User login
Can mood stabilizers reduce chronic pain in patients with bipolar disorder?
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).
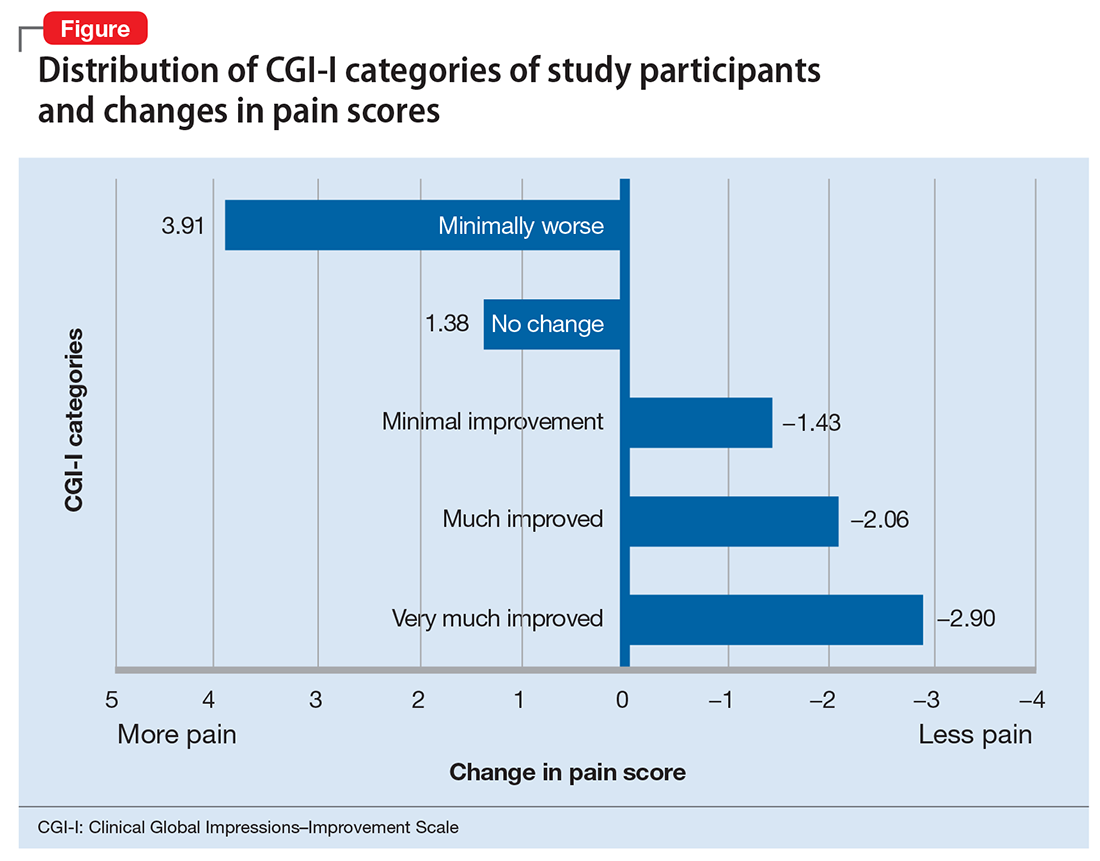
Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).

Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
Misuse of prescription opioids has led to a staggering number of patients developing addiction, which the National Institutes of Health (NIH) and Department of Health and Human Services (HHS) have identified as a health care crisis. In the United States, approximately 29% of patients prescribed an opioid misuse it, and approximately 80% of heroin users started with prescription opioids.1,2 The NIH and HHS have outlined 5 priorities to help resolve this crisis:
- Improve access to prevention, treatment, and recovery support services
- Increase availability and distribution of overdose-reversing medications
- As the epidemic changes, strengthen what we know with improved public health surveillance
- Support research that advances the understanding of pain and addiction and that develops new treatments and interventions
- Improve pain management by utilizing evidence-based practices and reducing opioid misuse and opiate-related harm.3
Treating chronic pain in patients with bipolar disorder
At the Missouri University Psychiatric Center, an inpatient psychiatric ward, we recently conducted a retrospective cohort study to identify effective alternatives for treating pain, and to decrease opioid-related harm. Our study focused on 73 inpatients experiencing exacerbation of bipolar I disorder who also had chronic pain. These patients were treated with mood stabilizers, including lithium and carbamazepine. Patients also were taking medications, as needed, for agitation and their home medications for various medical problems. Selection of mood stabilizer therapy was non-random by standard of care based on best clinical practices. Dosing was based on blood-level monitoring adjusted to maintain therapeutic levels while receiving inpatient care. The average duration of inpatient treatment was approximately 1 to 5 weeks.
Pain was measured at baseline and compared with daily pain scores after mood stabilizer therapy using a 10-point scale, with 0 for no pain to 10 for worse pain, for the duration of the admission As expected based on the findings of previous research, carbamazepine resulted in a decrease in average daily pain score by 1.25 points after treatment (P = .048; F value = 4.3; F-crit = 4.23; calculated by one-way analysis of variance). However, patients who received lithium experienced a greater decrease in average daily pain score, by 2.17 points after treatment (P = .00035; F value = 14.56; F-crit = 4.02).
To further characterize the relationship between bipolar disorder and chronic pain, we looked at change in pain scores for mixed, manic, and depressive episodes of bipolar disorder by Clinical Global Impressions—Improvement (CGI-I) Scale categories (Figure). Participants who experienced the greatest clinical improvement also experienced the highest degree of analgesia. Those in the “Very much improved” CGI-I category experienced an almost 3-point decrease in average daily pain scores, with significance well below threshold (P = .0000967; F value = 19.83; F-crit = 4.11). Participants who showed no change in their bipolar I disorder symptoms or experienced exacerbation of their symptoms showed a significant increase in pain scores (P = .037; F value = 6.24; F-crit = 5.32).

Our data show that lithium and carbamazepine provide clinically and statistically significant analgesia in patients with bipolar I disorder and chronic pain. Furthermore, exacerbation of bipolar I disorder symptoms was associated with an increase of approximately 4 points on a 10-point chronic pain scale.
Acknowledgments
We would like to acknowledge contributions of Yajie Yu, MD, Sailaja Bysani, MD, Emily Leary, PhD, and Oluwole Popoola, MD, for their work in this study.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
1. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569-576.
2. Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Rev. 2013.
3. National Institutes of Health. Department of Health and Human Services. Opiate crisis. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-crisis. Updated January 2018. Accessed February 5, 2018.
ACIP vaccine update
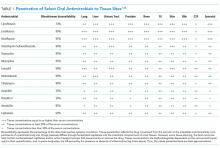
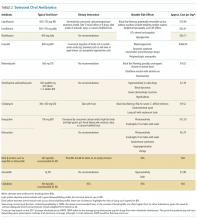
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
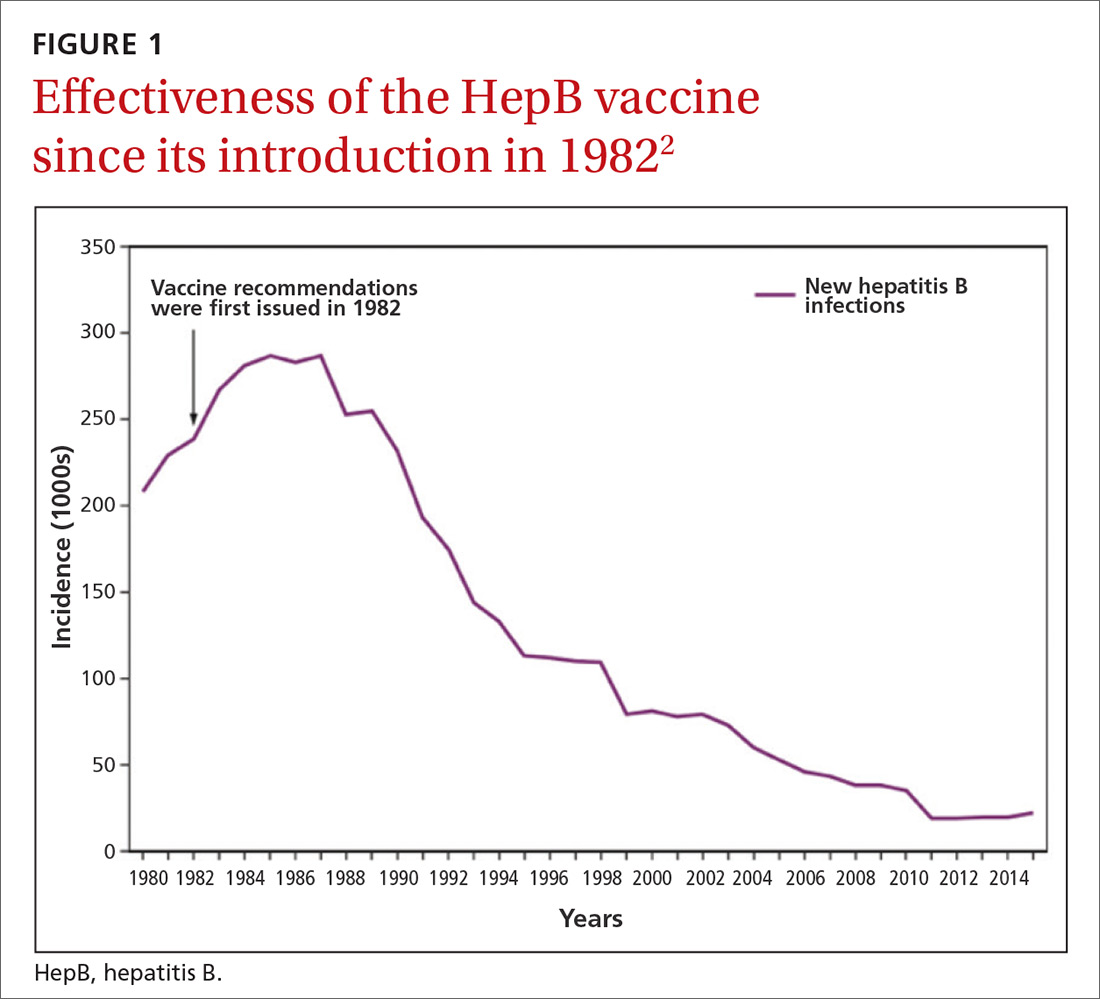
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
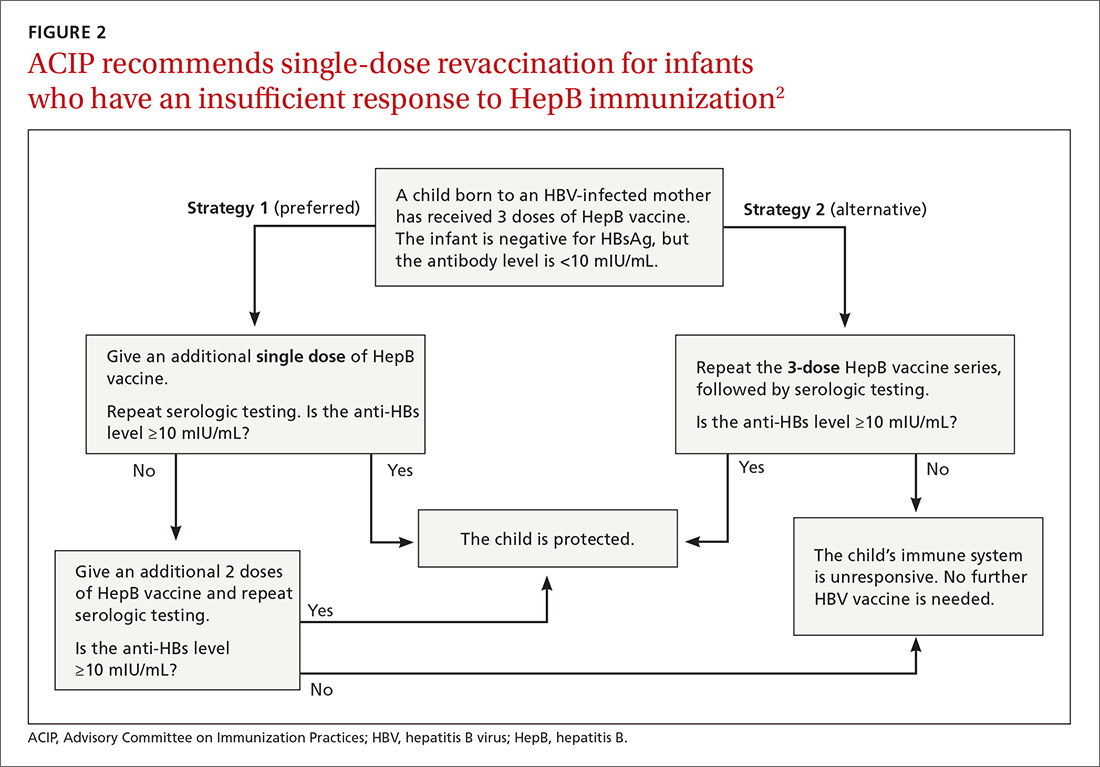
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
When are Oral Antibiotics a Safe and Effective Choice for Bacterial Bloodstream Infections? An Evidence-Based Narrative Review
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
Bacterial bloodstream infections (BSIs) are a major cause of morbidity and mortality in the United States. Approximately 600,000 BSI cases occur annually, resulting in 85,000 deaths,1 at a cost exceeding $1 billion.2 Traditionally, BSIs have been managed with intravenous antimicrobials, which rapidly achieve therapeutic blood concentrations, and are viewed as more potent than oral alternatives. Indeed, for acutely ill patients with bacteremia and sepsis, timely intravenous antimicrobials are lifesaving.3
However, whether intravenous antimicrobials are essential for the entire treatment course in BSIs, particularly for uncomplicated episodes, is controversial. Patients that are clinically stable or have been stabilized after an initial septic presentation may be appropriate candidates for treatment with oral antimicrobials. There are costs and risks associated with extended courses of intravenous agents, such as the necessity for long-term intravenous catheters, which entail risks for procedural complications, secondary infections, and thrombosis. A prospective study of 192 peripherally inserted central catheter (PICC) episodes reported an overall complication rate of 30.2%, including central line-associated BSIs (CLABSI) or venous thrombosis.4 Other studies also identified high rates of thrombosis5 and PICC-related CLABSI, particularly in patients with malignancy, where sepsis-related complications approach 25%.6 Additionally, appropriate care of indwelling catheters requires significant financial and healthcare resources.
Oral antimicrobial therapy for bacterial BSIs offers several potential benefits. Direct economic and healthcare workforce savings are expected to be significant, and procedural and catheter-related complications would be eliminated.7 Moreover, oral therapy provides antimicrobial stewardship by reducing the use of broad-spectrum intravenous agents.8 Recent infectious disease “Choosing Wisely” initiatives recommend clinicians “prefer oral formulations of highly bioavailable antimicrobials whenever possible”,9 and this approach is supported by the Centers for Disease Control and Prevention antibiotic stewardship program.10 However, the expected savings and benefits of oral therapy would be lost should they be less effective and result in treatment failure or relapse of the primary BSI. Pathogen susceptibility, gastrointestinal absorption, oral bioavailability, patient tolerability, and adherence with therapy need to be carefully considered before choosing oral antimicrobials. Thus, oral antimicrobial therapy for BSI should be utilized in carefully selected circumstances.
In this narrative review, we highlight areas where oral therapy is safe and effective in treating bloodstream infections, as well as offer guidance to clinicians managing patients experiencing BSI. Given the lack of robust clinical trials on this subject, the evidence for performing a systematic review was insufficient. Thus, the articles and recommendations cited in this review were selected based on the authors’ experiences to represent the best available evidence.
Infection Source Control
Pathogen and Antimicrobial Factors
Patient Factors
Evidence Regarding Bloodstream Infections due to Gram-Negative Rods
BSIs due to gram-negative rods (GNRs) are common and cause significant morbidity and mortality. GNRs represent a broad and diverse array of pathogens. We focus on the Enterobacteriaceae family and Pseudomonas aeruginosa, because they are frequently encountered in clinical practice.1
Gram-Negative Rods, Enterobacteriaceae Family
The Enterobacteriaceae family includes Escherichia coli, Klebsiella, Salmonella, Proteus, Enterobacter, Serratia, and Citrobacter species. The range of illnesses caused by Enterobacteriaceae is as diverse as the family, encompassing most body sites. Although most Enterobacteriaceae are intrinsically susceptible to antibiotics, there is potential for significant multi-drug resistance. Of particular recent concern has been the emergence of Enterobacteriaceae that produce extended-spectrum β-lactamases (ESBL) and even carbapenem-resistant strains.14
However, Enterobacteriaceae species susceptible to oral antimicrobials are often suitable candidates for oral BSI therapy. Among 106 patients with GNR BSI treated with a highly bioavailable oral antibiotic (eg, levofloxacin), the treatment failure rate was only 2% (versus 14% when an antimicrobial with only moderate or low bioavailability was selected).15 Oral treatment of Enterobacteriaceae BSIs secondary to urinary tract infection has been best studied. A prospective randomized, controlled trial evaluated oral versus intravenous ciprofloxacin amongst 141 patients with severe pyelonephritis or complicated urinary tract infections, in which the rate of bacteremia was 38%.16 Notably, patients with obstruction or renal abscess were excluded from the trial. No significant differences in microbiological failure or unsatisfactory clinical responses were found between the IV and oral treatment groups. Additionally, a Cochrane review reported that oral antibiotic therapy is no less effective than intravenous therapy for severe UTI, although data on BSI frequency were not provided.17
Resistance to fluoroquinolones such as ciprofloxacin has been identified as a risk factor for GNR BSI oral treatment failure, highlighting the importance of confirming susceptibilities before committing to an oral treatment plan.18,19 Even ESBL Enterobacteriaceae can be considered for treatment with fluoroquinolones if susceptibilities allow.20
The ideal duration of therapy for GNR BSI is an area of active research. A recent retrospective trial showed no difference in all-cause mortality or recurrent BSI in GNR BSI treated for 8 versus 15 days.21 A recent meta-analysis suggested that 7 days of therapy was noninferior to a longer duration therapy (10–14 days) for pyelonephritis, in which a subset was bacteremic.22 However, another trial reported that short course therapy for GNR BSI (<7 days) is associated with higher risk of treatment failure.22 Further data are needed.
Gram-Negative Rods, Pseudomonas aeruginosa
Pseudomonas aeruginosa is a common pathogen, intrinsically resistant to many antimicrobials, and readily develops antimicrobial resistance during therapy. Fluoroquinolones (such as ciprofloxacin, levofloxacin, and delafloxacin) are the only currently available oral agents with antipseudomonal activity. However, fluoroquinolones may not achieve blood concentrations appropriate for P. aeruginosa treatment at standard doses, while higher dose regimens may be associated with increased risk for undesirable side effects.24,25 Currently, given the minimal trial data comparing oral versus intravenous therapy for P. aeruginosa BSIs, and multiple studies indicating increased mortality when P. aeruginosa is treated inappropriately,26,27 we prefer a conservative approach and consider oral therapy a less-preferred option.
Evidence Regarding Bloodstream Infections due to Gram-Positive Cocci
The majority of bloodstream infections in the United States, and the resultant morbidity and mortality, are from gram-positive cocci (GPCs) such as Staphylococcus, Streptococcus, and Enterococcus species.1
Gram-Positive Cocci, Streptococcus pneumoniae
Of the approximately 900,000 annual cases of S. pneumoniae infection in the United States, approximately 40,000 are complicated by BSI, with 70% of those cases being secondary to pneumococcal pneumonia.28 In studies on patients with pneumococcal pneumonia, bacteremic cases generally fare worse than those without bacteremia.29,30 However, several trials demonstrated comparable outcomes in the setting of bacteremic pneumococcal pneumonia when switched early (within 3 days) from intravenous to oral antibiotics to complete a 7-day course.31,32 Before pneumococcal penicillin resistance became widespread, oral penicillin was shown to be effective, and remains an option for susceptible strains.33 It is worth noting, however, that other trials have shown a mortality benefit from treating bacteremic pneumococcal pneumonia initially with dual-therapy including a β-lactam and macrolide such as azithromycin. This observation highlights the importance of knowing the final susceptibility data prior to consolidating to monotherapy with an oral agent, and that macrolides may have beneficial anti-inflammatory effects, though further research is needed.34,35
Although the evidence for treating bacteremic pneumococcal pneumonia using a highly active and absorbable oral agent is fairly robust, S. pneumoniae BSI secondary to other sites of infection sites is less well studied and may require a more conservative approach.
Gram-Positive Cocci, β-hemolytic Streptococcus species
β-Hemolytic Streptococci include groups A to H, of which groups A (S. pyogenes) and B (S. agalactiae) are the most commonly implicated in BSIs.36 Group A Streptococcus (GAS) is classically associated with streptococcal pharyngitis and Group B Streptococcus (GBS) is associated with postpartum endometritis and neonatal meningitis, though both are virulent organisms with a potential to cause invasive infection throughout the body and in all age-groups. Up to 14% of GAS and 41% GBS BSIs have no clear source;37,38 given these are skin pathogens, such scenarios likely represent invasion via microabrasion. As β-hemolytic streptococcal BSI is often observed in the context of necrotizing skin and soft tissue infections, surgical source control is particularly important.39 GAS remains exquisitely susceptible to penicillin, and intravenous penicillin remains the mainstay for invasive disease; GBS has higher penicillin resistance rates than GAS.40 Clindamycin should be added when there is concern for severe disease or toxic shock.41 Unfortunately, oral penicillin is poorly bioavailable (approximately 50%), and there has been recent concern regarding inducible clindamycin resistance in GAS.42 Thus, oral penicillin V and/or clindamycin is a potentially risky strategy, with no clinical trials supporting this approach; however, they may be reasonable options in selected patients with source control and stable hemodynamics. Amoxicillin has high bioavailability (85%) and may be effective; however, there is lack of supporting data. Highly bioavailable agents such as levofloxacin and linezolid have GAS and GBS activity43 and might be expected to produce satisfactory outcomes. Because no clinical trials have compared these agents with intravenous therapy for BSI, caution is advised. Although bacteriostatic against Staphylococcus, linezolid is bactericidal against Streptococcus.44 Fluoroquinolone resistance amongst β-hemolytic Streptococcus is rare (approximately 0.5%) but does occur.45
Gram-Positive Cocci, Staphylococcus Species
Staphylococcus species include S. aureus (including methicillin susceptible and resistant strains: MSSA and MRSA, respectively) and coagulase-negative species, which include organisms such as S. epidermidis. S. aureus is the most common cause of BSI mortality in the United States,1 with mortality rates estimated at 20%–40% per episode.46 Infectious disease consultation has been associated with decreased mortality and is recommended.47 The guidelines of the Infectious Diseases Society of America for the treatment of MRSA recommend the use of parenteral agents for BSI.48 It is important to consider if a patient with S. aureus BSI has infective endocarditis.
Oral antibiotic therapy for S. aureus BSI is not currently standard practice. Although trimethoprim-sulfamethoxazole (TMP-SMX) has favorable pharmacokinetics and case series of using it successfully for BSI exist,49 TMP-SMX showed inferior outcomes in a randomized trial comparing oral TMP-SMX with intravenous vancomycin in a series of 101 S. aureus infections.50 This observation has been replicated.51 Data on doxycycline or clindamycin for S. aureus BSI are limited, and IDSA guidelines advise against their use in this setting because they are predominantly bacteriostatic.48 Linezolid has favorable pharmacokinetics, with approximately 100% bioavailability, and S. aureus resistance to linezolid is rare.52 Several randomized trials have compared oral linezolid with intravenous vancomycin for S. aureus BSI; for instance, Stevens et al. randomized 460 patients with S. aureus infection (of whom 18% had BSI) to linezolid versus vancomycin and observed similar clinical cure rates.53 A pooled analysis showed oral linezolid was noninferior to vancomycin specifically for S. aureus BSI.54 However, long-term use is often limited by hematologic toxicity, peripheral or optic neuropathy (which can be permanent), and induced serotonin syndrome. Additionally, linezolid is bacteriostatic, not bactericidal against S. aureus. Using oral linezolid as a first-line option for S. aureus BSI would not be recommended; however, it may be used as a second-line treatment option in selected cases. Tedizolid has similar pharmacokinetics and spectrum of activity with fewer side effects; however, clinical data on its use for S. aureus BSI are lacking.55 Fluoroquinolones such as levofloxacin and the newer agent delafloxacin have activity against S. aureus, including MRSA, but on-treatment emergence of fluoroquinolone resistance is a concern, and data on delafloxacin for BSI are lacking.56,57 Older literature suggested the combination of ciprofloxacin and rifampin was effective against right-sided S. aureus endocarditis,58 and other oral fluoroquinolone-rifamycin combinations have also been found to be effective59 However, this approach is currently not a standard therapy, nor is it widely used. Decisions on the duration of therapy for S. aureus BSI should be made in conjunction with an infectious diseases specialist; 14 days is currently regarded as a minimum.47,48
Published data regarding oral treatment of coagulase-negative Staphylococcus (CoNS) BSI are limited. Most CoNS bacteremia and up to 80% Staphylococcus epidermidis bacteremia represent blood culture contamination, though true infection from CoNS is not uncommon, particularly in patients with indwelling catheters.60 An exception is the CoNS species Staphylococcus lugdunensis, which is more virulent, and bacteremia with this organism usually warrants antibiotics. Oral antimicrobial therapy is currently not a standard treatment practice for CoNS BSI that is felt to represent true infection; however, linezolid has been successfully used in case series.61
Gram-Positive Cocci, Enterococcus
E. faecium and E. faecalis are commonly implicated in BSI.1 Similar to S. aureus, infective endocarditis must be ruled out when treating enterococcus BSI; a scoring system has been proposed to assist in deciding if such patients require echocardiography.62 Intravenous ampicillin is a preferred, highly effective agent for enterococci treatment when the organism is susceptible.44 However, oral ampicillin has poor bioavailability (50%), and data for its use in BSI are lacking. For susceptible strains, amoxicillin has comparable efficacy for enterococci and enhanced bioavailability (85%); high dose oral amoxicillin could be considered, but there is minimal clinical trial data to support this approach. Fluoroquinolones exhibit only modest activity against enterococci and would be an inferior choice for BSI.63 Although often sensitive to oral tetracyclines, data on their use in enterococcal BSI are insufficient. Nitrofurantoin can be used for susceptible enterococcal urinary tract infection; however, it does not achieve high blood concentrations and should not be used for BSI.
There is significant data comparing oral linezolid with intravenous daptomycin for vancomycin-resistant enterococci (VRE) BSI. In a systematic review including 10 trials using 30-day all-cause mortality as the primary outcome, patients treated with daptomycin demonstrated higher odds of death (OR 1.61, 95% CI 1.08–2.40) compared with those treated with linezolid.64 However, more recent data suggested that higher daptomycin doses than those used in these earlier trials resulted in improved VRE BSI outcomes.65 A subsequent study reported that VRE BSI treatment with linezolid is associated with significantly higher treatment failure and mortality compared with daptomycin therapy.66 Further research is needed, but should the side-effect profile of linezolid be tolerable, it remains an effective option for oral treatment of enterococcal BSIs.
Evidence Regarding Anaerobic Bacterial Blood Stream Infection
Anaerobic bacteria include Bacteroides, Prevotella, Porphyromonas, Fusobacterium, Peptostreptococcus, Veillonella, and Clostridium. Anaerobes account for approximately 4% of bacterial BSIs, and are often seen in the context of polymicrobial infection.67 Given that anaerobes are difficult to recover, and that antimicrobial resistance testing is more labor intensive, antibiotic therapy choices are often made empirically.67 Unfortunately, antibiotic resistance amongst anaerobes is increasing.68 However, metronidazole remains highly active against a majority of anaerobes, with only a handful of treatment failures reported,69 and has a highly favorable pharmacokinetic profile for oral treatment. Oral metronidazole remains an effective choice for many anaerobic BSIs. Considering the polymicrobial nature of many anaerobic infections, source control is important, and concomitant GNR infection must be ruled out before using metronidazole monotherapy.
Clindamycin has significant anaerobic activity, but Bacteroides resistance has increased significantly in recent years, as high as 26%-44%.70 Amoxicillin-clavulanate has good anaerobic coverage, but bioavailability of clavulanate is limited (50%), making it inferior for BSI. Bioavailability is also limited for cephalosporins with anaerobic activity, such as cefuroxime. Moxifloxacin is a fluoroquinolone with some anaerobic coverage and a good oral pharmacokinetic profile, but Bacteroides resistance can be as high as 50%, making it a risky empiric choice.68
Conclusions
Bacterial BSIs are common and result in significant morbidity and mortality, with high associated healthcare costs. Although BSIs are traditionally treated with intravenous antimicrobials, many BSIs can be safely and effectively cured using oral antibiotics. When appropriately selected, oral antibiotics offer lower costs, fewer side effects, promote antimicrobial stewardship, and are easier for patients. Ultimately, the decision to use oral versus intravenous antibiotics must consider the characteristics of the pathogen, patient, and drug.
Disclosures
None of the authors report any conflicts of interest.
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
1. Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19(6):501-509. PubMed
2. Kilgore M, Brossette S. Cost of bloodstream infections. Am J Infect Control. 2008;36(10):S172.e1-3. PubMed
3. Youkee D, Hulme W, Roberts T, Daniels R, Nutbeam T, Keep J. Time Matters: Antibiotic Timing in Sepsis and Septic Shock. Crit Care Med. 2016;44(10):e1016-1017. PubMed
4. Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;28;6:18. PubMed
5. Allen AW, Megargell JL, Brown DB, Lynch FC, Singh H, Singh Y, Waybill PN. Venous Thrombosis Associated with the Placement of Peripherally Inserted Central Catheters. J Vasc Interv Radiol. 2000;11(10):1309-1314. PubMed
6. Cheong K, Perry D, Karapetis C, Koczwara B. High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234-238. PubMed
7. Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006;90(6):1197-1222. PubMed
8. Cyriac JM, James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014;5(2):83-87. PubMed
9. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
10. Lehmann C, Berner R, Bogner JR, et al. The “Choosing Wisely” initiative in infectious diseases. Infection. 2017;45(3):263-268. PubMed
11. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
12. Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54(3):393-407. PubMed
13. Cua YM, Kripalani S. Medication Use in the Transition from Hospital to Home. Ann Acad Med Singapore. 2008;37(2):136. PubMed
14. Paterson DL. Resistance in Gram-Negative Bacteria: Enterobacteriaceae. Am J Med. 2006; 119(6):S20-28.
15. Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents. 2016;48(5):498-503. PubMed
16. Mombelli G, Pezzoli R, Pinoja-Lutz G, Monotti R, Marone C, Franciolli M Oral vs Intravenous Ciprofloxacin in the Initial Empirical Management of Severe Pyelonephritis or Complicated Urinary Tract Infections: A Prospective Randomized Clinical Trial. Arch Intern Med. 1999;159(1):53-58. PubMed
17. Pohl A. Modes of administration of antibiotics for symptomatic severe urinary tract infections. Cochrane Database Syst Rev. 2007;(4):CD003237. PubMed
18. Brigmon MM, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Impact of fluoroquinolone resistance in Gram-negative bloodstream infections on healthcare utilization. Clin Microbiol Infect. 2015;21(9):843-849. PubMed
19. Ortega M, Marco F, Soriano A, et al. Analysis of 4758 Escherichia coli bacteraemia episodes: predictive factors for isolation of an antibiotic-resistant strain and their impact on the outcome. J Antimicrob Chemother. 2009;63(3):568-574. PubMed
20. Lo CL, Lee CC, Li CW, et al. Fluoroquinolone therapy for bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2017;50(3):355-361. PubMed
21. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the Outcomes of Adults With Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score–Matched Cohort. Clin Infect Dis. 2017; cix767. doi.org/10.1093/cid/cix767 PubMed
22. Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection-- 7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. PubMed
23. Nelson AN, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Optimal duration of antimicrobial therapy for uncomplicated Gram-negative bloodstream infections. Infection. 2017;45(5):613-620. PubMed
24. Zelenitsky S, Ariano R, Harding G, Forrest A. Evaluating Ciprofloxacin Dosing for Pseudomonas aeruginosa Infection by Using Clinical Outcome-Based Monte Carlo Simulations. Antimicrob Agents Chemother. 2005;49(10):4009-4014. PubMed
25. Cazaubon Y, Bourguignon L, Goutelle S, Martin O, Maire P, Ducher M. Are ciprofloxacin dosage regimens adequate for antimicrobial efficacy and prevention of resistance? Pseudomonas aeruginosa bloodstream infection in elderly patients as a simulation case study. Fundam Clin Pharmacol. 2015;29(6):615-624. PubMed
26. Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob Agents Chemother. 2005;49(4):1306-1311. PubMed
27. Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of Combination Antimicrobial Therapy for Pseudomonas aeruginosa Bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756-2764. PubMed
28. The Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) Emerging Infections Program Network Streptococcus pneumoniae, 2013. https://www.cdc.gov/abcs/reports-findings/survreports/spneu13.pdf. Published November, 2014. Accessed September 26, 2017.
29. Brandenburg JA, Marrie TJ, Coley CM, et al. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15(9):638-646. PubMed
30. Musher DM, Alexandraki I, Graviss EA, et al. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210-221. PubMed
31. Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001; 161(6):848-850. PubMed
32. Oosterheert JJ, Bonten MJM, Schneider MME, et al. Effectiveness of early switch from intravenous to oral antibiotics in severe community acquired pneumonia: multicentre randomised trial. BMJ. 2006;333(7580):1193. PubMed
33. Austrian R, Winston AL. The efficacy of penicillin V (phenoxymethyl-penicillin) in the treatment of mild and of moderately severe pneumococcal pneumonia. Am J Med Sci. 1956;232(6):624-628. PubMed
34. Waterer GW, Somes GW, Wunderink RG. Monotherapy May Be Suboptimal for Severe Bacteremic Pneumococcal Pneumonia. Arch Intern Med. 2001; 161(15):1837-1842. PubMed
35. Baddour LM, Yu VL, Klugman KP, et al. International Pneumococcal Study Group. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440-444. PubMed
36. Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Bacteremia due to beta-hemolytic streptococcus group g: increasing incidence and clinical characteristics of patients. Am J Med. 2002;112(8):622-626. PubMed
37. Davies HD, McGeer A, Schwartz B, Green, et al; Ontario Group A Streptococcal Study Group. Invasive Group A Streptococcal Infections in Ontario, Canada. N Engl J Med. 1996;335(8):547-554. PubMed
38. Farley MM, Harvey C, Stull T, et al. A Population-Based Assessment of Invasive Disease Due to Group B Streptococcus in Nonpregnant Adults. N Engl J Med. 1993;328(25):1807-1811. PubMed
39. Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478-486. PubMed
40. Betriu C, Gomez M, Sanchez A, Cruceyra A, Romero J, Picazo JJ. Antibiotic resistance and penicillin tolerance in clinical isolates of group B streptococci. Antimicrob Agents Chemother. 1994;38(9):2183-2186. PubMed
41. Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100. PubMed
42. Chen I, Kaufisi P, Erdem G. Emergence of erythromycin- and clindamycin-resistant Streptococcus pyogenes emm 90 strains in Hawaii. J Clin Microbiol. 2011;49(1):439-441. PubMed
43. Biedenbach DJ, Jones RN. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25(1):47–51. PubMed
44. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS, Pavia AT. Sanford Guide To Antimicrobial Therapy 2017. Dallas, TX. Antimicrobial Theapy, Inc, 2017.
45. Biedenbach DJ, Toleman MA, Walsh TR, Jones RN. Characterization of fluoroquinolone-resistant beta-hemolytic Streptococcus spp. isolated in North America and Europe including the first report of fluoroquinolone-resistant Streptococcus dysgalactiae subspecies equisimilis: report from the SENTRY Antimicrobial Surveillance Program (1997-2004). Diagn Microbiol Infect Dis. 2006;55(2):119-127. PubMed
46. Shurland S, Zhan M, Bradham DD, Roghmann M-C. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):2739. PubMed
47. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis. 2013;56(4):527-535. PubMed
48. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-55. PubMed
49. Adra M, Lawrence KR. Trimethoprim/Sulfamethoxazole for Treatment of Severe Staphylococcus aureus Infections. Ann Pharmacother. 2004;38(2):338-341. PubMed
50. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117(5):390-398. PubMed
51. Paul M, Bishara J, Yahav D, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ. 2015;350:h2219. PubMed
52. Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260-2264. PubMed
53. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481-1490. PubMed
54. Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother. 2005;56(5):923-929. PubMed
55. Kisgen JJ, Mansour H, Unger NR, Childs LM. Tedizolid: a new oxazolidinone antimicrobial. Am J Health-Syst Pharm. 2014;71(8):621-633. PubMed
56. Gade ND, Qazi MS. Fluoroquinolone Therapy in Staphylococcus aureus Infections: Where Do We Stand? J Lab Physicians. 2013;5(2):109-112. PubMed
57. Kingsley J, Mehra P, Lawrence LE, et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother. 2016;71(3):821-829. PubMed
58. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet. 1989;2(8671):1071-1073. PubMed
59. Schrenzel J, Harbarth S, Schockmel G, et al. A Randomized Clinical Trial to Compare Fleroxacin-Rifampicin with Flucloxacillin or Vancomycin for the Treatment of Staphylococcal Infection. Clin Infect Dis. 2004;39(9):1285-1292. PubMed
60. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788-802. PubMed
61. Antony SJ, Diaz-Vasquez E, Stratton C. Clinical experience with linezolid in the treatment of resistant gram-positive infections. J Natl Med Assoc. 2001;93(10):386-391. PubMed
62. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis. 2015;60(4):528-535. PubMed
63. Martínez-Martínez L, Joyanes P, Pascual A, Terrero E, Perea EJ. Activity of eight fluoroquinolones against enterococci. Clin Microbiol Infect. 1997;3(4):497-499. PubMed
64. Balli EP, Venetis CA, Miyakis S. Systematic Review and Meta-Analysis of Linezolid versus Daptomycin for Treatment of Vancomycin-Resistant Enterococcal Bacteremia. Antimicrob Agents Chemother. 2014;58(2):734-739. PubMed
70. Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51(5):1649-1655. PubMed
69. Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis. 2010;50 Suppl 1:S26-33. PubMed
68. Karlowsky JA, Walkty AJ, Adam HJ, Baxter MR, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistance among clinical isolates of Bacteroides fragilis group in Canada in 2010-2011: CANWARD surveillance study. Antimicrob Agents Chemother. 2012;56(3):1247-1252. PubMed
67. Salonen JH, Eerola E, Meurman O. Clinical significance and outcome of anaerobic bacteremia. Clin Infect Dis. 1998;26(6):1413-1417. PubMed
66. Britt NS, Potter EM, Patel N, Steed ME. Comparison of the Effectiveness and Safety of Linezolid and Daptomycin in Vancomycin-Resistant Enterococcal Bloodstream Infection: A National Cohort Study of Veterans Affairs Patients. Clin Infect Dis. 2015;61(6):871-878. PubMed
65. Chuang YC, Lin HY, Chen PY, et al. Effect of Daptomycin Dose on the Outcome of Vancomycin-Resistant, Daptomycin-Susceptible Enterococcus faecium Bacteremia. Clin Infect Dis. 2017;64(8):1026-1034. PubMed
© 2018 Society of Hospital Medicine
Immunotherapy-Induced Colitis: An Emerging Problem for the Hospitalist
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
Immune checkpoint inhibitors (ICIs), a form of immunotherapy, have changed the management of cancer since their introduction in 2011.1 They were initially tested on melanoma.2 Their use in the advanced stages of the disease demonstrated a 2-year survival of 18% compared with 5% by using other therapies.3 Similar results were observed in nonsmall cell lung carcinoma (NSCLC); the overall survival benefit was 3 months with the use of ICIs compared with traditional chemotherapy (42% and 24% at 1 year, respectively).4 Antitumor activity has also been seen in the treatment of other malignancies, including renal cell carcinoma,5 bladder carcinoma,6,7 head and neck carcinoma,8 colorectal cancer,9 Hodgkin lymphoma,10 and, more recently, hepatocellular carcinoma.11 The use of ICIs has also been linked to serious complications.12 Although the skin, kidneys, lungs, and endocrine and nervous systems may be affected, complications of the gastrointestinal (GI) tract are frequent and can be life-threatening.12-16 We performed a thorough review of the literature to familiarize hospitalists with the mechanism of action and uses of ICIs, the clinical presentation of their GI toxicity, and the current recommendations regarding diagnosis and treatment.
CASE PRESENTATION
A 66-year-old man was admitted to our institution with a 1-week history of severe, diffuse abdominal pain and profuse watery diarrhea. He reported having more than 8 watery bowel movements per day and denied fever, recent travel, ill contacts, or ingestion of undercooked food. He had a history of metastatic melanoma and was undergoing treatment with both nivolumab and ipilimumab; the drugs were started 6 weeks prior to presentation. Physical examination revealed a heart rate of 110 beats/minute while supine and 123 beats/minute while standing, blood pressure of 112/69 mm Hg while supine and 92/62 mm Hg while standing, and a temperature of 37.2°C. He was in mild distress and had dry oral mucosa. Abdominal examination revealed hyperactive bowel sounds and mild diffuse abdominal tenderness with no guarding or rebound. His extremities were cool, but peripheral pulses were present. Initial laboratory results included a hemoglobin level of 15.3 g/dL (range 12.0-16.0 mg/dL), white blood cell count 14.2 × 109/L (range 4.5-11.0 × 109/L), and platelet count 236 × 109/L (range 150-400 × 109/L); other test results included a sodium level of 130 mmol/L (range 135-145 mmol/L), potassium 2.3 mmol/L (range 3.5-5.5 mmol/L), serum creatinine 2.2 mg/dL (range 0.8-1.3 mg/dL), blood urea nitrogen 72 mg/dL (range 8-21 mg/dL), and serum venous lactate 5.9 mmol/L (range 0.9-1.7 mmol/L).
MECHANISM OF ACTION AND USES OF ICIS
T-cell lymphocytes play a pivotal role in acquired immunity, but their function requires an appropriate balance between stimulatory and inhibitory signals to prevent autoimmunity.17 Immune checkpoint molecules are used by the immune system to assist with this balance.18 Although several of these molecules exist, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) are among the most widely studied.12
Ipilimumab is a monoclonal antibody directed against CTLA-4.24 After demonstrating survival benefits in patients with unresectable and metastatic melanoma, ipilimumab was the first ICI approved for use by the US Food and Drug Administration (FDA).1,3 Another monoclonal antibody directed against CTLA-4, tremelimumab, is not currently approved for use by the FDA.
TOXIC PROFILE
Because of the sustained T-cell activation, ICIs have been associated with autoimmune-like toxicities known as immune-related adverse events (irAEs).19,31 Because the PD-1/PD-L1 pathway is more tumor-specific than the CTLA-4 pathway,21-23 there is a higher incidence of serious irAEs seen with ipilimumab, reported to be around 27%.18,22 Furthermore, the risk of developing irAEs is dose-dependent and can increase up to 55% when anti-CTLA-4 are used with other ICIs such as nivolumab.13,32-34
The skin and GI tract are the most commonly involved organs.14-16 Skin is affected in 50% of patients receiving ipilimumab and 40% of patients on nivolumab or pembrolizumab, often in the form of a rash or pruritus.12,35-37 The rash is often described as faintly erythematous, reticular, and maculopapular and typically affects the trunk and extremities.38 Importantly, these events usually occur within the first 2 weeks of treatment, and fewer than 5% are severe.12,36,39 A higher percentage of severe adverse events occurs in the GI tract, with a reported incidence of 12%.3,14,36,39
CLINICAL PRESENTATION
Colitis, defined by either the presence of symptoms or radiologic findings suggestive of inflammation, occurs less often than diarrhea alone, with a reported incidence of 2.3%.37,43 This incidence increases to almost 12% when anti-CTLA-4 and anti-PD-1/PD-L1 are combined.32 Colitis symptoms include abdominal pain (20%), nausea and vomiting (15%), fever (12%), and, less often, bloody diarrhea or rectal bleeding.19,20 Colitis severity is graded according to the CTCAE (Table 2).42 Most patients have mild colitis (grade 1 or 2).19 The risk for developing severe colitis (grade 3 or higher) is almost 10 times higher with the use of anti-CTLA-4 compared with anti-PD-1/PD-L1 agents.43 Patients with severe disease are at risk of developing life-threatening complications, such as ileus, toxic megacolon, bowel ischemia, necrosis, or even perforation, which has been reported in up to 5% of patients with colitis because of ipilimumab.13,17
CASE APPROACH STRATEGY
Based on the patient’s symptoms, physical findings, and temporal relationship to ICI therapy, he was believed to have immune-mediated colitis. Stool studies, including those looking for ova and parasites, C
DIAGNOSIS
In a patient undergoing ICI treatment who has diarrhea, the initial assessment should exclude C. difficile and Salmonella by stool culture, PCR, or pathogenic antigens.19 Cytomegalovirus reactivation should also be considered. Immune-mediated colitis and infection can coexist; thus, a positive infectious etiology does not rule out the presence of immune colitis or vice versa.44 Fecal calprotectin, a marker of neutrophil-associated inflammation, is nonspecific for ICI-induced colitis; however, it may help to distinguish inflammatory from noninflammatory diarrhea.33,45
No clear guideline exists for the use of abdominal imaging. Some experts suggest using computed tomography in patients with severe, persistent, or progressive symptoms in order to exclude bowel obstruction, toxic megacolon, or perforation.19,46
In patients with typical symptoms, and after infectious etiologies are ruled out, empiric use of corticosteroids can be initiated without an endoscopic evaluation, which is not necessary to establish a diagnosis and rarely changes management.12,37,47 In patients with atypical presentations or for whom the diagnosis remains in question, endoscopic evaluation with biopsies may be required. Macroscopic findings may be similar to those seen with inflammatory bowel disease (IBD), including erythema, edema, ulceration, granularity, or loss of vascular pattern. Although immune-mediated colitis affects the descending colon more often than IBD, this feature and any macroscopic findings are insufficient to make this distinction.20,36 Furthermore, the lack of macroscopic abnormalities does not rule out immune-mediated colitis.20
When endoscopic biopsies are obtained, histologic findings for anti-CTLA-4 medications (eg, ipilimumab) usually follow 3 patterns: neutrophilic infiltrate (46%), lymphocytic infiltrate (15%), and mixed infiltrate (38%).41 Other findings include crypt abscesses and tissue destruction.20 No biopsy-specific pattern has been described with anti-PD-1/PD-L1 medications, such as nivolumab or pembrolizumab.18 A normal colonic tissue does not exclude the presence of an irAE, as cases of isolated ileitis48 or enteritis49 without colitis can also occur.
CASE MANAGEMENT STRATEGY
The patient was started on intravenous (IV) methylprednisolone 2 mg/kg twice a day. After 48 hours, he still had more than 7 episodes of diarrhea per day, so he was treated with 1 dose of infliximab 5 mg/kg without stopping corticosteroids. Within 72 hours, the patient’s abdominal pain improved and his diarrhea stopped. He was discharged on an 8-week taper of prednisone starting at 1 mg/kg/day, pneumocystis pneumonia (PCP) prophylaxis was started, and ICI therapy was discontinued indefinitely.
MANAGEMENT OF COLITIS
Management of grade 1 and 2 colitis is mainly supportive, consisting of fluid and electrolyte replacement, the American Dietetic Association colitis diet, and antimotility agents, such as loperamide, oral diphenoxylate hydrochloride, or atropine sulfate.36,37 Persistent grade 2 symptoms (lasting >3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral prednisone or an equivalent.19 If symptoms do not improve with oral corticosteroids, patient hospitalization for IV corticosteroids should be considered.37 Importantly, opioids and antidiarrheals may mask the pain and severity of symptoms and, therefore, should be used cautiously.19
Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require the use of systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or an equivalent.15 Patients who fail to respond to prednisone alone may benefit from the addition of oral budesonide at a dose of 9 to 12 mg/day.50 In severe cases of colitis, hospitalization may be necessary for IV hydration, electrolyte replacement, and IV methylprednisolone at a starting dose of 2 mg/kg twice a day for 1 to 2 days before transitioning to oral corticosteroids.12,15 Though improvement is usually noted within the first 2 weeks of treatment, prednisone should be slowly tapered over a period of 4 to 8 weeks to ensure complete healing and prevent relapse.20,36 Patients who receive an equivalent dose of prednisone 20 mg daily during a period of 4 weeks or more should receive PCP prophylaxis.51 Some patients fail to respond to IV corticosteroids despite adequate dosing. Many of these patients have severe disease, possibly because of delayed recognition and initiation of treatment.19 As with IBD, the addition of infliximab to corticosteroids at 5 mg/kg as a single dose is usually successful for this population subset.52-54 Although a response is seen within 1 to 3 days,41 some patients benefit from an additional dose of infliximab 2 weeks after the initial dose.19 If sepsis or perforation is suspected at any point, corticosteroids or infliximab should be avoided and antibiotics should be started immediately.15,19 Patients with a medically unresponsive disease may require partial or complete colectomy.20 The use of prophylactic budesonide to prevent diarrhea or colitis has not been proven effective and should not be used.55 Despite complications, mortality from colitis has markedly decreased given the increased awareness of this adverse event, reduction in the time to recognition and treatment, and increased adherence to corticosteroids.12
Treating physicians may be delayed in starting appropriate therapy because patients are concerned that using corticosteroids will negatively impact immunotherapy efficacy. Current evidence shows that the use of temporary immunosuppression to treat irAEs does not affect overall survival, efficacy, or time to treatment failure of the ICI.12,56 Restarting ICI therapy is a complex decision and should always be individualized. In grade 1 and 2 colitis, ICI therapy is typically restarted after symptoms have improved.5 In grade 3 and 4 colitis, ICI therapy is often permanently discontinued.20
CONCLUSION
ICIs have not only increased our understanding of the biology of cancer, but they have also improved survival in advanced stages of malignancies like melanoma, NSCLC, and renal cell carcinoma. The expanding use of these medications increases the likelihood that healthcare providers will encounter patients experiencing their adverse events.
Immune-mediated GI adverse events include a wide range of symptoms, from mild diarrhea to severe colitis complicated by perforation and death. Diagnosis requires exclusion of an infectious process. Early recognition and treatment with corticosteroids or another immunosuppressant such as infliximab hastens recovery and decreases complications and mortality. Treatment should be started within 5 days of symptom onset. Corticosteroids should be slowly tapered for no less than 4 weeks to prevent relapse and PCP prophylaxis administered in appropriate patients. Restarting ICI therapy may be considered in cases of mild colitis, but in severe cases, ICI therapy is usually discontinued.
Disclosure
Julian Marin-Acevedo, Dana Harris, and M. Caroline Burton have no conflicts of interest or funding sources to declare.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
1. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. PubMed
2. Ribas A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin Oncol. 2010;37(5):450-454. PubMed
3. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. PubMed
4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135. PubMed
5. Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430-1437. PubMed
6. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-562. PubMed
7. Massard C, Gordon MS, Sharma S, et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34(26):3119-3125. PubMed
8. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856-1867. PubMed
9. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-2520. PubMed
10. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. PubMed
11. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088)2492-2502. PubMed
12. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016;2(10):1346-1353. PubMed
13. Heinzerling L, Goldinger SM. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr Opin Oncol. 2017;29(2):136-144. PubMed
14. Kahler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277-286. PubMed
15. Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist. 2016;21(10):1230-1240. PubMed
16. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211-224. PubMed
17. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi:10.1371/journal.pone.0160221 PubMed
18. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. PubMed
19. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-417. PubMed
20. Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264-268. PubMed
21. Kamata T, Suzuki A, Mise N, et al. Blockade of programmed death-1/programmed death ligand pathway enhances the antitumor immunity of human invariant natural killer T cells. Cancer Immunol Immunother. 2016;65(12):1477-1489. PubMed
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. PubMed
23. Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206-210. PubMed
24. Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100(14):8372-8377. PubMed
25. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (urothelial carcinoma). https://www.genentech-access.com/content/dam/gene/accesssolutions/brands/tecentriq/Appeals%20Tips/TECENTRIQ-FDA-Approval-Letter-Metastatic-Urothelial-Carcinoma-First-Line-Therapy.pdf. Accessed September 30, 2017.
26. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imfinzi (durvalumab) approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761069Orig1s000ltr.pdf. Accessed September 30, 2017.
27. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) accelerated approval letter - urothelial carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761078Orig1s000ltr.pdf. Accessed May 16, 2017.
28. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter (NSCLC).
29. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Bavencio (avelumab) approval letter - Merkel cell carcinoma. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/761049Orig1s000ltr.pdf. Accessed April 27, 2017.
30. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Atezolizumab BLA 761041 approval letter. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000Approv.pdf. Accessed April 6, 2017.
31. Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi:10.1371/journal.pone.0053745. PubMed
32. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23-34. PubMed
33. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. PubMed
34. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560-575. PubMed
35. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697. PubMed
36. Kahler KC, Hassel JC, Heinzerling L, et al. Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016;14(7):662-681. PubMed
37. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76-83. PubMed
38. Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71(1):161-169. PubMed
39. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521-2532. PubMed
40. Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58(5):823-830. PubMed
41. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289. PubMed
42. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
43. De Velasco G, Je Y, Bosse D, et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5(4):312-318. PubMed
44. McCutcheon JL, McClain CM, Puzanov I, Smith TA. Infectious Colitis Associated With Ipilimumab Therapy. Gastroenterology Res. 2014;7(1):28-31. PubMed
45. Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11-20. PubMed
46. Reynolds K, Ananthakrishnan A, Dougan M, Bardia A. Immune-Related Adverse Events (irAEs) in Cancer Patients. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York: McGraw-Hill Education; 2017.
47. Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunol Res. 2017;5(4):286-291. PubMed
48. Venditti O, De Lisi D, Caricato M, et al. Ipilimumab and immune-mediated adverse events: a case report of anti-CTLA4 induced ileitis. BMC Cancer. 2015;15:87-91. PubMed
49. Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced Enteritis without Colitis: A New Challenge. Case Rep Oncol. 2016;9(3):705-713. PubMed
50. De Felice KM, Gupta A, Rakshit S, et al. Ipilimumab-induced colitis in patients with metastatic melanoma. Melanoma Res. 2015;25(4):321-327. PubMed
51. Baden LR, Swaminathan S, Angarone M, et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Newt. 2017;14(7):882-913. PubMed
52. Minor DR, Chin K, Kashani-Sabet M. Infliximab in the treatment of anti-CTLA4 antibody (ipilimumab) induced immune-related colitis. Cancer Biother Radiopharm. 2009;24(3):321-325. PubMed
53. Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806-810. PubMed
54. Pages C, Gornet JM, Monsel G, et al. Ipilimumab-induced acute severe colitis treated by infliximab. Melanoma Res. 2013;23(3):227-230. PubMed
55. Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15(17):5591-5598. PubMed
56. Horvat TZ, Adel NG, Dung TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193-3198. PubMed
57. Cancer Therapy Evaluation Program, National Cancer Institute (NCI). Common terminology criteria for adverse events v3.0 (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed April 9, 2017.
© 2018 Society of Hospital Medicine
Transient neurologic syndromes: A diagnostic approach
Many patients present to their primary care physicians, urgent care centers, and emergency rooms because of neurologic symptoms lasting seconds to hours. Their problems can be a cause for concern and a challenge to diagnose, as in many cases their symptoms have returned to baseline by the time of evaluation. Referral to a neurologist may not be practical for all of them, particularly given that a consultation may take a long time to obtain.
Understanding the causes of transient neurologic syndromes and their phenomenology may help the clinician diagnose, triage, and treat such conditions effectively.
Here, we outline several transient neurologic syndromes—transient ischemic attack (TIA), migraine with aura, partial seizures, hypoglycemic encephalopathy, hyperventilation syndrome, transient global amnesia, narcolepsy, parasomnias, and some rarer conditions— focusing on their diagnostic elements. Others, such as drug-induced transient neurologic syndromes, vertigo, and dizziness, have been well discussed elsewhere.1–3
THE BIG 3: TIA, MIGRAINE, SEIZURES
A 45-year-old woman with a history of tobacco use and headaches presents to the emergency department with a 4-month history of episodic numbness and tingling of her right arm and face. She reports a prodromal state of anxiety and irritability 24 to 48 hours before symptom onset.
The sensory symptoms begin on her face and gradually progress down the arm and eventually to her fingers. They fully resolve within 2 hours without sequelae. Family members have noted some “slurred speech” during the episodes, and the episodes are occasionally preceded by a unilateral, throbbing headache that improves with rest.
What are the possible causes of her symptoms?
Transient ischemic attack
If a patient reports transient neurologic symptoms and has vascular risk factors, TIA is often the default diagnostic consideration. The risk of stroke is 9.9% in the 2 days after a TIA, 13.4% at 30 days, and 17.3% at 90 days.4 Rapid recognition offers a crucial period to minimize the possibility of permanent impairment. Interventions include modifying risk factors (hypertension, diabetes, and smoking) and starting an antiplatelet drug, an anticoagulant drug, or both, and possibly a statin.
It can be difficult to determine if this workup needs to be completed in the inpatient or outpatient setting. There is no clear consensus, but the ultimate goal is timely evaluation (within 24 to 48 hours). The ABCD2 (Age, Blood pressure, Clinical features, Duration of symptoms, and Diabetes) risk factor calculator was developed to help triage patients, though it has limitations.5,6
One should assess a patient’s history of a possible TIA in a stepwise fashion. First, analyze the patient’s age and demographics for known vascular risk factors or central embolic sources (eg, atrial fibrillation). Then consider the symptoms. TIA symptoms have rapid onset, usually within seconds7; symptoms with a more gradual crescendo suggest a nonvascular cause.8 TIA manifestations should resolve within 1 hour, and most studies suggest symptom resolution within 10 minutes is specific for a TIA.9–11 TIA symptoms are negative neurologic phenomena that denote a loss of function, such as loss of vision, motor weakness, or sensory numbness.
Symptoms should also correlate with a defined vascular territory:
- The middle cerebral artery is commonly involved; its blockage is associated with aphasia, weakness of the face and arm, and homonymous visual field impairment (loss of one-half of the visual fields in both eyes)
- Blockage in the posterior circulation generally causes symptoms localized to the brainstem, cerebellum, and occipital cortex. The symptoms are usually grouped together as the “5Ds”: dizziness, diplopia, dysarthria, dysphagia, and dystaxia/ataxia. Brainstem involvement classically produces “crossed” findings, with ipsilateral cranial findings and contralateral motor or sensory findings.
- Lacunar strokes involve the subcortical white matter and produce typical patterns including pure motor or sensory syndromes.
Loss of consciousness is rarely a symptom of TIA and should suggest another etiology.
The definition of TIA has evolved from an operational one, ie, symptoms lasting less than 24 hours, to a tissue-based one, ie, focal cerebral ischemia not associated with permanent cerebral infarction.12 Though imperfect, this pathophysiology should help reinforce the most common features of TIA, including a sudden onset of negative symptoms that are localized to a defined vascular territory.13,14
Migraine with aura
Migraine with aura is common in patients ages 25 to 55 who have a long-standing history of headache. The pathophysiologic mechanism of an aura is believed to be a disseminating wave of cortical depression, which is a self-propagating wave of neural depression and then activation. Ultimately, this leads to a cascade of inflammatory and pain signals, resulting in a headache.
This background helps explain the positive (superimposed) symptoms associated with the aura. Positive symptoms are produced by excessive neuronal discharges stimulating the visual (flashing lights, zigzag lines), sensory (paresthesias), or motor (limb movements) pathways.
Common symptoms associated with aura include visual disturbances such as scintillating scotoma (a blind spot), sensory changes such as tingling, or auditory disruption with tinnitus. Symptoms may evolve over the course of 5 to 20 minutes, first affecting vision and then other senses. In contrast, in a TIA, symptoms usually begin simultaneously and are confined to a vascular territory.7,15 Symptoms of an aura usually resolve within an hour, but there is evidence showing a substantial number of patients have an aura lasting much longer.16 Focal weakness is uncommon during an aura but is reported in specific migraine conditions such as hemiplegic migraine and migraine with unilateral motor symptoms. The vast majority of patients experience other neurologic symptoms during this prodrome.17,18
The prodromal period (2 to 48 hours leading up to the onset of migraine) is a commonly overlooked feature of migraine.19 Common symptoms during this time include fatigue, mood change, and gastrointestinal symptoms.20 One study demonstrated that patients generally had good intuition concerning these nonspecific prodromal symptoms and could predict the onset of migraine 72% of the time.21
In addition, a myriad of possible triggers and exacerbating factors can be identified (and sometimes avoided) such as visual stimuli, weather changes, nitrates, sleep disturbances, menstruation, foods, and stressors.22
Although headache is often the cardinal manifestation of migraine, some patients experience aura without headache—acephalgic migraine.23 This can be a diagnostic challenge, especially in an older population with multiple vascular risk factors. New-onset acephalgic migraine may be a cause for concern but is not uncommon and is not associated with a significantly increased risk of stroke.24 Focusing on the character of the neurologic symptoms in regard to timing, progression, and resolution will help differentiate this disease from other transient neurologic syndromes.25
Partial seizure
Partial seizure produces a diverse range of stereotypical symptoms due to focal abnormal neuronal activation. The aberrant electrical firing generates positive symptoms involving the motor, sensory, or visual pathway. A history of trauma, neurosurgical intervention, central nervous system infection, stroke, or other seizure foci can suggest this diagnosis. Other prodromal clues include abdominal discomfort, sense of detachment, déjà vu, or jamais vu.26
During a seizure, there may be a progression of positive symptoms similar to what happens in migraine aura, because both represent cortical spread and depression.
Involvement of the motor pathway may produce tonic (stiffening) or clonic (twitching) movement. Other common motor abnormalities include automatisms such as lip smacking, chewing, and hand gestures (picking, fidgeting, fumbling).27
Epileptic discharges in the sensory cortex commonly cause paresthesias or distortion of a sensory input. Visual symptoms may be more complex. In occipital epilepsy, circular phenomena with a colored pattern are common, which contrasts with the photopsia (flashes of light) or fortification (a bright zigzag of lines resembling a fort) seen in migraines.28
Autonomic or somatosensory symptoms can also occur.
Todd paralysis, also called transient postictal paralysis, occurs in only 13% of seizures but can linger for 0.5 to 36 hours.29,30 This weakness is most pronounced within the affected region after a partial seizure.
In general, focal seizures are often stereotyped with positive neurologic features, usually last a few minutes, and resolve fully. These episodes may cause an arrest in activity but not usually loss of consciousness unless the epileptic discharge secondarily generalizes into the adjacent hemisphere.
A common differential diagnosis encountered during an epilepsy workup is psychogenic nonepileptic seizures. Nonepileptic seizures consist of transient, abnormal movements, sensation, or cognition but lack ictal electroencephalographic changes. This is a specifically challenging patient population, with high healthcare utilization and high risk for iatrogenic harm. In addition, on average, diagnosis can take years to establish and usually requires referral to a tertiary care facility.31,32
The big 3: Back to our patient
Our patient’s vascular risk factors, transient symptoms, and language involvement support the diagnosis of TIA. A feature that points away from the diagnosis of TIA is the gradual onset of positive neurologic symptoms. This pattern is not consistent with neuronal ischemia.
Also, our patient had a repetitive, stereotypical pattern of symptoms, which supports including partial seizures in the differential diagnosis. On the other hand, her lack of risk factors for seizure (a history of febrile seizures, developmental delay, trauma, or infection) would make this diagnosis less likely. Also pointing away from the diagnosis of seizures are her lack of typical prodromal symptoms, the length of the events, and the postevent headache.
Table 1 summarizes the clinical findings associated with TIA, migraine, and partial seizure.
EPISODES OF CONFUSION
A 35-year-old woman with a history of depression, anxiety, and poorly controlled type 1 diabetes presents to the clinic after several weeks of episodes of confusion, usually accompanied by paresthesias in both hands, dizziness, and palpitations. In each episode, soon after the symptoms began, she had painful cramps in her hand. The symptoms fully resolved within 10 minutes without sequelae.
Questioned further, the patient describes the confusion as a “mental haze” but denies frank disorientation. She has not kept a log of her blood sugar levels but has not noticed a temporal relationship with regard to her meals or insulin injections.
What are the possible causes of these episodes?
Hypoglycemic encephalopathy
Hypoglycemia is common in most people with diabetes, who have been reported to suffer from 62 to 320 severe hypoglycemic episodes in their lifetime.33,34 The neurologic consequences can be devastating in these severe cases.
During mild to moderate drops in the glucose level, generalized symptoms stem from sympathetic activation. These include generalized anxiety, tremor, palpitations, and sweating. Focal symptoms such as unilateral weakness have also been reported.35,36
Unfortunately, people with long-standing diabetes have a blunted response to epinephrine that reduces their sensitivity to hypoglycemia, placing them at high risk of permanent neurologic damage. This can lead to seizures and coma, as the hypoglycemia has a greater effect on cortical and subcortical structures (highly metabolic areas) than on the brainstem. Thus, respiratory and cardiovascular function is maintained but cerebral function is abnormal. If this state is prolonged, brain death can occur.37,38
Hyperventilation syndrome
Hyperventilation syndrome is not well characterized. Most think of it as synonymous with an underlying psychopathology, but there is evidence to suggest it can occur without underlying anxiety.
There is no clear mechanism, but it is hypothesized that diminished carbon dioxide levels lead to cerebral vasoconstriction. This may lead to reduced cerebral blood flow, causing dizziness, lightheadedness, or vertigo.39 Appendicular symptoms including paresthesias, carpopedal spasm, or tetany have been core features since the syndrome was first described in the early 1900s.40
Though the disorder has rather nonspecific features, it can be easily reproduced in the clinical setting by asking the patient to breathe deeply and rapidly. This can help confirm the underlying diagnosis and also reassure the patient that the underlying pathology is not life-threatening and that he or she has some control over the disease.
Transient global amnesia
Transient global amnesia usually strikes older patients (50 to 70 years old) in the setting of an acute physical or emotional stressor. There is also a correlation between transient global amnesia and migraine, with studies showing migraineurs are at higher risk than the general population.41 Despite common clinical concerns, there is no relationship between transient global amnesia and stroke.42
Transient global amnesia is defined by acute transient anterograde amnesia (coding of new memories). To try to reorient themselves, patients will repeatedly ask questions such as “What day is it?” or “Why are we here?” Retrograde memories, especially long-standing ones, are usually well preserved. The patient’s cognition is otherwise intact, and there are no other focal neurologic symptoms. The event usually lasts 2 to 24 hours and resolves without sequelae.43,44 Afterward, patients remember the event only poorly, which supports the notion that they cannot code new memories.
Confusional episodes: Discussion
Evaluating confusional episodes can be time-consuming and vexing. The subjective nature of the symptoms and the vast differential diagnosis can be overwhelming. Subtle clinical details can help formulate an appropriate evaluation.
Hypoglycemia can produce bizarre neurologic symptoms. Most cases of hypoglycemia produce an exaggerated sympathetic response, though this is blunted in people with longstanding diabetes. In addition, there should be a temporal association with meals, insulin doses, or both.
Transient global amnesia usually occurs with acute stressors and produces a confusional state. These episodes rarely recur, and the patient cannot provide much history regarding the episode secondary to the anterograde amnesia.
Table 2 summarizes the clinical findings associated with hypoglycemic encephalopathy, hyperventilation syndrome, and transient global amnesia.
Back to our patient
In our patient, the likely diagnosis is hyperventilation syndrome, even though we don’t know if her respiratory rate is increased during attacks. Some patients lack awareness of their breathing or are too distracted by the vague symptoms to have insight into the true cause. The cramps and contractions in the hands are a specific feature of the disease and can be accompanied by confusion.
SLEEP DISORDERS
A 17-year-old boy with a history of depression and anxiety presents to his pediatrician because he has had difficulty staying awake in school over the past year. His sleepiness has gradually worsened over the last few months and has taken a toll on his grades, leading to discord in his family. Over the past month he has had some difficulty holding his head up during arguments with friends. He does not lose consciousness during these events but is described as “unresponsive.” He describes vivid dreams when going to sleep that have startled him awake at times. His family history is positive for somnambulism on his father’s side.
Does this patient have a sleep disorder, and if so, which one?
Narcolepsy
Narcolepsy is defined by excessive daytime sleepiness, cataplexy, hypnagogic hallucination, and sleep paralysis. It is more common in men but its prevalence varies widely by geographic region, supporting an underlying interplay between genetics and environment.45
Sleep attacks or excessive daytime sleepiness are the cardinal features of narcolepsy. The dissociation between the sleep-wake cycle is evident with rapid transition into rapid-eye-movement (REM) sleep during these sleep attacks. This results in a “refreshing nap” that commonly involves vivid dreams. These episodes occur about 3 to 5 times per day, varying in duration from a few minutes to hours.46
Cataplexy is very specific feature of narcolepsy. Triggered by strong emotion, the body loses skeletal muscle tone except for the diaphragm and ocular muscles. The patient does not lose consciousness and remains aware of his or her environment. Of note, the loss of tone does not need to be dramatic. The hypotonia can manifest as jaw-dropping or head-nodding. The paralysis is related to prolonged REM atonia and impaired transition from sleep to wakefulness.47 Hypnagogic hallucination and sleep paralysis can occur, together with vivid visual hallucinations.
Parasomnias: Somnambulism and night terrors
Most non-REM parasomnias occur in childhood and diminish in adulthood. Two of the most common disorders are sleepwalking (somnambulism) and night terrors. Both are characterized by arousal from slow-wave sleep and are commonly associated with sedating medication, sleep deprivation, or psychopathology.
In somnambulism, patients exhibit complex motor behavior without interaction with their environment. Most have little recollection of the event.48 Sleep terrors produce a more intense reaction. The patient erupts out of sleep with profound terror, confusion, and autonomic changes. Interestingly, the patient can normally fall right back into sleep after the event.49–51
Back to our patient
Excessive daytime sleepiness and generalized fatigue are commonly encountered in outpatients. They can be frustrating because in many cases, no clear etiology can be discovered.52
This patient has several risk factors for parasomnias. His history of anxiety and depression in the setting of recent stressors sets the stage for night terrors. In addition, like many patients with parasomnias, he has a family history of sleep disorders. His vivid dreams make night terrors possible, but without the stark sympathetic activation it is a less likely diagnosis. It also does not account for the other symptoms he describes.
Our patient’s excessive daytime sleepiness interfering with daily activities, cataplexy, and hypnagogic hallucinations support the diagnosis of narcolepsy. This case highlights the variable weakness experienced during a cataplexy attack. It can range from a simple head droop to complete paralysis. Subtle findings require specific probing by the clinician. Patients with narcolepsy typically present in their late teens to early adulthood, but the cataplexy attacks may develop later in the disease course.
Table 3 summarizes the clinical findings associated with night terrors, somnambulism, and narcolepsy.
RARE CAUSES OF TRANSIENT NEUROLOGIC SYMPTOMS
Transient (paroxysmal) neurologic events in multiple sclerosis
A less well-known phenomenon in multiple sclerosis is termed “transient” (paroxysmal) neurologic events. These are typically stereotyped episodes lasting seconds, occurring sometimes hundreds of times a day. They are thought to arise from spontaneous electrical activity in an area of demyelination (ephaptic transmission), creating a wide range of symptoms. Some common events include positive sensory symptoms, alteration of the motor system such as spasms, or brainstem symptoms.53
Channelopathy
Two prototypical channelopathies are hyperkalemic and hypokalemic periodic paralysis. They are rare conditions, usually inherited in an autosomal dominant pattern.54 Both produce episodic, flaccid weakness in the setting of activity or other stressors (fasting, pregnancy, an emotionally charged episode). The attacks last a few minutes to hours and affect proximal skeletal muscles, with very little respiratory or bulbar involvement.
Hyperkalemic periodic paralysis is also associated with myotonia, which is the inability to voluntarily relax after stimulation. This can be evident after shaking a patient’s hand, as he or she would be unable to release because of the sustained activation. The myotonia is evident between attacks and may help cue a physician to the diagnosis even if the weakness has abated.55
As the name implies, potassium levels can vary during the attack, though hyperkalemic periodic paralysis can be seen with normal levels of serum potassium. The underlying pathology is tied to a voltage-gated sodium channel or calcium channel necessary for action potential generation.56
Paroxysmal dyskinesias
Paroxysmal dyskinesias encompass a rare group of movement disorders characterized by attacks without alterations in consciousness. Patients have reported dystonic, choreoathetotic, or ballistic movements. The attacks can be triggered by stress, eating, or even other types of movements. Most reported cases have a strong family history and are inherited in an autosomal dominant pattern. The exact pathophysiology is unclear. When paroxysmal dyskinesia was initially discovered, many thought it was a form of epilepsy, but the lack of electroencephalographic changes and postictal events argues against this etiology.
Transient focal neurologic episodes in cerebral amyloid angiopathy
Cerebral amyloid angiopathy is a degenerative condition in which amyloid is deposited in cerebral vessels, making them friable and at risk of bleeding. Most patients have no symptoms whatsoever, and the diagnosis is made by magnetic resonance imaging. Small microbleeds are common, but lobar intraparenchymal hemorrhage is the most feared complication.
Transient focal neurologic episodes, sometimes termed “amyloid spells,” are recurrent, stereotyped neurologic events that are spurred by cortical superficial siderosis (deposition of iron). Unfortunately, these events are difficult to characterize by their clinical morphology. The events can involve the visual, motor, and sensory pathways with both positive and negative symptoms, making the diagnosis difficult without imaging. These events may precede a symptomatic intraparenchymal hemorrhage, offering a unique window to reconsider the decision to continue an antiplatelet or anticoagulant drug.57,58
- Vuadens P, Regli F. Drug-induced neurological complications in a hospital cohort. Schweiz Med Wochenschr 1995; 125:1625–1633. French.
- Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract 2001; 51:666–671.
- Brignole M. Diagnosis and treatment of syncope. Heart 2007; 93:130–136.
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6:1063–1072.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015; 85:373–380.
- Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol 2014; 14:23–31.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am 2011; 21:303–313.
- Kimura K, Minematsu K, Yasaka M, Wada K, Yamaguchi T. The duration of symptoms in transient ischemic attack. Neurology 1999; 52:976–980.
- Lewandowski CA, Rao CP, Silver B. Transient ischemic attack: definitions and clinical presentations. Ann Emerg Med 2008; 52:S7–S16.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007; 298:2877–2885.
- van Rooij FG, Vermeer SE, Goraj BM, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol 2015; 78:1005–1010.
- Silberstein SD, Young WB. Migraine aura and prodrome. Semin Neurol 1995; 15:175–182.
- Viana M, Sprenger T, Andelova M, Goadsby PJ. The typical duration of migraine aura: a systematic review. Cephalalgia 2013; 33:483–490.
- Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry 2007; 78:600–604.
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125:1379–1391.
- Buzzi MG, Cologno D, Formisano R, Rossi P. Prodromes and the early phase of the migraine attack: therapeutic relevance. Funct Neurol 2005; 20:179–183.
- Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 2004; 44:865–872.
- Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003; 60:935–940.
- Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001; 85:911–941.
- Naeije G, Gaspard N, Legros B, Mavroudakis N, Pandolfo M. Transient CNS deficits and migrainous auras in individuals without a history of headache. Headache 2014; 54:493–499.
- Tuna MA, Mehta Z, Rothwell PM; Stroke Prevention Research Unit, Neuroscience Department, John Radcliffe Hospital, Oxford University. Stroke risk after a first late–onset migraine–like transient neurological attack (TNA): Oxford vascular study TNA cohort. J Neurol Neurosurg Psychiatry 2013; 84:e2.
- Fisher CM. Late-life migraine accompaniments—further experience. Stroke 1986; 17:1033–1042.
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Wyllie E, Rothner AD, Luders H. Partial seizures in children: clinical features, medical treatment, and surgical considerations. Pediatr Clin North Am 1989; 36:343–364.
- Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord 1999; 1:205–216.
- Gallmetzer P, Leutmezer F, Serles W, Assem-Hilger E, Spatt J, Baumgartner C. Postictal paresis in focal epilepsies—incidence, duration, and causes: a video-EEG monitoring study. Neurology 2004; 62:2160–2164.
- Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd’s post-epileptic paralysis. J Neurol Neurosurg Psychiatry 1992; 55:63–64.
- Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003; 4:205–216.
- LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50:1140–1147.
- Cryer PE, Axelrod L, Grossman AB, et al; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, Itoh H. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci 2012; 117:347–351.
- Lee SH, Kang CD, Kim SS, et al. Lateralization of hypoglycemic encephalopathy: evidence of a mechanism of selective vulnerability. J Clin Neurol 2010; 6:104–108.
- Siegel GJ, Agranoff BW. Basic neurochemistry: molecular, cellular, and medical aspects. 6th ed. Philadelphia, PA: Lippincott-Williams & Wilkins; 1999.
- Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia—a prospective population-based study. Exp Clin Endocrinol Diabetes 2003; 111:364–369.
- Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke 1972; 3:566–575.
- Kerr WJ, Gliebe PA, Dalton JW. Physical phenomena associated with anxiety states: the hyperventilation syndrome. Cal West Med 1938; 48:12–16.
- Lin KH, Chen YT, Fuh JL, et al. Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol 2014; 21:718–724.
- Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 2017; 92:399–405.
- Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc 2015; 90:264–272.
- Bartsch T, Butler C. Transient amnesic syndromes. Nat Rev Neurol 2013; 9:86–97.
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373:2654–2662.
- Ahmed I, Thorpy M. Clinical features, diagnosis and treatment of narcolepsy. Clin Chest Med 2010; 31:371–381.
- Leschziner G. Narcolepsy: a clinical review. Pract Neurol 2014; 14:323–331.
- Hughes JR. A review of sleepwalking (somnambulism): the enigma of neurophysiology and polysomnography with differential diagnosis of complex partial seizures. Epilepsy Behav 2007; 11:483–491.
- Gremmo M, Blanchi I, Costa B, et al. An abilitative approach to the premature infant in neonatal intensive care unit (NICU). J Perinat Med 1994; 22(suppl 1):102–105.
- Howell MJ. Parasomnias: an updated review. Neurotherapeutics 2012; 9:753–775.
- Giglio P, Undevia N, Spire JP. The primary parasomnias. A review for neurologists. Neurologist 2005; 11:90–97.
- Viner R, Christie D. Fatigue and somatic symptoms. BMJ 2005; 330:1012–1015.
- Rae-Grant AD. Unusual symptoms and syndromes in multiple sclerosis. Continuum (Minneap Minn) 2013; 19:992–1006.
- Fontaine B. Periodic paralysis. Adv Genet 2008; 63:3–23.
- Jurkat-Rott K, Lehmann-Horn F. Paroxysmal muscle weakness: the familial periodic paralyses. J Neurol 2006; 253:1391–1398.
- Lehmann-Horn F, Jurkat-Rott K, Rudel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep 2002; 2:61–69.
- Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007; 68:457–460.
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012; 43:2324–2330.
Many patients present to their primary care physicians, urgent care centers, and emergency rooms because of neurologic symptoms lasting seconds to hours. Their problems can be a cause for concern and a challenge to diagnose, as in many cases their symptoms have returned to baseline by the time of evaluation. Referral to a neurologist may not be practical for all of them, particularly given that a consultation may take a long time to obtain.
Understanding the causes of transient neurologic syndromes and their phenomenology may help the clinician diagnose, triage, and treat such conditions effectively.
Here, we outline several transient neurologic syndromes—transient ischemic attack (TIA), migraine with aura, partial seizures, hypoglycemic encephalopathy, hyperventilation syndrome, transient global amnesia, narcolepsy, parasomnias, and some rarer conditions— focusing on their diagnostic elements. Others, such as drug-induced transient neurologic syndromes, vertigo, and dizziness, have been well discussed elsewhere.1–3
THE BIG 3: TIA, MIGRAINE, SEIZURES
A 45-year-old woman with a history of tobacco use and headaches presents to the emergency department with a 4-month history of episodic numbness and tingling of her right arm and face. She reports a prodromal state of anxiety and irritability 24 to 48 hours before symptom onset.
The sensory symptoms begin on her face and gradually progress down the arm and eventually to her fingers. They fully resolve within 2 hours without sequelae. Family members have noted some “slurred speech” during the episodes, and the episodes are occasionally preceded by a unilateral, throbbing headache that improves with rest.
What are the possible causes of her symptoms?
Transient ischemic attack
If a patient reports transient neurologic symptoms and has vascular risk factors, TIA is often the default diagnostic consideration. The risk of stroke is 9.9% in the 2 days after a TIA, 13.4% at 30 days, and 17.3% at 90 days.4 Rapid recognition offers a crucial period to minimize the possibility of permanent impairment. Interventions include modifying risk factors (hypertension, diabetes, and smoking) and starting an antiplatelet drug, an anticoagulant drug, or both, and possibly a statin.
It can be difficult to determine if this workup needs to be completed in the inpatient or outpatient setting. There is no clear consensus, but the ultimate goal is timely evaluation (within 24 to 48 hours). The ABCD2 (Age, Blood pressure, Clinical features, Duration of symptoms, and Diabetes) risk factor calculator was developed to help triage patients, though it has limitations.5,6
One should assess a patient’s history of a possible TIA in a stepwise fashion. First, analyze the patient’s age and demographics for known vascular risk factors or central embolic sources (eg, atrial fibrillation). Then consider the symptoms. TIA symptoms have rapid onset, usually within seconds7; symptoms with a more gradual crescendo suggest a nonvascular cause.8 TIA manifestations should resolve within 1 hour, and most studies suggest symptom resolution within 10 minutes is specific for a TIA.9–11 TIA symptoms are negative neurologic phenomena that denote a loss of function, such as loss of vision, motor weakness, or sensory numbness.
Symptoms should also correlate with a defined vascular territory:
- The middle cerebral artery is commonly involved; its blockage is associated with aphasia, weakness of the face and arm, and homonymous visual field impairment (loss of one-half of the visual fields in both eyes)
- Blockage in the posterior circulation generally causes symptoms localized to the brainstem, cerebellum, and occipital cortex. The symptoms are usually grouped together as the “5Ds”: dizziness, diplopia, dysarthria, dysphagia, and dystaxia/ataxia. Brainstem involvement classically produces “crossed” findings, with ipsilateral cranial findings and contralateral motor or sensory findings.
- Lacunar strokes involve the subcortical white matter and produce typical patterns including pure motor or sensory syndromes.
Loss of consciousness is rarely a symptom of TIA and should suggest another etiology.
The definition of TIA has evolved from an operational one, ie, symptoms lasting less than 24 hours, to a tissue-based one, ie, focal cerebral ischemia not associated with permanent cerebral infarction.12 Though imperfect, this pathophysiology should help reinforce the most common features of TIA, including a sudden onset of negative symptoms that are localized to a defined vascular territory.13,14
Migraine with aura
Migraine with aura is common in patients ages 25 to 55 who have a long-standing history of headache. The pathophysiologic mechanism of an aura is believed to be a disseminating wave of cortical depression, which is a self-propagating wave of neural depression and then activation. Ultimately, this leads to a cascade of inflammatory and pain signals, resulting in a headache.
This background helps explain the positive (superimposed) symptoms associated with the aura. Positive symptoms are produced by excessive neuronal discharges stimulating the visual (flashing lights, zigzag lines), sensory (paresthesias), or motor (limb movements) pathways.
Common symptoms associated with aura include visual disturbances such as scintillating scotoma (a blind spot), sensory changes such as tingling, or auditory disruption with tinnitus. Symptoms may evolve over the course of 5 to 20 minutes, first affecting vision and then other senses. In contrast, in a TIA, symptoms usually begin simultaneously and are confined to a vascular territory.7,15 Symptoms of an aura usually resolve within an hour, but there is evidence showing a substantial number of patients have an aura lasting much longer.16 Focal weakness is uncommon during an aura but is reported in specific migraine conditions such as hemiplegic migraine and migraine with unilateral motor symptoms. The vast majority of patients experience other neurologic symptoms during this prodrome.17,18
The prodromal period (2 to 48 hours leading up to the onset of migraine) is a commonly overlooked feature of migraine.19 Common symptoms during this time include fatigue, mood change, and gastrointestinal symptoms.20 One study demonstrated that patients generally had good intuition concerning these nonspecific prodromal symptoms and could predict the onset of migraine 72% of the time.21
In addition, a myriad of possible triggers and exacerbating factors can be identified (and sometimes avoided) such as visual stimuli, weather changes, nitrates, sleep disturbances, menstruation, foods, and stressors.22
Although headache is often the cardinal manifestation of migraine, some patients experience aura without headache—acephalgic migraine.23 This can be a diagnostic challenge, especially in an older population with multiple vascular risk factors. New-onset acephalgic migraine may be a cause for concern but is not uncommon and is not associated with a significantly increased risk of stroke.24 Focusing on the character of the neurologic symptoms in regard to timing, progression, and resolution will help differentiate this disease from other transient neurologic syndromes.25
Partial seizure
Partial seizure produces a diverse range of stereotypical symptoms due to focal abnormal neuronal activation. The aberrant electrical firing generates positive symptoms involving the motor, sensory, or visual pathway. A history of trauma, neurosurgical intervention, central nervous system infection, stroke, or other seizure foci can suggest this diagnosis. Other prodromal clues include abdominal discomfort, sense of detachment, déjà vu, or jamais vu.26
During a seizure, there may be a progression of positive symptoms similar to what happens in migraine aura, because both represent cortical spread and depression.
Involvement of the motor pathway may produce tonic (stiffening) or clonic (twitching) movement. Other common motor abnormalities include automatisms such as lip smacking, chewing, and hand gestures (picking, fidgeting, fumbling).27
Epileptic discharges in the sensory cortex commonly cause paresthesias or distortion of a sensory input. Visual symptoms may be more complex. In occipital epilepsy, circular phenomena with a colored pattern are common, which contrasts with the photopsia (flashes of light) or fortification (a bright zigzag of lines resembling a fort) seen in migraines.28
Autonomic or somatosensory symptoms can also occur.
Todd paralysis, also called transient postictal paralysis, occurs in only 13% of seizures but can linger for 0.5 to 36 hours.29,30 This weakness is most pronounced within the affected region after a partial seizure.
In general, focal seizures are often stereotyped with positive neurologic features, usually last a few minutes, and resolve fully. These episodes may cause an arrest in activity but not usually loss of consciousness unless the epileptic discharge secondarily generalizes into the adjacent hemisphere.
A common differential diagnosis encountered during an epilepsy workup is psychogenic nonepileptic seizures. Nonepileptic seizures consist of transient, abnormal movements, sensation, or cognition but lack ictal electroencephalographic changes. This is a specifically challenging patient population, with high healthcare utilization and high risk for iatrogenic harm. In addition, on average, diagnosis can take years to establish and usually requires referral to a tertiary care facility.31,32
The big 3: Back to our patient
Our patient’s vascular risk factors, transient symptoms, and language involvement support the diagnosis of TIA. A feature that points away from the diagnosis of TIA is the gradual onset of positive neurologic symptoms. This pattern is not consistent with neuronal ischemia.
Also, our patient had a repetitive, stereotypical pattern of symptoms, which supports including partial seizures in the differential diagnosis. On the other hand, her lack of risk factors for seizure (a history of febrile seizures, developmental delay, trauma, or infection) would make this diagnosis less likely. Also pointing away from the diagnosis of seizures are her lack of typical prodromal symptoms, the length of the events, and the postevent headache.
Table 1 summarizes the clinical findings associated with TIA, migraine, and partial seizure.
EPISODES OF CONFUSION
A 35-year-old woman with a history of depression, anxiety, and poorly controlled type 1 diabetes presents to the clinic after several weeks of episodes of confusion, usually accompanied by paresthesias in both hands, dizziness, and palpitations. In each episode, soon after the symptoms began, she had painful cramps in her hand. The symptoms fully resolved within 10 minutes without sequelae.
Questioned further, the patient describes the confusion as a “mental haze” but denies frank disorientation. She has not kept a log of her blood sugar levels but has not noticed a temporal relationship with regard to her meals or insulin injections.
What are the possible causes of these episodes?
Hypoglycemic encephalopathy
Hypoglycemia is common in most people with diabetes, who have been reported to suffer from 62 to 320 severe hypoglycemic episodes in their lifetime.33,34 The neurologic consequences can be devastating in these severe cases.
During mild to moderate drops in the glucose level, generalized symptoms stem from sympathetic activation. These include generalized anxiety, tremor, palpitations, and sweating. Focal symptoms such as unilateral weakness have also been reported.35,36
Unfortunately, people with long-standing diabetes have a blunted response to epinephrine that reduces their sensitivity to hypoglycemia, placing them at high risk of permanent neurologic damage. This can lead to seizures and coma, as the hypoglycemia has a greater effect on cortical and subcortical structures (highly metabolic areas) than on the brainstem. Thus, respiratory and cardiovascular function is maintained but cerebral function is abnormal. If this state is prolonged, brain death can occur.37,38
Hyperventilation syndrome
Hyperventilation syndrome is not well characterized. Most think of it as synonymous with an underlying psychopathology, but there is evidence to suggest it can occur without underlying anxiety.
There is no clear mechanism, but it is hypothesized that diminished carbon dioxide levels lead to cerebral vasoconstriction. This may lead to reduced cerebral blood flow, causing dizziness, lightheadedness, or vertigo.39 Appendicular symptoms including paresthesias, carpopedal spasm, or tetany have been core features since the syndrome was first described in the early 1900s.40
Though the disorder has rather nonspecific features, it can be easily reproduced in the clinical setting by asking the patient to breathe deeply and rapidly. This can help confirm the underlying diagnosis and also reassure the patient that the underlying pathology is not life-threatening and that he or she has some control over the disease.
Transient global amnesia
Transient global amnesia usually strikes older patients (50 to 70 years old) in the setting of an acute physical or emotional stressor. There is also a correlation between transient global amnesia and migraine, with studies showing migraineurs are at higher risk than the general population.41 Despite common clinical concerns, there is no relationship between transient global amnesia and stroke.42
Transient global amnesia is defined by acute transient anterograde amnesia (coding of new memories). To try to reorient themselves, patients will repeatedly ask questions such as “What day is it?” or “Why are we here?” Retrograde memories, especially long-standing ones, are usually well preserved. The patient’s cognition is otherwise intact, and there are no other focal neurologic symptoms. The event usually lasts 2 to 24 hours and resolves without sequelae.43,44 Afterward, patients remember the event only poorly, which supports the notion that they cannot code new memories.
Confusional episodes: Discussion
Evaluating confusional episodes can be time-consuming and vexing. The subjective nature of the symptoms and the vast differential diagnosis can be overwhelming. Subtle clinical details can help formulate an appropriate evaluation.
Hypoglycemia can produce bizarre neurologic symptoms. Most cases of hypoglycemia produce an exaggerated sympathetic response, though this is blunted in people with longstanding diabetes. In addition, there should be a temporal association with meals, insulin doses, or both.
Transient global amnesia usually occurs with acute stressors and produces a confusional state. These episodes rarely recur, and the patient cannot provide much history regarding the episode secondary to the anterograde amnesia.
Table 2 summarizes the clinical findings associated with hypoglycemic encephalopathy, hyperventilation syndrome, and transient global amnesia.
Back to our patient
In our patient, the likely diagnosis is hyperventilation syndrome, even though we don’t know if her respiratory rate is increased during attacks. Some patients lack awareness of their breathing or are too distracted by the vague symptoms to have insight into the true cause. The cramps and contractions in the hands are a specific feature of the disease and can be accompanied by confusion.
SLEEP DISORDERS
A 17-year-old boy with a history of depression and anxiety presents to his pediatrician because he has had difficulty staying awake in school over the past year. His sleepiness has gradually worsened over the last few months and has taken a toll on his grades, leading to discord in his family. Over the past month he has had some difficulty holding his head up during arguments with friends. He does not lose consciousness during these events but is described as “unresponsive.” He describes vivid dreams when going to sleep that have startled him awake at times. His family history is positive for somnambulism on his father’s side.
Does this patient have a sleep disorder, and if so, which one?
Narcolepsy
Narcolepsy is defined by excessive daytime sleepiness, cataplexy, hypnagogic hallucination, and sleep paralysis. It is more common in men but its prevalence varies widely by geographic region, supporting an underlying interplay between genetics and environment.45
Sleep attacks or excessive daytime sleepiness are the cardinal features of narcolepsy. The dissociation between the sleep-wake cycle is evident with rapid transition into rapid-eye-movement (REM) sleep during these sleep attacks. This results in a “refreshing nap” that commonly involves vivid dreams. These episodes occur about 3 to 5 times per day, varying in duration from a few minutes to hours.46
Cataplexy is very specific feature of narcolepsy. Triggered by strong emotion, the body loses skeletal muscle tone except for the diaphragm and ocular muscles. The patient does not lose consciousness and remains aware of his or her environment. Of note, the loss of tone does not need to be dramatic. The hypotonia can manifest as jaw-dropping or head-nodding. The paralysis is related to prolonged REM atonia and impaired transition from sleep to wakefulness.47 Hypnagogic hallucination and sleep paralysis can occur, together with vivid visual hallucinations.
Parasomnias: Somnambulism and night terrors
Most non-REM parasomnias occur in childhood and diminish in adulthood. Two of the most common disorders are sleepwalking (somnambulism) and night terrors. Both are characterized by arousal from slow-wave sleep and are commonly associated with sedating medication, sleep deprivation, or psychopathology.
In somnambulism, patients exhibit complex motor behavior without interaction with their environment. Most have little recollection of the event.48 Sleep terrors produce a more intense reaction. The patient erupts out of sleep with profound terror, confusion, and autonomic changes. Interestingly, the patient can normally fall right back into sleep after the event.49–51
Back to our patient
Excessive daytime sleepiness and generalized fatigue are commonly encountered in outpatients. They can be frustrating because in many cases, no clear etiology can be discovered.52
This patient has several risk factors for parasomnias. His history of anxiety and depression in the setting of recent stressors sets the stage for night terrors. In addition, like many patients with parasomnias, he has a family history of sleep disorders. His vivid dreams make night terrors possible, but without the stark sympathetic activation it is a less likely diagnosis. It also does not account for the other symptoms he describes.
Our patient’s excessive daytime sleepiness interfering with daily activities, cataplexy, and hypnagogic hallucinations support the diagnosis of narcolepsy. This case highlights the variable weakness experienced during a cataplexy attack. It can range from a simple head droop to complete paralysis. Subtle findings require specific probing by the clinician. Patients with narcolepsy typically present in their late teens to early adulthood, but the cataplexy attacks may develop later in the disease course.
Table 3 summarizes the clinical findings associated with night terrors, somnambulism, and narcolepsy.
RARE CAUSES OF TRANSIENT NEUROLOGIC SYMPTOMS
Transient (paroxysmal) neurologic events in multiple sclerosis
A less well-known phenomenon in multiple sclerosis is termed “transient” (paroxysmal) neurologic events. These are typically stereotyped episodes lasting seconds, occurring sometimes hundreds of times a day. They are thought to arise from spontaneous electrical activity in an area of demyelination (ephaptic transmission), creating a wide range of symptoms. Some common events include positive sensory symptoms, alteration of the motor system such as spasms, or brainstem symptoms.53
Channelopathy
Two prototypical channelopathies are hyperkalemic and hypokalemic periodic paralysis. They are rare conditions, usually inherited in an autosomal dominant pattern.54 Both produce episodic, flaccid weakness in the setting of activity or other stressors (fasting, pregnancy, an emotionally charged episode). The attacks last a few minutes to hours and affect proximal skeletal muscles, with very little respiratory or bulbar involvement.
Hyperkalemic periodic paralysis is also associated with myotonia, which is the inability to voluntarily relax after stimulation. This can be evident after shaking a patient’s hand, as he or she would be unable to release because of the sustained activation. The myotonia is evident between attacks and may help cue a physician to the diagnosis even if the weakness has abated.55
As the name implies, potassium levels can vary during the attack, though hyperkalemic periodic paralysis can be seen with normal levels of serum potassium. The underlying pathology is tied to a voltage-gated sodium channel or calcium channel necessary for action potential generation.56
Paroxysmal dyskinesias
Paroxysmal dyskinesias encompass a rare group of movement disorders characterized by attacks without alterations in consciousness. Patients have reported dystonic, choreoathetotic, or ballistic movements. The attacks can be triggered by stress, eating, or even other types of movements. Most reported cases have a strong family history and are inherited in an autosomal dominant pattern. The exact pathophysiology is unclear. When paroxysmal dyskinesia was initially discovered, many thought it was a form of epilepsy, but the lack of electroencephalographic changes and postictal events argues against this etiology.
Transient focal neurologic episodes in cerebral amyloid angiopathy
Cerebral amyloid angiopathy is a degenerative condition in which amyloid is deposited in cerebral vessels, making them friable and at risk of bleeding. Most patients have no symptoms whatsoever, and the diagnosis is made by magnetic resonance imaging. Small microbleeds are common, but lobar intraparenchymal hemorrhage is the most feared complication.
Transient focal neurologic episodes, sometimes termed “amyloid spells,” are recurrent, stereotyped neurologic events that are spurred by cortical superficial siderosis (deposition of iron). Unfortunately, these events are difficult to characterize by their clinical morphology. The events can involve the visual, motor, and sensory pathways with both positive and negative symptoms, making the diagnosis difficult without imaging. These events may precede a symptomatic intraparenchymal hemorrhage, offering a unique window to reconsider the decision to continue an antiplatelet or anticoagulant drug.57,58
Many patients present to their primary care physicians, urgent care centers, and emergency rooms because of neurologic symptoms lasting seconds to hours. Their problems can be a cause for concern and a challenge to diagnose, as in many cases their symptoms have returned to baseline by the time of evaluation. Referral to a neurologist may not be practical for all of them, particularly given that a consultation may take a long time to obtain.
Understanding the causes of transient neurologic syndromes and their phenomenology may help the clinician diagnose, triage, and treat such conditions effectively.
Here, we outline several transient neurologic syndromes—transient ischemic attack (TIA), migraine with aura, partial seizures, hypoglycemic encephalopathy, hyperventilation syndrome, transient global amnesia, narcolepsy, parasomnias, and some rarer conditions— focusing on their diagnostic elements. Others, such as drug-induced transient neurologic syndromes, vertigo, and dizziness, have been well discussed elsewhere.1–3
THE BIG 3: TIA, MIGRAINE, SEIZURES
A 45-year-old woman with a history of tobacco use and headaches presents to the emergency department with a 4-month history of episodic numbness and tingling of her right arm and face. She reports a prodromal state of anxiety and irritability 24 to 48 hours before symptom onset.
The sensory symptoms begin on her face and gradually progress down the arm and eventually to her fingers. They fully resolve within 2 hours without sequelae. Family members have noted some “slurred speech” during the episodes, and the episodes are occasionally preceded by a unilateral, throbbing headache that improves with rest.
What are the possible causes of her symptoms?
Transient ischemic attack
If a patient reports transient neurologic symptoms and has vascular risk factors, TIA is often the default diagnostic consideration. The risk of stroke is 9.9% in the 2 days after a TIA, 13.4% at 30 days, and 17.3% at 90 days.4 Rapid recognition offers a crucial period to minimize the possibility of permanent impairment. Interventions include modifying risk factors (hypertension, diabetes, and smoking) and starting an antiplatelet drug, an anticoagulant drug, or both, and possibly a statin.
It can be difficult to determine if this workup needs to be completed in the inpatient or outpatient setting. There is no clear consensus, but the ultimate goal is timely evaluation (within 24 to 48 hours). The ABCD2 (Age, Blood pressure, Clinical features, Duration of symptoms, and Diabetes) risk factor calculator was developed to help triage patients, though it has limitations.5,6
One should assess a patient’s history of a possible TIA in a stepwise fashion. First, analyze the patient’s age and demographics for known vascular risk factors or central embolic sources (eg, atrial fibrillation). Then consider the symptoms. TIA symptoms have rapid onset, usually within seconds7; symptoms with a more gradual crescendo suggest a nonvascular cause.8 TIA manifestations should resolve within 1 hour, and most studies suggest symptom resolution within 10 minutes is specific for a TIA.9–11 TIA symptoms are negative neurologic phenomena that denote a loss of function, such as loss of vision, motor weakness, or sensory numbness.
Symptoms should also correlate with a defined vascular territory:
- The middle cerebral artery is commonly involved; its blockage is associated with aphasia, weakness of the face and arm, and homonymous visual field impairment (loss of one-half of the visual fields in both eyes)
- Blockage in the posterior circulation generally causes symptoms localized to the brainstem, cerebellum, and occipital cortex. The symptoms are usually grouped together as the “5Ds”: dizziness, diplopia, dysarthria, dysphagia, and dystaxia/ataxia. Brainstem involvement classically produces “crossed” findings, with ipsilateral cranial findings and contralateral motor or sensory findings.
- Lacunar strokes involve the subcortical white matter and produce typical patterns including pure motor or sensory syndromes.
Loss of consciousness is rarely a symptom of TIA and should suggest another etiology.
The definition of TIA has evolved from an operational one, ie, symptoms lasting less than 24 hours, to a tissue-based one, ie, focal cerebral ischemia not associated with permanent cerebral infarction.12 Though imperfect, this pathophysiology should help reinforce the most common features of TIA, including a sudden onset of negative symptoms that are localized to a defined vascular territory.13,14
Migraine with aura
Migraine with aura is common in patients ages 25 to 55 who have a long-standing history of headache. The pathophysiologic mechanism of an aura is believed to be a disseminating wave of cortical depression, which is a self-propagating wave of neural depression and then activation. Ultimately, this leads to a cascade of inflammatory and pain signals, resulting in a headache.
This background helps explain the positive (superimposed) symptoms associated with the aura. Positive symptoms are produced by excessive neuronal discharges stimulating the visual (flashing lights, zigzag lines), sensory (paresthesias), or motor (limb movements) pathways.
Common symptoms associated with aura include visual disturbances such as scintillating scotoma (a blind spot), sensory changes such as tingling, or auditory disruption with tinnitus. Symptoms may evolve over the course of 5 to 20 minutes, first affecting vision and then other senses. In contrast, in a TIA, symptoms usually begin simultaneously and are confined to a vascular territory.7,15 Symptoms of an aura usually resolve within an hour, but there is evidence showing a substantial number of patients have an aura lasting much longer.16 Focal weakness is uncommon during an aura but is reported in specific migraine conditions such as hemiplegic migraine and migraine with unilateral motor symptoms. The vast majority of patients experience other neurologic symptoms during this prodrome.17,18
The prodromal period (2 to 48 hours leading up to the onset of migraine) is a commonly overlooked feature of migraine.19 Common symptoms during this time include fatigue, mood change, and gastrointestinal symptoms.20 One study demonstrated that patients generally had good intuition concerning these nonspecific prodromal symptoms and could predict the onset of migraine 72% of the time.21
In addition, a myriad of possible triggers and exacerbating factors can be identified (and sometimes avoided) such as visual stimuli, weather changes, nitrates, sleep disturbances, menstruation, foods, and stressors.22
Although headache is often the cardinal manifestation of migraine, some patients experience aura without headache—acephalgic migraine.23 This can be a diagnostic challenge, especially in an older population with multiple vascular risk factors. New-onset acephalgic migraine may be a cause for concern but is not uncommon and is not associated with a significantly increased risk of stroke.24 Focusing on the character of the neurologic symptoms in regard to timing, progression, and resolution will help differentiate this disease from other transient neurologic syndromes.25
Partial seizure
Partial seizure produces a diverse range of stereotypical symptoms due to focal abnormal neuronal activation. The aberrant electrical firing generates positive symptoms involving the motor, sensory, or visual pathway. A history of trauma, neurosurgical intervention, central nervous system infection, stroke, or other seizure foci can suggest this diagnosis. Other prodromal clues include abdominal discomfort, sense of detachment, déjà vu, or jamais vu.26
During a seizure, there may be a progression of positive symptoms similar to what happens in migraine aura, because both represent cortical spread and depression.
Involvement of the motor pathway may produce tonic (stiffening) or clonic (twitching) movement. Other common motor abnormalities include automatisms such as lip smacking, chewing, and hand gestures (picking, fidgeting, fumbling).27
Epileptic discharges in the sensory cortex commonly cause paresthesias or distortion of a sensory input. Visual symptoms may be more complex. In occipital epilepsy, circular phenomena with a colored pattern are common, which contrasts with the photopsia (flashes of light) or fortification (a bright zigzag of lines resembling a fort) seen in migraines.28
Autonomic or somatosensory symptoms can also occur.
Todd paralysis, also called transient postictal paralysis, occurs in only 13% of seizures but can linger for 0.5 to 36 hours.29,30 This weakness is most pronounced within the affected region after a partial seizure.
In general, focal seizures are often stereotyped with positive neurologic features, usually last a few minutes, and resolve fully. These episodes may cause an arrest in activity but not usually loss of consciousness unless the epileptic discharge secondarily generalizes into the adjacent hemisphere.
A common differential diagnosis encountered during an epilepsy workup is psychogenic nonepileptic seizures. Nonepileptic seizures consist of transient, abnormal movements, sensation, or cognition but lack ictal electroencephalographic changes. This is a specifically challenging patient population, with high healthcare utilization and high risk for iatrogenic harm. In addition, on average, diagnosis can take years to establish and usually requires referral to a tertiary care facility.31,32
The big 3: Back to our patient
Our patient’s vascular risk factors, transient symptoms, and language involvement support the diagnosis of TIA. A feature that points away from the diagnosis of TIA is the gradual onset of positive neurologic symptoms. This pattern is not consistent with neuronal ischemia.
Also, our patient had a repetitive, stereotypical pattern of symptoms, which supports including partial seizures in the differential diagnosis. On the other hand, her lack of risk factors for seizure (a history of febrile seizures, developmental delay, trauma, or infection) would make this diagnosis less likely. Also pointing away from the diagnosis of seizures are her lack of typical prodromal symptoms, the length of the events, and the postevent headache.
Table 1 summarizes the clinical findings associated with TIA, migraine, and partial seizure.
EPISODES OF CONFUSION
A 35-year-old woman with a history of depression, anxiety, and poorly controlled type 1 diabetes presents to the clinic after several weeks of episodes of confusion, usually accompanied by paresthesias in both hands, dizziness, and palpitations. In each episode, soon after the symptoms began, she had painful cramps in her hand. The symptoms fully resolved within 10 minutes without sequelae.
Questioned further, the patient describes the confusion as a “mental haze” but denies frank disorientation. She has not kept a log of her blood sugar levels but has not noticed a temporal relationship with regard to her meals or insulin injections.
What are the possible causes of these episodes?
Hypoglycemic encephalopathy
Hypoglycemia is common in most people with diabetes, who have been reported to suffer from 62 to 320 severe hypoglycemic episodes in their lifetime.33,34 The neurologic consequences can be devastating in these severe cases.
During mild to moderate drops in the glucose level, generalized symptoms stem from sympathetic activation. These include generalized anxiety, tremor, palpitations, and sweating. Focal symptoms such as unilateral weakness have also been reported.35,36
Unfortunately, people with long-standing diabetes have a blunted response to epinephrine that reduces their sensitivity to hypoglycemia, placing them at high risk of permanent neurologic damage. This can lead to seizures and coma, as the hypoglycemia has a greater effect on cortical and subcortical structures (highly metabolic areas) than on the brainstem. Thus, respiratory and cardiovascular function is maintained but cerebral function is abnormal. If this state is prolonged, brain death can occur.37,38
Hyperventilation syndrome
Hyperventilation syndrome is not well characterized. Most think of it as synonymous with an underlying psychopathology, but there is evidence to suggest it can occur without underlying anxiety.
There is no clear mechanism, but it is hypothesized that diminished carbon dioxide levels lead to cerebral vasoconstriction. This may lead to reduced cerebral blood flow, causing dizziness, lightheadedness, or vertigo.39 Appendicular symptoms including paresthesias, carpopedal spasm, or tetany have been core features since the syndrome was first described in the early 1900s.40
Though the disorder has rather nonspecific features, it can be easily reproduced in the clinical setting by asking the patient to breathe deeply and rapidly. This can help confirm the underlying diagnosis and also reassure the patient that the underlying pathology is not life-threatening and that he or she has some control over the disease.
Transient global amnesia
Transient global amnesia usually strikes older patients (50 to 70 years old) in the setting of an acute physical or emotional stressor. There is also a correlation between transient global amnesia and migraine, with studies showing migraineurs are at higher risk than the general population.41 Despite common clinical concerns, there is no relationship between transient global amnesia and stroke.42
Transient global amnesia is defined by acute transient anterograde amnesia (coding of new memories). To try to reorient themselves, patients will repeatedly ask questions such as “What day is it?” or “Why are we here?” Retrograde memories, especially long-standing ones, are usually well preserved. The patient’s cognition is otherwise intact, and there are no other focal neurologic symptoms. The event usually lasts 2 to 24 hours and resolves without sequelae.43,44 Afterward, patients remember the event only poorly, which supports the notion that they cannot code new memories.
Confusional episodes: Discussion
Evaluating confusional episodes can be time-consuming and vexing. The subjective nature of the symptoms and the vast differential diagnosis can be overwhelming. Subtle clinical details can help formulate an appropriate evaluation.
Hypoglycemia can produce bizarre neurologic symptoms. Most cases of hypoglycemia produce an exaggerated sympathetic response, though this is blunted in people with longstanding diabetes. In addition, there should be a temporal association with meals, insulin doses, or both.
Transient global amnesia usually occurs with acute stressors and produces a confusional state. These episodes rarely recur, and the patient cannot provide much history regarding the episode secondary to the anterograde amnesia.
Table 2 summarizes the clinical findings associated with hypoglycemic encephalopathy, hyperventilation syndrome, and transient global amnesia.
Back to our patient
In our patient, the likely diagnosis is hyperventilation syndrome, even though we don’t know if her respiratory rate is increased during attacks. Some patients lack awareness of their breathing or are too distracted by the vague symptoms to have insight into the true cause. The cramps and contractions in the hands are a specific feature of the disease and can be accompanied by confusion.
SLEEP DISORDERS
A 17-year-old boy with a history of depression and anxiety presents to his pediatrician because he has had difficulty staying awake in school over the past year. His sleepiness has gradually worsened over the last few months and has taken a toll on his grades, leading to discord in his family. Over the past month he has had some difficulty holding his head up during arguments with friends. He does not lose consciousness during these events but is described as “unresponsive.” He describes vivid dreams when going to sleep that have startled him awake at times. His family history is positive for somnambulism on his father’s side.
Does this patient have a sleep disorder, and if so, which one?
Narcolepsy
Narcolepsy is defined by excessive daytime sleepiness, cataplexy, hypnagogic hallucination, and sleep paralysis. It is more common in men but its prevalence varies widely by geographic region, supporting an underlying interplay between genetics and environment.45
Sleep attacks or excessive daytime sleepiness are the cardinal features of narcolepsy. The dissociation between the sleep-wake cycle is evident with rapid transition into rapid-eye-movement (REM) sleep during these sleep attacks. This results in a “refreshing nap” that commonly involves vivid dreams. These episodes occur about 3 to 5 times per day, varying in duration from a few minutes to hours.46
Cataplexy is very specific feature of narcolepsy. Triggered by strong emotion, the body loses skeletal muscle tone except for the diaphragm and ocular muscles. The patient does not lose consciousness and remains aware of his or her environment. Of note, the loss of tone does not need to be dramatic. The hypotonia can manifest as jaw-dropping or head-nodding. The paralysis is related to prolonged REM atonia and impaired transition from sleep to wakefulness.47 Hypnagogic hallucination and sleep paralysis can occur, together with vivid visual hallucinations.
Parasomnias: Somnambulism and night terrors
Most non-REM parasomnias occur in childhood and diminish in adulthood. Two of the most common disorders are sleepwalking (somnambulism) and night terrors. Both are characterized by arousal from slow-wave sleep and are commonly associated with sedating medication, sleep deprivation, or psychopathology.
In somnambulism, patients exhibit complex motor behavior without interaction with their environment. Most have little recollection of the event.48 Sleep terrors produce a more intense reaction. The patient erupts out of sleep with profound terror, confusion, and autonomic changes. Interestingly, the patient can normally fall right back into sleep after the event.49–51
Back to our patient
Excessive daytime sleepiness and generalized fatigue are commonly encountered in outpatients. They can be frustrating because in many cases, no clear etiology can be discovered.52
This patient has several risk factors for parasomnias. His history of anxiety and depression in the setting of recent stressors sets the stage for night terrors. In addition, like many patients with parasomnias, he has a family history of sleep disorders. His vivid dreams make night terrors possible, but without the stark sympathetic activation it is a less likely diagnosis. It also does not account for the other symptoms he describes.
Our patient’s excessive daytime sleepiness interfering with daily activities, cataplexy, and hypnagogic hallucinations support the diagnosis of narcolepsy. This case highlights the variable weakness experienced during a cataplexy attack. It can range from a simple head droop to complete paralysis. Subtle findings require specific probing by the clinician. Patients with narcolepsy typically present in their late teens to early adulthood, but the cataplexy attacks may develop later in the disease course.
Table 3 summarizes the clinical findings associated with night terrors, somnambulism, and narcolepsy.
RARE CAUSES OF TRANSIENT NEUROLOGIC SYMPTOMS
Transient (paroxysmal) neurologic events in multiple sclerosis
A less well-known phenomenon in multiple sclerosis is termed “transient” (paroxysmal) neurologic events. These are typically stereotyped episodes lasting seconds, occurring sometimes hundreds of times a day. They are thought to arise from spontaneous electrical activity in an area of demyelination (ephaptic transmission), creating a wide range of symptoms. Some common events include positive sensory symptoms, alteration of the motor system such as spasms, or brainstem symptoms.53
Channelopathy
Two prototypical channelopathies are hyperkalemic and hypokalemic periodic paralysis. They are rare conditions, usually inherited in an autosomal dominant pattern.54 Both produce episodic, flaccid weakness in the setting of activity or other stressors (fasting, pregnancy, an emotionally charged episode). The attacks last a few minutes to hours and affect proximal skeletal muscles, with very little respiratory or bulbar involvement.
Hyperkalemic periodic paralysis is also associated with myotonia, which is the inability to voluntarily relax after stimulation. This can be evident after shaking a patient’s hand, as he or she would be unable to release because of the sustained activation. The myotonia is evident between attacks and may help cue a physician to the diagnosis even if the weakness has abated.55
As the name implies, potassium levels can vary during the attack, though hyperkalemic periodic paralysis can be seen with normal levels of serum potassium. The underlying pathology is tied to a voltage-gated sodium channel or calcium channel necessary for action potential generation.56
Paroxysmal dyskinesias
Paroxysmal dyskinesias encompass a rare group of movement disorders characterized by attacks without alterations in consciousness. Patients have reported dystonic, choreoathetotic, or ballistic movements. The attacks can be triggered by stress, eating, or even other types of movements. Most reported cases have a strong family history and are inherited in an autosomal dominant pattern. The exact pathophysiology is unclear. When paroxysmal dyskinesia was initially discovered, many thought it was a form of epilepsy, but the lack of electroencephalographic changes and postictal events argues against this etiology.
Transient focal neurologic episodes in cerebral amyloid angiopathy
Cerebral amyloid angiopathy is a degenerative condition in which amyloid is deposited in cerebral vessels, making them friable and at risk of bleeding. Most patients have no symptoms whatsoever, and the diagnosis is made by magnetic resonance imaging. Small microbleeds are common, but lobar intraparenchymal hemorrhage is the most feared complication.
Transient focal neurologic episodes, sometimes termed “amyloid spells,” are recurrent, stereotyped neurologic events that are spurred by cortical superficial siderosis (deposition of iron). Unfortunately, these events are difficult to characterize by their clinical morphology. The events can involve the visual, motor, and sensory pathways with both positive and negative symptoms, making the diagnosis difficult without imaging. These events may precede a symptomatic intraparenchymal hemorrhage, offering a unique window to reconsider the decision to continue an antiplatelet or anticoagulant drug.57,58
- Vuadens P, Regli F. Drug-induced neurological complications in a hospital cohort. Schweiz Med Wochenschr 1995; 125:1625–1633. French.
- Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract 2001; 51:666–671.
- Brignole M. Diagnosis and treatment of syncope. Heart 2007; 93:130–136.
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6:1063–1072.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015; 85:373–380.
- Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol 2014; 14:23–31.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am 2011; 21:303–313.
- Kimura K, Minematsu K, Yasaka M, Wada K, Yamaguchi T. The duration of symptoms in transient ischemic attack. Neurology 1999; 52:976–980.
- Lewandowski CA, Rao CP, Silver B. Transient ischemic attack: definitions and clinical presentations. Ann Emerg Med 2008; 52:S7–S16.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007; 298:2877–2885.
- van Rooij FG, Vermeer SE, Goraj BM, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol 2015; 78:1005–1010.
- Silberstein SD, Young WB. Migraine aura and prodrome. Semin Neurol 1995; 15:175–182.
- Viana M, Sprenger T, Andelova M, Goadsby PJ. The typical duration of migraine aura: a systematic review. Cephalalgia 2013; 33:483–490.
- Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry 2007; 78:600–604.
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125:1379–1391.
- Buzzi MG, Cologno D, Formisano R, Rossi P. Prodromes and the early phase of the migraine attack: therapeutic relevance. Funct Neurol 2005; 20:179–183.
- Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 2004; 44:865–872.
- Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003; 60:935–940.
- Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001; 85:911–941.
- Naeije G, Gaspard N, Legros B, Mavroudakis N, Pandolfo M. Transient CNS deficits and migrainous auras in individuals without a history of headache. Headache 2014; 54:493–499.
- Tuna MA, Mehta Z, Rothwell PM; Stroke Prevention Research Unit, Neuroscience Department, John Radcliffe Hospital, Oxford University. Stroke risk after a first late–onset migraine–like transient neurological attack (TNA): Oxford vascular study TNA cohort. J Neurol Neurosurg Psychiatry 2013; 84:e2.
- Fisher CM. Late-life migraine accompaniments—further experience. Stroke 1986; 17:1033–1042.
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Wyllie E, Rothner AD, Luders H. Partial seizures in children: clinical features, medical treatment, and surgical considerations. Pediatr Clin North Am 1989; 36:343–364.
- Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord 1999; 1:205–216.
- Gallmetzer P, Leutmezer F, Serles W, Assem-Hilger E, Spatt J, Baumgartner C. Postictal paresis in focal epilepsies—incidence, duration, and causes: a video-EEG monitoring study. Neurology 2004; 62:2160–2164.
- Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd’s post-epileptic paralysis. J Neurol Neurosurg Psychiatry 1992; 55:63–64.
- Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003; 4:205–216.
- LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50:1140–1147.
- Cryer PE, Axelrod L, Grossman AB, et al; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, Itoh H. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci 2012; 117:347–351.
- Lee SH, Kang CD, Kim SS, et al. Lateralization of hypoglycemic encephalopathy: evidence of a mechanism of selective vulnerability. J Clin Neurol 2010; 6:104–108.
- Siegel GJ, Agranoff BW. Basic neurochemistry: molecular, cellular, and medical aspects. 6th ed. Philadelphia, PA: Lippincott-Williams & Wilkins; 1999.
- Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia—a prospective population-based study. Exp Clin Endocrinol Diabetes 2003; 111:364–369.
- Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke 1972; 3:566–575.
- Kerr WJ, Gliebe PA, Dalton JW. Physical phenomena associated with anxiety states: the hyperventilation syndrome. Cal West Med 1938; 48:12–16.
- Lin KH, Chen YT, Fuh JL, et al. Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol 2014; 21:718–724.
- Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 2017; 92:399–405.
- Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc 2015; 90:264–272.
- Bartsch T, Butler C. Transient amnesic syndromes. Nat Rev Neurol 2013; 9:86–97.
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373:2654–2662.
- Ahmed I, Thorpy M. Clinical features, diagnosis and treatment of narcolepsy. Clin Chest Med 2010; 31:371–381.
- Leschziner G. Narcolepsy: a clinical review. Pract Neurol 2014; 14:323–331.
- Hughes JR. A review of sleepwalking (somnambulism): the enigma of neurophysiology and polysomnography with differential diagnosis of complex partial seizures. Epilepsy Behav 2007; 11:483–491.
- Gremmo M, Blanchi I, Costa B, et al. An abilitative approach to the premature infant in neonatal intensive care unit (NICU). J Perinat Med 1994; 22(suppl 1):102–105.
- Howell MJ. Parasomnias: an updated review. Neurotherapeutics 2012; 9:753–775.
- Giglio P, Undevia N, Spire JP. The primary parasomnias. A review for neurologists. Neurologist 2005; 11:90–97.
- Viner R, Christie D. Fatigue and somatic symptoms. BMJ 2005; 330:1012–1015.
- Rae-Grant AD. Unusual symptoms and syndromes in multiple sclerosis. Continuum (Minneap Minn) 2013; 19:992–1006.
- Fontaine B. Periodic paralysis. Adv Genet 2008; 63:3–23.
- Jurkat-Rott K, Lehmann-Horn F. Paroxysmal muscle weakness: the familial periodic paralyses. J Neurol 2006; 253:1391–1398.
- Lehmann-Horn F, Jurkat-Rott K, Rudel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep 2002; 2:61–69.
- Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007; 68:457–460.
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012; 43:2324–2330.
- Vuadens P, Regli F. Drug-induced neurological complications in a hospital cohort. Schweiz Med Wochenschr 1995; 125:1625–1633. French.
- Hanley K, O’Dowd T, Considine N. A systematic review of vertigo in primary care. Br J Gen Pract 2001; 51:666–671.
- Brignole M. Diagnosis and treatment of syncope. Heart 2007; 93:130–136.
- Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007; 6:1063–1072.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369:283–292.
- Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015; 85:373–380.
- Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol 2014; 14:23–31.
- Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008; 26:630–635.
- Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am 2011; 21:303–313.
- Kimura K, Minematsu K, Yasaka M, Wada K, Yamaguchi T. The duration of symptoms in transient ischemic attack. Neurology 1999; 52:976–980.
- Lewandowski CA, Rao CP, Silver B. Transient ischemic attack: definitions and clinical presentations. Ann Emerg Med 2008; 52:S7–S16.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009; 40:2276–2293.
- Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007; 298:2877–2885.
- van Rooij FG, Vermeer SE, Goraj BM, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol 2015; 78:1005–1010.
- Silberstein SD, Young WB. Migraine aura and prodrome. Semin Neurol 1995; 15:175–182.
- Viana M, Sprenger T, Andelova M, Goadsby PJ. The typical duration of migraine aura: a systematic review. Cephalalgia 2013; 33:483–490.
- Young WB, Gangal KS, Aponte RJ, Kaiser RS. Migraine with unilateral motor symptoms: a case-control study. J Neurol Neurosurg Psychiatry 2007; 78:600–604.
- Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002; 125:1379–1391.
- Buzzi MG, Cologno D, Formisano R, Rossi P. Prodromes and the early phase of the migraine attack: therapeutic relevance. Funct Neurol 2005; 20:179–183.
- Kelman L. The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 2004; 44:865–872.
- Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003; 60:935–940.
- Martin VT, Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001; 85:911–941.
- Naeije G, Gaspard N, Legros B, Mavroudakis N, Pandolfo M. Transient CNS deficits and migrainous auras in individuals without a history of headache. Headache 2014; 54:493–499.
- Tuna MA, Mehta Z, Rothwell PM; Stroke Prevention Research Unit, Neuroscience Department, John Radcliffe Hospital, Oxford University. Stroke risk after a first late–onset migraine–like transient neurological attack (TNA): Oxford vascular study TNA cohort. J Neurol Neurosurg Psychiatry 2013; 84:e2.
- Fisher CM. Late-life migraine accompaniments—further experience. Stroke 1986; 17:1033–1042.
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston, MA: Butterworths; 1990.
- Wyllie E, Rothner AD, Luders H. Partial seizures in children: clinical features, medical treatment, and surgical considerations. Pediatr Clin North Am 1989; 36:343–364.
- Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord 1999; 1:205–216.
- Gallmetzer P, Leutmezer F, Serles W, Assem-Hilger E, Spatt J, Baumgartner C. Postictal paresis in focal epilepsies—incidence, duration, and causes: a video-EEG monitoring study. Neurology 2004; 62:2160–2164.
- Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd’s post-epileptic paralysis. J Neurol Neurosurg Psychiatry 1992; 55:63–64.
- Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003; 4:205–216.
- LaFrance WC Jr, Baird GL, Barry JJ, et al; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007; 50:1140–1147.
- Cryer PE, Axelrod L, Grossman AB, et al; Endocrine Society. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728.
- Yoshino T, Meguro S, Soeda Y, Itoh A, Kawai T, Itoh H. A case of hypoglycemic hemiparesis and literature review. Ups J Med Sci 2012; 117:347–351.
- Lee SH, Kang CD, Kim SS, et al. Lateralization of hypoglycemic encephalopathy: evidence of a mechanism of selective vulnerability. J Clin Neurol 2010; 6:104–108.
- Siegel GJ, Agranoff BW. Basic neurochemistry: molecular, cellular, and medical aspects. 6th ed. Philadelphia, PA: Lippincott-Williams & Wilkins; 1999.
- Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia—a prospective population-based study. Exp Clin Endocrinol Diabetes 2003; 111:364–369.
- Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke 1972; 3:566–575.
- Kerr WJ, Gliebe PA, Dalton JW. Physical phenomena associated with anxiety states: the hyperventilation syndrome. Cal West Med 1938; 48:12–16.
- Lin KH, Chen YT, Fuh JL, et al. Migraine is associated with a higher risk of transient global amnesia: a nationwide cohort study. Eur J Neurol 2014; 21:718–724.
- Arena JE, Brown RD, Mandrekar J, Rabinstein AA. Long-term outcome in patients with transient global amnesia: a population-based study. Mayo Clin Proc 2017; 92:399–405.
- Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc 2015; 90:264–272.
- Bartsch T, Butler C. Transient amnesic syndromes. Nat Rev Neurol 2013; 9:86–97.
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373:2654–2662.
- Ahmed I, Thorpy M. Clinical features, diagnosis and treatment of narcolepsy. Clin Chest Med 2010; 31:371–381.
- Leschziner G. Narcolepsy: a clinical review. Pract Neurol 2014; 14:323–331.
- Hughes JR. A review of sleepwalking (somnambulism): the enigma of neurophysiology and polysomnography with differential diagnosis of complex partial seizures. Epilepsy Behav 2007; 11:483–491.
- Gremmo M, Blanchi I, Costa B, et al. An abilitative approach to the premature infant in neonatal intensive care unit (NICU). J Perinat Med 1994; 22(suppl 1):102–105.
- Howell MJ. Parasomnias: an updated review. Neurotherapeutics 2012; 9:753–775.
- Giglio P, Undevia N, Spire JP. The primary parasomnias. A review for neurologists. Neurologist 2005; 11:90–97.
- Viner R, Christie D. Fatigue and somatic symptoms. BMJ 2005; 330:1012–1015.
- Rae-Grant AD. Unusual symptoms and syndromes in multiple sclerosis. Continuum (Minneap Minn) 2013; 19:992–1006.
- Fontaine B. Periodic paralysis. Adv Genet 2008; 63:3–23.
- Jurkat-Rott K, Lehmann-Horn F. Paroxysmal muscle weakness: the familial periodic paralyses. J Neurol 2006; 253:1391–1398.
- Lehmann-Horn F, Jurkat-Rott K, Rudel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep 2002; 2:61–69.
- Katoh M, Yoshino M, Asaoka K, et al. A restricted subarachnoid hemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 2007; 68:457–460.
- Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012; 43:2324–2330.
KEY POINTS
- Transient ischemic attack, migraine aura, and partial seizures are common and often can be differentiated by their distinctive symptoms.
- Episodes of confusion in a patient with diabetes raise the possibility of hypoglycemic encephalopathy; other possibilities include hyperventilation syndrome and transient global amnesia.
- Daytime sleepiness in a young patient may be due to narcolepsy or parasomnias.
Medication management in older adults
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
Medications started for appropriate indications in middle age may need to be monitored more closely as the patient ages. Some drugs may become unnecessary or even dangerous as the patient ages, functional status and renal function decline, and goals of care change.
Older adults tend to have multiple illnesses and therefore take more drugs, and polypharmacy increases the risk of poor outcomes. The number of medications a person uses is a risk factor for adverse drug reactions, nonadherence, financial burden, drug-drug interactions, and worse outcomes.1
The prevalence of polypharmacy increased from an estimated 8.2% to 15% from 1999 to 2011 based on the National Health and Nutrition Examination Survey.2 Guideline-based therapy for specific diseases may lead to the addition of more medications to reach disease targets.3 Most older adults in the United States compound the risk of prescribed medications by also taking over-the-counter medications and dietary supplements.4
In addition, medications are often used in older adults based on studies of younger persons without significant comorbidities. Applying clinical guidelines based on these studies to older adults with comorbidity and functional impairment is challenging.5 Age-related pharmacokinetic and pharmacodynamic changes increase the risk of adverse drug reactions.6
In this article, we review commonly used medications that are potentially inappropriate based on clinical practice. We also review tools to evaluate appropriate drug therapy in older adults.
DRUGS THAT ARE COMMONLY USED, BUT POTENTIALLY INAPPROPRIATE
Statins
Statins are effective when used as secondary prevention in older adults,7 but their efficacy when used as primary prevention of atherosclerotic cardiovascular disease in people age 75 and older is questionable.8 Nevertheless, they are widely used for this purpose. For example, before the 2013 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) were released, 22% of patients age 80 and older in the Geisinger health system were taking a statin for primary prevention.9
The 2013 ACC/AHA guidelines included a limited recommendation for statins for primary prevention of atherosclerotic cardiovascular disease in adults age 75 and older.10 The guideline noted, however, that few data were available to support this recommendation.10
In a systematic review of 18 randomized clinical trials of statins for primary prevention of atherosclerotic cardiovascular disease, the mean age was 57, yet conclusions were extrapolated to an older patient population.11 The estimated 10-year risk of atherosclerotic cardiovascular disease based on pooled cohort risk equations of adults age 75 and older always exceeds the 7.5% treatment threshold recommended by the guidelines.8
Myopathy is a common adverse effect of statins. In addition, statins interact with other drugs that inhibit the cytochrome P450 3A4 isoenzyme, such as amlodipine, amiodarone, and diltiazem.8,12 If statin therapy caused no functional limitation due to muscle pain or weakness, statins for primary prevention would be cost-effective, but even a small increase in adverse effects in an elderly patient can offset the cardiovascular benefit.13 A recent post hoc secondary analysis found no benefit of pravastatin for primary prevention in adults age 75 and older.14
Thus, statin treatment for primary prevention in older patients should be individualized, based on life expectancy, function, and cardiovascular risk. Statin therapy does not replace modification of other risk factors.
Anticholinergics
Drugs with anticholinergic properties are commonly prescribed in the elderly for conditions such as muscle spasm, overactive bladder, psychiatric disorders, insomnia, extrapyramidal symptoms, vertigo, pruritus, peptic ulcer disease, seasonal allergies, and even the common cold,15 and many of the drugs often prescribed have strong anticholinergic properties (Table 1). Taking multiple medications with anticholinergic properties results in a high “anticholinergic burden,” which is associated with falls, impulsive behavior, poor physical performance, loss of independence, dementia, delirium, and brain atrophy.15–18
The 2014 American College of Physicians guideline on nonsurgical management of urinary incontinence in women recommends pharmacologic treatment for urgency and stress urinary incontinence after failure of nonpharmacologic therapy,19 and many drugs for these urinary symptoms have anticholinergic properties. If an anticholinergic is necessary, an agent that results in a lower anticholinergic burden should be considered in older patients.
A pharmacist-initiated medication review and intervention may be another way to adjust medications to reduce the patient’s anticholinergic burden.20,21 The common use of anticholinergic drugs in older adults reminds us to monitor their use closely.22
Benzodiazepines and nonbenzodiazepines
Benzodiazepines are among the most commonly prescribed psychotropics in developed countries and are prescribed mainly by primary care physicians rather than psychiatrists.23
In 2008, 5.2% of US adults ages 18 to 80 used a benzodiazepine, and long-term use was more prevalent in older patients (ages 65–80).23
Benzodiazepines are prescribed for anxiety,24 insomnia,25 and agitation. They can cause withdrawal26 and have potential for abuse.27 Benzodiazepines are associated with cognitive decline,28 impaired driving,29 falls,30 and hip fractures31 in older adults.
In addition, use of nonbenzodiazepine hypnotics (eg, zolpidem) is on the rise,32 and these drugs are known to increase the risk of hip fracture in nursing home residents.33
The American Geriatrics Society, through the American Board of Internal Medicine’s Choosing Wisely campaign, recommends avoiding benzodiazepines as a first-line treatment for insomnia, agitation, or delirium in older adults.34 Yet prescribing practices with these drugs in primary care settings conflict with guidelines, partly due to lack of training in constructive strategies regarding appropriate use of benzodiazepines.35 Educating patients on the risks and benefits of benzodiazepine treatment, especially long-term use, has been shown to reduce the rate of benzodiazepine-associated secondary events.36
Antipsychotics
Off-label use of antipsychotics is common and is increasing in the United States. In 2008, off-label use of antipsychotic drugs accounted for an estimated $6 billion.37 A common off-label use is to manage behavioral symptoms of dementia, despite a black-box warning about an increased risk of death in patients with dementia who are treated with antipsychotics.38,39 The Choosing Wisely campaign recommends against prescribing antipsychotics as a first-line treatment of behavioral and psychological symptoms of dementia.34
Antipsychotic drugs are associated with risk of acute kidney injury,40 as well as increased risk of falls and fractures (eg, a 52% higher risk of a serious fall, and a 50% higher risk of a nonvertebral osteoporotic fracture).41
Patients with dementia often exhibit aggression, resistance to care, and other challenging or disruptive behaviors. In such instances, antipsychotic drugs are often prescribed, but they provide limited and inconsistent benefits, while causing oversedation and worsening of cognitive function and increasing the likelihood of falling, stroke, and death.38,39,41
Because pharmacologic treatments for dementia are only modestly effective, have notable risks, and do not treat some of the behaviors that family members and caregivers find most distressing, nonpharmacologic measures are recommended as first-line treatment.42 These include caregiver education and support, training in problem-solving, and targeted therapy directed at the underlying causes of specific behaviors (eg, implementing nighttime routines to address sleep disturbances).42 Nonpharmacologic management of behavioral symptoms in dementia can significantly improve quality of life for patients and caregivers.42 Use of antipsychotic drugs in patients with dementia should be limited to cases in which nonpharmacologic measures have failed and patients pose an imminent threat to themselves or others.43
Proton pump inhibitors
Proton pump inhibitors are among the most commonly prescribed medications in the United States, and their use has increased significantly over the decade. It has been estimated that between 25% and 70% of these prescriptions have no appropriate indication.44
There is considerable excess use of acid suppressants in both inpatient and outpatient settings.45,46 In one study, at discharge from an internal medicine service, almost half of patients were taking a proton pump inhibitor.47
Evidence-based guidelines recommend these drugs to treat gastroesophageal reflux disease, nonerosive reflux disease, erosive esophagitis, dyspepsia, and peptic ulcer disease. However, long-term use (ie, beyond 8 weeks) is recommended only for patients with erosive esophagitis, Barrett esophagus, a pathologic hypersecretory condition, or a demonstrated need for maintenance treatment for reflux disease.48
Although proton pump inhibitors are highly effective and have low toxicity, there are reports of an association with Clostridium difficile infection,49 community-acquired pneumonia,50 hip fracture,51 vitamin B12 deficiency,52 atrophic gastritis,53 kidney disease,54 and dementia.55
Nondrug therapies such as weight loss and elevation of the head of the bed may improve esophageal pH levels and reflux symptoms.56
Deprescribing.org has practical advice for healthcare providers, patients, and caregivers on how to discontinue proton pump inhibitors, including videos, algorithms, and guidelines.
TOOLS TO EVALUATE APPROPRIATE DRUG THERAPY
Beers criteria
The Beers criteria (Table 2), developed in 1991 by a geriatrician as an approach to safer, more effective drug therapy in frail elderly nursing home patients,57 were updated by the American Geriatrics Society in 2015 for use in any clinical setting.58 (The criteria are also available as a smartphone application through the American Geriatrics Society at www.americangeriatrics.org.)
The Beers criteria offer evidence-based recommendations on drugs to avoid in the elderly, along with the rationale for use, the quality of evidence behind the recommendation, and the graded strength of the recommendation. The Beers criteria should be viewed through the lens of clinical judgment to offer safer nonpharmacologic and pharmacologic treatments.
The Joint Commission recommends medication reconciliation at every transition of care.59 The Beers criteria are a good starting point for a comprehensive medication review.
STOPP/START criteria
Another tool to aid safe prescribing in older adults is the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), used in conjuction with the Screening Tool to Alert Doctors to Right Treatment (START). The STOPP/START criteria60,61 are based on an up-to-date literature review and consensus (Table 3).
THE BOTTOM LINE
Physicians caring for older adults need to diligently weigh the benefits of drug therapy and consider the patient’s care goals, current level of functioning, life expectancy, values, and preferences. Statin therapy for primary prevention, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely used without proper indications, pointing to the need for a periodic comprehensive review of medications to reevaluate the risks vs the benefits of the patient’s medications. The Beers criteria and the STOPP/ START criteria can be useful tools for this purpose.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
- Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med 2016; 176:482–483.
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015; 314:1818–1831.
- Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016; 176:473–482.
- Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294:716–724.
- Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging 1999; 14:141–152.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016; 338:2532–2561.
- Gurwitz JH, Go AS, Fortman SP. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016; 316:1971–1972.
- Chokshi NP, Messerli FH, Sutin D, Supariwala AA, Shah NR. Appropriateness of statins in patients aged ≥ 80 years and comparison to other age groups. Am J Cardiol 2012; 110:1477–1481.
- Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816.
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf 2010; 33:171–187.
- Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–541.
- Han BH, Sutin D, Williamson JD, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults. The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017; 177:955–965.
- Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015; 175:401–407.
- Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008; 168:508–513.
- Hilmer SN, Mager DE, Simonsick EM, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007; 167:781–787.
- Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016; 73:721–732.
- Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Nonsurgical management of urinary incontinence in women: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014; 161:429–440.
- Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013; 61:1624–1625.
- Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013; 68:271–278.
- Curtis LH, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med 2004; 164:1621–1625.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015; 72:136–142.
- Martin JL, Sainz-Pardo M, Furukawa TA, Martín-Sánchez E, Seoane T, Galán C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol 2007; 21:774–782.
- Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med 2007; 22:1335–1350.
- Rickels K, Schweizer E, Case WG, Greenblatt DJ. Long-term therapeutic use of benzodiazepines, I. Effects of abrupt discontinuation. Arch Gen Psychiatry 1990; 47:899–907.
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am J Psychiatry 2010; 167:1247–1253.
- Billoti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014; 349:g5205.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systemic literature review. CNS Drugs 2010; 24:639–653.
- Tinett, ME, Speechley M, Ginter S. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988; 319:1701–1707.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010; 19:1248–1255.
- Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Medicare Part D’s exclusion of benzodiazepines and fracture risk in nursing homes. Arch Intern Med 2010; 170:693–698.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013; 173:754–761.
- American Geriatrics Society. Choosing Wisely. Ten things clinicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society/. Accessed December 3, 2017.
- Cook JM, Marshall R, Masci C, Coyne JC. Physicians’ perspectives on prescribing benzodiazepines for older adults: a qualitative study. J Gen Intern Med 2007; 22:303–307.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174:890–898.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Phamacoepidemiol Drug Saf 2011; 20:177–184.
- Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786.
- US Food and Drug Administration (FDA). Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Accessed December 4, 2017.
- Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults. Ann Intern Med 2014; 161:242–248.
- Fraser L, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med 2015; 175:450–452.
- Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029.
- Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 2006; 355:1525–1538.
- Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ 2008; 336:2–3.
- Mazer-Amirshahi M, Mullins PM, van den Anker J, Meltzer A, Pines JM. Rising rates of proton pump inhibitor prescribing in US emergency departments. Am J Emerg Med 2014; 32:618–622.
- Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care 2010; 16:e228–e324.
- Pham CQ, Regal RE, Bostwich TR, Knauf KS. Acid suppressive therapy used on an inpatient internal medicine service. Ann Pharmacother 2006; 40:1261–1266.
- Kahrilas PJ, Shaheen NJ, Vaezi MF, et al; American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology 2008; 135:1383–1391.e1–e5.
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170:784–790.
- Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med 2007; 167:950–955.
- Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006; 296:2947–2953.
- Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013; 310:2435–2442.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996; 334:1018–1022.
- Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176:238–246.
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73:410–416.
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006; 166:965–971.
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151:1825–1832.
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63:2227–2246.
- Joint Commission. Sentinel event alert, Issue 35: using medication reconciliation to prevent errors. www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed August 18, 2017.
- Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46:72–83.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44:213–218.
KEY POINTS
- Statins, anticholinergics, benzodiazepines, antipsychotics, and proton pump inhibitors are widely prescribed.
- In older patients, a periodic comprehensive medication review is needed to reevaluate the risks and the benefits of current medications in light of goals of care, life expectancy, and the patient’s preferences.
- The Beers criteria and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions provide valuable guidance for safe prescribing in older adults.
Benzodiazepines: Sensible prescribing in light of the risks
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.
Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.
A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.
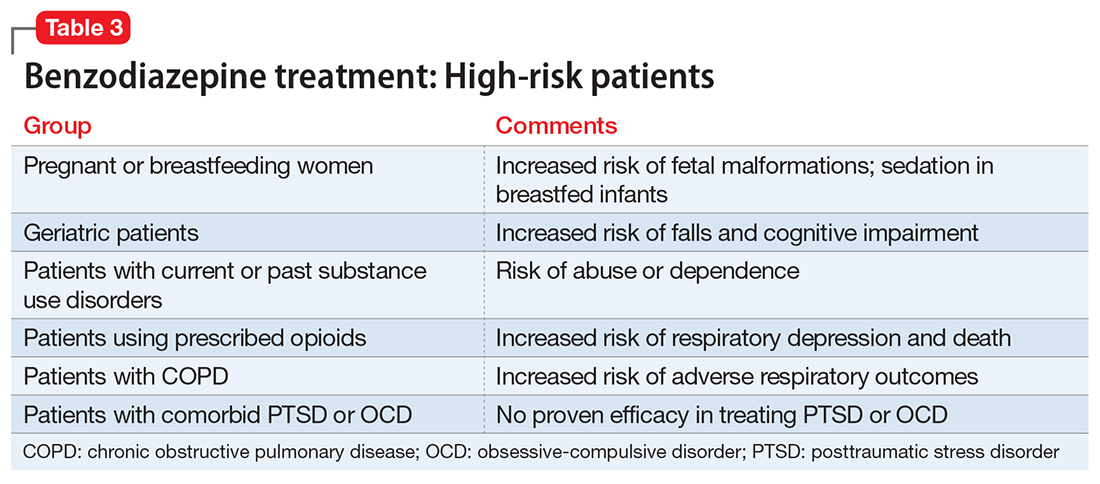
Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).
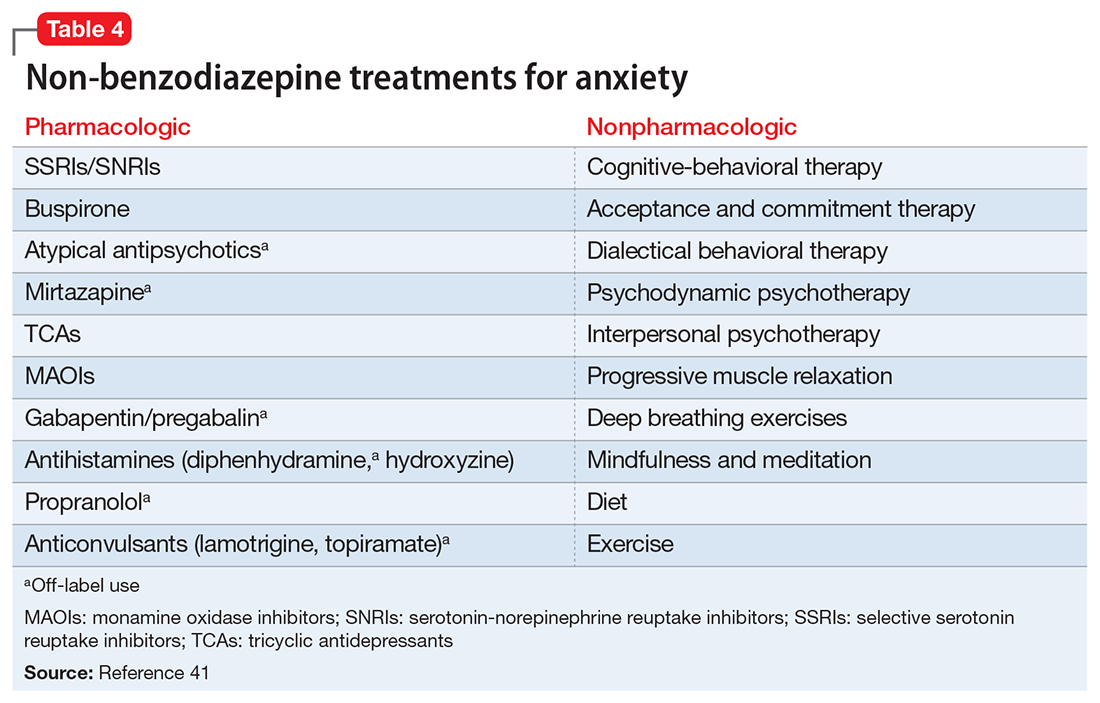
Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.

Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.

A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.

Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).

Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
As a group, anxiety disorders are the most common mental illness in the Unites States, affecting 40 million adults. There is a nearly 30% lifetime prevalence of anxiety disorders in the general population.1 DSM-5 anxiety disorders include generalized anxiety disorder, social anxiety disorder (social phobia), panic disorder, specific phobia, and separation anxiety disorder. Although DSM-IV-TR also classified obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD) as anxiety disorders, these diagnoses were reclassified in DSM-5. Anxiety also is a frequent symptom of many other psychiatric disorders, especially major depressive disorder.

Although benzodiazepines have many potential uses, they also carry risks that prescribers should recognize. This article reviews some of the risks of benzodiazepine use, identifies patients with higher risks of adverse effects, and presents a practical approach to prescribing these medications.

A wide range of risks
Abuse and addiction. Perhaps the most commonly recognized risk associated with benzodiazepine use is the potential for abuse and addiction.4 Prolonged benzodiazepine use typically results in physiologic tolerance, requiring higher dosing to achieve the same initial effect.5 American Psychiatric Association practice guidelines recognize the potential for benzodiazepine use to result in symptoms of dependence, including cravings and withdrawal, stating that “with ongoing use, all benzodiazepines will produce physiological dependence in most patients.”6 High-potency, short-acting compounds such as alprazolam have a higher risk for dependence, toxicity, and abuse.7 However, long-acting benzodiazepines (such as clonazepam) also can be habit-forming.8 Because of these properties, it is generally advisable to avoid prescribing benzodiazepines (and short-acting compounds in particular) when treating patients with current or past substance use disorders, except when treating withdrawal.9
Limited efficacy for other disorders. Although benzodiazepines can help reduce anxiety in patients with anxiety disorders, they have shown less promise in treating other disorders in which anxiety is a common symptom. Treating PTSD with benzodiazepines does not appear to offer any advantage over placebo, and may even result in increased symptoms over time.10,11 There is limited evidence supporting the use of benzodiazepines to treat OCD.12,13 Patients with borderline personality disorder who are treated with benzodiazepines may experience an increase in behavioral dysregulation.14
Physical ailments. Benzodiazepines can affect comorbid physical ailments. One study found that long-term benzodiazepine use among patients with comorbid pain disorders was correlated with high utilization of medical services and high disability levels.15 Benzodiazepine use also has been associated with an increased risk of exacerbating respiratory conditions, such as chronic obstructive pulmonary disease,16 and increased risk of pneumonia.17,18
Pregnancy and breastfeeding. Benzodiazepines carry risks for women who are pregnant or breastfeeding. Benzodiazepine use during pregnancy may increase the relative risk of major malformations and oral clefts. It also may result in neonatal lethargy, sedation, and weight loss. Benzodiazepine withdrawal symptoms can occur in the neonate.19 Benzodiazepines are secreted in breast milk and can result in sedation among breastfed infants.20
Geriatric patients. Older adults may be particularly vulnerable to the adverse effects of benzodiazepines. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends against prescribing benzodiazepines to geriatric patients.21 Benzodiazepine use has been associated with an increased risk for falls among older adults,22,23 with an increased risk of fractures24 that can be fatal.25 Benzodiazepines also have been associated with an increased risk of cognitive dysfunction and dementia.26,27 Despite the documented risks of using benzodiazepines in geriatric patients, benzodiazepines continue to be frequently prescribed to this age group.28,29 One study found that the rate of prescribing benzodiazepines by primary care physicians increased from 2003 to 2012, primarily among older adults with no diagnosis of pain or a psychiatric disorder.30
Mortality. Benzodiazepine use also carries an increased risk of mortality. Benzodiazepine users are at increased risk of motor vehicle accidents because of difficulty maintaining road position.31 Some research has shown that patients with schizophrenia treated with benzodiazepines have an increased risk of death compared with those who are prescribed antipsychotics or antidepressants.32 Another study showed that patients with schizophrenia who were prescribed benzodiazepines had a greater risk of death by suicide and accidental poisoning.33 Benzodiazepine use has been associated with suicidal ideation and an increased risk of suicide.34 Prescription opioids and benzodiazepines are the top 2 causes of overdose-related deaths (benzodiazepines are involved in approximately 31% of fatal overdoses35), and from 2002 to 2015 there was a 4.3-fold increase in deaths from benzodiazepine overdose in the United States.36 CDC guidelines recommend against co-prescribing opioids and benzodiazepines because of the risk of death by respiratory depression.37 As of August 2016, the FDA required black-box warnings for opioids and benzodiazepines regarding the risk of respiratory depression and death when these agents are used in combination, noting that “If these medicines are prescribed together, limit the dosages and duration of each drug to the minimum possible while achieving the desired clinical effect.”38,39
A sensible approach to prescribing
Given the risks posed by benzodiazepines, what would constitute a sensible approach to their use? Clearly, there are some patients for whom benzodiazepine use should be minimized or avoided (Table 3). In a patient who is deemed a good candidate for benzodiazepines, a long-acting agent may be preferable because of the increased risk of dependence associated with short-acting compounds. Start with a low dose, and use the lowest dose that adequately treats the patient’s symptoms.40 Using scheduled rather than “as-needed” dosing may help reduce behavioral escape patterns that reinforce anxiety and dependence in the long term.

Before starting a patient on a benzodiazepine, discuss with him (her) the risks of use and an exit plan to discontinue the medication. For example, a benzodiazepine may be prescribed at the same time as a selective serotonin reuptake inhibitor (SSRI), with the goal of weaning off the benzodiazepine once the SSRI has achieved efficacy.6 Inform the patient that prescribing or treatment may be terminated if it is discovered that the patient is abusing or diverting the medication (regularly reviewing the state prescription monitoring program database can help determine if this has occurred). Strongly consider using non-benzodiazepine treatments for anxiety with (or eventually in place of) benzodiazepines (Table 441).

Reducing or stopping benzodiazepines can be challenging.42 Patients often are reluctant to stop such medications, and abrupt cessation can cause severe withdrawal. Benzodiazepine withdrawal symptoms can be severe or even fatal. Therefore, a safe and collaborative approach to reducing or stopping benzodiazepines is necessary. A starting point might be to review the risks associated with benzodiazepine use with the patient and ask about the frequency of use. Discuss with the patient a slow taper, perhaps reducing the dose by 10% to 25% increments weekly to biweekly.43,44 Less motivated patients may require a slower taper, more time, or repeated discussions. When starting a dose reduction, notify the patient that some rebound anxiety or insomnia are to be expected. With any progress the patient makes toward reducing his usage, congratulate him on such progress.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Balon R, Fava GA, Rickels K. Need for a realistic appraisal of benzodiazepines. World Psychiatry. 2015;14(2):243-244.
3. Ashton CH. Benzodiazepine equivalence table. http://www.benzo.org.uk/bzequiv.htm. Revised April 2007. Accessed May 3, 2017.
4. National Institute on Drug Abuse. Commonly abused drugs. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/commonly_abused_drugs_3.pdf. Revised January 2016. Accessed January 9, 2018.
5. Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90(1):74-89.
6. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, second edition. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed May 3, 2017.
7. Salzman C. The APA Task Force report on benzodiazepine dependence, toxicity, and abuse. Am J Psychiatry. 1991;148(2):151-152.
8. Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755.
9. O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223-230.
10. Mellman TA, Bustamante V, David D, et al. Hypnotic medication in the aftermath of trauma. J Clin Psychiatry. 2002;63(12):1183-1184.
11. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry. 1996;57(9):390-394.
12. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed May 3, 2017.
13. Abdel-Ahad P, Kazour F. Non-antidepressant pharmacological treatment of obsessive compulsive disorder: a comprehensive review. Curr Clin Pharmacol. 2015;10(2):97-111.
14. Gardner DL, Cowdry RW. Alprazolam-induced dyscontrol in borderline personality disorder. Am J Psychiatry. 1985;142(1):98-100.
15. Ciccone DS, Just N, Bandilla EB, et al. Psychological correlates of opioid use in patients with chronic nonmalignant pain: a preliminary test of the downhill spiral hypothesis. J Pain Symptom Manage. 2000;20(3):180-192.
16. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J. 2014;44(2):332-340.
17. Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-170.
18. Taipale H, Tolppanen AM, Koponen M, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ. 2017;189(14):E519-E529.
19. Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatric Serv. 2002;53:39-49.
20. U.S. National Library of Medicine, TOXNET Toxicology Data Network. Lactmed: alprazolam. http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+335. Accessed May 3, 2017.
21. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
22. Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48(6):682-685.
23. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952-1960.
24. Bolton JM, Morin SN, Majumdar SR, et al. Association of mental disorders and related medication use with risk for major osteoporotic fractures. JAMA Psychiatry. 2017;74(6):641-648.
25. Pariente A, Dartiques JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25(1):61-70.
26. Lagnaoui R, Tournier M, Moride Y, et al. The risk of cognitive impairment in older community-dwelling women after benzodiazepine use. Age Ageing. 2009;38(2):226-228.
27. Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231.
28. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
29. Maust DT, Kales HC, Wiechers IR, et al. No end in sight: benzodiazepine use in older adults in the United States. J Am Geriatr Soc. 2016;64(12):2546-2553.
30. Maust DT, Blow FC, Wiechers IR, et al. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363-e371.
31. Rapoport MJ, Lanctôt KL, Streiner DL, et al. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70(5):663-673.
32. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
33. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry. 2016;77(5):661-667.
34. McCall WV, Benca RM, Rosenguist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25.
35. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688.
36. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Updated September 2017. Accessed January 8, 2018.
37. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. MMWR Recomm Rep 2016;65(1):1-49.
38. U.S. Food and Drug Administration. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use [press release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518697.htm. Published August 31, 2016. Accessed May 3, 2017.
39. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. http://www.fda.gov/Drugs/DrugSafety/ucm518473.htm. Published August 31, 2016. Accessed May 3, 2017.
40. National Institute for Health and Care Excellence. Controlled drugs: safe use and management. https://www.nice.org.uk/guidance/ng46/evidence/full-guideline-pdf-2427186353. Published April 2016. Accessed July 25, 2017.
41. Stahl SM. Anxiety disorders and anxiolytics. In: Stahl’s essential psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008:721-772.
42. Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13(7):919-934.
43. Nardi AE, Freire RC, Valença AM, et al. Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol. 2010;30(3):290-293.
44. Tampi R. How to wean geriatric patients off benzodiazepines. Psychiatric News. http://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2016.PP3b6. Published March 18, 2016. Accessed May 3, 2017.
Compulsive sexual behavior: A nonjudgmental approach
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
Compulsive sexual behavior (CSB), also referred to as sexual addiction or hypersexuality, is characterized by repetitive and intense preoccupations with sexual fantasies, urges, and behaviors that are distressing to the individual and/or result in psychosocial impairment. Individuals with CSB often perceive their sexual behavior to be excessive but are unable to control it. CSB can involve fantasies and urges in addition to or in place of the behavior but must cause clinically significant distress and interference in daily life to qualify as a disorder.
Because of the lack of large-scale, population-based epidemiological studies assessing CSB, its true prevalence among adults is unknown. A study of 204 psychiatric inpatients found a current prevalence of 4.4%,1 while a university-based survey estimated the prevalence of CSB at approximately 2%.2 Others have estimated that the prevalence is between 3% to 6% of adults in the United States,3,4 with males comprising the majority (≥80%) of affected individuals.5
CSB usually develops during late adolescence/early adulthood, and most who present for treatment are male.5 Mood states, including depression, happiness, and loneliness, may trigger CSB.6 Many individuals report feelings of dissociation while engaging in CSB-related behaviors, whereas others report feeling important, powerful, excited, or gratified.
Why CSB is difficult to diagnose
Although CSB may be common, it usually goes undiagnosed. This potentially problematic behavior often is not diagnosed because of:
- Shame and secrecy. Embarrassment and shame, which are fundamental to CSB, appear to explain, in part, why few patients volunteer information regarding this behavior unless specifically asked.1
- Patient lack of knowledge. Patients often do not know that their behavior can be successfully treated.
- Clinician lack of knowledge. Few health care professionals have education or training in CSB. A lack of recognition of CSB also may be due to our limited understanding regarding the limits of sexual normality. In addition, the classification of CSB is unclear and not agreed upon (Box7-9), and moral judgments often are involved in understanding sexual behaviors.10
Box
Classifying compulsive sexual behavior
No consensus on diagnostic criteria
Accurately diagnosing CSB is difficult because of a lack of consensus about the diagnostic criteria for the disorder. Christenson et al11 developed an early set of criteria for CSB as part of a larger survey of impulse control disorders. They used the following 2 criteria to diagnose CSB: (1) excessive or uncontrolled sexual behavior(s) or sexual thoughts/urges to engage in behavior, and (2) these behaviors or thoughts/urges lead to significant distress, social or occupational impairment, or legal and financial consequences.11,12
During the DSM-5 revision process, a second approach to the diagnostic criteria was proposed for hypersexuality disorder. Under the proposed criteria for hypersexuality, a person would meet the diagnosis if ≥3 of the following were endorsed over a 6-month period: (a) time consumed by sexual fantasies, urges, or behaviors repetitively interferes with other important (non-sexual) goals, activities, and obligations; (b) repetitively engaging in sexual fantasies, urges, or behaviors in response to dysphoric mood states; (c) repetitively engaging in sexual fantasies, urges, or behaviors in response to stressful life events; (d) repetitive but unsuccessful efforts to control or significantly reduce these sexual fantasies, urges, or behaviors; and (e) repetitively engaging in sexual behaviors while disregarding the risk for physical or emotional harm to self or others.9
These 2 proposed approaches to diagnosis are somewhat similar. Both suggest that the core underlying issues involve sexual urges or behaviors that are difficult to control and that lead to psychosocial dysfunction. Differences in the criteria, however, could result in different rates of CSB diagnosis; therefore, further research will need to determine which diagnostic approach reflects the neurobiology underlying CSB.
Avoid misdiagnosis
Before making a diagnosis of CSB, it is important for clinicians to consider whether they are stigmatizing “negative consequences,” distress, or social impairment based on unconscious bias toward certain sexual behaviors. In addition, we need to ensure that we are not holding sex to different standards than other behaviors (for example, there are many things in life we do that result in negative consequences and yet do not classify as a mental disorder, such as indulging in less healthy food choices). Furthermore, excessive sexual behaviors might be associated with the normal coming out process for LGBTQ individuals, partner relationship problems, or sexual/gender identity. Therefore, the behavior needs to be assessed in the context of these psychosocial environmental factors.
Differential diagnosis
Various psychiatric disorders also may include excessive sexual behavior as part of their clinical presentation, and it is important to differentiate that behavior from CSB.
Bipolar disorder. Excessive sexual behavior can occur as part of a manic episode in bipolar disorder. If the problematic sexual behavior also occurs when the person’s mood is stable, the individual may have CSB and bipolar disorder. This distinction is important because the treatment for bipolar disorder is often different for CSB, because anticonvulsants have only case reports attesting to their use in CSB.
Substance abuse. Excessive sexual behavior can occur when a person is abusing substances, particularly stimulants such as cocaine and amphetamines.13 If the sexual behavior does not occur when the person is not using drugs, then the appropriate diagnosis would not likely be CSB.
Obsessive-compulsive disorder (OCD). Individuals with OCD often are preoccupied with sexual themes and feel that they think about sex excessively.14 Although patients with OCD may be preoccupied with thoughts of sex, the key difference is that persons with CSB report feeling excited by these thoughts and derive pleasure from the behavior, whereas the sexual thoughts of OCD are perceived as unpleasant.
Other disorders that may give rise to hypersexual behavior include neurocognitive disorders, attention-deficit/hyperactivity disorder, autism spectrum disorders, and depressive disorders.
Adverse effects of medication. It is important to ask the patient whether he (she)developed CSB after starting a medication. Certain medications (eg, medications for Parkinson’s disease or restless leg syndrome, or aripiprazole to treat depression or psychosis) may cause patients to engage in problematic sexual behavior.15,16 If the sexual behavior decreases or stops when the medication dosage is reduced or the medication is stopped, a diagnosis of CSB would not be appropriate.
Comorbidity is common
Research suggests that approximately one-half of adults with CSB meet criteria for at least 1 other psychiatric disorder, such as mood, anxiety, substance use, impulse control, or personality disorders. A study of men with CSB (N = 103) found that 71% met criteria for a mood disorder, 40% for an anxiety disorder, 41% for a substance use disorder, and 24% for an impulse control disorder such as gambling disorder.17 Therefore, to successfully treat CSB, clinicians also may need to focus on how and to what extent these co-occurring disorders drive the sexual behavior.
Co-occurring medical conditions also are common among individuals with CSB. Medical concerns may include unwanted pregnancy, sexually transmitted infections, and HIV/AIDS. Thus, treating psychiatric comorbidities and providing education about sexual health, with referrals to primary care specialists, often are part of CSB treatment.
Neuroimaging and cognition
One imaging study that compared participants with and without CSB found that participants with CSB had higher activity in the ventral striatum, anterior cingulate cortex, and amygdala relative to controls during a cue-reactivity functional MRI task.18 These findings show notable similarities to the patterns of activation seen in patients addicted to drugs when assessed using drug-craving paradigms. An additional neuroimaging study assessing patients with hypersexuality using diffusion tensor imaging noted that diffusivity in a prefrontal white matter tract within a superior frontal region was greater in patients with CSB.18 This study also indicated that there was a negative correlation between observed diffusion in the noted location and overall severity score for CSB symptoms such as frequency of urges or behaviors.
In terms of cognition, a preliminary assessment of young adults with CSB compared with healthy controls did not find any differences between groups across several tasks, although the previously mentioned diffusion tensor imaging study reported elevated impulsivity in CSB.18
Approaches to treatment
Most people with CSB are reluctant to mention it to their health care providers, and most physicians are generally uncomfortable talking about sex with their patients, in part, because of a lack of training.19 Patients are more likely to bring up the topic when they are receiving treatment for anxiety, depression, or substance abuse. Therefore, clinicians must consider that sexual behavior might be associated with a coping mechanism, distressing outcome, or comorbid condition in these patients.
Pharmacologic treatment
Evidence for the pharmacologic treatment of CSB consists primarily of small, open-label studies, case series, or retrospective analyses, except for 1 double-blind, placebo-controlled study. Based on this evidence, there may be several pharmacologic treatment options for patients with CSB; however, there are no FDA-approved medications for CSB.
Antidepressants. One of the most thoroughly documented categories of pharmacologic treatment for CSB is selective serotonin reuptake inhibitors (SSRIs). Several retrospective analyses and case series have reported on the general efficacy of SSRIs in reducing symptoms of CSB.20-23 Citalopram, the only treatment for CSB that has been assessed using a double-blind, placebo-controlled methodology, was associated with significant decreases in CSB symptoms, including sexual desire/drive, frequency of masturbation, and pornography use.24
In addition to SSRIs, several additional case reports have suggested that other classes of antidepressants, such as serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants, or stimulants may be beneficial when treating CSB.25 Several case reports have indicated significant improvement of CSB symptoms using clomipramine.22 A retrospective study of nefazodone also has suggested that it may be an option for treating CSB. Patients reported notable reductions in the frequency of sexual obsessions/compulsions while taking nefazodone and reported no notable sexual adverse effects.26 One branded version of nefazodone, Serzone, was associated with rare but severe liver problems and was withdrawn from the U.S. market in 2004.
Although some initial evidence regarding antidepressant use, particularly SSRIs, to treat CSB has suggested that these medications may be potentially beneficial, the findings are far from conclusive, with only 1 controlled trial and only single-subject case reports for many of the medications studied.
Naltrexone, an opioid antagonist, has received support from available cases, open-label studies, and retrospective analyses.17,27 Although evidence for the use of naltrexone in CSB is limited to case reports and retrospective analyses, results have been positive. Naltrexone has shown notable decreases in CSB symptom severity when used as monotherapy and when used in combination with other treatments.
Anticonvulsants. Several case reports have suggested that certain anticonvulsants may be beneficial for treating CSB.
Psychotherapy
Evidence supporting specific types of psychotherapy for CSB is limited and largely drawn from uncontrolled studies and case reports.
Cognitive-behavioral therapy (CBT) is one of the more common psychotherapeutic options used for CSB. Several uncontrolled studies and case reports have found that CBT is beneficial for CSB, although methodologies have varied.
Several cases found that combining CBT with motivational interviewing was associated with significant reductions in sexual behaviors, such as frequency of sexual partners and amount of time spent online during work hours.29,30 Group CBT also has been shown to be effective for CSB.31
Acceptance and commitment therapy (ACT) has received some initial support, with 1 uncontrolled study and 1 controlled study.32,33 The controlled study used 12 sessions of individual ACT compared with a wait-list condition.32 Improvements in CSB symptoms were maintained for 3 months. The overall reduction in problematic Internet pornography use was reported as 92% immediately after the study ended, and 86% after 3 months.
Marital/relationship therapy has been used successfully in several case series and case reports, although no studies have assessed its efficacy in treating CSB using a randomized protocol. In 1 case report, the researcher found that participation in marital sex therapy elicited notable improvements over the course of 1 year and 20 sessions.34
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
1. Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am J Psychiatry. 2005;162(11):2184-2188.
2. Odlaug BL, Lust K, Schreiber LR, et al. Compulsive sexual behavior in young adults. Ann Clin Psychiatry. 2013;25(3):193-200.
3. Black DW. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4(4):219-229.
4. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatr Ann. 1992;22(6):320-325.
5. Kaplan MS, Krueger RB. Diagnosis, assessment, and treatment of hypersexuality. J Sex Res. 2010;47(2):181-198.
6. Black DW, Kehrberg LL, Flumerfelt DL, et al. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154(2):243-249.
7. McElroy SL, Phillips KA, Keck PE Jr. Obsessive compulsive spectrum disorder. J Clin Psychiatry. 1994;(suppl 55):33-51; discussion 52-53.
8. McElroy SL, Pope HG Jr, Keck PE Jr, et al. Are impulse-control disorders related to bipolar disorder? Compr Psychiatry. 1996;37(4):229-240.
9. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39(2):377-400.
10. Levine SB. What is sexual addiction? J Sex Marital Ther. 2010;36(3):261-275.
11. Christenson GA, Faber RJ, de Zwaan M, et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J Clin Psychiatry. 1994;55(1):5-11.
12. Grant JE. Impulse control disorders: a clinician’s guide to understanding and treating behavioral addictions. New York, NY: W.W. Norton & Company, Inc.; 2008.
13. Frohmader KS, Lehman MN, Laviolette SR, et al. Concurrent exposure to methamphetamine and sexual behavior enhances subsequent drug reward and causes compulsive sexual behavior in male rats. J Neurosci. 2011;31(45):16473-16482.
14. Grant JE, Pinto A, Gunnip M, et al. Sexual obsessions and clinical correlates in adults with obsessive-compulsive disorder. Compr Psychiatry. 2006;47(5):325-329.
15. Mété D, Dafreville C, Paitel V, et al. Aripiprazole, gambling disorder and compulsive sexuality [in French]. Encephale. 2016;42(3):281-283.
16. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
17. Kraus SW, Meshberg-Cohen S, Martino S, et al. Treatment of compulsive pornography use with naltrexone: a case report. Am J Psychiatry. 2015;172(12):1260-1261.
18. Derbyshire KL, Grant JE. Compulsive sexual behavior: a review of the literature. J Behav Addict. 2015;4(2):37-43.
19. Levine SB, Scott DL. Sexual education for psychiatric residents. Acad Psychiatry. 2010;34(5):349-352.
20. Alsughier N. Compulsive masturbation treated with selective serotonin reuptake inhibitors. African J Psychiatry (Johannesbg). 2015;18:299.
21. Elmore JL. SSRI reduction of nonparaphilic sexual addiction. CNS Spectr. 2000;5(11);53-56.
22. Stein DJ, Hollander E, Anthony DT, et al. Serotonergic medications for sexual obsessions, sexual addictions, and paraphilias. J Clinical Psychiatry. 1992;53(8):267-271.
23. Kafka M. Psychopharmacologic treatments for nonparaphilic compulsive sexual behaviors. CNS Spectr. 200;5(1):49-59.
24. Wainberg ML, Muench F, Morgenstern J, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67(12):1968-1973.
25. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry. 2000;61(9):664-670.
26. Coleman E, Raymond N, McBean A. Assessment and treatment of compulsive sexual behavior. Minn Med. 2003;86(7):42-47.
27. Raymond NC, Grant JE, Coleman E. Augmentation with naltrexone to treat compulsive sexual behavior: a case series. Ann Clin Psychiatry. 2010;22(1):56-62.
28. Fong TW, De La Garza R 2nd, Newton TF. A case report of topiramate in the treatment of nonparaphilic sexual addiction. J Clin Psychopharmacol. 2005;25(5):512-514.
29. Del Giudice MJ, Kutinsky J. Applying motivational interviewing to the treatment of sexual compulsivity and addiction. Sex Addict Comp. 2007;14(4):303-319.
30. Shepherd L. Cognitive behavior therapy for sexually addictive behavior. Clin Case Stud. 2010;9(1):18-27.
31. Sadiza J, Varma R, Jena SPK, et al. Group cognitive behaviour therapy in the management of compulsive sex behaviour. International Journal of Criminal Justice Sciences. 2011;6(1-2):309-325.
32. Crosby JM, Twohig MP. Acceptance and commitment therapy for problematic Internet pornography use: a randomized trial. Behav Ther. 2016;47(3):355-366.
33. Twohig MP, Crosby JM. Acceptance and commitment therapy as a treatment for problematic internet pornography viewing. Behav Ther. 2010;41(3):285-295.
34. Sprenkle DH. Treating a sex addict through marital sex therapy. Fam Relat. 1987;36(1):11-14.
4 Ways to help your patients with schizophrenia quit smoking
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
Tobacco-related cardiovascular disease is the primary reason adults with schizophrenia die on average 28 years earlier than their peers in the U.S. general population.1 To address this, clinicians need to prioritize smoking cessation and emphasize to patients with schizophrenia that quitting is the most important change they can make to improve their health. Here are 4 ways to help patients with schizophrenia quit smoking.
Provide hope, but be realistic. Most patients with schizophrenia who smoke want to quit; however, patients and clinicians alike have been discouraged by low quit rates and high relapse rates. Smoking often is viewed as one of the few remaining personal freedoms, as a lower priority than active psychiatric symptoms, or even as neuroprotective. By perpetuating these falsehoods and avoiding addressing smoking cessation, we are failing our patients.
With persistent engagement and use of effective pharmacotherapeutic interventions, smoking cessation is attainable and does not worsen psychiatric symptoms. Additionally, smoking cessation could save patients >$4,000 a year. It is crucial to make smoking cessation a priority at every appointment, and to offer patients hope and practical guidance through repeated attempts to quit.
Offer varenicline. For patients with schizophrenia, cessation counseling or behavioral interventions alone have a poor efficacy rate of approximately 5% (compared with 15% to 20% in the general population).2 Varenicline is the most effective smoking cessation treatment; it increases cessation rates 5-fold among patients with schizophrenia.3 As demonstrated by the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES),4 varenicline does not lead to an increased risk of suicidality or serious neuropsychiatric adverse effects.
When starting a patient on varenicline, set a quit date 4 weeks from medication initiation. Individuals with schizophrenia often have a greater smoking burden and experience more intense symptoms of nicotine withdrawal. A 4-week period between medication initiation and the quit date will allow these patients to gradually experience reduced cravings and separate minor adverse effects of the medication from those of nicotine withdrawal. Concurrent prescription of nicotine replacement therapy (eg, patch, gum, lozenge, inhaler) also is safe and can assist in quit attempts.
Consider varenicline maintenance therapy. After a successful quit attempt, increase the likelihood of sustained cessation by continuing varenicline beyond 12 weeks. Varenicline can be used as a maintenance medication to prevent smoking relapse in patients with schizophrenia; when prescribed to these patients for an additional 3 months, it can reduce the relapse rate similarly to that seen in smokers in the general population.5
Adjust antipsychotic dosages. Tobacco smoke increases the activity of cytochrome P450 1A2, which metabolizes several antipsychotics. Thus, after successful smoking cessation, concentrations of clozapine, fluphenazine, haloperidol, and olanzapine may increase, and dose reduction may be warranted. Conversely, if a patient resumes smoking, dosages of these medications may need to be increased.
Acknowledgments
The authors thank Anne Eden Evins, MD, MPH, and Corinne Cather, PhD, for their input on this article.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.
1. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
2. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2(2):CD007253.
3. Evins AE, Benowitz N, West R, et al. Neuropsychiatric safety and efficacy of varenicline and bupropion vs. nicotine patch and placebo in the psychiatric cohort of the EAGLES trial. Paper presented at: Society for Research on Nicotine and Tobacco, 22nd Annual Meeting; March 2-5, 2016; Chicago, IL.
4. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
5. Evins AE, Hoeppner SS, Schoenfeld DA, et al. Maintenance pharmacotherapy normalizes the relapse curve in recently abstinent tobacco smokers with schizophrenia and bipolar disorder. Schizophr Res. 2017;183:124-129.