin but more potent and with a much longer half-life.2 Case report data suggest the plasma half-life of these chemicals is 20 to 62 days.3
Most commercial rat poisons are made of brodifacoum, which has a chemical structure similar to warfarin but with an additional long polycyclic hydrocarbon side chain (Figure 1). The potency of brodifacoum compared with warfarin is approximately 100 to 1.4-6 The chemical is highly lipophilic and can stay in the body for an extended period.4-6 Lab tests can measure serum brodifacoum levels.3
After Ms. M identifies the brand name of the rat poison she ingested, we contact the American Association of Poison Control Centers and verify the agent she used was brodifacoum. This explains why her INR was not decreasing—but does not explain the increase.
A drug interaction? Because Ms. M’s liver function is within normal limits, the next theory to investigate is if brodifacoum is interacting with any medications she is taking. I could not find any medical journal articles, programs, or Web sites describing brodifacoum’s interactions with medications. After all, brodifacoum is a pesticide, not a medication.
I considered that because brodifacoum and warfarin have a similar structure and function, they may interact with medications in a similar manner. After another literature search, only acetaminophen had evidence of interaction with warfarin that could explain her rising INR.
Documentation of interactions between warfarin and acetaminophen are sparse. In one case, a 74-year-old man receiving warfarin for atrial fibrillation experienced an abrupt increase in INR after taking acetaminophen.7 In a double-blind, placebo controlled, randomized trial of patients taking warfarin, INR rose rapidly after the start of acetaminophen and was significantly increased within 1 week compared with patients receiving placebo.8
Figure 1 Chemical structures of warfarin and rat poison
Most commercial rat poisons are made of brodifacoum, which is chemically similar to warfarin but has an additional long polycyclic hydrocarbon side chain.
FOLLOW-UP: Analgesic substitution
We suggest to the physician that Ms. M’s INR may be increasing because of an interaction between brodifacoum and acetaminophen, which she took several times a day. On day 8 of Ms. M’s hospitalization, the physician discontinues acetaminophen and prescribes ibuprofen, 400 mg tid as needed for pain, and pantoprazole, 40 mg/d, to prevent GI bleeding from possible irritation caused by ibuprofen. The team continues to monitor Ms. M’s coagulation values.
Within a day of discontinuing acetaminophen, Ms. M’s INR decreased as expected (Figure 2). The rest of her medication regimen is continued, and her INR levels continued to decrease.
One-to-one observation is discontinued. However, because of the patient’s continued determination to end her life and no significant improvement in her depression, Ms. M is considered a danger to herself and involuntary inpatient hospitalization is continued.
Figure 2 Ms. M’s INR values during hospitalization
The patient’s INR values began to rise mysteriously after she was transferred to the inpatient psychiatric unit. Acetaminophen was discontinued on day 8, and within a day her INR values began to drop.
INR: International normalized ratio
The author’s observations
Poisoning is a common method of attempting suicide, patients may use substances that clinicians rarely encounter. For most toxic, nonmedication substances, data on interactions with medications are sparse. if you suspect your patient has ingested a toxic substance with which the treatment team has little experience, contact the American Association of Poison Control Centers at 800-222-1222.
Suspect superwarfarin poisoning in suicidal patients with coagulopathy, prolonged prothrombin time, and elevated INR that does not respond to large amounts of vitamin K.9,10 These patients are at high risk of successfully completing suicide because of superwarfarins’ long half-life and daily maintenance required to keep coagulation levels within a safe range for at least several weeks.
The most serious complication these patients face is intracranial hemorrhage, which occurs in 2% of patients with excessive warfarin-based coagulation and is associated with a 77% mortality rate.11 GI bleeding occurs in 67% of these patients.2
Also take into account medical conditions—such as hypertension or hepatic disease—and medication side effects that can increase bleeding risk. When treating pain in these patients, consider avoiding acetaminophen but be aware of the risks of NSAIDs, such as gastritis or GI bleeding.
Related resource
- The American Association of Poison Control Centers. 800-222-1222; www.aapcc.org.
Drug brand names
- Escitalopram • Lexapro
- Lamotrigine • Lamictal
- Pantoprazole • Protonix
- Warfarin • Coumadin
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Unusual suicide attempt
After a friend calls 911, Ms. M, age 20, is brought to an emergency room (ER) complaining of severe leg and abdominal pain. The ER physician finds she is bleeding from her vagina and nose and has severe ecchymosis anemia. After Ms. M is admitted, clinicians discover these conditions are secondary to a suicide attempt—she ingested 15 to 16 pellets of rat poison daily for approximately 2 months.
While hospitalized, Ms. M is treated with several transfusions of fresh frozen plasma, packed red blood cells, and phytonadione (vitamin K). A consultation-liaison psychiatrist diagnoses bipolar disorder and starts Ms. M on lamotrigine, 25 mg once daily. (The justification for this diagnosis was not documented.) After physicians judge her to be medically stable, Ms. M is involuntarily committed to a short-term psychiatric care facility. Her vital signs and coagulation values are stable.
At the psychiatric facility, our team determines that her symptoms and history are consistent with major depressive disorder, recurrent. For 5 months, Ms. M had depressed mood for most of the day, diminished interest in activities, and feelings of worthlessness. She also experienced weight loss—10 lbs in 2 months—with decreased appetite and low energy for most of the day. She denies past symptoms of mania or psychosis. She says she does not know why she was diagnosed with bipolar disorder. She admits to multiple previous suicide attempts via hanging and ingesting cleaning fluid or rat poison.
We place Ms. M on suicide precautions and prescribe escitalopram, 10 mg/d, in addition to lamotrigine, 50 mg once daily. We continue lamotrigine despite a lack of documentation for Ms. M’s bipolar diagnosis because evidence suggests the drug may be an effective augmentation to antidepressants in patients with treatment-resistant depression.1
The author’s observations
Any patient transferred from a medical floor to a psychiatric inpatient unit should have documentation that clarifies any need for further medical treatment. Ms. M’s physicians told us that she was medically stable and should require little if any further treatment for her ingestion of rat poison.
TREATMENT: Coagulation concerns
We request a medical consult to monitor possible complications from the rat poison. The physician advises that rat poison essentially is the same as the anticoagulant warfarin and its effects should steadily decrease over time because its half-life is 20 to 60 hours. However, for safety reasons, we closely follow Ms. M’s coagulation values and order daily vitamin K injections, 5 mg SC.
Further medical investigation shows no evidence of complications, but Ms. M continues to request medication for pain in her left leg. The physician prescribes acetaminophen, 650 mg every 6 hours as needed for pain, which the patient takes at almost every opportunity, often 4 times a day. The physician does not choose a nonsteroidal anti-inflammatory drug (NSAID) for pain to avoid the possibility of gastrointestinal (GI) irritation that could lead to bleeding.
In the psychiatric facility, the patient’s international normalized ratio (INR) is found to be rising, indicating a lack of clotting and a risk of uncontrolled bleeding. The physician states that given the half-life of warfarin, Ms. M’s INR should be decreasing. Liver function testing does not show that liver dysfunction is contributing to the increasing INR.
Because we assume the vitamin K the patient received has been absorbed, we hypothesize that Ms. M has continued to surreptitiously ingest rat poison or another anticoagulant, which she denies. We search Ms. M and her room. She is placed on 1-to-1 observation 24 hours a day. Ms. M’s visitors also are searched, and visits are observed. We find no evidence of an anticoagulant agent.
Ms. M’s INR continues to rise. We search the facility to rule out the possibility that the patient had hidden a supply of anticoagulant outside her room. The search finds nothing. At this point we consider performing an abdominal x-ray to rule out the possibility that Ms. M may have a supply of medication hidden in her gastrointestinal tract.
The author’s observations
Patients hiding and using contraband is a common problem in involuntary inpatient units. It seemed that Ms. M was secretly ingesting rat poison. Her history showed she was determined to end her life, and she ingested rat poison daily for months. However, because an exhaustive search for contraband and 1-to-1 observation yielded no positive results, the evidence did not support this theory. Some team members thought we were not searching hard enough. I decided we needed to pursue other theories.
I was skeptical that escitalopram could be contributing to Ms. M’s rising anticoagulation values. Selective serotonin reuptake inhibitors have antiplatelet effects, but platelet function does not affect INR to the degree we were observing.
‘Superwarfarins’
Physicians had advised us that Ms. M’s INR should decrease under the assumption that rat poison is for all practical purposes the same as warfarin, but we had not investigated distinctions between the 2 compounds. A literature search revealed that several rat poisons are not simply warfarin repackaged as a pesticide. Most are “superwarfarins”—chemicals similar to warfarin but more potent and with a much longer half-life.2 Case report data suggest the plasma half-life of these chemicals is 20 to 62 days.3
Most commercial rat poisons are made of brodifacoum, which has a chemical structure similar to warfarin but with an additional long polycyclic hydrocarbon side chain (Figure 1). The potency of brodifacoum compared with warfarin is approximately 100 to 1.4-6 The chemical is highly lipophilic and can stay in the body for an extended period.4-6 Lab tests can measure serum brodifacoum levels.3
After Ms. M identifies the brand name of the rat poison she ingested, we contact the American Association of Poison Control Centers and verify the agent she used was brodifacoum. This explains why her INR was not decreasing—but does not explain the increase.
A drug interaction? Because Ms. M’s liver function is within normal limits, the next theory to investigate is if brodifacoum is interacting with any medications she is taking. I could not find any medical journal articles, programs, or Web sites describing brodifacoum’s interactions with medications. After all, brodifacoum is a pesticide, not a medication.
I considered that because brodifacoum and warfarin have a similar structure and function, they may interact with medications in a similar manner. After another literature search, only acetaminophen had evidence of interaction with warfarin that could explain her rising INR.
Documentation of interactions between warfarin and acetaminophen are sparse. In one case, a 74-year-old man receiving warfarin for atrial fibrillation experienced an abrupt increase in INR after taking acetaminophen.7 In a double-blind, placebo controlled, randomized trial of patients taking warfarin, INR rose rapidly after the start of acetaminophen and was significantly increased within 1 week compared with patients receiving placebo.8
Figure 1 Chemical structures of warfarin and rat poison
Most commercial rat poisons are made of brodifacoum, which is chemically similar to warfarin but has an additional long polycyclic hydrocarbon side chain.
FOLLOW-UP: Analgesic substitution
We suggest to the physician that Ms. M’s INR may be increasing because of an interaction between brodifacoum and acetaminophen, which she took several times a day. On day 8 of Ms. M’s hospitalization, the physician discontinues acetaminophen and prescribes ibuprofen, 400 mg tid as needed for pain, and pantoprazole, 40 mg/d, to prevent GI bleeding from possible irritation caused by ibuprofen. The team continues to monitor Ms. M’s coagulation values.
Within a day of discontinuing acetaminophen, Ms. M’s INR decreased as expected (Figure 2). The rest of her medication regimen is continued, and her INR levels continued to decrease.
One-to-one observation is discontinued. However, because of the patient’s continued determination to end her life and no significant improvement in her depression, Ms. M is considered a danger to herself and involuntary inpatient hospitalization is continued.
Figure 2 Ms. M’s INR values during hospitalization
The patient’s INR values began to rise mysteriously after she was transferred to the inpatient psychiatric unit. Acetaminophen was discontinued on day 8, and within a day her INR values began to drop.
INR: International normalized ratio
The author’s observations
Poisoning is a common method of attempting suicide, patients may use substances that clinicians rarely encounter. For most toxic, nonmedication substances, data on interactions with medications are sparse. if you suspect your patient has ingested a toxic substance with which the treatment team has little experience, contact the American Association of Poison Control Centers at 800-222-1222.
Suspect superwarfarin poisoning in suicidal patients with coagulopathy, prolonged prothrombin time, and elevated INR that does not respond to large amounts of vitamin K.9,10 These patients are at high risk of successfully completing suicide because of superwarfarins’ long half-life and daily maintenance required to keep coagulation levels within a safe range for at least several weeks.
The most serious complication these patients face is intracranial hemorrhage, which occurs in 2% of patients with excessive warfarin-based coagulation and is associated with a 77% mortality rate.11 GI bleeding occurs in 67% of these patients.2
Also take into account medical conditions—such as hypertension or hepatic disease—and medication side effects that can increase bleeding risk. When treating pain in these patients, consider avoiding acetaminophen but be aware of the risks of NSAIDs, such as gastritis or GI bleeding.
Related resource
- The American Association of Poison Control Centers. 800-222-1222; www.aapcc.org.
Drug brand names
- Escitalopram • Lexapro
- Lamotrigine • Lamictal
- Pantoprazole • Protonix
- Warfarin • Coumadin
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sharma V, Khan M, Corpse C. Role of lamotrigine in the management of treatment-resistant bipolar II depression: a chart review. J Affect Disord Epub 2008 Mar 1.
2. Su M, Hoffman R. Anticoagulants. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s toxicologic emergencies. 8th ed. New York, NY: McGraw-Hill Medical Publishing; 2006:891-4.
3. Chua JD, Friedenberg WR. Superwarfarin poisoning. Anaesth Intensive Care 1997;25:707-9.
4. Leck JB, Park BK. A comparative study of the effect of warfarin and brodifacoum on the relationship between vitamin K1 metabolism and clotting factor activity in warfarin susceptible and warfarin resistant rats. Biochem Pharmacol 1981;30:123-9.
5. Lund M. Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg 1981;87:101-7.
6. Park BK, Scott AK, Wilson AC, et al. Plasma disposition of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol 1982;31:3535-639.
7. Gebauer MG, Nyfort-Hansen K, Henschke PJ, Gallus AS. Warfarin and acetaminophen interaction. Pharmacotherapy 2003;23(1):109-12.
8. Mahe I, Bertrand N, Drouet L, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica 2006;91(12):1621-7.
9. Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet 2005;365:552-3.
10. Scully M. Warfarin therapy: rat poison and the prevention of thrombosis. Biochemist 2002;24:15-7.
11. Mathiesen T, Benediktsdottir K, Johnsson H, Lindqvist M. Intracranial traumatic and nontraumatic haemorrhagic complications of warfarin treatment. Acta Neurol Scan 1995;91:208-14.
1. Sharma V, Khan M, Corpse C. Role of lamotrigine in the management of treatment-resistant bipolar II depression: a chart review. J Affect Disord Epub 2008 Mar 1.
2. Su M, Hoffman R. Anticoagulants. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, et al, eds. Goldfrank’s toxicologic emergencies. 8th ed. New York, NY: McGraw-Hill Medical Publishing; 2006:891-4.
3. Chua JD, Friedenberg WR. Superwarfarin poisoning. Anaesth Intensive Care 1997;25:707-9.
4. Leck JB, Park BK. A comparative study of the effect of warfarin and brodifacoum on the relationship between vitamin K1 metabolism and clotting factor activity in warfarin susceptible and warfarin resistant rats. Biochem Pharmacol 1981;30:123-9.
5. Lund M. Comparative effect of the three rodenticides warfarin, difenacoum and brodifacoum on eight rodent species in short feeding periods. J Hyg 1981;87:101-7.
6. Park BK, Scott AK, Wilson AC, et al. Plasma disposition of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol 1982;31:3535-639.
7. Gebauer MG, Nyfort-Hansen K, Henschke PJ, Gallus AS. Warfarin and acetaminophen interaction. Pharmacotherapy 2003;23(1):109-12.
8. Mahe I, Bertrand N, Drouet L, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica 2006;91(12):1621-7.
9. Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet 2005;365:552-3.
10. Scully M. Warfarin therapy: rat poison and the prevention of thrombosis. Biochemist 2002;24:15-7.
11. Mathiesen T, Benediktsdottir K, Johnsson H, Lindqvist M. Intracranial traumatic and nontraumatic haemorrhagic complications of warfarin treatment. Acta Neurol Scan 1995;91:208-14.