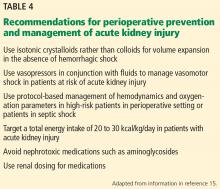
User login
Navigating travel with diabetes
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
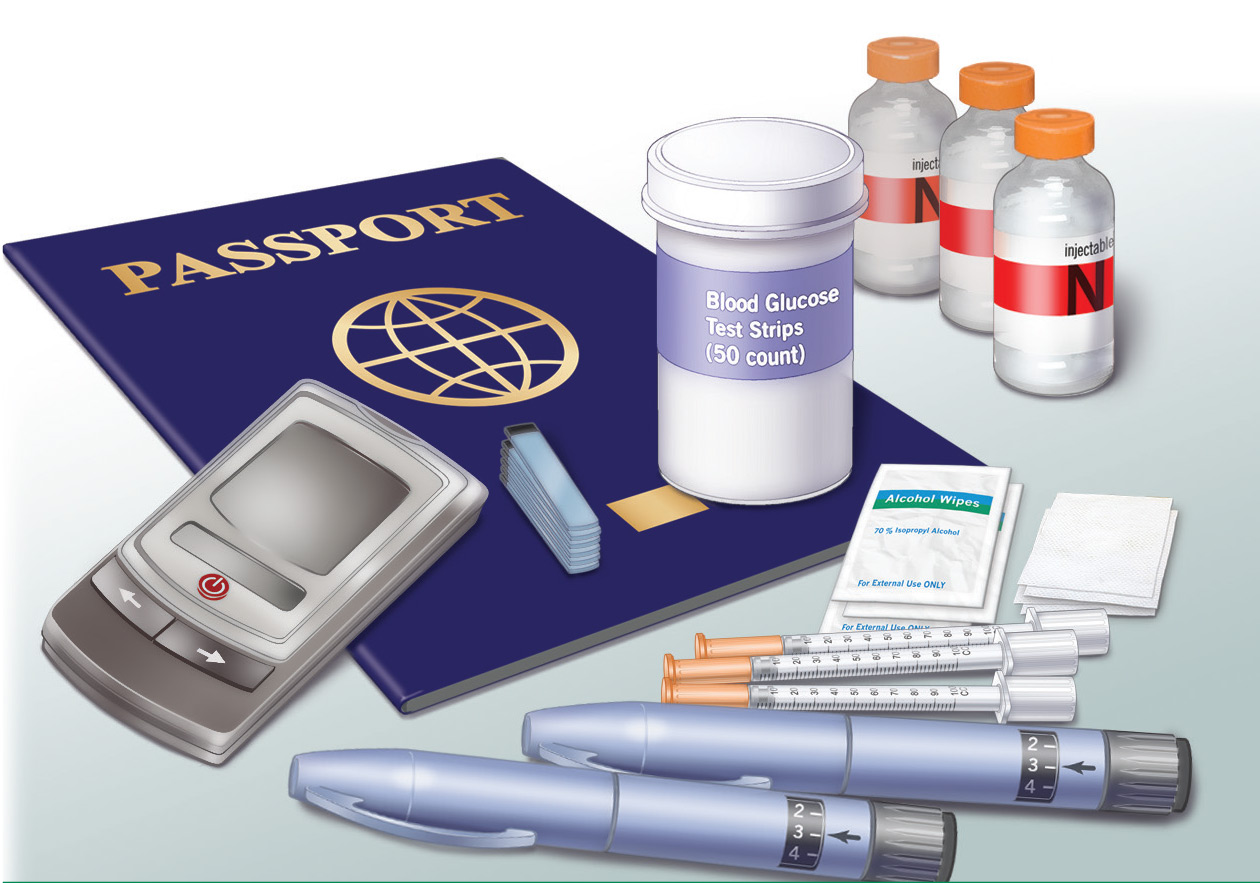
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
Travel, once reserved for wealthy vacationers and high-level executives, has become a regular experience for many people. The US Travel and Tourism Overview reported that US domestic travel climbed to more than 2.25 billion person-trips in 2017.1 The US Centers for Disease Control and Prevention (CDC) and the US Travel Association suggest that, based on this frequency and the known rate of diabetes, 17 million people with diabetes travel annually for leisure and 5.6 million for business, and these numbers are expected to increase.2
It stands to reason that as the number of people who travel continues to increase, so too will the number of patients with diabetes seeking medical travel advice. Despite resources available to travelers with diabetes, researchers at the 2016 meeting of the American Diabetes Association noted that only 30% of patients with diabetes who responded to a survey reported being satisfied with the resources available to help them manage their diabetes while traveling.2 This article discusses how clinicians can help patients manage their diabetes while traveling, address common travel questions, and prepare patients for emergencies that may arise while traveling.
PRE-TRIP PREPARATION
Provider visit before travel: Checking the bases
Advise patients to schedule an appointment 4 to 6 weeks before their trip.3 At this appointment, give the patient a healthcare provider travel letter (Figure 1) and prescriptions that the patient can hand-carry en route.3 The provider letter should state that the patient has diabetes and should list all supplies the patient needs. The letter should also include specific medications used by the patient and the devices that deliver these medications, eg, Humalog insulin and U-100 syringes4 to administer insulin, as well as any food and medication allergies.
Prescriptions should be written for patients to use in the event of an emergency during travel. Prescriptions for diabetes medications should be written with generic names to minimize confusion for those traveling internationally. Additionally, all prescriptions should provide enough medication to last throughout the trip.4
Advise patients that rules for filling prescriptions may vary between states and countries.3 Also, the strength of insulin may vary between the United States and other countries. Patients should understand that if they fill their insulin prescription in a foreign country, they may need to purchase new syringes to match the insulin dose. For example, if patients use U-100 syringes and purchase U-40 insulin, they will need to buy U-40 syringes or risk taking too little of a dose.
Remind patients that prescriptions are not necessary for all diabetes supplies but are essential for coverage by insurance companies. Blood glucose testing supplies, ketone strips, and glucose tablets may be purchased in a pharmacy without a prescription. Human insulin may also be purchased over the counter. However, oral medications, glucagon, and analog insulins require a prescription. We suggest that patients who travel have their prescriptions on file at a chain pharmacy rather than an independent one. If they are in the United States, they can go to any branch of the chain pharmacy and easily fill a prescription.
Work with the patient to compile a separate document that details the medication dosing, correction-scale instructions, carbohydrate-to-insulin ratios, and pump settings (basal rates, insulin sensitivity, active insulin time).4 Patients who use an insulin pump should record all pump settings in the event that they need to convert to insulin injections during travel.4 We suggest that all patients with an insulin pump have an alternate insulin method (eg, pens, vials) and that they carry this with them along with basal insulin in case the pump fails. This level of preparation empowers the patient to assume responsibility for his or her own care if a healthcare provider is not available during travel.
Like all travelers, patients with diabetes should confirm that their immunizations are up to date. Encourage patients to the CDC’s page (wwwnc.cdc.gov/travel) to check the list of vaccines necessary for their region of travel.4,5 Many special immunizations can be acquired only from a public health department and not from a clinician’s office.
Additionally, depending on the region of travel, prescribing antibiotics or antidiarrheal medications may be necessary to ensure patient safety and comfort. We also recommend that patients with type 1 diabetes obtain a supply of antibiotics and antidiarrheals because they can become sick quickly.
Packing with diabetes: Double is better
The American Diabetes Association recommends that patients pack at least twice the medication and blood-testing supplies they anticipate needing.3 Reinforce to patients the need to pack all medications and supplies in their carry-on bag and to keep this bag in their possession at all times to avoid damage, loss, and extreme changes in temperature and air pressure, which can adversely affect the activity and stability of insulin.
Ask patients about the activities they plan to participate in and how many days they will be traveling, and then recommend shoes that will encourage appropriate foot care.4 Patients with diabetes should choose comfort over style when selecting footwear. All new shoes should be purchased and “broken in” 2 to 3 weeks before the trip. Alternating shoes decreases the risk of blisters and calluses.4
Emergency abroad: Planning to be prepared
It is crucial to counsel patients on how to respond in an emergency.
Encourage patients with diabetes, especially those who use insulin, to obtain a medical identification bracelet, necklace, or in some cases, a tattoo, that states they use insulin and discloses any allergies.3 This ensures that emergency medical personnel will be aware of the patient’s condition when providing care. Also suggest that your patients have emergency contact information available on their person and their cell phone to expedite assistance in an emergency (Table 2).
Urge patients to determine prior to their departure if their health coverage will change once they leave the state or the country. Some insurance companies require patients to go to a specific healthcare system while others regulate the amount of time a patient can be in the hospital before being transferred home. It is important for patients to be aware of these terms in the event of hospitalization.4 Travel insurance should be considered for international travel.
AIRPORT SECURITY: WHAT TO EXPECT WITH DIABETES
The American Diabetes Association works with the US Transportation Security Administration (TSA) to ensure that passengers with diabetes have access to supplies. Travelers with diabetes are allowed to apply for an optional disability notification card, which discreetly informs officers that the passenger has a condition or device that may affect screening procedures.6
The TSA suggests that, before going through airport screening, patients with diabetes separate their diabetes supplies from their luggage and declare all items.6 Including prescription labels for medications and medical devices helps speed up the security process. Advise patients to carry glucose tablets and other solid foods for treating hypoglycemia when passing through airport security checkpoints.7
Since 2016, the TSA has allowed all diabetes-related supplies, medications, and equipment, including liquids and devices, through security after they have been screened by the x-ray scanner or by hand.7 People with diabetes are allowed to carry insulin and other liquid medications in amounts greater than 3.4 ounces (100 mLs) through airport security checkpoints.
Insulin can pass safely through x-ray scanners, but if patients are concerned, they may request that their insulin be inspected by hand.7 Patients must inform airport security of this decision before the screening process begins. A hand inspection may include swabbing for explosives.
Patients with an insulin pump and a continuous glucose monitoring device may feel uncomfortable during x-ray screening and special security screenings. Remind patients that it is TSA policy that patients do not need to disconnect their devices and can request screening by pat-down rather than x-ray scanner.6 It is the responsibility of the patient to research whether the pump can pass through x-ray scanners.
All patients have the right to request a pat-down and can opt out of passing through the x-ray scanner.6 However, patients need to inform officers about a pump before screening and must understand that the pump may be subject to further inspection. Usually, this additional inspection includes swabbing the patient’s hands to check for explosive material and a simple pat-down of the insulin pump.7
IN-FLIGHT TIPS
Time zones and insulin dosing
Diabetes management is often based on a 24-hour medication schedule. Travel can disrupt this schedule, making it challenging for patients to determine the appropriate medication adjustments. With some assistance, the patient can determine the best course of action based on the direction of travel and the number of time zones crossed.
According to Chandran and Edelman,7 medication adjustments are needed only when the patient is traveling east or west, not north or south. As time zones change, day length changes and, consequently, so does the 24-hour regimen many patients follow. As a general rule, traveling east results in a shortened day, requiring a potential reduction in insulin, while traveling west results in a longer day, possibly requiring an increase in insulin dose.7 However, this is a guideline and may not be applicable to all patients.7
Advise patients to follow local time to administer medications beginning the morning after arrival.7 It is not uncommon, due to changes in meal schedules and dosing, for patients to experience hyperglycemia during travel. They should be prepared to correct this if necessary.
Patients using insulin injections should plan to adjust to the new time zone as soon as possible. If the time change is only 1 or 2 hours, they should take their medications before departure according to their normal home time.7 Upon arrival, they should resume their insulin regimen based on the local time.
Westward travel. If the patient is traveling west with a time change of 3 or more hours, additional changes may be necessary. Advise patients to take their insulin according to their normal home time before departure. The change in dosing and schedule will depend largely on current glucose control, time of travel, and availability of food and glucose during travel. Encourage patients to discuss these matters with you in advance of any long travel.
Eastward travel. When the patient is traveling east with a time change greater than 3 hours, the day will be consequently shortened. On the day of travel, patients should take their morning dose according to home time. If they are concerned about hypoglycemia, suggest that they decrease the dose by 10%.6 On arrival, they should adhere to the new time zone and base insulin dosing on local time.
Advice for insulin pump users. Patients with an insulin pump need make only minimal changes to their dosing schedule. They should continue their routine of basal and bolus doses and change the time on their insulin pump to local time when they arrive. Insulin pump users should bring insulin and syringes as backup; in the event of pump malfunction, the patient should continue to use the same amount of bolus insulin to correct glucose readings and to cover meals.7 As for the basal dose, patients can administer a once-daily injection of long-acting insulin, which can be calculated from their pump or accessed from the list they created as part of their pre-travel preparation.7
Advice for patients on oral diabetes medications
If a patient is taking an oral medication, it is less crucial to adhere to a time schedule. In fact, in some cases it may be preferable to skip a dose and risk slight hyperglycemia for a few hours rather than take medication too close in time and risk hypoglycemia.7
Remind patients to anticipate a change in their oral medication regimen if they travel farther than 5 time zones.7 Encourage patients to research time changes and discuss the necessary changes in medication dosage on the day of travel as well as the specific aspects of their trip. A time-zone converter can be found at www.timeanddate.com.8
WHAT TO EXPECT WHILE ON LAND
Insulin 101
Storing insulin at the appropriate temperature may be a concern. Insulin should be kept between 40°F and 86°F (4°C–30°C).4 Remind patients to carry their insulin with them at all times and to not store it in a car glove compartment or backpack where it can be exposed to excessive sun. The Frio cold pack (ReadyCare, Walnut Creek, CA) is a helpful alternative to refrigeration and can be used to cool insulin when hiking or participating in activities where insulin can overheat. These cooling gel packs are activated when exposed to cold water for 5 to 7 minutes5 and are reusable.
Alert patients that insulin names and concentrations may vary among countries. Most insulins are U-100 concentration, which means that for every 1 mL of liquid there are 100 units of insulin. This is the standard insulin concentration used in the United States. There are U-200, U-300, and U-500 insulins as well. In Europe, the standard concentration is U-40 insulin. Syringe sizes are designed to accommodate either U-100 or U-40 insulin. Review these differences with patients and explain the consequences of mixing insulin concentration with syringes of different sizes. Figure 2 shows how to calculate equivalent doses.
Resort tips: Food, drinks, and excursions
A large component of travel is indulging in local cuisine. Patients with diabetes need to be aware of how different foods can affect their diabetes control. Encourage them to research the foods common to the local cuisine. Websites such as Calorie King, MyFitnessPal, Lose it!, and Nutrition Data can help identify the caloric and nutritional makeup of foods.9
Advise patients to actively monitor how their blood glucose is affected by new foods by checking blood glucose levels before and after each meal.9 Opting for vegetables and protein sources minimizes glucose fluctuations. Remind patients that drinks at resorts may contain more sugar than advertised. Patients should continue to manage their blood glucose by checking levels and by making appropriate insulin adjustments based on the readings. We often advise patients to pack a jar of peanut butter when traveling to ensure a ready source of protein.
Patients who plan to participate in physically challenging activities while travelling should inform all relevant members of the activity staff of their condition. In case of an emergency, hotel staff and guides will be better equipped to help with situations such as hypoglycemia. As noted above, patients should always carry snacks and supplies to treat hypoglycemia in case no alternative food options are available during an excursion. Also, warn patients to avoid walking barefoot. Water shoes are a good alternative to protect feet from cuts and sores.
Patients should inquire about the safety of high-elevation activities. With many glucose meters, every 1,000 feet of elevation results in a 1% to 2% underestimation of blood glucose,10 which could result in an inaccurate reading. If high-altitude activities are planned, advise patients to bring multiple meters to cross-check glucose readings in cases where inaccuracies (due to elevation) are possible.
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
- US Travel Association. US travel and tourism overview. www.ustravel.org/system/files/media_root/document/Research_Fact-Sheet_US-Travel-and-Tourism-Overview.pdf. Accessed June 14, 2018.
- Brunk D. Long haul travel turbulent for many with type 1 diabetes. Clinical Endocrinology News 2016. www.mdedge.com/clinicalendocrinologynews/article/109866/diabetes/long-haul-travel-turbulent-many-type-1-diabetes. Accessed June 14, 2018.
- American Diabetes Association. When you travel. www.diabetes.org/living-with-diabetes/treatment-and-care/when-you-travel.html?utm_source=DSH_BLOG&utm_medium=BlogPost&utm_content=051514-travel&utm_campaign=CON. Accessed June 14, 2018.
- Kruger DF. The Diabetes Travel Guide. How to travel with diabetes-anywhere in the world. Arlington, VA: American Diabetes Association; 2000.
- Centers for Disease Control and Prevention. Travelers’ health. wwwnc.cdc.gov/travel/. Accessed June 14, 2018.
- American Diabetes Association. What special concerns may arise? www.diabetes.org/living-with-diabetes/know-your-rights/discrimination/public-accommodations/air-travel-and-diabetes/what-special-concerns-may.html. Accessed June 14, 2018.
- Chandran M, Edelman SV. Have insulin, will fly: diabetes management during air travel and time zone adjustment strategies. Clinical Diabetes 2003; 21(2):82–85. doi:10.2337/diaclin.21.2.82
- Time and Date AS. Time zone converter. timeanddate.com. Accessed March 19, 2018.
- Joslin Diabetes Center. Diabetes and travel—10 tips for a safe trip. www.joslin.org/info/diabetes_and_travel_10_tips_for_a_safe_trip.html. Accessed June 14, 2018.
- Jendle J, Adolfsson P. Impact of high altitudes on glucose control. J Diabetes Sci Technol 2011; 5(6):1621–1622. doi:10.1177/193229681100500642
KEY POINTS
- Patients should pack all diabetes medications and supplies in a carry-on bag and keep it in their possession at all times.
- A travel letter will facilitate easy transfer through security and customs.
- Patients should always take more supplies than needed to accommodate changes in travel plans.
- If patients will cross multiple time zones during their travel, they will likely need to adjust their medication and food schedules.
‘Dry drowning’ and other myths
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
In June 2017, a 4-year-old boy died 1 week after being knocked over and briefly submerged while playing in knee-deep water. This story was widely reported as a case of a rare occurrence called “dry” or “secondary” drowning, depending on the source.1 The media accounts went viral, spreading fear in parents and others learning about these alleged conditions from the news and social media.
Many alleged cases of dry drowning are reported every year, but each has been found to have a recognized medical source that has a legitimate medically recognized diagnosis (which dry and secondary drowning are not).
Drowning is one of the most common causes of death in children, and so we ought to make sure that the information we share about it is accurate, as it is vital to effective prevention, rescue, and treatment.
Unfortunately, medical providers, medical journals, and the mass media continue to disseminate misinformation on drowning.2 These reports often prevail over updated information and hinder accurate understanding of the drowning problem and its solutions.
Every death is tragic, especially the death of a child, and our heartfelt sympathies go out to the family in this alleged drowning case, as well as to all families suffering the loss of a loved one to drowning. However, in the 2017 case, the cause of death was found on autopsy to be myocarditis not related in any way to drowning. As often happens in such situations, this clarification did not receive any media attention, despite the wide reporting and penetration of the original, erroneous story.
We hope our review will reduce misunderstanding among the public and healthcare providers, contribute to improved data collection, and help to promote interventions aimed at prevention, rescue, and mitigation of drowning incidents.
WHAT IS DROWNING?
A consensus committee of the World Health Organization defined drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”3 The process begins when the victim’s airway goes below the surface of the liquid (submersion) or when water splashes over the face (immersion). If the victim is rescued at any time, the process is interrupted, and this is termed a nonfatal drowning. If the victim dies at any time, this is a fatal drowning. Any water-distress incident without evidence of respiratory impairment (ie, without aspiration) should be considered a water rescue and not a drowning.
Rarely do minimally symptomatic cases progress to death, just as most cases of chest pain do not progress to cardiac arrest.4 Nonetheless, rescued drowning victims can deteriorate, which is why we encourage people to seek medical care immediately upon warning signs, as we do with chest pain. For drowning, such warning signs are any water distress followed by difficulty breathing, excessive coughing, foam in the mouth, or abnormal behavior.
A SERIOUS PUBLIC HEALTH ISSUE
Drowning is a serious and neglected public health issue, claiming the lives of 372,000 people a year worldwide.5 It is a leading cause of death in children ages 1 to 14. The toll continues largely unabated, and in low- and middle-income nations it does not attract the levels of funding that go to other forms of injury prevention, such as road safety.
Nonfatal drowning—with symptoms ranging from mild cough to severe pulmonary edema, and complications ranging from none to severe neurologic impairment—is far more common than fatal drowning.6 For every fatal drowning, there are at least 5 nonfatal drowning incidents in which medical care is needed, and 200 rescues are performed.7–10
In the United States, drowning accounts for almost 13,000 emergency department visits per year and about 3,500 deaths.7,8
In Brazil, with two-thirds the population of the United States, drowning accounts for far fewer hospital visits but about twice as many deaths. In Rio de Janeiro, where a highly effective and specialized prehospital service is provided at 3 drowning resuscitation centers staffed by medical doctors, an analysis of the 46,060 cases of rescue in 10 years from 1991 to 2000 showed that medical assistance was needed in only 930 cases (2%).10 The preventive and rescue actions of parents, bystanders, lifeguards, and prehospital rescue services significantly reduce the number of drowning deaths, but these groups do not consistently gather data on nonfatal drowning that can be included in a comprehensive database.
DROWNING IS A PROCESS
When a person in the water can no longer keep the airway clear, water that enters the mouth is voluntarily spit out or swallowed. Within a few seconds to minutes, the person can no longer clear the airways and water is aspirated, stimulating the cough reflex. Laryngospasm, another myth concerning drowning, is presumed to protect the airways but does not, as it is rare, occurring in less than 2% of cases.11,12
If the person is not rescued, aspiration of water continues, and hypoxemia leads to loss of consciousness and apnea within seconds to a few minutes, followed by cardiac arrest. As a consequence, hypoxemic cardiac arrest generally occurs after a period of tachycardia followed by bradycardia and pulseless electrical activity, usually leading to asystole.13,14
The entire drowning process, from water distress to cardiac arrest, usually takes a few minutes, but in rare situations, such as rapid hypothermia, it can go on for up to an hour.15 Most drowning patients have an otherwise healthy heart, and the apnea and hypoxemia precede the cardiac arrest by only a few seconds to minutes; thus, cardiac arrest is caused by the hypoxemic insult and not by ventricular dysrhythmias.6,16
Drowning can be interrupted at any point between distress and death. If the person is rescued early, the clinical picture is determined by the reactivity of the airway and the amount of water that has been aspirated, but not by the type of water (salt or fresh).
Another myth is that drowning in salt water is different from drowning in fresh water. Both salt water and fresh water cause similar surfactant destruction and washout and disrupt the alveolar-capillary membrane. Disruption of the alveolar-capillary membrane increases its permeability and exacerbates shifting of fluid, plasma, and electrolytes into the alveoli.13 The clinical picture of the damage is one of regional or generalized pulmonary edema, which interferes with gas exchange in the lungs.6,13,17
Animal studies by Modell et al showed that aspiration of just 2.2 mL of water per kilogram of body weight is sufficient to cause severe disturbances in oxygen exchange,17 reflected in a rise in arterial pH and a drop in partial pressure of oxygen. The situation must be similar in humans. In a 70-kg person, this is only about 154 mL of water—about two-thirds of a cup.
The combined effects of fluid in the lungs, the loss of surfactant, and the increase in capillary-alveolar permeability can result in decreased lung compliance, increased right-to-left shunting in the lungs, atelectasis, alveolitis, hypoxemia, and cerebral hypoxia.13
If the victim needs cardiopulmonary resuscitation, the possibility of neurologic damage is similar to that in other cardiac arrest situations, but exceptions exist. For example, in rare cases, hypothermia provides a protective mechanism that allows victims to survive prolonged submersion.4,15
The duration of submersion is the best predictor of death.18 Underwater, people are not taking in oxygen, and cerebral hypoxia causes both morbidity and death. For this reason, reversing cerebral hypoxia with effective ventilation, oxygen, and chest compression is the priority of treatment.
MYTHS AND SLOPPY TERMINOLOGY
“Near drowning,” “dry drowning,” “wet drowning,” “delayed drowning,” and “secondary drowning” are not medically accepted diagnoses,3,4,19 and many organizations and lifesaving institutions around the world discourage the use of these terms.19,20 Unfortunately, these terms still slip past the editors of medical journals and are thus perpetuated. The terms are most pervasive in the nonmedical media, where drowning seems to be synonymous with death.3,19,21 We urge all authors and stakeholders to abandon these terms in favor of understanding and communicating drowning as a process that can vary in severity and have a fatal or nonfatal outcome.
Near-drowning
Historically, drowning meant death, while near-drowning meant the victim survived, at least initially (usually for at least 24 hours).
Before 2002, there were 13 different published definitions of near-drowning.21,22 This variability has caused a great deal of confusion when trying to describe and monitor drowning.
A person can drown and survive, just as a person can have cardiac arrest and survive.4,21 Just as there is no recognized condition of “near-cardiac arrest,” there is also no condition of near-drowning. Using near-drowning as a medical diagnosis hides the true burden of drowning and consequently amplifies difficulties in developing effective prevention, rescue, and treatment programs.
Dry drowning
Dry drowning has never been an accepted medical term, although it has been used to describe different parts of the drowning process. While many authors use it as a synonym for secondary drowning (described below), in the past it was usually used in cases in which no water was found in the lungs at autopsy in persons who were found dead in the water.2–4,21 This occurred in about 10% to 15% of cases and was also called drowning “without water aspiration.”
Perhaps some victims suffer sudden cardiac death. It happens on land—why not in the water? Modell et al stated, “In the absence of the common finding of significant pulmonary edema in the victim’s respiratory system, to conclude his or her death was caused by ‘drowning without aspiration’ is unwise.”23
Laryngospasm is another proposed explanation. It could play a role in the fewer than 2% of cases in which no other cause of death is found on clinical examination or autopsy,11,12,19,23 but it does not occur in most cases of drowning, or it is brief and is terminated by the respiratory movements that allow the air in the lung to escape and water to be inhaled.
The problem with the term dry drowning is the harm caused by misdiagnosing cases of sudden death as drowning, when an alternative cause is present. Most importantly, the management is the same if small amounts of water are present or not; therefore, no clinical distinction is made between wet and dry drowning.
Secondary drowning
Secondary drowning, sometimes called delayed drowning, is another term that is not medically accepted. The historical use of this term reflects the reality that some patients may worsen due to pulmonary edema after aspirating small amounts of water.
Drowning starts with aspiration, and few or only mild symptoms may be present as soon as the person is removed from the water. Either the small amount of water in the lungs is absorbed and causes no complications or, rarely, the patient’s condition becomes progressively worse over the next few hours as the alveoli become inflamed and the alveolar-capillary membrane is disrupted. But people do not unexpectedly die of drowning days or weeks later with no preceding symptoms. The lungs and heart do not “fill up with water,” and water does not need to be pumped out of the lungs.
There has never been a case published in the medical literature of a patient who underwent clinical evaluation, was initially without symptoms, and later deteriorated and died more than 8 hours after the incident.6,10,21 People who have drowned and have minimal symptoms get better (usually) or worse (rarely) within 4 to 8 hours. In a study of more than 41,000 lifeguard rescues, only 0.5% of symptomatic patients died.6
Drowning secondary to injury or sudden illness
Any injury, trauma, or sudden illness that can cause loss of consciousness or mental or physical weakness can lead to drowning. Physicians need to recognize these situations to treat them appropriately. Drowning that is secondary to other primary insults can be classified as24:
- Drowning caused by injury or trauma (eg, a surfing, boating, or a hang-gliding accident)
- Drowning caused by a sudden illness such as cardiac disease (eg, myocardial ischemia, arrhythmias, prolonged QT syndrome, hypertrophic cardiomyopathy) or neurologic disease (eg, epilepsy, stroke)
- Diving disease (eg, decompression sickness, pulmonary overpressurization syndrome, compression barotrauma, narcosis [“rapture of the deep”], shallow water blackout, immersion pulmonary edema).
PREVENTION IS BEST
Drowning is a leading and preventable cause of death worldwide and for people of all ages. The danger is real, not esoteric or rare, and healthcare providers should use any opportunity to discuss with patients, parents, and the media the most important tool for treating drowning: primary prevention.
For example, small children should be continuously and uninterruptedly supervised within arm’s reach while in the water, even if a lifeguard is present. Other preventive measures are lifejackets, fences completely enclosing pools or ponds, and swimming and water safety lessons. Drowning often occurs in a deceptively pleasant environment that may not seem dangerous.
RECOGNIZE DISTRESS
When preventive measures fail, responders (usually a health professional is involved) need to be able to perform the necessary steps to interrupt the drowning process.
The first challenge is to recognize when someone in the water is at risk of drowning and needs to be rescued.25 Early self-rescue or rescue by others may stop the drowning process and prevent most cases of initial and subsequent water aspiration, respiratory distress, and medical complications.
DON’T BECOME A VICTIM
Rescuers must take care not to become victims themselves. Panicked swimmers can thrash about and injure the rescuer or clutch at anything they encounter, dragging the rescuer under. And the rescuer can succumb to the same hazards that got the victim into trouble, such as strong currents, deep water, or underwater hazards.
Certified lifeguards are trained to get victims out of the water safely. The American Red Cross slogan “Reach or throw, don’t go” means “Reach out with a pole or other object or throw something that floats; don’t get in the water yourself.”
WHAT TO TELL THE PUBLIC
While some journalists acknowledge that the terms dry drowning and secondary drowning are medically discredited, they still use them in their reports. The novelty of this story—and its appeal to media outlets—is precisely the unfamiliarity of these terms to the general public and the perceived mysterious, looming threat.
We often hear that these terms are more familiar to the public, which is likely true. More concerning, some physicians continue to use them (and older definitions of drowning that equate it with death) in media interviews, clinical care, and publications. The paradox is that we, the medical community, invented these terms, not patients or the media.
As clinicians and researchers, we should drive popular culture definitions, not the other way around. Rather than dismiss these terms as “semantics” or “technicalities,” we should take the opportunity to highlight the dangers of drowning and the importance of prevention, and to promote simpler language that is easier for us and our patients to understand.19,21
Healthcare providers should understand and share modern drowning science and best practices, which will reduce fear, improve resource utilization, and prevent potentially deadly consequences due to misunderstanding or misinterpretation of incorrect terminology.
WHEN PATIENTS SHOULD SEEK CARE
Anyone who experiences cough, breathlessness, or other worrisome symptoms such as abnormal mentation within 8 hours of a drowning incident (using the modern definition above) should seek medical advice immediately.
We tell people to seek care if symptoms seem any worse than the experience of a drink “going down the wrong pipe” at the dinner table.21 But symptoms can be minimal. Careful attention should be given to mild symptoms that get progressively worse during that time. These cases can rarely progress to acute respiratory distress syndrome.
Table 1 explores who needs further medical help after being rescued from the water.26
In most of these cases, it is most appropriate to call an ambulance, but care may involve seeing a doctor depending on the severity of the symptoms.6,21 Usually, drowning patients are observed for 4 to 8 hours in an emergency department and are discharged if normal. Symptoms that are more significant include persistent cough, foam at the mouth or nose, confusion, or abnormal behavior, and these require further medical evaluation.
Patients should also seek medical care even if they are 100% normal upon exiting the water but develop worrisome symptoms more than 8 hours later, and providers should consider diagnoses other than primary drowning. Spontaneous pneumothorax, chemical pneumonitis, bacterial or viral pneumonia, head injury, asthma, chest trauma, and acute respiratory distress syndrome have been mislabeled as delayed, dry, or secondary drowning.3,4,19,21
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
- Buffington B. Texas boy dies from ‘dry drowning’ days after swimming. USA Today, June 8, 2017. www.usatoday.com/story/news/nation-now/2017/06/08/texas-boy-dies-dry-drowning-days-after-swimming/379944001.
- Schmidt AC, Sempsrott JR, Szpilman D, et al. The use of non-uniform drowning terminology: a follow-up study. Scand J Trauma Resusc Emerg Med 2017; 25(1):72. doi:10.1186/s13049-017-0405-x
- van Beeck EF, Branche CM, Szpilman D, Modell JH, Bierens JJ. A new definition of drowning: towards documentation and prevention of a global public health problem. Bull World Health Organ 2005; 83(11):853–856. pmid:16302042
- Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med 2012; 366(22):2102–2110. doi:10.1056/NEJMra1013317
- World Health Organization. Global report on drowning: preventing a leading killer. www.who.int/violence_injury_prevention/global_report_drowning/en. Accessed June 13, 2018.
- Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest 1997; 112(3):660–665. pmid:9315798
- Centers for Disease Control and Prevention. Welcome to WISQARS. www.cdc.gov/injury/wisqars. Accessed June 13, 2018.
- Centers for Disease Control and Prevention. WONDER. https://wonder.cdc.gov. Accessed June 13, 2018.
- Cummings P, Quan L. Trends in unintentional drowning: the role of alcohol and medical care. JAMA 1999; 281(23):2198–2202. pmid:10376572
- Szpilman D, Elmann J, Cruz-Filho FES. Drowning classification: a revalidation study based on the analysis of 930 cases over 10 years. World Congress on Drowning, Netherlands 2002. www.researchgate.net/publication/267981062_DROWNING_CLASSIFICATION_a_revalidation_study_based_on_the_analysis_of_930_cases_over_10_years. Accessed June 13, 2018.
- Szpilman D, Elmann J, Cruz-Filho FES. Dry-drowning—fact or myth? World Congress on Drowning. Netherlands, 2002. www.researchgate.net/publication/267981164_Dry-drowning_-Fact_or_Myth. Accessed June 13, 2018.
- Lunetta P, Modell JH, Sajantila A. What is the incidence and significance of "dry-lungs" in bodies found in water? Am J Forensic Med Pathol 2004; 25(4):291–301. pmid:15577518
- Orlowski JP, Abulleil MM, Phillips JM. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann Emerg Med 1989; 18:1044–1049. pmid:2802278
- Grmec S, Strnad M, Podgorsek D. Comparison of the characteristics and outcome among patients suffering from out-of-hospital primary cardiac arrest and drowning victims in cardiac arrest. Int J Emerg Med 2009; 2(1):7–12. doi:10.1007/s12245-009-0084-0
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011; 82(7):819–824. doi:10.1016/j.resuscitation.2011.02.021
- Orlowski JP, Szpilman D. Drowning. Rescue, resuscitation, and reanimation. Pediatr Clin North Am 2001; 48(3):627–646. pmid:11411297
- Modell JH, Moya F, Newby EJ, Ruiz BC, Showers AV. The effects of fluid volume in seawater drowning. Ann Intern Med 1967; 67(1):68–80. pmid:6028660
- Quan L, Wentz KR, Gore EJ, Copass MK. Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990; 86(4):586–593. pmid:2216625
- Szpilman D, Orlowski JP, Cruz-Filho FES. Hey “Near-drowning,” you’ve been messing up our minds! World Congress on Drowning. Amsterdam, 2002. www.researchgate.net/publication/267981173_HEY_Near-drowning_YOU%27VE_BEEN_MESSING_UP_OUR_MINDS. Accessed June 13, 2018.
- American College of Emergency Physicians. Death after swimming is extremely rare—and is not “dry drowning.” http://newsroom.acep.org/2017-07-11-Death-After-Swimming-Is-Extremely-Rare-And-Is-NOT-Dry-Drowning. Accessed June 13, 2018.
- Hawkins SC, Sempsrott J, Schmidt A. “Drowning” in a sea of misinformation. Emergency Medicine News 2017; 39*8):1. http://journals.lww.com/em-news/blog/BreakingNews/pages/post.aspx?PostID=377. Accessed June 5, 2018.
- Szpilman D, Tipton M, Sempsrott J, et al. Drowning timeline: a new systematic model of the drowning process. Am J Emerg Med 2016; 34(11):2224–2226. doi:10.1016/j.ajem.2016.07.063
- Modell JH, Bellefleur M, Davis JH. Drowning without aspiration: is this an appropriate diagnosis? J Forensic Sci 1999; 44(6):1119–1123. pmid:10582353
- Szpilman D, Orlowski JP. Sports related to drowning. Eur Respir Rev 2016; 25(141):348–359. doi:10.1183/16000617.0038-2016
- Szpilman D, Webber J, Quan L, et al. Creating a drowning chain of survival. Resuscitation 2014; 85(9):1149–1152. doi:10.1016/j.resuscitation.2014.05.034
- International Life Saving Federation. Who needs further medical help after rescue from the water. Medical Position Statement - MPS 06, 2016. www.ilsf.org/file/3916/download?token=pDnPDCrk. Accessed June 13, 2018.
KEY POINTS
- Drowning is a process of aspiration leading to hypoxia and eventually cardiac arrest. However, it is not synonymous with death: it can be interrupted.
- Patients who have been rescued from drowning and who have minimal symptoms generally get better within 4 to 8 hours of the event.
- Rescued victims should be warned that, although a rare condition, if they develop cough, breathlessness, or any other worrisome symptom within 8 hours of being in the water, they should seek medical attention immediately.
Optimizing calcium and vitamin D intake through diet and supplements
Although calcium and vitamin D are often recommended for prevention and treatment of osteoporosis, considerable controversy exists in terms of their safety and efficacy.1 This article highlights the issues, referring readers to reviews and meta-analyses for details and providing some practical advice for patients requiring supplementation.
CALCIUM INTAKE AND BONE DENSITY
Calcium enters the body through diet and supplementation. If intake is low, blood calcium levels fall, resulting in secondary hyperparathyroidism, which has 3 main effects:
- Increased fractional absorption of the calcium that is consumed
- Reduced urinary excretion of calcium
- Increased bone resorption, which releases calcium into the blood,2 which explains the potential for the deleterious effect of deficient intake of calcium on bone.3
Based on the simple physiology outlined above, it seems logical that insufficient intake of calcium over time could lead to mobilization of calcium from bone, lower bone mineral density, and higher fracture risk.3 This topic has been reviewed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and the International Foundation for Osteoporosis.1
Many lines of evidence suggest that low calcium intake adversely affects bone mineral density.1 Low calcium intake has been associated with lower bone density in some cross-sectional studies,4–6 though not all.7 Interventions to increase calcium intake in postmenopausal women have shown beneficial effects on bone density,8–10 though in some studies the benefit was small and nonprogressive.11 The question is whether this improvement in bone mineral density translates into fewer fractures.
Results from individual studies looking at fracture prevention through calcium supplementation have been conflicting,10,12–14 and reviews and meta-analyses have summarized the data.1,3,15 A recent review of these meta-analyses showed a small but significant reduction in some types of fracture.1
Some speculate that the difficulty in demonstrating fracture efficacy might be due to imperfect compliance with calcium intake, and that the participants in the placebo groups often had fairly robust calcium intake from diet and off-study supplemental intake, which could reduce the sensitivity of studies to demonstrate the fracture benefit.1,16
The US Preventive Services Task Force17 recommends that the general public not take supplemental calcium for skeletal health, but emphasizes that this recommendation does not apply to patients with osteoporosis. Most other official guidelines (eg, those of the Endocrine Society,18 American Association of Clinical Endocrinologists,19 Institute of Medicine,20 and National Osteoporosis Foundation21) recommend adequate calcium intake to optimize skeletal health.
CALCIUM INTAKE AND CORONARY ARTERY DISEASE
Patients often wonder if the calcium in their supplements ends up in their coronary arteries rather than their bones. Although we once dismissed such concerns, several studies and meta-analyses have reported higher rates of cardiovascular disease with supplemental calcium use.22–24 A proposed mechanism to explain this increased risk is that taking calcium supplements transiently raises the serum calcium level, resulting in calcium deposition in coronary arteries, accelerating atherosclerosis formation.25
On the other hand, some studies and meta-analyses have not shown any increased risk of cardiovascular disease with calcium and vitamin D supplementation.26,27 This subject has been reviewed by Harvey et al.1
Our conclusions are as follows:
Patients should be told that the National Osteoporosis Foundation and the American Society for Preventive Cardiology released a statement in 2016 adopting the position that calcium intake from food and supplements should be considered safe from a cardiovascular perspective.28
If patients want to avoid the possible increase in risk of cardiovascular disease due to calcium supplementation, they can optimize their calcium intake with dietary calcium. Observational studies that showed increased risk with supplemental calcium found no such increase in cardiovascular disease with a robust dietary intake of calcium.29
This is not to say that patients should be encouraged to boost their dietary calcium intake and avoid heart disease by eating more cheese and ice cream, as these foods are high in saturated fats and cholesterol. Many dairy and nondairy sources of calcium do not contain these undesirable nutrients.
CALCIUM SUPPLEMENTATION AND NEPHROLITHIASIS
High dietary calcium intake has not been shown to increase the risk of kidney stones.
In the Nurses’ Health Study, the multivariate relative risk of stone formation was 0.65 (95% confidence interval [CI] 0.5–0.83) in those in the highest vs the lowest quintiles of dietary calcium intake.30 In contrast, the relative risk of stones in those taking calcium supplements was 1.2 (CI 1.02–1.41),30 although this higher risk was not seen in younger women (ages 27 to 44).31
Similar results were seen in the Women’s Health Initiative, in which calcium carbonate and vitamin D supplements resulted in a relative increased risk of stone formation of 1.17 (95% CI 1.02–1.34) compared with women on placebo.12
Data from male stone-formers also suggests that high dietary calcium intake does not increase the risk of stones.32
A theory to explain the difference between dietary and supplemental calcium with respect to stone formation is that dietary calcium binds to oxalate in the gut and reduces its absorption. The most common type of kidney stones are composed of calcium oxalate, and the oxalate, not the calcium, may be the real culprit. In contrast, calcium supplements are often taken between meals and therefore do not exert this protective effect and may be absorbed more rapidly and raise the serum calcium level more, which could lead to higher urinary calcium excretion.33
CALCIUM INTAKE IN PATIENTS TAKING ANTIRESORPTIVE DRUGS
Patients often mistakenly think that calcium and vitamin D supplements are given for mild cases of bone loss, and that if their bone loss is significant enough to require a medication, then they no longer need calcium and vitamin D supplements. Most clinical trials showing bone mineral density and fracture benefit from antiresorptive therapy were in patients who were taking enough calcium and vitamin D, so the efficacy of antiresorptive therapy is most clear only when taking enough calcium and vitamin D.34,35
Furthermore, patients with inadequate calcium and vitamin D intake essentially maintain their serum calcium levels by mobilizing calcium from bone; the combination of insufficient calcium intake and administration of agents that interfere with the ability to mobilize calcium from bone may put patients at risk of hypocalcemia.36
OPTIMIZING INTAKE OF CALCIUM AND VITAMIN D
Diet is key to calcium intake. People should consume adequate amounts of calcium-rich foods regardless of whether they have a history of kidney stones, since robust dietary intake of calcium does not increase the risk of cardiovascular disease or kidney stones and may actually have a protective effect. We also remain skeptical of the concern that supplemental calcium increases the risk of cardiovascular disease.
We recommend a target total calcium intake from diet, and if necessary, supplements, of 1,000 to 1,200 mg daily, and not to worry about cardiovascular disease or kidney stones. A patient or clinician reluctant to push calcium intake that high with supplements might opt for a more conservative goal of 800 mg of calcium daily. This recommendation is based on data suggesting that in the presence of vitamin D sufficiency, calcium supplementation with 500 mg of calcium citrate does little for patients whose calcium intake is above 400 mg/day.37
Vitamin D: How much do we need?
Regarding vitamin D intake, the Institute of Medicine recommends 600 to 800 IU to achieve a 25-hydroxyvitamin D level of 20 to 40 ng/mL.20
The Endocrine Society recommends “at least” 600 to 800 IU, but says that 1,500 to 2,000 IU may be needed to get the 25-hydroxyvitamin D level to 30 to 60 ng/mL.18
The Institute of Medicine based its recommendation on randomized controlled trials that showed fewer fractures with vitamin D intakes of 600 to 800 IU/day.13,14 Also, observational studies show little further reduction in fracture risk when the 25-hydroxyvitamin D levels rise above 20 ng/mL.38 A case-control study found an association between 25-hydroxyvitamin D levels higher than 40 ng/mL and pancreatic cancer.39
The Endocrine Society guidelines recommended higher intakes and levels of vitamin D because there are data suggesting that vitamin D levels higher than 30 ng/mL suppress parathyroid hormone levels further, which should favor less mobilization of bone.40
Levels of 25-hydroxyvitamin D in people exposed to plenty of sunlight rarely go above 60 ng/mL, suggesting 60 ng/mL should be the upper limit of levels to target, and it is unlikely that such levels are harmful.41
Implementing either recommendation—a target 25-hydroxyvitamin D level of 20 to 40 ng/mL or 30 to 60 ng/mL—is reasonable.
CALCULATING A PATIENT’S DIETARY CALCIUM INTAKE
A detailed dietary history can be obtained by a dietitian, or by using the Calcium Calculator app supported by the International Osteoporosis Foundation.43 However, dietary calcium intake can be assessed quickly. To approximate a patient’s total dietary calcium intake (in milligrams), we multiply the number of servings of dietary calcium by 300. A serving of dietary calcium is found in:
- 1 cup of milk, yogurt, calcium-fortified juice, almonds, cooked spinach, or collard greens
- 1.5 ounces of hard cheese
- 2 cups of ice cream, cottage cheese, or beans
- 4 ounces of tofu or canned fish with bones such as salmon or sardines.
Therefore, if a patient consumes 1 cup of milk daily and 1 cup of yogurt 3 times a week, she takes in an estimated 1.5 servings of dietary calcium daily, or 450 mg. What the patient does not receive in the diet should be made up with supplemental calcium.
CALCIUM SUPPLEMENTS
Calcium citrate has certain advantages as a supplement. Calcium carbonate requires gastric acidity to be absorbed and is therefore better absorbed if taken with meals; however, calcium citrate is equally well absorbed in the fasting or fed state and so can be taken without regard for achlorhydria or timing of meals.44
Another potential advantage of calcium citrate is that it has never been shown to increase the risk of kidney stones the way calcium carbonate has.12 Further, potassium citrate is a treatment for certain types of kidney stones,45 and it is possible that when calcium is given as citrate there is less danger of kidney stones.46 For these reasons, we generally recommend calcium citrate over other forms of calcium.
The brand of calcium citrate most readily available is Citracal, but any version of calcium citrate is acceptable.
SOURCES OF CONFUSION
Labels that describe calcium content of supplements are often misleading, and this lack of clarity can interfere with the patient’s ability to correctly identify how much calcium is in each pill.
Serving size. Whereas 1 serving of Caltrate is 1 pill, 1 serving of Tums or Citracal is 2 pills; for other brands a serving may be 3 or 4 pills.
Calcium salt vs elemental calcium. The amount of elemental calcium contained in different calcium salts varies according to the molecular weight of the salt: 1,000 mg of calcium carbonate has 400 mg of elemental calcium, while 1,000 mg of calcium citrate has 200 mg of elemental calcium.
When we recommend 1,000 to 1,200 mg of calcium daily, we mean the amount of elemental calcium. The label on calcium supplements usually indicates the amount of elemental calcium, but some have confusing information about the amount of calcium salt they contain. For instance, Tums lists the amount of calcium carbonate per pill on the top of the label, but elsewhere lists the amount of elemental calcium.
Same brand, different preparation. Some brands of calcium have more than 1 formulation, each with a different amount of calcium. For instance, Citracal has a maximum-strength 315-mg tablet and a “petite” 200-mg tablet. Careful reading of the label is required to make sure that the patient is getting the amount of calcium she thinks she is getting.
OPTIONS FOR THOSE WITH DIFFICULTY SWALLOWING LARGE PILLS
Many calcium pills are large and difficult to swallow. Patients often ask if calcium pills can be crushed, and the answer is that they certainly can, but this approach is cumbersome and usually results in patients eventually stopping calcium in frustration.
CALCIUM SUPPLEMENTS AND CONSTIPATION
Constipation is a common side effect of calcium supplementation.47 Many patients report that they cannot take a calcium supplement because of constipation, or ask if there are calcium preparations that are less constipating than others.
There are ways of overcoming the constipating effects of calcium. Osmotic laxatives and stool softeners such as polyethylene glycol, magnesium citrate, and docusate sodium are safe and effective, although patients are often reluctant to take a medicine to combat the side effects from another medicine.
In such circumstances patients are often amenable to taking a combination product such as calcium with magnesium, since the cathartic effects of magnesium nicely counteract the constipating effects of calcium. This idea is exploited in antacids such as Rolaids, which are combinations of calcium carbonate and magnesium oxide that usually have no net effect on stool consistency.47
Many patients believe that calcium must be combined with magnesium to be absorbed. Although there are no data to support this idea, a patient already harboring this misconception may be more amenable to calcium-magnesium combinations for the purpose of avoiding constipation.
If a patient cannot find a calcium preparation that she can take at the full recommended doses, we often suggest starting with a very small dose for 2 weeks, and then adjusting the dose upward every 2 weeks until reaching the maximum dose that the patient can tolerate. Even if the dose is well below recommended doses, most of the benefit of calcium is obtained by bringing total intake to more than 500 mg daily,37 so continued use should be encouraged even when optimal targets cannot be sustained.
For patients who cannot tolerate enough calcium, we recommend being especially sure to optimize the vitamin D levels, since there are studies that suggest that secondary hyperparathyroidism mostly occurs in states of low calcium intake if vitamin D levels are insufficient.48
If the patient has secondary hyperparathyroidism despite best attempts at supplementation with calcium and vitamin D, consider prescribing calcitriol (activated vitamin D), which stimulates gut absorption of whatever calcium is taken.49 If calcitriol is given, the patient must undergo cumbersome monitoring for hypercalcemia and hypercalciuria. Fortunately, it is unusual to require calcitriol unless the patient has significant structural gastrointestinal abnormalities such as gastric bypass or Crohn disease.
- Harvey NC, Bilver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 2017; 28(2):447–462. doi:10.1007/s00198-016-3773-6
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005; 115(12):3318–3325. doi.10.1172/JCI27071
- Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med 2013; 369(16):1537–154 doi:10.1056/NEJMcp1210380
- Choi MJ, Park EJ, Jo HJ. Relationship of nutrient intakes and bone mineral density of elderly women in Daegu, Korea. Nutr Res Pract 2007; 1(4):328–33 doi:10.4162/nrp.2007.1.4.328
- Kim KM, Choi SH, Lim S, et al. Interactions between dietary calcium intake and bone mineral density or bone ge6ometry in a low calcium intake population (KNHANES IV 2008–2010). J Clin Endocrinol Metab 2014; 99(7):2409–2417. doi:10.1210/jc.2014-1006
- Joo NS, Dawson-Hughes B, Kim YS, Oh K, Yeum KJ. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 2013; 28(4):764–770. doi:10.1002/jbmr.1790
- Anderson JJ, Roggenkamp KJ, Suchindran CM. Calcium intakes and femoral and lumbar bone density of elderly US men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J Clin Endocrinol Metab 2012; 97(12):4531–4539. doi:10.1210/jc.2012-1407
- Gui JC, Brašic JR, Liu XD, et al. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow’s milk. Osteoporos Int 2012; 23(5):1563–1570. doi:10.1007/s00198-012-1895-z
- Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr 2010; 104(1):100–107. doi:10.1017/S000711451000019X
- Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996; 11(12):1961–1966. doi:10.1002/jbmr.5650111218
- Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015; 351:h4183. doi:10.1136/bmj.h4183
- Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006; 354(7):669–683. doi:10.1056/NEJMoa055218
- Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327(23):1637–1642. doi:10.1056/NEJM199212033272305
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337(10):670–676. doi:10.1056/NEJM199709043371003
- Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ 2015; 351:h4580. doi:10.1136/bmj.h4580
- Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med 2012; 367(1):77–78. doi:10.1056/NEJMe1206858
- Moyer VA; US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158(9):691–696. doi:10.7326/0003-4819-158-9-201305070-00603
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7):1911–1930. doi:10.1210/jc.2011-0385
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologist and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract 2016; 22(suppl 4):1–42. doi:10.4158/EP161435.ESGL
- Ross AC, Manson JE, Abrams SA et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Cosman F, De Beur SJ, Leboff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25(10):2359–2381. doi:10.1007/s00198-014-2794-2
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336(7638):262–266. doi:10.1136/bmj.39440.525752.BE
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011; 342:d2040. doi:10.1136/bmj.d2040
- Mao PJ, Zhang C, Tang L, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 169(2):106–111. doi:10.1016/j.ijcard.2013.08.055
- Reid IR, Bolland MJ. Calcium supplementation and vascular disease. Climacteric 2008; 11(4):280–286. doi:10.1080/13697130802229639
- Lewis JR, Radavelli-Bagatini S, Rejnmark L, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015; 30(1):165–175. doi:10.1002/jbmr.2311
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115(19):846–854. doi:10.1161/CIRCULATIONAHA.106.673491
- Kopecky SL, Bauer DC, Gulati M, et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med 2016; 165(12):867–868. doi:10.7326/M16-1743
- Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc 2016; 5(10):e003815. doi:10.1161/JAHA.116.003815
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126(7):497–504. pmid:9092314
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164(8):885–891. doi:10.1001/archinte.164.8.885
- Borhi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346(2):77–84. doi:10.1056/NEJMoa010369
- Prochaska ML, Taylor EN, Curhan GC. Insights into nephrolithiasis from the Nurses’ Health Studies. Am J Public Health 2016; 106(9):1638–1643. doi:10.2105/AJPH.2016.303319
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Chen J, Smerdely P. Hypocalcaemia after denosumab in older people following fracture. Osteoporos Int 2017; 28(2):517–522. doi:10.1007/s00198-016-3755-8
- Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990; 323(13):878–883. doi:10.1056/NEJM199009273231305
- Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 2010; 95(6):2637–2645. doi:10.1210/jc.2009-2699
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1):81–93. doi:10.1093/aje/kwq120
- Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 2012; 97(11):3989–3995. doi:10.1210/jc.2012-2276
- Binkley N, Novotny R, Krueger T, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92(6):2130–2135. doi:10.1210/jc.2006-2250
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA food composition databases. https://ndb.nal.usda.gov/ndb/search/list. Accessed May 7, 2018.
- International Osteoporosis Foundation. IOF calcium calculator version 1.10. Apple App Store. https://itunes.apple.com/us/app/iof-calcium-calculator/id956198268?mt=8. Accessed June 11, 2018.
- Recker RR. Calcium absorption and achlorhydria. N Engl J Med 1985; 313(2):70–73. doi:10.1056/NEJM198507113130202
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115(10):2598–2608. doi:10.1172/JCI26662
- Sakhaee K, Poindexter JR, Griffith CS, Pak CY. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol 2004; 172(3):958–961. doi:10.1097/01.ju.0000136400.14728.cd
- Kitchin B. Nutrition counseling for patients with osteoporosis: a personal approach. J Clin Densitom 2013; 16(4):426–431. doi:10.1016/j.jocd.2013.08.013
- Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005; 294(18):2336–2341. doi:10.1001/jama.294.18.2336
- Need AG, Horowitz M, Philcox JC, Nordin BE. 1,25-dihydroxycalciferol and calcium therapy in osteoporosis with calcium malabsorption. Dose response relationship of calcium absorption and indices of bone turnover. Miner Electrolyte Metab 1985; 11(1):35–40. pmid:3838358
Although calcium and vitamin D are often recommended for prevention and treatment of osteoporosis, considerable controversy exists in terms of their safety and efficacy.1 This article highlights the issues, referring readers to reviews and meta-analyses for details and providing some practical advice for patients requiring supplementation.
CALCIUM INTAKE AND BONE DENSITY
Calcium enters the body through diet and supplementation. If intake is low, blood calcium levels fall, resulting in secondary hyperparathyroidism, which has 3 main effects:
- Increased fractional absorption of the calcium that is consumed
- Reduced urinary excretion of calcium
- Increased bone resorption, which releases calcium into the blood,2 which explains the potential for the deleterious effect of deficient intake of calcium on bone.3
Based on the simple physiology outlined above, it seems logical that insufficient intake of calcium over time could lead to mobilization of calcium from bone, lower bone mineral density, and higher fracture risk.3 This topic has been reviewed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and the International Foundation for Osteoporosis.1
Many lines of evidence suggest that low calcium intake adversely affects bone mineral density.1 Low calcium intake has been associated with lower bone density in some cross-sectional studies,4–6 though not all.7 Interventions to increase calcium intake in postmenopausal women have shown beneficial effects on bone density,8–10 though in some studies the benefit was small and nonprogressive.11 The question is whether this improvement in bone mineral density translates into fewer fractures.
Results from individual studies looking at fracture prevention through calcium supplementation have been conflicting,10,12–14 and reviews and meta-analyses have summarized the data.1,3,15 A recent review of these meta-analyses showed a small but significant reduction in some types of fracture.1
Some speculate that the difficulty in demonstrating fracture efficacy might be due to imperfect compliance with calcium intake, and that the participants in the placebo groups often had fairly robust calcium intake from diet and off-study supplemental intake, which could reduce the sensitivity of studies to demonstrate the fracture benefit.1,16
The US Preventive Services Task Force17 recommends that the general public not take supplemental calcium for skeletal health, but emphasizes that this recommendation does not apply to patients with osteoporosis. Most other official guidelines (eg, those of the Endocrine Society,18 American Association of Clinical Endocrinologists,19 Institute of Medicine,20 and National Osteoporosis Foundation21) recommend adequate calcium intake to optimize skeletal health.
CALCIUM INTAKE AND CORONARY ARTERY DISEASE
Patients often wonder if the calcium in their supplements ends up in their coronary arteries rather than their bones. Although we once dismissed such concerns, several studies and meta-analyses have reported higher rates of cardiovascular disease with supplemental calcium use.22–24 A proposed mechanism to explain this increased risk is that taking calcium supplements transiently raises the serum calcium level, resulting in calcium deposition in coronary arteries, accelerating atherosclerosis formation.25
On the other hand, some studies and meta-analyses have not shown any increased risk of cardiovascular disease with calcium and vitamin D supplementation.26,27 This subject has been reviewed by Harvey et al.1
Our conclusions are as follows:
Patients should be told that the National Osteoporosis Foundation and the American Society for Preventive Cardiology released a statement in 2016 adopting the position that calcium intake from food and supplements should be considered safe from a cardiovascular perspective.28
If patients want to avoid the possible increase in risk of cardiovascular disease due to calcium supplementation, they can optimize their calcium intake with dietary calcium. Observational studies that showed increased risk with supplemental calcium found no such increase in cardiovascular disease with a robust dietary intake of calcium.29
This is not to say that patients should be encouraged to boost their dietary calcium intake and avoid heart disease by eating more cheese and ice cream, as these foods are high in saturated fats and cholesterol. Many dairy and nondairy sources of calcium do not contain these undesirable nutrients.
CALCIUM SUPPLEMENTATION AND NEPHROLITHIASIS
High dietary calcium intake has not been shown to increase the risk of kidney stones.
In the Nurses’ Health Study, the multivariate relative risk of stone formation was 0.65 (95% confidence interval [CI] 0.5–0.83) in those in the highest vs the lowest quintiles of dietary calcium intake.30 In contrast, the relative risk of stones in those taking calcium supplements was 1.2 (CI 1.02–1.41),30 although this higher risk was not seen in younger women (ages 27 to 44).31
Similar results were seen in the Women’s Health Initiative, in which calcium carbonate and vitamin D supplements resulted in a relative increased risk of stone formation of 1.17 (95% CI 1.02–1.34) compared with women on placebo.12
Data from male stone-formers also suggests that high dietary calcium intake does not increase the risk of stones.32
A theory to explain the difference between dietary and supplemental calcium with respect to stone formation is that dietary calcium binds to oxalate in the gut and reduces its absorption. The most common type of kidney stones are composed of calcium oxalate, and the oxalate, not the calcium, may be the real culprit. In contrast, calcium supplements are often taken between meals and therefore do not exert this protective effect and may be absorbed more rapidly and raise the serum calcium level more, which could lead to higher urinary calcium excretion.33
CALCIUM INTAKE IN PATIENTS TAKING ANTIRESORPTIVE DRUGS
Patients often mistakenly think that calcium and vitamin D supplements are given for mild cases of bone loss, and that if their bone loss is significant enough to require a medication, then they no longer need calcium and vitamin D supplements. Most clinical trials showing bone mineral density and fracture benefit from antiresorptive therapy were in patients who were taking enough calcium and vitamin D, so the efficacy of antiresorptive therapy is most clear only when taking enough calcium and vitamin D.34,35
Furthermore, patients with inadequate calcium and vitamin D intake essentially maintain their serum calcium levels by mobilizing calcium from bone; the combination of insufficient calcium intake and administration of agents that interfere with the ability to mobilize calcium from bone may put patients at risk of hypocalcemia.36
OPTIMIZING INTAKE OF CALCIUM AND VITAMIN D
Diet is key to calcium intake. People should consume adequate amounts of calcium-rich foods regardless of whether they have a history of kidney stones, since robust dietary intake of calcium does not increase the risk of cardiovascular disease or kidney stones and may actually have a protective effect. We also remain skeptical of the concern that supplemental calcium increases the risk of cardiovascular disease.
We recommend a target total calcium intake from diet, and if necessary, supplements, of 1,000 to 1,200 mg daily, and not to worry about cardiovascular disease or kidney stones. A patient or clinician reluctant to push calcium intake that high with supplements might opt for a more conservative goal of 800 mg of calcium daily. This recommendation is based on data suggesting that in the presence of vitamin D sufficiency, calcium supplementation with 500 mg of calcium citrate does little for patients whose calcium intake is above 400 mg/day.37
Vitamin D: How much do we need?
Regarding vitamin D intake, the Institute of Medicine recommends 600 to 800 IU to achieve a 25-hydroxyvitamin D level of 20 to 40 ng/mL.20
The Endocrine Society recommends “at least” 600 to 800 IU, but says that 1,500 to 2,000 IU may be needed to get the 25-hydroxyvitamin D level to 30 to 60 ng/mL.18
The Institute of Medicine based its recommendation on randomized controlled trials that showed fewer fractures with vitamin D intakes of 600 to 800 IU/day.13,14 Also, observational studies show little further reduction in fracture risk when the 25-hydroxyvitamin D levels rise above 20 ng/mL.38 A case-control study found an association between 25-hydroxyvitamin D levels higher than 40 ng/mL and pancreatic cancer.39
The Endocrine Society guidelines recommended higher intakes and levels of vitamin D because there are data suggesting that vitamin D levels higher than 30 ng/mL suppress parathyroid hormone levels further, which should favor less mobilization of bone.40
Levels of 25-hydroxyvitamin D in people exposed to plenty of sunlight rarely go above 60 ng/mL, suggesting 60 ng/mL should be the upper limit of levels to target, and it is unlikely that such levels are harmful.41
Implementing either recommendation—a target 25-hydroxyvitamin D level of 20 to 40 ng/mL or 30 to 60 ng/mL—is reasonable.
CALCULATING A PATIENT’S DIETARY CALCIUM INTAKE
A detailed dietary history can be obtained by a dietitian, or by using the Calcium Calculator app supported by the International Osteoporosis Foundation.43 However, dietary calcium intake can be assessed quickly. To approximate a patient’s total dietary calcium intake (in milligrams), we multiply the number of servings of dietary calcium by 300. A serving of dietary calcium is found in:
- 1 cup of milk, yogurt, calcium-fortified juice, almonds, cooked spinach, or collard greens
- 1.5 ounces of hard cheese
- 2 cups of ice cream, cottage cheese, or beans
- 4 ounces of tofu or canned fish with bones such as salmon or sardines.
Therefore, if a patient consumes 1 cup of milk daily and 1 cup of yogurt 3 times a week, she takes in an estimated 1.5 servings of dietary calcium daily, or 450 mg. What the patient does not receive in the diet should be made up with supplemental calcium.
CALCIUM SUPPLEMENTS
Calcium citrate has certain advantages as a supplement. Calcium carbonate requires gastric acidity to be absorbed and is therefore better absorbed if taken with meals; however, calcium citrate is equally well absorbed in the fasting or fed state and so can be taken without regard for achlorhydria or timing of meals.44
Another potential advantage of calcium citrate is that it has never been shown to increase the risk of kidney stones the way calcium carbonate has.12 Further, potassium citrate is a treatment for certain types of kidney stones,45 and it is possible that when calcium is given as citrate there is less danger of kidney stones.46 For these reasons, we generally recommend calcium citrate over other forms of calcium.
The brand of calcium citrate most readily available is Citracal, but any version of calcium citrate is acceptable.
SOURCES OF CONFUSION
Labels that describe calcium content of supplements are often misleading, and this lack of clarity can interfere with the patient’s ability to correctly identify how much calcium is in each pill.
Serving size. Whereas 1 serving of Caltrate is 1 pill, 1 serving of Tums or Citracal is 2 pills; for other brands a serving may be 3 or 4 pills.
Calcium salt vs elemental calcium. The amount of elemental calcium contained in different calcium salts varies according to the molecular weight of the salt: 1,000 mg of calcium carbonate has 400 mg of elemental calcium, while 1,000 mg of calcium citrate has 200 mg of elemental calcium.
When we recommend 1,000 to 1,200 mg of calcium daily, we mean the amount of elemental calcium. The label on calcium supplements usually indicates the amount of elemental calcium, but some have confusing information about the amount of calcium salt they contain. For instance, Tums lists the amount of calcium carbonate per pill on the top of the label, but elsewhere lists the amount of elemental calcium.
Same brand, different preparation. Some brands of calcium have more than 1 formulation, each with a different amount of calcium. For instance, Citracal has a maximum-strength 315-mg tablet and a “petite” 200-mg tablet. Careful reading of the label is required to make sure that the patient is getting the amount of calcium she thinks she is getting.
OPTIONS FOR THOSE WITH DIFFICULTY SWALLOWING LARGE PILLS
Many calcium pills are large and difficult to swallow. Patients often ask if calcium pills can be crushed, and the answer is that they certainly can, but this approach is cumbersome and usually results in patients eventually stopping calcium in frustration.
CALCIUM SUPPLEMENTS AND CONSTIPATION
Constipation is a common side effect of calcium supplementation.47 Many patients report that they cannot take a calcium supplement because of constipation, or ask if there are calcium preparations that are less constipating than others.
There are ways of overcoming the constipating effects of calcium. Osmotic laxatives and stool softeners such as polyethylene glycol, magnesium citrate, and docusate sodium are safe and effective, although patients are often reluctant to take a medicine to combat the side effects from another medicine.
In such circumstances patients are often amenable to taking a combination product such as calcium with magnesium, since the cathartic effects of magnesium nicely counteract the constipating effects of calcium. This idea is exploited in antacids such as Rolaids, which are combinations of calcium carbonate and magnesium oxide that usually have no net effect on stool consistency.47
Many patients believe that calcium must be combined with magnesium to be absorbed. Although there are no data to support this idea, a patient already harboring this misconception may be more amenable to calcium-magnesium combinations for the purpose of avoiding constipation.
If a patient cannot find a calcium preparation that she can take at the full recommended doses, we often suggest starting with a very small dose for 2 weeks, and then adjusting the dose upward every 2 weeks until reaching the maximum dose that the patient can tolerate. Even if the dose is well below recommended doses, most of the benefit of calcium is obtained by bringing total intake to more than 500 mg daily,37 so continued use should be encouraged even when optimal targets cannot be sustained.
For patients who cannot tolerate enough calcium, we recommend being especially sure to optimize the vitamin D levels, since there are studies that suggest that secondary hyperparathyroidism mostly occurs in states of low calcium intake if vitamin D levels are insufficient.48
If the patient has secondary hyperparathyroidism despite best attempts at supplementation with calcium and vitamin D, consider prescribing calcitriol (activated vitamin D), which stimulates gut absorption of whatever calcium is taken.49 If calcitriol is given, the patient must undergo cumbersome monitoring for hypercalcemia and hypercalciuria. Fortunately, it is unusual to require calcitriol unless the patient has significant structural gastrointestinal abnormalities such as gastric bypass or Crohn disease.
Although calcium and vitamin D are often recommended for prevention and treatment of osteoporosis, considerable controversy exists in terms of their safety and efficacy.1 This article highlights the issues, referring readers to reviews and meta-analyses for details and providing some practical advice for patients requiring supplementation.
CALCIUM INTAKE AND BONE DENSITY
Calcium enters the body through diet and supplementation. If intake is low, blood calcium levels fall, resulting in secondary hyperparathyroidism, which has 3 main effects:
- Increased fractional absorption of the calcium that is consumed
- Reduced urinary excretion of calcium
- Increased bone resorption, which releases calcium into the blood,2 which explains the potential for the deleterious effect of deficient intake of calcium on bone.3
Based on the simple physiology outlined above, it seems logical that insufficient intake of calcium over time could lead to mobilization of calcium from bone, lower bone mineral density, and higher fracture risk.3 This topic has been reviewed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and the International Foundation for Osteoporosis.1
Many lines of evidence suggest that low calcium intake adversely affects bone mineral density.1 Low calcium intake has been associated with lower bone density in some cross-sectional studies,4–6 though not all.7 Interventions to increase calcium intake in postmenopausal women have shown beneficial effects on bone density,8–10 though in some studies the benefit was small and nonprogressive.11 The question is whether this improvement in bone mineral density translates into fewer fractures.
Results from individual studies looking at fracture prevention through calcium supplementation have been conflicting,10,12–14 and reviews and meta-analyses have summarized the data.1,3,15 A recent review of these meta-analyses showed a small but significant reduction in some types of fracture.1
Some speculate that the difficulty in demonstrating fracture efficacy might be due to imperfect compliance with calcium intake, and that the participants in the placebo groups often had fairly robust calcium intake from diet and off-study supplemental intake, which could reduce the sensitivity of studies to demonstrate the fracture benefit.1,16
The US Preventive Services Task Force17 recommends that the general public not take supplemental calcium for skeletal health, but emphasizes that this recommendation does not apply to patients with osteoporosis. Most other official guidelines (eg, those of the Endocrine Society,18 American Association of Clinical Endocrinologists,19 Institute of Medicine,20 and National Osteoporosis Foundation21) recommend adequate calcium intake to optimize skeletal health.
CALCIUM INTAKE AND CORONARY ARTERY DISEASE
Patients often wonder if the calcium in their supplements ends up in their coronary arteries rather than their bones. Although we once dismissed such concerns, several studies and meta-analyses have reported higher rates of cardiovascular disease with supplemental calcium use.22–24 A proposed mechanism to explain this increased risk is that taking calcium supplements transiently raises the serum calcium level, resulting in calcium deposition in coronary arteries, accelerating atherosclerosis formation.25
On the other hand, some studies and meta-analyses have not shown any increased risk of cardiovascular disease with calcium and vitamin D supplementation.26,27 This subject has been reviewed by Harvey et al.1
Our conclusions are as follows:
Patients should be told that the National Osteoporosis Foundation and the American Society for Preventive Cardiology released a statement in 2016 adopting the position that calcium intake from food and supplements should be considered safe from a cardiovascular perspective.28
If patients want to avoid the possible increase in risk of cardiovascular disease due to calcium supplementation, they can optimize their calcium intake with dietary calcium. Observational studies that showed increased risk with supplemental calcium found no such increase in cardiovascular disease with a robust dietary intake of calcium.29
This is not to say that patients should be encouraged to boost their dietary calcium intake and avoid heart disease by eating more cheese and ice cream, as these foods are high in saturated fats and cholesterol. Many dairy and nondairy sources of calcium do not contain these undesirable nutrients.
CALCIUM SUPPLEMENTATION AND NEPHROLITHIASIS
High dietary calcium intake has not been shown to increase the risk of kidney stones.
In the Nurses’ Health Study, the multivariate relative risk of stone formation was 0.65 (95% confidence interval [CI] 0.5–0.83) in those in the highest vs the lowest quintiles of dietary calcium intake.30 In contrast, the relative risk of stones in those taking calcium supplements was 1.2 (CI 1.02–1.41),30 although this higher risk was not seen in younger women (ages 27 to 44).31
Similar results were seen in the Women’s Health Initiative, in which calcium carbonate and vitamin D supplements resulted in a relative increased risk of stone formation of 1.17 (95% CI 1.02–1.34) compared with women on placebo.12
Data from male stone-formers also suggests that high dietary calcium intake does not increase the risk of stones.32
A theory to explain the difference between dietary and supplemental calcium with respect to stone formation is that dietary calcium binds to oxalate in the gut and reduces its absorption. The most common type of kidney stones are composed of calcium oxalate, and the oxalate, not the calcium, may be the real culprit. In contrast, calcium supplements are often taken between meals and therefore do not exert this protective effect and may be absorbed more rapidly and raise the serum calcium level more, which could lead to higher urinary calcium excretion.33
CALCIUM INTAKE IN PATIENTS TAKING ANTIRESORPTIVE DRUGS
Patients often mistakenly think that calcium and vitamin D supplements are given for mild cases of bone loss, and that if their bone loss is significant enough to require a medication, then they no longer need calcium and vitamin D supplements. Most clinical trials showing bone mineral density and fracture benefit from antiresorptive therapy were in patients who were taking enough calcium and vitamin D, so the efficacy of antiresorptive therapy is most clear only when taking enough calcium and vitamin D.34,35
Furthermore, patients with inadequate calcium and vitamin D intake essentially maintain their serum calcium levels by mobilizing calcium from bone; the combination of insufficient calcium intake and administration of agents that interfere with the ability to mobilize calcium from bone may put patients at risk of hypocalcemia.36
OPTIMIZING INTAKE OF CALCIUM AND VITAMIN D
Diet is key to calcium intake. People should consume adequate amounts of calcium-rich foods regardless of whether they have a history of kidney stones, since robust dietary intake of calcium does not increase the risk of cardiovascular disease or kidney stones and may actually have a protective effect. We also remain skeptical of the concern that supplemental calcium increases the risk of cardiovascular disease.
We recommend a target total calcium intake from diet, and if necessary, supplements, of 1,000 to 1,200 mg daily, and not to worry about cardiovascular disease or kidney stones. A patient or clinician reluctant to push calcium intake that high with supplements might opt for a more conservative goal of 800 mg of calcium daily. This recommendation is based on data suggesting that in the presence of vitamin D sufficiency, calcium supplementation with 500 mg of calcium citrate does little for patients whose calcium intake is above 400 mg/day.37
Vitamin D: How much do we need?
Regarding vitamin D intake, the Institute of Medicine recommends 600 to 800 IU to achieve a 25-hydroxyvitamin D level of 20 to 40 ng/mL.20
The Endocrine Society recommends “at least” 600 to 800 IU, but says that 1,500 to 2,000 IU may be needed to get the 25-hydroxyvitamin D level to 30 to 60 ng/mL.18
The Institute of Medicine based its recommendation on randomized controlled trials that showed fewer fractures with vitamin D intakes of 600 to 800 IU/day.13,14 Also, observational studies show little further reduction in fracture risk when the 25-hydroxyvitamin D levels rise above 20 ng/mL.38 A case-control study found an association between 25-hydroxyvitamin D levels higher than 40 ng/mL and pancreatic cancer.39
The Endocrine Society guidelines recommended higher intakes and levels of vitamin D because there are data suggesting that vitamin D levels higher than 30 ng/mL suppress parathyroid hormone levels further, which should favor less mobilization of bone.40
Levels of 25-hydroxyvitamin D in people exposed to plenty of sunlight rarely go above 60 ng/mL, suggesting 60 ng/mL should be the upper limit of levels to target, and it is unlikely that such levels are harmful.41
Implementing either recommendation—a target 25-hydroxyvitamin D level of 20 to 40 ng/mL or 30 to 60 ng/mL—is reasonable.
CALCULATING A PATIENT’S DIETARY CALCIUM INTAKE
A detailed dietary history can be obtained by a dietitian, or by using the Calcium Calculator app supported by the International Osteoporosis Foundation.43 However, dietary calcium intake can be assessed quickly. To approximate a patient’s total dietary calcium intake (in milligrams), we multiply the number of servings of dietary calcium by 300. A serving of dietary calcium is found in:
- 1 cup of milk, yogurt, calcium-fortified juice, almonds, cooked spinach, or collard greens
- 1.5 ounces of hard cheese
- 2 cups of ice cream, cottage cheese, or beans
- 4 ounces of tofu or canned fish with bones such as salmon or sardines.
Therefore, if a patient consumes 1 cup of milk daily and 1 cup of yogurt 3 times a week, she takes in an estimated 1.5 servings of dietary calcium daily, or 450 mg. What the patient does not receive in the diet should be made up with supplemental calcium.
CALCIUM SUPPLEMENTS
Calcium citrate has certain advantages as a supplement. Calcium carbonate requires gastric acidity to be absorbed and is therefore better absorbed if taken with meals; however, calcium citrate is equally well absorbed in the fasting or fed state and so can be taken without regard for achlorhydria or timing of meals.44
Another potential advantage of calcium citrate is that it has never been shown to increase the risk of kidney stones the way calcium carbonate has.12 Further, potassium citrate is a treatment for certain types of kidney stones,45 and it is possible that when calcium is given as citrate there is less danger of kidney stones.46 For these reasons, we generally recommend calcium citrate over other forms of calcium.
The brand of calcium citrate most readily available is Citracal, but any version of calcium citrate is acceptable.
SOURCES OF CONFUSION
Labels that describe calcium content of supplements are often misleading, and this lack of clarity can interfere with the patient’s ability to correctly identify how much calcium is in each pill.
Serving size. Whereas 1 serving of Caltrate is 1 pill, 1 serving of Tums or Citracal is 2 pills; for other brands a serving may be 3 or 4 pills.
Calcium salt vs elemental calcium. The amount of elemental calcium contained in different calcium salts varies according to the molecular weight of the salt: 1,000 mg of calcium carbonate has 400 mg of elemental calcium, while 1,000 mg of calcium citrate has 200 mg of elemental calcium.
When we recommend 1,000 to 1,200 mg of calcium daily, we mean the amount of elemental calcium. The label on calcium supplements usually indicates the amount of elemental calcium, but some have confusing information about the amount of calcium salt they contain. For instance, Tums lists the amount of calcium carbonate per pill on the top of the label, but elsewhere lists the amount of elemental calcium.
Same brand, different preparation. Some brands of calcium have more than 1 formulation, each with a different amount of calcium. For instance, Citracal has a maximum-strength 315-mg tablet and a “petite” 200-mg tablet. Careful reading of the label is required to make sure that the patient is getting the amount of calcium she thinks she is getting.
OPTIONS FOR THOSE WITH DIFFICULTY SWALLOWING LARGE PILLS
Many calcium pills are large and difficult to swallow. Patients often ask if calcium pills can be crushed, and the answer is that they certainly can, but this approach is cumbersome and usually results in patients eventually stopping calcium in frustration.
CALCIUM SUPPLEMENTS AND CONSTIPATION
Constipation is a common side effect of calcium supplementation.47 Many patients report that they cannot take a calcium supplement because of constipation, or ask if there are calcium preparations that are less constipating than others.
There are ways of overcoming the constipating effects of calcium. Osmotic laxatives and stool softeners such as polyethylene glycol, magnesium citrate, and docusate sodium are safe and effective, although patients are often reluctant to take a medicine to combat the side effects from another medicine.
In such circumstances patients are often amenable to taking a combination product such as calcium with magnesium, since the cathartic effects of magnesium nicely counteract the constipating effects of calcium. This idea is exploited in antacids such as Rolaids, which are combinations of calcium carbonate and magnesium oxide that usually have no net effect on stool consistency.47
Many patients believe that calcium must be combined with magnesium to be absorbed. Although there are no data to support this idea, a patient already harboring this misconception may be more amenable to calcium-magnesium combinations for the purpose of avoiding constipation.
If a patient cannot find a calcium preparation that she can take at the full recommended doses, we often suggest starting with a very small dose for 2 weeks, and then adjusting the dose upward every 2 weeks until reaching the maximum dose that the patient can tolerate. Even if the dose is well below recommended doses, most of the benefit of calcium is obtained by bringing total intake to more than 500 mg daily,37 so continued use should be encouraged even when optimal targets cannot be sustained.
For patients who cannot tolerate enough calcium, we recommend being especially sure to optimize the vitamin D levels, since there are studies that suggest that secondary hyperparathyroidism mostly occurs in states of low calcium intake if vitamin D levels are insufficient.48
If the patient has secondary hyperparathyroidism despite best attempts at supplementation with calcium and vitamin D, consider prescribing calcitriol (activated vitamin D), which stimulates gut absorption of whatever calcium is taken.49 If calcitriol is given, the patient must undergo cumbersome monitoring for hypercalcemia and hypercalciuria. Fortunately, it is unusual to require calcitriol unless the patient has significant structural gastrointestinal abnormalities such as gastric bypass or Crohn disease.
- Harvey NC, Bilver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 2017; 28(2):447–462. doi:10.1007/s00198-016-3773-6
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005; 115(12):3318–3325. doi.10.1172/JCI27071
- Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med 2013; 369(16):1537–154 doi:10.1056/NEJMcp1210380
- Choi MJ, Park EJ, Jo HJ. Relationship of nutrient intakes and bone mineral density of elderly women in Daegu, Korea. Nutr Res Pract 2007; 1(4):328–33 doi:10.4162/nrp.2007.1.4.328
- Kim KM, Choi SH, Lim S, et al. Interactions between dietary calcium intake and bone mineral density or bone ge6ometry in a low calcium intake population (KNHANES IV 2008–2010). J Clin Endocrinol Metab 2014; 99(7):2409–2417. doi:10.1210/jc.2014-1006
- Joo NS, Dawson-Hughes B, Kim YS, Oh K, Yeum KJ. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 2013; 28(4):764–770. doi:10.1002/jbmr.1790
- Anderson JJ, Roggenkamp KJ, Suchindran CM. Calcium intakes and femoral and lumbar bone density of elderly US men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J Clin Endocrinol Metab 2012; 97(12):4531–4539. doi:10.1210/jc.2012-1407
- Gui JC, Brašic JR, Liu XD, et al. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow’s milk. Osteoporos Int 2012; 23(5):1563–1570. doi:10.1007/s00198-012-1895-z
- Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr 2010; 104(1):100–107. doi:10.1017/S000711451000019X
- Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996; 11(12):1961–1966. doi:10.1002/jbmr.5650111218
- Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015; 351:h4183. doi:10.1136/bmj.h4183
- Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006; 354(7):669–683. doi:10.1056/NEJMoa055218
- Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327(23):1637–1642. doi:10.1056/NEJM199212033272305
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337(10):670–676. doi:10.1056/NEJM199709043371003
- Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ 2015; 351:h4580. doi:10.1136/bmj.h4580
- Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med 2012; 367(1):77–78. doi:10.1056/NEJMe1206858
- Moyer VA; US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158(9):691–696. doi:10.7326/0003-4819-158-9-201305070-00603
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7):1911–1930. doi:10.1210/jc.2011-0385
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologist and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract 2016; 22(suppl 4):1–42. doi:10.4158/EP161435.ESGL
- Ross AC, Manson JE, Abrams SA et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Cosman F, De Beur SJ, Leboff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25(10):2359–2381. doi:10.1007/s00198-014-2794-2
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336(7638):262–266. doi:10.1136/bmj.39440.525752.BE
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011; 342:d2040. doi:10.1136/bmj.d2040
- Mao PJ, Zhang C, Tang L, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 169(2):106–111. doi:10.1016/j.ijcard.2013.08.055
- Reid IR, Bolland MJ. Calcium supplementation and vascular disease. Climacteric 2008; 11(4):280–286. doi:10.1080/13697130802229639
- Lewis JR, Radavelli-Bagatini S, Rejnmark L, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015; 30(1):165–175. doi:10.1002/jbmr.2311
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115(19):846–854. doi:10.1161/CIRCULATIONAHA.106.673491
- Kopecky SL, Bauer DC, Gulati M, et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med 2016; 165(12):867–868. doi:10.7326/M16-1743
- Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc 2016; 5(10):e003815. doi:10.1161/JAHA.116.003815
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126(7):497–504. pmid:9092314
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164(8):885–891. doi:10.1001/archinte.164.8.885
- Borhi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346(2):77–84. doi:10.1056/NEJMoa010369
- Prochaska ML, Taylor EN, Curhan GC. Insights into nephrolithiasis from the Nurses’ Health Studies. Am J Public Health 2016; 106(9):1638–1643. doi:10.2105/AJPH.2016.303319
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Chen J, Smerdely P. Hypocalcaemia after denosumab in older people following fracture. Osteoporos Int 2017; 28(2):517–522. doi:10.1007/s00198-016-3755-8
- Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990; 323(13):878–883. doi:10.1056/NEJM199009273231305
- Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 2010; 95(6):2637–2645. doi:10.1210/jc.2009-2699
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1):81–93. doi:10.1093/aje/kwq120
- Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 2012; 97(11):3989–3995. doi:10.1210/jc.2012-2276
- Binkley N, Novotny R, Krueger T, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92(6):2130–2135. doi:10.1210/jc.2006-2250
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA food composition databases. https://ndb.nal.usda.gov/ndb/search/list. Accessed May 7, 2018.
- International Osteoporosis Foundation. IOF calcium calculator version 1.10. Apple App Store. https://itunes.apple.com/us/app/iof-calcium-calculator/id956198268?mt=8. Accessed June 11, 2018.
- Recker RR. Calcium absorption and achlorhydria. N Engl J Med 1985; 313(2):70–73. doi:10.1056/NEJM198507113130202
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115(10):2598–2608. doi:10.1172/JCI26662
- Sakhaee K, Poindexter JR, Griffith CS, Pak CY. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol 2004; 172(3):958–961. doi:10.1097/01.ju.0000136400.14728.cd
- Kitchin B. Nutrition counseling for patients with osteoporosis: a personal approach. J Clin Densitom 2013; 16(4):426–431. doi:10.1016/j.jocd.2013.08.013
- Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005; 294(18):2336–2341. doi:10.1001/jama.294.18.2336
- Need AG, Horowitz M, Philcox JC, Nordin BE. 1,25-dihydroxycalciferol and calcium therapy in osteoporosis with calcium malabsorption. Dose response relationship of calcium absorption and indices of bone turnover. Miner Electrolyte Metab 1985; 11(1):35–40. pmid:3838358
- Harvey NC, Bilver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 2017; 28(2):447–462. doi:10.1007/s00198-016-3773-6
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 2005; 115(12):3318–3325. doi.10.1172/JCI27071
- Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med 2013; 369(16):1537–154 doi:10.1056/NEJMcp1210380
- Choi MJ, Park EJ, Jo HJ. Relationship of nutrient intakes and bone mineral density of elderly women in Daegu, Korea. Nutr Res Pract 2007; 1(4):328–33 doi:10.4162/nrp.2007.1.4.328
- Kim KM, Choi SH, Lim S, et al. Interactions between dietary calcium intake and bone mineral density or bone ge6ometry in a low calcium intake population (KNHANES IV 2008–2010). J Clin Endocrinol Metab 2014; 99(7):2409–2417. doi:10.1210/jc.2014-1006
- Joo NS, Dawson-Hughes B, Kim YS, Oh K, Yeum KJ. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 2013; 28(4):764–770. doi:10.1002/jbmr.1790
- Anderson JJ, Roggenkamp KJ, Suchindran CM. Calcium intakes and femoral and lumbar bone density of elderly US men and women: National Health and Nutrition Examination Survey 2005–2006 analysis. J Clin Endocrinol Metab 2012; 97(12):4531–4539. doi:10.1210/jc.2012-1407
- Gui JC, Brašic JR, Liu XD, et al. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow’s milk. Osteoporos Int 2012; 23(5):1563–1570. doi:10.1007/s00198-012-1895-z
- Moschonis G, Katsaroli I, Lyritis GP, Manios Y. The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr 2010; 104(1):100–107. doi:10.1017/S000711451000019X
- Recker RR, Hinders S, Davies KM, et al. Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 1996; 11(12):1961–1966. doi:10.1002/jbmr.5650111218
- Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015; 351:h4183. doi:10.1136/bmj.h4183
- Jackson RD, LaCroix AZ, Gass M, et al; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006; 354(7):669–683. doi:10.1056/NEJMoa055218
- Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992; 327(23):1637–1642. doi:10.1056/NEJM199212033272305
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 1997; 337(10):670–676. doi:10.1056/NEJM199709043371003
- Bolland MJ, Leung W, Tai V, et al. Calcium intake and risk of fracture: systematic review. BMJ 2015; 351:h4580. doi:10.1136/bmj.h4580
- Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med 2012; 367(1):77–78. doi:10.1056/NEJMe1206858
- Moyer VA; US Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158(9):691–696. doi:10.7326/0003-4819-158-9-201305070-00603
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96(7):1911–1930. doi:10.1210/jc.2011-0385
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologist and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract 2016; 22(suppl 4):1–42. doi:10.4158/EP161435.ESGL
- Ross AC, Manson JE, Abrams SA et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96(1):53–58. doi:10.1210/jc.2010-2704
- Cosman F, De Beur SJ, Leboff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25(10):2359–2381. doi:10.1007/s00198-014-2794-2
- Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008; 336(7638):262–266. doi:10.1136/bmj.39440.525752.BE
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011; 342:d2040. doi:10.1136/bmj.d2040
- Mao PJ, Zhang C, Tang L, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 169(2):106–111. doi:10.1016/j.ijcard.2013.08.055
- Reid IR, Bolland MJ. Calcium supplementation and vascular disease. Climacteric 2008; 11(4):280–286. doi:10.1080/13697130802229639
- Lewis JR, Radavelli-Bagatini S, Rejnmark L, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015; 30(1):165–175. doi:10.1002/jbmr.2311
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115(19):846–854. doi:10.1161/CIRCULATIONAHA.106.673491
- Kopecky SL, Bauer DC, Gulati M, et al. Lack of evidence linking calcium with or without vitamin D supplementation to cardiovascular disease in generally healthy adults: a clinical guideline from the National Osteoporosis Foundation and the American Society for Preventive Cardiology. Ann Intern Med 2016; 165(12):867–868. doi:10.7326/M16-1743
- Anderson JJ, Kruszka B, Delaney JA, et al. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc 2016; 5(10):e003815. doi:10.1161/JAHA.116.003815
- Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997; 126(7):497–504. pmid:9092314
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004; 164(8):885–891. doi:10.1001/archinte.164.8.885
- Borhi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346(2):77–84. doi:10.1056/NEJMoa010369
- Prochaska ML, Taylor EN, Curhan GC. Insights into nephrolithiasis from the Nurses’ Health Studies. Am J Public Health 2016; 106(9):1638–1643. doi:10.2105/AJPH.2016.303319
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356(18):1809–1822. doi:10.1056/NEJMoa067312
- Chen J, Smerdely P. Hypocalcaemia after denosumab in older people following fracture. Osteoporos Int 2017; 28(2):517–522. doi:10.1007/s00198-016-3755-8
- Dawson-Hughes B, Dallal GE, Krall EA, Sadowski L, Sahyoun N, Tannenbaum S. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N Engl J Med 1990; 323(13):878–883. doi:10.1056/NEJM199009273231305
- Melhus H, Snellman G, Gedeborg R, et al. Plasma 25-hydroxyvitamin D levels and fracture risk in a community-based cohort of elderly men in Sweden. J Clin Endocrinol Metab 2010; 95(6):2637–2645. doi:10.1210/jc.2009-2699
- Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010; 172(1):81–93. doi:10.1093/aje/kwq120
- Valcour A, Blocki F, Hawkins DM, Rao SD. Effects of age and serum 25-OH-vitamin D on serum parathyroid hormone levels. J Clin Endocrinol Metab 2012; 97(11):3989–3995. doi:10.1210/jc.2012-2276
- Binkley N, Novotny R, Krueger T, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007; 92(6):2130–2135. doi:10.1210/jc.2006-2250
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA food composition databases. https://ndb.nal.usda.gov/ndb/search/list. Accessed May 7, 2018.
- International Osteoporosis Foundation. IOF calcium calculator version 1.10. Apple App Store. https://itunes.apple.com/us/app/iof-calcium-calculator/id956198268?mt=8. Accessed June 11, 2018.
- Recker RR. Calcium absorption and achlorhydria. N Engl J Med 1985; 313(2):70–73. doi:10.1056/NEJM198507113130202
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest 2005; 115(10):2598–2608. doi:10.1172/JCI26662
- Sakhaee K, Poindexter JR, Griffith CS, Pak CY. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol 2004; 172(3):958–961. doi:10.1097/01.ju.0000136400.14728.cd
- Kitchin B. Nutrition counseling for patients with osteoporosis: a personal approach. J Clin Densitom 2013; 16(4):426–431. doi:10.1016/j.jocd.2013.08.013
- Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005; 294(18):2336–2341. doi:10.1001/jama.294.18.2336
- Need AG, Horowitz M, Philcox JC, Nordin BE. 1,25-dihydroxycalciferol and calcium therapy in osteoporosis with calcium malabsorption. Dose response relationship of calcium absorption and indices of bone turnover. Miner Electrolyte Metab 1985; 11(1):35–40. pmid:3838358
KEY POINTS
- We advise modest targets for total calcium intake, maximizing dietary calcium intake and making up the deficit with calcium citrate supplements.
- Gastrointestinal complaints are common with calcium supplements and can be mitigated with osmotic cathartics (mixed in the same pill or not) or with dose adjustment.
- Vitamin D levels should be optimized to help prevent secondary hyperparathyroidism.
Cardiac rehabilitation: A class 1 recommendation
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
Cardiac rehabilitation has a class 1 indication (ie, strong recommendation) after heart surgery, myocardial infarction, or coronary intervention, and for stable angina or peripheral artery disease. It has a class 2a indication (ie, moderate recommendation) for stable systolic heart failure. Yet it is still underutilized despite its demonstrated benefits, endorsement by most recognized cardiovascular societies, and coverage by the US Centers for Medicare and Medicaid Services (CMS).
Here, we review cardiac rehabilitation—its benefits, appropriate indications, barriers to referral and enrollment, and efforts to increase its use.
EXERCISE: SLOW TO BE ADOPTED
In 1772, William Heberden (also remembered today for describing swelling of the distal interphalangeal joints in osteoarthritis) described1 a patient with angina pectoris who “set himself a task of sawing wood for half an hour every day, and was nearly cured.”
Despite early clues, it would be some time before the medical community would recognize the benefits of exercise for cardiovascular health. Before the 1930s, immobilization and extended bedrest were encouraged for up to 6 weeks after a cardiovascular event, leading to significant deconditioning.2 Things slowly began to change in the 1940s with Levine’s introduction of up-to-chair therapy,3 and short daily walks were introduced in the 1950s. Over time, the link between a sedentary lifestyle and cardiovascular disease was studied and led to greater investigation into the benefits of exercise, propelling us into the modern era.4,5
CARDIAC REHABILITATION: COMPREHENSIVE RISK REDUCTION
The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) defines cardiac rehabilitation as the provision of comprehensive long-term services involving medical evaluation, prescriptive exercise, cardiac risk-factor modification, education, counseling, and behavioral interventions.6 CMS defines it as a physician-supervised program that furnishes physician-prescribed exercise, cardiac risk-factor modification (including education, counseling, and behavioral intervention), psychosocial assessment, outcomes assessment, and other items and services.7
In general, most cardiac rehabilitation programs provide medically supervised exercise and patient education designed to improve cardiac health and functional status. Risk factors are targeted to reduce disability and rates of morbidity and mortality, to improve functional capacity, and to alleviate activity-related symptoms.
FROM HOSPITAL TO SELF-MAINTENANCE
Phase 1: Inpatient rehabilitation
Phase 1 typically takes place in the inpatient setting, often after open heart surgery (eg, coronary artery bypass grafting, valve repair or replacement, heart transplant), myocardial infarction, or percutaneous coronary intervention. This phase may last only a few days, especially in the current era of short hospital stays.
During phase 1, patients discuss their health situation and goals with their primary provider or cardiologist and receive education about recovery and cardiovascular risk factors. Early mobilization to prepare for discharge and to resume simple activities of daily living is emphasized. Depending on the institution, phase 1 exercise may involve simple ambulation on the ward or using equipment such as a stationary bike or treadmill.6 Phase 2 enrollment ideally is set up before discharge.
Phase 2: Limited-time outpatient rehabilitation
Phase 2 traditionally takes place in a hospital-based outpatient facility and consists of a physician-supervised multidisciplinary program. Growing evidence shows that home-based cardiac rehabilitation may be as effective as a medical facility-based program and should be an option for patients who have difficulty getting access to a traditional program.8
A phase 2 program takes a threefold approach, consisting of exercise, aggressive risk-factor modification, and education classes. A Cochrane review9 included programs that also incorporated behavioral modification and psychosocial support as a means of secondary prevention, underscoring the evolving definition of cardiac rehabilitation.
During the initial phase 2 visit, an individualized treatment plan is developed, incorporating an exercise prescription and realistic goals for secondary prevention. Sessions typically take place 3 times a week for up to 36 sessions; usually, options are available for less frequent weekly attendance for a longer period to achieve a full course. In some cases, patients may qualify for up to 72 sessions, particularly if they have not progressed as expected.
Exercise. As part of the initial evaluation, AACVPR guidelines6 suggest an exercise test—eg, a symptom-limited exercise stress test, a 6-minute walk test, or use of a Rating of Perceived Exertion scale. Prescribed exercise generally targets moderate activity in the range of 50% to 70% of peak estimated functional capacity. In the appropriate clinical context, high-functioning patients can be offered high-intensity interval training instead of moderate exercise, as they confer similar benefits.10
Risk-factor reduction. Comprehensive risk-factor reduction can address smoking, hypertension, high cholesterol, diabetes, obesity, and diet, as well as psychosocial issues such as stress, anxiety, depression, and alcohol use. Sexual activity counseling may also be included.
Education classes are aimed at helping patients understand cardiovascular disease and empowering them to manage their medical treatment and lifestyle modifications.6
Phase 3: Lifetime maintenance
In phase 3, patients independently continue risk-factor modification and physical activity without cardiac monitoring. Most cardiac rehabilitation programs offer transition-to-maintenance classes after completion of phase 2; this may be a welcome option, particularly for those who have developed a good routine and rapport with the staff and other participants. Others may opt for an independent program, using their own home equipment or a local health club.
EXERCISE: MOSTLY SAFE, WITH PROVEN BENEFITS
The safety of cardiac rehabilitation is well established, with a low risk of major cardiovascular complications. A US study in the early 1980s of 167 cardiac rehabilitation programs found 1 cardiac arrest for every 111,996 exercise hours, 1 myocardial infarction per 293,990 exercise hours, and 1 fatality per 783,972 exercise hours.11 A 2006 study of more than 65 cardiac rehabilitation centers in France found 1 cardiac event per 8,484 exercise tests and 1.3 cardiac arrests per 1 million exercise hours.12
The benefits of cardiac rehabilitation are numerous and substantial.9,13–17 A 2016 Cochrane review and meta-analysis of 63 randomized controlled trials with 14,486 participants found a reduced rate of cardiovascular mortality (relative risk [RR] 0.74, 95% confidence interval [CI] 0.64–0.86), with a number needed to treat of 37, and fewer hospital readmissions (RR 0.82, 95% CI 0.70–0.96).9
Reductions in mortality rates are dose-dependent. A study of more than 30,000 Medicare beneficiaries who participated in cardiac rehabilitation found that those who attended more sessions had a lower rate of morbidity and death at 4 years, particularly if they participated in more than 11 sessions. Those who attended the full 36 sessions had a mortality rate 47% lower than those who attended a single session.17 There was a 15% reduction in mortality for those who attended 36 sessions compared with 24 sessions, a 28% lower risk with attending 36 sessions compared with 12. After adjustment, each additional 6 sessions was associated with a 6% reduction in mortality. The curves continued to separate up to 4 years.
The benefits of cardiac rehabilitation go beyond risk reduction and include improved functional capacity, greater ease with activities of daily living, and improved quality of life.9 Patients receive structure and support from the management team and other participants, which may provide an additional layer of friendship and psychosocial support for making lifestyle changes.
Is the overall mortality rate improved?
In the modern era, with access to optimal medical therapy and drug-eluting stents, one might expect only small additional benefit from cardiac rehabilitation. The 2016 Cochrane review and meta-analysis found that although cardiac rehabilitation contributed to improved cardiovascular mortality rates and health-related quality of life, no significant reduction was detected in the rate of death from all causes.8 But the analysis did not necessarily support removing the claim of reduced all-cause mortality for cardiac rehabilitation: only randomized controlled trials were examined, and the quality of evidence for each outcome was deemed to be low to moderate because of a general paucity of reports, including many small trials that followed patients for less than 12 months.
A large cohort analysis15 with more than 73,000 patients who had undergone cardiac rehabilitation found a relative reduction in mortality rate of 58% at 1 year and 21% to 34% at 5 years, with elderly women gaining the most benefit. In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, with more than 2,300 patients followed for a median of 2.5 years, exercise training for heart failure was associated with reduced rates of all-cause mortality or hospitalization (HR 0.89, 95% CI 0.81–0.99; P = .03) and of cardiovascular mortality or heart failure hospitalization (HR 0.85, 95% CI 0.74–0.99; P = .03).18
Regardless of the precise reduction in all-cause mortality, the cardiovascular and health-quality outcomes of cardiac rehabilitation clearly indicate benefit. More trials with follow-up longer than 1 year are needed to definitively determine the impact of cardiac rehabilitation on the all-cause mortality rate.
WHO SHOULD BE OFFERED CARDIAC REHABILITATION?
The 2006 CMS coverage criteria listed the indications for cardiac rehabilitation as myocardial infarction within the preceding 12 months, coronary artery bypass surgery, stable angina pectoris, heart valve repair or replacement, percutaneous coronary intervention, and heart or heart-lung transplant.
In 2014, stable chronic systolic heart failure was added to the list (Table 2). Qualifications include New York Heart Association class II (mild symptoms, slight limitation of activity) to class IV (severe limitations, symptoms at rest), an ejection fraction of 35% or less, and being stable on optimal medical therapy for at least 6 weeks.
In 2017, CMS approved supervised exercise therapy for peripheral arterial disease. Supervised exercise has a class 1 recommendation by the American Heart Association and American College of Cardiology for treating intermittent claudication. Supervised exercise therapy can increase walking distance by 180% and is superior to medical therapy alone. Unsupervised exercise has a class 2b recommendation.19,20
Other patients may not qualify for phase 2 cardiac rehabilitation according to CMS or private insurance but could benefit from an exercise prescription and enrollment in a local phase 3 or home exercise program. Indications might include diabetes, obesity, metabolic syndrome, atrial fibrillation, postural orthostatic tachycardia syndrome, and nonalcoholic steatohepatitis. The benefits of cardiac rehabilitation after newer, less-invasive procedures for transcatheter valve repair and replacement are not well established, and more research is needed in this area.
WHEN TO REFER
Ades et al have defined cardiac rehabilitation referral as a combination of electronic medical records order, patient-physician discussion, and receipt of an order by a cardiac rehabilitation program.21
Ideally, referral for outpatient cardiac rehabilitation should take place at the time of hospital discharge. The AACVPR endorses a “cardiovascular continuum of care” model that emphasizes a smooth transition from inpatient to outpatient programs.6 Inpatient referral is a strong predictor of cardiac rehabilitation enrollment, and lack of referral in phase 1 negatively affects enrollment rates.
Depending on the diagnosis, US and Canadian guidelines recommend cardiac rehabilitation starting within 1 to 4 weeks of the index event, with acceptable wait times up to 60 days.6,22 In the United Kingdom, referral is recommended within 24 hours of patient eligibility; assessment for a cardiovascular prevention and rehabilitation program, with a defined pathway and individual goals, is expected to be completed within 10 working days of referral.23 Such a standard is difficult to meet in the United States, where the time from hospital discharge to cardiac rehabilitation program enrollment averages 35 days.24,25
After an uncomplicated myocardial infarction or percutaneous coronary intervention, patients with a normal or mildly reduced left ventricular ejection fraction should start outpatient cardiac rehabilitation within 14 days of the index event. For such cases, cardiac rehabilitation has been shown to be safe within 1 to 2 weeks of hospital discharge and is associated with increased participation rates.
REHABILITATION IS STILL UNDERUSED
Despite its significant benefits, cardiac rehabilitation is underused for many reasons.
Referral rates vary
A study using the 1997 Medicare claims database showed national referral rates of only 14% after myocardial infarction and 31% after coronary artery bypass grafting.31
A later study using the National Cardiovascular Data Registry between 2009 and 2017 found that the situation had improved, with a referral rate of about 60% for patients undergoing percutaneous coronary intervention.32 Nevertheless, referral rates for cardiac rehabilitation remain highly variable and still lag behind other CMS quality measures for optimal medical therapy after acute myocardial infarction (Figure 1). Factors associated with higher referral rates included ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, care in a high-volume center for percutaneous coronary intervention, and care in a private or community hospital in a Midwestern state. Small Midwestern hospitals generally had referral rates of over 80%, while major teaching hospitals and hospital systems on the East Coast and the West Coast had referral rates of less than 20%. Unlike some studies, this study found that insurance status had little bearing on referral rates.
Other studies found lower referral rates for women and patients with comorbidities such as previous coronary artery bypass grafting, diabetes, and heart failure.33,34
In the United Kingdom, patients with heart failure made up only 5% of patients in cardiac rehabilitation; only 7% to 20% of patients with a heart failure diagnosis were referred to cardiac rehabilitation from general and cardiology wards.35
Enrollment, completion rates even lower
Rates of referral for cardiac rehabilitation do not equate to rates of enrollment or participation. Enrollment was 50% in the United Kingdom in 2016.35 A 2015 US study evaluated 58,269 older patients eligible for cardiac rehabilitation after acute myocardial infarction; 62% were referred for cardiac rehabilitation at the time of discharge, but only 23% of the total attended at least 1 session, and just 5% of the total completed 36 or more sessions.36
BARRIERS, OPPORTUNITIES TO IMPROVE
The underuse of cardiac rehabilitation in the United States has led to an American Heart Association presidential advisory on the referral, enrollment, and delivery of cardiac rehabilitation.34 Dozens of barriers are mentioned, with several standing out as having the largest impact: lack of physician referral, weak endorsement by the prescribing provider, female sex of patients, lack of program availability, work-related hardship, low socioeconomic status, and lack of or limited healthcare insurance. Copayments have also become a major barrier, often ranging from $20 to $40 per session for patients with Medicare.
The Million Hearts Initiative has established a goal of 70% cardiac rehabilitation compliance for eligible patients by 2022, a goal they estimate could save 25,000 lives and prevent 180,000 hospitalizations annually.21
Lack of physician awareness and lack of referral may be the most modifiable factors with the capacity to have the largest impact. Increasing physician awareness is a top priority not only for primary care providers, but also for cardiologists. In 2014, CMS made referral for cardiac rehabilitation a quality measure that is trackable and reportable. CMS has also proposed models that would incentivize participation by increasing reimbursement for services provided, but these models have been halted.
Additional efforts to increase cardiac rehabilitation referral and participation include automated order sets, increased caregiver education, and early morning or late evening classes, single-sex classes, home or mobile-based exercise programs, and parking and transportation assistance.34 Grace et al37 reported that referral rates rose to 86% when a cardiac rehabilitation order was integrated into the electronic medical record and combined with a hospital liaison to educate patients about their need for cardiac rehabilitation. Lowering patient copayments would also be a good idea. We have recently seen some creative ways to reduce copayments, including philanthropy and grants.
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
- Herberden W. Classics in cardiology: description of angina pectoris by William Herberden. Heart Views 2006; 7(3):118–119. www.heartviews.org/text.asp?2006/7/3/118/63927. Accessed May 9, 2018.
- Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012; 2(1):38–49. doi:10.3978/j.issn.2223-3652.2012.01.02
- Levine SA, Lown B. The “chair” treatment of acute thrombosis. Trans Assoc Am Physicians 1951; 64:316–327. pmid:14884265
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet 1980; 2(8206):207–210. pmid:6108391
- Morris JN, Heady JA. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Ind Med 1953; 10(4):245–254. pmid:13106231
- American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for cardiac rehabilitation and secondary prevention programs/American Association of Cardiovascular and Pulmonary Rehabilitation. 5th ed. Champaign, IL: Human Kinetics; 2013.
- Department of Health & Human Services (DHHS); Centers for Medicare & Medicaid Services (CMS). CMS manual system. Cardiac rehabilitation and intensive cardiac rehabilitation. www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r126bp.pdf. Accessed May 9, 2018.
- Anderson L, Sharp GA, Norton RJ, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2017; 6:CD007130. doi:10.1002/14651858.CD007130.pub4
- Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016; 67(1):1–12. doi:10.1016/j.jacc.2015.10.044
- Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med 2012; 42(7):587–605. doi:10.2165/11631910-000000000-00000
- Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986; 256(9):1160–1163. pmid:3735650
- Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006; 166(21):2329–2334. doi:10.1001/archinte.166.21.2329
- Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after a myocardial infarction: The National Exercise and Heart Disease Project. Am J Cardiol 1981; 48(1):39–46. pmid:6972693
- Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015; 65(4):389–395. doi:10.1016/j.jacc.2014.10.059
- Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol 2009; 54(1):25–33. doi:10.1016/j.jacc.2009.01.078
- Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation 2011: 123(21):2344–2352. doi:10.1161/CIRCULATIONAHA.110.983536
- Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010; 121(1):63–70. doi:10.1161/CIRCULATIONAHA.109.876383
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301(14):1439–1450. doi:10.1001/jama.2009.454
- Hirsch A, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006; 113(11):463–654. doi:10.1161/CIRCULATIONAHA.106.174526
- Ambrosetti M. Advances in exercise rehabilitation for patients with lower extremity peripheral artery disease. Monaldi Arch Chest Dis 2016; 86(1–2):752. doi:10.4081/monaldi.2016.752
- Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc 2017; 92(2):234–242. doi:10.1016/j.mayocp.2016.10.014
- Dafoe W, Arthur H, Stokes H, Morrin L, Beaton L; Canadian Cardiovascular Society Access to Care Working Group on Cardiac Rehabilitation. Universal access: but when? Treating the right patient at the right time: access to cardiac rehabilitation. Can J Cardiol 2006; 22(11):905–911. pmid:16971975
- The British Association for Cardiovascular Prevention and Rehabilitation. The BACPR standards and core components for cardiovascular disease prevention and cardiac rehabilitation 2017. www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed May 9, 2018.
- Zullo MD, Jackson LW, Whalen CC, Dolansky MA. Evaluation of the recommended core components of cardiac rehabilitation practice: an opportunity for quality improvement. J Cardiopulm Rehabil Prev 2012; 32(1):32–40. doi:10.1097/HCR.0b013e31823be0e2
- Russell KL, Holloway TM, Brum M, Caruso V, Chessex C, Grace SL. Cardiac rehabilitation wait times: effect on enrollment. J Cardiopulm Rehabil Prev 2011; 31(6):373–377. doi:10.1097/HCR.0b013e318228a32f
- Soga Y, Yokoi H, Ando K, et al. Safety of early exercise training after elective coronary stenting in patients with stable coronary artery disease. Eur J Cardiovasc Prev Rehabil 2010; 17(2):230–234. doi:10.1097/HJR.0b013e3283359c4e
- Scheinowitz M, Harpaz D. Safety of cardiac rehabilitation in a medically supervised, community-based program. Cardiology 2005; 103(3):113–117. doi:10.1159/000083433
- Goto Y, Sumida H, Ueshima K, Adachi H, Nohara R, Itoh H. Safety and implementation of exercise testing and training after coronary stenting in patients with acute myocardial infarction. Circ J 2002; 66(10):930–936. pmid:12381088
- Parker K, Stone JA, Arena R, et al. An early cardiac access clinic significantly improves cardiac rehabilitation participation and completion rates in low-risk ST-elevation myocardial infarction patients. Can J Cardiol 2011; 27(5):619–627. doi:10.1016/j.cjca.2010.12.076
- Pack QR, Mansour M, Barboza JS, et al. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation: a randomized, single-blind, controlled trial. Circulation 2013; 127(3):349–355. doi:10.1161/CIRCULATIONAHA.112.121996
- Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007; 116(15):1653–1662. doi:10.1161/CIRCULATIONAHA.107.701466
- Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol 2015; 65(19):2079–2088. doi:10.1016/j.jacc.2015.02.063
- Bittner V, Sanderson B, Breland J, Green D. Referral patterns to a university-based cardiac rehabilitation program. Am J Cardiol 1999; 83(2):252–255, A5. pmid:10073829
- Balady GJ, Ades PA, Bittner VA, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond. A presidential advisory from the American Heart Association. Circulation 2011; 124(25):2951–2960. doi:10.1161/CIR.0b013e31823b21e2
- British Heart Foundation. The national audit of cardiac rehabilitation annual statistical report 2016. www.cardiacrehabilitation.org.uk/docs/BHF_NACR_Report_2016.pdf. Accessed April 12, 2018.
- Doll JA, Hellkamp A, Ho PM, et al. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med 2015; 175(10):1700–1702. doi:10.1001/jamainternmed.2015.3819
- Grace SL, Russell KL, Reid RD, et al. Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011; 171(3):235–241. doi:10.1001/archinternmed.2010.501
KEY POINTS
- Cardiac rehabilitation should begin in the hospital after heart surgery or myocardial infarction, should continue with a hospital-centered 36-session program, and should be maintained independently by the patient for life.
- Exercise in a cardiac rehabilitation program entails little risk and many proven benefits.
- Cardiac rehabilitation is indicated and covered by the Centers for Medicare and Medicaid Services (CMS) for a number of cardiovascular conditions.
- Utilization of cardiac rehabilitation could be improved through CMS reimbursement incentives, electronic medical record prompts, lower copayments for participation, and home-based programs for patients who live far from medical centers.
Renal disease and the surgical patient: Minimizing the impact
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
Chronic kidney disease (CKD) is estimated to affect 14% of Americans, but it is likely underdiagnosed because it is often asymptomatic.1,2 Its prevalence is even higher in patients who undergo surgery—up to 30% in cardiac surgery.3 Its impact on surgical outcomes is substantial.4 Importantly, patients with CKD are at higher risk of postoperative acute kidney injury (AKI), which is also associated with adverse outcomes. Thus, it is important to recognize, assess, and manage abnormal renal function in surgical patients.
WHAT IS THE IMPACT ON POSTOPERATIVE OUTCOMES?
Cardiac surgery outcomes
Moreover, in patients undergoing coronary artery bypass grafting (CABG), the worse the renal dysfunction, the higher the long-term mortality rate. Patients with moderate (stage 3) CKD had a 3.5 times higher odds of in-hospital mortality compared with patients with normal renal function, rising to 8.8 with severe (stage 4) and to 9.6 with dialysis-dependent (stage 5) CKD.11
The mechanisms linking CKD with negative cardiac outcomes are unclear, but many possibilities exist. CKD is an independent risk factor for coronary artery disease and shares underlying risk factors such as hypertension and diabetes. Cardiac surgery patients with CKD are also more likely to have diabetes, left ventricular dysfunction, and peripheral vascular disease.
Noncardiac surgery outcomes
CKD is also associated with adverse outcomes in noncardiac surgery patients, especially at higher levels of renal dysfunction.12–14 For example, in patients who underwent major noncardiac surgery, compared with patients in stage 1 (estimated GFR > 90 mL/min/1.73 m2), the odds ratios for all-cause mortality were as follows:
- 0.8 for patients with stage 2 CKD
- 2.2 in stage 3a
- 2.8 in stage 3b
- 11.3 in stage 4
- 5.8 in stage 5.14
The association between estimated GFR and all-cause mortality was not statistically significant (P = .071), but statistically significant associations were observed between estimated GFR and major adverse cardiovascular events (P < .001) and hospital length of stay (P < .001).
The association of CKD with major adverse outcomes and death in both cardiac and noncardiac surgical patients demonstrates the importance of understanding this risk, identifying patients with CKD preoperatively, and taking steps to lower the risk.
WHAT IS THE IMPACT OF ACUTE KIDNEY INJURY?
AKI is a common and serious complication of surgery, especially cardiac surgery. It has been associated with higher rates of morbidity, mortality, and cardiovascular events, longer hospital length of stay, and higher cost.
Several groups have proposed criteria for defining AKI and its severity; the KDIGO criteria are the most widely accepted.15 These define AKI as an increase in serum creatinine concentration of 0.3 mg/dL or more within 48 hours or at least 1.5 times the baseline value within 7 days, or urine volume less than 0.5 mL/kg/hour for more than 6 hours. There are 3 stages of severity:
- Stage 1—an increase in serum creatinine of 1.5 to 1.9 times baseline, an absolute increase of at least 0.3 mg/dL, or urine output less than 0.5 mL/kg/hour for 6 to 12 hours
- Stage 2—an increase in serum creatinine of 2.0 to 2.9 times baseline or urine output less than 0.5 mmL/kg/hour for 12 or more hours
- Stage 3—an increase in serum creatinine of 3 times baseline, an absolute increase of at least 4 mg/dL, initiation of renal replacement therapy, urine output less than 0.3 mL/kg/hour for 24 or more hours, or anuria for 12 or more hours.15
Multiple factors associated with surgery may contribute to AKI, including hemodynamic instability, volume shifts, blood loss, use of heart-lung bypass, new medications, activation of the inflammatory cascade, oxidative stress, and anemia.
AKI in cardiac surgery
The incidence of AKI is high in cardiac surgery. In a meta-analysis of 46 studies (N = 242,000), its incidence in cardiopulmonary bypass surgery was about 18%, with 2.1% of patients needing renal replacement therapy.16 However, the incidence varied considerably from study to study, ranging from 1% to 53%, and was influenced by the definition of AKI, the type of cardiac surgery, and the patient population.16
Cardiac surgery-associated AKI adversely affects outcomes. Several studies have shown that cardiac surgery patients who develop AKI have higher rates of death and stroke.16–21 More severe AKI confers higher mortality rates, with the highest mortality rate in patients who need renal replacement therapy, approximately 37%.17 Patients with cardiac surgery-associated AKI also have a longer hospital length of stay and significantly higher costs of care.17,18
Long-term outcomes are also negatively affected by AKI. In cardiac surgery patients with AKI who had completely recovered renal function by the time they left the hospital, the 2-year incidence rate of CKD was 6.8%, significantly higher than the 0.2% rate in patients who did not develop AKI.19 The 2-year survival rates also were significantly worse for patients who developed postoperative AKI (82.3% vs 93.7%). Similarly, in patients undergoing CABG who had normal renal function before surgery, those who developed AKI postoperatively had significantly shorter long-term survival rates.20 The effect does not require a large change in renal function. An increase in creatinine as small as 0.3 mg/dL has been associated with a higher rate of death and a long-term risk of end-stage renal disease that is 3 times higher.21
WHAT ARE THE RISK FACTORS FOR ACUTE KIDNEY INJURY?
Cardiac surgery
CKD is a risk factor not only after cardiac surgery but also after percutaneous procedures. In a meta-analysis of 4,992 patients with CKD who underwent transcatheter aortic valve replacement, both moderate and severe CKD increased the odds of AKI, early stroke, the need for dialysis, and all-cause and cardiovascular mortality at 1 year.22,23 Increased rates of AKI also have been found in patients with CKD undergoing CABG surgery.24 These results point to a synergistic effect between AKI and CKD, with outcomes much worse in combination than alone.
In cardiac surgery, the most important patient risk factors associated with a higher incidence of postoperative AKI are age older than 75, CKD, preoperative heart failure, and prior myocardial infarction.19,25 Diabetes is an additional independent risk factor, with type 1 conferring higher risk than type 2.26 Preoperative use of angiotensin-converting enzyme (ACE) inhibitors may or may not be a risk factor for cardiac surgery-associated AKI, with some studies finding increased risk and others finding reduced rates.27,28
Anemia, which may be related to either patient or surgical risk factors (eg, intraoperative blood loss), also increases the risk of AKI in cardiac surgery.29,30 A retrospective study of CABG surgery patients found that intraoperative hemoglobin levels below 8 g/dL were associated with a 25% to 30% incidence of AKI, compared with 15% to 20% with hemoglobin levels above 9 g/dL.29 Additionally, having severe hypotension (mean arterial pressure < 50 mm Hg) significantly increased the AKI rates in the low-hemoglobin group.29 Similar results were reported in a later study.30
Among surgical factors, several randomized controlled trials have shown that off-pump CABG is associated with a significantly lower risk of postoperative AKI than on-pump CABG; however, this difference did not translate into any long-term difference in mortality rates.31,32 Longer cardiopulmonary bypass time is strongly associated with a higher incidence of AKI and postoperative death.33
Noncardiac surgery
AKI is less common after noncardiac surgery; however, outcomes are severe in patients in whom it occurs. In a study of 15,102 noncardiac surgery patients, only 0.8% developed AKI and 0.1% required renal replacement therapy.34
Risk factors after noncardiac surgery are similar to those after cardiac surgery (Table 3).34–36 Factors with the greatest impact are older age, peripheral vascular occlusive disease, chronic obstructive pulmonary disease necessitating chronic bronchodilator therapy, high-risk surgery, hepatic disease, emergent or urgent surgery, and high body mass index.
Surgical risk factors include total vasopressor dose administered, use of a vasopressor infusion, and diuretic administration.34 In addition, intraoperative hypotension is associated with a higher risk of AKI, major adverse cardiac events, and 30-day mortality.37
Noncardiac surgery patients with postoperative AKI have significantly higher rates of 30-day readmissions, 1-year progression to end-stage renal disease, and mortality than patients who do not develop AKI.35 Additionally, patients with AKI have significantly higher rates of cardiovascular complications (33.3% vs 11.3%) and death (6.1% vs 0.9%), as well as a significantly longer length of hospital stay.34,36
CAN WE DECREASE THE IMPACT OF RENAL DISEASE IN SURGERY?
Before surgery, practitioners need to identify patients at risk of AKI, implement possible risk-reduction measures, and, afterward, treat it early in its course if it occurs.
The preoperative visit is the ideal time to assess a patient’s risk of postoperative renal dysfunction. Laboratory tests can identify risks based on surgery type, age, hypertension, the presence of CKD, and medications that affect renal function. However, the basic chemistry panel is abnormal in only 8.2% of patients and affects management in just 2.6%, requiring the clinician to target testing to patients at high risk.38
Patients with a significant degree of renal dysfunction, particularly those previously undiagnosed, may benefit from additional preoperative testing and medication management. Perioperative management of medications that could adversely affect renal function should be carefully considered during the preoperative visit. In addition, the postoperative inpatient team needs to be informed about potentially nephrotoxic medications and medications that are renally cleared. Attention needs to be given to the renal impact of common perioperative medications such as nonsteroidal anti-inflammatory drugs, antibiotics, intravenous contrast, low-molecular-weight heparins, diuretics, ACE inhibitors, and angiotensin II receptor blockers. With the emphasis on opioid-sparing analgesics, it is particularly important to assess the risk of AKI if nonsteroidal anti-inflammatory drugs are part of the pain control plan.
Nephrology referral may help, especially for patients with a GFR less than 45 mL/min. This information enables more informed decision-making regarding the risks of adverse outcomes related to kidney disease.
WHAT TOOLS DO WE HAVE TO DIAGNOSE RENAL INJURY?
Several risk-prediction models have been developed to assess the postoperative risk of AKI in both cardiac and major noncardiac surgery patients. Although these models can identify risk factors, their clinical accuracy and utility have been questioned.
Biomarkers
Early diagnosis is the first step in managing AKI, allowing time to implement measures to minimize its impact.
Serum creatinine testing is widely used to measure renal function and diagnose AKI; however, it does not detect small reductions in renal function, and there is a time lag between renal insult and a rise in creatinine. The result is a delay to diagnosis of AKI.
Biomarkers other than creatinine have been studied for early detection of intraoperative and postoperative renal insult. These novel renal injury markers include the following:
Neutrophil gelatinase-associated lipocalin (NGAL). Two studies looked at plasma NGAL as an early marker of AKI in patients with CKD who were undergoing cardiac surgery.39,40 One study found that by using NGAL instead of creatinine, postoperative AKI could be diagnosed an average of 20 hours earlier.39 In addition, NGAL helped detect renal recovery earlier than creatinine.40 The diagnostic cut-off values of NGAL were different for patients with CKD than for those without CKD.39,40
Other novel markers include:
- Kidney injury marker 1
- N-acetyl-beta-D-glucosaminidase
- Cysteine C.
Although these biomarkers show some ability to detect renal injury, they provide only modest discrimination and are not widely available for clinical use.41 Current evidence does not support routine use of these markers in clinical settings.
CAN WE PROTECT RENAL FUNCTION?
Interventions to prevent or ameliorate the impact of CKD and AKI on surgical outcomes have been studied most extensively in cardiac surgery patients.
Aspirin. A retrospective study of 3,585 cardiac surgery patients with CKD found that preoperative aspirin use significantly lowered the incidence of postoperative AKI and 30-day mortality compared with patients not using aspirin.42 Aspirin use reduced 30-day mortality in CKD stages 1, 2, and 3 by 23.3%, 58%, and 70%, respectively. On the other hand, in the Perioperative Ischemic Evaluation (POISE) trial, in noncardiac surgery patients, neither aspirin nor clonidine started 2 to 4 hours preoperatively and continued up to 30 days after surgery altered the risk of AKI significantly more than placebo.43
Statins have been ineffective in reducing the incidence of AKI in cardiac surgery patients. In fact, a meta-analysis of 8 interventional trials found an increased incidence of AKI in patients in whom statins were started perioperatively.44 Erythropoietin was also found to be ineffective in the prevention of perioperative AKI in cardiac surgery patients in a separate study.45
The evidence regarding other therapies has also varied.
N-acetylcysteine in high doses reduced the incidence of AKI in patients with CKD stage 3 and 4 undergoing CABG.46 Another meta-analysis of 10 studies in cardiac surgery patients published recently did not show any benefit of N-acetylcysteine in reducing AKI.47
Human atrial natriuretic peptide, given preoperatively to patients with CKD, reduced the acute and long-term creatinine rise as well as the number of cardiac events after CABG; however, it did not reduce mortality rates.48
Renin-angiotensin system inhibitors, given preoperatively to patients with heart failure was associated with a decrease in the incidence of AKI in 1 study.49
Dexmedetomidine is a highly selective alpha 2 adrenoreceptor agonist. A recent meta-analysis of 10 clinical trials found it beneficial in reducing the risk of perioperative AKI in cardiac surgery patients.50 An earlier meta-analysis had similar results.51
Levosimendan is an inotropic vasodilator that improves cardiac output and renal perfusion in patients with systolic heart failure, and it has been hypothesized to decrease the risk of AKI after cardiac surgery. Previous data demonstrated that this drug reduced AKI and mortality; however, analysis was limited by small sample size and varying definitions of AKI.52 A recent meta-analysis showed that levosimendan was associated with a lower incidence of AKI but was also associated with an increased incidence of atrial fibrillation and no reduction in 30-day mortality.53
Remote ischemic preconditioning is a procedure that subjects the kidneys to brief episodes of ischemia before surgery, protecting them when they are later subjected to prolonged ischemia or reperfusion injury. It has shown initial promising results in preventing AKI. In a randomized controlled trial in 240 patients at high risk of AKI, those who received remote ischemic preconditioning had an AKI incidence of 37.5% compared with 52.5% for controls (P = .02); however, the mortality rate was the same.54 Similarly, remote ischemic preconditioning significantly lowered the incidence of AKI in nondiabetic patients undergoing CABG surgery compared with controls.55
Fluid management. Renal perfusion is intimately related to the development of AKI, and there is evidence that both hypovolemia and excessive fluid resuscitation can increase the risk of AKI in noncardiac surgery patients.56 Because of this, fluid management has also received attention in perioperative AKI. Goal-directed fluid management has been evaluated in noncardiac surgery patients, and it did not show any benefit in preventing AKI.57 However, in a more recent retrospective study, postoperative positive fluid balance was associated with increased incidence of AKI compared with zero fluid balance. Negative fluid balance did not appear to have a detrimental effect.58
RECOMMENDATIONS
No prophylactic therapy has yet been shown to definitively decrease the risk of postoperative AKI in all patients. Nevertheless, it is important to identify patients at risk during the preoperative visit, especially those with CKD. Many patients undergoing surgery have CKD, placing them at high risk of developing AKI in the perioperative period. The risk is particularly high with cardiac surgery.
Serum creatinine and urine output should be closely monitored perioperatively in at-risk patients. If AKI is diagnosed, practitioners need to identify and ameliorate the cause as early as possible.
Recommendations from KDIGO for perioperative prevention and management of AKI are listed in Table 4.15 These include avoiding additional nephrotoxic medications and adjusting the doses of renally cleared medications. Also, some patients may benefit from preoperative counseling and specialist referral.
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
- Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298(17):2038–2047. doi:10.1001/jama.298.17.2038
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed June 11, 2018.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1(1):19–32. doi:10.2215/CJN.00240605
- Meersch M, Schmidt C, Zarbock A. Patient with chronic renal failure undergoing surgery. Curr Opin Anaesthesiol 2016; 29(3):413–420. doi:10.1097/ACO.0000000000000329
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67(6):2089–2100. doi:10.1111/j.1523-1755.2005.00365.x
- Saitoh M, Takahashi T, Sakurada K, et al. Factors determining achievement of early postoperative cardiac rehabilitation goal in patients with or without preoperative kidney dysfunction undergoing isolated cardiac surgery. J Cardiol 2013; 61(4):299–303. doi:10.1016/j.jjcc.2012.12.014
- Minakata K, Bando K, Tanaka S, et al. Preoperative chronic kidney disease as a strong predictor of postoperative infection and mortality after coronary artery bypass grafting. Circ J 2014; 78(9):2225–2231. doi:10.1253/circj.CJ-14-0328
- Domoto S, Tagusari O, Nakamura Y, et al. Preoperative estimated glomerular filtration rate as a significant predictor of long-term outcomes after coronary artery bypass grafting in Japanese patients. Gen Thorac Cardiovasc Surg 2014; 62(2):95–102. doi:10.1007/s11748-013-0306-5
- Hedley AJ, Roberts MA, Hayward PA, et al. Impact of chronic kidney disease on patient outcome following cardiac surgery. Heart Lung Circ 2010; 19(8):453–459. doi:10.1016/j.hlc.2010.03.005
- Boulton BJ, Kilgo P, Guyton RA, et al. Impact of preoperative renal dysfunction in patients undergoing off-pump versus on-pump coronary artery bypass. Ann Thorac Surg 2011; 92(2):595–601. doi:10.1016/j.athoracsur.2011.04.023
- Prowle JR, Kam EP, Ahmad T, Smith NC, Protopapa K, Pearse RM. Preoperative renal dysfunction and mortality after non-cardiac surgery. Br J Surg 2016; 103(10):1316–1325. doi:10.1002/bjs.10186
- Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg 2013; 258(1):169–177. doi:10.1097/SLA.0b013e318288e18e
- Mases A, Sabaté S, Guilera N, et al. Preoperative estimated glomerular filtration rate and the risk of major adverse cardiovascular and cerebrovascular events in non-cardiac surgery. Br J Anaesth 2014; 113(4):644–651. doi:10.1093/bja/aeu134
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice 2012; 120(4):c179–c184. doi:10.1159/000339789
- Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015; 65(2):283–293. doi:10.1053/j.ajkd.2014.09.008
- Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23(6):1970-1974. doi:10.1093/ndt/gfm908
- Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery focus on modifiable risk factors. Circulation 2009; 119(4):495–502. doi:10.1161/CIRCULATIONAHA.108.786913
- Xu JR, Zhu JM, Jiang J, et al. Risk factors for long-term mortality and progressive chronic kidney disease associated with acute kidney injury after cardiac surgery. Medicine (Baltimore) 2015; 94(45):e2025. doi:10.1097/MD.0000000000002025
- Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M. The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 2013; 43(3):555–559. doi:10.1093/ejcts/ezs329
- Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation 2014; 130(23):2005–2011. doi:10.1161/CIRCULATIONAHA.114.010622
- Gargiulo G, Capodanno D, Sannino A, et al. Impact of moderate preoperative chronic kidney disease on mortality after transcatheter aortic valve implantation. Int J Cardiol 2015; 189:77–78. doi:10.1016/j.ijcard.2015.04.077
- Gargiulo G, Capodanno D, Sannino A, et al. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation meta-analysis of 4,992 patients. Circ Cardiovasc Interv 2015; 8(2):e002220. doi:10.1161/CIRCINTERVENTIONS.114.002220
- Han SS, Shin N, Baek SH, et al. Effects of acute kidney injury and chronic kidney disease on long-term mortality after coronary artery bypass grafting. Am Heart J 2015; 169(3):419–425. doi:10.1016/j.ahj.2014.12.019
- Aronson S, Fontes ML, Miao Y, Mangano DT; Investigators of the Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation 2007; 115(6):733–742. doi:10.1161/CIRCULATIONAHA.106.623538
- Hertzberg D, Sartipy U, Holzmann MJ. Type 1 and type 2 diabetes mellitus and risk of acute kidney injury after coronary artery bypass grafting. Am Heart J 2015; 170(5):895–902. doi:10.1016/j.ahj.2015.08.013
- Benedetto U, Sciarretta S, Roscitano A, et al. Preoperative angiotensin-converting enzyme inhibitors and acute kidney injury after coronary artery bypass grafting. Ann Thorac Surg 2008; 86(4):1160–1165. doi:10.1016/j.athoracsur.2008.06.018
- Arora P, Rajagopalam S, Ranjan R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 2008; 3(5):1266–1273. doi:10.2215/CJN.05271107
- Haase M, Bellomo R, Story D, et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrol Dial Transplant 2012; 27(1):153–160. doi:10.1093/ndt/gfr275
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013; 41(2):464-471. doi:10.1097/CCM.0b013e31826ab3a1
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010; 5(10):1734–1744. doi:10.2215/CJN.02800310
- Garg AX, Devereaux PJ, Yusuf S, et al; CORONARY Investigators. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA 2014; 311(21):2191–2198. doi:10.1001/jama.2014.4952
- Kumar AB, Suneja M, Bayman EO, Weide GD, Tarasi M. Association between postoperative acute kidney injury and duration of cardiopulmonary bypass: a meta-analysis. J Cardiothorac Vasc Anesth 2012; 26(1):64–69. doi:10.1053/j.jvca.2011.07.007
- Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007; 107(6):892–902. doi:10.1097/01.anes.0000290588.29668.38
- Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
- Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014: 207(1):53–59. doi:10.1016/j.amjsurg.2013.04.006
- Gu W-J, Hou B-L, Kwong JS, et al. Association between intraoperative hypotension and 30-day mortality, major adverse cardiac events, and acute kidney injury after non-cardiac surgery: a meta-analysis of cohort studies. Int J Cardiol 2018; 258:68–73. doi:10.1016/j.ijcard.2018.01.137
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87(1):7–40. pmid:12575882
- Perrotti A, Miltgen G, Chevet-Noel A, et al. Neutrophil gelatinase-associated lipocalin as early predictor of acute kidney injury after cardiac surgery in adults with chronic kidney failure. Ann Thorac Surg 2015; 99(3):864–869. doi:10.1016/j.athoracsur.2014.10.011
- Doi K, Urata M, Katagiri D, et al. Plasma neutrophil gelatinase-associated lipocalin in acute kidney injury superimposed on chronic kidney disease after cardiac surgery: a multicenter prospective study. Crit Care 2013; 17(6):R270. doi:10.1186/cc13104
- Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
- Yao L, Young N, Liu H, et al. Evidence for preoperative aspirin improving major outcomes in patients with chronic kidney disease undergoing cardiac surgery: a cohort study. Ann Surg 2015; 261(1):207–212. doi:10.1097/SLA.0000000000000641
- Garg AX, Kurz A, Sessler DI, et al; POISE-2 Investigators. Aspirin and clonidine in non-cardiac surgery: acute kidney injury substudy protocol of the perioperative ischaemic evaluation (POISE) 2 randomised controlled trial. BMJ open 2014; 4(2):e004886. doi:10.1136/bmjopen-2014-004886
- He SJ, Liu Q, Li HQ, Tian F, Chen SY, Weng JX. Role of statins in preventing cardiac surgery-associated acute kidney injury: an updated meta-analysis of randomized controlled trials. Ther Clin Risk Manag 2018; 14:475–482. doi:10.2147/TCRM.S160298
- Tie HT, Luo MZ, Lin D, Zhang M, Wan JY, Wu QC. Erythropoietin administration for prevention of cardiac surgery-associated acute kidney injury: a meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg 2015; 48(1):32–39. doi:10.1093/ejcts/ezu378
- Santana-Santos E, Gowdak LH, Gaiotto FA, et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg 2014; 97(5):1617–1623. doi:10.1016/j.athoracsur.2014.01.056
- Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: a meta-analysis study. J Invest Surg 2018; 31(1):14–23. doi:10.1080/08941939.2016.1269853
- Sezai A, Hata M, Niino T, et al. Results of low-dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose HANP infusion therapy during cardiac surgery) trial for CKD. J Am Coll Cardiol 2011; 58(9):897–903. doi:10.1016/j.jacc.2011.03.056
- Xu N, Long Q, He T, et al. Association between preoperative renin-angiotensin system inhibitor use and postoperative acute kidney injury risk in patients with hypertension. Clin Nephrol 2018; 89(6):403–414. doi:10.5414/CN109319
- Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol 2018; 18(1):7. doi:10.1186/s12871-018-0472-1
- Shi R, Tie H-T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: a meta-analysis. Critical Care 2017; 21(1):198. doi:10.1186/s13054-017-1776-0
- Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2016; 67(3):408–416. doi:10.1053/j.ajkd.2015.09.015
- Elbadawi A, Elgendy IY, Saad M, et al. Meta-analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg 2018; 105(5):1403–1410. doi:10.1016/j.athoracsur.2017.11.027
- Zarbock A, Schmidt C, Van Aken H, et al; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313(21):2133–2141. doi:10.1001/jama.2015.4189
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis 2010; 56(6):1043–1049. doi:10.1053/j.ajkd.2010.07.014
- Futier E, Constantin JM, Petit A, et al. Conservative vs restrictive individualized goal-directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Arch Surg 2010; 145(12):1193–1200. doi:10.1001/archsurg.2010.275
- Patel A, Prowle JR, Ackland GL. Postoperative goal-directed therapy and development of acute kidney injury following major elective noncardiac surgery: post-hoc analysis of POM-O randomized controlled trial. Clin Kidney J 2017; 10(3):348–356. doi:10.1093/ckj/sfw118
- Shen Y, Zhang W, Cheng X, Ying M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: a retrospective cohort study. J Crit Care 2018; 44:273–277. doi:10.1016/j.jcrc.2017.11.041
KEY POINTS
- Many patients undergoing surgery have CKD—up to 30% in some cardiac surgery populations.
- CKD is a risk factor for perioperative complications including acute kidney injury and death.
- Although challenging, early detection of renal injury is crucial to improving outcomes in this patient population. New biomarkers are being investigated.
- Preoperative assessment and perioperative management of renal dysfunction may reduce the risk of adverse postoperative outcomes.
Long-acting injectable antipsychotics: What to do about missed doses
Antipsychotic agents are the mainstay of treatment for patients with schizophrenia,1-3 and when taken regularly, they can greatly improve patient outcomes. Unfortunately, many studies have documented poor adherence to antipsychotic regimens in patients with schizophrenia, which often leads to an exacerbation of symptoms and preventable hospitalizations.4-8 In order to improve adherence, many clinicians prescribe long-acting injectable antipsychotics (LAIAs).
LAIAs help improve adherence, but these benefits are seen only in patients who receive their injections within a specific time frame.9-11 LAIAs administered outside of this time frame (missed doses) can lead to reoccurrence or exacerbation of symptoms. This article explains how to adequately manage missed LAIA doses.
First-generation long-acting injectable antipsychotics
Two first-generation antipsychotics are available as a long-acting injectable formulation: haloperidol decanoate and fluphenazine decanoate. Due to the increased risk of extrapyramidal symptoms, use of these agents have decreased, and they are often less preferred than second-generation LAIAs. Furthermore, unlike many of the newer second-generation LAIAs, first-generation LAIAs lack literature on how to manage missed doses. Therefore, clinicians should analyze the pharmacokinetic properties of these agents (Table 112-28), as well as the patient’s medical history and clinical presentation, in order to determine how best to address missed doses.
Haloperidol decanoate plasma concentrations peak approximately 6 days after the injection.12 The medication has a half-life of 3 weeks. One study found that haloperidol plasma concentrations were detectable 13 weeks after the discontinuation of haloperidol decanoate.17 This same study also found that the change in plasma levels from 3 to 6 weeks after the last dose was minimal.17 Based on these findings, Figure 1 summarizes our recommendations for addressing missed haloperidol decanoate doses.
Fluphenazine decanoate levels peak 24 hours after the injection.18 An estimated therapeutic range for fluphenazine is 0.2 to 2 ng/mL.21-25 One study that evaluated fluphenazine decanoate levels following discontinuation after reaching steady state found there was no significant difference in plasma levels 6 weeks after the last dose of fluphenazine, but a significant decrease in levels 8 to 12 weeks after the last dose.26 Other studies found that fluphenazine levels were detectable 21 to 24 weeks following fluphenazine decanoate discontinuation.27,28 Based on these findings, Figure 2 summarizes our recommendations for addressing missed fluphenazine decanoate doses.
Continue to: Second-generation LAIAs
Second-generation LAIAs
Six second-generation LAIAs are available in the United States. Compared with the first-generation LAIAs, second-generation LAIAs have more extensive guidance on how to address missed doses.
Risperidone long-acting injection. When addressing missed doses of risperidone long-acting injection, first determine whether the medication has reached steady state. Steady state occurs approximately after the fourth consecutive injection (approximately 2 months).29
If a patient missed a dose but has not reached steady state, he or she should receive the next dose as well as oral antipsychotic supplementation for 3 weeks.30 If the patient has reached steady state and if it has been ≤6 weeks since the last injection, give the next injection as soon as possible. However, if steady state has been reached and it has been >6 weeks since the last injection, give the next injection, along with 3 weeks of oral antipsychotic supplementation (Figure 3).
Paliperidone palmitate monthly long-acting injection. Once the initiation dosing phase of paliperidone palmitate monthly long-acting injection (PP1M) is completed, the maintenance dose is administered every 4 weeks. When addressing missed doses of PP1M, first determine whether the patient is in the initiation or maintenance dosing phase.31
Initiation phase. Patients are in the initiation dosing phase during the first 2 injections of PP1M. During the initiation phase, the patient first receives 234 mg and then 156 mg 1 week later, both in the deltoid muscle. One month later, the patient receives a maintenance dose of PP1M (in the deltoid or gluteal muscle). The second initiation injection may be given 4 days before or after the scheduled administration date. The initiation doses should be adjusted in patients with mild renal function (creatinine clearance 50 to 80 mL/min).31 Figure 4 summarizes the guidance for addressing a missed or delayed second injection during the initiation phase.
Continue to: Maintenance phase
Maintenance phase. During the maintenance phase, PP1M can be administered 7 days before or after the monthly due date. If the patient has missed a maintenance injection and it has been <6 weeks since the last dose, the maintenance injection can be given as soon as possible (Figure 5).31 If it has been 6 weeks to 6 months since the last injection, the patient should receive their prescribed maintenance dose as soon as possible and the same dose 1 week later, with both injections in the deltoid muscle. Following the second dose, the patient can resume their regular monthly maintenance schedule, in either the deltoid or gluteal muscle. For example, if the patient was maintained on 117 mg of PP1M and it had been 8 weeks since the last injection, the patient should receive 117 mg immediately, then 117 mg 1 week later, then 117 mg 1 month later. An exception to this is if a patient’s maintenance dose is 234 mg monthly. In this case, the patient should receive 156 mg of PP1M immediately, then 156 mg 1 week later, and then 234 mg 1 month later.31 If it has been >6 months since the last dose, the patient should start the initiation schedule as if he or she were receiving a new medication.31
Paliperidone palmitate 3-month long-acting injection (PP3M) should be administered every 3 months. This injection can be given 2 weeks before or after the date of the scheduled dose.32
If the patient missed an injection and it has been <4 months since the last dose, the next scheduled dose should be given as soon as possible.32 If it has been 4 to 9 months since the last dose, the patient must return to PP1M for 2 booster injections 1 week apart. The dose of these PP1M booster injections depends on the dose of PP3M that the patient had been stabilized on:
- 78 mg if stabilized on 273 mg
- 117 mg if stabilized on 410 mg
- 156 mg if stabilized on 546 mg or 819 mg.32
After the second booster dose, PP3M can be restarted 1 month later.32 If it has been >9 months since the last PP3M dose, the patient should be restarted on PP1M. PP3M can be reconsidered once the patient has been stabilized on PP1M for ≥4 months (Figure 6).32
Continue to: Aripiprazole long-acting injection
Aripiprazole long-acting injection is administered every 4 weeks. If a patient misses an injection, first determine how many consecutive doses he or she has received.33 If the patient has missed the second or third injection, and it has been <5 weeks since the last dose, give the next injection as soon as possible. If it has been >5 weeks, give the next injection as soon as possible, plus oral aripiprazole supplementation for 2 weeks (Figure 7).
If the patient has received ≥4 consecutive doses and misses a dose and it has been <6 weeks since the last dose, administer an injection as soon as possible. If it has been >6 weeks since the last dose, give the next injection as soon as possible, plus with oral aripiprazole supplementation for 2 weeks.
Aripiprazole lauroxil long-acting injection. Depending on the dose, aripiprazole lauroxil can be administered monthly, every 6 weeks, or every 2 months. Aripiprazole lauroxil can be administered 14 days before or after the scheduled dose.34
The guidance for addressing missed or delayed doses of aripiprazole lauroxil differs depending on the dose the patient is stabilized on, and how long it has been since the last injection. Figure 8 summarizes how missed injections should be managed. When oral aripiprazole supplementation is needed, the following doses should be used:
- 10 mg/d if stabilized on 441 mg every month
- 15 mg/d if stabilized on 662 mg every month, 882 mg every 6 weeks, or 1,064 mg every 2 months
- 20 mg/d if stabilized on 882 mg every month.34
Olanzapine pamoate long-acting injection is a unique LAIA because it requires prescribers and patients to participate in a risk evaluation and mitigation strategies (REMS) program due the risk of post-injection delirium/sedation syndrome. It is administered every 2 to 4 weeks, with loading doses given for the first 2 months of treatment (Table 235). After 2 months, the patient can proceed to the maintenance dosing regimen.
Continue to: Currently, there is no concrete guidance...
Currently, there is no concrete guidance on how to address missed doses of olanzapine long-acting injection; however, the pharmacokinetics of this formulation allow flexibility in dosing intervals. Therapeutic levels are present after the first injection, and the medication reaches steady-state levels in 3 months.35-37 As a result of its specific formulation, olanzapine pamoate long-acting injection provides sustained olanzapine pamoate plasma concentrations between injections, and has a half-life of 30 days.35 Consequently, therapeutic levels of the medication are still present 2 to 4 weeks after an injection.37 Additionally, clinically relevant plasma concentrations may be present 2 to 3 months after the last injection.36
In light of this information, if a patient has not reached steady state and has missed an injection, he or she should receive the recommended loading dose schedule. If the patient has reached steady state and it has been ≤2 months since the last dose, he or she should receive the next dose as soon as possible. If steady state has been reached and it has been >2 months since the last injection, the patient should receive the recommended loading dosing for 2 months (Figure 9). Because of the risk of post-injection delirium/sedation syndrome, and because therapeutic levels are achieved after the first injection, oral olanzapine supplementation is not recommended.
Use a stepwise approach
In general, clinicians can use a stepwise approach to managing missed doses of LAIAs (Figure 10). First, establish the number of LAIA doses the patient had received prior to the last dose, and whether these injections were administered on schedule. This will help you determine if the patient is in the initiation or maintenance phase and/or has reached steady state. The second step is to establish the date of the last injection. Use objective tools, such as pharmacy records or the medical chart, to determine the date of the last injection, rather than relying on patient reporting. For the third step, calculate the time that has passed since the last LAIA dose. Once you have completed these steps, use the specific medication recommendations described in this article to address the missed dose.
Continue to: Address barriers to adherence
Address barriers to adherence
When addressing missed LAIA doses, be sure to identify any barriers that may have led to a missed injection. These might include:
- bothersome adverse effects
- transportation difficulties
- issues with insurance/medication coverage
- comorbidities (ie, alcohol/substance use disorders)
- cognitive and functional impairment caused by the patient’s illness
- difficulty with keeping track of appointments.
Clinicians can work closely with patients and/or caregivers to address any barriers to ensure that patients receive their injections in a timely fashion.
The goal: Reducing relapse
LAIAs improve medication adherence. Although nonadherence is less frequent with LAIAs than with oral antipsychotics, when a LAIA dose is missed, it is important to properly follow a stepwise approach based on the unique properties of the specific LAIA prescribed. Proper management of LAIA missed doses can prevent relapse and reoccurrence of schizophrenia symptoms, thus possibly avoiding future hospitalizations.
Acknowledgments
The authors thank Brian Tschosik, JD, Mary Collen O’Rourke, MD, and Amanda Holloway, MD, for their assistance with this article.
Bottom Line
Although long-acting injectable antipsychotics (LAIAs) greatly assist with adherence, these agents are effective only when missed doses are avoided. When addressing missed LAIA doses, use a stepwise approach that takes into consideration the unique properties of the specific LAIA prescribed.
Related Resources
- Haddad P, Lambert T, Lauriello J, eds. Antipsychotic long-acting injections. 2nd ed. Oxford, UK: Oxford University Press; 2016.
- Diefenderfer LA. When should you consider combining 2 long-acting injectable antipsychotics? Current Psychiatry. 2017;16(10):42-46.
Drug Brand Names
Aripiprazole long-acting injection • Abilify Maintena
Aripiprazole lauroxil long-acting injection • Aristada
Fluphenazine decanoate • Prolixin decanoate
Haloperidol decanoate • Haldol decanoate
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month long-acting injection • Invega Trinza
Risperidone long-acting injection • Risperdal Consta
1. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;195(52):S63-S67.
4. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
5. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
6. Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346(8973):477-481.
7. de Sena EP, Santos-Jesus R, Miranda-Scippa Â, et al. Relapse in patients with schizophrenia: a comparison between risperidone and haloperidol. Rev Bras Psiquiatr. 2003;25(4):220-223.
8. Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr Dis Treat. 2007;3(1):13-39.
9. Lafeuille MH, Frois C, Cloutier M, et al. Factors associated with adherence to the HEDIS Quality Measure in medicaid patients with schizophrenia. Am Health Drug Benefits. 2016;9(7):399-410.
10. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
11. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
12. Haldol Decanoate injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
13. Magliozzi JR, Hollister LE, Arnold KV, et al. Relationship of serum haloperidol levels to clinical response in schizophrenic patients. Am J Psychiatry. 1981;138(3):365-367.
14. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology (Berl). 1983;81(4):354-356.
15. Reyntigens AJ, Heykants JJ, Woestenborghs RJ, et al. Pharmacokinetics of haloperidol decanoate. A 2-year follow-up. Int Pharmacopsychiatry. 1982;17(4):238-246.
16. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
17. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
18. Ereshefsky L, Saklad SR, Jann MW. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry.1984;45(5 pt 2):50-58.
19. Marder SR, Hawes EM, Van Putten T, et al. Fluphenazine plasma levels in patients receiving low and conventional doses of fluphenazine decanoate. Psychopharmacology (Berl). 1986;88(4):480-483.
20. Marder SR, Hubbard JW, Van Putten T, et al. Pharmacokinetics of long-acting injectable neuroleptic drugs: clinical implications. Psychopharmacology (Berl). 1989;98(4):433-439.
21. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Fluphenazine plasma levels and clinical response. J Clin Psychiatry. 1984;45(9):370-373.
22. Balant-Gorgia AE, Balant LP, Andreoli A. Pharmacokinetic optimisation of the treatment of psychosis. Clin Pharmacokinet. 1993;25(3):217-236.
23. Van Putten T, Marder SR, Wirshing WC, et al. Neuroleptic plasma levels. Schizophr Bull. 1991;17(2):197-216.
24. Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet. 1986;11(1):36-61.
25. Miller RS, Peterson GM, McLean S, et al. Monitoring plasma levels of fluphenazine during chronic therapy with fluphenazine decanoate. J Clin Pharm Ther. 1995;20(2):55-62.
26. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
27. Wistedt B, Wiles D, Kolakowska T. Slow decline of plasma drug and prolactin levels after discontinuation of chronic treatment with depot neuroleptics. Lancet. 1981;1(8230):1163.
28. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology (Berl). 1982;78(4):301-304.
29. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
30. Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry. 2003;64(suppl 16):41-46.
31. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
32. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
33. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; December 2016.
34. Artistada [package insert]. Waltham, MA: Alkermes, Inc.; June 2017.
35. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; February 2017.
36. Heres S, Kraemer S, Bergstrom RF, et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299-312.
37. Detke HC, Zhao F, Garhyan P, et al. Dose correspondence between olanzapine long-acting injection and oral olanzapine: recommendations for switching. Int Clin Psychopharmacol. 2011;26(1):35-42.
Antipsychotic agents are the mainstay of treatment for patients with schizophrenia,1-3 and when taken regularly, they can greatly improve patient outcomes. Unfortunately, many studies have documented poor adherence to antipsychotic regimens in patients with schizophrenia, which often leads to an exacerbation of symptoms and preventable hospitalizations.4-8 In order to improve adherence, many clinicians prescribe long-acting injectable antipsychotics (LAIAs).
LAIAs help improve adherence, but these benefits are seen only in patients who receive their injections within a specific time frame.9-11 LAIAs administered outside of this time frame (missed doses) can lead to reoccurrence or exacerbation of symptoms. This article explains how to adequately manage missed LAIA doses.
First-generation long-acting injectable antipsychotics
Two first-generation antipsychotics are available as a long-acting injectable formulation: haloperidol decanoate and fluphenazine decanoate. Due to the increased risk of extrapyramidal symptoms, use of these agents have decreased, and they are often less preferred than second-generation LAIAs. Furthermore, unlike many of the newer second-generation LAIAs, first-generation LAIAs lack literature on how to manage missed doses. Therefore, clinicians should analyze the pharmacokinetic properties of these agents (Table 112-28), as well as the patient’s medical history and clinical presentation, in order to determine how best to address missed doses.
Haloperidol decanoate plasma concentrations peak approximately 6 days after the injection.12 The medication has a half-life of 3 weeks. One study found that haloperidol plasma concentrations were detectable 13 weeks after the discontinuation of haloperidol decanoate.17 This same study also found that the change in plasma levels from 3 to 6 weeks after the last dose was minimal.17 Based on these findings, Figure 1 summarizes our recommendations for addressing missed haloperidol decanoate doses.
Fluphenazine decanoate levels peak 24 hours after the injection.18 An estimated therapeutic range for fluphenazine is 0.2 to 2 ng/mL.21-25 One study that evaluated fluphenazine decanoate levels following discontinuation after reaching steady state found there was no significant difference in plasma levels 6 weeks after the last dose of fluphenazine, but a significant decrease in levels 8 to 12 weeks after the last dose.26 Other studies found that fluphenazine levels were detectable 21 to 24 weeks following fluphenazine decanoate discontinuation.27,28 Based on these findings, Figure 2 summarizes our recommendations for addressing missed fluphenazine decanoate doses.
Continue to: Second-generation LAIAs
Second-generation LAIAs
Six second-generation LAIAs are available in the United States. Compared with the first-generation LAIAs, second-generation LAIAs have more extensive guidance on how to address missed doses.
Risperidone long-acting injection. When addressing missed doses of risperidone long-acting injection, first determine whether the medication has reached steady state. Steady state occurs approximately after the fourth consecutive injection (approximately 2 months).29
If a patient missed a dose but has not reached steady state, he or she should receive the next dose as well as oral antipsychotic supplementation for 3 weeks.30 If the patient has reached steady state and if it has been ≤6 weeks since the last injection, give the next injection as soon as possible. However, if steady state has been reached and it has been >6 weeks since the last injection, give the next injection, along with 3 weeks of oral antipsychotic supplementation (Figure 3).
Paliperidone palmitate monthly long-acting injection. Once the initiation dosing phase of paliperidone palmitate monthly long-acting injection (PP1M) is completed, the maintenance dose is administered every 4 weeks. When addressing missed doses of PP1M, first determine whether the patient is in the initiation or maintenance dosing phase.31
Initiation phase. Patients are in the initiation dosing phase during the first 2 injections of PP1M. During the initiation phase, the patient first receives 234 mg and then 156 mg 1 week later, both in the deltoid muscle. One month later, the patient receives a maintenance dose of PP1M (in the deltoid or gluteal muscle). The second initiation injection may be given 4 days before or after the scheduled administration date. The initiation doses should be adjusted in patients with mild renal function (creatinine clearance 50 to 80 mL/min).31 Figure 4 summarizes the guidance for addressing a missed or delayed second injection during the initiation phase.
Continue to: Maintenance phase
Maintenance phase. During the maintenance phase, PP1M can be administered 7 days before or after the monthly due date. If the patient has missed a maintenance injection and it has been <6 weeks since the last dose, the maintenance injection can be given as soon as possible (Figure 5).31 If it has been 6 weeks to 6 months since the last injection, the patient should receive their prescribed maintenance dose as soon as possible and the same dose 1 week later, with both injections in the deltoid muscle. Following the second dose, the patient can resume their regular monthly maintenance schedule, in either the deltoid or gluteal muscle. For example, if the patient was maintained on 117 mg of PP1M and it had been 8 weeks since the last injection, the patient should receive 117 mg immediately, then 117 mg 1 week later, then 117 mg 1 month later. An exception to this is if a patient’s maintenance dose is 234 mg monthly. In this case, the patient should receive 156 mg of PP1M immediately, then 156 mg 1 week later, and then 234 mg 1 month later.31 If it has been >6 months since the last dose, the patient should start the initiation schedule as if he or she were receiving a new medication.31
Paliperidone palmitate 3-month long-acting injection (PP3M) should be administered every 3 months. This injection can be given 2 weeks before or after the date of the scheduled dose.32
If the patient missed an injection and it has been <4 months since the last dose, the next scheduled dose should be given as soon as possible.32 If it has been 4 to 9 months since the last dose, the patient must return to PP1M for 2 booster injections 1 week apart. The dose of these PP1M booster injections depends on the dose of PP3M that the patient had been stabilized on:
- 78 mg if stabilized on 273 mg
- 117 mg if stabilized on 410 mg
- 156 mg if stabilized on 546 mg or 819 mg.32
After the second booster dose, PP3M can be restarted 1 month later.32 If it has been >9 months since the last PP3M dose, the patient should be restarted on PP1M. PP3M can be reconsidered once the patient has been stabilized on PP1M for ≥4 months (Figure 6).32
Continue to: Aripiprazole long-acting injection
Aripiprazole long-acting injection is administered every 4 weeks. If a patient misses an injection, first determine how many consecutive doses he or she has received.33 If the patient has missed the second or third injection, and it has been <5 weeks since the last dose, give the next injection as soon as possible. If it has been >5 weeks, give the next injection as soon as possible, plus oral aripiprazole supplementation for 2 weeks (Figure 7).
If the patient has received ≥4 consecutive doses and misses a dose and it has been <6 weeks since the last dose, administer an injection as soon as possible. If it has been >6 weeks since the last dose, give the next injection as soon as possible, plus with oral aripiprazole supplementation for 2 weeks.
Aripiprazole lauroxil long-acting injection. Depending on the dose, aripiprazole lauroxil can be administered monthly, every 6 weeks, or every 2 months. Aripiprazole lauroxil can be administered 14 days before or after the scheduled dose.34
The guidance for addressing missed or delayed doses of aripiprazole lauroxil differs depending on the dose the patient is stabilized on, and how long it has been since the last injection. Figure 8 summarizes how missed injections should be managed. When oral aripiprazole supplementation is needed, the following doses should be used:
- 10 mg/d if stabilized on 441 mg every month
- 15 mg/d if stabilized on 662 mg every month, 882 mg every 6 weeks, or 1,064 mg every 2 months
- 20 mg/d if stabilized on 882 mg every month.34
Olanzapine pamoate long-acting injection is a unique LAIA because it requires prescribers and patients to participate in a risk evaluation and mitigation strategies (REMS) program due the risk of post-injection delirium/sedation syndrome. It is administered every 2 to 4 weeks, with loading doses given for the first 2 months of treatment (Table 235). After 2 months, the patient can proceed to the maintenance dosing regimen.
Continue to: Currently, there is no concrete guidance...
Currently, there is no concrete guidance on how to address missed doses of olanzapine long-acting injection; however, the pharmacokinetics of this formulation allow flexibility in dosing intervals. Therapeutic levels are present after the first injection, and the medication reaches steady-state levels in 3 months.35-37 As a result of its specific formulation, olanzapine pamoate long-acting injection provides sustained olanzapine pamoate plasma concentrations between injections, and has a half-life of 30 days.35 Consequently, therapeutic levels of the medication are still present 2 to 4 weeks after an injection.37 Additionally, clinically relevant plasma concentrations may be present 2 to 3 months after the last injection.36
In light of this information, if a patient has not reached steady state and has missed an injection, he or she should receive the recommended loading dose schedule. If the patient has reached steady state and it has been ≤2 months since the last dose, he or she should receive the next dose as soon as possible. If steady state has been reached and it has been >2 months since the last injection, the patient should receive the recommended loading dosing for 2 months (Figure 9). Because of the risk of post-injection delirium/sedation syndrome, and because therapeutic levels are achieved after the first injection, oral olanzapine supplementation is not recommended.
Use a stepwise approach
In general, clinicians can use a stepwise approach to managing missed doses of LAIAs (Figure 10). First, establish the number of LAIA doses the patient had received prior to the last dose, and whether these injections were administered on schedule. This will help you determine if the patient is in the initiation or maintenance phase and/or has reached steady state. The second step is to establish the date of the last injection. Use objective tools, such as pharmacy records or the medical chart, to determine the date of the last injection, rather than relying on patient reporting. For the third step, calculate the time that has passed since the last LAIA dose. Once you have completed these steps, use the specific medication recommendations described in this article to address the missed dose.
Continue to: Address barriers to adherence
Address barriers to adherence
When addressing missed LAIA doses, be sure to identify any barriers that may have led to a missed injection. These might include:
- bothersome adverse effects
- transportation difficulties
- issues with insurance/medication coverage
- comorbidities (ie, alcohol/substance use disorders)
- cognitive and functional impairment caused by the patient’s illness
- difficulty with keeping track of appointments.
Clinicians can work closely with patients and/or caregivers to address any barriers to ensure that patients receive their injections in a timely fashion.
The goal: Reducing relapse
LAIAs improve medication adherence. Although nonadherence is less frequent with LAIAs than with oral antipsychotics, when a LAIA dose is missed, it is important to properly follow a stepwise approach based on the unique properties of the specific LAIA prescribed. Proper management of LAIA missed doses can prevent relapse and reoccurrence of schizophrenia symptoms, thus possibly avoiding future hospitalizations.
Acknowledgments
The authors thank Brian Tschosik, JD, Mary Collen O’Rourke, MD, and Amanda Holloway, MD, for their assistance with this article.
Bottom Line
Although long-acting injectable antipsychotics (LAIAs) greatly assist with adherence, these agents are effective only when missed doses are avoided. When addressing missed LAIA doses, use a stepwise approach that takes into consideration the unique properties of the specific LAIA prescribed.
Related Resources
- Haddad P, Lambert T, Lauriello J, eds. Antipsychotic long-acting injections. 2nd ed. Oxford, UK: Oxford University Press; 2016.
- Diefenderfer LA. When should you consider combining 2 long-acting injectable antipsychotics? Current Psychiatry. 2017;16(10):42-46.
Drug Brand Names
Aripiprazole long-acting injection • Abilify Maintena
Aripiprazole lauroxil long-acting injection • Aristada
Fluphenazine decanoate • Prolixin decanoate
Haloperidol decanoate • Haldol decanoate
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month long-acting injection • Invega Trinza
Risperidone long-acting injection • Risperdal Consta
Antipsychotic agents are the mainstay of treatment for patients with schizophrenia,1-3 and when taken regularly, they can greatly improve patient outcomes. Unfortunately, many studies have documented poor adherence to antipsychotic regimens in patients with schizophrenia, which often leads to an exacerbation of symptoms and preventable hospitalizations.4-8 In order to improve adherence, many clinicians prescribe long-acting injectable antipsychotics (LAIAs).
LAIAs help improve adherence, but these benefits are seen only in patients who receive their injections within a specific time frame.9-11 LAIAs administered outside of this time frame (missed doses) can lead to reoccurrence or exacerbation of symptoms. This article explains how to adequately manage missed LAIA doses.
First-generation long-acting injectable antipsychotics
Two first-generation antipsychotics are available as a long-acting injectable formulation: haloperidol decanoate and fluphenazine decanoate. Due to the increased risk of extrapyramidal symptoms, use of these agents have decreased, and they are often less preferred than second-generation LAIAs. Furthermore, unlike many of the newer second-generation LAIAs, first-generation LAIAs lack literature on how to manage missed doses. Therefore, clinicians should analyze the pharmacokinetic properties of these agents (Table 112-28), as well as the patient’s medical history and clinical presentation, in order to determine how best to address missed doses.
Haloperidol decanoate plasma concentrations peak approximately 6 days after the injection.12 The medication has a half-life of 3 weeks. One study found that haloperidol plasma concentrations were detectable 13 weeks after the discontinuation of haloperidol decanoate.17 This same study also found that the change in plasma levels from 3 to 6 weeks after the last dose was minimal.17 Based on these findings, Figure 1 summarizes our recommendations for addressing missed haloperidol decanoate doses.
Fluphenazine decanoate levels peak 24 hours after the injection.18 An estimated therapeutic range for fluphenazine is 0.2 to 2 ng/mL.21-25 One study that evaluated fluphenazine decanoate levels following discontinuation after reaching steady state found there was no significant difference in plasma levels 6 weeks after the last dose of fluphenazine, but a significant decrease in levels 8 to 12 weeks after the last dose.26 Other studies found that fluphenazine levels were detectable 21 to 24 weeks following fluphenazine decanoate discontinuation.27,28 Based on these findings, Figure 2 summarizes our recommendations for addressing missed fluphenazine decanoate doses.
Continue to: Second-generation LAIAs
Second-generation LAIAs
Six second-generation LAIAs are available in the United States. Compared with the first-generation LAIAs, second-generation LAIAs have more extensive guidance on how to address missed doses.
Risperidone long-acting injection. When addressing missed doses of risperidone long-acting injection, first determine whether the medication has reached steady state. Steady state occurs approximately after the fourth consecutive injection (approximately 2 months).29
If a patient missed a dose but has not reached steady state, he or she should receive the next dose as well as oral antipsychotic supplementation for 3 weeks.30 If the patient has reached steady state and if it has been ≤6 weeks since the last injection, give the next injection as soon as possible. However, if steady state has been reached and it has been >6 weeks since the last injection, give the next injection, along with 3 weeks of oral antipsychotic supplementation (Figure 3).
Paliperidone palmitate monthly long-acting injection. Once the initiation dosing phase of paliperidone palmitate monthly long-acting injection (PP1M) is completed, the maintenance dose is administered every 4 weeks. When addressing missed doses of PP1M, first determine whether the patient is in the initiation or maintenance dosing phase.31
Initiation phase. Patients are in the initiation dosing phase during the first 2 injections of PP1M. During the initiation phase, the patient first receives 234 mg and then 156 mg 1 week later, both in the deltoid muscle. One month later, the patient receives a maintenance dose of PP1M (in the deltoid or gluteal muscle). The second initiation injection may be given 4 days before or after the scheduled administration date. The initiation doses should be adjusted in patients with mild renal function (creatinine clearance 50 to 80 mL/min).31 Figure 4 summarizes the guidance for addressing a missed or delayed second injection during the initiation phase.
Continue to: Maintenance phase
Maintenance phase. During the maintenance phase, PP1M can be administered 7 days before or after the monthly due date. If the patient has missed a maintenance injection and it has been <6 weeks since the last dose, the maintenance injection can be given as soon as possible (Figure 5).31 If it has been 6 weeks to 6 months since the last injection, the patient should receive their prescribed maintenance dose as soon as possible and the same dose 1 week later, with both injections in the deltoid muscle. Following the second dose, the patient can resume their regular monthly maintenance schedule, in either the deltoid or gluteal muscle. For example, if the patient was maintained on 117 mg of PP1M and it had been 8 weeks since the last injection, the patient should receive 117 mg immediately, then 117 mg 1 week later, then 117 mg 1 month later. An exception to this is if a patient’s maintenance dose is 234 mg monthly. In this case, the patient should receive 156 mg of PP1M immediately, then 156 mg 1 week later, and then 234 mg 1 month later.31 If it has been >6 months since the last dose, the patient should start the initiation schedule as if he or she were receiving a new medication.31
Paliperidone palmitate 3-month long-acting injection (PP3M) should be administered every 3 months. This injection can be given 2 weeks before or after the date of the scheduled dose.32
If the patient missed an injection and it has been <4 months since the last dose, the next scheduled dose should be given as soon as possible.32 If it has been 4 to 9 months since the last dose, the patient must return to PP1M for 2 booster injections 1 week apart. The dose of these PP1M booster injections depends on the dose of PP3M that the patient had been stabilized on:
- 78 mg if stabilized on 273 mg
- 117 mg if stabilized on 410 mg
- 156 mg if stabilized on 546 mg or 819 mg.32
After the second booster dose, PP3M can be restarted 1 month later.32 If it has been >9 months since the last PP3M dose, the patient should be restarted on PP1M. PP3M can be reconsidered once the patient has been stabilized on PP1M for ≥4 months (Figure 6).32
Continue to: Aripiprazole long-acting injection
Aripiprazole long-acting injection is administered every 4 weeks. If a patient misses an injection, first determine how many consecutive doses he or she has received.33 If the patient has missed the second or third injection, and it has been <5 weeks since the last dose, give the next injection as soon as possible. If it has been >5 weeks, give the next injection as soon as possible, plus oral aripiprazole supplementation for 2 weeks (Figure 7).
If the patient has received ≥4 consecutive doses and misses a dose and it has been <6 weeks since the last dose, administer an injection as soon as possible. If it has been >6 weeks since the last dose, give the next injection as soon as possible, plus with oral aripiprazole supplementation for 2 weeks.
Aripiprazole lauroxil long-acting injection. Depending on the dose, aripiprazole lauroxil can be administered monthly, every 6 weeks, or every 2 months. Aripiprazole lauroxil can be administered 14 days before or after the scheduled dose.34
The guidance for addressing missed or delayed doses of aripiprazole lauroxil differs depending on the dose the patient is stabilized on, and how long it has been since the last injection. Figure 8 summarizes how missed injections should be managed. When oral aripiprazole supplementation is needed, the following doses should be used:
- 10 mg/d if stabilized on 441 mg every month
- 15 mg/d if stabilized on 662 mg every month, 882 mg every 6 weeks, or 1,064 mg every 2 months
- 20 mg/d if stabilized on 882 mg every month.34
Olanzapine pamoate long-acting injection is a unique LAIA because it requires prescribers and patients to participate in a risk evaluation and mitigation strategies (REMS) program due the risk of post-injection delirium/sedation syndrome. It is administered every 2 to 4 weeks, with loading doses given for the first 2 months of treatment (Table 235). After 2 months, the patient can proceed to the maintenance dosing regimen.
Continue to: Currently, there is no concrete guidance...
Currently, there is no concrete guidance on how to address missed doses of olanzapine long-acting injection; however, the pharmacokinetics of this formulation allow flexibility in dosing intervals. Therapeutic levels are present after the first injection, and the medication reaches steady-state levels in 3 months.35-37 As a result of its specific formulation, olanzapine pamoate long-acting injection provides sustained olanzapine pamoate plasma concentrations between injections, and has a half-life of 30 days.35 Consequently, therapeutic levels of the medication are still present 2 to 4 weeks after an injection.37 Additionally, clinically relevant plasma concentrations may be present 2 to 3 months after the last injection.36
In light of this information, if a patient has not reached steady state and has missed an injection, he or she should receive the recommended loading dose schedule. If the patient has reached steady state and it has been ≤2 months since the last dose, he or she should receive the next dose as soon as possible. If steady state has been reached and it has been >2 months since the last injection, the patient should receive the recommended loading dosing for 2 months (Figure 9). Because of the risk of post-injection delirium/sedation syndrome, and because therapeutic levels are achieved after the first injection, oral olanzapine supplementation is not recommended.
Use a stepwise approach
In general, clinicians can use a stepwise approach to managing missed doses of LAIAs (Figure 10). First, establish the number of LAIA doses the patient had received prior to the last dose, and whether these injections were administered on schedule. This will help you determine if the patient is in the initiation or maintenance phase and/or has reached steady state. The second step is to establish the date of the last injection. Use objective tools, such as pharmacy records or the medical chart, to determine the date of the last injection, rather than relying on patient reporting. For the third step, calculate the time that has passed since the last LAIA dose. Once you have completed these steps, use the specific medication recommendations described in this article to address the missed dose.
Continue to: Address barriers to adherence
Address barriers to adherence
When addressing missed LAIA doses, be sure to identify any barriers that may have led to a missed injection. These might include:
- bothersome adverse effects
- transportation difficulties
- issues with insurance/medication coverage
- comorbidities (ie, alcohol/substance use disorders)
- cognitive and functional impairment caused by the patient’s illness
- difficulty with keeping track of appointments.
Clinicians can work closely with patients and/or caregivers to address any barriers to ensure that patients receive their injections in a timely fashion.
The goal: Reducing relapse
LAIAs improve medication adherence. Although nonadherence is less frequent with LAIAs than with oral antipsychotics, when a LAIA dose is missed, it is important to properly follow a stepwise approach based on the unique properties of the specific LAIA prescribed. Proper management of LAIA missed doses can prevent relapse and reoccurrence of schizophrenia symptoms, thus possibly avoiding future hospitalizations.
Acknowledgments
The authors thank Brian Tschosik, JD, Mary Collen O’Rourke, MD, and Amanda Holloway, MD, for their assistance with this article.
Bottom Line
Although long-acting injectable antipsychotics (LAIAs) greatly assist with adherence, these agents are effective only when missed doses are avoided. When addressing missed LAIA doses, use a stepwise approach that takes into consideration the unique properties of the specific LAIA prescribed.
Related Resources
- Haddad P, Lambert T, Lauriello J, eds. Antipsychotic long-acting injections. 2nd ed. Oxford, UK: Oxford University Press; 2016.
- Diefenderfer LA. When should you consider combining 2 long-acting injectable antipsychotics? Current Psychiatry. 2017;16(10):42-46.
Drug Brand Names
Aripiprazole long-acting injection • Abilify Maintena
Aripiprazole lauroxil long-acting injection • Aristada
Fluphenazine decanoate • Prolixin decanoate
Haloperidol decanoate • Haldol decanoate
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month long-acting injection • Invega Trinza
Risperidone long-acting injection • Risperdal Consta
1. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;195(52):S63-S67.
4. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
5. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
6. Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346(8973):477-481.
7. de Sena EP, Santos-Jesus R, Miranda-Scippa Â, et al. Relapse in patients with schizophrenia: a comparison between risperidone and haloperidol. Rev Bras Psiquiatr. 2003;25(4):220-223.
8. Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr Dis Treat. 2007;3(1):13-39.
9. Lafeuille MH, Frois C, Cloutier M, et al. Factors associated with adherence to the HEDIS Quality Measure in medicaid patients with schizophrenia. Am Health Drug Benefits. 2016;9(7):399-410.
10. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
11. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
12. Haldol Decanoate injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
13. Magliozzi JR, Hollister LE, Arnold KV, et al. Relationship of serum haloperidol levels to clinical response in schizophrenic patients. Am J Psychiatry. 1981;138(3):365-367.
14. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology (Berl). 1983;81(4):354-356.
15. Reyntigens AJ, Heykants JJ, Woestenborghs RJ, et al. Pharmacokinetics of haloperidol decanoate. A 2-year follow-up. Int Pharmacopsychiatry. 1982;17(4):238-246.
16. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
17. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
18. Ereshefsky L, Saklad SR, Jann MW. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry.1984;45(5 pt 2):50-58.
19. Marder SR, Hawes EM, Van Putten T, et al. Fluphenazine plasma levels in patients receiving low and conventional doses of fluphenazine decanoate. Psychopharmacology (Berl). 1986;88(4):480-483.
20. Marder SR, Hubbard JW, Van Putten T, et al. Pharmacokinetics of long-acting injectable neuroleptic drugs: clinical implications. Psychopharmacology (Berl). 1989;98(4):433-439.
21. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Fluphenazine plasma levels and clinical response. J Clin Psychiatry. 1984;45(9):370-373.
22. Balant-Gorgia AE, Balant LP, Andreoli A. Pharmacokinetic optimisation of the treatment of psychosis. Clin Pharmacokinet. 1993;25(3):217-236.
23. Van Putten T, Marder SR, Wirshing WC, et al. Neuroleptic plasma levels. Schizophr Bull. 1991;17(2):197-216.
24. Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet. 1986;11(1):36-61.
25. Miller RS, Peterson GM, McLean S, et al. Monitoring plasma levels of fluphenazine during chronic therapy with fluphenazine decanoate. J Clin Pharm Ther. 1995;20(2):55-62.
26. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
27. Wistedt B, Wiles D, Kolakowska T. Slow decline of plasma drug and prolactin levels after discontinuation of chronic treatment with depot neuroleptics. Lancet. 1981;1(8230):1163.
28. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology (Berl). 1982;78(4):301-304.
29. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
30. Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry. 2003;64(suppl 16):41-46.
31. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
32. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
33. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; December 2016.
34. Artistada [package insert]. Waltham, MA: Alkermes, Inc.; June 2017.
35. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; February 2017.
36. Heres S, Kraemer S, Bergstrom RF, et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299-312.
37. Detke HC, Zhao F, Garhyan P, et al. Dose correspondence between olanzapine long-acting injection and oral olanzapine: recommendations for switching. Int Clin Psychopharmacol. 2011;26(1):35-42.
1. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
2. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;195(52):S63-S67.
4. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306-324.
5. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
6. Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet. 1995;346(8973):477-481.
7. de Sena EP, Santos-Jesus R, Miranda-Scippa Â, et al. Relapse in patients with schizophrenia: a comparison between risperidone and haloperidol. Rev Bras Psiquiatr. 2003;25(4):220-223.
8. Chue P. Long-acting risperidone injection: efficacy, safety, and cost-effectiveness of the first long-acting atypical antipsychotic. Neuropsychiatr Dis Treat. 2007;3(1):13-39.
9. Lafeuille MH, Frois C, Cloutier M, et al. Factors associated with adherence to the HEDIS Quality Measure in medicaid patients with schizophrenia. Am Health Drug Benefits. 2016;9(7):399-410.
10. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
11. Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754-768.
12. Haldol Decanoate injection [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
13. Magliozzi JR, Hollister LE, Arnold KV, et al. Relationship of serum haloperidol levels to clinical response in schizophrenic patients. Am J Psychiatry. 1981;138(3):365-367.
14. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Clinical response and plasma haloperidol levels in schizophrenia. Psychopharmacology (Berl). 1983;81(4):354-356.
15. Reyntigens AJ, Heykants JJ, Woestenborghs RJ, et al. Pharmacokinetics of haloperidol decanoate. A 2-year follow-up. Int Pharmacopsychiatry. 1982;17(4):238-246.
16. Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10(4):315-333.
17. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
18. Ereshefsky L, Saklad SR, Jann MW. Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches. J Clin Psychiatry.1984;45(5 pt 2):50-58.
19. Marder SR, Hawes EM, Van Putten T, et al. Fluphenazine plasma levels in patients receiving low and conventional doses of fluphenazine decanoate. Psychopharmacology (Berl). 1986;88(4):480-483.
20. Marder SR, Hubbard JW, Van Putten T, et al. Pharmacokinetics of long-acting injectable neuroleptic drugs: clinical implications. Psychopharmacology (Berl). 1989;98(4):433-439.
21. Mavroidis ML, Kanter DR, Hirschowitz J, et al. Fluphenazine plasma levels and clinical response. J Clin Psychiatry. 1984;45(9):370-373.
22. Balant-Gorgia AE, Balant LP, Andreoli A. Pharmacokinetic optimisation of the treatment of psychosis. Clin Pharmacokinet. 1993;25(3):217-236.
23. Van Putten T, Marder SR, Wirshing WC, et al. Neuroleptic plasma levels. Schizophr Bull. 1991;17(2):197-216.
24. Dahl SG. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin Pharmacokinet. 1986;11(1):36-61.
25. Miller RS, Peterson GM, McLean S, et al. Monitoring plasma levels of fluphenazine during chronic therapy with fluphenazine decanoate. J Clin Pharm Ther. 1995;20(2):55-62.
26. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
27. Wistedt B, Wiles D, Kolakowska T. Slow decline of plasma drug and prolactin levels after discontinuation of chronic treatment with depot neuroleptics. Lancet. 1981;1(8230):1163.
28. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology (Berl). 1982;78(4):301-304.
29. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
30. Marder SR, Conley R, Ereshefsky L, et al. Clinical guidelines: dosing and switching strategies for long-acting risperidone. J Clin Psychiatry. 2003;64(suppl 16):41-46.
31. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
32. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; February 2017.
33. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; December 2016.
34. Artistada [package insert]. Waltham, MA: Alkermes, Inc.; June 2017.
35. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; February 2017.
36. Heres S, Kraemer S, Bergstrom RF, et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299-312.
37. Detke HC, Zhao F, Garhyan P, et al. Dose correspondence between olanzapine long-acting injection and oral olanzapine: recommendations for switching. Int Clin Psychopharmacol. 2011;26(1):35-42.
Neuropsychiatric symptoms of dementia: Monotherapy, or combination therapy?
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors
Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors
Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
More than 5 million older Americans are living with Alzheimer’s disease and related dementias—and this number is estimated to rise to almost 14 million by 2050.1 Dementia is associated with high costs for the patient, family, and society. In 2017, nearly 16.1 million caregivers assisted older adults with dementia, devoting more than 18.2 billion hours per year in care.1 In the United States, the cost of caring for individuals with dementia is expected to reach $277 billion in 2018. Additionally, Medicare and Medicaid are expected to pay 67% of the estimated 2018 cost, and 22% is expected to come out of the pockets of patients and their caregivers.1
Although dementia is often viewed as a memory loss disease, neuropsychiatric symptoms (NPS) are common. NPS includes distressing behaviors, such as aggression and wandering, that increase caregiver burden, escalate the cost of care, and contribute to premature institutionalization. This article examines the evidence for the use of a combination of a cholinesterase inhibitor and memantine vs use of either medication alone for treating NPS of Alzheimer’s disease and other types of dementia.
First, rule out reversible causes of NPS
There are no disease-modifying treatments for dementia1; therefore, clinicians focus on decreasing patients’ suffering and improving their quality of life. Nearly all patients with dementia will develop at least one NPS. These commonly include auditory and visual hallucinations, delusions, depression, anxiety, psychosis, psychomotor agitation, aggression, apathy, repetitive questioning, wandering, socially or sexually inappropriate behaviors, and sleep disturbances.2 The underlying cause of these behaviors may be neurobiological,3 an acute medical condition, unmet needs or a pre-existing personality disorder, or other psychiatric illness.2 Because of this complexity, there is no specific treatment for NPS of dementia. Treatment should begin with an assessment to rule out potentially reversible causes of NPS, such as a urinary tract infection, environmental triggers, unmet needs, or untreated psychiatric illness. For mild to moderate NPS, short-term behavioral interventions, followed by pharmacologic interventions, are used. For moderate to severe NPS, pharmacologic interventions and behavioral interventions are often used simultaneously.
Pharmacologic options for treating NPS
The classes of medications frequently used to treat NPS include antidepressants, antipsychotics, mood stabilizers, and memory-enhancing, dementia-specific agents (cholinesterase inhibitors and the N-methyl-
Antipsychotic medications are typically reserved for treating specific non-cognitive NPS, such as psychosis and/or severe agitated behavior that causes significant distress. Atypical antipsychotics,
The mood stabilizers valproate
Continue to: Evidence for dementia-specific medications
Evidence for dementia-specific medications
An alternative to the above pharmacologic options is treatment with a cholinesterase inhibitor and/or memantine. Among cholinesterase inhibitors
Few randomized controlled trials (RCTs) of cholinesterase inhibitors or memantine have focused on improvement of NPS as a primary outcome measure, but some RCTs have used treatment of NPS as a secondary outcome.4 Most RCT data for using medications for NPS have come from small studies that lasted 17 days to 28 weeks and had design limitations. Most meta-analyses and review articles exclude trials if they do not evaluate NPS as a primary outcome, and most RCTs have only included NPS as a secondary outcome. We hypothesize that this is because NPS is conceptualized as a psychiatric condition, while dementia is codified as a neurologic condition. The reality is that dementia is a neuropsychiatric condition. This artificial divergence complicates both the evaluation and treatment of patients with dementia, who almost always have NPS. Medication trials focused on the neurologic components for primary outcomes contribute to the confusion and difficulty of building an evidence base around the treatment of NPS in Alzheimer’s disease. Patients with severe NPS are seldom included in RCTs.
A cholinesterase inhibitor, memantine, or both?
In the absence of extended RCTs, attention turns to the opinions of panels of experts examining available data.
The 2012 Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia12 recommended a trial of a cholinesterase inhibitor in most patients with Alzheimer’s disease or Alzheimer’s disease combined with another type of dementia. The panel did not find enough evidence to recommend for or against the use of cholinesterase inhibitors and/or memantine for the treatment of NPS as a primary indication. However, they warned of the risks of discontinuing a cholinesterase inhibitor and suggested a slow taper and monitoring, with consideration of restarting the medication if there is notable functional or behavioral decline.
Continue to: In 2015, the European Neurological Society and the European Federation of Neurological Societies...
In 2015, the European Neurological Society and the European Federation of Neurological Societies (now combined into the European Academy of Neurology) found a moderate benefit for using cholinesterase inhibitors to treat problematic behaviors in patients with Alzheimer’s disease.13 They found the evidence weak only when they included consideration of cognitive benefits. For patients with moderate to severe Alzheimer’s disease, the Academy endorsed the combination of cholinesterase inhibitors and memantine.13
The United Kingdom National Institute for Clinical Excellence (NICE) guideline on dementia is updated every 1 to 3 years based on evolving evidence for the treatment of Alzheimer’s disease and related symptoms. In 2016, NICE updated its guideline to recommend the use of a cholinesterase inhibitor for patients with mild to severe Alzheimer’s disease and memantine for those with severe Alzheimer’s disease.14 NICE specifically noted that it could not endorse the use of a cholinesterase inhibitor for severe dementia because that indication is not approved in the United Kingdom, even though there is evidence for this use. The NICE guidelines recommend use of cholinesterase inhibitors for the non-cognitive and/or behavioral symptoms of Alzheimer’s disease, vascular dementia, or mixed dementia after failure or intolerance of an antipsychotic medication. They recommend memantine if there is a failure to respond or intolerance of a cholinesterase inhibitor. The NICE guideline did not address concomitant use of a cholinesterase inhibitor with memantine.
The 2017 guideline published by the British Association for Psychopharmacology states that combination therapy (a cholinesterase inhibitor plus memantine) “may” be beneficial. The group noted that while studies were well-designed, sample sizes were small and not based on clinically representative samples.15
Both available evidence and published guidelines suggest that combination treatment for moderate to severe Alzheimer’s disease may slow the worsening of symptoms or prevent the emergence of NPS better than either medication could accomplish alone. Slowing symptom progression could potentially decrease the cost of in-home care and delay institutionalization.
For a patient prescribed combination therapy, the cost of treatment with generics (as of June 2018) could range from approximately $120 per year for donepezil, 10 mg/d, and approximately $180 per year for memantine, 10 mg twice daily, taken by mouth.16 The cost of a once-daily capsule that contains a combination pill of donepezil and memantine is much more because this product is not available generically.
The Donepezil and Memantine in Moderate to Severe Alzheimer’s disease (DOMINO-AD) trial assessed the effect of combination therapy on cognition, activities of daily living, and health-related quality of life, as well as the cost efficacy of the combined treatment.17 In the 52-week study, researchers found that combined donepezil and memantine was not more cost-effective than donepezil alone. However, a post hoc analysis of the DOMINO-AD data combined with the Memantine Clinical Trial Program data found benefits across multiple clinical domains.18
Continue to: Don't overlook nonpharmacologic interventions
Don’t overlook nonpharmacologic interventions
Families caring for a loved one with Alzheimer’s disease face many decisions. Regardless of when in the course of the disease the diagnosis occurs, its pronouncement is followed by a complex and often emotional negotiation process that includes identifying community resources, making care arrangements, and legal and financial planning. This work may take place concurrently with the exhausting physical care that often comes with the job of a caregiver. As the disease progresses, the physical, emotional, and financial stress on the family increases.
Because they may be pressed for time, have limited staff support, or have limited knowledge of community resources, physicians unfamiliar with the treatment of Alzheimer’s disease may focus on prescribing pharmacologic interventions rather than providing education, resources, and referrals. This approach may lead caregivers to unrealistic expectations of medications in lieu of beneficial environmental and behavioral interventions for NPS. For a family attempting to provide home care for a patient with Alzheimer’s disease, improved behavior may lead to improved quality of life—both for those with dementia and their caregivers. Further, environmental and behavioral interventions could also slow the speed of functional decline and decrease NPS.
Despite the quality of the small studies we examined, without replication in diverse populations that reflect patients seen in everyday clinical practice, it is difficult to know which patients will benefit from combination therapy. The goal of evidence-based medicine is to use evidence gathered from patients who are similar to those that the physician is treating. To evaluate the evidence base around the use of dementia-specific medications and the impact on patients with dementia, additional RCTs, longitudinal data, and secondary outcomes are needed. However, even without this evidence, currently available data should not be ignored. This is part of the evolution of the evidence base.
Bottom Line
For treatment of neuropsychiatric symptoms (NPS) in patients with dementia, evidence supports monotherapy with a cholinesterase inhibitor for patients with mild to moderate dementia, and memantine for those with moderate to severe dementia. The use of these agents results in moderate improvements in NPS. Combination of a cholinesterase inhibitor and memantine increasingly appears to offer benefit.
Related Resources
- Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing textbook of geriatric psychiatry, 5th ed. Arlington, Virginia: American Psychiatric Association; 2015.
- Jacobson SA. Clinical manual of geriatric psychopharmacology, 2nd ed. Washington, DC: American Psychiatric Publishing; 2014.
- Fitzpatrick JL. Cruising through caregiving. Austin, Texas: Greenleaf Book Press; 2016.
- Snow T. Positive approach to care. Techniques and training for families and professionals working with persons with cognitive impairment. www.teepasnow.com.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Donepezil • Aricept
Donepezil/memantine • Namzaric
Fluoxetine • Prozac, Sarafem
Galantamine • Razadyne
Lithium • Eskalith, Lithobid
Memantine • Namenda
Olanzapine • Zyprexa
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Valproate • Depakote
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
1. Alzheimer’s Association Report. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367-429.
2. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi:10.1136/bmj.h369.
3. Nowrangi MA, Lyketsos CG, Rosenberg PB. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res Ther. 2015;7(1):12. doi: 10.1186/s13195-015-0096-3.
4. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia. JAMA. 2005;293(5):596-608.
5. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
6. Aricept [package insert]. Woodcliff Lak, NJ: Eisai Inc.; 2016.
7. Razadyne [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2016.
8. Exelon [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016.
9. Namenda [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
10. Namenda XR [package insert]. Irvine, CA: Allergan USA, Inc.; 2016.
11. Atri A, Hendrix SB, Pejovic
12. Gauthier S, Patterson C, Chertkow H, et al; CCCDTD4 participants. 4th Canadian consensus conference on the diagnosis and treatment of dementia. Can J Neurol Sci. 2012;39(6 suppl 5):S1-S8.
13. Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol. 2015;22(6):889-898.
14. National Collaborating Centre for Mental Health (UK). Dementia: supporting people with dementia and their carers in health and social care. www.nice.org.uk/guidance/cg42. Updated September 2016. Accessed May 31, 2018.
15. O’Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147-168.
16. GoodRx. https://www.goodrx.com. Accessed May 31, 2018.
17. Knapp M, King D, Romeo R, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry. 2017;32(12):1205-1216.
18. Hendrix S, Ellison N, Stanworth S, et al. Post hoc evidence for an additive effect of memantine and donepezil: consistent findings from DOMINO-AD study and Memantine Clinical Trial Program. J Prev Alzheimers Dis. 2015;2(3):165-171.
Complementary treatments for anxiety: Beyond pharmacotherapy and psychotherapy
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.
Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.
Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.
Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
10 Myths about ECT
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
As evidence supporting the use of electroconvulsive therapy (ECT) to treat patients with depression and other psychiatric illnesses continues to grow, myths about this treatment persist. In light of these myths, patients might be reluctant to receive ECT. As clinicians, we need to educate patients about the safety and effectiveness of this treatment. Here are 10 of the most commonly held myths about ECT, and why each is a misconception.
1. It is a barbaric treatment. ECT is conducted in a controlled medical environment, either during a hospitalization or as an outpatient procedure, by a team consisting of a psychiatrist, anesthesiologist, and nurse. Patients receive a short-acting intravenous anesthetic to ensure that they are unaware of the procedure, and a muscle relaxant to help prevent physical injury. Vital signs and brain waves are monitored throughout the procedure, which typically lasts 15 to 20 minutes. Patients remain relaxed, are unaware that they are having a seizure, and experience no pain. Following ECT, the patient is taken to a recovery area, where he or she is closely monitored as the medications wear off.
2. It causes brain damage. Studies using MRI to look at the brain before and after ECT have found no evidence that ECT causes negative changes in the brain’s structural anatomy.1 To the contrary, there is evidence that there is neuroplasticity in the brain in response to ECT, and the neurotrophin brain-derived neurotrophic factor also may be increased.2,3
3. It causes permanent memory loss. ECT can result in both anterograde and retrograde memory impairment; however, anterograde amnesia typically lasts only days to weeks. Retrograde amnesia is much less common, but when it occurs, it tends to be loss of memory of events that took place in the weeks leading up to and during treatment. Using an ultrabrief (as opposed to standard brief) pulse, as well as right unilateral (as opposed to bilateral) electrode placement, substantially reduces the risk of cognitive and memory adverse effects.4
4. It is a treatment of last resort. Typically, ECT is used for patients who have not responded to other interventions. However, ECT can be used as a first-line treatment for patients if a rapid or higher likelihood of response is necessary, such as when a patient is suicidal, catatonic, or malnourished as a result of severe depression.5
5. It only works for depression. Evidence shows ECT is efficacious for several psychiatric conditions, not just unipolar depressive disorder. It can effectively treat bipolar depression, mania, catatonia, and acute psychosis associated with schizophrenia and schizoaffective disorders.6 ECT also has been demonstrated to be effective in acute and maintenance treatment of Parkinson’s disease.7
6. It is not safe. Death associated with ECT is extremely rare. A recent analysis estimated that the rate of ECT-related mortality is 2.1 deaths per 100,000 treatments. In comparison, the mortality rate of general anesthesia used during surgery has been reported as 3.4 deaths per 100,000 procedures.8 Evidence also suggests ECT can be safely administered to patients who are pregnant.9
Continue to: 7. It cannot be given to patients with epilepsy
7. It cannot be given to patients with epilepsy. There are no absolute contraindications to using ECT for these patients. Most patients with epilepsy can be successfully treated with ECT without requiring an adjustment to the dose of their antiepileptic medications.10
8. It will change one’s personality. ECT has not been found to cause any alterations in personality. Patients who are treated with ECT may describe feeling more like themselves once their chronic symptoms of depression have improved. However, ECT has not been shown to effectively treat the symptoms or underlying illness of personality disorders, and it may not be an effective treatment for depression associated with borderline personality disorder.11
9. Its success rate is low. ECT has the highest response and remission rates of any form of treatment used for depression. An estimated 70% to 90% of patients with depression who are treated with ECT show improvement.12
10. It is a permanent cure. ECT is not likely a permanent solution for severe depression. The likelihood of relapse in patients with severe depression who are helped by ECT can be reduced by receiving ongoing antidepressant treatment, and some patients may require continuation or maintenance ECT.13
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.
1. Scott AI, Turnbull LW. Do repeated courses of ECT cause brain damage detectable by MRI? Am J Psychiatry. 1990;147(3):371-372.
2. Sartorius A, Demirakca T, Böhringer A, et al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26(3)506-517.
3. Bocchio-Chiavetto L, Zanardini R, Bortolomasi M et al. Electroconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620-624.
4. Sackeim HA, Prudic J, Nobler MS, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71-83.
5. American Psychiatric Association. The practice of electroconvulsive therapy: recommendations for treatment, training, and privileging: a task force report of the American Psychiatric Association, 2nd edition. Washington, DC: American Psychiatric Association; 2001.
6. Fontenelle LF, Coutinho ES, Lins-Martins NM, et al. Electroconvulsive therapy for obsessive-compulsive disorder: a systematic review. J Clin Psychiatry. 2015;76(7):949-957.
7. Narang P, Glowacki A, Lippmann S. Electroconvulsive therapy intervention for Parkinson’s disease. Innov Clin Neurosci. 2015;12(9-10):25-28.
8. Tørring N, Sanghani SN, Petrides G, et al. The mortality rate of electroconvulsive therapy: a systematic review and pooled analysis. Acta Psychiatr Scand. 2017;135(5):388-397.
9. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88.
10. Lunde ME, Lee EK, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
11. Feske U, Mulsant BH, Pilkonis PA, et al. Clinical outcome of ECT in patients with major depression and comorbid borderline personality disorder. Am J Psychiatry. 2004;161(11):2073-2080.
12. Kellner CH, McClintock SM, McCall WV, et al; CORE/PRIDE Group. Brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy (ECT) for major depression: efficacy, effectiveness, and cognitive effects. J Clin Psychiatry. 2014;75(7):777.
13. Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474.